Abstract
Mutations in mitochondrial DNA (mtDNA) have been reported in cancer and are involved in the pathogenesis of many mitochondrial diseases. Uracil-DNA glycosylase, encoded by the UNG1 gene in Saccharomyces cerevisiae, repairs uracil in DNA formed due to deamination of cytosine. Our study demonstrates that inactivation of the UNG1 gene leads to at least a 3-fold increased frequency of mutations in mtDNA compared with the wild-type. Using a Ung1p–green fluorescent protein (GFP) fusion construct, we demonstrate that yeast yUng1–GFP protein localizes to both mitochondria and the nucleus, indicating that Ung1p must contain both a mitochondrial localization signal (MLS) and a nuclear localization signal. Our study reveals that the first 16 amino acids at the N-terminus contain the yUng1p MLS. Deletion of 16 amino acids resulted in the yUng1p–GFP fusion protein being transported to the nucleus. We also investigated the intracellular localization of human hUng1p–GFP in yeast. Our data indicate that hUng1p–GFP predominately localizes to the mitochondria. Further analysis identified the N-terminal 16 amino acids as important for localization of hUng1 protein into the mitochondria. Expression of both yeast and human UNG1 cDNA suppressed the frequency of mitochondrial mutation in UNG1-deficient cells. However, expression of yUNG1 in wild-type cells increased the frequency of mutations in mtDNA, suggesting that elevated expression of Ung1p is mutagenic. An increase in the frequency of mitochondrial mutants was also observed when hUNG1 site-directed mutants (Y147C and Y147S) were expressed in mitochondria. Our study suggests that deamination of cytosine is a frequent event in S.cerevisiae mitochondria and both yeast and human Ung1p repairs deaminated cytosine in mitochondria.
INTRODUCTION
Mitochondrial dysfunction is increasingly recognized as an important cause of adult and childhood human pathology. Mitochondrial dysfunction has been found in adult diseases as diverse as cancer, migraine, infertility, diabetes, heart disease, blindness, deafness, kidney disease, liver disease, stroke and the toxicity of HIV drugs. Mitochondrial dysfunction is also associated with aging and neurodegenerative diseases such as Parkinson’s and Alzheimer’s dementia. Thus mitochondria appear to be central players in many diseases that can affect any organ, at any age (1,2). Many different mutations of mitochondrial DNA (mtDNA) have been found to be pathogenic in humans. Individuals with childhood mitochondrial diseases either inherit their mutation from the mother or experience a mutation during oogenesis or early in embryogenesis. Several general classes of mutations in mtDNA have been reported. These include point mutations, deletions, duplications, insertions and rearrangements. To date over 100 pathogenic point mutations and 200 deletions, insertions and rearrangements have been reported (3–5).
Mutation in mtDNA can be produced by many cellular processes, such as replication and repair errors (20), spontaneous depurination or deamination and by endogenous and environmental mutagens (6). Spontaneous deamination of cytosine in DNA results in the formation of uracil. Uracil in DNA may also arise by misincorporation of dUMP opposite adenine during DNA replication. If uracil is not repaired, it can lead to a GC→AT transition mutation during replication. The UNG1 gene encodes the major uracil-DNA glycosylase (UDG) that excises uracil from DNA. Bacterial, yeast and mouse cells deficient in Ung1 protein (Ung1p) exhibit increased levels of spontaneous mutation frequencies in the nuclear genome (6–10). Human cells contain two isoforms of UDG protein; one localizes to the nucleus and the other to the mitochondria. These isoforms are generated by transcription from different promoters and alternative splicing of the mRNA (11). Interestingly, mouse cells also contain a nuclear and mitochondrial isoform of the Udg protein (12). These studies indicate that Udg present in mitochondria must repair uracil in mtDNA. Although a Udg-deficient mouse has been generated (10), to date it is not clear whether the Ung1p-deficient mouse accumulates mutations in mtDNA. Furthermore, a previous study showed that the ung1 yeast null mutant shows a mutator phenotype in nuclear DNA but not in mtDNA (9).
In this paper we demonstrate that Ung1p localizes to yeast mitochondria (and to the nucleus) and that inactivation of the UNG1 gene leads to an increased frequency of mutations in mtDNA. We also describe the functional role of human Ung1p in yeast mitochondria. Our study also reveals that mutant hUng1p causes mutations in mtDNA when expressed in yeast mitochondria.
MATERIALS AND METHODS
Strains, media and reagents
The yeast strains used in this study were wild-type MKP-0 (MATα, can1-100, ade2-1, lys2-1, ura3-52, leu2-3, 112his3-Δ200, trp1-Δ901), its isogenic derivative ung1 null mutant PY32 (MATα, can1-100, ade2-1, lys2-1, ura3-52, leu2-3, 112his3-Δ200, trp1-Δ901, UNG1::URA3), YF250 (MATα, ura3-52, hisΔ200, leu2Δ1, trp1Δ63) and YPH500 (MATα, his3-Δ200, leu2-Δ1, ura3-52, trp1-Δ63, lys2-801, ade2-100). All yeast strains used in this study were grown in YPD medium (1% Yeast Extract, 2% Bacto Peptone, 2% dextrose and 2% agar for plates). At the same time the strains were also grown in YPG (1% Yeast Extract, 2% Bacto Peptone, 3% v/v glycerol and 2% agar for plates) for detection of respiratory incompetent colonies. Synthetic Complete (SC) medium (0.67% Yeast Nitrogen Base without amino acids, 2% dextrose, 0.08% Drop Out mix and 2% agar for the plates) lacking uracil was used for transformation of yeast plasmids. SC medium lacking uracil and methionine was used for maintaining GFP-C-Fus constructs.
All restriction enzymes and DNA-modifying enzymes were obtained from Life Technologies. Bacterial strain DH10B was used in all cloning steps. Luria–Bertani (LB) medium (1% Yeast Extract, 0.5% Bacto Tryptone, 1% NaCl and 2% agar for plates) was used for growth of Escherichia coli. Ampicillin was added to LB at 100 mg/ml for the growth of plasmid-containing strains.
Plasmid construction
The plasmid pUNG15 containing the full-length cDNA encoding human UDG was used. The four UNG mutants used in this study, Tyr147Ala(Y174A), Tyr147Cys(Y147C), Tyr147Ser(Y147S) and Asn204Asp(N204D), were kindly provided by Dr Hans E. Krokan (UNIGEN Center for Molecular Biology, University of Trondheim, Norway; 13).
Yeast UNG1 (yUNG1) was excised from plasmid Kp201 using HindIII, treated with calf intestinal phosphatase, purified by gel electrophoresis and ligated to the HindIII site of vector p426ADH (a 7.456 kb yeast vector) containing a URA3 yeast selectable marker. The vector was obtained from ATCC. The orientation of yUDG was confirmed by digestion with KpnI. This plasmid was named pAC7.
The site-directed mutants Y147A, Y147C, Y147S and N204D were subcloned into the NcoI site of pUNG15. The sequence was confirmed by DNA sequencing. The resulting construct had both mitochondrial and nuclear signals and the constructs were named pAC10, pAC11, pAC12 and pAC13, respectively. The coding region of the gene was generated by PCR from the plasmids pUNG15, pAC10, pAC11, pAC12 and pAC13 using primers 5′-CGG ACT AGT ATG GGC GTC TTC TGC CTT G-3′ and 5′-GCA TCG ATC AGC TCC TTC CAG TCA ATG-3′. The PCR products were digested with SpeI and ClaI, purified from the gel and cloned into the SpeI and ClaI sites of vector p426ADH and the resulting constructs were named pAC20 (wild-type hUNG1), pAC21 (Y147A), pAC22 (Y147C), pAC23 (Y147S) and pAC24 (N204D), respectively.
The wild-type or ung1 mutant yeast strain was transformed with plasmid constructs by the lithium acetate method (14).
Measurement of mitochondrial mutants
Single yeast colonies were grown to saturation (for 24 h) in 5 ml of YPD. The cells were washed, resuspended in 1 ml of sterile water, diluted and plated on YPD plates, incubated at 30°C for 3–4 days and the number of red (wild-type) and white (petite) colonies were scored (14). The frequency of erythromycin-resistant (Eryr) colonies was also determined from 15 independent cultures of MKP-0 and pY32. Cells were cultured to log phase (OD600 = 1.0), washed and plated on YPEG (1% Yeast Extract, 2% Bacto peptone, 3% v/v glycerol, 3% v/v ethanol and 2% agar) plates containing 2 mg/ml erythromycin. Erythromycin-resistant colonies were scored after incubation at 30°C for 10 days. The cultures were diluted and plated on YPD plates to determine the viable cell count. Mutation frequency was calculated per 108 surviving cells. Statistical significance was calculated using Student’s t-test assuming unequal variance.
Intracellular localization of hUng1p and yUng1p
The pGFP-C-Fus expression vector was employed for the generation of human and yeast UDG–GFP fusion protein (15). A 941 bp insert containing the entire hUNG1 gene and a 1.08 kb insert containing the yUNG1 gene was generated by PCR from plasmids pUNG15 and Kp201, respectively, using primers SetI (5′-GCG GAA TTC ATG GGC GTC TTC TGC CTT G-3′ and 5′-GCA TCG ATC AGC TCC TTC CAG TCA ATG-3′) and SetII (5′-GCG CGG ATC CGA AGC TGT ACA CAA GCC G-3′ and 5′-CCA GGA ATT CCT CTA AGC GAG CAT TTG CCT CC-3′), respectively. To generate yUNG1 and hUNG1 lacking the mitochondrial signal, 16 amino acids from the N-terminus of each of the gene were deleted by PCR using primers Set I (5′-CCG GAA TTC ATG AGG AAG CGA CAC GCC CCC-3′ and 5′-GCA TCG ATC AGC TCC TTC CAG TCA ATG-3′) and Set II (5′-CGC GGA TCC ATG CGA AAG AGA AAG CAA ACT ACG-3′ and 5′-CCA GGA ATT CCT CTA AGC GAG CAT TTG CCT CC-3′).
The PCR products were digested with EcoRI + ClaI and BamHI + EcoRI for hUNG1 and yUNG1, respectively, and cloned in-frame into the sites of the plasmid pGFP-C-Fus, giving rise to the constructs pAC14 (hUNG1), pAC6 (yUNG1), pAC41 [hUNG1(–MLS)] and pAC42 [yUNG1(–MLS)], respectively. Yeast strain FY250 was transformed with the above mentioned plasmids and the cells were selected on SC plates lacking methionine and uracil. The cells were then resuspended in 500 µl of 0.1 µg/ml DAPI/H2O and incubated for 10 min in the dark. The cells were pelleted, washed twice with 1 ml of H2O and once with 1× PBS. Fluorescence was examined using a green fluorescent protein (GFP) optimized filter and a DAPI optimized filter using a Zeiss-Axiovert 135 TV inverted microscope equipped with a PXl camera (SEBSYS Photometrica).
RESULTS
Inactivation of the UNG1 gene leads to mutation in mtDNA
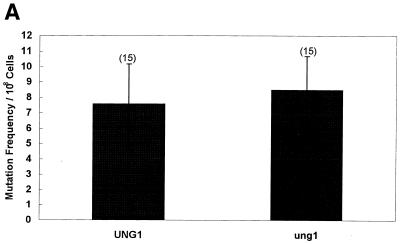
A previous study by Burgers and Klein (9) has reported that inactivation of the Saccharomyces cerevisiae UNG1 gene results in a ‘mutator phenotype for nuclear but not for mitochondrial genes’. The antibiotic erythromycin or chloramphenicol selectively inhibits mitochondrial protein synthesis. However, cells can acquire resistance to erythromycin or chloramphenicol due to a mutation in the 21S rRNA gene encoded by the mtDNA. This phenotype of eukaryotic cells has frequently been used to determine the frequency of mutations in the mitochondrial genome (16–20). In their study Burgers and Klein (9) measured the frequency of mutation in the mitochondrial 21S rRNA gene. We also used this method to measure the frequency of mutation in the 21S rRNA gene and found no difference between the wild-type and the ung1 null mutant (Fig. 1A).
Figure 1.
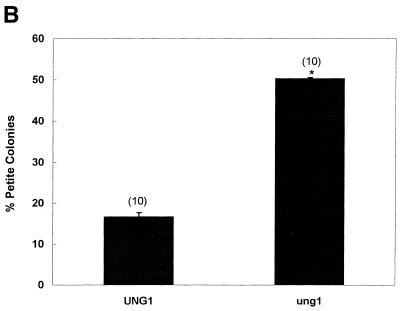
(A) Spontaneous mutation frequency in an ung1 null mutant (erythromycin method). The wild-type (UNG1) and its mutant (ung1) derivative were grown, washed and plated on YPD (at the required dilution) and erythromycin plates. The number of erythromycin-resistant colonies was scored and the mutation frequency per 108 viable cells was obtained. The number of independent cultures used in the study is shown in parentheses. (B) Spontaneous mutation frequency in an ung1 null mutant (colony color method). The wild-type (UNG1) and its mutant (ung1) derivative were grown in YPD, washed and plated on YPD plates. The ade2 auxotroph forms red colonies on YPD that grow on YPG (Yeast Extract, Bacto Peptone, glycerol) plates. When red colonies loose their mitochondrial function they turn white and appear as petite colonies that do not grow on YPG plates (14). The number of independent cultures used in the study is shown in parentheses. Statistical significance was calculated using Student’s t-test assuming unequal variance. The P value relevant to statistical significance was P < 0.001.
We recently described a colony color assay that proved to be useful in analyzing mtDNA mutations because it provides a target size of the entire mitochondrial genome rather than of the 21S rRNA gene (14). Using the colony color assay, we compared mutation frequencies between the wild type and a mutant lacking the UNG1 gene. Results of this analysis revealed at least 3-fold more mitochondrial mutants in ung1 when compared with wild-type cells (Fig. 1B). We conclude that inactivation of the UNG1 gene leads to increased frequency of mitochondrial mutants.
Ung1p localizes to mitochondria
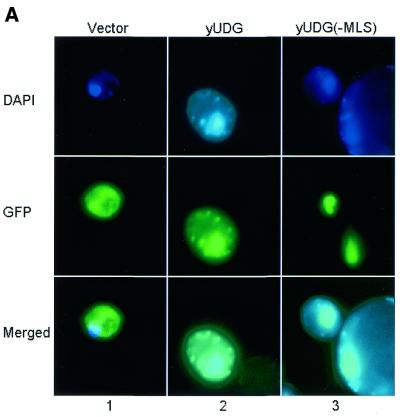
Yeast Ung1p contains an N-terminus which appears to contain the typical features of a mitochondrial localization signal (MLS). MitoProt II (http://psort.nibb.ac.jp/index.html) analysis of 24 amino acids from N-terminal sequences revealed a net charge of +9, with six basic and no acidic residues, and predicted a cleavage site at position 21. We therefore tested whether yUng1p localizes to mitochondria. Figure 2A shows that when the entire UNG1 coding sequence was expressed in-frame with GFP, yUng1p–GFP localized to both the nucleus and the mitochondria (panel 2). However, deletion of 16 amino acids containing the putative MLS led to Ung1p being localized exclusively in the nucleus (panel 3). DAPI was used as a control to determine the nuclear and mitochondrial compartments (Fig. 2, panels 1–3). When the DAPI and Ung1p–GFP expression images were merged a nuclear localization of the yUng1p–GFP fusion protein is clearly apparent. These results demonstrate that Ung1p indeed localizes to mitochondria (and to the nucleus).
Figure 2.
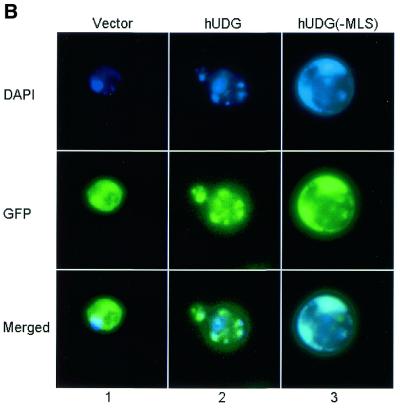
(A) Subcellular localization of the yUDG–GFP fusion protein. Yeast YF250 cells expressing GFP–yUNG1 were grown in selection media lacking uracil and methionine (see Materials and Methods). DAPI was used to locate the mitochondria and the nucleus of the cells (see DAPI panel). Merged images show that in cells expressing GFP–yUNG1 the fusion protein localized to both the nucleus and the mitochondria, whereas in cells expressing GFP–yUNG1(–MLS) it localized only to the nucleus. (B) Subcellular localization of the hUNG1–GFP fusion protein. Yeast YF250 cells expressing GFP–hUNG1 were grown in selection media lacking uracil and methionine (see Materials and Methods). DAPI was used to locate the mitochondria and the nucleus of the cells (see DAPI panel). Merged images show that in cells expressing GFP–hUNG1 the fusion protein localized to both the nucleus and the mitochondria, whereas in cells expressing GFP–hUNG1(–MLS) it localized mainly to the nucleus and to a lesser extent to the mitochondria.
Two alternative spliced forms of human UDG protein exist in human cells; one resides in the nucleus and the other in the mitochondria. We cloned the hUNG1 cDNA sequence containing the MLS in-frame with GFP and analyzed the subcellular localization in yeast. Figure 2B demonstrates that hUng1 when expressed in yeast predominantly localizes to mitochondria (panel 2). However, when 16 amino acids containing the hUng1p MLS were removed, the fusion protein was located in the nucleus (Fig. 2B, panel 3). We conclude that yUng1p is a mitochondrial (as well as nuclear) protein. Furthermore, hUng1p when expressed in yeast localizes to the mitochondria, suggesting that the hUng1p MLS functions well in transport of the protein into yeast mitochondria.
The ung1 gene defect is suppressed by expression of yeast and human Ung1p
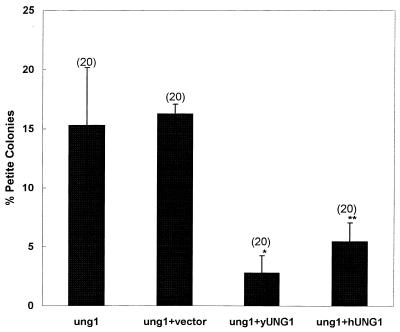
Analysis of DNA repair in the nucleus has revealed that the function of both yeast and human Ung1p protein is conserved. We therefore investigated whether Ung1p when expressed in yeast protects cells from the ung1 mitochondrial phenotype. We cloned the hUNG1 cDNA (encoding the MLS) under the control of a constitutively expressed ADH promoter. As a control, yeast cDNA was also cloned under the ADH promoter. Figure 3 shows that expression of the hUNG1 gene in yeast suppresses the ung1 gene defect in mitochondria. The ung1 null mutant and the null mutant containing the vector alone showed high frequencies of mitochondrial mutants. Interestingly, expression of either yeast or human Ung1p reduced the frequency of mitochondrial mutants to the wild-type level.
Figure 3.
Expression of yeast or human UNG1 reduces the frequency of mitochondrial mutation. The yeast ung1 mutant (MATα, can1-100, ade2-1, lys2-1, ura3-52, leu2-3, 112his3-Δ200, trp1Δ901, UNG1::URA3) was transformed with a control vector p(426ADH) and with a vector carrying either yUNG1 or hUNG1. The cultures were grown in selection media, washed and plated on YPD plates. Red and white (petite) colonies were scored after 3–4 days (14). Statistical significance was calculated using Student’s t-test assuming unequal variance. The P values relevant to statistical significance were *P < 0.004 and **P < 0.009. The number of independent cultures used in the study is shown in parentheses.
Constitutive expression of the yeast UNG1 gene is mutagenic
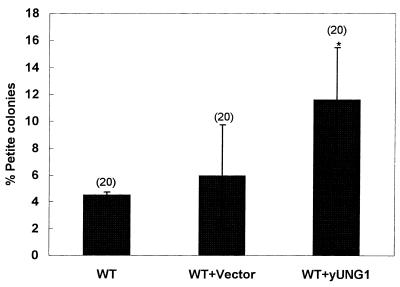
We previously demonstrated that expression of the Ogg1 protein in wild-type yeast cells reduced the frequency of spontaneously arising mutations in mtDNA (14). These studies indicated that Ogg1p expression was a limiting factor in the mitochondria and that 8-oxoguanine was a major contributor to mutation in mtDNA. To test whether Ung1p expression was a limiting factor and that deamination of cytosine plays a significant role in spontaneous mtDNA mutagenesis in wild-type cells, we transformed wild-type cells with a plasmid containing the yUNG1 gene cloned under the control of the ADH promoter. Figure 4 demonstrates that cells expressing the yUNG1 gene constitutively induce a higher frequency of mitochondrial mutants than the vector alone control. We conclude that constitutive expression of yUNG1 is mutagenic in mitochondria.
Figure 4.
Constitutive expression of the UNG1 gene is mutagenic. The yeast UNG1 gene was cloned under the control of the constitutive ADH promoter (see Materials and Methods) and transformed in the wild-type strain YPH500 (MATα, his3-Δ200, leu2-Δ1, ura3-52, trp1-Δ63, lys2-801, ade2-100). The percentage of petite colonies was scored by the color screening method (see Materials and Methods). Statistical significance was calculated using Student’s t-test assuming unequal variance. The P values relevant to statistical significance was P < 0.01. The number of independent cultures used in the study is shown in parentheses.
Mutant hUNG1 gene expression increases the frequency of mutations in mtDNA
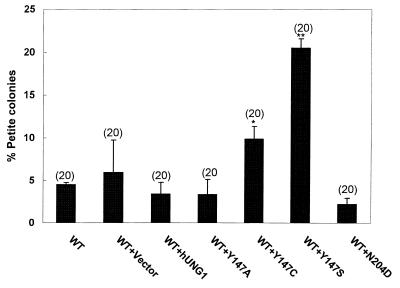
Mutational analysis of hUng1p protein revealed that replacement of Asn204 by Asp or Tyr147 by Ala, Cys or Ser results in enzymes that have cytosine-DNA (CDG) and thymine-DNA glycosylase (TDG) activity, respectively (13). Expression of CDG and TDG in E.coli caused 4–100-fold increases in the frequency of rifampicin-resistant mutants. These results indicated that single amino acid substitutions in UDG confer a mutator phenotype (13). We sought to test the effect of hUng1p mutants in yeast mitochondria. Figure 5 shows that expression of hUng1p mutants Y147C and Y147S in mitochondria leads to elevated levels of mitochondrial mutants compared to wild-type hUng1p or vector alone. Figure 5 also indicates that expression of hUng1p mutants N204D and Y147A did not increase the frequency of mtDNA mutations. These results suggest that replacement of Tyr147 by Cys or Ser in hUng1p is mutagenic in mitochondria.
Figure 5.
Expression of a mutant hUNG1 gene increases the frequency of mitochondrial mutations. Site-directed mutants Y147A, Y147C, Y147S and N204D, along with human wild-type hUNG1 and the control vector, were transformed in the wild-type strain YPH500 (MATα, his3-Δ200, leu2-Δ1, ura3-52, trp1-Δ63, lys2-801, ade2-100). The percentage of mitochondrial mutants was determined by colony color assay (14). Statistical significance was calculated using Student’s t-test assuming unequal variance. The P values relevant to statistical significance were *P < 0.0008 and **P < 0.0001 in comparison with wild-type hUNG1. The number of independent cultures used in the study is shown in parentheses.
DISCUSSION
Mutation of mtDNA is involved in the pathogenesis of many diseases, including cancer (1–5). Improvements in understanding the underlying pathogenetic mechanisms largely depend on perspective genetic models and molecular approaches. One such model is the yeast S.cerevisiae, whose entire genome has been sequenced (21). We are using S.cerevisiae as a genetic model to identify proteins that repair DNA in mitochondria. The sequence of UDG is well conserved between bacteria, yeast, human and other eurkaryotes (10). A previous study to identify the role of Ung1p in yeast concluded that UNG1 inactivation does not lead to a mitochondrial mutator phenotype (9). Using the colony color assay, our analysis revealed significant differences in the frequency of mitochondrial mutants between the wild-type and ung1 mutant. The colony color assay is more sensitive than the antibiotic resistance assay used in the previous study, resulting in the detection of more mitochondrial mutants in the ung1 null mutant. It is important to note that a change in colony color may arise due to random mutation (point mutation, insertion, deletion or loss of the entire mitochondrial genome) in the mitochondrial genome that leads to mitochondrial dysfunction. In comparison, resistance to antibiotics arises due to specific point mutations in two particular genes, rib2 and rib3, both encoded by the mitochondrial genome. In addition, the target size for a mutation resulting in antibiotic resistance (∼4 kb) is very small compared to the mitochondrial genome (∼85 kb) (14). We performed mutational analysis of several petite mutants arising due to UNG1 deficiency. Our results demonstrate that UNG1 deficiency does not lead to deletions in the mtDNA molecule or to complete loss of the mitochondrial genome (data not shown). Since the colony color assay also measures mitochondrial dysfunction arising due to point mutations, it is plausible that a point mutation in any of the multiple transcriptional control sites may render the cell deficient in mitochondrial function. It is also conceivable that a GC→AT conversion in the mitochondrial genome results in a dominant mutation. Indeed, dominant mutations in the mitochondrial genome have been described (22,23). However, further study is required to identify the exact nature of the mutation in the mitochondrial genome of the ung1 mutant.
The studies described in this paper clearly establish an in vivo function of yeast and human Ung1p proteins in mitochondria. Using yUng1p–GFP we have shown that full-length yeast Ung1p localizes to both the mitochondria and the nuclear compartment. Deletion analysis of the N-terminus revealed that the first 16 amino acids are important for mitochondrial import of Ung1p because this deletion abolished mitochondrial localization. Interestingly, deleted yUng1p was transported to the nucleus. Deleted Ung1p retained the nuclear localization signal (NLS) (located at amino acids 17–20 and 38–44) that is likely responsible for its nuclear transport. Strikingly, similar results were obtained with yOgg1p protein that lacked the MLS (14). Our results suggest that hUng1p primarily localizes in yeast mitochondria. These results are consistent with a previous report demonstrating preferential localization of hUng1p into human cell mitochondria (24). The MLS in hUng1p is located within 28 amino acids of the N-terminus (24). When these 28 amino acids of hUng1p were deleted it reduced mitochondrial translocation but did not completely abolish it (24). Consistently, removal of the N-terminal 16 amino acids from hUng1p led to a decreased level of hUng1p in yeast mitochondria. Since truncated hUngp1p contained the NLS (located at amino acids 17–20 and 48–51) it was localized to the nucleus. In spite of the similarities, differences clearly exist between the mechanisms for mitochondrial localization of the human and yeast Ung1p. The mitochondrial and nuclear isoforms of hUng1p are encoded by the same gene but differ in their N-terminal sequences because of alternative splicing and the use of alternative promoters (24). In contrast, our study suggests that S.cerevisiae contains a single isoform of Ung1p that contains both a NLS and a MLS. Our study of localization of hUng1p in yeast mitochondria by its own MLS provides evidence that yeast can be used as a model for functional and genetic analyses of human DNA repair mechanisms in mitochondria. Our analysis also shows that expression of either the yeast or human UNG1 gene rescues ung1 cells from the mitochondrial mutator phenotype, confirming that yeast should aid in analysis of UNG1 gene polymorphisms in mitochondrial metabolism (25).
Our analysis revealed that an elevated level of yUng1p in wild-type cells causes a mitochondrial mutator phenotype. This is likely due to an imbalance in the base excision repair pathway, as described for nuclear DNA (26). Interestingly, expression of wild-type hUng1p does not lead to a mutator phenotype in yeast mitochondria. Both Ung1 proteins are capable of undergoing post-translational modifications. It is conceivable that yeast proteins that modify yUng1p are incapable of modifying hUng1p. Differences in levels of post-translational modification may also contribute to differences in the mutator phenotype. For example yUng1p contains a tyrosine phosphorylation site (317KMINDWLY324) but hUng1p does not (see expasy.ch/cgi-bin/scanprosite.scanprosite?1). Additionally, yUng1p contains seven potential protein kinase C phosphorylation sites, whereas hUng1p contains only four.
Uracil binds in a pocket at the base of the DNA-binding groove of hUng1p and the specificity for uracil over structurally related DNA bases is conferred by shape complemetarity as well as by main chain and Asn204 side chain hydrogen bonds (13). Asn204 is critical for substrate binding and Tyr147 lines the active site uracil-binding pocket and contributes to shape complementarity (13). Replacement of Asn204 by Asp or Tyr147 by Ala, Cys or Ser results in enzymes that have CDG and TDG activity, respectively (13). Expression of CDG or TDG in E.coli causes an increase in rifampicin-resistant mutants. Our study demonstrates that expression of a site-directed Tyr147 but not Asp204 catalytic hUng1p mutant leads to a mutator phenotype in yeast mitochondria. The Asp204 mutation converts hUng1p to CDG, excising cytosine from DNA. The yeast mtDNA is rich in T and extremely poor in C. Therefore, TDG activity is likely to play a significant role in yeast mtDNA mutagenesis. Studies are underway to identify the mechanisms of mtDNA mutagenesis by mutant hUng1 proteins in human cells.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Dr Hans Krokan for generously providing us with the hUNG1 cDNAs used in this study. We also thank Dr Peter Burgers for the MKP-0 and PY3210 strains. We thank the members of our laboratory for critical reading of this manuscript. Research in our laboratory is supported by grants from the National Institutes of Health (RO1-097714, P50 CA88843 and P20 CA86346) and an American Heart Association Scientist Development Award (9939223N).
REFERENCES
- 1.Singh K.K. (2000) Mitochondrial me and the mitochondrion journal. Mitochondrion, 1, 1–2. [Google Scholar]
- 2.Naviaux R.K. (2000) Mitochondrial DNA disorders. Eur. J. Pediatr., 159, S219–S226. [DOI] [PubMed] [Google Scholar]
- 3.Singh K.K. (1998) Mitochondrial DNA Mutations in Aging, Disease, and Cancer. Springer, New York, NY.
- 4.Scheffler I.E. (2000) A century of mitochondrial research: achievements and perspectives. Mitochondrion, 1, 3–33. [DOI] [PubMed] [Google Scholar]
- 5.Wallace D.C. (1999) Mitochondrial diseases in man and mouse. Science, 283, 1482–1488. [DOI] [PubMed] [Google Scholar]
- 6.Krokan H.E., Nilsen,H., Skorpen,F., Otterlei,M. and Slupphaug,G. (2000) Base excision repair of DNA in mammalian cells. FEBS Lett., 476, 73–77. [DOI] [PubMed] [Google Scholar]
- 7.Impellizzeri K.J., Anderson,B. and Burgers,P.M. (1991) The spectrum of spontaneous mutations in a Saccharomyces cerevisiae uracil-DNA-glycosylase mutant limits the function of this enzyme to cytosine deamination repair. J. Bacteriol., 173, 6807–6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Percival K.J., Klein,M.B. and Burgers,P.M. (1989) Molecular cloning and primary structure of the uracil-DNA-glycosylase gene from Saccharomyces cerevisiae. J. Biol. Chem., 264, 2593–2598. [PubMed] [Google Scholar]
- 9.Burgers P.M. and Klein,M.B. (1986) Selection by genetic transformation of a Saccharomyces cerevisiae mutant defective for the nuclear uracil-DNA-glycosylase. J. Bacteriol., 166, 905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nilsen H., Rosewell,I., Robins,P., Skjelbred,C.F., Andersen,S., Slupphaug,G., Daly,G., Krokan,H.E., Lindahl,T. and Barnes,D.E. (2000) Uracil-DNA glycosylase (UNG)-deficient mice reveal a primary role of the enzyme during DNA replication. Mol. Cell, 5, 1059–1065. [DOI] [PubMed] [Google Scholar]
- 11.Nilsen H., Otterlei,M., Haug,T., Solum,K., Nagelhus,T.A., Skorpen,F. and Krokan,H.E. (1997) Nuclear and mitochondrial uracil-DNA glycosylases are generated by alternative splicing and transcription from different positions in the UNG gene. Nucleic Acids Res., 25, 750–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nilsen H., Steinsbekk,K.S., Otterlei,M., Slupphaug,G., Aas,P.A. and Krokan,H.E. (2000) Analysis of uracil-DNA glycosylases from the murine Ung gene reveals differential expression in tissues and in embryonic development and a subcellular sorting pattern that differs from the human homologues. Nucleic Acids Res., 28, 2277–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kavli B., Slupphaug,G., Mol,C.D., Arvai,A.S., Peterson,S.B., Tainer,J.A. and Krokan,H.E. (1996) Excision of cytosine and thymine from DNA by mutants of human uracil-DNA glycosylase. EMBO J., 15, 3442–3447. [PMC free article] [PubMed] [Google Scholar]
- 14.Singh K.K., Sigala,B., Sikder,H.A. and Schwimmer,C. (2001) Inactivation of Saccharomyces cerevisiae OGG1 DNA repair gene leads to an increased frequency of mitochondrial mutants. Nucleic Acids Res., 29, 1381–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niedenthal R.K., Riles,L., Johnston,M. and Hegemann,J.H. (1996) Green fluorescent protein as a marker for gene expression and subcellular localization in budding yeast. Yeast, 12, 773–786. [DOI] [PubMed] [Google Scholar]
- 16.Foury F. (1989) Cloning and sequencing of the nuclear gene MIP1 encoding the catalytic subunit of the yeast mitochondrial DNA polymerase. J. Biol. Chem., 264, 20552–20560. [PubMed] [Google Scholar]
- 17.Cui Z. and Mason,T. (1989) A single nucleotide substitution at the rib2 locus of the yeast mitochondrial gene for 21S rRNA confers resistance to erythromycin and cold sensitive ribosome assembly. Curr. Genet., 16, 273–279. [DOI] [PubMed] [Google Scholar]
- 18.Foury F. and Vanderstraeten,S. (1992) Yeast mitochondrial DNA mutators with deficient proofreading exonucleolytic activity. EMBO J., 11, 2717–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sor F. and Fukuhara,H. (1983) Complete DNA sequence coding for the large ribosomal RNA of yeast mitochondria. Nucleic Acids Res., 11, 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chi N.-W. and Kolodner,R.D. (1994) Purification and characterization of MSH1, a yeast mitochondrial proteins that binds to DNA mismatches. J. Biol. Chem., 269, 29984–29992. [PubMed] [Google Scholar]
- 21.Bassett D.E.,Jr, Boguski,M.S. and Hieter,P. (1996) Yeast genes and human disease. Nature, 379, 589–590. [DOI] [PubMed] [Google Scholar]
- 22.Yoneda M., Chomyn,A., Martinuzzi,A., Hurko,O. and Attardi,G. (1992) Marked replicative advantage of human mtDNA carrying a point mutation that causes the MELAS encephalomyopathy. Proc. Natl Acad. Sci. USA, 89, 11164–11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fangman W.L. and Dujon,B. (1984) Yeast mitochondrial genomes consisting of only A.T base pairs replicate and exhibit suppressiveness. Proc. Natl Acad. Sci. USA, 81, 7156–7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otterlei M., Haug,T., Nagelhus,T.A., Slupphaug,G., Lindmo,T. and Krokan,H.E. (1998) Nuclear and mitochondrial splice forms of human uracil-DNA glycosylase contain a complex nuclear localisation signal and a strong classical mitochondrial localisation signal, respectively. Nucleic Acids Res., 26, 4611–4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kvaloy K., Nilsen,H., Steinsbekk,K.S., Nedal,A., Monterotti,B., Akbari,M. and Krokan,H.E. (2001) Sequence variation in the human uracil-DNA glycosylase (UNG) gene. Mutat. Res., 461, 325–338. [DOI] [PubMed] [Google Scholar]
- 26.Glassner B.J., Rasmussen,L.J., Najarian,M.T., Posnick,L.M. and Samson,L.D. (1998) Generation of a strong mutator phenotype in yeast by imbalanced base excision repair. Proc. Natl Acad. Sci. USA, 95, 9997–10002. [DOI] [PMC free article] [PubMed] [Google Scholar]