Abstract
Background:
Children born extremely preterm (EP) are at increased risk of cognitive deficits that persist into adulthood. Few large cohort studies have examined differential impairment of cognitive function in EP-born adolescents in relation to early life risk factors, including maternal social disadvantage, gestational age at delivery, and neonatal morbidities prevalent among EP neonates.
Objectives:
To assess cognitive abilities in relation to early life risk factors in an EP-born cohort at 15 years of age.
Methods:
681 of 1198 surviving participants (57%) enrolled from 2002 to 2004 in the Extremely Low Gestational Age Newborn Study returned at age 15 years for an assessment of cognitive abilities with the Wechsler Abbreviated Scale of Intelligence-II and the NIH Toolbox Cognition Battery (NTCB) verbal cognition and fluid processing composites, the latter of which measured executive functions and processing speed. Three cognitive outcomes, WASI-II IQ, NTCB verbal cognition, and NTCB fluid processing, were analyzed for associations with maternal social disadvantage and gestational age. Mediation of maternal social disadvantage by gestational age and mediation of gestational age by neonatal morbidities were also examined.
Results:
Test scores were lower for NTCB fluid processing relative to IQ and NTCB verbal abilities. Social disadvantage and gestational age were associated with all three cognitive outcomes. Mediation analyses indicated partial mediation of gestational age associations with all three outcomes by neonatal morbidities but did not support mediation by gestational age of social risk associations with cognitive outcomes.
Conclusions:
Greater maternal social disadvantage and lower gestational age are associated with less favorable cognitive outcomes among EP-born adolescents at 15 years of age. Neonatal morbidities partially mediate associations between lower gestational age and cognitive outcomes. These findings highlight the need for improved medical and remedial interventions to mitigate risk of poor cognitive outcomes among EP-born adolescents.
Keywords: extremely preterm birth, adolescent, cognition, neuropsychological tests, risk factors, cohort studies
1. BACKGROUND
Children born extremely preterm (EP; <28 weeks’ gestation) are at increased risk of adverse cognitive outcomes1,2 that persist into adulthood,3–5 including impairment of general intelligence (IQ),6–8 language,9,10 attention,11,12 executive function,13–16 and processing speed.16–19 Among individuals born EP, cognitive impairment is the most prevalent developmental disability.20,21 To prevent cognitive impairments related to extreme prematurity, a better understanding is needed of early life factors that contribute to risk of cognitive impairment among EP-born individuals.
Early life factors associated with cognitive impairment in EP-born individuals include social disadvantage,22,23 lower gestational age,6,7 and medical disorders associated with lower gestational age, most prominently, EP-related brain injury24,25 and neonatal morbidities (bronchopulmonary dysplasia,26 necrotizing enterocolitis,27 retinopathy of prematurity,28 and bacteremia29). Existing studies of the relationships between early life factors and cognitive impairment among individuals born preterm have included relatively few individuals born EP7 and seldom were based on cohorts born in the current millennium. Further, only two large studies of individuals born EP5,22 provide information about cognitive function during adolescence, when large changes in brain structure occur. In addition, most studies have focused on general intelligence, and few have analyzed associations between early life risk factors and more specific verbal learning and fluid information processing outcomes.
The objectives of this study were to evaluate, in a large multi-center cohort of adolescents born EP, the relationships between two early life factors, social disadvantage and gestational age at delivery, and 3 cognitive outcomes: verbal ability, fluid information processing, and full-scale IQ. The rationale for focusing on socioeconomic risk and gestational age is that these two factors were strongly associated with cognitive outcomes in the ELGAN cohort at 10 years of age21 but were not highly correlated and thus might convey distinct information about risk. The rationale for conducting separate analyses of verbal ability, fluid information processing, and full-scale IQ is to provide more specificity about what cognitive abilities are most vulnerable to adverse early life factors in adolescent EP-born children.
2. METHODS
2.1. Participants
Participants were recruited from the ELGAN Study cohort, a prospective longitudinal study in which 1506 neonates born extremely preterm, selected based on gestational age (23–27 completed weeks of gestation), were enrolled at birth from 14 hospitals in five states in the U.S. between 2002–2004. At age 15 years, of 1198 surviving ELGAN participants, 700 adolescents were enrolled, of whom 681 (57%) accompanied their parent/guardian for a cognitive assessment.
2.2. Maternal and newborn characteristics
Maternal age, race and ethnicity, education, health insurance status, supplemental nutrition assistance, and marital/partnered status were self-reported at the time of the child’s birth. From the latter 4 variables, we derived a cumulative composite index of maternal social disadvantage, which increased by one point for each of the following: maternal education less than high school, lack of private health insurance, receipt of government-provided supplemental nutritional assistance, and single marital/unpartnered status.30
Newborn characteristics (sex, gestational age, birthweight) and neonatal medical illnesses (bronchopulmonary dysplasia requiring ventilation, necrotizing enterocolitis requiring surgery, retinopathy of prematurity, definite bacteremia) were identified by review of medical records.31 Structural evidence of neonatal brain injuries (echolucent lesions of cerebral white matter, ventriculomegaly) were identified by cranial ultrasound.32 White matter damage was defined as the presence of either echolucent (hypoechoic) lesions in white matter or ventricular enlargement persisting after the second postnatal week. We derived a morbidity index by summing the number of these 5 morbidities.
2.3. Cognitive assessments
Intelligence quotient (IQ).
IQ was assessed with Wechsler Abbreviated Scale of Intelligence - II (WASI-II)33 two-subtest form consisting of Vocabulary and Matrix Reasoning subtests. The WASI-II yields an estimate of full-scale IQ.
Neuropsychological abilities.
The iPad version of the NIH Toolbox Cognition Battery (NTCB)34 was administered to assess more specific cognitive abilities. The NTCB consists of 2 measures of verbal or “crystallized cognition,” Picture Vocabulary (receptive vocabulary) and Oral Reading Recognition (oral reading), and 5 measures of “fluid” cognition, Flanker Inhibitory Control and Attention (sustained attention/inhibition), Dimensional Change Card Sort (set switching/cognitive flexibility), Pattern Comparison Processing Speed (processing speed), List Sorting Working Memory (verbal working memory), and Picture Sequence Memory (episodic memory). A recent study35 yielded different factor-based composites from the original NTCB composites for and adolescents, such that the Picture Vocabulary, Oral Reading Recognition, and List Sorting Working Memory tests loaded onto one factor (“verbal cognition”), and the Flanker Inhibitory Control and Attention, Dimensional Change Card Sort, and Pattern Comparison Processing Speed tests loaded onto a second factor (“fluid cognition”). Principal components factor analysis with orthogonal rotation of the ELGAN NTCB data set yielded the same solution, with Cronbach’s alphas of .72 and .71 for the 3-subtest verbal/crystalized and fluid composites, respectively (Supporting Information Table 1). We used these two factor-based composites as the main NTCB outcomes in data analyses, which we refer to as NTCB “verbal” versus “fluid information processing” abilities or “verbal” versus “fluid” factor composite scores below. We note that the NTCB “crystallized” and “fluid” cognition composites and the “verbal” and “fluid” factor scores that we extracted from the 7 NTCB subtests are not in conformity with the contemporary Cattell–Horn–Carroll (CHC) theory of crystallized and fluid cognitive abilities. From the perspective of the CHC theory of cognition, the NTCB verbal and fluid information processing factors (executive control, processing speed) that served as cognitive outcomes in this study are considered to represent broader and distinct cognitive abilities.36,37
2.4. Statistical analysis
We examined distributions of cognitive test scores for the ELGAN age 15 sample relative to corresponding test norms by converting participants’ test scores to z-scores using the normative means and SDs for each cognitive subtest. To assess differential cognitive impairment, we calculated the mean difference between the WASI-II Vocabulary and Matrix Reasoning scores, and between the NTCB verbal and fluid factor composite scores. SAS V9.4 survey procedures were used to account for correlation between multiple birth sibships and missing data from the original cohort through inverse probability weighting.
To assess associations of early risk factors with cognitive outcomes, we conducted causal mediation analyses.38 Causal mediation is based on counterfactual theory and accounts for exposure-mediator interactions. We separately assessed associations of social disadvantage and gestational age with the three main cognitive outcomes. Potential confounders were identified using directed acyclic graphs (Supporting Information Figures 1 and 2). First, for social disadvantage, we examined the total effects of the number of social disadvantages separately for each of the three cognitive outcomes, controlling for maternal age. We then assessed a possible mediating effect of weeks of gestational age on these associations by estimating natural direct, indirect, and interaction effects, controlling for maternal age. Second, we assessed the association of gestational age with each cognitive outcome, controlling for social disadvantage and maternal age. We then examined mediation of associations between weeks of gestational age and cognitive outcomes by the morbidity composite index. All exposure, outcome, and mediator variables included in the causal mediation analyses are continuous variables, and effects are interpreted as slopes from regression models, reflecting the expected change in the outcome measure for a unit change in the exposure. Causal mediation analyses were performed using the SAS V9.4 causalmed procedure, accounting for missing data from the original cohort through inverse probability weighting and using bootstrap-based confidence intervals based on 10,000 bootstrap samples. This procedure examines a single mediator of the exposure – outcome association and does not account for clustering within the sample. As a sensitivity analysis of the ignored clustering due to including sibships in the sample, we repeated the causal mediation analysis on a sample with only one randomly selected participant from each sibship (reducing the sample from n=667 to n=562). Estimates of natural effects from causal mediation are based on four assumptions about confounding, and we performed an E-value assessment for unmeasured confounding of the exposure – outcome association, the mediator – outcome association, and the exposure – mediator association.39
Missing data
Of the 681 participants who presented for a cognitive assessment, 667 completed the assessment for at least one of the three cognitive outcomes (n = 653 for IQ, n = 600 for the NTCB verbal composite, and n = 603 for the fluid composite). Of the 14 participants not included in the final sample, 7 had uncorrectable functional blindness, and 7 were too severely cognitively impaired to comply with the cognitive assessments. Those participants who did not complete the NTCB assessments had lower WASI-II IQ scores, with a mean IQ of 75.3 (SD = 33.0) and of 75.3 (SD = 33.5) for those without NTCB verbal and fluid factor composite scores, respectively.
Inverse probability weighting (IPW) was used to account for missing cognitive data from the surviving ELGAN sample (n=1198) relative to age 15 participants with at least one main cognitive outcome (n=667). The probability of being in the age 15 data set was modelled from gestational age, sex, birthweight-for-gestational age category, multiple gestation, neonatal morbidities, maternal social risk at birth, and birth hospital. Separate IPW weights were created for WASI-II IQ and NTCB verbal and fluid measures. IPW weights were accounted for by SAS survey procedures, which yielded weighted analyses adjusted for clustering through Taylor series linearization.
There were minimal missing data for study predictor variables and covariates. For the variables comprising the social disadvantage composite, 19 participants were missing maternal education data and 11 were missing data for health insurance status and supplemental nutrition status. For neonatal morbidities, 10 participants were missing data for retinopathy of prematurity and bacteremia, and 3 for bronchopulmonary dysplasia.
2.5. Ethics approval
Procedures for this study were approved by the institutional review boards of all participating institutions at the time of enrollment and for the age 15 year follow up.
3. RESULTS
3.1. Sample description
Of the 1198 participants who survived to 15 years, those not included in our study sample (n = 531) were more likely to have indicators of social disadvantage at birth but did not differ from age 15 study participants in neonatal/postnatal characteristics (Table 1).
Table 1.
1198 children eligible for recruitment classified by whether they received a cognitive assessment at age 15
| Assessed | ||
|---|---|---|
| Yes (n = 667) | No (n = 531) | |
| % (n) | % (n) | |
| Maternal characteristics | ||
| Age, years | ||
| < 21 | 12.0 (80) | 16.9 (90) |
| 21–35 | 66.3 (442) | 67.8 (360) |
| > 35 | 21.7 (145) | 15.2 (81) |
| Racial identity | ||
| White | 66.5 (442) | 52.1 (272) |
| Black | 23.6 (157) | 31.6 (165) |
| Other | 9.9 (66) | 16.3 (85) |
| Hispanic | ||
| Yes | 9.3 (62) | 16.1 (85) |
| No | 90.7 (603) | 83.9 (443) |
| Education, years | ||
| < 12 | 13.0 (84) | 21.4 (108) |
| ≤ 12 (high school) | 24.1 (156) | 31.3 (158) |
| > 12, < 16 | 23.0 (149) | 24.0 (121) |
| ≥ 16 (≥ college) | 40.0 (259) | 23.2 (117) |
| Private health insurance | ||
| Yes | 68.6 (450) | 51.5 (266) |
| No | 31.4 (206) | 48.5 (251) |
| Supplemental nutritional assistance | ||
| Yes | 11.0 (72) | 18.2 (94) |
| No | 89.0 (584) | 81.8 (421) |
| Married/partnered | ||
| Yes | 81.3 (542) | 73.1 (388) |
| No | 18.7 (125) | 26.9 (143) |
| Social disadvantage composite1 | ||
| 0 | 61.3 (409) | 41.1 (218) |
| 1 | 15.0 (100) | 23.0 (122) |
| 2 | 14.4 (96) | 21.3 (113) |
| 3–4 | 9.3 (62) | 14.7 (78) |
| Newborn birth characteristics | ||
| Sex | ||
| Male | 51.3 (342) | 52.5 (279) |
| Female | 48.7 (325) | 47.5 (252) |
| Gestational age, full weeks | ||
| 23 | 5.1 (34) | 4.5 (24) |
| 24 | 15.9 (106) | 15.2 (81) |
| 25 | 21.1 (141) | 20.2 (107) |
| 26 | 24.7 (165) | 26.4 (140) |
| 27 | 33.1 (221) | 33.7 (179) |
| Birthweight, grams | ||
| ≤ 750 | 36.6 (244) | 36.2 (192) |
| 751–1000 | 43.6 (291) | 43.1 (229) |
| > 1000 | 19.8 (132) | 20.7 (110) |
| Birthweight for gestational age z-score | ||
| < −2 | 6.3 (42) | 3.8 (20) |
| ≥ −2, < −1 | 12.3 (82) | 13.4 (71) |
| ≥ −1 | 81.4 (543) | 82.9 (440) |
| Multiple birth | ||
| Yes | 36.7 (245) | 27.9 (148) |
| No | 63.3 (422) | 72.1 (383) |
| Neonatal morbidities | ||
| Intraventricular hemorrhage | ||
| Yes | 22.5 (150) | 17.8 (94) |
| No | 77.5 (517) | 82.2 (435) |
| White matter damage | ||
| Yes | 13.8 (92) | 13.0 (69) |
| No | 86.2 (575) | 87.0 (460) |
| Bronchopulmonary dysplasia | ||
| None | 46.5 (309) | 53.6 (281) |
| Oxygen only | 44.1 (293) | 39.1 (205) |
| Ventilation | 9.3 (62) | 7.3 (38) |
| Necrotizing enterocolitis | ||
| None | 92.2 (615) | 92.8 (493) |
| Medical | 0.7 (5) | 1.1 (6) |
| Surgical | 4.2 (28) | 3.0 (16) |
| Perforation | 2.9 (19) | 3.0 (16) |
| Retinopathy of prematurity | ||
| Yes | 13.5 (89) | 12.9 (67) |
| No | 86.5 (568) | 87.1 (453) |
| Bacteremia | ||
| None | 36.7 (241) | 37.6 (204) |
| Suspected | 34.5 (227) | 34.1 (185) |
| Definite | 28.8 (189) | 28.2 (153) |
| Number of neonatal morbidities2 | ||
| 0 | 50.8 (333) | 52.6 (271) |
| 1 | 35.0 (229) | 34.2 (176) |
| 2 | 11.2 (73) | 9.5 (49) |
| 3 | 3.0 (20) | 3.7 (19) |
Maternal education less than high school, lack of private health insurance, receipt of government-provided supplemental nutritional assistance, and single marital/unpartnered status
White matter damage, bronchopulmonary dysplasia requiring ventilation, necrotizing enterocolitis requiring surgery, retinopathy of prematurity, definite bacteremia
3.2. Distributions of cognitive test scores
The distribution of WASI-II IQ and NTCB fluid factor composite scores were lower than normative expectation; this was not true for NTCB verbal factor composite scores, which corresponded to normative expectation (Table 2, Figure 1). On the WASI-II, the proportion of the sample scoring one or more SDs below test norms (expected normative rate of 15.9%) was 26.4%, 23.5% and 27.3% on full-scale IQ, and the Vocabulary and Matrix Reasoning subtests, respectively. On the NTCB verbal factor composite, 15.2% of the sample scored one or more SDs below the test mean, consistent with normative expectation (15.9%). In contrast, on the NTCB fluid factor composite, 52.1% of the sample scored one or more SDs below normative expectation. However, a substantial proportion of the sample scored at the normative mean or above (IPW-weighted z-score of 0 or above corresponding to a normative expectation of 50.0%) on WASI-II IQ (48.4%) and the NTCB verbal factor composite (53.3%). Fewer participants scored in this range on the NTCB fluid factor composite (16.6%).
Table 2.
Means and distributions of z-transformed neurocognitive test scores1
| Distribution of z-scores (relative to normative expectation) |
||||||
|---|---|---|---|---|---|---|
| ≤ −2 (2.3%) |
> −2, ≤ −1 (13.6%) |
> −1, ≤ 1 (68.3%) |
> 1 (15.9%) |
|||
| M (SD) | % (n) | % (n) | % (n) | % (n) | Row n | |
| WASI-II IQ | ||||||
| Full-scale IQ | 95.9 (23.4) | 14.5 (91) | 11.9 (74) | 55.4 (358) | 18.2 (130) | 653 |
| Vocabulary | 49.5 (14.0) | 10.1 (64) | 13.4 (79) | 57.2 (368) | 19.3 (142) | 653 |
| Matrix Reasoning | 46.5 (12.6) | 12.9 (86) | 14.4 (94) | 60.3 (388) | 12.4 (85) | 653 |
| NIH Toolbox Cognition Battery | ||||||
| Verbal factor composite3 | 100.5 (14.8) | 1.6 (9) | 13.8 (82) | 69.6 (414) | 15.0 (95) | 600 |
| Picture Vocabulary | 99.7 (18.3) | 4.8 (27) | 16.3 (91) | 64.0 (385) | 14.9 (97) | 600 |
| Oral Reading Recognition | 106.4 (21.1) | 3.3 (21) | 12.9 (73 | 50.6 (311) | 33.1 (195) | 600 |
| List Sorting Working Memory | 95.5 (16.7) | 4.0 (24) | 23.3 (139) | 60.9 (362) | 11.8 (75) | 600 |
| Fluid factor composite4 | 85.5 (14.4) | 13.4 (80) | 38.8 (228) | 45.3 (281) | 2.5 (14) | 603 |
| Flanker Inhibitory Control and Attention | 78.6 (12.2) | 20.3 (121) | 58.3 (351) | 20.6 (126) | 0.9 (5) | 603 |
| Dimensional Change Card Sort | 89.8 (19.9) | 15.2 (87) | 34.9 (208) | 39.0 (243) | 10.8 (65) | 603 |
| Pattern Comparison Processing Speed | 88.0 (23.7) | 18.5 (115) | 23.2 (131) | 47.0 (289) | 11.3 (68) | 603 |
| Picture Sequence Memory5 | 97.5 (16.4) | 1.4 (8) | 25.7 (155) | 61.5 (361) | 11.4 (74) | 603 |
Sample means and norm-based z-scores percentages are adjusted with inverse proportional weighting used to account for missing data from the surviving ELGAN sample. WASI-II full-scale IQ and all NIH Toolbox Cognition scores have a normative mean = 100, SD = 15, and WASI-II subtest scores have a mean = 50, SD = 10.
Factor-analyzed composite of NCTB Picture Recognition, Oral Reading Recognition, and List Sort Working Memory
Factor-analyzed composite of NTCB Flanker Inhibitory Control and Attention, Dimensional Change Card Sort, and Pattern Comparison Processing Speed
Picture Sequence Memory did not load onto either factor in NTCB factor analyses
Figure 1.
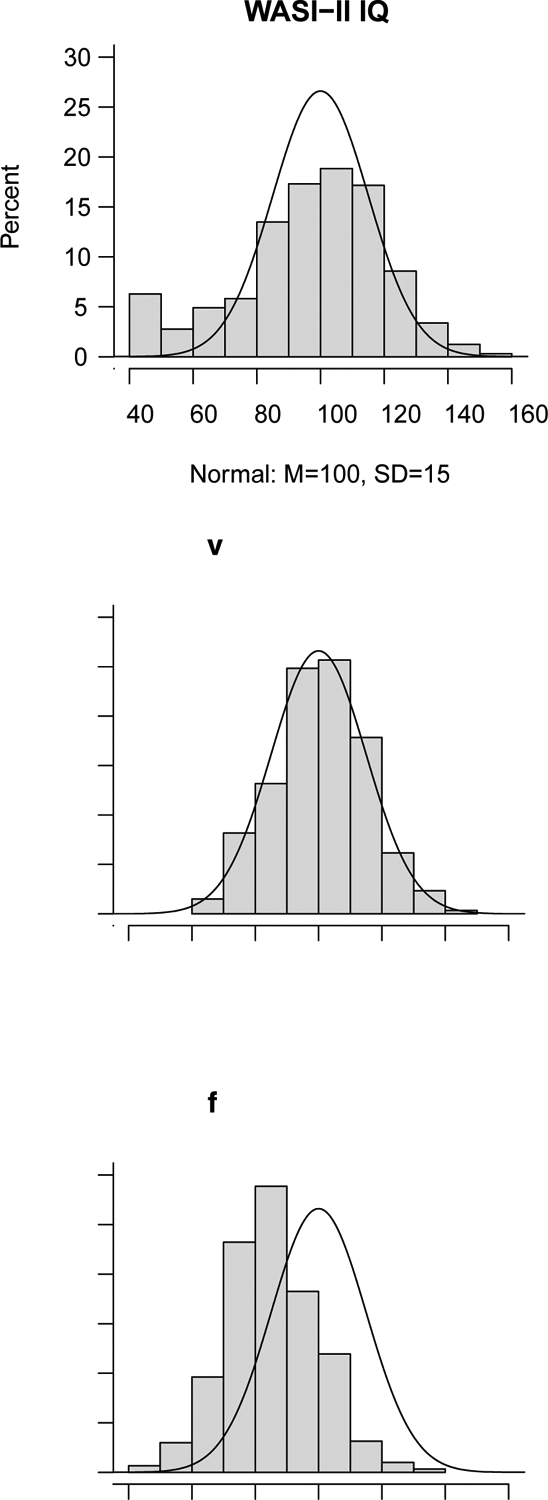
Distributions of scores for WASI-II IQ, NTCB verbal composite factor, and NTCB fluid composite factor
3.3. Differences between cognitive abilities
Weighted means and standard deviations of neurocognitive test scores are presented in Table 2. WASI-II Matrix Reasoning scores were on average 3.0 (95% CI 2.0, 4.0) points lower than WASI-II Vocabulary scores (Cohen’s d = .20). Similarly, but to a much more striking degree, NTCB fluid factor scores were on average 15.1 (95% CI 13.8, 16.5) points lower than NTCB verbal factor scores (Cohen’s d = 1.0).
3.4. Associations between social disadvantage, gestational age, and cognitive outcomes
Social disadvantage had a negative total effect on IQ and NTCB verbal factor scores (Table 3), with a greater effect on IQ (expected difference of 5.5 points) than NTCB verbal scores (expected difference of 3.2 points). Causal mediation analyses of gestational age as a mediator of the association between social disadvantage and cognitive outcome showed no evidence of interaction between social disadvantage and gestational age, with less than 1% of total effects explained by interaction. We found no evidence that gestational age mediated the associations between social disadvantage and cognitive outcomes, with 2% or less of the association between social disadvantage and cognitive outcomes mediated through gestational age. Results of a sensitivity analysis to examine the effect of siblings in the sample showed similar results both in terms of effect estimates and whether confidence intervals included the null (Supporting Information Table 2). An E-value assessment of unmeasured confounding was conducted for the total effect of social disadvantage on cognitive outcomes. An E-value is the minimum strength of association, on the risk ratio scale, needed between an unmeasured confounder and both social disadvantage and a cognitive outcome to fully explain the observed association. The social disadvantage – IQ association had an E-value of 3.31, and the social disadvantage – NTCB verbal factor association had an E-value of 2.31 (Supporting Information Table 3).
TABLE 3.
Causal mediation analysis of gestational age as mediator of the association between social disadvantage and cognitive outcome, controlling for maternal age
| Cognitive Outcome | |||
|---|---|---|---|
| WASI-II IQ | NTCB verbal factor | NTCB fluid factor | |
| Total effect of social disadvantage1 | −5.5 (−7.3 −3.7) |
−3.2 (−4.4, −2.0) |
−1.0 (−2.4, 0.4) |
| Controlled direct effect of social disadvantage | −5.5 (−7.3, −3.7) |
−3.2 (−4.4, −2.0) |
−1.0 (−2.3, 0.4) |
| Natural direct effect of social disadvantage | −5.5 (−7.3, −3.7) |
−3.2 (−4.4, −2.0) |
−1.0 (−2.3, 0.4) |
| Natural indirect effect through gestational age | 0.0 (−0.3, 0.4) |
0.0 (−0.1, 0.1) |
−0.0 (−0.2, 0.1) |
| Percentage mediated by gestational age | −0.0% (−7.2%, 5.9%) |
−0.1% (−4.3%, 2.9%) |
2.0% (−37.1%, 57.4%) |
| Percentage due to interaction | 0.0% (−1.9%, 1.8%) |
0.0% (−4.3%, 4.6%) |
−0.4% (−35.0%, 13.2%) |
| Percentage eliminated | −0.0% (−7.3%, 6.2%) |
−0.1% (−5.9%, 5.4%) |
2.3% (−53.5%, 59.2%) |
Effects correspond to expected difference in outcome for each additional social disadvantage
3.5. Associations between gestational age, morbidities at birth, and cognitive outcomes
Gestational age had a negative total effect on all cognitive outcomes (Table 4), with an expected difference in IQ of 3.1 points, in NTCB verbal scores of 1.3 points, and in NTCB fluid scores of 1.7 for each week lower in gestational age in the study range from 27 to 23 weeks. We found no evidence of exposure-mediator interactions, with less than 2.5% of the effect of prematurity on cognition due to interaction. The number of neonatal morbidities partially mediated associations between prematurity and all three cognitive measures, explaining 37.2%, 36.7%, and 33.9% of the total effect of gestational age on IQ and NTCB verbal and fluid scores, respectively. Direct effects of gestational age, not explained by neonatal morbidities, remained for IQ and NTCB fluid scores, whereas the effect of gestational age on NTCB verbal scores was attenuated when neonatal morbidities were considered. Results of a sensitivity analysis to examine the effect of siblings in the sample showed similar results both in terms of effect estimates and whether confidence intervals included the null (Supporting Information Table 4). An E-value assessment of the assumption of no unmeasured confounding of causal mediation yielded E-values ranging from 2.05 to 4.02 for these analyses (Supporting Information Table 5).
TABLE 4.
Causal mediation analysis of co-morbidities at birth as mediator of the association between gestational age and cognitive outcome, controlling for maternal age and social disadvantage
| WASI-II IQ | NTCB verbal factor | NTCB fluid factor | |
|---|---|---|---|
| Total effect of gestational age1 | −3.1 (−4.5, −1.6) |
−1.3 (−2.2, −0.3) |
−1.7 (−2.6, −0.7) |
| Controlled direct effect of gestational age | −2.0 (−3.5, −0.5) |
−0.7 (−1.7, 0.3) |
−1.1 (−2.0, −0.2) |
| Natural direct effect of gestational age | −1.9 (−3.4, −0.4) |
−0.8 (−1.8, 0.2) |
−1.1 (−2.0, −0.2) |
| Natural indirect effect through morbidities | −1.1 (−1.7, −0.7) |
−0.5 (−0.8, −0.2) |
−0.6 (−0.9, −0.3) |
| Percentage mediated by morbidities | 37.2% (20.1%, 76.2%) |
36.7% (12.1%, 163.5%) |
33.9% (16.3%, 80.4%) |
| Percentage due to interaction | 2.1% (−3.0%, 10.5%) |
−2.3% (−27.3%, 5.9%) |
−1.0% (−9.4%, 5.5%) |
| Percentage eliminated | 35.0% (17.6%, 74.5%) |
39.1% (13.6%, 175.2%) |
34.9% (17.1%, 79.7%) |
Effects correspond to expected difference in the outcome for each additional week lower in gestational age
4. COMMENT
4.1. Principal findings
Among 667 EP-born children assessed at age 15 years, NTCB verbal factor composite scores were within normative expectation, whereas WASI-II IQ and NTCB fluid processing scores were lower than normative expectation. Higher maternal social risk at birth was negatively associated with IQ and NTCB verbal ability, but not with NTCB fluid processing. Gestational age was positively associated with scores on all three cognitive outcomes. Associations between gestational age and cognitive outcomes were partially mediated by the number of neonatal morbidities. The effect of gestational age on NTCB verbal scores was attenuated when neonatal morbidities were taken into account.
4.2. Strengths of the study
Strengths of this study include a large sample size of EP-born adolescents and selection of the cohort using gestational age rather than birthweight to minimize bias arising from factors associated with being small for gestational age.40 In addition, we used a cognitive test battery that allowed an assessment of differential cognitive outcomes at age 15 in relation to a comprehensive and well-characterized set of birth and early life risk factors for poorer cognitive outcomes.
4.3. Limitations of the data
The main limitation of this study was the lack of a term-born control group, which required us to compare ELGAN cognitive test results to norms from test standardization samples which generally have more advantageous sociodemographic characteristics than EP-born children.1 Thus, our cognitive test results may have underestimated our cohort’s cognitive abilities, particularly verbal abilities, as compared to peers with similar socioeconomic risk. In addition, approximately 10% of participants did not complete the NTCB verbal and fluid information composite measures. These participants tended to have lower IQ than the sample mean, suggesting that the reported NTCB composite factor scores may have somewhat overestimated verbal and fluid information processing skills. Our causal mediation analyses did not account for clustering due to sibships from multiple births, but sensitivity analyses suggested clustering had little impact on results. Indirect effects from causal mediation analyses are based on four assumptions about confounding: 1) there is no unmeasured confounding of the exposure on the outcome, 2) there is no unmeasured confounding of the mediator on the outcome, 3) no unmeasured cofounding of the exposure – mediator scenario, and 4) no unmeasured confounding of the mediator – outcome that is caused by the exposure. We only controlled for maternal age (for analyses of social disadvantage and cognitive outcome) or maternal age and social disadvantage (for analyses of gestational age and cognitive outcome. However, E-value analyses suggest our results are somewhat robust against unmeasured confounding, with unmeasured confounders needing associations corresponding to risk ratios between 2.00 and 4.00 to explain observed effects. Finally, as our original cohort was not population based, our findings may be limited in their generalizability to EP-born adolescents in the U.S. and other countries.
4.4. Interpretation
The degree of cognitive impairment differed considerably between NTCB composite measures of verbal and fluid information processing. NTCB verbal factor composite scores did not differ from normative expectation and were associated with maternal social risk and to a lesser degree with gestational age. The development of verbal cognitive abilities is understood to be strongly influenced by experience, including early intervention, formal education opportunities, and advantageous familial and social environmental factors.34 These factors potentially mitigate the adverse effects of EP-birth, including lower gestational age and neonatal morbidities, on the development of verbal knowledge and abilities, consistent with findings from individuals who are born at term,41,42 and with research indicating that social-environmental factors are more strongly associated with verbal IQ among EP- and VP-born children as they become older.22,23,41 However, our social disadvantage composite served mainly as an indirect proxy for more proximal factors that are associated with poorer cognitive outcomes, such as air pollution, household toxins, community violence, parental psychosocial stress and isolation, quality of caregiving, racial discrimination, and access to health care and remedial learning and educational opportunities.43 Research on these more proximal factors remains a priority to understand and modify the detrimental effects of social disadvantage on prenatal and postnatal development of preterm born children.44
Scores on the NTCB fluid information processing factor composite, comprised by executive control measures of inhibition and cognitive flexibility and processing speed, were one standard deviation below those for the NTCB verbal factor composite, consistent with prior evidence of executive control and processing speed deficits in EP- and VP-born adolescents.13,16 NTCB fluid processing abilities were associated with social risk at birth, but to a lesser degree than NTCB verbal abilities. Fluid processing abilities were also associated with gestational age, and this association was partially mediated by the number of neonatal comorbidities. Fluid processing of novel information is less likely to be scaffolded by formal education, and may be more affected by perturbations of distributed brain networks and white matter connectivity, including disruptions in the development of pre-myelinating oligodendrocytes, aberrant apoptosis of neurons, and disturbances in neuronal migration, synapse and axonal formation, and myelination.24,45,46 Among VP-born children and adults, neuroimaging studies have shown that abnormalities in gray and white matter development and connectivity are associated with poorer cognitive outcome.47,48 Early aberrations in white matter development and brain connectivity may lay the foundation for weaknesses particularly in fluid information processing. Our findings that fluid information processing is most impaired among EP-born adolescents suggests a potential positive impact of early identification and remediation of executive control abilities essential to the development of adaptive cognitive and behavioral self-regulation.49,50
Partial mediation of gestational age effects on fluid information processing by neonatal morbidities is consistent with recent meta-analytic findings showing that bronchopulmonary dysplasia is the most frequently observed predictor of lower IQ among EP- and VP-born children.26 One potential mechanism linking bronchopulmonary dysplasia to poorer cognitive function is neonatal systemic inflammation, which is increased among EP infants who develop bronchopulmonary dysplasia.51 This mechanism might also apply to necrotizing enterocolitis, also associated with neonatal systemic inflammation.52
In contrast to the NTCB fluid information processing scores, but consistent with NTCB verbal scores, WASI-II IQ scores were considerably less impaired. IQ scores were associated with both maternal social risk and gestational age, and the association with gestational age was partially mediated by the number of neonatal morbidities. With respect to level of cognitive function, while it is tempting to suggest that WASI-II IQ is representative of general intellectual functioning, it is important to note that it comprises only two subtests as an abbreviated IQ measure. Nonetheless, the WASI-II IQ scores were more in line with the NTCB verbal than the fluid factor composite, perhaps because of the broader sampling of cognitive functions by the fluid factor when compared to the WASI-II. Additionally, although comprised of only two subtests, WASI-II IQ contains elements of both verbal (Vocabulary) and fluid reasoning (Matrix Reasoning), and the early life contributors to this combined IQ score mirror the early life predictor results that were obtained separately on the NTCB verbal and fluid factors (i.e., both maternal risk and gestational age).
Our finding that gestational age was associated with IQ and fluid information processing at age 15 differs from other studies of EP- and VP-born adolescents reporting a lack of association between gestational age and IQ, executive function, and processing speed.16,23 One exception is a study by Kroll et al.41 who in a longitudinal study of EP- and VP-born individuals from 8 to 31 years of age found a stable association of nonverbal IQ (but not verbal IQ) with higher gestational age. However, exclusion of the lowest stratum of gestational age attenuated this association between nonverbal IQ and gestational age.
It is important to note that approximately one-half of our sample scored at normative expectation or above on WASI-II IQ and NTCB verbal ability. Our findings that decreased social risk was associated with both IQ and verbal ability suggest that favorable social-environmental circumstances and associated experiences and exposures may foster the development of general intelligence and verbal abilities among EP-born individuals as they age into adolescence and adulthood.22,23,41
5. CONCLUSIONS
Adolescents born extremely preterm remain at heightened risk for cognitive deficits, in general intelligence and particularly fluid information processing involving executive control abilities and processing speed, which are associated with poorer academic attainment, general quality of life, psychological well-being, and vocational success. Poorer cognitive outcomes were associated with maternal social risk at birth as well as lower gestational age. The association with gestational age was partially mediated by the number of neonatal morbidities, including ultrasound-identified cerebral white matter damage and bronchopulmonary dysplasia. Efforts to reduce the frequency of these morbidities could lead to long-term benefits for individuals born extremely preterm. In addition, our finding that social disadvantage measured at birth was associated with cognitive outcomes at age 15 years indicates the importance of investigating the proximal factors that mediate relationships between perinatal socioeconomic variables and cognitive function among individuals born extremely preterm.
Supplementary Material
Supporting Information Table 1. NIH Toolbox Cognition Battery (NTCB) factor analysis
Supporting Information Figure 1. Directed acyclic graph illustrating analyses of association of social disadvantage, adjusted for maternal age, with cognitive outcomes
Supporting Information Figure 2. Directed acyclic graph illustrating analyses of gestational age, adjusted for maternal age and social disadvantage, with cognitive outcomes
Supporting Information Table 2. Sensitivity analysis for causal mediation models examining gestational age as a mediator of the association between social disadvantage and cognitive outcome, controlling for maternal age, with only one randomly selected participant from each multiple birth
Supporting Information Table 3. E-value analysis of causal mediation analysis of gestational age as a mediator of the association between social disadvantage and cognitive outcome, controlling for maternal age
Supporting Information Table 4. Sensitivity analysis for causal mediation models examining the number of neonatal morbidities as a mediator of the association between gestational age and cognitive outcome, controlling for maternal age and social disadvantage, with only one randomly selected participant from each multiple birth
Supporting Information Table 5. E-value analysis of causal mediation analysis of the number of neonatal morbidities as a mediator of the association between gestational age and cognitive outcome, controlling for maternal age and social disadvantage
Synopsis.
1. Study Questions
Are early maternal social disadvantage and gestational age associated with cognitive outcomes of extremely preterm (EP) born adolescents? Are EP-born adolescents differentially impaired in verbal learning versus fluid information processing skills?
2. What’s Already Known
EP-born children are at risk of longstanding cognitive deficits associated with endogenous child biological factors and social environmental exposures.
3. What This Study Adds
Early life risk factors, including maternal social disadvantage and lower gestational age, are associated with cognitive outcomes at age 15. Associations of IQ and verbal and fluid information processing with lower gestational age are partially mediated by the number of neonatal morbidities, including cerebral white matter damage and bronchopulmonary dysplasia. EP-born adolescents are more impaired in fluid information processing than verbal learning abilities.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the invaluable contributions of the participants and their families in this study as well as those of the ELGAN Study Investigators listed below.
Project Lead:
Julie V. Rollins
Site Principal Investigators:
Baystate Medical Center, Springfield, MA: Bhavesh Shah, Rachana Singh, Ruben Vaidya
Boston Children’s Hospital, Boston, MA: Linda Van Marter, Camilla Martin, Janice Ware, Caitlin Rollins
Tufts Medical Center, Boston, MA: Cynthia Cole, Ellen Perrin, Christina Sakai
University of Massachusetts Medical School, Worcester, MA: Frank Bednarek (deceased); Jean Frazier
Yale University School of Medicine, New Haven, CT: Richard Ehrenkranz (deceased), Jennifer Benjamin, Angela Montgomery
Wake Forest University, Winston-Salem, NC: T. Michael O’Shea, Lisa Washburn, Semsa Gogcu
University of North Carolina, Chapel Hill, NC: Carl Bose, Diane Warner, T. Michael O’Shea
East Carolina University, Greenville, NC: Steve Engelke, Amanda Higginson, Jason Higginson, Kelly Bear
Helen DeVos Children’s Hospital, Grand Rapids, MI: Mariel Poortenga, Steve Pastyrnak
Sparrow Hospital, Lansing, MI and Michigan State University, East Lansing, MI: Padu Karna, Nigel Paneth, Madeleine Lenski
University of Chicago Medical Center, Chicago, IL: Michael Schreiber, Scott Hunter, Michael Msall
William Beaumont Hospital, Royal Oak, MI: Danny Batton, Judith Klarr, Young Ah Lee, Rawad Obeid
Site Study Coordinators:
Baystate Medical Center, Springfield, MA: Karen Christianson, Deborah Klein, Katie Wagner
Boston Children’s Hospital, Boston MA: Maureen Pimental, Collen Hallisey, Taryn Coster, Maddie Dolins, Maggie Mittleman, Hannah Haile, Julia Rohde, Kaysi Herrera Pujols
Tufts Medical Center, Boston, MA: Ellen Nylen, Emily Neger, Kathryn Mattern, Catherine Ma, Deanna Toner, Elizabeth Vitaro
University of Massachusetts Medical School, Worcester, MA: Beth Powers Taylor Merk
Yale University School of Medicine, New Haven, CT: Joanne Williams, Elaine Romano, Christine Henry
Wake Forest University, Winston-Salem, NC: Debbie Hiatt (deceased); Nancy Peters, Patricia Brown, Emily Ansusinha, Jazmyne James, MS, Nou Yang
University of North Carolina, Chapel Hill, NC: Gennie Bose, Janice Wereszczak, Janice Bernhardt
East Carolina University, Greenville, NC: Joan Adams (deceased), Donna Wilson, Nancy Darden-Saad, Bree Williams, Emily Jones, Hannah Morris
Helen DeVos Children’s Hospital, Grand Rapids, MI: Dinah Sutton, Julie Rathbun, Stephanie Fagerman, William Boshoven, Jalen Johnson, Brandon James; Cynthia Gile
Sparrow Hospital, Lansing, MI and Michigan State University, East Lansing, MI: Karen Miras, Carolyn Solomon, Deborah Weiland
University of Chicago Medical Center, Chicago, IL: Grace Yoon, Rugile Ramoskaite, Suzanne Wiggins, Krissy Washington, Ryan Martin, Barbara Prendergast, Emma Lynch, Sabina Hajdarovic
William Beaumont Hospital, Royal Oak, MI: Beth Kring
Funding
This study was supported by grants from the National Institute of Neurological Disorders and Stroke (5U01NS040069-05; 2R01NS040069-06A2), the Office of the NIH Director (5UH3OD023348-05) the National Institute of Child Health and Human Development (5P30HD018655-28; 5R01HD092374-04), and the National Institute of Nursing Research (5R01NR019245-02).
REFERENCES
- 1.Anderson PJ. Neuropsychological outcomes of children born very preterm. Seminars in fetal & neonatal medicine. 2014;19(2):90–96. [DOI] [PubMed] [Google Scholar]
- 2.Johnson S, Marlow N. Early and long-term outcome of infants born extremely preterm. Archives of disease in childhood. 2017;102(1):97–102. [DOI] [PubMed] [Google Scholar]
- 3.Ekeus C, Lindstrom K, Lindblad F, Rasmussen F, Hjern A. Preterm birth, social disadvantage, and cognitive competence in Swedish 18- to 19-year-old men. Pediatrics. 2010;125(1):e67–73. [DOI] [PubMed] [Google Scholar]
- 4.Eryigit Madzwamuse S, Baumann N, Jaekel J, Bartmann P, Wolke D. Neuro-cognitive performance of very preterm or very low birth weight adults at 26 years. Journal of child psychology and psychiatry, and allied disciplines. 2015;56(8):857–864. [DOI] [PubMed] [Google Scholar]
- 5.Linsell L, Johnson S, Wolke D, et al. Cognitive trajectories from infancy to early adulthood following birth before 26 weeks of gestation: a prospective, population-based cohort study. Archives of disease in childhood. 2018;103(4):363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson S, Fawke J, Hennessy E, et al. Neurodevelopmental disability through 11 years of age in children born before 26 weeks of gestation. Pediatrics. 2009;124(2):e249–257. [DOI] [PubMed] [Google Scholar]
- 7.Kerr-Wilson C, Mackay D, Smith G, Pell J. Meta-analysis of the association between preterm delivery and intelligence. Journal of Public Health. 2012;34(2):209–216. [DOI] [PubMed] [Google Scholar]
- 8.Orchinik LJ, Taylor HG, Espy KA, et al. Cognitive outcomes for extremely preterm/extremely low birth weight children in kindergarten. Journal of the International Neuropsychological Society : JINS. 2011;17(6):1067–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barre N, Morgan A, Doyle LW, Anderson PJ. Language abilities in children who were very preterm and/or very low birth weight: a meta-analysis. The Journal of pediatrics. 2011;158(5):766–774.e761. [DOI] [PubMed] [Google Scholar]
- 10.Vohr B. Speech and language outcomes of very preterm infants. Seminars in fetal & neonatal medicine. 2014;19(2):78–83. [DOI] [PubMed] [Google Scholar]
- 11.Anderson PJ, De Luca CR, Hutchinson E, et al. Attention problems in a representative sample of extremely preterm/extremely low birth weight children. Developmental neuropsychology. 2011;36(1):57–73. [DOI] [PubMed] [Google Scholar]
- 12.Wilson-Ching M, Molloy CS, Anderson VA, et al. Attention difficulties in a contemporary geographic cohort of adolescents born extremely preterm/extremely low birth weight. Journal of the International Neuropsychological Society : JINS. 2013;19(10):1097–1108. [DOI] [PubMed] [Google Scholar]
- 13.Burnett AC, Scratch SE, Anderson PJ. Executive function outcome in preterm adolescents. Early human development. 2013;89(4):215–220. [DOI] [PubMed] [Google Scholar]
- 14.Mulder H, Pitchford NJ, Hagger MS, Marlow N. Development of executive function and attention in preterm children: a systematic review. Developmental neuropsychology. 2009;34(4):393–421. [DOI] [PubMed] [Google Scholar]
- 15.Taylor HG, Clark CA. Executive function in children born preterm: Risk factors and implications for outcome. Seminars in perinatology. 2016;40(8):520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brydges CR, Landes JK, Reid CL, Campbell C, French N, Anderson M. Cognitive outcomes in children and adolescents born very preterm: a meta-analysis. Developmental medicine and child neurology. 2018;60(5):452–468. [DOI] [PubMed] [Google Scholar]
- 17.Anderson P, Doyle LW, Victorian Infant Collaborative Study G. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. Jama. 2003;289(24):3264–3272. [DOI] [PubMed] [Google Scholar]
- 18.Mulder H, Pitchford NJ, Marlow N. Processing speed and working memory underlie academic attainment in very preterm children. Archives of Disease in Childhood-Fetal and Neonatal Edition. 2010:fetalneonatal167965. [DOI] [PubMed] [Google Scholar]
- 19.Rose SA, Feldman JF. Memory and processing speed in preterm children at eleven years: A comparison with full-terms. Child development. 1996;67(5):2005–2021. [PubMed] [Google Scholar]
- 20.Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. New England journal of medicine. 2005;352(1):9–19. [DOI] [PubMed] [Google Scholar]
- 21.Joseph RM, O’Shea TM, Allred EN, et al. Neurocognitive and Academic Outcomes at Age 10 Years of Extremely Preterm Newborns. Pediatrics. 2016;137(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doyle LW, Cheong JL, Burnett A, et al. Biological and Social Influences on Outcomes of Extreme-Preterm/Low-Birth Weight Adolescents. Pediatrics. 2015;136(6):e1513–1520. [DOI] [PubMed] [Google Scholar]
- 23.Linsell L, Malouf R, Morris J, Kurinczuk JJ, Marlow N. Prognostic Factors for Poor Cognitive Development in Children Born Very Preterm or With Very Low Birth Weight: A Systematic Review. JAMA Pediatr. 2015;169(12):1162–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Back SA, Miller SP. Brain injury in premature neonates: A primary cerebral dysmaturation disorder? Ann Neurol. 2014;75(4):469–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hintz SR, Vohr BR, Bann CM, et al. Preterm Neuroimaging and School-Age Cognitive Outcomes. Pediatrics. 2018;142(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Twilhaar ES, Wade RM, de Kieviet JF, van Goudoever JB, van Elburg RM, Oosterlaan J. Cognitive Outcomes of Children Born Extremely or Very Preterm Since the 1990s and Associated Risk Factors: A Meta-analysis and Meta-regression. JAMA Pediatr. 2018;172(4):361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hintz SR, Kendrick DE, Stoll BJ, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005;115(3):696–703. [DOI] [PubMed] [Google Scholar]
- 28.Bassler D, Stoll BJ, Schmidt B, et al. Using a count of neonatal morbidities to predict poor outcome in extremely low birth weight infants: added role of neonatal infection. Pediatrics. 2009;123(1):313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. Jama. 2004;292(19):2357–2365. [DOI] [PubMed] [Google Scholar]
- 30.Santos HP Jr., Bhattacharya A, Martin EM, et al. Epigenome-wide DNA methylation in placentas from preterm infants: association with maternal socioeconomic status. Epigenetics. 2019;14(8):751–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Shea TM, Allred EN, Dammann O, et al. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early human development. 2009;85(11):719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuban K, Adler I, Allred EN, et al. Observer variability assessing US scans of the preterm brain: the ELGAN study. Pediatric radiology. 2007;37(12):1201–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wechsler D. Wechsler Abbreviated Scale of Intelligence Second Edition (WASI-II). San Antonio, TX: The Psychological Corporation. 2011. [Google Scholar]
- 34.Akshoomoff N, Beaumont JL, Bauer PJ, et al. VIII. NIH Toolbox Cognition Battery (CB): composite scores of crystallized, fluid, and overall cognition. Monogr Soc Res Child Dev. 2013;78(4):119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akshoomoff N, Brown TT, Bakeman R, Hagler DJ. Developmental differentiation of executive functions on the NIH Toolbox Cognition Battery. Neuropsychology. 2018;32(7):777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneider WJ, McGrew KS. The Cattell–Horn–Carroll theory of cognitive abilities. 2018.
- 37.Jewsbury PA, Bowden SC, Strauss ME. Integrating the switching, inhibition, and updating model of executive function with the Cattell-Horn-Carroll model. J Exp Psychol Gen. 2016;145(2):220–245. [DOI] [PubMed] [Google Scholar]
- 38.Ananth CV, Brandt JS. A principled approach to mediation analysis in perinatal epidemiology. American journal of obstetrics and gynecology. 2022;226(1):24–32. e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haneuse S, VanderWeele TJ, Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. Jama. 2019;321(6):602–603. [DOI] [PubMed] [Google Scholar]
- 40.Arnold CC, Kramer MS, Hobbs CA, McLean FH, Usher RH. Very low birth weight: a problematic cohort for epidemiologic studies of very small or immature neonates. American journal of epidemiology. 1991;134(6):604–613. [DOI] [PubMed] [Google Scholar]
- 41.Kroll J, Karolis V, Brittain PJ, et al. Systematic assessment of perinatal and sociodemographic factors associated with IQ from childhood to adult life following very preterm birth. Intelligence. 2019;77:101401. [Google Scholar]
- 42.Von Stumm S, Plomin R. Socioeconomic status and the growth of intelligence from infancy through adolescence. Intelligence. 2015;48:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lorch SA, Enlow E. The role of social determinants in explaining racial/ethnic disparities in perinatal outcomes. Pediatric research. 2016;79(1):141–147. [DOI] [PubMed] [Google Scholar]
- 44.Rubin LP. Maternal and pediatric health and disease: integrating biopsychosocial models and epigenetics. Pediatric research. 2016;79(1–2):127–135. [DOI] [PubMed] [Google Scholar]
- 45.Durston S, Hulshoff Pol HE, Casey BJ, Giedd JN, Buitelaar JK, van Engeland H. Anatomical MRI of the developing human brain: what have we learned? Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(9):1012–1020. [DOI] [PubMed] [Google Scholar]
- 46.Leviton A, Gressens P. Neuronal damage accompanies perinatal white-matter damage. Trends in neurosciences. 2007;30(9):473–478. [DOI] [PubMed] [Google Scholar]
- 47.Thompson DK, Lee KJ, Egan GF, et al. Regional white matter microstructure in very preterm infants: predictors and 7 year outcomes. Cortex. 2014;52:60–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nosarti C, Giouroukou E, Healy E, et al. Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain. 2008;131(Pt 1):205–217. [DOI] [PubMed] [Google Scholar]
- 49.Diamond A, Lee K. Interventions shown to aid executive function development in children 4 to 12 years old. Science. 2011;333(6045):959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zelazo PD, Forston JL, Masten AS, Carlson SM. Mindfulness Plus Reflection Training: Effects on Executive Function in Early Childhood. Frontiers in Psychology. 2018;9(208). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bose C, Laughon M, Allred EN, et al. Blood protein concentrations in the first two postnatal weeks that predict bronchopulmonary dysplasia among infants born before the 28th week of gestation. Pediatric research. 2011;69(4):347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin CR, Bellomy M, Allred EN, Fichorova RN, Leviton A. Systemic inflammation associated with severe intestinal injury in extremely low gestational age newborns. Fetal and pediatric pathology. 2013;32(3):222–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table 1. NIH Toolbox Cognition Battery (NTCB) factor analysis
Supporting Information Figure 1. Directed acyclic graph illustrating analyses of association of social disadvantage, adjusted for maternal age, with cognitive outcomes
Supporting Information Figure 2. Directed acyclic graph illustrating analyses of gestational age, adjusted for maternal age and social disadvantage, with cognitive outcomes
Supporting Information Table 2. Sensitivity analysis for causal mediation models examining gestational age as a mediator of the association between social disadvantage and cognitive outcome, controlling for maternal age, with only one randomly selected participant from each multiple birth
Supporting Information Table 3. E-value analysis of causal mediation analysis of gestational age as a mediator of the association between social disadvantage and cognitive outcome, controlling for maternal age
Supporting Information Table 4. Sensitivity analysis for causal mediation models examining the number of neonatal morbidities as a mediator of the association between gestational age and cognitive outcome, controlling for maternal age and social disadvantage, with only one randomly selected participant from each multiple birth
Supporting Information Table 5. E-value analysis of causal mediation analysis of the number of neonatal morbidities as a mediator of the association between gestational age and cognitive outcome, controlling for maternal age and social disadvantage


