Abstract
Trauma and chronic pain frequently co-occur, but the underlying neurological mechanisms are poorly understood. The current study investigated the neural correlates of stress and physical symptoms in trauma patients using functional magnetic resonance imaging (fMRI) and follow-up smartphone surveys. Participants were 10 patients diagnosed with Trauma- and Stressor-Related Disorders and 18 demographically-matched healthy controls who completed a fMRI stress provocation task in which they viewed stressful and neutral-relaxing images. Subsequently, participants completed daily smartphone surveys which prospectively monitored their stress and physical symptoms for 30 days. The trauma group experienced a significantly higher frequency of physical symptoms than controls during the follow-up period. During stress, trauma patients exhibited increased activity in the hippocampus, insula, and sensorimotor areas, but decreased activity in the ventromedial prefrontal cortex (vmPFC), lateral prefrontal cortex (LPFC), and dorsal striatum relative to controls. In all participants, higher physical symptom frequency was significantly associated with a hyperactive left hippocampal response to stress. The current study reports that trauma is characterized by greater physical symptoms and decreased prefrontal but increased limbic responses to stress. Our findings suggest that trauma may increase physical health symptoms by compromising hippocampal function, which could also increase vulnerability to stress- and pain-related disorders.
Keywords: trauma, stress, pain, physical symptoms, fMRI, hippocampus
1. Introduction
Trauma, although primarily conceptualized as a psychiatric disorder, is often accompanied by pain and physical health symptoms (Pacella et al., 2013; Ryder et al., 2018). In fact, trauma and chronic pain are highly co-occurring conditions, with comorbidity rates in the United States reaching as high as 50% in veteran populations (Fishbain et al., 2017). It has previously been suggested that chronic stress and pain are mutually reinforcing wherein the symptoms of one can exacerbate the symptoms of the other, and vice versa (Asmundson et al., 2002; Sharp & Harvey, 2001). As a potential explanation, prior studies have suggested the framework of allostatic load (Abdallah & Geha, 2017; Lunde & Sieberg, 2020), which refers to the “wear and tear” induced by chronic stress on bodily systems which can eventually lead to physical health problems (McEwen, 1998, 2007). Consistent with this, a national epidemiologic survey conducted in the U.S. found that Post-Traumatic Stress Disorder (PTSD) patients had disproportionately high rates of various medical conditions compared to controls (Pietrzak et al., 2011).
However, there remain significant gaps in our understanding of the neural mechanisms underlying the link between stress, trauma, and pain. A prior study from our group with healthy community individuals found that cumulative trauma and adversity were associated with a greater likelihood of physical health symptoms and an altered neural response to stress (Seo, Tsou, et al., 2014). Specifically, hypoactivity in the ventromedial prefrontal cortex (vmPFC) and hyperactivity in the hippocampus explained the link between high cumulative stress and physical symptoms (Seo, Tsou, et al., 2014). The vmPFC is known to be involved in emotion regulation (Golkar et al., 2012; Ochsner et al., 2002), while the hippocampus is involved in stress, memory (Kim & Diamond, 2002; Lupien & Lepage, 2001), and modulation of immune function (Devi et al., 2004; Xiong et al., 2016). These findings suggest that an overactive hippocampal response to stress resulting from decreased vmPFC regulatory control may underlie the link between trauma and physical symptoms. Although our prior study provided insight into the neural mechanisms of stress and physical pain in healthy individuals, it did not examine this phenomenon in trauma patients who are more likely to display stronger stress-related symptoms.
Furthermore, prior trauma studies measuring pain have been constrained by a retrospective approach to tracking pain and physical symptoms (Langford et al., 2018; Noel et al., 2016). A prospective approach where participants are tracked in real-time and in real-life settings can minimize participants’ recall bias (Shiffman et al., 2008) and yield more accurate data on stress and health-related symptoms (Smyth & Stone, 2003). These studies emphasize the need for prospective measures of physical symptoms to better understand the link between trauma and pain.
To extend our findings in community individuals from Seo, Tsou, et al. (2014) to clinical populations, the present study examined the associations between neural correlates of stress and prospectively measured physical symptoms in trauma patients and demographically-matched healthy controls. We used functional magnetic resonance imaging (fMRI) combined with a prospective follow-up method that tracked daily stress and physical symptoms in real-life settings via smartphone surveys. For stress manipulation during fMRI, we used a sustained stress provocation task that has been well-validated in prior neuroimaging studies (Goldfarb et al., 2020; Goldfarb et al., 2019; Sinha et al., 2016). Based on our prior results (Seo, Tsou, et al., 2014), we hypothesized that trauma patients would experience greater physical symptoms than healthy controls and exhibit altered activation of the vmPFC and hippocampus in response to stress exposure. In addition, we predicted that the degree of the altered vmPFC and hippocampal responses to stress would be associated with greater physical health symptoms.
2. Methods
2.1. Participants
Participants were 28 community adults (aged 19–48), including 10 individuals with trauma and 18 demographically-matched healthy controls (Table 1). No significant differences in age, sex, race, education, or intelligence were found between trauma patients and healthy controls (all p’s > 0.05). Participants were recruited through advertisements placed on social-networking sites and in local newspapers as well as flyers posted on community bulletin boards in the greater New Haven area.
Table 1.
Demographic and clinical characteristics.
| Trauma | Control | |
|---|---|---|
| (n = 10) | (n = 18) | |
| Demographics | ||
| Age (years) | 28.9 (11.1) | 27.9 (7.2) |
| Sex, female | 9 (90.0%) | 15 (83.3%) |
| Race, Caucasian | 6 (60.0%) | 10 (55.6%) |
| Education (years) | 14.3 (2.4) | 16.0 (2.3) |
| Shipley (IQ) | 113.0 (5.4) | 112.4 (8.0) |
| DSM-5 Trauma Diagnoses | ||
| PTSD (current) | 7 (70.0%) | 0 (0%) |
| Other Trauma Disorder (current) | 2 (20.0%) | 0 (0%) |
| PTSD (lifetime) | 1 (10.0%) | 0 (0%) |
| DSM-5 Comorbid Diagnoses | ||
| Anxiety (current) | 3 (30.0%) | 0 (0%) |
| Substance (current) | 1 (10.0%) | 0 (0%) |
Note: DSM-5 = Diagnostic and Statistical Manual of Mental Disorders 5; PTSD = Post-Traumatic Stress Disorder; Other Trauma Disorder = Other Specified Trauma- and Stressor-Related Disorder; Anxiety = Anxiety Disorders; Substance = Substance-Related and Addictive Disorders. Mean values (and standard deviations) are denoted for age, education, and Shipley IQ scores. Frequency (and percentages) are denoted for sex, race, and DSM-5 diagnoses. There were no significant group differences in any of these characteristics.
Participants in the control group were healthy individuals with no past or current Diagnostic and Statistical Manual of Mental Disorders 5 (DSM-5) diagnoses for any mental health disorders. Participants in the trauma group met current DSM-5 criteria for Trauma- and Stressor-Related Disorders, as determined by the Structured Clinical Interview for DSM-5 (SCID-5; First (2015)). One participant in the trauma group met lifetime—but not current—criteria for a DSM-5 trauma-related disorder. However, this participant exhibited significant trauma-related symptoms and had last experienced a traumatic event approximately one year prior to study participation and was thus included in the study. Given the high rate of comorbidity between trauma and other psychiatric disorders (Brady et al., 2000), the presence of other DSM-5 psychiatric diagnoses was non-exclusionary. All comorbid non-trauma-related DSM-5 diagnoses are presented in Table 1.
In order to ensure that results would not be influenced by the presence of physical disorders, potential participants were excluded if they had any significant medical conditions (e.g., seizures, thyroid disorders, cardiovascular diseases). Other exclusion criteria included MRI-related issues (e.g., claustrophobia, metal in body), history of head trauma, current pregnancy, and use of psychiatric medications. Additional eligibility criteria included the ability to read English and provide informed written and verbal consent. All study procedures were reviewed and approved by the Human Investigation Committee of the Yale School of Medicine.
2.2. Study procedure
Interested participants were initially screened over the phone to determine their eligibility. Eligible participants completed 3–4 intake appointments where they provided informed consent and completed additional in-person intake screenings. Participants also completed the Shipley Institute of Living Scale (Shipley, 1940) to assess their cognitive aptitude and intelligence (IQ). The Structured Clinical Interview for DSM-5 (SCID-5; First (2015)) was administered to determine whether participants met DSM-5 criteria for any current Trauma- and Stressor-Related Disorders. Upon completion of intake procedures, participants were scheduled for a functional magnetic resonance imaging (fMRI) scan session from 8:00am to 10:00am. All participants were instructed to abstain from using any drugs and/or medications for 48 hours prior to their fMRI scan to minimize drug-related confounding factors. Alcohol and drug abstinence were verified by breathalyzer and urine tests administered shortly before each scan. During the fMRI scan session, participants completed a well-validated stress provocation task (adapted from Sinha et al. (2016)) in which they were exposed to stressful and neutral-relaxing scenes. After completion of the fMRI scan, participants were prospectively followed with daily smartphone surveys that monitored their daily stress and physical symptoms for 30 consecutive days.
2.3. fMRI experimental task
During the fMRI scan, participants completed a stress provocation task (adapted from Sinha et al. (2016); see Figure 1), which was administered with E-Prime 2.0 (Psychology Software Tools, Inc.). The stress provocation task included stressful and neutral-relaxing visual stimuli in a fMRI block design, with each condition presented in a separate block (Figure 1). Condition order was randomized across participants. Stressful images (e.g., scenes of violence, injury, and terror) were selected from the International Affective Picture System (IAPS; Lang et al. (1997)). Neutral-relaxing images (e.g., scenes of nature and people relaxing) were selected from validated pictures sets (Blaine et al., 2020; Goldfarb et al., 2020; Sinha et al., 2016). Pre-task ratings for mean valence (1 = negative to 9 = positive) and arousal (1 = calm/relaxed to 9 = aroused/excited) were Mvalence = 2.34 (SD = 0.63) and Marousal = 6.00 (SD = 0.83) for stress images, and Mvalence = 6.07 (SD = 0.40) and Marousal = 3.63 (SD = 0.47) for neutral-relaxing images.
Figure 1. fMRI task design.
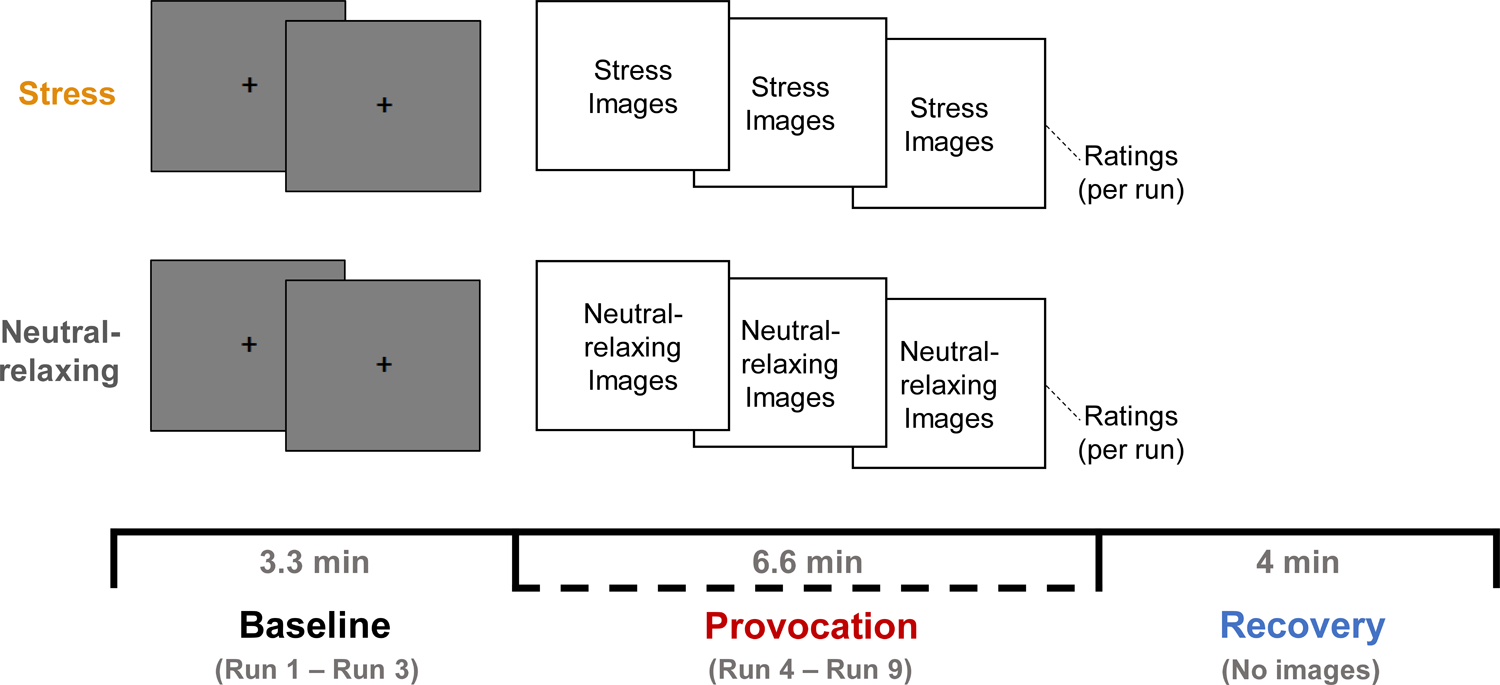
The fMRI stress provocation task (adapted from Sinha et al. (2016)) consisted of two blocks representing the stress and neutral-relaxing conditions. Condition order was counterbalanced across participants. Each block consisted of three phases: baseline, provocation, and recovery. During the baseline phase, blank gray images with central fixation cross were displayed for three runs (Runs 1–3). During the provocation phase, participants viewed either stressful or neutral-relaxing images for six runs (Runs 4–9). During each run, 11 images (or gray blanks) were shown in succession for 5 seconds each with a 1-second interstimulus interval (ISI), for a total of 66 seconds (1.1 minutes) per run. During the recovery phase (4 minutes), participants were asked to relax and were not shown any images. Participants were asked to rate their stress and arousal using two-button fMRI response pads. Stress (1 = not at all stressed to 9 = extremely stressed) and arousal (1 = calm/relaxed to 9 = aroused/excited) ratings were collected after each baseline and provocation run and after each recovery phase.
The fMRI stress provocation task (Goldfarb et al., 2020; Goldfarb et al., 2019; Sinha et al., 2016) consisted of two randomly ordered blocks in which participants were exposed to either stressful or neutral-relaxing scenes (Figure 1). In each block, participants completed three sequential phases: baseline, provocation, and recovery. During the baseline phase, blank gray images with central fixation cross were displayed for three runs (Runs 1–3). During the provocation phase, participants viewed either stressful or neutral-relaxing images for six runs (Runs 4–9). In each run, 11 images were shown in succession for 5 seconds each with a 1-second interstimulus interval (ISI), for a total of 66 seconds (1.1 minutes) per run. The level of emotional intensity of images was matched across the six provocation runs in each condition, verified by no statistical differences in IAPS normative ratings of valence or arousal, as previously described (Blaine et al., 2020; Sinha et al., 2016). During the recovery phase (4 minutes), participants were asked to stay still and relax and were not shown any images as they were scanned. In-between blocks (stress followed by neutral-relaxing, or vice versa), a 2-minute progressive relaxation recording was played to ensure that physiological levels returned to baseline, as described in previous work (Seo et al., 2013; Sinha et al., 2016). After each baseline/provocation run and after the recovery phase, participants were asked to provide ratings of their stress (1 = not at all stressed to 9 = extremely stressed) and arousal (1 = calm/relaxed to 9 = aroused/excited) using the Self-Assessment Manikin method (SAM; Bradley and Lang (1994)) and two-button fMRI response pads.
2.4. Daily smartphone surveys
After the fMRI scan session, participants completed daily smartphone surveys over a 30-day follow-up period where they reported their daily stress and physical symptoms. The surveys were programmed and administered using a HIPAA-compliant smartphone application (MetricWire Inc., ON, Canada). Participants received each daily survey at 5:00pm (EST) on their smartphone devices. This time of day was selected to sync up with the end of the standard business day to maximize compliance rates. Participants were given a seven-hour window from 5:00pm to 2:00am of the next calendar day to complete each daily survey, which required approximately 10–15 minutes of their time. On the daily survey, participants were asked whether they had experienced any physical symptoms since completing the previous day’s survey including: “headache,” “stomachache/indigestion,” “chest pain,” “allergy symptoms,” “dizziness/fainting feeling,” “shortness of breath,” “fatigue/weakness,” “muscle aches/pain,” “cold symptoms/flu,” and “other.” Choosing “other” prompted participants to specify any additional physical symptoms. Frequency of physical symptoms was indexed by the number of days on which at least one physical symptom was reported (mean: 10.9 days, range: 0–29 days). Participants were also asked to rate their daily stress levels using a sliding scale ranging from 1 to 100 (1 = not at all stressed; 100 = extremely stressed). The daily protocol achieved a very high rate of survey compliance (91.4%). No significant differences in compliance rates were observed between trauma patients and healthy controls, t(26) = 0.46, p = 0.65.
2.5. fMRI data acquisition
MRI data were acquired using a T2*-sensitive gradient-recalled single-shot echo-planar pulse sequence on a 3T Siemens Prisma MRI system equipped with a standard quadrature head coil. For functional MRI data, 75 axial slices parallel to the AC-PC line covering the whole brain were acquired with the following acquisition parameters: TR = 1000 ms, TE = 30 ms, bandwidth = 1894 Hz/pixel, flip angle = 55°, field of view = 220 × 220 mm, slice thickness = 2 mm with no gap. Blood-oxygen-level-dependent (BOLD) signals were acquired with a 64-channel head coil with a multiband accelerated echo planar imaging (EPI) sequence. A high-resolution 3D magnetization-prepared rapid gradient-echo (MP-RAGE) sequence was used to acquire sagittal anatomical images.
2.6. fMRI data analysis
fMRI data processing and analysis followed the procedures described previously (Sinha et al., 2016). XMedCon (Nolf et al., 2003) was used to convert fMRI data from Digital Imaging and Communication in Medicine (DICOM) format to Analyze format. During conversion, the first 3 images of every functional run were discarded to enable the signal to achieve steady-state equilibrium between radiofrequency pulsing and relaxation. Images were motion corrected for three translational and three rotational directions (Friston et al., 1996), and any trials with linear motion exceeding 1.5 mm or rotation exceeding 2° were discarded. To address possible temporal autocorrelation in multiband imaging, a Restricted Maximum Likelihood (ReML) pre-whitening procedure was applied to the data (Olszowy et al., 2019).
An individual-level General Linear Model (GLM) analysis was performed on each voxel for the entire brain volume with a regressor (provocation – baseline) specific to each experimental condition (stress, neutral-relaxing) using BioImage Suite (Duncan et al., 2004). All functional images for each condition were first spatially smoothed with a 6 mm Gaussian kernel, and individually normalized beta maps were generated in the acquired space (3.44 mm × 3.44 mm × 4 mm). To account for variations in brain anatomy across individuals, three sequential registrations were applied to the individually normalized beta maps, as previously described (Seo et al., 2013; Sinha et al., 2016).
For group level analysis, data were converted to Analysis of Functional NeuroImages (AFNI) format (Cox, 1996). Then, whole-brain voxel-based group analysis was conducted using AFNI’s 3dLME program (Chen et al., 2013). Linear mixed-effects (LME) models were estimated specifying a random intercept varying by subject. The fixed effects included Group (trauma, control) as a between-subjects factor and Condition (stress, neutral-relaxing) as a within-subjects factor. Family-wise error (FWE) rate correction for multiple comparisons was conducted using Monte Carlo simulations (Xiong et al., 1995) on AFNI’s 3dClustSim program (version 16.0.09). Finally, the voxel-wise threshold was set at p = 0.001 and cluster-level threshold at α < 0.05 FWE-corrected.
To examine associations between fMRI responses and prospective physical symptoms, we used two data analytic approaches: (1) whole-brain voxel-based regression analysis and (2) regions of interest (ROI) analysis using two hypothesized a priori regions (vmPFC, hippocampus; based on Seo, Tsou, et al. (2014)). Whole-brain voxel-based analysis was conducted with AFNI’s 3dLME regression program. For ROI analysis, two a priori regions—the vmPFC (BA11) and the hippocampus—were selected based on a previous work in a community sample reporting associations between physical symptoms and brain responses to stress in these regions (Seo, Tsou, et al., 2014). ROIs were defined using the Yale-Brodmann atlas in the Yale BioImage Suite application (Lacadie et al., 2008). To examine the relationship between activation in the a priori brain regions (vmPFC and hippocampus) and physical symptoms, beta values were first extracted from each ROI and then associated with frequency of physical symptoms over the entire 30-day follow-up period using Ordinary Least Squares (OLS) regression analysis. The Cook’s distance statistic (Cook’s D) was used to check for extreme and/or influential cases. No outliers were identified in the associations reported in the current study.
3. Results
3.1. fMRI task ratings
To evaluate group differences in stress and arousal ratings for each condition across time, a 2 × 3 × 2 mixed factorial analysis of variance (ANOVA) was conducted with Group (trauma, control) as the between-subjects factor and Time (baseline, provocation, recovery) and Condition (stress, neutral-relaxing) as within-subjects factors.
The results indicated significant main effects of Condition and Time and a significant Condition × Time interaction for stress and arousal ratings. The stress condition elicited higher stress and arousal ratings during provocation than at baseline, which was not observed in the neutral-relaxing condition. These findings are described in greater detail as follows. A significant main effect of Condition was found for stress (F(1,26) = 27.14, p < 0.001) and arousal ratings (F(1,26) = 35.66, p < 0.001); the stress provocation elicited higher stress (t(27) = 1.63, p < 0.001) and arousal ratings (t(27) = 1.23, p < 0.001) than the neutral-relaxing condition. A main effect of Time indicated higher stress ratings in the provocation (t(27) = 1.75, p < 0.001) and recovery (t(27) = 0.74, p < 0.05) phases than in baseline, as well as higher stress ratings in provocation than in recovery (t(27) = 1.00, p < 0.001). Arousal ratings were also higher in the provocation phase compared to baseline (t(27) = 1.12, p < 0.001) and recovery (t(27) = 0.65, p < 0.01), but no difference was observed between recovery and baseline arousal. A significant Condition × Time interaction for stress (F(2,52) = 30.57, p < 0.001) and arousal ratings (F(2,52) = 27.03, p < 0.001) indicated that time-related effects were only significant in the stress condition but not in the neutral-relaxing condition. During the stress condition, the provocation phase induced higher stress and arousal ratings than both the baseline (stress: t(27) = 3.55, p < 0.001; arousal: t(27) = 2.55, p < 0.001) and recovery (stress: t(27) = 2.10, p < 0.001; arousal: t(27) = 1.37, p < 0.001) phases. Higher stress and arousal ratings were also found in the recovery phase relative to baseline (stress: t(27) = 1.45, p < 0.01; arousal: t(27) = 1.18, p < 0.01).
There were no significant Group or Group × Time effects. A significant Group × Condition interaction (F(1,26) = 9.75, p < 0.01) was found only for arousal. Arousal ratings of the trauma group tended to be higher in the stress condition, while arousal ratings of the control group tended to be higher in the neutral-relaxing condition. Despite the significant interaction effect, simple effect t-test comparisons of group differences in each condition did not reach significance at p < 0.05.
The three-way Group × Condition × Time interaction effects on stress and arousal ratings were not statistically significant, probably due to the relatively small sample size and resulting lack of statistical power in our study to detect three-way interaction effects. However, time-related stress responses during stress provocation were predominantly observed in the trauma group. After the fMRI stress provocation, the trauma group’s stress ratings in the recovery phase remained significantly higher than baseline (t(9) = 1.97, p < 0.05), which was not observed in the control group (Figure 2a).
Figure 2. fMRI task ratings.
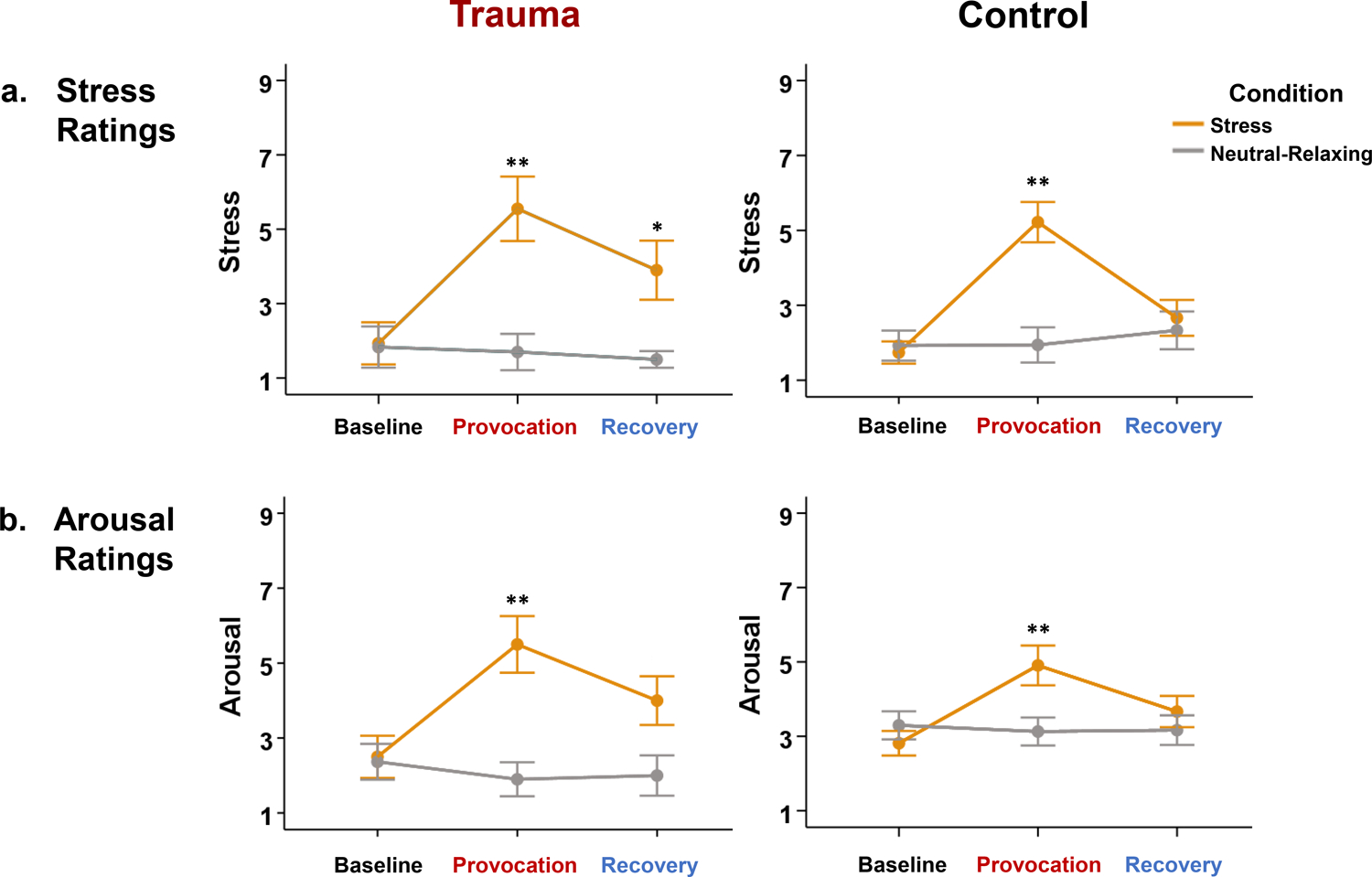
(a) Stress Ratings: Both trauma patients and healthy controls reported significantly higher stress ratings during stress provocation than at baseline (p < 0.01). However, only trauma patients exhibited elevated stress ratings after stress recovery relative to baseline (p < 0.05). (b) Arousal Ratings: Both trauma patients and healthy controls reported significantly higher arousal ratings during stress provocation than at baseline (p < 0.01). Note: *p < 0.05, **p < 0.01. Error bars represent ± 1 standard error (SE) of the mean.
3.2. Physical symptoms and stress
Group differences in prospective physical symptoms were examined using data collected via daily smartphone surveys. Overall, trauma patients experienced significantly more frequent physical symptoms during the 30-day follow-up period than healthy controls (Figure 3a; t(26) = 3.57, p < 0.01), reporting physical health-related symptoms on an average of 17.7 days (trauma) versus 7.1 days (control). The trauma group also reported significantly higher daily stress levels over the follow-up period than the control group (t(26) = 2.27, p < 0.05), with a mean stress level of 42.3 (trauma) versus 28.0 (control) out of 100.
Figure 3. Physical symptoms and stress.
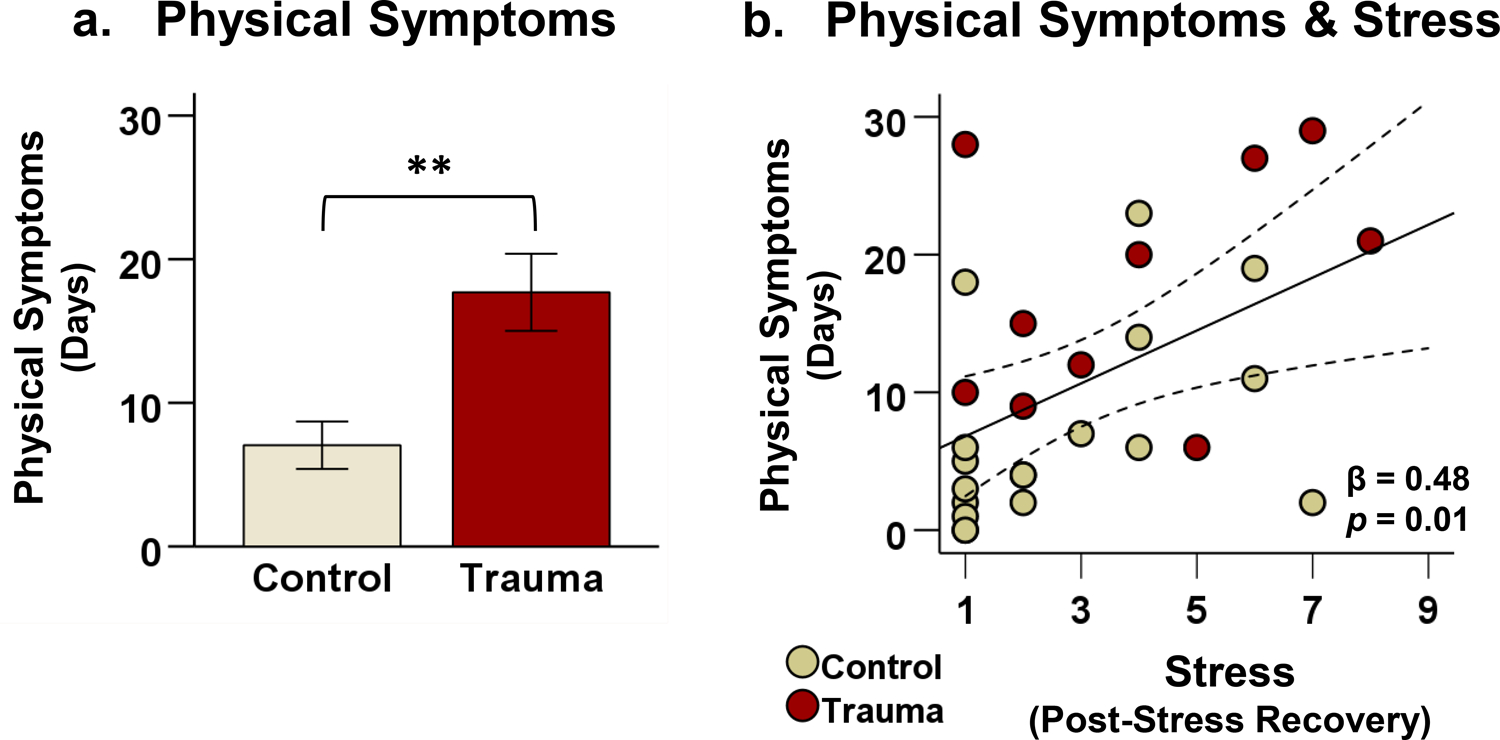
After the fMRI scan, participants completed daily smartphone surveys monitoring their stress and physical symptoms over a 30-day follow-up period. (a) Trauma patients experienced a higher frequency of physical symptoms (indexed by the number of days on which at least one physical symptom was reported) compared to healthy controls, t(26) = 3.57, p < 0.01. (b) In all participants, higher fMRI stress ratings after stress recovery were associated with a greater number of days with physical symptoms in the follow-up period (β = 0.48, p = 0.01). Note: **p < 0.01. Error bars represent ± 1 standard error (SE) of the mean.
Furthermore, individual differences in stress ratings in the recovery phase after fMRI stress provocation prospectively predicted the frequency of physical symptoms in follow-up, such that increased post-recovery stress ratings were associated with more days with physical symptoms (Figure 3b; β = 0.48, p = 0.01). This association remained significant even after controlling for psychiatric comorbidities as a covariate.
However, physical symptom frequency was not associated with stress ratings from baseline or provocation of the stress condition, nor with any stress ratings from the neutral-relaxing condition. In addition, no significant associations were found between physical symptoms and arousal ratings from either condition.
Table 2 displays a breakdown by category of every daily physical symptom reported by each group during the smartphone follow-up. Trauma patients reported a significantly greater quantity of daily physical symptoms over the follow-up period than controls (t(26) = 3.61, p < 0.01). In all participants, the total quantity of daily physical symptoms was positively associated with stress ratings in the recovery phase after fMRI stress provocation (β = 0.42, p < 0.05).
Table 2.
Daily physical symptoms reported during the follow-up period by category.
| Physical Symptom | Trauma (n = 10) |
Control (n = 18) |
|---|---|---|
| Headache | 61 | 53 |
| Stomachache/Indigestion | 16 | 11 |
| Chest pain | 3 | 3 |
| Allergy symptoms | 25 | 6 |
| Dizziness/Fainting feeling | 27 | 3 |
| Shortness of breath | 7 | 1 |
| Fatigue/Weakness | 56 | 9 |
| Muscle aches/pain | 66 | 43 |
| Cold symptoms/Flu | 20 | 16 |
| Other | ||
| Bursitis | 0 | 1 |
| Clenched jaw | 2 | 0 |
| Shakiness | 1 | 0 |
| Itching/Eczema | 3 | 0 |
| Unspecified | 2 | 1 |
| Total** | 289 | 147 |
Note: **p < 0.01. Trauma patients endorsed a significantly higher quantity of daily physical symptoms than controls. Each count denotes each instance that a physical symptom was reported on a daily survey. The overall survey compliance rate over the 30-day follow-up period was 91.4%, with no significant difference observed between trauma patients and healthy controls.
3.3. fMRI results
3.3.1. Group differences in neural response to stress
Whole-brain voxel-based analysis indicated significant group differences in the fMRI BOLD response during the stress condition but not in the neutral-relaxing condition. During stress provocation, trauma patients exhibited decreased activity relative to controls in the ventromedial prefrontal cortex (vmPFC), lateral prefrontal cortex (LPFC), temporal lobe (superior, middle, and inferior temporal gyri), and dorsal striatum (caudate and globus pallidus) but increased activity in the left hippocampus, right insula, and parts of the occipital cortex (visual association areas), fusiform gyrus, parietal lobe (angular and supramarginal gyri), primary motor cortex, and midcingulate cortex (Figure 4a; p < 0.001, α < 0.05, whole-brain FWE-corrected).
Figure 4. fMRI results.
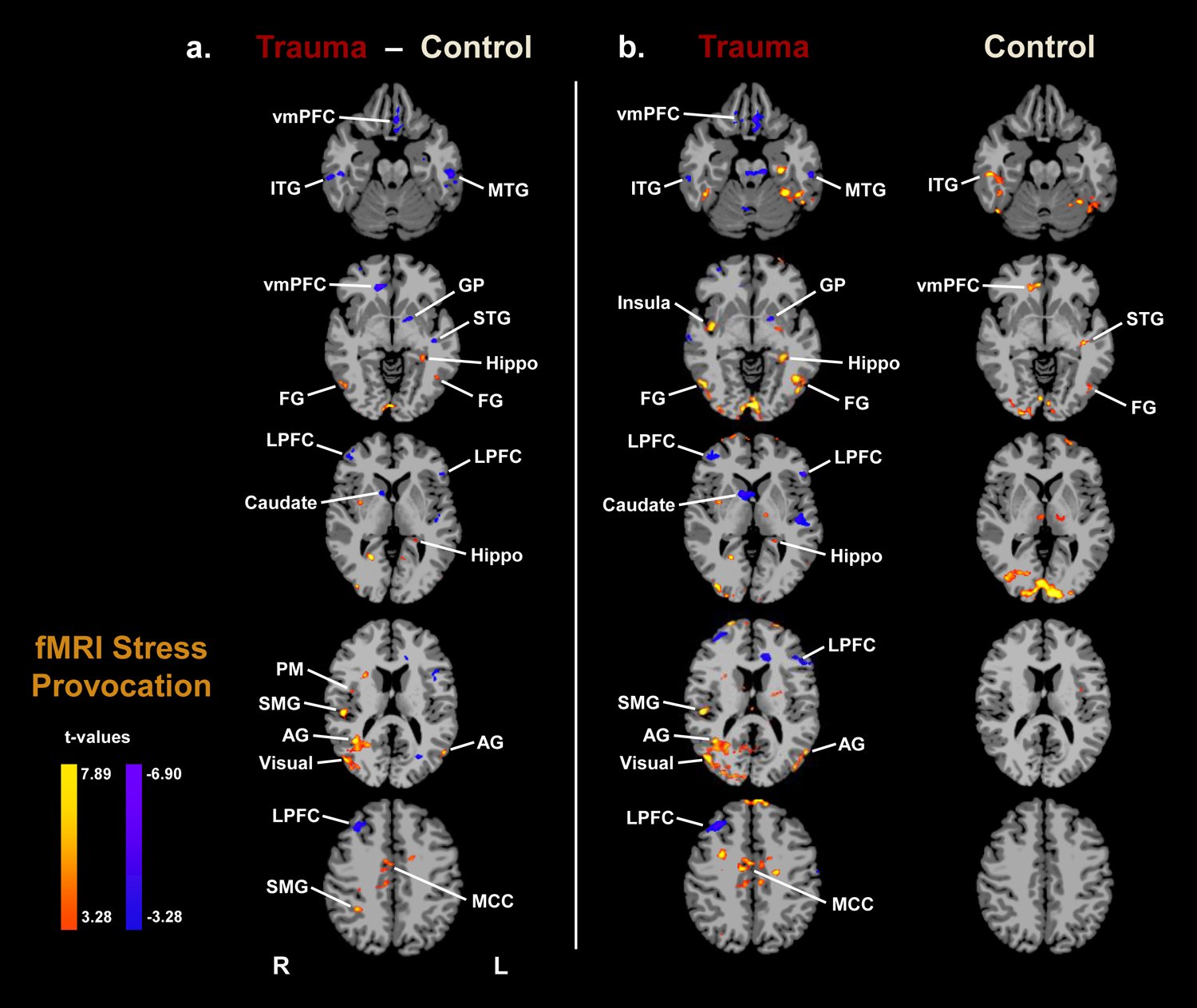
Whole-brain voxel-based analysis indicated significant group differences in the fMRI BOLD response to stress. (a) Group differences during stress provocation (Trauma − Control): Trauma patients, relative to controls, showed decreased activity in the ventromedial prefrontal cortex (vmPFC), lateral prefrontal cortex (LPFC), temporal lobe (superior, middle, and inferior temporal gyri), and dorsal striatum (caudate and globus pallidus) but increased activity in the left hippocampus, right insula, occipital cortex (visual association areas), parietal lobe (angular and supramarginal gyri), fusiform gyrus, primary motor cortex, and midcingulate cortex (p < 0.001, α < 0.05, whole-brain family-wise error corrected). A blue/purple color denotes decreased brain activity (Trauma < Control), while a yellow/red color denotes increased brain activity (Trauma > Control). (b) The fMRI BOLD response to stress provocation relative to baseline are displayed by group (p < 0.001, α < 0.05, whole-brain family-wise error corrected).
Note: R = Right; L = Left; vmPFC = ventromedial prefrontal cortex; LPFC = lateral prefrontal cortex; Hippo = hippocampus; GP = globus pallidus; ITG = inferior temporal gyrus; MTG = middle temporal gyrus; STG = superior temporal gyrus; FG = fusiform gyrus; SMG = supramarginal gyrus; AG = angular gyrus; Visual = visual association areas; PM = primary motor cortex; MCC = midcingulate cortex.
3.3.2. Neural correlates of physical symptoms and stress
To understand the relationship between brain activity and physical symptoms, we used two approaches: whole-brain and regions of interest (ROI) analysis. Using whole-brain voxel-based regression analysis, no significant associations were found between task-related activity and frequency of physical symptoms at p < 0.001 (α < 0.05, whole-brain FWE-corrected), which may have been due to the small sample size. Given the small number of participants in the study (n = 28), we also used an ROI approach based on Seo, Tsou, et al. (2014) and explored the associations between a priori regions (vmPFC and hippocampus) and frequency of physical symptoms during the prospective follow-up period. ROI analyses detected significant associations in the hippocampus but not in the vmPFC. In all participants, BOLD activity in the left hippocampus during stress provocation was positively associated with a greater frequency of physical symptoms (indexed by number of days with physical symptoms) in the follow-up period (Figure 5a; β = 0.42, p < 0.05). However, no significant association was found between left hippocampal activity and total quantity of physical symptoms. In addition, left hippocampal hyperactivity was associated with significantly higher stress ratings in the recovery phase after fMRI stress provocation (Figure 5b; β = 0.51, p < 0.01). Both of these associations (Figure 5) remained significant even after controlling for psychiatric comorbid diagnoses as covariates, indicating that psychiatric comorbidities did not significantly affect the findings.
Figure 5. Neural correlates of physical symptoms and stress.
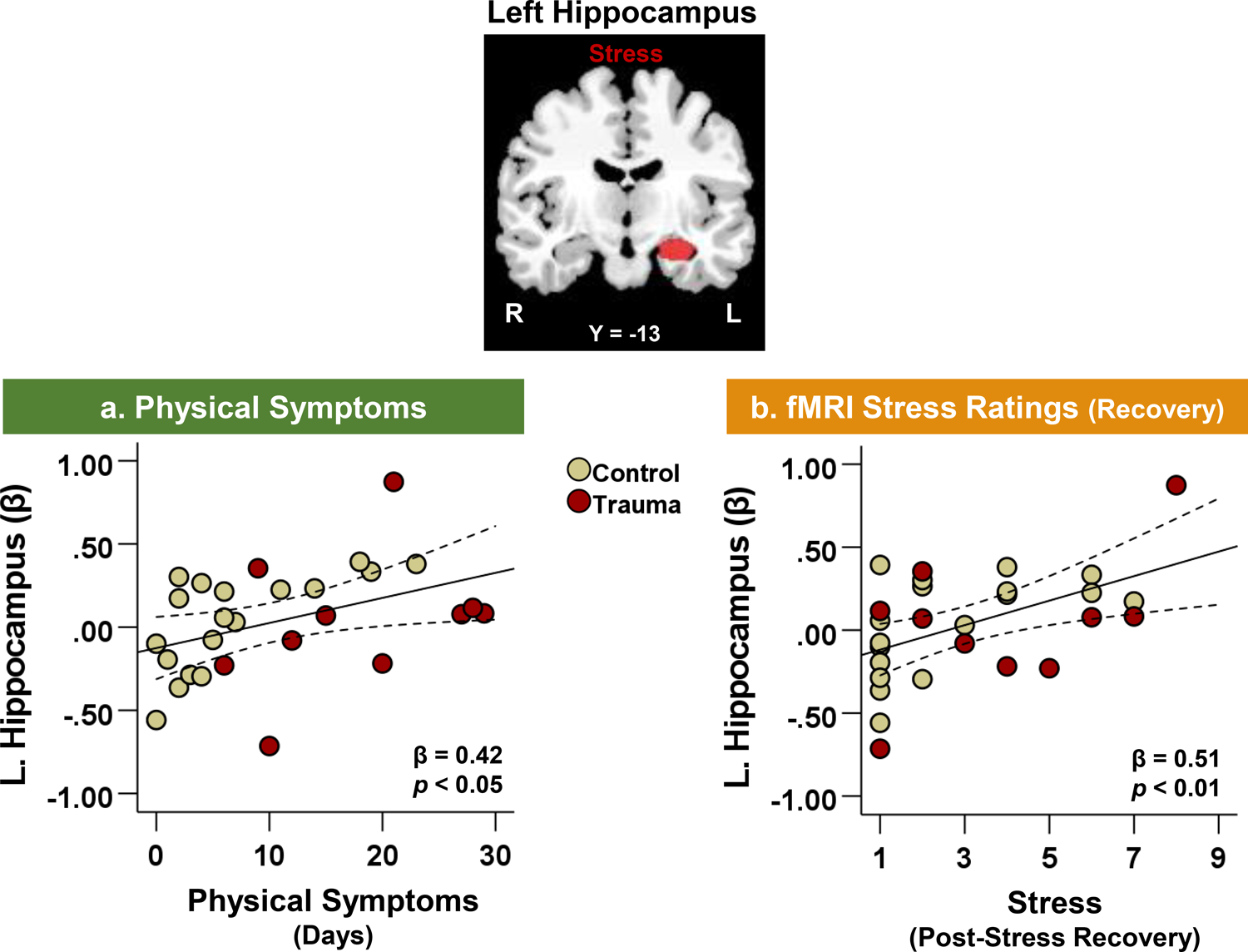
Left hippocampal activity during fMRI stress provocation was positively associated with (a) number of days with physical symptoms over the 30-day follow-up period (β = 0.42, p < 0.05) and (b) fMRI stress ratings after stress task recovery (β = 0.51, p < 0.01).
4. Discussion
4.1. Summary
We investigated the neural correlates of trauma and physical symptoms using fMRI and a prospective daily follow-up. Trauma patients experienced more frequent physical symptoms over the 30-day follow-up when compared to healthy controls. In addition, trauma patients exhibited an altered neural response to stress, marked by hypoactivity in emotion regulation regions (vmPFC, LPFC, temporal lobe, dorsal striatum) and hyperactivity in regions of emotional reactivity (insula, hippocampus) and sensorimotor processing (occipito-parietal, motor, and midcingulate cortices). In particular, hyperactivity in the left hippocampus during stress provocation was associated with more frequent physical symptoms in the follow-up period. Our findings suggest that trauma patients may have altered function in brain regions involved in emotion and pain regulation, which may increase their vulnerability to co-occurring stress- and pain-related disorders.
4.2. Trauma, stress, and physical symptoms
In the present study, trauma patients exhibited more frequent physical symptoms than controls during the follow-up period. This finding is in accord with prior studies reporting a greater incidence of physical symptoms and pain in trauma patients (Asmundson et al., 2002; Fishbain et al., 2017; Noel et al., 2016; Pietrzak et al., 2011; Woods et al., 2008). The greater physical pain symptoms displayed in trauma patients may be understood within the framework of allostatic load, wherein trauma and chronic stress progressively burden bodily systems, which significantly increases the odds of developing a range of physical and psychiatric conditions (McEwen, 1998, 2008). In support of this, a recent meta-analysis found that patients who experienced greater acute post-traumatic stress symptoms were at increased risk of later developing other adverse health symptoms including physical pain (Garfin et al., 2018).
In addition, the trauma group tended to have higher stress after recovery from stress exposure than controls. Elevated stress levels after removal of stressful stimuli suggest that the trauma patients had difficulties with properly extinguishing their negative emotions. Fear extinction difficulties are a key feature of stress- and trauma-related disorders (Zuj et al., 2016). Both preclinical and clinical studies have observed lasting negative affect resulting from improper extinction in highly stressed rodents (Knox et al., 2012; Maren & Holmes, 2016) and in trauma patients (Wessa & Flor, 2007; Wicking et al., 2016).
Furthermore, our data showed an association between more frequent physical symptoms and elevated post-stress recovery ratings, suggesting a potential link between physical pain and an inability to extinguish emotional distress in trauma patients. An association between pain and lasting distress can be explained by neuroscience literature indicating a shared neural circuit of pain and negative emotion (Alba-Delgado et al., 2013; Cai et al., 2018; Vogt, 2005). Consistent with this, recent studies have reported associations between increased physical pain and greater PTSD symptom severity (Ahmadian et al., 2019; Langford et al., 2018). The dysregulation of overlapping neural circuits of emotion and pain may contribute to an increased susceptibility to stress and physical symptoms over time.
4.3. Neural correlates of stress in trauma patients
During stress exposure, trauma participants exhibited hypoactivity in regions of emotion regulation (prefrontal cortex, temporal lobe, and dorsal striatum) and hyperactivity in the insula, hippocampus, and regions of sensorimotor processing (occipito-parietal, motor, and midcingulate cortices). These neural patterns suggest that compromised regulatory control over limbic and sensorimotor regions may underlie emotional dysregulation during acute stress in trauma patients.
The prefrontal cortex (PFC) plays a major role in the regulation of stress and negative emotion, with the ventromedial part of the PFC being involved more in the emotional aspect (Hiser & Koenigs, 2018; Maier & Watkins, 2010; Seo et al., 2013; Sinha et al., 2016), while the lateral PFC is involved more in the cognitive aspect (Etkin et al., 2015; Moodie et al., 2020; Morawetz et al., 2017) of emotion regulation. The vmPFC also plays a role in the extinction of fear and negative emotion (Delgado et al., 2008; Milad et al., 2007). Hypoactivity in the vmPFC has previously been observed in trauma patients exposed to negative stimuli (Etkin & Wager, 2007; Hughes & Shin, 2011; Liberzon & Sripada, 2008) as well as in PTSD patients with fear extinction deficits (Milad et al., 2009). The LPFC is crucial in the cognitive control of emotion, specifically in diminishing negative affect under stress (Wager et al., 2008). Past studies have shown that PTSD patients exhibited decreased LPFC activity during stress exposure (Lanius et al., 2007) and that lesions to the LPFC led to heightened negative emotion in primates (Agustin-Pavon et al., 2012), highlighting the importance of PFC integrity in stress regulation. Consistent with previous studies, the decreased PFC response to stress in our trauma patients may indicate compromised regulatory control during stress provocation.
Trauma patients also showed stress-induced hypoactivity in the temporal lobe and dorsal striatum. Prior studies have reported the involvement of the temporal lobe in perceiving and processing emotion (Robins et al., 2009; Rosen et al., 2006). The temporal lobe also plays a role in emotion regulation by assisting the PFC in properly perceiving and appraising emotional stimuli (Seo, Olman, et al., 2014). In the present study, activity in the temporal lobe was decreased in the trauma group during stress provocation, whereas it was increased in the control group. This suggests that trauma patients may have difficulties with recruiting the necessary neural resources for properly appraising their emotional distress in response to stressful stimuli. The dorsal striatum is a region involved in action-oriented responses and habitual behavior (Balleine et al., 2007; Graybiel & Grafton, 2015; Yin, Knowlton, et al., 2005; Yin, Ostlund, et al., 2005). Activity in the dorsal striatum typically increases in response to stressful stimuli in healthy individuals (Seo et al., 2011; Sinha & Li, 2007), suggesting that the dorsal striatal hypoactivity observed in our trauma patients may reflect more habitual—and less proactive—responses to stress. Taken together, trauma patients’ decreased activity in the temporal lobe and dorsal striatum may reflect compromised emotional appraisal and habitual emotional responses in the face of stressful events.
In contrast to these regions, hyperactivity was observed in trauma patients during stress exposure in regions of emotional reactivity (insula, hippocampus) and sensorimotor processing (occipito-parietal, motor, and midcingulate cortices). The insula is involved in interoceptive signaling and perception of bodily arousal (Craig, 2009; Critchley et al., 2004). In PTSD patients, increased activity in the insula has been linked to heightened interoception and hypersensitivity to threat and negative emotion (Lanius et al., 2015; Pitman et al., 2012). This is consistent with prior findings of insular hyperactivity in trauma patients in response to emotionally distressing stimuli (Hopper et al., 2007; Mazza et al., 2013). It follows that the increased insular response to stress observed in our trauma patients may indicate an increased sensitivity to stress-related bodily arousal and heightened negative affect. The hippocampus is involved in the processing of emotionally distressing stimuli (McEwen & Magarinos, 2001) and emotional memory storage (Desmedt et al., 2015; Phelps et al., 2004). It is a region that is particularly vulnerable to chronic stress (McEwen, 1999, 2001), and altered hippocampal function is commonly associated with trauma (Kunimatsu et al., 2020). Prior studies have shown that PTSD patients exhibited increased hippocampal activity while encoding negative stimuli (Brohawn et al., 2010; Thomaes et al., 2013; Thomaes et al., 2009) and when viewing trauma-related images (Tural et al., 2018). Thus, the hyperactive hippocampal response to stress observed in our study may indicate increased stress sensitivity and excessive stress-related processing in trauma patients.
Trauma patients also exhibited increased stress-induced activity in regions of sensorimotor processing including parts of the occipito-parietal, primary motor, and midcingulate cortices. Hyperactivity in the occipital lobe has been associated with greater visual processing (Press et al., 2001), while hyperactivity in the parietal lobe (angular and supramarginal gyrus) has been associated with greater sensory awareness (Ben-Shabat et al., 2015; Farrer et al., 2008). It follows that the trauma group’s increased sensitivity to emotionally distressing stimuli may be related to the higher activity observed in these visuosensory processing regions. The trauma group also showed hyperactivity in the primary motor and midcingulate cortices. Among other functions, the primary motor cortex is also involved in emotional-affective processing (Hajcak et al., 2007; Leite et al., 2017). Using paired transcranial magnetic stimulation (pTMS), a prior study observed increased excitability in the motor cortex of trauma patients, which may underlie the development of common PTSD symptoms such as hyperarousal (Centonze et al., 2005). The midcingulate cortex (MCC) is involved in the monitoring of sensorimotor processing (Vogt, 2016). It has been suggested that emotional information from the parietal cortex is first projected to the MCC and then transmitted from the MCC to the motor cortex to enhance arousal and initiate action (Rolls, 2019). In addition, increased MCC activity has been implicated in the experiencing of pain and negative affect (Tolomeo et al., 2016). Taken together, these studies suggest that the increased activity in these regions in our trauma patients reflects a greater engagement in sensorimotor processing, monitoring, and arousal than controls when viewing stressful images.
In sum, trauma patients exhibited stress-related hypoactivity in emotion regulation regions but hyperactivity in limbic and sensorimotor processing regions, which may reflect compromised prefrontal control over their emotional and sensory responses to stress. The neural alterations observed in trauma patients may underlie their difficulties with stress and emotion regulation.
4.4. Neural correlates of physical symptoms
In our study, we found that altered hippocampal function was associated with greater emotional and physical difficulties. Specifically, a hyperactive left hippocampal response to stress was associated with emotional distress even after removal of stressful stimuli during the fMRI task and with more frequent physical symptoms during the prospective follow-up. This suggests that left hippocampal hyperactivity may underlie the greater negative emotion and physical symptoms experienced by trauma patients. The hippocampus is highly vulnerable to chronic stress and trauma (McEwen, 1999, 2001), and it has been well established that PTSD is associated with altered hippocampal function and structure (Bremner et al., 1995; Gilbertson et al., 2002; Kunimatsu et al., 2020; Logue et al., 2018). While previous studies have also observed associations between hippocampal hyperactivity and emotional difficulties in trauma patients (Huang et al., 2014; Koch et al., 2016; Sachinvala et al., 2000), this is the first finding to extend this relationship to their physical symptoms.
In addition to well-established hippocampal alterations, prior studies have consistently observed immune dysfunction in trauma patients (Ayaydin et al., 2016; Neigh & Ali, 2016). For example, PTSD patients have exhibited disproportionately high rates of chronic inflammation (Gill et al., 2009; O’Donovan, 2016; O’Donovan et al., 2017; Speer et al., 2018), which is often accompanied by pain symptoms and an increased susceptibility to physical disease (Bennett et al., 2018; Couzin-Frankel, 2010). Therefore, a possible link between trauma-related hippocampal alterations and physical pain may lie in the hippocampus’ involvement in neuroimmune processes (Williamson & Bilbo, 2013). In particular, the hippocampus plays a key role in regulating the HPA axis (Jacobson & Sapolsky, 1991; Radley & Sawchenko, 2011) which is involved in modulation of the immune system via glucocorticoids (Bellavance & Rivest, 2014; Pierre et al., 2016). This is consistent with prior studies reporting assocations between hippocampal alterations and increased inflammatory biomarkers in healthy individuals (Marsland et al., 2008; Schmidt et al., 2016). Taken together, these studies along with our findings suggest that traumatic experiences may sensitize the hippocampus and disrupt its modulation over neuroimmune systems, leading to compromised immune function and a higher incidence of pain and physical symptoms in trauma patients.
5. Conclusion
To summarize, the present study identified patterns of stress-related neural alterations and neural correlates of physical symptoms in trauma patients. In response to stress, trauma patients exhibited neural alterations characterized by hypoactivity in prefrontal regulatory regions and hyperactivity in regions of emotional and sensorimotor processing. These findings suggest that compromised regulatory control and disinhibited emotional reactivity may underlie the emotional difficulties that trauma patients often experience under stress. Notably, we extended our findings from a prior study of healthy community individuals (Seo, Tsou, et al., 2014) to a clinical sample; we identified stress-related activity in the hippocampus, a modulator of chronic stress and the immune system, as a neural correlate of physical health symptoms in trauma patients. It is of note that the relationship between hippocampal hyperactivity and greater physical symptoms was found in both trauma patients and healthy individuals, which suggests that a dimensional approach may be appropriate when translating hippocampal responses to stress and physical health symptoms. It is likely that high-intensity stress experiences may alter hippocampal function and related modulation over immune processes, thus increasing susceptibility to physical dysfunction across healthy and clinical populations.
Although our study provides important insights into the neural link between stress, trauma, and physical symptoms, it has some limitations. It should be noted that our study had a relatively small sample size and a majority of female participants. Future investigations with more male participants may be able to uncover the role of sex in the relationship between stress, trauma, and pain. In addition, the potential impact of comorbid psychiatric conditions should be considered in understanding the associations between stress, trauma, and physical pain. Our main findings on these associations remained significant even after controlling for psychiatric comorbid disorders. However, given that our sample size was small, it is possible that the presence of comorbid conditions might differentially influence physical pain symptoms in a large population. As trauma-related psychiatric comorbidities are well-documented (Brady et al., 2000; Fox et al., 2020), more study is warranted to better isolate the effects of trauma on physical pain. Furthermore, we note that our findings are largely correlational and cannot be relied upon to determine the specific directionality and causality of the relationship between trauma and hippocampal alterations; it may be that pre-existing (non-trauma-related) individual differences in hippocampal function increase the risk of developing stress-related psychological and physical sequelae in response to traumatic events. Despite these limitations, our study findings may still have important clinical implications for trauma- and stressor-related disorders as we report potential neural correlates of stress and physical symptoms in trauma patients. These correlates may be useful in the early identification of trauma-related complications, such as chronic pain and other comorbid physical disorders. Future studies should further investigate the utility of these correlates, especially their associations with immune system dysfunction in trauma patients.
In conclusion, the neural correlates of stress and physical symptoms found in our study may serve as potential neural markers of comorbid psychological and physical dysfunction in trauma patients. These markers may help identify trauma patients who may be vulnerable to chronic, pain-like physical symptoms as well as inform the development of novel treatments tailored for comorbid trauma and chronic pain. In addition, detecting and treating physical symptoms may be a useful adjunct to standard trauma treatment.
Acknowledgments
This research was funded by grants from the National Institutes of Health (R01-AA026844; R01-AA013892) and Peter F. McManus Charitable Trust. We thank Cheryl Lacadie, Katie Kim, Chloe Larkin, and Ryan Douglas for their assistance with recruitment, data collection, and data processing.
Footnotes
Declaration of Competing Interest: All authors declare no conflicts of interest or financial relationships with commercial interests.
References
- Abdallah CG, & Geha P (2017). Chronic Pain and Chronic Stress: Two Sides of the Same Coin? Chronic Stress (Thousand Oaks), 1. 10.1177/2470547017704763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agustin-Pavon C, Braesicke K, Shiba Y, Santangelo AM, Mikheenko Y, Cockroft G, Asma F, Clarke H, Man MS, & Roberts AC (2012). Lesions of ventrolateral prefrontal or anterior orbitofrontal cortex in primates heighten negative emotion. Biol Psychiatry, 72(4), 266–272. 10.1016/j.biopsych.2012.03.007 [DOI] [PubMed] [Google Scholar]
- Ahmadian AJ, Neylan TC, Metzler T, & Cohen BE (2019). Longitudinal association of PTSD symptoms and self-reported physical functioning among Veterans. J Affect Disord, 250, 1–8. 10.1016/j.jad.2019.02.048 [DOI] [PubMed] [Google Scholar]
- Alba-Delgado C, Llorca-Torralba M, Horrillo I, Ortega JE, Mico JA, Sanchez-Blazquez P, Meana JJ, & Berrocoso E (2013). Chronic pain leads to concomitant noradrenergic impairment and mood disorders. Biol Psychiatry, 73(1), 54–62. 10.1016/j.biopsych.2012.06.033 [DOI] [PubMed] [Google Scholar]
- Asmundson GJ, Coons MJ, Taylor S, & Katz J (2002). PTSD and the experience of pain: research and clinical implications of shared vulnerability and mutual maintenance models. Can J Psychiatry, 47(10), 930–937. 10.1177/070674370204701004 [DOI] [PubMed] [Google Scholar]
- Ayaydin H, Abali O, Akdeniz NO, Kok BE, Gunes A, Yildirim A, & Deniz G (2016). Immune system changes after sexual abuse in adolescents. Pediatr Int, 58(2), 105–112. 10.1111/ped.12767 [DOI] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, & Hikosaka O (2007). The role of the dorsal striatum in reward and decision-making. J Neurosci, 27(31), 8161–8165. 10.1523/JNEUROSCI.1554-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellavance MA, & Rivest S (2014). The HPA - Immune Axis and the Immunomodulatory Actions of Glucocorticoids in the Brain. Front Immunol, 5, 136. 10.3389/fimmu.2014.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shabat E, Matyas TA, Pell GS, Brodtmann A, & Carey LM (2015). The Right Supramarginal Gyrus Is Important for Proprioception in Healthy and Stroke-Affected Participants: A Functional MRI Study. Front Neurol, 6, 248. 10.3389/fneur.2015.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JM, Reeves G, Billman GE, & Sturmberg JP (2018). Inflammation-Nature’s Way to Efficiently Respond to All Types of Challenges: Implications for Understanding and Managing “the Epidemic” of Chronic Diseases. Front Med (Lausanne), 5, 316. 10.3389/fmed.2018.00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine SK, Wemm S, Fogelman N, Lacadie C, Seo D, Scheinost D, & Sinha R (2020). Association of Prefrontal-Striatal Functional Pathology With Alcohol Abstinence Days at Treatment Initiation and Heavy Drinking After Treatment Initiation. Am J Psychiatry, 177(11), 1048–1059. 10.1176/appi.ajp.2020.19070703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, & Lang PJ (1994). Measuring emotion: the Self-Assessment Manikin and the Semantic Differential. J Behav Ther Exp Psychiatry, 25(1), 49–59. 10.1016/0005-7916(94)90063-9 [DOI] [PubMed] [Google Scholar]
- Brady KT, Killeen TK, Brewerton T, & Lucerini S (2000). Comorbidity of psychiatric disorders and posttraumatic stress disorder. J Clin Psychiatry, 61 Suppl 7, 22–32. https://www.ncbi.nlm.nih.gov/pubmed/10795606 [PubMed] [Google Scholar]
- Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, Delaney RC, McCarthy G, Charney DS, & Innis RB (1995). MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry, 152(7), 973–981. 10.1176/ajp.152.7.973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohawn KH, Offringa R, Pfaff DL, Hughes KC, & Shin LM (2010). The neural correlates of emotional memory in posttraumatic stress disorder. Biol Psychiatry, 68(11), 1023–1030. 10.1016/j.biopsych.2010.07.018 [DOI] [PubMed] [Google Scholar]
- Cai YQ, Wang W, Paulucci-Holthauzen A, & Pan ZZ (2018). Brain Circuits Mediating Opposing Effects on Emotion and Pain. J Neurosci, 38(28), 6340–6349. 10.1523/JNEUROSCI.2780-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Palmieri MG, Boffa L, Pierantozzi M, Stanzione P, Brusa L, Marciani M, Siracusano A, Bernardi G, & Caramia M (2005). Cortical hyperexcitability in post-traumatic stress disorder secondary to minor accidental head trauma: a neurophysiologic study. J Psychiatry Neurosci, 30(2), 127–132. https://www.ncbi.nlm.nih.gov/pubmed/15798788 [PMC free article] [PubMed] [Google Scholar]
- Chen G, Saad ZS, Britton JC, Pine DS, & Cox RW (2013). Linear mixed-effects modeling approach to FMRI group analysis. Neuroimage, 73, 176–190. 10.1016/j.neuroimage.2013.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzin-Frankel J (2010). Inflammation bares a dark side. Science, 330(6011), 1621. 10.1126/science.330.6011.1621 [DOI] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res, 29(3), 162–173. 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Craig AD (2009). How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci, 10(1), 59–70. 10.1038/nrn2555 [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, & Dolan RJ (2004). Neural systems supporting interoceptive awareness. Nat Neurosci, 7(2), 189–195. 10.1038/nn1176 [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, Ledoux JE, & Phelps EA (2008). Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron, 59(5), 829–838. 10.1016/j.neuron.2008.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt A, Marighetto A, Richter-Levin G, & Calandreau L (2015). Adaptive emotional memory: the key hippocampal-amygdalar interaction. Stress, 18(3), 297–308. 10.3109/10253890.2015.1067676 [DOI] [PubMed] [Google Scholar]
- Devi RS, Sivaprakash RM, & Namasivayam A (2004). Rat hippocampus and primary immune response. Indian J Physiol Pharmacol, 48(3), 329–336. https://www.ncbi.nlm.nih.gov/pubmed/15648405 [PubMed] [Google Scholar]
- Duncan JS, Papademetris X, Yang J, Jackowski M, Zeng X, & Staib LH (2004). Geometric strategies for neuroanatomic analysis from MRI. Neuroimage, 23 Suppl 1, S34–45. 10.1016/j.neuroimage.2004.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Buchel C, & Gross JJ (2015). The neural bases of emotion regulation. Nat Rev Neurosci, 16(11), 693–700. 10.1038/nrn4044 [DOI] [PubMed] [Google Scholar]
- Etkin A, & Wager TD (2007). Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry, 164(10), 1476–1488. 10.1176/appi.ajp.2007.07030504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer C, Frey SH, Van Horn JD, Tunik E, Turk D, Inati S, & Grafton ST (2008). The angular gyrus computes action awareness representations. Cereb Cortex, 18(2), 254–261. 10.1093/cercor/bhm050 [DOI] [PubMed] [Google Scholar]
- First MB (2015). Structured Clinical Interview for the DSM (SCID) [ 10.1002/9781118625392.wbecp351]. The Encyclopedia of Clinical Psychology, 1–6. 10.1002/9781118625392.wbecp351 [DOI] [Google Scholar]
- Fishbain DA, Pulikal A, Lewis JE, & Gao J (2017). Chronic Pain Types Differ in Their Reported Prevalence of Post -Traumatic Stress Disorder (PTSD) and There Is Consistent Evidence That Chronic Pain Is Associated with PTSD: An Evidence-Based Structured Systematic Review. Pain Med, 18(4), 711–735. 10.1093/pm/pnw065 [DOI] [PubMed] [Google Scholar]
- Fox R, Hyland P, McHugh Power J, & Coogan AN (2020). Patterns of comorbidity associated with ICD-11 PTSD among older adults in the United States. Psychiatry Res, 290, 113171. 10.1016/j.psychres.2020.113171 [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, & Turner R (1996). Movement-related effects in fMRI time-series. Magn Reson Med, 35(3), 346–355. 10.1002/mrm.1910350312 [DOI] [PubMed] [Google Scholar]
- Garfin DR, Thompson RR, & Holman EA (2018). Acute stress and subsequent health outcomes: A systematic review. J Psychosom Res, 112, 107–113. 10.1016/j.jpsychores.2018.05.017 [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, & Pitman RK (2002). Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci, 5(11), 1242–1247. 10.1038/nn958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JM, Saligan L, Woods S, & Page G (2009). PTSD is associated with an excess of inflammatory immune activities. Perspect Psychiatr Care, 45(4), 262–277. 10.1111/j.1744-6163.2009.00229.x [DOI] [PubMed] [Google Scholar]
- Goldfarb EV, Rosenberg MD, Seo D, Constable RT, & Sinha R (2020). Hippocampal seed connectome-based modeling predicts the feeling of stress. Nat Commun, 11(1), 2650. 10.1038/s41467-020-16492-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb EV, Seo D, & Sinha R (2019). Sex differences in neural stress responses and correlation with subjective stress and stress regulation. Neurobiol Stress, 11, 100177. 10.1016/j.ynstr.2019.100177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golkar A, Lonsdorf TB, Olsson A, Lindstrom KM, Berrebi J, Fransson P, Schalling M, Ingvar M, & Ohman A (2012). Distinct contributions of the dorsolateral prefrontal and orbitofrontal cortex during emotion regulation. PLoS One, 7(11), e48107. 10.1371/journal.pone.0048107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM, & Grafton ST (2015). The striatum: where skills and habits meet. Cold Spring Harb Perspect Biol, 7(8), a021691. 10.1101/cshperspect.a021691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Molnar C, George MS, Bolger K, Koola J, & Nahas Z (2007). Emotion facilitates action: a transcranial magnetic stimulation study of motor cortex excitability during picture viewing. Psychophysiology, 44(1), 91–97. 10.1111/j.1469-8986.2006.00487.x [DOI] [PubMed] [Google Scholar]
- Hiser J, & Koenigs M (2018). The Multifaceted Role of the Ventromedial Prefrontal Cortex in Emotion, Decision Making, Social Cognition, and Psychopathology. Biol Psychiatry, 83(8), 638–647. 10.1016/j.biopsych.2017.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper JW, Frewen PA, van der Kolk BA, & Lanius RA (2007). Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. J Trauma Stress, 20(5), 713–725. 10.1002/jts.20284 [DOI] [PubMed] [Google Scholar]
- Huang MX, Yurgil KA, Robb A, Angeles A, Diwakar M, Risbrough VB, Nichols SL, McLay R, Theilmann RJ, Song T, Huang CW, Lee RR, & Baker DG (2014). Voxel-wise resting-state MEG source magnitude imaging study reveals neurocircuitry abnormality in active-duty service members and veterans with PTSD. Neuroimage Clin, 5, 408–419. 10.1016/j.nicl.2014.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes KC, & Shin LM (2011). Functional neuroimaging studies of post-traumatic stress disorder. Expert Rev Neurother, 11(2), 275–285. 10.1586/ern.10.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L, & Sapolsky R (1991). The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev, 12(2), 118–134. 10.1210/edrv-12-2-118 [DOI] [PubMed] [Google Scholar]
- Kim JJ, & Diamond DM (2002). The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci, 3(6), 453–462. 10.1038/nrn849 [DOI] [PubMed] [Google Scholar]
- Knox D, George SA, Fitzpatrick CJ, Rabinak CA, Maren S, & Liberzon I (2012). Single prolonged stress disrupts retention of extinguished fear in rats. Learn Mem, 19(2), 43–49. 10.1101/lm.024356.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch SB, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, & Olff M (2016). Aberrant Resting-State Brain Activity in Posttraumatic Stress Disorder: A Meta-Analysis and Systematic Review. Depress Anxiety, 33(7), 592–605. 10.1002/da.22478 [DOI] [PubMed] [Google Scholar]
- Kunimatsu A, Yasaka K, Akai H, Kunimatsu N, & Abe O (2020). MRI findings in posttraumatic stress disorder. J Magn Reson Imaging, 52(2), 380–396. 10.1002/jmri.26929 [DOI] [PubMed] [Google Scholar]
- Lacadie CM, Fulbright RK, Rajeevan N, Constable RT, & Papademetris X (2008). More accurate Talairach coordinates for neuroimaging using non-linear registration. Neuroimage, 42(2), 717–725. 10.1016/j.neuroimage.2008.04.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P, Bradley M, & Cuthbert B (1997). International affective picture system (IAPS): Technical manual and affective ratings. NIMH Center for the Study of Emotion and Attention, 1, 39–58. [Google Scholar]
- Langford DJ, Theodore BR, Balsiger D, Tran C, Doorenbos AZ, Tauben DJ, & Sullivan MD (2018). Number and Type of Post-Traumatic Stress Disorder Symptom Domains Are Associated With Patient-Reported Outcomes in Patients With Chronic Pain. J Pain, 19(5), 506–514. 10.1016/j.jpain.2017.12.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Frewen PA, Girotti M, Neufeld RW, Stevens TK, & Densmore M (2007). Neural correlates of trauma script-imagery in posttraumatic stress disorder with and without comorbid major depression: a functional MRI investigation. Psychiatry Res, 155(1), 45–56. 10.1016/j.pscychresns.2006.11.006 [DOI] [PubMed] [Google Scholar]
- Lanius RA, Frewen PA, Tursich M, Jetly R, & McKinnon MC (2015). Restoring large-scale brain networks in PTSD and related disorders: a proposal for neuroscientifically-informed treatment interventions. Eur J Psychotraumatol, 6, 27313. 10.3402/ejpt.v6.27313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite J, Carvalho S, Battistella LR, Caumo W, & Fregni F (2017). Editorial: The Role of Primary Motor Cortex as a Marker and Modulator of Pain Control and Emotional-Affective Processing. Front Hum Neurosci, 11, 270. 10.3389/fnhum.2017.00270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, & Sripada CS (2008). The functional neuroanatomy of PTSD: a critical review. Prog Brain Res, 167, 151–169. 10.1016/S0079-6123(07)67011-3 [DOI] [PubMed] [Google Scholar]
- Logue MW, van Rooij SJH, Dennis EL, Davis SL, Hayes JP, Stevens JS, Densmore M, Haswell CC, Ipser J, Koch SBJ, Korgaonkar M, Lebois LAM, Peverill M, Baker JT, Boedhoe PSW, Frijling JL, Gruber SA, Harpaz-Rotem I, Jahanshad N, Koopowitz S, Levy I, Nawijn L, O’Connor L, Olff M, Salat DH, Sheridan MA, Spielberg JM, van Zuiden M, Winternitz SR, Wolff JD, Wolf EJ, Wang X, Wrocklage K, Abdallah CG, Bryant RA, Geuze E, Jovanovic T, Kaufman ML, King AP, Krystal JH, Lagopoulos J, Bennett M, Lanius R, Liberzon I, McGlinchey RE, McLaughlin KA, Milberg WP, Miller MW, Ressler KJ, Veltman DJ, Stein DJ, Thomaes K, Thompson PM, & Morey RA (2018). Smaller Hippocampal Volume in Posttraumatic Stress Disorder: A Multisite ENIGMA-PGC Study: Subcortical Volumetry Results From Posttraumatic Stress Disorder Consortia. Biol Psychiatry, 83(3), 244–253. 10.1016/j.biopsych.2017.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunde CE, & Sieberg CB (2020). Walking the Tightrope: A Proposed Model of Chronic Pain and Stress. Front Neurosci, 14, 270. 10.3389/fnins.2020.00270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, & Lepage M (2001). Stress, memory, and the hippocampus: can’t live with it, can’t live without it. Behav Brain Res, 127(1–2), 137–158. 10.1016/s0166-4328(01)00361-8 [DOI] [PubMed] [Google Scholar]
- Maier SF, & Watkins LR (2010). Role of the medial prefrontal cortex in coping and resilience. Brain Res, 1355, 52–60. 10.1016/j.brainres.2010.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, & Holmes A (2016). Stress and Fear Extinction. Neuropsychopharmacology, 41(1), 58–79. 10.1038/npp.2015.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, & Hariri AR (2008). Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry, 64(6), 484–490. 10.1016/j.biopsych.2008.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M, Tempesta D, Pino MC, Catalucci A, Gallucci M, & Ferrara M (2013). Regional cerebral changes and functional connectivity during the observation of negative emotional stimuli in subjects with post-traumatic stress disorder. Eur Arch Psychiatry Clin Neurosci, 263(7), 575–583. 10.1007/s00406-013-0394-3 [DOI] [PubMed] [Google Scholar]
- McEwen BS (1998). Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci, 840, 33–44. 10.1111/j.1749-6632.1998.tb09546.x [DOI] [PubMed] [Google Scholar]
- McEwen BS (1999). Stress and hippocampal plasticity. Annu Rev Neurosci, 22, 105–122. 10.1146/annurev.neuro.22.1.105 [DOI] [PubMed] [Google Scholar]
- McEwen BS (2001). Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci, 933, 265–277. 10.1111/j.1749-6632.2001.tb05830.x [DOI] [PubMed] [Google Scholar]
- McEwen BS (2007). Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev, 87(3), 873–904. 10.1152/physrev.00041.2006 [DOI] [PubMed] [Google Scholar]
- McEwen BS (2008). Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol, 583(2–3), 174–185. 10.1016/j.ejphar.2007.11.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, & Magarinos AM (2001). Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum Psychopharmacol, 16(S1), S7–S19. 10.1002/hup.266 [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, & Rauch SL (2009). Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry, 66(12), 1075–1082. 10.1016/j.biopsych.2009.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, & Rauch SL (2007). Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry, 62(5), 446–454. 10.1016/j.biopsych.2006.10.011 [DOI] [PubMed] [Google Scholar]
- Moodie CA, Suri G, Goerlitz DS, Mateen MA, Sheppes G, McRae K, Lakhan-Pal S, Thiruchselvam R, & Gross JJ (2020). The neural bases of cognitive emotion regulation: The roles of strategy and intensity. Cogn Affect Behav Neurosci, 20(2), 387–407. 10.3758/s13415-020-00775-8 [DOI] [PubMed] [Google Scholar]
- Morawetz C, Bode S, Derntl B, & Heekeren HR (2017). The effect of strategies, goals and stimulus material on the neural mechanisms of emotion regulation: A meta-analysis of fMRI studies. Neurosci Biobehav Rev, 72, 111–128. 10.1016/j.neubiorev.2016.11.014 [DOI] [PubMed] [Google Scholar]
- Neigh GN, & Ali FF (2016). Co-morbidity of PTSD and immune system dysfunction: opportunities for treatment. Curr Opin Pharmacol, 29, 104–110. 10.1016/j.coph.2016.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel M, Wilson AC, Holley AL, Durkin L, Patton M, & Palermo TM (2016). Posttraumatic stress disorder symptoms in youth with vs without chronic pain. Pain, 157(10), 2277–2284. 10.1097/j.pain.0000000000000642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolf E, Voet T, Jacobs F, Dierckx R, & Lemahieu I (2003). XMedCon: an open-source medical image conversion toolkit. Eur J Nuclear Med, 30, S246. [Google Scholar]
- O’Donovan A (2016). PTSD is associated with elevated inflammation: any impact on clinical practice? Evid Based Ment Health, 19(4), 120. 10.1136/eb-2016-102376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Ahmadian AJ, Neylan TC, Pacult MA, Edmondson D, & Cohen BE (2017). Current posttraumatic stress disorder and exaggerated threat sensitivity associated with elevated inflammation in the Mind Your Heart Study. Brain Behav Immun, 60, 198–205. 10.1016/j.bbi.2016.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, & Gabrieli JD (2002). Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci, 14(8), 1215–1229. 10.1162/089892902760807212 [DOI] [PubMed] [Google Scholar]
- Olszowy W, Aston J, Rua C, & Williams GB (2019). Accurate autocorrelation modeling substantially improves fMRI reliability. Nat Commun, 10(1), 1220. 10.1038/s41467-019-09230-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacella ML, Hruska B, & Delahanty DL (2013). The physical health consequences of PTSD and PTSD symptoms: A meta-analytic review. Journal of Anxiety Disorders, 27(1), 33–46. 10.1016/j.janxdis.2012.08.004 [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, & LeDoux JE (2004). Extinction learning in humans: role of the amygdala and vmPFC. Neuron, 43(6), 897–905. 10.1016/j.neuron.2004.08.042 [DOI] [PubMed] [Google Scholar]
- Pierre K, Schlesinger N, & Androulakis IP (2016). The role of the hypothalamic-pituitary-adrenal axis in modulating seasonal changes in immunity. Physiol Genomics, 48(10), 719–738. 10.1152/physiolgenomics.00006.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak RH, Goldstein RB, Southwick SM, & Grant BF (2011). Medical comorbidity of full and partial posttraumatic stress disorder in US adults: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom Med, 73(8), 697–707. 10.1097/PSY.0b013e3182303775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, & Liberzon I (2012). Biological studies of post-traumatic stress disorder. Nature reviews. Neuroscience, 13(11), 769–787. 10.1038/nrn3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press WA, Brewer AA, Dougherty RF, Wade AR, & Wandell BA (2001). Visual areas and spatial summation in human visual cortex. Vision Res, 41(10–11), 1321–1332. 10.1016/s0042-6989(01)00074-8 [DOI] [PubMed] [Google Scholar]
- Radley JJ, & Sawchenko PE (2011). A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. J Neurosci, 31(26), 9683–9695. 10.1523/JNEUROSCI.6040-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins DL, Hunyadi E, & Schultz RT (2009). Superior temporal activation in response to dynamic audio-visual emotional cues. Brain Cogn, 69(2), 269–278. 10.1016/j.bandc.2008.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET (2019). The cingulate cortex and limbic systems for emotion, action, and memory. Brain Struct Funct, 224(9), 3001–3018. 10.1007/s00429-019-01945-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Wilson MR, Schauer GF, Allison S, Gorno-Tempini ML, Pace-Savitsky C, Kramer JH, Levenson RW, Weiner M, & Miller BL (2006). Neuroanatomical correlates of impaired recognition of emotion in dementia. Neuropsychologia, 44(3), 365–373. 10.1016/j.neuropsychologia.2005.06.012 [DOI] [PubMed] [Google Scholar]
- Ryder AL, Azcarate PM, & Cohen BE (2018). PTSD and Physical Health. Curr Psychiatry Rep, 20(12), 116. 10.1007/s11920-018-0977-9 [DOI] [PubMed] [Google Scholar]
- Sachinvala N, Kling A, Suffin S, Lake R, & Cohen M (2000). Increased regional cerebral perfusion by 99mTc hexamethyl propylene amine oxime single photon emission computed tomography in post-traumatic stress disorder. Mil Med, 165(6), 473–479. https://www.ncbi.nlm.nih.gov/pubmed/10870367 [PubMed] [Google Scholar]
- Schmidt MF, Freeman KB, Windham BG, Griswold ME, Kullo IJ, Turner ST, & Mosley TH Jr. (2016). Associations Between Serum Inflammatory Markers and Hippocampal Volume in a Community Sample. J Am Geriatr Soc, 64(9), 1823–1829. 10.1111/jgs.14283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Jia Z, Lacadie CM, Tsou KA, Bergquist K, & Sinha R (2011). Sex differences in neural responses to stress and alcohol context cues. Hum Brain Mapp, 32(11), 1998–2013. 10.1002/hbm.21165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Lacadie CM, Tuit K, Hong KI, Constable RT, & Sinha R (2013). Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatry, 70(7), 727–739. 10.1001/jamapsychiatry.2013.762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Olman CA, Haut KM, Sinha R, MacDonald AW 3rd, & Patrick CJ (2014). Neural correlates of preparatory and regulatory control over positive and negative emotion. Soc Cogn Affect Neurosci, 9(4), 494–504. 10.1093/scan/nst115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Tsou KA, Ansell EB, Potenza MN, & Sinha R (2014). Cumulative adversity sensitizes neural response to acute stress: association with health symptoms. Neuropsychopharmacology, 39(3), 670–680. 10.1038/npp.2013.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp TJ, & Harvey AG (2001). Chronic pain and posttraumatic stress disorder: mutual maintenance? Clin Psychol Rev, 21(6), 857–877. 10.1016/s0272-7358(00)00071-4 [DOI] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, & Hufford MR (2008). Ecological momentary assessment. Annu Rev Clin Psychol, 4, 1–32. 10.1146/annurev.clinpsy.3.022806.091415 [DOI] [PubMed] [Google Scholar]
- Shipley W (1940). A Self-Administering Scale for Measuring Intellectual Impairment and Deterioration. Journal of Psychology: Interdisciplinary and Applied, 9, 371–377. 10.1080/00223980.1940.9917704 [DOI] [Google Scholar]
- Sinha R, Lacadie CM, Constable RT, & Seo D (2016). Dynamic neural activity during stress signals resilient coping. Proc Natl Acad Sci U S A, 113(31), 8837–8842. 10.1073/pnas.1600965113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, & Li CS (2007). Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev, 26(1), 25–31. 10.1080/09595230601036960 [DOI] [PubMed] [Google Scholar]
- Smyth JM, & Stone AA (2003). Ecological Momentary Assessment Research in Behavioral medicine. Journal of Happiness Studies, 4(1), 35–52. 10.1023/A:1023657221954 [DOI] [Google Scholar]
- Speer K, Upton D, Semple S, & McKune A (2018). Systemic low-grade inflammation in post-traumatic stress disorder: a systematic review. J Inflamm Res, 11, 111–121. 10.2147/JIR.S155903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomaes K, Dorrepaal E, Draijer N, de Ruiter MB, Elzinga BM, Sjoerds Z, van Balkom AJ, Smit JH, & Veltman DJ (2013). Increased anterior cingulate cortex and hippocampus activation in Complex PTSD during encoding of negative words. Soc Cogn Affect Neurosci, 8(2), 190–200. 10.1093/scan/nsr084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomaes K, Dorrepaal E, Draijer NP, de Ruiter MB, Elzinga BM, van Balkom AJ, Smoor PL, Smit J, & Veltman DJ (2009). Increased activation of the left hippocampus region in Complex PTSD during encoding and recognition of emotional words: a pilot study. Psychiatry Res, 171(1), 44–53. 10.1016/j.pscychresns.2008.03.003 [DOI] [PubMed] [Google Scholar]
- Tolomeo S, Christmas D, Jentzsch I, Johnston B, Sprengelmeyer R, Matthews K, & Douglas Steele J (2016). A causal role for the anterior mid-cingulate cortex in negative affect and cognitive control. Brain, 139(Pt 6), 1844–1854. 10.1093/brain/aww069 [DOI] [PubMed] [Google Scholar]
- Tural U, Aker AT, Onder E, Sodan HT, Unver H, & Akansel G (2018). Neurotrophic factors and hippocampal activity in PTSD. PLoS One, 13(5), e0197889. 10.1371/journal.pone.0197889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA (2005). Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci, 6(7), 533–544. 10.1038/nrn1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA (2016). Midcingulate cortex: Structure, connections, homologies, functions and diseases. J Chem Neuroanat, 74, 28–46. 10.1016/j.jchemneu.2016.01.010 [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, & Ochsner KN (2008). Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron, 59(6), 1037–1050. 10.1016/j.neuron.2008.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessa M, & Flor H (2007). Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. Am J Psychiatry, 164(11), 1684–1692. 10.1176/appi.ajp.2007.07030525 [DOI] [PubMed] [Google Scholar]
- Wicking M, Steiger F, Nees F, Diener SJ, Grimm O, Ruttorf M, Schad LR, Winkelmann T, Wirtz G, & Flor H (2016). Deficient fear extinction memory in posttraumatic stress disorder. Neurobiol Learn Mem, 136, 116–126. 10.1016/j.nlm.2016.09.016 [DOI] [PubMed] [Google Scholar]
- Williamson LL, & Bilbo SD (2013). Chemokines and the hippocampus: a new perspective on hippocampal plasticity and vulnerability. Brain Behav Immun, 30, 186–194. 10.1016/j.bbi.2013.01.077 [DOI] [PubMed] [Google Scholar]
- Woods SJ, Hall RJ, Campbell JC, & Angott DM (2008). Physical health and posttraumatic stress disorder symptoms in women experiencing intimate partner violence. J Midwifery Womens Health, 53(6), 538–546. 10.1016/j.jmwh.2008.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H, Zhang J, & Liu J (2016). Hippocampus, spatial memory and neuroimmunomodulation. In Neuroimmune Pharmacology (pp. 69–79). 10.1007/978-3-319-44022-4_6 [DOI] [Google Scholar]
- Xiong J, Gao J-H, Lancaster JL, & Fox PT (1995). Clustered pixels analysis for functional MRI activation studies of the human brain [ 10.1002/hbm.460030404]. Human Brain Mapping, 3(4), 287–301. 10.1002/hbm.460030404 [DOI] [Google Scholar]
- Yin HH, Knowlton BJ, & Balleine BW (2005). Blockade of NMDA receptors in the dorsomedial striatum prevents action-outcome learning in instrumental conditioning. Eur J Neurosci, 22(2), 505–512. 10.1111/j.1460-9568.2005.04219.x [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, & Balleine BW (2005). The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci, 22(2), 513–523. 10.1111/j.1460-9568.2005.04218.x [DOI] [PubMed] [Google Scholar]
- Zuj DV, Palmer MA, Lommen MJ, & Felmingham KL (2016). The centrality of fear extinction in linking risk factors to PTSD: A narrative review. Neurosci Biobehav Rev, 69, 15–35. 10.1016/j.neubiorev.2016.07.014 [DOI] [PubMed] [Google Scholar]


