Abstract
Studies of the pathogenesis of enterotoxigenic Escherichia coli (ETEC) have largely centered on extrachromosomal determinants of virulence, in particular the plasmid-encoded heat-labile (LT) and heat-stable enterotoxins and the colonization factor antigens. ETEC causes illnesses that range from mild diarrhea to severe cholera-like disease. These differences in disease severity are not readily accounted for by our current understanding of ETEC pathogenesis. Here we demonstrate that Tia, a putative adhesin of ETEC H10407, is encoded on a large chromosomal element of approximately 46 kb that shares multiple features with previously described E. coli pathogenicity islands. Further analysis of the region downstream from tia revealed the presence of several candidate open reading frames (ORFs) in the same transcriptional orientation as tia. The putative proteins encoded by these ORFs bear multiple motifs associated with bacterial secretion apparatuses. An in-frame deletion in one candidate gene identified here as leoA (labile enterotoxin output) resulted in marked diminution of secretion of the LT enterotoxin and lack of fluid accumulation in a rabbit ileal loop model of infection. Although previous studies have suggested that E. coli lacks the capacity to secrete LT, our studies show that maximal release of LT from the periplasm of H10407 is dependent on one or more elements encoded on a pathogenicity island.
Enterotoxigenic Escherichia coli (ETEC) causes diarrheal diseases that range in severity from mild, self-limited illness to more severe cases that are clinically indistinguishable from cholera (21, 55, 56). ETEC infections remain a significant cause of childhood morbidity and mortality in developing countries, causing one-fifth of all severe diarrheal illnesses (35), and accounting for nearly three quarters of a million deaths per year in children under the age of 5 years (67). Worldwide, this pathogen causes an estimated 600 million cases of diarrheal illness each year. It is the most common cause of diarrhea in travelers (3) and in soldiers deployed to developing countries (7, 34).
Studies to date of the pathogenesis of ETEC infection and vaccine development have largely focused on the known plasmid-encoded virulence factors (33, 36). These include a diverse repertoire of fimbrial colonization factor antigens (CFAs), critical for colonization of the small intestine (25), and heat-labile (LT) and heat-stable (ST) enterotoxins, responsible for net secretion of fluid into the intestine and diarrhea (2, 59). LT is a multimeric protein composed of an A subunit and a B subunit pentamer and is structurally similar to cholera toxin (CT) (62). Both toxins can translocate across the outer membrane of Vibrio cholerae via the two-step general secretion pathway (GSP). In the first step, the N-terminal signal peptides of the subunits are cleaved during sec-dependent (66) transport across the inner membrane to the periplasm, where the proteins combine to form the holotoxin (30). Secretion across the outer membrane requires a second system known as the main terminal branch of the GSP (50, 57). Homologues of the eps genes, which comprise the GSP in Vibrio cholerae, can be found in E. coli K-12 (5). However, the gps operon of E. coli appears to be cryptic (23), and previous studies found that a K-12 derivative was incapable of exporting either CT or LT beyond the periplasm (31, 51). The presence of LT in supernatants of some wild-type ETEC strains (38) and the occurrence of cholera-like diarrhea in infections caused by these organisms suggest that they may possess previously uncharacterized virulence factors required for enterotoxin export.
In previous studies with ETEC H10407, a strain isolated from a patient with severe cholera-like disease in Bangladesh (20), we identified a chromosomal locus that encodes the putative adhesin Tia (22). Here we report that this locus resides on a pathogenicity island that shares many features with those previously described in other pathogenic E. coli strains. Importantly, we have identified a gene encoded on the island that is required for export of the heat-labile toxin, a finding that may relate to the cholera-like disease caused by some ETEC infections.
MATERIALS AND METHODS
Bacterial strains.
The E. coli K-12 strain MG1655 (5) was obtained from the E. coli Genome Center, University of Wisconsin, Madison. Pathogenic E. coli strains were obtained from collections of clinical isolates maintained at the Walter Reed Army Institute of Research or the Memphis Veterans Affairs Medical Center. The strains and plasmids used are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Reference |
|---|---|---|
| Strains | ||
| H10407 | ETEC serotype O78:H11, LT+ | 19 |
| EDL903 | ETEC serotype O88:H25, LT+ | Fleckenstein et al.a |
| DS7-3 | ETEC serotype O8:H9, LT+ | Fleckenstein et al. |
| MG1655 | Prototypical E. coli K-12 strain | 5 |
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10 (Tetr)] | Stratagene |
| Plasmids | ||
| pET101 | pHC79-based cosmid containing tia locus from H10407 | 17 |
| pET102 | pHC79-based cosmid containing tia locus from H10407 | 17 |
| pGEM-7zf(+) | Cloning vector Apr | Promega |
| pQE-30 | Expression vector, Apr | Qiagen |
| pREP4 | lacIq Kmr repressor plasmid | Qiagen |
| pT7Blue-3 | Multipurpose PCR cloning vector, Apr Kmr | Novagen |
| pCVD442 | sacB-containing suicide vector, Apr | 13 |
| pPIR-K | Temperature-sensitive pir helper plasmid, Kmr | 53 |
| pES009 | 1,680-bp insert containing 822-bp in-frame leoA deletion cloned into EcoRV site of pT7Blue-3 | This study |
| pES010 | XbaI insert fragment from pES009 cloned into pCVD442 | This study |
| pES011 | pQE-30-based leoA expression plasmid | This study |
J. M. Fleckenstein, N. J. Snellings, E. A. Elsinghorst, and L. E. Lindler, Abstr. 1997 Meet. Microb. Pathog. Host Resp., p. 37.
DNA sequencing of the tia locus.
Two overlapping tia+ pHC79-based cosmids, pET101 and pET102, served as the initial templates for DNA sequencing using the Applied Biosystems 373 automated sequencer. Sequence was determined by several methods. To locate the flanking ends of the island, the ends of each of the cosmid inserts were sequenced by primer walking, starting with primers pHC79.314-331 (5′-CTC GCT TCG CTA CTT GGA-3′) and pHC79.517-500 (5′-CAT ACC CAC GCC GAA ACA-3′), which flank the BamHI site of pHC79. To facilitate sequencing of the 12-kb region downstream from tia, the entire region was amplified from pET101 using the proofreading polymerase rTth, XL (Perkin Elmer), and primers pHC79.314-331 and 12209601 (5′-GCA CGA AAT GTA ACT GGA-3′). The amplicon was then partially digested with Sau3AI to produce a series of small fragments (average size, ≤2 kb), which were then randomly cloned into the BamHI site of pGEM-7zf(+). Conserved SP6 and T7 vector primers were then used to sequence the insert regions of the subclones in both directions. The data obtained in this fashion were then used to construct primers to obtain additional sequence by primer walking. Sequence data were edited and assembled into contiguous sequence with the Sequencher program (Gene Codes, Ann Arbor, Mich.). Further analysis employed the ORF Finder and BLAST (1) programs available at the National Center for Biotechnology Information website (www.ncbi.nlm.nih.gov). Translations of the candidate open reading frames (ORFs) were then searched against databases to identify putative motifs not identified by BLAST using the sites www.http://dna.stanford.edu/identify (48) and http://www.motif.genome.ad.jp.
DNA hybridization studies.
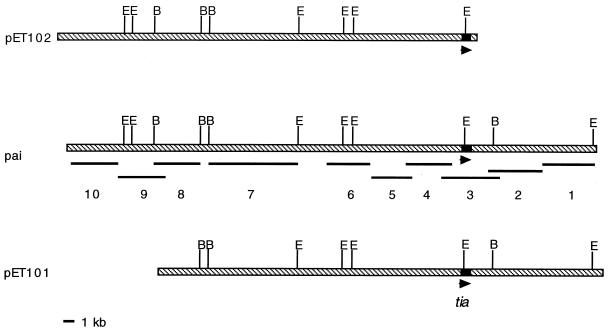
Genomic DNA was prepared from E. coli strains by established protocols (22). The resulting DNA was then dotted onto nylon membranes and hybridized with digoxigenin-labeled DNA probes made by random primer labeling of either PCR products or restriction digest fragments from the original cosmids (Fig. 1). Hybridizations were done under high-stringency conditions at 68°C without formamide, and posthybridization washes were performed with 2× SSC (1× SSC is 0.15 M NaCl, 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate (SDS) at room temperature followed by 0.1× SSC–0.1% SDS at 68°C.
FIG. 1.
Alignment of tia+ cosmids pET101 and pET102 with the pathogenicity island and locations of probes used in hybridization studies. Restriction endonuclease sites are indicated (B, BamHI; E, EcoRI). Arrows indicate the location and direction of transcription of tia.
Multiprimer PCR.
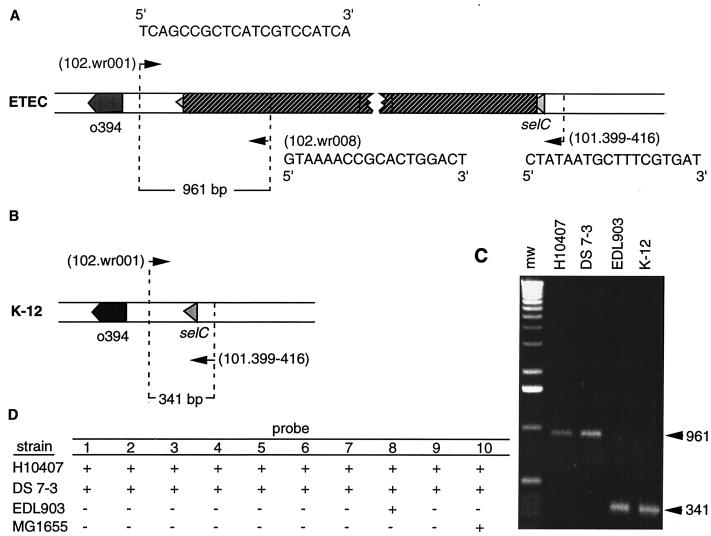
PCR was used to demonstrate interruption of the selC tRNA gene by using primers flanking the selC gene (designated 102.wr001 and 101.399-416) and a third primer based on sequence internal to the island (102.wr008). Genomic DNA from either E. coli K-12 (MG1655) or clinical ETEC strains was used as the template. Reactions were done in a Perkin Elmer 2400 thermal cycler, with denaturing for 5 min at 94°C, followed by 35 cycles of 30 s at 94°C, 30 s of 50°C, and 1 min at 72°C, and a final extension for 7 min at 72°C.
Construction of an in-frame deletion in leoA.
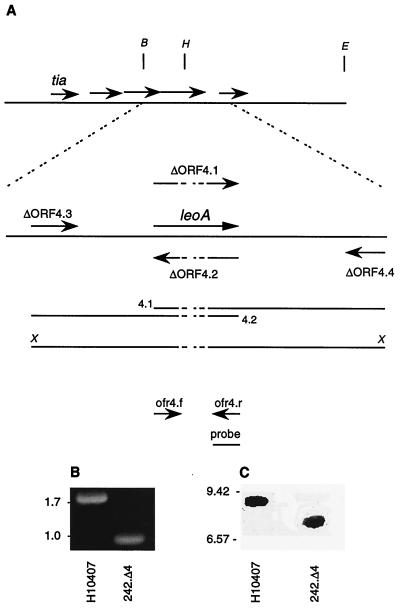
To construct an isogenic ΔleoA mutant (see Fig. 4), four-primer PCR was first used to engineer a product containing an in-frame 822-bp deletion in leoA (14, 41). Oligonucleotide Δorf4.1 was constructed as a 34-bp fragment with nucleotides 1 to 17 and 18 to 34 corresponding to bases 152 to 168 and 991 to 1007 of leoA, respectively. Oligonucleotide Δorf4.2 is the reverse complement of Δorf4.1. Primers Δorf4.3 and Δorf4.4 flank the orf4 sequence and contain XbaI restriction sites at their 5′ ends. Primers Δorf4.1 and Δorf4.4 and primers Δorf4.2 and Δorf4.3 were combined in separate PCRs with H10407 genomic DNA as the template. Products from these reactions were then combined in a third reaction mixture to generate a 1,680-bp amplicon containing the deletion, which was then cloned into pT7blue-3 to yield pES009. The XbaI insert from pES009 was subsequently cloned into the XbaI site of pCVD442 (13) to yield plasmid pES010, which was introduced into H10407(pPIR-K) (53) to perform suicide vector-directed double homologous recombination. Loss of the leoA sequence from the resulting strain (242.Δ4) was confirmed by PCR and Southern blot hybridization. To complement the ΔleoA deletion, 242.Δ4(pREP4) was transformed to ampicillin resistance with the pQE-30-based leoA expression plasmid pES011.
FIG. 4.
Construction of an in-frame deletion in orf4 (leoA). (A) Locations of restriction sites for BamHI (B), HindIII (H), and EcoRI (E), primers, leoA 3′ probe, ORFs, and PCR amplicons relative to leoA. Intermediate amplicons from the first round of PCR are labeled 4.1 and 4.2. (amplicon 4.1 is the product of the PCR with primers ΔORF4.1 and ΔORF4.4; amplicon 4.2 is the product obtained with primers ΔORF4.2 and ΔORF4.3). The final PCR product is shown with flanking XbaI sites (X). (B) PCR products generated from H10407 and the ΔleoA strain (242.Δ4) genomic DNA using primers orf4.f (5′-GAACAATTCAAACAGTTCA-3′) and orf4.r (5′-CTATCTGGCAGTATGGTTT-3′), representing bases 4 to 22 and 1734 to 1716 of the gene, respectively. (C) Southern blot confirming the deletion in leoA. BamHI- and EcoRI-digested genomic DNA from parent strain H10407 and 242.Δ4, probed with biotinylated DNA probe consisting of a 675-bp fragment from the 3′ end of leoA. (B and C) Molecular size markers (in kilobases) are shown to the left of each panel.
LT production assays.
Subcultures from frozen bacterial stocks maintained at −20°C were grown overnight in CYE-glucose medium (54) at 37°C with vigorous aeration. Cells were pelleted by centrifugation at 16,000 × g for 15 min. The resulting clarified bacterial supernatants were frozen at −80°C. To measure LT retained in the periplasm, bacterial pellets from mid-logarithmic growth phase cultures were washed twice in 1 ml of cold phosphate-buffered saline and resuspended in an equal volume of buffer containing 25 mM Tris HCl (pH 8.0), 10 mM EDTA, lysozyme (3 mg/ml), polymyxin B (2 mg/ml), and phenylmethylsulfonyl fluoride (8 μg/ml). After incubation on ice for 10 min, pellets were subjected to repeated freeze-thaw cycles to release any LT remaining in the periplasm (38, 51). After centrifugation at 4°C and 16,000 × g for 15 min, clarified lysates were frozen at −80°C until used in LT assays. Measurement of LT was performed with the mixed-ganglioside enzyme-linked immunosorbent assay (10).
Analysis of extracellular and periplasmic proteins.
To exclude the possibility of LT release on the basis of nonspecific lysis of the bacteria, supernatant and periplasmic fractions were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) (39) and assayed for activity of the periplasmic enzyme alkaline phosphatase using the chromogenic substrate p-nitrophenyl phosphate (PNPP) as previously described (45). Hydrolysis of PNPP was detected at an optical density at 420 nm, and the values were adjusted for the optical density at 600 nm of the respective cultures. Extracellular proteins for SDS-PAGE analysis were concentrated by precipitation with 10% (wt/vol) ice-cold trichloroacetic acid and washed with ice-cold acetone. Samples were resuspended in buffer containing 2-mercaptoethanol and heated at 95°C for 10 min, and proteins were separated by SDS-PAGE. Proteins were visualized by colloidal Coomassie staining (47).
Rabbit ileal loop assay.
Rabbit ileal loop studies were performed as previously described (12, 49) with 2-kg male New Zealand White rabbits. Rabbits were fasted for 48 h prior to surgery. Laparotomy was performed to externalize the intestine by aseptic technique under anesthesia with intramuscularly administered ketamine (35 mg/kg) and xylazine (5 mg/kg). Loops were created in the jejunum by placing ligatures at 10-cm intervals and separating loops with a 1-cm interposing loop. Test strains were grown from single colonies in 2 ml of LB on the morning of the assay, and the number of cells was adjusted to approximately 108 CFU/ml. A 1-ml inoculum was injected into each loop in random fashion, the intestine was internalized, and the incision was closed. After approximately 18 h, the volume of fluid in each loop was measured.
Statistical analysis.
Assay results were compared by using the unpaired Student t test with MultiStat software (Biosoft, Cambridge, United Kingdom).
Nucleotide sequence accession number.
The leoA sequence has been assigned GenBank accession number AF170971.
RESULTS
Localization of the tia locus to selC.
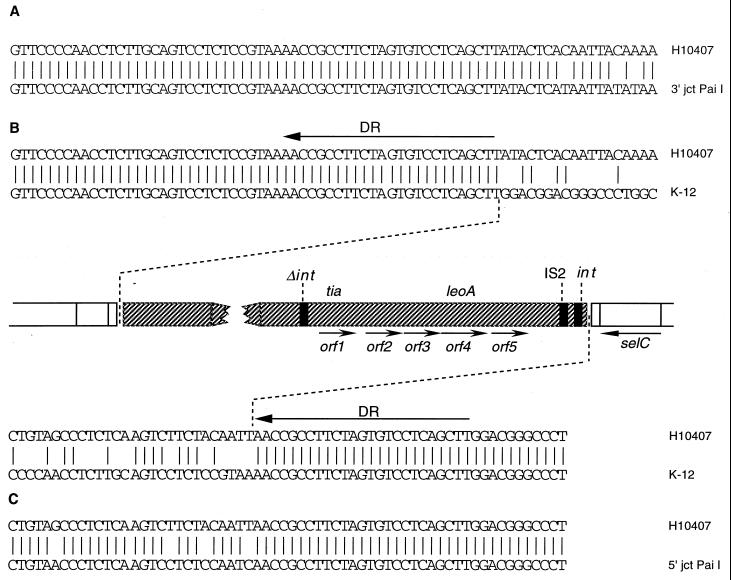
Initial sequencing of the inserts of H10407 genomic cosmid clones pET101 and pET102 by primer walking revealed that the flanking ends of these cosmids contained DNA sequence identical to that of E. coli K-12 (Fig. 2). However, further DNA sequence analysis of the end of the pET102 insert far upstream from tia revealed that this sequence homology with E. coli K-12 ends abruptly with the 25-bp sequence 5′-TTCGACTCCTGTGATCTTCCGCCAA-3′. Sequencing the end of pET101, far downstream from tia, demonstrated the same 25-bp sequence arranged in a direct orientation. These direct repeat (DR) elements, which are identical to those flanking pathogenicity island 1 (PAI 1) from the uropathogenic E. coli strain 536 (37), flank a region of approximately 46 kb inserted at the 3′ end of the selC tRNA gene at precisely the same location as PAI 1 and the locus of enterocyte effacement from enteropathogenic E. coli (6, 46). This is also identical to the point of insertion of a pathogenicity island in Salmonella enterica subsp. enterica serovar Typhimurium, which contains genes mediating the survival of the organism in macrophages (4).
FIG. 2.
Features of the pathogenicity island and localization within the E. coli genome. (A) Alignment of upstream (relative to tia) flank with 3′-flanking region of PAI 1 of uropathogenic E. coli 536. (B) Locations of ORFs, DRs, and mobility elements (represented as thick black vertical bars) relative to selC. Orf1 is tia. The direction of transcription is indicated by the arrows. (C) Alignment of downstream flank with 5′-flanking region of PAI 1.
Forty-six geographically diverse human clinical ETEC strains comprising a diverse collection of serotypes, CFA types, and toxin types were examined by DNA hybridization studies. While slightly more than half (57%) of these strains hybridized to six or more island probes, we noted considerable heterogeneity in the hybridization patterns. Genomic DNA from some strains such as DS7-3 yielded hybridization patterns identical to that of H10407, whereas others such as strain EDL903 hybridized with a single PAI probe (Fig. 3). When genomic DNA from a subset of 11 clinical ETEC strains was subjected to three-primer PCR (Fig. 3), we noted similar heterogeneity. Some strains (e.g., EDL903) yielded an amplicon of approximately 341 bp, such as would be predicted for an intact selC region. Conversely, PCR with DNA from H10407 and other ETEC strains resulted in the 961-bp product predicted for a selC region that has been interrupted by the island. We were unable to amplify either product from some ETEC strains, suggesting the presence of insertions in selC that differ from the H10407 island. Still other strains such as DS67-1 (LT−) hybridized to 10 of 10 PAI probes but yielded the 341-bp amplicon, indicating that in this strain the island is inserted at a location other than selC. There was no clear correlation of presence or absence of the island with CFA type or toxin genotype.
FIG. 3.
Multiprimer PCR assay and results of DNA hybridization studies. (A) Locations of primers relative to pathogenicity island and selC. A 961-bp amplicon is predicted for the H10407 island and similar islands. (B) The 341-bp amplicon predicted for an intact selC locus. (C) PCR results for clinical ETEC strains and E. coli K-12. (D) Results of hybridization studies with island probes. Probes represent the entire island and are numbered sequentially starting with the probe closest to selC. (The locations of these probes are indicated in Fig. 1.)
A number of other diarrheagenic E. coli strains also yielded genomic DNA which hybridized to multiple island probes, including enteropathogenic E. coli B170 (nine of nine probes) and enterohemorrhagic E. coli EDL931 (eight of nine probes). Laboratory strains and normal intestinal isolates of E. coli did not hybridize with the majority of the probes tested.
tia locus shares structural features with pathogenicity islands.
In addition to the presence of DR elements and insertion in a tRNA gene, the tia locus has additional features in common with previously described PAIs (26). Analysis of the sequence immediately upstream from the tia gene revealed the presence of a candidate ORF encoding a putative protein of 77 amino acids (aa). Comparison of this ORF translation with known sequences in GenBank demonstrated that it contains a high degree of homology to the last 54 aa of the E. coli prophage P4 integrase (70% identity, 79% similarity) (8), suggesting that this ORF represents a nonfunctional remnant of an integrase gene. Downstream from tia, the first candidate ORF inside the island encodes a putative peptide of 397 aa that is nearly identical to the 393-aa CP4-like integrase found at the end of the EDL933 locus of enterocyte effacement (52). Also situated at this end of the island in close proximity to the CP4-like integrase are two ORFs which encode predicted peptides with 96 and 99% identity to hypothetical proteins of 34.4 and 13 kDa from IS2, respectively. This follows a pattern similar to that seen in EDL933, in which ORFs L0004 and L0006 adjacent to the CP4-like integrase appear to be part of an insertion sequence element (52). Finally, the G+C content of this large insertion is approximately 43.7% (based on 37 kb of sequence data), versus 51% for the surrounding E. coli chromosome, supporting horizontal transfer of the island into the genome of H10407.
Maximal secretion of LT from ETEC H10407 requires a gene encoded on the pathogenicity island.
The region downstream from the tia gene was sequenced in its entirety in both directions. Analysis of this region demonstrated the presence of at least four candidate ORFs in the same transcriptional orientation as tia (Fig. 2B). One of these, orf4, encodes a putative protein of 578 aa with considerable homology to the putative 573-aa protein encoded by the Helicobacter pylori gene designated HP0731 (64). While no function has been assigned to HP0731, the deduced amino acid sequence of orf4 contains several motifs shared by proteins from bacterial secretion apparatuses (Table 2). These include an ATP/GTP binding site, [AG]-x(4)-G-K-[ST] (65), and a second motif in common with the HlyD hemolysin translocator protein (60). Finally, this putative protein contains a third element found in some type II secretion proteins, including EpsE, a component of the general secretion pathway required for export of CT through the outer membrane of Vibrio cholerae (58).
TABLE 2.
Analysis of candidate ORFs downstream from tia
| H10407 protein | Closest homologue | Organism | BLASTPc | % of aa
|
Motif(s) | Location (putative) | |
|---|---|---|---|---|---|---|---|
| Identical | Similar | ||||||
| Orf2 | HP0733a | H. pylori | 3 × 10−10 | 38 | 56 | ATP/GTP binding | Inner membrane |
| Orf3 | HP0733b | H. pylori | 5 × 10−12 | 24 | 39 | SecA, FHIPEP | Inner membrane |
| Orf4 (leoA) | HP0731 | H. pylori | 1 × 10−68 | 30 | 52 | ATP/GTP binding, HlyD, type II secretion | Cytosol |
| Orf5 | YHBX | E. coli | 0 | 61 | 74 | Ion channel/porin/flagellar transport (FliP family) | Integral membrane |
aa 4 to 91.
aa 128 to 433.
Probability values obtained in BLAST alignment of predicted amino acid sequence and closest homologue.
Although the candidate protein encoded by orf4 has no overt homology to those involved in the secretion of CT, most proteins containing ATP-binding cassette (ABC) motifs are involved in transport of substances across the bacterial membrane (42). Thus, we chose to begin to assess the contribution of this putative PAI to the virulence of ETEC H10407 by focusing on orf4.
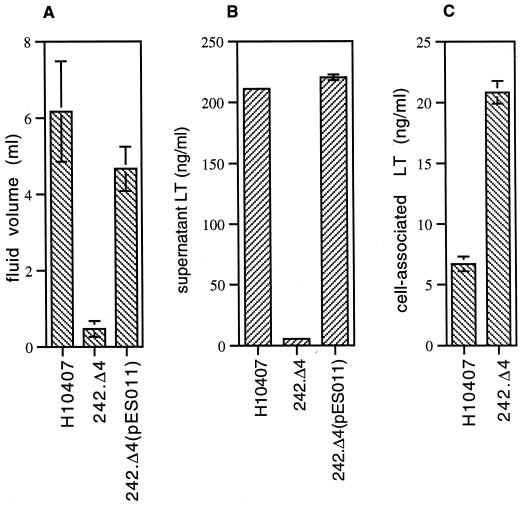
An in-frame deletion in orf4 was constructed. Testing of the resultant mutant strain, 242.Δ4, in the rabbit ileal loop model revealed that it caused little or no fluid accumulation (0.47 ± 0.21 ml) in comparison to the parent (6.17 ± 1.31 ml) (P = 0.0001) (Fig. 5). Complementation of 242.Δ4 with the gene in trans on the expression plasmid pES011 restored virulence in this model to nearly wild-type levels (4.67 ± 0.58 ml) (P = 0.21).
FIG. 5.
(A) Rabbit ileal loop results. A total of four loops (for each strain) were examined in two different rabbit experiments. (B) LT in supernatants after overnight growth at 37°C in CYE-G medium. (C) Cell-associated LT recovered from pellets after growth to mid-log phase in CYE-G. Error bars indicate standard deviations.
Previous investigators observed that LT+ clinical ETEC strains exhibit marked strain-to-strain variation in enterotoxin activity as assessed in vitro using measurements of cyclic AMP and in vivo in the rabbit ileal loop model (15). These findings could at least in part account for the variation observed in the severity of ETEC-associated diarrhea. Because of the effects of the orf4 deletion in the rabbit ileal loop model, we questioned whether orf4 might play a role in export of LT. ETEC H10407 secreted a significant amount of LT into culture supernatants (211.28 ± 0.52 ng/ml), while the mutant strain 242.Δ4 did not (5.64 ± 0.64 ng/ml) (P = 0.0025) (Fig. 5B). Finally, the amount of toxin secreted by the complemented strain 242.Δ4(pES011) (220.4 ± 2.4 ng/ml) was not significantly different from the amount released by the parent strain (P = 0.14). Together, these data suggest that toxin release is dependent on orf4. Conversely, in mid-log-phase cultures, the amount of LT retained in the periplasmic space of the 242.Δ4 mutant strain was significantly greater (20.85 ± 0.93) than that of the wild type (6.71 ± 0.61 ng/ml) (P = 0.04), indicating that interruption of orf4 results in defective export of LT rather than a decrease in its synthesis. The amount of LT retained in the periplasm of the complemented mutant 242.Δ4(pES011) was not appreciably different from the amount in the parent strain. Orf4 was subsequently designated leoA, for labile enterotoxin output A. Two additional ETEC strains were also evaluated for LT secretion and the presence of the PAI. The LT+ clinical ETEC strain EDL903 secreted very little LT into the supernatant and retained the majority of the enterotoxin in the periplasmic space. Interestingly, this strain did not contain the PAI (Fig. 3C and D). Strain DS7-3, which contained the entire PAI in selC, secreted LT at a level comparable to that in H10407 (data not shown).
To exclude the possibilities that release of LT from the periplasm of H10407 is actually the result of autolysis and that the leoA mutant is deficient in nonspecific release of proteins from the periplasm, we analyzed the periplasmic extracts and culture supernatants of both strains using an established assay for alkaline phosphatase (45). We found no appreciable difference in the amounts of alkaline phosphatase activity in periplasmic fractions from the parent and mutant. We were not able to detect enzyme activity in supernatants from either strain. Furthermore, SDS-PAGE analysis of whole-cell lysates, periplasmic extracts, and trichloroacetic acid-precipitated proteins in the supernatants of the wild type and mutant failed to show any difference between the two strains.
Identification of a putative secretion apparatus downstream from tia.
The majority of ABC domains are found in the context of multicomponent membrane transport systems (42). Analysis of the deduced translation products of the other candidate genes downstream from tia revealed multiple motifs commonly associated with bacterial secretion apparatuses (Table 2). Following tia are two additional closely spaced candidate ORFs that share homology with a candidate gene of unknown function in the genome of H. pylori strain 26695 immediately adjacent to HP0731 (64). Translation of the first ORF downstream from tia (orf2) yields a predicted protein of 220 aa that shares homology with 87 aa of the amino terminus of the putative 521-aa protein encoded by HP0733. It also contains a potential ATP/GTP binding motif, A [AG]-x(4)-G-K-[ST] (65), and shares limited homology with a number of ABC membrane transporter proteins. The 571-aa putative protein encoded by orf3 also has HP0733 as its closest homologue, but the region of homology differs, extending from aa 128 to aa 433 of HP0733. This protein shares limited homology with a number of ATPases and at its amino terminus possesses a SecA motif characteristic of proteins that couple the hydrolysis of ATP to the transfer of proteins across the membrane (40). Interestingly, the carboxy terminus of this same protein possesses a putative FHIPEP (flagella/hypersensitive response/invasion proteins/export pore) motif common to a number of molecules such as InvA from Salmonella serovar Typhimurium involved in the transfer of virulence factors across the bacterial cell membrane (24). The protein encoded by the last ORF, orf5, shares a high degree of homology with YHBX, a putative transmembrane protein of unknown function in E. coli K-12 (5). Searches of this protein yielded potential ion channel and flagellar transport (FliP family) motifs.
DISCUSSION
ETEC is associated with diseases that vary in severity, ranging from mild illness to severe cholera-like presentations characterized by profuse watery diarrhea (21, 56). These differences are not easily accounted for by our current understanding of the pathogenesis of ETEC infections. While Echeverria et al. had previously suggested that variable amounts of LT produced by clinical ETEC strains could at least in part account for differences in the severity of ETEC-associated diarrhea (15), the mechanism(s) underlying this variation was unclear.
As with other bacterial pathogens, the ultimate outcome of infection may relate to the presence or absence of genes encoded on pathogenicity islands. Previous studies with H. pylori have suggested that variations in the cag pathogenicity island contribute to differences in clinical presentations of this disease that range from asymptomatic infection to peptic ulcer disease (9, 61). Moreover, Conner et al. have suggested that maintenance of an “archipelago” of pathogenicity islands is required for full virulence of Salmonella serovar Typhimurium (11). Interestingly, one of these islands bears iviVI-A (28), which encodes a close homologue to the product of the tia gene located within the ETEC island described here. Our experiments also suggest that manifestation of the cholera-like disease initially associated with ETEC H10407 (19, 20) requires the complement of genes encoded on the PAI described herein.
Previous studies have suggested that LT remains largely in the periplasm of E. coli (31, 32). However, in experiments performed by Hirst et al., laboratory strains of E. coli were used as hosts for recombinant plasmids bearing the LT genes, and they concluded that LT was not necessarily confined to the periplasm of wild-type ETEC (32). Other investigators have demonstrated that significant amounts of LT are in fact released from wild-type ETEC strains when grown under the appropriate conditions (38). Our studies suggest that export of the LT occurs with at least some strains of ETEC and that this process is dependent on factors not found in E. coli K-12 and other laboratory derivatives.
Although the precise role of LeoA in the release of LT from the periplasm of H10407 awaits further study, we found multiple motifs associated with bacterial secretion clustered among the proteins encoded by leoA and the neighboring genes. This raised the possibility that these ORFs encode orthologous elements in H10407. This region contains two putative ABC motifs, most of which are found in proteins involved in transport of substances across the bacterial membrane (42). The presence of multidomain proteins, presumably the result of gene fusion, is a major feature of the ABC transporters (42). This could explain the appearance of multiple potential motifs often found in individual proteins of other secretion systems within LeoA. Although these data support a role for the involvement of leoA in the secretion of LT from H10407, this region of the PAI does not correspond to any of the bacterial secretion systems (types I to IV) that have been described to date. Secretion across the outer membrane of H10407, and of gram-negative pathogens in general, could ultimately prove to be more complex than has been appreciated.
The putative products of the leoA gene and candidate ORFs 2 and 3 each share homology with predicted peptides encoded by a region of the H. pylori genome to which no function has been assigned (64). Therefore, further study of H10407 may provide additional insight into the pathogenesis of ETEC as well as H. pylori.
The role of tia in the pathogenesis of these organisms has not yet been determined. However, close homologues of Tia have now been described in two pathogenicity islands, PAI V from the uropathogenic E. coli strain J96 (63), as well as a putative PAI in Salmonella serovar Typhimurium and other pathogenic Salmonella serovars, including Typhi (11, 28, 29, 44). Another tia homologue was cloned as a hemagglutinin from a porcine ETEC strain by Lutwyche et al. (43). In experiments not presented here, genomic DNA from this same strain hybridized to all of the probes from the H10407 island, suggesting that some human and porcine strains bear closely related islands.
The present evidence suggests that this island is largely distinct from other previously described PAIs in E. coli, including PAI 1 (37) and the locus of enterocyte effacement (16), both located in the selC genes of uropathogenic E. coli 536 and enteropathogenic E. coli, respectively. Hacker et al. noted that PAI 1 is unstable and is spontaneously lost at a frequency of approximately 10−3 to 10−4 due to the presence of DR elements flanking the island (37). While identical DRs flank the H10407 island, its stability in the chromosome of this strain has not yet been directly assessed. However, the marked heterogeneity seen in hybridization patterns among different ETEC strains raises the possibility that, like many PAIs, this island may undergo deletions or other rearrangements following integration (27). The presence of a Tia homologue in close proximity to a P4 integrase in PAI V described by Swenson et al. (63) raised the possibility that PAI V and the H10407 island are similar. However, we were unable to amplify cnf-1, a gene contained on PAI V, from either H10407 genomic DNA or the tia cosmids, and sequencing to date has not revealed the presence of a cnf-1 homologue.
While the PAI bearing tia is the first pathogenicity island to be described in an ETEC strain, the heterogeneity of these pathogens in general and the presence of multiple PAIs in uropathogenic E. coli (26) may herald the finding of additional PAIs among these organisms. Indeed, another putative adhesin, referred to as Tib (18), appears to be encoded on a large chromosomal element of low G+C content in the H10407 genome distinct from the tia locus. Similar to that of Salmonella serovar Typhimurium (11), the overall fitness of an individual ETEC strain as a pathogen is likely a cumulative reflection of the compilation of virulence genes maintained in its arsenal.
ACKNOWLEDGMENTS
This work was supported in part by grants from the Walter Reed Army Institute of Research In-House Laboratory Independent Research fund (J.M.F.) and the Department of Veterans Affairs (to J.B.D. and J.M.F.) and institutional funds from the University of Tennessee, Research, Inc., and the University of Kansas General Research Fund.
We thank T. Mandrell and M. Randolf for help with the animal model, J. T. Holland and K. Park for technical assistance, and J. Hacker, D. Hasty, M. Donnenberg, and G. Pósfai for helpful suggestions and discussions.
REFERENCES
- 1.Altschul S, Madden T, Schaffer A, Zhang J, Miller W, Lipman D. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banwell J, Gorbach S, Pierce N, Mitra R, Mondal A. Acute undifferentiated human diarrhea in the tropics. II. Alterations in intestinal fluid and electrolyte movements. J Clin Investig. 1971;50:890–900. doi: 10.1172/JCI106561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black R. Epidemiology of diarrhoeal disease: implications for control by vaccines. Vaccine. 1993;11:100–106. doi: 10.1016/0264-410x(93)90002-f. [DOI] [PubMed] [Google Scholar]
- 4.Blanc-Potard A, Groisman E. The Salmonella selC locus contains a pathogenicity island mediating intramacrophage survival. EMBO J. 1997;16:5376–5385. doi: 10.1093/emboj/16.17.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blattner F R, Plunkett III G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 6.Blum G, Ott M, Lischewski A, Ritter A, Imrich H, Tschäpe H, Hacker J. Excision of large DNA regions termed pathogenicity islands form tRNA-specific loci in the chromosome of an Escherichia coli wild-type pathogen. Infect Immun. 1994;62:606–614. doi: 10.1128/iai.62.2.606-614.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourgeois A, Gardiner C, Thornton S, Batchelor R, Burr D, Escamilla J, Echeverria P, Blacklow N, Herrmann J, Hyams K. Etiology of acute diarrhea among United States military personnel deployed to South America and West Africa. Am J Trop Med Hyg. 1993;48:243–248. doi: 10.4269/ajtmh.1993.48.243. [DOI] [PubMed] [Google Scholar]
- 8.Burland V, Plunkett G, 3rd, Sofia H J, Daniels D L, Blattner F R. Analysis of the Escherichia coli genome. VI. DNA sequence of the region from 92.8 through 100 minutes. Nucleic Acids Res. 1995;23:2105–2119. doi: 10.1093/nar/23.12.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Censini S, Lange C, Xiang Z, Crabtree J, Ghiara P, Bordovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type 1-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clements J. Construction of a nontoxic fusion peptide for immunization against Escherichia coli strains that produce heat-labile and heat-stable enterotoxins. Infect Immun. 1990;58:1159–1166. doi: 10.1128/iai.58.5.1159-1166.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conner C, Heithoff D, Julio S, Sinsheimer R, Mahan M. Differential patterns of acquired virulence genes distinguish Salmonella strains. Proc Natl Acad Sci USA. 1998;95:4641–4645. doi: 10.1073/pnas.95.8.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De S, Chatterje D. An experimental study of the mechanism of action of Vibrio cholerae on the intestinal mucous membrane. J Pathol Bacteriol. 1953;66:559–562. doi: 10.1002/path.1700660228. [DOI] [PubMed] [Google Scholar]
- 13.Donnenberg M, Kaper J. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donnenberg M, Yu J, Kaper J B. A second chromosomal gene necessary for intimate attachment of enteropathogenic Escherichia coli to epithelial cells. J Bacteriol. 1993;175:4670–4680. doi: 10.1128/jb.175.15.4670-4680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Echeverria P, Louria C J, Smith A L, Smith D. Variation in enterotoxigenicity of Escherichia coli. J Infect Dis. 1977;135:195–200. doi: 10.1093/infdis/135.2.195. [DOI] [PubMed] [Google Scholar]
- 16.Elliott S, Wainwright L, McDaniel T, Jarvis K, Deng Y, Lai L, McNamara B, Donnenberg M, Kaper J. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic E. coli E2348/69. Mol Microbiol. 1998;28:1–4. doi: 10.1046/j.1365-2958.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- 17.Elsinghorst E A, Kopecko D J. Molecular cloning of epithelial cell invasion determinants from enterotoxigenic Escherichia coli. Infect Immun. 1992;60:2409–2417. doi: 10.1128/iai.60.6.2409-2417.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elsinghorst E A, Weitz J A. Epithelial cell invasion and adherence directed by the enterotoxigenic Escherichia coli tib locus is associated with a 104-kilodalton outer membrane protein. Infect Immun. 1994;62:3463–3471. doi: 10.1128/iai.62.8.3463-3471.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans D G, Evans D, Jr, DuPont H L. Virulence factors of enterotoxigenic Escherichia coli. J Infect Dis. 1977;136:S118–S123. doi: 10.1093/infdis/136.supplement.s118. [DOI] [PubMed] [Google Scholar]
- 20.Evans D G, Silver R P, Evans D J, Jr, Chase D G, Gorbach S L. Plasmid-controlled colonization factor associated with virulence in Escherichia coli enterotoxigenic for humans. Infect Immun. 1975;12:656–667. doi: 10.1128/iai.12.3.656-667.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finkelstein R, Vasil M, Jones J, Anderson R, Barnard T. Clinical cholera caused by enterotoxigenic Escherichia coli. J Clin Microbiol. 1976;3:382–384. doi: 10.1128/jcm.3.3.382-384.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fleckenstein J, Kopecko D, Warren R, Elsinghorst E. Molecular characterization of the tia invasion locus from enterotoxigenic Escherichia coli. Infect Immun. 1996;64:2256–2265. doi: 10.1128/iai.64.6.2256-2265.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Francetic O, Pugsley A P. The cryptic general secretory pathway (gsp) operon of Escherichia coli K-12 encodes functional proteins. J Bacteriol. 1996;178:3544–3549. doi: 10.1128/jb.178.12.3544-3549.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galan J E, Ginocchio C, Costeas P. Molecular and functional characterization of the Salmonella invasion gene invA: homology of InvA to members of a new protein family. J Bacteriol. 1992;174:4338–4349. doi: 10.1128/jb.174.13.4338-4349.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorbach S, Banwell J, Chatterjee B, Jacobs B, Sack R. Acute undifferentiated human diarrhea in the tropics. I. Alterations in the intestinal microflora. J Clin Investig. 1971;50:881–889. doi: 10.1172/JCI106560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hacker J, Blum-Oehler G, Mühldorfer I, Tschäpe H. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol Microbiol. 1997;23:1089–1097. doi: 10.1046/j.1365-2958.1997.3101672.x. [DOI] [PubMed] [Google Scholar]
- 27.Hacker J, Kaper J B. The concept of pathogenicity islands. In: Kaper J B, Hacker J, editors. Pathogenicity islands and other mobile virulence elements. Vol. 1. Washington, D.C.: ASM Press; 1999. pp. 1–11. [Google Scholar]
- 28.Heithoff D M, Conner C P, Hanna P C, Julio S M, Hentschel U, Mahan M J. Bacterial infection as assessed by in vivo gene expression. Proc Natl Acad Sci USA. 1997;94:934–939. doi: 10.1073/pnas.94.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heithoff D M, Conner C P, Hentschel U, Govantes F, Hanna P C, Mahan M J. Coordinate intracellular expression of Salmonella genes induced during infection. J Bacteriol. 1999;181:799–807. doi: 10.1128/jb.181.3.799-807.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirst T, Holmgren J. Conformation of protein secreted across bacterial outer membranes: a study of enterotoxin translocation from Vibrio cholerae. Proc Natl Acad Sci USA. 1987;84:7418–7422. doi: 10.1073/pnas.84.21.7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirst T, Sanchez J, Kaper J, Hardy S, Holmgren J. Mechanism of toxin secretion by Vibrio cholerae investigated in strains harboring plasmids that encode heat-labile enterotoxins of Escherichia coli. Proc Natl Acad Sci USA. 1984;81:7752–7756. doi: 10.1073/pnas.81.24.7752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirst T R, Randall L L, Hardy S J. Cellular location of heat-labile enterotoxin in Escherichia coli. J Bacteriol. 1984;157:637–642. doi: 10.1128/jb.157.2.637-642.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holmgren J, Svennerholm A. Development of oral vaccines against cholera and enterotoxigenic Escherichia coli diarrhea. Scand J Infect Dis. 1990;S76:47–53. [PubMed] [Google Scholar]
- 34.Hyams K, Bourgeois A, Merrell B, Rozmajzl P, et al. Diarrheal disease during Operation Desert Shield. N Engl J Med. 1991;325:1423–1428. doi: 10.1056/NEJM199111143252006. [DOI] [PubMed] [Google Scholar]
- 35.Institute of Medicine. New vaccine development: establishing priorities, vol. II: diseases of importance in developing countries. Washington, D.C.: National Academy Press; 1986. [PubMed] [Google Scholar]
- 36.Kaper J B, Levine M M. Progress towards a vaccine against enterotoxigenic Escherichia coli. Vaccine. 1988;6:197–199. doi: 10.1016/s0264-410x(88)80028-8. [DOI] [PubMed] [Google Scholar]
- 37.Knapp S, Hacker J, Jarchau T, Goebel W. Large, unstable inserts in the chromosome affect virulence properties of uropathogenic Escherichia coli O6 strain 536. J Bacteriol. 1986;168:22–30. doi: 10.1128/jb.168.1.22-30.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kunkel S, Robertson D. Factors affecting release of heat-labile enterotoxin by enterotoxigenic Escherichia coli. Infect Immun. 1979;23:652–659. doi: 10.1128/iai.23.3.652-659.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laemmli U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 40.Lill R, Cunningham K, Brundage L, Ito K, Oliver D, Wicker W. SecA protein hydrolyzes ATP and is an essential component of the protein translocation ATPase of Escherichia coli. EMBO J. 1989;8:961–966. doi: 10.1002/j.1460-2075.1989.tb03458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Link A J, Phillips D, Church G M. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J Bacteriol. 1997;179:6228–6237. doi: 10.1128/jb.179.20.6228-6237.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Linton K J, Higgins C F. The Escherichia coli ATP-binding cassette (ABC) proteins. Mol Microbiol. 1998;28:5–13. doi: 10.1046/j.1365-2958.1998.00764.x. [DOI] [PubMed] [Google Scholar]
- 43.Lutwyche P, Rupps R, Cavanagh J, Warren R A, Brooks D E. Cloning, sequencing, and viscometric adhesion analysis of heat-resistant agglutinin 1, an integral membrane hemagglutinin from Escherichia coli O9:H10:K99. Infect Immun. 1994;62:5020–5026. doi: 10.1128/iai.62.11.5020-5026.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahan M J, Slauch J M, Mekalanos J J. Selection of bacterial virulence genes that are specifically induced in host tissues. Science. 1993;259:686–688. doi: 10.1126/science.8430319. [DOI] [PubMed] [Google Scholar]
- 45.Maloy S R, Stewart V J, Taylor R K. Enzyme assays: alkaline phosphate assay in permeabilized cells. In: Maloy S R, Stewart V J, Taylor R K, editors. Genetic analysis of pathogenic bacteria: a laboratory manual. Vol. 1. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 496–498. [Google Scholar]
- 46.McDaniel T K, Jarvis K, Donnenberg M S, Kaper J B. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci USA. 1995;92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neuhoff V, Arold N, Taube D, Ehrhardt W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis. 1988;9:255–262. doi: 10.1002/elps.1150090603. [DOI] [PubMed] [Google Scholar]
- 48.Nevill-Manning C, Wu T, Brutlag D. Highly specific protein sequence motifs for genome analysis. Proc Natl Acad Sci USA. 1998;95:5865–5871. doi: 10.1073/pnas.95.11.5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishibuchi M, Fasano A, Russell R, Kaper J. Enterotoxigenicity of Vibrio parahaemolyticus with and without genes encoding thermostable direct hemolysin. Infect Immun. 1992;60:3539–3545. doi: 10.1128/iai.60.9.3539-3545.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Overbye L J, Sandkvist M, Bagdasarian M. Genes required for extracellular secretion of enterotoxin are clustered in Vibrio cholerae. Gene. 1993;132:101–106. doi: 10.1016/0378-1119(93)90520-d. [DOI] [PubMed] [Google Scholar]
- 51.Pearson D, Mekalanos J. Molecular cloning of Vibrio cholerae enterotoxin genes in Escherichia coli K-12. Proc Natl Acad Sci USA. 1982;79:2976–2980. doi: 10.1073/pnas.79.9.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perna N T, Mayhew G F, Posfai G, Elliott S, Donnenberg M S, Kaper J B, Blattner F R. Molecular evolution of a pathogenicity island from enterohemorrhagic Escherichia coli O157:H7. Infect Immun. 1998;66:3810–3817. doi: 10.1128/iai.66.8.3810-3817.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Posfai G, Koob M D, Kirkpatrick H A, Blattner F R. Versatile insertion plasmids for targeted genome manipulations in bacteria: isolation, deletion, and rescue of the pathogenicity island LEE of the Escherichia coli O157:H7 genome. J Bacteriol. 1997;179:4426–4428. doi: 10.1128/jb.179.13.4426-4428.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sack D, Huda S, Neogi P, Daniel R, Spira W. Microtiter ganglioside enzyme-linked immunosorbent assay for Vibrio and Escherichia coli heat-labile enterotoxins and antitoxin. J Clin Microbiol. 1980;11:35–40. doi: 10.1128/jcm.11.1.35-40.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sack D A, McLaughlin J C, Sack R B, Orskov F, Orskov I. Enterotoxigenic Escherichia coli isolated from patients at a hospital in Dacca. J Infect Dis. 1977;135:275–280. doi: 10.1093/infdis/135.2.275. [DOI] [PubMed] [Google Scholar]
- 56.Sack R, Gorbach S, Banwell J, Jacobs B, Chatterjee B, Mitra R. Enterotoxigenic Escherichia coli isolated from patients with severe cholera-like disease. J Infect Dis. 1971;123:378–385. doi: 10.1093/infdis/123.4.378. [DOI] [PubMed] [Google Scholar]
- 57.Sandkvist M, Michel L O, Hough L P, Morales V M, Bagdasarian M, Koomey M, DiRita V J. General secretion pathway (eps) genes required for toxin secretion and outer membrane biogenesis in Vibrio cholerae. J Bacteriol. 1997;179:6994–7003. doi: 10.1128/jb.179.22.6994-7003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sandkvist M, Morales V, Bagdasarian M. A protein required for secretion of cholera toxin through the outer membrane of Vibrio cholerae. Gene. 1993;123:81–86. doi: 10.1016/0378-1119(93)90543-c. [DOI] [PubMed] [Google Scholar]
- 59.Schlager T, Guerrant R. Seven possible mechanisms for Escherichia coli diarrhea. Infect Dis Clin N Am. 1988;2:607–624. [PubMed] [Google Scholar]
- 60.Schulein R, Gentschev I, Mollenkopf H, Goebel W. A topological model for the haemolysin translocator protein HlyD. Mol Gen Genet. 1992;234:155–163. doi: 10.1007/BF00272357. [DOI] [PubMed] [Google Scholar]
- 61.Segal E, Lange C, Covacci A, Tompkins L, Falkow S. Induction of host signal transduction pathways by Helicobacter pylori. Proc Natl Acad Sci USA. 1997;94:7595–7599. doi: 10.1073/pnas.94.14.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spangler B D. Structure and function of the cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev. 1992;56:622–647. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Swenson D L, Bukanov N, Berg D, Welch R. Two pathogenicity islands in uropathogenic Escherichia coli J96: cosmid cloning and sample sequencing. Infect Immun. 1996;64:3736–3743. doi: 10.1128/iai.64.9.3736-3743.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzegerald L M, Lee N, Adams M D, Venter J C, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 65.Walker J E, Saraste M, Runswick M J, Gay N J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wandersman C. Secretion across the bacterial outer membrane. In: Neidhardt F C, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: American Society for Microbiology; 1996. pp. 955–966. [Google Scholar]
- 67.World Health Organization. The work of the WHO 1992–1993. Biennial report to the Director-General to the World Health Assembly and to the United Nations. Geneva, Switzerland: World Health Organization; 1994. pp. 14–72. [Google Scholar]