Abstract
The deacetylase sirtuin 1 (Sirt1), activated by calorie restriction and fasting, exerts several complementary effects on cellular function that are favourable to healthspan; it is often thought of as an ‘anti-aging’ enzyme. Practical measures which might boost Sirt1 activity are therefore of considerable interest. A number of nutraceuticals have potential in this regard. Nutraceuticals reported to enhance Sirt1 synthesis or protein expression include ferulic acid, tetrahydrocurcumin, urolithin A, melatonin, astaxanthin, carnosic acid and neochlorogenic acid. The half-life of Sirt1 protein can be enhanced with the natural nicotinamide catabolite N1-methylnicotinamide. The availability of Sirt1’s obligate substrate NAD+ can be increased in several ways: nicotinamide riboside and nicotinamide mononucleotide can function as substrates for NAD+ synthesis; activators of AMP-activated kinase—such as berberine—can increase expression of nicotinamide phosphoribosyltransferase, which is rate limiting for NAD+ synthesis; and nutraceutical quinones such as thymoquinone and pyrroloquinoline quinone can boost NAD+ by promoting oxidation of NADH. Induced ketosis—as via ingestion of medium-chain triglycerides—can increase NAD+ in the brain by lessening the reduction of NAD+ mediated by glycolysis. Post-translational modifications of Sirt1 by O-GlcNAcylation or sulfonation can increase its activity, suggesting that administration of glucosamine or of agents promoting hydrogen sulfide synthesis may aid Sirt1 activity. Although resveratrol has poor pharmacokinetics, it can bind to Sirt1 and activate it allosterically—as can so-called sirtuin-activating compound drugs. Since oxidative stress can reduce Sirt1 activity in multiple ways, effective antioxidant supplementation that blunts such stress may also help preserve Sirt1 activity in some circumstances. Combination nutraceutical regimens providing physiologically meaningful doses of several of these agents, capable of activating Sirt1 in complementary ways, may have considerable potential for health promotion. Such measures may also amplify the benefits of sodium-glucose cotransporter-2 (SGLT2) inhibitors in non-diabetic disorders, as these benefits appear to reflect upregulation of Sirt1 and AMP-activated protein kinase activities.
Keywords: carotid artery diseases, peripheral vascular diseases, atrial fibrillation
Health promotion via sirtuin 1 activation
The type III deacetylase sirtuin 1 (Sirt1) has aroused considerable interest, as its activity has been linked to enhanced healthspan.1–3 Sirt1 is particularly intriguing for its wide-ranging modulatory activities—enhancing autophagy, mitophagy, mitochondrial biogenesis (MB), DNA repair, antioxidant enzyme expression, osteoblast generation and endothelial nitric oxide synthase expression and activity, while inhibiting apoptosis, senescence, de novo lipogenesis, atherogenesis and—via suppression of canonical NF-κB activity—inflammation.4–9 In aggregate, these effects may account for the favourable impact of Sirt1 on healthspan.
With respect to cardiovascular health, Sirt1 opposes atherogenesis both by favourable effects on endothelial function—downregulating inflammation via NF-κB suppression and promotion of endothelial nitric oxide synthase activity—and by opposing foam cell formation by decreasing low-density lipoprotein (LDL) uptake while boosting reverse cholesterol transport.6 7 9–12 Moreover, measures which increase Sirt1 activity have shown benefit in rodent models of ventricular hypertrophy and heart failure.13–20
It is therefore of importance to devise clinical strategies—preferably involving safe nutraceuticals appropriate for use in primary prevention—for enhancing Sirt1 activity. A growing literature suggests that several phytochemicals, natural metabolites and approved drugs have potential in this regard. The following brief review cites the pertinent literature on these agents and attempts to define their likely mechanisms of action. An understanding of these mechanisms may aid the development of complex nutraceutical regimens that can support Sirt1 activity in complementary ways.
Nutraceuticals for increasing Sirt1 synthesis or half-life
Certain nutraceuticals have the potential to increase protein expression of Sirt1 by promoting its synthesis. These include ferulic acid, tetrahydrocurcumin, urolithin A, melatonin, carnosic acid, neochlorogenic acid and astaxanthin. Ferulic acid, tetrahydrocurcumin and urolithin A may be the absorbed metabolites mainly responsible for the health benefits of orally administered anthocyanins, curcumin and pomegranate ellagitanins, respectively.21–23 Sodium ferulate has long been used in Chinese cardiovascular medicine.24 Carnosic acid is a prominent constituent of rosemary extract, and neochlorogenic acid is found in mulberry leaves.25 26 Ferulic acid and tetrahydrocurcumin can boost Sirt1 expression at both the mRNA and protein level; how they accomplish this remains obscure.27–32 Melatonin likewise can enhance mRNA and protein expression of Sirt1; its activity in this regard has been traced to activation of the clock transcription factor Bmal1, which binds to the promoter of the Sirt1 gene and drives its transcription.33–37 Bmal1 activation, in turn, may reflect melatonin’s interaction with its M1 membrane receptor.38 39 Urolithin A, carnosic acid and neochlorogenic acid appear to exert their upregulatory impacts on Sirt1 synthesis by suppressing expression of miR-34a, which binds to the 3’UTR of Sirt1 mRNA and promotes its degradation.40–47 Agents of this type may be of particular interest in cardiovascular medicine, inasmuch as upregulation of miR-34a, in addition to its suppressive effect on Sirt1 expression, works in additional ways to compromise vascular health.48 Astaxanthin is reported to increase protein expression of Sirt1 in a range of rodent tissues; the mechanism responsible remains unclear.49–54
Of ancillary interest is evidence that treadmill exercise training in rodents induces Sirt1 expression at the mRNA and protein level in brain and various other tissues.55–62 In the brain, the effect is mediated at least in part by lactate; the molecular biology underlying this remains obscure.61 It is reasonable to suspect that Sirt1 induction is a key mediator of the broad-ranging neuroprotective effects of aerobic exercise training—effects documented both in rodents and via epidemiology.63–65 Among other benefits, Sirt1 promotes expression of brain-derived neurotrophic factor.61 62
Sirt1 protein expression may also be increased by prolonging its half-life. The stress-inducible MAP kinase c-Jun N-terminal kinase 1 (JNK1) can confer a phosphorylation on Sirt1 (Ser-46) that promotes its ubiquitination and subsequent proteasomal degradation.66 67 N1-methylnicotinamide (MNA) is a natural catabolite of nicotinamide known to have anti-inflammatory properties.68 MNA has been shown to boost Sirt1 protein expression by slowing proteasomal degradation of Sirt1, and this may be traceable to its ability to inhibit phosphorylation of Ser-46.69–72
Nutraceutical enhancement of Sirt1’s substrate NAD+
Sirt1 has an obligate requirement for NAD+ as a substrate. Hence, measures which either increase NAD+ synthesis or increase the NAD+/NADH ratio, can boost Sirt1 activity. With respect to the latter possibility, fasting or calorie restriction can activate Sirt1 by reducing the availability of oxidisable substrate that drives metabolic reduction of NAD+.73 Quinones susceptible to reversible reduction, notably thymoquinone (from the oil of black cumin seed—Nigella sativa) and pyrroloquinoline quinone (PQQ—a vitamin-like compound found in many foods) can boost Sirt1 activity by oxidising NADH. The reduction of thymoquinone is catalysed by the Nrf2-inducible enzyme (NQO1), and PQQ’s high-affinity binding to lactate dehydrogenase promotes PQQ’s reduction by NADH.74–78
The brain readily employs ketones—chiefly β-hydroxybutyrate (BHB)—as an alternative substrate to glucose during fasting. Oxidation of BHB in the brain is associated with a compensatory reduction in glucose uptake.79 When a molecule of glucose passes down the glycolytic pathway to generate two molecules of acetyl-CoA, two molecules of NAD+ are reduced to NADH in the cytoplasm; when a molecule of BHB is converted to two acetyl-CoAs, no reduction of cytoplasmic NAD+ is induced. For this reason, the brain NAD+/NADH ratio is higher during ketosis than during normal glucose-based metabolism.80 This effect has been directly demonstrated in the brain of healthy volunteers following administration of medium-chain triglycerides (MCTs).81 This effect can be expected to be associated with increased brain Sirt1 activity, and it has been suggested that this phenomenon—and perhaps other consequences of an elevated NAD+/NADH ratio—may help to explain the neuroprotective properties of ketogenic diets.80 Moreover, there is recent evidence that exposure of neurons to BHB in vitro increases their expression of Sirt1 at both the mRNA and protein level.82
Evidently, fasting for the purpose of inducing ketogenesis is only a temporary expedient. Diets very high in fats and low in both carbohydrates and protein can be used to achieve ketosis while maintaining an adequate calorie intake, but such diets are too monotonous for most people to practise indefinitely. The most practical approach to boosting plasma BHB levels is through administration of MCTs; the short-chain fatty acids which these supply are not stored in triglycerides, but rather are either oxidised quickly or converted to ketone bodies in the liver.83 Hence, ingestion of MCTs can be employed to enhance brain Sirt1 activity.
De novo synthesis of NAD+ can be enhanced by precursors such as nicotinamide riboside or nicotinamide mononucleoside, each of which are Sirt1 activators.84–89 AMP-activated protein kinase (AMPK) boosts NAD+ synthesis via induction of the enzyme nicotinamide ribosylphosphotransferase (NAMPT), rate limiting for conversion of nicotinamide to NAD+.90–92 Since nicotinamide is a product of Sirt1 activity that inhibits Sirt1, NAMPT also promotes Sirt1 activity by alleviating this inhibition. While the therapeutic utility of the drug metformin in diabetes reflects its ability to activate AMPK, this activity is shared by the phytochemical berberine, a component of many Chinese medicinal herbs, that has long been used for management of type II diabetes in China.93–95 Both metformin and berberine boost Sirt1 activity.96–100
CD38 functions to degrade NAD+ to generate two molecules which can regulate intracellular calcium, ADP-ribose and cyclic ADP-ribose; its expression is most notable in immune cells, but other cells can express it. CD38 can be inhibited by the flavonoids apigenin and quercetin, with Kis of about 12 and 13 µM, respectively; this inhibition can boost Sirt1 activity by boosting NAD+.101 102 Although intraperitoneal administration of an ample dose (100 mg/kg) of apigenin has been reported to alleviate metabolic syndrome in obese mice, presumably via CD38 inhibition, it seems unlikely that this effect could be replicated with oral administration of apigenin or quercetin, for which absorption is inefficient and conjugation rapid.102
Nutraceuticals can activate Sirt1 allosterically or via post-translational modifications
Certain post-translational modifications of Sirt1 can enhance its activity. O-GlcNAcylation of Sirt1 at Ser-549 boosts its enzymatic activity—an effect which possibly contributes to the anti-inflammatory activity of supplemental glucosamine.103 104 It is possible that the favourable effects of glucosamine supplementation on human mortality and lifespan of mice reflect, to some extent, Sirt1 activation.105–108 Sirt1 activity is also enhanced by covalent interaction with hydrogen sulfide—suggesting a Sirt1 activating role for nutraceuticals or drugs which promote hydrogen sulfide generation.109–111 N-acetylcysteine can serve as a substrate for H2S generation, whereas taurine has been shown to induce two key enzymes for H2S synthesis, cystathionine β-synthase and cystathionine γ-lyase, in vascular tissues.111
Sirt1 can also be allosterically activated by certain agents. The lignin phytochemical resveratrol has this potential, and studies with resveratrol in cell cultures and rodents drew early attention to the health-protective potential of Sirt1 activation.112–114 Unfortunately, the clinical utility of resveratrol is impaired by poor pharmacokinetics—inefficient absorption and rapid conjugation in the intestinal mucosa and liver.115 Presumably for this reason, clinical evaluations with supplemental resveratrol have produced inconsistent results.116 Nonetheless, a meta-analysis of clinical studies with resveratrol in type 2 diabetics has concluded that it has useful effects on systolic blood pressure, haemoglobin A1c and creatinine.117 Drugs with better pharmacokinetics which can allosterically activate Sirt1—known as sirtuin-activating compounds—may have greater potential if and when they are approved.118 119
Countering oxidative stress may support Sirt1 activity
On the other hand, oxidant stress can oppose Sirt1 activity via multiple mechanisms. Reactive oxidant species (ROS) can increase miR-34a expression via upregulation of NF-κB and p53 activities.120 In that regard, a report that treatment with the drug salsalate can elevate Sirt1 levels in endothelial cells and monocytes may reflect the ability of salicylic acid to suppress activation of NF-κB via IκB kinase-β.121 122 (Salsalate is a dimer of the anti-inflammatory phytochemical salicylic acid; esterase activity in the intestinal tract cleaves it to release free salicylic acid. In multigram daily doses, it exerts anti-inflammatory activity useful in rheumatoid arthritis.123 Unlike its derivative acetylsalicylic acid, it only mildly and reversibly inhibits cyclo-oxygenase activity, and hence is comparatively safe; however, its clinical utility is compromised by the fact that it induces fully reversible ototoxicity in a fairly high proportion of patients.)
ROS can decrease NAD+ levels via DNA damage and consequent PARP activation.124 And ROS can also promote Sirt1 proteolysis by boosting JNK1 activity; as noted, the latter can confer a phosphorylation on Sirt1 that enables its ubiquitination and subsequent proteasomal degradation (an effect opposed by MNA).66 67 Hence, effective antioxidant measures may help support Sirt1 activity in the context of oxidative stress. Moreover, antioxidant supplementation could be expected to complement the anti-inflammatory activity of Sirt1, as reversible oxidation of sulfhydryl groups by hydrogen peroxide works in various ways to upregulate activation of NF-κB and MAP kinases, mediators of the synthesis and activity of many pro-inflammatory cytokines.125–127
Summing up and future research prospects
Figure 1 depicts the various mechanisms whereby the nutraceuticals discussed above are believed to promote Sirt1 activity. It is almost surely the case that future research will identify further phytochemicals or metabolites with potential for Sirt1 activation. It is reasonable to expect that nutraceutical combinations which promote Sirt1 activity by multiple complementary mechanisms may have considerable potential for health promotion. Various combinations of nutraceuticals, thought to boost Sirt1 activity in ways that are potentially complementary or synergistic, could be evaluated in preclinical research to determine which might be most appropriate for clinical study. Although support of Sirt1 activity is likely to benefit cardiovascular health in a great many ways, studying Sirt1-activating regimens in the context of heart failure—the leading overall cause of death—may be of particular interest; Sirt1 supports mitophagy and MB—known to be protective but defective in heart failure128–132—while suppressing inflammation. Curiously, the SGLT2 inhibitory drugs used to treat diabetes—by diminishing renal retention of glucose and hence moderating glycaemia—have been found to be therapeutically useful in heart and renal failure, even in patients who are normoglycaemic.133 This effect has been traced to their ability to boost Sirt1 and AMPK activity, thereby promoting autophagy, mitophagy and MB.20 134–137 While blunting postprandial rises in glucose may boost Sirt1 and AMPK activity via a reduction in oxidisable substrate—rather like caloric restriction does135 138—there is evidence that these drugs can exert this effect on cells in vitro, including in heart tissue, which does not express SGLT2.20 137 139 140 The molecular biology underlying this latter effect remains unclear. It is reasonable to suspect that the nutraceutical strategies outlined above could complement the benefits of SGLT2 inhibitors for non-diabetic health disorders. These drugs tend to be well tolerated aside from a moderate increase in risk of bladder and genital infections reflecting the increased glucose content of urine.133
Figure 1.
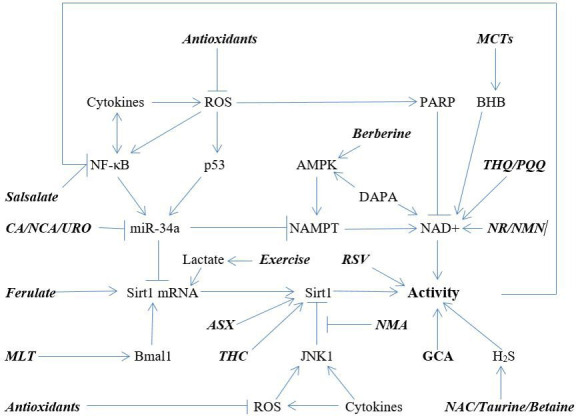
Mechanisms that regulate Sirt1 activity, as modulated by nutraceuticals. Also depicted: exercise-induced lactic acid boosts Sirt1 expression in the brain. AMPK, AMP-activated protein kinase; ASX, astaxanthin; BHB, β-hydroxybutyrate; CA, carnosic acid; DAPA, dapaflogazin; GCA, glucosamine; MCTs, medium-chain triglycerides; MLT, melatonin; NAC, N-acetylcysteine; NAMPT, nicotinamide ribosylphosphotransferase; NCA, neochlorogenic acid; NMA, N1-methylnicotinamide; NMN, nicotinamide mononucleotide; NR, nicotinamide riboside; PQQ, pyrroloquinoline quinone; ROS, reactive oxidant species; RSV, resveratrol; THC, tetrahydrocurcumin; THQ, thymoquinone; URO, urolithin A.
As noted, promotion of autophagy and of mitophagy/MB is a key mechanism whereby effective Sirt1 activity can maintain or restore health. It is therefore appropriate to comment on ancillary nutraceuticals which may complement Sirt1 in aiding these processes. Two recent essays have addressed this issue.141 142 Stimulation of AMPK, in addition to its role in boosting Sirt1 activity, can promote these processes in independent ways—pointing to the potential value of berberine in this regard.143–145 The dietary polyamine spermidine—recently available as a nutraceutical—can aid autophagy, mitophagy and MB by promoting efficient translation of the mRNA coding for transcription factor EB.146–149 In at least some tissues, nitric oxide aids MB by boosting PGC-1α expression and half-life via cGMP-PKC-p38 MAP kinase signalling; recoupling endothelial nitric oxide synthase with citrulline, or directly stimulating soluble guanylate cyclase with high-dose biotin, represents nutraceutical strategies for achieving these effects.150–156 The xanthophyll carotenoid astaxanthin can aid MB by acting as an agonist for the PPAR-α transcription factor, and phase 2 activating nutraceuticals, such as lipoic acid or sulforaphane, can analogously aid MB by boosting expression of the transcription factor NRF-1, another key mediator of MB.157–162 Hence, administration of berberine, spermidine, citrulline, biotin, astaxanthin, lipoic acid and/or sulforaphane may complement the utility of Sirt1 activators for aiding autophagy, mitophagy and MB.
Footnotes
Contributors: All authors contributed to the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: JJD is affiliated with companies that sell supplements. MM and JO own companies that sell supplements.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Not applicable.
References
- 1.Satoh A, Stein L, Imai S. The role of mammalian sirtuins in the regulation of metabolism, aging, and longevity. Handb Exp Pharmacol 2011;206:125–62. 10.1007/978-3-642-21631-2_7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baur JA, Ungvari Z, Minor RK, et al. Are sirtuins viable targets for improving healthspan and lifespan? Nat Rev Drug Discov 2012;11:443–61. 10.1038/nrd3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol 2012;13:225–38. 10.1038/nrm3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiNicolantonio JJ, McCarty MF, Assanga SI, et al. Ferulic acid and berberine, via SIRT1 and AMPK, may act as cell cleansing promoters of healthy longevity. Open Heart 2022;9:e001801. 10.1136/openhrt-2021-001801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ponugoti B, Kim D-H, Xiao Z, et al. Sirt1 deacetylates and inhibits SREBP-1c activity in regulation of hepatic lipid Metabolism*. J Biol Chem 2010;285:33959–70. 10.1074/jbc.M110.122978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattagajasingh I, Kim C-S, Naqvi A, et al. Sirt1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A 2007;104:14855–60. 10.1073/pnas.0704329104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeung F, Hoberg JE, Ramsey CS, et al. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. Embo J 2004;23:2369–80. 10.1038/sj.emboj.7600244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim H-N, Ponte F, Warren A, et al. A decrease in NAD+ contributes to the loss of osteoprogenitors and bone mass with aging. NPJ Aging Mech Dis 2021;7:8. 10.1038/s41514-021-00058-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winnik S, Stein S, M. Matter C. Matter cm. Si. Curr Vasc Pharmacol 2012;10:693–6. 10.2174/157016112803520756 [DOI] [PubMed] [Google Scholar]
- 10.Ministrini S, Puspitasari YM, Beer G, et al. Sirtuin 1 in endothelial dysfunction and cardiovascular aging. Front Physiol 2021;12:733696. 10.3389/fphys.2021.733696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Man AWC, Li H, Xia N. The role of Sirtuin1 in regulating endothelial function, arterial remodeling and vascular aging. Front Physiol 2019;10:1173. 10.3389/fphys.2019.01173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stein S, Matter CM. Protective roles of SIRT1 in atherosclerosis. Cell Cycle 2011;10:640–7. 10.4161/cc.10.4.14863 [DOI] [PubMed] [Google Scholar]
- 13.Li P, Song X, Zhang D, et al. Resveratrol improves left ventricular remodeling in chronic kidney disease via Sirt1‐mediated regulation of FoxO1 activity and MnSOD expression. Biofactors 2020;46:168–79. 10.1002/biof.1584 [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, He T, Zhang Z, et al. Activation of SIRT1 by resveratrol alleviates pressure overload-induced cardiac hypertrophy via suppression of TGF-β1 signaling. Pharmacology 2021;106:667–81. 10.1159/000518464 [DOI] [PubMed] [Google Scholar]
- 15.Sundaresan NR, Pillai VB, Gupta MP. Emerging roles of SIRT1 deacetylase in regulating cardiomyocyte survival and hypertrophy. J Mol Cell Cardiol 2011;51:614–8. 10.1016/j.yjmcc.2011.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanno M, Kuno A, Horio Y, et al. Emerging beneficial roles of sirtuins in heart failure. Basic Res Cardiol 2012;107:273. 10.1007/s00395-012-0273-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu XS, Wang ZB, Ye Z, et al. Resveratrol, an activator of SIRT1, upregulates AMPK and improves cardiac function in heart failure. Genet Mol Res 2014;13:323–35. 10.4238/2014.January.17.17 [DOI] [PubMed] [Google Scholar]
- 18.Gorski PA, Jang SP, Jeong D, et al. Role of SIRT1 in Modulating Acetylation of the Sarco-Endoplasmic Reticulum Ca 2+ -ATPase in Heart Failure. Circ Res 2019;124:e63–80. 10.1161/CIRCRESAHA.118.313865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin B, Zhao H, Li L, et al. Sirt1 improves heart failure through modulating the NF-κB p65/microRNA-155/BNDF signaling cascade. Aging 2021;13:14482–98. 10.18632/aging.103640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Packer M. Cardioprotective effects of sirtuin-1 and its downstream effectors: potential role in mediating the heart failure benefits of SGLT2 (sodium-glucose cotransporter 2) inhibitors. Circ Heart Fail 2020;13:e007197. 10.1161/CIRCHEARTFAILURE.120.007197 [DOI] [PubMed] [Google Scholar]
- 21.McCarty MF, Assanga SBI. Ferulic acid may target MyD88-mediated pro-inflammatory signaling – implications for the health protection afforded by whole grains, anthocyanins, and coffee. Med Hypotheses 2018;118:114–20. 10.1016/j.mehy.2018.06.032 [DOI] [PubMed] [Google Scholar]
- 22.Pari L, Amali DR. Protective role of tetrahydrocurcumin (THC) an active principle of turmeric on chloroquine induced hepatotoxicity in rats. J Pharm Pharm Sci 2005;8:115–23. [PubMed] [Google Scholar]
- 23.Raimundo AF, Ferreira S, Tomás-Barberán FA, et al. Urolithins: diet-derived bioavailable metabolites to tackle diabetes. Nutrients 2021;13:4285. 10.3390/nu13124285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X-X, Zhao D-S, Wang J, et al. The treatment of cardiovascular diseases: a review of ferulic acid and its derivatives. Pharmazie 2021;76:55–60. 10.1691/ph.2021.0958 [DOI] [PubMed] [Google Scholar]
- 25.Satoh T, Trudler D, Oh C-K, et al. Potential therapeutic use of the rosemary diterpene carnosic acid for Alzheimer's disease, Parkinson's disease, and Long-COVID through Nrf2 activation to counteract the NLRP3 inflammasome. Antioxidants 2022;11:124. 10.3390/antiox11010124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao X-H, Zhang S-D, Wang L-T, et al. Anti-Inflammatory Effects of Neochlorogenic Acid Extract from Mulberry Leaf (Morus alba L.) Against LPS-Stimulated Inflammatory Response through Mediating the AMPK/Nrf2 Signaling Pathway in A549 Cells. Molecules 2020;25:1385. 10.3390/molecules25061385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Mesallamy HO, Gawish RA, Sallam A-AM, et al. Ferulic acid protects against radiation-induced testicular damage in male rats: impact on SIRT1 and PARP1. Environ Sci Pollut Res Int 2018;25:6218–27. 10.1007/s11356-017-0873-6 [DOI] [PubMed] [Google Scholar]
- 28.Moghadam FH, Mesbah-Ardakani M, Nasr-Esfahani MH. Ferulic acid exerts concentration-dependent anti-apoptotic and neuronal differentiation-inducing effects in PC12 and mouse neural stem cells. Eur J Pharmacol 2018;841:104–12. 10.1016/j.ejphar.2018.10.003 [DOI] [PubMed] [Google Scholar]
- 29.Hou T, Zhang L, Yang X. Ferulic acid, a natural polyphenol, protects against osteoporosis by activating SIRT1 and NF-κB in neonatal rats with glucocorticoid-induced osteoporosis. Biomed Pharmacother 2019;120:109205. 10.1016/j.biopha.2019.109205 [DOI] [PubMed] [Google Scholar]
- 30.Xu T, Song Q, Zhou L, et al. Ferulic acid alleviates lipotoxicity-induced hepatocellular death through the SIRT1-regulated autophagy pathway and independently of AMPK and Akt in AML-12 hepatocytes. Nutr Metab 2021;18:13. 10.1186/s12986-021-00540-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li K, Zhai M, Jiang L, et al. Tetrahydrocurcumin ameliorates diabetic cardiomyopathy by attenuating high glucose-induced oxidative stress and fibrosis via activating the SIRT1 pathway. Oxid Med Cell Longev 2019;2019:1–15. 10.1155/2019/6746907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, Liu X, Li S, et al. Tetrahydrocurcumin protects against sepsis-induced acute kidney injury via the SIRT1 pathway. Ren Fail 2021;43:1028–40. 10.1080/0886022X.2021.1942915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cristòfol R, Porquet D, Corpas R, et al. Neurons from senescence-accelerated SAMP8 mice are protected against frailty by the sirtuin 1 promoting agents melatonin and resveratrol. J Pineal Res 2012;52:271–81. 10.1111/j.1600-079X.2011.00939.x [DOI] [PubMed] [Google Scholar]
- 34.Yu L, Sun Y, Cheng L, et al. Melatonin receptor-mediated protection against myocardial ischemia/reperfusion injury: role of SIRT1. J Pineal Res 2014;57:228–38. 10.1111/jpi.12161 [DOI] [PubMed] [Google Scholar]
- 35.Yang Y, Jiang S, Dong Y, et al. Melatonin prevents cell death and mitochondrial dysfunction via a SIRT1-dependent mechanism during ischemic-stroke in mice. J Pineal Res 2015;58:61–70. 10.1111/jpi.12193 [DOI] [PubMed] [Google Scholar]
- 36.Zhou B, Zhang Y, Zhang F, et al. Clock/Bmal1 regulates circadian change of mouse hepatic insulin sensitivity by SIRT1. Hepatology 2014;59:2196–206. 10.1002/hep.26992 [DOI] [PubMed] [Google Scholar]
- 37.Liu J, Zhou B, Yan M, et al. Clock and BMAL1 regulate muscle insulin sensitivity via SIRT1 in male mice. Endocrinology 2016;157:2259–69. 10.1210/en.2015-2027 [DOI] [PubMed] [Google Scholar]
- 38.Jilg A, Moek J, Weaver DR, et al. Rhythms in clock proteins in the mouse pars tuberalis depend on MT1 melatonin receptor signalling. Eur J Neurosci 2005;22:2845–54. 10.1111/j.1460-9568.2005.04485.x [DOI] [PubMed] [Google Scholar]
- 39.Fu S, Kuwahara M, Uchida Y, et al. Circadian production of melatonin in cartilage modifies rhythmic gene expression. J Endocrinol 2019:161–73. 10.1530/JOE-19-0022 [DOI] [PubMed] [Google Scholar]
- 40.Chen P, Chen F, Lei J, et al. Activation of the miR-34a-mediated SIRT1/mTOR signaling pathway by urolithin a attenuates d-galactose-induced brain aging in mice. Neurotherapeutics 2019;16:1269–82. 10.1007/s13311-019-00753-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghosh N, Das A, Biswas N, et al. Urolithin a augments angiogenic pathways in skeletal muscle by bolstering NAD+ and SIRT1. Sci Rep 2020;10:20184. 10.1038/s41598-020-76564-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J, Jiang J, Qiu J, et al. Urolithin a protects dopaminergic neurons in experimental models of Parkinson's disease by promoting mitochondrial biogenesis through the SIRT1/PGC-1α signaling pathway. Food Funct 2022;13:375–85. 10.1039/D1FO02534A [DOI] [PubMed] [Google Scholar]
- 43.Shi P-Z, Wang J-W, Wang P-C, et al. Urolithin a alleviates oxidative stress-induced senescence in nucleus pulposus-derived mesenchymal stem cells through SIRT1/PGC-1α pathway. World J Stem Cells 2021;13:1928–46. 10.4252/wjsc.v13.i12.1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shan W, Gao L, Zeng W, et al. Activation of the SIRT1/p66shc antiapoptosis pathway via carnosic acid-induced inhibition of miR-34a protects rats against nonalcoholic fatty liver disease. Cell Death Dis 2015;6:e1833. 10.1038/cddis.2015.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu M-H, Hung T-W, Wang C-C, et al. Neochlorogenic acid attenuates hepatic lipid accumulation and inflammation via regulating miR-34a in vitro. Int J Mol Sci 2021;22:13163. 10.3390/ijms222313163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee J, Padhye A, Sharma A, et al. A pathway involving farnesoid X receptor and small heterodimer partner positively regulates hepatic sirtuin 1 levels via microRNA-34a inhibition. J Biol Chem 2010;285:12604–11. 10.1074/jbc.M109.094524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tabuchi T, Satoh M, Itoh T, et al. Microrna-34A regulates the longevity-associated protein SIRT1 in coronary artery disease: effect of statins on SIRT1 and microRNA-34a expression. Clin Sci 2012;123:161–71. 10.1042/CS20110563 [DOI] [PubMed] [Google Scholar]
- 48.Hua C-C, Liu X-M, Liang L-R, et al. Targeting the microRNA-34a as a novel therapeutic strategy for cardiovascular diseases. Front Cardiovasc Med 2021;8:784044. 10.3389/fcvm.2021.784044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang X, Lu Y, Wu Q, et al. Astaxanthin mitigates subarachnoid hemorrhage injury primarily by increasing sirtuin 1 and inhibiting the Toll‐like receptor 4 signaling pathway. Faseb J 2019;33:722–37. 10.1096/fj.201800642RR [DOI] [PubMed] [Google Scholar]
- 50.Zhang J, Wang Q-Z, Zhao S-H, et al. Astaxanthin attenuated pressure overload-induced cardiac dysfunction and myocardial fibrosis: partially by activating SIRT1. Biochim Biophys Acta Gen Subj 2017;1861:1715–28. 10.1016/j.bbagen.2017.03.007 [DOI] [PubMed] [Google Scholar]
- 51.Gao D, Wang H, Xu Y, et al. Protective effect of astaxanthin against contrast-induced acute kidney injury via SIRT1-p53 pathway in rats. Int Urol Nephrol 2019;51:351–8. 10.1007/s11255-018-2027-2 [DOI] [PubMed] [Google Scholar]
- 52.Xu Y, Yao S, Gao D, et al. Effect of astaxanthin on apoptosis of rat renal tubular epithelial cells induced by iohexol. Am J Transl Res 2019;11:3039–47. [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X-S, Lu Y, Li W, et al. Astaxanthin ameliorates oxidative stress and neuronal apoptosis via SIRT1/NRF2/Prx2/ASK1/p38 after traumatic brain injury in mice. Br J Pharmacol 2021;178:1114–32. 10.1111/bph.15346 [DOI] [PubMed] [Google Scholar]
- 54.Shatoor AS, Al Humayed S. Astaxanthin ameliorates high-fat diet-induced cardiac damage and fibrosis by upregulating and activating SIRT1. Saudi J Biol Sci 2021;28:7012–21. 10.1016/j.sjbs.2021.07.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bayod S, Del Valle J, Canudas AM, et al. Long-Term treadmill exercise induces neuroprotective molecular changes in rat brain. J Appl Physiol 2011;111:1380–90. 10.1152/japplphysiol.00425.2011 [DOI] [PubMed] [Google Scholar]
- 56.Steiner JL, Murphy EA, McClellan JL, et al. Exercise training increases mitochondrial biogenesis in the brain. J Appl Physiol 2011;111:1066–71. 10.1152/japplphysiol.00343.2011 [DOI] [PubMed] [Google Scholar]
- 57.Koo J-H, Kang E-B, Oh Y-S, et al. Treadmill exercise decreases amyloid-β burden possibly via activation of SIRT-1 signaling in a mouse model of Alzheimer's disease. Exp Neurol 2017;288:142–52. 10.1016/j.expneurol.2016.11.014 [DOI] [PubMed] [Google Scholar]
- 58.Muñoz A, Corrêa CL, Lopez-Lopez A, et al. Physical exercise improves aging-related changes in angiotensin, IGF-1, SIRT1, SIRT3, and VEGF in the substantia nigra. J Gerontol A Biol Sci Med Sci 2018;73:1594–601. 10.1093/gerona/gly072 [DOI] [PubMed] [Google Scholar]
- 59.Kim T-W, Park S-S, Shin M-S, et al. Treadmill exercise ameliorates social isolation-induced memory impairment by enhancing silent information regulator-1 expression in rats. J Exerc Rehabil 2020;16:227–33. 10.12965/jer.2040400.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li F, Geng X, Lee H, et al. Neuroprotective effects of exercise postconditioning after stroke via SIRT1-mediated suppression of endoplasmic reticulum (ER) stress. Front Cell Neurosci 2021;15:598230. 10.3389/fncel.2021.598230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.El Hayek L, Khalifeh M, Zibara V, et al. Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF). J Neurosci 2019;39:2369–82. 10.1523/JNEUROSCI.1661-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lang X, Zhao N, He Q, et al. Treadmill exercise mitigates neuroinflammation and increases BDNF via activation of SIRT1 signaling in a mouse model of T2DM. Brain Res Bull 2020;165:30–9. 10.1016/j.brainresbull.2020.09.015 [DOI] [PubMed] [Google Scholar]
- 63.Mahalakshmi B, Maurya N, Lee S-D, et al. Possible neuroprotective mechanisms of physical exercise in neurodegeneration. Int J Mol Sci 2020;21:5895. 10.3390/ijms21165895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arida RM, Teixeira-Machado L. The contribution of physical exercise to brain resilience. Front Behav Neurosci 2020;14:626769. 10.3389/fnbeh.2020.626769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jiao F, Gong Z. The beneficial roles of SIRT1 in Neuroinflammation-Related diseases. Oxid Med Cell Longev 2020;2020:1–19. 10.1155/2020/6782872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hwang JS, Ham SA, Yoo T, et al. Upregulation of MKP-7 in response to rosiglitazone treatment ameliorates lipopolysaccharide-induced destabilization of SIRT1 by inactivating JNK. Pharmacol Res 2016;114:47–55. 10.1016/j.phrs.2016.10.014 [DOI] [PubMed] [Google Scholar]
- 67.Kim M, Kwon YE, Song JO, et al. Chfr negatively regulates SIRT1 activity upon oxidative stress. Sci Rep 2016;6:37578. 10.1038/srep37578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gebicki J, Sysa-Jedrzejowska A, Adamus J, et al. 1-Methylnicotinamide: a potent anti-inflammatory agent of vitamin origin. Pol J Pharmacol 2003;55:109–12. [PubMed] [Google Scholar]
- 69.Hong S, Moreno-Navarrete JM, Wei X, et al. Nicotinamide N-methyltransferase regulates hepatic nutrient metabolism through SIRT1 protein stabilization. Nat Med 2015;21:887–94. 10.1038/nm.3882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takeuchi K, Yokouchi C, Goto H, et al. Alleviation of fatty liver in a rat model by enhancing N1-methylnicotinamide bioavailability through aldehyde oxidase inhibition. Biochem Biophys Res Commun 2018;507:203–10. 10.1016/j.bbrc.2018.11.008 [DOI] [PubMed] [Google Scholar]
- 71.Zhang J, Chen Y, Liu C, et al. N1-Methylnicotinamide Improves Hepatic Insulin Sensitivity via Activation of SIRT1 and Inhibition of FOXO1 Acetylation. J Diabetes Res 2020;2020:1–11. 10.1155/2020/1080152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen Y, Zhang J, Li P, et al. N1‑methylnicotinamide ameliorates insulin resistance in skeletal muscle of type 2 diabetic mice by activating the SIRT1/PGC‑1α signaling pathway. Mol Med Rep 2021;23. 10.3892/mmr.2021.11909 [DOI] [PubMed] [Google Scholar]
- 73.Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol 2005;6:298–305. 10.1038/nrm1616 [DOI] [PubMed] [Google Scholar]
- 74.Tsvetkov P, Adler J, Strobelt R, et al. Nqo1 binds and supports SIRT1 function. Front Pharmacol 2021;12:671929. 10.3389/fphar.2021.671929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qiu D, Song S, Wang Y, et al. NAD(P)H: quinone oxidoreductase 1 attenuates oxidative stress and apoptosis by regulating Sirt1 in diabetic nephropathy. J Transl Med 2022;20:44. 10.1186/s12967-021-03197-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Akagawa M, Minematsu K, Shibata T, et al. Identification of lactate dehydrogenase as a mammalian pyrroloquinoline quinone (PQQ)-binding protein. Sci Rep 2016;6:26723. 10.1038/srep26723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang H, Li J, Cao C, et al. Pyrroloquinoline quinone inhibits the production of inflammatory cytokines via the SIRT1/NF-κB signal pathway in weaned piglet jejunum. Food Funct 2020;11:2137–53. 10.1039/C9FO02609F [DOI] [PubMed] [Google Scholar]
- 78.Saihara K, Kamikubo R, Ikemoto K, et al. Pyrroloquinoline quinone, a redox-active o-quinone, stimulates mitochondrial biogenesis by activating the SIRT1/PGC-1α signaling pathway. Biochemistry 2017;56:6615–25. 10.1021/acs.biochem.7b01185 [DOI] [PubMed] [Google Scholar]
- 79.Courchesne-Loyer A, Croteau E, Castellano C-A, et al. Inverse relationship between brain glucose and ketone metabolism in adults during short-term moderate dietary ketosis: a dual tracer quantitative positron emission tomography study. J Cereb Blood Flow Metab 2017;37:2485–93. 10.1177/0271678X16669366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Elamin M, Ruskin DN, Masino SA, et al. Ketone-Based metabolic therapy: is increased NAD+ a primary mechanism? Front Mol Neurosci 2017;10:377. 10.3389/fnmol.2017.00377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xin L, Ipek Özlem, Beaumont M, et al. Nutritional Ketosis Increases NAD+/NADH Ratio in Healthy Human Brain: An in Vivo Study by 31P-MRS. Front Nutr 2018;5:62. 10.3389/fnut.2018.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dabke P, Das AM. Mechanism of action of ketogenic diet treatment: impact of decanoic acid and beta-hydroxybutyrate on sirtuins and energy metabolism in hippocampal murine neurons. Nutrients 2020;12:2379. 10.3390/nu12082379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McCarty MF, DiNicolantonio JJ. Lauric acid-rich medium-chain triglycerides can substitute for other oils in cooking applications and may have limited pathogenicity. Open Heart 2016;3:e000467. 10.1136/openhrt-2016-000467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cantó C, Houtkooper RH, Pirinen E, et al. The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab 2012;15:838–47. 10.1016/j.cmet.2012.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leduc-Gaudet J-P, Dulac M, Reynaud O, et al. Nicotinamide riboside supplementation to improve skeletal muscle mitochondrial health and whole-body glucose homeostasis: does it actually work in humans? J Physiol 2020;598:619–20. 10.1113/JP279280 [DOI] [PubMed] [Google Scholar]
- 86.Martens CR, Denman BA, Mazzo MR, et al. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat Commun 2018;9:1286. 10.1038/s41467-018-03421-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yoshino J, Mills KF, Yoon MJ, et al. Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab 2011;14:528–36. 10.1016/j.cmet.2011.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Caton PW, Kieswich J, Yaqoob MM, et al. Nicotinamide mononucleotide protects against pro-inflammatory cytokine-mediated impairment of mouse islet function. Diabetologia 2011;54:3083–92. 10.1007/s00125-011-2288-0 [DOI] [PubMed] [Google Scholar]
- 89.Liu X, Li D, Liu Z, et al. Nicotinamide mononucleotide promotes pancreatic islet function through the SIRT1 pathway in mice after severe burns. Burns 2022;48:1922–32. 10.1016/j.burns.2022.01.013 [DOI] [PubMed] [Google Scholar]
- 90.Fulco M, Cen Y, Zhao P, et al. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of NAMPT. Dev Cell 2008;14:661–73. 10.1016/j.devcel.2008.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Costford SR, Bajpeyi S, Pasarica M, et al. Skeletal muscle NAMPT is induced by exercise in humans. Am J Physiol Endocrinol Metab 2010;298:E117–26. 10.1152/ajpendo.00318.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Revollo JR, Grimm AA, Imai S-ichiro. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem 2004;279:50754–63. 10.1074/jbc.M408388200 [DOI] [PubMed] [Google Scholar]
- 93.Lee YS, Kim WS, Kim KH, et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 2006;55:2256–64. 10.2337/db06-0006 [DOI] [PubMed] [Google Scholar]
- 94.Kim WS, Lee YS, Cha SH, et al. Berberine improves lipid dysregulation in obesity by controlling central and peripheral AMPK activity. Am J Physiol Endocrinol Metab 2009;296:E812–9. 10.1152/ajpendo.90710.2008 [DOI] [PubMed] [Google Scholar]
- 95.Hawley SA, Ross FA, Chevtzoff C, et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab 2010;11:554–65. 10.1016/j.cmet.2010.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li Q, Jia S, Xu L, et al. Metformin‐induced autophagy and irisin improves INS-1 cell function and survival in high-glucose environment via AMPK/SIRT1/PGC-1α signal pathway. Food Sci Nutr 2019;7:1695–703. 10.1002/fsn3.1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zheng Z, Bian Y, Zhang Y, et al. Metformin activates AMPK/SIRT1/NF-κB pathway and induces mitochondrial dysfunction to drive caspase3/GSDME-mediated cancer cell pyroptosis. Cell Cycle 2020;19:1089–104. 10.1080/15384101.2020.1743911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gomes AP, Duarte FV, Nunes P, et al. Berberine protects against high fat diet-induced dysfunction in muscle mitochondria by inducing SIRT1-dependent mitochondrial biogenesis. Biochim Biophys Acta 2012;1822:185–95. 10.1016/j.bbadis.2011.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yu Y, Zhao Y, Teng F, et al. Berberine improves cognitive deficiency and muscular dysfunction via activation of the AMPK/SIRT1/PGC-1a pathway in skeletal muscle from naturally aging rats. J Nutr Health Aging 2018;22:710–7. 10.1007/s12603-018-1015-7 [DOI] [PubMed] [Google Scholar]
- 100.Shan Y, Zhang S, Gao B, et al. Adipose tissue SIRT1 regulates insulin sensitizing and anti-inflammatory effects of berberine. Front Pharmacol 2020;11:591227. 10.3389/fphar.2020.591227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kellenberger E, Kuhn I, Schuber F, et al. Flavonoids as inhibitors of human CD38. Bioorg Med Chem Lett 2011;21:3939–42. 10.1016/j.bmcl.2011.05.022 [DOI] [PubMed] [Google Scholar]
- 102.Escande C, Nin V, Price NL, et al. Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes 2013;62:1084–93. 10.2337/db12-1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Han C, Gu Y, Shan H, et al. O-Glcnacylation of SIRT1 enhances its deacetylase activity and promotes cytoprotection under stress. Nat Commun 2017;8:1491. 10.1038/s41467-017-01654-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McCarty MF, O'Keefe JH, DiNicolantonio JJ. Glucosamine for the treatment of osteoarthritis: the time has come for Higher-Dose trials. J Diet Suppl 2019;16:179–92. 10.1080/19390211.2018.1448920 [DOI] [PubMed] [Google Scholar]
- 105.Bell GA, Kantor ED, Lampe JW, et al. Use of glucosamine and chondroitin in relation to mortality. Eur J Epidemiol 2012;27:593–603. 10.1007/s10654-012-9714-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li Z-H, Gao X, Chung VC, et al. Associations of regular glucosamine use with all-cause and cause-specific mortality: a large prospective cohort study. Ann Rheum Dis 2020;79:829–36. 10.1136/annrheumdis-2020-217176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Weimer S, Priebs J, Kuhlow D, et al. D-Glucosamine supplementation extends life span of nematodes and of ageing mice. Nat Commun 2014;5:3563. 10.1038/ncomms4563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.King DE, Xiang J. Glucosamine/Chondroitin and mortality in a US NHANES cohort. J Am Board Fam Med 2020;33:842–7. 10.3122/jabfm.2020.06.200110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Suo R, Zhao Z-ZHI, TANG ZHI-HAN, et al. Hydrogen sulfide prevents H2O2-induced senescence in human umbilical vein endothelial cells through SIRT1 activation. Mol Med Rep 2013;7:1865–70. 10.3892/mmr.2013.1417 [DOI] [PubMed] [Google Scholar]
- 110.Du C, Lin X, Xu W, et al. Sulfhydrated sirtuin-1 increasing its deacetylation activity is an essential epigenetics mechanism of Anti-Atherogenesis by hydrogen sulfide. Antioxid Redox Signal 2019;30:184–97. 10.1089/ars.2017.7195 [DOI] [PubMed] [Google Scholar]
- 111.DiNicolantonio JJ, OKeefe JH, McCarty MF. Boosting endogenous production of vasoprotective hydrogen sulfide via supplementation with taurine and N-acetylcysteine: a novel way to promote cardiovascular health. Open Heart 2017;4:e000600. 10.1136/openhrt-2017-000600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Howitz KT, Bitterman KJ, Cohen HY, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003;425:191–6. 10.1038/nature01960 [DOI] [PubMed] [Google Scholar]
- 113.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006;444:337–42. 10.1038/nature05354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 2008;8:157–68. 10.1016/j.cmet.2008.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chimento A, De Amicis F, Sirianni R, et al. Progress to improve oral bioavailability and beneficial effects of resveratrol. Int J Mol Sci 2019;20:1381. 10.3390/ijms20061381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Smoliga JM, Baur JA, Hausenblas HA. Resveratrol and health--a comprehensive review of human clinical trials. Mol Nutr Food Res 2011;55:1129–41. 10.1002/mnfr.201100143 [DOI] [PubMed] [Google Scholar]
- 117.Hausenblas HA, Schoulda JA, Smoliga JM. Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus-systematic review and meta-analysis. Mol Nutr Food Res 2015;59:147–59. 10.1002/mnfr.201400173 [DOI] [PubMed] [Google Scholar]
- 118.Bonkowski MS, Sinclair DA. Slowing ageing by design: the rise of NAD+ and sirtuin-activating compounds. Nat Rev Mol Cell Biol 2016;17:679–90. 10.1038/nrm.2016.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schultz MB, Rinaldi C, Lu Y, et al. Molecular and cellular characterization of SIRT1 allosteric activators. Methods Mol Biol 2019;1983:133–49. 10.1007/978-1-4939-9434-2_8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li J, Wang K, Chen X, et al. Transcriptional activation of microRNA-34a by NF-kappa B in human esophageal cancer cells. BMC Mol Biol 2012;13:4. 10.1186/1471-2199-13-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jung TW, Park HS, Jeong JH, et al. Salsalate ameliorates the atherosclerotic response through HO-1- and SIRT1-mediated suppression of ER stress and inflammation. Inflamm. Res. 2019;68:655–63. 10.1007/s00011-019-01248-6 [DOI] [PubMed] [Google Scholar]
- 122.Yin M-J, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IκB kinase-β. Nature 1998;396:77–80. 10.1038/23948 [DOI] [PubMed] [Google Scholar]
- 123.McCarty MF. Salsalate may have broad utility in the prevention and treatment of vascular disorders and the metabolic syndrome. Med Hypotheses 2010;75:276–81. 10.1016/j.mehy.2009.12.027 [DOI] [PubMed] [Google Scholar]
- 124.Giansanti V, Donà F, Tillhon M, et al. Parp inhibitors: new tools to protect from inflammation. Biochem Pharmacol 2010;80:1869–77. 10.1016/j.bcp.2010.04.022 [DOI] [PubMed] [Google Scholar]
- 125.Korbecki J, Baranowska-Bosiacka I, Gutowska I, et al. The effect of reactive oxygen species on the synthesis of prostanoids from arachidonic acid. J Physiol Pharmacol 2013;64:409–21. [PubMed] [Google Scholar]
- 126.Li Q, Spencer NY, Oakley FD, et al. Endosomal Nox2 facilitates redox-dependent induction of NF-kappaB by TNF-alpha. Antioxid Redox Signal 2009;11:1249–63. 10.1089/ars.2008.2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tobiume K, Matsuzawa A, Takahashi T, et al. Ask1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep 2001;2:222–8. 10.1093/embo-reports/kve046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Marquez J, Lee SR, Kim N, et al. Rescue of heart failure by mitochondrial recovery. Int Neurourol J 2006;20:5–12. 10.5213/inj.1632570.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sheeran FL, Pepe S. Mitochondrial bioenergetics and dysfunction in failing heart. Adv Exp Med Biol 2017;982:65–80. 10.1007/978-3-319-55330-6_4 [DOI] [PubMed] [Google Scholar]
- 130.Sanz M-N, Grimbert L, Moulin M, et al. Inducible cardiac-specific deletion of SIRT1 in male mice reveals progressive cardiac dysfunction and sensitization of the heart to pressure overload. Int J Mol Sci 2019;20:5005. 10.3390/ijms20205005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Qiu Z, Wei Y, Song Q, et al. The role of myocardial mitochondrial quality control in heart failure. Front Pharmacol 2019;10:1404. 10.3389/fphar.2019.01404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen L, Qin Y, Liu B, et al. PGC-1α-Mediated Mitochondrial Quality Control: Molecular Mechanisms and Implications for Heart Failure. Front Cell Dev Biol 2022;10:871357. 10.3389/fcell.2022.871357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tsai W-C, Hsu S-P, Chiu Y-L, et al. Cardiovascular and renal efficacy and safety of sodium-glucose cotransporter-2 inhibitors in patients without diabetes: a systematic review and meta-analysis of randomised placebo-controlled trials. BMJ Open 2022;12:e060655. 10.1136/bmjopen-2021-060655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Packer M. Autophagy stimulation and intracellular sodium reduction as mediators of the cardioprotective effect of sodium-glucose cotransporter 2 inhibitors. Eur J Heart Fail 2020;22:618–28. 10.1002/ejhf.1732 [DOI] [PubMed] [Google Scholar]
- 135.Hoong CWS, Chua MWJ. Sglt2 inhibitors as calorie restriction mimetics: insights on longevity pathways and age-related diseases. Endocrinology 2021;162:bqab079. 10.1210/endocr/bqab079 [DOI] [PubMed] [Google Scholar]
- 136.Lee J-Y, Lee M, Lee JY, et al. Ipragliflozin, an SGLT2 inhibitor, ameliorates high-fat diet-induced metabolic changes by upregulating energy expenditure through activation of the AMPK/ SIRT1 pathway. Diabetes Metab J 2021;45:921–32. 10.4093/dmj.2020.0187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ren F-F, Xie Z-Y, Jiang Y-N, et al. Dapagliflozin attenuates pressure overload-induced myocardial remodeling in mice via activating SIRT1 and inhibiting endoplasmic reticulum stress. Acta Pharmacol Sin 2022;43:1721–32. 10.1038/s41401-021-00805-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Miller RA, Harrison DE, Allison DB, et al. Canagliflozin extends life span in genetically heterogeneous male but not female mice. JCI Insight 2020;5. 10.1172/jci.insight.140019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang X, Liu Q, Li Y, et al. The diabetes medication canagliflozin promotes mitochondrial remodelling of adipocyte via the AMPK-Sirt1-Pgc-1α signalling pathway. Adipocyte 2020;9:484–94. 10.1080/21623945.2020.1807850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li X, Flynn ER, do Carmo JM, et al. Direct cardiac actions of sodium-glucose cotransporter 2 inhibition improve mitochondrial function and attenuate oxidative stress in pressure overload-induced heart failure. Front Cardiovasc Med 2022;9:859253. 10.3389/fcvm.2022.859253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lewis Luján LM, McCarty MF, Di Nicolantonio JJ, et al. Nutraceuticals/Drugs promoting mitophagy and mitochondrial biogenesis may combat the mitochondrial dysfunction driving progression of dry age-related macular degeneration. Nutrients 2022;14:1985. 10.3390/nu14091985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.McCarty MF. Nutraceutical and dietary strategies for up-regulating macroautophagy. Int J Mol Sci 2022;23:2054. 10.3390/ijms23042054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhao M, Klionsky DJ. Ampk-Dependent phosphorylation of ULK1 induces autophagy. Cell Metab 2011;13:119–20. 10.1016/j.cmet.2011.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lee JW, Park S, Takahashi Y, et al. The association of AMPK with ULK1 regulates autophagy. PLoS One 2010;5:e15394. 10.1371/journal.pone.0015394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cantó C, Gerhart-Hines Z, Feige JN, et al. Ampk regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009;458:1056–60. 10.1038/nature07813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wolff EC, Kang KR, Kim YS, et al. Posttranslational synthesis of hypusine: evolutionary progression and specificity of the hypusine modification. Amino Acids 2007;33:341–50. 10.1007/s00726-007-0525-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang H, Alsaleh G, Feltham J, et al. Polyamines control eIF5A Hypusination, TFEB translation, and autophagy to reverse B cell senescence. Mol Cell 2019;76:110–25. 10.1016/j.molcel.2019.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Theeuwes WF, Gosker HR, Schols AMWJ, et al. Regulation of PGC-1α expression by a GSK-3β-TFEB signaling axis in skeletal muscle. Biochim Biophys Acta Mol Cell Res 2020;1867:118610. 10.1016/j.bbamcr.2019.118610 [DOI] [PubMed] [Google Scholar]
- 149.Zhao E, Czaja MJ. Transcription factor EB: a central regulator of both the autophagosome and lysosome. Hepatology 2012;55:1632–4. 10.1002/hep.25619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nisoli E, Falcone S, Tonello C, et al. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc Natl Acad Sci U S A 2004;101:16507–12. 10.1073/pnas.0405432101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bhargava P, Janda J, Schnellmann RG. Elucidation of cGMP-dependent induction of mitochondrial biogenesis through PKG and p38 MAPK in the kidney. Am J Physiol Renal Physiol 2020;318:F322–8. 10.1152/ajprenal.00533.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr 2011;93:884S–90. 10.3945/ajcn.110.001917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.McCarty MF. Asymmetric dimethylarginine is a well established mediating risk factor for cardiovascular morbidity and Mortality-Should patients with elevated levels be supplemented with citrulline? Healthcare 2016;4:40. 10.3390/healthcare4030040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vesely DL. Biotin enhances guanylate cyclase activity. Science 1982;216:1329–30. 10.1126/science.6123152 [DOI] [PubMed] [Google Scholar]
- 155.Watanabe-Kamiyama M, Kamiyama S, Horiuchi K, et al. Antihypertensive effect of biotin in stroke-prone spontaneously hypertensive rats. Br J Nutr 2008;99:756–63. 10.1017/S0007114507841122 [DOI] [PubMed] [Google Scholar]
- 156.McCarty MF, DiNicolantonio JJ. Neuroprotective potential of high-dose biotin. Med Hypotheses 2017;109:145–9. 10.1016/j.mehy.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 157.Jia Y, Wu C, Kim J, et al. Astaxanthin reduces hepatic lipid accumulations in high-fat-fed C57BL/6J mice via activation of peroxisome proliferator-activated receptor (PPAR) alpha and inhibition of PPAR gamma and Akt. J Nutr Biochem 2016;28:9–18. 10.1016/j.jnutbio.2015.09.015 [DOI] [PubMed] [Google Scholar]
- 158.Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta 2011;1813:1269–78. 10.1016/j.bbamcr.2010.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Piantadosi CA, Carraway MS, Babiker A, et al. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res 2008;103:1232–40. 10.1161/01.RES.0000338597.71702.ad [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Koriyama Y, Nakayama Y, Matsugo S, et al. Protective effect of lipoic acid against oxidative stress is mediated by Keap1/Nrf2-dependent heme oxygenase-1 induction in the RGC-5 cellline. Brain Res 2013;1499:145–57. 10.1016/j.brainres.2012.12.041 [DOI] [PubMed] [Google Scholar]
- 161.Kyung S, Lim JW, Kim H. α-Lipoic Acid Inhibits IL-8 Expression by Activating Nrf2 Signaling in Helicobacter pylori-infected Gastric Epithelial Cells. Nutrients 2019;11:2524. 10.3390/nu11102524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kensler TW, Egner PA, Agyeman AS, et al. Keap1-Nrf2 signaling: a target for cancer prevention by sulforaphane. Top Curr Chem 2013;329:163–77. 10.1007/128_2012_339 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data are available.


