
Keywords: executive function, intranasal insulin, memory, mitochondrial function, physical activity
Abstract
Exercise has systemic health benefits in people, in part, through improving whole body insulin sensitivity. The brain is an insulin-sensitive organ that is often underdiscussed relative to skeletal muscle, liver, and adipose tissue. Although brain insulin action may have only subtle impacts on peripheral regulation of systemic glucose homeostasis, it is important for weight regulation as well as mental health. In fact, brain insulin signaling is also involved in processes that support healthy cognition. Furthermore, brain insulin resistance has been associated with age-related declines in memory and executive function as well as Alzheimer’s disease pathology. Herein, we provide an overview of brain insulin sensitivity in relation to cognitive function from animal and human studies, with particular emphasis placed on the impact exercise may have on brain insulin sensitivity. Mechanisms discussed include mitochondrial function, brain growth factors, and neurogenesis, which collectively help combat obesity-related metabolic disease and Alzheimer’s dementia.
INTRODUCTION
Approximately 42% of American adults were defined as obese according to their body mass index (BMI) in 2017–2018, and it is projected that this will increase to 48.9% by 2030 (1, 2). The rise in obesity is alarming because it coincides with a greater risk for type 2 diabetes (T2D), in part, through insulin resistance. Traditionally, insulin resistance is defined as an inability of insulin to exert regulation of blood glucose via “below the neck” mechanisms. For instance, decreased skeletal muscle glucose uptake and/or elevated hepatic glucose production as well as lipolysis are considered to promote stress on the pancreas to overproduce insulin in an effort to maintain circulating glucose within normal ranges. If this underlying insulin resistance persists, the pancreas becomes “exhausted” to the point of generating little or no insulin, thus creating the hyperglycemic state seen in T2D (3). More recently, elevated glucose levels and insulin resistance have been linked to neuropsychological and neurologic diseases in humans, including major depression, cognitive decline, and Alzheimer’s disease (AD) (4–6). In fact, AD has even been coined “type 3 diabetes” (7). These observations have led the field to examine the role of insulin and glucose homeostasis beyond T2D to elucidate dynamic bidirectional pathways between the brain and periphery that have been implicated in declines in cardiovascular and cognitive health.
Given that ∼5 million Americans are living with AD and almost 35 million with T2D, understanding the interaction between these two diseases has clinical implications for treatment and prevention (8, 9). Indeed, although the brain only comprises ∼2% of total adult body weight, it uses upward of 20% of blood glucose as an energy source (10). Much of this glucose utilization by the brain is noninsulin dependent, leading many to believe the brain is not reliant on insulin action for general health. However, more recent work in rodents and humans highlights that the brain is an insulin-sensitive organ as evident by the presence of insulin receptors/signaling cascades (11, 12). This action of insulin in the brain is also important for roles in cognition and feeding behavior (13, 14). Interestingly, exercise has gained attention as an effective behavioral prevention and/or treatment not only for T2D but also for minimizing cognitive decline in neurotypical aging and among patients with AD (15–17). Herein, we discuss the relationship between peripheral and brain insulin resistance and recent evidence that examines the effect of insulin on mitochondrial metabolism, brain structure and function, and cognition. In this review, we focus on how insulin and other growth factor-related hormones [i.e., insulin-like growth factor (IGF-1) and brain-derived neurotrophic factor (BDNF)] influence the brain to modulate both glucose homeostasis as well as cognition. We also review the literature on the effect of exercise on brain structure and function as it relates to age-related cognitive decline and AD from rodent and human studies, with particular emphasis on how brain insulin sensitivity may be targeted as a novel mechanism for promoting healthy cognitive function through exercise therapy (Fig. 1). Finally, we discuss the clinical implications of using medications including intranasal insulin and insulin sensitizers as an alternative or adjunct to exercise for overall well-being.
Figure 1.
A novel mechanism for promoting healthy cognitive function with exercise. Created with BioRender.com with permission. AD, Alzheimer’s disease; ROS, reactive oxygen species.
“TRADITIONAL” INSULIN RESISTANCE
Blood glucose regulation is traditionally considered to be controlled through cross talk interactions of the pancreas, liver, skeletal muscle, adipose tissue, and vasculature (18). Under fasting conditions, the α cells of the pancreas secrete glucagon, a glucoregulatory peptide hormone, responsible for stimulating endogenous glucose production to maintain normoglycemia. The liver, with secondary influences from the kidney and to a lesser extent the small intestine, is primarily responsible (e.g., ∼80%–85%) for maintaining fasting plasma glucose (18). Thus, fasting plasma glucose is clinically used to crudely estimate hepatic glucose metabolism. Under fed conditions though, insulin levels typically rise after carbohydrate absorption, thereby leading to reductions in hepatic glucose production and lipolysis while increasing skeletal muscle blood flow for insulin-mediated glucose uptake (18). Subsequently, hyperglycemia is believed to develop when pancreatic insulin secretion fails to overcome insulin resistance in either the liver, skeletal muscle, or adipose tissue. How exactly this peripheral insulin resistance develops is an area of intense investigation, but it is often attributable to excess nutrients promoting oxidative stress/inflammation that, in turn, impair the action of insulin to promote glucose uptake (19). Research from the past several years has also shown the key roles of insulin and insulin signaling in the brain (20) (see INSULIN ACTION ON BRAIN METABOLISM). The notion that the pancreas is also key for brain insulin action is worth noting as the debate has occurred regarding the ability of neurons and glial cells to produce insulin in the nervous system, although clear evidence is currently lacking. Thus, insulin transport from the blood into the brain becomes relevant and passes through the blood-brain barrier (BBB) and/or blood-cerebrospinal fluid barrier (21). However, studies have questioned diffusion through the use of radiolabeled insulin and identified that insulin localizes to brain endothelial cells in various regions (e.g., hippocampus, hypothalamus, and frontal cortex) (22, 23). In fact, insulin receptors on endothelial cells seem to have a key role in the regulation of insulin transport throughout the body. This is believed to occur in a variety of tissues (e.g., skeletal muscle and brown fat) with continuous or tight endothelial barriers when compared with other tissues with discontinuous endothelial barriers (e.g., liver) (21). The brain is unique though in that the BBB is a combination of endothelial cells, pericytes, and glial cells that collectively form tight junctions between endothelial cells and limit insulin delivery. To this extent, it is of interest that in a knockout model of endothelial insulin receptors, insulin stimulation had delayed responses for insulin signaling in olfactory bulb compared with hypothalamic, hippocampal, or prefrontal cortex neurons (21). This is clinically and scientifically germane for human health in understanding the relation of pancreatic insulin section and insulin action on the brain for regulating food intake, glucose metabolism, and cognitive benefit.
INSULIN ACTION ON BRAIN METABOLISM
Although the central nervous system regulates energy homeostasis (24), less attention has been directed toward the action of insulin in regulating brain function. Notably, the insulin receptor is expressed widely in the brain with the highest levels seen in the cerebellum, cortex, and hypothalamus (25). Upon entry into the brain, insulin is known to activate canonical pathways including IRS-1 and IRS-2 thereby leading to the activation of PI3K, AKT, and so forth. In particular, activation of AKT is known to stimulate the mammalian target of rapamycin c (mTOR) that impacts synaptic plasticity, neurotransmitter trafficking, and neuronal survival (26–28). A key difference in insulin action in the brain relates to glucose uptake. First, peripheral insulin receptors are mainly in the α isoform compared with predominantly β isoforms in the brain, which is important considering the higher insulin binding affinity in the former isoform (21). Second, neurons, glial cells, and brain endothelial cells rely on GLUT1 and GLUT3 transporters compared with mostly GLUT4 transporters in skeletal muscle under insulin-stimulated states (29). Importantly, brain glucose transporters are mainly insulin-independent compared with GLUT4. Although this makes good evolutionary sense for having a low affinity for glucose to be utilized by the central nervous system in times of need, GLUT4 transporters are located heavily in the cerebellum, hypothalamus, and hippocampus (30). Thus, the combined work indicates that most of the glucose uptake in the brain per se is not regulated by insulin signaling. This points toward the notion that insulin resistance in the brain is related more to impaired insulin signaling pathways per se. Indeed, peripheral insulin resistance often leads to “below the neck” hyperinsulinemia that can downregulate central nervous system insulin signaling and contribute to the formation of tau phosphorylation, amyloid-β toxicity, and oxidative stress/inflammation that jointly, or independently, raise cognitive decline risk (31). In addition, deficient brain insulin signaling may interfere with lipid transportation (notably cholesterol) as well as protein expression (e.g., GABA, NMDA, etc.) linked to depression, cognitive decline, and dementia (31). Together, it seems insulin plays a unique role in modifying brain metabolism pathways. In fact, there is good evidence from rodent studies that insulin acts on PI3K to stimulate mTOR, which is a known regulator of PGC1-α and nuclear respiratory factors 1 and 2 for mitochondrial biogenesis and metabolism as well (21). mTOR is known to stimulate mitochondrial proteins involved in the TCA cycle, fatty acid β-oxidation, as well as the electron transport chain (32). This is important as mitochondria are a key producer of cellular ATP, oxidative stress, and energy homeostasis. Given that obesity and T2D promote mitochondrial dysfunction, it is not surprising that insulin resistance is a related characteristic.
INSULIN REGULATES BRAIN SYSTEMIC PHYSIOLOGY
One of the established effects of insulin on the brain relates to the regulation of feeding. Insulin is known to reduce food intake in part through the binding on insulin receptors of the pro-opiomelanocortin (POMc) and agouti-related protein (AgRP) neurons in the arcuate nucleus of the hypothalamus to induce satiety as well as blunt orexigenic behaviors, respectively (33, 34). Indeed, using intranasal insulin as a treatment has been shown to induce fat loss in men, but not in women, over an 8-wk timeframe (35). Although this work suggests potential sex-based differences in insulin regulation of food intake, the work highlights that brain insulin signaling influences peripheral homeostasis. In addition to this classic action of insulin, it is also recognized to stimulate sympathetic outflow to regulate body temperature as well as increase postprandial thermogenesis (12). Insulin also affects peripheral tissues in maintaining substrate concentration levels. Indeed, insulin acts on the neurons that express AgRP neurons from rats to regulate hepatic glucose production (36, 37), while at the same time influencing POMc neurons to inhibit adipose tissue lipolysis in mice (38). In fact, decreases in hypothalamic lipid oxidation in the fasted state inhibit glucose production in the liver by activating ATP-sensitive potassium (KATP) channels in the ventromedial hypothalamus (VMH). From a neural perspective, this partly explains why hepatic glucose production decreases under fed conditions since the preferred metabolic fuel source switches from fat to carbohydrates in the brain. It is important to note that the VMH, along with the arcuate hypothalamic nucleus (ARC), also influences glucose uptake in skeletal muscle and adipose tissue. Toda et al. (39) demonstrated that leptin injection into the VMH increased glucose uptake in skeletal muscle, brown adipose tissue, and the heart in lean mice. However, the effects of insulin on hepatic glucose production and insulin sensitivity have been controversial with mixed effects reported. To further elucidate the effect of insulin on the central nervous system in women, acute intranasal insulin administration (160 IU) reduced appetite in the postprandial state (40) despite no significant differences in blood glucose concentrations. This is consistent with previous studies showing no significant decrease in hepatic glucose production following acute intranasal insulin administration in humans (41–43). However, Kishore et al. (44) showed that KATP activation via acute oral administration of diazoxoide (a known hypothalamic KATP activator) using the pancreatic euglycemic-hyperinsulinemic method significantly decreased hepatic glucose production by ∼30% in humans. Dash et al. (45) reported similar findings in healthy men where acute intranasal insulin administration (40 IU) during a pancreatic clamp significantly decreased hepatic glucose production by ∼35% compared with placebo. However, in a follow-up study with overweight/obese insulin-resistant men receiving the same intranasal insulin dose, others report no differences in hepatic glucose production during a pancreatic clamp between the experimental insulin and control group (43). The Memory Advancement with Intranasal Insulin in Type 2 Diabetes (MemAID Trial) demonstrated that intranasal insulin treatment for 24 wk increased normal and dual task walking, raised cerebral blood flow, and lowered insulin resistance as estimated by Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) in people with T2D (46). Taken together, and despite controversial studies of the brain regulating hepatic glucose production, the combined literature highlights complex interactions between insulin, the brain, and peripheral tissues.
INSULIN AND COGNITIVE FUNCTION
Cerebral glucose uptake is an important mechanism supporting cognition (20). In turn, it is not surprising that raising systemic insulin levels would favor brain glucose uptake and support memory, executive function, and cognition. Conversely, reduced glucose uptake via insulin resistance has been suggested to be an underlying contributor to accelerated brain aging (47). This notion was supported in a recent study where acute intravenous insulin administration (80 mU/m2/min) during a euglycemic-hyperinsulinemic clamp improved working memory and cognition in healthy older adults (48) as evidenced by increased blood oxygen level-dependent (BOLD) signaling via functional MRI (fMRI). Interestingly, it was also reported that diminished task-related activation was associated with decreased insulin sensitivity. In addition, Craft et al. (49) reported that regular intranasal administration of insulin (40 IU/day) for 16 wk improved memory, brain volume through structural MRI, and AD biomarkers in cerebrospinal fluid after just 2 and 4 mo in adults with mild cognitive impairment (MCI). However, not all areas of the brain may respond to insulin. For instance, insulin does not appear to increase BOLD signaling in primary visual cortex (50, 51). This follows earlier work conducted by Banks and Kastin (52) in mice who showed endogenous insulin crosses the BBB and several forebrain structures (e.g., hypothalamus, hippocampus, parietal cortex, and frontal cortex) but does not cross midbrain structures or the occipital cortex (location of primary visual cortex). As a result, the effect of insulin on the brain seems to be localized to regions responsible for higher-level cognitive processes, including executive functions (e.g., frontotemporal regions).
In addition to prefrontal-dependent executive processes, insulin is also a key component of hippocampal-dependent memory processes. For instance, acute (i.e., 1 day) and/or habitual (i.e., 21 days) intranasal insulin has been reported to improve memory for visuospatial and odor-cued spatial memory (53, 54) as well as verbal fluency tasks in adults with obesity and T2D (53, 55). These findings parallel work in young adults, whereby intranasal insulin increased working verbal memory performance 75 min after administration (56). In older adults with T2D, acute intranasal insulin has been reported to modify the functional connectivity among brain regions regulating memory and sensory and affective processing to higher-level cognition (57, 58). However, not all studies support cognitive benefits from habitual intranasal insulin in people with MCI (59), and it is possible that cognitive task specificity as well as the dose and delivery method of insulin could contribute to the observed differences. In either case, brain insulin action is emerging as a reasonable target to improve neurocognitive outcomes and overall quality of life.
BRAIN INSULIN RESISTANCE
The etiology of brain insulin resistance awaits to be fully elucidated, although several mechanisms have been proposed (Fig. 2A). Like obesity-induced insulin resistance in tissues such as skeletal muscle and the vasculature, it is not surprising that a high fat/sugar diet, lack of physical activity, and genetic predisposition have been suggested as modifiable and nonmodifiable risk factors for brain insulin resistance. Indeed, many of these risk factors are related to developing cognitive decline and AD (28). A unifying conceptual theme of these risk factors may relate to and include inflammation and oxidative stress. The elevated nutrient availability (i.e., fatty acids or glucose) may overload mitochondrial flux, such that there is an increase in reactive oxygen species (ROS). Although ROS itself is a key player in cell signaling, energy sensing, and antioxidant processes, the genesis of excessive ROS can be problematic for brain health (60–62). The brain, compared with skeletal muscle, has relatively low antioxidant activity levels (63–65). It is therefore not surprising that brain insulin resistance is linked to oxidative stress as well as elevated levels of protein, lipid, and DNA oxidation byproducts (66), suggesting heightened sensitivity of the brain to oxidative stress than peripheral tissues. In turn, these mechanisms may help to explain the associated declines in insulin receptor expression in brains of patients with AD compared with age-matched controls (67), as well as lower levels of insulin signaling cascade proteins (68). Although the pathogenesis of AD is complex and awaits to be further elucidated, extracellular plaques composed of β-amyloid and accretion of phosphorylated τ proteins into intracellular tangles is a core feature of AD. In turn, it is of interest that insulin receptor knockout animals have an accumulation of τ protein (69). Furthermore, treatment of AD mouse models through insulin pathways increases the clearance of β-amyloid (70). Interestingly, individuals with T2D have mild to moderate reductions in cognition and have an approximate 50% elevated risk of developing dementia compared with nondiabetic controls (71). This evidence insinuates that brain insulin resistance may be an important mechanism underlying cognitive decline in obesity and T2D. Indeed, Baker et al. (72) compared insulin resistance (HOMA-IR), working memory (memory encoding task), and brain activation ([F-18]FDG PET) between nondiabetic adults and adults with prediabetes/early T2D. Not only was insulin resistance associated with reduced glucose uptake in the frontal lobe, like patients with AD, but subjects with prediabetes/early T2D recalled fewer items during a memory recall task and showed diffuse neural activation and hyperactivation of neural areas not typically engaged during cognitive task performance compared with nondiabetic controls. Therefore, it appears that factors linked to insulin resistance play a complex role in cognitive function and cognitive decline.
Figure 2.
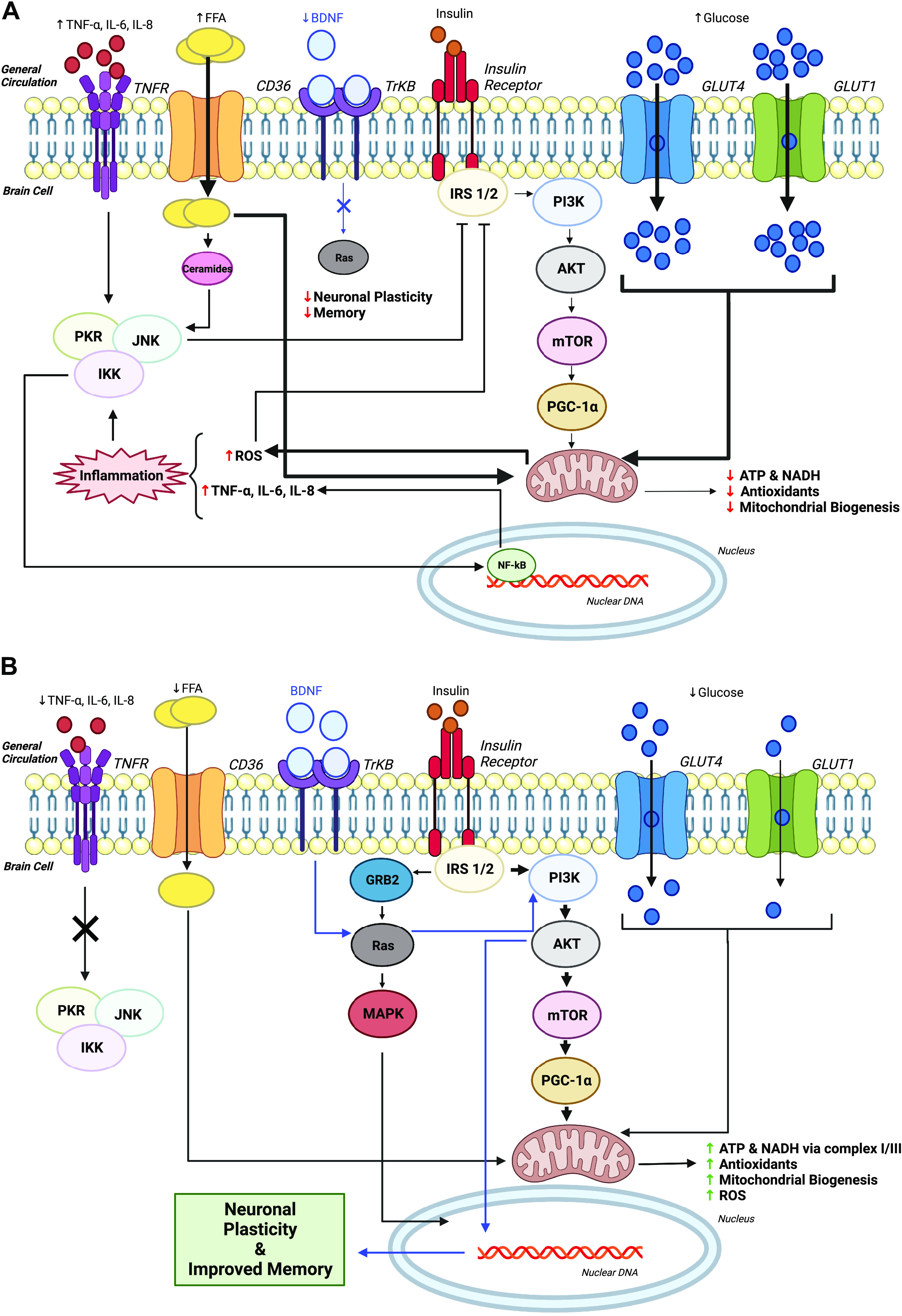
Cellular mechanism(s) by which proper peripheral insulin signaling through sedentary lifestyle (A) compared with exercise (B) may impact brain insulin signaling. AKT, serine/threonine protein kinase; ATP, adenosine triphosphate; BDNF, brain-derived neurotrophic factor; CD36, cluster of differentiation 36; CREB, cAMP response element-binding protein; FFA, free-fatty acids; GLUT1, Glucose transporter type 1; GLUT4, Glucose transporter type 4; GRB2, growth factor receptor bound protein 2; IKK, ikappaB kinase; IL-6, interleukin 6; IL-8, interleukin 8; IRS 1/2, insulin receptor substrate 1/insulin receptor substrate 2; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinases; mTOR, mammalian target of rapamycin; NADH, nicotinamide adenine dinucleotide hydrogen; NF-κB, Nuclear factor kappa-light-chain-enhancer of activated B cells; PGC-1α, proliferator-activated receptor-gamma coactivator-1alpha; PI3K, phosphoinositide 3-kinase; PKR, protein kinase R; Ras, guanosine-nucleotide-binding protein; ROS, reactive oxygen species; TNF-α, tumor necrosis factor α; TNFR, tumor necrosis factor receptor; TrKB, tropomyosin receptor kinase B. Created with BioRender with permission.
EXERCISE AND BRAIN INSULIN RESISTANCE
Aerobic and resistance exercise improve aerobic fitness and muscular strength and endurance. These changes in muscle function are clinically relevant because they are associated with decreased cognitive decline and brain degeneration (e.g., decreased gray matter volume) (73–78). In fact, slower walking speed is associated with hypoperfusion in adults with T2D and can predict cognitive impairment (58, 79). Interestingly, nonleisure time physical activity has also shown to be neuroprotective in adults, although this activity may need to be of moderate to high intensity compared with light intensity (80). This latter observation is noteworthy given interest in breaking up extended periods of sedentary behavior and identification of how movement, independent of exercise, can favorably impact health. Indeed, recent work in children and college-aged adults suggest that breaking up classroom sitting with physical activity may influence cognitive function and cerebral blood flow (81, 82). However, similar results were not observed in adults with excess body weight (83). In either case, aerobic or resistance movement improves muscle function through reduced systemic inflammation as well as favorable mitochondrial function and protein metabolism (84). However, few data exist specifically determining the effects of exercise (and/or physical activity) on brain insulin sensitivity. In rodent models, exercise has been reported to favorably impact insulin signaling in the brain (Fig. 2B). High-fat feeding in rodents is often used to induce insulin resistance and has been shown to decrease phosphorylation of insulin receptor-B, IRS-1, PI3-K, and AKT in the hippocampus (85). This decline in insulin signaling, however, was rescued with treadmill-based exercise, although not to comparable levels of exercise plus chow (carbohydrate-based) diet. Excitingly, exercise increased hippocampal neurogenesis during high-fat feeding as well. This points toward the ability of exercise to support insulin action on memory, learning, and tissue health, and to protect against obesity-related cognitive dysfunction. These findings parallel recent work in rodents showing that aerobic exercise increases brain insulin sensitivity, in part, through improved mitochondrial function (86). Gusdon et al. (87) further demonstrated that ∼3 wk of treadmill exercise training in 24-wk-old mice was associated with increased coupled complex I to complex III enzymatic activity in the brain. This increased enzymatic activity may improve brain mitochondrial function via the electron transport chain, ultimately leading to improvements in memory and cognitive function. Marosi et al. (88) reported that high-fat feeding elevated mitochondrial ROS that contributes to impaired insulin signaling in rodents. However, exercise was able to upregulate antioxidant pathways to protect against modifications of ROS on brain insulin sensitivity, a potential key deleterious factor in the aging hippocampus (88). Similarly, in a rodent model of AD where memory impairments exist, exercise was able to raise insulin signaling (e.g., IRS-1, AKT, and glycogen synthase 3-α) as well as reduce β-amyloid in conjunction with improved cognitive function (85). Improved insulin signaling may also relate to the preservation or deterioration of brain structure and function. For instance, Dietrich et al. (89) demonstrated in mice that exercise induces uncoupling protein 2 (UCP2) mRNA expression and mitochondrial oxygen consumption in the hippocampus. UCP2 is a mitochondrial protein that uncouples substrate oxidation from ATP synthesis. These changes in mitochondrial metabolism supported the increased bioenergetic adaptation to neurogenesis and synaptogenesis in response to exercise. Although these studies highlight the utility of exercise to positively impact brain insulin signaling, additional work in humans is warranted to understand how gains in brain insulin sensitivity relate to mitochondrial function as well as memory and executive function processes.
Exercise increases skeletal muscle (90), liver (91), and adipose tissue (92) insulin sensitivity to favorably regulate systemic glucose homeostasis in humans. Although few studies have focused specifically on the brain in humans, Kullmann et al. (93) recently showed that lifestyle interventions, consisting of exercise and diet counseling for 24 mo, increased brain insulin sensitivity following intranasal insulin administration in middle-aged people with prediabetes. Moreover, Honkala et al. (94) demonstrated that 2 wk of sprint interval training (SIT) in insulin-resistant, middle-aged adults decreased insulin-stimulated glucose uptake in cortical gray matter and all brain regions except the occipital lobe. Interestingly, these changes in brain insulin-stimulated glucose metabolism were not seen in adults who underwent moderate-intensity continuous training (MICT). This intensity-based effect on the brain is interesting since both SIT and MICT raised whole body insulin sensitivity via the euglycemic-hyperinsulinemic clamp. Despite this latter study suggesting exercise dose may impact tissue-specific insulin action on glucose metabolism, limited data exist to understand or support optimal mode, intensity, or timing of exercise on brain insulin sensitivity in people at risk for or with metabolic or brain disease.
EXERCISE AND COGNITION
The notion that various lifestyle behaviors such as exercise, diet, and social engagement impact cognitive functioning has continued to garner scientific attention (95). Early cross-sectional studies indicated that cognitive slowing associated with age were offset by greater amounts of leisure-time exercise and increased cardiorespiratory fitness (96, 97). Although these seminal studies were unable to establish a causal relationship between exercise and cognitive function, they provided foundational evidence to support the influence of physical activity and exercise for promoting cognitive function.
Kramer et al. (98) conducted one of the initial randomized controlled behavioral intervention trials of aerobic exercise (walking) on cognitive function among 124 previously sedentary 60- to 75-yr-old adults. Older adults assigned to aerobic exercise showed substantial improvements in performance on executive function tasks at 6 mo relative to those assigned to a stretching and toning comparator group. Interestingly, there was also a 5.1% increase in cardiorespiratory fitness over the course of the 6-mo intervention among adults in the walking condition compared with a 2.8% decrease in maximal aerobic capacity among the stretching and toning group. Their findings also pointed to the potential for task specificity in the effects of exercise on cognition, such that exercise resulted in larger effects for cognitive tasks involving executive function. Executive functions refer to higher-order cognitive functions that are supported by prefrontal and parietal brain regions. Subsequent meta-analyses (99, 100) confirmed a beneficial influence of exercise on executive functioning. Furthermore, combined aerobic and resistance exercise programs tend to result in larger effects than aerobic exercise performed alone, highlighting the potential cognitive benefit of alternative modes of exercise (e.g., resistance exercise). Interestingly, although adults with excess weight (101) and/or T2D (102) have demonstrated mild-to-moderate decrements in executive functioning, it may be possible to mitigate or reverse these deficits with exercise. Exercise has also been shown to influence cognitive domains of short- and long-term memory (103) and this may be one mechanism through which exercise decreases risk for age-related dementia and AD (104). Based on the collective evidence to date, the 2018 US Physical Activity Guidelines Committee concluded that sufficient evidence exists to indicate exercise favorably influences cognitive function in individuals across the lifespan, including those with various comorbid health conditions (105). Unfortunately, there are no clear public health guidelines for prescribing exercise to optimize its cognitive enhancing effects (106) and future research is warranted to examine the mode and dose of exercise to improve cognitive function, particularly among those at increased risk for accelerated cognitive decline.
INSULIN, EXERCISE, AND BRAIN PLASTICITY
Brain plasticity is the ability of the brain to undergo structural and functional changes in response to learning, life experience, and memory formation. This phenomenon has been studied in relation to sensory stimuli, diet, pharmaceuticals, and gonadal hormones. Still, few studies have examined the role of other hormones, such as insulin, on brain plasticity. The strength of signaling at the level of the synapse has been identified as a key underlying mechanism to facilitate changes in brain structure and function. Thus, understanding the effect of insulin at the synapse and on neurotransmitters may give insight into brain plasticity. Lee et al. (107) tested this mechanism in rodent models and found that insulin increases basal neurotransmitter release from the presynaptic terminals through activation of the PI3K/AKT/mTOR and Rac1 signaling pathways and promotes dendritic spine formations in the hippocampus. As previously mentioned, the hippocampus supports memory encoding and retrieval processes as well as various forms of learning. However, systemic hyperinsulinemia has been linked to decreased spatial memory and learning deficits in insulin-resistant, obese Zucker rats which further supports that too much insulin in the brain may have negative consequences (108). However, it is important to acknowledge that some evidence suggests that systematic hyperinsulinemia may contribute to decreased abundance and transport of insulin into the brain by downregulating the number of insulin receptors, thereby creating brain hypoinsulinemia (23). These latter findings highlight a need for additional work examining how insulin enters the brain across populations. Interestingly, Kamal et al. (108) reported that presynaptic structure and function were not the cause of impairment under hyperinsulinemic conditions in obese Zucker rats, but that diminished long-term potentiation (persistent strengthening of synapses) and decreased synaptic plasticity were associated with hyperinsulinemia.
Exercise, particularly aerobic exercise, improves brain structure and function across age groups and health states (109, 110). In one of the first studies to examine the influence of cardiorespiratory fitness on brain structure, Colcombe et al. (111) examined high-resolution magnetic resonance imaging (MRI) among 55 older adults and assessed differences in gray and white matter tissue density as a function of age and fitness. Consistent with the aging and brain structure literature, they reported age-related declines in the frontal, parietal, and temporal cortices. However, increased cardiorespiratory fitness had a sparing effect on gray matter in prefrontal, superior parietal, and temporal cortices. These brain regions are clinically relevant as they subserve aspects of higher-order cognition and executive functioning, such as working memory, cognitive flexibility, and inhibitory control. Colcombe et al. (75) later assigned sedentary older adults to either a moderate-intensity aerobic exercise group or a nonaerobic stretching and toning control group for 6 mo. Participants in both groups attended three 60-min sessions per week across the 6-mo intervention period. Using high spatial resolution MRI, increased gray matter volume was observed in prefrontal, parietal, and lateral temporal brain regions and increased white matter volume was found in the genu of the corpus callosum among the aerobic exercise group. These structural MRI data highlight the potential for aerobic exercise to attenuate the normal trajectory of age-related loss in brain tissue and enhance brain plasticity, particularly in regions critical for higher order cognition.
Erickson et al. (112) investigated whether individuals with higher levels of aerobic fitness displayed greater volume of the hippocampus and better spatial memory performance relative to their lesser fit counterparts. Their findings revealed that higher fitness levels were associated with larger left and right hippocampi after controlling for age, sex, and years of education among 165 nondemented older adults. In addition, larger hippocampi and higher fitness levels were correlated with better spatial memory performance. In a subsequent randomized control trial (77), Erickson et al. assigned 120 sedentary older adults without dementia to a moderate-intensity aerobic exercise intervention or a stretching and toning control group for one year. The aerobic exercise intervention was effective at offsetting the normal age-related deterioration in the hippocampus. In fact, there was a 2% increase in hippocampal volume following the one-year exercise program relative to a 1.4% decline in the control group. Increased hippocampal volume was associated with increased serum levels of brain-derived neurotrophic factor (BDNF) and improved memory performance. These neurogenic effects of exercise on the hippocampus have been supported by two meta-analyses of exercise on hippocampal volume. Firth et al. (113) reviewed 14 available studies and found a significant positive effect of exercise on left hippocampal volume in comparison to control conditions [g = 0.265, 95% confidence interval (CI) = 0.1–0.44]. A more recent meta-analysis demonstrated a small but significant effect of exercise on total hippocampal volume, with an effect size of g = 0.13 (114). This overall effect was driven by a significant decrease in hippocampal volume (−0.72%) in control or comparator groups, whereas an increase in hippocampal volume (1.2%) was observed across exercise treatment groups. The findings from both meta-analyses indicate that most randomized controlled trials to date have been performed on individuals over 65 years of age, suggesting that additional evidence is required before definitive statements can be made regarding the effect of exercise on the hippocampus across the lifespan or in individuals with insulin resistance, obesity, or T2D. Interestingly, individuals with overweight and obesity exhibit widespread alterations in the structure and function of the prefrontal cortex (115, 116) and hippocampus (117, 118). Behavioral weight loss interventions, including those that incorporate exercise, have been shown to improve cognitive functioning (119), highlighting that accelerated cognitive aging associated with obesity and T2D in mid-life may be reversible.
EXERCISE AND GROWTH FACTORS INFLUENCING THE BRAIN
Studies using rodent models have been instrumental in providing insight into the mechanisms through which exercise influences brain function. It is important to note that many of the neurobiological mechanisms that have been studied in animal models have been restricted to the hippocampus, although more recent work highlights influences across the brain and multiple organ systems (120). In the mid to late 1990s, evidence began to emerge indicating that the adult brain was capable of neuroplasticity and that new neurons were capable of being produced in the dentate gyrus of the hippocampus. Although the precise role of neural progenitor cells has long been debated (119), exercise emerged as an early and promising behavioral manipulation that resulted in an increased number and proliferation of new neurons in the hippocampus. Exercise may even be the critical component of the early environmental enrichment studies on neurogenesis (121). In addition to neurogenesis, exercise increases the number of connections or synapses between neurons (synaptogenesis) as well as blood supply through the proliferation of new vasculature (angiogenesis) in the cerebellum, hippocampus, motor cortex, frontal cortex, and basal ganglia (17, 122). Exercise also improves energy metabolism and neuroimmune modulation in the brain, and these neurotrophic processes may be mediated by increased production of principal growth factors (110, 122). Some of the most widely studied growth factors known to mediate the effects of exercise on brain health include BDNF, insulin-like growth factor (IGF-1), and vascular endothelial growth factor (VEGF). However, because of the widespread effects of exercise on whole body physiology, including all of the major organ systems (e.g., neuromuscular, skeletal, endocrine, cardiovascular, and respiratory), there are likely a host of different biologically plausible pathways through which exercise influences the brain and cognition (120).
BDNF is a neurotrophic factor that is considered essential for hippocampal function, synaptic plasticity, growth and differentiation of neurons, neuroprotection, as well as learning and memory. In a classic study, Neeper et al. (123) showed that physical exercise affects BDNF mRNA production not necessarily in brain regions involved in movement per se, but rather in the hippocampus and caudal cortex of rats. This is clinically relevant as these brain regions are essential to higher-level cognitive functions. Subsequent studies have confirmed that exercise increases BDNF levels in the hippocampus, cerebellum, and frontal cortex of mice, rats, and humans (124–126). Induction of BDNF in the hippocampus following exercise is regulated by other neuroendocrine systems, growth factors such as IGF-1, and neurotransmitter systems. Furthermore, BDNF signaling (by binding to its receptor) is a crucial mechanism underlying improved hippocampal-dependent learning in response to exercise (127) and blocking BDNF in animals by infusing an antibody that blocks BDNF activation of its receptor eliminates the benefit of exercise on learning (128). Inhibiting BDNF action also blocks the influence of exercise on downstream neurobiological pathways important for synaptic plasticity and cell proliferation (122). Collectively, these findings highlight a central role of BDNF in the effects of exercise on hippocampal plasticity, function, and learning.
In addition to BDNF, exercise also influences IGF-1 gene expression and protein levels, both in the periphery and in several brain regions (129). IGF-1 is a growth factor that influences neurogenic processes and reduced IGF-1 levels may contribute to age-associated cognitive decline (130). In addition, circulating IGF-1 levels are associated with hippocampal volume and performance on verbal learning and memory tasks in older adults (131). Circulating levels of IGF-1 are increased following exercise (132), can cross the BBB, and result in increased IGF-1 levels in the brain, particularly in the hippocampus (133). If circulating levels of IGF-1 are prevented from entering the central nervous system, many of the benefits from exercise, including neurogenesis, angiogenesis, and improved memory do not occur (127, 133). Together, the existing evidence suggests IGF-1 is another neurobiological mediator of the salutary effects of exercise on learning and memory.
VEGF is a potent angiogenic factor that is produced by skeletal muscles in response to exercise. Similar to IGF-1, circulating levels of VEGF cross the BBB into the central nervous system and contribute to exercise-dependent stimulation of neurogenesis and angiogenesis (127), although not all studies in humans support systemic effects of VEGF (134–136). In either case, studies in mice using antibodies that block entry of peripheral VEGF into the brain have shown that VEGF may be an important mediator of exercise-induced hippocampal neurogenesis (137).
The molecular mechanisms that underlie the beneficial effects of exercise remain poorly understood and recent work has suggested the influence of “exerkines,” a broad variety of signaling moieties released in response to acute and/or chronic exercise that exert their effects across multiple organ systems through endocrine, paracrine, and/or autocrine pathways (120). Consistent with the mechanisms discussed herein, it is possible that exercise results in complex yet critical interactions between insulin, IGF, VEGF, and BDNF on brain and body systems. Recent studies have begun to shed light on these potential interactions (138), and interestingly, have framed these purported interactions within the context of inflammation, stress, and resilience. Related to neurocognitive mechanisms of acute and chronic exercise, it is also important to examine regional versus global effects of these “exerkines” on the brain. Much of the research evidence of IGF, VEGF, and BDNF has focused on the hippocampus while exercise-related changes in insulin seem to have a broader cortical effect. Such findings may advance exercise therapy from a precision medicine approach.
FUTURE DIRECTION AND CONCLUSIONS
Exercise has emerged as a promising behavioral approach for positively influencing cognitive function and reducing the risk of age-related cognitive decline (Fig. 1). Aspects of cognitive function that may be improved include processing speed, attention, executive function, memory, and academic performance among youth. In addition, exercise reduces the risk of age-related neurodegeneration, dementia, and AD (104). Based on the available scientific evidence, experts have recently concluded that “exercise unequivocally influences the brain” (110). The research evidence from humans on exercise-dependent influences on cognition has largely focused on frontal-brain-dependent tasks (executive function and cognitive control), whereas animal studies have primarily assessed the effects of exercise on hippocampal-dependent learning and plasticity (122). Although unequivocal evidence supports the influence of exercise on the prefrontal cortex and hippocampus, additional evidence exists to support the effects of exercise on numerous structural and functional aspects of the brain. How exercise influences the brain is an area that warrants further attention though, as we present the hypothesis that insulin resistance is a key factor involved in cognitive improvement. In fact, several studies support that excess nutrients and energy surplus can lead to elevations in inflammation and oxidative stress generated by mitochondria that decrease brain insulin signaling. Thus, increased energy metabolism and decreased oxidative stress that accompany exercise represent fundamental mechanisms through which exercise may promote brain insulin signaling for cognitive gain. Interestingly, the role of brain insulin action is further evidenced by pharmaceutical intervention with metformin as insulin-sensitizing agent showing in some, but not all studies, favorable effects on cognitive function that may be modified by age, sex, and glycemia status (139–142). Furthermore, the sodium glucose transporter 2 inhibitors have gained recent attention for their glucose lowering benefit as well as cognitive function preservation effects in people with T2D (143). At the same time, the insulin secretagogue class of drugs referred to as GLP-1 agonists have also been shown to reduce the risk of developing AD in people with diabetes (144). However, caution should be used when considering the influence of these agents for promoting healthy lifestyles. It is also worth noting that a combination of exercise with diet and mental health activities should be considered as the FINGER study (145) demonstrated that cognitive and social activities as well as cardiometabolic risk were improved with this intensive lifestyle modification in middle-aged to older adults. Future prospective studies designed to tease out exercise, diet, and medication are warranted to understand the underlying issues and mechanisms related to cognitive health and brain insulin resistance. In turn, better understanding how insulin action on the brain affects cognitive health may promote new therapeutic approaches to combat obesity, T2D, CVD, and neurodegenerative diseases.
GRANTS
This work was supported by, in part, by the National Institutes of Health Grant RO1-HL130296 (to S. K. Malin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.K.M. and N.R.S. conceived and designed research; S.K.M. and N.R.S. prepared figures; S.K.M., N.R.S., A.A.U., and B.L.A. drafted manuscript; S.K.M., N.R.S., A.A.U., and B.L.A. edited and revised manuscript; S.K.M., N.R.S., A.A.U., and B.L.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Christoph Buettner, for constructive feedback on the manuscript. Figures 1 and 2 and Graphical Abstract were created with BioRender.com with permission.
REFERENCES
- 1. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief 360: 1–8, 2020. [PubMed] [Google Scholar]
- 2. Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, Long MW, Gortmaker SL. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med 381: 2440–2450, 2019. doi: 10.1056/NEJMsa1909301. [DOI] [PubMed] [Google Scholar]
- 3. Robertson RP, Harmon J, Tran PO, Tanaka Y, Takahashi H. Glucose toxicity in β-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes 52: 581–587, 2003. doi: 10.2337/diabetes.52.3.581. [DOI] [PubMed] [Google Scholar]
- 4. Janson J, Laedtke T, Parisi JE, O'Brien P, Petersen RC, Butler PC. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes 53: 474–481, 2004. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- 5. Huang C-C, Chung C-M, Leu H-B, Lin L-Y, Chiu C-C, Hsu C-Y, Chiang C-H, Huang P-H, Chen T-J, Lin S-J, Chen J-W, Chan W-L. Diabetes mellitus and the risk of Alzheimer’s disease: a nationwide population-based study. PLoS One 9: e87095, 2014. doi: 10.1371/journal.pone.0087095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haroon NN, Austin PC, Shah BR, Wu J, Gill SS, Booth GL. Risk of dementia in seniors with newly diagnosed diabetes: a population-based study. Diabetes Care 38: 1868–1875, 2015. doi: 10.2337/dc15-0491. [DOI] [PubMed] [Google Scholar]
- 7. de la Monte SM, Wands JR. Alzheimer’s disease is type 3 diabetes—evidence reviewed. J Diabetes Sci Technol 2: 1101–1113, 2008. doi: 10.1177/193229680800200619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. What is Alzheimer’s Disease? (Online). https://www.cdc.gov/aging/aginginfo/alzheimers.htm [2022 Jun 24].
- 9.Centers for Disease Control and Prevention. The Facts, Stats, and Impacts of Diabetes (Online). https://www.cdc.gov/diabetes/library/spotlights/diabetes-facts-stats.html [2022 Jun 24].
- 10. Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci 36: 587–597, 2013. doi: 10.1016/j.tins.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Plum L, Schubert M, Brüning JC. The role of insulin receptor signaling in the brain. Trends Endocrinol Metab 16: 59–65, 2005. doi: 10.1016/j.tem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 12. Kleinridders A, Ferris HA, Cai W, Kahn CR. Insulin action in brain regulates systemic metabolism and brain function. Diabetes 63: 2232–2243, 2014. doi: 10.2337/db14-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kullmann S, Kleinridders A, Small DM, Fritsche A, Häring H-U, Preissl H, Heni M. Central nervous pathways of insulin action in the control of metabolism and food intake. Lancet Diabetes Endocrinol 8: 524–534, 2020. doi: 10.1016/S2213-8587(20)30113-3. [DOI] [PubMed] [Google Scholar]
- 14. Arvanitakis Z, Wang H-Y, Capuano AW, Khan A, Taïb B, Anokye-Danso F, Schneider JA, Bennett DA, Ahima RS, Arnold SE. Brain insulin signaling, Alzheimer disease pathology, and cognitive function. Ann Neurol 88: 513–525, 2020. doi: 10.1002/ana.25826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Du Z, Li Y, Li J, Zhou C, Li F, Yang X. Physical activity can improve cognition in patients with Alzheimer’s disease: a systematic review and meta-analysis of randomized controlled trials. Clin Interv Aging 13: 1593–1603, 2018. doi: 10.2147/CIA.S169565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sampath Kumar A, Maiya AG, Shastry BA, Vaishali K, Ravishankar N, Hazari A, Gundmi S, Jadhav R. Exercise and insulin resistance in type 2 diabetes mellitus: a systematic review and meta-analysis. Ann Phys Rehabil Med 62: 98–103, 2019. doi: 10.1016/j.rehab.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 17. Kramer AF, Erickson KI, Colcombe SJ. Exercise, cognition, and the aging brain. J Appl Physiol (1985) 101: 1237–1242, 2006. doi: 10.1152/japplphysiol.00500.2006. [DOI] [PubMed] [Google Scholar]
- 18. Malin SK, Liu Z, Barrett EJ, Weltman A. Exercise resistance across the prediabetes phenotypes: impact on insulin sensitivity and substrate metabolism. Rev Endocr Metab Disord 17: 81–90, 2016. doi: 10.1007/s11154-016-9352-5. [DOI] [PubMed] [Google Scholar]
- 19. Heiston EM, Malin SK. Impact of exercise on inflammatory mediators of metabolic and vascular insulin resistance in type 2 diabetes. Adv Exp Med Biol 1134: 271–294, 2019. doi: 10.1007/978-3-030-12668-1_15. [DOI] [PubMed] [Google Scholar]
- 20. Arnold SE, Arvanitakis Z, Macauley-Rambach SL, Koenig AM, Wang H-Y, Ahima RS, Craft S, Gandy S, Buettner C, Stoeckel LE, Holtzman DM, Nathan DM. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol 14: 168–181, 2018. doi: 10.1038/nrneurol.2017.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Milstein JL, Ferris HA. The brain as an insulin-sensitive metabolic organ. Mol Metab 52: 101234, 2021. doi: 10.1016/j.molmet.2021.101234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Konishi M, Sakaguchi M, Lockhart SM, Cai W, Li ME, Homan EP, Rask-Madsen C, Kahn CR. Endothelial insulin receptors differentially control insulin signaling kinetics in peripheral tissues and brain of mice. Proc Natl Acad Sci USA 114: E8478–E8487, 2017. doi: 10.1073/pnas.1710625114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gray SM, Barrett EJ. Insulin transport into the brain. Am J Physiol Cell Physiol 315: C125–C136, 2018. doi: 10.1152/ajpcell.00240.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hetherington AW, Ranson SW. Hypothalamic lesions and adiposity in the rat. Anat Rec 78: 149–172, 1940. doi: 10.1002/ar.1090780203. [DOI] [Google Scholar]
- 25. Margolis S, Saudek CD. The John Hopkins White Papers. Baltimore, MD: Johns Hopkins Medical Institutions, 1997, p. 59. [Google Scholar]
- 26. Goodner CJ, Hom FG, Berrie MA. Investigation of the effect of insulin upon regional brain glucose metabolism in the rat in vivo. Endocrinology 107: 1827–1832, 1980. doi: 10.1210/endo-107-6-1827. [DOI] [PubMed] [Google Scholar]
- 27. Shymko RM, DE Meyts P, Thomas R. Logical analysis of timing-dependent receptor signalling specificity: application to the insulin receptor metabolic and mitogenic signalling pathways. Biochem J 326: 463–469, 1997. doi: 10.1042/bj3260463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Talbot K, Wang H-Y, Kazi H, Han L-Y, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 122: 1316–1338, 2012. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McEwen BS, Reagan LP. Glucose transporter expression in the central nervous system: relationship to synaptic function. Eur J Pharmacol 490: 13–24, 2004. doi: 10.1016/j.ejphar.2004.02.041. [DOI] [PubMed] [Google Scholar]
- 30. Sankar R, Thamotharan S, Shin D, Moley KH, Devaskar SU. Insulin-responsive glucose transporters—GLUT8 and GLUT4 are expressed in the developing mammalian brain. Brain Res Mol Brain Res 107: 157–165, 2002. doi: 10.1016/S0169-328X(02)00487-4. [DOI] [PubMed] [Google Scholar]
- 31. Nguyen TT, Ta QTH, Nguyen TTD, Le TT, Vo VG. Role of insulin resistance in the Alzheimer’s disease progression. Neurochem Res 45: 1481–1491, 2020. doi: 10.1007/s11064-020-03031-0. [DOI] [PubMed] [Google Scholar]
- 32. Ruegsegger GN, Creo AL, Cortes TM, Dasari S, Nair KS. Altered mitochondrial function in insulin-deficient and insulin-resistant states. J Clin Invest 128: 3671–3681, 2018. doi: 10.1172/JCI120843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Varela L, Horvath TL. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO Rep 13: 1079–1086, 2012. doi: 10.1038/embor.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dodd GT, Kim SJ, Méquinion M, Xirouchaki CE, Brüning JC, Andrews ZB, Tiganis T. Insulin signaling in AgRP neurons regulates meal size to limit glucose excursions and insulin resistance. Sci Adv 7: eabf4100, 2021. doi: 10.1126/sciadv.abf4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hallschmid M, Benedict C, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin reduces body fat in men but not in women. Diabetes 53: 3024–3029, 2004. doi: 10.2337/diabetes.53.11.3024. [DOI] [PubMed] [Google Scholar]
- 36. Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci 5: 566–572, 2002. doi: 10.1038/nn0602-861. [DOI] [PubMed] [Google Scholar]
- 37. Pocai A, Obici S, Schwartz GJ, Rossetti L. A brain-liver circuit regulates glucose homeostasis. Cell Metab 1: 53–61, 2005. doi: 10.1016/j.cmet.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 38. Shin AC, Filatova N, Lindtner C, Chi T, Degann S, Oberlin D, Buettner C. Insulin receptor signaling in POMC, but not AgRP, neurons controls adipose tissue insulin action. Diabetes 66: 1560–1571, 2017. doi: 10.2337/db16-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Toda C, Shiuchi T, Lee S, Yamato-Esaki M, Fujino Y, Suzuki A, Okamoto S, Minokoshi Y. Distinct effects of leptin and a melanocortin receptor agonist injected into medial hypothalamic nuclei on glucose uptake in peripheral tissues. Diabetes 58: 2757–2765, 2009. doi: 10.2337/db09-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hallschmid M, Higgs S, Thienel M, Ott V, Lehnert H. Postprandial administration of intranasal insulin intensifies satiety and reduces intake of palatable snacks in women. Diabetes 61: 782–789, 2012. doi: 10.2337/db11-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Spanswick D, Smith MA, Mirshamsi S, Routh VH, Ashford MLJ. Insulin activates ATP-sensitive K + channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosci 3: 757–758, 2000. doi: 10.1038/77660. [DOI] [PubMed] [Google Scholar]
- 42. Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 8: 1376–1382, 2002. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- 43. Xiao C, Dash S, Stahel P, Lewis GF. Effects of intranasal insulin on endogenous glucose production in insulin-resistant men. Diabetes Obes Metab 20: 1751–1754, 2018. doi: 10.1111/dom.13289. [DOI] [PubMed] [Google Scholar]
- 44. Kishore P, Boucai L, Zhang K, Li W, Koppaka S, Kehlenbrink S, Schiwek A, Esterson YB, Mehta D, Bursheh S, Su Y, Gutierrez-Juarez R, Muzumdar R, Schwartz GJ, Hawkins M. Activation of KATP channels suppresses glucose production in humans. J Clin Invest 121: 4916–4920, 2011. doi: 10.1172/JCI58035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dash S, Xiao C, Morgantini C, Koulajian K, Lewis GF. Intranasal insulin suppresses endogenous glucose production in humans compared with placebo in the presence of similar venous insulin concentrations. Diabetes 64: 766–774, 2015. doi: 10.2337/db14-0685. [DOI] [PubMed] [Google Scholar]
- 46. Novak V, Mantzoros CS, Novak P, McGlinchey R, Dai W, Lioutas V, Buss S, Fortier CB, Khan F, Aponte Becerra L, Ngo LH. MemAID: memory advancement with intranasal insulin vs. placebo in type 2 diabetes and control participants: a randomized clinical trial. J Neurol 269: 4817–4835, 2022. doi: 10.1007/s00415-022-11119-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wahl D, Cavalier AN, LaRocca TJ. Novel strategies for healthy brain aging. Exerc Sport Sci Rev 49: 115–125, 2021. doi: 10.1249/JES.0000000000000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Williams VJ, Trombetta BA, Jafri RZ, Koenig AM, Wennick CD, Carlyle BC, Ekhlaspour L, Ahima RS, Russell SJ, Salat DH, Arnold SE. Task-related fMRI BOLD response to hyperinsulinemia in healthy older adults. JCI Insight 4: e129700, 2019. doi: 10.1172/jci.insight.129700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Craft S, Claxton A, Baker LD, Hanson AJ, Cholerton B, Trittschuh EH, Dahl D, Caulder E, Neth B, Montine TJ, Jung Y, Maldjian J, Whitlow C, Friedman S. Effects of regular and long-acting insulin on cognition and Alzheimer’s disease biomarkers: a pilot clinical trial. J Alzheimers Dis 57: 1325–1334, 2017. doi: 10.3233/JAD-161256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Benedict L, Nelson CA, Schunk E, Sullwold K, Seaquist ER. Effect of insulin on the brain activity obtained during visual and memory tasks in healthy human subjects. Neuroendocrinology 83: 20–26, 2006. doi: 10.1159/000093338. [DOI] [PubMed] [Google Scholar]
- 51. Seaquist ER, Chen W, Benedict LE, Ugurbil K, Kwag J-H, Zhu X-H, Nelson CA. Insulin reduces the BOLD response but is without effect on the VEP during presentation of a visual task in humans. J Cereb Blood Flow Metab 27: 154–160, 2007. doi: 10.1038/sj.jcbfm.9600316. [DOI] [PubMed] [Google Scholar]
- 52. Banks WA, Kastin AJ. Differential permeability of the blood–brain barrier to two pancreatic peptides: insulin and amylin. Peptides 19: 883–889, 1998. doi: 10.1016/S0196-9781(98)00018-7. [DOI] [PubMed] [Google Scholar]
- 53. Novak V, Milberg W, Hao Y, Munshi M, Novak P, Galica A, Manor B, Roberson P, Craft S, Abduljalil A. Enhancement of vasoreactivity and cognition by intranasal insulin in type 2 diabetes. Diabetes Care 37: 751–759, 2014. doi: 10.2337/dc13-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brünner YF, Kofoet A, Benedict C, Freiherr J. Central insulin administration improves odor-cued reactivation of spatial memory in young men. J Clin Endocrinol Metab 100: 212–219, 2015. [Erratum in J Clin Endocrinol Metab 101: 3863, 2016] doi: 10.1210/jc.2014-3018. [DOI] [PubMed] [Google Scholar]
- 55. Claxton A, Baker LD, Hanson A, Trittschuh EH, Cholerton B, Morgan A, Callaghan M, Arbuckle M, Behl C, Craft S. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia. J Alzheimers Dis 44: 897–906, 2015. [Erratum in J Alzheimers Dis 45: 1269–1270, 2015]. doi: 10.3233/JAD-141791. [DOI] [PubMed] [Google Scholar]
- 56. Benedict C, Kern W, Schultes B, Born J, Hallschmid M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J Clin Endocrinol Metab 93: 1339–1344, 2008. doi: 10.1210/jc.2007-2606. [DOI] [PubMed] [Google Scholar]
- 57. Schilling TM, Ferreira de Sá DS, Westerhausen R, Strelzyk F, Larra MF, Hallschmid M, Savaskan E, Oitzl MS, Busch H-P, Naumann E, Schächinger H. Intranasal insulin increases regional cerebral blood flow in the insular cortex in men independently of cortisol manipulation. Hum Brain Mapp 35: 1944–1956, 2014. doi: 10.1002/hbm.22304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang H, Hao Y, Manor B, Novak P, Milberg W, Zhang J, Fang J, Novak V. Intranasal insulin enhanced resting-state functional connectivity of hippocampal regions in type 2 diabetes. Diabetes 64: 1025–1034, 2015. doi: 10.2337/db14-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Craft S, Raman R, Chow TW, Rafii MS, Sun C-K, Rissman RA, Donohue MC, Brewer JB, Jenkins C, Harless K, Gessert D, Aisen PS. Safety, efficacy, and feasibility of intranasal insulin for the treatment of mild cognitive impairment and Alzheimer disease dementia: a randomized clinical trial. JAMA Neurol 77: 1099–1109, 2020. doi: 10.1001/jamaneurol.2020.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 279: L1005–L1028, 2000. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 61. Zhang Y, Lee JH, Paull TT, Gehrke S, D’Alessandro A, Dou Q. Mitochondrial redox sensing by the kinase ATM maintains cellular antioxidant capacity. Sci Signal 11: eaaq0702, 2018. doi: 10.1126/scisignal.aaq0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sun Y, Lu Y, Saredy J, Wang X, Drummer Iv C, Shao Y, Saaoud F, Xu K, Liu M, Yang WY, Jiang X, Wang H, Yang X. ROS systems are a new integrated network for sensing homeostasis and alarming stresses in organelle metabolic processes. Redox Biol 37: 101696, 2020. doi: 10.1016/j.redox.2020.101696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lyn-Cook LE, Lawton M, Tong M, Silbermann E, Longato L, Jiao P, Mark P, Wands JR, Xu H, de la Monte SM. Hepatic ceramide may mediate brain insulin resistance and neurodegeneration in type 2 diabetes and non-alcoholic steatohepatitis. J Alzheimers Dis 16: 715–729, 2009. doi: 10.3233/JAD-2009-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gandhi S, Abramov AY. Mechanism of oxidative stress in neurodegeneration. Oxid Med Cell Longev 2012: 428010, 2012. doi: 10.1155/2012/428010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Maciejczyk M, Żebrowska E, Zalewska A, Chabowski A. Redox balance, antioxidant defense, and oxidative damage in the hypothalamus and cerebral cortex of rats with high fat diet-induced insulin resistance. Oxid Med Cell Longev 2018: e6940515, 2018. doi: 10.1155/2018/6940515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Maciejczyk M, Żebrowska E, Chabowski A. Insulin resistance and oxidative stress in the brain: what’s new? Int J Mol Sci 20: 874, 2019. doi: 10.3390/ijms20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease—is this type 3 diabetes? J Alzheimers Dis 7: 63–80, 2005. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 68. Liu Y, Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Deficient brain insulin signalling pathway in Alzheimer’s disease and diabetes. J Pathol 225: 54–62, 2011. doi: 10.1002/path.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schubert M, Gautam D, Surjo D, Ueki K, Baudler S, Schubert D, Kondo T, Alber J, Galldiks N, Küstermann E, Arndt S, Jacobs AH, Krone W, Kahn CR, Brüning JC. Role for neuronal insulin resistance in neurodegenerative diseases. Proc Natl Acad Sci USA 101: 3100–3105, 2004. doi: 10.1073/pnas.0308724101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. de la Monte SM. Brain insulin resistance and deficiency as therapeutic targets in Alzheimer’s disease. Curr Alzheimer Res 9: 35–66, 2012. doi: 10.2174/156720512799015037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 5: 64–74, 2006. [Erratum in Lancet Neurol 5: 113, 2006] doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 72. Baker LD, Cross D, Minoshima S, Belongia D, Watson GS, Craft S. Insulin resistance is associated with Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with pre-diabetes or early type 2 diabetes. Arch Neurol 68: 51–57, 2011. doi: 10.1001/archneurol.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Landrigan JF, Bell T, Crowe M, Clay OJ, Mirman D. Lifting cognition: a meta-analysis of effects of resistance exercise on cognition. Psychol Res 84: 1167–1183, 2020. doi: 10.1007/s00426-019-01145-x. [DOI] [PubMed] [Google Scholar]
- 74. Paillard T. Preventive effects of regular physical exercise against cognitive decline and the risk of dementia with age advancement. Sports Med Open 1: 20, 2015. doi: 10.1186/s40798-015-0016-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci 61: 1166–1170, 2006. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 76. Broadhouse KM, Singh MF, Suo C, Gates N, Wen W, Brodaty H, Jain N, Wilson GC, Meiklejohn J, Singh N, Baune BT, Baker M, Foroughi N, Wang Y, Kochan N, Ashton K, Brown M, Li Z, Mavros Y, Sachdev PS, Valenzuela MJ. Hippocampal plasticity underpins long-term cognitive gains from resistance exercise in MCI. Neuroimage Clin 25: 102182, 2020. doi: 10.1016/j.nicl.2020.102182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 108: 3017–3022, 2011. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kelty TJ, Schachtman TR, Mao X, Grigsby KB, Childs TE, Olver TD, Michener PN, Richardson RA, Roberts CK, Booth FW. Resistance-exercise training ameliorates LPS-induced cognitive impairment concurrent with molecular signaling changes in the rat dentate gyrus. J Appl Physiol (1985) 127: 254–263, 2019. [Erratum in J Appl Physiol (1985) 128: 462, 2020] doi: 10.1152/japplphysiol.00249.2019. [DOI] [PubMed] [Google Scholar]
- 79. Chung C-C, Pimentel Maldonado DA, Jor'dan AJ, Alfaro FJ, Lioutas V-A, Núñez MZ, Novak V. Lower cerebral vasoreactivity as a predictor of gait speed decline in type 2 diabetes mellitus. J Neurol 265: 2267–2276, 2018. doi: 10.1007/s00415-018-8981-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Engeroff T, Ingmann T, Banzer W. Physical activity throughout the adult life span and domain-specific cognitive function in old age: a systematic review of cross-sectional and longitudinal data. Sports Med 48: 1405–1436, 2018. doi: 10.1007/s40279-018-0920-6. [DOI] [PubMed] [Google Scholar]
- 81. Mazzoli E, Salmon J, Teo W-P, Pesce C, He J, Ben-Soussan TD, Barnett LM. Breaking up classroom sitting time with cognitively engaging physical activity: behavioural and brain responses. PLoS One 16: e0253733, 2021. doi: 10.1371/journal.pone.0253733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Paulus M, Kunkel J, Schmidt SCE, Bachert P, Wäsche H, Neumann R, Woll A. Standing breaks in lectures improve university students’ self-perceived physical, mental, and cognitive condition. Int J Environ Res Public Health 18: 4204, 2021. doi: 10.3390/ijerph18084204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wanders L, Cuijpers I, Kessels RPC, van de Rest O, Hopman MTE, Thijssen DHJ. Impact of prolonged sitting and physical activity breaks on cognitive performance, perceivable benefits, and cardiometabolic health in overweight/obese adults: the role of meal composition. Clin Nutr 40: 2259–2269, 2021. doi: 10.1016/j.clnu.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 84. Oudbier SJ, Goh J, Looijaard SMLM, Reijnierse EM, Meskers CGM, Maier AB. Pathophysiological mechanisms explaining the association between low skeletal muscle mass and cognitive function. J Gerontol A Biol Sci Med Sci 77: 1959–1968, 2022. doi: 10.1093/gerona/glac121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Park HS, Park SS, Kim CJ, Shin MS, Kim TW. Exercise alleviates cognitive functions by enhancing hippocampal insulin signaling and neuroplasticity in high-fat diet-induced obesity. Nutrients 11: 1603, 2019. doi: 10.3390/nu11071603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ruegsegger GN, Vanderboom PM, Dasari S, Klaus KA, Kabiraj P, McCarthy CB, Lucchinetti CF, Nair KS. Exercise and metformin counteract altered mitochondrial function in the insulin-resistant brain. JCI Insight 4: 130681, 2019. doi: 10.1172/jci.insight.130681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gusdon AM, Callio J, Distefano G, O'Doherty RM, Goodpaster BH, Coen PM, Chu CT. Exercise increases mitochondrial complex I activity and DRP1 expression in the brains of aged mice. Exp Gerontol 90: 1–13, 2017. doi: 10.1016/j.exger.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Marosi K, Bori Z, Hart N, Sárga L, Koltai E, Radák Z, Nyakas C. Long-term exercise treatment reduces oxidative stress in the hippocampus of aging rats. Neuroscience 226: 21–28, 2012. doi: 10.1016/j.neuroscience.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 89. Dietrich MO, Andrews ZB, Horvath TL. Exercise-induced synaptogenesis in the hippocampus is dependent on UCP2-regulated mitochondrial adaptation. J Neurosci 28: 10766–10771, 2008. doi: 10.1523/JNEUROSCI.2744-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Stanford KI, Goodyear LJ. Exercise and type 2 diabetes: molecular mechanisms regulating glucose uptake in skeletal muscle. Adv Physiol Educ 38: 308–314, 2014. doi: 10.1152/advan.00080.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Basu R, Chandramouli V, Dicke B, Landau B, Rizza R. Obesity and type 2 diabetes impair insulin-induced suppression of glycogenolysis as well as gluconeogenesis. Diabetes 54: 1942–1948, 2005. doi: 10.2337/diabetes.54.7.1942. [DOI] [PubMed] [Google Scholar]
- 92. Ibañez J, Izquierdo M, Argüelles I, Forga L, Larrión JL, García-Unciti M, Idoate F, Gorostiaga EM. Twice-weekly progressive resistance training decreases abdominal fat and improves insulin sensitivity in older men with type 2 diabetes. Diabetes Care 28: 662–667, 2005. doi: 10.2337/diacare.28.3.662. [DOI] [PubMed] [Google Scholar]
- 93. Kullmann S, Valenta V, Wagner R, Tschritter O, Machann J, Häring H-U, Preissl H, Fritsche A, Heni M. Brain insulin sensitivity is linked to adiposity and body fat distribution. Nat Commun 11: 1841, 2020. doi: 10.1038/s41467-020-15686-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Honkala SM, Johansson J, Motiani KK, Eskelinen J-J, Virtanen KA, Löyttyniemi E, Knuuti J, Nuutila P, Kalliokoski KK, Hannukainen JC. Short-term interval training alters brain glucose metabolism in subjects with insulin resistance. J Cereb Blood Flow Metab 38: 1828–1838, 2018. [Erratum in J Cereb Blood Flow Metab 38: 1848, 2018] doi: 10.1177/0271678X17734998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Walsh R. Lifestyle and mental health. Am Psychol 66: 579–592, 2011. doi: 10.1037/a0021769. [DOI] [PubMed] [Google Scholar]
- 96. Spirduso WW. Reaction and movement time as a function of age and physical activity level 1. J Gerontol 30: 435–440, 1975. doi: 10.1093/geronj/30.4.435. [DOI] [PubMed] [Google Scholar]
- 97. Spirduso WW, Clifford P. Replication of age and physical activity effects on reaction and movement time. J Gerontol 33: 26–30, 1978. doi: 10.1093/geronj/33.1.26. [DOI] [PubMed] [Google Scholar]
- 98. Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A. Ageing, fitness and neurocognitive function. Nature 400: 418–419, 1999. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- 99. Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci 14: 125–130, 2003. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 100. Verburgh L, Königs M, Scherder EJA, Oosterlaan J. Physical exercise and executive functions in preadolescent children, adolescents and young adults: a meta-analysis. Br J Sports Med 48: 973–979, 2014. doi: 10.1136/bjsports-2012-091441. [DOI] [PubMed] [Google Scholar]
- 101. Yang Y, Shields GS, Guo C, Liu Y. Executive function performance in obesity and overweight individuals: a meta-analysis and review. Neurosci Biobehav Rev 84: 225–244, 2018. doi: 10.1016/j.neubiorev.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 102. Vincent C, Hall PA. Executive function in adults with type 2 diabetes: a meta-analytic review. Psychosom Med 77: 631–642, 2015. doi: 10.1097/PSY.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 103. Roig M, Nordbrandt S, Geertsen SS, Nielsen JB. The effects of cardiovascular exercise on human memory: a review with meta-analysis. Neurosci Biobehav Rev 37: 1645–1666, 2013. doi: 10.1016/j.neubiorev.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 104. Etnier JL, Karper WB, Labban JD, Piepmeier AT, Shih C-H, Dudley WN, Henrich VC, Wideman L. The physical activity and Alzheimer’s disease (PAAD) study: cognitive outcomes. Ann Behav Med 52: 175–185, 2018. doi: 10.1093/abm/kax035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Department of Health and Human Services. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: U.S. Department of Health and Human Services, 2018, p. 779. [Google Scholar]
- 106. Erickson KI, Donofry SD, Sewell KR, Brown BM, Stillman CM. Cognitive aging and the promise of physical activity. Annu Rev Clin Psychol 18: 417–442, 2022. doi: 10.1146/annurev-clinpsy-072720-014213. [DOI] [PubMed] [Google Scholar]
- 107. Lee CC, Huang CC, Hsu KS. Insulin promotes dendritic spine and synapse formation by the PI3K/Akt/mTOR and Rac1 signaling pathways. Neuropharmacology 61: 867–879, 2011. doi: 10.1016/j.neuropharm.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 108. Kamal A, Ramakers GMJ, Gispen WH, Biessels GJ, Al Ansari A. Hyperinsulinemia in rats causes impairment of spatial memory and learning with defects in hippocampal synaptic plasticity by involvement of postsynaptic mechanisms. Exp Brain Res 226: 45–51, 2013. [Erratum in Exp Brain Res 227: 421, 2013]. doi: 10.1007/s00221-013-3409-4. [DOI] [PubMed] [Google Scholar]
- 109. Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc 86: 876–884, 2011. doi: 10.4065/mcp.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Stillman CM, Esteban-Cornejo I, Brown B, Bender CM, Erickson KI. Effects of exercise on brain and cognition across age groups and health states. Trends Neurosci 43: 533–543, 2020. doi: 10.1016/j.tins.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci 58: 176–180, 2003. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- 112. Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, White SM, Wójcicki TR, McAuley E, Kramer AF. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus 19: 1030–1039, 2009. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Firth J, Stubbs B, Vancampfort D, Schuch F, Lagopoulos J, Rosenbaum S, Ward PB. Effect of aerobic exercise on hippocampal volume in humans: a systematic review and meta-analysis. NeuroImage 166: 230–238, 2018. doi: 10.1016/j.neuroimage.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 114. Wilckens KA, Stillman CM, Waiwood AM, Kang C, Leckie RL, Peven JC. Exercise interventions preserve hippocampal volume: a meta-analysis. Hippocampus 31: 335–347, 2021. doi: 10.1002/hipo.23292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Brooks SJ, Cedernaes J, Schiöth HB. Increased prefrontal and parahippocampal activation with reduced dorsolateral prefrontal and insular cortex activation to food images in obesity: a meta-analysis of fMRI studies. PLoS One 8: e60393, 2013. doi: 10.1371/journal.pone.0060393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Marqués-Iturria I, Pueyo R, Garolera M, Segura B, Junqué C, García-García I, José Sender-Palacios M, Vernet-Vernet M, Narberhaus A, Ariza M, Jurado MÁ. Frontal cortical thinning and subcortical volume reductions in early adulthood obesity. Psychiatry Res 214: 109–115, 2013. doi: 10.1016/j.pscychresns.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 117. Donofry SD, Stillman CM, Erickson KI. A review of the relationship between eating behavior, obesity and functional brain network organization. Soc Cogn Affect Neurosci 15: 1157–1181, 2020. doi: 10.1093/scan/nsz085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Yokum S, Ng J, Stice E. Relation of regional gray and white matter volumes to current BMI and future increases in BMI: a prospective MRI study. Int J Obes 36: 656–664, 2012. doi: 10.1038/ijo.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Espeland MA, Rapp SR, Bray GA, Houston DK, Johnson KC, Kitabchi AE, Hergenroeder AL, Williamson J, Jakicic JM, van Dorsten B, Kritchevsky SB; Action for Health In Diabetes (Look AHEAD) Movement and Memory Subgroup, Look AHEAD Research Group. Long-term impact of behavioral weight loss intervention on cognitive function. J Gerontol A Biol Sci Med Sci 69: 1101–1108, 2014. doi: 10.1093/gerona/glu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Chow LS, Gerszten RE, Taylor JM, Pedersen BK, van Praag H, Trappe S, Febbraio MA, Galis ZS, Gao Y, Haus JM, Lanza IR, Lavie CJ, Lee C-H, Lucia A, Moro C, Pandey A, Robbins JM, Stanford KI, Thackray AE, Villeda S, Watt MJ, Xia A, Zierath JR, Goodpaster BH, Snyder MP. Exerkines in health, resilience and disease. Nat Rev Endocrinol 18: 273–289, 2022. doi: 10.1038/s41574-022-00641-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2: 266–270, 1999. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 122. Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci 30: 464–472, 2007. [Erratum in Trends Neurosci 30: 489, 2007] doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 123. Neeper SA, Gómez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res 726: 49–56, 1996. doi: 10.1016/0006-8993(96)00273-9. [DOI] [PubMed] [Google Scholar]
- 124. Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience 133: 853–861, 2005. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 125. Erickson KI, Miller DL, Roecklein KA. The aging hippocampus: interactions between exercise, depression, and BDNF. Neuroscientist 18: 82–97, 2012. doi: 10.1177/1073858410397054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Praag H. V, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci 25: 8680–8685, 2005. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Berchtold NC, Cotman CW. Exercise and cognitive function: neurobiological mechanisms. In: Routledge Handbook of Physical Activity and Mental Health, edited by Ekkekakis P. London: Routledge, 2013. [Google Scholar]
- 128. Vaynman S, Ying Z, Gomez‐Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci 20: 2580–2590, 2004. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 129. Duzel E, van Praag H, Sendtner M. Can physical exercise in old age improve memory and hippocampal function? Brain 139: 662–673, 2016. doi: 10.1093/brain/awv407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Nishijima T, Torres-Aleman I, Soya H. Exercise and cerebrovascular plasticity. Prog Brain Res 225: 243–268, 2016. doi: 10.1016/bs.pbr.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 131. Maass A, Düzel S, Brigadski T, Goerke M, Becke A, Sobieray U, Neumann K, Lövdén M, Lindenberger U, Bäckman L, Braun-Dullaeus R, Ahrens D, Heinze H-J, Müller NG, Lessmann V, Sendtner M, Düzel E. Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. NeuroImage 131: 142–154, 2016. doi: 10.1016/j.neuroimage.2015.10.084. [DOI] [PubMed] [Google Scholar]
- 132. Schwarz AJ, Brasel JA, Hintz RL, Mohan S, Cooper DM. Acute effect of brief low- and high-intensity exercise on circulating insulin-like growth factor (IGF) I, II, and IGF-binding protein-3 and its proteolysis in young healthy men. J Clin Endocrinol Metab 81: 3492–3497, 1996. doi: 10.1210/jcem.81.10.8855791. [DOI] [PubMed] [Google Scholar]
- 133. Trejo JL, Carro E, Torres-Alemán I. Circulating insulin-like growth factor i mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci 21: 1628–1634, 2001. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Danzig V, Míková B, Kuchynka P, Benáková H, Zima T, Kittnar O, Škrha J, Linhart A, Kalousová M. Levels of circulating biomarkers at rest and after exercise in coronary artery disease patients. Physiol Res 59: 385–392, 2010. doi: 10.33549/physiolres.931764. [DOI] [PubMed] [Google Scholar]
- 135. Sandri M, Beck EB, Adams V, Gielen S, Lenk K, Höllriegel R, Mangner N, Linke A, Erbs S, Möbius-Winkler S, Scheinert D, Hambrecht R, Schuler G. Maximal exercise, limb ischemia, and endothelial progenitor cells. Eur J Cardiovasc Prev Rehabil 18: 55–64, 2011. doi: 10.1097/HJR.0b013e32833ba654. [DOI] [PubMed] [Google Scholar]
- 136. Schlager O, Giurgea A, Schuhfried O, Seidinger D, Hammer A, Gröger M, Fialka-Moser V, Gschwandtner M, Koppensteiner R, Steiner S. Exercise training increases endothelial progenitor cells and decreases asymmetric dimethylarginine in peripheral arterial disease: a randomized controlled trial. Atherosclerosis 217: 240–248, 2011. doi: 10.1016/j.atherosclerosis.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 137. Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci 18: 2803–2812, 2003. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- 138. Inoue DS, Antunes BM, Maideen MFB, Lira FS. Pathophysiological features of obesity and its impact on cognition: exercise training as a non-pharmacological approach. Curr Pharm Des 26: 916–931, 2020. doi: 10.2174/1381612826666200114102524. [DOI] [PubMed] [Google Scholar]
- 139. Imfeld P, Bodmer M, Jick SS, Meier CR. Metformin, other antidiabetic drugs, and risk of Alzheimer’s disease: a population-based case-control study. J Am Geriatr Soc 60: 916–921, 2012. doi: 10.1111/j.1532-5415.2012.03916.x. [DOI] [PubMed] [Google Scholar]
- 140. Campbell JM, Stephenson MD, de Courten B, Chapman I, Bellman SM, Aromataris E. Metformin use associated with reduced risk of dementia in patients with diabetes: a systematic review and meta-analysis. J Alzheimers Dis 65: 1225–1236, 2018. doi: 10.3233/JAD-180263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Sluggett JK, Koponen M, Bell JS, Taipale H, Tanskanen A, Tiihonen J, Uusitupa M, Tolppanen AM, Hartikainen S. Metformin and risk of Alzheimer’s disease among community-dwelling people with diabetes: a national case-control study. J Clin Endocrinol Metab 105: dgz234, 2020. doi: 10.1210/clinem/dgz234. [DOI] [PubMed] [Google Scholar]
- 142. Chaudhari K, Reynolds CD, Yang SH. Metformin and cognition from the perspectives of sex, age, and disease. GeroScience 42: 97–116, 2020. doi: 10.1007/s11357-019-00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Wium-Andersen IK, Osler M, Jørgensen MB, Rungby J, Wium-Andersen MK. Antidiabetic medication and risk of dementia in patients with type 2 diabetes: a nested case-control study. Eur J Endocrinol 181: 499–507, 2019. doi: 10.1530/EJE-19-0259. [DOI] [PubMed] [Google Scholar]
- 144. Cukierman-Yaffe T, Gerstein HC, Colhoun HM, Diaz R, García-Pérez L-E, Lakshmanan M, Bethel A, Xavier D, Probstfield J, Riddle MC, Rydén L, Atisso CM, Hall S, Rao-Melacini P, Basile J, Cushman WC, Franek E, Keltai M, Lanas F, Leiter LA, Lopez-Jaramillo P, Pirags V, Pogosova N, Raubenheimer PJ, Shaw JE, Sheu WH-H, Temelkova-Kurktschiev T. Effect of dulaglutide on cognitive impairment in type 2 diabetes: an exploratory analysis of the REWIND trial. Lancet Neurol 19: 582–590, 2020. doi: 10.1016/S1474-4422(20)30173-3. [DOI] [PubMed] [Google Scholar]
- 145. Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, Bäckman L, Hänninen T, Jula A, Laatikainen T, Lindström J, Mangialasche F, Paajanen T, Pajala S, Peltonen M, Rauramaa R, Stigsdotter-Neely A, Strandberg T, Tuomilehto J, Soininen H, Kivipelto M. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 385: 2255–2263, 2015. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]


