Abstract
Background
Perfluoroalkylated substances (PFAS) are man-made, persistent organic compounds with immune-modulating potentials. Given that pregnancy itself represents an altered state of immunity, PFAS exposure-related immunotoxicity is an important environmental factor to consider in SARS-CoV-2 infection during pregnancy as it may further affect humoral immune responses.
Aim
To investigate the relationship between maternal plasma PFAS concentrations and SARS-CoV-2 antibody levels in a NYC-based pregnancy cohort.
Methods
Maternal plasma was collected from 72 SARS-CoV-2 IgG + participants of the Generation C Study, a birth cohort established at the beginning of the COVID-19 pandemic in New York City. Maternal SARS-CoV-2 anti-spike IgG antibody levels were measured using ELISA. A panel of 16 PFAS congeners were measured in maternal plasma using a targeted UHPLC-MS/MS-based assay. Spearman correlations and linear regressions were employed to explore associations between maternal IgG antibody levels and plasma PFAS concentrations. Weighted quantile sum (WQS) regression was also used to evaluate mixture effects of PFAS. Models were adjusted for maternal age, gestational age at which SARS-CoV-2 IgG titer was measured, COVID-19 vaccination status prior to IgG titer measurement, maternal race/ethnicity, parity, type of insurance and pre-pregnancy BMI.
Results
Our study population is ethnically diverse with an average maternal age of 32 years. Of the 16 PFAS congeners measured, nine were detected in more than 60% samples. Importantly, all nine congeners were negatively correlated with SARS-CoV-2 anti-spike IgG antibody levels; n-PFOA and PFHxS, PFHpS, and PFHxA reached statistical significance (p < 0.05) in multivariable analyses. When we examined the mixture effects using WQS, a quartile increase in the PFAS mixture-index was significantly associated with lower maternal IgG antibody titers (beta [95% CI] = −0.35 [-0.52, −0.17]). PFHxA was the top contributor to the overall mixture effect.
Conclusions
Our study results support the notion that PFAS, including short-chain emerging PFAS, act as immunosuppressants during pregnancy. Whether such compromised immune activity leads to downstream health effects, such as the severity of COVID-19 symptoms, adverse obstetric outcomes or neonatal immune responses remains to be investigated.
Keywords: PFAS, SARS-CoV-2 IgG, COVID19, Immunotoxicity, Pregnancy
1. Introduction
Per-/Poly-Fluoroalkyl Substances (PFAS) are persistent organic compounds that are resistant to degradation due to their stable structure (De Silva et al., 2020; Wang et al., 2017). These man-made compounds are ubiquitous in the environment (i.e., soil, groundwater) and in consumer goods (i.e., food packaging, textiles, non-stick cookware). Thus, humans are continuously exposed through contaminated drinking water and consumer products (Agency for Toxic Substances and Disease Registry - ATSDR 2020; Centers for Disease Control – CDC, 2019; Worley et al., 2017). Although the long-chain (i.e., congeners with a carbon-backbone greater than seven carbons, such as PFOA and PFOS) “legacy” PFAS have been voluntarily phased out by industrial manufacturers, short-chain or emerging PFAS (i.e., GenX, PFBS) are now readily used as replacements (Ateia et al., 2019; Kaboré et al., 2018; Wang et al., 2017). Short-chain PFAS are rising in detection frequency in drinking water and in human biological samples (Kaboré et al., 2018). Despite having shorter half-lives than their legacy counterparts (Xu et al., 2020), emerging PFAS still demonstrate deleterious effects to human health, ranging from metabolic perturbation to carcinogenic potential (Chang et al., 2022; Coperchini et al., 2020; Shearer et al., 2021).
Given the influence of the immune system on a myriad of organs and tissues, the immunotoxic impact of PFAS exposure is a chief public health concern and has been investigated over the past decade (Grandjean et al., 2012; Grandjean et al., 2017; Grandjean et al., 2017; Meng et al., 2018; National Toxicology Program, 2016; Stein et al., 2016; Zhang et al., 2015). These studies have established that elevated PFAS exposure reduces humoral immune responses (Smith and Cunningham-Rundles, 2019). In the midst of the emerging COVID-19 pandemic, worsened clinical outcome of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) infection has been linked with elevated PFAS exposure in a Danish population-based cohort (Grandjean et al., 2020). More recently, two other studies reported that individuals from PFAS-polluted regions in China and Italy demonstrated a greater risk of COVID-19 infection susceptibility and mortality (Catelan et al., 2021; Ji et al., 2021). The link between higher PFAS exposure and the risk of worsened COVID-19 prognosis is also under investigation by the CDC, which published a statement in conjunction with the ATSDR, underscoring the need for further research on this topic (ATSDR 2020). The statement recognizes that high levels of PFAS exposure may affect immune function and antibody responses to vaccines (Looker et al., 2014, National Toxicology Program, 2016) highlighting the need to study COVID-19 severity in this context.
Pregnancy represents an altered state of immunity and SARS-CoV-2 infection can further perturb the immune system, therefore PFAS exposure is another important factor to consider given that it has been shown to suppress immune responses (Granum et al., 2013). Pregnant individuals infected with SARS-CoV-2 appear to have compromised SARS-CoV-2-specific placental antibody transfer and altered glycosylation profiles (Ateyo, 2021). Compared to non-pregnant individuals, pregnant individuals have lower SARS-CoV-2 antibody titers after the first dose of the vaccine (Ateyo, 2021). Pregnant individuals who have higher SARS-CoV-2 IgG levels tend to be those who are diagnosed at an earlier gestational age and have shorter intervals between diagnosis and blood draw (Buckley, 2022). Due to its novel nature, it is currently unknown if elevated maternal PFAS exposure during the perinatal time period may further impact COVID-19-related health outcomes.
As of the end of September 2022, more than 94 million COVID-19 cases and upwards of 1 million deaths have been attributed to SARS-CoV-2 infection in the US alone, and over 225,000 cases and 300 deaths were amongst pregnant individuals (CDC Data Tracker, 2022). More than two million people have been infected in New York City (NYC) by SARS-CoV-2 as of September 2022 (Tracking Coronavirus in New York: Latest Map and Case Count, 2022). To explore the impacts of gestational SARS-CoV-2 infection in pregnancy, The Generation C (Gen C) cohort was established in 2020 at the Mount Sinai Hospital in NYC (CDC grant 75D30120C08186). Our pilot study presented here leveraged a subset of the on-going Gen C study to investigate possible links between PFAS exposure and immune responses to SARS-CoV-2 infection in pregnant women.
2. Methods
2.1. Study population
The Gen C cohort study was established in April 2020 to investigate the effects of SARS-CoV-2 infection during pregnancy on birth outcomes as described in detail previously (Janevic et al., 2022; Lesseur et al., 2022; Molenaar et al., 2022). This cohort recruited 3121 pregnant participants in NYC from the Mount Sinai Health System (MSHS) between April 20, 2020 and February 24, 2022. As a part of routine obstetrical care, enrolled participants provided blood samples in each trimester. Maternal plasma samples were collected between April 23, 2020 and May 21, 2021. Past SARS-CoV-2 infection was confirmed via serological SARS-CoV-2 IgG antibody test (described in section 2.3) and electronic medical record (EMR) review. Vaccination history was ascertained through the EMR of the MSHS, which is linked to the New York Citywide Immunization Registry (CIR) and consolidates all immunization information across NYC into a centralized database (further details can be found in the supplemental section). Pre-pregnancy BMI was gathered from EMR review and race/ethnicity were self-reported in participant questionnaires. All participants provided written informed consent as per the institutional review board (IRB) at the Icahn School of Medicine at Mount Sinai (protocol IRB-20-03352, April 15, 2020). This pilot study included 72 participants who were SARS-CoV-2 IgG anti-spike protein positive (IgG+). These participants were enrolled in the early phase of the study, i.e. between April 2020 and May 2021, and had plasma samples available for PFAS measurements. Amongst this subset, seven individuals received at least one dose of the COVID-19 vaccine before blood collection.
2.2. Maternal plasma PFAS measurements
Maternal plasma, stored at −80 °C, was used to quantify a panel of 16 PFAS congeners [Supplemental Table 1] at the Icahn School of Medicine at Mount Sinai's Human Health Exposure Analysis Resource (HHEAR) Targeted Analysis Laboratory, a continuation of Children's Health Exposure Analysis Resource (CHEAR) (Balshaw et al., 2017). A low-volume (100 μL) and high-sensitivity (0.2 ng/mL median Limit of Detection - LOD) targeted assay using isotope-dilution liquid chromatography with tandem mass spectrometry (LC-MS/MS) was followed based on a CDC method (Kato et al., 2018) with modifications (Coggan et al., 2019; Reagen et al., 2008). The assay includes the most widely studied PFAS (n-PFOS, Sm-PFOS, n-PFOA, PFHxS, PFDA, PFNA), emerging PFAS (PFBS, PFHpS, PFHpA, PFHxA, PFUndA, PFDoDA), and replacements (such as N-EtFOSAA, N-MeFOSAA, among others) (Poothong et al., 2017). Additional analytical details are provided in the supplementary section. Matrix-based LODs were determined using a laboratory QC serum pool (product # BP2525100) from Fisher Scientific (Hampton, NH, USA), which ranged from 0.04 to 0.8 ng/mL for the study PFAS congeners. Quality controls (QCs) included in this study were experimental blanks (reagent-and matrix-based), matrix spikes in the range of assay validation, NIST standard reference material (SRM 1957: Organic Contaminants in Non-Fortified Hu et al., 2019: Organic Contaminants in Fortified Human Serum), and archived proficiency testing material. HHEAR/CHEAR includes additional internal quality assurance and QC protocols, including pooled sample analysis to assess analytical precision (Kannan et al., 2021). The Mount Sinai HHEAR/CHEAR Network Laboratory Hub has participated and qualified in proficiency testing programs for PFAS in serum by G-EQUAS (http://www.g-equas.de/) (Göen et al., 2012) and CTQ-AMAP (https://www.inspq.qc.ca/en/ctq/eqas/amap/description) (CTQ, AMAP: AMAP Ring Test for Persistent Organic Pollutants in Hu et al., 2019 , CTQ, 2022).
2.3. SARS-CoV-2 IgG antibody quantification
Serological testing for IgG antibodies against the SARS-CoV-2 spike (S) protein, an enzyme-linked immunosorbent assay (ELISA) developed at the Icahn School of Medicine at Mount Sinai (Stadlbauer et al., 2020) was used. IgG antibodies were measured in the same maternal plasma samples in which PFAS were measured during pregnancy.
2.4. Statistical analyses
The analyses were conducted in two stages; first, multiple linear regression models were used to assess the associations between maternal SARS-CoV-2 IgG antibody titers and individual PFAS congener concentrations in maternal plasma. Second, weighted quantile sum (WQS) regression was implemented to account for the joint mixture effects of the individual PFAS pollutants using the “gWQS: generalized Weighted Quantile Sum regression” package in R (Carrico et al., 2015; Czarnota et al., 2015; Renzetti et al., 2016). Both the linear and WQS regression models were adjusted for maternal age, gestational age at which SARS-CoV-2 IgG titer was measured, COVID-19 vaccination status prior to IgG titer measurement, maternal race/ethnicity, parity, type of insurance, and pre-pregnancy BMI. The covariates were selected as they have been associated with PFAS exposure in previous studies (Boronow et al., 2019; Nelson, 2012).
PFAS concentrations below LOD were imputed by the formula LOD/(√2). Further, PFAS analytes that were below LOD in more than 40% samples were excluded from analyses; thus the final analytical panel includes nine PFAS congeners (PFOS, PFHxA, PFHxS, n-PFOA, PFHpS, PFDA, PFNA, PFBS, and PFUnDA). Maternal plasma-PFAS concentrations and SARS-CoV-2 spike IgG antibody titer values were log10-transformed prior to running statistical analysis models due to skewed distributions. Correlations between maternal PFAS concentrations and IgG titers were assessed using Spearman correlations. All analyses were performed in R version 4.2.0 (R Core Team, 2022). Any two-tailed p-value <0.05 is considered statistically significant.
3. Results
Sociodemographic and clinical characteristics of the of 72 Gen C participants in the current study are shown in Table 1 . Our NYC-based study population is comprised of 38.9% White, 29.2% Hispanic and 22.2% Black participants, which is reflective of the catchment area of our health system. A large fraction of our study participants had obesity (45.8%) prior to pregnancy and were nulliparous (51.4%) with an average age of 32.9 years at delivery. On average, blood samples were collected at 27 weeks of gestation to measure SARS-CoV-2 spike IgG titers, which varied widely with an average titer of 1060 arbitrary antibody units (AU) per milliliter (SD: 1480 AU/mL, range: 50–64,000 AU/mL).
Table 1.
Sociodemographic and clinical characteristics of our study population.
| n (%) | Mean (SD) | [Range] | |
|---|---|---|---|
| Maternal characteristics | |||
| Age at delivery (years) | 32.9 (4.6) | [24–46] | |
| BMI (kg/m2) | 30.4 (7.9) | [18.9–59.8] | |
| SARS-CoV-2 Spike IgG titer | 1060 (1408) | [50–64,000] | |
| Gestational age at blood draw (weeks) | 27 (10.8) | [4.7–39.9] | |
| BMI category | |||
| Normal | 18 (25%) | ||
| Overweight | 21 (29.2%) | ||
| Obese | 33 (45.8%) | ||
| Race ethnicity | |||
| White | 28 (38.9%) | ||
| Hispanic | 21 (29.2%) | ||
| Black | 16 (22.2%) | ||
| Asian | 3 (4.2%) | ||
| Other | 4 (5.6%) | ||
| Parity | |||
| Multiparous | 35 (48.6%) | ||
| Nulliparous | 37 (51.4%) | ||
| Delivery mode | |||
| C-Section | 28 (38.9%) | ||
| Vaginal | 43 (59.7%) | ||
| Insurance Category | |||
| Private | 48 (66.7%) | ||
| Public | 22 (30.6%) | ||
| No insurance | 2 (2.7%) | ||
| Infant characteristics | |||
| Gestational age at delivery (weeks) | 38.4 (2.1) | [32.1–41.6] | |
| Birthweight (g) | 3138.4 (539.9) | [1545–4090] | |
| Infant sex | |||
| Female | 43 (59.7%) | ||
| Male | 29 (40.3%) | ||
Categorical variables are frequencies and percentages (%). Continuous variables summarized with mean (standard deviation) and [range].
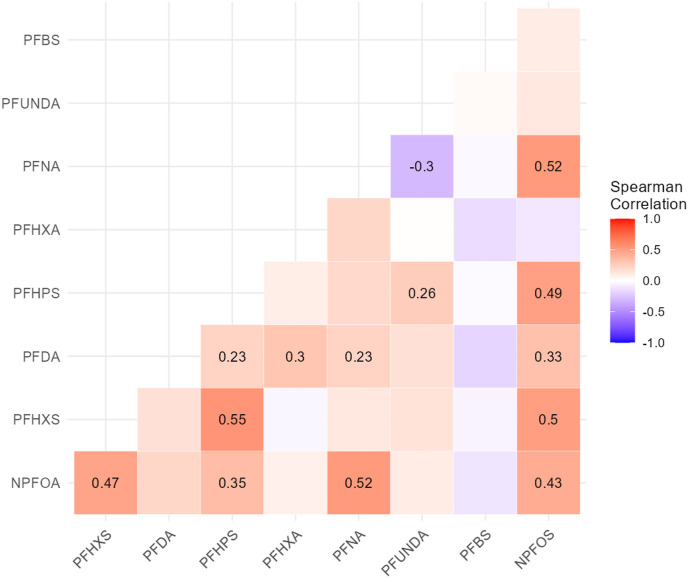
The summary statistics of plasma-PFAS concentrations from the 72 participants are shown in Table 2 . Out of the 16 total PFAS congeners measured, nine were detected in more than 60% of the study samples; three of them (i.e., PFOS, PFHxA and PFHxS) were detected in all samples. We compared the levels detected in our study population with those reported in 977 female participants in 2017–2018 cycle of National Health and Nutrition Examination Survey (NHANES) [Table 2]. While levels of “legacy” PFAS, such as PFOA and PFOS, in our study population were lower than those of NHANES (median: 0.91 vs. 1.27 ng/mL, respectively for PFOA; 0.41 vs. 3.30 ng/mL, respectively for PFOS), median levels of the “emerging” PFAS, such as PFHxS (0.23 ng/mL) and PFBS (0.38 ng/mL) were higher in comparison to below LOD levels of both compounds reported in NHANES. Among the nine congeners detected in a majority of our study samples, several of them demonstrated mostly positive, moderate correlations (rho > |0.20|), as shown by the Spearman correlation coefficients in Fig. 1 . The only exception is between PFNA and PFUnDA, which were negatively correlated. We also examined associations between IgG levels and potential confounding variables including pre-pregnancy BMI, infant sex and race/ethnicity in bivariate analyses. No significant associations were observed for these variables [Supplementary Fig. 1].
Table 2.
Summary statistics of the levels of PFAS (ng/mL) in maternal plasma (n = 72; 2020–2021) from our study population and in comparison to levels reported in NHANES female serum (n = 977, survey years 2017–2018).
| PFAS Congener | Gen C Maternal Plasma (n = 72) |
NHANESc Female Serum (n = 977) |
||||||
|---|---|---|---|---|---|---|---|---|
| LOD | % <LOD | Mean | SD | Min. | Median | Max. | Median | |
| PFOSa | 0.2 | 0 | 1.84 | 1.13 | 1.52 | 0.41 | 6.15 | 3.30 |
| PFHxA | 0.1 | 0 | 0.32 | 0.24 | 0.16 | 0.23 | 1.62 | < LOD |
| PFHxS | 0.04 | 0 | 0.46 | 0.28 | 0.15 | 0.39 | 1.29 | 0.80 |
| n-PFOA | 0.2 | 1.4 | 1.10 | 0.60 | 0.14 | 0.91 | 2.96 | 1.27 |
| PFHPS | 0.04 | 5.6 | 0.10 | 0.04 | 0.03 | 0.10 | 0.24 | 0.20 |
| PFDA | 0.1 | 8.3 | 0.49 | 0.22 | 0.07 | 0.45 | 1.02 | 0.20 |
| PFNA | 0.2 | 15.3 | 0.51 | 0.40 | 0.14 | 0.40 | 1.81 | 0.40 |
| PFBS | 0.2 | 20.8 | 0.39 | 0.23 | 0.14 | 0.38 | 1.32 | < LODb |
| PFUnDA | 0.3 | 22.2 | 0.56 | 0.38 | 0.21 | 0.42 | 2.03 | 0.10 |
Sum of linear (n-PFOS) and branched (Sm-PFOS) isomers of PFOS.
Data only listed for years 2013–2014, n = 1136 females for PFBS.
Data from CDC Exposure Report, 2021 (www.cdc.gov/exposurereport).
Fig. 1.
Spearman correlations between nine PFAS in final analytical panel measured in maternal plasma. Spearman coefficient (rho) with p-value <0.05 are displayed in the corresponding block.
Linear regression models reported in Table 3 demonstrate the relationship between maternal plasma PFAS levels and SARS-CoV-2 IgG titers. These models are adjusted for maternal age, gestational age at which SARS-CoV-2 IgG titer was measured, COVID-19 vaccination status prior to IgG titer measurement, maternal race/ethnicity, parity, type of insurance, and pre-pregnancy BMI. The beta estimates for all nine PFAS congeners are negative, indicating inverse associations between PFAS and IgG levels. For four out of the nine PFAS (n-PFOA, PFHxS, PFHpS, and PFHxA), the associations are statistically significance (p < 0.05).
Table 3.
Associations between maternal SARS-CoV-2 anti-spike IgG titers and PFAS exposure.
| PFAS Congener | Beta | SE | p-value | 95%CI |
|---|---|---|---|---|
| Linear Regression of Individual PFAS | ||||
| n-PFOA | −0.62 | 0.25 | 0.017* | (-1.11, −0.12) |
| PFHxS | −0.68 | 0.25 | 0.008* | (-1.18, −0.18) |
| PFHpS | −0.81 | 0.31 | 0.011* | (-1.42, −0.19) |
| PFHxA | −0.54 | 0.25 | 0.037* | (-1.04, −0.04) |
| PFNA | −0.22 | 0.20 | 0.269 | (-0.62, 0.18) |
| PFOS | −0.33 | 0.26 | 0.209 | (-0.85, 0.20) |
| PFUnDA | −0.20 | 0.22 | 0.388 | (-0.63, 0.25) |
| PFBS | −0.24 | 0.25 | 0.339 | (-0.74, 0.26) |
| PFDA | −0.15 | 0.22 | 0.490 | (-0.60, 0.29) |
| WQS regression of PFAS Mixture | ||||
| PFAS Mixture | −0.35 | 0.09 | 0.0003* | (-0.52, −0.17) |
SE: standard error; CI: confidence intervals.
*p-value <0.05.
Linear and WQS regression models are both adjusted for maternal age, gestational age at which SARS-CoV-2 IgG titer was measured, COVID-19 vaccination status prior to IgG titer measurement, maternal race/ethnicity, parity, type of insurance and pre-pregnancy BMI.
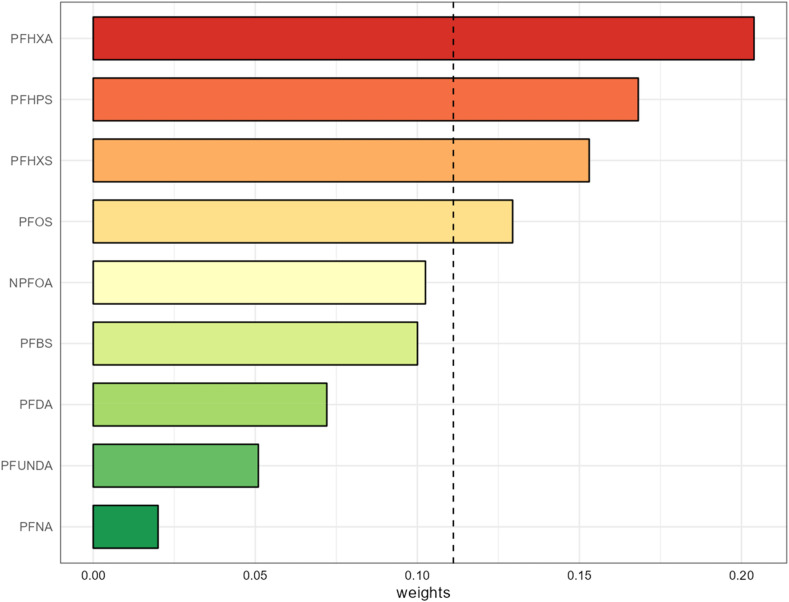
We also examined the mixture effect of PFAS using a WQS regression model to account for simultaneous co-pollutant exposures. This model demonstrated that a quartile increase in the plasma PFAS mixture index is associated with reduced maternal IgG antibody titer (beta [95% CI] = −0.35 [−0.52, −0.17]) [Table 3]. Fig. 2 depicts the contribution of individual PFAS congeners to the overall mixture index, where PFHxA had the highest weight among all the other PFAS.
Fig. 2.
WQS regression model weights of each individual PFAS contributing to the overall co-pollutant mixture effect (Beta [95% CI] = −0.35 [-0.52, −0.17], p-value = 0.0003) in the index. The black dotted line shows the selection threshold (1/9). Adjusted for maternal age, gestational age at which SARS-CoV-2 IgG titer was measured, COVID-19 vaccination status prior to IgG titer measurement, maternal race/ethnicity, parity, type of insurance and pre-pregnancy BMI.
4. Discussion
PFAS-induced immunotoxicity has been documented for years within the literature in both animal and epidemiological studies (Gaballah et al., 2020; Granum et al., 2013; Shane et al., 2020; Stein et al., 2016; Timmermann et al., 2020). Most recently, studies in human populations have demonstrated that COVID-19 may be exacerbated by increasing levels of PFAS exposure (Catelan et al., 2021; Grandjean et al., 2020; Ji et al., 2021). Although these studies have indicated a link between SARS-CoV-2 risk or clinical severity and PFAS exposure, no study to date has investigated such an effect in a pregnant population. To our best knowledge, this is the first study to examine the relationship between maternal plasma-PFAS concentrations and SARS-CoV-2 spike IgG antibody titers in pregnant individuals. Since pregnancy is an immune-altered state, exposure to immune-altering toxicants (i.e., PFAS) may pose a greater threat during gestation.
The exposure levels of legacy PFAS in our study population are lower than the general US population in 2017–2018 as reported in the latest NHANES survey data (CDC Data Tracker, 2022). As per the ATSDR, blood-PFOA and -PFOS levels have declined by more than 70% and 85%, respectively, between 1999 and 2018. This may be attributed to the EPA PFOA Stewardship Program to promote voluntary industrial phase-out of legacy PFAS (2010/15 PFOA Stewardship Program: Guidance on Reporting Emissions and Product Content 2006; Kato et al., 2011). However, manufacturers have replaced long-chain with short-chain PFAS (i.e., GenX, PFBS), and there is a concurrent rise in exposure to these new-age PFAS congeners of which toxicity is less known (Calafat et al., 2019; Hu et al., 2019; Kato et al., 2011). It has been reported that both legacy (PFOA) and emerging (PFNA) were detected in tap water samples collected from 1989 to 1990 from a nationwide prospective cohort of US women and they were significant predictors of plasma PFAS concentrations (Hu et al., 2019). In our study population, we have similarly observed higher levels of emerging PFAS in maternal plasma when compared to historical NHANES levels.
All nine PFAS congeners (n-PFOA, PFHxS, PFHpS, PFHxA, PFNA, n-PFOS, PFUnDA, PFBS, PFDA) measured in our study population demonstrate negative associations with SARS-CoV-2 IgG levels. This finding is in line with multiple previous studies that have found inverse associations between PFAS exposure and IgG antibody level in other infectious diseases (Abraham et al., 2020; Goudarzi et al., 2017; Liu et al., 2020; Looker et al., 2014; Timmermann et al., 2020) and implicates PFAS as immunomodulators and immunosuppressants. The results of our linear regression models also demonstrate similar findings for four PFAS congeners, n-PFOA, PFHxS, PFHpS and PFHxA; notably three of them are short-chain PFAS that can also impact immune responses. Timmermann et al., reported that elevated PFAS serum concentration were associated with decrements in measles antibody concentrations and higher morbidity, and that a doubling of both serum-PFOS and serum-PFDA was associated with lower measles antibody concentrations in children who received a measles vaccine (Timmermann et al., 2020). Immunosuppressive effects of prenatal exposure to PFOS and PFHxS were associated with increased childhood infections (Goudarzi et al., 2017). It is also important to point out that innate immune responses following natural infection are also attenuated by PFAS exposure and associated with a higher burden of persistent infections (Bulka et al., 2021). Both legacy and emerging PFAS demonstrated immunotoxicity in these studies.
Of note, our WQS regression model reveals that PFHxA contributes most to the overall mixture effect, which is also significantly associated with lower maternal SARS-CoV-2 IgG antibody titers. PFHpS, PFHxS and PFOS are lesser contributors to the mixture effect. Within the literature, the three PFAS identified in our WQS model have been found to potentially impact a myriad of health parameters. Liu et al., found that PFHxS and PFHxA perturb human mesenchymal stem cells and adipogenic differentiation (Liu et al., 2020). PFHxS has been associated with immunosuppression in childhood (Goudarzi et al., 2017) and correlated with thyroid autoantibodies in cases of congenital hypothyroidism (Kim et al., 2016). Low-dose PFHxS exposure was also found to be associated with higher prospective odds of actual infertility (Vélez et al., 2015). PFHxA is a persistent short-chain fluorinated organic compound in the environment making dietary exposure a concern due to its accumulation in fruits and vegetables (Felizeter et al., 2012, 2014; Krippner et al., 2014). Only two epidemiologic studies report significant associations with serum PFHxA, one found an inverse association with testosterone levels in teenage boys (Zhou et al., 2016) and a positive association with biomarkers of thyroid autoimmune disease in a Chinese population (Li et al., 2017). Our results and the aforementioned studies suggest that the emerging PFAS can play a varied role in health outcomes and should be investigated further, especially when considering the multifaceted physiological influence of the immune system.
Because the current study population consists of Gen C participants recruited in the early period of pandemic (April 2020–May 2021) only seven of our participants had received any vaccine prior to blood collection. While we adjusted for vaccine status in our statistical models, we also performed sensitivity analysis by excluding these seven individuals and observed no significant differences [Supplementary Table 2 and Supplementary Fig. 2]. PFAS have been detected in cord blood serum as well as breast milk, indicating they are transferred from mother to fetus (Chen et al., 2017; Mamsen et al., 2019; Needham et al., 2011; Sunderland et al., 2019; Wang et al., 2019). Prior cohort studies of the Faroese fishery population, where dietary PFAS exposure is higher than other populations (Grandjean et al., 2017a, 2017b, 2012; Eriksson et al., 2013) have found that antibody titers to routine childhood vaccines (i.e., tetanus, diphtheria, measles) fell below the clinical level of protection during childhood in association with higher PFAS exposure, and these effects persisted into adolescence despite booster shot administration (Grandjean et al., 2017b). We plan to investigate the effects of PFAS exposure on COVID-19 vaccine efficacy in our full cohort where the power is sufficient.
We acknowledge some notable limitations in this study. First, this is a small study of only 72 individuals, thus the power of the study is limited and the interpretation of our study results warrants caution until it can be validated in a larger population. However, our results are in line with the literature indicating PFAS-related immunotoxicity and also reflect the increasing exposure to short-chain PFAS. Second, the timing of maternal infection during pregnancy was difficult to pinpoint given the nature of early pandemic quarantining, testing availability and the ambiguity of infection symptoms. Though we do not know the exact amount of time elapsed between infection and the collection of the plasma sample for IgG titer measurements, we would expect that this interval would be randomly distributed over the time-course of IgG levels. This would be more likely to drive the PFAS-IgG association towards the null given that IgG levels rise sharply within days after infection, reaching peaks in two weeks before waning (Ruggero, 2021). Third, plasma-PFAS and IgG were measured cross-sectionally and may not precisely depict PFAS exposure at the time of infection. However, PFAS are generally stable within the human body, with half-lives ranging typically between 3 and 5 years (Olsen et al., 2007). Lastly, information on some potential confounders, such asbreastfeeding history or socioeconomic status, were incomplete within this population. However, we did include other covariates such as parity and the type of insurance as proxy measures to account for potential confounding.
5. Conclusion
This pilot study is the first to demonstrate that maternal PFAS exposure may negatively impact maternal SARS-CoV-2 IgG antibody titers following SARS-CoV-2 infection during pregnancy. This study adds to the weight-of-evidence that PFAS, including the short-chain “emerging” congeners, are immunotoxic. Whether such an exposure may influence disease progression/severity for the mother or antibody transfer in utero upon infection leading to an immunomodulatory impact on the offspring warrants further investigation.
Credit author statement
K.K. and J.C. were involved in study conceptualization. K.K., J.C., A.S.R. and S.S.A. were involved in funding acquisition and methodology. S.S.A., S.N. and D.P. were involved in performing experiments. Formal data analyses were carried out by K.K., C.L. and V.M. For the Gen C population, E.I., F.G., M.L. were involved in data curation. T.J., W.L., L.D.D.W., V.B., A.S.R. and J.C. were involved in supervising the Gen C study. K.K. was responsible for manuscript original writing and preparation with support from L.C. and J.C. Manuscript critical review and editing was done by J.C., C.L., T.J., S.S.A. and A.S.R. All authors read and approved of the final manuscript.
Funding
This project was partially supported by the CDC (BAA 75D30120C08186) for the Gen C study. The NIEHS Human Health Exposure Analysis Resource (HHEAR) NIH 2U2CES026561 funded the PFAS measurements, which were carried out within the Senator Frank R. Lautenberg Environmental Health Sciences Laboratory at the Icahn School of Medicine at Mount Sinai. The NIEHS T32HD049311 funded K. Kaur.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank the Gen C team, especially Sophie Orn, for collecting and processing biospecimens; Dr. Florian Krammer and his laboratory members for the COVID-19 antibody assay and results; the Chen lab members for their lab support and data feedback (Drs. James Wetmur, Hachem Saddiki and Qian Li); the HHEARS's program manager; Tracy Spangler for assisting in the application process; and the CDC's COVID-19 response and epidemiology task force and Pregnancy and Infant Linked Outcomes Team (PILOT).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2022.115067.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Abraham K., Mielke H., Fromme H., Völkel W., Menzel J., Peiser M., Zepp F., Willich S.N., Weikert C. Internal exposure to perfluoroalkyl substances (PFASs) and biological markers in 101 healthy 1-year-old children: associations between levels of perfluorooctanoic acid (PFOA) and vaccine response. Arch. Toxicol. 2020;94(6):2131–2147. doi: 10.1007/s00204-020-02715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ateia M., Maroli A., Tharayil N., Karanfil T. The overlooked short- and ultrashort-chain poly- and perfluorinated substances: a review. Chemosphere. 2019;220:866–882. doi: 10.1016/j.chemosphere.2018.12.186. [DOI] [PubMed] [Google Scholar]
- Ateyo, et al. COVID-19 mRNA vaccines drive differential Fc-functional profiles in pregnant, lactating, and nonpregnant women. Sci. Transl. Med. 2021;12 doi: 10.1126/scitranslmed.abi8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balshaw D.M., Collman G.W., Gray K.A., Thompson C.L. The Children's Health Exposure Analysis Resource: enabling research into the environmental influences on children's health outcomes. Curr. Opin. Pediatr. 2017;29(3):385–389. doi: 10.1097/MOP.0000000000000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boronow K., et al. Serum concentrations of PFASs and exposure-related behaviors in African American and non-Hispanic white women. J. Expo. Sci. Environ. Epidemiol. 2019;29:206–217. doi: 10.1038/s41370-018-0109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley, et al. SARS-CoV-2 antibody response among women infected during pregnancy. Am. J. Perinatol. 2022;39(9):707–713. doi: 10.1055/s-0041-1739469. [DOI] [PubMed] [Google Scholar]
- Bulka C.M., Avula V., Fry R.C. Associations of exposure to perfluoroalkyl substances individually and in mixtures with persistent infections: recent findings from NHANES 1999-2016. Environ. Pollut. 2021;275 doi: 10.1016/j.envpol.2021.116619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat A.M., Kato K., Hubbard K., Jia T., Botelho J.C., Wong L.Y. Legacy and alternative per- and polyfluoroalkyl substances in the U.S. general population: paired serum-urine data from the 2013-2014 National Health and Nutrition Examination Survey. Environ. Int. 2019;131 doi: 10.1016/j.envint.2019.105048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico C., Gennings C., Wheeler D.C., Factor-Litvak P. Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. J. Agric. Biol. Environ. Stat. 2015;20(1):100–120. doi: 10.1007/s13253-014-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catelan D., Biggeri A., Russo F., Gregori D., Pitter G., Da Re F., Fletcher T., Canova C. Exposure to perfluoroalkyl substances and mortality for COVID-19: a spatial ecological analysis in the veneto region (Italy) Int. J. Environ. Res. Publ. Health. 2021;18(5) doi: 10.3390/ijerph18052734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC Data Tracker (2022).
- Chang C.J., Barr D.B., Ryan P.B., Panuwet P., Smarr M.M., Liu K., Kannan K., Yakimavets V., Tan Y., Ly V., Marsit C.J., Jones D.P., Corwin E.J., Dunlop A.L., Liang D. Per- and polyfluoroalkyl substance (PFAS) exposure, maternal metabolomic perturbation, and fetal growth in African American women: a meet-in-the-middle approach. Environ. Int. 2022;158 doi: 10.1016/j.envint.2021.106964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Yin S., Kelly B.C., Liu W. Isomer-specific transplacental transfer of perfluoroalkyl acids: results from a survey of paired maternal, cord sera, and placentas. Environ. Sci. Technol. 2017;51(10):5756–5763. doi: 10.1021/acs.est.7b00268. [DOI] [PubMed] [Google Scholar]
- Coggan T.L., Anumol T., Pyke J., Shimeta J., Clarke B.O. A single analytical method for the determination of 53 legacy and emerging per- and polyfluoroalkyl substances (PFAS) in aqueous matrices. Anal. Bioanal. Chem. 2019;411(16):3507–3520. doi: 10.1007/s00216-019-01829-8. [DOI] [PubMed] [Google Scholar]
- Coperchini F., Croce L., Ricci G., Magri F., Rotondi M., Imbriani M., Chiovato L. Thyroid disrupting effects of old and new generation PFAS. Front. Endocrinol. 2020;11 doi: 10.3389/fendo.2020.612320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ctq A.M.A.P. CTQ; 2022. AMAP Ring Test for Persistent Organic Pollutants in Human Serum 2022.https://www.inspq.qc.ca/en/ctq/eqas/amap/description [Google Scholar]
- Czarnota J., Gennings C., Wheeler D.C. Assessment of weighted quantile sum regression for modeling chemical mixtures and cancer risk. Cancer Inf. 2015;14(Suppl. 2):159–171. doi: 10.4137/cin.S17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva A, Armitage J, Burton T, Dassuncao C, Heiger-Bernays W, Hu X, Karrman A, Kelly B, Ng C, Robuck A, Sun M, Webster T, Sunderland E. PFAS exposure pathways for humans and wildlife: A synthesis of current knowledge and key gaps in understanding. Environmental Toxicology and Chemistry. 2020;40(3):631–657. doi: 10.1002/etc.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson U, Karrman A, Rotander A, Mikkelsen B, Dam M. Perfluoroalkyl substances (PFASs) in food and water from Faroe Islands. Environ. Sci. Pollut. Res. Int. 2013;20(11):7940–7948. doi: 10.1007/s11356-013-1700-3. [DOI] [PubMed] [Google Scholar]
- Felizeter S., McLachlan M.S., De Voogt P. Uptake of perfluorinated alkyl acids by hydroponically grown lettuce (Lactuca sativa) Environ. Sci. Technol. 2012;46(21):11735–11743. doi: 10.1021/es302398u. [DOI] [PubMed] [Google Scholar]
- Felizeter S., McLachlan M.S., De Voogt P. Root uptake and translocation of perfluorinated alkyl acids by three hydroponically grown crops. J. Agric. Food Chem. 2014;62(15):3334–3342. doi: 10.1021/jf500674j. [DOI] [PubMed] [Google Scholar]
- Gaballah S., Swank A., Sobus J.R., Howey X.M., Schmid J., Catron T., McCord J., Hines E., Strynar M., Tal T. Evaluation of developmental toxicity, developmental neurotoxicity, and tissue dose in zebrafish exposed to GenX and other PFAS. Environ. Health Perspect. 2020;128(4) doi: 10.1289/EHP5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göen T., Schaller K.-H., Drexler H. External quality assessment of human biomonitoring in the range of environmental exposure levels. Int. J. Hyg Environ. Health. 2012;215(2):229–232. doi: 10.1016/j.ijheh.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Goudarzi H., Miyashita C., Okada E., Kashino I., Chen C.-J., Ito S., Araki A., Kobayashi S., Matsuura H., Kishi R. Prenatal exposure to perfluoroalkyl acids and prevalence of infectious diseases up to 4 years of age. Environ. Int. 2017;104:132–138. doi: 10.1016/j.envint.2017.01.024. [DOI] [PubMed] [Google Scholar]
- Grandjean P., Andersen E.W., Budtz-Jorgensen E., Nielsen F., Molbak K., Weihe P., Heilmann C. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA. 2012;307(4):391–397. doi: 10.1001/jama.2011.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P., Heilmann C., Weihe P., Nielsen F., Mogensen U.B., Budtz-Jorgensen E. Serum vaccine antibody concentrations in adolescents exposed to perfluorinated compounds. Environ. Health Perspect. 2017;125(7) doi: 10.1289/EHP275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P., Heilmann C., Weihe P., Nielsen F., Mogensen U.B., Timmermann A., Budtz-Jorgensen E. Estimated exposures to perfluorinated compounds in infancy predict attenuated vaccine antibody concentrations at age 5-years. J. Immunot. 2017;14(1):188–195. doi: 10.1080/1547691X.2017.1360968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P., Timmermann C.A.G., Kruse M., Nielsen F., Vinholt P.J., Boding L., Heilmann C., Molbak K. Severity of COVID-19 at elevated exposure to perfluorinated alkylates. 2020. medRxiv. [DOI] [PMC free article] [PubMed]
- Granum B., Haug L.S., Namork E., Stolevik S.B., Thomsen C., Aaberge I.S., van Loveren H., Lovik M., Nygaard U.C. Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J. Immunot. 2013;10(4):373–379. doi: 10.3109/1547691X.2012.755580. [DOI] [PubMed] [Google Scholar]
- Hu X.C., Tokranov A.K., Liddie J., Zhang X., Grandjean P., Hart J.E., Laden F., Sun Q., Yeung L.W.Y., Sunderland E.M. Tap water contributions to plasma concentrations of poly- and perfluoroalkyl substances (PFAS) in a nationwide prospective cohort of U.S. Women. Environ. Health Perspect. 2019;127(6) doi: 10.1289/EHP4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janevic T., Lieb W., Ibroci E., Lynch J., Lieber M., Molenaar N.M., Rommel A.S., de Witte L., Ohrn S., Carreno J.M., Krammer F., Zapata L.B., Snead M.C., Brody R.I., Jessel R.H., Sestito S., Adler A., Afzal O., Gigase F., Missall R., Carrion D., Stone J., Bergink V., Dolan S.M., Howell E.A., Krammer Serology Core Study G. The influence of structural racism, pandemic stress, and SARS-CoV-2 infection during pregnancy with adverse birth outcomes. Am J Obstet Gynecol MFM. 2022;4(4) doi: 10.1016/j.ajogmf.2022.100649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J., Song L., Wang J., Yang Z., Yan H., Li T., Yu L., Jian L., Jiang F., Li J., Zheng J., Li K. Association between urinary per- and poly-fluoroalkyl substances and COVID-19 susceptibility. Environ. Int. 2021;153 doi: 10.1016/j.envint.2021.106524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaboré H.A., Duy S.V., Munoz G., Méité L., Desrosiers M., Liu J., Sory T.K., Sauvé S. Worldwide drinking water occurrence and levels of newly-identified perfluoroalkyl and polyfluoroalkyl substances. Sci. Total Environ. 2018;616:1089–1100. doi: 10.1016/j.scitotenv.2017.10.210. [DOI] [PubMed] [Google Scholar]
- Kannan K., Stathis A., Mazzella M.J., Andra S.S., Barr D.B., Hecht S.S., Merrill L.S., Galusha A.L., Parsons P.J. Quality assurance and harmonization for targeted biomonitoring measurements of environmental organic chemicals across the Children's Health Exposure Analysis Resource laboratory network. Int. J. Hyg Environ. Health. 2021;234 doi: 10.1016/j.ijheh.2021.113741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K., Wong L.Y., Jia L.T., Kuklenyik Z., Calafat A.M. Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999-2008. Environ. Sci. Technol. 2011;45(19):8037–8045. doi: 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- Kato K., Kalathil A.A., Patel A.M., Ye X., Calafat A.M. Per- and polyfluoroalkyl substances and fluorinated alternatives in urine and serum by on-line solid phase extraction-liquid chromatography-tandem mass spectrometry. Chemosphere. 2018;209:338–345. doi: 10.1016/j.chemosphere.2018.06.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.-H., Kim U.-J., Kim H.-Y., Choi S.-D., Oh J.-E. Perfluoroalkyl substances in serum from South Korean infants with congenital hypothyroidism and healthy infants–Its relationship with thyroid hormones. Environ. Res. 2016;147:399–404. doi: 10.1016/j.envres.2016.02.037. [DOI] [PubMed] [Google Scholar]
- Krippner J., Brunn H., Falk S., Georgii S., Schubert S., Stahl T. Effects of chain length and pH on the uptake and distribution of perfluoroalkyl substances in maize (Zea mays) Chemosphere. 2014;94:85–90. doi: 10.1016/j.chemosphere.2013.09.018. [DOI] [PubMed] [Google Scholar]
- Lesseur C., Jessel R.H., Ohrn S., Ma Y., Li Q., Dekio F., Brody R.I., Wetmur J.G., Gigase F.A.J., Lieber M., Lieb W., Lynch J., Afzal O., Ibroci E., Rommel A.S., Janevic T., Stone J., Howell E.A., Galang R.R., Dolan S.M., Bergink V., De Witte L.D., Chen J. Gestational SARS-CoV-2 infection is associated with placental expression of immune and trophoblast genes. Placenta. 2022;126:125–132. doi: 10.1016/j.placenta.2022.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Cheng Y., Xie Z., Zeng F. Perfluorinated alkyl substances in serum of the southern Chinese general population and potential impact on thyroid hormones. Sci. Rep. 2017;7(1):1–10. doi: 10.1038/srep43380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Yang R., Yin N., Faiola F. The short-chain perfluorinated compounds PFBS, PFHxS, PFBA and PFHxA, disrupt human mesenchymal stem cell self-renewal and adipogenic differentiation. J. Environ. Sci. 2020;88:187–199. doi: 10.1016/j.jes.2019.08.016. [DOI] [PubMed] [Google Scholar]
- Looker C., Luster M.I., Calafat A.M., Johnson V.J., Burleson G.R., Burleson F.G., Fletcher T. Influenza vaccine response in adults exposed to perfluorooctanoate and perfluorooctanesulfonate. Toxicol. Sci. 2014;138(1):76–88. doi: 10.1093/toxsci/kft269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamsen L.S., Bjorvang R.D., Mucs D., Vinnars M.T., Papadogiannakis N., Lindh C.H., Andersen C.Y., Damdimopoulou P. Concentrations of perfluoroalkyl substances (PFASs) in human embryonic and fetal organs from first, second, and third trimester pregnancies. Environ. Int. 2019;124:482–492. doi: 10.1016/j.envint.2019.01.010. [DOI] [PubMed] [Google Scholar]
- Meng Q., Inoue K., Ritz B., Olsen J., Liew Z. Prenatal exposure to perfluoroalkyl substances and birth outcomes; an updated analysis from the Danish national birth cohort. Int. J. Environ. Res. Publ. Health. 2018;15(9) doi: 10.3390/ijerph15091832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar N.M., Rommel A.S., de Witte L., Dolan S.M., Lieb W., Ibroci E., Ohrn S., Lynch J., Capuano C., Stadlbauer D., Krammer F., Zapata L.B., Brody R.I., Pop V.J., Jessel R.H., Sperling R.S., Afzal O., Gigase F., Missall R., Janevic T., Stone J., Howell E.A., Bergink V. SARS-CoV-2 during pregnancy and associated outcomes: results from an ongoing prospective cohort. Paediatr. Perinat. Epidemiol. 2022;36(4):466–475. doi: 10.1111/ppe.12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Toxicology Program . Monograph on Immunotoxicity Associated with Exposure to Perfluorooctanoic acid (PFOA) and Perfluorooctane Sulfonate (PFOS; 2016. [Google Scholar]
- Needham L.L., Grandjean P., Heinzow B., Jørgensen P.J., Nielsen F., Patterson D.G., Jr., Sjödin A., Turner W.E., Weihe P. Partition of environmental chemicals between maternal and fetal blood and tissues. Environ. Sci. Technol. 2011;45(3):1121–1126. doi: 10.1021/es1019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, et al. Social disparities in exposures to bisphenol A and polyfluoroalkyl chemicals: a cross-sectional study within NHANES 2003-2006. Environ. Health. 2012;11:10. doi: 10.1186/1476-069X-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen G.W., Burris J.M., Ehresman D.J., Froehlich J.W., Seacat A.M., Butenhoff J.L., Zobel L.R. Half-life of serum elimination of perfluorooctanesulfonate,perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 2007;115(9):1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFOA Stewardship Program . 2006. Guidance on Reporting Emissions and Product Content.https://www.epa.gov/sites/default/files/2015-10/documents/pfoaguidance.pdf 2010/15. [Google Scholar]
- Poothong S., Thomsen C., Padilla-Sanchez J.A., Papadopoulou E., Haug L.S. Distribution of novel and well-known poly- and perfluoroalkyl substances (PFASs) in human serum, plasma, and whole blood. Environ. Sci. Technol. 2017;51(22):13388–13396. doi: 10.1021/acs.est.7b03299. [DOI] [PubMed] [Google Scholar]
- R Core Team, 2022. In.
- Reagen W.K., Ellefson M.E., Kannan K., Giesy J.P. Comparison of extraction and quantification methods of perfluorinated compounds in human plasma, serum, and whole blood. Anal. Chim. Acta. 2008;628(2):214–221. doi: 10.1016/j.aca.2008.09.029. [DOI] [PubMed] [Google Scholar]
- Renzetti S., Curtin P., Just A.C., Gennings C. Gwqs: generalized weighted quantile sum regression. R package version. 2016;1 [Google Scholar]
- Ruggero, et al. Antibodies against SARS-CoV-2 time course in patients and vaccinated subjects: an evaluation of the harmonization of two different methods. Diagnostics. 2021;11(9):1709. doi: 10.3390/diagnostics11091709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane H.L., Baur R., Lukomska E., Weatherly L., Anderson S.E. Immunotoxicity and allergenic potential induced by topical application of perfluorooctanoic acid (PFOA) in a murine model. Food Chem. Toxicol. 2020;136 doi: 10.1016/j.fct.2020.111114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer J.J., Callahan C.L., Calafat A.M., Huang W.Y., Jones R.R., Sabbisetti V.S., Freedman N.D., Sampson J.N., Silverman D.T., Purdue M.P., Hofmann J.N. Serum concentrations of per- and polyfluoroalkyl substances and risk of renal cell carcinoma. J. Natl. Cancer Inst. 2021;113(5):580–587. doi: 10.1093/jnci/djaa143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T., Cunningham-Rundles C. Primary B-cell immunodeficiencies. Hum. Immunol. 2019;80(6):351–362. doi: 10.1016/j.humimm.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadlbauer D., Amanat F., Chromikova V., Jiang K., Strohmeier S., Arunkumar G.A., Tan J., Bhavsar D., Capuano C., Kirkpatrick E., Meade P., Brito R.N., Teo C., McMahon M., Simon V., Krammer F. SARS-CoV-2 seroconversion in humans: a detailed protocol for a serological assay, antigen production, and test setup. Curr Protoc Microbiol. 2020;57(1):e100. doi: 10.1002/cpmc.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C.R., McGovern K.J., Pajak A.M., Maglione P.J., Wolff M.S. Perfluoroalkyl and polyfluoroalkyl substances and indicators of immune function in children aged 12-19 y: National Health and Nutrition Examination Survey. Pediatr. Res. 2016;79(2):348–357. doi: 10.1038/pr.2015.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland E.M., Hu X.C., Dassuncao C., Tokranov A.K., Wagner C.C., Allen J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019;29(2):131–147. doi: 10.1038/s41370-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermann C.A.G., Jensen K.J., Nielsen F., Budtz-Jorgensen E., van der Klis F., Benn C.S., Grandjean P., Fisker A.B. Serum perfluoroalkyl substances, vaccine responses, and morbidity in a cohort of Guinea-bissau children. Environ. Health Perspect. 2020;128(8) doi: 10.1289/EHP6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracking Coronavirus in New York: Latest Map and Case Count. (2022). https://www.nytimes.com/interactive/2021/us/new-york-covid-cases.html.
- Vélez M., Arbuckle T., Fraser W. Maternal exposure to perfluorinated chemicals and reduced fecundity: the MIREC study. Hum. Reprod. 2015;30(3):701–709. doi: 10.1093/humrep/deu350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., DeWitt J.C., Higgins C.P., Cousins I.T. A never-ending story of per- and polyfluoroalkyl substances (PFASs)? Environ. Sci. Technol. 2017;51(5):2508–2518. doi: 10.1021/acs.est.6b04806. [DOI] [PubMed] [Google Scholar]
- Wang Y., Han W., Wang C., Zhou Y., Shi R., Bonefeld-Jørgensen E.C., Yao Q., Yuan T., Gao Y., Zhang J. Efficiency of maternal-fetal transfer of perfluoroalkyl and polyfluoroalkyl substances. Environ. Sci. Pollut. Control Ser. 2019;26(3):2691–2698. doi: 10.1007/s11356-018-3686-3. [DOI] [PubMed] [Google Scholar]
- Worley R, Moore S, Tierney B, Ye X, Calafat A, Campbell S, Woudneh M, Fisher J. Per- and polyfluoroalkyl substances in human serum and urine samples from a residentially exposed community. Environ Int. 2017;106:135–143. doi: 10.1016/j.envint.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Fletcher T, Pineda D, Lindh C, Nilsson C, Glynn A, Vogs C, Norstrom K, Lilja K, Jakobsson K, Li Y. Serum half-lives of short- and long- chain perfluoroalkyl acids after ceasing exposure from drinking water contaminated by firefighting foams. Environ Health Prespect. 2020;128(7):77004. doi: 10.1289/EHP6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Sundaram R., Maisog J., Calafat A.M., Barr D.B., Louis G.M.B. A prospective study of prepregnancy serum concentrations of perfluorochemicals and the risk of gestational diabetes. Fertil. Steril. 2015;103(1):184–189. doi: 10.1016/j.fertnstert.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Hu L.-W., Qian Z.M., Chang J.-J., King C., Paul G., Lin S., Chen P.-C., Lee Y.L., Dong G.-H. Association of perfluoroalkyl substances exposure with reproductive hormone levels in adolescents: by sex status. Environ. Int. 2016;94:189–195. doi: 10.1016/j.envint.2016.05.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.