Abstract
Specific major surface protein 2 (MSP2) variants are expressed by Anaplasma marginale within the tick salivary gland and, following transmission, are expressed during acute rickettsemia. In previous work, we have shown that a restricted pattern of MSP2 variants is expressed in the salivary glands of Dermacentor andersoni ticks infected with the South Idaho strain of A. marginale. Now we demonstrate that the identical restriction does not apply to two other strains of A. marginale, and that different variants are also expressed when the same strain is transmitted by different Dermacentor spp. This indicates that antigenic diversity among strains is maintained in tick transmission and may be a significant constraint to MSP2 vaccine development.
Anaplasma marginale is a tick-borne pathogen of cattle that causes severe anemia during acute rickettsemia (13). Individuals that survive acute disease remain persistently infected and serve as reservoirs for transmission (4, 25). Persistent infection is characterized by sequential cycles of rickettsemia, each composed of a progressive, logarithmic increase in rickettsemia followed by a precipitous decrease (3, 4, 9). In each cycle A. marginale that express novel structural and antigenic variants of the immunodominant outer membrane protein major surface protein 2 (MSP2) emerge (7, 8). These variants, typified by amino acid substitutions, deletions, and insertions in the central hydrophilic region of MSP2, express unique B-cell epitopes that are recognized, not at the time of emergence, but only following control of each rickettsemic cycle (7). Thus, the antigenic structure of the A. marginale populations continually changes throughout persistent infection and ixodid ticks feeding during persistence ingest a heterogeneous population of variants that differ over time and among individual animals within a herd (15).
Following ingestion in the bloodmeal by feeding ticks, A. marginale undergoes a complex developmental cycle of replication within midgut epithelium and gut muscle cells, culminating in the development of infective stages in the tick salivary gland (10, 11, 22). In studies using Dermacentor andersoni acquisition and transmission of the South Idaho strain of A. marginale, we discovered that a restricted set of MSP2 variants were expressed within the salivary gland and transmitted to naïve cattle (19). The same MSP2 salivary gland variants (SGV) were expressed within ticks that had acquired A. marginale infection by feeding on different individual calves at different time points, feeding during both acute and persistent rickettsemia, and feeding on rickettsemic blood containing distinctly different MSP2 variants (19). The restriction of MSP2 variant heterogeneity in the salivary gland is significant, as A. marginale expressing these variants were transmitted to cattle and subsequently composed the acute rickettsemia (19). This suggested that, in contrast to the antigenic heterogeneity in persistently infected cattle, the restricted set of transmitted MSP2 SGV could provide a stable target for vaccine development.
A. marginale strains isolated from acute disease outbreaks can be distinguished genetically and differ in the antigenic structure of the major surface proteins, virulence, and tick transmissibility (1, 5, 14, 21, 24). However, all examined strains contained the polymorphic msp2 multigene family and expressed structurally variant MSP2 during each of the rickettsemic cycles in persistent infection (2, 6, 7, 15–19). Vaccine development based on a restricted set of MSP2 SGV would require that only these variants, or at least a limited number of variants, be expressed by the salivary gland stages of multiple, and ideally all, A. marginale strains. Do different strains of A. marginale express identical MSP2 SGV within the tick? We addressed this question by comparing the sequences of msp2 transcripts expressed in the salivary glands of D. andersoni ticks fed on cattle infected with the St. Maries (Idaho) strain with the MSP2 SGV1 and SGV2 expressed by the South Idaho strain of A. marginale. Both strains are naturally transmitted by D. andersoni ticks and have been shown to be experimentally transmitted by the D. andersoni laboratory stock isolated in Idaho and used in this experiment (4, 5). Calf 787 was infected by intravenous inoculation of a stabilate containing 1010 erythrocytes infected with the St. Maries strain. Giemsa-stained blood smears were examined daily to monitor the development of acute rickettsemia, and when rickettsemia levels reached 109 infected erythrocytes per ml, 250 laboratory-reared adult male D. andersoni ticks were placed in an orthopedic stockinette and allowed to attach and acquisition-feed for 7 days. The ticks were removed and incubated for an additional 7 days at 26°C with 90 to 98% relative humidity and a 14-h photo period. To stimulate development of the infective stage in the salivary gland (10, 11, 22), the ticks were allowed to attach and feed on an uninfected calf, 789, for 3 days. Ticks were then removed and total RNA was extracted from isolated salivary glands, as previously described (19). Transmission to calf 789 was confirmed by microscopic detection of A. marginale-infected erythrocytes, and total RNA was extracted from whole blood collected on the first day of microscopically detectable rickettsemia, using Trizol (Bio-Rad Laboratories), as described previously (8). Total RNA was reverse transcribed with random hexamers, and msp2 cDNA was amplified by using PCR (2, 8). The full-length transcript was amplified by using forward and reverse primers from the conserved 5′ and 3′ ends (7, 19). To amplify only the msp2 hypervariable region, primers derived from the conserved regions that flank the central, hypervariable 595-bp region of msp2 were used (7, 8). The primer sequences, amplification conditions, cloning into pCR2.1, and sequencing were all as previously reported (7, 8, 19). D. andersoni adult male ticks of the same stock were acquisition-fed on an uninfected calf and were handled identically and served as negative controls. No msp2-specific amplicons were identified with salivary gland RNA from these control ticks.
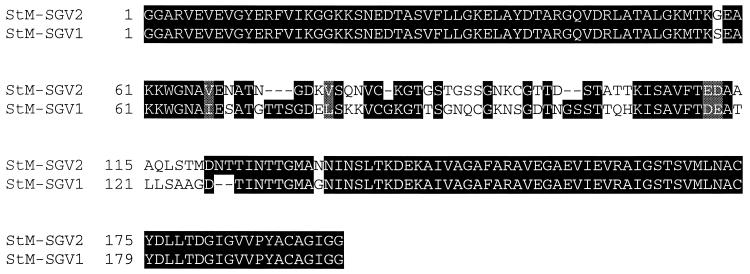
Variant msp2-sgv full-length transcripts were identified in the St. Maries strain-infected salivary glands by sequencing 37 independently derived cDNA clones. Consistent with previous results from studies with the Florida and South Idaho strains, MSP2 polymorphism in transcripts of the St. Maries strain was localized to the central hypervariable region (amino acids 185 to 280, based on the predicted amino acid sequence of pCKR11.2 msp2 [16]). The MSP2 SGV hypervariable region sequences encoded by the two predominant transcripts, defined as composing more than 10% of the cDNA clones, were designated St. Maries MSP2 SGV1 and SGV2 (Fig. 1). Neither these nor the minor variants (fewer than 10% of the total clones sequenced) encoded proteins identical to the previously reported South Idaho strain MSP2 SGV1 and SGV2 (19). The most similar are the St. Maries MSP2 SGV2 and the South Idaho MSP2 SGV1, which share 90% identity in the approximately 200 amino acids composing the central hypervariable region. Thus, two strains, both isolated from acute outbreaks in Idaho and naturally transmitted by D. andersoni, expressed distinctly different MSP2 SGV in the same stock of D. andersoni. In addition, the St. Maries strain expressed multiple heterogeneous variants, unlike the restricted expression of only two closely related variants by the South Idaho strain (19).
FIG. 1.
Amino acid sequence alignment of the hypervariable regions from MSP2 SGV1 and SGV2 from the St. Maries (StM) strain of A. marginale. Areas of amino acid substitutions, insertions, and deletions are indicated by a white background, areas of amino acid identity have a black background, and grey shading indicates conservative amino acid substitutions.
Analysis of the A. marginale transcripts expressed during acute rickettsemia of calf 789, following tick transmission of the St. Maries strain, revealed that 10 of 11 clones had the St. Maries MSP2 SGV1 sequence. Expression of identical MSP2 in both the salivary gland and in the bloodstream also occurs in the South Idaho strain (19). This pattern is notably different from that shown by tick-transmitted Borrelia hermsii, in which there is a switch in the expressed surface coat between organisms in the salivary gland and those in the blood of the mammalian host following transmission (20). For A. marginale, expression of new variants of MSP2 is not seen until later in acute rickettsemia, presumably reflecting immune selection of MSP2 variants (15, 19).
The differences in MSP2 SGV between the South Idaho and St. Maries strains raised the question of whether specific hypervariable region sequences in each strain were associated with development within the tick salivary gland. If so, the MSP2 SGV hypervariable regions would be expected to cluster by strain. To increase the number of strain-specific sequences for analysis, the acquisition feeding and development of infective A. marginale stages in the salivary gland was repeated with a third D. andersoni-transmissible strain. Calf 794 was infected by intravenous inoculation of 1010 erythrocytes infected with the Virginia strain and was monitored, as described above, for development of rickettsemia. Adult male D. andersoni ticks were acquisition-fed on calf 794, and following incubation and transmission feeding to stimulate development of infectivity, RNA was isolated from the salivary glands. The msp2-specific cDNA clones were obtained and sequenced, as described previously for the South Idaho strain (19). Again, none of the Virginia strain MSP2 SGV was identical to any of those expressed by either the South Idaho or the St. Maries strain. Comparison of the two South Idaho MSP2 SGV, four St. Maries MSP2 SGV (major and minor variants), and the six Virginia MSP2 SGV by using a phylogram based on the hypervariable region amino acid sequences revealed that the expressed MSP2 SGV do not segregate by strain (Fig. 2). This is illustrated by comparison of Virginia MSP2 SGV1, which is more similar to the South Idaho MSP2 SGV1 and SGV2 and to St. Maries MSP2 SGV2 than to any other Virginia MSP2 SGV (Fig. 2). Furthermore, examination of the MSP2 SGV encoded by each strain using BestFit analysis to detect small regions of conservation and the Genetics Computer Group shuffle program to test significance of conserved oligopeptides (program manual for the Genetics Computer Group package, Genetics Computer Group, Madison, Wis.) failed to identify any strain-specific oligopeptide motifs within the hypervariable region (data not shown).
FIG. 2.
A phylogram of MSP2 SGV types expressed by the South Idaho (SI), St. Maries (StM), and Virginia (VA) strains in D. andersoni, based on predicted amino acid sequences. The SEQBOOT, PROTDIST, NEIGHBOR, and CONSENSE programs in the PHYLIP phylogenetic inference package were used for the derivation of the data used in the phylogram (6). Bootstrap values from 100 analyses are shown at the branch points of the tree.
Overall, the polymorphism encoded within the SGV-MSP2 hypervariable regions of the three tick-transmitted strains examined is similar to that expressed by a single strain during rickettsemic cycles in persistently infected cattle (7, 8, 19). The MSP2 central hypervariable region has been shown to encode surface-exposed B-cell epitopes (2, 7, 8), and even very closely related MSP2 proteins, sharing more than 90% identity in amino acid sequence, are antigenically distinct (2). Furthermore, these MSP2 epitopes are highly immunogenic and development of variant-specific antibody correlates with clearance of the expressed variant (7, 17, 23). Although all expressed MSP2 variants, including the MSP2 SGV, have highly conserved N- and C-terminal regions (2, 7, 19), these regions are hydrophobic membrane domains with minimal surface exposure (7). Consequently, the central region diversity in expressed MSP2 SGV indicates that a vaccination strategy targeted solely against the MSP2 SGV is unlikely to protect against the numerous strains transmitted by ticks within endemic regions.
To test whether the vector tick species affects the specific MSP2 SGV expressed by an A. marginale strain, the sequences of the msp2-sgv transcripts expressed by the Virginia strain within the salivary glands of adult male Dermacentor variabilis were compared to those expressed by this strain within the salivary glands of adult male D. andersoni. Like D. andersoni, D. variabilis is a natural vector of A. marginale, and the Oklahoma State University laboratory stock used in this experiment has been shown to transmit the Virginia strain (12, 22). Using separate stockinettes, 250 adult males of each D. andersoni and D. variabilis were acquisition-fed for the same 7-day period on calf 794, infected with the Virginia strain as described above. In vitro incubation, stimulation of infectivity by transmission feeding, isolation of total RNA from isolated salivary glands, generation of msp2 cDNA clones, and sequencing were done as described previously (8, 19). Three expressed MSP2 SGV were identified in the Virginia strain within D. variabilis and were designated MSP2 SGVDv 1, 2, and 3. None of these was identical to any of the six Virginia strain MSP2 SGV expressed within D. andersoni (Fig. 3). This observation is consistent with the selective or inductive role of the tick vector and suggests that the influence of the tick may differ between vector species. However, no tick species-specific motifs were identified by comparison of the Virginia strain MSP2 SGV expressed in D. andersoni and D. variabilis. Furthermore, comparison of the Virginia strain MSP2 SGV sequences in D. variabilis with all the MSP2 SGV sequences from the three strains (St. Maries, South Idaho, and Virginia) in D. andersoni indicated that expressed MSP2 SGV sequences did not cluster by tick species (Fig. 4).
FIG. 3.
Amino acid sequence alignment of the MSP2 SGV hypervariable regions expressed by the Virginia (VA) strain in D. andersoni (SGV1 to SGV6) and D. variabilis (SGVDv1 to SGVDv3). Areas of amino acid substitutions, insertions, and deletions are indicated by a white background, areas of amino acid identity have a black background, and grey shading indicates conservative amino acid substitutions.
FIG. 4.
A phylogram of MSP2 SGV types expressed by the A. marginale South Idaho (SI), St. Maries (StM), and Virginia (VA) strains in D. andersoni (SGV1 to SGV4) and in D. variabilis (SGVDV1 to SGVDV3). The distance value determination, tree construction, and calculation of the bootstrap values were done as described for Fig. 2.
In contrast to the findings in our original study, using the South Idaho strain, which has restricted expression of only two very closely related MSP2 SGV (19), in this study both the St. Maries and Virginia strains expressed multiple, heterogeneous MSP2 SGV. This heterogeneity and lack of tight restriction was observed in the Virginia strain in both vector species examined. The basis for this difference among strains is currently unknown but may reflect strain-specific selection for certain MSP2 SGV sequences or differences in regulation of gene expression. Using the sequences and methodology reported here, we were unable to identify specific hypervariable region sequences common to multiple MSP2 SGV that could associate with a required function in the salivary gland and we could not detect clustering of the expressed MSP2 SGV by either organism strain or vector species. The regulation of msp2 gene expression has not been completely defined. Recently, msp2 genes have been shown to be expressed as part of a four-gene operon (A. F. Barbet, A. Lundgren, J. Yi, F. R. Rurangirwa, and G. H. Palmer, submitted for publication). Whether A. marginale msp2 can also be expressed individually under the direct control of a msp2-specific promoter is unknown; however, this has been reported for the msp2 orthologue (p44) in the closely related agent of human granulocytic ehrlichiosis (26). This raises the possibility that expression of specific A. marginale msp2 genes may be regulated either individually or as part of an operon. Determining whether differential regulation of gene expression occurs within the tick vector and if it varies between strains is important for understanding the basis of MSP2 expression within the tick salivary gland.
Nucleotide sequence accession numbers.
The msp2 nucleotide sequences have been assigned the GenBank accession numbers AF107766 to AF107767 and AF227261 to AF227271.
Acknowledgments
This work was supported by U.S. Department of Agriculture grant 96-37204-3610, NIH grant R01 AI44005, and the Agricultural Research Service of the U.S.D.A.
We thank Ralph Horn, Yvonne McGehee, and Susan Roberts for excellent technical assistance.
REFERENCES
- 1.Allred D R, McGuire T C, Palmer G H, Leib S R, Harkins T M, McElwain T F, Barbet A F. Molecular basis for surface antigen size polymorphisms and conservation of a neutralization-sensitive epitope in Anaplasma marginale. Proc Natl Acad Sci USA. 1990;87:3220–3224. doi: 10.1073/pnas.87.8.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eid G, French D M, Lundgren A M, Barbet A F, McElwain T F, Palmer G H. Expression of major surface protein 2 antigenic variants during acute Anaplasma marginale rickettsemia. Infect Immun. 1996;64:836–841. doi: 10.1128/iai.64.3.836-841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eriks I S, Palmer G H, McGuire T C, Allred D R, Barbet A F. Detection and quantitation of Anaplasma marginale in carrier cattle by using a nucleic acid probe. J Clin Microbiol. 1989;27:279–284. doi: 10.1128/jcm.27.2.279-284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eriks I S, Stiller D, Palmer G H. Impact of persistent Anaplasma marginale rickettsemia on tick infection and transmission. J Clin Microbiol. 1993;31:2091–2096. doi: 10.1128/jcm.31.8.2091-2096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eriks I S, Stiller D, Goff W L, Panton M, Parish S M, McElwain T F, Palmer G H. Molecular and biological characterization of a new isolated Anaplasma marginale strain. J Vet Diagn Investig. 1994;6:435–441. doi: 10.1177/104063879400600406. [DOI] [PubMed] [Google Scholar]
- 6.Felsenstein J. PHYLIP (phylogency inference package), version 3.57. Seattle: University of Washington; 1995. [Google Scholar]
- 7.French D M, Brown W C, Palmer G H. Emergence of Anaplasma marginale antigenic variants during persistent rickettsemia. Infect Immun. 1999;67:5834–5840. doi: 10.1128/iai.67.11.5834-5840.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.French D M, McElwain T F, McGuire T C, Palmer G H. Expression of Anaplasma marginale major surface protein 2 variants during persistent cyclic rickettsemia. Infect Immun. 1998;66:1200–1207. doi: 10.1128/iai.66.3.1200-1207.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kieser S T, Eriks I S, Palmer G H. Cyclic rickettsemia during persistent Anaplasma marginale infection of cattle. Infect Immun. 1990;58:1117–1119. doi: 10.1128/iai.58.4.1117-1119.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kocan K M. Development of Anaplasma marginale in ixodid ticks: coordinated development of a rickettsial organism and its tick host. In: Sauer J R, Hair J A, editors. Morphology, physiology and behavioral ecology of ticks. Chichester, United Kingdom: Horwood; 1986. pp. 472–505. [Google Scholar]
- 11.Kocan K M, Golf W L, Stiller D, Edwards W, Ewing S A, Claypool P L, McGuire T C, Hair J A, Barron S J. Development of Anaplasma marginale in salivary glands of male Dermacentor andersoni. Am J Vet Res. 1993;54:107–112. [PubMed] [Google Scholar]
- 12.Kocan K M, Hair J A, Ewing S A, Stratton L G. Transmission of Anaplasma marginale Theiler by Dermacentor andersoni Stiles and Dermacentor variabilis Say. Am J Vet Res. 1981;54:15–18. [PubMed] [Google Scholar]
- 13.Losos G J. Anaplasmosis. In: Losos G J, editor. Infectious tropical diseases of domestic animals. Essex, United Kingdom: Longman House; 1986. pp. 743–795. [Google Scholar]
- 14.McGuire T C, Palmer G H, Goff W L, Johnson M I, Davis W C. Common and isolate restricted antigens of Anaplasma marginale detected with monoclonal antibodies. Infect Immun. 1984;45:697–700. doi: 10.1128/iai.45.3.697-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmer, G. H., W. C. Brown, and F. R. Rurangirwa. Antigenic variation in the persistence and transmission of the ehrlichia Anaplasma marginale. Microbes Infect., in press. [DOI] [PubMed]
- 16.Palmer G H, Eid G, Barbet A F, McGuire T C, McElwain T F. The immunoprotective Anaplasma marginale major surface protein 2 is encoded by a polymorphic multigene family. Infect Immun. 1994;62:3808–3816. doi: 10.1128/iai.62.9.3808-3816.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmer G H, Oberle S M, Barbet A F, Davis W C, Goff W L, McGuire T C. Immunization with a 36-kilodalton surface protein induces protection against homologous and heterologous Anaplasma marginale challenge. Infect Immun. 1988;56:1526–1531. doi: 10.1128/iai.56.6.1526-1531.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer G H, Rurangirwa F R, Kocan K M, Brown W C. Molecular basis for vaccine development against the ehrlichial pathogen Anaplasma marginale. Parasitol Today. 1999;15:281–286. doi: 10.1016/s0169-4758(99)01469-6. [DOI] [PubMed] [Google Scholar]
- 19.Rurangirwa F R, Stiller D, French D M, Palmer G H. Restriction of major surface protein 2 (MSP2) variants during tick transmission of the ehrlichia Anaplasma marginale. Proc Natl Acad Sci USA. 1999;96:3171–3176. doi: 10.1073/pnas.96.6.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwan T G, Hinnebusch B J. Bloodstream- versus tick-associated variants of a relapsing fever bacterium. Science. 1998;280:1938–1940. doi: 10.1126/science.280.5371.1938. [DOI] [PubMed] [Google Scholar]
- 21.Smith R D, Hungerford L L, Armstrong C T. Epidemiologic investigation and control of an epizootic of anaplasmosis in cattle in winter. J Am Vet Med Assoc. 1989;195:476–480. [PubMed] [Google Scholar]
- 22.Stiller D, Kocan K M, Edwards W, Ewing S A, Hair J A, Barron S J. Detection of colonies of Anaplasma marginale Theiler in salivary glands of three Dermacentor spp. infected as nymphs or adults. Am J Vet Res. 1989;50:1281–1386. [PubMed] [Google Scholar]
- 23.Tebele N, McGuire T C, Palmer G H. Induction of protective immunity using Anaplasma marginale initial body membranes. Infect Immun. 1991;59:3199–3204. doi: 10.1128/iai.59.9.3199-3204.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wickwire K B, Kocan K M, Barron S J. Infectivity of three Anaplasma marginale isolates for Dermacentor andersoni. Am J Vet Res. 1987;48:96–99. [PubMed] [Google Scholar]
- 25.Zaugg J L, Stiller D, Coan M E, Lincoln S D. Transmission of Anaplasma marginale Theiler by males of Dermacentor andersoni Stiles fed on an Idaho field infected, chronic carrier cow. Am J Vet Res. 1986;47:2269–2271. [PubMed] [Google Scholar]
- 26.Zhi N, Ohashi N, Rikihisa Y. Multiple p44 genes encoding major outer membrane proteins are expressed in the human granulocytic ehrlichiosis agent. J Biol Chem. 1999;274:17828–17836. doi: 10.1074/jbc.274.25.17828. [DOI] [PubMed] [Google Scholar]