Abstract
Background:
Although nausea and vomiting of pregnancy (NVP) is common, the secular and demographic trends of NVP and its treatments are not well-studied.
Objectives:
To describe the prevalence and patterns of first-trimester NVP and selected treatments among controls in the National Birth Defects Prevention Study (NBDPS).
Methods:
National Birth Defects Prevention Study is a population-based case–control study of birth defects in the United States (1997-2011). We collected self-reported data about NVP and use of commonly reported pharmacological and herbal/natural treatments (ondansetron, promethazine, pyridoxine, metoclopramide, doxylamine succinate, ginger, phosphorated carbohydrate solution, and prochlorperazine) from mothers of non-malformed control infants. We estimated the prevalence of NVP and selected treatments and examined secular and demographic trends (education, race/ethnicity, and maternal age) for such use, adjusting for study centre.
Results:
Among 10 540 mothers of controls, 7393 women (70.1%) reported first-trimester NVP, and 12.2% of those used one or more of the commonly reported treatments. Specific treatment use varied after adjustment for study centre (ondansetron: 3.4%; promethazine: 4.2%; pyridoxine: 3.2%; metoclopramide: 0.7%; doxylamine succinate: 1.7%; ginger: 1.0%; phosphorated carbohydrate solution: 0.4%; and prochlorperazine: 0.3%). Treatment use increased for each agent over the study period. Women with more years of education reported more NVP and treatment use. White (72%), Hispanic (71%), and other race (73%) women reported more NVP than Black women (67%); White women used selected NVP treatments most frequently, and Black women used them more than Hispanic women. Though women aged 25-34 years reported more NVP (72%) than younger (69%) or older (67%) women, the frequency of medication use was similar among women aged 25-34 and ≥35, and lower among women aged <25 years.
Conclusions:
National Birth Defects Prevention Study controls reported NVP at frequencies similar to those previously reported. Of note, we observed an increase in use of selected treatments over time, and variations in NVP and treatments by study site and demographic factors.
Keywords: antiemetics, morning sickness, nausea, pregnancy, vomiting
1 ∣. BACKGROUND
A majority of women experience nausea in pregnancy, and about half experience vomiting, most often between 5 and 18 weeks of pregnancy.1-3 The causes of nausea and vomiting of pregnancy (NVP) include increased human chorionic gonadotropin, increased oestrogen and progesterone, or an evolutionary adaptation to reduce risk to the fetus from dangerous foods.1,4-6
Nausea and vomiting of pregnancy is associated with both favourable and adverse pregnancy outcomes. Compared to women with no NVP, women with NVP have a reduced risk of miscarriage, low birth-weight, small for gestational age, preterm birth, fetal death, and some birth defects.7-10 In contrast, NVP is associated with increased risks of pelvic girdle pain, proteinuria, high blood pressure, and preeclampsia.7 Both the presence and severity of NVP can increase health care costs and reduce quality of life and work productivity among pregnant women.2,11-14 Thus, treatment for NVP is highly sought.
Although NVP is very common, there is a lack of consensus on best treatment.2,15 Between 1983 and 2013, after the voluntary withdrawal of Bendectin (doxylamine succinate and vitamin B6) by the manufacturer following reports of birth defects, there were no there were no US Food and Drug Administration (FDA)-approved treatments for NVP, so treatment typically involved off-label, over-the-counter, or herbal/natural products, and as many as 97% of women took medications not labelled for use in pregnancy or not indicated for NVP.15-17 Safety information about many commonly used treatments is incomplete. Clinical guidelines recommend both non-pharmacological (eg dietary changes, avoiding triggers) and pharmacological therapies for NVP, including pyridoxine with or without doxylamine, dopamine antagonists, antihistamines, and serotonin 5-hydroxytryptamine type 3 receptor antagonists (5-HT3). Steroids are recommended for severe vomiting.1,3
The secular time and demographic trends of NVP and its treatments are not well-studied. Reports of associations between NVP and demographic factors suggest that increasing age and Black race are associated with a decrease in NVP, but also with persistence of symptoms into the second trimester.4,18 Increasing education is associated with a slight increase in NVP, but income is not associated with NVP, independent of other socioeconomic, regional, or demographic factors.4 These studies did not examine any associations with or patterns in treatments.
Variations in pharmacologic treatments are likely due to regional and cultural factors, and secular trends in pharmacologic treatments are likely driven by practice guidelines, introduction of new anti-emetics for other indications, and drug costs. To our knowledge, no studies have examined the secular and demographic trends of other common treatments for NVP in a US population. This has important implications for research concerning the effects of treatments for NVP on adverse pregnancy and birth outcomes. To examine secular trends and demographic patterns in NVP and self-reported treatments, we used data from 1997 to 2011 from the National Birth Defects Prevention Study (NBDPS), a large case–control study of birth defects in the United States.
2 ∣. METHODS
National Birth Defects Prevention Study is a multi-site, case–control study designed to investigate risk factors, including medication use, for birth defects. From 1997 to 2011, the NBDPS identified 30 major birth defects among livebirths, stillbirths, and (at selected sites) elective terminations through surveillance programs in Arkansas, California, Georgia, Iowa, Massachusetts, New Jersey, New York, North Carolina, Texas, and Utah. Mothers of both case and control infants were asked to complete a computer-assisted telephone interview up to 24 months after delivery. The telephone interview included questions on medical and pregnancy history, medication use during pregnancy, demographic characteristics, and pregnancy outcomes. Detailed information on the study design is published elsewhere.19
2.1 ∣. Case–Control Selection
Birth defect diagnoses were confirmed by clinical geneticists. A random sample of liveborn infants without birth defects identified from birth certificates or birth hospitals in the same geographic regions and time periods as malformed infants served as controls.
Mothers of non-malformed control infants comprise the present analysis. Of 11 829 controls, we excluded multiple pregnancies (n = 358), controls missing birth outcome (n = 37), and controls who did not complete the standardized telephone interview (n = 358). New Jersey participated in NBDPS only until 2002; because one of our aims was to establish trends in exposures over time, we excluded controls from New Jersey (n = 578). Of the remaining controls, 7393 women experienced first-trimester NVP, comprising the analytic sample for analyses of treatment trends.
2.2 ∣. Exposures
The occurrence of NVP was self-reported via the standardized telephone interview. Specifically, women were asked, “During this pregnancy, did you have morning sickness or nausea?” If they answered “yes,” women were asked to describe the frequency of nausea and vomiting, separately, in a given month of pregnancy (never, less than once a week, once a week, several times a week, every day, constantly, refused, or don't know). In addition, women were asked if they had any medical treatment or took any medications for their nausea or vomiting. Finally, in a separate portion of the interview, women were asked what, if any, medications they took during their pregnancy, including timing and indication (selected from a standard list of indications, including “morning sickness”). However, for many reported drugs, indication was not recorded. Women also reported their educational attainment, race/ethnicity, and age. We restricted this analysis to women reporting NVP in the first trimester, defined as the 90 days after the estimated date of conception.
Among women with NVP, the standardized interview captured self-reported treatments with prescription and non-prescription medications, herbal products, and supplements. Treatments were coded and classified using the Slone Drug Dictionary.20 Information on drug use was obtained in NBDPS by asking women who reported a particular condition (eg diabetes, sore throat, seizures), if they used any treatment for that condition. Then, women were asked if they took any other medications, but were not asked for the indications. Because of this limitation, we selected the top 10 prescription and over-the-counter (including herbal) treatments for NVP based on the frequency of use for NVP among all cases and all controls in NBDPS. Thus, the exposures in this analysis reflect agents most commonly used for NVP. Drugs that have indications other than use as an anti-emetic (eg doxylamine for insomnia) were included. Therefore, the population included as exposed to a particular agent includes both women who reported taking that agent for NVP and women who reported NVP and reported taking that specific agent (but not necessarily for NVP).
Prescription exposures of interest included ondansetron (Zofran and generics), promethazine (eg Phenergan and generics), metoclopramide (Reglan and generics), and prochlorperazine (Compro and generics). Non-prescription exposures of interest included ginger (as ginger, ginger extract, ginger teas, ginger beer, and herbal remedies marketed for NVP), pyridoxine (as vitamin B6 alone or vitamin B complex), doxylamine succinate (eg Unisom Sleep Tabs and generics), and phosphorated carbohydrate solution (eg Emetrol, Nauzene, and generics). We excluded use of ginger and pyridoxine included in multivitamins. Though calcium carbonate (eg Tums, Rolaids Softchews, and generics) and diphenhydramine (eg Benadryl, Tylenol PM, and generics) were frequently reported in both cases and controls in NBDPS as a treatment for NVP, in the NBDPS, the large majority of exposures were as a component of multivitamins (calcium carbonate) or sleep aids and cold/flu medications (diphenhydramine). Among women reporting use of calcium carbonate or diphenhydramine, only 7% (calcium carbonate) and 12% (diphenhydramine) reported using the drug to treat NVP. Therefore, we excluded calcium carbonate and diphenhydramine from this analysis.
2.3 ∣. Statistical analysis
We measured the proportion of women with NVP descriptively according to several sociodemographic characteristics. While there is no agreed-upon definition of severity of NVP, as a proxy, we evaluated the proportion of women who reported nausea alone, compared to nausea with vomiting, as well as treatment use by symptom, according to month of pregnancy. We examined secular trends in NVP and treatments in five-year increments based on the estimated date of delivery (1997-2001, 2002-2006, and 2007-2011). Because NVP prevalence and treatments varied substantially by study site (see below), we standardized for study site according to the geographic distribution of the entire population of cases and controls in NBDPS (n = 44 029). We also examined patterns of NVP and treatments by age, self-reported race/ethnicity, and educational attainment, all standardized for study site. We calculated weights based on the distribution of all participants in NBDPS subject to the same exclusion criteria.
To evaluate the impact of time to interview on report of symptoms and treatment use, we examined the proportion of women experiencing first-trimester NVP and any treatment use according to time to interview. We defined time to interview as the time from the infant's date of birth to the date the interview was completed. We then considered time to interview categorically (<6, 7-12, 13-18, and 19-24 months).
2.4 ∣. Missing data
There were no data missing for study site, estimated date of delivery, or maternal age. The data missing for other variables were minimal; 13 women were missing data on education or first-trimester NVP (0.001%), and 141 women were missing data on race/ethnicity (1.3%).
2.5 ∣. Ethics approval
All participants provided informed consent, and Institutional Review Board approval was obtained at all participating study sites.
3 ∣. RESULTS
Among 10 540 eligible subjects, 7393 (70.1%) reported first-trimester NVP. NVP was more common in the second and third month of pregnancy, and more women experienced nausea and vomiting (range by pregnancy months 28.2%-39.6%) than nausea alone (range 16.0%-21.9%). Treatment use was also higher among women experiencing nausea with vomiting (range 14.1%-16.0%) than nausea alone (range 6.6%-7.6%) (Table 1). Site-specific NVP prevalence ranged from 66.0% in Massachusetts to 81.0% in Utah. The proportion of women reporting NVP increased from 67.3% to 72.0% over the three study periods. Specific treatment use varied by study site (Figure 1).
TABLE 1.
Prevalence of NVP and treatment of interest use by timing and symptom, geographic location, estimated date of delivery, and demographic factors among NBDPS controls (1997-2011)
| N | Women with NVP (%) |
Treatment of interest use among women with NVP (n = 7393) (%) |
|
|---|---|---|---|
| Total | 10 540 | 7393 (70.1%) | 907 (12.3%) |
| Month 1 | |||
| Nausea only | 1689 (16.0%) | 111 (6.6%) | |
| Nausea with vomiting | 2 974(28.2%) | 420 (14.1%) | |
| Month 2 | |||
| Nausea only | 2310 (21.9%) | 165 (7.1%) | |
| Nausea with vomiting | 4169 (39.6%) | 640 (15.4%) | |
| Month 3 | |||
| Nausea only | 2215 (21.0%) | 168 (7.6%) | |
| Nausea with vomiting | 3943 (37.4%) | 630 (16.0%) | |
| Location | |||
| Arkansas | 1408 | 964 (68.5%) | 189 (19.6%) |
| California | 1210 | 824 (68.1%) | 56 (6.8%) |
| Georgia | 1190 | 866 (72.8%) | 93 (10.7%) |
| Iowa | 1226 | 846 (69.0%) | 109 (12.9%) |
| Massachusetts | 1305 | 861 (66.0%) | 75 (8.7%) |
| New York | 937 | 640 (68.3%) | 42 (6.6%) |
| North Carolina | 935 | 683 (73.1%) | 116 (17.0%) |
| Texas | 1274 | 865 (67.9%) | 45 (5.2%) |
| Utah | 1045 | 844 (80.8%) | 182 (21.6%) |
| Year of delivery | |||
| 1997-2001 | 2081 | 1884 (67.3%) | 136 (7.2%) |
| 2002-2006 | 3953 | 2793 (70.7%) | 337 (12.1%) |
| 2007-2011 | 3773 | 2716 (72.0%) | 434 (16.0%) |
| Education (y) | |||
| <12 | 1772 | 1184 (66.8%) | 51 (4.3%) |
| 12 | 2525 | 1735 (68.7%) | 154 (8.9%) |
| 13-15 | 2841 | 2038 (71.7%) | 315 (15.5%) |
| ≥16 | 3376 | 2428 (71.9%) | 386 (15.9%) |
| Race/ethnicity | |||
| White | 6135 | 4408 (71.4%) | 671 (15.7%) |
| Black | 1131 | 754 (66.7%) | 101 (12.0%) |
| Hispanic | 2580 | 1746 (67.7%) | 71 (4.1%) |
| Other | 679 | 485 (71.4%) | 51 (11.2%) |
| Missing | 141 | 91 (64.5%) | 13 (9.2%) |
| Age (y) | |||
| <25 | 3478 | 2384 (68.5%) | 226 (9.5%) |
| 25-34 | 5626 | 4050 (72.0%) | 557 (13.7%) |
| ≥35 | 1423 | 959 (67.4%) | 124 (12.9%) |
FIGURE 1.
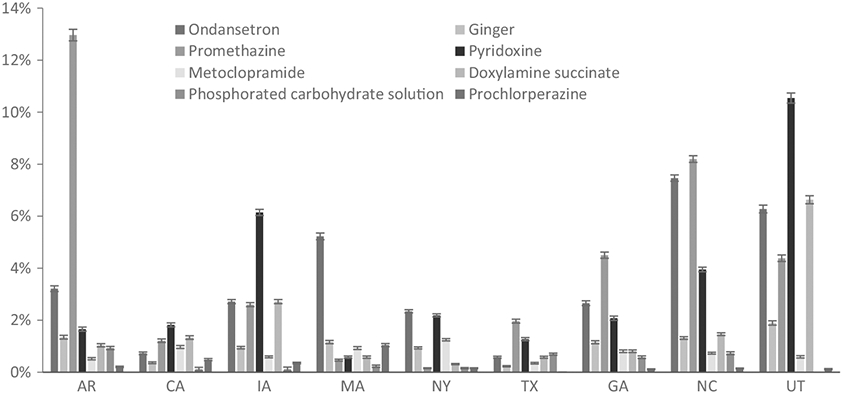
Prevalence of common treatments and 95% confidence intervals among those with nausea and vomiting of pregnancy (NVP) in the first trimester among controls, by study site, in the National Birth Defects Prevention Study, 1997-2011
Overall, 15.4% of women reported any treatment for NVP, and 12.2% of women with NVP used at least one of the medications of interest, after standardizing by site. Among these medications, indication was missing for 1.2% (pyridoxine) to 10.1% (doxylamine succinate). NVP was reported as the indication for 100% of phosphorated carbohydrate solution, 97.2% of ondansetron, 91% of promethazine, 90.9% of prochlorperazine, 90.7% of metoclopramide, 67.4% of doxylamine succinate, 55.8% of ginger, and 38.1% of vitamin B6 use. Site-specific use of at least one of the studied medications ranged from 6.3% in California to 21.4% in Utah (Table 1). After standardizing by site, the prevalence of specific drugs used for NVP was promethazine (4.2%), ondansetron (3.4%), pyridoxine (3.2%), doxylamine (1.7%), ginger (1.0%), metoclopramide (0.7%), phosphorated carbohydrate solution (0.4%), and prochlorperazine (0.3%).
The majority of controls completed the interview within one year of delivery (34.2% at <6 months, 41.6% at 7-12 months, 15.9% at 13-18 months, and 8.3% at 19-24 months). The proportion of women reporting first-trimester NVP decreased with longer time to interview (71.5% at <6 months, 70.8% at 7-12 months, 67.8% at 13-18 months, and 66.4% at 19-24 months). Self-report of treatment use among those with NVP was highest among women interviewed <6 months after delivery (13.0%), similar among women interviewed 7-12 months and 19-24 months after delivery (11.3% and 11.5%, respectively), and lowest among women interviewed 13-18 months after delivery (8.8%).
3.1 ∣. Secular trends
Use of ondansetron, ginger, promethazine, pyridoxine, metoclopramide, and doxylamine succinate increased between 1997 and 2011. Use of prochloperazine and phosphorated carbohydrate solution decreased slightly over the study period, but use of these drugs was infrequent overall (Figure 2). There was a marked increase in ondansetron use after 2006. While pyridoxine use increased steadily across study periods, this did not correspond with a similar increase in doxylamine succinate use, which increased slightly between the first and second study periods and then plateaued.
FIGURE 2.
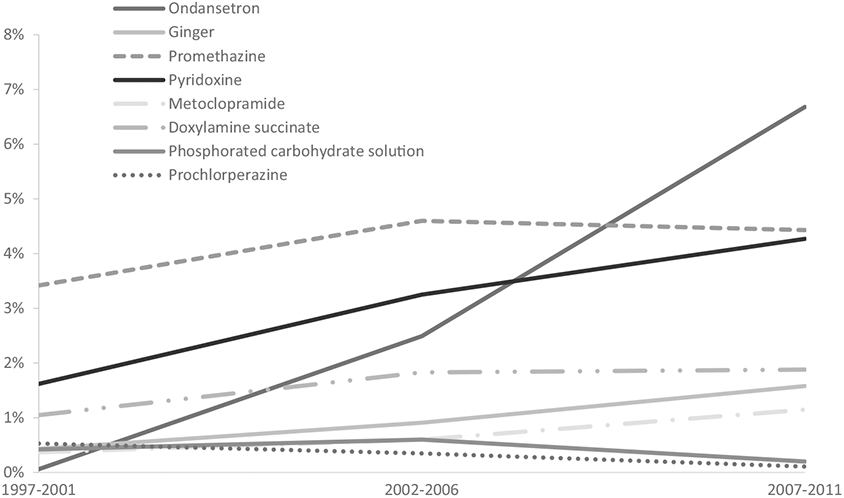
Secular trends in use of selected treatments among controls with nausea and vomiting of pregnancy (NVP) in NBDPS, standardized by site location, 1997-2011
More women with ≥16 years of education (corresponding to a four-year college degree) reported NVP (72.1%) than women with fewer years of education (67.3% among 13-15 years, 68.8% among 12 years, and 67.0% among <12 years). Similarly, there was an increasing trend in medication use with more years of education for all agents except metoclopramide, for which use was fairly consistent across categories of educational attainment (Figure 3).
FIGURE 3.
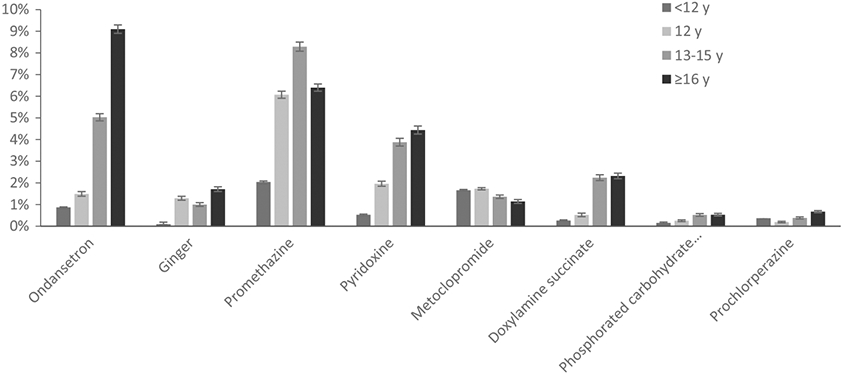
Use of selected treatments among NBDPS controls with first-trimester nausea and vomiting of pregnancy (NVP) in NBDPS, by educational attainment, standardized by site location, 1997-2011
Sixty-seven per cent of Black women reported NVP, compared to 71% of White women, 68% of Hispanic women, and 71% of women of other races/ethnicities. Hispanic women were less likely to use the studied medications than women of other races. White women had the highest frequency of use for each of the studied medications except promethazine, phosphorated carbohydrate solution, and prochlorperazine, for which use was more frequent among Black women (Figure 4).
FIGURE 4.
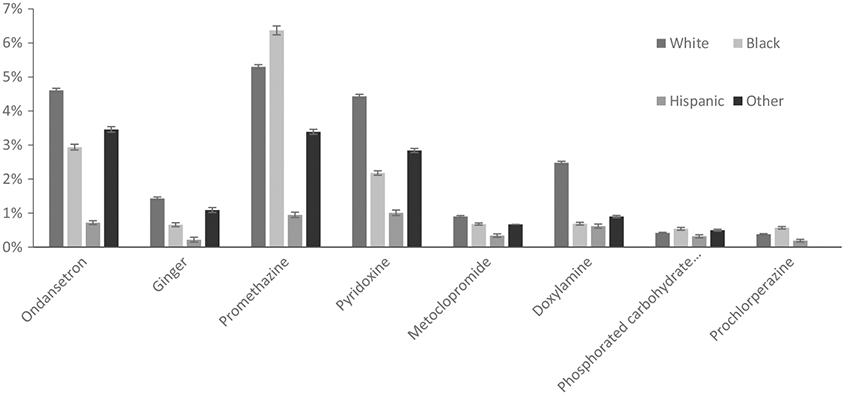
Use of selected treatments among NBDPS controls with first-trimester nausea and vomiting of pregnancy (NVP) in NBDPS, by race/xethnicity, standardized by site location, 1997-2011
More women aged 25-34 reported NVP (72%) than both older (67%) and younger (69%) women, and women <25 years were less likely to use ondansetron, ginger, pyridoxine, metoclopramide, and doxylamine than older women. Promethazine use was lowest among women ≥35 years; otherwise, use did not differ appreciably between women 25-34 years and ≥35 years (Figure 5).
FIGURE 5.
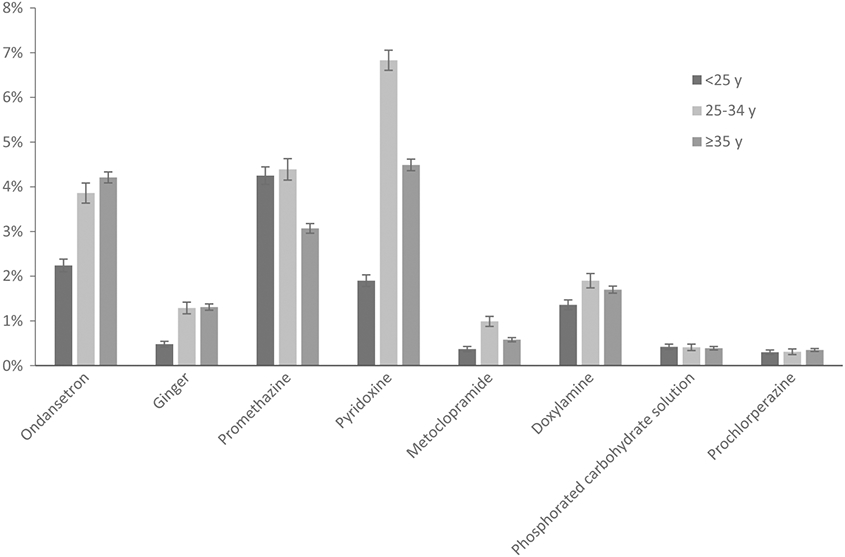
Use of selected treatments among NBDPS controls with first-trimester nausea and vomiting of pregnancy (NVP) in NBDPS, by age at expected delivery date, standardized by site location, 1997-2011
3.2 ∣. Comment
3.2.1 ∣. Principal findings
More than two-thirds of mothers of controls in NBDPS reported NVP, which is similar to previous reports.7,21,22 Overall, 15.4% of women reported any treatment for NVP, and 12.2% of women with NVP used at least one of the medications of interest, but treatment use varied by geographic location, over time, and by demographic factors. Prescription drug use is more common than non-prescription drug and herbal/natural treatment use. White women, older women, and women with more education report using treatments more frequently than Black and Hispanic women, younger women, and women with less education.
3.2.2 ∣. Strengths of the study
Strengths of this analysis include the fact that NBDPS is a large study that captured NVP among pregnant women in the United States. Over the long study period, NVP and treatments were consistently measured via standardized questions in computer-assisted interviews. Nausea and especially vomiting in pregnancy are likely to be recalled with accuracy because NVP tends to persist over several weeks.
3.2.3 ∣. Limitations of the data
Our study has several limitations. The retrospective design may lead to incomplete recall, especially of treatments, which could potentially differ by demographic factors. Furthermore, we limited our analysis to livebirths, which inherently exclude spontaneous or therapeutic abortions. Women with pregnancies ending in spontaneous abortion are known to experience less NVP than women with pregnancies carried to term, which would result in an overestimation of the true proportion of women experiencing NVP.9 Women with extremely severe NVP (ie hyperemesis gravidarum) may terminate their pregnancies due to intolerance of their NVP symptoms.23-25 Any resulting downward bias in our estimations of the proportion of women experiencing NVP or the use of specific treatments due to the exclusion of terminations is likely to be small. Additionally, we inferred treatment indication by identifying drugs used commonly to treat NVP among the entire NBDPS population of cases and controls and then assuming that use of these drugs among control women in the analytic data set with first-trimester NVP represents use for NVP. Certain treatments we examined have multiple indications and may have been used for reasons other than NVP (eg doxylamine succinate for insomnia, pyridoxine in a B complex supplement for fatigue). This is especially true of non-prescription treatments. Because of these limitations, our observations regarding medications used for NVP are limited to the specific medications included in this analysis.
3.2.4 ∣. Interpretation
Our results agree with a previous study that suggested older women and Black women report less NVP, while those with more years of education report more NVP than their respective counterparts.4 In an earlier NBDPS analysis, data from 1997 to 2004 suggested Hispanic women were less likely than women of other race/ethnicities to use NVP treatments10; this finding persisted in the later study period (2005-2011).
Our results are also consistent with other studies in identifying an increase in ondansetron use over time, especially after the availability of generic ondansetron in 2007.26 However, we did not observe a concomitant decrease in promethazine and metoclopramide use, as other studies have reported.15,27 One report noted that the withdrawal of Bendectin from the market in 1983 was followed by an increase in hospitalizations for NVP, which may have been attributable to severe NVP being under-treated due to an increase in fear of teratogenicity of NVP treatments.17 In the current study, the increase over time in the use of all treatments, both prescription and non-prescription, may reflect both a decrease in fear of teratogenicity and an increase in health care provider willingness to treat NVP to prevent severe complications that might require hospitalization. Should this trend continue, it is possible that future research may be better able to detect potential teratogenic effects of treatments for NVP.
Our observation that increasing education was associated with increases in both NVP prevalence and use of treatments is unlikely to represent an underlying biological mechanism, but more likely reflects differences in perceptions of NVP, since these data were based on self-report. More years of education is associated with higher socio-economic status, which may correspond to greater access to medical care, differing cultural approaches to illness, or the ability to afford more expensive over-the-counter or prescription treatments.
Despite adjusting for regional variations in treatment practices, we found that though Hispanic women report more NVP than Black women, a greater proportion of Black women treat their NVP with the studied medications than Hispanic women. White women report both more NVP and more common treatment of their NVP than Black and Hispanic women. As with education, there are potential cultural differences that may explain this discrepancy. It is also possible that preferred treatments vary by racial/ethnic group.
The noted sociodemographic differences in NVP prevalence and treatment have clinical implications. While self-report of symptoms of NVP likely varies due to differences in perception, differences in treatment use may reflect both cultural differences and provider recommendations. While there are guidelines for treatment of NVP by respected professional organizations (eg the American College of Obstetricians and Gynecologists), the regional variation in treatment practices suggests these recommendations are not universally adopted across geographic regions.
4 ∣. CONCLUSIONS
In this large, diverse sample of mothers of non-malformed infants, we identified regional variations in NVP treatments and found secular and demographic trends in both the prevalence of NVP and its treatments that do not appear to be explained by regional variations in treatments. This has important implications for research concerning the association of NVP or its treatments with adverse pregnancy and birth outcomes. Researchers should consider whether there are secular or demographic trends in the outcome of interest, and control for such factors as appropriate.
Synopsis.
Study question
What is the prevalence of nausea and vomiting of pregnancy (NVP) and use of treatments for NVP, and are there secular or demographic variations in NVP and treatment use?
What is already known
70.1% of women experience NVP. Older women, Black women, and women with less education are less likely to report NVP than their counterparts.
What this study adds
A minority of women treat their NVP with medication, but treatment use varies by geographic location, over time, and by demographic factors. Prescription drug use is more common than non-prescription drug and herbal/natural treatment use. White women, older women, and women with more education report using treatments more frequently than Black and Hispanic women, younger women, and women with less education.
Funding information
This study was supported in part by cooperative agreements U01DD000493 and U01DD001037 between the Birth Defects Branch of the Centers for Disease Control and Prevention and the Massachusetts Department of Public Health. Coding of drug information in the National Birth Defects Prevention Study used the Slone Drug Dictionary under licence from the Slone Epidemiology Center of Boston University, MA.
REFERENCES
- 1.Erick M, Cox JT, Mogensen KM. ACOG Practice Bulletin 189: nausea and vomiting of pregnancy. Obstet Gynecol. 2018;131:935. [DOI] [PubMed] [Google Scholar]
- 2.Matthews A, Haas DM, O'Mathuna DP, Dowswell T. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev. 2015;(4):CD007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith JA, Refuerzo JS, Fox KA. Patient education: nausea and vomiting of pregnancy. UpToDate. 2018. [Google Scholar]
- 4.Louik C, Hernandez-Diaz S, Werler MM, Mitchell AA. Nausea and vomiting in pregnancy: maternal characteristics and risk factors. Paediatr Perinat Epidemiol. 2006;20:270–278. [DOI] [PubMed] [Google Scholar]
- 5.Cardwell MS. Pregnancy sickness: a biopsychosocial perspective. Obstet Gynecol Surv. 2012;67:642–652. [DOI] [PubMed] [Google Scholar]
- 6.Lee NM, Saha S. Nausea and vomiting of pregnancy. Gastroenterol Clin North Am. 2011;40:309–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chortatos A, Haugen M, Iversen PO, et al. Pregnancy complications and birth outcomes among women experiencing nausea only or nausea and vomiting during pregnancy in the Norwegian Mother and Child Cohort Study. BMC Pregnancy Childbirth. 2015;15:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrell HE. Nausea and vomiting of pregnancy. Am Fam Physician. 2014;89:965–970. [PubMed] [Google Scholar]
- 9.Koren G, Madjunkova S, Maltepe C. The protective effects of nausea and vomiting of pregnancy against adverse fetal outcome–a systematic review. Reprod Toxicol. 2014;47:77–80. [DOI] [PubMed] [Google Scholar]
- 10.Anderka M, Mitchell AA, Louik C, et al. Medications used to treat nausea and vomiting of pregnancy and the risk of selected birth defects. Birth Defects Res A Clin Mol Teratol. 2012;94:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai G, Korfage IJ, Groen EH, Jaddoe VW, Mautner E, Raat H. Associations between nausea, vomiting, fatigue and health-related quality of life of women in early pregnancy: the generation R study. PLoS One. 2016;11:e0166133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacasse A, Rey E, Ferreira E, Morin C, Berard A. Nausea and vomiting of pregnancy: what about quality of life? BJOG. 2008;115:1484–1493. [DOI] [PubMed] [Google Scholar]
- 13.Munch S, Korst LM, Hernandez GD, Romero R, Goodwin TM. Health-related quality of life in women with nausea and vomiting of pregnancy: the importance of psychosocial context. J Perinatol. 2011;31:10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Locock L, Alexander J, Rozmorits L. Women's responses to nausea and vomiting in pregnancy. Midwifery. 2008;24:143–152. [DOI] [PubMed] [Google Scholar]
- 15.Koren G. Treating morning sickness in the United States–changes in prescribing are needed. Am J Obstet Gynecol. 2014;211:602–606. [DOI] [PubMed] [Google Scholar]
- 16.Thorpe PG, Gilboa SM, Hernandez-Diaz S, et al. Medications in the first trimester of pregnancy: most common exposures and critical gaps in understanding fetal risk. Pharmacoepidemiol Drug Saf. 2013;22:1013–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slaughter SA, Hearns-Stokes R, van der Vlugt T, Joffe HV. FDA approval of doxylamine-pyridoxine therapy for use in pregnancy. N Engl J Med. 2014;370:1081–1083. [DOI] [PubMed] [Google Scholar]
- 18.Chan RL, Olshan AF, Savitz DA, et al. Maternal influences on nausea and vomiting in early pregnancy. Matern Child Health J. 2011;15:122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reefhuis J, Gilboa SM, Anderka M, et al. The national birth defects prevention study: a review of the methods. Birth Defects Res A Clin Mol Teratol. 2015;103:656–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelley KE, Kelley T, Kaufman D, Mitchell AA. The slone drug dictionary: a research driven pharmacoepidemiology tool. Pharmacoepidemiol Drug Saf. 2003;12:S168–S169. [Google Scholar]
- 21.LaCroix R, Eason E, Melzack R. Nausea and vomiting during pregnancy: a prospective study of its frequency, intensity, and patterns of change. Am J Obstet Gynecol. 2000;182:931–937. [DOI] [PubMed] [Google Scholar]
- 22.Emilianova S, Mazzota P, Einarson A, Koren G. Prevalence and severity of nausea and vomiting of pregnancy and the effect of vitamin supplementation. Clin Invest Med. 1999;22:106–110. [PubMed] [Google Scholar]
- 23.Havnen GC, Truong MB, Do MH, Heitmann K, Holst L, Nordeng H. Women's perspectives on the management and consequences of hyperemesis gravidarum - a descriptive interview study. Scand J Prim Health Care. 2019;37:30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heitmann K, Nordeng H, Havnen GC, Solheimsnes A, Holst L. The burden of nausea and vomiting during pregnancy: severe impacts on quality of life, daily life functioning and willingness to become pregnant again - results from a cross-sectional study. BMC Pregnancy Childbirth. 2017;17:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broussard CN, Richter JE. Nausea and vomiting of pregnancy. Gastroenterol Clin North Am. 1998;27:123–151. [DOI] [PubMed] [Google Scholar]
- 26.Parker SE, Van Bennekom C, Anderka M, Mitchell AA, Study NBDP. Ondansetron for treatment of nausea and vomiting of pregnancy and the risk of specific birth defects. Obstet Gynecol. 2018;132:385–394. [DOI] [PubMed] [Google Scholar]
- 27.Taylor LG, Bird ST, Sahin L, et al. Antiemetic use among pregnant women in the United States: the escalating use of ondansetron. Pharmacoepidemiol Drug Saf. 2017;26:592–596. [DOI] [PubMed] [Google Scholar]


