Abstract
Face-processing deficits, while not required for the diagnosis of Autism Spectrum Disorder (ASD), have been associated with impaired social skills—a core feature of ASD; however, the strength and prevalence of this relationship remains unclear. Across 445 participants from the NIMH Data Archive, we examined the relationship between Benton Face Recognition Test (BFRT) performance and Autism Diagnostic Observation Schedule-Social Affect (ADOS-SA) scores. Lower BFRT scores (worse face-processing performance) were associated with higher ADOS-SA scores (higher ASD severity)–a relationship that held after controlling for other factors associated with face processing, i.e., age, sex, and IQ. These findings underscore the utility of face discrimination, not just recognition of facial emotion, as a key covariate for the severity of symptoms that characterize ASD.
Keywords: face perception, face processing, face discrimination, social interaction, social cognition, autism spectrum disorder
Autism Spectrum Disorder (ASD) is a heterogeneous neurodevelopmental disability that affects one in 54 people in the United States (Maenner 2020) and is characterized by persistent impairments in two broad domains: (1) social communication and interaction, as well as (2) the presence of restricted and repetitive patterns of behavior, interests, or activities (American Psychiatric Association 2013). Although assessment of deficits in these two domains guide diagnosis and are used as measures of clinical severity, it is clear that they do not capture the full scope of actual impairment in everyday function that individuals with ASD experience. Comorbid symptoms, such as intellectual delay, language deficits, impaired attention, aggression, anxiety, and depression can often have a greater impact on function than do the core ASD symptoms themselves (Constantino and Charman 2016; Senland and Higgins-D’Alessandro 2016).
One symptom of particular interest, though not part of the DSM-5 criteria for ASD, is difficulty with face processing, i.e., the ability to recognize or utilize facial information such as identity, emotion, and gaze cueing (Dawson et al. 2004, 2005; McPartland et al. 2004). Most notably, individuals with ASD often demonstrate difficulty interpreting emotional expressions from faces, which likely contributes to impairments in social function (Ashwin et al. 2006; Lindner and Rosén 2006; Philip et al. 2010; Wallace et al. 2008). Similarly, individuals with developmental prosopagnosia, typically defined as isolated face-processing deficits in the absence of ASD, also describe difficulty recognizing close friends and relatives, which patients report can lead to traumatic social interactions, and avoidance of social situations, in turn resulting in an increased dependence on others, restricted social circles, reduced employment opportunities, and loss of self-confidence (Yardley et al. 2008).
While recognizing and understanding emotional content has clear ramifications for social development, difficulty with face identity recognition/discrimination, separate from recognition of facial emotion, also appears to play a role, with an extensive literature examining the face-processing abilities in ASD which has documented behavioral and neurological differences among individuals with ASD when compared to normative populations (Weigelt et al. 2012). While controversial, this literature appears to indicate larger quantitative differences in face perception/identity discrimination than qualitative differences in how individuals with ASD perform face perception (Dawson et al. 2005; Golarai et al. 2006; Jemel et al. 2006; Marcus and Nelson 2001; Pierce and Courchesne 2000; Sasson 2006; Schultz 2005; Simmons et al. 2009; Tang et al. 2015; Weigelt et al. 2012). As one example, a consistent finding appears to be that individuals with ASD perform significantly worse during face memory tasks, even with minimal demands, e.g., sequential vs simultaneous presentation, and that this difference is specific for face vs other visual stimuli (Boucher and Lewis 1992; Gelder et al. 1991; Hauck et al. 1998; McPartland et al. 2011).
Differences in face recognition/perception also appear to arise quite early in development (Baron-Cohen et al. 1996; Marcus and Nelson 2001; Osterling et al. 2002; Osterling and Dawson 1994). While difficult to study due to delay in diagnosis, ASD-related group-level differences in face processing have been observed as early as two years of age (Corbett et al. 2014; Harms et al. 2010; Klin et al. 1999; Tanaka et al. 2008), and even earlier using electrophysiological techniques (McCleery et al. 2009). Two non-exclusive explanations have been put forward to explain this linkage: that underlying face-processing deficits may lead to a failure to attribute a special status to facial stimuli, and that a reduced underlying social interest leads to the lack of reinforcement-based training for face discrimination (Sasson 2006). Regardless of the directionality of the initial developmental perturbation, it is very likely that face-processing deficits influence early communication and social-skills development in ASD, and face-processing impairment may represent a potential treatment target for ASD in addition to potentially serving as a biomarker for early ASD diagnosis (McPartland et al. 2020; Webb et al. 2020).
To date, many studies that have examined face-processing abilities in ASD have focused on group-level comparisons, with heterogeneous results across tasks and modalities as noted above (Tang et al. 2015; Weigelt et al. 2012). As such, the strength of the relationship between face recognition/discrimination ability and social skills in ASD, as well as the overall prevalence of face-processing difficulties in ASD has been difficult to characterize (Weigelt et al. 2012). It also remains unclear: 1) to what degree performance on face recognition/discrimination tasks is associated with social affect independently of other important variables such as age, sex, and IQ, since each of these variables also impacts sociability, and 2) whether this relationship is specific to social affect or whether face-processing ability correlates with overall ASD symptom severity. Thanks to the efforts of the National Institute of Mental Health (NIMH) and countless hours of collaborative work, a large-scale federally funded database now exists that consolidates acquired data from thousands of research participants in a secure and de-identified fashion: the National Database for Autism Research (NDAR), which is part of the NIMH Data Archive (NDA), (Hall et al. 2012). In this study, we leverage the availability of these data to determine the strength of the relationship between face-processing difficulties, as measured by the Benton Face Recognition Test (BFRT), (Benton and Van Allen 1968), and social affect, as measured by the Autism Diagnostic Observation Schedule Social Affect score (ADOS-SA), (Lord et al. 1989), both available in a large number of participants across a broad spectrum of ages. We then assess whether this relationship is specific to the social-affect domain of the ADOS and whether any identified relationships are independent of age, sex, and IQ.
Methods
Participants
We obtained de-identified phenotypic and behavioral data from datasets assessing ASD-related symptoms from the NIMH Data Archive (NDA, 2019), an NIH-funded research data repository. Our study utilized data from three independent NDA datasets: collections #2179, PI: James McPartland (Dataset 1), #6, PI: Nancy Minshew (Dataset 2), and #2312, PI: James McPartland (Dataset 3). These datasets were chosen due to the depth of information available for each participant, including results from the Autism Diagnostic Observation Scale (ADOS), the Benton Face Recognition Test (BFRT), as well as standardized measures of general intellectual ability.
Behavioral Measures
The following measures were downloaded, stored, and analyzed in accordance with the NDA Data Use Certification:
The ADOS-2 is a semi-structured diagnostic tool administered by trained individuals who utilize behavioral observation and interaction to quantify ASD-related symptoms (Lord et al. 2012). The Social Affect sub-score of the ADOS-2 (ADOS-SA) is an aggregate item that measures social affect through joint attention, eye contact, initiation of social communication, reciprocal interaction, and other age-related social behavior items. The other core sub-score of the ADOS-2 assesses the presence of Repetitive and Restrictive Behaviors (ADOS-RRB), such as hand flapping, fixation with specific aspects of objects, and restricted interests (Kim and Lord 2010; Lord et al. 2012). Taken together, ADOS-SA and ADOS-RRB can be combined to generate an ADOS-Total score that correlates with autism severity and is often used to support a clinical ASD diagnosis (Gotham et al. 2009). Because the underlying studies were performed during the timeframe in which the ADOS-2 entered widespread adoption, ADOS-Total, ADOS-SA, and RRB subscale scores from either the ADOS or ADOS-2 (modules 1–3) were utilized as they were available (Lord et al. 2012). Terminology for ASD diagnoses varied across each dataset and participants with descriptions of “Autism,” “Autism Spectrum,” “Asperger’s Syndrome,” and “PDD-NOS (Pervasive Development Disorder-Not Otherwise Specified),” were considered to have clinical diagnoses of ASD.
In the Benton Face Recognition Test (BFRT), participants match a presented photograph of a target to a photograph of the same target from a field of distractors; for each item, participants are required to discriminate the target individual from six faces presented simultaneously. The angle of the faces presented in the photographs is varied throughout three conditions to show alternate facial perspectives. Two versions of the BFRT exist: a short form which is composed of 13 items with a total of 27 possible points (Levin et al. 1975), and a long form of 22 items with a total of 54 possible points (Duchaine and Nakayama 2004). This task measures perceptual discrimination and matching of unfamiliar faces, which are distinct from tasks that measure the recognition of known facial identity (Benton et al. 1994; Benton and Van Allen 1968; Duchaine and Nakayama 2004). Here, we used the long form of the BFRT as a measure of “faceprocessing” performance, where, per prior literature, a score above 40 out of 54 is considered as evidence for typical face-matching ability.
Finally, intellectual ability was measured by intelligence-quotient (IQ) scores, obtained either from the Differential Ability Scales second edition (DAS-II) in Dataset 1 or from the Wechsler Abbreviated Scale of Intelligence (WASI) obtained from Datasets 2 and 3. The DAS-II has been validated for use in participants 2.5–18 years of age and is composed of 20 cognitive subtests, 17 of which originate from the original DAS (Elliott 1990, 2007). The DAS-II provides a General Conceptual Ability (GCA) score based on verbal ability, non-verbal reasoning, and spatial ability (Elliott 2007). The spatial ability subset of the DAS-II (DAS-Spatial) measures the ability to construct patterns by replicating designs and the capacity to copy a figure, and was used in this analysis as an alternative, non-facial, task requiring visual discrimination (Elliott et al. 2018). The WASI has been validated for participants 6–90 years of age and yields composite scores spanning comprehension and reasoning across multiple domains (Wechsler 1999). Here, we used the Full-Scale Intelligence Quotient (FSIQ-4), based on four domain sub-scores including the Verbal Comprehension Index (VCI), Perceptual Reasoning Index (PRI), Working Memory Index (WMI), and Processing Speed Index (PSI).
Statistical Analysis
We first assessed the overall relationship between face-discrimination performance and ADOS-Total in our combined study sample using simple linear regression. Then, we assessed the relationship between BFRT performance and ADOS-SA by constructing a family of multivariate linear models in which we sequentially added salient independent covariates, namely, age, sex, and IQ, to determine whether this relationship persisted when controlling for these variables. We repeated this analysis for ADOS-RRB to assess the degree to which BFRT performance is associated with either or both symptom domains of ASD as measured by the ADOS across a sample of individuals with and without clinical diagnoses of ASD.
Second, we examined the effect of age, sex, and IQ on the relationship between BFRT performance and ADOS-SA. Age and IQ, obtained from GCA and FSIQ-4, were considered as continuous variables. For both age and IQ, we then conducted post-hoc analyses to determine whether there was an interaction between age or IQ z-scores and the relationship between BFRT and ADOS-SA. For age, data was discretized into four age groups, chosen to approximate prior studies focusing on children, adolescents, young adults, and adults in general: (1) 0–12 years old, (2) 12–18 years old, (3) 18–26 years old, and (4) older than 26 years old. This generated groups of 85, 124, 129, and 124 participants respectively. IQ scores, obtained from GCA and FSIQ-4, were converted into z-scores and divided into quartiles, generating groups of 111 participants each.
Third, to examine for potential batch effects as well as to determine whether our findings would be generalizable in smaller subsets of individuals, we tested whether any of the relationships observed across our combined sample were consistent within each of the three underlying datasets. This involved performing the analysis noted above for each dataset, i.e., first identifying whether a relationship between face-processing task performance and social-affect scores existed in each dataset and then constructed multivariate linear models for each dataset to evaluate the influence of the described independent variables.
Finally, to explore the question of whether any relationship observed between BFRT performance and ADOS-SA was the result of impaired visual-spatial processing, we incorporated an available measure that also required visual discrimination but was not specific to face discrimination, i.e., the DAS-Spatial subscore from Dataset 1. We compared two multivariate linear models, one of which related ADOS-SA to DAS-Spatial, age, sex, and IQ, and a second model which was identical, but additionally included BFRT performance as an independent predictor. This analysis was repeated for ADOS-Total as a dependent variable to assess for alteration of the relationship between face processing and autism severity in general.
All data were analyzed using R v4.0.0 with RStudio v1.1.456 (RStudio Inc., Boston, MA). Code is available upon request and data are available directly from the NIMH NDA with an appropriately signed Data Use Agreement.
Results
Demographics and BFRT vs. ADOS-Total
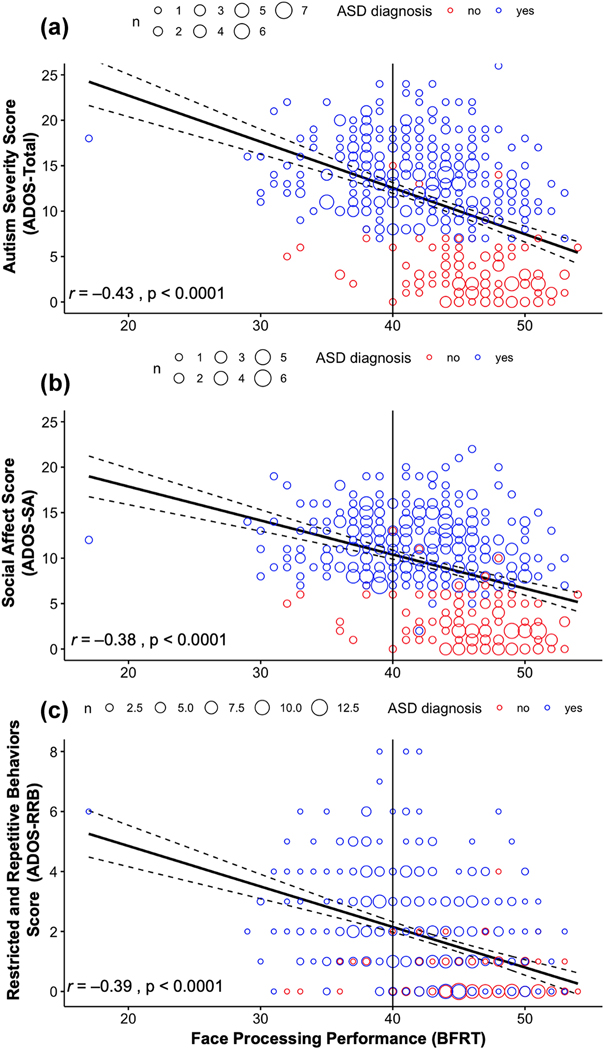
We identified 445 unique participants with all available measures of interest, derived from the three NDA collections, referred to here as: Dataset 1 (n = 92), Dataset 2 (n = 224), and Dataset 3 (n = 135). Of the study sample, 325 (73%) received a diagnosis of ASD. The average age of this sample was 21.28 years old (SD = 9.99; ages = 6.17–59.5 years old), and 79% were male, consistent with the M:F prevalence of ASD diagnosis (Table 1). Additionally, the sample had a mean score on the BFRT of 42.88 (SD = 5.18; ages = 17–54 years old), a mean ADOS-SA score of 9.33 (SD = 5.09, ages = 0–22 years old), and a mean ADOS-RRB of 1.76 (SD = 1.77, ages = 0–8 years old). Figure 1 shows the relationship between face processing performance as measured by the BFRT (abscissa) and ADOS-RRB, ADOS-SA, and ADOS-Total, an aggregate score of ADOS-SA and ADOS-RRB (ordinate). As can be seen in the top panel (a), BFRT scores and ADOS-Total are inversely related: a decrease in face discrimination ability was associated with an increase in symptom severity across all participants (r = −0.43; p < 0.001). Additionally, 32% of all participants scored below the typically used cut-off for normal performance on the BFRT; 40% of the participants with clinical ASD diagnoses and 8% of participants without clinical ASD diagnoses. As depicted in Figure 1, a participant with a BFRT score of 17 and an ADOS-Total score of 18 was included in our sample; regression analyses performed with and without this participant did not change the significance or magnitude of any reported effects.
Table 1.
Descriptive statistics for participants in the combined study sample and in each dataset
| Study Sample | Dataset 1 | Dataset 2 | Dataset 3 | |
|---|---|---|---|---|
| Total participants, n | 445 | 92 | 224 | 135 |
| Participants with ASD diagnosis, n (%) | 325 (73) | 87 (95) | 190 (85) | 53 (39) |
| Male sex, n (%) | 352 (79) | 69 (75) | 192 (86) | 96 (71) |
| Mean age at ADOS testing | ||||
| Years (SD) | 21.28 (9.99) | 12.99 (2.76) | 21.88 (11.52) | 25.79 (6.06) |
| Range | 6.17–59.5 | 7.17–17.92 | 6.17–59.5 | 17.92–48.0 |
| Mean IQ scorea | ||||
| Score (SD) | n/a | 100.4 (19.98) | 110.5 (13.19) | 102.9 (17.43) |
| Range | n/a | 48–161 | 75–140 | 62–142 |
| Mean BFRT | ||||
| Score (SD) | 42.88 (5.18) | 40.49 (5.14) | 42.25 (5.18) | 45.53 (4.00) |
| Range | 17–54 | 17–53 | 29–53 | 33–54 |
| Mean DAS-II-Spatial | ||||
| Score (SD) | n/a | 97.95 (17.19) | n/a | n/a |
| Range | n/a | 58–142 | n/a | n/a |
| Mean ADOS-Total | ||||
| Score (SD) | 11.09 (6.10) | 12.62 (4.24) | 13.12 (5.76) | 6.62 (5.25) |
| Range | 0–26 | 3–24 | 0–26 | 0–21 |
| Mean ADOS-SA | ||||
| Score (SD) | 9.33 (5.09) | 10.46 (3.76) | 10.77 (4.81) | 6.14 (4.85) |
| Range | 0–22 | 2–19 | 0–22 | 0–19 |
| Mean ADOS-RRB | ||||
| Score (SD) | 1.76 (1.77) | 2.16 (1.91) | 2.35 (1.73) | 0.47 (0.84) |
| Range | 0–8 | 0–8 | 0–8 | 0–5 |
ADOS Autism Diagnostic Observation Schedule, ADOS-RRB, ADOS Repetitive and Restrictive Behaviors, ADOS-SA ADOS Social Affect, ASD Autism Spectrum Disorder, BFRT Benton Facial Recognition Test, DAS-II Differential Ability Scales second edition, IQ Intelligence Quotient
as measured using the General Conceptual Ability (GCA) score of the DAS-II in Dataset 1 or the Full-Scale Intelligence Quotient, four subsets (FSIQ-4) of the Wechsler Abbreviated Scale of Intelligence (WASI) in Datasets 2 and 3
Fig 1:
Face-processing performance compared to ADOS-Total, ADOS-SA, and ADOS-RRB scores across the entire study cohort.
BFRT vs. ADOS-SA and ADOS-RRB
Similarly, using our combined dataset, the middle panel (b) indicates a negative correlation between BFRT performance and ADOS-SA (r = −0.38, p < 0.001), linear regression identified a 0.38-point decrease in ADOS-SA for each one-point increase in BFRT (Table 2, Model 1), with a smaller but still significant relationship evident after controlling for age, sex, and IQ (b = −0.25, p < 0.001, i.e., a 0.25-point decrease in ADOS-SA for each one-point increase in BFRT) (Table 2, Model 4). Finally, BFRT performance was also negatively correlated with ADOS-RRB (r = −0.39, p < 0.001; Figure 1c), with linear regression finding a 0.14-point decrease in ADOS-RRB for each one-point increase in BFRT, which also decreased slightly after all independent covariates were included in a model to a 0.11-point decrease for each one-point increase in BFRT (Appendix B, Model 1 and 4).
Table 2:
Family of linear regression models relating ADOS-SA to BFRT (Model 1), ADOS-SA to BFRT and Age (Model 2), ADOS-SA to BFRT, Age, and Sex (Model 3), and ADOS-SA to BFRT, Age, Sex, and IQ (Model 4), across 445 Age participants
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| BFRT | ||||
| Estimate [95% CI] | −0.38 [−0.46, −0.29] | −0.29 [−0.39, −0.20] | −0.28 [−0.37, −0.18] | −0.25 [−0.34, −0.15] |
| P | < 0.0001*** | < 0.0001*** | < 0.0001*** | < 0.0001*** |
| Age | ||||
| Estimate [95% CI] | — | −0.09 [−0.14, −0.04] | −0.09 [−0.14, −0.04] | −0.08 [−0.13, −0.04] |
| P | — | 2.45×10−4 ** | 1.75×10−4 ** | 4.69×10−4 ** |
| Sex (male) | ||||
| Estimate [95% CI] | — | — | 2.05 [0.99, 3.10] | 1.76 [0.71, 2.81] |
| P | — | — | 1.55×10−4 ** | 1.09×10−3 * |
| IQ (z-scores) | ||||
| Estimate [95% CI] | — | — | — | −0.84 [−1.28, −0.40] |
| p | — | — | — | 1.84×10−4 ** |
| AIc | 2646.702 | 2635.147 | 2622.687 | 2610.529 |
| Adjusted R 2 | 0.1383 | 0.1661 | 0.191 | 0.2145 |
| r | −0.38 | −0.41 | −0.44 | −0.47 |
ADOS-SA Autism Diagnostic Observation Schedule Social Affect, BFRT Benton Facial
Recognition Test, CI Confidence Interval
p < 0.01
p < 0.001
p < 0.0001
BFRT performance explained a similar amount of the variation in both ADOS-SA (13.8%) and ADOS-RRB (15.4%) when independent covariates were ignored; however, models including major identifiable independent covariates accounted for a slightly greater proportion of the variance in ADOS-SA (21.5%) than in ADOS-RRB (16.7%). BFRT performance and age were always statistically significant independent predictors of ADOS-SA (Table 2) and ADOS-RRB (Appendix B), while sex and IQ were not independent predictors of ADOS-RRB.
Effect of Specific Covariates on Social Affect
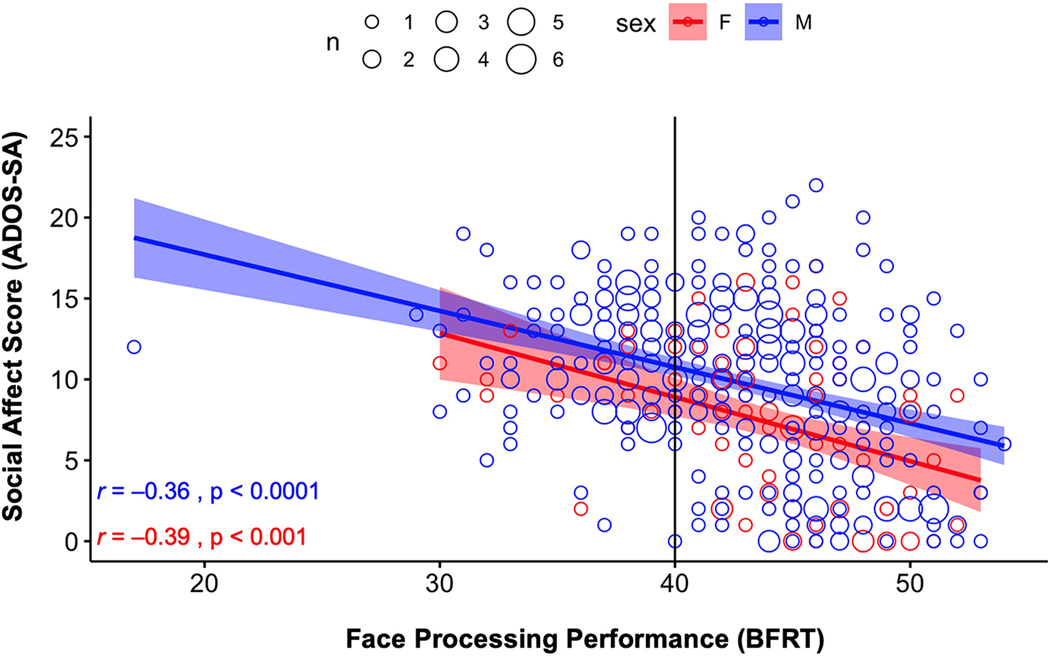
As can be seen in Figure 2, the relationship between face discrimination and social affect was similar for males (r = −0.36, p < 0.001) and females (r = −0.39, p < 0.001); however, males generally demonstrated lower face-processing scores (worse task-performance) and social-affect scores (more severe social affect deficits) than did females (BFRT average = 42.59 vs. 43.99, p = 0.0125; ADOS-SA average = 9.85 vs. 7.32, p < 0.001). Age also accounted for an additional slight but independent variance in ADOS-SA scores (a 0.09-point decrease in ADOS-SA for every one-year increase in age). Inclusion of age in a multiple linear regression model also resulted in an increase in Adjusted R2 from 0.138 to 0.166 (Table 2, Model 2). Post-hoc analyses also revealed an interaction effect, such that the relationship between face-processing performance and ADOS-SA was greater in children and adult participants than in adolescents.
Fig 2:
Face-processing performance compared to ADOS-SA scores for male and female participants.
Finally, as shown in Figure 3, the relationship between face-processing performance and IQ was also statistically significant across all participants (r = 0.23, p < 0.001; Figure 3a); however, this relationship was more than twice as strong in females as compared to males (r = 0.39, p < 0.001 vs. r = 0.18, p < 0.001; respectively; Figure 3b). When combined into a model relating face-processing performance, age, sex, and IQ to ADOS-SA, a 0.84-point decrease in ADOS-SA was associated with each one-point increase in IQ (Table 2, Model 4). Post-hoc analyses also revealed an interaction between face-processing performance and IQ such that the relationship between face processing and ADOS-SA was weaker among participants with lower IQ scores and greater in those with higher IQ scores.
Fig 3:
Face-processing performance compared to standardized IQ scores for all participants and for male and female participants.
Assessing Consistency Across Subsets
The relationship between face-processing performance and social-affect score was also statistically significant in independent models generated from two of the three datasets (Dataset 2 and Dataset 3), with and without controlling for the independent covariates identified above, i.e., age, sex, and IQ. In Dataset 1, with the smallest sample size, the relationship between BFRT and ADOS-SA was not statistically significant across models; however, neither were any of the other independent predictors (Appendix A).
Assessing Visuospatial Ability as a Predictor of ADOS-SA and ADOS-Total
In Dataset 1, the relationship between DAS-Spatial and ADOS-SA was also not statistically significant after controlling for age, sex, and IQ (p = 0.615; Appendix C, Model 1). In a model that included these independent covariates and added BFRT performance, DAS-Spatial was again not a significant predictor of ADOS-SA (p = 0.720; Appendix C, Model 2). However, in a model controlling for all independent covariates predicting ADOS-Total (ADOS-SA and ADOS-RRB combined), BFRT performance was a significant predictor of ADOS-Total (p = 0.0421), whereas DAS-Spatial was not (p = 0.615) (Appendix C, Model 4).
Discussion
Here, we identified that decreased performance on a commonly used face-discrimination task are consistently correlated with higher ADOS scores, and the Social Affect subscale in particular, in an independent manner from a number of important covariates. We also empirically confirm that: 1) a substantial proportion of individuals with ASD have difficulty with face processing and 2) this difficulty is consistently correlated with overall symptom severity, impaired social skills, and increased restrictive and repetitive behaviors. The implications of this study are discussed below.
Face-Processing Impairment Correlates with Symptom Severity
The ASD literature has numerous reports of atypical face processing in individuals with ASD; yet it has been unclear whether this is a finding in all individuals with ASD or whether these group-level effects are driven by a subgroup of individuals that are more significantly affected (Dawson et al. 2004; McPartland et al. 2004; Weigelt et al. 2012). Leveraging the large sample sizes and high-quality phenotypic data available through the NDA, we observed that face-processing impairment affected a substantial proportion (40%) of individuals with clinical ASD diagnoses vs only 8% of the assessed individuals without clinical ASD diagnoses, consistent with Benton’s original study of 286 typically developing adults also finding ~8% scoring below the same cutoff of 40 (Benton et al. 1994). Furthermore, the distribution of face-discrimination scores, both above and below this cutoff, was negatively correlated with ADOS severity scores, empirically establishing an association between measures of autism severity and face-processing ability that does not include emotional assessment. We also found that the relationship between face-processing performance and ADOS-SA was significant even in models including the effects of age, sex, or IQ. Our findings were separately identifiable in two of our three individual datasets, derived from different NDA collections with differing ages, reinforcing the need for large cohorts to effectively examine multifactor models. Of note, while the observed relationship was stronger for the ADOS Social Affect subscale, it was also present for the ADOS Restrictive and Repetitive Behaviors subscale (discussed below).
ADOS-SA and ADOS-RRB are Both Correlated with Face-Processing Impairment
We hypothesized that face-processing measures would be correlated with social skill development and not necessarily with the presence of repetitive and restricted behaviors. However, we found that face-processing performance was also the independent factor with the strongest association with ADOS-RRB, although ADOS-RRB would be expected, by definition, to correlate to some degree with ADOS-SA in participants with ASD. RRBs include not only atypical movements such as stereotypies and restricted interests, but also sensory processing deficits including hypo-reactivity to sensory input or focused interest in sensory aspects of the environment (American Psychiatric Association 2013). As such, we speculate that the same neuropsychological differences that result in impaired face-processing ability also likely affect other sensory processing circuits and behaviors. Regardless, after controlling for independent covariates, BFRT performance explains a higher proportion of variance of ADOS-SA than ADOS-RRB. Below, we discuss our analysis of BFRT vs ADOS-SA in further detail; however parallel results for ADOS-RRB are presented in Appendix B.
The Effect of Age on the Relationship Between Face Processing and Social Affect
Across our study cohort, with an age range of 5.17 years old–64.5 years old, mean face-processing scores increased in a relatively linear fashion; however, the relationship between face processing and social affect varied across age. Specifically, while BFRT and social-affect scores were negatively associated in children and adults, no correlation was detected for 12–18-year-olds. This ‘flattening’ of the relationship during adolescence may represent a number of factors, consistent with the observation that different components of face processing appear to develop at differing rates throughout this time of rapid biological and psychological change (Mamashli et al. 2018; O’Hearn et al. 2010, 2014). For instance, sensitivity to complex facial expressions appears to improve later during childhood when compared with the recognition of basic facial expressions that are critical in infancy (Motta-Mena and Scherf 2016). Studies that have examined the development of face recognition across child maturation have also postulated that there is a bi-directional relationship between the development of face-processing skills and experiences that are crucial for shaping behaviors (Scherf and Scott 2012). Physical maturation, i.e., hormonal changes and altered cognitive neuroplasticity, and changes in social dynamics that occur during adolescence likely also reinforce changes in interest, motivation, and social abilities that have a complex interaction with face-processing ability. Some research, in typically developing children, has emphasized that changes in hormones might increase motivation to master new peer-oriented development, which in turn may prompt the emergence of new socialaffective components of face processing (Scherf and Scott 2012). These developmental changes, occurring at different times and at different rates for different people, likely lead to increased variability both across studied individuals and within them in regard to social affect, or a latent factor, e.g., besides IQ, sex, and age, that may disproportionately affect adolescents. Furthermore, there may be increased testing variability during adolescence, e.g., due to inconsistent social perception among participants and administrators, or that the social interactions tested by the ADOS are less consistently reflective of actual affect ability during these years. However, while meta-analytic research has demonstrated a high level of heterogeneity in ADOS scores across studies measuring the social-affect subdomain, there is also strong group-wise temporal/developmental stability, and the lack of a persistent correlation between BFRT and ADOS-SA score during adolescence is unlikely to be due to methodological issues alone (Bieleninik et al. 2017).
The Effects of Sex Differences on the Relationship Between Face Processing and Social Affect
While not a huge effect, males demonstrated higher (more severe autism) ADOS-SA scores than did their female peers. Sex differences in social affect are not restricted to the ASD population, and there is a long line of evidence demonstrating higher performance on empathy tasks in females as well as greater accuracy decoding emotion from facial expressions (Simon Baron-Cohen and Wheelwright 2004; Biele and Grabowska 2006; Mancini et al. 2013; Montagne et al. 2005). These findings have been linked to sex differences in the structure of the amygdala-hippocampal complex (Campbell et al. 2002) as well as increased levels of oxytocin, thought to promote social bonding, while others have postulated that females make greater efforts to decode the emotions of others due to increased social pressure to ‘fit in’(Carter 2007; Rynkiewicz et al. 2016; Rynkiewicz and Łucka 2018). This, in turn, may contribute to the finding that females receive lower ADOS scores in relation to their ASD severity when compared to males — the female protective effect (Ferri et al. 2018). Relatedly, males demonstrated lower face-processing task scores (lower performance) when compared to females, consistent with findings in typically developing participants (Wang et al. 2020); however, the relationship between face processing and social affect was indeed similar for both males and females.
The Effect of Measured Intelligence Quotient on the Relationship Between Face Processing and Social Affect
In addition to identifying a significant correlation between measured IQ and BFRT performance, we also found a stronger correlation between BFRT and social affect in participants with higher IQs than in participants with the lowest IQs, i.e., an IQ by BFRT interaction effect for predicting ADOS-SA. This may be due to participants with lower IQs demonstrating increased variability in their scores and thus a weaker relationship between BFRT and ADOS-SA; however, one could also speculate that participants with higher IQ may utilize face-processing information more to support their social abilities. The correlation between BFRT and IQ was also more than twice as strong in females, potentially indicating that the sex-related differences noted above involve strategies that rely heavily on cognition.
In contrast, no significant relationship was identified between ADOS-RRB and IQ, despite prior reports demonstrating an inverse relationship between the two (Gabriels et al. 2005). Research using latent factor analysis has established two subtypes of RRBs, repetitive sensorimotor behaviors (RSB) and insistence on sameness (IS; resistance to change; (Cuccaro et al. 2003; Szatmari et al. 2006), and identified an inverse relationship between IQ and RSB, which may be stronger for older children (Bishop et al. 2006). Conversely, other groups have found that there is no correlation, or a positive correlation, between IQ and IS subscores (Bishop et al. 2013; Richler et al. 2010), and it is possible that these two effects play a role in the lack of interaction between IQ and ADOS-RRB seen here.
Unlike Face-Processing Performance, Visuospatial Skills do not Correlate with ADOS Severity
An alternative interpretation of the above results is that the association between BFRT performance and ADOS scores merely reflects decreased visuospatial performance. While a matched/comparable object-recognition task was not administered in any of these datasets, an alternative task requiring visuospatial processing, the DAS-Spatial subscore of the DAS-II (Elliott 2007), was acquired for Dataset 1. The DAS-Spatial was not predictive of ADOS severity when the additional covariates of age, sex, and IQ were accounted for, nor did inclusion of DAS-Spatial score in a multivariate linear model reduce the relationship between BFRT and ADOS-SA described above. This supports the conclusion that our findings are unlikely to represent pure visuospatial impairment, but a closer comparison to other object recognition tasks will be required to assess the specificity of the relationship between face recognition and ADOS-SA.
The Importance of Face Discrimination and Recognition in Social Function and Shared Neuroanatomy
Face processing represents a critical element of human social cognition and shared experience that is supported by specialized neural circuitry (Haxby et al. 2000). Face recognition is typically considered to be the result of a holistic pattern-matching process occurring early in visual-processing streams, versus a more cognitive process analyzing separable features that would, by definition, occur later in visual processing (Tanaka and Farah 1993; Yin 1969). There is significant evidence for face-specific neural topography from numerous lines of evidence including seminal fMRI neuroimaging studies (Haxby et al. 1999; Kanwisher et al. 1997; Kanwisher and Yovel 2006) and investigations into the ‘critical’ neuroanatomy of face processing, i.e., where brain injury or disruption results in impaired face processing (Barton 2008; Ellis and Florence 1990; Hecaen and Angelergues 1962; Mattson et al. 2000; Steeves et al. 2009). More recently, transcranial magnetic stimulation studies have demonstrated that stimulation of the right occipital face area and the right fusiform face area indeed disrupt face perception ability (Jonas et al. 2015; Maurer et al. 2017; Pitcher et al. 2008; Rangarajan et al. 2014; Tsao 2006), while recent lesion network mapping work has identified that brain injury leading to new-onset face-processing deficits, i.e., acquired prosopagnosia, typically impacts brain regions connected to both the right fusiform face area and the left frontal regions of the frontoparietal control network (Cohen et al. 2019). Interestingly, these final regions have been shown to demonstrate group-level activation differences in both developmental prosopagnosia and in ASD (Bookheimer et al. 2008; Herrington et al. 2015; Towler et al. 2017).
While atypical face processing is apparent in many disorders that affect social-emotional functioning and has been used as a general marker for social-information processing (Evans et al. 2008; Kohler et al. 2010; Stuhrmann et al. 2011), impaired face recognition/discrimination does appear to be a central and consistent role in ASD. Neurophysiologic studies have demonstrated that participants with high levels of autistic traits exhibit longer N170 and P100 evoked potential latencies when viewing faces than do individuals who show low levels of autistic traits (Stavropoulos et al. 2018) while children with ASD and tuberous sclerosis complex (TSC) demonstrated longer N290 latencies but similar P1s when compared to individuals with TSC, but without ASD, consistent with the premise that slower face processing in ASD is not driven by lower-level global deficits in visual processing (Jeste et al. 2013).
Limitations and Future Directions
While the current work represents data from 445 participants and multiple analyses, there are several limitations. First, given the large cohort size and the breadth of participants’ age, scores from multiple modules of the ADOS were necessarily combined both across and within datasets. While batch effects are certainly possible in any multi-cohort study, we did not detect group-level differences in age where the datasets overlapped, and identifiable relationships in the overall cohort were consistently replicated in each dataset separately, i.e., they were not driven by batch effects between the datasets. Second, although ADOS sub-scores have been shown to be independent of child characteristics–such as language skills relative to raw total severity scores–they do not necessarily capture all aspects of social skills that might be impacted by face processing (Hus et al. 2014; Catherine Lord et al. 2006).
Third, while the BFRT has been widely used as an assessment of face-processing impairment and has a low false-positive rate, scoring in the normal range does not necessarily imply ‘intact’ face processing. In fact, individuals with developmental prosopagnosia often score in the normal range on the BFRT (Duchaine and Nakayama 2004) and report utilizing feature-matching techniques to circumnavigate their difficulty with holistic face recognition (Duchaine and Nakayama 2004; Farah et al. 1998), highlighting the limitations of utilizing a single instrument to assess face ‘processing’. Despite this, our analyses indicate a significant and specific relationship between BFRT task performance and ASD severity. Future studies incorporating a battery of face-processing tasks may find that additional variance in ASD severity can be explained by distinct components of face processing and/or be able to differentiate whether other components of face processing are more closely linked to ASD severity than the discriminative task utilized here.
Of note, while our secondary analysis to evaluate whether our findings generalize to the DAS-spatial demonstrated that the effects shown here are not present for all visual spatial processing tasks, it should not be interpreted as indicating specificity of face vs object recognition. This more focused determination will require data from participants with consistent and comparable face- and object-recognition tasks, e.g., houses, which was not currently available in these datasets. This should be incorporated into future studies.
Finally, the inclusion of additional covariates, such as language use, environmental factors, history of therapy, and genetic information as well as inclusion of non-ASD populations may also allow for further delineation of the relationships described here and elucidate whether the relationships described here are unique to ASD or represent a general principle of face-discrimination supporting social-skill development, which we believe is likely.
Conclusions
Here, we identified that the commonly presumed inverse relationship between face processing and autism severity extended to pure face discrimination, not just to the recognition of facial emotion. This inverse relationship was independent of the effects of age, sex, and IQ. Furthermore, this relationship was not limited to social affect, but was present for both total ADOS score as well as for restricted and repetitive behaviors. This study benefited from aggregating NDAR datasets to create a large sample (N = 445), highlighting the necessity for future research to consider face processing as a key covariate for ASD severity.
These findings also provide additional support for using face-processing-related measures, such as the N170 response and eye-tracking of orientation to faces, as potential biomarkers for ASD; however, given the strength of the relationship noted here, improving face recognition and discrimination may in fact represent an important and high-yield treatment target for behavioral therapy to improve social skill development.
Supplementary Material
Acknowledgments:
We thank Drs. Simon Warfield, PhD and Charles Nelson, PhD for their comments. This work was funded in part by a Shields Research grant from the Child Neurology Foundation to AC, and by NIH/NIMH K23MH120510.
Data and/or research tools used in the preparation of this manuscript were obtained from the National Institute of Mental Health (NIMH) Data Archive (NDA). NDA is a collaborative informatics system created by the National Institutes of Health to provide a national resource to support and accelerate research in mental health. Dataset identifier(s): Collection #1055, https://doi.org/10.15154/1520631. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or of the Submitters submitting original data to NDA.
This work was funded in part by a Shields Research grant from the Child Neurology Foundation and by NIH/NIMH K23MH120510, to AC. We thank Drs. Simon Warfield, PhD and Charles Nelson, PhD for their comments. The authors declare that they have no conflicts of interest. IZO and AC contributed to the study conception and design. IZO performed the data analyses with the help of a statistician (LS) and guidance of AC. IZO and MK drafted the manuscript, while AC critically revised the manuscript for important intellectual content. All authors read and approved the final manuscript. Part of this analysis was presented at the Annual International Society for Autism Research (INSAR) Conference, June 3rd, 2020. Data and/or research tools used in the preparation of this manuscript were obtained from the National Institute of Mental Health (NIMH) Data Archive (NDA). NDA is a collaborative informatics system created by the National Institutes of Health to provide a national resource to support and accelerate research in mental health. Dataset identifier(s): Collection #1055, https://doi.org/10.15154/1520631. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or of the Submitters submitting original data to NDA.
While completing this research, Ivry Zagury-Orly was affiliated with the Department of Neurology, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA; Mallory R. Kroeck was affiliated with the Department of Neurology, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA; Louis Soussand was affiliated with at the Department of Neurology, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA; and Alexander Li Cohen was affiliated with the Department of Neurology, Boston Children’s Hospital, Harvard Medical School, and the Computational Radiology Laboratory, Department of Radiology, Boston Children’s Hospital, Harvard Medical School—all of which are located in Boston, MA, USA.
IZO is now affiliated with the Faculty of Medicine, Université de Montréal, Montreal, QC, CA. LS is now affiliated with the Assistance Publique – Hôpitaux de Paris: the Greater Paris University Hospitals in France and the French Banque Nationale de Données Maladies Rares.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th Edition: DSM-5. Washington, DC: American Psychiatric Association. [Google Scholar]
- Ashwin C, Chapman E, Colle L, & Baron-Cohen S. (2006). Impaired recognition of negative basic emotions in autism: A test of the amygdala theory. Social Neuroscience, 1(3–4), 349–363. 10.1080/17470910601040772 [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Cox A, Baird G, Swettenham J, Nightingale N, Morgan K, et al. (1996). Psychological markers in the detection of autism in infancy in a large population. The British Journal of Psychiatry: The Journal of Mental Science, 168(2), 158–163. 10.1192/bjp.168.2.158 [DOI] [PubMed] [Google Scholar]
- Baron-Cohen Simon, & Wheelwright S. (2004). The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. Journal of Autism and Developmental Disorders, 34(2), 163–175. 10.1023/B:JADD.0000022607.19833.00 [DOI] [PubMed] [Google Scholar]
- Barton JJS (2008). Structure and function in acquired prosopagnosia: lessons from a series of 10 patients with brain damage. Journal of Neuropsychology, 2(1), 197–225. [DOI] [PubMed] [Google Scholar]
- Benton AL, & Van Allen MW (1968). Impairment in facial recognition in patients with cerebral disease. Transactions of the American Neurological Association, 93, 38–42. 10.1016/S0010-9452(68)80018-8 [DOI] [PubMed] [Google Scholar]
- Benton A, Sivan A, Hamsher K, Varney N, & Spreen O. (1994). Contributions to Neuropsychological Assessment. New York: Oxford University Press. [Google Scholar]
- Biele C, & Grabowska A. (2006). Sex differences in perception of emotion intensity in dynamic and static facial expressions. Experimental Brain Research, 171(1), 1–6. 10.1007/s00221-005-0254-0 [DOI] [PubMed] [Google Scholar]
- Bieleninik Ł, Posserud M-B, Geretsegger M, Thompson G, Elefant C, & Gold C. (2017). Tracing the temporal stability of autism spectrum diagnosis and severity as measured by the Autism Diagnostic Observation Schedule: A systematic review and meta-analysis. PLOS ONE, 12(9), e0183160. 10.1371/journal.pone.0183160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SL, Hus V, Duncan A, Huerta M, Gotham K, Pickles A, et al. (2013). Subcategories of restricted and repetitive behaviors in children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 43(6), 1287–1297. 10.1007/s10803-012-1671-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SL, Richler J, & Lord C. (2006). Association between restricted and repetitive behaviors and nonverbal IQ in children with autism spectrum disorders. Child Neuropsychology, 12(4–5), 247–267. 10.1080/09297040600630288 [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Wang AT, Scott A, Sigman M, & Dapretto M. (2008). Frontal contributions to face processing differences in autism: evidence from fMRI of inverted face processing. Journal of the International Neuropsychological Society : JINS, 14(6), 922–932. 10.1017/S135561770808140X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher J, & Lewis V. (1992). Unfamiliar face recognition in relatively able autistic children. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 33(5), 843–859. 10.1111/j.1469-7610.1992.tb01960.x [DOI] [PubMed] [Google Scholar]
- Campbell R, Elgar K, Kuntsi J, Akers R, Terstegge J, Coleman M, & Skuse D. (2002). The classification of ‘fear’ from faces is associated with face recognition skill in women. Neuropsychologia, 40(6), 575–584. 10.1016/S0028-3932(01)00164-6 [DOI] [PubMed] [Google Scholar]
- Carter C. (2007). Sex differences in oxytocin and vasopressin: Implications for autism spectrum disorders? Behavioural Brain Research, 176(1), 170–186. 10.1016/j.bbr.2006.08.025 [DOI] [PubMed] [Google Scholar]
- Cohen AL, Soussand L, Corrow SL, Martinaud O, Barton JJS, & Fox MD (2019). Looking beyond the face area: Lesion network mapping of prosopagnosia. Brain, 142(12), 3975–3990. 10.1093/brain/awz332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, & Charman T. (2016). Diagnosis of autism spectrum disorder: reconciling the syndrome, its diverse origins, and variation in expression. The Lancet Neurology, 15(3), 279–291. 10.1016/S1474-4422(15)00151-9 [DOI] [PubMed] [Google Scholar]
- Corbett BA, Newsom C, Key AP, Qualls LR, & Edmiston EK (2014). Examining the relationship between face processing and social interaction behavior in children with and without autism spectrum disorder. Journal of Neurodevelopmental Disorders, 6(1), 35. 10.1186/1866-1955-6-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuccaro ML, Shao Y, Grubber J, Slifer M, Wolpert CM, Donnelly SL, et al. (2003). Factor analysis of restricted and repetitive behaviors in autism using the Autism Diagnostic Interview-R. Child Psychiatry and Human Development, 34(1), 3–17. 10.1023/a:1025321707947 [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, Carver L, Panagiotides H, & McPartland J. (2004). Young children with autism show atypical brain responses to fearful versus neutral facial expressions of emotion. Developmental Science, 7(3), 340–359. 10.1111/j.1467-7687.2004.00352.x [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, & McPartland J. (2005). Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Developmental Neuropsychology, 27(3), 403–424. 10.1207/s15326942dn2703_6 [DOI] [PubMed] [Google Scholar]
- Duchaine BC, & Nakayama K. (2004). Developmental prosopagnosia and the Benton Facial Recognition Test. Neurology, 62(7), 1219–1220. 10.1212/01.WNL.0000118297.03161.B3 [DOI] [PubMed] [Google Scholar]
- Elliott CD (1990). DAS technical handbook. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Elliott CD (2007). Differential Ability Scales (2nd ed.). San Antonio, TX: Harcourt Assessment . Canadian Journal of School Psychology, 22(1), 128–132. 10.1177/0829573507302967 [DOI] [Google Scholar]
- Elliott CD, Salerno JD, Dumont R, & Willis JO (2018). The Differential Ability Scales—Second Edition. In Contemporary intellectual assessment: Theories, tests, and issues, 4th ed. (pp. 360–382). New York, NY, US: The Guilford Press. [Google Scholar]
- Ellis HD, & Florence M. (1990). Bodamer’s (1947) paper on prosopagnosia. Cognitive Neuropsychology, 7(2), 81–105. 10.1080/02643299008253437 [DOI] [Google Scholar]
- Evans KC, Wright CI, Wedig MM, Gold AL, Pollack MH, & Rauch SL (2008). A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depression and Anxiety, 25(6), 496–505. 10.1002/da.20347 [DOI] [PubMed] [Google Scholar]
- Farah MJ, Wilson KD, Drain M, & Tanaka JN (1998). What is “special” about face perception? Psychological Review, 105(3), 482–498. 10.1037/0033-295X.105.3.482 [DOI] [PubMed] [Google Scholar]
- Ferri SL, Abel T, & Brodkin ES (2018). Sex differences in autism spectrum disorder: A review. Current Psychiatry Reports, 20(2), 9. 10.1007/s11920-018-0874-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriels RL, Cuccaro ML, Hill DE, Ivers BJ, & Goldson E. (2005). Repetitive behaviors in autism: relationships with associated clinical features. Research in Developmental Disabilities, 26(2), 169–181. 10.1016/j.ridd.2004.05.003 [DOI] [PubMed] [Google Scholar]
- Gelder B. de, Vroomen J, & Heide L. van der. (1991). Face recognition and lip-reading in autism. European Journal of Cognitive Psychology, 3(1), 69–86. 10.1080/09541449108406220 [DOI] [Google Scholar]
- Golarai G, Grill-Spector K, & Reiss AL (2006). Autism and the development of face processing. Clinical Neuroscience Research, 6(3), 145–160. 10.1016/j.cnr.2006.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Pickles A, & Lord C. (2009). Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism and Developmental Disorders, 39(5), 693–705. 10.1007/s10803-008-0674-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D, Huerta MF, McAuliffe MJ, & Farber GK (2012). Sharing heterogeneous data: the national database for autism research. Neuroinformatics, 10(4), 331–339. 10.1007/s12021-012-9151-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms MB, Martin A, & Wallace GL (2010). Facial emotion recognition in autism spectrum disorders: a review of behavioral and neuroimaging studies. Neuropsychology Review, 20(3), 290–322. 10.1007/s11065-010-9138-6 [DOI] [PubMed] [Google Scholar]
- Hauck M, Fein D, Maltby N, Waterhouse L, & Feinstein C. (1998). Memory for faces in children with autism. Child Neuropsychology, 4(3), 187–198. 10.1076/chin.4.3.187.3174 [DOI] [Google Scholar]
- Haxby JV, Hoffman EA, & Gobbini MI (2000). The distributed human neural system for face perception. Trends in Cognitive Sciences, 4(6), 223–233. 10.1016/S1364-6613(00)01482-0 [DOI] [PubMed] [Google Scholar]
- Haxby JV, Ungerleider LG, Clark VP, Schouten JL, Hoffman EA, & Martin A. (1999). The effect of face inversion on activity in human neural systems for face and object perception. Neuron, 22(1), 189–199. 10.1016/S0896-6273(00)80690-X [DOI] [PubMed] [Google Scholar]
- Hecaen H, & Angelergues R. (1962). Agnosia for faces (prosopagnosia). Archives of Neurology, 7(2), 92–100. 10.1001/archneur.1962.04210020014002 [DOI] [PubMed] [Google Scholar]
- Herrington JD, Riley ME, Grupe DW, & Schultz RT (2015). Successful face recognition is associated with increased prefrontal cortex activation in autism spectrum disorder. Journal of Autism and Developmental Disorders, 45(4), 902–910. 10.1007/s10803-014-2233-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hus V, Gotham K, & Lord C. (2014). Standardizing ADOS domain scores: Separating severity of social affect and restricted and repetitive behaviors. Journal of Autism and Developmental Disorders, 44(10), 2400–2412. 10.1007/s10803-012-1719-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemel B, Mottron L, & Dawson M. (2006). Impaired face processing in autism: fact or artifact? Journal of Autism and Developmental Disorders, 36(1), 91–106. 10.1007/s10803-005-0050-5 [DOI] [PubMed] [Google Scholar]
- Jeste SS, Hirsch S, Vogel-Farley V, Norona A, Navalta MC, Gregas MC, et al. (2013). Atypical face processing in children with tuberous sclerosis complex. Journal of Child Neurology, 28(12), 1569–1576. 10.1177/0883073812465122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas J, Rossion B, Brissart H, Frismand S, Jacques C, Hossu G, et al. (2015). Beyond the core face-processing network: Intracerebral stimulation of a face-selective area in the right anterior fusiform gyrus elicits transient prosopagnosia. Cortex, 72, 140–155. 10.1016/j.cortex.2015.05.026 [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, & Chun MM (1997). The fusiform face area: a module in human extrastriate cortex specialized for face perception. Journal of Neuroscience, 17(11), 4302–4311. 10.1523/JNEUROSCI.17-11-04302.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, & Yovel G. (2006). The fusiform face area: a cortical region specialized for the perception of faces. Philosophical Transactions of the Royal Society B: Biological Sciences, 361(1476), 2109–2128. 10.1098/rstb.2006.1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, & Lord C. (2010). Restricted and repetitive behaviors in toddlers and preschoolers with autism spectrum disorders based on the Autism Diagnostic Observation Schedule (ADOS). Autism Research, 3(4), 162–173. 10.1002/aur.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Sparrow SS, de Bildt A, Cicchetti DV, Cohen DJ, & Volkmar FR (1999). A normed study of face recognition in autism and related disorders. Journal of Autism and Developmental Disorders, 29(6), 499–508. 10.1023/a:1022299920240 [DOI] [PubMed] [Google Scholar]
- Kohler CG, Walker JB, Martin EA, Healey KM, & Moberg PJ (2010). Facial emotion perception in schizophrenia: a meta-analytic review. Schizophrenia Bulletin, 36(5), 1009–1019. 10.1093/schbul/sbn192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin HS, Hamsher K. de S., & Benton AL (1975). A short form of the Test of Facial Recognition for clinical use. The Journal of Psychology, 91(2), 223–228. 10.1080/00223980.1975.9923946 [DOI] [Google Scholar]
- Lindner JL, & Rosén LA (2006). Decoding of emotion through facial expression, prosody and verbal content in children and adolescents with Asperger’s syndrome. Journal of Autism and Developmental Disorders, 36(6), 769–777. 10.1007/s10803-006-0105-2 [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, & Bishop S. (2012). Autism diagnostic observation schedule, second edition. Torrance, CA: Western Psychological Services. [Google Scholar]
- Lord Catherine, Risi S, DiLavore PS, Shulman C, Thurm A, & Pickles A. (2006). Autism from 2 to 9 years of age. Archives of General Psychiatry, 63(6), 694–701. 10.1001/archpsyc.63.6.694 [DOI] [PubMed] [Google Scholar]
- Lord Catherine, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, & Schopler E. (1989). Austism diagnostic observation schedule: A standardized observation of communicative and social behavior. Journal of Autism and Developmental Disorders, 19(2), 185–212. 10.1007/BF02211841 [DOI] [PubMed] [Google Scholar]
- Maenner MJ (2020). Prevalence of autism spectrum disorder among children aged 8 years — autism and developmental disabilities monitoring network, 11 Sites, united states, 2016. MMWR. Surveillance Summaries, 69(4), 1–12. 10.15585/mmwr.ss6904a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamashli F, Khan S, Bharadwaj H, Losh A, Pawlyszyn SM, Hämäläinen MS, & Kenet T. (2018). Maturational trajectories of local and long-range functional connectivity in autism during face processing. Human Brain Mapping, 39(10), 4094–4104. 10.1002/hbm.24234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G, Agnoli S, Baldaro B, Ricci Bitti PE, & Surcinelli P. (2013). Facial expressions of emotions: recognition accuracy and affective reactions during late childhood. The Journal of Psychology, 147(6), 599–617. 10.1080/00223980.2012.727891 [DOI] [PubMed] [Google Scholar]
- Marcus DJ, & Nelson CA (2001). Neural bases and development of face recognition in autism. CNS spectrums, 6(1), 36–59. 10.1017/s1092852900022872 [DOI] [PubMed] [Google Scholar]
- Mattson AJ, Levin HS, & Grafman J. (2000). A case of prosopagnosia following moderate closed head injury with left hemisphere focal lesion. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 36(1), 125–137. 10.1016/s0010-9452(08)70841-4 [DOI] [PubMed] [Google Scholar]
- Maurer S, Giglhuber K, Sollmann N, Kelm A, Ille S, Hauck T, et al. (2017). Noninvasive mapping of face processing by navigated transcranial magnetic stimulation. Frontiers in Human Neuroscience, 11. 10.3389/fnhum.2017.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleery JP, Akshoomoff N, Dobkins KR, & Carver LJ (2009). Atypical face versus object processing and hemispheric asymmetries in 10-month-old infants at risk for autism. Biological Psychiatry, 66(10), 950–957. 10.1016/j.biopsych.2009.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland JC, Bernier RA, Jeste SS, Dawson G, Nelson CA, Chawarska K, et al. (2020). The Autism Biomarkers Consortium for Clinical Trials (ABC-CT): Scientific Context, Study Design, and Progress Toward Biomarker Qualification. Frontiers in Integrative Neuroscience, 14. 10.3389/fnint.2020.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland JC, Webb SJ, Keehn B, & Dawson G. (2011). Patterns of visual attention to faces and objects in autism spectrum disorder. Journal of Autism and Developmental Disorders, 41(2), 148–157. 10.1007/s10803-010-1033-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland J, Dawson G, Webb SJ, Panagiotides H, & Carver LJ (2004). Event-related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 45(7), 1235–1245. 10.1111/j.1469-7610.2004.00318.x [DOI] [PubMed] [Google Scholar]
- Montagne B, Kessels RPC, Frigerio E, de Haan EHF, & Perrett DI (2005). Sex differences in the perception of affective facial expressions: Do men really lack emotional sensitivity? Cognitive Processing, 6(2), 136–141. 10.1007/s10339-005-0050-6 [DOI] [PubMed] [Google Scholar]
- Motta-Mena NV, & Scherf KS (2016). Pubertal development shapes perception of complex facial expressions. Developmental Science, 20(4), e12451. 10.1111/desc.12451 [DOI] [PubMed] [Google Scholar]
- O’Hearn K, Schroer E, Minshew N, & Luna B. (2010). Lack of developmental improvement on a face memory task during adolescence in autism. Neuropsychologia, 48(13), 3955–3960. 10.1016/j.neuropsychologia.2010.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hearn K, Tanaka J, Lynn A, Fedor J, Minshew N, & Luna B. (2014). Developmental plateau in visual object processing from adolescence to adulthood in autism. Brain and Cognition, 90, 124–134. 10.1016/j.bandc.2014.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterling JA, Dawson G, & Munson JA (2002). Early recognition of 1-year-old infants with autism spectrum disorder versus mental retardation. Development and Psychopathology, 14(2), 239–251. 10.1017/s0954579402002031 [DOI] [PubMed] [Google Scholar]
- Osterling J, & Dawson G. (1994). Early recognition of children with autism: a study of first birthday home videotapes. Journal of Autism and Developmental Disorders, 24(3), 247–257. 10.1007/BF02172225 [DOI] [PubMed] [Google Scholar]
- Philip RCM, Whalley HC, Stanfield AC, Sprengelmeyer R, Santos IM, Young AW, et al. (2010). Deficits in facial, body movement and vocal emotional processing in autism spectrum disorders. Psychological Medicine, 40(11), 1919–1929. 10.1017/S0033291709992364 [DOI] [PubMed] [Google Scholar]
- Pierce K, & Courchesne E. (2000). Exploring the neurofunctional organization of face processing in autism. Archives of General Psychiatry, 57(4), 344–346. 10.1001/archpsyc.57.4.344 [DOI] [PubMed] [Google Scholar]
- Pitcher D, Garrido L, Walsh V, & Duchaine BC (2008). Transcranial magnetic stimulation disrupts the perception and embodiment of facial expressions. Journal of Neuroscience, 28(36), 8929–8933. 10.1523/JNEUROSCI.1450-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan V, Hermes D, Foster BL, Weiner KS, Jacques C, Grill-Spector K, & Parvizi J. (2014). Electrical stimulation of the left and right human fusiform gyrus causes different effects in conscious face perception. Journal of Neuroscience, 34(38), 12828–12836. 10.1523/JNEUROSCI.0527-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richler J, Huerta M, Bishop SL, & Lord C. (2010). Developmental trajectories of restricted and repetitive behaviors and interests in children with autism spectrum disorders. Development and Psychopathology, 22(1), 55–69. 10.1017/S0954579409990265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rynkiewicz A, & Łucka I. (2018). Autism spectrum disorder (ASD) in girls. Co-occurring psychopathology. Sex differences in clinical manifestation. Psychiatria Polska, 52(4), 629–639. 10.12740/PP/OnlineFirst/58837 [DOI] [PubMed] [Google Scholar]
- Rynkiewicz A, Schuller B, Marchi E, Piana S, Camurri A, Lassalle A, & Baron-Cohen S. (2016). An investigation of the ‘female camouflage effect’ in autism using a computerized ADOS-2 and a test of sex/gender differences. Molecular Autism, 7. 10.1186/s13229-016-0073-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson NJ (2006). The development of face processing in autism. Journal of Autism and Developmental Disorders, 36(3), 381–394. 10.1007/s10803-006-0076-3 [DOI] [PubMed] [Google Scholar]
- Scherf KS, & Scott LS (2012). Connecting developmental trajectories: Biases in face processing from infancy to adulthood. Developmental Psychobiology, 54(6), 643–663. 10.1002/dev.21013 [DOI] [PubMed] [Google Scholar]
- Schultz RT (2005). Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience: The Official Journal of the International Society for Developmental Neuroscience, 23(2–3), 125–141. 10.1016/j.ijdevneu.2004.12.012 [DOI] [PubMed] [Google Scholar]
- Senland AK, & Higgins-D’Alessandro A. (2016). Sociomoral reasoning, empathy, and meeting developmental tasks during the transition to adulthood in autism spectrum disorder. Journal of Autism and Developmental Disorders, 46(9), 3090–3105. 10.1007/s10803-016-2849-7 [DOI] [PubMed] [Google Scholar]
- Simmons DR, Robertson AE, McKay LS, Toal E, McAleer P, & Pollick FE (2009). Vision in autism spectrum disorders. Vision Research, 49(22), 2705–2739. 10.1016/j.visres.2009.08.005 [DOI] [PubMed] [Google Scholar]
- Stavropoulos KKM, Viktorinova M, Naples A, Foss-Feig J, & McPartland JC (2018). Autistic traits modulate conscious and nonconscious face perception. Social Neuroscience, 13(1), 40–51. 10.1080/17470919.2016.1248788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeves J, Dricot L, Goltz HC, Sorger B, Peters J, Milner AD, et al. (2009). Abnormal face identity coding in the middle fusiform gyrus of two brain-damaged prosopagnosic patients. Neuropsychologia, 47(12), 2584–2592. 10.1016/j.neuropsychologia.2009.05.005 [DOI] [PubMed] [Google Scholar]
- Stuhrmann A, Suslow T, & Dannlowski U. (2011). Facial emotion processing in major depression: a systematic review of neuroimaging findings. Biology of Mood & Anxiety Disorders, 1, 1–17. 10.1186/2045-5380-1-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari P, Georgiades S, Bryson S, Zwaigenbaum L, Roberts W, Mahoney W, et al. (2006). Investigating the structure of the restricted, repetitive behaviours and interests domain of autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 47(6), 582–590. 10.1111/j.1469-7610.2005.01537.x [DOI] [PubMed] [Google Scholar]
- Tanaka JW, & Farah MJ (1993). Parts and wholes in face recognition. The Quarterly Journal of Experimental Psychology Section A, 46(2), 225–245. 10.1080/14640749308401045 [DOI] [PubMed] [Google Scholar]
- Tanaka JW, Wolf JM, Klaiman C, Cockburn J, Herlihy L, Brown C, et al. (2008). Specific impairment of face-processing abilities in children with autism spectrum disorder using the Let’s Face it! Skills Battery. Autism Research, 1(6), 329–340. 10.1002/aur.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Falkmer M, Horlin C, Tan T, Vaz S, & Falkmer T. (2015). Face Recognition and Visual Search Strategies in Autism Spectrum Disorders: Amending and Extending a Recent Review by Weigelt et al. PloS one, 10(8), e0134439–e0134439. 10.1371/journal.pone.0134439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler J, Fisher K, & Eimer M. (2017). The cognitive and neural basis of developmental prosopagnosia. Quarterly Journal of Experimental Psychology (2006), 70(2), 316–344. 10.1080/17470218.2016.1165263 [DOI] [PubMed] [Google Scholar]
- Tsao DY (2006). A cortical region consisting entirely of face-selective cells. Science, 311(5761), 670–674. 10.1126/science.1119983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace S, Coleman M, & Bailey A. (2008). An investigation of basic facial expression recognition in autism spectrum disorders. Cognition & Emotion, 22(7), 1353–1380. 10.1080/02699930701782153 [DOI] [Google Scholar]
- Wang LAL, Herrington JD, Tunç B, & Schultz RT (2020). Bayesian regression-based developmental norms for the Benton Facial Recognition Test in males and females. Behavior Research Methods. 10.3758/s13428-019-01331-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Shic F, Murias M, Sugar CA, Naples AJ, Barney E, et al. (2020). Biomarker acquisition and quality control for multi-site studies: The autism biomarkers consortium for clinical trials. Frontiers in Integrative Neuroscience, 13, 71. 10.3389/fnint.2019.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. (1999). Wechsler Abbreviated Scale of Intelligence (WASI). San Antonio, TX: Psychological Corporation. [Google Scholar]
- Weigelt S, Koldewyn K, & Kanwisher N. (2012). Face identity recognition in autism spectrum disorders: A review of behavioral studies. Neuroscience & Biobehavioral Reviews, 36(3), 1060–1084. 10.1016/j.neubiorev.2011.12.008 [DOI] [PubMed] [Google Scholar]
- Yardley L, McDermott L, Pisarski S, Duchaine B, & Nakayama K. (2008). Psychosocial consequences of developmental prosopagnosia: A problem of recognition. Journal of Psychosomatic Research, 65(5), 445–451. [DOI] [PubMed] [Google Scholar]
- Yin RK (1969). Looking at upside-down faces. Journal of Experimental Psychology, 81(1), 141. 10.1037/h0027474 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.