Abstract
Streptococcus pyogenes is a globally prominent human-specific pathogen responsible for an enormous burden of human illnesses, including >600 million pharyngeal and >100 million skin infections each year. Despite intensive efforts that focus on invasive indications, much remains unknown about this bacterium in its natural state during colonization of the nasopharynx and skin. Using acute experimental infection models in HLA-transgenic mice, we evaluated how the hyaluronic acid (HA) capsule contributes to S. pyogenes MGAS8232 infection within these limited biological niches. Herein, we demonstrate that HA capsule expression promotes bacterial burden in murine nasal turbinates and skin lesions by resisting neutrophil-mediated killing. HA capsule production is encoded by the hasABC operon and compared to wildtype S. pyogenes infections, mice infected with a ΔhasA mutant exhibited over a 1000-fold CFU reduction at 48-hours post-nasal challenge, and a 10,000-fold CFU reduction from skin lesions 72-hours post-skin challenge. HA capsule expression contributed substantially to skin lesion size development following subdermal inoculations. In the absence of capsule expression, S. pyogenes revealed drastically impeded growth in whole human blood and increased susceptibility to killing by isolated neutrophils ex vivo, highlighting its important role in resisting phagocytosis. Furthermore, we establish that neutrophil depletion in mice recovered the reduced burden by the ΔhasA mutant in both the nasopharynx and skin. Together, this work confirms that the HA capsule is a key virulence determinant during acute infections by S. pyogenes and demonstrates that its predominant function is to protect S. pyogenes against neutrophil-mediated killing.
Author summary
Streptococcus pyogenes is a globally disseminated and human-adapted bacterial pathogen that has evolved an arsenal of evasion strategies to overcome and escape host immune clearing mechanisms. Many strains of S. pyogenes are covered by a polysaccharide capsule composed of hyaluronic acid (HA) that is widely recognized to promote severe infections. In this study, we demonstrate using the encapsulated S. pyogenes MGAS8232 strain that the HA capsule is a key virulence factor that facilitates non-invasive infections of the nasopharynx and skin. Although bacterial adhesion and entry into host cells was impeded by HA capsule expression, we show that the key function for both nasal and skin infections is to protect S. pyogenes from neutrophil-mediated killing. Depletion of neutrophils recovered the low bacterial burden by unencapsulated S. pyogenes at both sites of infection. Our findings outline an important interaction between the HA capsule and neutrophils in the establishment of acute upper respiratory and skin infections by S. pyogenes.
Introduction
Streptococcus pyogenes (the group A Streptococcus) is a globally prominent, human-adapted bacterial pathogen responsible for an enormous burden of disease [1]. While S. pyogenes exists primarily as an asymptomatic commensal in up to 12% of school-aged children [2], more than 600 million cases of pharyngitis and 100 million cases of skin infections are recorded each year, and at least 18.1 million people worldwide currently suffer from serious post-infection sequelae resulting in over 500,000 annual deaths [1].
Many strains of S. pyogenes produce a high molecular weight hyaluronic acid (HA) polysaccharide capsule that presents distinct mucoid colony morphology when grown on solid media. The HA capsule is composed of repeating disaccharide units of glucuronic acid and N-acetylglucosamine (GlcNAc) and is structurally identical to HA found within the human extracellular matrix (ECM), and therefore, is immunologically inert [3]. Capsule production is encoded by the hasABC genetic locus involved in HA biosynthesis [4–6]. The first gene in the operon, hasA, encodes hyaluronate synthase [7,8]; the second gene, hasB, encodes UDP-glucose 6-dehydrogenase [9]; and the third gene, hasC, encodes UDP-glucose pyrophosphorylase [10]. Although proteins encoded by hasB and hasC are enzymatically active, they are not individually essential for HA capsule synthesis [11,12]. Interestingly, expression of the hasA gene is the only fundamental gene required for the production of the HA polymer from UDP-glucuronic acid and UDP-GlcNAc sugar precursors [7]. The amount of capsule produced can vary widely among individual strains, regulated by growth conditions and in response to changes in the host environment. Maximal HA capsule production occurs during early and mid-exponential phase in vitro, followed by capsule shedding during stationary phase [5]. These observations are further supported in vivo as introduction of S. pyogenes into the pharynx of non-human primates or into the mouse peritoneum induces high levels of HA capsule gene transcription within 1–2 hours of inoculation [13], suggesting that capsule expression has an important function during initial stages of colonization.
Early pioneering studies using encapsulated M18 and M24 serotypes revealed that transposon mutants lacking the HA capsule had ~100-fold increases in the LD50 using invasive intraperitoneal infections in CD1 mice [14–16], and further discovered that capsule expression was strongly selected for during pharyngeal colonization of BALB/c mice [15]. Following intratracheal inoculation of the mouse-adapted B514 S. pyogenes strain, the HA capsule promoted chronic throat colonization, pneumonia, and secondary systemic infections in C57BL/10SnJ mice [17]. Furthermore, encapsulation was shown to enhance persistent colonization of S. pyogenes in the pharynx of baboons as unencapsulated mutants were cleared more quickly [18]. Together, encapsulation appears to offer S. pyogenes a powerful survival advantage for colonization and dissemination.
Prior studies have shown that the HA capsule binds to the cell surface ligand CD44 to mediate adherence to epithelium [19,20], which can induce host cell signaling events that disrupts tight junctions to promote invasion [21]. Furthermore, the HA capsule can also specifically bind to lymphatic vessel endothelial receptor-1 (LYVE-1) expressed in lymph node sinuses and lymphatic vessles to promote dissemination to draining lymph nodes via the lymphatic system using a mouse thigh muscle infection model [22]. However, reduced binding efficiencies by encapsulated strains have also been observed [19,23]. Removal of the capsule by genetic inactivation of the has operon can also promote robust invasion of cultured epithelial cells, although once internalized, S. pyogenes is rapidly killed [24]. By producing a molecule ubiquitously expressed by its host, molecular mimicry enables S. pyogenes to avoid detection by host immune surveillance and increases resistance to phagocytic-mediated killing. In several experiments, unencapsulated mutants display significant susceptibility to complement-dependent phagocytic killing by human blood compared to their encapsulated parental strains [16,25,26].
Although nearly all S. pyogenes strains encode the has operon, the capsule is not universally present in all isolates. For example, M4 and M22 serotypes, and some M89 serotypes, do not contain the hasABC operon and thus cannot express HA capsule [27,28], suggesting that capsule expression is not essential for pathogenicity across all serotypes. Furthermore, studies in human carriers and other primate models have also identified mutations that reduced or eliminated capsule production in long-term carriage isolates [29,30]. Therefore, differential regulation of the HA capsule may consequently offer an important survival adaptation in specific host environments. Thus, while it is recognized that encapsulation may be advantageous for bacterial virulence, mechanisms whereby it promotes acute S. pyogenes infections in vivo merit further investigation [31]. In this work, we aimed to further evaluate the role of the HA capsule in two non-invasive murine infection models using a precise genetic deletion of the hasA gene. Herein, we demonstrate using the encapsulated S. pyogenes MGAS8232 strain that the HA capsule is a key virulence factor for non-invasive nasopharyngeal and skin infections. Though removal of the capsule permitted bacterial invasion into host cells, we demonstrate that the key function for both in vivo nasal and skin infections is to protect S. pyogenes from neutrophil-mediated killing.
Results
The S. pyogenes HA capsule promotes nasopharyngeal infection and enhances a cytokine response that supports the recruitment of neutrophils
In order to evaluate the influence of the HA capsule during experimental infections, we used the pG+host5 integration plasmid [32] (Table 1) to generate a markerless 1,212-bp in-frame deletion of hasA in the M18 serotype rheumatic fever isolate S. pyogenes MGAS8232 [33] (Table 1). M18 serotypes are well known for being highly encapsulated, a phenotype that has been traced to the mutations within the RocA regulatory protein [34]. The isogenic nature of the hasA mutant was determined by PCR and DNA sequencing with primers that flanked the deleted hasA region (S1 Table) and whole genome sequencing analysis. The correct ΔhasA deletion was confirmed, but compared to the wildtype MGAS8232 strain, two non-synonymous single nucleotide polymorphisms (SNPs) within pstI gene encoding the cytosolic protein enzyme I in the phosphoenolpyruvate phosphotransferase system (PTS) were also identified and resulted in two amino acid substitutions (Leu194Phe and Ser306Phe) (S2 Table). Thus, we generated a complementation strain using the pDCerm plasmid [35] (S1 Table) by expressing the hasA gene and its native hasA promoter in the ΔhasA mutant background (MGAS8232 ΔhasA + hasA). As predicted, MGAS8232 ΔhasA lost the large mucoid colony phenotype on sheep blood agar compared to wildtype MGAS8232, and capsule production was restored in the hasA-complemented strain (Fig 1A).
Table 1. Bacterial strains and plasmids used in this study.
| Strain/plasmid | Description | Source |
|---|---|---|
| Streptococcus pyogenes | ||
| MGAS8232 | M18 serotype isolated from a patient with acute rheumatic fever (GenBank accession: NC_003485.1) | [33] |
| MGAS8232 ΔhasA | hasA deletion mutant derived from MGAS8232 | This study |
| MGAS8232 ΔhasA + hasA | MGAS8232 ΔhasA containing complementation plasmid pDCerm expressing the hasA gene | This study |
| Escherichia coli | ||
| XL1-Blue | General cloning strain | Stratagene |
| Plasmids | ||
| pG+host5 | Temperature-sensitive Gram-positive/E. coli shuttle vector; Ermr | [32] |
| pG+host5::ΔhasA | pG+host5 with hasA flanking regions inserted | This study |
| pDCerm | Ermr derivative of streptococcus-E. coli shuttle vector pDC123 (erm of Tn916ΔE) replacing cat | [35] |
| pDCerm::hasA | pDCerm expressing hasA gene for plasmid-based complementation | This study |
Ermr—erythromycin resistance
cat—chloramphenicol acetyltransferase gene
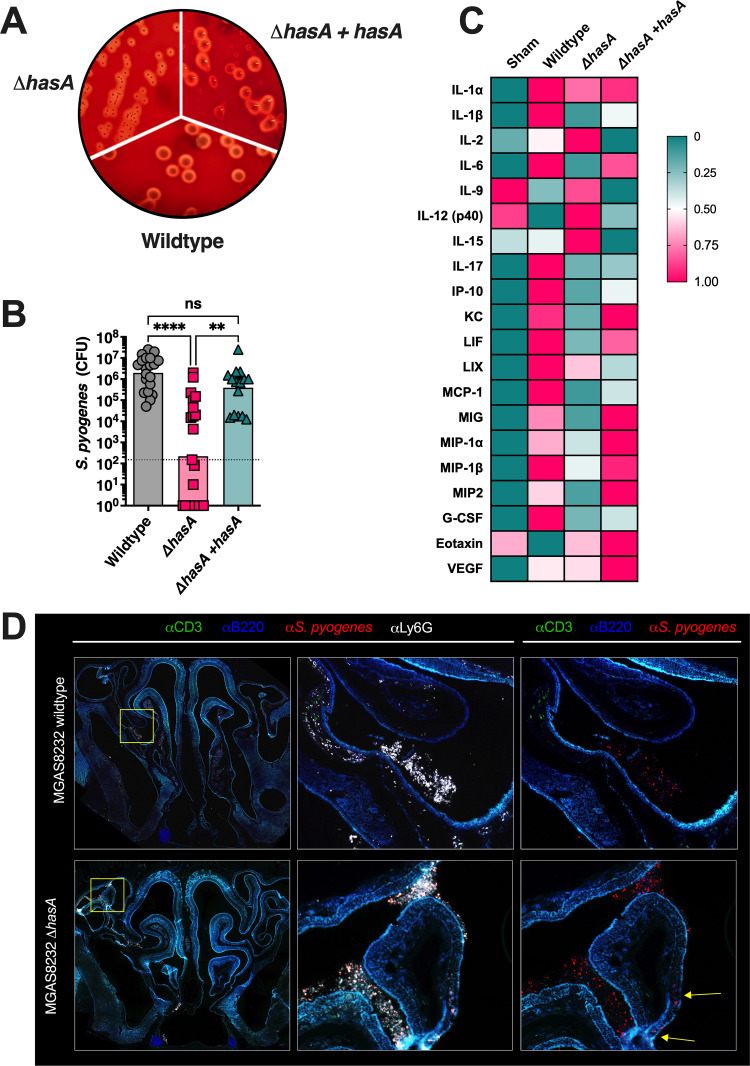
Fig 1. Hyaluronic acid expression by Streptococcus pyogenes promotes nasal infection in B6HLA mice.
(A) S. pyogenes constructs streaked onto TSA + 5% sheep blood agar plates. The plate figure are representative images of the wild-type MGAS8232, ΔhasA mutant and the hasA complemented strain. (B) B6HLA mice were administered ~1×108 CFUs of S. pyogenes MGAS8232 wildtype, ΔhasA, or ΔhasA +hasA strains intranasally and sacrificed 48 h later. Data points represent CFUs from cNTs of individual B6HLA mice. Horizontal bars represent the geometric mean. Significance was determined by Kruskal Wallis one-way ANOVA with Dunn’s multiple comparisons test (****, P < 0.0001; **, P < 0.01). The horizontal dotted line indicates the theoretical limit of detection. (C) Heat-map of cytokine responses in cNTs of B6HLA mice during S. pyogenes infection. Data shown represent normalized median cytokine responses from cNTs (n ≥ 3 per group). (D) Immunohistochemistry of infected cNTs at 24 h post-infection with wildtype S. pyogenes MGAS8232 and the ΔhasA mutant. Sections were stained with α-S. pyogenes (red), αB220 (blue), αCD3 (green), and αLy6G (white) antibodies. Panels are a close-up view from the boxed section. Arrows indicate regions with internalized S. pyogenes.
The human-specific tropism of S. pyogenes represents a major challenge when conducting experimental infection models. We previously demonstrated that the use of transgenic mice that express human MHC class II molecules (herein referred to as B6HLA mice) greatly enhances S. pyogenes nasopharyngeal infection due to the selective specificity of superantigens for human MHC class II molecules [36]. Using this infection model, we examined the influence of HA capsule expression on bacterial burden during acute nasopharyngeal infection through nasal inoculation (~1×108 CFUs) of B6HLA mice using the three MGAS8232 strains. The ΔhasA mutant resulted in a >1000-fold reduction in bacterial CFUs from the nasal mucosa at 48 hours compared to infection by wildtype S. pyogenes MGAS8232 (Fig 1B). We confirmed that capsule expression was specifically required for the nasopharyngeal infection phenotype as the complemented strain with restored capsule expression phenocopied the wildtype infection (Fig 1B). Consistent with the non-invasive nature of the model [36], mean bacterial dissemination of wildtype S. pyogenes remained below the limit of detection in the lungs, liver, spleen, heart, and kidneys (S1 Fig). We conclude that HA capsule expression improves experimental S. pyogenes nasopharyngeal infection in B6HLA mice, and removal of the capsule does not increase bacterial dissemination of S. pyogenes MGAS8232.
To further assess the nasopharyngeal environment during wildtype S. pyogenes and ΔhasA infections, we conducted a multiplex cytokine and chemokine array using infected nasal turbinate homogenates [37]. Quantitative data is shown in supplemental figures (S2 Fig) and summarized as a heatmap showing normalized cytokine responses for any cytokine with an average concentration above 20 pg ml-1 within a treatment group (Fig 1C). Uninfected mice demonstrated no apparent inflammatory signature from the complete nasal turbinate (cNT) homogenates whereas robust cytokine responses were evident in wildtype-infected mice and included: Th1-type cytokines (IL-1α and IL-1β); Th17-type cytokines (IL-6 and IL-17); chemokines (KC, IP-10, MCP-1, MIP-1α, MIP-1β, MIG, MIP-2, LIF and LIX); and growth factors (G-CSF) (Figs 1C and S2). In contrast, ΔhasA-infected cNTs presented a reduced inflammatory signature and revealed similar cytokine expression profiles as uninfected mice. Particularly, reductions were detected with pro-inflammatory cytokines, such as IL-1β, IL-6, and IL-17, and those involved in monocyte and neutrophil recruitment, including KC, IP-10, MCP-1, MIP-1β, MIG, and G-CSF (Figs 1C and S2). Restoring capsule expression in the ΔhasA mutant background induced a moderately inflamed environment, trending for greater concentrations of KC, MIP-1α, MIG, MIP-2, and G-CSF compared to ΔhasA mutant infections. Interestingly, IL-2, IL-12 (p40), IL-15, and IL-9 concentrations were higher in cNTs challenged with ΔhasA mutant compared to wildtype or ΔhasA + hasA strains; however, concentrations for these cytokines did not drastically differ from uninfected murine cNTs (S2 Fig). There were also numerous cytokines that did not have average concentrations above 20 pg ml-1 in any treatment groups, were not different between treatment groups, or did not conform to any obvious trends (S2 Fig). These cytokine trends suggest that at 48 hours post-nasal inoculation with S. pyogenes, HA capsule expression is associated with higher concentrations of cytokines and chemokines that support inflammation and monocyte and neutrophil function.
To gain further insight into the interaction between S. pyogenes and host immune cells during nasopharyngeal infection, wildtype MGAS8232 and ΔhasA-infected cNTs were harvested and cryopreserved for immunohistochemistry. Due to the possibility that ΔhasA mutant could be completely cleared by 48 hours post-infection (Fig 1B), infected cNTs were collected at 24 hours post-infection and sections were stained with α-S. pyogenes (red), α-B220 (blue), α-CD3 (green), and α-Ly6G (white) fluorescent antibodies where indicated. Sections revealed that S. pyogenes was present with robust α-Ly6G neutrophil signals in both wildtype and ΔhasA-infected cNTs (Fig 1D). By 24 hours, neutrophils infiltrated to the central areas where S. pyogenes resided, whether the HA capsule was expressed or not (Fig 1D). Although few differences in immune cell percentages have been detected within the cNTs of wildtype-infected B6HLA mice by 48 hours [36], infected nasal passages have demonstrated increased trends for neutrophil populations (GR1+) during wildtype S. pyogenes MGAS8232 infection [36], entirely consistent with these immunofluorescence experiments. Notably, S. pyogenes ΔhasA, but not wildtype, was detected within the epithelial cell layer in some areas (Fig 1D, denoted by arrows in the right panel). Together, these observations are consistent with a model whereby neutrophils are recruited to murine cNTs by 24 hours post-nasal infection with either wildtype MGAS8232 or ΔhasA strains; however, by 48 hours ΔhasA mutants are rapidly cleared and cannot remodel the nasopharynx to express favourable inflammatory responses.
The hyaluronic acid capsule blocks S. pyogenes invasion of pharyngeal cells and protects against killing by neutrophils in vitro
If S. pyogenes successfully escapes mucocilliary clearance, it proceeds to target and adhere to the underlying epithelial surface. Multiple studies have described the HA capsule as an important adhesin [19,20], and we next sought to evaluate and compare adherence capabilities of wildtype S. pyogenes MGAS8232 with its unencapsulated mutant. Collagen type IV and fibronectin make up a significant portion of the nasopharyngeal ECM, and therefore, bacterial binding to these structures may contribute to streptococcal infection [38–41]. Collagen type IV is the primary component of the ECM basement membrane that underlays epithelial cells, and fibronectin, while only a minor component, is frequently secreted to mediate adhesion and migration of host cells [42,43]. Bacterial binding was assessed by inoculating S. pyogenes onto wells pre-coated with either collage type IV or fibronectin. A decline in both fibronectin binding and collagen type IV binding was observed for the ΔhasA mutant (Fig 2A and 2B) demonstrating that under in vitro growth conditions, S. pyogenes HA capsule can likely adhere to the ECM through interactions with both collagen type IV and fibronectin.
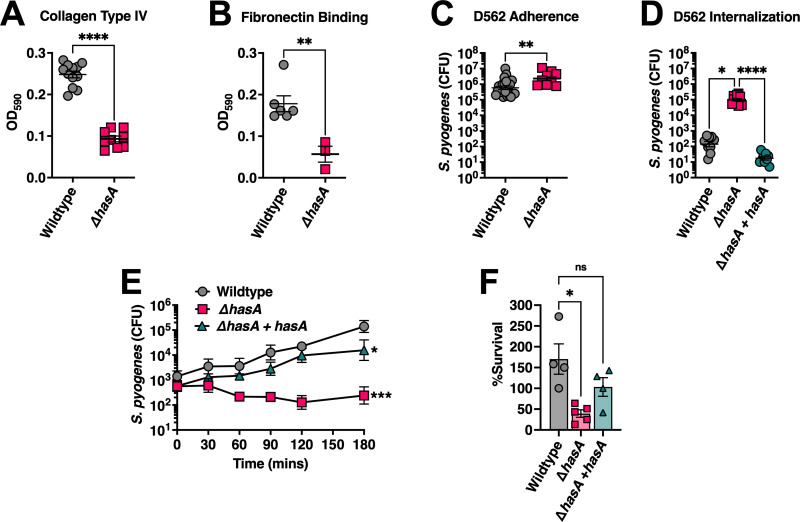
Fig 2. The S. pyogenes HA capsule inhibits host cell invasion but promotes survival from neutrophil-mediated killing.
Binding of S. pyogenes to wells pre-coated with 1 μg of human ECM components (A) fibronectin and (B) collagen type IV. (C) Adhesion of S. pyogenes to D562 pharyngeal epithelial cells. Confluent cell monolayers were cultured with S. pyogenes (MOI of 100) for 2 h at 37°C + 5% CO2. Cells were washed with PBS and lysed with Triton X-100 for enumerating remaining adherent bacteria. (D) Internalization of S. pyogenes into D562 cells. Confluent D562 cells were cultured with S. pyogenes (MOI of 100) for 2 h at 37°C + 5% CO2 followed by 1 h in media supplemented with 100 μg mL-1 of gentamycin. Bars represent mean CFUs ± SEM and each dot represents a biological replicate. Statistical differences were evaluated by unpaired t-test (A–C) (**, P < 0.01; ****, P < 0.0001) or (D) one-way ANOVA (*, P < 0.05; ****, P < 0.0001). (E) Whole human blood survival assay. Heparinized blood from human donors were inoculated with ~103 CFUs of S. pyogenes MGAS8232 at 37°C with rotation for 3 h. Data points represent geometric mean CFUs ± SD at each timepoint (n ≥ 3). Statistical significance was determined using one-way ANOVA with Friedman test (***, P < 0.001). (F) Neutrophil survival assay. Neutrophils were isolated from human blood by density centrifugation and inoculated with opsonized S. pyogenes at a MOI of 10. Surviving bacteria were enumerated after 60 mins at 37°C with rotation and calculated as the difference between survival in the no neutrophil control and in the presence of neutrophils. Each data point represents S. pyogenes CFUs from an individual donor. Data shown are the means of percent survival ± SD. Statistical analyses were performed using one-way ANOVA with Kruskal-Wallis test (*, P < 0.05).
Bacterial adherence was also evaluated using the pharyngeal cell line Detroit-562 (D562) due to its similarity of surface molecules with non-transformed pharyngeal cells [43], and its ability to induce streptococcal superantigens and DNAses that are otherwise weakly expressed by S. pyogenes [44,45]. There was a slight but statistically significant increase in the amount of S. pyogenes that adhered to D562 cells when HA capsule expression was absent (Fig 2C). Despite the ability to bind collagen type IV and fibronectin, these results conflict with reports indicating the HA capsule contributes substantially to bacterial adhesion properties, and suggests that the HA capsule may function in part to mask adhesins on the bacterial cell wall and obstruct adherence, at least with S. pyogenes MGAS8232 [15,20]. Next, we aimed to characterize the capacity of these constructs for epithelial cell internalization. We found a dramatic ~1000-fold increase in ΔhasA mutants recovered from lysed D562 cells following gentamycin treatment (Fig 2D). Given this dramatic phenotype, we also evaluated the capsule complemented strain (ΔhasA + hasA), which completely lost the invasion phenotype (Fig 2D). These data demonstrate that HA capsule expression by S. pyogenes MGAS8232 inhibits adhesion and represses internalization into pharyngeal epithelial cells.
Following attachment to epithelial cell surfaces, a critical mechanism during early colonization stages is to evade host immune responses. S. pyogenes MGAS8232 resistance to bacteriolysis was investigated to determine if the HA capsule improves immune evasion. Compared with wildtype S. pyogenes MGAS8232, growth and survival in whole human blood was markedly attenuated (~3 logs) in the absence of capsule expression (Fig 2E). Upon earlier histological assessment of infected nasal turbinates, neutrophils had accumulated in regions surrounding both wildtype S. pyogenes and ΔhasA at 24 hours post-infection, yet the ΔhasA strain had significantly less bacterial burden by 48 hours (Fig 1B and 1C). Furthermore, a significant decline in cytokines and chemokines involved in recruiting, modulating, and activating neutrophils were detected by 48 hours with ΔhasA infection (Figs 1C and S2). Therefore, we next sought to examine if the HA capsule resists neutrophil activity specifically. Unencapsulated bacteria were more susceptible to neutrophil-mediated killing demonstrated by a significant decline in ΔhasA mutants that survived in the presence of freshly isolated human neutrophils (Fig 2F). Indeed, the reduced bacterial survival in each condition was rescued with complementation of capsule expression in the MGAS8232 ΔhasA + hasA strain (Fig 2E and 2F). These results confirm an important role for the capsule in promoting resistance to killing by neutrophils, and thus, may provide a protective role against innate immune responses during early stages of acute infection.
Depletion of neutrophils restores S. pyogenes ΔhasA bacterial load during nasopharyngeal infection
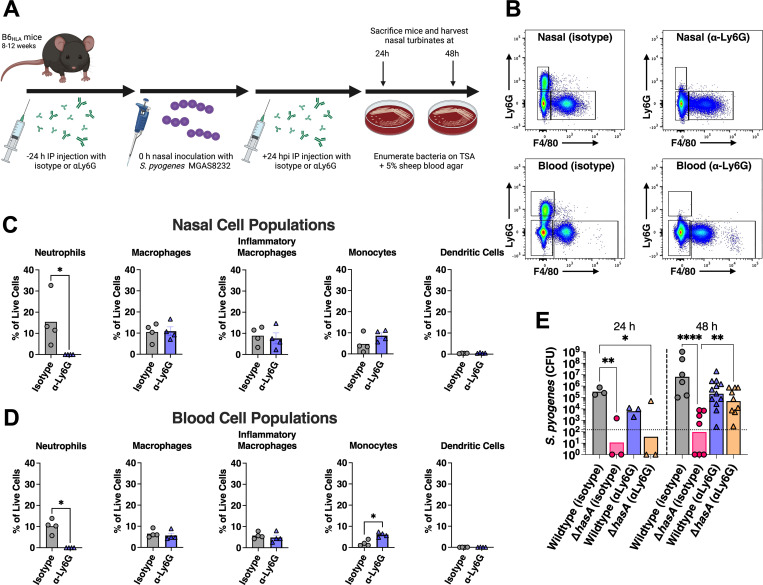
Preventing opsonophagocytic bacterial clearance is one of the main proposed mechanisms for the HA capsule and has been repeatedly investigated using various in vitro bacterial survival assays [16,25,26]. Since neutrophil influx is a major feature of our experimental nasopharyngeal model and during natural infections [46], we aimed to explore the importance of neutrophils during nasopharyngeal infection and determine whether preventing phagocyte-mediated killing is a key molecular process by which HA capsule functions in this model. For this purpose, mice were depleted of neutrophils by administering the αLy6G monoclonal antibody (Fig 3A), which effectively depletes neutrophils from the peripheral blood of mice [47], with rat IgG2a used as an isotype control. To confirm the neutrophil depletions, we assessed innate immune cell populations from both the nasal passages and blood at 48 hours in wildtype MGAS8232 infected mice. This protocol successfully eliminated virtually all Ly6G+ cells from both sites (Fig 3B) and did not alter macrophage, monocyte or dendritic cell populations in the nasal passage (Fig 3C), however, a small but statistical increase in monocyte population was observed in the blood (Fig 3D). We next examined the effect of depleting neutrophils on nasal challenges with wildtype MGAS8232 or ΔhasA strains at both 24- and 48-hours post-infection. Although there were trends for reductions in CFUs, there were no statistical differences in the amount of wildtype S. pyogenes recovered from neutrophil depleted mice compared to control mice at either 24- or 48-hours post-infection (24 h, p = 0.5802; 48 h, p = 0.2803), indicating that wildtype S. pyogenes MGAS8232 infection is not impacted by neutrophil depletion in this model (Fig 3E). As expected, isotype treated mice showed a reduction for the ΔhasA mutant at both 24- (p < 0.05) and 48-hours (p < 0.0001) compared to wildtype-infected control mice (Fig 3E). While there was no difference in ΔhasA mutant recovery between control and neutrophil depleted mice at 24 hours post-infection (p = 0.9808), ΔhasA mutant burden substantially increased (~3 logs) at 48 hours post-infection in neutrophil depleted mice compared to control mice (p < 0.05). By 48 hours post-infection, no statistical differences were observed between the amount of ΔhasA mutants recovered from neutrophil depleted mice and control mice receiving wildtype S. pyogenes (p = 0.0989). Overall, these findings suggest neutrophil-mediated clearance mechanisms contribute substantially to the lower burden of unencapsulated S. pyogenes in the nasopharynx.
Fig 3. Early clearance of the HA capsule-deficient mutant from murine nasal turbinates is due to enhanced susceptibility to neutrophil-mediated killing.
(A) Schematic outline for in vivo depletion of neutrophils with injections of 250 μg (500 μg total) of αLy6G or isotype control rat IgG2a 24 h prior to and 24 h post-intranasal challenge with 108 CFUs of S. pyogenes wildtype or ΔhasA mutant strains. (B) Representative flow cytometric analyses of nasal and blood innate immune cells from the neutrophil depletion experiments at 48 h. Flow plots show live cells that were negative for CD4, CD45R and CD19, and gates were set on Ly6G+ and F4/80- cells for neutrophils, and Ly6G- and F4/80+ for macrophage populations. Percentage of innate immune cell populations from either nasal cell extracts (C) or blood (D) for the indicated treatment groups as percentage of live cells. Data points represent individual mice and the bars represent the mean. Significance was determined by Mann-Whitney test (*, P < 0.05) (E) Neutrophil effects on S. pyogenes survival in the nasopharynx. Data points represent CFUs from cNTs of individual mice 24 and 48 h post-infection. Horizontal bars represent the geometric mean. The horizontal dotted line indicates limit of detection. Significance was determined by two-way ANOVA with Tukey’s multiple comparisons (*, P < 0.05; **, P < 0.01; ****, P < 0.0001). Fig 3A was created using Biorender.com.
Bacterial burden and lesion pathology during skin infection are enhanced by S. pyogenes hyaluronic acid capsule expression
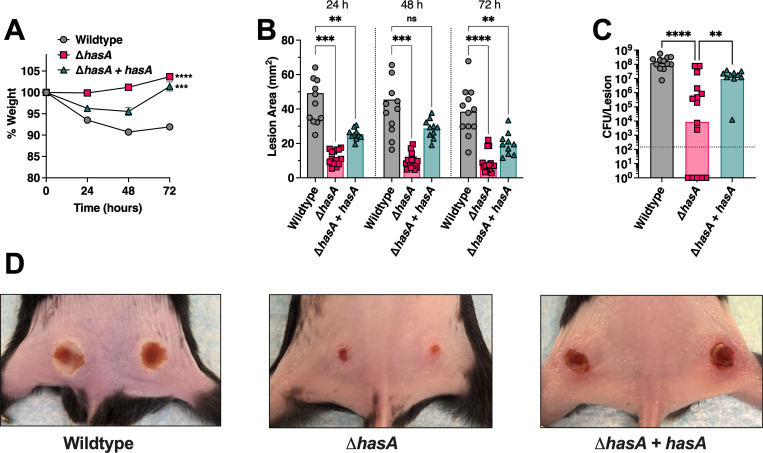
Pharyngeal colonization by S. pyogenes is believed to be the major reservoir for this pathogen in developed countries, yet skin infections (impetigo) tend to be more prevalent in resource-poor settings [48]. Since nasopharyngeal infection by S. pyogenes MGAS8232 was notably compromised by the loss of the HA capsule, we next performed a novel skin infection model to further assess whether capsule expression could promote experimental skin infection. To address this, B6HLA mice were intradermally injected in each hind flank with 2.5×107 CFUs of wildtype, ΔhasA, or ΔhasA + hasA strains. There was a ~10% decline in the weights of mice infected with wildtype S. pyogenes, a striking contrast to mice infected with the ΔhasA strain that gained weight over the 72 hour infection period (Fig 4A). The ΔhasA mutant strain revealed a clear reduction in virulence through considerably smaller lesions and less inflamed tissue over the infection period compared to wildtype-infected mice (Fig 4B and 4D). Significantly less bacterial CFUs were also recovered from each ΔhasA-infected lesion compared to wildtype-infected lesions (Fig 4C). Though weight loss and lesion sizes were only partially restored in ΔhasA + hasA-infected mice, bacterial CFUs recovered were fully complemented and did not differ from wildtype-infected mice (Fig 4B–4D). The moderate restoration of weight loss and lesion sizes in ΔhasA + hasA-infected mice may be due to the incomplete complementation of the hasA gene expressed in trans using the pDCerm plasmid, as erythromycin was not used topically to maintain plasmid expression and replication over the 72 hour infection period. This is supported by the observation of some capsule deficient reversion colonies on plated skin homogenates. Overall, these data demonstrate that the expression of the HA capsule in S. pyogenes supports virulence during acute skin infections in B6HLA mice.
Fig 4. Hyaluronic acid capsule expression by Streptococcus pyogenes promotes skin infection in B6HLA mice.
B6HLA mice were administered ~5 × 107 CFUs of wildtype S. pyogenes MGAS8232, or the hasA mutant, or the ΔhasA + hasA complemented strain by intradermal injections in each hind flank. (A) Weights of B6HLA mice at 24, 48, and 72 h following skin challenge. Data is represented as a percentage of day 0 weight. Data points represent the weight means ± SEM (n ≥ 5). (B) Skin lesion areas of mice at 24, 48, and 72 h after skin challenge. Data points represent individual lesion areas (2 per mouse), and the bars represent the mean. Significance was determined by two-way ANOVA with Geisser’s Greenhouse correction and Dunnett’s multiple comparisons test (*, P < 0.05, **, P < 0.01, ***, P < 0.001;) for panels A and B. (C) Data points represent CFUs from individual infected skin lesions from mice at 72 h. Horizontal bars represent the geometric mean. Significance was determined by one-way ANOVA with Kruskal-Wallis test (****, P < 0.0001; **, P < 0.01). The horizontal dotted line indicates the theoretical limit of detection. (D) Representative skin lesion images from B6HLA mice 72 h following skin challenge.
Depletion of neutrophils recovers S. pyogenes ΔhasA bacterial load during skin challenge
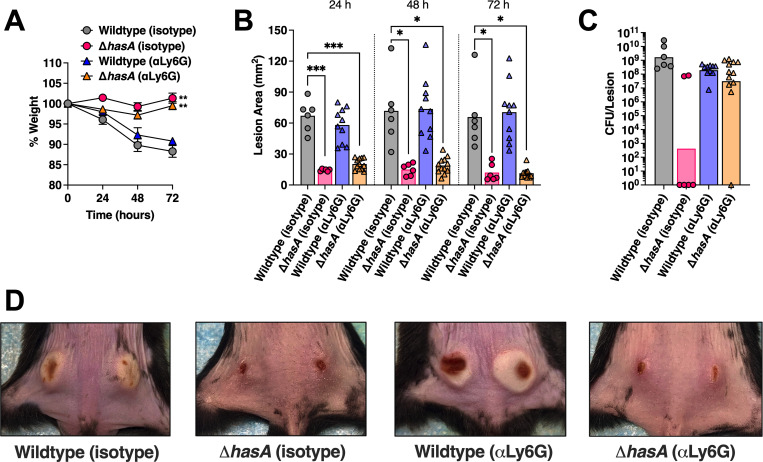
To examine whether a lack of neutrophils would similarly enhance infection by unencapsulated S. pyogenes in the skin, B6HLA mice were depleted of neutrophils as described above and challenged with subdermal infections with 2.5×107 CFUs wildtype or ΔhasA S. pyogenes MGAS8232. Irrespective of depletion status, mice infected with the ΔhasA mutant revealed significantly less weight loss and considerably smaller lesions compared to wildtype-infected mice over the infection period (Fig 5A, 5B and 5D). Neutrophil depletion did not impact weight loss, lesion sizes, or the amount of wildtype S. pyogenes CFUs retrieved from each infected lesion (Fig 5A–5D). As expected, control mice receiving the isotype antibody showed a significant reduction of ΔhasA mutants recovered within lesions at 72 hours (p < 0.0001) compared to wildtype-infected control mice (Fig 5C). In contrast, neutrophil depleted mice displayed a sharp increase in ΔhasA CFUs recovered from each lesion compared to control mice that received infections with the ΔhasA mutant (p < 0.001), despite presenting similar lesion sizes (Fig 5C and 5D). Overall, these data support that S. pyogenes HA capsule expression is important for resisting bacterial killing by neutrophils during experimental skin infection.
Fig 5. The hyaluronic acid capsule is important for resisting neutrophil-mediated killing in the skin.
B6HLA mice were administered ~5 × 107 CFUs of wildtype S. pyogenes MGAS8232 or the ΔhasA or ΔhasA + hasA complemented strain intradermally in each hind flank. Mice received αLy6G or rat IgG2a isotype control antibodies intraperitoneally 24 h preceding and 24 h after skin infections. (A) Weights of B6HLA mice at 24, 48, and 72 h following S. pyogenes skin challenge. Data is represented as a percentage of day 0 weight. Data points represent the weight means ± SEM (n ≥ 3). (B) Skin lesion areas of mice following at 24, 48, and 72 h after skin challenge. Data points represent individual lesion areas (2 per mouse), and the bars represent the mean. (C) Data points represent CFUs from individual infected skin lesions from mice at 72 h. Horizontal bars represent the geometric mean. The horizontal dotted line indicates limit of detection. Significance was determined by two-way ANOVA with Geisser’s Greenhouse correction and Dunnett’s multiple comparisons test (*, P < 0.05, **, P < 0.01, ***, P < 0.001;) for panels A and B, or one-way ANOVA with Kruskal Wallis (***, P < 0.001; ****, P < 0.0001) for panel C. (D) Representative skin lesion images from B6HLA mice 72 h following skin challenge.
Discussion
S. pyogenes is a human-specific bacterial pathogen and we previously demonstrated that mice that express human MHC-II molecules (B6HLA mice) were dramatically more susceptible to experimental nasopharyngeal infection, denoting MHC-II as an important host factor for the adaptation of S. pyogenes to the human host [36]. It was further demonstrated that host sensitivity to superantigen-mediated T cell activation induces an excessive inflammatory signature within the nasopharyngeal environment that promoted the infection [49] and additionally, IL-1β-mediated inflammation mediated by the SpeB protease can similarly promote S. pyogenes colonization of the nasopharynx [47]. Consequently, S. pyogenes must remodel its external environment and balance superantigen- and SpeB-mediated inflammation while tempering host immune clearance mechanisms at various stages of infection, each of which may be influenced by strain-specific differences and tissue-specific cues that can affect the outcome of infection. Herein, we leveraged the B6HLA mouse model to investigate the role of the HA capsule of S. pyogenes MGAS8232 during acute infections and provide evidence that the S. pyogenes capsule functions in vivo to inhibit neutrophil-mediated clearance in both experimental nasopharyngeal and skin infections.
The findings presented here illustrate an important role of the HA capsule during the pathogenesis of acute upper respiratory and skin infections by S. pyogenes; however, this may appear inconsistent with some previous investigations for other encapsulated bacterial pathogens. For example, reduced or eliminated capsule production appears to have advantages for the invasive potential or persistence at mucosal surfaces across multiple bacterial species, including Streptococcus agalactiae (Group B Streptococcus) [50], Streptococcus pneumoniae [51], Neisseria meningitidis [52,53], and Haemophilus influenzae [54]. S. pneumoniae, for example, varies capsule expression from its initial abundance to prevent mucus-mediated clearance [55], yet it is subsequently downregulated to expose underlying adherence molecules [56] and to promote biofilm formation [57,58]. Although the ΔhasA mutant did have enhanced invasion of epithelial cells (Figs 1D and 2D), we did not detect an increase in the dissemination to other organs in vivo (S1 Fig). A caveat to this conclusion however is that if the capsule deficient (ΔhasA) mutant did gain access to the circulatory system, this strain would likely be rapidly eliminated. Furthermore, our findings also contradict some previous reports where acapsular S. pyogenes infected the pharynx as effectively as the parental strain in a baboon model of pharyngeal infection [18], and that frameshift inactivating mutations in the hasA or hasB genes that deplete capsule production contributed to persistence during asymptomatic carriage [18,29]. The use of different strains and different infections models have likely contributed to these disparate findings, and since not all S. pyogenes strains encode the has operon [59], it is clear that the HA capsule is not an essential virulence factor for all S. pyogenes isolates. Nevertheless, our work is entirely consistent with other prior work demonstrating an important selective advantage of the HA capsule for survival within the nasopharynx [15,17,18,24].
During infection of the nasopharynx, the epithelium and mucus layer form the frontline barrier against invading pathogens where adherence to epithelial cells or exposed ECM may be exploited to prevent mucosal-mediated removal. In this study, we report that unencapsulated S. pyogenes display reduced binding to collagen type IV and fibronectin ECM components (Fig 2A and 2B), yet pharyngeal epithelial cell adhesion and internalization were significantly greater compared to the encapsulated wildtype strain (Fig 2C and 2D). Although this may appear to be paradoxical, prior studies have shown that once internalized within epithelial cells, S. pyogenes is rapidly killed [24]. Thus, entry into cells is unlikely a virulence mechanism, but rather a failure of S. pyogenes to avoid ingestion by host cells. Therefore, encapsulation helps resist internalization and enhances the capacity to invade tissues by an extracellular route to promote S. pyogenes infection. Future work is needed to clarify adherence properties of encapsulated and unencapsulated S. pyogenes, however, an impairment in adherence appears to be less important throughout the overall course of infection compared to the capsule’s potent protective effect from ingestion and killing by host phagocytes.
Upon infection with S. pyogenes, the immune system launches a complex innate response that largely depends on the recruitment and activity of neutrophils, macrophages, and dendritic cells [60–64]. Although we attempted to also deplete macrophage populations using clodronate containing liposomes from the nasal passages using established protocols for systemic macrophage depletion [65], including direct nasal administration of clodronate, these protocols were not successful. Nevertheless, as resident and inflammatory macrophage populations were intact following the neutrophil depletions (Fig 3C and 3D), and the hasA mutant could proficiently infect these neutrophil-depleted mice, this suggests that protection against phagocytosis by macrophages is not a key mechanism by which the HA capsule functions during experimental nasopharyngeal infection. In contrast, we have demonstrated the the HA capsule is a key structure that promotes resistance to neutrophil mediated killing. Neutrophils are the most abundant leukocyte involved in innate host responses, acting as both professional detectors that release inflammatory alarms to invading bacteria as well as direct killers via phagocytosis, degranulation, and the formation of neutrophil extracellular traps (NETs). While neutrophil influx during severe infections is protective against S. pyogenes [63], we show that depleting neutrophils did not affect wildtype S. pyogenes MGAS8232 acute infections. These results are in contrast to findings where neutrophils are key for pathogenesis and that neutrophil ablation by αLy6G administration reduces S. pyogenes infection of the nasopharynx [47,66]. However, conventional C57BL/6 mice were used in these studies with superantigen-mediated inflammation absent. In the presence of a superantigen-driven inflammatory response capable of promoting infection [36], our results indicate that neutrophils are not essential for S. pyogenes to establish nasopharyngeal or skin infections. Instead, expression of the HA capsule offered a clear survival advantage that promoted a strong resistance to bacterial clearance by neutrophils. Since innate immune cells are thought to participate in host protection against S. pyogenes, more research is needed to define specific roles, to examine crosstalk, and to address redundancy in responses between individual cell types.
Interestingly, both encapsulated and unencapsulated type 18 S. pyogenes are equally opsonized by C3 in either plasma or serum [25], suggesting that the HA capsule does not inhibit complement activation or deposition of complement fragments on the bacterial cell wall. Since opsonization does not necessarily lead to phagocytic ingestion, the HA capsule may serve as a physical barrier that interferes with leukocyte access to opsonic complement proteins deposited on the bacterial surface [25]. More recently, the HA capsule has been shown to promote bacterial survival within NETs by resisting a major component and antimicrobial effector, cathelicidin antimicrobial peptide LL-37 [67]. As different strains of S. pyogenes harbour variations in global virulence factor expression, and consequently express varying amounts of HA capsule, it is likely that distinct strategies to prevent phagocytic ingestion and killing are exploited among individual strains. For example, mutations that produce a truncated RocA (regulator of Cov) protein have amplified expression of the has operon through transcriptional activation of the repressor covR, and have been identified in S. pyogenes types emm18 and emm3 [34,68]. Thus, no single strain of S. pyogenes should be considered representative of the population as a whole and future studies using additional encapsulated strains are recommended to draw general conclusions on the mechanisms utilized by the HA capsule. Although various strains may vary greatly in their degree of encapsulation, the results presented here provide evidence that HA capsule expression by S. pyogenes MGAS8232 promotes a strong resistance to killing by neutrophils during acute infection models. Defining strategies by which neutrophils can counteract HA capsule resistance is warranted to combat this leading bacterial pathogen.
Materials & methods
Ethics statement
Human venous blood was taken from healthy volunteer donors in accordance with human subject protocol 110859. The full study protocol was approved by the London Health Sciences Centre Research Ethics Board (University of Western Ontario, London, ON, Canada). Volunteers were recruited by a passive advertising campaign within the Department of Microbiology and Immunology at the University of Western Ontario, and following an outline of the risks, written consent was obtained from each volunteer before samples were taken. Following blood collection, samples were fully anonymized and no information regarding the identity of the donor, including sex and age, were retained.
All mouse experiments were conducted in accordance with the Canadian Council on Animal Care Guide to the Care and Use of Experimental Animals. The Animal Use Protocol (AUP) number 2020–041 was approved by the Animal Use Subcommittee at the University of Western Ontario (London, ON, Canada).
Bacterial strains, media, and growth conditions
Bacterial strains used in this study are listed in Table 1. The main bacterial model strain for our work is S. pyogenes MGAS8232, an M18 serotype and pharyngeal isolate from a patient with acute rheumatic fever [33]. S. pyogenes strains were grown statically in Todd Hewitt broth (BD Biosciences; Franklin Lakes, NJ, USA) supplemented with 1% (w/v) yeast extract (BD Biosciences) (THY) and 1 μg mL-1 erythromycin when appropriate. For solid media preparation, 1.5% agar and/or 1 μg mL-1 erythromycin were added to the media when applicable. Molecular cloning experiments utilized the E. coli XL1-Blue strain cultured in Luria-Bertani (LB) broth (Thermo Fisher Scientific, Waltham, MA, USA) aerobically at 37°C, or Brain Heart Infusion (BHI; BD Biosciences, Franklin Lakes, NJ, USA) media containing 1.5% (w/v) agar (Thermo Fisher Scientific). Media was supplemented with 150 μg mL-1 erythromycin (Sigma-Aldrich Canada, Oakville, ON, Canada) as necessary. A complete list of plasmids used in this study can be found in Table 1.
Construction of recombinant S. pyogenes strains
References to genomic loci are based on the genome of S. pyogenes MGAS8232 [33]. In-frame genetic deletion in the hasA gene was generated using the Gram-positive-E. coli shuttle vector, pG+host5 [32] (Table 1). Using appropriate PCR amplification primers listed in S1 Table, the upstream and downstream regions flanking the hasA gene were amplified from the MGAS8232 genome. Allelic replacement of the wildtype hasA gene with the deletion mutant ΔhasA via homologous recombination was conducted as described previously [36]. For complementation of the hasA genetic deletion, DNA fragments containing the hasA open reading frame and its native promoter were amplified from the S. pyogenes MGAS8232 genome using complementation primers listed in S1 Table, and cloned into the XhoI and SpeI restriction sites of the plasmid pDCerm [35]. This construct (pDCerm::hasA) was electroporated into the MGAS8232 ΔhasA mutant strain to produce the hasA complementation strain (ΔhasA + hasA).
Genomic sequencing analysis
Genomic DNA preparations from S. pyogenes MGAS8232 wildtype and ΔhasA strains were sent for paired end Illumina sequencing at the John P. Robarts Research Institute sequencing facility (University of Western Ontario, London, Ontario). Illumina short-read sequence data were used to generate de novo assemblies using SPAdes v3.15 [69], which were annotated using Prokka v1.12 [70]. The assemblies for the isogenic MGAS8232 and MGAS8232 ΔhasA have been deposited at NCBI (Biosamples SAMN27522080 and SAMN27522081 [Bioproject: PRJNA825546]). Any sequence differences between the strains were determined using Snippy v4.1 (https://github.com/tseemann/snippy). The publicly available S. pyogenes MGAS8232 sequence was used as a reference (Bioproject: PRJNA286). SNPs unique to the ΔhasA mutant were reported (S2 Table).
Extracellular matrix binding assay
Corning Costar 9018 high-binding 96-well plates (Corning; Kennebuck, ME, USA) were coated with 1 μg of collagen type IV (Sigma-Aldrich) or fibronectin (Calbiochem, EMD Millipore Corporation; Temecula, CA, USA) dissolved in carbonate-bicarbonate buffer (0.2M sodium carbonate anhydrous, 0.2M sodium bicarbonate, pH = 9.6) and left overnight at 4°C. The following day, the plates were washed three times using PBS with 0.05% (v/v) tween-20 and blocked for two hours with 5% (w/v) skim milk at room temperature and then washed as described. Afterwards, 100 μl of bacteria containing 107 CFUs were added in triplicate to pre-coated wells and left for 2.5 hours at 37°C. Plates were washed and fixed with 10% neutral buffered formalin (VWR International; Randor PA, USA) for 40 minutes and then washed again. Wells were incubated with 50 μl of 0.5% (w/v) crystal violet (Sigma-Aldrich) in 80% (v/v) sterile MilliQ water and 20% (v/v) methanol for 5 minutes at room temperature before being washed five times. Stain was solubilized in 5% (v/v) acetic acid with mild agitation for 10 minutes. Colorimetric analysis was measured at OD590 using Synergy HTX Multi-Mode Microplate Reader (Biotek).
Human cell culturing
The Detroit-562 (ATCC CCL-138) human pharyngeal cell line was grown and maintained at 37°C in 5% CO2 in minimal essential medium (MEM) and passaged every 2–3 days. All tissue culture basal media was supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO, USA), 100 IU penicillin (Gibco, Life Technologies Inc, Carlsbad, CA, USA), and 100 μg mL-1 streptomycin (Gibco-BRL, Life Technologies, Grand Island, N.Y.), all filtered through a 0.2 μm PES filter (Nalgene, Thermo Scientific, Waltham, MA, USA).
Epithelial cell adhesion and invasion
Detroit-562 cells were grown to confluence on 12 or 24-well TC-treated plates (Falcon, Corning). On the day of infection, cells were washed with PBS and maintained in their respective serum-free media for at least 1 h prior to infection. The average number of cells per well was calculated and used to determine the number of bacteria required for a multiplicity of infection (MOI) of 100. During this time, overnight streptococcal cultures were subcultured into pre-warmed media and grown to early exponential phase. Tissue culture plate wells were washed three times with PBS and inoculated with 500 μl of the resuspended bacterial dose per well and left for 2–3 hours at 37°C in a 5% CO2 incubator to allow bacteria to adhere to the cells. Background adherence levels were measured by inoculating bacteria onto uncoated wells. Following incubation, wells were washed three times with PBS to remove non-adherent bacteria, and cell monolayers were lysed with 500 μl of 0.01% Triton X-100 for 5 minutes, followed by disruption of the wells by scraping with a 1 mL pipette tip. Solubilized wells were serially diluted 10-fold and plated onto TSA 5% sheep blood agar plates (BD Biosciences) to enumerate bacteria present.
For invasion experiments, bacterial were inoculated onto cell monolayers for 2–3 hours at 37°C with 5% CO2 and washed as above, followed by the addition of media containing 100 μg mL-1 of gentamicin for 1 hour at 37°C and 5% CO2 to kill extracellular bacteria. Wells were then extensively washed to remove the gentamicin media. Cells were lysed, serially diluted, and plated as described above to estimate the number of intracellular bacteria.
Whole blood survival assay
Lancefield bactericidal assays were performed as previously described [71] with some modifications. Briefly, a volume of 10 μl containing 1000 bacterial CFUs was added to 990 μl heparinized whole human blood in 1.5 mL Eppendorf tubes and allowed to incubate at 37°C with rotation. After 30, 60, 90, 120, and 180 minutes, samples were serially diluted 10-fold and drop plated in triplicate onto 5% TSA blood agar plates to enumerate the surviving bacteria in whole blood at indicated time points.
Isolation of human polymorphonuclear neutrophils
Polymorphonuclear neutrophils (PMNs) were isolated using a Ficoll (GE-Healthcare) Histopaque (Sigma-Aldrich) density gradient centrifugation method. Briefly, heparinized blood from human donors was diluted with an equal volume of PBS (Wisent Bioproducts Inc.) and layered carefully onto a dual Ficoll-Histopaque gradient and centrifuged at 396 × g for 20 minutes without braking. The PMN layer was collected and washed with cold RPMI containing 0.05% human serum albumin (RPMI-HSA) and with addition of 1 mL ice-cold water to lyse residual erythrocytes. PMNs were then collected in RPMI-HSA following centrifugation and adjusted to 4 × 106 cells mL-1.
PMN survival assay
Overnight streptococcal cultures were grown to early exponential phase and diluted to 104 CFU mL-1 in RPMI containing 10% (v/v) normal serum for 30 minutes to assist with bacterial opsonization. A volume of 0.225 mL opsonized bacteria were co-cultured with 0.025 mL of isolated human PMNs at 4 × 106 cells mL-1 (1:10 bacterial CFUs to neutrophils) at 37°C with vigorous shaking. Control samples had no PMNs added to control for bacterial growth. Viable bacteria in each reaction mixture were measured after 60 mins by lysing cells with 750 μl of 0.025% Triton X100, followed by serial dilution and plating onto 5% TSA blood agar plates overnight at 37°C. Percent bacterial survival was calculated as average bacterial CFUs in the presence of neutrophils divided by bacterial CFUs in no PMN control samples.
Mice
Mice were bred in a barrier facility at the University of Western Ontario and genotyped routinely for appropriate transgene expression. Human MHC class II transgenic (B6HLA) mice [72] were bred from McCormick laboratory colonies specifically for this study and were on a C57BL/6 (B6) background. During all breeding and experiments, mice were provided food and water ad libitum and appropriate enrichment was provided in all cages.
Nasopharyngeal infection model
Preparation of S. pyogenes MGAS8232 for nasal inoculation has been previously described [36,37,49]. Briefly, bacteria were grown to early exponential phase (OD600 of 0.2–0.3), cells were centrifuged, washed, and resuspended in Hank’s buffered saline solution (HBSS) at ∼1 × 108 CFUs per 15 μL. Mice were given 2 mg ml-1 neomycin sulphate ad libitum in their drinking water two days prior to infection to reduce the nasal microbiota. Mice were anesthetized using FORANE (isoflurane, USP; Baxter Corporation; Mississauga, ON, Canada) and 7.5 μL of bacterial inoculum was administered into each nostril. Mice were sacrificed 24 or 48 hours post-infection and their complete nasal turbinates (cNTs), including the nasal-associated lymphoid tissue, nasal turbines, and maxillary sinuses, were extracted and homogenized using a glass homogenizer [37]. Murine lungs, liver, spleen, kidneys, and heart were also removed for bacterial enumeration where indicated. Organs were serially diluted in HBSS and plated on TSA supplemented with 5% sheep’s blood for bacterial enumeration. Counts less than 30 CFUs per 100 μL of tissue supernatant were considered below the theoretical limit of detection.
Skin infection model
The fur on the lower backs of B6HLA mice between 8–12 weeks old was removed by shaving and hair removal cream the day prior to infection. S. pyogenes was grown and prepared as stated above and resuspended to 5 × 108 CFU per mL in HBSS. Mice were anesthetized and a 50 μL dose containing ~2.5 × 107 CFU was injected intradermally into each lower flank. On each day following infection, mice were weighed and lesions at the injection sites were measured using calipers. At 72 hours post-infection, mice were sacrificed and the skin around each injection site was harvested, homogenized, and plated on TSA with 5% sheep’s blood overnight at 37°C for bacterial enumeration. Bacterial burden was presented as CFUs from individual lesions.
Immunofluorescent histology
At the previously identified endpoint, cNTs were collected as described [37] and tissues were fixed in periodate-lysine-paraformaldehyde (PLP) and prepared for sectioning as previously [73]. Following fixation, cNT were passed through sucrose gradients and frozen in OCT (TissueTek) media. Serial sections (7 μm) were cut using a cryostat. Prior to staining, all slide-mounted tissue sections were blocked with PBS containing 1% BSA, 0.1% Tween-20, and 10% rat serum. Sections were stained with the following antibodies: anti-Streptococcus pyogenes Group A Carbohydrate (Abcam, ab9191), anti-B220 (Biolegend, RA3-6B2), anti-CD3 (Biolegend, 17A2), and anti-Ly6G (Biolegend, 1A8). After staining, sections were mounted with ProLong Gold Antifade Reagent (Invitrogen). Tiled images of whole cNT sections were collected using a DM5500B fluorescence microscope (Leica) at 10× and 20×.
Neutrophil depletion in vivo
The function of neutrophils during acute infections by S. pyogenes were examined in B6HLA mice between 8–12 weeks old. Neutrophils were depleted in vivo by intraperitoneally injecting mice with 250 μg mAb αLy6G clone 1A8 (BioXcell, NH, USA) 24 hours before and 24 hours following nasopharyngeal and skin infections. Control mice received rat IgG2a clone 2A3 (BioXCell, NH, USA). Depletion of circulating neutrophils has been confirmed in previous studies using flow cytometry of Ly6G+ expressing populations in blood [47,74,75]. Bacterial burden in nasal turbinates was examined at both 24 and 48 hours following infection.
Flow cytometry analyses
To assess the neutrophil depletion experiments, we phenotyped innate immune cells from both the nasal tubinates and blood. Murine nasal turbinates were isolated as previously described [37] and collected in R10 media. Nasal turbinates were treated with 0.3 mg ml-1 collagenase D (Sigma-Aldrich) in R10 media at 37°C for 30 minutes and then pushed through a 0.7-μm cell strainer. The single-cell suspension was then treated with ACK lysis buffer (Gibco) and washed with PBS containing 2% FBS. Blood was collected from mice via cardiac puncture. Following isolation, blood was treated with ACK lysis buffer (Gibco) and washed with PBS containing 2% FBS. Following isolation, both blood and nasal cells were stained and analysed as follows. Cell viability was first determined using Fixable Viability Dye eFluor506 (Thermo Fisher) and then subsequently stained anti-CD4-PE-Cy5 (clone RM4-5, Thermo Fisher) anti-CD45r(B220)-V450 (clone RA3-6B2, BD), anti-CD19-BV711 (clone 1D3, Thermo Fisher), anti-F4/80-A647 (clone BM8, Biolegend), anti-Ly6G-A700 (clone RB6-8C5, Biolegend), anti-Ly6C-PE (clone HK1.4, Biolegend), anti-CD11b-A488 (clone M1/70, Biolegend), and anti-CD11c-APC-Cy7 (clone HL3, BD). Cells were fixed overnight with 1% paraformaldehyde prior to analysis. Events were acquired using a LSR II (BD Biosciences), and data were analyzed using FlowJo v10.7.1 (TreeStar).
Cells were gated first on whether they were alive or dead, and the live population was subsequently gated for the singlet population. Single cells were checked for the expression of CD4, CD45r, and CD19 and the triple negative population was gated for further analysis. Cells were then assessed for expression of F4/80 and Ly6G. F4/80 positive and Ly6G negative cells were classed as macrophages. Cells that were subsequently high for Ly6G but negative for F4/80 were assessed for CD11b and Ly6C expression and classed as neutrophils. F4/80 and Ly6G double negative cells were gated and assessed for CD11b and Ly6C expression, with double positive cells classed as monocytes. Ly6G and F4/80 double negative cells were also gated and assessed for CD11c and Ly6C expression, with CD11c positive and Ly6C negative cells classed as dendritic cells. Data is presented as the percentage of live cells.
Detection of cytokines and chemokines in vivo
Cytokine and chemokine concentrations were determined from cNT homogenates of B6HLA mice infected with S. pyogenes MGAS8232. Uninfected murine cNT homogenates were measured as a background control. Mutiplex cytokine array (Mouse Cytokine/Chemokine Array 32-Plex) was performed by Eve Technologies (Calgary, AB, Canada) and data on heat maps is presented as normalized median cytokine responses (Xnormalized = [(x—xmin)/(xmax−xmin)]) from cNT homogenates.
Statistical analysis
All statistical analysis was completed using GraphPad Prism 9.3.1. Significance was calculated using the Student’s t test or one-way ANOVA with Dunnett’s or Tukey’s multiple comparisons post hoc test where indicated. A P value less than 0.05 was determined to be statistically significant.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank members of the McCormick laboratory for helpful discussions.
Data Availability
The assemblies for the isogenic MGAS8232 and MGAS8232 ΔhasA have been deposited at NCBI (Biosamples SAMN27522080 and SAMN27522081 [Bioproject:PRJNA825546]). All other relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by Project Grant 461623 from the Canadian Institutes of Health Research (CIHR) to JKM. JRH was supported by an RGE Murray Graduate Scholarship and a Queen Elizabeth II Graduate Scholarship in Science and Technology. BAS was supported by an Ontario Graduate Scholarship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5: 685–94. doi: 10.1016/S1473-3099(05)70267-X [DOI] [PubMed] [Google Scholar]
- 2.Shaikh N, Leonard E, Martin JM. Prevalence of streptococcal pharyngitis and streptococcal carriage in children: a meta-analysis. Pediatrics. 2010;126: e557–64. doi: 10.1542/peds.2009-2648 [DOI] [PubMed] [Google Scholar]
- 3.Kendall FE, Heidelberger M, Dawson MH. A serologically inactive polysaccharide elaborated by mucoid strains of group a hemolytic Streptococcus. J Biol Chem. 1937;118: 61–69. doi: 10.1016/S0021-9258(18)74517-1 [DOI] [Google Scholar]
- 4.Dougherty BA, van de Rijn I. Molecular characterization of a locus required for hyaluronic acid capsule production in group A streptococci. J Exp Med. 1992/05/01. 1992;175: 1291–1299. doi: 10.1084/jem.175.5.1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crater DL, Van de Rijn I. Hyaluronic acid synthesis operon (has) expression in group A streptococci. J Biol Chem. 1995;270: 18452–18458. doi: 10.1074/jbc.270.31.18452 [DOI] [PubMed] [Google Scholar]
- 6.DeAngelis PL, Papaconstantinou J, Weigel PH. Isolation of a Streptococcus pyogenes gene locus that directs hyaluronan biosynthesis in acapsular mutants and in heterologous bacteria. J Biol Chem. 1993;268: 14568–71. doi: 10.1016/s0021-9258(18)82366-3 [DOI] [PubMed] [Google Scholar]
- 7.Dougherty BA, Van De Rijn I. Molecular characterization of hasA from an operon required for hyaluronic acid synthesis in group A streptococci. J Biol Chem. 1994;269: 169–175. [PubMed] [Google Scholar]
- 8.DeAngelis PL, Papaconstantinou J, Weigel PH. Molecular cloning, identification, and sequence of the hyaluronan synthase gene from group A Streptococcus pyogenes. J Biol Chem. 1993;268: 19181–19184. doi: 10.1016/s0021-9258(19)36494-4 [DOI] [PubMed] [Google Scholar]
- 9.Dougherty BA, Van de Rijn I. Molecular characterization of hasB from an operon required for hyaluronic acid synthesis in group A streptococci. Demonstration of UDP-glucose dehydrogenase activity. J Biol Chem. 1993;268: 7118–7124. doi: 10.1016/s0021-9258(18)53153-7 [DOI] [PubMed] [Google Scholar]
- 10.Crater DL, Dougherty BA, Van de Rijn I. Molecular characterization of hasC from an operon required for hyaluronic acid synthesis in group A streptococci. Demonstration of UDP-glucose pyrophosphorylase activity. J Biol Chem. 1995;270: 28676–28680. doi: 10.1074/jbc.270.48.28676 [DOI] [PubMed] [Google Scholar]
- 11.Ashbaugh CD, Albertí S, Wessels MR. Molecular analysis of the capsule gene region of group A Streptococcus: the hasAB genes are sufficient for capsule expression. J Bacteriol. 1998;180: 4955–9. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole JN, Aziz RK, Kuipers K, Timmer AM, Nizet V, van Sorge NM. A conserved UDP-glucose dehydrogenase encoded outside the hasABC operon contributes to capsule biogenesis in group A Streptococcus. J Bacteriol. 2012;194: 6154–61. doi: 10.1128/JB.01317-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gryllos I, Cywes C, Shearer MH, Cary M, Kennedy RC, Wessels MR. Regulation of capsule gene expression by group A Streptococcus during pharyngeal colonization and invasive infection. Mol Microbiol. 2001/10/27. 2001;42: 61–74. doi: 10.1046/j.1365-2958.2001.02635.x [DOI] [PubMed] [Google Scholar]
- 14.Wessels MR, Moses AE, Goldberg JB, DiCesare TJ. Hyaluronic acid capsule is a virulence factor for mucoid group A streptococci. Proc Natl Acad Sci. 1991;88: 8317–8321. doi: 10.1073/pnas.88.19.8317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wessels MR, Bronze MS. Critical role of the group A streptococcal capsule in pharyngeal colonization and infection in mice. Proc Natl Acad Sci U S A. 1994/December/06. 1994;91: 12238–12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wessels MR, Goldberg JB, Moses AE, DiCesare TJ. Effects on virulence of mutations in a locus essential for hyaluronic acid capsule expression in group A streptococci. Infect Immun. 1994;62: 433–441. doi: 10.1128/iai.62.2.433-441.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Husmann LK, Yung DL, Hollingshead SK, Scott JR. Role of putative virulence factors of Streptococcus pyogenes in mouse models of long-term throat colonization and pneumonia. Infect Immun. 1997;65: 1422–1430. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashbaugh CD, Moser TJ, Shearer MH, White GL, Kennedy RC, Wessels MR. Bacterial determinants of persistent throat colonization and the associated immune response in a primate model of human group A streptococcal pharyngeal infection. Cell Microbiol. 2000;2: 283–292. doi: 10.1046/j.1462-5822.2000.00050.x [DOI] [PubMed] [Google Scholar]
- 19.Schrager HM, Albertí S, Cywes C, Dougherty GJ, Wessels MR. Hyaluronic acid capsule modulates M protein-mediated adherence and acts as a ligand for attachment of group A Streptococcus to CD44 on human keratinocytes. J Clin Invest. 1998;101: 1708–1716. doi: 10.1172/JCI2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cywes C, Stamenkovic I, Wessels MR. CD44 as a receptor for colonization of the pharynx by group A Streptococcus. J Clin Invest. 2000;106: 995–1002. doi: 10.1172/JCI10195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cywes C, Wessels MR. Group A Streptococcus tissue invasion by CD44-mediated cell signalling. Nature. 2001;414: 648–652. doi: 10.1038/414648a [DOI] [PubMed] [Google Scholar]
- 22.Lynskey NN, Banerji S, Johnson LA, Holder KA, Reglinski M, Wing PAC, et al. Rapid lymphatic dissemination of encapsulated group A streptococci via lymphatic vessel endothelial receptor-1 interaction. PLOS Pathog. 2015;11: e1005137. doi: 10.1371/journal.ppat.1005137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hollands A, Pence MA, Timmer AM, Osvath SR, Turnbull L, Whitchurch CB, et al. Genetic switch to hypervirulence reduces colonization phenotypes of the globally disseminated group A Streptococcus M1T1 clone. J Infect Dis. 2010;202: 11–9. doi: 10.1086/653124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schrager HM, Rheinwald JG, Wessels MR. Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infection. J Clin Invest. 1996/November/01. 1996;98: 1954–1958. doi: 10.1172/JCI118998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dale JB, Washburn RG, Marques MB, Wessels MR. Hyaluronate capsule and surface M protein in resistance to opsonization of group A streptococci. Infect Immun. 1996;64: 1495–501. doi: 10.1128/iai.64.5.1495-1501.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moses AE, Wessels MR, Zalcman K, Albertí S, Natanson-Yaron S, Menes T, et al. Relative contributions of hyaluronic acid capsule and M protein to virulence in a mucoid strain of the group A Streptococcus. Infect Immun. 1997;65: 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flores AR, Jewell BE, Fittipaldi N, Beres SB, Musser JM. Human disease isolates of serotype M4 and M22 group A Streptococcus lack genes required for hyaluronic acid capsule biosynthesis. MBio. American Society for Microbiology; 2012;3: e00413–12. doi: 10.1128/mBio.00413-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner CE, Abbott J, Lamagni T, Holden MTG, David S, Jones MD, et al. Emergence of a new highly successful acapsular group A Streptococcus clade of genotype emm89 in the United Kingdom. MBio. 2015;6: 1–11. doi: 10.1128/mBio.00622-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flores AR, Jewell BE, Olsen RJ, Shelburne SA, Fittipaldi N, Beres SB, et al. Asymptomatic carriage of group A Streptococcus is associated with elimination of capsule production. Infect Immun. 2014;82: 3958–67. doi: 10.1128/IAI.01788-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shea PR, Beres SB, Flores AR, Ewbank AL, Gonzalez-Lugo JH, Martagon-Rosado AJ, et al. Distinct signatures of diversifying selection revealed by genome analysis of respiratory tract and invasive bacterial populations. Proc Natl Acad Sci U S A. 2011;108: 5039–5044. doi: 10.1073/pnas.1016282108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wessels MR. Capsular polysaccharide of group A Streptococcus. Gram-Positive Pathogens. Washington, DC, USA: ASM Press; 2019. pp. 45–54. doi: 10.1128/9781683670131.ch4 [DOI] [Google Scholar]
- 32.Biswas I, Gruss A, Ehrlich SD, Maguin E. High-efficiency gene inactivation and replacement system for gram-positive bacteria. J Bacteriol. 1993;175: 3628–3635. doi: 10.1128/jb.175.11.3628-3635.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smoot JC, Barbian KD, Van Gompel JJ, Smoot LM, Chaussee MS, Sylva GL, et al. Genome sequence and comparative microarray analysis of serotype M18 group A Streptococcus strains associated with acute rheumatic fever outbreaks. Proc Natl Acad Sci U S A. 2002;99: 4668–4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lynskey NN, Goulding D, Gierula M, Turner CE, Dougan G, Edwards RJ, et al. RocA truncation underpins hyper-encapsulation, carriage longevity and transmissibility of serotype M18 group A streptococci. PLoS Pathog. 2013;9: e1003842. doi: 10.1371/journal.ppat.1003842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeng A, Sakota V, Li Z, Datta V, Beall B, Nizet V. Molecular genetic analysis of a group A Streptococcus operon encoding serum opacity factor and a novel fibronectin-binding protein, SfbX. J Bacteriol. 2003;185: 1208–17. doi: 10.1128/JB.185.4.1208-1217.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kasper KJ, Zeppa JJ, Wakabayashi AT, Xu SX, Mazzuca DM, Welch I, et al. Bacterial superantigens promote acute nasopharyngeal infection by Streptococcus pyogenes in a human MHC Class II-dependent manner. PLoS Pathog. 2014;10: e1004155. doi: 10.1371/journal.ppat.1004155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeppa JJ, Wakabayashi AT, Kasper KJ, Xu SX, Haeryfar SMM, McCormick JK. Nasopharyngeal infection of mice with Streptococcus pyogenes and in vivo detection of superantigen activity. Methods Mol Biol. 2016;1396: 95–107. doi: 10.1007/978-1-4939-3344-0_8 [DOI] [PubMed] [Google Scholar]
- 38.LeBleu VS, MacDonald B, Kalluri R. Structure and Function of Basement Membranes. Exp Biol Med. 2007;232: 1121–1129. doi: 10.3181/0703-MR-72 [DOI] [PubMed] [Google Scholar]
- 39.Kreikemeyer B, Oehmcke S, Nakata M, Hoffrogge R, Podbielski A. Streptococcus pyogenes fibronectin-binding protein F2: expression profile, binding characteristics, and impact on eukaryotic cell interactions. J Biol Chem. 2004;279: 15850–9. doi: 10.1074/jbc.M313613200 [DOI] [PubMed] [Google Scholar]
- 40.Hanski E, Caparon M. Protein F, a fibronectin-binding protein, is an adhesin of the group A Streptococcus Streptococcus pyogenes. Proc Natl Acad Sci. 1992;89: 6172–6176. doi: 10.1073/pnas.89.13.6172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nyberg P, Sakai T, Cho KH, Caparon MG, Fässler R, Björck L. Interactions with fibronectin attenuate the virulence of Streptococcus pyogenes. EMBO J. 2004;23: 2166–2174. doi: 10.1038/sj.emboj.7600214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hymes JP, Klaenhammer TR. Stuck in the middle: Fibronectin-binding proteins in Gram-positive bacteria. Front Microbiol. 2016;7: 1504. doi: 10.3389/fmicb.2016.01504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kosmehl H, Berndt A, Strassburger S, Borsi L, Rousselle P, Mandel U, et al. Distribution of laminin and fibronectin isoforms in oral mucosa and oral squamous cell carcinoma. Br J Cancer. 1999;81: 1071–1079. doi: 10.1038/sj.bjc.6690809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Broudy TB, Pancholi V, Fischetti VA. Induction of lysogenic bacteriophage and phage-associated toxin from group a streptococci during coculture with human pharyngeal cells. Infect Immun. 2001;69: 1440–3. doi: 10.1128/IAI.69.3.1440-1443.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Broudy TB, Pancholi V, Fischetti VA. The in vitro interaction of Streptococcus pyogenes with human pharyngeal cells induces a phage-encoded extracellular DNase. Infect Immun. 2002;70: 2805–11. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker MJ, Barnett TC, McArthur JD, Cole JN, Gillen CM, Henningham A, et al. Disease manifestations and pathogenic mechanisms of Group A Streptococcus. Clin Microbiol Rev. 2014;27: 264–301. doi: 10.1128/CMR.00101-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.LaRock DL, Russell R, Johnson AF, Wilde S, LaRock CN. Group A Streptococcus infection of the nasopharynx requires proinflammatory signaling through the interleukin-1 receptor. Infect Immun. 2020;88. doi: 10.1128/IAI.00356-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bessen DE. Tissue tropisms in group A Streptococcus. Curr Opin Infect Dis. 2016;29: 295–303. doi: 10.1097/QCO.0000000000000262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeppa JJ, Kasper KJ, Mohorovic I, Mazzuca DM, Haeryfar SMM, McCormick JK. Nasopharyngeal infection by Streptococcus pyogenes requires superantigen-responsive Vβ-specific T cells. Proc Natl Acad Sci. 2017;114: 10226–10231. doi: 10.1073/pnas.1700858114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davies HD, Adair C, McGeer A, Ma D, Robertson S, Mucenski M, et al. Antibodies to capsular polysaccharides of group B Streptococcus in pregnant Canadian women: Relationship to colonization status and infection in the neonate. J Infect Dis. 2001;184: 285–291. doi: 10.1086/322029 [DOI] [PubMed] [Google Scholar]
- 51.Hanage WP, Kaijalainen T, Saukkoriipi A, Rickcord JL, Spratt BG. A successful, diverse disease-associated lineage of nontypeable pneumococci that has lost the capsular biosynthesis locus. J Clin Microbiol. 2006;44: 743–749. doi: 10.1128/JCM.44.3.743-749.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoang LMN, Thomas E, Tyler S, Pollard AJ, Stephens G, Gustafson L, et al. Rapid and fatal meningococcal disease due to a strain of Neisseria meningitidis containing the capsule null locus. Clin Infect Dis. 2005;40. doi: 10.1086/427875 [DOI] [PubMed] [Google Scholar]
- 53.Johswich KO, Zhou J, Law DKS, St Michael F, McCaw SE, Jamieson FB, et al. Invasive potential of nonencapsulated disease isolates of Neisseria meningitidis. Infect Immun. 2012;80: 2346–53. doi: 10.1128/IAI.00293-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nizet V, Colina KF, Almquist JR, Rubens CE, Smith AL. A virulent nonencapsulated Haemophilus influenzae. J Infect Dis. 1996;173: 180–186. doi: 10.1093/infdis/173.1.180 [DOI] [PubMed] [Google Scholar]
- 55.Nelson AL, Roche AM, Gould JM, Chim K, Ratner AJ, Weiser JN. Capsule enhances pneumococcal colonization by limiting mucus-mediated clearance. Infect Immun. 2007;75: 83–90. doi: 10.1128/IAI.01475-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Novick S, Shagan M, Blau K, Lifshitz S, Givon-Lavi N, Grossman N, et al. Adhesion and invasion of Streptococcus pneumoniae to primary and secondary respiratory epithelial cells. Mol Med Rep. 2017;15: 65–74. doi: 10.3892/mmr.2016.5996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muñoz-Elías EJ, Marcano J, Camilli A. Isolation of Streptococcus pneumoniae biofilm mutants and their characterization during nasopharyngeal colonization. Infect Immun. 2008;76: 5049–5061. doi: 10.1128/IAI.00425-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanchez CJ, Kumar N, Lizcano A, Shivshankar P, Hotopp JCD, Jorgensen JH, et al. Streptococcus pneumoniae in biofilms are unable to cause invasive disease due to altered virulence determinant production. PLoS One. 2011;6. doi: 10.1371/journal.pone.0028738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Flores AR, Jewell BE, Fittipaldi N, Beres SB, Musser JM. Human disease isolates of serotype M4 and M22 group A Streptococcus lack genes required for hyaluronic acid capsule biosynthesis. MBio. 2012; doi: 10.1128/mBio.00413-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goldmann O, Rohde M, Chhatwal GS, Medina E. Role of macrophages in host resistance to group A streptococci. Infect Immun. 2004/April/23. 2004;72: 2956–2963. doi: 10.1128/IAI.72.5.2956-2963.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mishalian I, Ordan M, Peled A, Maly A, Eichenbaum MB, Ravins M, et al. Recruited macrophages control dissemination of group A Streptococcus from infected soft tissues. J Immunol. 2011;187: 6022–31. doi: 10.4049/jimmunol.1101385 [DOI] [PubMed] [Google Scholar]
- 62.Loof TG, Rohde M, Chhatwal GS, Jung S, Medina E. The contribution of dendritic cells to host defenses against Streptococcus pyogenes. J Infect Dis. Oxford Academic; 2007;196: 1794–1803. doi: 10.1086/523647 [DOI] [PubMed] [Google Scholar]
- 63.Zinkernagel AS, Timmer AM, Pence MA, Locke JB, Buchanan JT, Turner CE, et al. The IL-8 protease SpyCEP/ScpC of group A Streptococcus promotes resistance to neutrophil killing. Cell Host Microbe. 2008;4: 170–8. doi: 10.1016/j.chom.2008.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Döhrmann S, Cole JN, Nizet V. Conquering Neutrophils. PLoS Pathog. 2016;12: e1005682. doi: 10.1371/journal.ppat.1005682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tuffs SW, Goncheva MI, Xu SX, Craig HC, Kasper KJ, Choi J, et al. Superantigens promote Staphylococcus aureus bloodstream infection by eliciting pathogenic interferon-gamma production. Proc Natl Acad Sci U S A. National Academy of Sciences; 2022;119. doi: 10.1073/pnas.2115987119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tanaka M, Kinoshita-Daitoku R, Kiga K, Sanada T, Zhu B, Okano T, et al. Group A Streptococcus establishes pharynx infection by degrading the deoxyribonucleic acid of neutrophil extracellular traps. Sci Rep. 2020;10: 3251. doi: 10.1038/s41598-020-60306-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cole JN, Pence MA, von Köckritz-Blickwede M, Hollands A, Gallo RL, Walker MJ, et al. M Protein and Hyaluronic Acid Capsule Are Essential for In Vivo Selection of covRS Mutations Characteristic of Invasive Serotype M1T1 Group A Streptococcus. MBio. 2010;1. doi: 10.1128/mBio.00191-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lynskey NN, Turner CE, Heng LS, Sriskandan S. A truncation in the regulator RocA underlies heightened capsule expression in serotype M3 group A streptococci. Infect Immun. 2015;83: 1732–1733. doi: 10.1128/IAI.02892-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19: 455–477. doi: 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seemann T. Genome analysis Prokka: rapid prokaryotic genome annotation. 2014;30: 2068–2069. doi: 10.1093/bioinformatics/btu153 [DOI] [PubMed] [Google Scholar]
- 71.Todd EW. A method of measuring the increase or decrease of the population of haemolytic streptococci in blood. Br J Exp Pathol. 1927;VIII: 1–5. [Google Scholar]
- 72.Nooh MM, El-Gengehi N, Kansal R, David CS, Kotb M. HLA transgenic mice provide evidence for a direct and dominant role of HLA class II variation in modulating the severity of streptococcal sepsis. J Immunol. 2007;178: 3076–3083. doi: 10.4049/jimmunol.178.5.3076 [pii] [DOI] [PubMed] [Google Scholar]
- 73.Kerfoot SM, Yaari G, Patel JR, Johnson KL, Gonzalez DG, Kleinstein SH, et al. Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity. 2011/June/04. 2011;34: 947–960. doi: 10.1016/j.immuni.2011.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Puerta-Arias JD, Pino-Tamayo PA, Arango JC, González Á. Depletion of neutrophils promotes the resolution of pulmonary inflammation and fibrosis in mice infected with Paracoccidioides brasiliensis. PLoS One. 2016;11. doi: 10.1371/journal.pone.0163985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moynihan KD, Opel CF, Szeto GL, Tzeng A, Zhu EF, Engreitz JM, et al. Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat Med. 2017;22: 1402–1410. doi: 10.1038/nm.4200.Eradication [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
The assemblies for the isogenic MGAS8232 and MGAS8232 ΔhasA have been deposited at NCBI (Biosamples SAMN27522080 and SAMN27522081 [Bioproject:PRJNA825546]). All other relevant data are within the manuscript and its Supporting Information files.