Abstract
Xanthine oxidase (XO) is a metalloflavoenzyme associated with the uric acid formation in purine metabolism. Serum XO has been suggested to be associated with liver and kidney dysfunction, diabetes and cardiovascular diseases. However, there is limited information on the relationship between serum XO levels and hypertension. This study aimed to assess the relationship between serum XO levels and hypertension in Bangladeshi adults. In this study, fasting blood samples were collected from 312 participants (225 males and 87 females), aged ≥ 20 years. Serum levels of XO were determined by ELISA and other biochemical parameters including serum uric acid (SUA) were measured by colorimetric methods. Hypertension was defined as SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg or self-reported recent use of anti-hypertensive medications. Association between serum XO levels and hypertension was evaluated by multinomial logistic regression analysis. The mean level of XO was significantly higher (p < 0.001) in females (5.8 ± 3.2 U/L) than in males (3.9 ± 2.5 U/L). When the participants were divided by blood pressure levels, the mean level of serum XO was significantly higher (p < 0.01) in the hypertensive group (5.0 ± 2.7 U/L) compared to the normotensive control group (4.0 ± 2.7 U/L). An increasing trend for SBP and DBP levels was observed across the XO quartiles (at least p < 0.01 for both cases). A significant positive correlation was found for XO with SBP and DBP (p < 0.01). In regression analysis, the serum levels of XO showed a significant and independent association with hypertension prevalence. In conclusion, the mean level of serum XO was significantly higher in hypertensive individuals and XO was independently associated with the prevalence of hypertension. Our results indicate that XO may have a potential role in the pathophysiology of elevated blood pressure through generating of reactive oxygen species. Further large-scale longitudinal studies are needed to determine the underlying mechanisms between XO and hypertension.
Subject terms: Biomarkers, Predictive markers
Introduction
Xanthine oxidase (XO) is an isoform of xanthine oxidoreductase (XOR) that catalyzes the oxidation of hypoxanthine to xanthine and xanthine to uric acid, the last two steps of purine metabolism in humans1. Another isoform of XOR is xanthine dehydrogenase (XDH). XDH is primarily synthesized as the 150-kDa protein from which XO is derived, either reversibly by limited proteolysis or reversibly by conformational changes2. XO is widely distributed throughout the different organs in mammals including the heart, liver, lung, kidney, brain, and gut as well as the plasma3. Apart from its role in uric acid production, XO is also involved in the formation of reactive oxygen species (ROS) in mammalian cells1,4. ROS are associated with increased blood pressure (BP) via endothelial dysfunction, vascular inflammation, and structural remodeling5. Nitric oxide (NO) is a potent vasodilator and its levels may be diminished upon reaction with ROS-like superoxide anion radical (O2•−) causing greater resistance of arterioles and finally leading to hypertension development6,7. Although there might be other sources of oxidants in the vasculature implicated in hypertension, XO has received greater attention in recent years. In animal studies, it has been shown that XO-derived oxidants exert a stronger effect on arterial blood pressure in spontaneously hypertensive rats8. XO has also been suggested to be associated with diabetes in the human population9,10. However, only a limited number of studies have assessed the relationship between circulating levels of XO and hypertension in human individuals.
The elevated level of serum uric acid (SUA) is associated with several disease conditions including obesity, hypertension, diabetes, liver and kidney dysfunction, metabolic syndrome, and cardiovascular diseases11–17. However, some studies also reported that both elevated and low levels of SUA are associated with cardiovascular disease18,19 and renal disease20,21. A recent umbrella review that included meta-analyses, Mendelian analyses and randomized controlled trials, indicated that SUA has a clear role only in gout development and nephrolithiasis22. Therefore, SUA has been suggested as a mere biomarker other than an actual risk factor for kidney and cardiovascular disease including elevated blood pressure22,23.
A recent publication showed a positive association between plasma XOR and elevated blood pressure (BP) in the Japanese population who were not using anti-hypertensive or anti-hyperuricemic medications24. The authors suggested that XOR may induce the pathophysiology of elevated BP through ROS other than uric acid production24. Another study conducted on Japanese adults in the same country reported an independent association of plasma XOR with the risk of hypertension in nondiabetic subjects who were not taking any medications25. An observational study in a patient cohort in Japan showed that the use of XO inhibitors was associated with a lower risk of cardiovascular morbidity and all-cause mortality in hypertensive individuals with reduced kidney function26. Furthermore, Feig and colleagues found that allopurinol, a potential inhibitor of XO decreased the blood pressure in adolescents with newly diagnosed hypertension27. Therefore, it remains unclear, whether, XO causes hypertension before its involvement in renal injury, diabetes, and cardiovascular disease. Moreover, several lines of evidence suggest that either uric acid or XO are associated with vascular injury through endocytosis by vascular endothelial cells28–30. Therefore, further studies are needed to examine the role of XO in hypertension development. Moreover, it is also important to examine the association between XO and hypertension in various age groups as well as in different ethnic populations. Considering these aspects, our present study aimed to investigate the relationship between XO and hypertension in an adult cohort in Bangladesh.
Methods
Study population
This cross-sectional study was conducted between October 2019 and July 2020 at the Department of Biochemistry and Molecular Biology, Shahjalal University of Science and Technology, Sylhet, Bangladesh. In total, 312 participants (age range 20–80 years) were enrolled from general people living in the Sylhet city region, university students and academic and non-academic staff. As inclusion criteria, both genders aged ≥ 20 years and willing to participate were included in the study. As exclusion criteria, participants who were in the pregnancy or lactating stage or individuals with a history of drug addiction, alcohol consumption or participants with anti-hyperuricemic drug intake were not included in the study. We further excluded subjects with self-reported liver and kidney diseases, hypothyroidism, malignant disease and any infectious diseases. The study protocol was approved by the internal ethics committee existed at the Department of Biochemistry and Molecular Biology, SUST (Reference no 02/BMB/2019). All methods of the study were carried out in accordance with relevant guidelines and regulations. Informed consent was obtained from all subjects before inclusion in the study.
Data collection
The participant’s anthropometric data like weight, height, waist circumferences (WC) and hip circumferences (HC) were measured according to standard procedure described elsewhere31–37. Individual health status-related information such as the presence of hypertension, diabetes mellitus, and other chronic diseases was also recorded in the questionnaire form. Body mass index (BMI) was calculated as body weight in kg divided by body height in meters squared (kg/m2). The systolic and diastolic blood pressure (SBP and DBP, respectively) were measured using digital blood pressure (BP) machine (Omron M10, Tokyo Japan).
Blood collection and laboratory measurements
Fasting blood samples (4 mL) were drawn from the antecubital vein by venipuncture after overnight fasting (10–12 h) and kept in the serum collection tube. The samples were collected from each participant in the morning by trained personnel. The blood samples were placed in the ice box immediately after collection and transported to the laboratory for serum separation. The blood samples were centrifuged by an ultracentrifuge machine (Sorvall ST 8R Centrifuge, Thermo Scientific, Germany) at 4400 rpm for 10 min. The isolated serum samples were collected and stored at − 80 °C at the Departmental laboratory until biochemical parameter measurements. The serum level of xanthine oxidase (XO) was measured by enzyme-linked immunosorbent assay (ELISA). The other biochemical parameters like fasting blood glucose (FBG), serum uric acid (SUA), serum creatinine, and lipid profile markers (TC: total cholesterol, TG: triglyceride, HDL-C: high-density lipoprotein cholesterol, and LDL-C: low-density lipoprotein cholesterol) were measured by colorimetric methods using commercially available kits (Human Diagnostic, Germany) with a biochemistry analyzer (Humalyzer 3000, USA). All the measurements were done according to the manufacturer’s protocol provided in the kits.
Estimation of serum xanthine oxidase (XO)
Xanthine Oxidase (XO) levels in serum were measured by ELISA using a commercially available ELISA kit, which was purchased from MyBioSource company, USA (Human XO ELISA Kit, Cat No: MBS774009). This kit was used to measure the levels of XO in serum, based on the principle of ELISA. Briefly, standards and serum samples were added to the wells that were pre-coated with specific antibodies and then HRP-Conjugate solution was added to the wells to form an immune complex. After incubation, the unbound enzyme was removed through washing. The substrates were added, then the solution colour turned blue, and finally changed into yellow. The colour intensity was positively correlated with XO levels present in the serum sample. The absorbance (OD) of each micro-well was measured at 450 nm within 15 min by an ELISA reader (Apollo 11 LB 913, Berthold, Germany). Finally, XO concentrations in the samples were calculated according to the standard graph prepared in the excel sheet.
Diagnostic criteria
Hypertension was defined as SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg38, and participants with self-reporting intake of antihypertensive medication. Prehypertension was defined as SBP 120–139 mmHg; and/or DBP 80–89 mmHg38. Hyperuricemia was defined as SUA concentration > 7.0 mg/dL in males and > 6.0 mg/dL in females11,12.
Statistical analysis
All statistical analyses were performed using IBM SPSS statistical software version 25.0 (SPSS Inc, Chicago, Illinois, USA). Descriptive data are presented as mean ± standard deviation (SD), whereas categorical variables are expressed as percentages. Differences in the baseline variables between the control and the hypertensive group were analyzed by independent sample t-test. Serum XO was divided into four quartiles based on the frequency test: Q1 (< 2.32 U/L), Q2 (2.32–3.76 U/L), Q3 (3.77–5.79 U/L), and Q4 (> 5.79 U/L). One-way ANOVA was used to determine the differences in variables in the XO quartiles and BP groups. The association between serum level of XO and hypertension was evaluated by multinomial logistic regression analysis. p-values were considered significant at p < 0.05.
Results
Baseline characteristics of the study subjects in the control and hypertensive groups
The basic characteristics of the participants by blood pressure groups are summarized in Table 1. Of the total 312 subjects, 181 were normotensive control and 131 were hypertensive individuals. The mean age of the participants was 43.3 ± 12.6 years with a significant difference between the normotensive (40.4 ± 12.3 years) and hypertensive (47.2 ± 12.0 years) groups (p < 0.001). The mean value of BMI was significantly higher in the hypertensive group (26.0 ± 3.7 kg/m2) compared to the normotensive (24.6 ± 3.7 kg/m2) group (p < 0.01). Subjects in the hypertensive group had a higher mean level of XO (5.0 ± 2.7 U/L) and SUA (8.1 ± 4.2 mg/dL) than subjects in the control group (4.0 ± 2.7 U/L, 5.2 ± 1.6 mg/dL, respectively) (p < 0.01). The mean concentration of FBG, TC, and HDL-C were also significantly higher in the hypertensive group compared to the control group (at least p < 0.05 for all cases).
Table 1.
Baseline characteristics of the participants in normotensive and hypertensive groups.
| Variables | Total (n = 312) | Normotensive (n = 181) | Hypertensive (n = 131) | p-value |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Gender, m/f | 225/87 | 130/51 | 95/36 | − |
| Age, year | 43.3 ± 12.6 | 40.4 ± 12.3 | 47.2 ± 12.0 | 0.000 |
| Height, cm | 162.1 ± 9.3 | 161.4 ± 9.3 | 160.8 ± 9.3 | 0.563 |
| Weight, kg | 65.2 ± 11.7 | 64 ± 10.3 | 66.9 ± 13.3 | 0.030 |
| BMI, kg/m2 | 25.2 ± 3.7 | 24.6 ± 3.7 | 26.0 ± 3.7 | 0.001 |
| WC, cm | 86.6 ± 10.4 | 84.8 ± 10 .0 | 88.9 ± 10.4 | 0.004 |
| HC, cm | 92.7 ± 8.7 | 90.9 ± 7.7 | 92.7 ± 8.7 | 0.110 |
| SBP, mmHg | 130.2 ± 17.9 | 119.5 ± 8.9 | 145 ± 16.5 | 0.000 |
| DBP, mmHg | 84.5 ± 10 .0 | 79.6 ± 7.2 | 91.3 ± 9.4 | 0.000 |
| FBG, mmol/dL | 7.3 ± 3.7 | 6.6 ± 3.2 | 8.0 ± 4.2 | 0.001 |
| SUA, mg/dL | 5.4 ± 1.8 | 5.2 ± 1.6 | 8.1 ± 4.2 | 0.004 |
| XO, U/L | 4.4 ± 2.8 | 4.0 ± 2.7 | 5.0 ± 2.7 | 0.002 |
| Creatinine, mg/dL | 0.9 ± 0.3 | 0.9 ± 0.3 | 0.8 ± 0.3 | 0.490 |
| TG, mg/dL | 199.7 ± 122.8 | 191 ± 112.4 | 210.8 ± 122.8 | 0.184 |
| TC, mg/dL | 217.7 ± 87.1 | 207.7 ± 80.7 | 230.6 ± 93.5 | 0.032 |
| HDL-C, mg/dL | 34.3 ± 13.6 | 32.1 ± 11.7 | 37.2 ± 15.0 | 0.000 |
| LDL-C, mg/dL | 144.2 ± 80.3 | 138.1 ± 74.9 | 152.1 ± 86.4 | 0.155 |
| Smoking, n (%) | 0.214 | |||
| Yes | 63 (20.3) | 48 (22.1) | 15 (15.9) | – |
| No | 248 (79.7) | 169 (77.9) | 79 (84.1) | – |
Values are presented as mean ± SD. p-values are obtained from the independent sample t-test.
BMI, body mass index; WC, waist circumference; HC, hip circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; XO, xanthine oxidase; SUA, serum uric acid; FBG, fasting blood glucose; TG, triglyceride; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Baseline characteristics of the participants based on sex
Table 2 shows the baseline characteristics according to sex. Among the study subjects, 225 (72.1%) were males and 87 (27.9%) were females. There was no significant difference in the mean age and BMI. The mean level of XO was higher in females (5.8 ± 3.2 U/L) than in male (3.9 ± 2.5 U/L) participants (p < 0.001), whereas, the mean SUA was higher in males (5.9 ± 1.6 mg/dL) than in female (4.4 ± 1.7 mg/dL) subjects (p < 0.001). The mean level of FBG was higher in females than in males (p < 0.05). Based on BP levels the participants were also divided into normotensive, prehypertensive and hypertensive groups (Fig. 1). The mean serum XO level was higher in females than in males (p < 0.01). No significant difference was found within the blood pressure groups in female subjects. However, the XO level was higher in the hypertensive group than in the normotensive and prehypertensive groups, especially in male subjects (p < 0.01). When participants were divided into three age groups (Fig. 2), an increasing trend in XO level was observed in the age groups, especially in females (p < 0.01).
Table 2.
Baseline characteristics of the participants based on gender.
| Variables | Total (n = 312) | Male (n = 225) | Female (n = 87) | p-value |
|---|---|---|---|---|
| Age, year | 43.3 ± 12.6 | 42.9 ± 12.6 | 44.3 ± 12.6 | 0.387 |
| Height, cm | 162.1 ± 9.3 | 164.8 ± 7.1 | 151.7 ± 7.5 | 0.000 |
| Weight, kg | 65.2 ± 11.7 | 68.1 ± 10.6 | 57.9 ± 11.4 | 0.000 |
| BMI, kg/m2 | 25.2 ± 3.7 | 25 ± 3.4 | 25.6 ± 4.5 | 0.249 |
| WC, cm | 86.6 ± 10.4 | 87.8 ± 10.2 | 84 ± 10.6 | 0.015 |
| HC, cm | 92.7 ± 8.7 | 92.2 ± 8.2 | 90.7 ± 8.2 | 0.203 |
| SBP, mmHg | 130.2 ± 17.9 | 130.1 ± 17.5 | 130.6 ± 18.8 | 0.830 |
| DBP, mmHg | 84.5 ± 10 | 84.3 ± 10.3 | 85 ± 9.4 | 0.559 |
| FBG, mmol/dL | 7.3 ± 3.7 | 6.9 ± 3.6 | 8.2 ± 4 | 0.024 |
| SUA, mg/dL | 5.4 ± 1.8 | 5.9 ± 1.6 | 4.4 ± 1.7 | 0.000 |
| XO, U/L | 4.4 ± 2.8 | 3.9 ± 2.5 | 5.8 ± 3.2 | 0.000 |
| Creatinine, mg/dL | 0.9 ± 0.3 | 0.9 ± 0.3 | 0.7 ± 0.2 | 0.000 |
| TG, mg/dL | 199.7 ± 122.8 | 201.6 ± 18.3 | 194.9 ± 134.4 | 0.703 |
| TC, mg/dL | 217.7 ± 87.1 | 213.6 ± 82.5 | 228.1 ± 97.9 | 0.248 |
| HDL-C, mg/dL | 34.3 ± 13.6 | 34.1 ± 14.4 | 36.4 ± 10.9 | 0.160 |
| LDL-C, mg/dL | 144.2 ± 80.3 | 140.1 ± 72.6 | 154.8 ± 97.4 | 0.170 |
| Hypertension, (%) | 41.9 | 42.2 | 41.4 | – |
Values are presented as mean ± SD. p-values are obtained from the independent sample t-test.
BMI, body mass index; WC, waist circumference; HC, hip circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; XO, xanthine oxidase; SUA, serum uric acid; FBG, fasting blood glucose; TG, triglyceride; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Figure 1.
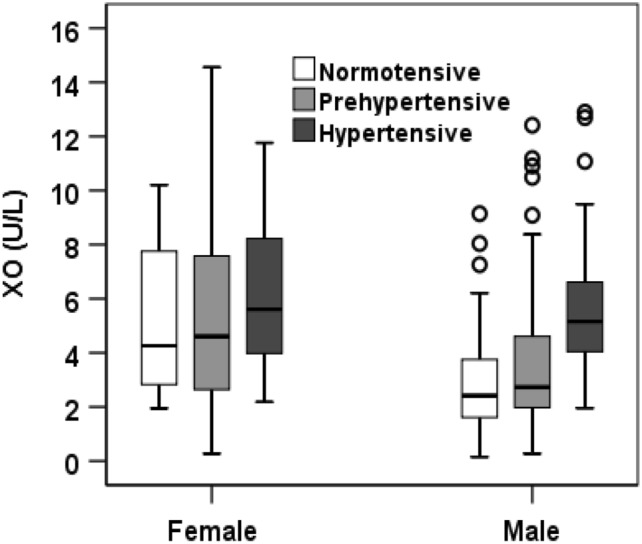
Levels of XO in the normotensive, prehypertensive, and hypertensive groups by gender. p < 0.01, when XO levels in normotensive, prehypertensive, and hypertensive groups were compared between male and female groups. p < 0.05, when XO levels were compared within the blood pressure groups in female subjects.
Figure 2.
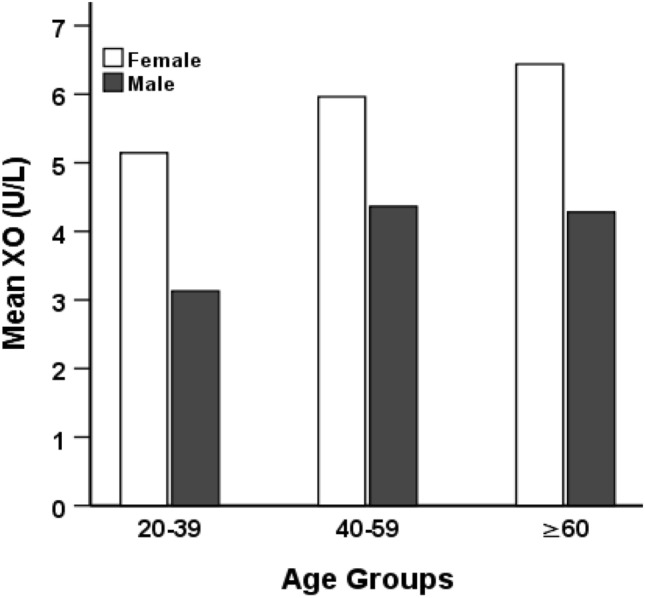
Levels of XO by age groups. p < 0.01, when XO level was compared within age groups in females. p-values were obtained from one-way ANOVA.
Baseline parameters in XO quartiles and correlation of XO with BP
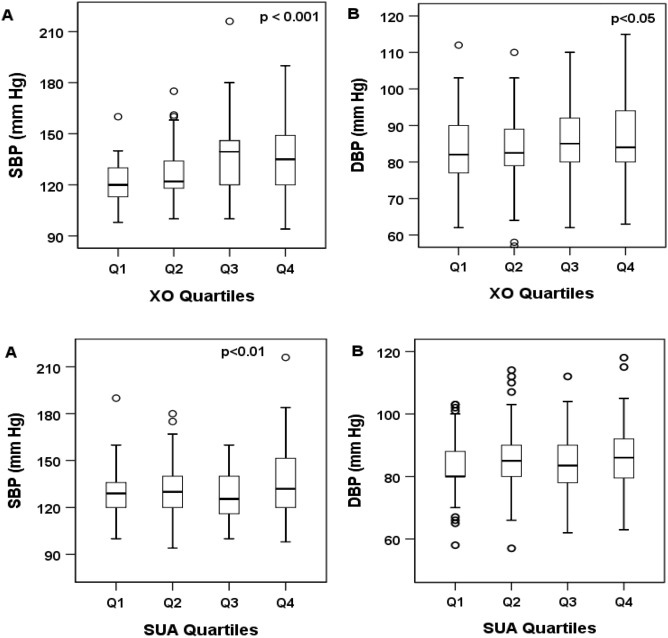
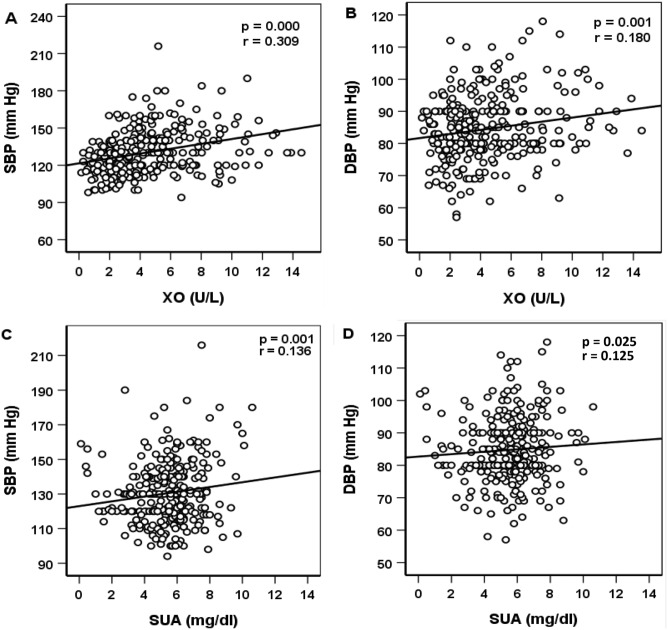
Serum XO levels were divided into four quartiles: Q1 (< 2.32 U/L), Q2 (2.32–3.76 U/L), Q3 (3.77–5.79 U/L), and Q4 (> 5.79 U/L). A significant increasing trend was observed for SBP and DBP across the XO quartiles (Table 3 and Fig. 3). In SUA quartile groups, the increasing trend of both SBP and DBP was inconsistent, however, the overall trend within the quartiles was significant, especially for SBP. The correlation of XO and SUA with SBP and DBP is depicted in Fig. 4. A significant positive correlation was observed for serum XO level with SBP and DBP (at least p < 0.01 for both cases). Similarly, SUA also showed a positive correlation with SBP and DBP (at least p < 0.05 for both cases).
Table 3.
Characteristics of the study subjects according to XO quartiles.
| Variables | Xanthine oxidase (U/L) | p-value | |||
|---|---|---|---|---|---|
| Q1 (< 2.32) | Q2 (2.32–3.76) | Q3 (3.77–5.79) | Q4 (> 5.79) | ||
| N | 78 | 78 | 78 | 78 | – |
| Age (year) | 38.2 ± 11.3 (65) | 42.2 ± 12.9 (70) | 46.6 ± 12.8 (85) | 43.3 ± 12.6 (85) | 0.000 |
| Height (cm) | 164.2 ± 9.9 (181) | 161.4 ± 8.6 (178) | 162.3 ± 7.6 (177) | 161.1 ± 9.3 (181) | 0.000 |
| Weight (kg) | 67.6 ± 8.9 (92) | 66.4 ± 11.1 (96.6) | 64.9 ± 13.4 (95) | 65.2 ± 11.7 (105) | 0.019 |
| BMI (kg/m2) | 25.2 ± 3.4 (38.4) | 25.4 ± 3.6 (33.9) | 24.9 ± 3.8 (40) | 25.2 ± 3.8 (40) | 0.862 |
| WC (cm) | 87.8 ± 8.4 (103) | 89 ± 11.8 (116) | 86.5 ± 9.5 (109) | 86.6 ± 10.4 (116) | 0.060 |
| HC (cm) | 95 ± 6.6 (108) | 93.3 ± 9.0 (113) | 90.5 ± 7.9 (110) | 91.7 ± 8.2 (113) | 0.003 |
| SBP (mmHg) | 120.9 ± 12.1 (160) | 126.5 ± 14.8 (175) | 137.1 ± 19.3 (216) | 136.2 ± 19.1 (216) | 0.000 |
| DBP (mmHg) | 82.9 ± 9.2 (112) | 82.7 ± 9.5 (110) | 85.6 ± 9.9 (110) | 84.5 ± 10 (118) | 0.019 |
| FBG (mmol/L) | 5.8 ± 2.9 (26.9) | 7.4 ± 4.3 (24) | 7.5 ± 3.2 (18.4) | 7.3 ± 3.7 (26.9) | 0.000 |
| SUA (mg/dL) | 5.6 ± 1.5 (7.9) | 5.4 ± 2 (10.1) | 5.6 ± 1.6 (10.6) | 5.4 ± 1.8 (10.6) | 0.304 |
| XO (U/L) | 1.6 ± 0.6 (2.3) | 3.0 ± 0.4 (3.8) | 4.7 ± 0.6 (5.8) | 8.4 ± 2.2 (14.6) | 0.000 |
| Creatinine (mg/dL) | 0.9 ± 0.3 (2.5) | 0.9 ± 0.2 (1.3) | 0.9 ± 0.3 (2) | 0.9 ± 0.3 (2.5) | 0.083 |
| TG (mg/dL) | 186.7 ± 114.2 (675.2) | 179.4 ± 115.4 (583.3) | 216.3 ± 131.9(726.6) | 199.7 ± 122.8 (812.6) | 0.185 |
| TC (mg/dL) | 209.2 ± 65.6 (463.5) | 234.9 ± 105.6 (562.8) | 215.1 ± 89.8 (584.0) | 217.7 ± 87.1 (584.0) | 0.325 |
| HDL-C (mg/dL) | 31.3 ± 9.1 (63.2) | 35.4 ± 14 (91) | 37.3 ± 17 (112.4) | 34.8 ± 13.6 (112.4) | 0.063 |
| LDL-C (mg/dL) | 141.4 ± 60.2 (324.6) | 165.6 ± 97.0 (517.2) | 135.3 ± 79.4 (506.5) | 144.2 ± 80.3 (517.2) | 0.099 |
Values are presented as mean ± SD (max.). p-values are obtained from one-way ANOVA.
BMI, body mass index; WC, waist circumference; HC, hip circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; XO, xanthine oxidase; SUA, serum uric acid; FBG, fasting blood glucose; TG, triglyceride; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Figure 3.
Systolic (A) and Diastolic (B) blood pressure across the XO and SUA quartiles. The scale in the Y-axis is not similar to the figures. p-values were obtained from one-way ANOVA.
Figure 4.
Correlation of XO level with SBP (A) and DBP (B) and SUA levels with SBP (C) and DBP (D). The scale in the X-axis is not similar in (A) and (C). p-values for XO and SUA with blood pressures were obtained from Pearson’s correlation coefficient.
Association between serum XO and hypertension
Table 4 represents the association between serum XO levels and hypertension. In logistic regression analysis, four models were applied to evaluate the relationship. After adjusting for age and sex in model 1, the odds ratios (ORs) and 95% CI were 1.030 (1.005–1.055), 1.067 (1.041–1.094), and 1.066 (1.040–1.093) respectively in Q2–Q4 compared to Q1. In model 2, after additionally adjusting BMI with model 1, the ORs (95% Cl) were 1.033 (1.007–1.059), 1.075 (1.047–1.103), and 1.073 (1.046–1.102) respectively in Q2–Q4 compared to Q1. In model 3, after adjustment of lipid profile (TG, TC, HDL-C, and LDL-C) with model 2, the ORs (95% Cl) were 1.029 (1.000–1.059), 1.085 (1.054–1.118), and 1.084 (1.052–1.117). In model 4, after adjustment of smoking status with model 3, the ORs (95% Cl) were 1.033 (0.988–1.081), 1.084 (1.037–1.134) and 1.079 (1.031–1.129). In all models, XO showed a positive and independent association with hypertension (p < 0.01).
Table 4.
Association between XO and hypertension.
| OR (95% CI) | p-values for trend | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Model 1 | 1 | 1.030 (1.005–1.055) | 1.067 (1.041–1.094) | 1.066 (1.040–1.093) | < 0.01 |
| Model 2 | 1 | 1.033 (1.007–1.059) | 1.075 (1.047–1.103) | 1.073 (1.046–1.102) | < 0.01 |
| Model 3 | 1 | 1.029 (1.000–1.059) | 1.085 (1.054–1.118) | 1.084 (1.052–1.117) | < 0.01 |
| Model 4 | 1 | 1.033 (0.988–1.081) | 1.084 (1.037–1.134) | 1.079 (1.031–1.129) | < 0.01 |
Multinomial logistic regression analysis was applied to evaluate the associations between XO and hypertension. Dependent variable was hypertension (yes) and independent variable was XO (U/L). OR, odds ratio; CI, confidence interval; SE, standard error. Model 1: adjusted for age and sex; Model 2: Model 1 plus BMI and SUA; Model 3: Model 2 plus TG, TC, HDL-C, and LDL-C; Model 4: Model 3 plus smoking status.
Discussion
The present study reports a positive and independent association between serum XO levels and hypertension. This association remained even after adjustment for several confounders such as age, gender, BMI, lipid markers and smoking status. To our knowledge, this study provides the first information on the relationship between serum XO levels and hypertension in Bangladeshi adults.
In our study, the female participants had a higher mean XO level than the male participants. On the other hand, the mean SUA was higher in males than in females. An increased level of SUA has been found in males in several studies11,14,39,40. Although there is no established consensus on sex differences in serum XO levels. The differences in the mean level of XO between the sex groups may be related to sex hormones. However, little is known about the relationship between sex hormones and serum XO levels. Visceral fat content adipose tissue is one of the major sources of XOR41. Considering that visceral fat mass is generally increased in the postmenopausal stage in women42, elderly female participants might have higher serum XO levels. However, further sex-specific mechanistic studies can reveal the exact mechanism for this physiological difference. When study participants were divided by BP levels, the mean level of serum XO was found to be significantly higher in the hypertensive group than in the normotensive group. An increasing trend was observed for SBP and DBP levels across the XO quartiles and XO showed an independent association with hypertension. Our findings are consistent with a recent study that showed also a significant association of plasma XOR with elevated BP in Japanese adults who were not taking antihypertensive or anti-hyperuricemic drugs24. This independent association between plasma XOR and hypertension was also found in nondiabetic individuals in another recent study in Japan25. However, these two studies could not provide information, on whether taking antihypertensive drugs affects XO levels. In the current study, we also included participants who were receiving antihypertensive medications and still our analysis showed a higher level of serum XO in hypertensive subjects than in normotensive subjects. Furthermore, in our study, some participants were diabetic, however, the presence of diabetes did not show any significant difference in serum XO level with participants who were non-diabetic. Along with the findings of a previous study24, our observations suggest that serum XO levels may increase the risk of elevated BP with or without comorbidities, including diabetes.
In the present study, we also observed an increasing trend of serum XO levels with increasing age in the groups. A study stated that the deterioration of the tissues is generally higher in older people43. This may be a cause of higher purine metabolism and elevated XO levels in older individuals than in the younger. However, the mechanism for the age-related relationship between serum XO and BP is yet to be explored.
Hyperuricemia is known to predict hypertension development and using an XOR inhibitor has been shown to lower BP in some studies44,45. On the other hand, several46–49 but not all studies have found no effect from using uricosuric drugs such as benzbromarone on BP. In cardiovascular patients, XO inhibitor has been proven to be effective in reducing the risk of cardiovascular disease50. Although in many studies a significant positive association was found between elevated SUA and hypertension, cardiovascular diseases and chronic kidney diseases13,15,51, some other studies found both elevated and low levels of SUA to be associated with cardiovascular disease18,19 and renal disease20,21. A recent umbrella review covering meta-analyses of observational studies, Mendelian analyses and randomized controlled trials, reported that SUA is truly linked only to gout development and nephrolithiasis22. Therefore, SUA has been suggested as a mere biomarker other than a true risk factor for cardiovascular and renal disease including hypertension22.
The underlying mechanisms between XO and hypertension are not well established yet. There are some possible ways through which elevated XO levels may increase the risk of hypertension. During purine metabolism, XO produces ROS as byproducts1,4 which may increase BP via endothelial dysfunction, vascular inflammation, and structural remodeling5. In endothelial cells, the redox state is regulated through a balance between NO and ROS-like superoxide anion radical (). NO is a potent vasodilator and its diminished levels upon reaction with cause increased resistance of arterioles, and eventually leads to hypertension development6,7. Furthermore, reduced activation of XO may also assist oxidative stress-related injury in kidney and endothelial cells23 which leads to elevated BP. However, the exact mechanisms between XO and the risk of hypertension is remained to explore.
The major strength of the present study was the adjustment for several well-known hypertension risk factors in the regression analysis. Moreover, the data and samples were collected following standard procedures and all the biochemical analyses were done in a single laboratory. Our study had also some limitations. First, the cross-sectional nature of this study may not prove the causal relationships between serum XO levels and hypertension. Therefore, a future longitudinal study may provide better insights into the link between XO levels and hypertension. Second, the sample size of this study was relatively small, which does not represent the whole Bangladeshi population. Third, the findings of our study may not be applied to other ethnic groups. Despite several limitations, this study findings may be a worthy reference for future investigations in this area.
Conclusion
In the present study, a higher mean serum XO level was found in hypertensive individuals and this elevation was higher in females than in males. Serum XO levels were positively correlated with both SBP and DBP. In regression analysis, serum levels of XO showed a positive and independent association with hypertension. Our findings indicate that XO may have an important role in the pathophysiology of elevated blood pressure through generating of ROS. However, more studies are needed to reveal the underlying mechanisms between XO and the risk of hypertension.
Acknowledgements
The authors are thankful to all participants for their active participation and cooperation in the study.
Author contributions
R.M. collected the samples, conducted the experiments, data analysis and drafted the primary manuscript. K.A.F., S.A.S. S.A., assisted in lab experiments and data analysis. M.H., A.D.M, Z.B., A.H., and N.C.M., helped in sample and data collection. N.A. contributed to the conception and design of the study, data interpretation, drafting and revision of the manuscript. All authors read and approved the submitted version.
Funding
This study did not receive any external grant rather than a small grant from the university as internal.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hille R, Nishino T. Xanthine oxidase and xanthine dehydrogenase. FASEB J. 1995;9:995–1003. doi: 10.1096/fasebj.9.11.7649415. [DOI] [PubMed] [Google Scholar]
- 2.Waud WR, Rajagopalan KV. The mechanism of conversion of rat liver xanthine dehydrogenase from an NAD+-dependent form (type D) to an O2-dependent form (type O) Arch. Biochem. Biophys. 1976;172:365–379. doi: 10.1016/0003-9861(76)90088-6. [DOI] [PubMed] [Google Scholar]
- 3.Pacher P, Nivorozhkin A, Szabó C. Therapeutic effects of xanthine oxidase inhibitors: Renaissance half a century after the discovery of allopurinol. Pharmacol. Rev. 2006;58:87–114. doi: 10.1124/pr.58.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Battelli MG, Polito L, Bolognesi A. Xanthine oxidoreductase in atherosclerosis pathogenesis: Not only oxidative stress. Atherosclerosis. 2014;237:562–567. doi: 10.1016/j.atherosclerosis.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Touyz RM, Briones AM. Reactive oxygen species and vascular biology: Implications in human hypertension. Hypertens. Res. 2011;34:5–14. doi: 10.1038/hr.2010.201. [DOI] [PubMed] [Google Scholar]
- 6.Lee CI, Liu X, Zweier JL. Regulation of xanthine oxidase by nitric oxide and peroxynitrite. J. Biol. Chem. 2000;275:9369–9376. doi: 10.1074/jbc.275.13.9369. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Cui H, Kundu TK, Alzawahra W, Zweier JL. Nitric oxide production from nitrite occurs primarily in tissues not in the blood. J. Biol. Chem. 2008;283:17855–17863. doi: 10.1074/jbc.M801785200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki H, et al. Xanthine oxidase activity associated with arterial blood pressure in spontaneously hypertensive rats. Proc. Natl. Acad. Sci. USA. 1998;95:4754–4759. doi: 10.1073/pnas.95.8.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, et al. Elevated serum xanthine oxidase activity is associated with the development of type 2 diabetes: A prospective cohort study. Diabetes Care. 2018;41:884–890. doi: 10.2337/dc17-1434. [DOI] [PubMed] [Google Scholar]
- 10.Hasan M, et al. Assessment of the relationship between serum xanthine oxidase levels and type 2 diabetes: A cross-sectional study. Sci. Rep. 2022;12:20816. doi: 10.1038/s41598-022-25413-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali N, et al. Prevalence of hyperuricemia and the relationship between serum uric acid and obesity: A study on Bangladeshi adults. PLoS ONE. 2018;13:e0206850. doi: 10.1371/journal.pone.0206850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali N, et al. Association between serum uric acid and metabolic syndrome: A cross-sectional study in Bangladeshi adults. Sci. Rep. 2020;10:1–7. doi: 10.1038/s41598-020-64884-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali N, et al. Relationship between serum uric acid and hypertension: A cross-sectional study in Bangladeshi adults. Sci. Rep. 2019;9:1–7. doi: 10.1038/s41598-019-45680-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ali N, et al. The relationship between serum uric acid and lipid profile in Bangladeshi adults. BMC Cardiovasc. Disord. 2019;19:1–7. doi: 10.1186/s12872-019-1026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borghi C, et al. Serum uric acid and the risk of cardiovascular and renal disease. J. Hypertens. 2015;33:1729–1741. doi: 10.1097/HJH.0000000000000701. [DOI] [PubMed] [Google Scholar]
- 16.Haque T, Rahman S, Islam S, Molla NH, Ali N. Assessment of the relationship between serum uric acid and glucose levels in healthy, prediabetic and diabetic individuals. Diabetol. Metab. Syndr. 2019;11:1–8. doi: 10.1186/s13098-019-0446-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molla NH, et al. Assessment of the relationship between serum uric acid levels and liver enzymes activity in Bangladeshi adults. Sci. Rep. 2021;11:1–9. doi: 10.1038/s41598-021-99623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verdecchia P, et al. Relation between serum uric acid and risk of cardiovascular disease in essential hypertension: The PIUMA study. Hypertension. 2000;36:1072–1078. doi: 10.1161/01.HYP.36.6.1072. [DOI] [PubMed] [Google Scholar]
- 19.Zhang W, et al. Serum uric acid and mortality form cardiovascular disease: EPOCH-JAPAN study. J. Atheroscler. Thromb. 2016;23:692–703. doi: 10.5551/jat.31591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mori K, et al. U-shaped relationship between serum uric acid level and decline in renal function during a 10-year period in female subjects: BOREAS-CKD2. Hypertens. Res. 2021;44:107–116. doi: 10.1038/s41440-020-0532-z. [DOI] [PubMed] [Google Scholar]
- 21.Wakasugi M, et al. Association between hypouricemia and reduced kidney function: A cross-sectional population-based study in Japan. Am. J. Nephrol. 2015;41:138–146. doi: 10.1159/000381106. [DOI] [PubMed] [Google Scholar]
- 22.Li X, et al. Serum uric acid levels and multiple health outcomes: Umbrella review of evidence from observational studies, randomised controlled trials, and Mendelian randomisation studies. BMJ. 2017;357:253. doi: 10.1136/bmj.j2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furuhashi M. New insights into purine metabolism in metabolic diseases: Role of xanthine oxidoreductase activity. Am. J. Physiol.-Endocrinol. Metab. 2020;319(5):E827–E834. doi: 10.1152/ajpendo.00378.2020. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida S, et al. Association of plasma xanthine oxidoreductase activity with blood pressure affected by oxidative stress level: MedCity21 health examination registry. Sci. Rep. 2020;10:4437. doi: 10.1038/s41598-020-61463-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furuhashi M, et al. Independent association of plasma xanthine oxidoreductase activity with hypertension in nondiabetic subjects not using medication. Hypertens. Res. 2021;44:1213–1220. doi: 10.1038/s41440-021-00679-1. [DOI] [PubMed] [Google Scholar]
- 26.Terawaki H, et al. Effect of allopurinol on cardiovascular incidence among hypertensive nephropathy patients: The Gonryo study. Clin. Exp. Nephrol. 2013;17:549–553. doi: 10.1007/s10157-012-0742-z. [DOI] [PubMed] [Google Scholar]
- 27.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: A randomized trial. JAMA. 2008;300:924–932. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houston M, et al. Binding of xanthine oxidase to vascular endothelium: Kinetic characterization and oxidative impairment of nitric oxide-dependent signaling. J. Biol. Chem. 1999;274:4985–4994. doi: 10.1074/jbc.274.8.4985. [DOI] [PubMed] [Google Scholar]
- 29.Kang D-H, Park S-K, Lee I-K, Johnson RJ. Uric acid–induced C-reactive protein expression: Implication on cell proliferation and nitric oxide production of human vascular cells. J. Am. Soc. Nephrol. 2005;16:3553–3562. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- 30.Radi R, Rubbo H, Bush K, Freeman BA. Xanthine oxidase binding to glycosaminoglycans: Kinetics and superoxide dismutase interactions of immobilized xanthine oxidase–heparin complexes. Arch. Biochem. Biophys. 1997;339:125–135. doi: 10.1006/abbi.1996.9844. [DOI] [PubMed] [Google Scholar]
- 31.Ali N, et al. Hypertension prevalence and influence of basal metabolic rate on blood pressure among adult students in Bangladesh. BMC Public Health. 2017;18:58. doi: 10.1186/s12889-017-4617-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kathak RR, et al. The association between elevated lipid profile and liver enzymes: A study on Bangladeshi adults. Sci. Rep. 2022;12:1711. doi: 10.1038/s41598-022-05766-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mou AD, et al. Prevalence of preeclampsia and the associated risk factors among pregnant women in Bangladesh. Sci. Rep. 2021;11:21339. doi: 10.1038/s41598-021-00839-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahman S, Islam S, Haque T, Kathak RR, Ali N. Association between serum liver enzymes and hypertension: A cross-sectional study in Bangladeshi adults. BMC Cardiovasc. Disord. 2020;20:128. doi: 10.1186/s12872-020-01411-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Islam S, et al. Prevalence of elevated liver enzymes and its association with type 2 diabetes: A cross-sectional study in Bangladeshi adults. Endocrinol. Diabetes Metab. 2020;3:e00116. doi: 10.1002/edm2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali N, Mohanto NC, Nurunnabi SM, Haque T, Islam F. Prevalence and risk factors of general and abdominal obesity and hypertension in rural and urban residents in Bangladesh: A cross-sectional study. BMC Public Health. 2022;22:1–14. doi: 10.1186/s12889-022-14087-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ali, N. et al. The prevalence of general obesity, abdominal obesity, and hypertension and its related risk factors among young adult students in Bangladesh. J. Clin. Hypertens. (2022). [DOI] [PMC free article] [PubMed]
- 38.Chobanian AV, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 39.Feig DI, Kang D-H, Johnson RJ. Uric acid and cardiovascular risk. N. Engl. J. Med. 2008;359:1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Multidisciplinary Expert Task Force on Hyperuricemia and Related Diseases. Chinese Multidisciplinary Expert Consensus on the Diagnosis and Treatment of Hyperuricemia and Related Diseases. Chin. Med. J. (Engl.)130, 2473–2488 (2017). [DOI] [PMC free article] [PubMed]
- 41.Tsushima Y, et al. Uric acid secretion from adipose tissue and its increase in obesity. J. Biol. Chem. 2013;288:27138–27149. doi: 10.1074/jbc.M113.485094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kozakowski J, Gietka-Czernel M, Leszczyńska D, Majos A. Obesity in menopause—our negligence or an unfortunate inevitability? Przeglad Menopauzalny Menopause Rev. 2017;16:61–65. doi: 10.5114/pm.2017.68594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willems L, Garnham B, Headrick JP. Aging-related changes in myocardial purine metabolism and ischemic tolerance. Exp. Gerontol. 2003;38:1169–1177. doi: 10.1016/j.exger.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Agarwal V, Hans N, Messerli FH. Effect of allopurinol on blood pressure: A systematic review and meta-analysis: Allopurinol and blood pressure. J. Clin. Hypertens. 2013;15:435–442. doi: 10.1111/j.1751-7176.2012.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qu L, Jiang H, Chen J. Effect of uric acid-lowering therapy on blood pressure: Systematic review and meta-analysis. Ann. Med. 2017;49:142–156. doi: 10.1080/07853890.2016.1243803. [DOI] [PubMed] [Google Scholar]
- 46.McMullan CJ, Borgi L, Fisher N, Curhan G, Forman J. Effect of uric acid lowering on renin-angiotensin-system activation and ambulatory BP: A randomized controlled trial. Clin. J. Am. Soc. Nephrol. 2017;12:807–816. doi: 10.2215/CJN.10771016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ogino K, et al. Uric acid-lowering treatment with benzbromarone in patients with heart failure: A double-blind placebo-controlled crossover preliminary study. Circ. Heart Fail. 2010;3:73–81. doi: 10.1161/CIRCHEARTFAILURE.109.868604. [DOI] [PubMed] [Google Scholar]
- 48.Ohta Y, et al. Effective uric acid-lowering treatment for hypertensive patients with hyperuricemia. Hypertens. Res. 2017;40:259–263. doi: 10.1038/hr.2016.139. [DOI] [PubMed] [Google Scholar]
- 49.Okuda C, et al. Serum CRP in patients with gout and effects of benzbromarone. Int. J. Clin. Pharmacol. Ther. 2011;49:191–197. doi: 10.5414/CP201425. [DOI] [PubMed] [Google Scholar]
- 50.Saito H, et al. Xanthine oxidase inhibitors are associated with reduced risk of cardiovascular disease. Sci. Rep. 2021;11:1380. doi: 10.1038/s41598-020-80835-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu AH, Gladden JD, Ahmed M, Ahmed A, Filippatos G. Relation of serum uric acid to cardiovascular disease. Int. J. Cardiol. 2016;213:4–7. doi: 10.1016/j.ijcard.2015.08.110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.