Abstract
Background
Killed whole-cell oral cholera vaccines (OCVs) are widely used for prevention of cholera in developing countries. However, few studies have evaluated the protection conferred by internationally recommended OCVs for durations beyond 2 years of follow-up.
Methods
In this study, we followed up the participants of a cluster-randomised controlled trial for 2 years after the end of the original trial. Originally, we had randomised 90 geographical clusters in Dhaka slums in Bangladesh in equal numbers (1:1:1) to a two-dose regimen of OCV alone (targeted to people aged 1 year or older), a two-dose regimen of OCV plus a water–sanitation–hygiene (WASH) intervention, or no intervention. There was no masking of group assignment. The WASH intervention conferred little additional protection to OCV and was discontinued at 2 years of follow-up. Surveillance for severe cholera was continued for 4 years. Because of the short duration and effect of the WASH intervention, we combined the two OCV intervention groups. The primary outcomes were OCV overall protection (protection of all members of the intervention clusters) and total protection (protection of individuals who got vaccinated in the intervention clusters) against severe cholera, which we assessed by multivariable survival models appropriate for cluster-randomised trials. This trial is registered on ClinicalTrials.gov, NCT01339845.
Findings
The study was done between April 17, 2011, and Nov 1, 2015. 268 896 participants were present at the time of the first dose, with 188 206 in the intervention group and 80 690 in the control group. OCV coverage of the two groups receiving OCV was 66% (123 659 of 187 214 participants). During 4 years of follow-up, 441 first episodes of severe cholera were detected (243 episodes in the vaccinated groups and as 198 episodes in the unvaccinated group). Overall OCV protection was 36% (95% CI 19 to 49%) and total OCV protection was 46% (95% CI 32 to 58). Cumulative total vaccine protection was notably lower for people vaccinated before the age of 5 years (24%; −30 to 56) than for people vaccinated at age 5 years or older (49%; 35 to 60), although the differences in protection for the two age groups were not significant (p=0·3308). Total vaccine protection dropped notably (p=0·0115) after 3 years in children vaccinated at 1–4 years of age.
Interpretation
These findings provide further evidence of long-term effectiveness of killed whole-cell OCV, and therefore further support for the use of killed whole-cell OCVs to control endemic cholera, but indicate that protection is shorter-lived in children vaccinated before the age of 5 years than in people vaccinated at the age of 5 years or older.
Funding
Bill & Melinda Gates Foundation.
Translation
For the Bengali translation of the abstract see Supplementary Materials section.
Introduction
It is clear that provision of clean water and adequate sanitation as well as the maintenance of good personal hygiene (WASH) stops the transmission of cholera. Affluent countries have implemented these adequate levels of WASH by major investments into municipal WASH infrastructure. Unfortunately, the financial costs of such improvements are currently beyond the reach of most low-income and middle-income countries (LMICs), and cholera continues to thrive in many of these settings, accounting for a major burden of morbidity and mortality globally.1 While awaiting such improvements in poorer countries, recent efforts to prevent cholera have focused largely on delivery of low cost, oral cholera vaccines (OCVs) based on killed whole cells (WCs), which have been shown to be safe and to confer substantial protection.2 Protection data for the vaccine Shanchol, which is used in the global OCV stockpile, have shown sustained protection for 4 years post-dosing in Haiti and 5 years post-dosing in Kolkata.2, 3 Follow-up of a mass immunisation programme with a different killed WC-OCV found protection lasting for 5 years in Vietnam.4 However, similar but not identical inactivated OCVs tested in the first efficacy trial of inactivated OCVs, done in Bangladesh in 1985, revealed protection lasting only 2–3 years.5
In 2011, we initiated a large-scale, cluster-randomised trial in urban Bangladesh to assess whether Shanchol, a WHO-prequalified, killed WC-only OCV, could protect against severe cholera in densely populated slums with intense cholera transmission, and whether concomitant provision of an inexpensive WASH intervention enhanced this OCV protection.6 As reported elsewhere, at 2 years of follow-up, we observed substantial protection by the OCV, but little difference in protection with the addition of the WASH intervention.6 The WASH intervention was thereby discontinued at 2 years of follow-up, but surveillance was maintained for an additional 2 years. In this Article, we report on the findings for vaccine protection during this extended follow-up.
Research in context.
Evidence before this study
Killed whole-cell (WC) oral cholera vaccines (OCV) are internationally licensed and recommended, as well as stockpiled for use in cholera epidemics, in cholera-endemic populations, and in humanitarian emergencies. However, more evidence is needed on the long-term protection of these vaccines. We searched PubMed using the terms “oral cholera vaccine”, “Dukoral”, “Shanchol”, “whole cell inactivated cholera vaccine”, “clinical trial”, “vaccine efficacy”, and “vaccine effectiveness” to identify reports published in English between Jan 1, 1985, and April 1, 2020, investigating the protective effects of WC OCV. We identified studies done in India, Bangladesh, Haiti, and Vietnam, which reported protective effects of OCV for 4 years or longer after vaccination.
Added value of this study
To better understand the performance of killed WC OCV under programmatic conditions, we did a cluster-randomised effectiveness trial of killed WC OCV in urban slums of Dhaka, Bangladesh, where the burden of cholera disease is high. Our study found that vaccine (ie, Shanchol) protection against severe cholera was moderately high and sustained for at least 4 years in people vaccinated when aged 5 years or older, but was lower and only demonstrable for 3 years in children vaccinated at 1–4 years of age.
Implications of all the available evidence
Our findings support the programmatic usefulness of the Shanchol vaccine, but underscore the need to devise ways to improve protection of young children against cholera.
Methods
Study design and participants
This large-scale, cluster-randomised trial was done in urban slums in the Mirpur district of Dhaka City (which have a population of approximately 3 million people) in Bangladesh.7 Households with low socioeconomic status from the six wards of Mirpur (2, 4, 5, 6, 14, and 16) were selected for this study. The study protocol was approved by the Research Review Committee and the Ethical Review Committee of the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b) and the Institutional Review Board of the International Vaccine Institute. Written informed consent was obtained from individuals older than 18 years and from the parents or guardians of individuals aged 1–17 years. Additional assent was obtained from individuals aged 12–17 years.
Randomisation and masking
The 90 geographical clusters in the area in Mirpur, of approximately equal size (average population was 2988; range 2288–4299), were randomised by a computer-generated sequence into three groups (1:1:1) of 30 clusters. Of the study's three groups, one group received a two-dose regimen of the Shanchol vaccine alone (VAX group), one group a two-dose regimen of the Shanchol vaccine plus a behaviour change WASH intervention (VBCC group), and one group no intervention (control). The participants and investigators were aware of the group assignments. Each cluster was separated from the adjoining cluster by at least a 30 m buffer area, to minimise transmission of cholera and diffusion of the WASH intervention between clusters.5
Procedures
Healthy, non-pregnant individuals aged 1 year or older from the VAX and VBCC clusters were invited to receive two doses of the bivalent WC inactivated OCV, Shanchol, at least 14 days apart.6 The vaccination campaign was done between Feb 17, 2011, and April 6, 2011. The behaviour change WASH intervention (BCC) intervention in the VBCC group was delivered by a non-governmental organisation and included installation of hand washing stations, distribution of soap and chlorine tablets, and community engagement and promotion. These interventions were continued up to August 2013. Details of the interventions can be found elsewhere.9
Outcomes
The primary outcomes were OCV overall protection and total protection against severe cholera. Passive diarrhoeal disease surveillance was done by specially trained study staff at the two hospitals of the icddr,b and ten other hospitals located either inside or nearby the study area between April 17, 2011, and Nov 1, 2015. A diarrhoeal visit was defined as having three or more loose stools or one-to-two or an indeterminate number of loose stools in the past 24 h before presentation with evidence of dehydration according to WHO criteria.10 Diarrhoeal visits for which the date of onset was 7 days or less from the date of discharge for the previous visit were treated as the same episode. The onset of a diarrhoeal episode was the day on which the patient first reported loose or liquid stools before the first diarrhoeal visit of the episode. Patients from the study area were identified in the treatment centres by use of a household ID card, which they had been given for the project, and by searching the ID in an onsite computerised data system. Physicians did the clinical examination and the project staff filled the data form and obtained faecal specimens after obtaining written informed consent. Faecal specimens were tested for Vibrio cholerae O1 and O139 serogroups and Inaba and Ogawa serotypes using conventional methods.6 Biotype was ascertained for all isolates.11 Severe cholera was defined as a diarrhoeal episode in which V cholerae 01 or 0139 was isolated and severe dehydration, defined elsewhere,6 was detected in at least one constituent diarrhoeal visit of the episode.
Statistical analysis
For several reasons, we decided to merge the two vaccine groups of the trial for this analysis. First, our interest was in extended vaccine protection over the 4-year period of cholera surveillance after vaccination indicated in the study protocol, and the high migration rate of this population substantially reduced the baseline population still under follow-up during the 3rd and 4th years. Second, vaccine protection for the two vaccine groups was similar during the first 2 years of follow-up. Third, the BCC WASH intervention was stopped after 2 years and was not found to confer significant additional protection against severe cholera.6
The start date for follow-up for analyses of vaccine protection was the date of the second dose for recipients of the second dose or the median date of the second dose in the cluster for non-recipients of the second dose in intervention clusters; for control clusters, the median date of the second dose in the nearest cluster of the intervention group was used. We evaluated overall vaccine effectiveness12 by comparing the occurrence of first episodes of severely dehydrating cholera during the 4-year post-vaccination period among all individuals present at zero time in the two intervention groups combined versus the control group. Zero time was defined as the date of dose one for vaccine recipients, and the median date of dose one in a manner analogous to the start date of follow-up. To evaluate total vaccine effectiveness, we compared the occurrence of first episodes of severely dehydrating cholera in recipients of a two-dose regimen of OCV in the combined intervention groups and age-eligible individuals (≥1 year) present at the start of follow-up in the control group. For both types of protection, we analysed only severely dehydrating cholera episodes whose onsets were at least 14 days after the second dose for two-dose OCV recipients, and 14 days after the median date of the second dose for others in the OCV clusters. In the control clusters, the start date of follow-up was 14 days after the median date of the second dose in the nearest intervention cluster.
Survival analyses were done censoring individuals who died or migrated out before the end of the follow-up period. In descriptive analyses, we fitted Kaplan-Meier curves. We then fitted unadjusted and adjusted Cox proportional hazards regression models after verifying that the proportionality assumptions were fulfilled for all independent variables.13, 14, 15 We then estimated the hazard ratios by exponentiating the coefficient of the group variable in these models. Vaccine protective effectiveness was calculated as (1–hazard ratio) x 100%. Robust sandwich variance estimates were used to account for the design effect of cluster randomisation, allowing inferences for vaccine protection at the individual level.16 The baseline variables that were found to be associated with time to event at p less than 0·10 in bivariate models were considered in backward-elimination models, retaining those variables that remained significant at p less than 0·10 for the final models. The stratification variable (distance to the hospitals) for randomisation was also included in the models for adjustment regardless of its level of statistical significance. We also analysed vaccine protection by age group at zero time (0·0–4·9 years and ≥5·0 years) and by year of follow-up. Heterogeneity of vaccine protection in these subgroups was assessed by analysing two-way interaction terms between the assigned group and subgroup variables in the models. The sample size for this cluster-randomised trial was calculated with the assumption of 80% power to detect vaccine total protection of 65%, a two-tailed p value smaller than 0·05, and an intra-cluster correlation coefficient (ICC) of 0·00014. The threshold of significance for individual estimates of protective effectiveness was p less than 0·05 with corresponding 95% CI (two-sided). All statistical analyses were done using SAS version 9.4. The study is registered at ClinicalTrials.gov number, NCT01339845.
Role of the funding source
The project was funded by the Bill & Melinda Gates Foundation, which provided inputs to the design and planning of the study. The funder had no role in data collection, data analysis, data interpretation, or writing of the report.
Results
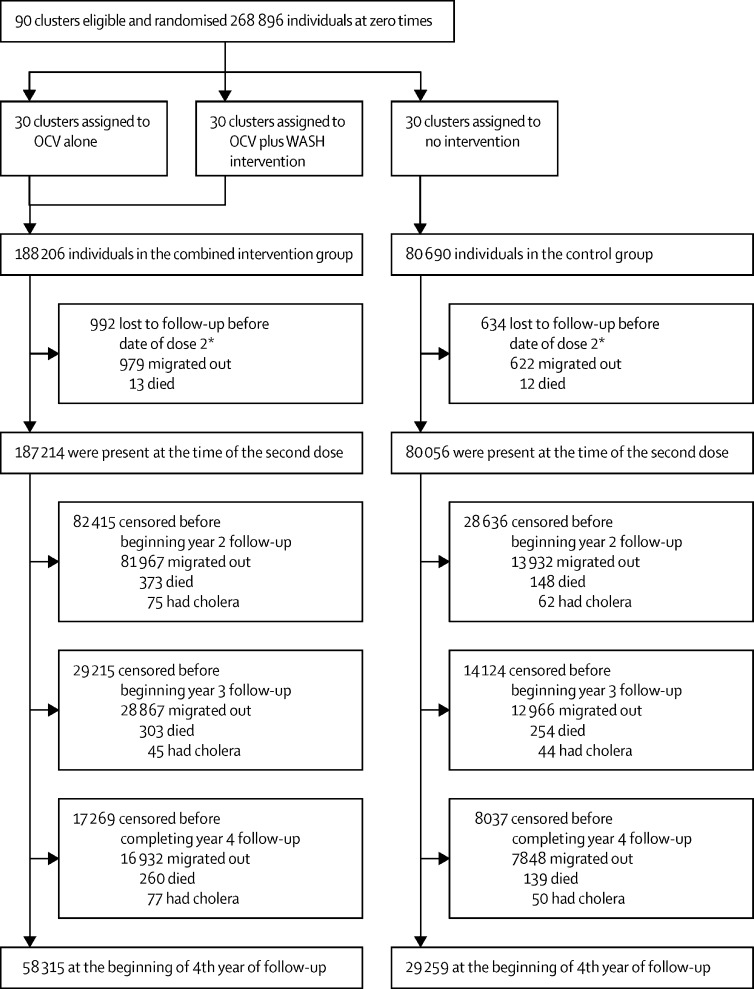
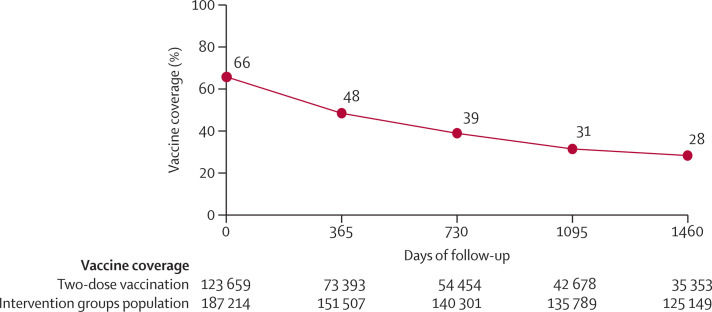
In the 90 clusters, there were 268 896 individuals at zero time and 267 270 individuals at the start of follow-up, as described in the methods. The coverage of the OCV (two-dose recipients) in the two intervention groups was 66% (123 659 of 187 214 individuals). Of the 267 270 residents at the start of follow-up, 179 696 (67%) migrated out or died before completion of the 4 years of follow-up (figure 1 ). Figure 2 shows that these migration patterns resulted in a decline of vaccine coverage to 28% (35 353 of 125 149 participants) by the end of the 4-year follow-up. Individual-level and cluster-level baseline characteristics, measured at baseline, were well balanced between the different groups of the study at zero time, as well as at the beginning of the 2nd, 3rd, and 4th years of follow-up (appendix 2 pp 3–10).
Figure 1.
Trial profile
OCV=oral cholera vaccine. WASH=water–sanitation–hygiene. *The date of dose 2 for the two-dose recipients or the median date of dose 2 of the cycle of vaccination for no-dose or one-dose recipients.
Figure 2.
Trend of two-dose oral cholera vaccine coverage (%) over 4 years of follow-up post-vaccination*
*In the calculation of vaccine coverage, the numerator was all participants who received two-dose vaccines and who were under follow-up at each time point noted on the x-axis. Days in the x-axis begin with the time of the second dose for the intervention clusters.
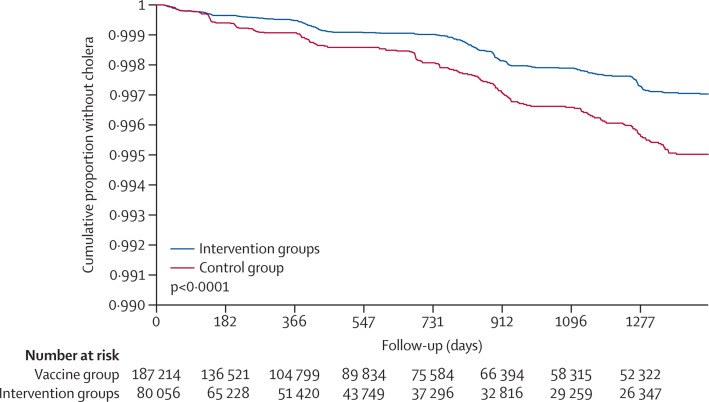
For the analysis of overall vaccine effectiveness, we detected 441 first episodes of cholera with severe dehydration during the 4 years of follow-up, which were observed as 243 episodes in the vaccinated groups and as 198 episodes in the unvaccinated group. All cases were V cholerae O1 El Tor biotype; only six isolates were Inaba serotype. Figure 3 shows that event-free survival curves in the analysis of overall vaccine effectiveness continued to diverge with time of follow-up (p<0·0001, favouring the vaccine group). Cumulative 4-year overall protective effectiveness, after adjusting for covariates, was 36% (95% CI 19 to 49; p=0·0002; table 1 ). Point estimates of overall vaccine effectiveness were similar for people younger than 5 years (30%; 95% CI −8 to 55) and for people aged 5 years or older (36%; 95% CI 19 to 50; p=0·8128) at zero time in the intervention clusters. Analysis of overall effectiveness by year of follow-up revealed adjusted estimates that varied between 21% (95% CI −18 to 47) and 46% (95% CI 12 to 67), with no apparent decline with time (p=0·4050 for heterogeneity of the yearly estimates).
Figure 3.
Kaplan-Meier plot
The plot shows the estimates of the cumulative risk of not having cholera with severe dehydration among the entire population at zero time during 4 years of follow-up post-vaccination (overall vaccine efficacy analysis). The p value (p<0·0001) is two-tailed and calculated by the log-rank test. Note that, for demonstration purposes, the y axis of this plot goes from 0·990 to 1, not from 0 to 1. Days on the x-axis begin with the time of the second dose (ie, the start of follow-up), as described in the text.
Table 1.
Incidence of severe cholera and cumulative overall protection by the killed whole-cell OCV during 4 years of follow-up
|
Intervention groups |
Control group |
Overall effectiveness* |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Cholera episodes; person-days of follow-up | Incidence per 100 000 person-days of follow-up (95% CI) | N | Cholera episodes; person-days of follow-up | Incidence per 100 000 person-days of follow-up (95% CI) | Crude estimate (95% CI) | Adjusted estimate (95% CI) | p value | |
| Participants | |||||||||
| All participants | 187 214 | 243; 125 913 808 | 0·19 (0·17 to 0·22) | 80 056 | 198; 61 010 881 | 0·32 (0·28 to 0·37) | 40% (23 to 54) | 36% (19 to 49)† | 0·0002 |
| Participants aged <5 years | 18 693 | 35; 11 938 147 | 0·29 (0·21 to 0·41) | 8081 | 28; 5 925 727 | 0·47 (0·33 to 0·68) | 38% (4 to 60) | 30% (−8 to 55)‡ | 0·8128§ |
| Participants aged ≥5 years | 168 521 | 208; 113 975 661 | 0·18 (0·16 to 0·21) | 71 975 | 170; 55 085 154 | 0·31 (0·27 to 0·36) | 41% (23 to 54) | 36% (19 to 50)¶ | .. |
| Year of follow-up | |||||||||
| 1st year | 187 214 | 75; 49 865 475 | 0·15 (0·12 to 0·19) | 80 056 | 62; 23 478 438 | 0·26 (0·21 to 0·34) | 43% (10 to 64) | 38% (5 to 60)‖ | 0·4050§ |
| 2nd year | 104 799 | 45; 32 589 219 | 0·14 (0·1 to 0·18) | 51 420 | 44; 15 885 613 | 0·28 (0·21 to 0·37) | 50% (15 to 71) | 46% (12 to 67)** | .. |
| 3rd year | 75 584 | 77; 24 336 059 | 0·32 (0·25 to 0·4) | 37 296 | 50; 12 027 110 | 0·42 (0·32 to 0·55) | 24% (−14 to 49) | 21% (−18 to 47)†† | .. |
| 4th year | 58 315 | 46; 19 123 055 | 0·24 (0·18 to 0·32) | 29 259 | 42; 9 619 720 | 0·44 (0·32 to 0·59) | 45% (13 to 65) | 43% (11 to 64)‡‡ | .. |
Two-tailed 95% CIs and p values are given for the analyses.
Adjusted for age at zero time (years), sex (male), individuals having reported diarrhoea within 48 h at the time of household registration, individuals living in a household using safe water source (household tap), individuals living in a household having only one room, individuals living in a household sharing water source with others, individuals living in a household using treated water (boiled, filtered, or chemical treatment), individuals living in a household knowing about cholera vaccine, closer distance from the household to the nearest ICDDR,B hospital, and percentage of individuals living in their own house in the cluster.
Adjusted for individuals living in a household having specific place for waste disposal, individuals living in a household having only one room, individuals living in a household that knows about cholera vaccine, closer distance from the household to the nearest ICDDR,B hospital, and percentage of individuals living in their own house in the cluster.
Calculated for overall interaction terms between the assigned group and subgroup variables in the model.
Adjusted for age at zero time (years), sex (male), individuals having reported diarrhoea within 48 h at the time of household registration, individuals living in a household using safe water source (household tap), individuals living in a household having only one room, individuals living in a household sharing water source with others, individuals living in a household using treated water (boiled, filtered, or chemical treatment), closer distance from the household to the nearest ICDDR,B hospital, and percentage of individuals living in their own house in the cluster.
Adjusted for age at zero time (years), individuals having reported diarrhoea within 6 months at the time of household registration, individuals living in a household using safe water source (household tap), individuals living in a household having only one room, individuals living in a household that knows about cholera vaccine, closer distance from the household to the nearest ICDDR,B hospital, percentage of individuals living in their own house in the cluster, and percentage of individuals using sanitary toilet in the cluster.
Adjusted for age at zero time (years), sex (male), individuals having reported diarrhoea within 48 h at the time of household registration, individuals living in a household using safe water source (household tap), individuals living in a household sharing water source with others, individuals living in a household using treated water (boiled, filtered, or chemical treatment), individuals living in a household that knows about cholera vaccine, and closer distance from the household to the nearest ICDDR,B hospital.
Adjusted for age at zero time (years), individuals having reported diarrhoea within 6 months at the time of household registration, individuals living in study area for less than 1 year, individuals living in a household sharing water source with others, individuals living in a household using treated water (boiled, filtered, or chemical treatment), individuals living in a household that knows about cholera vaccine, closer distance from the household to the nearest ICDDR,B hospital, and percentage of individuals using sanitary toilet in the cluster.
Adjusted for age at zero time (years), individuals living in a household having only one room, individuals living in a household using treated water (boiled, filtered, or chemical treatment), and closer distance from the household to the nearest ICDDR,B hospital.
202 177 individuals were included in the analyses for assessing total protective effectiveness (appendix 2 p 1). Similar to the analysis of overall vaccine protection, in this analysis the two compared groups were well balanced with respect to baseline characteristics during each of the 4 years of follow-up (appendix 2 pp 11–18). For this analysis, there were 140 episodes of severely dehydrating cholera in the vaccinated groups and 192 episodes of severely dehydrating cholera in the control group. The event-free survival curves for patients assigned to the different groups of the study for evaluating total effectiveness are shown in the appendix 2 (p 2). The curves, which differed significantly (p<0·0001), continued to diverge over the entire period of follow-up. As shown in table 2 , the adjusted value for cumulative total vaccine protection over the 4 years was 46% (95% CI 32 to 58; p<0·0001). Adjusted, cumulative total vaccine protection was notably lower for people vaccinated before the age of 5 years (24%, 95% CI −30 to 56) than for people vaccinated aged 5 years or older (49%, 95% CI 35 to 60), although the differences in protection for the two age groups were not significant (p=0·3308). Adjusted total vaccine protection for all age groups combined varied by year of follow-up, from 25% to 68%, but point estimates suggested no trend with time, and the differences in these yearly values were not significant (p=0·1159). When adjusted total vaccine protection was examined by year of follow-up within age groups (table 3 ), there was significant variation of protection among children vaccinated before the age of 5 years (p=0·0115), attributable in part to a notable drop in the 4th year of follow-up. By contrast, adjusted total vaccine protection in people vaccinated aged 5 years or older showed no decline during the follow-up. Finally, as expected, there was little difference in total cumulative protective effectiveness for the group receiving OCV only versus the group receiving OCV and WASH (total cumulative protection 42%, 21–57, for the vaccine only group and 51%, 36–63, for the vaccine plus WASH group).
Table 2.
Incidence of severe cholera and cumulative total vaccine protection by the killed OCV during the 4 years of follow-up
|
Intervention groups |
Control group |
Total effectiveness* |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Cholera episodes; person-days of follow-up | Incidence per 100 000 person-days of follow-up (95% CI) | N | Cholera episodes; person-days of follow-up | Incidence per 100 000 person-days of follow-up (95% CI) | Crude estimate (95% CI) | Adjusted estimate (95% CI) | p value | |
| Participants | |||||||||
| All participants | 123 659 | 140; 88 958 822 | 0·16 (0·13 to 0·19) | 78 518 | 192; 59 875 658 | 0·32 (0·28 to 0·37) | 51% (36 to 62) | 46% (32 to 58)† | <0·0001 |
| Participants vaccinated aged <5 years | 12 371 | 24; 8 358 297 | 0·29 (0·19 to 0·43) | 6543 | 22; 4 790 504 | 0·46 (0·3 to 0·7) | 37% (−9 to 64) | 24% (−30 to 56)‡ | 0·3308§ |
| Participants vaccinated aged ≥5 years | 111 288 | 116; 80 600 525 | 0·14 (0·12 to 0·17) | 71 975 | 170; 55 085 154 | 0·31 (0·27 to 0·36) | 53% (39 to 64) | 49% (35 to 60)¶ | .. |
| Year of follow-up | |||||||||
| 1st year | 123 659 | 44; 33 980 854 | 0·13 (0·1 to 0·17) | 78 518 | 61; 23 025 351 | 0·26 (0·21 to 0·34) | 51% (22 to 70) | 47% (17 to 66)‖ | 0·1159§ |
| 2nd year | 73 373 | 20; 23 188 785 | 0·09 (0·06 to 0·13) | 50 443 | 44; 15 590 105 | 0·28 (0·21 to 0·38) | 69% (43 to 83) | 68% (42 to 82)** | .. |
| 3rd year | 54 454 | 46; 17 682 090 | 0·26 (0·19 to 0·35) | 36 616 | 45; 11 810 799 | 0·38 (0·28 to 0·51) | 32% (−4 to 55) | 25% (−13 to 51)†† | .. |
| 4th year | 42 675 | 30; 14 107 093 | 0·21 (0·15 to 0·3) | 28 734 | 42; 9 449 403 | 0·44 (0·33 to 0·6) | 52% (23 to 70) | 48% (16 to 67)‡‡ | .. |
ICDDR,B=International Centre for Diarrhoeal Disease Research, Bangladesh.
Two-tailed 95% CIs and p values are given for the analyses.
Adjusted for age at zero time (years), individuals having reported diarrhoea within 6 months at the time of household registration, individuals living in a household using safe water source (household tap), individuals living in a household having only one room, individuals living in a household sharing water source with others, individuals living in a household using treated water (boiled, filtered, or chemical treatment), closer distance from the household to the nearest ICDDR,B hospital, percentage of individuals living in their own house in the cluster.
Adjusted for individuals living in study area for less than 1 year, individuals living in a household having specific place for waste disposal, closer distance from the household to the nearest ICDDR,B hospital, percentage of individuals living in their own house in the cluster, and percentage of individuals using sanitary toilet in the cluster.
Calculated for overall interaction terms between the assigned group and subgroup variables in the model.
Adjusted for age at zero time (years), individuals having reported diarrhoea within 48 h at the time of household registration, individuals living in a household using safe water source (household tap), individuals living in a household having only one room, individuals living in a household sharing water source with others, individuals living in a household using treated water (boiled, filtered, or chemical treatment), closer distance from the household to the nearest ICDDR,B hospital, and percentage of individuals living in their own house in the cluster.
Adjusted for age at zero time (years), individuals having reported diarrhoea within 6 months at the time of household registration, individuals living in a household having only one room, closer distance from the household to the nearest ICDDR,B hospital, and percentage of individuals living in their own house in the cluster.
Adjusted for individuals having reported diarrhoea within 48 h at the time of household registration, individuals living in study area less than 1 year, individuals living in their own house, individuals living in a household using treated water (boiled, filtered, or chemical treatment), and closer distance from the household to the nearest ICDDR,B hospital.
Adjusted for age at zero time (years), individuals living in study area for less than 1 year, individuals living in a household having specific place for waste disposal, individuals living in a household using treated water (boiled, filtered, or chemical treatment), closer distance from the household to the nearest ICDDR,B hospital, and percentage of individuals using sanitary toilet in the cluster.
Adjusted for age at zero time (years), individuals having reported diarrhoea within 48 h at the time of household registration, individuals living in a household having only one room, individuals living in their own house, individuals living in a household sharing kitchen with others, individuals living in a household using treated water (boiled, filtered, or chemical treatment), closer distance from the household to the nearest ICDDR,B hospital, and percentage of individuals living in their own house in the cluster.
Table 3.
Incidence of severe cholera and cumulative total vaccine protection by the killed OCV by follow-up period and age at vaccination
|
Intervention groups |
Control group |
Total effectiveness* |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Cholera episodes; person-days of follow-up | Incidence per 100 000 person-days of follow-up (95% CI) | N | Cholera episodes; person-days of follow-up | Incidence per 100 000 person-days of follow-up (95% CI) | Crude estimate (95% CI) | Adjusted estimate (95% CI) | p value | |
| Among participants vaccinated aged <5 years | |||||||||
| 1st year | 12 371 | 12; 3 271 192 | 0·37 (0·21 to 0·65) | 6543 | 7; 1 879 013 | 0·37 (0·18 to 0·78) | 2 (−158 to 63) | 2 (−158 to 63)† | 0·0115‡ |
| 2nd year | 6862 | 3; 2 163 197 | 0·14 (0·04 to 0·43) | 4032 | 10; 1 229 466 | 0·81 (0·44 to 1·51) | 83 (38 to 95) | 83 (38 to 95)† | .. |
| 3rd year | 5033 | 2; 1 630 257 | 0·12 (0·03 to 0·49) | 2882 | 4; 935 564 | 0·43 (0·16 to 1·14) | 71 (−45 to 94) | 71 (−45 to 94)† | .. |
| 4th year | 3920 | 7; 1 293 651 | 0·54 (0·26 to 1·14) | 2265 | 1; 746 461 | 0·13 (0·02 to 0·95) | −305 (−3000 to 47) | −305 (−3000 to 47)† | .. |
| Among participants vaccinated aged ≥5 years | |||||||||
| 1st year | 111 288 | 32; 30 709 662 | 0·10 (0·07 to 0·15) | 71 975 | 54; 21 146 338 | 0·26 (0·2 to 0·33) | 59 (33 to 75) | 55 (29 to 72)§ | 0·0963‡ |
| 2nd year | 66 511 | 17; 21 025 588 | 0·08 (0·05 to 0·13) | 46 411 | 34; 14 360 639 | 0·24 (0·17 to 0·33) | 66 (30 to 83) | 64 (29 to 82)¶ | .. |
| 3rd year | 49 421 | 44; 16 051 833 | 0·27 (0·2 to 0·37) | 33 734 | 41; 10 875 235 | 0·38 (0·28 to 0·51) | 27 (−11 to 53) | 23 (−16 to 49)‖ | .. |
| 4th year | 38 755 | 23; 12 813 442 | 0·18 (0·12 to 0·27) | 26 469 | 41; 8 702 942 | 0·47 (0·35 to 0·64) | 62 (37 to 77) | 59 (32 to 75)** | .. |
ICDDR,B=International Centre for Diarrhoeal Disease Research, Bangladesh.
Two-tailed 95% CIs are given for the analyses.
The number of outcomes was insufficient for the adjusted model.
Calculated for overall interaction terms between the assigned group and subgroup variables in the model.
Adjusted for age at zero time (years), individuals having reported diarrhoea within 6 months at the time of household registration, individuals living in a household having only one room, closer distance from the household to the nearest ICDDR,B hospital, percentage of individuals living in their own house in the cluster, and percentage of individuals using sanitary toilet in the cluster.
Adjusted for age at zero time (years), individuals having reported diarrhoea within 6 months at the time of household registration, individuals living in study area for less than 1 year, individuals living in a household sharing water source with others, and closer distance from the household to the nearest ICDDR,B hospital.
Adjusted for age at zero time (years), individuals living in study area for less than 1 year, individuals living in a household using treated water (boiled, filtered, or chemical treatment), closer distance from the household to the nearest ICDDR,B hospital, and percentage of individuals using sanitary toilet in the cluster.
Adjusted for age at zero time (years), individuals living in a household having only one room, individuals living in a household sharing kitchen with others, individuals living in a household using treated water (boiled, filtered, or chemical treatment), and closer distance from the household to the nearest ICDDR,B hospital.
Discussion
The findings of our study show that a moderate level of both overall and total OCV protection against severe cholera was sustained over the 4 years of follow-up for the study population in this trial. For reasons mentioned in the methods section, we combined the two vaccinated groups of the trial for this analysis, but in fact there was little difference in vaccine protection between the two groups. Point estimates for overall vaccine protection were similar for people younger than 5 years and for people aged 5 years or older. By contrast, point estimates for total vaccine protection were notably lower for young children than for older people, although the difference did not reach statistical significance. Both types of vaccine protection remained stable over the entire 4-year period of follow-up for the entire study population, although a marked decline in total vaccine protection in the 4th year was evident in children vaccinated before the age of 5 years.
Before discussing the implications of these findings, it is important to address several limitations of the study. First, this trial was not a blinded study. However, the similarity of our estimates of total vaccine protection to the estimates for protective effectiveness in a randomised, blinded, placebo-controlled trial of Shanchol in Kolkata, India, is reassuring.2 Second, 67% of the baseline population died or migrated out during the 4-year follow-up period. Fortunately, the losses to follow-up were unlikely to have biased our estimates because they were quantitatively similar in magnitude in the intervention and control groups of the study, and the groups remained qualitatively similar in respect to demographical and clinical predictors of cholera throughout the follow-up period. However, the losses did diminish the statistical power of the study, particularly in the last years of the study. Third, we did not use a comparator agent for OCV in the control group. Although this absence would not be expected to bias our analyses of overall vaccine protection, analyses of total vaccine protection were based on the comparison of people who accepted the vaccine in the intervention group versus all people in the control group, including people who would have refused a control agent had it been offered. Because people who refuse a control agent might be at higher risk of cholera than people who do not refuse a control agent, inclusion of these people might have resulted in an overestimation of total vaccine protection.17 Fourth, and conversely, the design of our trial, which enrolled clusters of people in the middle of a densely populated slum population, permitted transmission of cholera from outside the clusters into the clusters. Such transmission has been shown in this trial to depress estimates of vaccine protection that include vaccine herd effects, such as total and overall vaccine protection.18 Fifth, because our analysis included only people present at baseline, overall OCV protection during each year of follow-up should be interpreted as an overall protection of those individuals in the vaccinated clusters who were present at baseline and who were followed up, rather than an overall protection of the entire population of the vaccinated clusters during follow-up. Finally, because our study was done in an endemic population that is regularly exposed to cholera, which might have acted to provide immunological boosting to people receiving the vaccine during post-vaccination follow-up, our results might not generalise to populations without endemic cholera.
Our findings support the findings of an earlier trial of Shanchol in Kolkata, India, which showed that total vaccine protection was long-lived (at least 5 years in that trial) in people vaccinated aged 5 years or older, but shorter lived in children vaccinated aged 1–4 years.2 Indeed, point estimates, by year of follow-up, of protective efficacy in per-protocol analyses of the Kolkata trial and of total vaccine protection in the present analyses are very similar, except for the 3rd year of follow-up, in which protective efficacy in the Kolkata trial was 66% (95% CI 40% to 81%) whereas total protection was 25% (95% CI −13% to 51%) in the present analysis. Analyses of total vaccine protection in individuals younger than 5 years in the current trial and of direct protection of individuals younger than 5 years in the Kolkata trial both gave point estimates suggesting protection in the 3rd year of follow-up, although the point estimates were not significant in either trial. Even if the results do not provide absolute certainty about optimal intervals before vaccine boosting of children vaccinated before the age of 5 years, the data of these two trials suggest that, for populations with endemic cholera, boosting after 3 years of follow-up with Shanchol is rational for individuals younger than 5 years, whereas boosting at a longer time interval might be needed for individuals aged 5 years or older.
In aggregate, these findings indicate that the protection conferred by a two-dose regimen of Shanchol is considerable, even in densely populated, south Asian urban slums in which the force of cholera infection is high. A global OCV stockpile funded by Gavi and coordinated by WHO contains Shanchol and an identical OCV marketed as Euvichol. As of the end of 2018, more than 55 million doses had been deployed to over 100 vaccine campaigns in 22 developing countries.19 Our findings are consistent with analyses of several of these deployments indicating that vaccine protection in diverse settings was remarkably consistent with those of the Kolkata trial.20 That said, the lower level and shorter duration of vaccine protection observed in young children in both the Kolkata and Dhaka trials indicates that research is needed to improve vaccine protection against cholera in this age group. Although analyses of the first 2 years of follow-up of the present trial did not show that the WASH intervention tested in this trial significantly improved OCV protection, and although a meta-analysis of trials of WASH interventions against cholera did not identify interventions with major protective effects,21 it seems plausible that more effective WASH interventions implementable with the limited resources of low-income countries might protect against cholera and complement the protection conferred by OCVs.
Data sharing
Data used for the analyses in this paper will be made available to all interested researchers following the published policies of the International Centre for Diarrhoeal Disease Research, Bangladesh and the International Vaccine Institute.
Declaration of interests
We declare no competing interests.
Contributors
JDC, MA, and FQ conceptualised the analytic approaches. JDC and MA wrote the Article. DRK and FA did the statistical analysis and created the figures. KZ, AIK, MTI, JI, FM, and JHK reviewed the Article. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. MA, FA, and DRK accessed and verified the data.
Supplementary Materials
References
- 1.Ali M, Nelson AR, Lopez AL, Sack DA. Updated global burden of cholera in endemic countries. PLoS Negl Trop Dis. 2015;9 doi: 10.1371/journal.pntd.0003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattacharya SK, Sur D, Ali M, et al. 5 year efficacy of a bivalent killed whole-cell oral cholera vaccine in Kolkata, India: a cluster-randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2013;13:1050–1056. doi: 10.1016/S1473-3099(13)70273-1. [DOI] [PubMed] [Google Scholar]
- 3.Franke MF, Ternier R, Jerome JG, Matias WR, Harris JB, Ivers LC. Long-term effectiveness of one and two doses of a killed, bivalent, whole-cell oral cholera vaccine in Haiti: an extended case-control study. Lancet Glob Health. 2018;6:e1028–e1035. doi: 10.1016/S2214-109X(18)30284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thiem VD, Deen JL, von Seidlein L, et al. Long-term effectiveness against cholera of oral killed whole-cell vaccine produced in Vietnam. Vaccine. 2006;24:4297–4303. doi: 10.1016/j.vaccine.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Clemens JD, Sack DA, Harris JR, et al. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet. 1990;335:270–273. doi: 10.1016/0140-6736(90)90080-o. [DOI] [PubMed] [Google Scholar]
- 6.Qadri F, Ali M, Chowdhury F, et al. Feasibility and effectiveness of oral cholera vaccine in an urban endemic setting in Bangladesh: a cluster randomised open-label trial. Lancet. 2015;386:1362–1371. doi: 10.1016/S0140-6736(15)61140-0. [DOI] [PubMed] [Google Scholar]
- 7.Khan IA, Saha A, Chowdhury F, et al. Coverage and cost of a large oral cholera vaccination program in a high-risk cholera endemic urban population in Dhaka, Bangladesh. Vaccine. 2013;31:6058–6064. doi: 10.1016/j.vaccine.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 9.Najnin N, Leder K, Qadri F, et al. Impact of adding hand-washing and water disinfection promotion to oral cholera vaccination on diarrhoea-associated hospitalization in Dhaka, Bangladesh: evidence from a cluster randomized control trial. Int J Epidemiol. 2017;46:2056–2066. doi: 10.1093/ije/dyx187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO The treatment of diarrhoea: a manual for physicians and other senior health workers (4th revision) https://apps.who.int/iris/bitstream/handle/10665/43209/9241593180.pdf?sequence=1&isAllowed=y
- 11.Ansaruzzaman M, Bhuiyan NA, Nair BG, et al. Cholera in Mozambique, variant of Vibrio cholerae. Emerg Infect Dis. 2004;10:2057–2059. doi: 10.3201/eid1011.040682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halloran ME, Struchiner CJ, Longini IM., Jr Study designs for evaluating different efficacy and effectiveness aspects of vaccines. Am J Epidemiol. 1997;146:789–803. doi: 10.1093/oxfordjournals.aje.a009196. [DOI] [PubMed] [Google Scholar]
- 13.Lin DY, Wei L-J, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80:557–572. [Google Scholar]
- 14.Lin DY, Wei L-J. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–1078. [Google Scholar]
- 15.Reid N, Cracpeau H. Influence functions for proportional hazards regression. Biometrika. 1985;72:1–9. [Google Scholar]
- 16.Lee EW, Wei LJ, Amato DA, Leurgans S. In: Survival analysis: state of the art. Klein JP, Goel PK, editors. Springer; Berlin: 1992. Cox-type regression analysis for large numbers of small groups of correlated failure time observations; pp. 237–247. [Google Scholar]
- 17.Clemens JD, van Loon FF, Rao M, et al. Nonparticipation as a determinant of adverse health outcomes in a field trial of oral cholera vaccines. Am J Epidemiol. 1992;135:865–874. doi: 10.1093/oxfordjournals.aje.a116382. [DOI] [PubMed] [Google Scholar]
- 18.Ali M, Qadri F, Kim DR, et al. Unmasking herd protection by an oral cholera vaccine in a cluster-randomized trial. Int J Epidemiol. 2019;48:1252–1261. doi: 10.1093/ije/dyz060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pezzoli L. Global oral cholera vaccine use, 2013–2018. Vaccine. 2020;38(suppl 1):A132–A140. doi: 10.1016/j.vaccine.2019.08.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin S, Lopez AL, Bellos A, et al. Post-licensure deployment of oral cholera vaccines: a systematic review. Bull World Health Organ. 2014;92:881–893. doi: 10.2471/BLT.14.139949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor DL, Kahawita TM, Cairncross S, Ensink JH. The impact of water, sanitation and hygiene interventions to control cholera: a systematic review. PLoS One. 2015;10 doi: 10.1371/journal.pone.0135676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Uncited References
- 8.Clemens J, Shin S, Ali M. New approaches to the assessment of vaccine herd protection in clinical trials. Lancet Infect Dis. 2011;11:482–487. doi: 10.1016/S1473-3099(10)70318-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used for the analyses in this paper will be made available to all interested researchers following the published policies of the International Centre for Diarrhoeal Disease Research, Bangladesh and the International Vaccine Institute.