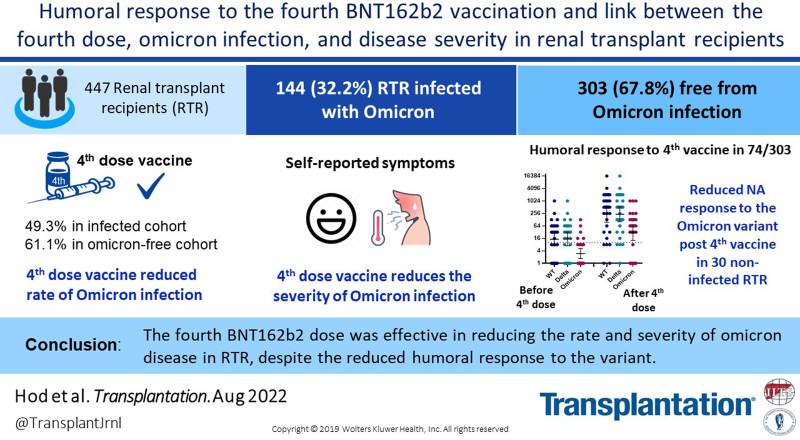
Background.
The effectiveness of the fourth BNT162b2 vaccination in reducing the rate and severity of coronavirus disease 2019 (COVID-19) caused by the Omicron variant in renal transplant recipients (RTRs) is unknown.
Methods.
Interviews were conducted with 447 RTRs regarding the status and timing of the fourth vaccination, prior vaccinations, and preceding COVID-19 infection. RTRs with polymerase chain reaction–confirmed COVID-19 infection from December 1, 2021, to the end of March 2022 were considered to have been infected with the Omicron variant and were interviewed to determine their disease severity. In a subgroup of 74 RTRs, the humoral response to the fourth dose was analyzed. In 30 RTRs, microneutralization assays were performed to reveal the humoral response to wild-type, Delta, and Omicron variant isolates before and after the fourth dose.
Results.
Of 447 RTRs, 144 (32.2%) were infected with the Omicron variant, with 71 (49.3%) of the infected RTRs having received the fourth vaccine dose. RTRs who did not receive the fourth dose before the infection had more serious illness. In a subgroup of 74 RTRs, the fourth dose elicited a positive humoral response in 94.6% (70/74), with a significant increase in geometric mean titer for receptor-binding domain immunoglobulin G and neutralizing antibodies (P < 0.001). The humoral responses to the Omicron variant before and after the fourth dose were significantly lower than the responses to the wild-type and the Delta variants.
Conclusions.
Overall, the fourth BNT162b2 dose was effective in reducing the rate and severity of Omicron disease in RTRs, despite the reduced humoral response to the variant.
INTRODUCTION
Following the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) waves of the Wuhan, Alpha, and then Delta variants, populations in many parts of the world had acquired immunity from previous infection and vaccination. The subsequently appearing Omicron variant nonetheless infected people having this acquired immunity, albeit with a significantly less severe clinical picture compared with that with the previous variants.1 Whether the clinical presentation observed upon infection with Omicron versus previous variants is indeed a result of preexisting host immunity or whether the variant is less virulent is still unknown. For example, in a recent study conducted in South Africa, which included 5144 patients from wave 4 and 11 609 patients from prior waves, severe coronavirus disease of 2019 (COVID-19) outcomes were indeed found to be reduced, but the intrinsically reduced virulence of the Omicron variant only accounted for about a 25% reduced risk of severe hospitalization or death compared with the Delta variant.2 Findings such as these are of particular concern to nonimmune or immunocompromised people with an impaired response to vaccination, who may be severely affected by the Omicron variant.
A few studies have reported a poor humoral response in renal transplant recipients (RTRs) after SARS-CoV-2 mRNA vaccinations.3-6 A study in the Czech Republic found that as many as 80% of RTRs failed to generate a positive humoral response after 2 doses of the BNT162b2 mRNA vaccine and that 16% of vaccinated kidney recipients became infected within 3 mo, with a high proportion of severe cases and significant mortality compared with the course of COVID-19 in unvaccinated RTRs.7 Similarly, we observed a humoral response rate of as little as 35% in 120 RTRs after 2 doses of the BNT162b2 mRNA vaccine.8 However, it was shown that administration of a third dose of an mRNA vaccine enhanced the immune response in immunocompromised patients.9 In our patients, 85.9% of RTRs developed neutralizing antibodies (NAs) after a third BNT162b2 dose.10 In immunocompetent populations in Israel, rates of confirmed COVID-19 infection and severe illness were substantially lower after a third dose than for individuals vaccinated with only 2 doses for adults aged ≥60 y11 and for younger healthcare workers.12
Following the success of the third vaccine and in light of the rapid spread of the Omicron variant and concerns regarding a significant increase in severe cases and hospitalizations, the State of Israel decided, on January 2, 2022, that a fourth dose of the BNT162b2 vaccine should be given to healthcare workers, adults aged >60 y, and immunosuppressed individuals for whom at least 4 mo had passed since the administration of the third vaccine dose.
In this study, we aimed to elucidate the response to the fourth vaccine dose (specifically, the response to the different SARS-CoV-2 variants, including the Omicron variant) and the correlation between the fourth vaccine dose and the rate and severity of morbidity because of the Omicron variant in RTRs. We also monitored the adverse events (AEs) after the fourth booster dose in our population.
MATERIALS AND METHODS
Study Population
The study cohort consisted of 447 RTRs followed up at the outpatient RTR clinic at the Sheba Medical Center. In the first part of the study, all 447 transplant recipients were interviewed by telephone after giving their consent to participate in the study. The questionnaire included questions regarding the status and timing of the fourth vaccination, prior vaccinations, and whether and when infected with SARS-CoV-2 (information was also collected for patients who had contracted the disease more than once). RTRs with confirmed COVID-19 (by a polymerase chain reaction [PCR]) from December 1, 2021, until the end of March 2022 were considered to have been infected with the Omicron variant because almost all sequenced infections during this period were Omicron validated. These patients were interviewed about the self-reported severity of their disease. Patients were asked whether they had been hospitalized during the disease course and whether they had experienced any of the symptoms listed in Table 4 (fever, chills, cough, etc). In addition, they were requested to rate their disease severity as mild, moderate, or severe and to report antiviral therapy (specifically molnupiravir and nirmatrelvir/ritonavir) given during the course of the disease.
TABLE 4.
Omicron infection self-reported disease severity assessed by questionnaire results in RTRs, stratified by preinfection fourth BNT162b2 vaccination status
| Variable, n (%) | Total cohort (N = 137) | Fourth vaccine administered (N = 68) | No fourth vaccine (N = 69) | P |
|---|---|---|---|---|
| Hospitalization | 7 (5.1) | 3 (4.4) | 4 (5.7) | 0.73 |
| Fever | 51 (37.2) | 20 (29.4) | 31 (44.9) | 0.06 |
| Chills | 45 (32.8) | 15 (22.1) | 30 (43.5) | 0.008** |
| Cough | 80 (58.4) | 34 (50) | 46 (66.7) | 0.048* |
| Sore throat | 46 (33.6) | 19 (27.9) | 27 (39.1) | 0.16 |
| Headache | 55 (40.1) | 23 (33.8) | 32 (46.4) | 0.13 |
| Fatigue/weakness | 78 (56.9) | 32 (47.1) | 46 (66.7) | 0.02* |
| Myalgia | 57 (41.6) | 23 (33.8) | 34 (49.3) | 0.06 |
| Nausea/vomiting | 12 (8.7) | 2 (2.9) | 10 (14.5) | 0.02* |
| Abdominal pain/diarrhea | 22 (16) | 5 (7.4) | 17 (24.6) | 0.006** |
| Back pain | 31 (22.6) | 12 (17.6) | 19 (27.5) | 0.17 |
| Loss of smell and taste | 18 (13.1) | 6 (8.8) | 12 (18.8) | 0.09 |
| N = 134 | N = 66 | N = 68 | ||
| Disease severity | ||||
| Mild | 86 (64.2) | 52 (78.8) | 37 (54.4) | 0.008** |
| Moderate | 21 (15.7) | 8 (12.1) | 13 (19.1) | |
| Severe | 24 (17.9) | 6 (9.1) | 18 (26.5) | |
| Received treatment | 31 (23.1) | 19 (28.8) | 12 (17.6) | 0.11 |
*P < 0.05; **P < 0.01.
RTR, renal transplant recipient.
In addition, we conducted a prospective study of 79 of 447 RTRs who had previously received 3 doses of the BNT162b2 vaccine and had subsequently been vaccinated with a homologous fourth dose of the vaccine. Patients with a positive SARS-CoV-2 PCR test before or after the full 3-dose vaccination were excluded from the study, as were patients vaccinated before transplant (given the stronger response to the BNT162b2 vaccine in patients vaccinated before kidney transplant). Five of these RTRs were subsequently excluded from the study because they were infected with COVID-19 before the post fourth vaccine serology assessment, leaving a cohort of 74 RTRs for further assessment. Vaccination was avoided during the first 3 mo after transplantation and during active treatment for rejection. On the day of the fourth vaccination, blood was drawn, before administration of the booster dose, for baseline serology assessment of receptor-binding domain (RBD) immunoglobulin (IgG) and NAs. Three to 4 wk after the fourth booster dose, testing for RBD IgG and NA was repeated to assess the humoral response to the vaccine.
For 56 of the subgroup of 74 RTRs, NA had been determined before and 3 wk after the third vaccine, which enabled a comparison between the antibody response to the fourth versus the third dose. In addition, to study the antibody response to the third vaccination over time, blood was drawn from those 56 patients 3 mo after the third vaccination. Furthermore, in 30 of 74 RTRs, blood was drawn on the day of the fourth vaccination and 3 wk after that vaccination for assessment of NA to 3 different SARS-CoV-2 variants (wild-type, Delta, and Omicron) with the aim to evaluate the humoral response to the different SARS-CoV-2 variants before and after the fourth vaccine dose. Written informed consent was obtained from all 79 participants. The protocol and informed consent were approved by our institutional review board (8314-21-SMC).
Immunosuppression
In our medical center, the standard maintenance immunosuppression regimen for RTRs comprises a calcineurin inhibitor (usually tacrolimus), an antimetabolite (usually a mycophenolate-based drug, mainly mycophenolic acid [MPA]), and prednisone, as described previously.10 For RTRs with a low immunological risk of rejection, early steroid withdrawal is implemented 5 to 8 d after transplant, and the maintenance regimen thus consists of tacrolimus and MPA. Conversion to a mammalian target of rapamycin inhibitor (sirolimus or everolimus) is instituted according to the patient’s risk of malignancy and lack of tolerance to calcineurin inhibitors.
Primary Outcome
For the retrospective study of 447 RTRs, the rate and severity of Omicron infection were evaluated in those who received the fourth vaccine versus those nonvaccinated with the fourth vaccine. For the prospective study of 74 RTRs, a positive response to the fourth booster dose of the BNT162b2 vaccine was defined as the presence of NA capable of reducing viral replication by at least 50% at a ≥16-fold dilution.
Data Extraction and Study Assessments
Patient information was obtained from the electronic patient records in the MDClone data acquisition system of the Sheba Medical Center, as described previously (Table 1).8 This system facilitated retrieval of relevant biochemical and clinical information, as described previously.8 For 10 patients, total daily mycophenolate dose was converted to the equivalent MPA dose by dividing the mycophenolate dose by 1.388. The use of cyclosporine, azathioprine, rapamycin, and everolimus on the day of the fourth vaccine was also retrieved from the MDClone system.
TABLE 1.
Demographic and clinical characteristics of RTRs, stratified by Omicron infection
| Variable | Total cohort (N = 447) | Noninfected patients (N = 303) | Infected patients (N = 144) | P |
|---|---|---|---|---|
| RTR characteristics | ||||
| Age, y, median (IQR) | 61.5 (50.5–70.4) | 63.2 (52.3–71.5) | 57.4 (47.5–67.8) | 0.0002** |
| Female sex, n (%) | 313 (70) | 218 (71.9) | 95 (66) | 0.19 |
| Time from transplant to December 1, 2021, y, median (IQR) | 4.6 (1.4–13.3) | 5.6 (1.7–13.5) | 3.5 (1.3–11.7) | 0.12 |
| ESRD cause, n (%) | ||||
| ADPKD | 70 (15.7) | 54 (17.8) | 16 (11.1) | 0.05 |
| Diabetic nephropathy | 74 (16.5) | 53 (17.5) | 21 (14.6) | |
| Glomerulonephritis | 132 (29.5) | 85 (28.1) | 47 (32.6) | |
| Nephrosclerosis | 55 (12.3) | 42 (13.9) | 13 (9) | |
| Other | 74 (16.5) | 41 (13.5) | 33 (22.9) | |
| Unknown | 42 (9.4) | 28 (9.2) | 14 (9.7) | |
| Dialysis pretransplant | 293 (65.5) | 193 (63.7) | 100 (69.4) | 0.44 |
| Transplant number, n (%) | ||||
| 1 | 424 (94.8) | 285 (94.1) | 139 (96.5) | 0.69 |
| 2 | 17 (3.8) | 13 (4.3) | 4 (2.8) | |
| 3 | 5 (1.1) | 4 (1.3) | 1 (0.7) | |
| 4 | 1 (0.2) | 1 (0.3) | 0 (0.0) | |
| BNT162b2 mRNA vaccination and COVID-19 infection | ||||
| COVID-19 infection in prior waves, n (%) | 28 (6.3) | 23 (7.6) | 5 (3.5) | 0.09 |
| Received fourth vaccination,n (%) | 256 (57.3) | 185 (61.1) | 71 (49.3) | 0.02* |
| Days from fourth vaccination to Omicron infection,median (IQR) | 33 (17.0–49.0) | 33 (17.0–49.0) | ||
| N = 392 | N = 270 | N = 122 | ||
| No. vaccines, n (%) | ||||
| 0 | 15 (3.8) | 12 (4.4) | 3 (2.5) | 0.16 |
| 1 | 6 (1.5) | 4 (1.5) | 2 (1.6) | |
| 2 | 19 (4.8) | 12 (4.4) | 7 (5.7) | |
| 3 | 96 (24.5) | 57 (21.1) | 39 (32) | |
| 4 | 256 (65.3) | 185 (68.5) | 71 (58.2) | |
*P < 0.05; **P < 0.01.
ADPKD, autosomal dominant polycystic kidney disease; COVID-19, coronavirus disease 2019; ESRD, end-stage renal disease; IQR, interquartile range; RTR, renal transplant recipient.
Patients were instructed to report (using a specific questionnaire) any systemic (fever, fatigue, headache, myalgia, chills, nausea/vomiting, paresthesia) and local (pain, redness, or swelling at the injection site) reactions occurring within 30 d after fourth vaccine dose and were actively screened for any other systemic and local complaints.
Antibody Detection Assays
RBD IgG was measured using the SARS-CoV-2 IgG II Quant (6S60, Abbott) test. These commercial tests were performed according to the manufacturer’s instructions. For determining NAs, a SARS-CoV-2 pseudovirus (psSARS-2) neutralization assay was performed13 using a propagation-competent vesicular stomatitis virus spike (kindly provided by Gert Zimmer, University of Bern, Switzerland).
Antibody Detection Assays for Different SARS-CoV-2 Variants
To enable a comparison of the humoral responses to different SARS-CoV-2 variants after the fourth vaccine dose, microneutralization assays with wild-type virus, B.1.617.2 (Delta), and Omicron variant isolates were performed on serum samples obtained from 30 RTRs immediately before and 3 wk after the fourth booster dose, as previously described.14
Statistical Analysis
Descriptive statistics were expressed as frequencies and percentages for categorical data and as median values with interquartile range (IQR) for continuous variables. All continuous variables were assessed for normality by the Kolmogorov-Smirnov test and log-transformed as appropriate. Differences in baseline characteristics between the groups were tested using the chi-square or Fisher exact test for the categorical variables or a t test for the continuous variables. To compare the humoral responses before and after the fourth vaccine dose, a paired t test and McNemar test were used.
Multivariable logistic regression analysis was used to identify factors associated with the vaccine-induced antibody response. The variables used in the multivariate analysis were those with a P value of <0.15 in the univariate analysis and those of clinical and biological relevance. Results are presented as odds ratio (OR), 95% confidence intervals (CIs), and P values.
The humoral responses to different SARS-CoV-2 variants before and after the fourth BNT162b2 mRNA vaccine in 30 RTRs were compared by a mixed model for repeated measures adjusted for multiple comparisons by the Tukey test.
All data analyses were performed with the SAS 9.4 software (Cary, NC). Scatter plots of log-transformed IgG and NA were obtained using GraphPad Prism 5.0 (GraphPad Software, Inc, San Diego, CA). A P value of <0.05 was considered as the cutoff for statistical significance.
RESULTS
Cohort Characteristics of 447 RTRs
Cohort characteristics, including the cause of end-stage renal disease, transplant number, dialysis pretransplant, and number of COVID-19 vaccines, are presented in Table 1. The median age was 61.5 y (IQR, 50.5–70.4); 313 (70%) were females; and the median time from transplant to December 1, 2021, was 4.6 y (IQR, 1.4–13.3). Of our RTR cohort, 28 patients (6.3%) had PCR-confirmed COVID-19 infection in waves that preceded the Omicron wave, and 256 patients (57.3%) received the fourth BNT162b2 dose.
Univariate and Multivariable Comparison of Noninfected Versus Omicron-infected RTRs
During the 4-mo period from December 1, 2021, until the end of March 2022, 144 of 447 RTRs (32.2%) fell ill with the Omicron infection. Infected patients were younger, with a median age of 57.4 y (IQR, 47.5–67.8), than noninfected RTRs, with a median age of 63.2 y (IQR, 52.3–71.5; P = 0.0002). Of the infected RTRs, 71 (49.3%) had received the fourth vaccine dose versus 185 (61.1%) noninfected RTRs who had been 4-dose vaccinated (P = 0.02; Table 1).
In a multivariable logistic regression analysis for Omicron infection in RTRs adjusted for age, gender, status of fourth vaccination before the infection, and COVID-19 infection in prior waves (Table 2), age ≤60 y increased the likelihood of Omicron infection by 83% compared with age >60 y (OR 1.83; 95% CI, 1.21-2.77; P = 0.04). In addition, the fourth vaccination and COVID-19 disease in preceding waves reduced the odds for contracting the infection by 37% and 67%, respectively (OR 0.63; 95% CI, 0.41-0.96; P = 0.03 and OR 0.33; 95% CI, 0.12-0.92; P = 0.03, respectively).
TABLE 2.
Multivariate logistic regression analysis for Omicron infection in RTRs
| Effect | Odds ratio (95% CI) | P |
|---|---|---|
| Age, ≤60 vs >60 y | 1.83 (1.21-2.77) | 0.004** |
| Gender, M vs F | 1.25 (0.81-1.94) | 0.31 |
| Fourth vaccine dose, yes vs no | 0.63 (0.41-0.96) | 0.03* |
| COVID-19 infection in prior waves, yes vs no | 0.33 (0.12-0.92) | 0.03* |
*P < 0.05; **P < 0.01
CI, confidence interval; COVID-19, coronavirus disease 2019; F, female; M, male; RTR, renal transplant recipient.
Univariate Comparison of RTRs Infected With the Omicron Variant Who Received the Fourth Vaccine Dose Versus Infected RTRs Who Did Not Receive the Fourth Dose
Of 144 RTRs infected with the Omicron variant, 71 (49.3%) had received the fourth dose. Infected patients who had received the fourth vaccine were older (median age 60.2 y; IQR, 47.3–70.6) than those who had not (median age 55.3 y; IQR, 47.6–64.4; P = 0.049). Of the female RTRs, 54 (76.1%) received the fourth vaccine versus 41 (56.2%) who did not (P = 0.01). The rate of COVID-19 infection in prior waves was higher in those who did not receive the fourth vaccine versus those who did (Table 3).
TABLE 3.
Demographic and clinical characteristics of RTRs infected with Omicron, stratified by preinfection fourth BNT162b2 vaccination status
| Variable | Total cohort (N = 144) | Fourth vaccine administered (N = 71) | No fourth vaccine (N = 73) | P |
|---|---|---|---|---|
| RTR characteristics | ||||
| Age, y, median (IQR) | 57.4 (47.5–67.8) | 60.2 (47.3–70.6) | 55.3 (47.6–64.4) | 0.049* |
| Female sex, n (%) | 95 (66) | 54 (76.1) | 41 (56.2) | 0.01* |
| Transplant to December 1, 2021, y,median (IQR) | 3.5 (1.3–11.7) | 3.8 (1.4–9.9) | 3.2 (0.9–13.4) | 0.49 |
| ESRD cause, n (%) | ||||
| ADPKD | 16 (11.1) | 9 (12.7) | 7 (9.6) | 0.91 |
| Diabetic nephropathy | 21 (14.6) | 10 (14.1) | 11 (15.1) | |
| Glomerulonephritis | 47 (32.6) | 23 (32.4) | 24 (32.9) | |
| Nephrosclerosis | 13 (9.03) | 8 (11.3) | 5 (6.8) | |
| Other | 33 (22.9) | 15 (21.1) | 18 (24.7) | |
| Unknown | 14 (9.7) | 6 (8.5) | 8 (11) | |
| Dialysis pretransplant, n (%) | 100 (69.4) | 51 (71.8) | 49 (67.1) | 0.38 |
| Transplant number, n (%) | ||||
| 1 | 139 (96.5) | 69 (97.2) | 70 (95.9) | 0.37 |
| 2 | 4 (2.8) | 1 (1.4) | 3 (4.1) | |
| 3 | 1 (0.7) | 1 (1.4) | 0 (0) | |
| COVID-19 infection in prior waves, n (%) | 5 (3.5) | 0 (0) | 5 (6.8) | 0.02* |
*P < 0.05; **P < 0.01.
ADPKD, autosomal dominant polycystic kidney disease; ESRD, end-stage renal disease; IQR, interquartile range; RTR, renal transplant recipient.
Univariate Comparison for Omicron Disease Severity Based on Questionnaire Results of RTR Infected With the Omicron Variant Who Received the Fourth Vaccine Dose Versus Infected RTRs Who Did Not
Of 137 RTRs infected with the Omicron variant who completed the questionnaire, 80 (58.4%) had a cough, 78 (56.9%) experienced fatigue or weakness, 57 (41.6%) had myalgia, 51 (37.2%) had fever, 46 (33.6%) complained of sore throat, and 45 (32.8%) had chills during the disease. RTRs who did not receive the fourth dose before the Omicron infection had higher rates of self-reported fever (44.9% not vaccinated versus 29.4% vaccinated, P = 0.06), chills (43.5% versus 22.1%; P = 0.008), cough (66.7% versus 50%; P = 0.048), fatigue or weakness (66.7% versus 47.1%; P = 0.02), myalgia (49.3% versus 33.8%; P = 0.06), nausea or vomiting (14.5% versus 2.9%; P = 0.02), and abdominal pain or diarrhea (24.6% versus 7.4%; P = 0.006) during the course of the disease. In addition, 18 (26.5%) of the nonvaccinated RTRs ranked their disease as severe compared with only 6 (9.1%) of those vaccinated before the infection, whereas 52 (78.8%) of vaccinated RTRs evaluated their disease as mild as opposed to only 37 (54.4%) of those not vaccinated (P = 0.008). A total of 31 RTRs (23.1%) received treatment with molnupiravir or nirmatrelvir/ritonavir during the disease, with no significant difference between vaccinated to nonvaccinated RTRs (Table 4).
Cohort Characteristics of a Subgroup of 74 RTRs Participating in a Prospective Study for Assessment of the Humoral Response to the Fourth BNT162b2 Vaccine Dose
Of 447 RTRs, 303 were not infected with the Omicron variant. In a subgroup of 74 of 303 noninfected RTRs, the humoral response before and after fourth vaccine was analyzed. For this subgroup, the median age was 60.2 y (IQR, 53.3–69.8); 24 (32.4%) were females; and median body mass index was 26.7 kg/m2 (IQR, 23.3–30.8). The median time from transplant was 3.1 y; 81.8% had received a living donor transplant; and 70.3% had undergone pretransplant dialysis. Past medical histories of hypertension (71.6%), diabetes (37.8%), ischemic heart disease (10.8%), and congestive heart failure (5.4%) were recorded for these patients (Table 5). Of these patients, 44.6% received the 3-drug immunosuppression regimen of tacrolimus-MPA-prednisone, whereas 14.9% were treated with the 2-dose protocol of tacrolimus and MPA (Table 6). Overall, 91.9% of these RTRs were treated with a calcineurin inhibitor (86.5% with tacrolimus and 5.4% with cyclosporine), 59.5% with MPA, and 71.6% with prednisone.
TABLE 5.
Demographic, clinical, and biochemical characteristics of RTRs, stratified by antibody response.
| Variable | Total cohort (N = 74) | Negative (N = 4) | Positive (N = 70) | P |
|---|---|---|---|---|
| RTR characteristics | ||||
| Age, y, median (IQR) | 60.2 (53.3–69.8) | 70 (62.9–78.4) | 59.8 (52.4–69.7) | 0.06 |
| Female sex, n (%) | 24 (32.4) | 1 (25) | 23 (32.9) | 0.74 |
| Transplant to fourth vaccine, y, median (IQR) | 3.1 (1.5–8.3) | 6.5 (3.1–11.4) | 3.1 (1.4–8.3) | 0.35 |
| Third to fourth vaccine date, d, median (IQR) | 173 (172–174) | 172 (146–175) | 173 (172–174) | 0.53 |
| Fourth vaccine to antibody testing date, d,median (IQR) | 21 (21–21) | 21 (21–21.5) | 21 (21–21) | 0.25 |
| ESRD cause, n (%) | ||||
| ADPKD | 9 (12.2) | 0 (0) | 9 (12.9) | 0.72 |
| DN | 16 (21.6) | 1 (25) | 15 (21.4) | |
| Glomerulonephritis | 19 (25.7) | 2 (50) | 17 (24.3) | |
| Nephrosclerosis | 11 (14.9) | 1 (25) | 10 (14.3) | |
| Other | 12 (16.2) | 0 (0) | 12 (17.1) | |
| Unknown | 7 (9.5) | 0 (0) | 7 (10) | |
| ESRD secondary to DN | 16 (21.6) | 1 (25) | 15 (21.4) | 0.87 |
| Dialysis pretransplant | 52 (70.3) | 1 (25) | 51 (72.8) | 0.14 |
| Transplant number, n (%) | ||||
| 1 | 69 (93.2) | 4 (100) | 65 (92.9) | 0.86 |
| 2 | 3 (4.1) | 0 (0) | 3 (4.3) | |
| 3 | 2 (2.7) | 0 (0) | 2 (2.86) | |
| Donor type, n (%) | ||||
| Living | 81 (81.8) | 3 (75) | 56 (80) | 0.81 |
| Deceased | 16 (16.2) | 1 (25) | 14 (20) | |
| Medical history | ||||
| Hypertension | 53 (71.6) | 2 (50) | 51 (72.9) | 0.32 |
| SBP 3-mo average, median (IQR) | 130.2 (121–148.6) | 159 (136.0–177.5) | 129 (119–144.7) | 0.047* |
| DBP 3-mo average, median (IQR) | 75.8 (71.0–80.0) | 77 (73.0–83.0) | 75.5 (70.3–80.0) | 0.57 |
| Ischemic heart disease | 8 (10.8) | 0 (0) | 8 (11.4) | 0.47 |
| Congestive heart failure | 4 (5.4) | 0 (0) | 4 (5.7) | 0.62 |
| Diabetes | 28 (37.8) | 2 (50) | 26 (37.1) | 0.61 |
| HbA1C 3-mo average, %, mean ± SD | 6.32 ± 1.01 | — | 6.32 ± 1.01 | |
| Weight, kg,median (IQR) | 80.3 (70–94) | 67 (63.5–81.6) | 80.5 (71–94.9) | 0.47 |
| BMI, kg/m2,median (IQR) | 26.7 (23.3–30.8) | 23.1 (19.3–30.1) | 26.9 (23.6–31) | 0.26 |
| Average laboratory results 1 mo before antibody testing day, median (IQR) | ||||
| White blood cells, K/μL | 7.1 (6.0–8.6) | 6.9 (4.9–7.3) | 7.2 (6.0–8.7) | 0.41 |
| Lymphocyte absolute, K/μL | 1.6 (1.3–2.0) | 1.5 (1.2–1.8) | 1.7 (1.3–2.1) | 0.57 |
| Neutrophils absolute, K/μL | 4.5 (3.6–5.5) | 4.5 (2.8–5.1) | 4.6 (3.6–5.5) | 0.61 |
| Hemoglobin, g/dL | 13.4 (12.2–14.2) | 13.9 (10.9–14.4) | 13.3 (12.2–14.2) | 0.85 |
| Platelets, K/μL | 186 (150–228) | 207 (78–224) | 183 (150–230) | 0.72 |
| Creatinine, mg/dL | 1.2 (1.0–1.4) | 2 (1.9–2.3) | 1.2 (1.0–1.3) | 0.006** |
| eGFR (CKD-EPI)a | 63.7 (50.8–78.7) | 34.2 (26.3–36.8) | 64.3 (51.0–80.7) | 0.008** |
| Glucose, mg/dL | 108 (98–142) | 108 (96–192) | 108.9 (98–142) | 0.79 |
| Albumin, g/dL | 4.1 (3.9–4.3) | 3.7 (3.6–3.8) | 4.1 (3.9–4.3) | 0.08 |
| Globulins, g/dL | 2.6 (2.5–2.8) | 2.6 (2.2–3.0) | 2.6 (2.5–2.8) | 0.82 |
| C-reactive protein, mg/L | 4.4 (1.7–10.8) | 2 (0.9–7.7) | 4.4 (1.7–10.9) | 0.39 |
*P < 0.05; ** P < 0.01.
eGFR was calculated according to the following CKD-EPI formula:
eGFR = 14 * min (Scr/k, 1)α * max(Scr/k, 1) – 1.209 * 0.993Age * 1.018 * 1.159 (if black)
(where Scr is standardized serum creatinine; k = 0.7 if female, 0.9 if male; α = –0.329 if female, –0.411 if male; min = the minimum of Scr/k of 1; max = the maximum of Scr/k or 1).
ADPKD, autosomal dominant polycystic kidney disease; BMI, body mass index; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; DBP, diastolic blood pressure; DN, diabetic nephropathy; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HbA1C, hemoglobin A1C; IQR, interquartile range; RTR, renal transplant recipients; SBP, systolic blood pressure.
TABLE 6.
RTR immunosuppression treatment on fourth vaccine day, stratified by antibody response
| Immunosuppressive therapy | Total cohort (N = 74) | Negative (N = 4) | Positive (N = 70) | P |
|---|---|---|---|---|
| Tacrolimus, n (%) | 67 (90.5) | 4 (100) | 63 (90) | 0.51 |
| Tacrolimus daily dose (mg) on fourth vaccine date, median, IQR | 2.5 (1.5–3.5) | 2.8 (2.3–3.0) | 2.3 (1.5–3.5) | 0.76 |
| Tacrolimus daily dose (mg) per weight (kg) on fourth vaccine day, median (IQR) | 0.03 (0.02–0.05) | 0.04 (0.03–0.05) | 0.03 (0.02–0.05) | 0.44 |
| Tacrolimus trough level, 1-mo average before fourth vaccine day, μg/L,median (IQR) | 6.9 (5.30–8.35) | 6.75 (3.30–7.10) | 6.9 (5.30–8.50) | 0.52 |
| MPA, n (%) | 50 (67.6) | 2 (50) | 48 (68.6) | 0.44 |
| MPA daily dose (mg) on fourth vaccine date, median (IQR) | 360 (0–720) | 180 (0–540) | 360 (0–720) | 0.35 |
| MPA daily dose (mg) per weight (kg) on fourth vaccine date, median (IQR) | 6 (0–8.37) | 2.57 (0–8.2) | 6.21 (0–8.37) | 0.61 |
| Prednisone, n (%) | 59 (79.7) | 4 (100) | 55 (78.6) | 0.29 |
| Prednisone daily dose (mg) on fourth vaccine date, median (IQR) | 5.0 (3.0–5.0) | 5.0 (5.0–7.5) | 5.0 (2.5–5.0) | 0.06 |
| Prednisone daily dose (mg) per weight (kg) on fourth vaccine date, median (IQR) | 0.05 (0.04–0.07) | 0.08 (0.06–0.12) | 0.05 (0.04–0.07) | 0.07 |
| Immunosuppressive regimen, n (%) | ||||
| Tacrolimus + MPA + prednisone | 33 (44.6) | 2 (50) | 31 (44.3) | 0.82 |
| Tacrolimus + MPA | 11 (14.9) | 0 (0) | 11 (15.7) | 0.39 |
| Tacrolimus + prednisone | 20 (27) | 2 (50) | 18 (25.7) | 0.28 |
| Cyclosporine | 4 (5.4) | 0 (0) | 4 (5.7) | 0.62 |
| Azathioprine | 3 (4) | 0 (0) | 3 (4.3) | 0.67 |
| mTORi (everolimus or sirolimus | 4 (5.4) | 0 (0) | 4 (5.7) | 0.62 |
IQR, interquartile range; MPA, mycophenolic acid; mTORi, mammalian target of rapamycin inhibitor; RTR, renal transplant recipient.
The median time from the fourth vaccine dose to antibody testing was 21 d (IQR, 21–21). Seventy RTRs (94.6%) had NAs ≥16 (positive-response group), and only 4 RTRs (5.4%) exhibited NAs <16 (negative-response group).
Univariate Comparison of Patients With a Positive Versus a Negative Response to the Fourth Vaccine Dose in the Subgroup of 74 RTRs
RTRs who responded to the fourth dose were younger, with a median age of 59.8 y (IQR, 52.4–69.7), as opposed to 70 y (IQR, 62.9–78.4) in nonresponders (P = 0.06). Average systolic blood pressure in the 3 mo before the fourth vaccination was lower in the responders than in the nonresponders (P = 0.047). Renal allograft function was significantly higher in the positive-response versus the negative-response patients (median estimated glomerular filtration rate of 64.3 mL/min, IQR [51–80.7] and 34.2 mL/min, IQR [26.3–36.8], respectively; P = 0.008). For all other demographic, clinical, and laboratory variables, the differences between the positive- and negative-response patients were not significant (Table 5). Given the small number of patients who did not respond to the vaccine, inferences cannot be made about the clinical significance of these findings (although statistically significant differences were found).
Different immunosuppressive medications and regimens, including the triple regimen containing MPA and the double regimen of tacrolimus and prednisone, were similar for the patients with positive and negative antibody responses (Table 6). The differences in the humoral response between the positive and negative responders to the fourth vaccine dose are shown in Table 7.
TABLE 7.
RBD IgG and NAs in RTRs before and after fourth vaccine, stratified by antibody response to the fourth dose
| Variable | Total cohort (N = 74) | Negative (N = 4) | Positive (N = 70) | P |
|---|---|---|---|---|
| Baseline immune status on fourth vaccine day | ||||
| Positive NAs on fourth vaccine day, n (%) | 58 (78.4) | 1 (25) | 57 (81.4) | 0.008** |
| IgG-RBD GMT on fourth vaccine day (95% CI) | 38.34 (20.8-70.8) | 1.77 (0.01-1029) | 45.72 (25.33-82.53) | 0.01* |
| NA GMT on fourth vaccine day, (95% CI) | 66.44 (41-107.7) | 6.73 (0.14-319.4) | 75.73 (49.96-122.1) | 0.02* |
| Response to fourth vaccine | ||||
| IgG-RBD GMT post fourth vaccine (95% CI) | 646.5 (360.6-1159) | 39.53 (0.02-103310) | 758.4 (440.9-1305) | 0.32 |
| NA GMT post fourth vaccine (95% CI) | 950.4 (550.3-1641) | 2.83 (0.94-8.52) | 1325 (833.1-2108) | <0.0001** |
*P < 0.05; **P < 0.001.
CI, confidence intervals; GMT, geometric mean titer; IgG, immunoglobulin; NA, neutralizing antibody; RBD, receptor-binding domain; RTR, renal transplant recipient.
For the 5 RTRs excluded from the study because they were infected with COVID-19 before the post fourth vaccine serology assessment, no significant differences in the humoral response were found between those infected before antibody testing and the other 74 members of the subgroup (Table 8).
TABLE 8.
Immune status before versus after the fourth vaccine dose in 5 RTRs who had COVID-19 infection before post fourth vaccine serology testing compared with the remainder of the cohort
| RTR infected with COVID-19 before post fourth vaccine serology testing (N = 5) | Remainder of cohort (N = 74) | P | |
|---|---|---|---|
| Before fourth vaccine | |||
| IgG-RBD GMT (95% CI) | 24.11 (0.41-1428) | 38.34 (20.8-70.8) | 0.71 |
| NA GMT (95% CI) | 64 (4.84-846.3) | 66.44 (41-107.7) | 0.97 |
| After fourth vaccine | |||
| IgG-RBD GMT (95% CI) | 878.1 (5.09-152E3) | 646.5 (360.6-1159) | 0.8 |
| NA GMT (95% CI) | 1463 (35.85-59731) | 950.4 (550.3-1641) | 0.69 |
CI, confidence interval; COVID-19, coronavirus disease 2019; GMT, geometric mean titer; IgG, immunoglobulin G; NA, neutralizing antibody; RBD, receptor-binding domain; RTR, renal transplant recipient.
Response to the Third Vaccine Dose Versus the Fourth Dose of the BNT162b2 mRNA Vaccine in RTRs
The positive-response rate based on NA titers increased from 78.4% immediately before the fourth vaccine dose to 94.6% (70/74) 3 wk (median 21 d, IQR [21–21]) after the fourth dose. Geometric mean titers (GMT) for RBD IgG and for NA increased from 38.34 (95% CI, 20.8-70.8) and 66.44 (95% CI, 41-107.7) on the day of the fourth vaccine, respectively, to 646.5 (95% CI, 360.6-1159) and 950.4 (95% CI, 550.3-1641), respectively, after the vaccine (P < 0.001). Both the rate and the intensity of response to the fourth dose were significantly higher than the values before the fourth dose (Table 7).
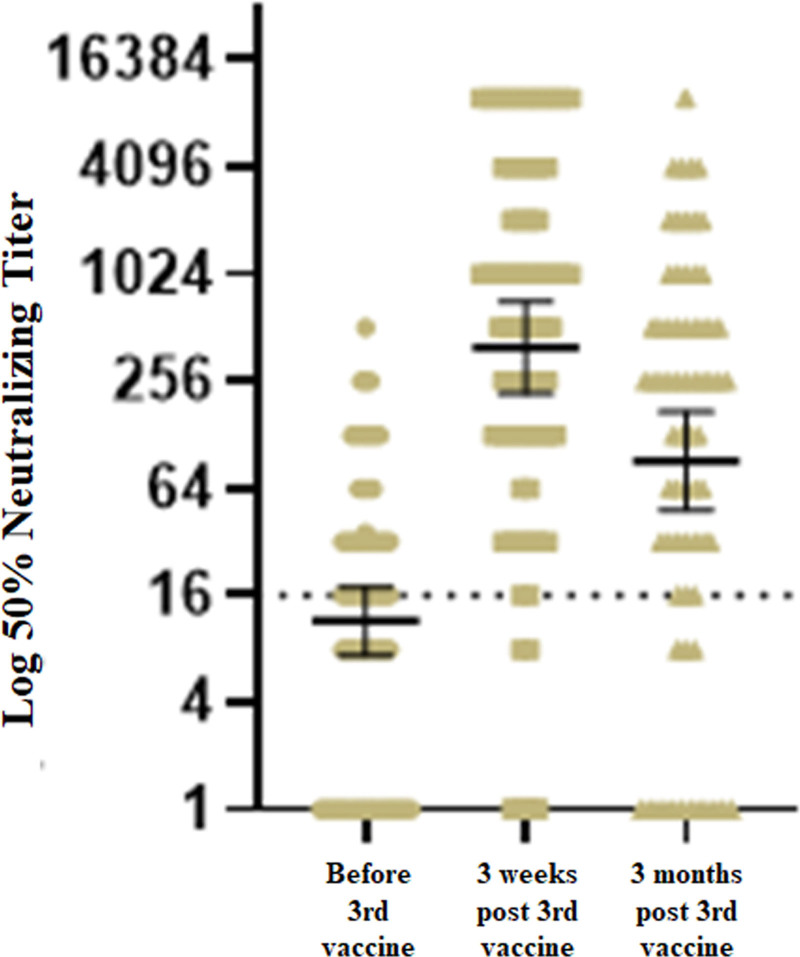
For 56 of 74 patients, we had assessed NAs before and 3 wk (median 21 d, IQR [21–21]) after the third dose. In those 56 RTRs, a positive-response rate, based on NA titers, increased from 58.9% (31/56) before the third vaccine to 91.1% (51/56) after the third dose. Three months after the third vaccine, we observed a decrease in GMT for NAs, as shown in Figure 1. The intensity of the response to the fourth dose was significantly higher than that observed after the third vaccine (GMT for NAs post third dose of 573.5 [95% CI, 299.9-1097] versus 1119 [95% CI, 627.1-1997] after the fourth dose, P = 0.0024).
FIGURE 1.
Neutralizing antibody levels before the third vaccine, and 3 wk and 3 mo after the third vaccine.
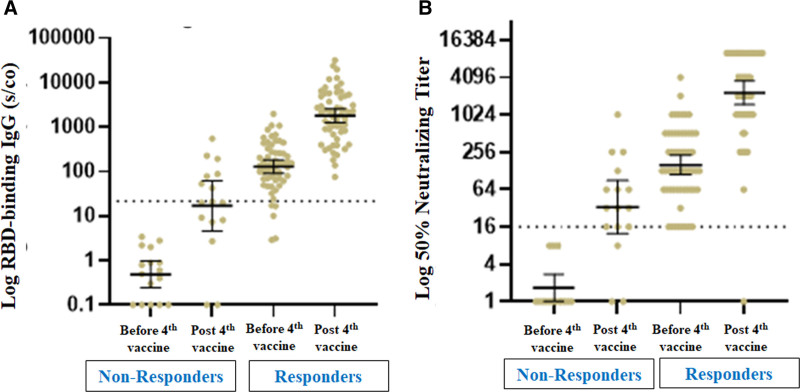
Of the 58 recipients with a positive humoral response before the fourth dose of the vaccine, 57 (98.3%) remained positive after the fourth dose, with a significant increase in GMTs for RBD IgG and NAs. Sixteen patients (21.6%) had a blunted antibody response before the fourth vaccine (GMTs for RBD IgG and NAs of 0.49 [95% CI, 0.24-0.97] and 1.68 [95% CI, 1.02-2.76], respectively); among those, 13 (81.3%) exhibited a positive antibody response after the fourth dose, with a significant increase in GMTs for RBD IgG and NAs (Figure 2).
FIGURE 2.
Antibody response before and after the fourth vaccine dose in RTRs with positive vs negative humoral responses before the fourth vaccine dose. A, GM of RBD IgG antibody levels. B, Neutralizing antibody titers. GM, geometrical mean; IgG, immunoglobulin G; RBD, receptor-binding domain; RTR, renal transplant recipient.
AEs in the Subgroup of 74 RTRs
AEs were common (82.4% of the subgroup) after administration of the fourth dose of the BNT162b2 vaccine. Local and systemic AEs were reported in 75.7% and 37.8% of the cohort, respectively, with 56 (75.7%) recipients experiencing pain at the injection site as the most frequent local adverse event. Systemic AEs, mainly fatigue, were reported for 20 RTRs (27%), with all other systemic AEs being experienced only by the positive responders. Recipients with a positive humoral response after the fourth dose were more likely to experience local and systemic AEs than nonresponders, but because of the low number of nonresponders, a statistically significant difference could not be detected (Table 9). No episodes of rejection were observed, and renal allograft function remained stable at a mean follow-up of 60 d after the third vaccine dose. No allergic responses were documented.
TABLE 9.
Local and systemic AEs in RTRs reported after the fourth dose of BNT162b2 vaccine, stratified by antibody response
| AEs | Total cohort (N = 74) | Negative (N = 4) | Positive (N = 70) | P |
|---|---|---|---|---|
| Local AEs, n (%) | ||||
| Pain at injection site | 56 (75.7) | 2 (50) | 54 (77.1) | 0.22 |
| Swelling | 7 (9.4) | 0 (0) | 7 (10) | 0.5 |
| Redness | 7 (6.8) | 0 (0) | 5 (7.1) | 0.58 |
| Systemic AEs, n (%) | ||||
| Fever | 3 (4.1) | 0 (0) | 3 (4.3) | 0.67 |
| Fatigue | 20 (27) | 1 (25) | 19 (27.1) | 0.92 |
| Headache | 8 (10.8) | 0 (0) | 8 (11.4) | 0.47 |
| Myalgia | 13 (17.6) | 0 (0) | 13 (18.6) | 0.34 |
| Chills | 4 (5.4) | 0 (0) | 4 (5.7) | 0.62 |
| Nausea/vomiting | 2 (2.7) | 0 (0) | 2 (2.9) | 0.73 |
| Paresthesia | 0 (0) | 0 (0) | 0 (0) | |
| Any local AE | 56 (75.7) | 2 (50) | 54 (77.1) | 0.22 |
| Any systemic AE | 28 (37.8) | 1 (25) | 27 (38.6) | 0.59 |
| Any AE | 61 (82.4) | 2 (50) | 59 (84.3) | 0.08 |
AE, adverse event; RTR, renal transplant recipient.
Humoral Response to Different SARS-CoV-2 Variants Before and After the Fourth BNT162b2 mRNA Vaccine in 30 RTRs
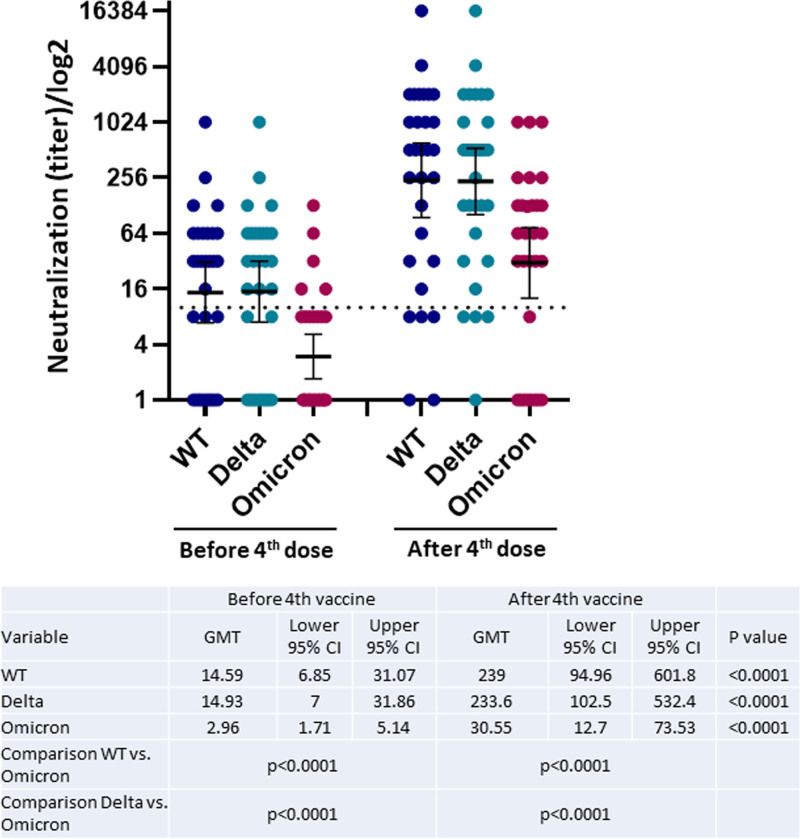
The humoral response to the Omicron variant before the fourth vaccine dose was significantly reduced compared with the response to the wild-type virus and the Delta variant (GMT 2.96; 95% CI, 1.71-5.14 versus GMT 14.59; 95% CI, 6.85-31.07 and GMT 14.93; 95% CI, 7-31.86, respectively; P < 0.001). The antibody titer to the Omicron variant (GMT 30.55; 95% CI, 12.7-73.53) 3 wk after the fourth vaccine dose was also significantly reduced compared with the increases for the wild-type virus (239; 95% CI, 94.96-601.8) and the Delta variant (233.6; 95% CI, 102.5-532.4; P < 0.001; Figure 3).
FIGURE 3.
Neutralizing antibody response before and after the fourth vaccine dose to the WT virus and the Delta and Omicron variants of SARS-CoV-2 in RTRs. CI, confidence interval; GMT, geometric mean titer; RTR, renal transplant recipient; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WT, wild type.
DISCUSSION
At the height of the Omicron wave of the epidemic, 144 of 447 RTRs (32.3%) in our study had contracted the infection, with 71 of the 144 (49.3%) of those infected with Omicron having received the fourth vaccine dose before the infection. Yet, a comparison of self-reported disease severity between nonvaccinated RTRs and those vaccinated with the fourth BNT162b2 dose before the infection showed that having received a fourth dose was associated with markedly reduced self-reported disease severity, both as assessed by self-report of the presence or absence of symptoms listed on a questionnaire and also by subjective report of mild/moderate/severe disease.
Of the 74 RTRs tested for the humoral response after the fourth vaccine dose, 70 (94.6%) responded to the vaccine. The rate of response increased from 78.4% before the fourth vaccine dose to 94.6% postvaccine, with a significant increase in RBD IgG and NA titers. In fact, the intensity of NA response to the vaccine was significantly stronger after the fourth dose than that observed after the third dose in a subgroup of 56 RTR. Given the high rate of Omicron infection after the fourth vaccination dose, we analyzed the humoral response to 3 different SARS-CoV-2 variants before and after the fourth dose. The antibody response to the Omicron variant was markedly lower than the responses to the wild-type virus and to the Delta variant before and after the fourth vaccine.
To the best of our knowledge, this is the first study showing the effect of the fourth BNT162b2 vaccine on the rate and severity of Omicron infection in a large cohort of RTRs. In addition, we demonstrated a strong humoral response to the fourth vaccine in a subgroup of 74 RTRs. However, the antibody response before and after the fourth vaccine dose differed significantly when tested against different SARS-CoV-2 variants, showing unequivocally a reduced humoral response to the Omicron variant. Our findings thus provide an explanation for the high rate of breakthrough Omicron infection after the third and fourth BNT162b2 vaccine doses in RTRs. Nonetheless, we have clearly shown that the fourth vaccine dose was associated with significantly reduced self-reported severity of Omicron disease in our RTR population.
In line with our data, a small cohort of 18 solid organ transplant recipients developed high antibody titers after a fourth injection to negative and positive responders to 3 doses of the vaccine.15 In another study, 43% of 49 RTRs with a negative serology after the third injection seroconverted after the fourth mRNA vaccination, although the response remained weak and was probably not protective against COVID-19.16 In a different study, a fourth dose of mRNA vaccine given to 92 RTRs with low IgG titers 1 mo after the third dose elicited a satisfactory antibody response in 48% and 52% of those who received the BNT162b2 and the mRNA-1273 vaccines, respectively.17 Moreover, a fourth mRNA-1273 dose significantly increased the neutralizing response against the Delta variant, with the rate of NA rising from 16% to 66%.18
Taken together, the data indicate that a fourth vaccine dose is beneficial in mounting an antibody response in transplant recipients. It is thought that the higher antibody titers detected post–COVID-19 infection versus postvaccination19 can be attributed to the higher antigen dose exposure with infection versus vaccination. Previous publications have reported that stronger immune stimuli with additional or higher vaccine doses are required to generate better immunogenicity in immunocompromised patients.20,21 Similarly, it can be assumed that additional doses of COVID-19 vaccine in RTRs increase the antigen load exposure, thereby stimulating a stronger humoral response.
Interestingly, an age >60 versus <60 y was protective of Omicron infection in multivariable analysis. This is most probably related to older patients being more careful about social distancing and masking. Among 144 RTRs infected with the Omicron variant, those who received the fourth vaccine were older, indicating that older patients are also more watchful about getting vaccinated.
Our data indicate a substantially reduced NA response to the Omicron variant after the third and fourth BNT162b2 vaccine doses, with a concomitant high rate of Omicron infection in vaccinated RTRs. Nonetheless, we also demonstrated robust protection against severe Omicron disease provided by the fourth vaccine dose. Similarly, in a study estimating the rate of confirmed Omicron infection and severe disease in >1 million participants >60 y, a fourth dose provided added short-term protection against confirmed infections and severe illness compared with 3 vaccine doses given at least 4 mo earlier. Interestingly, protection from confirmed Omicron infection waned rapidly from a peak in the fourth week after vaccination, whereas protection against severe illness had not decreased by the sixth week after the fourth dose.11 In addition, BNT162b2 vaccine was shown to provide 70% protection against hospitalization with Omicron in South Africa.22
Because the multiple spike (S) protein mutations in the Omicron variant contribute to reduced vaccine-elicited antibody neutralization23-26 and reduced protection from infection—as reflected in the attenuated humoral response to the Omicron variant shown in our study—it seems likely that cellular immunity plays an important role in ameliorating the severity of COVID-19 caused by the Omicron variant. It has been shown that the BNT162b2 vaccine stimulated extensive crossreactive cellular immunity, manifested in specific CD8+ and CD4+ T-cell responses against SARS-CoV-2 variants, including the Omicron variant, with minimal escape at the T-cell level.26,27 Similarly, in a study of 70 participants, comprising individuals vaccinated with Ad26.CoV2.S or BNT162b2 and unvaccinated but convalescent COVID-19 individuals, 70% to 80% of the CD4+ and CD8+ T-cell response to the spike was maintained, and the magnitude of Omicron crossreactive T cells was similar to that for the Beta and Delta variants.28 In addition, greater crossreactivity of vaccine-induced cellular immune responses compared with humoral responses against the Alpha, Beta, and Gamma variants, consistent with T-cell responses targeting multiple regions in the spike protein and hence contributing to the preserved cellular immune responses to Omicron, has previously been shown.29 Taken together, the abovementioned data confirm robust CD4+ and CD8+ T-cell responses that largely crossreact with Omicron after vaccination and infection.
The retained T-cell immunity to the Omicron variant as shown in the abovementioned studies, despite a significant reduction of the Omicron-specific humoral response, thus may explain the marked protection against severe disease, whereas the infection rate with Omicron of 4-dose vaccinated RTRs was relatively high. It can be postulated that the number of NAs in vaccinated RTRs is insufficient to prevent the infection, but the cellular defense, specifically CD8+ cells capable of clearing viral infections, probably provides protection against severe disease.
Certain limitations should be taken into consideration in interpreting our results. The use of patients’ self-assessed severity of illness introduces an element of subjectivity, which is a limitation relative to the assessments that can be performed on inpatients under direct observation. It is not known whether vaccination status might influence how patients report their severity of illness. Nonetheless, given the acknowledged limitations of a survey study in this regard, we feel this information is still of value to clinicians. Omicron infection was confirmed by PCR to SARS-CoV-2 but not by a specific PCR to the Omicron variant. Nonetheless, the PCR testing was performed during a period in which the B.1.1.529 (Omicron) variant of SARS-CoV-2 was predominant. Longer follow-up is needed to evaluate the protection afforded by the fourth dose against confirmed infection and severe illness over time. Confounding factors may exist, such as personal and barrier measures differences between RTRs who received the fourth dose and those who did not. In addition, for severe illness, differences in the prevalence of coexisting medical conditions could potentially have affected the results. Finally, the study was not designed as an efficacy trial (there was no control group), but a significant correlation was demonstrated between NAs and protection from SARS-CoV-2. The implications of our findings for the subgroups of 74 and 30 RTRs are limited by the small number of patients and the short follow-up period after vaccination. Antibodies may wane over time, and the half-life of the neutralizing response cannot be predicted. Furthermore, cellular immunity was not assessed.
In conclusion, a fourth BNT162b2 vaccination should be encouraged for RTRs to prevent the undesirable consequences of severe Omicron illness in this vulnerable population.
Supplementary Material
Footnotes
The authors declare no funding or conflicts of interest.
T.H. contributed in conception and design, data acquisition, data interpretation, writing, and revising. A.B.-D., R.H., and P.B. contributed in data collection. E.M. and E.G. contributed in revising. L.O. contributed in data analysis. V.I., R.D., K.A., and N.A. contributed in data acquisition. O.B. and M.M. contributed in data interpretation. Y.L. contributed in conception and design and data interpretation. G.R. contributed in conception and design, data interpretation, and revising.
Supplemental Visual Abstract; http://links.lww.com/TP/C599.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Fall A, Eldesouki RE, Sachithanandham J, et al. A quick displacement of the SARS-CoV-2 variant Delta with Omicron: unprecedented spike in COVID-19 cases associated with fewer admissions and comparable upper respiratory viral loads. medRxiv. 2022; 2022.01.26.22269927 [Google Scholar]
- 2.Davies MA, Kassanjee R, Rousseau P, et al. Outcomes of laboratory-confirmed SARS-CoV-2 infection in the Omicron-driven fourth wave compared with previous waves in the Western Cape Province, South Africa. Preprint. Posted January 12, 2022. medRxiv. doi:10.1101/2022.01.12.22269148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rincon-Arevalo H, Choi M, Stefanski AL, et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci Immunol. 2021;6:eabj1031. [DOI] [PubMed] [Google Scholar]
- 4.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sattler A, Schrezenmeier E, Weber UA, et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J Clin Invest. 2021;131:150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cucchiari D, Egri N, Bodro M, et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant. 2021;21:2727–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reischig T, Kacer M, Vlas T, et al. Insufficient response to mRNA SARS-CoV-2 vaccine and high incidence of severe COVID-19 in kidney transplant recipients during pandemic. Am J Transplant. 2022;22:801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hod T, Ben-David A, Olmer L, et al. Humoral response of renal transplant recipients to the BNT162b2 SARS-CoV-2 mRNA vaccine using both RBD IgG and neutralizing antibodies. Transplantation. 2021;105:e234–e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385:1244–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hod T, Ben-David A, Olmer L, et al. BNT162b2 third booster dose significantly increases the humoral response assessed by both RBD IgG and neutralizing antibodies in renal transplant recipients. Transpl Int. 2022;35:10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bar-On YM, Goldberg Y, Mandel M, et al. Protection against COVID-19 by BNT162b2 booster across age groups. N Engl J Med. 2021;385:2421–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spitzer A, Angel Y, Marudi O, et al. Association of a third dose of BNT162b2 Vaccine with incidence of SARS-CoV-2 infection among health care workers in Israel. JAMA. 2022;327:341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lustig Y, Sapir E, Regev-Yochay G, et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med. 2021;9:999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lustig Y, Nemet I, Kliker L, et al. Neutralizing response against variants after SARS-CoV-2 infection and one dose of BNT162b2. N Engl J Med. 2021;384:2453–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alejo JL, Mitchell J, Chiang TP, et al. Antibody response to a fourth dose of a SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Transplantation. 2021;105:e280–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masset C, Benotmane I, Dantal J, et al. A fourth SARS-CoV-2 mRNA vaccine in strictly seronegative kidney transplant recipients. Kidney Int. 2022;101:825–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caillard S, Thaunat O, Benotmane I, et al. Antibody response to a fourth messenger RNA COVID-19 vaccine dose in kidney transplant recipients: a case series. Ann Intern Med. 2022;175:455–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benotmane I, Bruel T, Planas D, et al. A fourth dose of the mRNA-1273 SARS-CoV-2 vaccine improves serum neutralization against the Delta variant in kidney transplant recipients. Kidney Int. 2022;101:1073–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall VJ, Foulkes S, Saei A, et al. ; SIREN Study Group. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397:1725–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pratt PK, Jr, Nunes D, Long MT, et al. Improved antibody response to three additional hepatitis B vaccine doses following primary vaccination failure in patients with inflammatory bowel disease. Dig Dis Sci. 2019;64:2031–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Natori Y, Shiotsuka M, Slomovic J, et al. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis. 2018;66:1698–1704. [DOI] [PubMed] [Google Scholar]
- 22.Collie S, Champion J, Moultrie H, et al. Effectiveness of BNT162b2 vaccine against Omicron variant in South Africa. N Engl J Med. 2022;386:494–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cameroni E, Bowen JE, Rosen LE, et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature. 2022;602:664–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cele S, Jackson L, Khoury DS, et al. ; NGS-SA; COMMIT-KZN Team. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2022;602:654–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao Y, Wang J, Jian F, et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. 2022;602:657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.GeurtsvanKessel CH, Geers D, Schmitz KS, et al. Divergent SARS-CoV-2 Omicron-reactive T and B cell responses in COVID-19 vaccine recipients. Sci Immunol. 2022;7:eabo2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Chandrashekar A, Sellers D, et al. Vaccines elicit highly conserved cellular immunity to SARS-CoV-2 Omicron. Nature. 2022;603:493–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keeton R, Tincho MB, Ngomti A, et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature. 2022;603:488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alter G, Yu J, Liu J, et al. Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans. Nature. 2021;596:268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.