Abstract
Chikungunya virus (CHIKV) and Zika virus (ZIKV) are mosquito-borne viruses that have caused several outbreaks worldwide. Aedes mosquitoes transmit these viruses mainly through sylvatic and urban transmission cycles. In the sylvatic cycle, nonhuman primates (NHPs) can be infected with CHIKV and ZIKV and may play an essential role as reservoirs for virus transmission. To improve our knowledge on the role of NHPs in the sylvatic cycle, we performed a systematic review and meta-analysis study on the seroprevalence of CHIKV and ZIKV worldwide in NHPs. According to the PRISMA guidelines, 17 CHIKV and 16 ZIKV seroprevalence studies in NHPs from 3 online databases: PubMed, Embase, and Scopus were selected. Data were extracted, including location and study year, type of NHP, sample size, serological tests, and seropositivity. All included studies have high-quality scores, between 5 and 8, corresponding to the grading criteria. Seroprevalence estimation was pooled using the ‘meta’ package in the R statistical software. The estimated pooled seroprevalence of CHIKV and ZIKV in NHP was 17% (95%CI: 5–34, I2: 99%, p < 0.05) and 6% (95% CI: 2–12, I2: 92%, p < 0.05), respectively. Most of the NHPs tested were wild Old World monkeys. The subgroup was analyzed by continents; high seropositive CHIKV and ZIKV were found in African NHPs at 35% (95% CI 9–66.0, I2 = 100) and 16% (95% CI 1–44, I2 = 97), respectively. While NHPs in America have 7% (95% CI 0-28, I2 = 99) and 2% (95% CI 1-3, I2 = 54) against CHIKV and ZIKV. In Asia, 6% (95% CI: 5–34, I2 = 96) CHIKV seroprevalence and 7% (95% CI 0–20, I2 = 98) ZIKV seroprevalence were found in NHP. This study provides a comprehensive overview of the seroprevalence of CHIKV and ZIKV among NHPs in various regions.
Keywords: Chikungunya virus, Zika virus, Seroprevalence, Nonhuman primates, Systematic review, Meta-analysis
1. Introduction
Mosquito-borne viruses such as chikungunya (CHIKV) and Zika viruses (ZIKV) have become public health concerns after causing numerous large outbreaks worldwide. First identified in Tanzania (East Africa) in 1954, CHIKV is an alphavirus belonging to the Togaviridae family that spreads over 100 countries [1]. Common symptoms such as acute onset fever with severe arthralgia occur in approximately 72% - 95% of infected patients. In particular, joint pain developed from CHIKV can last from a few days to months or years [2]. Whereas ZIKV, a flavivirus belonging to the Flaviviridae family, was first isolated in 1947, it is estimated that 1.62 million people are infected in >70 countries [3]. Most ZIKV infection is asymptomatic or presents only mild clinical disease that resolves within a few days; however, several studies have shown that ZIKV is associated with congenital disorders during pregnancy and Guillain-Barre syndrome (GBS) [4,5]. There is currently no specific antiviral drug treatment or vaccine prevention for these viruses; therefore, only supportive care can be provided when symptoms develop. CHIKV and ZIKV circulate in two transmission cycles, the sylvatic and urban cycles. In the sylvatic cycle, CHIKV and ZIKV spread between NHPs and other wild animals in forest habitats through arboreal mosquito bites without causing symptoms [6]. A recent study of seroprevalence in NHPs in Senegal suggests that NHPs play a role as an amplification host for viral replication [7], highlighting the probability that the viral load is amplified before transmission to other hosts. Humans are an incidental host for many arboviruses, and urban transmission was formed based on the viral adaptation of the human population and the preference for vector mosquito feeding [8]. For example, the dengue virus (DENV) has fully adapted to the urban cycle and no longer requires NHP for virus maintenance in some locations [9]. Unlike DENV, the role of NHPs in the CHIKV and ZIKV transmission cycle remains unclear. Insufficient knowledge of these has challenged the potential risk to the NHP community. To delineate a public health control program, investigating the NHPs involved in the CHIKV and ZIKV transmission cycles is essential. Although several studies have investigated evidence of CHIKV and ZIKV infection in NHPs, there is a high heterogeneity between studies with respect to the study site, sampling year and sample size, NHP species and laboratory testing method. In addition, infected NHPs have no disease symptoms and relatively short arbovirus viremia; serological assays are essential to investigate possible CHIKV and ZIKV sylvatic transmission in NHPs [10]. Therefore, this systematic review and meta-analysis study aims to gather all the available evidence for CHIKV and ZIKV infection in NHPs investigated by serological examination to evaluate and compare CHIKV and ZIKV seroprevalence in NHPs on three continents: Asia, Africa, and America.
2. Materials and methods
2.1. Search strategy
This study was carried out according to the guidelines of the preferred reporting items for systematic review and meta-analysis (PRISMA) [11]. To prevent the same objective from being achieved with previous publications, the keywords ‘systematic review’ and ‘chikungunya virus’ or ‘zika virus’ were searched in the database. A publication search was performed on the Embase, PubMed, and Scopus databases. The following keywords: chikungunya, CHIKV, Zika, ZIKV, arbovirus, mosquito-borne, seroprevalence, serosurvey, seroepidemiology, prevalence, antibody, animal, NHPs, monkeys, and macaques were established for exploration. References from selected papers were selected for additional studies that may not be included in the database. The import of references and the removal of duplicates were performed using the Endnote version X9 bibliographic software package (Thomas Reuters, New York, NY, USA).
2.2. Inclusion and exclusion criteria
The focus was on publications of the seroprevalence of CHIKV and ZIKV in NHPs. Two independent reviewers screened the title and abstract of all selected studies. Original articles with a full text published in English were included. Exclusion criteria included studies with duplicate articles, review articles, short reports, clinical studies in animal models, vaccine trials, case reports, and abstracts alone. The total number of studies searched was compared between two reviewers.
2.3. Data extraction and evaluation of the quality of studies
The full text of all eligible studies was reviewed by two study authors, after which the following information was extracted and recorded in excel: author, year of sampling, year of publication, geographical region, NHP species, serological test, sample size, number of seropositive samples, and percentage of seropositivity. The data were re-checked and confirmed by two reviewers. If a difference between the two reviewers occurred, the result was determined by a third-party reviewer. The quality of each eligible study was evaluated using our grading system. Four criteria were used to evaluate the quality of the publication. For each criterion, eligible studies were assigned a score of 2. The classification criteria used in this study include the objective and the research question (clear = 2, unclear = 1), the details of the sampling method (clear = 2, unclear = 1), sample size (>100 = 2, <100 = 1), and the validation of the serological test (neutralization test/ plaque reduction neutralization test = 2, hemagglutination test/ ELISA and another test = 1). These grading criteria were adapted from other published studies [12]. Two reviewers graded and recorded each included study's quality and total scores. The quality score results of two reviewers were compared, and a third-party reviewer adjusted inconsistent results.
2.4. Statistical analysis
A meta-analysis was performed to combine 1) CHIKV seroprevalence and 2) ZIKV seroprevalence between studies. Heterogeneity was observed at the collection site, sample size, species of NHPs, and serological test. Consequently, we applied the random effects model with Freeman-Turkey double arcsine transformation to obtain variance stability [13]. The pooled seroprevalence with a 95% confidence interval of CHIKV and ZIKV in NHPs was presented in forest plots using the ‘meta’ package in the R statistical software version 4.0.2. Publication bias was evaluated using Egger's test and presented in funnel plots. An asymmetry funnel plot with p < 0.05 in Egger's test indicates evidence of publication bias [14].
3. Results
3.1. Characteristics of included studies
3.1.1. CHIKV seroprevalence in NHPs
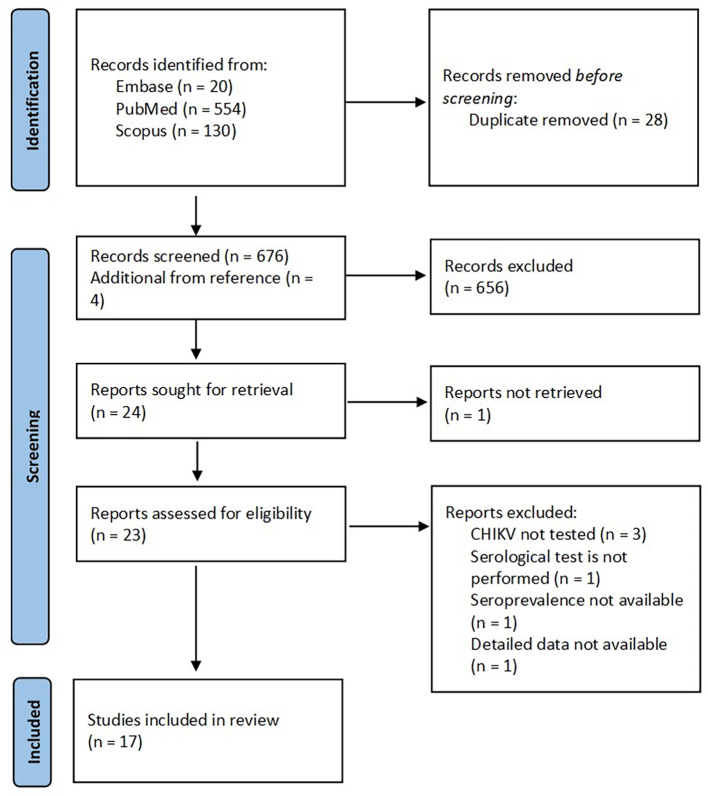
A total of 704 studies were found during our database search for CHIKV seroprevalence in NHPs. Twenty-eight duplicate studies were removed and 687 articles were excluded due to conflicting study objectives and unclear methodology. The remaining 17 studies were reviewed. The schematic flow of the study selection process is presented in Fig. 1, and the characteristics of the included studies are presented in Table 1. All 17 included studies published between 1964 and 2021 were conducted on three continents: Africa (Southern Rhodesia [15], Uganda [16], Congo [17], Reunion Island, Mauritius, and Mayotte [18], Kenya [19], Senegal [7,20], Cameroon and Congo [21]), America (United States of America [22], Brazil [23], Saint Kitts [24]), and Asia (Sri Lanka [25], Malaysia [26,27], Philippines [28], Thailand [10,29]). Most of the studies were of high quality, with scores of 7 and 8. Ten studies used a plaque reduction neutralization test (PRNT) to measure CHIKV antibodies. The remaining studies detected CHIKV antibodies with a Hemagglutination Inhibition Test (HI), Enzyme-Linked Immunosorbent Assay (ELISA), Luminex beads, and immunofluorescence Assay (IFA). The NHPs tested were Old World monkeys (96%) belonging to the genus Allenopithecus, Cercopithecus, Chlorocebus, Erythrocebus, Eulemur, Gorilla, Macaca, Mandrillus, Pan, Papio, and orangutans (Pongo).
Fig. 1.
Flow diagram for search and selection process of CHIKV seroprevalence in NHPs.
Table 1.
Seroprevalence of chikungunya (CHIKV) and zika virus (ZIKV) among non-human primates.
| Region | Year of study | Non-human primates | Assay | Seropositive(n) | Sample size(n) | % seropositive | References |
|---|---|---|---|---|---|---|---|
| CHIKV seroprevalence studies among non-human primates | |||||||
| Rhodesia | 1962 | Vervet Monkey (Cercopithecus aethiops pygerythrus), Baboon (Papio ursinus) |
HI | 12 | 15 | 80 | [15] |
| USA | Not present | Chimpanzee (Pan troglodytes), Gorilla (Gorilla gorilla), Macaca mulatta, Cercopithecus sp., Baboon (Papio sp.) | Plaque-inhibition, CF, HI | 36 | 136 | 26.47 | [46] |
| Uganda | 1969 | Vervet monkeys (Cercopithecus aethiops spp.), Redtail monkeys (Cercopithecus ascanius sups.schmidti Matschie) | HI | 25 | 36 | 69.44 | [16] |
| Sri Lanka | 1987 | Toque macaques (Macaca sinica) | PRNT | 0 | 115 | 0 | [25] |
| Malaysia | 1996, 1997 | Orangutans (Pongo) | PRNT | 0 | 71 | 0 | [41] |
| Philippines | 1999 | Macaca fascicularis | ELISA | 32 | 54 | 59.3 | [28] |
| Congo | 1991 and 2009 2001 and 2009 |
Mandrills (Mandrillus sphinx), Mountain gorillas (Gorilla beringei beringei), Grauer's gorillas (Gorilla beringei graueri), L'Hoest's monkeys (Cercopithecus lhoesti), Golden monkeys (Cercopithecus kandti), Chimpanzees (Pan troglodytes) |
PRNT | 2 | 69 | 2.89 | [17] |
| Reunion Island, Mauritius and Mayotte. | 2006–2007 | Brown lemur (Eulemur fulvus) Crab-eating macaques (Macaca fascicularis) Hamadryas Baboon (Papio hamadryas) Southern Pig-tailed Macaque (Macaca nemestrina) Campbell's Monkey (Cercopithecus campbelli) |
ELISA, IFA | 4 | 181 | 2.21 | [18] |
| Northern Thailand | 2008–2009 | Northern pig-tailed macaques (Macaca nemestrina leonina) | PRNT | 4 | 38 | 10 | [29] |
| Malaysia | 2009 and 2010 | Long-tailed macaques (Macaca fascicularis) | IFA | 1 | 147 | 0.7 | [27] |
| Kenya | 1985–2000 and 2014 | Olive baboon (Papio anubis), Vervet monkeys (Chlorocebus aethiops), Blue monkey (Cercopithecus mitis), red-tailed monkey (Cercopithecus ascanius), Yellow baboon (Papio cynocephalus) |
PRNT | 43 | 319 | 13.4 | [19] |
| Senegal | 2010 | African green monkeys (Chlorocebus sabaeus), Patas monkeys (Erythrocebus patas), Guinea baboons (Papio papio) | PRNT | 96 | 116 | 82.76 | [20] |
| Senegal | 2010–2012 | Chlorocebus sabaeus, Erythrocebus patas, Papio papio | PRNT | 479 | 667 | 72 | [7] |
| Brazil | 2013–2014 | Aotidae, Atelidae, Callitrichidae, Cebidae, Pitheciidae | PRNT | 11 | 207 | 5.3 | [23] |
| Caribbean island | 2013, 2019 | African green monkey (Chlorocebus sabaeus) | ELISA, PRNT | 0 | 858 | 0 | [24] |
| Thailand | 2018 | Northern pig-tailed macaques (Macaca leonina), Stump-tailed macaques (Macaca arctoides), Long-tailed macaques (Macaca fascicularis) |
PRNT | 1 | 62 | 1.6 | [10] |
| Cameroon and Congo | 1999 and 2016 | Allan's swamp monkey (Allenopithecus nigroviridis), Agile mangabey (Cercocebus agilis), Red capped mangabey (Cercocebus torquatus), Angolan colobus (Colobus angolensis), Mantled guereza (Colobus guereza), Black colobus (Colobus satanas), Tshuapa red colobus (Piliocolobus tholloni), Red tailed monkey (Cercopithecus ascanius), moustached monkey (Cercopithecus cephus), Hamlyn's monkey (Cercopithecus hamlyni), L'Hoest's monkey (Allochrocebus lhoesti), Blue monkey (Cercopithecus mitis), Mona monkey (Cercopithecus mona), De Brazza's monkey (Cercopithecus neglectus), Greater spot-nosed monkey (Cercopithecus nictitans), Crested mona monkey (Cercopithecus mona), Preuss's monkey (Allochrocebus preussi), Wolf's monkey (Cercopithecus wolfi), Tantalus monkey (Chlorocebus tantalus), Patas monkey (Erythrocebus patas), Grey-cheecked mangabey (Lophocebus albigena), Black mangabey (Lophocebus aterrimus), Mandrill (Mandrillus leucophaeus), Northern talapoin (Miopithecus spp.), Olive baboon (Papio anubis) |
IgG Luminex beads assay | 67 | 2100 | 3.2 | [21] |
| ZHIKV seroprevalence studies among non-human primates | |||||||
| Nigeria | 1971, 1972 | Monkeys (Data not show) | HI, NT | 20 | 30 | 67 | [30] |
| Borneo, Malaysia | 1996, 1997 | Orangutans | PRNT | 6 | 71 | 8.5 | [26] |
| South Africa (Gambia, Tanzania, Zambia) |
Tanzania in 1985,1986 Gambia, Zambia in 2010,2014 |
Chacma-Kinda hybrid baboons (Papio kindae x Papio ursinus griseipes), Yellow baboon (Papio cynocephalus), African green monkey (Chlorocebus sabaeus) | ELISA | 6 | 239 | 3 | [31] |
| Brazil | 2006 through 2014 | Leontopithecus chrysomelas, Sapajus xanthosternos | HI | 6 | 110 | 5.45 | [34] |
| Brazil | 2012 through 2017 | Family Aotidae, Atelidae, Callitrichidae, Cebidae, Pitheciidae | PRNT | 6 | 207 | 2.9 | [23] |
| Brazil | June 2015 and January 2016 | Capuchin monkeys (Sapajus libidinosus), Free-ranging monkey (Sapajus flavius) | PRNT | 2 | 49 | 4.08 | [35] |
| West-Central Brazil | February 2017 to March 2018 | Ateles marginatus, Sapajus cay | PRNT | 3 | 78 | 3.8 | [36] |
| Northeast Brazil | June 2015 to December 2016 | Capuchin monkeys (Sapajus libidinosus), Marmosets (Callithrix jacchus), Common squirrel monkeys (Saimiri sciureus), Common woolly monkey (Lagothrix lagotricha), Spider monkey (Ateles paniscus), Night monkey (Aotus sp.) | PRNT | 2 | 117 | 1.7 | [37] |
| Zambia | 2009,2010 |
Chacma baboons (Papio ursinus), Zambian malbrouck monkeys (Chlorocebus cynosuros), Yellow baboons (Papio cynocephalus) |
PRNT | 33 | 96 | 34.4 | [32] |
| Malaysia | 2009 through 2010,2016 | Long-tailed macaques (Macaca fascicularis) | PRNT | 3 | 234 | 1.3 | [40] |
| Southeast Brazil | 2015 and 2018 | Callithrix jacchus, Alouatta g. clamitans, Leontopithecus rosalia, Brachyteles arachnoides | PRNT | 0 | 118 | 0 | [38] |
| Thailand | 2018 | Northern pig-tailed macaques (Macaca leonina), Stump-tailed macaques (Macaca arctoides), Long-tailed macaques (Macaca fascicularis) | PRNT | 6 | 62 | 9.67 | [10] |
| St. Kitts, West Indies Caribbean island | 2013 and 2019 | Chlorocebus aethiops sabeus | ELISA, PRNT | 0 | 590 | 0 | [24] |
| Costa Rica | 2000 to 2008, 2014 to 2015 | howler monkeys (Alouatta palliata), spider monkeys (Ateles geoffroyi), squirrel monkeys (Saimiri oerstedii), white-faced monkey (Cebus imitator) | micro-PRNT | 0 | 86 | 0 | [39] |
| Cameroon and Congo | 1999 to 2006 |
Allan's swamp monkey (Allenopithecus nigroviridis), Agile mangabey (Cercocebus agilis), Red capped mangabey (Cercocebus torquatus), Angolan colobus (Colobus angolensis), Mantled guereza (Colobus guereza), Black colobus (Colobus satanas), Tshuapa red colobus (Piliocolobus tholloni), Red tailed monkey (Cercopithecus ascanius), moustached monkey (Cercopithecus cephus), Hamlyn's monkey (Cercopithecus hamlyni), L'Hoest's monkey (Allochrocebus lhoesti), Blue monkey (Cercopithecus mitis), Mona monkey (Cercopithecus mona), De Brazza's monkey (Cercopithecus neglectus), Greater spot-nosed monkey (Cercopithecus nictitans), Crested mona monkey (Cercopithecus mona), Preuss's monkey (Allochrocebus preussi), Wolf's monkey (Cercopithecus wolfi), Tantalus monkey (Chlorocebus tantalus), Patas monkey (Erythrocebus patas), Grey-cheecked mangabey (Lophocebus albigena), Black mangabey (Lophocebus aterrimus), Mandrill (Mandrillus leucophaeus), Northern talapoin (Miopithecus spp.), Olive baboon (Papio anubis) |
Luminex assay | 65 | 2100 | 3 | [21] |
| Côte d'Ivoire | 2006 and 2016 | King colobus (Colobus polycomos), Western red colobus (Piliocolobus badius), Sooty mangabey (Cercocebus atys), Chimpanzees (Pan troglodytes verus) | ECLIA, PRNT | 3 | 48 | 6.25 | [33] |
HI: hemagglutination inhibition, CF: complement fixation test, PRNT: Plaque reduction neutralization test, ELISA: Enzyme linked immunosorbent assay, IFA: Immunofluorescence assay, NT: Neutralization test, ECLIA:Electrochemiluminescence immunoassay.
3.1.2. ZIKV seroprevalence in NHPs
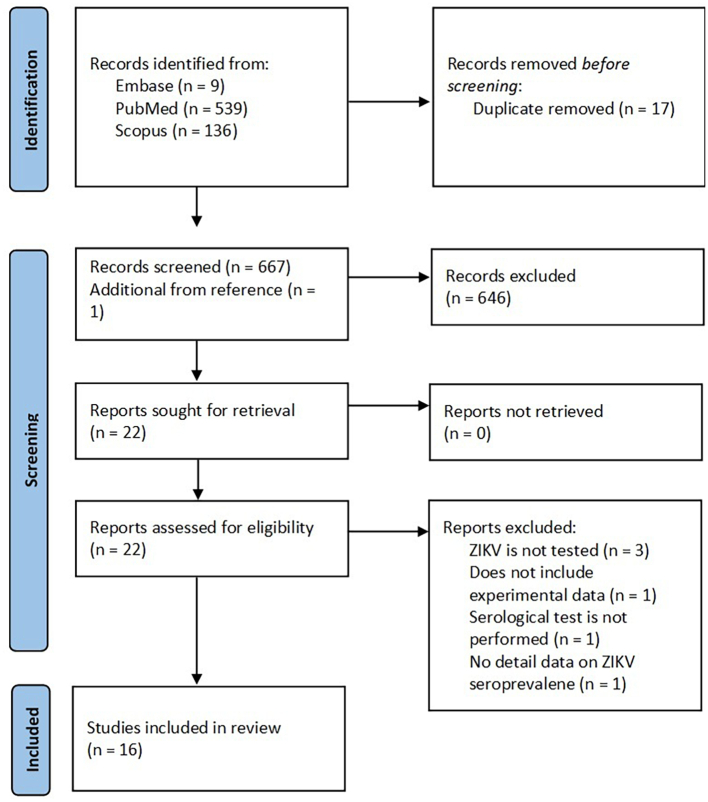
Regarding the seroprevalence of ZIKV in NHPs, 684 articles were found in the database search. We excluded 17 duplicate studies and 646 articles after screening the titles and abstracts. Furthermore, a study was added to the screened article reference list that was consistent with our study objective. The full text of the 22 remaining articles was examined and 6 articles were excluded due to the absence of serological tests and unclear experimental data. The schematic flow of the ZIKV study selection process is presented in Fig. 2. Data from the 16 included studies were extracted (Table 1). Most eligible studies have met the quality criteria. The scores obtained from these studies ranged from 5 to 8. All 16 studies published between 1977 and 2022 were carried out in Africa (Nigeria [30], Gambia, Tanzania, Zambia [31,32], Cameroon and Congo [21], Côte d'Ivoire [33]), Americas (Brazil [23,[34], [35], [36], [37], [38]], Saint Kitts [24], Costa Rica [39]), and Asia (Malaysia [26,40], Thailand [10]). Old World monkeys were the most recruited NHP species (76%), and nearly all studies measured ZIKV antibodies by PRNT. Other serological tests, such as HI, ELISA, Electrochemiluminescence Immunoassay (ECLIA), and the Luminex assay, were also performed.
Fig. 2.
Flow diagram for search and selection process of ZIKV seroprevalence in NHPs.
3.2. The pooled and subgroup seroprevalence of CHIKV and ZIKV in NHPs
3.2.1. Pooled CHIKV seroprevalence in NHP
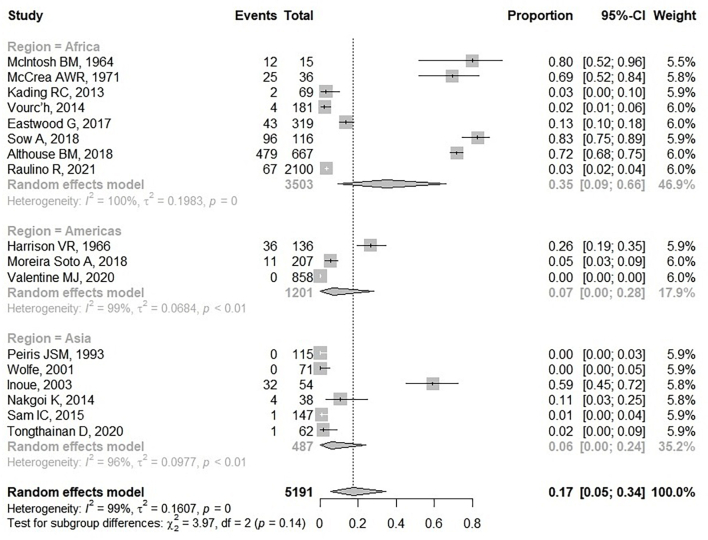
In the 17 CHIKV seroprevalence studies included in NHP studies, 5191 NHPs were collected between 1962 and 2019 for the detection of anti-CHIKV antibodies. Eight hundred and thirteen (15.67%) NHPs were seropositive for CHIKV ranging from 0.7 to 82.76% (Table 1). Studies in Senegal, Rhodesia, Uganda and the Philippines presented higher levels of CHIKV seropositivity than in other countries. CHIKV seronegative NHPs were reported in three included studies from Polonnaruwa in Sri Lanka [25], Borneo in Malaysia [41] and St. Kitts in the West Indies [24]. Sixteen per cent (802/4984) of Old World monkeys of the genus Cercopithecus, Chlorocebus, Erythrocebus, Macaca, Mandrillus, Pan, Papio and Patas were positive for the anti-CHIKV antibody. Cercopithecus and Chlorocebus monkeys were the main NHPs tested. High CHIKV seropositive rates were found in the genus Papio (474/547, 86.65%), Erythrocebus (75/101, 74.26%) and Pan (23/37, 62.16%). However, Old World monkeys in the genus Allenopithecus, Allochrocebus, and Miopithecus were CHIKV seronegative. Two hundred and seven monkeys from the New World were captured and tested for the CHIKV antibody. Of these, 11 monkeys (11/207, 5.3%) of the genus Atelidae, Callitrichidae, and Cebidae were CHIKV seropositive. The pool CHIKV seroprevalence in worldwide NHPs was presented in a forest plot (Fig. 3). Meta-analysis of these 17 included studies showed that the combined seroprevalence of CHIKV in NHP was 17% (95%CI: 5–34). There was significant heterogeneity in CHIKV seroprevalence in NHP (I2: 99%, p < 0.05). A subgroup analysis based on different study regions was performed. The result showed that the highest CHIKV seroprevalence was observed in African NHPs at 35% (95% CI: 9–66; I2 = 100%; p < 0.05). In the Americas and Asia, NHPs presented low CHIKV seroprevalence; 7% (95% CI 0–28; I2 = 99%; p < 0.05) and 6% (95% CI: 0–24; I2 = 96%; p < 0.01), respectively.
Fig. 3.
Forest plot of pooled CHIKV seroprevalence among NHPs.
3.2.2. Pooled ZIKV seroprevalence in NHP
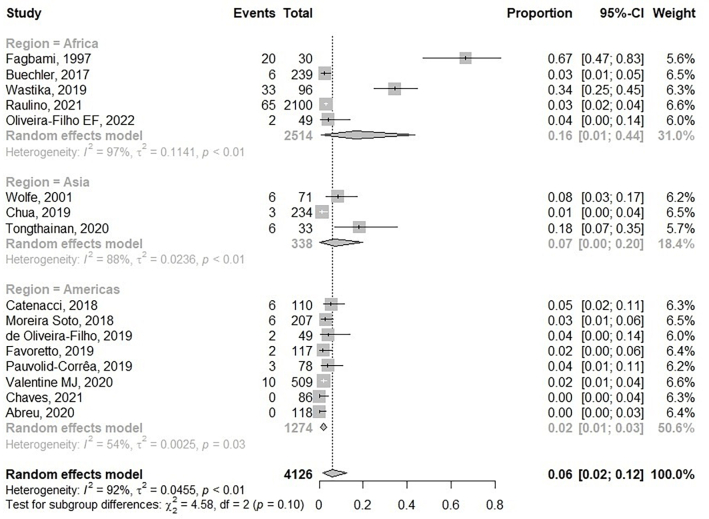
Of the 4235 NHPs tested, 161 were found to be ZIKV seropositive (3.8%). ZIKV seropositive NHP ranged from 1.3 to 67% (Table 1). Old World monkeys of the genera Cercopithecus, Papio, Pongo, Macaca, Chlorocebus, Allenopithecus, Cercocebus, Colobus, Lophocebus, and Miopithecus were reported to have seropositive ZIKV ranging from 1 to 8.5%. The highest rate of ZIKV seropositives was in the genus Papio (19/119, 15.97%). In contrast, the seropositive ZIKV in New World monkeys was 4.5% (48/1062). Sapajus and Atelidae monkeys had high ZIKV seropositive rates at 14.29% and 12.50%, accordingly. Most of the New World monkeys tested had low ZIKV ranging from 0 to 5%. A high seropositivity for ZIKV in NHP at 67% was observed in Nigeria. According to the meta-analysis, the pooled seroprevalence of ZIKV among NHP was 6% (95% CI: 2–12), with high heterogeneity between studies (I2: 92%, p < 0.01) (Fig. 4). In the subgroup analysis, the highest ZIKV seroprevalence was also present in Africa at 16% (95% CI 1–44; I2 = 97; p < 0.01). In Asia, the pooled seroprevalence of ZIKV in NHP was 7% (95% CI 0–20; I2 = 88; p < 0.01), which was higher than in America at 2% (95% CI 1–3; I2 = 54: p < 0.05).
Fig. 4.
Forest plot of pooled ZIKV seroprevalence among NHPs.
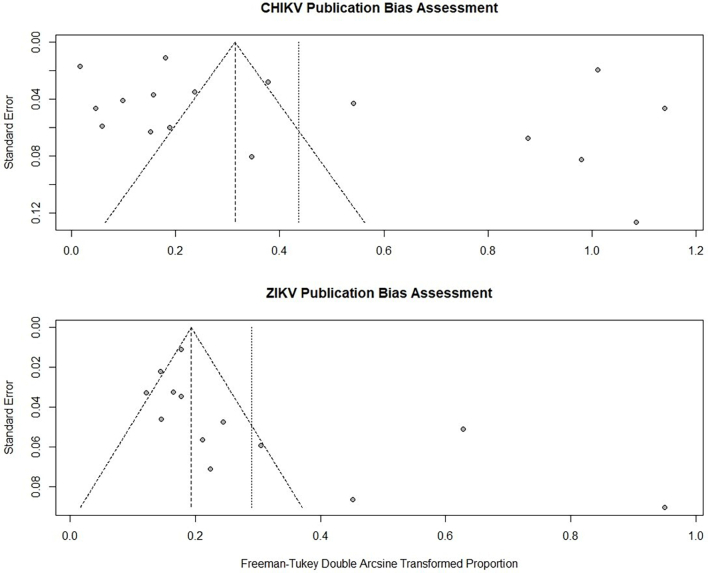
3.3. Publication bias evaluation
We evaluated the publication bias of all included studies using Egger's test and presented it with funnel plots. No evidence of publication bias was observed in the CHIKV studies (pegger = 0.36, Fig. 5 top). On the other hand, an asymmetrical funnel plot of ZIKV was observed (pegger < 0.05, Fig. 5 lower panel), indicating publication bias in included studies of ZIKV seroprevalence in NHPs.
Fig. 5.
Funnel plots for publication bias evaluation by Egger's test in CHIKV (top) and ZIKV (lower panel) seropositive study in NHPs.
4. Discussion
CHIKV and ZIKV are emerging mosquito-borne viruses. The human population in several areas, including Asia, Europe and the Americas, has been affected by CHIKV and ZIKV infection. These viruses circulate in forest habitats by the sylvatic cycle and in urban areas by the urban cycle. In the sylvatic cycle, CHIKV and ZIKV can infect NHP by feeding arboreal mosquitoes [3,42]. Although several studies have evidence that NHPs are susceptible to CHIKV and ZIKV infection, the potential role of NHPs in the transmission of CHIKV and ZIKV remains ambiguous. To better understand the role of NHPs in emerging viruses, CHIKV and ZIKV, we performed a systematic review and meta-analysis of the seroprevalence of CHIKV and ZIKV in NHPs. We evaluated 17 and 16 studies worldwide for evidence of CHIKV and ZIKV infection in NHP, respectively. Based on this review, CHIKV and ZIKV can infect multiple NHPs, as illustrated by the pooled seroprevalence, which was 17% and 6%, respectively. We also discovered that NHPs in developing countries, especially Africa, have a high seroprevalence of CHIKV and ZIKV in NHPs. Like Africa, an endemic area for CHIKV circulation has a higher pooled CHIKV seroprevalence in NHP than in the Americas or Asia. The seroprevalence of CHIKV in NHPs has been investigated since 1962 in southern Rhodesia [15] and since 1971 in Uganda [15]. The outcome has suggested that NHPs in these regions have a high CHIKV seropositive rate of up to 70–80%. Evidence of CHIKV seroprevalence studies in Senegal shows that NHPs in these areas were highly infected with CHIKV with a high level of anti-CHIKV antibody titer [7]. CHIKV has been isolated from multiple species of NHP and forest-dwelling mosquitoes in Senegal between 1972 and 1983 [43]. These results suggest that monkeys may serve as amplification hosts in the CHIKV sylvatic cycle rather than reservoirs [7,20]. Although the study in Rhodesia, Uganda, and Senegal presented a high CHIKV seroprevalence in NHP, low CHIKV seropositive rates have been reported in Cameroon, Congo, Reunion Island, Mauritius, and Mayotte. These contradictory results were also observed in a recent systematic review and meta-analysis evaluating the seroprevalence of CHIKV and ZIKV worldwide in humans by Li et al. [1]. The study reported that the highest CHIKV seroprevalence in humans was found in the South-East Asian region rather than Africa. In Africa, the highest CHIKV seroprevalence in humans was found in Cameroon (68%), and the lowest CHIKV seroprevalence was found in Gabon, Mali, and Senegal, ranging from 1 to 3% [1]. For the seroprevalence of ZIKV in NHPs, our results show that high ZIKV seropositive NHPs were found in Nigeria in 1971 and Zambia in 2009. However, these results may be unreliable because NHPs that are ZIKV seropositive in the study in Nigeria also showed DENV-2 infection [30]. A recent publication mentioned that African ZIKV strains have higher transmissibility in mosquito vectors compared to Asian strains, possibly explaining the higher seroprevalence of ZIKV in Africa [44]. Although ZIKV was first isolated from a caged rhesus macaque and a forest mosquito, Aedes Africanus, in Africa [44]. But low seropositive NHPs with ZIKV were observed in some areas such as Tanzania, Cameroon and Congo. In the Americas, CHIKV and ZIKV were introduced in 2013 and 2015, respectively [44,45]. Our study showed that low CHIKV and ZIKV seroprevalence was found in NHPs on this continent. A study conducted in 1966 in the United States reported a high rate of seropositive CHIKV in NHP (26.47%). However, most of the NHPs were imported from Africa, India, Malaysia, Borneo, and Ceylon (Sri Lanka) [46]. Therefore, the high CHIKV seropositivity in these NHPs may result from prior infection in highly endemic areas before immigration. On the other hand, most of the included publications on ZIKV seroprevalence in the Americas were performed in Brazil (6 publications). The differences between the seroprevalence of ZIKV in NHP ranged from 0 to 5.45%. The low seroprevalence of ZIKV in NHPs in Brazil, Costa Rica, and the Caribbean Islands may indicate that the sylvatic cycle of ZIKV in these regions is untraceable. CHIKV was introduced to Asia in 1958 [42]. According to our analysis, CHIKV seropositive NHPs in this region ranged between 0 and 59%. Although CHIKV was reported to cause a significant epidemic in Sri Lanka in 1965, CHIKV seronegative in NHPs was reported in a study in 1987 in Sri Lanka [25]. In Malaysia, NHPs have a low seroprevalence of CHIKV, suggesting that CHIKV may not be an enzootic disease in this area [26,27]. Only three publications performed ZIKV studies in NHPs in Asia. In Malaysia and Thailand, a high seroprevalence of ZIKV in NHP was presented, and previous research in Malaysia highlighted that orangutans could be infected with ZIKV [26]. The ZIKV antibody was also detected in stump-tailed macaques living in Thailand's national parks [10], which could be a consequence of spillover infection from human populations. Old World and New World monkeys were tested for CHIKV and ZIKV antibodies. Of all monkeys tested, the Old World monkeys had a higher percentage of seroprevalence for CHIKV and ZIKV than those found in the New World monkeys. Several factors, including group size, movement between groups, sexual selection (animal mating) in NHP, mosquito vector distribution and laboratory assay, were discovered to influence the seroprevalence rate [47]. PRNT is the gold standard method due to its high specificity and sensitivity [48]. However, ELISA and HI, which are less specific in reducing the cross-reaction between ZIKV and other flaviviruses, were used in many studies that may affect the precision of the results.
5. Limitations
The limitations of this investigation may include the availability of data, the high heterogeneity between searches, and current assessable serological tests. First, the CHIKV and ZIKV seroprevalence data can only be evaluated from publications in online databases. Therefore, unpublished data may be missing from the analysis. Second, this study shows a high heterogeneity in factors such as date and location of study and sampling, serological tests, NHP species, sample size, and so on. Third, there is the issue of cross-reaction between the ZIKV and DENV antibodies. The current gold standard for ZIKV antibody detection recommended by the WHO is the PRNT test with identification criteria. However, the laboratory technique used to differentiate between ZIKV and DENV is less specific. For that reason, the high rate of ZIKV seropositive in NHP reported in some studies may result from cross-reaction with DENV. Finally, limitations in the study, laboratory testing, and sample collection can lead to an underestimating of the seroprevalence of CHIKV and ZIKV infection.
6. Conclusions
This is the first systematic review and meta-analysis of the seroprevalence of CHIKV and ZIKV in NHPs. Evidence of NHPs infected with CHIKV and ZIKV suggests the involvement of NHPs in the transmission cycle and viral maintenance in the environment. Therefore, understanding the reservoirs of these viruses is essential for public health control programs.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
All the data generated or analyzed during this study are included in this published article.
Funding statement
The National Science and Technology Development Agency (NSTDA), Thailand, financially supported the study through the Asia joint research program (grant number FDA-CO-2562-9880-TH). Additionally, FW was supported by the German Academic Exchange Service (DAAD), and NM was supported by a Siam University scholarship.
Authors' contributions
The conception of the research idea and the design of the research protocol (KB, PC and NM), literature review (NM and FW), publication search (NM, FW, SS and OL), publication screening and data extraction (NM and FW), third-party reviewer (KB), statistical analysis (FW and PC), data analysis and interpretation of results (NM, FW, KB and PC), manuscript drafting (NM and FW), and review of the final draft of the manuscript (KB and PC).
Credit authorship contribution statement
Nanthanida Mongkol: The conception of the research idea and the design of the research protocol, literature review, publication search, publication screening and data extraction, data analysis and interpretation of results, manuscript drafting.
Fanny Sae Wang: literature review, publication search, publication screening and data extraction, statistical analysis, data analysis and interpretation of results, manuscript drafting.
Sarocha Suthisawat: publication search.
Oranit Likhit: publication search.
Pimphen Charoen: The conception of the research idea and the design of the research protocol, statistical analysis, data analysis and interpretation of results, review of the final draft of the manuscript.
Kobporn Boonnak: The conception of the research idea and the design of the research protocol, third-party reviewer, data analysis and interpretation of results, review of the final draft of the manuscript.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Kobporn Boonnak reports financial support was provided by The National Science and Technology Development Agency (NSTDA), Thailand. Fanny Sae Wang reports financial support was provided by German Academic Exchange Service (DAAD). Nanthanida Mongkol reports financial support was provided by Siam University.
Data availability
Data will be made available on request.
References
- 1.Li Z., Wang J., Cheng X., Hu H., Guo C., et al. The worldwide seroprevalence of DENV, CHIKV and ZIKV infection: a systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2021;15(4) doi: 10.1371/journal.pntd.0009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matusali G., Colavita F., Bordi L., Lalle E., Ippolito G., et al. Tropism of the chikungunya virus. Viruses. 2019;11(2) doi: 10.3390/v11020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pielnaa P., Al-Saadawe M., Saro A., Dama M.F., Zhou M., et al. Zika virus-spread, epidemiology, genome, transmission cycle, clinical manifestation, associated challenges, vaccine and antiviral drug development. Virology. 2020;543:34–42. doi: 10.1016/j.virol.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Dos Santos T., Rodriguez A., Almiron M., Sanhueza A., Ramon P., et al. Zika Virus and the Guillain-Barré syndrome - case series from seven countries. N. Engl. J. Med. 2016;37516:1598–1601. doi: 10.1056/NEJMc1609015. [DOI] [PubMed] [Google Scholar]
- 5.Brasil P., Pereira J.P., Jr., Moreira M.E., Ribeiro Nogueira R.M., Damasceno L., et al. Zika virus infection in pregnant women in Rio de Janeiro. N. Engl. J. Med. 2016;375(24):2321–2334. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valentine M.J., Murdock C.C., Kelly P.J. Sylvatic cycles of arboviruses in non-human primates. Parasit. Vectors. 2019;12(1):463. doi: 10.1186/s13071-019-3732-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Althouse B.M., Guerbois M., Cummings D.A.T., Diop O.M., Faye O., et al. Role of monkeys in the sylvatic cycle of chikungunya virus in Senegal. Nat. Commun. 2018;9(1):1046. doi: 10.1038/s41467-018-03332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollidge B.S., Gonzalez-Scarano F., Soldan S.S. Arboviral encephalitides: transmission, emergence, and pathogenesis. J. NeuroImmune Pharmacol. 2010;5(3):428–442. doi: 10.1007/s11481-010-9234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gubler D.J. The global emergence/resurgence of arboviral diseases as public health problems. Arch. Med. Res. 2002;33(4) doi: 10.1016/s0188-4409(02)00378-8. [DOI] [PubMed] [Google Scholar]
- 10.Tongthainan D., Mongkol N., Jiamsomboon K., Suthisawat S., Sanyathitiseree P., et al. Seroprevalence of Dengue, Zika, and Chikungunya viruses in wild monkeys in Thailand. Am. J. Trop. Med. Hyg. 2020;103(3):1228–1233. doi: 10.4269/ajtmh.20-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int. J. Surg. 2021;88 doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 12.Ding H., Gao Y.M., Deng Y., Lamberton P.H., Lu D.B. A systematic review and meta-analysis of the seroprevalence of toxoplasma gondii in cats in mainland China. Parasit. Vectors. 2017;10(1):27. doi: 10.1186/s13071-017-1970-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barendregt J.J., Doi S.A., Lee Y.Y., Norman R.E., Vos T. Meta-analysis of prevalence. J. Epidemiol. Community Health. 2013;67(11):974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 14.Egger M., Davey S.G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McIntosh B.M., Paterson H.E., McGillivray G., Desousa J. Further Studies on the Chikungunya Outbreak in Southern Rhodesia in 1962. I. Mosquitoes, Wild Primates and Birds in Relation to the Epidemic. Ann. Trop. Med. Parasitol. 1964;58:45–51. doi: 10.1080/00034983.1964.11686213. [DOI] [PubMed] [Google Scholar]
- 16.McCARE A.W.R., Henderson B.E., Kirya B.G., Sempala S.D.K. Chikungunya virus in the entebbe area of Uganda:Isolations and epidemiology. Trans. R. Soc. 1971;65 doi: 10.1016/0035-9203(71)90212-4. [DOI] [PubMed] [Google Scholar]
- 17.Kading R.C., Borland E.M., Cranfield M., Powers A.M. Prevalence of antibodies to alphaviruses and flaviviruses in free-ranging game animals and nonhuman primates in the greater Congo basin. J. Wildl. Dis. 2013;49(3):587–599. doi: 10.7589/2012-08-212. [DOI] [PubMed] [Google Scholar]
- 18.Vourc’h G., Halos L., Desvars A., Boué F., Pascal M., et al. Chikungunya antibodies detected in non human primates and rats in three Indian Ocean islands after the 2006 ChikV outbreak. Vet. Res. 2014;45 doi: 10.1186/1297-9716-45-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eastwood G., Sang R.C., Guerbois M., Taracha E.L.N., Weaver S.C. Enzootic circulation of chikungunya virus in East Africa: serological evidence in non-human Kenyan Primates. Am. J. Trop. Med. Hyg. 2017;97(5):1399–1404. doi: 10.4269/ajtmh.17-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sow A., Faye O., Diallo M., Diallo D., Chen R., et al. Chikungunya outbreak in Kedougou, southeastern Senegal in 2009-2010. Open Forum. Infect. Dis. 2018;5(1):ofx259. doi: 10.1093/ofid/ofx259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raulino R., Thaurignac G., Butel C., Villabona-Arenas C.J., Foe T., et al. Multiplex detection of antibodies to chikungunya, O’nyong-nyong, Zika, dengue, West Nile and Usutu viruses in diverse non-human primate species from Cameroon and the Democratic Republic of Congo. PLoS Negl. Trop. Dis. 2021;15(1) doi: 10.1371/journal.pntd.0009028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrison V.R., Marshall J.D., Guilloud N.B. The presence of antibody to chikungunya and other serologically related viruses in the sera of sub-human primate imports to the United States. J. Immunol. 1967;98(5):979–981. [PubMed] [Google Scholar]
- 23.Moreira-Soto A., Carneiro I.O., Fische R.C., Feldmann M., Kümmerer B.M., et al. Limited evidence for infection of urban and peri-urban nonhuman primates with Zika and Chikungunya viruses in Brazil. mSphere. 2018;3 doi: 10.1128/mSphere.00523-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valentine M.J., Ciraola B., Aliota M.T., Vandenplas M., Marchi S., et al. No evidence for sylvatic cycles of chikungunya, dengue and Zika viruses in African green monkeys (Chlorocebus aethiops sabaeus) on St. Kitts, West Indies. Parasit. Vectors. 2020;13(1):540. doi: 10.1186/s13071-020-04419-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peiris J.S.M., Dittus W.P.J., Ratnayake C.B. Seroepidemiology of dengue and other arboviruses in a natural population of toque macaques (Macaca sinica) at Polonnaruwa, Sri Lanka. J. Med. Primatol. 1993;22:240–245. [PubMed] [Google Scholar]
- 26.Wolfe N.D., Kilbourn A.M., Karesh W.B., Rahman H.A., Bosi E.J., et al. Sylvatic transmission of arboviruses among Bornean orangutans. Am. J. Trop. Med. Hyg. 2001;64:310–316. doi: 10.4269/ajtmh.2001.64.310. [DOI] [PubMed] [Google Scholar]
- 27.Sam I.C., Chua C.L., Rovie-Ryan J.J., Fu J.Y., Tong C., et al. Chikungunya virus in Macaques, Malaysia. Emerg. Infect. Dis. 2015;21(9):1683–1685. doi: 10.3201/eid2109.150439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inoue S., Morita K., Matias R.R., Tuplano J.V., Resuello R.R.G., et al. Distribution of three arbovirus antibodies among monkeys (Macaca fascicularis) in the Philippines. J. Med. Primatol. 2003;32:89–94. doi: 10.1034/j.1600-0684.2003.00015.x. [DOI] [PubMed] [Google Scholar]
- 29.Nakgoi K., Nitatpattana N., Wajjwalku W., Pongsopawijit P., Kaewchot S., et al. Dengue, Japanese encephalitis and chikungunya virus antibody prevalence among captive monkey (Macaca nemestrina) colonies of northern Thailand. Am. J. Primatol. 2014;76(1):97–102. doi: 10.1002/ajp.22213. [DOI] [PubMed] [Google Scholar]
- 30.Fagbami A.H., Monath T.P., Fabiyi A. Dengue virus infections in Nigeria: a survey for antibodies in monkeys and humans. Trans. R. Soc. Trop. Med. Hyg. 1977;71(1):60–65. doi: 10.1016/0035-9203(77)90210-3. [DOI] [PubMed] [Google Scholar]
- 31.Buechler C.R., Bailey A.L., Weiler A.M., Barry G.L., Breitbach M.E., et al. Seroprevalence of Zika Virus in Wild African Green Monkeys and Baboons. mSphere. 2017;2(2) doi: 10.1128/mSphere.00392-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wastika C.E., Sasaki M., Yoshii K., Anindita P.D., Hang’ombe B.M., et al. Serological evidence of Zika virus infection in non-human primates in Zambia. Arch. Virol. 2019;164(8):2165–2170. doi: 10.1007/s00705-019-04302-0. [DOI] [PubMed] [Google Scholar]
- 33.Oliveira-Filho E.F., Carneiro I.O., Fischer C., Kuhne A., Postigo-Hidalgo I., et al. Evidence against Zika virus infection of pets and peri-domestic animals in Latin America and Africa. J. Gen. Virol. 2022;103(1) doi: 10.1099/jgv.0.001709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Catenacci L.S., Ferreira M., Martins L.C., De Vleeschouwer K.M., Cassano C.R., et al. Surveillance of arboviruses in primates and sloths in the Atlantic Forest, Bahia, Brazil. EcoHealth. 2018;15(4):777–791. doi: 10.1007/s10393-018-1361-2. [DOI] [PubMed] [Google Scholar]
- 35.de Oliveira-Filho E.F., Oliveira R.A.S., Ferreira D.R.A., Laroque P.O., Pena L.J., et al. Seroprevalence of selected flaviviruses in free-living and captive capuchin monkeys in the state of Pernambuco, Brazil, Transbound. Emerg. Dis. 2018;65(4):1094–1097. doi: 10.1111/tbed.12829. [DOI] [PubMed] [Google Scholar]
- 36.Pauvolid-Correa A., Goncalves Dias H., Marina Siqueira Maia L., Porfirio G., Oliveira Morgado T., et al. Zika virus surveillance at the human-animal Interface in west-Central Brazil, 2017-2018. Viruses. 2019;11(12) doi: 10.3390/v11121164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Favoretto S.R., Araujo D.B., Duarte N.F.H., Oliveira D.B.L., da Crus N.G., et al. Zika virus in peridomestic neotropical primates, Northeast Brazil. EcoHealth. 2019;16(1):61–69. doi: 10.1007/s10393-019-01394-7. [DOI] [PubMed] [Google Scholar]
- 38.Abreu F.V.S., Ferreira-de-Brito A., Azevedo A.S., Linhares J.H.R., de Oliveira Santos V., et al. Survey on non-human Primates and mosquitoes does not provide evidences of spillover/spillback between the urban and sylvatic cycles of yellow fever and Zika viruses following severe outbreaks in Southeast Brazil. Viruses. 2020;12(4) doi: 10.3390/v12040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaves A., Piche-Ovares M., Ibarra-Cerdena C.N., Corrales-Aguilar E., Suzan G., et al. Serosurvey of nonhuman Primates in Costa Rica at the human-wildlife Interface reveals high exposure to Flaviviruses. Insects. 2021;12(6) doi: 10.3390/insects12060554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chua C.L., Chan Y.F., Andu E., Rovie-Ryan J.J., Sitam F.T., et al. Little evidence of Zika virus infection in wild long-tailed macaques, peninsular Malaysia. Emerg. Infect. Dis. 2019;25(2):374–376. doi: 10.3201/eid2502.180258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolfe N.D., Kilbourn A.M., Karesh W.B., Rahman H.A., Bosi E.J., et al. Sylvatic transmission of arboviruses among Bornean orangutans. Am. J. Trop. Med. Hyg. 2001;64:310–316. doi: 10.4269/ajtmh.2001.64.310. [DOI] [PubMed] [Google Scholar]
- 42.Weaver S.C., Forrester N.L. Chikungunya: evolutionary history and recent epidemic spread. Antivir. Res. 2015;120:32–39. doi: 10.1016/j.antiviral.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 43.Diallo M., Thonnon J., Traore-Lamizana M., Fontenille D. Vectors of chikungunya virus in Senegal: current data and transmission cycles. Am. J. Trop. Med. Hyg. 1999;60(2):281–286. doi: 10.4269/ajtmh.1999.60.281. [DOI] [PubMed] [Google Scholar]
- 44.Musso D., Gubler D.J. Zika Virus. Clin. Microbiol. Rev. 2016;29(3):487–524. doi: 10.1128/CMR.00072-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yactayo S., Staples J.E., Millot V., Cibrelus L., Ramon-Pardo P. Epidemiology of chikungunya in the Americas. J. Infect. Dis. 2016;214(Suppl. 5) doi: 10.1093/infdis/jiw390. S441-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harrison V.R., Marshall D., Guilloud N.B. The presence of antibody to chikungunya and other serologically related viruses in the sera of sub-human primate imports to the United States. J. Immunol. 1967;98:979–981. [PubMed] [Google Scholar]
- 47.Altizer S., Nunn C.L., Thrall P.H., Gittleman J.L., Antonovics J., et al. Social organization and parasite risk in mammals: integrating theory and empirical studies. Annu. Rev. Ecol. Evol. Syst. 2003;34(1):517–547. [Google Scholar]
- 48.Roehrig J.T., Hombach J., Barrett A.D. Guidelines for plaque-reduction neutralization testing of human antibodies to dengue viruses. Viral Immunol. 2008;21(2):123–132. doi: 10.1089/vim.2008.0007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data generated or analyzed during this study are included in this published article.
Data will be made available on request.