Abstract
Background
Anti-melanoma differentiation-associated protein 5 (MDA5) associated idiopathic inflammatory myopathy (IIM) often manifests with minimal muscle weakness and rapidly progressive interstitial lung disease (RP-ILD) with a poor prognosis. The clinical presentation may be varied in different ethnic groups. The ongoing coronavirus disease (COVID-19) pandemic has made management even more challenging as certain manifestations may be difficult to diagnose remotely.
Aim of the work
To throw light on the rare association of CMV infection in established anti-MDA5 myositis with severe consequences. Similar cases were presented and compared.
Case report
A 42-year-old lady presented with heliotrope rash, periorbital edema, ulcerated Gottron’s papules, proximal muscle weakness and intermittent fever of six-month duration. Anti-MDA5 antibodies were positive. Active disease, including myocarditis and RP-ILD, were challenging to diagnose on teleconsultation. Upon initiating tofacitinib, cytomegalovirus (CMV) polymerized chain reaction (PCR) came positive. Ganciclovir was started with the possibility of viral activation being the potential driving force for interferon pathway activation and dermatomyositis (DM) flare, but the patient succumbed to the illness.
Conclusion
Viral triggers are known to induce autoimmune disease in the genetically predisposed. However, CMV infection in established anti-MDA5 myositis is uncommon and further association with myocarditis is a rare occurrence. Ulcerated Gottron’s and periorbital oedema may carry a sinister connotation in Indians with anti-MDA5 DM, with worse manifestations such as myocarditis– which albeit rare, can be fatal.
Keywords: Myositis, Dermatomyositis, Cytomegalovirus, Teleconsultation, Immunodeficiency
1. Introduction:
Dermatomyositis (DM) presents with florid muscle weakness and varied cutaneous manifestations and comprises skin features as heliotrope rash, gottron’s papules, macular erythema, and occasionally palmar papules and skin ulceration [1], [2]. In contrast, anti-melanoma differentiation-associated protein 5 (MDA5) positive DM is a rare subset which oftentimes manifest with minimal muscle weakness and rapidly progressive interstitial lung disease (RP-ILD) with a poor prognosis unless diagnosed by testing for specific Myositis-specific antibodies (MSAs) [3], [4]. Classic cutaneous manifestations vary in different ethnic groups and may be important pointers towards diagnosis [5], [6]. Cutaneous ulceration is reported in 50% of anti-MDA5 associated idiopathic inflammatory myopathies (IIM), most frequently over the elbows, knuckles, knees, lateral nail folds and also seen over sun exposed areas as chest or back [1], [3].
Interstitial lung disease (ILD) among Asians is generally rapidly progressive compared to Westerners and frequently culminates in fatal outcomes with up to 70% mortality in patients with anti-MDA5 IIM [4], [5]. It is crucial to initiate prompt intensive treatment for RP-ILD with anti-MDA5 antibody due to its poor prognosis. The measurement of titer of anti-MDA5 antibodies takes several days and this can delay the necessary intervention. Consequently, identifying key clinical signs in suspects can offer important guidance for initiating early aggressive therapy. The ongoing coronavirus disease (COVID-19) pandemic has made management challenging as certain manifestations may be difficult to diagnose remotely.
As we are in the midst of an unprecedented pandemic, we can only speculate the long-term effects and implications of COVID-19 [6]. A negative impact of the pandemic on the QoL of RA patients has been described [7]. Among persons with COVID-19, the development of fibromyalgia syndrome was anticipated as a consequence to pandemic-associated stressors [6]. A causal relationship between vasculitis and viral infections has been postulated. Vasculitis is potentially one of COVID-19′s presenting symptoms and prompt diagnosis and treatment is crucial in improving outcome of patients [8]. Viral triggers are known to induce autoimmune disease in the genetically predisposed. However, the hypothesis that viral infections can drive autoimmunity in established anti-MDA5 myositis is proposed but poorly studied. Myocarditis is another cause of mortality in IIM though not typically described in anti MDA5 IIM.
A case is presented with anti MDA5 positive IIM with CMV infection associated flare of dermatomyositis (DM), who succumbed to RP-ILD and myocarditis. Albeit rare, myocarditis should be actively looked out for in patient with MDA5 positive IIM.
2. Methods
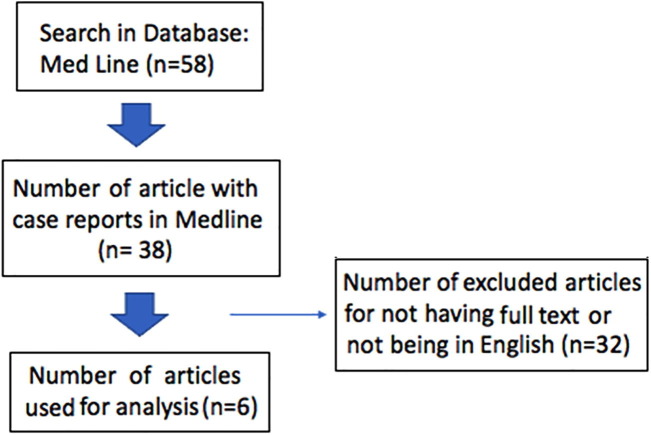
A search was conducted using the terms (“Cytomegalovirus and myositis”) published on MEDLINE any time. Of fifty-eight articles obtained, 38 case reports were identified. After excluding articles in languages other than English and those without available full-text, six reports were used for further analysis. Written consent was obtained from the patient described (Fig. 1 ).
Fig. 1.
Flow chart for the search strategy of the terms (“Cytomegalovirus and myositis”) published on MEDLINE any time.
3. Case presentation
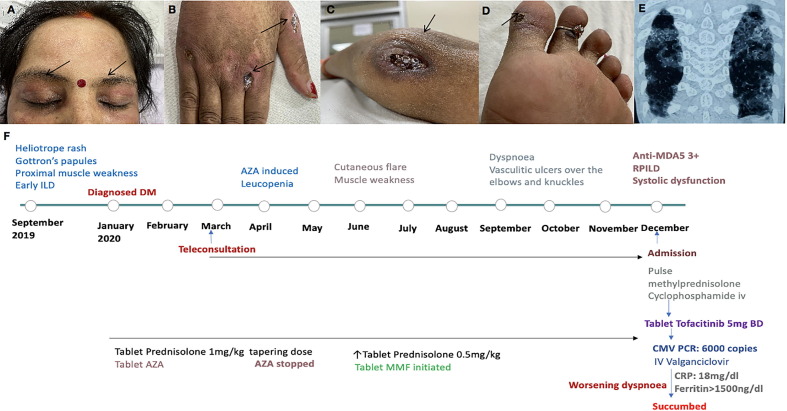
A 42-year-old lady presented with heliotrope rash and periorbital edema, ulcerated Gottron’s papules, proximal muscle weakness, and intermittent fever of six-month duration. Computerized Tomography (CT) of the chest suggested early ILD. Myositis specific autoantibodies were sent, and azathioprine (AZA) initiated for DM. The patient developed cytopenia with AZA which improved on discontinuing the drug though the patient’s condition rapidly deteriorated during the imposed lockdown period. She returned with symptoms of easy fatiguability, dyspnea, and vasculitis ulcers over the Gottron’s rashes, elbow, and feet (Fig. 2 a–d) requiring in-patient admission nine months after initial presentation and 15 months into the illness. Anti- MDA5 antibodies tested positive. 2D-Echocardiography confirmed systolic dysfunction and CT revealed rapid progression of interstitial lung disease (RP-ILD), both of which were missed on teleconsultations (Fig. 2e, f).
Fig. 2.
A: Heliotrope rash with periorbital puffiness; B: Vasculitis ulcer over knuckles; C: Vasculitis ulcer involving elbow; D: Vasculitis ulcer involving feet; E: Both the lung fields having patchy consolidations with ground glass opacities suggestive of an organizing pneumonia pattern; F: Timeline of the patient's clinical manifestations along with treatment.
As she continued to worsen despite pulse methylprednisolone and cyclophosphamide, tofacitinib was initiated. However, it had to be discontinued after CMV polymerized chain reaction (PCR) turned positive. She was treated with ganciclovir with the possibility of viral activation being the potential driving force for interferon pathway activation and flare of DM. Despite continuing high dose glucocorticoids and antivirals, CRP (18 mg/dl) and high ferritin (>1500 ng/ml) remained high, and patient succumbed to her illness over a fortnight of in-patient management.
4. Discussion
Ulcerated Gottron’s and periorbital edema can be important clues to underlying anti-MDA5 DM and viral activation could be the underlying driver of autoimmunity [9]. Six other cases were identified in literature reporting the intersection of CMV with IIM (Table 1 ). A few cases were successfully managed with ganciclovir though many succumbed despite treatment. High mortality of anti-MDA5 positive DM and association with CMV infections and seasonality of the former opens the case to explore underlying inborn immune pathway defects which may predispose these individuals to viral infections [10]. Recently identified similarity between COVID-19 and anti-MDA5 positive IIM further highlights an important role of viruses in triggering and sustaining autoimmunity. MDA5 being the cytoplasmic sensor for viral RNA could potentially predispose to infections when defective or lead to autoimmunity from hyperfunctioning gain-of-function mutations. Autoimmunity and primary immunodeficiency are now known to be along a continuum, with the recent recognition of heritable digenic and polygenic defects in several autoimmune disorders with widespread availability of whole exome sequencing [11].Therefore, it seems plausible that greater insight into the area may potentially classify anti-MDA5 DM as an adult-onset primary immunodeficiency with autoimmunity with important therapeutic implications [12]. Milder disease course in another subset of anti-MDA5 IIM suggests the disease may be bi-phenotypic with two distinct variants in the Indian population [9].
Table 1.
Association of cytomegalovirus (CMV) infection with myositis case reports.
| Case Report | Country/year | Caseage/sex | Risk factors | With CMV | MSA | Other organs involved | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| Gupta et al | India/2021 | 42/F | DM | Yes | MDA5 | Myocarditis, ILD | AZA, MP, CYC, tofacitinib, GCV | Death |
| Sakthivadivel et al [14] | India/2020 | 29/M | GBS | Yes | None | Oral ulcer, Myonecrosis | P, valganciclovir, plasmapheresis | Improved with plasmapheresis. |
| Zhang et al [15] | China/2020 | 61/M | DM | Yes | None | Pneumonia, Bronchitis | MP, moxifloxacin, TZP, oseltamivir, voriconazole, GCV. | Resolved after 4 days |
| Lange et al [16] | USA/2018 | Middle aged/F | MAS | Yes | anti-RNP anti-Ro | Endocarditis, DPI, GNB, UTI | Valganciclovir, DX, anakinra etoposide | Death |
| Hozumi et al [17] | Japan/2012 | 62/F | PM | Yes | None | Ulcerating bronchitis | IV GCV | Improved after 3 wks |
| Hashimoto et al [18] | Japan/2006 | 67/F | DM | Yes | None | ILD | CYC, P, GCV | Death |
| 72/F | DM | Yes | None | P, GCV | Improved after 3 mo | |||
| Naylor et al [19] | UK/1987 | 65/F | – | Yes | None | ARF | Peritoneal dialysis | Ms. strength recovery 4 mo after discharge |
| 69/F | – | No | None | subclinical HT | Corticosteroids |
DM: Dermatomyositis, GBS: Guillain-Barre syndrome, MAS: macrophage activation syndrome, CMV: cytomegalovirus, MSA: myositis-specific autoantibodies, PM: Polymyositis, MTX: methotrexate, anti-RNP: anti-ribonucleoprotein, UTI: urinary tract infection, ILD: Interstitial Lung disease, DPI: Diffuse pulmonary infiltrates, GNB: gram-ve bacteremia, ARF: acute renal failure, HT: Hashimoto's thyroiditis, AZA: azathioprine, MP: methylprednisolone, TZP: Piperacillin/Tazobactam, GCV: Ganciclovir, DX: dexamethasone, CYC: cyclophosphamide, P: Prednisolone, TMP-SMX: Trimethoprim-sulfamethoxazole, Ms: muscle.
Teleconsultation has assumed the center stage in management of chronic diseases during the COVID-19 pandemic [13]. Although the preferred means of communication in these unusual times, there is felt need to develop means of better clinical examination and identify key aspects of poor health like cardiac or muscle disease through audiovisual consultations [11]. The rare occurrence of myocarditis in anti-MDA5 DM may be fatal as in this case, and greater attention to cardiac function may be considered in management.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are thankful to the Connecting Researchers and Dr Malke Asaad for their help with this work.
Footnotes
Peer review under responsibility of Egyptian Society of Rheumatic Diseases.
References:
- 1.Narang N.S., Casciola-Rosen L., Li S., Chung L., Fiorentino D.F. Cutaneous ulceration in dermatomyositis: Association with anti–melanoma differentiation–associated gene 5 antibodies and interstitial lung disease. Arthritis Care Res. 2015;67(5):667–672. doi: 10.1002/acr.22498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li L., Wang Q., Yang F., Wu C., Chen S., Wen X., et al. Anti-MDA5 antibody as a potential diagnostic and prognostic biomarker in patients with dermatomyositis. Oncotarget. 2017;8(16):26552–26564. doi: 10.18632/oncotarget.15716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tansley S.L., Betteridge Z.E., Gunawardena H., Jacques T.S., Owens C.M., Pilkington C., et al. Anti-MDA5 autoantibodies in juvenile dermatomyositis identify a distinct clinical phenotype: a prospective cohort study. Arthritis Res Ther. 2014;16(4):R138. doi: 10.1186/ar4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawasumi H., Gono T., Kawaguchi Y., Yamanaka H. Recent treatment of interstitial lung disease with idiopathic inflammatory myopathies. Clin Med Insights Circ Respir Pulm Med. 2015;9:9–17. doi: 10.4137/CCRPM.S23313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chino H., Sekine A., Baba T., Kitamura H., Iwasawa T., Okudela K., et al. Interstitial lung disease with anti-melanoma differentiation-associated protein 5 antibody: rapidly progressive perilobular opacity. Intern Med. 2019;58(18):2605–2613. doi: 10.2169/internalmedicine.2328-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gheita T.A., Fathi H.M., ElAdle S.S., Eesa N.N., Hammam N.H. Coronavirus disease 2019 (COVID-19) an emerging trigger for primary fibromyalgia syndrome: A tale of three cases post-COVID-19. Int J Clin Rheumatol. 2021;16(4):129–135. [Google Scholar]
- 7.Zomalheto Z., Assogba C., Dossou-yovo H. Impact of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) infection and disease-2019 (COVID-19) on the quality of life of rheumatoid arthritis patients in Benin. Egyptan Rheumatol. 2021;43(1):23–27. doi: 10.1016/j.ejr.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assar S., Pournazari M., Soufivand P., Mohamadzadeh D., Sanaee S. Microscopic polyangiitis associated with coronavirus disease-2019 (COVID-19) infection in an elderly male. Egypt Rheumatol. 2021;43(3):225–228. doi: 10.1016/j.ejr.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta L., Naveen R., Gaur P., Agarwal V., Aggarwal R. Myositis-specific and myositis-associated autoantibodies in a large Indian cohort of inflammatory myositis. Semin Arthritis Rheum. 2021;51(1):113–120. doi: 10.1016/j.semarthrit.2020.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Mehta P., Agarwal V., Gupta L. High early mortality in idiopathic inflammatory myopathies: Results from the inception cohort at a tertiary care center in northern India. Rheumatology (Oxford) 2021:keab001. doi: 10.1093/rheumatology/keab001. [DOI] [PubMed] [Google Scholar]
- 11.Gupta L., Chinoy H. Monitoring disease activity and damage in adult and juvenile idiopathic inflammatory myopathy. Curr Opin Rheumatol. 2020;32(6):553–561. doi: 10.1097/BOR.0000000000000749. [DOI] [PubMed] [Google Scholar]
- 12.Rathore U., Haldule S., Gupta L. Psoriasiform rashes as the first manifestation of anti-MDA5 associated myositis. Rheumatology (Oxford) 2020:keaa821. doi: 10.1093/rheumatology/keaa821. [DOI] [PubMed] [Google Scholar]
- 13.Gupta L., Lilleker J.B., Agarwal V., Chinoy H., Aggarwal R. COVID-19 and myositis - unique challenges for patients. Rheumatology (Oxford) 2021;60(2):907–910. doi: 10.1093/rheumatology/keaa610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakthivadivel V., Naveenraj P., Kachhwaha A., Kumar D., Anne P.B., Elhence P., et al. Concurrent acute myositis and Guillain-Barre syndrome in cytomegalovirus infection – a rare case report. BMC Infect Dis. 2020;20(1) doi: 10.1186/s12879-020-05506-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang K., Yu C., Li Y., Wang Y. Next-generation sequencing technology for detecting pulmonary fungal infection in bronchoalveolar lavage fluid of a patient with dermatomyositis: a case report and literature review. BMC Infect Dis. 2020;20 doi: 10.1186/s12879-020-05341-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lange AV, Kazi S, Chen W, Barnes A. Fatal case of macrophage activation syndrome (MAS) in a patient with dermatomyositis and cytomegalovirus viraemia. BMJ Case Rep.2018. 2018:bcr2018225231. [DOI] [PMC free article] [PubMed]
- 17.Hozumi H., Fujisawa T., Kuroishi S., Inui N., Nakamura Y., Suda T., et al. Ulcerating bronchitis caused by cytomegalovirus in a patient with polymyositis. Intern Med. 2012;51(20):2933–2936. doi: 10.2169/internalmedicine.51.8497. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto A., Okuyama R., Watanabe H., Tagami H., Aiba S. Cytomegalovirus infection complicating immunosuppressive therapy for dermatomyositis. Acta Derm Venereol. 2006;86(6):535–537. doi: 10.2340/00015555-0152. [DOI] [PubMed] [Google Scholar]
- 19.Naylor C.D., Jevnikar A.M., Witt N.J. Sporadic viral myositis in two adults. CMAJ. 1987;137:819–821. [PMC free article] [PubMed] [Google Scholar]