ABSTRACT
Alzheimer’s disease (AD) and other neurodegenerative dementias have a progressive course, impairing cognition, functional capacity, and behavior. Most studies have focused on AD. Severe dementia is associated with increased age, higher morbidity-mortality, and rising costs of care. It is fundamental to recognize that severe dementia is the longest period of progression, with patients living for many years in this stage. It is the most heterogeneous phase in the process, with different abilities and life expectancies. This practice guideline focuses on severe dementia to improve management and care in this stage of dementia. As it is a long period in the continuum of dementia, clinical practice should consider non-pharmacological and pharmacological approaches. Multidisciplinary interventions (physical therapy, speech therapy, nutrition, nursing, and others) are essential, besides educational and support to caregivers.
Keywords: Dementia, Palliative Care, Behavior, Cognition
RESUMO
A doença de Alzheimer (DA) e outras demências neurodegenerativas têm um curso progressivo com comprometimento da cognição, capacidade funcional e comportamento. A maioria dos estudos enfocou a DA. A demência grave está associada ao aumento da idade, maior morbimortalidade e aumento dos custos de cuidados. É fundamental reconhecer que a demência grave é o período mais longo de progressão, com o paciente vivendo muitos anos nesta fase. É a fase mais heterogênea do processo, com diferentes habilidades e expectativa de vida. Esta diretriz de prática concentra-se na demência grave para melhorar o manejo e o cuidado nessa fase da demência. Como um longo período no continuum da demência, as abordagens não farmacológicas e farmacológicas devem ser consideradas. Intervenções multidisciplinares (fisioterapia, fonoaudiologia, nutrição, enfermagem, entre outras) são essenciais, além de educacionais e de apoio aos cuidadores.
Palavras-chave: Demência, Cuidados Paliativos, Comportamento, Cognição
INTRODUCTION
A lzheimer’s disease (AD) and other neurodegenerative dementias have a progressive course, impairing cognition, functional capacity, and behavior. Most studies have focused on AD. Severe dementia is associated with increased age, higher morbidity-mortality, and rising costs of care. Severe stages could account for 70 to 80% of total treatment expenses 1 ),( 2 . Degenerative dementias slowly and progressively worsen. In its severe stage, patients show a higher dependency for all basic activities of daily living and incapacity for instrumental activities of daily living.
Around 30 to 60% of patients with dementia (PWD) and 90% of individuals residing in long-term care facilities are in its moderate-late stage 3 )-( 5 .
The progression of dementia is associated with a progressive dependency on caregivers, with loss of capacity to provide self-care in basic activities of daily living. Usually, severe AD patients will score below ten on mini mental examination (MMSE) and moderately severely with 10 to 15 5 . Severe dementia could cause complications such as immobility, swallowing disorders, malnutrition, and fragility. This situation can increase the risk of pneumonia, which has been found as a common cause of death in PWD 5 )-( 9 .
It is fundamental to recognize that severe dementia is the longest period of progression, with patients living for many years in this stage. It is the most heterogeneous phase in the process, with different abilities and life expectancies 7 . Around 17% of PWD older than 75 years are living with a very severe stage of dementia 8 .
This practice guideline focuses on severe dementia to improve management and care in this stage of the disease. As a long period in the continuum of dementia, clinical practice should consider non-pharmacological and pharmacological approaches. Multidisciplinary interventions (physical therapy, speech therapy, nutrition, nursing, and others) are essential, besides educational and support to caregivers. Primary, secondary, and tertiary care center professionals could use this practice guideline .
STAGING SEVERE DEMENTIA
We find many different situations and stages of severe dementia. A bedridden patient with dysphagia is different from a walking and dependent but communicative and without dysphagia patient.
The Clinical Dementia Rating (CDR) permits rating the severity of AD and other dementias on a five-point scale from 0 (normal) to 3 (severe stage) 10 . The final score is obtained after interviews with PWD and informants. It evaluates memory, orientation, judgment, problem solving, community affairs, home, hobbies, self-care. Chaves et al. 11 ) validated this study for Brazilian Portuguese.
The Alzheimer Disease Cooperative Study of activities of daily living (ADCS-ADL sev) is a good measure of activities of daily living (ADL) which strongly correlates with cognition and severity of dementia in moderate to severe AD. Its scores vary from 0 to 54 points, divided into 19 items 12 .
A simple scale that assesses gradual severity and progression of the disability during follow-up is the Global Deterioration Scale (GDS) 13 . This scale has seven global stages, from normality to severe impairment; stage 6 represents severe dementia, and stage 7, very severe 13 .
The Functional Assessment Staging (FAST) assesses decline from normal (stage 1) to severe stages (stage 7); the latter is subdivided into seven situations (A to G) (Box 1) 14 .
Box 1. Functional Assessment Staging (FAST).
| 1 | No difficulty either subjectively or objectively. |
| 2 | Complains of forgetting location of objects. Subjective work difficulties. |
| 3 | Decreased job functioning evident to co-workers. Difficulty in traveling to new locations. Decreased organizational capacity. |
| 4 | Decreased ability to perform complex tasks, e.g., planning dinner for guests, handling personal finances (such as forgetting to pay bills), difficulty shopping, etc. |
| 5 | Requires assistance in choosing proper clothing. |
| 6a | Improperly puts on clothes without assistance. |
| 6b | Unable to properly bathe (shower) (e.g., difficulty adjusting bathwater (shower) temperature. |
| 6c | Inability to handle toileting mechanics. |
| 6d | Urinary incontinence. |
| 6e | Fecal incontinence. |
| 7a | Ability to speak limited to approximately a half a dozen intelligible different words or fewer in the course of an average day or in the course of an intensive interview. |
| 7b | Speech ability limited to the use of a single intelligible word in an average day or in the course of an interview. |
| 7c | Loss of ambulatory ability (unable to walk without personal assistance). |
| 7d | Unable to sit up without assistance. |
| 7e | Loss of ability to smile. |
| 7f | Loss of ability to independently hold up their head. |
In advanced dementia, we observe impairment to basic daily activities. The Katz Scale is often used to measure performance in bathing, dressing, toileting, transferring, continence, and feeding; characterizing severe dementia. Table 1 summarizes all these instruments.
Table 1. Instruments to evaluate severe dementia.
| Test | Measure | Score | Answered by | Comments |
|---|---|---|---|---|
| SMMSE | Global cognition | 0-30 points | Patient | Good for measuring cognition |
| CDR | 0 none | Caregiver and patient | Good for follow-up | |
| Global State | 0.5 questionable | |||
| Domains: memory, orientation, judgment, problem solving, community affairs, home and hobbies, and personal care | 1 mild | |||
| 2 moderate | ||||
| 3 severe | ||||
| SIB | Global cognition | 0-100 points (<63 severe impairment) | Patient | Allows to evaluate very severe patients |
| ADCS-ADL | ADL | 0-54 points | Caregiver | Allows to evaluate severe to very severe patients |
| 19 ADL of moderate to severe dementia | ||||
| FAST | Functionality | 1-7 | Health professional | Stages 6-7: divided into incapacity progression |
COGNITIVE EVALUATION
Cognitive evaluation in advanced dementia is relevant to measure pharmacologic and non-pharmacologic management and interventions. MMSE is the most frequently used instrument in brief cognitive evaluation in dementia. However, for moderate-late stages, we could observe a floor-effect, with few modifications over time below 10 points. At this point, we must consider other tests. The GDS described above could be an instrument for cognitive evaluation but severe-MMSE (SMMSE) or Severe Impairment Battery (SIB) 15 ),( 16 ) could offer more detailed and objective assessments.
The SIB, consisting of 40 questions, evaluates nine areas of cognition: social interaction, memory, orientation, language, attention, praxis, visuospatial ability, construction, and orientation to name. Scores vary from 0 to 100. 15
SMMSE is a brief test requiring minimal training and no special materials, but educational attainment influences it. Its scores vary from 0 to 30 and its items are divided into autobiographical knowledge, executive function, language, verbal fluency, and spelling 16 ),( 17 (Table 1).
If only one physician monitors cognitive status, they may ask the same questions to observe the progression of deterioration, such as age, date of birth, names of children or spouse, food or soccer team preferences.
TREATMENT OF BEHAVIOR AND THE PSYCHOLOGICAL SYMPTOMS OF DEMENTIA
Behavior and psychological symptoms of dementia (BPSD) comprehends the neuropsychiatric manifestations besides cognition, affecting between 60 and 90% of people with dementia 18 . BPSD includes a wide spectrum of conditions: apathy, depression, anxiety, sleep disorders, psychosis, agitation, aggression, wandering and motor manifestations, disinhibition, and many others. Generally, BPSD prevalence increases with disease severity although epidemiologic studies in this topic mostly include small sample sizes, especially among those with AD 18 ),( 19 . Moreover, severe forms of BPSD like delusions, hallucinations, agitation, aggression, and aberrant motor conditions are more common in moderate and severe dementia 20 . Multiple adverse outcomes have been associated with BPSD, such as cognitive and functional impairment, caregiver burnout, nursing home placement, and mortality 21 )-( 25 . However, research has scarcely explored outcomes in severe forms of dementia.
The occurrence and maintenance of BPSD relies on three factors, namely patients (e.g., hunger, pain, and acute medical conditions), caregivers (e.g., stress, lack of politeness, and communication skills), and the environment (e.g., under/overstimulation and lack of routine and activities). Consequently, it is rational and evidence-based that the first treatment step against BPSD includes non-pharmacological measures 26 . A systematic review and meta-analysis points to an effect size at least equivalent to those of psychotropic drugs, and it is safer 26 . However, in clinical practice, non-pharmacological measures are yet to be fully implemented due to several economic and cultural reasons. The next session will better explore this specific topic. Clinical practice recommends the observation of BPSD symptoms at different hours, with varying caregivers, and multiple environments. Moreover, research has also suggested using BPSD measurement instruments, such as the Neuropsychiatric Inventory (including the clinician version or NPI-C) and the BEHAVE-AD, due to multiple concurrent and sometimes complex symptoms.
After non-pharmacological measures or concomitantly to them, pharmacological treatment is possible and common due to safety reasons or BPSD severity. In general, response rates to different classes of medications are heterogenous and of small therapeutic effect. The overall evidence points to a small number of psychotropic drugs which reasonably improve BPSD. We recommend that these drugs be prescribed at low doses and that clinicians avoid, if possible, polypharmacotherapy. Table 2 summarizes our expert consensus-based recommendation for the use of psychotropic medication in BPSD associated with AD.
Table 2. Consensus-based recommendation for the use of psychotropic medication in specific behavior and psychological symptoms of dementia (BPSD) associated with severe Alzheimer’s dementia.
| BPSD | Suggested dose range* | Side effects | |
|---|---|---|---|
| Agitation or aggression | Citalopram** | 10-20mg/day, single dose | nausea, diarrhea, headache, increased risk of falls |
| Sertraline | 50-100mg/day, single dose | ||
| Trazodone*** | 25-100mg/day, single/partial doses | ||
| Risperidone | 0.25-1mg/day, single dose | extrapyramidal side effects, weight gain, metabolic abnormalities, hyperprolactinemia | |
| Psychosis | Risperidone | 1-3mg/day, single dose | extrapyramidal side effects, weight gain, metabolic abnormalities, hyperprolactinemia |
| Quetiapine | 100-200mg/day, single/ partial doses | drowsiness, weight gain, metabolic abnormalities | |
| Aripiprazole | 10-30mg/day, single dose | nausea, weight gain, headache, somnolence, akathisia | |
*Doses are recommended based on clinical trials and personal experience, considering the pharmacological properties of these agents; **May consider escitalopram due to its greater safety and better cognitive profile. It is also more commonly prescribed in Brazil than citalopram; ***Although it has less evidence than the other agents, this drug shows a good balance between safety and effectiveness in clinical practice. Extended-release formulation is better during the day to avoid somnolence.
Treating cognitive impairment with cholinesterase inhibitors and memantine shows slight improvement in early and moderate stages of AD, according to a recent systematic review and meta-analysis 27 . Effects are small and benefits to which family members and caregivers referred may amount to a placebo effect. The benefits of these drugs for severe dementia are questionable but may exist and thus, clinicians must weigh the decision of maintaining or ceasing such treatment 27 . In moderate to severe AD, low to insufficient evidence suggested that cholinesterase inhibitors and add-on memantine inconsistently improved cognition and global clinical impression, compared to placebo. However, the evidence is questionable and deserves further exploration in clinical trials. Cholinesterase inhibitors and memantine also slightly improve BPSD, especially in the early and moderate stages of AD and include apathy, anxiety, and depressive symptoms. In total, three systematic reviews with meta-analysis 28 )-( 30 have summarized this evidence. In general, attending physicians may consider prescribing and maximizing both cholinesterase inhibitors and memantine to improve cognitive function and BPSD before considering other psychotropic medication. Nonetheless, we emphasize that evidence is insufficient to decide in favor or against this position.
Citalopram and risperidone have produced favorable evidence for agitation in AD. Citalopram improved moderate agitation, if administered between 10-30mg a day 31 . Other antidepressant agents, such as sertraline and trazodone, improved agitation 32 . However, we must stress their potential limitations as one review included fewer patients with severe AD, still showing cognitive performance and a prolonged QT interval in patients receiving citalopram 30mg 31 . Ongoing S-CitAD, which evaluates escitalopram for agitation, may show greater safety and a better cognitive profile. Risperidone, mostly at low doses (0.5-1mg), and selective serotonin reuptake inhibitors (SSRI), as a class, alleviated agitation in patients with dementia, according to a systematic review and meta-analysis 32 )-( 34 .
The evidence for the use of antipsychotics for BPSD is limited even for agitation and aggression. Data from a systematic review showed that aripiprazole was the safest and most effective antipsychotic versus placebo, and it was associated with improved outcomes on the NPI, the Brief Psychiatric Rating Scale (BPRS), and the Cohen-Mansfield Agitation Inventory (CMAI) ( 35 . Quetiapine improved outcomes on the BPRS, and risperidone was associated with improved outcomes on the CMAI. Differences between atypical antipsychotics were insignificant for effectiveness, death, or cardiovascular adverse events 35 .
Apathy is a common BPSD, especially in early-stage dementia. The literature reports pooled evidence (small sample size from three studies) favoring the use of methylphenidate to reduce this symptom in the Apathy Evaluation Scale 36 . However, apathy is part of the phenotype of more severe dementia, and the overall cognitive decline summed with other bothersome BPSD in this stage results in a questionable pharmacological treatment of apathy.
A systematic review and meta-analysis 37 ) was unable to recommend the use of antidepressants to treat depression in BPSD based on its lack of efficacy in respond to or reduce depressive symptomatology. Benzodiazepines, anticonvulsants, and cannabidiols are also ineffective in pharmacologically controlling BPSD due to their lack of efficiency or adverse reactions, including cognitive decline. Finally, dextromethorphan/quinidine and prazosin showed evidence of improving agitation in AD 38 ),( 39 and pimavanserin reduced psychosis in AD 40 . However, these three agents are unavailable in Brazil.
Box 2 summarizes our overall treatment recommendation for cognitive dysfunction and BPSD. In conclusion, taken together, current evidence shows low certainty for prescribing cholinesterase inhibitors and memantine in severe dementia. The use of agents such as SSRIs, risperidone, and aripiprazole for agitation, aggression, and psychosis also showed a small or uncertain effect when we consider severe dementia. Non-pharmacological measures, including activities of daily living and care routines, proper feeding, pain control, music therapy, physical therapies, and caregiver education and support, seem to be safer and more effective 41 . The use of pharmacological agents should consist of single agents, in small doses, and for a short period to control the targeted BPSD (Table 2).
Box 2. Overall recommendation for the non-pharmacological and pharmacological treatment of cognitive dysfunction and behavior and psychological symptoms of dementia (BPSD) in severe Alzheimer’s dementia.
| 1. Consider withdrawing cholinesterase inhibitors and memantine in severe dementia in the absence of clear benefits to cognition or BPSD |
| 2. Education and support for caregivers |
| 3. Well established routine of daily care and activities |
| 4. If possible, consider music therapy and any form of physical activity |
| 5. Investigate causes for cognitive fluctuations or BPSD |
| 6. Consider pain control before prescribing psychotropic agents |
| 7. If agitated or aggressive, consider citalopram, sertraline or trazodone |
| 8. If agitation or aggression persists with antidepressants, consider antipsychotics such as risperidone, aripiprazole or quetiapine |
| 9. Always reevaluate withdrawing psychotropics for BPSD after symptom control |
NON-PHARMACOLOGICAL TREATMENT
Nonverbal communication
“Perhaps it’s the most important part of caring. Because it leads to reflection, because it provides the manifestation of human nature, present in each of the professionals involved with care. It is the deepest empathy and the need to show affection, affection and, above all, respect for the other. Holding the hands of someone who may have their mind lost, in another dimension perhaps, gently touching the thin skin of an already weakened body, looking deeply into their eyes and letting the feelings present in this exchange of glances lead to the understanding of what is needed to do. It is the meeting of the human and bioethics, a feeling of lightness and of certainty that the task is being carried out with respect and with the dignity that every human being deserves.” (Ceres Ferretti)
The literature is very rich in papers discussing non-pharmacological approaches to psychological and behavioral symptoms in dementia - BPSD, which arise with the evolution of different dementia syndromes 42 ),( 43 . However, unfortunately, the literature still lacks studies discussing the possibilities and effectiveness of these approaches in severe dementia, or even the difficulty of conceptualizing and adequately treating people with it 44 )-( 46 . Some behaviors are more prevalent in the initial phase of the disease, others in its intermediate phase and some in its severe phase. Its last phase signals a possible combination of factors intrinsic to patients (comorbidities) and caregivers (stress) and extrinsic (environmental) ones, which require multi and interdisciplinary assessment and conduct to make the best decision, case by case 47 .
Family doctors and multidisciplinary teams (previously trained by specialists) in primary health care units can theoretically recognize the needs and monitor patients with severe dementia who were discharged from the secondary care service. However, professionals working in this sector still face difficulties, related to their knowledge to perform diagnoses and administer pharmacological and non-pharmacological treatment, especially in mild and severe AD phases 48 .
Due to the problems in the health, economic, and social sectors in Brazil and the need for biopsychosocial support (especially during this serious health crisis 49 , reference and assistance centers have created projects which aim to minimize the direct and indirect social costs of PWD caregivers 50 ),( 51 . These projects are in line with most guidelines from international consensus meetings of associations 52 )-( 57 , universities 58 ),( 59 , and scientific committees 57 ),( 60 .
All expert consensuses discuss the need for psychoeducational programs and suggest recommendations which contribute to the education of caregivers about systematized and appropriate models for different behavioral disorders. The great gap, however, is still the approach to the severe phase of AD. Knowing the causes of SCPD in this phase is the objective, and solving them, or better yet, preventing them is the goal 61 . The starting point is understanding possible social and more individual family barriers to the construction and practice of these models, those aimed at reducing these behaviors and, consequently, the comfort and quality of life of patients with severe dementia and their caregivers 62 .
Assessment Models
In Brazil, the nursing model in dementia care includes all stages of disease evolution 63 . Research has shown that the protocol is useful in its main objective, which is to assess patients and their caregivers. Composed of two stages, patients and caregivers focus on patient-centered care models, in combination with the caregiver-centered care model 64 .
Kales (2015) ( 58 offers a review with an interesting conceptual model of factors which require evaluation, leading to the reflection on the union of disciplines combining different areas of knowledge such as neurology, geriatrics, psychiatry, and gerontology in a multidisciplinary view (Figure 1). The same review shows “DICE - Describe, Investigate, Create, and Evaluate,” proposed by the University of Michigan in partnership with the Johns Hopkins Alzheimer’s Disease Research Center and the Center for Innovative Care in Aging with guidelines to identify needs for the practice of care 58 .
Figure 1. Depiction of a conceptual model for different interactions resulting in BPSD. Adapted from Kales (2015) ( 58 .
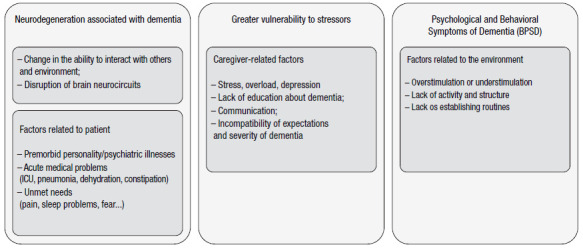
As mentioned above, clinical practice still lacks a consensus on the definition of severe dementia, sometimes confused with moderate dementia. However, studies agree on possible causes that give rise to common behaviors, such as agitation, apathy, hallucinations, delusions, “unmotivated” crying, fear, anxiety, contracted body, and resistance to care, among others 58 ),( 62 .
Several studies report that most factors responsible for SPCD in severe dementia originate from patients’ comorbidities, caregivers’ lack of knowledge and stress, and the environment. The inability to verbally communicate can lead to the behaviors in the same way that the rapid recognition of any change in the pattern of patients’ usual behavior can prevent progressing of the SPCD and interfering with the quality of life of both patients and caregivers (Table 3) ( 58 ),( 65 .
Table 3. Recognition and Management Recommendations for Psychological and Behavioral Symptoms in Severe Dementia. Adapted of Kales (2015) 58 .
| In the patient | In the caregiver | In the environment | Recommendations |
|---|---|---|---|
| Pain | Caregivers’ training; | ||
| Health education; | |||
| Lack of knowledge | Poor positioning | Identification and elimination of causal factors in patients and the environment; | |
| Health education | Excessive noise | Promotion of physical comfort; | |
| Lack of education in dementia | Abrupt movements | Information to the responsible physician and members of the health team; | |
| Elimination of excessive noise; | |||
| Gentle patient mobilization. | |||
| Urinary infection | Inadequate hygiene | Caregivers’ training; | |
| Health education; | |||
| Lack of knowledge | Optimization of fractional hydration (1.5-2L/day) or at medical discretion; | ||
| Stress | Double incontinence; | ||
| Hygiene, body, and environmental measures. | |||
| Respiratory Infection | Lack of knowledge | Dark, closed or dirty environment | Caregivers’ training; |
| Cold temperature | Preventive measures; | ||
| Passive exercises. | |||
| Constipation | Lack of knowledge | Adequate bathroom hygiene, security, and privacy | Training in hygiene measures and health education to caregivers; |
| Medical and nutritional approach; | |||
| Fractioned hydration. | |||
| Refusal of care | Inadequate communication and conducts | Environment adaptation and care | Training of caregivers and health education; |
| Caregivers must be careful with all stimulus and approaches. | |||
| Sleep disturbances | Lack of recognition concerning pain, hunger, coldness, heat, mobilization in bed, and previous sleep problems | Observation | Training and health education; |
| Change in patients’ behavioral patterns | Adequacy of auditory environmental stimuli; | ||
| Medical evaluation. |
* All caregivers must be trained in all activities proposed by the health team to offer their consent and understand the importance of each proposed intervention. Elaborated by Ferretti (2021).
Comorbidities in advanced dementia
In general, older adults, and especially those with dementia, face exclusion from clinical trials on pharmacological interventions and the medical literature is scarce in good-quality data regarding the advanced stages of dementia since the focus of guidelines and randomized, controlled studies and intervention are aimed at premorbid, very mild, and mild stages of dementias 66 ),( 67 .
Although research has suggested the possibility of AD patients being healthier 68 , current evidence points to the opposite: people with dementia (PWD) show a significantly higher prevalence and medication use 69 )-( 73 .
Clinical diseases impact quality of life (by, for example, worsening mobility) and patient survival regardless of dementia, both in institutionalized individuals and those who remain in the community, increasing the number of searches for emergency rooms, hospitalizations, hospital costs, and health expenditures 69 ),( 70 .
Older adults with dementia are three times more likely to have four or more concurrent chronic diseases and annual expenses, up to 3.3 times higher than in older adults without this disease. These comorbidities impact survival throughout patients’ later years 74 ),( 75 (Table 4).
Table 4. Conditions associated with higher mortality among subjects.
| Number of diagnoses |
| Male |
| Age |
| Lung infections |
| Parkinsonism |
| Previous Stroke |
| Atrial fibrillation |
| Malignancy |
As for the severity of dementia, results pointed to a significantly lower two-year survival rate among most dependent patients, regardless of their number of comorbidities. We find a positive correlation between number of comorbidities and dementia severity independently but not the impact of the interaction between them; the more advanced phases compete with higher health expenditures 73 .
Schubert et al. highlight the possibility that the lesser search for diagnoses and treating comorbidities in PWD on an outpatient basis may be one of the conditions responsible for its aggravation, showing the need to search for advanced care units, which could be avoided under a less nihilistic outlook 72 ),( 76 ),( 77 (Box 3).
Box 3. Chronic conditions most frequently associated in PWD.
| • Heart failure |
| • Hypertension |
| • Diabetes mellitus |
| • Neoplasms |
| • Kidney failure |
| • Cerebral and coronary artery diseases |
| • Atrial fibrillation |
| • Lung diseases |
Dementia in comorbidities
As previously mentioned, dementia directly impacts the survival of individuals regardless of the presence of comorbidities 75 . Dementia decreases survival after acute myocardial infarction, with greater renal decompensation, higher rate of acute lung edema, showing the delay in hospitalization and the lesser prescription of antiplatelet agents and beta-blockers at hospital discharge 77 . Dementia also predicted higher mortality in patients with heart failure 78 .
The frequent coexistence between Advanced Dementia and Frailty syndrome could be one of the ways to explain, at least partially, the worse prognosis of comorbidities among PWD 79 . As it is known, although the definition of frailty is still a matter of debate in the current medical literature, frail individuals, especially the elderly, have less functional reserve of physiological organs and systems and less capacity to face challenges and organic overload, having greater difficulty in maintaining homeostasis when faced with acute illnesses, interventions of any kind, diagnostic or therapeutic 80 .
The management of comorbidities in dementia, on the other hand, faces several difficulties regardless of what has been exposed so far: starting with making a diagnosis in these individuals, who, due to the progressive impairment of language, memory, and criticism the efficiency in reporting symptoms and complaints is lower, less valued or commented upon and dependent on a third party not always attentive or accurate (the caregiver). Adherence to medication and non-pharmacological guidelines is also erratic and variable, by patients and caregivers 69 ),( 81 . Moreover, once undiagnosed and addressed with an adequate care plan at an outpatient level, such illnesses become more complicated and patients end up being taken to emergency rooms, wards, and Intensive Care Units, often with treatments that are disproportionate to their condition. advanced disease 69 ),( 81 .
Thus, often, despite having a high number of comorbidities, patients with dementia are less likely to have their health problems diagnosed and treated, including those that may directly impact their functionality, such as auditory and visual sensory alterations 71 . Often, by the simple mention of the diagnosis of dementia, especially if advanced (albeit moderately), such individuals are deprived of beneficial proportional treatments due to discrimination since the diagnosis suggests that these patients would fail to obtain the benefit of a certain procedure, even though they might live long enough to experience the complications of the disease, thus sometimes imposing suffering which could have been avoided 72 ),( 82 .
The reduction in survival expectancy should limit the administration of treatments unable to improve patients’ symptoms or quality of life. Thus, clinical practice should rethink primary preventive treatments in light of the prognosis to avoid the unnecessary use of medications and polypharmacy and control diseases such as hypertension and diabetes mellitus, and dyslipidemia. Moreover, other secondary prevention measures must comply with current guidelines indicating less strict goals for older adults with advanced ages and those with lower survival expectations 83 .
Comorbidities of dementia
The more advanced stages of dementias, especially in its terminal stages, induce the increase of morbid conditions such as lung infections due to bronchial aspiration or other causes, immobility, dysphagia, urinary tract and skin infections, pressure injuries, falls, malnutrition, and various dental problems 84 .
Maintaining patients’ dignity, regardless of their cognitive status, treating and preventing reversible complications (considering individual and family values), and establishing, as early as possible, a plan to consistently care for these patients with the best technical-scientific knowledge, are the pillars to treat individuals with any diagnosis, including dementia, whatever their etiology, patients’ socioeconomic condition or stage 81 ),( 82 ),( 84 ),( 85 .
Cancer and dementia
Both cancer and dementia have increased prevalence with age and lead to very complex health care needs and worse outcomes in people suffering from these diseases than those without these comorbidities 86 . Estimates of the prevalence of the association between the two diseases vary in the literature. An estimate suggests that 7.5% of individuals over 75 years old live with both diagnoses 86 ),( 87 . People living with this dual diagnosis are less likely to receive screening, staging, curative treatment, and adequate pain management than cancer patients without dementia 86 ),(87. Moreover, they have late diagnoses, a lower survival rate after it, and a greater number of comorbidities than those living only with cancer or dementia 87 .
Oncology services poorly identify dementia and its patients often receive limited therapeutic options. Oncology teams feel insecure about managing these patients since dementia carries more complex decision-making. Finally, studies highlight the important role families play in promoting greater success in treating and managing cancer in patients with dementia 86 .
Cancer diagnosis and treatment for patients in very advanced stages of dementia refrain from administering screening and very invasive measures. Even so, clinical practice must establish a care plan to ensure quality at the end of life for people who have aged with these two diseases: pain management, prevention of avoidable complications, and family support.
Approach to pain in advanced dementia
Older adults with dementia shown a 32-53% estimated prevalence of pain, higher among those with diseases known to cause pain, such as osteoarthritis, fractures, peripheral arterial disease, and cancer. This prevalence can reach 83%¨in those living in ILPIs. No study has shown that dementia affects pain sensitivity; rather, what it alters is individuals’ ability to report what they are feeling 88 .
Research considers self-reports as the gold standard in pain diagnosis. Patients with dementia show an unacceptable underdiagnosis and undertreated pain 88 )-( 90 . Among institutionalized older adults, 25% of those who daily complained of pain had received no analgesics. Among those with hip fractures and dementia, a student found that the prescribed opioid was a third of the normal dose and, therefore, insufficient ( 89 ),( 91 .
Untreated pain manifests itself via secondary symptoms in patients with dementia, such as sleep disturbances, agitation, depression, weight loss, and decreased mobility 90 . Studies have shown that analgesics better control agitation in PWD and pain than neuroleptics, especially in moderate-advanced dementia. They also reduce aggressiveness and pain without worsening patients’ cognition 91 ),( 92 .
To optimize pain assessment in PWD and, therefore, its identification, a study developed a specific pain scale for severely demented patients, later validated for Brazilian Portuguese 90 . The instrument (PAINAD) evaluates five items: breathing; negative vocalizations; facial expressions; body language; and comfortability, observing the patient for five minutes in different daily situations: during rest, in pleasurable activities and moments of care; and 30 minutes after analgesic medication. Multidisciplinary teams can use it after training without impeding its complexity.
Thus, evaluating and treating pain in patients with advanced dementia is a challenge that clinical practice must face and, whenever suspected due to its manifestations, approach and treat it.
Oral health
People with dementia have the same oral problems as the general population. Good oral health positively influences individuals’ overall health, dignity, self-esteem, social integration, and nutrition. Studies show the effects of oral problems in patients with dementia with difficulty chewing due to missing dental elements and consequent refusal to eat, behavioral changes (such as withdrawal and aggressiveness due to pain - as previously mentioned), and other changes. The nature of dementia and its severity, social functioning, behavioral aspects, adherence to oral cavity care, and caregivers’ ability of caregivers to replace them in this care may compromise the conditions for maintaining oral health 93 ) (Table 5).
Table 5. The main changes seen in the oral cavity of patients with dementia are:
| • Poor hygiene |
| • Gingivitis with accumulation of bacterial plaque, calculi, and bleeding |
| • Caries |
| • Fractures with remaining roots and eventual infection |
| • Ulcers, gingival hyperplasia, and lack of taste due to psychotropic drugs often used to control symptoms in these patients. |
Overall health and comfort are closely linked to oral health in the terminal stages of neurodegenerative diseases 94 (Table 6). Oral diseases worsen general comfort, cause pain, affect cognition and behavior, and alter quality and life expectancy of people with dementia. The risk of aspiration pneumonia increases in the presence of oral factors such as poor hygiene, meager coronal and cervical teeth, periodontal disease, and the presence of microbes in saliva, whereas a clean and healthy oral cavity significantly reduces its occurrence 95 ),( 96 .
Table 6. Factors which negatively influence oral health 93 .
| • The severity of dementia |
| • Previous dental history - care and diseases |
| • Ability to receive / consent to oral hygiene care by caregivers or dental teams |
| • Knowledge of patients or their caregivers about the importance of oral health |
| • Lack of patient/caregiver motivation |
| • Impacts of medications on the oral cavity (xerostomia) |
| • Lack of information on how to access teams |
| • Degree of knowledge/training of oral health teams regarding dementia and aging |
| • Teams’ failure to develop strategies and long-term care plans. |
| • Scarcity of appropriate care facilities for small/medium dental surgeries, day centers, and at home. |
Caregivers play a central role in the oral health of patients with advanced dementia. The quality of hygiene care and the perception of problems which may arise depend almost exclusively on them. Studies have shown a poor understanding of the importance of oral health and its subsequent problems, such as patients’ (often aggressive) resistance further complicate the adherence of caregivers to these demands. Education, motivation, and the offer of strategies was associated with improved oral hygiene in patients with severe dementia 97 . Regular odonatological visits, caregivers’ education and access to care facilities in day centers, outpatient clinics, and home services are strategies which can ensure better oral health for these patients, benefitting their global health and quality of life increasing their expectations of survival.
NUTRITIONAL ISSUES IN ADVANCED DEMENTIA
Undernutrition in advanced dementia
As dementia advances into a severe stage, feeding difficulties become more common and bring about important problems such as undernutrition and weight loss, which are associated with more rapidly progressing cognitive impairment and increased mortality 98 . Therefore, all PWD should receive routine assessments of their nutritional problems. Several instruments can identify whether patients are undernourished, such as the Mini Nutritional Assessment (MNA) and the Malnutrition Universal Screening Tool (MUST) 99 ),( 100 . An unstructured evaluation is, however, also a useful option, particularly under limited time, common in Brazilian primary care consultations. These evaluations should include anthropometric measurements (weight, height, and body mass index) and questions about food and fluid intake, dietary habits, and adversative feeding behaviors. Laboratory tests are also helpful to better evaluate patients’ nutritional status, including full blood count, electrolytes, B12, urea, creatinine, glucose, albumin, and ferritin.
Individuals with advanced dementia may show adversative feeding behaviors that can importantly contribute to undernutrition, such as refusal to eat, wandering, and agitation 101 . Research has little evidence that interventions to modify mealtime environments can improve food and fluid intake for these patients 102 ),( 103 . However, given their favorable risk-benefit and the possibility of improving patients’ quality of life, employing such measures is reasonable 104 . Box 4 describes our suggested interventions.
Box 4. Measures to improve mealtime environment in advanced dementia.
| Refrain from rushing; good interaction between patients and caregivers increases food intake |
| Offer food patients like |
| If possible, the same caregiver should always be responsible for assisting patient’s meals |
| Try to set a pleasant, homelike environment with improved lighting and familiar music |
| Although it is useful to maintain a routine, it is advisable to wait until patients are calm before offering them food and fluids. |
| Use high-contrast colored tableware |
| Consider offering regular snacks and small meals |
Oral Nutritional Supplements (ONS) can increase macronutrient intake and avoid undernutrition in older adults, including persons with advanced dementia. As a meta-analysis shows, ONS can increase weight in persons with dementia, showing few gastrointestinal side effects. Note, however, that the literature lacks enough evidence to aclaimffirm that ONS decrease mortality or cognitive deterioration among persons with dementia 105 .
Dysphagia in advanced dementia
Oropharyngeal dysphagia is a serious and common problem in advanced dementia, as it may be an important cause of undernutrition and increase the risk of respiratory infections and death 106 . Early stages of dementia may show subtle aspirations and even go unnoticed by patients or caregivers 107 . Therefore, speech and language therapists should take part in managing PWD as early as possible, even in the absence of swallowing complaints. The most important instrument for diagnosing dysphagia on a day-to-day basis is the Clinical Swallow Evaluation (CSE). it includes both questionnaires on swallowing problems and a motor and sensory examination of all oral structures involved in bolus formation 108 ),( 109 . More specific situations (e.g., to diagnose more accurately aspiration) may require employing an instrumental assessment, such as videofluoroscopic swallow studies (VFSS) and fiberoptic endoscopic evaluation of swallowing (FEES) 108 )-( 110 .
The use of thickeners to change the consistency of fluids offered to patients with advanced dementia can help to mitigate the consequences of dysphagia. In 2018, a meta-analysis concluded that thickening the fluids offered to patients with advanced dementia may have an immediate positive effect on swallowing and may decrease the three-month incidence of pneumonia 111 . It noted, however, that long-term benefits are uncertain due to scarce evidence 111 . Research also lacks enough evidence to recommend a specific thickness (i.e., nectar- or honey-thick) over the other 112 . Adopting a chin-down posture while drinking liquids is another useful intervention in dysphagia and may be as effective as fluid thickening to decrease the incidence of pneumonia, especially in individuals with milder dysphagia 112 ),( 113 .
Tube feeding in advanced dementia
Using or withholding tube feeding remains one of the most controversial topics in the management of advanced dementia. Caregivers often misinterpret the recommendation to not insert a percutaneous endoscopic gastrostomy (PEG) as a recommendation to withdraw all types of care, which might compromise ongoing treatment and even harm the confidence in healthcare providers. Of note, such recommendations are based mostly on observational studies and not on randomized controlled trials, as these are unavailable due to ethical reasons 114 ),( 115 .
Most of the available evidence suggest that tube feeding fails to benefit patients with advanced dementia and may even harm them. The use of PEG tubes seems to fail to improve mortality in individuals with advanced dementia 116 )-( 118 , may lead to higher levels of discomfort 119 , and its complications are responsible for almost half of all emergency department visits among patients with advanced dementia 120 . Tube feeding also seems to increase the risk of pressure ulcers and are unhelpful to heal existing pressure ulcers 121 ),( 122 . Finally, enteral feeding with a PEG tube fails to decrease caregivers’ burden 121 and raise a perception of better end-of-life care among relatives of individuals with advanced dementia 123 . Current evidence still remains unclear, however, on whether such adverse outcomes are related to the late use of PEG tubes and if patients in less severe stages of advanced dementia could benefit from it ( 117 ),( 124 .
The decision to withhold tube feeding in advanced dementia is ethical and based on current scientific evidence but should not be viewed as a one-size-fits-all way to approach the matter. The discussion should include families and, whenever possible, patients, and consider their social, cultural, and religious values. In other words, the decision to use or withhold use PEG tube feeding must be individualized. Whenever tube feeding is contraindicated, assisted oral feeding is encouraged. The concept of “comfort feeding only” 125 ) is useful in such situations: it is based on feeding with comfort as its main goal, i.e., focusing on satisfaction and stopping whenever feeding is distressing.
END-OF-LIFE CARE IN DEMENTIA
People with advanced dementia may possibly include a great part of the patients living with dementia in Brazil. However, trustful estimates are still impossible due to the lack of epidemiological data and the profile of this population. Advanced dementia patients show a low income, associated with a high disease burden profile, often lacking the assistance of formal caregivers 126 . Delivery of care to PWD is essentially provided by family members in Brazil 127 , unlike European countries with a public health system, such as the United Kingdom 128 . Thus, unique characteristics of the Brazilian people and its continental-size health system demand adapted recommendations for appropriate end-of-life care of people with dementia.
End-of-life care, or palliative care, is a therapeutic strategy to maintain a person’s quality of life by relieving discomfort or stress in a life-limiting condition. This subtopic details a few steps to aid general practitioners and primary care physicians: (1) accurately identify the moment of defining end-of-life care, (2) plan next steps with patients and their family members, and (3) provide mental and physical assistance to patients with terminal illness.
Identifying the moment of end-of-life caring
Recognizing individuals with dementia in advanced disease stage is a cornerstone to provide adequate strategies. In Brazil, almost 80% of individuals with dementia are still undiagnosed 129 . A sensitive, accurate feeling that forgetfulness may not be solely associated with the aging process is the first step to provide proper care of patients with severe dementia. Ideally, the moment of discussing palliative care is as early as dementia is diagnosed, focusing on patients’ wishes. Family members are essentially involved in deliberating decisions when individuals no longer may take complex decisions 130 .
Primary care physicians should have a high suspicion of dementia even in patients without cognitive complaints 131 . Once they identify the cognitive decline, the next step is defining its stage. Clinical features vary in severe stage dementia, including mutism, impaired per os, and severe gait disturbances. Recurrent hospital admissions or demanding frequent medical assistance may be early signs of end-of-life disease, especially involving a recent diagnosis of cancer, heart failure or chronic obstructive pulmonary disease. Primary healthcare professionals should also pay attention to mental status of patients in home care appointments, as a great number of individuals were home assisted, lacking appropriate follow-up 132 .
Planning the approach to patients and their families
The Brazilian public healthcare system has a major infra-structure to provide an interdisciplinary approach to families with patients in the end-of-life dementia stage. Primary care facilities are strategically located close to the local community, which increases confidence and access to healthcare. However, most public facilities lack psychologists, physical therapists, speech therapists or social workers.
Once the diagnosis of end-of-life dementia is established, the next priority is to discuss an interdisciplinary approach to provide optimal assistance to loved ones. Only a minority of older adults have access to long-term care institutions 133 . Although interdisciplinary care is essential to mitigate end-of-life discomforts involving different healthcare professionals, Clinical practice should implement a broad care for those with access to private physical, occupational, psychological, and speech therapy.
For most patients with limited access to these services, we must discuss a few points. Firstly, family members must immediately increase awareness of end-of-life care. They should raise a community spirit, reach local policies, and increase their voice on a nationwide level. Philanthropic institutions such as “Associação Brasileira de Alzheimer” (ABraz) may provide information about local hospices or low-cost services, though they often have limited regional actions. All levels of care should also address spiritual needs, as sect leaders should aid planning at the time of diagnosis 134 .
Providing mental and physical assistance for patients (and caregivers)
Assisting people with terminal dementia should consider several levels of care 135 . The relatively weak healthcare structure for patients with terminal diseases compels families to establish a plan of care for persons with dementia. The whole primary care team should perform timely planning discussions, including stratifying care demanded by both patients (when cognitively able to take decisions) and caregivers 136 .
Ideally, they shall create a plan of care whenever patients receive a diagnosis of end-of-life dementia. They must assess patients’ understanding and judgement to evaluate their wishes; if impossible, this decision should involve their families or caregivers. Patients with end-of-life dementia benefit from care levels divided into five stages, ranging from critical care including invasive procedures to supportive care and comfort measures only 137 ),( 138 . Care level must consider patients’ wishes regarding invasive procedures in general, including orotracheal tube, central venous catheter, cardiopulmonary resuscitation, and ICU admission (Table 7). Caring should be individualized and performed together with families.
Table 7. Medical Orders for Scope of Treatment (MOST), in Portuguese, adapted to patients with end-of-life dementia stage. Staging must consider wishes to admission in a critical care unit, orotracheal intubation and cardiac resuscitation.
| Plan demanded by the patient | Medical record | Plan of care | Level of care |
|---|---|---|---|
| I want my life to be preserved using all measures of care available, when indicated | Full intensive care | ICU admission, including orotracheal intubation and CPR | Critical care: |
| I want my life to be preserved, except when my heart stops. I accept ICU admission and all measures, including intubation, except CPR if my heart stops. | Intensive care without CPR | ICU admission. Excluding: Defibrillation and CPR | - Attempt to support life with artificial measures in ICUs. |
| I want my life to be preserved, except when my heart stops. I accept ICU admission and all measures, except CPR if my heart stops or in case of intubation. | Intensive care without orotracheal intubation or CPR | ICU admission Non-invasive ventilation and high-flow O2 Excluding: Intubation, defibrillation, and CPR | - These orders in general are not used in case of natural end-of-life outcomes. |
| I want to be treated for reversible causes but without measures of artificial life support. If I show an irreversible condition, I want to receive the treatment available, except for invasive procedures (intubation or CPR). I want to receive this care in this ward. | Advanced ward care | Ward care, including treatment for reversible causes. Excluding: ICU admission, intubation or CPR | Ward care: |
| I understand death as a natural, expected event. I accept receiving care to decrease pain or other symptoms. I would like to receive this set of care whenever available. | Basic ward care | Exclusive symptoms management and comfort measures | - Support artificial measures will not be initiated if patients’ heart or breathing stops (i.e., intubation or CPR) |
Education for caregivers and family members is a fundamental point that must be thoroughly discussed. Instructions such as available sources of information about end-of-life dementia, where to ask for help, when to ask for assistance (or be taken to the ER), and what is expected and what is not. Assistant physicians may provide adequate end-of-life care, including adequate management of pain, agitation/aggression, risk of aspiration, and need for a feeding tube, dyspnea, and pneumonia 139 .
Caregivers’ mental health must also be addressed. Since they usually are a family member, they often show a psychological distress associated with the caregivers’ burden 140 ),( 141 . Caregiver assistance should focus on providing a better quality of life for the entire family. Avoiding psychological distress should be a priority, and we must suggest them to perform rotation shifts between caregivers (other family members or half-time support caregivers). Mental health hotlines have also been progressively implemented to assist those with psychological needs in Brazil 142 , especially after the COVID-19 pandemic 143 .
Thus, end-of-care dementia demands unique, individualized strategies which assistant physicians should recognize. Early identification, conjoint planning with families, and with level of care staging are cornerstones of adequate dementia care.
ETHICAL AND LEGAL ASPECTS
Countless ethical and legal issues arise and evolve regarding the stage and severity of neurodegenerative diseases. These disorders compromise the psychological well-being and behavior of patients, impacting their quality of life, and physically and emotionally harming their families. Dementias generate loss of autonomy and inability to make decisions, pillars of medical ethics which support the management of diagnostic or therapeutic medical approaches 144 .
In its advanced stage, PWD are unable to care for themselves 145 . Advance care planning (ACP) involves a dynamic process based on a dialogue between individuals, close ones, and healthcare providers and concern future preferences about their medical treatment. Although referring to advanced dementia and contemplating favorable intentions, they are unable to predict all possible scenarios for the most appropriate decision-making. Patients have their own characteristics; diseases have different courses, and the future is unpredictable 146 .
Over the past few years, several countries have implemented laws regulating care for the dying. Without a doubt, the issue is controversial, especially regarding shortening patients’ life; colorful debates address euthanasia, though very timidly in Brazil. In patients with severe dementia, the situation becomes more complex due to patients’ inability to make decisions and report their feelings and suffering 147 .
The literature on severe dementia is significantly reduced, containing few studies with reliable indicators and guidelines directing healthcare providers in this moment of innumerable doubts and uncertainties. The lack of scientific evidence predisposes the prescription of aggressive therapies without concern for their insignificance, especially in intercurrent diseases, such as the lack of results showing the effectiveness of artificial nutrition and hydration. Thus, to avoid procedures incompatible with the dignity of the human life, we need to advance concepts such as which prognostic criteria best fit survival, how to assess suffering and quality of life in this special population, and for how long the medications available could prolong life 148 .
In this sense, evoking the Hippocratic maxim of “primum non nocere,” i.e., above all do no harm, is always an appropriate judgment 145 .
It is essential to reflect on the development of technologies that prolong life. Clinical practice may falsely perceive that this has been accompanied by an improvement in the quality of life. Living longer fails to necessarily mean patients have had an adequate quality of life, as the opposite is more often the case 149 . Therefore, when questioned, people claim that living with quality of life is more relevant than living longer but the measures adopted in many assistance services may be incompatible with this desire. Living without a minimum of quality is unacceptable for a considerable portion of the cognitively competent population.
In severe dementia, cardiopulmonary resuscitation and other life support measures may seem futile and should be carefully evaluated according to the patients’ previous wishes. Refusal to eat and dysphagia are late manifestations and uncomfortable ethical dilemmas for doctors who care for older adults. The decision to insert food via an alternative route is complex and dependent on a multidimensional understanding 144 .
In the end, our approach must be proportional to patients’ needs and based on bioethical principles. A bioethical perspective that studies life not only from a biological point of view but also from a biographical one, with the maintenance of life as a right rather than obligation 149 .
Decision-making capacity
Ethically, screening and evaluating individuals for skills such as decision-making capacity (DMC) should be patient-centered and based on a functional assessment, rather than on expectations from an instrument or scale and its quantitative analyses. In dementia, substitute decision makers (SDM) are authorized to have frank conversations to predict and document patients’ desire prior to their disability. Even when a SDM is appointed to provide legally effective consent or refusal, this fails to render patients’ preferences ethically irrelevant 150 .
In accordance with the DMC issue, doctors must understand legal consequences, as they are responsible for patients and will always be asked, when necessary, to report information officially attesting the disease, as well as briefly describing patients’ conditions within expectations. One of these situations is the possibility of a guardianship procedure for individuals with dementia, depriving them of the legal capacity to make decisions and manage their assets. The Brazilian judicial determination of disability (a legal institution provided for in the legislation) finds only a small number of interdicted patients at an advanced stage of dementia. The main intention of the interdiction is to protect individuals with a legally significant disability. The Civil Procedure Code regulates these guardianship processes, “interdiction” in the Brazilian legal language. This means that Brazil faces a significant lack of legal responsibility in severe dementia, probably resulting in numerous inappropriate measures which fial to reflect patients’ real needs and interests 151 .
Topics to deal with ethical dilemmas in the advanced stages of chronic diseases such as dementias
The concept called “Jonsen’s 4-topic” is a practical approach and structure used by many ethics committees to resolve clinical ethical dilemmas. It includes a categorization into four similarly weighted quadrants composed of information, facts, and descriptions (Box 5) 144 ),( 145 ),( 151 ),( 152 .
Box 5. Bioethical topics.
| Medical indication | Patient preference |
|---|---|
| benefits X harms/favoring patients | principle of autonomy/honor and respect the patients’ wishes |
| It refers to the practice of doing good and benefiting others against acts which may be harmful, helping health professionals choose available treatments and examining how each alternative raises the possibility of success and favors patients. | It applies the ethical principle of autonomy and examines patients’ previously expressed or assumed beliefs and preferences. It aims to honor and respect patients’ wishes as much as possible within acceptable limits. In severe dementia, it would be supported by an advance directive, with the need for a SDM as a family member or friend to help with decision-making. In the absence of advanced directives, it is essential to request the presence of an SDM. This enables caregivers to determine how patients would like to be cared for if they were able to decide, whatever it may be. It would involve their morals, hopes, aspirations, values, principles, and spirituality. Therefore, sensitivity of those responsible is paramount, which is practically the current rule in Brazil. |
| Quality of life | Contextual features |
| Dignity/safety and comfort | socio-economic factors/political and religious preferences |
| The various treatments available should provide a better quality of life (QOL). Dimensioning QOL is complex and unique for everyone, even with the help of family members’ judgment, care must be taken not to prolong life with suffering. Dignity, safety, and comfort must prevail. | This last quadrant refers to the exacerbation of ethical dilemmas due to socio-economic, political, cultural, and religious factors that frequently appear in advanced dementia and are reflected in care and decision-making. |
In conclusion, the general approach to patients with advanced dementia is, as can be seen from the above, quite complex. The range of information, difficulties in diagnosis, peculiarities in treatments, and the mandatory inclusion of family members in decision-making impose extensive and frequent care to guarantee these individuals the best care based on the best evidence and, in the absence of such, the best experience and common sense available. To this end, we suggest that patients and their caregivers are evaluated very closely, at least every three or four months, to maintain their best possible functionality and maximum comfort, and that the following aspects receive attention. Respectful care, based on the best evidence, individual and family values, and the search to clarify any remaining doubts should guide the medical practice before patients and their loved ones.
ACKNOWLEDGEMENTS
PC, LCS and RN are funded by CNPq, Brazil (bolsa de produtividade em pesquisa).
Referências
- 1.Wimo A, Ljunggren G, Winblad B. Costs of dementia and dementia care: a review. Int J Geriatr Psychiatry. 1997;12(8):841–856. doi: 10.1002/(SICI)1099-1166(199708)12:8<841::AID-GPS652>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 2.Ferretti C, Sarti FM, Nitrini R, Ferreira FF, Brucki SMD. An assessment of direct and indirect costs of dementia in Brazil. PLoS ONE. 2018;13(3):1–15. doi: 10.1371/journal.pone.0193209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canadian Study of Health and Aging Study methods and prevalence of dementia. Can Med Assoc J. 1994;150:899–913. [PMC free article] [PubMed] [Google Scholar]
- 4.Schmitt FA, Wichems CH. A systematic review of assessment and treatment of moderate to severe Alzheimer's disease. J Clin Psychiatry. 2006;8(3):158–159. doi: 10.4088/pcc.v08n0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herrmann N, Gauthier S. Diagnosis and treatment of dementia: 6. Management of severe Alzheimer's disease. Can Med Assoc J. 2008;179(12):1279–1287. doi: 10.1503/cmaj.070804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldman HH, Woodward M. The staging and assessment of moderate to severe Alzheimer disease. Neurology. 2005;65(6suppl3):S10–S17. doi: 10.1212/WNL.65.6_suppl_3.S10. [DOI] [Google Scholar]
- 7.Volicer L. Management of Alzheimer's Disease and End-of-life issues. Clin Geriatr Med. 2001;17(2):377–391. doi: 10.1016/S0749-0690(05)70074-4. [DOI] [PubMed] [Google Scholar]
- 8.Voisin T, Vellas B. Diagnosis and treatment of patients with Severe Alzheimer's disease. Drugs Aging. 2009;26:135–144. doi: 10.2165/0002512-200926020-00005. [DOI] [PubMed] [Google Scholar]
- 9.Arrighi HM, Neumann PJ, Lieberburg IM, Townsend RJ. Lethality of Alzheimer's disease and its impact on nursing home placement. Alzheimer Dis Assoc Disord. 2010;24(1):90–95. doi: 10.1097/WAD.0b013e31819fe7d1. [DOI] [PubMed] [Google Scholar]
- 10.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 11.Chaves MLF, Camozzato AL, Godinho C, Kochhann R, Schuh A, Almeida VL. Validity of clinical dementia rating scale for the detection and stasging dementia in Brazilian patients. Alzheimer Dis Assoc Disord. 2007;21(3):210–217. doi: 10.1097/WAD.0b013e31811ff2b4. [DOI] [PubMed] [Google Scholar]
- 12.Galasko D, Schmitt F, Thomas R, Jin S, Bennett D, Ferris S. Detailed assessment of activities of daily living in moderate to severe Alzheimer's disease. J Int Neuropsychol Soc. 2005;11(4):446–453. doi: 10.1017/S1355617705050502. [DOI] [PubMed] [Google Scholar]
- 13.Reisberg B, Ferris SH, Leon MJ, Crook T. The Global deterioration scale (GDS) for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139(9):1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 14.Reiberg B. Functional Assessment Staging (FAST) Psychopharmacol Bull. 1988;24(4):653–659. [PubMed] [Google Scholar]
- 15.Saxton J, McGonigle-Gibson KL, Swihart AA, Miller VJ, Boller F. Assessment of the severely impaired patient: description and validation of a new neuropsychological test battery. Psychol Assess. 1990;2(3):298–303. doi: 10.1037/1040-3590.2.3.298. [DOI] [Google Scholar]
- 16.Harrel LE, Marson D, Chatterjee A, Parrish JA. The Severe Mini-Mental State Examination: a new neuropsychologic instrument for the bedside assessment of severely impaired patients with Alzheimer disease. Alzheimer Dis Assoc Disord. 2000;14(3):168–175. doi: 10.1097/00002093-200007000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Wajman JR. Bertolucci PHF: Comparison between neuropsychological evaluation instruments for severe dementia Arq. Neuropsiquiatr. 2006;64(3b):736–740. doi: 10.1590/S0004-282X2006000500007. [DOI] [PubMed] [Google Scholar]
- 18.Lyketsos CG, Steinberg M, Tschanz JT, Norton MC, Steffens DC, Breitner JC. Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry. 2000;157(5):708–714. doi: 10.1176/appi.ajp.157.5.708. [DOI] [PubMed] [Google Scholar]
- 19.Fossey J, Ballard C, Juszczak E, James I, Alder N, Jacoby R, Howard R. Effect of enhanced psychosocial care on antipsychotic use in nursing home residents with severe dementia cluster randomised trial. Br Med J. 2006;332(7544):756–761. doi: 10.1136/bmj.38782.575868.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selbaek G, Kirkevold Ø, Engedal K. The prevalence of psychiatric symptoms and behavioural disturbances and the use of psychotropic drugs in Norwegian nursing homes. Int J Geriatr Psychiatry. 2007;22(9):843–849. doi: 10.1002/gps.1749. [DOI] [PubMed] [Google Scholar]
- 21.Siafarikas N, Selbaek G, Fladby T, Šaltyt BJ, Auning E, Aarsland D. Frequency and subgroups of neuropsychiatric symptoms in mild cognitive impairment and different stages of dementia in Alzheimer’s disease. Int Psychogeriatr. 2018;30(1):103–113. doi: 10.1017/S1041610217001879. [DOI] [PubMed] [Google Scholar]
- 22.Cerejeira J, Lagarto L, Mukaetova-Ladinska EB. Behavioral and psychological symptoms of dementia. Front Neurol. 2012;3(73):1–21. doi: 10.3389/fneur.2012.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toot S, Swinson T, Devine M, Challis D, Orrell M. Causes of nursing home placement for older people with dementia: A systematic review and meta-analysis. Int Psychogeriatr. 2017;29(2):195–208. doi: 10.1017/S1041610216001654. [DOI] [PubMed] [Google Scholar]
- 24.Kamiya M, Sakurai T, Ogama N, Maki Y, Toba K. Factors associated with increased caregivers' burden in several cognitive stages of Alzheimer's disease. Geriatr Gerontol Int. 2014;14(52):45–55. doi: 10.1111/ggi.12260. [DOI] [PubMed] [Google Scholar]
- 25.Bränsvik V, Granvik E, Minthon L, Nordström P, Nägga K. Mortality in patients with behavioural and psychological symptoms of dementia: a registry-based study. Aging Ment Health. 2021;25(6):1101–1109. doi: 10.1080/13607863.2020.1727848. [DOI] [PubMed] [Google Scholar]
- 26.Brodaty H, Arasaratnam C. Meta-analysis of nonpharmacological interventions for neuropsychiatric symptoms of dementia. Am J Psychiatry. 2012;169(9):946–953. doi: 10.1176/appi.ajp.2012.11101529. [DOI] [PubMed] [Google Scholar]
- 27.Fink HA, Linskens EJ, MacDonald R, Silverman PC, McCarten JR, Talley KMC. Benefits and Harms of Prescription Drugs and Supplements for Treatment of Clinical Alzheimer-Type Dementia. Ann Intern Med. 2020;172(10):656–668. doi: 10.7326/M19-3887. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy RE, Cutter GR, Fowler ME, Schneider LS. Association of Concomitant Use of Cholinesterase Inhibitors or Memantine With Cognitive Decline in Alzheimer Clinical Trials: A Meta-analysis. JAMA Netw Open. 2018;1(7):1–10. doi: 10.1001/jamanetworkopen.2018.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen R, Chan PT, Chu H, Lin YC, Chang PC, Chen CY, Chou KR. Treatment effects between monotherapy of donepezil versus combination with memantine for Alzheimer disease: A meta-analysis. PLoS One. 2017;12(8):1–14. doi: 10.1371/journal.pone.0183586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Yu JT, Wang HF, Meng XF, Wang C, Tan CC, Tan L. Pharmacological treatment of neuropsychiatric symptoms in Alzheimer's disease a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2015;86(1):101–109. doi: 10.1136/jnnp-2014-308112. [DOI] [PubMed] [Google Scholar]
- 31.Trinh NH, Hoblyn J, Mohanty S, Yaffe K. Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer disease: a meta-analysis. JAMA. 2003;289(2):210–216. doi: 10.1001/jama.289.2.210. [DOI] [PubMed] [Google Scholar]
- 32.Porsteinsson AP, Drye LT, Pollock BG, Devanand DP, Frangakis C, Ismail Z. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682–691. doi: 10.1001/jama.2014.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henry G, Williamson D, Tampi RR. Efficacy and tolerability of antidepressants in the treatment of behavioral and psychological symptoms of dementia: a literature review of evidence. Am J Alzheimers Dis Other Demen. 2011;26(3):169–183. doi: 10.1177/1533317511402051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ehrhardt S, Porsteinsson AP, Munro CA, Rosenberg PB, Pollock BG, Devanand DP. Escitalopram for agitation in Alzheimer's disease (S-CitAD): Methods and design of an investigator-initiated, randomized, controlled, multicenter clinical trial. Alzheimers Dement. 2019;15(11):1427–1436. doi: 10.1016/j.jalz.2019.06.4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kongpakwattana K, Sawangjit R, Tawankanjanachot I, Bell JS, Hilmer SN, Chaiyakunapruk N. Pharmacological treatments for alleviating agitation in dementia: a systematic review and network meta-analysis. Br J Clin Pharmacol. 2018;84(7):1445–1456. doi: 10.1111/bcp.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yunusa I, Alsumali A, Garba AE, Regestein QR, Eguale T. Assessment of Reported Comparative Effectiveness and Safety of Atypical Antipsychotics in the Treatment of Behavioral and Psychological Symptoms of Dementia: A Network Meta-analysis. JAMA Netw Open. 2019;2(3):1–14. doi: 10.1001/jamanetworkopen.2019.0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruthirakuhan MT, Herrmann N, Abraham EH, Chan S, Lanctôt KL. Pharmacological interventions for apathy in Alzheimer's disease. Cochrane Database Syst Rev. 2018;5(5):CD012197–CD012197. doi: 10.1002/14651858.CD012197.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orgeta V, Tabet N, Nilforooshan R, Howard R. Efficacy of Antidepressants for Depression in Alzheimer's Disease: Systematic Review and Meta-Analysis. J Alzheimers Dis. 2017;58(3):725–733. doi: 10.3233/JAD-161247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cummings JL, Lyketsos CG, Peskind ER, Porsteinsson AP, Mintzer JE, Scharre DW. Effect of Dextromethorphan-Quinidine on Agitation in Patients With Alzheimer Disease Dementia: A Randomized Clinical Trial. JAMA. 2015;314(12):1242–1254. doi: 10.1001/jama.2015.10214. [DOI] [PubMed] [Google Scholar]
- 40.Wang LY, Shofer JB, Rohde K, Hart KL, Hoff DJ, McFall YH. Prazosin for the treatment of behavioral symptoms in patients with Alzheimer disease with agitation and aggression. Am J Geriatr Psychiatry. 2009;17(9):744–751. doi: 10.1097/JGP.0b013e3181ab8c61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ballard C, Youakim JM, Coate B, Stankovic S. Pimavanserin in Alzheimer's Disease Psychosis: Efficacy in Patients with More Pronounced Psychotic Symptoms. J Prev Alzheimers Dis. 2019;6(1):27–33. doi: 10.14283/jpad.2018.30. [DOI] [PubMed] [Google Scholar]
- 42.Kales HC, Lyketsos CG, Miller EM, Ballard C. Management of behavioral and psychological symptoms in people with Alzheimer's disease: an international Delphi consensus. Int Psychogeriatr. 2019;31(1):83–90. doi: 10.1017/S1041610218000534. [DOI] [PubMed] [Google Scholar]
- 43.Bessey LJ, Walaszek A. Management of Behavioral and Psychological Symptoms of Dementia. Curr Psychiatry Rep. 2019;21(8):66–66. doi: 10.1007/s11920-019-1049-5. [DOI] [PubMed] [Google Scholar]
- 44.Reese TR, Thiel DJ, Cocker KE. Behavioral Disorders in Dementia: Appropriate Nondrug Interventions and Antipsychotic Use. Am Fam Physician. 2016;94(4):276–282. [PubMed] [Google Scholar]
- 45.Vellas B, Gauthier S, Allain H, Andrieu S, Aquino J-P, Berrut G. Consensus sur la démence de type Alzheimer au stade severe. Presse Med. 2005;34(20):1545–1555. doi: 10.1016/S0755-4982(05)84221-6. [DOI] [PubMed] [Google Scholar]
- 46.Rongen S, Krammers C, O´mahony D, Feuth TB, Rikkert MGMO, Ahmed AIA. Potentially inappropriate prescribing in older patients admitted to psychiatric hospital. Int J Geriatr Psychiatry. 2016;31(2):137–145. doi: 10.1002/gps.4302. [DOI] [PubMed] [Google Scholar]
- 47.McGilton KS, Rochon E, Sidani S, Shaw A, Ben-David BM, Saragosa M. Can we help care providers communicate more effectively with persons having dementia living in long-term care homes? Am J Alzheimers Dis Other Demen. 2017 Feb;32(1):41–50. doi: 10.1177/1533317516680899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hickey JV. Neurological and Neurosurgical Nursing. 5. Philadelphia: Lippincott Williams & Wilkins; 2003. pp. 1–816. [Google Scholar]
- 49.New hope for advancing neuropalliative care. Lancet Neurol. 2021;20(6):409–409. doi: 10.1016/S1474-4422(21)00142-3. [DOI] [PubMed] [Google Scholar]
- 50.Tsolaki M, Papaliagkas V, Anogeianakis G, Bernabei R, Emre M, Frolich L. Consensus statement on dementia education and training in Europe. J Nutr Health Aging. 2010;14(2):131–135. doi: 10.1007/s12603-009-0238-z. [DOI] [PubMed] [Google Scholar]
- 51.Costa GD, Santos OG, Oliveira MAC. Knowledge, attitudes, and qualification needs of primary health care professionals in the care of dementia. Rev Bras Enferm. 2020;73(Suppl 3):1–9. doi: 10.1590/0034-7167-2020-0330. [DOI] [PubMed] [Google Scholar]
- 52.Keng A, Brown EE, Rostas A, Rajji TK, Pollock BG, Mulsant BH. Effectively Caring for Individuals with Behavioral and Psychological Symptoms of Dementia During the Covid-19 Pandemic. Front Psychiatry. 2020;11:1–9. doi: 10.3389/fpsyt.2020.573367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferretti C, Nitrini R, Brucki SMD. Virtual Support in Dementia: A possible viable strategy for caregivers. Front Neurol. 2021;12:1–7. doi: 10.3389/fneur.2021.662253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Egan KJ, Pinto-Bruno AC, Bighelli I, Berg-Weger M, van Straten A, Albanese E. Online Training Support Programs Designed to Improve Mental Health and Reduce Burden Among Caregivers of People With Dementia: A Systematic Review. J Am Med Dir Assoc. 2018;19(3):200–206. doi: 10.1016/j.jamda.2017.10.023. [DOI] [PubMed] [Google Scholar]
- 55.Reus VI, Forchtmann LJ, Eyler E, Hilty DM, Horvitz-Lennon M, Jibson MD. The American Psychiatric Association Practice Guideline on the Use of Antipsychotics to Treat Agitation or Psychosis in Patients With Dementia. Am J Psychiatry. 2016;173(5):543–546. doi: 10.1176/appi.ajp.2015.173501. [DOI] [PubMed] [Google Scholar]
- 56.National Institute for Health and Care Excellence . Dementia: A NICE.Suportting people with dementia and their carers.in health and social care. Leicester (UK): British Psychological Society; 2012. pp. 1–392. [Google Scholar]
- 57.International Psychogeriatric Association. Milwalkee (WI): IPA; 2015. [citado 23 ago. 2022]. Disponível em: https://www.ipa-online.org/ [Google Scholar]
- 58.American Geriatrics Society and American Association for Geriatric Psychiatry Consensus Statement on Improving the Quality of Mental Health Care in U S. Nursing homes: management of depression and behavioral symptoms associated with dementia. J Am Geriatr Soc. 2003;51(9):1287–1298. doi: 10.1046/j.1532-5415.2003.51415.x. [DOI] [PubMed] [Google Scholar]
- 59.American Medical Association. American Academy of Neurology Institute. American Psychiatric Association . Dementia management quality measurement set update. Chicago (IL): American Medical Association; 2018. [citado 23 ago. 2022]. Disponível em: https://www.aan.com/siteassets/home-page/policy-and-guidelines/quality/quality-measures/2018-dementia-management-measures.pdf . [Google Scholar]
- 60.Ismail Z, Black SE, Camicioli R, Chertkow H, Herrmann N, Laforce R. Recommendations of the 5th Canadian Consensus Conference on the diagnosis and treatment of dementia. Alzheimers Dement. 2020;16(8):1182–1195. doi: 10.1002/alz.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. Br Med J. 2015;350:1–16. doi: 10.1136/bmj.h369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brodaty H, Arasaratnam C. Meta-Analysis of Non-Pharmacological Interventions for Neuropsychiatric Symptoms of Dementia. Am J Psychiatry. 2012;169(9):946–953. doi: 10.1176/appi.ajp.2012.11101529. [DOI] [PubMed] [Google Scholar]
- 63.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S. Dementia prevention, intervention, and care: 2020 report of the Lancet Comission. Lancet. 2020;396:413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caramelli P, Bottino CMC. Tratando os sintomas comportamentais e psicológicos da demência (SCPD). Conferência Clínica. J Bras Psiquiatr. 2007;56(2):83–87. doi: 10.1590/S0047-20852007000200002. [DOI] [Google Scholar]
- 65.Eisenmann Y, Golla H, Schmidt H, Voltz R, Perrar KM. Palliative Care in Advanced Dementia. Front Psychiatry. 2020;11:1–13. doi: 10.3389/fpsyt.2020.00699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ferretti CEL. Koizume MS, Diccini S, compilers. In: Enfermagem em Neurociência: Fundamentos para a prática clínica. São Paulo (SP): Atheneu; 2006. Intervenções de Enfermagem nas Doenças Neurodegenerativas; pp. 1–672. [Google Scholar]
- 67.Bessey LJ, Walaszek A. Management of behavioral and psychological symptoms of dementia. Curr Psychiatry Rep. 2019;21(8):66–66. doi: 10.1007/s11920-019-1049-5. [DOI] [PubMed] [Google Scholar]
- 68.de Vugt ME, Stevens F, Aalten P, Lousberg R, Jaspers N, Winkens I. Do caregiver management strategies influence patient behavior? Int J Geriatr Psychiatry. 2004;19(1):85–92. doi: 10.1002/gps.1044. [DOI] [PubMed] [Google Scholar]
- 69.Abreu W, Tolson D, Jackson GA, Staines H, Costa N. The relationship between frailty, functional dependence, and healthcare needs among community-dwelling people with moderate to severe dementia. Health Soc Care Community. 2018;27(3):642–653. doi: 10.1111/hsc.12678. [DOI] [PubMed] [Google Scholar]
- 70.Aguero-Torres H, Fratiglioni L, Guo Z, Viitanen M, Winblad B. Prognostic Factors in Very Old Demented Adults: A Seven-Year Follow-Up From a Population-Based Survey in Stockholm. J Am Geriatr Soc. 1988;46(4):444–452. doi: 10.1111/j.1532-5415.1998.tb02464.x. [DOI] [PubMed] [Google Scholar]
- 71.Allen RS, Thorn BE, Fisher EF, Gerstle J, Quarles K, Bourgeois MS. Prescription and Dosage of Analgesic Medication in Relation to Resident Behaviors in the Nursing Home. J Am Geriatr Soc. 2003;51(4):534–538. doi: 10.1046/j.1532-5415.2003.51164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bauer K, Schwarzkopf L, Graessel E, Holle R. A claims data-based comparison of comorbidity in individual with and without dementia. BMC Geriatrics. 2014;14(10):1–13. doi: 10.1186/1471-2318-14-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bynum JP, Rabins PV, Weller W, Niefeld M, Anderson GF, Wu AW. The Relationship Between a Dementia Diagnosis, Chronic Ilness, Medical Expenditures and Hospital use. J Am Geriatr Soc. 2004;52(2):187–194. doi: 10.1111/j.1532-5415.2004.52054.x. [DOI] [PubMed] [Google Scholar]
- 74.Cintra MTG, Rezende NA, Torres HOG. Advanced Dementia in a sample of Brazilian elderly: Sociodemographic and morbidity analysis. Rev Assoc Med Bras. 2016;62(8):735–741. doi: 10.1590/1806-9282.62.08.735. [DOI] [PubMed] [Google Scholar]
- 75.Doraiswamy PM, Leon J, Cummings J, Marin D, Neumann P. Prevalence and Impact of Medical Comorbity in Alzheimer's Disease. J Gerontol A Biol Sci Med Sci. 2002;57(3):173–177. doi: 10.1093/gerona/57.3.m173. [DOI] [PubMed] [Google Scholar]
- 76.Downs M, Small N, Froggatt K. Burns A, Winblad B, compilers. In: Severe Dementia. New York: Wiley; 2007. Person-centred Care for People with Severe Dementia; pp. 1–260. [Google Scholar]
- 77.Fiske J, Griffiths J, Jamieson R, Manger D. Guidelines for oral health care for long-stay patients and residents. Gerodontology. 2008;17(1):55–64. doi: 10.1111/j.1741-2358.2000.00055.x. [DOI] [PubMed] [Google Scholar]
- 78.Fulton AT, Rhodes-Kropf J, Corcoran AM, Chau D, Castillo EH. Palliative Care for Patitents With Dementia in Long-Term Care. Clin Geriatr Med. 2011;27(2):153–170. doi: 10.1016/j.cger.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 79.Gauthier S, Patterson C, Chertkow H, Gordon M, Herrmann N, Rockood K, Rosa-Neto K, Soucy JP. 4th Canadian Consensus Conference on the Diagnosis and Treatment of Dementia. Can J Neurol Sci. 2012;39(6Suppl5):1–8. doi: 10.1017/s0317167100015183. [DOI] [PubMed] [Google Scholar]
- 80.Herrera E, Caramelli P, Silveira ASB, Nitrini R. Epidemiologic survey of Dementia in a community-dwelling Brazilian population. Alzheimer Dis Assoc Disord. 2002;16(2):103–108. doi: 10.1097/00002093-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 81.Herrman N. Gauthier S Diagnosis and treatment of dementia: 6 Management of severe Alzheimer disease. Can Med Am J. 2008;179(12):1279–1287. doi: 10.1503/cmaj.070804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hill JW, Futterman R, Duttagupta S, Mastey V, Lloyd JR, Fillit H. Alzheimer's disease and related dementias increase costs of comorbidities in managed Medicare. Neurology. 2002;58(1):62–70. doi: 10.1212/WNL.58.1.62. [DOI] [PubMed] [Google Scholar]
- 83.Horgas AL, Elliott A, Marsiske M. Pain Assessment in Persons with Dementia Relationship between Self-report and Behavioral Observation. J Am Geriatr Soc. 2009;57(1):126–132. doi: 10.1111/j.1532-5415.2008.02071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Husebo B, Ballard C, Sandvik R, Nilsen OB, Aarsland D. Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: cluster randomised clinical trial. Br Med J. 2011;343:1–11. doi: 10.1136/bmj.d4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hyland K, Fiske J, Matthews N. Nutritional and dental health management in Parkinson's disease. J Community Nurs. 2000;14(1):28–32. [Google Scholar]
- 86.James PA, Oparil S, Carter BL, William CC, Handler J, Lackland DT. Evidence-Based Guideline for the Management of High Blood Pressure in Adults - Report From the Panel Members Appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 87.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting Mortality Among Hospitalized Patients for Heart Failure - derivation and variation of a clinical model. JAMA. 2003;290(19):2581–2587. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 88.Lyketsos CG, Colenda CC, Beck C, Blank K, Doraiswamy MP, Kalunian DA. Position Statement of the American Association for Geriatric Psychiatry Regarding Principles of Care for Patients With Dementia Resulting From Alzheimer Disease. Am J Geriatr Psychiatry. 2006;14(7):561–573. doi: 10.1097/01.JGP.0000221334.65330.55. [DOI] [PubMed] [Google Scholar]
- 89.McWillimas L, Farrell C, Grande G, Keady J, Swarbrick C, Yorke J. A systematic review of the prevalence of comorbid cancer and dementia and its implication for cancer-related care. Aging Ment Health. 2017;22(10):1254–1271. doi: 10.1080/13607863.2017.1348476. [DOI] [PubMed] [Google Scholar]
- 90.Manchery N, Subbiah GK, Nagappan N, Premnath P. Are oral health education for carers effective in the oral hygiene management of elderly with dementia? A systematic review. Dent Res J. 2020;17(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- 91.Mitchell SL. Advanced Dementia. N Engl J Med. 2015;372:2533–2540. doi: 10.1056/NEJMcp1412652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morrison RS, Siu AL. A comparison of pain and its treatment in advanced dementia and cognitively intact patients with hip fracture. J Pain Symptom Manag. 2000;19(4):240–248. doi: 10.1016/S0885-3924(00)00113-5. [DOI] [PubMed] [Google Scholar]
- 93.Reisberg B, Wegiel J, Franssen E, Kadiyala S, Auer S, Souren L. Burns A, Winblad B, compilers. In: Severe Dementia. Hoboken: John Wiley & Sons Ltd; 2006. Clinical Features of Severe Dementia: Staging; pp. 1–260. [Google Scholar]
- 94.Schubert CC, Bustani M, Callahan CM, Perkins AJ, Carney CP, Fox C. Comorbidity Profile of Dementia Patients in Primary Care: Are They Sicker? J Am Geriatr Soc. 2006;54(1):104–109. doi: 10.1111/j.1532-5415.2005.00543.x. [DOI] [PubMed] [Google Scholar]
- 95.Sloan FA, Trogdon JG, Curtis LH, Schulman KA. The Effect of Dementia on Outcomes and Process of Care for Medicare Beneficiaries Admitted with Acute Myocardial Infarction. J Am Geriatr Soc. 2004;52(2):173–181. doi: 10.1111/j.1532-5415.2004.52052.x. [DOI] [PubMed] [Google Scholar]
- 96.Surr C, Griffiths AW, Kelley R, Ashley L, Cowdell F, Henry A. Navigating cancer treatment and care when living with comorbid dementia: an ethnographic study. Support Care Cancer. 2021;29:2571–2579. doi: 10.1007/s00520-020-05735-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Terpenning MS, Taylor GW, Lopatin DE, Kerr CK, Dominguez BL, Loesche WJ. Aspiration pneumonia: dental and oral risk factors in an older veteran population. J Am Geriatr Soc. 2001;49(5):557–589. doi: 10.1046/j.1532-5415.2001.49113.x. [DOI] [PubMed] [Google Scholar]
- 98.Fiske J, Frenkel H, Griffiths J, Jones V. British Society of Gerodontology. British Society for Disability and Oral Health Guidelines for the development of local standards of oral health care for people with dementia. Gerodontology. 2006;23(1):3–32. doi: 10.1111/j.1741-2358.2006.00140.x. [DOI] [PubMed] [Google Scholar]
- 99.van Dijk PTM, Dippell DWJ, Meulen JHP, Habbema JDF. Comorbidity and Its Effects on Mortality in Nursing Home Patients with Dementia. J Nerv Ment Dis. 1996;184(3):180–187. doi: 10.1097/00005053-199603000-00007. [DOI] [PubMed] [Google Scholar]
- 100.Volicer L, McKee A, Hewitt S. Dementia. Neurol Clin. 2001;19(4):867–885. doi: 10.1016/S0733-8619(05)70051-7. [DOI] [PubMed] [Google Scholar]
- 101.Warden V, Hurley AC, Volicer L. Development and Psychometric Evaluation of the Pain Assessment in Advanced Dementia (PAINAD) Scale J Am Med Dir Assoc. 2003;4:9–15. doi: 10.1097/01.JAM.0000043422.31640.F7. [DOI] [PubMed] [Google Scholar]
- 102.Wolf-Klein GP, Silverstone F, Brod MS, Levy A, Foley CJ, Termotto V, Breuer J. Are Alzheimer Patients Healthier? J Am Geriatr Soc. 1988;36(3):219–224. doi: 10.1111/j.1532-5415.1988.tb01804.x. [DOI] [PubMed] [Google Scholar]
- 103.Xue QI. The Frailty Syndrome: Definition and Natural History. Clin Geriatr Med. 2011;27(1):1–15. doi: 10.1016/j.cger.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhu CW, Consentino S, Ornstein KA, Gu Y, Andrews H, Stern Y. Interactive Effect of Dementia Severity and Comorbidities on Medical Expenditures. J Alzheimers Dis. 2017;57(1):301–315. doi: 10.3233/JAD-161077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.White H, Pieper C, Schmader K. The association of weight change in Alzheimer's disease with severity of disease and mortality: A longitudinal analysis. J Americ Geriatr Soc. 1998;46(10):1223–1227. doi: 10.1111/j.1532-5415.1998.tb04537.x. [DOI] [PubMed] [Google Scholar]
- 106.Hanson LC, Ersek M, Lin FC, Carey TS. Outcomes of feeding problems in advanced dementia in a nursing home population. J Am Geriatr Soc. 2013;61(10):1692–1697. doi: 10.1111/jgs.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rubenstein LZ, Harker JO, Salvà A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: Developing the short-form mini-nutritional assessment (MNA-SF) J Geront Series A Biolog Sci Med Sci. 2001;56(6):M366–M372. doi: 10.1093/gerona/56.6.m366. [DOI] [PubMed] [Google Scholar]
- 108.Stratton RJ, Hackston A, Longmore D, Dixon R, Price S, Stroud M. Malnutrition in hospital outpatients and inpatients: Prevalence, concurrent validity and ease of use of the "malnutrition universal screening tool" ('MUST') for adults. British J Nut. 2004;92(5):799–808. doi: 10.1079/bjn20041258. [DOI] [PubMed] [Google Scholar]
- 109.Volkert D, Chourdakis M, Faxen-Irving G, Frühwald T, Landi F, Suominen ESPEN guidelines on nutrition in dementia. Clin Nutr. 2015;34(6):1052–1073. doi: 10.1016/j.clnu.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 110.Herke M, Fink A, Langer G, Wustmann T, Watzke S, Hanff AM. Environmental and behavioural modifications for improving food and fluid intake in people with dementia. Cochrane Database Syst Rev. 2018;7(7):CD011542–CD011542. doi: 10.1002/14651858.CD011542.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu W, Cheon J, Thomas SA. Interventions on mealtime difficulties in older adults with dementia: A systematic review. Int J Nurs Studies. 2014;51(1):14–27. doi: 10.1016/j.ijnurstu.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 112.Abdelhamid A, Bunn D, Copley M, Cowap V, Dickinson A, Gray L. Effectiveness of interventions to directly support food and drink intake in people with dementia: Systematic review and meta-analysis. BMC Geriatrics. 2016;16(1):26–26. doi: 10.1186/s12877-016-0196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Prince M, Albanese E, Guerchet M, Prina M. Nutrition and dementia: A review of available research. Chertsey: Compass group; 2014. [Google Scholar]
- 114.Espinosa-Val MC, Martín-Martínez A, Graupera M, Arias O, Elvira A, Cabré M. Prevalence, Risk Factors, and Complications of Oropharyngeal Dysphagia in Older Patients with Dementia. Nutrients. 2020;12(3):1–15. doi: 10.3390/nu12030863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Özsürekci C, Arslan SS, Demir N, Çalikan H, engül Ayçiçek G, Kilinç HE. Timing of Dysphagia Screening in Alzheimer's Dementia. J Parenter Enteral Nutr. 2020;44(3):516–524. doi: 10.1002/jpen.1664. [DOI] [PubMed] [Google Scholar]
- 116.Alagiakrishnan K, Bhanji RA, Kurian M. Evaluation and management of oropharyngeal dysphagia in different types of dementia: A systematic review. Archives Gerontology Geriatrics. 2013;56(1):1–9. doi: 10.1016/j.archger.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 117.Boccardi V., Ruggiero C., Patriti A., Marano L. Diagnostic Assessment and Management of Dysphagia in Patients with Alzheimer's Disease. J Alzheimers Dis. 2016;50(4):947–955. doi: 10.3233/JAD-150931. [DOI] [PubMed] [Google Scholar]
- 118.Egan A, Andrews C, Lowit A. Dysphagia and mealtime difficulties in dementia: Speech and language therapists' practices and perspectives. Int J Lang Commun Disord. 2020;55(5):777–792. doi: 10.1111/1460-6984.12563. [DOI] [PubMed] [Google Scholar]
- 119.Flynn E, Smith CH, Walsh CD, Walshe M. Modifying the consistency of food and fluids for swallowing difficulties in dementia. Cochrane Database System Rev. 2018;9(9):CD011077–CD011077. doi: 10.1002/14651858.CD011077.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Robbins J, Gensler G, Hind J, Logemann JA, Lindblad AS, Brandt D. Comparison of 2 interventions for liquid aspiration on pneumonia incidence: A randomized trial. Ann Intern Med. 2008;148(7):509–518. doi: 10.7326/0003-4819-148-7-200804010-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Saconato M., Chiari B. M., Lederman H. M., Gonçalves M. I. R. Effectiveness of Chin-tuck Maneuver to Facilitate Swallowing in Neurologic Dysphagia. Int Arch Otorhinolaryngol. 2016;20(1):13–17. doi: 10.1055/s-0035-1564721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ijaopo EO, Ijaopo RO. Tube feeding in individuals with advanced dementia: a review of its burdens and perceived benefits. J Aging Res. 2019:7272067–7272067. doi: 10.1155/2019/7272067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Orlandoni P, Jukic Peladic N, Cherubini A. Enteral nutrition in advanced dementia: An unresolved dilemma in clinical practice. Eur Geriatr Med. 2020;11(2):191–194. doi: 10.1007/s41999-020-00292-4. [DOI] [PubMed] [Google Scholar]
- 124.Murphy LM, Lipman TO. Percutaneous endoscopic gastrostomy does not prolong survival in patients with dementia. Arch Intern Med. 2003;163(11):1351–1353. doi: 10.1001/archinte.163.11.1351. [DOI] [PubMed] [Google Scholar]
- 125.Ribeiro Salomon AL, Novaes MR. Outcomes of enteral nutrition for patients with advanced dementia: A systematic review. J Nutr Health Aging. 2015;19(2):169–177. doi: 10.1007/s12603-014-0517-1. [DOI] [PubMed] [Google Scholar]
- 126.Sanders DS, Carter MJ, D'Silva J, James G, Bolton RP, Bardhan KD. Survival analysis in percutaneous endoscopic gastrostomy feeding: A worse outcome in patients with dementia. Am J Gastroenterol. 2000;95(6):1472–1475. doi: 10.1111/j.1572-0241.2000.02079.x. [DOI] [PubMed] [Google Scholar]
- 127.Pasman HRW, Onwuteaka-Philipsen BD, Kriegsman DMW, Ooms ME, Ribbe MW. van der Wal G Discomfort in nursing home patients with severe dementia in whom artificial nutrition and hydration is forgone. Arch Intern Med. 2005;165(15):1729–1735. doi: 10.1001/archinte.165.15.1729. [DOI] [PubMed] [Google Scholar]
- 128.Givens JL, Selby K, Goldfeld KS, Mitchell SL. Hospital transfers of nursing home residents with advanced dementia. J Am Geriatr Soc. 2012;60(5):905–909. doi: 10.1111/j.1532-5415.2012.03919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cintra MTG, Rezende NA, Torres HO, Cintra MTG, Rezende NA, Torres OG. Advanced dementia in a sample of Brazilian elderly: Sociodemographic and morbidity analysis. Rev Assoc Méd Bras. 2016;62(8):735–741. doi: 10.1590/1806-9282.62.08.735. [DOI] [PubMed] [Google Scholar]
- 130.Teno JM, Gozalo P, Mitchell SL, Kuo S, Fulton AT, Mor V. Feeding Tubes and the Prevention or Healing of Pressure Ulcers. Arch Intern Med. 2012;172(9):697–701. doi: 10.1001/archinternmed.2012.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Teno JM, Mitchell SL, Kuo SK, Gozalo PL, Rhodes RL, Lima JC. Decision-making and outcomes of feeding tube insertion: A five-state study. J Am Geriatr Soc. 2011;59(5):881–886. doi: 10.1111/j.1532-5415.2011.03385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Goldberg LS, Altman KW. The role of gastrostomy tube placement in advanced dementia with dysphagia: A critical review. Clin Interv Aging. 2014;9:1733–1739. doi: 10.2147/CIA.S53153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Palecek EJ, Teno JM, Casarett DJ, Hanson LC, Rhodes RL, Mitchell SL. Comfort Feeding Only: A Proposal to Bring Clarity to Decision-Making Regarding Difficulty with Eating for Persons with Advanced Dementia. J Am Geriatr Soc. 2010;58(3):580–584. doi: 10.1111/j.1532-5415.2010.02740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Azevedo LVDS, Calandri IL, Slachevsky A, Graviotto HG, Vieira MCS, Andrade CB. Impact of Social Isolation on People with Dementia and Their Family Caregivers. J Alzheimers Dis. 2021;81(2):607–617. doi: 10.3233/JAD-201580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bamford C, Lee R, McLellan E, Poole M, Harrison-Dening K, Hughes J. What enables good end of life care for people with dementia? A multi-method qualitative study with key stakeholders. BMC Geriatr. 2018;18(1):302–302. doi: 10.1186/s12877-018-0983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Borelli WV, Augustin MC, Oliveira PBF, Reggiani LC, Bandeira-de-Mello RG, Schumacher-Schuh AF. Neuropsychiatric Symptoms in Patients with Dementia Associated with Increased Psychological Distress in Caregivers During the COVID-19 Pandemic. J Alzheimers Dis. 2021;80(4):1705–1712. doi: 10.3233/JAD-201513. [DOI] [PubMed] [Google Scholar]
- 137.Cintra MT, Rezende NA, Torres HO. Advanced dementia in a sample of Brazilian elderly: Sociodemographic and morbidity analysis. Rev Assoc Méd Bras. 2016;62(8):735–741. doi: 10.1590/1806-9282.62.08.735. [DOI] [PubMed] [Google Scholar]
- 138.Citko J, Moss AH, Carley M, Tolle S. The National POLST Paradigm Initiative. J Palliat Med. 2011;14(2):241–242. doi: 10.1089/jpm.2010.9730. [DOI] [PubMed] [Google Scholar]
- 139.Dezorzi LW, Raymundo MM, Goldim JR, Oliveira CAV. Spirituality in the continuing education of healthcare professionals: An approach to palliative care. Palliat Support Care. 2019;17(6):662–667. doi: 10.1017/S1478951519000117. [DOI] [PubMed] [Google Scholar]
- 140.Durgante H, Contreras ML, Backhouse T, Mavrodaris A, Ferreira MG, Paulo DLV. Challenges in dementia care: comparing key issues from Brazil and the United Kingdom. Dement Neuropsychol, 2020;14(3):216–222. doi: 10.1590/1980-57642020dn14-030003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Harzheim E, Gonçalves MR, Umpierre RN, Silva Siqueira AC, Katz N, Agostinho MR. Telehealth in Rio Grande do Sul, Brazil: Bridging the Gaps. Telemed E-Health. 2016;22(11):938–944. doi: 10.1089/tmj.2015.0210. [DOI] [PubMed] [Google Scholar]
- 142.Hickman SE, Keevern E, Hammes BJ. Use of the physician orders for life-sustaining treatment program in the clinical setting: a systematic review of the literature. J Am Geriatr Soc. 2015;63(2):341–350. doi: 10.1111/jgs.13248. [DOI] [PubMed] [Google Scholar]
- 143.Marcucci FCI, Cabrera MAS, Perilla AB, Brun MM, Barros EML, Martins VM, Rosenberg JP, Yates P. Identification and characteristics of patients with palliative care needs in Brazilian primary care. BMC Palliative Care. 2016;15(1):1–10. doi: 10.1186/s12904-016-0125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nakamura AE, Opaleye D, Tani G, Ferri CP. Dementia underdiagnosis in Brazil. Lancet, 2015;385(9966):418–419. doi: 10.1016/S0140-6736(15)60153-2. [DOI] [PubMed] [Google Scholar]
- 145.Nitrini R, Barbosa MT, Brucki SMD, Yassuda MS, Caramelli P. Current trends and challenges on dementia management and research in Latin America. J Global Health, 2020;10(1):1–10. doi: 10.7189/jogh.10.010362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sampson EL, Candy B, Davis S, Gola AB, Harrington J, King M. Living and dying with advanced dementia: A prospective cohort study of symptoms, service use and care at the end of life. Palliat Med, 2017;32(3):668–681. doi: 10.1177/0269216317726443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schafirovits-Morillo L, Suemoto CK. Severe dementia: A review on diagnoses, therapeutic management and ethical issues. Dement Neuropsychol, 2010;4(3):158–164. doi: 10.1590/S1980-57642010DN40300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Viana BM, Bicalho MAC, Moraes EN, Romano-Silva MA. Twenty-four-year demographic trends of a Brazilian long-term care institution for the aged. J Am Med Dir Assoc. 2015;16(2):174–174. doi: 10.1016/j.jamda.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 149.Wittmann-Vieira R, Goldim JR. Bioética e cuidados paliativos: tomada de decisões e qualidade de vida. Acta Paul Enferm. 2012;25(3):334–339. doi: 10.1590/S0103-21002012000300003. [DOI] [Google Scholar]
- 150.Low JA, Ho E. Managing ethical dilemmas in end-stage neurodegenerative diseases. Geriatrics. 2017;2(1):1–7. doi: 10.3390/geriatrics2010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Passmore MJ, Ho A, Gallagher R. Behavioral and psychological symptoms in moderate to severe Alzheimer's disease: a palliative care approach emphasizing recognition of personhood and preservation of dignity. J Alzheimers Dis. 2012;29(1):1–13. doi: 10.3233/JAD-2012-111424. [DOI] [PubMed] [Google Scholar]
- 152.Piers R, Albers G, Gilissen J, De Lepeleire J, Steyaert J, van Mechelen W. Advance care planning in dementia: recommendations for healthcare professionals. BMC Palliat Care. 2018;17(1):88–88. doi: 10.1186/s12904-018-0332-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cipriani G, Di Fiorino M. Euthanasia and other end of life in patients suffering from dementia. Leg Med. 2019;40:54–59. doi: 10.1016/j.legalmed.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 154.Congedo M, Causarano R, Alberti F, Bonito V, Borghi L, Colombi L. Ethical issues in end of life treatments for patients with dementia. Eur J Neurol. 2010;17:774–779. doi: 10.1111/j.1468-1331.2010.02991.x. [DOI] [PubMed] [Google Scholar]
- 155.Fontaneda AJD, Sainz MIL. Problemas éticos y legales en la demência severa. El derecho a morir en paz. Rev Esp Ger Gerontol. 2009;44(2):43–47. doi: 10.1016/j.regg.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 156.Allen W. Medical ethics issues in dementia and end of life. Curr Psychiatry Rep. 2020;22(6):31–31. doi: 10.1007/s11920-020-01150-7. [DOI] [PubMed] [Google Scholar]
- 157.Bello DVME, Schultz RR. Dementia and legal determination of capacity. Arq Neuro-Psiquiatr. 2017;75(6):349–353. doi: 10.1590/0004-282X20170061. [DOI] [PubMed] [Google Scholar]
- 158.Toh HJ, Low JA, Lim ZY, Lim Y, Siddiqui S, Tan L. Jonsen's four topics approach as a framework for clinical ethics consultation. Asian Bioeth. 2018;10(1):37–51. doi: 10.1007/s41649-018-0047-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


