Abstract
DTNBP1 is a gene associated with schizophrenia. Postmortem studies found a reduced expression of DTNBP1 in regions associated with schizophrenia in patients’ brains. Sandy (Sdy) mice have a loss-of-function mutation in Dtnbp1 gene, resulting in behavioral deficits and brain changes similar to those seen in patients with schizophrenia. We previously showed that exposing adult Sdy mice to circadian disruption led to an exacerbation of schizophrenia-relevant behaviors. Here we asked whether the interaction between this genetic risk factor and circadian disruption occurs during adolescence, a period when environmental insults can promote schizophrenia symptoms, and whether sex affects this interaction. Starting at postnatal day 21, wild-type (WT) and Sdy males and females were housed for 4 weeks either in a 12 h light:12 h dark (LD 12:12) cycle or under chronic jetlag (CJL). Then, after 2 weeks in LD 12:12, behavioral assessments were conducted, including elevated plus maze (EPM), novel object recognition (NOR), social interaction, and prepulse inhibition (PPI) of acoustic startle. NOR and social novelty tests showed that, surprisingly, CJL during adolescence had opposite effects on WT and Sdy males, that is, behavioral deficits in WT males while rescuing preexisting deficits in Sdy mice. CJL led to decreased sociability in WT and Sdy mice while decreasing PPI only in females. Sdy mice showed decreased anxiety-like behavior compared with wild-type (WT), which was further accentuated by CJL in males. Thus, circadian disruption during adolescence, on its own or in association with Dtnbp1 mutation, can influence cognition, sociability, sensorimotor gating, and anxiety-like behaviors in a sex-dependent manner.
Keywords: circadian disruption, schizophrenia, adolescence, Sandy mice, chronic jetlag, behavior
Schizophrenia is a neurodevelopmental disorder with symptoms including hallucinations, delusion, social and emotional deficits, as well as cognitive impairments (McCutcheon et al., 2020). Adolescence is a critical period in the development of the disease as the onset of symptoms is typically during late adolescence or early adulthood (McGrath et al., 2004; Patel et al., 2021; van Os and Kapur, 2009). It is generally believed that the development of schizophrenia is guided by an interaction between genetic and environmental factors, including viral infections in utero, childhood trauma, as well as adolescent substance abuse and social isolation (Buka et al., 2001; van Os et al., 2002; Varese et al., 2012; Wicks et al., 2005).
We previously reported that circadian disruption could act as another environmental risk factor for schizophrenia (Bhardwaj et al., 2015; Delorme et al., 2020). Circadian disruptions are disturbances of circadian rhythms, which are near 24 h endogenous rhythms governing metabolism, body temperature, hormone secretion, and many more physiological variables and systems (Bollinger and Schibler, 2014). These endogenous rhythms arise from a central clock located in the suprachiasmatic nucleus, as well as clocks located in all other tissues and organs. Furthermore, clocks, including the central clock, can be entrained by external cues, the most potent one being light (Golombek and Rosenstein, 2010). Irregular schedules of exposure to light can lead to altered circadian rhythms, or to a misalignment between endogenous rhythms and behavioral activities (Baron and Reid, 2014).
Studies have found that up to 80% of patients with schizophrenia exhibit sleep and circadian disruptions (Wulff et al., 2012). Indeed, patients with schizophrenia display reduced total sleep time, reduced sleep quality and reduced slow-wave sleep (Hofstetter et al., 2005; van Kammen et al., 1988). Using mouse models to study schizophrenia, multiple reports have also shown abnormal circadian phenotypes, such as decreased dark phase activity and increased daytime activity (Bhardwaj et al., 2015; Delorme et al., 2020, 2021; Ingiosi et al., 2019; Lee et al., 2018).
The interaction between schizophrenia and circadian disruption seems bidirectional. Circadian disruption leads to a dysregulation of the midbrain dopamine pathways, which results in altered reward-motivated behaviors (Acosta et al., 2020). Dopamine pathways and behaviors are critical parts of schizophrenia pathophysiology (Howes et al., 2012). Furthermore, an increase in psychotic episodes was found following sleep disruption in humans (Waters et al., 2018).
Family, twin and adoption studies have consistently demonstrated an important genetic component to schizophrenia, with 80% heritability (Gejman et al., 2010). Sandy (Sdy) mice are used to study aspects of schizophrenia given their loss of function mutation in the schizophrenia-associated gene Dtnbp1 (Dysbindin-1). This mutation in Sdy mice leads to behavioral and cognitive deficits analogous to those seen in the patients, for example, reduced prepulse inhibition (PPI) of acoustic startle, reduced auditory-evoked response adaptation, reduced social interaction, and reduced spatial and declarative memory (Bhardwaj et al., 2009; Lamont et al., 2007; Li et al., 2003; Talbot, 2009). Although DTNBP1 is not among the top genes found in genome-wide association studies (Harrison, 2015), its reduced expression in the prefrontal cortex, superior temporal gyrus, and hippocampus of patients with schizophrenia, together with studies in Sdy mice, have established this gene as a potentially important gene implicated in schizophrenia (Talbot et al., 2004, 2011; Tang et al., 2009; Wang et al., 2017; Weickert et al., 2004, 2008). We have previously shown that exposing adult Sdy mice to constant light, leading to circadian disruption, resulted in behavioral changes including an increase in locomotor activity, a “resistance” to anxiety-like behaviors, and reduced prepulse inhibition of acoustic startle (Bhardwaj et al., 2015).
However, our previous study was conducted in adult mice, which were older than 2 months at the time of environmental circadian disruption, an age when many important neurodevelopmental changes in the brain have already taken place (Agoglia et al., 2017; Hammelrath et al., 2016; Konrad et al., 2013). To our knowledge, there are no studies examining the interaction between genetic risk factors of schizophrenia and circadian disruption during adolescence, which is a critical period for the onset in the development of abnormal behavior in schizophrenia (Androutsos, 2012). Given our previous study showing the impact of constant light on schizophrenia-relevant behaviors and the importance of adolescence in the development of the disease, we hypothesized that circadian disruption during adolescence can act as a risk factor for schizophrenia, leading to the development and/or worsening of the symptoms. To test this, we subjected Sdy and WT males and females either to chronic jetlag (CJL) or to a regular 12 h light:12 h dark cycle (LD 12:12) during pre-adolescence and adolescence (3-7 weeks old). Cognitive behaviors of WT mice were negatively impacted by circadian disruption, whereas Sdy mice had a rescue of their cognitive decifits. We also found multiple effects of circadian disruption during adolescence, leading to decreased sociability in males and females, reduced sensorimotor gating in females and decreased anxiety-like behavior in males. Our data uncover a novel interaction between the genetic risk factor, Dtnbp1, and circadian disruption.
Materials and Methods
Animals
Sandy mice were originally on a DBA/2J genetic background. We previously backcrossed these mice to the C57BL/6J background (Jackson Laboratories) for over 10 generations. Animals used in this project were bred from heterozygous parents in the lab. They were genotyped using a duplex polymerase chain reaction procedure as previously described (Bhardwaj et al., 2015; Cox et al., 2009). Homozygous WT and Sdy males and females were used in the experiments. Animals used as strangers during the 3-chamber social interaction test were all on a C57BL/6 genetic background. These strangers were either homozygous WT mice from other colonies in the lab or ordered 1 week prior testing from Charles River Laboratories (Saint-Constant, QC, Canada), age- and sex-matched with the experimental mice. Animal use was in accordance with the guidelines of the Canadian Council of Animal Care and was approved by the McGill University Animal Care Committee.
Chronic Jetlag During Adolescence
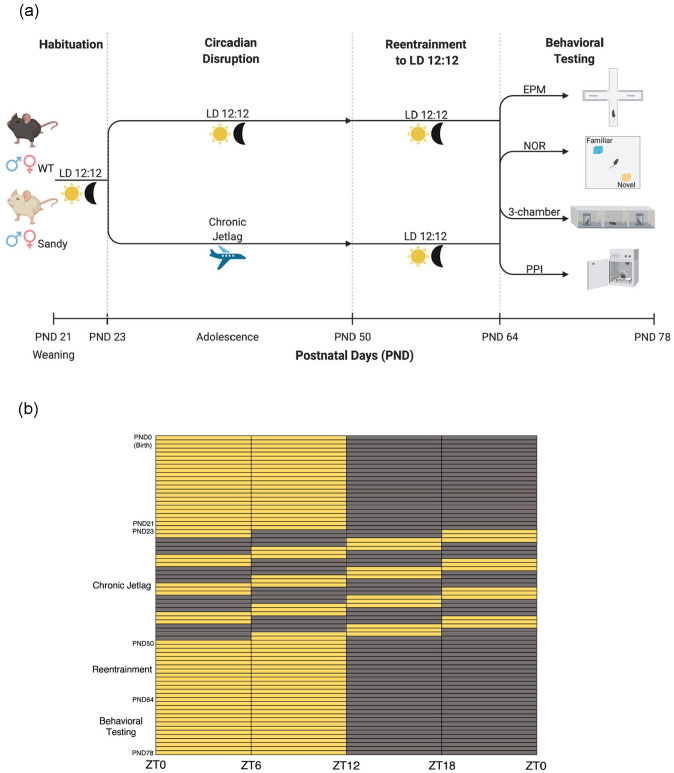
At postnatal day 21 (beginning of pre-adolescence) (Brust et al., 2015), mice were weaned and placed in ventilated light-proof cabinets (Actimetrics, Wilmette, IL, USA) having a 12 hour light: 12 hour dark (LD 12:12) cycle. The light intensity was between 150 and 200 lux. At postnatal day 23, some mice started a CJL procedure known to disrupt circadian rhythms: a 6-h dark phase advance every 2 days, for 4 weeks (Casiraghi et al., 2012). A control group of mice remained in a LD 12:12 cycle. Males and females were housed in different cabinets. To ensure that all mice were tested at the same circadian time, they were then reentrained to LD 12:12 for 2 weeks before undergoing behavioral assessment (Figure 1). Indeed, we previously showed that Sdy mice do not show circadian alterations under LD 12:12 (Bhardwaj et al., 2015). Therefore, any phenotypes would likely not be caused by altered phase and/or sleep-wake states.
Figure 1.
Protocol for the circadian disruption and behavioral testing during adolescence. (a) Wild-type (WT) and Sandy mice underwent either a 12 h light:12 h dark (LD 12:12) light cycle or a chronic jetlag starting at postnatal day (PND) 23 for 4 weeks. At the end of these 4 weeks, mice were returned to LD 12:12 for 2 weeks before undergoing behavioral tests: elevated plus maze (EPM), novel object recognition (NOR), 3-chamber social interaction test (3-chamber), prepulse inhibition (PPI) of acoustic startle. Figure created with Biorender.com. (b) Light cycles experienced by mice of the chronic jetlag group from birth up to the end of the experiment. Yellow boxes indicate lights on, and dark gray boxes indicate lights off. Zeitgeber times (ZT) refer to the times under the initial and final LD 12:12 conditions. Color version of the figure is available online.
Behavioral Testing
Given the small litter sizes in the Sdy colony, a number of cohorts had to be successively raised under CJL (or control LD 12:12) and tested. We ensured consistency throughout CJL and behavioral testing by having the same person conduct the experiments. Behavioral testing was performed during a restricted time slot every day (details for each test, below). Statistical tests specific for each of the behavioral tests (described for each test, below), were redone by removing each cohort individually to confirm that the effects observed were not driven by 1 litter.
The tests were performed in order of least to most stressful: EPM, NOR, social interaction, and PPI. Mice had 2 days of rest between EPM and NOR, and between NOR and social interaction. Three days separated the 3-chamber social interaction test from PPI. All behavioral tests were started at ZT2 and were performed until ZT6 or ZT8 depending on the duration of each test, that is, tests that are longer to perform ended later in the day (for animals under an LD cycle, zeitgeber time (ZT) represents the number of hours since lights were turned on (ZT0)).
Elevated Plus Maze (EPM)
An elevated plus maze was used to measure anxiety-like behavior (Walf and Frye, 2007). The test was performed as previously described (Bhardwaj et al., 2015), in dim light (10 lux), between ZT2 and ZT6. The time spent and number of entries in open arms, closed arms, and central area were scored using the recordings made using an overhead camera. The ratio of time in the open arms versus closed arms and ratio of entries in open versus closed arms were used as a measure of anxiety-like behavior:
Mice that jumped out of the maze were excluded from the analysis (nmales: Sdy LD 12:12 = 3, Sdy CJL = 3; nfemales: Sdy LD 12:12 = 1). One WT CJL male was also excluded from the analysis given that it spent the entire test grooming in the closed arm and was a significant outlier according to the ROUT test. One Sdy CJL female was also excluded for being a significant outlier in the ROUT test.
Novel Object Recognition (NOR)
Open field Sentra gray boxes were used to measure recognition memory (Şık et al., 2003). The test was performed as previously described (Srikanta et al., 2021) between ZT2 and ZT8 in dim light. On Day 1 of testing, mice were habituated to the empty open-field boxes for 10 min. On the morning of Day 2 (training segment of the test), mice were allowed to explore 2 identical objects for 10 min. Four hours later (NOR segment of the test), mice were allowed to expore the familiar object and the novel object for 10 min. Tracking was done using a stopwatch program coded for this experiment using the Wing Python IDE, Wing101 (version 2.7.16 for MacOS, Cambridge, Massachusetts USA, https://wingware.com/). We used the ratio of time spent with novel over familiar object as a measure of recognition memory:
For the training segment, the discrimination ratio was calculated using time spent exploring the object that will subsequently be changed/time spent exploring the object that will remain in place (“familiar object” in the next segment).
One WT CJL male was a significant outlier according to the ROUT test and had a preference for one of the objects during training, which led to its removal from the test analysis.
Three-chamber Social Interaction Test
Three chambered boxes were used to assess sociability and social novelty preference (Yang et al., 2011). The apparatus is made of 3 chambers separated by walls, each having a vertical opening allowing the mice to travel between chambers. The left and right chambers have weighted cups in the middle of the chamber. The test was divided into 3 segments, each lasting 10 min: habituation, sociability, and social novelty preference. The mouse started each segment in the middle chamber. During habituation, both cups were empty. During the sociability segment, one of the cups had a conspecific sex- and age-matched stranger and the other one had an object about the same size as a mouse. During the social novelty preference segment, the object was replaced by a novel stranger. Testing was done in dim light, between ZT2 and ZT8.
The night before the experiment, stranger mice were habituated to the apparatus. During the habituation of stranger mice, a mouse was put under each weighted cup for 10 min. The mouse under the left cup was then allowed to discover the apparatus for 10 min. This mouse was put back under the cup and the other mouse was allowed to discover the apparatus for 10 min.
Tracking was done using a stopwatch program coded using Wing101, for this experiment. The time spent interacting with the weighted cups, defined as time at which the mouse is directly interacting with the cups by sniffing or walking on them, was used to assess sociality and social novelty preference:
Mice that jumped out of the apparatus were excluded from the analysis, starting from the trial during which they jumped out (nmales: WT CJL = 6, Sdy CJL = 5; nfemales: Sdy LD 12:12 = 1, Sdy CJL = 1). Furthermore, significant outliers according to the ROUT test during sociability were excluded from both the sociability and social novelty analysis (nmales: WT CJL = 1, Sdy LD 12:12 = 1, Sdy CJL = 1; nfemales: WT LD 12:12 = 2, Sdy CJL = 1).
Prepulse Inhibition of Acoustic Startle
Prepulse inhibition (PPI) of acoustic startle was used to measure sensorimotor gating (Shoji and Miyakawa, 2018). The test was performed between ZT2 and ZT6 with the lights on, using SR lab system (San Diego Instruments, San Diego, CA), as previously described (Bhardwaj et al., 2015). SR lab’s GPPIDR1 session was used to generate sound pulses. Mice were first habituated to the enclose for 5 min. To habituate the animal to the experimental procedures, two 120 dB pulses were generated. These pulses were followed by 30 trials. A startle pulse lasting 30 msec was presented either alone or 100 msec after a prepulse lasting 20 msec. These prepulses were presented randomly 5 times each and had intensities of 6, 9, 12, or 15 dB above background noise. To measure sensorimotor gating, we calculated the percentage PPI:
Mice showing abnormal acoustic startle response (startle amplitude close to zero or higher than 300) were excluded from the analysis (nmales: WT CJL = 2, Sdy LD 12:12 = 3, Sdy CJL = 5; nfemales: WT CJL = 1, Sdy LD 12:12 = 3) (Manning et al., 2021). Indeed, these mice were excluded because baseline acoustic startle close to zero or above 300 show abnormal responses to the test either due to recording errors, impaired hearing capacities or impaired capacity to process sounds in the brain (Lauer et al., 2017). Although most excluded mice were Sdy mice, a previous study did not report differences in startle responses between Sdy mice and WT mice (Carlson et al., 2011). Furthermore, in the same study, Sdy mice on the C57BL/6 background were shown to not have auditory deficits unlike Sdy mice on the previous DBA/2 J genetic background (Carlson et al., 2011).
Statistical Analysis
GraphPad Prism (version 9.3.0 for Windows, GraphPad Software, San Diego, California USA) was used to perform statistical analysis and graphing. Assumptions of normality and homogeneity were verified using both GraphPad Prism and JASP (version 0.13.1 for Mac OS, JASP Team, Amsterdam, The Netherlands, https://jasp-stats.org). Interaction between two factors (genotype and lighting) during nonparametric Kruskal-Wallis (KW) tests was verified using R Studio (version 2021.9.1.372 for macOS, RStudio Team, RStudio: Integrated Development Environment for R, Boston, Massachusetts, USA, http://www.rstudio.com/).
The ROUT test was used to find significant outliers in data sets. A value is considered an outlier if it is significantly different (Q = 1%) from the entire data set.
Two-way between-factor analysis of variance (ANOVA) and 3-way between-factor ANOVA were performed on normally distributed data sets with equal variance across groups. JASP was used to perform simple main effects as post hoc. Upon violation of normality during 2-way ANOVAs, nonparametric Kruskal-Wallis tests were done using JASP and R Studio (ratio of time spend in open/close arm for males; ratio of time spend with novel/familiar object for males and females during NOR; sniffing time during sociability for males; sniffing time during social novelty for females; acoustic startle response for males and females). Upon violation of normality, a nonparametric post hoc was done by using the multiple Mann-Whitney test (sniffing time during sociability for males). One-tailed paired Student t tests were used to assess differences between training and NOR during the NOR test. We used paired Student t tests as the direction of the difference was expected; mice with proper recognition memory were expected to show a higher ratio of time spent with novel/familiar object during NOR. One sample Student t tests were performed to see deviations from expected mean of 1 (i.e. showing no recognition memory during NOR). Student t tests and 1 sample Student t tests are necessary during the analysis of NOR to ensure no preference for any object during the training phase of the test and to verify an expected significant difference between NOR and the training phase, indicating proper recognition. This is important as abnormal learning of the objects during training, that is, a training ratio above or below 1, showing a preference for 1 of the 2 identical objects, could lead to false positives. Levene’s test was done using R Studio to determine differences in variability between males and females during NOR. The average PPI across different prepulse intensities was studied using a 3-way ANOVA to see any interaction of genotype, lighting or prepulse intensity. Differences were considered significant when p < 0.05 and considered trending when p < 0.1.
Results
Anxiety-like Behavior
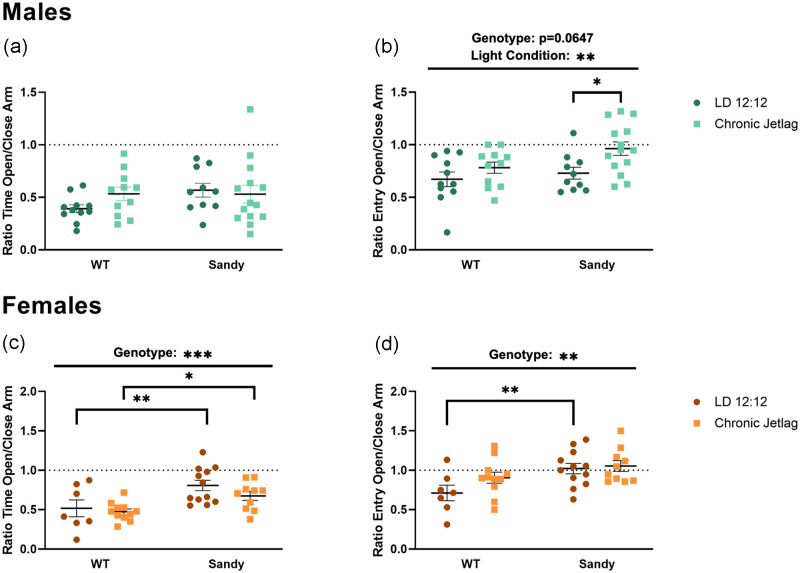
Anxiety-like behavior was assessed using the elevated plus maze (EPM) test. There was no main effect of genotype, H(1) = 0.774, p = 0.379, lighting, H(1) = 0.368, p = 0.544, or interaction between genotype and lighting, H(3) = 3.857, p = 0.2773, for the time spent in the open compared with the close arms for males (Figure 2a). In contrast, there was a main effect of lighting, F(1,42, = 7.531, p = 0.0089, for the number of entries in the open compared with closed arms for males, with a decreased anxiety-like behavior for CJL compared with LD 12:12 males (Figure 2b). Although we did not find any interaction, it is interesting to look at whether the main effect of lighting is significant for both WT and Sdy mice. To answer this question, we used a simple main effect test, which revealed that this decrease in anxiety-like behavior was mainly significant for Sdy CJL males compared with Sdy LD 12:12 males (p = 0.0106). There was also a trend toward an effect of genotype for males for the number of entries, F(1,42 = 3.600, p = 0.0647, with Sdy males having lower anxiety-like behavior compared with WT (Figure 2b). Therefore, CJL males, especially Sdy, showed reduced anxiety-like behavior.
Figure 2.
Reduced anxiety-like behaviors in Sandy male and female mice and additional effects of chronic jetlag in males. The elevated plus maze test was used to assess anxiety-like behaviors. Proportion of time spent in the open compared with close arms for (a) males and (c) females. Proportion of entries in open compared with close arms for males (b) and females (d). Individual data points represent independent mice (nmales: WT LD 12:12 = 11, WT CJL = 11, Sdy LD 12:12 = 10, Sdy CJL = 14; nfemales: WT LD 12:12 = 7, WT CJL = 11, Sdy LD 12:12 = 12, Sdy CJL = 10) and data are represented as mean ± SEM. Two-way between-factor ANOVA with simple main effect post hoc (c and d). Kruskal-Wallis test (a). Abbreviations: WT = wild-type; LD = light-dark cycle; CJL = chronic jetlag; ANOVA = analysis of variance.
*p < 0.05. **p < 0.01. ***p < 0.001.
No main effect of lighting was found in female mice for the ratio of entries, F(1, 36) = 2.301, p = 0.1380. However, a main effect of genotype was found in female for both the ratio of time spent in the open arms, F(1, 36) = 14.09, p = 0.0006 (Figure 2c), and the ratio of entries, F(1, 36) = 9.355, p = 0.0042, with Sdy females showing lower anxiety-like behavior compared with WT (Figure 2d). Although no interaction was found, we wanted to know whether these differences in genotypes are seen in both lighting condition. We found that differences between Sdy and WT were seen after both LD 12:12 (p = 0.005) and CJL (p = 0.030). There was no significant interaction between genotype and lighting for the ratio of time spent in the open arms, F(1,36) = 0.4780, p = 0.4937, and the ratio of entries, F(1,36) = 1.168, p = 0.2869. This shows that Sdy females, in contrast to males, were not affected by CJL during adolescence, whereas Dtnbp1 mutation led to a reduction in anxiety-like behavior compared with WT mice.
Recognition Memory
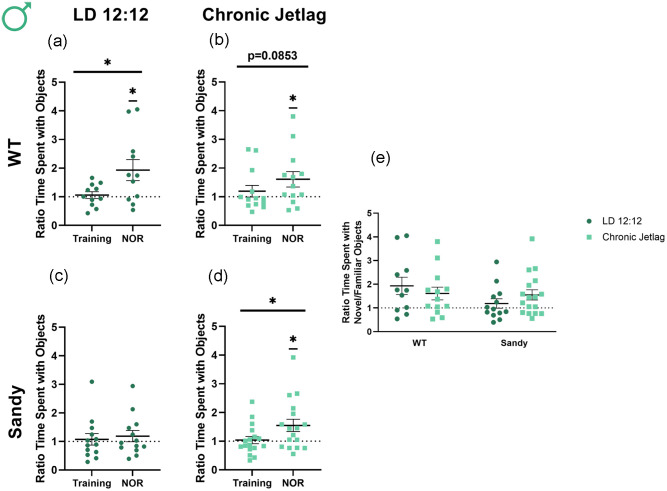
Recognition memory was assessed using the NOR test. A paired Student t test showed a significant difference between the ratio of time spent with novel/familiar object during NOR compared with the ratio during training for LD 12:12 WT males, t(10) = 2.745, p = 0.0103. This indicates a proper recognition of the novel object (Figure 3a). In contrast, the difference in ratios between NOR and training was trending but not significant in WT CJL males, indicating a reduction in recognition memory by CJL, t(13 = 1.458, p = 0.0853 (Figure 3b). This result is in concordance with our hypothesis that circadian disruption would lead to the development and/or worsening of schizophrenia-relevant behaviors including cognitive deficits. Sdy LD 12:12 males also did not show differences between NOR and training, t(12) = 0.3473, p = 0.3672) (Figure 3c), consistent with previous reports (Al-Shammari et al., 2018; Bhardwaj et al., 2009). Surprisingly, there was a significant difference between training and NOR for Sdy CJL males, t(16) = 2.238, p = 0.0199 (Figure 3d). However, an abnormal training ratio, such as a mouse that has a preference for 1 of the 2 objects during training (but not during NOR), could lead to a significant difference between training and NOR without showing proper recognition of the novel object. Thus, to verify whether the male mice differed from the expected ratio of 1 during training, a 1-sample Student t test was used and showed no significant difference for these Sdy CJL males during the training phase of testing, t(16) = 0.2954, p = 0.7715. However, the ratio was significantly different from 1 for these mice during NOR, showing proper recognition of the object, t(16) = 2.558, p = 0.0211) (Figure 3d). Thus, CJL during adolescence rescued impairments in recognition memory previously seen in the Sdy mouse model. When comparing the NOR segment for all groups, Kruskal-Wallis tests found no significant main effect of genotype, H(1) = 1.659, p = 0.198, or lighting, H(1) = 0.291, p = 0.589. There was no significant interaction between genotype and lighting using nonparametric and parametric tests, KW: H(3) = 3.264, p = 0.3527, ANOVA: F(1,50) = 1.731, p = 0.1943 (Figure 3e). Therefore, WT males’ cognitive behavior was negatively impacted by CJL, whereas under these altered lighting conditions Sdy males had a rescue in their preexisting cognitive deficits.
Figure 3.
Opposite impact of chronic jetlag on recognition memory of WT and Sdy male mice. Novel object recognition was used to assess recognition memory in male mice. Proportion of time spent for WT LD 12:12 (a), WT CJL (b), Sdy LD 12:12 (c) Sdy CJL (d) with the novel compared with the familiar object. (e) Ratio time familiar/novel object during the NOR phase of testing for all groups. Individual data points represent independent mice (nmales: WT LD 12:12 = 11, WT CJL = 13, Sdy LD 12:12 = 13, Sdy CJL = 17), and data are represented as mean ± SEM. One-tailed paired Student t test and 1-sample t test for (a-d). Kruskal-Wallis test (e). Abbreviations: WT = wild-type; LD = light-dark cycle; NOR = novel object recognition; CJL = chronic jetlag.
*p < 0.05.
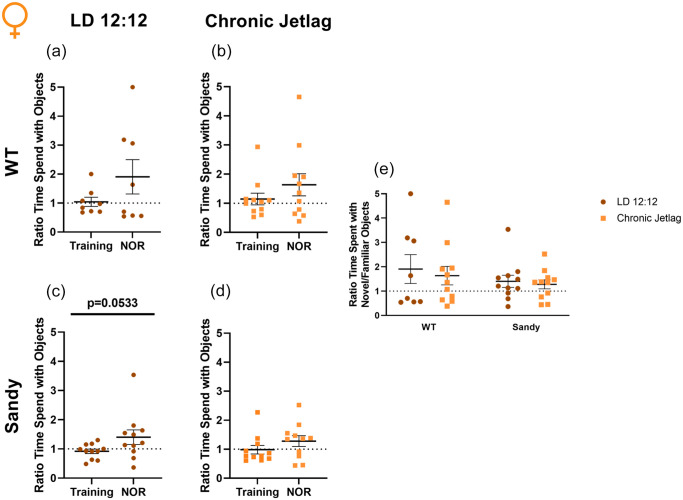
For all females groups, we did not find evidence of proper recognition memory. Indeed, none of the differences between NOR and training were significant, WT LD 12:12: t(7) = 1.342, p = 0.1108; WT CJL: t(10) = 1.036, p = 0.1622; Sdy CJL: t(10) = 1.331, p = 0.1064 (Figure 4a, 4b and 4d). There was a trend toward an increased ratio of time novel/familiar during NOR compared with the training for Sdy LD 12:12 mice, t(10) = 1.773, p = 0.0533) (Figure 4c). However, the ratio of time spent with the object did not differ from 1, confirming that there was not a proper recognition of the novel object, t(10) = 1.616, p = 0.1372. Furthermore, 1-sample Student t tests were not significant for all the other groups, WT LD 12:12: t(7) = 1.535, p = 0.1687; WT CJL: t(10) = 1.672, p = 0.1255; Sdy CJL: t(10) = 1.499, p = 0.1649. Finaly, when comparing the NOR segment for all groups, there was no significant main effect of genotype, H(1) = 0.186, p = 0.666, or lighting, H(1) = 0.006, p = 0.937. There was also no significant interaction between genotype and lighting using nonparametric and parametric tests, KW: H(3) = 0.231, p = 0.9724, ANOVA: F(1,37) = 0.04372, p = 0.8355) (Figure 4e). The lack of differences found during the 2-way ANOVA confirms that all the mice groups behaved similarly. However, no significant differences were found using Student t tests and the 1-sample Student t tests, indicating a lack of recognition of the novel object. This apparent absence of recognition memory in females will be addressed in the discussion.
Figure 4.
Impaired recognition memory in female mice. Novel object recognition was used to assess recognition memory in female mice. Proportion of time spent for WT LD 12:12 (a), WT CJL (b), Sdy LD 12:12 (c) Sdy CJL (d) with the novel compared with the familiar object. (e) Ratio time familiar/novel object during the NOR phase of testing for all groups. Individual data points represent independent mice (nfemales: WT LD 12:12 = 8, WT CJL = 11, Sdy LD 12:12 = 11, Sdy CJL = 11) and data are represented as mean ± SEM. One-tailed paired Student t test and 1-sample t test (a-d). Kruskal-Wallis test (e). Abbreviations: WT = wild-type; LD = light-dark cycle; NOR = novel object recognition; CJL = chronic jetlag.
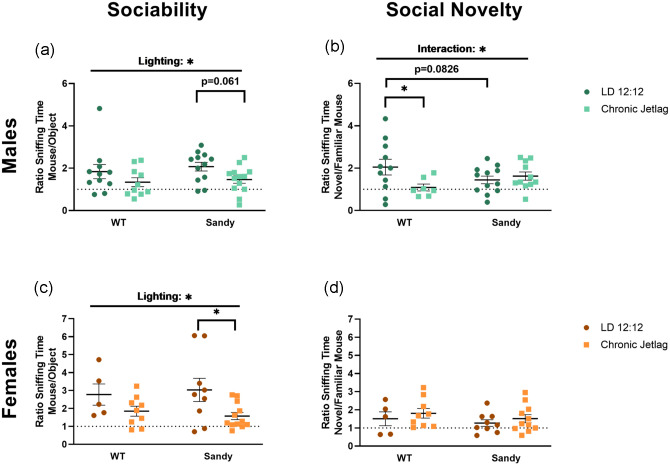
Sociability and Social Recognition Memory
The 3-chamber test was used to measure sociability and social recognition memory. As hypothesized, there was a main effect of lighting on the sociability of male mice, H(1) = 4.636, p = 0.031, with CJL mice having a reduced sociability compared with LD 12:12 mice (Figure 5a). During the social novelty preference phase, an interaction between genotype and lighting was found, F(1,37) = 4.855, p = 0.0339. Interestingly, post hoc analysis showed that the differences lied between LD 12:12 and WT CJL mice (p = 0.0192). WT mice that underwent a CJL during their adolescence spent less time with the novel conspecific compared with WT LD 12:12 males (Figure 5b). These differences indicate social recognition deficits are similar to the trends observed in NOR (Figure 3e).
Figure 5.
Reduced sociability after jetlag and opposite impact of jetlag on social recognition memory for WT and Sdy mice. The 3-chamber social interaction test was used to assess sociability and social recognition in mice. Sociability indicated by the proportion of time spent with the mouse compared with the object for males (a) and females (c) (nmales: WT LD 12:12 = 11, WT CJL = 10, Sdy LD 12:12 = 12, Sdy CJL = 13; nfemales: WT LD 12:12 = 5, WT CJL = 9, Sdy LD 12:12 = 9, Sdy CJL = 12). Social novelty preferences indicated by the proportion of time spent with the novel mouse compared with the familiar mouse for males (b) and females (d) (nmales: WT LD 12:12 = 11, WT CJL = 7, Sdy LD 12:12 = 12, Sdy CJL = 11; nfemales: WT LD 12:12 = 5, WT CJL = 9, Sdy LD 12:12 = 9, Sdy CJL = 11). Individual data points represent independent mice and data are represented as mean ± SEM. Two-way between subject ANOVA with simple main effect post hoc (b-c). Kruskal-Wallis test (a and d) with multiple Mann-Whitney post hoc (a). Abbreviations: WT = wild-type; LD = light-dark cycle; CJL = chronic jetlag; ANOVA = analysis of variance.
*p > 0.05.
Similar to the males, female mice showed an effect of lighting during the sociability portion of the test, F(1,31) = 7.282, p = 0.0112 (Figure 5c). Females from the CJL group showed reduced sociability compared with females staying in LD 12:12. No significant main effects of genotype, H(1) = 1.416, p = 0.234, or lighting, H(1) = 1.333, p = 0.248, were found for females during the social novelty preference phase (Figure 5d). Therefore, although sex-specific differences are seen for social novelty, sociability was negatively affected by CJL for both sexes and genotypes.
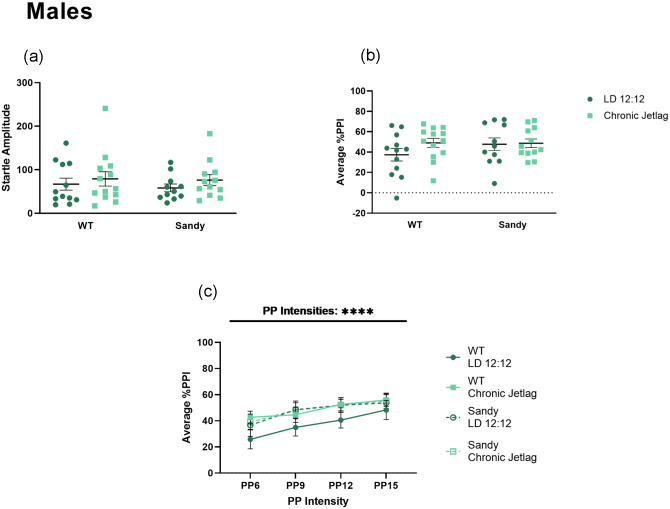
Sensorimotor Gating
Sensorimotor gating was assessed using the PPI test. A Kruskal-Wallis test showed no main effect of genotype, H(1) = 0.038, p = 0.845, or lighting, H(1) = 1.312, p = 0.252, for acoustic startle response, showing that all male groups had similar baseline startles and no hearing impairments (Figure 6a). Furthermore, we did not find any main effect of genotype, lighting or interaction for PPI of acoustic startle for males, F(1,44) = 0.8935, 1.407 and 1.019, p values > 0.2 (Figure 6b). No more differences between genotypes and lighting conditions were uncovered when looking across the different PP intensities using a 3-way ANOVA (Figure 6c).
Figure 6.
No effect of chronic jetlag or Dtnbp1 mutation on sensorimotor gating in male mice. Prepulse inhibition of acoustic startle was used to assess sensorimotor gating in male mice. (a) Acoustic baseline startle response for males. (b) Percentage PPI averaged across all prepulse (PP) intensities. (c) Average prepulse inhibition across different prepulse intensities. PP intensities are indicated as the number of decibels above background noise. Three-way 2 between (genotype and lighting), 1 within (decibel intensities) ANOVA. Individual data points represent independent mice (nmales: WT LD 12:12 = 12, WT CJL = 13, Sdy LD 12:12 = 11, Sdy CJL = 12) and data are represented as mean ± SEM. Two-way between subject ANOVA (b). Kruskal-Wallis test (a). Abbreviations: WT = wild-type; LD = light-dark cycle; PPI = prepulse inhibition; PP = prepulse; ANOVA = analysis of variance; CJL = chronic jetlag.
****p < 0.0001.
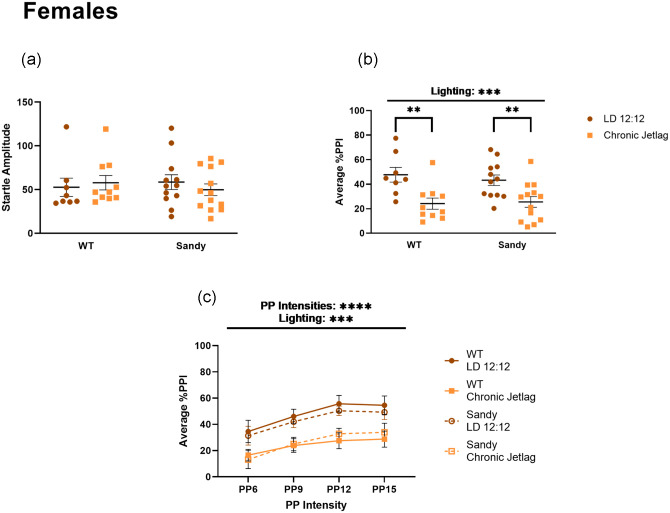
There were no main effects of genotype, H(1) = 1.515 × 10–4, p = 0.990 or lighting, H(1) = 0.043, p = 0.836, for baseline acoustic startle response in the females (Figure 7a). When analyzing the average percentage PPI, there was a significant main effect of lighting, F(1,39) = 18.61, p = 0.0001 (Figure 7b); female mice that underwent CJL had a reduced average percentage PPI compared with females that stayed in LD 12:12. As an exploratory analysis, a post hoc test was done to see whether differences could be found under both genotypes. It revealed that this reduction in % PPI was significant for both WT (p = 0.003) and Sdy (p = 0.007) females. This effect of lighting was also seen using a 3-way ANOVA, when looking across PP intensities (main effect of lighting: F(1, 39) = 18.36, p = 0.0001, main effect of PP intensities: F(1.771, 69.06) = 15.33, p < 0.0001 and no interaction between lighting and PP intensities: F(3,117) = 0.2197, p = 0.8825) (Figure 7c). Therefore, CJL had a sex-dependent effect leading to a decrease in sensorimotor gating in females.
Figure 7.
Reduced sensorimotor gating after chronic jetlag in female mice. Prepulse inhibition of acoustic startle was used to assess sensorimotor gating in female mice. (a) Acoustic startle response for females. (b) Percentage PPI averaged across all prepulse. (c) Average prepulse inhibition across different prepulse intensities. PP intensities are indicated as the number of decibels above background noise. Three-way 2 between (genotype and lighting), 1 within (decibel intensities) ANOVA. Individual data points represent independent mice (nfemales: WT LD 12:12 = 8, WT CJL = 10, Sdy LD 12:12 = 12, Sdy CJL = 13) and data are represented as mean ± SEM. Two-way between subject ANOVA with simple main effect post hoc (b). Kruskal-Wallis test (a). Abbreviations: WT = wild-type; LD = light-dark cycle; PPI = prepulse inhibition; PP = prepulse; ANOVA = analysis of variance; CJL = chronic jetlag.
**p < 0.01. ***p < 0.001. ****p < 0.0001.
Evaluating Sex Differences
To confirm that the effects seen above are sex-dependent, we performed a 3-way ANOVA (factors sex, genotype, lighting) for each of the behavioral tests. Results from this 3-way ANOVA are found in Supplementary Table S1. For the elevated plus maze, we found a significant main effect of sex when looking at both the ratio of time in the open/close arms, F(1,78) = 5.471, p = 0.0219, and the ratio of entries in the open/close arms, F(1,78) = 7.952, p = 0.0061, indicating that male and female mice are indeed behaving differently during this test. Males and females were also significantly different during the sociability test, F(1,73) = 7.216, p = 0.0089, and during prepulse inhibition of acoustic startle, F(1,83) = 8.492, p = 0.0046. In addition, for PPI, there was a significant sex × lighting interaction, F(1,83) = 13.95, p = 0.0003. However, interestingly, males and females did not show any difference on the recognition memory tests: social novelty, F(1,67) = 0.01948, p = 0.8894; NOR, F(1,87) = 0.004156, p = 0.9487. However, a sex × lighting interaction was trending during social novelty, F(1,67) = 3.152, p = 0.0804. The lack of significant difference between sexes regarding cognition might be due to the lower number of females, reducing statistical power. This analysis further emphasizes sex differences found after CJL and/or Dtnbp1 mutation.
Discussion
Here, we report an interaction between a known risk factor of schizophrenia, a mutation in Dtnbp1, and circadian disruption during adolescence in mice. We found clear sex-dependent effects, with circadian disruption and Dtnbp1 mutation affecting male and female mice differentially. In particular, we found that the interaction between CJL and Dtnbp1 mutation led to further reduction in anxiety-like behaviors and cognitive alterations in males. Furthermore, these effects were persistent for 2 weeks after a return to LD 12:12. Therefore, these are not acute effects of CJL, which indicates that the impacts on behavior are long lasting and might be due to neurodevelopmental alterations. This work is, to our knowledge, the first demonstration of an interaction between a genetic risk factor of schizophrenia and exposure to circadian disruption during adolescence. It also represents a significant contribution to the knowledge on sex-specific differences in Sdy mice, as to our knowledge, only 1 paper previously reported behavioral sex differences in this strain, in the rotarod and EPM (Cox et al., 2009).
Previous studies have shown a decrease in anxiety-like behavior in Sdy mice (Al-Shammari et al., 2018; Cox et al., 2009). This phenotype has also been shown in other mouse models of schizophrenia (Lee et al., 2018; Takashima et al., 2011). We previously observed that Sdy mice, unlike WT mice, did not show an increase in anxiety-like behaviors after exposure to constant light during adulthood, thus showing a resistance to anxiety-like behaviors (Bhardwaj et al., 2015). We wondered if CJL during adolescence could further accentuate this decrease in anxiety-like behaviors. Surprisingly, females were not impacted by CJL. In contrast, although both WT and Sdy males were affected by CJL during adolescence, Sdy males seem to have a further decrease in anxiety-like behaviors as they enter the open arm more often compared with Sdy LD 12:12 mice. It would be interesting to look at other paradigms of anxiety-related behaviors in future experiments to see whether they are similarly affected.
We also uncovered an effect of jetlag on sociability in WT and Sdy male and female mice. Mice that underwent repeated jetlag during their adolescence were less sociable compared with the mice that stayed in a regular LD 12:12. Decreased sociability is a trait known to be a negative symptom of schizophrenia (Goldberg and Schmidt, 2001). This impairment in sociability by CJL is consistent with a previous study where disruption of circadian rhythms by housing mice under 20 h light-dark cycles resulted in shorter dendrites and decreased neuronal complexity in the prefrontal cortex (Karatsoreos et al., 2011). We did not find any differences in sociability between Sdy and WT mice that stayed in LD 12:12, unlike previous studies (Cox et al., 2009; Feng et al., 2008). However, these studies used Sdy mice on a DBA/2 J genetic background, which is known to have several mutations, 4 of which are associated with neuronal impairments (Talbot et al., 2009). Studies on Sdy mice on the C57BL/6 genetic background, like the mice used in this study, have not looked at sociability. Instead, a study found that Sdy/C57BL/6 mice are a good model of schizophrenia due to their hyperactivity and cognitive deficits (Cox et al., 2009). Indeed, there is no mouse model directly aiming to model schizophrenia itself. Instead, mouse models like the Sdy mice allow to model known human risk factors like the mutation in DTNBP1. Even in humans, symptoms of the disease are very diverse and having a single genetic risk factor for the disease is not likely to cause schizophrenia. The disease will rather occur upon the addition or interaction between risk factors, which mouse models are well suited to investigate. In this study, we are therefore using the Sdy mouse model to study such interactions between risk factors. Furthermore, we have shown that although Sdy/C57BL6 do not show social impairments unlike previously seen on the DBA/2 J background, CJL can lead to a decrease in sociability for both Sdy and WT mice. Therefore, showing a very negative impact of CJL during adolescence on schizophrenia-associated behaviors.
Prepulse inhibition is a translational paradigm often conducted in mice and in humans to assess sensorimotor deficits in schizophrenia. It is known that men with schizophrenia have a lower PPI compared with healthy men (Kumari et al., 2004). However, this difference between patients with schizophrenia and healthy controls is not seen in women. Surprisingly, we have seen the opposite in our PPI data: female Sdy mice exhibited a reduced sensorimotor gating following CJL during adolescence, which was not the case for males. We therefore uncovered a potential neurodevelopmental impact of jetlag on sensorimotor gating functions for females. Behavioral studies using female mice modeling aspects of schizophrenia are sparse; studies in some transgenic mice have shown no difference in PPI between female WT and transgenic mice overexpressing Neuregulin 1 Type III (Olaya et al., 2018). Interestingly, in the present study we did not observe a significant effect of genotype on PPI in the male mice, which is unexpected given that previous results showed a reduction in percentage PPI in Sdy males compared with WT after 4 weeks housing in constant light (Bhardwaj et al., 2015). This suggests that this reduction in percentage PPI in Sdy males was an acute effect of constant light since 3 weeks of LD 12:12 partially rescued this phenotype (Bhardwaj et al., 2015).
When investigating object recognition memory, there was a genotype by lighting interaction in males while females did not have any significant differences. In fact, we have found that WT males that underwent a CJL during their adolescence had a reduction in recognition memory compared with WT LD 12:12 mice. This result follows our hypothesis that exposure to circadian disruption during adolescence could be a risk factor for the disease. Furthermore, a previous study has shown that exposing rats to an advance jetlag procedure (a 6 h advance each week, for 8 weeks) resulted in cognitive impairment during NOR due to a decrease in hippocampal neurogenesis (Horsey et al., 2020). These findings support our results that jetlag leads to detrimental effect on cognition.
Unlike our previous study using constant light, we opted for CJL because it can better model irregular light exposure that teenagers are exposed to daily. Indeed, during adolescence, circadian rhythms are delayed, resulting in later bedtimes and wake up times. These “night owls” are therefore greatly affected by early morning awakening for school. Indeed, their light phase is advanced during weekdays compared with weekends since they wake up earlier than their internal clock to go to school (Hasler and Clark, 2013). Circadian misalignment can thus be a consequence of a mismatch between internal rhythms and social activities. Our study indicates that such circadian misalignment could results in cognitive deficits, adding to the list of deficits associated with misalignments during adolescence in humans: poor executive function, deficits in impulse control and altered neural response to reward (Logan et al., 2018). Such cognitive deficits during adolescence and early adulthood have been associated with an increased risk for psychosis later in life for men (MacCabe et al., 2013). As previously shown, Sdy males that stayed in a regular LD cycle during their adolescence also showed such cognitive deficits (Al-Shammari et al., 2018; Bhardwaj et al., 2009). Surprisingly though, CJL in male Sdy mice did not result in such recognition memory impairments. This is the opposite of what we would expect given the reduced cognition seen in WT mice. Furthermore, it has been shown that deficits in DTNBP1 in the Sdy mouse model leads to a reduced neuronal differentiation (Ma et al., 2011; Nihonmatsu-Kikuchi et al., 2011). Thus, given the impact of CJL on recognition memory of WT mice, and preexisting cognitive deficits in Sdy mice, we expected CJL and Dtnbp1 mutation to interact, resulting in greater deficit in recognition memory, which is the opposite of what we are seeing. It would be interesting to look at other learning and memory paradigms after CJL and at neurogenesis in the brains of WT and Sdy mice after CJL or LD 12:12. The latter study would allow to study this interaction at the cellular level by seeing whether Sdy CJL mice show differences in hippocampal neurogenesis compared with WT CJL mice.
The differences in response to CJL in WT compared with Sdy males were also seen during social novelty in males, confirming the opposite responses to jetlag between WT and Sdy mice. Indeed, CJL in WT mice once again resulted in cognitive deficits. However, CJL did not have an impact on Sdy mice, which already had cognitive impairments. This lack of effect from CJL could potentially be due to a floor effect since the ratio of time spent with the novel/familiar mouse is already very low for Sdy mice that stayed in LD. Furthermore, Sdy mice social recognition memory was not rescued as seen during NOR. Although possible impairments in the hippocampus of Sdy mice might be rescued by CJL leading to a rescue in cognitive deficits as supposed during NOR, other brain regions related to social cognition might be affected leading to the continuation of deficits in proper recognition in Sdy mice during social novelty.
As mentioned, in the NOR test none of the female groups showed proper recognition of the novel object. Although this result was surprising at first, it has been shown in rats that the exploration of novel objects (novelty-seeking behaviors) is different between males and females during adolescence. Indeed, female rats during mid-adolescence showed less preference for the novel object compared with the males (Cyrenne and Brown, 2011). Late adolescence in mice is defined as the time at which mice start to disperse, that is, time at which mice leave their birthplace to pursue reproduction, which is around postnatal day 49 for males and postnatal day 71 for females (Groó et al., 2013). Furthermore, there are many known sex differences in rodent brain development during adolescence (Juraska et al., 2013). Indeed, the volume of the anteroventral periventricular nucleus (AVPv) in the hippocampus, an important region for cognition, increases significantly between PND30 and 60 in female rats, but was already stable at PDN30 in male rats (Davis et al., 1996). Our mice were tested around postnatal days 65-66. This is approximately early adulthood for male mice. On the other hand, the female mice might still have been in their late adolescence at the time of NOR; this is the period at which males showed more recognition memory by having a higher novelty-seeking behavior compared with females in rats (Cyrenne and Brown, 2011). This might explain why females did not show proper recognition memory even in the control group (WT LD 12:12). Another explanation could be related to increased variability in females, due to the estrous cycle. Indeed, it was previously shown that the phase of the estrous cycle has an impact on NOR memory in mice, where females in the Metestrus/Diestrus phase of the cycle had a lower recognition memory compared with mice in the Proestrus/Estrus phase of the cycle (Cordeira et al., 2018). However, we did not find a difference in variability between males and females using a Levene’s test, F(1, 93) = 0.0643, p = 0.8003. Therefore, although we cannot fully exclude the estrous cycle as a factor preventing seeing recognition memory, there might be additional factors. Further studies analyzing recognition memory in female throughout development and the estrous cycle would be necessary to understand this phenomenon.
Women with schizophrenia have been shown to have fewer cognitive deficits compared with men (Zhang et al., 2017). Our observation of the lack of difference on cognitive tasks between WT and Sdy females was therefore consistent and seen again during the social novelty recognition test. In a previous study in females of a mouse model used to study schizophrenia, WT and neuregulin 1 type III-overexpressing females failed to recognize the novel mouse (Olaya et al., 2018).
Although CJL during adolescence caused or accentuated behavioral alterations, it is unclear whether these effects are the result of circadian disruption occurring specifically during adolescence. An interesting follow-up study would be to subject adult Sdy mice to CJL to see whether these changes in behaviors are still seen or whether they are restricted to disruption during adolescence. In a previous study using a similar CJL procedure, male mice showed an increased depression-like behavior but no changes found in anxiety-like behavior in the elevated plus maze (Chen et al., 2021). This is in contrast to our mice exposed to CJL during adolescence, which show alterations of anxiety-like behavior. This suggest that at least some of the behavioral alterations depend on circadian disruption at a time when neurodevelopment is still going on.
In conclusion, we have uncovered a behavioral interaction between a mutation in Dtnbp1 and circadian disruption during adolescence, leading to consequences on cognition. We have also shown that female mice were less affected by the Dtnbp1 mutation than male mice in most behavioral tests. We expected to see an interaction leading to additive effects of these 2 hits and found such interactions. However, we also found more complex interactions where the effects of the 2 factors act in opposite directions. Given that behavioral tests are dependent upon many brain regions, it is not so surprising that the effects of CJL be different between behavioral tests. CJL might therefore have distinct impacts on different brain regions, leading to additive effects with the Sdy mutation in some cases, or in contrast, to the rescue in recognition memory for Sdy mice but reduction in recognition memory for WT mice. These complex interactions warrant more studies on how circadian disruption or abnormal lighting impact cognition. A large proportion of teenagers are experiencing social jetlag, with earlier awakening on school days compared with weekends (Cetiner et al., 2021; Goldin et al., 2020; Hasler and Clark, 2013; Hena and Garmy, 2020; Mathew et al., 2019). Furthermore, exposure to light at night is an important source of circadian misalignment experienced by teenagers (Hena and Garmy, 2020). It is therefore important to study the effects of circadian disruption during adolescence on mental health. Research on potential new risk factors for schizophrenia and other mental disorders is therefore essential to find new prevention strategies and treatments. Indeed, circadian-based therapies combined with other therapies as well as prevention strategies, such as later school start in the morning, can be interesting avenues to treat or prevent neurodevelopmental disorders like schizophrenia.
Supplemental Material
Supplemental material, sj-docx-1-jbr-10.1177_07487304221125363 for Exposure to Circadian Disruption During Adolescence Interacts With a Genetic Risk Factor to Modify Schizophrenia-relevant Behaviors in a Sex-dependent Manner by Marie-Ève Cloutier, Lalit K. Srivastava and Nicolas Cermakian in Journal of Biological Rhythms
Acknowledgments
We thank all members of the Cermakian lab for discussions. We thank Shashank Srikanta, Henrik Larsen, Tara Delorme, Guylaine Gadoury, Geneviève Dubeau-Laramée, and Christine Kirady for technical help and for assistance in the behavioral tests; Henrik Larsen, Yu Ding, and Camila Chacon for data analysis; and Sanjeev Bhardwaj for breeding and genotyping of Sandy mice.
The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by grants from the Canadian Institutes of Health Research (PJT-153299) and Velux Stiftung (Project 927). Marie-Ève Cloutier was funded by a McGill Integrated Program in Neuroscience Internal Student Award
Supplementary material is available for this article online.
Footnotes
The author(s) have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Nicolas Cermakian  https://orcid.org/0000-0003-4517-9131
https://orcid.org/0000-0003-4517-9131
Data Availability Statement: All data points are included in figures of the article. Further inquiries about the data can be directed to the corresponding authors.
References
- Acosta J, Bussi IL, Esquivel M, Höcht C, Golombek DA, Agostino PV. (2020) Circadian modulation of motivation in mice. Behav Brain Res 382:112471. [DOI] [PubMed] [Google Scholar]
- Agoglia AE, Holstein SE, Small AT, Spanos M, Burrus BM, Hodge CW. (2017) Comparison of the adolescent and adult mouse prefrontal cortex proteome. PLoS ONE 12:e0178391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shammari AR, Bhardwaj SK, Musaelyan K, Srivastava LK, Szele FG. (2018) Schizophrenia-related dysbindin-1 gene is required for innate immune response and homeostasis in the developing subventricular zone. NPJ Schizophr 4:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Androutsos C. (2012) [Schizophrenia in children and adolescents: relevance and differentiation from adult schizophrenia]. Psychiatrike 23:82-93. [PubMed] [Google Scholar]
- Baron KG, Reid KJ. (2014) Circadian misalignment and health. Int Rev Psychiatry 26:139-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj SK, Baharnoori M, Sharif-Askari B, Kamath A, Williams S, Srivastava LK. (2009) Behavioral characterization of dysbindin-1 deficient sandy mice. Behav Brain Res 197:435-441. [DOI] [PubMed] [Google Scholar]
- Bhardwaj SK, Stojkovic K, Kiessling S, Srivastava LK, Cermakian N. (2015) Constant light uncovers behavioral effects of a mutation in the schizophrenia risk gene Dtnbp1 in mice. Behav Brain Res 284:58-68. [DOI] [PubMed] [Google Scholar]
- Bollinger T, Schibler U. (2014) Circadian rhythms—from genes to physiology and disease. Swiss Med Wkly 144:w13984. [DOI] [PubMed] [Google Scholar]
- Brust V, Schindler PM, Lewejohann L. (2015) Lifetime development of behavioural phenotype in the house mouse (Mus musculus). Front Zool 12:S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Bernstein D, Yolken RH. (2001) Maternal infections and subsequent psychosis among offspring. Arch Gen Psychiatry 58:1032-1037. [DOI] [PubMed] [Google Scholar]
- Carlson GC, Talbot K, Halene TB, Gandal MJ, Kazi HA, Schlosser L, Phung QH, Gur RE, Arnold SE, Siegel SJ. (2011) Dysbindin-1 mutant mice implicate reduced fast-phasic inhibition as a final common disease mechanism in schizophrenia. Proc Natl Acad Sci USA 108:E962-E970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casiraghi LP, Oda GA, Chiesa JJ, Friesen WO, Golombek DA. (2012) Forced desynchronization of activity rhythms in a model of chronic jet lag in mice. J Biol Rhythms 27:59-69. [DOI] [PubMed] [Google Scholar]
- Cetiner O, Yildirim G, Kalyoncu ZB. (2021) Social jetlag is associated with the frequency of consumption of sugar-sweetened beverages and a high BMI percentile in adolescents: results of the cross-sectional Family Life, Activity, Sun, Health, and Eating (FLASHE) study. J Acad Nutr Diet 121:1721-1731.e1. [DOI] [PubMed] [Google Scholar]
- Chen R, Weitzner AS, McKennon LA, Fonken LK. (2021) Chronic circadian phase advance in male mice induces depressive-like responses and suppresses neuroimmune activation. Brain Behav Immun Heal 17:100337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeira J, Kolluru SS, Rosenblatt H, Kry J, Strecker RE, McCarley RW. (2018) Learning and memory are impaired in the object recognition task during metestrus/diestrus and after sleep deprivation. Behav Brain Res 339:124-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MM, Tucker AM, Tang J, Talbot K, Richer DC, Yeh L, Arnold SE. (2009) Neurobehavioral abnormalities in the dysbindin-1 mutant, sandy, on a C57BL/6J genetic background. Genes Brain Behav 8:390-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyrenne DLM, Brown GR. (2011) Ontogeny of sex differences in response to novel objects from adolescence to adulthood in Lister-hooded rats. Dev Psychobiol 53:670-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EC, Shryne JE, Gorski RA. (1996) Structural sexual dimorphisms in the anteroventral periventricular nucleus of the rat hypothalamus are sensitive to gonadal steroids perinatally, but develop peripubertally. Neuroendocrinology 63:142-148. [DOI] [PubMed] [Google Scholar]
- Delorme TC, Srivastava LK, Cermakian N. (2020) Are circadian disturbances a core pathophysiological component of schizophrenia? J Biol Rhythms 35:325-339. [DOI] [PubMed] [Google Scholar]
- Delorme TC, Srivastava LK, Cermakian N. (2021) Altered circadian rhythms in a mouse model of neurodevelopmental disorders based on prenatal maternal immune activation. Brain Behav Immun 93:119-131. [DOI] [PubMed] [Google Scholar]
- Feng YQ, Zhou ZY, He X, Wang H, Guo XL, Hao CJ, Guo Y, Zhen XC, Li W. (2008) Dysbindin deficiency in sandy mice causes reduction of snapin and displays behaviors related to schizophrenia. Schizophr Res 106:218-228. [DOI] [PubMed] [Google Scholar]
- Gejman PV, Sanders AR, Duan J. (2010) The role of genetics in the etiology of schizophrenia. Psychiatr Clin North Am 33:35-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JO, Schmidt LA. (2001) Shyness, sociability, and social dysfunction in schizophrenia. Schizophr Res 48:343-349. [DOI] [PubMed] [Google Scholar]
- Goldin AP, Sigman M, Braier G, Golombek DA, Leone MJ. (2020) Interplay of chronotype and school timing predicts school performance. Nat Hum Behav 4:387-396. [DOI] [PubMed] [Google Scholar]
- Golombek DA, Rosenstein RE. (2010) Physiology of circadian entrainment. Physiol Rev 90:1063-1102. [DOI] [PubMed] [Google Scholar]
- Groó Z, Szenczi P, Bánszegi O, Altbäcker V. (2013) Natal dispersal in two mice species with contrasting social systems. Behav Ecol Sociobiol 67:235-242. [Google Scholar]
- Hammelrath L, Škokić S, Khmelinskii A, Hess A, van der Knaap N, Staring M, Lelieveldt BPF, Wiedermann D, Hoehn M. (2016) Morphological maturation of the mouse brain: an in vivo MRI and histology investigation. NeuroImage 125:144-152. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. (2015) Recent genetic findings in schizophrenia and their therapeutic relevance. J Psychopharmacol 29:85-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Clark DB. (2013) Circadian misalignment, reward-related brain function, and adolescent alcohol involvement. Alcohol Clin Exp Res 37:558-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hena M, Garmy P. (2020) Social jetlag and its association with screen time and nighttime texting among adolescents in Sweden: a cross-sectional study. Front Neurosci 14:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstetter JR, Lysaker PH, Mayeda AR. (2005) Quality of sleep in patients with schizophrenia is associated with quality of life and coping. BMC Psychiatry 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsey EA, Maletta T, Turner H, Cole C, Lehmann H, Fournier NM. (2020) Chronic jet lag simulation decreases hippocampal neurogenesis and enhances depressive behaviors and cognitive deficits in adult male rats. Front Behav Neurosci 13:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, Kapur S. (2012) The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry 69:776-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingiosi AM, Schoch H, Wintler T, Singletary KG, Righelli D, Roser LG, Medina E, Risso D, Frank MG, Peixoto L. (2019) Shank3 modulates sleep and expression of circadian transcription factors. eLife 8:e42819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juraska JM, Sisk CL, DonCarlos LL. (2013) Sexual differentiation of the adolescent rodent brain: hormonal influences and developmental mechanisms. Horm Behav 64:203-210. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS. (2011) Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci 108:1657-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad K, Firk C, Uhlhaas PJ. (2013) Brain development during adolescence: neuroscientific insights into this developmental period. Dtsch Arztebl Int 110:425-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Aasen I, Sharma T. (2004) Sex differences in prepulse inhibition deficits in chronic schizophrenia. Schizophr Res 69:219-235. [DOI] [PubMed] [Google Scholar]
- Lamont EW, Legault-Coutu D, Cermakian N, Boivin DB. (2007) The role of circadian clock genes in mental disorders. Dialogues Clin Neurosci 9:333-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer AM, Behrens D, Klump G. (2017) Acoustic startle modification as a tool for evaluating auditory function of the mouse: progress, pitfalls, and potential. Neurosci Biobehav Rev 77:194-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FY, Wang HB, Hitchcock ON, Loh DH, Whittaker DS, Kim YS, Aiken A, Kokikian C, Dell’Angelica EC, Colwell CS, et al. (2018) Sleep/wake disruption in a mouse model of BLOC-1 deficiency. Front Neurosci 12:759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zhang Q, Oiso N, Novak EK, Gautam R, O’Brien EP, Tinsley CL, Blake DJ, Spritz RA, Copeland NG, et al. (2003) Hermansky-Pudlak syndrome type 7 (HPS-7) results from mutant dysbindin, a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1). Nat Genet 35:84-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan RW, Shafer AR, Ngari W, Hasler BP. (2018) Circadian rhythms, impulsivity, and adolescence: effects of circadian misalignment on the neural response during the go/no-go task in human adolescents. Sleep 41:A99. [Google Scholar]
- Ma X, Fei E, Fu C, Ren H, Wang G. (2011) Dysbindin-1, a schizophrenia-related protein, facilitates neurite outgrowth by promoting the transcriptional activity of p53. Mol Psychiatry 16:1105-1116. [DOI] [PubMed] [Google Scholar]
- MacCabe JH, Wicks S, Löfving S, David AS, Berndtsson Gustafsson ÅJE, Allebeck P, Dalman C. (2013) Decline in cognitive performance between ages 13 and 18 years and the risk for psychosis in adulthood: a Swedish longitudinal cohort study in males. JAMA Psychiatry 70:261-270. [DOI] [PubMed] [Google Scholar]
- Manning EE, Wang AY, Saikali LM, Winner AS, Ahmari SE. (2021) Disruption of prepulse inhibition is associated with compulsive behavior severity and nucleus accumbens dopamine receptor changes in Sapap3 knockout mice. Sci Rep 11:9442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew GM, Li X, Hale L, Chang AM. (2019) Sleep duration and social jetlag are independently associated with anxious symptoms in adolescents. Chronobiol Int 36:461-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon RA, Reis Marques T, Howes OD. (2020) Schizophrenia—an overview. JAMA Psychiatry 77:201-210. [DOI] [PubMed] [Google Scholar]
- McGrath J, Saha S, Welham J, El Saadi O, MacCauley C, Chant D. (2004) A systematic review of the incidence of schizophrenia: the distribution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC Med 2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nihonmatsu-Kikuchi N, Hashimoto R, Hattori S, Matsuzaki S, Shinozaki T, Miura H, Ohota S, Tohyama M, Takeda M, Tatebayashi Y. (2011) Reduced rate of neural differentiation in the dentate gyrus of adult dysbindin null (sandy) mouse. PLoS ONE 6:e15886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaya JC, Heusner CL, Matsumoto M, Shannon Weickert C, Karl T. (2018) Schizophrenia-relevant behaviours of female mice overexpressing neuregulin 1 type III. Behav Brain Res 353:227-235. [DOI] [PubMed] [Google Scholar]
- Patel PK, Leathem LD, Currin DL, Karlsgodt KH. (2021) Adolescent neurodevelopment and vulnerability to psychosis. Biol Psychiatry 89:184-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji H, Miyakawa T. (2018) Relationships between the acoustic startle response and prepulse inhibition in C57BL/6J mice: a large-scale meta-analytic study. Mol Brain 11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Şık A, van Nieuwehuyzen P, Prickaerts J, Blokland A. (2003) Performance of different mouse strains in an object recognition task. Behav Brain Res 147:49-54. [DOI] [PubMed] [Google Scholar]
- Srikanta SB, Stojkovic K, Cermakian N. (2021) Behavioral phenotyping of mice lacking the deubiquitinase USP2. PLoS ONE 16:e0241403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima N, Odaka YS, Sakoori K, Akagi T, Hashikawa T, Morimura N, Yamada K, Aruga J. (2011) Impaired cognitive function and altered hippocampal synapse morphology in mice lacking Lrrtm1, a gene associated with schizophrenia. PLoS ONE 6:e22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot K. (2009) The sandy (Sdy) mouse: a dysbindin-1 mutant relevant to schizophrenia research. Prog Brain Res 179:87-94. [DOI] [PubMed] [Google Scholar]
- Talbot K, Eidem WL, Tinsley CL, Benson MA, Thompson EW, Smith RJ, Hahn CG, Siegel SJ, Trojanowski JQ, Gur RE, et al. (2004) Dysbindin-1 is reduced in intrinsic, glutamatergic terminals of the hippocampal formation in schizophrenia. J Clin Invest 113:1353-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot K, Louneva N, Cohen JW, Kazi H, Blake DJ, Arnold SE. (2011) Synaptic dysbindin-1 reductions in schizophrenia occur in an isoform-specific manner indicating their subsynaptic location. PLoS ONE 6:e16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot K, Ong WY, Blake DJ, Tang J, Louneva N, Carlson GC, Arnold SE. (2009) Dysbindin-1 and its protein family. In: Lajtha A, Javitt D, Kantrowitz J, editors. Handbook of neurochemistry and molecular neurobiology. Boston (MA): Springer. p. 107-241. [Google Scholar]
- Tang J, LeGros RP, Louneva N, Yeh L, Cohen JW, Hahn CG, Blake DJ, Arnold SE, Talbot K. (2009) Dysbindin-1 in dorsolateral prefrontal cortex of schizophrenia cases is reduced in an isoform-specific manner unrelated to dysbindin-1 mRNA expression. Hum Mol Genet 18:3851-3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kammen DP, van Kammen WB, Peters J, Goetz K, Neylan T. (1988) Decreased slow-wave sleep and enlarged lateral ventricles in schizophrenia. Neuropsychopharmacology 1:265-271. [PubMed] [Google Scholar]
- van Os J, Kapur S. (2009) Schizophrenia. Lancet 374:635-645. [DOI] [PubMed] [Google Scholar]
- van Os J, Bak M, Hanssen M, Bijl RV, de Graaf R, Verdoux H. (2002) Cannabis use and psychosis: a longitudinal population-based study. Am J Epidemiol 156:319-327. [DOI] [PubMed] [Google Scholar]
- Varese F, Smeets F, Drukker M, Lieverse R, Lataster T, Viechtbauer W, Read J, van Os J, Bentall RP. (2012) Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr Bull 38:661-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. (2007) The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2:322-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Xu J, Lazarovici P, Zheng W. (2017) Dysbindin-1 involvement in the etiology of schizophrenia. Int J Mol Sci 18:2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters F, Chiu V, Atkinson A, Blom JD. (2018) Severe sleep deprivation causes hallucinations and a gradual progression toward psychosis with increasing time awake. Front Psychiatry 9:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert CS, Rothmond DA, Hyde TM, Kleinman JE, Straub RE. (2008) Reduced DTNBP1 (dysbindin-1) mRNA in the hippocampal formation of schizophrenia patients. Schizophr Res 98:105-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert CS, Straub RE, McClintock BW, Matsumoto M, Hashimoto R, Hyde TM, Herman MM, Weinberger DR, Kleinman JE. (2004) Human dysbindin (DTNBP1) gene expression in normal brain and in schizophrenic prefrontal cortex and midbrain. Arch Gen Psychiatry 61:544-555. [DOI] [PubMed] [Google Scholar]
- Wicks S, Hjern A, Gunnell D, Lewis G, Dalman C. (2005) Social adversity in childhood and the risk of developing psychosis: a national cohort study. Am J Psychiatry 162:1652-1657. [DOI] [PubMed] [Google Scholar]
- Wulff K, Dijk DJ, Middleton B, Foster RG, Joyce EM. (2012) Sleep and circadian rhythm disruption in schizophrenia. Br J Psychiatry 200:308-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Silverman JL, Crawley JN. (2011) Automated three-chambered social approach task for mice. Curr Protoc Neurosci Chapter 8:Unit 8.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Han M, Tan S, De Yang F, Tan Y, Jiang S, Zhang X, Huang XF. (2017) Gender differences measured by the MATRICS consensus cognitive battery in chronic schizophrenia patients. Sci Rep 7:11821. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jbr-10.1177_07487304221125363 for Exposure to Circadian Disruption During Adolescence Interacts With a Genetic Risk Factor to Modify Schizophrenia-relevant Behaviors in a Sex-dependent Manner by Marie-Ève Cloutier, Lalit K. Srivastava and Nicolas Cermakian in Journal of Biological Rhythms