Abstract
Cardiovascular disease (CVD), the leading cause of death among US adults, is more prevalent in menopausal females compared with age-matched males. Vasomotor symptoms of menopause (VMS; hot flashes/flushes and night sweats) are common among females undergoing menopausal transition and have been associated with elevated blood pressure (BP) and increased CVD risk. Autonomic dysregulation of BP has been posited as a contributing factor to the elevated CVD risk in menopausal females with VMS. This review includes 1) a brief overview of the relationship between VMS and CVD, 2) mechanisms of hot flushes and their potential impact on short- and long-term BP regulation, and 3) how the disruption of autonomic function associated with VMS might provide a mechanistic pathway to CVD development. Finally, this review will highlight knowledge gaps and future directions toward better understanding of hot flush physiology and VMS contributions to CVD.
Keywords: blood pressure, hot flushes, menopause, night sweats, sex differences
INTRODUCTION
The prevalence of hypertension (HTN) is lower in females compared with males until about the fifth decade of life (1), at which point HTN risk increases more drastically with aging in females. In addition, HTN is more likely to be uncontrolled in females older than 60 yr compared with males (2). Hypertension among females warrants attention as they have unique conditions throughout life that can be associated with HTN that males do not experience. These conditions include the menstrual cycle, pregnancy, and menopause, and each is associated with substantial changes in several hormonal pathways that influence blood pressure (BP) regulation. With the 2017 American College of Cardiology/American Heart Association (ACC/AHA) guidelines for HTN, the prevalence of HTN in females was 19% for ages 20–44 yr, 44% for ages 45–54 yr, and 75% for ages of 65–74 yr (3). In females over the age of 75 yr, the prevalence is 85%, which is higher than age-matched males. The influence of sex and age on HTN prevalence is due to true biological sex differences, a greater proportion of older females than older males, and disparate access to health care (4, 5). Females between the ages of 45 and 64 yr, which captures the menopausal transition for most individuals, appear to have the greatest rise in prevalence of HTN as defined by the 2017 ACC/AHA guidelines for diagnosis of HTN (which codified the current BP classifications), increasing from 44 to 63% (3). In addition to females being at a greater risk of HTN, their risks for cardiovascular disease (CVD) and adverse cardiac events are also elevated after menopause compared with males (6), yet mechanisms contributing to these increased risks remain unclear.
The risk of CVD for European Americans is lower than for other ethnic and racial groups. For example, Black females in the United States have one of the highest rates of HTN in the world (7). Although there are likely several mediating factors including atherosclerosis, hyperlipidemia, obesity, physical inactivity, and lifestyle choices, it is also important to acknowledge the impact of psychosocial factors that disproportionately impact non-European American females such as trauma, stress, access to healthcare, socioeconomic status, etc. (8). Collectively, literature supports that the greater CVD risk in non-European American racial and ethnic groups is complex and multifactorial. Although it is important to note that the CVD risk is different for females of diverse backgrounds, the mechanisms and factors associated with these differences are not within the scope of this review and are reviewed elsewhere (9–12).
Because menopause occurs naturally with age and the perimenopause stage can last up to 1–4 yr and varies across racial and ethnic groups (13), the impact of menopause (cessation of the menstrual cycle, reduced estradiol and progesterone, and other hormonal changes) versus the influence of aging on BP is not well understood. For example, a recent article from the Study of Women’s Health Across the Nation (SWAN) demonstrated that the trajectory of BP may not be similar throughout the menopause transition for all females (14). The authors stratified participants into three groups based on BP changes over the menopause transition: one in which BP rose steadily before and after menopause, a second that demonstrated an accelerated BP increase after the final menstrual period, and a third in which BP rose before menopause completion but declined sharply after menopause (14). The group with a steady increase in BP appeared to demonstrate a natural aging effect. The low-accelerated BP trajectory appeared to be more linked to the changes associated with menopause, as females in this group were more likely to have experienced early menopause. Regardless of the trajectory of BP, the presence of vasomotor symptoms of menopause (VMS, hot flushes, or night sweats) was a significant predictor of high BP (14).
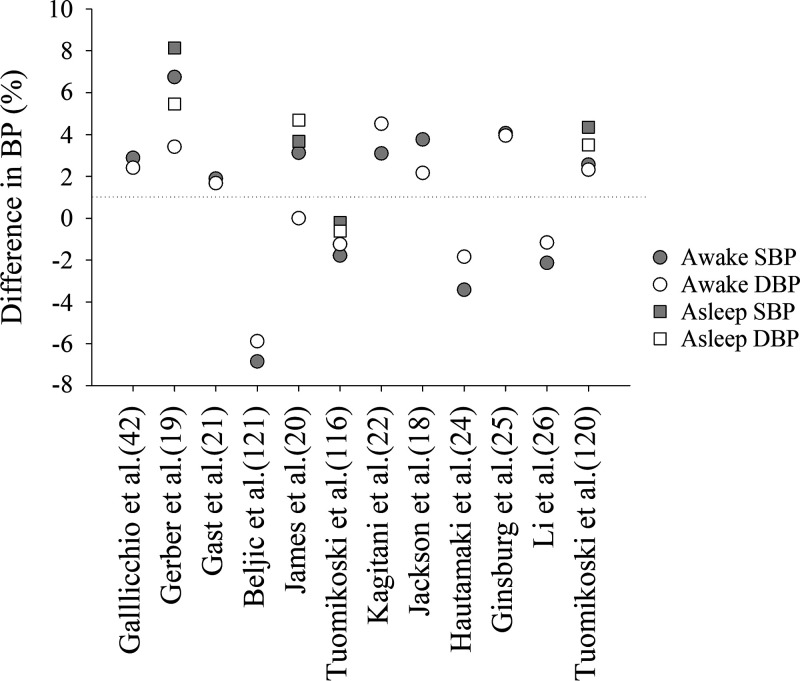
Vasomotor symptoms are experienced by 60–80% of females during and/or after the menopause transition. In some females, VMS may only last for 6 mo, but a meta-analysis demonstrated that symptoms persisted for 4 yr after menopause in 50% of females, with symptoms lasting for up to 12 yr after menopause in 10% of females (15). Another study documented that VMS can persist for up to 20 yr postmenopause (16). Because an episode of VMS can last up to several minutes (17), we refer to these episodes as “hot flushes” rather than “hot flashes” throughout this review. These symptoms are not only debilitating and interfere with quality of life, but are also associated with higher BP levels and clinical HTN (14, 18). Indeed, the SWAN reported that more frequent VMS at baseline were associated with elevated BP and HTN in a multiethnic cohort of 1,353 females (18). In a study of midlife females (n = 154), those who reported VMS had significantly higher daytime and nocturnal systolic BP compared with females without VMS (19). Both studies were well-controlled for age, race/ethnicity, body mass index (BMI), and menopause status (18, 19). Other studies have reported similar findings (20–22). However, not all studies report increased BP in females with hot flushes (23–26). Figure 1 demonstrates the percent difference in BP noted between females who experience hot flushes compared with those who do not. The discrepancy in findings between these studies is likely due to different methodologies of recording BP (24-h ambulatory BP vs. collecting one or limited BP measures), various inclusion criteria with respect to smoking and comorbidities, and varying approaches to documenting hot flushes. Thus, although a majority of the literature suggests that VMS is associated with elevated BP, this relationship is not consistent and may require additional research to be fully elucidated.
Figure 1.
Difference in systolic (SBP) and diastolic (DBP) blood pressure in females with hot flushes compared with females without hot flushes. The dotted horizontal line at 0 represents no change. Data points above 0 indicate a greater increase in females with hot flushes compared with females without hot flushes. Percent differences were calculated based on the absolute data provided in each of the publications indicated. For example: [(BP in females with VMS − BP in females without VMS)/(BP in females without VMS) × 100]. Citations are in parentheses on the x-axis. BP, blood pressure; VMS, vasomotor symptoms.
Vasomotor symptoms are also experienced differently by females of non-European American backgrounds and diverse life experiences. For example, Black females have the highest prevalence, longest duration, and greatest severity of VMS, whereas females of Asian descent have reported the lowest VMS prevalence (16, 27, 28). Females of Hispanic and European American backgrounds appear to fall somewhere between these two groups, though there are differences for Hispanic females based on their country or region of origin (29), and Mayan females from the Yucatan have reported no history of hot flush symptoms (30). Females who identify with a lower socioeconomic status are more likely to have VMS, independent of race/ethnicity (16). In addition, several other demographic and psychological factors can be associated with VMS, which may impact CVD risk. The SWAN identified that less education, smoking, greater depressive symptoms, greater anxiety, and more symptom sensitivity (i.e., sensitivity to hunger, cold, heat, noise, pain, and bodily sensations) are all related to subsequent reporting of VMS (16). Females with a history of childhood abuse or neglect are also more likely to report VMS over the menopause transition (31). In summary, both physiological and psychosocial factors should be considered when addressing the complex relationship between VMS and CVD.
It is important to note that the limited knowledge of relationships between VMS and CVD is, in part, due to the dearth of research on female populations in both preclinical and clinical studies. For example, a meta-analysis suggests that only 10% of preclinical cardiovascular research focuses on female populations (32) and recruitment of females into clinical trials remains low (33). This is despite the National Institutes of Health 1993 Revitalization Act to establish guidelines for inclusion of females and minorities in clinical research, as well as the 2016 call requiring biological sex be factored into both clinical and preclinical study designs to improve rigor and translation of science to care of patients (34). In response to the lack of research including females, the American Journal of Physiology-Heart and Circulatory Physiology has called for publications that consider sex as a biological variable in cardiovascular research (35). This has included a number of recent studies focused on sex differences in physiology (36, 37) and autonomic dysfunction (38, 39) as it relates to cardiovascular health and risk. In this capacity, we focused this female-centered review on the influence of VMS on autonomic function.
Vasomotor Symptoms and Cardiovascular Disease
Traditional CVD risk factors include HTN, dyslipidemia, insulin resistance, diabetes mellitus, older age, smoking, poor diet, higher adiposity and BMI, and lack of physical activity (40, 41). Numerous studies demonstrate that females who experience VMS are more likely to have adverse CVD risk profiles compared with individuals without VMS (18, 21, 22, 26, 42–46). The SWAN reports that females with frequent VMS (at least six of the last 14 days) had a 77% higher risk of developing CVD compared with females with no or less frequent VMS (47). Other studies using more direct indicators of cardiovascular health report physiological differences between females who do and do not have VMS, such as lower flow-mediated dilation (indicating endothelial dysfunction) (48), reduced forearm blood flow (25), greater aortic calcification (43, 48), higher carotid intima-media thickness (49), and altered blood factors involved in clotting and fibrinolysis (50) in females with VMS. In contrast, females with VMS have also demonstrated lower pulse wave velocity with a vasodilatory stimulus than those without VMS (23), suggesting a healthy cardiovascular profile. From this body of literature, it appears that having VMS, particularly hot flushes, is associated with higher CVD risk, although the exact nature of this relationship remains unclear.
In contrast, a limited number of studies have reported a lack of association, or even an inverse relationship, between VMS and traditional CVD risk factors. Hitchcock and colleagues (51) reported that among female participants with daytime VMS, BP and BMI were lower compared with participants with fewer and/or nighttime VMS; however, nocturnal VMS was associated with higher BP but lower levels of circulating inflammatory markers. Such contradictions may be related to variability of the participant profiles, including the timing of VMS onset and VMS duration. In the context of CVD risk, Tuomikoski and Savolainen-Peltonen (52) suggest that VMS duration may be more clinically meaningful than VMS presence. Furthermore, adjustment for factors such as smoking, BMI, cholesterol-lowering medications, antihypertensive medications, age, and alcohol use can attenuate relationships between VMS and measures of cardiovascular health (19, 42), although this is not always observed (45). When considering whether VMS increases a person’s risk of developing CVD, the issue of confounding factors between CVD risk and VMS remains a concern.
The autonomic nervous system (ANS), comprised both the sympathetic and parasympathetic nervous systems, may be involved in the mechanisms connecting VMS to CVD (Fig. 2). Multiple chronic diseases, including CVD, are associated with an imbalance between sympathetic nerve activity (SNA) and parasympathetic nerve activity (PNA), with an excess of SNA relative to PNA (53, 54). Muscle sympathetic nerve activity (MSNA), the gold-standard measure of direct postganglionic SNA, increases with age in both sexes, but rises more sharply in females than males throughout the lifespan (55, 56). In addition, postmenopausal females have demonstrated autonomic dysregulation of BP (57) as well as elevated sympathetic reactivity compared with age-matched males and younger females (58). Such dysfunction may contribute to the overall increase in CVD risk that occurs in females after the age of menopause. However, VMS may also be linked to adverse changes in autonomic function, which would augment the risk of CVD in postmenopausal females who experience VMS. This review will focus on recent literature related to how VMS of menopause might contribute to BP dysregulation and autonomic dysfunction, thus representing a potential mechanistic link between VMS and CVD.
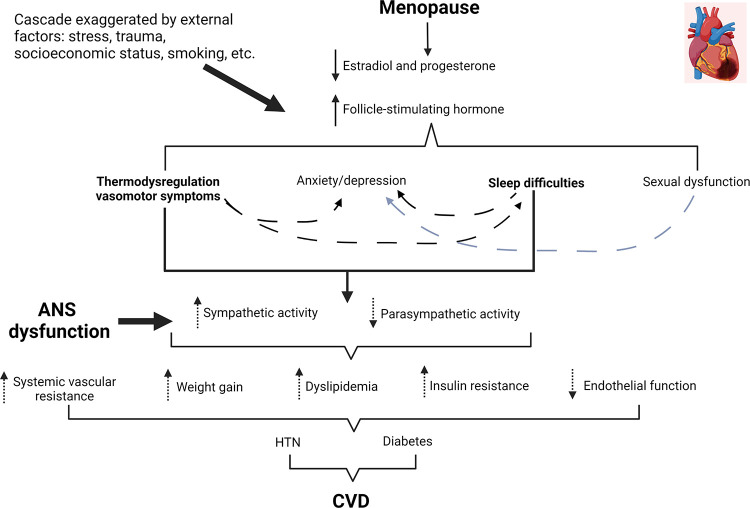
Figure 2.
Conceptual model and proposed pathways of how vasomotor symptoms (VMS) of menopause can influence autonomic (ANS) function and cardiovascular disease (CVD) risk. The loss of sex hormones, particularly estradiol, triggers thermodysregulation and VMS in some females. Although there are other mechanisms for anxiety/depression in menopausal females, VMS are also a suspected contributor. Sleep difficulties often occur with menopause and may be exaggerated by nighttime hot flushes or night sweats. Finally, this cascade of events can be exaggerated by external stressors, such as stress, trauma, and low socioeconomic status. Image was created with Biorender.com and published with permission.
Menopause and Sex Hormones
Menopause is characterized by the permanent cessation of menstruation and the progressive loss of endogenous sex hormones (59). Although the timing of physiological changes is different (60, 61) following menopausal transition or surgical menopause (60, 61), a person’s risk of developing HTN and CVD rises dramatically, and the incidence of premature and early menopause increases this risk (60). The menopausal process begins with the loss of ovarian function, and as a consequence of ovarian follicle depletion, the ovary is unable to respond to pituitary hormones such as follicle-stimulating hormone and luteinizing hormone (62). As a result, menses cease to occur. Estrone (E1), estradiol (E2), and progesterone levels decline, with E1 becoming the predominant bioactive form of estrogen after menopause (63). In contrast to estrogens, testosterone levels remain stable across the menopausal transition but gradually decline during the subsequent postmenopausal period (64). Furthermore, over 600,000 females per year undergo a bilateral oophorectomy, which causes immediate cessation of the production of sex hormones (65, 66). These individuals often experience VMS along with other menopause symptoms and are at the highest risk for CVD (67).
Bioactive estrogens (E1, E2) have numerous protective effects on cardiovascular function by modulating vascular function, metabolism, and cardiac hypertrophy (68). In addition, estrogens play an important role in autonomic function by increasing cardiovagal and sympathetic baroreflex sensitivity (69), suppressing MSNA in postmenopausal females (70–72), and preventing cell death in autonomic nuclei (73). Progesterone and testosterone may also play a role in autonomic function. In the midluteal phase, when progesterone levels are at their highest, resting heart rate (HR) and MSNA increase from the early follicular phase (low progesterone) of the menstrual cycle (74), whereas vagal activity decreases (75). Finally, testosterone is the most biologically active androgenic sex hormone and mediates mood, development of reproductive tissues, vascular function, and thermoregulation (76). Testosterone deficiency in females has been linked to sexual dysfunction, anxiety, and depression (77, 78), which collectively increase CVD risk (79); however, the effects of low testosterone on cardiovascular health in females are equivocal (80). As hormone levels decline following the menopausal transition, VMS incidence increases (81). However, there is substantial evidence to suggest that while the decline in sex hormones may be the trigger for VMS, it is not the direct cause (82, 83).
Hot Flush Physiology and Mechanisms
Hot flushes are the most common VMS experienced in menopausal females (8), and typically emerge as estrogen levels decline beginning 1–2 yr before menopause (84). Hot flushes are also a frequent result of menopause caused by bilateral oophorectomy and medical management of breast cancer (e.g., chemotherapy, radiation, and gonadotropin-releasing hormones), and the use of selective estrogen receptor modulators (SERM) can also induce hot flushes (85). There are significant interindividual variations in hot flushes, but in general, they are characterized as a transient internal sensation of heat that spreads caudally from the chest to the neck and face, resulting in profuse sweating (82). Hot flushes are often accompanied by an increase in skin SNA (86), increased heart rate, and decreased mean arterial pressure (87), which is similar to the physiological changes that occur during passive heating (86). Skin conductance, a measurement of the electrodermal response (i.e., sweat), can be used to objectively measure a hot flush. A hot flush is often accompanied by a ≥1.5–2 µΩ−1 increase of sternal skin conductivity within 30 s of onset (84, 87). It is currently unclear whether the mechanism of or the physiological response to a hot flush is associated with BP dysregulation and/or CVD.
The increased CVD risk in females with VMS could be related to changes occurring in the hypothalamic-pituitary-gonadal axis that contribute to the occurrence of VMS. Hot flushes have been attributed to changes in levels of estrogens, luteinizing hormone, and follicle-stimulating hormone; however, hot flushes have not been consistently linked with plasma concentrations or fluctuations of these hormones (88). Instead, hot flushes appear to be more directly linked to the sudden withdrawal or deprivation of estrogens (89), and to concentrations of brain norepinephrine, discussed in Autonomic Regulation in Menopausal Females with Hot Flushes. Warm ambient temperatures and peripheral body heating can trigger hot flushes in postmenopausal females who experience VMS, suggesting that the upper-temperature threshold of the thermoneutral zone is lower than in postmenopausal females who do not experience hot flushes (90).
Furthermore, ɑ2-adrenergic receptor antagonists, such as yohimbine, have been shown to elevate brain norepinephrine and trigger hot flush symptoms in symptomatic females without triggering similar responses in asymptomatic females. Yohimbine has a high affinity for ɑ2-adrenergic receptors and antagonizes ɑ-adrenergic inhibition of adrenergic transmission, increasing plasma norepinephrine (83). Conversely, clonidine, an ɑ2-adrenergic agonist, will decrease the incidence of hot flushes (83). In addition, estrogens are a known modulator of ɑ2-adrenergic receptors (91). For example, the administration of estrogen has been shown to decrease hot flush intensity and frequency by raising the sweating threshold (92), and estrogen withdrawal may play a role in hot flush initiation related to elevated sympathetic activation (83).
Despite the influence of ɑ2-adrenergic receptor antagonists and agonists on hot flushes, it appears that β2-adrenergic mechanisms likely contribute to age-related alterations in the sympathetic control of BP in females, and the positive relationship between MSNA and vascular resistance that appears in postmenopausal females is related to declining function in β2-adrenergic vasodilatory mechanisms (93). Furthermore, reductions in β2-adrenergic receptor sensitivity to norepinephrine in postmenopausal females may be reflected in altered neurovascular transduction due to decreased sensitivity to norepinephrine (94). Although estrogen withdrawal may influence hot flush initiation, it is unclear whether changes in neurovascular transduction are related to reductions in female sex hormones associated with menopause or are due to the effects of aging (94, 95).
Thermoregulatory dysfunction has been linked to CVD (96), which may be one of several reasons why high levels of bioactive estrogens (E1, E2) have been associated with a lower risk of coronary heart disease (97). Bioactive estrogens influence cardiovascular function through modulation of vascular function, metabolism, and cardiac hypertrophy (68). Estradiol in particular has been found to promote vasodilation and sweating, thereby decreasing BP and body temperature through increased heat dissipation (98). Progesterone may also contribute to thermoregulation and consequently VMS incidence. Although the thermoregulatory actions of progesterone are not well understood, it has been suggested that progesterone promotes heat conservation and higher body temperatures, opposing the heat-dissipating effects of estrogens (98). Progesterone may also increase the central operating point of thermoregulation, thereby increasing peripheral cutaneous vasoconstriction (99). Finally, although the ratio of testosterone to estrogen has been implicated in increased VMS frequency and severity (97), reports from the SWAN and others indicate that testosterone levels may be protective and an unreliable predictor of VMS in postmenopausal females (100).
Hot Flushes, Night Sweats, and Sleep
Night sweats, a component of VMS, remain poorly defined due to their close association with the hot flush. It is unclear if night sweats are hot flushes that occur at night or are a distinct phenomenon (101). Similar to the prevalence of hot flushes in racial and ethnic groups, the SWAN observed the highest night-sweat complaints in Black females (102). In another study examining night sweats, 36% of females reported night sweats alone, whereas 30% experienced hot flushes and night sweats, and perimenopausal females seemed to report night sweats more than postmenopausal females (103).
Evidence to support VMS disruption of sleep remains equivocal. Early epidemiological studies suggested there was no relationship between objective sleep measures and self-reported VMS (104, 105). Freedman and colleagues identified that when controlling for sleep apnea, drug use, and obesity, females with hot flushes did not demonstrate increased nighttime awakening or reduced sleep efficiency when compared with asymptomatic females (104). These studies contradict data from the SWAN, which reported that moderate to severe hot flushes were associated with more nocturnal waking (106). In addition, an experimental study that induced hot flushes in premenopausal females (age range: 18–45 yr) via gonadotropin-releasing agonist therapy reported a significant reduction in objective sleep efficiency, increased nocturnal wake time after sleep onset, and longer latency to sleep in the hot-flush group (107).
A recent review highlights several studies that collectively indicate that hot flushes occurring at night are associated with sleep disturbance, and particularly with frequent nocturnal awakenings (108). Zambotti and colleagues (109) reported that among perimenopausal females, hot-flush-associated wake time was related to 27% of wake time after sleep onset, and that the majority (69%) of reported hot flushes with awakenings were associated with altered polysomnography. The discrepancies across various studies are likely due to limited sample sizes and variations in controlling for sleep disorders or other confounding factors (104–109). Nonetheless, there is growing evidence supporting chronic sleep disturbance as a risk factor for HTN and CVD (110, 111), suggesting that additional studies into VMS-related sleep disruption and its link to CVD are warranted. It is important to acknowledge that a variety of other factors during menopause may also contribute to poor sleep, including poor health, chronic pain or illness, anxiety, depression, and medications; these factors should be accounted for in future research.
Mood disorders such as anxiety and depression have been associated with menopause independent of sleep disturbances or VMS (112). There is also evidence linking VMS and sleep disturbance as factors that contribute to and/or exacerbate mood disorders (113–115) (Fig. 2). It would be ideal to examine VMS, sleep disturbance, and mood disorders separately in future research to determine the independent contribution of each factor to CVD risk. However, such experimental designs have their own limitations and would diminish study feasibility and generalizability. Nevertheless, the interactions of these factors complicate the potential mechanisms underlying how VMS may contribute to CVD, and future research should account for mood disorders as a potential confounder in studies examining the complex relationship between sleep, VMS, and CVD risk.
Autonomic Regulation in Menopausal Females with Hot Flushes
During an episode of VMS, there is a consistent rise in body temperature and HR that is thought to be due, in part, to an increase in SNA (54, 116). Chronic sympathoexcitation has the potential to increase sympathetic tone and contribute to HTN, dyslipidemia, insulin resistance, and central adiposity, all hallmarks of the metabolic syndrome, an established risk factor for CVD (26). Several investigators have suggested that people who experience VMS have a higher basal level of SNA than those without VMS (23, 24). Moreover, a shift toward higher SNA and lower PNA with menopause has been demonstrated (117), which may partly account for the age-related increase in CVD risk in females, regardless of the presence of VMS. In this section, we will explore some of the known autonomic mechanisms of hot flushes, and how VMS may influence autonomic function as a potential link to CVD.
Increased SNA and/or decreased PNA can lead to elevated BP and CVD risk (118). To date, studies have demonstrated that BP is either elevated (18–22, 119, 120) or not changed (24, 25, 121, 122) in females who experience hot flushes compared with those who do not experience hot flushes (Fig. 1). For example, when calculating the average increase in systolic and diastolic BP in females with hot flushes, compared with those who do not experience hot flushes in the studies presented in Fig. 1, there was an increase of 5.3 mmHg for those who experience hot flushes and 2.7 mmHg for those who do not experience hot flushes. This finding incorporates both daytime and nocturnal recordings. In some cases, the differences remained significant when adjusting for confounders (19), but in other studies, when adjusted for smoking, age, and other CVD risk factors, the significant difference between groups was no longer detectable (42) or was mitigated (22). Overall, it appears that physiological changes that occur during hot flushes may contribute to BP dysregulation, but the mechanism remains unclear.
It is important to note that when BP is measured during a hot flush, measurable decreases are often observed (86, 123). This is because when a hot flush occurs, there is peripheral vasodilation that increases cutaneous blood flow (124) that acutely reduces BP in some, but not all, hot flushes (86, 123, 124). Such vasodilation and associated drop in BP would suggest a compensatory increase in SNA to raise BP back to baseline levels during a hot flush episode. Skin SNA has been found to rise during a hot flush (124), providing support for a greater sympathoexcitatory response to a hot flush. Further studies on the autonomic adjustments that occur with hot flushes, particularly changes in MSNA, remain unexplored and are necessary to provide mechanistic insight into the link between VMS and CVD.
There remain some inconsistencies regarding overall SNA in females with hot flushes compared with those without hot flushes (125–127). Most studies demonstrate that brain norepinephrine, particularly in the preoptic nucleus, measured by a plasma metabolite (3-methoxy-4-hydroxyphenylglycol) (127), is higher in females with hot flushes, which narrows the thermoneutral zone and contributes to triggering a hot flush (127). This elevated brain norepinephrine, however, does not always translate into greater peripheral SNA, measured by plasma norepinephrine (128) or pre-ejection period with impedance cardiography (129). Nevertheless, Cignarelli et al. (130) observed an increase in plasma norepinephrine, along with luteinizing hormone and free fatty acids, during a hot flush. Notably, some of these measures are estimates of SNA and lack adequate temporal resolution. Moreover, these prior investigations on SNA and hot flushes have not adequately investigated females of diverse racial/ethnic backgrounds. Thus, whether peripheral SNA is elevated in females who experience VMS is not yet elucidated, and SNA responses to a hot flush using gold-standard microneurographic techniques require further investigation. Baroreflex function during VMS also merits consideration, as the baroreflex regulates SNA and contributes to both short- and long-term control of BP (131). Because BP decreases during some hot flushes, it is likely that the baroreflex responds homeostatically, sending signals to increase SNA and restore BP to baseline levels. Therefore, females with more frequent or severe hot flushes may experience more sympathetic surges (Fig. 2) compared with individuals who do not experience hot flushes, although this theory has yet to be definitively explored.
As previously addressed, the literature evaluating the ANS in females with hot flushes often report surrogate markers of autonomic function. One common proxy is heart rate variability (HRV), which quantifies the variation in the intervals between heartbeats and, if interpreted correctly, can be used as a reliable tool to assess cardiac autonomic function from a risk-stratification perspective (132) and predict clinical outcomes in patients with cardiovascular disease (133). In HRV analysis, there are two domains (time and frequency). The most common clinical method is power spectral analysis of the frequency domain (132). The frequency domain consists of high-frequency (HF), low-frequency (LF), and LF/HF components; the former two provide information about autonomic function when expressed in normalized units. High-frequency HRV reflects PNA, whereas LF power and the LF/HF ratio are associated with both SNA and PNA (134). Over the past 3 decades, HRV has become a common tool to assess autonomic function (135).
Profound reductions in HF power have been associated with postmenopausal status, anxiety, and older age (136, 137), which indicates attenuated parasympathetic function in peri- and postmenopausal females. Because females who experience VMS are at a higher risk of CVD, a number of studies have focused on the assessment of HRV in females with VMS. In a cross-sectional study involving perimenopausal females, Akiyoshi and colleagues (46) reported that cardiac parasympathetic function was positively related to estrogen levels after adjusting for age. Furthermore, females who reported VMS demonstrated lower resting HRV (total power, LF power, and HF power) than participants without VMS (46). Thurston et al. (53) reported similar findings in a study involving peri- and postmenopausal females in which an acute reduction in cardiac vagal control (i.e., low HF power) was observed during objective hot flushes compared with subjective hot flushes after adjustment for age, education, race, ethnicity, age of menopause, anxiety level, and physical activity. Thus, VMS are associated with both acute and chronic attenuation of HRV (Fig. 2), which may contribute to an elevated CVD risk.
Whether HRV is altered in association with hot flushes that occur during sleep remains equivocal. During nighttime hot flushes, Freedman and coworkers (138) reported an acute increase in LF and very-low-frequency (VLF) power, and no change in HF power. On the contrary, a decrease in HF power and an increase in LF and VLF power with overnight hot flushes were observed by Hokiala et al. (139), and a profound reduction in HF power during nighttime hot flushes was observed when compared with daytime hot flushes (53, 136). Although HF power is thought to reflect vagal cardiac control, the physiological contributions of LF and VLF power remain controversial (140). Collectively, these findings may suggest that nighttime hot flushes result in an inadequate restoration of vagal tone during sleep, contributing to autonomic imbalance.
Role of Hormone Therapy in Autonomic Function
Low estradiol levels have been implicated in adverse CVD outcomes associated with menopause, and hormone replacement therapy (HT) can reduce CVD-related mortality in some groups of females (141). Reports from the Women’s Health Initiative (WHI) initially suggested that HT may be linked to breast cancer and CVD risk; however, subsequent analyses of WHI data support the timing hypothesis, which is that HT use early in the menopause transition may in fact be cardioprotective (142). Furthermore, postmenopausal females using HT have demonstrated improved vagal and reduced sympathetic indices compared with individuals not using HT, suggesting a role for estrogen in modulating both PNA and SNA in females (117). However, one randomized controlled trial observed that 6 mo of HT did not alter HF or LF power in females with hot flushes, regardless of the route of HT administration (143). Gibson and colleagues (129) reported that females with more frequent moderate or severe hot flushes had higher cardiac PNA than those with fewer VMS, which is the opposite of what might be hypothesized. However, the authors note that all participants in their study experienced VMS to some degree, and they postulate that ANS function could differentiate between females with and without VMS (129). A key question that merits further exploration is whether the mechanisms underlying VMS, or the physiological responses that comprise VMS, contribute to the CVD risk that is associated with experiencing VMS.
Gaps in the Literature and Future Directions
Although most epidemiological and observational studies have broadly investigated the relationship between VMS and CVD, mechanisms specific to how VMS can contribute to CVD remain unclear. This link could be the result of VMS-associated sleep disruption, as disturbed sleep is known to be detrimental to autonomic function (Fig. 2). Alternatively or additionally, this connection between VMS and CVD could be due to greater sympathetic neural drive with frequent or severe hot flushes, contributing to long-term ANS dysfunction (54).
Females who progress through menopause commonly report difficulty sleeping (144), and this sleep disruption is often associated with the presence of VMS (87). Sleep disruption is linked to elevated risks for HTN (145) and CVD (146), as well as greater SNA reactivity (147). Sleep disruption may represent an important causal factor in how VMS are associated with an increased risk of CVD. However, many laboratory experiments and epidemiological studies do not differentiate between hot flushes and night sweats. This distinction is important because hot flushes and night sweats may influence CVD risk independently of one another (21). Night sweats were also associated with poor sleep and nighttime awakenings (87), which independently increase the risk of CVD (148) and can exaggerate sympathetic reactivity (147). Future research should interrogate the relationships between VMS, ANS, and CVD in postmenopausal females by investigating the distinct contributions of hot flushes, night sweats, and sleep difficulty to ANS and CVD risk.
Finally, we hypothesize that chronic accumulation of acute sympathoexcitation during hot flushes may cause long-term ANS dysfunction. Although the evidence is sparse, skin SNA and brain norepinephrine increase during a hot flush, suggesting greater sympathoexcitation in females experiencing VMS. However, the impact of hot flushes on plasma norepinephrine and other estimates of sympathetic activity has been inconsistent. Repeated episodes of acute sympathoexcitation during hot flushes over time could lead to broader ANS dysfunction, contributing to the increased risk of CVD. As epidemiological evidence suggests a link between VMS and CVD, there is a need for rigorous experimental studies to identify the mechanistic pathway(s) to better understand how hot flushes and night sweats could lead to HTN and CVD risk.
GRANTS
This work was funded by National Institutes of Health Grants K01AG064038-01A1 (to M.L.K.-R.), F32HL160012-01A1 (to E.L.), and R01AA024892-01A1 (to J.R.C.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Jason Carter is an editor of American Journal of Physiology-Heart and Circulatory Physiology and was not involved and did not have access to information regarding the peer-review process or final disposition of this article. An alternate editor oversaw the peer-review and decision-making process for this article.
AUTHOR CONTRIBUTIONS
M.L.K.-R. conceived and designed research; M.L.K.-R. prepared figures; E.L., M.A., C.T.T., M.V.N., W.S., J.R.C., and M.L.K.-R. drafted manuscript; E.L., M.A., C.T.T., M.V.N., W.S., J.R.C., and M.L.K.-R. edited and revised manuscript; E.L., M.A., C.T.T., M.V.N., W.S., J.R.C., and M.L.K.-R. approved final version of manuscript.
REFERENCES
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R , et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 135: e146–e603, 2017. [Erratum in Circulation 135: e646, 2017, and in Circulation 136: e196, 2017]. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lloyd-Jones DM, Evans JC, Levy D. Hypertension in adults across the age spectrum: current outcomes and control in the community. JAMA 294: 466–472, 2005. doi: 10.1001/jama.294.4.466. [DOI] [PubMed] [Google Scholar]
- 3. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr.. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 71: 1269–1324, 2018. [Erratum in Hypertension 71: e136–e139, 2018, and in Hypertension 72: e33, 2018]. doi: 10.1161/HYP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 4. Abramson BL, Srivaratharajah K, Davis LL, Parapid B. Women and Hypertension: Beyond the 2017 Guideline for Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. American College of Cardiology, 2018. https://www.acc.org/latest-in-cardiology/articles/2018/07/27/09/02/women-and-hypertension [Accessed Sept 2022]. [Google Scholar]
- 5. McSweeney JC, Rosenfeld AG, Abel WM, Braun LT, Burke LE, Daugherty SL, Fletcher GF, Gulati M, Mehta LS, Pettey C, Reckelhoff JF; American Heart Association Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council on Hypertension, Council on Lifestyle and Cardiometabolic Health, and Council on Quality of Care and Outcomes Research. Preventing and experiencing ischemic heart disease as a woman: state of the science: a scientific statement from the American Heart Association. Circulation 133: 1302–1331, 2016. doi: 10.1161/CIR.0000000000000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS , et al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation 145: e153–e639, 2022. [Erratum in Circulation 146: e141, 2022]. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 7. Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM , et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women–2011 update: a guideline from the American Heart Association. Circulation 123: 1243–1262, 2011. [Erratum in Circulation 123: e624, 2011, and in Circulation 124: e427, 2011]. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Divens LL, Chatmon BN. Cardiovascular disease management in minority women: special considerations. Crit Care Nurs Clin North Am 31: 39–47, 2019. doi: 10.1016/j.cnc.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 9. Williams RA. Cardiovascular disease in African American women: a health care disparities issue. J Natl Med Assoc 101: 536–540, 2009. doi: 10.1016/s0027-9684(15)30938-x. [DOI] [PubMed] [Google Scholar]
- 10. Juarbe TC. Risk factors for cardiovascular disease in Latina women. Prog Cardiovasc Nurs 13: 17–27, 1998. [PubMed] [Google Scholar]
- 11. Struthers R, Savik K, Hodge FS. American Indian women and cardiovascular disease: response behaviors to chest pain. J Cardiovasc Nurs 19: 158–163, 2004. doi: 10.1097/00005082-200405000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Finkelstein EA, Khavjou OA, Mobley LR, Haney DM, Will JC. Racial/ethnic disparities in coronary heart disease risk factors among WISEWOMAN enrollees. J Womens Health (Larchmt) 13: 503–518, 2004. doi: 10.1089/1540999041280963. [DOI] [PubMed] [Google Scholar]
- 13. Chan S, Gomes A, Singh RS. Is menopause still evolving? Evidence from a longitudinal study of multiethnic populations and its relevance to women’s health. BMC Womens Health 20: 74, 2020. doi: 10.1186/s12905-020-00932-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Samargandy S, Matthews KA, Brooks MM, Barinas-Mitchell E, Magnani JW, Thurston RC, El Khoudary SR. Trajectories of blood pressure in midlife women: does menopause matter? Circ Res 130: 312–322, 2022. doi: 10.1161/CIRCRESAHA.121.319424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Politi MC, Schleinitz MD, Col NF. Revisiting the duration of vasomotor symptoms of menopause: a meta-analysis. J Gen Intern Med 23: 1507–1513, 2008. doi: 10.1007/s11606-008-0655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gold EB, Colvin A, Avis N, Bromberger J, Greendale GA, Powell L, Sternfeld B, Matthews K. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women’s health across the nation. Am J Public Health 96: 1226–1235, 2006. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sturdee DW. The menopausal hot flush—anything new? Maturitas 60: 42–49, 2008. doi: 10.1016/j.maturitas.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 18. Jackson EA, El Khoudary SR, Crawford SL, Matthews K, Joffe H, Chae C, Thurston RC. Hot flash frequency and blood pressure: data from the study of women’s health across the nation. J Womens Health (Larchmt) 25: 1204–1209, 2016. doi: 10.1089/jwh.2015.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gerber LM, Sievert LL, Warren K, Pickering TG, Schwartz JE. Hot flashes are associated with increased ambulatory systolic blood pressure. Menopause 14: 308–315, 2007. doi: 10.1097/01.gme.0000236938.74195.c6. [DOI] [PubMed] [Google Scholar]
- 20. James GD, Sievert LL, Flanagan E. Ambulatory blood pressure and heart rate in relation to hot flash experience among women of menopausal age. Ann Hum Biol 31: 49–58, 2004. doi: 10.1080/03014460310001636561. [DOI] [PubMed] [Google Scholar]
- 21. Gast G-CM, Grobbee DE, Pop VJM, Keyzer JJ, Wijnands-van Gent CJM, Samsioe GN, Nilsson PM, van der Schouw YT. Menopausal complaints are associated with cardiovascular risk factors. Hypertension 51: 1492–1498, 2008. doi: 10.1161/HYPERTENSIONAHA.107.106526. [DOI] [PubMed] [Google Scholar]
- 22. Kagitani H, Asou Y, Ishihara N, Hoshide S, Kario K. Hot flashes and blood pressure in middle-aged Japanese women. Am J Hypertens 27: 503–507, 2014. doi: 10.1093/ajh/hpt125. [DOI] [PubMed] [Google Scholar]
- 23. Tuomikoski P, Ebert P, Groop P-H, Haapalahti P, Hautamäki H, Rönnback M, Ylikorkala O, Mikkola TS. Evidence for a role of hot flushes in vascular function in recently postmenopausal women. Obstet Gynecol 113: 902–908, 2009. doi: 10.1097/AOG.0b013e31819cac04. [DOI] [PubMed] [Google Scholar]
- 24. Hautamäki H, Piirilä P, Haapalahti P, Tuomikoski P, Sovijärvi ARA, Ylikorkala O, Mikkola TS. Cardiovascular autonomic responsiveness in postmenopausal women with and without hot flushes. Maturitas 68: 368–373, 2011. doi: 10.1016/j.maturitas.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 25. Ginsburg J, Hardiman P, O'Reilly B. Peripheral blood flow in menopausal women who have hot flushes and in those who do not. BMJ 298: 1488–1490, 1989. doi: 10.1136/bmj.298.6686.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li J, Liu B, Tang R, Luo M, Li HJ, Peng Y, Wang Y, Liu G, Lin S, Chen R. Relationship between vasomotor symptoms and metabolic syndrome in Chinese middle-aged women. Climacteric 24: 151–156, 2021. doi: 10.1080/13697137.2020.1789094. [DOI] [PubMed] [Google Scholar]
- 27. Avis NE, Crawford SL, Greendale G, Bromberger JT, Everson-Rose SA, Gold EB, Hess R, Joffe H, Kravitz HM, Tepper PG, Thurston RC; Study of Women's Health Across the Nation. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med 175: 531–539, 2015. doi: 10.1001/jamainternmed.2014.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thurston RC, Bromberger JT, Joffe H, Avis NE, Hess R, Crandall CJ, Chang Y, Green R, Matthews KA. Beyond frequency. Menopause 15: 841–847, 2008. doi: 10.1097/gme.0b013e318168f09b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Green R, Polotsky AJ, Wildman RP, McGinn AP, Lin J, Derby C, Johnston J, Ram KT, Crandall CJ, Thurston R, Gold E, Weiss G, Santoro N. Menopausal symptoms within a Hispanic cohort: SWAN, the Study of Women’s Health Across the Nation. Climacteric 13: 376–384, 2010. doi: 10.3109/13697130903528272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beyene Y, Martin MC. Menopausal experiences and bone density of Mayan women in Yucatan, Mexico. Am J Hum Biol 13: 505–511, 2001. doi: 10.1002/ajhb.1082. [DOI] [PubMed] [Google Scholar]
- 31. Thurston RC, Bromberger J, Chang Y, Goldbacher E, Brown C, Cyranowski JM, Matthews KA. Childhood abuse or neglect is associated with increased vasomotor symptom reporting among midlife women. Menopause 15: 16–22, 2008. [PMC free article] [PubMed] [Google Scholar]
- 32. Ramirez FD, Motazedian P, Jung RG, Di Santo P, MacDonald Z, Simard T, Clancy AA, Russo JJ, Welch V, Wells GA, Hibbert B. Sex bias is increasingly prevalent in preclinical cardiovascular research: implications for translational medicine and health equity for women: A systematic assessment of leading cardiovascular journals over a 10-year period. Circulation 135: 625–626, 2017. doi: 10.1161/CIRCULATIONAHA.116.026668. [DOI] [PubMed] [Google Scholar]
- 33. López-Vilella R, Marqués-Sulé E, Laymito Quispe R. D P, Sánchez-Lázaro I, Donoso Trenado V, Martínez Dolz L, Almenar Bonet L. The female sex confers different prognosis in heart failure: same mortality but more readmissions. Front Cardiovasc Med 8: 618398, 2021. doi: 10.3389/fcvm.2021.618398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arnegard ME, Whitten LA, Hunter C, Clayton JA. Sex as a biological variable: a 5-year progress report and call to action. J Womens Health (Larchmt) 29: 858–864, 2020. doi: 10.1089/jwh.2019.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lindsey ML, LeBlanc AJ, Ripplinger CM, Carter JR, Kirk JA, Keehan KH, Brunt KR, Kleinbongard P, Kassiri Z. Reinforcing rigor and reproducibility expectations for use of sex and gender in cardiovascular research. Am J Physiol Heart Circ Physiol 321: H819–H824, 2021. doi: 10.1152/ajpheart.00418.2021. [DOI] [PubMed] [Google Scholar]
- 36. Badrov MB, Keir DA, Notarius CF, O’Donnell E, Millar PJ, Kimmerly DS, Shoemaker JK, Floras JS. Influence of sex and age on the relationship between aerobic fitness and muscle sympathetic nerve activity in healthy adults. Am J Physiol Heart Circ Physiol 323: H934–H940, 2022. doi: 10.1152/ajpheart.00450.2022. [DOI] [PubMed] [Google Scholar]
- 37. Harada E, Mizuno Y, Ishii M, Ishida T, Yamada T, Kugimiya F, Yasue H. β-Blockers are associated with increased B-type natriuretic peptide levels differently in men and women in heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol 323: H276–H284, 2022. doi: 10.1152/ajpheart.00029.2022. [DOI] [PubMed] [Google Scholar]
- 38. Castro PM, Santos R, Freitas J, Panerai RB, Azevedo E. Autonomic dysfunction affects dynamic cerebral autoregulation during Valsalva maneuver: comparison between healthy and autonomic dysfunction subjects. J Appl Physiol (1985) 117: 205–213, 2014. doi: 10.1152/japplphysiol.00893.2013. [DOI] [PubMed] [Google Scholar]
- 39. Zwack CC, McDonald R, Tursunalieva A, Cooray A, Lambert GW, Lambert EA. Does autonomic nervous system dysfunction influence cardiovascular disease risk in young adults with intellectual disability? Am J Physiol Heart Circ Physiol 320: H891–H900, 2021. doi: 10.1152/ajpheart.00807.2020. [DOI] [PubMed] [Google Scholar]
- 40. Mozaffarian D, Wilson PWF, Kannel WB. Beyond established and novel risk factors. Circulation 117: 3031–3038, 2008. doi: 10.1161/CIRCULATIONAHA.107.738732. [DOI] [PubMed] [Google Scholar]
- 41. Gast KB, Tjeerdema N, Stijnen T, Smit JWA, Dekkers OM. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: meta-analysis. PLoS One 7: e52036, 2012. doi: 10.1371/journal.pone.0052036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gallicchio L, Miller SR, Zacur H, Flaws JA. Hot flashes and blood pressure in midlife women. Maturitas 65: 69–74, 2010. doi: 10.1016/j.maturitas.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thurston RC, Kuller LH, Edmundowicz D, Matthews KA. History of hot flashes and aortic calcification among postmenopausal women. Menopause 17: 256–261, 2010. doi: 10.1097/gme.0b013e3181c1ad3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thurston RC, El Khoudary SR, Sutton-Tyrrell K, Crandall CJ, Sternfeld B, Joffe H, Gold EB, Selzer F, Matthews KA. Vasomotor symptoms and insulin resistance in the Study of Women’s Health Across the Nation. J Clin Endocrinol Metab 97: 3487–3494, 2012. doi: 10.1210/jc.2012-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thurston RC, El Khoudary SR, Sutton-Tyrrell K, Crandall CJ, Gold E, Sternfeld B, Joffe H, Selzer F, Matthews KA. Vasomotor symptoms and lipid profiles in women transitioning through menopause. Obstet Gynecol 119: 753–761, 2012. doi: 10.1097/AOG.0b013e31824a09ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Akiyoshi M, Kato K, Owa Y, Sugiyama M, Miyasaka N, Obayashi S, Kubota T, Aso T, Kimura T, Moritani T, Sato K. Relationship between estrogen, vasomotor symptoms, and heart rate variability in climacteric women. J Med Dent Sci 58: 49–59, 2011. [PubMed] [Google Scholar]
- 47. Thurston RC, Aslanidou Vlachos HE, Derby CA, Jackson EA, Brooks MM, Matthews KA, Harlow S, Joffe HE, Khoudary SR. Menopausal vasomotor symptoms and risk of incident cardiovascular disease events in SWAN. J Am Heart Assoc 10: e017416, 2021. doi: 10.1161/JAHA.120.017416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Matthews KA. Hot flashes and subclinical cardiovascular disease. Circulation 118: 1234–1240, 2008. doi: 10.1161/CIRCULATIONAHA.108.776823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thurston RC, El Khoudary SR, Guo Tepper P, Jackson EA, Joffe H, Chen H-Y, Matthews KA, Harlow S, Sowers M, Finkelstein J, Neer R, Kravitz H, Powell L, Gold E, Greendale G, Derby C, Wildman R, Santoro N, Weiss G, Rossi W, Sherman S, Ory M, McConnell D, Mori Brooks M, Sutton-Tyrrell K, McKinlay S, Johnson S, Gallagher C; Appendix. Trajectories of vasomotor symptoms and carotid intima media thickness in the Study of Women’s Health Across the Nation. Stroke 47: 12–17, 2016. doi: 10.1161/STROKEAHA.115.010600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thurston RC, El Khoudary SR, Sutton-Tyrrell K, Crandall CJ, Gold E, Sternfeld B, Selzer F, Matthews KA. Are vasomotor symptoms associated with alterations in hemostatic and inflammatory markers? Findings from the Study of Women’s Health Across the Nation. Menopause 18: 1044–1051, 2011. doi: 10.1097/gme.0b013e31821f5d39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hitchcock CL, Elliott TG, Norman EG, Stajic V, Teede H, Prior JC. Hot flushes and night sweats differ in associations with cardiovascular markers in healthy early postmenopausal women. Menopause 19: 1208–1214, 2012. doi: 10.1097/gme.0b013e31825541cc. [DOI] [PubMed] [Google Scholar]
- 52. Tuomikoski P, Savolainen-Peltonen H. Vasomotor symptoms and metabolic syndrome. Maturitas 97: 61–65, 2017. doi: 10.1016/j.maturitas.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 53. Thurston RC, Christie IC, Matthews KA. Hot flashes and cardiac vagal control during women’s daily lives. Menopause 19: 406–412, 2012. doi: 10.1097/gme.0b013e3182337166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gorodeski EZ. Autonomic dysfunction: a common mechanism for heart failure and hot flashes? Menopause 19: 382–383, 2012. doi: 10.1097/gme.0b013e31824c7a3b. [DOI] [PubMed] [Google Scholar]
- 55. Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension 45: 522–525, 2005. doi: 10.1161/01.HYP.0000160318.46725.46. [DOI] [PubMed] [Google Scholar]
- 56. Keir DA, Badrov MB, Tomlinson G, Notarius CF, Kimmerly DS, Millar PJ, Shoemaker JK, Floras JS. Influence of sex and age on muscle sympathetic nerve activity of healthy normotensive adults. Hypertension 76: 997–1005, 2020. doi: 10.1161/HYPERTENSIONAHA.120.15208. [DOI] [PubMed] [Google Scholar]
- 57. Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J, Joyner MJ. Sex and ageing differences in resting arterial pressure regulation: the role of the β-adrenergic receptors. J Physiol 589: 5285–5297, 2011. doi: 10.1113/jphysiol.2011.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Keller-Ross ML, Cunningham HA, Carter JR. Impact of age and sex on neural cardiovascular responsiveness to cold pressor test in humans. Am J Physiol Regul Integr Comp Physiol 319: R288–R295, 2020. doi: 10.1152/ajpregu.00045.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stuenkel CA. Menopausal hormone therapy and the role of estrogen. Clin Obstet Gynecol 64: 757–771, 2021. doi: 10.1097/GRF.0000000000000648. [DOI] [PubMed] [Google Scholar]
- 60. Barton M, Meyer MR. Postmenopausal hypertension: mechanisms and therapy. Hypertension 54: 11–18, 2009. doi: 10.1161/HYPERTENSIONAHA.108.120022. [DOI] [PubMed] [Google Scholar]
- 61. Mercuro G, Zoncu S, Saiu F, Mascia M, Melis GB, Rosano GMC. Menopause induced by oophorectomy reveals a role of ovarian estrogen on the maintenance of pressure homeostasis. Maturitas 47: 131–138, 2004. doi: 10.1016/S0378-5122(03)00252-4. [DOI] [PubMed] [Google Scholar]
- 62. Orlowski M, Sarao MS. Physiology, follicle stimulating hormone. In: StatPearls. Treasure Island, FL: StatPearls Publishing, 2021. [PubMed] [Google Scholar]
- 63. Davis SR, Martinez-Garcia A, Robinson PJ, Handelsman DJ, Desai R, Wolfe R, Bell RJ; ASPREE Investigator Group. Estrone is a strong predictor of circulating estradiol in women age 70 years and older. J Clin Endocrinol Metab 105: e3348–e3354, 2020. doi: 10.1210/clinem/dgaa429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.North American Menopause Society. The role of testosterone therapy in postmenopausal women: position statement of The North American Menopause Society. Menopause 12: 496–511, 2005. doi: 10.1097/01.gme.0000177709.65944.b0. [DOI] [PubMed] [Google Scholar]
- 65. Parker WH, Broder MS, Liu Z, Shoupe D, Farquhar C, Berek JS. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol 106: 219–226, 2005. doi: 10.1097/01.AOG.0000167394.38215.56. [DOI] [PubMed] [Google Scholar]
- 66. Rivera CM, Grossardt BR, Rhodes DJ, Brown RD Jr, Roger VL, Melton LJ 3rd, Rocca WA. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause 16: 15–23, 2009. doi: 10.1097/gme.0b013e31818888f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rocca WA, Shuster LT, Grossardt BR, Maraganore DM, Gostout BS, Geda YE, Melton LJ 3rd.. Long-term effects of bilateral oophorectomy on brain aging: unanswered questions from the Mayo Clinic Cohort Study of Oophorectomy and Aging. Womens Health (Lond) 5: 39–48, 2009. doi: 10.2217/17455057.5.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Murphy E. Estrogen signaling and cardiovascular disease. Circ Res 109: 687–696, 2011. doi: 10.1161/CIRCRESAHA.110.236687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101: 862–868, 2000. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- 70. Fadel PJ, Wang Z, Watanabe H, Arbique D, Vongpatanasin W, Thomas GD. Augmented sympathetic vasoconstriction in exercising forearms of postmenopausal women is reversed by oestrogen therapy. J Physiol 561: 893–901, 2004. doi: 10.1113/jphysiol.2004.073619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vongpatanasin W, Tuncel M, Mansour Y, Arbique D, Victor RG. Transdermal estrogen replacement therapy decreases sympathetic activity in postmenopausal women. Circulation 103: 2903–2908, 2001. doi: 10.1161/01.cir.103.24.2903. [DOI] [PubMed] [Google Scholar]
- 72. Weitz G, Elam M, Born J, Fehm HL, Dodt C. Postmenopausal estrogen administration suppresses muscle sympathetic nerve activity. J Clin Endocrinol Metab 86: 344–348, 2001. doi: 10.1210/jcem.86.1.7138. [DOI] [PubMed] [Google Scholar]
- 73. Saleh TM, Connell BJ. Role of oestrogen in the central regulation of autonomic function. Clin Exp Pharmacol Physiol 34: 827–832, 2007. doi: 10.1111/j.1440-1681.2007.04663.x. [DOI] [PubMed] [Google Scholar]
- 74. Carter JR, Fu Q, Minson CT, Joyner MJ. Ovarian cycle and sympathoexcitation in premenopausal women. Hypertension 61: 395–399, 2013. doi: 10.1161/HYPERTENSIONAHA.112.202598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. de Zambotti M, Nicholas CL, Colrain IM, Trinder JA, Baker FC. Autonomic regulation across phases of the menstrual cycle and sleep stages in women with premenstrual syndrome and healthy controls. Psychoneuroendocrinology 38: 2618–2627, 2013. doi: 10.1016/j.psyneuen.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Smith T, Batur P. Prescribing testosterone and DHEA: the role of androgens in women. Cleve Clin J Med 88: 35–43, 2021. doi: 10.3949/ccjm.88a.20030. [DOI] [PubMed] [Google Scholar]
- 77. Hoeger KM, Guzick DS. The use of androgens in menopause. Clin Obstet Gynecol 42: 883–894, 1999. doi: 10.1097/00003081-199912000-00016. [DOI] [PubMed] [Google Scholar]
- 78. Sands R, Studd J. Exogenous androgens in postmenopausal women. Am J Med 98: 76S–79S, 1995. doi: 10.1016/s0002-9343(99)80062-x. [DOI] [PubMed] [Google Scholar]
- 79. Silverman AL, Herzog AA, Silverman DI. Hearts and minds: stress, anxiety, and depression: unsung risk factors for cardiovascular disease. Cardiol Rev 27: 202–207, 2019. doi: 10.1097/CRD.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 80. Moreau KL, Donato AJ, Tanaka H, Jones PP, Gates PE, Seals DR. Basal leg blood flow in healthy women is related to age and hormone replacement therapy status. J Physiol 547: 309–316, 2003. doi: 10.1113/jphysiol.2002.032524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Al-Azzawi F, Palacios S. Hormonal changes during menopause. Maturitas 63: 135–137, 2009. doi: 10.1016/j.maturitas.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 82. Freedman RR. Hot flashes: behavioral treatments, mechanisms, and relation to sleep. Am J Med 118, Suppl 12B: 124–130, 2005. doi: 10.1016/j.amjmed.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 83. Freedman RR, Woodward S, Sabharwal SC. Alpha 2-adrenergic mechanism in menopausal hot flushes. Obstet Gynecol 76: 573–578, 1990. [PubMed] [Google Scholar]
- 84. Freedman RR. Menopausal hot flashes: mechanisms, endocrinology, treatment. J Steroid Biochem Mol Biol 142: 115–120, 2014. doi: 10.1016/j.jsbmb.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Stearns V, Ullmer L, López JF, Smith Y, Isaacs C, Hayes D. Hot flushes. Lancet 360: 1851–1861, 2002. doi: 10.1016/s0140-6736(02)11774-0. [DOI] [PubMed] [Google Scholar]
- 86. Low DA, Davis SL, Keller DM, Shibasaki M, Crandall CG. Cutaneous and hemodynamic responses during hot flashes in symptomatic postmenopausal women. Menopause 15: 290–295, 2008. doi: 10.1097/gme.0b013e3180ca7cfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Baker FC, Forouzanfar M, Goldstone A, Claudatos SA, Javitz H, Trinder J, De Zambotti M. Changes in heart rate and blood pressure during nocturnal hot flashes associated with and without awakenings. Sleep 42: zsz175, 2019. doi: 10.1093/sleep/zsz175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Aksel S, Schomberg DW, Tyrey L, Hammond CB. Vasomotor symptoms, serum estrogens, and gonadotropin levels in surgical menopause. Am J Obstet Gynecol 126: 165–169, 1976. doi: 10.1016/0002-9378(76)90270-2. [DOI] [PubMed] [Google Scholar]
- 89. Vilar-González S, Pérez-Rozos A, Cabanillas-Farpón R. Mechanism of hot flashes. Clin Transl Oncol 13: 143–147, 2011. doi: 10.1007/s12094-011-0633-x. [DOI] [PubMed] [Google Scholar]
- 90. Freedman RR, Woodward S, Norton DAM. Laboratory and ambulatory monitoring of menopausal hot flushes: comparison of symptomatic and asymptomatic women. Maturitas 16: 211, 1993. doi: 10.1016/0378-5122(93)90085-V. [DOI] [Google Scholar]
- 91. Etgen AM, Ansonoff MA, Quesada A. Mechanisms of ovarian steroid regulation of norepinephrine receptor-mediated signal transduction in the hypothalamus: implications for female reproductive physiology. Horm Behav 40: 169–177, 2001. doi: 10.1006/hbeh.2001.1676. [DOI] [PubMed] [Google Scholar]
- 92. Freedman RR, Blacker CM. Estrogen raises the sweating threshold in postmenopausal women with hot flashes. Fertil Steril 77: 487–490, 2002. doi: 10.1016/s0015-0282(01)03009-6. [DOI] [PubMed] [Google Scholar]
- 93. Klassen SA, Joyner MJ, Baker SE. The impact of ageing and sex on sympathetic neurocirculatory regulation. Semin Cell Dev Biol 116: 72–81, 2021. doi: 10.1016/j.semcdb.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Briant LJB, Charkoudian N, Hart EC. Sympathetic regulation of blood pressure in normotension and hypertension: when sex matters. Exp Physiol 101: 219–229, 2016. doi: 10.1113/EP085368. [DOI] [PubMed] [Google Scholar]
- 95. Baker SE, Limberg JK, Ranadive SM, Joyner MJ. Neurovascular control of blood pressure is influenced by aging, sex, and sex hormones. Am J Physiol Regul Integr Comp Physiol 311: R1271–R1275, 2016. doi: 10.1152/ajpregu.00288.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Balmain BN, Jay O, Sabapathy S, Royston D, Stewart GM, Jayasinghe R, Morris NR. Altered thermoregulatory responses in heart failure patients exercising in the heat. Physiol Rep 4: e13022, 2016. doi: 10.14814/phy2.13022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhao D, Guallar E, Ouyang P, Subramanya V, Vaidya D, Ndumele CE, Lima JA, Allison MA, Shah SJ, Bertoni AG, Budoff MJ, Post WS, Michos ED. Endogenous sex hormones and incident cardiovascular disease in post-menopausal women. J Am Coll Cardiol 71: 2555–2566, 2018. doi: 10.1016/j.jacc.2018.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Charkoudian N, Hart ECJ, Barnes JN, Joyner MJ. Autonomic control of body temperature and blood pressure: influences of female sex hormones. Clin Auton Res 27: 149–155, 2017. doi: 10.1007/s10286-017-0420-z. [DOI] [PubMed] [Google Scholar]
- 99. Charkoudian N, Stachenfeld N. Sex hormone effects on autonomic mechanisms of thermoregulation in humans. Auton Neurosci 196: 75–80, 2016. doi: 10.1016/j.autneu.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 100. Øverlie I, Moen MH, Holte A, Finset A. Androgens and estrogens in relation to hot flushes during the menopausal transition. Maturitas 41: 69–77, 2002. doi: 10.1016/s0378-5122(01)00256-0. [DOI] [PubMed] [Google Scholar]
- 101. Mold JW, Holtzclaw BJ, McCarthy L. Night sweats: a systematic review of the literature. J Am Board Fam Med 25: 878–893, 2012. doi: 10.3122/jabfm.2012.06.120033. [DOI] [PubMed] [Google Scholar]
- 102. Freeman EW, Sherif K. Prevalence of hot flushes and night sweats around the world: a systematic review. Climacteric 10: 197–214, 2007. doi: 10.1080/13697130601181486. [DOI] [PubMed] [Google Scholar]
- 103. Leidy Sievert L, Makhlouf Obermeyer C, Price K. Determinants of hot flashes and night sweats. Ann Hum Biol 33: 4–16, 2006. doi: 10.1080/03014460500421338. [DOI] [PubMed] [Google Scholar]
- 104. Freedman RR, Roehrs TA. Lack of sleep disturbance from menopausal hot flashes. Fertil Steril 82: 138–144, 2004. doi: 10.1016/j.fertnstert.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 105. Thurston RC, Blumenthal JA, Babyak MA, Sherwood A. Association between hot flashes, sleep complaints, and psychological functioning among healthy menopausal women. Int J Behav Med 13: 163–172, 2006. doi: 10.1207/s15327558ijbm1302_8. [DOI] [PubMed] [Google Scholar]
- 106. Kravitz HM, Joffe H. Sleep during the perimenopause: a SWAN story. Obstet Gynecol Clin North Am 38: 567–586, 2011. doi: 10.1016/j.ogc.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Joffe H, White DP, Crawford SL, McCurnin KE, Economou N, Connors S, Hall JE. Adverse effects of induced hot flashes on objectively recorded and subjectively reported sleep: results of a gonadotropin-releasing hormone agonist experimental protocol. Menopause 20: 905–914, 2013. doi: 10.1097/GME.0b013e31828292d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Baker FC, Lampio L, Saaresranta T, Polo-Kantola P. Sleep and sleep disorders in the menopausal transition. Sleep Med Clin 13: 443–456, 2018. doi: 10.1016/j.jsmc.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. de Zambotti M, Colrain IM, Javitz HS, Baker FC. Magnitude of the impact of hot flashes on sleep in perimenopausal women. Fertil Steril 102: 1708–1715.e1, 2014. doi: 10.1016/j.fertnstert.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Nagai M, Hoshide S, Kario K. Sleep duration as a risk factor for cardiovascular disease- a review of the recent literature. Curr Cardiol Rev 6: 54–61, 2010. doi: 10.2174/157340310790231635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J 32: 1484–1492, 2011. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 112. Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry 63: 375–382, 2006. doi: 10.1001/archpsyc.63.4.375. [DOI] [PubMed] [Google Scholar]
- 113. Sassarini DJ. Depression in midlife women. Maturitas 94: 149–154, 2016. doi: 10.1016/j.maturitas.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 114. Avis NE, Stellato R, Crawford S, Bromberger J, Ganz P, Cain V, Kagawa-Singer M. Is there a menopausal syndrome? Menopausal status and symptoms across racial/ethnic groups. Soc Sci Med 52: 345–356, 2001. doi: 10.1016/S0277-9536(00)00147-7. [DOI] [PubMed] [Google Scholar]
- 115. Avis NE, Brambilla D, McKinlay SM, Vass K. A longitudinal analysis of the association between menopause and depression. Results from the Massachusetts Women’s Health Study. Ann Epidemiol 4: 214–220, 1994. doi: 10.1016/1047-2797(94)90099-X. [DOI] [PubMed] [Google Scholar]
- 116. Tuomikoski P, Ylikorkala O, Mikkola TS. Menopausal hot flushes and vascular health. Ann Med 43: 283–291, 2011. doi: 10.3109/07853890.2010.546364. [DOI] [PubMed] [Google Scholar]
- 117. Liu CC, Kuo TBJ, Yang CCH. Effects of estrogen on gender-related autonomic differences in humans. Am J Physiol Heart Circ Physiol 285: H2188–H2193, 2003. doi: 10.1152/ajpheart.00256.2003. [DOI] [PubMed] [Google Scholar]
- 118. Schwartz PJ, De Ferrari GM. Sympathetic-parasympathetic interaction in health and disease: abnormalities and relevance in heart failure. Heart Fail Rev 16: 101–107, 2011. doi: 10.1007/s10741-010-9179-1. [DOI] [PubMed] [Google Scholar]
- 119. Chiang C, Gallicchio L, Zacur H, Miller S, Flaws JA, Smith RL. Hormone variability and hot flash experience: results from the midlife women’s health study. Maturitas 119: 1–7, 2019. doi: 10.1016/j.maturitas.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Tuomikoski P, Haapalahti P, Sarna S, Ylikorkala O, Mikkola TS. Vasomotor hot flushes and 24-hour ambulatory blood pressure in normotensive women: a placebo-controlled trial on post-menopausal hormone therapy. Ann Med 42: 334–343, 2010. doi: 10.3109/07853891003796760. [DOI] [PubMed] [Google Scholar]
- 121. Beljic T, Babic D, Marinkovic J, Prelevic GM. Effect of estrogen replacement therapy on cardiac function in postmenopausal women with and without flushes. Gynecol Endocrinol 13: 104–112, 1999. doi: 10.3109/09513599909167541. [DOI] [PubMed] [Google Scholar]
- 122. Tuomikoski P, Haapalahti P, Ylikorkala O, Mikkola TS. Vasomotor hot flushes and 24-hour ambulatory blood pressure in recently post-menopausal women. Ann Med 42: 216–222, 2010. doi: 10.3109/07853891003657319. [DOI] [PubMed] [Google Scholar]
- 123. Nelesen R, Krohn P, Dimsdale JE. Hot-flash hypotension. N Engl J Med 351: 1577–1579, 2004. doi: 10.1056/NEJM200410073511521. [DOI] [PubMed] [Google Scholar]
- 124. Low DA, Hubing KA, Del Coso J, Crandall CG. Mechanisms of cutaneous vasodilation during the postmenopausal hot flash. Menopause 18: 359–365, 2011. doi: 10.1097/gme.0b013e3181f7a17a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Freedman RR, Krell W. Reduced thermoregulatory null zone in postmenopausal women with hot flashes. Am J Obstet Gynecol 181: 66–70, 1999. doi: 10.1016/s0002-9378(99)70437-0. [DOI] [PubMed] [Google Scholar]
- 126. Freedman RR, Dinsay R. Clonidine raises the sweating threshold in symptomatic but not in asymptomatic postmenopausal women. Fertil Steril 74: 20–23, 2000. doi: 10.1016/S0015-0282(00)00563-X. [DOI] [PubMed] [Google Scholar]
- 127. Freedman RR. Physiology of hot flashes. Am J Hum Biol 13: 453–464, 2001. doi: 10.1002/ajhb.1077. [DOI] [PubMed] [Google Scholar]
- 128. Kronenberg F, Cote LJ, Linkie DM, Dyrenfurth I, Downey JA. Menopausal hot flashes: thermoregulatory, cardiovascular, and circulating catecholamine and LH changes. Maturitas 6: 31–43, 1984. doi: 10.1016/0378-5122(84)90063-x. [DOI] [PubMed] [Google Scholar]
- 129. Gibson CJ, Mendes WB, Schembri M, Grady D, Huang AJ. Cardiac autonomic function and hot flashes among perimenopausal and postmenopausal women. Menopause 24: 756–761, 2017. doi: 10.1097/GME.0000000000000843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Cignarelli M, Cicinelli E, Corso M, Cospite MR, Garruti G, Tafaro E, Giorgino R, Schonauer S. Biophysical and endocrine-metabolic changes during menopausal hot flashes: increase in plasma free fatty acid and norepinephrine levels. Gynecol Obstet Invest 27: 34–37, 1989. doi: 10.1159/000293612. [DOI] [PubMed] [Google Scholar]
- 131. Lohmeier TE, Iliescu R. The baroreflex as a long-term controller of arterial pressure. Physiology (Bethesda) 30: 148–158, 2015. doi: 10.1152/physiol.00035.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 93: 1043–1065, 1996. doi: 10.1161/01.CIR.93.5.1043. [DOI] [PubMed] [Google Scholar]
- 133. Wolf MM, Varigos GA, Hunt D, Sloman JG. Sinus arrhythmia in acute myocardial infarction. Med J Aust 2: 52–53, 1978. doi: 10.5694/j.1326-5377.1978.tb131339.x. [DOI] [PubMed] [Google Scholar]
- 134. Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol 141: 122–131, 2010. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- 135. Villareal RP, Liu BC, Massumi A. Heart rate variability and cardiovascular mortality. Curr Atheroscler Rep 4: 120–127, 2002. doi: 10.1007/s11883-002-0035-1. [DOI] [PubMed] [Google Scholar]
- 136. Thurston RC, Christie IC, Matthews KA. Hot flashes and cardiac vagal control: a link to cardiovascular risk? Menopause 17: 456–461, 2010. doi: 10.1097/gme.0b013e3181c7dea7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Friedman BH. An autonomic flexibility–neurovisceral integration model of anxiety and cardiac vagal tone. Biol Psychol 74: 185–199, 2007. doi: 10.1016/j.biopsycho.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 138. Freedman RR, Kruger ML, Wasson SL. Heart rate variability in menopausal hot flashes during sleep. Menopause 18: 897–900, 2011. doi: 10.1097/gme.0b013e31820ac941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Hoikkala H, Haapalahti P, Viitasalo M, Väänänen H, Sovijärvi ARA, Ylikorkala O, Mikkola TS. Association between vasomotor hot flashes and heart rate variability in recently postmenopausal women. Menopause 17: 315–320, 2010. doi: 10.1097/gme.0b013e3181c2bb6d. [DOI] [PubMed] [Google Scholar]
- 140. Berntson GG, Bigger JT Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology 34: 623–648, 1997. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 141. Stampfer MJ, Colditz GA, Willett WC, Manson JE, Rosner B, Speizer FE, Hennekens CH. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses’ health study. N Engl J Med 325: 756–762, 1991. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- 142. Clarkson TB, Meléndez GC, Appt SE. Timing hypothesis for postmenopausal hormone therapy: its origin, current status, and future. Menopause 20: 342–353, 2013. doi: 10.1097/GME.0b013e3182843aad. [DOI] [PubMed] [Google Scholar]
- 143. Hautamäki H, Mikkola TS, Sovijärvi ARA, Piirilä P, Haapalahti P. Menopausal hot flushes do not associate with changes in heart rate variability in controlled testing: a randomized trial on hormone therapy. Acta Obstet Gynecol Scand 92: 902–908, 2013. doi: 10.1111/aogs.12164. [DOI] [PubMed] [Google Scholar]
- 144. Kravitz HM, Ganz PA, Bromberger J, Powell LH, Sutton-Tyrrell K, Meyer PM. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause 10: 19–28, 2003. doi: 10.1097/00042192-200310010-00005. [DOI] [PubMed] [Google Scholar]
- 145. Matthews KA, Chang Y, Kravitz HM, Bromberger JT, Owens JF, Buysse DJ, Hall MH. Sleep and risk for high blood pressure and hypertension in midlife women: the SWAN (Study of Women’s Health Across the Nation) Sleep Study. Sleep Med 15: 203–208, 2014. doi: 10.1016/j.sleep.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Thurston RC, Chang Y, von Känel R, Barinas-Mitchell E, Jennings JR, Hall MH, Santoro N, Buysse DJ, Matthews KA. Sleep characteristics and carotid atherosclerosis among midlife women. Sleep 40: zsw052, 2017. doi: 10.1093/sleep/zsw052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Carter JR, Fonkoue IT, Greenlund IM, Schwartz CE, Mokhlesi B, Smoot CA. Sympathetic neural responsiveness to sleep deprivation in older adults: sex differences. Am J Physiol Heart Circ Physiol 317: H315–H322, 2019. doi: 10.1152/ajpheart.00232.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Gottlieb DJ, Redline S, Nieto FJ, Baldwin CM, Newman AB, Resnick HE, Punjabi NM. Association of usual sleep duration with hypertension: the sleep heart health study. Sleep 29: 1009–1014, 2006. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]