Highlights
-
•
With no deaths, the study data contrast with the death rates reported in Brazil.
-
•
27.5% of hospitalized children were classified as severe/critical cases.
-
•
Most hospitalizations occurred in healthy children; risk was higher among those with comorbidities.
-
•
The leading determinants of hospitalization were pulmonary disease, cancer and obesity.
-
•
Children with multi-system inflammatory syndrome in this study had a lower median age compared with high-income countries.
Keywords: SARS-CoV-2, COVID-19, MIS-C, PIMS-TS, Hospital-based surveillance, Paediatric, Brazil
Abstract
Background
In 2020, Brazil became the epicentre of the coronavirus disease (COVID-19) pandemic in Latin America, resulting in an unparalleled health catastrophe. Nevertheless, comprehensive clinical reports in Brazilian children are not available.
Methods
This retrospective, hospital-based, active surveillance study was performed to identify paediatric patients with COVID-19 who presented at a private academic medical centre in a large urban area between March 2020 and March 2021. Clinical and demographic information was analysed for those requiring hospitalization, those with severe illness and those with clinical syndromes.
Results
In total, 964 symptomatic cases were evaluated; of these, 17.7% required hospitalization, and 27.5% of hospitalized cases were classified as severe/critical. Acute bronchiolitis and pneumonia were the most common causes of hospitalization among the severe cases. Twenty-seven hospitalized children fulfilled the diagnostic criteria for multi-system inflammatory syndrome (median age 29 months; 85.2% cases were non-severe). A significant co-existing condition was present in 29% of hospitalized children. The risk of hospitalization was higher in children with at least one comorbidity, children aged <2 years and obese children. Increased risk of severe disease was described among those with leukopenia, leukocytosis or any significant comorbidity. No deaths occurred among the study population.
Conclusion
Although most children with COVID-19 experienced mild disease, and no deaths occurred among the study population, a significant proportion of cases required hospitalization and developed severe illness. Obesity, young age, underlying comorbidity, leukopenia and leukocytosis were risk factors for hospitalization or severe disease.
Introduction
The coronavirus disease (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), is the most severe global public health threat since the influenza A H1N1 pandemic in 1918. With a population of 210 million individuals, Brazil, an upper-middle-income country, was the first Latin American country to report confirmed cases of COVID-19 (late February 2020) [1]. As a large urban area of more than 12 million inhabitants, São Paulo has been the epicentre of Brazil's coronavirus pandemic.
In the first half of 2020, Brazil became the epicentre of the COVID-19 pandemic in Latin America, accounting for the second highest death toll in the world [2,3]. Starting in late 2020, the country suffered from a second wave associated with the emergence of a new variant of concern – the Gamma variant (also known as lineage P.1) [4]. Subsequently, Brazil has plunged into an unparalleled health catastrophe: despite Brazil accounting for <3% of the world's population, nearly one in every nine deaths worldwide occurred in Brazil [5]. Since the start of the pandemic, there have been more than 34.5 million confirmed cases of COVID-19 in Brazil, including 3.2 million hospitalizations and 686,000 deaths associated with COVID-19 [6]. In addition, by June 2022, children and adolescents aged <19 years accounted for >41,000 hospitalizations and >3000 deaths [7]. These numbers indicate a case fatality rate as high as 7.2% among children and adolescents hospitalized with COVID-19 (data from SIVEP-Influenza Epidemiological Surveillance Information System, Brazilian Ministry of Health) and multi-system inflammatory syndrome in children (MIS-C) [3], which is up to four times higher than that observed in the USA [8].
Extensive worldwide data suggest lower rates of severe or critical COVID-19, significantly lower risk of death from the disease, and higher rates of asymptomatic infection in children than in adults [9], [10], [11], [12], [13]. The underlying reasons for these considerable differences remain unclear, but age-related differences in expression of angiotensin-converting enzyme 2 receptors and differences in innate and adaptive immunity may play a role [14]. Despite the lower risk for severe outcomes in children, it is essential to acknowledge the consequences of the disease burden on children, including hospitalizations, deaths, long COVID and MIS-C, particularly in low-and-middle-income countries.
Contrasting with the abundant data on adult and paediatric cases of COVID-19 worldwide, comprehensive reports about Brazilian children are not available. Understanding the risk factors associated with hospitalization and severity of COVID-19 could guide clinicians to better diagnostic and management strategies. This article presents the clinical, laboratory and radiographic characteristics of symptomatic paediatric patients with confirmed SARS-CoV-2 infection presenting to a large private paediatric referral hospital in the heart of the pandemic in Brazil.
Materials and methods
Setting
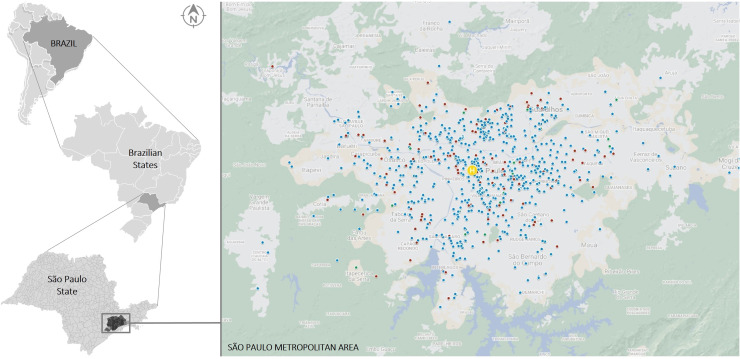
This hospital-based descriptive surveillance analysis defines the epidemiology of paediatric COVID-19 at Hospital Infantil Sabará (Sabará Children's Hospital; HIS), a 145-bed private hospital providing tertiary care in metropolitan São Paulo, Brazil (Figure 1). With approximately 100,000 urgent consultations and >6000 admissions every year, HIS is the second largest private paediatric medical centre in the country [15].
Figure 1.
Map of São Paulo metropolitan area (population in 2018: 21,571,281) [51] with the approximate geographic location of residence of outpatients (blue) and hospitalized patients (red). The location of Hospital Infantil Sabará is shown in yellow.
Study design
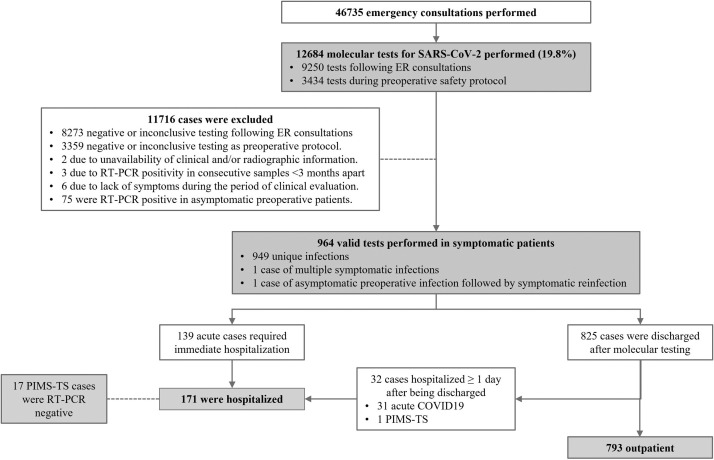
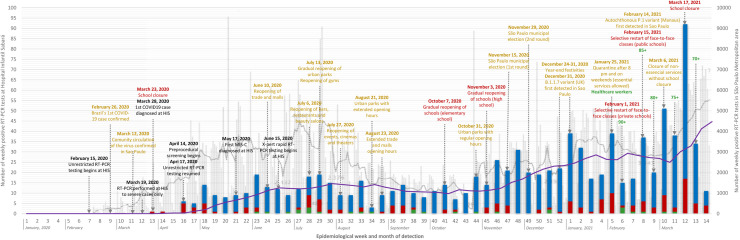
All patients aged <18 years with SARS-CoV-2 detected in nasopharyngeal swab specimens using reverse transcription polymerase chain reaction (RT-PCR) and symptoms consistent with COVID-19 from 28 March 2020 to 31 March 2021 were included in this study (Figure 2). As per hospital protocol, a nasal swab was only performed on symptomatic children if clinical and epidemiological information raised suspicion of SARS-CoV-2 infection. RT-PCR testing began on 15 February 2020, with the first positive test on 28 March 2020 (Figure 3). Asymptomatic children were not tested, even if they had been in close contact with a confirmed case of COVID-19. To determine an association between recent SARS-CoV-2 infection and post-infection manifestations (herein ‘MIS-C’), immunological testing was performed using an anti-SARS-CoV-2 enzyme-linked immunosorbent assay for the detection of immunogloblin (Ig) A/IgM, IgG and total antibodies [16], independent of the molecular evaluation.
Figure 2.
Flow chart of study population selection, including patient recruitment and exclusion criteria. RT-PCR, reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome; ER, emergency room; PIMS-TS, paediatric inflammatory multi-system syndrome.
Figure 3.
Number of positive reverse transcription polymerase chain reaction tests among outpatients (blue), hospitalized patients (red) and cases of multi-system inflammatory syndrome in children (MIS-C) (green) according to epidemiological week [52] among 964 children with coronavirus disease 2019 (COVID-19) at Hospital Infantil Sabará (HIS) (left axis). Textboxes present the relevant events occurring at HIS during the COVID-19 pandemic (black), main non-pharmaceutical interventions (yellow), school-related interventions (red), and vaccination against COVID-19 by age in São Paulo State (green). The grey columns in the background represent COVID-19 cases of all ages in the São Paulo metropolitan area (right axis) [53]. By overlapping the paediatric cases from HIS with overall cases (i.e. paediatric and non-paediatric cases) in the same geographic area, a disproportionate number of infections in adults was identified during the first months of the pandemic, which flattened as non-pharmacological measures and restrictions took place. An apparent increase in infections occurred in mid–late October 2020, coinciding with the gradual reopening of elementary and high schools. This pattern persisted during the remaining weeks of the study, which included the initial period of circulation of the Gamma variant. RT-PCR, reverse transcription polymerase chain reaction.
Evaluation of SARS-CoV-2 infection
Laboratory testing was requested upon individual medical assessments under hospital protocols. All laboratory evaluations were conducted at Diagnósticos da America S.A. RT-PCR testing was performed according to the Charité-Berlin protocol [17]. In addition, Xpert Xpress SARS-CoV-2 (Cepheid, Sunnyvale, CA, USA) was used in limited samples.
Evaluation of other respiratory pathogens
The evaluation of additional respiratory viruses [through rapid respiratory syncytial virus (RSV) and influenza A/B immunochromatographic tests] and group A streptococcus in the nasopharynx (through rapid immunoenzymatic test) is not standardized by HIS, and was requested exclusively upon individual medical assessment. Such tests are not covered by private medical care. Even more expensive multiplex RT-PCR is requested more frequently following hospitalization (see online supplementary material for technical information regarding this RT-PCR assay).
Radiologic evaluation
Chest radiographs and computed tomography (CT) scans were requested upon individual medical decisions. When performed, the examinations were analysed by unblinded radiologists who interpreted the results independently.
Data sources and analysis
Clinical and demographic information was extracted systematically from electronic medical records for each case, and included age, sex, underlying medical conditions that posed risk for severe outcomes, exposure to cases of COVID-19 (confirmed, suspected or unknown – when no symptomatic disease was identified among close contacts irrespective of laboratory confirmation), symptoms experienced, radiologic evaluation, laboratory evaluation, hospitalization, need for intensive care, supportive treatments, pharmacologic therapy and outcomes. Race and ethnicity were self-reported based on fixed categories. For children requiring serial laboratory evaluations, the most altered value was considered.
Disease severity among inpatients and outpatients was classified [18] as mild (individuals who had any signs or symptoms of COVID-19, except shortness of breath, dyspnoea or abnormal chest imaging), moderate (individuals who showed evidence of lower respiratory disease during clinical assessment or imaging, and had SpO2 ≥94% on room air), severe (individuals who had SpO2 <94% on room air or lung infiltrates >50% on chest CT), or critical (individuals who had respiratory failure, septic shock and/or multiple organ dysfunction). MIS-C was defined using the World Health Organization case definition for multi-system inflammatory disorders [19].
Statistical data analysis
Due to the large number of predictor variables to be analysed for each outcome, pre-selection was performed in bivariate analyses. Chi-squared test or Fisher's exact test was used to evaluate the association between categorical variables and the binary outcomes, as appropriate, and Student's t-test or Mann–Whitney test was used to compare the categorical variables and binary outcomes. Significant predictors with P≤0.2 on bivariate analysis were exported to the multi-variable logistic regression model, where three separate analyses were conducted for each outcome: (i) factors associated with hospitalization; (ii) factors associated with clinical severity among hospitalized children (i.e. non-severe cases vs severe cases); and (iii) factors associated with predefined clinical syndromes among hospitalized children (i.e. ‘respiratory syndrome’, ‘MIS-C’ and ‘other clinical syndromes’). Multi-variate logistic regression analysis was carried out using the stepwise forward technique to find the combined effects of the variables. Odds ratios (ORs) and 95% confidence intervals (CIs) were determined; the significance level was set at 0.05 (two-tailed test) for all tests. Cases with unknown/blank information were excluded from the analysis. Categorical variables were reported as absolute numbers and percentages, and continuous variables were expressed as medians and interquartile ranges (IQRs), if distributed non-normally. Statistical analyses were performed using SPSS Statistics Version 13 (IBM Corp., Armonk, NY, USA).
Ethics
Study approval was obtained from the Research Ethics Committee of José Luiz Egydio Setúbal Foundation Review Board (Approval No. 42080620.5.0000.5567). Given the study's purely descriptive and retrospective nature, written informed consent was waived. Data were collected anonymously, and analysed and reported in aggregate form.
Results
Study population
From the first detected case of COVID-19 at HIS, a total of 964 symptomatic SARS-CoV-2-related conditions from 962 unique patients were evaluated. Of these, 17.7% (n=171) were hospitalized for at least 1 day (Figure 2), and 95.3% (n=163) of hospitalized cases stayed for ≥48 h. Geographical case distribution is depicted in Figure 1. Overall, the median age was 44.7 (IQR 16.4–104.7) months, and 55.4% (n=534) were male. White ethnicity was the most prevalent (74.5%, n=718), followed by black/African descent (11.9%, n=115) and Asian (3.6%, n=35). Case distribution was inversely related to age: children aged <2 years accounted for more than one-third of all infections (34.2%, n=330), followed by children aged 2–5 years (24.8%, n=239), 5–10 years (21.6%, n=208) and >10 years (19.4%, n=187). Known exposure to SARS-CoV-2 was documented in 80.7% (n=778) of cases, and a family contact was the source in 75.7% (n=730) of cases. Marked differences in most of these epidemiological variables were seen when comparing inpatients and outpatients (Table 1).
Table 1.
Clinical, demographic and laboratory characteristics of paediatric patients with coronavirus disease 2019 (COVID-19) evaluated at Hospital Infantil Sabará between March 2020 and March 2021.
| All patients |
Clinical syndrome among hospitalized patients |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Hospitalized |
Outpatient |
P-value | MIS-C |
Respiratory |
Other syndromes |
P-value | |||||
| Number of patients (%) | 171 | 17.7% | 793 | 82.3% | 27 | 15.8% | 86 | 50.3% | 58 | 33.9% | ||
| Age, median (IQR), months | 21.6 | (7.2-53.8) | 51.5 | (19.3-108.3) | < 0.001 | 29 | (19-69) | 16 | (4-47) | 21.6 | (3-54) | < 0.001 |
| Age distribution, no. (%), years | < 0.001 | 0.444 | ||||||||||
| <2 | 91 | 53.2% | 239 | 30.1% | 10 | 37.0% | 50 | 58.1% | 31 | 53.4% | ||
| 2-5 | 40 | 23.4% | 199 | 25.1% | 8 | 29.6% | 18 | 20.9% | 14 | 24.1% | ||
| 5-10 | 16 | 9.4% | 192 | 24.2% | 5 | 18.5% | 7 | 8.1% | 4 | 6.9% | ||
| >10 | 24 | 14.0% | 163 | 20.6% | 4 | 14.8% | 11 | 12.8% | 9 | 15.5% | ||
| Sex, no. (%) | 0.217 | 0.318 | ||||||||||
| Female | 69 | 40.4% | 361 | 45.5% | 10 | 37.0% | 38 | 44.2% | 21 | 36.2% | ||
| Male | 102 | 59.6% | 432 | 54.5% | 17 | 63.0% | 48 | 55.8% | 37 | 63.8% | ||
| Race/ethnicity, no. (%) | 0.693 | 0.421 | ||||||||||
| Black | 1 | 0.6% | 5 | 0.6% | 0 | 0.0% | 1 | 1.2% | 0 | 0.0% | ||
| Afrodescent | 13 | 7.6% | 96 | 12.1% | 1 | 3.7% | 8 | 9.3% | 4 | 6.9% | ||
| White | 137 | 80.1% | 581 | 73.3% | 25 | 92.6% | 66 | 76.7% | 46 | 79.3% | ||
| Asian | 6 | 3.5% | 29 | 3.7% | 0 | 0.0% | 6 | 7.0% | 0 | 0.0% | ||
| NA | 14 | 8.2% | 82 | 10.3% | 1 | 3.7% | 5 | 5.8% | 8 | 13.8% | ||
| Significant underlying medical conditions, no. (%) | 50 | 29% | 153 | 19% | 0.001 | < 0.001 | ||||||
| Asthma or recurrent wheezing | 25 | 14.6% | 82 | 10.3% | 0 | 0.0% | 22 | 25.6% | 3 | 5.2% | < 0.001 | |
| Prematurity | 10 | 5.8% | 7 | 0.9% | 0 | 0.0% | 6 | 7.0% | 4 | 6.9% | 0.392 | |
| Chronic neurologic disease | 11 | 6.4% | 28 | 3.5% | 0 | 0.0% | 7 | 8.1% | 4 | 6.9% | 0.099 | |
| Genetic/chromosomal disease | 6 | 3.5% | 7 | 0.9% | 1 | 3.7% | 4 | 4.7% | 1 | 1.7% | 0.423 | |
| Onocologic disease | 3 | 1.8% | 0 | 0.0% | 0 | 0.0% | 1 | 1.2% | 2 | 3.4% | 0.49 | |
| Congenital cardiopathy | 5 | 2.9% | 12 | 1.5% | 1 | 3.7% | 3 | 3.5% | 1 | 1.7% | 0.524 | |
| Chronic pulmonary disease (not asthma) | 5 | 2.9% | 4 | 0.5% | 0 | 0.0% | 5 | 5.8% | 0 | 0.0% | 0.14 | |
| Obesity | 3 | 1.8% | 1 | 0.1% | 0 | 0.0% | 3 | 3.5% | 0 | 0.0% | 0.382 | |
| Severity of illness and outcomes | ||||||||||||
| Severe and critical | 47 | 27.5% | - | - | - | 4 | 14.8% | 40 | 46.5% | 3 | 5.2% | < 0.001 |
| Required intensive care | 57 | 33.3% | - | - | - | 14 | 51.9% | 39 | 45.3% | 4 | 6.9% | < 0.001 |
| Length of stay in PICU, median (IQR), days | 4.5 | (2.3-8) | - | - | - | 6 | (3.3-8.0) | 4 | (2-8) | 4 | (3.5-6) | - |
| Length of stay, hospital, median (IQR), days | 4 | (3-6) | - | - | - | 6 | (4.5-8.5) | 4 | (3-7) | 3 | (2-4) | - |
| Symptoms presented during disease course, no. (%) | ||||||||||||
| Median duration of symptoms/signs at ER evaluation (IQR), days | 3.0 | (2-5) | 3.0 | (2-4) | 0.004 | 7.0 | (4-9) | 3.0 | (2-5) | 2.0 | (1-4) | 0.004 |
| Fever | 131 | 76.6% | 525 | 66.2% | 0.008 | 27 | 100.0% | 61 | 70.9% | 43 | 74.1% | < 0.001 |
| Cough | 72 | 42.1% | 416 | 52.5% | 0.014 | 3 | 11.1% | 62 | 72.1% | 7 | 12.1% | < 0.001 |
| Rhinorrhoea/nasal congestion | 65 | 38.0% | 469 | 59.1% | < 0.001 | 5 | 18.5% | 54 | 62.8% | 6 | 10.3% | 0.127 |
| Sneezing | 13 | 7.6% | 113 | 14.2% | 0.019 | 0 | 0.0% | 11 | 12.8% | 2 | 3.4% | < 0.001 |
| Tachypnoea on admission | 41 | 24.0% | 21 | 2.6% | < 0.001 | 1 | 3.7% | 38 | 44.2% | 2 | 3.4% | 0.083 |
| Tachycardia on admission | 18 | 10.5% | 7 | 0.9% | < 0.001 | 5 | 18.5% | 11 | 12.8% | 2 | 3.4% | < 0.001 |
| Shortness of breath | 48 | 28.1% | 26 | 3.3% | < 0.001 | 0 | 0.0% | 47 | 54.7% | 1 | 1.7% | < 0.001 |
| Hypoxia (O2 <92% as measured by pulse oximetry) | 31 | 18.1% | 4 | 0.5% | < 0.001 | 1 | 3.7% | 28 | 32.6% | 2 | 3.4% | 0.329 |
| Cyanosis | 9 | 5.3% | 0 | 0.0% | < 0.001 | 0 | 0.0% | 6 | 7.0% | 3 | 5.2% | 0.023 |
| Reduced feeding or difficulty feeding | 68 | 39.8% | 169 | 21.3% | 0.01 | 16 | 59.3% | 28 | 32.6% | 24 | 41.4% | 0.023 |
| Abdominal pain | 28 | 16.4% | 72 | 9.1% | 0.197 | 13 | 48.1% | 2 | 2.3% | 13 | 22.4% | < 0.001 |
| Diarrhoea | 43 | 25.1% | 164 | 20.7% | < 0.001 | 9 | 33.3% | 10 | 11.6% | 24 | 41.4% | 0.020 |
| Nausea or vomiting | 53 | 31.0% | 119 | 15.0% | < 0.001 | 13 | 48.1% | 15 | 17.4% | 25 | 43.1% | 0.002 |
| Dehydration | 25 | 14.6% | 14 | 1.8% | < 0.001 | 7 | 25.9% | 5 | 5.8% | 13 | 22.4% | 0.004 |
| Fatigue/mialgia | 21 | 12.3% | 114 | 14.4% | 0.351 | 10 | 37.0% | 7 | 8.1% | 4 | 6.9% | < 0.001 |
| Drowsiness/Irritability | 44 | 25.7% | 51 | 6.4% | < 0.001 | 10 | 37.0% | 16 | 18.6% | 18 | 31.0% | 0.018 |
| Headache | 16 | 9.4% | 181 | 22.8% | < 0.001 | 4 | 14.8% | 6 | 7.0% | 6 | 10.3% | 0.322 |
| Sore throat | 16 | 9.4% | 144 | 18.2% | 0.002 | 6 | 22.2% | 6 | 7.0% | 4 | 6.9% | 0.018 |
| Anosmia | 4 | 2.3% | 39 | 4.9% | 0.072 | 0 | 0.0% | 4 | 4.7% | 0 | 0.0% | 0.401 |
| Ageusia | 4 | 2.3% | 39 | 4.9% | 0.072 | 0 | 0.0% | 4 | 4.7% | 0 | 0.0% | 0.401 |
| Neurologic symptoms | 9 | 5.3% | 6 | 0.8% | < 0.001 | 1 | 3.7% | 0 | 0.0% | 8 | 13.8% | < 0.001 |
| Meningeal signs | 1 | 0.6% | 0 | 0.0% | 0.178 | 0 | 0.0% | 0 | 0.0% | 1 | 1.7% | 0.122 |
| Rash | 24 | 14.0% | 33 | 4.2% | < 0.001 | 15 | 55.6% | 4 | 4.7% | 5 | 8.6% | < 0.001 |
| Cervical adenopathy | 5 | 2.9% | 1 | 0.1% | 0.001 | 5 | 18.5% | 0 | 0.0% | 0 | 0.0% | < 0.001 |
| Oral abnormalities | 17 | 9.9% | 48 | 6.1% | 0.069 | 12 | 44.4% | 2 | 2.3% | 3 | 5.2% | < 0.001 |
| Non-supurative conjunctivitis | 10 | 5.8% | 5 | 0.6% | < 0.001 | 10 | 37.0% | 0 | 0.0% | 0 | 0.0% | < 0.001 |
| Extreme abnormalities | 11 | 6.4% | 0 | 0.0% | < 0.001 | 10 | 37.0% | 0 | 0.0% | 1 | 1.7% | < 0.001 |
| General laboratory evaluation | ||||||||||||
| Age-matched anaemia | 50/158 | 31.6% | 6/85 | 7.1% | < 0.001 | 19/25 | 76.0% | 17/81 | 21.0% | 14/52 | 26.9% | < 0.001 |
| WBC per µL, median | 8330 | (5925-12,950) | 7815 | (5925-10,175) | 0.172 | 13000 | (8040-19,200) | 7600 | (5700-12,100) | 7920 | (5140-12,200) | - |
| Leukopenia (<4000 cells/μL) | 14/156 | 9.0% | 3/88 | 3.4% | 0.101 | 2/25 | 8.0% | 8/78 | 10.3% | 2/26 | 7.7% | 0.898 |
| Left shift | 17/158 | 10.8% | 2/85 | 2.4% | 0.017 | 6/25 | 24.0% | 6/81 | 7.4% | 5/52 | 9.6% | 0.063 |
| Absolute neutrophil count (cells/μL), median | 3525 | (1890-6440) | 2998 | (1815-5965) | 6198 | (3450-8532) | 3300 | (1890-5800) | 3745 | (1880-6485) | - | |
| Absolute lymphocyte count (cells/μL), median | 2595 | (1630-4220) | 3170 | (2045-4525) | 0.246 | 2553 | (1311-3387) | 2500 | (1490-4300) | 2930 | (1800-4275) | - |
| Neutrophil to lymphocyte ratio, median (rv not established) | 1.4 | (0.6-3.2) | 1.2 | (0.4-2.1) | - | 1.8 | (1.1-4.6) | 1.5 | (0.6-3.2) | 1.3 | (0.5-2.7) | - |
| Age-matched lymphopenia (cells/µL) | 57/153 | 37.3% | 15/90 | 16.7% | 0.001 | 7/21 | 33.3% | 33/80 | 41.3% | 17/52 | 32.7% | 0.227 |
| Atypical lymphocytes | 92/158 | 58.2% | 43/84 | 51.2% | 0.178 | 16/25 | 64.0% | 43/81 | 53.1% | 33/52 | 63.5% | 0.597 |
| Platelets/µL, median | 284000 | (210,000-383,000) | 269000 | (217,000-3,432,500) | 0.184 | 180000 | (82,000-434,000) | 296000 | (249,000-371,000) | 283000 | (211,000-350,000) | |
| Increased platelets count (>450,000/µL) | 20/157 | 12.7% | 5/84 | 6.0% | 0.050 | 6/25 | 24.0% | 10/81 | 12.3% | 4/51 | 7.8% | 0.405 |
| Reduced platelets count (<150,000/µL) | 136/157 | 86.6% | 79/84 | 94.0% | 0.937 | 19/25 | 76.0% | 70/81 | 86.4% | 47/51 | 92.2% | < 0.001 |
| AST (U/L), median | 39 | (29-65) | 44 | (27-43) | 0.424 | 48 | (32-81) | 35 | (31-64) | 28 | (24-41) | 0.153 |
| ALT (U/L), median | 24 | (17-44) | 22 | (17-32) | 0.999 | 30 | (21-57) | 24 | (17-44) | 20 | (17-24) | 0.323 |
| Laboratory evidence of cardiac involvement | ||||||||||||
| Increased CPK (rv 30-135 U/L) | 1/19 | 5.3% | - | - | - | 1/12 | 8.3% | 0/5 | 0.0% | 0/2 | 0.0% | 0.767 |
| Increased troponin I (rv ≤53 ng/L) | 8/22 | 36.4% | - | - | - | 6/15 | 40.0% | 2/6 | 33.3% | 0/1 | 0.0% | 0.119 |
| Increased proBNP (rv <100 pg/mL) | 8/21 | 38.1% | - | - | - | 7/15 | 46.7% | 1/4 | 25.0% | 0/2 | 0.0% | 0.517 |
| Laboratory evidence of systemic inflammation | ||||||||||||
| Median C-reactive protein level (mg/dL) | 1.5 | (0.3-6.8) | 0.6 | (0.1-2.4) | - | 12.6 | (5.9-20.8) | 0.8 | (0.2-2.6) | 1.2 | (0.2-4.7) | - |
| Elevated C-reactive protein (>3.0 mg/dL) | 56/157 | 35.7% | 11/63 | 17.5% | 0.002 | 21/26 | 80.8% | 17/80 | 21.3% | 18/51 | 35.3% | < 0.001 |
| Elevated C-reactive protein (>10.0 mg/dL) | 26/157 | 16.6% | 3/63 | 4.8% | 0.028 | 15/26 | 57.7% | 4/80 | 5.0% | 7/51 | 13.7% | < 0.001 |
| ESR (mm/h), median | 53.0 | (25-81) | 11.0 | (9-13) | 0.004 | 72 | (63-105) | 23 | (15-36) | 30 | (23-42) | - |
| Elevated ESR (≥40 mm/h) | 27 | 58.1% | 0 | 0.0% | 0.041 | 21/21 | 100.0% | 3/15 | 20.0% | 3/7 | 42.9% | < 0.001 |
| Leukocytosis (≥15,000 mm³) | 26/158 | 16.5% | 5/85 | 5.9% | 0.01 | 9/25 | 36.0% | 12/81 | 14.8% | 5/52 | 9.6% | 0.003 |
| Elevated LDH (>237 UI/L) | 35/41 | 85.4% | - | - | - | 18/20 | 90.0% | 12/13 | 92.3% | 5/8 | 62.5% | 0.002 |
| Elevated D-dimer (> 0.50 µg/mL) | 122/122 | 100.0% | - | - | - | 5/5 | 100.0% | 65/65 | 100.0% | 52/52 | 100.0% | 0.006 |
| Altered fibrinogen (rv 200-400 mg/dL) | 24/38 | 63.2% | - | - | - | 19/21 | 90.5% | 3/12 | 25.0% | 2/5 | 40.0% | 0.001 |
| Altered coagulopathy | 7/35 | 20.0% | - | - | - | 5/15 | 33.3% | 1/14 | 7.1% | 1/6 | 16.7% | 0.081 |
| Albumin level ≤3.5 g/dL | 108/108 | 100.0% | - | - | - | 5/5 | 100.0% | 57/57 | 100.0% | 46/46 | 100.0% | 0.215 |
| Increased IL-6 (rv <7 pg/mL) | 15/20 | 75.0% | - | - | - | 14/14 | 100.0% | 1/5 | 20.0% | 0/1 | 0.0% | 0.001 |
| Exposure to SARS-CoV-2, no. (%) | < 0.001 | 0.689 | ||||||||||
| Family cluster | 109 | 63.7% | 621 | 78.3% | 17 | 63.0% | 58 | 67.4% | 34 | 58.6% | ||
| Contact with other suspected case | 6 | 3.5% | 42 | 5.3% | 1 | 3.7% | 4 | 4.7% | 1 | 1.7% | ||
| Unidentified source of infection | 56 | 32.7% | 130 | 16.4% | 9 | 33.3% | 24 | 27.9% | 23 | 39.7% | ||
| Laboratory evidence of COVID-19, contact with SARS-CoV-2 and detection of other respiratory pathogens | ||||||||||||
| Positive test for any other respiratory pathogen | 22/82 | 26.8% | 2/81 | 2.5% | < 0.001 | 2/12 | 16.7% | 20/51 | 39.2% | 0/19 | 0.0% | - |
| Positive multiplex RT-PCR respiratory panel | 15/35 | 42.9% | 0/0 | #DIV/0! | - | 2/4 | 50.0% | 13/27 | 48.1% | 0/4 | 0.0% | - |
| RSV (rapid test) | 10/22 | 35.7% | 1/10 | 10.0% | - | 0/1 | 0.0% | 10/24 | 41.7% | 0/3 | 0.0% | - |
| RSV (any test) | 15/22 | 68.2% | 1/2 | 50.0% | - | 0/2 | 0.0% | 15/20 | 75.0% | 0/0 | #DIV/0! | - |
| Influenza A/B (rapid test) | 0/23 | 0.0% | 0/17 | 0.0% | - | 0/2 | 0.0% | 0/17 | 0.0% | 0/4 | 0.0% | - |
| Group A streptococcus (rapid test) | 0/28 | 0.0% | 1/66 | 1.5% | - | 0/7 | 0.0% | 0/9 | 0.0% | 0/12 | 0.0% | - |
| Radiologic evaluation, no. (%) | ||||||||||||
| No radiologic evaluation | 40 | 23.4% | 575 | 72.5% | - | 6 | 22.2% | 0 | 0.0% | 34 | 58.6% | - |
| Chest CT performed | 18 | 10.5% | 7 | 0.9% | - | 2 | 7.4% | 14 | 16.3% | 2 | 3.4% | - |
| Chest CT abnormalities | 14/18 | 77.8% | 1/7 | 14.3% | - | 0/2 | 0.0% | 12/14 | 85.7% | 2/2 | 100.0% | - |
| Ground-glass opacity | 11 | 61.1% | 1 | 14.3% | - | 0 | 0.0% | 9 | 64.3% | 2 | 100.0% | - |
| Consolidations | 9 | 50.0% | 0 | 0.0% | - | 0 | 0.0% | 8 | 57.1% | 1 | 50.0% | - |
| Other | 5 | 27.8% | 0 | 0.0% | - | 0 | 0.0% | 4 | 28.6% | 1 | 50.0% | - |
| Chest CT abnormalities with normal X-ray | 4/14 | 0/1 | - | 0/0 | 3/12 | 1/2 | - | |||||
| X-ray performed | 130 | 76.0% | 213 | 26.9% | - | 21 | 77.8% | 85 | 98.8% | 24 | 41.4% | - |
| X-ray performed, no chest CT | 113 | 86.9% | 211 | 99.1% | - | 19 | 90.5% | 72 | 84.7% | 22 | 91.7% | - |
| Any X-ray abnormalities | 78/130 | 60.0% | 75/213 | 35.2% | < 0.001 | 11/21 | 52.4% | 54/85 | 63.5% | 13/24 | 54.2% | 0.271 |
| Perihilar peribroncovascular thickening | 65/130 | 50.0% | 65/213 | 30.5% | - | 9/21 | 42.9% | 44/85 | 51.8% | 12/24 | 50.0% | - |
| Pulmonary opacities | 24/130 | 18.5% | 11/213 | 5.2% | - | 3/21 | 14.3% | 19/85 | 22.4% | 2/24 | 8.3% | - |
| Atelectasis | 8/130 | 6.2% | 4/213 | 1.9% | - | 1/21 | 4.8% | 5/85 | 5.9% | 2/24 | 8.3% | - |
| Maximum respiratory and vasoactive support | ||||||||||||
| None | 124 | 72.5% | - | - | - | 23 | 85.2% | 46 | 53.5% | 55 | 94.8% | < 0.001 |
| Supplemental oxygen | 25 | 14.6% | - | - | - | 1 | 3.7% | 23 | 26.7% | 1 | 1.7% | 0.002 |
| High-flow nasal cannula | 13 | 7.6% | - | - | - | 0 | 0.0% | 12 | 14.0% | 1 | 1.7% | 0.016 |
| CPAP or BiPAP | 4 | 2.3% | - | - | - | 0 | 0.0% | 4 | 4.7% | 0 | 0.0% | 0.275 |
| Intubation/tracheostomy ventilation | 5 | 2.9% | - | - | - | 3 | 11.1% | 1 | 1.2% | 1 | 1.7% | 0.016 |
| Vasoactive support | 4 | 2.3% | - | - | - | 3 | 11.1% | 0 | 0.0% | 1 | 1.7% | 0.002 |
| Advanced therapy (iNO, ECMO, prone ventilation) | 2 | 1.2% | - | - | - | 1 | 3.7% | 0 | 0.0% | 1 | 1.7% | 0.122 |
| Pharmacologic therapy | ||||||||||||
| None | 74 | 43.3% | 723 | 91.2% | < 0.001 | 4 | 14.8% | 28 | 32.6% | 42 | 72.4% | < 0.001 |
| Macrolide | 29 | 17.0% | 19 | 2.4% | < 0.001 | 2 | 7.4% | 26 | 30.2% | 1 | 1.7% | < 0.001 |
| Tocilizumab | 3 | 1.8% | 0 | 0.0% | - | 3 | 11.1% | 0 | 0.0% | 0 | 0.0% | 0.002 |
| Intravenous immunoglobulin | 19 | 11.1% | 0 | 0.0% | - | 16 | 59.3% | 1 | 1.2% | 2 | 3.4% | < 0.001 |
| Oseltamivir | 19 | 11.1% | 1 | 0.1% | < 0.001 | 2 | 7.4% | 17 | 19.8% | 0 | 0.0% | 0.083 |
| Systemic glucocorticoids | 54 | 31.6% | 24 | 3.0% | < 0.001 | 14 | 51.9% | 36 | 41.9% | 4 | 6.9% | < 0.001 |
| Antibacterial therapy (other than macrolide) | 65 | 38.0% | 30 | 3.8% | < 0.001 | 17 | 63.0% | 34 | 39.5% | 14 | 24.1% | 0.017 |
ALT, alanine transaminase; AST, aspartate aminotransferase; BiPAP, bilevel positive airway pressure; CPAP, continuous positive airway pressue; CPK, creatine phosphokinase; CT, computed tomography; ECMO, extracorporeal membrane oxygenation; ER, emergency room; ESR, erythrocyte sedimentation rate; IL-6, interleukin-6; iNO, inhaled nitric oxide; IQR, interquartile range; PICU, paediatric intensive care unit; proBNP, pro B-type natriuretic peptide; RT-PCR, reverse transcription polymerase chain reaction; RSV, respiratory syncytial virus; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; WBC, white blood cells. LDH, lactate dehydrogenase; rv, reference value.
Reference values and ranges for parameters were provided by the local laboratory
Clinical characteristics and outcomes
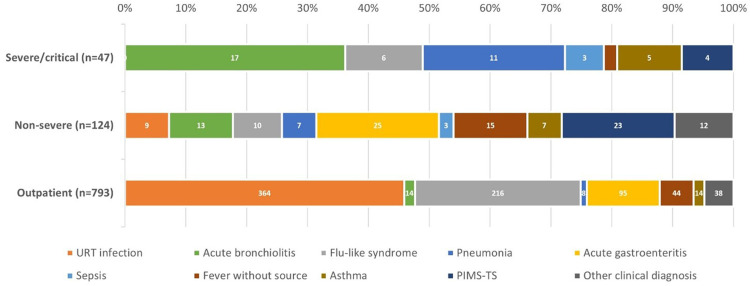
A co-existing condition was present in 21.1% (n=203) of all cases. Asthma/recurrent wheezing, chronic neurologic disease, and prematurity were disproportionately represented among both outpatients and hospitalized children (Table 1). Twenty-five of 57 patients (43.8%) admitted to a paediatric intensive care unit (PICU) reported at least one significant underlying disease. The additional relevant clinical conditions are described in Table 2. Among the outpatients, upper respiratory tract infection (URTI) (n=364/793) and flu-like syndrome (n=216/793) were the leading presentations, comprising 73.1% of all discharged children. Acute gastroenteritis (12%, n=95/793) and fever without source (5.5%, n=44/793) were less common presentation syndromes.
Table 2.
Significant underlying chronic conditions according to disease severity among paediatric patients with coronavirus disease 2019 evaluated at Hospital Infantil Sabará between March 2020 and March 2021.
Bacterial agents causing carbapenemase-producing carbapenem-resistant Enterobacterales infection found in clinical specimens at Debre Berhan Comprehensive Specialized Hospital from January to June 2021
| Bacterial isolate | Clinical sample | CPE infection (n=164) |
Total |
|
|---|---|---|---|---|
| Yes (%) | No (%) | |||
| E. coli | Blood | 3 (1.8) | 14 (8.5) | 17 (10.4) |
| Urine | 1 (0.6) | 30 (18.3) | 31 (18.9) | |
| Wound | 4 (2.4) | 10 (6.1) | 14 (8.5) | |
| Sputum | - | 2 (1.2) | 2 (1.2) | |
| Stool | - | 10 (6.1) | 10 (6.1) | |
| Total | 8 (4.9) | 66 (40.2) | 74 (45.1) | |
| K. pneumoniae | Blood | 3 (1.8) | 10 (6.1) | 13 (7.9) |
| Stool | 1 (0.6) | 2 (1.2) | 3 (1.8) | |
| Urine | 4 (2.4) | 19 (11.6) | 23 (14.0) | |
| Wound | - | 8 (4.9) | 8 (4.9) | |
| Total | 8 (4.9) | 39 (23.8) | 47 (28.7) | |
| K. oxytoca | Blood | 2 (1.2) | 2 (1.2) | 4 (2.4) |
| Stool | 1 (0.6) | 2 (1.2) | 3 (1.8) | |
| Total | 3 (1.8) | 4 (2.4) | 7 (4.3) | |
| C. koseri | Wound | - | 1 (0.6) | 1 (0.6) |
| Stool | - | 1 (0.6) | 1 (0.6) | |
| Blood | 2 (1.2) | 4 (2.4) | 6 (3.7) | |
| Urine | - | 2 (1.2) | 2 (1.2) | |
| Total | 2 (1.2) | 8 (4.9) | 10 (6.1) | |
| K. aerogenes | Wound | 1 (0.6) | - | 1 (0.6) |
| Urine | - | 3 (1.8) | 3 (1.8) | |
| Stool | - | 3 (1.8) | 3 (1.8) | |
| Total | 1 (0.6) | 6 (3.7) | 7 (4.3) | |
| M. morganii | Urine | - | 1 (0.6) | 1 (0.6) |
| Stool | - | 1 (0.6) | 1 (0.6) | |
| Total | - | 2 (1.2) | 2 (1.2) | |
| K. ozaenae | Blood | - | 3 (1.8) | 3 (1.8) |
| Urine | - | 1 (0.6) | 1 (0.6) | |
| Total | - | 4 (2.4) | 4 (2.4) | |
| P. stuartii | Urine | 1 (0.6) | 2 (1.2) | 3 (1.8) |
| Blood | 1 (0.6) | - | 1 (0.6) | |
| Stool | - | 1 (0.6) | 1 (0.6) | |
| Total | 2 (1.2) | 3 (1.8) | 5 (3.0) | |
| E. cloacae | Urine | - | 4 (2.4) | 4 (2.4) |
| Stool | - | 2 (1.2) | 2 (1.2) | |
| Total | 6 (3.7) | 6 (3.7) | ||
| Citrobacter spp. | Wound | - | 1 (0.6) | 1 (0.6) |
| C. freundii | Stool | - | 1 (0.6) | 1 (0.6) |
| Total | 24 (14.6) | 140 (85.4) | 164(100) | |
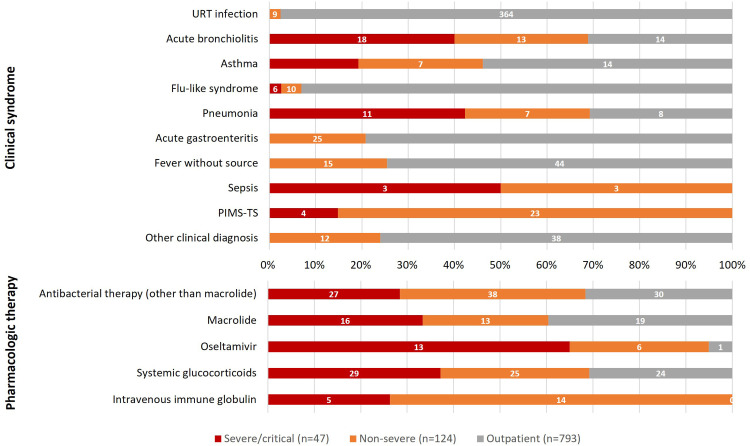
Children requiring hospitalization were divided into two groups according to the clinical course: non-severe hospitalized cases (72.5%, n=124/171) and severe/critical cases (27.5%, n=47/171) requiring PICU care (Table 1). The clinical syndromes in this subgroup were distributed evenly among nine conditions (Figure 4). A significant number of severe/critical patients experienced respiratory disease: acute bronchiolitis (38.3%, n=18/47) and pneumonia (23.4%, n=11/47) were the most common causes of hospitalization, followed by flu-like syndrome (12.7%, n=6/47), asthma exacerbation (10.6%, n=5/47) and MIS-C (8.5%, n=4/47). No deaths related to COVID-19 or MIS-C occurred at HIS prior to the acceptance date of this article.
Figure 4.
Distribution of clinical syndromes according to disease severity among 964 children with coronavirus disease 2019 (COVID-19) at Hospital Infantil Sabará. Outpatients were considered mild. URT, upper respiratory tract; PIMS-TS, paediatric inflammatory multi-system syndrome.
COVID-19 in very young infants
Thirty-four symptomatic infections occurred in children aged <2 months, and 26 were hospitalized (15.2% of all inpatients); of these, four were late preterm babies and all had significant comorbidities. The most common clinical syndromes causing hospitalization were acute bronchiolitis (n=6), URTI (n=5) and fever without source (n=8). One-quarter (23.5%, n=8) of cases were severe, and RSV was co-detected in two of these babies. URTI (n=3) and exanthaematous disease (n=2) were the most common presentations among outpatients.
Viral co-detections
Testing for other respiratory pathogens was performed in 17.0% of all children (n=164/964), including 10.3% of outpatients (n=82/793) and 48% of inpatients (n=82/171). The crude positivity rate was 16.5% (n=27/164), but differed significantly among hospitalized cases [26.8% (n=22/82) vs 6.1% (5/82); P=0.001]. RSV was detected exclusively in inpatients, found in 75% (n=15/20) of those hospitalized with respiratory symptoms. RSV was also the leading co-pathogen in acute bronchiolitis, found in 15 of 28 tests irrespective of disease severity, and in 10 of 16 severe cases tested for additional pathogens.
Among the 29 children with severe respiratory disease, three viruses were identified: 11 RSV (one co-detection, rhinovirus) and five rhinoviruses (two co-detection: RSV and adenovirus). Seven positive tests in patients hospitalized with non-severe infection detected RSV (n=4) and three rhinoviruses (one co-detection, adenovirus).
Clinical markers for hospitalization
Factors that increased the risk of hospitalization are described in Table 1, and included chronic pulmonary disease, but not asthma or recurrent wheezing (P=0.002), genetic or chromosomal disease (P=0.003), oncologic disease (P=0.006) and obesity (P=0.019). Symptomatic infections occurring after identification of the Gamma variant did not result in a higher risk of hospitalization (P=0.434). The adjusted odds of hospitalization were 2.0 (95% CI 1.23–3.47) and 22.9 (95% CI 1.67–313.8) among children with at least one significant comorbidity and obesity, respectively, compared with those with no underlying medical conditions. Additional conditions associated with increased risk of hospitalization included age <2 years (OR 6.6, 95% CI 4.0–11.0), nausea or vomiting (OR 4.5, 95% CI 2.2–9.2), abdominal pain (OR 4.07, 95% CI 2.1–10.1) and rash (OR 3.5, 95% CI 1.7–7.3).
Risk markers for severe clinical illness
The clinical factors associated with increased risk of severe disease included asthma/recurrent wheezing (P<0.001), presence of any significant comorbidity (P<0.001), prematurity (P=0.013), any gastrointestinal symptoms (P=0.009) and age <2 years (P=0.033). The only laboratory markers differentiating between severe cases and non-severe cases were higher erythrocyte sedimentation rate (median 62 vs 21 mm/h, respectively; P=0.007) and increased white blood cell count (median 10,500 vs 7640/mm3, respectively; P=0.036). The adjusted OR of severe disease was 3.0 (95% CI 1.09–8.6) among cases with at least one significant comorbidity compared with cases without any significant comorbidities. Additional laboratory markers associated with increased severity included leukopenia (<4000 cells/μL) (OR 7.3, 95% CI 1.4–37.5) and leukocytosis (OR 6.0, 95% CI 1.7–21.2).
Radiologic findings
A radiologic evaluation was performed in 36.2% of all children (n=349) (Table 1). Any pulmonary abnormality was present in 35.2% of discharged patients, in contrast with 60% of inpatients (P<0.001). The most common findings in both subgroups were perihilar peribroncovascular thickening, pulmonary opacities and atelectasis. A chest CT scan was performed in 25 children (2.6%): 60% (n=15) had a significant underlying condition, and 18 were hospitalized. Ground-glass opacity with, at most, moderate involvement (25–50%) of the lung parenchyma was the most common finding among those with an abnormal report (n=12, 48.0%), followed by consolidations (n=9, 36%). However, these abnormalities differed significantly between inpatients [n=11 (61.1%) and n=9 (50%), respectively] and outpatients [n=1 (14.3%) and n=0, respectively]. Radiologic signs of complicated pneumonia (i.e. pleural effusion or empyema, necrotizing pneumonia or intrapulmonary abscesses) were not described in any patients.
Administered therapy
Most non-hospitalized patients were discharged from the emergency room with a prescription for symptomatic treatment alone (Table 1 and Figure 5). Forty-nine outpatients (6.2%) were prescribed an oral β-lactam or macrolide antibiotic. Antibiotic justifications were acute otitis media (n=10), presumed urinary tract infection (n=3), ‘abnormalities’ seen on chest X-ray (n=2), and treatment of group A streptococcal pharyngotonsillitis (n=1). Only 7.7% of all children presenting with flu-like syndrome or pneumonia (n=20/258) and 32.5% of children with severe respiratory illness (n=13/40) were prescribed oseltamivir. Overall, 8.1% of patients (n=78/964) were prescribed a systemic glucocorticoid: 3% of outpatients (n=24/793) and 31.6% of hospitalized patients (n=54/171), of whom 29 had severe clinical presentation. No patients received specific SARS-CoV-2 antiviral therapy (e.g. remdesivir) or chloroquine/hydroxychloroquine.
Figure 5.
Distribution of clinical syndromes and pharmacologic therapies according to disease severity among 964 children with coronavirus disease 2019 at Hospital Infantil Sabará. Outpatients were considered mild.
MIS-C
Twenty-seven hospitalized children fulfilled the diagnostic criteria for MIS-C. The median duration of fever was 7 (range 3–15) days, and the most common clinical findings are described in Table 1. RT-PCR-confirmed COVID-19 and serologic evidence of SARS-CoV-2 infection was seen in 29.6% (n=8) and 59.3% (n=16) of patients tested, respectively. Only three cases (11.1%) showed a positive epidemiologic link and both tests were negative. Three of the four severe/critical cases were characterized by intense clinical courses including mechanical ventilation in a prone position, vasoactive support, dialysis (n=1) and therapy with tocilizumab (a monoclonal antibody against the interleukin-6 receptor). Six (22.2%) children were treated with high-dose intravenous immunoglobulin (IVIG) alone, four (14.8%) children were treated with systemic glucocorticoids alone, 10 (37%) children were treated with both medications in combination, and seven (26%) children did not receive any treatment.
All MIS-C patients had echocardiographic evaluations. Abnormal findings were seen in two-thirds of cases (n=18), while two or more abnormalities occurred in 22.2% (n=6) of cases. As expected, the presence of any abnormal finding was significantly more common in children with MIS-C than in children with acute COVID-19 (n=29; P=0.001): pericardial effusion (PE) was the most common finding [37.0% (n=10) vs 6.9% (n=2)], followed by coronary artery dilation [22.2% (n=6) vs 3.4% (n=1)], mitral valve regurgitation [14.8% (n=4) vs 3.4% (n=1)] and left ventricular dilation [7.4% (n=2) vs n=0]. Coronary artery aneurysms were not detected.
Discussion
Globally, the paediatric population represents approximately 15% of all cases of COVID-19 [20], and <1% of documented critical and fatal cases [11,21]. However, low-and-middle-income countries, including several Latin American countries, experience higher numbers of paediatric deaths than high-income nations [22], [23], [24]. Brazil, for example, has one of the highest paediatric COVID-19 death rates worldwide, accounting for almost one in every four paediatric COVID-19 deaths worldwide before the Omicron variant [25]. Despite these concerning numbers, limited comprehensive data on SARS-CoV-2 infection in Brazilian children have been published since the start of the COVID-19 pandemic.
To the best of the authors’ knowledge, this is the largest study to date to evaluate the clinical, laboratory and radiographic characteristics of paediatric COVID-19 in a Brazilian population. As the disease epicentre in Brazil and the largest paediatric hospital in the country's south-east region, the high prevalence of SARS-CoV-2 infection in metropolitan São Paulo offered a unique opportunity to describe the varied paediatric forms of the disease.
This study had two remarkable findings. First, before the predominance of the Delta and Omicron variants, paediatric COVID-19 resulted in hospitalization rates ranging from 0.1% to 1.5% in the USA and some European Union countries [20,26]; however, the rates in Brazil were more than 10–15-fold higher. Even higher rates were reported by a multi-centre paediatric research network, comprising five Latin American countries, where an overwhelming 47% of paediatric COVID-19 cases were admitted to hospital [23]. The present data support the current evidence of more severe disease in Latin/Hispanic children in one of the largest and most populated areas in the world. Similarly, PICU admission occurred in <13% of hospitalized paediatric patients in Europe and North America [26], [27], [28], [29], compared with the present finding of 27.5% in Brazil. However, the authors believe the clinical severity is a more correct measurement of the need for intensive care, as almost one-fifth of the PICU admissions at HIS were required due to IVIG administration.
This study strengthens the concept that the risk of hospitalization and severe COVID-19 outcome is substantially elevated for children with underlying risk factors [27,30]. Asthma, neurodevelopmental disorders, congenital cardiac anomalies, prematurity (particularly in young children), leukopenia and lymphocytopenia are among the strongest risk factors for hospitalization and severe disease [31]. This is the first study from Latin America to report paediatric obesity as a risk factor for COVID-19 hospitalization. Persistent immune dysregulation (including dysfunctional innate T cells), chronic inflammation and endothelial dysfunction are among the postulated underlying mechanisms [32], [33], [34].
This study found that thrombocytosis is an additional risk factor infrequently described in children. While thrombocytopenia is a well-recognized negative prognostic factor in COVID-19 and MIS-C, a platelet count ≥450 × 109/L has been reported in <5% of cases [35,36] and is usually associated with reduced overall in-hospital mortality and need for mechanical ventilation [36]. Additionally, atypical lymphocytes in the peripheral blood (regarded as immunologically activated T cells) were associated with pneumonia and need for supplemental oxygen [37]. However, despite being documented in almost 60% of children requiring hospitalization, this did not differ between hospitalized and non-hospitalized patients, or between clinical syndromes in the present study.
In contrast with the high hospitalization and PICU rates, the absence of fatal outcomes was the second and probably most intriguing finding. No deaths directly or indirectly related to SARS-CoV-2 infection had been documented at HIS by November 2022, even during the emergence of the Omicron BA.1/BA.2 and BA.5 variants. While this information could be seen as reassurance of the low severity of COVID-19 among children, the numbers diverge from the expected case fatality rates from national and international data [7,25]. Additionally, common cardiovascular complications such as shock and cardiac collapse, considered cardinal acute cardiovascular manifestations in classic episodes of MIS-C [38], were rarely found in the study patients. Such numbers contrast with the Brazilian Ministry of Health data, where the overall case fatality rate of 7% is among the highest in the world [3]; this could be related to discrepancies in quality and timely medical care in most Brazilian cities. It is unclear why the severity of MIS-C differs between countries and populations, but viral characteristics and low specificity of case definitions may be involved. The present data suggest that the diagnostic criteria in use may not be specific enough to predict severe or lethal cases [39,40].
Despite early promotion of ineffective drugs against COVID-19 (including hydroxychloroquine, azithromycin and ivermectin) by the Brazilian Government, none of the patients at HIS received these medications for COVID-19. However, despite the exceptionally low frequency of antibacterial prescriptions for outpatients, disproportionate use of macrolide derivatives, such as azithromycin, was found among those who received antibiotics. Not shown to improve survival in patients with COVID-19 [41,42], recurrent macrolide use affects the gut resistome (the body's largest reservoir of antimicrobial resistance genes), and has the potential to propagate antibiotic resistance in young children [43].
The findings of this study are subject to several limitations. HIS is a private hospital that is not associated with Brazil's free and universal Unified Health System, limiting access to those with supplementary private health insurance. In Brazil, where >70% of the population do not have private medical insurance [44], poverty, inequality and social determinants of health create favourable conditions for the transmission of infectious diseases, including COVID-19 [45,46]. Therefore, as a population with disproportionate access to high-quality medical care, this study may have had selection bias [47]. Second, a small number of Black participants were included in this analysis, which is consistent with the racial profile of the population who attended HIS. Ethnicity was not found to be a predictor for hospitalization or death among the study population; this is in contrast to previous studies, which found that race, particularly Black, Indigenous and Hispanic, was an independent risk factor for negative outcomes in paediatric COVID-19 and MIS-C [31,46,[48], [49], [50]]. Third, data collection through in-depth abstraction of routine clinical documentation is subject to incomplete reporting. As most clinical data were recovered from electronic records, and attending clinicians did not use standardized forms, some inputs were excluded from the dataset. This drawback was minimized by reviewing all available medical records carefully. Fourth, as this study evaluated COVID-19 in a broad but well-defined region in São Paulo, the findings may underestimate the extent of the disease in other regions. Marked climatic diversity and latitudinal differences in the pattern of viral circulation may differ substantially in a country such as Brazil. Moreover, testing strategies may differ regionally, resulting in geographic differences in disease hospitalization rates and outcomes. Fifth, it was not possible to evaluate vaccine effectiveness against severe disease or hospitalization, as immunization of children aged 5–11 years only became available in Brazil in late January 2022. Finally, these findings describe the epidemiology and characteristics of COVID-19 during the circulation of two primary SARS-CoV-2 variants. Continuous hospital-based surveillance of new variants should bring new information on COVID-19 in children, including the circulation of highly transmissible strains (such as Omicron) and the effect of paediatric vaccination.
With absolute and proportionate COVID-19 cases in children increasing since late December 2021, paediatric disease is cause for increased concern given the vulnerabilities of this population group. Multi-centre studies to detect regional disparities in the disease course and replicate the present findings in different settings are needed urgently, especially following the Omicron wave of infections.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Acknowledgments
The authors wish to thank Erika Tiemi Fukunaga for providing statistical advice.
Disclaimer
The findings and conclusions are those of the authors, and do not necessarily represent the views of the institutions cited in the article.
Funding
None.
Author contributions
DJ, FJA and MAPS contributed to the conception and design of the study. All authors contributed to data collection. DJ organized the database. DJ wrote the first draft and the definitive version of the manuscript. MAPS and FJA wrote sections of the manuscript. All authors contributed to manuscript revision, and read and approved the submitted version.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijregi.2022.12.003.
Appendix. Supplementary materials
References
- 1.Rodriguez-Morales AJ, Gallego V, Escalera-Antezana JP, Méndez CA, Zambrano LI, Franco-Paredes C, et al. COVID-19 in Latin America: the implications of the first confirmed case in Brazil. Travel Med Infect Dis. 2020;35 doi: 10.1016/j.tmaid.2020.101613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. COVID-19 situation reports. Geneva: WHO; n.d. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (Accessed 27 April 2020).
- 3.Ministério da Saúde . Vol. 24. Ministério da Saúde do Brasil; Brasilia: 2021. (Secretaria de Vigilância em Saúde. Boletim Epidemiológico Especial COVID19 - Semana Epidemiológica). 07 a 13/06/2021. [Google Scholar]
- 4.World Health Organization. Tracking SARS-CoV-2 variants. Geneva: WHO; n.d. Available at: https://www.who.int/emergencies/emergency-health-kits/trauma-emergency-surgery-kit-who-tesk-2019/tracking-SARS-CoV-2-variants (Accessed 16 August 2021).
- 5.Mathieu E, Ritchie H, Rodés-Guirao L, Appel C, Giattino C, Hasell J, et al. Brazil: coronavirus pandemic country profile. Our World in Data; 2020. Available at: https://ourworldindata.org/coronavirus/country/brazil (accessed 21 December 2022).
- 6.Ministério da Saúde . Vol. 23. Ministério da Saúde do Brasil; Brasilia: 2022. (Secretaria de Vigilância em Saúde. Boletim Epidemiológico Especial COVID19 - Semana Epidemiológica). 05 a 11/06/2022. [Google Scholar]
- 7.Ministério da Saúde. Boletins Epidemiológicos COVID-19, Ministério da Saúde do Brasil, Brasilia. 2022. Available at: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/boletins-epidemiologicos/covid-19/boletins-epidemiologicos-covid-19 (accessed 18 April 2022).
- 8.Martins-Filho PR, Barberia LG. The unjustified and politicized battle against vaccination of children and adolescents in Brazil. Lancet Reg Health Am. 2022;8 doi: 10.1016/j.lana.2022.100206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tagarro A, Epalza C, Santos M, Sanz-Santaeufemia FJ, Otheo E, Moraleda C, et al. Screening and severity of coronavirus disease 2019 (COVID-19) in children in Madrid, Spain. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145 doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 11.Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. SARS-CoV-2 infection in children. N Engl J Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmermann P, Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J. 2020;39:355–368. doi: 10.1097/INF.0000000000002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 14.Patel AB, Verma A. Nasal ACE2 levels and COVID-19 in children. JAMA. 2020;323:2386–2387. doi: 10.1001/jama.2020.8946. [DOI] [PubMed] [Google Scholar]
- 15.Fundação José Luiz Egydio Setúbal . Fundação José Luiz Egydio Setúbal; São Paulo: 2019. Relatório de Atividades 2018 – Hospital Infantil Sabará.https://fundacaojles.org.br/relatorio-de-atividades-2018/sabara-hospital-infantil/ Available at. accessed 19 June 2020. [Google Scholar]
- 16.Beavis KG, Matushek SM, Abeleda APF, Bethel C, Hunt C, Gillen S, et al. Evaluation of the EUROIMMUN Anti-SARS-CoV-2 ELISA assay for detection of IgA and IgG antibodies. J Clin Virol. 2020;129 doi: 10.1016/j.jcv.2020.104468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Institutes of Health . NIH; Bethesda, MD: 2020. Clinical spectrum of SARS-CoV-2 infection. [Google Scholar]
- 19.World Health Organization . WHO; Geneva: 2020. Multisystem inflammatory syndrome in children and adolescents with COVID-19. [Google Scholar]
- 20.American Academy of Pediatrics . AAP; Itasca, IL: 2022. Children and COVID-19: state-level data report.http://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/ Available at. accessed 3 March 2022. [Google Scholar]
- 21.Zachariah P, Johnson CL, Halabi KC, Ahn D, Sen AI, Fischer A, et al. Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a children's hospital in New York City, New York. JAMA Pediatr. 2020;174 doi: 10.1001/jamapediatrics.2020.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitano T, Kitano M, Krueger C, Jamal H, Al Rawahi H, Lee-Krueger R, et al. The differential impact of pediatric COVID-19 between high-income countries and low- and middle-income countries: a systematic review of fatality and ICU admission in children worldwide. PLoS One. 2021;16 doi: 10.1371/journal.pone.0246326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antúnez-Montes OY, Escamilla MI, Figueroa-Uribe AF, Arteaga-Menchaca E, Lavariega-Saráchaga M, Salcedo-Lozada P, et al. COVID-19 and multisystem inflammatory syndrome in Latin American children: a multinational study. Pediatr Infect Dis J. 2021;40:e1–e6. doi: 10.1097/INF.0000000000002949. [DOI] [PubMed] [Google Scholar]
- 24.Madani S, Shahin S, Yoosefi M, Ahmadi N, Ghasemi E, Koolaji S, et al. Red flags of poor prognosis in pediatric cases of COVID-19: the first 6610 hospitalized children in Iran. BMC Pediatr. 2021;21:563. doi: 10.1186/s12887-021-03030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.United Nations Children's Fund . UNICEF; New York: 2022. Child mortality and COVID-19.https://data.unicef.org/topic/child-survival/covid-19/ Available at. accessed 20 April 2022. [Google Scholar]
- 26.Bundle N, Dave N, Pharris A, Spiteri G, Deogan C, Suk JE, et al. COVID-19 trends and severity among symptomatic children aged 0–17 years in 10 European Union countries, 3 August 2020 to 3 October 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.50.2101098. pii=2101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward JL, Harwood R, Smith C, Kenny S, Clark M, Davis PJ, et al. Risk factors for PICU admission and death among children and young people hospitalized with COVID-19 and PIMS-TS in England during the first pandemic year. Nat Med. 2022;28:193–200. doi: 10.1038/s41591-021-01627-9. [DOI] [PubMed] [Google Scholar]
- 28.Shekerdemian LS, Mahmood NR, Wolfe KK, Riggs BJ, Ross CE, McKiernan CA, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020;174:868–873. doi: 10.1001/jamapediatrics.2020.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uka A, Buettcher M, Bernhard-Stirnemann S, Fougère Y, Moussaoui D, Kottanattu L, et al. Factors associated with hospital and intensive care admission in paediatric SARS-CoV-2 infection: a prospective nationwide observational cohort study. Eur J Pediatr. 2022;181:1245–1255. doi: 10.1007/s00431-021-04276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Q, Wang Z, Liu J, Wang X, Zhou Q, Li Q, et al. Risk factors for poor prognosis in children and adolescents with COVID-19: a systematic review and meta-analysis. EClinMed. 2021;41 doi: 10.1016/j.eclinm.2021.101155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodruff RC, Campbell AP, Taylor CA, Chai SJ, Kawasaki B, Meek J, et al. Risk factors for severe COVID-19 in children. Pediatrics. 2022;149 doi: 10.1542/peds.2021-053418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korakas E, Ikonomidis I, Kousathana F, Balampanis K, Kountouri A, Raptis A, et al. Obesity and COVID-19: immune and metabolic derangement as a possible link to adverse clinical outcomes. Am J Physiol Endocrinol Metab. 2020;319:E105–E109. doi: 10.1152/ajpendo.00198.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frydrych LM, Bian G, O'Lone DE, Ward PA, Delano MJ. Obesity and type 2 diabetes mellitus drive immune dysfunction, infection development, and sepsis mortality. J Leukoc Biol. 2018;104:525–534. doi: 10.1002/JLB.5VMR0118-021RR. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Woods K, Parry-Strong A, Anderson RJ, Capistrano C, Gestin A, et al. Distinct dysfunctional states of circulating innate-like T cells in metabolic disease. Front Immunol. 2020;11:448. doi: 10.3389/fimmu.2020.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wool GD, Miller JL. The impact of COVID-19 disease on platelets and coagulation. Pathobiol J Immunopathol Mol Cell Biol. 2021;88:15–27. doi: 10.1159/000512007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lucijanic M, Krecak I, Soric E, Sedinic M, Sabljic A, Derek L, et al. Thrombocytosis in COVID-19 patients without myeloproliferative neoplasms is associated with better prognosis but higher rate of venous thromboembolism. Blood Cancer J. 2021;11:189. doi: 10.1038/s41408-021-00585-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugihara J, Shibata S, Doi M, Shimmura T, Inoue S, Matsumoto O, et al. Atypical lymphocytes in the peripheral blood of COVID-19 patients: a prognostic factor for the clinical course of COVID-19. PLoS One. 2021;16 doi: 10.1371/journal.pone.0259910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valverde I, Singh Y, Sanchez-de-Toledo J, Theocharis P, Chikermane A, Di Filippo S, et al. Acute cardiovascular manifestations in 286 children with multisystem inflammatory syndrome associated with COVID-19 infection in Europe. Circulation. 2021;143:21–32. doi: 10.1161/CIRCULATIONAHA.120.050065. [DOI] [PubMed] [Google Scholar]
- 39.Godfred-Cato S. COVID-19-associated multisystem inflammatory syndrome in children — United States, March–July 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rhys-Evans S. Call for a universal PIMS-TS/MIS-C case definition. Arch Dis Child. 2022;107:e10. doi: 10.1136/archdischild-2021-322829. [DOI] [PubMed] [Google Scholar]
- 41.RECOVERY Collaborative Group Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:605–612. doi: 10.1016/S0140-6736(21)00149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.PRINCIPLE Trial Collaborative Group Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021;397:1063–1074. doi: 10.1016/S0140-6736(21)00461-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doan T, Worden L, Hinterwirth A, Arzika AM, Maliki R, Abdou A, et al. Macrolide and nonmacrolide resistance with mass azithromycin distribution. N Engl J Med. 2020;383:1941–1950. doi: 10.1056/NEJMoa2002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Instituto Brasileiro de Geografia e Estatística . Instituto Brasileiro de Geografia e Estatística (IBGE); Rio de Janeiro: 2019. Pesquisa Nacional de Saúde 2019.https://www.ibge.gov.br/estatisticas/sociais/saude/9160-pesquisa-nacional-de-saude.html?=&t=microdados Available at. accessed 22 October 2021. [Google Scholar]
- 45.Quinn SC, Kumar S. Health inequalities and infectious disease epidemics: a challenge for global health security. Biosecurity Bioterrorism Biodefense Strategy Pract Sci. 2014;12:263–273. doi: 10.1089/bsp.2014.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oliveira EA, Colosimo EA, Simões E, Silva AC, Mak RH, Martelli DB, Silva LR, et al. Clinical characteristics and risk factors for death among hospitalised children and adolescents with COVID-19 in Brazil: an analysis of a nationwide database. Lancet Child Adolesc Health. 2021;5:559–568. doi: 10.1016/S2352-4642(21)00134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore JT. Disparities in incidence of COVID-19 among underrepresented racial/ethnic groups in counties identified as hotspots during June 5–18, 2020 — 22 States, February–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1122–1126. doi: 10.15585/mmwr.mm6933e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreira A, Chorath K, Rajasekaran K, Burmeister F, Ahmed M, Moreira A. Demographic predictors of hospitalization and mortality in US children with COVID-19. Eur J Pediatr. 2021;180:1659–1663. doi: 10.1007/s00431-021-03955-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee EH, Kepler KL, Geevarughese A, Paneth-Pollak R, Dorsinville MS, Ngai S, et al. Race/ethnicity among children with COVID-19-associated multisystem inflammatory syndrome. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.30280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alsaied T, Tremoulet AH, Burns JC, Saidi A, Dionne A, Lang SM, et al. Review of cardiac involvement in multisystem inflammatory syndrome in children. Circulation. 2021;143:78–88. doi: 10.1161/CIRCULATIONAHA.120.049836. [DOI] [PubMed] [Google Scholar]
- 51.Empresa Paulista de Planejamento Metropolitano S/A (EMPLASA) Governo do Estado de São Paulo . Empresa Paulista de Planejamento Metropolitano S/A (EMPLASA); São Paulo: 2019. Região Metropolitana de São Paulo.https://www.emplasa.sp.gov.br/RMSP Available at. accessed 19 June 2020. [Google Scholar]
- 52.Sistema de Informação de Agravos de Notificação (SINAN) Ministério da Saúde do Brasil; Brasilia: 2021. Ministério da Saúde do Brasil. SINANWEB - Calendários Epidemiológicos.http://portalsinan.http://portalsinan.saude.gov.br/calendario-epidemiologico/171-calendario-epidemiologico-2021 Available at. accessed 19 June 2022. [Google Scholar]
- 53.e-SUS VE/DVE/COVISA/SMS-SP. TabNet Win32 2.7 - COVID19 e-SUS-VE Síndrome Gripal, Ministério da Saúde do Brasil, Brasilia. n.d. Available at: http://tabnet.saude.prefeitura.sp.gov.br/cgi/tabcgi.exe?secretarias/saude/TABNET/RCOVID19/covid19.def (accessed 19 June 2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.