Abstract
Soil carbon dynamics is strongly controlled by depth globally, with increasingly slow dynamics found at depth. The mechanistic basis remains however controversial, limiting our ability to predict carbon cycle-climate feedbacks. Here we combine radiocarbon and thermal analyses with long-term incubations in absence/presence of continuously 13C/14C-labelled plants to show that bioenergetic constraints of decomposers consistently drive the depth-dependency of soil carbon dynamics over a range of mineral reactivity contexts. The slow dynamics of subsoil carbon is tightly related to both its low energy density and high activation energy of decomposition, leading to an unfavourable ‘return-on-energy-investment’ for decomposers. We also observe strong acceleration of millennia-old subsoil carbon decomposition induced by roots (‘rhizosphere priming’), showing that sufficient supply of energy by roots is able to alleviate the strong energy limitation of decomposition. These findings demonstrate that subsoil carbon persistence results from its poor energy quality together with the lack of energy supply by roots due to their low density at depth.
Subject terms: Carbon cycle, Carbon cycle, Ecosystem ecology, Ecosystem ecology
The high persistence of deep soil carbon is controlled by bioenergetic constraints of decomposers resulting from the poor energy quality of soil carbon together with the lack of energy supply by roots due to their low density at depth
Introduction
Earth’s soils store more carbon in soil organic matter (SOM) than the global vegetation and atmosphere combined1. The fate of this large terrestrial reservoir of carbon under global change remains however a major uncertainty limiting our ability to accurately predict carbon cycle-climate feedbacks2,3. While most of our research effort has so far focused on soil organic carbon (SOC) located up to 30 cm deep (topsoil), a major portion corresponding to around half of the global SOC stock is stored in deeper soil layers (subsoil)1,4. Deep SOC formation can derive from dissolved organic matter and colloidal organo-mineral particles percolating downward through the soil profile, as well as organic matter inputs by deep plant roots, accumulation of eroded soil downhill and bioturbation by soil fauna such as earthworms4–7. Depth is the primary driver of SOC dynamics globally, with SOC turnover time increasing with depth from decades or centuries in topsoil to millennia in subsoil8,9. There is still much controversy about the underlying mechanisms explaining the slow dynamics of deep SOC5,10. Unravelling the mechanistic basis of the depth-dependency of SOC dynamics is critical not only for improving our fundamental knowledge about SOC dynamics, but also to enhance our ability to forecast the response of the large reservoir of SOC to global change.
The high persistence of deep SOC has been largely attributed to stabilisation mechanisms reducing its accessibility to microbial decomposers and their extracellular enzymes11: either by mineral protection related to its large proportion associated with reactive minerals7,12–14, or by physical separation related to its sparse density and associated high spatial heterogeneity in the soil matrix15–17. Suboptimal environmental conditions such as low temperature and anaerobic conditions have additionally been mentioned10,11, and obviously represent key drivers of deep SOC persistence for permafrost and peatlands. Empirical evidence supporting their importance for mineral well-aerated soils remains however limited12,14,16. Because energy limitation of microbial metabolism is a fundamental property of deep environments on Earth18, it has also been proposed that the high persistence of deep SOC could be related to a strong energy limitation of decomposers at depth17,19,20.
Some theoretical models suggest that microbes decomposing persistent SOC are mostly limited by the metabolic energy needed for the biosynthesis of exoenzymes catalysing SOC depolymerisation and solubilisation, which are the major bottlenecks restricting microbial uptake and respiration of SOC21,22. Metabolising SOC must indeed yield greater energy to decomposers than they invest in exoenzyme production to acquire this energy. If the resulting ‘return-on-energy-investment’ is negative or does not reach maintenance energy needs, decomposers then starve and their growth and exoenzyme production eventually stop, thereby allowing SOC to persist23,24. Consistent with this idea, a bioenergetic framework has been proposed to assess the energy quality of SOC related to its ‘return-on-energy-investment’ for decomposers25–28. Deep SOC has previously been shown to feature a smaller energy density29 and larger thermal stability30 than surface SOC, suggesting a decline in SOC energy quality with depth.
Energy limitation of deep SOC decomposition could also provide a mechanistic underpinning to the large destabilization of deep SOC associated with fresh organic matter supply20. Plant living roots have for instance been shown to induce an acceleration of deep SOC decomposition17, corresponding to a ‘rhizosphere priming’ phenomenon31. Rhizodeposition indeed represents a major source of fresh bioavailable and energy-rich substrates for soil decomposers32,33. Rhizodeposition could thereby trigger the decomposition of persistent SOC having poor energy quality via microbial co-metabolism because it can subsidise the energy cost of exoenzyme production21,22. Rhizosphere priming could also be driven by rhizosphere processes increasing SOC accessibility such as root exudation of organic ligands disrupting organo-mineral associations34–36, or root uptake of water and nutrients intensifying drying-rewetting cycles and breakage of soil aggregates31. The lack of supply of energy in different forms, either metabolic, chemical or mechanical, by plant roots due to their low density at depth could thus be at play in explaining deep SOC persistence4,20.
Direct experimental evidence linking deep SOC dynamics to both its bioenergetic signature and sensitivity to rhizosphere priming is still lacking to date. We thus investigate here the bioenergetic control of the depth-dependency of SOC dynamics in temperate well-aerated mineral soils. Specifically, we hypothesise that the slow dynamics of deep SOC is related to its poor energy quality together with the lack of energy supply by plant roots in subsoil. To test this hypothesis, we combine a characterisation of soil biogeochemical properties, including radiocarbon and thermal analyses, with a complementary experiment involving long-term soil incubations under controlled conditions in presence or absence of continuously 13C/14C-labelled plants. To assess the robustness of our hypothesis according to soil mineral reactivity context, we replicate our study in three soil profiles with contrasting mineralogy: a cambisol, a vertisol and an andosol which are respectively characterised by moderate, high and very high mineral reactivity and deep SOC persistence based on a priori knowledge14. Our results demonstrate that the slowing of SOC dynamics with depth is consistently linked to a degradation of SOC energy quality, that is a smaller energy density and larger activation energy of decomposition in connection with its chemistry and interactions with soil minerals, together with a decline in the density of roots and their energy supply. These findings provide insights into the bioenergetic control of SOC persistence and indicate that an increase in plant rooting depth induced by global change could threaten the storage of millennia-old SOC in deep layers.
Results
Bioenergetic control of SOC dynamics across depth
Our biogeochemical characterisation of soil showed a clear in situ differentiation of SOM properties across depth that was consistently found for each of the three soil profiles studied despite their contrasting mineralogy (Fig. 1, RC1). Relative to topsoil, the subsoil was characterised by smaller concentration ([SOC]) and radiocarbon signature (Δ14C) of SOC, as well as lesser proportion of particulate organic matter (fPOM, Table 1). Subsoil was in contrast characterised by higher δ13C signature of SOC indicative of greater microbial processing37, as well as greater proportion of mineral-associated organic matter (fMAOM). Microbial biomass per unit soil mass and SOC were both smaller for subsoil than topsoil. A modelling approach was used to estimate SOC dynamics from radiocarbon (Δ14C) measurements of SOC and we found a large increase in SOC turnover time with depth, increasing on average 6.9-fold from 1096 to 7567 years.
Fig. 1. Variation in soil biogeochemical properties among soil types and layers.
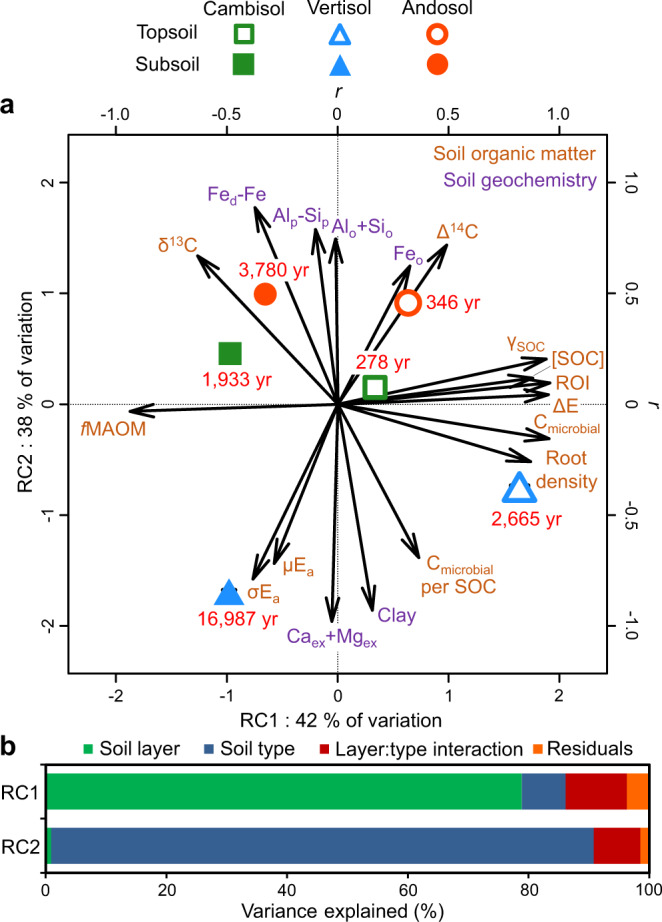
a ordination by rotated principal component analysis (n = 24 soil cores). Arrows indicate correlations between soil properties and rotated component (RC) axes. Large symbols with error bars show treatment means ± standard errors. The numbers in red indicate the turnover time of soil organic carbon (SOC) based on radiocarbon signature for each treatment. The subscripts ex, d, o, and p indicate exchangeable, dithionite, oxalate, and pyrophosphate extracts, respectively. See Table 1 for other abbreviations. b variance partitioning of RC axis values across experimental factors.
Table 1.
Depth variation in soil organic carbon (SOC) properties averaged across the three soil types
| Topsoil | Subsoil | Depth effect size | Partial η² | |
|---|---|---|---|---|
| SOC concentration ([SOC], g SOC kg−1 soil) | 85.5 ± 5.6 | 34.7 ± 5.6 | −59.4%a | 0.57 |
| fPOM (% SOC) | 8.3 ± 0.6 | 2.7 ± 0.3 | −67.9%a | 0.82 |
| fMAOM (% SOC) | 91.7 ± 0.6 | 97.3 ± 0.3 | +6.1%a | 0.82 |
| δ13C (‰) | −26.85 ± 0.12 | −25.88 ± 0.27 | +0.98b | 0.80 |
| Δ14C (‰) | −75.8 ± 34.2 | −375.8 ± 61.1 | −300b | 0.88 |
| SOC turnover time (τ, year) | 1096 ± 340 | 7566 ± 2033 | +590%a | 0.56 |
| Energy density (ΔE, kJ mol−1 SOM) | 175.1 ± 4.5 | 126.7 ± 4.5 | −27.6%a | 0.85 |
| Degree of reduction of SOC (γSOC) | 3.05 ± 0.06 | 2.26 ± 0.08 | −26.0%a | 0.83 |
| Hydrogen Index (HI, g HxCy kg−1 SOC) | 229.1 ± 16.9 | 114.5 ± 10.6 | −50.0%a | 0.68 |
| Oxygen Index (OI, g O2 kg−1 SOC) | 180.1 ± 9.6 | 222.2 ± 19.1 | +23.4%a | 0.55 |
| Mean activation energy (µEa, kJ mol−1 SOM) | 159.5 ± 0.4 | 162.0 ± 0.9 | +2.4b | 0.61 |
| SD of activation energy (σEa, kJ mol−1 SOM) | 16.10 ± 0.06 | 16.80 ± 0.28 | +0.70b | 0.43 |
| T90-HxCy-pyrolysis (°C) | 521.3 ± 0.7 | 532.8 ± 0.9 | +11.5b | 0.82 |
| T50-CO2-pyrolysis (°C) | 387.2 ± 0.9 | 394.3 ± 2.6 | +7.1b | 0.51 |
| T50-CO2-oxidation (°C) | 422.5 ± 2.1 | 437.8 ± 5.2 | +15.3b | 0.56 |
| Return-on-energy-investment (ROI, ΔE:µEa) | 1.10 ± 0.03 | 0.78 ± 0.03 | −28.7%a | 0.85 |
| Microbial biomass (Cmicrobial, g C kg−1 soil) | 1.25 ± 0.17 | 0.32 ± 0.03 | −74.1%a | 0.73 |
| Microbial biomass per unit SOC (g C kg−1 SOC) | 15.2 ± 0.9 | 10.9 ± 1.4 | −28.1%a | 0.37 |
| Root density (g dm−3) | 3.21 ± 0.82 | 0.14 ± 0.03 | −95.6%a | 0.52 |
fPOM fraction of particulate organic matter; fMAOM, fraction of mineral-associated organic matter; µEa and σEa, mean and standard deviation of the activation energy of soil organic carbon (SOC) decomposition; Return-on-energy-investment (ROI) is the ratio of ΔE to µEa; T90-HxCy-pyrolysis, temperature at which 90 % of HxCy was evolved during pyrolysis; T50-CO2-pyrolysis and T50-CO2-oxidation, temperatures at which 50 % of CO2 was evolved during pyrolysis and oxidation, respectively. Mean ± standard errors (n = 12 soil cores, including 3 soil types with 4 replicates each).
aRelative change in soil properties for subsoil relative to topsoil: this effect size was computed as (x̄subsoil − x̄topsoil)/x̄topsoil.
bAbsolute change in soil properties for subsoil relative to topsoil: this effect size was computed as x̄subsoil − x̄topsoil. Partial η² indicates the proportion of variance associated with the depth effect after accounting for soil type effect. See Supplementary Table 1 for depth variation in soil properties for each soil type, and Supplementary Table 2 for statistical results.
We adopted a bioenergetic framework to assess how SOC dynamics could be related to its energy quality for microbial decomposers25–28, defined here as the net energy gain that decomposers could realise for a given energy investment in SOC acquisition. The energy quality of SOC was quantified using the return-on-energy-investment (ROI) parameter26, which is the ratio between (i) the energy density of SOC (ΔE, energy content per unit SOC) representing the amount of energy that decomposers could gain by SOC catabolism38, and (ii) the mean activation energy (µEa) of SOC decomposition representing the energetic barriers to its acquisition by decomposers39. The energy density of SOC and the activation energy of its decomposition were respectively measured by differential scanning calorimetry and evolved gas analysis during ramped combustion25,28.
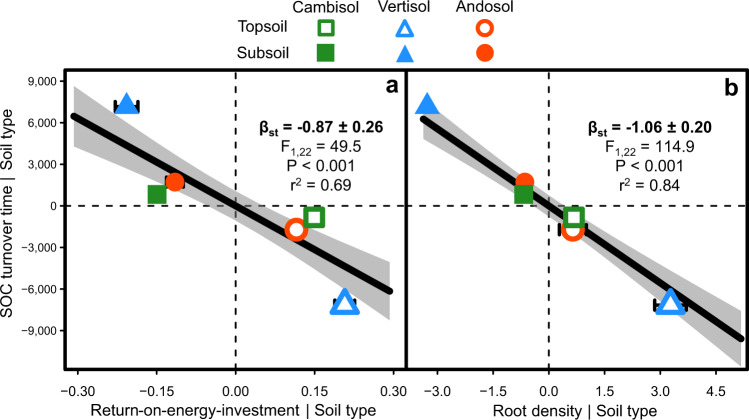
This approach allowed us to reveal an important shift in SOC bioenergetic signature with depth (Fig. 1, Table 1, Supplementary Fig. 1). The energy density of SOC (ΔE) was indeed 27.6% smaller for subsoil than topsoil (decline from 175.1 to 126.7 kJ mol−1 SOM with depth). The degree of reduction of SOC (γSOC) was accordingly also lower at depth (decline from 3.05 to 2.26 with depth), consistent with the smaller hydrogen content (HI) and larger oxygen content (OI) of SOM. In contrast, the mean and standard deviation of the activation energy of SOC thermal decomposition were greater for subsoil than topsoil (µEa, +2.4 kJ mol−1 SOM; σEa, +0.70 kJ mol−1 SOM), consistent with the larger thermal stability of deep SOC (higher T90-HxCy-pyrolysis, T50-CO2-pyrolysis and T50-CO2-oxidation). Accordingly, the return-on-energy-investment parameter (ROI) declined with depth from 1.10 to 0.78, which was strongly related to the increase in SOC turnover time with depth (Fig. 2a). We also observed a very strong decline in root density with depth from 3.21 to 0.14 g dm–3 (Table 1), which was tightly related to the increase in SOC turnover time with depth as well (Fig. 2b).
Fig. 2. Energy quality and root density as drivers of the depth-dependency of soil carbon dynamics.
Partial regression plots relating the depth variation in the turnover time of soil organic carbon (SOC) with those of the return-on-energy-investment parameter (a) and root density (b, n = 24 soil cores). The residuals of linear models fitting each variable with ‘soil type’ as a fixed effect were used here so that each variable was normalised for variation across soil types. The return-on-energy-investment parameter was calculated as the ratio between the energy density of SOC and its mean activation energy as determined by thermal analyses, and was used here as an indicator of SOC energy quality. Symbols with error bars show treatment means ± standard errors (n = 4 replicate soil cores). Polygons around regression lines represent 95% confidence intervals. βst are regression slope coefficients ± 95% confidence intervals standardised by range. Significant βst are in bold (two-sided F-test).
Root effects on SOC decomposition across depth
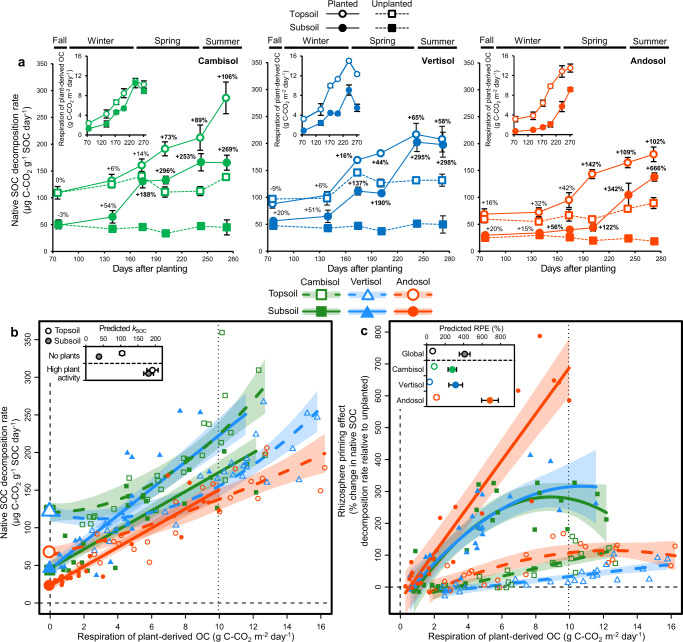
In order to investigate the consequences of the variation in root density across depth for SOC decomposition, we performed a complementary experiment involving long-term soil incubations in presence or absence of continuously 13C/14C-labelled plants. This dual-labelling allowed us to simultaneously partition soil and plant sources into C fluxes and pools, and determine the mean age of native SOC respired17,20. In an effort to decouple the natural covariation in the vertical distribution of root density and SOC properties4, we grew a deep-rooting grass species (Dactylis glomerata) in soil columns made of intact soil cores derived exclusively from either topsoil or subsoil (Supplementary Fig. 2). As SOC dynamics can be very sensitive to physical disturbance, especially for subsoil16,17, we took care to preserve the soil structure as undisturbed as possible during the sampling and incubations. We performed two series of incubations under moisture and temperature-controlled conditions. The first series of incubations performed throughout plant growth consisted of regularly measuring the respiration of the plant-soil system across time (Fig. 3a). The second series of incubations performed at the end of the experiment consisted of retrieving the original soil cores and measuring the respiration of the root-soil system at different depths in the soil column and thus at different root densities (Fig. 4a). The density of roots indeed declined from 3.57 to 0.51 g dm-3 with depth in the soil column, these values being close to the decline in root density with depth observed in the field.
Fig. 3. Root effects on the decomposition of native soil organic carbon (SOC) across time.
a Temporal variation in native SOC decomposition rate (kSOC) and respiration of plant-derived organic carbon (OC, insets) at the microcosm scale in the first series of incubations (n = 288 microcosm incubations). The respiration of plant-derived OC includes both plant autotrophic respiration and soil microbial heterotrophic respiration derived from rhizodeposits. Symbols with error bars show treatment means ± standard errors (n = 4 replicate microcosms). Numbers above the symbols represent rhizosphere priming effects (RPEs, in % change in kSOC for planted soils relative to the unplanted control). Significant RPEs are in bold (two-sided t-test). b, c Response of native SOC decomposition rate (b, kSOC, n = 288 microcosm incubations) and rhizosphere priming effect (c, RPE, n = 144 planted microcosm incubations) to the variation in respiration of plant-derived OC across time in the first incubation series. Symbols with error bars along the vertical dashed line in (b) show treatment means ± standard errors of unplanted controls (n = 24 microcosm incubations). Polygons around regression lines represent 95% confidence intervals. The inset in (b) show kSOC values averaged across soil types in the absence of roots and at high level of plant activity as predicted for a common high value of the predictor shown by the vertical dotted line. The insets in (c) show predicted RPE values across treatments at high level of plant activity. Statistical results are shown in Supplementary Table 3.
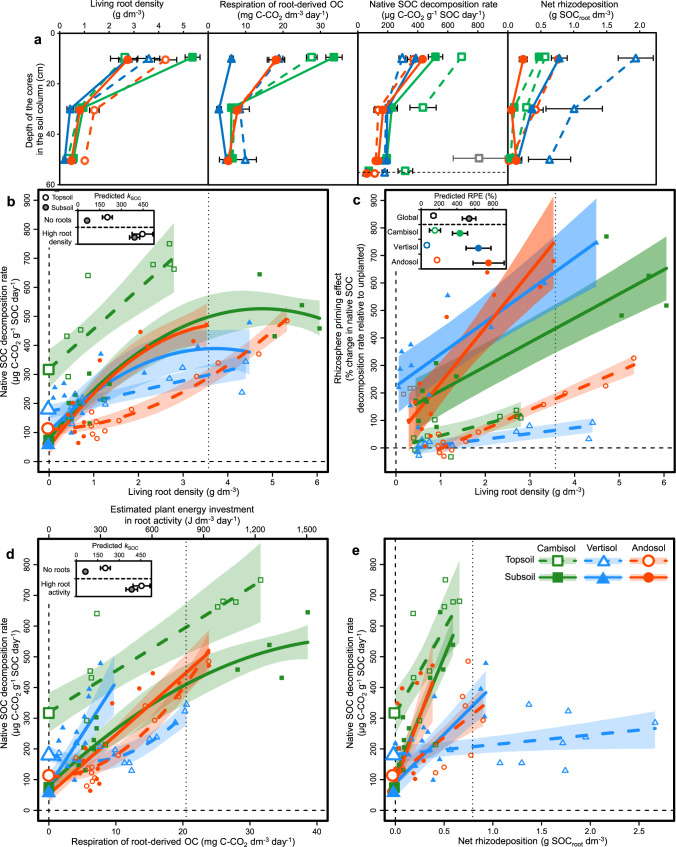
Fig. 4. Root effects on the decomposition of native soil organic carbon (SOC) across soil column depth.
a Depth variation in living root density, respiration of root-derived organic carbon (OC), native SOC decomposition rate and net rhizodeposition at the soil core scale in the second incubation series 279 days after planting (n = 144 soil core incubations). Symbols with error bars show treatment means ± standard errors (n = 4 replicate soil cores). b–e Response of native SOC decomposition rate (b, d, e, kSOC, n = 144 soil core incubations) and rhizosphere priming effect (c, RPE, n = 72 soil core incubations) to the variation in living root density (b, c), respiration of root-derived OC (d) and net rhizodeposition (e) across soil column depth in the second incubation series. The respiration of root-derived OC includes both root autotrophic respiration and soil microbial heterotrophic respiration derived from rhizodeposits, and was used here to estimate plant energy supply to soil by root activity (Methods section). Symbols with error bars along the horizontal dashed line in (a) and along the vertical dashed line in (b, d, e) show treatment means ± standard errors of unplanted controls (n = 24 soil core incubations). Polygons around regression lines represent 95% confidence intervals. Grey symbols represent outliers. Statistical results are shown in Supplementary Table 4.
For both incubation series, we found that the native SOC decomposition rate (kSOC, respiration of pre-existing SOC per unit SOC stock) in the absence of roots was on average 3.0-fold smaller for subsoil relative to topsoil across the three soil types (Figs. 3b, 4b), consistent with the slower SOC dynamics found for subsoil than topsoil based on radiocarbon measurements. Native SOC decomposition included here the respiration of pre-existing SOM but also root litter. However, the amount of OC in pre-existing root litter represented on average only 2.6 and 0.3% of the amount of native SOC respectively for the topsoil and subsoil at the beginning of the experiment (Supplementary Table 6). By the end of the experiment, 90.1% of pre-existing root masses were lost on average, and pre-existing dead roots OC represented on average 0.27% of the amount of native SOC for the topsoil. This indicates that our estimation of kSOC largely reflects the decomposition of native SOM, rather than pre-existing root litter.
The radiocarbon signature of respired CO2 in unplanted subsoil cores at the end of the experiment revealed that the mean age of native SOC respired by decomposers was within the range of initial SOC dominated by millennia-old pools, that were around 1950 and 2900 years respectively for the cambisol and andosol (Fig. 5). This result indicates that the small amounts of young and labile organic matter present in subsoil (root litter and particulate organic matter, Table 1) were probably largely exhausted after nearly 1 year of incubation, leading microbes to decompose old SOC pools17.
Fig. 5. Root effects on the mean age of subsoil organic carbon respired by decomposers.
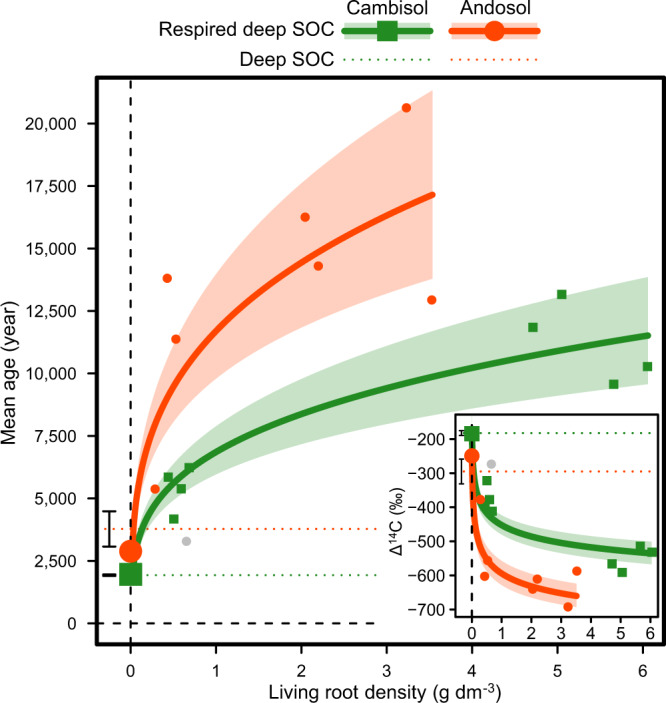
Response of radiocarbon-based mean age of respired CO2 derived from the decomposition of native deep soil organic carbon (SOC) to the variation in living root density across soil column depth at the soil core scale in the second incubation series 279 days after planting (n = 24 soil core incubations). The inset show radiocarbon signatures (Δ14C) of soil-respired CO2 used to determine the mean age of respired native SOC. Since turnover time and mean age are identical under our model assumption of SOC being a homogeneous one-pool reservoir at steady-state, the mean ages were estimated using the same modelling approach used for turnover time as described in the methods section. Symbols with error bars along the vertical dashed line show treatment means ± standard errors of unplanted controls (n = 4 soil core incubations). Horizontal dotted lines with error bars show the means ± standard errors of mean ages (turnover times) and radiocarbon signatures of initial deep SOC. Polygons around regression lines represent 95% confidence intervals. The grey symbol is an outlier not included in regression model. Statistical results are shown in Supplementary Table 5.
The presence of roots induced an acceleration of kSOC corresponding to a rhizosphere priming effect (RPE) for both topsoil and subsoil (Figs. 3b, c, 4b, c). In the first incubation series, we observed an increase in kSOC that was associated with an increase in the respiration of plant-derived OC related to plant growth over time (Fig. 3a). In the second incubation series, we observed a concomitant increase in kSOC, living root density, respiration of root-derived OC and net rhizodeposition with decreasing soil column depth (Fig. 4a). Consequently, we found strong positive relationships of kSOC with respiration of plant-derived OC in the first incubation series (Fig. 3b), as well as with living root density, respiration of root-derived OC and net rhizodeposition in the second incubation series (Fig. 4b, d, e).
For both incubation series, we observed that the kSOC of topsoil and subsoil converged toward high levels at high plant activity or root density (Figs. 3b, 4b), so that the acceleration of kSOC induced by roots (RPE) was proportionally much higher for subsoil than topsoil (Figs. 3c, 4c). In the first incubation series, the average RPEs across soil types were for instance +64% for topsoil and +411% for subsoil at a given high level of respiration of plant-derived OC (9.94 g C–CO2 m−2 day−1), corresponding to a 6.4-fold difference (Fig. 3c). In the second incubation series, we similarly found that the average RPEs across soil types were +138% for topsoil and +535% for subsoil at a given high level of living root density (3.57 g dm−3), corresponding to a 3.9-fold difference (Fig. 4c). We also observed a strong asymptotic increase toward very high mean age of native deep SOC respired by decomposers with increasing living root density at the end of the experiment, reaching around 9,000 and 17,000 years respectively for the cambisol and andosol at high root density (Fig. 5).
Overall, we found that SOC bioenergetic signature properties and root density were as good or better predictors of SOC dynamics parameters than more classical predictors of SOC stability such as fPOM versus fMOAM, δ13C or [SOC] (Supplementary Figs. 3, 4).
Mineral control of SOC dynamics across depth
The field characterisation of soil also confirmed strong differences in mineralogy among soil types (Fig. 1, RC2). The vertisol and andosol contained high amounts of reactive secondary minerals of different mineral composition. The andosol was characterised by high concentrations of organically complexed metals (Alp-Sip), as well as short-range-ordered (SRO) metal oxyhydroxides (Feo, e.g. ferrihydrite) and aluminosilicates (Alo and Sio, e.g. allophane) forming covalent bonds with SOC through ligand exchange. The vertisol was characterized by high concentrations of phyllosilicate minerals (clay) such as smectite and halloysite based on XRD analyses (Supplementary Table 1), as well as divalent metal cations (Caex + Mgex) involved in organo-mineral associations through cation bridging. The cambisol was characterized by smaller concentrations of reactive minerals, mostly composed of Fe oxides in both crystalline (Fed–Feo) and SRO oxyhydroxide (Feo) forms as well as divalent metal cations along with kaolinite and vermiculite clay minerals. We concomitantly observed higher turnover time of subsoil SOC for the andosol (3780 years) and vertisol (16,987 years) than the cambisol (1933 years, Fig. 1).
The vertisol SOC also showed high thermal stability (T50-CO2-pyrolysis, T50-CO2-oxidation), low radiocarbon signature (Δ14C) and oxygen content (OI) for both the topsoil and subsoil. This suggests a substantial abundance of pyrogenic SOC that could partly explained the unusually high turnover time of vertisol SOC40, though a partial contribution of fossil C from volcanic CO2 emission or inherited from the parent material could also possibly be involved7.
Overall, the smaller kSOC in the absence of roots and larger RPE for subsoil than topsoil was a pattern that has been consistently found for each of the three soil types with contrasting mineralogy studied here (Figs. 3c, 4c). However, we observed important differences in RPE among soil types for the subsoil. Indeed, the RPE at high plant activity was much higher for the andosol than the vertisol and cambisol in the first incubations series (Fig. 3c), while the RPE at high root density was higher for the andosol and to a lesser extent the vertisol than for the cambisol (Fig. 4c).
Discussion
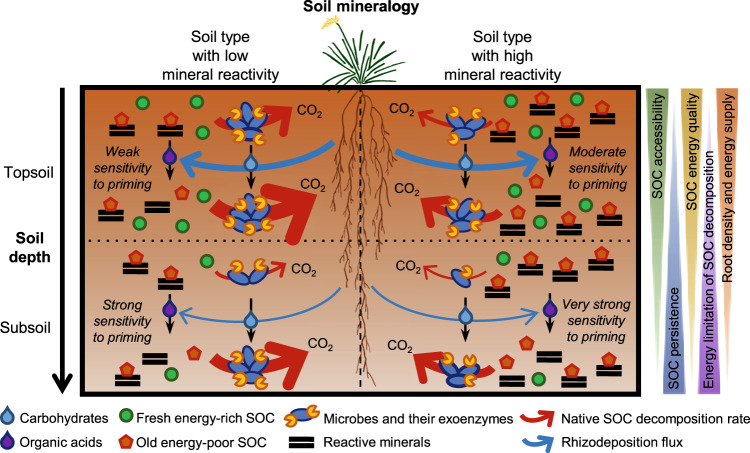
Our study combining radiocarbon and multiple thermal analyses methods along with plant isotopic labelling and long-term soil incubations provides a robust set of observations supporting the idea of a bioenergetic control of the depth-dependency of SOC dynamics (Fig. 6). The high persistence of deep SOC was indeed related to its poor energy quality (Fig. 2a). More precisely, deep SOC decomposition required higher energy inputs to proceed (activation energy) while yielding less energy available for biosynthesis (energy density), leading to a less favorable ‘return-on-energy-investment’ for microbes decomposing this persistent SOC25–28 (Table 1). These results provide evidence of an increasing energy limitation of SOC decomposition with depth. The slow dynamics of deep SOC was also strongly related to the low density of plant roots in subsoil (Fig. 2b). Our soil incubation experiment showed that the decomposition rate of native SOC was much lower for subsoils than topsoils in the absence of roots, while subsoil and topsoil SOC decomposition converged toward greater levels at high root density typically found in topsoil (Figs. 3, 4). Such acceleration of deep SOC decomposition by root activity (rhizosphere priming) led to the respiration of millennia-old SOC (Fig. 5). These results demonstrates that the persistent SOC found in subsoil could be destabilised by rhizosphere priming when root density and activity are high enough to provide the supply of energy able to alleviate the energy limitation of SOC decomposition17,20. This implies that the energy limitation of deep SOC decomposition related to its poor energy quality is exacerbated by the lack of energy supply by plant roots due to their low density at depth, thus contributing of deep SOC persistence. These results also contribute to the growing body of evidence showing the greater sensitivity to priming of SOC with higher persistence because of a greater energy limitation of decomposition41.
Fig. 6. Conceptual model illustrating the bioenergetic control of SOC dynamics and its sensitivity to rhizosphere priming across soil depth and mineral reactivity contexts.
The width of native SOC decomposition arrows is proportional to its rate. The rhizosphere priming intensity represents the magnitude in the acceleration of native SOC decomposition induced by a given density and energy supply of plant roots. Plant image by Alice Trotel.
The shift in SOC bioenergetic signature with depth could be related to both its chemistry and interactions with soil minerals. Deep SOC had broader distributions of activation energy than surface SOC (Table 1), and this larger bond-strength diversity indicates larger formation of high-energy bonds with reactive minerals contributing to its persistence by mineral protection39. Accordingly, the smaller energy density of deep SOC could be partly ascribed to the greater proportion of MAOM requiring the breaking of organo-mineral bonds before decomposition, and conversely to the lesser proportion of energy-richer POM42. From a chemical point of view, deep SOC had smaller degree of reduction (γSOC, Table 1), which was related to its reduced content of highly energetic C–H bonds and often increased content of less energetic C–O bonds25,43. This is consistent with the idea of SOC becoming increasingly oxidised (less reduced) as decomposition proceed44. The γSOC represents a measure of the chemical energy stored in organic matter, corresponding to the number of electron equivalents per SOC amount38,43. The γSOC is widely known to control microbial carbon-use efficiency38,45,46, that is the ratio of growth over C uptake. When γSOC decreases below the degree of reduction of C in biomass of microbial decomposers (γ ~ 4.2), their biosynthesis become increasingly energy limited. We found here that γSOC decreased with depth on average from 3.05 for topsoil to 2.26 for subsoil. This result highlights that the smaller energy density of deep SOC could also be ascribed to the fact that deep SOC is more oxidised47.
Despite their contrasting mineralogy and deep SOC persistence, the three soil types studied showed a similar pattern of slower SOC dynamics with depth that was related to a decline in both SOC energy quality and root density (Fig. 2). This provides evidence that bioenergetic constraints of decomposers consistently drive the depth-dependency of SOC dynamics over a range of mineral reactivity contexts. It remained so far uncertain how mineral reactivity modulates the sensitivity of deep SOC to rhizosphere priming35,36,48, despite soil mineralogy being largely recognised as a key control over deep SOC persistence12,14. We observed here important mineral control of the depth-dependency over root effects on SOC decomposition. In contrast with the idea that strong mineral protection of SOC provided by high mineral reactivity would lead to weak rhizosphere priming because of the low accessibility of SOC to microbial metabolism48, our results interestingly showed that a greater mineral protection of deep SOC also involves a greater sensitivity to rhizosphere priming (Figs. 3c, 4c, 6). This was especially clear for the andosol rich in SRO minerals forming covalent bonds with SOC through ligand exchange14,49. This is consistent with recent studies showing that root exudation of organic acids acting as ligands can enhance SOC accessibility to decomposers by disruption of protective organo-mineral associations36,50,51, thereby promoting greater rhizosphere priming for soil types with larger mineral reactivity35.
Suboptimal environmental conditions have been mentioned as potentially important drivers of deep SOC persistence10,11. Our results largely confirmed empirical studies showing that their importance for mineral well-aerated soils remains limited12,14,16. The smaller decomposition rate of native SOC under temperature and moisture-controlled conditions for subsoil than topsoil in the absence of roots indeed demonstrates that the slowing of SOC dynamics with depth cannot be merely driven by abiotic conditions such as cold or waterlogged pedoclimate and oxygen limitation at depth. Furthermore, our result of a decline in γSOC with depth contrasts with the idea that increasingly anaerobic conditions within microaggregates with depth can lead to deep SOC persistence even for well-aerated soils52. This is because it should theoretically involve an increase in γSOC by preferential preservation of highly reduced organic matter. This mechanism may however be central in permanently waterlogged soils in wetlands and peatlands43,53.
The mechanisms controlling SOC dynamics are still actively debated. One of the leading paradigm of recent years postulates that SOC dynamics is largely controlled by stabilisation mechanisms reducing its accessibility to microbial decomposers and their extracellular enzymes10,11,44. Though energy flow through living systems has long been considered a major driver of biogeochemical cycling processes54, there has been comparatively little interest until recently about energy limitation of SOC dynamics55. Our findings support the emerging view that SOC dynamics could also be controlled by bioenergetic constraints experienced by decomposers20,25,56. Bioenergetic constraints have often been viewed as a competing mechanism of SOC stabilisation relative to those restricting SOC accessibility such as physical separation and mineral protection36,48. We propose here that these mechanisms are not mutually exclusive. We even suggest that stabilisation mechanisms restricting SOC accessibility can often be interpreted based on bioenergetic constraints. For instance, physical separation of SOC from its decomposers implies large diffusion distance between them and enhanced probability of exoenzyme inactivation and decomposition product loss by diffusion away from decomposers or immobilisation by reactive minerals and cheater microbes15,17,23. For this reason, greater physical separation between SOC and its decomposers can lead to lower return-on-energy-investment in exoenzymes57. However, physical separation could often be overcome by mobility for flagellated bacteria or hyphal growth for fungi. Similarly, mineral protection restricts exoenzyme access to SOC, but microbial decomposers can produce organic ligands34,50 or reactive oxygen species through oxidative (exo)enzymes and reactive metal intermediates58,59. These compounds are able to disrupt organo-mineral associations, thereby enhancing microbial access to previously mineral-protected SOC. Nevertheless, both of these microbial strategies to access SOC imply metabolic costs, so that reduced accessibility could lead SOC to persist ultimately because of the associated exacerbation of energy limitation for decomposers. Our study demonstrates that persistent SOC can still be decomposed by rhizosphere priming provided that plants invest energy to (i) subsidise these metabolic costs for decomposers through rhizodeposition of fresh energy-rich compounds, and/or (ii) directly increase SOC accessibility through rhizosphere processes leading to the breakdown of organo-mineral associations and aggregates. We therefore propose that the role of mechanisms controlling SOC accessibility for SOC dynamics cannot be fully understood without explicit consideration of the bioenergetic constraints of decomposers and vice versa. Considering their interactive effects could thus prove valuable in our research effort towards developing a unified theory of biogeochemical controls on organic matter decomposition across Earth’s environments60.
There is growing evidence that global change drivers such as elevated CO261,62 as well as climate warming and reduced precipitation63,64 are increasing plant rooting depth in terrestrial ecosystems. The breeding and use of deep-rooting plant species resistant to drought has also been promoted as a land management adaptation to climate change in cropping systems65. Our results indicate that an increased root biomass allocation to deep soil layers previously featuring low root density can greatly enhance the decomposition of millennia-old SOC, which could threaten the storage of persistent SOC and potentially lead to a positive feedback on global warming2. This could however also promote SOC formation31, but elevated CO2 experiments focusing on topsoil SOC storage have shown that the loss of old SOC associated to rhizosphere priming usually largely offsets SOC formation66,67, and can even lead to a decline in SOC storage when plant biomass is strongly stimulated68. Further research including a comprehensive SOC budget accounting for the balance between SOC formation and loss will therefore be crucial to evaluate the net effect of plant rooting depth on SOC storage in deep layers. Our results are at least questioning the efficiency of using deep-rooting plant species as a SOC sequestration strategy65,69.
Methods
Further details about radiocarbon and thermal analysis, isotopic partitioning procedures and quantification of their uncertainty, and statistical analyses can be found in Supplementary Methods.
Study soils, experimental design and soil sampling
We selected three soil types: eutric cambisol, chromic vertisol and silandic andosol70. The three soil profiles studied were found in long-term semi-natural grasslands located relatively close to each other (<100 km) in Auvergne, France. They developed under a similar temperate semi-continental climate and mainly differed by their parent materials: granite, basalt, and trachyandesite for the cambisol, vertisol and andosol, respectively (Supplementary Table 7). The three soil profiles studied were well-aerated since none showed reductimorphic layers, and redoximorphic features were present only in the vertisol.
The experiment had a factorial design of two crossed factors: two soil layers including topsoil versus subsoil, and three soil types for a total of six treatments each including four replicates. We collected 20 cm high soil cores from the two layers for each soil profile. Intact soil columns of 8 cm diameter were extracted using a percussion core drill that can be opened from sideways. Topsoil cores were taken in the 5–25 cm depth part of the A horizon, which allowed us to remove both the native vegetation and a large proportion of their fresh litter. Subsoil cores were taken in the top 20 cm of the B horizon, that are at 40–60, 55–75 and 35–55 cm depth respectively for the cambisol, vertisol and andosol (Supplementary Fig. 5).
Soil biogeochemical analyses
Four soil cores of each treatment were used to characterise soil biogeochemical properties. These soil cores were first sieved at 2 mm and a portion was air-dried at room temperature. A portion of the air-dried soil was also ground (<250 μm) to homogeneity.
Soil C and N concentrations and δ13C was determined on ground soil containing ~1 mg C using an elemental analyser (EA, Carlo Erba, Rodana, Italy) coupled to an isotope-ratio mass spectrometer (IRMS; Elementar, Langenselbold, Hesse, Germany). None of the soil profiles contained carbonates, and total soil C content is interpreted as SOC only here. To determine the relative contribution of mineral-associated organic matter versus particulate organic matter to SOC, each soil sample was fractionated by particle size (50 μm) after full soil dispersion71. Briefly, 5 g of air-dried soil was shaken for 18 h in sodium hexametaphosphate (0.5%) with beads to completely disperse the soil. The dispersed soil was then rinsed onto a 50 μm sieve and the fraction passing through (<50 μm) was collected as MAOM, while the fraction remaining on the sieve was collected as POM. Each fraction was then analysed for C concentration using an EA.
The radiocarbon signature (Δ14C) of SOC was determined by Accelerator Mass Spectroscopy (AMS) on ground soil containing respectively ~1 and 0.14 mg C for topsoil and subsoil with a Mini Carbon Dating System (ECHoMICADAS) operated at LSCE (Climate and Environment Sciences Laboratory) in Gif-sur-Yvette, France. To assess SOC dynamics, we estimated SOC turnover time based on radiocarbon measurements using a modelling approach72,73. The following time-dependent, homogeneous one-pool model was used:
| 1 |
where t is the time (in year), F14CSOC is the 14C content of SOC, F14Catm is the 14C content of CO2 in the local atmosphere, τ is the mean SOC turnover time and λ is the radioactive decomposition constant for 14C (1.21 × 10−4 year−1). We ran the model from 50 kyr BP until the year 2016 to calculate the predicted SOC Δ14C at the year of sampling for a range of τ values (1 to 30,000 years). We then derived τ values from our Δ14C measurements for each sample based on the relationship between τ and predicted Δ14C (Supplementary Fig. 6). Though the model assumption of SOC as a homogeneous one-pool is clearly an oversimplification, our approach remains useful to compare SOC dynamics across soil samples9.
Thermal analyses were used to characterise the bioenergetic signature of SOM. The activation energy (Ea) of SOC thermal decomposition was measured by evolved gas analysis during ramped combustion using Rock-Eval® thermal analysis. Sequential ramping by pyrolysis and oxidation was performed on ~60 mg of ground soil using a Rock-Eval® 6 Turbo device (Vinci Technologies, France). Hydrocarbon effluents were quantified by flame ionisation detection during the pyrolysis phase, while CO and CO2 were quantified by infra-red detection during both ramping phases. Based on evolved SOC kinetics, a regularised inverse method was used to determine the continuous distribution of Ea that best predicts the profile of SOC decay measured during ramped combustion74 (Supplementary Fig. 1a, b). The distribution of Ea was then integrated to calculate the mean (µEa) and standard deviation (σEa) of activation energy. Additionally, the temperature at which 90% of hydrocarbons was evolved during pyrolysis (T90-HxCy-pyrolysis, °C), and the temperatures at which 50% of CO2 was evolved during pyrolysis and oxidation (T50-CO2-pyrolysis and T50-CO2-oxidation, °C) were used as indices of SOC thermal stability75. The hydrogen index (HI) and oxygen index (OI) providing information about elemental H:C and O:C ratios of SOM were calculated respectively as the amount of hydrocarbons and the amount of CO2 and CO formed during pyrolysis divided by SOC concentration.
Energy density of SOC (ΔE) was measured by differential scanning calorimetry (DSC)28,76. Oxidation ramping was performed on ~60 mg of ground soil using a DSC thermal analyser (TGA-DSC 3+ model, Mettler-Toledo, Greifensee, Switzerland) to measure the net energy released by SOM combustion (enthalpy of combustion), knowing that some of the energy applied to the sample is consumed by the breakdown of the organo-mineral associations. The ΔE was calculated as the net energy released determined by integration of heat flux over the exothermic region associated to SOC combustion (185–600 °C, Supplementary Fig. 1c), divided by SOC concentration and multiplied by SOC molar mass (Supplementary Table 8, Supplementary Methods). Calorimetry also allows to estimate the degree of reduction of SOC (γSOC)77, which was calculated here as ΔE divided by Q0, the oxycaloric quotient representing the ratio between the enthalpy of combustion and the degree of reduction76. We used a Q0 value of 109.04 kJ mol−1 SOC degree of reduction−1, obtained from the average of the heat of combustion of a large set of organic compounds78. Additionally, a return-on-energy-investment (ROI) parameter was calculated as ΔE divided by µEa26 and was used here as an index of SOC energy quality27,28.
Soil microbial biomass was measured on fresh sieved soil by the chloroform-fumigation-extraction method79. We performed an extraction with 50 mL 0.5 M K2SO4 on 10 g of fresh soil. A second set of samples was placed in a vacuum desiccator and fumigated with chloroform for 24 h prior to K2SO4 extraction as above. Extractable C was analysed using an automated analyser (TOC-L analyser, Shimadzu, Milton Keynes, UK). Microbial biomass C was calculated as the difference between the fumigated and unfumigated C extracts and a correction for extraction efficiency was applied by dividing with a coefficient of 0.4580. Initial soil mineral N content was measured from 25 g of fresh soil after extraction in 2 M KCl using a continuous-flow analyser (AA3, Bran + Luebbe, Norderstedt, Germany). All roots retained by sieving of soil cores and visible roots in sieved fresh soil were handpicked and washed with tap water. After drying at 60 °C for 48 h, roots were weighted and root density was calculated as root dry mass divided by the soil core volume.
We also characterized the mineral composition of soil samples. Soil pHwater was measured in a 1:5 soil:solution ratio after 1-h end-over-end shaking. Soil clay content was measured using the pipette method81. Phyllosilicate mineralogy was determined by X-ray diffraction82. Random oriented powders with a Philips PW 3710 X-ray diffractometer with Cu-Kα radiation at 40 kV and 40 mA were used to obtained spectra (Supplementary Fig. 7). A counting time of 13 s per 0.02° step was used for 2θ in the range 3.5–80°. Cation exchange capacity (CEC) as well as major exchangeable cations (Ca, Mg, Na, K, Al, Mn, Fe) were determined determined using the cobalt hexamine exchange method83. The concentrations of divalent cations involved in organo-mineral cation bridging was calculated as the sum of exchangeable calcium and magnesium (Caex+Mgex). Pedogenic reactive metals were quantify by selective dissolution procedures of Fe, Al and Si using standard methods of citrate–dithionite (d), acid ammonium oxalate (o), and sodium pyrophosphate (p) extractions84. Crystalline Fe minerals was then calculated as the difference between Fed and Feo (Fed–o). We used Feo to quantify short-range-ordered Fe-oxyhydroxides such as ferrihydrite, while Alo+Sio were used to quantify organo-metal complexes and short-range-ordered aluminosilicates such as allophane. Alp allows to quantify organo-metal complexes, but pyrophosphate is not completely selective and can also extract Al from silicates, which could be indicated by Sip84. Organo-metal complexes was thus calculated as Alp-xSip, where x is the Al:Si ratio of the dominant clay mineral (x = 0.5 for subsoil of the vertisol dominated by montmorillonite, and x = 1 for all other layers dominated by vermiculite, kaolinite or halloysite).
Plant isotopic labelling and soil incubation experiment
Soil cores collected for the experiment were immediately proceeded in the field following sampling to establish microcosms with a new soil column made of intact soil cores derived exclusively from either topsoil or subsoil (Supplementary Fig. 2). For each microcosm, three soil cores of the same layer were gently stacked vertically and tightly sealed together within a polyethylene sheath before transfer into a 60 cm high PVC pot (diameter 10 cm, height 60 cm, with a permeable bottom-cap to allow drainage). For each the six treatments including the three soil types and the two layers, we included four planted replicates and four unplanted controls for a total of 48 microcosms. The experiment started within 2 months after sampling and microcosm were stored at 4 °C until then.
The experiment was performed for 279 days, from late August 2016 until early July 2017. Two weeks before starting the incubation experiment, the microcosms were transferred at ambient temperature, irrigated until water saturation and weighed after 48 h of water percolation to measure the soil water-holding capacity (WHC). Planted microcosms were sown (1400 seeds m−2) with Dactylis glomerata, a fast-growing grass species with a dense and deep root system commonly found in temperate grassland85. After germination, microcosms were transferred to a greenhouse exposed to natural light and temperature conditions (Clermont‐Ferrand, temperate semi‐continental climate). The greenhouse was coupled to a continuous dual-labelling (13C/14C) system86,87. Labelled air depleted in both 13C and 14C was produced by injecting fossil fuel-derived CO2 (δ13C = −35.23 ± 0.02 ‰, Δ14C~0‰) in CO2-free air ([CO2] < 20 ppm) up to reach ambient CO2 concentration (400 ppm), and the greenhouse was continuously supplied with labelled air during daytime, with a flow renewing the greenhouse volume once every 2 min to maintain constant CO2 concentration, δ13C and Δ14C86. Soil water content was monitored daily using soil moisture sensors (ECH2O EC-5, Decagon®, USA) inserted at 5 cm and drip irrigation was adjusted individually for each treatment as to maintain moisture around 85 ± 5% of WHC. In order to compensate for the low nutrient availability and plant growth potential expected in subsoil relative to topsoil, we fertilised the planted subsoil microcosms for each soil type on day 51 after planting. Unplanted subsoil microcosms were kept unfertilised to avoid excessive concentrations of mineral nutrients, which already tend to accumulate in soils in absence of rhizodeposition and nutrient uptake by plant roots41,88. The fertilisation solution was composed of inorganic N (NH4NO3), P, S, K and Mg, with a dose of 11.5, 0.6, 1.0, 1.5 and 0.7 g m−2, respectively. The fertilization was adjusted to compensate for initial differences in measured soil mineral N concentrations compared with planted topsoil microcosms, with a single dose added for each soil type and a second dose including only N applied to the andosol (Supplementary Table 9). This allowed to reach similar soil mineral N concentrations, plant N concentration and biomass between planted topsoil and subsoil microcosms following fertilization (Supplementary Tables 9, 10).
For the first incubation series, we measured CO2 fluxes of each microcosm 76, 139, 174, 201, 242 and 272 days after planting, from late fall until early summer (n = 288 incubations). Microcosms were sealed in opaque airtight chambers and incubated for 24 h at 21.5 °C. Chamber gas was sampled at the end of incubation, and its CO2 concentration and δ13C were measured using a gas chromatograph (Clarus 480, Perkin Elmer, Waltham, MA, USA) and an isotopic analyser (G2201-i, Picarro, Santa Clara, CA, USA). The amount and δ13C of CO2 derived from plant-soil respiration were corrected for background atmospheric CO2.
For the second incubation series performed 279 days after planting, the soil column was gently extracted from each microcosm. Shoots were cut at the soil surface for planted microcosms, and the soil column was vertically sliced to recover the three original soil cores. Each soil core was transferred into a 3 L flask as gently as possible to maintain the soil core structure intact (n = 144 incubations). After a preincubation period of 24 h at 21.5 °C, flasks were airtight‐sealed and incubated for 24 h at 21.5 °C. The evolved CO2 was trapped in NaOH and its concentration was measured using an automated analyser (TOC-L analyser, Shimadzu, Milton Keynes, UK). After carbonate precipitation with BaCl2 and filtration, the δ13C of evolved CO2 was analysed by an EA-IRMS. We also measured the Δ14C of evolved CO2 by AMS analyses as described above for planted subsoil cores of 0–20 and 40–60 cm depth, and unplanted subsoil cores of 40–60 cm depth. Given the high cost of AMS analyses, Δ14C–CO2 measurements were restricted to the cambisol and andosol that a priori featured the most contrasting mineral reactivity (n = 24 incubations).
After the incubation, each planted soil core was sieved at 2 mm. Roots retained by sieve and all visible roots in sieved soil were handpicked and washed with tap water. Shoot, root and soil materials were dried at 60 °C, weighed, grounded and analysed separately for C and N concentrations and δ13C using an EA-IRMS as described above. For the cambisol and andosol, Δ14C of root biomass in subsoil cores was measured as described above.
Isotopic partitioning and calculations
For both incubation series, the continuous isotopic labelling of plants with 13C-depleted air allowed us to partition total respiration into its soil and plant/root sources (~25‰ average difference in δ13C). It was calculated using the following equations:
| 2 |
| 3 |
where Rtotal and δ13Ctotal are respectively the total CO2 flux and its δ13C from plant/root-soil respiration at the microcosm/core scale; Rsoil and δ13Csoil are respectively the CO2 flux and its δ13C from microbial respiration of unlabelled native (pre-existing) SOC and root litter; and Rplant and δ13Cplant are respectively the CO2 flux and its δ13C from respiration of labelled (recently fixed) plant/root-derived organic carbon (OC). For the first incubation series, we used as δ13Cplant the mass-weighted δ13C of the mesocosm shoot and living root biomass, assuming negligible 13C fractionation during whole-plant respiration89. A parallel experiment running during the same period and using a common labelling system found that the δ13C of plant biomass was constant through time86, ensuring that the labelling remained homogeneous throughout the experiment. For the second incubation series, we used as δ13Cplant the δ13C of living root biomass corrected by a δ13C fractionation factor of root respiration, which was assumed to be −0.61‰ for grass species based a previous study87. For both series of incubations, we used as δ13Csoil the δ13C of CO2 derived from the respiration of native SOC in unplanted controls.
In the second incubation series, the continuous dual-labelling (13C/14C) of plants allowed us to quantify the radiocarbon signature of native SOC respired by decomposers (Δ14Csoil) by partitioning the Δ14C signature of total respiration into its soil and root sources17,20. It was calculated using the following equation:
| 4 |
where Rtotal and Δ14Ctotal are respectively the total CO2 flux and its Δ14C of root-soil respiration at the core scale, Δ14Cplant is the Δ14C of root-derived OC respiration, and Rplant and Rsoil are the CO2 fluxes of respectively root-derived and soil-derived OC respiration as calculated in Eqs. 2, 3. Assuming no fractionation of 14C during root respiration, we used the Δ14C of living root biomass as Δ14Cplant.
As the root material harvested for topsoil was composed of both pre-existing root litter (unlabelled) and living roots (labelled) that could not be clearly visually sorted, we used an isotopic partitioning method to estimate living root biomass (Rootliving) for each planted topsoil core using the following equation:
| 5 |
where Roottotal and δ13Ctotal are respectively the biomass and δ13C of both dead and living roots; and δ13Cliving and δ13Cdead are the δ13C of respectively living and dead roots.
We also quantified the net rhizodeposition corresponding to root-derived OC remaining in the soil after microbial utilisation. It was estimated for each planted soil core using the following equation:
| 6 |
where SOCtotal and δ13CSOC-final are respectively the concentration and δ13C of SOC in planted soil core at the end of the experiment, δ13CSOC-initial is the average δ13C of SOC from initial soil cores, and δ13Croot is the δ13C of living root biomass in planted microcosm.
In order to evaluate the uncertainty associated with our isotopic mixing model assumptions, we performed sensitivity analyses where we quantified the error in source proportion related to a 1‰ variation in the δ13C of both sources for each isotopic partitioning (Supplementary Methods). Low levels of uncertainty were found for every isotopic partitionings, providing evidence that our results were robust (Supplementary Tables 10–14, see Supplementary Methods for further details).
Assuming first-order decomposition kinetics, native SOC decomposition rate (kSOC) was calculated as native SOC respiration (Rsoil) divided by initial SOC concentration. The corresponding rhizosphere priming effect (RPE, in % of change in kSOC for planted relative to unplanted soils) was calculated using the following equation:
| 7 |
The respiration of root-derived OC (Rplant) in the second incubation series includes both the autotrophic respiration of roots and the heterotrophic respiration of soil microbial decomposers derived from rhizodeposits. We thus used it here as a proxy of plant C allocation belowground to root activity, including root maintenance, growth and uptake of water and nutrients, as well as rhizodeposition of fresh OC to soil which is then quickly taken up and metabolised by rhizosphere microbes33. This allowed us to assess how root effects on SOC decomposition could depend on plant inputs to soil of energy that could take different forms, including food substrates for microbes (metabolic energy), ligand exudates desorbing SOC from minerals (chemical energy) and root uptake of water and nutrients breaking up soil aggregates by physical disturbances (physical energy). After converting Rplant from an amount of C in CO2 (mg) into an amount of glucose (mol), we calculated the plant energy investment into root activity (J dm−3 day−1) as Rplant multiplied by the heat of combustion of glucose, that is 2807 kJ per mol78. This estimation relies on the assumption that glucose composed most of the fresh photosynthate-C metabolised by both roots and soil microbes32.
Statistical analyses
All analyses were performed using R v3.4.3. We used a rotated principal component analysis (rPCA) to explore soil properties covariance and divergence between treatments. Analyses of variance were used to partition the variance explained by factors ‘soil layer’, ‘soil type’ and their interaction for the two first axis scores and soil properties.
For each incubation series, the responses of kSOC, RPE and Δ14Csoil to predictors were assessed by regression for each treatment. We tested linear (Y = a + bX), polynomial (Y = a + bX + cX2) and power (Y = aXb) regression functions, where Y is the response variable and X is the predictor. The kSOC and RPE values were standardised for each treatment to a common high value of the following predictors: ‘respiration of plant-derived OC’ for the first incubation series, ‘living root density’ and ‘plant-derived OC respiration’ for the second incubation series. Additionally, we used analyses of covariance including the quantitative explanatory variables, the factors ‘soil layers’ and ‘soil type’, and their interactions as fixed factors to test their effects and quantify the proportion of variance they explain. To deal with the repeated measures design in both incubation series, we used linear mixed-effect models including ‘microcosm’ as random factor in regression and analyses of covariance.
We explored bivariate relationships between variation in SOC dynamics and SOM properties across depth using partial regression and correlation analyses controlling for soil types. Additionally, we performed an ordination of SOC dynamics variables constrained by soil biogeochemical predictors using a redundancy analysis.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This work is dedicated to Jerôme Balesdent, Research Director at INRAE, who died prematurely at the age of 63. This study was financially supported by the ANR project DEDYCAS (ANR 14-CE01-0004) led by J. Balesdent, and the Programme EJP Soil of the European Commission (project AgroSeqC). We thank S. Revaillot, F. Savignac, L. Andanson, and A. Salcedo for their technical support. We also thank V. Genevois, K. Klumpp, F. Louault, and J. Reymond for their help in soil sampling and access to the sites, including the AnaEE-France (ANR-11- INBS-0001) in natura SOERE ACBB permanent grassland experimental sites of Theix and Laqueuille. We thank the certified facility in Functional Ecology (PTEF OC 081) from UMR 1137 EEF and UR 1138 BEF in research centre INRAE Nancy-Lorraine for its contribution to isotopic analysis of plant and soil samples. The PTEF facility is supported by the French National Research Agency through the Laboratory of Excellence ARBRE (ANR-11-LABX-0002-01). The TGA-DSC thermal analyser (ISTerre) was partially funded by a grant from Labex OSUG@2020 (Investissements d’Avenir, ANR10-LABX56).
Source data
Author contributions
L.H. and S.F. conceived the ideas and designed methodology. L.H., G.A. and S.F. performed the soil sampling. L.H. and S.F. performed most of the soil biogeochemical analyses, except for radiocarbon analyses performed by C.H., Rock-Eval thermal analyses performed by P.B., F.B. and L.C., DSC thermal analyses performed by A.F.M., and X-ray diffraction and pedogenic reactive metals analyses performed by I.B.D. and J.B. L.H. and S.F. performed the sampling and all analyses of CO2 during the incubations, except for radiocarbon analyses performed by C.H. L.H. analysed the data and led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Peer review
Peer review information
Nature Communications thanks Ellen Fry and Alain Plante for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
All data generated or analysed during this study are provided in the Supplementary Information (Supplementary Data). Additional data that support the findings of this study are available from the corresponding author (L.H.) upon request. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Deceased: Jerôme Balesdent.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-34951-w.
References
- 1.Hiederer, R. & Köchy, M. Global soil organic carbon estimates and the harmonized world soil database. Report No. EUR 25225 EN (Publications Office of the European Union, 2011).
- 2.Heimann M, Reichstein M. Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature. 2008;451:289–292. doi: 10.1038/nature06591. [DOI] [PubMed] [Google Scholar]
- 3.Bradford MA, et al. Managing uncertainty in soil carbon feedbacks to climate change. Nat. Clim. Change. 2016;6:751–758. doi: 10.1038/nclimate3071. [DOI] [Google Scholar]
- 4.Jobbágy EG, Jackson RB. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000;10:423–436. doi: 10.1890/1051-0761(2000)010[0423:TVDOSO]2.0.CO;2. [DOI] [Google Scholar]
- 5.Rumpel C, Kögel-Knabner I. Deep soil organic matter—a key but poorly understood component of terrestrial C cycle. Plant Soil. 2011;338:143–158. doi: 10.1007/s11104-010-0391-5. [DOI] [Google Scholar]
- 6.Roth, V.-N. et al. Persistence of dissolved organic matter explained by molecular changes during its passage through soil. Nat. Geosci. 12, 755–761 (2019).
- 7.Schrumpf M, et al. Storage and stability of organic carbon in soils as related to depth, occlusion within aggregates, and attachment to minerals. Biogeosciences. 2013;10:1675–1691. doi: 10.5194/bg-10-1675-2013. [DOI] [Google Scholar]
- 8.Balesdent J, et al. Atmosphere–soil carbon transfer as a function of soil depth. Nature. 2018;559:599–602. doi: 10.1038/s41586-018-0328-3. [DOI] [PubMed] [Google Scholar]
- 9.Shi, Z. et al. The age distribution of global soil carbon inferred from radiocarbon measurements. Nat. Geosci. 13, 555–559 (2020).
- 10.Schmidt MWI, et al. Persistence of soil organic matter as an ecosystem property. Nature. 2011;478:49–56. doi: 10.1038/nature10386. [DOI] [PubMed] [Google Scholar]
- 11.Dungait JAJ, Hopkins DW, Gregory AS, Whitmore AP. Soil organic matter turnover is governed by accessibility not recalcitrance. Glob. Change Biol. 2012;18:1781–1796. doi: 10.1111/j.1365-2486.2012.02665.x. [DOI] [Google Scholar]
- 12.Chen L, et al. Soil carbon persistence governed by plant input and mineral protection at regional and global scales. Ecol. Lett. 2021;24:1018–1028. doi: 10.1111/ele.13723. [DOI] [PubMed] [Google Scholar]
- 13.Jackson RB, et al. The Ecology of Soil Carbon: Pools, Vulnerabilities, and Biotic and Abiotic Controls. Annu. Rev. Ecol. Evol. Syst. 2017;48:419–445. doi: 10.1146/annurev-ecolsys-112414-054234. [DOI] [Google Scholar]
- 14.Mathieu JA, Hatté C, Balesdent J, Parent É. Deep soil carbon dynamics are driven more by soil type than by climate: a worldwide meta-analysis of radiocarbon profiles. Glob. Change Biol. 2015;21:4278–4292. doi: 10.1111/gcb.13012. [DOI] [PubMed] [Google Scholar]
- 15.Don A, Rödenbeck C, Gleixner G. Unexpected control of soil carbon turnover by soil carbon concentration. Environ. Chem. Lett. 2013;11:407–413. doi: 10.1007/s10311-013-0433-3. [DOI] [Google Scholar]
- 16.Salomé C, Nunan N, Pouteau V, Lerch TZ, Chenu C. Carbon dynamics in topsoil and in subsoil may be controlled by different regulatory mechanisms. Glob. Change Biol. 2010;16:416–426. doi: 10.1111/j.1365-2486.2009.01884.x. [DOI] [Google Scholar]
- 17.Shahzad T, et al. Root penetration in deep soil layers stimulates mineralization of millennia-old organic carbon. Soil Biol. Biochem. 2018;124:150–160. doi: 10.1016/j.soilbio.2018.06.010. [DOI] [Google Scholar]
- 18.Hoehler TM, Jørgensen BB. Microbial life under extreme energy limitation. Nat. Rev. Microbiol. 2013;11:83–94. doi: 10.1038/nrmicro2939. [DOI] [PubMed] [Google Scholar]
- 19.Ahrens B, et al. Combination of energy limitation and sorption capacity explains 14C depth gradients. Soil Biol. Biochem. 2020;148:107912. doi: 10.1016/j.soilbio.2020.107912. [DOI] [Google Scholar]
- 20.Fontaine S, et al. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature. 2007;450:277–U10. doi: 10.1038/nature06275. [DOI] [PubMed] [Google Scholar]
- 21.Fontaine S, Barot S. Size and functional diversity of microbe populations control plant persistence and long-term soil carbon accumulation. Ecol. Lett. 2005;8:1075–1087. doi: 10.1111/j.1461-0248.2005.00813.x. [DOI] [Google Scholar]
- 22.Schimel JP, Weintraub MN. The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol. Biochem. 2003;35:549–563. doi: 10.1016/S0038-0717(03)00015-4. [DOI] [Google Scholar]
- 23.Ekschmitt K, Liu M, Vetter S, Fox O, Wolters V. Strategies used by soil biota to overcome soil organic matter stability — why is dead organic matter left over in the soil? Geoderma. 2005;128:167–176. doi: 10.1016/j.geoderma.2004.12.024. [DOI] [Google Scholar]
- 24.Malik AA, Puissant J, Goodall T, Allison SD, Griffiths RI. Soil microbial communities with greater investment in resource acquisition have lower growth yield. Soil Biol. Biochem. 2019;132:36–39. doi: 10.1016/j.soilbio.2019.01.025. [DOI] [Google Scholar]
- 25.Barré P, et al. The energetic and chemical signatures of persistent soil organic matter. Biogeochemistry. 2016;130:1–12. doi: 10.1007/s10533-016-0246-0. [DOI] [Google Scholar]
- 26.Harvey OR, et al. Discrimination in Degradability of Soil Pyrogenic Organic Matter Follows a Return-On-Energy-Investment Principle. Environ. Sci. Technol. 2016;50:8578–8585. doi: 10.1021/acs.est.6b01010. [DOI] [PubMed] [Google Scholar]
- 27.Rovira P, Kurz-Besson C, Coûteaux M-M, Ramón Vallejo V. Changes in litter properties during decomposition: A study by differential thermogravimetry and scanning calorimetry. Soil Biol. Biochem. 2008;40:172–185. doi: 10.1016/j.soilbio.2007.07.021. [DOI] [Google Scholar]
- 28.Williams, E. K. & Plante, A. F. A Bioenergetic Framework for Assessing Soil Organic Matter Persistence. Front. Earth Sci. 6, 143 (2018).
- 29.Stone MM, Plante AF. Relating the biological stability of soil organic matter to energy availability in deep tropical soil profiles. Soil Biol. Biochem. 2015;89:162–171. doi: 10.1016/j.soilbio.2015.07.008. [DOI] [Google Scholar]
- 30.Soucémarianadin LN, et al. Environmental factors controlling soil organic carbon stability in French forest soils. Plant Soil. 2018;426:267–286. doi: 10.1007/s11104-018-3613-x. [DOI] [Google Scholar]
- 31.Dijkstra FA, Zhu B, Cheng W. Root effects on soil organic carbon: a double-edged sword. N. Phytol. 2020;230:60–65. doi: 10.1111/nph.17082. [DOI] [PubMed] [Google Scholar]
- 32.Jones DL, Hodge A, Kuzyakov Y. Plant and Mycorrhizal Regulation of Rhizodeposition. N. Phytol. 2004;163:459–480. doi: 10.1111/j.1469-8137.2004.01130.x. [DOI] [PubMed] [Google Scholar]
- 33.Pausch J, Kuzyakov Y. Carbon input by roots into the soil: Quantification of rhizodeposition from root to ecosystem scale. Glob. Change Biol. 2018;24:1–12. doi: 10.1111/gcb.13850. [DOI] [PubMed] [Google Scholar]
- 34.Clarholm M, Skyllberg U, Rosling A. Organic acid induced release of nutrients from metal-stabilized soil organic matter—The unbutton model. Soil Biol. Biochem. 2015;84:168–176. doi: 10.1016/j.soilbio.2015.02.019. [DOI] [Google Scholar]
- 35.Finley BK, et al. Soil mineral assemblage and substrate quality effects on microbial priming. Geoderma. 2018;322:38–47. doi: 10.1016/j.geoderma.2018.01.039. [DOI] [Google Scholar]
- 36.Keiluweit M, et al. Mineral protection of soil carbon counteracted by root exudates. Nat. Clim. Change. 2015;5:588–595. doi: 10.1038/nclimate2580. [DOI] [Google Scholar]
- 37.Menichetti L, et al. Increase in soil stable carbon isotope ratio relates to loss of organic carbon: results from five long-term bare fallow experiments. Oecologia. 2015;177:811–821. doi: 10.1007/s00442-014-3114-4. [DOI] [PubMed] [Google Scholar]
- 38.Chakrawal, A., Calabrese, S., Herrmann, A. M. & Manzoni, S. Interacting Bioenergetic and Stoichiometric Controls on Microbial Growth. Front. Microbiol. 13, 859063 (2022). [DOI] [PMC free article] [PubMed]
- 39.Hemingway JD, et al. Mineral protection regulates long-term global preservation of natural organic carbon. Nature. 2019;570:228–231. doi: 10.1038/s41586-019-1280-6. [DOI] [PubMed] [Google Scholar]
- 40.Chassé M, et al. Long-term bare-fallow soil fractions reveal thermo-chemical properties controlling soil organic carbon dynamics. Biogeosciences. 2021;18:1703–1718. doi: 10.5194/bg-18-1703-2021. [DOI] [Google Scholar]
- 41.Zhang, Q. et al. A distinct sensitivity to the priming effect between labile and stable soil organic carbon. New Phytol. 10.1111/nph.18458 (2022). [DOI] [PubMed]
- 42.Williams EK, Fogel ML, Berhe AA, Plante AF. Distinct bioenergetic signatures in particulate versus mineral-associated soil organic matter. Geoderma. 2018;330:107–116. doi: 10.1016/j.geoderma.2018.05.024. [DOI] [Google Scholar]
- 43.LaRowe DE, Van Cappellen P. Degradation of natural organic matter: A thermodynamic analysis. Geochim. Cosmochim. Acta. 2011;75:2030–2042. doi: 10.1016/j.gca.2011.01.020. [DOI] [Google Scholar]
- 44.Lehmann J, Kleber M. The contentious nature of soil organic matter. Nature. 2015;528:60–68. doi: 10.1038/nature16069. [DOI] [PubMed] [Google Scholar]
- 45.Manzoni S, Taylor P, Richter A, Porporato A, Ågren GI. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. N. Phytol. 2012;196:79–91. doi: 10.1111/j.1469-8137.2012.04225.x. [DOI] [PubMed] [Google Scholar]
- 46.Gunina A, Smith AR, Kuzyakov Y, Jones DL. Microbial uptake and utilization of low molecular weight organic substrates in soil depend on carbon oxidation state. Biogeochemistry. 2017;133:89–100. doi: 10.1007/s10533-017-0313-1. [DOI] [Google Scholar]
- 47.Nunan N, et al. Metabolising old soil carbon: Simply a matter of simple organic matter? Soil Biol. Biochem. 2015;88:128–136. doi: 10.1016/j.soilbio.2015.05.018. [DOI] [Google Scholar]
- 48.Chen L, et al. Regulation of priming effect by soil organic matter stability over a broad geographic scale. Nat. Commun. 2019;10:5112. doi: 10.1038/s41467-019-13119-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rasmussen C, et al. Beyond clay: towards an improved set of variables for predicting soil organic matter content. Biogeochemistry. 2018;137:297–306. doi: 10.1007/s10533-018-0424-3. [DOI] [Google Scholar]
- 50.Li H, et al. Simple Plant and Microbial Exudates Destabilize Mineral-Associated Organic Matter via Multiple Pathways. Environ. Sci. Technol. 2021;55:3389–3398. doi: 10.1021/acs.est.0c04592. [DOI] [PubMed] [Google Scholar]
- 51.Jilling A, Keiluweit M, Gutknecht JLM, Grandy AS. Priming mechanisms providing plants and microbes access to mineral-associated organic matter. Soil Biol. Biochem. 2021;158:108265. doi: 10.1016/j.soilbio.2021.108265. [DOI] [Google Scholar]
- 52.Keiluweit M, Wanzek T, Kleber M, Nico P, Fendorf S. Anaerobic microsites have an unaccounted role in soil carbon stabilization. Nat. Commun. 2017;8:1771. doi: 10.1038/s41467-017-01406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boye K, et al. Thermodynamically controlled preservation of organic carbon in floodplains. Nat. Geosci. 2017;10:415–419. doi: 10.1038/ngeo2940. [DOI] [Google Scholar]
- 54.Falkowski PG, Fenchel T, Delong EF. The Microbial Engines That Drive Earth’s Biogeochemical Cycles. Science. 2008;320:1034–1039. doi: 10.1126/science.1153213. [DOI] [PubMed] [Google Scholar]
- 55.Currie WS. Relationships between carbon turnover and bioavailable energy fluxes in two temperate forest soils. Glob. Change Biol. 2003;9:919–929. doi: 10.1046/j.1365-2486.2003.00637.x. [DOI] [Google Scholar]
- 56.Gunina A, Kuzyakov Y. From energy to (soil organic) matter. Glob. Change Biol. 2022;28:2169–2182. doi: 10.1111/gcb.16071. [DOI] [PubMed] [Google Scholar]
- 57.Lehmann J, et al. Persistence of soil organic carbon caused by functional complexity. Nat. Geosci. 2020;13:529–534. doi: 10.1038/s41561-020-0612-3. [DOI] [Google Scholar]
- 58.Jones ME, et al. Enzymes, Manganese, or Iron? Drivers of Oxidative Organic Matter Decomposition in Soils. Environ. Sci. Technol. 2020;54:14114–14123. doi: 10.1021/acs.est.0c04212. [DOI] [PubMed] [Google Scholar]
- 59.Yu G-H, Kuzyakov Y. Fenton chemistry and reactive oxygen species in soil: Abiotic mechanisms of biotic processes, controls and consequences for carbon and nutrient cycling. Earth Sci. Rev. 2021;214:103525. doi: 10.1016/j.earscirev.2021.103525. [DOI] [Google Scholar]
- 60.Kothawala DN, Kellerman AM, Catalán N, Tranvik LJ. Organic Matter Degradation across Ecosystem Boundaries: The Need for a Unified Conceptualization. Trends Ecol. Evol. 2021;36:113–122. doi: 10.1016/j.tree.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 61.Nie M, Lu M, Bell J, Raut S, Pendall E. Altered root traits due to elevated CO2: a meta-analysis. Glob. Ecol. Biogeogr. 2013;22:1095–1105. doi: 10.1111/geb.12062. [DOI] [Google Scholar]
- 62.Iversen CM. Digging deeper: fine-root responses to rising atmospheric CO2 concentration in forested ecosystems. N. Phytol. 2010;186:346–357. doi: 10.1111/j.1469-8137.2009.03122.x. [DOI] [PubMed] [Google Scholar]
- 63.Liu H, et al. Shifting plant species composition in response to climate change stabilizes grassland primary production. Proc. Natl Acad. Sci. 2018;115:4051. doi: 10.1073/pnas.1700299114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang J, et al. Fine-root functional trait responses to experimental warming: a global meta-analysis. N. Phytol. 2021;230:1856–1867. doi: 10.1111/nph.17279. [DOI] [PubMed] [Google Scholar]
- 65.Kell, D. B. Breeding crop plants with deep roots: their role in sustainable carbon, nutrient and water sequestration. Ann. Bot. 108, 407–418 (2011). [DOI] [PMC free article] [PubMed]
- 66.Sulman BN, Phillips RP, Oishi AC, Shevliakova E, Pacala SW. Microbe-driven turnover offsets mineral-mediated storage of soil carbon under elevated CO2. Nat. Clim. Change. 2014;4:1099–1102. doi: 10.1038/nclimate2436. [DOI] [Google Scholar]
- 67.van Groenigen KJ, Qi X, Osenberg CW, Luo Y, Hungate BA. Faster Decomposition Under Increased Atmospheric CO2 Limits Soil Carbon Storage. Science. 2014;344:508–509. doi: 10.1126/science.1249534. [DOI] [PubMed] [Google Scholar]
- 68.Terrer C, et al. A trade-off between plant and soil carbon storage under elevated CO2. Nature. 2021;591:599–603. doi: 10.1038/s41586-021-03306-8. [DOI] [PubMed] [Google Scholar]
- 69.Thorup-Kristensen K, et al. Digging Deeper for Agricultural Resources, the Value of Deep Rooting. Trends Plant Sci. 2020;25:406–417. doi: 10.1016/j.tplants.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 70.FAO. World reference base for soil resources: international soil classification system for naming soils and creating legends for soil maps. (2014).
- 71.Poeplau C, et al. Isolating organic carbon fractions with varying turnover rates in temperate agricultural soils—A comprehensive method comparison. Soil Biol. Biochem. 2018;125:10–26. doi: 10.1016/j.soilbio.2018.06.025. [DOI] [Google Scholar]
- 72.Trumbore, S. E., Sierra, C. A. & Hicks Pries, C. E. Radiocarbon Nomenclature, Theory, Models, and Interpretation: Measuring Age, Determining Cycling Rates, and Tracing Source Pools. in Radiocarbon and Climate Change: Mechanisms, Applications and Laboratory Techniques (eds. Schuur, E. A. G., Druffel, E. & Trumbore, S. E.) 45–82 (Springer International Publishing, 2016).
- 73.Torn, M. S., Swanston, C. W., Castanha, C. & Trumbore, S. Storage and Turnover of Organic Matter in Soil. in Biophysico-Chemical Processes Involving Natural Nonliving Organic Matter in Environmental Systems (eds. Senesi, N., Xing, B. & Huang, P. M.) vol. 2 219–272 (Wiley & Sons Inc, 2009).
- 74.Hemingway JD, Rothman DH, Rosengard SZ, Galy VV. Technical note: An inverse method to relate organic carbon reactivity to isotope composition from serial oxidation. Biogeosciences. 2017;14:5099–5114. doi: 10.5194/bg-14-5099-2017. [DOI] [Google Scholar]
- 75.Cécillon L, et al. Partitioning soil organic carbon into its centennially stable and active fractions with statistical models based on Rock-Eval® thermal analysis (PARTYSOCv2.0 and PARTYSOCv2.0EU) Geosci. Model Dev. Discuss. 2021;2021:1–35. [Google Scholar]
- 76.Barros, N. Thermodynamics of Soil Microbial Metabolism: Applications and Functions. Appl. Sci. 11, 4962 (2021).
- 77.Masiello, C. A., Gallagher, M. E., Randerson, J. T., Deco, R. M. & Chadwick, O. A. Evaluating two experimental approaches for measuring ecosystem carbon oxidation state and oxidative ratio. J. Geophys. Res. Biogeosciences113, G03010 (2008).
- 78.Sandler SI, Orbey H. On the thermodynamics of microbial growth processes. Biotechnol. Bioeng. 1991;38:697–718. doi: 10.1002/bit.260380704. [DOI] [PubMed] [Google Scholar]
- 79.Vance ED, Brookes PC, Jenkinson DS. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987;19:703–707. doi: 10.1016/0038-0717(87)90052-6. [DOI] [Google Scholar]
- 80.Joergensen RG. The fumigation-extraction method to estimate soil microbial biomass: Calibration of the kEC value. Soil Biol. Biochem. 1996;28:25–31. doi: 10.1016/0038-0717(95)00102-6. [DOI] [Google Scholar]
- 81.Pansu, M. & Gautheyrou, J. Handbook of soil analysis: mineralogical, organic and inorganic methods. (Springer Science & Business Media, 2007).
- 82.Zhou X, et al. XRD-based quantitative analysis of clay minerals using reference intensity ratios, mineral intensity factors, Rietveld, and full pattern summation methods: A critical review. Solid Earth Sci. 2018;3:16–29. doi: 10.1016/j.sesci.2017.12.002. [DOI] [Google Scholar]
- 83.Ciesielski H, Sterckeman T. Determination of cation exchange capacity and exchangeable cations in soils by means of cobalt hexamine trichloride. Eff. Exp. Cond. Agronomie. 1997;17:9–15. doi: 10.1051/agro:19970102. [DOI] [Google Scholar]
- 84.Rennert T. Wet-chemical extractions to characterise pedogenic Al and Fe species—a critical review. Soil Res. 2019;57:1–16. doi: 10.1071/SR18299. [DOI] [Google Scholar]
- 85.Zwicke M, Picon-Cochard C, Morvan-Bertrand A, Prud’homme M-P, Volaire F. What functional strategies drive drought survival and recovery of perennial species from upland grassland? Ann. Bot. 2015;116:1001–1015. doi: 10.1093/aob/mcv037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cros C, Alvarez G, Keuper F, Fontaine S. A new experimental platform connecting the rhizosphere priming effect with CO2 fluxes of plant-soil systems. Soil Biol. Biochem. 2019;130:12–22. doi: 10.1016/j.soilbio.2018.11.022. [DOI] [Google Scholar]
- 87.Henneron L, Cros C, Picon-Cochard C, Rahimian V, Fontaine S. Plant economic strategies of grassland species control soil carbon dynamics through rhizodeposition. J. Ecol. 2020;108:528–545. doi: 10.1111/1365-2745.13276. [DOI] [Google Scholar]
- 88.Henneron L, Kardol P, Wardle DA, Cros C, Fontaine S. Rhizosphere control of soil nitrogen cycling: a key component of plant economic strategies. N. Phytol. 2020;228:1269–1282. doi: 10.1111/nph.16760. [DOI] [PubMed] [Google Scholar]
- 89.Schnyder H, Lattanzi FA. Partitioning Respiration of C3-C4 Mixed Communities Using the Natural Abundance 13 C Approach - Testing Assumptions in a Controlled Environment. Plant Biol. 2005;7:592–600. doi: 10.1055/s-2005-872872. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are provided in the Supplementary Information (Supplementary Data). Additional data that support the findings of this study are available from the corresponding author (L.H.) upon request. Source data are provided with this paper.