Summary
Background
COVID-19 convalescent plasma (CCP) contains neutralising anti-SARS-CoV-2 antibodies that may be useful as COVID-19 passive immunotherapy in patients at risk of developing severe disease. Such plasma from convalescent patients may also have additional immune-modulatory properties when transfused to COVID-19 patients.
Methods
CCP (n = 766) was compared to non-convalescent control plasma (n = 166) for soluble inflammatory markers, ex-vivo inflammatory bioactivity on endothelial cells, neutralising auto-Abs to type I IFNs and reported adverse events in the recipients.
Findings
CCP exhibited a statistically significant increase in IL-6 and TNF-alpha levels (0.531 ± 0.04 vs 0.271 ± 0.04; (95% confidence interval [CI], 0.07371–0.4446; p = 0.0061) and 0.900 ± 0.07 vs 0.283 ± 0.07 pg/mL; (95% [CI], 0.3097–0.9202; p = 0.0000829) and lower IL-10 (0.731 ± 0.07 vs 1.22 ± 0.19 pg/mL; (95% [CI], −0.8180 to −0.1633; p = 0.0034) levels than control plasma. Neutralising auto-Abs against type I IFNs were detected in 14/766 (1.8%) CCPs and were not associated with reported adverse events when transfused. Inflammatory markers and bioactivity in CCP with or without auto-Abs, or in CCP whether or not linked to adverse events in transfused patients, did not differ to a statistically significant extent.
Interpretation
Overall, CCP exhibited moderately increased inflammatory markers compared to the control plasma with no discernible differences in ex-vivo bioactivity. Auto-Abs to type I IFNs detected in a small fraction of CCP were not associated with reported adverse events or differences in inflammatory markers. Additional studies, including careful clinical evaluation of patients treated with CCP, are required in order to further define the clinical relevance of these findings.
Funding
French National Blood Service—EFS, the Association “Les Amis de Rémi” Savigneux, France, the “Fondation pour la Recherche Médicale (Medical Research Foundation)–REACTing 2020”.
Keywords: Transfusion, Endothelial cells, Convalescent plasma, COVID-19, Inflammation, Adverse events
Research in context.
Evidence before this study
COVID-19 convalescent plasma (CCP) contains neutralising anti-SARS-CoV-2 antibodies that may be useful as COVID-19 passive immunotherapy in patients at risk of developing severe disease. Such plasma from convalescent patients may also have additional immune-modulatory properties when transfused to COVID-19 patients. In addition, auto-Abs (antibodies) against type I interferons (IFNs) were found to underlie severe COVID-19 in at least 15% of previously healthy patients. They appeared to precede the disease and may therefore be present in plasma donors who previously presented a hypoxemic form of COVID-19 pneumonia.
Added value of this study
This study seeks to quantify soluble inflammatory molecules as well as bioactivity in CCP and compare it to non-convalescent FFP. Furthermore, we also assessed the presence of auto-Abs to type I IFNs in CCP. Finally, we investigated whether the inflammatory characteristics of CCP differed in the presence or absence of auto-Abs or in CCP involved in transfusion-related adverse events. CCP (COVID-19 convalescent plasma) exhibited significantly higher IL-6 and TNF alpha concentrations than control plasma while the IL-10 concentration was lower. Other soluble inflammatory mediators as well as ex-vivo inflammatory bioactivity on endothelial cells did not differ between CCP and control plasma. Auto-Abs neutralising type I IFNs were detected in 1.8 percent of CCP. Auto-Abs neutralising type I IFNs were mostly found in donors who had been hospitalised. CCP-associated adverse events (n = 18 patients, involving 48 CCP versus 400 CCP—without ARs), were not associated with significant alterations in inflammatory markers or ex-vivo bioactivity in CCP involved in transfusion-related adverse events.
Implications of all the available evidence
Overall, CCP exhibited moderately increased inflammatory markers compared to the control plasma with no discernible differences in ex-vivo bioactivity. Auto-Abs to type I IFNs detected in a small fraction of CCP were not associated with reported adverse events or differences in inflammatory markers. We also believe that it would be interesting for future clinical studies involving CCP to evaluate whether this heterogeneity could contribute to differences in CCP efficacy.
Introduction
COVID-19 convalescent plasma (CCP) provided by previously infected individuals to passively transfer anti-SARS-CoV-2 antibodies and potentially prevent or treat COVID-19.1
Fresh Frozen Plasma (FPP) has proved effective in correcting clotting factor deficiencies2 and is therefore frequently transfused in patients with active bleeding, as well as those with abnormal coagulation tests to prevent bleeding.3,4 However, FFP transfusion can be associated with adverse outcomes, including transfusion-related acute lung injury (TRALI) as well as transfusion-associated circulatory overload.5 An inflammatory response, mediated at least in part by factors present in the transfused FPP, may contribute to plasma-related adverse events. A causal relationship between inflammation and transfusion-related adverse events has been clearly described in the platelet transfusion context.6,7 In comparison, the role of inflammation in ARs associated with FFP transfusion has been poorly explored to date.8,9
FFP cytokines and chemokines are powerful modulators of a broad spectrum of immune responses, including inflammation. In addition to interfering with coagulation, such molecules promote a pro-coagulant state, which effectively maintains inflammation.10 Furthermore, when used as passive immunotherapy, the inflammatory content of CCP may interact with COVID-19 neutralising antibodies, which may be present in the plasma or already present in the recipient.11 In addition, auto-Abs against type I interferons (IFNs) were detected in severe cases of COVID-19 in at least 15% of previously healthy patients.12 This study therefore seeks to quantify soluble inflammatory molecules as well as bioactivity in CCP and compare it to non-convalescent FFP. We also assessed the presence of auto-Abs to type I IFNs in CCP. Finally, we investigated whether the inflammatory characteristics of CCP differed in the presence or absence of auto-Abs or in CCP involved in transfusion-related adverse events.
Methods
Donor recruitment and sample collection
Convalescent patients eligible for plasma donation underwent plasma apheresis, as described earlier, at least 14 days after symptom resolution.13 After undergoing pathogen reduction (INTERCEPT Blood System–Cerus Corp, Concord, CA), anti–SARS-CoV-2 antibody content was assessed in each donation, with a SARS-CoV-2 seroneutralisation titre requirement ≥40 and/or an immunoglobulin G (IgG) enzyme-linked immunosorbent assay (EUROIMMUN, Bussy-Saint-Martin, France) ratio >5.6.14 The plasma was cryopreserved (in 200–250 mL units) and made available for clinical use.
These CCPs were used to treat COVID-19 patients in observational studies under close monitoring15 and in a prospective randomised trial.14,16 The patients generally received two units of 200 mL CCP on day 1 and two additional units on day 2, i.e. a total of 4 units provided by 4 different donors. According to the French National Guideline on Haemovigilance Practices, the type, severity (grade 1, minor; grade 2, moderate; grade 3, severe; or grade 4, death), and outcomes of adverse events occurring during or after CCP or FFP transfusion (within 7d) were recorded. FFP and CCP were prepared with pathogen reduction (INTERCEPT Blood System–Cerus Corp, Concord, CA). FFP is considered as the gold standard in our study.
Ethics
Consecutive control plasma apheresis (FFP) was provided by non-convalescent donors eligible for plasma donation and who similarly underwent pathogen reduction. All CCP and control plasma donors provided written informed consent, and enrolled in a multiple site trial with similar preparation and collection procedures between May and November 2020. The COVID-19 CP donor collection was approved by the French National Ethics Committee. Written informed consent was obtained from all the patients or their trusted persons. Data collection from the PLASMACOV cohort was approved by the French National Ethics Committee (2020-A00728-31).15,16 No study protocol is available.
Sample size collection
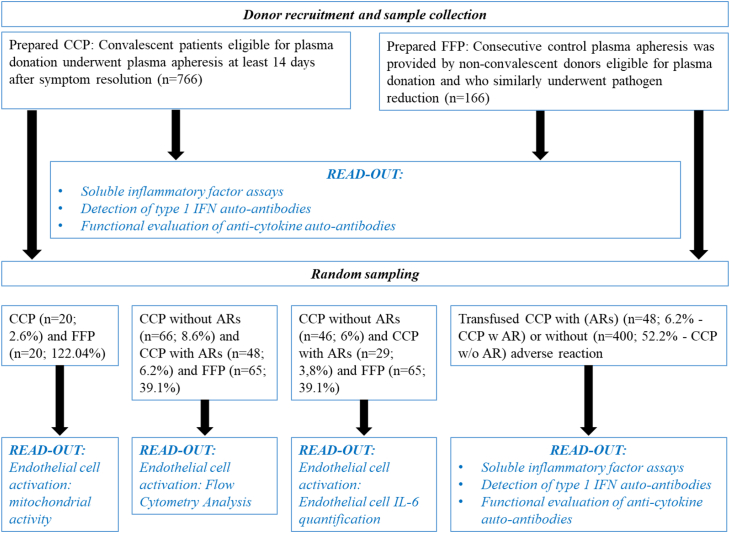
Sample size refers to the number of participants enrolled in a study between May and November 2020. CCP (n = 766) was compared to non-convalescent control plasma (n = 166) for soluble inflammatory markers, detection of type 1 IFN auto-antibodies and functional evaluation of anti-cytokine auto-antibodies. We identified that 400 out of 766 CCPs in our cohort had been transfused. A total of 18 patients who had been transfused with 2–4 CCPs experienced adverse reactions (AR) (n = 48 CCP). The adverse reactions reported in patients (n = 18) were divided as follows: fever, 44%; allergic transfusion reactions, 17%; dyspnoea not related to pulmonary oedema, 11%; transfusion-associated circulating overload (TACO), 6%; transient aggravation, 11% and undetermined, 11%. The severity of adverse transfusion reactions was as follows: grade 1, 39%; grade 2, 22%; grade 3, 28% and undetermined, 11%. Transfused CCP with ARs (n = 48) were compared to transfused CCP without ARs (n = 400) for soluble inflammatory markers, detection of type 1 IFN auto-antibodies and functional evaluation of anti-cytokine auto-antibodies. Random sampling was used for endothelial cell activation: mitochondrial activity, flow cytometry analysis or endothelial cell IL-6 quantification as described in Fig. 1.
Fig. 1.
Flow chart for the study. Sampling at random (n = number of samples, % of sampling at random vs. sample collection).
Soluble inflammatory factor assays in CP
Levels of various soluble inflammatory factors (sCD40 Ligand, IFN-alpha, IFN-beta, IFN-gamma, IL-1 beta, IL-6, IL-8, IL-10, IL-18, and TNF-alpha) were quantified in CP using Luminex Technology (RRID:SCR_018025), according to the manufacturer's instructions (Bio-Techne, Minneapolis, US). Absorbance at 450 nm was determined using an enzyme-linked immunosorbent assay reader (Magellan Sunrise software, Tecan Group Ltd., Lyon, France).
Detection of type 1 IFN autoantibodies
CCP and FFP samples were screened for auto-Abs by Gyros: cytokines, recombinant human (rh)IFN-α2 (Milteny Biotec, Paris, France) or rhIFN-ω (Merck, St. Quentin Fallavier, France), were first biotinylated with EZ-Link Sulfo-NHS-LC-Biotin (Thermo Fisher Scientific, Illkirch, France), according to the manufacturer's instructions, with a biotin-to-protein molar ratio of 1:12. The detection reagent contained a secondary antibody (Alexa Fluor 647 goat anti-human IgG (Thermo Fisher Scientific Cat# A-21445, RRID:AB_2535862) diluted in Rexip F (Gyros Protein Technologies, ref. number P0004825; 1/500 dilution of the 2 mg/mL stock to yield a final concentration of 4 μg/mL). 0.01% PBS-T buffer and Gyros Wash buffer (Gyros Protein Technologies, Uppsala, Sweden) were prepared according to the manufacturer's instructions. Plasma or serum samples were then diluted 1/100 in 0.01% PBS-T and tested with Bioaffy 1000 CD (Gyros Protein Technologies) and Gyrolab X-Pand (Gyros Protein Technologies). Cleaning cycles were performed in 20% ethanol.12
Functional evaluation of anti-cytokine auto-antibodies
Luciferase reporter assays: The blocking activity of anti-IFN-α2 and anti-IFN-ω auto-Abs was determined with a reporter luciferase activity.12 Briefly, HEK293T cells (RRID:CVCL_0063) were transfected with a plasmid containing the firefly luciferase gene under the control of the human ISRE promoter in the pGL4.45 backbone, and a plasmid constitutively expressing Renilla luciferase for normalisation (pRL-SV40P - RRID:Addgene_27163). Cells were transfected in the presence of the X-tremeGene 9 transfection reagent (Sigma Aldrich, St. Quentin Fallavier, France) for 36 h. Cells in Dulbecco's modified Eagle medium (DMEM, Thermo Fisher Scientific) supplemented with 2% foetal calf serum (FCS) and 10% healthy control or patient serum/plasma were stimulated with IFN-α2 (Milteny Biotec, Paris, France), IFN-ω (Merck, St. Quentin Fallavier, France), at 10 ng/mL or 100 pg/mL, or IFN-β (Milteny Biotech) at 10 ng/mL, for 16 h at 37 °C. Each sample was tested once for each cytokine and dose. Finally, cells were lysed for 20 min at room temperature and luciferase levels were measured with the Dual-Luciferase® Reporter 1000 assay system (Promega, Charbonnières-les-Bains, France), according to the manufacturer's protocol. Luminescence intensity was measured with a VICTOR X Multilabel Plate Reader (PerkinElmer Life Sciences, USA). Firefly luciferase activity values were normalised against Renilla luciferase activity values. These values were then normalised against the median induction level for non-neutralising samples, and expressed as a percentage. Samples were considered to be neutralising if luciferase induction, normalised against Renilla luciferase activity, was below 15% of the median values for controls tested on the same day.
Endothelial cell culture
EA.hy926 (RRID:CVCL_3901) is a permanent cell line derived by fusing human umbilical vein endothelial cells (HUVEC) with cell line A549.17 EA.hy926 was cultured as described.18 Cells were cultured in 6-well and 96-well plates in order to obtain approximately 106 cells/mL. The cell count was quantified with a TC10 Automated Cell Counter (Bio-Rad, Marnes-la-Coquette, France). Cells were grown until confluent then passaged with 0.25% trypsin (Sigma-Aldrich, Saint-Quentin-Fallavier, France). Using cultured human pulmonary microvascular endothelial cells (HPMVEC), we assessed whether CCP and FFP could trigger endothelial damage in vitro as described previously.19
Endothelial cell activation
Flow cytometry analysis
The marker surface expression of EA.hy926 endothelial cells was determined by flow cytometry. Cells were fixed beforehand as described above. Direct labelling was performed for the following markers: endoglin-CD105 (BD Biosciences Cat# 563264, RRID:AB_2738104), ICAM-1-CD54 (BD Biosciences Cat# 740978, RRID:AB_2740602) and Platelet endothelial cell adhesion molecule PECAM1-CD31 (BD Biosciences Cat# 744077, RRID:AB_2741979). CD54 and CD31 were two principal adhesion molecules, which determine changes in endothelial permeability, activation and transendothelial leukocyte migration. The cells were incubated for 30 min with antibodies. In all of the experiments, background labelling was assessed using the relevant fluorochrome-conjugated mouse IgG isotype control (BD Biosciences, Le Pont de Claix, France). Cells were centrifuged at 300 g for 5 min at 22 °C and then washed in PBS. Data acquisition was performed using a Guava easyCyte HT Flow Cytometer (Merck Millipore, Molsheim, France) and the data were analysed using the Incyte programme. At least 10,000 events were collected for each sample.
Mycoplasma testing was performed to confirm the absence of contamination according to the recommended protocol (MycoStrip™–Mycoplasma Detection Kit # rep-mys-50 InvivoGen).
Endothelial cell IL-6 quantification
The production of soluble cytokines in culture supernatants of EA.hy926 endothelial cells, with and without convalescent plasma stimulation, was measured using the specific enzyme-linked immunosorbent assays (ELISA). IL-6 levels were measured using the commercially available ELISA kit (DuoSet–R&D Systems, Lille, France) according to the manufacturer's instructions. Absorbance at 450 nm was determined with an ELISA reader (Magellan Sunrise software, Tecan Group Ltd., Lyon, France).
Mitochondrial activity
The cytotoxicity of plasma samples on HPMVEC was evaluated by assessing mitochondrial activity (WST-1 test) 1 h after incubating cells with plasma as previously described.19 The cytotoxicity of convalescent/non-convalescent plasma on HPMVEC was assessed by means of a colorimetric assay using 4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1.3-benzene disulfonate (WST-1), which in viable cells is cleaved by mitochondrial dehydrogenase. After incubation, the cells were washed with phosphate buffered saline and incubated with WST-1 (Roche, Basel, Switzerland) at a dilution of 1:10 (10 μL) for 2 h at 37 °C. As a positive control for endothelial cell injury, Shigatoxin 145, spiked in plasma from healthy adults, statistically significant decreased HPMVEC viability. Absorbance was measured using a multiwell plate reader (Synergy HTX multi-mode plate reader, BioTek Instruments, Highland Park, VT) at 450 nm with a reference wavelength of 620 nm.19
Statistical analysis
Manually collected data were entered into a database and the results are presented as median values ± standard error to the median (sem) or as scatter dot plots. The red line denotes the median of raw data. Statistical analyses were performed using unpaired t-tests (GraphPad Prism (RRID:SCR_002798)). Statistically significant differences required a p value < 0.05 (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001). Group comparisons were made using one-way analysis of variance (ANOVA), a parametric test, and Tukey's post-hoc test. The two-tailed t-test was used to compare two groups for parametric data (GraphPad Prism (RRID:SCR_002798)). No allowance for multiplicity has been applied. For some readout analyses, we performed simple random sampling where a select number of individuals from an entire population is randomly selected.
Role of funders
The funder had no role in the study design, data collection, data analyses, interpretation, or writing of this report. The authors are solely responsible for the study design, data collection, interpretation, manuscript preparation, and decision to submit the manuscript.
Results
Concentrations of soluble factors in COVID-19 convalescent plasma vs. control plasma
CCP (n = 766) and FFP (n = 166) donor characteristics are provided in Table 1 and Fig. 1. CCP and FFP donors were of a similar age, namely 38.3 and 39.7 years, respectively. The sex ratio (F/M) in terms of CCP and FFP was 1.71–3.74 respectively, differing statistically significant with regard to blood group distribution. The blood group balance among FFP donors is explained by the preferential recruitment of AB plasma (FFP) donors. Only 1.7 percent of convalescent donors reported they had spent some time in hospital during their illness, and none said they had spent any time in the intensive care unit.
Table 1.
Donor demographic characteristics for CCP (n = 766) and FFP (n = 166).
| Donor characteristics | CCP donors (n = 766) mean, [SD] | FFP donors (n = 166) mean, [SD] |
|---|---|---|
| Age | 38.3 [ ± 13.6] | 39.7 [ ± 14.2] |
| CCP donors (n = 766) n, (%) | FFP donors (n = 166) n, (%) | |
| Sex | ||
| Female | 282 (36.8) | 35 (21.1) |
| Male | 484 (63.2) | 131 (78.9) |
| Group | ||
| A | 356 (46.5) | 4 (2.4) |
| B | 52 (6.8) | 1 (0.6) |
| AB | 53 (6.9) | 156 (94) |
| O | 305 (39.8) | 5 (3) |
| Rhesus | ||
| Negative | 138 (18) | 28 (16.9) |
| Positive | 628 (82) | 138 (83.1) |
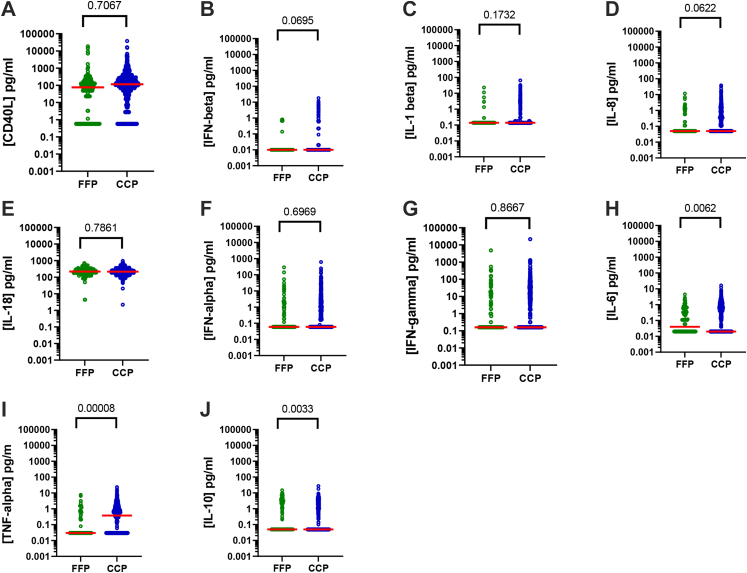
The concentration of several soluble inflammatory factors sCD40L, IFN-beta, IL-1 beta, IL-8, IL-18, IFN-alpha, IFN-gamma, IL-6, TNF-alpha and IL-10 were assessed in CCP (n = 766) and FFP (n = 166) (Fig. 2 and Supplemental Table S1). sCD40L was tested as a reference inflammatory marker due to its consistent association with ARs in both our experience and that of other groups.20, 21, 22, 23, 24, 25 While most factors were comparable in terms of CCP and control FFP, IL-6 and TNF-alpha concentrations were found to be elevated to a statistically significant extent (unpaired t-tests) in CCP compared to control plasma 0.531 ± 0.04 versus 0.271 ± 0.04 pg/mL; (95% confidence interval [CI], 0.07371–0.4446; p = 0.0061) and 0.900 ± 0.07 versus 0.283 ± 0.07 pg/mL; (95% confidence interval [CI], 0.3097–0.9202; p = 0.0000829), respectively, and IL-10 concentrations had decreased (0.731 ± 0.007 versus 1.22 ± 0.19 pg/mL; (95% confidence interval [CI], −0.8180 to −0.1633; p = 0.0034)). It is important to note that there was no correlation between inflammatory characteristics and ABO blood groups for both CCP and FFP (unpublished data).
Fig. 2.
Inflammatory characteristics of COVID-19 convalescent plasma. Concentrations of 10 soluble factors in the FFP (n = 166) considered as the gold standard versus the CCP (n = 766)—A) sCD40L (pg/mL), B) IFN-beta (pg/mL), C) IL-1 beta (pg/mL), D) IL-8 (pg/mL), E) IL-18 (pg/mL), F) IFN-alpha (pg/mL), G) IFN-gamma (pg/mL), H) IL-6 (pg/mL), I) TNF-alpha (pg/mL) and J) IL-10 (pg/mL). Scatter dot plots represent data and the red line denotes the median of raw data. Statistical analyses were performed using unpaired t-tests.
Bioactivity of COVID-19 convalescent plasma versus fresh frozen plasma assessed on endothelial cells
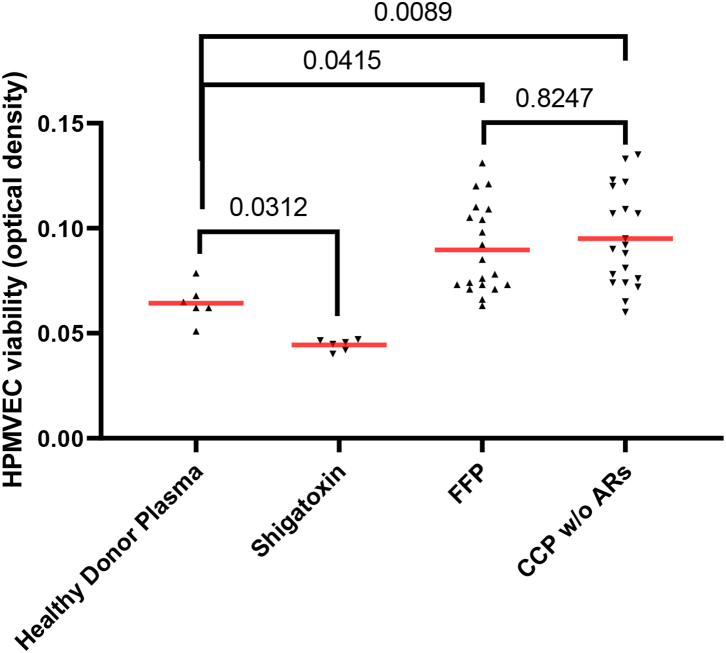
CCP (n = 20) and FFP (n = 20) (random sampling) were added to HPMVECs to assess the effects of CCP versus FFP in terms of endothelial viability. Compared to FFP, CCP did not alter HPMVEC viability (Fig. 3 and Supplemental Table S2). As a positive control for endothelial cell injury, Shigatoxin 145 was spiked in plasma from healthy adults, incubated at 37 °C for 15 min, before being added to HPMVECs with a statistically significant decrease in HPMVEC viability as described previously.19
Fig. 3.
Endothelial cell cytotoxicity. HPMVEC cytotoxicity induced by FFP (n = 20) considered as the gold standard versus the CCP without ARs (n = 20). HPMVEC cytotoxicity induced by negative and positive controls. Shigatoxin 145 spiked in healthy adult plasma was considered as the positive control (n = 6) and Healthy Donor Plasma-stimulated HPMVEC cells were the negative control (n = 6). Scatter dot plots represent data and the red line denotes the median of raw data. Statistical analyses were performed using one-way Anova.
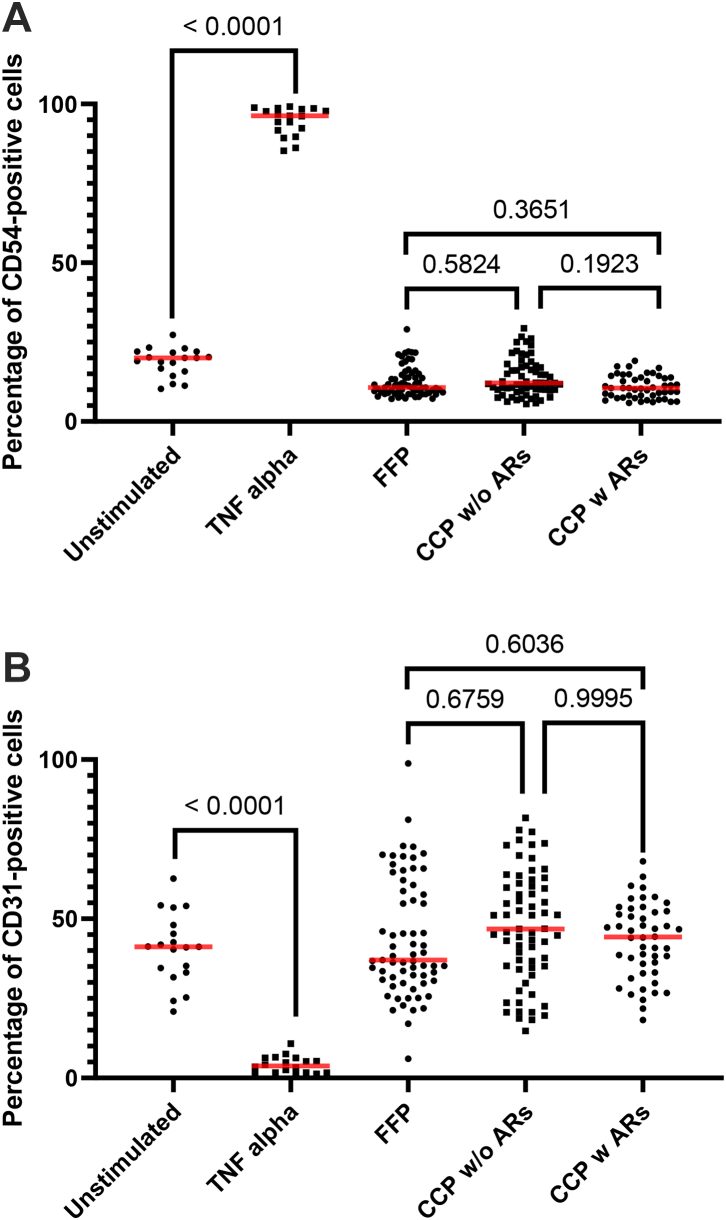
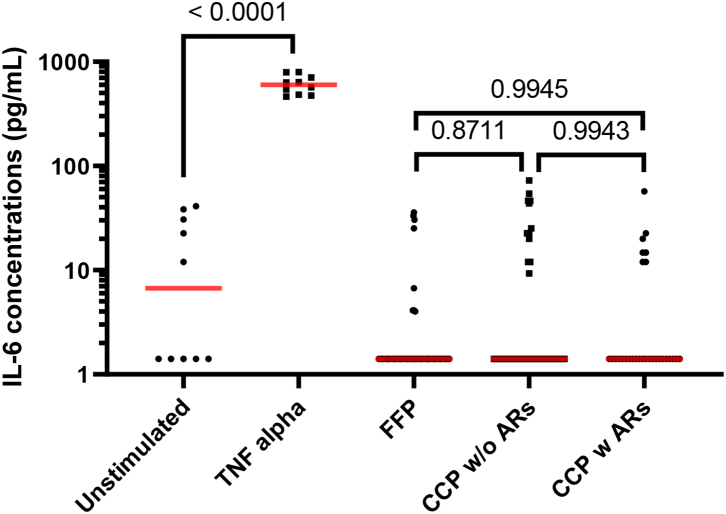
Moreover, to investigate whether or not CCP was involved in adverse reactions (ARs) versus FFP on endothelial cell activation, FFP (n = 65–random sampling), CCP without ARs (n = 66–random sampling) and CCP with ARs (n = 48) were added to the EA.hy926 cell. EA.hy926 cell monolayer membrane expression markers were then measured. CD54 (Fig. 4A and Supplemental Table S3) and CD31 (Fig. 4B and Supplemental Table S3) were not modulated in the presence of CCP without ARs and CCP with ARs compared to FFP, contrary to TNF-alpha stimulation as the positive control (n = 18) (Fig. 4A, B). The percentage expression for CD105 (Supplemental Fig. S1) was not modulated in the presence of CCP without ARs and CCP with ARs compared to FFP or the positive control (n = 18) (recombinant human TNF-alpha at a final concentration of 100 pg/mL). As expected and discussed earlier, TNF-alpha stimulation can reduce the expression of PECAM-1 (CD31) from endothelial cell junctions and increase the expression of ICAM (CD54).26
Fig. 4.
Endothelial cell activation focusing on membrane expression. EA.hy926 cells were stimulated with FFP (n = 65) considered as the gold standard versus the CCP without (n = 65) or with ARs (n = 48). EA.hy926 cells were stimulated with negative and positive controls. TNF-α stimulation was considered the positive control (n = 18) and unstimulated EA.hy926 cells were viewed as the negative control (n = 19). Bioactivity in EA.hy926 endothelial cells was measured with FCM focusing on membrane expression of CD54 (A) and CD31 (B) with or without stimulation. Scatter dot plots represent the data and the red line denotes the median of raw data. Statistical analyses were performed using one-way Anova with multiple comparisons.
Similar findings were observed with the EA.hy926 cell soluble factor released after activation with CCP without ARs (n = 46) and CCP with ARs (n = 29) with no statistically significant modulation of IL-6 released by EA.hy926 compared to FFP (n = 65) (Fig. 5 and Supplemental Table S4) contrary to TNF-alpha stimulation as the positive control (n = 18) (Fig. 5). As expected, TNF-alpha stimulation can trigger the release of IL-6 from endothelial cells.27
Fig. 5.
Endothelial cell activation focusing on IL-6 release. EA.hy926 cells were stimulated with FFP (n = 65) considered as the gold standard versus the CCP without (n = 46) or with ARs (n = 29). EA.hy926 cells were stimulated with negative and positive controls. TNF-α stimulation was considered the positive control (n = 10) and non-stimulated EA.hy926 cells were viewed as the negative control (n = 10). Bioactivity in EA.hy926 endothelial cells was measured with IL-6 release, with or without stimulation. Scatter dot plots represent the data and the red line denotes the median of raw data. Statistical analyses were performed using one-way Anova.
Collectively, these data indicate that CCP (without ARs) versus FFP, CCP (with ARs) versus FFP and CCP (without ARs) versus CCP (with ARs) do not activate or affect the viability of various endothelial cells lines (HPMVECs and EA.hy926).
Auto-Abs against type I IFNs
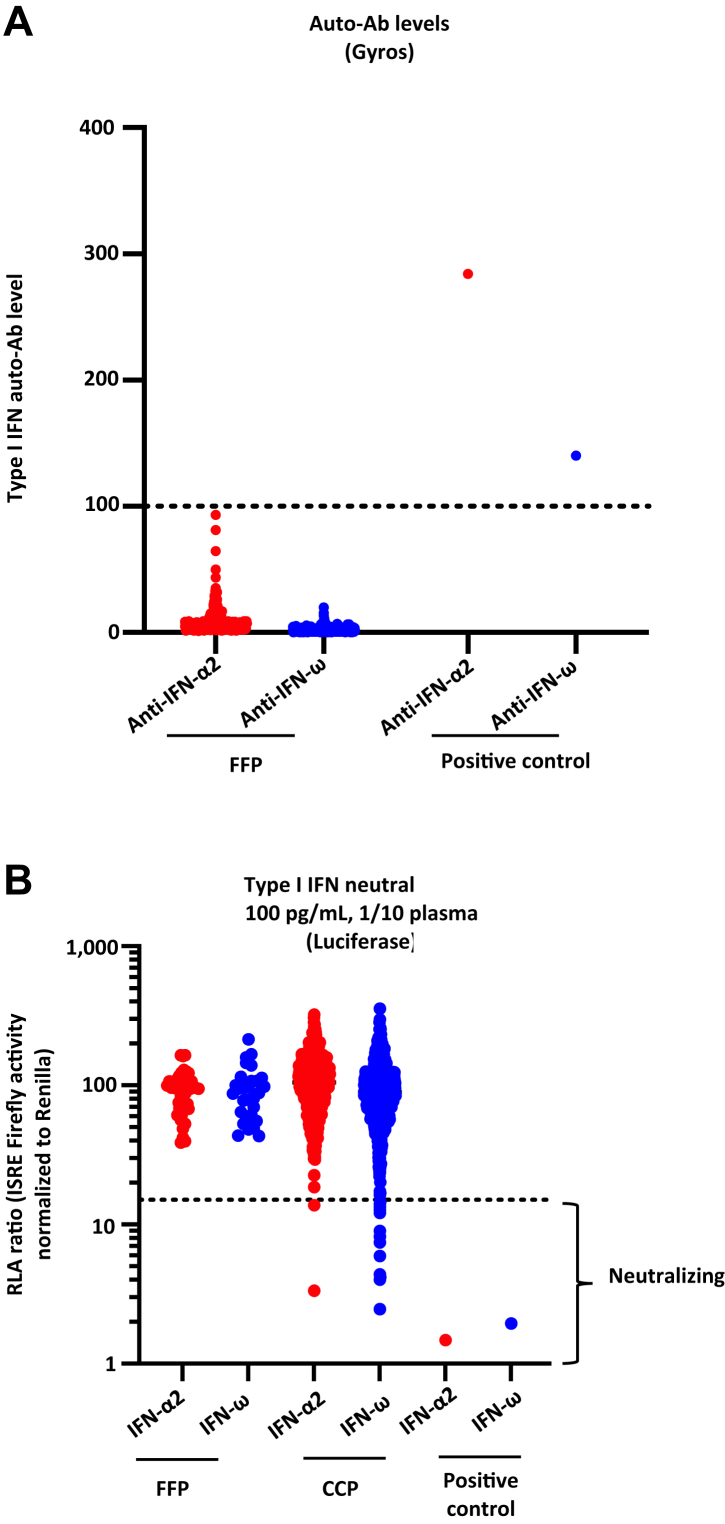
CCP (n = 766) and FFP (n = 166) samples were screened for auto-Abs to type I IFNs. We screened all the patients for IgG auto-Abs neutralising type I IFNs, using a high-throughput luciferase-based assay, as described previously12,28 (Fig. 6A and Supplemental Table S5).
Fig. 6.
Detection and neutralising auto-Abs against IFN-α2 and/or IFN-ω in FFP versus CCP.(A) Gyros (high-throughput automated ELISA) results for auto-Abs against IFN-α2 and IFN-ω in the FFP (n = 166) considered as the reference versus CCP (n = 766). (B) Results for the neutralisation of 100 pg/mL IFN-α2 or IFN-ω in the presence of 10% plasma in the FFP (n = 166) versus CCP (n = 766). Relative luciferase activity is shown (ISRE dual luciferase activity, with normalisation against Renilla luciferase activity) after stimulation with 100 pg/mL IFN-α2 or IFN-ω in the presence of 10% plasma. RLU: relative luciferase units. ISRE: interferon-sensitive response elements. Dotted lines indicate neutralising levels, defined as induction levels below 15% of the median value for controls tested on the same day.
We assessed the neutralising activity against IFN-α2, IFN-ω, and IFN-β. At the dose of 10 ng/mL in 10% plasma, none of the samples tested was found to have neutralising auto-Abs (unpublished data). At the dose of 100 pg/mL in 10% plasma, 1.8% of the CCPs tested (14 of 766) were found to have neutralising auto-Abs against 100 pg/mL of type I IFNs. One was neutralising against IFN-α2 and IFN-ω at 100 pg/mL, while one was neutralising against IFN-α2 only and 12 against IFN-ω only at 100 pg/mL. Under the same conditions, none of the (FPP) samples tested was found to have neutralising auto-Abs against 100 pg/mL of type I IFNs (Fig. 6B and Supplemental Table S5). It is important to note that no ARs were reported in the 14 COVID-19 patients who were transfused with CCP containing neutralising auto-Abs to type 1 IFN.
The CCPs with (n = 14) or without Anti-IFN auto-Abs (n = 752) were tested for the same 10 soluble inflammatory factors: sCD40 Ligand (Supplemental Fig. S2A), IFN-beta (Supplemental Fig. S2B), IL-1 beta (Supplemental Fig. S2C), IL-8 (Supplemental Fig. S2D), IL-18 (Supplemental Fig. S2E), IFN-alpha (Supplemental Fig. S2F), IFN-gamma (Supplemental Fig. S2G), IL-6 (Supplemental Fig. S2H), TNF-alpha (Supplemental Fig. S2I), and IL-10 (Supplemental Fig. S2J). Interestingly, we found no statistically significant differences in the markers assessed in the CCPs with or without Anti-IFN auto-Abs.
Concentrations of inflammatory factors in transfused COVID-19 convalescent plasma involved in adverse reactions
No statistically significant differences in sCD40 Ligand, IFN-beta, IL-1 beta, IL-8, IL-18, IFN-alpha, IFN-gamma, IL-6, TNF-alpha and IL-10 concentrations were observed between CCPs with or without AR involvement (Supplemental Table S6) or between ARs of differing severity even when the analysis was limited to ARs known to be linked to immune reactivity. Moreover, we observed no statistically significant differences in sCD40 Ligand, IFN-beta, IL-1 beta, IL-8, IL-18, IFN-alpha, IFN-gamma, IL-6, TNF-alpha and IL-10 concentrations in CPPs involved in ARs (n = 48) depending on the severity (grade 1, minor (n = 22); grade 2, moderate (n = 12); grade 3, severe (n = 7) or undetermined (n = 7) (Supplemental Fig. S3). Similar results were observed regarding the same soluble immunomodulatory factors on CPPs involved in ARs (n = 48) depending on the clinical signs or symptoms: fever (n = 18); atypical allergic transfusion reactions—AATR (n = 11); dyspnoea not related to pulmonary oedema—DNRPE (n = 6); TACO (n = 3); transient aggravation—TA (n = 5); and undetermined—n = 5) (Supplemental Fig. S4).
Discussion
There is increasing evidence to suggest a link between immune response and disease progression in COVID-1929,30 as well as other viral infections such as MERS-CoV, SARS-CoV and influenza. A cytokine storm, hyperinflammation and multi-organ failure have been identified in COVID-19 patients with severe disease.29 This is because the effects of an overactive immune response are far more damaging than the infection per se, resulting in massive and irreversible organ damage.30 COVID-19 convalescent plasma therapy (CCP) has emerged as a potential treatment for COVID-19, having been studied in a number of clinical trials around the world.31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57 The findings of several randomised, controlled clinical trials shed little light on any noteworthy clinical benefits of CCP32,33,39,41,43, 44, 45,49,50,58,59 while others have reported a favourable outcome, notably when CCP is administered very early after disease onset60,61 and/or in patients with underlying immunodeficiencies.16,44,58,59
Finally, the findings of several randomised, controlled clinical trials and meta-analyses shed light on any noteworthy clinical benefits of COVID-19 convalescent plasma therapy. Focosi D et al.62 recently analysed efficacy-related variables such as clinical settings, disease severity, CCP SARS-CoV-2 antibody levels and the function, dose and timing of administration, various outcomes, CCP origin and collection time along with efficacy criteria. This review of 30 available RCTs demonstrated that efficacy indicators (including reduced mortality rates) were more likely to occur if the CCP neutralising titre was >160 and the time to randomisation was less than 9 days following early, high-titre administration. CCP transfusion is reported to be very safe.63 Nguyen FT et al. did find a statistically significant higher incidence of febrile non-haemolytic transfusion reaction in their CCP-transfused patients versus FFP-transfused patients, but they attribute this to the various clinical settings in which these studies were conducted.60
A cytokine storm may drive pro-inflammatory and procoagulatory processes to greater extent across the phases of COVID-19, resulting in systemic endotheliitis and a high prevalence of thrombi.61,64 Such processes may still be underway early in convalescence. Furthermore, immune-complexes and antibody-dependent infection of macrophages—mediated may worsen inflammation.65,66 It is therefore meaningful to investigate COVID-19 convalescent plasma from an inflammatory perspective and to assess its potential role in exacerbating the recipient's clinical situation.
As mentioned earlier, we recently demonstrated that circulating sCD40L and sCD62P levels are statistically significant modulated throughout the disease course of COVID-19 and differ statistically significant from those measured in convalescent patients. Here, we find that IL-6 and TNF-alpha levels were statistically significant higher in CCP vs FFP, whereas IL-10 levels were statistically significant lower in CCP vs FFP. Evidence that IL-6 drives immune dysregulation and respiratory failure in the COVID-19-related cytokine storm syndrome is rapidly increasing.67 Elevated serum IL-6 levels are associated with lymphopenia, impaired lymphocyte cytotoxicity and endothelial activation.67 In a multivariate analysis, Del Valle et al. found that elevated plasma levels of IL-6 and TNF-α was strongly correlated with poor prognosis in COVID-19 patients.68 IL-6 concentrations were found to be elevated to a statistically significant extent in CCP compared to control plasma (0.531 ± 0.04 versus 0.271 ± 0.04 pg/mL; p = 0.0061), but this concentration is very low compared to serum samples from hospitalised COVID-19 patients (70 pg/mL for IL-668 or 21.55 pg/mL69). Moreover, IL-6 levels increased with disease severity (>100–120 pg/mL).70
One of the most significant anti-inflammatory cytokines for limiting inflammatory Th cells and immunopathology while maintaining tissue homeostasis is IL-10.71 IL-10 is also elevated in COVID-19 patients with poor prognosis.72 This could represent a “danger signal”, an alarm triggered by the host, given the immuno-regulatory properties of this cytokine.72
Among the pro or anti-inflammatory factors quantified in CCP in our study, IL-6 levels were found to statistically significant correlate positively with the anti-SARS-CoV-2 neutralisation titres (unpublished data). Of note, IFN beta and IL-18 levels appeared to correlate as well while not reaching statistical significance. Moreover, no statistically significant association between inflammatory characteristics and the ELISA test was observed. Moreover, Tania S Bonny et al. described that only median IL-6 levels differed statisticallysignificant by hospitalization status after adjusting for multiple comparisons.73
Brox et al.74 investigated whether CCP contained additional immunoregulatory constituents compared to healthy control plasma, CCP statistically significant reduced pro-inflammatory cytokine production triggered by various TLR ligands in healthy donors. Authors described that the production of IL-6, MCP-1, and IFN-γ were most frequently downregulated by CCP convalescent plasma and concluded that CCP may have a novel, SARS-CoV-2-independent immunomodulatory activity that could be beneficial to COVID-19 patients.
In our study, in vitro exposure of endothelial cells to CCP did not result in endothelial damage or activation cell activation. Dupont A et al.75 reported a link between endothelial damage and the severity of immune inflammatory responses, suggesting that inflammatory-driven processes are most likely to be the primary drivers of endothelial damage in COVID-19, contributing to microvascular thrombosis and organ failure. Furthermore, when convalescent patient plasma was tested post-intensive care unit (ICU) discharge and compared to plasma from the same patients at the time of ICU admission or to control healthy individuals, the authors found that HPMVEC viability was only partially restored to the control levels.19 Difference in the methods used to assess endothelial damage and/or differences in the patients assessed may contribute the differing results. When comparing CCP to FFP, it is worth noting that FFP may have its own biological effects as highlighted by Estcourt et al.58 In particular, pre-existing humoral immunity to human common cold coronaviruses has been found to negatively impact the protective SARS-CoV-2 antibody response.76
Auto-Abs neutralising type I IFNs have been detected in at least 15% of patients with critical COVID-19 and are a major immunological determinant of disease severity12,77,78 despite full knowledge of the clinical impact.12,79 Vazquez et al.80 described 116 convalescent plasma samples from unique donors who had previously been hospitalised for COVID-19, and found that 3% (n = 4/116) of plasma samples tested positive for type 1 IFN autoantibodies, among which 2 were capable of neutralising 10 ng/mL IFN α and ω in 10% plasma. In our study, 1.8% CCP were found to harbour neutralising auto-Abs against type I IFNs, mostly against IFN ω and none neutralized 10 ng/mL IFN α or ω. Of note, only 1.7% of convalescent donors in our study reported a history of brief hospitalisation during their illness with no ICU admissions. Moreover, contrary Vazquez et al.80 study, our CCPs underwent pathogen reduction (Amotosalen + UVA; Intercept blood system, Cerus).
Another study by Raadsen et al.81 confirmed the presence of neutralising auto-Abs against type I IFNs in the most severely ill COVID-19 patients and a link to delayed viral clearance. These findings were not evident in samples collected from convalescent plasma donors. It should be noted that, out of the 118 donors screened, auto-Abs to IFN- α2 was detected by ELISA in 3 individuals but were found to be non-neutralising under the test conditions. Lastly, Van der Wijst et al.82 preprint studied CCP involving 175 donors. None of the donors tested positive for anti-IFN-α2 autoantibodies, which further suggests the rarity of these auto-antibodies in COVID-19 convalescent donors.
Taken together, these findings may suggest that CCP from previously hospitalised patients with severe COVID-19 may require screening for type I interferon auto-antibodies.
Nevertheless it is important to note that no transfusion-related adverse events were reported in the 14 COVID-19 patients transfused with type 1 IFN auto-antibodies containing CCP. In France, transfusion-related adverse events must be reported to the health authorities. However, it is important to note that the current haemovigilance programmes may not necessarily view a worsening of COVID-19 or the occurrence of a de novo herpes infection potentially arising from the transfusion of type 1 IFN neutralising antibodies, as a transfusion-related event.
The CCP with type 1 IFN auto-antibodies did not differ from those without auto-antibodies with regard to all soluble inflammatory factors tested. The limited number of CCP samples with auto-antibodies must, however, be emphasised (n = 14).
This study shows that, in contrast to platelet concentrate transfusion,83,84 the inflammatory component of CCPs is unlikely to contribute to reactions observed post-CCP transfusion. This report focuses on differences between CCP and FFP, but the differences highlighted were minor and limited to quantitative differences in a few non-matching cytokines.
This observation reinforces the concept that the CCP active substance is a specific antibody and that higher-titre CCP is more effective than lower-titre CCP, probably regardless of the inflammatory content (cytokines/chemokines) of convalescent plasma. However, in specific clinical situations, we cannot rule out the impact of the inflammatory component on changes in patient pathology, as observed with other labile blood products such as platelet concentrates with a major inflammatory component.
There is increasing evidence to suggest a link between the immune response and disease progression in COVID-19, and abnormalities in immune cells, inflammatory markers and the cytokine storm implicated in disease severity and outcome. Cytokine storms are common in viral infections caused by MERS-CoV, SARS-CoV, influenza and other viruses. Cytokine storm, hyperinflammation, and multi-organ failure have also been identified in patients with severe disease. Even in convalescent patients eligible for plasma donation, we note considerable heterogeneity from all donors in terms of inflammatory markers, at least 14 days after symptom resolution. We also believe that it would be interesting for future clinical studies involving CCP to evaluate whether this heterogeneity could contribute to differences in CCP efficacy. Moreover, the SARS-CoV-2- independent immunomodulatory activity might or might not be beneficial to COVID-19 patients. The correlation of CCP inflammatory markers and clinical efficacy should be investigated using a similar strategy.
In conclusion, inflammatory markers were found to be moderately increased in CCP when compared to control plasma with no discernible differences in ex-vivo bioactivity as assessed by endothelial toxicity and/or activation. Auto-Abs to type I IFNs were detected in a small fraction of CCP and were not associated with reported adverse events or differences in inflammatory markers. Additional studies, including careful clinical evaluation of patients treated with CCP, are required in order to further define the clinical relevance of these findings.
Caveats and limitations
The possible confounding effect could be caused by the heterogeneous distribution of CCPs and FFP, the time period and absence of donor characteristics (sex, age, body mass index (BMI), ABO group, rhesus, time from symptom onset, time from end of symptoms, duration of symptoms, fever, breathing difficulties and loss of taste and smell). One study limitation may be linked to the lack of sample size calculation, depending solely on one time period. Finally, clinical aspects were less detailed in our study but the efficacy of Convalescent Plasma to Treat COVID-19 Patients, a Nested Trial in the CORIMUNO-19 Cohort (CORIPLASM) (NCT04345991), is under analysis.
Contributors
Contribution: F.C. and H.H.C. supervised all experiments and wrote the manuscript; M.R., D.C., B.B., C.P., J.H., C.A.A., M.A.E., A.P., A.C.D., T.E., M.H., E.A.C., Q.P., and T.L.V., performed experiments and analysis. O.H., A.M.F., P.C., D.L., P.R., F.P., P.G., J.L.C., S.S., P.M., K.L., P.B., and P.T. supervised the studies and amended the manuscript. The underlying data were verified by F.C., H.H.C., and P.T. All authors have read and approved the final version of the manuscript.
Data sharing statement
Dr Fabrice COGNASSE had full access to all of the study data and takes responsibility for the integrity of the data and the accuracy of the data analyses. The data are available for further research. Supplemental data are available at the journal's website. The data used in this study can be obtained by request to Fabrice Cognasse (fabrice.cognasse@efs.sante.fr).
Declaration of interests
The authors have no competing financial interests to declare.
Acknowledgments
We would like to thank the medical staff and personnel of the Etablissement Français du Sng, France, for medical and technical support throughout our investigations. We are extremely grateful to the blood donors for taking part in this study. This work was supported by grants from the French National Blood Service—EFS, the Association “Les Amis de Rémi” Savigneux, France and the “Fondation pour la Recherche Médicale (Medical Research Foundation) - REACTing 2020”. We wish to warmly thank teams from both branches of the Laboratory of Human Genetics of Infectious Diseases. The latter is supported by the Howard Hughes Medical Institute, the Rockefeller University, the St. Giles Foundation, the National Institutes of Health (NIH) (R01AI088364), the National Centre for Advancing Translational Sciences (NCATS), the NIH Clinical and Translational Science Award (CTSA) programme (UL1 TR001866), a Fast Grant from Emergent Ventures, the Mercatus Center at George Mason University, the Yale Centre for Mendelian Genomics and the GSP Coordinating Centre funded by the National Human Genome Research Institute (NHGRI) (UM1HG006504 and U24HG008956), the Yale High Performance Computing Centre (S10OD018521), the Fisher Centre for Alzheimer’s Research Foundation, the Meyer Foundation, the French National Research Agency (ANR) under the “Investments for the Future” programme (ANR-10-IAHU-01), the Integrative Biology of Emerging Infectious Diseases Laboratory of Excellence (ANR-10-LABX-62-IBEID), the French Foundation for Medical Research (FRM) (EQU201903007798), the FRM and ANR GENCOVID project, the ANRS-COV05, ANR GENVIR (ANR-20-CE93-003) and ANR AABIFNCOV (ANR-20-CO11-0001) projects, the European Union’s Horizon 2020 research and innovation programme under grant agreement No 824110 (EASI-genomics), the Square Foundation, Grandir - Fonds de solidarité pour l'enfance, the Fondation du Souffle (Children's Aid Foundation), the SCOR Corporate Foundation for Science, the Institut National de la Santé et de la Recherche Médicale (INSERM) (National Institute for Health and Medical Research) and the University of Paris. PB was supported by the Imagine Institute MD-PhD programme (with the support of the Fondation Bettencourt-Schueller).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2022.104414.
Appendix A. Supplementary data
References
- 1.Casadevall A., Pirofski L.A. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130(4):1545–1548. doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Shaughnessy D.F., Atterbury C., Bolton Maggs P., et al. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. Br J Haematol. 2004;126(1):11–28. doi: 10.1111/j.1365-2141.2004.04972.x. [DOI] [PubMed] [Google Scholar]
- 3.Parker R.I. Transfusion in critically ill children: indications, risks, and challenges. Crit Care Med. 2014;42(3):675–690. doi: 10.1097/CCM.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 4.Dara S.I., Rana R., Afessa B., Moore S.B., Gajic O. Fresh frozen plasma transfusion in critically ill medical patients with coagulopathy. Crit Care Med. 2005;33(11):2667–2671. doi: 10.1097/01.ccm.0000186745.53059.f0. [DOI] [PubMed] [Google Scholar]
- 5.Cho M.S., Modi P., Sharma S. StatPearls Publishing LLC; Treasure Island, FL: 2021. Transfusion-related acute lung injury. [PubMed] [Google Scholar]
- 6.Cognasse F., Hally K., Fauteux-Daniel S., et al. Effects and side effects of platelet transfusion. Hamostaseologie. 2021;41(2):128–135. doi: 10.1055/a-1347-6551. [DOI] [PubMed] [Google Scholar]
- 7.Hally K., Fauteux-Daniel S., Hamzeh-Cognasse H., Larsen P., Cognasse F. Revisiting platelets and toll-like receptors (TLRs): at the interface of vascular immunity and thrombosis. Int J Mol Sci. 2020;21(17) doi: 10.3390/ijms21176150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandey S., Vyas G.N. Adverse effects of plasma transfusion. Transfusion. 2012;52(Suppl 1):65S–79S. doi: 10.1111/j.1537-2995.2012.03663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watson G.A., Sperry J.L., Rosengart M.R., et al. Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. J Trauma. 2009;67(2):221–227. doi: 10.1097/TA.0b013e3181ad5957. discussion 8-30. [DOI] [PubMed] [Google Scholar]
- 10.Levi M., van der Poll T. Coagulation and sepsis. Thromb Res. 2017;149:38–44. doi: 10.1016/j.thromres.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bastard P., Gervais A., Le Voyer T., et al. Autoantibodies neutralizing type I IFNs are present in ∼4% of uninfected individuals over 70 years old and account for ∼20% of COVID-19 deaths. Sci Immunol. 2021;6(62) doi: 10.1126/sciimmunol.abl4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tiberghien P., de Lamballerie X., Morel P., Gallian P., Lacombe K., Yazdanpanah Y. Collecting and evaluating convalescent plasma for COVID-19 treatment: why and how? Vox Sang. 2020;115(6):488–494. doi: 10.1111/vox.12926. [DOI] [PubMed] [Google Scholar]
- 14.Hueso T., Pouderoux C., Péré H., et al. Convalescent plasma therapy for B-cell-depleted patients with protracted COVID-19. Blood. 2020;136(20):2290–2295. doi: 10.1182/blood.2020008423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ClinicalTrialsgov Efficacy of convalescent plasma to treat COVID-19 patients, a nested trial in the CORIMUNO-19 cohort (CORIPLASM), ClinicalTrials.gov, Identifier: NCT04345991. 2021. https://clinicaltrials.gov/ct2/show/NCT04345991 Available at:
- 16.Hueso T., Godron A.S., Lanoy E., et al. Convalescent plasma improves overall survival in patients with B-cell lymphoid malignancy and COVID-19: a longitudinal cohort and propensity score analysis. Leukemia. 2022;36:1–10. doi: 10.1038/s41375-022-01511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edgell C.J., McDonald C.C., Graham J.B. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci U S A. 1983;80(12):3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sut C., Hamzeh-Cognasse H., Arthaud C.A., et al. Platelet concentrate supernatants alter endothelial cell mRNA and protein expression patterns as a function of storage length. Transfusion. 2018;58(11):2635–2644. doi: 10.1111/trf.14973. [DOI] [PubMed] [Google Scholar]
- 19.Rauch A., Dupont A., Goutay J., et al. Endotheliopathy is induced by plasma from critically ill patients and associated with organ failure in severe COVID-19. Circulation. 2020;142(19):1881–1884. doi: 10.1161/CIRCULATIONAHA.120.050907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urner M., Herrmann I.K., Buddeberg F., et al. Effects of blood products on inflammatory response in endothelial cells in vitro. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0033403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufman J., Spinelli S.L., Schultz E., Blumberg N., Phipps R.P. Release of biologically active CD154 during collection and storage of platelet concentrates prepared for transfusion. J Thromb Haemost. 2007;5(4):788–796. doi: 10.1111/j.1538-7836.2007.02412.x. [DOI] [PubMed] [Google Scholar]
- 22.Stolla M., Refaai M.A., Heal J.M., et al. Platelet transfusion - the new immunology of an old therapy. Front Immunol. 2015;6:28. doi: 10.3389/fimmu.2015.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sut C., Aloui C., Tariket S., et al. Assessment of soluble platelet CD40L and CD62P during the preparation process and the storage of apheresis platelet concentrates: absence of factors related to donors and donations. Transfus Clin Biol. 2018;25(3):192–196. doi: 10.1016/j.tracli.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Tariket S., Hamzeh-Cognasse H., Laradi S., et al. Evidence of CD40L/CD40 pathway involvement in experimental transfusion-related acute lung injury. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-49040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cognasse F., Sut C., Fromont E., Laradi S., Hamzeh-Cognasse H., Garraud O. Platelet soluble CD40-ligand level is associated with transfusion adverse reactions in a mixed threshold-and-hit model. Blood. 2017;130(11):1380–1383. doi: 10.1182/blood-2017-03-773945. [DOI] [PubMed] [Google Scholar]
- 26.Bui T.M., Wiesolek H.L., Sumagin R. ICAM-1: a master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J Leukoc Biol. 2020;108(3):787–799. doi: 10.1002/JLB.2MR0220-549R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khaedir Y., Kartika R. Perspectives on targeting IL-6 as a potential therapeutic strategy for COVID-19. J Interferon Cytokine Res. 2021;41(2):37–43. doi: 10.1089/jir.2020.0135. [DOI] [PubMed] [Google Scholar]
- 28.Bastard P., Michailidis E., Hoffmann H.H., et al. Auto-antibodies to type I IFNs can underlie adverse reactions to yellow fever live attenuated vaccine. J Exp Med. 2021;218(4):e20202486. doi: 10.1084/jem.20202486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leisman D.E., Ronner L., Pinotti R., et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8(12):1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong R.S.Y. Inflammation in COVID-19: from pathogenesis to treatment. Int J Clin Exp Pathol. 2021;14(7):831–844. [PMC free article] [PubMed] [Google Scholar]
- 31.Yoon H.A., Bartash R., Gendlina I., et al. Treatment of severe COVID-19 with convalescent plasma in Bronx, NYC. JCI Insight. 2021;6(4):e142270. doi: 10.1172/jci.insight.142270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simonovich V.A., Burgos Pratx L.D., Scibona P., et al. A randomized trial of convalescent plasma in covid-19 severe pneumonia. N Engl J Med. 2021;384(7):619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sekine L., Arns B., Fabro B.R., et al. Convalescent plasma for COVID-19 in hospitalised patients: an open-label, randomised clinical trial. Eur Respir J. 2022;59(2) doi: 10.1183/13993003.01471-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Donnell M.R., Grinsztejn B., Cummings M.J., et al. A randomized double-blind controlled trial of convalescent plasma in adults with severe COVID-19. J Clin Invest. 2021;131(13) doi: 10.1172/JCI150646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Libster R., Pérez Marc G., Wappner D., et al. Early high-titer plasma therapy to prevent severe covid-19 in older adults. N Engl J Med. 2021;384(7):610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Körper S., Weiss M., Zickler D., et al. Results of the CAPSID randomized trial for high-dose convalescent plasma in patients with severe COVID-19. J Clin Invest. 2021;131(20):e152264. doi: 10.1172/JCI152264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klassen S.A., Senefeld J.W., Senese K.A., et al. Convalescent plasma therapy for COVID-19: a graphical mosaic of the worldwide evidence. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.684151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klassen S.A., Senefeld J.W., Johnson P.W., et al. The effect of convalescent plasma therapy on mortality among patients with COVID-19: systematic review and meta-analysis. Mayo Clin Proc. 2021;96(5):1262–1275. doi: 10.1016/j.mayocp.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirenga B., Byakika-Kibwika P., Muttamba W., et al. Efficacy of convalescent plasma for treatment of COVID-19 in Uganda. BMJ Open Respir Res. 2021;8(1):e001017. doi: 10.1136/bmjresp-2021-001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan T.N.S., Mukry S.N., Masood S., et al. Usefulness of convalescent plasma transfusion for the treatment of severely ill COVID-19 patients in Pakistan. BMC Infect Dis. 2021;21(1):1014. doi: 10.1186/s12879-021-06451-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janiaud P., Axfors C., Schmitt A.M., et al. Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. JAMA. 2021;325(12):1185–1195. doi: 10.1001/jama.2021.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamilton F.W., Lee T., Arnold D.T., Lilford R., Hemming K. Is convalescent plasma futile in COVID-19? A Bayesian re-analysis of the RECOVERY randomized controlled trial. Int J Infect Dis. 2021;109:114–117. doi: 10.1016/j.ijid.2021.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gharbharan A., Jordans C.C.E., GeurtsvanKessel C., et al. Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection. Nat Commun. 2021;12(1):3189. doi: 10.1038/s41467-021-23469-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Estcourt L.J., Turgeon A.F., McQuilten Z.K., et al. Effect of convalescent plasma on organ support-free days in critically ill patients with COVID-19: a randomized clinical trial. JAMA. 2021;326(17):1690–1702. doi: 10.1001/jama.2021.18178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Devos T., Van Thillo Q., Compernolle V., et al. Early high antibody-titre convalescent plasma for hospitalised COVID-19 patients: DAWn-plasma. Eur Respir J. 2021;59:2101724. doi: 10.1183/13993003.01724-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Casadevall A., Dragotakes Q., Johnson P.W., et al. Convalescent plasma use in the USA was inversely correlated with COVID-19 mortality. Elife. 2021:10. doi: 10.7554/eLife.69866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cao H., Ming L., Chen L., Zhu X., Shi Y. The effectiveness of convalescent plasma for the treatment of novel corona virus disease 2019: a systematic review and meta-analysis. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.641429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Briggs N., Gormally M.V., Li F., et al. Early but not late convalescent plasma is associated with better survival in moderate-to-severe COVID-19. PLoS One. 2021;16(7) doi: 10.1371/journal.pone.0254453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bégin P., Callum J., Jamula E., et al. Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial. Nat Med. 2021;27(11):2012–2024. doi: 10.1038/s41591-021-01488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balcells M.E., Rojas L., Le Corre N., et al. Early versus deferred anti-SARS-CoV-2 convalescent plasma in patients admitted for COVID-19: a randomized phase II clinical trial. PLoS Med. 2021;18(3) doi: 10.1371/journal.pmed.1003415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Avendaño-Solá C., Ramos-Martínez A., Muñez-Rubio E., et al. A multicenter randomized open-label clinical trial for convalescent plasma in patients hospitalized with COVID-19 pneumonia. J Clin Invest. 2021;131(20):e152740. doi: 10.1172/JCI152740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rasheed A.M., Fatak D.F., Hashim H.A., et al. The therapeutic potential of convalescent plasma therapy on treating critically-ill COVID-19 patients residing in respiratory care units in hospitals in Baghdad, Iraq. Infez Med. 2020;28(3):357–366. [PubMed] [Google Scholar]
- 53.Liu S.T.H., Lin H.M., Baine I., et al. Convalescent plasma treatment of severe COVID-19: a propensity score-matched control study. Nat Med. 2020;26(11):1708–1713. doi: 10.1038/s41591-020-1088-9. [DOI] [PubMed] [Google Scholar]
- 54.Li L., Zhang W., Hu Y., et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324(5):460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Joyner M.J., Bruno K.A., Klassen S.A., et al. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin Proc. 2020;95(9):1888–1897. doi: 10.1016/j.mayocp.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duan K., Liu B., Li C., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Agarwal A., Mukherjee A., Kumar G., Chatterjee P., Bhatnagar T., Malhotra P. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ. 2020;371:m3939. doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Estcourt L., Callum J. Convalescent plasma for covid-19–making sense of the inconsistencies. N Engl J Med. 2022;386(18):1753–1754. doi: 10.1056/NEJMe2204332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thompson M.A., Henderson J.P., Shah P.K., et al. Association of convalescent plasma therapy with survival in patients with hematologic cancers and COVID-19. JAMA Oncol. 2021;7(8):1167–1175. doi: 10.1001/jamaoncol.2021.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nguyen F.T., van den Akker T., Lally K., et al. Transfusion reactions associated with COVID-19 convalescent plasma therapy for SARS-CoV-2. Transfusion. 2021;61(1):78–93. doi: 10.1111/trf.16177. [DOI] [PubMed] [Google Scholar]
- 61.von Meijenfeldt F.A., Thålin C., Lisman T. Sustained prothrombotic changes in convalescent patients with COVID-19. Lancet Haematol. 2021;8(7):e475. doi: 10.1016/S2352-3026(21)00146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Focosi D., Franchini M., Pirofski L.A., et al. COVID-19 convalescent plasma and clinical trials: understanding conflicting outcomes. Clin Microbiol Rev. 2022;35 doi: 10.1128/cmr.00200-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Piechotta V., Iannizzi C., Chai K.L., et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst Rev. 2021;5(5):CD013600. doi: 10.1002/14651858.CD013600.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leentjens J., van Haaps T.F., Wessels P.F., Schutgens R.E.G., Middeldorp S. COVID-19-associated coagulopathy and antithrombotic agents-lessons after 1 year. Lancet Haematol. 2021;8(7):e524–e533. doi: 10.1016/S2352-3026(21)00105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Junqueira C., Crespo Â., Ranjbar S., et al. FcγR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature. 2022;606(7914):576–584. doi: 10.1038/s41586-022-04702-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee W.S., Wheatley A.K., Kent S.J., DeKosky B.J. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat Microbiol. 2020;5(10):1185–1191. doi: 10.1038/s41564-020-00789-5. [DOI] [PubMed] [Google Scholar]
- 67.Chen L.Y.C., Hoiland R.L., Stukas S., Wellington C.L., Sekhon M.S. Confronting the controversy: interleukin-6 and the COVID-19 cytokine storm syndrome. Eur Respir J. 2020;56(4) doi: 10.1183/13993003.03006-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Del Valle D.M., Kim-Schulze S., Huang H.H., et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang J., Hao Y., Ou W., et al. Serum interleukin-6 is an indicator for severity in 901 patients with SARS-CoV-2 infection: a cohort study. J Transl Med. 2020;18(1):406. doi: 10.1186/s12967-020-02571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zizzo G., Tamburello A., Castelnovo L., et al. Immunotherapy of COVID-19: inside and beyond IL-6 signalling. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.795315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ouyang W., O'Garra A. IL-10 family cytokines IL-10 and IL-22: from basic science to clinical translation. Immunity. 2019;50(4):871–891. doi: 10.1016/j.immuni.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 72.Albini A., Calabrone L., Carlini V., et al. Preliminary evidence for IL-10-induced ACE2 mRNA expression in lung-derived and endothelial cells: implications for SARS-cov-2 ARDS pathogenesis. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.718136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bonny T.S., Patel E.U., Zhu X., et al. Cytokine and chemokine levels in coronavirus disease 2019 convalescent plasma. Open Forum Infect Dis. 2021;8(2):ofaa574. doi: 10.1093/ofid/ofaa574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brox R., Achenbach S., Hackstein H. Detection of SARS-CoV-2-independent immunoregulatory activity of COVID-19 convalescent plasma. Transfusion. 2021;61:3087–3093. doi: 10.1111/trf.16685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dupont A., Rauch A., Staessens S., et al. Vascular endothelial damage in the pathogenesis of organ injury in severe COVID-19. Arterioscler Thromb Vasc Biol. 2021;41(5):1760–1773. doi: 10.1161/ATVBAHA.120.315595. [DOI] [PubMed] [Google Scholar]
- 76.Lin C.Y., Wolf J., Brice D.C., et al. Pre-existing humoral immunity to human common cold coronaviruses negatively impacts the protective SARS-CoV-2 antibody response. Cell Host Microbe. 2022;30(1):83–96.e4. doi: 10.1016/j.chom.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Manry J., Bastard P., Gervais A., et al. The risk of COVID-19 death is much greater and age-dependent with type I IFN autoantibodies. Proc Natl Acad Sci U S A. 2022;119(21) doi: 10.1073/pnas.2200413119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bastard P., Rosen L.B., Zhang Q., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515):eabd4585. doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meisel C., Akbil B., Meyer T., et al. Mild COVID-19 despite autoantibodies against type I IFNs in autoimmune polyendocrine syndrome type 1. J Clin Invest. 2021;131(14):e150867. doi: 10.1172/JCI150867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vazquez S.E., Bastard P., Kelly K., et al. Neutralizing autoantibodies to type I interferons in COVID-19 convalescent donor plasma. J Clin Immunol. 2022;41(6):1169–1171. doi: 10.1007/s10875-021-01060-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Raadsen M.P., Gharbharan A., Jordans C.C.E., et al. Interferon-α2 auto-antibodies in convalescent plasma therapy for COVID-19. J Clin Immunol. 2022;42(2):232–239. doi: 10.1007/s10875-021-01168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van der Wijst M.G.P., Vazquez S.E., Hartoularos G.C., et al. Longitudinal single-cell epitope and RNA-sequencing reveals the immunological impact of type 1 interferon autoantibodies in critical COVID-19. bioRxiv. 2021 [Google Scholar]
- 83.Cognasse F., Hamzeh-Cognasse H. Platelet-derived immune-modulatory mediators and transfusion: time to consider their effects? Blood Transfus. 2022;20(3):177–179. doi: 10.2450/2022.0322-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cognasse F., Laradi S., Berthelot P., et al. Platelet inflammatory response to stress. Front Immunol. 2019;10:1478. doi: 10.3389/fimmu.2019.01478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.