ABSTRACT
Background
Females with a history of gestational diabetes mellitus (GDM) are at higher risk of developing type 2 diabetes mellitus (T2D) later in life.
Objective
This study prospectively examined whether greater habitual coffee consumption was related to a lower risk of T2D among females with a history of GDM.
Methods
We followed 4522 participants with a history of GDM in the NHS II for incident T2D between 1991 and 2017. Demographic, lifestyle factors including diet, and disease outcomes were updated every 2–4 y. Participants reported consumption of caffeinated and decaffeinated coffee on validated FFQs. Fasting blood samples were collected in 2012–2014 from a subset of participants free of diabetes to measure glucose metabolism biomarkers (HbA1c, insulin, C-peptide; n = 518). We used multivariable Cox regression models to calculate adjusted HRs and 95% CIs for the risk of T2D. We estimated the least squares mean of glucose metabolic biomarkers according to coffee consumption.
Results
A total of 979 participants developed T2D. Caffeinated coffee consumption was inversely associated with the risk of T2D. Adjusted HR (95% CI) for ≤1 (nonzero), 2–3, and 4+ cups/d compared with 0 cup/d (reference) was 0.91 (0.78, 1.06), 0.83 (0.69, 1.01), and 0.46 (0.28, 0.76), respectively (P-trend = 0.004). Replacement of 1 serving/d of sugar-sweetened beverage and artificially sweetened beverage with 1 cup/d of caffeinated coffee was associated with a 17% (risk ratio [RR] = 0.83, 95% CI: 0.75, 0.93) and 9% (RR = 0.91, 95% CI: 0.84, 0.99) lower risk of T2D, respectively. Greater caffeinated coffee consumption was associated with lower fasting insulin and C-peptide concentrations (all P-trend <0.05). Decaffeinated coffee intake was not significantly related to T2D but was inversely associated with C-peptide concentrations (P-trend = 0.003).
Conclusions
Among predominantly Caucasian females with a history of GDM, greater consumption of caffeinated coffee was associated with a lower risk of T2D and a more favorable metabolic profile.
Keywords: individuals with a history of gestational diabetes mellitus, type 2 diabetes risk, coffee consumption, diet, nutrition
See corresponding editorial on page 1468.
Introduction
Gestational diabetes mellitus (GDM) is a common pregnancy complication currently affecting ∼8% of pregnancies in the USA (1) and ≤30% worldwide (2). Compared with the general population, individuals with a history of GDM have a nearly 10-fold higher risk of developing type 2 diabetes (T2D) (3). GDM is considered to be an early marker for cardiometabolic health, offering an opportunity for early intervention on modifiable risk factors to lower the subsequent risk of T2D among these individuals (2).
Coffee is among the most widely consumed beverages worldwide (4). In the USA between 2011 and 2012, an average coffee consumption was 1.5 cups/d for adults, with coffee as the main contributor to caffeine intake (5). In 2015 and reaffirmed in 2020 (6, 7), the Scientific Report of the Dietary Guidelines Advisory Committee concluded that moderate coffee consumption (3–5 cups/d) can be part of a healthy diet. A wide range of long-term health benefits of coffee consumption has been reported in the general population, including a reduction in the risk of T2D via the consumption of both caffeinated and decaffeinated coffee (8).
Several biological mechanisms have been reported for multiple bioactive compounds of coffee (e.g. caffeine, phenolics) for an improved metabolic profile, including improved insulin and glucose homeostasis (9), antioxidative (10), thermogenic (11), and anti-inflammatory effects (12), and improved gut microbiota diversity (4, 8, 13). Findings from clinical trials focusing on the effect of caffeine on glucose metabolism have suggested the effect may depend on follow-up duration; in the short term, caffeine was related to insulin resistance (4) whereas, in the long term, coffee consumption improved glucose metabolism in general (8). However, the associations of coffee consumption with subsequent risk of T2D or the profile of glucose metabolism among high-risk females with a history of GDM remained unexplored. To address these knowledge gaps, we sought to evaluate the associations between coffee consumption and the risk of incident T2D and biomarker profile of glucose metabolism among individuals with a history of GDM in a large prospective female cohort.
Methods
Study population
The study population consists of participants with a history of GDM in the NHS II, as part of the Diabetes & Women's Health (DWH) Study (14), which aims to identify determinants for progression from GDM to T2D. The NHS II, established in 1989, is an ongoing prospective cohort study of 116,429 female nurses aged 24–44 y at study initiation (15). Participants received a biennial questionnaire to update information on health-related behaviors and disease outcomes. The study protocol was approved by the Institutional Review Boards of the Brigham and Women's Hospital and Harvard T. H. Chan School of Public Health, with participants’ consent implied by the return of the questionnaires.
For the current investigation, 1991 was the start of follow-up, i.e. the first follow-up cycle when detailed information on diet and lifestyle was collected. Participants were included in the study if they self-reported GDM in 1991. They also became eligible if they reported incident GDM at any time during the follow-up period from 1991 to 2001, after which the majority of the NHS II participants had passed reproductive age. In a prior validation study, 94% of self-reported GDM cases were confirmed to have a physician diagnosis of GDM either by the National Diabetes Data Group criteria or evidence of abnormal glucose homeostasis on record review (16). A high level of GDM surveillance was found in a random sample of parous participants without GDM in the NHS II (16). We excluded participants from the analysis if they had missing birthday or missing baseline dietary data, a multiple-birth pregnancy (i.e. twins or triplets), a GDM-complicated pregnancy before the age of 18, a history of T2D, cardiovascular disease (i.e. MI or stroke), or cancer except nonmelanoma skin cancers prior to the GDM pregnancy or before the return of their first post-GDM questionnaire (Supplementary Figure 1). The final analytic sample consisted of 4522 participants with a history of GDM (follow-up rate as of the end of follow-up, June 2017: 88%).
Assessment of coffee, caffeine, and other beverage intakes
The NHS II participants reported their usual intakes of food and beverages through a validated semi-quantitative food frequency questionnaire (FFQ) administered every 4 y starting in 1991. Participants were asked how often, on average, they had consumed a standard portion size of each food item during the previous year (never to ≥6 times/d) (17). Frequency of consumption for both caffeinated and decaffeinated coffee (“1 cup”) was separately assessed on the FFQ. Intake of caffeine (mg/d) was derived by summing the caffeine content of contributing items (137 mg/cup from coffee, 47 mg/cup from tea, 46 mg/bottle or can from cola beverage, and 7 mg/serving from chocolate candy) according to the USDA food composition data, weighted by the frequency of its use reported on the FFQ (18). The questionnaire items on the other beverage intakes, including sugar-sweetened beverages (SSBs), artificially sweetened beverages (ASBs), fruit juices, caffeinated and decaffeinated tea, and dairy, have been described elsewhere (17). A validation study conducted in a subsample of the participants found a correlation coefficient of 0.78 for coffee intake between the FFQ and 4 1-wk multiple dietary records collected over a 1-y period; intakes of the other beverages were validated in the same study (correlation coefficient: 0.93 for tea, 0.84 for SSBs and ASBs, and 0.56 for fruit juices) (19).
Ascertainment of covariates
At baseline and during follow-up, information on demographics, lifestyle, other risk factors for chronic diseases, and reproductive events was self-reported and updated biennially through self-administered questionnaires. Family history of diabetes in first-degree relatives was assessed in 1989,1997, 2001, and 2005. We used the Alternate Healthy Eating Index (AHEI) derived from the FFQ as a measure of overall diet quality (20). Alcohol intake (g/d) was assessed on the same FFQs (21). Leisure-time physical activity was self-reported every 2–6 y starting from 1991 (22). Self-reported body weight and physical activity have been validated in previous studies (23, 24).
Ascertainment of T2D
Participants who reported physician-diagnosed T2D on any biennial questionnaire received a supplementary questionnaire regarding symptoms, diagnostic tests, and hypoglycemic therapy to confirm the T2D diagnoses. A case was considered confirmed if ≥1 of the following was reported on the supplementary questionnaire according to the American Diabetes Association criteria (25): 1) 1 or more classic symptoms (excessive thirst, polyuria, weight loss, hunger, pruritus, or coma) plus elevated glucose concentrations (fasting plasma glucose concentration ≥7.0 mmol/L or random plasma glucose ≥11.1 mmol/L); or 2) no symptoms reported but 2 or more elevated plasma glucose concentrations on >1 occasion (fasting ≥7.0 mmol/L, random ≥11.1 mmol/L, 2-h OGTT ≥11.1 mmol/L); or 3) treatment with insulin or oral hypoglycemic agent. Before 1998, fasting plasma glucose ≥7.8 mmol/L was used instead of ≥7.0 mmol/L for the diagnosis of diabetes according to the criteria of National Diabetes Data Group (26). A high accuracy (98%) (27) and a low frequency (0.5%) (28) of underreporting were observed compared with medical records.
Biospecimen collection and biomarker assessment
Between 2012 and 2014, the DWH Study invited NHS II participants with a history of GDM for biospecimen collection. Details on the collection and processing can be found elsewhere (14, 29). Briefly, a phlebotomy kit including instructions for fasting blood sample collection was mailed to these participants. Blood samples were shipped overnight back to a central laboratory for processing according to the standardized procedures and storage (at −80°C). HbA1c was assessed using a nonporous ion exchange HPLC assay (Tosoh Automated Analyzer HLC- 723G8; Tosoh Bioscience, Inc.). C-peptide concentration was measured using C-peptide micro-ELISA (Quansys Biosciences). Insulin concentrations were assessed using the cobas 6000 chemistry analyzer (Roche Diagnostics). The inter-assay CVs ranged from 1.2% to 10.0% for all assays. A total of 743 participants provided fasting blood samples.
Statistical analysis
For the current analysis, baseline was defined as the questionnaire period when a participant first reported a pregnancy complicated by GDM during 1991–2001. We computed person-time years from the date of GDM diagnosis to the date of T2D diagnosis, death, last biennial questionnaire response, or the end of the follow-up (June 2017), whichever occurred first. As coffee consumption during pregnancy may not reflect habitual coffee consumption (4), questionnaire data and person-time were excluded from analysis when a participant reported being pregnant during a follow-up cycle.
Given the biological plausibility of caffeine and other compounds of coffee for T2D etiology, we considered the consumption of total coffee, including both caffeinated and decaffeinated coffee, as the main exposure of interest; we also examined caffeinated and decaffeinated coffee separately. With respect to coffee intake and other dietary factors, in order to represent long-term intake and to minimize within-person variation, we calculated time-updated cumulative averages of dietary data reported from the first post-GDM follow-up cycle (i.e. baseline) through the time of censoring events (e.g. 1995 cumulative average was calculated as the average of intake reported in 1991 and 1995) (30). If dietary data was missing at a given questionnaire cycle or participants had unusual FFQs (i.e. >70 items left blank or total energy intake <500 or >3500 kcal/d), values were carried forward from the previous questionnaire for which the data was reported; update of dietary factors ceased if a participant reported incident diagnosis of cancer or CVD during the follow-up, since such outcomes may change dietary behaviors (30).
For the main analysis on coffee consumption and the risk of T2D incidence, participants were divided into 4 coffee categories: 0 cup/d (reference), ≤1 cup/d (nonzero), 2–3 cups/d, or 4+ cups/d, consistent with an earlier NHS II analysis (18). We used multivariable Cox proportional hazards models to calculate HR and 95% CI for the risk of T2D; we checked proportional hazard assumptions by stratifying on age (<50, ≥50 y) and conducting likelihood ratio tests to test the interaction terms between exposure and age. We did not find any evidence that the proportional hazards assumption was violated. The models were stratified by age and calendar period (Model 1). The models were then additionally adjusted for race, family history of diabetes, history of hypertension, history of hypercholesterolemia, parity, oral contraceptive use, menopausal status, smoking status, physical activity, and other dietary factors (i.e. total energy intake, diet quality [modified AHEI excluding the alcohol component], alcohol intake in quartiles, and consumption of tea, SSBs, ASBs, fruit juices, and dairy). Since BMI may be a mediator and/or a confounder for the associations of interest, we presented the models before (Model 2) and after (Model 2 + BMI) adjusting for BMI. Covariates were time-updated except race; the value from the previous cycle was carried forward if a covariate was missing at a given follow-up cycle. Median values of each exposure category were entered into the model to estimate P value for trend. The risk of T2D associated with per additional cup/d coffee was estimated. We subsequently examined caffeinated and decaffeinated coffee separately on T2D risk, mutually adjusting for each other. We evaluated the associations of hypothetically replacing various beverages per serving/d, including tea, SSB, ASB, and fruit juice with coffee (17, 30, 31). We additionally examined caffeine intake by quartiles with the risk of T2D; the same set of covariates excluding caffeine-contained dietary factors (i.e. SSB, ASB, and caffeinated tea) were adjusted in the models.
In addition, we evaluated whether coffee consumption may be differentially associated with T2D risk among individuals with certain lifestyle factors or medical history by stratifying on major risk factors of T2D, including BMI (<30 kg/m2 compared with ≥30), diet quality (below compared with above AHEI median [46.6]), alcohol intake (<0.5 g/d compared with ≥0.5 g/d), physical activity [<10 metabolic equivalents (MET)]-h/wk compared with ≥10 MET-h/wk], family history of diabetes (yes compared with no), history of hypertension (yes compared with no), history of hypercholesterolemia (yes compared with no), and smoking status (never compared with current or past); all the stratified risk factors were time updated. Furthermore, we examined coffee consumption by alternatively modeling time-updated coffee consumption reported at the most recent cycle with T2D risk (30). To address potential residual confounding due to socioeconomic status and sugar intakes, we additionally adjusted for household income reported in 2001 and intakes of total added sugar (tablespoons/d). We further estimated the associations of coffee with T2D risk within 10 y and within 20 y since the start of the follow-up to evaluate whether the associations may differ over time.
For the investigation of coffee consumption and glucose metabolism biomarkers, glucose metabolic biomarkers of interest were HbA1c (%, mmol/mol), C-peptide (ng/mL), and insulin (pmol/L). Analysis was restricted to participants who: 1) had nonmissing values for the biomarkers of interest (n = 622), 2) were free of T2D based on the diagnostic criteria (n = 610), and 3) fasting HbA1c <6.5% (48 mmol/mol) at the time of blood collection (n = 518). Baseline population characteristics were overall comparable in participants who had available biomarkers of interest (n = 622) compared with those who did not. For each biomarker, distribution was assessed for normality first; natural log transformation was performed for skewed biomarkers. Multivariable generalized linear models were used to estimate the least square mean of each biomarker according to categories of coffee consumption calculated as the cumulative average through the eligible follow-up period(s) up to the time of the blood draw. All statistical analyses were performed with SAS software (version 9.3; SAS Institute Inc.); a P value <0.05 was considered statistically significant.
Results
With a median follow-up of 23.9 y, we documented 979 T2D cases over a total of 88,809.5 person-years. At baseline, compared to individuals with no coffee consumption, individuals with higher coffee consumption were more likely to be leaner and be a current or past smoker; they were less likely to have a history of hypertension or hypercholesterolemia; they had higher intakes of alcohol, caffeine, and dairy, on average (Table 1). During the follow-up, the mean and median of total coffee consumption was 1.2 cups/d and 0.7 cup/d over the entire study sample, respectively; the consumption of caffeinated coffee was higher than that of decaffeinated coffee (mean: 1.0 cup/d compared with 0.2 cup/d; median: 0.5 cup/d compared with 0 cup/d; correlation between caffeinated compared with decaffeinated coffee: 0.28) (Supplementary Table 1). The consumption of decaffeinated coffee was uncommon, with 52.5%, 44.0%, and 3.5% of total person-years allocated to 0 cup/d, ≤ 1 cup/d, and 2+ cups/d, respectively (Table 2).
TABLE 1.
Baseline population characteristics by total coffee consumption among individuals with a history of gestational diabetes mellitus, NHS II (n = 4522)1
| Total coffee intake | ||||
|---|---|---|---|---|
| 0 cup/d (n = 1634, 36.1%) | ≤1 cup/d (n = 1289, 28.5%) | 2–3 cups/d (n = 1240, 27.4%) | 4+ cups/d (n = 359, 7.9%) | |
| Age, mean (SD), y | 37.8 (5.2) | 38.2 (5.4) | 38.9 (5.1) | 38.4 (4.4) |
| Age at GDM incidence, mean (SD), y | 30.5 (5.3) | 30.4 (5.1) | 30.7 (5.2) | 29.3 (5.4) |
| White, no. (%) | 1486 (90.9) | 1141 (88.5) | 1167 (94.1) | 343 (95.5) |
| BMI, mean (SD), kg/m2 | 27.0 (6.5) | 26.2 (6.1) | 25.9 (5.7) | 26.3 (5.7) |
| Age at first birth, mean (SD), y | 27.5 (4.8) | 27.5 (4.7) | 27.6 (4.9) | 26.4 (4.9) |
| Parity, median (IQR) | 2 (2,3) | 2 (2,3) | 2 (2,3) | 2 (2,3) |
| Status of oral contraceptive use, no. (%) | ||||
| - Current user | 138 (8.4) | 103 (8.0) | 75 (6.0) | 18 (5.2) |
| - Past user | 1270 (77.7) | 1006 (78.0) | 997 (80.4) | 299 (83.3) |
| - Never user | 226 (13.8) | 180 (14.0) | 168 (13.5) | 42 (11.7) |
| Family history of diabetes, no. (%) | 764 (46.8) | 663 (51.4) | 589 (47.5) | 184 (51.3) |
| Ever had hypertension, no. (%) | 148 (9.1) | 121 (9.4) | 84 (6.8) | 20 (5.6) |
| Ever had hypercholesterolemia, no. (%) | 330 (20.2) | 242 (18.8) | 235 (19.0) | 52 (14.5) |
| Menopausal status, no. (%) | ||||
| - Premenopausal | 1581 (96.8) | 1218 (94.5) | 1192 (96.1) | 342 (95.3) |
| - Postmenopausal | 53 (3.2) | 71 (5.5) | 48 (3.9) | 17 (4.7) |
| Leisure-time physical activity, mean (SD), MET-h/wk2 | 17.7 (25.6) | 17.9 (27.2) | 18.3 (23.4) | 17.0 (21.7) |
| Total energy intake, mean (SD), kcal/d | 1893 (581) | 1891 (579) | 1941 (563) | 1959 (561) |
| AHEI score, mean (SD) | 45.3 (10.7) | 47.8 (10.9) | 49.6 (10.7) | 47.6 (11) |
| Total caffeine intake, mean (SD), mg/d | 96.6 (103.7) | 133.5 (87.1) | 345.0 (129.7) | 640.4 (216.1) |
| Total tea consumption, mean (SD), serving/d | 0.8 (1.2) | 0.8 (1.1) | 0.6 (1.0) | 0.6 (1.2) |
| Sugar-sweetened beverage, mean (SD), serving/d | 0.5 (1.0) | 0.5 (0.9) | 0.4 (0.7) | 0.4 (0.8) |
| Artificially sweetened beverage, mean (SD), serving/day | 1.1 (1.7) | 1.0 (1.5) | 1.0 (1.3) | 1.1 (1.5) |
| Fruit juice, mean (SD), serving/d | 0.5 (0.7) | 0.7 (0.8) | 0.6 (0.8) | 0.6 (0.7) |
| Dairy consumption, mean (SD), serving/d | 1.1 (1.2) | 1.3 (1.2) | 1.5 (1.3) | 1.5 (1.5) |
| Alcohol intake, mean (SD), g/d | 1.5 (4.1) | 1.9 (3.7) | 3.5 (6) | 3.6 (6.4) |
| Smoking status, no. (%) | ||||
| - Never smoker | 1266 (77.5) | 897 (69.6) | 701 (56.5) | 137 (38.2) |
| - Past smoker | 244 (14.9) | 295 (22.9) | 381 (30.7) | 111 (30.9) |
| - Current smoker | 124 (7.6) | 97 (7.5) | 158 (12.7) | 111 (30.9) |
Baseline was defined as the first post-GDM pregnancy diet questionnaire cycle during follow-up period between 1991 and 2001. AHEI, Alternate Healthy Eating Index; GDM, gestational diabetes mellitus; MET, metabolic equivalents.
Metabolic equivalents were calculated from weekly leisure-time moderate- or vigorous-intensity physical activities.
TABLE 2.
Habitual coffee consumption and risk of subsequent type 2 diabetes (T2D) among individuals with a history of gestational diabetes mellitus, NHS II (n = 4,522,979 T2D cases with 88,809.5 person-years)1
| Risk of type 2 diabetes, HR (95% CI) | |||||
|---|---|---|---|---|---|
| Cumulative average of coffee intake | Median (cup/d) | Case/person-years | Model 12 | Model 23 | Model 2 + BMI |
| Total coffee | |||||
| 0 cup/d | 0 | 290/22,792.5 | Ref (HR = 1.00) | Ref (HR = 1.00) | Ref (HR = 1.00) |
| ≤1 cup/d | 0.6 | 417/36,679.3 | 0.85 (0.73, 0.98) | 0.90 (0.77, 1.06) | 0.93 (0.79, 1.09) |
| 2–3 cups/d | 2.5 | 239/24,336.7 | 0.75 (0.63, 0.89) | 0.92 (0.76, 1.11) | 0.95 (0.78, 1.15) |
| 4+ cups/d | 4.5 | 33/5001.0 | 0.52 (0.36, 0.74) | 0.57 (0.39, 0.83) | 0.60 (0.41, 0.88) |
| P-trend4 | <0.001 | 0.04 | 0.07 | ||
| Per additional 1 cup/d | 0.88 (0.83, 0.93) | 0.92 (0.87, 0.98) | 0.93 (0.88, 0.99) | ||
| Caffeinated coffee | |||||
| 0 cup/d | 0 | 344/26,973.6 | Ref (HR = 1.00) | Ref (HR = 1.00) | Ref (HR = 1.00) |
| ≤1 cup/d | 0.5 | 428/37,945.6 | 0.83 (0.72, 0.96) | 0.91 (0.78, 1.06) | 0.91 (0.78, 1.06) |
| 2–3 cups/d | 2.5 | 190/20,745.6 | 0.70 (0.58, 0.83) | 0.85 (0.70, 1.03) | 0.83 (0.69, 1.01) |
| 4+ cups/d | 4.5 | 17/3144.8 | 0.44 (0.27, 0.71) | 0.46 (0.28, 0.76) | 0.46 (0.28, 0.76) |
| P-trend | <0.001 | 0.005 | 0.004 | ||
| Per additional 1 cup/d | 0.87 (0.82, 0.92) | 0.92 (0.86, 0.98) | 0.91 (0.86, 0.98) | ||
| Decaffeinated coffee | |||||
| 0 cup/d | 0 | 556/46,601.7 | Ref (HR = 1.00) | Ref (HR = 1.00) | Ref (HR = 1.00) |
| ≤1 cup/d | 0.1 | 394/39,095.5 | 0.80 (0.70, 0.91) | 0.89 (0.78, 1.02) | 0.98 (0.86, 1.13) |
| 2+ cups/d | 2.5 | 29/3112.3 | 0.79 (0.54, 1.16) | 0.90 (0.62, 1.32) | 1.06 (0.73, 1.56) |
| P-trend | 0.27 | 0.65 | 0.74 | ||
| Per additional 1 cup/d | 0.89 (0.77, 1.02) | 0.94 (0.82, 1.08) | 1.02 (0.90, 1.17) | ||
| Caffeine intake5 | Range, median (mg/d) | ||||
| Quartile 1 | 0–74.1, 31.0 | 253/22,217.4 | Ref (HR = 1.00) | Ref (HR = 1.00) | Ref (HR = 1.00) |
| Quartile 2 | 74.2–161.0,118.3 | 279/22,025.5 | 1.12 (0.94, 1.33) | 1.11 (0.94, 1.32) | 1.11 (0.94, 1.33) |
| Quartile 3 | 161.1–296.5,222.0 | 238/22,161.3 | 0.93 (0.78, 1.11) | 0.97 (0.81, 1.17) | 0.97 (0.80, 1.16) |
| Quartile 4 | 296.6–1181,390 | 209/22,405.3 | 0.80 (0.66, 0.96) | 0.84 (0.69, 1.03) | 0.81 (0.66, 0.99) |
| P-trend | 0.002 | 0.03 | 0.008 | ||
AHEI, Alternate Healthy Eating Index; ASB, artificially sweetened beverage; GDM, gestational diabetes mellitus; MET, metabolic equivalents; SSB, sugar-sweetened beverage.
Model 1 was stratified by age (y) and calendar time period (indicators).
Model 2 was additionally adjusted for race (white, nonwhite), family history of diabetes (yes, no), history of hypertension (yes/no), history of hypercholesterolemia (yes/no), parity (1, 2+), oral contraceptive use (current, past, never), menopausal status (premenopausal, postmenopausal), smoking (never, current, past), physical activity (MET-h/wk), total calorie intake (kcal/d), AHEI score excluding alcohol, alcohol intake (g/d) in quartiles, continuous beverage intakes including tea, SSB, ASB, and juice, and dairy intake. Caffeinated and decaffeinated coffee were mutually adjusted for each other depending on the model.
Medium values for each category were entered into the model to estimate P value for trend.
Caffeine intake was derived by summing the caffeine content for a specific amount multiplied by a weight proportional to the frequency of its use from contributing items, including coffee (137 mg/cup), tea (47 mg/cup), cola beverage (46 mg/can or bottle), and chocolate candy (7 mg/serving), according to the USDA food composition data. Caffeine-containing beverages (i.e. SSB, ASB, and tea) were not adjusted in the models for caffeine.
Coffee consumption and T2D risk
After adjusting for major diet and other lifestyle covariates, the consumption of total coffee was inversely associated with the risk of T2D (Table 2). Compared with individuals who did not drink coffee, the adjusted HR (95% CI) was 0.90 (0.77, 1.06), 0.92 (0.76, 1.11), and 0.57 (0.39, 0.83) for ≤1 cup/d, 2–3 cups/d, and 4+ cups/d of total coffee, respectively (P-trend = 0.04); each additional 1 cup/d of total coffee was associated with an 8% lower risk of T2D (HR = 0.92, 95% CI: 0.87, 0.98). Additional adjustment for BMI attenuated the associations (P-trend = 0.07), but the adjusted HR for consumption of 4+ cups/d remained significant (HR = 0.60, 95% CI: 0.41, 0.88).
When examining the associations by coffee type and mutually adjusting for each other, we observed an inverse association between the consumption of caffeinated coffee and the risk of T2D: compared with individuals who did not drink caffeinated coffee, the adjusted HR (95% CI) for ≤1 cup/d, 2–3 cups/d, and 4+ cups/d of caffeinated coffee was 0.91 (0.78, 1.06), 0.85 (0.70, 1.03), and 0.46 (0.28, 0.76), respectively (P-trend = 0.005); each additional 1 cup/d of caffeinated coffee was associated with an 8% lower risk of T2D (HR = 0.92, 95% CI: 0.86, 0.98) (Table 2). The inverse associations remained significant after further adjusting for BMI (HR [95% CI] for ≤1 cup/d, 2–3 cups/d, and 4+ cups/d: 0.91 [0.78, 1.06], 0.83 [0.69, 1.01], and 0.46 [0.28, 0.76], respectively (P-trend = 0.004); each additional 1 cup/d of caffeinated coffee was associated with a 9% lower risk of T2D (HR = 0.91, 95% CI: 0.86, 0.98). No evidence of associations was observed for decaffeinated coffee with T2D risk overall.
The intake of caffeine was significantly associated with a lower risk of T2D (Table 2). Compared with the lowest quartile (Q1), the adjusted HR (95% CI) for Q2, Q3, and Q4 was 1.11 (0.94, 1.32), 0.97 (0.81, 1.17), and 0.84 (0.69, 1.03), respectively (P-trend = 0.03). The association remained significant after additional adjustment for BMI: the HR (95% CI) for Q2, Q3, and Q4 was 1.11 (0.94, 1.33), 0.97 (0.80, 1.16), and 0.81 (0.66, 0.99), respectively (P-trend = 0.008).
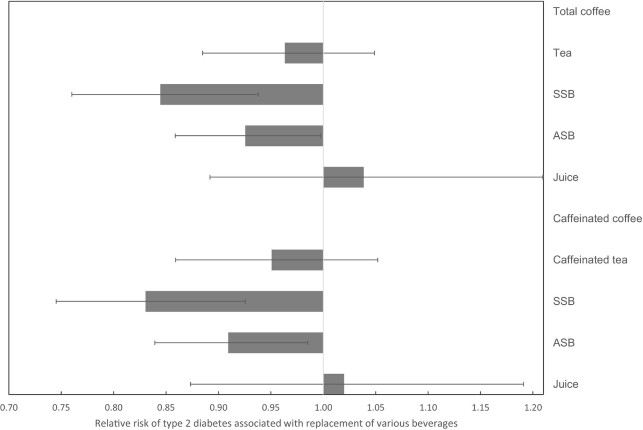
Substituting the consumption of various beverages with total coffee was associated with a reduced risk of T2D. Specifically, a hypothetical replacement of 1 serving/d of SSBs and ASBs with 1 cup/d of total coffee was associated with 16% (risk ratio [RR] = 0.84, 95% CI: 0.76, 0.94) and 7% (RR = 0.93, 95% CI: 0.86, 1.00) lower risk of T2D, respectively (Figure 1). The replacement of 1 serving/d of SSBs and ASBs with 1 cup/d of caffeinated coffee was associated with 17% (RR = 0.83, 95% CI: 0.75, 0.93) and 9% (RR = 0.91, 95% CI: 0.84, 0.99) lower risk of T2D, respectively.
FIGURE 1.
RR and 95% CI for risk of type 2 diabetes associated with hypothetical replacement of various beverages 1 serving/d with coffee among individuals with a history of gestational diabetes mellitus, the NHS II. Footnote: Model was adjusted for age, calendar time period, race, family history of diabetes, BMI, history of hypertension, history of hypercholesterolemia, parity, oral contraceptive use, menopausal status, smoking, physical activity, total energy intake, AHEI score excluding alcohol, alcohol intake, and dairy intake. Consumption of all the beverages (total coffee or caffeinated coffee, total tea or caffeinated tea for caffeinated coffee, SSB, ASB, and juice) were entered into the model as continuous variables. Mean prevalence of individual beverage intake over follow-up ≥1 serving/d: 43.7%, 37.6%, 6.4%, 23.6%, 9.8%, 28.1%, and 13.2% for total coffee, caffeinated coffee, decaffeinated coffee, tea, SSB, ASB, and juice, respectively, (n = 4522). AHEI, Alternate Healthy Eating Index; ASB, artificially sweetened beverage; SSB, sugar-sweetened beverage.
In the stratified analyses by major risk factors of T2D, significant heterogeneity was observed for BMI (P-heterogeneity <0.05): the inverse association for caffeinated coffee was stronger among participants with BMI ≥30 compared with those with BMI <30 (Supplementary Table 2). No significant heterogeneity was seen for the other risk factors. Results of time-updated recent coffee consumption with T2D risk were, overall, consistent with the primary findings (Supplementary Table 3). Additional adjustment for household income or total added sugar did not influence the results: compared with nondrinkers, the adjusted HR (95% CI) was 0.91 (0.78, 1.06), 0.83 (0.68, 1.01), and 0.46 (0.28, 0.76) for ≤1, 2–3, and 4+ cups of caffeinated coffee, respectively (P-trend = 0.003), for additional adjustment of household income, and the adjusted HR (95% CI) was 0.90 (0.78, 1.05), 0.82 (0.67, 1.00), and 0.44 (0.27, 0.73) for ≤1, 2–3, and 4+ cups of caffeinated coffee, respectively (P-trend = 0.002), for additional adjustment of total added sugar. When examining the associations with restricting follow-up time, we observed stronger inverse associations of caffeinated coffee with T2D risk within the first 10 y compared to within the first 20 y since the start of the follow-up (adjusted HR [95% CI] for each additional cup/d caffeinated coffee: 0.79 [0.68, 0.91] compared with 0.90 [0.83, 0.97]; both P-trend<0.01).
Coffee consumption and glucose metabolic biomarkers
Among 518 individuals who were T2D-free at the time of blood draw (median age: 57.7 y), after accounting for major covariates, the consumption of total coffee or caffeinated coffee was inversely associated with concentrations of C-peptide and fasting insulin concentrations, respectively (all P-trend <0.05) (Table 3). For decaffeinated coffee, lower concentrations of C-peptide and fasting insulin concentrations were seen with a higher intake, respectively, but the P-trend was only significant for C-peptide concentrations (P-trend = 0.003).
TABLE 3.
Least squares mean of glucose metabolic biomarker concentrations according to coffee consumption among individuals with a history of gestational diabetes mellitus who were free of type 2 diabetes at the time of blood collection, NHS II (n = 518)1
| Total coffee consumption | |||||
|---|---|---|---|---|---|
| 0 cup/d | ≤ 1 cup/d | 2–3 cups/d | 4+ cups/d | ||
| (n, percent) | 116, 22.4% | 197, 38.0% | 176, 34.0% | 29, 5.6% | P-trend |
| HbA1c, %; mmol/mol | — | — | — | — | — |
| Model 12 | 5.62 (5.56, 5.69) | 5.67 (5.62, 5.72) | 5.60 (5.55, 5.65) | 5.60 (5.47, 5.72) | 0.18 |
| Model 23 | 5.61 (5.55, 5.68) | 5.67 (5.62, 5.71) | 5.62 (5.50, 5.75) | 5.62 (5.49, 5.75) | 0.38 |
| Model 2 + BMI | 5.62 (5.55, 5.68) | 5.66 (5.62, 5.71) | 5.61 (5.56, 5.66) | 5.61 (5.49, 5.74) | 0.34 |
| C-peptide, ng/mL | — | — | — | — | — |
| Model 1 | 3.85 (3.58, 4.13) | 3.65 (3.46, 3.86) | 3.32 (3.14, 3.52) | 3.11 (2.69, 3.59) | <0.001 |
| Model 2 | 3.78 (3.50, 4.08) | 3.65 (3.45, 3.85) | 3.36 (3.17, 3.57) | 3.16 (2.73, 3.66) | 0.005 |
| Model 2 + BMI | 3.80 (3.54, 4.07) | 3.63 (3.45, 3.82) | 3.38 (3.20, 3.57) | 3.10 (2.71, 3.54) | 0.002 |
| Insulin, pmol/L | — | — | — | — | — |
| Model 1 | 64.3 (57.4, 72.1) | 60.9 (55.8, 66.5) | 49.0 (44.7, 53.8) | 48.8 (38.8, 61.3) | <0.001 |
| Model 2 | 61.2 (54.4, 68.8) | 60.3 (55.4, 65.6) | 50.7 (46.3, 55.5) | 52.2 (41.7, 65.2) | 0.006 |
| Model 2 + BMI | 61.7 (55.6, 68.5) | 59.8 (55.5, 64.5) | 51.1 (47.1, 55.4) | 50.5 (41.3, 61.6) | 0.002 |
| Caffeinated coffee consumption | |||||
| 0 cup/d | ≤ 1 cup/d | 2–3 cups/d | 4+ cups/d | — | |
| (n, percent) | 133, 25.7% | 227, 43.8% | 144, 27.8% | 14, 2.7% | P-trend |
| HbA1c, %; mmol/mol | — | — | — | — | — |
| Model 1 | 5.63 (5.58, 5.69) | 5.65 (5.60, 5.69) | 5.61 (5.55, 5.66) | 5.61 (5.43, 5.79) | 0.36 |
| Model 2 | 5.62 (5.56, 5.68) | 5.65 (5.60, 5.69) | 5.62 (5.57, 5.68) | 5.63 (5.46, 5.82) | 0.84 |
| Model 2 + BMI | 5.62 (5.56, 5.68) | 5.64 (5.60, 5.68) | 5.62 (5.57, 5.68) | 5.64 (5.47, 5.82) | 0.85 |
| C-peptide, ng/mL | — | — | — | — | — |
| Model 1 | 3.74 (3.50, 4.00) | 3.63 (3.45, 3.82) | 3.29 (3.09, 3.51) | 3.19 (2.59, 3.93) | 0.003 |
| Model 2 | 3.61 (3.36, 3.88) | 3.65 (3.46, 3.84) | 3.37 (3.15, 3.60) | 3.28 (2.66, 4.03) | 0.05 |
| Model 2 + BMI | 3.65 (3.42, 3.90) | 3.62 (3.45, 3.79) | 3.37 (3.18, 3.58) | 3.31 (2.74, 4.01) | 0.04 |
| Insulin, pmol/L | — | — | — | — | — |
| Model 1 | 61.8 (55.5, 68.8) | 60.0 (55.3, 65.1) | 48.2 (43.5, 53.4) | 49.1 (35.3, 68.3) | <0.001 |
| Model 2 | 57.9 (51.9, 64.7) | 59.9 (55.3, 64.9) | 50.8 (45.9, 56.2) | 54.2 (39.4, 74.7) | 0.04 |
| Model 2 + BMI | 59.1 (53.4, 65.2) | 59.1 (53.5, 65.2) | 50.9 (46.5, 55.7) | 55.3 (41.5, 73.6) | 0.02 |
| Decaffeinated coffee consumption | |||||
| 0 cup/d | ≤ 1 cup/d | 2–3 cups/d4 | — | — | |
| (n, percent) | 215, 41.5% | 277, 53.4% | 26, 5.0% | — | P-trend |
| HbA1c, %; mmol/mol | — | — | — | — | — |
| Model 1 | 5.64 (5.59, 5.68) | 5.63 (5.59, 5.67) | 5.65 (5.51, 5.78) | — | 0.83 |
| Model 2 | 5.64 (5.59, 5.69) | 5.63 (5.59, 5.67) | 5.61 (5.48, 5.74) | — | 0.87 |
| Model 2 + BMI | 5.63 (5.58, 5.68) | 5.63 (5.60, 5.67) | 5.61 (5.48, 5.74) | — | 0.73 |
| C-peptide, ng/mL | — | — | — | — | — |
| Model 1 | 3.82 (3.62, 4.02) | 3.39 (3.24, 3.56) | 3.10 (2.66, 3.61) | — | 0.04 |
| Model 2 | 3.79 (3.59, 4.00) | 3.43 (3.27, 3.59) | 2.98 (2.56, 3.47) | — | 0.01 |
| Model 2 + BMI | 3.72 (3.54, 3.91) | 3.48 (3.34, 3.64) | 2.90 (2.52, 3.34) | — | 0.003 |
| Insulin, pmol/L | — | — | — | — | — |
| Model 1 | 63.0 (57.9, 68.6) | 52.3 (48.6, 56.4) | 54.0 (41.6, 67.7) | — | 0.43 |
| Model 2 | 61.6 (56.7, 66.9) | 53.6 (49.8, 57.6) | 49.9 (39.4, 63.2) | — | 0.23 |
| Model 2 + BMI | 59.8 (55.5, 64.5) | 55.0 (51.6, 58.7) | 47.7 (38.7, 59.0) | — | 0.09 |
Analysis was restricted to participants with a history of GDM who were: 1) free of diabetes at the time of blood collection (defined as HbA1c <6.5% [48 mmol/mol]) in addition to the diagnostic criteria of T2D, and 2) provided fasting blood sample in 2012–2014 assayed for HbA1c, insulin, and C-peptide. Analysis was performed by modeling the cumulative average of total coffee consumption reported from baseline up to the time of blood collection (median age: 57.7 y, [IQR: 54.6, 60.8]). AHEI, Alternate Healthy Eating Index; ASB, artificially sweetened beverage; GDM, gestational diabetes mellitus; MET, metabolic equivalents; SSB, sugar-sweetened beverage; T2D, type 2 diabetes.
Model 1 was adjusted for age at time of blood draw (y).
Model 2 was additionally adjusted for race (white, nonwhite), family history of diabetes (yes, no), history of hypertension (yes/no), history of hypercholesterolemia (yes/no), parity (1, 2+), oral contraceptive use (current, past, never), menopausal status (premenopausal, postmenopausal), smoking (never, current, past), physical activity (MET-h/wk), total calorie intake (kcal/d), AHEI score excluding alcohol, alcohol intake (g/d) in quartiles, continuous beverage intakes including tea, SSB, ASB, and juice, and dairy intake. Caffeinated and decaffeinated coffee were mutually adjusted for each other depending on the model.
For decaffeinated coffee, results of 2+ cups/d are presented in this column.
Discussion
In this longitudinal cohort of US female nurses with a history of GDM with 24 y of follow-up, the greater consumption of caffeinated coffee was associated with a lower risk of T2D. Replacement of SSBs or ASBs with total or caffeinated coffee was associated with a meaningful risk reduction in T2D. We also noted that a greater consumption of total or caffeinated coffee was associated with a more favorable glucose metabolic profile among these individuals who were free of T2D.
Levels of coffee consumption observed in this female cohort with a history of GDM were comparable to those reported in the 2016 US national data (1.2 cups/d compared with 1.5 cups/d) (5). Overall, epidemiological studies across diverse populations have consistently reported the benefits of coffee, including both caffeinated and decaffeinated coffee, on lowering T2D risk (8, 32). A previous meta-analysis (8) pooling 30 prospective cohort studies summarized an inverse association between coffee consumption and risk of T2D: per 1 cup/d of coffee was associated with a 6% lower risk of T2D in a dose-response manner (RR = 0.94, 95% CI: 0.93, 0.95). When examining the consumption by coffee type, they reported a 7% and a 6% significantly lower T2D risk associated with an increment in per 1 cup/d of caffeinated and decaffeinated coffee, respectively (8). This current study presents complementary findings on the inverse associations of coffee, particularly caffeinated coffee, with T2D progression for high-risk individuals with a history of GDM. However, our observation on the null findings for decaffeinated coffee was in contrast to the literature evidence, possibly due to the overall low consumption levels in this cohort. We also noted a stronger inverse association of caffeinated coffee in an earlier follow-up period, which may possibly be due to an increased risk of T2D with age and altered T2D risk in the context of other major risk factors that may have changed over time. We evaluated the associations of coffee consumption with T2D risk by modeling cumulative average and the most recent level of consumption. The associations of caffeinated coffee consumption appeared to be stronger when it was modeled as cumulative average compared with the most recent level. The use of cumulative average may reduce measurement error due to within-person variations over time and therefore, produce a more robust estimate of the association. It is also possible that the beneficial association of coffee consumption is due to its cumulative exposure which is better captured by cumulative averages versus a measurement at a single timepoint.
Individuals with a history of GDM are at exceptionally higher risk of T2D than the general population (3). Although past studies examined coffee consumption pre- (34) or during pregnancy (33, 34) with risk of GDM or coffee consumption with risk of T2D in the general population (8), we are unaware of any studies examining post-GDM coffee consumption with long-term T2D risk among these individuals at high risk. Our primary results suggest a nonsignificant 9% and 17% and a significant 54% reduction in the risk of T2D for the consumption of ≤1, 2–3 cups/d and 4+ cups/d of caffeinated coffee, respectively, compared with no consumption. In a study on coffee consumption and risk of T2D in the general population of the NHS II (n = 88,259), the authors reported that 2–3 cups/d and 4+ cups/d of caffeinated coffee was associated with a significant 38% and 39% lower T2D risk, respectively, compared with no consumption (18). The present investigation examined the associations of interest in the NHS II participants with a history of GDM, a high-risk group for T2D incidence, with longer follow-up. Our findings on the lower risk reduction associated with 2–3 cups/d for caffeinated coffee are in slight contrast compared with the results from the general NHS II study. Several reasons might explain the differences. Compared with the general population, individuals with a history of GDM are at higher risk of developing T2D, and as such, a greater dose of caffeinated coffee might be required to confer similar protection on T2D progression. The distributions of other major risk factors for T2D (e.g. BMI, diet, and other lifestyle factors) may also vary between individuals with a history of GDM and the general population.
Total or caffeinated coffee was associated with a more favorable metabolic profile indicated by lower concentrations of fasting insulin and C-peptide among individuals with a history of GDM. Prior studies have examined different markers of metabolism in relation to short-term or habitual coffee consumption (35–39). Two earlier studies among predominantly Caucasian populations respectively reported lower fasting insulin concentration with habitual coffee consumption (35) and lower plasma C-peptide with the habitual consumption of caffeinated coffee (37), consistent with our findings.
We did not observe evidence of associations between decaffeinated coffee and T2D risk among individuals with a history of GDM. This finding differs from the literature as well as the general population of the NHS II where similar magnitudes of the benefits for decaffeinated coffee were reported as compared with caffeinated coffee (8, 18). In our study, due to the relatively low consumption levels and thus few T2D cases accrued in the group of 2+ cups/d (n = 29), statistical power evaluating T2D risk with consumption of decaffeinated coffee was limited. On the other hand, examining continuous glucose-related markers may provide mechanistic insights into T2D development with greater statistical power. In the analyses of glucose metabolism biomarkers, decaffeinated coffee was inversely associated with C-peptide, and a similar pattern was seen for fasting insulin. Lower concentrations of C-peptide have been reported with greater consumption of decaffeinated coffee from an early study (37). Overall, current literature suggests that habitual coffee consumption is favorably linked with glucose metabolism and T2D likely due to caffeine and other bioactive compounds from coffee. Such bioactive compounds include polyphenols (e.g. chlorogenic acid, lignans), trigonelline, and several key vitamins (e.g. magnesium, potassium, and vitamin B-3), which may reduce oxidative stress and inflammation, improve gut microbiome diversity, and modulate glucose and fat metabolism (4).
In the analyses stratified by major covariates, significant heterogeneity by BMI status (<30, ≥30) was observed; among participants consuming caffeinated coffee 4+ cups/d, T2D risk was lower for those with BMI ≥30 compared to those with BMI<30. Individuals with higher BMI are, in general, more likely to have a greater degree of insulin resistance and impaired glucose metabolism (40), and given the beneficial associations we observed between caffeinated coffee consumption and biomarkers of glucose metabolism, this may partly explain the stronger inverse associations we observed among participants with BMI≥30. However, the observed interaction by BMI needs to be interpreted with caution due to the relatively low person-years of follow-up and T2D events in the 4+ cups/d category and warrants further investigation in larger populations with comparable degrees of coffee consumption. Smoking is a strong confounder for the associations between coffee consumption and T2D risk. Both current smoking and past smoking have been associated with higher risks of T2D (41). We adjusted for smoking status for the associations of coffee consumption with T2D. In the analyses stratified by smoking status, a lower risk of T2D with greater caffeinated coffee consumption among never smokers suggested that the inverse associations of caffeinated coffee consumption with T2D was likely to be independent of smoking.
Consistent with findings among the general population (17), this study supports coffee as a healthier alterative to SSB and ASB in preventing T2D for individuals with a history of GDM. In line with the 2020–2025 Dietary Guidelines for Americans (7), given the epidemiological evidence on the detrimental effects of added sugar (42) and the moderately protective associations between low-fat dairy consumption and T2D risk (43), coffee, when consumed properly at moderate levels (3–5 cups/d), may be considered as a healthier beverage option compared with the other less healthy beverages among individuals with a history of GDM.
The strengths of this study include the focus of the research questions among a high-risk population with a large sample size, the prospective study design with long-term follow-up and high follow-up rates, and large number of T2D events leading to great statistical precision. We had detailed repeated measures of both caffeinated and decaffeinated coffee consumption, as well as other dietary and lifestyle factors, which enabled us to adjust for a wide range of lifestyle characteristics. To the best of our knowledge, we are the first study evaluating long-term coffee consumption with the profile of glucose metabolism biomarkers in this high-risk population.
This study has some potential limitations. First, measurement error due to self-reported coffee intake and other dietary factors was inevitable. However, the FFQs used in the present study have been validated against dietary records (19), and such misclassifications would be nondifferential with respect to the outcomes, diluting the associations. Second, similar to other observational studies, although we adjusted for major risk factors of T2D, residual confounding was possible. To assess the potential influence of unmeasured confounding using the E-value (44), in order to explain the observed RR (HR = 0.46) for 4+ cups/d caffeinated coffee, an unmeasured confounder that was associated with caffeinated coffee consumption and with T2D by an RR of 3.77 each, above and beyond the measured confounders, could explain it, but weaker confounding (i.e. RR <3.77) could not. We did not have data on coffee preparation methods (instant, filtered, or unfiltered) or its consumption with sugar and/or dairy; we adjusted for intakes of total added sugar to address the concern related to sugar. The consumption of added sugar or artificial sweeteners with coffee may likely attenuate the true underlying inverse associations of coffee with T2D. For the biochemical analyses, we did not evaluate insulin sensitivity by the gold standard of the hyperinsulinemic clamp or HOMA measures for practical reasons. We assumed that lower concentrations of fasting insulin and C-peptide may reflect greater insulin sensitivity (37). Both fasting insulin (45) and C-peptide (46) have been demonstrated to be well correlated with the gold standard or HOMA measures. Finally, our study was conducted among a predominantly Caucasian cohort of registered nurses, which may not be generalizable to populations of other racial/ethnic or socioeconomic groups. However, the use of a homogenous cohort may help address unmeasured confounding. Studies of diverse populations or more contemporary cohorts are warranted to confirm our findings.
In conclusion, in this large prospective study with 24 y of follow-up, the consumption of caffeinated coffee was inversely associated with the risk of subsequent T2D among individuals with a history of GDM. A higher intake of coffee was related to a more favorable glucose metabolic profile. Coffee might be promoted as an alternative to other less healthy beverages and be incorporated into a healthy lifestyle to prevent T2D progression for individuals with a history of GDM.
Supplementary Material
Acknowledgements
We would like to acknowledge the Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA, as the home of the NHS I and II. The NHS II would like to thank the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The authors’ responsibilities were as follows—JY, DKT, and CZ: designed the study; JY: performed data analysis; DKT and SL: provided initial programming support; JY: drafted the manuscript; CZ: designed and provided oversight of the DWH Study; DKT: has verified the underlying data and the analysis; CZ: is responsible for the decision to submit the manuscript; and all authors interpreted the data, critically revised the manuscript for important intellectual content, and read and approved the final version of the manuscript, and had final responsibility for the decision to submit for publication. DKT is an Editor of the American Journal of Clinical Nutrition and played no role in the journal's evaluation of the manuscript.
Notes
CZ and JEC received research support from the NIH during the conduct of the study. DKT received grants from the American Diabetes Association. CZ was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Natitonal Instutitdes of Health (NIH) (contract # HHSN275201000020C; HHSN275201100002I). The NHS II was funded by the NIH grants U01 CA176726, U01 HL145386, and R01 CA67262. JEC received grants from NIH (U01HL145386 and P30 DK046200). DKT received grants from the American Diabetes Association. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author disclosures: The authors report no conflicts of interest.
Supplementary Figure 1 and Supplementary Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AHEI, Alternate Healthy Eating Index; ASB, artificially sweetened beverage; DWH, Diabetes & Women's Health Study; GDM, gestational diabetes mellitus; MET, metabolic equivalents; RR, risk ratio; SSB, sugar-sweetened beverage; T2D, type 2 diabetes.
Contributor Information
Jiaxi Yang, Global Center for Asian Women's Health, Yong Loo Lin School of Medicine, National University of Singapore, Singapore; Department of Obstetrics and Gynaecology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore; Bia-Echo Asia Centre for Reproductive Longevity & Equality (ACRLE), Yong Loo Lin School of Medicine, National University of Singapore, Singapore; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Deirdre K Tobias, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Division of Preventive Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
Shanshan Li, Department of Medicine, Boston University School of Medicine, Boston, MA, USA.
Shilpa N Bhupathiraju, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Sylvia H Ley, Department of Epidemiology, Tulane University School of Public Health and Tropical Medicine, New Orleans, LA, USA.
Stefanie N Hinkle, Department of Biostatistics, Epidemiology and Informatics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Frank Qian, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA.
Zhangling Chen, Department of Epidemiology, Erasmus Medical Center, University Medical Center Rotterdam, Rotterdam, The Netherlands.
Yeyi Zhu, Division of Research, Kaiser Permanente Northern California, Oakland, CA, USA.
Wei Bao, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, China.
Jorge E Chavarro, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
Frank B Hu, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
Cuilin Zhang, Global Center for Asian Women's Health, Yong Loo Lin School of Medicine, National University of Singapore, Singapore; Department of Obstetrics and Gynaecology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore; Bia-Echo Asia Centre for Reproductive Longevity & Equality (ACRLE), Yong Loo Lin School of Medicine, National University of Singapore, Singapore; Epidemiology Branch, Division of Population Health Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, Rockville, MD, USA.
Data Availability
Analytic code used for the present analysis may be made available on a case-by-case basis with approval from the senior author of this manuscript. Data described in the manuscript will not be made publicly available. Further information including the procedures for obtaining and accessing data from the NHS II is described at www.nurseshealthstudy.org/researchers (e-mail: nhsaccess@channing.harvard.edu).
References
- 1. Tobias DK. Prediction and prevention of type 2 diabetes in women with a history of GDM. Curr Diab Rep. 2018;18(10):1–5. [DOI] [PubMed] [Google Scholar]
- 2. McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5(1):1–19. [DOI] [PubMed] [Google Scholar]
- 3. Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: Systematic review and meta-analysis. BMJ. 2020;369:m1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Dam RM, Hu FB, Willett WC. Coffee, caffeine, and health. N Engl J Med. 2020;383(4):369–78. [DOI] [PubMed] [Google Scholar]
- 5. Drewnowski A, Rehm CD. Sources of caffeine in diets of US children and adults: Trends by beverage type and purchase location. Nutrients. 2016;8(3):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. U.S. Department of Health and Human Services and U.S . Department of Agriculture. 2015 – 2020 Dietary Guidelines for Americans. 8th Edition. December 2015. Available from: https://health.gov/our-work/food-nutrition/previous-dietary-guidelines/2015 [Google Scholar]
- 7. U.S. Department of Agriculture and U.S. Department of Health and Human Services . Dietary Guidelines for Americans, 2020–2025. 9th Edition. 2020. [Internet].Available from: DietaryGuidelines.gov. [Google Scholar]
- 8. Carlström M, Larsson SC. Coffee consumption and reduced risk of developing type 2 diabetes: A systematic review with meta-analysis. Nutr Rev. 2018;76(6):395–417. [DOI] [PubMed] [Google Scholar]
- 9. Sarriá B, Martínez-López S, Mateos R, Bravo-Clemente L. Long-term consumption of a green/roasted coffee blend positively affects glucose metabolism and insulin resistance in humans. Food Res Int. 2016;89:1023–8. [Google Scholar]
- 10. Godos J, Pluchinotta FR, Marventano S, Buscemi S, Li Volti G, Galvano Fet al. Coffee components and cardiovascular risk: Beneficial and detrimental effects. Int J Food Sci Nutr. 2014;65(8):925–36. [DOI] [PubMed] [Google Scholar]
- 11. Greenberg JA, Boozer CN, Geliebter A. Coffee, diabetes, and weight control. Am J Clin Nutr. 2006;84(4):682–93. [DOI] [PubMed] [Google Scholar]
- 12. Koloverou E, Panagiotakos D, Pitsavos C, Chrysohoou C, Georgousopoulou E, Laskaris Aet al. The evaluation of inflammatory and oxidative stress biomarkers on coffee–diabetes association: Results from the 10-year follow-up of the ATTICA Study (2002–2012). Eur J Clin Nutr. 2015;69(11):1220–5. [DOI] [PubMed] [Google Scholar]
- 13. Zhernakova A, Kurilshikov A, Bonder MJ, Tigchelaar EF, Schirmer M, Vatanen Tet al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016;352(6285):565–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang C, Hu FB, Olsen SF, Vaag A, Gore-Langton R, Chavarro JEet al. Rationale, design, and method of the Diabetes & Women's Health study—a study of long-term health implications of glucose intolerance in pregnancy and their determinants. Acta Obstet Gynecol Scand. 2014;93(11):1123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bao Y, Bertoia ML, Lenart EB, Stampfer MJ, Willett WC, Speizer FEet al. Origin, methods, and evolution of the three Nurses' Health Studies. Am J Public Health. 2016;106(9):1573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Solomon CG, Willett WC, Carey VJ, Rich-Edwards J, Hunter DJ, Colditz GAet al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA. 1997;278(13):1078–83. [PubMed] [Google Scholar]
- 17. Bhupathiraju SN, Pan A, Malik VS, Manson JE, Willett WC, van Dam RMet al. Caffeinated and caffeine-free beverages and risk of type 2 diabetes. Am J Clin Nutr. 2013;97(1):155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Dam RM, Willett WC, Manson JE, Hu FB. Coffee, caffeine, and risk of type 2 diabetes: A prospective cohort study in younger and middle-aged U.S. women. Diabetes Care. 2006;29(2):398–403. [DOI] [PubMed] [Google Scholar]
- 19. Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner Bet al. Food-based validation of a dietary questionnaire: The effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18(4):858–67. [DOI] [PubMed] [Google Scholar]
- 20. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang Met al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wannamethee SG, Camargo CA, Manson JE, Willett WC, Rimm EB. Alcohol drinking patterns and risk of type 2 diabetes mellitus among younger women. Arch Intern Med. 2003;163(11):1329–36. [DOI] [PubMed] [Google Scholar]
- 22. Bao W, Tobias DK, Bowers K, Chavarro J, Vaag A, Grunnet LGet al. Physical activity and sedentary behaviors associated with risk of progression from gestational diabetes mellitus to type 2 diabetes mellitus: A prospective cohort study. JAMA Intern Med. 2014;174(7):1047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1(6):466–73. [DOI] [PubMed] [Google Scholar]
- 24. Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KAet al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991–9. [DOI] [PubMed] [Google Scholar]
- 25. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus . Report of the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20(7):1183–97. [DOI] [PubMed] [Google Scholar]
- 26. National Diabetes Data Group . Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28(12):1039–57. [DOI] [PubMed] [Google Scholar]
- 27. Manson JE, Rimm EB, Stampfer MJ, Colditz GA, Willett WC, Krolewski ASet al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet North Am Ed. 1991;338(8770):774–8. [DOI] [PubMed] [Google Scholar]
- 28. Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WHet al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161(13):1581–6. [DOI] [PubMed] [Google Scholar]
- 29. Ley SH, Chavarro JE, Li M, Bao W, Hinkle SN, Wander PLet al. Lactation duration and long-term risk for incident type 2 diabetes in women with a history of gestational diabetes mellitus. Diabetes Care. 2020;43(4):793–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pan A, Malik VS, Schulze MB, Manson JE, Willett WC, Hu FB. Plain-water intake and risk of type 2 diabetes in young and middle-aged women. Am J Clin Nutr. 2012;95(6):1454–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman Det al. Dietary fat and coronary heart disease: A comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149(6):531–40. [DOI] [PubMed] [Google Scholar]
- 32. Mirmiran P, Carlström M, Bahadoran Z, Azizi F. Long-term effects of coffee and caffeine intake on the risk of pre-diabetes and type 2 diabetes: Findings from a population with low coffee consumption. Nutr Metab Cardiovasc Dis. 2018;28(12):1261–6. [DOI] [PubMed] [Google Scholar]
- 33. Adeney KL, Williams MA, Schiff MA, Qiu C, Sorensen TK. Coffee consumption and the risk of gestational diabetes mellitus. Acta Obstet Gynecol Scand. 2007;86(2):161–6. [DOI] [PubMed] [Google Scholar]
- 34. Hinkle SN, Laughon SK, Catov JM, Olsen J, Bech BH. First trimester coffee and tea intake and risk of gestational diabetes mellitus: A study within a national birth cohort. BJOG: An International Journal of Obstetrics & Gynaecology. 2015;122(3):420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Van Dam R, Dekker J, Nijpels G, Stehouwer C, Bouter L, Heine R. Coffee consumption and incidence of impaired fasting glucose, impaired glucose tolerance, and type 2 diabetes: the Hoorn Study. Diabetologia. 2004;47(12):2152–9. [DOI] [PubMed] [Google Scholar]
- 36. Gao F, Zhang Y, Ge S, Lu H, Chen R, Fang Pet al. Coffee consumption is positively related to insulin secretion in the Shanghai High-risk Diabetic Screen (SHiDS) study. Nutr Metab (Lond). 2018;15:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu T, Willett WC, Hankinson SE, Giovannucci E. Caffeinated coffee, decaffeinated coffee, and caffeine in relation to plasma C-peptide levels, a marker of insulin secretion, in US women. Diabetes Care. 2005;28(6):1390–6. [DOI] [PubMed] [Google Scholar]
- 38. van Dam RM, Pasman WJ, Verhoef P. Effects of coffee consumption on fasting blood glucose and insulin concentrations: Randomized controlled trials in healthy volunteers. Diabetes Care. 2004;27(12):2990–2. [DOI] [PubMed] [Google Scholar]
- 39. Ärnlöv J, Vessby B, Risérus U. Coffee consumption and insulin sensitivity. JAMA. 2004;291(10):1199–a-1201. [DOI] [PubMed] [Google Scholar]
- 40. Singla P, Bardoloi A, Parkash AA. Metabolic effects of obesity: A review. World Journal of Diabetes. 2010;1(3):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pan A, Wang Y, Talaei M, Hu FB, Wu T. Relation of active, passive, and quitting smoking with incident type 2 diabetes: A systematic review and meta-analysis. The Lancet Diabetes & Endocrinology. 2015;3(12):958–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Imamura F, O'Connor L, Ye Z, Mursu J, Hayashino Y, Bhupathiraju SNet al. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ. 2015;351:h3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mitri J, Yusof B-NM, Maryniuk M, Schrager C, Hamdy O, Salsberg V. Dairy intake and type 2 diabetes risk factors: A narrative review. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2019;13(5):2879–87. [DOI] [PubMed] [Google Scholar]
- 44. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: Introducing the E-value. Ann Intern Med. 2017;167(4):268–74. [DOI] [PubMed] [Google Scholar]
- 45. Singh B, Saxena A. Surrogate markers of insulin resistance: A review. World Journal of Diabetes. 2010;1(2):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Khan HA, Sobki SH, Ekhzaimy A, Khan I, Almusawi MA. Biomarker potential of C-peptide for screening of insulin resistance in diabetic and non-diabetic individuals. Saudi Journal of Biological Sciences. 2018;25(8):1729–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Analytic code used for the present analysis may be made available on a case-by-case basis with approval from the senior author of this manuscript. Data described in the manuscript will not be made publicly available. Further information including the procedures for obtaining and accessing data from the NHS II is described at www.nurseshealthstudy.org/researchers (e-mail: nhsaccess@channing.harvard.edu).