OBJECTIVES:
To determine the effect of the awake prone position (APP) on gas exchange and the work of breathing in spontaneously breathing patients with COVID-19–associated acute hypoxemic respiratory failure (AHRF) supported by high-flow nasal oxygen.
DESIGN:
Prospective randomized physiologic crossover multicenter trial.
SETTINGS:
Four ICUs in Marseille, France.
PATIENTS:
Seventeen patients with laboratory-confirmed COVID-19 pneumonia and Pao2/Fio2 less than or equal to 300 mm Hg while treated with high-flow nasal cannula oxygen therapy.
INTERVENTIONS:
Periods of APP and semirecumbent position (SRP) were randomly applied for 2 hours and separated by a 2-hour washout period.
MEASUREMENTS AND MAIN RESULTS:
Arterial blood gases, end-tidal CO2. and esophageal pressure were recorded prior to and at the end of each period. Inspiratory muscle effort was assessed by measuring the esophageal pressure swing (∆PES) and the simplified esophageal pressure–time product (sPTPES). The other endpoints included physiologic dead space to tidal volume ratio (VD/VT) and the transpulmonary pressure swing. The APP increased the Pao2/Fio2 from 84 Torr (61–137 Torr) to 208 Torr (114–226 Torr) (p = 0.0007) and decreased both the VD/VT and the respiratory rate from 0.54 (0.47–0.57) to 0.49 (0.45–0.53) (p = 0.012) and from 26 breaths/min (21–30 breaths/min) to 21 breaths/min (19–22 breaths/min), respectively (p = 0.002). These variables remained unchanged during the SRP. The ∆PES and sPTPES per breath were unaffected by the position. However, the APP reduced the sPTPES per minute from 225 cm H2O.s.m–1 (176–332 cm H2O.s.m–1) to 174 cm H2O.s.m–1 (161–254 cm H2O.s.m–1) (p = 0.049).
CONCLUSIONS:
In spontaneously breathing patients with COVID-19–associated AHRF supported by high-flow nasal oxygen, the APP improves oxygenation and reduces the physiologic dead space, respiratory rate, and work of breathing per minute.
Keywords: COVID-19, prone position, respiratory distress syndrome, respiratory insufficiency, work of breathing
KEY POINTS
Question: Does the awake prone position (APP) reduce the work of breathing in spontaneously breathing patients with COVID-19–associated acute hypoxemic respiratory failure supported by high-flow nasal oxygen?
Findings: In this randomized multicenter crossover trial, a 2-hour period of the APP significantly reduced the physiologic dead space, respiratory rate, and work of breathing per minute but not per breath compared with a 2-hour period of the semirecumbent position.
Meanings: APP effectively reduces the work of breathing mainly by decreasing the respiratory rate in spontaneously breathing patients with COVID-19–associated acute hypoxemic respiratory failure supported by high-flow nasal oxygen.
Severe COVID-19 is associated with acute hypoxemic respiratory failure (AHRF), which frequently progresses toward acute respiratory distress syndrome (ARDS) and may require invasive mechanical ventilation (1). Although hypoxemia is a hallmark of the disease, the respiratory pattern may vary substantially between individuals, ranging from quiet breathing (i.e., silent hypoxemia) to tachypnea and respiratory distress (2).
High-flow nasal oxygen (HFNO) therapy and continuous positive airway pressure (CPAP) are effective noninvasive respiratory techniques to support patients with COVID-19–associated AHRF (3–5). In addition, the awake prone position (APP) has attracted increasing interest during the COVID-19 pandemic, as it markedly improves oxygenation, reduces the respiratory rate, and decreases the risk of endotracheal intubation and death (3, 6). The mechanisms underlying these benefits may involve changes in the distribution of ventilation/perfusion (VA/Q) (7, 8) and a reduction in the work of breathing (WOB). In patients supported by CPAP, the APP failed to reduce inspiratory muscle effort but decreased the respiratory rate and WOB (9). As the effect of the APP on respiratory mechanics has not yet been determined in patients supported by HFNO, we investigated the short-term effects of the APP on gas exchange and the WOB.
MATERIALS AND METHODS
This physiologic randomized crossover study was conducted in four ICUs in Marseille, France. The protocol was approved by an independent national review board on June 11, 2020 (Comité de Protection des Personnes Nord Ouest, ID 20.05.26.63610; title: “Effect of Prone Positioning Combined With High Flow Oxygen Therapy on Oxygenation During Acute Respiratory Failure Due to COVID-19”) and was registered on ClinicalTrials.gov (NCT04543760). Each patient signed an informed consent form prior to inclusion. All procedures performed in the present study were in accordance with the Declaration of Helsinki.
Patients
All adult patients admitted to the ICUs for less than 72 hours with a laboratory-confirmed diagnosis of COVID-19 pneumonia were screened. Patients were eligible for enrollment if they were spontaneously breathing and fulfilled the criteria for AHRF, as defined by a Pao2/Fio2 ratio less than or equal to 300 mm Hg while receiving HFNO, had evidence of bilateral pulmonary infiltrates on a chest radiograph or a CT scan, and had an acute onset (< 1 wk) of respiratory distress. The exclusion criteria are presented in the Supplemental Digital Content (http://links.lww.com/CCX/B95).
Interventions
The settings of HFNO are detailed in the Supplemental Digital Content (http://links.lww.com/CCX/B95). Each patient was placed in semirecumbent position (SRP) and prone position (PP) for 2 hours, and the sequence order was determined by randomization. A washout period of 2 hours was applied to prevent a carryover effect. The use of sedative or analgesic agents that may interfere with the breathing pattern was not allowed during the study period. Further details on the interventions are available in the Supplemental Digital Content (http://links.lww.com/CCX/B95).
Prior to randomization, an esophageal balloon catheter (Cooper Surgical, Trumbull, CT, USA) was inserted to measure esophageal pressure (PES) (10, 11). End-tidal CO2 (ETCO2) was obtained by capnometry while the patients breathed through a mouthpiece using a mainstream CO2 sensor (CAPNOSTAT 5; Hamilton Medical AG, Bonaduz, Switzerland) connected to the ventilator.
Measurements
Demographics and clinically relevant data were collected at inclusion. All available chest CT scans were reviewed to determine the CT-based lung extension severity (Supplemental Digital Content, http://links.lww.com/CCX/B95). Physiologic variables and self-assessed symptoms (dyspnea, discomfort, and pain) were assessed at baseline and at the end of each intervention (PP and SRP) in the following order: clinical data (respiratory rate, Spo2, arterial pressure, and heart rate); self-assessed symptoms (through adapted Visual Analog Scales ranging from 0 to 100 points) (10); and a 2-minute continuous recording of PES. Subsequently, arterial blood gases were analyzed, and the ETCO2 was recorded over ten breathing cycles. Additionally, intermediary arterial blood gases were sampled at 30 minutes and 1 hour after the beginning of each intervention.
We measured the following PES-related variables: the respiratory rate, the inspiratory effort (∆PES), the simplified PES–time product (sPTPES) as a surrogate of the WOB per breath and per minute, and the dynamic transpulmonary driving pressure (∆PL). From ETCO2 and Paco2, we computed the Paco2 to ETCO2 difference (Paco2–ETCO2) to estimate the physiologic dead space to tidal volume ratio (VD/VT) (12) as an index of ventilatory inefficiency (13). Additional details are provided in the Supplemental Digital Content (http://links.lww.com/CCX/B95).
Clinical Follow-Up
After the study procedure, the patients were followed for 2 months to record the vital status (60-d mortality), the need for intubation, and the duration of mechanical ventilation.
Endpoints
The primary endpoint of this study was the difference in the Pao2/Fio2 ratio between positions at the end of the period. The secondary objectives were the absolute and relative variations in the Pao2/Fio2 while patients were lying in the different positions. The proportion of responders, as defined by a relative increase in Pao2/Fio2 greater than or equal to 20% during the PP, was evaluated. The other endpoints included the absolute and relative variations in blood gas variables, esophageal-related variables, self-assessed symptoms, and adverse events (see Supplemental Digital Content, http://links.lww.com/CCX/B95).
In an exploratory analysis, we evaluated whether some physiologic variables that were measured prior to any interventions (i.e., at study entry) would differentiate intubated from nonintubated patients.
Statistical Analysis
Details regarding the sample size calculation are provided in the Supplemental Digital Content (http://links.lww.com/CCX/B95). Qualitative data are presented as counts and proportions (%), and quantitative data are presented as medians and interquartile ranges. Referring to the crossover design, we used a mixed model analysis with nested random effects to simultaneously test the effect of the sequence (PP-SRP and SRP-PP), period (first and second), and position (PP and SRP) on the quantitative variables. Crude comparisons within positions (baseline vs 120 min) were performed using the Wilcoxon signed-rank test, which did not account for the crossover effect. We used a Kruskal-Wallis analysis to compare the changes in Pao2/Fio2 during the PP at multiple time points with Conover post hoc comparisons. The proportions of patients breathing with high inspiratory efforts were compared using the chi-square test. Correlations between the variables were assessed by calculating Pearson’s correlation coefficient (r). A univariate analysis between intubated and nonintubated patients was performed at study entry using the Mann-Whitney U test. We then analyzed the receiver operating characteristic curve to determine the area under the curve, and the optimal cutoff value was obtained by calculating Youden’s index. All tests were two-sided, and p values less than or equal to 0.05 were considered significant. Statistical analyses were performed using SAS V9.4 (SAS Institute, Cary, NC), and graphics were created with MedCalc v20.015 (MedCalc Software Ltd., Ostend, Belgium).
RESULTS
From October 2020 to January 2021, 18 patients were included (eFig. 1, http://links.lww.com/CCX/B95). One patient had to be intubated immediately after randomization and was subsequently excluded from the study. The demographics and most relevant clinical characteristics of the patients are presented in Table 1. The CT-based lung extension severity is presented in eTable 1 (http://links.lww.com/CCX/B95). Among the 17 patients who completed the trial, nine received PP first, and eight received SRP first. No differences in the studied variables were observed at baseline. Five patients (29%) were intubated during their ICU stay, and one of those patients died. The main physiologic variables that were recorded during the study are displayed in Table 2.
TABLE 1.
Main Characteristics of the Study Population
| Variables, Units | Overall, N = 17 | Prone Position First, N = 9 | Semirecumbent Position First, N = 8 |
|---|---|---|---|
| Age, yr | 61 (57–71) | 61 (55–77) | 63 (56–68) |
| Sex, male, n (%) | 15 (88) | 8 (89) | 7 (88) |
| Body mass index, kg/m² | 27 (25–31) | 27 (26–31) | 27 (25–31) |
| Simplified Acute Physiologic Score II, at inclusion | 29 (22–33) | 27 (22–33) | 30 (24–33) |
| Comorbidities, n (%) | |||
| Cancer | 7 (41) | 4 (44) | 3 (38) |
| Diabetes | 2 (12) | 1 (11) | 1 (13) |
| Hypertension | 10 (59) | 6 (67) | 4 (50) |
| Chronic obstructive pulmonary disease | 1 (6) | 1 (11) | 0 |
| Chronic heart diseases | 3 (18) | 3 (33) | 0 |
| Chronic liver diseases | 0 | 0 | 0 |
| Chronic renal failure | 1 (6) | 0 | 1 (13) |
| Immunodepression | 3 (18) | 2 (22) | 1 (13) |
| Time from symptom onset to hospital admission, d | 9 (5–10) | 9 (6–10) | 9 (3–10) |
| Time from symptom onset to ICU admission, d | 9 (6–11) | 9 (6–11) | 9 (7–10) |
| Time from symptom onset to enrollment, d | 10 (8–12) | 10 (8–12) | 11 (8–12) |
| High-flow nasal O2 gas flow at enrollment, L/min | 30 (30–50) | 30 (30–50) | 30 (30–55) |
| Arterial pH at enrollment | 7.45 (7.44–7.5) | 7.44 (7.44–7.5) | 7.47 (7.44–7.5) |
| Paco2 at enrollment, mm Hg | 32 (30–33) | 32 (29–33) | 33 (31–34) |
| Pao2/Fio2 at enrollment, mm Hg/% | 115 (88–139) | 130 (107–151) | 96 (74–128) |
| Respiratory rate at enrollment, breaths/min | 25 (20–33) | 22 (20–26) | 32 (23–39) |
| Heart rate at enrollment, beats/min | 75 (69–88) | 75 (67–89) | 76 (71–89) |
| Mean arterial pressure at enrollment, mm Hg | 76 (58–99) | 90 (63–132) | 66 (58–77) |
| Length of ICU stay, d | 15 (9–27) | 14 (9–31) | 16 (11–23) |
| Need for endotracheal intubation after enrollment, n (%) | 5 (29) | 2 (22) | 3 (38) |
| Length of mechanical ventilation after intubation, d | 24 (17–36) | 36 (20–51) | 24 (11–29) |
| 60-d mortality, n (%) | |||
| Overall | 1 (6) | 0 | 1 (13) |
| Intubated patients | 1 (6) | 0 | 1 (13) |
| Nonintubated patients | 0 | 0 | 0 |
Data are expressed as the median (interquartile range, 25–75%) unless otherwise specified.
TABLE 2.
Main Physiologic Variables During the Semirecumbent and Prone Periods
| Variables, Units | Semirecumbent Position | Prone Position | ||||||
|---|---|---|---|---|---|---|---|---|
| Base | End | Absolute Difference | Relative Difference | Base | End | Absolute Difference | Relative Difference | |
| Gas exchange | ||||||||
| Arterial pH | 7.45 (7.44–7.48) | 7.47 (7.44–7.49) | 0.01 (–0.01 to 0.01) | 0.1 (–0.1 to 0.1) | 7.46 (7.44–7.49) | 7.44 (7.43–7.48) | –0.01 (–0.02 to 0.01) | –0.1 (–0.3 to 0.1) |
| Pao2, torr | 79 (64–90) | 64 (56–82) | –6 (–19 to 4) | –8 (–22 to 3) | 70 (61–96) | 130 (103–180)a | 37 (16–96)b | 34 (16–147)c |
| Fio2, torr | 0.8 (0.7–0.9) | 0.8 (0.7–0.95) | 0 (0–0) | 0 (0–0) | 0.8 (0.7–0.95) | 0.8 (0.7–1) | 0 (0–0) | 0 (0–0) |
| Pao2/Fio2, torr | 95 (73–135) | 91 (64–120) | –8 (–26 to 4) | –8 (–22 to 3) | 84 (61–137) | 208 (114–226)a | 41 (20–124)b | 34 (12–147)c |
| Paco2, torr | 32 (30–35) | 31 (28–34) | 0 (–3 to 1.4) | 0 (–9 to 5) | 32 (29–34) | 33 (30–35) | 1.2 (–1 to 2) | 4 (–3 to 7) |
| End-tidal CO2 tension, torr | 30 (27–31) | 28 (26–32) | 0.1 (–1.3 to 0.8) | 0.5 (–4 to 3) | 29 (26–31) | 31 (29–33) | 2.8 (0–3.7)b | 9 (0–14)c |
| Physiologic dead space to tidal volume ratio | 0.51 (0.48–0.58) | 0.52 (0.5–0.56) | 0 (–0.03 to 0.02) | –0.8 (–5.5 to 3.8) | 0.54 (0.48–0.57) | 0.49 (0.45–0.52) | –0.03 (–0.06 to –0.02)b | –5 (–11.5 to –3.7)c |
| Respiratory mechanics | ||||||||
| Respiratory rate, breaths/min | 26 (21–32) | 28 (20–33) | 0 (–4 to 2) | 0 (–14 to 8) | 26 (21–30) | 21 (19–22)a | –5 (–9 to 1)b | –17 (–29 to 6)c |
| Delta of the esophageal pressure between the end-expiratory and end-inspiratory values, cm H2O | 11.3 (8.1–14.7) | 11.6 (9–16.9) | 0.2 (–0.8 to 4.1) | 1 (–9 to 31) | 10.6 (7.4–17.7) | 9.7 (7.5–13.1) | –1.5 (–3.5 to 1.2) | –11 (–19 to 14) |
| Delta of the transpulmonary pressure (airway minus esophageal) between the end-inspiratory and end-expiratory values, cm H2O | 11.3 (8–14.7) | 11.6 (9–16.9) | 0.2 (–0.8 to 4.2) | 1 (–9 to 30) | 10.6 (6.8–17.7) | 9.7 (7.5–13.1) | –1.5 (–3.5 to 1.2) | –11 (–19 to 14) |
| sPTPES/breath, cm H2O.s | 6.1 (4.8–10.8) | 6.6 (4.9–12.1) | 0.1 (–0.3 to 1.1) | 1 (–4 to 13) | 7 (5.1–11.2) | 8.2 (5.2–10.3) | –0.6 (–1.9 to 1.5) | –6 (–23 to 26) |
| sPTPES/min, cm H2O.s.min-1 | 213 (161–267) | 217 (161–331) | 20 (7–50) | 10 (3–20) | 225 (176–332) | 174 (161–254) | –29 (–54 to 7)b | –10 (–22 to 2)c |
| Self-assessed symptoms | ||||||||
| Dyspnea, VAS | 29 (20–45) | 20 (13–40) | –5 (–12 to 0) | –24 (–38 to 0) | 30 (8–40) | 20 (0–40) | 0 (–10 to 5) | –9 (–80 to 18) |
| Discomfort, VAS | 30 20–34) | 20 (18–40) | 0 (–17 to 5) | 0 (–34 to 18) | 30 (15–42) | 31 (25–62) | 0 (–5 to 25)a | 5 (–17 to 75) |
| Pain, VAS | 0 (0–15) | 0 (0–20) | 0 (0–0) | 0 (0–0) | 10 (0–20) | 15 (0–20) | 5 (0–17) | 0 (0–0) |
| Hemodynamics | ||||||||
| Heart rate, beats/min | 72 (65–84) | 75 (68–88) | –1 (–2 to 3) | –1 (–2 to 4) | 77 (71–84) | 78 (64–84) | –1 (–10 to 6) | –1 (–14 to 8) |
| Mean arterial pressure, mm Hg | 91 (85–97) | 91 (89–98) | 0 (–2 to 3) | 0 (–2 to 7) | 91 (88–93) | 93 (83–95) | –2 (–4 to 9) | –2 (–4 to 10) |
sPTPES = simplified esophageal pressure time product, VAS = Visual Analog Scales with values ranging from 0 (minimal) to 100 (maximal).
p < 0.05 vs the end value of the semirecumbent position (SRP).
p < 0.05 vs the absolute difference of the SRP.
p < 0.05 vs the relative difference of the SRP.
The absolute difference corresponds to the crude difference between the values at the end of the period minus the baseline. The relative difference is calculated as ([end value–baseline value]/baseline value) × 100 and is expressed as a percentage. Between-group comparisons (prone position vs SRP) were performed by a mixed model analysis. Data are expressed as the median (interquartile range 25–75%) unless otherwise specified.
Gas Exchange
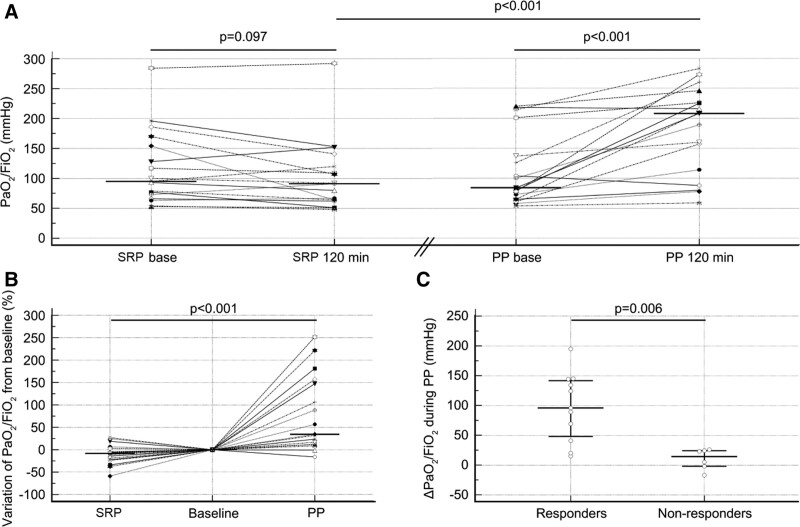
The Pao2/Fio2 ratio increased during the PP from 84 Torr (61–137 Torr) to 208 Torr (114–226 Torr) (p < 0.001) but did not change during the SRP. The Pao2/Fio2 at the end of the periods was significantly higher in patients placed in the PP than in the SRP (208 [114–226] vs 91 Torr [64–120 Torr]; p < 0.001) (Fig. 1A). The relative variations during the SRP and PP were –8% (–22% to 3%) and 34% (12–147%), respectively (p < 0.001) (Fig. 1B). During the PP, Pao2/Fio2 increased above 20% in 11 patients (65%), and these patients were classified as responders. The time course of the Pao2/Fio2 ratio among responders in the PP is presented in eFigure 2 (http://links.lww.com/CCX/B95). Further data on oxygenation in responders are available in the Supplemental Digital Content (http://links.lww.com/CCX/B95).
Figure 1.
Effects of the semirecumbent position (SRP) and prone position (PP) on the Pao2/Fio2 ratio. A, Dot plots and lines of Pao2/Fio2 at baseline and the end (120 min) of each period during the SRP and PP. The horizontal line indicates the median value. B, Dot plots and lines of the relative variation in Pao2/Fio2 during the SRP and PP, which was computed as 100 × (end value–baseline value)/baseline value. The baseline of each period was normalized to the reference level (zero). The horizontal line indicates the median value. C, Dot plots of the absolute variation in Pao2/Fio2 during the PP in the responders and nonresponders, which was computed as the end value–baseline value. The horizontal lines indicate the median values and the 25–75th percentiles. Among the six nonresponders, three had a ∆Pao2/Fio2 greater than 20 mm Hg.
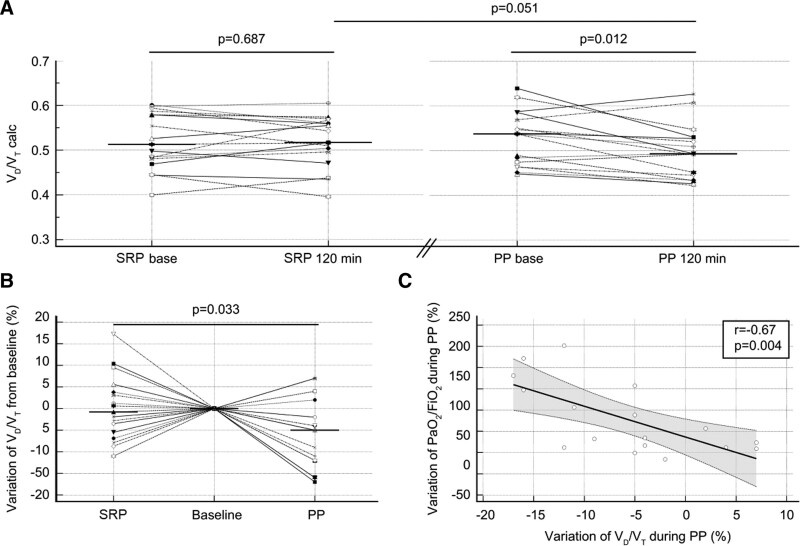
Paco2 remained remarkably constant and did not differ between the different positions. In contrast, ETCO2 increased during the PP by 2.8 mm Hg (0.3–7 mm Hg) but did not change during the SRP (0.1 mm Hg [–1.3 to 0.8 mm Hg]). Thus, the VD/VT ratio decreased during the PP from 0.54 (0.47–0.57) to 0.49 (0.45–0.53) (p = 0.012) but did not change during the SRP (Fig. 2A), and the relative variations were significantly different between the positions (Fig. 2B). Notably, the decrease in VD/VT was correlated with the increase in Pao2/Fio2 during the PP session (r = –0.67; p = 0.004) (Fig. 2C).
Figure 2.
Effects of the semirecumbent position (SRP) and prone position (PP) on the physiologic dead space to tidal volume ratio (VD/VT). A, Dot plots and lines of VD/VT at baseline and the end (120 min) of each period during the SRP and PP. The horizontal line indicates the median value. B, Dot plots and lines of the relative variation in VD/VT during the SRP and PP, which was computed as 100 × (end value–baseline value)/baseline value. The baseline of each period was normalized to the reference level (zero). The horizontal line indicates the median value. C, Scatter plot and regression analysis of the relative variation in VD/VT and the relative variation in Pao2/Fio2. The dashed lines indicate the 95% CI of the regression line.
Esophageal-Related Variables
The PES measurements were available for 16 of the 17 patients (one patient had an inaccurate PES signal). The gas flow applied during HFNO and the resulting airway pressure are reported in the Supplemental Digital Content (http://links.lww.com/CCX/B95).
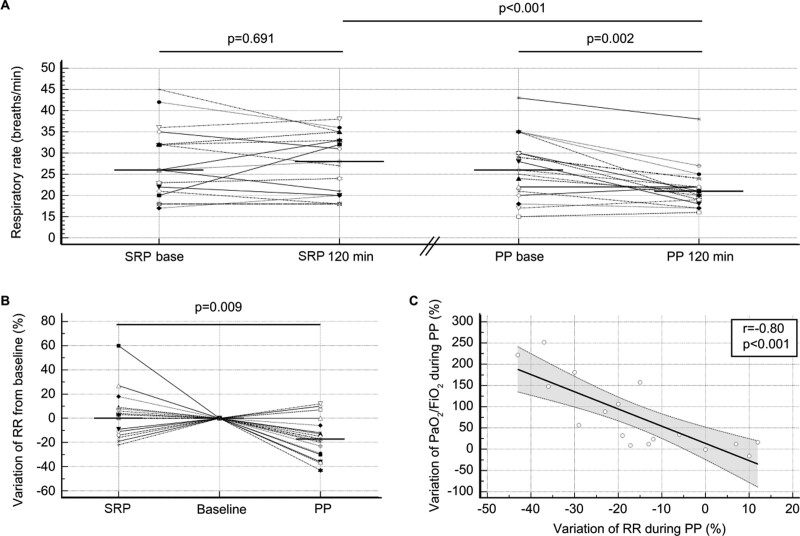
The respiratory rate decreased during the PP from 26 breaths/min (21–30 breaths/min) to 21 breaths/min (19–22 breaths/min) (p = 0.002) but did not change during the SRP. The respiratory rate was significantly lower at the end of the PP than at the end of the SRP (21 [19–22] vs 28 breaths/min [20–33 breaths/min]; p < 0.001) (Fig. 3A). The relative respiratory rate variations were also significantly different (Fig. 3B). At the individual level, 13 patients (76%) decreased their respiratory rate during the PP period with a median reduction of –5 breaths/min (–10 to –4 breaths/min). The change in the respiratory rate during the PP was inversely correlated (r = –0.74; p < 0.001) with the baseline respiratory rate level. We also observed a correlation (r = –0.8; p < 0.001) between the relative variations in the respiratory rate and Pao2/Fio2 during the PP session (p < 0.001) (Fig. 3C).
Figure 3.
Effects of the semirecumbent position (SRP) and prone position (PP) on the respiratory rate (RR). A, Dot plots and lines of the RR at the baseline and the end (120 min) of each period during the SRP and PP. The horizontal line indicates the median value. B, Dot plots and lines of the relative variation in the RR during the SRP and PP, which was computed as 100 × (end value–baseline value)/baseline value. The baseline of each period was normalized to the reference level (zero). The horizontal line indicates the median value. C, Scatter plot and regression analysis of the relative variation in the RR and the relative variation in Pao2/Fio2. The dashed lines indicate the 95% CI of the regression line.
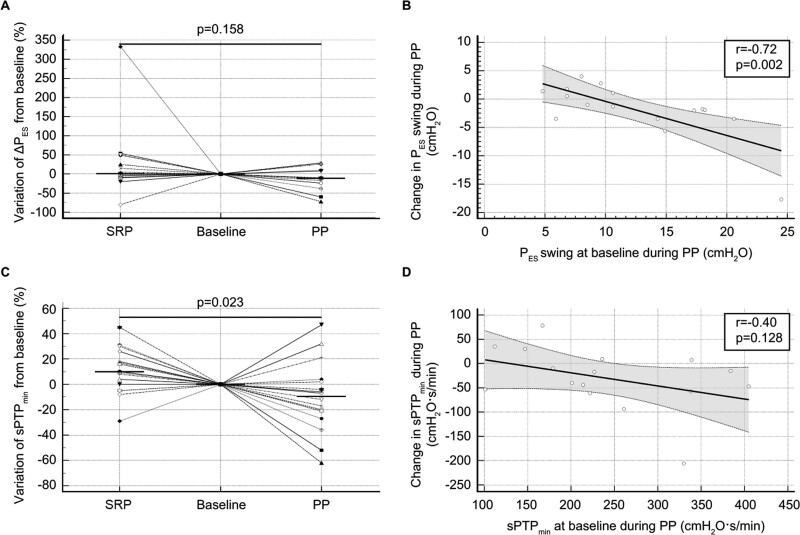
The inspiratory effort per breath, as assessed by calculating the ∆PES and sPTPES, did not significantly change with the position (Fig. 4A; and eFig. 3a, http://links.lww.com/CCX/B95). However, the changes in these variables during the PP were inversely correlated with their corresponding baseline values (r = –0.72 and p = 0.002 for both) (Fig. 4B; and eFig. 3b, http://links.lww.com/CCX/B95). The proportion of patients breathing with an inspiratory effort above the median baseline level (10.9 cm H2O) was not significantly lower at the end of the APP compared with the SRP (38% vs 56%; p = 0.18). The ∆PL displayed a similar pattern to the ∆PES (eFig. 3, c and d, http://links.lww.com/CCX/B95). The sPTPES per minute decreased during the PP from 225 cm H2O.s.min–1 (176–332 cm H2O.s.min–1) to 174 cm H2O.s.min–1 (161–254 cm H2O.s.min–1) (p = 0.049) but did not change during the SRP, resulting in significantly different variations with the positions (Fig. 4C). However, the change in the sPTPES per minute during the PP was not correlated with the baseline level (Fig. 4D). Finally, we did not observe any significant correlations between PP-induced variations in the esophageal-related variables and the change in the Pao2/Fio2 ratio.
Figure 4.
Effects of the semirecumbent position (SRP) and prone position (PP) on inspiratory muscle effort. A, Dot plots and lines of the relative variation in the esophageal pressure swing (∆PES) during SRP and PP, which was computed as 100 × (end value–baseline value)/baseline value. The baseline of each period was normalized to the reference level (zero). The horizontal line indicates the median value. B, Scatter plot and regression analysis of the baseline ∆PES and the relative variation in ∆PES during the PP. The dashed lines indicate the 95% CI of the regression line. C, Dot plots and lines of the relative variation in the simplified esophageal pressure–time product per minute (sPTPmin) during the SRP and PP, which was computed as 100 × (end value–baseline value)/baseline value. The baseline of each period was normalized to the reference level (zero). The horizontal line indicates the median value. D, Scatter plot and regression analysis of the baseline sPTPmin and the relative variation in sPTPmin during the PP. The dashed lines indicate the 95% CI of the regression line.
Hemodynamics, Self-Assessed Symptoms, and Adverse Events
The hemodynamic variables, dyspnea, and pain were not affected by the position. Discomfort was significantly lower at the end of the SRP than the PP (20 [18–40] vs 31 [25–62]; p = 0.023).
We did not observe any adverse events, except for one episode of desaturation, in each of the positions.
The results for respiratory variables associated with the need for intubation are available in the Supplemental Digital Content (eFigures 4 and 5, http://links.lww.com/CCX/B95).
DISCUSSION
The main findings of this study are as follows: 1) APP improves oxygenation in two thirds of patients and 2) APP decreases the physiologic dead space, respiratory rate, and WOB.
In nonintubated patients with COVID-19–associated AHRF, several studies have reported an increase in oxygenation during the APP, but discrepancies have been documented regarding the oxygenation endpoints, the duration of the PP, and the underlying respiratory support devices (6, 14–18). In the present study, we confirmed a significant increase in Pao2/Fio2 after 2 hours of the APP compared with the SRP. Consistent with other studies, the increase in oxygenation during the APP was not related to the baseline level of Pao2/Fio2, suggesting that the implementation of the APP should not be guided by the level of Pao2/Fio2. Furthermore, a clinically significant increase in Pao2/Fio2 (≥ 20%) was observed during the APP in only 65% of the population. A higher proportion of responders (85%) was reported when the patients were supported by helmet CPAP with a median positive end-expiratory pressure (PEEP) of 10 cm H2O (9); however, the level of PEEP provided by the HFNO in our study was much lower. The present study also provides original results for the temporal variation in Pao2/Fio2 over the 2 hours of the APP. Among the responders, Pao2/Fio2 was significantly higher after 30 minutes of the APP, suggesting that a rapid improvement is awaited. However, the time at which the Pao2/Fio2 reached its maximum level varied substantially between individuals.
Arterial hypoxemia is the main feature of severe COVID-19 pneumonia and seems to result more from the hyperperfusion of poorly ventilated lung areas (providing low VA/Q regions) than from true shunts. On the other hand, many patients had perfusion defects on CT angiography. These multiple occlusions of vessels provide high VA/Q regions with increased dead space ventilation (19–22). In the present study, we confirmed that severe hypoxemia in the context of COVID-19 pneumonia is accompanied by a high level of physiologic dead space (23). Most importantly, we found that the APP decreases both the physiologic dead space and the respiratory rate without affecting the Paco2 level, which supports an effective reduction in ventilatory inefficiency (i.e., wasted minute ventilation) (13). As the improvement in oxygenation correlated with the reduction in physiologic dead space, we hypothesize that the APP acts mainly through the homogenization of the VA/Q ratio.
The effect of the APP on the WOB in nonintubated patients is poorly described. In children with severe bronchiolitis who are supported by nasal CPAP, 1 hour of the APP decreased not only the PES–time product (PTP) per minute but also the PES swing (∆PES) and the esophageal PTP per breath (24). In adult patients with severe COVID-19 pneumonia who were supported by helmet CPAP, 3 hours of the APP reduced the modified esophageal PTP per minute but not the ∆PES (9). To the best of our knowledge, the present study is the first to investigate the effect of the APP on the WOB in spontaneously breathing patients with COVID-19 supported by HFNO. We report a reduction in the esophageal PTP per minute but no significant variation in the inspiratory muscle activity per breath.
The inspiratory effort of patients with COVID-19–related AHRF seems to be lower than that of patients with other causes of AHRF, as suggested by a retrospective propensity-matched analysis (25). In our population, the median ∆PES at the study entry was low (10.9 cm H2O), despite evidence of an increased respiratory drive (respiratory alkalosis), and this result was concordant with the value of 12.5 cm H2O reported by Tonelli et al (25) in a similar population. However, our patients were already supported by HFNO at the time of study entry, which per se yields a reduction in inspiratory effort and respiratory rate (26). Nevertheless, the low levels of dyspnea and inspiratory effort corroborate the concept of silent hypoxemia during COVID-19 (2, 27).
In the present study, the APP did not reduce the proportion of patients breathing with an inspiratory effort above the median baseline level (10.9 cm H2O). However, the patients with the highest inspiratory effort at baseline achieved the greatest reduction in inspiratory effort. Nevertheless, the APP effectively reduces the PTP per minute, which is a surrogate of the energy that is dissipated by the respiratory muscles over time. This finding is mainly attributed to the reduction in the respiratory rate, which was also consistently observed in other studies (6, 9). Similarly, the patients with the highest respiratory rate at baseline were those who achieved the greatest reduction during the APP, suggesting that despite tachypnea or high inspiratory effort, the APP should be attempted with close monitoring of respiratory function and maintained, provided a rapid clinical improvement is observed.
In patients with non-COVID-19–related AHRF, the need to switch from noninvasive support to invasive mechanical ventilation seems to be correlated with the magnitude of the inspiratory effort (28). In our population, the five patients (29%) who required subsequent intubation already had a significantly higher inspiratory effort and PTP per minute at the time of study entry. A ∆PES greater than 11.4 cm H2O best predicts the need for intubation. Although this finding strengthens the relevance of monitoring the inspiratory efforts of patients with AHRF, further confirmation in a larger population is needed.
Our study has some limitations. The sample size was calculated to detect an improvement in oxygenation, but it seems underpowered to detect a reduction in the inspiratory effort per breath. Furthermore, the measurements of the respiratory mechanic parameters relied on some assumptions that may be subject to errors. First, the airway pressure was not measured but was estimated from the HFNO settings (29) and was presumed to be constant during tidal ventilation. Second, similar to other studies (10, 26), we neglected the WOB and the inspiratory effort due to the elastic recoil of the chest wall. Nevertheless, we did not measure the transdiaphragmatic pressure and thus cannot exclude the possibility that expiratory muscle activity affected our results (30). Additionally, we used a predictive equation to estimate the VD/VT (12) that was not validated in nonintubated patients. Nevertheless, the range of physiologic dead space that we reported is consistent with those of mechanically ventilated patients with COVID-19 (31), and the significant reduction observed during the APP was not achieved with the SRP. Finally, the results of this study should not be extrapolated to patients receiving respiratory support other than HFNO.
CONCLUSIONS
In patients with AHRF related to severe COVID-19 pneumonia who are supported by HFNO, the APP improves oxygenation and reduces ventilatory inefficiency, resulting in a decrease in the WOB, which mainly occurs by lowering the respiratory rate. The inspiratory effort was unaltered by the APP, but approximately half of the patients had a low baseline ∆PES. In contrast, the patients with the highest respiratory rate or inspiratory effort at baseline were those who achieved the greatest reduction during the APP. Our findings provide novel pathophysiologic insights into the short-term effect of the APP and enhance the rationale for its early use in the process of care of patients with COVID-19–related AHRF.
ACKNOWLEDGMENTS
We thank Dr. Anne Marie Dols (Faculté de Médecine, Université Grenoble Alpes, Grenoble, France) for her advice on the study design. The English language of this article was edited by American Journal Experts.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
The authors have disclosed that they do not have any potential conflicts of interest.
Drs. Lehingue and Allardet-Servent are cofirst authors.
Registered on ClinicalTrials.gov (NCT04543760).
REFERENCES
- 1.COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators: Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: A prospective cohort study. Intensive Care Med 2021; 47:60–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tobin MJ, Laghi F, Jubran A: Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med 2020; 202:356–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alhazzani W, Møller MH, Arabi YM, et al. : Surviving sepsis campaign: Guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med 2020; 46:854–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ospina-Tascón GA, Calderón-Tapia LE, García AF, et al. ; HiFLo-Covid Investigators: Effect of high-flow oxygen therapy vs conventional oxygen therapy on invasive mechanical ventilation and clinical recovery in patients with severe COVID-19: A randomized clinical trial. JAMA 2021; 326:2161–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nasa P, Azoulay E, Khanna AK, et al. : Expert consensus statements for the management of COVID-19-related acute respiratory failure using a Delphi method. Crit Care 2021; 25:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehrmann S, Li J, Ibarra-Estrada M, et al. ; Awake Prone Positioning Meta-Trial Group: Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: A randomised, controlled, multinational, open-label meta-trial. Lancet Respir Med 2021; 9:1387–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nyrén S, Mure M, Jacobsson H, et al. : Pulmonary perfusion is more uniform in the prone than in the supine position: scintigraphy in healthy humans. J Appl Physiol (1985) 1999; 86:1135–1141 [DOI] [PubMed] [Google Scholar]
- 8.Henderson AC, Sá RC, Theilmann RJ, et al. : The gravitational distribution of ventilation-perfusion ratio is more uniform in prone than supine posture in the normal human lung. J Appl Physiol (1985) 2013; 115:313–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiumello D, Chiodaroli E, Coppola S, et al. : Awake prone position reduces work of breathing in patients with COVID-19 ARDS supported by CPAP. Ann Intensive Care 2021; 11:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grieco DL, Menga LS, Raggi V, et al. : Physiological comparison of high-flow nasal cannula and helmet noninvasive ventilation in acute hypoxemic respiratory failure. Am J Respir Crit Care Med 2020; 201:303–312 [DOI] [PubMed] [Google Scholar]
- 11.Mojoli F, Chiumello D, Pozzi M, et al. : Esophageal pressure measurements under different conditions of intrathoracic pressure. An in vitro study of second generation balloon catheters. Minerva Anestesiol 2015; 81:10. [PubMed] [Google Scholar]
- 12.Frankenfield DC, Alam S, Bekteshi E, et al. : Predicting dead space ventilation in critically ill patients using clinically available data. Crit Care Med 2010; 38:288–291 [DOI] [PubMed] [Google Scholar]
- 13.López R, Caviedes I, Graf J: Minute ventilation to carbon dioxide production ratio is a simple and non-invasive index of ventilatory inefficiency in mechanically ventilated patients: proof of concept. Intensive Care Med 2017; 43:1542–1543 [DOI] [PubMed] [Google Scholar]
- 14.Coppo A, Bellani G, Winterton D, et al. : Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): A prospective cohort study. Lancet Respir Med 2020; 8:765–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrando C, Mellado-Artigas R, Gea A, et al. ; COVID-19 Spanish ICU Network: Awake prone positioning does not reduce the risk of intubation in COVID-19 treated with high-flow nasal oxygen therapy: A multicenter, adjusted cohort study. Crit Care 2020; 24:597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perez-Nieto OR, Escarraman-Martinez D, Guerrero-Gutierrez MA, et al. ; APRONOX Group: Awake prone positioning and oxygen therapy in patients with COVID-19: The APRONOX study. Eur Respir J 2022; 59:2100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Padrão EMH, Valente FS, Besen BAMP, et al. ; COVIDTEAM: Awake prone positioning in COVID-19 hypoxemic respiratory failure: Exploratory findings in a single-center retrospective cohort study. Acad Emerg Med 2020; 27:1249–1259 [DOI] [PubMed] [Google Scholar]
- 18.Jouffroy R, Darmon M, Isnard F, et al. : Impact of prone position in non-intubated spontaneously breathing patients admitted to the ICU for severe acute respiratory failure due to COVID-19. J Crit Care 2021; 64:199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Busana M, Giosa L, Cressoni M, et al. : The impact of ventilation-perfusion inequality in COVID-19: A computational model. J Appl Physiol (1985) 2021; 130:865–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel BV, Arachchillage DJ, Ridge CA, et al. : Pulmonary angiopathy in severe COVID-19: Physiologic, imaging, and hematologic observations. Am J Respir Crit Care Med 2020; 202:690–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang M, Som A, Mendoza DP, et al. : Hypoxaemia related to COVID-19: Vascular and perfusion abnormalities on dual-energy CT. Lancet Infect Dis 2020; 20:1365–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrmann J, Mori V, Bates JHT, et al. : Modeling lung perfusion abnormalities to explain early COVID-19 hypoxemia. Nat Commun 2020; 11:4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L, Xie J, Wang C, et al. : Prone position improves lung ventilation–perfusion matching in non-intubated COVID-19 patients: A prospective physiologic study. Crit Care 2022; 26:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baudin F, Emeriaud G, Essouri S, et al. : Physiological effect of prone position in children with severe bronchiolitis: A randomized cross-over study (BRONCHIO-DV). J Pediatr 2019; 205:112–119.e4 [DOI] [PubMed] [Google Scholar]
- 25.Tonelli R, Busani S, Tabbì L, et al. : Inspiratory effort and lung mechanics in spontaneously breathing patients with acute respiratory failure due to COVID-19: A matched control study. Am J Respir Crit Care Med 2021; 204:725–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mauri T, Turrini C, Eronia N, et al. : Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med 2017; 195:1207–1215 [DOI] [PubMed] [Google Scholar]
- 27.Simonson TS, Baker TL, Banzett RB, et al. : Silent hypoxaemia in COVID-19 patients. J Physiol 2021; 599:1057–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tonelli R, Fantini R, Tabbì L, et al. : Early inspiratory effort assessment by esophageal manometry predicts noninvasive ventilation outcome in de novo respiratory failure. A pilot study. Am J Respir Crit Care Med 2020; 202:558–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parke RL, McGuinness SP: Pressures delivered by nasal high flow oxygen during all phases of the respiratory cycle. Respir Care 2013; 58:1621–1624 [DOI] [PubMed] [Google Scholar]
- 30.Doorduin J, Roesthuis LH, Jansen D, et al. : Respiratory muscle effort during expiration in successful and failed weaning from mechanical ventilation. Anesthesiology 2018; 129:490–501 [DOI] [PubMed] [Google Scholar]
- 31.Vasques F, Sanderson B, Formenti F, et al. : Physiological dead space ventilation, disease severity and outcome in ventilated patients with hypoxaemic respiratory failure due to coronavirus disease 2019. Intensive Care Med 2020; 46:2092–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.