Abstract
We previously established a monoclonal antibody (MAb), designated H9, which reacts with the heat shock protein 60 (HSP60) homologue of Helicobacter pylori as well as with other bacterial and human HSP60s. To determine the importance of a cross-reactive epitope on H. pylori HSP60 in H. pylori immunopathogenesis, we performed (i) mapping of an epitope on H. pylori HSP60 recognized by the H9 MAb, (ii) analysis of immunoglobulin G responses of patients with or without H. pylori infection to its epitope region, and (iii) studies of the protective effect of immunization with its epitope region on H. pylori infection in mice. The epitope recognized by the H9 MAb was mapped to the sequence of amino acids 189 to 203 (VEGMQFDRGYLSPYF) on the H. pylori HSP60 molecule. It was confirmed that the synthesized peptide designated pH9 was recognized by the H9 MAb. Enzyme-linked immunosorbent assay analysis showed that patients with H. pylori infection (n = 349) had significantly lower titers of pH9 antibody than did uninfected patients (n = 200) (P < 0.001), but this was not the case with purified H. pylori HSP60 recombinant Escherichia coli GroEL, or recombinant human HSP60. In C57BL/6 mice immunized with the pH9 peptide with Freund's complete adjuvant (FCA), the number of H. pylori organisms colonizing the stomach was significantly lower than that in mice immunized with pCont plus FCA (P < 0.0001) or FCA only (P < 0.005). The results suggest that the immune response to the cross-reactive epitope (pH9 region) on H. pylori HSP60 is unique and might be associated with protection against H. pylori infection.
Helicobacter pylori is associated with the occurrence of chronic gastritis, and infection with H. pylori is implicated in the pathogenesis of peptic ulcer disease and adenocarcinoma of the stomach (5, 13, 25, 33, 34, 37, 51). Although several virulence factors of H. pylori have been reported (7, 10, 11, 16, 22, 26, 32, 39), the relationship between these antigens of H. pylori and the pathogenic mechanism by which H. pylori persists in the stomach is not fully understood.
Heat shock proteins (HSPs), highly conserved proteins found in all prokaryotic and eukaryotic cells, are induced by a variety of environmental stresses, such as temperature change, inflammation, viral infection, and malignant transformation (6, 23, 52). The HSP60 family of chaperonins, such as GroEL of Escherichia coli and the 65-kDa antigen of Mycobacterium spp., are thought to be immunodominant antigens and to facilitate folding, unfolding, and translocation of polypeptides as well as the assembly and disassembly of oligomeric protein complexes (8, 12, 18). It has also been reported that H. pylori HSP60 is a chaperonin for urease, one of the putative virulence factors of H. pylori (9, 15, 35).
Recently, Huesca et al. reported that expression of H. pylori HSP60 is related to recognition of sulfated glycolipid on gastric cells by H. pylori (20). We also demonstrated that H. pylori HSP60 is expressed on the bacterial surface (45, 46) and that the H. pylori HSP60 homologue is associated with adhesion to human gastric epithelial cells (47). Moreover, Vanet and Labigne reported the possibility that expression of HSP60 and urease on the bacterial surface of H. pylori is induced by a specific secretion system (44). In contrast, Phadnis et al. indicated that surface expression of H. pylori HSP60 is induced through bacterial autolysis (38). Moreover, Ferrero et al. reported that the GroEL homologue of H. pylori is associated with the induction of protective immunity against H. pylori infection (17). These reports indicate that H. pylori HSP60 is located on the bacterial surface and that H. pylori HSP60 might be a target for bacterial elimination by immunity raised by H. pylori infection.
On the other hand, several investigators reported that H. pylori induces autoantibodies that play a crucial role in the pathogenesis of gastritis and gastric atrophy (29, 30). Autoimmunity may also play a role in the pathogenesis of H. pylori-linked chronic gastritis and carcinoma (1, 33, 34). It is known that the humoral immune response against H. pylori HSP60 is strongly induced in patients with H. pylori infection (27, 36). Recently, we reported the cross-reactivities between H. pylori HSP60 and human gastric epithelial cells by histochemical immunostaining with monoclonal antibodies (MAbs) directed against the HSP60 homologue of H. pylori (45, 48). The findings indicate that the immune response against the cross-reactive epitope between H. pylori HSP60 and human HSP60 might induce tissue damage.
These facts imply that immune responses against H. pylori HSP60 may be associated with protective immunity and tissue damage. Therefore, to confirm the region on H. pylori HSP60 associated with either protection or damage is of importance for understanding the pathogenesis of H. pylori infection and for the development of a vaccine against H. pylori infection.
We previously established a MAb designated H9 directed against the HSP60 homologue of H. pylori, which reacts not only with H. pylori HSP60 but also with other bacterial HSP60s (48). To understand the possible role of the immune response against a cross-reactive epitope on H. pylori HSP60, we determined the epitope recognized by the H9 MAb on H. pylori HSP60 and analyzed the human humoral immune response against its epitope region. The protective effect of immunizing mice with the epitope region on H. pylori infection was also investigated.
MATERIALS AND METHODS
Sera.
Serum specimens from 549 patients undergoing gastroendoscopic examination were obtained from S. Arakawa (Tokyo Dental College Hospital). To confirm H. pylori infection, urea breath tests and cultivation of biopsy specimens from all patients were performed. Patients who tested positive in either urea breath tests or biopsy-based cultivation tests were considered to be infected with H. pylori. Out of 549 patients, 349 were positive and 200 were negative for H. pylori infection. Diagnosis of individual patients was made by gastroendoscopic analysis as follows (number of patients with/without H. pylori infection): gastritis (23/23), gastritis with atrophy (52/29), gastric ulcer (100/57), duodenal ulcer (103/39), gastric ulcer and duodenal ulcer (49/18), gastric cancer (10/11), and patients without gastroduodenal disease (12/23).
Antigens.
H. pylori HSP60 was affinity purified from clinical isolate H. pylori strain TK1029 (vacA+ cagA+), which was obtained from a patient with a gastric ulcer by a specific MAb (H20) directed against H. pylori HSP60, as described previously (45). Recombinant E. coli GroEL (rGroEL) and recombinant human HSP60 (rHSP60) were purchased from Sigma Chemical Co. (St. Louis, Mo.). Purified protein derivative (PPD) was purchased from JAPAN BCG Products (Tokyo, Japan).
Construction of E. coli expressing H. pylori HSP60.
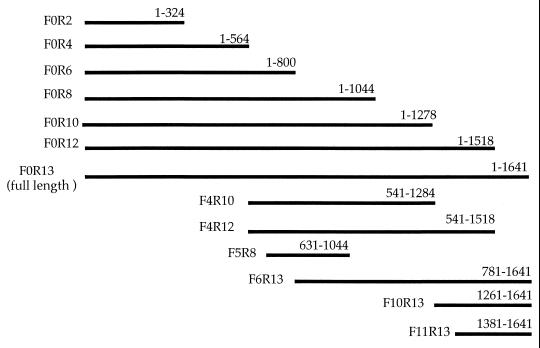
PCR was performed using the primer sets indicated in Table 1, and several DNA fragments of different length encoding H. pylori HSP60 were amplified from H. pylori TK1029 genomic DNA (Fig. 1). The unrelated sequence ACGT and the EcoRI recognition sequence GAATTC were added to both sides of the 5′ primers (upstream) before PCR for subcloning the resultant cDNA into a plasmid pEX. The resultant PCR products were integrated into a plasmid, pEX, capable of producing a fused protein with β-galactosidase (42). The sequences of cloned PCR fragments inserted into plasmid pEX were confirmed by direct sequencing. The constructed plasmids were transformed into E. coli pop2136, and each bacterium was designated HY3-15 or PEX (Table 2). Fusion proteins expressed by the pEX vector in E. coli pop2136 containing the cI ts857 repressor accounted for >30% of the total sodium dodecyl sulfate (SDS)-extracted bacterial proteins (42). These bacteria were grown at 30°C and then were shifted to 42°C for 2 h to induce expression of the recombinant protein (fusion protein). Bacteria were recovered after centrifugation, and bacterial pellets were stored at −80°C until used for SDS-polyacrylamide gel electrophoresis (PAGE). E. coli pop2136 and protein expression vector pEX were a generous gift from K. Ohsumi (Mitubishi Chemical Laboratory Co. Ltd., Tokyo, Japan).
TABLE 1.
Oligonucleotide primers used for PCR
| Primer | Sequence (5′ to 3′)a | Position no.b |
|---|---|---|
| Forward | ||
| F0 | ATGGCAAAAGAAATCAAATT | 1–20 |
| F4 | GGCATTGAAGATGAATTGGA | 541–560 |
| F5 | ACCGCTCAATTGGATAATGC | 631–650 |
| F6 | CTAGTGGTGAATAAATTAAG | 781–800 |
| F10 | ATTCGCGCGGCTCAAAAAGT | 1261–1280 |
| F11 | GTGGTCGTGAATGAAGTAGA | 1381–1400 |
| Reverse | ||
| R2 | TGCCGTGATATTCCTCAAACCTTC | 324–301 |
| R4 | GACATCCAATTCATCTTCAATGCC | 564–541 |
| R6 | CTTAATTTATTCACCACTAG | 800–781 |
| R8 | GATCTGCGCGATCCTGTCTTTAAC | 1044–1021 |
| R10 | ATGCACTTTTTGAGCCGGCGAAT | 1284–1255 |
| R12 | CGAAACCGAAACCGCATTTTGTAG | 1518–1493 |
| R13 | TTACATCATGCCGCCCATGC | 1641–1622 |
The unrelated nucleotide sequence ACGT and the EcoRI recognition sequence GAATTC were added on the 5′ side (upstream).
Position relative to the H. pylori HSP60 genomic sequence described by Macchia et al. (27).
FIG. 1.
Schematic diagram of DNA fragments encoding H. pylori HSP60 amplified from H. pylori genomic DNA by PCR. The numbers are the nucleotide positions relative to the H. pylori HSP60 genomic sequence described by Macchia et al. (27).
TABLE 2.
Bacterial strains used in the present study
| Strain | Descriptiona |
|---|---|
| H. pylori TK1029 | Used for PCR amplification of H. pylori HSP60 or the part of the gene encoding this molecule |
| E. coli pop2136 | Parent strain for transformation |
| HY3 | Pop2136 harboring pEXF0R13 |
| HY4 | Pop2136 harboring pEXF0R2 |
| HY5 | Pop2136 harboring pEXF0R4 |
| HY6 | Pop2136 harboring pEXF0R6 |
| HY7 | Pop2136 harboring pEXF0R8 |
| HY8 | Pop2136 harboring pEXF0R10 |
| HY9 | Pop2136 harboring pEXF0R12 |
| HY10 | Pop2136 harboring pEXF4R10 |
| HY11 | Pop2136 harboring pEXF4R12 |
| HY12 | Pop2136 harboring pEXF5R8 |
| HY13 | Pop2136 harboring pEXF6R13 |
| HY14 | Pop2136 harboring pEXF10R13 |
| HY15 | Pop2136 harboring pEXF11R13 |
| PEX | Pop2136 harboring protein expression vector pEX |
Protein expression plasmids (pEX plasmids) contain PCR products (Table 1) cloned into the EcoRI cloning site.
Preparation of MAb.
MAb H9 (immunoglobulin G2a [IgG2a]), which reacts with H. pylori HSP60, was previously established (48). The 60-kDa antigen derived from H. pylori strain TK1029 was partially purified by extraction from separating gels after SDS-PAGE. BALB/c mice were intraperitoneally (i.p.) immunized with the antigen mixed with Freund's complete adjuvant (FCA) (Difco Laboratories, Detroit, Mich.) three times at intervals of 10 days. Ten days after the last i.p. injection, the mice were administered the partially purified antigen intravenously. Three days after the last immunization, the spleens were removed for cell fusion between spleen cells and mouse myeloma cells (P3-X63-Ag8-U1). The hybridoma cells producing MAb, which reacts with affinity-purified H. pylori HSP60 and the sonicated MKN45 cells (human gastric cancer cell line), were collected by enzyme-linked immunosorbent assay (ELISA). The hybridoma cells with apparent specific antibody production were cloned by limiting dilution. Cells (106) were inoculated i.p. into a BALB/c mouse that had been pretreated by i.p. administration of 0.5 ml of pristane (Wako Pure Chemical Ltd.) 4 days previously. Approximately 2 weeks later, ascites fluids were obtained from the mouse. Immunoglobulins in the ascites fluids were purified using an Immunoglobulin-Easy-Separation kit (Pharmacia Biotech Co., Tokyo, Japan). The purified MAb was used for ELISA and immunoblotting analysis.
Peptides.
The amino acid sequences corresponding to residues 189 to 203 (VEGMQFDRGYLSPYF) and 463 to 477 (VNEVEKHEGHFGFNA) on the H. pylori HSP60 molecule, which were designated pH9 and pCont, respectively, were synthesized by Sawady Technology Co. Ltd. (Tokyo, Japan). The peptide pCont was used as a negative control. The molecular weights of synthetic peptides pH9 and pCont were confirmed to be 1,808.0 and 1,712.5, respectively, by high-pressure liquid chromatography and mass spectrum analysis.
SDS-PAGE and immunoblotting.
SDS-PAGE using 8 or 10% gels was carried out according to Laemmli (24). Each bacterial pellet stored at −80°C (corresponding to 1 ml of cultured bacterial cells expressing fusion proteins) was suspended in 300 μl of phosphate-buffered saline (PBS). The bacterial solutions were mixed with 300 μl of 0.12 M Tris buffer (pH 6.8) containing 20% (vol/vol) glycerol, 0.015 M SDS, and 0.4 mM 2-mercaptoethanol. The solutions were heated for 10 min at 100°C. Finally, 20 μl of the bacterial lysates was loaded per lane on 8% separating gels. Affinity-purified H. pylori HSP60 and human rHSP60 were loaded (1.25 μg of protein per lane) on 10% separating gels. Immunoblot analysis was carried out as described by Towbin et al. (43). After electrophoresis, the separated proteins were transferred to nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany) at 0.25 A overnight. After blocking with PBS (pH 7.4) containing 1% (wt/vol) skim milk (Yukijirushi Nyugyo Co. Ltd, Sapporo, Japan) (PBS-S) for 1 h at room temperature, the membranes were incubated for 1 h with 1 μg of H9 MAb/ml diluted in PBS-S. They were then incubated for 1 h with goat anti-mouse IgG peroxidase conjugate diluted 1:500 with PBS-S. Immunoblots were developed in 50 mM Tris-HCl buffer (pH 7.4) containing 0.12% (vol/vol) H2O2 and 1 mM 3,3′-diaminobenzidine tetrahydrochloride (Dojinkagaku Co., Kumamoto, Japan).
Conventional ELISA (cELISA).
ELISA was performed as previously described (48). Ninety-six-well microplates were coated for 2 h with affinity-purified H. pylori HSP60, E. coli rGroEL, human rHSP60, or PPD at a concentration of 0.1 μg/well. After a washing with PBS containing 0.05% (vol/vol) Tween 20 (Wako Pure Chemical Ltd.) (PBS-T), the plates were incubated with 150 μl of PBS-S for 1 h at room temperature. The plates were then incubated for 1 h at room temperature with patients' sera diluted 1:200 or mouse sera diluted 1:40 in PBS-S. After a washing with PBS-T, the plates were incubated with either goat anti-human IgG peroxidase conjugate (Capel Research Products, West Chester, Pa.) diluted 1:500 in PBS-S or anti-mouse IgG peroxidase conjugate (Biosource International, Vacaville, Calif.). The plates were then developed with OPD buffer, pH 5.0 (0.1 M citric acid, 0.07 M sodium phosphate dibasic 12-hydrate, 0.015% [vol/vol] H2O2) containing 0.1% (vol/vol) o-phenylenediamine. After 5 min, the reaction was stopped with 2 N H2SO4 and the developed color was measured at 490 nm. Data are shown as the means of ELISA values ± the standard errors (SE).
Peptide ELISA (pELISA).
AquaBind 96-well microplates (Iwaki Glass Co. Ltd., Tokyo, Japan) were used for immobilizing synthesized peptides. The 96-well microplates were coated for 1 h with either pH9 or pCont solubilized in binding buffer (0.03 M Na2CO3 and 0.069 M NaHCO3, pH 9.6) at a concentration of 0.3 to 10 μg/well. After a washing with washing buffer (pH 7.2) (0.5 M NaCl, 0.0026 M KCl, 0.0014 M KH2PO4, 0.0082 M Na2HPO4 · H2O, and 1% [vol/vol] Triton X-100 [Wako Pure Chemical Ltd.]), each well was incubated with 150 μl of blocking buffer (pH 9.6) (7.7% [wt/vol] PEG8000 [Sigma Chemical Co.], 0.5% [wt/vol] bovine serum albumin, 0.012 M Na2CO3, 0.0275 M NaHCO3, and 3% [vol/vol] 2-aminoethanol) for 18 h at room temperature. After a washing, the plates were incubated for 2 h at room temperature with H9 MAb diluted to 10 μg/ml or patients' sera diluted 1:200 in dilution buffer (pH 7.2) (3% [vol/vol] bovine serum albumin, 1% [vol/vol] dextran [molecular weight, 70,000] [Sigma Chemical Co.], 20% [vol/vol] fetal calf serum, 0.45 M NaCl, 0.0023 M KCl, 0.0012 M KH2PO4, 0.0073 M Na2HPO4 · H2O, and 0.9% [vol/vol] Triton X-100). After further washing, the plates were incubated with goat anti-mouse IgG or anti-human IgG peroxidase conjugate (Capel Research Products) diluted 1:500 with dilution buffer for 1 h at room temperature. After a washing, the plates were developed using the method described above for cELISA. Data shown are mean values of pELISA ± SE.
Animal experiment.
Specific-pathogen-free C57BL/6 mice (5-week-old females) were purchased from Nihon CLEA Co. Ltd., Tokyo, Japan. Mice were i.p. immunized five times on a weekly schedule with either pH9 peptide plus FCA (Difco Laboratories) (n = 11), pCont peptide plus FCA (n = 10), or FCA only (n = 10). Nonimmunized mice without H. pylori infection were used as controls in ELISA analysis (n = 7). A solution of each antigen, prepared at a concentration of 1 mg/ml in PBS, was mixed with an equal volume of FCA. Finally, 100 μl of solution (50 μg/mouse) was used to immunize the mice. One week after the last immunization, the mice were orally infected three times daily with 5 × 108 cells of the H. pylori clinical isolate TK1402 (vacA+ cagA+) isolated from a patient with gastritis. It has already been confirmed that this strain can colonize the stomach and induce mild gastritis in specific-pathogen-free C57BL/6 mice (data not shown). Two weeks after infection, the mice were sacrificed. Serum was obtained from each mouse for ELISAs, and the number of bacteria colonizing the stomach was determined.
Assessment of H. pylori colonization in mouse stomach.
Whole gastric mucosa obtained from a mouse stomach with a small spatula (width, 3 mm; thickness, 0.5 mm) was suspended in 400 μl of Hanks' balanced salt solution (Nikken Seibutu Co., Ltd.) and vortexed (Scientific Industries, Inc., New York, N.Y.) until the mucosa was disrupted, and 100 μl of this suspension was spread on plates containing a selective agar medium (M-BHI PYLORI agar plates; Nikken Seibutu Co., Ltd.). After cultivation for 4 days at 37°C under microaerophilic conditions, colonies with a gold color were counted. Data shown are the mean number of colonies ± SE per stomach.
Statistical methods.
The statistical significance of difference was assessed by Welch's unpaired t test.
RESULTS
Determination of an epitope recognized by H9 MAb on H. pylori HSP60.
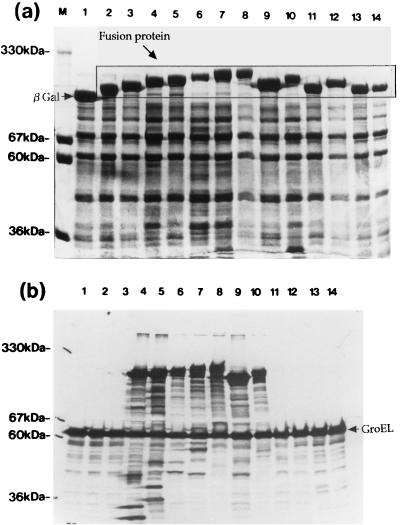
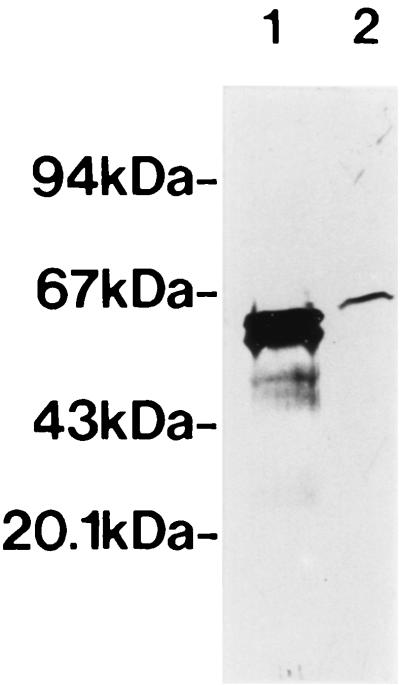
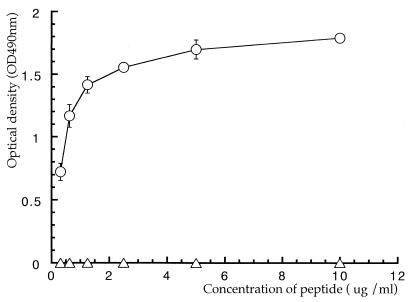
An E. coli strain expressing H. pylori HSP60, designated HY3-HY13, was constructed. After cells had been heat shocked at 42°C for 2 h, fusion proteins of β-galactosidase and H. pylori HSP60 in E. coli lysates were confirmed by carrying out SDS-PAGE and visualizing the bands by staining with Coomassie brilliant blue R-250 (Wako Chemical Ltd.) (Fig. 2a). H9 MAb reacted with fusion proteins derived from HY3, HY6, HY7, HY8, HY9, HY10, and HY11 (Fig. 2b, lanes 4 to 10) but not with other fusion proteins or PEX (expressing β-galactosidase only) (Fig. 2b). Bands migrating in the region of 60 kDa in all lanes (Fig. 2b) were thought to be GroEL protein derived from E. coli pop2136, and the observed weakly staining ladder bands were thought to be due to partially degraded target molecules reacting with H9 MAb (Fig. 2b). These results indicated that the epitope recognized by H9 MAb was related to the sequence of amino acids 181 to 229 on H. pylori HSP60. We previously showed that H9 MAb reacts with several H. pylori strains and other organisms (Helicobacter mustelae, Pseudomonas aeruginosa, Vibrio cholerae, Serratia marcescens, E. coli, and Shigella sonnei), indicating that the epitope recognized by the MAb is conserved on HSP60 in a broad range of bacteria (48). In addition, H9 MAb reacted with human rHSP60 by immunoblot analysis (Fig. 3, lane 2). The amino acid sequences of two regions (residues 181 to 188 and 204 to 229) on H. pylori HSP60 were different from the amino acid sequences of other bacterial HSP60s, and the amino acid region of residues 189 to 203 on H. pylori HSP60 was conserved among several bacterial HSP60s and human HSP60 (P1 protein) (Fig. 4). The sequence of amino acids 189 to 203 on H. pylori HSP60 was thus considered a candidate for the epitope region recognized by H9 MAb. This pH9 peptide was synthesized, and the reaction between pH9 and H9 MAb was studied by pELISA. H9 MAb reacted strongly with the peptide pH9 (Fig. 5), whereas it did not react with the peptide pCont, which contains the unrelated sequence of amino acids 463 to 477 on the H. pylori HSP60 molecule (VNEVEKHEGHFGFNA).
FIG. 2.
Profiles of SDS-PAGE (a) and immunoblotting with H9 MAb recognizing bacterial HSP60 (b) of fusion proteins of H. pylori HSP60 and β-galactosidase overexpressed in E. coli pop2136. The bacterial lysates were loaded as follows: lanes 1, PEX; lanes 2, HY4; lanes 3, HY5; lanes 4, HY6; lanes 5, HY7; lanes 6, HY8; lanes 7, HY9; lanes 8, HY3; lanes 9, HY10; lanes 10, HY11; lanes 11, HY12; lanes 12, HY13; lanes 13, HY14; lanes 14, HY15. M, molecular weight markers.
FIG. 3.
Immunoblot analysis of the reaction between affinity-purified H. pylori HSP60 (lane 1) or recombinant human HSP60 (lane 2) and H9 MAb diluted to 1 μg/ml. Each lane contained 1.25 μg of protein.
FIG. 4.
Comparison of amino acid sequences of the mapped epitope region on H. pylori HSP60 (27) with E. coli GroEL (18), Y. enterocolitica HSP60 (49), P. aeruginosa (41), Legionella pneumophila (19), and human HSP60 (P1 protein) (21). The deduced amino acid sequence of the epitope region recognized by H9 MAb is underlined.
FIG. 5.
The reaction between the synthesized peptide pH9 (circles) or pCont as a negative control (triangles) and H9 MAb by pELISA. Values indicate means ± standard deviations of the means from triplicate experiments.
Human humoral immune response against pH9.
The reaction between pH9 and patient sera was analyzed by pELISA (Table 3). The reactivities with affinity-purified H. pylori HSP60, E. coli rGroEL and human rHSP60 were also studied by cELISA. The serum samples from H. pylori-infected patients had significantly higher levels of serum IgG antibodies recognizing affinity-purified H. pylori HSP60 than did the sera of uninfected persons (0.365 ± 0.013 versus 0.223 ± 0.015, P < 0.0001) (Table 3). However, serum samples from infected subjects had significantly lower titers of pH9 antibody than did those of uninfected subjects (0.278 ± 0.012 versus 0.353 ± 0.021, P < 0.001), indicating that the reactivities with the amino acid sequence of pH9 conserved on H. pylori HSP60 were different from those with H. pylori HSP60. There was no significant difference in the reactivities with E. coli GroEL between the infected and uninfected sera (Table 3). Moreover, the reaction with human rHSP60 was not observed in all sera from patients with or without H. pylori infection (Table 3). No significant difference in the reactivity against pH9 among the group of patients classified by diagnosis was observed (data not shown).
TABLE 3.
Serum IgG responses to HSP60 homologues, synthesized peptide pH9, and H. pylori whole antigen as measured by ELISA
| Antigenb | Optical densitya
|
P valuec | |
|---|---|---|---|
| H. pylori infected (n = 349) | H. pylori uninfected (n = 200) | ||
| H. pylori | |||
| HSP60 | 0.365 ± 0.013 | 0.223 ± 0.015 | <0.0001 |
| pH9 | 0.278 ± 0.012 | 0.353 ± 0.021 | <0.001 |
| E. coli GroEL | 0.405 ± 0.019 | 0.410 ± 0.025 | NSd |
| Human HSP60 | 0.039 ± 0.003 | 0.040 ± 0.003 | NS |
Values are means ± standard errors of the means. n, number of patients.
Each antigen was used at 100 ng/well.
Determined by Welch's unpaired t test.
NS, not significant.
Protective effect of immunizing mice with the pH9 peptide on H. pylori infection.
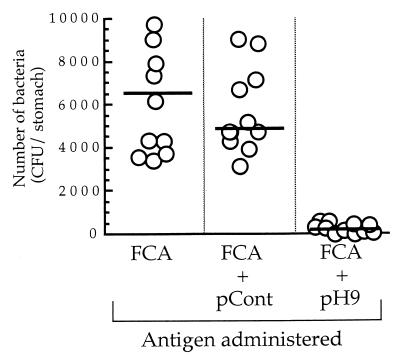
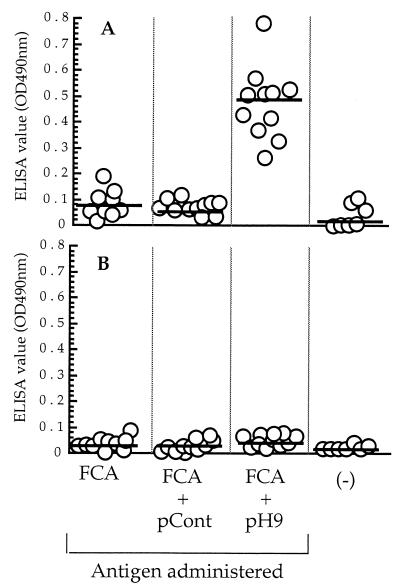
The number of H. pylori organisms colonizing the stomach mucosa of mice immunized with pH9 plus FCA was significantly lower than that in mice immunized with either pCont plus FCA or FCA only (259 ± 65 versus 4,939 ± 827 [P < 0.0001] or versus 6,663 ± 2,068 [P < 0.005]) (Fig. 6). As shown in Fig. 7A, values of IgG against pH9 in the mice immunized with pH9 plus FCA were significantly higher than in mice immunized with pCont plus FCA, mice immunized with FCA only, or control mice (0.479 ± 0.042 versus 0.088 ± 0.006 [P < 0.0001], versus 0.079 ± 0.015 [P < 0.0001], or versus 0.036 ± 0.014 [P < 0.0001]). In contrast, no significant IgG immune response against PPD was observed in any of the mice groups (Fig. 7B).
FIG. 6.
Protective effect of immunization of mice with the pH9 peptide on H. pylori infection. C57BL/6 mice were i.p. immunized five times with either FCA only, pCont plus FCA, or pH9 plus FCA prior to oral inoculation with the H. pylori TK1402 strain. Two weeks after infection, the mice were sacrificed and the number of H. pylori colonizing gastric mucosa was quantified. Horizontal lines represent mean numbers of colonizing bacteria.
FIG. 7.
Measurement by ELISA of serum IgG antibodies in mice immunized with FCA only, pCont plus FCA, or pH9 plus FCA or in untreated mice [(−)] against the pH9 peptide (A) or PPD (B). Horizontal lines represent mean values obtained by cELISA or pELISA.
DISCUSSION
In this study, we showed that the epitope recognized by H9 MAb, which reacts with both bacterial and human HSP60s, is related to the amino acid sequences of residues 189 to 203 on the H. pylori HSP60 molecule (VEGMQFDRGYLSPYF). As shown in Fig. 4, this epitope region was broadly conserved in various other bacterial species and human HSP60. We have also shown that the IgG antibody against the synthesized peptide (pH9) corresponding to the above sequence was strongly induced in patients without H. pylori infection. In addition, immunization of C57BL/6 mice with pH9 induced protection against H. pylori infection.
It is known that an IgG immune response to H. pylori HSP60 is strongly induced in H. pylori-infected patients (27, 36, 40). Our data also indicate that the antibody response to H. pylori HSP60 was high in patients with H. pylori infection compared to that in uninfected patients. These findings indicate that the immune response against HSP60 in H. pylori infection is dominant. However, interestingly, the reactivity with pH9 was low in H. pylori-infected patients compared with that in uninfected patients. This suggests that the humoral immune response to a cross-reactive epitope recognized by H9 MAb might be different from the response to other epitopes on H. pylori HSP60. It is very difficult to explain the difference in the reactivity of the sera to pH9 between H. pylori-infected and uninfected patients. It is possible that in the process of eliminating H. pylori colonizing the stomach, the immune response against the cross-reactive epitope on the bacterial HSP60 might be gradually induced, since the uninfected patients might have had a previous H. pylori infection. We also found that there was no significant difference in humoral immune response to E. coli rGroEL in patients with or without H. pylori infection, indicating that the high level of IgG response to pH9 in uninfected patients is not associated with the humoral immune response to HSP60s induced by natural infection with other bacteria.
Human HSP60 is thought to be a target molecule for induction of local inflammation caused by stimulation of bacterial HSP60 (8, 12, 18, 23). However, in the present study, the human humoral immune response to human rHSP60 in patients with or without H. pylori infection was very low compared with the response to other antigens used. Although the synthesized peptide pH9 contains the homologous sequence of human HSP60 (Fig. 4), an IgG immune response to pH9 was detected. These findings suggest that humoral immune response induced by cross-reactive region antigen among H. pylori HSP60 and human HSP60 in H. pylori infection could not react with native human HSP60 distributed in human tissues. Similarly, a negative immune reaction to human HSP60 in H. pylori infection has also been reported by Sharma et al. (40).
Previous reports have shown that certain bacterial GroEL proteins are able to induce a protective immune response against infections by Legionella spp. (3, 4). Noll et al. also reported the protective role of a cross-reactive epitope on HSP60 of Yersinia enterocolitica in murine yersiniosis (31). A continuous B-cell response against Chlamydia HSP60 in chlamydial infection was thought to induce protective immunity (50). Mattsson et al. demonstrated that H. pylori infection induces strong antibody responses in human gastric mucosa associated with the elucidating of the pathogen (28). The association between the protection against H. pylori infection and the humoral immune response against urease and GroES and GroEL homologues in H. pylori has also been reported (17). These reports suggest that the strong humoral immune response induced by bacterial HSPs is important for the elimination of these bacteria.
To determine whether the immune response against pH9, which is a part of the H. pylori HSP60 molecule, is associated with the elimination of H. pylori, we assessed the protective effect of immunizing mice with the pH9 peptide on H. pylori infection. As shown in Fig. 6, the number of H. pylori colonizing the stomachs of the pH9-immunized mice was significantly lower than that in the control mice treated with either pCont plus FCA or FCA only. These results strongly suggest that the cross-reactive region (VEGMQFDRGYLSPYF) is associated with protection against H. pylori infection.
Several reports have indicated the mechanism of protection against H. pylori infection (2, 14, 17, 40). Ferrero et al. reported that protection against H. pylori infection is mediated by a predominantly Th2-type immune response to the urease, GroES, and GroEL homologues of H. pylori (17). However, Sharma et al. reported that the Th2-type immune response to H. pylori HSP60 was dominant in patients with H. pylori infection after analysis of cytokine-induction levels (interleukin 2 [IL-2], IL-4, gamma interferon, and IL-10) from peripheral blood mononuclear cells, indicating that the Th2-type immune response is associated with gastric inflammation from H. pylori infection (40). On the other hand, Blanchard et al. (2) and Ermark et al. (14) indicated antibody-independent protective mucosal immunity against H. pylori infection in a mouse; and the phenomenon of protection against H. pylori infection being mediated by a major histocompatibility complex class II-restricted response was also reported (14). It appears that the mechanism of protection against H. pylori infection is very complicated.
In the present study, we mapped the epitope recognized by H9 MAb on H. pylori HSP60 and also showed that the humoral immune response against the pH9 peptide is dominant in uninfected patients, indicating that the humoral immune response to a cross-reactive epitope recognized by H9 MAb might be different from that to other epitopes on H. pylori HSP60. Moreover, using an animal experiment, we demonstrated that the immune response against a cross-reactive region on H. pylori HSP60 is associated with protection against H. pylori infection. Although the mechanism by which protective immunization against H. pylori is induced by pH9 remains to be determined, cross-reactive pH9 might be a useful tool as a vaccine component for prevention of H. pylori infection.
ACKNOWLEDGMENT
This study was supported in part by a grant for scientific research from the Ministry of Education, Science, Sport and Culture of Japan.
REFERENCES
- 1.Appelmelk B J, Simoons-Smit I, Negrini R, Moran A P, Aspinall G O, Forte J G, De Vries T, Quan H, Verboom T, Maaskant J J, Ghiara P, Kuipers E J, Bloemena E, Tadema T M, Townsend R R, Tyagarajan K, Crothers J M, Jr, Monteiro M A, Savio A, De Graaff J. Potential role of molecular mimicry between Helicobacter pylori lipopolysaccharide and host Lewis blood group antigens in autoimmunity. Infect Immun. 1996;64:2031–2040. doi: 10.1128/iai.64.6.2031-2040.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanchard T G, Czinn S J, Redline R W, Sigmund N, Harriman G, Nedrud J G. Antibody-independent protective mucosal immunity to gastric Helicobacter infection in mice. Cell Immunol. 1999;191:74–80. doi: 10.1006/cimm.1998.1421. [DOI] [PubMed] [Google Scholar]
- 3.Blander S J, Horwitz M A. Vaccination with major secretory protein of Legionella induces humoral and cell-mediated immune responses and protective immunity across different serogroups of Legionella pneumophila and different species of Legionella. J Immunol. 1991;147:285–291. [PubMed] [Google Scholar]
- 4.Blander S J, Horwitz M A. Major cytoplasmic membrane protein of Legionella pneumophila, genus common antigen and member of the hsp60 family of heat shock proteins, induces protective immunity in a guinea pig model of Legionnaires' disease. J Clin Investig. 1993;91:717–723. doi: 10.1172/JCI116253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaser M J. Hypotheses on the pathogenesis and natural history of Helicobacter pylori induced inflammation. Gastroenterology. 1992;102:720–727. doi: 10.1016/0016-5085(92)90126-j. [DOI] [PubMed] [Google Scholar]
- 6.Born W, Hall L, Dallas A, Boymel J, Shinnick T, Young D, Brennan P, O'Brien R. Recognition of a peptide antigen by heat shock-reactive γδ T lymphocytes. Science. 1990;249:67–69. doi: 10.1126/science.1695022. [DOI] [PubMed] [Google Scholar]
- 7.Censini S, Lange C, Xiang Z, Crabtree J E, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Creighton T E. Unfolding protein folding. Nature. 1991;352:17–18. doi: 10.1038/352017a0. [DOI] [PubMed] [Google Scholar]
- 9.Dunn B E, Campbell G P, Perez-Perez G I, Blaser M J. Purification and characterization of urease from Helicobacter pylori. J Biol Chem. 1990;265:9464–9469. [PubMed] [Google Scholar]
- 10.Eaton K A, Krakowka S. Effect of gastric pH on urease-dependent colonization of gnotobiotic piglets by Helicobacter pylori. Infect Immun. 1994;62:3604–3607. doi: 10.1128/iai.62.9.3604-3607.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eaton K A, Morgan D R, Krakowka S. Campylobacter pylori virulence factors in gnotobiotic piglets. Infect Immun. 1989;57:1119–1125. doi: 10.1128/iai.57.4.1119-1125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellis R J. The molecular chaperone concept. Semin Cell Biol. 1990;1:1–9. [PubMed] [Google Scholar]
- 13.Engstrand L, Gustavsson S, Schwan A, Scheynius A. Local and systemic immune response in Helicobacter pylori-associated chronic gastritis before and after treatment. Scand J Gastroenterol. 1993;28:1105–1111. doi: 10.3109/00365529309098317. [DOI] [PubMed] [Google Scholar]
- 14.Ermark T H, Giannasca P J, Nichols R, Myers G A, Nedrud J, Weltzin R, Lee C K, Kleanthous H, Monath T P. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J Exp Med. 1998;188:2277–2288. doi: 10.1084/jem.188.12.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans D J, Jr, Evans D G, Engstrand L, Graham D Y. Urease-associated heat shock protein of Helicobacter pylori. Infect Immun. 1992;60:2125–2127. doi: 10.1128/iai.60.5.2125-2127.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans D G, Evans D J, Jr, Moulds J J, Graham D Y. N-Acetylneuraminyllactose-binding fibrillar hemagglutinin of Campylobacter pylori: a putative colonization factor antigen. Infect Immun. 1988;56:2896–2906. doi: 10.1128/iai.56.11.2896-2906.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrero R L, Jean-Michel T, Imada K, Wuscher N, Huerre M, Labigne A. The GroES homolog of Helicobacter pylori confers protective immunity against mucosal infection in mice. Proc Natl Acad Sci USA. 1995;92:6499–6503. doi: 10.1073/pnas.92.14.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hemmingsen S M, Woolford C, van der Vies S M, Tilly K, Dennis D T, Georgopoulos C P, Hendrix R W, Ellis R J. Homologous plant and bacterial proteins chaperone protein assembly. Nature. 1988;333:330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman P S, Houston L, Butler C A. Legionella pneumophila htpAB heat shock operon: nucleotide sequence and expression of the 60-kilodalton antigen in L. pneumophila-infected HeLa cells. Infect Immun. 1990;58:3380–3387. doi: 10.1128/iai.58.10.3380-3387.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huesca M, Borgia S, Hoffman P, Lingwood C A. Acidic pH changes receptor binding specificity of Helicobacter pylori: a binary adhesion model in which surface heat shock (stress) proteins mediate sulfatide recognition in gastric colonization. Infect Immun. 1996;64:2643–2648. doi: 10.1128/iai.64.7.2643-2648.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jindal S, Dudani A K, Singh B, Harley C B, Gupta R S. Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen. Mol Cell Biol. 1989;9:2279–2283. doi: 10.1128/mcb.9.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamiya S, Kai M, Ozawa A, Kobayashi H, Shirai T, Harasawa S, Miwa T. Characteristics of vacuolating toxin produced by Helicobacter pylori. Eur J Gastroenterol Hepatol. 1994;6(Suppl. 1):S23–S27. [PubMed] [Google Scholar]
- 23.Kaufman S H E, Schoel B, Wand-Wurttenburger A, Steinhoff U, Munk M E, Koga T. T-cells, stress proteins, and pathogenesis of mycobacterial infections. Curr Top Microbiol Immunol. 1990;155:125–141. doi: 10.1007/978-3-642-74983-4_9. [DOI] [PubMed] [Google Scholar]
- 24.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Lee A, Fox J, Hazell S. Pathogenicity of Helicobacter pylori: a perspective. Infect Immun. 1993;61:1601–1610. doi: 10.1128/iai.61.5.1601-1610.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leunk R D, Johnson P T, David B C, Kraft W G, Morgan D R. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J Med Microbiol. 1988;26:93–99. doi: 10.1099/00222615-26-2-93. [DOI] [PubMed] [Google Scholar]
- 27.Macchia G, Massone A, Burroni D, Covacci A, Rappuoli R. The Hsp60 protein of Helicobacter pylori: structure and immune response in patients with gastroduodenal diseases. Mol Microbiol. 1993;9:645–652. doi: 10.1111/j.1365-2958.1993.tb01724.x. [DOI] [PubMed] [Google Scholar]
- 28.Mattsson A, Quiding-Järbrink M, Lönroth H, Hamlet A, Ahlstedt I, Svennerholm A-M. Antibody-secreting cells in the stomachs of symptomatic and asymptomatic Helicobacter pylori-infected subjects. Infect Immun. 1998;66:2705–2712. doi: 10.1128/iai.66.6.2705-2712.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Negrini R, Savio A, Graffeo M, Rolfi F, Ghielmi S. Autoantibodies and gastric Helicobacter pylori infection: does autoimmunity affect progression to atrophic gastritis? Eur J Gastroenterol Hepatol. 1993;5(Suppl.2):S27–S29. [Google Scholar]
- 30.Negrini R, Lisato L, Zanella I, Cavazzini S, Guillini S, Villanacci V, Poiesi C, Albertini A, Ghielmi S. Helicobacter pylori infection induces antibodies cross-reacting with human gastric mucosa. Gastroenterology. 1991;101:437–445. doi: 10.1016/0016-5085(91)90023-e. [DOI] [PubMed] [Google Scholar]
- 31.Noll A, Roggenkamp A, Heesemann J, Autenrieth I B. Protective role for heat shock protein-reactive αβ T cells in murine yersiniosis. Infect Immun. 1994;62:2784–2791. doi: 10.1128/iai.62.7.2784-2791.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Toole P W, Kostrzynska M, Trust T J. Non-motile mutants of Helicobacter pylori and Helicobacter mustelae defective in flagellar hook production. Mol Microbiol. 1994;14:691–703. doi: 10.1111/j.1365-2958.1994.tb01307.x. [DOI] [PubMed] [Google Scholar]
- 33.Parsonnet J, Friedman G D, Vandersteen D P, Chang Y, Vogelman J H, Sibley R K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 34.Parsonnet J, Hansen S, Rodriguez L, Gelb A B, Warnke R A, Jellum E, Vogelman J H, Friedman G D. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 35.Pérez-Pérez G I, Olivares A Z, Cover T L, Blaser M J. Characteristics of Helicobacter pylori variants selected for urease deficiency. Infect Immun. 1992;60:3658–3663. doi: 10.1128/iai.60.9.3658-3663.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pérez-Pérez G I, Brown W R, Cover T L, Dunn B E, Cao P, Blaser M J. Correlation between serological and mucosal inflammatory responses to Helicobacter pylori. Clin Diagn Lab Immunol. 1994;1:325–329. doi: 10.1128/cdli.1.3.325-329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peterson W L. Helicobacter pylori and peptic ulcer disease. N Engl J Med. 1991;324:1043–1048. doi: 10.1056/NEJM199104113241507. [DOI] [PubMed] [Google Scholar]
- 38.Phadnis S H, Parlow M H, Levy M, Ilver D, Caulkins C M, Connors J B, Dunn B E. Surface localization of Helicobacter pylori urease and a heat shock protein homolog requires bacterial autolysis. Infect Immun. 1996;64:905–912. doi: 10.1128/iai.64.3.905-912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segal E D, Shon J, Tompkins L S. Characterization of Helicobacter pylori urease mutants. Infect Immun. 1992;60:1883–1889. doi: 10.1128/iai.60.5.1883-1889.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma S A, Miller G G, Peek R A, Jr, Pérez-Pérez G I, Blaser M J. T-cell, antibody, and cytokine responses to homologs of the 60-kilodalton heat shock protein in Helicobacter pylori infection. Clin Diagn Lab Immunol. 1997;4:440–446. doi: 10.1128/cdli.4.4.440-446.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sipos A, Klocke M, Frosch M. Cloning and sequencing of the genes coding for the 10- and 60-kDa heat shock proteins from Pseudomonas aeruginosa and mapping of a species-specific epitope. Infect Immun. 1991;59:3219–3226. doi: 10.1128/iai.59.9.3219-3226.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stanly K K, Luzio J P. Construction of a new family of high efficiency bacterial expression vectors: identification of cDNA clones coding for human liver proteins. EMBO J. 1984;3:1429–1434. doi: 10.1002/j.1460-2075.1984.tb01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vanet A, Labigne A. Evidence for specific secretion rather than autolysis in the release of some Helicobacter pylori proteins. Infect Immun. 1998;66:1023–1027. doi: 10.1128/iai.66.3.1023-1027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamaguchi H, Osaki T, Taguchi H, Hanawa T, Yamamoto T, Kamiya S. Flowcytometric analysis of the heat shock protein 60 expressed on the cell surface of Helicobacter pylori. J Med Microbiol. 1996;45:270–277. doi: 10.1099/00222615-45-4-270. [DOI] [PubMed] [Google Scholar]
- 46.Yamaguchi H, Osaki T, Taguchi H, Hanawa T, Yamamoto T, Fukuda M, Kawakami H, Hirano H, Kamiya S. Growth inhibition of Helicobacter pylori by monoclonal antibody to heat-shock protein 60. Microbiol Immunol. 1997;41:909–916. doi: 10.1111/j.1348-0421.1997.tb01949.x. [DOI] [PubMed] [Google Scholar]
- 47.Yamaguchi H, Osaki T, Kurihara N, Taguchi H, Hanawa T, Yamamoto T, Kamiya S. Heat-shock protein 60 homologue of Helicobacter pylori is associated with adhesion of H. pylori to human gastric epithelial cells. J Med Microbiol. 1997;46:825–831. doi: 10.1099/00222615-46-10-825. [DOI] [PubMed] [Google Scholar]
- 48.Yamaguchi H, Osaki T, Taguchi H, Hanawa T, Yamamoto T, Kamiya S. Production and characterisation of monoclonal antibodies to heat-shock protein 60 of Helicobacter pylori. J Med Microbiol. 1997;46:819–824. doi: 10.1099/00222615-46-10-819. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto T, Miura H, Ohsumi K, Yamaguchi H, Taguchi H, Ogata S. Cloning and nucleotide sequence analysis of immunodominant heat-shock protein of Yersinia enterocolitica. Res Microbiol. 1993;144:691–701. doi: 10.1016/0923-2508(93)90033-x. [DOI] [PubMed] [Google Scholar]
- 50.Yi Y, Zhong G, Brunham R C. Continuous B-cell epitopes in Chlamydia trachomatis heat shock protein 60. Infect Immun. 1993;61:1117–1120. doi: 10.1128/iai.61.3.1117-1120.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshida N, Granger D N, Evans D J, Evans D G, Graham D Y, Anderson D C, Wolf R E, Kvietys P R. Mechanisms involved in Helicobacter pylori-induced inflammation. Gastroenterology. 1993;105:1431–1440. doi: 10.1016/0016-5085(93)90148-6. [DOI] [PubMed] [Google Scholar]
- 52.Young D B. Chaperonins and the immune response. Semin Cell Biol. 1990;1:27–35. [PubMed] [Google Scholar]