Abstract
Stress-associated disruptions in the development of frontolimbic regions may play a critical role in the emergence of adolescent-onset depression. These regions are particularly sensitive to Hypothalamic-Pituitary-Adrenal (HPA) axis signaling. The HPA axis is hyperactive in adolescent depression, and interventions that attenuate such hyperactivity hold promise as potential treatments. The Microbiome-Gut-Brain (MGB) axis is an important pathway through which stress dysregulates HPA-axis activity and thus exerts deleterious effects on the adolescent brain. Probiotic agents, which alter the gut microbiota composition by introducing bacterial strains with beneficial physiological effects, normalize aberrant HPA-axis activity and reduce depressive symptoms in both animal studies and adult clinical trials. While the potential utility of such agents in treating or preventing adolescent depression remains largely unexplored, recent data suggest the existence of an adolescent sensitive window during which probiotics may be especially efficacious in reducing depressive symptoms compared to effects observed in adult populations. In this review, we outline evidence that probiotic use may attenuate stress effects on frontolimbic development, providing a novel means of improving depressive symptoms among adolescent populations.
Keywords: Adolescent, Depression, Stress, HPA axis, Neurodevelopment, Microbiota, MGB axis, Probiotics
Highlights
-
•
Stress effects on neurodevelopment may be critical to adolescent-onset depression.
-
•
Chronic HPA-axis hyperactivation adversely impacts frontolimbic development.
-
•
The gut microbiota is a pathway by which stress causes chronic HPA hyperactivation.
-
•
Probiotics normalize HPA activity, and reduce depressive symptoms, in adult trials.
-
•
Probiotics may be a novel means of improving depressive symptoms among adolescents.
1. Introduction
The prevalence of depressive disorders increases sharply during adolescence(Thapar and Riglin, 2020; Rao and Chen, 2022; Davey et al., 2008). Depression is the primary psychiatric risk factor for suicide, the second leading cause of death among adolescents(National Institute of Mental Health, 2021), and adolescent depression increases risk for poor long-term clinical and behavioral outcomes, including a three-fold higher risk for adult depression(Rao and Chen, 2022). Prevalence rates of depressive symptoms among adolescents have sharply increased over the last 10 years(Mayne et al., 2021; Ho et al., 2022), such that an estimated 17% of U.S. adolescents (ages 1217 years) experienced a major depressive episode in 2020 (Substance Abuse and Mental Health Services Administration, 2021). These trends have been further accelerated in the context of psychosocial stressors surrounding the COVID-19 pandemic(Mayne et al., 2021), highlighting the need for more research focused on how stress contributes to the pathophysiology of adolescent-onset depression.
While adolescent depression rates continue to increase, options for treating depression in this age-group remain limited. Notably, antidepressant agents across multiple classes are less effective in adolescents compared to adults(Thapar and Riglin, 2020), and are often ineffective in achieving clinical remission(Kennard et al., 2006), likely reflecting differences between age groups in the neuropathophysiological underpinnings of depression. In particular, the neuropathophysiological processes underlying adolescent-onset depression may be characterized by more prominent hormonal (Shaw et al., 2020) and neurodevelopmental (Ho and King, 2021) components relative to adult-onset depression. Elucidating how hormonal and neurodevelopmental processes contribute to the emergence of depressive symptoms during adolescence may be a key step in identifying more effective therapies for adolescents with a clinical depressive disorder. As the effects of early adversity on neurodevelopment also likely contribute to the etiology of childhood-onset depressive disorders(Teicher and Samson, 2013), the conceptual model we outline below may also be applicable to such disorders; however, a detailed discussion of childhood depression is outside the scope of this review.
A critical feature underlying normative maturation of the brain during adolescence is its increased responsivity to hormonal signals, which contribute to shaping the structure and function of the developing brain(Shaw et al., 2020). Consequently, environmental inputs that impact hormonal signaling, such as dietary intake and exposure to psychosocial stressors, drive deviations from normative neurodevelopmental trajectories. The Hypothalamic-Pituitary-Adrenal (HPA) axis is the primary neuroendocrine pathway underlying the hormonal stress response; it has been hypothesized to mediate the effects of environmental inputs on both aberrant neurodevelopment and adolescent-onset depression(Guerry and Hastings, 2011; Koss and Gunnar, 2018; Raymond et al., 2018), although the mechanisms by which it may do so remain incompletely defined. The Microbiome-Gut-Brain (MGB) axis may be one important channel by which the effects of stress are transmitted to the HPA axis and, thus, to the developing brain(Tremblay et al., 2021; Misiak et al., 2020; Cowan et al., 2020; Flannery et al., 2019). The MGB axis consists of bidirectional communication between the gut (including the enteric nervous system) and the brain via signals from the gut microbiota, a complex ecosystem increasingly recognized as important to host physiological homeostasis(Gentile and Weir, 2018). Beneficial gut bacterial strains contribute to a range of physiological functions, including regulation of microglia homeostasis(Erny et al., 2015) and oligodendrocyte maturation(Hoban et al., 2016; Keogh et al., 2021), that are implicated in adolescent neurodevelopmental processes, such as prefrontal cortex (PFC) myelination(Lynch et al., 2021). Conversely, gut microbiota disturbances (broadly defined as a change in the gut microbiota community associated with deleterious physiological effects) have been linked to the emergence of neurodevelopmental and neuropsychiatric disorders including depression(Cowan et al., 2020; Flannery et al., 2019).
In this review, we summarize recent evidence indicating that the gut microbiota may be an important factor in the association between stress exposure and adolescent-onset depression, and we suggest that targeting the gut microbiota may provide a novel means of improving or preventing depressive symptoms among adolescents by attenuating stress effects on frontolimbic development. In making this argument we integrate evidence across five related domains as discussed below.
We first discuss evidence demonstrating the impact of psychosocial stress on the emergence of adolescent depressive disorders, in terms of stress-induced effects on key neurodevelopmental processes that occur during adolescence (Section 2). We then emphasize the particular importance of chronic HPA-axis dysregulation in disrupting frontolimbic maturation, and precipitating depression onset via dysfunction of neural circuitry involved with stress responsivity, emotional regulation, and reward-processing (Section 3). Next, we present a conceptual framework for considering the MGB axis as an important pathway by which stress exposure contributes to long-term HPA-axis dysregulation, adversely impacting frontolimbic development and contributing to the emergence of adolescent-onset depressive disorders (Section 4). This framework unifies two distinct bodies of work; the first (Sections 2, 3) which has shown the importance of stress-sensitive neurodevelopmental plasticity in frontolimbic circuitry as an etiological factor in the increased incidence of depressive disorders during adolescence, and the second (Section 5) which has indicated that adolescence is a particularly sensitive period in development of the MGB axis. We lastly highlight how, consistent with our proposal that gut microbiota disturbances potentiate stress effects on HPA-axis dysregulation and disrupted frontolimbic development, the MGB axis may provide a target for conceptually novel treatments (Section 6). Specifically, we discuss the potential utility of probiotics—agents which may help maintain MGB axis homeostasis, by introducing to the gut microbiota community strains which exert beneficial effects—as a low-risk means of attenuating the downstream effects of stress, and altered MGB and HPA functioning, on adolescent frontolimbic development and depression.
2. A neurodevelopmental framework for understanding the relationship between stress, frontolimbic circuitry, and adolescent-onset depression
Depression incidence peaks in adolescence(Thapar and Riglin, 2020), paralleling neurodevelopmental processes that are distinct to this developmental timeframe(Thapar and Riglin, 2020; Ho et al., 2022; Shaw et al., 2020; Ho and King, 2021). These processes are most pronounced in frontal regions and frontolimbic circuits involved with emotional regulation and reward-based processing, the structural and functional alterations of which are implicated in the etiology of depressive disorders(Davey et al., 2008; Shaw et al., 2020; Ho and King, 2021; Vanes et al., 2020; van Velzen et al., 2020). MRI studies performed by members of our group and others suggest that frontolimbic structural dysconnectivity may predispose to(LeWinn et al., 2014) – while reduced frontolimbic functional connectivity may serve as a biomarker for(Connolly et al., 2017) – adolescent depressive disorders. Neuroplastic changes in and between these regions are particularly sensitive to the effects of stress hormones(van der Kooij et al., 2016), which interact with sex hormones during adolescence in shaping long-term brain structure and function(Ho and King, 2021; Herting et al., 2012; Rowson et al., 2019). Here we outline results supporting a neurodevelopmental framework in which adolescent depression develops partially as a response to stress-induced deviations in normative, hormone-sensitive neurodevelopmental processes(Ho et al., 2022; Ho and King, 2021).
Adolescent neurodevelopment is characterized by maturation of frontal regions, highlighted by synaptogenesis and synaptic pruning, cytoskeletal organization, and myelin-related changes such as oligodendrocyte maturation with increased myelin sheath thickness(Shaw et al., 2020). These processes are experience-dependent throughout adolescence, a time when exposure to psychosocial stressors normally increases(Davey et al., 2008; Pfeifer et al., 2011). Rodent studies suggest that adolescence may represent a sensitive neuroplastic window, during which the trajectory of frontal synaptic refinement and myelin maturation is dependent on experiential learning for normative long-term development, but also vulnerable to stress-induced deviations. In mid-adolescent rats, social stress is associated with greater long-term deficits in mPFC neuron intrinsic excitability (a contributor to synaptic inputs)(Urban and Valentino, 2017), and deficiencies in mPFC apical dendritic branching (a contributor to synaptic connectivity)(Urban et al., 2019), compared to effects seen in stress-exposed adult rats. Furthermore, rats isolated in early (but not later) adolescence show irreversible deficits in mPFC myelination and oligodendrocyte maturation(Makinodan et al., 2012). That stress exposure in adolescent (compared to adult) animals produces enduring frontal synaptic and myelin-related dysfunction associated with depression-like behaviors(Shaw et al., 2020), supports the etiological relevance of stress-associated disturbances in frontolimbic gray matter volume and white matter microstructure observed among clinical cohorts of adolescents with depressive disorders(Vanes et al., 2020; van Velzen et al., 2020; Kircanski et al., 2019; Hanson et al., 2015).
Adolescence is psychosocially defined by behavioral adaptations, including the formation of emotional regulatory behaviors(Pfeifer et al., 2011), and the development of complex social behaviors associated with changes in reward processing(Davey et al., 2008), that both respond to and underlie the ongoing maturation of frontolimbic circuits. Members of our group identified alterations in frontolimbic white matter (WM) microstructure during adolescence as a potential predisposing factor to depression(LeWinn et al., 2014). Structural deficits in these WM tracts early in the course of adolescent-onset depression may contribute to the reduced amygdala-prefrontal functional connectivity(Connolly et al., 2017), both at rest and during emotion-regulatory tasks(Perlman et al., 2012), known to characterize adolescent depressive disorders. Subsequent studies have identified early adversity as a risk factor for aberrant maturation of frontolimbic WM tracts (including the corpus callosum, uncinate fasciculus, and cingulum bundles(Vanes et al., 2020; Kircanski et al., 2019; Hanson et al., 2015)), structural and functional changes in regions connected by these tracts (including the amygdala, hippocampus, prefrontal cortex, and striatum(Raymond et al., 2018; Kircanski et al., 2019)), and the development of adolescent-onset depression(Ho and King, 2021; Vanes et al., 2020; Kircanski et al., 2019). Furthermore, Ojha et al. demonstrated that lower gray matter volume in the amygdala, ventral striatum, and anterior cingulate cortex (ACC) is associated with past social stress exposure and mediates longitudinal depressive symptom trajectories among adolescents(Ojha, 2022), while cingulum bundle fiber density in early adolescence longitudinally predicts future stress and depressive symptoms(Chahal et al., 2022). Together, these observations support the idea that stress-induced changes in early structural development of frontolimbic circuitry may predispose individuals to later functional deficits in stress responsivity, emotional regulation, and reward-processing characteristic of adolescent depression.
3. Dysregulation of the Hypothalamic-Pituitary-Adrenal (HPA) axis as a critical component of this neurodevelopmental framework
Changes in frontolimbic circuitry during adolescence are closely linked with ongoing neuroendocrine development(Shaw et al., 2020; Guerry and Hastings, 2011; Koss and Gunnar, 2018; Raymond et al., 2018; Herting et al., 2012; Romeo, 2013). The Hypothalamic-Pituitary Adrenal (HPA) axis is the primary driver of the neuroendocrine stress response. In response to stressful internal or external stimuli, hypophysiotropic neurons in the hypothalamic paraventricular nucleus (PVN) synthesize corticotropin-releasing factor (CRF), leading to release of Adrenocorticotropic Hormone (ACTH) from the pituitary which signals the adrenal cortex to produce glucocorticoids (such as cortisol) released into the circulation. In the brain, glucocorticoids exert a wide range of short- and long-term effects, including acute changes in neuronal activity and (with repeated signaling) chronic effects on dendritic remodeling and myelination(McEwen et al., 2015; Hall et al., 2015; Liston and Gan, 2011). Throughout adolescence, HPA-axis activity modulates the function of neural circuitry implicated in emotional regulation(Raymond et al., 2018; Herting et al., 2012) and reward processing(Davey et al., 2008; Raymond et al., 2018); as a result, factors influencing adolescent HPA-axis activity may shape the development of neural circuitry underlying long-term emotional regulatory and reward behaviors (as detailed in Section 4 below). In support of this possibility, dysregulation of the HPA axis underlies the effects of early adversity on later emotional dysregulation and depression(Koss and Gunnar, 2018; Raymond et al., 2018; Kircanski et al., 2019). Furthermore, HPA-axis dysregulation precedes the initial development and recurrence of depression in adolescent populations(Guerry and Hastings, 2011; Morris et al., 2012; Colich et al., 2015). Among adolescents with a diagnosis of – or at high risk for – clinical depression, depressive symptom severity is cross-sectionally associated with cortisol stress reactivity(Morris et al., 2017), a measure which also longitudinally predicts both increases in(Morris et al., 2012) and onset of(Colich et al., 2015) depressive symptoms. More explicitly connecting stress exposure, HPA-axis dysregulation, and subsequent depression-onset: adversity-related baseline cortisol abnormalities in youth have been associated with subsequent negative affect and social withdrawal(Alink et al., 2012), while maltreatment history interacts with subsequent depressive symptom severity to predict cortisol reactivity in adolescents(Harkness et al., 2011).
The interactive effects of stress and sex hormones on developing neurocircuitry may be critical to understanding the effects of HPA-axis dysfunction on adolescent depression risk. Consistent with this formulation is evidence from female adolescents that cortisol hyperreactivity to a stressor predicts future depression onset (independent of familial depression-risk status) only among those later in pubertal development at the time of stress assessment(Colich et al., 2015), while estradiol predicts future depressive symptoms only among participants with elevated baseline cortisol – and in opposite directions depending on time of pubertal onset(Chafkin et al., 2021). Relatedly, one MRI study found that greater gray matter volume of the pituitary gland (which arguably reflects HPA-axis hyperactivity) mediates the association between earlier pubertal timing and subsequent depressive symptoms(Whittle et al., 2012). Such interactions may help explain the published observation that increased prevalence of depression among females arises only after mid-adolescence (prior to which males and females are equally affected)(Thapar and Riglin, 2020; Rao and Chen, 2022). The enduring and sex-specific effects of stress on the adolescent brain (and long-term behavioral outcomes, including depression risk) may be due in part to the hormonally driven alterations in neural circuits underlying emotion regulation and reward behaviors during adolescence(Davey et al., 2008; Shaw et al., 2020). In particular, the development of brain regions underlying the formation of emotional regulatory and reward behaviors are not only associated with HPA-axis functioning but are also especially responsive to hormonal inputs(Koss and Gunnar, 2018; Raymond et al., 2018; van der Kooij et al., 2016; Herting et al., 2012; Rowson et al., 2019; Kircanski et al., 2019). Moreover, given that hormonal activity is itself sensitive to environmental inputs, substantial changes in hormone levels (e.g., in sex hormones due to puberty(Herting et al., 2012; Colich et al., 2015; Chafkin et al., 2021), or in stress hormones due to new psychosocial stressors associated with enhanced risk of anticipated reward disappointment(Davey et al., 2008)) and expression of hormonal receptors make adolescence a timepoint during which the brain is especially and highly susceptible to environmental insults (e.g., psychosocial stressors).
Changes in neuroendocrine function underlie the effects of stress on both frontolimbic development and adolescent depressive disorders. In the brain, chronic stress exposure leads to excessive glucocorticoid signaling (a marker of HPA-axis dysregulation), which can adversely affect dynamic neural processes including myelination and dendritic spine formation in frontolimbic regions expressing glucocorticoid receptors (GRs)(McEwen et al., 2015; Hall et al., 2015; Liston and Gan, 2011). HPA-axis dysregulation (as measured by alterations in magnitude of cortisol reactivity to a stressor) correlates with frontolimbic white matter alterations in adolescents exposed to early adversity; furthermore, such alterations longitudinally predict depressive symptoms(Kircanski et al., 2019) and internalizing symptomatology(Hanson et al., 2015) in such populations. In addition, adolescents with and without depression show opposite associations between intrinsic frontolimbic connectivity (as measured using resting-state fMRI) and cortisol reactivity(Thai et al., 2021), supporting a close link between frontolimbic neural and neuroendocrine systems in the pathophysiology of adolescent-onset depression. Given that chronic HPA-axis activation has been hypothesized to directly alter frontolimbic activity (and consequently alter myelination of frontolimbic circuits)(Vanes et al., 2020; Kircanski et al., 2019), these findings suggest a conceptual model in which the development of frontolimbic circuits involved in emotional regulation and reward behaviors are adversely impacted by HPA-axis hyperactivity, and thus serve as an intermediary through which stress exposure potentiates adolescent emotional dysregulation and depression.
Several possible mechanisms could explain how HPA-axis hyperactivity contributes, through the actions of glucocorticoids, to pathophysiological changes in structure and function of frontolimbic circuitry associated with the development of depressive symptoms. In animal models such glucocorticoid actions influence a wide range of neural processes, including synaptic and dendritic remodeling, neurogenesis, and myelination(McEwen et al., 2015; Hall et al., 2015; Liston and Gan, 2011). Excessive exposure to glucocorticoids leads to dendritic process atrophy and reduced synaptic plasticity in regions which express a relatively high density of glucocorticoid receptors (GR), including the hippocampus, PFC, amygdala, and striatum(Raymond et al., 2018; McEwen et al., 2015; Hall et al., 2015; Liston and Gan, 2011); furthermore, glucocorticoids modulates microglia activity in corticolimbic regions, impacting myelination within and between these areas(McEwen et al., 2015). Long-term consequences of glucocorticoid-associated impairments on the brain and behavior may be more pronounced among adolescents compared to adults, due to multiple factors: 1) the HPA axis of adolescents may show greater and more protracted activation in response to stress, such that a similar stressor may cause stronger HPA axis signaling (greater glucocorticoid exposure) among adolescents(Romeo, 2013; McCormick et al., 2017); 2) frontolimbic structures may be more responsive to glucocorticoid signaling during adolescence, such that equivalent glucocorticoid exposure may have differential effects on hippocampal expression of NMDA receptor subunit genes(Lee et al., 2003); 3) frontolimbic regions most sensitive to the effects of glucocorticoid signaling (namely the hippocampus, PFC, and amygdala) continue to develop throughout adolescence, such that glucocorticoid-associated changes that occur during adolescence may be less reversible(Raymond et al., 2018; van der Kooij et al., 2016; Rowson et al., 2019; Romeo, 2013). As a result, glucocorticoid-driven alterations of frontolimbic activity produce stress effects on the developing brain which are disproportionate to those seen in adult brain and lead to enduring structural and behavioral changes. For example, activity-dependent myelination may lead to inappropriately accelerated maturation of frontolimbic white matter tracts and premature closure of developmental windows in neurocircuitry associated with emotion-regulation(Ho and King, 2021; Callaghan and Tottenham, 2016). Consequently, early stress exposure precipitates functional deficits in social-affective (emotional regulatory and reward-based) networks(Shaw et al., 2020; Ho and King, 2021; Raymond et al., 2018; Vanes et al., 2020; van Velzen et al., 2020; Kircanski et al., 2019; Hanson et al., 2015; Romeo, 2013; Hall et al., 2015) during adolescence associated with increased depression-risk extending into adulthood.
4. Effects of the HPA and MGB axes on neurodevelopment and adolescent-onset depression
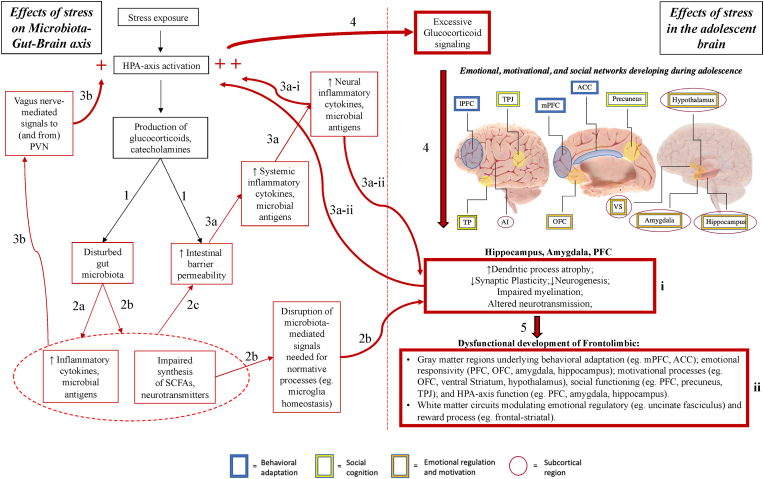
The mechanisms by which stress exposure leads to HPA axis dysregulation, aberrant frontolimbic development, and adolescent depression(Guerry and Hastings, 2011; Koss and Gunnar, 2018; Raymond et al., 2018; Vanes et al., 2020; van Velzen et al., 2020; Kircanski et al., 2019; Morris et al., 2012; Colich et al., 2015; Thai et al., 2021) remain only partially defined. We propose the Microbiota-Gut-Brain (MGB) axis as a key pathway by which stress-related HPA-axis dysregulation impacts adolescent frontolimbic development and associated behaviors (poorer emotion regulation, altered reward behaviors, and other depressive symptoms). Recently, gut microbiota signaling has been recognized as an important factor in modulating host physiology(Gentile and Weir, 2018), including brain structure and function through the “microbiota-gut-brain” (MGB) axis which involves neuroimmune, neuroendocrine, and direct neural pathways(Tremblay et al., 2021; Misiak et al., 2020; Cowan et al., 2020; Flannery et al., 2019; Cruz-Pereira et al., 2020). The HPA and MGB axes mature in parallel and have a bi-directional relationship; on one hand, activation of the HPA axis impacts microbiota composition and function, while on the other hand microbiota-derived signals appear critical to normative HPA axis development(Tremblay et al., 2021; Misiak et al., 2020). This relationship may provide an important pathway by which external stimuli (e.g., psychosocial stressors) are converted to internal stimuli (e.g., taxonomic and/or functional gut microbiota disturbances), which may in turn contribute to long-term HPA axis dysregulation, subsequently altering neurophysiology including neurodevelopmental processes involved in emotional regulation and reward processing (Fig. 1).
Fig. 1.
Effects of stress on Microbiota-Gut-Brain (MGB) axis, and the resulting impact of these effects on adolescent frontolimbic development. Red arrows indicate processes leading to physiological dysfunction. Red boxes indicate disturbance in normative processes. Red (+) indicate direct causes of HPA-axis hyperactivation.
- Anatomical image adapted from Society for Neuroscience (2017) interactive brain model tool.
- Abbreviations: PVN (Paraventricular nucleus of the hypothalamus); SCFA (Short-chain fatty acid); ACC (Anterior cingulate cortex); PFC (Prefrontal cortex); OFC (Orbitofrontal cortex); VS (Ventral striatum); TPJ (Temporoparietal junction); AI (Anterior insula). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
-
1)HPA-axis activation by stress exposure leads to production of stress hormones (glucocorticoids, catecholamines), which: increase intestinal barrier permeability (“leaky gut”)(Zheng et al., 2013; Ait-Belgnaoui et al., 2012) and disturb the gut microbiota composition(Bailey et al., 2011; Jang et al., 2018; Galley et al., 2014; Gareau et al., 2007; Moussaoui et al., 2017; Marin et al., 2017; Bharwani et al., 2016).
-
2)The disturbed microbiota composition:
-
a)Increases local inflammatory signaling (e.g, bacterial production of inflammatory cytokines including IL-1, IL-6, and TNF-α, in addition to release of lipopolysaccharide [LPS] and peptidoglycan from bacterial cell walls)(Bailey et al., 2011; Jang et al., 2018);
-
b)Produces fewer of the bioactive substances (SCFAs; e.g., butyrate, propionate)(Moussaoui et al., 2017; Bharwani et al., 2016) and neurotransmitters (e.g., serotonin, dopamine, norepinephrine)(Bharwani et al., 2016) necessary for functional gut-brain signaling (disrupting processes such as PFC synaptogenesis and myelination(Lynch et al., 2021), as well as hippocampal neurogenesis(Jang et al., 2018; Ait-Belgnaoui et al., 2014; Murray et al., 2020; Sudo et al., 2004; Liao et al., 2019; Wei et al., 2019; Liu et al., 2016));
-
c)Interacts with initial stress hormone-driven effects to further increase intestinal barrier permeability, via multiple pathways including microbiota-induced epigenetic programming of the intestinal epithelium(Ansari et al., 2020).
-
a)
-
3)Disruptions in gut microbiota structure and function, and increased intestinal barrier permeability, interact synergistically to potentiate the down-stream effects of stress exposure on HPA-axis through multiple pathways:
-
a)Inflammatory cytokines (IL-1, IL-6, and TNF-α) and microbial antigens (lipopolysaccharide [LPS]) enter the systemic circulation through the impaired intestinal barrier and penetrate the blood-brain-barrier, leading to subclinical neuroinflammation(Ait-Belgnaoui et al., 2012, 2014) which:
-
i)directly enhance HPA-axis excitability (e.g., increased increased neuronal activity and synaptogenesis in the paraventricular nucleus (PVN)(Ait-Belgnaoui et al., 2014)), and
-
ii)indirectly activate the HPA axis, by impairing function(Jang et al., 2018) of neural regions critical to HPA axis negative feedback mechanisms (such as the PFC and hippocampus)(Cruz-Pereira et al., 2020).
-
i)
-
b)Independent of penetration through the intestinal barrier, stress-associated gut microbiota changes can activate the HPA axis through direct effects on gastrointestinal vagal afferents (e.g., modulation of enteric neuron excitability via effects on ion channels(Kunze et al., 2009; Bercik et al., 2011)), which terminate in the nucleus tracTus solitarius (NTS) – and project to regions including the amygdala, hippocampus, ventral tegmental area, and paraventricular nucleus (PVN) of the hypothalamus(Fülling et al., 2019).
-
a)
-
4)Chronic HPA-axis activation leads to excessive glucocorticoid signaling, which influences dendritic and synaptic remodeling, myelination, and neurogenesis in regions expressing Glucocorticoid Receptors (GRs)(McEwen et al., 2015; Hall et al., 2015; Liston and Gan, 2011) – including the amygdala, hippocampus, and PFC(Raymond et al., 2018).
-
5)Prolonged disruption of these neural processes (box i) during adolescence(van der Kooij et al., 2016; Rowson et al., 2019; Urban and Valentino, 2017; Urban et al., 2019; Makinodan et al., 2012) may lead to impaired development of neurocircuitry underlying long-term emotional regulatory and reward processes (box ii), increasing depression risk(Davey et al., 2008; Shaw et al., 2020; Ho and King, 2021; Vanes et al., 2020; Kircanski et al., 2019).
While more research is needed to elucidate the exact mechanisms linking the HPA axis with the gut microbiota (hereafter, we will specify whether findings were associated with changes in gut microbial taxonomic distribution, functional effects, or both), multiple pathways appear to connect the two in a bidirectional, mutually-dependent relationship. Stress exposure activates the HPA axis, triggering systemic release of glucocorticoids, as well as intestinal secretion of stress-mediators (e.g., corticotropin releasing factor, CRF), immune factors (e.g., cytokine and antibody production) and neurotransmitters (e.g., acetylcholine)(Tremblay et al., 2021; Misiak et al., 2020; Cruz-Pereira et al., 2020). While stress alters local intestinal conditions in several important ways, we will focus here on two: disruptions in gut microbiota structure and function, and increased intestinal barrier permeability. These alterations synergistically potentiate the downstream effects of chronic stress exposure on the HPA axis (Fig. 1).
First, disruptions in gut microbiota structure and function are seen in rodents exposed to social(Bailey et al., 2011)-, restraint(Jang et al., 2018; Galley et al., 2014)-, maternal separation (Gareau et al., 2007; Fukui et al., 2018)-, and unpredictable(Moussaoui et al., 2017; Marin et al., 2017)- stressors. The gut microbiota taxonomic distribution shifts toward a greater relative abundance of pathogenic bacteria including those from the genus Clostridium(Bailey et al., 2011) and family Enterobacter(Jang et al., 2018), with decreased prevalence of beneficial Lactobacilli(Jang et al., 2018; Galley et al., 2014; Gareau et al., 2007; Moussaoui et al., 2017; Marin et al., 2017) and Bifidobacteria(Jang et al., 2018) species. In addition to phylum-level shifts in the microbiota community, stress exposure can also induce more subtle changes including decreased overall species diversity (richness, or total number of species present, and evenness, a measure of species distribution)(Moussaoui et al., 2017; Bharwani et al., 2016). Stress signaling can also exert distinct structural effects by microbiota niche, as comparison of colonic tissue (analyzing mucosa-associated microbiota community) and fecal contents (luminally-associated microbiota) revealed a decrease in relative Lactobacillus abundance only among the mucosa-associated community(Galley et al., 2014).
Stress-induced structural microbiota alterations are associated with functional disruptions, including: reduced abundance of pathways involved in synthesis of short chain fatty acids (SCFAs; e.g., butyrate, propionate)(Moussaoui et al., 2017; Bharwani et al., 2016) and neurotransmitters (e.g., serotonin, dopamine, norepinephrine)(Bharwani et al., 2016), as well as increased local- and downstream-inflammatory signaling (eg. bacterial production of inflammatory cytokines including IL-1, IL-6, and TNF-α, in addition to release of lipopolysaccharide [LPS] and peptidoglycan from bacterial cell walls)(Bailey et al., 2011; Jang et al., 2018). Jang et al. demonstrated that stress-associated shifts in microbiota community play a causative role in these functional changes, as oral administration of fecal microbiota from stress-exposed mice is sufficient to reproduce functional effects, including increased colonic NF-κB activation and microglia/monocyte population in the colon and increased LPS in systemic circulation(Jang et al., 2018).
Second, increased intestinal barrier permeability occurs independent of microbiota-associated changes. CRH- and glucocorticoid-mediated signals impair production of epithelial tight junction proteins such as occludin and zona-occludens-1 (ZO-1), compromising intestinal- and blood-brain-barrier integrity in a manner which can be blocked by corticoid receptor antagonists(Zheng et al., 2013). These initial HPA-axis effects then interact with microbiota-driven effects to further increase intestinal barrier permeability, via multiple pathways including microbiota-induced epigenetic programming of the intestinal epithelium(Ansari et al., 2020). As a result, inflammatory cytokines (e.g., IL-1, IL-6, TNF-α) and microbial antigens (e.g., LPS) are able to cross the intestinal barrier, enter the systemic circulation, and pass through the blood-brain-barrier, contributing to neuroinflammation which influences the HPA axis via direct and indirect mechanisms. With regard to direct effects on the HPA axis, rodents exposed to water avoidance(Ait-Belgnaoui et al., 2014)- and partial restraint(Ait-Belgnaoui et al., 2012)- stress show functional changes in the paraventricular nucleus (PVN) of the hypothalamus including: increased pro-inflammatory cytokine (IL-1, IL-6, and TNF-α) expression(Ait-Belgnaoui et al., 2012), increased neuronal activity (measured by cFos positive nuclei)(Ait-Belgnaoui et al., 2014), and altered expression of genes implicated in neurotransmission and synaptogenesis – associated with enhanced HPA-axis excitability(Ait-Belgnaoui et al., 2014). Moreover, pretreatment with Lactobacillus(Ait-Belgnaoui et al., 2012, 2014) and Bifidobacterium(Ait-Belgnaoui et al., 2014) strains prevented these changes, specifically by preventing stress-induced deficits in colonic mucosa tight junction protein expression (e.g., occludin, ZO-1) and associated LPS translocation from the intestinal lumen(Ait-Belgnaoui et al., 2012). The neuroinflammatory cascade (e.g., NF-κB activation and microglia/monocyte populations in the hippocampus), initiated by stress-induced gut microbiota changes and barrier permeability, also indirectly activates the HPA-axis due to impaired function of regions critical to HPA negative feedback(Cruz-Pereira et al., 2020) (e.g., decreased hippocampal BDNF expression(Jang et al., 2018), doublecortin (DCX)-positive cells(Ait-Belgnaoui et al., 2014)).
Independent of systemic effects, stress-associated gut microbiota changes can activate the HPA axis through direct effects on gastrointestinal vagal afferents, which terminate in the Nucleus Tracus Solitarius (NTS) and project to regions including the amygdala, hippocampus, ventral tegmental area, and paraventricular nucleus (PVN) of the hypothalamus(Fülling et al., 2019). Lactobacillus-mediated signals in mice are able to alter mesenteric vagal afferent fiber firing, and induce neuronal activation (assessed by c-Fos expression) – in brain regions that receive vagal afferents – which is prevented by sub-diaphragmatic vagotomy (SDV)(Bharwani et al., 2020). One important mechanism for vagal-dependent microbiota signaling may be the ability of bacterial species (eg. Lactobacillus Reuteri, and Bifidobacterium Longum) to directly modulate enteric neuron excitability by exerting differential effects on ion channels(Kunze et al., 2009; Bercik et al., 2011). The downstream effects of stress on the brain and behavior, via altered microbiota composition, appear to be in-part vagally dependent: SDV blocks the microbiota of rats exposed to chronic social defeat stress (delivered via FMT to non-stressed rats with antibiotic-depleted microbiomes) from causing decreased PFC synaptic protein expression, and development of depressive behaviors(Wang et al., 2020). Protective effects of bacterial strains are also transmitted through the vagus nerve, as Bravo et al. showed that the ability of Lactobacillus Rhamnosus to prevent stress-induced glucocorticoid elevations, GABA mRNA expression changes in frontal and limbic areas, and onset of depression-like behaviors, is absent in vagotomized mice(Bravo et al., 2011). Importantly, the vagus nerve can also transmit effects to the microbiota: systemic administration of LPS produces abnormalities in gut microbiota diversity, elevations in systemic inflammatory markers, downregulation of mPFC synaptic proteins, and onset of depressive behaviors in a vagally-dependent manner(Zhang et al., 2020). That these effects are not observed among mice who have undergone SDV, suggests that the vagus serves as a bidirectional link between the gut and brain; it both carries stress signals that alter the microbiota,and transmits stress-induced microbiota changes to the brain's stress circuitry (Fig. 1).
5. Adolescence as a sensitive period in development of the microbiome-gut-brain (MGB) axis: potential implications for development of adolescent depression
Dysfunction of the MGB axis during development has been implicated in the emergence of neuropsychiatric disorders, including depression, across both animal models and clinical studies(Misiak et al., 2020; Cowan et al., 2020; Knudsen et al., 2021; Lach et al., 2020; Alli et al., 2022). In rodents, stress-associated microbiota alterations early in life have been shown to directly impact neurobiology and behavior into adulthood, including: HPA-axis dysregulation, systemic and neural inflammation, impaired neurogenesis, altered frontolimbic synaptogenesis and myelination, and anhedonic behaviors(Tremblay et al., 2021; Misiak et al., 2020; Lynch et al., 2021; Cruz-Pereira et al., 2020). While evidence in humans remains preliminary, recent studies suggest that these observations may have translational relevance. For example, one longitudinal study(Querdasi et al., 2022) found that higher postnatal adversity exposure was associated with reduced gut microbiota phylogenetic diversity and differential abundance of Parabacteriodes at 2 years of age, and microbiota compositional profiles among 2 year-olds were associated with concurrent (at year 2) and prospective (at year 4) socioemotional functioning. Stress-associated gut microbiota alterations continue at least into adolescence: stress measures, including negative event exposure and parasympathetic activity, are cross-sectionally associated with lower alpha diversity and differential bacterial abundance at the phylum-level (eg. lower Firmicutes, higher Bacteroides, Parabacteroides) among 8–16 year-olds(Michels et al., 2019), while adult women who report having experienced two or more adverse childhood events (ACEs) have distinct gut microbiota profiles (eg. greater differential abundance of Prevotella) compared to adults with fewer ACEs(Hantsoo et al., 2019). Together, these results suggest that gut microbiota composition remains plastic to stress signaling throughout adolescence, with effects that may persist into adulthood. Among human adults, observational studies have consistently identified depression-associated fecal microbiota composition phenotypes(Nikolova et al., 2021; Stevens et al., 2021), and gut microbiota sequencing has been found to reliably differentiate patients with depression from healthy subjects(Nikolova et al., 2021; Stevens et al., 2021). Notably, transplantation of the microbiota from depressed patients to microbiota-depleted rats induces depressive behaviors and physiology (while transplantation of the microbiota from healthy subjects reduces depressive symptoms in rats previously subjected to stress)(Knudsen et al., 2021), suggesting a possible causative role for gut microbiota signaling in the etiology of depression.
One notable aspect of the microbiota as a pathway for communication between the gut and the brain is that microbiota community functionality appears to be adapted to age-specific developmental processes(Cowan et al., 2020; Flannery et al., 2019; Jašarević et al., 2016). For example, Hollister et al. found differences in gut microbiota compositional and functional profile between healthy pre-adolescent children (ages 7–12) and healthy adults, such that children harbored a greater prevalence of Bifidobacterium and Faecalibacterium species, associated with a greater relative abundance of genes involved in metabolic pathways that promote neural development (e.g., vitamin B12 and folate synthesis) (Hollister et al., 2015). As seen in studies of germ-free (GF) rodents, presence of the gut microbiota is necessary for normative neural and behavioral development, and microbial colonization is sufficient to restore normative developmental trajectories only if delivered within limited windows. As an example, Hoban et al. (2016) identified alterations in PFC myelin-related gene expression among GF mice at 10 weeks of age, which could be prevented by microbial colonization post-weaning (at P21); however, ultrastructural changes in myelin sheath thickness at week 10 were not normalized by colonization. Furthermore, deficits in mPFC dendritic spine remodeling and fear-extinction learning among GF mice are reversible only if microbiota colonization occurs prior to weaning(Chu et al., 2019), while alterations in anxiety-like behavior associated with striatal PSD-95 and synaptophysin expression (markers of developmental synaptogenesis) in GF mice are normalized only by colonization early in life(Diaz Heijtz et al., 2011). Combined with the observation that periods of peak microbiota change parallel periods of heightened neurodevelopmental plasticity in both rodents and humans(Cowan et al., 2020; Flannery et al., 2019; Jašarević et al., 2016), these results suggest the existence of “sensitive windows” in the MGB axis: periods of heightened microbiota and brain plasticity, during which microbiota-derived signals are critical to determining long-term neural and behavioral trajectories.
Late childhood through adolescence may be the last sensitive window of MGB axis development. Longitudinal studies suggest that, in contrast to the relative long-term stability of the adult microbiota composition(Faith et al., 2013), adolescence may represent a distinct period of microbiota function tailored to age-specific developmental changes. For example, healthy adolescent (ages 11–18) and adult (ages 19+) human distal gut microbiota samples can be distinguished by relative species abundances(Agans et al., 2011), including differential abundance of genera Bifidobacterium and Clostridium. Supporting the involvement of gut microbiota signaling in adolescent neurodevelopment, transient antibiotic-depletion of the microbiota in adolescent (but not adult) rodents leads to enduring effects on microbiota composition, amygdala gene expression and anxiety-like behavior(Lach et al., 2020). A potential factor underlying sensitivity of the MGB axis during adolescence is the involvement of the gut microbiota in hormonal signaling, including interactions with stress hormones (heightened in the setting of new complex social behaviors) and sex hormones (peaking in the setting of pubertal development)(Jašarević et al., 2016; Cowan and Richardson, 2019; Murray et al., 2020; Yurkovetskiy et al., 2013). Among rats, the gut microbiota has been implicated in the stress-induced changes in pubertal onset previously observed among human adolescents(Cowan and Richardson, 2019). In particular, exposing rats to early stress via maternal separation leads to sex-dependent changes in pubertal timing (precocious in females, delayed in males), which are preventable with administration of probiotics containing Lactobacillus strains for the duration of maternal separation (P2–P14)(Cowan and Richardson, 2019). During puberty (at 6 weeks of age), the rat gut microbiota composition is more malleable to stress-induced change via LPS challenge compared to adult rats (at 10 weeks of age), including more signification depletion of beneficial Lactobacillus genera; furthermore, these changes are associated with enduring, sex-dependent effects on anxiety-behavior and hippocampal c-Fos expression into adulthood, which are preventable via Lactobacillus Reuteri administration during puberty(Murray et al., 2020). These findings support the possibility that microbiota-derived signals during adolescence serve as one possible pathway by which stress effects interact with gonadal hormones to shape long-term brain structure and function.
Gut microbiota disturbances during adolescent development have been associated with dysfunction (while gut microbiota normalization prior to adulthood has been associated with functional restoration) of frontolimbic processes implicated in both the adolescent stress-response and adolescent-onset depression(Hoban et al., 2016; Keogh et al., 2021; Lach et al., 2020; Diaz Heijtz et al., 2011; Sudo et al., 2004). Notably, restoration of microbiota signaling in GF rodents at various developmental stages suggests a limited adolescent window in which microbiota-derived signals can normalize both: 1) HPA-axis function(Sudo et al., 2004); and 2) neurodevelopmental processes known to be uniquely vulnerable to stress exposure, including PFC myelination(Keogh et al., 2021). Sudo et al. demonstrated that the increased sensitivity of the HPA-axis stress response (assessed via cortisol reactivity to restraint stress, associated with reduced BDNF expression in the cortex and hippocampus) observed in GF mice can be partially attenuated following microbiota colonization during adolescence (at 6 weeks old), but not during adulthood (at 14 weeks old)(Sudo et al., 2004). Furthermore, normative PFC myelination during adolescence in rodents is dependent on the microbiota via production of the short-fatty acid (SCFA) butyrate(Keogh et al., 2021), a process known to differ by stress-induced changes in microbiota function (Bharwani et al., 2016). Supplementation with butyrate during adolescence rescues behavioral and PFC myelin-related deficits associated with gut microbiota compositional changes resulting from early-life antibiotic exposure(Keogh et al., 2021). Notably, the profile of long-term microbiota changes associated with antibiotic exposure during postnatal development resembled the effects of behavioral stress on the microbiota(Jang et al., 2018; Galley et al., 2014; Gareau et al., 2007; Moussaoui et al., 2017; Marin et al., 2017), including reduced diversity and phylum-level changes in the Bacteroides:Firmicutes ratio with decreased Lactobacillaceae abundance. Immune- or behavioral-stress induced microbiota changes in rodents are also associated with other processes implicated in maturation of emotion-relevant neural circuitry, including synaptic plasticity in the PFC(Zhang et al., 2020) and amygdala(Bravo et al., 2011). By attenuating the effects of stress on these neural processes, targeting the gut microbiota during adolescence may be an effective intervention for development of depressive symptoms.
6. Probiotics as an MGB-targeted treatment for adolescent depression
Animal models support the concept that normalization of gut microbiota disturbances during adolescence represents a unique opportunity to attenuate long-term effects of stress on the brain and behavior. For example, immune challenge (via LPS treatment, a model of immune stress) in rodents during the adolescent period is associated with short-term microbiota compositional changes, and long-term neural and behavioral alterations, which are prevented by consumption of probiotics during adolescence(Murray et al., 2019, 2020; Yahfoufi et al., 2021). Notably, Murray et al. (2019) showed that one-time LPS exposure at 6 weeks was associated with enduring microbiota and behavioral changes, including phylum-level differences in relative Firmicutes:Proteobacteria ratio and increased anxious and depressive behaviors at 10 weeks. Treatment from weeks 5–7 with a probiotic containing multiple Lactobacillus strains prevented these changes, and normalized toll-like receptor-4 activity in the hypothalamic PVN in response to a novel stressor(Murray et al., 2019). As demonstrated by Smith et al., long-term effects of adolescent gut microbiota disturbances on depression- and anxiety-like behaviors appear to be primarily driven by maladaptive changes to the HPA axis, with pubertal LPS exposure decreasing PVN glucocorticoid receptor (GR) expression into adulthood, and pubertal probiotic (composed of multiple Lactobacillus strains) treatment blocking this LPS-induced programing(Smith et al., 2021). These results highlight the importance of adolescence as a developmental period during which long-term HPA-axis function, and neural and behavioral sequalae of prolonged HPA-axis hyperactivity, remain both vulnerable to microbiota disturbances and amenable to interventions targeting the microbiota.
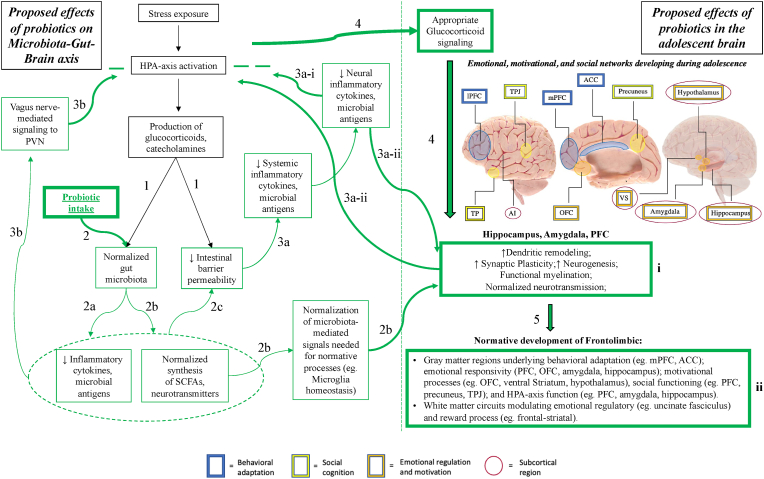
Probiotic interventions that attenuate HPA-axis hyperactivity may be particularly useful to treat depressive symptoms among adolescent populations (Fig. 2). Supporting this possibility, administration of probiotics to stress-exposed adolescent rodents attenuates HPA-axis hyperactivity, thereby reducing later depressive-like behaviors and ameliorating functional alterations in depression-associated regions(Liao et al., 2019; Wei et al., 2019; Liu et al., 2016), regardless of whether the stress occurred in early life (Liao et al., 2019; Liu et al., 2016) or during adolescence(Wei et al., 2019). For example, Lactobacillus Paracasei PS23 (provided starting on PD29) improves anxiety- and depression-like behaviors, while reducing serum corticosterone levels and normalizing hippocampal dopaminergic metabolite concentrations, in juvenile rodents who previously underwent a maternal separation paradigm (P2-14)(Liao et al., 2019). Furthermore, in mice exposed to maternal-separation stress(PD2-14), Lactobacillus Plantarum PS128 (administered starting on PD29) reduces anxiety- and depression-like behaviors while reducing serum corticosterone and increasing dopamine and serotonin levels in the PFC(Liu et al., 2016). Among mice treated with corticosterone throughout early and middle adolescence (from PD17 to 37), treatment with Lactobacillus Paracasei PS23 until day 41: reverses chronic corticosterone-induced anxiety- and depression-like behaviors, and normalizes BDNF and glucocorticoid receptor levels in the hippocampus, in addition to serotonin levels in the hippocampus, PFC, and striatum(Wei et al., 2019).
Fig. 2.
Hypothesized model of the effects of probiotics on stress-associated Microbiota-Gut-Brain (MGB) axis dysfunction which, in turn, impact adolescent frontolimbic development and associated behaviors (e.g., depressive symptoms). Green arrows indicate normal physiological processes. Green boxes indicate normative function. Green (−) indicate attenuation of HPA-axis hyperactivity.
- Anatomical image adapted from Society for Neuroscience (2017) interactive brain model tool.
- Abbreviations: PVN (Paraventricular nucleus of the hypothalamus); SCFA (Short-chain fatty acid); ACC (Anterior cingulate cortex); PFC (Prefrontal cortex); OFC (Orbitofrontal cortex); VS (Ventral striatum); TPJ (Temporoparietal junction); AI (Anterior insula). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
-
1)HPA-axis activation by stress exposure leads to production of stress hormones (glucocorticoids, catecholamines), which: increase intestinal barrier permeability (“leaky gut”)(Zheng et al., 2013; Ait-Belgnaoui et al., 2012) and disturb the gut microbiota composition(Bailey et al., 2011; Jang et al., 2018; Galley et al., 2014; Gareau et al., 2007; Moussaoui et al., 2017; Marin et al., 2017; Bharwani et al., 2016).
-
2)Probiotic use populates the gut microbiota with beneficial bacterial strains, a process which:
-
a)Reduces local and systemic release of inflammatory cytokines and microbial antigens(Jang et al., 2018; Murray et al., 2019; Liu et al., 2016);
-
b)Normalizes synthesis of bioactive substances (e.g. SCFAs, neurotransmitters)(Erny et al., 2015; Keogh et al., 2021; Yadav et al., 2013; Srivastav et al., 2019) necessary for functional gut-brain signaling (facilitating processes such as PFC myelination, via restoration of microglia homeostasis(Erny et al., 2015), oligodendrocyte maturation(Hoban et al., 2016; Keogh et al., 2021));
-
c)Restores the production of intestinal epithelial cell tight junction proteins, improving intestinal barrier function(Gareau et al., 2007; Fukui et al., 2018; Ait-Belgnaoui et al., 2012; Vanhaecke et al., 2017).
-
a)
-
3)Probiotics may attenuate HPA-axis hyperactivity through multiple pathways:
-
a)Restoring intestinal barrier function(Gareau et al., 2007; Vanhaecke et al., 2017), thus preventing the entrance of inflammatory cytokines and microbial antigens into the systemic circulation, and reducing downstream subclinical neuroinflammation(Ait-Belgnaoui et al., 2012, 2014);
-
b)Transmitting vagal afferent signals associated with the normalized microbiota, leading to normalized Paraventricular Nucleus (PVN) signaling and HPA-axis activity(Bercik et al., 2011; Bravo et al., 2011).
-
a)
-
4)Prevention of chronic HPA-axis activation prevents excessive glucocorticoid signaling(Ait-Belgnaoui et al., 2012, 2014; Liao et al., 2019; Wei et al., 2019; Liu et al., 2016), which normalizes dendritic and synaptic remodeling, myelination, and neurogenesis in regions expressing Glucocorticoid Receptors (GRs)(McEwen et al., 2015; Hall et al., 2015; Liston and Gan, 2011) – including the amygdala, hippocampus, and PFC(Raymond et al., 2018).
-
5)Normalization of these neural processes (box i) during adolescence may lead to more appropriate development of neurocircuitry underlying long-term emotional regulatory and reward processes (box ii)(Davey et al., 2008; Shaw et al., 2020; Ho and King, 2021; Vanes et al., 2020; Kircanski et al., 2019), potentially reducing(Liao et al., 2019; Wei et al., 2019; Liu et al., 2016) or preventing(Kato-Kataoka et al., 2016a, 2016b; Takada et al., 2016; Nishida et al., 2017; Sawada et al., 2017) depressive symptoms.
Preliminary clinical trials in humans suggest that these effects may have translational relevance, as Lactobacillus-containing probiotics have been shown to prevent stress-induced HPA-axis hyperactivity (e.g., normalizing increased salivary cortisol)(Kato-Kataoka et al., 2016a, 2016b; Takada et al., 2016; Nishida et al., 2017; Sawada et al., 2017) and attenuate development of anxiety and depressive symptoms in non-clinical youth populations(Nishida et al., 2017; Sawada et al., 2017). Kata-Kataoka et al. and Sawada et al. demonstrated that Lactobacillus Casei strain Shirota(Kato-Kataoka et al., 2016a) and Lactobacillus gasseri CP2305(Sawada et al., 2017) respectively are able to suppress stress-induced salivary cortisol increases, as well as stress-associated changes in fecal microbiota profiles (eg. relative prevalence of Bacteroidaceae(Kato-Kataoka et al., 2016a) and Enterobacteriaceae(Sawada et al., 2017), and altered alpha diversity(Kato-Kataoka et al., 2016a)) among healthy medical students exposed to stress. Furthermore, attenuation of these stress effects by Lactobacillus gasseri CP2305 was also associated with improvements in depressive symptoms, anxiety, and global sleep quality(Sawada et al., 2017), while Nishida et al. found that the same strain attenuated escalations of both salivary cortisol levels and anxiety scores among healthy medical students preparing to take an exam(Nishida et al., 2017). In a similar population of Japanese medical students exposed to exam stress, (Takada et al., 2016; Kato-Kataoka et al., 2016a, Kato-Kataoka et al., 2016b) found that Lactobacillus Casei strain Shirota (compared to placebo) prevented stress-induced increases in salivary cortisol and physical symptoms. These studies demonstrate the ability of Lactobacillus-probiotics to not only ameliorate stress-associated physical and mood symptoms(Nishida et al., 2017; Sawada et al., 2017), but also to prevent stress-induced increases in HPA axis signaling (and the development of stress-associated symptoms) among young adults given probiotics prior to or in the course of stress exposure (see Table 1 for study details).
Table 1.
Preventive effects of probiotics in stress-exposed young adults.
| Intervention | Participants | Results |
|---|---|---|
| 8 week RCT; probiotic as a stand-alone agent for preventing stress-induced changes. | 49 healthy medical students (<30 years) preparing to take nationwide examination for academic advancement. |
|
| 100-ml bottle of either L. casei strain Shirota-fermented milk (L. casei strain Shirota at more than 1.0 × 1011 CFU per 100-ml bottle) or placebo milk taken daily prior to standardized examination. | Placebo group: 22.8 ± 0.3 yo | |
| Kato-Kataoka et al. (2016a) | Probiotic group: 22.8 ± 0.4 yo | |
| 8 week RCT; probiotic as a stand-alone agent for preventing stress-induced changes. | 51 healthy medical students (<30 years) preparing to take nationwide examination for academic advancement. |
|
| 100-ml bottle of either L. casei strain Shirota-fermented milk (L. casei strain Shirota at more than 1.0 × 109 CFU/ml) or placebo milk taken daily prior to standardized examination. | Placebo group: 22.7 ± 0.4 yo | |
| Kato-Kataoka et al. (2016b) | Probiotic group: 23.0 ± 0.4 yo | |
| 8 week RCT; probiotic as a stand-alone agent for preventing stress-induced changes. | 149 healthy medical students (<30 years) preparing to take nationwide examination for academic advancement. |
|
| 100-ml bottle of either L. casei strain Shirota-fermented milk (L. casei strain Shirota at more than 1.0 × 109 CFU/ml) or placebo milk taken daily prior to standardized examination. | Placebo group: 22.8 ± 0.2 yo | |
| Rats given feed w/or w/o LcS for 2 weeks, prior to Water avoidance stress (WAS). | Probiotic group: 23.0 ± 0.2 yo | |
| Takada et al. (2016) | Rats aged 8–9 weeks old | |
| 12 week RCT; probiotic as a stand-alone agent for preventing stress-induced changes. | 69 healthy medical students (aged 22–35) preparing to take nationwide examination for academic advancement. |
|
| 200 mL beverage containing containing Lactobacillus gasseri strain CP2305 (CP2305) or placebo beverage daily prior to standardized examination. | Placebo group: 25.1 ± 0.4 (22–35) yo | |
| Nishida et al. (2017) | Probiotic group: 24.9 ± 0.3 (23–32) yo | |
| 11 week placebo-controlled, crossover-designed trial (3 week washout). Probiotic as a stand-alone agent for preventing stress-induced changes. | Participants in CP2305 group had:
|
|
| Daily consumption of Lactobacillus gasseri CP2305 (CP2305) at 1.0 × 1010 CFU dissolved in water, compared to placebo dissolved in water. | ||
| Sawada et al. (2017) | 24 healthy medical students taking cadaver dissection course. |
Clinical trials have also shown probiotic-associated improvements in depressive symptoms among adults with clinical depression (as reviewed by Liu et al. (2019) and Alli et al. (2022)) although results have been inconsistent and difficult to generalize due to inter-study differences with respect to probiotic strains and dosages used. Among patients with Major Depressive Disorder (MDD) taking probiotics as a stand-alone treatment, Akkasheh et al. (2016), Majeed et al. (2018), Kazemi et al. (2019), and Wallace et al., (2021), found that probiotic intake (compared to placebo) was associated with significant improvement in depressive symptoms (see Table 2 for study details). Probiotics have also been found to reduce depressive symptoms (compared to placebo) when taken as adjunctive agents to standard clinical treatment among patients with MDD. Furthermore, both Miyaoka et al. (2018) and Bambling et al. (2017) found that probiotic agents taken as adjuncts to Selective Serotonin Reuptake Inhibitors (SSRIs) provided symptomatic improvement among patients with previously treatment-resistant depression (see Table 2 for study details). The mechanistic underpinnings of these effects have primarily been studied in rodent models, but several clinical trials among adults suggest that probiotic-associated mood and anxiety improvements may be mechanistically linked to 1) attenuated HPA axis signaling, and 2) normalized frontolimbic neural function. For example, Messaoudi et al. found improvements in psychological distress measures and significantly lower urinary cortisol levels (compared to controls) among healthy adults administered a combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175(Messaoudi et al., 2011); furthermore, Allen et al. found that Bifidobacterium longum 1714 consumption among healthy subjects: attenuated cortisol and subjective anxiety in response to cold stressor test, reduced daily reported stress, and enhanced frontal midline electroencephalographic mobility(Allen et al., 2016). Lastly, among Irritable Bowel Syndrome (IBS) patients, Pinto-Sanchez et al. demonstrated that depressive symptom improvements after Bifidobacterium longum NCC3001 intake were associated with reduced amygdala activation to negative emotional stimuli (Pinto-Sanchez et al., 2017) (see Table 3 for study details). While probiotics have rarely been studied in adolescent clinical populations, their unique benefits in postnatal/adolescent (compared to adult) rodents, as well as their utility in preventing the development of stress-associated effects in young adults and ameliorating depressive symptoms among clinically depressed adults, suggests the possibility that probiotics may be an efficacious intervention in adolescent-onset depression.
Table 2.
Effect of probiotics on depressive symptoms in adults with MDD.
| Intervention | Participants | Results |
|---|---|---|
| 8 week RCT; Probiotic as stand-alone intervention. | ||
| One daily capsule containing: | 40 adults with MDD, aged 20–55 years. |
|
| Lactobacillus acidophilus (2 × 109 CFU/g); | ||
| Lactobacillus casei (2 × 109 CFU/g), Bifidobacterium bifidum (2 × 109 CFU/g). | Placebo group: 36.2 ± 8.2 yo | |
| Akkasheh et al. (2016) | Probiotic group: 38.3 ± 12.1 yo | |
| 90 day RCT; Probiotic as stand-alone intervention. | 40 adults with MDD experiencing IBS symptoms, aged 20–65 years. |
|
| One daily capsule containing: B. coagulans MTCC 5856 (2 × 109 CFU/g). | Placebo group: 43.88 ± 9.85 yo | |
| Majeed et al. (2018) | Probiotic group: 40.36 ± 10.28 yo | |
| 110 adults with MDD, aged 18–50 years |
|
|
| 8 week RCT; Probiotic as stand-alone intervention. | Placebo group: 36 ± 8.47 yo | |
| Probiotic (L. helveticus R0052 and B. longum R0175, 10 × 109 CFU per 5 g sachet), prebiotic (galactooligosaccharide) or placebo. | Probiotic group: 36.15 ± 7.8) yo | |
| Kazemi et al. (2019) | Prebiotic group: 37.35 ± 7.97 yo | |
| 8 week open-label pilot study; Probiotic as stand-alone intervention. |
|
|
| L. helveticus R0052 (90%) and B. longum R0175 (10%) | 10 treatment-naïve MDD patients, aged 18–65 yo | |
| 1.5 g sachets at a dose of 3 × 109 CFU per sachet | ||
| Wallace et al. (2021) | Mean participant age: 25.2 ± 7 yo | |
| Probiotics as adjunctive agents in adults with treatment-resistant depression (TRD) | ||
| 8-week open-label study; probiotic as adjuvant treatment to antidepressant. | 40 adults with TRD – defined as inadequate or nonresponse to 2 or more 8-week trials with 2 different classes of antidepressants. |
|
| C. butyricum MIYAIRI 588 (CBM588).20 mg orally BID ×1 week, 20 mg orally TID from weeks 2–8. | CBM588 group (probiotic + antidepressant): 44.2 ± 15.6 yo | |
| Miyaoka et al. (2018) | Control group (antidepressant alone): 41.9 ± 14.2 yo | |
| 8-week open label study; probiotic as adjuvant treatment to antidepressant. |
|
|
| Combination of probiotic (Lactobacillus acidophilus, Bifidobacterium bifidum, Streptoccocus thermophiles, 2 × 1010 CFU) and magnesium orotate 1600 mg divided in two daily doses. | 12 adults with TRD; mean age = 49.3 ± 10.9 yo | |
| Bambling et al. (2017) | Mean years of MDD = 19.8 yo | |
Table 3.
Potential mechanisms underlying probiotic-associated mood and anxiety improvements in human participants.
| Intervention | Participants | Results |
|---|---|---|
| 30-day double-blind, placebo controlled RCT | 55 healthy adults, aged 30–60 years |
|
| Once daily probiotic (one stick 1.5g combination of L. helveticus R0052 and B. longum R0175, 3 × 109 CFU/stick) | Probiotic group: 42.4 ± 7.5 yo | |
| Messaoudi et al. (2011) | Placebo group: 43.2 ± 8.5 yo | |
| 8-week repeated measures, placebo-controlled design. Placebo for 4 weeks, followed by probiotic (1 × 109 CFU stick of Bifidobacterium longum 1714) for 4 weeks. | 22 healthy adults, aged 18–40 |
|
| Allen et al. (2016) | Mean age: 25.5 ± 1.2 yo | |
| 6-week double-blind, placebo controlled RCT | 44 adults with IBS and mild to moderate anxiety and/or depression (HAD-A or HAD-D score 8–14) |
|
| Once daily probiotic (B. longum NCC 3001, 1 × 1010 CFU/1g powder) or placebo for 6 weeks. | Probiotic group median age: 46.5 (30–58)yo | |
| Pinto et al. (2017) | Placebo group median age: 40.0 (26–57) yo |
7. Discussion
As we have described above, gut microbiota disturbances (e.g., taxonomic and/or functional alterations) represent an important internal pathway through which external stimuli (e.g., psychosocial stressors) induce long-term HPA-axis dysregulation, adversely impacting development of frontolimbic circuits and contributing to the emergence of adolescent-onset clinical depressive disorders. Probiotic agents – which may help maintain gut microbiota homeostasis by introducing beneficial bacterial strains, such as those from the genus Lactobacillus – have been shown to prevent stress-induced HPA axis hyperactivity(Kato-Kataoka et al., 2016a, 2016b; Takada et al., 2016; Nishida et al., 2017; Sawada et al., 2017) and the development of stress-associated mood and physical symptoms among young adults(Nishida et al., 2017; Sawada et al., 2017). Furthermore, clinical trials in adults with(Akkasheh et al., 2016; Majeed et al., 2018; Kazemi et al., 2019; Wallace and Milev, 2021; Miyaoka et al., 2018; Bambling et al., 2017) and without MDD(Messaoudi et al., 2011; Allen et al., 2016; Pinto-Sanchez et al., 2017) have demonstrated that probiotic-associated improvements in depressive symptoms may be mechanistically linked to 1) attenuated HPA axis signaling, and 2) normalized frontolimbic neural function. However, despite evidence that probiotics may provide a novel means of reducing or preventing depressive symptoms among adolescents with or at risk for depressive disorders, their potential utility in adolescent populations remains largely unexplored. Below, we discuss several open questions regarding the potential utility of probiotics as therapeutic agents for depressive disorders.
The heterogeneity of previous randomized controlled trials (RCTs) assessing probiotics for the treatment of depressive symptoms limits the reproducibility and comparability of current evidence(Liu et al., 2019). Sources of variability include inter-study discrepancies with respect to probiotic dosing, method of delivery (e.g., capsule vs. yogurt), and specific probiotic bacterial species/strains utilized, as well as differences in mechanisms assessed (e.g., systemic inflammatory markers, measures of HPA-axis activity). The inconsistency of bacterial species/strains used in probiotic formulations across studies is a particularly important impediment to developing treatment guidelines, as different gut bacterial strains from within the same species may exert distinct physiologic effects(Ouwehand et al., 2018). Furthermore, while multi-strain and multi-species formulations may exert a greater gut health benefit (and thus may more effectively ameliorate neuropsychiatric symptoms) relative to mono-strain probiotics, the potentially interactive effects in such formulations remain poorly defined(Ouwehand et al., 2018). For example, while Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 show greater efficacy in regulating stress-induced negative feedback on the HPA axis compared to either species alone(Ait-Belgnaoui et al., 2018), it remains unclear whether this synergistic effect would be reproducible between different strains of Lactobacillus helveticus and Bifidobacterium longum – as well as whether such synergy would survive if incorporated into a probiotic formulation including additional species or strains. More broadly, improving the precision with which probiotic treatments target depression (and other microbiota-associated disorders) may require defining the optimal gut microbiota profile necessary to rescue normative physiologic function (e.g., HPA axis activity) from the adverse effects of gut microbiota disturbances, including whether this profile may differ according to factors such as host age, sex, and clinical condition.
An important question to resolve prior to the widespread implementation of probiotic agents as antidepressant therapies is about their most appropriate role in depressive symptom management. Initial evidence suggests that while some probiotic formulations may be efficacious as stand-alone therapies in reducing depressive symptoms(Akkasheh et al., 2016; Majeed et al., 2018; Wallace and Milev, 2021), they may be most effective as adjuncts to current first-line antidepressant agents such as Selective Serotonin Reuptake Inhibitors(SSRIs)(Miyaoka et al., 2018; Bambling et al., 2017). In particular, probiotics may be especially effective in normalizing hippocampal functioning(Jang et al., 2018; Ait-Belgnaoui et al., 2014; Murray et al., 2020; Sudo et al., 2004; Liao et al., 2019; Wei et al., 2019; Liu et al., 2016), which may be only partially improved by current first-line antidepressant treatments and may be necessary for antidepressant-associated normalization of HPA-axis activity (Cruz-Pereira et al., 2020). An important limitation in considering probiotics as first-line antidepressant therapies is the heterogeneity of symptoms associated with depressive disorders and the importance of targeting specific outcomes associated with depression. For example, while some studies suggest that probiotics may reduce overall depressive symptoms with a magnitude comparable to that of SSRIs(Akkasheh et al., 2016; Majeed et al., 2018; Wallace and Milev, 2021), there is limited evidence with respect to the effect of stand-alone probiotic treatments on suicidal behaviors in high-risk populations. However, an important strength of probiotics as adjunctive treatments when first-line therapies fail to achieve clinical remission (as is the case for a significant number of adolescents diagnosed with a clinical depressive disorder, with up to half of depressed youth who were classified as treatment-responsive continuing to experience residual symptoms after 12 weeks of treatment(Kennard et al., 2006)) is that probiotic treatments have not been associated with the adverse effects that contribute to treatment non-adherence and discontinuation(Bet et al., 2013). Such considerations may be particularly relevant among adolescents, who cite weight gain and loss of sex drive as primary factors deterring use of pharmacological interventions(Bradley et al., 2010). That probiotic use has not been associated with any significant adverse effects and may in fact confer health benefits(Gentile and Weir, 2018), suggests that in addition to low-risk adjunctive therapies(Miyaoka et al., 2018; Bambling et al., 2017), probiotics may also be appropriate as: 1) maintenance therapy to prevent relapse among individuals with depressive disorders in clinical remission, and 2) an addition to safe early interventions for high-risk individuals with subclinical depressive symptoms. Given that early-life fecal microbiota disruptions (e.g., reduced diversity) have been associated with altered HPA-axis function(Keskitalo et al., 2021), which has been shown to precede the initial onset of depressive symptoms(Guerry and Hastings, 2011), fecal microbiota testing(Nikolova et al., 2021; Stevens et al., 2021) may be a non-invasive method of screening for individuals at high-risk of developing depression (e.g., exposure to early adversity and/or first-degree relative with a clinical depressive disorder). Furthermore, as probiotic pretreatment prior to or during stress exposure prevents the deleterious effects of stress on HPA axis activity in both animal studies(Ait-Belgnaoui et al., 2014, 2018) and stress-exposed young adults(Kato-Kataoka et al., 2016a, 2016b; Takada et al., 2016; Nishida et al., 2017; Sawada et al., 2017), probiotics may be a low-risk intervention for delaying – or preventing – the onset of depression in high-risk individuals.
Probiotics may also exert different effects on depressive symptoms among specific populations, based on differences between populations in the impact of stress on gut microbiota composition – and the effect of gut microbiota disturbances on the brain. In support of this concept, probiotics administered to adolescent rodents prevent enduring neural effects (hippocampal c-Fos expression evident only in males (Murray et al., 2020); hippocampal 5HT1A expression evident only in females(Yahfoufi et al., 2021)) and behavioral (anxiety-like behavior evident only in males(Murray et al., 2020); depressive-like behavior evident only in females(Yahfoufi et al., 2021)) effects associated with immune stress exposure in a sex-dependent manner(Murray et al., 2019, 2020; Yahfoufi et al., 2021). Yurkovetskiy et al. showed that sexual dimorphism in the rodent gut microbiota emerges during puberty, a trend which is eliminated by male castration(Yurkovetskiy et al., 2013). Notably, sex differences exist in the gut microbiota profile associated with depressive symptoms in adult clinical populations – including different phylotype predominance in adult men and women with depressive disorders(Chen et al., 2018). However, further research is needed to identify precise mechanisms underlying sex-based differences in the relationship between gut microbiota disturbances and depression.
Potential differences in probiotic efficacy may also extend to different depressive disorder subtypes. For example, depressed patients with glucocorticoid resistance (elevated cortisol) and subclinical inflammation may be more likely to experience treatment-resistance or minimal responsivity to first-line antidepressants(Haroon et al., 2018; Miller and Raison, 2016), highlighting the therapeutic potential of targeting the inflammatory response in treatment-resistant depression. In light of the potential anti-inflammatory properties demonstrated by probiotic agents(Cruz-Pereira et al., 2020), as well as preliminary evidence that probiotics may be effective adjuvant therapies among individuals with treatment-resistant MDD and elevated inflammatory markers(Miyaoka et al., 2018; Bambling et al., 2017), an important area of future inquiry would be to further investigate their potential utility as adjuncts to first-line antidepressants in clinical cohorts enriched with individuals displaying an inflammatory subtype of depression. Furthermore, given the evidence that adolescent-onset depression may represent a depressive subtype(Thapar and Riglin, 2020; Ho and King, 2021; Teicher and Samson, 2013) defined by the impact of early adversity on neurodevelopmental factors in communication with the MGB axis, an important next step would be to conduct a double-blinded RCT with placebo and probiotic assessing the utility of probiotics for both 1) attenuating stress-associated neurohormonal changes, and 2) reducing depressive symptoms among adolescents diagnosed with clinical depression.
In conclusion, we described evidence that: stress-induced deviations in normative, experience-dependent and hormone sensitive neurodevelopmental processes may help explain the increased incidence of depressive disorders onset during adolescence; the MGB axis is critical for normative programing of the developing adolescent brain, and gut microbiota disturbances provide an important pathway by which stress signals contribute to long-term HPA-axis dysregulation, adversely impacting development of frontolimbic circuitry; and probiotics, which may help maintain gut microbiota homeostasis, protect against adolescent-onset depression by attenuating stress-induced changes in HPA-axis function and frontolimbic development. While other work has previously described adolescence as a period of stress-sensitive neurodevelopmental plasticity, and proposed adolescence as a sensitive period for development of the MGB axis, we outline here the first framework to comprehensively describe how these distinct literatures provide overlapping support for the utility of probiotics as a novel means of treating or preventing depressive symptoms among adolescents.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by the National Center for Complementary and Integrative Health (NCCIH) R21AT009173and R61AT009864 to OT and TTY; by the UCSF Weill Institute for Neurosciences Weill Award for Junior Investigators in the Neurosciences Impacted by COVID-19 Setbacks to OT and TTY; by the National Center for Advancing Translational Sciences (CTSI), National Institutes of Health, through UCSF-CTSI UL1TR001872 to OT and TTY; by the American Foundation for Suicide Prevention (AFSP) SRG-1-141-18 to OT and TTY; by the National Institute of Mental Health (NIMH) R01MH085734 and the Brain and Behavior Research Foundation (formerly NARSAD) to TTY; by the National Institute of Mental Health K01MH117442 and R01MH127176 to TCH; by the Ray and Dagmar Dolby Family Fund to TCH; by the UCSF Research Evaluation and Allocation Committee to TCH; by the Raschen-Tiedeman Fund to TCH, and the Moffitt Memorial Fund to TCH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funding agencies.
Data availability
No data was used for the research described in the article.
References
- Agans R., et al. Distal gut microbiota of adolescent children is different from that of adults. FEMS Microbiol. Ecol. 2011;77(2):404–412. doi: 10.1111/j.1574-6941.2011.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait-Belgnaoui A., et al. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology. 2012;37(11):1885–1895. doi: 10.1016/j.psyneuen.2012.03.024. [DOI] [PubMed] [Google Scholar]
- Ait-Belgnaoui A., et al. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neuro Gastroenterol. Motil. 2014;26(4):510–520. doi: 10.1111/nmo.12295. [DOI] [PubMed] [Google Scholar]
- Ait-Belgnaoui A., et al. Bifidobacterium longum and Lactobacillus helveticus synergistically suppress stress-related visceral hypersensitivity through hypothalamic-pituitary-adrenal Axis modulation. J. Neurogastroenterol. Motil. 2018;24(1):138–146. doi: 10.5056/jnm16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkasheh G., et al. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition. 2016;32(3):315–320. doi: 10.1016/j.nut.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Alink L.R., et al. Longitudinal associations among child maltreatment, social functioning, and cortisol regulation. Dev. Psychol. 2012;48(1):224–236. doi: 10.1037/a0024892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A.P., et al. Bifidobacterium longum 1714 as a translational psychobiotic: modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl. Psychiatry. 2016;6(11):e939. doi: 10.1038/tp.2016.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alli S.R., et al. The gut microbiome in depression and potential benefit of prebiotics, probiotics and synbiotics: a systematic review of clinical trials and observational studies. Int. J. Mol. Sci. 2022;23(9) doi: 10.3390/ijms23094494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari I., et al. The microbiota programs DNA methylation to control intestinal homeostasis and inflammation. Nat. Microbiol. 2020;5(4):610–619. doi: 10.1038/s41564-019-0659-3. [DOI] [PubMed] [Google Scholar]
- Bailey M.T., et al. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav. Immun. 2011;25(3):397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambling M., et al. A combination of probiotics and magnesium orotate attenuate depression in a small SSRI resistant cohort: an intestinal anti-inflammatory response is suggested. Inflammopharmacology. 2017;25(2):271–274. doi: 10.1007/s10787-017-0311-x. [DOI] [PubMed] [Google Scholar]
- Bercik P., et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neuro Gastroenterol. Motil. 2011;23(12):1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bet P.M., et al. Side effects of antidepressants during long-term use in a naturalistic setting. Eur. Neuropsychopharmacol. 2013;23(11):1443–1451. doi: 10.1016/j.euroneuro.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Bharwani A., et al. Structural & functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinology. 2016;63:217–227. doi: 10.1016/j.psyneuen.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Bharwani A., et al. The vagus nerve is necessary for the rapid and widespread neuronal activation in the brain following oral administration of psychoactive bacteria. Neuropharmacology. 2020;170 doi: 10.1016/j.neuropharm.2020.108067. [DOI] [PubMed] [Google Scholar]
- Bradley K.L., et al. Adolescents' attitudes and opinions about depression treatment. Community Ment. Health J. 2010;46(3):242–251. doi: 10.1007/s10597-009-9224-5. [DOI] [PubMed] [Google Scholar]
- Bravo J.A., et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. U. S. A. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan B.L., Tottenham N. The Stress Acceleration Hypothesis: effects of early-life adversity on emotion circuits and behavior. Curr. Opin. Behav. Sci. 2016;7:76–81. doi: 10.1016/j.cobeha.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chafkin J.E., et al. Gonadal and adrenal hormones interact with pubertal maturation to predict depressive symptoms in a group of high-school females. Dev. Psychopathol. 2021:1–15. doi: 10.1017/S0954579420001935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahal R., et al. Sex-specific vulnerability to depressive symptoms across adolescence and during the COVID-19 pandemic: the role of the cingulum bundle. JCPP Adv. 2022;2(1) doi: 10.1002/jcv2.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.J., et al. Sex differences in gut microbiota in patients with major depressive disorder. Neuropsychiatric Dis. Treat. 2018;14:647–655. doi: 10.2147/NDT.S159322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C., et al. The microbiota regulate neuronal function and fear extinction learning. Nature. 2019;574(7779):543–548. doi: 10.1038/s41586-019-1644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colich N.L., et al. HPA-axis reactivity interacts with stage of pubertal development to predict the onset of depression. Psychoneuroendocrinology. 2015;55:94–101. doi: 10.1016/j.psyneuen.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly C.G., et al. Resting-state functional connectivity of the amygdala and longitudinal changes in depression severity in adolescent depression. J. Affect. Disord. 2017;207:86–94. doi: 10.1016/j.jad.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan C.S.M., Richardson R. Early-life stress leads to sex-dependent changes in pubertal timing in rats that are reversed by a probiotic formulation. Dev. Psychobiol. 2019;61(5):679–687. doi: 10.1002/dev.21765. [DOI] [PubMed] [Google Scholar]
- Cowan C.S.M., Dinan T.G., Cryan J.F. Annual Research Review: critical windows - the microbiota-gut-brain axis in neurocognitive development. J. Child Psychol. Psychiatry. 2020;61(3):353–371. doi: 10.1111/jcpp.13156. [DOI] [PubMed] [Google Scholar]
- Cruz-Pereira J.S., et al. Depression's unholy trinity: dysregulated stress, immunity, and the microbiome. Annu. Rev. Psychol. 2020;71:49–78. doi: 10.1146/annurev-psych-122216-011613. [DOI] [PubMed] [Google Scholar]
- Davey C.G., Yücel M., Allen N.B. The emergence of depression in adolescence: development of the prefrontal cortex and the representation of reward. Neurosci. Biobehav. Rev. 2008;32(1):1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Diaz Heijtz R., et al. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. U. S. A. 2011;108(7):3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erny D., et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015;18(7):965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faith J.J., et al. The long-term stability of the human gut microbiota. Science. 2013;341(6141) doi: 10.1126/science.1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery J., et al. Is adolescence the missing developmental link in Microbiome-Gut-Brain axis communication? Dev. Psychobiol. 2019;61(5):783–795. doi: 10.1002/dev.21821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui H., et al. Effect of probiotic Bifidobacterium bifidum G9-1 on the relationship between gut microbiota profile and stress sensitivity in maternally separated rats. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-30943-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fülling C., Dinan T.G., Cryan J.F. Gut Microbe to brain signaling: what Happens in vagus…. Neuron. 2019;101(6):998–1002. doi: 10.1016/j.neuron.2019.02.008. [DOI] [PubMed] [Google Scholar]
- Galley J.D., et al. The structures of the colonic mucosa-associated and luminal microbial communities are distinct and differentially affected by a prolonged murine stressor. Gut Microb. 2014;5(6):748–760. doi: 10.4161/19490976.2014.972241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau M.G., et al. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut. 2007;56(11):1522–1528. doi: 10.1136/gut.2006.117176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile C.L., Weir T.L. The gut microbiota at the intersection of diet and human health. Science. 2018;362(6416):776–780. doi: 10.1126/science.aau5812. [DOI] [PubMed] [Google Scholar]
- Guerry J.D., Hastings P.D. In search of HPA axis dysregulation in child and adolescent depression. Clin. Child Fam. Psychol. Rev. 2011;14(2):135–160. doi: 10.1007/s10567-011-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B.S., Moda R.N., Liston C. Glucocorticoid mechanisms of functional connectivity changes in stress-related neuropsychiatric disorders. Neurobiol. Stress. 2015;1:174–183. doi: 10.1016/j.ynstr.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., et al. Lower structural integrity of the uncinate fasciculus is associated with a history of child maltreatment and future psychological vulnerability to stress. Dev. Psychopathol. 2015;27(4 Pt 2):1611–1619. doi: 10.1017/S0954579415000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantsoo L., et al. Childhood adversity impact on gut microbiota and inflammatory response to stress during pregnancy. Brain Behav. Immun. 2019;75:240–250. doi: 10.1016/j.bbi.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness K.L., Stewart J.G., Wynne-Edwards K.E. Cortisol reactivity to social stress in adolescents: role of depression severity and child maltreatment. Psychoneuroendocrinology. 2011;36(2):173–181. doi: 10.1016/j.psyneuen.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Haroon E., et al. Antidepressant treatment resistance is associated with increased inflammatory markers in patients with major depressive disorder. Psychoneuroendocrinology. 2018;95:43–49. doi: 10.1016/j.psyneuen.2018.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting M.M., et al. The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cerebr. Cortex. 2012;22(9):1979–1992. doi: 10.1093/cercor/bhr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T.C., King L.S. Mechanisms of neuroplasticity linking early adversity to depression: developmental considerations. Transl. Psychiatry. 2021;11(1):517. doi: 10.1038/s41398-021-01639-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T.C., Gifuni A.J., Gotlib I.H. Psychobiological risk factors for suicidal thoughts and behaviors in adolescence: a consideration of the role of puberty. Mol. Psychiatr. 2022;27(1):606–623. doi: 10.1038/s41380-021-01171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoban A.E., et al. Regulation of prefrontal cortex myelination by the microbiota. Transl. Psychiatry. 2016;6(4):e774. doi: 10.1038/tp.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister E.B., et al. Structure and function of the healthy pre-adolescent pediatric gut microbiome. Microbiome. 2015;3:36. doi: 10.1186/s40168-015-0101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang H.M., et al. Immobilization stress-induced Escherichia coli causes anxiety by inducing NF-κB activation through gut microbiota disturbance. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-31764-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jašarević E., Morrison K.E., Bale T.L. Sex differences in the gut microbiome-brain axis across the lifespan. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016;371(1688) doi: 10.1098/rstb.2015.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato-Kataoka A., et al. Fermented milk containing Lactobacillus casei strain Shirota preserves the diversity of the gut microbiota and relieves abdominal dysfunction in healthy medical students exposed to academic stress. Appl. Environ. Microbiol. 2016;82(12):3649–3658. doi: 10.1128/AEM.04134-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato-Kataoka A., et al. Fermented milk containing Lactobacillus casei strain Shirota prevents the onset of physical symptoms in medical students under academic examination stress. Benef. Microbes. 2016;7(2):153–156. doi: 10.3920/BM2015.0100. [DOI] [PubMed] [Google Scholar]
- Kazemi A., et al. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: a randomized clinical trial. Clin. Nutr. 2019;38(2):522–528. doi: 10.1016/j.clnu.2018.04.010. [DOI] [PubMed] [Google Scholar]
- Kennard B., et al. Remission and residual symptoms after short-term treatment in the treatment of adolescents with depression study (TADS) J. Am. Acad. Child Adolesc. Psychiatry. 2006;45(12):1404–1411. doi: 10.1097/01.chi.0000242228.75516.21. [DOI] [PubMed] [Google Scholar]
- Keogh C.E., et al. Myelin as a regulator of development of the microbiota-gut-brain axis. Brain Behav. Immun. 2021;91:437–450. doi: 10.1016/j.bbi.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keskitalo A., et al. Gut microbiota diversity but not composition is related to saliva cortisol stress response at the age of 2.5 months. Stress. 2021;24(5):551–560. doi: 10.1080/10253890.2021.1895110. [DOI] [PubMed] [Google Scholar]
- Kircanski K., et al. Early life stress, cortisol, frontolimbic connectivity, and depressive symptoms during puberty. Dev. Psychopathol. 2019;31(3):1011–1022. doi: 10.1017/S0954579419000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen J.K., et al. Faecal microbiota transplantation from patients with depression or healthy individuals into rats modulates mood-related behaviour. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-01248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss K.J., Gunnar M.R. Annual Research Review: early adversity, the hypothalamic-pituitary-adrenocortical axis, and child psychopathology. J. Child Psychol. Psychiatry. 2018;59(4):327–346. doi: 10.1111/jcpp.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze W.A., et al. Lactobacillus reuteri enhances excitability of colonic AH neurons by inhibiting calcium-dependent potassium channel opening. J. Cell Mol. Med. 2009;13(8b):2261–2270. doi: 10.1111/j.1582-4934.2009.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lach G., et al. Enduring neurobehavioral effects induced by microbiota depletion during the adolescent period. Transl. Psychiatry. 2020;10(1):382. doi: 10.1038/s41398-020-01073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P.R., Brady D., Koenig J.I. Corticosterone alters N-methyl-D-aspartate receptor subunit mRNA expression before puberty. Brain Res. Mol. Brain Res. 2003;115(1):55–62. doi: 10.1016/s0169-328x(03)00180-3. [DOI] [PubMed] [Google Scholar]
- LeWinn K.Z., et al. White matter correlates of adolescent depression: structural evidence for frontolimbic disconnectivity. J. Am. Acad. Child Adolesc. Psychiatry. 2014;53(8):899–909. doi: 10.1016/j.jaac.2014.04.021. 909.e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J.F., et al. Lactobacillus paracasei PS23 reduced early-life stress abnormalities in maternal separation mouse model. Benef. Microbes. 2019;10(4):425–436. doi: 10.3920/BM2018.0077. [DOI] [PubMed] [Google Scholar]
- Liston C., Gan W.B. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc. Natl. Acad. Sci. U. S. A. 2011;108(38):16074–16079. doi: 10.1073/pnas.1110444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.W., et al. Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naïve adult mice. Brain Res. 2016;1631:1–12. doi: 10.1016/j.brainres.2015.11.018. [DOI] [PubMed] [Google Scholar]
- Liu R.T., Walsh R.F.L., Sheehan A.E. Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci. Biobehav. Rev. 2019;102:13–23. doi: 10.1016/j.neubiorev.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch C.M.K., et al. Wrapping things up: recent developments in understanding the role of the microbiome in regulating myelination. Curr. Opin. Physiol. 2021;23 [Google Scholar]
- Majeed M., et al. Bacillus coagulans MTCC 5856 for the management of major depression with irritable bowel syndrome: a randomised, double-blind, placebo controlled, multi-centre, pilot clinical study. Food Nutr. Res. 2018;62 doi: 10.29219/fnr.v62.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinodan M., et al. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science. 2012;337(6100):1357–1360. doi: 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin I.A., et al. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci. Rep. 2017;7 doi: 10.1038/srep43859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayne S.L., et al. COVID-19 and adolescent depression and suicide risk screening outcomes. Pediatrics. 2021;148(3) doi: 10.1542/peds.2021-051507. [DOI] [PubMed] [Google Scholar]
- McCormick C.M., Green M.R., Simone J.J. Translational relevance of rodent models of hypothalamic-pituitary-adrenal function and stressors in adolescence. Neurobiol. Stress. 2017;6:31–43. doi: 10.1016/j.ynstr.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., et al. Mechanisms of stress in the brain. Nat. Neurosci. 2015;18(10):1353–1363. doi: 10.1038/nn.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi M., et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011;105(5):755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- Michels N., et al. Gut microbiome patterns depending on children's psychosocial stress: reports versus biomarkers. Brain Behav. Immun. 2019;80:751–762. doi: 10.1016/j.bbi.2019.05.024. [DOI] [PubMed] [Google Scholar]
- Miller A.H., Raison C.L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016;16(1):22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiak B., et al. The HPA axis dysregulation in severe mental illness: can we shift the blame to gut microbiota? Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2020;102 doi: 10.1016/j.pnpbp.2020.109951. [DOI] [PubMed] [Google Scholar]
- Miyaoka T., et al. Clostridium butyricum MIYAIRI 588 as adjunctive therapy for treatment-resistant major depressive disorder: a prospective open-label trial. Clin. Neuropharmacol. 2018;41(5):151–155. doi: 10.1097/WNF.0000000000000299. [DOI] [PubMed] [Google Scholar]
- Morris M.C., Rao U., Garber J. Cortisol responses to psychosocial stress predict depression trajectories: social-evaluative threat and prior depressive episodes as moderators. J. Affect. Disord. 2012;143(1–3):223–230. doi: 10.1016/j.jad.2012.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M.C., et al. Depressive symptom composites associated with cortisol stress reactivity in adolescents. J. Affect. Disord. 2017;210:181–188. doi: 10.1016/j.jad.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussaoui N., et al. Chronic early-life stress in rat pups alters basal corticosterone, intestinal permeability, and fecal microbiota at weaning: influence of sex. J. Neurogastroenterol. Motil. 2017;23(1):135–143. doi: 10.5056/jnm16105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E., et al. Probiotic consumption during puberty mitigates LPS-induced immune responses and protects against stress-induced depression- and anxiety-like behaviors in adulthood in a sex-specific manner. Brain Behav. Immun. 2019;81:198–212. doi: 10.1016/j.bbi.2019.06.016. [DOI] [PubMed] [Google Scholar]
- Murray E., et al. Pubertal probiotic blocks LPS-induced anxiety and the associated neurochemical and microbial outcomes, in a sex dependent manner. Psychoneuroendocrinology. 2020;112 doi: 10.1016/j.psyneuen.2019.104481. [DOI] [PubMed] [Google Scholar]
- Nikolova V.L., et al. Perturbations in gut microbiota composition in psychiatric disorders: a review and meta-analysis. JAMA Psychiatr. 2021;78(12):1343–1354. doi: 10.1001/jamapsychiatry.2021.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida K., et al. Daily administration of paraprobiotic Lactobacillus gasseri CP2305 ameliorates chronic stress-associated symptoms in Japanese medical students. J. Funct.Foods. 2017;36:112–121. [Google Scholar]
- Ojha, A., Psychol. Med., 2022(In press).
- Ouwehand A.C., et al. Effectiveness of multistrain versus single-strain probiotics: current status and recommendations for the future. J. Clin. Gastroenterol. 2018;52(Suppl. 1) doi: 10.1097/MCG.0000000000001052. Proceedings from the 9th Probiotics, Prebiotics and New Foods, Nutraceuticals and Botanicals for Nutrition & Human and Microbiota Health Meeting, held in Rome, Italy from September 10 to 12, 2017: p. S35-s40. [DOI] [PubMed] [Google Scholar]
- Perlman G., et al. Amygdala response and functional connectivity during emotion regulation: a study of 14 depressed adolescents. J. Affect. Disord. 2012;139(1):75–84. doi: 10.1016/j.jad.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., et al. Entering adolescence: resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron. 2011;69(5):1029–1036. doi: 10.1016/j.neuron.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto-Sanchez M.I., et al. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology. 2017;153(2):448–459. doi: 10.1053/j.gastro.2017.05.003. e8. [DOI] [PubMed] [Google Scholar]
- Querdasi F.R., et al. 2022. Multigenerational Adversity Impacts on Gut Microbiome Composition and Socioemotional Functioning in Early Childhood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao U., Chen L.-A. Characteristics, correlates, and outcomes of childhood and adolescent depressive disorders. Dialogues Clin. Neurosci. 2022 doi: 10.31887/DCNS.2009.11.1/urao. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond C., et al. Early child adversity and psychopathology in adulthood: HPA axis and cognitive dysregulations as potential mechanisms. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2018;85:152–160. doi: 10.1016/j.pnpbp.2017.07.015. [DOI] [PubMed] [Google Scholar]
- Romeo R.D. The teenage brain: the stress response and the adolescent brain. Curr. Dir. Psychol. Sci. 2013;22(2):140–145. doi: 10.1177/0963721413475445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowson S.A., et al. Chronic adolescent stress sex-specifically alters the hippocampal transcriptome in adulthood. Neuropsychopharmacology. 2019;44(7):1207–1215. doi: 10.1038/s41386-019-0321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada D., et al. Daily intake of Lactobacillus gasseri CP2305 improves mental, physical, and sleep quality among Japanese medical students enrolled in a cadaver dissection course. J. Funct.Foods. 2017;31:188–197. [Google Scholar]
- Shaw G.A., Dupree J.L., Neigh G.N. Adolescent maturation of the prefrontal cortex: role of stress and sex in shaping adult risk for compromise. Gene Brain Behav. 2020;19(3) doi: 10.1111/gbb.12626. [DOI] [PubMed] [Google Scholar]
- Smith K.B., et al. Pubertal probiotics mitigate lipopolysaccharide-induced programming of the hypothalamic-pituitary-adrenal axis in male mice only. Brain Res. Bull. 2021;177:111–118. doi: 10.1016/j.brainresbull.2021.09.017. [DOI] [PubMed] [Google Scholar]
- Srivastav S., et al. Probiotics mixture increases butyrate, and subsequently rescues the nigral dopaminergic neurons from MPTP and rotenone-induced neurotoxicity. J. Nutr. Biochem. 2019;69:73–86. doi: 10.1016/j.jnutbio.2019.03.021. [DOI] [PubMed] [Google Scholar]
- Stevens B.R., et al. Depression phenotype identified by using single nucleotide exact amplicon sequence variants of the human gut microbiome. Mol. Psychiatr. 2021;26(8):4277–4287. doi: 10.1038/s41380-020-0652-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, S . CBHSQ Data; 2021. National Survey on Drug Use and Health. [Google Scholar]
- Sudo N., et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004;558(Pt 1):263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suicide. National Institute of Mental Health (NIMH).
- Takada M., et al. Probiotic Lactobacillus casei strain Shirota relieves stress-associated symptoms by modulating the gut-brain interaction in human and animal models. Neuro Gastroenterol. Motil. 2016;28(7):1027–1036. doi: 10.1111/nmo.12804. [DOI] [PubMed] [Google Scholar]
- Teicher M.H., Samson J.A. Childhood maltreatment and psychopathology: a case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am. J. Psychiatr. 2013;170(10):1114–1133. doi: 10.1176/appi.ajp.2013.12070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai M., et al. Coordination between frontolimbic resting state connectivity and hypothalamic-pituitary-adrenal axis functioning in adolescents with and without depression. Psychoneuroendocrinology. 2021;125 doi: 10.1016/j.psyneuen.2020.105123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A., Riglin L. The importance of a developmental perspective in Psychiatry: what do recent genetic-epidemiological findings show? Mol. Psychiatr. 2020;25(8):1631–1639. doi: 10.1038/s41380-020-0648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay A., et al. The effects of psychobiotics on the microbiota-gut-brain axis in early-life stress and neuropsychiatric disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2021;105 doi: 10.1016/j.pnpbp.2020.110142. [DOI] [PubMed] [Google Scholar]
- Urban K.R., Valentino R.J. Age- and sex-dependent impact of repeated social stress on intrinsic and synaptic excitability of the rat prefrontal cortex. Cerebr. Cortex. 2017;27(1):244–253. doi: 10.1093/cercor/bhw388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban K.R., et al. Age- and sex-dependent impact of repeated social stress on morphology of rat prefrontal cortex pyramidal neurons. Neurobiol. Stress. 2019;10 doi: 10.1016/j.ynstr.2019.100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooij M.A., et al. The stressed cytoskeleton: how actin dynamics can shape stress-related consequences on synaptic plasticity and complex behavior. Neurosci. Biobehav. Rev. 2016;62:69–75. doi: 10.1016/j.neubiorev.2015.12.001. [DOI] [PubMed] [Google Scholar]
- van Velzen L.S., et al. White matter disturbances in major depressive disorder: a coordinated analysis across 20 international cohorts in the ENIGMA MDD working group. Mol. Psychiatr. 2020;25(7):1511–1525. doi: 10.1038/s41380-019-0477-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanes L.D., et al. White matter tract myelin maturation and its association with general psychopathology in adolescence and early adulthood. Hum. Brain Mapp. 2020;41(3):827–839. doi: 10.1002/hbm.24842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaecke T., et al. fermentum CECT L. 5716 prevents stress-induced intestinal barrier dysfunction in newborn rats. Neuro Gastroenterol. Motil. 2017;29(8) doi: 10.1111/nmo.13069. [DOI] [PubMed] [Google Scholar]
- Wallace C.J.K., Milev R.V. The efficacy, safety, and tolerability of probiotics on depression: clinical results from an open-label pilot study. Front. Psychiatr. 2021;12 doi: 10.3389/fpsyt.2021.618279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., et al. Ingestion of Lactobacillus intestinalis and Lactobacillus reuteri causes depression- and anhedonia-like phenotypes in antibiotic-treated mice via the vagus nerve. J. Neuroinflammation. 2020;17(1):241. doi: 10.1186/s12974-020-01916-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C.L., et al. Antidepressant-like activities of live and heat-killed Lactobacillus paracasei PS23 in chronic corticosterone-treated mice and possible mechanisms. Brain Res. 2019;1711:202–213. doi: 10.1016/j.brainres.2019.01.025. [DOI] [PubMed] [Google Scholar]
- Whittle S., et al. Pituitary volume mediates the relationship between pubertal timing and depressive symptoms during adolescence. Psychoneuroendocrinology. 2012;37(7):881–891. doi: 10.1016/j.psyneuen.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Yadav H., et al. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J. Biol. Chem. 2013;288(35):25088–25097. doi: 10.1074/jbc.M113.452516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahfoufi N., et al. Adolescent use of potential novel probiotic Rouxiella badensis subsp. acadiensis (Canan SV-53) mitigates pubertal LPS-Induced behavioral changes in adulthood in a sex-specific manner by modulating 5HT1A receptors expression in specific brain areas. Comprehensive Psychoneuroendocrinol. 2021;7 doi: 10.1016/j.cpnec.2021.100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurkovetskiy L., et al. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39(2):400–412. doi: 10.1016/j.immuni.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., et al. A key role of the subdiaphragmatic vagus nerve in the depression-like phenotype and abnormal composition of gut microbiota in mice after lipopolysaccharide administration. Transl. Psychiatry. 2020;10(1):186. doi: 10.1038/s41398-020-00878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G., et al. Corticosterone mediates stress-related increased intestinal permeability in a region-specific manner. Neuro Gastroenterol. Motil. 2013;25(2):e127–e139. doi: 10.1111/nmo.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.