Abstract
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and the fourth most common cancer among men in South Korea, where the prevalence of chronic hepatitis B infection is high in middle and old age. The current practice guidelines will provide useful and sensible advice for the clinical management of patients with HCC. A total of 49 experts in the fields of hepatology, oncology, surgery, radiology, and radiation oncology from the Korean Liver Cancer Association-National Cancer Center Korea Practice Guideline Revision Committee revised the 2018 Korean guidelines and developed new recommendations that integrate the most up-to-date research findings and expert opinions. These guidelines provide useful information and direction for all clinicians, trainees, and researchers in the diagnosis and treatment of HCC.
Keywords: Diagnosis, Guidelines, Hepatocellular carcinoma, Management
INTRODUCTION
Intent of Revision
The Korean Liver Cancer Study Group (KLCSG)-National Cancer Center (NCC) Korea practice guidelines for the management of hepatocellular carcinoma (HCC) were first announced in 2003 and have been revised three times; first in 2009, second in 2014, and then in 2018. Since then, an abundance of new research findings and therapies for HCC have been presented and published in South Korea and around the globe. As many studies have been conducted, a substantial amount of knowledge have been accumulated on the diagnosis, staging, and treatment of HCC specific to Asia, with the study results showing different clinical behaviors from the West, especially in South Korea; these new research findings have provided clinicians with various action plans and measures related to HCC. Accordingly, in the summer of 2021, the Korean Liver Cancer Association (KLCA, formerly KLCSG)-NCC Korea Practice Guideline Revision Committee (KPGRC) initiated the revision of the guidelines to develop a new recommendation plan that integrates the most up-to-date research findings and expert opinions after the release of the 2018 guidelines.
Target Population
The primary targets of these new guidelines are patients with suspicious or newly diagnosed HCC. The key to treatment according to these guidelines is the initial treatment of patients with newly diagnosed HCC; however, for the first time, we extensively reviewed and discussed residual, progressive, or recurrent cancer after initial treatment and provided relevant recommendations since the 2018 guidelines. Moreover, these guidelines can be applied more usefully in actual clinical practice as it described the prevention methods, surveillance tests, a treatment overview, preventive antiviral treatment of underlying chronic hepatitis, management of cancer pain, and an assessment of the tumor response after treatment.
Intended Users
These guidelines are intended to provide useful clinical information and direction for all clinicians in charge of the diagnosis and treatment of HCC in South Korea and other countries with similar conditions. They are also intended to provide specific and practical information for medical residents in training, specialists, and their instructors.
Developers and Funding Source
The KLCA-NCC KPGRC, organized by the consensus of the KLCA and NCC, consists of hepatologists, oncologists, surgeons, radiologists, and radiation oncologists. All required funding was provided by the NCC (#1731510-1). Each member of the KPGRC collected, analyzed relevant evidence, and wrote the manuscript. Conflicts of interests among the members are summarized in Appendix 2.
Literature Search for Evidence Collection
The 2022 KPGRC (Appendix 1) collected and analyzed the Korean and international literature published on HCC since the announcement of the 2018 guidelines through a PubMed search for revision of the guidelines based on the latest updated evidence. Only English and Korean literature were searched, and the keywords included HCC and other keywords specific to related sub-topics. The sub-topics encompassed a wide range of clinically important items, such as epidemiology, prevention, diagnosis, staging, treatment, and response assessment of HCC.
Literature collected for evidence was analyzed through systematic review, and levels of evidence were classified by the revised Grading of Recommendations, Assessment, Development and Evaluation (GRADE) (Table 1) [1,2,3,4]. The levels of evidence were categorized based on the possibility of changes in the assessment through further research and were defined as follows: high (A), with lowest possibility; moderate (B), with certain possibility; and low (C), with highest possibility. For example, level A evidence is similar but not identical to that from one or more randomized controlled trials (RCTs). When there is no possibility of a change in the level of evidence since further RCTs are unlikely to be conducted, such evidence could be considered level A. In contrast, RCTs that have a small population of target patients and need further research or have been published only in abstracts were regarded as a lower level evidence. The GRADE system was implemented for classifying the grades of recommendation as strong (1) and weak (2) collectively, considering not only the level of evidence but also the quality, patient benefit-risk, and socioeconomic aspects of each study. Therefore, each recommendation was graded based on the level of evidence (A-C) and grade of recommendation (1 or 2) as follows: A1, A2, B1, B2, C1, or C2 (Table 1). These guidelines avoided giving C2 grades as much as possible. For the first time, the D-grade recommendation was described as the opinions of experts only.
Table 1. Grading of Recommendations, Assessment, Development and Evaluation (GRADE).
| Criteria | ||
|---|---|---|
| Quality of evidence | ||
| High (A) | Further research is unlikely to change confidence in the estimate of the clinical effect | |
| Moderate (B) | Further research may change confidence in the estimate of the clinical effect | |
| Low (C) | Further research is very likely to impact confidence on the estimate of clinical effect | |
| Very low (D) | Any estimate of effect is uncertain | |
| Strength of recommendation | ||
| Strong (1) | Factors influencing the strength of the recommendation included the quality of the evidence, presumed patient important outcomes, and cost | |
| Weak (2) | Variability in preferences and values, or more uncertainty. Recommendation is made with less certainty, higher cost or resource consumption | |
Evidence level was graded down if there was only an abstract, poor quality or inconsistency between studies; level was graded up if there was a large effect size.
List of Clinical Questions
The KPGRC selected sub-topics and clinical questions from four departments regarding the revision of the guidelines (Appendix 3), reviewed the evidence of each item, and suggested recommendations through discussion with each subcommittee (Table 2).
Table 2. Recommendations at a Glance of 2022 KLCA-NCC Korea Practice Guidelines for Management of Hepatocellular Carcinoma.
| Topic | Recommendations | |
|---|---|---|
| Prevention | 1. All newborns (A1) and seronegative (negative for all of HBsAg, anti-HBs, and anti-HBc) children and adults should be vaccinated against HBV (B1) to prevent HCC. | |
| 2. General HCC preventive measures include the following: prevention of HBV/HCV transmission (A1); avoidance of alcohol abuse; and control of metabolic disorders, such as obesity and diabetes (C1). | ||
| 3. Antiviral therapy as a secondary prevention of HCC should follow the KASL guidelines for the management of chronic hepatitis B or C (A1). | ||
| 4. The risk of HCC can be reduced if HBV replication is persistently suppressed in patients with chronic hepatitis B (A1), and if an SVR is achieved by interferon therapy (A2) or DAA therapy (B1) in patients with chronic hepatitis C. | ||
| 5. Among patients with chronic liver disease, the risk of developing HCC is lower in patients receiving statin therapy for the management of dyslipidemia compared to those undergoing no treatment (B1). | ||
| 6. Among patients with chronic liver disease, the risk of developing HCC is lower in patients receiving aspirin therapy for the purpose of preventing cardiovascular complications or managing pain and inflammation compared to those undergoing no treatment. However, the administration of aspirin for patients with liver cirrhosis should be considered with caution as the risk of gastrointestinal bleeding may increase (B2). | ||
| 7. Coffee consumption in patients with chronic liver disease can lower the risk of HCC (B1). | ||
| 8. After curative treatment of HBV-associated HCC, antiviral therapy should be considered to reduce the risk of HCC recurrence in patients with detectable serum HBV DNA (B1). | ||
| 9. After curative treatment of HCV-associated HCC, the association of DAA therapy with the risk or prevention of HCC recurrence remains unclear (C1). | ||
| Surveillance | 1. Surveillance for HCC should be performed in high-risk groups; patients with chronic hepatitis B (A1), chronic hepatitis C (B1), and liver cirrhosis (A1). | |
| 2. Surveillance test for HCC should be performed with liver US plus serum AFP measurement every 6 months (A1). | ||
| 3. When liver US cannot be performed adequately, dynamic contrast-enhanced CT or dynamic contrast-enhanced MRI can be performed as an alternative (C1). | ||
| Diagnosis | 1. The diagnosis of HCC can be made pathologically or using the typical hallmarks of HCC obtained by non-invasive imaging in high-risk groups (chronic hepatitis B [A1], chronic hepatitis C [B1], or cirrhosis [A1]). | |
| 2. For a new liver nodule ≥ 1 cm detected by surveillance tests in high-risk patients, multiphasic CT, or multiphasic MRI (extracellular contrast agents or hepatocyte-specific contrast agents) should be performed as a first-line imaging study for the diagnosis of HCC (A1). If first-line imaging study is inconclusive for the diagnosis of HCC, second-line imaging study including multiphasic CT, multiphasic MRI (extracellular contrast agents or hepatocyte-specific contrast agents), and contrast-enhanced US (blood-pool contrast agents or Kupffer cell-specific contrast agents) can be applied (B1). | ||
| 3. Imaging diagnosis of “definite” HCC can be made for the nodule ≥ 1 cm detected by surveillance tests in high-risk patients based on the following radiological hallmarks: | ||
| (1) The radiological hallmarks in multiphasic CT or MRI with extracellular contrast agents are APHE with washout appearance in the portal venous or delayed phases (A1). | ||
| (2) The radiological hallmarks in multiphasic MRI with hepatocyte-specific contrast agents are APHE with washout appearance in the portal venous, delayed, or hepatobiliary phases; these criteria should be applied only to a lesion which does not show either marked T2 hyperintensity or targetoid appearances on diffusion-weighted images or contrast-enhanced images (B1). | ||
| (3) The radiological hallmarks in contrast-enhanced US (blood-pool contrast agents or Kupffer cell-specific contrast agents) performed as a second-line imaging study are APHE with late (≥ 60 seconds) and mild washout or washout appearance in the Kupffer phase; these criteria should be applied only to a lesion which does not show either rim or peripheral globular enhancement on arterial phase (B1). | ||
| 4. In nodules ≥ 1 cm that do not meet the radiologic diagnosis criteria of “definite” HCC, a diagnosis of “probable” HCC can be assigned by applying ancillary imaging features of HCC (B1). There are two categories of ancillary imaging features including imaging features favoring malignancy in general (mild-to-moderate T2 hyperintensity, restricted diffusion, threshold growth) and those favoring HCC in particular enhancing or non-enhancing capsule, mosaic architecture, nodule-in-nodule appearance, fat or blood products in the mass). For nodules without APHE, “probable” HCC can be assigned only when the lesion fulfills at least one item from each of the two categories of ancillary imaging features. For nodules with APHE but without washout appearance, “probable” HCC can be assigned when the lesion fulfills at least one of the aforementioned ancillary imaging features. | ||
| 5. For “probable” HCC, follow-up imaging study within 3 months or biopsy should be considered (C1). For “indeterminate” nodules that cannot be diagnosed as “definite” or “probable” HCC by imaging, follow-up imaging study within 6 months or biopsy should be considered (B1). Follow-up study should be performed using one of the first-line imaging modalities. | ||
| 6. For subcentimeter nodules newly detected on HCC surveillance in high-risk patients, follow-up surveillance test within 6 months is recommended (C1). | ||
| 7. Newly detected or growing nodules in the follow-up study of patients with a history of prior HCC can be diagnosed as recurrent HCC regardless of size if they show the radiological hallmarks of HCC or ancillary imaging features with an increase in size (C1). | ||
| 8. Although it is not recommended to strictly limit the radiation dose for the diagnosis and follow-up evaluation of HCC, unnecessary CT examinations should be avoided. To optimize radiation exposure, the use of dose reduction techniques as well as alternative imaging modalities should to be considered in HCC patients (C1). | ||
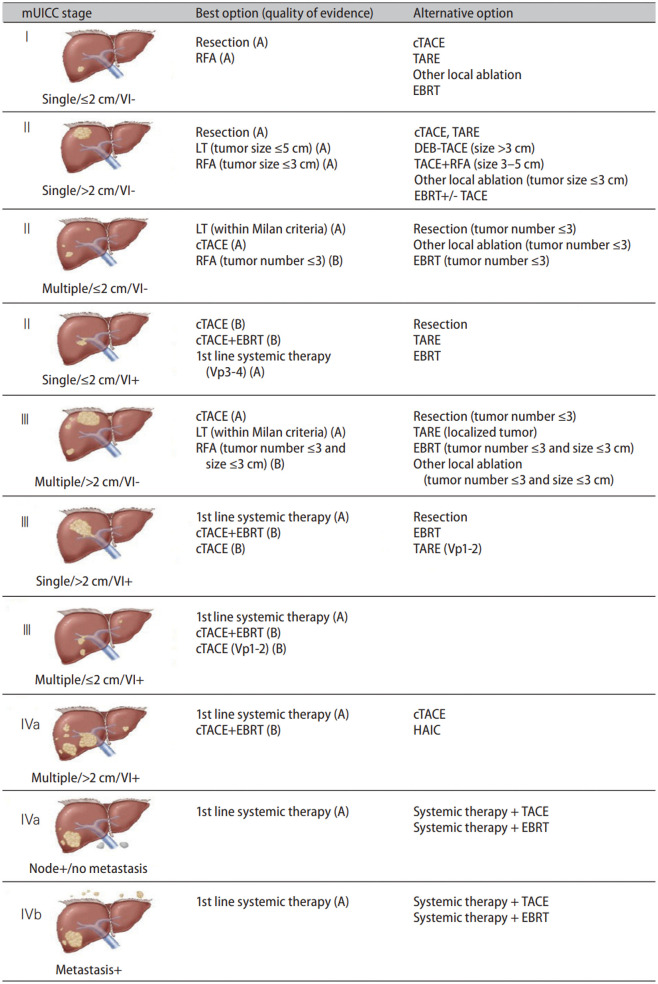
| Staging | 1. This guideline adopts the mUICC stages as the primary staging system, with the BCLC staging system and the AJCC/UICC TNM staging system serving as complementary systems (B1). | |
| 2. FDG PET-CT can be utilized for staging prior to treatments with curative intent, such as hepatic resection or LT (C1). | ||
| 3. Chest CT, pelvis CT, and bone scan can be used for HCC staging workup if extrahepatic metastasis of HCC is suspected (C1). | ||
| Hepatic resection | 1. Hepatic resection is the primary treatment modality for single HCC limited to the liver in Child-Pugh grade A patients without portal hypertension and hyperbilirubinemia (A1). | |
| 2. Limited hepatic resection can be selectively performed for Child-Pugh A or B7 single HCC with mild portal hypertension or hyperbilirubinemia (C1). | ||
| 3. Hepatic resection may be considered even in the cases of HCC with invasion to the portal vein, hepatic vein, or bile duct if the main portal trunk is not invaded in patients with well-preserved liver function (C2). | ||
| 4. Hepatic resection may be considered for three or less multiple HCCs in patients with well-preserved liver function (C2). | ||
| 5. LLR for HCC located in the left lateral section and anterolateral segments can be selectively performed (B2). | ||
| 6. LLR for HCC located in the posterosuperior segments or caudate lobe can be selectively performed depending on the location and size of the tumor (C2). | ||
| 7. For recurrent HCC after being cured by hepatic resection, the retreatment method can be selected considering the timing of recurrence, remnant liver function, performance status, and the size, location, number of recurrent tumors (C1). | ||
| Liver transplantation | 1. LT is the primary treatment modality for patients with HCC unsuitable for resection but within the Milan criteria (a single tumor ≤ 5 cm or small multinodular tumors [≤ 3 nodules, ≤ 3 cm]) (A1). | |
| 2. In LT candidates with HCC, loco-regional therapies or TACE are recommended if the timing of transplantation is unpredictable (B1). | ||
| 3. If the HCC tumor stage is downgraded to meet the Milan criteria by loco-regional therapies, including TACE and RFA, in patients initially exceeding the Milan criteria, LT shows superior outcomes compared to other treatments (B1). | ||
| 4. Expanded indications beyond the Milan criteria for LT may be considered in limited cases without definitive vascular invasion or extrahepatic spread if other effective treatment options are not applicable (C2). | ||
| 5. Salvage transplantation can be indicated for recurrent HCC after resection according to the same criteria as for first-line transplantation (B1). | ||
| 6. For recurrent HCC after being cured by LT, the retreatment method can be selected considering the time to recurrence, liver function, performance status, size, location, and the number of recurrent tumors (C1). | ||
| Local ablation therapies | 1. RFA has an equivalent survival rate, a higher LTP rate, and a lower complication rate compared to hepatic resection in patients with a single nodular HCC ≤ 3 cm in diameter (A1). | |
| 2. Combined therapy with TACE and RFA or microwave ablation increases the survival rate in patients with 3–5 cm HCCs that are not amenable to hepatic resection compared to RFA or microwave ablation alone (A2). | ||
| 3. In the treatment of HCC, microwave ablation and cryoablation are expected to produce comparable rates of survival, recurrence, and complications to those of RFA (B2). | ||
| 4. Contrast-enhanced US and fusion imaging improve the detection rate and the technical success rate of local ablation therapy for HCCs ≤ 2 cm (B1). | ||
| TACE and radioembolization | 1. cTACE is recommended for HCC patients with a good performance status without major vascular invasion or extrahepatic spread who are ineligible for hepatic resection, LT, or local ablation therapies (A1). | |
| 2. cTACE should be performed through tumor-feeding arteries using selective/superselective techniques to maximize antitumor activity and minimize hepatic damage (B1). | ||
| 3. In cases of HCC with portal vein invasion, cTACE alone (B2) or cTACE combined with external beam radiation therapy (EBRT) (B1) can be considered for patients with intrahepatic localized tumors and well-preserved liver function. | ||
| 4. Compared with cTACE, DEB-TACE has similar clinical outcomes in ≥3 cm HCCs; therefore, it can be considered as an alternative treatment to cTACE (A2). | ||
| 5. Compared with cTACE, TARE results in a better quality of life and lower occurrence of PES; therefore, it can be considered an alternative treatment to cTACE when the remnant liver function is expected to be sufficient after the TARE treatment (B2). | ||
| 6. When developing one or more of the following conditions after two or more sessions of on-demand TACE within 6 months from the first TACE, a switch to other treatments should be considered: (1) absence of objective response, (2) new appearance of vascular invasion (3) the new appearance of extrahepatic spread (C1). | ||
| External beam radiation therapy | 1. EBRT is recommended for patients with HCC unsuitable for hepatic resection, transplantation, local ablation treatments, or TACE (C1). | |
| 2. EBRT is performed when the liver function is Child-Pugh grade A or B7 and when the volume to be irradiated with ≤ 30 Gy is ≥ 40% of the total liver volume in the computerized treatment plan (B1). | ||
| 3. EBRT can be combined for HCCs that are expected to have an incomplete response after TACE (B2). | ||
| 4. EBRT can be performed for the treatment of HCC with portal vein invasion (B2). | ||
| 5. EBRT can be combined with systemic therapy for HCC treatment (C2). | ||
| 6. EBRT is recommended for palliating symptoms of HCC (B1). | ||
| 7. PBT is not inferior in the local control rate and shows no difference in survival and toxicity rates compared to RFA in treating recurrent or residual HCCs ≤3 cm in size (A2); SBRT may not be inferior in the local control rate compared to RFA for the treatment of HCCs ≤3 cm in size (C2). | ||
| Systemic therapies | [First-line therapies] | |
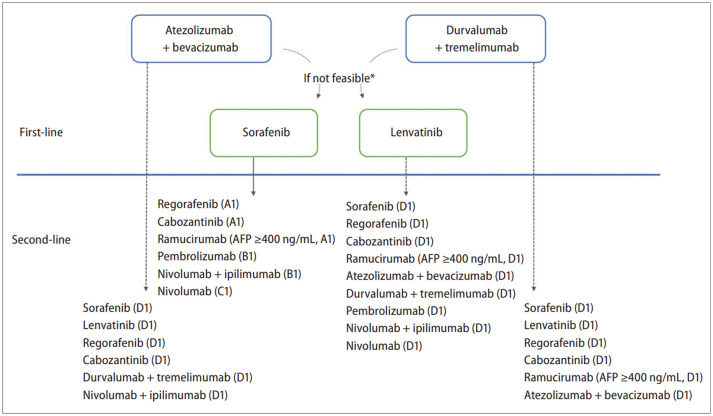
| 1. Atezolizumab plus bevacizumab or durvalumab plus tremelimumab is recommended for systemic treatment-naïve patients with locally advanced unresectable or metastatic HCC not amenable to curative or loco-regional therapy who have Child-Pugh class A and ECOG performance status 0–1 (A1). If these two combination therapies cannot be applied, sorafenib or lenvatinib is recommended (A1). | ||
| 2. Sorafenib is considered for patients with HCC who have Child-Pugh class B7 (B1) or B8–9 (B2) if other conditions listed in Recommendation 1 are met. | ||
| [Second-line therapies] | ||
| 1. Regorafenib is recommended for patients with progressive HCC after at least 3 weeks of sorafenib (≥ 400 mg/day) treatment and with Child-Pugh class A and good performance status (ECOG score 0–1) (A1). | ||
| 2. Cabozantinib is recommended for patients with progressive HCC after first-line sorafenib or second-line systemic treatment and with Child-Pugh class A and good performance status (ECOG score 0–1) (A1). | ||
| 3. Ramucirumab is recommended for patients with progressive HCC after sorafenib or intolerance to sorafenib and with Child-Pugh class A, good performance status (ECOG score 0–1), and serum AFP level ≥ 400 ng/mL (A1). | ||
| 4. Pembrolizumab is recommended for patients with progressive HCC after sorafenib or intolerance to sorafenib and with Child-Pugh class A and good performance status (ECOG score 0–1) (B1). | ||
| 5. Either nivolumab plus ipilimumab combination therapy (B1) or nivolumab monotherapy (C1) can be considered for patients with progressive HCC after sorafenib or intolerance to sorafenib and with Child-Pugh class A and good performance status (ECOG score 0–1). | ||
| 6. Sorafenib, regorafenib, cabozantinib, ramucirumab (if serum AFP level ≥ 400 ng/mL), atezolizumab-bevacizumab, durvalumab-tremelimumab, pembrolizumab, nivolumab-ipilimumab, or nivolumab treatment can be tried for patients with progressive HCC after lenvatinib (D1). | ||
| 7. Sorafenib, lenvatinib, regorafenib, cabozantinib, durvalumab-tremelimumab, or nivolumab-ipilimumab can be tried for patients with progressive HCC after combination therapy with atezolizumab plus bevacizumab (D1). | ||
| 8. Sorafenib, lenvatinib, regorafenib, cabozantinib, ramucirumab (if serum AFP level ≥ 400 ng/mL), or atezolizumabbevacizumab can be tried for patients with progressive HCC after combination therapy with durvalumab plus tremelimumab (D1). | ||
| [Cytotoxic chemotherapy and hepatic arterial infusion chemotherapy] | ||
| 1. HAIC may be considered for advanced HCC patients with preserved liver function and portal vein invasion without extrahepatic spread for whom first-line or second-line systemic therapies, such as atezolizumab-bevacizumab, durvalumab-tremelimumab, sorafenib, lenvatinib, regorafenib, cabozantinib, ramucirumab, nivolumab-ipilimumab, or pembrolizumab, have failed or cannot be used (C2). | ||
| Adjuvant therapy | 1. Adjuvant immunotherapy with CIK cells can be considered after curative treatment (resection, RFA, or PEI) in patients with HCC ≤ 2 cm without lymph node or distant metastasis (A2). | |
| 2. Adjuvant therapy with TACE, sorafenib, or cytotoxic chemotherapy is not recommended for patients with HCC after curative treatment (B1). | ||
| Preventive antiviral therapy | 1. HCC Patients should be tested for hepatitis B surface antigen before starting HCC treatment (A1). | |
| 2. In HCC patients with HBV, antiviral therapy should be initiated if serum HBV DNA is detected (A1). | ||
| 3. In HBsAg-positive HCC patients with undetectable serum HBV DNA, preventive antiviral therapy is recommended before cytotoxic chemotherapy (A1), TACE (A2), HAIC (A2), hepatic resection (A2), EBRT (B1), RFA (C1), tyrosine kinase inhibitor, or immune checkpoint inhibitor (C1) treatment. | ||
| 4. Antiviral agents for the prevention of HBV reactivation should be selected based on the KASL clinical practice guidelines for management of chronic hepatitis B (A1). | ||
| 5. There is still no evidence to recommend preventive antiviral therapy with DAAs for HCC patients who are HCV RNA positive (C1). | ||
| Drug treatment for cancer pain in HCC | 1. In HCC patients, pain control using drugs requires a careful approach with consideration of the underlying liver disease, and type of the drug, dose, and interval of administration should be determined according to liver function (C1). | |
| 2. In patients with HCC accompanied by chronic liver disease, a reduced dose of acetaminophen should be considered (C1), and NSAIDs should be used with caution (B1). | ||
| 3. In patients with HCC accompanied by chronic liver disease, the selection of opioid analgesics, and adjustments in the dosage and interval of administration should be carefully considered based on drug metabolism and liver function (C1). | ||
| Assessment of tumor response and post-treatment follow-up | 1. Assessment of tumor response to treatment should be done using the RECIST v.1.1 according to the change in tumor size and the mRECIST according to the change in viable tumor by dynamic contrast-enhanced CT or MRI (B1). | |
| Management of patients with HCC during COVID-19 pandemic | 1. Even during the COVID-19 pandemic, the management of chronic liver disease, the surveillance of at-risk patients, and the treatment of HCC should be continued (D1). | |
| 2. COVID-19 vaccination is recommended in patients with HCC, as the benefits of vaccination outweigh the risks (C1). Meanwhile, it is necessary to monitor the occurrence of adverse events after vaccination. | ||
| 3. Patients with chronic liver disease and HCC should strictly adhere to the infection precautionary measures even after COVID-19 vaccination since they may have a low antibody titer (D1). | ||
KLCA = Korean Liver Cancer Association, NCC = National Cancer Center, HBsAg = HBV surface antigen, anti-HBs = HBV surface antibody, anti-HBc = HBV core antibody, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, KASL = Korean Association for the Study of Liver, SVR = sustained virologic response, DAA = direct-acting antiviral, HCV = hepatitis C virus, US = ultrasonography, AFP = alpha-fetoprotein, CT = computed tomography, MRI = magnetic resonance imaging, APHE = arterial phase hyperenhancement, mUICC = modified Union for International Cancer Control, AJCC = American Joint Committee on Cancer, TNM = tumor-node-metastasis, PET = positron emission tomography, LLR = laparoscopic liver resection, LT = liver transplantation, TACE = transarterial chemoembolization, RFA = radiofrequency ablation, cTACE = conventional TACE, DEB = drug-eluting bead, TARE = transarterial radioembolization, PES = postembolization syndrome, SBRT = stereotactic body radiotherapy, ECOG = Eastern Cooperative Oncology Group, HAIC = hepatic arterial infusion chemotherapy, CIK = cytokine induced killer, PEI = percutaneous ethanol injection, NSAID = nonsteroidal anti-inflammatory drug, RECIST = Response Evaluation Criteria in Solid Tumors, mRECIST = modified RECIST.
Manuscript Review
Recommendation drafts were made through several intradepartmental meetings after the initial meeting of the KPGRC and two interdepartmental meetings attended by all members of the committee. The drafts were then thoroughly reviewed through several online discussions and three department head meetings. In addition to the integrity of the contents, methodological validity of the manuscript was also evaluated on the basis of the Appraisal of Guidelines for Research and Evaluation II [5,6]. The complete draft was then reviewed by the advisory board and through a public meeting, and was modified further at the KPGRC department head meeting. The advisory board consisted of nine clinical specialists in liver cancer. The guidelines made through this process were endorsed by the open meeting, board of directors of the KLCA, and the NCC (Appendix 4).
Release of Guidelines
The revised guidelines were presented at the 16th conference of the KLCA on June 24, 2022 (Appendix 5). The Korean version is available at the KLCA and NCC websites (http://livercancer.or.kr; http://ncc.re.kr).
Plan for Updates
The KLCA and NCC Korea will update part or all of these guidelines when new test methods, drugs, or treatments regarding HCC are developed and new significant research fndings are made, and thus, the revision of the guidelines is deemed necessary for promoting the national health of Korea. The schedule for this plan will be posted as needed.
EPIDEMIOLOGY
Metrics of Disease Burden From Liver Cancer (Mortality vs. Incidence, Crude Rate vs. Age-Standardized Rate)
The disease burden of cancer is commonly described as the incidence or cause-specific mortality. Of these, cause-specific mortality is the most important and standard measure in assessing the disease burden of cancer. Mortality due to a specific disease is useful for determining priorities in public healthcare policies and research. The latest data on disease-specific mortalities are used to determine whether current healthcare policies and research can effectively reduce the burden of a disease and whether new measures must be taken [7,8].
Mortality and incidence are reported as crude rates and age-standardized rates. Cancer mortality in South Korea is reported with both crude and age-standardized rates (revised by the resident registration data in 2005), and the incidence of cancer is reported with crude rates based on the Korean Central Cancer Registry (KCCR) and age-standardized rates (revised by the resident registration data in 2000). It has been reported that age-standardized rates do not differ significantly according to which population they are adjusted for. However, age-standardized rates must be carefully interpreted, as they sometimes differ from crude rates (Fig. 1), especially more so if the population is rapidly aging, as is the case in South Korea. The United States Centers for Disease Control and Prevention (CDC) recommends choosing between crude rates and age-standardized rates depending on the purpose of use (https://www.cdc.gov/cancer/npcr). It is recommended to use crude rates for estimating the magnitude of resources needed to overcome the social burden of the disease and the disease itself. Age-standardized rates are recommended for determining whether the difference between countries, regions, or time periods are attributable to the age distribution within different population groups.
Fig. 1. Crude death rate and age-standardized death rate in South Korea in calendar years 2010 to 2020.

Given this background, the current guideline considers crude death rate as the most important indicator of the disease burden of liver cancer. This guideline additionally considers crude incidence rate, age-standardized death rate, and age-standardized incidence rate as supplementary indicators.
Liver Cancer Mortality and Economic Burden
Malignant neoplasm (cancer) is the main cause of death among South Koreans. According to Statistics Korea (KOSTAT), cancer was the number one cause of death in 2020, with cancer mortality reported as 160.1 persons per 100000 population. This was 2.5 times higher than that of cardiac diseases, the second most common cause of death, which had a mortality of 63.0 persons per 100000 population. Liver cancer was the second most common cause of cancer-related death in 2020, with a mortality of 20.6 persons per 100000 population, following lung cancer with a mortality of 36.4 persons per 100000 population. However, liver cancer was the number one cause of death among people aged 40–59 years, the most economically productive age group, and the second and fourth highest cause of death among men (30.5 persons per 100000) and women (10.7 persons per 100000), respectively (2020 Cause of Death Statistics, Statistics Korea https://kostat.go.kr/portal/korea/kor_nw/1/1/index.board?bmode=read&aSeq=403046).
In 2015, the yearly economic burden caused by liver cancer in South Korea was 2266100000 USD (approximately 2.7 trillion Korean Won), the highest among all types of cancer. It also showed a steady increase from 2065000000 USD (approximately 2.3 trillion Korean Won) reported in 2000 [9,10]. In other words, liver cancer has the highest disease burden among all types of cancer in South Korea.
Trends in Liver Cancer Mortality and Incidence
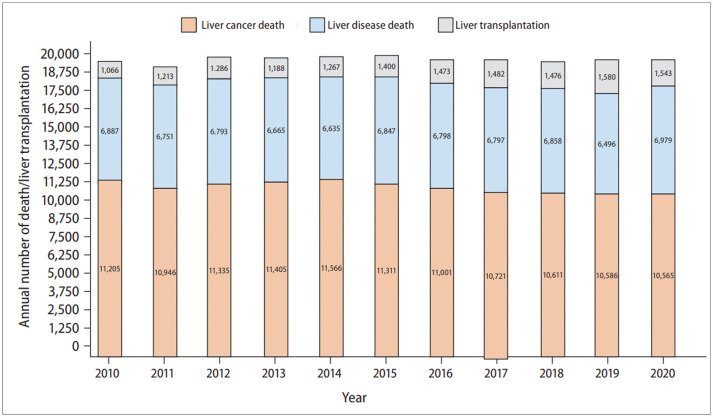
The yearly crude death rate of liver cancer began to plateau in the last 5 years, after having shown a consistent increase in the previous years. The yearly crude death rate of liver cancer (in unit of deaths per 100000 population) drastically increased from 16.2 in 1984 to 20.5 in 1999 and 22.5 in 2010, plateaued after 2015, and then settled at 20.6 in 2019 and 2020 (Fig. 1). The yearly absolute number of deaths has also increased over the last two decades; it increased by 19.4% from 9682 in 1999 to 11566 in 2014, and then decreased by 8.6% to 10565 in 2020 (Fig. 2). The yearly crude incidence rate of liver cancer has also increased over the last two decades; it consistently increased from 28.1 in 1999 to its peak at 32.8 in 2010 and 31–32 in 2015, and has been maintained at 30.4 since 2019.
Fig. 2. Annual number of liver cancer deaths, liver disease deaths and liver transplantations in South Korea during calendar years 2010 to 2020.
In contrast to the yearly crude death and incidence rates of liver cancer, which started to plateau recently after having consistently increased in the last two decades, the yearly age-standardized death and incidence rates of liver cancer have decreased. The age-standardized death rate of liver cancer significantly decreased from 24.7 in 1999 to 16.4 in 2014 and 11.5 in 2020. The age-standardized incidence of liver cancer also significantly decreased from 28.9 in 1999 to 19.7 in 2014 and 16.1 in 2019 (Korea Central Cancer Registry. Annual Report of Cancer Statistics in South Korea [2018], Ministry of Health and Welfare, 2021) [11]. The different trends between the crude and age-standardized rates on the yearly death and incidence rates of liver cancer can be attributed to the rapid aging of the Korean population, including the patients with liver cancer. The general elderly population aged ≥ 65 years increased from 3394896 in 2000 (7.2% of the total population) to 8571347 in 2021 (16.5% of the total population), contributing to a considerable increase in the mean age of the total population and the proportion of the elderly (2021 Elderly Statistics, Statistics Korea). There was a greater increase in age among liver cancer patients compared to the general population, making it appear as if the age-standardized rates have decreased significantly.
Summary
To summarize, although liver cancer has the second-highest crude death rate across all age groups, it ranks first among the working-age group and causes the highest economic burden among all types of cancer. Although the age-standardized death and incidence rates of liver cancer appear to have decreased, this is not due to an actual decrease in the disease burden of liver cancer but due to the rapid aging of the general population. In addition, the crude death and incidence rates of liver cancer are not decreasing but rather have remained constant in recent years, suggesting that liver cancer requires the most urgent attention among all types of cancer in South Korea.
PREVENTION
Causes and Prevention of HCC
HCC occurs almost exclusively in patients with risk factors, such as chronic hepatitis B, chronic hepatitis C, or liver cirrhosis. The most important cause of HCC in South Korea is chronic hepatitis B virus (HBV) infection. According to the results of a random selection registry study of the KLCA and the KCCR, 59.1% of patients diagnosed with HCC between 2012 and 2014 were infected with HBV and 10.7% with hepatitis C virus (HCV). Unknown causes accounted for the remaining 30.3% [12]. It is presumed that liver cirrhosis caused by alcoholic and/or nonalcoholic fatty liver disease would be the main underlying disease for the unknown causes. A cohort study from a single center (2010–2015) reported that 74.0% of patients diagnosed with HCC were with HBV infection [13]. Since about 90% of patients with HCC have cirrhosis or chronic hepatitis B at diagnosis, it is difficult to perform radical treatment, and the risk of recurrence continues even 5 or 10 years after treatment, which worsens the overall prognosis of the patients. According to the National Cancer Registry released by the KCCR in 2017, the 5-year survival rate of patients with HCC was 33.6% and the 10-year survival rate was as low as 20% [14]. These data suggest that preventive measures against HCC are of the utmost importance.
Primary prevention of HCC is to prevent the risk of HCC through measures such as vaccination against HBV and abstinence from alcohol consumption. Secondary prevention is to reduce the risk of developing HCC in patients who already have a risk of HCC, using measures such as antiviral treatment for HBV and HCV to prevent the progression of chronic inflammation and fibrosis of the liver. Tertiary prevention is to prevent the development of new HCC in the remaining liver after curative treatment in patients who have already developed HCC [15].
Primary Prevention of HCC
The most important preventive measure for HCC in South Korea is the universal neonatal vaccination against HBV, since most HBV infections are caused by vertical transmission of the virus from mother to child in the neonatal period [16]. Since the majority of HBV infection cases worldwide were reported as mother-to-child transmission during the neonatal period, HBV vaccination should be given as early as possible within 24 hours after birth. The World Health Organization (WHO) recommends HBV vaccination for all newborns regardless of maternal HBV status [17]. In South Korea, the prevalence of chronic hepatitis B infection is about 3%–4%, with a high risk of transmission even in adults. Therefore, adults who do not have antibodies to the HBV surface antigen (HBsAg) and have never been exposed to the virus (negative for all HBsAg, HBV surface antibody [anti-HBs], and immunoglobulin (Ig) G HBV core antibody [anti-HBc]) should be vaccinated against HBV [18,19]. In particular, people at high risk of HBV infection (family members of chronic hepatitis B patients, healthcare workers, travelers traveling to areas with high HBV prevalence, persons who inject drugs, and people with multiple sexual partners, etc.) should also be vaccinated against HBV.
No vaccine has yet been developed to prevent HCV infection. Since HCV is transmitted almost entirely through contaminated blood, infection must be prevented by avoiding unsanitary invasive procedures (such as multiple use of acupuncture needles, capping, tattooing, or needle sharing).
Excessive alcohol intake over an extended period of time is an independent cause of liver cirrhosis and HCC, and can further increase the risk of liver cirrhosis and HCC in patients with preexisting chronic liver disease. In South Korea, alcoholic liver cirrhosis is one of the leading causes of HCC, together with chronic hepatitis B and C. Therefore, efforts should be made to lower the risk of developing HCC by limiting excessive alcohol consumption. A systematic review with meta-analysis has shown that continuous consumption of even a relatively low level of alcohol (≥ 1 drink/day for female, ≥ 2 drinks/day for male) increases the risk of developing HCC [20].
Metabolic syndrome and fatty liver disease are associated with obesity and diabetes mellitus, and are also known to increase the incidence of HCC [21,22,23]. Therefore, efforts to reduce obesity and metabolic syndrome are necessary to prevent the development of HCC. Statins for treating hyperlipidemia have been extensively studied for an association with the reduction of HCC risk. Large-scale meta-analyses involving earlier studies have reported that statin use was associated with a reduction in the incidence of HCC by 37% [24]; however, in the RCTs that were included in the meta-analyses, a reduction of HCC incidence was not shown with statin therapy. It is of note that this finding was derived from post-hoc analysis of the RCTs, of which the primary outcome focused on the effect of statins on cardiovascular mortality. Moreover, the study subjects included in the RCTs were at a low risk for developing HCC and not regularly monitored under surveillance program for HCC; therefore, the negative results from RCTs should be interpreted with caution. Recent prospective studies involving large European population-based cohorts revealed that statins had a higher chemopreventive effect on HCC occurrence [25,26]. Studies of Korean public database as well as a hospital-based cohort of Korean patients with chronic hepatitis B also reported that statins were associated with a lower risk for HCC [27,28]. Recent meta-analyses of large-scale cohort studies also showed a significant reduction in the risk of HCC (relative risk [RR], 0.54; hazard ratio [HR], 0.57) with statin use [29,30]. Based on the published data, the potential hepatotoxicity or myopathy of statins was not a cause for concern (less than 3% of all patients taking statins) [29]. However, caution is still required as the long-term safety of statins has not been well-documented in patients with cirrhosis [31]. Another study reported that along with statins, metformin reduced HCC development in type 2 diabetes [32], and this should be further confirmed through additional studies.
Aspirin and other antiplatelet agents have also been suggested to reduce the risk of developing HCC in large prospective population-based observational studies [33,34]. A Swedish study of nationwide patient registries observing 50275 patients with HBV or HCV for 7.9 years reported that treatment with low-dose aspirin (< 160 mg/day) was associated with a significantly reduced risk of HCC (adjusted HR [aHR], 0.69; 95% confidence interval [CI], 0.62–0.76) [35]. In the study, it was noted that the preventive effects of aspirin on HCC incidence appeared to be treatment duration-dependent. A retrospective cohort study involving Korean patients with chronic hepatitis B on antiviral therapy showed similar results regarding the beneficial effects of aspirin on HCC [36]. Recent meta-analyses of population-based cohorts or at-risk patients with chronic liver disease revealed that aspirin was associated with a significantly decreased risk of HCC development (HR, 0.51–0.59; RR, 0.73) [37,38,39]. However, aspirin use was reported to slightly increase the risk (RR, 1.15–1.32) of gastrointestinal bleeding as a major adverse event [37,38]; therefore, the potential benefits from aspirin must be weighed against the potential for bleeding in patients with chronic liver disease. Particularly, the benefits from aspirin use regarding lowering HCC risk were reportedly lacking (aHR, 1.00; 95% CI, 0.85–1.18) in patients with HBV-related cirrhosis in a recent analysis of Korean population-based administrative database [40]. Thus, the use of aspirin or anti-platelet agents for the prevention of HCC is not uniformly recommended in routine practice for managing patients with cirrhosis. The optimal dose and duration of aspirin effective for preventing HCC occurrence are yet to be determined, and the chemopreventive effect of other nonsteroidal anti-inflammatory drugs (NSAIDs), excluding aspirin, on HCC also remains uncertain.
Coffee is the only food or drink that has shown evidence for reducing the risk of HCC occurrence. In recent meta-analyses and large-scale cohort studies, coffee consumption significantly reduced the risk of HCC, regardless of the consumption amount, as well as the severity and cause of underlying liver disease [41,42,43,44]. In most studies, the reported amount of coffee consumed per day was more than 2–3 cups or more, or was not clearly described.
Secondary Prevention of HCC
Continued high-level viremia in patients with chronic hepatitis B or C is an independent risk factor for the development of HCC. Therefore, the inhibition of HBV or HCV proliferation by antiviral therapy is expected to reduce the incidence of HCC. Regarding the antiviral therapy of chronic hepatitis B and chronic hepatitis C, we recommend following the clinical practice guidelines of the Korean Association for the Study of Liver (KASL) [45,46].
Oral antiviral agents, such as tenofovir and entecavir, are preferred as the first-line treatment for chronic HBV infection. There is no RCT to determine whether interferon therapy reduces the incidence of HCC in chronic hepatitis B patients. Lamivudine, the first oral antiviral agent for patients with chronic hepatitis B, has shown to reduce the incidence of HCC in patients with advanced hepatic fibrosis in a RCT (32 months of follow-up: lamivudine vs. control, 3.9% vs. 7.4%; p = 0.047) [47]. Large-scale observational studies have consistently shown that long-term therapy with entecavir and tenofovir, potent antiviral agents that have a strong inhibitory effect on HBV proliferation, significantly reduces the incidence of HCC compared with the untreated control group [48,49,50].
Recently, a number of active studies have been performed to compare the difference in HCC prevention between antiviral drugs for chronic hepatitis B, particularly in South Korea. The first study analyzed the National Health Insurance Service database of 24156 patients and in-hospital cohort of 2701 Korean patients with chronic hepatitis B and showed that tenofovir significantly decreased the risk of HCC occurrence by 32% compared to entecavir [51]. However, other two large cohort studies, involving 2897 and 3022 Korean patients, revealed no difference in the incidence of HCC between groups on tenofovir and entecavir therapy [52,53]. Another Korean study including the largest cohort of 55473 patients with chronic hepatitis B showed no difference in the occurrence of HCC in the entire cohort, but a lower incidence of HCC among patients on tenofovir than those on entecavir in the subgroup analysis of patients enrolled between 2012 and 2014 [54]. According to reports from Asian as well as Western countries, there have been huge controversies regarding the chemopreventive effects between tenofovir and entecavir on the development of HCC, mostly showing the superior preventive effects with tenofovir than with entecavir, or no difference between the two drugs. The results of a systematic literature review or meta-analysis also showed conflicting results. In a meta-analysis of 14 relevant studies, there was no difference between the two drugs in the overall HCC risk (RR, 1.28; 95% CI, 0.99–1.66), and the analysis of seven studies that adjusted for clinical variables reported a reduction in HCC risk among patients treated with tenofovir compared to those treated with entecavir (95% CI, 1.01–1.60, p = 0.04) [55]. In another meta-analysis including a total of 119053 patients from 31 studies, no difference in the occurrence of HCC was observed between patients treated with tenofovir and entecavir, in both the propensity score-matching analysis (5-year HCC incidence of 3.44% for entecavir vs. 3.39% for tenofovir) and the analysis after adjustment for clinical variables (aHR, 0.88; 95% CI, 0.73–1.07) [56]. On the other hand, several retrospective studies that evaluated the chemopreventive effects of tenofovir tenofovir alafenamide (TAF), which improved the side effects of tenofovir disoproxil fumarate (TDF), were also conducted and showed no difference in the incidence of HCC between patients on TAF and TDF, or TAF and entecavir [57,58].
Based on the aforementioned studies, it commonly appears that the preventive effects on HCC was more apparent for an antiviral drug with a shorter observation period, and thus, the follow-up duration may function as a determinant of preventive effects of antiviral drugs [59]. In general, in-hospital cohort studies report no difference between drugs, whereas studies of administrative, public database suggest a superiority of tenofovir to entecavir in lowering the risk of HCC occurrence [56]. These database studies have an advantage of including a large sample size, but also have some disadvantages, such as potential unbalanced distribution of HCC risk factors between drugs and different periods of ETV and TDF adminstration [60]. For these reasons, patients with favorable prognosis are more likely to be included in the tenofovir group than in the entecavir group. There could be additional confounders that are unable to be corrected for by any sophisticated statistical methods [60]. Therefore, the overall reliability of the comparative studies appears low, since each study is quite heterogeneous in terms of patient characteristics, severity of liver disease, study period, the time of drug availability, imbalance in the number of patients between drugs, and the analytical methods used [55]. The aforementioned studies on the chemopreventive effect of anti-HBV drugs represent mostly short observation period of less than 5 years. In theory, given the expected tumor doubling time during the development of HCC, it takes an average of 9–10 years for a single malignant transformed cell to grow to a clinically detectable size (~1 cm) [61]. Therefore, well-designed, large-scale randomized studies with longer follow-up duration are needed to determine the true difference in the prevention of HCC between antiviral drugs. Most importantly, before discussing the differential efficacy between drugs, it has to be emphasized that the risk of HCC does not completely disappear despite long-term antiviral treatment [62,63]. It is because, apart from inflammation caused by viral hepatitis, various other non-viral factors, such as underlying liver disease, demographic characteristics such as age and sex, alcohol, as well as metabolic diseases, can also contribute to hepatocarcinogenesis. In conclusion, secondary prevention of HCC through antiviral therapy in chronic hepatitis B is not complete [64].
The primary aim of chronic hepatitis C treatment is to achieve a sustained virologic response (SVR) that is defined as undetectable HCV RNA using polymerase chain reaction (PCR) at 12 or 24 weeks after the end of treatment. The HCV recurrence rate after an SVR is only about 1% in the long term, so it is regarded as a virological cure. The achievement of an SVR can prevent progression to cirrhosis and the development of HCC. However, patients with preexisting hepatic fibrosis should undergo regular surveillance for HCC, since there is a continuing risk of developing HCC even after achieving an SVR [50].
Interferon therapy has been consistently reported to reduce the incidence of HCC in chronic hepatitis C patients compared with untreated controls. In a meta-analysis of 20 studies (4700 patients), the HCC risk was significantly reduced in the interferon treatment group (RR, 0.43; 95% CI, 0.33–0.56) and to a greater extent in patients with an SVR (RR, 0.35; 95% CI, 0.26–0.46) compared to the control group [65]. Another meta-analysis of 30 studies (approximately 25000 patients) reported a 76% reduction in the incidence of HCC in patients with an SVR compared with those without an SVR [66]. These results were consistent regardless of the degree of hepatic fibrosis or the presence of cirrhosis. Direct-acting antivirals (DAAs) against HCV have recently been introduced successively, leading to an SVR achievement rate as high as 98%–100%. A prospective cohort study recruiting 9895 French patients with chronic HCV infection showed that exposure to DAA was associated with a significantly reduced risk for HCC (HR, 0.66; 95% CI, 0.46–0.93) [67]. Other two large-scale independent studies revealed consistent results from DAA treatment: in a study involving a prospective cohort of 2249 Italian cirrhotic patients, the absence of an SVR (HR, 3.40; 95% CI, 1.89–6.12) was independently associated with an increased risk for HCC [68]; another cohort study that prospectively recruited 1760 patients with chronic hepatitis C in Latin America showed that attaining an SVR (HR, 0.2; 95% CI, 0.1–0.8) significantly reduced the risk of de novo occurrence of HCC [69]. In a meta-analysis comparing the risk of HCC between DAA treatment and interferon therapy, the incidence and recurrence rates of HCC were not different between the two treatments after adjusting the follow-up period and patient age [70]. In summary, acquisition of SVR, whether treated with interferon or DAA, leads to a reduced risk of HCC by 70%–75% [70,71]. Therefore, achieving SVR is an important immediate therapeutic goal to reduce the risk of HCC.
Tertiary Prevention of HCC
HCC is associated with a high rate of recurrence even after curative treatment. In fact, the 5-year recurrence rate is as high as 50%–70%; therefore, tertiary prevention is very important. Recurrence within 2 years after curative treatment is highly likely to be metastasis of the primary tumor, and adjuvant cytotoxic chemotherapy has previously been attempted without proving reduction in recurrence or prolongation of survival [50].
There has been a paucity of well-designed RCTs that determined whether antiviral treatment could reduce the incidence of HCC after hepatic resection in patients with chronic HBV or HCV infection. However, many observational studies have reported that oral antiviral therapy after curative treatment of HBV-associated HCC can significantly reduce recurrence of HCC by up to 50% (HR, 0.48; 95% CI, 0.32–0.70) [72]. A meta-analysis that compared HCC recurrence between antiviral-treated and untreated patients after curative treatments (i.e., hepatic resection, radiofrequency ablation [RFA], and percutaneous ethanol injection [PEI]) showed that antiviral treatment for HBV significantly reduced the recurrence of HCC (odds ratio [OR], 0.59; 95% CI, 0.35–0.97), liver-related mortality (OR, 0.13; 95% CI, 0.02–0.69), and overall mortality (OR, 0.27; 95% CI, 0.14–0.50) [73,74]. In a meta-analysis of studies investigating post-operative recurrence of HBV-related HCC, antiviral treatment led to a significant reduction in the overall mortality (HR, 0.69; 95% CI, 0.52–0.92) and recurrence (HR, 0.58; 95% CI, 0.49–0.70) in patients with high-level viremia (HBV DNA ≤ 20000 IU/mL), but this effect was not observed in patients with low-level viremia (HBV DNA < 20000 IU/mL) [75]. There have also been some studies that focused on the differential post-operative recurrence between antiviral drugs. However, it remains inconclusive whether one drug is more effective than the other in reducing the recurrence of HCC, due to an ongoing controversy over the results observed between antiviral drugs [76,77,78]. Rather than comparing the preventive effects between individual antiviral drugs, it is more important to consider various factors beside the viral factor such as tumor factors (tumor size and number, vascular invasion, degree of tumor differentiation), techniques and types of curative treatment, and underlying liver disease which play important roles in the recurrence of HCC after treatment.
In a meta-analysis of interferon therapy after curative treatment for HCV-associated HCC that observed 665 patients for 2 to 7 years, the achievement of an SVR was associated with a 74% reduction in the HCC recurrence rate and a 60% reduction in the mortality rate [79]. In another meta-analysis, HCC recurrence was significantly lower in the interferon-treated group than in the non-treated group after hepatic resection (ORs of 0.52, 0.23, 0.41, 0.37 at 1, 2, 3, and 5 years, respectively) [74]. Earlier reports of cases series suggested that HCC recurrence occurred earlier and more commonly after DAA treatment [80,81]. Regarding such phenomenon, it has been hypothesized that rapid reduction in the HCV viral load with DAAs may cause a decrease in immune surveillance against intrahepatic microscopic tumor clones, leading to an enhanced early recurrence [82,83]. However, recent analyses yielded contradictory results. In a large-scale prospective cohort study of the French Agency for AIDS and Viral hepatitis Research, the recurrence rate after the curative treatment of HCC was not significantly different between the DAA-treated group and the no-treatment group; nevertheless, there was a significantly higher HCC recurrence rate in the no-treatment group in the presence of compensated cirrhosis [84]. In addition, among liver transplant recipients, there was no difference in the incidence of HCC between the DAA-treated and non-treated groups. In a prospective cohort study conducted in Italy, DAA was not associated with HCC recurrence after curative treatment; however, the acquisition of SVR resulted in a significant reduction of HCC recurrence (HR, 0.25; 95% CI, 0.11–0.57) [85]. A series of systematic review and meta-analysis investigating the relationship between DAA treatment and HCC recurrence also showed that DAA treatment did not increase HCC recurrence, but rather appeared to decrease the recurrence of HCC when an SVR was achieved [70,83,86]. Nevertheless, there is considerable heterogeneity among studies in terms of patient characteristics, the timing of DAA administration, duration of follow-up, and the interval or method of surveillance for HCC. Therefore, it is still difficult to conclude whether DAA increases or decreases recurrence after curative treatment of HCC, which remains an open question to be answered in future studies.
There have been some studies that explored the potential effects of NSAIDs, including aspirin, on recurrence in patients with HCC undergoing hepatic resection. The two meta-analyses suggested that only the non-aspirin NSAIDs were associated with significant risk reduction in the recurrence of HCC, unlike aspirin which showed unclear preventive effects against post-treatment recurrence [38,87]. However, these results should be interpreted with caution since the studies represented only a small sample size and conflicting results, together with significant heterogeneity in methodology. It was also reported that the use of these drugs was associated with a non-negligible risk of hemorrhagic complications in patients with HCC. Therefore, the administration of NSAIDs, including aspirin, or antiplatelet agents for the purpose of preventing recurrence should be decided carefully. On the other hand, several retrospective cohort studies have suggested a preventive effect of statin on recurrence after curative treatment of HCC [88,89]. In agreement with the results, two Korean studies involving transplant recipients also showed that statin use was associated with a significant risk reduction of HCC recurrence after liver transplantation (LT) [90,91]. Large-scale prospective studies are needed to confirm the preventive roles of these medications on recurrence after curative treatment of HCC.
[Recommendations]
1. All newborns (A1) and seronegative (negative for all of HBsAg, anti-HBs, and anti-HBc) children and adults should be vaccinated against HBV (B1) to prevent HCC.
2. General HCC preventive measures include the following: prevention of HBV/HCV transmission (A1); avoidance of alcohol abuse; and control of metabolic disorders, such as obesity and diabetes (C1).
3. Antiviral therapy as a secondary prevention of HCC should follow the KASL guidelines for the management of chronic hepatitis B or C (A1).
4. The risk of HCC can be reduced if HBV replication is persistently suppressed in patients with chronic hepatitis B (A1), and if an SVR is achieved by interferon therapy (A2) or DAA therapy (B1) in patients with chronic hepatitis C.
5. Among patients with chronic liver disease, the risk of developing HCC is lower in patients receiving statin therapy for the management of dyslipidemia compared to those undergoing no treatment (B1).
6. Among patients with chronic liver disease, the risk of developing HCC is lower in patients receiving aspirin therapy for the purpose of preventing cardiovascular complications or managing pain and inflammation compared to those undergoing no treatment. However, the administration of aspirin for patients with liver cirrhosis should be considered with caution as the risk of gastrointestinal bleeding may increase (B2).
7. Coffee consumption in patients with chronic liver disease can lower the risk of HCC (B1).
8. After curative treatment of HBV-associated HCC, antiviral therapy should be considered to reduce the risk of HCC recurrence in patients with detectable serum HBV DNA (B1).
9. After curative treatment of HCV-associated HCC, the association of DAA therapy with the risk or prevention of HCC recurrence remains unclear (C1).
SURVEILLANCE
The major purpose of intensive surveillance for cancer is to reduce disease-related mortality. There are two RCTs on the efficacy of surveillance programs in reducing HCC-related mortality among individuals at risk of HCC. In a Chinese study of 5581 chronic hepatitis B patients recruited in the early 1990s, surveillance for HCC using only 6-monthly alpha-fetoprotein (AFP) assays resulted in an earlier diagnosis of HCC; however, the gain in lead time did not result in a significant reduction in overall mortality due to ineffective treatments for HCC [92]. In contrast, a large-scale randomized trial involving 18816 Chinese patients with chronic hepatitis B demonstrated that, despite poor study adherence (58.2%), a strategy of surveillance with ultrasonography (US) and AFP measurement every 6 months significantly reduced HCC-related mortality by 37% compared to no surveillance. In addition, the surveillance strategy was associated with a higher rate of detection of small HCC and surgically amenable HCC, as well as better overall survival (OS) after the diagnosis of HCC [93]. Several non-randomized cohort studies and meta-analyses have also found that surveillance has led to the detection of more early stage HCCs, provided a higher rate of curative treatments, and a significantly better OS than that found in the control group, indicating the compelling justification for HCC surveillance in at-risk patients [94,95,96,97,98]. In a meta-analysis of 32 HCC surveillance studies with a total of 13367 cirrhotic patients, the sensitivity for detecting all stages of HCC was 84% (47% in early stage) with US alone, whereas combining serum AFP and US increased the RR of HCC detection at all stages and early stage to 0.88 and 0.81, respectively [99]. US and serum AFP measurement was reported to be cost-effective as an HCC surveillance tool. In a study using Markov model of 1 million cirrhotic patients, three groups of US alone, US and serum AFP measurement and no surveillance were compared. With the assumption of HCC incidence ≥ 0.4%/year, adherence to surveillance test > 19.5%, and willingness-topay threshold of 100000 USD, performing the combination of US and serum AFP measurement every 6 months was the most cost-effective [100].
Unlike other malignancies, HCC has well-established risk factors that allow the identification of an at-risk patient group. Since approximately 90% of HCC cases are associated with a well-known risk factor, most of the international guidelines have been adapted to perform HCC surveillance in the population at risk of HCC development [95]. Patients with cirrhosis derived from any etiology are regarded as the most important targets to undergo a surveillance program, since more than 80% of patients diagnosed with HCC have underlying cirrhosis. Viral hepatitis is also one of the most important causal risk factors for HCC. Chronic HBV infection is responsible for around 70% of all patients diagnosed with HCC in East Asia, including Korea, whereas chronic HCV infection accounts for around 30% of HCC patients in Western countries, with most of the HCV-associated HCC patients having either cirrhosis or advanced fibrosis at diagnosis. However, one Korean study on patients who underwent hepatic resection shown that 32.5% of HCV-related HCCs were not associated with underlying cirrhosis, indicating a lower rate of HCV-related HCC accompanying cirrhosis than that reported in Western countries [101]. In addition, the risk of HCC also increases with the patient’s age, excessive alcohol drinking, male sex, and diabetes mellitus, and risk is higher among Asian HBV carriers with high viral activity and family history of the disease, and chronic hepatitis B patients with cirrhosis or advanced fibrosis [102,103]. Based on a cost-effectiveness study, it is generally accepted that an annual incidence of HCC surpassing 1.5% would warrant a surveillance scheme of HCC in cirrhotic patients [104]. However, patients with chronic HBV infection can develop HCC in the absence of underlying cirrhosis. Therefore, expert opinion indicates that HCC surveillance for chronic HBV carriers is deemed to be cost-effective if the annual incidence exceeds 0.2% [105]. Given this definition, patients with liver cirrhosis of all etiologies, chronic HBV infection, or chronic HCV infection with cirrhosis or advanced fibrosis are the major target population for surveillance as a high risk group for HCC. From a pooled analysis of previously published studies on the natural history of various liver diseases, patients with liver cirrhosis are at the highest risk of developing HCC, irrespective of etiology. Patients with chronic HBV infection and those with HCV-related cirrhosis or advanced fibrosis are also at a high risk of HCC, of which annual incidences exceed 0.2% and 1.5%, respectively [95,105].
In particular, HCV-infected patients with cirrhosis or advanced liver fibrosis (≥ F3) need to receive HCC surveillance even after they achieve SVR by DAA treatment. Transient elastography is known to well predict the risk of HCC development in treatment-naïve HCV-infected patients. However, data are scarce regarding the performance of transient elastography in predicting the risk of HCC in HCV patients who achieved SVR after antiviral therapy. In addition, since patients who achieved SVR may still develop HCC, if cirrhosis or advanced fibrosis had not been ruled out by biopsy, patients should be in the HCC surveillance program [81,106,107,108].
According to the increasing availability of non-invasive bio-markers or imaging which assess liver fibrosis, it has been suggested that patients with NAFLD who were found to have cirrhosis or advanced fibrosis by these tests should receive HCC surveillance. For example, if FIB-4, which is a non-invasive liver fibrosis marker using age, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and platelet count, is more than 2.67, there is a high probability of cirrhosis or bridging fibrosis. Thus, in this case, a patient with NAFLD needs to receive HCC surveillance [109].
In general, US with or without AFP is widely used as a tool for HCC surveillance in high-risk patients. However, globally there are some regional discrepancies regarding the recommended surveillance methods. Among tumor markers relevant to HCC, information on surveillance are mostly limited to AFP, and therefore almost all studies focusing on the effectiveness of a surveillance program have implemented only AFP as a tumor marker for HCC. The sensitivity of detecting an early stage HCC in high-risk patients is reportedly approximately 60% when performing surveillance using US with or without serum AFP measurement [110,111,112]. The sensitivity and specificity of US as a surveillance tool for HCC in patients with chronic HBV infection were reported to be 65%–80% and over 90%, respectively, with a higher sensitivity compared to serum markers such as AFP [98,113]. While AFP measurement and US are imperfect tools, they appear to be mutually complementary [103]. In a meta-analysis of 16 relevant studies, the combined use of US and AFP measurement yielded a higher sensitivity for HCC detection compared to US alone (0.79 [95% CI, 0.57–0.91] vs. 0.69 [95% CI, 0.46–0.85]), although it was not statistically significant [98]. In another meta-analysis of 13 selected studies, the pooled sensitivity for detecting early-stage HCC increased from 63% with US alone to 70% with US combined with AFP measurement [94]. A pooled analysis of seven studies on patients with cirrhosis showed that US with and without AFP measurement detected early-stage HCC with 63% sensitivity (95% CI, 48%–75%) and 45% sensitivity (95% CI, 30%–62%), respectively, indicating a higher sensitivity by US combined with AFP measurement than by US only [99]. The performance of surveillance varies depending on the cut-off levels of biomarkers and the prevalence of HCC among the general population in the region. In the United States and Europe, where the prevalence of HCC is relatively low, only the US examination is often recommended as a surveillance method. On the other hand, in South Korea and Japan, where the HCC prevalence is high, it is recommended to perform US with serum AFP measurement for HCC surveillance in the high-risk population [114,115,116].
The interval of cancer surveillance should be determined based on tumor doubling time, time to stage migration to enable curative treatments at diagnosis, cost-effectiveness, and its impact on patient survival. Although the optimal surveillance intervals for patients at risk of HCC are yet to be clearly determined, the intervals of HCC surveillance recommended by most of the regional guidelines range from 3 to 12 months [105,114,115,116,117]. An Italian study that compared 6- vs. 12-month surveillance failed to increase the detection rate of a single nodular tumor with 6-month surveillance compared to 12-month surveillance [118]. A RCT that evaluated more intense surveillance of 3- vs. 6-month intervals also provided similar results in detecting small HCCs [119]. In contrast, another Italian study on the performance of semiannual surveillance showed that it increased the detection rate of early-stage HCC and patient survival compared to an annual program [97]. Another randomized trial evaluating US as a surveillance tool in Taiwanese patients with viral hepatitis demonstrated that a 4-month interval scheme performed better in detecting very early stage HCC compared to a 12-month interval, although it did not provide a survival benefit [120]. Moreover, the pooled sensitivity of detecting HCC increased from 50% with the annual scheme to 70% with the semiannual surveillance [94]. In a cost-effective study, a semiannual US surveillance program in cirrhotic patients also resulted in improved clinical outcomes at a reasonable cost [121]. The mean tumor doubling time of small HCCs (< 5 cm) is estimated to be around 4–7 months, ranging between 136 and 204 days [122,123], and semiannual surveillance was the interval employed in the only RCT that showed a survival benefit with an HCC surveillance scheme [93]. Thus, taken together, a 6-month interval for an HCC surveillance program would be considered a preferable and reasonable strategy.
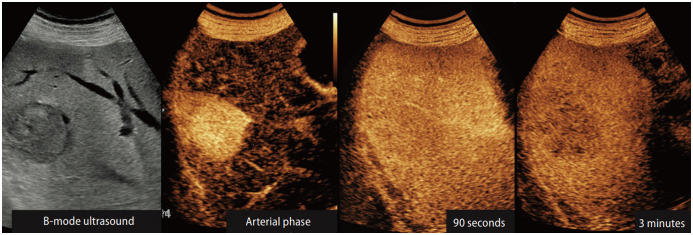
Given that the incidence of HCC varies according to the cause of underlying liver disease and the degree of cirrhosis even in the high-risk group, some groups may be at a higher risk of HCC than others. Under circumstances in which HCC is highly suspected, contrast-enhanced US (CEUS), liver dynamic computed tomography (CT), or contrast-enhanced magnetic resonance imaging (MRI) can be performed as an alternative to US when an US examination fails to detect nodules or is incomplete due to poor visualization. With the advantage of being able to assess the blood supply and vascular invasion of tumors, CEUS has been found to be more cost-effective in surveillance for HCC than US alone [124].
A recent randomized trial that compared biannual US with yearly contrast CT has shown the former to be marginally more sensitive and less expensive for the detection of early HCC in patients with compensated cirrhosis. Recently, MRI with liver-specific contrast in a surveillance setting of cirrhotic patients has resulted in a higher detection rate of HCC and lower false-positive findings compared to US [125]. Due to the incomplete performance of US as a surveillance tool, the need for an alternative imaging test which can avoid radiation exposure and contrast agent is increasing. An abbreviated MRI with or without contrast agent reduced the scanning time and images acquired, and it is gaining attention as an alternative tool to US in HCC surveillance. In three prospective studies and 12 retrospective studies, 917 patients developed HCC among 2807 patients who received surveillance with abbreviated MRI or US. In a meta-analysis of these 15 studies, the sensitivity and specificity of non-contrast abbreviated MRI were similar to those of contrast-enhanced abbreviated MRI (86% vs. 94%; 87% vs. 94%, respectively). Also, the sensitivity of abbreviated MRI was higher compared to US (82% vs. 53%) [126]. However, the information on the alternative surveillance imaging strategies is very limited and should be interpreted carefully. Study results regarding the diagnostic performance of CT or MRI for HCC cannot be directly extrapolated to the setting of cancer surveillance. Regarding abbreviated MRI, most studies were retrospective and non-randomized. Particularly, the safety of MRI contrast has not been guaranteed in a surveillance setting, which might be another limitation of contrast-enhanced MRI as a surveillance tool. In addition, the risks, accessibility, and cost-effectiveness of these alternative imaging methods should be meticulously evaluated. Therefore, further studies on the accuracy, costs, and potential harms regarding these new radiological modalities are needed before the wide implementation of the alternative imaging surveillance strategies.
[Recommendations]
1. Surveillance for HCC should be performed in high-risk groups; patients with chronic hepatitis B (A1), chronic hepatitis C (B1), and liver cirrhosis (A1).
2. Surveillance test for HCC should be performed with liver US plus serum AFP measurement every 6 months (A1).
3. When liver US cannot be performed adequately, dynamic contrast-enhanced CT or dynamic contrast-enhanced MRI can be performed as an alternative (C1).
DIAGNOSIS
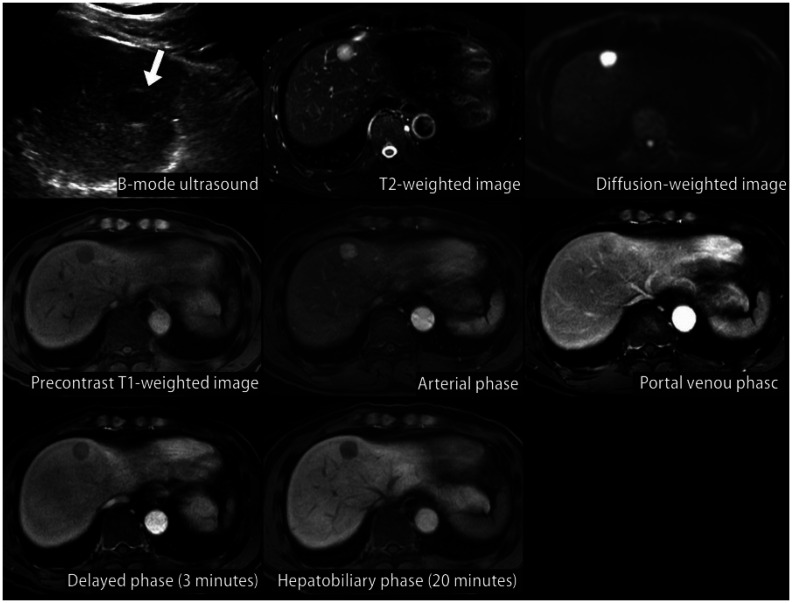
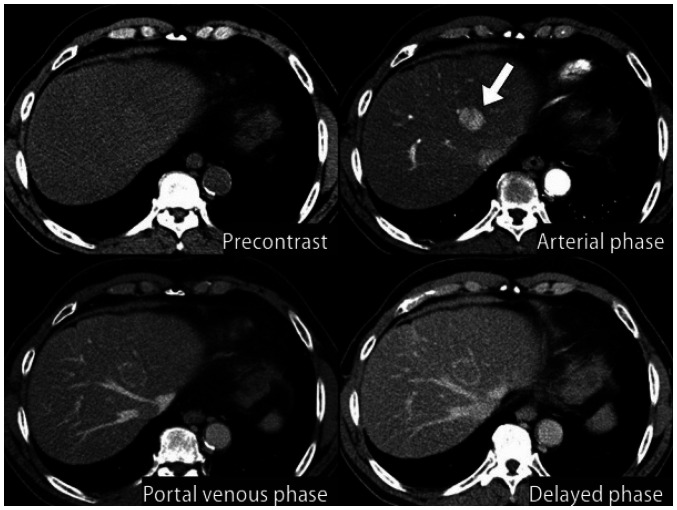
HCC can be diagnosed either pathologically by biopsy or clinically by the use of non-invasive imaging in high-risk groups (chronic hepatitis B, chronic hepatitis C, or cirrhosis) [127,128,129,130,131,132,133]. If a new liver nodule ≥ 1 cm in size is detected by surveillance test in high-risk patients, a first-line imaging study, such as dynamic contrast-enhanced CT or dynamic contrast-enhanced MRI with extracellular contrast agents or hepatocyte-specific contrast agents like gadoxetic acid (gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid; Gd-EOBDTPA), should be performed for the imaging diagnosis of HCC (Fig. 3). Since imaging-based diagnosis of HCC relies on the dynamic contrast enhancement characteristics on multiphasic CT or MRI, single phase CT or MRI may not be used as a diagnostic tool. The etiology of cirrhosis does not infuence the imaging diagnosis of HCC but it should be applied with caution in patients with cirrhosis due to vascular disorders, such as Budd-Chiari syndrome, or due to Fontan-associated liver disease, as such conditions are often accompanied with benign hyperplastic nodules that can mimic HCC on imaging [134,135].
Fig. 3. Diagnostic algorithm.
*The radiological hallmarks for diagnosing “definite” HCC on multiphasic contrast-enhanced CT or magnetic resonance imaging (MRI) are arterial phase hyperenhancement (APHE) with washout appearance in the portal venous, delayed, or hepatobiliary phases. These criteria should be applied only to a lesion that does not show either marked T2 hyperintensity or targetoid appearances on diffusion-weighted images or contrast-enhanced images. For a second-line imaging modality, the radiologic hallmarks of contrast-enhanced ultrasonography (blood-pool contrast agent or Kupffer cell-specific contrast agent) for a “definite” diagnosis of HCC are APHE with mild and late (≥60 seconds) washout. These criteria should be applied only to a lesion that does not show either rim or peripheral globular enhancement in the arterial phase. †For the diagnosis of “probable” HCC, ancillary imaging features are applied as follows: there are two categories of ancillary imaging features, including imaging features favoring malignancy in general (mild-to-moderate T2 hyperintensity, restricted diffusion, threshold growth) and those favoring HCC in particular (enhancing or non-enhancing capsule, mosaic architecture, nodule-in-nodule appearance, fat or blood products in the mass). For nodules without APHE, “probable” HCC can be assigned only when the lesion fulfills at least one item from each of the two categories of ancillary imaging features. For nodules with APHE but without washout appearance, “probable” HCC can be assigned when the lesion fulfills at least one of the aforementioned ancillary imaging features. HCC = hepatocellular carcinoma, CHB = chronic hepatitis B, CHC = chronic hepatitis C, CT = computed tomography
A recent meta-analysis regarding the imaging diagnosis of HCC showed a per-lesion sensitivity of 66% (95% CI, 60%–72%) for multiphasic CT and 82% (95% CI, 75%–87%) for multiphasic MRI (extracellular contrast agents or hepatocyte-specific contrast agents), and a per-lesion specificity of 92% (95% CI, 84%–96%) for multiphasic CT and 91% (95% CI, 82%–95%) for multiphasic MRI [136]. Using the 2018 KLCA-NCC imaging criteria for HCC diagnosis, recent retrospective studies reported that MRI using hepatocyte-specific contrast agent had a per-lesion sensitivity of 87% and a per-lesion specificity of 86% [137], and MRI using hepatocyte-specific contrast agent had a higher sensitivity than extracellular contrast agent (79% vs. 69%), but similar specificity (96% vs. 94%) [138].
When an imaging diagnosis of HCC cannot be made on a first-line imaging study, a second-line imaging study can be applied to enhance the sensitivity of HCC diagnosis [139,140]. Imaging modalities for second-line studies include multiphasic CT, multiphasic MRI with extracellular contrast agents or hepatocyte-specific contrast agents, and CEUS with blood-pool contrast agents or Kupffer cell-specific contrast agents (Fig. 3). CEUS with blood-pool contrast agents showed high specificity for HCC diagnosis in a recent large multi-center retrospective study [141]. Moreover, a meta-analysis found that CEUS had a sensitivity of 84% (95% CI, 79%–87%) and a positive predictive value of 89% (95% CI, 86%–93%), which was comparable to multiphasic CT and MRI with extracellular contrast agents [142]. However, considering that the purpose of diagnostic imaging study also includes determining the tumor extent and staging, CEUS has limitations in these aspects, and therefore, is not recommended as a first-line imaging study. Instead, it can be used as one of second-line imaging studies if the first-line imaging study is inconclusive.
Non-invasive diagnosis of “definite” HCC is based on the radiological hallmarks on multiphasic CT or multiphasic MRI (extracellular contrast agents or hepatocyte-specific contrast agents) for a liver nodule ≥ 1 cm detected in high-risk patients. The radiological hallmarks for diagnosing “definite” HCC are arterial phase hyperenhancement (APHE) with washout appearance in the portal venous, delayed, or hepatobiliary phases (hepatobiliary phase finding is included if hepatocyte-specific contrast agents are used) (Table 3, Fig. 4). The definition of each imaging feature used for HCC diagnosis in this guideline adopts the latest Liver Imaging Reporting and Data System (LI-RADS) lexicon (https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS).
Table 3. Diagnosis of Hepatocellular Carcinoma.
| Imaging Modality | Role in HCC Diagnosis | Assessment of “Washout” Appearance | ||
|---|---|---|---|---|
| Timing | Degree | Preconditions | ||
| Multiphasic contrast-enhanced CT | First- and second-line imaging study | Portal venous phase or delayed phase | All | No targetoid appearance on contrast-enhanced images |
| Multiphasic MRI using extracellular contrast agent | First- and second-line imaging study | Portal venous phase or delayed phase | All | Neither marked T2 hyperintensity nor targetoid appearances on diffusion-weighted images or contrast-enhanced images |
| Multiphasic MRI using hepatocyte-specific contrast agent | First- and second-line imaging study | Portal venous phase or delayed phase or hepatobiliary phase | All | |
| Contrast-enhanced US using blood-pool contrast agent | Second-line imaging study | Late vascular phase (≥ 60 seconds) | Mild | No rim or peripheral globular enhancement on arterial phase; no early washout (<60 seconds); no punch-out pattern washout within 120 seconds |
| Contrast-enhanced US using Kupffer cell-specific contrast agent | Second-line imaging study | Late vascular phase (≥ 60 seconds) or Kupffer phase | Mild (if late vascular phase) | |
1. Imaging diagnosis: in high-risk patients (chronic hepatitis B, chronic hepatitis C, and cirrhosis), a liver nodule ≥ 1 cm detected by surveillance test can be diagnosed as an HCC if it shows radiological hallmarks of HCC. When an imaging diagnosis of HCC cannot be made with confidence on a first-line imaging study, an additional second-line imaging study can be applied. (1) Major imaging features are defined as arterial phase hyperenhancement and washout appearance on portal venous, delayed, or hepatobiliary phases on dynamic contrast-enhanced CT or dynamic contrast-enhanced MRI (extracellular contrast agent or hepatocyte-specific contrast agent). These criteria should be applied only to a lesion that does not show either marked T2 hyperintensity or targetoid appearances on diffusion-weighted images or contrast-enhanced images. (2) When contrast-enhanced ultrasound (blood-pool contrast agent or Kupffer cell-specific contrast agent) is performed as a second-line imaging study, arterial phase hyperenhancement and mild and late (≥ 60 seconds) washout are radiological hallmarks of HCC. These criteria should be applied only to a lesion that does not show rim or peripheral globular enhancement on the arterial phase.
2. Pathologic diagnosis: if the patient does not have any risk factor for HCC or the nodule does not show typical radiological hallmarks of HCC, a biopsy can be performed for confirmative diagnosis.
HCC = hepatocellular carcinoma, CT = computed tomography, MRI = magnetic resonance imaging, US = ultrasonography.
Fig. 4. Definite hepatocellular carcinoma (HCC) on multiphasic magnetic resonance imaging (MRI) with hepatocyte-specific contrast agent.
A 1.7-cm liver nodule (arrow) is detected on surveillance ultrasound in a patient with liver cirrhosis. The lesion shows the radiological hallmarks of HCC, i.e., arterial phase hyperenhancement and washout appearance (portal venous phase, delayed phase, and hepatobiliary phase) on multiphasic MRI using hepatocyte-specific contrast agent (gadoxetic acid) but does not show marked T2 hyperintensity or targetoid appearances on diffusion-weighted images and contrast-enhanced images. Therefore, this nodule can be noninvasively diagnosed as “definite” HCC.
Prospective studies have demonstrated that the imaging criteria of APHE with washout appearance on portal venous or delayed phases on multiphasic CT or MRI resulted in sensitivities of 65%–89% and specificities of 91%–100% [139,140]. Following these criteria provides high specificity but limited sensitivity, especially for nodules less than 2 cm in diameter (sensitivity, 41%–62%) [143,144]. However, when hypointensity in the hepatobiliary phase is also considered equal to washout appearance, sensitivity is increased [145,146,147]. Given the medical environments in South Korea where hepatocyte-specific contrast agent is commonly used for liver MRI and pursues early detection and treatment of HCC, high sensitivity is preferred for the diagnosis of HCC. Therefore, since the previous version (ver. 2018), KLCA-NCC guidelines have defined washout appearances in not only the portal venous and delayed phases but also the hepatobiliary phase. It should be noted that this principle carries the risk of misdiagnosis of hemangioma and intrahepatic cholangiocarcinoma (CCA) as an HCC [147]. Therefore, in order to exclude hemangioma and intrahepatic CCA, these diagnostic criteria should not be applied in lesions showing marked T2 hyperintensity or targetoid appearances on diffusion-weighted images or contrast-enhanced images. In addition, focal eosinophilic liver diseases are relatively common in South Korea, which can mimic HCC on imaging, especially on MRI using hepatocyte-specific contrast agents. To avoid a false-positive diagnosis, the peripheral eosinophil count should be checked before making an imaging diagnosis of HCC [148]. For the assessment of APHE, the use of arterial subtraction images can increase the sensitivity of HCC diagnosis by detecting more APHEs, especially for nodules with precontrast T1 hyperintensity or with equivocal enhancement on arterial phase images [149,150,151]. However, to avoid false-positive diagnosis, the use of arterial subtraction imaging to detect APHE is recommended only in lesions without rim APHE. In addition, for the imaging diagnosis of HCC, recent studies have reported that the combination of imaging findings from multiphasic CT and multiphasic MRI may improve diagnostic performance compared to CT or MRI alone [152,153].
If there is a tumor thrombus in the portal vein or hepatic vein, which is often associated with HCC, HCC can be diagnosed based on imaging findings of the contiguous parenchymal mass. In cases of HCC with tumor thrombus, the parenchymal mass frequently shows atypical imaging features, and sometimes only tumor thrombi are present without a visible parenchymal mass, making it difficult to diagnose HCC [154]. Since non-HCC malignancies, including intrahepatic CCA or combined hepatocellular-cholangiocarcinoma (combined HCC-CCA), may also rarely be accompanied by tumor thrombus [155,156], it would be inappropriate to diagnose HCC with the sole finding of tumor thrombus on imaging.
When CEUS (blood-pool contrast agents or Kupffer cell-specific contrast agents) is performed as a second-line imaging study for a nodule ≥ 1 cm detected in high-risk patients, the radiological hallmarks for diagnosing “definite” HCC are APHE with late (≥ 60 seconds) and mild washout or washout appearance in the Kupffer phase (Kupffer phase finding is included if Kupffer cell-specific contrast agents are used) (Fig. 5). If a nodule shows early washout (< 60 seconds) or punched-out pattern washout within 120 seconds after contrast injection, it should be excluded due to the possibility of non-HCC malignancies, such as intrahepatic CCA or metastasis [157]. In addition, these criteria should not be applied to lesions presenting with rim or peripheral globular enhancement on arterial phase, which are typical imaging features of intrahepatic CCA and hemangioma, respectively [157]. As discussed above, CEUS with blood-pool contrast agent showed comparable diagnostic performance to multiphasic CT or MRI [142]. Moreover, regarding CEUS with Kupffer cell-specific contrast agents, a recent meta-analysis found a good overall diagnostic performance, with a sensitivity of 90% (95% CI, 82%–95%) and a specificity of 97% (95% CI, 93%–98%) [158]. A prospective intra-individual comparative study reported that CEUS with Kupffer cell-specific contrast agents had a significantly higher sensitivity compared to CEUS with blood-pool contrast agents (79% [95% CI, 64%–90%] vs. 54% [95% CI, 38%–67%]), without difference in specificity (100% [95% CI, 79%–100%] vs. 100% [95% CI, 79%–100%]) [159]. In another prospective study, CEUS with Kupffer cell-specific contrast agents demonstrated diagnostic performances similar to multiphasic CT or multiphasic MRI [160].
Fig. 5. Definite hepatocellular carcinoma (HCC) on contrast-enhanced ultrasound.
A 3.5-cm liver nodule is detected in a patient with chronic hepatitis B. On contrast-enhanced ultrasound using blood-pool contrast agent, the nodule shows arterial phase hyperenhancement and mild washout on 3 minutes delayed image. Therefore, it can be noninvasively diagnosed as “definite” HCC.
In nodules ≥ 1 cm that do not meet the non-invasive diagnostic criteria of “definite” HCC, a diagnosis of “probable” HCC can be assigned by applying ancillary imaging features (Table 4, Figs. 6, 7) [161]. There are two categories of ancillary imaging features: i) imaging features favoring malignancy in general (mild-to-moderate T2 hyperintensity, restricted diffusion, threshold growth) and ii) those favoring HCC in particular (enhancing or non-enhancing capsule, mosaic architecture, nodule-in-nodule appearance, fat or blood products in the mass). For nodules without APHE, “probable” HCC can be assigned only when the lesion fulfills at least one item from each of the two categories of ancillary imaging features. For nodules with APHE but without washout appearance, “probable” HCC can be assigned when the lesion fulfills at least one of aforementioned ancillary imaging features. Like “definite” HCC, the diagnosis of “probable” HCC should be applied only to a lesion which does not show either marked T2 hyperintensity or targetoid appearances on diffusion-weighted images or contrast-enhanced images to rule out the possibility of hemangioma or intrahepatic CCA. “Probable” HCC in this guideline corresponds to the concept of LR-4 (probably HCC) of the LI-RADS. In a recent meta-analysis, the pooled percentages of LR-4 nodules confirmed as HCC and overall malignancy were 74% and 80%, respectively [162]. During follow-up, 20%–34% of LR-4 nodules progressed to LR-5 (definitely HCC) within 3 months and 37%–75% to LR-5 within 6 months [163,164,165]. For “probable” HCC, therefore, a follow-up imaging study within 3 months or biopsy should be considered, and a treatment plan for the lesion may be determined through multidisciplinary discussion.
Table 4. Imaging Diagnosis of Probable HCC.
| Diagnostic Criteria for Probable HCC | |
|---|---|
| In nodules ≥ 1 cm that do not meet the major imaging features of HCC, a diagnosis of “probable” HCC can be assigned by applying ancillary imaging features: 1) nodule without APHE: at least one each of the ancillary features of group A and group B; 2) nodule with APHE but without washout appearance: at least one of the ancillary features in group A or B. | |
| These criteria should be applied only to a lesion that does not show either marked T2 hyperintensity or targetoid appearances on diffusion-weighted images or contrast-enhanced images. | |
| Ancillary Imaging Features of HCC | |
| Ancillary features suggesting malignancy in general (group A) | Ancillary features favoring HCC in particular (group B) |
| ·Mild-to-moderate T2 hyperintensity | ·Enhancing or non-enhancing capsule |
| ·High signal intensity on diffusion-weighted imaging | ·Mosaic architecture |
| ·Threshold growth* | ·Nodule-in-nodule |
*Threshold growth is defined as a size growth of the nodule of at least 50% in the longest dimension in ≤ 6 months on computed tomography or magnetic resonance imaging [165]. HCC = hepatocellular carcinoma, APHE = arterial phase hyperenhancement
Fig. 6. Probable hepatocellular carcinoma (HCC) on dynamic contrast-enhanced computed tomography (CT).
On dynamic contrast-enhanced CT in a patient with chronic hepatitis B, there is a 2-cm liver nodule (arrow) with arterial phase hyperenhancement. This nodule does not show a washout appearance in the portal venous phase or delayed phase, so it cannot be non-invasively diagnosed as “definite” HCC. However, based on the presence of enhancing capsule in the portal venous phase and delayed phase, an ancillary imaging feature of HCC, this nodule can be diagnosed as “probable” HCC.
Fig. 7. Probable hepatocellular carcinoma (HCC) on multiphasic magnetic resonance imaging (MRI) with hepatocyte-specific contrast agent.
On multiphasic MRI with hepatocyte-specific contrast agent (gadoxetic acid), a 2.5-cm nodule (arrows) is found in segment VII of the liver in a patient with liver cirrhosis. This lesion is indistinguishable from surrounding liver parenchyma on precontrast T1-weighted image and arterial phase image but shows hypointensity on portal venous phase, delayed phase, and hepatobiliary phase images. Since it does not show arterial phase hyperenhancement, an imaging diagnosis of “definite” HCC cannot be made. However, it shows mild-to-moderate T2 hyperintensity and focal signal drop on the opposed phase image in comparison with an in-phase image, which suggests the presence of intra-tumoral fat. Therefore, based on MRI ancillary imaging features, this nodule can be diagnosed as a “probable” HCC.
For nodules detected by surveillance, if imaging studies cannot make a diagnosis of “definite” or “probable” HCC, they can be assigned as an “indeterminate” nodule. The category of “indeterminate” corresponds to the concept of LR-3 (indeterminate probability of malignancy) of the LI-RADS. In a recent meta-analysis, the pooled percentages of LR-3 nodules confirmed as HCC and overall malignancy were 38% and 40%, respectively [162]. During follow-up, 0%–25.8% of LR-3 lesions progressed to LR-5 (definitely HCC) within 6 months and 8.9%–57.3% to LR-5 in 6–12 months [163,164,165]. In addition, according to a Korean domestic study on the prediction of progression to HCC, among 474 indeterminate nodules ≤ 2 cm in HBV-related cirrhosis, 17% progressed to HCC during a median follow-up of 36 months. In this study, old age, presence of APHE, large nodule size (> 1 cm), low serum albumin level (≤ 3.5 g/dL), and high serum AFP level (≥ 100 ng/mL) were identified as independent risk factors for progression to HCC [166]. For an “indeterminate” nodule, a follow-up imaging study within 6 months or biopsy should be considered, taking into account the probability of HCC and its potential future progression to HCC. The International Liver Cancer Association recommends follow-up of up to 2 years for indeterminate nodules, considering the doubling time of HCC [167].
For subcentimeter nodules detected on HCC surveillance in high-risk patients, follow-up surveillance within 6 months is recommended. With recent advances in imaging techniques, subcentimeter nodules with characteristic imaging features of HCC are more commonly found. Some HCC guidelines from Asian countries allow the imaging diagnosis of subcentimeter HCC [116,129,168]. In addition, recent studies have revealed that the use of ancillary imaging features may improve the diagnostic performances for subcentimeter HCCs [148,169,170,171,172]. However, the sensitivity of imaging diagnosis for subcentimeter HCCs is reported to be lower than that of HCCs ≥ 1 cm (< 1 cm vs. ≥ 1 cm: 31% vs. 82%, p < 0.001 for CT; 48% vs. 88%, p = 0.02 for MRI) [173]. Even MRI with hepatocyte-specific contrast agents showed a significantly lower per-lesion sensitivity (46%) and positive predictive value (48%) for subcentimeter HCCs than those for HCCs ≥ 1 cm (sensitivity, 95%; positive predictive value, 78%) [171]. In a retrospective study of subcentimeter nodules showing typical imaging features on MRI with hepatocyte-specific contrast agents, the specificity for HCC diagnosis was reported to be 50% [174], which was very low compared to the specificity of approximately 90% in nodules ≥ 1 cm [137]. These results suggest that the probability of a false positive diagnosis is high for subcentimeter nodules. Therefore, a conservative approach is preferred in subcentimeter nodules, with close monitoring of interval growth or changes in the imaging features in follow-up studies within 6 months.
For the pathologic diagnosis of HCC, biopsy is considered a relatively safe procedure. However, in clinical practice, it is often difficult to perform a biopsy due to the presence of ascites, bleeding risk associated with poor hepatic function, concerns for needle track seeding, or challenges in tumor targeting. Biopsy techniques for the liver nodules in cirrhotic patients include core needle biopsy, fine needle aspiration cytology, and fine needle aspiration biopsy. Among them, only core needle biopsy is recommended for the diagnosis of early HCC or dysplastic nodule, as it enables the observation of cellular and structural atypia. Cytology examination methods, such as fine needle aspiration cytology and fine needle aspiration biopsy, can be helpful in the diagnosis of advanced HCC with moderate or poor differentiation. The sensitivity of the pathologic diagnosis for HCC has been reported to be about 72%; however, it varies depending on the tumor location, size, and degree of differentiation. Its sensitivity is lower in small HCCs of < 2 cm [175,176], or when tumors that are difficult to target are included [175]. As the risk of tumor seeding due to biopsy has been reported to be 0.6%–5.1%, there is considerable objection to the biopsy procedure in patients who are likely to be cured by surgery or LT [177,178]. Moreover, with biopsy, it is difficult to detect stromal invasion which is a critical clue to differentiate early HCC from dysplastic nodule, and the false negativity of biopsy was reported to be approximately 33% [175,176]. Hence, the majority of HCCs are non-invasively diagnosed using imaging studies in clinical practice.
Clinical interests in the pathologic diagnosis in addition to the imaging diagnosis have recently been increasing in order to diagnose HCC at an earlier phase. Since the majority of early HCC consists of well-differentiated tumor, histologic analysis (a combination of small cell change and increased cell density [> 2 times that of the surrounding tissue], pseudo-glandular pattern, unpaired arteries and frequent absence of portal veins, and stromal invasion), together with immune-histochemical staining of the relevant markers (marker panel; heat shock protein 70, glypican 3, and glutamine synthetase) are useful for its diagnosis. In particular, when two of the above markers are positive, the sensitivity and specificity for diagnosing early HCC were reported to be 60% and 100%, respectively [179]. Given that imaging studies sometimes fail to differentiate between HCC and less common primary liver cancers, including combined HCC-CCA and intrahepatic CCA, biopsy is required when an accurate diagnosis is difficult due to atypical imaging features or an atypical clinical course. Confirmatory biopsy should also be considered for differential diagnosis of tumors that are refractory to the best standard treatment. For HCC or CCA with poor differentiation, it is hard to differentiate them only by histological findings; therefore, the diagnosis should be made by integrating the results of various immune-histochemical staining to identify hepatocyte differentiation (arginase-1, Hep Par-1, polyclonal carcinoembryonic antigen (CEA), CD10, glypican-3, and AFP, etc.) or cholangiocyte differentiation (K7, K19, and EpCAM, etc.) [180]. In addition, K19-expression, which is found in 4%–28% of HCCs, is associated with poor prognosis, and in some cases it is necessary to differentiate the tumor from intrahepatic CCA when it is positive [181]. Based on the recent molecular and histopathologic findings, approximately 35% of HCCs can now be classified into specific subtypes. It has been reported that, in comparison to the conventional HCC, macrotrabecular-massive, neutrophil-rich, and vessel encapsulating tumor clusters (VETC) subtypes show worse prognosis; lymphocyte-rich and clear cell subtypes show relatively favorable prognosis; fibrolamellar, steatohepatitic, and chromophobe subtypes show similar prognosis; and scirrhous subtype shows similar or worse prognosis [182]. Recent advances in pathogenetic studies have suggested several categories according to the histopathologic features of HCC, which seem to be helpful in predicting the treatment response or prognosis in clinical practice or identifying therapeutic targets [183]. However, there are still no histological biomarkers that can directly guide treatment decisions. Therefore, in HCCs that can be diagnosed by imaging, it is necessary to further evaluate the role and value of biopsy in the upcoming era of precision medicine [184,185]
The role of serological biomarkers in diagnosing HCC is limited due to their high false-positive and false-negative rates [186]. Serum AFP levels remain within the normal range in 35% of patients with small HCCs, whereas the levels can be elevated not only in HCC patients but also in non-specific conditions, such as aggravation of hepatitis and active regeneration of hepatocytes [112,187,188]. Therefore, AFP alone is insufficient to make a diagnosis of HCC. Although recent retrospective multi-center studies have reported that serum AFP levels could improve the performance of distinguishing HCC from other diagnoses when combined with imaging features [189,190], the practical interpretation and application of these results have not been established yet.
To date, the criteria for diagnostic imaging on recurrent intrahepatic HCC are not well-established. However, in patients previously diagnosed with HCC, high sensitivity should be pursued since the pre-test probability of HCC is higher than those without [191,192]. Therefore, newly detected or growing nodules in a follow-up study of patients with a history of prior HCC can be diagnosed as recurrent HCC regardless of size, if they show radiological hallmarks of HCC or ancillary imaging features of HCC with an increase in size.
Radiation Exposure Dose and the Risk of CT Examination in HCC Patients
The International Commission on Radiological Protection (ICRP) reported that the cancer risk after radiation exposure exhibits a linear no-threshold dose-response relationship [193,194]; therefore, it is critical to minimize the medical radiation exposure. However, there has been no report on the direct risk of diagnostic radiation exposure to patients. The dose of radiation exposure from four-phase liver CT is approximately 20–30 mSv. Moreover, according to the Biological Effects of Ionizing Radiation VII phase 2, the additional lifetime attributable risk of incidence and mortality of solid cancer or leukemia were reported as 0.148% and 0.09%, respectively, in a 50-year-old man exposed to 25 mSv of medical radiation [195,196]. The ICRP 2007 recommendations on radiological protection included the following: “Dose limits do not apply to medical exposures. If they did, the effectiveness of diagnosis or treatment might be reduced, doing more harm than good for the patient. The emphasis is on justification of medical procedures and optimization of protection” [197]. In addition, the risk of radiation-associated malignancy is considered less significant in patients with decreased life expectancy, such as elderly or severely ill patients [198]. For this reason, it is not recommended to strictly limit the radiation dose for the diagnosis and follow-up evaluation of HCC. However, unnecessary CT examinations should be avoided, and alternative imaging studies should be considered particularly in patients with long life expectancy. Recently, various dose reduction techniques that do not impair the image quality or diagnostic ability for focal liver lesions, such as iterative reconstruction or deep learning-based reconstruction combined with low tube voltage, are being developed [199,200,201,202,203]. To optimize radiation exposure, the use of low-dose CT techniques as well as alternative imaging modalities, such as MRI, should to be considered in HCC patients.
[Recommendations]
1. The diagnosis of HCC can be made pathologically or using the typical hallmarks of HCC obtained by non-invasive imaging in high-risk groups (chronic hepatitis B [A1], chronic hepatitis C [B1], or cirrhosis [A1]).
2. For a new liver nodule ≥ 1 cm detected by surveillance tests in high-risk patients, multiphasic CT, or multiphasic MRI (extracellular contrast agents or hepatocyte-specific contrast agents) should be performed as a first-line imaging study for the diagnosis of HCC (A1). If first-line imaging study is inconclusive for the diagnosis of HCC, second-line imaging study including multiphasic CT, multiphasic MRI (extracellular contrast agents or hepatocyte-specific contrast agents), and contrast-enhanced US (blood-pool contrast agents or Kupffer cell-specific contrast agents) can be applied (B1).
3. Imaging diagnosis of “definite” HCC can be made for the nodule ≥ 1 cm detected by surveillance tests in high-risk patients based on the following radiological hallmarks: (1) the radiological hallmarks in multiphasic CT or MRI with extracellular contrast agents are APHE with washout appearance in the portal venous or delayed phases (A1). (2) The radiological hallmarks in multiphasic MRI with hepatocyte-specific contrast agents are APHE with washout appearance in the portal venous, delayed, or hepatobiliary phases; these criteria should be applied only to a lesion which does not show either marked T2 hyperintensity or targetoid appearances on diffusion-weighted images or contrast-enhanced images (B1). (3) The radiological hallmarks in contrast-enhanced US (blood-pool contrast agents or Kupffer cell-specific contrast agents) performed as a second-line imaging study are APHE with late (≥ 60 seconds) and mild washout or washout appearance in the Kupffer phase; these criteria should be applied only to a lesion which does not show either rim or peripheral globular enhancement on arterial phase (B1).
4. In nodules ≥ 1 cm that do not meet the radiologic diagnosis criteria of “definite” HCC, a diagnosis of “probable” HCC can be assigned by applying ancillary imaging features of HCC (B1). There are two categories of ancillary imaging features including imaging features favoring malignancy in general (mild-to-moderate T2 hyperintensity, restricted diffusion, threshold growth) and those favoring HCC in particular (enhancing or non-enhancing capsule, mosaic architecture, nodulein-nodule appearance, fat or blood products in the mass). For nodules without APHE, “probable” HCC can be assigned only when the lesion fulfills at least one item from each of the two categories of ancillary imaging features. For nodules with APHE but without washout appearance, “probable” HCC can be assigned when the lesion fulfills at least one of the aforementioned ancillary imaging features.
5. For “probable” HCC, follow-up imaging study within 3 months or biopsy should be considered (C1). For “indeterminate” nodules that cannot be diagnosed as “definite” or “probable” HCC by imaging, follow-up imaging study within 6 months or biopsy should be considered (B1). Follow-up study should be performed using one of the first-line imaging modalities.
6. For subcentimeter nodules newly detected on HCC surveillance in high-risk patients, follow-up surveillance test within 6 months is recommended (C1).
7. Newly detected or growing nodules in the follow-up study of patients with a history of prior HCC can be diagnosed as recurrent HCC regardless of size if they show the radiological hallmarks of HCC or ancillary imaging features with an increase in size (C1).
8. Although it is not recommended to strictly limit the radiation dose for the diagnosis and follow-up evaluation of HCC, unnecessary CT examinations should be avoided. To optimize radiation exposure, the use of dose reduction techniques as well as alternative imaging modalities should to be considered in HCC patients (C1).
STAGING
Cancer staging plays a pivotal role in predicting the prognosis as well as in selecting the treatment modality to maximize survival. It also facilitates the exchange of information and trial design. Since HCC mostly develops in patients with cirrhosis or chronic liver disease, not only the tumor burden but also the underlying liver function affects prognosis [204,205]. In the treatment of HCC, liver function is an important factor influencing the OS [206]. Therefore, an ideal HCC staging should include both tumor staging and liver function, which makes it complicated. This is the reason why although several staging systems for HCC have been devised, there is still no global consensus [207].
The American Joint Committee on Cancer (AJCC) has led a collaborative effort with the Union for International Cancer Control (UICC) to maintain a cancer staging system (https://www.uicc.org/resources/tnm). This system classifies the extent of disease mostly based on anatomic information regarding the primary tumor, regional lymph nodes, and distant metastases (i.e., tumor-node-metastasis [TNM] staging system), and has been modified repeatedly. The 8th edition was proposed in 2017. Compared to the 7th edition, the 8th edition was revised to classify tumors less than 2 cm as T1a regardless of the presence of microvascular invasion, and T4 if there is an invasion of the portal vein or major branches of the hepatic vein. However, recent studies have shown that prognosis is not well-reflected in the 8th edition, as the presence of vascular invasion in tumors less than 2 cm was not considered [208,209]; therefore, further validation studies are warranted for the 8th edition. The KLCA-NCC guidelines had adopted the 5th version of the modified UICC (mUICC) staging system as a primary staging system for HCC in 2003 [210,211]. Thus, the continued use of this staging system may facilitate consistency in the analyses of registry data (Table 5). A recent Korean study reported that the mUICC staging system better reflects the OS and disease-free survival (DFS) compared to the AJCC staging system [212]. However, the mUICC staging system lacks international validation and has limitations, such as difficulty in the exchange of extensive information internationally, since it differs from the AJCC/UICC TNM staging system. In addition, the revised mUICC staging system [211] has defined biliary tract invasion and vascular involvement as same stages. However, the reason for this is unclear, and biliary tract invasion is different from vascular invasion in terms of the indication for surgery and prognosis following treatment; therefore, further research to validate this guideline is necessary. For the staging of HCC, chest CT, bone scan, positron emission tomography (PET) CT scans may be required in addition to dynamic CT or MRI of the primary liver tumor. The risk of distant metastasis is low for patients with early-stage HCC; therefore, tests for the evaluation of extrahepatic metastasis should be carefully selected. Gastroscopic examination is also required to confirm the presence of portal hypertension, which is important in the treatment decision process.
Table 5. Modified UICC Stage.
| Stage | T | N | M | |
|---|---|---|---|---|
| I | T1 | N0 | M0 | |
| II | T2 | N0 | M0 | |
| III | T3 | N0 | M0 | |
| IV A | T4 | N0 | M0 | |
| T1, T2, T3, T4 | N1 | M0 | ||
| IV B | T1, T2, T3, T4 | N0, N1 | M1 | |
| Criteria | T1 | T2 | T3 | T4 |
| (1) Number of tumors: solitary (2) Diameter of the largest tumor ≤ 2 cm (3) No vascular or bile duct invasion: Vp0, Vv0, B0 |
All three criteria are fulfilled | Two of the three criteria are fulfilled | One of the three criteria is fulfilled | None of the three criteria are fulfilled |
| ||||
The Barcelona Clinic Liver Cancer (BCLC) staging system, which includes factors related to tumor stage, liver function, and performance status of the patient, was last updated in 2022 [213]. Preserved liver function status was defined as Child-Pugh grade A and the absence of ascites. It suggests the most recommendable treatment modality for each stage, and is endorsed by the American Association for the Study of Liver Diseases (AASLD), the European Association for the Study of the Liver (EASL), and the European Organization for Research and Treatment of Cancer (EORTC). However, the use of the BCLC staging system is limited in a way as it contains a subjective component (i.e., performance status), crude evaluation of liver function (i.e., Child-Pugh class), and unduly simplified recommendations for treatment modality [127,214]. The Hong Kong Liver Cancer (HKLC) staging system was developed for Asian patients, most of whom were diagnosed with hepatitis B. Patients with intermediate or advanced stage disease according to the BCLC staging system were more likely to undergo more active treatment than the BCLC staging system, and the survival rate was increased when the patients followed the HKLC staging system [215]. In a follow-up study, validation was performed by changing the 9-stage system to a 5-stage system. However, further validation is required for non-Asian populations and liver cancer from other causes [216].
The evaluation of extrahepatic metastasis is critical for the accurate determination of cancer stage and treatment strategy. Common sites of HCC metastasis include the lung, lymph nodes, bone, adrenal gland, and peritoneum [217]. However, the indications and methods to detect these metastatic lesions have not yet been established. The recently revised National Comprehensive Cancer Network (NCCN) guidelines recommend chest CT and CT or MRI of the pelvis as routine staging workups, and bone scan and/or specific bone imaging in those with bone pain or suspicion of bone metastases on cross-sectional images [218]. Several meta-analyses and retrospective studies have found that 18F-fluorodeoxyglucose (FDG) PET-CT was useful in detecting extrahepatic metastasis in patients with HCC [219,220,221]. In a prospective Korean study including 35 metastatic HCC patients, the sensitivity of FDG PET-CT for extrahepatic HCC lesions was reported to be 85.7% [219]. In particular, the detection rates of lung and bone metastases, which were the most common types of HCC metastases, were 80% and 100%, respectively. Another Korean study also demonstrated that 5% of BCLC stage A (six of 119) and 1.4% of BCLC stage B (one of 71) HCC patients were shifted to BCLC stage C after identifying extrahepatic lesions using FDG PET-CT [222]. An U.S. cohort study of 101 treatment-naïve patients reported changes of BCLC staging and treatment strategy in 5.9% and 9.9%, respectively, of the patients by adding FDG PET-CT after initial staging with contrast-enhanced CT or MRI [223]. Also, dual tracer PET-CT (18F-fluorocholine and FDG PET-CT) detected new lesions in 26 patients (21%), updated the BCLC stage in 14 (11%), and modified treatment strategy in 17 (14%), compared to conventional imaging alone, in a retrospective cohort of 122 HCC patients from France [224]. Hence, FDG PET-CT may be selectively considered for patients with HCC prior to curative surgical treatments, such as hepatic resection and LT.
[Recommendations]
1. This guideline adopts the mUICC stages as the primary staging system, with the BCLC staging system and the AJCC/UICC TNM staging system serving as complementary systems (B1).
2. FDG PET-CT can be utilized for staging prior to treatments with curative intent, such as hepatic resection or LT (C1).
3. Chest CT, pelvis CT, and bone scan can be used for HCC staging workup if extrahepatic metastasis of HCC is suspected (C1).
TREATMENT OVERVIEW
The goal of HCC treatment may vary according to the stage of cancer, underlying liver function, and performance status of the patient. However, the ultimate goal is to increase the OS and improve the quality of life. In order to achieve this, establishing multidisciplinary treatment plans by various experts, including hepatologist, gastroenterologist, surgeon, radiologist, interventional radiologist, oncologist, radiation oncologist, pathologist, and other related medical practitioners is necessary [225]. It would be effective to make personalized treatment plans based on the opinions of relevant experts as there is a wide range of treatment options available for HCC, including hepatic resection, LT, locoregional ablative therapies, transarterial therapies, external-beam radiation therapy, and systemic therapies. Furthermore, unlike other types of cancer, HCC often develops in the presence of underlying liver cirrhosis and its complications may occur during cancer treatment [218,226]. Although there has been no large-scale prospective study on the effectiveness of multidisciplinary approach in patients with HCC conducted to date, a number of retrospective studies have consistently reported improvements in the early diagnosis rates, the likelihood of patients actively receiving cancer treatments, and the OS [227,228,229,230,231]. In subgroup analyses, significant improvements in OS were particularly observed in difficult-to-treat cases, such as patients with liver dysfunction, and intermediate or advanced HCCs [227,228]. These results may indicate that multidisciplinary approaches allow medical specialists from different fields to actively communicate with one another, share patient’s clinical information without delay, and apply the latest treatment strategies, including clinical trials. Therefore, a multidisciplinary approach may play a key role in improving patient satisfaction, reducing tumor progression, and prolonging patient survival [229,232,233,234,235,236,237,238]. Multidisciplinary approaches for HCC began developing in the early 2000s, but there are still no clear guidelines regarding the optimal frequency, format, and management, including necessary expenses. In addition, more evidence is still required on clinical outcomes and cost-effectiveness. Prospective studies are needed for the precise assessment of clinical benefits and to establish detailed guidelines on multidisciplinary approach in HCC patients.
The choice of treatment method should be as evidence-based as possible, and the best evidence is a meta-analysis targeting RCTs or prospective controlled studies, and prospective large-scale cohort studies to confirm survival. Although these studies are gradually increasing, the best evidence such as RCT for the treatment of HCC is still lacking, so much of the treatment plan is based on moderate evidence.
Therefore, much understanding and attention are needed in the treatment application. It is difficult to establish a balanced multidisciplinary treatment plan in clinical practice because there is a lack of objectivity in the treatment indications and results claimed by each department that directly performs patient treatment, so a more objective evaluation is needed through collective discussion by expert groups such as this guideline revision committee.
The best treatments recommended in this guideline are the results of evidence-based medicine. Prerequisites to adequately apply these recommendations include actual facilities and trained personnel to provide all possible treatments for the patients, as well as the financial condition of patients and cooperation from patients and guardians. Therefore, considering the various aforementioned requirements, these guidelines first provided both the best and alternative treatments for each mUICC staging in 2014, and the same manner is used in the revised guidelines (Fig. 8). However, as different treatments may be selected for HCC depending on the underlying liver function, performance status, and symptoms in addition to staging, not all possible cases could be listed and summarized in the guidelines. Recommendations for specific treatments are made based on medical evidence and expert opinions for various HCC conditions, and they are described in detail in each treatment section of these guidelines.
Fig. 8. First-line treatment of 2022 Korean Liver Cancer Association-National Cancer Center Korea practice guidelines for patients with hepatocellular carcinoma, Child-Pugh class A, no portal hypertension, and Eastern Cooperative Oncology Group performance status 0–1.
mUICC = modified Union for International Cancer Control, VI = vascular or bile duct invasion, RFA = radiofrequency ablation, cTACE = conventional transarterial chemoembolization, TARE = transarterial radioembolization, Other local ablation = percutaneous ethanol injection (PEI), microwave ablation (MWA), and cryoablation, EBRT = external beam radiation therapy, Vp = portal vein invasion, LT = liver transplantation, DEB-TACE= drug eluting bead-TACE, TACE = cTACE and DEB-TACE, HAIC = hepatic arterial infusion chemotherapy
This overview summarizes the treatments for HCC patients with various mUICC disease stages with good liver function (Child-Pugh A level) and good performance status (Eastern Cooperative Oncology Group [ECOG] performance 0–1) without any complications of portal hypertension to promote understanding of treatments in general. These guidelines have separately dealt with second-line treatment for the first time, but this management overview provides information only on the initial treatment. Second-line treatments for residual, recurred, or progressed cancer after the initial treatment are later described separately, along with the recommendations.
HEPATIC RESECTION
Hepatic resection is not only a primary treatment modality for patients with solitary HCC unaccompanied by liver cirrhosis [239], but also a preferentially considered option for cirrhotic patients with sufficient hepatic functional reserve [240,241]. The outcomes of hepatic resection for HCC have markedly improved following recent advances in preoperative tests and surgical skills, as well as the accumulation of experience in postoperative management [242]. Recent studies have shown that postoperative mortality after HCC resection is less than 1%–3%. In addition, the 5-year OS and DFS rates are 46% to 69.5% and 23% to 56.3%, respectively [243,244,245,246]. The 5-year recurrence rate after hepatic resection of HCC ranges from 43.7% to 77%, and about 80% to 95% of postoperative recurrences are intrahepatic [247]. Intrahepatic recurrences are divided into intrahepatic metastasis and de novo HCC by multicentric carcinogenesis. The two recurrence entities can be differentiated by the means of genomic hybridization, DNA fingerprinting, DNA microarray, or HBV integration pattern [248]. However, no clinical definition of either entity has been established. In general, late recurrence more than 2 years after primary resection is considered as a de novo HCC [249]. Risk factors associated with recurrence after resection are classified as either tumor-related or underlying disease-related. Tumor-related factors, which are usually related to early recurrence, include the tumor size and number, microvascular invasion, poor tumor differentiation, high serum AFP and prothrombin induced by vitamin K absence II (PIVKA-II) levels, and positivity of 18F-FDG PET. Meanwhile, underlying disease-related risk factors, which influence late recurrence, include cirrhosis, high serum HBV DNA levels, and active hepatitis [219,249,250,251,252,253,254,255]. Nevertheless, no association between risk factors and timing of recurrence is evident in many cases, since this time-dependent classification does not actually reflect the tumor-pathologic mechanism of HCC recurrence.
Imaging modalities, such as CT and MRI, as well as serum tumor markers, are the recommended surveillance tools during follow-up. Serum AFP, a traditional tumor marker of HCC, is also an effective marker for recurrence when liver function is normalized after resection in cases with preoperatively elevated AFP levels [256]. PIVKA-II is another HCC marker with increasing utility for diagnosis, follow-up, and prognostication of HCC [250,257].
Preoperative Evaluation
Child-Pugh classification is conventionally used to preoperatively assess the safety of hepatic resection (Table 6) [258]. Hepatic resection is commonly performed in patients with Child-Pugh class A with ECOG performance status 0–2 (Table 7).
Table 6. Child-Pugh Classification.
| 1 | 2 | 3 | |
|---|---|---|---|
| Albumin (g/dL) | > 3.5 | 2.8–3.5 | < 2.8 |
| Bilirubin (mg/dL) | < 2.0 | 2.0–3.0 | > 3.0 |
| Prothrombin time prolonged (seconds) | < 4 | 4–6 | > 6 |
| Ascites | None | Slight | Moderate |
| Encephalopathy (grade) | None | 1–2 | 3–4 |
Class A, ≤ 6 points; class B, 7–9 points; class C, ≥ 10 points
Table 7. Eastern Cooperative Oncology Group (ECOG) Performance Status*.
| Grade | ECOG |
|---|---|
| 0 | Fully active, able to carry on all pre-disease performance without restriction |
| 1 | Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, e.g., light housework, office work |
| 2 | Ambulatory and capable of all selfcare but unable to carry out any work activities. Up and about more than 50% of waking hours |
| 3 | Capable of only limited selfcare, confined to bed or chair more than 50% of waking hours |
| 4 | Completely disabled. Cannot carry on any selfcare. Totally confined to bed or chair |
| 5 | Dead |
*Oken MM, et al. Toxicity And Response Criteria Of The Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649-655, 1982.
However, Child-Pugh classification is an insufficient preoperative indicator of operability as many patients’ liver function can remain in Child-Pugh class A despite advanced cirrhosis [259,260]. Therefore, the indocyanine green 15-minute retention rate (ICG-R15), which was suggested for use in Japan, is utilized at many Korean institutions as a preoperative test for the prediction of residual liver function [261].
Although major hepatic resection is recommended only for patients with ICG-R15 ≤ 10%, a recent study reported safe right hemihepatectomy even in patients with an ICG-R15 of up to 14% [262]. In contrast, portal hypertension and serum bilirubin level have been suggested as the criteria to determine resectability in Europe and the United States, in which portal hypertension is defined as a hepatic venous pressure gradient ≥ 10 mm Hg [263].
Esophageal varix and thrombocytopenia < 100000/mm3 accompanied by splenomegaly and ascites are also indicators of portal hypertension, and thrombocytopenia is considered the most clinically relevant criterion.
In patients with portal hypertension, the post-hepatectomy complication rate is high and long-term prognosis is poor [263,264,265]. However, some recent studies reported comparable outcomes even in patients with portal hypertension [266,267,268,269].
Minor hepatic resection instead of major hepatic resection should be considered in patients with mild portal hypertension, as resection volume is closely associated with the risk of postoperative hepatic insufficiency. HCC is usually accompanied by chronic liver disease in most cases. In order to predict postoperative hepatic insufficiency, the assessment of future liver volume or remnant liver volume after resection is as important as the hepatic reservoir function test. Although 70% to 80% of the volume can be resected in healthy liver, a much lower resection volume is allowed for diseased or cirrhotic liver. There have been few studies about the safe remnant liver volume in patients with cirrhosis. Nevertheless, a remnant liver volume ≥ 40% is generally recommended in cirrhotic patients for safety [270]. Recently, several noninvasive tests to measure the severity of hepatic fibrosis have been developed. Among them, liver stiffness measurement (LSM) with transient elastography was recently reported to be effective for predicting postoperative hepatic failure and recurrence [271,272,273,274]. The optimal LSM cut-off value varies according to background liver condition and measurement methods [271,274,275,276,277]. Recently, a meta-analysis study and the EASL guidelines reported that significant risk of posthepatectomy liver failure can be predicted by liver stiffness above 11.3–14.2 kPa and 12–14 kPa, respectively [278].
Dynamic contrast-enhanced CT is the basic test utilized as a preoperative radiologic study to assess the possibility of resection. MRI using a hepatic cell-specific contrast medium is superior to CT for HCC detection, especially for small HCCs < 1 cm [279,280], and may be a useful method to assess resectability and to formulate resection plans. Gadolinium-EOB-DTPA MRI was also proposed for the evaluation of liver function, like ICG-R15. Several studies reported that it could be used as a novel tool to assess or monitor liver function during perioperative period [281,282,283,284].
Further examinations may be necessary to find extrahepatic metastases before hepatic resection in patients with HCC. 18F-FDG PET-CT may be effective for investigating extrahepatic metastasis [285], although its sensitivity is very low for the diagnosis of intrahepatic HCC [219]. In addition, chest CT and bone scan may also be helpful [286].
Basic Principles of Hepatic Resection
One reason why hepatic resection has recently become safer is the reduction in the amount of intraoperative hemorrhage, which minimizes the amount of transfusion required. Blood transfusion has been reported to compromise anticancer immunologic mechanisms and increase postoperative recurrence [287]. However, a recent meta-analysis study reported that intraoperative or postoperative blood transfusion was not associated with DFS [288,289]. Recent transfusion rates in hepatic resection are ≤ 10% owing to selective hepatic blood flow occlusion, maintenance of low central venous pressure, and precise transection of the hepatic parenchyma [290]. However, a recent prospective randomized study reported that goal-directed fluid therapy based on the stroke volume was sufficient to minimize bleeding, without the need to unconditionally lower the central venous pressure during surgery [291]. In addition, although the Pringle’s maneuver is a useful method for lowering intraoperative bleeding, caution is still required as a meta-analysis reported that it may increase early recurrence [292,293].
The debate regarding anatomical and non-anatomical HCC resection continues. Several retrospective studies [294,295,296,297,298,299] and a meta-analysis [300] suggested that anatomical resection may be superior to non-anatomical resection in terms of securing the resection margin and removing micro-metastases. A recent prospective randomized trial showed that anatomical resection decreased the early recurrence rate within 2 years after hepatic resection, but did not affect 5-year DFS or OS [301]. In two recent meta-analysis studies, anatomical resection showed no difference in surgical complications compared to non-anatomical resection, while showing superior results in DFS and OS [302,303,304]. Therefore, it is desirable to consider anatomical resection, if possible, for HCC resection.
Securing a tumor-free resection margin is critical to improve long-term prognosis. One prospective randomized trial showed that a resection margin > 2 cm led to better outcomes after HCC resection [305]. However, according to recent meta-analyses, it was reported that a resection margin of 1 cm or more is sufficient [306,307]. Therefore as excessive hepatic resection is closely associated with complications in patients with cirrhosis, determining the appropriate extent of resection with patient safety as the top priority is important although a sufficient margin from the tumor and anatomical resection are recommended [308,309,310].
Transarterial chemoembolization (TACE), performed before hepatic resection for the purpose of improving postoperative prognosis, is not recommended [311,312,313]. Patients with liver cirrhosis need more sufficient remnant liver volume than patients with normal liver, since the remnant liver volume after hepatic resection is an important prognostic factor for hepatic insufficiency [314,315]. When insufficient remnant liver volume is expected, portal vein embolization before hepatic resection or portal vein ligation during hepatic resection may enable extensive hepatic resection by inducing compensatory hypertrophy of the residual liver [316,317,318]. Recently, resection using Associated Liver Partition and Portal vein Ligation for Staged hepatectomy (ALPPS) has been reported for cases of insufficient remnant liver volume even in HCC patients, but it has not been universalized yet [319].
The hanging maneuver is frequently used during hepatic resection, although there is no report on its effect on survival or recurrence after HCC resection. Nevertheless, the hanging maneuver can shorten surgical time and reduce the amount of bleeding [320]. The anterior approach, which is often used for the resection of large tumors, is associated with less bleeding, a lower transfusion rate, and better survival, according to a meta-analysis [321].
Minimally Invasive Hepatic Resection
Techniques of laparoscopic liver resection (LLR) have evolved rapidly, and its indications have extended. LLR can be applied for HCC located in the posterosuperior segments and caudate lobe as well as in the left lateral section and anterolateral segments. Compared to open liver resection (OLR), LLR has led to less postoperative pain, complications, and shorter postoperative hospital stays although the overall recurrence rate and survival rate were not significantly different between the two groups [322,323,324].
With the development of laparoscopic techniques and surgical instruments, especially laparoscopic imaging system (4K, 3D, and indocyanine green fluorescence images), major hepatectomy, hepatectomy for recurrent HCC, hepatectomy for HCC in patients with liver dysfunction have also gradually increased [325,326,327]. A recent study showed that compared to OLR, LLR for patients with Child-Pugh B7 cirrhosis or portal hypertension was associated with less perioperative bleeding, postoperative pain, complications, and shorter postoperative hospital stays. However, the overall recurrence rate and survival rate were not significantly different between the two groups [328,329,330].
Techniques of robotic liver resection has also evolved, and its indications have extended. However, robotic liver resection for HCC is still performed only in highly experienced centers, and further comparative studies with OLR and LLR should be performed in the near future [331].
Indication of Hepatic Resection
In general, hepatic resection shows a good prognosis when performed for one or two tumors of small sizes. As the size of the tumor increases, the frequency of vascular invasion also increases which leads to poor prognosis. However, according to recent studies, microvascular invasion was not observed in about one-third of patients with tumors sized more than 10 cm, and surgical treatment showed better results compared to non-surgical treatment even in those patients [332,333,334]. Accordingly, hepatic resection can be favorably considered when operable in patients with a large sized tumor. For multiple tumors, surgical treatment may be limited in its indication. As recent reports have shown that liver resection was more effective than non-surgical treatment for ≤ 3 tumors [335,336,337], hepatic resection can be considered even for multiple liver tumors that are ≤ 3 in number and not indicated for LT. With the development of surgical techniques and improvement in patient management, even elderly patients have shown similar short-term and long-term results after hepatic resection as in other age groups, whereas major hepatic resection should still be performed with caution due to the decreased regenerative capacity of the liver in elderly patients [338,339,340].
Although the long-term outcome of ruptured HCC is inferior to that of unruptured HCC [341,342,343], patients who received hepatic resection after emergency transarterial embolization for hemostasis revealed better survival rates compared to those who only underwent TACE [344]. Although primary hepatic resection was performed effectively in patients with good liver function in some reports [345,346] it is more safe and effective, when the patient is hemodynamically unstable, to perform transarterial embolization first followed by elective surgery after an accurate evaluation of residual liver function [347,348].
Generally, tumor invasion to the major hepatic veins or major portal veins has been considered as a contraindication of hepatic resection. However, recent retrospective studies have shown that the OS of hepatic resection was better than that of non-surgical treatment modalities, such as TACE, radiation therapy, or sorafenib, unless the main portal trunk or contralateral branch was involved [349,350,351,352,353,354]. In addition, according to a Korean multicenter study and a Korea-Japan joint study, the 5-year survival rate after hepatic resection for HCC with bile duct invasion was 32.0%–43.6%, which was fairly appreciable, and aggressive hepatic resection including bile duct resection was helpful to improve survival [355,356]. Therefore, even for HCC that has invaded blood vessels or bile ducts, hepatic resection can be selectively considered if the patient’s general condition is tolerable.
[Recommendations]
1. Hepatic resection is the primary treatment modality for single HCC limited to the liver in Child-Pugh grade A patients without portal hypertension and hyperbilirubinemia (A1).
2. Limited hepatic resection can be selectively performed for Child-Pugh A or B7 single HCC with mild portal hypertension or hyperbilirubinemia (C1).
3. Hepatic resection may be considered even in the cases of HCC with invasion to the portal vein, hepatic vein, or bile duct if the main portal trunk is not invaded in patients with well-preserved liver function (C2).
4. Hepatic resection may be considered for three or less multiple HCCs in patients with well-preserved liver function (C2).
5. LLR for HCC located in the left lateral section and anterolateral segments can be selectively performed (B2).
6. LLR for HCC located in the posterosuperior segments or caudate lobe can be selectively performed depending on the location and size of the tumor (C2).
Treatment of Intrahepatic Metastasis After Hepatic Resection
The rate of postoperative recurrence with intrahepatic metastasis owing to local dissemination or de novo carcinogenesis is about 50%–60% at 5 years after hepatic resection [296,357]. Recurrence of the tumor with intrahepatic metastasis usually presents as multiple intrahepatic recurrences. In such cases, it is often impossible to repeat curative treatment, and the risk of recurrence after treatment is high [358]. In contrast, de novo recurrence can be the target of curative re-operation or local treatment [249,263,359,360,361,362,363]. Typically, recurrence within 2 years after surgery is classified as early recurrence, and recurrence after 2 years is classified as late recurrence. The risk factors for recurrence can be divided into tumor-related factors and underlying liver disease-related factors. Tumor-related risk factors include the tumor size, number, degree of differentiation, vascular involvement, serum AFP level (elevated before surgery), serum PIVKA-II level, lack of adequate resection margin, and non-anatomical resection, which are mainly associated with early recurrence [249,253,362,363,364,365,366,367,368]. The risk factors related to underlying liver disease are high serum HBV DNA levels before and after surgery for chronic hepatitis B [254,369,370,371] and persistent active inflammation and degree of hepatic fibrosis for chronic hepatitis C [371,372]; these are associated with late recurrence. In a randomized prospective study of repeated hepatic resection and RFA for intrahepatic recurrence, no statistically significant differences were found in the 5-year DFS and OS between the repeated hepatic resection group and the RFA group (36.2% and 43.6% in the repeat hepatic resection group vs. 30.2% and 38.5% in the RFA group, respectively). In this study, RFA had a higher early recurrence rate compared to repeated hepatic resection. In subgroup analysis, the survival rate of repeat hepatic resection was statistically higher than that of RFA when the tumor size was 3 cm or more and AFP was 200 ng/mL or higher. According to previous retrospective studies, the incidence of complications after repeated hepatic resection was higher than that of RFA [247,364,373]. Salvage LT for recurrent intrahepatic HCC after hepatic resection requires a cautious approach [374]. If intrahepatic recurrence after hepatic resection does not progress after locoregional therapies, such as RFA, TACE, or radiation therapy, salvage LT is the most effective treatment to increase the DFS and OS rates compared to repeated hepatic resection or other local treatments. Salvage LT should be determined by carefully considering the shortage of liver grafts from deceased donors or the problems related to living donors [359,375]. However, the patients who undergo repeated resection are limited in clinical practice, since they have small residual liver parenchyma after resection and are at risk of additional recurrence [376]. For recurrent HCC which is not indicated for repeated hepatic resection, non-surgical local treatments, such as RFA and TACE, can be applied. RFA has been extensively performed as a minimally invasive treatment for small relapsing HCCs [360,377]. TACE is the most widely used treatment for multiple HCC recurrences [378,379,380]. The meta-analysis that compared the effects of each of the above-mentioned treatments revealed that there was no difference in survival benefit among the treatment modalities for recurrent tumors after surgery. Therefore, appropriate treatment option should be selected considering the remnant liver function, the location and the number of recurrent tumors [377].
[Recommendations]
1. For recurrent HCC after being cured by hepatic resection, the retreatment method can be selected considering the timing of recurrence, remnant liver function, performance status, and the size, location, number of recurrent tumors (C1).
LIVER TRANSPLANTATION
LT is the treatment of choice for HCC within Milan criteria (a single tumor ≤ 5 cm or small multinodular tumors [≤ 3 nodules, ≤ 3 cm]), if unsuitable for resection. LT involves the complete removal of a diseased liver, including HCC, and replacement with a new liver. Theoretically, it is the ideal and the most effective treatment method providing excellent and unparallel long-term survival outcomes. However, there are limitations in its application due to insufficient deceased organ donation and living donor liver transplantation (LDLT) is currently the main type of LT for HCC in South Korea.
The Milan Group in Italy reported an excellent result (i.e., a 4-year survival rate of 75% and a DFS rate of 83%) after LT in HCC patients with the following conditions: (1) no extrahepatic metastasis and no vascular infiltration in the radiologic study before transplantation; (2) a single nodule of 5 cm or less; and (3) three or fewer nodules in cases with multiple nodules and each nodule being 3 cm or less [381]. Since then, the Milan criteria have been widely used for LT in patients with HCC. A recent systematic review of 90 studies, comprising a total of 17780 patients over 15 years, identified the Milan criteria as an independent prognostic factor for a favorable outcome after LT. The overall 5-year survival of patients meeting the Milan criteria (65% to 78%) was similar to that of non-HCC patients, according to the European and American transplant registries [382,383].
Recent advances in imaging technologies have enabled non-invasive diagnosis of HCC with higher accuracy. However, small lesions, which could not be detected with imaging studies at the time of the establishment of the Milan criteria, can be detected on imaging studies with current technologies, and can cause confusion regarding whether or not a patient meets the Milan criteria. A recent meta-analysis including 22392 patients concluded that the size of the largest tumor and the total diameter of nodules were the best predictors of outcome, while number of tumors was not associated with the outcome of LT [384]. Sugimachi et al. [385] also reported poor diagnostic accuracy of imaging for small (< 1 cm) HCCs and the limited effect of preoperatively unobserved tumors on prognosis after LT. Therefore, lesions ≤ 10 mm or with atypical findings should not be used to decide for or against transplantation.
Before transplantation, HCC patients undergo tests for staging in addition to general whole-body examination. In addition to dynamic contrast enhancement CT or MRI, extra-hepatic staging should include CT of the chest, and CT or MRI of the abdomen and pelvis. Imaging of the brain, bone scintigraphy, and 18F-FDG PET-CT may be performed [386]. 18F-FDG PET-CT can help characterizing the biology of HCC, since PET-positive tumors more frequently display unfavorable histological features (e.g., high cellular dedifferentiation and microvascular invasion) [387], resulting in poorer recurrence-free survival (RFS) after LT [388,389]. There has been no specific study nor consensus on the optimal timing or modality for evaluation of patients on the waiting list to monitor whether they remain within the acceptability criteria for LT, although dynamic CT or MRI and AFP measurement at a 3-month interval is commonly used [383].
Deceased Donor LT
Although LT is a very effective treatment for HCC, the risk of waiting-list mortality is very high due to the gap between the demand and supply in deceased organ donation regardless of underlying liver disease. Especially in South Korea, the risk of dropping out from the waiting list due to tumor progression is very high owing to the low rate of deceased organ donation. Many countries have developed their own organ allocation systems according to their donation situations. Each system tried to balance the risk of drop-out between HCC and non-HCC patients, and developed various rules of bonus points for HCC patients [390,391]. The National Institute of Organ, Tissue, and Blood Management operates the Korean Network for Organ Sharing (KONOS), has adopted the model for end-stage liver disease (MELD) score in June 2016. When fulfilling the Milan criteria, patients with a MELD score of 0 to 13 receive an additional 4 points; patients with a MELD score of 14 to 20 also receive an additional 5 points, while those with a MELD score of 21 or higher do not. Nevertheless, deceased donor liver transplantation (DDLT) in South Korea is mostly performed when the MELD score is above 30, and it is very unlikely that a graft liver from a deceased donor is to be allocated to an HCC patient without underlying decompensated liver disease. The annual case number of DDLT in South Korea reached its peaked in 2016 at 508 cases; and since then, it has decreased to 391 cases in 2019 [392]. Such decrease in the deceased organ donation rate and relative disadvantage in organ allocation to HCC patients have led the proportion of HCC patients to account for only 2%–5% of the total DDLT cases in South Korea [392].
Bridging and Downstaging Therapy
The dropout rate at which LT becomes infeasible due to tumor progression while waiting for LT is reported to be 15%–30% per year [393,394], and bridging therapy using loco-regional therapy is reported to reduce the dropout rate to 0%–25% [395,396,397]. However, these figures are based on Western studies, and may not be applicable to South Korea. A recent report showed promising results after LT when the waiting period prior to LT was within 6 to 18 months in HCC patients [398]. Since the possibility of HCC progression is high when the waiting period for transplantation is prolonged, HCC treatment prior to transplantation is recommended if the waiting period for transplantation of more than 6 months is expected [395,398,399].
Many studies have been conducted on the effects of pretransplant HCC treatments on the outcomes of LT; and so far, many studies have reported that treatment using loco-regional therapy in patients within the Milan criteria is not related to a reduction in recurrence of HCC after LT and an increase in the survival rate [368,400,401,402,403,404,405]. However, a recent study using Organ Procurement and Transplantation Network (OPTN)/Scientific Registry of Transplant Recipients (SRTR) in the United States showed that patients who received loco-regional therapy before LT had higher survival rates than those who did not, and that the longer the waiting period for transplantation, the higher the survival rate after transplantation [406]. Therefore, further research is needed.
Loco-regional therapies, including TACE, RFA, and stereo-tactic radiotherapy, along with hepatic resection are generally used to treat patients before transplantation [395,396,407,408,409,410], and they are implemented not only to reduce the dropout rate due to tumor progression during the waiting period but also for downstaging after planning LT in HCC patients who are not initially indicated for LT.
The most commonly used loco-regional therapy for HCC prior to LT is TACE, which can downgrade the stage of HCC by 24%–63% [406,411,412]. Downstaging is known to be more effective when the tumor size is smaller than 7 cm or there are less than three tumors [22], but there are no restrictions [413]. No difference has been reported on the outcomes of LT following transarterial radioembolization (TARE) using Yttrium-90 (90Y) and conventional TACE (cTACE) for downstaging [414,415,416]; however, further research is required.
There has been no large-scale prospective study on the outcomes of patients who initially did not meet the indications for LT but were downstaged to meet the Milan or University of California San Francisco (UCSF) criteria using loco-regional therapy for the purpose of LT. However, an Italian group recently conducted a RCT of 45 patients with HCC who effectively downgraded their stage to meet the Milan criteria through loco-regional therapy, and the patients who received LT after downstaging showed significantly higher DFS and OS rates compared to the patients who did not undergo LT [417]. In addition, several previous small-scale prospective studies have shown that in patients outside the Milan or UCSF criteria, their 5-year survival rate were similar to that of patients within the Milan or UCSF criteria when successful downstaging had been achieved to meet the Milan or UCSF criteria using loco-regional therapy prior to LT [405,409,418,419,420,421]. Therefore, when patients with HCC outside the Milan criteria, who are not indicated for LT, show therapeutic response to loco-regional therapies including TACE, RFA, and stereotactic radiotherapy to meet the Milan criteria, LT is recommended.
In patients with HCC outside of the Milan criteria that deviates from indication for LT, stage reduction was successfully acquired in more than 40% but recurred in 16% after LT, and in other reports, more than 80% of them were transplanted with successful stage reduction. Evaluating the therapeutic response of loco-regional therapy before transplantation can be used to select subjects for LT in patients with HCC outside the Milan criteria [415,416,422]. In evaluating the prognosis after LT, not only the pathological findings of the extracted liver tissue but also the changes in biological indicators, such as levels of tumor markers, are used [422,423]. In recently conducted studies, the recurrence rate of HCC after LT was low when a complete remission by loco-regional therapy was identified in the extracted liver tissue [368,423,424]. Also, the DFS and OS rates were higher in patients with a significant decrease in the levels of tumor markers after loco-regional therapy compared to those without [425,426].
Living Donor LT
The number of DDLTs are increasing in South Korea recently due to changes in the society’s perception on organ donation and the revision of laws to promote organ donation [427,428]. However, LDLT is still the main type of LT in South Korea due to a shortage of deceased donor organs in the country. In 2019, there were 1579 cases of LT, including 1188 LDLTs (75.2%) and 391 DDLTs (24.8%), in South Korea [429]. Following the revision of the allocation system on DDLT, the number of deceased donors has increased for a few years but is recently on the decrease again. Therefore, the number of people on the waiting list for DDLT had decreased from 6334 in 2013 to 4969 in 2016 but increased to 5734 in 2020.
According to the KONOS regulation for registration and allocation in South Korea, recipient candidates with HCC can gain a higher priority on the waiting list. However, in real clinical settings, patients with HCC in South Korea have a very low chance of receiving DDLT since most deceased donor livers are allocated to patients with a high MELD score (> 30). These findings suggest that currently DDLT is not a feasible treatment modality for HCC patients in South Korea. Therefore, LDLT from a healthy donor has emerged as an alternative to DDLT as a treatment modality for HCC and a significant proportion of the LT recipients with HCC have received transplantations from living donors in South Korea. The comparative outcome of LDLT versus DDLT for patients with HCC is controversial. A meta-analysis of 633 LDLTs and 1232 DDLTs indicated that LDLT is an acceptable option without compromising the survival rates [430]. However, the DFS was worse with LDLT than with DDLT [430]. Another meta-analysis of 1310 patients who underwent LDLT and DDLT for HCC showed no difference in the OS and DFS [431]. A recent meta-analysis of 40495 cases reported no statistically difference in the recurrence of HCC between LDLT and DDLT (17% vs. 14%, respectively) [432].
Patients undergoing LDLT have a short wait time and are unlikely to drop out, whereas a dropout rate of 5%–30% is reported in DDLT patients. Given that an intention-to-treat (ITT) analysis includes patients who drops out of the waiting list, it is an ideal method for the comparison of LT outcomes according to the difference in donation patterns. In ITT analysis, there was no difference in the rates of OS and DFS between the two groups according to donation patterns [433,434]. The higher recurrence rates observed after LDLT in some reports is likely due to the differences in tumor characteristics, pretransplant HCC management, and wait time [435,436,437]. In order to compare the outcomes of LT for HCC according to the type of graft, well-designed studies are needed to reflect bias and the effects of tumor biology.
In the DDLT program, the selection criteria have been set to maximize the efficacy-efficiency of donor organs. In contrast to DDLT, the indications for LDLT in HCC patients are decided based on the balance between donor risks and recipient benefits. Several eligibility criteria besides the Milan criteria for LDLTs have been adopted by many high-volume LDLT centers. At Samsung Medical Center, patient selection according to tumor size < 5 cm and AFP < 400 ng/mL without limitation in the tumor number expanded patient selection; 1-, 3-, and 5-year survival rates were reported to be 92.2%, 82.6%, and 79.9%, respectively [438]. At Seoul National University Hospital, the 3-year survival rate was reported to be 86.2% if vascular invasion was absent in preoperative radiological studies and preoperative AFP was < 400 ng/mL [439]. At Catholic Medical Center, LDLT was considered the preferred therapeutic option in patients with an AFP level < 100 ng/mL and a tumor diameter < 5 cm. The 5-year DFS and OS after LDLT were 80.9% and 76.4%, respectively [440]. At Asan Medical Center, patients with ≤6 HCCs each sized ≤ 5 cm and without gross vascular invasion were considered eligible for LT, and such patients had a 5-year survival rate of 81.6% [441]. In the selection of HCC patients for LT, the University of Tokyo has adopted the 5-5 rule, i.e., HCC ≤ 5 cm and ≤ 5 in number, and a RFS rate of 94% after LT was achieved [442]. Kyoto University further extended the number of tumors to 10 with serum PIVKA-II levels ≤ 400 mAU/mL; the resultant 5-year survival rate was 86.7% [443]. At Kyushu University, a 5-year survival rate of 82.7% was achieved in patients with HCCs ≤ 5 cm and serum PIVKAII levels < 300 mAU/mL [444]. In a study involving 49 centers and 653 patients in Japan, patients with HCCs beyond the Milan criteria but with serum AFP levels ≤ 200 ng/mL and serum PIVKA-II levels ≤ 100 mAU/mL had a 5-year DFS rate of 84.3% [445]. Most of these expanded criteria were made after modifying tumor size and number in the Milan criteria. However, the selection criteria have recently been amended to include biological markers, such as AFP and PIVKA-II [446]. Criteria based on tumor biology, including FDG-uptake, led to the accurate prediction of prognosis and risk factors in LT recipients with HCC [388,447,448,449]. European multicenter studies have shown that AFP-containing criteria better predict tumor recurrence after LT compared to criteria based on the number and size of tumors. There have been reports that even if patients with HCC exceed the Milan criteria, they can achieve good results when they fulfil the criteria including AFP [450,451,452]. LDLT has been proposed as an ideal setting for exploring expanded indications for HCC, considering the lack of graft allocation and priority policies for patients with HCC. Moreover, special personal relationship between the living donor and the recipient should be taken into account. Therefore, if the posttransplant outcomes of several eligible criteria beyond the Milan criteria for LDLTs are comparable to that of the Milan criteria, expanded indications can be accepted as long as the safety of the liver donor is ensured.
The safety of the liver donor is of paramount importance in the LDLT. The outcomes of living donors from South Korea are excellent [453,454,455,456,457,458]. According to the Korean Organ Transplantation Registry study including 832 living liver donors, major complication (including bile leakage, biliary stricture, portal vein stricture, wound dehiscence, and pulmonary edema) rates were 1.9%, and there was no mortality [459]. Recent literature reported similar outcomes and decrease in hospital stay and wound owing to the advance in laparoscopic surgery [460]. Robotic donor hepatectomy also reported good satisfaction for scar and recovery without increase in complication, establishing the safety and satisfaction of minimal invasive surgery [461]. However, in the early days of LDLT, the probabilities of death and life-threatening complications in healthy donors have been reported to be 0.2%–0.3% and about 2% globally, respectively [458,462,463,464,465]. Recent long-term outcomes of 12372 donors also reported higher mortality and disease prevalence in liver donors compared to the healthy control group (mortality rate 0.91 in 1000 population) [466]. Due to the complexity of the procedure, LDLT must be restricted to centers of expertise in hepatic surgery and LT to minimize donor risk and maximize recipient outcome. Careful attention should be given to the psychosocial well-being of liver donors.
Immunosuppression After LT
Immunosuppressants, such as calcineurin inhibitors (cyclosporine, tacrolimus) and the mammalian target of rapamycin inhibitors (mTORi; sirolimus, everolimus), are used for patients with HCC after LT [467]. Recent studies have shown that the use of mTORi may be helpful for reducing recurrence and prolonging survival in HCC patients after LT, but further studies are needed [468,469,470]. Recent meta-analysis reported better outcomes in mTORi group than non-mTORi groups in the 5-year RFS rate (ratio, 1.13; 95% CI, 1.02–1.26 in RCT and ratio, 1.17; 95 CI, 1.10–1.24 in cohort study) [471]. Therefore, if there are no significant adverse events related to drugs, mTORi may be considered in LT recipients with HCC.
[Recommendations]
1. LT is the primary treatment modality for patients with HCC unsuitable for resection but within the Milan criteria (a single tumor ≤ 5 cm or small multinodular tumors [≤ 3 nodules, ≤ 3 cm]) (A1).
2. In LT candidates with HCC, loco-regional therapies or TACE are recommended if the timing of transplantation is unpredictable (B1).
3. If the HCC tumor stage is downgraded to meet the Milan criteria by loco-regional therapies, including TACE and RFA, in patients initially exceeding the Milan criteria, LT shows superior outcomes compared to other treatments (B1).
4. Expanded indications beyond the Milan criteria for LT may be considered in limited cases without definitive vascular invasion or extrahepatic spread if other effective treatment options are not applicable (C2).
5. Salvage transplantation can be indicated for recurrent HCC after resection according to the same criteria as for first-line transplantation (B1).
Treatment of Intrahepatic Recurrence After LT
LT within the Milan criteria is known to have a recurrence rate of 8%–20% in HCC patients [472]. Due to the effects of immunosuppressants, the prognosis of HCC that recur after LT is poor, with a median survival of < 12 months after the diagnosis of recurrence and a 5-year survival rate of only 22% [472,473]. In 119 patients with HCC who underwent LT, recurrence occurred in 16 patients (13.4%) during a 17.2-month median follow-up period, and intrahepatic recurrence was the most common type [474]. In another study of 857 patients with HCC who underwent LT, recurrence occurred in 106 patients (12.4%) during a median follow-up period of 15.8 months after transplantation, and the median survival period after recurrence was 10.6 months. The recurrence sites were in the order of lung (55.7%), transplanted liver (37.8%), abdominal cavity (37.7%), and bone (25.5%) [475]. The prognosis of patients with HCC who have relapsed after LT depends not only on the stage before transplantation or the pathological findings of the removed liver, but also on the time to recurrence after transplantation and whether it has invaded multiple organs. Furthermore, the treatment method for recurrent cancer is an important factor; hence, it is necessary to apply the appropriate individualized treatment to patients [476].
Even if HCC recurs after LT, the survival rate can increase if curative treatment is available. In 121 patients who had cancer recurrence after LT, 38 (31.4%) patients received hepatic resection or local therapy, 51 (42.1%) received palliative care, and the remaining 32 (26.4%) received conservative treatment [477]. Among these patients, the median survival period of those who could receive radical treatment was significantly longer than those who received other treatments. A study performed in Japan included 101 patients who underwent LDLT for HCC between 1996 and 2007, of which 17 patients with recurrence were analyzed. Nine patients underwent surgical treatment, including hepatic resection (six cases), resection of lung metastasis (10 cases), and resection of lymph node metastasis (three cases); and eight patients received non-surgical treatment. The survival rates for 1, 3, and 5 years in patients with hepatic resection were 100%, 87.5%, and 87.5%, respectively, whereas the survival rates in patients with non-surgical treatment were 50%, 12.5%, and 0%, respectively, showing significant differences [478].
When the recurrent HCC after LT is confined within the liver and hepatic resection is unviable, RFA may provide a good prognosis. In one study, of the 486 patients who underwent LT, HCC recurred in 78 patients (16%) and 15 patients underwent hepatic resection, 11 patients RFA, and 52 patients received conservative treatment. The survival rates for 1, 3, and 5 years in the surgical group were 92%, 51%, and 35%, respectively, and the survival rates in the RFA group were 87%, 51%, and 28%, showing no significant difference between the two groups (p = 0.879). The RFS rates for 1, 3, and 5 years in the surgical group were 83%, 16%, and 16%, respectively, and the RFS rates in the RFA group were 76%, 22%, and 0%, respectively, with no difference between the two groups (p = 0.745) [479]. Since HCC that recur after LT is often multiple or accompanied by extrahepatic metastases, it is not common to apply radical hepatic resection or RFA. Although there are limited studies on the efficacy and safety of TACE when recurrence occurs after LT, a study of 14 patients with intrahepatic or intrahepatic and extrahepatic recurrent HCC reported that the partial response (PR) after TACE was 57%, stable disease (SD) was 28%, and the disease progressed in 14% of patients. The survival rates at 6, 12, and 24 months after recurrence in patients who received TACE were 64.3%, 50%, and 22.2%, respectively, while the survival rates of 14 patients who received systemic chemotherapy were 35.7%, 21.4%, and 10.7%, respectively (p = 0.034) [480]. The Child-Pugh score did not significantly increase after TACE, there was no severe adverse event, and the degree of postembolization syndrome (PES) was not different from that of patients who did not undergo LT. In a study conducted in Taiwan, 11 patients with recurrent multiple HCCs after LT underwent TACE, and the median survival rate was 6.6 months (0.3–12.7 months) with a 1-year survival rate of 12.5% [481].
Sorafenib may be used when hepatic resection, RFA, or TACE cannot be performed due to extensive recurrence, or in cases when the disease progresses after local therapy; however, there has been no well-designed RCT to verify its efficacy and safety. In a case-control study of 39 patients, 24 patients were treated with best supportive care and 15 patients were treated with sorafenib, and the median survival period was 21.3 months from the time of recurrence in the sorafenib group, which was significantly longer compared to the 11.8 months in the supportive care group (HR, 5.2; p = 0.0009), and no severe adverse event was observed after sorafenib [482]. However, another study reported that sorafenib is more toxic after LT [483]. In particular, a case of death due to gastrointestinal hemorrhage was reported when sorafenib and everolimus, an mTORi, were combined to increase the anti-cancer effect [484]. Since there has been a report of severe side effects and a high rate of dose reduction, continuous monitoring of mTORi from the beginning is essential [485]. In another study, among 34 patients with recurrent HCC after LT, 17 patients were treated with sorafenib and the remaining 17 patients received conservative treatment, with the survival rates at 3 months and 12 months in the two groups being 100% and 62%, and 73% and 23%, respectively, showing a significant difference. Adverse events occurred in the order of diarrhea (18%), elevated transaminase (11%), fatigue (11%), hand-foot skin reaction (HFSR) (6%), and nausea (6%) [486].
There has been a report on the use of regorafenib as a second-line treatment after sorafenib failure in patients with recurrence after LT. According to a multicenter retrospective study in Europe, in 28 patients who received LT, the median OS from regorafenib initiation was 12.9 months and 38.4 months since sorafenib administeration [487]. There were only common side effects in patients who received LT. Another multicenter retrospective study showed that among 132 patients who were administered sorafenib after LT, those who used regorafenib as second-line treatment had a significantly higher survival rate compared to those who received only supportive care after the failure of sorafenib, and multivariate analysis showed that regorafenib independently lowered mortality [488].
The use of other tyrosine kinase inhibitors such as lenvatinib, cabozantinib, and ramicirumab, a monoclonal antibody, may also be considered, but evidence is still insufficient to verify the safety and efficacy of their use after LT. Recently, reports on the use of immune checkpoint inhibitors targeting cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) and programmed cell death (PD)-1/programmed cell death-ligand 1 (PD-L1) in patients with HCC who have undergone LT have been released [489,490,491]. According to these reports, rejections due to the immune checkpoint inhibitors may occur in up to half of them, and immediately after 1–2 weeks of commencing immunotherapy. Treatment strategies commonly used for rejections, such as steroids, may work; however, the use of immune checkpoint inhibitors in patients who receive LT requires much attention.
[Recommendations]
1. For recurrent HCC after being cured by LT, the retreatment method can be selected considering the time to recurrence, liver function, performance status, size, location, and the number of recurrent tumors (C1).
LOCAL ABLATION THERAPIES
Local ablation therapies are widely performed as non-surgical treatments for HCC, as they are easy to perform and induce tumor necrosis with minimal damage to the normal hepatic parenchyma. Among various local ablation therapies, RFA and PEI are accepted as standard treatments. In recent years, microwave ablation and cryoablation have been considered as effective local ablation therapies, while clinical trials are underway for other modalities, such as laser ablation therapy and high-intensity focused US.
The indications for local ablation therapies include patients with a single HCC ≤ 5 cm or up to three nodules ≤ 3 cm, although minor discrepancies exist across different investigators and studies. Efforts to apply local ablation therapies to larger HCCs have been made; however, the treatment outcomes are closely associated with the tumor size. If the corrected platelet count is less than 50000/mm3, the prothrombin time is less than 50%, or the international normalized ratio (INR) is equal to or higher than 1.5–1.8, then the risk of tract bleeding following the ablation procedure may be high [492,493].
RFA
RFA is the most widely used local ablation therapy for HCC. Very fast alternating currents (460–500 kHz) flow in the vicinity of radiofrequency electrodes, inducing internal friction among molecules. The internal heat generated by the internal friction can evoke tissue necrosis. Exposure to temperatures higher than 60℃ causes almost immediate protein denaturation and destruction of cell membranes, followed by coagulative necrosis. Similar necrotic effects can also be obtained by maintaining the temperature of 45℃–50℃ for ≥ 3 minutes. The main advantage of RFA compared with PEI is that fewer treatment sessions are required to achieve complete tumor necrosis. For HCC nodules ≤ 2 cm, RFA results in a higher complete tumor necrosis rate compared to PEI [494,495,496,497]. Most procedures are performed via a percutaneous approach; however, a laparoscopic or open surgical approach may sometimes be required.
The initial complete tumor necrosis rates, which were evaluated by CT or MRI within 1 day to 1 week after RFA, were reported to exceed 95%. If RFA procedures are repeated for residual viable tumors, a complete tumor necrosis rate of almost 100% can be achieved [496,498]. However, the 3-year local tumor progression (LTP) rate after RFA ranges widely from 0.9% to 21.4% [453,498,499]. According to Shiina et al. [498], the 10-year LTP rate after RFA was 3.2%. However, Kim et al. [453] reported a 10-year LTP rate of 38.2% after RFA, and there is a big difference in LTP rate across institutions. The independent factors associated with the OS after RFA include initial complete tumor necrosis, Child-Pugh score, number and size of tumors, and pre-operative serum AFP level. The best outcome after RFA can be achieved in patients with a single HCC < 2 cm in diameter and Child-Pugh class A function. If the tumor location is ideal for performing RFA, the efficacy of RFA is comparable to that of hepatic resection. Hence, some reports suggest that RFA should be considered a primary treatment [214,499]. The treatment outcome after RFA of HCC is affected by the location of the tumor. The best results can be expected when the tumor is not attached to the hepatic capsule, intrahepatic blood vessels, or central bile duct [500]. Subphrenic HCCs have a high risk of LTP after US-guided RFA, and the frequency of peritoneal seeding has been reported to be up to 9.5% [501,502]. In addition, when 3 mm or more of the tumor surface is in contact with the portal vein or hepatic vein, RFA may not be effective due to the heat-sink effect, and the risk of complication increases due to blood vessel or bile duct damage [501,503,504].
The long-term survival outcomes after RFA of HCC patients are dependent on the tumor size. For Child-Pugh class A patients with tumors < 2 cm, the 3- and 5-year OS after RFA are approximately 90% and 65%–70%, respectively [453,498,499]. Meanwhile, the corresponding OS for 2–5 cm tumors are 65%–75% and 50%, respectively [453,498]. The therapeutic efficacy of RFA has been improved with the introduction of antiviral treatment [505], and the 5-year rates of OS were 83.7%–85.1% in the recent RFA studies from South Korea in HCC patients within the Milan criteria [501,506].
No-touch RFA has recently been performed after placing multiple electrodes outside the tumor. It showed a lower LTP rate compared to conventional tumor-puncturing RFA [507,508]. A prospective multicenter study also found improved local tumor control after no-touch RFA for HCC [509]. However, further investigation is warranted to evaluate whether no-touch RFA would also enhance the survival outcomes after treating patients with HCCs.
Most studies comparing RFA with hepatic resection for HCC were not RCTs; even with RCTs, their sample size was not big enough to make a definite conclusion [510]. Three RCTs, including a recently published study, showed no significant difference in survival rate between the two treatments [511,512,513]. In a RCT that reported a significant difference in survival rates between the two therapies, the number of patients included in the single HCC < 3 cm group was too small, and the 1-year survival rate of RFA was 91%, which was substantially lower than the 100% survival rate of hepatic resection [514]. A meta-analysis of eight RCTs showed that the 5-year OS and DFS were not significantly different between the hepatic resection and RFA groups for HCC patients within the Milan criteria [515]. In a prospective controlled study recently published in South Korea [516], there was no difference in the survival rates between hepatic resection and RFA; however, the DFS was longer in the hepatic resection group. Other non-RCTs reported no significant difference in survival rates between hepatic resection and RFA in treating HCC ≤ 3 cm in diameter [517,518,519]. Hepatic resection had a higher incidence of complications and a longer hospital stay of 8 to 9 days on average [520].
A RCT comparing repeat hepatic resection and RFA in HCC patients who relapsed within the Milan criteria after hepatic resection also showed the same results as patients with treatment-naïve HCC. However, in patients with recurrent HCCs > 3 cm and AFP levels ≥ 200 ng/mL, repeat hepatic resection showed better OS and DFS rates compared to RFA.
It is well-known that MRI findings, serum levels of tumor markers, and tumor size are related to microvascular invasion of HCC. HCCs with a high risk of microvascular invasion have shown poor prognosis after RFA [366,521,522]. However, since no RCT or meta-analysis has been performed yet, additional studies are needed.
For HCCs > 3 cm, the local recurrence rates after RFA range from 30% to 50% [453], and combined treatment with TACE and RFA can be considered for these tumors. In three or fewer HCCs of ≤ 3 cm in diameter, the survival rate and recurrence rate were not significantly different between the combined treatment and RFA alone groups [523]. In contrast, when the size of HCC ranged from 3 cm to 5 cm, the LTP rate and survival rate were better in the combined treatment group [524,525]. A meta-analysis of seven RCTs showed better survival in the combined treatment group than the RFA monotherapy group; however, the subgroup comparison of tumors < 3 cm in size showed no significant difference in survival rate between the combined treatments and RFA alone groups [526]. In a meta-analysis of eight RCTs comparing RFA alone and combined TACE and RFA, the combined treatment group showed better survival and recurrence rates; however, there was no significant difference in the major complication rates between the two groups [527,528]. Considering the results above, the combination of TACE and RFA in treating HCCs with 3–5 cm in size showed a higher survival rate and lower recurrence rate compared to RFA alone, with no significant difference in the incidence of complications between the two treatments.
Despite these favorable outcomes, RFA has some disadvantages. First, the risk of major adverse events is usually higher than that of PEI, particularly when the tumors are located near the liver hilum or major abdominal organs, such as the colon. In addition, the heat-sink effect may hinder the effective transmission of heat energy to a tumor adjacent to relatively large intrahepatic vessels [497,529,530]. Sometimes, however, the risk of thermal injury to the adjacent abdominal organs can be overcome by inducing artificial ascites [531]. Another major limitation of RFA is that HCCs < 2 cm may not be visible on the conventional US. However, recent applications of US contrast agents and fusion imaging techniques have broadened the indications for RFA in such cases [532,533]. In a prospective study of 216 patients with HCCs < 5 cm conducted in South Korea, 30 (39.5%) of 76 HCCs not visible on the B-mode US were recognizable on fusion imaging [532]. Also, for 60 HCCs untreatable with RFA under B-mode US guidance, all of them could be treated when fusion imaging was applied. In this study, the technical success rate was 97.1% after fusion imaging-guided RFA. On the other hand, for small HCCs which are challenging to detect on the B-mode US, the detection rate was improved when the CEUS was performed [533]. In particular, the detection rate of HCC was higher when CEUS was performed with fusion imaging than when the CEUS was used alone.
The mortality rate due to procedure-related complications after RFA is reported to be 0.1%–0.5%, and the major complication rate after RFA is less than 5% [499,529,530]. Major complications include needle tract seeding, hemoperitoneum, hemothorax, liver abscess, massive infarction of liver parenchyma, intestinal perforation, and pneumoperitoneum [498].
In conclusion, for HCCs within the Milan criteria, hepatic resection has shown a lower recurrence rate than RFA and a higher postoperative complication rate; however, further studies are warranted to verify the difference in the survival rate. For single nodular HCCs < 3 cm in diameter, RFA has an equivalent survival rate, higher LTP rate, and lower complication rate than hepatic resection. Therefore, it can be used as an alternative treatment for surgery if the location of HCC is ideal to perform RFA.
PEI
PEI was widely used in treating HCC, since it is relatively simple to perform and adverse reactions are infrequent. However, it has to be performed repetitively in contrast to RFA, and it is difficult to obtain complete necrosis for tumors > 3 cm as the diffusion of injected ethanol may be blocked by the fibrous septum or tumor capsule, resulting in a decreased therapeutic effect. Therefore, PEI has been largely replaced by RFA. The tumor necrosis rate of PEI was reported to be 66%–100% [495,496,497,534]. Tumor size is important; tumors < 2 cm in diameter have more than a 90% tumor necrosis rate. However, as the tumor size increases, the necrosis rate decreases, and the tumor necrosis rate is only 50% for tumors 3–5 cm in size. LTP rates after PEI range between 24% and 34%, but it was reported to be as high as 43% for HCCs ≥ 3 cm [535,536,537,538]. For patients with Child-Pugh class A function and a solitary HCC < 2 cm, the 3- and 5-year OS are 70%–80% and ≥ 50%, respectively. For HCCs 2–3 cm in diameter, the 3-year OS ranges from 47% to 64% [495,534].
Among the RCTs comparing RFA and PEI in patients with HCC [495,496,497,534,539,540], except for those published in Italy [539,540], RFA showed a significantly lower LTP rate and a higher survival rate. In particular, in a meta-analysis of four RCTs, the 3-year survival rate of RFA was significantly higher than that of PEI [541,542,543,544]. However, there was no significant difference in the survival rates between the subgroups of HCCs < 2 cm in diameter [543]. These results suggest that the RFA group has a lower LTP rate and a higher survival rate compared to the PEI group; however, further study is needed. In HCCs < 2 cm in diameter, studies have reported a similar OS, and PEI can be considered if RFA is not feasible [545]. PEI can be performed to treat perivascular tumors to reduce the heat-sink effect of RFA. However, the risk of biliary stricture also exists with PEI if the tumors are located in the liver hilum [546,547].
Microwave Ablation and Cryoablation
Recently, the use of microwave ablation and cryoablation are increasing. The advantage of microwave ablation over RFA is that effective ablation can be expected even for tissues with low electrical conductivity, and an ablation temperature over 100℃ can be achieved rapidly [548]. Therefore, the treatment efficacy of microwave ablation is less affected by blood vessels located near the tumor, and the size of the ablation zone is larger. For these reasons, microwave ablation is frequently used for HCCs ≥ 2 cm instead of RFA. Meanwhile, monitoring the ablation zone during cryoablation is relatively easy since the ice ball shows a clear margin under the US, non-enhanced CT, or MRI guidance. Moreover, cryoablation has less procedure-related pain [548,549]. However, cryoablation with a single probe generates a small ablation zone, requiring multiple cryoprobes in most cases, and it is rather time-consuming compared to other thermal ablations.
In Child-Pugh class A and B patients with up to three HCCs and a tumor size ≤ 4 cm, a RCT showed no significant differences in the 2-year LTP rate between RFA and microwave ablation [550]. In a RCT comparing the RFA and the microwave ablation for HCC ≤ 3 in number and up to 5 cm in size in Child-Pugh class A and B patients, there were no significant differences in the OS, DFS, and complication rate between the two groups. However, the total ablation time of microwave ablation was shorter than that of RFA [551]. A meta-analysis comparing RFA and other ablation therapies revealed no significant difference in the OS and major complication rate between RFA and microwave ablation [544,552,553,554]. On the other hand, combined TACE and microwave ablation showed a higher OS and lower recurrence rate than microwave ablation alone in a RCT for treating HCCs that are 3–5 cm in size [555].
In patients with Child-Pugh class A and B liver cirrhosis and one or two HCCs, a multicenter RCT showed no significant difference in the 1-, 3-, and 5-year OS, DFS, and major complication rate between RFA and cryoablation [556]. However, cryoablation has been reported to have a lower complication rate compared to RFA in treating HCCs located near the bile duct or intrahepatic vessels [557,558].
In the limited RCTs and meta-analyses mentioned above, microwave ablation and cryoablation showed similar results in terms of the OS, recurrence rate, and major complication rate compared to RFA. Currently, in South Korea, the cost of cryoablation is higher than that for RFA. Additional large-scale prospective RCTs are needed to confirm the difference in therapeutic efficacy among various local ablation therapies.
Other Local Ablation Therapies
Clinical trials on other local ablation therapies, such as high-intensity focused US and laser ablation, are underway. However, as there are few comparative studies with standard treatment, further technological developments and outcomes from the ongoing clinical trials are required to verify their efficacy in managing HCC.
Treatment of Intrahepatic Recurrence After RFA
LTP was reported to be higher in patients who underwent RFA than in those who underwent hepatic resection [514,559]. LTP is defined as recurrence of tumor at the treatment site or margins in which complete response (CR) was verified after initial local ablation therapy. The 3-year LTP rate after RFA has been reported to be 14.5% for HCC patients within the Milan criteria [560].
A large-scale retrospective study at a single institution in South Korea reported that the 5- and 10-year cumulative recurrence rates were 73.1% and 88.5%, respectively, after RFA for HCC patients within the Milan criteria [453]. RFA showed the best therapeutic efficacy for patients with small single nodular HCC (especially tumors ≤ 2 cm) and well-preserved liver function with a 5-year survival rate of 70% [499]. Since repeated RFA for recurred HCC after RFA can improve survival if it achieves a CR, an early detection of recurrence is essential [561]. Surgical treatment, such as hepatic resection and salvage LT, for recurrent HCC after RFA, showed similar therapeutic efficacy compared to repeated RFA [562,563]. If surgical treatment or RFA is not feasible, TACE can be applied [564].
[Recommendations]
1. RFA has an equivalent survival rate, a higher LTP rate, and a lower complication rate compared to hepatic resection in patients with a single nodular HCC ≤ 3 cm in diameter (A1).
2. Combined therapy with TACE and RFA or microwave ablation increases the survival rate in patients with 3–5 cm HCCs that are not amenable to hepatic resection compared to RFA or microwave ablation alone (A2).
3. In the treatment of HCC, microwave ablation and cryoablation are expected to produce comparable rates of survival, recurrence, and complications to those of RFA (B2).
4. Contrast-enhanced US and fusion imaging improve the detection rate and the technical success rate of local ablation therapy for HCCs ≤ 2 cm (B1).
TACE AND RADIOEMBOLIZATION
The majority of HCCs are unresectable at the time of diagnosis due to portal hypertension, poor liver function, multiplicity of tumors, portal vein tumor invasion, inability to secure sufficient resection margin, old age, and severe comorbidities [565]. TACE is the most commonly used non-surgical treatment modality for these patients; tumor necrosis can be achieved by the combined effects of antitumor chemotherapy and selective ischemia of tumor tissue [427,565,566,567]. TACE can be classified as cTACE using lipiodol and drug-eluting bead (DEB)-TACE. TARE is an internal radiation therapy in which the microspheres containing radioactive isotopes are infused into the hepatic artery. As safe and effective methods of delivering radiation to tumors are established, TARE is increasingly being used for the management of HCC.
cTACE
The cTACE procedure involves the injection of a mixture of chemotherapeutic agents, such as doxorubicin, cisplatin, and mitomycin, with iodized oil into the tumor-feeding artery as an emulsion, followed by embolization using gelatin sponge particles, polyvinyl alcohol particles, or microspheres, which induce tumor ischemia. In order to maximize the anticancer effect and minimize liver damage, TACE should be performed as selectively as possible through the tumor-feeding arteries [568,569]. Superselective TACE through the tumor-feeding arteries can significantly increase the tumor necrosis and the local control rate [570,571,572]. In addition, cone-beam CT during TACE can help demonstrate tumors, tumor-feeding arteries, and iodized oil accumulation at the tumor during procedure more precisely and also detect occult lesions, thereby resulting in a better therapeutic effect [573,574,575,576]. Regarding the repetition strategy of TACE, on-demand repetitions to treat the residual or recurrent tumors can minimize the incidence of procedure-related liver toxicity, which is therefore preferable to on-schedule regular repetitions every 1–2 months. Although TACE has been used in clinical practice for a long time, its detailed techniques are not standardized, and the differences according to chemotherapeutic agents and embolic materials are still insufficiently known [577]. In a recent multicenter RCT conducted in Japan, there was no significant difference in tumor response rate and survival rate between miriplatin and epirubicin in cTACE [578].
Compared with best supportive care, several RCTs and meta-analyses have confirmed that cTACE results in a more favorable tumor response, time to progression (TTP), and survival outcomes in patients with unresectable HCC [579,580,581,582,583]. A prospective cohort study by the Japanese Liver Cancer Study Group reported that the 1-, 3-, 5-, and 7-year survival rates of 8510 patients who underwent TACE were 82%, 47%, 26%, and 16%, respectively; for tumors larger than 5 cm, the 1-, 3-, and 5-year survival rates were 63%, 30%, and 16%, respectively [581]. In a prospective multicenter study performed in 27 Japanese and South Korean centers, the complete or partial remission rate according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria was 73% and the 2-year OS was 75%; these figures were higher than those previously reported in the literature [584]. These results were supported by a recent systematic review of 101 articles on cTACE published over the last 30 years, which showed that the OS was 70.3% at 1 year, 51.8% at 2 years, 40.4% at 3 years, and 32.4% at 5 years [583]. This outcome was similar to those of published RCTs.
Local tumor response after cTACE can vary substantially according to the size and number of tumors, as well as the patterns of tumor growth, such as tumor encapsulation and vascular invasion [581,582]. The complete remission rate is quite low for large or multiple tumors despite multiple TACE sessions. However, in small tumors, complete tumor necrosis can be obtained in more than 50% of cases after superselective cTACE [569]. A prospective cohort study conducted in South Korea comparing hepatic resection after primary cTACE with cTACE monotherapy reported that the survival rates were similar between the two treatment groups for stage T3 HCC. Moreover, the survival rate of the TACE group for stage T1 and T2 HCC was similar to that of the hepatic resection group if iodized oil was compactly retained within the tumor [585]. In a prospective cohort study of BCLC stage A HCC patients in whom resection or ablation could not be performed, the 1-month complete remission rate according to the mRECIST criteria was 67%, and the 3-year OS was 80% [586]. In three retrospective studies conducted in South Korea on patients with small HCC within the Milan criteria, there was no significant difference in the long-term (> 5 years) OS among hepatic resection, RFA, and cTACE, although TTP was the shortest in the cTACE group [587,588]. Given the potential selection bias of the studies mentioned above, cTACE can be considered as an alternative to treatments with curative intent when a patient refuses surgical treatment or is at a high risk for undergoing surgery, or HCC is unsuitable for local ablation therapy.
Portal vein tumor invasion is found in approximately 30% of patients with HCCs at initial diagnosis in South Korea [566]. Systemic chemotherapy is the standard primary treatment for HCC with portal vein invasion [213]. However, in real-world practice, more aggressive treatment and various kinds of combination therapy are used, since the expected survival benefits of systemic therapies are modest and no study has yet compared systemic therapy and locoregional treatment, such as cTACE [567,589]. cTACE can be safely performed in advanced HCC patients with portal vein tumor invasion and preserved liver function, without significant risk of liver function deterioration [590,591,592,593,594]. In patients with unresectable HCC with portal vein invasion, survival outcomes were more favorable in the TACE-treated groups than in the supportive treatment groups [595,596,597]. The prognosis was better for tumors localized in one or two hepatic segments, tumors with nodular growth pattern [590,591], or when only segmental portal vein was involved [581,594]. According to a single-center retrospective analysis of cTACE for HCC with segmental portal vein invasion, the median survival was 26.9 months in patients with Child-Pugh class A, ECOG 0, and no extrahepatic spread [598]. The therapeutic effectiveness can be improved by combining cTACE and radiation therapy [49,599,600]. Recently, a Korean single-center RCT reported that cTACE combined with radiation therapy significantly increased the OS, the objective response rate (ORR), and TTP compared to sorafenib monotherapy in patients with HCC and portal vein invasion [601]. Furthermore, a few retrospective studies showed that TACE is associated with survival gain when intrahepatic HCC is treated with TACE in patients with extrahepatic spread [602,603,604].
There have been several studies on the combination of cTACE with systemic therapy to increase the therapeutic effectiveness compared to cTACE alone [605]. Recently, in a multi-center prospective phase 2 randomized study conducted in Japan, the combination therapy of cTACE and sorafenib showed better progression-free survival (PFS) compared to cTACE alone in HCC patients without portal invasion and extrahepatic spread [606]. Studies on combination with lenvatinib, a targeted therapy introduced into clinical practice after sorafenib, is ongoing [607], but its benefits compared to TACE alone has not yet been fully demonstrated. Further studies are needed to select the patients who would benefit most from cTACE combined with systemic therapy compared to other treatments.
The most common complication after cTACE is PES, which is a complex of symptoms, including fever, abdominal pain, nausea, and vomiting. Serious liver-related complications, including irreversible hepatic failure, hepatic infarction, abscess, and biliary injury, can occur. Sepsis, pulmonary oil embolism, cholecystitis, gallbladder infarction, and gastrointestinal complications may also occur [608]. The frequency and severity of complications are related to the tumor size, hepatic functional reserve, portal vein invasion, extent of chemoembolization, and the dose of chemoembolic agents. According to a systematic review, the most common complication after TACE was fever (57.8%), followed by liver enzyme abnormalities (52.0%), PES (47.7%), abdominal pain (42.5%), fatigue/malaise (39.9%), anorexia (38.0%), vomiting (34.2%), nausea (32.4%), and hematological/bone marrow toxicity (28.6%). Hepatic failure occurred in only 1% of the patients, and no new or unexpected safety concerns were identified [583]. The use of anti-inflammatory drugs, such as dexamethasone or parecoxib, to reduce PES before and after TACE has been reported in RCTs [609,610,611], but caution is still required due to the risk of adverse effects, such as worsening of viral hepatitis or diabetes.
In conclusion, cTACE is expected to have the best efficacy and safety when it is selectively performed through tumor-feeding arteries in patients with preserved liver function and good performance status to HCCs localized in the liver with nodular tumor growth and no vascular invasion.
DEB-TACE
Drug-eluting microspheres or DEBs refer to microspheres loaded with high-dose doxorubicin, which can embolize tumor feeders. Embolization of the tumor feeders with DEBs has several benefits, such as tumor ischemia, higher intratumor drug concentration, and lower serum drug concentration due to the slow release of doxorubicin from the DEBs [612].
Prospective RCTs did not show a significant difference in the tumor response rate, time-to-recurrence, and OS between the DEB-TACE group and cTACE group [613,614,615]. In a prospective multicenter study conducted in Europe on 173 patients who underwent DEB-TACE, the 5-year OS rate was 22.5% [616].
A prospective multicenter registry including 152 Korean patients showed a complete remission and ORR of 40.1% and 91.4% at 1 month, and 43.0% and 55.4% at 6 months, respectively [617]. PFS was 9.3 months and the 2-year OS was 79.7% [618]. There was no mortality related to complications including liver abscess. In subgroup analysis, the best tumor response was shown in 2–5 cm tumors, and the tumor response was lower in < 2 cm tumors [618]. The same trend was also observed in a retrospective study conducted at a Korean single center; and in particular, the objective tumor response of DEB-TACE was significantly lower than that of cTACE in < 3 cm tumors [619]. This is presumed to be because DEBs cannot reach the small tumor sufficiently. Recently, DEBs that are sized < 100 µm have been introduced into clinical practice, and several small scaled studies have been reported [620,621]. Further studies regarding their safety and therapeutic efficacy are needed.
At the beginning of the introduction of DEB-TACE, since it is pharmacokinetically superior to cTACE, it was expected to have less hepatic or systemic toxicity and be more useful for patients with liver dysfunction or poor performance [622]. However, in the prospective studies, there was no significant difference in hepatotoxicity or deterioration of liver function after DEB-TACE compared to cTACE [614,615]. Pain after the procedure was less severe and less frequent, and the length of hospital stay was also shorter by 1 day in the DEB-TACE group [615,619]. Since DEBs are small permanent embolic materials, global damage to the liver parenchyma and biliary tree was reported to be two times more common compared to cTACE [623]. However, in case of superselective infusion through tumor-feeding arteries, the clinically relevant damage to the liver parenchyma and biliary tree in DEB-TACE was not significantly different from that in cTACE [619].
In conclusion, DEB-TACE has similar long-term survival, less PES, and shorter hospital stay than cTACE. Therefore, further studies are needed to establish optimal indications for DEBTACE, considering its cost-effectiveness and the lower response rates in small tumors.
TARE Using 90Y Microspheres
TARE involves the injection of implantable radioactive microspheres into tumor-feeding arteries to expose the tumor to highly concentrated radiation while protecting the normal parenchyma. 90Y is the most commonly used radioisotope that emits high-energy and pure β-rays with a half-life of 64.2 hours, and the mean and maximum tissue penetration of 2.5 mm and 11 mm, respectively. The microspheres available for 90Y infusion are 20–60 µm in diameter and are made of resin or glass. The small size of the injected microspheres and their concentration in hypervascular HCC minimize the embolic effect on the surrounding tissue. Preprocedural angiography and 99mTc-labeled macroaggregated albumin scans are required to determine the treatment site, radiation dose and the degree of shunting to the lungs and any other extrahepatic organs. In particular, assessing the lung dose via hepatopulmonary shunt is important, as exceeding the permitted lung dose can increase the risk of radiation pneumonitis [624]. Recently, in a retrospective analysis of 448 patients with HCC within the Milan criteria who underwent 90Y TARE, it was reported that the estimation of lung shunt may be eliminated in these patients since the tumor burden is not large, and the required radiation dose and the degree of hepatopulmonary shunt is not high except in patients with transjugular intrahepatic portosystemic shunt [625].
According to the results of a phase 2 study of 90Y TARE conducted in the United States and Europe between 2010 and 2013, the median survival period was 24.4–26.9 months in BCLC stage A, 16.4–18 months in BCLC stage B, and 7.3–13 months in BCLC stage C [626,627,628,629]. There has been no large-scale prospective RCT comparing TACE and 90Y TARE to date, and according to the meta-analysis of three small RCTs, there was no significant difference in the survival rates and safety between the two treatments [630,631,632,633]. Two phase 3 RCTs did not demonstrate the OS of 90Y TARE to be superior to sorafenib in HCC with portal vein invasion, although it had a higher tumor response rate and fewer side effects [634,635]. Also, in a multi-center prospective RCT comparing the combination therapy of 90Y TARE and sorafenib with sorafenib monotherapy, there was no significant difference in the OS [636].
Recently, improved outcomes were reported by using a higher radiation dose than the standard dose (absorbed tumor dose, 100–150 Gy) [637]. In a multicenter retrospective study of 90Y TARE using a high radiation dose (median absorbed dose, 410 Gy) for a single HCC sized less than 8 cm, the complete remission rate was 84% and the 3-year survival rate was 86.6% [638]. In a multicenter prospective RCT conducted in France on patients with BCLC stage B and C with tumors larger than 7 cm, the standard dosimetry arm applied to deliver 120 ± 20 Gy to the perfused lobe had a median survival period of 10.7 months, while the personalized dosimetry arm applied to deliver at least 205 Gy to the tumor had a median survival period of 26.6 months [639]. In a Korean single-center study of 90Y TARE with over 150 Gy delivered to ≥ 5 cm HCC, the complete remission rate was 80% [640]. According to a retrospective cohort study of patients with a single HCC sized ≥ 5 cm at two Korean centers, 90Y TARE showed similar OS and PFS compared to hepatic resection, with fewer side effects and superior safety [641]. Therefore, 90Y TARE, like TACE, can minimize liver damage and maximize the therapeutic efficacy when the procedure is selectively performed through the tumor-feeding artery. Further studies are needed to select appropriate patients and to compare 90Y TARE with other treatments.
The most common side effect of 90Y TARE is temporary fatigue and it can be safely performed even in the elderly or patients with large tumors due to less PES and better quality of life compared to TACE [642,643]. Radioembolization-induced liver disease (REILD) usually occurs 4–8 weeks after 90Y TARE, and the risk factors include small liver (< 1.5 L), small functional liver volume associated with liver cirrhosis, systemic therapy within 2 months, and extensive infusion of 90Y micro-spheres to both lobes of the liver [644,645,646]. In some patients, delayed hepatotoxicity may occur 6 months after TARE, and it may not be recognized as REILD [647]. Tumor involvement of greater than 50% of the liver and cirrhosis have been reported to be predisposing factors for delayed REILD. Therefore, 90Y TARE should be performed when the tumor is localized and the remnant liver function is expected to be sufficient after the treatment. When 90Y microspheres are delivered to organs other than the liver, more serious complications than TACE, such as radiation pneumonitis and gastric ulcer, can occur; therefore, special attention is required.
In conclusion, 90Y TARE did not show an increase in the OS compared to standard treatments, such as TACE or sorafenib, in RCTs. However, considering the improved therapeutic efficacy when using a higher radiation dose and less PES, 90Y TARE can be an alternative treatment to cTACE in select patient groups, such as those with a single HCC.
[Recommendations]
1. cTACE is recommended for HCC patients with a good performance status without major vascular invasion or extrahepatic spread who are ineligible for hepatic resection, LT, or local ablation therapies (A1).
2. cTACE should be performed through tumor-feeding arteries using selective/superselective techniques to maximize antitumor activity and minimize hepatic damage (B1).
3. In cases of HCC with portal vein invasion, cTACE alone (B2) or cTACE combined with external beam radiation therapy (EBRT) (B1) can be considered for patients with intrahepatic localized tumors and well-preserved liver function.
4. Compared with cTACE, DEB-TACE has similar clinical outcomes in ≥ 3 cm HCCs; therefore, it can be considered as an alternative treatment to cTACE (A2).
5. Compared with cTACE, TARE results in a better quality of life and lower occurrence of PES; therefore, it can be considered an alternative treatment to cTACE when the remnant liver function is expected to be sufficient after the TARE treatment (B2).
Refractoriness to cTACE
cTACE has proven its survival benefit in patients with unresectable HCC [205,580]; therefore, it is recommended as a standard treatment for intermediate-stage HCC according to the BCLC staging system or HCC without major vessel invasion and extrahepatic metastasis, which is unsuitable for hepatic resection, LT, and other local treatments [648]. cTACE is generally considered as a palliative treatment and requires multiple sessions of treatment [649]. However, disease progression is frequently observed during repeated treatment with cTACE, and therefore the concepts of cTACE-refractoriness or cTACE-failure have been proposed [580,650]. In general, cTACE-refractoriness is defined as an insufficient response owing to tumor biology, and cTACE-failure is defined as a technical failure or an inappropriate indication [651,652].
Systemic treatment is considered as a standard treatment for advanced HCC with vascular invasion and/or extrahepatic metastasis. However, due to recent improvements in systemic therapies, an early switch to systemic therapies instead of repeated cTACE or an initial systemic therapy can be considered in patients with intermediate stage HCC who are expected to have a poor prognosis with cTACE. For this reason, it became critical to define the cTACE-refractoriness, and several studies to define the cTACE-refractoriness have been published recently. In a Korean single-center study, when the stage progression during repeated cTACE was set as a surrogate endpoint, the requirement of three or more sessions of cTACE or disease progression during first 6 months after the first session of TACE was associated with short PFS, which was consequently proposed as a predictor of cTACE-refractoriness [653]. These criteria may enable prompt switching to other treatments. However, there are some limitations as the deterioration of liver function after cTACE was not accounted for in the study, and the result is still not fully validated.
The Assessment for Retreatment with TACE (ART) score developed by researchers from Austria integrated post-cTACE elevation of AST, Child-Pugh score and the absence of radiological tumor response. The ART score of ≥ 2.5 after the first TACE was proposed as an indicator for early switching to sorafenib or other treatment, as it was associated with poor survival and significant adverse event after the second session of TACE [654]. Likewise, a French group developed the ABCR (AFP, BCLC, Child-Pugh, and response) score, which combined AFP, tumor stage, change in liver function, and radiologic tumor response, suggesting that patients with ABCR scores ≥ 4 may not benefit from further sessions of TACE [655].
Recently, another Korean multicenter study reported that the change in MoRAL score calculated using the two serum tumor markers AFP and PIVKA-II (= 11 × √PIVKA-II + 2 × √AFP) may indicate TACE-refractoriness. In patients with intermediate-stage HCC, an increase of MoRAL score by 5% or more after the initial session of cTACE showed significantly shorter median OS compared to the control group (18.8 vs. 37.8 months; HR, 2.18; 95% CI, 1.37–34.6; p = 0.001). Specifically, patients who had high pretreatment MoRAL score (≥ 89.5) and an increase in MoRAL score after the initial cTACE showed a median OS below 10 months and these patients were defined as the very poor prognosis group [656]. In addition, SNACOR score (utilizing size and number of tumors, serum AFP level, Child-Pugh score, and radiological response after the first session of cTACE) and ABRAS score (utilizing ALBI score, BCLC stage, radiological response after the first session of cTACE, serum level of AFP, and sex) were reported to predict poor prognosis after cTACE among Korean patients [657,658].
The 2012 European guidelines defined treatment stage migration as no response to at least two sessions of cTACE, and recommended switching to sorafenib [114]. The 2014 KLCA-NCC guidelines defined stage migration following repeated cTACE as cTACE-refractoriness, and recommended switching to sorafenib [116]. The 2014 Japanese guidelines provided the following criteria for TACE refractoriness: i) consecutive insufficient tumor response (≥ 2 sessions) in ≥ 50% of lesions; ii) two or more consecutive progressions in tumor number; iii) development of vascular invasion or extrahepatic spread; or iii) continuous elevation of tumor markers [659]. The 2018 KLCANCC guidelines defined i) no objective response (CR or PR) after two or more sessions of on-demand cTACE during 6 months, ii) development of vascular invasion, or iii) development of extrahepatic metastasis as cTACE-refractoriness, and recommended to switch treatment [660].
To date, various definitions of TACE refractoriness exist, and a treatment strategy to overcome such a condition has not been well-established. Systemic treatments with proven efficacy for advanced HCC, including sorafenib, lenvatinib, atezolizumab/bevacizumab, and durvalumab/tremelimumab, have been proposed as a treatment option to overcome cTACErefractoriness. Although switching to systemic treatment should be recommended if HCC progresses to an advanced stage with extrahepatic spread or vascular invasion, evidence for patients with cTACE-refractoriness presenting only with intrahepatic progression is limited. A sub-analysis of the Phase III Study of Sorafenib in Patients With Advanced Hepatocellular Carcinoma (SHARP) trial showed the survival benefit of sorafenib in patients with prior TACE compared with placebo [661]. However, it remains questionable whether sorafenib is the optimal treatment for cTACE-refractoriness, as there has been no study comparing sorafenib and locoregional therapies. Two retrospective studies conducted in Japan demonstrated that a switch to sorafenib was associated with longer OS and slower hepatic functional deterioration compared to continued cTACE in patients with TACE refractoriness [662,663]. In a retrospective study on patients with TACE refractoriness in Japan, hepatic artery infusion chemotherapy (HAIC) showed promising results in terms of tumor response and survival [664]. It is warranted to evaluate the therapeutic role of various systemic agents that were recently introduced for patients with cTACE-refractoriness.
On the other hand, given the potential ischemic injury due to tissue ischemia following TACE, combination treatment strategies are under investigation, such as TACE plus systemic agents with antiangiogenic property (e.g., sorafenib) [665]. However, the patients enrolled in these clinical trials appear heterogeneous in terms of tumor stage [666], which indicates that a clinical trial designed solely for TACE-refractoriness has not yet been conducted. Several recent studies on combination treatments have shown mixed results. A systematic review with meta-analysis reported that prolonged TTP without significant improvement in OS was achieved with a combination of TACE and sorafenib, compared to TACE alone [667]. A global SPACE trial on combination of sorafenib and DEB-TACE failed to reach clinical significance in terms of TTP [668]. Another large-scale European study comparing combination of DEB-TACE with sorafenib vs. TACE with placebo did not improve PFS in unresectable, liver-confined HCC [669]. In the ORIENTAL study, an Asian multicenter study comparing orantinib vs. placebo combined with TACE, orantinib combined with TACE failed to prolong OS, which was the primary endpoint, in patients with unresectable HCC [670]. In conclusion, the amount of current evidence supporting combination treatment of TACE and systemic agents is insufficient.
[Recommendations]
1. When developing one or more of the following conditions after two or more sessions of on-demand TACE within 6 months from the first TACE, a switch to other treatments should be considered: (1) absence of objective response, (2) new appearance of vascular invasion (3) the new appearance of extrahepatic spread (C1).
EXTERNAL BEAM RADIATION THERAPY
The role of EBRT for HCC is gradually expanding. It is mainly performed when the liver function is Child-Pugh grade A or B7, and a 40%–90% tumor response rate and a median survival period of 10–25 months are reported [671,672,673,674]. For EBRT, a computerized treatment plan using CT is required. In a dose-volume analysis based on a three-dimensional treatment plan, the volume irradiated with < 30 Gy should be≥ 40% of the total liver volume in cases with liver function of Child-Pugh grade A or B7 [675]. Regarding hypofractionated radiation therapy with less than 10 fractions, the volume of normal liver irradiated with < 15 Gy should be at least 700 mL [676], and the mean dose irradiated to normal liver should be ≤ 28 Gy (bioequivalent dose converted to 2 Gy per fraction) [677]. Re-irradiation for recurrent intrahepatic tumors can be performed on the same dose-volume basis as the initial treatment, if the liver function is Child-Pugh grade A or B7 [678,679,680]. When liver function is worse than Child-Pugh grade B7, it is necessary to apply more stringent dose-volume criteria in the computerized treatment plan [681].
EBRT can be performed for HCC patients with difficulties undergoing hepatic resection, transplantation, or other local treatments. The 3-year local control and survival rate of EBRT (including hypofractionated radiation therapy, stereotactic body radiation therapy, and particle radiation therapy) ranged from 81% to 100% and 60%–87%, respectively, and the 5-year local control rate and survival rate ranged from 69%–97% and 43%–78%, respectively [681,682,683,684,685,686,687,688,689,690,691,692,693,694,695,696,697,698,699,700,701,702,703,704,705,706,707,708,709,710,711]. In a meta-analysis, the combination treatment of TACE and EBRT showed a significantly better response rate as well as the 1- and 3-year survival rates, compared to TACE alone [712]. In cases where TACE was infeasible due to severe arteriovenous shunt, vascular occlusion was induced in about 20% of patients after EBRT, thereby enabling subsequent TACE [713]. Response rates of 63%–88% were reported after applying EBRT for HCCs with incomplete response after TACE [714,715,716]. Sequential combination of EBRT after 2 weeks of TACE may cause deterioration of liver function, but liver dysfunction of grade ≥ 3 in the Common Terminology Criteria of Adverse Event (CTCAE) was less than 2.5% [717].
EBRT can be safely performed in advanced HCC with macrovascular invasion. After EBRT, the overall tumor response rate was reported to be 30%–96%, and the median survival time was 7–34.4 months [681,686,692,718,719,720,721,722,723,724,725,726,727,728,729,730,731,732,733,734,735,736,737]. The response rates varied depending on the location of tumors; 30%–83% for portal vein tumor invasion and 43%–96% for inferior vena cava and right atrium tumor invasion. The median survival period after EBRT in HCC with inferior vena cava and right atrium invasion was 12.1 months and 9.3 months, respectively, which was significantly improved from those reported in previous cohort studies [735]. In a Korean multicenter retrospective cohort analysis, 67% of patients who received EBRT for HCC with portal vein invasion received combined treatment with TACE or HAIC [738]. A recent meta-analysis reported that the combination treatment of TACE or HAIC and EBRT significantly improved the objective response and OS of HCC patients with portal vein invasion compared to those treated with TACE, HAIC [739], or sorafenib monotherapy [740]. In retrospective series analyses [599,600,741] and a recent prospective RCT [601], the combination treatment of TACE and EBRT for HCC patients with portal vein invasion significantly improved the survival rates compared to sorafenib monotherapy.
A Korean multicenter cohort study reported that concurrent administration of sorafenib and EBRT improved survival [742]. According to a Taiwan National Cancer Registry cohort study and a Korean retrospective analysis, OS was significantly improved with the addition of EBRT, even after discontinuation or failure of sorafenib [743,744]. A phase 3, multicenter RCT comparing the combination treatment of EBRT and sorafenib versus sorafenib monotherapy (ClinicalTrials.gov: NCT 01730937) is currently underway in the United States, and the results will be noteworthy. There have been several small series reporting that EBRT induces an immune response and improves the treatment outcome when combined with immunotherapy, but the evidence is insufficient at present [745,746]. Several prospective clinical studies are currently underway to investigate the effects of combination treatment of EBRT and systemic therapy.
The combination treatment of EBRT and TACE or HAIC for locally advanced HCC resulted in a median survival period of 13 to 25 months [720,737,747]. In locally advanced HCC, hepatic resection or LT can be considered when downstaging of the disease is achieved by EBRT, and these surgical treatments have been safe and effective among EBRT responders [748,749,750,751,752]. It has also been reported that OS was significantly improved by neoadjuvant EBRT for HCC with portal vein invasion [689,753]. In addition, EBRT can be considered as a bridging treatment for patients awaiting LT [410,754,755,756], or as a second-line treatment for recurrent HCC after treatments such as hepatic resection, RFA, PEI, or TACE [757,758,759,760,761,762].
EBRT is also effective in relieving symptoms caused by tumors, such as cancer pain [763,764]. When jaundice occurs due to obstruction of the biliary tract by HCCs, EBRT could relieve obstruction and jaundice by reducing the tumors, which prolongs the survival [765,766]. In cases of abdominal lymph node metastasis, EBRT showed a tumor response rate of 75%–95%, and prolongation of survival was also reported [767,768,769,770,771,772,773]. In patients with adrenal metastases, EBRT achieved disease control in more than 90% [774]. EBRT for lung metastases showed a tumor response rate in 65%–75% of the patients, and symptom improvement in 90% of the patients [771,775]. EBRT for bone metastases relieved pain in 75%–99% of the patients, and the symptom relief was more significant with higher radiation dose [776,777,778,779,780]. EBRT for spinal metastases accompanying spinal cord compression prevented neurologic dysfunction in 63%–83% of the patients [781]. EBRT can be performed to relieve symptoms of brain metastases [782]. Prolongation of PFS and OS can be expected when EBRT is performed for oligometastasis [783].
In a recent phase 3 RCT, proton beam radiotherapy (PBT) for recurrent or residual HCC of ≤ 3 cm in size was not inferior to RFA in local control rate, and there was no difference in the PFS, OS, and toxicity rates; therefore, PBT can be considered as one of the curative therapeutic options for patients with small HCC [784]. In a single-group study in which proton therapy was applied as the initial treatment, the 5-year local control rate and OS were 94% and 69% in BCLC stage 0/A [700,711]. In other retrospective series, SBRT for recurrent tumors of ≤ 3 cm in size had similar local control rates to RFA [689,785,786,787,788,789]; SBRT was reported to have a superior local control rate than RFA for tumors sized > 3 cm [453,689,785,787].
[Recommendations]
1. EBRT is recommended for patients with HCC unsuitable for hepatic resection, transplantation, local ablation treatments, or TACE (C1).
2. EBRT is performed when the liver function is Child-Pugh grade A or B7 and when the volume to be irradiated with ≤ 30 Gy is ≥ 40% of the total liver volume in the computerized treatment plan (B1).
3. EBRT can be combined for HCCs that are expected to have an incomplete response after TACE (B2).
4. EBRT can be performed for the treatment of HCC with portal vein invasion (B2).
5. EBRT can be combined with systemic therapy for HCC treatment (C2).
6. EBRT is recommended for palliating symptoms of HCC (B1).
7. PBT is not inferior in the local control rate and shows no difference in survival and toxicity rates compared to RFA in treating recurrent or residual HCCs ≤ 3 cm in size (A2); SBRT may not be inferior in the local control rate compared to RFA for the treatment of HCCs ≤ 3 cm in size (C2).
SYSTEMIC THERAPIES
Systemic therapy refers to any drug treatment that travels the bloodstream to reach cancer cells throughout the body. Molecular targeted therapy is regarded as a therapy that targets the intracellular signals involved in the growth and metastasis of cancer cells, while immunotherapy stimulates the host immune system to fight cancer cells. Currently, conventional cytotoxic chemotherapeutic agents, molecular targeted agents, and immune checkpoint inhibitors (a type of cancer immunotherapy) are utilized as systemic therapies for HCC. The primary endpoint of phase 3 clinical trial of systemic therapy is the improvement of OS in most cases and the improvement of PFS in some cases.
First-Line Therapies (Table 8)
Table 8. Summary of Clinical Outcomes of First-Line Key Trials.
| SHARP [790] | REFLECT [796] | IMbrave150 [797,831] | HIMALAYA [828] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SOR | PBO | LEN | SOR | ATZ/BEV | SOR | DURV/TREM | DURV | SOR | |
| Number of patients allocated | 299 | 303 | 478 | 476 | 336 | 165 | 393 | 389 | 389 |
| Median OS (months) | 10.7 | 7.9 | 13.6 | 12.3 | NR (19.2)† | 13.2 (13.4)† | 16.4 | 16.6 | 13.8 |
| HR (95% CI) | 0.69 (0.55–0.87); p < 0.001 | 0.92 (0.79–1.06) | 0.58 (0.42–0.79); p < 0.001 | 0.78 (0.65–0.92) for D/T vs. SOR | |||||
| 0.86 (0.73–1.03) for D vs. SOR | |||||||||
| Median PFS (months) | NA | NA | 7.4 | 3.7 | 6.8 | 4.3 | 3.78 | 3.65 | 4.07 |
| HR (95% CI) | NA | 0.66 (0.57–0.77); p < 0.0001 | 0.59 (0.47–0.76); p < 0.001 | 0.90 (0.77–1.05) for D/T vs. SOR | |||||
| 1.02 (0.88–1.19) for D vs. SOR | |||||||||
| Median TTP (months) | 5.5 | 2.8 | 8.9 | 3.7 | NA | NA | 5.42 | 3.75 | 5.55 |
| HR (95% CI) | 0.58 (0.45–0.74); p < 0.001 | 0.63 (0.53–0.73); p < 0.0001 | NA | NA | |||||
| ORR/CR (%) | 2.0/0.0 | 1.0/0.0 | 24.1/1 | 9.2/< 1 | 27.3/5.5 | 11.9/0.0 | 20.1/3.1 | 17.0/1.5 | 5.1/0.0 |
| DCR (%) | 43 (73*) | 32 (68*) | 75.5 | 60.5 | 73.6 | 55.3 | 60.1 | 54.8 | 60.7 |
| Median duration of treatment (months) | 5.3 | 4.3 | 5.7 | 3.7 | 7.4 for A | 2.8 | NA | NA | NA |
| 6.9 for B | |||||||||
| Median duration of response (months) | NA | NA | NA | NA | (18.1)† | (14.9)† | 22.34 | 16.82 | 18.43 |
| Response evaluation | RECIST v1.1 | mRECIST | RECIST v1.1 | RECIST v1.1 | |||||
*In the SHARP trial, the disease-control rate was presented as the percentage of patients who had a best-response rating of complete or partial response or stable disease that was maintained for at least 28 days after the first demonstration of that rating on independent radiologic review. Numbers in parenthesis indicate the percentage of patients showing complete or partial response or stable disease by independent radiologic review, †Updated analysis of IMbrave150 trial was performed 12 months after the primary analysis and presented. SHARP, A Phase III Study of Sorafenib in Patients With Advanced Hepatocellular Carcinoma; REFLECT, A phase III, multinational, randomized, non-inferiority trial compared the efficacy and safety of lenvatinib (LEN) and sorafenib for the treatment of unresectable hepatocellular carcinoma; HIMALAYA, Study of Durvalumab and Tremelimumab as First-line Treatment in Patients With Advanced Hepatocellular Carcinoma; SOR = sorafenib, PBO = placebo, LEN = lenvatinib, ATZ = atezolizumab, BEV = bevacizumab, DURV = durvalumab, TREM = tremelimumab, OS = overall survival, NR = not reached, HR = hazard ratio, CI = confidence interval, D = durvalumab, T = tremelimumab, PFS = progression-free survival, NA = not available, TTP = time-to-progression, ORR = objective response rate, CR = complete response, DCR = disease control rate, A = atezolizumab, B = bevacizumab, RECIST v1.1 = Response Evaluation Criteria in Solid Tumors version 1.1, mRECIST = modified Response Evaluation Criteria in Solid Tumors
Sorafenib
Sorafenib is a multi-tyrosine kinase inhibitor that targets vascular endothelial growth factor receptor-2 (VEGFR-2), platelet-derived growth factor receptor (PDGFR), Raf-1, and c-kit. Sorafenib is the first molecular targeted agent which proved the survival benefit for advanced HCC in 2007. In the SHARP trial, a global phase 3 RCT, the median survival of HCC patients with portal vein tumor invasion or extrahepatic metastasis treated with sorafenib (400 mg, twice daily) was 10.7 months, which was significantly longer than the 7.9-month survival of patients who received a placebo (HR, 0.69; 95% CI, 0.55–0.87; p = 0.0006) [790]. The TTP in the sorafenib group was 5.5 months, which was also significantly longer than the 2.8 months in the control group [790]. In the phase 3 RCT conducted in the Asia-Pacific region, including Korean patients with unresectable HCC (Asia-Pacific trial), the patients who received sorafenib had a significantly longer median survival period (6.5 months) compared to patients in the control group (4.2 months; HR, 0.68; 95% CI, 0.50–0.93; p = 0.01) [791]. Both phase 3 trials (SHARP and Asia-Pacific trials) enrolled patients with preserved liver function (Child-Pugh class A) and adequate performance status (ECOG performance status of 0 to 2). Thereafter, sorafenib was given as a comparator in seven global RCTs for advanced HCC. The median OS of sorafenib-treated patients was more than 10 months (range, 8.5–14.7 months), longer than that of earlier studies [792,793,794,795,796,797,798].
Sorafenib was administered only for Child-Pugh class A patients; however, real-world retrospective studies have reported comparable TTP and safety between Child-Pugh class A and Child-Pugh class B patients [799,800,801,802,803,804,805,806]. The OS was shorter in Child-Pugh class B patients, and the presence of ascites was significantly associated with worse prognosis among the Child-Pugh class B patients [807]. Underlying liver function may have contributed to the shorter OS in Child-Pugh class B patients compared to Child-Pugh class A patients since they showed similar TTP. According to a large-scale observational study on 3371 sorafenib-treated patients from 39 countries, the overall serious adverse events (SAEs) occurred more frequently in Child-Pugh class B patients (60%) than in Child-Pugh class A patients (36%). Within Child-Pugh B patients, Child-Pugh class B8–9 patients (69%, 67%) experienced SAEs more frequently than Child-Pugh class B7 patients (54%). However, the incidence of treatment-related SAEs was not significantly different between Child-Pugh class A (9%) and Child-Pugh class B patients (14%) [808]. The median OS was different according to Child-Pugh class: 13.6 months for class A, 6.2 months for B7, 4.8 months for B8, and 3.7 months for B9 [808]. Collectively, sorafenib can be considered with caution for patients with liver dysfunction (i.e., Child-Pugh B patients). However, meticulous follow-up is required, since liver-related adverse events tend to occur frequently in Child-Pugh class B patients [801,803]. Careful selection and close monitoring of Child-Pugh class B8/9 patients are necessary, as only limited studies are available so far. Further interventional studies are warranted to determine the optimal use of sorafenib in these patients.
The most common adverse events related to sorafenib treatment are HFSR and diarrhea; other common adverse events include fatigue, skin rash, hypertension, dysphonia, anorexia, weight loss, constipation, and alopecia. HFSR tends to resolve spontaneously after 3 months of treatment; therefore, it is important to continue therapy with patient education and proper management [809]. Since HFSR and hypertension have been reported as potential surrogate predictors of a good response to sorafenib, the management of adverse events needs to be emphasized to clinicians and patients [810]. Creams containing urea may help prevent dryness of the hands and feet. It is recommended that patients remove thick calluses, wear comfortable shoes with cushioning, avoid bathing with hot water, and take analgesics, if necessary, to mitigate and alleviate the symptoms associated with HFSR [809]. An open randomized controlled study reported that urea-containing cream significantly decreased the incidence of HFSR in sorafenib-treated patients [811]; however, another randomized placebo-controlled trial failed to reach statistical significance [812].
Lenvatinib
Lenvatinib is an oral multi-kinase inhibitor targeting VEGFR-1/2/3, fibroblast growth factor receptor (FGFR)-1/2/3/4, PDGFRα, RET, and C-kit. In a global randomized controlled non-inferiority phase 3 trial (REFLECT trial), lenvatinib demonstrated non-inferior OS to sorafenib in advanced HCC patients with a tumor occupying less than 50% of the liver and no bile duct or main portal vein invasion, who had preserved liver function (Child-Pugh class A) and ECOG performance status of 0 or 1 (HR, 0.92; 95% CI, 0.79–1.06) [796]. It was the first drug in 10 years since sorafenib to be approved for the treatment of advanced HCC. Median OS was 13.6 months (95% CI, 12.1–14.9 months) for patients taking lenvatinib (12 mg [weight ≥ 60 kg] or 8 mg [weight < 60 kg] once daily) and 12.3 months (95% CI, 10.4–13.9 months) for patients taking sorafenib. PFS and TTP, both secondary endpoints, were significantly longer in the lenvatinib group than in the sorafenib group (PFS: 7.4 vs. 3.7 months; HR, 0.66; 95% CI, 0.57–0.77; p < 0.00001; TTP: 8.9 vs. 3.7 months; HR, 0.63; 95% CI, 0.53–0.73; p < 0.0001). In the masked independent imaging review according to RECIST 1.1, the ORR was significantly higher in the lenvatinib group (18.8%; CR, < 1%; PR, 18%) than in the sorafenib group (6.5%; CR, < 1%, PR, 6%) (OR, 3.34; 95% CI, 2.17–5.14; p < 0.0001).
SAEs were significantly more frequent in the lenvatinib group than in the sorafenib group (43% vs. 30%) [796]. HFSR was less frequent in the lenvatinib group (27%) than in the sorafenib group (54%), and hypertension was more frequent in the lenvatinib group (42%) than in the sorafenib group (30%). Other adverse events frequently observed in the lenvatinib group were diarrhea (39%), anorexia (34%), weight loss (31%), fatigue (30%), proteinuria (25%), and hypothyroidism (16%). It is recommended to interrupt lenvatinib if 24-hour urinary protein is ≥ 2 g. If a dipstick proteinuria result of 2+ or more is detected, a random urinary protein to creatinine ratio can be used to monitor proteinuria before further testing with the 24-hour urinary protein [813,814]. Thyroid stimulating hormone (TSH) levels should be monitored. If the TSH level is higher than 10 mIU/L or higher than 5 mIU/L on two separate occasions, consultation with an endocrinologist should be considered [814,815]. Hypertension or HFSR has been reported as a predictor of better prognosis, and the OS in patients who discontinued lenvatinib due to SAEs was significantly shorter than those who continuously received treatment [816]. When patients were divided into those with objective response and those without, relative dose intensity was significantly higher in patients showing objective response to lenvatinib [817]. Patients with low relative dose intensity (≤ 70%) demonstrated significantly shorter PFS [818]; therefore, proper management of adverse events is important to continue systemic therapy.
Real-world studies included patients who did not meet the REFLECT criteria, and PFS or ORRs were comparable between patients who met the REFLECT criteria and those who did not [819,820,821,822]. No significant differences were observed in the PFS or ORRs for patients receiving lenvatinib as a first-line or a later-line therapy [819,823]. Meanwhile, in another study, ORRs were lower in patients with Child-Pugh class B, and patients with Child-Pugh class B or beyond the REFLECT criteria showed shorter OS regardless of objective response [823,824]. Some studies reported comparable incidence of adverse events in those patients [820,821,822]; however, others reported that adverse events, such as liver-related adverse events, were more frequent in patients with Child-Pugh class B [819,824]. Collectively, lenvatinib can be considered for patients who do not meet the REFLECT criteria (Child-Pugh class B, tumor occupying > 50% of liver, invasion of main portal vein or bile duct, history of prior systemic therapy, etc.); however, careful monitoring of Child-Pugh class B patients is required. Further studies are warranted.
Atezolizumab Plus Bevacizumab
Atezolizumab is an immune checkpoint inhibitor and a humanized IgG1 monoclonal antibody binding to PD-L1 that can be administered intravenously. Bevacizumab is a molecular targeted agent, an intravenous IgG1 monoclonal antibody binding to VEGF. In a global phase 3 RCT (IMbrave150) comparing atezolizumab plus bevacizumab (atezolizumab 1200 mg + bevacizumab 15 mg/kg every 3 weeks) and sorafenib in patients with advanced HCC, atezolizumab plus bevacizumab significantly improved the OS and PFS [797]. The IMbrave150 study enrolled patients with treatment-naïve advanced HCC who had Child-Pugh class A and ECOG performance status 0 or 1; however, it excluded patients with autoimmune diseases (except autoimmune-related hypothyroidism on thyroid-replacement hormone, type 1 diabetes mellitus on insulin therapy, and autoimmune-related skin diseases with dermatologic manifestations only), treatment with immunosuppressive medication, history of organ or allogeneic stem cell transplantation, inadequately controlled hypertension, gastroesophageal varices incompletely treated or with high-risk for bleeding, and current or recent use of anti-platelet agents, anti-coagulants, or thrombolytic agents for therapeutic purpose.
The median PFS, a co-primary endpoint, was significantly longer with atezolizumab plus bevacizumab (6.8 months; 95% CI, 5.7–8.3) than sorafenib (4.3 months; 95% CI, 4.0–5.6; HR, 0.59; 95% CI, 0.47–0.76; p < 0.001) [797]. The median OS, another co-primary endpoint, was also significantly improved by atezolizumab plus bevacizumab (not evaluable) compared to sorafenib (13.2 months; 95% CI, 10.4 to not evaluable; HR, 0.58; 95% CI, 0.42–0.79; p < 0.001). The median OS of the atezolizumab plus bevacizumab group was not reached at the time of publication. The ORR, a secondary endpoint, was 27.3% (CR, 5.5%; PR, 21.8%) in the atezolizumab plus bevacizumab group, significantly higher than that in the sorafenib group (11.9%; CR, 0%; PR, 11.9%). The disease control rate (DCR) was 73.6% and 55.3% in the atezolizumab plus bevacizumab group and the sorafenib group, respectively [797].
The most frequent adverse event of the atezolizumab plus bevacizumab group was hypertension (29.8% vs. 24.4% in the sorafenib group). Fatigue (20.4% vs. 18.6%), proteinuria (20.1% vs. 18.6%), elevated AST (19.5% vs. 16.7%), and pruritus (19.5% vs. 9.5%) were more frequently observed in the atezolizumab plus bevacizumab group than in the sorafenib group, while diarrhea (18.8% vs. 49.4%) and anorexia (17.6% vs. 24.4%) were less frequently observed in the atezolizumab plus bevacizumab group than in the sorafenib group [797]. Hypothyroidism (10.9%) and pneumonitis (1.2%) were also reported. Although patients at high risk for bleeding were excluded from the IMbrave150 trial, the incidence of upper gastrointestinal bleeding was high in the atezolizumab plus bevacizumab group (7% vs. 4.5%) [797]. Therefore, patients at high risk for bleeding should be evaluated for gastroesophageal varices by esophagogastroduodenoscopy and be managed before initiating atezolizumab plus bevacizumab therapy.
SAEs occurred more frequently in the atezolizumab plus bevacizumab group than in the sorafenib group (38.0% vs. 30.8%); however, treatment-related grade 5 adverse events were less frequent in the atezolizumab plus bevacizumab group (4.6% vs. 5.8%).
A recent real-world study reported that a history of prior systemic therapy did not have a significant effect on the incidence of adverse events; however, additional studies are warranted since there are conflicting results on the treatment response [825,826].
Durvalumab Plus Tremelimumab
Tremelimumab is an immune checkpoint inhibitor and an intravenous fully human IgG2 monoclonal antibody that binds to CTLA-4 expressed on T cells. Durvalumab is another immune checkpoint inhibitor, a fully human IgG1 monoclonal antibody binding to PD-L1 that can be administered intravenously. A global, multicenter, open-label phase 1/2 trial evaluated the safety and efficacy of tremelimumab plus durvalumab, tremelimumab monotherapy, and durvalumab monotherapy. The ORRs were relatively high, 24.0% in patients receiving tremelimumab (300 mg, one dose) plus durvalumab (1500 mg every 4 weeks) and 10.6% in patients receiving durvalumab monotherapy (1500 mg every 4 weeks), respectively [827]. Dermatologic adverse events, such as pruritus and rash, were frequently observed (pruritus, 32.4% in the tremelimumab plus durvalumab group and 10.9% in the durvalumab monotherapy group; rash, 32.4% in the tremelimumab plus durvalumab group and 6.9% in the durvalumab monotherapy group) [827].
In a global multicenter phase 3 RCT (HIMALAYA), the primary endpoint was met, and it was demonstrated that tremelimumab (300 mg, one dose) plus durvalumab (1500 mg every 4 weeks) significantly improved the OS over sorafenib (median, 16.43 vs. 13.77 months; HR, 0.78; 96% CI, 0.65–0.92; p = 0.0035). OS with durvalumab monotherapy (1500 mg every 4 weeks) was noninferior to sorafenib (median, 16.56 vs. 13.77 months; HR, 0.86; 96% CI, 0.73–1.03). The median PFS was not significantly different between the groups: 3.78 months in the tremelimumab plus durvalumab group, 3.65 months in the durvalumab monotherapy group, and 4.07 months in the sorafenib group. The ORRs were 20.1% in the tremelimumab plus durvalumab group, 17.0% in the durvalumab monotherapy group, and 5.1% in the sorafenib group. The DCRs were 60.1% in the tremelimumab plus durvalumab group, 54.8% in the durvalumab mono-therapy group, and 60.7% in the sorafenib group. Treatment-related grade 3/4 adverse events occurred in 17.5% of the tremelimumab plus durvalumab group, 8.2% of the durvalumab monotherapy group, and 9.4% of the sorafenib group. Adverse events, such as esophageal variceal bleeding, did not occur [828].
Others
Nivolumab is an immune checkpoint inhibitor, a human IgG4 monoclonal antibody binding to programmed cell death protein-1 (PD-1) receptor expressed on T cells that can be intravenously administered and restore impaired anticancer activity. In a global phase 3 RCT comparing nivolumab and sorafenib (CheckMate 459) in patients with advanced HCC, the primary endpoint was not met with the median OS of 16.4 months (95% CI, 13.9–18.4) in the nivolumab group and 14.7 months (95% CI, 11.9–17.2) in the sorafenib group (HR, 0.85; 95% CI, 0.72–1.02; p = 0.075) [798]. Nivolumab monotherapy can be considered for patients with contraindications for tyrosine kinase inhibitor, high-risk of bleeding, or anticoagulant users; however, with the success of tremelimumab plus durvalumab therapy, nivolumab monotherapy is expected to play a very limited role.
Donafenib is a multikinase inhibitor and a modified sorafenib derivative. In an open-label phase 2/3 RCT, donafenib significantly improved the OS, the primary endpoint, over sorafenib (12.1 vs. 10.3 months; HR, 0.831; 95% CI, 0.699–0.988; p = 0.0245); however, there was no significant difference between donafenib and sorafenib in the PFS (3.7 vs. 3.6 months, p = 0.0570) and ORR (4.6% vs. 2.7%, p = 0.2448) [829]. Drug-related grade 3 or more adverse events occurred in significantly fewer patients who received donafenib than in patients who received sorafenib (38% vs. 50%, p = 0.0018); however, this trial was limited as it was conducted in a single country.
Another randomized, open-label, multicenter, phase 2–3 study demonstrated that sintilimab (PD-1 inhibitor) plus bevacizumab biosimilar (IBI305) significantly improved the median PFS (4.6 vs. 2.3 months; HR, 0.56; 95% CI, 0.46–0.70; p < 0.0001) and OS (median not reached; HR, 0.57; 95% CI, 0.43–0.75; p < 0.0001) compared to sorafenib. However, the trial was also limited in that it was conducted in a single country [829].
An interim analysis of a global multicenter phase 3 RCT comparing atezolizumab plus cabozantinib and sorafenib reported that PFS, the primary endpoint, was significantly longer with atezolizumab plus cabozantinib (6.8 vs. 4.2 months; HR, 0.64; 95% CI, 0.44–0.91; p = 0.0012) compared to sorafenib; however, there was no statistically significant difference in the OS between the two groups (15.4 vs. 15.5 months; HR, 0.90; 95% CI, 0.69–1.18; p = 0.438). Results of the final analysis are awaited [830].
Considerations in First-Line Therapies
Atezolizumab plus bevacizumab is recommended as a preferred first-line option since it proved superior efficacy over sorafenib. However, atezolizumab is an immune checkpoint inhibitor and patients with a history of stem cell or solid organ transplantation, and autoimmune diseases were excluded from the IMbrave150 trial [797,831]. Therefore there is no evidence for its use in such patients. Considering the adverse events of bevacizumab, a VEGF inhibitor, high-risk varices and inadequately controlled hypertension should be managed before initiating atezolizumab plus bevacizumab. Other first-line systemic agents should be considered for patients who are not adequately managed for varices, current or recent use of anti-platelet agents, anti-coagulants, or thrombolytic agents for therapeutic purposes. Durvalumab and tremelimumab are also immune checkpoint inhibitors, and caution should be taken for patients with a history of transplantation or autoimmune diseases as rejection occurred in 37.5% of LT recipients who were treated with immune checkpoint inhibitors, 75% of whom progressed to end-stage organ failure [832,833,834]. However, durvalumab plus tremelimumab appears to be safe, as it did not increase the risk of bleeding in the phase 3 RCT.
All phase 3 RCTs of first-line systemic therapy have been conducted in patients with Child-Pugh class A. Evidence is lacking for systemic therapy in patients with Child-Pugh class B; however, TTP or safety profiles have been reported to be comparable between patients with Child-Pugh class A and B in real-world studies [800,801,802,803,804,805,806,808]. Sorafenib can be considered for patients with Child-Pugh class B, and liver-related adverse events should be closely monitored for patients with Child-Pugh class B8–9.
HCC can be generally divided into virus-related and non-virus-related types, according to the etiology. A meta-analysis of three randomized controlled phase 3 clinical trials on immune checkpoint inhibitors found that patients with non-viral HCC did not benefit from immune checkpoint inhibitor therapy [797,798,835,836]. On the contrary, the response to molecular targeted therapy did not differ between patients with viral HCC and non-viral HCC [835]. The results of this meta-analysis may support the stratification of patients according to the etiology for systemic therapy; however, it was derived from a post-hoc analysis, and the survival benefit was also observed in patients with non-viral HCC in phase 3 clinical trial of durvalumab plus tremelimumab. Further prospective studies are warranted to confirm these findings. Sorafenib improved the OS in patients with HCV-related HCC [661,837]. Although the survival benefit was not observed in patients with HBV-related HCC who received sorafenib treatment, it should be taken into consideration that baseline HBV DNA titer was not investigated and antiviral therapy was not mandatory in those studies. Lenvatinib demonstrated longer PFS in patients with HBV-related HCC; however, the results of the post-hoc analysis should be carefully interpreted [796].
[Recommendations]
1. Atezolizumab plus bevacizumab or durvalumab plus tremelimumab is recommended for systemic treatment-naïve patients with locally advanced unresectable or metastatic HCC not amenable to curative or loco-regional therapy who have Child-Pugh class A and ECOG performance status 0–1 (A1). If these two combination therapies cannot be applied, sorafenib or lenvatinib is recommended (A1).
2. Sorafenib is considered for patients with HCC who have Child-Pugh class B7 (B1) or B8–9 (B2) if other conditions listed in Recommendation 1 are met.
Second-Line or Subsequent Systemic Therapy After Failure of First-Line Treatment (Table 9)
Table 9. Summary of Clinical Outcomes of Second-Line Key Trials.
| RESORCE [844] | CELESTIAL [845] | REACH-2 [847] | CheckMate-040 [853] | CheckMate-040 [855] | KEYNOTE-394 [846] | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| REG | PBO | CAB | PBO | RAM | PBO | NIV | NIV + IPI | PEM | PBO | |
| Number of patients allocated | 379 | 194 | 470 | 237 | 197 | 95 | 214* | 50† | 300 | 153 |
| Median OS (months) | 10.6 | 7.8 | 10.2 | 8.0 | 8.5 | 7.3 | 9-month 74% | 22.8 | 14.6 | 13.0 |
| HR (95% CI) | 0.63 (0.50–0.79); p < 0.0001 | 0.76 (0.63–0.92); p = 0.005 | 0.710 (0.531–0.949); p = 0.0199 | NA | NA | 0.79 (0.63–0.99); p = 0.0180 | ||||
| Median PFS (months) | 3.1 | 1.5 | 5.2 | 1.9 | 2.8 | 1.6 | 4.0 | NA | 2.6 | 2.3 |
| HR (95% CI) | 0.44 (0.37–0.56); p < 0.0001 | 0.44 (0.36–0.52); p < 0.001 | 0.452 (0.339–0.603); p < 0.0001 | NA | NA | 0.74 (0.60–0.92); p = 0.0032 | ||||
| Median TTP (months) | 3.2 | 1.5 | NA | NA | 3.0 | 1.6 | 4.1 | NA | 3.8 | 2.8 |
| HR (95% CI) | 0.44 (0.36–0.55); p < 0.0001 | NA | 0.427 (0.313–0.582); p < 0.0001 | NA | NA | 0.69 (0.54–0.88); p = 0.0011 | ||||
| ORR/CR (%) | 11.0/1.0 | 4.0/0.0 | 4.0/0.0 | < 1.0/0.0 | 5.0/0.0 | 1.0/0.0 | 20.0/1.0 | 32.0/8.0 | 12.7/2.0 | 1.3/0.7 |
| DCR (%) | 65.0 | 36.0 | 64.0 | 33.0 | 59.9 | 38.9 | 64.0 | 54.0 | 51.0 | 47.1 |
| Median duration of treatment (months) | 3.6 | 1.9 | 3.8 | 2.0 | 12 weeks | 8 weeks | NA | 5.1 | NA | NA |
| Median duration of response (months) | NA | NA | NA | NA | NA | NA | 9.9 | 17.5 | 23.9 | 5.6 |
| Response evaluation | mRECIST | RECIST v1.1 | RECIST v1.1 | RECIST v1.1 | RECIST v1.1 | RECIST v1.1 | ||||
*214 patients in the dose expansion phase, †Nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks (4 doses) followed by nivolumab 240 mg intravenously every 2 weeks.
REG = regorafenib, PBO = placebo, CAB = cabozantinib, RAM = ramucirumab, NIV = nivolumab, IPI = ipilimumab, PEM = pembrolizumab, OS = overall survival, HR = hazard ratio, CI = confidence interval, NA = not available, PFS = progression-free survival, TTP = time-to-progression, ORR = objective response rate, CR = complete response, DCR = disease control rate, mRECIST = modified Response Evaluation Criteria in Solid Tumors, RECIST v1.1 = Response Evaluation Criteria in Solid Tumors version 1.1
It has been approximately 15 years since sorafenib first demonstrated survival benefits over placebo in patients with unresectable HCC in 2007. Since then, there have been several prospective studies on the second-line or third-line treatments after sorafenib failure; and regorafenib, nivolumab plus ipilimumab, cabozantinib, ramucirumab, and pembrolizumab obtained the final approval, conditional approval, or prior authorization from the U.S. Food and Drug Administration (FDA) or the Korean Ministry of Food and Drug Safety. Meanwhile, there have been few studies on effective second-line treatment after the failure of lenvatinib, and atezolizumab plus bevacizumab, as these first-line treatments were approved more recently. Also, as the superiority of durvalumab plus tremelimumab treatment compared to sorafenib was reported very recently, there has been no study on the second-line treatment after durvalumab plus tremelimumab failure. Herein, second-line treatments after failure of first-line treatments, including sorafenib, lenvatinib, atezolizumab plus bevacizumab, and durvalumab plus tremelimumab, are described (Fig. 9).
Fig. 9. Treatment algorithm of systemic therapy for hepatocellular carcinoma.
*If patients have absolute or relative contraindications for immune-checkpoint inhibitors or bevacizumab, multiple tyrosine kinase inhibitors such as sorafenib or lenvatinib should be recommended. AFP = alpha-fetoprotein
Second-Line Systemic Therapies After Sorafenib Failure
Sorafenib failure is generally defined as a progression of the pre-existing disease or an appearance of a new intrahepatic or extrahepatic lesion during sorafenib treatment, and various patterns of disease progression after sorafenib failure are associated with the prognosis [838]. As long-term administration of sorafenib is often limited by disease progression, adverse events, or deterioration in liver function, the median duration of sorafenib administration is reportedly as short as 12 weeks [800,839].
To develop a second-line systemic therapy for HCC patients who stopped sorafenib due to disease progression or adverse events, several phase 3 clinical trials have been conducted using targeted agents, such as brivanib, which inhibits FGF and VEGF [840]; everolimus, which is an mTORi [841]; ramucirumab, which blocks VEGF-2 [842]; and tivantinib, which is a non-selective c-Met inhibitor [843]. However, all of these new agents failed to show improved survival compared to placebo. Recently, several agents, including regorafenib, cabozantinib, pembrolizumab, and ramucirumab (only in patients with serum AFP ≥ 400 ng/mL), have shown survival benefits over placebo after sorafenib-failure [844,845,846,847,848,849].
Regorafenib
Regorafenib is an oral multikinase inhibitor that blocks the activity of protein kinases involved in angiogenesis, oncogenesis, metastasis, and tumor immunity. Although regorafenib has a similar molecular structure to sorafenib, it has a distinct molecular target profile [850,851,852]. An international phase 3 RCT was conducted to validate the efficacy and safety of regorafenib as a second-line therapy for HCC patients with Child-Pugh A liver function and an ECOG score 0–1 who progressed after sorafenib treatment. Only the participants who had tolerated sorafenib (≥ 400 mg/day for ≥ 20 days of last 28 days of treatment) were enrolled. They were randomly assigned to receive either regorafenib or placebo at a 2:1 ratio. Regorafenib improved OS with an HR of 0.63 (95% CI, 0.50–0.79; p < 0.0001); median survival was 10.6 months (95% CI, 9.1–12.1 months) for regorafenib vs. 7.8 months (6.3–8.8 months) for placebo. Based on this result, regorafenib was the first drug to show an improvement in survival as a second-line systemic therapy [844]. The regorafenib group showed significantly longer median PFS by mRECIST compared to the placebo group (3.1 months [95% CI, 2.8–4.2 months] vs. 1.5 months [95% CI 1.4–1.6 months]; p < 0.001). Median TTP by mRECIST was also significantly longer in the regorafenib group (3.2 months; 95% CI, 2.9–4.2 months) than in the placebo group (1.5 months; 95% CI, 1.4–1.6 months; p < 0.001). The mean duration of regorafenib administration was 5.9 months, and that of sorafenib was 3.3 months. Grade 3 or 4 adverse events associated with regorafenib were hypertension (15%), HFSR (13%), fatigue (9%), and diarrhea (3%) [844].
Cabozantinib
Cabozantinib is an oral, molecular targeted agent which blocks MET, VEGFR-2, and RET. An international phase 3 RCT was conducted to validate the efficacy and safety of cabozantinib as a second- or third-line therapy in patients with advanced HCC who progressed on sorafenib treatment and had Child-Pugh A liver function and ECOG score 0–1. The enrolled patients had shown progressive diseases (PDs) despite undergoing one or two systemic therapies including sorafenib, prior to participating in the study. The primary endpoint was OS, and the secondary endpoint was PFS and ORR according to RECIST v1.1. Among all participants, 27% received two systemic therapies including sorafenib. The median OS in the cabozantinib group was 10.2 months, which was significantly longer than the 8.0 months in the control group (HR, 0.76; 95% CI, 0.63–0.92; p = 0.0049). Thus, the clinical trial met the primary endpoint [845]. In subgroup analysis, among patients who experienced sorafenib only, the median OS in the cabozantinib group was 11.3 months, which was also significantly longer than the 7.2 months in the control group (stratified HR, 0.70; 95% CI, 0.55–0.88). According to RECIST v.1.1 criteria, the median PFS was longer in the cabozantinib group (5.2 months) than in the control group (1.9 months) (HR, 0.44; 95% CI, 0.36–0.52; p < 0.001), and ORR was also higher in the cabozantinib group than in the control group (4% vs. 0.4%, p = 0.009) [845]. The median duration of cabozantinib therapy was 3.8 months. The grade 3 or 4 adverse events observed were HFSR (17%), hypertension (16%), elevation of transaminase levels (12%), fatigue (10%), and diarrhea (10%) [845].
Ramucirumab
Ramucirumab is an intravenous monoclonal antibody targeting VEGFR-2. A phase 3 REACH RCT of ramucirumab as a second-line therapy for patients with advanced HCC who progressed on sorafenib treatment was conducted, but it failed to meet the primary endpoint of improvement in OS compared with control [842]. However, in a post-hoc subgroup analysis, the OS in patients with a serum AFP level ≥ 400 ng/mL was 7.8 months, which was significantly longer than the 4.2 months in the placebo group (HR, 0.67; 95% CI, 0.51–0.90). Based on this result, a subsequent phase 3 REACH-2 RCT with 2:1 assignment to ramucirumab or placebo for patients with serum AFP levels of ≥ 400 ng/mL was conducted [847]. The enrolled patients had progressive HCC even after sorafenib, or had stopped sorafenib due to adverse events. All patients had Child-Pugh class A liver function, ECOG score of 0–1, and serum AFP level of ≥ 400 ng/mL. The primary endpoint of the study was OS. The OS in patients who received 8 mg/kg of ramucirumab every 2 weeks was 8.5 months, which was significantly longer than the 7.3 months in the placebo group (HR, 0.71; 95% CI, 0.531–0.949; p = 0.0199). Therefore, the trial met the primary endpoint. By RECIST v.1.1 criteria, the median PFS in the ramucirumab group was 2.8 months, which was also significantly longer than the 1.6 months in the control group (HR, 0.452; 95% CI, 0.339–0.603; p < 0.0001). The DCR in the ramucirumab and control group was 59.9% and 38.9%, respectively (p = 0.0006); however, there was no difference in ORR between the two groups. The median duration of ramucirumab administration was 12 weeks, and the most common grade 3 or 4 adverse event was hypertension (12.2%). Other adverse events included hyponatremia (5.6%). Gastrointestinal bleeding occurred in 6% of the ramucirumab group, but it did not significantly differ from the placebo group (5%).
Nivolumab/Ipilimumab
Nivolumab, a checkpoint inhibitor, is a fully human IgG4-type, monoclonal inhibitory antibody against PD-1. An international phase 1/2 uncontrolled trial on nivolumab for advanced HCC (CheckMate-040) involved patients with histologically confirmed HCC, compensated liver function (i.e., Child-Pugh score ≤ 6 for the dose expansion study and Child-Pugh score ≤ 7 for dose-escalation study), ECOG 0–1, and low serum HBV DNA level below 100 IU/mL (in case of HBV-related HCC) [853]. CheckMate-040 trial included a cohort, in which the primary endpoint was ORR (by RECIST v.1.1) and secondary endpoint included OS and DCR during intravenous nivolumab treatment (3 mg/kg, every 2 weeks) to 145 patients with either sorafenib failure or intolerance (132 patients with sorafenib failure and 12 patients with sorafenib intolerance). In this cohort, ORR was 20% (95% CI, 15%–26%), median duration of response (DOR) was 9.9 months, and 12-month survival rate was 60% (95% CI, 51.4%–67.5%). Grade 3 or 4 AEs, including fatigue, pruritis, rash, and diarrhea, occurred in less than 2% of the patients [853]. In another cohort (cohort 5) of CheckMate-040 trial, when a fixed dose (240 mg every 2 weeks) of nivolumab was administered to 49 patients (25 sorafenib-naïve and 24 sorafenib-experienced patients) with advanced HCC and Child-Pugh class B7–8, ORR was 12% (6 of 49; 95% CI, 5%–25%) and DCR was 55% (95% CI, 40%–69%). Twenty-five patients (51%) reported treatment-related adverse event (TRAE) and two (4%) discontinued treatment owing to TRAE, which were comparable results to those in Child-Pugh class A patients [854].
Another cohort of CheckMate-040 trial evaluated the efficacy of nivolumab in combination with ipilimumab, an inhibitor of CTLA-4, as a second-line treatment for patients with Child-Pugh class A liver function and ECOG 0–1 status who progressed on sorafenib treatment. In group A (n = 49) to whom intravenous nivolumab 1 mg/kg and ipilimumab 3 mg/kg were administered every 3 weeks for four times and then nivolumab 240 mg was administered every 2 weeks, ORR by RECIST v.1.1 was 33% (n = 16; 95% CI, 20%–48%), median DOR (95% CI, 8.3 months–longer than 33.7 months) was not reached, and rates of TRAE was 94%, including one death by pneumonia [855]. Based on these results, the U.S. FDA conditionally approved the combination therapy with nivolumab 1 mg/kg and ipilimumab 3 mg/kg every 3 weeks for four times followed by nivolumab 240 mg every 2 weeks as the second-line treatment after sorafenib.
Pembrolizumab
Pembrolizumab is a humanized IgG4 anti-PD-1 monoclonal antibody that inhibits interaction between PD-1 and PD-L1/PD-L2. A phase 3 multicenter RCT (KEYNOTE-240) compared the OS and PFS between intravenous pembrolizumab (200 mg every 3 weeks), and placebo. This trial included 413 Child-Pugh class A and ECOG 0–1 patients who had previously underwent sorafenib treatment for advanced HCC. Patients were randomly assigned to the pembrolizumab or placebo group in a 2:1 ratio. Pembrolizumab treatment improved both the median OS (13.9 vs. 10.6 months; HR, 0.781; 95% CI, 0.611–0.998; p = 0.0238) and PFS by RECIST v.1.1 (3.0 vs. 2.8 months; HR, 0.718; 95% CI, 0.570–0.904; p = 0.0022) compared to the placebo, which, however, failed to reach the prespecified superiority margin (p = 0.002 in the final analysis). ORR was significantly higher in the pembrolizumab group than in the placebo group (18.3% vs 4.4%, p = 0.00007). Grade 3/4 AEs occurred in 52.7% in the pembrolizumab group and 46.3% in the placebo group. Common grade 3/4 AEs in the pembrolizumab group included elevations of AST (13.3%), bilirubin (7.5%), and ALT (6.1%), which occurred in 7.5%, 5.2%, and 3.0% of the placebo group, respectively [836]. In a post hoc analysis of KEYNOTE-240 trial including Asian patients, the pembrolizumab group showed significantly longer OS (median, 13.8 vs. 8.3 months; HR, 0.55; 95% CI, 0.37–0.88; p = 0.0009) and PFS (median, 2.8 vs. 1.4 months; HR, 0.48; 95% CI, 0.32–0.72; p < 0.0001). ORR was significantly higher in the pembrolizumab group (20.6% vs. 2.0%; p = 0.0014) [854]. The U.S. FDA conditionally approved pembrolizumab as a second-line treatment for HCC.
Recently, the abstract of KEYNOTE-394 trial, which investigated the efficacy and safety of intravenous pembrolizumab (300 mg every 3 weeks, n = 300) versus placebo (n = 153) in 453 Asian patients was presented. The indication criteria of this trial were patients who had baseline Child-Pugh A liver function and ECOG score 0–1, and progression on oxaliplatinbased cytotoxic chemotherapy or sorafenib treatment for BCLC stage C HCC, HCC ineligible for curative treatment or HCC ineligible or refractory to local treatment. The primary endpoint was OS, and pembrolizumab treatment significantly improved OS (median, 14.6 vs. 13.0 months; HR, 0.79; 95% CI, 0.63–0.99; p = 0.018). TTP by RECIST v.1.1 was significantly longer in the pembrolizumab group (median, 2.7 vs. 1.7 months; HR, 0.72; 95% CI, 0.58–0.90). ORR was 13.7% in the pembrolizumab group and 1.3% in the placebo group. The median DOR was 23.9 months in the pembrolizumab group and 5.6 months in the placebo group [846].
Miscellaneous Agents: Apatinib and Camrelizumab
Apatinib is a tyrosine kinase inhibitor that inhibits VEGFR-2. In a Chinese phase 3 RCT (AHELP trial), 400 HCC patients who failed one or more systemic therapies (including oxaliplatinbased cytotoxic chemotherapy as well as molecularly targeted agent, such as sorafenib) were assigned to the apatinib group (oral apatinib 750 mg everyday) or placebo group in a 2:1 ratio. As patients were stratified according to sorafenib treatment, the proportions of patients who had experienced sorafenib were identical (41%) between the two groups. Both OS (median, 8.7 vs. 6.8 months; HR, 0.785; 95% CI, 0.617– 0.998; p = 0.048) and PFS (median, 4.5 vs. 1.9 months; HR, 0.471; 95% CI, 0.369–0.601; p < 0.0001) were significantly longer in the apatinib group. ORR was 11% in the apatinib group and 2% in the placebo group. The most common grade 3/4 AEs were hypertension (28%), HFSR (18%), and thrombocytopenia (13%) in the apatinib group, which developed in 2%, 0%, and 1%, respectively, in the placebo group [856]. In this trial, 9% of the apatinib group and 10% of the placebo group died of AEs, although the investigators regarded all deaths as being unrelated to treatment.
Camrelizumab is a humanized anti-PD-1 monoclonal antibody. In a multicenter phase 2 open-label RCT, 220 Chinese patients who failed previous systemic treatment were assigned to intravenous camrelizumab 3 mg/kg every 2 weeks or every 3 weeks in a 1:1 ratio. ORR was 14.7% (95% CI, 10.3–20.2%), and the 6-month survival rate was 74.4% (95% CI, 68.0–79.7 months) [857].
Selection of Second-Line Treatment
There has been no head-to-head comparison of the efficacy among second-line treatments after sorafenib failure. Instead, a network meta-analysis of previous phase 3 trials indirectly compared the efficacy of four second-line agents (regorafenib, cabozantinib, pembrolizumab, and ramucirumab). In the network meta-analysis, all of the included agents showed significantly longer PFS compared to the placebo (for regorafenib: HR, 0.46 [95% CI, 0.37–0.57]; for cabozantinib: HR, 0.44 [95% CI, 0.37–0.53]; for pembrolizumab: HR, 0.72 [95% CI, 0.57–0.90]; and for ramucirumab: HR, 0.62 [95% CI, 0.52–0.74]). However, only regorafenib (HR, 0.62; 95% CI, 0.51–0.75) and cabozantinib (HR, 0.76; 95% CI, 0.63–0.92) significantly prolonged OS [848]. In comparison of each of the agents, regorafenib had significantly longer PFS than either pembrolizumab (HR, 0.64; 95% CI, 0.47–0.87) or ramucirumab (HR, 0.74; 95% CI, 0.56–0.98). Cabozantinib showed significantly longer PFS than either pembrolizumab (HR, 0.61; 95% CI, 0.46–0.82) or ramucirumab (HR, 0.71; 95% CI, 0.55–0.92) [848]. There was no significant difference in PFS between the other agents. In terms of OS, regorafenib was superior to ramucirumab (HR, 0.71; 95% CI, 0.54–0.93). There was no significant difference in OS between the other agents. However, among patients with serum AFP ≥ 400 ng/mL, in whom ramucirumab is indicated, either regorafenib or cabozantinib was not superior to ramucirumab in terms of both PFS and OS [848].
Several retrospective studies comparing the efficacy of second-line treatments after sorafenib failure were conducted in South Korea. A single-center study involving 102 patients treated with regorafenib and 48 patients with nivolumab as a second-line treatment after sorafenib failure reported that nivolumab treatment was an independent prognostic factor for longer survival (aHR, 0.54; 95% CI, 0.30–0.96; p = 0.04) in multivariable analysis, although there was no significant difference in OS (6.9 vs. 5.9 months, log-rank p = 0.88) in univariable analysis [858]. In contrast, another single-center retrospective study involving 223 patients treated with regorafenib and 150 with nivolumab as a second-line treatment after sorafenib failure reported that there was no difference in both PFS (HR, 0.85; 95% CI, 0.69–1.06; p = 0.15) and OS (HR, 0.83; 95% CI, 0.64–1.07; p = 0.15) between the two treatments. The results were consistent in multivariable analysis, propensity score-matching analysis, and inverse probability treatment weighting analysis [859].
To select second-line or subsequent systemic treatments, physicians may refer to the aforementioned studies. However, further studies are warranted.
Second-Line Treatment After Lenvatinib Failure
As lenvatinib has been used as a first-line treatment for unresectable HCC in clinical practice from late 2018, only a few small-scale retrospective studies on the second-line treatment after lenvatinib failure are available.
A post hoc analysis of phase 3 REFLECT study reported that, at the time of discontinuation of lenvatinib in 451 patients, 36.6%, 48.8%, 9.3%, and 4.9% were ECOG 0, 1, 2, and 3 or 4, respectively, and 75.2%, 21.5%, and 2.9% were Child-Pugh class A, B, and C, respectively. In 156 patients who underwent any subsequent systemic therapy after lenvatinib treatment, the median OS was 20.8 months. Among them, 43 responders to lenvatinib showed a median OS of 25.7 months. Subsequent anticancer medications included sorafenib (32.6%), fluorouracil (4.2%), cisplatin (3.8%), investigational immunotherapies (3.1%), and oxaliplatin (2.9%). In contrast, in 332 patients who underwent no systemic treatment or were not able to receive any systemic treatment, the median OS was merely 11.5 months. These findings support that subsequent systemic treatment may be associated with longer OS [860].
In a retrospective study, among 105 patients who received lenvatinib treatment as a first-line treatment for HCC, 28 patients underwent second-line treatment. In this study, subsequent treatment with molecular targeted agent was an independent prognostic factor for longer OS (aHR, 0.299; 95% CI, 0.120–0.746; p = 0.012) [861]. In another Japanese multicenter retrospective study involving 69 patients who underwent second-line treatment after lenvatinib failure, 53 patients (76.8%) received sorafenib and 22 patients received regorafenib as a second- or third-line treatment. In sorafenib-treated patients, the median PFS was 1.8 months and the ORR was 1.8%. In regorafenib-treated patients, the median PFS was 3.2 months and the ORR was 13.6% [862]. In a retrospective study, 28 patients who underwent ramucirumab treatment after lenvatinib failure in 16 centers in Japan were included. Among them, 14, 9, and 5 patients utilized ramucirumab as a second-, third-, and fourth-line treatment, retrospectively. Their median PFS was 2.0 months, ORR was 3.8% and, DCR was 42.3% [863].
Based on the results of aforementioned retrospective studies, for patients with lenvatinib failure, sorafenib and some second-line agents approved for sorafenib failure (i.e., regorafenib, cabozantinib, and ramucirumab [for patients with serum AFP ≥ 400 ng/mL]) can be considered. Although further studies are required, theoretically, treatments including immune checkpoint inhibitors (i.e., atezolizumab plus bevacizumab, nivolumab plus ipilimumab, nivolumab, and pembrolizumab) can also be considered. In addition, participation in the clinical trials on second-line treatment after lenvatinib failure may be considered.
Second-Line Treatment After Atezolizumab/Bevacizumab Failure
As combination therapy with atezolizumab and bevacizumab has been used as a first-line treatment for unresectable HCC in clinical practice from early 2020, only a few small-scale retrospective studies on second-line treatment after atezolizumab/bevacizumab failure are available.
A recent multinational retrospective study involved 49 patients who underwent second-line treatment after atezolizumab/bevacizumab combination therapy. All the included patients received multikinase inhibitors: 29 patients, 19 patients, and one patient received sorafenib, lenvatinib, and cabozantinib, respectively. Their median PFS was 3.4 months, and the median OS was 14.7 months. The lenvatinib group had significantly longer PFS compared to the sorafenib group (6.1 vs. 2.5 months, p = 0.004), but showed comparable OS (16.6 vs. 11.2 months, p = 0.347) [784].
For patients with atezolizumab/bevacizumab failure, sorafenib, lenvatinib, some second-line agents approved for sorafenib failure (i.e., regorafenib and cabozantinib), and immune checkpoint inhibitors with different targets (i.e., combination therapies with nivolumab plus ipilimumab and durvalumab plus tremelimumab) can be considered, although further studies are required. Regorafenib and cabozantinib, which demonstrated survival benefits as a second- or third-line treatment in patients previously exposed to VEGF inhibitors (e.g., sorafenib), may be theoretically preferred after atezolizumab/bevacizumab failure over sorafenib and lenvatinib, which are proven first-line systemic therapies in patients who are VEGF inhibitors-naïve. However, further studies are warranted [848]. In addition, participation in the clinical trials on second-line treatment after atezolizumab/bevacizumab failure may be considered.
Second-Line Treatment After Durvalumab/Tremelimumab Failure
As a recent phase 3 RCT reported that the combination therapy with durvalumab plus tremelimumab resulted in a longer OS compared to sorafenib as a first-line treatment for unresectable HCC [828], approval by the U.S. FDA for commercial use is expected. Although there has been no report so far, sorafenib, lenvatinib, regorafenib, cabozantinib, ramucirumab (patients with serum AFP ≥ 400 ng/mL), and atezolizumab/bevacizumab can be considered as a second-line treatment for patients with durvalumab/tremelimumab failure. In addition, participation in the clinical trials on second-line treatment after durvalumab/tremelimumab failure may be considered.
[Recommendations]
1. Regorafenib is recommended for patients with progressive HCC after at least 3 weeks of sorafenib (≥ 400 mg/day) treatment and with Child-Pugh class A and good performance status (ECOG score 0–1) (A1).
2. Cabozantinib is recommended for patients with progressive HCC after first-line sorafenib or second-line systemic treatment and with Child-Pugh class A and good performance status (ECOG score 0–1) (A1).
3. Ramucirumab is recommended for patients with progressive HCC after sorafenib or intolerance to sorafenib and with Child-Pugh class A, good performance status (ECOG score 0–1), and serum AFP level ≥ 400 ng/mL (A1).
4. Pembrolizumab is recommended for patients with progressive HCC after sorafenib or intolerance to sorafenib and with Child-Pugh class A and good performance status (ECOG score 0–1) (B1).
5. Either nivolumab plus ipilimumab combination therapy (B1) or nivolumab monotherapy (C1) can be considered for patients with progressive HCC after sorafenib or intolerance to sorafenib and with Child-Pugh class A and good performance status (ECOG score 0–1).
6. Sorafenib, regorafenib, cabozantinib, ramucirumab (if serum AFP level ≥ 400 ng/mL), atezolizumab-bevacizumab, durvalumab-tremelimumab, pembrolizumab, nivolumab-ipilimumab, or nivolumab treatment can be tried for patients with progressive HCC after lenvatinib (D1).
7. Sorafenib, lenvatinib, regorafenib, cabozantinib, durvalumab-tremelimumab, or nivolumab-ipilimumab can be tried for patients with progressive HCC after combination therapy with atezolizumab plus bevacizumab (D1).
8. Sorafenib, lenvatinib, regorafenib, cabozantinib, ramucirumab (if serum AFP level ≥ 400 ng/mL), or atezolizumab-bevacizumab can be tried for patients with progressive HCC after combination therapy with durvalumab plus tremelimumab (D1).
Cytotoxic Chemotherapy and Hepatic Arterial Infusion Chemotherapy
Cytotoxic chemotherapy can be considered for patients with HCC [864,865,866]. However, in most cases, HCC is accompanied by liver cirrhosis, which affects the absorption and metabolism of anticancer drugs, making it impossible to administer a therapeutic dose, and resulting in an increased risk of cytotoxic chemotherapy-related toxicity [867,868]. Fluorouracil, leucovorin, and oxaliplatin (FOLFOX) combination therapy has been studied in a multicenter RCT (EACH study) including 317 Asian patients, but the control arm was doxorubicin monotherapy [869]. To date, there has been no cytotoxic chemotherapy regimen that showed superiority or non-inferiority to sorafenib, lenvatinib, or atezolizumab-bevacizumab combination therapy, which are the currently available options for first-line treatment. Cytotoxic chemotherapy has been studied as a rescue regimen for patients who progressed on first-line sorafenib treatment [870]; and yet, there has been no cytotoxic chemotherapy regimen that demonstrated superiority or non-inferiority to regorafenib or cabozantinib, which have shown benefits for patients who failed the first- or second-line systemic treatment in RCTs. Hence, cytotoxic chemotherapy should be considered for patients with preserved liver function and good performance status who failed or cannot use first- or second-line systemic treatments, such as sorafenib, lenvatinib, regorafenib, cabozantinib, ramucirumab, nivolumab-ipilimumab, or pembrolizumab after careful individualized assessment on the risk and benefit of cytotoxic chemotherapy. Care must be taken to avoid inadvertently worsening the patient’s quality of life.
HAIC is a type of cytotoxic chemotherapy that involves direct injection of the cytotoxic anticancer drugs into the hepatic artery, thereby causing fewer adverse systemic reactions, while exposing HCC to high concentrations of anticancer drugs. The most commonly used drug in HAIC therapy is 5-fluorouracil, which is used alone or in combination with cisplatin. The ORR of HAIC is 3.8%–38.5%, with a PR of 7%–81% and a median survival period of 5.0–19.5 months [871,872,873,874,875]. In observational studies that compared the efficacy of HAIC to sorafenib in advanced HCC, HAIC showed better outcomes compared to sorafenib in some studies [876,877,878,879,880], while other studies showed no difference between HAIC and sorafenib therapies [881]. In a RCT conducted in South Korea that directly compared HAIC and sorafenib in 58 patients with advanced HCC and major portal vein invasion (PVI), the OS was better in the HAIC group than in the sorafenib group (14.9 vs. 7.2 months; HR, 0.32; 95% CI, 0.15–071) [882]. However, the sample size was small and only advanced patients with major PVI were included. Recently, a RCT conduced in China was reported (FOHAIC-1), in which 262 patients with advanced HCC were assigned HAIC and sorafenib in a 1:1 ratio. In this RCT, HAIC showed better OS compared to sorafenib (13.9 vs. 8.2 months; HR, 0.408; 95% CI, 0.301–0.552) [883]. In a multicenter retrospective observational study conducted in South Korea, HAIC was compared to lenvatinib in 244 patients with advanced HCC, and the results showed no difference in the OS between the HAIC and lenvatinib group (9.4 vs. 9.3 months, p = 0.489) [884].
There have been several RCTs on the treatment outcomes of combination treatment of HAIC with sorafenib in advanced HCC, but the findings were inconsistent. In a phase 2 RCT of 108 patients with advanced HCC, the HAIC and sorafenib combination treatment had a longer OS compared to sorafenib monotherapy (10.6 vs. 8.7 months; HR, 0.60; 95% CI, 0.38–0.96) [885]. Meanwhile, in a phase 3 RCT of 205 patients with advanced HCC (SILIUS study), there was no difference in the OS between the HAIC and sorafenib combination treatment and the sorafenib monotherapy group (11.8 vs. 11.5 months; HR, 1.009; 95% CI, 0.743–1.371) [886]. In another RCT of 68 patients with advanced HCC (SCOOP-2 study), there was no difference in the OS between the sequential HAIC followed by sorafenib group and the sorafenib monotherapy group (10.0 vs. 15.2 months; HR, 1.08; 95% CI, 0.63–1.86) [887]. In a RCT of 247 patients with advanced HCC with PVI, survival was better in the HAIC and sorafenib combination group compared to the sorafenib monotherapy group (13.4 vs. 7.1 months; HR, 0.35; 95% CI, 0.26–0.48) [888]. In another RCT of 64 patients with advanced HCC with PVI, survival was better in the HAIC and sorafenib combination group compared to the sorafenib monotherapy group (16.3 vs. 6.5 months; HR, 0.28; 95% CI 0.15–0.53) [889]. There has been no RCT comparing the efficacy and safety of HAIC combination therapy to other systemic therapies. In a retrospective analysis of 170 patients with PD-L1 expressing unresectable HCC, combined treatment with pembrolizumab-lenvatinib and HAIC showed better survival compared to pembrolizumab-lenvatinib therapy [890]; and in another retrospective study of 157 patients with advanced HCC, the OS was better with lenvatinib-toripalimab and HAIC combination therapy compared to lenvatinib monotherapy [891]. Although HAIC is mainly used for the treatment of advanced HCC, HAIC was also studied in a RCT involving 315 unresectable HCC with maximal tumor size > 7 cm but without major vascular invasion or extrahepatic spread (BCLC stage A or B). In this RCT, HAIC using FOLFOX showed better OS compared to TACE (23.1 vs. 16.1 months; HR, 0.58; 95% CI, 0.45–0.75) [892]. Therefore, there may be a group of patients for whom HAIC can be considered as a treatment option [636]; however, studies comparing the efficacy or safety of HAIC to first- or second-line systemic option, such as atezolizumab-bevacizumab, durvalumab-tremelimumab, lenvatinib, regorafenib, cabozantinib, ramucirumab, nivolumab-ipilimumab, and pembrolizumab, are still lacking. Therefore, HAIC might be considered on an individual basis for advanced HCC patients with portal vein invasion, preserved liver function, and without extrahepatic spread for whom first-line or second-line systemic treatment have failed or cannot be used.
[Recommendations]
1. HAIC may be considered for advanced HCC patients with preserved liver function and portal vein invasion without extrahepatic spread for whom first-line or second-line systemic therapies, such as atezolizumab-bevacizumab, durvalumab-tremelimumab, sorafenib, lenvatinib, regorafenib, cabozantinib, ramucirumab, nivolumab-ipilimumab, or pembrolizumab, have failed or cannot be used (C2).
Combination of Local and Systemic Treatment for Advanced HCC
There have been several RCTs on whether combining local and systemic treatment can improve the outcome of patients with advanced HCC. In a RCT that compared TARE and sorafenib combination treatment to sorafenib in 424 patients with advanced HCC, there was no difference in the OS between the two groups (12.1 vs. 11.4 months; HR, 1.01; 95% CI, 0.81–1.25) [636]. In a multicenter phase 3 RCT conducted in South Korea involving 339 patients with advanced HCC (STAH trial), sorafenib with concurrent cTACE failed to prolong the OS of advanced HCC patients compared to sorafenib (12.8 vs. 10.8 months; HR, 0.91; 95% CI, 0.69–1.21) [893]. However, combination treatment with sorafenib and concurrent cTACE significantly improved the secondary outcomes such as PFS, TTP, and tumor response rate compared to sorafenib monotherapy. Post hoc analysis showed that the OS was longer in the combination treatment group than in the sorafenib group if the patients received more than two sessions of cTACE (18.6 vs. 10.8 months; HR, 0.58; 95% CI, 0.40–0.82; p = 0.006) [893]. There may be a subgroup of advanced HCC patients in which the combination of local and systemic treatment may offer survival benefits compared to systemic treatment only. However, further studies are needed to identify candidates for combination therapy and decide what would be the best combination out of many systemic treatment options and local treatment modalities. Recently, a phase 3 RCT comparing lenvatinib plus cTACE and lenvatinib monotherapy for patients with advanced HCC was presented as a meeting abstract [894], and reported better OS, better PFS, and ORR in the combination group. The final announcement is awaited. To date, no final data from a RCT has been reported on the efficacy and safety of combination treatment of systemic agents, other than sorafenib, and various local treatment modalities (cTACE, TARE, EBRT).
Management of Patients With CR After Systemic Treatment
Due to the development of systemic treatments, CR is often observed after systemic treatment for advanced HCC. In a global phase 3 trial (IMbrave150 study), 0% of patients in the sorafenib group and 5.5% in the atezolizumab-bevacizumab group achieved a CR by the RECIST 1.1 criteria, and 1.9% in the sorafenib group and 10.2% in the atezolizumab-bevacizumab group achieved a CR by the mRECIST criteria [797]. In patients with malignant melanoma treated by immunotherapy, durable CR after discontinuation of immunotherapy have been reported [895,896]. This suggests that the discontinuation of immune checkpoint inhibitor-based treatment may be possible for patients achieving CR. However, to date, there is no study that reported whether systemic treatment can be discontinued after achieving CR in advanced HCC patients. Considering the medical resources related to systemic treatment, additional studies on the management of patients with CR after systemic treatment are required.
Adjuvant Therapy
Adjuvant therapy usually refers to additional treatment after curative therapy to prevent recurrence. As the 5-year recurrence rate even after curative resection for HCC is as high as 50%–70%, effective adjuvant therapy is urgently required [249,897,898]. Although TACE [898,899], iodine-131 infusion therapy via the hepatic artery [900], vitamin K2 [901], or vitamin A analogues [902] have been tested as adjuvant therapies after curative treatment for HCC, no therapy has been validated. Cytotoxic chemotherapy or sorafenib also has failed to provide clinical evidence for adjuvant therapy [903,904]. Recently, randomized controlled phase 3 studies on adjuvant therapies after curative treatment using immune checkpoint inhibitors are underway, and the results are awaited [905].
After a Japanese study reported that adjuvant therapy of cytokine induced killer (CIK) cells reduced the 3-year HCC recurrence rate by up to 15% compared with control [906], several prospective RCTs have been conducted [907,908,909,910,911]. In a Korean phase 3 RCT, adjuvant therapy with CIK cells significantly improved the RFS (HR, 0.63; 95% CI, 0.43–0.94) and OS (HR, 0.21; 95% CI, 0.06–0.75) in patients with AJCC stage I or II HCC after curative resection or local ablative therapy (RFA or PEI) [908]. A subgroup analysis demonstrated that RFS was significantly improved only in patients with AJCC stage I HCC. An extended follow-up study (median, 68.5 months; interquartile range, 45.0–82.2) also showed a sustained improvement in both RFS (HR, 0.67; 95% CI, 0.48–0.94; p = 0.009) and OS (HR, 0.33; 95% CI, 0.15–0.76, p = 0.006) [912]. In a Korean real-world study using propensity score analysis, CIK adjuvant therapy significantly improved RFS (HR, 0.42; 95% CI, 0.22–0.80, p = 0.006) [913]. In a cost-effectiveness analysis study based on the results of the randomized controlled study and the real-world study, the incremental cost-effectiveness ratio were $33,077/QALY (quality-adjusted life-year) and$25,107/QALY, respectively [814]. In a Chinese randomized controlled phase 3 trial, CIK cell treatment significantly prolonged the time-to-recurrence (13.6 months in the CIK group and 7.8 months in the control group, p = 0.01); however, in this study, no statistically significant differences were observed in either RFS or OS [907]. A meta-analysis of the RCTs reported that adjuvant CIK cell therapy significantly improved RFS and OS up to 3 years in patients after curative treatment [914].
Although TACE can be applied prior to resection as a neoadjuvant therapy in patients with resectable HCC, no robust evidence support that TACE followed by resection improves the OS or DFS compared to resection only [313].
[Recommendations]
1. Adjuvant immunotherapy with CIK cells can be considered after curative treatment (resection, RFA, or PEI) in patients with HCC ≤ 2 cm without lymph node or distant metastasis (A2).
2. Adjuvant therapy with TACE, sorafenib, or cytotoxic chemotherapy is not recommended for patients with HCC after curative treatment (B1).
PREVENTIVE ANTIVIRAL THERAPY
HBV-Related HCC
The rate of HBV reactivation following cytotoxic chemotherapy for HCC ranges widely from 30% to 60% [915,916], and the subsequent mortality rate is reported to be approximately 30% after HBV reactivation. Therefore, the test for HBsAg must be performed in all patients with HCC before cytotoxic chemotherapy. In patients with positive HBsAg, preventive antiviral drug should be administered before cytotoxic chemotherapy and maintained for at least 6 months after the end of cancer treatment. Interferon is not recommended as a preventive therapy due to the risk of bone marrow suppression and transient aggravation of hepatitis, and oral antiviral drugs are recommended instead. HBV reactivation has been reported in patients with HCC who test negative for HBsAg but positive for anti-HBc [917]; however, there is no strong evidence to recommend uniform preventive therapy for such cases. Preventive antiviral therapy during tyrosine kinase inhibitor treatment is currently controversial. A Korean retrospective study reported no HBV reactivation during sorafenib treatment [800], while another study reported a higher risk of HBV reactivation [918], suggesting the need for additional research. Immune checkpoint inhibitors increase immune responses against HBV, and thus may cause acute aggravation of hepatitis. Therefore, to maintain low HBV viral load during immune checkpoint inhibitor treatment, an effective antiviral drug should be co-administered. For this reason, clinical trials on immune checkpoint inhibitors have only included patients with low serum levels of HBV DNA [797,836,853]. A recent retrospective study of 60 HBV-related HCC patients who received immune checkpoint inhibitor treatments reported HBV reactivation and hepatitis in one out of six patients who did not receive preventive antiviral drugs [919].
Many studies have evaluated HBV reactivation during TACE, and it has been reported to occur in 4.3%–40.5% of patients [920,921,922,923]. In a RCT that compared preemptive lamivudine treatment to an untreated control group during TACE, significant differences were observed with respect to HBV reactivation (2.8% and 40.5%), as well as the consequent occurrence of hepatitis (2.8% and 29.7%) and liver failure (0.0% and 8.1%) [923]. Another randomized trial reported a higher rate of undetected HBV DNA in the preventive lamivudine group compared to that in the control (45.6% vs. 11.2%, p < 0.001), as well as longer TTP (8.2 vs. 4.3 months, p = 0.005) and OS (RR, 0.423; 95% CI, 0.248–0.721; p = 0.002) in the preventive lamivudine group [924]. An observational study compared preventive entecavir therapy with an untreated group and showed significant differences in the rates of virus-related events (6.8% vs. 54.4%, p = 0.001) and acute decompensation (0% vs. 11.6%, p = 0.039) between the two groups [925]. A recent retrospective propensity score-matching study involving 1547 patients reported 1-, 2-, and 3-year HBV reactivation rates of 28.6%, 37.9%, and 44.2%, respectively, after TACE in patients who did not receive preventive antiviral therapy, and a significantly higher 10-year survival rate in the preventive antiviral therapy group (26.5% vs. 12.8%, p < 0.0001) [926]. Therefore, the preventive use of antiviral drugs is necessary for HBV-related HCC patients who receive TACE.
HBV reactivation rates after HAIC for HCC (24% to 67%) are reported to be higher than those after TACE, which is possibly due to the higher dose of chemotherapeutic agents, as HAIC is carried out in shorter intervals [916,927,928]. However, more research is needed to support the claim that HAIC has a higher reactivation rate compared to TACE, as only a few studies with a limited number of participants have been reported and no comparative study with TACE has been performed.
Following the hepatic resection of HCC, HBV reactivation with concomitant elevation in the HBV DNA level or an abnormal biochemical liver function test was observed in 14%–32% of the patients [929]. In a RCT that compared preventive telbivudine administration to an untreated control group from the day of resection, the HBV reactivation rates were 2.5% and 31.8%, respectively. In this study, 57.1% of the reactivation developed within 1 week after hepatic resection [930]. Also, in a RCT that compared preventive adefovir therapy to a control group after R0 resection, the 1-, 3-, and 5-year RFS rates were superior in the adefovir group compared to the control group (85.0%, 50.3%, and 46.1% vs. 84.0%, 37.9%, and 27.1%, respectively) [931]. The corresponding OS rates were also superior in the adefovir group (96.0%, 77.6%, and 63.1% vs. 94.0%, 67.4%, and 41.5%, respectively). The RRs of recurrence and death for antiviral treatment were 0.65 and 0.42, respectively. Antiviral therapy was an independent predictive factor of late tumor recurrence.
A study that compared preventive lamivudine administration and an untreated control group following EBRT for HCC reported the HBV reactivation rates to be 0% and 21.8%, respectively; meanwhile, ALT elevation occurred in 2.3% and 12.5% of the patients, respectively [932]. It has also been reported that the rate of HBV reactivation increases two-fold if TACE is performed in conjunction with EBRT, compared to TACE [933]. A recent retrospective study involving 133 patients reported HBV reactivation rates of 12.7% and 0% in the untreated and preventive antiviral group, respectively, following EBRT and 50% and 16.7%, respectively, following TACE plus EBRT [934].
There are limited studies on HBV reactivation following PEI or RFA; nonetheless, the HBV reactivation rates after RFA have been reported to be 5.6%–9.1% [935,936].
Even in patients with positive HBsAg and undetectable HBV DNA, a few retrospective studies have reported a significant increase in the HBV reactivation rates following hepatic resection and TACE, and reactivation was shown to be associated with HCC recurrence [937] and OS [938]. A recent systemic review on HBV reactivation following HCC treatment classified TACE (19%), hepatectomy (16%), and EBRT (14%) as high-risk procedures with HBV reactivation rates greater than 10%, and tyrosine kinase inhibitor or immune checkpoint inhibitor therapy (7%) and RFA (7%) as moderate-risk procedures [939].
In patients with HBV-related HCC, HBV reactivation frequently develops after cancer treatment, and preventive antiviral treatment has been shown to effectively reduce the risk of reactivation, hepatitis, decompensation, and death. Therefore, the preventive use of oral nucleos(t)ide analogues should be actively considered before HCC treatment in patients with HBV-related HCC.
HCV-Related HCC
In the case of HCV-related HCC, HCV reactivation and the resultant hepatitis may occur after HCC treatment; however, liver failure and death due to HCV reactivation are extremely rare. In a retrospective observational study reporting on HCV- or HBV-related HCC, the rates of reactivation, hepatitis, and liver failure were 26.5%, 10.2%, and 0% in the HCV group and 32.6%, 34.8%, and 10.9% in the HBV group, respectively [940]. Although there was no difference in the reactivation rate after TACE between the two groups, the HCV group had significantly lower rates of hepatitis and liver failure compared to the HBV group. Therefore, it is necessary to monitor patients with HCV-related HCC for HCV reactivation and hepatitis. However, since no study has assessed the effectiveness of preventive antiviral therapy using DAA in patients with HCV-related HCC, there is no evidence yet to recommend preventive antiviral therapy.
[Recommendations]
1. HCC Patients should be tested for hepatitis B surface antigen before starting HCC treatment (A1).
2. In HCC patients with HBV, antiviral therapy should be initiated if serum HBV DNA is detected (A1).
3. In HBsAg-positive HCC patients with undetectable serum HBV DNA, preventive antiviral therapy is recommended before cytotoxic chemotherapy (A1), TACE (A2), HAIC (A2), hepatic resection (A2), EBRT (B1), RFA (C1), tyrosine kinase inhibitor, or immune checkpoint inhibitor (C1) treatment.
4. Antiviral agents for the prevention of HBV reactivation should be selected based on the KASL clinical practice guidelines for management of chronic hepatitis B (A1).
5. There is still no evidence to recommend preventive antiviral therapy with DAAs for HCC patients who are HCV RNA positive (C1).
DRUG TREATMENT FOR CANCER PAIN IN HCC
Types of Pain
Patients with HCC who experience cancer pain have a poorer quality of life and prognosis compared to those without cancer pain [941]. Understanding pain caused by HCC is not only important for the patient’s quality of life but also the prognosis. There are three types of pain caused by HCC: parietal or visceral pain, pain caused by metastasis to bone, and pain that occurs after HCC treatment.
First, parietal or visceral pain is caused by inflammation along the intestinal walls. It manifests as abdominal pain that occurs due to the infiltration of the primary or metastatic lesion to the intestinal wall. Although such pain is reported to be induced by the interactions between the immune system, central and peripheral nerves, and tumor cells, the relative contribution of this pathophysiology to cancer pain is unknown. Peripheral inflammation and recurrent acute pain contribute to visceral hypersensitivity, while recurrent acute pain also induces the formation of synaptic connections and reinforces existing connections in the brain regions associated with pain. These structural and functional changes in the peripheral and central nervous systems induce chronic abdominal pain [942].
Second, nociceptive pain occurs as cancer cells metastasize to the bones. Nociceptive pain is accompanied by the complicated characteristics of inflammatory and neuropathic pain [943]. Rather than damaging the bones, cancer cells induce osteoclastic activation. Osteoclasts and the acidic environment of bones activate sensory nerves through the acid-sensing ion channels and transient receptor potential vanilloid receptor 1, thereby inducing pain. Chemical substances released by cancer cells, such as prostaglandins, and nerve growth factors stimulate and sensitize pain receptors in the bones, and tumors directly pressurize sensory nerve fibers to induce pain.
Third, treatment-induced pain includes PES, which occurs after hepatic artery embolization, as well as pain that occurs during or after RFA.
The prevalence of cancer pain is reported to be 45%–53% [944,945,946]. Active palliative care including pain management from an early stage improves the quality of life [947,948,949] and survival [943,950] of patients with cancer. Although research on pain caused by HCC is rare, the prevalence of pain among patients with HCC is reported at 22%–66.8% [941,945,951], indicating the need to consider pain management as an important part of palliative care for HCC. As HCC is mostly accompanied by liver disease or cirrhosis, patients with HCC may experience changes in their drug metabolism and more serious side effects from pain analgesics depending on the severity of liver dysfunction [952]. However, there is a lack of research on pain management for patients with liver disease [953] or HCC. Therefore, standard cancer treatment principles should be followed [954,955,956], but it is necessary to select the appropriate medications, and adjust doses and administration intervals with considerations for the patient’s underlying liver disease.
Principles of Pain Management
The fundamental principles of the analgesic ladder for pain management proposed by the WHO are to give drugs “by the clock,” “by the mouth,” and “by the ladder.” The same principles are commonly followed to manage cancer pain; patients are initiated on nonopioid analgesics, followed by weak opioids and stronger opioids [954,955,956]. Nonopioid analgesics, such as acetaminophen and NSAIDs, are commonly prescribed for mild pain (numerical pain score: 1–3). Weak opioids, such as codeine, hydrocodone, and tramadol, are used for moderate pain (numerical pain score: 4–6). Strong opioids, such as morphine, oxycodone, hydromorphone, fentanyl, and their analogs, are used for severe pain (numerical pain score: 7–10). Patients with severe pain should not start from the bottom of the analgesic ladder; they may immediately start with strong opioids and then step down the ladder if the cause of pain is deemed resolved. By using these three steps of pain management, approximately 80%–90% of pain can be managed with drugs.
Mild Pain
Although acetaminophen can cause fulminant hepatic failure [957,958], amounts of less than 4 g per day are very unlikely to cause clinically significant hepatotoxicity [959]. However, when other analgesics are added as a fixed dose combination, the dose of acetaminophen should be limited to ≤ 325 mg per dosage unit (tablet, capsule) in order to reduce liver manage induced by acetaminophen [959]. Although acetaminophen-induced hepatic failure has been reported at doses ≤ 4 g in chronic alcohol users [960], a number of studies have reported no noticeable hepatotoxicity for a daily dose of 4 g [961,962], while one study reported a small but significant increase in ALT levels [963]. A daily dose of 2–3 g of acetaminophen was reported to have no association with decompensation in patients with liver cirrhosis [964]. Although the half-life of acetaminophen is increased several folds in patients with liver cirrhosis compared to that in healthy individuals [965], studies have reported that ≤ 4 g of acetaminophen did not cause meaningful side effects in patients with decompensated cirrhosis or chronic liver disease [965,966]. However, a daily dose of 2–3 g is generally recommended for acetaminophen, as patients with liver cirrhosis are at risk of metabolic disorder and prolonged half-life of acetaminophen [967,968].
NSAIDs prescribed to patients with liver disease have a higher concentration of free compounds and are, thus, more likely to cause side effects and toxicity [969]. They are responsible for 10% of cases of drug-induced hepatitis [970] and are reported to cause hepatotoxicity [957,971]. Furthermore, NSAIDs can cause side effects such as nephrotoxicity [972], gastric ulcers or bleeding [973,974], and decompensation [964] in patients with liver cirrhosis; therefore, their use must be avoided as much as possible. In patients with bone metastasis, COX-2 inhibitors (rofecoxib, celecoxib, valdecoxib) are used to alleviate pain by inhibiting prostaglandin synthesis.
Moderate Pain
Drug options are limited for the management of moderate pain before patients move on to take strong opioids, such as morphine. Major drugs used for moderate pain are tramadol and codeine. Tramadol is a nonopioid analgesic that acts on the central nervous system. It alleviates pain by binding with µ-opioid receptors. However, since tramadol is mainly metabolized in the liver, its bioavailability may increase two to three-fold in patients with liver cirrhosis; for these patients, no more than 50 mg of tramadol should be administered within 12 hours [975]. Additionally, tramadol should not be used in conjunction with adjuvant medications that interact with it to affect serotonin metabolism and lower the seizure threshold (e.g., selective serotonin reuptake inhibitor, serotonin-norepinephrine reuptake inhibitor, tricyclic antidepressants, and anticonvulsants).
Codeine is a weak opioid analgesic with 1/10 the potency of morphine and is metabolized via the P450 pathway. The use of codeine must be avoided in patients with liver cirrhosis since its metabolites may accumulate in the liver, causing side effects such as respiratory depression.
Severe Pain
Strong opioids are the main method of treatment for severe pain. Among the known strong opioids, morphine is the most widely used type. Although the effectiveness of strong opioids is acknowledged across many countries, the access to strong opioids is limited. Strong opioids used in hospitals include morphine, oxycodone, hydromorphone, and fentanyl. They are usually administered orally, and intravenously when faster analgesic effects are necessary. Long-acting opioids are administered every 8–12 hours, and short-acting opioids are administered every 3–4 hours for breakthrough pain. Table 10 summarizes the doses and durations of action of oral and intravenous opioids, and considerations for patients with liver cirrhosis [976]. It is difficult to manage cancer pain by a single type of drug as the pain may develop from many causes. At least two different drugs should be used in combination after considering the intensity, frequency, and location of the pain.
Table 10. Opioid Agonist in Patients with Cirrhosis [976].
| Opioid Agonist | Brand Name | Impairment in Metabolism | Dose Adjustment for Cirrhotic Patients | Onset of Action | Duration of Action | Phase I Metabolism | Phase II Metabolism |
|---|---|---|---|---|---|---|---|
| Morphine | Morphine 5/10/30/100 mg | Decreased intrinsic hepatic clearance (reduction in the enzyme activity or intrahepatic shunting) | Dosing interval should be increased 1.5- to 2-fold in cirrhotic patients. The dose should also be reduced. |
5 minutes (IV) 15 minutes (IM) 20 minutes (oral) |
3–7 hours | None | Glucuronidation via UGT2B7 |
| Oxycodone, semi-synthetic m-opioid agonist | Oxycontin CR 10 mg IR codon 10 mg IR codon 5 mg Oxynorm inj 10 mg Oxynorm inj 20 mg Targin CR 5/2.5 mg Targin CR 10/5 mg Targin CR 20/10 mg Targin CR 40/20 mg |
Decreased intrinsic hepatic clearance (reduction in the enzyme activity or intrahepatic shunting) | Oral oxycodone should be initiated at lower doses. | 10–30 minutes (IR, oral) 1 hour (CR, oral) |
3–6 hours (IR) 10–12 hours (CR) |
CYP3A4 CYP2D6 |
None |
| Hydromorphone, semi-synthetic opioid | Dilid 2 mg Jurnista PR 8 mg Jurnista SR 4 mg |
Possible decreases in the metabolizing capacity of conjugating enzymes | A reduction of dose with standard interval is necessary. It should be avoided in patients with hepatorenal syndrome due to accumulation of the neuroexcitatory metabolite. |
15–30 minutes | 4–5 hours | None | Glucuronidation via UGT2B7 |
| Fentanyl, synthetic opioid from the phenylpiperidine class | Fentanyl 50/100/500/1000 mcg Abstral SL tab 100/200 mcg Actiq 200/400 mcg Matrifen patch 12/25/50/100 mcg Instanyl nasal spray 50/100 mcg Durogesic D-trans 25/50/100 mcg |
Affected by changes in hepatic blood flow | It is a first-choice opioid in patients with hepatorenal syndrome, but dose reduction might be necessary to avoid accumulation. | 5 minutes (SL or IV) | 30–60 minutes (IV) 6–7 hours (IN) 20–27 hours (TD) 2–13 hours (SL/buccal) |
CYP3A4 | None |
IV = intravenous, IM = intramuscular, CR = controlled-release, IR = immediate-release, inj = injection, PR = prolonged-release, SR = sustained-release, SL = sublingual, IN = intranasal, TD = transdermal
Considerations for Patients With Liver Cirrhosis
As liver is the major organ responsible for the metabolism of opioids, HCC patients with liver dysfunction may experience increased side effects from opioids, which can be a major cause of hepatic encephalopathy [966]. For this reason, it is necessary to select drugs and adjust their doses and administration intervals according to the liver-related metabolic characteristics of each opioid [968,977]. Morphine has an analgesic effect of its own, and over 90% is excreted via the kidney after being metabolized by conjugation in the liver. Its half-life is increased by about two-fold in patients with liver cirrhosis [978,979], and its bioavailability is four-fold in patients with HCC (68%) compared to that in healthy individuals (17%) [980]. A study reported that oxycodone is metabolized into several metabolites including oxymorphone, which has an analgesic effect, and that estimating the analgesic effect of oxycodone may be difficult since the blood concentrations of its metabolites vary. Moreover, it has been reported that oxycodone has a longer half-life, lower clearance, and greater potency for respiratory depression before LT compared to after transplantation [981]. Hydromorphone has an analgesic effect of its own, and its half-life is reported to be stable even in patients with liver dysfunction as it is metabolized and excreted by conjugation [982]. Fentanyl is metabolized by cytochromes, but it does not produce toxic metabolites. Its blood concentration remains unchanged in patients with liver cirrhosis and is not dependent on renal function [968,977,983]. Recently, the EASL recommended the use of paracetamol, morphine, and hydromorphone for pain control, while NSAIDs, tramadol, codeine, and oxycodone were suggested to be avoided in patients with end-stage liver disease [984].
In addition to medications, there are procedures available for pain management. Radiation therapy is widely performed for pain resulting from bone or lymph node metastasis and is highly effective. It is recommended for managing pain from metastatic HCC, although the level of evidence is low [127]. Depending on the location of metastasis or the affected tissue, RFA or transarterial embolization may also be used to manage pain effectively [985,986].
A multidisciplinary approach involving experts in palliative care is needed to effectively manage acute, recurrent, and chronic pain. As HCC is often accompanied by liver cirrhosis, drug doses must be adjusted after considering the therapeutic and side effects. Further research on pain management is needed to improve the quality of life and increase the survival of patients with HCC.
[Recommendations]
1. In HCC patients, pain control using drugs requires a careful approach with consideration of the underlying liver disease, and type of the drug, dose, and interval of administration should be determined according to liver function (C1).
2. In patients with HCC accompanied by chronic liver disease, a reduced dose of acetaminophen should be considered (C1), and NSAIDs should be used with caution (B1).
3. In patients with HCC accompanied by chronic liver disease, the selection of opioid analgesics, and adjustments in the dosage and interval of administration should be carefully considered based on drug metabolism and liver function (C1).
ASSESSMENT OF TUMOR RESPONSE AND POST-TREATMENT FOLLOW-UP
Tumor Response
The primary purpose of research on HCC treatment is to verify the superiority of a treatment based on the OS. However, tumor response and TTP have also been used as alternative measures of assessing the therapeutic effect. In the field of oncology, tumor response has been traditionally assessed using the criteria by the WHO in 1979 (Table 11) [987]. However, this criterion poses a few problems; discrepancies in the measurement of changes in tumor size between researchers especially the short-axis diameter of the tumor, the number of tumors, and the different definitions of tumor progression, resulted in a lack of uniformity. For instance, whereas some researchers defined tumor progression based on change in the size of a single tumor, others defined it as the sum of the changes in all tumors. Additionally, the criteria do not account for the recent advances in imaging technologies, such as CT and MRI, which have enabled three-dimensional examination of changes in tumor size. To overcome these limitations, the RECIST and RECIST v1.1 were proposed in 2000 and 2009, respectively, which recommend assessing the overall response based on the treatment responses of both target and non-target lesions [988,989]. However, these criteria were designed to assess the outcome of cytotoxic chemotherapy and, thus, had limitations in assessing responses to treatments that do not affect the tumor size. Additionally, the RECIST criteria had some ambiguities regarding the assessment of treatment responses in cases where the best outcome was SD. Especially for molecular targeted therapy and TACE, which do not affect the tumor size, the RECIST is unsuitable to assess the treatment response [990]. Several studies have found that the RECIST does not appropriately account for tumor necrosis resulting from an intervention or a novel molecular targeted drug [790,991]. Theoretically, a viable tumor should be assessed by CT or MRI, and tumor viability should be defined according to the uptake of contrast agent in the arterial phase of dynamic imaging studies. Since the extent of tumor necrosis that occurs after local treatment of HCC is not proportional to a decrease in the diameter of the lesion, the EASL proposed a new definition of treatment response for HCC that considers the extent of tumor necrosis [992], and it was followed by the release of mRECIST criteria [990,993]. These proposals were based on the consensus that the diameter of a remnant tumor at the target site should be used to assess the treatment response. The assessment criteria for vascular invasion, lymph nodes, ascites, and pleural effusions were additionally revised in the mRECIST with a summary of the changes from the previous versions. However, since the mRECIST may be affected by the quality of CT and MRI used to locate tumors and the subjective judgment of the physician interpreting the imaging results, phase 3 clinical trials assessing the treatment response to molecular targeted therapy or immunotherapy tend to use the RECIST rather than the mRECIST.
Table 11. Assessment of Tumor Response.
| RECIST v1.1 | mRECIST | iRECIST | ||
|---|---|---|---|---|
| Target lesion response | ||||
| CR | Disappearance of all target lesions | Disappearance of any intratumoral arterial enhancement in all target lesions | ||
| PR | At least a 30% decrease in the sum of the diameters of target lesions, taking as reference the baseline sum of the diameters of target lesions | At least a 30% decrease in the sum of the diameters of viable (enhancement in the arterial phase) target lesions, taking as reference the baseline sum of the diameters of target lesions | ||
| SD | Any cases that do not qualify for either PR or PD | Any cases that do not qualify for either PR or PD | ||
| PD | An increase of at least 20% in the sum of the diameters of target lesions, taking as reference the smallest sum of the diameters of target lesions recorded since treatment started | An increase of at least 20% in the sum of the diameters of viable (enhancing) target lesions, taking as reference the smallest sum of the diameters of viable (enhancing) target lesions recorded since treatment started | iUPD: ≥ 20% increase of the sum of the longest diameters compared to nadir (minimum 5 mm) or progression of non-target lesions or new lesion; confirmation of progression recommended minimum 4 weeks after the first iUPD assessment iCPD: increased size of target or non-target lesions; increase in the sum of new target lesions > 5 mm; progression of new non-target lesions; appearance of another; new lesion | |
| Non-target lesion response | ||||
| CR | Disappearance of all non-target lesions | Disappearance of any intratumoral arterial enhancement in all non-target lesions | ||
| IR/SD | Persistence of one or more non-target lesions | Persistence of intratumoral arterial enhancement in one or more non-target lesions | ||
| PD | Appearance of one or more new lesions and/or unequivocal progression of existing non-target lesions | Appearance of one or more new lesions and/or unequivocal progression of existing non-target lesions | ||
| mRECIST recommendations | ||||
| Pleural effusion and ascites | Cytopathologic confirmation of the neoplastic nature of any effusion that appears or worsens during treatment is required to declare PD. | |||
| Porta hepatis lymph node | Lymph nodes detected at the porta hepatis can be considered malignant if the lymph node short axis is at least 2 cm. | |||
| Portal vein invasion | Malignant portal vein invasion should be considered as a non-measurable lesion and thus included in the non-target lesion group. | |||
| New lesion | A new lesion can be classified as HCC if its longest diameter is at least 1 cm and the enhancement pattern is typical for HCC. A lesion with atypical radiological pattern can be diagnosed as HCC by evidence of at least 1 cm interval growth. | |||
| Target lesion | Non-target lesion | New lesion | Overall response | |
| CR | CR | No | CR | |
| CR | IR/SD | No | PR | |
| PR | Non-PD | No | PR | |
| SD | Non-PD | No | SD | |
| PD | Any | Yes or no | PD | |
| Any | PD | Yes or no | PD | |
| Any | Any | Yes | PD | |
Adapted from European Association For The Study Of The Liver and European Organisation For Research And Treatment Of Cancer [114], Lencioni and Llovet [990], and Tazdait et al. [1007]. RECIST v1.1 = Response Evaluation Criteria in Solid Tumors version 1.1, mRECIST = modified Response Evaluation Criteria in Solid Tumors, CR = complete response, PR = partial response, SD = stable disease, PD = progressive disease, iUPD = immune unconfirmed progressive disease, iCPD = immune confirmed progressive disease, IR = incomplete response, HCC = hepatocellular carcinoma
When assessing treatment responses to recently introduced immunotherapy, pseudo-progression should be considered, which refers to a temporary increase in tumor size before showing a response to immunotherapy. A tumor undergoing pseudo-progression may be misdiagnosed as PD by the RECIST, resulting in a patient not being able to continue with the appropriate treatment. Pseudo-progression is a phenomenon in which tumor size temporarily increases due to inflammatory reactions such as inflammatory cell infiltration, swelling, and necrosis. It is also a phenomenon in which a delayed decrease in tumor size is observed as a result of the delayed immune response [994]. Pseudo-progression was first observed in melanomas. Approximately 2.8%–11% of patients were reported to experience pseudo-progression following immunotherapy [995]. The iRECIST for assessing the responses to immunotherapy has been recently revised. It differs from the RECIST in that it divides PDs into unconfirmed PDs (UPDs) and confirmed PDs (CPDs). A PD that is suspected for the first time is classified as a UPD, and cases in which a tumor shows a consistent increase in size in follow-up tests or cases in which new lesions persistently emerge are classified as CPDs. A recent study that retrospectively analyzed patients with HCC who underwent nivolumab treatment reported that, of the 22 patients classified as having UPDs in the initial response assessment, 21 (95.5%) were classified as having CPDs in the second response assessment, while UPD was maintained in only one patient; in other words, pseudo-progression was not observed in any case in this study [996]. If the rate of pseudo-progression turns out to be very low for HCC [997], it may be more advantageous and cost-effective to switch patients with HCC over to new drugs immediately when PD is observed after immunotherapy. However, a large-scale prospective study is necessary and the new response assessment criteria must be continuously verified and revised for the new immunotherapy drugs [998].
Assessing radiologic responses and disease progression is important for maintaining objectivity in the interpretation of clinical research results on HCC as new drugs are being developed. A recent meta-analysis reported a clear correlation between the mRECIST criteria and PFS and OS, and reported ORR as an independent predictor of survival [999,1000,1001,1002]. Although several retrospective studies have shown that the results of these tumor response assessment methods reflect the prognoses of patients with HCC, the efficacies of these criteria for patients with advanced HCC are yet to be assessed through prospective research. Since it is not yet clear as to which response assessment methods are superior, treatment decisions should be made based on appropriate methods according to the stage of HCC and the treatment modality. Serum tumor markers can assist in assessing treatment responses when it is difficult to measure the tumor size. When there are no increase in AST/ALT levels, without positive radiologic findings of recurrence, an elevated AFP could support diagnosing recurrence [256]. However, serum tumor markers alone should not be used to assess the treatment response [1003].
Follow-Up Interval for Tumor Response
After the RECIST v1.1 was published in 2009, follow-up assessment of treatment response in solid tumors were recommended every 6–8 weeks in clinical studies [989]. Most of the recent phase 3 clinical trials on target therapies followed the 6–8 week interval. However, some of the recent studies on immune checkpoint inhibitors or immunotherapy had CT or MRI examinations performed every 8–12 weeks to assess the treatment response [853,1004]. A possible theoretical explanation is that there are more delayed responses to immunotherapy compared to targeted molecular therapy, and albeit rare, pseudo-progression may be misinterpreted as disease progression [1005]. To prevent such errors, a second imaging test is suggested to be performed 4 weeks after a lesion is initially classified as an UPD to determine whether the lesion is a CPD [998,1000,1006].
[Recommendations]
1. Assessment of tumor response to treatment should be done using the RECIST v.1.1 according to the change in tumor size and the mRECIST according to the change in viable tumor by dynamic contrast-enhanced CT or MRI (B1).
Follow-Up After CR
There are only few studies on the follow-up evaluation after CR to HCC treatment. Complete response to curative treatment such as hepatic resection, LT, and percutaneous local ablation should be monitored with dynamic contrast-enhanced CT or MRI, serum tumor markers, and biochemical tests. Appropriate follow-up intervals are to be determined based on the pretreatment risk factors and the treatment-specific risk of recurrence.
Recurrence usually develops within 2 years after potentially curative treatments. Since early detection of recurrence increases the possibility of reapplication of curative treatment, posttreatment monitoring should be performed frequently enough to detect recurrence as early as possible [1008]. However, as the risk of recurrence varies depending on the stage of HCC, underlying risk factors, and the patient’s remnant liver function, it is difficult to suggest a uniform recommendation. In general, it is recommended to perform a follow-up assessment with dynamic contrast-enhanced CT or MRI, or MRI using liver-specific contrast agents in conjunction with serum tumor markers every 2–6 months for the first 2 years and every 6 months thereafter if no recurrence develops for 2 years [105,114,1009]. It is also important to note that patients may experience simultaneous or sequential metastases to other organs even after a curative treatment if the initial stage was advanced, vascular invasion was present, or serum AFP level was high [1010]. The lungs, lymph nodes, bones, and adrenal glands are common sites of extrahepatic metastasis. Although restriction of radiation dose for follow-up CTs is not recommended, patients who are expected to have a long survival period should avoid unnecessary CT exams, and alternative tests should also be considered. In addition, the monitoring interval should be individualized on the basis of patient-specific risk factors according to the tumor biology and the underlying liver diseases [1011,1012,1013].
[Recommendations]
1. HCC patients with a CR after treatment should be followed up with imaging studies (i.e., dynamic contrast-enhanced CT/MRI or MRI with liver-specific contrast agents) and serum tumor markers every 2 to 6 months in the first 2 years; after that, patients should be followed via regular checkups at individualized intervals (B1).
MANAGEMENT OF PATIENTS WITH HCC DURING COVID-19 PANDEMIC
The COVID-19 pandemic situation that began in early 2020, caused by infection with a type of SARS-CoV2 virus, currently continues, and it is unclear when it will end. Thus, we aimed to provide brief information on treating patients with HCC during the COVID-19 pandemic. Considering that most patients diagnosed with HCC have underlying liver diseases, the treatment of HCC during a pandemic should take into account both the recommendations for the treatment of underlying liver disease and the general principles for other solid malignancies.
Prognosis of COVID-19 in Patients With Chronic Liver Disease and HCC
In meta-analyses, chronic liver disease was reported to increase the severity (OR, 1.48–1.52) as well as the mortality (OR, 1.36–1.78) of COVID-19, although it did not affect the probability of hospitalizations due to COVID-19 [1014,1015]. Patients with HCC were also shown to have an increased mortality risk from COVID-19 [1016,1017]. Specifically, COVID-19-related deaths in advanced liver disease were strongly associated with decompensated cirrhosis [1017]. In short, both underlying chronic liver disease and HCC are risk factors that increase the severity and mortality of COVID-19 compared to the general population. This suggests that the treatment and surveillance of chronic liver disease and HCC are still crucial and should be maintained during the COVID-19 pandemic.
Prevention
COVID-19 Vaccination
Although the effectiveness of the COVID-19 vaccine varies depending on the type of SARS-CoV2 mutation, clinical trials have reported that mRNA vaccines are effective in preventing infection in up to 94.1%–95.0% of cases [1018,1019]. Real-world clinical data showed that more than 80% of the overall infection and 90% of the symptomatic infection have been prevented by vaccination [1020]. Anaphylaxis, one of the serious adverse effects of mRNA vaccine, occurred in 2.5–4.5 cases per million doses, which was similar to influenza vaccines (1.4 cases per million doses), pneumococcal vaccines (2.5 cases per million doses), and shingles vaccines (9.6 cases per million doses) [1021]. Meanwhile, the incidence of myocarditis or pericarditis after the second jab of mRNA vaccine was estimated to be 10.6 cases per million doses. However, despite these adverse events, the Advisory Committee on Immunization Practices of US CDC still recommends vaccination, as the benefits outweigh the risks [1021].
The NCCN recommends patients with solid malignancies, such as HCC, to receive the COVID-19 vaccination as soon as possible, unless they have contraindications to the vaccine’s component [1022]. It has been demonstrated that cytotoxic chemotherapy-induced granulocytopenia does not affect the effectiveness of vaccines. Theoretically, immunotherapy including immune checkpoint inhibitors, could increase the risk of immune-related adverse events, but early studies have shown that the immune-related adverse events were not significantly higher in patients undergoing immunotherapy [1023]. However, an interval of at least 2–3 days between surgery and vaccination is recommended in order to determine which of them is responsible for symptoms, such as fever; and in the case of surgery, such as splenectomy, which causes a loss of immune function, vaccination should be delayed for approximately 2 weeks [1024]. The CDC recommends the use of mRNA vaccines, such as Pfizer-BioNtech BNT162b2 and Moderna mRNA-1273 [1020].
Although there have been no comparative studies of COVID-19 vaccines in patients with chronic liver disease, a phase 2/3 study with the Pfizer BNT162b2 vaccine included approximately 20.5% of patients with underlying conditions, including liver disease, and it showed no difference in the effectiveness of vaccine between healthy subjects and patients with underlying diseases (95.3% vs. 94.7%) [1019]. In a phase 3 study of the mRNA-1273 vaccine involving 196 patients with liver disease (0.6%), 100 of whom received the vaccination and 96 of whom received a placebo, no patient was infected with COVID-19, making it impossible to compare the two groups [1024]. As described above, it is still unclear whether the effectiveness of the COVID-19 vaccine varies depending on the presence of underlying liver disease, but the frequency of adverse reactions is not expected to differ significantly [1025]. Meanwhile, there have been several reports of occurrence and activation of autoimmune hepatitis in South Korea and other countries following COVID-19 vaccination, and further research is warranted since the causal relationship has not been established [1025,1026]. Vaccination should be decided based on the history of adverse events after vaccination and the underlying liver disease of the patient.
In moderate to severe immunocompromised patients, including those who have received treatment for cancer, the FDA and NCCN recommend administering a booster shot using mRNA vaccine within 3 months of COVID-19 vaccination [1024].
Adherence to Precautionary Measures for Infection Prevention
As patients with chronic liver disease and HCC have a higher risk of COVID-19 infection due to compromised immunity, they should adhere to routine infection control precautionary measures, such as wearing a face mask that fits properly [1027] and washing their hands frequently, even after being vaccinated [19,1028].
[Recommendations]
1. Even during the COVID-19 pandemic, the management of chronic liver disease, the surveillance of at-risk patients, and the treatment of HCC should be continued (D1).
2. COVID-19 vaccination is recommended in patients with HCC, as the benefits of vaccination outweigh the risks (C1). Meanwhile, it is necessary to monitor the occurrence of adverse events after vaccination.
3. Patients with chronic liver disease and HCC should strictly adhere to the infection precautionary measures even after COVID-19 vaccination since they may have a low antibody titer (D1).
Acknowledgments
The authors would like to thank Dr. Sung Won Lee (Catholic Univ. of Korea, Internal Medicine) and Dr. Yuri Cho (NCC Korea, Internal Medicine) for proofreading and revising the English-version manuscript, and Suhyun Chae for improving the images of staging.
Abbreviations
- 90Y
Yttrium-90
- AASLD
American Association for the Study of Liver Diseases
- ABCR
AFP, BCLC, Child-Pugh, and response
- ABRAS score
utilizing ALBI score, BCLC stage, radiological response after the first session of cTACE, serum level of AFP, and sex
- AFP
alpha-fetoprotein
- aHR
adjusted HR
- AJCC
American Joint Committee on Cancer
- ALPPS
Associated Liver Partition and Portal vein Ligation for Staged hepatectomy
- ALT
alanine aminotransferase
- anti-HBc
HBV core antibody
- anti-HBs
HBV surface antibody
- APHE
arterial phase hyperenhancement
- ART
Assessment for Retreatment with TACE
- AST
aspartate aminotransferase
- BCLC
Barcelona Clinic Liver Cancer
- CCA
cholangiocarcinoma
- CDC
Centers for Disease Control and Prevention
- CEA
carcinoembryonic antigen
- CEUS
contrast-enhanced US
- CI
confidence interval
- CIK
cytokine induced killer
- combined HCC-CCA
combined hepatocellular-cholangiocarcinoma
- CPD
confirmed PD
- CR
complete response
- CT
computed tomography
- cTACE
conventional TACE
- CTCAE
Common Terminology Criteria of Adverse Event
- CTLA-4
cytotoxic T lymphocyte-associated antigen-4
- DAA
direct-acting antiviral
- DCR
disease control rate
- DDLT
deceased donor liver transplantation
- DEB
drug-eluting bead
- DFS
disease-free survival
- DOR
duration of response
- EASL
European Association for the Study of the Liver
- EBRT
external beam radiation therapy
- ECOG
Eastern Cooperative Oncology Group
- EORTC
European Organization for Research and Treatment of Cancer
- FDA
Food and Drug Administration
- FDG
fluorodeoxyglucose
- FGFR
fibroblast growth factor receptor
- FOLFOX
fluorouracil, leucovorin, and oxaliplatin
- Gd-EOB-DTPA
gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid
- GRADE
Grading of Recommendations, Assessment, Development and Evaluation
- HAIC
hepatic artery infusion chemotherapy
- HBsAg
HBV surface antigen
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HFSR
hand-foot skin reaction
- HIMALAYA
FULL NAME
- HKLC
Hong Kong Liver Cancer
- HR
hazard ratio
- ICG-R15
indocyanine green 15-minute retention rate
- ICRP
International Commission on Radiological Protection
- Ig
immunoglobulin
- INR
international normalized ratio
- ITT
intention-to-treat
- KASL
Korean Association for the Study of Liver
- KCCR
Korean Central Cancer Registry
- KLCA
Korean Liver Cancer Association
- KLCSG
Korean Liver Cancer Study Group
- KONOS
Korean Network for Organ Sharing
- KPGRC
Korea Practice Guideline Revision Committee
- LDLT
living donor liver transplantation
- LI-RADS
Liver Imaging Reporting and Data System
- LLR
laparoscopic liver resection
- LSM
liver stiffness measurement
- LT
liver transplantation
- LTP
local tumor progression
- MELD
model for end-stage liver disease
- mRECIST
modified Response Evaluation Criteria in Solid Tumors
- MRI
magnetic resonance imaging
- mTORi
mammalian target of rapamycin inhibitors
- mUICC
modified UICC
- NCC
National Cancer Center
- NCCN
National Comprehensive Cancer Network
- NSAID
nonsteroidal anti-inflammatory drug
- OLR
open liver resection
- OPTN
Organ Procurement and TransplantationNetwork
- OR
odds ratio
- ORR
objective response rate
- OS
overall survival
- PBT
proton beam radiotherapy
- PCR
polymerase chain reaction
- PES
postembolization syndrome
- PD-1
programmed cell death protein-1
- PD-L1
programmed cell death-ligand 1
- PD
progressive disease
- PDGFR
platelet-derived growth factor receptor
- PEI
percutaneous ethanol injection
- PET
positron emission tomography
- PFS
progression-free survival
- PIVKA-II
prothrombin induced by vitamin K absence II
- PR
partial response
- PVI
portal vein invasion
- QALY
quality-adjusted life-year
- RCT
randomized controlled trial
- REILD
radioembolizationinduced liver disease
- RFA
radiofrequency ablation
- RFS
recurrence-free survival
- RR
relative risk
- SAE
serious adverse event
- SBRT
stereotactic body radiotherapy
- SD
stable disease
- SRTR
Scientific Registry of Transplant Recipients
- SVR
sustained virologic response
- TACE
transarterial chemoembolization
- TAF
tenofovir alafenamide
- TARE
transarterial radioembolization
- TDF
tenofovir disoproxil fumarate
- TNM
tumor-node-metastasis
- TSH
thyroid stimulating hormone
- TTP
time to progression
- UCSF
University of California San Francisco
- UICC
Union for International Cancer Control
- UPD
unconfirmed PD
- US
ultrasonography
- VEGFR-2
vascular endothelial growth factor receptor-2
- VETC
vessel encapsulating tumor cluster
- WHO
World Health Organization
Appendix 1
2022 KLCA-NCC Korea Practice Guideline Revision Committee (KPGRC)
Chairman
Joong-Won Park (Hepatology, National Cancer Center Korea)
Head of Department: Moon Suk Choi (Hepatology, Sungkyunkwan University), Kyung-Suk Suh (Surgery, Seoul National University), Jin Wook Chung (Radiology, Seoul National University), Jinsil Seong (Radiation Oncology, Yonsei University)
Members
Internal Medicine: Do Young Kim (Hepatology, Yonsei University), Bo Hyun Kim (Hepatology, National Cancer Center Korea), Ji Hoon Kim (Hepatology, Korea University), Baek-Yeol Ryoo (Hepatology, Ulsan University), Dong Hyun Sinn (Hepatology, Sungkyunkwan University), Joo Hyeon Shim (Hepatology, Ulsan University), Sung Won Lee (Hepatology, Catholic University of Korea), Jeong Hoon Lee (Hepatology, Seoul National University), Hyun Woong Lee (Hepatology, Yonsei University), Ho Yeong Lim (Oncology, Sungkyunkwan University), Jeong Won Jang (Hepatology, Catholic University of Korea), Yuri Cho (Hepatology, National Cancer Center Korea)
Surgery: Dong-Sik Kim (Korea University), Jong Man Kim (Sungkyunkwan University), Ji Hoon Kim (National Cancer Center Korea), Jae Do Yang (Jeonbuk National University), Young In Yoon (Ulsan University), Jae Keun Lee (Yonsei University), Hae Won Lee (Seoul National University), Ho Joong Choi (Catholic University of Korea), Suk Kyun Hong (Seoul National University)
Radiology: Young Hwan Koh (National Cancer Center, Korea), Min Woo Lee (Sungkyunkwan University), Sunyoung Lee (Yonsei University), In Joon Lee (National Cancer Center Korea), Jeong Min Lee (Seoul National University), Ijin Joo (Seoul National University), Ho Jong Chun (Catholic University of Korea), Sang Hyun Choi (Ulsan University)
Radiation Oncology: Tae Hyun Kim (National Cancer Center Korea), Hee Chul Park (Sungkyunkwan University), Sun Hyeon Bae (Soon Chun Hyang University), Sang Min Yoon (Ulsan University), Chai Hong Rim (Korea University), Won Il Jang (Korea Cancer Center Hospital)
Appendix 2
Disclosure of Conflict of Interest in the Past 2 Years
Chairman: Joong-Won Park reports sponsored lectures for Roche, Ipsen, Bayer, Eisai; consultant/advisory roles for Astra-Zeneca, Roche, Genetech, Bigene, Bayer, Eutilex, Genexine, Onconic; and research grants from AstraZeneca, Roche, BMS, ONO, Exelixis, MSD-Merck.
Internal Medicine: Moon Suk Choi reports none. Do Young Kim reports none. Bo Hyun Kim reports an advisory role for Eisai, Roche; receiving honoraria from Eisai; and participating in research sponsored by Ono-BMS, Hanmi. Ji Hoon Kim reports sponsored lectures for Gilead, Abbvie, Yuhan, Dong-A ST, Daewoong; advisory role for Gilead, AbbVie, Roche, Dong-A ST; and research grants from Gilead, Celltrion, NOVONO, Daewoong, BMS. Baek-Yeol Ryoo reports none. Dong Hyun Sinn reports sponsored lectures for Bayer and participation in research sponsored by Bayer. Joo Hyeon Shim reports none. Sung Won Lee reports consultant and advisory roles for Eisai, Gilead, Abbvie, GC Cell. Jeong Hoon Lee reports sponsored lectures for BMS Korea, Eisai Korea, Samil Pharmaceutical Company and research grants from Yuhan Pharmaceutical Company, GreenCrossCell. Hyun Woong Lee reports sponsored lectures for Gilead, BMS, Abbvie, Chong Kun Dang, Yuhan, Dong-A, Daewoong; consultant/advisory roles for GC Cell; and participation in clinical trials from Gilead, Abbvie, Chong Kun Dang, Yuhan, Dong-A. Ho Yeong Lim reports advisory roles for Bayer, Eisai, Roche, Ipsen and participation in clinical trials from Bayer, Eisai, MSD, Roche, Ipsen, BMS. Jeong Won Jang reports honoraria from Gilead, Abbvie, Bayer, Roche, Sysmex, Ipsen and grants from Sysmex, Gilead, Yuhan, Dong-A ST, Hanmi. Yuri Cho reports none.
Surgery: Kyung-Suk Suh reports none. Dong-Sik Kim reports none. Jong Man Kim reports sponsored lecture for Chong Kun Dang, Johnson & Johnson, Greencross, Astellas, Novartis; advisory role for Roche; and participation in clinical trials from Chon Kun Dang, Greencross, Greencross Labcell, SK Plasma. Ji Hoon Kim reports none. Jae Do Yang reports none. Young In Yoon reports none. Jae Keun Lee reports none. Hae Won Lee reports none. Ho Joong Choi reports none. Suk Kyun Hong is a shareholder and an advisory board member of GENOME INSIGHT Inc.
Radiology: Jin Wook Chung reports sponsored lecture for Guerbet and participation in clinical trials from MSD, Parmicell. Young Hwan Koh reports none. Min Woo Lee reports sponsored lectures for Bracco, Boston scientific, RF medical; consultant/advisory role for STARrmed; and participation in a clinical trial from STARmed. Sunyoung Lee reports none. In Joon Lee reports sponsored lectures for Guerbet Korea. Jeong Min Lee reports grants from Bayer Healthcare, Canon Healthcare, Philips Healthcare, GE Healthcare, CMS, Dongkuk Pharma, Guerbet, Samsung Medison, Bracco, RF Medical, Starmed and personal fees from Bayer Healthcare, Siemens Healthineer, Samsung Medison, Guerbet, Philips, outside the submitted work. Ijin Joo reports sponsored lecture for Samsung Medicine, Bayer, Bracco and participation in clinical trials from Samsung Medicine. Ho Jong Chun reports none. Sang Hyun Choi reports none.
Radiation Oncology: Jinsil Seong reports none. Tae Hyun Kim reports none. Hee Chul Park reports none. Sun Hyeon Bae reports none. Sang Min Yoon reports none. Chai Hong Rim reports none. Won Il Jang reports none.
Delphi Committee: Yoon Jun Kim sponsored lectures for BMS, MSD, Abbvie, Bayer; consultant/advisory roles for Celltrion, Bukwang; research grant support from BTG, Boston Scientific, AstraZeneca, Gilead Sciences, Samjin, BL&H, Bayer; and participation in clinical trials from Gilead, BTG, GSK, MSD, Cymabay, Roche, Boehringer Ingelheim, Galectin, Intercept, Pfizer, Arbutus, Abbvie, Ildong, Hanmi, Creagen, AstraZeneca, PharmaKing. Tae-Yong Kim reports none. Soo Young Park reports none. Changhoon Yoo reports none. Young-Suk Lim serves as advisor/consultant/speaker for AbbVie, Arbutus Biopharma, Assembly Biosciences, Brii Biosciences, Bayer Healthcare, GlaxoSmithKline, Gilead Sciences, Janssen, Olix Phamaceuticals, Roche, Vaccitech, Vir Biotechnology and received grant/research support from Gilead Sciences. Hyung Joon Yim reports sponsored lectures for Gilead, Abbvie, Han-mi, Yuhan and participation in clinical trials from Gilead, Daewoong, GSK, Hanmi, Il Dong Pharm. Jae Young Jang reports none. Hong Jae Chon reports sponsored lecture for Roche, Bayer, BMS; consultant/advisory roles for Roche, ONO, BMS, Eisai, Bayer, MSD, AstraZeneca; research grants from Roche; and participation in clinical trials from, Roche, BMS, ONO, MSD, Amgen. Jung Yong Hong reports none.
Footnotes
This article is being published jointly in Clinical and Molecular Hepatology, Korean Journal of Radiology, and Journal of Liver Cancer.
Conflicts of Interest: See Appendix 2.
Author Contributions: See Appendix 1.
Funding Statement: All required funding was provided by the NCC Korea (grant number. 2112570-2).
Supplement
The Supplement is available with this article at https://doi.org/10.3348/kjr.2022.0822.
References
- 1.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ, et al. What is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336:995–998. doi: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guyatt GH, Oxman AD, Kunz R, Falck-Ytter Y, Vist GE, Liberati A, et al. Going from evidence to recommendations. BMJ. 2008;336:1049–1051. doi: 10.1136/bmj.39493.646875.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336:1106–1110. doi: 10.1136/bmj.39500.677199.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. Development of the AGREE II, part 1: performance, usefulness and areas for improvement. CMAJ. 2010;182:1045–1052. doi: 10.1503/cmaj.091714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. Development of the AGREE II, part 2: assessment of validity of items and tools to support application. CMAJ. 2010;182:E472–E478. doi: 10.1503/cmaj.091716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee KS, Chang HS, Lee SM, Park EC. Economic burden of cancer in Korea during 2000-2010. Cancer Res Treat. 2015;47:387–398. doi: 10.4143/crt.2014.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim YA, Lee YR, Park J, Oh IH, Kim H, Yoon SJ, et al. Socioeconomic burden of cancer in Korea from 2011 to 2015. Cancer Res Treat. 2020;52:896–906. doi: 10.4143/crt.2019.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korean Central Cancer Registry. Annual report of Korean central cancer registry (2019) Goyang: Korea Central Cancer Registry; 2021. [Google Scholar]
- 12.Chon YE, Lee HA, Yoon JS, Park JY, Kim BH, Lee IJ, et al. Hepatocellular carcinoma in Korea between 2012 and 2014: an analysis of data from the Korean nationwide cancer registry. J Liver Cancer. 2020;20:135–147. doi: 10.17998/jlc.20.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi SI, Cho Y, Ki M, Kim BH, Lee IJ, Kim TH, et al. Better survival of patients with hepatitis B virus-related hepatocellular carcinoma in South Korea: changes in 16-years cohorts. PLoS One. 2022;17:e0265668. doi: 10.1371/journal.pone.0265668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korean Central Cancer Registry. Annual report of Korean central cancer registry (2015) Goyang: Korea Central Cancer Registry; 2017. [Google Scholar]
- 15.Fujiwara N, Friedman SL, Goossens N, Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol. 2018;68:526–549. doi: 10.1016/j.jhep.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang MH, You SL, Chen CJ, Liu CJ, Lee CM, Lin SM, et al. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst. 2009;101:1348–1355. doi: 10.1093/jnci/djp288. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization (WHO) Hepatitis B vaccines: WHO position paper. Geneva: WHO; 2017. [DOI] [PubMed] [Google Scholar]
- 18.European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park H, Shin SK, Joo I, Song DS, Jang JW, Park JW, et al. Systematic review with meta-analysis: low-level alcohol consumption and the risk of liver cancer. Gut Liver. 2020;14:792–807. doi: 10.5009/gnl19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marrero JA, Fontana RJ, Fu S, Conjeevaram HS, Su GL, Lok AS. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J Hepatol. 2005;42:218–224. doi: 10.1016/j.jhep.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 22.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460–468. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 23.Lee YB, Moon H, Lee JH, Cho EJ, Yu SJ, Kim YJ, et al. Association of metabolic risk factors with risks of cancer and all-cause mortality in patients with chronic hepatitis B. Hepatology. 2021;73:2266–2277. doi: 10.1002/hep.31612. [DOI] [PubMed] [Google Scholar]
- 24.Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology. 2013;144:323–332. doi: 10.1053/j.gastro.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Simon TG, Duberg AS, Aleman S, Hagstrom H, Nguyen LH, Khalili H, et al. Lipophilic statins and risk for hepatocellular carcinoma and death in patients with chronic viral hepatitis: results from a nationwide Swedish population. Ann Intern Med. 2019;171:318–327. doi: 10.7326/M18-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tran KT, McMenamin ÚC, Coleman HG, Cardwell CR, Murchie P, Iversen L, et al. Statin use and risk of liver cancer: evidence from two population-based studies. Int J Cancer. 2020;146:1250–1260. doi: 10.1002/ijc.32426. [DOI] [PubMed] [Google Scholar]
- 27.Kim G, Jang SY, Nam CM, Kang ES. Statin use and the risk of hepatocellular carcinoma in patients at high risk: a nationwide nested case-control study. J Hepatol. 2018;68:476–484. doi: 10.1016/j.jhep.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Goh MJ, Sinn DH, Kim S, Woo SY, Cho H, Kang W, et al. Statin use and the risk of hepatocellular carcinoma in patients with chronic hepatitis B. Hepatology. 2020;71:2023–2032. doi: 10.1002/hep.30973. [DOI] [PubMed] [Google Scholar]
- 29.Islam MM, Poly TN, Walther BA, Yang HC, Li YCJ. Statin use and the risk of hepatocellular carcinoma: a meta-analysis of observational studies. Cancers (Basel) 2020;12:671. doi: 10.3390/cancers12030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong YJ, Qiu TY, Ng GK, Zheng Q, Teo EK. Efficacy and safety of statin for hepatocellular carcinoma prevention among chronic liver disease patients: a systematic review and meta-analysis. J Clin Gastroenterol. 2021;55:615–623. doi: 10.1097/MCG.0000000000001478. [DOI] [PubMed] [Google Scholar]
- 31.Choi J, Roberts LR. Statins and metformin for chemoprevention of hepatocellular carcinoma. Clin Liver Dis (Hoboken) 2016;8:48–52. doi: 10.1002/cld.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen HP, Shieh JJ, Chang CC, Chen TT, Lin JT, Wu MS, et al. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2013;62:606–615. doi: 10.1136/gutjnl-2011-301708. [DOI] [PubMed] [Google Scholar]
- 33.Sahasrabuddhe VV, Gunja MZ, Graubard BI, Trabert B, Schwartz LM, Park Y, et al. Nonsteroidal anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J Natl Cancer Inst. 2012;104:1808–1814. doi: 10.1093/jnci/djs452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simon TG, Ma Y, Ludvigsson JF, Chong DQ, Giovannucci EL, Fuchs CS, et al. Association between aspirin use and risk of hepatocellular carcinoma. JAMA Oncol. 2018;4:1683–1690. doi: 10.1001/jamaoncol.2018.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon TG, Duberg AS, Aleman S, Chung RT, Chan AT, Ludvigsson JF. Association of aspirin with hepatocellular carcinoma and liver-related mortality. N Engl J Med. 2020;382:1018–1028. doi: 10.1056/NEJMoa1912035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee M, Chung GE, Lee JH, Oh S, Nam JY, Chang Y, et al. Antiplatelet therapy and the risk of hepatocellular carcinoma in chronic hepatitis B patients on antiviral treatment. Hepatology. 2017;66:1556–1569. doi: 10.1002/hep.29318. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Wu S, Yu Y. Aspirin use and the incidence of hepatocellular carcinoma in patients with hepatitis B virus or hepatitis C virus infection: a meta-analysis of cohort studies. Front Med (Lausanne) 2021;7:569759. doi: 10.3389/fmed.2020.569759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan RZH, Lockart I, Abdel Shaheed C, Danta M. Systematic review with meta-analysis: the effects of non-steroidal anti-inflammatory drugs and anti-platelet therapy on the incidence and recurrence of hepatocellular carcinoma. Aliment Pharmacol Ther. 2021;54:356–367. doi: 10.1111/apt.16515. [DOI] [PubMed] [Google Scholar]
- 39.Wang S, Yu Y, Ryan PM, Dang M, Clark C, Kontogiannis V, et al. Association of aspirin therapy with risk of hepatocellular carcinoma: a systematic review and dose-response analysis of cohort studies with 2.5 million participants. Pharmacol Res. 2020;151:104585. doi: 10.1016/j.phrs.2019.104585. [DOI] [PubMed] [Google Scholar]
- 40.Jang H, Lee YB, Moon H, Chung JW, Nam JY, Cho EJ, et al. Aspirin use and risk of hepatocellular carcinoma in patients with chronic hepatitis B with or without cirrhosis. Hepatology. 2022;76:492–501. doi: 10.1002/hep.32380. [DOI] [PubMed] [Google Scholar]
- 41.Bravi F, Bosetti C, Tavani A, Gallus S, La Vecchia C. Coffee reduces risk for hepatocellular carcinoma: an updated meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1413–1421.e1. doi: 10.1016/j.cgh.2013.04.039. [DOI] [PubMed] [Google Scholar]
- 42.Inoue M, Yoshimi I, Sobue T, Tsugane S. Influence of coffee drinking on subsequent risk of hepatocellular carcinoma: a prospective study in Japan. J Natl Cancer Inst. 2005;97:293–300. doi: 10.1093/jnci/dji040. [DOI] [PubMed] [Google Scholar]
- 43.Gelatti U, Covolo L, Franceschini M, Pirali F, Tagger A, Ribero ML, et al. Coffee consumption reduces the risk of hepatocellular carcinoma independently of its aetiology: a case-control study. J Hepatol. 2005;42:528–534. doi: 10.1016/j.jhep.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 44.Setiawan VW, Wilkens LR, Lu SC, Hernandez BY, Le Marchand L, Henderson BE. Association of coffee intake with reduced incidence of liver cancer and death from chronic liver disease in the US multiethnic cohort. Gastroenterology. 2015;148:118–125. doi: 10.1053/j.gastro.2014.10.005. quiz e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Korean Association for the Study of the Liver (KASL) KASL clinical practice guidelines for management of chronic hepatitis B. Clin Mol Hepatol. 2022;28:276–331. doi: 10.3350/cmh.2022.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Korean Association for the Study of the Liver (KASL) 2017 KASL clinical practice guidelines management of hepatitis C: treatment of chronic hepatitis C. Clin Mol Hepatol. 2018;24:169–229. doi: 10.3350/cmh.2018.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 48.Wong GL, Chan HL, Mak CW, Lee SK, Ip ZM, Lam AT, et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology. 2013;58:1537–1547. doi: 10.1002/hep.26301. [DOI] [PubMed] [Google Scholar]
- 49.Kim WR, Loomba R, Berg T, Aguilar Schall RE, Yee LJ, Dinh PV, et al. Impact of long-term tenofovir disoproxil fumarate on incidence of hepatocellular carcinoma in patients with chronic hepatitis B. Cancer. 2015;121:3631–3638. doi: 10.1002/cncr.29537. [DOI] [PubMed] [Google Scholar]
- 50.Colombo M, Iavarone M. Role of antiviral treatment for HCC prevention. Best Pract Res Clin Gastroenterol. 2014;28:771–781. doi: 10.1016/j.bpg.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 51.Choi J, Kim HJ, Lee J, Cho S, Ko MJ, Lim YS. Risk of hepatocellular carcinoma in patients treated with entecavir vs tenofovir for chronic hepatitis B: a Korean nationwide cohort study. JAMA Oncol. 2019;5:30–36. doi: 10.1001/jamaoncol.2018.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim SU, Seo YS, Lee HA, Kim MN, Lee YR, Lee HW, et al. A multicenter study of entecavir vs. tenofovir on prognosis of treatment-naïve chronic hepatitis B in South Korea. J Hepatol. 2019;71:456–464. doi: 10.1016/j.jhep.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 53.Lee SW, Kwon JH, Lee HL, Yoo SH, Nam HC, Sung PS, et al. Comparison of tenofovir and entecavir on the risk of hepatocellular carcinoma and mortality in treatment-naïve patients with chronic hepatitis B in Korea: a large-scale, propensity score analysis. Gut. 2020;69:1301–1308. doi: 10.1136/gutjnl-2019-318947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choi HK, Seo GH. Entecavir versus tenofovir for the prevention of hepatocellular carcinoma in treatment-naïve chronic hepatitis B patients in Korea. J Korean Med Sci. 2021;36:e89. doi: 10.3346/jkms.2021.36.e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dave S, Park S, Murad MH, Barnard A, Prokop L, Adams LA, et al. Comparative effectiveness of entecavir versus tenofovir for preventing hepatocellular carcinoma in patients with chronic hepatitis B: a systematic review and meta-analysis. Hepatology. 2021;73:68–78. doi: 10.1002/hep.31267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tseng CH, Hsu YC, Chen TH, Ji F, Chen IS, Tsai YN, et al. Hepatocellular carcinoma incidence with tenofovir versus entecavir in chronic hepatitis B: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:1039–1052. doi: 10.1016/S2468-1253(20)30249-1. [DOI] [PubMed] [Google Scholar]
- 57.Lee HW, Cho YY, Lee H, Lee JS, Kim SU, Park JY, et al. Effect of tenofovir alafenamide vs. tenofovir disoproxil fumarate on hepatocellular carcinoma risk in chronic hepatitis B. J Viral Hepat. 2021;28:1570–1578. doi: 10.1111/jvh.13601. [DOI] [PubMed] [Google Scholar]
- 58.Lee HW, Cho YY, Lee H, Lee JS, Kim SU, Park JY, et al. Impact of tenofovir alafenamide vs. entecavir on hepatocellular carcinoma risk in patients with chronic hepatitis B. Hepatol Int. 2021;15:1083–1092. doi: 10.1007/s12072-021-10234-2. [DOI] [PubMed] [Google Scholar]
- 59.Li M, Lv T, Wu S, Wei W, Wu X, Ou X, et al. Tenofovir versus entecavir in lowering the risk of hepatocellular carcinoma development in patients with chronic hepatitis B: a critical systematic review and meta-analysis. Hepatol Int. 2020;14:105–114. doi: 10.1007/s12072-019-10005-0. [DOI] [PubMed] [Google Scholar]
- 60.Lee SW, Choi J, Kim SU, Lim YS. Entecavir versus tenofovir in patients with chronic hepatitis B: enemies or partners in the prevention of hepatocellular carcinoma. Clin Mol Hepatol. 2021;27:402–412. doi: 10.3350/cmh.2021.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Friberg S, Mattson S. On the growth rates of human malignant tumors: implications for medical decision making. J Surg Oncol. 1997;65:284–297. doi: 10.1002/(sici)1096-9098(199708)65:4<284::aid-jso11>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 62.Lim YS, Han S, Heo NY, Shim JH, Lee HC, Suh DJ. Mortality, liver transplantation, and hepatocellular carcinoma among patients with chronic hepatitis B treated with entecavir vs lamivudine. Gastroenterology. 2014;147:152–161. doi: 10.1053/j.gastro.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 63.Lok AS, McMahon BJ, Brown RS, Jr, Wong JB, Ahmed AT, Farah W, et al. Antiviral therapy for chronic hepatitis B viral infection in adults: a systematic review and meta-analysis. Hepatology. 2016;63:284–306. doi: 10.1002/hep.28280. [DOI] [PubMed] [Google Scholar]
- 64.Choi J, Han S, Kim N, Lim YS. Increasing burden of liver cancer despite extensive use of antiviral agents in a hepatitis B virus-endemic population. Hepatology. 2017;66:1454–1463. doi: 10.1002/hep.29321. [DOI] [PubMed] [Google Scholar]
- 65.Singal AG, Volk ML, Jensen D, Di Bisceglie AM, Schoenfeld PS. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin Gastroenterol Hepatol. 2010;8:280–288.e1. doi: 10.1016/j.cgh.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 66.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329–337. doi: 10.7326/0003-4819-158-5-201303050-00005. [DOI] [PubMed] [Google Scholar]
- 67.Carrat F, Fontaine H, Dorival C, Simony M, Diallo A, Hezode C, et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet. 2019;393:1453–1464. doi: 10.1016/S0140-6736(18)32111-1. [DOI] [PubMed] [Google Scholar]
- 68.Calvaruso V, Cabibbo G, Cacciola I, Petta S, Madonia S, Bellia A, et al. Incidence of hepatocellular carcinoma in patients with HCV-associated cirrhosis treated with direct-acting antiviral agents. Gastroenterology. 2018;155:411–421.e4. doi: 10.1053/j.gastro.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 69.Mendizabal M, Piñero F, Ridruejo E, Herz Wolff F, Anders M, Reggiardo V, et al. Disease progression in patients with hepatitis C virus infection treated with direct-acting antiviral agents. Clin Gastroenterol Hepatol. 2020;18:2554–2563.e3. doi: 10.1016/j.cgh.2020.02.044. [DOI] [PubMed] [Google Scholar]
- 70.Waziry R, Hajarizadeh B, Grebely J, Amin J, Law M, Danta M, et al. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: a systematic review, meta-analyses, and meta-regression. J Hepatol. 2017;67:1204–1212. doi: 10.1016/j.jhep.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 71.Delgado Martínez C, Gómez-Rubio M, Gómez-Domínguez C. Is hepatitis C direct-acting antiviral therapy a risk factor for the development and recurrence of hepatocellular carcinoma? Narrative literature review and clinical practice recommendations. Ann Hepatol. 2021;21:100225. doi: 10.1016/j.aohep.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 72.Yin J, Li N, Han Y, Xue J, Deng Y, Shi J, et al. Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma: a two-stage longitudinal clinical study. J Clin Oncol. 2013;31:3647–3655. doi: 10.1200/JCO.2012.48.5896. [DOI] [PubMed] [Google Scholar]
- 73.Wong JS, Wong GL, Tsoi KK, Wong VW, Cheung SY, Chong CN, et al. Meta-analysis: the efficacy of anti-viral therapy in prevention of recurrence after curative treatment of chronic hepatitis B-related hepatocellular carcinoma. Aliment Pharmacol Ther. 2011;33:1104–1112. doi: 10.1111/j.1365-2036.2011.04634.x. [DOI] [PubMed] [Google Scholar]
- 74.Miao RY, Zhao HT, Yang HY, Mao YL, Lu X, Zhao Y, et al. Postoperative adjuvant antiviral therapy for hepatitis B/C virus-related hepatocellular carcinoma: a meta-analysis. World J Gastroenterol. 2010;16:2931–2942. doi: 10.3748/wjg.v16.i23.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen XX, Cheng JW, Huang A, Zhang X, Wang J, Fan J, et al. The effect of antiviral therapy on patients with hepatitis B virus-related hepatocellular carcinoma after curative resection: a systematic review and meta-analysis. Onco Targets Ther. 2017;10:5363–5375. doi: 10.2147/OTT.S150281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choi J, Jo C, Lim YS. Tenofovir versus entecavir on recurrence of hepatitis B virus-related hepatocellular carcinoma after surgical resection. Hepatology. 2021;73:661–673. doi: 10.1002/hep.31289. [DOI] [PubMed] [Google Scholar]
- 77.Huang G, Yang Y, Shen F, Pan ZY, Fu SY, Lau WY, et al. Early viral suppression predicts good postoperative survivals in patients with hepatocellular carcinoma with a high baseline HBV-DNA load. Ann Surg Oncol. 2013;20:1482–1490. doi: 10.1245/s10434-012-2803-7. [DOI] [PubMed] [Google Scholar]
- 78.Lee JH, Kim BK, Park SY, Tak WY, Park JY, Kim DY, et al. The efficacies of entecavir and tenofovir in terms of enhancing prognosis after curative treatment of hepatitis B virus-related hepatocellular carcinoma. Eur J Intern Med. 2021;89:48–55. doi: 10.1016/j.ejim.2021.02.019. [DOI] [PubMed] [Google Scholar]
- 79.Singal AK, Freeman DH, Jr, Anand BS. Meta-analysis: interferon improves outcomes following ablation or resection of hepatocellular carcinoma. Aliment Pharmacol Ther. 2010;32:851–858. doi: 10.1111/j.1365-2036.2010.04414.x. [DOI] [PubMed] [Google Scholar]
- 80.Reig M, Mariño Z, Perelló C, Iñarrairaegui M, Ribeiro A, Lens S, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016;65:719–726. doi: 10.1016/j.jhep.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 81.Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016;65:727–733. doi: 10.1016/j.jhep.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 82.Reig M, Boix L, Mariño Z, Torres F, Forns X, Bruix J. Liver cancer emergence associated with antiviral treatment: an immune surveillance failure? Semin Liver Dis. 2017;37:109–118. doi: 10.1055/s-0037-1601349. [DOI] [PubMed] [Google Scholar]
- 83.Saraiya N, Yopp AC, Rich NE, Odewole M, Parikh ND, Singal AG. Systematic review with meta-analysis: recurrence of hepatocellular carcinoma following direct-acting antiviral therapy. Aliment Pharmacol Ther. 2018;48:127–137. doi: 10.1111/apt.14823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.ANRS collaborative study group on hepatocellular carcinoma (ANRS CO22 HEPATHER, CO12 CirVir and CO23 CUPILT cohorts) Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: data from three ANRS cohorts. J Hepatol. 2016;65:734–740. doi: 10.1016/j.jhep.2016.05.045. [DOI] [PubMed] [Google Scholar]
- 85.Cabibbo G, Celsa C, Calvaruso V, Petta S, Cacciola I, Cannavò MR, et al. Direct-acting antivirals after successful treatment of early hepatocellular carcinoma improve survival in HCV-cirrhotic patients. J Hepatol. 2019;71:265–273. doi: 10.1016/j.jhep.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 86.Sahakyan Y, Lee-Kim V, Bremner KE, Bielecki JM, Krahn MD. Impact of direct-acting antiviral regimens on mortality and morbidity outcomes in patients with chronic hepatitis C: systematic review and meta-analysis. J Viral Hepat. 2021;28:739–754. doi: 10.1111/jvh.13482. [DOI] [PubMed] [Google Scholar]
- 87.Pang Q, Jin H, Qu K, Man Z, Wang Y, Yang S, et al. The effects of nonsteroidal anti-inflammatory drugs in the incident and recurrent risk of hepatocellular carcinoma: a meta-analysis. Onco Targets Ther. 2017;10:4645–4656. doi: 10.2147/OTT.S143154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li X, Liu L, Hu Y. Statin use and the prognosis of patients with hepatocellular carcinoma: a meta-analysis. Biosci Rep. 2020;40:BSR20200232. doi: 10.1042/BSR20200232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nishio T, Taura K, Nakamura N, Seo S, Yasuchika K, Kaido T, et al. Impact of statin use on the prognosis of patients with hepatocellular carcinoma undergoing liver resection: a subgroup analysis of patients without chronic hepatitis viral infection. Surgery. 2018;163:264–269. doi: 10.1016/j.surg.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 90.Cho Y, Kim MS, Nam CM, Kang ES. Statin use is associated with decreased hepatocellular carcinoma recurrence in liver transplant patients. Sci Rep. 2019;9:1467. doi: 10.1038/s41598-018-38110-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee HL, Lee SW, Jang JW, Bae SH, Choi JY, Yoon SK, et al. Anticancer effect of statins in patients undergoing liver transplantation for hepatocellular carcinoma. Liver Transpl. 2022;28:397–406. doi: 10.1002/lt.26258. [DOI] [PubMed] [Google Scholar]
- 92.Chen JG, Parkin DM, Chen QG, Lu JH, Shen QJ, Zhang BC, et al. Screening for liver cancer: results of a randomised controlled trial in Qidong, China. J Med Screen. 2003;10:204–209. doi: 10.1258/096914103771773320. [DOI] [PubMed] [Google Scholar]
- 93.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417–422. doi: 10.1007/s00432-004-0552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30:37–47. doi: 10.1111/j.1365-2036.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhao C, Nguyen MH. Hepatocellular carcinoma screening and surveillance: practice guidelines and real-life practice. J Clin Gastroenterol. 2016;50:120–133. doi: 10.1097/MCG.0000000000000446. [DOI] [PubMed] [Google Scholar]
- 96.Sangiovanni A, Del Ninno E, Fasani P, De Fazio C, Ronchi G, Romeo R, et al. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology. 2004;126:1005–1014. doi: 10.1053/j.gastro.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 97.Santi V, Trevisani F, Gramenzi A, Grignaschi A, Mirici-Cappa F, Del Poggio P, et al. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol. 2010;53:291–297. doi: 10.1016/j.jhep.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 98.Kim HD, Lim YS, Han S, An J, Kim GA, Kim SY, et al. Evaluation of early-stage hepatocellular carcinoma by magnetic resonance imaging with gadoxetic acid detects additional lesions and increases overall survival. Gastroenterology. 2015;148:1371–1382. doi: 10.1053/j.gastro.2015.02.051. [DOI] [PubMed] [Google Scholar]
- 99.Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Gastroenterology. 2018;154:1706–1718.e1. doi: 10.1053/j.gastro.2018.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Parikh ND, Singal AG, Hutton DW, Tapper EB. Cost-effectiveness of hepatocellular carcinoma surveillance: an assessment of benefits and harms. Am J Gastroenterol. 2020;115:1642–1649. doi: 10.14309/ajg.0000000000000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shim CW, Park JW, Kim SH, Kim JS, Kim BH, Kim SH, et al. Noncirrhotic hepatocellular carcinoma: etiology and occult hepatitis B virus infection in a hepatitis B virus-endemic area. Therap Adv Gastroenterol. 2017;10:529–536. doi: 10.1177/1756283X17710247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hartke J, Johnson M, Ghabril M. The diagnosis and treatment of hepatocellular carcinoma. Semin Diagn Pathol. 2017;34:153–159. doi: 10.1053/j.semdp.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 103.Dulku G, Dhillon R, Goodwin M, Cheng W, Kontorinis N, Mendelson R. The role of imaging in the surveillance and diagnosis of hepatocellular cancer. J Med Imaging Radiat Oncol. 2017;61:171–179. doi: 10.1111/1754-9485.12568. [DOI] [PubMed] [Google Scholar]
- 104.Sarasin FP, Giostra E, Hadengue A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class a cirrhosis. Am J Med. 1996;101:422–434. doi: 10.1016/S0002-9343(96)00197-0. [DOI] [PubMed] [Google Scholar]
- 105.Bruix J, Sherman M American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zanetto A, Shalaby S, Vitale A, Mescoli C, Ferrarese A, Gambato M, et al. Dropout rate from the liver transplant waiting list because of hepatocellular carcinoma progression in hepatitis C virus-infected patients treated with direct-acting antivirals. Liver Transpl. 2017;23:1103–1112. doi: 10.1002/lt.24790. [DOI] [PubMed] [Google Scholar]
- 107.Ogata F, Kobayashi M, Akuta N, Osawa M, Fujiyama S, Kawamura Y, et al. Outcome of all-oral direct-acting antiviral regimens on the rate of development of hepatocellular carcinoma in patients with hepatitis C virus genotype 1-related chronic liver disease. Oncology. 2017;93:92–98. doi: 10.1159/000470910. [DOI] [PubMed] [Google Scholar]
- 108.Calleja JL, Crespo J, Rincón D, Ruiz-Antorán B, Fernandez I, Perelló C, et al. Effectiveness, safety and clinical outcomes of direct-acting antiviral therapy in HCV genotype 1 infection: results from a spanish real-world cohort. J Hepatol. 2017;66:1138–1148. doi: 10.1016/j.jhep.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 109.Loomba R, Lim JK, Patton H, El-Serag HB. AGA clinical practice update on screening and surveillance for hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: expert review. Gastroenterology. 2020;158:1822–1830. doi: 10.1053/j.gastro.2019.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chou R, Cuevas C, Fu R, Devine B, Wasson N, Ginsburg A, et al. Imaging techniques for the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Ann Intern Med. 2015;162:697–711. doi: 10.7326/M14-2509. [DOI] [PubMed] [Google Scholar]
- 111.Singal AG, Nehra M, Adams-Huet B, Yopp AC, Tiro JA, Marrero JA, et al. Detection of hepatocellular carcinoma at advanced stages among patients in the HALT-C trial: where did surveillance fail? Am J Gastroenterol. 2013;108:425–432. doi: 10.1038/ajg.2012.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Colli A, Fraquelli M, Casazza G, Massironi S, Colucci A, Conte D, et al. Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol. 2006;101:513–523. doi: 10.1111/j.1572-0241.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- 113.Aghoram R, Cai P, Dickinson JA. Alpha-foetoprotein and/or liver ultrasonography for screening of hepatocellular carcinoma in patients with chronic hepatitis B. Cochrane Database Syst Rev. 2012;2012:CD002799. doi: 10.1002/14651858.CD002799.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 115.Kokudo N, Makuuchi M. Evidence-based clinical practice guidelines for hepatocellular carcinoma in Japan: the J-HCC guidelines. J Gastroenterol. 2009;44 Suppl 19:119–121. doi: 10.1007/s00535-008-2244-z. [DOI] [PubMed] [Google Scholar]
- 116.Korean Liver Cancer Study Group (KLCSG); National Cancer Center, Korea (NCC) 2014 KLCSG-NCC Korea practice guideline for the management of hepatocellular carcinoma. Gut Liver. 2015;9:267–317. doi: 10.5009/gnl14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Korean Liver Cancer Study Group and National Cancer Center, Korea. Practice guidelines for management of hepatocellular carcinoma 2009. Korean J Hepatol. 2009;15:391–423. doi: 10.3350/kjhep.2009.15.3.391. [DOI] [PubMed] [Google Scholar]
- 118.Santagostino E, Colombo M, Rivi M, Rumi MG, Rocino A, Linari S, et al. A 6-month versus a 12-month surveillance for hepatocellular carcinoma in 559 hemophiliacs infected with the hepatitis C virus. Blood. 2003;102:78–82. doi: 10.1182/blood-2002-10-3310. [DOI] [PubMed] [Google Scholar]
- 119.Trinchet JC, Chaffaut C, Bourcier V, Degos F, Henrion J, Fontaine H, et al. Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3- and 6-month periodicities. Hepatology. 2011;54:1987–1997. doi: 10.1002/hep.24545. [DOI] [PubMed] [Google Scholar]
- 120.Wang JH, Chang KC, Kee KM, Chen PF, Yen YH, Tseng PL, et al. Hepatocellular carcinoma surveillance at 4- vs. 12-month intervals for patients with chronic viral hepatitis: a randomized study in community. Am J Gastroenterol. 2013;108:416–424. doi: 10.1038/ajg.2012.445. [DOI] [PubMed] [Google Scholar]
- 121.Andersson KL, Salomon JA, Goldie SJ, Chung RT. Cost effectiveness of alternative surveillance strategies for hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2008;6:1418–1424. doi: 10.1016/j.cgh.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Barbara L, Benzi G, Gaiani S, Fusconi F, Zironi G, Siringo S, et al. Natural history of small untreated hepatocellular carcinoma in cirrhosis: a multivariate analysis of prognostic factors of tumor growth rate and patient survival. Hepatology. 1992;16:132–137. doi: 10.1002/hep.1840160122. [DOI] [PubMed] [Google Scholar]
- 123.Sheu JC, Sung JL, Chen DS, Yang PM, Lai MY, Lee CS, et al. Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications. Gastroenterology. 1985;89:259–266. doi: 10.1016/0016-5085(85)90324-5. [DOI] [PubMed] [Google Scholar]
- 124.Tanaka H, Iijima H, Nouso K, Aoki N, Iwai T, Takashima T, et al. Cost-effectiveness analysis on the surveillance for hepatocellular carcinoma in liver cirrhosis patients using contrast-enhanced ultrasonography. Hepatol Res. 2012;42:376–384. doi: 10.1111/j.1872-034X.2011.00936.x. [DOI] [PubMed] [Google Scholar]
- 125.Pocha C, Dieperink E, McMaken KA, Knott A, Thuras P, Ho SB. Surveillance for hepatocellular cancer with ultrasonography vs. computed tomography -- a randomised study. Aliment Pharmacol Ther. 2013;38:303–312. doi: 10.1111/apt.12370. [DOI] [PubMed] [Google Scholar]
- 126.Gupta P, Soundararajan R, Patel A, Kumar-M P, Sharma V, Kalra N. Abbreviated MRI for hepatocellular carcinoma screening: a systematic review and meta-analysis. J Hepatol. 2021;75:108–119. doi: 10.1016/j.jhep.2021.01.041. [DOI] [PubMed] [Google Scholar]
- 127.European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 128.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 129.Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kokudo N, Takemura N, Hasegawa K, Takayama T, Kubo S, Shimada M, et al. Clinical practice guidelines for hepatocellular carcinoma: the Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res. 2019;49:1109–1113. doi: 10.1111/hepr.13411. [DOI] [PubMed] [Google Scholar]
- 131.Moctezuma-Velázquez C, Lewis S, Lee K, Amodeo S, Llovet JM, Schwartz M, et al. Non-invasive imaging criteria for the diagnosis of hepatocellular carcinoma in non-cirrhotic patients with chronic hepatitis B. JHEP Rep. 2021;3:100364. doi: 10.1016/j.jhepr.2021.100364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lewis S, Roayaie S, Ward SC, Shyknevsky I, Jibara G, Taouli B. Hepatocellular carcinoma in chronic hepatitis C in the absence of advanced fibrosis or cirrhosis. AJR Am J Roentgenol. 2013;200:W610–W616. doi: 10.2214/AJR.12.9151. [DOI] [PubMed] [Google Scholar]
- 133.Kim SE, Lee HC, Shim JH, Park HJ, Kim KM, Kim PN, et al. Noninvasive diagnostic criteria for hepatocellular carcinoma in hepatic masses >2 cm in a hepatitis B virus-endemic area. Liver Int. 2011;31:1468–1476. doi: 10.1111/j.1478-3231.2011.02529.x. [DOI] [PubMed] [Google Scholar]
- 134.Van Wettere M, Purcell Y, Bruno O, Payancé A, Plessier A, Rautou PE, et al. Low specificity of washout to diagnose hepatocellular carcinoma in nodules showing arterial hyperenhancement in patients with budd-chiari syndrome. J Hepatol. 2019;70:1123–1132. doi: 10.1016/j.jhep.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 135.Wells ML, Hough DM, Fidler JL, Kamath PS, Poterucha JT, Venkatesh SK. Benign nodules in post-Fontan livers can show imaging features considered diagnostic for hepatocellular carcinoma. Abdom Radiol (NY) 2017;42:2623–2631. doi: 10.1007/s00261-017-1181-9. [DOI] [PubMed] [Google Scholar]
- 136.Roberts LR, Sirlin CB, Zaiem F, Almasri J, Prokop LJ, Heimbach JK, et al. Imaging for the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Hepatology. 2018;67:401–421. doi: 10.1002/hep.29487. [DOI] [PubMed] [Google Scholar]
- 137.Lee SM, Lee JM, Ahn SJ, Kang HJ, Yang HK, Yoon JH. Diagnostic performance of 2018 KLCA-NCC practice guideline for hepatocellular carcinoma on gadoxetic acid-enhanced MRI in patients with chronic hepatitis B or cirrhosis: comparison with LI-RADS version 2018. Korean J Radiol. 2021;22:1066–1076. doi: 10.3348/kjr.2020.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee S, Kim SS, Chang DR, Kim H, Kim MJ. Comparison of LI-RADS 2018 and KLCA-NCC 2018 for noninvasive diagnosis of hepatocellular carcinoma using magnetic resonance imaging. Clin Mol Hepatol. 2020;26:340–351. doi: 10.3350/cmh.2020.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sangiovanni A, Manini MA, Iavarone M, Romeo R, Forzenigo LV, Fraquelli M, et al. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut. 2010;59:638–644. doi: 10.1136/gut.2009.187286. [DOI] [PubMed] [Google Scholar]
- 140.Khalili K, Kim TK, Jang HJ, Haider MA, Khan L, Guindi M, et al. Optimization of imaging diagnosis of 1-2 cm hepatocellular carcinoma: an analysis of diagnostic performance and resource utilization. J Hepatol. 2011;54:723–728. doi: 10.1016/j.jhep.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 141.Terzi E, Iavarone M, Pompili M, Veronese L, Cabibbo G, Fraquelli M, et al. Contrast ultrasound LI-RADS LR-5 identifies hepatocellular carcinoma in cirrhosis in a multicenter restropective study of 1,006 nodules. J Hepatol. 2018;68:485–492. doi: 10.1016/j.jhep.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 142.Hanna RF, Miloushev VZ, Tang A, Finklestone LA, Brejt SZ, Sandhu RS, et al. Comparative 13-year meta-analysis of the sensitivity and positive predictive value of ultrasound, CT, and MRI for detecting hepatocellular carcinoma. Abdom Radiol (NY) 2016;41:71–90. doi: 10.1007/s00261-015-0592-8. [DOI] [PubMed] [Google Scholar]
- 143.Yoon SH, Lee JM, So YH, Hong SH, Kim SJ, Han JK, et al. Multiphasic MDCT enhancement pattern of hepatocellular carcinoma smaller than 3 cm in diameter: tumor size and cellular differentiation. AJR Am J Roentgenol. 2009;193:W482–W489. doi: 10.2214/AJR.08.1818. [DOI] [PubMed] [Google Scholar]
- 144.Bolondi L, Gaiani S, Celli N, Golfieri R, Grigioni WF, Leoni S, et al. Characterization of small nodules in cirrhosis by assessment of vascularity: the problem of hypovascular hepatocellular carcinoma. Hepatology. 2005;42:27–34. doi: 10.1002/hep.20728. [DOI] [PubMed] [Google Scholar]
- 145.Kierans AS, Kang SK, Rosenkrantz AB. The diagnostic performance of dynamic contrast-enhanced MR imaging for detection of small hepatocellular carcinoma measuring up to 2 cm: a meta-analysis. Radiology. 2016;278:82–94. doi: 10.1148/radiol.2015150177. [DOI] [PubMed] [Google Scholar]
- 146.Choi SH, Byun JH, Lim YS, Yu E, Lee SJ, Kim SY, et al. Diagnostic criteria for hepatocellular carcinoma ≤3 cm with hepatocyte-specific contrast-enhanced magnetic resonance imaging. J Hepatol. 2016;64:1099–1107. doi: 10.1016/j.jhep.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 147.Joo I, Lee JM, Lee DH, Jeon JH, Han JK, Choi BI. Noninvasive diagnosis of hepatocellular carcinoma on gadoxetic acid-enhanced MRI: can hypointensity on the hepatobiliary phase be used as an alternative to washout? Eur Radiol. 2015;25:2859–2868. doi: 10.1007/s00330-015-3686-3. [DOI] [PubMed] [Google Scholar]
- 148.Ahn SJ, Choi JY, Kim KA, Kim MJ, Baek SE, Kim JH, et al. Focal eosinophilic infiltration of the liver: gadoxetic acid-enhanced magnetic resonance imaging and diffusion-weighted imaging. J Comput Assist Tomogr. 2011;35:81–85. doi: 10.1097/RCT.0b013e3181f39f30. [DOI] [PubMed] [Google Scholar]
- 149.Kim DH, Choi SH, Byun JH, Kang JH, Lim YS, Lee SJ, et al. Arterial subtraction images of gadoxetate-enhanced MRI improve diagnosis of early-stage hepatocellular carcinoma. J Hepatol. 2019;71:534–542. doi: 10.1016/j.jhep.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 150.Choi SH, Kim SY, Lee SS, Shim JH, Byun JH, Baek S, et al. Subtraction images of gadoxetic acid-enhanced MRI: effect on the diagnostic performance for focal hepatic lesions in patients at risk for hepatocellular carcinoma. AJR Am J Roentgenol. 2017;209:584–591. doi: 10.2214/AJR.16.17211. [DOI] [PubMed] [Google Scholar]
- 151.Kim SS, Lee S, Bae H, Chung YE, Choi JY, Park MS, et al. Extended application of subtraction arterial phase imaging in LI-RADS version 2018: a strategy to improve the diagnostic performance for hepatocellular carcinoma on gadoxetate disodium-enhanced MRI. Eur Radiol. 2021;31:1620–1629. doi: 10.1007/s00330-020-07229-2. [DOI] [PubMed] [Google Scholar]
- 152.Basha MAA, AlAzzazy MZ, Ahmed AF, Yousef HY, Shehata SM, El Sammak DAEA, et al. Does a combined CT and MRI protocol enhance the diagnostic efficacy of LI-RADS in the categorization of hepatic observations? A prospective comparative study. Eur Radiol. 2018;28:2592–2603. doi: 10.1007/s00330-017-5232-y. [DOI] [PubMed] [Google Scholar]
- 153.Min JH, Kim JM, Kim YK, Cha DI, Kang TW, Kim H, et al. Magnetic resonance imaging with extracellular contrast detects hepatocellular carcinoma with greater accuracy than with gadoxetic acid or computed tomography. Clin Gastroenterol Hepatol. 2020;18:2091–2100.e7. doi: 10.1016/j.cgh.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 154.Reynolds AR, Furlan A, Fetzer DT, Sasatomi E, Borhani AA, Heller MT, et al. Infiltrative hepatocellular carcinoma: what radiologists need to know. Radiographics. 2015;35:371–386. doi: 10.1148/rg.352140114. [DOI] [PubMed] [Google Scholar]
- 155.Joo I, Lee JM, Lee SM, Lee JS, Park JY, Han JK. Diagnostic accuracy of liver imaging reporting and data system (LI-RADS) v2014 for intrahepatic mass-forming cholangiocarcinomas in patients with chronic liver disease on gadoxetic acid-enhanced MRI. J Magn Reson Imaging. 2016;44:1330–1338. doi: 10.1002/jmri.25287. [DOI] [PubMed] [Google Scholar]
- 156.Zhou C, Wang Y, Ma L, Qian X, Yang C, Zeng M. Combined hepatocellular carcinoma-cholangiocarcinoma: MRI features correlated with tumor biomarkers and prognosis. Eur Radiol. 2022;32:78–88. doi: 10.1007/s00330-021-08188-y. [DOI] [PubMed] [Google Scholar]
- 157.Wilson SR, Lyshchik A, Piscaglia F, Cosgrove D, Jang HJ, Sirlin C, et al. CEUS LI-RADS: algorithm, implementation, and key differences from CT/MRI. Abdom Radiol (NY) 2018;43:127–142. doi: 10.1007/s00261-017-1250-0. [DOI] [PubMed] [Google Scholar]
- 158.Yang Y, Liu C, Yan J, Liu K. Perfluorobutane contrast-enhanced ultrasonography for the diagnosis of HCC: a systematic review and meta-analysis. Abdom Radiol (NY) 2021;46:4619–4628. doi: 10.1007/s00261-021-03141-5. [DOI] [PubMed] [Google Scholar]
- 159.Kang HJ, Lee JM, Yoon JH, Lee K, Kim H, Han JK. Contrast-enhanced US with sulfur hexafluoride and perfluorobutane for the diagnosis of hepatocellular carcinoma in individuals with high risk. Radiology. 2020;297:108–116. doi: 10.1148/radiol.2020200115. [DOI] [PubMed] [Google Scholar]
- 160.Hsiao CY, Chen PD, Huang KW. A prospective assessment of the diagnostic value of contrast-enhanced ultrasound, dynamic computed tomography and magnetic resonance imaging for patients with small liver tumors. J Clin Med. 2019;8:1353. doi: 10.3390/jcm8091353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cerny M, Chernyak V, Olivié D, Billiard JS, Murphy-Lavallée J, Kielar AZ, et al. LI-RADS version 2018 ancillary features at MRI. Radiographics. 2018;38:1973–2001. doi: 10.1148/rg.2018180052. [DOI] [PubMed] [Google Scholar]
- 162.van der Pol CB, Lim CS, Sirlin CB, McGrath TA, Salameh JP, Bashir MR, et al. Accuracy of the liver imaging reporting and data system in computed tomography and magnetic resonance image analysis of hepatocellular carcinoma or overall malignancy-a systematic review. Gastroenterology. 2019;156:976–986. doi: 10.1053/j.gastro.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 163.Tanabe M, Kanki A, Wolfson T, Costa EA, Mamidipalli A, Ferreira MP, et al. Imaging outcomes of liver imaging reporting and data system version 2014 category 2, 3, and 4 observations detected at CT and MR imaging. Radiology. 2016;281:129–139. doi: 10.1148/radiol.2016152173. [DOI] [PubMed] [Google Scholar]
- 164.Kim YY, Choi JY, Kim SU, Lee M, Park MS, Chung YE, et al. MRI ancillary features for LI-RADS category 3 and 4 observations: improved categorization to indicate the risk of hepatic malignancy. AJR Am J Roentgenol. 2020;215:1354–1362. doi: 10.2214/AJR.20.22802. [DOI] [PubMed] [Google Scholar]
- 165.Vernuccio F, Cannella R, Choudhury KR, Meyer M, Furlan A, Marin D. Hepatobiliary phase hypointensity predicts progression to hepatocellular carcinoma for intermediate-high risk observations, but not time to progression. Eur J Radiol. 2020;128:109018. doi: 10.1016/j.ejrad.2020.109018. [DOI] [PubMed] [Google Scholar]
- 166.Cho HJ, Kim B, Lee JD, Kang DR, Kim JK, Lee JH, et al. Development of risk prediction model for hepatocellular carcinoma progression of indeterminate nodules in hepatitis B virus-related cirrhotic liver. Am J Gastroenterol. 2017;112:460–470. doi: 10.1038/ajg.2016.480. [DOI] [PubMed] [Google Scholar]
- 167.Singal AG, Hoshida Y, Pinato DJ, Marrero J, Nault JC, Paradis V, et al. International liver cancer association (ILCA) white paper on biomarker development for hepatocellular carcinoma. Gastroenterology. 2021;160:2572–2584. doi: 10.1053/j.gastro.2021.01.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y, et al. Surveillance and diagnostic algorithm for hepatocellular carcinoma proposed by the liver cancer study group of Japan: 2014 update. Oncology. 2014;87 Suppl 1:7–21. doi: 10.1159/000368141. [DOI] [PubMed] [Google Scholar]
- 169.Park MJ, Kim YK, Lee MW, Lee WJ, Kim YS, Kim SH, et al. Small hepatocellular carcinomas: improved sensitivity by combining gadoxetic acid-enhanced and diffusion-weighted MR imaging patterns. Radiology. 2012;264:761–770. doi: 10.1148/radiol.12112517. [DOI] [PubMed] [Google Scholar]
- 170.Park MJ, Kim YK, Lee MH, Lee JH. Validation of diagnostic criteria using gadoxetic acid-enhanced and diffusion-weighted MR imaging for small hepatocellular carcinoma (≤ 2.0 cm) in patients with hepatitis-induced liver cirrhosis. Acta Radiol. 2013;54:127–136. doi: 10.1258/ar.2012.120262. [DOI] [PubMed] [Google Scholar]
- 171.Yu MH, Kim JH, Yoon JH, Kim HC, Chung JW, Han JK, et al. Small (≤1-cm) hepatocellular carcinoma: diagnostic performance and imaging features at gadoxetic acid-enhanced MR imaging. Radiology. 2014;271:748–760. doi: 10.1148/radiol.14131996. [DOI] [PubMed] [Google Scholar]
- 172.Jang KM, Kim SH, Kim YK, Choi D. Imaging features of subcentimeter hypointense nodules on gadoxetic acid-enhanced hepatobiliary phase MR imaging that progress to hypervascular hepatocellular carcinoma in patients with chronic liver disease. Acta Radiol. 2015;56:526–535. doi: 10.1177/0284185114534652. [DOI] [PubMed] [Google Scholar]
- 173.Lee YJ, Lee JM, Lee JS, Lee HY, Park BH, Kim YH, et al. Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology. 2015;275:97–109. doi: 10.1148/radiol.14140690. [DOI] [PubMed] [Google Scholar]
- 174.Park CJ, An C, Park S, Choi JY, Kim MJ. Management of subcentimetre arterially enhancing and hepatobiliary hypointense lesions on gadoxetic acid-enhanced MRI in patients at risk for HCC. Eur Radiol. 2018;28:1476–1484. doi: 10.1007/s00330-017-5088-1. [DOI] [PubMed] [Google Scholar]
- 175.Roskams T, Kojiro M. Pathology of early hepatocellular carcinoma: conventional and molecular diagnosis. Semin Liver Dis. 2010;30:17–25. doi: 10.1055/s-0030-1247129. [DOI] [PubMed] [Google Scholar]
- 176.Forner A, Vilana R, Ayuso C, Bianchi L, Solé M, Ayuso JR, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47:97–104. doi: 10.1002/hep.21966. [DOI] [PubMed] [Google Scholar]
- 177.Stigliano R, Marelli L, Yu D, Davies N, Patch D, Burroughs AK. Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. What is the risk and the outcome? Seeding risk for percutaneous approach of HCC. Cancer Treat Rev. 2007;33:437–447. doi: 10.1016/j.ctrv.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 178.Silva MA, Hegab B, Hyde C, Guo B, Buckels JA, Mirza DF. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut. 2008;57:1592–1596. doi: 10.1136/gut.2008.149062. [DOI] [PubMed] [Google Scholar]
- 179.Tremosini S, Forner A, Boix L, Vilana R, Bianchi L, Reig M, et al. Prospective validation of an immunohistochemical panel (glypican 3, heat shock protein 70 and glutamine synthetase) in liver biopsies for diagnosis of very early hepatocellular carcinoma. Gut. 2012;61:1481–1487. doi: 10.1136/gutjnl-2011-301862. [DOI] [PubMed] [Google Scholar]
- 180.WHO Classification of Tumours Editorial Board. WHO classification of tumors: digestive system tumours. 5th ed. Lyon: International Agency for Research on Cancer; 2019. [Google Scholar]
- 181.Rhee H, Kim H, Park YN. Clinico-radio-pathological and molecular features of hepatocellular carcinomas with keratin 19 expression. Liver Cancer. 2020;9:663–681. doi: 10.1159/000510522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kim H, Jang M, Park YN. Histopathological variants of hepatocellular carcinomas: an update according to the 5th edition of the WHO classification of digestive system tumors. J Liver Cancer. 2020;20:17–24. doi: 10.17998/jlc.20.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Calderaro J, Ziol M, Paradis V, Zucman-Rossi J. Molecular and histological correlations in liver cancer. J Hepatol. 2019;71:616–630. doi: 10.1016/j.jhep.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 184.Sherman M, Bruix J. Biopsy for liver cancer: how to balance research needs with evidence-based clinical practice. Hepatology. 2015;61:433–436. doi: 10.1002/hep.27563. [DOI] [PubMed] [Google Scholar]
- 185.Chen VL, Sharma P. Role of biomarkers and biopsy in hepatocellular carcinoma. Clin Liver Dis. 2020;24:577–590. doi: 10.1016/j.cld.2020.07.001. [DOI] [PubMed] [Google Scholar]
- 186.Tateishi R, Yoshida H, Matsuyama Y, Mine N, Kondo Y, Omata M. Diagnostic accuracy of tumor markers for hepatocellular carcinoma: a systematic review. Hepatol Int. 2008;2:17–30. doi: 10.1007/s12072-007-9038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wong RJ, Ahmed A, Gish RG. Elevated alpha-fetoprotein: differential diagnosis - hepatocellular carcinoma and other disorders. Clin Liver Dis. 2015;19:309–323. doi: 10.1016/j.cld.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 188.Lok AS, Sterling RK, Everhart JE, Wright EC, Hoefs JC, Di Bisceglie AM, et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010;138:493–502. doi: 10.1053/j.gastro.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hwang SH, Hong SB, Han K, Seo N, Choi JY, Lee JH, et al. A new reporting system for diagnosis of hepatocellular carcinoma in chronic hepatitis B with clinical and gadoxetic acid-enhanced MRI features. J Magn Reson Imaging. 2022;55:1877–1886. doi: 10.1002/jmri.27962. [DOI] [PubMed] [Google Scholar]
- 190.Joo I, Kim SY, Kang TW, Kim YK, Park BJ, Lee YJ, et al. Radiologic-pathologic correlation of hepatobiliary phase hypointense nodules without arterial phase hyperenhancement at gadoxetic acid-enhanced MRI: a multicenter study. Radiology. 2020;296:335–345. doi: 10.1148/radiol.2020192275. [DOI] [PubMed] [Google Scholar]
- 191.Mehta N, Heimbach J, Harnois DM, Sapisochin G, Dodge JL, Lee D, et al. Validation of a risk estimation of tumor recurrence after transplant (RETREAT) score for hepatocellular carcinoma recurrence after liver transplant. JAMA Oncol. 2017;3:493–500. doi: 10.1001/jamaoncol.2016.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261:947–955. doi: 10.1097/SLA.0000000000000710. [DOI] [PubMed] [Google Scholar]
- 193.Gilbert ES. Invited commentary: studies of workers exposed to low doses of radiation. Am J Epidemiol. 2001;153:319–322. doi: 10.1093/aje/153.4.319. discussion 323-324. [DOI] [PubMed] [Google Scholar]
- 194.Upton AC National Coluncil on Radiation Protection and Measurements Scientific Committee 1-6. The state of the art in the 1990’s: NCRP report No. 136 on the scientific bases for linearity in the dose-response relationship for ionizing radiation. Health Phys. 2003;85:15–22. doi: 10.1097/00004032-200307000-00005. [DOI] [PubMed] [Google Scholar]
- 195.Huda W, Ogden KM, Khorasani MR. Converting dose-length product to effective dose at CT. Radiology. 2008;248:995–1003. doi: 10.1148/radiol.2483071964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.National Research Council. Health risks from exposure to low levels of ionizing radiation: BEIR VII phase 2. Washington, D.C.: National Academy of Sciences; 2006. [Google Scholar]
- 197.ICRP publication 103. The 2007 recommendations of the International Commission on Radiological Protection. Ann ICRP. 2007;37:1–332. doi: 10.1016/j.icrp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 198.Brenner DJ, Shuryak I, Einstein AJ. Impact of reduced patient life expectancy on potential cancer risks from radiologic imaging. Radiology. 2011;261:193–198. doi: 10.1148/radiol.11102452. [DOI] [PubMed] [Google Scholar]
- 199.Takahashi H, Okada M, Hyodo T, Hidaka S, Kagawa Y, Matsuki M, et al. Can low-dose CT with iterative reconstruction reduce both the radiation dose and the amount of iodine contrast medium in a dynamic CT study of the liver? Eur J Radiol. 2014;83:684–691. doi: 10.1016/j.ejrad.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 200.Pregler B, Beyer LP, Teufel A, Niessen C, Stroszczynski C, Brodoefel H, et al. Low tube voltage liver MDCT with sinogram-affirmed iterative reconstructions for the detection of hepatocellular carcinoma. Sci Rep. 2017;7:9460. doi: 10.1038/s41598-017-10095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Nakamura Y, Narita K, Higaki T, Akagi M, Honda Y, Awai K. Diagnostic value of deep learning reconstruction for radiation dose reduction at abdominal ultra-high-resolution CT. Eur Radiol. 2021;31:4700–4709. doi: 10.1007/s00330-020-07566-2. [DOI] [PubMed] [Google Scholar]
- 202.Yoon JH, Chang W, Lee ES, Lee SM, Lee JM. Double low-dose dual-energy liver CT in patients at high-risk of HCC: a prospective, randomized, single-center study. Invest Radiol. 2020;55:340–348. doi: 10.1097/RLI.0000000000000643. [DOI] [PubMed] [Google Scholar]
- 203.Park S, Yoon JH, Joo I, Yu MH, Kim JH, Park J, et al. Image quality in liver CT: low-dose deep learning vs standard-dose model-based iterative reconstructions. Eur Radiol. 2022;32:2865–2874. doi: 10.1007/s00330-021-08380-0. [DOI] [PubMed] [Google Scholar]
- 204.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 205.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 206.Lee HW, Sinn DH, Kang W, Gwak GY, Paik YH, Choi MS, et al. Cause of mortality for hepatocellular carcinoma patients who were diagnosed within the milan criteria. J Liver Cancer. 2016;16:101–107. [Google Scholar]
- 207.Meier V, Ramadori G. Clinical staging of hepatocellular carcinoma. Dig Dis. 2009;27:131–141. doi: 10.1159/000218345. [DOI] [PubMed] [Google Scholar]
- 208.Park S, Choi S, Cho YA, Sinn DH, Kim JM, Park CK, et al. Evaluation of the American Joint Committee on Cancer (AJCC) 8th edition staging system for hepatocellular carcinoma in 1,008 patients with curative resection. Cancer Res Treat. 2020;52:1145–1152. doi: 10.4143/crt.2020.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Shindoh J, Kobayashi Y, Kawamura Y, Akuta N, Kobayashi M, Suzuki Y, et al. Microvascular invasion and a size cutoff value of 2 cm predict long-term oncological outcome in multiple hepatocellular carcinoma: reappraisal of the American joint committee on cancer staging system and validation using the surveillance, epidemiology, and end-results database. Liver Cancer. 2020;9:156–166. doi: 10.1159/000504193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ueno S, Tanabe G, Nuruki K, Hamanoue M, Komorizono Y, Oketani M, et al. Prognostic performance of the new classification of primary liver cancer of Japan (4th edition) for patients with hepatocellular carcinoma: a validation analysis. Hepatol Res. 2002;24:395–403. doi: 10.1016/s1386-6346(02)00144-4. [DOI] [PubMed] [Google Scholar]
- 211.Nihon Kangan K. General rules for the clinical and pathological study of primary liver cancer. Tokyo: Kanehara& Co.; 2010. [Google Scholar]
- 212.Kim IG, Hu XG, Wang HJ, Kim BW, Hong SY, Shen XY. The 7th/8th American Joint Committee on Cancer and the modified union for international cancer control staging system for hepatocellular carcinoma. Yonsei Med J. 2019;60:140–147. doi: 10.3349/ymj.2019.60.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bruix J, Sherman M Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 215.Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong liver cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146:1691–1700.e3. doi: 10.1053/j.gastro.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 216.Sohn JH, Duran R, Zhao Y, Fleckenstein F, Chapiro J, Sahu S, et al. Validation of the Hong Kong liver cancer staging system in determining prognosis of the north American patients following intra-arterial therapy. Clin Gastroenterol Hepatol. 2017;15:746–755.e4. doi: 10.1016/j.cgh.2016.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Uchino K, Tateishi R, Shiina S, Kanda M, Masuzaki R, Kondo Y, et al. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer. 2011;117:4475–4483. doi: 10.1002/cncr.25960. [DOI] [PubMed] [Google Scholar]
- 218.Benson AB, D’Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:541–565. doi: 10.6004/jnccn.2021.0022. [DOI] [PubMed] [Google Scholar]
- 219.Park JW, Kim JH, Kim SK, Kang KW, Park KW, Choi JI, et al. A prospective evaluation of 18F-FDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma. J Nucl Med. 2008;49:1912–1921. doi: 10.2967/jnumed.108.055087. [DOI] [PubMed] [Google Scholar]
- 220.Lee JE, Jang JY, Jeong SW, Lee SH, Kim SG, Cha SW, et al. Diagnostic value for extrahepatic metastases of hepatocellular carcinoma in positron emission tomography/computed tomography scan. World J Gastroenterol. 2012;18:2979–2987. doi: 10.3748/wjg.v18.i23.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sugiyama M, Sakahara H, Torizuka T, Kanno T, Nakamura F, Futatsubashi M, et al. 18F-FDG PET in the detection of extrahepatic metastases from hepatocellular carcinoma. J Gastroenterol. 2004;39:961–968. doi: 10.1007/s00535-004-1427-5. [DOI] [PubMed] [Google Scholar]
- 222.Cho Y, Lee DH, Lee YB, Lee M, Yoo JJ, Choi WM, et al. Does 18F-FDG positron emission tomography-computed tomography have a role in initial staging of hepatocellular carcinoma? PLoS One. 2014;9:e105679. doi: 10.1371/journal.pone.0105679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.John BV, Aubuchon S, Dahman B, Konjeti VR, Heuman D, Hubert J, et al. Addition of [18F]fluorodeoxyglucose positron emission tomography with computed tomography to cross-sectional imaging improves staging and alters management in hepatocellular carcinoma. Liver Transpl. 2020;26:774–784. doi: 10.1002/lt.25743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Chalaye J, Costentin CE, Luciani A, Amaddeo G, Ganne-Carrié N, Baranes L, et al. Positron emission tomography/computed tomography with 18F-fluorocholine improve tumor staging and treatment allocation in patients with hepatocellular carcinoma. J Hepatol. 2018;69:336–344. doi: 10.1016/j.jhep.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 225.Rilling WS, Drooz A. Multidisciplinary management of hepatocellular carcinoma. J Vasc Interv Radiol. 2002;13:S259–S263. doi: 10.1016/s1051-0443(07)61794-1. [DOI] [PubMed] [Google Scholar]
- 226.Colombo M, Raoul JL, Lencioni R, Galle PR, Zucman-Rossi J, Bañares R, et al. Multidisciplinary strategies to improve treatment outcomes in hepatocellular carcinoma: a European perspective. Eur J Gastroenterol Hepatol. 2013;25:639–651. doi: 10.1097/MEG.0b013e32835e33bb. [DOI] [PubMed] [Google Scholar]
- 227.Sinn DH, Choi GS, Park HC, Kim JM, Kim H, Song KD, et al. Multidisciplinary approach is associated with improved survival of hepatocellular carcinoma patients. PLoS One. 2019;14:e0210730. doi: 10.1371/journal.pone.0210730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Yopp AC, Mansour JC, Beg MS, Arenas J, Trimmer C, Reddick M, et al. Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Ann Surg Oncol. 2014;21:1287–1295. doi: 10.1245/s10434-013-3413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Serper M, Taddei TH, Mehta R, D’Addeo K, Dai F, Aytaman A, et al. Association of provider specialty and multidisciplinary care with hepatocellular carcinoma treatment and mortality. Gastroenterology. 2017;152:1954–1964. doi: 10.1053/j.gastro.2017.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Charriere B, Muscari F, Maulat C, Bournet B, Bonnet D, Bureau C, et al. Outcomes of patients with hepatocellular carcinoma are determined in multidisciplinary team meetings. J Surg Oncol. 2017;115:330–336. doi: 10.1002/jso.24500. [DOI] [PubMed] [Google Scholar]
- 231.Chang TT, Sawhney R, Monto A, Davoren JB, Kirkland JG, Stewart L, et al. Implementation of a multidisciplinary treatment team for hepatocellular cancer at a veterans affairs medical center improves survival. HPB (Oxford) 2008;10:405–411. doi: 10.1080/13651820802356572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Cohen GS, Black M. Multidisciplinary management of hepatocellular carcinoma: a model for therapy. J Multidiscip Healthc. 2013;6:189–195. doi: 10.2147/JMDH.S41206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Burak KW, Kneteman NM. An evidence-based multidisciplinary approach to the management of hepatocellular carcinoma (HCC): the Alberta HCC algorithm. Can J Gastroenterol. 2010;24:643–650. doi: 10.1155/2010/410574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Chan AC, Poon RT, Ng KK, Lo CM, Fan ST, Wong J. Changing paradigm in the management of hepatocellular carcinoma improves the survival benefit of early detection by screening. Ann Surg. 2008;247:666–673. doi: 10.1097/SLA.0b013e31816a747a. [DOI] [PubMed] [Google Scholar]
- 235.Taylor C, Munro AJ, Glynne-Jones R, Griffith C, Trevatt P, Richards M, et al. Multidisciplinary team working in cancer: what is the evidence? BMJ. 2010;340:c951. doi: 10.1136/bmj.c951. [DOI] [PubMed] [Google Scholar]
- 236.Fennell ML, Das IP, Clauser S, Petrelli N, Salner A. The organization of multidisciplinary care teams: modeling internal and external influences on cancer care quality. J Natl Cancer Inst Monogr. 2010;2010:72–80. doi: 10.1093/jncimonographs/lgq010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Gish RG, Lencioni R, Di Bisceglie AM, Raoul JL, Mazzaferro V. Role of the multidisciplinary team in the diagnosis and treatment of hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2012;6:173–185. doi: 10.1586/egh.11.105. [DOI] [PubMed] [Google Scholar]
- 238.Litton G, Kane D, Clay G, Kruger P, Belnap T, Parkinson B. Multidisciplinary cancer care with a patient and physician satisfaction focus. J Oncol Pract. 2010;6:e35–e37. doi: 10.1200/JOP.2010.000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lang H, Sotiropoulos GC, Dömland M, Frühauf NR, Paul A, Hüsing J, et al. Liver resection for hepatocellular carcinoma in non-cirrhotic liver without underlying viral hepatitis. Br J Surg. 2005;92:198–202. doi: 10.1002/bjs.4763. [DOI] [PubMed] [Google Scholar]
- 240.Capussotti L, Muratore A, Massucco P, Ferrero A, Polastri R, Bouzari H. Major liver resections for hepatocellular carcinoma on cirrhosis: early and long-term outcomes. Liver Transpl. 2004;10(2 Suppl 1):S64–S68. doi: 10.1002/lt.20035. [DOI] [PubMed] [Google Scholar]
- 241.Poon RT, Fan ST, Lo CM, Ng IO, Liu CL, Lam CM, et al. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63–70. doi: 10.1097/00000658-200107000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Andreou A, Vauthey JN, Cherqui D, Zimmitti G, Ribero D, Truty MJ, et al. Improved long-term survival after major resection for hepatocellular carcinoma: a multicenter analysis based on a new definition of major hepatectomy. J Gastrointest Surg. 2013;17:66–77. doi: 10.1007/s11605-012-2005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Huang J, Zhang Y, Peng Z, Gao H, Xu L, Jiao LR, et al. A modified TNM-7 staging system to better predict the survival in patients with hepatocellular carcinoma after hepatectomy. J Cancer Res Clin Oncol. 2013;139:1709–1719. doi: 10.1007/s00432-013-1497-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lee EC, Kim SH, Park H, Lee SD, Lee SA, Park SJ. Survival analysis after liver resection for hepatocellular carcinoma: a consecutive cohort of 1002 patients. J Gastroenterol Hepatol. 2017;32:1055–1063. doi: 10.1111/jgh.13632. [DOI] [PubMed] [Google Scholar]
- 245.Torzilli G, Belghiti J, Kokudo N, Takayama T, Capussotti L, Nuzzo G, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: An observational study of the HCC east-west study group. Ann Surg. 2013;257:929–937. doi: 10.1097/SLA.0b013e31828329b8. [DOI] [PubMed] [Google Scholar]
- 246.Kim JH, Choi DW, Kim SB. Saftey and long-term outcome following major hepatectomy for epatocellular carcinoma combined with compensated liver cirrhosis. J Korean Surg Soc. 2006;70:445–450. [Google Scholar]
- 247.Minagawa M, Makuuchi M, Takayama T, Kokudo N. Selection criteria for repeat hepatectomy in patients with recurrent hepatocellular carcinoma. Ann Surg. 2003;238:703–710. doi: 10.1097/01.sla.0000094549.11754.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Finkelstein SD, Marsh W, Demetris AJ, Swalsky PA, Sasatomi E, Bonham A, et al. Microdissection-based allelotyping discriminates de novo tumor from intrahepatic spread in hepatocellular carcinoma. Hepatology. 2003;37:871–879. doi: 10.1053/jhep.2003.50134. [DOI] [PubMed] [Google Scholar]
- 249.Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–207. doi: 10.1016/s0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 250.Kaibori M, Ishizaki M, Matsui K, Kwon AH. Predictors of microvascular invasion before hepatectomy for hepatocellular carcinoma. J Surg Oncol. 2010;102:462–468. doi: 10.1002/jso.21631. [DOI] [PubMed] [Google Scholar]
- 251.Li SH, Guo ZX, Xiao CZ, Wei W, Shi M, Chen ZY, et al. Risk factors for early and late intrahepatic recurrence in patients with single hepatocellular carcinoma without macrovascular invasion after curative resection. Asian Pac J Cancer Prev. 2013;14:4759–4763. doi: 10.7314/apjcp.2013.14.8.4759. [DOI] [PubMed] [Google Scholar]
- 252.Nathan H, Schulick RD, Choti MA, Pawlik TM. Predictors of survival after resection of early hepatocellular carcinoma. Ann Surg. 2009;249:799–805. doi: 10.1097/SLA.0b013e3181a38eb5. [DOI] [PubMed] [Google Scholar]
- 253.Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243:229–235. doi: 10.1097/01.sla.0000197706.21803.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Wu JC, Huang YH, Chau GY, Su CW, Lai CR, Lee PC, et al. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol. 2009;51:890–897. doi: 10.1016/j.jhep.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 255.Zhou L, Rui JA, Wang SB, Chen SG, Qu Q. Prognostic factors of solitary large hepatocellular carcinoma: the importance of differentiation grade. Eur J Surg Oncol. 2011;37:521–525. doi: 10.1016/j.ejso.2011.03.137. [DOI] [PubMed] [Google Scholar]
- 256.Kim YI, Kim HS, Park JW. Higher ratio of serum alpha-fetoprotein could predict outcomes in patients with hepatitis B virus-associated hepatocellular carcinoma and normal alanine aminotransferase. PLoS One. 2016;11:e0157299. doi: 10.1371/journal.pone.0157299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kim DY, Paik YH, Ahn SH, Youn YJ, Choi JW, Kim JK, et al. PIVKA-II is a useful tumor marker for recurrent hepatocellular carcinoma after surgical resection. Oncology. 2007;72 Suppl 1:52–57. doi: 10.1159/000111707. [DOI] [PubMed] [Google Scholar]
- 258.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 259.Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. doi: 10.1016/s1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 260.Farges O, Malassagne B, Flejou JF, Balzan S, Sauvanet A, Belghiti J. Risk of major liver resection in patients with underlying chronic liver disease: a reappraisal. Ann Surg. 1999;229:210–215. doi: 10.1097/00000658-199902000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Makuuchi M, Sano K. The surgical approach to HCC: our progress and results in Japan. Liver Transpl. 2004;10(2 Suppl 1):S46–S52. doi: 10.1002/lt.20044. [DOI] [PubMed] [Google Scholar]
- 262.Fan ST, Lai EC, Lo CM, Ng IO, Wong J. Hospital mortality of major hepatectomy for hepatocellular carcinoma associated with cirrhosis. Arch Surg. 1995;130:198–203. doi: 10.1001/archsurg.1995.01430020088017. [DOI] [PubMed] [Google Scholar]
- 263.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 264.An M, Park J, Shin JA, Choi JI, Kim TH, Kim S, et al. The adverse effect of indirectly diagnosed portal hypertension on the complications and prognosis after hepatic resection of hepatocellular carcinoma. Korean J Hepatol. 2006;12:553. [PubMed] [Google Scholar]
- 265.Choi GH, Park JY, Hwang HK, Kim DH, Kang CM, Choi JS, et al. Predictive factors for long-term survival in patients with clinically significant portal hypertension following resection of hepatocellular carcinoma. Liver Int. 2011;31:485–493. doi: 10.1111/j.1478-3231.2010.02436.x. [DOI] [PubMed] [Google Scholar]
- 266.Capussotti L, Ferrero A, Viganò L, Muratore A, Polastri R, Bouzari H. Portal hypertension: contraindication to liver surgery? World J Surg. 2006;30:992–999. doi: 10.1007/s00268-005-0524-9. [DOI] [PubMed] [Google Scholar]
- 267.Cucchetti A, Ercolani G, Vivarelli M, Cescon M, Ravaioli M, Ramacciato G, et al. Is portal hypertension a contraindication to hepatic resection? Ann Surg. 2009;250:922–928. doi: 10.1097/SLA.0b013e3181b977a5. [DOI] [PubMed] [Google Scholar]
- 268.He W, Zeng Q, Zheng Y, Chen M, Shen J, Qiu J, et al. The role of clinically significant portal hypertension in hepatic resection for hepatocellular carcinoma patients: a propensity score matching analysis. BMC Cancer. 2015;15:263. doi: 10.1186/s12885-015-1280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908–1916. doi: 10.1053/j.gastro.2008.02.091. [DOI] [PubMed] [Google Scholar]
- 270.Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Iacono C. How much remnant is enough in liver resection? Dig Surg. 2012;29:6–17. doi: 10.1159/000335713. [DOI] [PubMed] [Google Scholar]
- 271.Cescon M, Colecchia A, Cucchetti A, Peri E, Montrone L, Ercolani G, et al. Value of transient elastography measured with FibroScan in predicting the outcome of hepatic resection for hepatocellular carcinoma. Ann Surg. 2012;256:706–712. doi: 10.1097/SLA.0b013e3182724ce8. discussion 712-713. [DOI] [PubMed] [Google Scholar]
- 272.Hu H, Han H, Han XK, Wang WP, Ding H. Nomogram for individualised prediction of liver failure risk after hepatectomy in patients with resectable hepatocellular carcinoma: the evidence from ultrasound data. Eur Radiol. 2018;28:877–885. doi: 10.1007/s00330-017-4900-2. [DOI] [PubMed] [Google Scholar]
- 273.Kim SU, Ahn SH, Park JY, Kim DY, Chon CY, Choi JS, et al. Prediction of postoperative hepatic insufficiency by liver stiffness measurement (FibroScan®) before curative resection of hepatocellular carcinoma: a pilot study. Hepatol Int. 2008;2:471–477. doi: 10.1007/s12072-008-9091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Wong JS, Wong GL, Chan AW, Wong VW, Cheung YS, Chong CN, et al. Liver stiffness measurement by transient elastography as a predictor on posthepatectomy outcomes. Ann Surg. 2013;257:922–928. doi: 10.1097/SLA.0b013e318269d2ec. [DOI] [PubMed] [Google Scholar]
- 275.Li C, Zhang JY, Zhang XY, Wen TF, Yan LN. FibroScan predicts ascites after liver resection for hepatitis B virus-related hepatocellular carcinoma: a prospective cohort study. Int J Surg. 2015;20:21–25. doi: 10.1016/j.ijsu.2015.05.047. [DOI] [PubMed] [Google Scholar]
- 276.Chong CC, Wong GL, Chan AW, Wong VW, Fong AK, Cheung YS, et al. Liver stiffness measurement predicts high-grade post-hepatectomy liver failure: a prospective cohort study. J Gastroenterol Hepatol. 2017;32:506–514. doi: 10.1111/jgh.13503. [DOI] [PubMed] [Google Scholar]
- 277.Rajakannu M, Cherqui D, Ciacio O, Golse N, Pittau G, Allard MA, et al. Liver stiffness measurement by transient elastography predicts late posthepatectomy outcomes in patients undergoing resection for hepatocellular carcinoma. Surgery. 2017;162:766–774. doi: 10.1016/j.surg.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 278.Huang Z, Huang J, Zhou T, Cao H, Tan B. Prognostic value of liver stiffness measurement for the liver-related surgical outcomes of patients under hepatic resection: a meta-analysis. PLoS One. 2018;13:e0190512. doi: 10.1371/journal.pone.0190512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Hammerstingl R, Huppertz A, Breuer J, Balzer T, Blakeborough A, Carter R, et al. Diagnostic efficacy of gadoxetic acid (primovist)-enhanced MRI and spiral CT for a therapeutic strategy: comparison with intraoperative and histopathologic findings in focal liver lesions. Eur Radiol. 2008;18:457–467. doi: 10.1007/s00330-007-0716-9. [DOI] [PubMed] [Google Scholar]
- 280.Kim SH, Kim SH, Lee J, Kim MJ, Jeon YH, Park Y, et al. Gadoxetic acid-enhanced MRI versus triple-phase MDCT for the preoperative detection of hepatocellular carcinoma. AJR Am J Roentgenol. 2009;192:1675–1681. doi: 10.2214/AJR.08.1262. [DOI] [PubMed] [Google Scholar]
- 281.Ippolito D, Famularo S, Giani A, Orsini EB, Pecorelli A, Pinotti E, et al. Estimating liver function in a large cirrhotic cohort: signal intensity of gadolinium-ethoxybenzyl-diethylenetriamine penta-acetic acid-enhanced MRI. Dig Liver Dis. 2019;51:1438–1445. doi: 10.1016/j.dld.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 282.Lin CY, Chang WC, Chou CT, Chen RC. Dynamic-contrast-enhanced magnetic resonance imaging of cirrhotic liver parenchyma: a comparison between gadolinium-diethylene-triamine pentaacetic acid and gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid. J Chin Med Assoc. 2015;78:666–672. doi: 10.1016/j.jcma.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 283.Kubota K, Tamura T, Aoyama N, Nogami M, Hamada N, Nishioka A, et al. Correlation of liver parenchymal gadolinium-ethoxybenzyl diethylenetriaminepentaacetic acid enhancement and liver function in humans with hepatocellular carcinoma. Oncol Lett. 2012;3:990–994. doi: 10.3892/ol.2012.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Haimerl M, Verloh N, Zeman F, Fellner C, Nickel D, Lang SA, et al. Gd-EOB-DTPA-enhanced MRI for evaluation of liver function: comparison between signal-intensity-based indices and T1 relaxometry. Sci Rep. 2017;7:43347. doi: 10.1038/srep43347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Lin CY, Chen JH, Liang JA, Lin CC, Jeng LB, Kao CH. 18F-FDG PET or PET/CT for detecting extrahepatic metastases or recurrent hepatocellular carcinoma: a systematic review and meta-analysis. Eur J Radiol. 2012;81:2417–2422. doi: 10.1016/j.ejrad.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 286.Koneru B, Teperman LW, Manzarbeitia C, Facciuto M, Cho K, Reich D, et al. A multicenter evaluation of utility of chest computed tomography and bone scans in liver transplant candidates with stages I and II hepatoma. Ann Surg. 2005;241:622–628. doi: 10.1097/01.sla.0000157267.27356.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Liu L, Wang Z, Jiang S, Shao B, Liu J, Zhang S, et al. Perioperative allogenenic blood transfusion is associated with worse clinical outcomes for hepatocellular carcinoma: a meta-analysis. PLoS One. 2013;8:e64261. doi: 10.1371/journal.pone.0064261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Peng T, Zhao G, Wang L, Wu J, Cui H, Liang Y, et al. No impact of perioperative blood transfusion on prognosis after curative resection for hepatocellular carcinoma: a propensity score matching analysis. Clin Transl Oncol. 2018;20:719–728. doi: 10.1007/s12094-017-1773-4. [DOI] [PubMed] [Google Scholar]
- 289.Wada H, Eguchi H, Nagano H, Kubo S, Nakai T, Kaibori M, et al. Perioperative allogenic blood transfusion is a poor prognostic factor after hepatocellular carcinoma surgery: a multi-center analysis. Surg Today. 2018;48:73–79. doi: 10.1007/s00595-017-1553-3. [DOI] [PubMed] [Google Scholar]
- 290.Tsujita E, Taketomi A, Kitagawa D, Itoh S, Harimoto N, Gion T, et al. Selective hepatic vascular exclusion for the hepatic resection of HCC. Hepatogastroenterology. 2007;54:527–530. [PubMed] [Google Scholar]
- 291.Jongerius IM, Mungroop TH, Uz Z, Geerts BF, Immink RV, Rutten MVH, et al. Goal-directed fluid therapy vs. low central venous pressure during major open liver resections (GALILEO): a surgeon- and patient-blinded randomized controlled trial. HPB (Oxford) 2021;23:1578–1585. doi: 10.1016/j.hpb.2021.03.013. [DOI] [PubMed] [Google Scholar]
- 292.Lin N, Li J, Ke Q, Xin F, Zeng Y, Wang L, et al. Does the intermittent pringle maneuver affect the recurrence following surgical resection for hepatocellular carcinoma? A systematic review. PLoS One. 2020;15:e0229870. doi: 10.1371/journal.pone.0229870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Khajeh E, Shafiei S, Al-Saegh SA, Ramouz A, Hammad A, Ghamarnejad O, et al. Meta-analysis of the effect of the pringle maneuver on long-term oncological outcomes following liver resection. Sci Rep. 2021;11:3279. doi: 10.1038/s41598-021-82291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Cucchetti A, Qiao GL, Cescon M, Li J, Xia Y, Ercolani G, et al. Anatomic versus nonanatomic resection in cirrhotic patients with early hepatocellular carcinoma. Surgery. 2014;155:512–521. doi: 10.1016/j.surg.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 295.Ishii M, Mizuguchi T, Kawamoto M, Meguro M, Ota S, Nishidate T, et al. Propensity score analysis demonstrated the prognostic advantage of anatomical liver resection in hepatocellular carcinoma. World J Gastroenterol. 2014;20:3335–3342. doi: 10.3748/wjg.v20.i12.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Kaibori M, Kon M, Kitawaki T, Kawaura T, Hasegawa K, Kokudo N, et al. Comparison of anatomic and non-anatomic hepatic resection for hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2017;24:616–626. doi: 10.1002/jhbp.502. [DOI] [PubMed] [Google Scholar]
- 297.Kudo A, Tanaka S, Ban D, Matsumura S, Irie T, Nakamura N, et al. Anatomic resection reduces the recurrence of solitary hepatocellular carcinoma ≤5 cm without macrovascular invasion. Am J Surg. 2014;207:863–869. doi: 10.1016/j.amjsurg.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 298.Sakoda M, Ueno S, Iino S, Hiwatashi K, Minami K, Kawasaki Y, et al. Survival benefits of small anatomical resection of the liver for patients with hepatocellular carcinoma and impaired liver function, based on new-era imaging studies. J Cancer. 2016;7:1029–1036. doi: 10.7150/jca.15174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Zhao H, Chen C, Gu S, Yan X, Jia W, Mao L, et al. Anatomical versus non-anatomical resection for solitary hepatocellular carcinoma without macroscopic vascular invasion: a propensity score matching analysis. J Gastroenterol Hepatol. 2017;32:870–878. doi: 10.1111/jgh.13603. [DOI] [PubMed] [Google Scholar]
- 300.Huang X, Lu S. A meta-analysis comparing the effect of anatomical resection vs. non-anatomical resection on the long-term outcomes for patients undergoing hepatic resection for hepatocellular carcinoma. HPB (Oxford) 2017;19:843–849. doi: 10.1016/j.hpb.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 301.Feng X, Su Y, Zheng S, Xia F, Ma K, Yan J, et al. A double blinded prospective randomized trial comparing the effect of anatomic versus non-anatomic resection on hepatocellular carcinoma recurrence. HPB (Oxford) 2017;19:667–674. doi: 10.1016/j.hpb.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 302.Moris D, Tsilimigras DI, Kostakis ID, Ntanasis-Stathopoulos I, Shah KN, Felekouras E, et al. Anatomic versus non-anatomic resection for hepatocellular carcinoma: a systematic review and meta-analysis. Eur J Surg Oncol. 2018;44:927–938. doi: 10.1016/j.ejso.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 303.Sun Z, Li Z, Shi XL, He XW, Chen J, Song JH. Anatomic versus non-anatomic resection of hepatocellular carcinoma with microvascular invasion: a systematic review and meta-analysis. Asian J Surg. 2021;44:1143–1150. doi: 10.1016/j.asjsur.2021.02.023. [DOI] [PubMed] [Google Scholar]
- 304.Famularo S, Ceresoli M, Giani A, Ciulli C, Pinotti E, Romano F, et al. Is it just a matter of surgical extension to achieve the cure of hepatocarcinoma? A meta-analysis of propensity-matched and randomized studies for anatomic versus parenchyma-sparing liver resection. J Gastrointest Surg. 2021;25:94–103. doi: 10.1007/s11605-019-04494-5. [DOI] [PubMed] [Google Scholar]
- 305.Shi M, Guo RP, Lin XJ, Zhang YQ, Chen MS, Zhang CQ, et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg. 2007;245:36–43. doi: 10.1097/01.sla.0000231758.07868.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Zhong FP, Zhang YJ, Liu Y, Zou SB. Prognostic impact of surgical margin in patients with hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore) 2017;96:e8043. doi: 10.1097/MD.0000000000008043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Lazzara C, Navarra G, Lazzara S, Barbera A, Saitta C, Raimondo G, et al. Does the margin width influence recurrence rate in liver surgery for hepatocellular carcinoma smaller than 5 cm? Eur Rev Med Pharmacol Sci. 2017;21:523–529. [PubMed] [Google Scholar]
- 308.Eguchi S, Kanematsu T, Arii S, Okazaki M, Okita K, Omata M, et al. Comparison of the outcomes between an anatomical subsegmentectomy and a non-anatomical minor hepatectomy for single hepatocellular carcinomas based on a Japanese nationwide survey. Surgery. 2008;143:469–475. doi: 10.1016/j.surg.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 309.Suh KS. Systematic hepatectomy for small hepatocellular carcinoma in Korea. J Hepatobiliary Pancreat Surg. 2005;12:365–370. doi: 10.1007/s00534-005-1002-3. [DOI] [PubMed] [Google Scholar]
- 310.Wakai T, Shirai Y, Sakata J, Kaneko K, Cruz PV, Akazawa K, et al. Anatomic resection independently improves long-term survival in patients with T1-T2 hepatocellular carcinoma. Ann Surg Oncol. 2007;14:1356–1365. doi: 10.1245/s10434-006-9318-z. [DOI] [PubMed] [Google Scholar]
- 311.Kim IS, Lim YS, Yoon HK, Sung KB, Jang MK, Choi WB, et al. The effect of preoperative transarterial chemoembolization on the patient’s outcome in resectable hepatocellular carcinoma. Korean J Med. 2005;69:614–621. [Google Scholar]
- 312.Wu CC, Ho YZ, Ho WL, Wu TC, Liu TJ, P’eng FK. Preoperative transcatheter arterial chemoembolization for resectable large hepatocellular carcinoma: a reappraisal. Br J Surg. 1995;82:122–126. doi: 10.1002/bjs.1800820141. [DOI] [PubMed] [Google Scholar]
- 313.Jianyong L, Jinjing Z, Lunan Y, Jingqiang Z, Wentao W, Yong Z, et al. Preoperative adjuvant transarterial chemoembolization cannot improve the long term outcome of radical therapies for hepatocellular carcinoma. Sci Rep. 2017;7:41624. doi: 10.1038/srep41624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Hayashi H, Beppu T, Okabe H, Kuroki H, Nakagawa S, Imai K, et al. Functional assessment versus conventional volumetric assessment in the prediction of operative outcomes after major hepatectomy. Surgery. 2015;157:20–26. doi: 10.1016/j.surg.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 315.Nishio T, Taura K, Koyama Y, Tanabe K, Yamamoto G, Okuda Y, et al. Prediction of posthepatectomy liver failure based on liver stiffness measurement in patients with hepatocellular carcinoma. Surgery. 2016;159:399–408. doi: 10.1016/j.surg.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 316.Beppu T, Okabe H, Okuda K, Eguchi S, Kitahara K, Taniai N, et al. Portal vein embolization followed by right-side hemihepatectomy for hepatocellular carcinoma patients: a Japanese multi-institutional study. J Am Coll Surg. 2016;222:1138–1148.e2. doi: 10.1016/j.jamcollsurg.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 317.Pandanaboyana S, Bell R, Hidalgo E, Toogood G, Prasad KR, Bartlett A, et al. A systematic review and meta-analysis of portal vein ligation versus portal vein embolization for elective liver resection. Surgery. 2015;157:690–698. doi: 10.1016/j.surg.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 318.Schadde E, Raptis DA, Schnitzbauer AA, Ardiles V, Tschuor C, Lesurtel M, et al. Prediction of mortality after ALPPS stage-1: an analysis of 320 patients from the international ALPPS registry. Ann Surg. 2015;262:780–785. doi: 10.1097/SLA.0000000000001450. discussion 785-786. [DOI] [PubMed] [Google Scholar]
- 319.Chan A, Zhang WY, Chok K, Dai J, Ji R, Kwan C, et al. ALPPS versus portal vein embolization for hepatitis-related hepatocellular carcinoma: a changing paradigm in modulation of future liver remnant before major hepatectomy. Ann Surg. 2021;273:957–965. doi: 10.1097/SLA.0000000000003433. [DOI] [PubMed] [Google Scholar]
- 320.Belghiti J, Guevara OA, Noun R, Saldinger PF, Kianmanesh R. Liver hanging maneuver: a safe approach to right hepatectomy without liver mobilization. J Am Coll Surg. 2001;193:109–111. doi: 10.1016/s1072-7515(01)00909-7. [DOI] [PubMed] [Google Scholar]
- 321.Tang JX, Li JJ, Weng RH, Liang ZM, Jiang N. Anterior vs conventional approach right hepatic resection for large hepatocellular carcinoma: a systematic review and meta-analysis. World J Gastroenterol. 2017;23:7917–7929. doi: 10.3748/wjg.v23.i44.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Han HS, Shehta A, Ahn S, Yoon YS, Cho JY, Choi Y. Laparoscopic versus open liver resection for hepatocellular carcinoma: case-matched study with propensity score matching. J Hepatol. 2015;63:643–650. doi: 10.1016/j.jhep.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 323.Kabir T, Tan ZZ, Syn NL, Wu E, Lin JD, Zhao JJ, et al. Laparoscopic versus open resection of hepatocellular carcinoma in patients with cirrhosis: meta-analysis. Br J Surg. 2021;109:21–29. doi: 10.1093/bjs/znab376. [DOI] [PubMed] [Google Scholar]
- 324.Cheung TT, Poon RT, Yuen WK, Chok KS, Jenkins CR, Chan SC, et al. Long-term survival analysis of pure laparoscopic versus open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a single-center experience. Ann Surg. 2013;257:506–511. doi: 10.1097/SLA.0b013e31827b947a. [DOI] [PubMed] [Google Scholar]
- 325.Wang X, Teh CSC, Ishizawa T, Aoki T, Cavallucci D, Lee SY, et al. Consensus guidelines for the use of fluorescence imaging in hepatobiliary surgery. Ann Surg. 2021;274:97–106. doi: 10.1097/SLA.0000000000004718. [DOI] [PubMed] [Google Scholar]
- 326.Benedetti Cacciaguerra A, Görgec B, Lanari J, Cipriani F, Russolillo N, Mocchegiani F, et al. Outcome of major hepatectomy in cirrhotic patients; does surgical approach matter? A propensity score matched analysis. J Hepatobiliary Pancreat Sci. 2021 Dec 02; doi: 10.1002/jhbp.1087. [Epub] [DOI] [PubMed] [Google Scholar]
- 327.Yoon YI, Kim KH, Kang SH, Kim WJ, Shin MH, Lee SK, et al. Pure laparoscopic versus open right hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a propensity score matched analysis. Ann Surg. 2017;265:856–863. doi: 10.1097/SLA.0000000000002072. [DOI] [PubMed] [Google Scholar]
- 328.Berardi G, Morise Z, Sposito C, Igarashi K, Panetta V, Simonelli I, et al. Development of a nomogram to predict outcome after liver resection for hepatocellular carcinoma in Child-Pugh B cirrhosis. J Hepatol. 2020;72:75–84. doi: 10.1016/j.jhep.2019.08.032. [DOI] [PubMed] [Google Scholar]
- 329.Troisi RI, Berardi G, Morise Z, Cipriani F, Ariizumi S, Sposito C, et al. Laparoscopic and open liver resection for hepatocellular carcinoma with Child-Pugh B cirrhosis: multicentre propensity score-matched study. Br J Surg. 2021;108:196–204. doi: 10.1093/bjs/znaa041. [DOI] [PubMed] [Google Scholar]
- 330.Ruzzenente A, Bagante F, Ratti F, Alaimo L, Marques HP, Silva S, et al. Minimally invasive versus open liver resection for hepatocellular carcinoma in the setting of portal vein hypertension: results of an international multi-institutional analysis. Ann Surg Oncol. 2020;27:3360–3371. doi: 10.1245/s10434-020-08444-3. [DOI] [PubMed] [Google Scholar]
- 331.Pesi B, Bencini L, Moraldi L, Tofani F, Batignani G, Bechi P, et al. Robotic versus open liver resection in hepatocarcinoma: surgical and oncological outcomes. Surg Laparosc Endosc Percutan Tech. 2021;31:468–474. doi: 10.1097/SLE.0000000000000904. [DOI] [PubMed] [Google Scholar]
- 332.Zhou YM, Li B, Xu DH, Yang JM. Safety and efficacy of partial hepatectomy for huge (≥10 cm) hepatocellular carcinoma: a systematic review. Med Sci Monit. 2011;17:RA76–RA83. doi: 10.12659/MSM.881443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Hwang S, Lee YJ, Kim KH, Ahn CS, Moon DB, Ha TY, et al. Long-term outcome after resection of huge hepatocellular carcinoma ≥ 10 cm: single-institution experience with 471 patients. World J Surg. 2015;39:2519–2528. doi: 10.1007/s00268-015-3129-y. [DOI] [PubMed] [Google Scholar]
- 334.Wei CY, Chen PC, Chau GY, Lee RC, Chen PH, Huo TI, et al. Comparison of prognosis between surgical resection and transarterial chemoembolization for patients with solitary huge hepatocellular carcinoma. Ann Transl Med. 2020;8:238. doi: 10.21037/atm.2019.12.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Yue YY, Zhou WL. Hepatic resection is associated with improved long-term survival compared to radio-frequency ablation in patients with multifocal hepatocellular carcinoma. Front Oncol. 2020;10:110. doi: 10.3389/fonc.2020.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Lu L, Zheng P, Wu Z, Chen X. Hepatic resection versus transarterial chemoembolization for intermediate-stage hepatocellular carcinoma: a cohort study. Front Oncol. 2021;11:618937. doi: 10.3389/fonc.2021.618937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Fukami Y, Kaneoka Y, Maeda A, Kumada T, Tanaka J, Akita T, et al. Liver resection for multiple hepatocellular carcinomas: a Japanese nationwide survey. Ann Surg. 2020;272:145–154. doi: 10.1097/SLA.0000000000003192. [DOI] [PubMed] [Google Scholar]
- 338.Iakova P, Awad SS, Timchenko NA. Aging reduces proliferative capacities of liver by switching pathways of C/EBPalpha growth arrest. Cell. 2003;113:495–506. doi: 10.1016/s0092-8674(03)00318-0. [DOI] [PubMed] [Google Scholar]
- 339.Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854–862. doi: 10.1016/j.jamcollsurg.2006.12.032. discussion 862-864. [DOI] [PubMed] [Google Scholar]
- 340.Nishikawa H, Kimura T, Kita R, Osaki Y. Treatment for hepatocellular carcinoma in elderly patients: a literature review. J Cancer. 2013;4:635–643. doi: 10.7150/jca.7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Aoki T, Kokudo N, Matsuyama Y, Izumi N, Ichida T, Kudo M, et al. Prognostic impact of spontaneous tumor rupture in patients with hepatocellular carcinoma: an analysis of 1160 cases from a nationwide survey. Ann Surg. 2014;259:532–542. doi: 10.1097/SLA.0b013e31828846de. [DOI] [PubMed] [Google Scholar]
- 342.Li J, Huang L, Liu CF, Cao J, Yan JJ, Xu F, et al. Risk factors and surgical outcomes for spontaneous rupture of BCLC stages A and B hepatocellular carcinoma: a case-control study. World J Gastroenterol. 2014;20:9121–9127. doi: 10.3748/wjg.v20.i27.9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Kwon JH, Song GW, Hwang S, Kim KH, Ahn CS, Moon DB, et al. Surgical outcomes of spontaneously ruptured hepatocellular carcinoma. J Gastrointest Surg. 2021;25:941–953. doi: 10.1007/s11605-020-04555-0. [DOI] [PubMed] [Google Scholar]
- 344.Lee HS, Choi GH, Choi JS, Han KH, Ahn SH, Kim DY, et al. Staged partial hepatectomy versus transarterial chemoembolization for the treatment of spontaneous hepatocellular carcinoma rupture: a multicenter analysis in Korea. Ann Surg Treat Res. 2019;96:275–282. doi: 10.4174/astr.2019.96.6.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Chan WH, Hung CF, Pan KT, Lui KW, Huang YT, Lin SY, et al. Impact of spontaneous tumor rupture on prognosis of patients with T4 hepatocellular carcinoma. J Surg Oncol. 2016;113:789–795. doi: 10.1002/jso.24245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Wu JJ, Zhu P, Zhang ZG, Zhang BX, Shu C, Mba’nbo-Koumpa AA, et al. Spontaneous rupture of hepatocellular carcinoma: optimal timing of partial hepatectomy. Eur J Surg Oncol. 2019;45:1887–1894. doi: 10.1016/j.ejso.2019.02.033. [DOI] [PubMed] [Google Scholar]
- 347.Schwarz L, Bubenheim M, Zemour J, Herrero A, Muscari F, Ayav A, et al. Bleeding recurrence and mortality following interventional management of spontaneous HCC rupture: results of a multicenter European study. World J Surg. 2018;42:225–232. doi: 10.1007/s00268-017-4163-8. [DOI] [PubMed] [Google Scholar]
- 348.Zhou C, Zhang C, Zu QQ, Wang B, Zhou CG, Shi HB, et al. Emergency transarterial embolization followed by staged hepatectomy versus emergency hepatectomy for ruptured hepatocellular carcinoma: a single-center, propensity score matched analysis. Jpn J Radiol. 2020;38:1090–1098. doi: 10.1007/s11604-020-01007-2. [DOI] [PubMed] [Google Scholar]
- 349.Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M, et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol. 2016;65:938–943. doi: 10.1016/j.jhep.2016.05.044. [DOI] [PubMed] [Google Scholar]
- 350.Lee JM, Jang BK, Lee YJ, Choi WY, Choi SM, Chung WJ, et al. Survival outcomes of hepatic resection compared with transarterial chemoembolization or sorafenib for hepatocellular carcinoma with portal vein tumor thrombosis. Clin Mol Hepatol. 2016;22:160–167. doi: 10.3350/cmh.2016.22.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M, et al. Liver resection for hepatocellular carcinoma associated with hepatic vein invasion: a Japanese nationwide survey. Hepatology. 2017;66:510–517. doi: 10.1002/hep.29225. [DOI] [PubMed] [Google Scholar]
- 352.Zhang ZY, Dong KS, Zhang EL, Zhang LW, Chen XP, Dong HH. Resection might be a meaningful choice for hepatocellular carcinoma with portal vein thrombosis: a systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e18362. doi: 10.1097/MD.0000000000018362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Mei J, Li SH, Wang QX, Lu LH, Ling YH, Zou JW, et al. Resection vs. sorafenib for hepatocellular carcinoma with macroscopic vascular invasion: a real world, propensity score matched analytic study. Front Oncol. 2020;10:573. doi: 10.3389/fonc.2020.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Govalan R, Lauzon M, Luu M, Ahn JC, Kosari K, Todo T, et al. Comparison of surgical resection and systemic treatment for hepatocellular carcinoma with vascular invasion: national cancer database analysis. Liver Cancer. 2021;10:407–418. doi: 10.1159/000515554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Moon DB, Hwang S, Wang HJ, Yun SS, Kim KS, Lee YJ, et al. Surgical outcomes of hepatocellular carcinoma with bile duct tumor thrombus: a Korean multicenter study. World J Surg. 2013;37:443–451. doi: 10.1007/s00268-012-1845-0. [DOI] [PubMed] [Google Scholar]
- 356.Kim DS, Kim BW, Hatano E, Hwang S, Hasegawa K, Kudo A, et al. Surgical outcomes of hepatocellular carcinoma with bile duct tumor thrombus: a Korea-Japan multicenter study. Ann Surg. 2020;271:913–921. doi: 10.1097/SLA.0000000000003014. [DOI] [PubMed] [Google Scholar]
- 357.Kim JM, Rhu J, Ha SY, Choi GS, Kwon CHD, Kim G, et al. Realization of improved outcomes following liver resection in hepatocellular carcinoma patients aged 75 years and older. Ann Surg Treat Res. 2021;101:257–265. doi: 10.4174/astr.2021.101.5.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Kim JM, Joh JW, Yi NJ, Choi GS, Kim K, Lee KW, et al. Predicting hepatocellular carcinoma recurrence beyond milan criteria after liver resection for solitary hepatocellular carcinoma. J Gastrointest Surg. 2020;24:2219–2227. doi: 10.1007/s11605-019-04363-1. [DOI] [PubMed] [Google Scholar]
- 359.Yoon YI, Song GW, Lee S, Moon D, Hwang S, Kang WH, et al. Salvage living donor liver transplantation versus repeat liver resection for patients with recurrent hepatocellular carcinoma and Child-Pugh class A liver cirrhosis: a propensity score-matched comparison. Am J Transplant. 2022;22:165–176. doi: 10.1111/ajt.16790. [DOI] [PubMed] [Google Scholar]
- 360.Xia Y, Li J, Liu G, Wang K, Qian G, Lu Z, et al. Long-term effects of repeat hepatectomy vs percutaneous radiofrequency ablation among patients with recurrent hepatocellular carcinoma: a randomized clinical trial. JAMA Oncology. 2020;6:255–263. doi: 10.1001/jamaoncol.2019.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Wang HL, Mo DC, Zhong JH, Ma L, Wu FX, Xiang BD, et al. Systematic review of treatment strategy for recurrent hepatocellular carcinoma: salvage liver transplantation or curative locoregional therapy. Medicine (Baltimore) 2019;98:e14498. doi: 10.1097/MD.0000000000014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Okada S, Shimada K, Yamamoto J, Takayama T, Kosuge T, Yamasaki S, et al. Predictive factors for postoperative recurrence of hepatocellular carcinoma. Gastroenterology. 1994;106:1618–1624. doi: 10.1016/0016-5085(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 363.Shirabe K, Kanematsu T, Matsumata T, Adachi E, Akazawa K, Sugimachi K. Factors linked to early recurrence of small hepatocellular carcinoma after hepatectomy: univariate and multivariate analyses. Hepatology. 1991;14:802–805. doi: 10.1002/hep.1840140510. [DOI] [PubMed] [Google Scholar]
- 364.Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg. 1999;229:216–222. doi: 10.1097/00000658-199902000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Adachi E, Maeda T, Matsumata T, Shirabe K, Kinukawa N, Sugimachi K, et al. Risk factors for intrahepatic recurrence in human small hepatocellular carcinoma. Gastroenterology. 1995;108:768–775. doi: 10.1016/0016-5085(95)90450-6. [DOI] [PubMed] [Google Scholar]
- 366.Lee S, Kang TW, Song KD, Lee MW, Rhim H, Lim HK, et al. Effect of microvascular invasion risk on early recurrence of hepatocellular carcinoma after surgery and radiofrequency ablation. Ann Surg. 2021;273:564–571. doi: 10.1097/SLA.0000000000003268. [DOI] [PubMed] [Google Scholar]
- 367.Jung SM, Kim JM, Choi GS, Kwon CHD, Yi NJ, Lee KW, et al. Characteristics of early recurrence after curative liver resection for solitary hepatocellular carcinoma. J Gastrointest Surg. 2019;23:304–311. doi: 10.1007/s11605-018-3927-2. [DOI] [PubMed] [Google Scholar]
- 368.Agopian VG, Harlander-Locke MP, Ruiz RM, Klintmalm GB, Senguttuvan S, Florman SS, et al. Impact of pretransplant bridging locoregional therapy for patients with hepatocellular carcinoma within Milan criteria undergoing liver transplantation: analysis of 3601 patients from the US multicenter HCC transplant consortium. Ann Surg. 2017;266:525–535. doi: 10.1097/SLA.0000000000002381. [DOI] [PubMed] [Google Scholar]
- 369.Kim BK, Park JY, Kim DY, Kim JK, Kim KS, Choi JS, et al. Persistent hepatitis B viral replication affects recurrence of hepatocellular carcinoma after curative resection. Liver Int. 2008;28:393–401. doi: 10.1111/j.1478-3231.2007.01625.x. [DOI] [PubMed] [Google Scholar]
- 370.Ohkubo K, Kato Y, Ichikawa T, Kajiya Y, Takeda Y, Higashi S, et al. Viral load is a significant prognostic factor for hepatitis B virus-associated hepatocellular carcinoma. Cancer. 2002;94:2663–2668. doi: 10.1002/cncr.10557. [DOI] [PubMed] [Google Scholar]
- 371.Hung IF, Poon RT, Lai CL, Fung J, Fan ST, Yuen MF. Recurrence of hepatitis B-related hepatocellular carcinoma is associated with high viral load at the time of resection. Am J Gastroenterol. 2008;103:1663–1673. doi: 10.1111/j.1572-0241.2008.01872.x. [DOI] [PubMed] [Google Scholar]
- 372.Kubo S, Yamamoto T, Ikebe T, Shuto T, Hirohashi K, Tanaka H, et al. Relationship between multicentric occurrence of hepatocellular carcinoma and histology of noncancerous hepatic tissue in patients with chronic hepatitis C. Jpn J Cancer Res. 1999;90:1076–1080. doi: 10.1111/j.1349-7006.1999.tb00680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Chan DL, Morris DL, Chua TC. Clinical efficacy and predictors of outcomes of repeat hepatectomy for recurrent hepatocellular carcinoma - a systematic review. Surg Oncol. 2013;22:e23–e30. doi: 10.1016/j.suronc.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 374.Kim JM, Joh JW, Yi NJ, Choi GS, Kwon CHD, Lee KW, et al. Living donor liver transplantation should be cautiously considered as initial treatment in recurrent hepatocellular carcinoma within the Milan criteria after curative liver resection. Ann Transl Med. 2020;8:288. doi: 10.21037/atm.2020.02.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Chan DL, Alzahrani NA, Morris DL, Chua TC. Systematic review of efficacy and outcomes of salvage liver transplantation after primary hepatic resection for hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29:31–41. doi: 10.1111/jgh.12399. [DOI] [PubMed] [Google Scholar]
- 376.Poon RT, Fan ST, O’Suilleabhain CB, Wong J. Aggressive management of patients with extrahepatic and intrahepatic recurrences of hepatocellular carcinoma by combined resection and locoregional therapy. J Am Coll Surg. 2002;195:311–318. doi: 10.1016/s1072-7515(02)01226-7. [DOI] [PubMed] [Google Scholar]
- 377.Erridge S, Pucher PH, Markar SR, Malietzis G, Athanasiou T, Darzi A, et al. Meta-analysis of determinants of survival following treatment of recurrent hepatocellular carcinoma. Br J Surg. 2017;104:1433–1442. doi: 10.1002/bjs.10597. [DOI] [PubMed] [Google Scholar]
- 378.Peng ZW, Zhang YJ, Liang HH, Lin XJ, Guo RP, Chen MS. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: a prospective randomized trial. Radiology. 2012;262:689–700. doi: 10.1148/radiol.11110637. [DOI] [PubMed] [Google Scholar]
- 379.Shim JH, Kim KM, Lee YJ, Ko GY, Yoon HK, Sung KB, et al. Complete necrosis after transarterial chemoembolization could predict prolonged survival in patients with recurrent intrahepatic hepatocellular carcinoma after curative resection. Ann Surg Oncol. 2010;17:869–877. doi: 10.1245/s10434-009-0788-7. [DOI] [PubMed] [Google Scholar]
- 380.Shimada K, Sakamoto Y, Esaki M, Kosuge T, Morizane C, Ikeda M, et al. Analysis of prognostic factors affecting survival after initial recurrence and treatment efficacy for recurrence in patients undergoing potentially curative hepatectomy for hepatocellular carcinoma. Ann Surg Oncol. 2007;14:2337–2347. doi: 10.1245/s10434-007-9415-7. [DOI] [PubMed] [Google Scholar]
- 381.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 382.Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, et al. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17 Suppl 2:S44–S57. doi: 10.1002/lt.22365. [DOI] [PubMed] [Google Scholar]
- 383.Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11–e22. doi: 10.1016/S1470-2045(11)70175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Germani G, Gurusamy K, Garcovich M, Toso C, Fede G, Hemming A, et al. Which matters most: number of tumors, size of the largest tumor, or total tumor volume? Liver Transpl. 2011;17 Suppl 2:S58–S66. doi: 10.1002/lt.22336. [DOI] [PubMed] [Google Scholar]
- 385.Sugimachi K, Shirabe K, Taketomi A, Soejima Y, Iguchi T, Takeishi K, et al. Prognostic significance of preoperative imaging in recipients of living donor liver transplantation for hepatocellular carcinoma. Transplantation. 2011;91:570–574. doi: 10.1097/TP.0b013e318208134e. [DOI] [PubMed] [Google Scholar]
- 386.Lee JM, Trevisani F, Vilgrain V, Wald C. Imaging diagnosis and staging of hepatocellular carcinoma. Liver Transpl. 2011;17 Suppl 2:S34–S43. doi: 10.1002/lt.22369. [DOI] [PubMed] [Google Scholar]
- 387.Lv J, Yin H, Mao W, Shi H. Investigating the value of pre-treatment 18F-FDG PET/CT in predicting the pathological characteristic of hepatocellular carcinoma and recurrence after liver transplantation. Abdom Radiol (NY) 2021;46:2490–2497. doi: 10.1007/s00261-020-02872-1. [DOI] [PubMed] [Google Scholar]
- 388.Kang YK, Choi JY, Paeng JC, Kim YI, Kwon HW, Cheon GJ, et al. Composite criteria using clinical and FDG PET/CT factors for predicting recurrence of hepatocellular carcinoma after living donor liver transplantation. Eur Radiol. 2019;29:6009–6017. doi: 10.1007/s00330-019-06239-z. [DOI] [PubMed] [Google Scholar]
- 389.Lim C, Salloum C, Chalaye J, Lahat E, Costentin CE, Osseis M, et al. 18F-FDG PET/CT predicts microvascular invasion and early recurrence after liver resection for hepatocellular carcinoma: a prospective observational study. HPB (Oxford) 2019;21:739–747. doi: 10.1016/j.hpb.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 390.Alcorn JB United Network for Organ Sharing. Changes to OPTN bylaws and policies from actions at November board of directors meeting. Richmond: United Network for Organ Sharing; 2016. [Google Scholar]
- 391.Heimbach JK. Evolution of liver transplant selection criteria and U.S. allocation policy for patients with hepatocellular carcinoma. Semin Liver Dis. 2020;40:358–364. doi: 10.1055/s-0040-1709492. [DOI] [PubMed] [Google Scholar]
- 392.Korean Organ Donation Agency. Korea Network for Organ Sharing (KONOS) Registry. 2020;2020:15–17. [Google Scholar]
- 393.Freeman RB, Edwards EB, Harper AM. Waiting list removal rates among patients with chronic and malignant liver diseases. Am J Transplant. 2006;6:1416–1421. doi: 10.1111/j.1600-6143.2006.01321.x. [DOI] [PubMed] [Google Scholar]
- 394.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 395.Llovet JM, Mas X, Aponte JJ, Fuster J, Navasa M, Christensen E, et al. Cost effectiveness of adjuvant therapy for hepatocellular carcinoma during the waiting list for liver transplantation. Gut. 2002;50:123–128. doi: 10.1136/gut.50.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Mazzaferro V, Battiston C, Perrone S, Pulvirenti A, Regalia E, Romito R, et al. Radiofrequency ablation of small hepatocellular carcinoma in cirrhotic patients awaiting liver transplantation: a prospective study. Ann Surg. 2004;240:900–909. doi: 10.1097/01.sla.0000143301.56154.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Pomfret EA, Washburn K, Wald C, Nalesnik MA, Douglas D, Russo M, et al. Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transpl. 2010;16:262–278. doi: 10.1002/lt.21999. [DOI] [PubMed] [Google Scholar]
- 398.Mehta N, Heimbach J, Lee D, Dodge JL, Harnois D, Burns J, et al. Wait time of less than 6 and greater than 18 months predicts hepatocellular carcinoma recurrence after liver transplantation: proposing a wait time “sweet spot”. Transplantation. 2017;101:2071–2078. doi: 10.1097/TP.0000000000001752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Kollmann D, Selzner N, Selzner M. Bridging to liver transplantation in HCC patients. Langenbecks Arch Surg. 2017;402:863–871. doi: 10.1007/s00423-017-1609-2. [DOI] [PubMed] [Google Scholar]
- 400.Decaens T, Roudot-Thoraval F, Bresson-Hadni S, Meyer C, Gugenheim J, Durand F, et al. Impact of pretransplantation transarterial chemoembolization on survival and recurrence after liver transplantation for hepatocellular carcinoma. Liver Transpl. 2005;11:767–775. doi: 10.1002/lt.20418. [DOI] [PubMed] [Google Scholar]
- 401.Lesurtel M, Müllhaupt B, Pestalozzi BC, Pfammatter T, Clavien PA. Transarterial chemoembolization as a bridge to liver transplantation for hepatocellular carcinoma: an evidence-based analysis. Am J Transplant. 2006;6:2644–2650. doi: 10.1111/j.1600-6143.2006.01509.x. [DOI] [PubMed] [Google Scholar]
- 402.Pelletier SJ, Fu S, Thyagarajan V, Romero-Marrero C, Batheja MJ, Punch JD, et al. An intention-to-treat analysis of liver transplantation for hepatocellular carcinoma using organ procurement transplant network data. Liver Transpl. 2009;15:859–868. doi: 10.1002/lt.21778. [DOI] [PubMed] [Google Scholar]
- 403.Kulik L, Heimbach JK, Zaiem F, Almasri J, Prokop LJ, Wang Z, et al. Therapies for patients with hepatocellular carcinoma awaiting liver transplantation: a systematic review and meta-analysis. Hepatology. 2018;67:381–400. doi: 10.1002/hep.29485. [DOI] [PubMed] [Google Scholar]
- 404.Si T, Chen Y, Ma D, Gong X, Guan R, Shen B, et al. Transarterial chemoembolization prior to liver transplantation for patients with hepatocellular carcinoma: a meta-analysis. J Gastroenterol Hepatol. 2017;32:1286–1294. doi: 10.1111/jgh.13727. [DOI] [PubMed] [Google Scholar]
- 405.Porrett PM, Peterman H, Rosen M, Sonnad S, Soulen M, Markmann JF, et al. Lack of benefit of pre-transplant locoregional hepatic therapy for hepatocellular cancer in the current MELD era. Liver Transpl. 2006;12:665–673. doi: 10.1002/lt.20636. [DOI] [PubMed] [Google Scholar]
- 406.Chapman WC, Majella Doyle MB, Stuart JE, Vachharajani N, Crippin JS, Anderson CD, et al. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg. 2008;248:617–625. doi: 10.1097/SLA.0b013e31818a07d4. [DOI] [PubMed] [Google Scholar]
- 407.Lee MW, Raman SS, Asvadi NH, Siripongsakun S, Hicks RM, Chen J, et al. Radiofrequency ablation of hepatocellular carcinoma as bridge therapy to liver transplantation: a 10-year intention-to-treat analysis. Hepatology. 2017;65:1979–1990. doi: 10.1002/hep.29098. [DOI] [PubMed] [Google Scholar]
- 408.Yao FY, Bass NM, Nikolai B, Davern TJ, Kerlan R, Wu V, et al. Liver transplantation for hepatocellular carcinoma: analysis of survival according to the intention-to-treat principle and dropout from the waiting list. Liver Transpl. 2002;8:873–883. doi: 10.1053/jlts.2002.34923. [DOI] [PubMed] [Google Scholar]
- 409.Yao FY, Kerlan RK, Jr, Hirose R, Davern TJ, 3rd, Bass NM, Feng S, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48:819–827. doi: 10.1002/hep.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Sapisochin G, Barry A, Doherty M, Fischer S, Goldaracena N, Rosales R, et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol. 2017;67:92–99. doi: 10.1016/j.jhep.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 411.De Luna W, Sze DY, Ahmed A, Ha BY, Ayoub W, Keeffe EB, et al. Transarterial chemoinfusion for hepatocellular carcinoma as downstaging therapy and a bridge toward liver transplantation. Am J Transplant. 2009;9:1158–1168. doi: 10.1111/j.1600-6143.2009.02576.x. [DOI] [PubMed] [Google Scholar]
- 412.Roayaie S, Frischer JS, Emre SH, Fishbein TM, Sheiner PA, Sung M, et al. Long-term results with multimodal adjuvant therapy and liver transplantation for the treatment of hepatocellular carcinomas larger than 5 centimeters. Ann Surg. 2002;235:533–539. doi: 10.1097/00000658-200204000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Yao FY, Hirose R, LaBerge JM, Davern TJ, 3rd, Bass NM, Kerlan RK, Jr, et al. A prospective study on downstaging of hepatocellular carcinoma prior to liver transplantation. Liver Transpl. 2005;11:1505–1514. doi: 10.1002/lt.20526. [DOI] [PubMed] [Google Scholar]
- 414.Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9:1920–1928. doi: 10.1111/j.1600-6143.2009.02695.x. [DOI] [PubMed] [Google Scholar]
- 415.Parikh ND, Waljee AK, Singal AG. Downstaging hepatocellular carcinoma: a systematic review and pooled analysis. Liver Transpl. 2015;21:1142–1152. doi: 10.1002/lt.24169. [DOI] [PubMed] [Google Scholar]
- 416.Mehta N, Frenette C, Tabrizian P, Hoteit M, Guy J, Parikh N, et al. Downstaging outcomes for hepatocellular carcinoma: results from the multicenter evaluation of reduction in tumor size before liver transplantation (MERITS-LT) consortium. Gastroenterology. 2021;161:1502–1512. doi: 10.1053/j.gastro.2021.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Mazzaferro V, Citterio D, Bhoori S, Bongini M, Miceli R, De Carlis L, et al. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): a randomised, controlled, phase 2b/3 trial. Lancet Oncol. 2020;21:947–956. doi: 10.1016/S1470-2045(20)30224-2. [DOI] [PubMed] [Google Scholar]
- 418.Chapman WC, Garcia-Aroz S, Vachharajani N, Fowler K, Saad N, Lin Y, et al. Liver transplantation for advanced hepatocellular carcinoma after downstaging without up-front stage restrictions. J Am Coll Surg. 2017;224:610–621. doi: 10.1016/j.jamcollsurg.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 419.Kim JH, Sinn DH, Gwak GY, Choi GS, Kim JM, Kwon CHD, et al. Factors determining long-term outcomes of hepatocellular carcinoma within the Milan criteria: liver transplantation versus locoregional therapy: a retrospective cohort study. Medicine (Baltimore) 2016;95:e4735. doi: 10.1097/MD.0000000000004735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Ravaioli M, Grazi GL, Piscaglia F, Trevisani F, Cescon M, Ercolani G, et al. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant. 2008;8:2547–2557. doi: 10.1111/j.1600-6143.2008.02409.x. [DOI] [PubMed] [Google Scholar]
- 421.Yao FY, Mehta N, Flemming J, Dodge J, Hameed B, Fix O, et al. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology. 2015;61:1968–1977. doi: 10.1002/hep.27752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Halazun KJ, Sapisochin G, von Ahrens D, Agopian VG, Tabrizian P. Predictors of outcome after liver transplantation for hepatocellular carcinoma (HCC) beyond Milan criteria. Int J Surg. 2020;82S:61–69. doi: 10.1016/j.ijsu.2020.07.029. [DOI] [PubMed] [Google Scholar]
- 423.DiNorcia J, Florman SS, Haydel B, Tabrizian P, Ruiz RM, Klintmalm GB, et al. Pathologic response to pretransplant locoregional therapy is predictive of patient outcome after liver transplantation for hepatocellular carcinoma: analysis from the US multicenter HCC transplant consortium. Ann Surg. 2020;271:616–624. doi: 10.1097/SLA.0000000000003253. [DOI] [PubMed] [Google Scholar]
- 424.Ho MH, Yu CY, Chung KP, Chen TW, Chu HC, Lin CK, et al. Locoregional therapy-induced tumor necrosis as a predictor of recurrence after liver transplant in patients with hepatocellular carcinoma. Ann Surg Oncol. 2011;18:3632–3639. doi: 10.1245/s10434-011-1803-3. [DOI] [PubMed] [Google Scholar]
- 425.Kardashian A, Florman SS, Haydel B, Ruiz RM, Klintmalm GB, Lee DD, et al. Liver transplantation outcomes in a U.S. multicenter cohort of 789 patients with hepatocellular carcinoma presenting beyond milan criteria. Hepatology. 2020;72:2014–2028. doi: 10.1002/hep.31210. [DOI] [PubMed] [Google Scholar]
- 426.Mehta N, Dodge JL, Roberts JP, Hirose R, Yao FY. Alpha-fetoprotein decrease from > 1,000 to < 500 ng/mL in patients with hepatocellular carcinoma leads to improved posttransplant outcomes. Hepatology. 2019;69:1193–1205. doi: 10.1002/hep.30413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Bargellini I, Florio F, Golfieri R, Grosso M, Lauretti DL, Cioni R. Trends in utilization of transarterial treatments for hepatocellular carcinoma: results of a survey by the Italian Society of Interventional Radiology. Cardiovasc Intervent Radiol. 2014;37:438–444. doi: 10.1007/s00270-013-0656-5. [DOI] [PubMed] [Google Scholar]
- 428.Korean Organ Donation Agency. Korea Network for Organ Sharing (KONOS) Registry. 2017;2017:15–22. [Google Scholar]
- 429.Cioni R KONOS. KONOS annual report. Seoul: KONOS; 2019. [Google Scholar]
- 430.Grant RC, Sandhu L, Dixon PR, Greig PD, Grant DR, McGilvray ID. Living vs. deceased donor liver transplantation for hepatocellular carcinoma: a systematic review and meta-analysis. Clin Transplant. 2013;27:140–147. doi: 10.1111/ctr.12031. [DOI] [PubMed] [Google Scholar]
- 431.Liang W, Wu L, Ling X, Schroder PM, Ju W, Wang D, et al. Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl. 2012;18:1226–1236. doi: 10.1002/lt.23490. [DOI] [PubMed] [Google Scholar]
- 432.Tan DJH, Wong C, Ng CH, Poh CW, Jain SR, Huang DQ, et al. A meta-analysis on the rate of hepatocellular carcinoma recurrence after liver transplant and associations to etiology, alpha-fetoprotein, income and ethnicity. J Clin Med. 2021;10:238. doi: 10.3390/jcm10020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Azoulay D, Audureau E, Bhangui P, Belghiti J, Boillot O, Andreani P, et al. Living or brain-dead donor liver transplantation for hepatocellular carcinoma: a multicenter, western, intent-to-treat cohort study. Ann Surg. 2017;266:1035–1044. doi: 10.1097/SLA.0000000000001986. [DOI] [PubMed] [Google Scholar]
- 434.Bhangui P, Vibert E, Majno P, Salloum C, Andreani P, Zocrato J, et al. Intention-to-treat analysis of liver transplantation for hepatocellular carcinoma: living versus deceased donor transplantation. Hepatology. 2011;53:1570–1579. doi: 10.1002/hep.24231. [DOI] [PubMed] [Google Scholar]
- 435.Kulik LM, Fisher RA, Rodrigo DR, Brown RS, Jr, Freise CE, Shaked A, et al. Outcomes of living and deceased donor liver transplant recipients with hepatocellular carcinoma: results of the A2ALL cohort. Am J Transplant. 2012;12:2997–3007. doi: 10.1111/j.1600-6143.2012.04272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Kulik L, Abecassis M. Living donor liver transplantation for hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S277–S282. doi: 10.1053/j.gastro.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 437.Sarasin FP, Majno PE, Llovet JM, Bruix J, Mentha G, Hadengue A. Living donor liver transplantation for early hepatocellular carcinoma: a life-expectancy and cost-effectiveness perspective. Hepatology. 2001;33:1073–1079. doi: 10.1053/jhep.2001.23311. [DOI] [PubMed] [Google Scholar]
- 438.Kwon CH, Kim DJ, Han YS, Park JB, Choi GS, Kim SJ, et al. HCC in living donor liver transplantation: can we expand the Milan criteria? Dig Dis. 2007;25:313–319. doi: 10.1159/000106911. [DOI] [PubMed] [Google Scholar]
- 439.Suh KS, Cho EH, Lee HW, Shin WY, Yi NJ, Lee KU. Liver transplantation for hepatocellular carcinoma in patients who do not meet the Milan criteria. Dig Dis. 2007;25:329–333. doi: 10.1159/000106913. [DOI] [PubMed] [Google Scholar]
- 440.Choi HJ, Kim DG, Na GH, Han JH, Hong TH, You YK. Clinical outcome in patients with hepatocellular carcinoma after living-donor liver transplantation. World J Gastroenterol. 2013;19:4737–4744. doi: 10.3748/wjg.v19.i29.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Lee SG, Hwang S, Moon DB, Ahn CS, Kim KH, Sung KB, et al. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl. 2008;14:935–945. doi: 10.1002/lt.21445. [DOI] [PubMed] [Google Scholar]
- 442.Sugawara Y, Tamura S, Makuuchi M. Living donor liver transplantation for hepatocellular carcinoma: Tokyo University series. Dig Dis. 2007;25:310–312. doi: 10.1159/000106910. [DOI] [PubMed] [Google Scholar]
- 443.Ito T, Takada Y, Ueda M, Haga H, Maetani Y, Oike F, et al. Expansion of selection criteria for patients with hepatocellular carcinoma in living donor liver transplantation. Liver Transpl. 2007;13:1637–1644. doi: 10.1002/lt.21281. [DOI] [PubMed] [Google Scholar]
- 444.Taketomi A, Sanefuji K, Soejima Y, Yoshizumi T, Uhciyama H, Ikegami T, et al. Impact of des-gamma-carboxy prothrombin and tumor size on the recurrence of hepatocellular carcinoma after living donor liver transplantation. Transplantation. 2009;87:531–537. doi: 10.1097/TP.0b013e3181943bee. [DOI] [PubMed] [Google Scholar]
- 445.Todo S, Furukawa H, Tada M Japanese Liver Transplantation Study Group. Extending indication: role of living donor liver transplantation for hepatocellular carcinoma. Liver Transpl. 2007;13(11 Suppl 2):S48–S54. doi: 10.1002/lt.21334. [DOI] [PubMed] [Google Scholar]
- 446.Lee JH, Cho Y, Kim HY, Cho EJ, Lee DH, Yu SJ, et al. Serum tumor markers provide refined prognostication in selecting liver transplantation candidate for hepatocellular carcinoma patients beyond the Milan criteria. Ann Surg. 2016;263:842–850. doi: 10.1097/SLA.0000000000001578. [DOI] [PubMed] [Google Scholar]
- 447.Lee SD, Lee B, Kim SH, Joo J, Kim SK, Kim YK, et al. Proposal of new expanded selection criteria using total tumor size and (18)F-fluorodeoxyglucose - positron emission tomography/computed tomography for living donor liver transplantation in patients with hepatocellular carcinoma: the National Cancer Center Korea criteria. World J Transplant. 2016;6:411–422. doi: 10.5500/wjt.v6.i2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Kornberg A, Witt U, Schernhammer M, Kornberg J, Ceyhan GO, Mueller K, et al. Combining 18F-FDG positron emission tomography with up-to-seven criteria for selecting suitable liver transplant patients with advanced hepatocellular carcinoma. Sci Rep. 2017;7:14176. doi: 10.1038/s41598-017-14430-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Takada Y, Kaido T, Shirabe K, Nagano H, Egawa H, Sugawara Y, et al. Significance of preoperative fluorodeoxyglucose-positron emission tomography in prediction of tumor recurrence after liver transplantation for hepatocellular carcinoma patients: a Japanese multicenter study. J Hepatobiliary Pancreat Sci. 2017;24:49–57. doi: 10.1002/jhbp.412. [DOI] [PubMed] [Google Scholar]
- 450.Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, et al. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143:986–994.e3. doi: 10.1053/j.gastro.2012.05.052. quiz e14-e15. [DOI] [PubMed] [Google Scholar]
- 451.Notarpaolo A, Layese R, Magistri P, Gambato M, Colledan M, Magini G, et al. Validation of the AFP model as a predictor of HCC recurrence in patients with viral hepatitis-related cirrhosis who had received a liver transplant for HCC. J Hepatol. 2017;66:552–559. doi: 10.1016/j.jhep.2016.10.038. [DOI] [PubMed] [Google Scholar]
- 452.Alim A, Erdogan Y, Dayangac M, Yuzer Y, Tokat Y, Oezcelik A. Living donor liver transplantation: the optimal curative treatment for hepatocellular carcinoma even beyond Milan criteria. Cancer Control. 2021;28:10732748211011960. doi: 10.1177/10732748211011960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Kim YS, Lim HK, Rhim H, Lee MW, Choi D, Lee WJ, et al. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013;58:89–97. doi: 10.1016/j.jhep.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 454.Shin M, Song S, Kim JM, Kwon CH, Kim SJ, Lee SK, et al. Donor morbidity including biliary complications in living-donor liver transplantation: single-center analysis of 827 cases. Transplantation. 2012;93:942–948. doi: 10.1097/TP.0b013e31824ad5de. [DOI] [PubMed] [Google Scholar]
- 455.Kim KH, Jung DH, Park KM, Lee YJ, Kim DY, Kim KM, et al. Comparison of open and laparoscopic live donor left lateral sectionectomy. Br J Surg. 2011;98:1302–1308. doi: 10.1002/bjs.7601. [DOI] [PubMed] [Google Scholar]
- 456.Hwang S, Lee SG, Lee YJ, Sung KB, Park KM, Kim KH, et al. Lessons learned from 1,000 living donor liver transplantations in a single center: how to make living donations safe. Liver Transpl. 2006;12:920–927. doi: 10.1002/lt.20734. [DOI] [PubMed] [Google Scholar]
- 457.Yi NJ, Suh KS, Cho JY, Lee HW, Cho EH, Yang SH, et al. Three-quarters of right liver donors experienced postoperative complications. Liver Transpl. 2007;13:797–806. doi: 10.1002/lt.21030. [DOI] [PubMed] [Google Scholar]
- 458.Kim SJ, Na GH, Choi HJ, Yoo YK, Kim DG. Surgical outcome of right liver donors in living donor liver transplantation: single-center experience with 500 cases. J Gastrointest Surg. 2012;16:1160–1170. doi: 10.1007/s11605-012-1865-y. [DOI] [PubMed] [Google Scholar]
- 459.Lee JG, Lee KW, Kwon CHD, Chu CW, Kim BW, Choi DL, et al. Donor safety in living donor liver transplantation: the Korean organ transplantation registry study. Liver Transpl. 2017;23:999–1006. doi: 10.1002/lt.24778. [DOI] [PubMed] [Google Scholar]
- 460.Yang JD, Lee KW, Kim JM, Kim MS, Lee JG, Kang KJ, et al. A comparative study of postoperative outcomes between minimally invasive living donor hepatectomy and open living donor hepatectomy: the Korean organ transplantation registry. Surgery. 2021;170:271–276. doi: 10.1016/j.surg.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 461.Rho SY, Lee JG, Joo DJ, Kim MS, Kim SI, Han DH, et al. Outcomes of robotic living donor right hepatectomy from 52 consecutive cases: comparison with open and laparoscopy-assisted donor hepatectomy. Ann Surg. 2022;275:e433–e442. doi: 10.1097/SLA.0000000000004067. [DOI] [PubMed] [Google Scholar]
- 462.Chan SC, Chan AC, Sharr WW, Chok KS, Cheung TT, Fan ST, et al. Perpetuating proficiency in donor right hepatectomy for living donor liver transplantation. Asian J Surg. 2014;37:65–72. doi: 10.1016/j.asjsur.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 463.Ghobrial RM, Freise CE, Trotter JF, Tong L, Ojo AO, Fair JH, et al. Donor morbidity after living donation for liver transplantation. Gastroenterology. 2008;135:468–476. doi: 10.1053/j.gastro.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Siegler M, Simmerling MC, Siegler JH, Cronin DC., 2nd Recipient deaths during donor surgery: a new ethical problem in living donor liver transplantation (LDLT) Liver Transpl. 2006;12:358–360. doi: 10.1002/lt.20670. [DOI] [PubMed] [Google Scholar]
- 465.Brown RS., Jr Live donors in liver transplantation. Gastroenterology. 2008;134:1802–1813. doi: 10.1053/j.gastro.2008.02.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Choi JY, Kim JH, Kim JM, Kim HJ, Ahn HS, Joh JW. Outcomes of living liver donors are worse than those of matched healthy controls. J Hepatol. 2022;76:628–638. doi: 10.1016/j.jhep.2021.10.031. [DOI] [PubMed] [Google Scholar]
- 467.Schwartz M, Roayaie S, Llovet J. How should patients with hepatocellular carcinoma recurrence after liver transplantation be treated? J Hepatol. 2005;43:584–589. doi: 10.1016/j.jhep.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 468.Liang W, Wang D, Ling X, Kao AA, Kong Y, Shang Y, et al. Sirolimus-based immunosuppression in liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl. 2012;18:62–69. doi: 10.1002/lt.22441. [DOI] [PubMed] [Google Scholar]
- 469.Kang I, Lee JG, Choi SH, Kim HJ, Han DH, Choi GH, et al. Impact of everolimus on survival after liver transplantation for hepatocellular carcinoma. Clin Mol Hepatol. 2021;27:589–602. doi: 10.3350/cmh.2021.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Lee SG, Jeng LB, Saliba F, Singh Soin A, Lee WC, De Simone P, et al. Efficacy and safety of everolimus with reduced tacrolimus in liver transplant recipients: 24-month results from the pooled analysis of 2 randomized controlled trials. Transplantation. 2021;105:1564–1575. doi: 10.1097/TP.0000000000003394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Yan X, Huang S, Yang Y, Lu Z, Li F, Jiang L, et al. Sirolimus or everolimus improves survival after liver transplantation for hepatocellular carcinoma: a systematic review and meta-analysis. Liver Transpl. 2022;28:1063–1077. doi: 10.1002/lt.26387. [DOI] [PubMed] [Google Scholar]
- 472.Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, et al. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004;10:534–540. doi: 10.1002/lt.20128. [DOI] [PubMed] [Google Scholar]
- 473.Hollebecque A, Decaens T, Boleslawski E, Mathurin P, Duvoux C, Pruvot FR, et al. Natural history and therapeutic management of recurrent hepatocellular carcinoma after liver transplantation. Gastroenterol Clin Biol. 2009;33:361–369. doi: 10.1016/j.gcb.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 474.Kim YS, Lim HK, Rhim H, Lee WJ, Joh JW, Park CK. Recurrence of hepatocellular carcinoma after liver transplantation: patterns and prognostic factors based on clinical and radiologic features. AJR Am J Roentgenol. 2007;189:352–358. doi: 10.2214/AJR.07.2088. [DOI] [PubMed] [Google Scholar]
- 475.Bodzin AS, Lunsford KE, Markovic D, Harlander-Locke MP, Busuttil RW, Agopian VG. Predicting mortality in patients developing recurrent hepatocellular carcinoma after liver transplantation: impact of treatment modality and recurrence characteristics. Ann Surg. 2017;266:118–125. doi: 10.1097/SLA.0000000000001894. [DOI] [PubMed] [Google Scholar]
- 476.Roh YN, Kwon CHD, Song S, Shin M, Kim JM, Kim S, et al. The prognosis and treatment outcomes of patients with recurrent hepatocellular carcinoma after liver transplantation. Clin Transplant. 2014;28:141–148. doi: 10.1111/ctr.12286. [DOI] [PubMed] [Google Scholar]
- 477.Sapisochin G, Goldaracena N, Astete S, Laurence JM, Davidson D, Rafael E, et al. Benefit of treating hepatocellular carcinoma recurrence after liver transplantation and analysis of prognostic factors for survival in a large Euro-American series. Ann Surg Oncol. 2015;22:2286–2294. doi: 10.1245/s10434-014-4273-6. [DOI] [PubMed] [Google Scholar]
- 478.Taketomi A, Fukuhara T, Morita K, Kayashima H, Ninomiya M, Yamashita Y, et al. Improved results of a surgical resection for the recurrence of hepatocellular carcinoma after living donor liver transplantation. Ann Surg Oncol. 2010;17:2283–2289. doi: 10.1245/s10434-010-0999-y. [DOI] [PubMed] [Google Scholar]
- 479.Huang J, Yan L, Wu H, Yang J, Liao M, Zeng Y. Is radiofrequency ablation applicable for recurrent hepatocellular carcinoma after liver transplantation? J Surg Res. 2016;200:122–130. doi: 10.1016/j.jss.2015.07.033. [DOI] [PubMed] [Google Scholar]
- 480.Zhou B, Shan H, Zhu KS, Jiang ZB, Guan SH, Meng XC, et al. Chemoembolization with lobaplatin mixed with iodized oil for unresectable recurrent hepatocellular carcinoma after orthotopic liver transplantation. J Vasc Interv Radiol. 2010;21:333–338. doi: 10.1016/j.jvir.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 481.Cheng YC, Chen TW, Fan HL, Yu CY, Chang HC, Hsieh CB. Transarterial chemoembolization for intrahepatic multiple recurrent HCC after liver resection or transplantation. Ann Transplant. 2014;19:309–316. doi: 10.12659/AOT.890505. [DOI] [PubMed] [Google Scholar]
- 482.Sposito C, Mariani L, Germini A, Flores Reyes M, Bongini M, Grossi G, et al. Comparative efficacy of sorafenib versus best supportive care in recurrent hepatocellular carcinoma after liver transplantation: a case-control study. J Hepatol. 2013;59:59–66. doi: 10.1016/j.jhep.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 483.Staufer K, Fischer L, Seegers B, Vettorazzi E, Nashan B, Sterneck M. High toxicity of sorafenib for recurrent hepatocellular carcinoma after liver transplantation. Transpl Int. 2012;25:1158–1164. doi: 10.1111/j.1432-2277.2012.01540.x. [DOI] [PubMed] [Google Scholar]
- 484.Bhoori S, Toffanin S, Sposito C, Germini A, Pellegrinelli A, Lampis A, et al. Personalized molecular targeted therapy in advanced, recurrent hepatocellular carcinoma after liver transplantation: a proof of principle. J Hepatol. 2010;52:771–775. doi: 10.1016/j.jhep.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 485.Invernizzi F, Iavarone M, Zavaglia C, Mazza S, Maggi U, Cesarini L, et al. Experience with early sorafenib treatment with mTOR inhibitors in hepatocellular carcinoma recurring after liver transplantation. Transplantation. 2020;104:568–574. doi: 10.1097/TP.0000000000002955. [DOI] [PubMed] [Google Scholar]
- 486.Waghray A, Balci B, El-Gazzaz G, Kim R, Pelley R, Narayanan Menon KV, et al. Safety and efficacy of sorafenib for the treatment of recurrent hepatocellular carcinoma after liver transplantation. Clin Transplant. 2013;27:555–561. doi: 10.1111/ctr.12150. [DOI] [PubMed] [Google Scholar]
- 487.Iavarone M, Invernizzi F, Czauderna C, Sanduzzi-Zamparelli M, Bhoori S, Amaddeo G, et al. Preliminary experience on safety of regorafenib after sorafenib failure in recurrent hepatocellular carcinoma after liver transplantation. Am J Transplant. 2019;19:3176–3184. doi: 10.1111/ajt.15551. [DOI] [PubMed] [Google Scholar]
- 488.Iavarone M, Invernizzi F, Ivanics T, Mazza S, Zavaglia C, Sanduzzi-Zamparelli M, et al. Regorafenib efficacy after sorafenib in patients with recurrent hepatocellular carcinoma after liver transplantation: a retrospective study. Liver Transpl. 2021;27:1767–1778. doi: 10.1002/lt.26264. [DOI] [PubMed] [Google Scholar]
- 489.Gassmann D, Weiler S, Mertens JC, Reiner CS, Vrugt B, Nägeli M, et al. Liver allograft failure after nivolumab treatment-a case report with systematic literature research. Transplant Direct. 2018;4:e376. doi: 10.1097/TXD.0000000000000814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.DeLeon TT, Salomao MA, Aqel BA, Sonbol MB, Yokoda RT, Ali AH, et al. Pilot evaluation of PD-1 inhibition in metastatic cancer patients with a history of liver transplantation: the Mayo Clinic experience. J Gastrointest Oncol. 2018;9:1054–1062. doi: 10.21037/jgo.2018.07.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Biondani P, De Martin E, Samuel D. Safety of an anti-PD-1 immune checkpoint inhibitor in a liver transplant recipient. Ann Oncol. 2018;29:286–287. doi: 10.1093/annonc/mdx548. [DOI] [PubMed] [Google Scholar]
- 492.Patel IJ, Rahim S, Davidson JC, Hanks SE, Tam AL, Walker TG, et al. Society of Interventional Radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions-part II: recommendations: endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol. 2019;30:1168–1184.e1. doi: 10.1016/j.jvir.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 493.Tripodi A, Primignani M, Mannucci PM, Caldwell SH. Changing concepts of cirrhotic coagulopathy. Am J Gastroenterol. 2017;112:274–281. doi: 10.1038/ajg.2016.498. [DOI] [PubMed] [Google Scholar]
- 494.Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234:961–967. doi: 10.1148/radiol.2343040350. [DOI] [PubMed] [Google Scholar]
- 495.Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or =4 cm. Gastroenterology. 2004;127:1714–1723. doi: 10.1053/j.gastro.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 496.Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122–130. doi: 10.1053/j.gastro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 497.Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235–240. doi: 10.1148/radiol.2281020718. [DOI] [PubMed] [Google Scholar]
- 498.Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107:569–577. doi: 10.1038/ajg.2011.425. quiz 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2008;47:82–89. doi: 10.1002/hep.21933. [DOI] [PubMed] [Google Scholar]
- 500.Lee DH, Kim JW, Lee JM, Kim JM, Lee MW, Rhim H, et al. Laparoscopic liver resection versus percutaneous radiofrequency ablation for small single nodular hepatocellular carcinoma: comparison of treatment outcomes. Liver Cancer. 2021;10:25–37. doi: 10.1159/000510909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Lee MW, Kang D, Lim HK, Cho J, Sinn DH, Kang TW, et al. Updated 10-year outcomes of percutaneous radiofrequency ablation as first-line therapy for single hepatocellular carcinoma < 3 cm: emphasis on association of local tumor progression and overall survival. Eur Radiol. 2020;30:2391–2400. doi: 10.1007/s00330-019-06575-0. [DOI] [PubMed] [Google Scholar]
- 502.Song KD, Lim HK, Rhim H, Lee MW, Kang TW, Paik YH, et al. Hepatic resection vs percutaneous radiofrequency ablation of hepatocellular carcinoma abutting right diaphragm. World J Gastrointest Oncol. 2019;11:227–237. doi: 10.4251/wjgo.v11.i3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Kang TW, Lim HK, Lee MW, Kim YS, Rhim H, Lee WJ, et al. Aggressive intrasegmental recurrence of hepatocellular carcinoma after radiofrequency ablation: risk factors and clinical significance. Radiology. 2015;276:274–285. doi: 10.1148/radiol.15141215. [DOI] [PubMed] [Google Scholar]
- 504.Lee S, Kang TW, Cha DI, Song KD, Lee MW, Rhim H, et al. Radiofrequency ablation vs. surgery for perivascular hepatocellular carcinoma: propensity score analyses of long-term outcomes. J Hepatol. 2018;69:70–78. doi: 10.1016/j.jhep.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 505.Sohn W, Kang TW, Choi SK, Jung SH, Lee MW, Lim HK, et al. Effect of oral antiviral treatment on long-term outcomes of radiofrequency ablation therapy for hepatitis B virus-related hepatocellular carcinoma. Oncotarget. 2016;7:47794–47807. doi: 10.18632/oncotarget.10026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Lee DH, Lee JM, Lee JY, Kim SH, Kim JH, Yoon JH, et al. Non-hypervascular hepatobiliary phase hypointense nodules on gadoxetic acid-enhanced MRI: risk of HCC recurrence after radiofrequency ablation. J Hepatol. 2015;62:1122–1130. doi: 10.1016/j.jhep.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 507.Park SJ, Cho EJ, Lee JH, Yu SJ, Kim YJ, Yoon JH, et al. Switching monopolar no-touch radiofrequency ablation using octopus electrodes for small hepatocellular carcinoma: a randomized clinical trial. Liver Cancer. 2021;10:72–81. doi: 10.1159/000512338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Suh YS, Choi JW, Yoon JH, Lee DH, Kim YJ, Lee JH, et al. Notouch vs. conventional radiofrequency ablation using twin internally cooled wet electrodes for small hepatocellular carcinomas: a randomized prospective comparative study. Korean J Radiol. 2021;22:1974–1984. doi: 10.3348/kjr.2021.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Lee DH, Lee MW, Kim PN, Lee YJ, Park HS, Lee JM. Outcome of no-touch radiofrequency ablation for small hepatocellular carcinoma: a multicenter clinical trial. Radiology. 2021;301:229–236. doi: 10.1148/radiol.2021210309. [DOI] [PubMed] [Google Scholar]
- 510.Majumdar A, Roccarina D, Thorburn D, Davidson BR, Tsochatzis E, Gurusamy KS. Management of people with early- or very early-stage hepatocellular carcinoma: an attempted network meta-analysis. Cochrane Database Syst Rev. 2017;3:CD011650. doi: 10.1002/14651858.CD011650.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Ng KKC, Chok KSH, Chan ACY, Cheung TT, Wong TCL, Fung JYY, et al. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br J Surg. 2017;104:1775–1784. doi: 10.1002/bjs.10677. [DOI] [PubMed] [Google Scholar]
- 512.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Feng K, Yan J, Li X, Xia F, Ma K, Wang S, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57:794–802. doi: 10.1016/j.jhep.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 514.Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903–912. doi: 10.1097/SLA.0b013e3181efc656. [DOI] [PubMed] [Google Scholar]
- 515.Wang Q, Tang M, Zhang S. Comparison of radiofrequency ablation and surgical resection for hepatocellular carcinoma conforming to the Milan criteria: a meta-analysis. ANZ J Surg. 2021;91:E432–E438. doi: 10.1111/ans.16560. [DOI] [PubMed] [Google Scholar]
- 516.Lee HW, Lee JM, Yoon JH, Kim YJ, Park JW, Park SJ, et al. A prospective randomized study comparing radiofrequency ablation and hepatic resection for hepatocellular carcinoma. Ann Surg Treat Res. 2018;94:74–82. doi: 10.4174/astr.2018.94.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Yang HJ, Lee JH, Lee DH, Yu SJ, Kim YJ, Yoon JH, et al. Small single-nodule hepatocellular carcinoma: comparison of transarterial chemoembolization, radiofrequency ablation, and hepatic resection by using inverse probability weighting. Radiology. 2014;271:909–918. doi: 10.1148/radiol.13131760. [DOI] [PubMed] [Google Scholar]
- 518.Kang TW, Kim JM, Rhim H, Lee MW, Kim YS, Lim HK, et al. Small hepatocellular carcinoma: radiofrequency ablation versus nonanatomic resection--propensity score analyses of long-term outcomes. Radiology. 2015;275:908–919. doi: 10.1148/radiol.15141483. [DOI] [PubMed] [Google Scholar]
- 519.Kim GA, Shim JH, Kim MJ, Kim SY, Won HJ, Shin YM, et al. Radiofrequency ablation as an alternative to hepatic resection for single small hepatocellular carcinomas. Br J Surg. 2016;103:126–135. doi: 10.1002/bjs.9960. [DOI] [PubMed] [Google Scholar]
- 520.Qi X, Tang Y, An D, Bai M, Shi X, Wang J, et al. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: a meta-analysis of randomized controlled trials. J Clin Gastroenterol. 2014;48:450–457. doi: 10.1097/MCG.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 521.Imai K, Yamashita YI, Yusa T, Nakao Y, Itoyama R, Nakagawa S, et al. Microvascular invasion in small-sized hepatocellular carcinoma: significance for outcomes following hepatectomy and radiofrequency ablation. Anticancer Res. 2018;38:1053–1060. doi: 10.21873/anticanres.12322. [DOI] [PubMed] [Google Scholar]
- 522.Bai S, Yang P, Xie Z, Li J, Lei Z, Xia Y, et al. Preoperative estimated risk of microvascular invasion is associated with prognostic differences following liver resection versus radiofrequency ablation for early hepatitis B virus-related hepatocellular carcinoma. Ann Surg Oncol. 2021;28:8174–8185. doi: 10.1245/s10434-021-09901-3. [DOI] [PubMed] [Google Scholar]
- 523.Shibata T, Isoda H, Hirokawa Y, Arizono S, Shimada K, Togashi K. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology. 2009;252:905–913. doi: 10.1148/radiol.2523081676. [DOI] [PubMed] [Google Scholar]
- 524.Morimoto M, Numata K, Kondou M, Nozaki A, Morita S, Tanaka K. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer. 2010;116:5452–5460. doi: 10.1002/cncr.25314. [DOI] [PubMed] [Google Scholar]
- 525.Peng ZW, Zhang YJ, Chen MS, Xu L, Liang HH, Lin XJ, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31:426–432. doi: 10.1200/JCO.2012.42.9936. [DOI] [PubMed] [Google Scholar]
- 526.Lu Z, Wen F, Guo Q, Liang H, Mao X, Sun H. Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: a meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2013;25:187–194. doi: 10.1097/MEG.0b013e32835a0a07. [DOI] [PubMed] [Google Scholar]
- 527.Wang X, Hu Y, Ren M, Lu X, Lu G, He S. Efficacy and safety of radiofrequency ablation combined with transcatheter arterial chemoembolization for hepatocellular carcinomas compared with radiofrequency ablation alone: a time-to-event meta-analysis. Korean J Radiol. 2016;17:93–102. doi: 10.3348/kjr.2016.17.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Ni JY, Liu SS, Xu LF, Sun HL, Chen YT. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2013;19:3872–3882. doi: 10.3748/wjg.v19.i24.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.de Baère T, Risse O, Kuoch V, Dromain C, Sengel C, Smayra T, et al. Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR Am J Roentgenol. 2003;181:695–700. doi: 10.2214/ajr.181.3.1810695. [DOI] [PubMed] [Google Scholar]
- 530.Rhim H, Yoon KH, Lee JM, Cho Y, Cho JS, Kim SH, et al. Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiographics. 2003;23:123–134. doi: 10.1148/rg.231025054. discussion 134-136. [DOI] [PubMed] [Google Scholar]
- 531.Song I, Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: safety and technical efficacy in 143 patients. Eur Radiol. 2009;19:2630–2640. doi: 10.1007/s00330-009-1463-x. [DOI] [PubMed] [Google Scholar]
- 532.Ahn SJ, Lee JM, Lee DH, Lee SM, Yoon JH, Kim YJ, et al. Real-time US-CT/MR fusion imaging for percutaneous radiofrequency ablation of hepatocellular carcinoma. J Hepatol. 2017;66:347–354. doi: 10.1016/j.jhep.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 533.Calandri M, Mauri G, Yevich S, Gazzera C, Basile D, Gatti M, et al. Fusion imaging and virtual navigation to guide percutaneous thermal ablation of hepatocellular carcinoma: a review of the literature. Cardiovasc Intervent Radiol. 2019;42:639–647. doi: 10.1007/s00270-019-02167-z. [DOI] [PubMed] [Google Scholar]
- 534.Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005;54:1151–1156. doi: 10.1136/gut.2004.045203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Ishii H, Okada S, Nose H, Okusaka T, Yoshimori M, Takayama T, et al. Local recurrence of hepatocellular carcinoma after percutaneous ethanol injection. Cancer. 1996;77:1792–1796. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1792::AID-CNCR6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 536.Vilana R, Bruix J, Bru C, Ayuso C, Solé M, Rodés J. Tumor size determines the efficacy of percutaneous ethanol injection for the treatment of small hepatocellular carcinoma. Hepatology. 1992;16:353–357. doi: 10.1002/hep.1840160212. [DOI] [PubMed] [Google Scholar]
- 537.Livraghi T, Bolondi L, Lazzaroni S, Marin G, Morabito A, Rapaccini GL, et al. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma in cirrhosis. A study on 207 patients. Cancer. 1992;69:925–929. doi: 10.1002/1097-0142(19920215)69:4<925::aid-cncr2820690415>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 538.Khan KN, Yatsuhashi H, Yamasaki K, Yamasaki M, Inoue O, Koga M, et al. Prospective analysis of risk factors for early intrahepatic recurrence of hepatocellular carcinoma following ethanol injection. J Hepatol. 2000;32:269–278. doi: 10.1016/s0168-8278(00)80072-0. [DOI] [PubMed] [Google Scholar]
- 539.Brunello F, Veltri A, Carucci P, Pagano E, Ciccone G, Moretto P, et al. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: a randomized controlled trial. Scand J Gastroenterol. 2008;43:727–735. doi: 10.1080/00365520701885481. [DOI] [PubMed] [Google Scholar]
- 540.Giorgio A, Di Sarno A, De Stefano G, Scognamiglio U, Farella N, Mariniello A, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma compared to percutaneous ethanol injection in treatment of cirrhotic patients: an Italian randomized controlled trial. Anticancer Research. 2011;31:2291–2295. [PubMed] [Google Scholar]
- 541.Cho YK, Kim JK, Kim MY, Rhim H, Han JK. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology. 2009;49:453–459. doi: 10.1002/hep.22648. [DOI] [PubMed] [Google Scholar]
- 542.Shen A, Zhang H, Tang C, Chen Y, Wang Y, Zhang C, et al. Systematic review of radiofrequency ablation versus percutaneous ethanol injection for small hepatocellular carcinoma up to 3 cm. J Gastroenterol Hepatol. 2013;28:793–800. doi: 10.1111/jgh.12162. [DOI] [PubMed] [Google Scholar]
- 543.Yang B, Zan RY, Wang SY, Li XL, Wei ML, Guo WH, et al. Radiofrequency ablation versus percutaneous ethanol injection for hepatocellular carcinoma: a meta-analysis of randomized controlled trials. World J Surg Oncol. 2015;13:96. doi: 10.1186/s12957-015-0516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Luo W, Zhang Y, He G, Yu M, Zheng M, Liu L, et al. Effects of radiofrequency ablation versus other ablating techniques on hepatocellular carcinomas: a systematic review and meta-analysis. World J Surg Oncol. 2017;15:126. doi: 10.1186/s12957-017-1196-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Ebara M, Okabe S, Kita K, Sugiura N, Fukuda H, Yoshikawa M, et al. Percutaneous ethanol injection for small hepatocellular carcinoma: therapeutic efficacy based on 20-year observation. J Hepatol. 2005;43:458–464. doi: 10.1016/j.jhep.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 546.Cha DI, Lee MW, Rhim H, Choi D, Kim YS, Lim HK. Therapeutic efficacy and safety of percutaneous ethanol injection with or without combined radiofrequency ablation for hepatocellular carcinomas in high risk locations. Korean J Radiol. 2013;14:240–247. doi: 10.3348/kjr.2013.14.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Lencioni R, Llovet JM. Percutaneous ethanol injection for hepatocellular carcinoma: alive or dead? J Hepatol. 2005;43:377–380. doi: 10.1016/j.jhep.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 548.Ahmed M, Brace CL, Lee FT, Jr, Goldberg SN. Principles of and advances in percutaneous ablation. Radiology. 2011;258:351–369. doi: 10.1148/radiol.10081634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Shi Y, Zhai B. A recent advance in image-guided locoregional therapy for hepatocellular carcinoma. Gastrointest Tumors. 2016;3:90–102. doi: 10.1159/000445888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Vietti Violi N, Duran R, Guiu B, Cercueil JP, Aubé C, Digklia A, et al. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018;3:317–325. doi: 10.1016/S2468-1253(18)30029-3. [DOI] [PubMed] [Google Scholar]
- 551.Chong CCN, Lee KF, Cheung SYS, Chu CCM, Fong AKW, Wong J, et al. Prospective double-blinded randomized controlled trial of microwave versus radiofrequency ablation for hepatocellular carcinoma (McRFA trial) HPB (Oxford) 2020;22:1121–1127. doi: 10.1016/j.hpb.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 552.Gupta P, Maralakunte M, Kumar-M P, Chandel K, Chaluvashetty SB, Bhujade H, et al. Overall survival and local recurrence following RFA, MWA, and cryoablation of very early and early HCC: a systematic review and Bayesian network meta-analysis. Eur Radiol. 2021;31:5400–5408. doi: 10.1007/s00330-020-07610-1. [DOI] [PubMed] [Google Scholar]
- 553.Tan W, Deng Q, Lin S, Wang Y, Xu G. Comparison of microwave ablation and radiofrequency ablation for hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2019;36:264–272. doi: 10.1080/02656736.2018.1562571. [DOI] [PubMed] [Google Scholar]
- 554.Yu Q, Liu C, Navuluri R, Ahmed O. Percutaneous microwave ablation versus radiofrequency ablation of hepatocellular carcinoma: a meta-analysis of randomized controlled trials. Abdom Radiol (NY) 2021;46:4467–4475. doi: 10.1007/s00261-021-03080-1. [DOI] [PubMed] [Google Scholar]
- 555.Zaitoun MMA, Elsayed SB, Zaitoun NA, Soliman RK, Elmokadem AH, Farag AA, et al. Combined therapy with conventional trans-arterial chemoembolization (cTACE) and microwave ablation (MWA) for hepatocellular carcinoma>3-<5 cm. Int J Hyperthermia. 2021;38:248–256. doi: 10.1080/02656736.2021.1887941. [DOI] [PubMed] [Google Scholar]
- 556.Wang C, Wang H, Yang W, Hu K, Xie H, Hu KQ, et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology. 2015;61:1579–1590. doi: 10.1002/hep.27548. [DOI] [PubMed] [Google Scholar]
- 557.Kim R, Kang TW, Cha DI, Song KD, Lee MW, Rhim H, et al. Percutaneous cryoablation for perivascular hepatocellular carcinoma: therapeutic efficacy and vascular complications. Eur Radiol. 2019;29:654–662. doi: 10.1007/s00330-018-5617-6. [DOI] [PubMed] [Google Scholar]
- 558.Ko SE, Lee MW, Rhim H, Kang TW, Song KD, Cha DI, et al. Comparison of procedure-related complications between percutaneous cryoablation and radiofrequency ablation for treating periductal hepatocellular carcinoma. Int J Hyperthermia. 2020;37:1354–1361. doi: 10.1080/02656736.2020.1849824. [DOI] [PubMed] [Google Scholar]
- 559.Zhou Y, Zhao Y, Li B, Xu D, Yin Z, Xie F, et al. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol. 2010;10:78. doi: 10.1186/1471-230X-10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Lee DH, Lee JM, Lee JY, Kim SH, Yoon JH, Kim YJ, et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology. 2014;270:900–909. doi: 10.1148/radiol.13130940. [DOI] [PubMed] [Google Scholar]
- 561.Rossi S, Ravetta V, Rosa L, Ghittoni G, Viera FT, Garbagnati F, et al. Repeated radiofrequency ablation for management of patients with cirrhosis with small hepatocellular carcinomas: a long-term cohort study. Hepatology. 2011;53:136–147. doi: 10.1002/hep.23965. [DOI] [PubMed] [Google Scholar]
- 562.Imai K, Beppu T, Chikamoto A, Mima K, Okabe H, Hayashi H, et al. Salvage treatment for local recurrence of hepatocellular carcinoma after local ablation therapy. Hepatol Res. 2014;44:E335–E345. doi: 10.1111/hepr.12313. [DOI] [PubMed] [Google Scholar]
- 563.Xie X, Jiang C, Peng Z, Liu B, Hu W, Wang Y, et al. Local recurrence after radiofrequency ablation of hepatocellular carcinoma: treatment choice and outcome. J Gastrointest Surg. 2015;19:1466–1475. doi: 10.1007/s11605-015-2850-z. [DOI] [PubMed] [Google Scholar]
- 564.Okuwaki Y, Nakazawa T, Kokubu S, Hidaka H, Tanaka Y, Takada J, et al. Repeat radiofrequency ablation provides survival benefit in patients with intrahepatic distant recurrence of hepatocellular carcinoma. Am J Gastroenterol. 2009;104:2747–2753. doi: 10.1038/ajg.2009.414. [DOI] [PubMed] [Google Scholar]
- 565.Sotiropoulos GC, Lang H, Frilling A, Molmenti EP, Paul A, Nadalin S, et al. Resectability of hepatocellular carcinoma: evaluation of 333 consecutive cases at a single hepatobiliary specialty center and systematic review of the literature. Hepatogastroenterology. 2006;53:322–329. [PubMed] [Google Scholar]
- 566.Kwak HW, Park JW, Nam BH, Yu A, Woo SM, Kim TH, et al. Clinical outcomes of a cohort series of patients with hepatocellular carcinoma in a hepatitis B virus-endemic area. J Gastroenterol Hepatol. 2014;29:820–829. doi: 10.1111/jgh.12470. [DOI] [PubMed] [Google Scholar]
- 567.Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int. 2015;35:2155–2166. doi: 10.1111/liv.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Matsui O, Kadoya M, Yoshikawa J, Gabata T, Arai K, Demachi H, et al. Small hepatocellular carcinoma: treatment with subsegmental transcatheter arterial embolization. Radiology. 1993;188:79–83. doi: 10.1148/radiology.188.1.8390073. [DOI] [PubMed] [Google Scholar]
- 569.Miyayama S, Matsui O, Yamashiro M, Ryu Y, Kaito K, Ozaki K, et al. Ultraselective transcatheter arterial chemoembolization with a 2-F tip microcatheter for small hepatocellular carcinomas: relationship between local tumor recurrence and visualization of the portal vein with iodized oil. J Vasc Interv Radiol. 2007;18:365–376. doi: 10.1016/j.jvir.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 570.Golfieri R, Cappelli A, Cucchetti A, Piscaglia F, Carpenzano M, Peri E, et al. Efficacy of selective transarterial chemoembolization in inducing tumor necrosis in small (<5 cm) hepatocellular carcinomas. Hepatology. 2011;53:1580–1589. doi: 10.1002/hep.24246. [DOI] [PubMed] [Google Scholar]
- 571.Yamakado K, Miyayama S, Hirota S, Mizunuma K, Nakamura K, Inaba Y, et al. Hepatic arterial embolization for unresectable hepatocellular carcinomas: do technical factors affect prognosis? Jpn J Radiol. 2012;30:560–566. doi: 10.1007/s11604-012-0088-1. [DOI] [PubMed] [Google Scholar]
- 572.Golfieri R, Renzulli M, Mosconi C, Forlani L, Giampalma E, Piscaglia F, et al. Hepatocellular carcinoma responding to superselective transarterial chemoembolization: an issue of nodule dimension? J Vasc Interv Radiol. 2013;24:509–517. doi: 10.1016/j.jvir.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 573.Iwazawa J, Ohue S, Hashimoto N, Muramoto O, Mitani T. Survival after C-arm CT-assisted chemoembolization of unresectable hepatocellular carcinoma. Eur J Radiol. 2012;81:3985–3992. doi: 10.1016/j.ejrad.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 574.Miyayama S, Yamashiro M, Hashimoto M, Hashimoto N, Ikuno M, Okumura K, et al. Comparison of local control in transcatheter arterial chemoembolization of hepatocellular carcinoma ≤6 cm with or without intraprocedural monitoring of the embolized area using cone-beam computed tomography. Cardiovasc Intervent Radiol. 2014;37:388–395. doi: 10.1007/s00270-013-0667-2. [DOI] [PubMed] [Google Scholar]
- 575.Pung L, Ahmad M, Mueller K, Rosenberg J, Stave C, Hwang GL, et al. The role of cone-beam CT in transcatheter arterial chemoembolization for hepatocellular carcinoma: a systematic review and meta-analysis. J Vasc Interv Radiol. 2017;28:334–341. doi: 10.1016/j.jvir.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 576.Wattanasatesiri T, Kim HC, Choi JW, Lee JH, Joo I, Hur S, et al. Cone-beam CT-guided chemoembolization in patients with complete response after previous chemoembolization but subsequent elevated α-fetoprotein without overt hepatocellular carcinoma. J Vasc Interv Radiol. 2019;30:1273–1280. doi: 10.1016/j.jvir.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 577.Young S, Craig P, Golzarian J. Current trends in the treatment of hepatocellular carcinoma with transarterial embolization: a cross-sectional survey of techniques. Eur Radiol. 2019;29:3287–3295. doi: 10.1007/s00330-018-5782-7. [DOI] [PubMed] [Google Scholar]
- 578.Ikeda M, Kudo M, Aikata H, Nagamatsu H, Ishii H, Yokosuka O, et al. Transarterial chemoembolization with miriplatin vs. epirubicin for unresectable hepatocellular carcinoma: a phase III randomized trial. J Gastroenterol. 2018;53:281–290. doi: 10.1007/s00535-017-1374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 580.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 581.Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461–469. doi: 10.1053/j.gastro.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 582.Takayasu K, Arii S, Kudo M, Ichida T, Matsui O, Izumi N, et al. Superselective transarterial chemoembolization for hepatocellular carcinoma. Validation of treatment algorithm proposed by Japanese guidelines. J Hepatol. 2012;56:886–892. doi: 10.1016/j.jhep.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 583.Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016;64:106–116. doi: 10.1002/hep.28453. [DOI] [PubMed] [Google Scholar]
- 584.Ikeda M, Arai Y, Park SJ, Takeuchi Y, Anai H, Kim JK, et al. Prospective study of transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: an Asian cooperative study between Japan and Korea. J Vasc Interv Radiol. 2013;24:490–500. doi: 10.1016/j.jvir.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 585.Lee HS, Kim KM, Yoon JH, Lee TR, Suh KS, Lee KU, et al. Therapeutic efficacy of transcatheter arterial chemoembolization as compared with hepatic resection in hepatocellular carcinoma patients with compensated liver function in a hepatitis B virus-endemic area: a prospective cohort study. J Clin Oncol. 2002;20:4459–4465. doi: 10.1200/JCO.2002.02.013. [DOI] [PubMed] [Google Scholar]
- 586.Bargellini I, Sacco R, Bozzi E, Bertini M, Ginanni B, Romano A, et al. Transarterial chemoembolization in very early and early-stage hepatocellular carcinoma patients excluded from curative treatment: a prospective cohort study. Eur J Radiol. 2012;81:1173–1178. doi: 10.1016/j.ejrad.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 587.Oh JH, Sinn DH, Choi GS, Kim JM, Joh JW, Kang TW, et al. Comparison of outcome between liver resection, radiofrequency ablation, and transarterial therapy for multiple small hepatocellular carcinoma within the Milan criteria. Ann Surg Treat Res. 2020;99:238–246. doi: 10.4174/astr.2020.99.4.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Yun BY, Lee HW, Min IK, Kim SU, Park JY, Kim DY, et al. Prognosis of early-stage hepatocellular carcinoma: comparison between trans-arterial chemoembolization and radiofrequency ablation. Cancers (Basel) 2020;12:2527. doi: 10.3390/cancers12092527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Sinn DH, Lee HW, Paik YH, Kim DY, Kim YJ, Kim KM, et al. Patterns and outcomes in hepatocellular carcinoma patients with portal vein invasion: a multicenter prospective cohort study. Dig Dis Sci. 2021;66:315–324. doi: 10.1007/s10620-020-06134-4. [DOI] [PubMed] [Google Scholar]
- 590.Chung JW, Park JH, Han JK, Choi BI, Han MC. Hepatocellular carcinoma and portal vein invasion: results of treatment with transcatheter oily chemoembolization. AJR Am J Roentgenol. 1995;165:315–321. doi: 10.2214/ajr.165.2.7618547. [DOI] [PubMed] [Google Scholar]
- 591.Lee HS, Kim JS, Choi IJ, Chung JW, Park JH, Kim CY. The safety and efficacy of transcatheter arterial chemoembolization in the treatment of patients with hepatocellular carcinoma and main portal vein obstruction. A prospective controlled study. Cancer. 1997;79:2087–2094. [PubMed] [Google Scholar]
- 592.Georgiades CS, Hong K, D’Angelo M, Geschwind JF. Safety and efficacy of transarterial chemoembolization in patients with unresectable hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol. 2005;16:1653–1659. doi: 10.1097/01.RVI.0000182185.47500.7A. [DOI] [PubMed] [Google Scholar]
- 593.Chung GE, Lee JH, Kim HY, Hwang SY, Kim JS, Chung JW, et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology. 2011;258:627–634. doi: 10.1148/radiol.10101058. [DOI] [PubMed] [Google Scholar]
- 594.Silva JP, Berger NG, Tsai S, Christians KK, Clarke CN, Mogal H, et al. Transarterial chemoembolization in hepatocellular carcinoma with portal vein tumor thrombosis: a systematic review and meta-analysis. HPB (Oxford) 2017;19:659–666. doi: 10.1016/j.hpb.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 595.Luo J, Guo RP, Lai EC, Zhang YJ, Lau WY, Chen MS, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011;18:413–420. doi: 10.1245/s10434-010-1321-8. [DOI] [PubMed] [Google Scholar]
- 596.Niu ZJ, Ma YL, Kang P, Ou SQ, Meng ZB, Li ZK, et al. Transarterial chemoembolization compared with conservative treatment for advanced hepatocellular carcinoma with portal vein tumor thrombus: using a new classification. Med Oncol. 2012;29:2992–2997. doi: 10.1007/s12032-011-0145-0. [DOI] [PubMed] [Google Scholar]
- 597.Xue TC, Xie XY, Zhang L, Yin X, Zhang BH, Ren ZG. Transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: a meta-analysis. BMC Gastroenterol. 2013;13:60. doi: 10.1186/1471-230X-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Choi JW, Kim HC, Lee JH, Yu SJ, Kim YJ, Yoon JH, et al. Transarterial chemoembolization of hepatocellular carcinoma with segmental portal vein tumour thrombus. Eur Radiol. 2017;27:1448–1458. doi: 10.1007/s00330-016-4511-3. [DOI] [PubMed] [Google Scholar]
- 599.Cho JY, Paik YH, Park HC, Yu JI, Sohn W, Gwak GY, et al. The feasibility of combined transcatheter arterial chemoembolization and radiotherapy for advanced hepatocellular carcinoma. Liver Int. 2014;34:795–801. doi: 10.1111/liv.12445. [DOI] [PubMed] [Google Scholar]
- 600.Kim GA, Shim JH, Yoon SM, Jung J, Kim JH, Ryu MH, et al. Comparison of chemoembolization with and without radiation therapy and sorafenib for advanced hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. J Vasc Interv Radiol. 2015;26:320–329.e6. doi: 10.1016/j.jvir.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 601.Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH, An JH, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol. 2018;4:661–669. doi: 10.1001/jamaoncol.2017.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Yoo DJ, Kim KM, Jin YJ, Shim JH, Ko GY, Yoon HK, et al. Clinical outcome of 251 patients with extrahepatic metastasis at initial diagnosis of hepatocellular carcinoma: does transarterial chemoembolization improve survival in these patients? J Gastroenterol Hepatol. 2011;26:145–154. doi: 10.1111/j.1440-1746.2010.06341.x. [DOI] [PubMed] [Google Scholar]
- 603.Jung SM, Jang JW, You CR, Yoo SH, Kwon JH, Bae SH, et al. Role of intrahepatic tumor control in the prognosis of patients with hepatocellular carcinoma and extrahepatic metastases. J Gastroenterol Hepatol. 2012;27:684–689. doi: 10.1111/j.1440-1746.2011.06917.x. [DOI] [PubMed] [Google Scholar]
- 604.Kim J, Sinn DH, Choi MS, Kang W, Gwak GY, Paik YH, et al. Hepatocellular carcinoma with extrahepatic metastasis: are there still candidates for transarterial chemoembolization as an initial treatment? PLoS One. 2019;14:e0213547. doi: 10.1371/journal.pone.0213547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Dai Y, Jiang H, Jiang H, Zhao S, Zeng X, Sun R, et al. Optimal timing of combining sorafenib with trans-arterial chemoembolization in patients with hepatocellular carcinoma: a meta-analysis. Transl Oncol. 2021;14:101238. doi: 10.1016/j.tranon.2021.101238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69:1492–1501. doi: 10.1136/gutjnl-2019-318934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Fu Z, Li X, Zhong J, Chen X, Cao K, Ding N, et al. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. Hepatol Int. 2021;15:663–675. doi: 10.1007/s12072-021-10184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Chung JW, Park JH, Han JK, Choi BI, Han MC, Lee HS, et al. Hepatic tumors: predisposing factors for complications of transcatheter oily chemoembolization. Radiology. 1996;198:33–40. doi: 10.1148/radiology.198.1.8539401. [DOI] [PubMed] [Google Scholar]
- 609.Ogasawara S, Chiba T, Ooka Y, Kanogawa N, Motoyama T, Suzuki E, et al. A randomized placebo-controlled trial of prophylactic dexamethasone for transcatheter arterial chemoembolization. Hepatology. 2018;67:575–585. doi: 10.1002/hep.29403. [DOI] [PubMed] [Google Scholar]
- 610.Yang H, Seon J, Sung PS, Oh JS, Lee HL, Jang B, et al. Dexamethasone prophylaxis to alleviate postembolization syndrome after transarterial chemoembolization for hepatocellular carcinoma: a randomized, double-blinded, placebo-controlled study. J Vasc Interv Radiol. 2017;28:1503–1511.e2. doi: 10.1016/j.jvir.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 611.Lv N, Kong Y, Mu L, Pan T, Xie Q, Zhao M. Effect of perioperative parecoxib sodium on postoperative pain control for transcatheter arterial chemoembolization for inoperable hepatocellular carcinoma: a prospective randomized trial. Eur Radiol. 2016;26:3492–3499. doi: 10.1007/s00330-016-4207-8. [DOI] [PubMed] [Google Scholar]
- 612.Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474–481. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 613.Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Sacco R, Bargellini I, Bertini M, Bozzi E, Romano A, Petruzzi P, et al. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2011;22:1545–1552. doi: 10.1016/j.jvir.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 615.Golfieri R, Giampalma E, Renzulli M, Cioni R, Bargellini I, Bartolozzi C, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111:255–264. doi: 10.1038/bjc.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Malagari K, Pomoni M, Moschouris H, Bouma E, Koskinas J, Stefaniotou A, et al. Chemoembolization with doxorubicin-eluting beads for unresectable hepatocellular carcinoma: five-year survival analysis. Cardiovasc Intervent Radiol. 2012;35:1119–1128. doi: 10.1007/s00270-012-0394-0. [DOI] [PubMed] [Google Scholar]
- 617.Lee M, Chung JW, Lee KH, Won JY, Chun HJ, Lee HC, et al. Korean multicenter registry of transcatheter arterial chemoembolization with drug-eluting embolic agents for nodular hepatocellular carcinomas: six-month outcome analysis. J Vasc Interv Radiol. 2017;28:502–512. doi: 10.1016/j.jvir.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 618.Lee M, Chung JW, Lee KH, Won JY, Chun HJ, Lee HC, et al. Prospective multi-center Korean registry of transcatheter arterial chemoembolization with drug-eluting embolics for nodular hepatocellular carcinoma: a two-year outcome analysis. Korean J Radiol. 2021;22:1658–1670. doi: 10.3348/kjr.2020.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Lee IJ, Lee JH, Lee YB, Kim YJ, Yoon JH, Yin YH, et al. Effectiveness of drug-eluting bead transarterial chemoembolization versus conventional transarterial chemoembolization for small hepatocellular carcinoma in Child-Pugh class A patients. Ther Adv Med Oncol. 2019;11:1758835919866072. doi: 10.1177/1758835919866072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Deipolyi AR, Oklu R, Al-Ansari S, Zhu AX, Goyal L, Ganguli S. Safety and efficacy of 70-150 µm and 100-300 µm drug-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2015;26:516–522. doi: 10.1016/j.jvir.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 621.Urbano J, Echevarria-Uraga JJ, Ciampi-Dopazo JJ, Sánchez-Corral JA, Cobos Alonso J, Anton-Ladislao A, et al. Multicentre prospective study of drug-eluting bead chemoembolisation safety using tightly calibrated small microspheres in non-resectable hepatocellular carcinoma. Eur J Radiol. 2020;126:108966. doi: 10.1016/j.ejrad.2020.108966. [DOI] [PubMed] [Google Scholar]
- 622.Vogl TJ, Lammer J, Lencioni R, Malagari K, Watkinson A, Pilleul F, et al. Liver, gastrointestinal, and cardiac toxicity in intermediate hepatocellular carcinoma treated with PRECISION TACE with drug-eluting beads: results from the PRECISION V randomized trial. AJR Am J Roentgenol. 2011;197:W562–W570. doi: 10.2214/AJR.10.4379. [DOI] [PubMed] [Google Scholar]
- 623.Monier A, Guiu B, Duran R, Aho S, Bize P, Deltenre P, et al. Liver and biliary damages following transarterial chemoembolization of hepatocellular carcinoma: comparison between drug-eluting beads and lipiodol emulsion. Eur Radiol. 2017;27:1431–1439. doi: 10.1007/s00330-016-4488-y. [DOI] [PubMed] [Google Scholar]
- 624.Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: technical and methodologic considerations. J Vasc Interv Radiol. 2006;17:1251–1278. doi: 10.1097/01.RVI.0000233785.75257.9A. [DOI] [PubMed] [Google Scholar]
- 625.Gabr A, Ranganathan S, Mouli SK, Riaz A, Gates VL, Kulik L, et al. Streamlining radioembolization in UNOS T1/T2 hepatocellular carcinoma by eliminating lung shunt estimation. J Hepatol. 2020;72:1151–1158. doi: 10.1016/j.jhep.2020.02.024. [DOI] [PubMed] [Google Scholar]
- 626.Mazzaferro V, Sposito C, Bhoori S, Romito R, Chiesa C, Morosi C, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013;57:1826–1837. doi: 10.1002/hep.26014. [DOI] [PubMed] [Google Scholar]
- 627.Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 628.Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54:868–878. doi: 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]
- 629.Hilgard P, Hamami M, Fouly AE, Scherag A, Müller S, Ertle J, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology. 2010;52:1741–1749. doi: 10.1002/hep.23944. [DOI] [PubMed] [Google Scholar]
- 630.Kolligs FT, Bilbao JI, Jakobs T, Iñarrairaegui M, Nagel JM, Rodriguez M, et al. Pilot randomized trial of selective internal radiation therapy vs. chemoembolization in unresectable hepatocellular carcinoma. Liver Int. 2015;35:1715–1721. doi: 10.1111/liv.12750. [DOI] [PubMed] [Google Scholar]
- 631.Pitton MB, Kloeckner R, Ruckes C, Wirth GM, Eichhorn W, Wörns MA, et al. Randomized comparison of selective internal radiotherapy (SIRT) versus drug-eluting bead transarterial chemoembolization (DEB-TACE) for the treatment of hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2015;38:352–360. doi: 10.1007/s00270-014-1012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 632.Salem R, Gordon AC, Mouli S, Hickey R, Kallini J, Gabr A, et al. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2016;151:1155–1163.e2. doi: 10.1053/j.gastro.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Casadei Gardini A, Tamburini E, Iñarrairaegui M, Frassineti GL, Sangro B. Radioembolization versus chemoembolization for unresectable hepatocellular carcinoma: a meta-analysis of randomized trials. Onco Targets Ther. 2018;11:7315–7321. doi: 10.2147/OTT.S175715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Chow PKH, Gandhi M, Tan SB, Khin MW, Khasbazar A, Ong J, et al. SIRveNIB: selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. J Clin Oncol. 2018;36:1913–1921. doi: 10.1200/JCO.2017.76.0892. [DOI] [PubMed] [Google Scholar]
- 635.Vilgrain V, Pereira H, Assenat E, Guiu B, Ilonca AD, Pageaux GP, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624–1636. doi: 10.1016/S1470-2045(17)30683-6. [DOI] [PubMed] [Google Scholar]
- 636.Ricke J, Klümpen HJ, Amthauer H, Bargellini I, Bartenstein P, de Toni EN, et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol. 2019;71:1164–1174. doi: 10.1016/j.jhep.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 637.Garin E, Lenoir L, Edeline J, Laffont S, Mesbah H, Porée P, et al. Boosted selective internal radiation therapy with 90Y-loaded glass microspheres (B-SIRT) for hepatocellular carcinoma patients: a new personalized promising concept. Eur J Nucl Med Mol Imaging. 2013;40:1057–1068. doi: 10.1007/s00259-013-2395-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Salem R, Johnson GE, Kim E, Riaz A, Bishay V, Boucher E, et al. Yttrium-90 radioembolization for the treatment of solitary, unresectable HCC: the LEGACY study. Hepatology. 2021;74:2342–2352. doi: 10.1002/hep.31819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Garin E, Tselikas L, Guiu B, Chalaye J, Edeline J, de Baere T, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6:17–29. doi: 10.1016/S2468-1253(20)30290-9. [DOI] [PubMed] [Google Scholar]
- 640.Kim HC, Kim YJ, Lee JH, Suh KS, Chung JW. Feasibility of boosted radioembolization for hepatocellular carcinoma larger than 5 cm. J Vasc Interv Radiol. 2019;30:1–8. doi: 10.1016/j.jvir.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 641.Kim J, Kim JY, Lee JH, Sinn DH, Hur MH, Hong JH, et al. Long-term outcomes of transarterial radioembolization for large single hepatocellular carcinoma: a comparison to resection. J Nucl Med. 2022;63:1215–1222. doi: 10.2967/jnumed.121.263147. [DOI] [PubMed] [Google Scholar]
- 642.Benguerfi S, Estrade F, Lescure C, Rolland Y, Palard X, Le Sourd S, et al. Selective internal radiation therapy in older patients with hepatocellular carcinoma: a retrospective analysis. Eur J Gastroenterol Hepatol. 2022;34:417–421. doi: 10.1097/MEG.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 643.Das A, Gabr A, O’Brian DP, Riaz A, Desai K, Thornburg B, et al. Contemporary systematic review of health-related quality of life outcomes in locoregional therapies for hepatocellular carcinoma. J Vasc Interv Radiol. 2019;30:1924–1933.e2. doi: 10.1016/j.jvir.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 644.Sangro B, Gil-Alzugaray B, Rodriguez J, Sola I, Martinez-Cuesta A, Viudez A, et al. Liver disease induced by radioembolization of liver tumors: description and possible risk factors. Cancer. 2008;112:1538–1546. doi: 10.1002/cncr.23339. [DOI] [PubMed] [Google Scholar]
- 645.Gil-Alzugaray B, Chopitea A, Iñarrairaegui M, Bilbao JI, Rodriguez-Fraile M, Rodriguez J, et al. Prognostic factors and prevention of radioembolization-induced liver disease. Hepatology. 2013;57:1078–1087. doi: 10.1002/hep.26191. [DOI] [PubMed] [Google Scholar]
- 646.Sangro B, Martínez-Urbistondo D, Bester L, Bilbao JI, Coldwell DM, Flamen P, et al. Prevention and treatment of complications of selective internal radiation therapy: expert guidance and systematic review. Hepatology. 2017;66:969–982. doi: 10.1002/hep.29207. [DOI] [PubMed] [Google Scholar]
- 647.Currie BM, Hoteit MA, Ben-Josef E, Nadolski GJ, Soulen MC. Radioembolization-induced chronic hepatotoxicity: a single-center cohort analysis. J Vasc Interv Radiol. 2019;30:1915–1923. doi: 10.1016/j.jvir.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 648.Kudo M, Kawamura Y, Hasegawa K, Tateishi R, Kariyama K, Shiina S, et al. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver Cancer. 2021;10:181–223. doi: 10.1159/000514174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.Vogl TJ, Trapp M, Schroeder H, Mack M, Schuster A, Schmitt J, et al. Transarterial chemoembolization for hepatocellular carcinoma: volumetric and morphologic CT criteria for assessment of prognosis and therapeutic success-results from a liver transplantation center. Radiology. 2000;214:349–357. doi: 10.1148/radiology.214.2.r00fe06349. [DOI] [PubMed] [Google Scholar]
- 650.Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010;52:762–773. doi: 10.1002/hep.23725. [DOI] [PubMed] [Google Scholar]
- 651.Yamanaka K, Hatano E, Kitamura K, Iida T, Ishii T, Machimito T, et al. Early evaluation of transcatheter arterial chemoembolization-refractory hepatocellular carcinoma. J Gastroenterol. 2012;47:343–346. doi: 10.1007/s00535-011-0511-x. [DOI] [PubMed] [Google Scholar]
- 652.Park JW, Amarapurkar D, Chao Y, Chen PJ, Geschwind JF, Goh KL, et al. Consensus recommendations and review by an International Expert Panel on Interventions in Hepatocellular Carcinoma (EPOIHCC) Liver Int. 2013;33:327–337. doi: 10.1111/liv.12083. [DOI] [PubMed] [Google Scholar]
- 653.Kim HY, Park JW, Joo J, Jung SJ, An S, Woo SM, et al. Severity and timing of progression predict refractoriness to transarterial chemoembolization in hepatocellular carcinoma. J Gastroenterol Hepatol. 2012;27:1051–1056. doi: 10.1111/j.1440-1746.2011.06963.x. [DOI] [PubMed] [Google Scholar]
- 654.Sieghart W, Hucke F, Pinter M, Graziadei I, Vogel W, Müller C, et al. The ART of decision making: retreatment with transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology. 2013;57:2261–2273. doi: 10.1002/hep.26256. [DOI] [PubMed] [Google Scholar]
- 655.Adhoute X, Penaranda G, Naude S, Raoul JL, Perrier H, Bayle O, et al. Retreatment with TACE: the ABCR SCORE, an aid to the decision-making process. J Hepatol. 2015;62:855–862. doi: 10.1016/j.jhep.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 656.Yoon JS, Sinn DH, Lee JH, Kim HY, Lee CH, Kim SW, et al. Tumor marker-based definition of the transarterial chemoembolization-refractoriness in intermediate-stage hepatocellular carcinoma: a multi-cohort study. Cancers (Basel) 2019;11:1721. doi: 10.3390/cancers11111721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657.Kim BK, Shim JH, Kim SU, Park JY, Kim DY, Ahn SH, et al. Risk prediction for patients with hepatocellular carcinoma undergoing chemoembolization: development of a prediction model. Liver Int. 2016;36:92–99. doi: 10.1111/liv.12865. [DOI] [PubMed] [Google Scholar]
- 658.Kim JH, Sinn DH, Lee JH, Hyun D, Cho SK, Shin SW, et al. Novel albumin-bilirubin grade-based risk prediction model for patients with hepatocellular carcinoma undergoing chemoembolization. Dig Dis Sci. 2018;63:1062–1071. doi: 10.1007/s10620-018-4934-6. [DOI] [PubMed] [Google Scholar]
- 659.Kudo M, Matsui O, Izumi N, Kadoya M, Okusaka T, Miyayama S, et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology. 2014;87 Suppl 1:22–31. doi: 10.1159/000368142. [DOI] [PubMed] [Google Scholar]
- 660.Korean Liver Cancer Association (KLCA); National Cancer Center (NCC), Goyang, Korea. 2018 Korean Liver Cancer Association-National Cancer Center Korea practice guidelines for the management of hepatocellular carcinoma. Korean J Radiol. 2019;20:1042–1113. doi: 10.3348/kjr.2019.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57:821–829. doi: 10.1016/j.jhep.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662.Ogasawara S, Chiba T, Ooka Y, Kanogawa N, Motoyama T, Suzuki E, et al. Efficacy of sorafenib in intermediate-stage hepatocellular carcinoma patients refractory to transarterial chemoembolization. Oncology. 2014;87:330–341. doi: 10.1159/000365993. [DOI] [PubMed] [Google Scholar]
- 663.Arizumi T, Ueshima K, Minami T, Kono M, Chishina H, Takita M, et al. Effectiveness of sorafenib in patients with transcatheter arterial chemoembolization (TACE) refractory and intermediate-stage hepatocellular carcinoma. Liver Cancer. 2015;4:253–262. doi: 10.1159/000367743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664.Takaki H, Yamakado K, Tsurusaki M, Yasumoto T, Baba Y, Nari-matsu Y, et al. Hepatic arterial infusion chemotherapy with fine-powder cisplatin and iodized-oil suspension in patients with intermediate-stage and advanced-stage (Barcelona Clinic Liver Cancer stage-B or stage-C) hepatocellular carcinoma: multicenter phase-II clinical study. Int J Clin Oncol. 2015;20:745–754. doi: 10.1007/s10147-014-0773-4. [DOI] [PubMed] [Google Scholar]
- 665.Lin J, Wu L, Bai X, Xie Y, Wang A, Zhang H, et al. Combination treatment including targeted therapy for advanced hepatocellular carcinoma. Oncotarget. 2016;7:71036–71051. doi: 10.18632/oncotarget.11954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666.Kim HY, Park JW. Clinical trials of combined molecular targeted therapy and locoregional therapy in hepatocellular carcinoma: past, present, and future. Liver Cancer. 2014;3:9–17. doi: 10.1159/000343854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 667.Liu L, Chen H, Wang M, Zhao Y, Cai G, Qi X, et al. Combination therapy of sorafenib and TACE for unresectable HCC: a systematic review and meta-analysis. PLoS One. 2014;9:e91124. doi: 10.1371/journal.pone.0091124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668.Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Guglielmi A, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J Hepatol. 2016;64:1090–1098. doi: 10.1016/j.jhep.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 669.Meyer T, Fox R, Ma YT, Ross PJ, James MW, Sturgess R, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2:565–575. doi: 10.1016/S2468-1253(17)30156-5. [DOI] [PubMed] [Google Scholar]
- 670.Kudo M, Cheng AL, Park JW, Park JH, Liang PC, Hidaka H, et al. Orantinib versus placebo combined with transcatheter arterial chemoembolisation in patients with unresectable hepatocellular carcinoma (ORIENTAL): a randomised, double-blind, placebo-controlled, multicentre, phase 3 study. Lancet Gastroenterol Hepatol. 2018;3:37–46. doi: 10.1016/S2468-1253(17)30290-X. [DOI] [PubMed] [Google Scholar]
- 671.Korean Liver Cancer Study Group (KLCSG); National Cancer Center, Korea (NCC) 2014 Korean Liver Cancer Study Group-National Cancer Center Korea practice guideline for the management of hepatocellular carcinoma. Korean J Radiol. 2015;16:465–522. doi: 10.3348/kjr.2015.16.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672.Korean Liver Cancer Association; National Cancer Center. 2018 Korean Liver Cancer Association-National Cancer Center Korea practice guidelines for the management of hepatocellular carcinoma. Gut Liver. 2019;13:227–299. doi: 10.5009/gnl19024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673.Choi SH, Seong J. Strategic application of radiotherapy for hepatocellular carcinoma. Clin Mol Hepatol. 2018;24:114–134. doi: 10.3350/cmh.2017.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 674.Park HC, Yu JI, Cheng JC, Zeng ZC, Hong JH, Wang ML, et al. Consensus for radiotherapy in hepatocellular carcinoma from the 5th Asia-Pacific Primary Liver Cancer Expert meeting (APPLE 2014): current practice and future clinical trials. Liver Cancer. 2016;5:162–174. doi: 10.1159/000367766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675.Kim TH, Kim DY, Park JW, Kim SH, Choi JI, Kim HB, et al. Dose-volumetric parameters predicting radiation-induced hepatic toxicity in unresectable hepatocellular carcinoma patients treated with three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2007;67:225–231. doi: 10.1016/j.ijrobp.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 676.Schefter TE, Kavanagh BD, Timmerman RD, Cardenes HR, Baron A, Gaspar LE. A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys. 2005;62:1371–1378. doi: 10.1016/j.ijrobp.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 677.Pan CC, Kavanagh BD, Dawson LA, Li XA, Das SK, Miften M, et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys. 2010;76:S94–S100. doi: 10.1016/j.ijrobp.2009.06.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678.Kimura T, Takeda A, Tsurugai Y, Kawano R, Doi Y, Oku Y, et al. A multi-institutional retrospective study of repeated stereotactic body radiation therapy for intrahepatic recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2020;108:1265–1275. doi: 10.1016/j.ijrobp.2020.07.034. [DOI] [PubMed] [Google Scholar]
- 679.McDuff SGR, Remillard KA, Zheng H, Raldow AC, Wo JY, Eyler CE, et al. Liver reirradiation for patients with hepatocellular carcinoma and liver metastasis. Pract Radiat Oncol. 2018;8:414–421. doi: 10.1016/j.prro.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 680.Oshiro Y, Mizumoto M, Okumura T, Fukuda K, Fukumitsu N, Abei M, et al. Analysis of repeated proton beam therapy for patients with hepatocellular carcinoma. Radiother Oncol. 2017;123:240–245. doi: 10.1016/j.radonc.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 681.Bae SH, Kim MS, Cho CK, Kim KB, Lee DH, Han CJ, et al. Feasibility and efficacy of stereotactic ablative radiotherapy for Barcelona Clinic Liver Cancer-C stage hepatocellular carcinoma. J Korean Med Sci. 2013;28:213–219. doi: 10.3346/jkms.2013.28.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682.Andolino DL, Johnson CS, Maluccio M, Kwo P, Tector AJ, Zook J, et al. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:e447–e453. doi: 10.1016/j.ijrobp.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 683.Honda Y, Kimura T, Aikata H, Kobayashi T, Fukuhara T, Masaki K, et al. Stereotactic body radiation therapy combined with transcatheter arterial chemoembolization for small hepatocellular carcinoma. J Gastroenterol Hepatol. 2013;28:530–536. doi: 10.1111/jgh.12087. [DOI] [PubMed] [Google Scholar]
- 684.Sanuki N, Takeda A, Oku Y, Mizuno T, Aoki Y, Eriguchi T, et al. Stereotactic body radiotherapy for small hepatocellular carcinoma: a retrospective outcome analysis in 185 patients. Acta Oncol. 2014;53:399–404. doi: 10.3109/0284186X.2013.820342. [DOI] [PubMed] [Google Scholar]
- 685.Yoon SM, Lim YS, Park MJ, Kim SY, Cho B, Shim JH, et al. Stereotactic body radiation therapy as an alternative treatment for small hepatocellular carcinoma. PLoS One. 2013;8:e79854. doi: 10.1371/journal.pone.0079854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 686.Xi M, Zhang L, Zhao L, Li QQ, Guo SP, Feng ZZ, et al. Effectiveness of stereotactic body radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombosis. PLoS One. 2013;8:e63864. doi: 10.1371/journal.pone.0063864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 687.Weiner AA, Olsen J, Ma D, Dyk P, DeWees T, Myerson RJ, et al. Stereotactic body radiotherapy for primary hepatic malignancies - report of a phase I/II institutional study. Radiother Oncol. 2016;121:79–85. doi: 10.1016/j.radonc.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 688.Takeda A, Sanuki N, Tsurugai Y, Iwabuchi S, Matsunaga K, Ebinuma H, et al. Phase 2 study of stereotactic body radiotherapy and optional transarterial chemoembolization for solitary hepatocellular carcinoma not amenable to resection and radiofrequency ablation. Cancer. 2016;122:2041–2049. doi: 10.1002/cncr.30008. [DOI] [PubMed] [Google Scholar]
- 689.Wahl DR, Stenmark MH, Tao Y, Pollom EL, Caoili EM, Lawrence TS, et al. Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma. J Clin Oncol. 2016;34:452–459. doi: 10.1200/JCO.2015.61.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 690.Kawashima M, Furuse J, Nishio T, Konishi M, Ishii H, Kinoshita T, et al. Phase II study of radiotherapy employing proton beam for hepatocellular carcinoma. J Clin Oncol. 2005;23:1839–1846. doi: 10.1200/JCO.2005.00.620. [DOI] [PubMed] [Google Scholar]
- 691.Fukumitsu N, Sugahara S, Nakayama H, Fukuda K, Mizumoto M, Abei M, et al. A prospective study of hypofractionated proton beam therapy for patients with hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2009;74:831–836. doi: 10.1016/j.ijrobp.2008.10.073. [DOI] [PubMed] [Google Scholar]
- 692.Sugahara S, Nakayama H, Fukuda K, Mizumoto M, Tokita M, Abei M, et al. Proton-beam therapy for hepatocellular carcinoma associated with portal vein tumor thrombosis. Strahlenther Onkol. 2009;185:782–788. doi: 10.1007/s00066-009-2020-x. [DOI] [PubMed] [Google Scholar]
- 693.Nakayama H, Sugahara S, Tokita M, Fukuda K, Mizumoto M, Abei M, et al. Proton beam therapy for hepatocellular carcinoma: the University of Tsukuba experience. Cancer. 2009;115:5499–5506. doi: 10.1002/cncr.24619. [DOI] [PubMed] [Google Scholar]
- 694.Komatsu S, Fukumoto T, Demizu Y, Miyawaki D, Terashima K, Sasaki R, et al. Clinical results and risk factors of proton and carbon ion therapy for hepatocellular carcinoma. Cancer. 2011;117:4890–4904. doi: 10.1002/cncr.26134. [DOI] [PubMed] [Google Scholar]
- 695.Bush DA, Kayali Z, Grove R, Slater JD. The safety and efficacy of high-dose proton beam radiotherapy for hepatocellular carcinoma: a phase 2 prospective trial. Cancer. 2011;117:3053–3059. doi: 10.1002/cncr.25809. [DOI] [PubMed] [Google Scholar]
- 696.Kim TH, Park JW, Kim YJ, Kim BH, Woo SM, Moon SH, et al. Phase I dose-escalation study of proton beam therapy for inoperable hepatocellular carcinoma. Cancer Res Treat. 2015;47:34–45. doi: 10.4143/crt.2013.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 697.Hong TS, Wo JY, Yeap BY, Ben-Josef E, McDonnell EI, Blasz-kowsky LS, et al. Multi-institutional phase II study of high-dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2016;34:460–468. doi: 10.1200/JCO.2015.64.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 698.Bush DA, Smith JC, Slater JD, Volk ML, Reeves ME, Cheng J, et al. Randomized clinical trial comparing proton beam radiation therapy with transarterial chemoembolization for hepatocellular carcinoma: results of an interim analysis. Int J Radiat Oncol Biol Phys. 2016;95:477–482. doi: 10.1016/j.ijrobp.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 699.Kasuya G, Kato H, Yasuda S, Tsuji H, Yamada S, Haruyama Y, et al. Progressive hypofractionated carbon-ion radiotherapy for hepatocellular carcinoma: combined analyses of 2 prospective trials. Cancer. 2017;123:3955–3965. doi: 10.1002/cncr.30816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 700.Fukuda K, Okumura T, Abei M, Fukumitsu N, Ishige K, Mizumoto M, et al. Long-term outcomes of proton beam therapy in patients with previously untreated hepatocellular carcinoma. Cancer Sci. 2017;108:497–503. doi: 10.1111/cas.13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 701.Feng M, Suresh K, Schipper MJ, Bazzi L, Ben-Josef E, Matuszak MM, et al. Individualized adaptive stereotactic body radiotherapy for liver tumors in patients at high risk for liver damage: a phase 2 clinical trial. JAMA Oncol. 2018;4:40–47. doi: 10.1001/jamaoncol.2017.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 702.Kim JW, Kim DY, Han KH, Seong J. Phase I/II trial of helical IMRT-based stereotactic body radiotherapy for hepatocellular carcinoma. Dig Liver Dis. 2019;51:445–451. doi: 10.1016/j.dld.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 703.Jang WI, Bae SH, Kim MS, Han CJ, Park SC, Kim SB, et al. A phase 2 multicenter study of stereotactic body radiotherapy for hepatocellular carcinoma: safety and efficacy. Cancer. 2020;126:363–372. doi: 10.1002/cncr.32502. [DOI] [PubMed] [Google Scholar]
- 704.Durand-Labrunie J, Baumann AS, Ayav A, Laurent V, Boleslawski E, Cattan S, et al. Curative irradiation treatment of hepatocellular carcinoma: a multicenter phase 2 trial. Int J Radiat Oncol Biol Phys. 2020;107:116–125. doi: 10.1016/j.ijrobp.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 705.Beaton L, Dunne EM, Yeung R, Rackley T, Weber B, Mar C, et al. Stereotactic body radiotherapy for large unresectable hepatocellular carcinomas - a single institution phase II study. Clin Oncol (R Coll Radiol) 2020;32:423–432. doi: 10.1016/j.clon.2020.01.028. [DOI] [PubMed] [Google Scholar]
- 706.Yoon SM, Kim SY, Lim YS, Kim KM, Shim JH, Lee D, et al. Stereotactic body radiation therapy for small (≤5 cm) hepatocellular carcinoma not amenable to curative treatment: results of a single-arm, phase II clinical trial. Clin Mol Hepatol. 2020;26:506–515. doi: 10.3350/cmh.2020.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 707.Kimura T, Takeda A, Sanuki N, Ariyoshi K, Yamaguchi T, Imagumbai T, et al. Multicenter prospective study of stereotactic body radiotherapy for previously untreated solitary primary hepatocellular carcinoma: the STRSPH study. Hepatol Res. 2021;51:461–471. doi: 10.1111/hepr.13595. [DOI] [PubMed] [Google Scholar]
- 708.Brunner TB, Bettinger D, Schultheiss M, Maruschke L, Sturm L, Bartl N, et al. Efficacy of stereotactic body radiotherapy in patients with hepatocellular carcinoma not suitable for transarterial chemoembolization (HERACLES: HEpatocellular Carcinoma Stereotactic RAdiotherapy CLinical Efficacy Study) Front Oncol. 2021;11:653141. doi: 10.3389/fonc.2021.653141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 709.Kim TH, Park JW, Kim BH, Oh ES, Youn SH, Moon SH, et al. Phase II study of hypofractionated proton beam therapy for hepatocellular carcinoma. Front Oncol. 2020;10:542. doi: 10.3389/fonc.2020.00542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710.Parzen JS, Hartsell W, Chang J, Apisarnthanarax S, Molitoris J, Durci M, et al. Hypofractionated proton beam radiotherapy in patients with unresectable liver tumors: multi-institutional prospective results from the Proton Collaborative Group. Radiat Oncol. 2020;15:255. doi: 10.1186/s13014-020-01703-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 711.Iwata H, Ogino H, Hattori Y, Nakajima K, Nomura K, Hashimoto S, et al. A phase 2 study of image-guided proton therapy for operable or ablation-treatable primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2021;111:117–126. doi: 10.1016/j.ijrobp.2021.03.049. [DOI] [PubMed] [Google Scholar]
- 712.Huo YR, Eslick GD. Transcatheter arterial chemoembolization plus radiotherapy compared with chemoembolization alone for hepatocellular carcinoma: a systematic review and meta-analysis. JAMA Oncol. 2015;1:756–765. doi: 10.1001/jamaoncol.2015.2189. [DOI] [PubMed] [Google Scholar]
- 713.Hsu HC, Chen TY, Chiu KW, Huang EY, Leung SW, Huang YJ, et al. Three-dimensional conformal radiotherapy for the treatment of arteriovenous shunting in patients with hepatocellular carcinoma. Br J Radiol. 2007;80:38–42. doi: 10.1259/bjr/55395102. [DOI] [PubMed] [Google Scholar]
- 714.Oh D, Lim DH, Park HC, Paik SW, Koh KC, Lee JH, et al. Early three-dimensional conformal radiotherapy for patients with unresectable hepatocellular carcinoma after incomplete transcatheter arterial chemoembolization: a prospective evaluation of efficacy and toxicity. Am J Clin Oncol. 2010;33:370–375. doi: 10.1097/COC.0b013e3181b0c298. [DOI] [PubMed] [Google Scholar]
- 715.Choi C, Koom WS, Kim TH, Yoon SM, Kim JH, Lee HS, et al. A prospective phase 2 multicenter study for the efficacy of radiation therapy following incomplete transarterial chemoembolization in unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2014;90:1051–1060. doi: 10.1016/j.ijrobp.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 716.Jacob R, Turley F, Redden DT, Saddekni S, Aal AK, Keene K, et al. Adjuvant stereotactic body radiotherapy following transarterial chemoembolization in patients with non-resectable hepatocellular carcinoma tumours of ≥ 3 cm. HPB (Oxford) 2015;17:140–149. doi: 10.1111/hpb.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 717.Yu JI, Park HC, Lim DH, Kim CJ, Oh D, Yoo BC, et al. Scheduled interval trans-catheter arterial chemoembolization followed by radiation therapy in patients with unresectable hepatocellular carcinoma. J Korean Med Sci. 2012;27:736–743. doi: 10.3346/jkms.2012.27.7.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 718.Yamada K, Izaki K, Sugimoto K, Mayahara H, Morita Y, Yoden E, et al. Prospective trial of combined transcatheter arterial chemoembolization and three-dimensional conformal radiotherapy for portal vein tumor thrombus in patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2003;57:113–119. doi: 10.1016/s0360-3016(03)00434-6. [DOI] [PubMed] [Google Scholar]
- 719.Kim DY, Park W, Lim DH, Lee JH, Yoo BC, Paik SW, et al. Three-dimensional conformal radiotherapy for portal vein thrombosis of hepatocellular carcinoma. Cancer. 2005;103:2419–2426. doi: 10.1002/cncr.21043. [DOI] [PubMed] [Google Scholar]
- 720.Han KH, Seong J, Kim JK, Ahn SH, Lee DY, Chon CY. Pilot clinical trial of localized concurrent chemoradiation therapy for locally advanced hepatocellular carcinoma with portal vein thrombosis. Cancer. 2008;113:995–1003. doi: 10.1002/cncr.23684. [DOI] [PubMed] [Google Scholar]
- 721.Shirai S, Sato M, Suwa K, Kishi K, Shimono C, Sonomura T, et al. Feasibility and efficacy of single photon emission computed tomography-based three-dimensional conformal radiotherapy for hepatocellular carcinoma 8 cm or more with portal vein tumor thrombus in combination with transcatheter arterial chemoembolization. Int J Radiat Oncol Biol Phys. 2010;76:1037–1044. doi: 10.1016/j.ijrobp.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 722.Koo JE, Kim JH, Lim YS, Park SJ, Won HJ, Sung KB, et al. Combination of transarterial chemoembolization and three-dimensional conformal radiotherapy for hepatocellular carcinoma with inferior vena cava tumor thrombus. Int J Radiat Oncol Biol Phys. 2010;78:180–187. doi: 10.1016/j.ijrobp.2009.07.1730. [DOI] [PubMed] [Google Scholar]
- 723.Yu JI, Park HC, Lim DH, Park W, Yoo BC, Paik SW, et al. Prognostic index for portal vein tumor thrombosis in patients with hepatocellular carcinoma treated with radiation therapy. J Korean Med Sci. 2011;26:1014–1022. doi: 10.3346/jkms.2011.26.8.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 724.Chuma M, Taguchi H, Yamamoto Y, Shimizu S, Nakanishi M, Ogawa K, et al. Efficacy of therapy for advanced hepatocellular carcinoma: intra-arterial 5-fluorouracil and subcutaneous interferon with image-guided radiation. J Gastroenterol Hepatol. 2011;26:1123–1132. doi: 10.1111/j.1440-1746.2011.06745.x. [DOI] [PubMed] [Google Scholar]
- 725.Yoon SM, Lim YS, Won HJ, Kim JH, Kim KM, Lee HC, et al. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys. 2012;82:2004–2011. doi: 10.1016/j.ijrobp.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 726.Hou JZ, Zeng ZC, Zhang JY, Fan J, Zhou J, Zeng MS. Influence of tumor thrombus location on the outcome of external-beam radiation therapy in advanced hepatocellular carcinoma with macrovascular invasion. Int J Radiat Oncol Biol Phys. 2012;84:362–368. doi: 10.1016/j.ijrobp.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 727.Park MS, Kim SU, Park JY, Kim DY, Ahn SH, Han KH, et al. Combination treatment of localized concurrent chemoradiation therapy and transarterial chemoembolization in locally advanced hepatocellular carcinoma with intrahepatic metastasis. Cancer Chemother Pharmacol. 2013;71:165–173. doi: 10.1007/s00280-012-1993-9. [DOI] [PubMed] [Google Scholar]
- 728.Tanaka Y, Nakazawa T, Komori S, Hidaka H, Okuwaki Y, Takada J, et al. Radiotherapy for patients with unresectable advanced hepatocellular carcinoma with invasion to intrahepatic large vessels: efficacy and outcomes. J Gastroenterol Hepatol. 2014;29:352–357. doi: 10.1111/jgh.12333. [DOI] [PubMed] [Google Scholar]
- 729.Lee SU, Park JW, Kim TH, Kim YJ, Woo SM, Koh YH, et al. Effectiveness and safety of proton beam therapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Strahlenther Onkol. 2014;190:806–814. doi: 10.1007/s00066-014-0604-6. [DOI] [PubMed] [Google Scholar]
- 730.Kim DY, Park JW, Kim TH, Kim BH, Moon SH, Kim SS, et al. Risk-adapted simultaneous integrated boost-proton beam therapy (SIB-PBT) for advanced hepatocellular carcinoma with tumour vascular thrombosis. Radiother Oncol. 2017;122:122–129. doi: 10.1016/j.radonc.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 731.Dutta D, Tatineni T, Yarlagadda S, Gupte A, Reddy SK, Madhavan R, et al. Hepatocellular carcinoma patients with portal vein thrombosis treated with robotic radiosurgery: interim results of a prospective study. Indian J Gastroenterol. 2021;40:389–401. doi: 10.1007/s12664-021-01172-w. [DOI] [PubMed] [Google Scholar]
- 732.Kodama K, Kawaoka T, Aikata H, Uchikawa S, Nishida Y, Inagaki Y, et al. Comparison of outcome of hepatic arterial infusion chemotherapy combined with radiotherapy and sorafenib for advanced hepatocellular carcinoma patients with major portal vein tumor thrombosis. Oncology. 2018;94:215–222. doi: 10.1159/000486483. [DOI] [PubMed] [Google Scholar]
- 733.Kosaka Y, Kimura T, Kawaoka T, Ogawa Y, Amioka K, Naruto K, et al. Hepatic arterial infusion chemotherapy combined with radiation therapy for advanced hepatocellular carcinoma with tumor thrombosis of the main trunk or bilobar of the portal vein. Liver Cancer. 2021;10:151–160. doi: 10.1159/000513706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 734.Lou J, Li Y, Liang K, Guo Y, Song C, Chen L, et al. Hypofractionated radiotherapy as a salvage treatment for recurrent hepatocellular carcinoma with inferior vena cava/right atrium tumor thrombus: a multi-center analysis. BMC Cancer. 2019;19:668. doi: 10.1186/s12885-019-5870-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 735.Rim CH, Jeong BK, Kim TH, Kim JH, Kang HC, Seong J. Effectiveness and feasibility of external beam radiotherapy for hepatocellular carcinoma with inferior vena cava and/or right atrium involvement: a multicenter trial in Korea (KROG 17-10) Int J Radiat Biol. 2020;96:759–766. doi: 10.1080/09553002.2020.1721607. [DOI] [PubMed] [Google Scholar]
- 736.Rim CH, Kim CY, Yang DS, Yoon WS. External beam radiation therapy to hepatocellular carcinoma involving inferior vena cava and/or right atrium: a meta-analysis and systemic review. Radiother Oncol. 2018;129:123–129. doi: 10.1016/j.radonc.2018.02.030. [DOI] [PubMed] [Google Scholar]
- 737.Kim BK, Kim DY, Byun HK, Choi HJ, Beom SH, Lee HW, et al. Efficacy and safety of liver-directed concurrent chemoradiotherapy and sequential sorafenib for advanced hepatocellular carcinoma: a prospective phase 2 trial. Int J Radiat Oncol Biol Phys. 2020;107:106–115. doi: 10.1016/j.ijrobp.2020.01.027. [DOI] [PubMed] [Google Scholar]
- 738.Im JH, Yoon SM, Park HC, Kim JH, Yu JI, Kim TH, et al. Radiotherapeutic strategies for hepatocellular carcinoma with portal vein tumour thrombosis in a hepatitis B endemic area. Liver Int. 2017;37:90–100. doi: 10.1111/liv.13191. [DOI] [PubMed] [Google Scholar]
- 739.Zhao Q, Zhu K, Yue J, Qi Z, Jiang S, Xu X, et al. Comparison of intra-arterial chemoembolization with and without radiotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: a meta-analysis. Ther Clin Risk Manag. 2016;13:21–31. doi: 10.2147/TCRM.S126181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 740.Li MF, Leung HW, Chan AL, Wang SY. Network meta-analysis of treatment regimens for inoperable advanced hepatocellular carcinoma with portal vein invasion. Ther Clin Risk Manag. 2018;14:1157–1168. doi: 10.2147/TCRM.S162898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 741.Nakazawa T, Hidaka H, Shibuya A, Okuwaki Y, Tanaka Y, Takada J, et al. Overall survival in response to sorafenib versus radiotherapy in unresectable hepatocellular carcinoma with major portal vein tumor thrombosis: propensity score analysis. BMC Gastroenterol. 2014;14:84. doi: 10.1186/1471-230X-14-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 742.Cho YY, Yu SJ, Lee HW, Kim DY, Kang W, Paik YH, et al. Clinical characteristics of long-term survivors after sorafenib treatment for unresectable hepatocellular carcinoma: a Korean national multicenter retrospective cohort study. J Hepatocell Carcinoma. 2021;8:613–623. doi: 10.2147/JHC.S304439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 743.Chu SS, Kuo YH, Liu WS, Wang SC, Ho CH, Chen YC, et al. Effect of radiotherapy on survival in advanced hepatocellular carcinoma patients treated with sorafenib: a nationwide cancer-registry-based study. Sci Rep. 2021;11:1614. doi: 10.1038/s41598-021-81176-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 744.Chang WI, Kim BH, Kim YJ, Yoon JH, Jung YJ, Chie EK. Role of radiotherapy in Barcelona Clinic Liver Cancer stage C hepatocellular carcinoma treated with sorafenib. J Gastroenterol Hepatol. 2022;37:387–394. doi: 10.1111/jgh.15722. [DOI] [PubMed] [Google Scholar]
- 745.Yu JI, Lee SJ, Lee J, Lim HY, Paik SW, Yoo GS, et al. Clinical significance of radiotherapy before and/or during nivolumab treatment in hepatocellular carcinoma. Cancer Med. 2019;8:6986–6994. doi: 10.1002/cam4.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 746.Zhong L, Wu D, Peng W, Sheng H, Xiao Y, Zhang X, et al. Safety of PD-1/PD-L1 inhibitors combined with palliative radiotherapy and anti-angiogenic therapy in advanced hepatocellular carcinoma. Front Oncol. 2021;11:686621. doi: 10.3389/fonc.2021.686621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 747.Chu HH, Kim JH, Shim JH, Yoon SM, Kim PH, Alrashidi I. Chemoembolization plus radiotherapy versus chemoembolization plus sorafenib for the treatment of hepatocellular carcinoma invading the portal vein: a propensity score matching analysis. Cancers (Basel) 2020;12:1116. doi: 10.3390/cancers12051116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 748.Lee HS, Choi GH, Choi JS, Kim KS, Han KH, Seong J, et al. Surgical resection after down-staging of locally advanced hepatocellular carcinoma by localized concurrent chemoradiotherapy. Ann Surg Oncol. 2014;21:3646–3653. doi: 10.1245/s10434-014-3652-3. [DOI] [PubMed] [Google Scholar]
- 749.Hamaoka M, Kobayashi T, Kuroda S, Iwako H, Okimoto S, Kimura T, et al. Hepatectomy after down-staging of hepatocellular carcinoma with portal vein tumor thrombus using chemoradiotherapy: a retrospective cohort study. Int J Surg. 2017;44:223–228. doi: 10.1016/j.ijsu.2017.06.082. [DOI] [PubMed] [Google Scholar]
- 750.Chong JU, Choi GH, Han DH, Kim KS, Seong J, Han KH, et al. Downstaging with localized concurrent chemoradiotherapy can identify optimal surgical candidates in hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol. 2018;25:3308–3315. doi: 10.1245/s10434-018-6653-9. [DOI] [PubMed] [Google Scholar]
- 751.Han DH, Joo DJ, Kim MS, Choi GH, Choi JS, Park YN, et al. Living donor liver transplantation for advanced hepatocellular carcinoma with portal vein tumor thrombosis after concurrent chemoradiation therapy. Yonsei Med J. 2016;57:1276–1281. doi: 10.3349/ymj.2016.57.5.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 752.Choi JY, Yu JI, Park HC, Kwon CHD, Kim JM, Joh JW, et al. The possibility of radiotherapy as downstaging to living donor liver transplantation for hepatocellular carcinoma with portal vein tumor thrombus. Liver Transpl. 2017;23:545–551. doi: 10.1002/lt.24729. [DOI] [PubMed] [Google Scholar]
- 753.Wei X, Jiang Y, Zhang X, Feng S, Zhou B, Ye X, et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized, open-label, multicenter controlled study. J Clin Oncol. 2019;37:2141–2151. doi: 10.1200/JCO.18.02184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 754.Katz AW, Chawla S, Qu Z, Kashyap R, Milano MT, Hezel AF. Stereotactic hypofractionated radiation therapy as a bridge to transplantation for hepatocellular carcinoma: clinical outcome and pathologic correlation. Int J Radiat Oncol Biol Phys. 2012;83:895–900. doi: 10.1016/j.ijrobp.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 755.Mannina EM, Cardenes HR, Lasley FD, Goodman B, Zook J, Althouse S, et al. Role of stereotactic body radiation therapy before orthotopic liver transplantation: retrospective evaluation of pathologic response and outcomes. Int J Radiat Oncol Biol Phys. 2017;97:931–938. doi: 10.1016/j.ijrobp.2016.12.036. [DOI] [PubMed] [Google Scholar]
- 756.Wong TC, Lee VH, Law AL, Pang HH, Lam KO, Lau V, et al. Prospective study of stereotactic body radiation therapy for hepatocellular carcinoma on waitlist for liver transplant. Hepatology. 2021;74:2580–2594. doi: 10.1002/hep.31992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 757.Park W, Lim DH, Paik SW, Koh KC, Choi MS, Park CK, et al. Local radiotherapy for patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:1143–1150. doi: 10.1016/j.ijrobp.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 758.Kim TH, Kim DY, Park JW, Kim YI, Kim SH, Park HS, et al. Three-dimensional conformal radiotherapy of unresectable hepatocellular carcinoma patients for whom transcatheter arterial chemoembolization was ineffective or unsuitable. Am J Clin Oncol. 2006;29:568–575. doi: 10.1097/01.coc.0000239147.60196.11. [DOI] [PubMed] [Google Scholar]
- 759.Huang WY, Jen YM, Lee MS, Chang LP, Chen CM, Ko KH, et al. Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012;84:355–361. doi: 10.1016/j.ijrobp.2011.11.058. [DOI] [PubMed] [Google Scholar]
- 760.Seong J, Park HC, Han KH, Lee DY, Lee JT, Chon CY, et al. Local radiotherapy for unresectable hepatocellular carcinoma patients who failed with transcatheter arterial chemoembolization. Int J Radiat Oncol Biol Phys. 2000;47:1331–1335. doi: 10.1016/s0360-3016(00)00519-8. [DOI] [PubMed] [Google Scholar]
- 761.Bae SH, Park HC, Lim DH, Lee JA, Gwak GY, Choi MS, et al. Salvage treatment with hypofractionated radiotherapy in patients with recurrent small hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012;82:e603–e607. doi: 10.1016/j.ijrobp.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 762.Cha H, Park HC, Yu JI, Kim TH, Nam TK, Yoon SM, et al. Clinical practice patterns of radiotherapy in patients with hepatocellular carcinoma: a Korean radiation oncology group study (KROG 14-07) Cancer Res Treat. 2017;49:61–69. doi: 10.4143/crt.2016.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 763.Soliman H, Ringash J, Jiang H, Singh K, Kim J, Dinniwell R, et al. Phase II trial of palliative radiotherapy for hepatocellular carcinoma and liver metastases. J Clin Oncol. 2013;31:3980–3986. doi: 10.1200/JCO.2013.49.9202. [DOI] [PubMed] [Google Scholar]
- 764.Yeung CSY, Chiang CL, Wong NSM, Ha SK, Tsang KS, Ho CHM, et al. Palliative liver radiotherapy (RT) for symptomatic hepatocellular carcinoma (HCC) Sci Rep. 2020;10:1254. doi: 10.1038/s41598-020-58108-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 765.Cheng SH, Lin YM, Chuang VP, Yang PS, Cheng JC, Huang AT, et al. A pilot study of three-dimensional conformal radiotherapy in unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 1999;14:1025–1033. doi: 10.1046/j.1440-1746.1999.01994.x. [DOI] [PubMed] [Google Scholar]
- 766.Huang JF, Wang LY, Lin ZY, Chen SC, Hsieh MY, Chuang WL, et al. Incidence and clinical outcome of icteric type hepatocellular carcinoma. J Gastroenterol Hepatol. 2002;17:190–195. doi: 10.1046/j.1440-1746.2002.02677.x. [DOI] [PubMed] [Google Scholar]
- 767.Yoon SM, Kim JH, Choi EK, Ahn SD, Lee SW, Yi BY, et al. Radioresponse of hepatocellular carcinoma-treatment of lymph node metastasis. Cancer Res Treat. 2004;36:79–84. doi: 10.4143/crt.2004.36.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 768.Zeng ZC, Tang ZY, Fan J, Qin LX, Ye SL, Zhou J, et al. Consideration of role of radiotherapy for lymph node metastases in patients with HCC: retrospective analysis for prognostic factors from 125 patients. Int J Radiat Oncol Biol Phys. 2005;63:1067–1076. doi: 10.1016/j.ijrobp.2005.03.058. [DOI] [PubMed] [Google Scholar]
- 769.Park YJ, Lim DH, Paik SW, Koh KC, Lee JH, Choi MS, et al. Radiation therapy for abdominal lymph node metastasis from hepatocellular carcinoma. J Gastroenterol. 2006;41:1099–1106. doi: 10.1007/s00535-006-1895-x. [DOI] [PubMed] [Google Scholar]
- 770.Yamashita H, Nakagawa K, Shiraishi K, Tago M, Igaki H, Nakamura N, et al. Radiotherapy for lymph node metastases in patients with hepatocellular carcinoma: retrospective study. J Gastroenterol Hepatol. 2007;22:523–527. doi: 10.1111/j.1440-1746.2006.04450.x. [DOI] [PubMed] [Google Scholar]
- 771.Jang JW, Kay CS, You CR, Kim CW, Bae SH, Choi JY, et al. Simultaneous multitarget irradiation using helical tomotherapy for advanced hepatocellular carcinoma with multiple extrahepatic metastases. Int J Radiat Oncol Biol Phys. 2009;74:412–418. doi: 10.1016/j.ijrobp.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 772.Yeung R, Hamm J, Liu M, Schellenberg D. Institutional analysis of stereotactic body radiotherapy (SBRT) for oligometastatic lymph node metastases. Radiat Oncol. 2017;12:105. doi: 10.1186/s13014-017-0820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 773.Kim Y, Park HC, Yoon SM, Kim TH, Lee J, Choi J, et al. Prognostic group stratification and nomogram for predicting overall survival in patients who received radiotherapy for abdominal lymph node metastasis from hepatocellular carcinoma: a multi-institutional retrospective study (KROG 15-02) Oncotarget. 2017;8:94450–94461. doi: 10.18632/oncotarget.21775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 774.Jung J, Yoon SM, Park HC, Nam TK, Seong J, Chie EK, et al. Radiotherapy for adrenal metastasis from hepatocellular carcinoma: a multi-institutional retrospective study (KROG 13-05) PLoS One. 2016;11:e0152642. doi: 10.1371/journal.pone.0152642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 775.Jiang W, Zeng ZC, Zhang JY, Fan J, Zeng MS, Zhou J. Palliative radiation therapy for pulmonary metastases from hepatocellular carcinoma. Clin Exp Metastasis. 2012;29:197–205. doi: 10.1007/s10585-011-9442-4. [DOI] [PubMed] [Google Scholar]
- 776.Seong J, Koom WS, Park HC. Radiotherapy for painful bone metastases from hepatocellular carcinoma. Liver Int. 2005;25:261–265. doi: 10.1111/j.1478-3231.2005.01094.x. [DOI] [PubMed] [Google Scholar]
- 777.He J, Zeng ZC, Tang ZY, Fan J, Zhou J, Zeng MS, et al. Clinical features and prognostic factors in patients with bone metastases from hepatocellular carcinoma receiving external beam radiotherapy. Cancer. 2009;115:2710–2720. doi: 10.1002/cncr.24300. [DOI] [PubMed] [Google Scholar]
- 778.Sakaguchi M, Maebayashi T, Aizawa T, Ishibashi N, Fukushima S, Saito T. Radiation therapy and palliative care prolongs the survival of hepatocellular carcinoma patients with bone metastases. Intern Med. 2016;55:1077–1083. doi: 10.2169/internalmedicine.55.6003. [DOI] [PubMed] [Google Scholar]
- 779.Jung IH, Yoon SM, Kwak J, Park JH, Song SY, Lee SW, et al. High-dose radiotherapy is associated with better local control of bone metastasis from hepatocellular carcinoma. Oncotarget. 2017;8:15182–15192. doi: 10.18632/oncotarget.14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 780.Kim TH, Park S, Rim CH, Choi C, Seong J. Improved oncologic outcomes by ablative radiotherapy in patients with bone metastasis from hepatocellular carcinoma. J Cancer Res Clin Oncol. 2021;147:2693–2700. doi: 10.1007/s00432-021-03553-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 781.Rades D, Dahlke M, Janssen S, Gebauer N, Bartscht T. Radiation therapy for metastatic spinal cord compression in patients with hepatocellular carcinoma. In Vivo. 2015;29:749–752. [PubMed] [Google Scholar]
- 782.Choi HJ, Cho BC, Sohn JH, Shin SJ, Kim SH, Kim JH, et al. Brain metastases from hepatocellular carcinoma: prognostic factors and outcome: brain metastasis from HCC. J Neurooncol. 2009;91:307–313. doi: 10.1007/s11060-008-9713-3. [DOI] [PubMed] [Google Scholar]
- 783.Kim K, Kim TH, Kim TH, Seong J. Efficacy of local therapy for oligometastatic hepatocellular carcinoma: a propensity score matched analysis. J Hepatocell Carcinoma. 2021;8:35–44. doi: 10.2147/JHC.S290197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 784.Yoo C, Kim JH, Ryu MH, Park SR, Lee D, Kim KM, et al. Clinical outcomes with multikinase inhibitors after progression on first-line atezolizumab plus bevacizumab in patients with advanced hepatocellular carcinoma: a multinational multicenter retrospective study. Liver Cancer. 2021;10:107–114. doi: 10.1159/000512781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 785.Kim N, Kim HJ, Won JY, Kim DY, Han KH, Jung I, et al. Retrospective analysis of stereotactic body radiation therapy efficacy over radiofrequency ablation for hepatocellular carcinoma. Radiother Oncol. 2019;131:81–87. doi: 10.1016/j.radonc.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 786.Hara K, Takeda A, Tsurugai Y, Saigusa Y, Sanuki N, Eriguchi T, et al. Radiotherapy for hepatocellular carcinoma results in comparable survival to radiofrequency ablation: a propensity score analysis. Hepatology. 2019;69:2533–2545. doi: 10.1002/hep.30591. [DOI] [PubMed] [Google Scholar]
- 787.Kim N, Cheng J, Jung I, Liang J, Shih YL, Huang WY, et al. Stereotactic body radiation therapy vs. radiofrequency ablation in Asian patients with hepatocellular carcinoma. J Hepatol. 2020;73:121–129. doi: 10.1016/j.jhep.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 788.Ueno M, Takabatake H, Itasaka S, Kayahara T, Morimoto Y, Yamamoto H, et al. Stereotactic body radiation therapy versus radiofrequency ablation for single small hepatocellular carcinoma: a propensity-score matching analysis of their impact on liver function and clinical outcomes. J Gastrointest Oncol. 2021;12:2334–2344. doi: 10.21037/jgo-21-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 789.Jeong Y, Lee KJ, Lee SJ, Shin YM, Kim MJ, Lim YS, et al. Radiofrequency ablation versus stereotactic body radiation therapy for small (≤ 3 cm) hepatocellular carcinoma: a retrospective comparison analysis. J Gastroenterol Hepatol. 2021;36:1962–1970. doi: 10.1111/jgh.15442. [DOI] [PubMed] [Google Scholar]
- 790.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 791.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 792.Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, Qin S, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31:4067–4075. doi: 10.1200/JCO.2012.45.8372. [DOI] [PubMed] [Google Scholar]
- 793.Johnson PJ, Qin S, Park JW, Poon RT, Raoul JL, Philip PA, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31:3517–3524. doi: 10.1200/JCO.2012.48.4410. [DOI] [PubMed] [Google Scholar]
- 794.Cainap C, Qin S, Huang WT, Chung IJ, Pan H, Cheng Y, et al. Linifanib versus sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33:172–179. doi: 10.1200/JCO.2013.54.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 795.Zhu AX, Rosmorduc O, Evans TR, Ross PJ, Santoro A, Carrilho FJ, et al. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33:559–566. doi: 10.1200/JCO.2013.53.7746. [DOI] [PubMed] [Google Scholar]
- 796.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 797.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 798.Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77–90. doi: 10.1016/S1470-2045(21)00604-5. [DOI] [PubMed] [Google Scholar]
- 799.Furuse J, Ishii H, Nakachi K, Suzuki E, Shimizu S, Nakajima K. Phase I study of sorafenib in Japanese patients with hepatocellular carcinoma. Cancer Sci. 2008;99:159–165. doi: 10.1111/j.1349-7006.2007.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 800.Shim JH, Park JW, Choi JI, Park BJ, Kim CM. Practical efficacy of sorafenib monotherapy for advanced hepatocellular carcinoma patients in a hepatitis B virus-endemic area. J Cancer Res Clin Oncol. 2009;135:617–625. doi: 10.1007/s00432-008-0496-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 801.Kim JE, Ryoo BY, Ryu MH, Chang HM, Suh DJ, Lee HC, et al. Sorafenib for hepatocellular carcinoma according to Child-Pugh class of liver function. Cancer Chemother Pharmacol. 2011;68:1285–1290. doi: 10.1007/s00280-011-1616-x. [DOI] [PubMed] [Google Scholar]
- 802.Hollebecque A, Cattan S, Romano O, Sergent G, Mourad A, Louvet A, et al. Safety and efficacy of sorafenib in hepatocellular carcinoma: the impact of the Child-Pugh score. Aliment Pharmacol Ther. 2011;34:1193–1201. doi: 10.1111/j.1365-2036.2011.04860.x. [DOI] [PubMed] [Google Scholar]
- 803.Chiu J, Tang YF, Yao TJ, Wong A, Wong H, Leung R, et al. The use of single-agent sorafenib in the treatment of advanced hepatocellular carcinoma patients with underlying Child-Pugh B liver cirrhosis: a retrospective analysis of efficacy, safety, and survival benefits. Cancer. 2012;118:5293–5301. doi: 10.1002/cncr.27543. [DOI] [PubMed] [Google Scholar]
- 804.Pressiani T, Boni C, Rimassa L, Labianca R, Fagiuoli S, Salvagni S, et al. Sorafenib in patients with Child-Pugh class A and B advanced hepatocellular carcinoma: a prospective feasibility analysis. Ann Oncol. 2013;24:406–411. doi: 10.1093/annonc/mds343. [DOI] [PubMed] [Google Scholar]
- 805.Ogasawara S, Chiba T, Ooka Y, Kanogawa N, Saito T, Motoyama T, et al. Sorafenib treatment in Child-Pugh A and B patients with advanced hepatocellular carcinoma: safety, efficacy and prognostic factors. Invest New Drugs. 2015;33:729–739. doi: 10.1007/s10637-015-0237-3. [DOI] [PubMed] [Google Scholar]
- 806.Suzuki E, Kaneko S, Okusaka T, Ikeda M, Yamaguchi K, Sugimoto R, et al. A multicenter phase II study of sorafenib in Japanese patients with advanced hepatocellular carcinoma and Child Pugh A and B class. Jpn J Clin Oncol. 2018;48:317–321. doi: 10.1093/jjco/hyy010. [DOI] [PubMed] [Google Scholar]
- 807.Kim HY, Park JW, Joo J, Kim H, Woo SM, Lee WJ, et al. Worse outcome of sorafenib therapy associated with ascites and Child-Pugh score in advanced hepatocellular carcinoma. J Gastroenterol Hepatol. 2013;28:1756–1761. doi: 10.1111/jgh.12310. [DOI] [PubMed] [Google Scholar]
- 808.Marrero JA, Kudo M, Venook AP, Ye SL, Bronowicki JP, Chen XP, et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: the GIDEON study. J Hepatol. 2016;65:1140–1147. doi: 10.1016/j.jhep.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 809.Brose MS, Frenette CT, Keefe SM, Stein SM. Management of sorafenib-related adverse events: a clinician’s perspective. Semin Oncol. 2014;41 Suppl 2:S1–S16. doi: 10.1053/j.seminoncol.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 810.Granito A, Marinelli S, Negrini G, Menetti S, Benevento F, Bolondi L. Prognostic significance of adverse events in patients with hepatocellular carcinoma treated with sorafenib. Therap Adv Gastroenterol. 2016;9:240–249. doi: 10.1177/1756283X15618129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 811.Ren Z, Zhu K, Kang H, Lu M, Qu Z, Lu L, et al. Randomized controlled trial of the prophylactic effect of urea-based cream on sorafenib-associated hand-foot skin reactions in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33:894–900. doi: 10.1200/JCO.2013.52.9651. [DOI] [PubMed] [Google Scholar]
- 812.Lee YS, Jung YK, Kim JH, Cho SB, Kim DY, Kim MY, et al. Effect of urea cream on sorafenib-associated hand-foot skin reaction in patients with hepatocellular carcinoma: a multicenter, randomised, double-blind controlled study. Eur J Cancer. 2020;140:19–27. doi: 10.1016/j.ejca.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 813.Evans TRJ, Kudo M, Finn RS, Han KH, Cheng AL, Ikeda M, et al. Urine protein:creatinine ratio vs 24-hour urine protein for proteinuria management: analysis from the phase 3 REFLECT study of lenvatinib vs sorafenib in hepatocellular carcinoma. Br J Cancer. 2019;121:218–221. doi: 10.1038/s41416-019-0506-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 814.Cho JY, Kwon SH, Lee EK, Lee JH, Kim HL. Cost-effectiveness of adjuvant immunotherapy with cytokine-induced killer cell for hepatocellular carcinoma based on a randomized controlled trial and real-world data. Front Oncol. 2021;11:728740. doi: 10.3389/fonc.2021.728740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 815.Jannin A, Penel N, Ladsous M, Vantyghem MC, Do Cao C. Tyrosine kinase inhibitors and immune checkpoint inhibitors-induced thyroid disorders. Crit Rev Oncol Hematol. 2019;141:23–35. doi: 10.1016/j.critrevonc.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 816.Shimose S, Iwamoto H, Niizeki T, Shirono T, Noda Y, Kamachi N, et al. Clinical significance of adverse events for patients with unresectable hepatocellular carcinoma treated with lenvatinib: a multicenter retrospective study. Cancers (Basel) 2020;12:1867. doi: 10.3390/cancers12071867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 817.Sasaki R, Fukushima M, Haraguchi M, Miuma S, Miyaaki H, Hidaka M, et al. Response to lenvatinib is associated with optimal relativedose intensity in hepatocellular carcinoma: experience in clinical settings. Cancers (Basel) 2019;11:1769. doi: 10.3390/cancers11111769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 818.Ohki T, Sato K, Kondo M, Goto E, Sato T, Kondo Y, et al. Impact of adverse events on the progression-free survival of patients with advanced hepatocellular carcinoma treated with lenvatinib: a multicenter retrospective study. Drugs Real World Outcomes. 2020;7:141–149. doi: 10.1007/s40801-020-00179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 819.Maruta S, Ogasawara S, Ooka Y, Obu M, Inoue M, Itokawa N, et al. Potential of lenvatinib for an expanded indication from the REFLECT trial in patients with advanced hepatocellular carcinoma. Liver Cancer. 2020;9:382–396. doi: 10.1159/000507022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 820.Sho T, Suda G, Ogawa K, Shigesawa T, Suzuki K, Nakamura A, et al. Lenvatinib in patients with unresectable hepatocellular carcinoma who do not meet the REFLECT trial eligibility criteria. Hepatol Res. 2020;50:966–977. doi: 10.1111/hepr.13511. [DOI] [PubMed] [Google Scholar]
- 821.Goh MJ, Oh JH, Park Y, Kim J, Kang W, Sinn DH, et al. Efficacy and safety of lenvatinib therapy for unresectable hepatocellular carcinoma in a real-world practice in Korea. Liver Cancer. 2021;10:52–62. doi: 10.1159/000512239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 822.Sho T, Suda G, Ogawa K, Kimura M, Shimazaki T, Maehara O, et al. Early response and safety of lenvatinib for patients with advanced hepatocellular carcinoma in a real-world setting. JGH Open. 2020;4:54–60. doi: 10.1002/jgh3.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 823.Cheon J, Chon HJ, Bang Y, Park NH, Shin JW, Kim KM, et al. Real-world efficacy and safety of lenvatinib in Korean patients with advanced hepatocellular carcinoma: a multi-center retrospective analysis. Liver Cancer. 2020;9:613–624. doi: 10.1159/000508901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 824.Ogushi K, Chuma M, Uojima H, Hidaka H, Numata K, Kobayashi S, et al. Safety and efficacy of lenvatinib treatment in Child-Pugh A and B patients with unresectable hepatocellular carcinoma in clinical practice: a multicenter analysis. Clin Exp Gastroenterol. 2020;13:385–396. doi: 10.2147/CEG.S256691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 825.Sho T, Suda G, Ogawa K, Kimura M, Kubo A, Tokuchi Y, et al. Early response and safety of atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma in patients who do not meet IMbrave150 eligibility criteria. Hepatol Res. 2021;51:979–989. doi: 10.1111/hepr.13693. [DOI] [PubMed] [Google Scholar]
- 826.Hayakawa Y, Tsuchiya K, Kurosaki M, Yasui Y, Kaneko S, Tanaka Y, et al. Early experience of atezolizumab plus bevacizumab therapy in Japanese patients with unresectable hepatocellular carcinoma in real-world practice. Invest New Drugs. 2022;40:392–402. doi: 10.1007/s10637-021-01185-4. [DOI] [PubMed] [Google Scholar]
- 827.Kelley RK, Sangro B, Harris W, Ikeda M, Okusaka T, Kang YK, et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: randomized expansion of a phase I/II study. J Clin Oncol. 2021;39:2991–3001. doi: 10.1200/JCO.20.03555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 828.Abou-Alfa GK, Chan SL, Kudo M, Lau G, Kelley RK, Furuse J, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;4_suppl:379 [Google Scholar]
- 829.Qin S, Bi F, Gu S, Bai Y, Chen Z, Wang Z, et al. Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: a randomized, open-label, parallel-controlled phase II-III trial. J Clin Oncol. 2021;39:3002–3011. doi: 10.1200/JCO.21.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 830.Kelley RK, Yau T, Cheng AL, Kaseb A, Qin S, Zhu AX, et al. VP10-2021: cabozantinib (C) plus atezolizumab (A) versus sorafenib (S) as first-line systemic treatment for advanced hepatocellular carcinoma (aHCC): results from the randomized phase III COSMIC-312 trial. Ann Oncol. 2022;33:114–116. [Google Scholar]
- 831.Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862–873. doi: 10.1016/j.jhep.2021.11.030. [DOI] [PubMed] [Google Scholar]
- 832.d’Izarny-Gargas T, Durrbach A, Zaidan M. Efficacy and tolerance of immune checkpoint inhibitors in transplant patients with cancer: a systematic review. Am J Transplant. 2020;20:2457–2465. doi: 10.1111/ajt.15811. [DOI] [PubMed] [Google Scholar]
- 833.Pinter M, Scheiner B, Peck-Radosavljevic M. Immunotherapy for advanced hepatocellular carcinoma: a focus on special subgroups. Gut. 2021;70:204–214. doi: 10.1136/gutjnl-2020-321702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 834.Rimassa L, Personeni N, Czauderna C, Foerster F, Galle P. Systemic treatment of HCC in special populations. J Hepatol. 2021;74:931–943. doi: 10.1016/j.jhep.2020.11.026. [DOI] [PubMed] [Google Scholar]
- 835.Pfister D, Núñez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592:450–456. doi: 10.1038/s41586-021-03362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 836.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 837.Jackson R, Psarelli EE, Berhane S, Khan H, Johnson P. Impact of viral status on survival in patients receiving sorafenib for advanced hepatocellular cancer: a meta-analysis of randomized phase III trials. J Clin Oncol. 2017;35:622–628. doi: 10.1200/JCO.2016.69.5197. [DOI] [PubMed] [Google Scholar]
- 838.Reig M, Rimola J, Torres F, Darnell A, Rodriguez-Lope C, Forner A, et al. Postprogression survival of patients with advanced hepatocellular carcinoma: rationale for second-line trial design. Hepatology. 2013;58:2023–2031. doi: 10.1002/hep.26586. [DOI] [PubMed] [Google Scholar]
- 839.Lencioni R, Kudo M, Ye SL, Bronowicki JP, Chen XP, Dagher L, et al. GIDEON (Global Investigation of therapeutic DEcisions in hepatocellular carcinoma and Of its treatment with sorafeNib): second interim analysis. Int J Clin Pract. 2014;68:609–617. doi: 10.1111/ijcp.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 840.Llovet JM, Decaens T, Raoul JL, Boucher E, Kudo M, Chang C, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol. 2013;31:3509–3516. doi: 10.1200/JCO.2012.47.3009. [DOI] [PubMed] [Google Scholar]
- 841.Zhu AX, Kudo M, Assenat E, Cattan S, Kang YK, Lim HY, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA. 2014;312:57–67. doi: 10.1001/jama.2014.7189. [DOI] [PubMed] [Google Scholar]
- 842.Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R, Pastorelli D, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16:859–870. doi: 10.1016/S1470-2045(15)00050-9. [DOI] [PubMed] [Google Scholar]
- 843.Rimassa L, Assenat E, Peck-Radosavljevic M, Pracht M, Zagonel V, Mathurin P, et al. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): a final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol. 2018;19:682–693. doi: 10.1016/S1470-2045(18)30146-3. [DOI] [PubMed] [Google Scholar]
- 844.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 845.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 846.Qin S, Chen Z, Fang W, Ren Z, Xu R, Ryoo BY, et al. Pembrolizumab plus best supportive care versus placebo plus best supportive care as second-line therapy in patients in Asia with advanced hepatocellular carcinoma (HCC): phase 3 KEY-NOTE-394 study. J Clin Oncol. 2022;4_suppl:383 [Google Scholar]
- 847.Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 848.Sonbol MB, Riaz IB, Naqvi SAA, Almquist DR, Mina S, Almasri J, et al. Systemic therapy and sequencing options in advanced hepatocellular carcinoma: a systematic review and network meta-analysis. JAMA Oncol. 2020;6:e204930. doi: 10.1001/jamaoncol.2020.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 849.Lim H, Ramjeesingh R, Liu D, Tam VC, Knox JJ, Card PB, et al. Optimizing survival and the changing landscape of targeted therapy for intermediate and advanced hepatocellular carcinoma: a systematic review. J Natl Cancer Inst. 2021;113:123–136. doi: 10.1093/jnci/djaa119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 850.Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245–255. doi: 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
- 851.Abou-Elkacem L, Arns S, Brix G, Gremse F, Zopf D, Kiessling F, et al. Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol Cancer Ther. 2013;12:1322–1331. doi: 10.1158/1535-7163.MCT-12-1162. [DOI] [PubMed] [Google Scholar]
- 852.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 853.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 854.Kudo M, Matilla A, Santoro A, Melero I, Gracián AC, Acosta-Rivera M, et al. CheckMate 040 cohort 5: a phase I/II study of nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh B cirrhosis. J Hepatol. 2021;75:600–609. doi: 10.1016/j.jhep.2021.04.047. [DOI] [PubMed] [Google Scholar]
- 855.Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 2020;6:e204564. doi: 10.1001/jamaoncol.2020.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 856.Qin S, Li Q, Gu S, Chen X, Lin L, Wang Z, et al. Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol Hepatol. 2021;6:559–568. doi: 10.1016/S2468-1253(21)00109-6. [DOI] [PubMed] [Google Scholar]
- 857.Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21:571–580. doi: 10.1016/S1470-2045(20)30011-5. [DOI] [PubMed] [Google Scholar]
- 858.Lee CH, Lee YB, Kim MA, Jang H, Oh H, Kim SW, et al. Effectiveness of nivolumab versus regorafenib in hepatocellular carcinoma patients who failed sorafenib treatment. Clin Mol Hepatol. 2020;26:328–339. doi: 10.3350/cmh.2019.0049n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 859.Choi WM, Choi J, Lee D, Shim JH, Lim YS, Lee HC, et al. Regorafenib versus nivolumab after sorafenib failure: real-world data in patients with hepatocellular carcinoma. Hepatol Commun. 2020;4:1073–1086. doi: 10.1002/hep4.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 860.Alsina A, Kudo M, Vogel A, Cheng AL, Tak WY, Ryoo BY, et al. Effects of subsequent systemic anticancer medication following first-line lenvatinib: a post hoc responder analysis from the phase 3 REFLECT study in unresectable hepatocellular carcinoma. Liver Cancer. 2020;9:93–104. doi: 10.1159/000504624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 861.Ando Y, Kawaoka T, Suehiro Y, Yamaoka K, Kosaka Y, Uchikawa S, et al. Analysis of post-progression survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib. Oncology. 2020;98:787–797. doi: 10.1159/000509387. [DOI] [PubMed] [Google Scholar]
- 862.Koroki K, Kanogawa N, Maruta S, Ogasawara S, Iino Y, Obu M, et al. Posttreatment after lenvatinib in patients with advanced hepatocellular carcinoma. Liver Cancer. 2021;10:473–484. doi: 10.1159/000515552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 863.Hiraoka A, Kumada T, Tada T, Ogawa C, Tani J, Fukunishi S, et al. Therapeutic efficacy of ramucirumab after lenvatinib for post-progression treatment of unresectable hepatocellular carcinoma. Gastroenterol Rep (Oxf) 2020;9:133–138. doi: 10.1093/gastro/goaa042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 864.Brandi G, de Rosa F, Agostini V, di Girolamo S, Andreone P, Bolondi L, et al. Metronomic capecitabine in advanced hepatocellular carcinoma patients: a phase II study. Oncologist. 2013;18:1256–1257. doi: 10.1634/theoncologist.2013-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 865.Mir O, Coriat R, Boudou-Rouquette P, Ropert S, Durand JP, Cessot A, et al. Gemcitabine and oxaliplatin as second-line treatment in patients with hepatocellular carcinoma pretreated with sorafenib. Med Oncol. 2012;29:2793–2799. doi: 10.1007/s12032-012-0208-x. [DOI] [PubMed] [Google Scholar]
- 866.Lee JE, Bae SH, Choi JY, Yoon SK, You YK, Lee MA. Epirubicin, cisplatin, 5-FU combination chemotherapy in sorafenib-refractory metastatic hepatocellular carcinoma. World J Gastroenterol. 2014;20:235–241. doi: 10.3748/wjg.v20.i1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 867.Thomas MB. Systemic therapy for hepatocellular carcinoma. Cancer J. 2008;14:123–127. doi: 10.1097/PPO.0b013e31816a6058. [DOI] [PubMed] [Google Scholar]
- 868.Petrelli F, Coinu A, Borgonovo K, Cabiddu M, Ghilardi M, Lonati V, et al. Oxaliplatin-based chemotherapy: a new option in advanced hepatocellular carcinoma. A systematic review and pooled analysis. Clin Oncol (R Coll Radiol) 2014;26:488–496. doi: 10.1016/j.clon.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 869.Qin S, Bai Y, Lim HY, Thongprasert S, Chao Y, Fan J, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31:3501–3508. doi: 10.1200/JCO.2012.44.5643. [DOI] [PubMed] [Google Scholar]
- 870.Patrikidou A, Sinapi I, Regnault H, Fayard F, Bouattour M, Fartoux L, et al. Gemcitabine and oxaliplatin chemotherapy for advanced hepatocellular carcinoma after failure of anti-angiogenic therapies. Invest New Drugs. 2014;32:1028–1035. doi: 10.1007/s10637-014-0100-y. [DOI] [PubMed] [Google Scholar]
- 871.Lim TY, Cheong JY, Cho SW, Sim SJ, Kim JS, Choi SJ, et al. Effect of low dose 5-fluorouracil and cisplatin intra-arterial infusion chemotherapy in advanced hepatocellular carcinoma with decompensated cirrhosis. Korean J Hepatol. 2006;12:65–73. [PubMed] [Google Scholar]
- 872.Woo HY, Bae SH, Park JY, Han KH, Chun HJ, Choi BG, et al. A randomized comparative study of high-dose and low-dose hepatic arterial infusion chemotherapy for intractable, advanced hepatocellular carcinoma. Cancer Chemother Pharmacol. 2010;65:373–382. doi: 10.1007/s00280-009-1126-2. [DOI] [PubMed] [Google Scholar]
- 873.Hamada A, Yamakado K, Nakatsuka A, Takaki H, Akeboshi M, Takeda K. Hepatic arterial infusion chemotherapy with use of an implanted port system in patients with advanced hepatocellular carcinoma: prognostic factors. J Vasc Interv Radiol. 2004;15:835–841. doi: 10.1097/01.RVI.0000128815.35555.0E. [DOI] [PubMed] [Google Scholar]
- 874.Ueshima K, Kudo M, Takita M, Nagai T, Tatsumi C, Ueda T, et al. Hepatic arterial infusion chemotherapy using low-dose 5-fluorouracil and cisplatin for advanced hepatocellular carcinoma. Oncology. 2010;78 Suppl 1:148–153. doi: 10.1159/000315244. [DOI] [PubMed] [Google Scholar]
- 875.Kudo M, Izumi N, Sakamoto M, Matsuyama Y, Ichida T, Nakashima O, et al. Survival analysis over 28 years of 173,378 patients with hepatocellular carcinoma in Japan. Liver Cancer. 2016;5:190–197. doi: 10.1159/000367775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 876.Kawaoka T, Aikata H, Hyogo H, Morio R, Morio K, Hatooka M, et al. Comparison of hepatic arterial infusion chemotherapy versus sorafenib monotherapy in patients with advanced hepatocellular carcinoma. J Dig Dis. 2015;16:505–512. doi: 10.1111/1751-2980.12267. [DOI] [PubMed] [Google Scholar]
- 877.Terashima T, Yamashita T, Arai K, Kawaguchi K, Kitamura K, Yamashita T, et al. Beneficial effect of maintaining hepatic reserve during chemotherapy on the outcomes of patients with hepatocellular carcinoma. Liver Cancer. 2017;6:236–249. doi: 10.1159/000472262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 878.Terashima T, Yamashita T, Arai K, Sunagozaka H, Kitahara M, Nakagawa H, et al. Feasibility and efficacy of hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma after sorafenib. Hepatol Res. 2014;44:1179–1185. doi: 10.1111/hepr.12266. [DOI] [PubMed] [Google Scholar]
- 879.Jeong SW, Jang JY, Lee JE, Lee SH, Kim SG, Cha SW, et al. The efficacy of hepatic arterial infusion chemotherapy as an alternative to sorafenib in advanced hepatocellular carcinoma. Asia Pac J Clin Oncol. 2012;8:164–171. doi: 10.1111/j.1743-7563.2012.01543.x. [DOI] [PubMed] [Google Scholar]
- 880.Song DS, Song MJ, Bae SH, Chung WJ, Jang JY, Kim YS, et al. A comparative study between sorafenib and hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. J Gastroenterol. 2015;50:445–454. doi: 10.1007/s00535-014-0978-3. [DOI] [PubMed] [Google Scholar]
- 881.Fukubayashi K, Tanaka M, Izumi K, Watanabe T, Fujie S, Kawasaki T, et al. Evaluation of sorafenib treatment and hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma: a comparative study using the propensity score matching method. Cancer Med. 2015;4:1214–1223. doi: 10.1002/cam4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 882.Choi JH, Chung WJ, Bae SH, Song DS, Song MJ, Kim YS, et al. Randomized, prospective, comparative study on the effects and safety of sorafenib vs. hepatic arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma with portal vein tumor thrombosis. Cancer Chemother Pharmacol. 2018;82:469–478. doi: 10.1007/s00280-018-3638-0. [DOI] [PubMed] [Google Scholar]
- 883.Lyu N, Wang X, Li JB, Lai JF, Chen QF, Li SL, et al. Arterial chemotherapy of oxaliplatin plus fluorouracil versus sorafenib in advanced hepatocellular carcinoma: a biomolecular exploratory, randomized, phase III trial (FOHAIC-1) J Clin Oncol. 2022;40:468–480. doi: 10.1200/JCO.21.01963. [DOI] [PubMed] [Google Scholar]
- 884.Lee J, Han JW, Sung PS, Lee SK, Yang H, Nam HC, et al. Comparative analysis of lenvatinib and hepatic arterial infusion chemotherapy in unresectable hepatocellular carcinoma: a multi-center, propensity score study. J Clin Med. 2021;10:4045. doi: 10.3390/jcm10184045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 885.Ikeda M, Shimizu S, Sato T, Morimoto M, Kojima Y, Inaba Y, et al. Sorafenib plus hepatic arterial infusion chemotherapy with cisplatin versus sorafenib for advanced hepatocellular carcinoma: randomized phase II trial. Ann Oncol. 2016;27:2090–2096. doi: 10.1093/annonc/mdw323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 886.Kudo M, Ueshima K, Yokosuka O, Ogasawara S, Obi S, Izumi N, et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): a randomised, open label, phase 3 trial. Lancet Gastroenterol Hepatol. 2018;3:424–432. doi: 10.1016/S2468-1253(18)30078-5. [DOI] [PubMed] [Google Scholar]
- 887.Kondo M, Morimoto M, Kobayashi S, Ohkawa S, Hidaka H, Nakazawa T, et al. Randomized, phase II trial of sequential hepatic arterial infusion chemotherapy and sorafenib versus sorafenib alone as initial therapy for advanced hepatocellular carcinoma: SCOOP-2 trial. BMC Cancer. 2019;19:954. doi: 10.1186/s12885-019-6198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 888.He M, Li Q, Zou R, Shen J, Fang W, Tan G, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5:953–960. doi: 10.1001/jamaoncol.2019.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 889.Zheng K, Zhu X, Fu S, Cao G, Li WQ, Xu L, et al. Sorafenib plus hepatic arterial infusion chemotherapy versus sorafenib for hepatocellular carcinoma with major portal vein tumor thrombosis: a randomized trial. Radiology. 2022;303:455–464. doi: 10.1148/radiol.211545. [DOI] [PubMed] [Google Scholar]
- 890.Chen S, Xu B, Wu Z, Wang P, Yu W, Liu Z, et al. Pembrolizumab plus lenvatinib with or without hepatic arterial infusion chemotherapy in selected populations of patients with treatment-naive unresectable hepatocellular carcinoma exhibiting PD-L1 staining: a multicenter retrospective study. BMC Cancer. 2021;21:1126. doi: 10.1186/s12885-021-08858-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 891.He MK, Liang RB, Zhao Y, Xu YJ, Chen HW, Zhou YM, et al. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma. Ther Adv Med Oncol. 2021;13:17588359211002720. doi: 10.1177/17588359211002720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 892.Li QJ, He MK, Chen HW, Fang WQ, Zhou YM, Xu L, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: a randomized phase III trial. J Clin Oncol. 2022;40:150–160. doi: 10.1200/JCO.21.00608. [DOI] [PubMed] [Google Scholar]
- 893.Park JW, Kim YJ, Kim DY, Bae SH, Paik SW, Lee YJ, et al. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: the phase III STAH trial. J Hepatol. 2019;70:684–691. doi: 10.1016/j.jhep.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 894.Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao C, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: a phase III, randomized clinical trial (LAUNCH) J Clin Oncol. 2022 Aug 03; doi: 10.1200/JCO.22.00392. [Epub] [DOI] [PubMed] [Google Scholar]
- 895.Robert C, Ribas A, Hamid O, Daud A, Wolchok JD, Joshua AM, et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol. 2018;36:1668–1674. doi: 10.1200/JCO.2017.75.6270. [DOI] [PubMed] [Google Scholar]
- 896.Jansen YJL, Rozeman EA, Mason R, Goldinger SM, Geukes Foppen MH, Hoejberg L, et al. Discontinuation of anti-PD-1 antibody therapy in the absence of disease progression or treatment limiting toxicity: clinical outcomes in advanced melanoma. Ann Oncol. 2019;30:1154–1161. doi: 10.1093/annonc/mdz110. [DOI] [PubMed] [Google Scholar]
- 897.Roayaie S, Blume IN, Thung SN, Guido M, Fiel MI, Hiotis S, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137:850–855. doi: 10.1053/j.gastro.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 898.Belghiti J, Panis Y, Farges O, Benhamou JP, Fekete F. Intrahepatic recurrence after resection of hepatocellular carcinoma complicating cirrhosis. Ann Surg. 1991;214:114–117. doi: 10.1097/00000658-199108000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 899.Liao M, Zhu Z, Wang H, Huang J. Adjuvant transarterial chemoembolization for patients after curative resection of hepatocellular carcinoma: a meta-analysis. Scand J Gastroenterol. 2017;52:624–634. doi: 10.1080/00365521.2017.1292365. [DOI] [PubMed] [Google Scholar]
- 900.Hong Y, Wu LP, Ye F, Zhou YM. Adjuvant intrahepatic injection iodine-131-lipiodol improves prognosis of patients with hepatocellular carcinoma after resection: a meta-analysis. Indian J Surg. 2015;77(Suppl 3):1227–1232. doi: 10.1007/s12262-015-1261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 901.Riaz IB, Riaz H, Riaz T, Rahman S, Amir M, Badshah MB, et al. Role of vitamin K2 in preventing the recurrence of hepatocellular carcinoma after curative treatment: a meta-analysis of randomized controlled trials. BMC Gastroenterol. 2012;12:170. doi: 10.1186/1471-230X-12-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 902.Chu KJ, Lai EC, Yao XP, Zhang HW, Lau WY, Fu XH, et al. Vitamin analogues in chemoprevention of hepatocellular carcinoma after resection or ablation--a systematic review and meta-analysis. Asian J Surg. 2010;33:120–126. doi: 10.1016/S1015-9584(10)60021-8. [DOI] [PubMed] [Google Scholar]
- 903.Zhong J, Xiang B, Ma L, Li L. Conventional oral systemic chemotherapy for postoperative hepatocellular carcinoma: a systematic review. Mol Clin Oncol. 2014;2:1091–1096. doi: 10.3892/mco.2014.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 904.Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344–1354. doi: 10.1016/S1470-2045(15)00198-9. [DOI] [PubMed] [Google Scholar]
- 905.Zhu XD, Li KS, Sun HC. Adjuvant therapies after curative treatments for hepatocellular carcinoma: current status and prospects. Genes Dis. 2020;7:359–369. doi: 10.1016/j.gendis.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 906.Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356:802–807. doi: 10.1016/S0140-6736(00)02654-4. [DOI] [PubMed] [Google Scholar]
- 907.Xu L, Wang J, Kim Y, Shuang ZY, Zhang YJ, Lao XM, et al. A randomized controlled trial on patients with or without adjuvant autologous cytokine-induced killer cells after curative resection for hepatocellular carcinoma. Oncoimmunology. 2016;5:e1083671. doi: 10.1080/2162402X.2015.1083671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 908.Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383–1391.e6. doi: 10.1053/j.gastro.2015.02.055. [DOI] [PubMed] [Google Scholar]
- 909.Yu X, Zhao H, Liu L, Cao S, Ren B, Zhang N, et al. A randomized phase II study of autologous cytokine-induced killer cells in treatment of hepatocellular carcinoma. J Clin Immunol. 2014;34:194–203. doi: 10.1007/s10875-013-9976-0. [DOI] [PubMed] [Google Scholar]
- 910.Hui D, Qiang L, Jian W, Ti Z, Da-Lu K. A randomized, controlled trial of postoperative adjuvant cytokine-induced killer cells immunotherapy after radical resection of hepatocellular carcinoma. Dig Liver Dis. 2009;41:36–41. doi: 10.1016/j.dld.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 911.Weng DS, Zhou J, Zhou QM, Zhao M, Wang QJ, Huang LX, et al. Minimally invasive treatment combined with cytokine-induced killer cells therapy lower the short-term recurrence rates of hepatocellular carcinomas. J Immunother. 2008;31:63–71. doi: 10.1097/CJI.0b013e31815a121b. [DOI] [PubMed] [Google Scholar]
- 912.Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, et al. Sustained efficacy of adjuvant immunotherapy with cytokine-induced killer cells for hepatocellular carcinoma: an extended 5-year follow-up. Cancer Immunol Immunother. 2019;68:23–32. doi: 10.1007/s00262-018-2247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 913.Yoon JS, Song BG, Lee JH, Lee HY, Kim SW, Chang Y, et al. Adjuvant cytokine-induced killer cell immunotherapy for hepatocellular carcinoma: a propensity score-matched analysis of real-world data. BMC Cancer. 2019;19:523. doi: 10.1186/s12885-019-5740-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 914.Wang H, Liu A, Bo W, Feng X, Hu Y, Tian L, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma patients after curative resection, a systematic review and meta-analysis. Dig Liver Dis. 2016;48:1275–1282. doi: 10.1016/j.dld.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 915.Yeo W, Lam KC, Zee B, Chan PS, Mo FK, Ho WM, et al. Hepatitis B reactivation in patients with hepatocellular carcinoma undergoing systemic chemotherapy. Ann Oncol. 2004;15:1661–1666. doi: 10.1093/annonc/mdh430. [DOI] [PubMed] [Google Scholar]
- 916.Nagamatsu H, Itano S, Nagaoka S, Akiyoshi J, Matsugaki S, Kurogi J, et al. Prophylactic lamivudine administration prevents exacerbation of liver damage in HBe antigen positive patients with hepatocellular carcinoma undergoing transhepatic arterial infusion chemotherapy. Am J Gastroenterol. 2004;99:2369–2375. doi: 10.1111/j.1572-0241.2004.40069.x. [DOI] [PubMed] [Google Scholar]
- 917.Jang JW, Kim YW, Lee SW, Kwon JH, Nam SW, Bae SH, et al. Reactivation of hepatitis B virus in HBsAg-negative patients with hepatocellular carcinoma. PLoS One. 2015;10:e0122041. doi: 10.1371/journal.pone.0122041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 918.Lim S, Han J, Kim GM, Han KH, Choi HJ. Hepatitis B viral load predicts survival in hepatocellular carcinoma patients treated with sorafenib. J Gastroenterol Hepatol. 2015;30:1024–1031. doi: 10.1111/jgh.12898. [DOI] [PubMed] [Google Scholar]
- 919.Lee PC, Chao Y, Chen MH, Lan KH, Lee IC, Hou MC, et al. Risk of HBV reactivation in patients with immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. J Immunother Cancer. 2020;8:e001072. doi: 10.1136/jitc-2020-001072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 920.Park JW, Park KW, Cho SH, Park HS, Lee WJ, Lee DH, et al. Risk of hepatitis B exacerbation is low after transcatheter arterial chemoembolization therapy for patients with HBV-related hepatocellular carcinoma: report of a prospective study. Am J Gastroenterol. 2005;100:2194–2200. doi: 10.1111/j.1572-0241.2005.00232.x. [DOI] [PubMed] [Google Scholar]
- 921.Lao XM, Luo G, Ye LT, Luo C, Shi M, Wang D, et al. Effects of antiviral therapy on hepatitis B virus reactivation and liver function after resection or chemoembolization for hepatocellular carcinoma. Liver Int. 2013;33:595–604. doi: 10.1111/liv.12112. [DOI] [PubMed] [Google Scholar]
- 922.Lao XM, Wang D, Shi M, Liu G, Li S, Guo R, et al. Changes in hepatitis B virus DNA levels and liver function after transcatheter arterial chemoembolization of hepatocellular carcinoma. Hepatol Res. 2011;41:553–563. doi: 10.1111/j.1872-034X.2011.00796.x. [DOI] [PubMed] [Google Scholar]
- 923.Jang JW, Choi JY, Bae SH, Yoon SK, Chang UI, Kim CW, et al. A randomized controlled study of preemptive lamivudine in patients receiving transarterial chemo-lipiodolization. Hepatology. 2006;43:233–240. doi: 10.1002/hep.21024. [DOI] [PubMed] [Google Scholar]
- 924.Xu X, Huang P, Tian H, Chen Y, Ge N, Tang W, et al. Role of lamivudine with transarterial chemoembolization in the survival of patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29:1273–1278. doi: 10.1111/jgh.12554. [DOI] [PubMed] [Google Scholar]
- 925.Li X, Zhong X, Chen ZH, Wang TT, Ma XK, Xing YF, et al. Efficacy of prophylactic entecavir for hepatitis B virus-related hepatocellular carcinoma receiving transcatheter arterial chemoembolization. Asian Pac J Cancer Prev. 2015;16:8665–8670. doi: 10.7314/apjcp.2015.16.18.8665. [DOI] [PubMed] [Google Scholar]
- 926.Jang JW, Yoo SH, Nam HC, Jang BH, Sung PS, Lee SW, et al. Association of prophylactic anti-hepatitis B virus therapy with improved long-term survival in patients with hepatocellular carcinoma undergoing transarterial therapy. Clin Infect Dis. 2020;71:546–555. doi: 10.1093/cid/ciz860. [DOI] [PubMed] [Google Scholar]
- 927.Tamori A, Nishiguchi S, Tanaka M, Kurooka H, Fujimoto S, Nakamura K, et al. Lamivudine therapy for hepatitis B virus reactivation in a patient receiving intra-arterial chemotherapy for advanced hepatocellular carcinoma. Hepatol Res. 2003;26:77–80. doi: 10.1016/s1386-6346(03)00002-0. [DOI] [PubMed] [Google Scholar]
- 928.Liu S, Lai J, Lyu N, Xie Q, Cao H, Chen D, et al. Effects of antiviral therapy on HBV reactivation and survival in hepatocellular carcinoma patients undergoing hepatic artery infusion chemotherapy. Front Oncol. 2020;10:582504. doi: 10.3389/fonc.2020.582504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 929.Kubo S, Nishiguchi S, Hamba H, Hirohashi K, Tanaka H, Shuto T, et al. Reactivation of viral replication after liver resection in patients infected with hepatitis B virus. Ann Surg. 2001;233:139–145. doi: 10.1097/00000658-200101000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 930.Huang L, Li J, Yan J, Sun J, Zhang X, Wu M, et al. Antiviral therapy decreases viral reactivation in patients with hepatitis B virus-related hepatocellular carcinoma undergoing hepatectomy: a randomized controlled trial. J Viral Hepat. 2013;20:336–342. doi: 10.1111/jvh.12036. [DOI] [PubMed] [Google Scholar]
- 931.Huang G, Lau WY, Wang ZG, Pan ZY, Yuan SX, Shen F, et al. Antiviral therapy improves postoperative survival in patients with hepatocellular carcinoma: a randomized controlled trial. Ann Surg. 2015;261:56–66. doi: 10.1097/SLA.0000000000000858. [DOI] [PubMed] [Google Scholar]
- 932.Kim JH, Park JW, Kim TH, Koh DW, Lee WJ, Kim CM. Hepatitis B virus reactivation after three-dimensional conformal radiotherapy in patients with hepatitis B virus-related hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2007;69:813–819. doi: 10.1016/j.ijrobp.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 933.Jang JW, Kwon JH, You CR, Kim JD, Woo HY, Bae SH, et al. Risk of HBV reactivation according to viral status and treatment intensity in patients with hepatocellular carcinoma. Antivir Ther. 2011;16:969–977. doi: 10.3851/IMP1840. [DOI] [PubMed] [Google Scholar]
- 934.Jun BG, Kim YD, Kim SG, Kim YS, Jeong SW, Jang JY, et al. Hepatitis B virus reactivation after radiotherapy for hepatocellular carcinoma and efficacy of antiviral treatment: a multicenter study. PLoS One. 2018;13:e0201316. doi: 10.1371/journal.pone.0201316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 935.Dan JQ, Zhang YJ, Huang JT, Chen MS, Gao HJ, Peng ZW, et al. Hepatitis B virus reactivation after radiofrequency ablation or hepatic resection for HBV-related small hepatocellular carcinoma: a retrospective study. Eur J Surg Oncol. 2013;39:865–872. doi: 10.1016/j.ejso.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 936.Yoshida H, Yoshida H, Goto E, Sato T, Ohki T, Masuzaki R, et al. Safety and efficacy of lamivudine after radiofrequency ablation in patients with hepatitis B virus-related hepatocellular carcinoma. Hepatol Int. 2008;2:89–94. doi: 10.1007/s12072-007-9020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 937.Lee JI, Kim JK, Chang HY, Lee JW, Kim JM, Chung HJ, et al. Impact of postoperative hepatitis B virus reactivation in hepatocellular carcinoma patients who formerly had naturally suppressed virus. J Gastroenterol Hepatol. 2014;29:1019–1027. doi: 10.1111/jgh.12472. [DOI] [PubMed] [Google Scholar]
- 938.Chang JI, Sinn DH, Cho H, Kim S, Kang W, Gwak GY, et al. Clinical outcomes of hepatitis B virus-related hepatocellular carcinoma patients with undetectable serum HBV DNA levels. Dig Dis Sci. 2022;67:4565–4573. doi: 10.1007/s10620-021-07312-8. [DOI] [PubMed] [Google Scholar]
- 939.Papatheodoridi M, Tampaki M, Lok AS, Papatheodoridis GV. Risk of HBV reactivation during therapies for HCC: a systematic review. Hepatology. 2022;75:1257–1274. doi: 10.1002/hep.32241. [DOI] [PubMed] [Google Scholar]
- 940.Sung PS, Bae SH, Jang JW, Song DS, Kim HY, Yoo SH, et al. Differences in the patterns and outcomes of enhanced viral replication between hepatitis C virus and hepatitis B virus in patients with hepatocellular carcinoma during transarterial chemolipiodolization. Korean J Hepatol. 2011;17:299–306. doi: 10.3350/kjhep.2011.17.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 941.Carr BI, Pujol L. Pain at presentation and survival in hepatocellular carcinoma. J Pain. 2010;11:988–993. doi: 10.1016/j.jpain.2010.01.265. [DOI] [PubMed] [Google Scholar]
- 942.Ibrahim NM, Abdelhameed KM, Kamal SMM, Khedr EMH, Kotb HIM. Effect of transcranial direct current stimulation of the motor cortex on visceral pain in patients with hepatocellular carcinoma. Pain Med. 2018;19:550–560. doi: 10.1093/pm/pnx087. [DOI] [PubMed] [Google Scholar]
- 943.Chwistek M. Recent advances in understanding and managing cancer pain. F1000Res. 2017;6:945. doi: 10.12688/f1000research.10817.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 944.Hong SH, Roh SY, Kim SY, Shin SW, Kim CS, Choi JH, et al. Change in cancer pain management in Korea between 2001 and 2006: results of two nationwide surveys. J Pain Symptom Manage. 2011;41:93–103. doi: 10.1016/j.jpainsymman.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 945.Kim JY, Jang WY, Hur MH, Lee KK, Do YR, Park KU, et al. Prevalence and management of pain by different age groups of Korean cancer patients. Am J Hosp Palliat Care. 2013;30:393–398. doi: 10.1177/1049909112452624. [DOI] [PubMed] [Google Scholar]
- 946.van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18:1437–1449. doi: 10.1093/annonc/mdm056. [DOI] [PubMed] [Google Scholar]
- 947.Grudzen CR, Richardson LD, Johnson PN, Hu M, Wang B, Ortiz JM, et al. Emergency department-initiated palliative care in advanced cancer: a randomized clinical trial. JAMA Oncol. 2016;2:591–598. doi: 10.1001/jamaoncol.2015.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 948.Bakitas MA, Tosteson TD, Li Z, Lyons KD, Hull JG, Li Z, et al. Early versus delayed initiation of concurrent palliative oncology care: patient outcomes in the ENABLE III randomized controlled trial. J Clin Oncol. 2015;33:1438–1445. doi: 10.1200/JCO.2014.58.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 949.Zimmermann C, Swami N, Krzyzanowska M, Hannon B, Leighl N, Oza A, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383:1721–1730. doi: 10.1016/S0140-6736(13)62416-2. [DOI] [PubMed] [Google Scholar]
- 950.Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 951.Ryu E, Kim K, Cho MS, Kwon IG, Kim HS, Fu MR. Symptom clusters and quality of life in Korean patients with hepatocellular carcinoma. Cancer Nurs. 2010;33:3–10. doi: 10.1097/NCC.0b013e3181b4367e. [DOI] [PubMed] [Google Scholar]
- 952.Verbeeck RK. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol. 2008;64:1147–1161. doi: 10.1007/s00228-008-0553-z. [DOI] [PubMed] [Google Scholar]
- 953.Radner H, Ramiro S, Buchbinder R, Landewé RB, van der Heijde D, Aletaha D. Pain management for inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and other spondylarthritis) and gastrointestinal or liver comorbidity. Cochrane Database Syst Rev. 2012;1:CD008951. doi: 10.1002/14651858.CD008951.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 954.World Health Organization (WHO) Cancer pain relief: with a guide to opioid availability. Geneva: WHO; 1996. [Google Scholar]
- 955.Ministry of Health & Welfare. Cancer pain management guideline. Seoul: Ministry of Health & Welfare; 2012. [Google Scholar]
- 956.National Comprehensive Cancer Network (NCCN) NCCN clinical practice guideline in oncology: adult cancer pain. Vol. 1. Fort Washington: NCCN; 2013. [Google Scholar]
- 957.Rossi S, Assis DN, Awsare M, Brunner M, Skole K, Rai J, et al. Use of over-the-counter analgesics in patients with chronic liver disease: physicians’ recommendations. Drug Saf. 2008;31:261–270. doi: 10.2165/00002018-200831030-00007. [DOI] [PubMed] [Google Scholar]
- 958.Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 959.UFaDAF UDoHaHS. Drugs: acetaminophen information. Washington, D.C.: US Department of Health and Human Services; 2017. [Google Scholar]
- 960.Mofredj A, Cadranel JF, Darchy B, Barbare JC, Cazier A, Pras V, et al. Hepatotoxicity caused by therapeutic doses of paracetamol in alcoholics. Report of 2 cases of fatal hepatitis in cirrhosis. Ann Med Interne (Paris) 1999;150:507–511. [PubMed] [Google Scholar]
- 961.Dart RC, Bailey E. Does therapeutic use of acetaminophen cause acute liver failure? Pharmacotherapy. 2007;27:1219–1230. doi: 10.1592/phco.27.9.1219. [DOI] [PubMed] [Google Scholar]
- 962.Kuffner EK, Green JL, Bogdan GM, Knox PC, Palmer RB, Heard K, et al. The effect of acetaminophen (four grams a day for three consecutive days) on hepatic tests in alcoholic patients--a multicenter randomized study. BMC Med. 2007;5:13. doi: 10.1186/1741-7015-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 963.Heard K, Green JL, Bailey JE, Bogdan GM, Dart RC. A randomized trial to determine the change in alanine aminotransferase during 10 days of paracetamol (acetaminophen) administration in subjects who consume moderate amounts of alcohol. Aliment Pharmacol Ther. 2007;26:283–290. doi: 10.1111/j.1365-2036.2007.03368.x. [DOI] [PubMed] [Google Scholar]
- 964.Khalid SK, Lane J, Navarro V, Garcia-Tsao G. Use of over-the-counter analgesics is not associated with acute decompensation in patients with cirrhosis. Clin Gastroenterol Hepatol. 2009;7:994–999. doi: 10.1016/j.cgh.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 965.Villeneuve JP, Raymond G, Bruneau J, Colpron L, Pomier-Layrargues G. Pharmacokinetics and metabolism of acetaminophen in normal, alcoholic and cirrhotic subjects. Gastroenterol Clin Biol. 1983;7:898–902. [PubMed] [Google Scholar]
- 966.Hirschfield GM, Kumagi T, Heathcote EJ. Preventative hepatology: minimising symptoms and optimising care. Liver Int. 2008;28:922–934. doi: 10.1111/j.1478-3231.2008.01816.x. [DOI] [PubMed] [Google Scholar]
- 967.Benson GD, Koff RS, Tolman KG. The therapeutic use of acetaminophen in patients with liver disease. Am J Ther. 2005;12:133–141. doi: 10.1097/01.mjt.0000140216.40700.95. [DOI] [PubMed] [Google Scholar]
- 968.Chandok N, Watt KD. Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc. 2010;85:451–458. doi: 10.4065/mcp.2009.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 969.Williams RL, Upton RA, Cello JP, Jones RM, Blitstein M, Kelly J, et al. Naproxen disposition in patients with alcoholic cirrhosis. Eur J Clin Pharmacol. 1984;27:291–296. doi: 10.1007/BF00542162. [DOI] [PubMed] [Google Scholar]
- 970.Bessone F. Non-steroidal anti-inflammatory drugs: what is the actual risk of liver damage? World J Gastroenterol. 2010;16:5651–5661. doi: 10.3748/wjg.v16.i45.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 971.Riley TR, 3rd, Smith JP. Ibuprofen-induced hepatotoxicity in patients with chronic hepatitis C: a case series. Am J Gastroenterol. 1998;93:1563–1565. doi: 10.1111/j.1572-0241.1998.00484.x. [DOI] [PubMed] [Google Scholar]
- 972.Ackerman Z, Cominelli F, Reynolds TB. Effect of misoprostol on ibuprofen-induced renal dysfunction in patients with decompensated cirrhosis: results of a double-blind placebo-controlled parallel group study. Am J Gastroenterol. 2002;97:2033–2039. doi: 10.1111/j.1572-0241.2002.05847.x. [DOI] [PubMed] [Google Scholar]
- 973.Castro-Fernández M, Sánchez-Muñoz D, Galán-Jurado MV, Larraona JL, Suárez E, Lamas E, et al. Influence of nonsteroidal antiinflammatory drugs in gastrointestinal bleeding due to gastroduodenal ulcers or erosions in patients with liver cirrhosis. Gastroenterol Hepatol. 2006;29:11–14. doi: 10.1157/13083251. [DOI] [PubMed] [Google Scholar]
- 974.Lee YC, Chang CH, Lin JW, Chen HC, Lin MS, Lai MS. Non-steroidal anti-inflammatory drugs use and risk of upper gastrointestinal adverse events in cirrhotic patients. Liver Int. 2012;32:859–866. doi: 10.1111/j.1478-3231.2011.02739.x. [DOI] [PubMed] [Google Scholar]
- 975.Kotb HI, Fouad IA, Fares KM, Mostafa MG, Abd El-Rahman AM. Pharmacokinetics of oral tramadol in patients with liver cancer. J Opioid Manag. 2008;4:99–104. doi: 10.5055/jom.2008.0014. [DOI] [PubMed] [Google Scholar]
- 976.Christian-Miller N, Frenette C. Hepatocellular cancer pain: impact and management challenges. J Hepatocell Carcinoma. 2018;5:75–80. doi: 10.2147/JHC.S145450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 977.Smith HS. Opioid metabolism. Mayo Clin Proc. 2009;84:613–624. doi: 10.1016/S0025-6196(11)60750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 978.Hasselström J, Eriksson S, Persson A, Rane A, Svensson JO, Säwe J. The metabolism and bioavailability of morphine in patients with severe liver cirrhosis. Br J Clin Pharmacol. 1990;29:289–297. doi: 10.1111/j.1365-2125.1990.tb03638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 979.Tegeder I, Lötsch J, Geisslinger G. Pharmacokinetics of opioids in liver disease. Clin Pharmacokinet. 1999;37:17–40. doi: 10.2165/00003088-199937010-00002. [DOI] [PubMed] [Google Scholar]
- 980.Kotb HI, El-Kady SA, Emara SE, Fouad EA, El-Kabsh MY. Pharmacokinetics of controlled release morphine (MST) in patients with liver carcinoma. Br J Anaesth. 2005;94:95–99. doi: 10.1093/bja/aei007. [DOI] [PubMed] [Google Scholar]
- 981.Tallgren M, Olkkola KT, Seppälä T, Höckerstedt K, Lindgren L. Pharmacokinetics and ventilatory effects of oxycodone before and after liver transplantation. Clin Pharmacol Ther. 1997;61:655–661. doi: 10.1016/S0009-9236(97)90100-4. [DOI] [PubMed] [Google Scholar]
- 982.Durnin C, Hind ID, Ghani SP, Yates DB, Molz KH. Pharmacokinetics of oral immediate-release hydromorphone (Dilaudid IR) in subjects with moderate hepatic impairment. Proc West Pharmacol Soc. 2001;44:83–84. [PubMed] [Google Scholar]
- 983.Haberer JP, Schoeffler P, Couderc E, Duvaldestin P. Fentanyl pharmacokinetics in anaesthetized patients with cirrhosis. Br J Anaesth. 1982;54:1267–1270. doi: 10.1093/bja/54.12.1267. [DOI] [PubMed] [Google Scholar]
- 984.Berry P, Kotha S. Improving end of life care in liver disease. J Hepatol. 2022;76:1225–1226. doi: 10.1016/j.jhep.2021.10.001. [DOI] [PubMed] [Google Scholar]
- 985.Kashima M, Yamakado K, Takaki H, Kaminou T, Tanigawa N, Nakatsuka A, et al. Radiofrequency ablation for the treatment of bone metastases from hepatocellular carcinoma. AJR Am J Roentgenol. 2010;194:536–541. doi: 10.2214/AJR.09.2975. [DOI] [PubMed] [Google Scholar]
- 986.Nagata Y, Nakano Y, Abe M, Takahashi M, Kohno S. Osseous metastases from hepatocellular carcinoma: embolization for pain control. Cardiovasc Intervent Radiol. 1989;12:149–153. doi: 10.1007/BF02577380. [DOI] [PubMed] [Google Scholar]
- 987.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 988.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 989.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 990.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 991.Forner A, Ayuso C, Varela M, Rimola J, Hessheimer AJ, de Lope CR, et al. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer. 2009;115:616–623. doi: 10.1002/cncr.24050. [DOI] [PubMed] [Google Scholar]
- 992.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 993.Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 994.Chiou VL, Burotto M. Pseudoprogression and immune-related response in solid tumors. J Clin Oncol. 2015;33:3541–3543. doi: 10.1200/JCO.2015.61.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 995.Jia W, Gao Q, Han A, Zhu H, Yu J. The potential mechanism, recognition and clinical significance of tumor pseudoprogression after immunotherapy. Cancer Biol Med. 2019;16:655–670. doi: 10.20892/j.issn.2095-3941.2019.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 996.Lee DH, Hwang S, Koh YH, Lee KH, Kim JY, Kim YJ, et al. Outcome of initial progression during nivolumab treatment for hepatocellular carcinoma: should we use iRECIST? Front Med (Lausanne) 2021;8:771887. doi: 10.3389/fmed.2021.771887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 997.Lewis S, Cedillo MA, Lee KM, Bane O, Hectors S, Ma W, et al. Comparative assessment of standard and immune response criteria for evaluation of response to PD-1 monotherapy in unresectable HCC. Abdom Radiol (NY) 2022;47:969–980. doi: 10.1007/s00261-021-03386-0. [DOI] [PubMed] [Google Scholar]
- 998.Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–e152. doi: 10.1016/S1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 999.Llovet JM, Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol. 2020;72:288–306. doi: 10.1016/j.jhep.2019.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1000.Arora A, Kumar A. Treatment response evaluation and follow-up in hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4(Suppl 3):S126–S129. doi: 10.1016/j.jceh.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1001.Lencioni R, Montal R, Torres F, Park JW, Decaens T, Raoul JL, et al. Objective response by mRECIST as a predictor and potential surrogate end-point of overall survival in advanced HCC. J Hepatol. 2017;66:1166–1172. doi: 10.1016/j.jhep.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 1002.Llovet JM, Montal R, Villanueva A. Randomized trials and endpoints in advanced HCC: role of PFS as a surrogate of survival. J Hepatol. 2019;70:1262–1277. doi: 10.1016/j.jhep.2019.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1003.Yamamoto K, Imamura H, Matsuyama Y, Kume Y, Ikeda H, Norman GL, et al. AFP, AFP-L3, DCP, and GP73 as markers for monitoring treatment response and recurrence and as surrogate markers of clinicopathological variables of HCC. J Gastroenterol. 2010;45:1272–1282. doi: 10.1007/s00535-010-0278-5. [DOI] [PubMed] [Google Scholar]
- 1004.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 1005.Llovet JM, Villanueva A, Marrero JA, Schwartz M, Meyer T, Galle PR, et al. Trial design and endpoints in hepatocellular carcinoma: AASLD consensus conference. Hepatology. 2021;73 Suppl 1:158–191. doi: 10.1002/hep.31327. [DOI] [PubMed] [Google Scholar]
- 1006.Hodi FS, Ballinger M, Lyons B, Soria JC, Nishino M, Tabernero J, et al. Immune-modified response evaluation criteria in solid tumors (imRECIST): refining guidelines to assess the clinical benefit of cancer immunotherapy. J Clin Oncol. 2018;36:850–858. doi: 10.1200/JCO.2017.75.1644. [DOI] [PubMed] [Google Scholar]
- 1007.Tazdait M, Mezquita L, Lahmar J, Ferrara R, Bidault F, Ammari S, et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer. 2018;88:38–47. doi: 10.1016/j.ejca.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 1008.Poon RT. Differentiating early and late recurrences after resection of HCC in cirrhotic patients: implications on surveillance, prevention, and treatment strategies. Ann Surg Oncol. 2009;16:792–794. doi: 10.1245/s10434-009-0330-y. [DOI] [PubMed] [Google Scholar]
- 1009.Liu D, Chan AC, Fong DY, Lo CM, Khong PL. Evidence-based surveillance imaging schedule after liver transplantation for hepatocellular carcinoma recurrence. Transplantation. 2017;101:107–111. doi: 10.1097/TP.0000000000001513. [DOI] [PubMed] [Google Scholar]
- 1010.Tanaka K, Shimada H, Matsuo K, Takeda K, Nagano Y, Togo S. Clinical features of hepatocellular carcinoma developing extrahepatic recurrences after curative resection. World J Surg. 2008;32:1738–1747. doi: 10.1007/s00268-008-9613-x. [DOI] [PubMed] [Google Scholar]
- 1011.Hyder O, Dodson RM, Weiss M, Cosgrove DP, Herman JM, Geschwind JH, et al. Trends and patterns of utilization in post-treatment surveillance imaging among patients treated for hepatocellular carcinoma. J Gastrointest Surg. 2013;17:1774–1783. doi: 10.1007/s11605-013-2302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1012.Zheng J, Chou JF, Gönen M, Vachharajani N, Chapman WC, Majella Doyle MB, et al. Prediction of hepatocellular carcinoma recurrence beyond Milan criteria after resection: validation of a clinical risk score in an international cohort. Ann Surg. 2017;266:693–701. doi: 10.1097/SLA.0000000000002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1013.Dioguardi Burgio M, Ronot M, Fuks D, Dondero F, Cauchy F, Gaujoux S, et al. Follow-up imaging after liver transplantation should take into consideration primary hepatocellular carcinoma characteristics. Transplantation. 2015;99:1613–1618. doi: 10.1097/TP.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 1014.Kovalic AJ, Satapathy SK, Thuluvath PJ. Prevalence of chronic liver disease in patients with COVID-19 and their clinical outcomes: a systematic review and meta-analysis. Hepatol Int. 2020;14:612–620. doi: 10.1007/s12072-020-10078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1015.Yang H, Xu J, Liang X, Shi L, Wang Y. Chronic liver disease independently associated with COVID-19 severity: evidence based on adjusted effect estimates. Hepatol Int. 2021;15:217–222. doi: 10.1007/s12072-020-10133-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1016.Mallet V, Beeker N, Bouam S, Sogni P, Pol S Demosthenes research group. Prognosis of French COVID-19 patients with chronic liver disease: a national retrospective cohort study for 2020. J Hepatol. 2021;75:848–855. doi: 10.1016/j.jhep.2021.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1017.Kim D, Adeniji N, Latt N, Kumar S, Bloom PP, Aby ES, et al. Predictors of outcomes of COVID-19 in patients with chronic liver disease: US multi-center study. Clin Gastroenterol Hepatol. 2021;19:1469–1479.e19. doi: 10.1016/j.cgh.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1018.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1019.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1020.Oliver SE, Wallace M, See I, Mbaeyi S, Godfrey M, Hadler SC, et al. Use of the Janssen (Johnson & Johnson) COVID-19 vaccine: updated interim recommendations from the Advisory Committee on Immunization Practices-United States, December 2021. MMWR Morb Mortal Wkly Rep. 2022;71:90–95. doi: 10.15585/mmwr.mm7103a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1021.Gargano JW, Wallace M, Hadler SC, Langley G, Su JR, Oster ME, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices - United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977–982. doi: 10.15585/mmwr.mm7027e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1022.National Comprehensive Cancer Network (NCCN) NCCN: cancer and COVID-19 vaccination version 7.0. Plymouth Meeting: NCCN; 2022. [Google Scholar]
- 1023.Waissengrin B, Agbarya A, Safadi E, Padova H, Wolf I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021;22:581–583. doi: 10.1016/S1470-2045(21)00155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1024.Blumenthal KG, Freeman EE, Saff RR, Robinson LB, Wolfson AR, Foreman RK, et al. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N Engl J Med. 2021;384:1273–1277. doi: 10.1056/NEJMc2102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1025.Bril F, Al Diffalha S, Dean M, Fettig DM. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: causality or casualty? J Hepatol. 2021;75:222–224. doi: 10.1016/j.jhep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1026.McShane C, Kiat C, Rigby J, Crosbie Ó. The mRNA COVID-19 vaccine - a rare trigger of autoimmune hepatitis? J Hepatol. 2021;75:1252–1254. doi: 10.1016/j.jhep.2021.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1027.Wang Y, Tian H, Zhang L, Zhang M, Guo D, Wu W, et al. Reduction of secondary transmission of SARS-CoV-2 in households by face mask use, disinfection and social distancing: a cohort study in Beijing, China. BMJ Glob Health. 2020;5:e002794. doi: 10.1136/bmjgh-2020-002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1028.Brooks JT, Butler JC. Effectiveness of mask wearing to control community spread of SARS-CoV-2. JAMA. 2021;325:998–999. doi: 10.1001/jama.2021.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.