Abstract
Cockroaches are one of the hardiest insects that have survived on this planet for millions of years. They thrive in unhygienic environments, are able to survive without food for up to 30 days, without air for around 45 min and being submerged under water for 30 min. Cockroaches are omnivorous and feed on a variety of foods, including cellulose and plastic, to name a few. It is intriguing that cockroaches are able to endure and flourish under conditions that are harmful to Homo sapiens. Given the importance of the gut microbiome on its’ host physiology, we postulate that the cockroach gut microbiome and/or its metabolites, may be contributing to their “hardiness”, which should be utilized for the discovery of biologically
active molecules for the benefit of human health. Herein, we discuss the biology, diet/habitat of cockroaches, composition of gut microbiome, cellular senescence, and resistance to infectious diseases and cancer. Furthermore, current knowledge of the genome and epigenome of these remarkable species is considered. Being one of the most successful and diverse insects, as well as their extensive use in traditional and Chinese medicine, the lysates/extracts and gut microbial metabolites of cockroaches may offer a worthy resource for novel bioactive molecule(s) of therapeutic potential for the benefit of human health and may be potentially used as probiotics.
Keywords: Cockroaches, Gut microbiome, Microbial metabolites, Probiotics
Introduction
Cockroaches are a fascinating and ancient species, and are hemimetabolous insects of the class Insecta, order Blattodea, which also includes termites, with their ancestors originating from the Carboniferous period, emerging approximately 300–350 million years ago (Tinker and Ottesen 2021; Wang et al. 2017; Zhao et al. 2017). To date, around 4700 species of cockroaches are known; however, it is speculated that at least twice this number is yet to be ascertained (Beccaloni 2014). Cockroaches partake in an important role in terrestrial ecosystems, through breakdown of organic materials and release of nutrients from recycling dead plants, dead animals, and it has been suggested that extinct cockroaches (Blattulidae) were probably involved in the clean-up of dinosaur excrements (Vršanský et al. 2013). On the contrary, Homo sapiens are merely one species in the midst of millions of others and are a relatively new addition to Earth (Harari 2014). In comparison, cockroaches have been able to adapt, evolve and survive successfully over millions of years, indicating that we should learn from these species. Of note, cockroaches have been utilized in traditional Chinese medicine. For example, extracts of the American cockroach (Periplaneta americana) which is usually considered a pest, has been utilised in traditional Chinese medicine (Lu et al. 2021; Xin et al. 2015). These extracts have been used to treat aches and pains, inflammation and even chronic heart failure for hundreds of years (Ma et al. 2018; Xin et al. 2015). Ethanol extract of P. americana, known as “Kangfuxin”, has been used to treat skin and mucosal injuries since the 1980s and has the approval of the Chinese Food and Drug Administration (Wang et al. 2020). Cockroaches are one of the “hardiest” insects and are able to survive without food for up to a month, without air for around 45 min and being submerged under water for 30 min (Lee et al. 2012; Wharton and Wharton 1959). Moreover, they can endure high doses of radiation: 15 times higher than humans (Lee et al. 2011; Wharton and Wharton 1959). Accordingly, it is plausible to suggest that cockroaches represent a rich and diverse source of novel molecules with biological activity originating either in their tissues or their gut microbiome contributing to their overall health and resilience (Akbar et al. 2018; Ali et al. 2017; Moshaeb et al. 2018). Several studies indicate the significance of the gut microbiome and its role in the overall health and immunity of the host (Malard et al. 2021; Siddiqui et al. 2021a, b, c; Zheng et al. 2020). Cockroaches represent an ideal organism for studying gut microbiome composition as well as host microbial interactions, as they have evolved to host a multifaceted gut microbiome comprising several species of microbes (Tinker and Otteson 2021). Here, we describe the cockroach biology, diet and habitat, gut microbiome composition, in relation to cellular senescence, the genome and epigenome, as well as resistance of cockroaches to infectious diseases and cancer. Preliminary studies investigating the anti-cancer and anti-bacterial effects of cockroach gut bacterial metabolites and the molecules elucidated are also discussed (Fig. 1). Due to their hardiness, diversity, and successful survival throughout history, we suggest that roaches need to be studied as they are an excellent resource that we can use to extract novel bioactive molecules that can act as antimicrobials, antibacterial, or anticancer drugs for the benefit of human health.
Fig. 1.
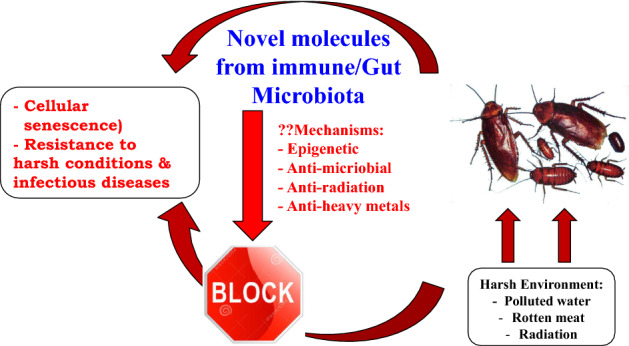
Novel molecules from immune/gut microbiota of cockroaches
Biology and classification
Life cycle of cockroaches may contribute to maximizing cockroaches’ reproductive fitness by reproducing a high number of eggs in order to compensate for constantly living under a high risk of death by external factors. After reaching sexual maturity, a female cockroach can reproduce an egg capsule every 9 days which contains around 14 embryos (Perrott and Miller 2010). After the juvenile cockroaches complete their molting stages, they will reach sexual maturity in 6 to 12 months and have a lifespan of up to a year and half (Perrott and Miller 2010) (Fig. 2). However, there are slight differences among different species. For instance, differences can be observed between the German and the Brown-Banded cockroaches. While the female German cockroaches are known to carry their egg cases until hatching, the female Brown-Banded cockroaches attach their egg cases to discrete locations (Malinoski 1999). The developmental period between egg to adulthood is around 95–276 days for the Brown-Banded cockroaches but only 55–68 days for the German cockroaches (Malinoski 1999). Some cockroaches can undergo parthenogenesis to reproduce and others can store sperm to reproduce at a later stage (Guzman et al. 2020). Cockroaches have incomplete metamorphosis, which refers to the nymphs being generally similar to adults, but with underdeveloped genitalia and wings. Some cockroaches have been able to survive in the laboratory for up to 4 years (Daly et al. 1978).
Fig. 2.
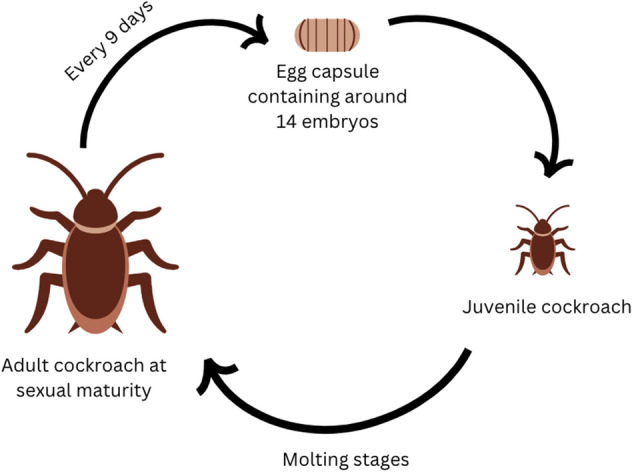
The life cycle of a cockroach through the three stages including (i) egg stage, (ii) nymph or juvenile cockroach, and (iii) adult cockroach
In the phylogenetic tree, Blattodea is divided into Corydioidea, Blaberoidea, and Blattoidea. Corydioidea includes sister groups called Nocticolidae and Corydiidae (Wang et al. 2017). Ectobius and Blaberidae are the sister groups of Blaberoidea (Evangelista et al. 2019; Wang et al. 2017). Blattoidea, a family of cockroaches and termites, includes Blattidae, Tryonicidae, Lamproblattidae, Anaplectidae, Cryptocercidae and Isoptera (Wang et al. 2017). Irrespective of the number of differences among the species, including behavioural and phenotypical differences, cockroaches are considered as monophyletic due to the results gathered by DNA sequencing of mitochondrial rRNA genes (Kambhampati 1995; Li et al. 2018). Recently used classifications are Princis, McKittrick, and Grandcolas (Roth 2003). The Princis classification is mostly based on differences in external structures of the cockroaches which gives rise to “4 suborders, 28 families, and 21 subfamilies” (Roth 2003) while McKittrick classification, the most acknowledged and accepted type, is based on the morphological characters of the male and female genitalia as well as their musculature, proventriculus, and oviposition behaviour (Kambhampati 1995; Roth 2003). The Grandcolas classification system “redescribed 6 families and sub-families” (Roth 2003) after analysing 221 genera and these classifications mostly coincide with McKittrick’s system but with a few exceptions.
The genome and epigenome of cockroaches
As previously mentioned, cockroaches are known to be able to survive in tough environments, variable climates, and have flexible feeding habits. These properties of cockroaches may have arisen from the epigenetic processes which allow them to regulate gene expression while maintaining the same DNA sequences without modifications and these processes may include DNA methylation as well as histone modifications (Qi et al. 2019; Villalba et al. 2021). Therefore, the outcome of epigenetic mechanisms may be key factors that contribute towards the cockroaches’ ability to adapt to several climates and thermal stress (Villalba et al. 2021). In addition, studies on several species showed that epigenetics may also contribute to the generation of new heritable variations in the phenotypic traits of the organism (Bird 2007; Villalba et al. 2021).
Another interesting hallmark about cockroaches is that their genome ranks as the second largest of all insect species, and is sized at about 3.38 Gb (Li et al. 2018), while containing a relatively lower number of species-specific genes, but the highest number of multicopy universal genes, whereby studies revealed a number of 13,555 genes in P. Americana in comparison to 7708 genes which was the highest number of universal genes in other insects (Li et al. 2018). The cockroaches’ large genome size is also a result of containing repetitive elements which constitute 60% of the roaches’ entire genome (Li et al. 2018). Additionally, the genome of P. Americana shares 90% of their genome with the rest of the Blattodea family and, therefore, contains some similar features to other insect species such as the exon number per gene; however, it differs from other insects when it comes to the length of introns which, too, is the second largest of all insect species numbered 3 Kb (Li et al. 2018). Remarkably, the American cockroaches’ genome shows around 80% homology with termites, Z. nevadensis and M. natalensis, which is more than the 75% homology with German cockroaches and this suggests that the American cockroaches are more related to termites than German cockroaches representing an evolutionarily significant finding (Li et al. 2018). Moreover, the 80% sequence identity between American cockroaches and termites are found in 29 pathophysiological pathways such as development and immunity while the 75% sequence identity with the German cockroaches are found in only 6 pathways including signal transduction (Li et al. 2018). Furthermore, in order to be able to survive and thrive in environments inhabited by humans, cockroaches contain a very distinctive genome which includes numerous genes regulating detoxification of xenobiotics through the function of P450s as well as the encoding of 154 olfactory receptors and 522 gustatory receptors (GRs) which far surpasses that of any other insect where 329 of these GRs are bitter receptors which benefit the roaches by allowing them to tolerate bitter food found and expanding the diversity of their diets (Li et al. 2018).
Diet and habitat
Cockroaches are omnivorous and generally feed on a variety of foods (McPherson et al. 2021; Schal et al. 1984; Lauprasert 2006). Notably, female cockroaches require more protein than males due to their high investment in egg development, while males preferred food with a high carbohydrate content (Lauprasert 2006). Cockroaches are also classified as omnivores due to having a varied diet which include any type of food eaten by humans or animals as well as their wastes such as fecal matter (Roth and Willis 1957), hair, hygienic products found in bathrooms, or even glue (Potter 2010). They also exhibit cannibalism during starvation (Guzman et al. 2020). As roaches eat items that are disposed of by humans, cockroaches have the ability to feed on certain types of plastic including plastic insulation and leather (Mogbo et al. 2013). These plastics vary in thickness but are still torn apart by the roaches’ strong mandibles (Weihmann et al. 2015). Cockroaches are able to digest cellulose and can utilise a diet of crystallisine cellulose (Slaytor 1992).
The ability of cockroaches to thrive in unhygienic environments possibly carrying infectious agents is one of the key factors that contribute to the cockroaches’ importance in the medical community (Akbar et al. 2019; Li et al. 2018; Koehler et al. 1999; Malinoski 1999; Roth and Willis 1957). Their habitats vary greatly as they can survive in forest canopy beds, arid land, water surfaces, etc. (Bell et al. 2007). With human interactions, cockroaches are mostly found in kitchens as a result of the presence of grease and also in bathrooms due to the readily available source of water, cracks in walls, large openings such as vents or pipes, as well as sewers (Koehler et al. 1999; Malinoski 1999). Cockroaches can also adapt and survive in a wide range of climates including tropical as well as polar temperatures by using glycerol as an anti-freeze and this results in different sizes of cockroaches (Channel, 2007). The differences in adaptations to temperatures also means that cockroaches have different tolerances to humidity. For example, while German, Brown-Banded, American, and Oriental cockroaches all prefer warm temperatures, only Brown-Banded and American cockroaches can be found in dry habitats, whereas German and Oriental cockroaches prefer moist environments (Malinoski 1999).
Gut bacteria and its role
Cockroaches are routinely associated with microbes in the environments in which they reside (Ali et al. 2017). They are associated with bacteria which are primary symbionts residing within specialized cells in the fat body and are involved in nitrogen metabolism, as well as facultative secondary symbionts that circulate in the gut (Domínguez-Santos et al. 2021; Guzman and Vilcinskas 2020). Gut microbiome of cockroaches has a major role in digestion, metabolism, absorption, immunity against pathogens, growth, behavior, etc. (Chen et al. 2020, Jandhyala et al. 2015; Tinker and Ottesen 2016). A number of studies have demonstrated the importance of gut microbiome and its role in the overall health and immunity of the host (Malard et al. 2021; Siddiqui et al. 2021a, b, c; Sieksmeyer 2021; Zheng et al. 2020). Cockroaches represent an ideal organism for studying gut microbiome composition as well as host microbial interactions, as they have evolved to host a multifaceted gut microbiome comprising hundreds of unique species of microbes, similar to a variety of omnivorous animals including humans and mice (Tinker and Otteson 2021). In a recent study, a weak but significant phylosymbiotic signature was detected, suggesting that cockroach phylogeny may have a role in structuring the gut microbiome over shorter evolutionary distances and possibly extended periods of evolutionary time (Tinker and Otteson 2020).
The developmental stages in lifecycle of cockroaches contribute to differences between species of microorganisms found in the gut microbiome of cockroaches (Chen et al. 2020). Nevertheless, there are certain bacteria that dominate throughout all three developmental stages of roaches indicating that some microorganisms found in the gut microbiome are inherited from mother to eggs which include Bacteroidetes, Firmicutes and Proteobacteria as these bacteria aid in the process of digestion, absorption and defence against pathogens (Chen et al. 2020; Tinker and Ottesen 2016). However, the diversity of the gut microbiome in eggs remain lower than that found in nymph or adult stages where major difference is the presence of the Blattabacterium and Lactobacillus which are also inherited from the mother (Chen et al. 2020). These dominant bacteria of the eggs are specifically beneficial since Blattabacterium has a significant role in nitrogen cycling as well as absorption and synthesis of important nutrients while Lactobacillus acts as a probiotic and protects the eggs from pathogens (Chen et al. 2020). On the other hand, Desulfovibrio and Parabacteroides are the dominant microorganisms in the nymph stages and Bacteroides, Dysgonomonas, Porphyromonadaceae and Alistipes dominate the adult stage of the cockroaches (Chen et al. 2020).
The gut microbiome of cockroaches does not only depend on which developmental stage they are moving through as it also depends on their diet. To begin with, it is important to understand that the gut microbiome of cockroaches differs from other insects and humans. This is mainly due to the diet habits of the species whereby cockroaches, which are considered as a typically gregarious or social species, feed on a big assortment of foods and have a varied diet while other insects, such as bees, who feed on a smaller array of food and are said to have more specialized diets (Tinker and Ottesen 2016). The varied diet of cockroaches enables this species to contain a highly diverse and complex gut microbiome which is comprised of a great number of microorganisms. This also means that roaches have evolved methods to maintain a stable gut microbiome regardless of any changes that may occur to their diet which is advantageous in their daily lives (Chen et al. 2020). Some of these advantages contribute to their ability to survive in various habitats as well as adapt to unfavourable conditions and as a result P. americana is observed to be found across continents (Tinker and Ottesen 2016). In addition, there is an obvious distinction in the abundance of the microorganisms depending on the division of the gut. For instance, the hindgut of the cockroaches contains the biggest and most diverse portion of the gut microbiome (Jahnes et al. 2021; Tinker and Ottesen, 2016). Therefore, the hindgut microbiome plays a significant role in the digestion of the materials that pass through the foregut and midgut as well as the promotion of social behaviour through the production of pheromones (Chen et al. 2020; Tinker and Ottesen 2016).
A recent study was conducted to compare gut microbial diversity between laboratory P. americana as well wild-caught populations of P. Americana and Periplaneta fuliginosa, prior to, during, and following a 2-week period in the laboratory environment (Tinker and Ottesen 2021). The data revealed that gut microbial changes were apparent, based on the species and environment and laboratory-based and wild-captured cockroaches from the same species depicted distinct gut microbiomes. Interestingly, being based in a laboratory environment led to decreased microbiome diversity for both species of wild-caught insects, suggesting that cockroaches could be used as a model to study changes in gut bacterial diversity as a result of various changes (Tinker and Otteson 2021). In another report, the hindgut microbiome of Blattella germanica cockroaches was analyzed for gut bacteria, fungi, archaea and viruses (Domínguez-Santos et al. 2021). In agreement with prior studies, the most abundant core genera were Bacteroides (Bacteroidetes), Desulfovibrio (Proteobacteria), Fusobacterium (Fusobacteria) and Clostridium (Firmicutes) and are known to participate in protein and polysaccharide digestion, protection versus pathogens and nitrogen fixation (Domínguez-Santos et al. 2021). In addition, 70 families of archaea were also detected, indicating a potential role of these microorganisms on cockroach physiology. The most abundant species in adults and nymphs were from the families: Methanobacteriaceae, Methanosarcinaceae, and Methanomassiliicoccaceae, which are methanogenic archaea that may be involved the hindgut nitrogen–carbon balance by nitrogen fixation (Domínguez-Santos et al. 2021).
German cockroach, Blatella germanica, is thought to be a vector of several enteric bacterial pathogens, including E. coli, among livestock and humans (Ray et al. 2020). In this study, B. germanica were orally infected with E. coli. The results revealed that E. coli is mostly cleared within 48 h, whereas one strain may persist in a majority of cockroaches for longer than 3 days with limited impact on cockroach longevity. The study also revealed that some strains of E. coli were greater in cockroach nymphs than adults. Interestingly, clearance of E. coli was significantly reduced in gnotobiotic cockroaches that were reared in the absence of environmental bacteria. This suggested a possible protective role for the microbiome versus bacterial pathogens (Ray et al. 2020).
Anti-microbial activity
Since roaches live in unhygienic and insanitary environments and niches, cockroaches have adapted, as a result of external stimuli, in ways by which they can protect themselves from exposure to contaminants or from microbial infections (Akbar et al. 2019; Ali et al. 2017; Latifi et al. 2015). This means that cockroaches may contain defence mechanisms and their “gut microbiota produce molecules to thwart invading pathogens” (Ali et al. 2017). Their ability to do this comes from the lectin proteins which identify the foreign or harmful bacteria and stimulate the innate immunity response against pathogens (Latifi et al. 2015). Another significant factor that may contribute to the cockroach immunity is the complex passageway found in their cavity which consists of antimicrobials that destroy the pathogens before reaching the haemocoel (Balasubramanian et al. 2017). Therefore, the cockroaches’ ability to be resistant to superbugs and other pathogens, due to the presence of lectin and antimicrobials in their cavities, indicates that their anti-bacterial properties need to be studied further in hopes of a new medical breakthrough such as the discovery of new antibiotics that could be useful for humanity.
It is not an uncommon practice for insects including cockroaches to be used therapeutically against some diseases such as malaria as well as asthma (Balasubramanian et al. 2017) since there are currently 50 anti-bacterial molecules being used in the medical field today that have been extracted from insects (Latifi et al. 2015). Furthermore, cockroaches have been observed to have anti-bacterial activities against Gram- positive and -negative bacteria as well as anti-amoebic properties (Akbar et al. 2018). In a study, some of the bacteria found in the gut microbiome of cockroaches were isolated which included Serratia marcescens, Escherichia coli, Klebsiella sp., Bacillus sp., and Streptococcus sp. This allowed for the development of conditioned media comprising the gut bacterial metabolites and the antibacterial activities of these metabolites were determined of various against pathogenic bacteria (Akbar et al. 2018). The gut microbial metabolites converse with the immune system and modulate immune responses, and play a profound role in cellular signaling, inflammation and interaction with the immune cells (Belkaid and Hand 2014; Kau et al. 2011). These gut bacterial metabolites were found to have bactericidal and inhibitory effects on methicillin-resistant Staphylococcus aureus (MRSA), Streptococcus pyogenes, Bacillus cereus, E. coli K1, P. aeruginosa, K. pneumoniae, Salmonella enterica and S. marcescens (Akbar et al. 2018). However, the extraordinary results did not stop there but continued when the conditioned media also indicated 40–60% amoebicidal effects when tested against the free-living amoebae A. castellanii (Akbar et al. 2018). In addition, other studies have also shown that roaches have antimicrobial properties against methicillin-susceptible Staphylococcus aureus (MSSA) (Billah et al. 2015), M. luteus (Basseri et al. 2016) Staphylococcus aureus and Bacillus subtilis (Mahboub et al. 2021). Another study conducted in Saudi Arabia on Blattella vaga extracted Bacillus licheniformis, Bacillus subtilis and Kocuria rosea from the cockroach’s gut microbiome and evaluated their anti-microbial properties against Salmonella enterica, MRSA, Streptococcus mutans and Candida albicans which are all considered as drug-resistant pathogens (Alkhalifah 2021). Their analysis showed that Bacillus subtilis does not demonstrate any antimicrobial activity while Bacillus licheniformis demonstrated inhibitory effects against Candida albicans and Kocuria rosea also showed antimicrobial properties against MRSA and Streptococcus mutans (Alkhalifah 2021). These studies and their results indicate that gut bacterial metabolites of cockroaches may be developed into effective antibiotics or probiotics to be utilized as a treatment or prevention against drug-resistant pathogens, which may solve a major public health crisis that we are facing today.
Nevertheless, the gut bacteria of cockroaches are not the only factor contributing to their antibacterial properties as cockroach brain extracts as well as hemolymph have demonstrated significant bactericidal activities against some pathogenic bacteria (Ali et al. 2017). Aspirated hemolymph demonstrated 35% antibacterial activity against MRSA and 20% against E. Coli K1 while brain lysates showed 90% antibacterial activity when tested on MRSA as well as E. Coli K1 (Ali et al. 2017). While the results clearly indicate that cockroach brain lysates are superior to hemolymph as antibacterial compounds, hemolymph have also exhibited potent anti-viral and anti-tumour properties, antimicrobial activities against parasitic worm embryos, aided in the treatment of several conditions and diseases such as diabetes (Ali et al. 2017). For instance, a study isolated the hemolymph of cockroaches and tested it against P. aeruginosa, P. mirabilis, S. aureus, E. coli and Salmonella typhi using a zone of inhibition test which exhibited antimicrobial properties against all 5 bacterial strains but with different degrees (Balasubramanian et al. 2017). At the same time, brain lysates have too exhibited positive outcomes in the treatment of viruses and tumours while also acting as anti-diabetic and anti-inflammatory compounds (Ali et al. 2017). The remarkable therapeutic effects of the cockroach hemolymph and brain lysates arise from the structure of their functional groups as well as the presence of other active compounds such as flavanones (Ali et al. 2017).
Cockroach anti-viral properties are significant as they act as their natural defense mechanism since roaches can be infected with viruses, which may cause behavioural changes, just as much as being biological vectors (Sieksmeyer 2021). Studies have shown that cockroaches’ anti-viral activity and antimicrobial peptides are effective against herpes simplex virus (Ali et al. 2017; Wang et al. 2011). Other studies have also demonstrated that cockroaches contain compounds found in their brain and hemolymph lysates which demonstrate anti-viral activity against Influenza A virus, Parainfluenza virus, HIV-1, Norovirus, retroviruses and several other types of viruses (Ali et al. 2017). Some studies have attributed the cockroaches’ anti-viral properties as well as other anti-microbial activities to 1, 2, 4-triazole compounds found in their hemolymph (Balasubramanian et al. 2017). This is understandable since 1, 2, 4-triazole compounds are found and used in several commercial drugs found in the market today including anti-viral and anti-fungal agents such as ribavirin and fluconazole, respectively (Balasubramanian et al. 2017).
Other than hemolymph, gut and brain extracts, antimicrobial properties in cockroaches can also originate from chitosan which is a polysaccharide as well as an antimicrobial agent found in the roaches’ exoskeleton (Balasubramanian et al. 2017; Mahboub et al. 2021). Interestingly, the activities and effects of chitosan found in the cockroaches depend upon the roaches’ species such that the antimicrobial properties associated with chitosan differs between the American cockroach and the German cockroach (Basseri et al. 2019). Chitosan can act as an antibacterial agent against Gram-positive bacteria, such as S. aureus and B. subtilis, where the minimum inhibitory concentration (MIC) was found to be 2000 μg/ml (Mahboub et al. 2021). Chitosan is also effective against Gram-negative bacteria where MIC of chitosan against E. coli was recorded to be 1000 μg/ml and MIC against Salmonella typhimurim was 2000 μg/ml (Mahboub et al. 2021). Yet, studies have shown different degrees of antibacterial activities in roaches and this can be a result of differences in nutrition and severity of the infections as well as other factors (Latifi et al. 2015).
Irrespective of its extensive antimicrobial properties, chitosan also exhibits anti-fungal activities against A. flavus and A. albican by inhibiting their growth (Basseri et al. 2019), but studies conducted by Mahboub et al. 2021 found that chitosan did not exhibit any anti-fungal activity against Candida albicans which agreed with research by Basseri et al. (2019) but was contradictory to other studies which revealed anti-fungal capabilities (Alburquenque et al. 2010). However, cockroaches contain another peptide, Periplanetasin-2, which demonstrates non-hemolytic anti-fungal properties (Yun et al. 2017). When Periplanetasin-2 was extracted and tested against mitochondria of Candida albicans, the results showed the stimulation of oxidative stress and lipid peroxidation resulted in inducing apoptosis that was brought about by the externalization of phosphatidylserine, DNA fragmentation, membrane depolarization and an increase in the calcium level and mitochondrial glutathione, but a decrease in cytosolic glutathione (Yun et al. 2017).
These results of the antimicrobial activity of cockroaches functioning as broad-spectrum antimicrobial peptides (Li et al. 2018) are all well and good but useless if they have potential harmful effects on humans and cannot be used. Hence, the Roche cytotoxicity detection kit was used to study the effects on human cells when infected with MRSA or E. coli K1 which resulted in 70% cytotoxicity (Ali et al. 2017). On the other hand, the detection kit demonstrated a minimal amount of cytotoxicity and cell damage when the infected human cells were treated with cockroach lysates (Ali et al. 2017). Therefore, this revealed that cockroach lysates contain antibacterial molecules, less than 10 kDa in molecular mass (Ali et al. 2017), that can treat multi-drug resistant infections and are safe to be used on human cells.
Anti-cancer activity
Cancer is established as one of the leading causes of mortality and morbidity and was ranked in 2020 as one of the top 3 causes of death, worldwide along with heart disease and COVID-19 (Ahmad et al. 2021). Western medicine has relied on chemotherapy, radiotherapy, and stem-cell therapy as well as other similar options for cancer treatment (Soopramanien et al. 2019; Zhao et al. 2017). However, due to drug resistance and several complications as well as harmful effects of the currently routinely used types of cancer treatment (Zhao et al. 2017), the medical community has been trying to innovate in hopes of improving healthcare quality and better patient care by discovering and developing novel anti-cancer agents and treatments without harming or compromising or suppressing the patient’s immune system (Mahboub et al. 2021). Therefore, in some parts of the world, cancer patients have resorted to Traditional Chinese Medicine (TCM) instead of Western medicine due to its lower cytotoxicity (Zhao et al. 2017). In particular, cancer patients benefit from TCM which uses plant derivatives along with insect secretions, including cockroaches’, which have proven to have anticancer properties (Seabrooks and Hu 2017; Wang et al. 2011). Aside from their anticancer properties, cockroach extracts also contain wound-healing activities and are also used in TCM (Zhu et al. 2018). The effects of these extracts vary from treating blood stasis to burns, tissue repair, or wounds from the first day of treatment (Li et al. 2018; Zhu et al. 2018).
A plausible explanation to the cockroach derivatives ability as inhibitors of tumour progression, maybe due to the roaches’ ability to withstand radiation as well as their living conditions in tough environments filled with pollutants such as chemicals, traces of heavy metals and infectious microorganisms (Soopramanien et al. 2021; Wang et al. 2011). Thus, traditional medicine introduced dried worms and adult cockroaches as potential treatments for several diseases due to their pharmacological effects such as blood pressure stabilization, detoxification, immunity enhancement and promotion of diuresis when necessary (Zhao et al. 2017). For example, cockroaches contain antimicrobial peptides that can be used in treating and stimulating liver recovery after hepatitis B infection (Zhao et al. 2017) and also in the treatment of Newcastle disease (Wang et al. 2011). Roaches are also involved in traditional medicine for numerous other health conditions which include heart diseases, asthma, digestive conditions, ulcers, burns and most importantly cancer (Seabrooks and Hu 2017; Wang et al. 2011) as a by-product of the interplay and effect of several components such as unsaturated fatty acids, ester, cyclic peptides, human essential and semi-essential amino acids, pheromones, polysaccharides and, once again, chitosan (Zhao et al. 2017). And since drug resistance is one of the major threats on public health including cancer treatments, it is important to note that one of the anti-cancer properties of cockroaches relates to their extracts targeting of multidrug resistance proteins and breast cancer resistance proteins which reverse the effects of drug resistance on cells and improve the possibility of successful cancer treatments (Zhao et al. 2017).
As mentioned above, cockroach brain and hemolymph have many therapeutic effects including anti-tumour activity against various forms of cancers such as ovarian, breast, lung and prostate cancers (Ali et al. 2017). In addition, chitosan, which is responsible for the roaches’ antibacterial properties, is similarly responsible for resulting in the cockroaches containing anticancer agents due to its role of acetylation and its molecular weight (Mahboub et al. 2021). Studies on this polysaccharide have shown to be an effective natural treatment for hepatoblastoma and breast cancer (Mahboub et al. 2021; Seabrooks and Hu 2017). Furthermore, there are several other researchers that have carried out studies on the anticancer properties of chitosan. For instance, it was shown that chitosan derived from shrimp can be an effective treatment for human bladder cell carcinoma (Younes et al. 2014). In another study, positive results against hepatocellular carcinoma cells using chitosan extracted from cockroaches were observed (Azuma et al. 2014). Furthermore, it was noted that chitosan is also effective against laryngeal cancer and human embryo rhabdomyosarcoma cells (Ganesan et al. 2020). Research has also revealed that chitosan found in cockroaches may be used against chronic myeloid leukemia by inducing G2/M phase arrest as well as acting as an EGFR-inhibiting agent (Seabrooks and Hu 2017).
Nevertheless, chitosan is not the only effective anticancer agent found in cockroaches since their gut microbial metabolites may also contribute to having anti-cancer molecules as a result of their need to protect themselves from diseases by the microorganism rich environments that they dwell in (Soopramanien et al. 2021). For instance, Staphylococcus xylosus extracted from the cockroach gut microbiome exhibited anti-cancer effects by reducing the growth of a human prostate cancer cell line (PC-3) (Soopramanien et al. 2019). Additionally, as with the action of chitosan on myeloid leukemia, cockroaches have the ability to prompt cell cycle arrest (Zhao et al. 2017). Some examples of the effects of cockroach extracts include the reduced growth of Lewis lung carcinoma due to blocking the G0/G1 phase of the cell cycle as well as reduced growth of endometrial cancer cells due to overexpression of p53 and reduced expression of C-erbB-2 as a result of cell cycle arrest. Cockroach extracts have been observed to also induce apoptosis in cancer cells such as in human hepatoma cells as well as in leukaemia cells which were brought about by the action of perplanetasin-5 (Kim et al. 2021; Zhao et al. 2017). Moreover, polypeptide extracts from cockroaches can reduce the tumour microvessel density and the expression of vascular endothelial growth factor and, therefore, have anti-angiogenic effects and impede tumour growth (Zhao et al. 2017). Therefore, it is clear that cockroaches may provide humanity with novel pharmacological drugs, such as anti-tumour agents, which would significantly help the medical community, but more research is still required for to develop a better understanding of the exact modes of action of the cockroaches’ anti-cancer compounds and the appropriate isolation of their chemical constituents to result in successful tumour suppression in humans (Soopramanien et al. 2019; Zhao et al. 2017).
Cellular senescence in cockroaches
While ageing is a continuous pathophysiological process, the mechanisms underlying this phenomenon are complex and vary between species. Cockroaches are typically a gregarious and social species and so may be useful model organisms to investigate the evolution of cellular senescence (Kramer et al. 2021; Tinker and Otteson 2020).
For instance, one of these mechanisms is the accumulation of cells that have undergone cellular senescence in body tissues (Collado et al. 2007) which refers to a permanent but stable arrest in the cell cycle due to the action of stressors which result in the limitation of cellular replication or cell proliferation (Ben-Porath and Weinberg 2005; Herranz and Gil 2018). Therefore, cellular senescence refers to the decline or deterioration of organisms as they age which may also be due to the build-up of physiological or oxidative damage causing what are commonly known as age-associated diseases (de Verges and Nehring 2016; Herranz and Gil 2018; Hseih and Hsu 2011; Lucas and Keller 2014).
In human somatic cells, one of the obvious stressors which lead to cellular senescence include the overexpression of oncoproteins or extensive cellular damage (Ben-Porath and Weinberg 2005). Another stressor which triggers senescence in humans is related to telomere shortening as a result of the inactivity of telomerase and this acts as our natural protection mechanism against cancer as this prevents the proliferation of cells (Greider 1998; Hornsby 2007). Therefore, in species where telomerase activity is organ-specific as that in humans (Sasaki and Fujiwara 2000), the ageing process is slowed down, attributable to cellular senescence reducing tumour growth (Hornsby 2007). In contrast, high non-tissue specific telomerase activity was found in cockroaches’ germ as well as somatic cells including fat bodies, neural tissue and muscles (Sasaki and Fujiwara 2000). This increased telomerase activity contributes to their shorter lifespan, in comparison to humans, and this results in the cockroaches portraying higher cell proliferation (Sasaki and Fujiwara 2000).
On the other hand, research on cellular senescence in honeybees is quite extensive (de Verges and Nehring 2016; Lucas and Keller 2014). Similar to humans, older honeybees demonstrate signs of ageing by becoming weaker and show a decline in their learning curve as well as jelly production and immune system activity (de Verges and Nehring 2016). Honeybees are considered as model organisms for cellular senescence research since honeybees are social insects which exhibit different phenotypes and behaviours during their lifetime depending on their role in the caste (Kramer et al. 2021). For instance, there is a clear difference in the lifespan of honeybees which are categorised as workers or foragers and reproductive individuals as well as the queen (Kramer et al. 2021). Workers are known to live a shorter life due to extrinsic as well as physiological damages which include protein carbonylation, possibly causing brain protein carbonylation damage, indicating the consequences of oxidative stress in relation to cell senescence (Kramer et al. 2021; Seehuus et al. 2006). The oxidative stress hypothesis states that cellular senescence is mainly due to the harmful effects of the accumulation of reactive oxygen species (ROS), and it is suggested that that senescence of cells in honeybees is also highly correlated with their social role (Hseih and Hsu 2011). Nevertheless, the oxidative stress hypothesis cannot be generalized to all social insects as it is considered as an inconsistent link when predicting cellular senescence in some species (Kramer et al. 2021) and this calls for using other age-related molecules or processes to determine senescence in honeybees. For example, biochemical assays of trophocytes and fat cells in honeybees are considered as good predictors of cellular senescence and illustrate that the age of honeybees is not entirely unrelated to their senescence. Old trophocytes and fat cells express higher levels of lipid peroxidation, protein oxidation, senescence-associated β galactosidase (SA-β-Gal) and lipofuscin granules where all act as indicators of ageing or cellular senescence (Hseih and Hsu 2011). However, unlike cockroaches and humans, the telomerase activity in trophocytes and fat cells of honeybees is not influenced by age and is not associated with cellular senescence as there are no significant differences in telomere length of newly emerged and old worker honeybees (Hseih and Hsu 2011). Another age-related molecule found in honeybees is vitellogenin (Vg) as these proteins are found in higher quantities in queens than workers which is one of the reasons pertaining to the significantly longer lifespan of queens and this is due to Vg acting as an anti-oxidant by providing protection against oxidative stress (Kramer et al. 2021). Additionally, Vg levels decrease during flying which is mostly performed by worker honeybees during foraging and this results in an increased risk of damages due oxidative stress, hence, relating social role and cellular senescence once again (Kramer et al. 2021). However, very few studies investigating cellular senescence in cockroaches have been accomplished and these are warranted, given the hardiness of these species.
Conclusions and future perspectives
Infectious diseases, cancer, and ageing are amongst the biological challenges affecting human health; thus understanding the precise mechanisms of senescence and how disease etiologies come about in model organisms such as cockroaches, locusts and other interesting species such as crocodiles is of immense value (Siddiqui et al. 2021b; Siddiqui et al. 2021c). Insects such as cockroaches have been on this earth for millions of years and are one of the hardiest insects, able to survive without food for up to a month, without air for around 45 min and being submerged under water for 30 min (Lee et al. 2012; Wharton and Wharton 1959). Moreover, they can endure high doses of radiation: 15 times higher than humans (Lee et al. 2012; Wharton and Wharton 1959). Accordingly, it is possible that cockroaches represent a rich and diverse source of novel molecules with biological activity originating either in their tissues or their gut microbiome playing a role in contributing to the overall health and resilience. Furthermore, cockroaches may be a tractable model for research on senescence in natural populations. Till date, various studies have been conducted to determine the gut microbial composition of cockroaches, however limited work has been accomplished to understand their gut bacterial metabolites and their potential as novel and active biological molecules for use in human health, and this warrants further study, although preliminary studies have isolated and identified potentially innovative as well as some already known molecules from cockroach gut bacterial metabolites which depict inhibition of cell metabolic activity or viability reduction, and cell survival inhibition in cancerous cell lines (Akbar et al. 2018; Ali et al. 2017; Soopramanien et al. 2021). Future studies on the microbiome and associated metabolites might allow for identification of novel therapeutic leads for clinical and pre-clinical investigations in invertebrate and animal models of ageing and/or disease. Another alternative strategy may be the direct implantation of select and biologically active gut microbiome species (portraying senloytic, anti-cancer/ anti- microbial effects) into mammalian models of disease or ageing. Although these notions may seem improbable, other studies such as the discovery of insulin was made when it was found that an aqueous pancreatic extract was able to normalize diabetes in a dog. Previously, insulin for clinical use was normally obtained from cows and pigs (Crasto et al. 2016). In another study, obesity was elevated with an increase in Firmicutes in a mouse model (Turnbaugh et al. 2008). Such studies could be emulated to utilize the unique microbiome of cockroaches for the benefit of Homo sapiens with in vivo work and clinical trials in the prospective years. Being one of the most successful and diverse insects, as well as their extensive use in traditional and Chinese medicine, the lysates and gut microbiome of cockroaches may offer a worthy resource for novel bioactive molecules of therapeutic potential.
Funding
RS and NAK are funded by the Air Force Office of Scientific Research (AFOSR), grant number: FA 8655-20-1-7004.
Declarations
Conflict of interest
No conflict of interest exists.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmad FB, Cisewski JA, Miniño A, Anderson RN. Provisional mortality data—United States, 2020. Morb Mortal Wkly Rep. 2021;70:519. doi: 10.15585/mmwr.mm7014e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbar N, Siddiqui R, Iqbal M, Sagathevan K, Khan NA. Gut bacteria of cockroaches are a potential source of antibacterial compound (s) Lett Appl Microbiol. 2018;66:416–426. doi: 10.1111/lam.12867. [DOI] [PubMed] [Google Scholar]
- Akbar N, Siddiqui R, Sagathevan KA, Khan NA. Gut bacteria of animals/pests living in polluted environments are a potential source of antibacterials. Appl Microbiol Biotechnol. 2019;103:3955–3964. doi: 10.1007/s00253-019-09783-2. [DOI] [PubMed] [Google Scholar]
- Alburquenque C, Bucarey SA, Neira-Carrillo A, Urzúa B, Hermosilla G, Tapia CV. Antifungal activity of low molecular weight chitosan against clinical isolates of Candida spp. Med Mycol J. 2010;48:1018–1023. doi: 10.3109/13693786.2010.486412. [DOI] [PubMed] [Google Scholar]
- Ali SM, Siddiqui R, Ong SK, Shah MR, Anwar A, Heard PJ, Khan NA. Identification and characterization of antibacterial compound (s) of cockroaches (Periplaneta americana) Appl Microbiol Biotechnol. 2017;101:253–286. doi: 10.1007/s00253-016-7872-2. [DOI] [PubMed] [Google Scholar]
- Alkhalifah DH. Evaluation of antimicrobial activity of bacterial symbionts isolated from wild field cockroach Blattella vaga from Saudi Arabia. Saudi J Biol Sci. 2021;28:6239–6244. doi: 10.1016/j.sjbs.2021.06.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma K, Ifuku S, Osaki T, Okamoto Y, Minami S (2014) Preparation and biomedical applications of chitin and chitosan nanofibers. J Biomed Nanotechnol 10(10):891–2920 [DOI] [PubMed]
- Balasubramanian S, Priya K, Revathi I, Revathi A, Venkatesh P, Gunasekaran G. Screening of antibacterial activity and biochemical assay from haemolymph of cockroach Blatta orientalis (Linnaeus, 1758) J Entomol Zool Stud. 2017;5:753–758. [Google Scholar]
- Basseri HR, Dadi-Khoeni A, Bakhtiari R, Abolhassani M, Hajihosseini-Baghdadabadi R. Isolation and purification of an antibacterial protein from immune induced haemolymph of American cockroach. Periplaneta Americana J Arthropod-Borne Dis. 2016;10:519. [PMC free article] [PubMed] [Google Scholar]
- Basseri H, Bakhtiyari R, Hashemi SJ, Baniardelani M, Shahraki H, Hosainpour L. Antibacterial/antifungal activity of extracted chitosan from American cockroach (Dictyoptera: Blattidae) and German cockroach (Blattodea: Blattellidae) J Med Entomol. 2019;56:1208–1214. doi: 10.1093/jme/tjz082. [DOI] [PubMed] [Google Scholar]
- Beccaloni GW (2014) Cockroach species file online.Publisher. http://cockroach.speciesfile.org/ Accessed 28 Nov 2021
- Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell WJ, Roth LM, Nalepa CA. Cockroaches: ecology, behavior, and natural history. Baltimore: JHU Press; 2007. [Google Scholar]
- Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005;37:961–976. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Billah MK, Pesewu GA, Otu H, Adetokunbo M. In vitro antibacterial activities of cockroach extracts against selected bacterial pathogens. Am J Res Commun. 2015;3:78–94. [Google Scholar]
- Bird A. Perceptions of epigenetics. Nature. 2007;447:396. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- Channel D. Animal planet the most extreme bugs. John Wiley & Sons; 2007. [Google Scholar]
- Chen Z, Yang B, Ou P, Jin X (2020) Differences in the Diversity and Structure of the Gut Microbiome in Different Life Stages of the American Cockroach (Periplaneta Americana).
- Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Crasto W, Jarvis J, Davies MJ. Handbook of insulin therapies. Springer; 2016. [Google Scholar]
- Daly HV, Doyen JT, Ehrlich PR. Introduction to insect biology and diversity. McGraw-Hill Book Company; 1978. [Google Scholar]
- De Verges J, Nehring V. A critical look at proximate causes of social insect senescence: damage accumulation or hyperfunction? Curr Opin Insect Sci. 2016;16:69–75. doi: 10.1016/j.cois.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Domínguez-Santos R, Pérez-Cobas AE, Cuti P, Pérez-Brocal V, García-Ferris C, Moya A, Latorre A, Gil R. Interkingdom gut microbiome and resistome of the cockroach Blattella germanica. mSystems. 2021;6:e01213–e1220. doi: 10.1128/mSystems.01213-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista DA, Wipfler B, Béthoux O, Donath A, Fujita M, Kohli MK, Simon S. An integrative phylogenomic approach illuminates the evolutionary history of cockroaches and termites (Blattodea) Proc R Soc B. 2019;286(1895):20182076. doi: 10.1098/rspb.2018.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan AR, Guru MS, Balasubramanian B, Mohan K, Liu WC, Arasu MV, Al-Dhabi NA, Duraipandiyan V, Ignacimuthu S, Sudhakar MP, Seedevi P. Biopolymer from edible marine invertebrates: a potential functional food. J King Saud Univ - Sci. 2020;32:1772–1777. doi: 10.1016/j.jksus.2020.01.015. [DOI] [Google Scholar]
- Greider CW. Telomeres and senescence: the history, the experiment, the future. Curr Biol. 1998;8:R178–R181. doi: 10.1016/S0960-9822(98)70105-8. [DOI] [PubMed] [Google Scholar]
- Guzman J, Vilcinskas A. Bacteria associated with cockroaches: health risk or biotechnological opportunity? Appl Microbiol Biotechnol. 2020;104:10369–10387. doi: 10.1007/s00253-020-10973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari YN. Sapiens: a brief history of humankind. New York: Random House; 2014. [Google Scholar]
- Herranz N, Gil J. Mechanisms and functions of cellular senescence. J Clin Investig. 2018;128:1238–1246. doi: 10.1172/JCI95148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornsby PJ. Telomerase and the aging process. Exp Gerontol. 2007;42:575–581. doi: 10.1016/j.exger.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh YS, Hsu CY. Honeybee trophocytes and fat cells as target cells for cellular senescence studies. Exp Gerontol. 2011;46:233–240. doi: 10.1016/j.exger.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Jahnes BC, Poudel K, Staats AM, Sabree ZL (2021) Microbial colonization promotes model cockroach gut tissue growth and development. J Insect Physiol 133:104274. [DOI] [PubMed]
- Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Reddy DN (2015) Role of the normal gut microbiota. World j gastroenterol: WJG 21(29):8787. [DOI] [PMC free article] [PubMed]
- Kambhampati S. A phylogeny of cockroaches and related insects based on DNA sequence of mitochondrial ribosomal RNA genes. Proc Natl Acad Sci. 1995;92:2017–2020. doi: 10.1073/pnas.92.6.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IW, Choi RY, Lee JH, Seo M, Lee HJ, Kim MA, Kim SH, Kim I, Hwang JS. Anticancer activity of periplanetasin-5, an antimicrobial peptide from the cockroach Periplaneta americana. J Microbiol Biotechnol. 2021;31:1343–1349. doi: 10.4014/jmb.2104.04040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler PG, Ol F, Branscome D. Cockroaches and their management. Florida: University of Florida Cooperative Extension Service, Institute of Food and Agriculture Sciences, EDIS; 1999. [Google Scholar]
- Kramer BH, Nehring V, Buttstedt A, Heinze J, Korb J, Libbrecht R, Meusemann K, Paxton RJ, Séguret A, Schaub F, Bernadou A. Oxidative stress and senescence in social insects: a significant but inconsistent link? Philos Trans R Soc B. 2021;376:20190732. doi: 10.1098/rstb.2019.0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latifi M, Alikhani MY, Salehzadeh A, Nazari M, Bandani AR, Zahirnia AH (2015) The antibacterial effect of American cockroach hemolymph on the nosocomial pathogenic bacteria.SID 0–0.
- Lauprasert P, Sitthicharoenchai D, Thirakhupt K, Pradatsudarasar AO. Food preference and feeding behavior of the German cockroach, Blattella germanica (Linnaeus) J Sci Res Chula Univ. 2006;31:121–126. [Google Scholar]
- Lee S, Siddiqui R, Khan NA. Animals living in polluted environments are potential source of antimicrobials against infectious agents. Pathog Glob Health. 2012;106:218–223. doi: 10.1179/2047773212Y.0000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zhu S, Jia Q, Yuan D, Ren C, Li K, Liu S, Cui Y, Zhao H, Cao Y, Fang G. The genomic and functional landscapes of developmental plasticity in the American cockroach. Nat Commun. 2018;9:1–1. doi: 10.1038/s41467-018-03281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K, Zhou J, Deng J, Li Y, Wu C, Bao J. Periplaneta americana oligosaccharides exert anti-inflammatory activity through immunoregulation and modulation of gut microbiota in acute colitis mice model. Molecules. 2021;26:1718. doi: 10.3390/molecules26061718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas ER, Keller L. Ageing and somatic maintenance in social insects. Curr Opin Insect Sci. 2014;5:31–36. doi: 10.1016/j.cois.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Ma X, Hu Y, Li X, Zheng X, Wang Y, Zhang J, Fu C, Geng F. Periplaneta americana ameliorates dextran sulfate sodium-induced ulcerative colitis in rats by Keap1/Nrf-2 activation, intestinal barrier function, and gut microbiota regulation. Front Pharmacol. 2018;9:944. doi: 10.3389/fphar.2018.00944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahboub MT, Hassan MI, Bream AS, Mohamed AF, Abdel-Samad MR. Evaluation of the antibacterial and antifungal activities of chitosan prepared from the American cockroach, (Periplaneta americana) Egypt Acad J Biol. 2021;13:39–46. [Google Scholar]
- Malard F, Dore J, Gaugler B, Mohty M. Introduction to host microbiome symbiosis in health and disease. Mucosal Immunol. 2021;14:547–554. doi: 10.1038/s41385-020-00365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinoski MK. Cockroaches. University of Maryland, College Park: University of Maryland Extension; 1999. [Google Scholar]
- McPherson S, Wada-Katsumata A, Hatano E, Silverman J, Schal C. Comparison of diet preferences of laboratory-reared and apartment-collected german cockroaches. J Econ Entomol. 2021;114:2189–2197. doi: 10.1093/jee/toab139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogbo TC, Okeke JJ, Nwosu MC, Ijuh II (2013) Assessment of ecofriendly methods of eliminating cockroaches. Adv Biosci Biotechnol 1(2):81–85
- Mosaheb MU, Khan NA, Siddiqui R. Cockroaches, locusts, and envenomating arthropods: a promising source of antimicrobials. Iran J Basic Med Sci. 2018;21:873. doi: 10.22038/IJBMS.2018.30442.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrott RC, Miller DM. American cockroach. Virginia: Virginia Cooperative Extension (VCE) Publications; 2010. [Google Scholar]
- Potter M. Cockroach elimination in homes & apartments. Lexington: University of Kentucky College of Agriculture; 2010. [Google Scholar]
- Qi C, Xu CJ, Koppelman GH. The role of epigenetics in the development of childhood asthma. Expert Rev Clin Immunol. 2019;15:1287–1302. doi: 10.1080/1744666X.2020.1686977. [DOI] [PubMed] [Google Scholar]
- Ray R, Potts R, Pietri JE. The persistence of Escherichia coli infection in German cockroaches (Blattodea: Blattellidae) varies between host developmental stages and is influenced by the gut microbiota. J Med Entomol. 2020;57:1964–1971. doi: 10.1093/jme/tjaa108. [DOI] [PubMed] [Google Scholar]
- Roth LM. Systematics and phylogeny of cockroaches (Dictyoptera: Blattaria) Orient inSects. 2003;37:1–86. doi: 10.1080/00305316.2003.10417344. [DOI] [Google Scholar]
- Roth LM, Willis ER. The medical and veterinary importance of cockroaches. SMC. 1957;134:1–147. [Google Scholar]
- Sasaki T, Fujiwara H. Detection and distribution patterns of telomerase activity in insects. Eur J Biochem. 2000;267:3025–3031. doi: 10.1046/j.1432-1033.2000.01323.x. [DOI] [PubMed] [Google Scholar]
- Schal C, Gautier JY, Bell WJ. Behavioural Ecology of Cockroaches. Biol. 1984;59:209–254. [Google Scholar]
- Seabrooks L, Hu L. Insects: an underrepresented resource for the discovery of biologically active natural products. Acta Pharm Sin B. 2017;7:409–426. doi: 10.1016/j.apsb.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seehuus SC, Krekling T, Amdam GV. Cellular senescence in honey bee brain is largely independent of chronological age. Exp Gerontol. 2006;41:1117–1125. doi: 10.1016/j.exger.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui R, Qaisar R, Goswami N, Khan NA, Elmoselhi A. Effect of microgravity environment on gut microbiome and angiogenesis. Life. 2021;11:1008. doi: 10.3390/life11101008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui R, Cruz Soares N, Khan NA. Crocodile gut microbiome is a potential source of novel bioactive molecules. ACS Pharmacol Transl Sci. 2021;4:1260–1261. doi: 10.1021/acsptsci.1c00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui R, Muhammad JS, Khan NA. Locust as an in vivo model. ACS Chem Neurosci. 2021;12:1469–1471. doi: 10.1021/acschemneuro.1c00190. [DOI] [PubMed] [Google Scholar]
- Sieksmeyer T (2021) Host-microbiome-pathogen interactions in cockroaches (Doctoral dissertation). https://refubium.fu-berlin.de/bitstream/handle/fub188/29499/Dissertation_Thorben_Sieksmeyer.pdf?sequence=4&isAllowed=y
- Slaytor M. Cellulose digestion in termites and cockroaches: what role do symbionts play? Comp Biochem Physiol Part B. 1992;103:775–784. doi: 10.1016/0305-0491(92)90194-V. [DOI] [Google Scholar]
- Soopramanien M, Mungroo MR, Sagathevan KA, Khan NA, Siddiqui R. Invertebrates living in polluted environments are potential source of novel anticancer agents. Marmara Pharm J. 2019;23:1079–1089. [Google Scholar]
- Soopramanien M, Khan NA, Siddiqui R. Gut microbiota of animals living in polluted environments are a potential resource of anticancer molecules. J Appl Microbiol. 2021;131:1039–1055. doi: 10.1111/jam.14981. [DOI] [PubMed] [Google Scholar]
- Tinker KA, Ottesen EA. The core gut microbiome of the American cockroach, Periplaneta americana, is stable and resilient to dietary shifts. Appl Environ Microbiol. 2016;82:6603–6610. doi: 10.1128/AEM.01837-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinker KA, Ottesen EA. Phylosymbiosis across deeply diverging lineages of omnivorous cockroaches (order Blattodea) Appl Environ Microbiol. 2020;86:e02513–e2519. doi: 10.1128/AEM.02513-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinker KA, Ottesen EA. Differences in gut microbiome composition between sympatric wild and allopatric laboratory populations of omnivorous cockroaches. Front Microbiol. 2021;12:703785. doi: 10.3389/fmicb.2021.703785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Marked alterations in the distal gut microbiome linked to diet-induced obesity. CELL HOST MICROBE. 2008;3:213. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba de la Pena M, Piskobulu V, Murgatroyd C, Hager R. DNA methylation patterns respond to thermal stress in the viviparous cockroach Diploptera punctata. Epigenetics. 2021;16:313–326. doi: 10.1080/15592294.2020.1795603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vršanský P, van de Kamp T, Azar D, Prokin A, Vidlička LU, Vagovič P. Cockroaches probably cleaned up after dinosaurs. PLoS ONE. 2013;8:e80560. doi: 10.1371/journal.pone.0080560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XY, He ZC, Song LY, Spencer S, Yang LX, Peng F, Liu GM, Hu MH, Li HB, Wu XM, Zeng S. Chemotherapeutic effects of bioassay-guided extracts of the American cockroach, Periplaneta americana. Integr Cancer Ther. 2011;10:NP12–23. doi: 10.1177/1534735411413467. [DOI] [PubMed] [Google Scholar]
- Wang Z, Shi Y, Qiu Z, Che Y, Lo N. Reconstructing the phylogeny of Blattodea: robust support for interfamilial relationships and major clades. Sci Rep. 2017;7:1–8. doi: 10.1038/s41598-017-04243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Liao Q, Wu Y, Wang X, Fu C, Geng F, Qu Y, Zhang J. A composite hydrogel loading natural polysaccharides derived from Periplaneta americana herbal residue for diabetic wound healing. Int J Biol Macromol. 2020;164:3846–3857. doi: 10.1016/j.ijbiomac.2020.08.156. [DOI] [PubMed] [Google Scholar]
- Weihmann T, Reinhardt L, Weißing K, Siebert T, Wipfler B. Fast and powerful: biomechanics and bite forces of the mandibles in the American cockroach Periplaneta americana. PLoS ONE. 2015;10:e0141226. doi: 10.1371/journal.pone.0141226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton DR, Wharton ML. The effect of radiation on the longevity of the cockroach, Periplaneta americana, as affected by dose, age, sex and food intake. Radiat Res. 1959;11:600–615. doi: 10.2307/3570814. [DOI] [PubMed] [Google Scholar]
- Xin L, Yu-Ping TA, Rui LI, Yi JI, Jian-Ming GU, Jin-Long ZH, Shao-Xiong DI, Xiang-Zhi LI, Ru-Rong LI, Jin-Ao DU. Antipyretic and anti-inflammatory activities of Thais luteostoma extracts and underlying mechanisms. Chin J Nat Med. 2015;13:192–198. doi: 10.1016/S1875-5364(15)30004-2. [DOI] [PubMed] [Google Scholar]
- Younes I, Hajji S, Frachet V, Rinaudo M, Jellouli K, Nasri M. Chitin extraction from shrimp shell using enzymatic treatment. Antitumor, antioxidant and antimicrobial activities of chitosan. Int J Biol Macromol. 2014;69:489–498. doi: 10.1016/j.ijbiomac.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Yun J, Hwang JS, Lee DG. The antifungal activity of the peptide, periplanetasin-2, derived from American cockroach Periplaneta americana. Biochem J. 2017;474:3027–3043. doi: 10.1042/BCJ20170461. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Yang A, Tu P, Hu Z. Anti-tumor effects of the American cockroach, Periplaneta americana. Chin Med. 2017;12:1–6. doi: 10.1186/s13020-017-0149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Yao S, Guo X, Yue BS, Ma XY, Li J (2018) Bioactivity-guided screening of wound-healing active constituents from American cockroach (Periplaneta americana). Molecules 23(1):101. [DOI] [PMC free article] [PubMed]


