Abstract
Among the many wonders of nature, the sense of smell of the fly Drosophila melanogaster might seem, at first glance, of esoteric interest. Nevertheless, for over a century, the ‘nose’ of this insect has been an extraordinary system to explore questions in animal behaviour, ecology and evolution, neuroscience, physiology and molecular genetics. The insights gained are relevant for our understanding of the sensory biology of vertebrates, including humans, and other insect species, encompassing those detrimental to human health. Here, I present an overview of our current knowledge of D. melanogaster olfaction, from molecules to behaviours, with an emphasis on the historical motivations of studies and illustration of how technical innovations have enabled advances. I also highlight some of the pressing and long-term questions.
Keywords: Drosophila melanogaster, olfaction, olfactory receptor, neural circuit, neurophysiology, animal behaviour
1. Introduction
Since they find their food with great certainty even in the dark, a habit that seemed to involve the sense of smell, I was led to take up an investigation of their reactions to odorous substances.
Barrows, 1907 [1]
Originating in the work of William Barrows over a century ago [1], how Drosophila melanogaster detects and responds to odours has intrigued an ever-increasing number of researchers. Many early studies focused on analysing olfactory contributions to mating rituals [2] and the formation of olfactory memories [3], two of the three behaviours subject to pioneering neurogenetic dissection by Seymour Benzer and collaborators (as compellingly described in his biography Time, love, memory [4]). Molecular and cellular dissection of the structure and function of the D. melanogaster olfactory system progressed only modestly in the last decades of the twentieth century [5,6], especially by comparison with advances in our understanding of visual system development and photoreception [7,8]. However, work in other insect species—dating back to the 1870s when Jean-Henri Fabre ‘discovered’ chemical communication in moths [9]—yielded many insights into how animals represent odours in the brain to evoke behaviour. Several principles defined in these species were subsequently confirmed, refined and extended in D. melanogaster and vertebrates [10].
A watershed in insect olfactory research occurred in the late 1990s with the identification of D. melanogaster genes encoding olfactory receptors [11–13]. Similar to the impact of the earlier discovery of mammalian olfactory receptor genes [14], this breakthrough provided the foundation for investigating how odours are detected and olfactory circuit organization and function. Drosophila melanogaster olfaction now represents a model for sensory coding that is relevant for understanding similar processes in vertebrate brains but also continues to inform (and be informed by) studies in other insect species. Here I highlight—admittedly superficially and subjectively—some of the historical work and current knowledge of the D. melanogaster olfactory system, as well as open questions. I do not discuss in any detail the vast body of research on the olfactory systems of other species [15] (and little on the D. melanogaster larval olfactory system [16,17]), nor how olfactory signals integrate with other sensory information [18] or are represented in memories [19]. Rather, the goal is to present a brisk, but holistic, tour of how studying the fly's nose advances biological knowledge.
2. Receptors
Olfactory receptors convert chemical signals in the environment into electrical signals in the nose. In insects, there are two main families of olfactory receptors: odorant receptors (Ors) [11–13] and the more recently discovered ionotropic receptors (Irs) [20]. Early work revealed several similarities to the peripheral olfactory system of mammals: receptor families are generally large (encoded by dozens to thousands of genes per species) and have high sequence divergence, reflecting their role in detecting a vast number of different chemicals [21]. Moreover, in both insects and vertebrates, the majority of olfactory sensory neurons (OSNs) in the nose—the antenna and maxillary palp in insects (figure 1)—express just one receptor [24,25], which is the principal determinant of the sensitivity and breadth of odour recognition [26]. As described in the ‘Circuitry’ and ‘Function’ sections, knowledge of the receptors was also key to characterize structural and physiological properties of the peripheral olfactory system of D. melanogaster, which further illuminated parallels with mammals.
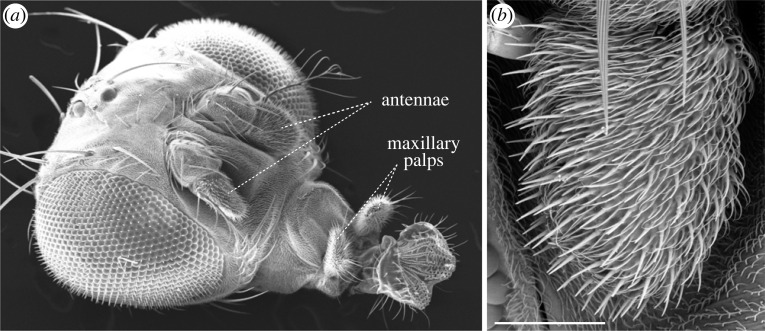
Figure 1.
Olfactory organs. (a) Scanning electron micrograph (SEM) of the head of adult D. melanogaster, showing the two bilaterally symmetric olfactory organs. Adapted from [22] (copyright © Cold Spring Harbor Laboratory Press). (b) SEM of a D. melanogaster antenna, illustrating the dense array of morphologically diverse sensilla (which house olfactory sensory neuron dendrites) covering the surface. Scale bar, 50 µm. Adapted from [23].
The similarities extend only so far. Unlike vertebrate olfactory receptors, which belong to the G-protein coupled receptor (GPCR) superfamily [27], insect Ors and Irs function as odour-gated ion channels [28–30]. Both classes of channels are composed of subunits of a uniquely expressed ‘tuning’ receptor (which recognises odours) and one or more broadly expressed, family specific ‘co-receptors’ (e.g. Orco for Ors), which are essential for localization and signalling [30–33]. Ors are seven transmembrane domain proteins, a feature leading to long-held, incorrect assumptions that they were GPCRs. Rather, these proteins define a novel family of ion channels (figure 2), apparently absent in vertebrates, but with distant relatives (of unknown function) across invertebrates as well as in plants and unicellular eukaryotes [41–44]. Irs have evolved in protostomes from ionotropic glutamate receptors (iGluRs), a widely conserved family of ligand-gated ion channels best-known for their roles at synapses in the central nervous system [20,45]. Why insects use ionotropic transduction mechanisms to encode odours—contrasting with the reliance of vertebrates on metabotropic pathways—is unknown [46]. It might, for example, reflect a need for rapid signalling in OSNs in insects, which often encounter and navigate through spatially complex odour plumes in flight. But it might simply be a chance of evolution that different types of ancestral membrane receptors were adopted as the principal mechanisms of olfactory transduction in different lineages.
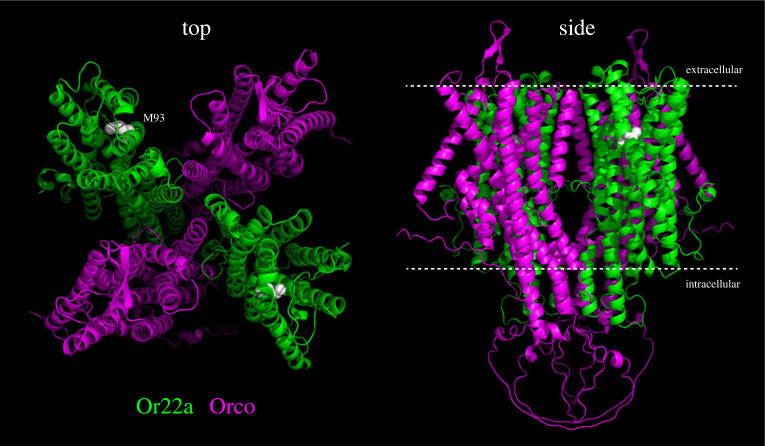
Figure 2.
Olfactory receptors. Model of a hypothetical heterotetrameric complex of D. melanogaster Or22a and the co-receptor Orco (two subunits each). The approximate position of the plasma membrane is indicated in the side view. In Or22a subunits, the residue highlighted in white (M93) is a major contributor to defining behaviourally relevant odour response differences between D. melanogaster and D. sechellia Or22a orthologues [34]; this residue is located within the putative odour-binding site [35]. The ion channel pore is formed at the interface of all four subunits [36]. Models of protein monomers were predicted by AlphaFold2 [37,38]; these exhibit very strong similarity to cryo-electron microscopic (cryoEM) structures of Ors from other insects [35,36]. Models were aligned to the cryoEM structure of the Orco homotetramer from the fig wasp (Apocrypta bakeri) [36] using Coot [39] and visualized in PyMol v. 2.5.4. Although the stoichiometry of Or/Orco complexes is unknown, evidence suggests that they contain at least two tuning Or subunits [32,40].
One outstanding question is how insect olfactory receptors interact with odour ligands and transduce this recognition into electrical signals. For Ors, advances were hampered by lack of similarity to known channels. A recent breakthrough came from the first cryo-electron microscopic structures of insect Ors—and indeed any animal olfactory receptor—that reveal important insights into how these proteins assemble into complexes and the mechanism by which odour binding gates the ion channel pore [35,36]. Of particular note are the first structural explanations for how an olfactory receptor can be activated by chemically diverse ligands [35]. Such structures—as well as remarkably similar de novo models of other receptors (figure 2) [42]—synergize with analysis of natural sequence variation and site-directed mutagenesis [34,35,47–51] to help define the molecular basis of receptor specificity and evolution. Irs may function similarly to their iGluR ancestors [30], and while no Ir structures are available, protein modelling in combination with mutational analyses have started to reveal principles of ligand-recognition and ion conduction of this family [30,52–54]. Beyond Ors and Irs, other types of proteins mediate peripheral sensation of ecologically important odours: CO2 is detected by two highly conserved members of the ‘Gustatory’ receptor (Gr) family (from which Ors evolved) [55,56], and an ammonium transporter is a sensor of ammonia [57].
Drosophila melanogaster has provided a facile experimental system for in vivo deorphanization of olfactory receptors. Odour-evoked activity can be easily measured by extracellular electrophysiological recordings of OSNs housed within porous sensory hairs (sensilla) on the surface of olfactory organs (figure 1) [22]. This ‘single sensillum recording’ method—initially developed in the mid-twentieth century for much larger insects [58,59]—has allowed identification of receptors/OSNs responding to, for example, food odours or pheromones [26,60–66] (figure 3a). Moreover, D. melanogaster mutants lacking specific tuning Ors or Irs have enabled use of the resultant ‘empty’ neurons as powerful in vivo heterologous expression systems for other receptors (including those from other insects) to determine their response profile [26,69–71] (figure 3b).
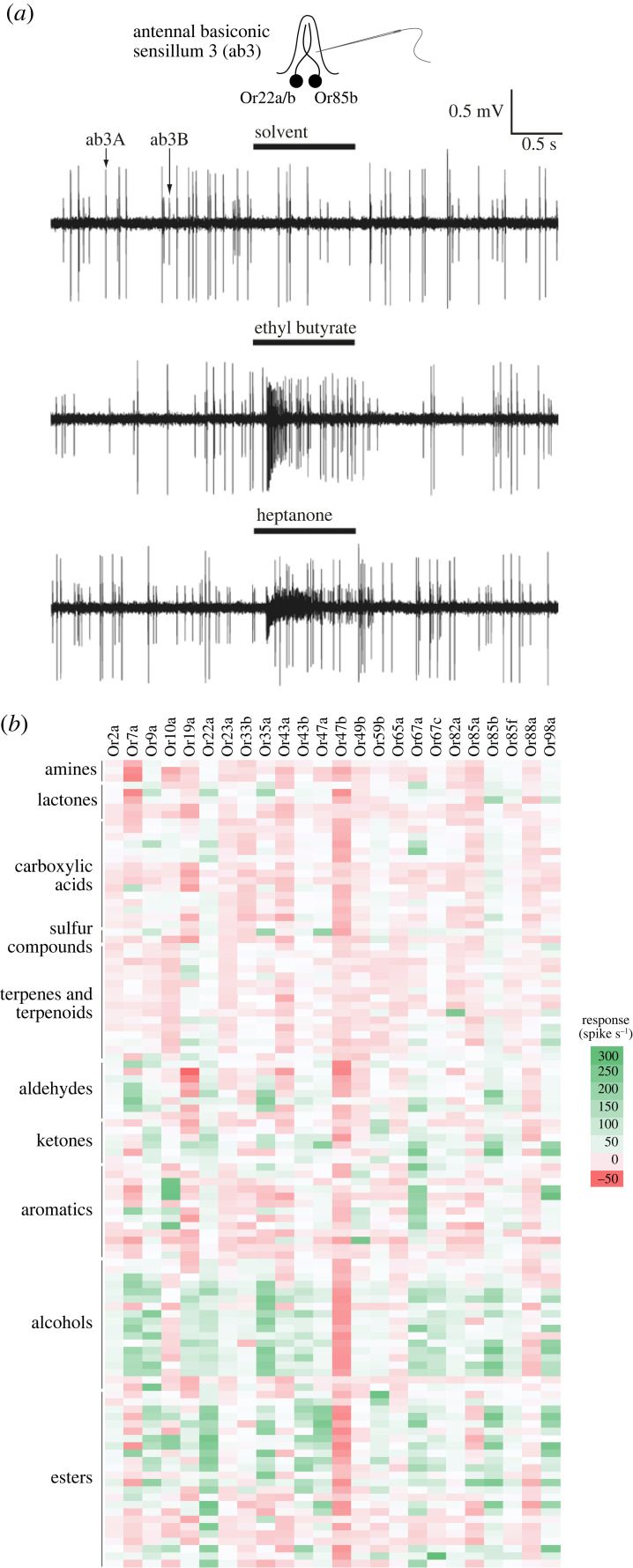
Figure 3.
Olfactory function. (a) Electrophysiological recordings from the antennal basiconic sensillum 3 (ab3), which houses two neurons (ab3A and ab3B) that express Or22a/b and Or85b, respectively. The neurons can be distinguished both by ‘spike’ (action potential) amplitude and their responses to different odours (diluted to 0.001% v/v in the paraffin oil solvent and presented during 1 s). Adapted from [22] (copyright © Cold Spring Harbor Laboratory Press). (b) Combinatorial coding of odours by Ors: the first large-scale profiling of responses of many Ors to a chemically diverse panel of stimuli. Here, Ors were transgenically expressed in the ‘empty’ Or22a/b neuron (lacking the endogenous receptors) to provide a consistent cellular background for comparison of receptor function. Data are replotted from [61]; the scale is shown on the right. Negative responses reflect odours that decrease the basal spiking frequency of neurons. Some receptors for which no strong agonists were identified (e.g. Or47b) were later found to respond to pheromones [67,68].
By contrast, ex vivo functional reconstitution of these receptors—in cultured mammalian or insect cells and frog oocytes—has been challenging: despite some successes [28–30,35,36,72,73], many Ors and Irs fail to exhibit odour-evoked current flow in other cell types. This limitation has constrained the aspirations of performing high-throughput ligand/receptor screening to identify novel (artificial) receptor agonists/antagonists, or perform large-scale, site-directed mutagenesis to define structure–activity relationships. The poor or absent activity of many olfactory receptors may be because such expression systems lack critical properties characteristic of these proteins' in vivo environment. Olfactory receptors localize to specialized sensory cilia of OSN dendrites that are bathed in an ion- and protein-rich lymph fluid within the sensillum [74,75]. Numerous perireceptor proteins (e.g. odorant binding proteins and odorant degrading enzymes) and accessory neuronal proteins (e.g. the CD36-related transporter Snmp1 or the ENaC-related channel Ppk25) contribute in diverse, often receptor-specific, ways to olfactory transduction [52,74,76–79]. Much remains to be discovered in the biochemistry and cell biology of how chemical cues are converted into electrical activity. Given the exceedingly small size of the sensory apparatus, many advances will depend upon technical innovations in cellular ultrastructural analysis [75,80].
3. Circuitry
The identification of olfactory receptor genes was also instrumental for neuroanatomical analysis of the D. melanogaster olfactory system. Receptor gene promoters form the basis of transgenic ‘drivers’ to visualize the innervations of the corresponding OSNs in the brain. A key principle is that OSNs expressing the same receptor project their axons to a common, discrete region of neuropil (a glomerulus) within the primary olfactory centre, the antennal lobe (figure 4a) [88,89]. This wiring pattern—originally observed in moths through dye back-filling of pheromone-sensing neurons [90]—is also a hallmark of the analogous olfactory bulb in vertebrates [91,92]. However, as OSN development in insects (see below) and in mammals [92] is very different, it is likely that glomerular segregation of OSNs is an evolutionary convergent, rather than conserved, property [15,93]. Drosophila melanogaster has excelled in olfactory circuit analysis because the combination of genetic tools and numerical simplicity (∼50 OSN classes) has enabled generation of an essentially complete receptor-to-glomerulus map [57,65,94–96]. This information synergizes powerfully with comprehensive knowledge of the odour-specificity of individual sensory channels (see ‘Function’ section) [26,61–66].
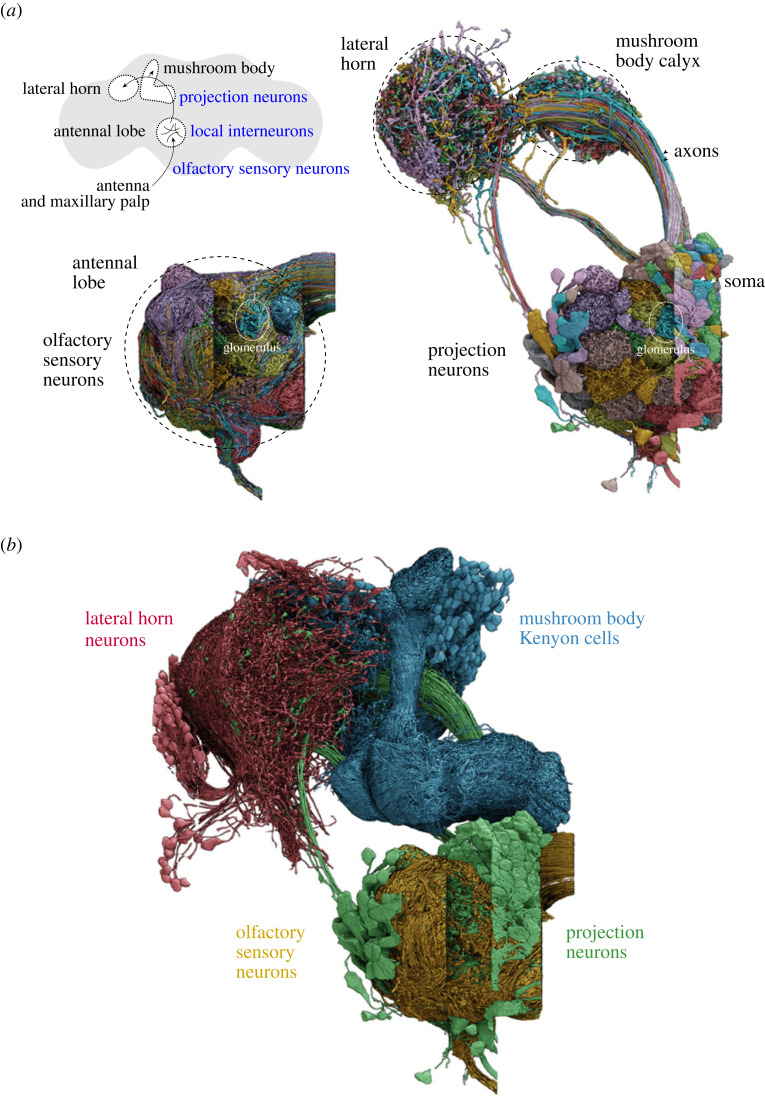
Figure 4.
Olfactory circuits. (a) Top left: schematic of the principal paths of olfactory information flow in the D. melanogaster brain (outlined in grey), indicating the main neuron classes and brain regions. The circuitry is bilaterally symmetric (and most olfactory sensory neurons (OSNs) project to both antennal lobes) but only one hemibrain pathway is illustrated. Below are electron microscopic-resolution connectomic reconstructions of OSNs (including some antennal hygrosensory and thermosensory neurons [81–86]; contralateral innervations are cut off on the right side) and uniglomerular projection neurons (PNs) in the antennal lobe. Partner OSNs and PNs, converging on a common glomerulus, are colour-matched; one such glomerulus is highlighted in both images. For this PN class, a subset of the soma (located outside the lobe) are indicated with white arrowheads; black arrowheads point to the axons that project to the higher brain centres. (b) Connectomic reconstructions of the indicated neuronal populations. Data in (a,b) are adapted from [87], prepared by P. Schlegel; note that missing surface ‘strips’ in the middle of the antennal lobe are due to absent data.
Within glomeruli, OSNs synapse with two types of interneurons: local interneurons (LNs) and projection neurons (PNs), whose anatomical properties were initially mostly studied using fortuitously identified enhancer-trap drivers and clonal labelling strategies [97–101]. More recently, electron microscopy-level analysis of the D. melanogaster brain [102] has prompted comprehensive synapse-resolution description of the olfactory system connectome [87,103–106]. LNs form connections between different glomeruli, exhibiting great diversity in the number and identity of glomeruli innervated, albeit with coarse-grained stereotypy [97]. Most PNs innervate single glomeruli (comprising an equivalent ∼50 classes as for OSNs) (figure 4a) and project their axons to higher brain centres: the lateral horn, which mediates innate responses to odours [107], and the mushroom body, a site of learned odour responses and sensory integration [19] (figure 4a,b). These centres are often considered insect analogues of the mammalian amygdala and piriform cortex, respectively.
The lateral horn does not exhibit the overt glomerular compartmentalization of the antennal lobe, but early work visualizing axons of single-labelled PNs and registering these images into a common ‘reference brain’, revealed spatially stereotyped PN innervations in this region [99–101] (figure 4a), concordant with its role in experience-independent odour-evoked behaviours. Further insights into how the antennal lobe glomerular map is transformed into the lateral horn neuronal map have required large-scale screening for sparsely expressed transgenic drivers [108,109], functional mapping of PN-lateral horn neuron connections using optogenetics [110], together with connectomics. These studies suggest the existence of a large number (greater than 250) of lateral horn neuron types [108]. Some of these neurons display only local innervations, while ‘output neurons’—which receive strong input from a stereotyped set of 1–10 PN classes [108]—project to other brain regions [108,110]. The unanticipated number and diversity of lateral horn neurons is likely to reflect the numerous (but largely unknown) ways in which olfactory inputs from PNs are integrated, processed and segregated to downstream circuits [107]. Lateral horn output neurons do not project directly to the ventral nerve cord—the insect analogue of the spinal cord—implying the existence of additional (though perhaps not many [111] and preprint [112]) layers of circuitry, before reaching motoneurons.
Within the mushroom body, PNs synapse with Kenyon cells (figure 4b), with up to ∼10 PNs converging onto individual mushroom body neurons. An important question is the degree to which PN connectivity in this brain region is stereotyped (as in the lateral horn) or whether random combinations of PN types synapse on common Kenyon cells. The latter scenario has been suggested to provide an anatomical basis to enhance odour discrimination and/or a template to permit ‘meaning’ to be imparted onto unpredictable olfactory stimuli through learning [104,113,114]. Initial low-resolution maps of PN innervations suggested the existence of a degree of zonal organization in the mushroom body [99,115,116], while subsequent single cell-resolution surveys of PN–Kenyon cell connectivity [114] (and related functional studies [117]) in a subset of the circuitry provided evidence that Kenyon cells receive input from random sets of PNs. Recent comprehensive analysis of the PN–Kenyon cell connectome revealed a more nuanced situation, where there is a degree of non-random structure [104]. For example, PNs transmitting food-related odour signals converge onto Kenyon cells at frequencies above those expected by chance.
Beyond these few examples, our understanding of olfactory circuit organization has been revolutionized by connectomics, which is uncovering many other features of known circuit elements (e.g. axo-axonic connections between PNs [103]) and new neuron types (e.g. those directly linking the lateral horn and mushroom body [118,119]). Such high-resolution anatomical data presents both opportunities and challenges [120]: although we can now trace sensory pathways from OSNs to deep within the brain, the wide dispersal of information across the network (e.g. preprints [112,121]) is daunting, as it remains non-trivial to investigate the physiological and behavioural relevance of such complex connectivity.
4. Development
Visualization of individual olfactory circuit elements reinvigorated historical interest (e.g. [122,123]) in olfactory system development, notably the specification and wiring of OSNs and PNs (study of LNs is still in its infancy [124,125]). The reproducible one-to-one matching in the antennal lobe of the ∼50 distinct classes of OSNs and PNs that are born at different times, in different places and in distinct ways during development is an incredible example of biological precision. These neuron types exemplify two emblematic strategies of insect nervous system development: OSNs derive from short lineages of sensory organ precursors (SOPs) in the late larval/early pupal antennal imaginal disc, while PNs are produced by a long series of asymmetric divisions of neuroblasts (stem cells) in the central brain during embryonic and larval stages [126–128]. Studies of how these neurons acquire their identity and wire up correctly both inform, and are informed by, knowledge of developmental algorithms and neural guidance molecules of many regions of the nervous system in D. melanogaster and other animals.
Thanks to a combination of genetic perturbations and spatio-temporally precise cell-labelling methods [126–129], we now have a good (albeit incomplete) understanding of OSN and PN development. For OSNs, a fate map of SOPs in the antennal disc has been defined: each SOP gives rise to a particular morphological type of sensillum (basiconic, intermediate, trichoid, coeloconic) housing a stereotyped combination of 1–4 OSNs [123,126,127,130–134]. How this map is established is unclear, but likely relies upon conserved disc patterning factors such as Wingless and Hedgehog [126,135]. OSN fate emerges through the action of a hierarchy of transcription factors—from ‘proneural’ factors demarcating the Or and Ir olfactory subsystems, to those defining sensillum type or individual OSN classes—and Notch signalling-dependent asymmetric cell divisions [133,134]. The identity of a given OSN class is realised by the expression of a specific olfactory receptor gene [25,136–139] and a (presumably unique) set of axon guidance molecules [140,141]. Within PN lineages, distinct PN classes are born in a highly stereotyped order during embryonic and larval development [98,142]. This birth order-dependent patterning relies, in part, upon a temporal gradient of a transcription factor (Chinmo) [143,144]. This protein is thought to regulate the expression of PN class-specific combinations of guidance molecules that control dendritic wiring in the antennal lobe and/or axonal projections in the higher olfactory centres [128,145,146].
How do the diverse types of OSNs and PNs assemble together to form the stereotyped glomerular structure of the antennal lobe? Several general principles are known [128,145,146]. First, PN dendrites enter the lobe first where they establish an initial map of protoglomeruli [147], using both long-range spatial information (e.g. a gradient of Semaphorin-1a [148–151]) and local cues (e.g. Capricious, which has heterogeneous glomerular expression [152]). Second, OSN axons from the antenna bifurcate into distinct tracts (requiring Semaphorin-2b [153]) before entering the antennal lobe, where they coarsely target the right region through cellular interactions with other OSNs and glia mediated by a multitude of neural guidance/adhesion molecules (e.g. Robos, Dscam, N-cadherin [154–157]). Third, OSN axons and PN dendrites—now positioned closely together in the lobe—wire up with high specificity using ‘match-maker’ molecules, which are expressed in partner OSNs and PNs (e.g. Teneurins [158]). Finally, OSN-PN synapses mature to render these connections functional (again using Teneurins [159]). Despite this framework, the wiring problem remains fantastically complex and still only superficially understood at the molecular and cellular levels.
Three technological advances promise to further enhance our understanding of olfactory circuit development. First, time-lapse imaging of the developing antennal lobe—using lattice light-sheet microscopy of antennal-brain explants—has permitted observation of OSN targeting behaviour that was previously interpreted only from snapshots of fixed tissue [160]. Second, ‘omics’ technologies, notably single-cell/nuclear RNA sequencing of OSNs and PNs [140,141,161–164] has revealed comprehensive lists of the molecular differences between populations that might explain their distinct properties both during development and in adults. Of course, determining the functional significance (if any) of differentially expressed genes requires substantial investment (e.g. [140,163]). Finally, proteomic profiling of cell-surface molecules in PNs has been particularly fruitful in identifying novel regulators of dendritic wiring [165]. This approach has also helped to make a causal link between a transcription factor (Acj6) defining neuron fate, the set of cell-surface proteins regulated by this transcription factor, and dendritic wiring specificity [166]. A goal of understanding olfactory circuit assembly—to decrypt and eventually reprogramme the successive combinatorial codes of transcription factors and their cellular executors that define wiring specificity—seems within reach.
5. Function
The anatomical map of olfactory circuitry provides a static picture of how olfactory information might be transmitted from the antenna to the brain. But understanding how odours are encoded as neuronal activity patterns requires physiological measurements and manipulations throughout this network [167,168]. Key initial insights into coding mechanisms came from surveys of odour-evoked activity in OSNs. These screens have mostly been via electrophysiological recordings in olfactory sensilla [26,60–66] (figure 3), but complemented by imaging OSN activity in their axon termini in the antennal lobe using genetically encoded sensors (e.g. the calcium reporter GCaMP) [169,170]. In the latter approach, the stereotypical organization of the lobe allows glomerular activity to be related to the corresponding receptors.
These efforts revealed a number of important observations that extended principles of odour coding originally proposed in other insects (notably, honeybees [171,172]) and mice [173]. First, olfactory receptor/OSN response profiles can vary from very narrow (such as those detecting a specific pheromone) to very broad (such as those detecting food-derived volatiles). Second, most individual odours activate multiple classes of OSNs. Third, the detection threshold of OSNs that respond to the same chemical can vary over several orders of magnitude. Fourth, OSNs exhibit a basal firing rate (potentially due to spontaneous receptor channel gating [29]) and many odours lead to a reduction in this activity [61]; basal firing can also be decreased indirectly through non-synaptic electrical interactions (ephaptic coupling) between OSNs housed in the same sensillum [174,175]. Lastly, there is substantial diversity in the temporal dynamics of odour responses, including onset latency upon stimulus presentation or persistence of firing after odour removal [26,176,177]. These properties have led to a model in which individual odours are represented as a ‘combinatorial code’ of OSN activity, whose spatio-temporal patterns of activation (and possibly inhibition) can inform the brain of the identity and intensity of a stimulus. This model, applicable to both insects and vertebrates, is compelling, not least for its ability to explain how olfactory systems might discriminate many more stimuli than there are sensory receptors. Surprisingly, it still remains unclear whether this model is valid for the encoding of the identity of individual odours. D. melanogaster responds behaviourally to almost every odour presented to it (e.g. [178]), but we do not know how many stimuli this species can discriminate. Explicit experimental tests of combinatorial coding have been rather limited (e.g. [179], or in larvae [180]). Moreover, there are now many examples of OSN classes that exhibit unique, narrow tuning to chemicals of particular ecological relevance (see ‘Ecology’ section). This species might therefore rely substantially on ‘labelled line’ olfactory coding, in which individual odours trigger specific behaviours through dedicated neural pathways [181,182]. Of course, natural odour sources (e.g. fermenting fruit) emit complex chemical blends, for which combinatorial neural representations are inherent to stimulus processing.
How the rich sensory information content that reaches the antennal lobe is transformed from OSNs to PNs has been subject to intensive investigations, through optical imaging at both of these neuronal layers, and electrophysiological recordings from PNs innervating specific glomeruli [168–170,183–185]. While OSNs provide the main excitatory drive to the PNs with which they directly synapse, the relationship between OSN and PN firing rates is nonlinear. In part, this relationship reflects the nature of OSN:PN synapses: the pooling of several-fold more OSNs than PNs in most glomeruli enables high sensitivity of PNs to low OSN activity (i.e. low odour concentrations) and highly reliable activation of PNs [186,187]. But transformation of signals from OSNs to PNs is also influenced by interactions between glomeruli. The most prominent of these is lateral inhibition, where strong OSN activity can suppress firing in other OSN populations via GABAergic local interneurons [188–190]. Inter-glomerular interactions can be global across the lobe but can also occur between certain combinations of glomeruli. Such inhibition is likely to underlie enhancement of the signal-to-noise ratio of glomerular odour representations, ensuring that strong signals through one pair of OSN/PN classes are not ‘muddied’ by weaker activation of others. Lateral excitation has also been reported, evident as responses of PNs to odours in the absence of activity in their partner OSNs [191,192], but the significance of this phenomenon is less clear.
The antennal lobe is also an important site of neuromodulation, influenced by the internal state of the animal. For example, when flies are starved, odour responses in a glomerulus detecting attractive food odours are increased, due to the integrated signalling of insulin and short neuropeptide F (a homologue of vertebrate NPY), which elevates calcium levels in OSN presynaptic termini [193]. Conversely, the responsiveness of a glomerulus detecting aversive odours is decreased through the action of a Tachykinin (a homologue of vertebrate Substance P) [194]. Many neuropeptides (or their receptors) have been detected in the antennal lobe [194–196], often at heterogeneous levels across glomeruli, suggesting that odour representation in this primary olfactory centre can be altered in a circuit-specific manner in response to many different external influences (e.g. mating, feeding and health status).
Studies of odour representations in higher olfactory centres are starting to reveal how these brain regions might direct an animal's behaviours. In the lateral horn, population-level calcium imaging, single-cell level electrophysiology and connectomics data suggest that odour-evoked activity in different PN classes is integrated by third-order neurons and categorized according to hedonic value (e.g. ‘attractive’ or ‘aversive’) [107,108,110,197]. While this framework is useful for exploration of how this centre directs innate odour-evoked behaviour, it is certainly simplistic, belying the anatomical complexity of this brain region (see the ‘Circuitry’ section above). An ‘attractive’ stimulus might manifest in many different behaviours (e.g. approach to the source, feeding, social aggregation or oviposition). Furthermore, an odour might be attractive or aversive to a fly depending upon the presence of other environmental cues or the animal's internal state (e.g. [198,199]). More generally, study of PN-to-lateral horn neuron neurotransmission has been a useful model to assess the functional significance of anatomical features revealed by the connectome, including synapse density and distance from the soma [200]; these insights should be pertinent throughout the nervous system.
Odour representations in the mushroom body of D. melanogaster (and other insects) are rather different to those in the lateral horn. Initial studies found that, in contrast to PNs, Kenyon cells display extremely sparse responses to odour stimulation, likely due to their high firing threshold and (at-the-time) presumed random connectivity with PNs [201–203]. Such properties might be advantageous for high capacity, non-overlapping neuronal representations of different stimuli in a centre where associative learning occurs. Recent volumetric calcium imaging has permitted a more comprehensive view of odour-induced activity in Kenyon cells [204]. Interestingly, this work has revealed greater spatial organization than previously appreciated—potentially matching the degree of non-random wiring indicated by connectomics [104]—as well as the existence of cells that respond to mixtures of odours, but not their individual components [204].
While global analyses of odour representations in the higher brain centres is clearly an exciting work-in-progress, focused attention over the past 15 years on the olfactory pathway detecting the sex pheromone cis-vaccenyl acetate is helping to relate its structural and functional properties to behaviour. This male-specific pheromone has multiple roles in D. melanogaster [205], notably its dual action in suppressing male courtship (to avoid homosexual advances) and promotion of female receptivity [206]. Unlike striking peripheral sexual dimorphisms in pheromone pathways in other insects [10], OSNs detecting cis-vaccenyl acetate (expressing Or67d) and the downstream PNs both display similar pheromone responsiveness in males and females [206–208]. Such similarity begs the question of how this pheromone evokes different behaviours in males and females. This pathway forms part of the so-called ‘Fruitless’ circuitry, which comprises sensory, inter- and moto- neurons that control courtship [209,210]. Using genetic drivers for this circuitry, it has been possible to anatomically and functionally characterize the cis-vaccenyl acetate pathway from the lateral horn through the ‘P1’ brain centre, which controls several sexually dimorphic behaviours, to descending neurons innervating the ventral nerve cord [111,211,212]. Importantly, these studies revealed sex-specific wiring between PNs and lateral horn neurons—offering a cellular explanation for the sex-specific behavioural responses to cis-vaccenyl acetate [212]—as well as other important insights into sensory coding, integration and plasticity [111,211,213] and preprint [121].
6. Behaviour
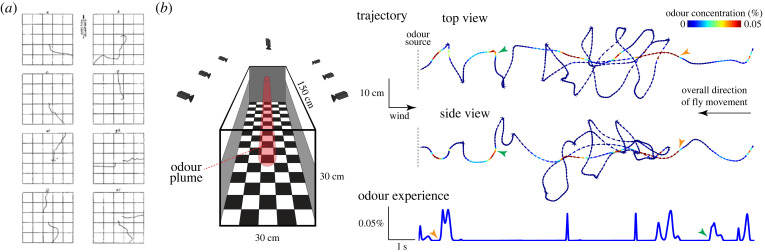
The raison d’être of the olfactory system is, of course, to tell the fly how to react when it encounters an odour. Typically, olfactory behaviours are assessed using simple assays in which flies can move toward or away from a chemical source, from which inferences about the ‘attractiveness’ or ‘aversiveness’ of an odour are made (figure 5a). Flies might also be offered a choice of two or more odours (or odour concentrations), to determine how well animals discriminate and/or value different stimuli [215]. Technological advancements in automated animal tracking [216–218], as well as more sophisticated methods for odour delivery and measurement [219–223], have enhanced the throughput, sensitivity and analytical objectivity of assays, as well as enabling study of navigation within odour gradients and plumes when walking or during flight [214,220,222,224,225] (figure 5b). Perhaps unsurprisingly, complex natural blends (such as vinegar or fruits) generally evoke the strongest behavioural responses. Consequently, to relate behaviours to specific receptor genes and circuits, there has been much interest in the innate actions, such as courtship or aggression, induced by single volatile pheromones (including cis-vaccenyl acetate) [67,68,206,210,226], as well as responses to other environmental chemicals that specifically activate single OSN populations (e.g. [57,227–230]).
Figure 5.
Olfactory behaviour. (a) Historical tracking: manually traced paths of individual walking D. ampelophila (the former name for D. melanogaster) from the edge of a 5 × 5 inch arena toward a piece of fermenting banana at the centre. Reproduced from [1], with permission. (b) State-of-the-art tracking: schematic of a wind tunnel through which a controlled odour plume can be introduced; a multi-camera system enables automated three-dimensional tracking of flies interacting with this plume in flight. On the right is the trajectory (top and side views) of D. melanogaster in a continuous plume of ethanol. Grey dashed lines on the left indicate the upwind wall of the tunnel towards which the animal flies. The trajectories are colour-coded for the instantaneous concentration of odour at a given point in the plume (as measured using a photoionization detector); the reconstructed odour experience of the animal over the course of its flight is plotted below the trajectories. Two time-synchronized reference points are indicated with orange and green arrowheads. Adapted from [214], with permission.
Many odour-evoked behaviours have been causally linked to specific olfactory pathways, typically through analysis of olfactory receptor mutants or animals in which specific OSN populations are ablated or artificially activated. The availability of driver lines for nearly all OSN populations has enabled systematic screening for behaviours evoked upon activation of individual pathways [179,231,232]. However, robust responses are rarely observed, indicating that meaningful perception of olfactory stimuli in the brain might require combinatorial activation of multiple sensory pathways with appropriate intensity and temporal precision. In this light, it is unsurprising that, deeper in the brain, olfactory behaviours have still mostly only been correlated with physiological representations (e.g. activation of putative ‘positive’ or ‘negative’ valence regions in the lateral horn [233] or sexually dimorphic pheromone responses [212]). However, some causal evidence has been obtained when selective driver lines permit targeted perturbation of higher order circuit elements (e.g. [197,234] and preprints [112,121]).
Interpreting behavioural responses can be tricky: subtle differences in culture conditions, genetic background and the assay itself can influence how animals behave, potentially masking effects of the desired experimental intervention. While frequently frustrating for researchers, behavioural variability is now being explored as an inherently interesting phenomenon, through examination of the individuality of olfactory responses of genetically identical individuals [235]. Conceptually, inter-individual variation in olfactory behaviour could be due to structural and functional differences at many levels of the olfactory circuitry [236]. Although the D. melanogaster olfactory system is considered to be largely genetically hard-wired, differences between isogenic animals [75,97,106] may result from imprecision in developmental processes [237] and/or fluctuations in the internal state of the animal impacted by a plethora of factors (e.g. feeding status, infection and prior sexual or social interactions). A recent preprint indicates that the sensory periphery is at least one site underlying inter-individual behavioural variation [238].
There is also growing appreciation that, as in humans, odours induce many other responses in D. melanogaster that do not directly involve locomotor circuitry, but rather impinge upon other neural or endocrine pathways. For example, olfactory perception (or lack of) can affect taste sensation (preprint [239]), modulate food ingestion and metabolism [240], contribute to maintenance and development of blood progenitors [241,242], as well as influence general stress responses and lifespan [243]. When flies do not visibly react to an odour, the olfactory system might still be meaningfully processing the sensory information.
7. Ecology
To keep pace with the progress in our understanding of D. melanogaster's olfactory neurobiology, increasing efforts aim to better appreciate the ecology of this species. Such knowledge is likely to help identify relevant ligands for sensory receptors and to describe naturalistic odour-evoked behaviours. It is unclear why entomologist Charles Woodworth chose to culture D. melanogaster at the beginning of the twentieth century [244], but the flourishing of the species in the laboratory was a natural extension of its cosmopolitan distribution and commensal relationship with humans [245]. The presence of D. melanogaster in orchards, wineries, household compost bins and fruit bowls attests to its ‘generalist’ ecology, capable of feeding and breeding on a wide range of fermenting vegetal substrates. Consequently, many early olfactory studies screened for physiologically and/or behaviourally active odour ligands among individual, off-the-shelf, synthetic chemicals found in the complex bouquet of ripe, over-ripe and rotting fruits as well as the microbial catalysts (notably yeasts) of fermentation [246].
More recently, the odorous world of D. melanogaster has been further probed by analysing volatiles produced by pathogenic bacteria [230] and fungi [247], predators (notably parasitoid wasps) [248], as well as the identification of pheromones controlling sexual and other social behaviours [67,68,249]. Adopting a fly's perspective of the chemical world has helped to discover potent ligands for previously ‘orphan’ receptors.
By contrast, there has still been very limited examination of the olfactory behaviours of D. melanogaster in nature. This may be understandable, given the challenges of interpreting odour-dependent behaviours even under well-controlled laboratory assays. However, the few field studies of D. melanogaster have proven to be illuminating, including description of social interactions of flies near fruits [250,251] or the discovery of a deceptive pollination system of the Solomon arum lily, which produces a remarkably faithful odour mimicry of fermentation to attract D. melanogaster as an unwitting courier for its pollen [252]. One ambitious investigation located a potential origin and ancestral chemical ecology of wild D. melanogaster—not associated with contemporary human settlements—within the balsam tree forests of Zimbabwe [253]. Here the wild flies appeared to exhibit seasonal specialism upon marula fruit (Sclerocarya birrea), an important food source for historical tribes of this region, leading to a plausible model for the transition of this species to human commensalism and the extant generalist ecology [253].
8. Evolution
Study of the development and function of D. melanogaster's olfactory circuits has benefited from their (mostly) stereotyped properties, enabling observation of reproducible phenotypes (both wild-type and mutant) across individuals. However, olfactory systems are highly dynamic over evolutionary timescales, shaped in part by changing environmental selection pressures, such as novel food odour signals when individuals colonize a new ecological niche. Given the enormous number of species and habitat diversity of insects, their olfactory systems have long been interesting models to explore the genetic, cellular and physiological basis of nervous system and behavioural evolution [129,254–257].
The deep foundation of knowledge of molecular, anatomical and physiological properties of the D. melanogaster olfactory system has made this a useful ‘anchor’ species for evolutionary comparisons at a range of evolutionary divergence times. Analysis of microevolution between geographically separated D. melanogaster strains, which may have diverged only a few thousand years ago has revealed that olfactory receptors (and other chemosensory protein families) display some of the strongest signals of recent selection, highlighting their potential roles as genetic ‘first-responders’ to new environments [47]. However, successful association of intraspecific genetic variation to physiological or behavioural differences has only rarely been achieved [49,258–260].
Macroevolutionary differences, across closely or more-distantly related drosophilid species, are widely documented, with particular interest in comparisons of the cosmopolitan generalist D. melanogaster with specialist species, such as the island endemic Drosophila sechellia, which feeds and breeds exclusively on the ‘noni’ fruit of the Morinda citrifolia shrub [34,54,70,257,261,262], the invasive agricultural pest Drosophila suzukii, which has evolved preference for ripe, rather than fermenting, fruit [263–267], or the herbivorous drosophilid Scaptomyza flava [268]. Together with comparative studies in many other insects, this work has revealed numerous examples of changes in odour response properties, reflecting in most cases evolution of olfactory receptors. In some cases, the causal molecular basis of functional changes in receptors has been mapped, demonstrating that while single amino acid substitutions can substantially modify odour responses (figure 2), species-specific tuning properties typically depend on multiple sites within (and beyond) the presumed ligand-binding site (e.g. [34,48,53,54]).
A second type of species-specific neuronal change is the increase (or decrease) in OSN population size, with commensurate changes in glomerular volume [34,54,262,269–271]. While expanded OSN classes are frequently those that detect ecologically important stimuli for a species, neither the developmental basis nor the functional significance of these presumed evolutionary adaptations are known. The non-pleiotropic functions of olfactory receptors (and the OSNs in which they are expressed) might explain why most described examples of evolutionary variation are within the sensory periphery. However, this bias probably largely reflects the experimental accessibility of this part of the olfactory system. We know very little about if and how other olfactory circuit elements have evolved, although subtle anatomical differences between species in PN innervations of higher brain centres have been observed [34]. Comparative studies of (contact) chemosensory pathways between drosophilids illustrate the potential for functional diversification of central circuit elements to explain species-specific behaviours [272].
The evolution of completely new olfactory circuits within the drosophilid phylogeny appears rare. One example exists in a class of pheromone-sensing sensilla, which houses a single neuron in the majority of drosophilids (the Or67d cis-vaccenyl acetate sensor) but a second, distinct neuron in a subset of species, including Drosophila mojavensis [273–275]. How new neurons appear during evolution is unclear, although work in D. melanogaster suggests that changes in the precise developmental patterning of programmed cell death—which removes many of the potential neurons in the olfactory sensory lineages—might be sufficient to create new OSN classes [273].
These studies highlight the core challenge of comparative neurobiology in balancing phylogenetic proximity and phenotypic divergence. Comparisons of closely related species facilitate identification of differences in traits and their experimental characterization, especially if these species produce fertile hybrids for genetic mapping, or genetic tools can be easily exported from D. melanogaster [34,257,272,276]. However, more dramatic changes in neuronal circuit structure and function may only be seen between species where it is harder to go beyond purely descriptive analysis. Of course, it is not always necessary (and probably often impossible) to determine the causal genetic basis of species differences. There is much current interest in harnessing genome-editing methods (notably CRISPR/Cas9) for molecular genetic characterization of the olfactory systems of both historically important species (e.g. moths [277–279]) and newer model systems (e.g. mosquitoes [280,281] and ants [282,283]). These studies can illuminate interesting properties of insect olfactory systems distinct from those of D. melanogaster, without necessarily providing a mechanistic basis for the evolutionary differences.
9. Applications
Since the discovery of olfactory receptors in D. melanogaster, the potential for application of this knowledge to combat the devastating impact of insect disease vectors (such as mosquitoes and tsetse flies) and agricultural pests (such as corn rootworm and locusts) has been widely recognized [284,285]. These harmful species depend heavily on their sense of smell for host animal- or host plant-seeking as well for reproductive and other social behaviours. It has been logically reasoned that characterization of peripheral olfactory receptors (and perireceptor proteins) in such species—following the lead in the benign D. melanogaster—could offer targets for pharmacological intervention to interfere with these odour-guided behaviours.
Soberingly, this ‘reverse chemical ecology’ approach has seen only limited progress. Technological advancements in sequencing have enabled identification of olfactory receptors in an enormous number of pest insect species [286,287]. However, only a minute fraction of these receptors has been functionally characterized, sometimes using D. melanogaster OSNs as a heterologous expression system [71,286–289]. Moreover, from information within at least the public domain, very few receptors have been subject to screens for artificial agonists or antagonists (e.g. [290–293]), although complementary chemoinformatic approaches to identify novel chemical modulators of receptors in silico show promise [294–296]. Some of these chemicals induce behaviours in laboratory-based assays [291–294,297], but there is little evidence of successful field trials.
Akin to the many ways in which drug development pipelines can fail, olfactory receptor-based efforts to identify new insect behavioural control molecules face numerous challenges. These include target specificity (many studies focused on (ant)agonists of the Or co-receptor Orco [290,294,297], which would likely affect all insect species), volatility, stability, non-toxicity to humans and cost of production. It remains to be seen whether reverse chemical ecology will ever yield more promising solutions than traditional, highly successful strategies of olfaction-based integrated pest management, which use natural semiochemicals with potent behavioural influence (e.g. pheromones) as the basis of lures or repellents [298,299]. Here, molecular knowledge of how a species detects a chemical cue is ultimately unnecessary.
A second potential avenue for practical application of insect olfactory receptors is in artificial chemosensors, with diverse applications, for example, in medical diagnostics, food quality assessment and environmental monitoring [73]. Theoretically, these proteins are ideal for this purpose: across millions of insect species there could be tens of millions of receptors with distinct chemical specificity and, in principle, they act autonomously as ligand-gated ion channels. Practically, however, progress has been slow, constrained by limitations in the reliability of heterologous functional expression of receptors. Moreover, despite some pioneering bioengineering efforts (e.g. [300]) integrating biological sensors into sensitive and robust optical- or electrical-based recording devices remains a huge technical challenge.
10. Perspectives
Barrows’ 1907 study [1] is remarkable for its foundational observations of D. melanogaster olfaction: the selectivity of odour responses and synergistic effect of odour mixtures, the rudiments of chemotaxis behaviour (in flying and walking animals), the importance of an animal's internal state, and the identification of the key sensory organs. Since that work, there have been enormous advances in our understanding of all of these—and many other—aspects of olfaction in this species. Yet, as illustrated by the open issues highlighted in this review (box 1), we cannot claim to be able to explain how the olfactory system is built or functions in much more than a partial way. Like many seemingly simple phenomena, the sense of smell of the fly is an extraordinarily complex problem, requiring integration of knowledge of genetics and molecular biology, cell and developmental biology, neuroscience and physiology, ecology and evolution. But this complexity offers the benefit that new insights have far-reaching significance in biology, from animal behaviour, neural circuit construction, ion channel function and gene regulation, to name just a few areas. More generally, during a research era where powerful new technologies, such as genome engineering and single-cell transcriptomics, open the doors to new questions and new model systems, the nose of D. melanogaster provides a useful reminder of the equal merits of focus. The novel, often surprising, scientific insights gained from study of the sense of smell of the fly, emerged because new results—whether a puzzling observation or a large dataset—could be better interpreted in a system where we already have a rich intellectual framework.
Box 1. Five areas of specific and open questions in D. melanogaster olfaction.
-
1.
How do olfactory receptors recognize odours with narrow or broad specificity? How do they collaborate with perireceptor proteins and sensillar structural properties to mediate sensitive and dynamic odour detection? What is the mechanistic basis by which sensory response profiles change over evolutionary timescales?
-
2.
What are the complete developmental pathways specifying the diversity in neuronal fate and wiring properties at different layers of the circuitry? How are synaptic partners matched up with precision? What are the molecular and cellular mechanisms underlying the evolution of modified—or completely new—olfactory pathways?
-
3.
How important is the combinatorial code for determining odour identity? What is the role of temporal properties of neuronal activity in odour coding? What are the physiologically important anatomical connections within the olfactory connectome? What is the logic of olfactory circuit organization underlying innate versus learned odour-driven responses?
-
4.
What are the natural pertinent odour signals in D. melanogaster's ecological niche? How are olfactory behaviours observed in the laboratory related to those in nature? How are olfactory cues integrated with other sensory information to drive complex behaviours, such as odour plume navigation? How and why are olfactory behaviours variable both between genetically identical individuals, and within an individual's lifetime?
-
5.
(How) can the information from studies of D. melanogaster be usefully exploited to control odour-driven behaviours of insect vectors of disease and agricultural pests, or in engineering artificial biosensors?
Acknowledgements
I regret being unable to cite many relevant studies due to space constraints. I thank Joel Butterwick, Christopher Dumayne, Hany Dweck, Matthieu Louis, Aleksandar Vjestica and members of my laboratory for comments on the manuscript, and Floris van Breugel and Philipp Schlegel for providing figures.
Data accessibility
This article has no additional data.
Conflict of interest declaration
I declare I have no competing interests.
Funding
Research in my laboratory is supported by the University of Lausanne, an ERC Advanced Grant (grant no. 833548) and the Swiss National Science Foundation.
References
- 1.Barrows WM. 1907. The reactions of the pomace fly, Drosophila ampelophila Loew, to odorous substances. J. Exp. Zool. 4, 515-537. ( 10.1002/jez.1400040403) [DOI] [Google Scholar]
- 2.Greenspan RJ, Ferveur JF. 2000. Courtship in Drosophila. Annu. Rev. Genet. 34, 205-232. ( 10.1146/annurev.genet.34.1.205) [DOI] [PubMed] [Google Scholar]
- 3.Quinn WG, Greenspan RJ. 1984. Learning and courtship in Drosophila: two stories with mutants. Annu. Rev. Neurosci. 7, 67-93. ( 10.1146/annurev.ne.07.030184.000435) [DOI] [PubMed] [Google Scholar]
- 4.Weiner J. 1999. Time, love, memory: a great biologist and his quest for the origins of behavior. New York, NY: Knopf. [Google Scholar]
- 5.Stocker RF. 1994. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 275, 3-26. ( 10.1007/BF00305372) [DOI] [PubMed] [Google Scholar]
- 6.Carlson JR. 1996. Olfaction in Drosophila: from odor to behavior. Trends Genet. 12, 175-180. ( 10.1016/0168-9525(96)10015-9). [DOI] [PubMed] [Google Scholar]
- 7.Cutforth T, Gaul U. 1997. The genetics of visual system development in Drosophila: specification, connectivity and asymmetry. Curr. Opin. Neurobiol. 7, 48-54. ( 10.1016/s0959-4388(97)80119-5). [DOI] [PubMed] [Google Scholar]
- 8.Hardie RC, Raghu P. 2001. Visual transduction in Drosophila. Nature 413, 186-193. ( 10.1038/35093002) [DOI] [PubMed] [Google Scholar]
- 9.Fabre J-H. 1912. Social life in the insect world. Translated by B Miall. London, UK: T. Fisher Unwin, LTD.
- 10.Hansson BS. 2002. A bug's smell—research into insect olfaction. Trends Neurosci. 25, 270-274. ( 10.1016/S0166-2236(02)02140-9) [DOI] [PubMed] [Google Scholar]
- 11.Vosshall LB, Amrein H, Morozov PS, Rzhetsky A, Axel R. 1999. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell 96, 725-736. ( 10.1016/S0092-8674(00)80582-6) [DOI] [PubMed] [Google Scholar]
- 12.Gao Q, Chess A. 1999. Identification of candidate Drosophila olfactory receptors from genomic DNA sequence. Genomics 60, 31-39. ( 10.1006/geno.1999.5894) [DOI] [PubMed] [Google Scholar]
- 13.Clyne PJ, Warr CG, Freeman MR, Lessing D, Kim J, Carlson JR. 1999. A novel family of divergent seven-transmembrane proteins: candidate odorant receptors in Drosophila. Neuron 22, 327-338. ( 10.1016/S0896-6273(00)81093-4) [DOI] [PubMed] [Google Scholar]
- 14.Buck L, Axel R. 1991. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell 65, 175-187. ( 10.1016/0092-8674(91)90418-X) [DOI] [PubMed] [Google Scholar]
- 15.Su CY, Menuz K, Carlson JR. 2009. Olfactory perception: receptors, cells, and circuits. Cell 139, 45-59. ( 10.1016/j.cell.2009.09.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerber B, Stocker RF, Tanimura T, Thum AS. 2009. Smelling, tasting, learning: Drosophila as a study case. Results Probl. Cell Differ. 47, 139-185. ( 10.1007/400_2008_9). [DOI] [PubMed] [Google Scholar]
- 17.Louis M. 2020. Mini-brain computations converting dynamic olfactory inputs into orientation behavior. Curr. Opin. Neurobiol. 64, 1-9. ( 10.1016/j.conb.2019.11.015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Currier TA, Nagel KI. 2020. Multisensory control of navigation in the fruit fly. Curr. Opin. Neurobiol. 64, 10-16. ( 10.1016/j.conb.2019.11.017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Modi MN, Shuai Y, Turner GC. 2020. The Drosophila mushroom body: from architecture to algorithm in a learning circuit. Annu. Rev. Neurosci. 43, 465-484. ( 10.1146/annurev-neuro-080317-0621333). [DOI] [PubMed] [Google Scholar]
- 20.Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. 2009. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 136, 149-162. ( 10.1016/j.cell.2008.12.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nei M, Niimura Y, Nozawa M. 2008. The evolution of animal chemosensory receptor gene repertoires: roles of chance and necessity. Nat. Rev. Genet. 9, 951-963. ( 10.1038/nrg2480) [DOI] [PubMed] [Google Scholar]
- 22.Benton R, Dahanukar A. 2011. Electrophysiological recording from Drosophila olfactory sensilla. Cold Spring Harb. Protoc. 2011, 824-838. ( 10.1101/pdb.prot5630) [DOI] [PubMed] [Google Scholar]
- 23.Scalzotto M, Ng R, Cruchet S, Saina M, Armida J, Su CY, Benton R. 2022. Pheromone sensing in Drosophila requires support cell-expressed Osiris 8. BMC Biol. 20, 230. ( 10.1186/s12915-022-01425-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monahan K, Lomvardas S. 2015. Monoallelic expression of olfactory receptors. Annu. Rev. Cell Dev. Biol. 31, 721-740. ( 10.1146/annurev-cellbio-100814-125308). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mika K, Benton R. 2021. Olfactory receptor gene regulation in insects: multiple mechanisms for singular expression. Front. Neurosci. 15, 738088. ( 10.3389/fnins.2021.738088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hallem EA, Ho MG, Carlson JR. 2004. The molecular basis of odor coding in the Drosophila antenna. Cell 117, 965-979. ( 10.1016/j.cell.2004.05.012) [DOI] [PubMed] [Google Scholar]
- 27.Mombaerts P. 1999. Seven-transmembrane proteins as odorant and chemosensory receptors. Science 286, 707-711. ( 10.1126/science.286.5440.707) [DOI] [PubMed] [Google Scholar]
- 28.Wicher D, Schafer R, Bauernfeind R, Stensmyr MC, Heller R, Heinemann SH, Hansson BS. 2008. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature 452, 1007-1011. ( 10.1038/nature06861) [DOI] [PubMed] [Google Scholar]
- 29.Sato K, Pellegrino M, Nakagawa T, Nakagawa T, Vosshall LB, Touhara K. 2008. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature 452, 1002-1006. ( 10.1038/nature06850) [DOI] [PubMed] [Google Scholar]
- 30.Abuin L, Bargeton B, Ulbrich MH, Isacoff EY, Kellenberger S, Benton R. 2011. Functional architecture of olfactory ionotropic glutamate receptors. Neuron 69, 44-60. ( 10.1016/j.neuron.2010.11.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. 2004. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43, 703-714. ( 10.1016/j.neuron.2004.08.019) [DOI] [PubMed] [Google Scholar]
- 32.Benton R, Sachse S, Michnick SW, Vosshall LB. 2006. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 4, e20. ( 10.1371/journal.pbio.0040020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vulpe A, Menuz K. 2021. Ir76b is a co-receptor for amine responses in Drosophila olfactory neurons. Front. Cell Neurosci. 15, 759238. ( 10.3389/fncel.2021.759238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Auer TO, et al. 2020. Olfactory receptor and circuit evolution promote host specialization. Nature 579, 402-408. ( 10.1038/s41586-020-2073-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Del Marmol J, Yedlin MA, Ruta V. 2021. The structural basis of odorant recognition in insect olfactory receptors. Nature 597, 126-131. ( 10.1038/s41586-021-03794-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butterwick JA, Del Marmol J, Kim KH, Kahlson MA, Rogow JA, Walz T, Ruta V. 2018. Cryo-EM structure of the insect olfactory receptor Orco. Nature 560, 447-452. ( 10.1038/s41586-018-0420-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varadi M, et al. 2022. AlphaFold Protein Structure Database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 50, D439-D444. ( 10.1093/nar/gkab1061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jumper J, et al. 2021. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583-589. ( 10.1038/s41586-021-03819-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emsley P, Lohkamp B, Scott WG, Cowtan K. 2010. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486-501. ( 10.1107/S0907444910007493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.German PF, van der Poel S, Carraher C, Kralicek AV, Newcomb RD.. 2013. Insights into subunit interactions within the insect olfactory receptor complex using FRET. Insect. Biochem. Mol. Biol. 43, 138-145. ( 10.1016/j.ibmb.2012.11.002) [DOI] [PubMed] [Google Scholar]
- 41.Robertson HM. 2015. The insect chemoreceptor superfamily is ancient in animals. Chem. Senses 40, 609-614. ( 10.1093/chemse/bjv046) [DOI] [PubMed] [Google Scholar]
- 42.Benton R, Dessimoz C, Moi D. 2020. A putative origin of the insect chemosensory receptor superfamily in the last common eukaryotic ancestor. Elife 9, e62507. ( 10.7554/eLife.62507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saina M, Busengdal H, Sinigaglia C, Petrone L, Oliveri P, Rentzsch F, Benton R. 2015. A cnidarian homologue of an insect gustatory receptor functions in developmental body patterning. Nat. Commun. 6, 6243. ( 10.1038/ncomms7243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benton R. 2015. Multigene family evolution: perspectives from insect chemoreceptors. Trends Ecol. Evol. 30, 590-600. ( 10.1016/j.tree.2015.07.009) [DOI] [PubMed] [Google Scholar]
- 45.Croset V, Rytz R, Cummins SF, Budd A, Brawand D, Kaessmann H, Gibson TJ, Benton R. 2010. Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS Genet. 6, e1001064. ( 10.1371/journal.pgen.1001064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silbering AF, Benton R. 2010. Ionotropic and metabotropic mechanisms in chemoreception: ‘chance or design'? EMBO Rep. 11, 173-179. ( 10.1038/embor.2010.8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arguello JR, Cardoso-Moreira M, Grenier JK, Gottipati S, Clark AG, Benton R. 2016. Extensive local adaptation within the chemosensory system following Drosophila melanogaster’s global expansion. Nat. Commun. 7, ncomms11855. ( 10.1038/ncomms11855) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leary GP, Allen JE, Bunger PL, Luginbill JB, Linn CE Jr, Macallister IE, Kavanaugh MP, Wanner KW. 2012. Single mutation to a sex pheromone receptor provides adaptive specificity between closely related moth species. Proc. Natl Acad. Sci. USA 109, 14081-14086. ( 10.1073/pnas.1204661109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pellegrino M, Steinbach N, Stensmyr MC, Hansson BS, Vosshall LB. 2011. A natural polymorphism alters odour and DEET sensitivity in an insect odorant receptor. Nature 478, 511-514. ( 10.1038/nature10438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hopf TA, Morinaga S, Ihara S, Touhara K, Marks DS, Benton R. 2015. Amino acid coevolution reveals three-dimensional structure and functional domains of insect odorant receptors. Nat. Commun. 6, 6077. ( 10.1038/ncomms7077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang K, Huang LQ, Ning C, Wang CZ. 2017. Two single-point mutations shift the ligand selectivity of a pheromone receptor between two closely related moth species. Elife 6, e29100. ( 10.7554/eLife.29100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ng R, Salem SS, Wu ST, Wu M, Lin HH, Shepherd AK, Joiner WJ, Wang JW, Su CY. 2019. Amplification of Drosophila olfactory responses by a DEG/ENaC channel. Neuron 104, 947-959. ( 10.1016/j.neuron.2019.08.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prieto-Godino LL, Schmidt HR, Benton R. 2021. Molecular reconstruction of recurrent evolutionary switching in olfactory receptor specificity. Elife 10, e69732. ( 10.7554/eLife.69732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prieto-Godino LL, Rytz R, Cruchet S, Bargeton B, Abuin L, Silbering AF, Ruta V, Dal Peraro M, Benton R. 2017. Evolution of acid-sensing olfactory circuits in drosophilids. Neuron 93, 661-676. ( 10.1016/j.neuron.2016.12.024) [DOI] [PubMed] [Google Scholar]
- 55.Jones WD, Cayirlioglu P, Grunwald Kadow I, Vosshall LB. 2007. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature 445, 86-90. ( 10.1038/nature05466) [DOI] [PubMed] [Google Scholar]
- 56.Kwon JY, Dahanukar A, Weiss LA, Carlson JR. 2007. The molecular basis of CO2 reception in Drosophila. Proc. Natl Acad. Sci. USA 104, 3574-3578. ( 10.1073/pnas.0700079104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vulpe A, et al. 2021. An ammonium transporter is a non-canonical olfactory receptor for ammonia. Curr. Biol. 31, 3382-3390. ( 10.1016/j.cub.2021.05.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schneider D, Boeckh J. 1962. Rezeptorptential und Nervenimpulse einzelner olfaktorischer Sensillen der Insektenantenne. Zeitschrift für vergleichende Physiologie 45, 405-412. ( 10.1007/BF00340462) [DOI] [Google Scholar]
- 59.Wolbarsht ML, Dethier VG. 1958. Electrical activity in the chemoreceptors of the blowfly. I. Responses to chemical and mechanical stimulation. J. Gen. Physiol. 42, 393-412. ( 10.1085/jgp.42.2.393) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clyne P, Grant A, O'Connell R, Carlson JR. 1997. Odorant response of individual sensilla on the Drosophila antenna. Invert. Neurosci. 3, 127-135. ( 10.1007/BF02480367) [DOI] [PubMed] [Google Scholar]
- 61.Hallem EA, Carlson JR. 2006. Coding of odors by a receptor repertoire. Cell 125, 143-160. ( 10.1016/j.cell.2006.01.050) [DOI] [PubMed] [Google Scholar]
- 62.de Bruyne M, Clyne PJ, Carlson JR.. 1999. Odor coding in a model olfactory organ: the Drosophila maxillary palp. J. Neurosci. 19, 4520-4532. ( 10.1523/JNEUROSCI.19-11-04520.1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Bruyne M, Foster K, Carlson JR.. 2001. Odor coding in the Drosophila antenna. Neuron 30, 537-552. ( 10.1016/S0896-6273(01)00289-6) [DOI] [PubMed] [Google Scholar]
- 64.Yao CA, Ignell R, Carlson JR. 2005. Chemosensory coding by neurons in the coeloconic sensilla of the Drosophila antenna. J. Neurosci. 25, 8359-8367. ( 10.1523/JNEUROSCI.2432-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Silbering AF, Rytz R, Grosjean Y, Abuin L, Ramdya P, Jefferis GS, Benton R. 2011. Complementary function and integrated wiring of the evolutionarily distinct Drosophila olfactory subsystems. J. Neurosci. 31, 13 357-13 375. ( 10.1523/JNEUROSCI.2360-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Munch D, Galizia CG. 2016. DoOR 2.0–comprehensive mapping of Drosophila melanogaster odorant responses. Sci. Rep. 6, 21841. ( 10.1038/srep21841) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dweck HK, et al. 2015. Pheromones mediating copulation and attraction in Drosophila. Proc. Natl Acad. Sci. USA 112, E2829-E2835. ( 10.1073/pnas.1504527112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin HH, et al. 2016. Hormonal modulation of pheromone detection enhances male courtship success. Neuron 90, 1272-1285. ( 10.1016/j.neuron.2016.05.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dobritsa AA, van der Goes van Naters W, Warr CG, Steinbrecht RA, Carlson JR.. 2003. Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron 37, 827-841. ( 10.1016/S0896-6273(03)00094-1) [DOI] [PubMed] [Google Scholar]
- 70.Prieto-Godino LL, Rytz R, Bargeton B, Abuin L, Arguello JR, Peraro MD, Benton R. 2016. Olfactory receptor pseudo-pseudogenes. Nature 539, 93-97. ( 10.1038/nature19824) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carey AF, Wang G, Su CY, Zwiebel LJ, Carlson JR. 2010. Odorant reception in the malaria mosquito Anopheles gambiae. Nature 464, 66-71. ( 10.1038/nature08834) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pitts RJ, Derryberry SL, Zhang Z, Zwiebel LJ. 2017. Variant ionotropic receptors in the malaria vector mosquito Anopheles gambiae tuned to amines and carboxylic acids. Sci. Rep. 7, 40297. ( 10.1038/srep40297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheema JA, Carraher C, Plank NOV, Travas-Sejdic J, Kralicek A. 2021. Insect odorant receptor-based biosensors: current status and prospects. Biotechnol. Adv. 53, 107840. ( 10.1016/j.biotechadv.2021.107840) [DOI] [PubMed] [Google Scholar]
- 74.Schmidt HR, Benton R. 2020. Molecular mechanisms of olfactory detection in insects: beyond receptors. Open Biol. 10, 200252. ( 10.1098/rsob.200252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nava GC, McKaughan Q, Bushong EA, Cauwenberghs K, Ng R, Madany M, Ellisman MH, Su CY. 2021. Systematic morphological and morphometric analysis of identified olfactory receptor neurons in Drosophila melanogaster. Elife 10, e69896. ( 10.7554/eLife.69896) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun JS, Xiao S, Carlson JR. 2018. The diverse small proteins called odorant-binding proteins. Open Biol. 8, 180208. ( 10.1098/rsob.180208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu P, Atkinson R, Jones DN, Smith DP. 2005. Drosophila OBP LUSH is required for activity of pheromone-sensitive neurons. Neuron 45, 193-200. ( 10.1016/j.neuron.2004.12.031) [DOI] [PubMed] [Google Scholar]
- 78.Benton R, Vannice KS, Vosshall LB. 2007. An essential role for a CD36-related receptor in pheromone detection in Drosophila. Nature 450, 289-293. ( 10.1038/nature06328) [DOI] [PubMed] [Google Scholar]
- 79.Jin X, Ha TS, Smith DP.. 2008. SNMP is a signaling component required for pheromone sensitivity in Drosophila. PNAS 105, 10 996-11 001. ( 10.1073/pnas.0803309105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tsang TK, Bushong EA, Boassa D, Hu J, Romoli B, Phan S, Dulcis D, Su CY, Ellisman MH. 2018. High-quality ultrastructural preservation using cryofixation for 3D electron microscopy of genetically labeled tissues. Elife 7, e35524. ( 10.7554/eLife.35524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Frank DD, Enjin A, Jouandet GC, Zaharieva EE, Para A, Stensmyr MC, Gallio M. 2017. Early integration of temperature and humidity stimuli in the Drosophila brain. Curr. Biol. 27, 2381-2388. ( 10.1016/j.cub.2017.06.077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Enjin A, Zaharieva EE, Frank DD, Mansourian S, Suh GS, Gallio M, Stensmyr MC. 2016. Humidity sensing in Drosophila. Curr. Biol. 26, 1352-1358. ( 10.1016/j.cub.2016.03.049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marin EC, et al. 2020. Connectomics analysis reveals first-, second-, and third-order thermosensory and hygrosensory neurons in the adult Drosophila brain. Curr. Biol. 30, 3167-3182. ( 10.1016/j.cub.2020.06.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ni L, Bronk P, Chang EC, Lowell AM, Flam JO, Panzano VC, Theobald DL, Griffith LC, Garrity PA. 2013. A gustatory receptor paralogue controls rapid warmth avoidance in Drosophila. Nature 500, 580-584. ( 10.1038/nature12390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Knecht ZA, et al. 2016. Distinct combinations of variant ionotropic glutamate receptors mediate thermosensation and hygrosensation in Drosophila. Elife 5, e17879. ( 10.7554/eLife.17879.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Knecht ZA, Silbering AF, Cruz J, Yang L, Croset V, Benton R, Garrity PA. 2017. Ionotropic receptor-dependent moist and dry cells control hygrosensation in Drosophila. Elife 6, e26654. ( 10.7554/eLife.26654) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schlegel P, et al. 2021. Information flow, cell types and stereotypy in a full olfactory connectome. Elife 10, e66018. ( 10.7554/eLife.66018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vosshall LB, Wong AM, Axel R. 2000. An olfactory sensory map in the fly brain. Cell 102, 147-159. ( 10.1016/S0092-8674(00)00021-0) [DOI] [PubMed] [Google Scholar]
- 89.Gao Q, Yuan B, Chess A. 2000. Convergent projections of Drosophila olfactory neurons to specific glomeruli in the antennal lobe. Nat. Neurosci. 3, 780-785. ( 10.1038/77680) [DOI] [PubMed] [Google Scholar]
- 90.Hansson BS, Ljungberg H, Hallberg E, Lofstedt C. 1992. Functional specialization of olfactory glomeruli in a moth. Science 256, 1313-1315. ( 10.1126/science.1598574) [DOI] [PubMed] [Google Scholar]
- 91.Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. 1996. Visualizing an olfactory sensory map. Cell 87, 675-686. ( 10.1016/S0092-8674(00)81387-2) [DOI] [PubMed] [Google Scholar]
- 92.Sakano H. 2020. Developmental regulation of olfactory circuit formation in mice. Dev. Growth Differ. 62, 199-213. ( 10.1111/dgd.12657) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ache BW, Young JM. 2005. Olfaction: diverse species, conserved principles. Neuron 48, 417-430. ( 10.1016/j.neuron.2005.10.022) [DOI] [PubMed] [Google Scholar]
- 94.Couto A, Alenius M, Dickson BJ. 2005. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr. Biol. 15, 1535-1547. ( 10.1016/j.cub.2005.07.034) [DOI] [PubMed] [Google Scholar]
- 95.Fishilevich E, Vosshall LB. 2005. Genetic and functional subdivision of the Drosophila antennal lobe. Curr. Biol. 15, 1548-1553. ( 10.1016/j.cub.2005.07.066) [DOI] [PubMed] [Google Scholar]
- 96.Grabe V, Baschwitz A, Dweck HKM, Lavista-Llanos S, Hansson BS, Sachse S. 2016. Elucidating the neuronal architecture of olfactory glomeruli in the Drosophila antennal lobe. Cell Rep. 16, 3401-3413. ( 10.1016/j.celrep.2016.08.063) [DOI] [PubMed] [Google Scholar]
- 97.Chou YH, Spletter ML, Yaksi E, Leong JC, Wilson RI, Luo L. 2010. Diversity and wiring variability of olfactory local interneurons in the Drosophila antennal lobe. Nat. Neurosci. 13, 439-449. ( 10.1038/nn.2489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jefferis GS, Marin EC, Stocker RF, Luo L. 2001. Target neuron prespecification in the olfactory map of Drosophila. Nature 414, 204-208. ( 10.1038/35102574) [DOI] [PubMed] [Google Scholar]
- 99.Jefferis GS, Potter CJ, Chan AM, Marin EC, Rohlfing T, Maurer CR Jr, Luo L. 2007. Comprehensive maps of Drosophila higher olfactory centers: spatially segregated fruit and pheromone representation. Cell 128, 1187-1203. ( 10.1016/j.cell.2007.01.040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marin EC, Jefferis GS, Komiyama T, Zhu H, Luo L. 2002. Representation of the glomerular olfactory map in the Drosophila brain. Cell 109, 243-255. ( 10.1016/S0092-8674(02)00700-6) [DOI] [PubMed] [Google Scholar]
- 101.Wong AM, Wang JW, Axel R. 2002. Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell 109, 229-241. ( 10.1016/S0092-8674(02)00707-9) [DOI] [PubMed] [Google Scholar]
- 102.Zheng Z, et al. 2018. A complete electron microscopy volume of the brain of adult Drosophila melanogaster. Cell 174, 730-743. ( 10.1016/j.cell.2018.06.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bates AS, et al. 2020. Complete connectomic reconstruction of olfactory projection neurons in the fly brain. Curr. Biol. 30, 3183-3199. ( 10.1016/j.cub.2020.06.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zheng Z, et al. 2022. Structured sampling of olfactory input by the fly mushroom body. Curr. Biol. 32, 3334-3349. ( 10.1016/j.cub.2022.06.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rybak J, Talarico G, Ruiz S, Arnold C, Cantera R, Hansson BS. 2016. Synaptic circuitry of identified neurons in the antennal lobe of Drosophila melanogaster. J. Comp. Neurol. 524, 1920-1956. ( 10.1002/cne.23966) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tobin WF, Wilson RI, Lee WA. 2017. Wiring variations that enable and constrain neural computation in a sensory microcircuit. Elife 6, e24838. ( 10.7554/eLife.24838) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chakraborty S Das, Sachse S. 2021. Olfactory processing in the lateral horn of Drosophila. Cell Tissue Res. 383, 113-123. ( 10.1007/s00441-020-03392-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Frechter S, Bates AS, Tootoonian S, Dolan MJ, Manton J, Jamasb AR, Kohl J, Bock D, Jefferis G. 2019. Functional and anatomical specificity in a higher olfactory centre. Elife 8, e44590. ( 10.7554/eLife.44590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dolan MJ, et al. 2019. Neurogenetic dissection of the Drosophila lateral horn reveals major outputs, diverse behavioural functions, and interactions with the mushroom body. Elife 8, e43079. ( 10.7554/eLife.43079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jeanne JM, Fisek M, Wilson RI. 2018. The organization of projections from olfactory glomeruli onto higher-order neurons. Neuron 98, 1198-1213. ( 10.1016/j.neuron.2018.05.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ruta V, Datta SR, Vasconcelos ML, Freeland J, Looger LL, Axel R. 2010. A dimorphic pheromone circuit in Drosophila from sensory input to descending output. Nature 468, 686-690. ( 10.1038/nature09554) [DOI] [PubMed] [Google Scholar]
- 112.Huoviala P, et al. 2020. Neural circuit basis of aversive odour processing in Drosophila from sensory input to descending output. bioRxiv, 394403. ( 10.1101/394403) [DOI]
- 113.Stevens CF. 2015. What the fly's nose tells the fly's brain. Proc. Natl Acad. Sci. USA 112, 9460-9465. ( 10.1073/pnas.1510103112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Caron SJ, Ruta V, Abbott LF, Axel R. 2013. Random convergence of olfactory inputs in the Drosophila mushroom body. Nature 497, 113-117. ( 10.1038/nature12063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lin HH, Lai JS, Chin AL, Chen YC, Chiang AS. 2007. A map of olfactory representation in the Drosophila mushroom body. Cell 128, 1205-1217. ( 10.1016/j.cell.2007.03.006) [DOI] [PubMed] [Google Scholar]
- 116.Tanaka NK, Awasaki T, Shimada T, Ito K. 2004. Integration of chemosensory pathways in the Drosophila second-order olfactory centers. Curr. Biol. 14, 449-457. ( 10.1016/j.cub.2004.03.006) [DOI] [PubMed] [Google Scholar]
- 117.Murthy M, Fiete I, Laurent G. 2008. Testing odor response stereotypy in the Drosophila mushroom body. Neuron 59, 1009-1023. ( 10.1016/j.neuron.2008.07.040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sayin S, et al. 2019. A neural circuit arbitrates between persistence and withdrawal in hungry Drosophila. Neuron 104, 544-558. ( 10.1016/j.neuron.2019.07.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dolan MJ, et al. 2018. Communication from learned to innate olfactory processing centers is required for memory retrieval in Drosophila. Neuron 100, 651-668. ( 10.1016/j.neuron.2018.08.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Galili DS, Jefferis G, Costa M. 2022. Connectomics and the neural basis of behaviour. Curr. Opin. Insect Sci. 54, 100968. ( 10.1016/j.cois.2022.100968) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Taisz I, et al. 2022. Generating parallel representations of position and identity in the olfactory system. bioRxiv, 2022.2005.2013.491877. ( 10.1101/2022.05.13.491877) [DOI]
- 122.Lienhard MC, Stocker RF. 1991. The development of the sensory neuron pattern in the antennal disc of wild-type and mutant (lz3, ssa) Drosophila melanogaster. Development 112, 1063-1075. ( 10.1242/dev.112.4.1063) [DOI] [PubMed] [Google Scholar]
- 123.Gupta BP, Rodrigues V. 1997. Atonal is a proneural gene for a subset of olfactory sense organs in Drosophila. Genes Cells 2, 225-233. ( 10.1046/j.1365-2443.1997.d01-312.x) [DOI] [PubMed] [Google Scholar]
- 124.Liou NF, et al. 2018. Diverse populations of local interneurons integrate into the Drosophila adult olfactory circuit. Nat. Commun. 9, 2232. ( 10.1038/s41467-018-04675-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang CJ, Tsai KT, Liou NF, Chou YH. 2019. Interneuron diversity: toward a better understanding of interneuron development in the olfactory system. J. Exp. Neurosci. 13, 1179069519826056. ( 10.1177/1179069519826056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Barish S, Volkan PC. 2015. Mechanisms of olfactory receptor neuron specification in Drosophila. Wiley Interdiscipl. Rev. Dev. Biol. 4, 609-621. ( 10.1002/wdev.197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rodrigues V, Hummel T. 2008. Development of the Drosophila olfactory system. Adv. Exp. Med. Biol. 628, 82-101. ( 10.1007/978-0-387-78261-4_6) [DOI] [PubMed] [Google Scholar]
- 128.Jefferis GS, Hummel T. 2006. Wiring specificity in the olfactory system. Semin. Cell Dev. Biol. 17, 50-65. ( 10.1016/j.semcdb.2005.12.002) [DOI] [PubMed] [Google Scholar]
- 129.Yan H, Jafari S, Pask G, Zhou X, Reinberg D, Desplan C. 2020. Evolution, developmental expression and function of odorant receptors in insects. J. Exp. Biol. 223, jeb208215. ( 10.1242/jeb.208215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chai PC, Cruchet S, Wigger L, Benton R. 2019. Sensory neuron lineage mapping and manipulation in the Drosophila olfactory system. Nat. Commun. 10, 643. ( 10.1038/s41467-019-08345-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.zur Lage PI, Prentice DR, Holohan EE, Jarman AP. 2003. The Drosophila proneural gene amos promotes olfactory sensillum formation and suppresses bristle formation. Development 130, 4683-4693. ( 10.1242/dev.00680) [DOI] [PubMed] [Google Scholar]
- 132.Goulding SE, zur Lage P, Jarman AP. 2000. amos, a proneural gene for Drosophila olfactory sense organs that is regulated by lozenge. Neuron 25, 69-78. ( 10.1016/S0896-6273(00)80872-7) [DOI] [PubMed] [Google Scholar]
- 133.Endo K, Karim MR, Taniguchi H, Krejci A, Kinameri E, Siebert M, Ito K, Bray SJ, Moore AW. 2011. Chromatin modification of Notch targets in olfactory receptor neuron diversification. Nat. Neurosci. 15, 224-233. ( 10.1038/nn.2998) [DOI] [PubMed] [Google Scholar]
- 134.Endo K, Aoki T, Yoda Y, Kimura K, Hama C. 2007. Notch signal organizes the Drosophila olfactory circuitry by diversifying the sensory neuronal lineages. Nat. Neurosci. 10, 153-160. ( 10.1038/nn1832) [DOI] [PubMed] [Google Scholar]
- 135.Li Q, Barish S, Okuwa S, Maciejewski A, Brandt AT, Reinhold D, Jones CD, Volkan PC. 2016. A functionally conserved gene regulatory network module governing olfactory neuron diversity. PLoS Genet. 12, e1005780. ( 10.1371/journal.pgen.1005780) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fuss SH, Ray A. 2009. Mechanisms of odorant receptor gene choice in Drosophila and vertebrates. Mol. Cell. Neurosci. 41, 101-112. ( 10.1016/j.mcn.2009.02.014) [DOI] [PubMed] [Google Scholar]
- 137.Ray A, van der Goes van Naters W, Shiraiwa T, Carlson JR.. 2007. Mechanisms of odor receptor gene choice in Drosophila. Neuron 53, 353-369. ( 10.1016/j.neuron.2006.12.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ray A, van der Goes van Naters W, Carlson J.. 2008. A regulatory code for neuron-specific odor receptor expression. PLoS Biol. 6, e125. ( 10.1371/journal.pbio.0060125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jafari S, Alkhori L, Schleiffer A, Brochtrup A, Hummel T, Alenius M. 2012. Combinatorial activation and repression by seven transcription factors specify Drosophila odorant receptor expression. PLoS Biol. 10, e1001280. ( 10.1371/journal.pbio.1001280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Arguello JR, Abuin L, Armida J, Mika K, Chai PC, Benton R. 2021. Targeted molecular profiling of rare olfactory sensory neurons identifies fate, wiring, and functional determinants. Elife 10, e63036. ( 10.7554/eLife.63036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McLaughlin CN, et al. 2021. Single-cell transcriptomes of developing and adult olfactory receptor neurons in Drosophila. Elife 10, e63856. ( 10.7554/eLife.63856) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yu HH, Kao CF, He Y, Ding P, Kao JC, Lee T. 2010. A complete developmental sequence of a Drosophila neuronal lineage as revealed by twin-spot MARCM. PLoS Biol. 8, e1000461. ( 10.1371/journal.pbio.1000461) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhu S, Lin S, Kao CF, Awasaki T, Chiang AS, Lee T. 2006. Gradients of the Drosophila Chinmo BTB-zinc finger protein govern neuronal temporal identity. Cell 127, 409-422. ( 10.1016/j.cell.2006.08.045) [DOI] [PubMed] [Google Scholar]
- 144.Kao CF, Yu HH, He Y, Kao JC, Lee T. 2012. Hierarchical deployment of factors regulating temporal fate in a diverse neuronal lineage of the Drosophila central brain. Neuron 73, 677-684. ( 10.1016/j.neuron.2011.12.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Brochtrup A, Hummel T. 2011. Olfactory map formation in the Drosophila brain: genetic specificity and neuronal variability. Curr. Opin. Neurobiol. 21, 85-92. ( 10.1016/j.conb.2010.11.001) [DOI] [PubMed] [Google Scholar]
- 146.Hong W, Luo L. 2014. Genetic control of wiring specificity in the fly olfactory system. Genetics 196, 17-29. ( 10.1534/genetics.113.154336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jefferis GS, Vyas RM, Berdnik D, Ramaekers A, Stocker RF, Tanaka NK, Ito K, Luo L. 2004. Developmental origin of wiring specificity in the olfactory system of Drosophila. Development 131, 117-130. ( 10.1242/dev.00896) [DOI] [PubMed] [Google Scholar]
- 148.Komiyama T, Sweeney LB, Schuldiner O, Garcia KC, Luo L. 2007. Graded expression of Semaphorin-1a cell-autonomously directs dendritic targeting of olfactory projection neurons. Cell 128, 399-410. ( 10.1016/j.cell.2006.12.028) [DOI] [PubMed] [Google Scholar]
- 149.Sweeney LB, Chou YH, Wu Z, Joo W, Komiyama T, Potter CJ, Kolodkin AL, Garcia KC, Luo L. 2011. Secreted semaphorins from degenerating larval ORN axons direct adult projection neuron dendrite targeting. Neuron 72, 734-747. ( 10.1016/j.neuron.2011.09.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lattemann M, Zierau A, Schulte C, Seidl S, Kuhlmann B, Hummel T. 2007. Semaphorin-1a controls receptor neuron-specific axonal convergence in the primary olfactory center of Drosophila. Neuron 53, 169-184. ( 10.1016/j.neuron.2006.12.024) [DOI] [PubMed] [Google Scholar]
- 151.Sweeney LB, Couto A, Chou YH, Berdnik D, Dickson BJ, Luo L, Komiyama T. 2007. Temporal target restriction of olfactory receptor neurons by Semaphorin-1a/PlexinA-mediated axon–axon interactions. Neuron 53, 185-200. ( 10.1016/j.neuron.2006.12.022) [DOI] [PubMed] [Google Scholar]
- 152.Hong W, Zhu H, Potter CJ, Barsh G, Kurusu M, Zinn K, Luo L. 2009. Leucine-rich repeat transmembrane proteins instruct discrete dendrite targeting in an olfactory map. Nat. Neurosci. 12, 1542-1550. ( 10.1038/nn.2442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Joo WJ, Sweeney LB, Liang L, Luo L. 2013. Linking cell fate, trajectory choice, and target selection: genetic analysis of Sema-2b in olfactory axon targeting. Neuron 78, 673-686. ( 10.1016/j.neuron.2013.03.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hummel T, Vasconcelos ML, Clemens JC, Fishilevich Y, Vosshall LB, Zipursky SL. 2003. Axonal targeting of olfactory receptor neurons in Drosophila is controlled by Dscam. Neuron 37, 221-231. ( 10.1016/S0896-6273(02)01183-2) [DOI] [PubMed] [Google Scholar]
- 155.Hummel T, Zipursky SL. 2004. Afferent induction of olfactory glomeruli requires N-cadherin. Neuron 42, 77-88. ( 10.1016/S0896-6273(04)00158-8) [DOI] [PubMed] [Google Scholar]
- 156.Zhu H, Hummel T, Clemens JC, Berdnik D, Zipursky SL, Luo L. 2006. Dendritic patterning by Dscam and synaptic partner matching in the Drosophila antennal lobe. Nat. Neurosci. 9, 349-355. ( 10.1038/nn1652) [DOI] [PubMed] [Google Scholar]
- 157.Zhu H, Luo L. 2004. Diverse functions of N-cadherin in dendritic and axonal terminal arborization of olfactory projection neurons. Neuron 42, 63-75. ( 10.1016/S0896-6273(04)00142-4) [DOI] [PubMed] [Google Scholar]
- 158.Hong W, Mosca TJ, Luo L. 2012. Teneurins instruct synaptic partner matching in an olfactory map. Nature 484, 201-207. ( 10.1038/nature10926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mosca TJ, Luo L. 2014. Synaptic organization of the antennal lobe and its regulation by the Teneurins. Elife 3, e03726. ( 10.7554/eLife.03726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li T, Fu TM, Wong KKL, Li H, Xie Q, Luginbuhl DJ, Wagner MJ, Betzig E, Luo L. 2021. Cellular bases of olfactory circuit assembly revealed by systematic time-lapse imaging. Cell 184, 5107-5121. ( 10.1016/j.cell.2021.08.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li H, et al. 2022. Fly Cell Atlas: a single-nucleus transcriptomic atlas of the adult fruit fly. Science 375, eabk2432. ( 10.1126/science.abk2432) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xie Q, et al. 2021. Temporal evolution of single-cell transcriptomes of Drosophila olfactory projection neurons. Elife 10, e63450. ( 10.7554/eLife.63450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li H, et al. 2020. Single-cell transcriptomes reveal diverse regulatory strategies for olfactory receptor expression and axon targeting. Curr. Biol. 30, 1189-1198. ( 10.1016/j.cub.2020.01.049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li H, Horns F, Wu B, Xie Q, Li J, Li T, Luginbuhl DJ, Quake SR, Luo L. 2017. Classifying Drosophila olfactory projection neuron subtypes by single-cell RNA sequencing. Cell 171, 1206-1220. ( 10.1016/j.cell.2017.10.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li J, et al. 2020. Cell-surface proteomic profiling in the fly brain uncovers wiring regulators. Cell 180, 373-386. ( 10.1016/j.cell.2019.12.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xie Q, et al. 2022. Transcription factor Acj6 controls dendrite targeting via a combinatorial cell-surface code. Neuron 110, 2299-2314. ( 10.1016/j.neuron.2022.04.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Grabe V, Sachse S. 2018. Fundamental principles of the olfactory code. Biosystems 164, 94-101. ( 10.1016/j.biosystems.2017.10.010) [DOI] [PubMed] [Google Scholar]
- 168.Wilson RI. 2013. Early olfactory processing in Drosophila: mechanisms and principles. Annu. Rev. Neurosci. 36, 217-241. ( 10.1146/annurev-neuro-062111-150533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. 2003. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell 112, 271-282. ( 10.1016/S0092-8674(03)00004-7) [DOI] [PubMed] [Google Scholar]
- 170.Ng M, Roorda RD, Lima SQ, Zemelman BV, Morcillo P, Miesenbock G. 2002. Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron 36, 463-474. ( 10.1016/S0896-6273(02)00975-3) [DOI] [PubMed] [Google Scholar]
- 171.Galizia CG, Sachse S, Rappert A, Menzel R. 1999. The glomerular code for odor representation is species specific in the honeybee Apis mellifera. Nat. Neurosci. 2, 473-478. ( 10.1038/8144) [DOI] [PubMed] [Google Scholar]
- 172.Sachse S, Rappert A, Galizia CG. 1999. The spatial representation of chemical structures in the antennal lobe of honeybees: steps towards the olfactory code. Eur. J. Neurosci. 11, 3970-3982. ( 10.1046/j.1460-9568.1999.00826.x) [DOI] [PubMed] [Google Scholar]
- 173.Malnic B, Hirono J, Sato T, Buck LB. 1999. Combinatorial receptor codes for odors. Cell 96, 713-723. ( 10.1016/S0092-8674(00)80581-4) [DOI] [PubMed] [Google Scholar]
- 174.Su CY, Menuz K, Reisert J, Carlson JR. 2012. Non-synaptic inhibition between grouped neurons in an olfactory circuit. Nature 492, 66-71. ( 10.1038/nature11712) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang Y, Tsang TK, Bushong EA, Chu LA, Chiang AS, Ellisman MH, Reingruber J, Su CY. 2019. Asymmetric ephaptic inhibition between compartmentalized olfactory receptor neurons. Nat. Commun. 10, 1560. ( 10.1038/s41467-019-09346-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Su CY, Martelli C, Emonet T, Carlson JR. 2011. Temporal coding of odor mixtures in an olfactory receptor neuron. Proc. Natl Acad. Sci. USA 108, 5075-5080. ( 10.1073/pnas.1100369108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gorur-Shandilya S, Demir M, Long J, Clark DA, Emonet T. 2017. Olfactory receptor neurons use gain control and complementary kinetics to encode intermittent odorant stimuli. Elife 6, e27670. ( 10.7554/eLife.27670) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Keller A, Vosshall LB. 2007. Influence of odorant receptor repertoire on odor perception in humans and fruit flies. Proc. Natl Acad. Sci. USA. 104, 5614-5619. ( 10.1073/pnas.0605321104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bell JS, Wilson RI. 2016. Behavior reveals selective summation and max pooling among olfactory processing channels. Neuron 91, 425-438. ( 10.1016/j.neuron.2016.06.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fishilevich E, Domingos AI, Asahina K, Naef F, Vosshall LB, Louis M. 2005. Chemotaxis behavior mediated by single larval olfactory neurons in Drosophila. Curr. Biol. 15, 2086-2096. ( 10.1016/j.cub.2005.11.016) [DOI] [PubMed] [Google Scholar]
- 181.Semmelhack JL, Wang JW. 2009. Select Drosophila glomeruli mediate innate olfactory attraction and aversion. Nature 459, 218-223. ( 10.1038/nature07983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Haverkamp A, Hansson BS, Knaden M. 2018. Combinatorial codes and labeled lines: how insects use olfactory cues to find and judge food, mates, and oviposition sites in complex environments. Front. Physiol. 9, 49. ( 10.3389/fphys.2018.00049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Fiala A, Spall T, Diegelmann S, Eisermann B, Sachse S, Devaud JM, Buchner E, Galizia CG. 2002. Genetically expressed cameleon in Drosophila melanogaster is used to visualize olfactory information in projection neurons. Curr. Biol. 12, 1877-1884. ( 10.1016/S0960-9822(02)01239-3) [DOI] [PubMed] [Google Scholar]
- 184.Wilson RI, Turner GC, Laurent G. 2004. Transformation of olfactory representations in the Drosophila antennal lobe. Science 303, 366-370. ( 10.1126/science.1090782) [DOI] [PubMed] [Google Scholar]
- 185.Root CM, Semmelhack JL, Wong AM, Flores J, Wang JW. 2007. Propagation of olfactory information in Drosophila. Proc. Natl Acad. Sci. USA 104, 11826-11 831. ( 10.1073/pnas.0704523104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kazama H, Wilson RI. 2008. Homeostatic matching and nonlinear amplification at identified central synapses. Neuron 58, 401-413. ( 10.1016/j.neuron.2008.02.030). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bhandawat V, Olsen SR, Gouwens NW, Schlief ML, Wilson RI. 2007. Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations. Nat. Neurosci. 10, 1474-1482. ( 10.1038/nn1976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wilson RI, Laurent G. 2005. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J. Neurosci. 25, 9069-9079. ( 10.1523/JNEUROSCI.2070-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Olsen SR, Bhandawat V, Wilson RI. 2010. Divisive normalization in olfactory population codes. Neuron 66, 287-299. ( 10.1016/j.neuron.2010.04.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Silbering AF, Okada R, Ito K, Galizia CG. 2008. Olfactory information processing in the Drosophila antennal lobe: anything goes? J. Neurosci. 28, 13 075-13 087. ( 10.1523/JNEUROSCI.2973-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Shang Y, Claridge-Chang A, Sjulson L, Pypaert M, Miesenbock G. 2007. Excitatory local circuits and their implications for olfactory processing in the fly antennal lobe. Cell 128, 601-612. ( 10.1016/j.cell.2006.12.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Olsen SR, Bhandawat V, Wilson RI. 2007. Excitatory interactions between olfactory processing channels in the Drosophila antennal lobe. Neuron 54, 89-103. ( 10.1016/j.neuron.2007.03.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Root CM, Ko KI, Jafari A, Wang JW. 2011. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell 145, 133-144. ( 10.1016/j.cell.2011.02.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ko KI, Root CM, Lindsay SA, Zaninovich OA, Shepherd AK, Wasserman SA, Kim SM, Wang JW. 2015. Starvation promotes concerted modulation of appetitive olfactory behavior via parallel neuromodulatory circuits. Elife 4, e08298. ( 10.7554/eLife.08298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ignell R, Root CM, Birse RT, Wang JW, Nassel DR, Winther AM. 2009. Presynaptic peptidergic modulation of olfactory receptor neurons in Drosophila. Proc. Natl Acad. Sci. USA 106, 13 070-13 075. ( 10.1073/pnas.0813004106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Carlsson MA, Diesner M, Schachtner J, Nassel DR. 2010. Multiple neuropeptides in the Drosophila antennal lobe suggest complex modulatory circuits. J. Comp. Neurol. 518, 3359-3380. ( 10.1002/cne.22405) [DOI] [PubMed] [Google Scholar]
- 197.Strutz A, et al. 2014. Decoding odor quality and intensity in the Drosophila brain. Elife 3, e04147. ( 10.7554/eLife.04147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Farhan A, Gulati J, Grobetae-Wilde E, Vogel H, Hansson BS, Knaden M. 2013. The CCHamide 1 receptor modulates sensory perception and olfactory behavior in starved Drosophila. Sci. Rep. 3, 2765. ( 10.1038/srep02765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Vogt K, et al. 2021. Internal state configures olfactory behavior and early sensory processing in Drosophila larvae. Sci. Adv. 7, abd6900. ( 10.1126/sciadv.abd6900) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Liu TX, Davoudian PA, Lizbinski KM, Jeanne JM. 2022. Connectomic features underlying diverse synaptic connection strengths and subcellular computation. Curr. Biol. 32, 559-569. ( 10.1016/j.cub.2021.11.056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Perez-Orive J, Mazor O, Turner GC, Cassenaer S, Wilson RI, Laurent G. 2002. Oscillations and sparsening of odor representations in the mushroom body. Science 297, 359-365. ( 10.1126/science.1070502) [DOI] [PubMed] [Google Scholar]
- 202.Honegger KS, Campbell RA, Turner GC. 2011. Cellular-resolution population imaging reveals robust sparse coding in the Drosophila mushroom body. J. Neurosci. 31, 11 772-11 785. ( 10.1523/JNEUROSCI.1099-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Turner GC, Bazhenov M, Laurent G. 2008. Olfactory representations by Drosophila mushroom body neurons. J. Neurophysiol. 99, 734-746. ( 10.1152/jn.01283.2007) [DOI] [PubMed] [Google Scholar]
- 204.Endo K, Tsuchimoto Y, Kazama H. 2020. Synthesis of conserved odor object representations in a random, divergent–convergent network. Neuron 108, 367-381. ( 10.1016/j.neuron.2020.07.029) [DOI] [PubMed] [Google Scholar]
- 205.Benton R. 2007. Sensitivity and specificity in Drosophila pheromone perception. Trends Neurosci. 30, 512-519. ( 10.1016/j.tins.2007.07.004) [DOI] [PubMed] [Google Scholar]
- 206.Kurtovic A, Widmer A, Dickson BJ. 2007. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature 446, 542-546. ( 10.1038/nature05672) [DOI] [PubMed] [Google Scholar]
- 207.Ha TS, Smith DP. 2006. A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. J. Neurosci. 26, 8727-8733. ( 10.1523/JNEUROSCI.0876-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Datta SR, et al. 2008. The Drosophila pheromone cVA activates a sexually dimorphic neural circuit. Nature 452, 473-477. ( 10.1038/nature06808) [DOI] [PubMed] [Google Scholar]
- 209.Stockinger P, Kvitsiani D, Rotkopf S, Tirian L, Dickson BJ. 2005. Neural circuitry that governs Drosophila male courtship behavior. Cell 121, 795-807. ( 10.1016/j.cell.2005.04.026) [DOI] [PubMed] [Google Scholar]
- 210.Auer T, Benton R. 2016. Sexual circuitry in Drosophila. Curr. Opin Neurobiol. 38, 18-26. ( 10.1016/j.conb.2016.01.004) [DOI] [PubMed] [Google Scholar]
- 211.Clowney EJ, Iguchi S, Bussell JJ, Scheer E, Ruta V. 2015. Multimodal chemosensory circuits controlling male courtship in Drosophila. Neuron 87, 1036-1049. ( 10.1016/j.neuron.2015.07.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kohl J, Ostrovsky AD, Frechter S, Jefferis GS. 2013. A bidirectional circuit switch reroutes pheromone signals in male and female brains. Cell 155, 1610-1623. ( 10.1016/j.cell.2013.11.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Keleman K, Vrontou E, Kruttner S, Yu JY, Kurtovic-Kozaric A, Dickson BJ. 2012. Dopamine neurons modulate pheromone responses in Drosophila courtship learning. Nature 489, 145-149. ( 10.1038/nature11345) [DOI] [PubMed] [Google Scholar]
- 214.van Breugel F, Dickinson MH.. 2014. Plume-tracking behavior of flying Drosophila emerges from a set of distinct sensory–motor reflexes. Curr. Biol. 24, 274-286. ( 10.1016/j.cub.2013.12.023) [DOI] [PubMed] [Google Scholar]
- 215.Zhang B, Freeman MR, Waddell S. 2010. Drosophila neurobiology: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- 216.Branson K, Robie AA, Bender J, Perona P, Dickinson MH. 2009. High-throughput ethomics in large groups of Drosophila. Nat. Methods 6, 451-457. ( 10.1038/nmeth.1328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Egnor SE, Branson K. 2016. Computational analysis of behavior. Annu. Rev. Neurosci. 39, 217-236. ( 10.1146/annurev-neuro-070815-013845) [DOI] [PubMed] [Google Scholar]
- 218.Kabra M, Robie AA, Rivera-Alba M, Branson S, Branson K. 2013. JAABA: interactive machine learning for automatic annotation of animal behavior. Nat. Methods 10, 64-67. ( 10.1038/nmeth.2281) [DOI] [PubMed] [Google Scholar]
- 219.Louis M, Huber T, Benton R, Sakmar TP, Vosshall LB. 2008. Bilateral olfactory sensory input enhances chemotaxis behavior. Nat. Neurosci. 11, 187-199. ( 10.1038/nn2031) [DOI] [PubMed] [Google Scholar]
- 220.Demir M, Kadakia N, Anderson HD, Clark DA, Emonet T. 2020. Walking Drosophila navigate complex plumes using stochastic decisions biased by the timing of odor encounters. Elife 9, e57524. ( 10.7554/eLife.57524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Steck K, Veit D, Grandy R, Badia SB, Mathews Z, Verschure P, Hansson BS, Knaden M. 2012. A high-throughput behavioral paradigm for Drosophila olfaction—the Flywalk. Sci. Rep. 2, 361. ( 10.1038/srep00361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Alvarez-Salvado E, Licata AM, Connor EG, McHugh MK, King BM, Stavropoulos N, Victor JD, Crimaldi JP, Nagel KI. 2018. Elementary sensory–motor transformations underlying olfactory navigation in walking fruit-flies. Elife 7, e37815. ( 10.7554/eLife.37815) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Gorur-Shandilya S, Martelli C, Demir M, Emonet T. 2019. Controlling and measuring dynamic odorant stimuli in the laboratory. J. Exp. Biol. 222, jeb.207787. ( 10.1242/jeb.207787) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Matheson AMM, Lanz AJ, Medina AM, Licata AM, Currier TA, Syed MH, Nagel KI. 2022. A neural circuit for wind-guided olfactory navigation. Nat. Commun. 13, 4613. ( 10.1038/s41467-022-32247-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Krishnan P, Duistermars BJ, Frye MA. 2011. Odor identity influences tracking of temporally patterned plumes in Drosophila. BMC Neurosci. 12, 62. ( 10.1186/1471-2202-12-62). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wang L, Anderson DJ. 2010. Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature 463, 227-231. ( 10.1038/nature08678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ronderos DS, Lin CC, Potter CJ, Smith DP. 2014. Farnesol-detecting olfactory neurons in Drosophila. J. Neurosci. 34, 3959-3968. ( 10.1523/JNEUROSCI.4582-13.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Grosjean Y, Rytz R, Farine JP, Abuin L, Cortot J, Jefferis GS, Benton R. 2011. An olfactory receptor for food-derived odours promotes male courtship in Drosophila. Nature 478, 236-240. ( 10.1038/nature10428) [DOI] [PubMed] [Google Scholar]
- 229.Ai M, Min S, Grosjean Y, Leblanc C, Bell R, Benton R, Suh GS. 2010. Acid sensing by the Drosophila olfactory system. Nature 468, 691-695. ( 10.1038/nature09537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Stensmyr MC, et al. 2012. A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell 151, 1345-1357. ( 10.1016/j.cell.2012.09.046) [DOI] [PubMed] [Google Scholar]
- 231.Chin SG, Maguire SE, Huoviala P, Jefferis G, Potter CJ. 2018. Olfactory neurons and brain centers directing oviposition decisions in Drosophila. Cell Rep. 24, 1667-1678. ( 10.1016/j.celrep.2018.07.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Tumkaya T, Burhanudin S, Khalilnezhad A, Stewart J, Choi H, Claridge-Chang A. 2022. Most primary olfactory neurons have individually neutral effects on behavior. Elife 11, e71238. ( 10.7554/eLife.71238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Das Chakraborty S, Chang H, Hansson BS, Sachse S. 2022. Higher-order olfactory neurons in the lateral horn support odor valence and odor identity coding in Drosophila. Elife 11, e74637. ( 10.7554/eLife.74637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Tanaka NK, Hirao T, Chida H, Ejima A. 2021. A sexually dimorphic olfactory neuron mediates fixed action transition during courtship ritual in Drosophila melanogaster. J. Neurosci. 41, 9732-9741. ( 10.1523/JNEUROSCI.1168-21.2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Honegger KS, Smith MA, Churgin MA, Turner GC, de Bivort BL.. 2019. Idiosyncratic neural coding and neuromodulation of olfactory individuality in Drosophila. Proc. Natl Acad. Sci. USA 117, 23 292-23 297. ( 10.1073/pnas.1901623116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Rihani K, Sachse S. 2022. Shedding light on inter-individual variability of olfactory circuits in Drosophila. Front. Behav. Neurosci. 16, 835680. ( 10.3389/fnbeh.2022.835680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hiesinger PR, Hassan BA. 2018. The evolution of variability and robustness in neural development. Trends Neurosci. 41, 577-586. ( 10.1016/j.tins.2018.05.007) [DOI] [PubMed] [Google Scholar]
- 238.Churgin MA, Lavrentovich D, Smith MA, Gao R, Boyden E, de Bivort B.2021. Neural correlates of individual odor preference in Drosophila. bioRxiv, 2021.2012.2024.474127. ( ) [DOI]
- 239.Junca P, Stanley M, Musso P-Y, Gordon MD. 2021. Modulation of taste sensitivity by the olfactory system in Drosophila. bioRxiv, 2021.2003.2030.437740. ( 10.1101/2021.03.30.437740) [DOI]
- 240.Lushchak OV, Carlsson MA, Nassel DR. 2015. Food odors trigger an endocrine response that affects food ingestion and metabolism. Cell. Mol. Life Sci. 72, 3143-3155. ( 10.1007/s00018-015-1884-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Madhwal S, Shin M, Kapoor A, Goyal M, Joshi MK, Ur Rehman PM, Gor K, Shim J, Mukherjee T. 2020. Metabolic control of cellular immune-competency by odors in Drosophila. Elife 9, e60376. ( 10.7554/eLife.60376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Shim J, Mukherjee T, Mondal BC, Liu T, Young GC, Wijewarnasuriya DP, Banerjee U. 2013. Olfactory control of blood progenitor maintenance. Cell 155, 1141-1153. ( 10.1016/j.cell.2013.10.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Libert S, Zwiener J, Chu X, Vanvoorhies W, Roman G, Pletcher SD. 2007. Regulation of Drosophila life span by olfaction and food-derived odors. Science 315, 1133-1137. ( 10.1126/science.1136610) [DOI] [PubMed] [Google Scholar]
- 244.Markow TA. 2015. The secret lives of Drosophila flies. Elife 4, e06793. ( 10.7554/eLife.06793). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Keller A. 2007. Drosophila melanogaster's history as a human commensal. Curr. Biol. 17, R77-R81. ( 10.1016/j.cub.2006.12.031) [DOI] [PubMed] [Google Scholar]
- 246.Mansourian S, Stensmyr MC. 2015. The chemical ecology of the fly. Curr. Opin. Neurobiol . 34C, 95-102. ( 10.1016/j.conb.2015.02.006) [DOI] [PubMed] [Google Scholar]
- 247.Yanagawa A, Chabaud MA, Imai T, Marion-Poll F. 2018. Olfactory cues play a significant role in removing fungus from the body surface of Drosophila melanogaster. J. Invertebr. Pathol. 151, 144-150. ( 10.1016/j.jip.2017.11.011) [DOI] [PubMed] [Google Scholar]
- 248.Ebrahim SA, et al. 2015. Drosophila avoids parasitoids by sensing their semiochemicals via a dedicated olfactory circuit. PLoS Biol. 13, e1002318. ( 10.1371/journal.pbio.1002318) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lebreton S, et al. 2017. A Drosophila female pheromone elicits species-specific long-range attraction via an olfactory channel with dual specificity for sex and food. BMC Biol. 15, 88. ( 10.1186/s12915-017-0427-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Dukas R. 2020. Natural history of social and sexual behavior in fruit flies. Sci. Rep. 10, 21932. ( 10.1038/s41598-020-79075-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Soto-Yeber L, Soto-Ortiz J, Godoy P, Godoy-Herrera R. 2018. The behavior of adult Drosophila in the wild. PLoS ONE 13, e0209917. ( 10.1371/journal.pone.0209917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Stökl J, Strutz A, Dafni A, Svatos A, Doubsky J, Knaden M, Sachse S, Hansson BS, Stensmyr MC. 2010. A deceptive pollination system targeting drosophilids through olfactory mimicry of yeast. Curr. Biol. 20, 1846-1852. ( 10.1016/j.cub.2010.09.033) [DOI] [PubMed] [Google Scholar]
- 253.Mansourian S, Enjin A, Jirle EV, Ramesh V, Rehermann G, Becher PG, Pool JE, Stensmyr MC. 2018. Wild African Drosophila melanogaster are seasonal specialists on marula fruit. Curr. Biol. 28, 3960-3968. ( 10.1016/j.cub.2018.10.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Ramdya P, Benton R. 2010. Evolving olfactory systems on the fly. Trends Genet. 26, 307-316. ( 10.1016/j.tig.2010.04.004) [DOI] [PubMed] [Google Scholar]
- 255.Zhao Z, McBride CS. 2020. Evolution of olfactory circuits in insects. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 206, 353-367. ( 10.1007/s00359-020-01399-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Hansson BS, Stensmyr MC. 2011. Evolution of insect olfaction. Neuron 72, 698-711. ( 10.1016/j.neuron.2011.11.003) [DOI] [PubMed] [Google Scholar]
- 257.Auer TO, Shahandeh MP, Benton R. 2021. Drosophila sechellia: a genetic model for behavioral evolution and neuroecology. Annu. Rev. Genet. 55, 527-554. ( 10.1146/annurev-genet-071719-020719) [DOI] [PubMed] [Google Scholar]
- 258.Rollmann SM, Wang P, Date P, West SA, Mackay TF, Anholt RR. 2010. Odorant receptor polymorphisms and natural variation in olfactory behavior in Drosophila melanogaster. Genetics 186, 687-697. ( 10.1534/genetics.110.119446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Shaw KH, Dent CI, Johnson TK, Anderson A, de Bruyne M, Warr CG.. 2021. Natural variation at the Drosophila melanogaster Or22 odorant receptor locus is associated with changes in olfactory behaviour. Open Biol. 11, 210158. ( 10.1098/rsob.210158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Shaw KH, Johnson TK, Anderson A, de Bruyne M, Warr CG. 2019. Molecular and functional evolution at the odorant receptor Or22 locus in Drosophila melanogaster. Mol. Biol. Evol. 36, 919-929 ( 10.1093/molbev/msz018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Ibba I, Angioy AM, Hansson BS, Dekker T. 2010. Macroglomeruli for fruit odors change blend preference in Drosophila. Naturwissenschaften 97, 1059-1066. ( 10.1007/s00114-010-0727-2) [DOI] [PubMed] [Google Scholar]
- 262.Dekker T, Ibba I, Siju KP, Stensmyr MC, Hansson BS. 2006. Olfactory shifts parallel superspecialism for toxic fruit in Drosophila melanogaster sibling D. sechellia. Curr. Biol. 16, 101-109. ( 10.1016/j.cub.2005.11.075) [DOI] [PubMed] [Google Scholar]
- 263.Keesey IW, Zhang J, Depetris-Chauvin A, Obiero GF, Gupta A, Gupta N, Vogel H, Knaden M, Hansson BS. 2019. Functional olfactory evolution in Drosophila suzukii and the subgenus Sophophora. iScience 25, 104212. ( 10.1016/j.isci.2022.104212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Crava CM, Sassu F, Tait G, Becher PG, Anfora G. 2019. Functional transcriptome analyses of Drosophila suzukii antennae reveal mating-dependent olfaction plasticity in females. Insect. Biochem. Mol. Biol. 105, 51-59. ( 10.1016/j.ibmb.2018.12.012) [DOI] [PubMed] [Google Scholar]
- 265.Karageorgi M, Bracker LB, Lebreton S, Minervino C, Cavey M, Siju KP, Grunwald Kadow IC, Gompel N, Prud'homme B. 2017. Evolution of multiple sensory systems drives novel egg-laying behavior in the fruit pest Drosophila suzukii. Curr. Biol. 27, 847-853. ( 10.1016/j.cub.2017.01.055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Hickner PV, Rivaldi CL, Johnson CM, Siddappaji M, Raster GJ, Syed Z. 2016. The making of a pest: Insights from the evolution of chemosensory receptor families in a pestiferous and invasive fly, Drosophila suzukii. BMC Genom. 17, 648. ( 10.1186/s12864-016-2983-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Keesey IW, Knaden M, Hansson BS. 2015. Olfactory specialization in Drosophila suzukii supports an ecological shift in host preference from rotten to fresh fruit. J. Chem. Ecol. 41, 121-128. ( 10.1007/s10886-015-0544-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Matsunaga T, Reisenman CE, Goldman-Huertas B, Brand P, Miao K, Suzuki HC, Verster KI, Ramirez SR, Whiteman NK. 2022. Evolution of olfactory receptors tuned to mustard oils in herbivorous Drosophilidae. Mol. Biol. Evol. 39, msab362. ( 10.1093/molbev/msab362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Linz J, Baschwitz A, Strutz A, Dweck HK, Sachse S, Hansson BS, Stensmyr MC. 2013. Host plant-driven sensory specialization in Drosophila erecta. Proc. R. Soc. B 280, 20130626. ( 10.1098/rspb.2013.0626) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Auer TO, Alvarez-Ocana R, Cruchet S, Benton R, Arguello JR. 2022. Copy number changes in co-expressed odorant receptor genes enable selection for sensory differences in drosophilid species. Nat. Ecol. Evol. 6, 1343-1353. ( 10.1038/s41559-022-01830-y). [DOI] [PubMed] [Google Scholar]
- 271.Dekker T, Revadi S, Mansourian S, Ramasamy S, Lebreton S, Becher PG, Angeli S, Rota-Stabelli O, Anfora G. 2015. Loss of Drosophila pheromone reverses its role in sexual communication in Drosophila suzukii. Proc. R. Soc. B 282, 20143018. ( 10.1098/rspb.2014.3018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Seeholzer LF, Seppo M, Stern DL, Ruta V. 2018. Evolution of a central neural circuit underlies Drosophila mate preferences. Nature 559, 564-569. ( 10.1038/s41586-018-0322-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Prieto-Godino LL, Silbering AF, Khallaf MA, Cruchet S, Bojkowska K, Pradervand S, Hansson BS, Knaden M, Benton R. 2020. Functional integration of ‘undead’ neurons in the olfactory system. Sci. Adv. 6, eaaz7238. ( 10.1126/sciadv.aaz7238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Khallaf MA, et al. 2020. Mate discrimination among subspecies through a conserved olfactory pathway. Sci. Adv. 6, eaba5279. ( 10.1126/sciadv.aba5279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Khallaf MA, Cui R, Weissflog J, Erdogmus M, Svatos A, Dweck HKM, Valenzano DR, Hansson BS, Knaden M. 2021. Large-scale characterization of sex pheromone communication systems in Drosophila. Nat. Commun. 12, 4165. ( 10.1038/s41467-021-24395-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Stern DL, et al. 2017. Genetic and transgenic reagents for Drosophila simulans D. mauritiana D. yakuba D. santomea and D. virilis. G3 (Bethesda) 7, 1339-1347. ( 10.1534/g3.116.038885) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Fandino RA, et al. 2019. Mutagenesis of odorant coreceptor Orco fully disrupts foraging but not oviposition behaviors in the hawkmoth Manduca sexta. Proc. Natl Acad. Sci. USA 116, 15677-15 685. ( 10.1073/pnas.1902089116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Shiota Y, Sakurai T, Ando N, Haupt SS, Mitsuno H, Daimon T, Kanzaki R. 2021. Pheromone binding protein is involved in temporal olfactory resolution in the silkmoth. iScience 24, 103334. ( 10.1016/j.isci.2021.103334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Sakurai T, et al. 2011. A single sex pheromone receptor determines chemical response specificity of sexual behavior in the silkmoth Bombyx mori. PLoS Genet. 7, e1002115. ( 10.1371/journal.pgen.1002115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Riabinina O, Task D, Marr E, Lin CC, Alford R, O'Brochta DA, Potter CJ. 2016. Organization of olfactory centres in the malaria mosquito Anopheles gambiae. Nat. Commun. 7, 13010. ( 10.1038/ncomms13010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Degennaro M, McBride CS, Seeholzer L, Nakagawa T, Dennis EJ, Goldman C, Jasinskiene N, James AA, Vosshall LB. 2013. orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature 498, 487-491. ( 10.1038/nature12206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Yan H, et al. 2017. An engineered orco mutation produces aberrant social behavior and defective neural development in ants. Cell 170, 736-747. ( 10.1016/j.cell.2017.06.051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Trible W, Olivos-Cisneros L, McKenzie SK, Saragosti J, Chang NC, Matthews BJ, Oxley PR, Kronauer DJC. 2017. orco mutagenesis causes loss of antennal lobe glomeruli and impaired social behavior in ants. Cell 170, 727-735. ( 10.1016/j.cell.2017.07.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Carey AF, Carlson JR. 2011. Insect olfaction from model systems to disease control. PNAS 108, 12987-12 995. ( 10.1073/pnas.1103472108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Clark JT, Ray A. 2016. Olfactory mechanisms for discovery of odorants to reduce insect–host contact. J. Chem. Ecol. 42, 919-930. ( 10.1007/s10886-016-0770-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Montagne N, de Fouchier A, Newcomb RD, Jacquin-Joly E.. 2015. Advances in the identification and characterization of olfactory receptors in insects. Prog. Mol. Biol. Transl. Sci. 130, 55-80. ( 10.1016/bs.pmbts.2014.11.003) [DOI] [PubMed] [Google Scholar]
- 287.Venthur H, Zhou JJ. 2018. Odorant receptors and odorant-binding proteins as insect pest control targets: a comparative analysis. Front. Physiol. 9, 1163. ( 10.3389/fphys.2018.01163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Hallem EA, Nicole Fox A, Zwiebel LJ, Carlson JR. 2004. Olfaction: mosquito receptor for human-sweat odorant. Nature 427, 212-213. ( 10.1038/427212a) [DOI] [PubMed] [Google Scholar]
- 289.de Fouchier A, et al. 2017. Functional evolution of Lepidoptera olfactory receptors revealed by deorphanization of a moth repertoire. Nat. Commun. 8, 15709. ( 10.1038/ncomms15709) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Jones PL, Pask GM, Rinker DC, Zwiebel LJ. 2011. Functional agonism of insect odorant receptor ion channels. Proc. Natl Acad. Sci. USA 108, 8821-8825. ( 10.1073/pnas.1102425108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Kythreoti G, et al. et al. 2021. Volatile allosteric antagonists of mosquito odorant receptors inhibit human-host attraction. J. Biol. Chem. 296, 100172. ( 10.1074/jbc.RA120.016557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Choo YM, Xu P, Hwang JK, Zeng F, Tan K, Bhagavathy G, Chauhan KR, Leal WS. 2018. Reverse chemical ecology approach for the identification of an oviposition attractant for Culex quinquefasciatus. Proc. Natl Acad. Sci. USA 115, 714-719. ( 10.1073/pnas.1718284115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Taylor RW, Romaine IM, Liu C, Murthi P, Jones PL, Waterson AG, Sulikowski GA, Zwiebel LJ. 2012. Structure–activity relationship of a broad-spectrum insect odorant receptor agonist. ACS Chem. Biol. 7, 1647-1652. ( 10.1021/cb300331z) [DOI] [PubMed] [Google Scholar]
- 294.Kepchia D, Xu P, Terryn R, Castro A, Schurer SC, Leal WS, Luetje CW. 2019. Use of machine learning to identify novel, behaviorally active antagonists of the insect odorant receptor co-receptor (Orco) subunit. Sci. Rep. 9, 4055. ( 10.1038/s41598-019-40640-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Boyle SM, McInally S, Ray A. 2013. Expanding the olfactory code by in silico decoding of odor-receptor chemical space. Elife 2, e01120. ( 10.7554/eLife.01120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Caballero-Vidal G, Bouysset C, Grunig H, Fiorucci S, Montagne N, Golebiowski J, Jacquin-Joly E. 2020. Machine learning decodes chemical features to identify novel agonists of a moth odorant receptor. Sci. Rep. 10, 1655. ( 10.1038/s41598-020-58564-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Kepchia D, Moliver S, Chohan K, Phillips C, Luetje CW. 2017. Inhibition of insect olfactory behavior by an airborne antagonist of the insect odorant receptor co-receptor subunit. PLoS ONE 12, e0177454. ( 10.1371/journal.pone.0177454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Sharma A, Sandhi RK, Reddy GVP. 2019. A review of interactions between insect biological control agents and semiochemicals. Insects 10, 439. ( 10.3390/insects10120439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Cook SM, Khan ZR, Pickett JA. 2007. The use of push–pull strategies in integrated pest management. Annu. Rev. Entomol. 52, 375-400. ( 10.1146/annurev.ento.52.110405.091407) [DOI] [PubMed] [Google Scholar]
- 300.Murugathas T, Hamiaux C, Colbert D, Kralicek AV, Plank NOV, Carraher C. 2020. Evaluating insect odorant receptor display formats for biosensing using graphene field effect transistors. ACS Appl. Electron. Mater. 2, 3610-3617. ( 10.1021/acsaelm.0c00677) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.