Abstract
Background
Steroid hormones play an essential role in many reproductive processes of vertebrates. Previous studies revealed that teleost-specific Cyp17a2 (cytochrome P450 family 17 subfamily a 2) might be required for the production of cortisol in the head-kidney and 17α,20β-dihydroxy-4-pregnen-3-one (DHP) in ovary during oocyte maturation. However, the role of Cyp17a2 in male reproduction remains to be largely unknown. The aim of this study was to explore the essentiality of cyp17a2 gene in male steroidogenesis, spermatogenesis, and male fertility.
Methods
A homozygous mutation line of cyp17a2 gene was constructed in tilapia by CRISPR/Cas9 gene editing technology. The expression level of germ cell and meiosis-related genes and steroidogenic enzymes were detected by qRT-PCR, IHC, and Western blotting. EIA and LC-MS/MS assays were used to measure the steroid production levels. And sperm quality was examined by Sperm Quality Analyzer software.
Results
In this study, cyp17a2 gene mutation resulted in the significant decline of serum DHP and cortisol levels. On the contrary, significant increases in intermediate products of cortisol and DHP were found in cyp17a2-/- male fish. The deficiency of cyp17a2 led to the arrest of meiotic initiation in male fish revealing as the reduction of the expression of germ cell-related genes (vasa, piwil, oct4) and meiosis-related genes (spo11 and sycp3) by 90 dah. Afterwards, spermatogenesis was gradually recovered with the development of testis in cyp17a2-/- males, but it showed a lower sperm motility and reduced fertility compared to cyp17a2+/+ XY fish. Deletion of cyp17a2 led to the abnormal upregulation of steroidogenic enzymes for cortisol production in the head-kidney. Moreover, unaltered serum androgens and estrogens, as well as unchanged related steroidogenic enzymes were found in the testis of cyp17a2-/- male fish.
Conclusion
This study proved that, for the fist time, Cyp17a2 is indispensable for cortisol and DHP production, and cyp17a2 deficiency associated curtailed meiotic initiation and subfertility suggesting the essentiality of DHP and cortisol in the male fertility of fish.
Keywords: Nile tilapia, Cyp17a2, DHP, cortisol, spermatogenesis, male fertility
Introduction
It is well known that steroid hormones play essential roles in gonadal development, gametogenesis, and fertility in vertebrates (1–5). In fish, 17α, 20β-dihydroxy-4-pregnen-3-one (17α, 20β-DP, DHP) and 17α, 20β, 21-trihydroxy-4-pregnen-3-one (17α, 20β, 21-P) are the two main maturation-inducing hormones (6, 7). Previous studies suggested that DHP might be involved in meiotic initiation and spermiation (8–11). Cortisol, the primary glucocorticoid in fish, regulates stress, immune response, energetic metabolism and osmoregulation, and recently being thought to be involved in testicular development and spermatogenesis in male fish (12–17).
In vertebrates, cytochrome P450s (17α-hydroxylase/C17–20 lyase, Cyp17) occupied the center of biosynthesis of steroid hormones. In mammals, Cyp17a had been proved to be required for the production of androgens and estrogens in gonadal tissues, and glucocorticoid in adrenal tissues (18, 19). In contrast, duplicated cyp17a genes, i.e. cyp17a1 and cyp17a2, were isolated from the genomes of several teleosts and suspected to synergistically catalyzing the synthesis of C18, C19 and C21 steroid hormones (20–22). Previous reports revealed that cyp17a1 gene mutation ceased both androgens and estrogens production, and caused spermiation disorder, infertility and all-male population production (23–25). Cyp17a2, a teleost-specific Cyp17a steroidogenic enzyme, possessed different tissue expression patterns and different steroidogenic activities from Cyp17a1 (20). Previous studies indicated that Cyp17a2 might be required for the biosynthesis of DHP during oocyte maturation as well as glucocorticoid production in the head-kidney (20, 26). However, the functions of Cyp17a2 in fish steroidogenesis and reproduction are still vague and require detailed investigation.
Nile tilapia (Oreochromis niloticus), a commercially important aquaculture fish across globe, is an ideal model to study the molecular mechanisms on fish steroidogenesis due to the well-established gene editing technology, availability of monosex fish and a short spawning cycle (14-day) (27, 28). Using tilapia, earlier we reported that Cyp17a2 was abundantly expressed in ovarian follicular cells, testicular interstitial cells and interrenal cells of head-kidney and possessed unique hydroxylase activity (20). In our present study, a null mutation line of cyp17a2 gene was constructed by CRISPR/Cas9 technology to assess the functional implication of Cyp17a2 in teleostean steroidogenesis and gonadal maintenance. Comparative analyses were conducted to assess the effects of cyp17a2 gene mutation on DHP and cortisol production, meiotic initiation, spermatogenesis, sperm health and fertility. Taken together, our findings provide important insights into the molecular mechanisms and endocrine regulation of male fish fertility.
Materials and methods
Animals
In this study, Nile tilapia (Oreochromis niloticus) were acclimatized in recirculating aerated freshwater tanks at 26 °C under a natural photoperiod before use. All animal experiments were conducted per the regulations of the Guide for Care and Use of Laboratory Animals approved by the Committee of Laboratory Animal Experimentation of Southwest University, China.
Production and characterization of Cyp17a2 polyclonal antibody
The recombinant construct of Cyp17a2 was prepared by cloning the 1566 bp cyp17a2 ORF sequences into a pCold I expression vector. The recombinant plasmid with His-tag at its N-terminal was transformed into Escherichia coli, and expressed with the induction of isopropyl β-D-l-thiogalactopyanoside (IPTG, 1 mmol/L). The His-Cyp17a2 recombinant proteins (25-30 µg) was purified with a Ni-NTA super flow cartridge (Qiagen, Hilden, Germany) and used as an antigen to immunize female rabbits three times at 15-day intervals. Ten days after the last immunization, rabbit serum was collected and purified by affinity chromatography on Sepharose 4B Fast Flow resin (Sigma, Darmstadt, Germany) combined with the Cyp17a2 recombinant protein. To confirm the specificity of the polyclonal antibody, total proteins extracted from testis, ovary, head-kidney, brain, and muscle from 180 dah tilapia were separated using 12.5% SDS-PAGE under reducing conditions. Western blotting was performed according to the methods described previously (29) and the antibody against tilapia Cyp17a2 was diluted at 1:1000.
Cellular localization of Cyp17a2 in gonads and head-kidney of tilapia by Immunohistochemistry
To determine the cellular localization of Cyp17a2 in the testis and head-kidney of tilapia, the testes and head-kidneys from wild-type males at different development stages were dissected and fixed in Bouin’s solution for 12 hours at room temperature, then dehydrated and embedded in paraffin. The tissue blocks were then sliced into 5-μm-thickness sections, then the sections were treated in a blocking solution (5% BSA diluted in 1x PBS) (Sangon Biotech, China), incubated with the primary antibody against Cyp17a2 overnight at 4°C and rinsed with 1x PBS five times for 5 min per wash. In this study, the anti-Cyp17a2 polyclonal antibody was diluted at 1:1000 before use. As a negative control, the primary antibody was replaced with normal rabbit serum. The slides were then incubated with anti-rabbit immunoglobulin G (diluted at 1:1000) at room temperature for 1 h, and then rinsed with 1x PBS three times for 5 min per wash. Immunoreactive signals were visualized using diaminobenzidine tetrachloride (DAB) (Sigma, Germany) as the substrate. Sections were then counterstained with hematoxylin. Finally, all the images for these sections were acquired with an Olympus BX5 light microscope (Olympus, Japan).
Knockout of the cyp17a2 gene by CRISPR/Cas9
A homozygous mutation line of cyp17a2 was constructed in tilapia to elucidate the functions of Cyp17a2 in fish steroidogenesis, spermatogenesis, and male fertility by using CRISPR/Cas9 technology. The guide RNA (gRNA) target site was selected from sequences corresponding to GGN18NGG on the sense strand of DNA (http://zifit.partners.org/ZiFiT/). The synthesis of gRNA and Cas9 mRNA, and the screening and establishment of homozygous mutation line were carried out according to previous report (25).
Histological observation and change of gene expression
Testes at different developmental stages (75, 90 and 180 dah) and head-kidneys (180 dah) from wild-type and cyp17a2 -/- fish were dissected and embedded in paraffin. IHC analysis was conducted as described above. Anti-Vasa (germ cells marker, diluted at 1:1000), -PCNA (Proliferating cell nuclear antigen, diluted at 1:500, Cusabio, Wuhan, China), -StAR1 (Steroidogenic acute regulatory protein 1, diluted at 1:500), -Cyp17a1 (cytochrome P450 family 17 subfamily a 1, Leydig cell marker, diluted at 1:1000), -Cyp11b2 (Cytochrome P450, family 11, subfamily B, polypeptide 2, Leydig cell marker, diluted at 1:1000), and -Cyp17a2 polyclonal antibodies were used to assess the impacts of cyp17a2 mutation on spermatogenesis and head-kidney development.
Quantitative real-time PCR
Gonads and head-kidneys were collected from wild-type and cyp17a2 -/- XY fish (n≥3 fish/genotype). Total RNA was isolated for each sample using RNAiso Plus (Takara, Dalian, China), and reverse transcribed using the Prime Script RT Master Mix Perfect Real Time Kit according to the manufacturer’s instructions (Takara, Dalian, China). All qRT-PCRs were carried out in an ABI-7500 fast Real-time PCR machine (Applied Biosystems, USA), and all experiments were performed according to the manufacturer’s instructions. The relative abundance of target genes was normalized to β-actin using R=2-ΔΔCt formula (30). The primer sequences used for the PCR reactions were listed in Table 1 .
Table 1.
Primers used in the present study.
| primer name | primer sequence (5'-3') | accession number | purpose |
|---|---|---|---|
| cyp17a2-Cas9-F | GGCAGTCTCCCCTGGCTTGG | NM_001279458 | gRNA amplification |
| cyp17a2-Cas9-R | TGTAAACATTTATGACAGTGGG | ||
| cyp17a2-T-F | TCGTTCCTGCCTCCTTCG | Sequencing and mutant screening | |
| cyp17a2-T-R | TGGGCTGATGAACATCACTCCT | ||
| cyp17a2-P-F | CTGAAACTGAACCCTCGGCT | ||
| cyp17a2-P-R | CTGTGGGACATCTGCGTGAA | ||
| cyp17a1-Q-F | TGTCATCAACCAGCATGTGCAC | NM_001279765 | qRT-PCR |
| cyp17a1-Q-R | ACTTCCACGTAGCACTGTAGTC | ||
| StAR1-Q-F | CTGAAACTGTTGCTGCGAATGGA | XM_003445605 | |
| StAR1 -Q-R | GGTCTCTGCGGATACCTCGTG | ||
| cyp11b2-Q-F | AAAGAAGTCCTCAGGTTGTACC | XM_003450906 | |
| cyp11b2-Q-R | GACCAAAGTTCCAGCAGGTATG | ||
| cyp21a2-Q-F | AATGCGGAACAACAACTATGG | XM_019365158 | |
| cyp21a2-Q-R | TATGTTGCAACCTCCCCCAG | ||
| vasa-Q-F | GGGAGCTGATCAACCAGATT | XM_019351278 | |
| vasa-Q-R | CTGGTGTTCCACACAACACA | ||
| piwil-Q-F | ACATCCCACAGCACAAGTTGAC | XM_003445546 | |
| piwil-Q-R | CTGCCTCAAGCTGACCATAAAG | ||
| cyp26b1-Q-F | CTTGCCCTTTCCCGTTGC | XM_005471225 | |
| cyp26b1-Q-R | GCGGTTCCCGAAGAGGTGT | ||
| spo11-Q-F | CGAGAAGGATGCGACGTTCCAGAG | XM_013274323 | |
| spo11-Q-R | GAGCGTCCTTGGGAACCCGC | ||
| sycp3-Q-F: | CTGACTTTGAGGAGGAGGCG | XM_003439369 | |
| sycp3-Q-R: | GCTTGTGTTGGCTTCCCTTC | ||
| β-actin-Q-F | GGCATCACACCTTCTACAACGA | XM_003443127 | |
| β-actin-Q-R | ACGCTCTGTCAGGATCTTCA |
F, Forward primer; R, Reverse primer, respectively. T, test; P, PAGE; Q, qRT-PCR.
Western blotting
Western blotting was performed to detect the expression of steroidogenic enzymes in the testes of cyp17a2 -/- XY tilapia. Total proteins were extracted from the testes of cyp17a2 +/+ XY (n=3) and cyp17a2 -/- XY (n=3) fish at 180 dah, diluted to a final concentration of 20 mg/mL and stored at -20 °C until use. Western blotting was performed as described previously (29). The primary antibodies against steroidogenic enzymes (anti-Cyp17a1 and -Cyp11b2) diluted at 1:1000. α-tubulin was used as a reference protein and its abundance was used to normalize protein sample loading. Densitometry was quantified and normalized using α-tubulin as a reference protein using the Fusion-CAPT software.
Germ cell counting
The cell count of spermatogenic cells at various stages (i.e. spermatogonia, primary spermatocyte and secondary spermatocyte) were calculated according to the methods described previously (31). Briefly, ten sections were randomly selected from each cyp17a2 +/+ (n=3) and cyp17a2 -/- (n=3) XY fish at 90 dah, and the sections were stained with conventional hematoxylin and eosin (H&E) staining. Then the image of each section at 40x magnification was acquired with an Olympus BX5 light microscope (Olympus, Japan) for germ cell counting.
Measurement of serum steroid hormone levels via EIA assay and LC-MS/MS
Blood samples were collected from the caudal veins of cyp17a2 +/+ XY (n≥3) and cyp17a2 -/- XY (n≥3) fish after anesthesia (250 mg/L, MS-222, Sigma, St Louis, USA) at 180 dah and kept at room temperature for 1 h. After centrifugation at 3000 rpm for 10 min, the supernatant was transferred to a clean centrifuge tube. The supernatant was then centrifuged at 12000 rpm for 10 min at 4 °C and frozen at -80 °C until use. Serum steroid levels were measured using the EIA (enzyme-linked immunosorbent assay) kits (Cayman, Michigan, USA) or detected by MetWare (http://www.metware.cn/) based on the AB Sciex QTRAP® 6500 LC-MS/MS system (AB Sciex, Framingham, USA). The steroid information was listed in Table 2 .
Table 2.
Key resources for steroids metabolism analysis.
| MW ID | Compound Name | CAS |
|---|---|---|
| MWS0782 | Testosterone | 58-22-0 |
| MWS0431 | Dihydrotestosterone | 521-18-6 |
| MWS0785 | Androstenedione | 63-05-8 |
| MWS0521 | Cortisol | 50-23-7 |
| MWS0958 | 11-Dehydrocorticosterone | 72-23-1 |
| MWS0523 | Deoxycorticosterone | 64-85-7 |
| MWS0797 | Cortisone | 53-06-5 |
| MWS0784 | 17β-Estradiol | 50-28-2 |
| MWS0485 | Estrone | 53-16-7 |
| MWS2130 | 2-Hydroxy Estrone | 362-06-1 |
| MWS0964 | 17α-Hydroxypregnenolone | 387-79-1 |
| MWS0833 | 5alpha-Pregnane-3,20-dione | 566-65-4 |
| MWS0799 | Progesterone | 57-83-0 |
| MWS0524 | 17α-Hydroxyprogesterone | 68-96-2 |
| MWS0834 | Pregnanediol | 80-92-2 |
| MWS0941 | 24-Hydroxycholesterol | 474-73-7 |
| MWS0936 | 7α,25-Dihydroxycholesterol | 64907-22-8 |
| MWS0138 | Cholesterol | 57-88-5 |
| MWS0723 | 7-Hydroxy-cholesten-3-one | 3862-25-7 |
| MWS0727 | 20α-Hydroxycholesterol | 516-72-3 |
| MWS0730 | 7-Ketocholesterol | 566-28-9 |
| MWS1082 | β-Sitosterol | 83-46-5 |
| MWS-20-102 | Pregnenolone sulfate sodium salt | 1852-38-6 |
| MWS-20-73 | Dehydroepiandrsterone | 651-48-9 |
Sperm characteristics and fertility assessment
To detect the impacts of cyp17a2 gene mutation on spermatogenesis and fertility of male tilapia, changes of sperm morphology, sperm motility, and fertility were compared between cyp17a2 -/- and cyp17a2 +/+ XY fish at 300 dah. Papanicolaou staining was used to detect the morphology of sperms according to the method which were used in previous reports (25, 31). Sperm motility, VCL (curvilinear velocity), VSL (straight line velocity) and sperm quality were examined via computer-assisted sperm analysis using the Sperm Quality Analyzer software according to the manufacturer’s instructions (Zoneking Software, China). The fertility of cyp17a2 +/+ and cyp17a2 -/- XY fish was assessed via artificial insemination. Eggs from wild-type XX female fish (n=3) were divided into six groups (approximately 300 eggs/group) and artificial insemination was performed using sperm obtained from the cyp17a2 +/+ and cyp17a2 -/- XY fish. Moreover, the survival rate of embryos was calculated from 1-9 days after fertilization.
Data analysis
All the data for qRT-PCR, Western blotting, cell count, LC-MS/MS and EIA assay are presented as the mean ± SD. And Student’s t-test was performed to determine the difference between the two groups. P<0.05 was considered statistically significant differences.
Results
Cellular localization of Cyp17a2 antibody in tilapia gonads and head-kidney
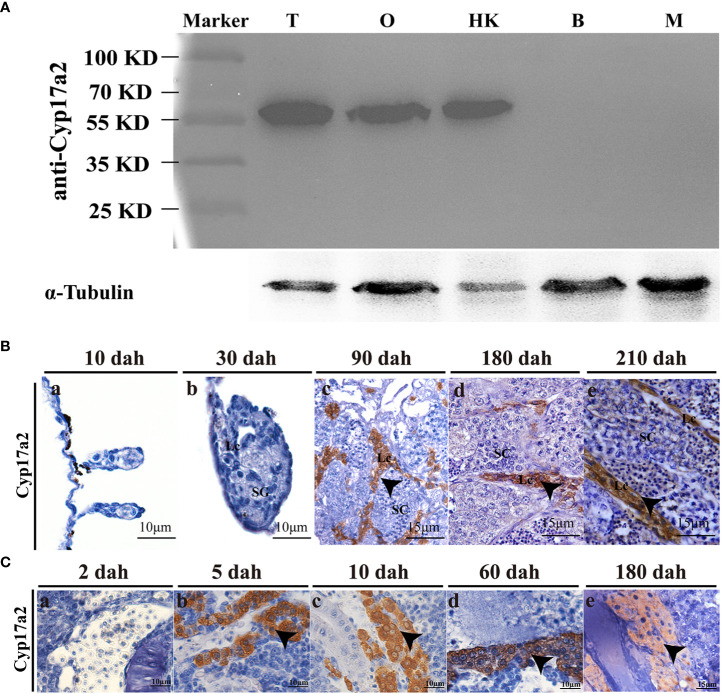
Total proteins extracted from gonads (ovary and testis), head-kidney, brain, and muscle were used to check the specificity of the Cyp17a2 polyclonal antibody by Western blotting. Western blotting showed that a single and specific band could be recognized in testis, ovary, and head-kidney, but not in brain and muscle by using tilapia Cyp17a2 antibody ( Figure 1A ). By IHC, positive Cyp17a2 immunostaining signals were observed in the Leydig cells of the testes in XY fish at 90, 180 and 210 dah ( Figure 1B ). In the head-kidney, Cyp17a2 was predominantly expressed in interrenal cells from 5 to 180 dah ( Figure 1C ).
Figure 1.
Expression profile of Cyp17a2 in tilapia. (A), The specificity of polyclonal antibody against Cyp17a2 (~60 KD) in the testis, ovary, head-kidney, brain, and muscle was verified by Western blotting. T, testis; O, ovary; HK, head-kidney; B, brain; M, muscle. (B), Cellular localization of Cyp17a2 in the testes was detected by IHC. Cyp17a2 was expressed in the Leydig cells of the testis at 90, 180, and 210 dah. SG, spermatogonia; SC, spermatocytes; Lc, Leydig cells. (C), Cyp17a2 expression in the head-kidneys was detected by IHC. Cyp17a2 positive signals were detected in the interrenal cells of the head-kidney at 5, 10, 60, and 180 dah. Arrowhead, positive signals of Cyp17a2.
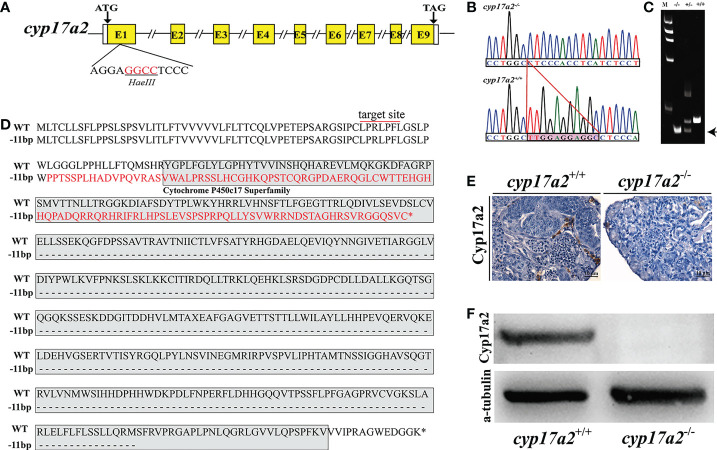
Generation of cyp17a2 homozygous mutation line
To investigate the potential roles of Cyp17a2 in fish steroidogenesis, a cyp17a2 homozygous mutation line was produced using CRISPR/Cas9 technology by targeting the first exon ( Figure 2A ). A frame-shift mutation with 11-bp (TTGGAGGAGGC) deletion in cyp17a2 homozygous mutant was obtained by Sanger sequencing ( Figure 2B ). A heteroduplex motility assay showed that heterozygous cyp17a2 +/− individual was identified as possessing both heteroduplex and homoduplex amplicons, while the cyp17a2 -/- and cyp17a2 +/+ individuals were found to be single band profile with only homoduplex amplicons ( Figure 2C ). The comparison of the predicted amino acid sequences demonstrated that the 11-bp deletion in the cyp17a2 gene resulted in the production of truncated protein due to frame shift and the occurrence of a premature stop codon ( Figure 2D ). IHC and Western blotting analyses also demonstrated that Cyp17a2 protein completely vanished in the testes of cyp17a2 -/- XY fish ( Figures 2E, F ).
Figure 2.
Establishment of cyp17a2-null mutation line by CRISPR/Cas9. (A), Gene structure of cyp17a2 gene with the target site and the underlined restriction enzyme (Hae III) cutting site. (B), A deletion of 11 bp (TTGGAGGAGGC) in the genome of cyp17a2 -/- fish compared with cyp17a2 +/+ fish was detected by Sanger sequencing analysis and the deletion region was highlighted by a pink shading. (C), Mutations in the cyp17a2 gene were detected by polyacrylamide gel electrophoresis. Wild type (+/+), heterozygous (+/-), and homozygous (-/-) fish were screened via PCR and PAGE gel electrophoresis. M, marker; Arrow, homoduplex of mutants (D), A putative truncated protein was produced in cyp17a2 -/- fish (-11 bp) compared with cyp17a2 +/+ fish (WT). Red line, target site; Red letters, putative amino acids in cyp17a2-/- fish; Gray shading, Cytochrome P450c17 Superfamily domain. Asterisk, termination of protein. (E, F) Cyp17a2 expression was not detected in the testes of cyp17a2 -/- fish according to IHC and Western blotting analyses.
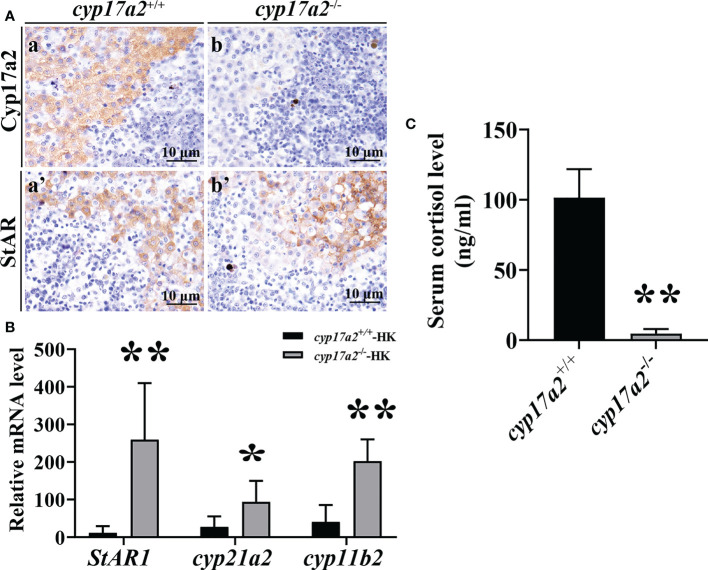
Decrease of cortisol production in cyp17a2 -/- fish
IHC results demonstrated that the expression of Cyp17a2 could not be detected in the head-kidney of cyp17a2 -/- fish ( Figure 3A ). The results of qRT-PCR showed that the expression of StAR1, cyp21a2 and cyp11b2 in the head-kidney was significantly increased in cyp17a2 -/- fish compared with wild-type fish ( Figure 3B ). EIA assay showed that serum cortisol level of cyp17a2 -/- XY fish was significantly reduced ( Figure 3C ).
Figure 3.
Effects of cyp17a2 deficiency on steroid production in head-kidney of tilapia. (A), Immunohistochemistry of Cyp17a2 (a and b) and StAR (a’ and b’) in the head-kidney of cyp17a2 +/+ and cyp17a2 -/- XY tilapia. (B), Expression level of StAR1, cyp21a2, and cyp11b2 in the head-kidney of cyp17a2 +/+ (n=3) and cyp17a2 -/- (n=3) fish detected by qRT-PCR. β-actin was used as a reference gene to normalize the expression values. (C), Serum cortisol level in cyp17a2 +/+ (n=5) and cyp17a2 -/- (n=5) fish measured using an EIA kit. The data are reported as the means ± SD. Asterisk above the error bar indicate significant differences between groups tested by Student’s t-test (*, P<0.05; **, P<0.01).
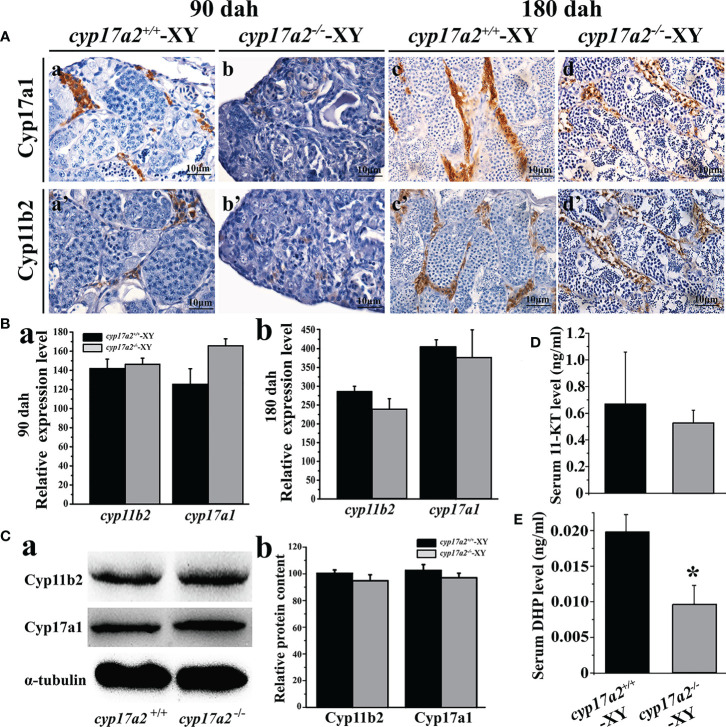
Cyp17a2 is responsible for DHP, but not 11-KT biosynthesis
EIA assay showed that cyp17a2 deficiency led to a significant decline in the biosynthesis of DHP, while no evident change of 11-KT production was detected between cyp17a2 -/- and wild-type XY fish ( Figures 4D, E ). Moreover, IHC analysis demonstrated that the positive signals of steroidogenic enzymes (Cyp17a1 and Cyp11b2) concerning androgen production were detected both in the testes of cyp17a2 -/- and wild-type XY fish ( Figure 4A ). Further analyses by qRT-PCR and Western blotting demonstrated that no significant difference in the expression level of androgen synthases (cyp17a1/Cyp17a1, cyp11b2/Cyp11b2) was found between cyp17a2 -/- XY fish and wild-type XY fish at 90 and 180 dah ( Figures 4B, C ).
Figure 4.
Effects of cyp17a2 deficiency on steroid production in testis of tilapia. (A), Immunohistochemistry of Cyp17a1 (a-d) and Cyp11b2 (a’-d’) in the testis of cyp17a2 +/+ and cyp17a2 -/- XY tilapia at 90 and 180 dah. (B), Expression level of cyp11b2 and cyp17a1 in the testis of cyp17a2 +/+ (n=3) and cyp17a2 -/- (n=3) fish at 90 (a) and 180 dah (b) detected by qRT-PCR. β-actin was used as a reference gene to normalize the expression values. (C), Expression of Cyp11b2 and Cyp17a1 analyzed by Western blotting (a) and quantification of protein level (b) in the testis of cyp17a2 +/+ and cyp17a2 -/- at 180 dah. (D, E), Serum 11-KT and DHP level in cyp17a2 +/+ (n=5) and cyp17a2 -/- (n=5) fish measured using an EIA kit. The data are reported as the means ± SD. Asterisk above the error bar indicate significant differences between groups tested by Student’s t-test (*, P<0.05).
The landscape of steroidogenesis in cyp17a2 -/- male fish
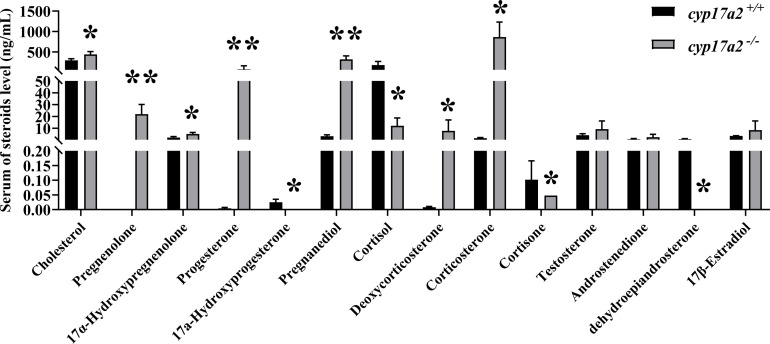
Consistently, the results of LC-MS/MS showed that cyp17a2 gene mutation led to significant changes in C21-steroid levels in cyp17a2 -/- XY fish compared with wild-type XY fish. The syntheses of 17α-hydroxyprogesterone, cortisol and cortisone were significantly reduced in cyp17a2 -/- XY fish, while cholesterol, pregnenolone, 17α-hydroxypregnenolone, progesterone, pregnanediol, deoxycorticosterone, and corticosterone were excessive accumulated ( Figure 5 ). However, compared with wild-type XY fish, the production levels of C19-steroids (testosterone, androstenedione) and C18-steroid (17β-estradiol) were not affected ( Figure 5 ).
Figure 5.
Mutation of cyp17a2 resulted in disordered steroidogenesis in male tilapia. Serum steroid levels of cyp17a2 +/+ (n=3) and cyp17a2 -/- (n=3) fish were measured by LC-MS/MS. The data are reported as the means ± SD. Asterisk above the error bar indicate significant differences between groups tested by Student’s t-test (*, P<0.05; **, P<0.01). .
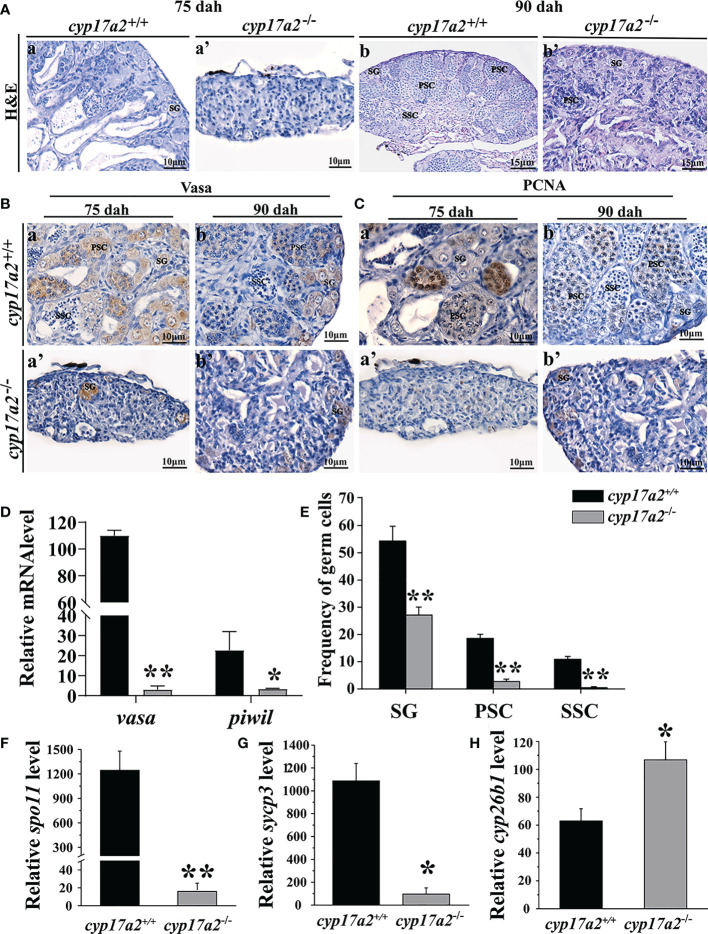
A deficiency of cyp17a2 resulted in arrested meiotic initiation
To detect the role of Cyp17a2 in the initiation of meiosis in the development of tilapia testes, the gonad phenotype was evaluated at 75 and 90 days, respectively. This period was proved to be the key period for the initiation of meiosis and the morphological differentiation of gonads in wild-type male tilapia. H&E staining reflected that only a few spermatogonia and few spermatocytes were found in cyp17a2 -/- XY fish compared with cyp17a2 +/+ XY fish at 75 and 90 dah ( Figure 6A ). Immunostaining showed that the number of Vasa (germ cell) and PCNA (proliferative cell) positive cells was significantly reduced in cyp17a2 -/- XY fish than that in wild-type XY fish at 75 and 90 dah ( Figures 6B, C ). And the relative expression level of vasa and piwil was significantly decreased in cyp17a2 -/- XY fish at 90 dah ( Figure 6D ). Consistently, a significant reduction of spermatogonia, primary spermatocytes, and secondary spermatocytes was detected in cyp17a2 -/- XY fish at 90 dah ( Figure 6E ). In addition, qRT-PCR demonstrated that compared with cyp17a2 +/+ XY fish, the expression of spo11, sycp3 (meiotic marker genes) were decreased while cyp26b1 (retinoic acid hydrolase) was increased significantly in cyp17a2 -/- XY fish at 90 dah ( Figures 6F-H ).
Figure 6.
Mutation of cyp17a2 resulted in delayed spermatogenesis. (A), H&E staining of testis from cyp17a2 +/+ (a and b) and cyp17a2 -/- XY fish (a’ and b’) at 75 and 90 dah. B and C, Immunohistochemistry of Vasa (B) and PCNA (C) in the testis of cyp17a2 +/+ and cyp17a2 -/- XY fish at 75 and 90 dah. SG, spermatogonia; PSC, primary spermatocytes; SSC, secondary spermatocytes; ST, Spermatids. (D), Expression level of vasa and piwil in the testis of cyp17a2 +/+ (n=3) and cyp17a2 -/- (n=3) fish at 90 dah detected by qRT-PCR. β-actin was used as a reference gene to normalize the expression values. (E), Spermatogenic cell proportion of cyp17a2 +/+ (n=3) and cyp17a2 -/- (n=3) XY fish at 90 dah. (F-H), Expression level of spo11, sycp3, and cyp26b1 in the testis of cyp17a2 +/+ (n=3) and cyp17a2 -/- (n=3) fish at 90 dah. β-actin was used as a reference gene to normalize the expression values. The data are reported as the means ± SD. Asterisk above the error bar indicate significant differences between groups tested by Student’s t-test (*, P<0.05; **, P<0.01).
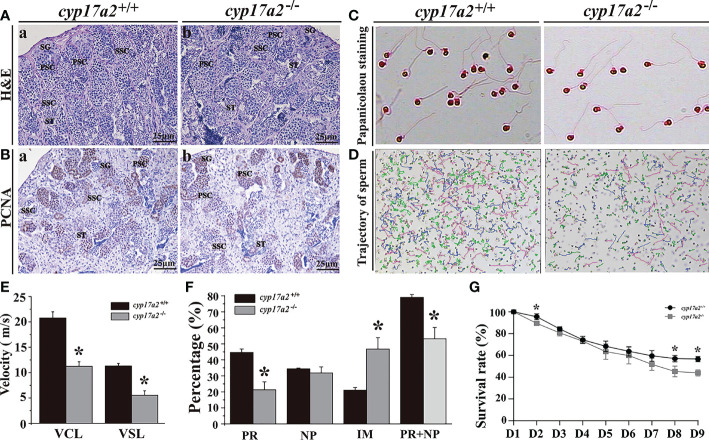
Deficiency of Cyp17a2 resulted in subfertility in male tilapia
Surprisingly, spermatogenesis was restored at 180 dah, as indicated by the existence of all stages of spermatogenic cells and normal proliferation ( Figures 7A, B ). Papanicolaou staining showed that sperm from cyp17a2 -/- XY fish showed normal sperm morphology ( Figure 7C ). However, low sperm motility was indicated by the trajectory and sperm tracking parameters (VCL and VSL) ( Figures 7D, E ). Quantification of the three sperm grades showed that dramatic attenuation of PR (progressive sperm) and NP (non-progressive sperm), while a significant increase in the frequency of IM (immotile sperm) was found in cyp17a2 -/- XY fish compared to their control counterparts ( Figure 7F ). The fertilization rate and survival rate (D8 and D9) of cyp17a2 -/- XY fish were significantly lower than those of cyp17a2 +/+ XY fish ( Figure 7G ).
Figure 7.
Mutation of cyp17a2 gene reduced sperm quality. (A), H&E staining of testis from cyp17a2 +/+ (a) and cyp17a2 -/- (b) XY fish at 180 dah. (B), Immunohistochemistry of PCNA in the testis of cyp17a2 +/+ (a) and cyp17a2 -/- (b) XY fish at 180 dah. SG, spermatogonia; PSC, primary spermatocytes; SSC, secondary spermatocytes; ST, spermatids. (C), Morphology of sperm from cyp17a2 +/+ (a) and cyp17a2 -/- (b) XY fish. (D), Trajectory of motile sperm from cyp17a2 +/+ (a) and cyp17a2 -/- XY (b) fish. (E), Average speed of VCL (curve movement speed) and VSL (straight line movement speed) in cyp17a2 +/+ and cyp17a2 -/- XY fish. (F), Quantification of different tracking parameters of PR (progressive sperm), PR+NP (non-progressive sperm) and IM (immotile sperm) in cyp17a2 +/+ and cyp17a2 -/- XY fish. (G), Survival rates of embryos that cyp17a2 -/- and cyp17a2 +/+ XY fish artificially inseminate with normal females (n=3). D, Days after fertilization. Data are expressed as the mean ± SD. Asterisk above the error bar indicate significant differences between groups tested by Student’s t-test (*, P<0.05).
Discussion
Previous studies revealed that steroid hormones, i.e. 11-KT, DHP and cortisol, might be involved in spermatogenesis and male fertility in fish (8, 16, 32). Due to the fish-specific genome duplication, duplicated copies of several steroidogenic enzymes (two StAR, two hsd3b, two cyp17, two cyp19a) had been identified in fish genomes (20, 21, 33–36). Therefore, clarification of the distinct roles of the duplicated steroidogenic enzymes will be helpful to understand the intricacy of fish steroidogenesis. In teleosts, two paralogous cyp17a genes, named cyp17a1 and cyp17a2, have been characterized (20–22). In this study, the homozygous mutation line of the cyp17a2 gene was constructed in tilapia. We found that a deficiency of Cyp17a2 led to the sharp decline of DHP and cortisol, which further impaired sperm motility and male fertility.
Steroidogenesis is regulated sequentially by a series of steroidogenic enzymes and co-factors (37). In mammalian species, a single Cyp17a was responsible for the catalyzation of the biosynthesis of C18, C19 and C21 steroids in both gonads and the adrenal gland (18, 38, 39). In contrast, it was documented that Cyp17a1 and 2 might be involved in the biosynthesis of androgen and estrogen in gonads, and glucocorticoids in head-kidney of fish (20). Previous reports revealed that both Cyp17a1 and 2 demonstrates the different spatiotemporal expression profile and different enzymatic activities. ISH (In situ hybridization) experiments showed that two cyp17a genes were colocalized in the granulosa cells in the ovaries, and Leydig cells in the testis, while cyp17a2 was specifically expressed in the interrenal cells in head-kidney in fish (20). In this study, an antibody against tilapia cyp17a2 gene was produced, and expression of Cyp17a2 in Leydig cells and interrenal cells of male fish were further evinced. Previous reports by in vitro assay revealed that Cyp17a1, with both of the 17α-hydroxylase and 17, 20 layse activities, was capable of converting from progesterone and pregnenolone to androstenedione and dehydroepiandrosterone (DHEA) through 17α-hydroxyprogesterone and 17α-hydroxypregnenolone. On the contrary, Cyp17a2, with only 17α-hydroxylase activity, was able to catalyze the biosynthesis from pregnenolone and progesterone to 17α-hydroxypregnenolone and 17α-hydroxyprogesterone, respectively (20). The different expression profiles and distinct enzymatic activities suggested that Cyp17a1 and Cyp17a2 might be involved in different steroidogenic pathway. Therefore, it is intriguing to explore the functions of Cyp17a1 and 2 in fish by loss of gene functions. In tilapia, zebrafish (Danio rerio) and common carp (Cyprinus carpio), mutation of cyp17a1 gene resulted in the blockage of androgen and estrogen synthesis, followed by sex reversal from female to male, suggesting that Cyp17a1 is essential for androgen and estrogen production in teleosts (23–25). On the contrary, we found that cyp17a2 gene mutation had no effects on the production of both androgens and estrogens. Significant declines of both DHP and cortisol were detected in cyp17a2 -/- XY fish indicated that cyp17a2 was indispensable for these two steroids production in fish. In the head-kidney of cyp17a2 -/- fish, an evident decrease of cortisol and striking up-regulation of StAR1, cyp21a2 and cyp11b2 was detected suggesting the critical role of Cyp17a2 in cortisol production. Taken together, functional analysis strongly emphasized that Cyp17a1 and 2 play distinct roles in androgen, estrogen and glucocorticoids production in fish. In the testis, Cyp17a1 was involved in the production of 11-KT in Leydig cells, which is required for both spermatogenesis and spermiation (25). Our present study by cyp17a2 gene editing further proved that the expression of Cyp17a2 in Leydig cells was responsible for DHP production in XY fish. Undoubtedly, this study has proved that specific expression of cyp17a2 in the interrenal cells of head-kidney was responsible for cortisol production in fish.
In fish, the production of 11-KT and DHP in Leydig cells in the testis were strictly controlled by gonadotropins from pituitary (40). In our present study, a significant decline in DHP biosynthesis was detected in serum of cyp17a2 -/- tilapia, indicating that Cyp17a2 is required for DHP production in male fish. However, a low DHP level was still detected in cyp17a2 -/- XY, so it is not excluded that the residual DHP production might be catalyzed by other enzymes via genetic compensation response in cyp17a2 -/- mutants. And the decline in DHP production did not affect 11-KT production. Furthermore, the stable expression of downstream steroidogenic genes (cyp17a1/Cyp17a1 and cyp11b2/Cyp11b2) was found in the testis of cyp17a2 -/- XY fish. These findings indicated the independence of DHP and 11-KT production in the testicular Leydig cells of tilapia. Consistently, our previous work found that RU486 treatment, an antagonist of DHP, had no effects on the biosynthesis of 11-KT and the expression of steroidogenic enzymes for 11-KT production (41).
For a long time, the involvements of 11-KT in spermatogenesis and testis development have been extensively studied by short-term culture experiments and gene editing tools (32, 42–44). Recent studies revealed that DHP and cortisol might also play essential roles in testicular differentiation. It was documented that cortisol treatment induced DNA replication in spermatogonia, and further enhanced 11-KT-induced spermatogonial proliferation (16). In fish, DHP had been proved to be a maturation-inducing hormone and it was increased sharply during oocytes maturation and ovulation (45). In Japanese ell (Anguilla japonica), using an in vitro testicular culture system, DHP was shown to induce DNA replication in spermatogonia (8). Furthermore, DHP treatment also induced the expression of meiosis-specific markers, such as Dmc1 and Spo11 highlighting their essentiality in meiotic initiation (8). Our previous work found that blockage of the DHP-Pgr signaling pathway by RU486 treatment led to the retardation of spermatogenesis and sperm maturation indicating the essential role of DHP production in male spermatogenesis and fertility (41). In fish, previous reports demonstrated Cyp17a2 might be one of the key enzymes to promote the production of both DHP and cortisol based on its expression profiles and enzymatic activities (20). In the present study, we found that cyp17a2 deficiency led to the significant decline of both DHP and cortisol, delay of meiotic initiation during the early stage of testicular differentiation revealing the decreased expression of meiosis related genes (spo11 and sycp3), and increased expression of retinoic acid hydrolase (cyp26b1). These findings indicated that DHP and cortisol production might be indispensable for the initiation of spermatogenesis in male tilapia.
It was also well documented that DHP played crucial roles in final sperm maturation, spermiation, and sperm motility (10). In male salmonid fish, a peak of DHP was found in blood plasma in the spawning season (46). In turbot (Scophthalmus maximus), males with higher sperm motility showed higher levels of DHP compared with males with lower sperm motility, which indicated that DHP might induce spermatogenesis and regulate sperm motility (47). Reports showed that DHP might be involved in seminal fluid production, spermatogonial proliferation and spermatogenesis (9, 48, 49). Knockout of the progesterone receptor gene, pgr, led to the decline of sperm count and sperm motility in male tilapia, indicating the importance of the DHP-Pgr signaling pathway in fish sperm maturation (31). Consistently, deficiency of cyp17a2 led to the decline of DHP and cortisol production, which resulted in the decline of sperm motility and subfertility in male tilapia. Through functional study, our present study further proved that DHP and cortisol played an essential role in spermiogenesis and fertility in male fish. In zebrafish, mutation of cyp17a2 gene resulted in the enlargement of interrenal gland, up-regulated expression of cyp11a1, cyp21a1, and hsd3b1 in the interrenal gland, and significant decrease of cortisol biosynthesis (50). However, zebrafish cyp17a2 gene mutation had no effects on fertility and secondary sex characteristics in males. Furthermore, abnormal increase of progesterone, testosterone, DHP, and 11-KT in the cyp17a2-deficient males than those in wild-type male fish was observed (50). We speculated that discrepancy of DHP production and male fertility between cyp17a2 deficiency zebrafish and tilapia might be due to the species difference.
Taken together, our findings, by using a homozygous mutation line of cyp17a2 gene, proved that Cyp17a2 was involved in the biosynthesis of DHP and cortisol in tilapia, which were essential for meiotic initiation, spermiogenesis and male fertility.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The animal study was reviewed and approved by Institutional Animal Care and Use Committee of Southwest University.
Author contributions
LZ and LY conceived and designed the experiments; LY and YW performed most of the experiments. YS and XZ maintained the fish stocks. LZ and LY analyzed the data, interpreted the results, and drafted the manuscript; LZ, DW, and TC edited the manuscript. All authors read and approved the final version of this manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (32072957, 31772825), Natural Science Foundation of Chongqing (cst2021jcjy-msxm0088), Scientific Research Innovation Project for Chongqing Postgraduates (CYS21105).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
11-KT, 11-ketotestosterone; Cyp11b2, Cytochrome P450, family 11, subfamily B, polypeptide 2; Cyp17a1, Cytochrome P450 family 17 subfamily A member 1; DHP, 17α, 20β-dihydroxy-4-pregnen-3-one.
References
- 1. Clelland E, Peng C. Endocrine/paracrine control of zebrafish ovarian development. Mol Cell Endocrinol (2009) 312(1-2):42–52. doi: 10.1016/j.mce.2009.04.009 [DOI] [PubMed] [Google Scholar]
- 2. Moore R, Scott A, Collins P. Circulating c-21 steroids in relation to reproductive condition of a viviparous marine teleost, sebastes rastrelliger (grass rockfish). Gen Comp Endocrinol (2000) 117(2):268–80. doi: 10.1006/gcen.1999.7422 [DOI] [PubMed] [Google Scholar]
- 3. Nagahama Y, Yamashita M. Regulation of oocyte maturation in fish. Dev Growth Differ (2008) 50:S195–219. doi: 10.1111/j.1440-169X.2008.01019.x [DOI] [PubMed] [Google Scholar]
- 4. Tokarz J, Moller G, de Angelis M, Adamski J. Steroids in teleost fishes: A functional point of view. Steroids (2015) 103:123–44. doi: 10.1016/j.steroids.2015.06.011 [DOI] [PubMed] [Google Scholar]
- 5. McCormick SD, Regish A, O'Dea M, Shrimpton J. Are we missing a mineralocorticoid in teleost fish? effects of cortisol, deoxycorticosterone and aldosterone on osmoregulation, gill Na+,K+-ATPase activity and isoform mRNA levels in Atlantic salmon. Gen Comp Endocrinol (2008) 157(1):35–40. doi: 10.1016/j.ygcen.2008.03.024 [DOI] [PubMed] [Google Scholar]
- 6. Nagahama Y, Adachi S. Identification of maturation-inducing steroid in a teleost, the amago salmon (Oncorhynchus-rhodurus). Dev Bio (1985) 109(2):428–35. doi: 10.1016/0012-1606(85)90469-5 [DOI] [PubMed] [Google Scholar]
- 7. Patino R, Thomas P. Gonadotropin stimulates 17 alpha,20 beta,21-trihydroxy-4-pregnen-3-one production from endogenous substrates in Atlantic croaker ovarian follicles undergoing final maturation in vitro . Gen Comp Endocrinol (1990) 78(3):474–8. doi: 10.1016/0016-6480(90)90036-l [DOI] [PubMed] [Google Scholar]
- 8. Miura T, Higuchi M, Ozaki Y, Ohta T, Miura C. Progestin is an essential factor for the initiation of the meiosis in spermatogenetic cells of the eel. P Natl Acad Sci USA (2006) 103(19):7333–8. doi: 10.1073/pnas.0508419103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schulz RW, de França L, Lareyre J, Le Gac F, Chiarini-Garcia H, Nobrega R, et al. Spermatogenesis in fish. Gen Comp Endocrinol (2010) 165(3):390–411. doi: 10.1016/j.ygcen.2009.02.013 [DOI] [PubMed] [Google Scholar]
- 10. Ueda H, Kambegawa A, Nagahama Y. Involvement of gonadotrophin and steroid hormones in spermiation in the amago salmon, Oncorhynchus rhodurus, and goldfish, Carassius auratus . Gen Comp Endocrinol (1985) 59(1):24–30. doi: 10.1016/0016-6480(85)90415-0 [DOI] [PubMed] [Google Scholar]
- 11. Baynes S, Scott A. Seasonal-variations in parameters of milt production and in plasma-concentration of sex steroids of Male rainbow-trout (Salmo-gairdneri). Gen Comp Endocrinol (1985) 57(1):150–60. doi: 10.1016/0016-6480(85)90211-4 [DOI] [PubMed] [Google Scholar]
- 12. Mommsen T, Vijayan M, Moon T. Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Rev Fish Biol Fisher (1999) 9(3):211–68. doi: 10.1023/A:1008924418720 [DOI] [Google Scholar]
- 13. Goikoetxea A, Todd EV, Gemmell N. Stress and sex: does cortisol mediate sex change in fish? Reproduction (2017) 154(6):R149–60. doi: 10.1530/REP-17-0408 [DOI] [PubMed] [Google Scholar]
- 14. Goos H, Consten D. Stress adaptation, cortisol and pubertal development in the male common carp, cyprinus carpio. Mol Cell Endocrinol (2002) 197(1-2):105–16. doi: 10.1095/biolreprod67.2.465 [DOI] [PubMed] [Google Scholar]
- 15. Shankar D, Kulkarni R. Effect of cortisol on testis of freshwater fish notopterus notopterus (Pallas). Indian J Exp Biol (2000) 38(12):1227–30. [PubMed] [Google Scholar]
- 16. Ozaki Y, Higuchi M, Miura C, Yamaguchi S, Tozawa Y, Miura T. Roles of 11 beta-hydroxysteroid dehydrogenase in fish spermatogenesis. Endocrinology (2006) 147(11):5139–46. doi: 10.1210/en.2006-0391 [DOI] [PubMed] [Google Scholar]
- 17. Tovo-Neto A, Matinez E, Melo A, Doretto L, Butzge A, Rodrigues M, et al. Cortisol directly stimulates spermatogonial differentiation, meiosis, and spermiogenesis in zebrafish (Danio rerio) testicular explants. Biomolecules (2020) 10(3):1–24. doi: 10.3390/biom10030429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miller W, Bose H. Early steps in steroidogenesis: intracellular cholesterol trafficking. J Lipid Res (2011) 52(12):2111–35. doi: 10.1194/jlr.R016675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miller W, Tee M. The post-translational regulation of 17,20 lyase activity. Mol Cell Endocrinol (2015) 408(C):99–106. doi: 10.1016/j.mce.2014.09.010 [DOI] [PubMed] [Google Scholar]
- 20. Zhou L, Wang D, Kobayashi T, Yano A, Paul-Prasanth B, Suzuki A, et al. A novel type of P450c17 lacking the lyase activity is responsible for C21-steroid biosynthesis in the fish ovary and head kidney. Endocrinology (2007) 148(9):4282–91. doi: 10.1210/en.2007-0487 [DOI] [PubMed] [Google Scholar]
- 21. Zhou L, Wang D, Shibata Y, Paul-Prasanth B, Suzuki A, Nagahama Y. Characterization, expression and transcriptional regulation of P450c17-I and -II in the medaka, Oryzias latipes . Biochem Bioph Res Co (2007) 362(3):619–25. doi: 10.1016/j.bbrc.2007.08.044 [DOI] [PubMed] [Google Scholar]
- 22. Jin G, Wen H, He F, Li J, Chen C, Zhang J, et al. Molecular cloning, characterization expression of P450c17-I and P450c17-II and their functions analysis during the reproductive cycle in males of barfin flounder (Verasper moseri). Fish Physiol Biochem (2012) 38(3):807–17. doi: 10.1007/s10695-011-9564-2 [DOI] [PubMed] [Google Scholar]
- 23. Zhai G, Shu T, Chen K, Lou Q, Jia J, Huang J, et al. Successful production of an all-female common carp (Cyprinus carpio l.) population using cyp17a1-deficient neomale carp. Engineering (2022) 8:181–9. doi: 10.1016/j.eng.2021.03.026 [DOI] [Google Scholar]
- 24. Zhai G, Shu T, Xia Y, Lu Y, Shang G, Jin X, et al. Characterization of sexual trait development in cyp17a1-deficient zebrafish. Endocrinology (2018) 159(10):3549–62. doi: 10.1210/en.2018-00551 [DOI] [PubMed] [Google Scholar]
- 25. Yang L, Zhang X, Liu S, Zhao C, Miao Y, Jin L, et al. Cyp17a1 is required for female sex determination and male fertility by regulating sex steroid biosynthesis in fish. Endocrinology (2021) 162(12):1–20. doi: 10.1210/endocr/bqab205 [DOI] [PubMed] [Google Scholar]
- 26. Zhou L, Li M, Wang D. Role of sex steroids in fish sex determination and differentiation as revealed by gene editing. Gen Comp Endocrinol (2021) 313:1–8. doi: 10.1016/j.ygcen.2021.113893 [DOI] [PubMed] [Google Scholar]
- 27. Sun L, Jiang X, Xie Q, Yuan J, Huang B, Tao W, et al. Transdifferentiation of differentiated ovary into functional testis by long-term treatment of aromatase inhibitor in Nile tilapia. Endocrinology (2014) 155(4):1476–88. doi: 10.1210/en.2013-1959 [DOI] [PubMed] [Google Scholar]
- 28. Li M, Yang H, Zhao J, Fang L, Shi H, Li M, et al. Efficient and heritable gene targeting in tilapia by CRISPR/Cas9. Genetics (2014) 197(2):591–U219. doi: 10.1534/genetics.114.163667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang L, Li Y, Wu Y, Sun S, Song Q, Wei J, et al. Rln3a is a prerequisite for spermatogenesis and fertility in male fish. J Steroid Biochem (2020) 197:105517. doi: 10.1016/j.jsbmb.2019.105517 [DOI] [PubMed] [Google Scholar]
- 30. Livak K, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-delta delta c) method. Methods (2001) 25(4):402–8. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 31. Fang X, Wu L, Yang L, Song L, Cai J, Luo F, et al. Nuclear progestin receptor (Pgr) knockouts resulted in subfertility in male tilapia (Oreochromis niloticus). J Steroid Biochem (2018) 182:62–71. doi: 10.1016/j.jsbmb.2018.04.011 [DOI] [PubMed] [Google Scholar]
- 32. Amer M, Miura T, Miura C, Yamauchi K. Involvement of sex steroid hormones in the early stages of spermatogenesis in Japanese huchen (Hucho perryi). Biol Reprod (2001) 65(4):1057–66. doi: 10.1095/biolreprod65.4.1057 [DOI] [PubMed] [Google Scholar]
- 33. Harvey S, Kwon J, Penman D. Physical mapping of the brain and ovarian aromatase genes in the Nile tilapia, Oreochromis niloticus, by fluorescence in situ hybridization. Anim Genet (2003) 34(1):62–4. doi: 10.1046/j.1365-2052.2003.00941.x [DOI] [PubMed] [Google Scholar]
- 34. Lin J, Hu S, Ho P, Hsu H, Postlethwait J, Chung B. Two zebrafish hsd3b genes are distinct in function, expression and evolution. Endocrinology (2016) 157(2):978–8. doi: 10.1210/en.2014-1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Trant J, Gavasso S, Ackers J, Chung B, Place A. Developmental expression of cytochrome P450 aromatase genes (CYP19a and CYP19b) in zebrafish fry (Danio rerio). J Of Exp Zoolo (2001) 290(5):475–83. doi: 10.1002/jez.1090 [DOI] [PubMed] [Google Scholar]
- 36. Yu X, Wu L, Xie L, Yang S, Charkraborty T, Shi H, et al. Characterization of two paralogous StAR genes in a teleost, Nile tilapia (Oreochromis niloticus). Mol Cell Endocrinol (2014) 392(1-2):152–62. doi: 10.1016/j.mce.2014.05.013 [DOI] [PubMed] [Google Scholar]
- 37. Rajakumar A, Senthilkumaran B. Steroidogenesis and its regulation in teleost-a review. Fish Physiol Biochem (2020) 46(3):803–18. doi: 10.1007/s10695-019-00752-0 [DOI] [PubMed] [Google Scholar]
- 38. Pandey A, Miller W. Regulation of 17,20 lyase activity by cytochrome b5 and by serine phosphorylation of P450c17. J Biol Chem (2005) 280(14):13265–71. doi: 10.1074/jbc.M414673200 [DOI] [PubMed] [Google Scholar]
- 39. Miller W, Auchus R. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocrine Rev (2011) 32(1):81–151. doi: 10.1210/er.2010-0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jin J, Yang W. Molecular regulation of hypothalamus-pituitary-gonads axis in males. Gene (2014) 551(1):15–25. doi: 10.1016/j.gene.2014.08.048 [DOI] [PubMed] [Google Scholar]
- 41. Liu G, Luo F, Song Q, Wu L, Qiu Y, Shi H, et al. Blocking of progestin action disrupts spermatogenesis in Nile tilapia (Oreochromis niloticus). J Mol Endocrinol (2014) 53(1):57–70. doi: 10.1530/JME-13-0300 [DOI] [PubMed] [Google Scholar]
- 42. Zheng Q, Xiao H, Shi H, Wang T, Sun L, Tao W, et al. Loss of Cyp11c1 causes delayed spermatogenesis due to the absence of 11-ketotestosterone. J Endocrinol (2020) 244(3):487–99. doi: 10.1530/JOE-19-0438 [DOI] [PubMed] [Google Scholar]
- 43. Zhang Q, Ye D, Wang H, Wang Y, Hu W, Sun Y. Zebrafish cyp11c1 knockout reveals the roles of 11-ketotestosterone and cortisol in sexual development and reproduction. Endocrinology (2020) 161(6):1–20. doi: 10.1210/endocr/bqaa048 [DOI] [PubMed] [Google Scholar]
- 44. Miura T, Yamauchi K, Takahashi H, Nagahama Y. Hormonal induction of all stages of spermatogenesis in vitro in the male Japanese eel (Anguilla japonica). P Natl Acad Sci USA (1991) 88(13):5. doi: 10.1073/pnas.88.13.5774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nagahama Y. 17 alpha,20 beta-dihydroxy-4-pregnen-3-one, a maturation-inducing hormone in fish oocytes: Mechanisms of synthesis and action. Steroids (1997) 62(1):190–6. doi: 10.1016/s0039-128x(96)00180-8 [DOI] [PubMed] [Google Scholar]
- 46. Scott A, Sumpter J. Seasonal-variations in testicular germ-cell stages and in plasma-concentrations of sex steroids in Male rainbow-trout (Salmo-gairdneri) maturing at 2 years old. Gen Comp Endocrinol (1989) 73(1):46–58. doi: 10.1016/0016-6480(89)90054-3 [DOI] [PubMed] [Google Scholar]
- 47. Feng C, Xu S, Liu Y, Wang Y, Wang W, Yang J, et al. Progestin is important for testicular development of male turbot (Scophthalmus maximus) during the annual reproductive cycle through functionally distinct progestin receptors. Fish Physiol Biochem (2018) 44(1):35–48. doi: 10.1007/s10695-017-0411-y [DOI] [PubMed] [Google Scholar]
- 48. Vizziano D, LeGac F, Fostier A. Effect of 17 beta-estradiol, testosterone, and 11-ketotestosterone on 17,20 beta-dihydroxy-4-pregnen-3-one production in the rainbow trout testis. Gen Comp Endocrinol (1996) 104(2):179–88. doi: 10.1006/gcen.1996.0160 [DOI] [PubMed] [Google Scholar]
- 49. Scott A, Sumpter J, Stacey N. The role of the maturation-inducing steroid, 17,20 beta-dihydroxypregn-4-en-3-one, in male fishes: a review. J Fish Biol (2010) 76(1):183–224. doi: 10.1111/j.1095-8649.2009.02483.x [DOI] [PubMed] [Google Scholar]
- 50. Shi S, Shu T, Li X, Lou Q, Jin X, He J, et al. Characterization of the interrenal gland and sexual traits development in cyp17a2-deficient zebrafish. Front Endocrinol (2022) 13:910639. doi: 10.3389/fendo.2022.910639 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.