Abstract
Exocyclic groups in the minor groove of DNA modulate the affinity and positioning of nucleic acids to the histone protein. The addition of exocyclic groups decreases the formation of this protein–DNA complex, while their removal increases nucleosome formation. On the other hand, recent theoretical results show a strong correlation between the BI/BII phosphate backbone conformation and the hydration of the grooves of the DNA. We performed a simulation of the d(CGCGAATTCGCG)2 Drew Dickerson dodecamer and one simulation of the d(CGCIAATTCGCG)2 dodecamer in order to investigate the influence of the exocyclic amino group of guanine. The removal of the amino group introduces a higher intrinsic flexibility to DNA supporting the suggestions that make the enhanced flexibility responsible for the enlarged histone complexation affinity. This effect is attributed to changes in the destacking interactions of both strands of the DNA. The differences in the hydration of the minor groove could be the explanation of this flexibility. The changed hydration of the minor groove also leads to a different BI/BII substate pattern. Due to the fact that the histone preferentially builds contacts with the backbone of the DNA, we propose an influence of these BI/BII changes on the nucleosome formation process. Thus, we provide an additional explanation for the enhanced affinity to the histone due to removal of exocyclic groups. In terms of BI/BII we are also able to explain how minor groove binding ligands could affect the nucleosome assembly without disrupting the structure of DNA.
INTRODUCTION
Knowledge of the detailed structural behavior of DNA is of extraordinary interest in order to understand sequence-specific DNA recognition by proteins and small ligands. Besides the direct recognition of nucleic acids mediated by contacts with the base pair edges, indirect readout (1) plays an important role in sequence-specific complexation. Indirect readout is mediated by the sequence-dependent conformational deformability of the DNA. Bending, unwinding and other recognition tools in the indirect readout have already been extensively investigated (2–16). An analysis (17) of protein–DNA complexes indicates that >50% of all contacts are between the amino acid side chain and the DNA backbone demonstrating the importance of DNA backbone conformations in recognition processes (18).
Experimental and theoretical studies showed that these backbone phosphates occupy either the BI or BII conformational substate (19–25). The BI/BII pattern is sequence dependent, thus transferring sequence information from the bases to the chemical degenerate phosphate groups. Hence, we surmise that these conformational substates of the phosphates influence the sequence-specific recognition of the DNA. The backbone angles ɛ and ζ are used to define the BI and BII states. In the BI state the corresponding ɛ and ζ angles are between 120–210° (trans) and 235–295°(gauche–), respectively, and for the BII state the ɛ and ζ angles are between 210–300° (gauche–) and 150–210° (trans), respectively (26–28).
A recently performed molecular dynamics simulation of the trp repressor–operator complex shows a strong correlation between the BI/BII phosphate substate and the number of interactions with this phosphate (Wellenzohn et al., submitted for publication). There is also evidence for the BI/BII equilibrium inducing a dynamic curvature in the NF-κB binding site playing an active role in DNA–protein recognition (29,30).
The role of water molecules in the mechanism of the BI→BII substate transitions were investigated by experimental (31) and theoretical (23,32) methods showing strong correlations with hydration patterns in the minor and major groove. Thus, such exocyclic groups should influence the BI→BII equilibrium. Recent experimental studies showed that the exocyclic groups modulate the affinity and positioning of DNA to the histone octamer (33–36). It was suggested that addition of exocyclic groups decrease and removal increase the local deformability of DNA (37) by maximizing and minimizing the steric resistance to bending. Inosine is a purine nucleoside, which differs from guanine only by the removal of the exocyclic amino group. It occurs naturally in the wobble position of some t-RNAs where it appears to pair also with adenosine in addition to cytidine and uridine (38–42). The X-ray structure of the d(CGCIAATTCGCG)2 (43) dodecamer, which is an inosine analog of the Drew Dickerson dodecamer d(CGCGAATTCGCG)2 (44–46), was derived recently.
We performed a 3 ns long molecular dynamics simulation of the d(CGCIAATTCGCG)2 dodecamer and compared it with a 10 ns long reference simulation of the Drew Dickerson dodecamer. The intention of this work was to investigate the influence which the exocyclic amino group in the minor groove exerts on DNA structure and dynamic. As a result of the strong correlation between the BI→BII equilibrium and the hydration pattern of the grooves the removal of the exocyclic amino group should also influence the phosphate conformations. An analysis of the simulation results indicates that the phosphates of the CpI steps have a preference for BII while in the reference simulation the respective CpG steps are mostly in BI. These results underline the importance of the hydration for the DNA conformations supporting theoretical (32) and experimental (31) work carried out previously. The removal of the amino group also enhances the flexibility (37) of the whole DNA, presumably because of altered base stacking interactions (33–36). The d(CGCIAATTCGCG)2 dodecamer shows no intrinsic curvature supporting the explanations that hold the enhanced flexibility responsible for the increased affinity to the histone octamer (33,47). In the nucleosome core particle the majority of the protein–DNA contacts are between charged amino acid side chains and the sugar phosphate backbone (48,49). Thus, we propose that besides the flexibility the change in the BI/BII pattern could also contribute to the altered histone binding. Observations at the trp repressor–operator complex in which BI→BII transitions occur synchronous to hydrogen bond breaking or formation (Wellenzohn et al., submitted for publication) and other experimental results (29,30), which attribute an active role in DNA–protein recognition to BI→BII support our suggestion. Additionally, minor groove binding ligands, which normally cause only small distortions in the DNA structure, also inhibit the formation of nucleosomes (50). Such ligands are changing the BI→BII (51,52) equilibrium, this being a possible explanation for this effect.
METHODS AND COMPUTATIONAL DETAILS
Molecular dynamics simulations of DNA (53–57) and DNA complexes (24,51,58–60) are providing complementary information to experiments and thus are of great interest in the field of structural biology. The inclusion of long-range interactions via the Ewald Summation in the form of the particle mesh Ewald method leads to stable B-form DNA trajectories (61–63). We performed one 3 ns long molecular dynamics simulation of the d(CGCIAATTCGCG)2 and one 10 ns long simulation of the d(CGCGAATTCGCG)2 (Drew Dickerson dodecamer; Fig. 1, top) dodecamer as reference system. The simulation parameters of the reference system are already described elsewhere (32). As a starting structure for the d(CGCIAATTCGCG)2 dodecamer (Fig. 1, bottom), the crystal structure with the PDB code 1D77 was used. Each strand of the DNA has 11 PO4– anions and in order to achieve electroneutrality 22 Na+ counterions were added using the program CION of the AMBER (64) package. Subsequently, solvation of the DNA with TIP3P Monte Carlo water boxes requiring a 12 Å solvent shell in all directions resulted in a system with dimensions 67.8695 × 48.7944 × 45.3952 Å3. The respective Γ-value = 176.6 (water/nucleotide). The force field parameters of inosine were selected in analogy to existing parameters in the force field of Cornell et al. (65) with the modifications of Cheatham et al. (25). The charges for inosine were derived by using the RESP standard procedure calculating the ab initio electrostatic potential with GAUSSIAN98 (66) at HF/6-31G* level of theory. As simulation protocol standard protocols (23,67–70) were adapted for our needs. The all atom force field of Cornell et al. (65) with the modifications of Cheatham et al. (25) was used. At the beginning, minimizations were carried out with harmonic restraints on DNA and counterion positions. The restraints were stepwise relaxed and at the end a 500 step minimization without restraints was performed. For equilibration the system was heated from 50 to 300 K during 10 ps under constant volume conditions and harmonic restraints. Subsequently, the restraints were once again relaxed and finally an unrestrained 5 ps constant temperature and pressure equilibration was carried out. Analysis of the resulting trajectories was performed with CARNAL, PTRAJ and different visualization programs (71,72). All calculations were performed on an SGI octane.
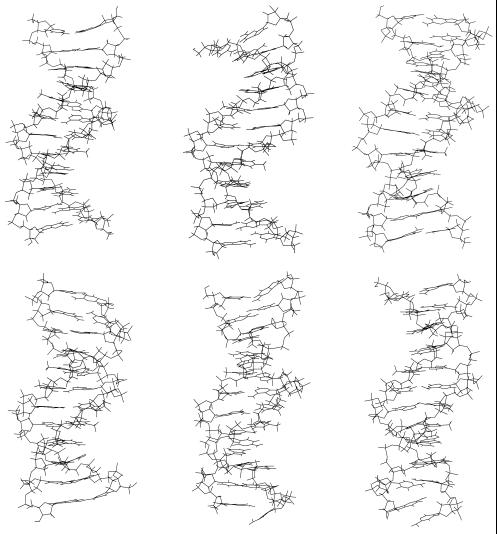
Figure 1.
The figures on the top show the starting (left) structure, one snapshot at the middle of the simulation (middle) and the structure at the end (right) of the molecular dynamics simulation of the d(CGCGAATTCGCG)2 dodecamer. The figures on the bottom show the respective snapshots of the d(CGCIAATTCGCG)2 dodecamer.
RESULTS
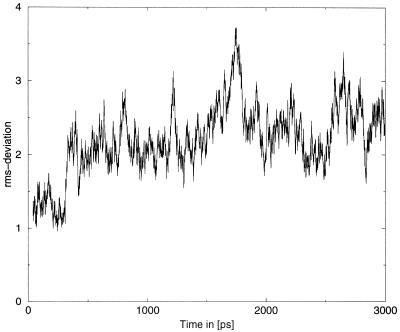
The total energy of the d(CGCIAATTCGCG)2 simulation is constant (not shown) during the simulation and the rms-deviation depicted in Figure 2 shows the normal fluctuations but no drift, indicating that the system is in the equilibrium. The mean value of the rms-deviation with respect to the X-ray starting structure is 2.2 Å.
Figure 2.
The rms-deviation with respect to the X-ray starting structure in Å as function of time. The mean value over the whole simultion is 2.2 Å, which is the normal range for such molecular dynamics simulations. The rms-values show only the normal fluctuation indicating that the system is in equilibrium.
In Figure 1 (bottom) it can be seen that one terminal C·G base pair is broken after ∼500 ps (is not responsible for the enhancement in the rms-deviation at 250 ps) and stays open for the rest of the simulation, thus they are not considered in this work. This occurs often in molecular dynamics simulations of DNA with explicit water molecules. The rest of the molecule remains unaffected.
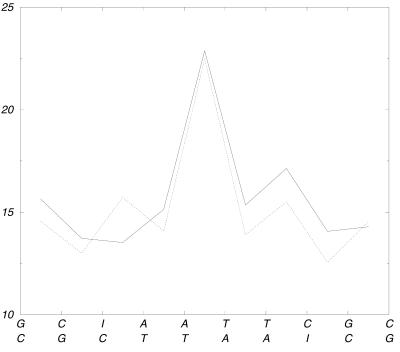
The wrapping of nucleic acids by the histone octamer is a mechanical deformation of the DNA, which is also influenced by the exocyclic groups that penetrate into the minor groove (33–36). Most of the contacts are between the sugar phosphate backbone and the amino acid side chains, thus it is unlikely that direct contacts with the minor grooves are influencing the protein–DNA interaction. Figure 3 shows the angles between the successive base pair planes for d(CGCGAATTCGCG)2 and d(CGCIAATTCGCG)2 in order to investigate whether the guanine → inosine transformation leads to an intrinsic curvature.
Figure 3.
The figure indicates the mean angle in (°) between the successive base pair planes. The most pronounced curvature exhibits at the AT step of the AATT tract. The solid line shows the values for the d(CGCGAATTCGCG)2 simulation and the dotted line gives the respective value for the d(CGCIAATTCGCG)2 dodecamer. Both end-standing base pairs are not shown in this graph because they are too flexible.
The most pronounced curvature exhibits the ApT step of the ApApTpT tract. The angles of the base pair planes of the CpIpA steps are neither significantly larger than in the case of the corresponding CpGpA steps nor do they show a distinctive absolute value. The sizes of the DNA molecules defined as the average distance (in the simulation) between the center of mass of the second base pair (first pair is opened as mentioned above) and the center of mass of the last base pair are also an indicator of the amount of bending. The value for the d(CGCGAATTCGCG)2 simulation is 33.5 Å and for d(CGCIAATTCGCG)2 the respective value is 34.3 Å which is <3% difference. These results show that inosine does not enlarge the bending of the dodecamer.
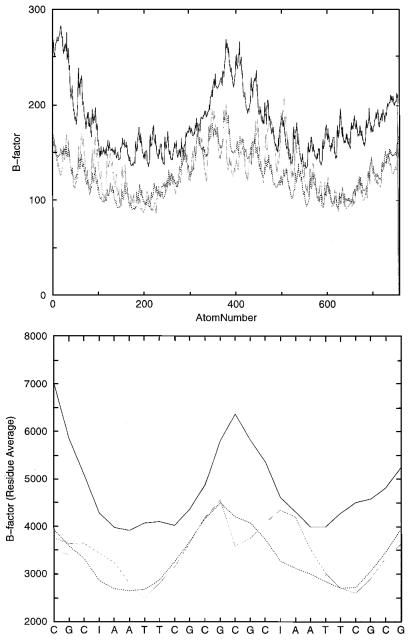
Buttinelly et al. (33) suggest that the removal of exocyclic groups of the bases increases the deformability of DNA by minimizing the steric resistance to bending. This enlarged deformability should be the explanation for the better binding to the histone octamer. The drug-induced inhibition of the inherent DNA flexibility is up to now also the most plausible mechanism for the fact that minor groove binders are able to inhibit the formation of the histone–DNA complex (50). We calculated the B-factors (Fig. 4) as an indication for the positional fluctuations for both simulations and for a recently published d(CGCGAATTCGCG)2–netropsin complex (24; Wellenzohn et al., submitted for publication). Netropsin is a widely studied (73–83) minor groove-binding ligand, which selectively recognizes AT-rich regions.
Figure 4.
The graphs show the B-factors estimated from the simulation of the d(CGCIAATTCGCG)2 (black line) dodecamer, the values of the d(CGCGAATTCGCG)2 (dotted line) DNA and of the d(CGCGAATTCGCG)2–netropsin complex (gray line). The figure on the top specifies the calculated values by each atom and the bottom picture gives the average for each residue. Larger peaks mean more motion.
Figure 4 clearly shows that the d(CGCIAATTCGCG)2 dodecamer exhibits the largest B-values and thus agrees with the above-mentioned suggestions. The peaks in the left, middle and right represent the ends of the DNA helices and the periodic peaks (top) represent the backbone phosphates. The largest fluctuation exhibits the d(CGCIAATTCGCG)2 dodecamer on the left side which is due to the above-mentioned opening of the base pair. The enlarged flexibility of the rest of the DNA seems not to be an artifact of this base pair opening because the other end of the DNA (middle), which is 12 bp distant from the opened one, also shows a higher positional fluctuation. The influence of the minor groove binding ligand netropsin is by far much less distinct than the guanine → inosine substitution. Netropsin does not substantially affect the flexibility, being in agreement with recent theoretical results which show that the DNA does not loose its pliability during complexation. A recently performed normal mode calculation (74) of a netropsin–DNA complex also finds that the frequency of the modes of the complex that are associated with the DNA are relatively unchanged from the frequencies found for the isolated helix.
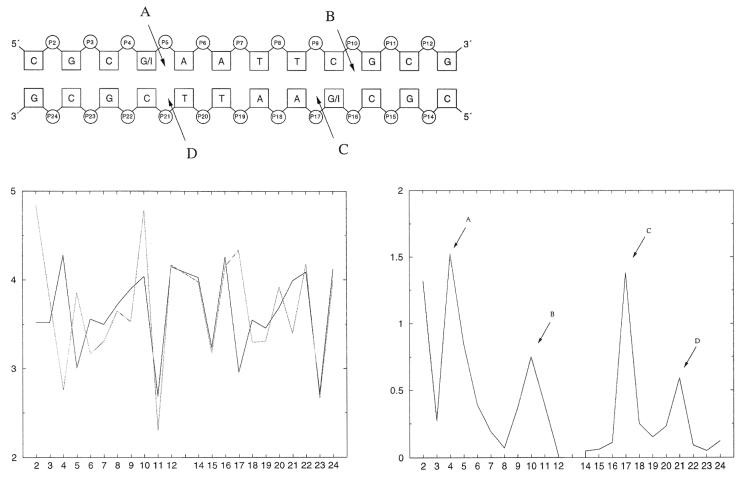
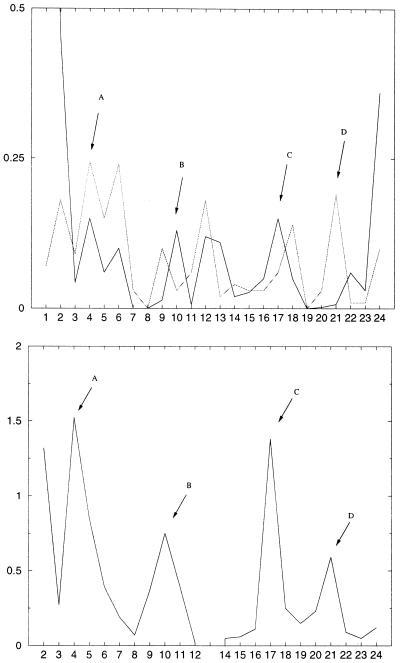
The change in the stacking interactions is often used (33–36) as an explanation for the high flexibility of the d(CGCIAATTCGCG)2 dodecamer. We calculated the projected C1′ (sugar carbon attached to the bases) distances (21) between the successive base pair steps as a destacking indicator (shown in Fig. 5).
Figure 5.
The graph in the lower left shows the mean values over the simulations of the projected C1′ distances for the Drew Dickerson dodecamer (black) and for d(CGCIAATTCGCG)2 (gray). The lower right curve gives the absolute values of the differences in these destacking distances between the Drew Dickerson dodecamer and the respecitve d(CGCIAATTCGCG)2 inosine analog. The schematic picture on the top demonstrates the places of the largest differences, indicated by A–D.
The largest differences in the destacking occur in the region where guanine is changed to inosine. Although the destacking of the strand where the base substitution occurs is changed more, both DNA strands are influenced. On the ends of the DNA the destacking seems to be normal with the exception of the peak on the left side in Figure 5 (bottom), which is an artifact because of the above-mentioned terminal base pair opening.
The changes in the hydration pattern of the minor grooves are shown in Figure 6. As an indication of the hydration, the mean value (over the simulation) of water oxygens closer than 3.2 Å to O4′ and closer than 3.6 Å to N3 were used according to Flader et al. (32).
Figure 6.
The top plot shows the difference (absolute value), between the Drew Dickerson dodecamer and d(CGIAATTCGCG)2, in the hydration of O4′ (dotted line) and N3 (solid line). The mean value of number of water oxygens closer than 3.2 Å to O4′ and closer than 3.6 Å to N3 were used for detecting the hydration pattern. The bottom graph gives again the difference in the destacking interactions.
The differences in the hydration between the Drew Dickerson dodecamer and d(CGCIAATTCGCG)2 DNA exhibit maxima also at the positions of the changed destacking (marked A–D). The numbers 1, 12, 13 and 24 are all terminal bases, explaining the large deviations. Thus, we conclude that the changes in the hydration are responsible for the destacking differences.
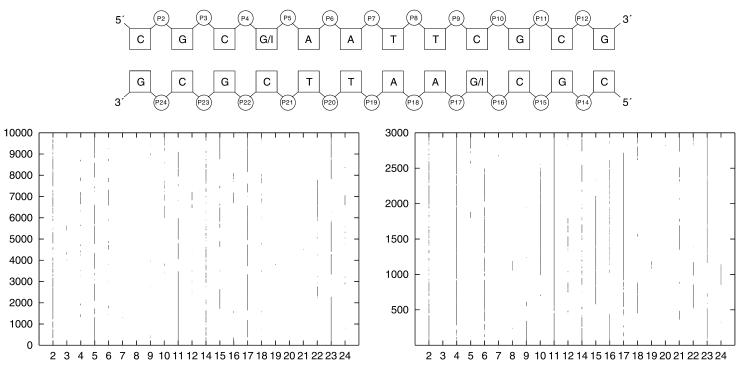
Although the phosphates are the most flexible part of the DNA there exist only two distinct conformations called BI and BII (see Introduction). Experimental and theoretical results showed that the hydration of the exocyclic groups in both the major and the minor groove are contributing to the BI→BII equilibrium. Thus, the removal of an amino group in the minor groove (= guanine → inosine transformation) should alter the conformational behavior of the phosphates. Figure 7 (top) shows the BI→BII pattern of the d(CGCGAATTCGCG)2 Drew Dickerson dodecamer and of the d(CGCIAATTCGCG)2 DNA.
Figure 7.
The graph on the lower right shows the BI/BII pattern of d(CGCIAATTCGCG)2 dodecamer and the lower left graph indicates the behaviour of the d(CGCGAATTCGCG)2 Drew Dickerson dodecamer. The time the phosphate is in BII is marked by a line/dot. The schematic picture on the top gives the numbering scheme of the phosphates.
The substitution from guanine to inosine changes the BI/BII equilibrium substantially. Phosphate number 4 is in the case of a CpG step nearly always in BI while the respective CpI step has a distinctive preference for the BII state. Phosphate 5 of the CpG step also changes the substate due to the guanine → inosine transformation. Phosphate 16, which also represents a CpI step, again shows an enhanced preference for the BII substate, but this phosphate very often interconverts between BI and BII. This may be because phosphate number 17 is most of the time in BII and it is very unlikely that two successive phosphates occupy the BII state at the same time. The results underline that the hydration of the grooves influences the backbone conformation of DNA and we propose again that a conversation between the major and minor groove could be mediated by these BI/BII substates.
SUMMARY AND CONCLUSIONS
We performed two molecular dynamics simulations; one of the d(CGCGAATTCGCG)2 Drew Dickerson dodecamer and one of the d(CGCIAATTCGCG)2 DNA. The transformation from guanine to inosine leads to the removal of an exocyclic amino group in the minor groove of the nucleic acid. Such exocyclic groups have already proven their influence on the binding of the DNA to the histone protein. In the complex, the DNA structure is strongly disrupted and the enlarged flexibility due to the removal of the amino group is used as an explanation for the enlarged complexation affinity. Our simulations show that the substitution of guanine by inosine in the Drew Dickerson dodecamer does not introduce an intrinsic bending but indeed introduces a higher flexibility to the whole DNA. The calculated B-factors are enhanced not only at the place of the guanine → inosine transformation and we propose that the differences in the hydration of the minor groove changes the stacking interactions that are responsible for this effect. The differences in the hydration also influence the conformation of the phosphate backbone according to Flader et al. (32) The phosphates of the CpI steps show a high preference for the BII state while the respective CpG step is mainly in BI. As a result of the fact that the histone preferentially contacts the backbone of the DNA, we suggest an influence of the BI/BII backbone substate pattern on the nucleosome formation process. Thus, the correlation between the BI/BII substrate pattern and the exocyclic amino groups provides an additional explanation (beside the fluctuation) for the above-mentioned enhanced affinity of DNA to the histone protein. In terms of BI/BII we are also able to give an explanation as to how minor groove binding ligands could influence the nucleosome assembly without disrupting the structure of DNA. Minor groove binding ligands are able to change the BI/BII pattern, thus they could alter the complexation affinity. Recently, experimental studies have shown that CpG methylation prevents the interaction of histone octamers with otherwise high affinity DNA sequences. The high correlation between the BI→BII equilibrium and the fine structure of the hydration could also be the explanation for this effect. Altogether the exact conformational behavior of the backbone phosphates could be of extraordinary interest for DNA recognition processes.
Acknowledgments
ACKNOWLEDGEMENT
This work was supported by a grant of the Austrian Science Fund (grant number P13845-TPH).
REFERENCES
- 1.Otwinowski Z., Schevitz,R.W., Zhang,R.-G., Lawson,C.L., Joachimiak,A., Marmorstein,R.Q., Luisi,B.F. and Sigler,P.B. (1988) Crystal structure of trp repressor/operator complex at atomic resolution. Nature, 335, 321–329. [DOI] [PubMed] [Google Scholar]
- 2.Bareketh-Samish A., Cohen,I. and Haran,T.E. (1998) Direct versus indirect readout in the interaction of the trp repressor with non-canonical binding sites. J. Mol. Biol., 277, 1071–1080. [DOI] [PubMed] [Google Scholar]
- 3.Dickerson R.E. and Chiu,T.K. (1998) Helix bending as a factor in protein/DNA recognition. Biopolymers, 44, 361–403. [DOI] [PubMed] [Google Scholar]
- 4.Giese K., Pagel,J. and Grosschedl,R. (1997) Functional analysis of DNA bending and unwinding by the high mobility group domain of lef-1. Proc. Natl Acad. Sci. USA, 94, 12845–12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi Y. and Berg,J.M. (1996) DNA unwinding induced by zinc finger protein binding. Biochemistry, 35, 3845–3848. [DOI] [PubMed] [Google Scholar]
- 6. von Hippel P.H. (1994) Protein-DNA recognition: new perspectives and underlying themes. Science, 263, 769–770. [DOI] [PubMed] [Google Scholar]
- 7.Grillo A.O., Brown,M.P. and Royer,C.A. (1999) Probing the physical basis for the trp repressor-operator recognition. J. Mol. Biol., 287, 539–554. [DOI] [PubMed] [Google Scholar]
- 8.Shakked Z., Guzikevich-Guerstein,G., Frolow,F., Rabinovich,D., Joachimiak,A. and Sigler,P.B. (1994) Determinants of repressor/operator recognition from the structure of the trp operator binding site. Nature, 368, 469–473. [DOI] [PubMed] [Google Scholar]
- 9.Steitz T.A. (1993) Structural Studies of Protein–Nucleic Acid Interaction. Cambridge University Press, Cambridge, UK.
- 10.Lilley D.M.J. (1995) DNA–Protein: Structural Interactions. Oxford University Press, Oxford, UK.
- 11.Travers A. (1993) DNA–Protein Interactions. Chapman and Hall, London, UK.
- 12.Bewley C.A., Grinenborn,A.M. and Clore,G.M. (1998) Minor groove-binding architectural proteins: structure, function and DNA recognition. Annu. Rev. Biophys. Biophys. Chem., 27, 105–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y.-Q., Gosh,S. and Gosh,G. (1998) A novel DNA recognition mode by the NF-κB p56 homodimer. Nature Struct. Biol., 5, 67–73. [DOI] [PubMed] [Google Scholar]
- 14.Ellenberger T. (1994) Getting a grip on DNA recognition: structures of the basic region leucine zipper and the basic region helix-loop-helix DNA-binding domains. Curr. Opin. Struct. Biol., 4, 12–21. [Google Scholar]
- 15.Neidle S. (1997) Crystallographic insights into DNA minor groove recognition by drugs. Biopolymers, 44, 105–121. [Google Scholar]
- 16.Pabo C.O. and Sauer,R.T. (1992) Transcription factors: structural families and principles of DNA recognition. Annu. Rev. Biochem., 61, 1053–1095. [DOI] [PubMed] [Google Scholar]
- 17.Mandel-Gutfreund Y., Schueler,O. and Margalit,H. (1995) Comprehensive analysis of hydrogen bonds in regulatory protein-DNA-complexes: in search of common principles. J. Mol. Biol., 253, 370–382. [DOI] [PubMed] [Google Scholar]
- 18.Smith S.A. and McLaughlin,L.W. (1997) Probing contacts to the DNA backbone in the trp repressor-operator sequence-specific protein-nucleic acid complex using diastereomeric methylphosphonate analogues. Biochemistry, 36, 6046–6058. [DOI] [PubMed] [Google Scholar]
- 19. van Dam L. and Levitt,M.H. (2000) BII nucleotides in the B and C form of natural-sequence polymeric DNA: a new model for the C form of DNA. J. Mol. Biol., 304, 541–561. [DOI] [PubMed] [Google Scholar]
- 20.Gorenstein D.G. (1994) Conformation and dynamics of DNA and protein-DNA complexes by 31P NMR. Chem. Rev., 94, 1315–1338. [Google Scholar]
- 21.Grzeskowiak K., Yanagi,K., Privé,G.G. and Dickerson,R.E. (1991) The structure of B-helical C-G-A-T-C-G-A-T-C-G and comparison with C-C-A-A-C-G-T-T-G-G. J. Biol. Chem., 266, 8861–8883. [DOI] [PubMed] [Google Scholar]
- 22.Pichler A., Rüdisser,S., Mitterböck,M., Huber,C.G., Winger,R.H., Liedl,K.R., Hallbrucker,A. and Mayer,E. (1999) Unexpected BII conformer substate population in unoriented hydrated films of the d(CGCGAATTCGCG)2 dodecamer and of native B-DNA from salmon testes. Biophys. J., 77, 398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winger R.H., Liedl,K.R., Rüdisser,S., Pichler,A., Hallbrucker,A. and Mayer,E. (1998) B-DNA’s BI→ BII conformer substate dynamics is coupled with water migration. J. Phys. Chem. B, 102, 8934–8940. [Google Scholar]
- 24.Wellenzohn B., Winger,R.H., Hallbrucker,A., Mayer,E. and Liedl,K.R. (2000) Simulation of EcoRI dodecamer netropsin complex confirms class I complexation mode. J. Am. Chem. Soc., 122, 3927–3931. [Google Scholar]
- 25.Cheatham T.E.III., Cieplak,P. and Kollman,P.A. (1999) A modified version of the Cornell et al. force field with improved sugar pucker phases and helical repeat. J. Biomol. Struct. Dyn., 16, 845–862. [DOI] [PubMed] [Google Scholar]
- 26.Schneider B., Neidle,S. and Berman,H.M. (1997) Conformations of the sugar-phosphate backbone in helical DNA crystal structures. Biopolymers, 42, 113–124. [DOI] [PubMed] [Google Scholar]
- 27.Berman H.M. (1997) Crystal studies of B-DNA: the answers and the questions. Biopolymers, 44, 23–44. [DOI] [PubMed] [Google Scholar]
- 28.Hartmann B. and Lavery,R. (1996) DNA structural forms. Q. Rev. Biophys., 29, 309–368. [DOI] [PubMed] [Google Scholar]
- 29.Tisné C., Hantz,E., Hartmann,B. and Delepierre,M. (1998) Solution structure of a non-palindromic 16 base-pair DNA related to the HIV-1κB site: evidence for BI-BII equilibrium inducing a global dynamic curvature of the duplex. J. Mol. Biol., 279, 127–142. [DOI] [PubMed] [Google Scholar]
- 30.Tisne C., Delepierre,M. and Hartmann,B. (1999) How NF-κB can be attracted by its cognate DNA. J. Mol. Biol., 293, 139–150. [DOI] [PubMed] [Google Scholar]
- 31.Pichler A., Rüdisser,S., Winger,R.H., Liedl,K.R., Hallbrucker,A. and Mayer,E. (2000) The role of water in B-DNAs BI to BII confomer substates interconversion: a combined study by calorimetry, FT-IR spectroscopy and computer simulation. Chem. Phys., 258, 391–404. [Google Scholar]
- 32.Flader W., Wellenzohn,B., Winger,R.H., Hallbrucker,A., Mayer,E. and Liedl,K.R. (2001) BI-BII substate transitions induce change in the hydration of B-DNA potentially mediating signal transduction from minor to major groove. J. Phys. Chem. B, 105, 10378–10387.
- 33.Buttinelli M., Minnock,A., Panetta,G., Waring,M. and Travers,A. (1998) The exocyclic groups of DNA modulate the affinity and positioning of the histone octamer. Proc. Natl Acad. Sci. USA, 95, 8544–8549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Travers A. and Drew,H. (1997) DNA recognition and nucleosome organization. Biopolymers, 44, 423–433. [DOI] [PubMed] [Google Scholar]
- 35.Marcotullio L.D., Buttinelli,M., Constanzo,G., Mauro,E.D. and Negri,R. (1998) Changing nucleosome positions in vivo through modifications of the DNA rotational information. Biochem. J., 333, 65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bailly C., Payet,D., Travers,A.A. and Waring,M.J. (1996) PCR-based development of DNA substrates containing modified bases: an efficient system for investigating the role of the exocyclic groups in chemical and structural recognition by minor groove binding drugs and proteins. Proc. Natl Acad. Sci. USA, 93, 13623–13628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bailly C., Waring,M.J. and Travers,A.A. (1995) Effect of base substitutions on the binding of a DNA-bending protein. J. Mol. Biol., 253, 1–7. [DOI] [PubMed] [Google Scholar]
- 38.Corfield P.W. R., Hunter,W.N., Brown,T., Robinson,P. and Kennard,O. (1987) Inosine.adenine base pairs in a B-DNA duplex. Nucleic Acids Res., 15, 7935–7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carter R.J., Bayens,K.J., SantaLucia,J., Hunter,D.H. and Holbrook,S.R. (1997) The crystal structure of an RNA oligomer incorporating tandem adenosine-inosine mismatches. Nucleic Acids Res., 25, 4117–4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leonard G.A., Booth,E., Hunter,W.N. and Brown,T. (1992) The conformational variability of an adenosine.inosine base-pair in a synthetic DNA dodecamer. Nucleic Acids Res., 20, 4753–4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen X., Mitra,S.N., Rao,S.T., Sekar,K. and Sundaralingam,M. (1998) A novel end-to-end binding of two netropsins to the DNA decamers d(CCCCIIII)2, d(CCCBr5CCIIIII)2 and d(CBr5CCCCIIII)2. Nucleic Acids Res., 26, 5464–5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar V.D., Harrison,R.W., Andrews,L.C. and Weber,I.T. (1992) Crystal structure at 1.5-Å resolution of d(CGCICICG), an octanucleotide containing inosine and its comparison with d(CGCG) and d(CGCGCG) structures. Biochemistry, 31, 1541–1550. [DOI] [PubMed] [Google Scholar]
- 43.Xuan J.-C. and Weber,I.T. (1992) Crystal structure of a B-DNA dodecamer containing inosine d(CGCIAATTCGCG), at 2.4 Å resolution and its comparison with other B-DNA dodecamers. Nucleic Acids Res., 20, 5457–5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dickerson R.E. and Drew,H.R. (1981) Structure of a B-DNA dodecamer: II. Influence of base sequence on helix structure. J. Mol. Biol., 149, 761–786. [DOI] [PubMed] [Google Scholar]
- 45.Dickerson R.E. and Drew,H.R. (1981) Kinematic model for B-DNA. Proc. Natl Acad. Sci. USA, 78, 7318–7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drew H.R., Wing,R.M., Takanao,T., Broka,C., Tanaka,S., Itakura,K. and Dickerson,R.E. (1981) Structure of a B-DNA dodecamer: conformation and dynamics. Proc. Natl Acad. Sci. USA, 78, 2179–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rossetti L., Cacchione,S., Fua,M. and Savino,M. (1998) Nucleosome assembly on telomeric sequences. Biochemistry, 37, 6727–6737. [DOI] [PubMed] [Google Scholar]
- 48.Luger K., Mäder,A.W., Richmond,R.K., Sargent,D.F. and Richmond,T.J. (1997) Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature, 389, 251–260. [DOI] [PubMed] [Google Scholar]
- 49.McGhee J.D. and Felsenfeld,G. (1982) Reconstitution of nucleosome core particles containing glucosylated DNA. J. Mol. Biol., 158, 685–697. [DOI] [PubMed] [Google Scholar]
- 50.Fitzgerald D.J. and Anderson,J.N. (1999) Selective nucleosome disruption by drugs that bind in the minor groove of DNA. J. Biol. Chem. 274, 27128–27138. [DOI] [PubMed] [Google Scholar]
- 51.Wellenzohn B., Flader,W., Winger,R.H., Hallbrucker,A., Mayer,E. and Liedl,K.R. (2001) Complex of B-DNA with polyamides freezes DNA backbone flexibility. J. Am. Chem. Soc., 123, 5044–5049. [DOI] [PubMed] [Google Scholar]
- 52.Wellenzohn B., Flader,W., Winger,R.H., Hallbrucker,A., Mayer,E. and Liedl,K.R. (2001) Significance of ligand tails for interaction with the minor groove of B-DNA. Biophys. J. 81, 1588–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. von Kitzing E. (1992) Modeling DNA structures: molecular mechanics and molecular dynamics. Methods Enzymol., 211, 449–467. [DOI] [PubMed] [Google Scholar]
- 54.Beveridge D.L. and Ravishanker,G. (1994) Molecular dynamics studies of DNA. Curr. Opin. Struct. Biol., 4, 246–255. [Google Scholar]
- 55.Beveridge D.L., Swaminathan,S., Ravishanker,G., Withka,J.M., Srinivasan,J., Prevost,C., Louise-May,S., Langley,D.R., DiCapua,F.M. and Bolton,P.H. (1993) Molecular dynamics simulations on the hydration, structure and motions of DNA oligomers. In Water and Biological Molecules. Macmillan Press Ltd, London, pp. 165–225.
- 56.Louise-May S., Auffinger,P. and Westhof,E. (1996) Calculations of nucleic acid conformations. Curr. Opin. Struct. Biol., 6, 289–298. [DOI] [PubMed] [Google Scholar]
- 57.Olson W.K. and Zhurkin,V.B. (2000) Modeling DNA deformations. Curr. Opin. Struct. Biol., 10, 286–297. [DOI] [PubMed] [Google Scholar]
- 58.Perree-Fauvet M. and Gresh,N. (1994) Structure and energetics in the complexes of a double-stranded B-DNA dodecamer with netropsin derivatives of a tricationic water-soluble porphyrin: a theoretical investigation. J. Biomol. Struct. Dyn., 11, 1203–1224. [DOI] [PubMed] [Google Scholar]
- 59.Ketterle C., Gabarro-Arpa,J.,M. Ouali,M.B., Auclair,C., Helissey,P., Giorgi-Renault,S. and Bret,M.L. (1996) Binding of NET-FLA, a netropsin-flavin hybrid molecule, to DNA: molecular mechanics and dynamics studies in vacuo and in water solution. J. Biomol. Struct. Dyn., 13, 963–977. [DOI] [PubMed] [Google Scholar]
- 60.Bifulco G., Galeone,A., Nicolaou,K., Chazin,W.J. and Gomez-Paloma,L. (1998) Solution structure of the complex between the head-to-tail dimer of calicheamicin γi1oligosaccharide and a DNA duplex containing d(ACCT) and d(TCCT) high-affinity binding sites. J. Am. Chem. Soc., 120, 7183–7191. [Google Scholar]
- 61.Cieplak P., Cheatham, III.,T.E. and Kollman,P.A. (1997) Molecular dynamics simulations find that 3′ phosphoramidate modified DNA duplexes undergo B to A transition and normal DNA duplexes an A to B transition. J. Am. Chem. Soc., 119, 6722–6730. [Google Scholar]
- 62.Young M.A., Nirmala,R., Srinivasan,J., McConnell,K.J., Ravishanker,G., Beveridge,D.L. and Berman,H.M. (1994) Analysis of helix bending in crystal structures and molecular dynamics simulations of DNA oligonucleotides. In Structural Biology: The State of the Art. Proceedings of the 8th Conversation. Adenine Press, Albany, NY, pp. 197–214.
- 63.Cheatham T.E.,III. and Kollman,P.A. (1996) Observation of the A-DNA to B-DNA transition during unrestrained molecular dynamics in aqueous solution. J. Mol. Biol., 259, 434–444. [DOI] [PubMed] [Google Scholar]
- 64.Case D.A., Pearlman,D.A., Caldwell,J.W., Cheatham,T.,III, Ross,W.S., Simmerling,C.L., Darden,T.A., Merz,K.M., Stanton,R.V., Cheng,A.L., Vincent,J.J., Crowley,M., Ferguson,D.M., Radmer,R.J., Seibel,G.L., Singh,U.C., Weiner,P.K. and Kollman,P.A. (1997) AMBER 5. University of California, San Francisco, CA.
- 65.Cornell W.D., Cieplak,P., Bayly,C.I., Gould,I.R., Merz,K.M.,Jr, Ferguson,D.M., Spellmeyer,D.C., Fox,T., Caldwell,J.W. and Kollman,P.A. (1995) A second generation force field for the simulation of proteins, nucleic acids and organic molecules. J. Am. Chem. Soc., 117, 5179–5197. [Google Scholar]
- 66.Frisch M.J., Trucks,G.W., Schlegel,H.B., Gill,P.M.W., Johnson,B.G., Robb,M.A., Cheeseman,J.R., Keith,T.A., Petersson,G.A., Montgomery,J.A., Raghavachari,K., Al-Laham,M.A., Zakrzewski,V.G., Ortiz,J.V., Foresman,J.B., Cioslowski,J., Stefanov,B.B., Nanayakkara,A., Challacombe,M., Peng,C.Y., Ayala,P.Y., Chen,W., Wong,M.W., Andres,J.L., Replogle,E.S., Gomperts,R., Martin,R.L., Fox,D.J., Binkley,J.S., Defrees,D.J., Baker,J., Stewart,J.P., Head-Gordon,M., Gonzalez,C. and Pople,J.A. (1995) Gaussian 94, Revision E.1. Gaussian, Inc., Pittsburgh, PA.
- 67.Young M.A., Ravishanker,G. and Beveridge,D.L. (1997) A 5-nanosecond molecular dynamics trajectory for B-DNA: analysis of structure, motions and solvation. Biophys. J., 73, 2313–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Young M.A., Jayaram,B. and Beveridge,D.L. (1997) Intrusion of counterions into the spine of hydration in the minor groove of B-DNA: fractional occupancy of electronegative pockets. J. Am. Chem. Soc., 119, 59–69. [Google Scholar]
- 69. de Souza O.N. and Ornstein,R.L. (1997) Effect of warmup protocol and sampling time on the convergence of molecular dynamics simulation of a DNA dodecamer using Amber 4.1 and particle-mesh ewald method. J. Biomol. Struct. Dyn., 14, 607–611. [DOI] [PubMed] [Google Scholar]
- 70. de Souza O.N. and Ornstein,R.L. (1997) Effect of periodic box size on aqueous molecular dynamics simulation of a DNA dodecamer with particle-mesh ewald method. Biophys. J., 72, 2395–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sayle R., Müller,A. and Bohne,A. (1999) rasmol2.6ab9. Molecular Modeling Group, German Cancer Research Center.
- 72.Laaksonen L. (1996) gOpenMol 1.21. Centre for Scientific Computing, Espoo (SF).
- 73.Lah J. and Vesnaver,G. (2000) Binding of dystamycin a and netropsin to the 12mer DNA duplexes containing mixed AT.GC sequences with at most five or three succesive AT base pairs. Biochemistry, 39, 9317–9327. [DOI] [PubMed] [Google Scholar]
- 74.Chen Y.Z. and Prohofsky,E.W. (1995) Normal mode calculation of a netropsin-DNA complex: effect of structural deformation on vibrational spectrum. Biopolymers, 35, 657–666. [DOI] [PubMed] [Google Scholar]
- 75.Singh S.B. and Kollman,P.A. (1999) Calculating the absolute free energy of association of netropsin and DNA. J. Am. Chem. Soc., 121, 3267–3271. [Google Scholar]
- 76.Zakrzewska K., Lavery,R. and Pullman,B. (1983) Theoretical studies of the selective binding to DNA of two non intercalating ligands:netropsin and SN18071. Nucleic Acids Res., 11, 8825–8839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zimmer C., Kakiuchi,N. and Guschlbauer,W. (1982) Differential stabilization by netropsin of inducible B-like conformations in deoxyribo-, ribo- and 2′-deoxy-2′-fluororibo-adenosine containing dublexes of (dA)n-(dT)n and (dA)n-(dU)n. Nucleic Acids Res., 10, 1721–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duong T.H. and Zakrzewska,K. (1997) Influence of drug binding on DNA flexibility: a normal mode analysis. J. Biomol. Struct. Dyn., 14, 691–701. [DOI] [PubMed] [Google Scholar]
- 79.Perez J.J. and Portugal,J. (1990) Molecular modelling study of changes induced by netropsin binding to nucleosome core particles. Nucleic Acids Res., 18, 3731–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coll M., Aymami,J., van der Marel,G.A., van Boom,J.H., Rich,A. and Wang,A.H.J. (1989) Molecular structure of the netropsin-d(CGCGATATCGCG) complex: DNA conformation in the alternating AT segment. Biochemistry, 28, 310–320. [DOI] [PubMed] [Google Scholar]
- 81.Kopka M.L., Yoon,C., Goodsell,D., Pjura,P. and Dickerson,R.E. (1985) The molecular origin of DNA-drug specificity in netropsin and distamycin. Proc. Natl Acad. Sci. USA, 82, 1376–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rentzeperis D., Marky,L.A., Dwyer,T.J., Geierstanger,B.H., Pelton,J.G. and Wemmer,D.E. (1995) Interaction of minor groove ligands to an AAATT/AATTT site: correlation of thermodynamic characterization and solution structure. Biochemistry, 34, 2937–2945. [DOI] [PubMed] [Google Scholar]
- 83.Sriram M., van der Marel,G.A., Roelen,H.L.P.F., van Boom,J.H. and Wang,A.H. J. (1992) Structural consequence of a carcinogen alkylation lesion on DNA: effect of E6-ethylguanine on the molecular structure of the d(CGC[E6]AATTCGCG)-netropsin complex. Biochemistry, 31, 11823–11834. [DOI] [PubMed] [Google Scholar]