Abstract
Background
Vitamin D is an essential nutrient in musculoskeletal function; however, its relationship to sarcopenia remains ambiguous, and the mechanisms and targets of vitamin D activity have not been elucidated. This study aimed to clarify the role of vitamin D in mature skeletal muscle and its relationship with sarcopenia.
Methods
This epidemiological study included 1653 community residents who participated in both the fifth and seventh waves of the National Institute for Longevity Sciences, Longitudinal Study of Aging and had complete background data. Participants were classified into two groups: vitamin D‐deficient (serum 25‐hydroxyvitamin D < 20 ng/mL) and non‐deficient (serum 25‐hydroxyvitamin D ≥ 20 ng/mL); they underwent propensity‐score matching for background factors (age, sex, height, weight, comorbidities, smoker, alcohol intake, energy intake, vitamin D intake, steps, activity, season and sarcopenia). Changes in muscle strength and mass over the 4‐year period were compared. For basic analysis, we generated Myf6 CreERT2 Vitamin D Receptor (VDR)‐floxed (Vdr mcKO) mice with mature muscle fibre‐specific vitamin D receptor knockout, injected tamoxifen into 8‐week‐old mice and analysed various phenotypes at 16 weeks of age.
Results
Grip strength reduction was significantly greater in the deficient group (−1.55 ± 2.47 kg) than in the non‐deficient group (−1.13 ± 2.47 kg; P = 0.019). Appendicular skeletal muscle mass reduction did not differ significantly between deficient (−0.05 ± 0.79 kg) and non‐deficient (−0.01 ± 0.74 kg) groups (P = 0.423). The incidence of new cases of sarcopenia was significantly higher in the deficient group (15 vs. 5 cases; P = 0.039). Skeletal muscle phenotyping of Vdr mcKO mice showed no significant differences in muscle weight, myofibre percentage or myofibre cross‐sectional area; however, both forelimb and four‐limb muscle strength were significantly lower in Vdr mcKO mice (males: forelimb, P = 0.048; four‐limb, P = 0.029; females: forelimb, P < 0.001; four‐limb, P < 0.001). Expression profiling revealed a significant decrease in expression of sarcoendoplasmic reticulum Ca2+‐ATPase (SERCA) 1 (P = 0.019) and SERCA2a (P = 0.049) genes in the Vdr mcKO mice. In contrast, expression of non‐muscle SERCA2b and myoregulin genes showed no changes.
Conclusions
Vitamin D deficiency affects muscle strength and may contribute to the onset of sarcopenia. Vitamin D‐VDR signalling has minimal influence on the regulation of muscle mass in mature myofibres but has a significant influence on muscle strength.
Keywords: Vitamin D deficiency, Sarcopenia, Muscle strength, Muscle mass
Introduction
Vitamin D deficiency is believed to be widespread worldwide. 1 Vitamin D has a critical contribution to the metabolism of calcium and the maintenance of musculoskeletal function. The relationship between blood vitamin D concentration and geriatric diseases, such as osteoporosis and sarcopenia, as well as functional disorders, such as falls, muscle strength loss and slow walking speed, has been reported recently. 2 , 3 , 4 , 5 Vitamin D supplementation in vitamin D‐deficient patients induces significant hip muscle strength 6 and helps prevent falls in older people over 65 years of age, 5 indicating a strong relationship between vitamin D and muscle and motor function. However, whereas lean limb skeletal muscle mass (SMI) and grip strength are used to diagnose sarcopenia, 7 the relationship between blood 25‐hydroxyvitamin D (25(OH)D) concentration and sarcopenia remains ambiguous, and further investigation, including evaluation of the mechanism of action of vitamin D, is needed. 3 , 8 , 9 , 10
One of the pathways for the effects of vitamin D on skeletal muscle is the genomic pathway, which is mediated via binding to vitamin D nuclear receptors (VDRs). Abnormalities in this signalling system, such as vitamin D deficiency or impaired utilization of vitamin D due to reduced VDR expression in muscle tissue, are assumed to have negative effects on muscles. 11 Notably, Vdr knockout mice and conditional knockout mice generated using HSA‐Cre, a myocyte‐specific Cre driver, show muscle atrophy, histological changes and muscle weakness. 12 , 13 However, the role of this signalling pathway in mature myofibres, which is the essence of sarcopenia, has not been accurately assessed in VDR‐deficient mice because the effects of tissue specificity and VDR deficiency on muscle during development cannot be ruled out.
Based on these findings, the present study combined epidemiological studies on serum vitamin D levels and age‐related changes in muscle mass and strength based on data from a long‐term longitudinal epidemiological survey of local residents and basic studies using mature skeletal muscle‐specific VDR‐deficient mice generated using genetic recombination technology. This study aimed to clarify the function and role of vitamin D in mature skeletal muscle in addition to its relationship with sarcopenia.
Subjects and methods
Epidemiological analysis
Participants
The National Institute for Longevity Sciences, Longitudinal Study of Aging (NILS‐LSA) is a dynamic cohort study that observes age‐related changes in middle‐aged and older community residents aged ≥40 years in Obu City (lat. 35°01′N) and Higashiura Town (lat. 34°58′N), Aichi Prefecture, Japan. In this project, the normal ageing process is assessed using detailed questionnaires and tests in the areas of medicine, body composition and measurements, motor function, nutrition and psychology. 14 Among the 1919 participants from both the 5th wave (total of 2419) from July 2006 to July 2008, and the 7th wave (total of 2330) from July 2010 to July 2012, we included 1653 participants with background factors (age, sex, height, weight, total caloric intake, alcohol intake, season of check‐up, total physical activity, vitamin D intake and daily steps measured in the 5th wave; and muscle strength, muscle mass, and walking speed measured in both 5th and 7th waves). Written informed consent was obtained from all participants. This study has passed an institutional ethics review (no. 1521‐2).
Methods
Measurement of physical performance
Grip strength (kg) was measured in the standing position using a digital hand dynamometer (T.K.K.4301a, Takei, Niigata, Japan). Normal walking speed over a 10‐m distance was measured using YW‐3 (Yagami, Aichi, Japan).
Measurement of blood vitamin D levels
Blood samples were collected from fasting or non‐fasting participants, and the serum obtained by centrifugation was stored at −80°C. Blood vitamin D concentration was determined by measuring the serum 25(OH)D concentration (ng/mL) using double antibody radioimmunoassay. Based on vitamin D concentration, participants were classified into two groups: vitamin D deficient (serum 25(OH)D concentration <20 ng/mL) and non‐deficient (serum 25(OH)D concentration ≥20 ng/mL). 15
Muscle mass measurement
Total body muscle mass was measured using QDR‐4500 dual‐energy X‐ray absorptiometry (DXA) unit (Hologic, Bedford, MA, USA). The total body soft tissue was divided into the extremities and trunk. The lean soft tissue mass of the arm and leg regions were combined to determine appendicular skeletal muscle mass (ASM). The SMI (kg/m2) was calculated as ASM divided by height2.
Background factors
The height (cm) and weight (kg) were measured using a digital scale. Body mass index (BMI) (kg/m2) was calculated by dividing weight by height2. Medical history and smoking habits were assessed using a questionnaire and confirmed by a physician during physical examination. Interviewers used a questionnaire to ask participants about their physical activity habits, frequency and exercise intensity (metabolic equivalent [MET] × min × 10−3/year) over the past 12 months. Nutritional intake was assessed using a 3‐day food record and daily averages of total caloric intake (kcal/day), alcohol intake (g/day) and vitamin D intake (μg/day) were calculated. Because serum vitamin D measurements are influenced by seasonality, we categorized participants into four seasons according to the season in which the test was performed. 1 The participants wore a uniaxial accelerometer sensor (Lifecorder, Suzuken, Aichi, Japan) constantly for 7 days, except during sleep or bathing, and the average number of steps was calculated from the records of 5 days, excluding the maximum and minimum.
Sarcopenia‐related classification
With reference to the Asian Working Group for Sarcopenia 2019 criteria, 16 the following criteria were used: dynapenia (muscle function loss: grip strength <28 kg in men, <18 kg in women and/or walking speed 1.0 m/s), presarcopenia (muscle mass loss: DXA 7.0 kg/m2 in men and 5.4 kg/m2 in women), sarcopenia (muscle mass loss and weak grip strength or slow walking speed) and severe sarcopenia (muscle mass loss and weak grip strength and slow walking speed).
Statistical analysis
Participants were classified into vitamin D‐deficient and non‐deficient groups. To adjust for baseline differences between the groups, matching was performed using a 1:1 matching protocol without replacement (greedy‐matching algorithm) with a calliper width equal to 0.2 of the standard deviation of the logit of the propensity score. Participants were matched using propensity scores for background factors [age, sex, height, weight, medical history (diabetes mellitus, hypertension, hyperlipidaemia, heart disease, stroke), smoking habit, alcohol intake, energy intake, vitamin D intake, total activity, steps, season, sarcopenia‐related classification]. Categorical data were compared between the two groups using Fisher's exact or chi‐square tests. All analyses of continuous variables were performed using the student's t‐test. All statistical tests were performed using SPSS version 27 (IBM Corp., Armonk, N.Y., USA). Statistical significance was set at P < 0.05.
Basic analysis
Subjects
To elucidate the function of the vitamin D‐VDR signalling pathway in mature skeletal muscle cells, Myf6 CreERT2 VDR‐floxed (Vdr mcKO ) mice were generated and analysed.
Materials and Methods
Animals
Myf6 CreERT2 mouse lines were obtained from the Jackson Laboratory, 17 , 18 and VDR‐floxed mice were obtained from the University of Tokyo. 19 These mice were crossed to generate Vdr mcKO mice. To induce Cre‐mediated recombination and mature muscle fibre‐specific vitamin D receptor deficiency, 8‐week‐old mice were injected intraperitoneally with 1 mg of tamoxifen per 10 g of body weight for 5 days. Various phenotypes were analysed at 16 weeks of age. Muscle strength (grip strength) was evaluated, and the tibialis anterior (TA) and soleus muscles were obtained to determine their wet weight, muscle fibre cross‐sectional area (CSA) and percentage of fast and slow muscle fibres. VDR expression was assessed in the extensor digitorum longus (EDL) and soleus muscles. Polymerase chain reaction (PCR) was performed using the gastrocnemius muscle. All mice were maintained, crossed, genotyped and sacrificed in accordance with the Institutional Animal Care and Use Committee of the National Center for Geriatrics and Gerontology (3‐55 and 4‐15).
Western blot analysis
Both the EDL and soleus muscles were dissected from control and Vdr mcKO mice. Snap‐frozen muscle tissues were crushed on an LN2‐cold mortar, and the crushed muscle powders were further homogenized in SDS‐HBS using a tissue homogenizer. Homogenates were subjected to electrophoresis (SDS‐PAGE) in Laemmli sample buffer. After transfer to the PVDF membrane, the VDR protein was detected by western blotting using the standard method. VDR was detected using anti‐VDR primary (9A7; Thermo Fisher Scientific, Waltham, MA, USA) and anti‐mouse IgG‐HRP secondary (Cell Signaling Technology, Danvers, MA, USA) antibodies.
Measurement of serum 25(OH)D level
To measure circulating the 25(OH)D level, blood was collected from the tail vein of Vdr‐cKO mice at 4 weeks after tamoxifen injection and then centrifuged at 2,000 rpm for 20 min to prepare the serum fraction. Serum samples were divided into 50 μL aliquots and stored at −80°C before use. Serum 25(OH)D levels were measured by ELISA (Cayman chemical, Ann Arbor, MI, USA) following the manufacturer's protocol.
Cultivation of C2C12 myotubes with vitamin D
C2C12 myoblasts (p5) were cultivated in 20% FBS/DMEM until they reached 100% confluence, after which the medium was replaced with 2% HS/DMEM to induce terminal differentiation. At 48 h, vitamin D (100 nM, calcitriol; Cayman Chemical, Ann Arbor, MI, USA) was added to the medium, and the myotubes were cultivated for a further 24 h.
Myofibre analysis (CSA and myofibre type)
Dyeing of slow‐twitch and fast‐twitch muscle fibres was performed using immunofluorescence staining for an anti‐slow myosin heavy chain antibody (clone NOQ7.5.4D; Merck‐Millipore, Burlington, MA, USA) (1:4000) and anti‐fast myosin heavy chain antibody (clone 8F72C8; Merck‐Millipore) (1:4000). An anti‐laminin antibody (Sigma, St Louis, MO, USA) was used to stain the myofibre basement membrane, and DAPI was used for nuclear staining. After staining, photographs were taken under a microscope, and the obtained images were used to calculate the CSA (μm2) and the percentage of each fibre type. The myofibre CSA was measured using ImageJ software (NIH).
qPCR
RNA was extracted from the gastrocnemius muscle of control and Vdr mcKO mice and C2C12 myotubes, and quantitative reverse transcription (RT)‐PCR was performed after cDNA synthesis. TRI reagent (Molecular Research Center, Cincinnati, OH, USA) was used for RNA extraction, and the PrimeScript II 1st strand cDNA synthesis kit (Takara‐Bio, Shiga, Japan) was used for cDNA synthesis. PCR was performed using a CFX96 real‐time PCR detection system (Bio‐Rad, Hercules, CA, USA). The PCR primer sequences were as follows: glyceraldehyde‐3‐phosphate dehydrogenase (Gapdh), sarcoendoplasmic reticulum Ca2+‐ ATPase (Serca) and myoregulin (Mln).
Gapdh; 5′‐ATCACTCGCCACCAGAGAGACT‐3′ and 5′‐CATGCCAGTGAGCTTCCCGTT‐3′
Serca1; 5′‐GCCCTGGACTTTACCCAGTG‐3′ and 5′‐CCTCCAGATAGTTC‐3′
Serca2a; 5′‐GATCCTCTACGTGGAACCTTTG‐3′ and 5′‐GGTAGATGTGTTGCTAACAACG‐3′
Serca2b; 5′‐GATCCTCTACGTGGAACCTTTG‐3′ and 5′‐CCACAGGGAGCAGGAAGAT‐3′
Mln; 5′‐CAACGTTGCTAGGAAAACACC‐3′ and GCTCTTGCCACTCATGTTCA‐3′
Number of satellite cells
Satellite cell numbers were measured in thin frozen sections of the TA and soleus muscles and compared between control and Vdr mcKO mice. Satellite cells were visualized using anti‐calcitonin receptor (CalR) antibody (Bio‐Rad) (1:50) and co‐stained with anti‐laminin antibody. Cells that were CalR positve and located just below the basement membrane were determined to be satellite cells.
Nuclear domain size
Thin frozen sections of TA muscle were subjected to immunofluorescence staining with anti‐laminin antibody, and the number of nuclei per myofibre was counted by nuclear co‐staining. Nuclei inside laminin were determined to be myofibre nuclei.
Muscle strength (grip strength) measurement
Grip strength was measured in both males and females 2 months after tamoxifen administration. It was also measured 14 months after tamoxifen administration in males only. The two measurements were obtained for the forelimb and four limbs, and the mean values were used for analyses. GPM‐101B/V (Melquest, Toyama, Japan) was used for measurements (in g).
Measurement of sarcoplasmic reticulum (SR) Ca2+ ATPase activity
TA muscle was harvested from 10‐month‐old female Vdr mcKO and control mice, and muscle homogenates were prepared after cryopreservation (n = 4 and n = 3, respectively). After treatment with saturated ammonium sulfate, the homogenates were centrifuged, and the pellet was resuspended in ice‐cold ATPase buffer. The homogenates were further treated with low (0.8 mM) or high (20 mM) concentrations of Ca2+ and then analysed using an ATPase assay kit (Abcam, Cambridge, UK) according to the manufacturer's protocol, and ATPase activity in the muscle was measured. Because a high concentration of Ca2+ immediately inhibits SR Ca2+ ATPase activity, 20 the difference in activity between the low and the high concentrations of Ca2+ was defined as the SR Ca2+ ATPase activity.
Statistical analysis
A two‐tailed Student's t‐test was used to compare data between the control and Vdr mcKO mice or vitamin D‐treated groups. All statistical analyses were performed using GraphPad Prism 7 and 9 software (GraphPad Software, San Diego, CA, USA).
Results
Epidemiological analysis
Using propensity‐score matching, 384 cases were selected in both the deficient and non‐deficient groups (Figure 1). The matched groups showed no significant differences in background factors (P > 0.05) except for serum 25(OH)D (P < 0.001) (Table 1). Therefore, the standardized difference was <0.1 for most variables except for season and serum 25 (OH)D, and the two groups were generally balanced by matching. The results for grip strength and muscle mass showed that the reduction in grip strength was significantly greater in the deficient group (−1.55 ± 2.47 kg) than in the non‐deficient group (−1.13 ± 2.47 kg) (P = 0.019) (Table 2). ASM and SMI did not differ significantly between the deficient and non‐deficient groups [−0.05 ± 0.79 kg, −0.01 ± 0.74 kg (P = 0.423); 0.02 ± 0.29 kg, 0.02 ± 0.29 kg (P = 0.743), respectively]. The incidence of new cases of dynapenia did not differ significantly between the deficient (15 cases, 3.9%) and non‐deficient groups (17 cases, 4.4%). The incidence of new cases of sarcopenia was significantly higher in the deficient group (15 cases, 3.9%) than in the non‐deficient group (5 cases, 1.3%) (P = 0.039).
Figure 1.
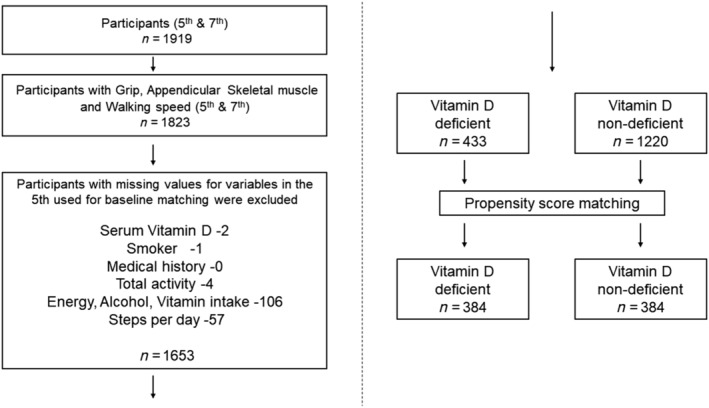
Flowchart of enrolled participants.
Table 1.
Comparison of the general characteristics at baseline (5th wave) of participants
| Unmatched cohort characteristics | Matched cohort characteristics | |||||||
|---|---|---|---|---|---|---|---|---|
| Total | Vitamin D non‐deficient | Vitamin D deficient | P value | Vitamin D non‐deficient | Vitamin D deficient | P value | Standard difference | |
| 1653 | 1220 | 433 | 384 | 384 | ||||
| Age (years) | 58.61 ± 11.44 | 59.78 ± 11.02 | 55.31 ± 11.96 | <0.001 | 56.49 ± 11.07 | 57.11 ± 11.51 | 0.453 | 0.054 |
| Sex men, (%) | 846 (51.2) | 705 (57.8) | 141 (32.6) | <0.001 | 140 (36.5) | 133 (34.6) | 0.651 | 0.038 |
| Body height (cm) | 160.3 ± 8.8 | 160.9 ± 8.8 | 158.4 ± 8.5 | <0.001 | 158.9 ± 8.2 | 158.4 ± 8.7 | 0.420 | 0.058 |
| Body weight (kg) | 58.80 ± 10.45 | 59.49 ± 10.33 | 56.85 ± 10.55 | <0.001 | 57.49 ± 11.05 | 57.24 ± 10.52 | 0.747 | 0.023 |
| Body mass index (kg/m2) | 22.79 ± 2.96 | 22.88 ± 2.90 | 22.54 ± 3.10 | 0.042 | 22.66 ± 3.26 | 22.71 ± 3.08 | 0.817 | 0.017 |
| Diabetes, n(%) | 113 (6.8) | 91 (7.5) | 22 (5.1) | 0.097 | 21 (5.5) | 22 (5.7) | 1.000 | 0.011 |
| Hypertension, n(%) | 421 (25.5) | 334 (27.4) | 87 (20.1) | 0.003 | 75 (19.5) | 86 (22.4) | 0.375 | 0.070 |
| Hyperlipidaemia, n(%) | 312 (18.9) | 246 (20.2) | 66 (15.2) | 0.027 | 69 (18.0) | 64 (16.7) | 0.703 | 0.034 |
| Heart disease, n(%) | 53 (3.2) | 41 (3.4) | 12 (2.8) | 0.635 | 12 (3.1) | 12 (3.1) | 1.000 | <0.001 |
| Stroke, n(%) | 44 (2.7) | 34 (2.8) | 10 (2.3) | 0.729 | 9 (2.3) | 10 (2.6) | 1.000 | 0.017 |
| Smoker, n(%) | 232 (14.0) | 173 (14.2) | 59 (13.6) | 0.810 | 49 (12.8) | 52 (13.5) | 0.831 | 0.023 |
| Alcohol intake (g/day) | 10.03 ± 15.93 | 11.00 ± 16.41 | 7.29 ± 14.14 | <0.001 | 7.84 ± 14.00 | 7.58 ± 14.65 | 0.803 | 0.018 |
| Total caloric intake (kcal/day) | 2065 ± 402 | 2097 ± 402 | 1976 ± 390 | <0.001 | 1998 ± 369 | 1976 ± 399 | 0.433 | 0.057 |
| Vitamin D intake (μg/day) | 8.38 ± 5.71 | 8.96 ± 5.92 | 6.74 ± 4.71 | <0.001 | 7.02 ± 4.26 | 6.98 ± 4.77 | 0.912 | 0.008 |
| Total physical activity (METs × min × 10−3/year) | 712.41 ± 75.80 | 709.81 ± 73.10 | 719.74 ± 82.59 | 0.019 | 718.59 ± 70.43 | 719.16 ± 83.88 | 0.919 | 0.007 |
| Steps per day | 8936 ± 3474 | 9109 ± 3514 | 8449 ± 3315 | 0.001 | 8752 ± 3292 | 8527 ± 3325 | 0.345 | 0.068 |
| Serum 25(OH)D (ng/mL) | 24.91 ± 8.72 | 28.41 ± 7.27 | 15.06 ± 3.11 | <0.001 | 26.94 ± 6.06 | 15.39 ± 2.90 | <0.001 | 2.433 |
| Grip (kg) | 31.74 ± 9.73 | 32.60 ± 9.76 | 29.30 ± 9.24 | <0.001 | 29.68 ± 9.35 | 29.38 ± 9.44 | 0.659 | 0.032 |
| SMI (kg/m2) | 6.73 ± 1.08 | 6.85 ± 1.07 | 6.40 ± 1.05 | <0.001 | 6.52 ± 1.12 | 6.46 ± 1.05 | 0.416 | 0.059 |
| ASM (kg) | 17.51 ± 4.21 | 17.95 ± 4.19 | 16.27 ± 4.01 | <0.001 | 16.68 ± 4.20 | 16.42 ± 4.05 | 0.383 | 0.063 |
| Season (%) | ||||||||
| Winter | 376 (22.7) | 218 (17.9) | 158 (36.5) | <0.001 | 135 (35.2) | 114 (29.7) | 0.208 | 0.154 |
| Spring | 433 (26.2) | 292 (23.9) | 141 (32.6) | 111 (28.9) | 136 (35.4) | |||
| Summer | 444 (26.9) | 363 (29.8) | 81 (18.7) | 87 (22.7) | 81 (21.1) | |||
| Autumn | 400 (24.2) | 347 (28.4) | 53 (12.2) | 51 (13.3) | 53 (13.8) | |||
| Sarcopenia classification (%) | ||||||||
| Normal | 1171 (70.8) | 874 (71.6) | 297 (68.6) | 0.713 | 276 (71.9) | 268 (69.8) | 0.696 | 0.087 |
| Dynapenia | 76 (4.6) | 56 (4.6) | 20 (4.6) | 13 (3.4) | 19 (4.9) | |||
| Presarcopenia | 342 (20.7) | 243 (19.9) | 99 (22.9) | 76 (19.8) | 80 (20.8) | |||
| Sarcopenia | 63 (3.8) | 46 (3.8) | 17 (3.9) | 19 (4.9) | 17 (4.4) | |||
| Severe sarcopenia | 1 (0.1) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
25(OH)D, 25‐hydroxyvitamin D; ASM, appendicular skeletal muscle mass; SMI, skeletal muscle mass.
Values are expressed as number (%).
Mean ± standard deviation.
P values were obtained using the t‐test for continuous data and the χ2 test and Fisher's exact test for categorical data.
Table 2.
Comparison of sarcopenia‐related changes in the two groups
| Vitamin D non‐deficient | Vitamin D deficient | P value | ||
|---|---|---|---|---|
| 384 | 384 | |||
| Grip 7th (kg) | 28.55 ± 9.14 | 27.83 ± 9.25 | 0.278 | |
| ASM 7th (kg) | 16.67 ± 4.24 | 16.37 ± 3.99 | 0.307 | |
| SMI 7th (kg/m2) | 6.55 ± 1.13 | 6.48 ± 1.03 | 0.367 | |
| ⊿Grip | −1.13 ± 2.47 | −1.55 ± 2.47 | 0.019 | |
| ⊿ASM | −0.01 ± 0.74 | −0.05 ± 0.79 | 0.423 | |
| ⊿SMI | 0.02 ± 0.29 | 0.02 ± 0.29 | 0.743 | |
| Sarcopenia classification 7th (%) | Normal | 266 (69.3) | 263 (68.5) | 0.299 |
| Dynapenia | 26 (6.8) | 29 (7.6) | ||
| Presarcopenia | 75 (19.5) | 64 (16.7) | ||
| Sarcopenia | 14 (3.6) | 26 (6.8) | ||
| Severe sarcopenia | 3 (0.8) | 2 (0.5) | ||
| New dynapenia (%) | 17 (4.4) | 15 (3.9) | 0.857 | |
| New presarcopenia (%) | 19 (4.9) | 13 (3.4) | 0.367 | |
| New sarcopenia (%) | 5 (1.3) | 15 (3.9) | 0.039 | |
| New severe sarcopenia (%) | 3 (0.8) | 2 (0.5) | 1.000 |
ASM, appendicular skeletal muscle mass; SMI, skeletal muscle mass.
Values are expressed as number (%).
Mean ± standard deviation.
P values were obtained using the t‐test for continuous data and Fisher's exact test for categorical data.
Basic analysis
When tamoxifen was administered to 8‐week‐old mice, a decrease in VDR expression was observed in both the slow‐ and fast‐twitch muscles of Vdr mcKO mice 2 months later. Thus, VDR is specifically defective in mature muscle fibres (Figure 2A). Skeletal muscle phenotyping of Vdr mcKO mice after 8 weeks of tamoxifen injection showed no significant differences in muscle weight, distribution of fast and slow fibres or myofibre CSA (Figure 2B–D). The number of satellite cells per myofibre and the number of myonuclei were compared, and these parameters also showed no significant differences between Vdr mcKO and control mice (Figure 3A and 3B). These results indicate that VDR deficiency did not cause morphological changes in mature skeletal muscles. Also, there was no significant difference in serum 25(OH)D levels between Vdr mcKO and control mice (Figure S1). Expression profiling of genes involved in muscle exertion in mature muscle fibres revealed a significant decrease in the expression of Serca1 (P = 0.019) and Serca2a (P = 0.049) in the gastrocnemius muscles of Vdr mcKO mice (Figure 4A). In contrast, no changes were observed in the expression of Serca2b, the non‐muscle type SERCA, and Mln. A similar phenomenon was observed when vitamin D was added to the mouse myogenic cell line C2C12 cells (Figure 4B). These results suggest that vitamin D and VDR contribute to muscle exertion by regulating calcium‐dependent ATPase expression. Notably, the Vdr mcKO mice showed a significant decrease in SR Ca2+ ATPase activity (P = 0.0031) (Figure 4C) and muscle strength. Two months after tamoxifen administration, both forelimb and four‐limb muscle strength were significantly lower in Vdr mcKO mice (males: forelimb, P = 0.048; four‐limb, P = 0.029; females: forelimb, P < 0.001; four‐limb, P < 0.001), and after 14 months, only four‐limb muscle strength was significantly lower in Vdr mcKO mice (P = 0.003) (Figure 5A and 5B).
Figure 2.
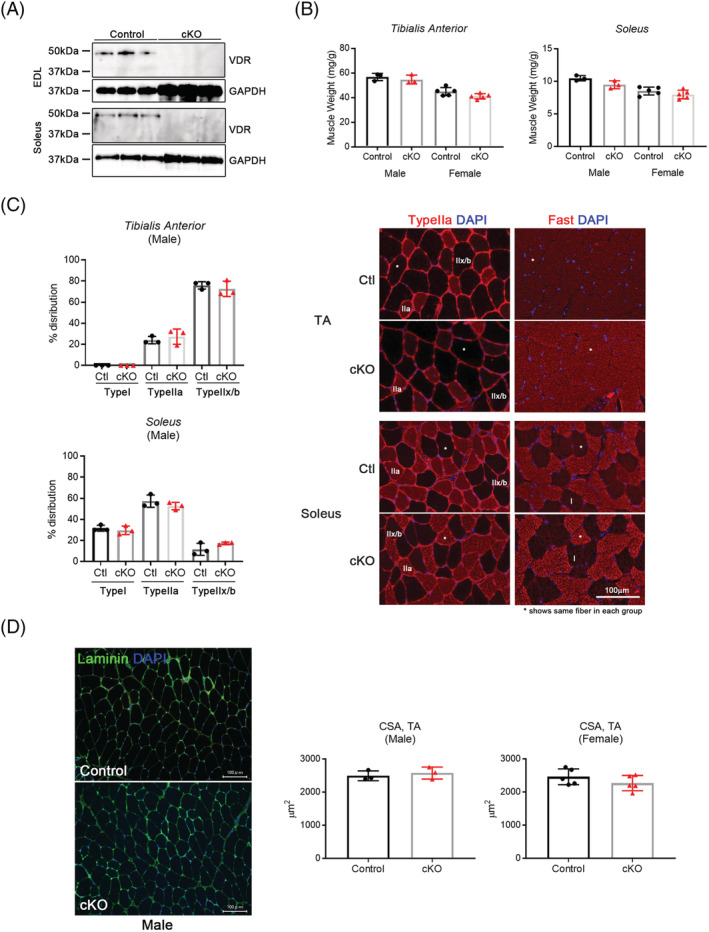
‘Matured’ myofibre‐specific Vdr‐deficient mice showed no obvious phenotypes in muscle morphology. (A) VDR expression was decreased in both fast (EDL) and slow (soleus) muscles of Vdr mcKO mice (cKO). n = 3 for each group. (B–D) There were no significant differences between age‐matched control (control or Ctl) and Vdr mcko mice in weight of muscles (fast and slow), fibre type, cross‐sectional area (CSA). Fibre typing was performed by immunofluorescence using anti‐Fast and anti‐Type IIa antibodies. Representative images were shown in (C), and the asterisk indicates the same myofibre in cross section. CSA was calculated in the images for laminin immunostaining (D). Muscle weight was calculated by division with body weight (mg/g). Values for control and cKO mice in each graph were shown as black circles and red triangles, respectively. n = 3 in male, and n = 5 in female. P < 0.05 was statistically significant.
Figure 3.
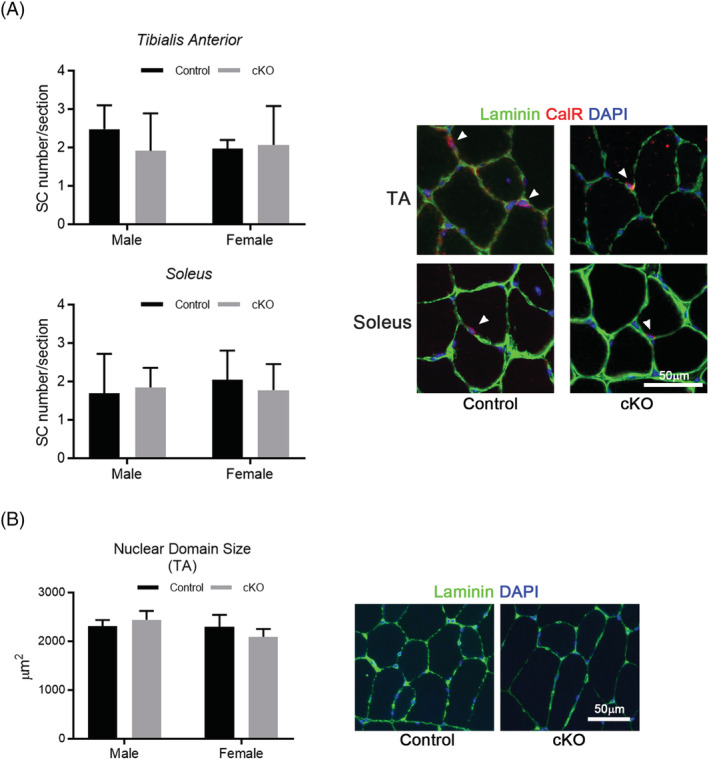
Vdr loss did not cause an alteration in muscle stem cell number and nuclear domain size. (A,B) Satellite cell number per fibre and nuclear domain size were calculated on muscle cross sections and compared between control (black bars) and cKO (grey bars) mice. Satellite cells were identified by immunostaining for calcitonin receptor (CalR) and laminin (A). Nuclear domain size was calculated in the images for laminin‐immunostaining (B). n = 3 in males, and n = 5 in females. P < 0.05 was statistically significant.
Figure 4.
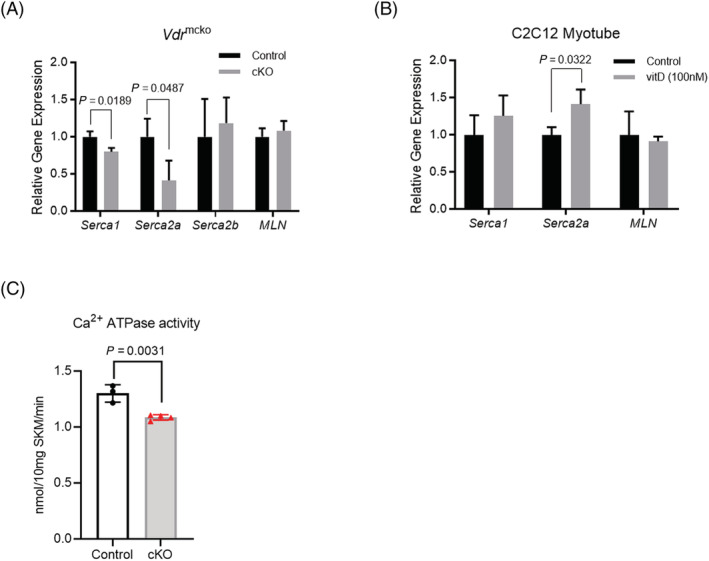
Fibre contraction‐related genes were significantly decreased in VDR‐deficient mice. (A) Quantitative RT‐PCR (qPCR) analysis was performed to compare expression of fibre contraction‐related genes, Serca1, Serca2a, Serca2b and MLN (myoregulin) in the gastrocnemius muscle (n = 3 for each group). Muscle type of SERCA genes (Serca1 and Serca2a) were significantly decreased in cKO mice (grey bars), whereas non‐muscle type SERCA gene (Serca2b) and MLN were not altered regardless of Vdr. (B) C2C12 myotubes were cultivated with vitamin D (100 nM) for 24 h, and then expression of muscle specific contraction‐related genes was analysed in qPCR analysis (n = 3 for each group). Expression of Serca2a was significantly increased in vitamin D‐treated myotubes (vitD; grey bars). (C) SR Ca2+ ATPase activity was assayed in TA muscle from control and cKO mice. VDR deficiency caused a significant decrease in muscle ATPase activity. N = 3 in control group, and n = 4 in cKO group. P < 0.05 was statistically significant.
Figure 5.
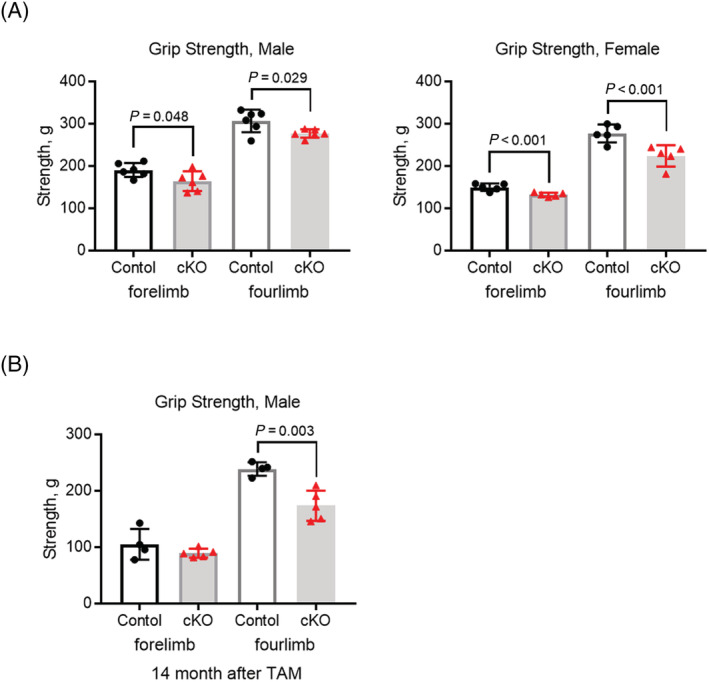
Muscle strength was significantly decreased in VDR‐deficient mice. (A) Grip strength (forelimb and four‐limbs) was measured at 2 months after tamoxifen administration and compared between control (black circles) and cKO (red triangles) mice. VDR loss caused a decrease in muscle strength in both male and female mice. n = 6 in males, and n = 5 in females. (B) Decreased muscle strength in Vdr loss was retained in older male mice. Muscle strength was measured at 14 months after tamoxifen administration. n = 4 in control group, and n = 5 in cKO group. P < 0.05 was statistically significant.
Discussion
In a relatively short longitudinal study (4 years) of community residents, vitamin D level at baseline had no significant effect on muscle mass after 4 years; however, it was significantly correlated with muscle strength. Phenotypic analysis of mature muscle fibre‐specific VDR‐deficient mice conducted in a basic study confirmed that inhibition of the genomic pathway of vitamin D leads to muscle weakness without affecting muscle mass. From both epidemiological and basic perspectives, the findings suggest that the effects of vitamin D deficiency in mature muscle fibres are particularly likely to affect muscle strength.
A 3‐year longitudinal study in the Netherlands found that low serum 25(OH)D levels of less than 10 ng/mL were associated with decreased grip strength. 3 A cross‐sectional study of older people in Japan also reported a relationship between low vitamin D levels and low grip strength. 10 These findings are consistent with that from our study, indicating that low serum vitamin D levels are closely related to muscle strength and may be associated with muscle weakness occurring in the near future (i.e. indicating the potential of serum vitamin D level as a predictor of muscle weakness). In addition, this epidemiological study evaluated changes in muscle mass and sarcopenia classification. As shown in Table 2, the 4‐year reduction in muscle mass was small, suggesting that the muscle‐strengthening effect of low serum vitamin D levels occurs through a different mechanism than the reduction in muscle mass. However, in the sarcopenia classification, vitamin D deficiency was significantly associated only with new onset of sarcopenia. It is unclear why, but it may be that vitamin D deficiency has a greater effect in the state of reduced muscle mass, as in presarcopenia, than in the normal state. Factors that cause muscle weakness without muscle mass loss include decreased single‐fibre contractile function, 21 impaired neuromuscular control such as lower motor neuron firing rate, 22 reduced nerve‐conduction velocities, 23 excitation–contraction uncoupling, 24 muscle fibrosis 25 a decline in voluntary neural drive 26 and poor muscle quality associated with fatty infiltration. 27 , 28 Using propensity‐score matching, we attempted to extract a clearer understanding of the effect of vitamin D deficiency status on mature muscle in middle‐aged and older people by matching participants for age, sex, caloric intake, vitamin D intake, activity, lifestyle, medical history and other background factors that may influence muscle. Based on these findings, we speculate that muscle weakness in this study was mainly caused by disturbances in the signalling pathway of vitamin D due to serum vitamin D deficiency. The mechanism of action of vitamin D can be divided into two main pathways: the genomic pathway via the nuclear receptor VDR 29 and the non‐genomic pathway in which vitamin D directly enters and acts on cells via caveolae on the cell surface. 30 Mature skeletal muscle functions as an aggregate of myofibres and many other cell types, and vitamin D target cells exist in addition to myofibers. 31 Therefore, vitamin D may act in a complex manner on various cells and tissues that make up skeletal muscle, thus affecting muscle strength. As a result, whether this muscle weakness is due to mature muscle fibres or to other cells and tissues, this mechanism is difficult to elucidate further by this epidemiological study alone.
We investigated the role of VDR signalling in mature myocytes by using Myf6, which is expressed in mature muscle fibres, as a driver of VDR deficiency and obtained a phenotype in which only muscle weakness was induced without muscle atrophy or obvious histological changes. This epidemiological analysis did not show correlation between low vitamin D levels and muscle mass, but with muscle weakness; these observations are similar to the findings obtained with Vdr mcKO mice. Vitamin D deficiency and decreased VDR expression may be the accelerating factor in the mechanism of muscle weakness. Previously, a relationship between vitamin D and muscle mass had been reported, including an association of vitamin D with muscle hypertrophy 32 and reduction of muscle fibre diameter in VDR‐null mice, 12 , 33 but no muscle mass changes occurred in the Vdr mcKO mice in the present study. This may be due to the different effects of vitamin D on developing (or growing) and matured muscle. An ageing‐related reduction in the expression of VDRs has been suggested to reduce functional responsiveness to vitamin D, resulting in loss of muscle mass and weakness. 34 A previous report showed a weak correlation between VDR expression and 25(OH)D or 1,25‐dihydroxyvitamin D. 11 Although VDR expression could not be analysed in this epidemiological study because muscle samples could not be obtained, the role of the vitamin D signalling system in muscle may be better clarified in the future by simultaneously measuring blood vitamin D levels and muscle VDR expression. In the present basic study, we also found that myofibre‐specific VDR deficiency did not influence circulating 25(OH)D levels, which is consistent with VDR‐null mice, 35 indicating that there was no correlation between VDR expression in myofibres and circulating 25(OH)D levels.
In addition, the present analysis of Vdr mcKO mice revealed that the vitamin D‐VDR pathway (genomic pathway) regulates muscle exertion by regulating calcium‐dependent ATPase expression. SERCA is a calcium pump in the sarcoplasmic reticulum and its membranes that concentrates calcium in the sarcoplasmic reticulum lumen. Three distinct genes encode SERCA 1, 2 and 3, which are known to produce more than 10 isoforms. The typical isoforms are as follows: SERCA1 is the fast‐twitch muscle isoform, SERCA2a is the slow‐twitch (cardiac) muscle isoform, and SERCA2b is the isoform that appears in all cell types in low abundance. 36 In this study, we analysed the gastrocnemius muscle, which is composed of fast‐twitch and slow‐twitch fibres and found that both SERCA1 and SERCA2a genes were downregulated in Vdr mcKO mice, whereas the expression of MLN gene, which antagonizes SERCA and regulates muscle contraction, 37 was unchanged in Vdr mcKO mice. Since reduced Serca2 expression was also observed in the skeletal muscle of myocyte‐specific Vdr‐deficient mice, which were driven by HSA‐Cre, 13 the findings suggest that vitamin D‐VDR signalling specifically targets muscle relaxation as a point of action. A decrease in serum vitamin D levels is expected to lead to inadequate VDR signalling in myofibres, causing excitation–contraction uncoupling. Notably, VDR deficiency reduced activity of SR Ca 2+ ATPase in mature myofibres, which is expected to be induced by decreased expression of SERCA genes. The present findings support this, because vitamin D alters the dynamics of muscle contraction by decreasing Ca2+ reuptake into the SR, thereby prolonging the relaxation phase of muscle contraction. 38 , 39 Although the mechanism of reduced grip strength in Vdr mcKO mice has not been completely clarified in the present study, we hypothesize that prolonged relaxation caused by SERCA inhibition is one of possible reasons for decrease of muscle strength in the mice and also elderly study participants. A similar idea has been discussed in previous study using myocyte‐specific Vdr knockout mice. 13 However, no correlation between relaxation and muscle strength has been reported in slow‐twitch muscles of Serca2‐conditional knockout mice, 40 indicating that further detailed investigations are needed to clarify the mechanism of muscle strength reduction due to prolonged relaxation.
Limitations
This was a relatively short epidemiological longitudinal study of only 4 years. Further long‐term studies on age‐related changes in vitamin D may clarify the details of the effects of vitamin D on muscles. We attempted to increase internal validity by matching background factors that are likely to affect serum vitamin D levels, such as vitamin D intake, physical activity, season and propensity‐score matching, in order to clarify the possible causal relationship between low vitamin D levels and muscle weakness. Some problems still exist with this method, such as the undeniable bias caused by unmeasured data and the possibility of reduced external validity compared to regular cohort studies. In terms of basic research, the knockout mice used in this study were slightly young in age; we were unable to examine the effects of vitamin D in older mice. In addition, we were unable to evaluate the effects of an active form of vitamin D (1,25‐dihydroxyvitamin D) in the blood and its interaction with non‐genomic pathways. More long‐term and comprehensive studies are needed.
Conclusion
In the present study, we attempted to determine the function and role of vitamin D on mature skeletal muscle from epidemiological and basic research perspectives. We evaluated the longitudinal effects of vitamin D levels on muscle strength and muscle mass over a period of 4 years through a cohort study in community residents and found that vitamin D deficiency affects future muscle strength and may be a factor related to the development of sarcopenia. In experiments using mature myofibre‐specific Vdr knockout mice, vitamin D nuclear receptor signalling had little effect on the regulation of muscle mass in mature myofibres, although it had a significant effect on muscle strength. We also proposed that vitamin D nuclear receptor signalling contributes to muscle strength through the regulation of muscle relaxation‐related factors and suggested that muscle weakness induced by vitamin D deficiency is possibly caused by excitation–contraction uncoupling.
Conflict of interest
The authors have no conflicts of interest to disclose.
Supporting information
Figure S1. Measurement of serum 25 (OH)D level in Vdr mcKO mice. 25 (OH)D level was measured in serum from female control and Vdr mcKO (cKO) mice. There was no significant difference in 25(OH) level between control and cKO mice. N = 3 for each group.
Acknowledgements
The authors certify that they complied with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 41 This work was supported in part by the Japanese Ministry of Health, Labor and Welfare (Research and Development Grant for Geriatrics and Gerontology: 21‐44 to T.H.) and the Japan Society for the Promotion and of Science (Grant‐in‐Aid for Scientific Research C: 21K09289 to T.H.).
Mizuno T., Hosoyama T., Tomida M., Yamamoto Y., Nakamichi Y., Kato S., Kawai‐Takaishi M., Ishizuka S., Nishita Y., Tange C., Shimokata H., Imagama S., and Otsuka R. (2022) Influence of vitamin D on sarcopenia pathophysiology: A longitudinal study in humans and basic research in knockout mice, Journal of Cachexia, Sarcopenia and Muscle, 13, 2961–2973, doi: 10.1002/jcsm.13102
Takafumi Mizuno and Tohru Hosoyama contributed equally to this work.
Contributor Information
Takafumi Mizuno, Email: takafumi.mizuno@med.nagoya-u.ac.jp.
Tohru Hosoyama, Email: toruhoso@ncgg.go.jp.
References
- 1. Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson‐Hughes B, Eisman JA, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int 2009;20:1807–1820. [DOI] [PubMed] [Google Scholar]
- 2. Sohl E, Van Schoor NM, De Jongh RT, Visser M, Deeg DJH, Lips P. Vitamin D status is associated with functional limitations and functional decline in older individuals. J Clin Endocrinol Metab 2013;98:1483–1490. [DOI] [PubMed] [Google Scholar]
- 3. Visser M, Deeg DJH, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): The longitudinal aging study Amsterdam. J Clin Endocrinol Metab 2003;88:5766–5772. [DOI] [PubMed] [Google Scholar]
- 4. Bischoff‐Ferrari HA, Dietrich T, Orav EJ, Hu FB, Zhang Y, Karlson EW, et al. Higher 25‐hydroxyvitamin D concentrations are associated with better lower‐extremity function in both active and inactive persons aged ≥60 y. Am J Clin Nutr 2004;80:752–758. [DOI] [PubMed] [Google Scholar]
- 5. Bischoff‐Ferrari HA, Dawson‐Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, et al. Fall prevention with supplemental and active forms of vitamin D: A meta‐analysis of randomised controlled trials. BMJ 2009;339:843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stockton KA, Mengersen K, Paratz JD, Kandiah D, Bennell KL. Effect of vitamin D supplementation on muscle strength: A systematic review and meta‐analysis. Osteoporos Int 2011;22:859–871. [DOI] [PubMed] [Google Scholar]
- 7. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Janssen HCJP, Emmelot‐Vonk MH, Verhaar HJJ, van der Schouw YT. Vitamin D and muscle function: Is there a threshold in the relation? J Am Med Dir Assoc 2013;14:627.e13–627.e18. [DOI] [PubMed] [Google Scholar]
- 9. Kim BJ, Kwak MK, Lee SH, Koh JM. Lack of association between vitamin D and hand grip strength in Asians: A nationwide population‐based study. Calcif Tissue Int 2019;104:152–159. [DOI] [PubMed] [Google Scholar]
- 10. Suzuki T, Kwon J, Kim H, Shimada H, Yoshida Y, Iwasa H, et al. Low serum 25‐hydroxyvitamin D levels associated with falls among Japanese community‐dwelling elderly. J Bone Miner Res 2008;23:1309–1317. [DOI] [PubMed] [Google Scholar]
- 11. Bischoff‐Ferrari HA, Borchers M, Gudat F, Dürmüller U, Stähelin HB, Dick W. Vitamin D receptor expression in human muscle tissue decreases with age. J Bone Miner Res 2004;19:265–269. [DOI] [PubMed] [Google Scholar]
- 12. Endo I, Inoue D, Mitsui T, Umaki Y, Akaike M, Yoshizawa T, et al. Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology 2003;144:5138–5144. [DOI] [PubMed] [Google Scholar]
- 13. Girgis CM, Cha KM, So B, Tsang M, Chen J, Houweling PJ, et al. Mice with myocyte deletion of vitamin D receptor have sarcopenia and impaired muscle function. J Cachexia Sarcopenia Muscle 2019;10:1228–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shimokata H, Ando F, Niino N. A new comprehensive study on aging‐the national institute for longevity sciences, longitudinal study of aging (NILS‐LSA). J Epidemiol 2000;10:1–9. [DOI] [PubMed] [Google Scholar]
- 15. Okazaki R, Ozono K, Fukumoto S, Inoue D, Yamauchi M, Minagawa M, et al. Assessment criteria for vitamin D deficiency/insufficiency in Japan: Proposal by an expert panel supported by the Research Program of Intractable Diseases, Ministry of Health, Labour and Welfare, Japan, the Japanese Society for Bone and Mineral Research and the Japan Endocrine Society [opinion]. J Bone Miner Metab 2017;35:1–5. [DOI] [PubMed] [Google Scholar]
- 16. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 2020;21:300–307.e2. [DOI] [PubMed] [Google Scholar]
- 17. Southard S, Low S, Li L, Rozo M, Harvey T, Fan CM, et al. A series of Cre‐ERT2 drivers for manipulation of the skeletal muscle lineage. Genesis 2014;52:759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hosoyama T. Possible application of muscle specific conditional mouse‐derived induced pluripotent stem cells for muscle research. Biochem Biophys Reports 2020;21:100744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yamamoto Y, Yoshizawa T, Fukuda T, Shirode‐Fukuda Y, Yu T, Sekine K, et al. Vitamin D receptor in osteoblasts is a negative regulator of bone mass control. Endocrinology 2013;154:1008–1020. [DOI] [PubMed] [Google Scholar]
- 20. Simonides WS, van Hardeveld C. An assay for sarcoplasmic reticulum Ca2+‐ATPase activity in muscle homogenates. Anal Biochem 1990;191:321–331. [DOI] [PubMed] [Google Scholar]
- 21. Larsson L, Li X, Frontera WR. Effects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. Am J Physiol Cell Physiol 1997;272:C638–C649. [DOI] [PubMed] [Google Scholar]
- 22. Connelly DM, Rice CL, Roos MR, Vandervoort AA. Motor unit firing rates and contractile properties in tibialis anterior of young and old men. J Appl Physiol 1999;87:843–852. [DOI] [PubMed] [Google Scholar]
- 23. Metter EJ, Conwit R, Metter B, Pacheco T, Tobin J. The relationship of peripheral motor nerve conduction velocity to age‐ associated loss of grip strength. Aging Clin Exp Res 1998;10:471–478. [DOI] [PubMed] [Google Scholar]
- 24. Payne AM, Delbono O. Neurogenesis of excitation‐contraction uncoupling in aging skeletal muscle. Exerc Sport Sci Rev 2004;32:36–40. [DOI] [PubMed] [Google Scholar]
- 25. Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 2007;317:807–810. [DOI] [PubMed] [Google Scholar]
- 26. Clark BC, Taylor JL. Age‐related changes in motor cortical properties and voluntary activation of skeletal muscle. Curr Aging Sci 2011;4:192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, et al. Attenuation of skeletal muscle and strength in the elderly: The health ABC study. J Appl Physiol 2001;90:2157–2165. [DOI] [PubMed] [Google Scholar]
- 28. Mizuno T, Matsui Y, Tomida M, Suzuki Y, Nishita Y, Tange C, et al. Differences in the mass and quality of the quadriceps with age and sex and their relationships with knee extension strength. J Cachexia Sarcopenia Muscle 2021;12:900–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Girgis CM, Clifton‐Bligh RJ, Hamrick MW, Holick MF, Gunton JE. The roles of vitamin D in skeletal muscle: Form, function, and metabolism. Endocr Rev 2013;34:33–83. [DOI] [PubMed] [Google Scholar]
- 30. Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)‐mediated actions of 1α,25(OH)2vitamin D3: Genomic and non‐genomic mechanisms. Best Pract Res Clin Endocrinol Metab 2011;25:543–559. [DOI] [PubMed] [Google Scholar]
- 31. Braga M, Simmons Z, Norris KC, Ferrini MG, Artaza JN. Vitamin D induces myogenic differentiation in skeletal muscle derived stem cells. Endocr Connect 2017;6:139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shuler FD, Wingate MK, Moore GH, Giangarra C. Sports health benefits of vitamin D. Sports Health 2012;4:496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ceglia L, Niramitmahapanya S, Da Silva Morais M, Rivas DA, Harris SS, Bischoff‐Ferrari H, et al. A Randomized study on the effect of vitamin d3 supplementation on skeletal muscle morphology and vitamin d receptor concentration in older women. J Clin Endocrinol Metab 2013;98:1927–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Campbell WW, Johnson CA, McCabe GP, Carnell NS. Dietary protein requirements of younger and older adults. Am J Clin Nutr 2008;88:1322–1329. [DOI] [PubMed] [Google Scholar]
- 35. Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet 1997;16:391–396. [DOI] [PubMed] [Google Scholar]
- 36. Periasamy M, Kalyanasundaram A. SERCA pump isoforms: Their role in calcium transport and disease. Muscle Nerve 2007;35:430–442. [DOI] [PubMed] [Google Scholar]
- 37. Anderson DM, Anderson KM, Chang CL, Makarewich CA, Nelson BR, McAnally JR, et al. A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. Cell 2015;160:595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rodman JS, Baker T. Changes in the kinetics of muscle contraction in vitamin D‐depleted rats. Kidney Int 1978;13:189–193. [DOI] [PubMed] [Google Scholar]
- 39. Curry OB, Basten JF, Francis MJO, Smith R. Calcium uptake by sarcoplasmic reticulum of muscle from vitamin D‐deficient rabbits. Nature 1974;249:83–84. [DOI] [PubMed] [Google Scholar]
- 40. Sjåland C, Lunde PK, Swift F, Munkvik M, Ericsson M, Lunde M, et al. Slowed relaxation and preserved maximal force in soleus muscles of mice with targeted disruption of the Serca2 gene in skeletal muscle. J Physiol 2011;589:6139–6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: Update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Measurement of serum 25 (OH)D level in Vdr mcKO mice. 25 (OH)D level was measured in serum from female control and Vdr mcKO (cKO) mice. There was no significant difference in 25(OH) level between control and cKO mice. N = 3 for each group.


