Abstract
The Kelch-like ECH-associated protein 1-NF-E2-related factor 2 (KEAP1-NRF2) system forms the major node of cellular and organismal defense against oxidative and electrophilic stresses of both exogenous and endogenous origins. KEAP1 acts as a cysteine thiol-rich sensor of redox insults, whereas NRF2 is a transcription factor that robustly transduces chemical signals to regulate a battery of cytoprotective genes. KEAP1 represses NRF2 activity under quiescent conditions, whereas NRF2 is liberated from KEAP1-mediated repression on exposure to stresses. The rapid inducibility of a response based on a derepression mechanism is an important feature of the KEAP1-NRF2 system. Recent studies have unveiled the complexities of the functional contributions of the KEAP1-NRF2 system and defined its broader involvement in biological processes, including cell proliferation and differentiation, as well as cytoprotection. In this review, we describe historical milestones in the initial characterization of the KEAP1-NRF2 system and provide a comprehensive overview of the molecular mechanisms governing the functions of KEAP1 and NRF2, as well as their roles in physiology and pathology. We also refer to the clinical significance of the KEAP1-NRF2 system as an important prophylactic and therapeutic target for various diseases, particularly aging-related disorders. We believe that controlled harnessing of the KEAP1-NRF2 system is a key to healthy aging and well-being in humans.
I. INTRODUCTION
A. Pre-NRF2 Era
Recognition of the concept that our environment has an impact on our health, especially in the case of carcinogenesis, was first described by Percival Pott in 1775 in his publication Chirurgical Observations: Relative to the Cataract, the Polypus of the Nose, the Cancer of the Scrotum, the Different Kinds of Ruptures, and the Mortification of the Toes and Feet (16). Pott noticed an unusually high incidence of scrotal cancers among chimney sweeps in London and suggested that coal soot is an environmental factor triggering carcinogenesis. In 1915, the causal link between environmental chemicals and carcinogenesis was clearly demonstrated by Yamagiwa and Ichikawa (257). They showed that chronic exposure of rabbit ears to coal tar induced skin cancers. Nowadays, it is well appreciated that avoiding and eliminating exposure to adverse environmental factors is critical in the prevention of chemical carcinogenesis in humans.
In the 1960s and 70s, it was demonstrated that coal tar carcinogens and carcinogens from other environmental and occupational settings typically required biotransformation to electrophilic metabolites that formed covalent adducts between “ultimate” carcinogens and proteins, RNA and DNA (34). This metabolic activation is typically catalyzed by the microsomal cytochrome P-450 system and other oxygenases, sometimes termed phase I enzymes (139). While increasing attention was being paid to chemicals as a cause of human cancers, others searched for effective inhibitors of chemical carcinogenesis, small molecules that inhibit the metabolic activation step(s) and/or enhance the detoxication of procarcinogens or their reactive intermediates. Initial interest in these “cancer chemopreventive” compounds focused on food antioxidants and natural products, such as flavonoids and isothiocyanates, which were presumed to be safe for humans. Pretreatment of rodents with the food antioxidant 2 (3)-tert-butyl-4-hydroxyanisole (BHA) effectively inhibited carcinogenesis, resulting from a wide variety of carcinogens that included benzo[a]pyrene, dimethyl-benz[a]anthracene, diethylnitrosamine, 4-nitroquinoline-N-oxide, urethane, and aflatoxin B1 (250).
Benson and colleagues (10) reported that BHA elevated the enzymatic activities of glutathione S-transferases, epoxide hydratase, NAD(P)H:quinone oxidoreductase, and other detoxication enzymes in multiple tissues of the mouse. Many classes of compounds (phenolic antioxidants, azo dyes, polycyclic aromatics, flavonoids, coumarins, cinnamates, indoles, isothiocyanates, 1,2-dithiole-3-thiones, and thiocarbamates) were identified as inducers of these enzymes. Several of these inducers contain a distinctive chemical feature, or acquired this feature after metabolism, that regulates the synthesis of these protective enzymes. Such inducers are Michael reaction acceptors, which are characterized by olefinic bonds that are rendered electrophilic (positively charged) by conjugation with electron-withdrawing substrates. The potency of inducers paralleled their efficiency in Michael reactions and shared the common property of modifying sulfhydryl groups by oxidation, reduction, or alkylation (229). Exposure of cells to low doses of “soft” (non-DNA reactive) sulfhydryl reactive electrophiles evoked a coordinated increase in these enzyme activities (historically termed phase II enzymes), thereby protecting cells against the toxicity of high doses of “hard” (DNA-reactive) electrophiles. This cellular adaptation is called the “electrophile counterattack response” (173). Stemming from these observations, the hypothesis emerged that the cellular “sensor” molecule that transduces the chemical signal of these inducers does so by virtue of unique and highly reactive sulfhydryl functions that recognize and react with them (41).
B. Discovery of NRF2: a Key Effector of the Electrophile Counterattack Response
The elevation of detoxication enzyme activities by electrophiles is now attributable primarily to the increased transcription of their respective genes. Cis-regulatory elements critical for the inducible expression of genes encoding phase II enzymes were identified and designated the antioxidant responsive element (ARE) or electrophile responsive element (EpRE) (55, 189, 190). However, trans regulatory elements encoding transcription factors for the ARE/EpRE were unknown until Itoh et al. established Nrf2-null mice and analyzed their expression of phase II enzymes (78). NRF2 (NF-E2-related factor 2) was originally isolated as a homolog of the hematopoietic transcription factor NF-E2 p45, but its function was unknown (77, 143). They found that the electrophile counterattack response was completely absent in Nrf2-null mice, as the induction of phase II enzymes by BHA was abrogated. Identification of NRF2 as a key regulator of the electrophile counterattack response opened a door in toxicology to a new stage where underlying regulatory mechanisms of various defense reactions and adaptations continue to be deciphered and understood at the molecular level.
C. Discovery of KEAP1: a Sensor for Electrophiles
It has been shown that reactive oxygen species (ROS), such as hydrogen peroxide and superoxide anion, and electrophiles of extrinsic and intrinsic origin exert beneficial and toxic effects on cellular functions. However, it has been a long-standing question as to how our body or cells sense these redox-disruptive stimuli and respond effectively to them. Although NRF2 was identified as a key regulator of the electrophile counterattack response, the mode of regulation of NRF2 activity in response to chemical insults was still a mystery.
However, the missing factor, the sensor of environmental insults and regulator of NRF2 activity, was soon discovered. In 1999, Itoh et al. (79) isolated, by yeast two-hybrid screening, a new thiol-rich protein and negative regulator of NRF2 and named it KEAP1 (Kelch-like ECH-associated protein 1). KEAP1 promotes NRF2 degradation in unstressed conditions, whereas redox-disrupting stimuli directly modify KEAP1 thiols, leading to inactivation of KEAP1 function, stabilization of NRF2, and induction of cytoprotective genes (32, 58, 108, 266). Thus KEAP1 is a biosensor for electrophiles and ROS.
D. Concise Overview of the KEAP1-NRF2 System
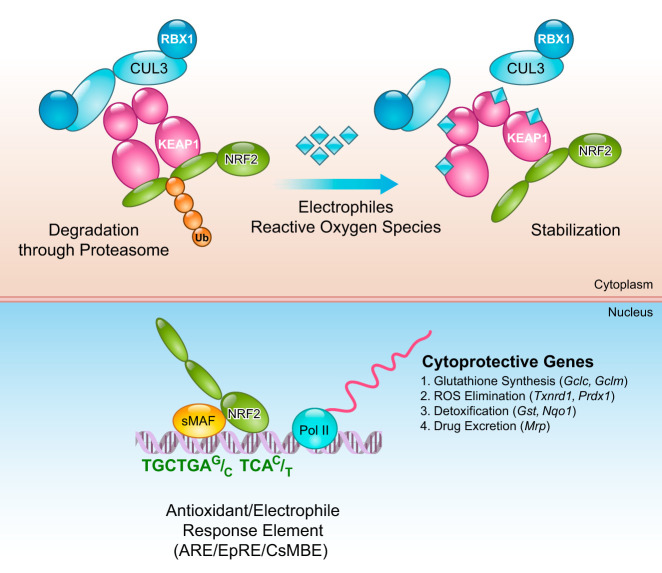
With these discoveries, the KEAP1-NRF2 system has become recognized as the body’s dominant defense mechanism against environmental insults. The KEAP1-NRF2 system is a typical two-component system: KEAP1 as a sensor for electrophiles, and NRF2 as an effector for the coordinated activation of cytoprotective genes (FIGURE 1). In the cytoplasm, KEAP1 forms a ubiquitin E3 ligase complex with CULLIN3 (CUL3) and polyubiquitinates NRF2, which marks NRF2 for rapid degradation through the proteasome system. Thus in unstressed conditions, NRF2 is synthesized, but constantly degraded. However, on exposure to electrophiles or ROS, the reactive cysteine residues of KEAP1 are directly modified, which reduces the ubiquitin E3 ligase activity of the KEAP1-CUL3 complex and results in NRF2 stabilization. Nascent NRF2 can then directly translocate into the nucleus, heterodimerize with one of the small musculo-aponeurotic fibrosarcoma (sMAF) proteins, bind to the ARE/EpRE, and robustly activate a battery of phase II genes, thereby acting as a master regulatory transcription factor.
FIGURE 1.
KEAP1-NRF2 is a two-component system. In the cytoplasm, NRF2 is ubiquitinated by the KEAP1-CUL3 ubiquitin E3 ligase complex to mark it for degradation by the proteasome. When cells are exposed to electrophiles or reactive oxygen species, KEAP1 is modified and the KEAP1-CUL3 ubiquitin E3 ligase activity declines, which results in the stabilization of NRF2. Stabilized and accumulated NRF2 translocates to the nucleus and activates a battery of cytoprotective genes.
Vigorous worldwide research on the KEAP1-NRF2 system led to substantial accumulation of evidence demonstrating the critical significance of NRF2 activity and its regulatory mechanisms to the maintenance of our health. Importantly, dysregulation of the KEAP1-NRF2 system underlies the pathogenesis of various human diseases. It is now widely recognized that the KEAP1-NRF2 system provides an attractive target for drug development. In this review, we provide a comprehensive overview of the KEAP1-NRF2 system. We discuss the molecular mechanisms by which the KEAP1-NRF2 system senses and responds to intrinsic and extrinsic redox disturbances and the roles the system plays in organismal defense mechanisms. We consider the generally mitigating, but sometimes exacerbating, contributions of the KEAP1-NRF2 system to various human pathological conditions, such as diabetes, inflammation, and cancer.
II. IDENTIFICATION OF CNC-sMAF TRANSCRIPTION FACTORS
A. Starting From cis-Regulatory Elements
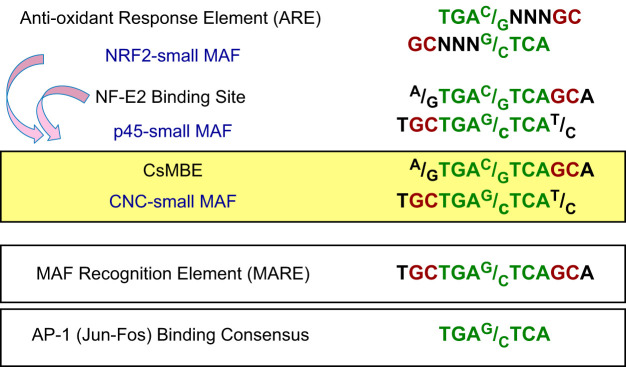
A cis-regulatory element, termed the ARE/EpRE (TGAG/CNNNGC), was first defined from studies in 1990 in the context of toxicology through studies on the mechanism of induction of a glutathione S-transferase gene in response to electrophiles (55, 189, 190). ARE/EpREs were also found in promoter regions of other cytoprotective genes within the phase II battery, confirming the inducible and concerted nature of this gene expression response. Although the importance of the ARE/EpRE was clearly shown, the transcription factors that bind to the element and elicit transcriptional activation remained unidentified.
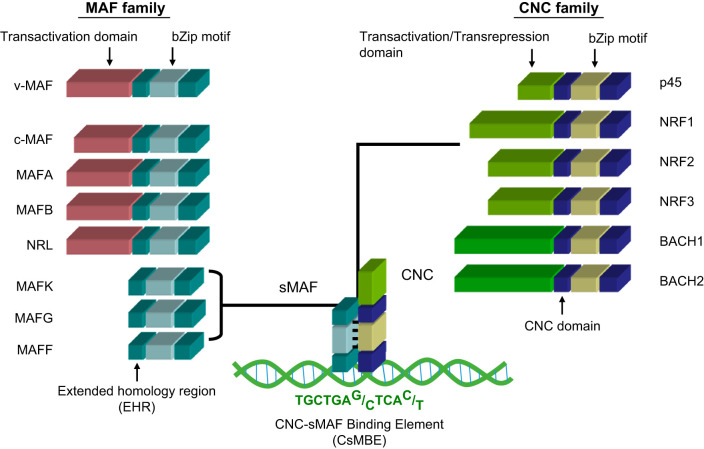
Meanwhile, researchers in the molecular biology field identified a critical cis-regulatory element for erythroid-specific transcriptional activation and named it the NF-E2 binding site (ATGAG/CTCAGCA) (138). The NF-E2 binding activity was successfully purified from a large amount (700 g) of cultured mouse erythroleukemia cells (4). NF-E2 p45 was identified and isolated and became a founding member of the mammalian cap ’n’ collar (CNC) transcription factor family. Following the discovery of p45, other members of the CNC family were identified as NRF1, NRF2, NRF3, BACH1, and BACH2 (21, 77, 107, 143, 169) (FIGURE 2).
FIGURE 2.
Members of Maf and CNC families of transcription factors. Both family members possess well-conserved basic region-leucine zipper motifs. The basic regions mediate DNA recognition, and the leucine zippers mediate dimerization. Small Maf proteins lacking canonical transactivation domains are obligatory heterodimeric partner molecules of CNC family proteins.
A seminal discovery related to the vertebrate CNC family of proteins is that they require sMAF factors as obligatory heterodimeric partner molecules for efficient binding to the ARE/EpRE/NF-E2 motif (68, 146). Both CNC proteins and sMAF factors possess a well-conserved basic region-leucine zipper (bZIP) motif. The CNC proteins also retain a characteristic CNC domain that was defined based on sequence homology of 43 amino acids with the Drosophila Cnc protein (142). The sMAF factors also retain a characteristic extended homology region (FIGURE 2). As the sMAF factors do not appear to possess any substantial extra-functional domains, including transactivation domains, the activity of the CNC-sMAF heterodimers seems to be defined mainly by that of the CNC factors. Of the CNC factors, NF-E2 p45, NRF1, NRF2, and NRF3 possess transactivation domains and are considered to be transcriptional activators (21, 77, 107, 143). In contrast, BACH1 and BACH2 possess characteristic broad complex-tramtrack-bric-a-brac (BTB) domains and lack any canonical transactivation domains, thus representing transcriptional repressors (39, 94, 153, 169). Consequently, based on the binding sequence similarity and related information, CNC-sMAF heterodimers emerged as strong candidates for the transcriptional activation of phase II enzyme genes.
B. sMAF as an Obligatory Partner of CNC Proteins
MAF family factors are DNA-binding transcription factors that possess a well-conserved bZIP motif and an extended homology region on the NH2-terminal side of the bZIP motif (FIGURE 2). MAF transcription factors can form homodimers and bind to the MAF recognition element, referred to as the MARE (TGCTGAG/CTCAGCA), that contains an activating protein-1 binding site known as the 12-O-tetradecanoylphorbol-13-acetate-responsive element (TRE) (TGAG/CTCA) (92, 102). MAF factors are divided into two groups: large MAF factors that possess transactivation domains, and sMAF factors that lack such domains (15, 89, 144, 146) (FIGURE 2). A founding member of the MAF family is v-MAF, which was isolated from avian musculo-aponeurotic fibrosarcoma virus (159). Other large MAF factors include the cellular counterpart of v-MAF called c-MAF and also MAFA, MAFB, and NRL. These large MAFs act as homodimers that activate transcription (9, 159). In contrast, the three mammalian sMAFs, named MAFF, MAFG, and MAFK, form homodimers that repress transcription (145, 148, 150, 154). Homodimers of sMAF are assumed to behave as competitive inhibitors of bZIP transcription factors acting on MAREs.
Another important role of sMAFs is that of obligatory heterodimeric partner molecules of CNC proteins, allowing them to bind to DNA and exert their function as transcription regulators (5, 68). Genetic evidence that sMAF deficiency recapitulates CNC deficiency strongly validates the critical contribution of sMAFs to the function of CNC proteins (261). For example, mice deficient in NF-E2 p45, NRF2, or NRF1 display severe thrombocytopenia, an impaired response to oxidative stress, and neuronal dysfunction, respectively (78, 110, 205). In good agreement to this observation, disruptions to the three sMAF factors in various combinations result in all of these phenotypes with various severities, depending on the extent of the sMAF reduction (96, 97, 147, 163, 199, 261). Thus CNC-sMAF heterodimers form a new functional unit of transcription factors that are distinct from other bZIP family transcription factors and regulate several biological processes, particularly those related to cell differentiation, maturation, and maintenance.
C. Unique Features of CNC-sMAF Binding Elements
The NF-E2 p45-sMAF heterodimer is a critical regulator of megakaryopoiesis through its binding to NF-E2 binding sites (24, 38, 56, 121, 228). Importantly, NRF2 also associates with the NF-E2 binding site (77, 143), which led us to recognize the sequence resemblance between the ARE/EpRE and the NF-E2 binding site and to then discover NRF2 as an ARE/EpRE binding transcription factor (FIGURE 3). This notion has been supported unequivocally by genetic analyses demonstrating that ARE/EpRE-regulated gene expression is severely impaired in Nrf2-null mice (78), as well as in sMAF-deficient mice (97). To date, genetic studies and other approaches have clearly established the NRF2-sMAF heterodimer as the trans-acting factor that interacts with the ARE/EpRE and activates the expression of cytoprotective genes.
FIGURE 3.
DNA binding consensus sequences of CNC and MAF family proteins. Core sequences common with the AP1 binding consensus sequence are indicated in green. Flanking “GC” dinucleotides unique to the MAF binding consensus are indicated in red.
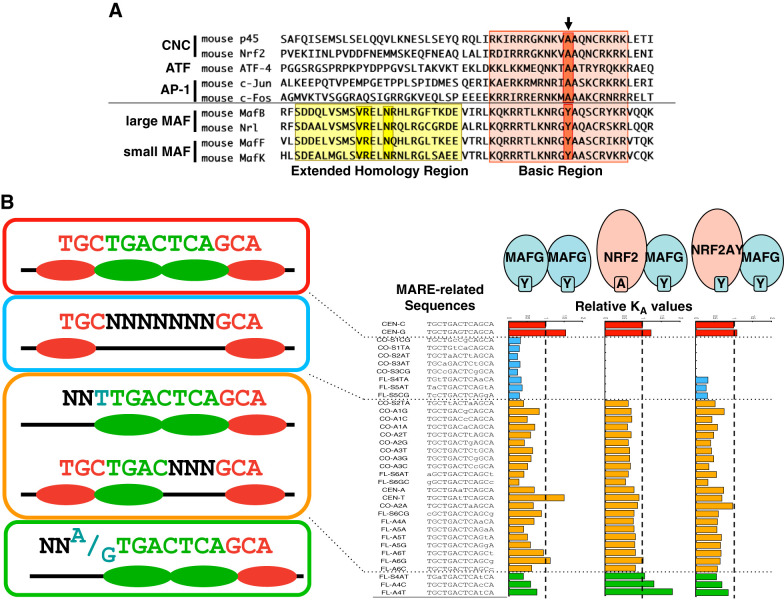
The NF-E2 binding site and the ARE/EpRE are collectively recognized as binding sites of CNC-sMAF heterodimers. Based on recent analyses of the genomewide distribution of CNC proteins, including NRF2, NF-E2 p45, NRF1, and BACH2 (6, 29, 56, 62, 65, 131), the sequence GCTGAG/CTCAC/T has arisen as a common consensus binding site and has been designated as the CNC-sMAF binding element (CsMBE) (168). Asymmetry in the CsMBE reflects the distinct binding preferences of CNC and sMAF proteins. Alignment of the DNA binding domains of various bZIP transcription factors (including those belonging to the CNC, ATF, JUN, FOS, and MAF families) revealed that a single amino acid in the basic region is a unique feature of MAF family proteins (FIGURE 4A). MAF proteins commonly possess a tyrosine (Tyr: Y) residue in their basic region, which corresponds to alanine (Ala: A) in the other bZIP transcription factors that include NRF2. The tyrosine residue in the basic region of MAF family proteins is a key to understanding the unique DNA binding preference of MAF family proteins.
FIGURE 4.
Unique DNA recognition preference of MAF family proteins. A: alignment of the DNA binding domains of various bZIP transcription factors. Arrow indicates a conserved tyrosine residue for the MAF family and a conserved alanine residue for the others. B, right: relative KA values of MAFG homodimer, NRF2-MAFG heterodimer, and NRF2A502Y-MAFG heterodimer to various MARE-related sequences. Left: categorization of the MARE-related sequences.
A comprehensive analysis revealed the DNA binding preferences of the MAFG homodimer and the NRF2-MAFG heterodimer (FIGURE 4B) (259). The “GC” dinucleotides on each side of the MARE binding site are sufficient for DNA binding of the MAFG homodimer (FIGURE 3). Instead, the “GC” dinucleotide on one side and one-half of the TRE on the other side of the binding site are required for DNA binding of the NRF2-MAFG heterodimer (FIGURE 3). As deduced from these observations, MAF proteins preferentially recognize the flanking “GC” dinucleotide, whereas CNC proteins recognize the core TRE. It should be noted that a single base outside the core TRE substantially influences the DNA binding affinity of the NRF2-MAFG heterodimer, suggesting that CNC proteins recognize a region that is one base wider than that recognized by JUN and FOS family proteins (104). Thus the “GC” in one-half of the CsMBE (underlined in GCTGAG/CTCAC/T) is recognized by sMAF, whereas the other one-half (underlined in GCTGAG/CTCAC/T) is recognized by CNC.
To understand the structural basis of the unique DNA recognition mode of MAF proteins, a DNA binding domain of MAFG was cocrystallized with the MARE-containing DNA duplex (117). Crystallization of the MAFG homodimer with the MARE revealed a critical role of the Tyr/Ala difference in the basic region that discriminates MAF proteins from other bZIP proteins, such as JUN, FOS, and ATF family proteins. The MAF-specific tyrosine residue is responsible for the unique DNA recognition mode. Swapping the unique tyrosine residue Y64 of MAFG and the corresponding alanine residue A502 of NRF2 resulted in the simultaneous swapping of DNA recognition specificity. MAFG Y64A did not require the “GC” dinucleotide for DNA binding. The NRF2 A502Y mutant partially mimicked the DNA binding preference of the MAFG homodimer. Thus the heterodimer of NRF2 A502Y and MafG acquired the ability to bind to MAF homodimer-specific binding sites (MARE) and lost their ability to bind to the CsMBE with high affinity (104). The significance of high-affinity binding to the CsMBE was highlighted in a study of mice expressing NRF2 A502Y instead of wild-type NRF2 (168). Mice homozygously expressing NRF2 A502Y recapitulated the major phenotypes of Nrf2-null mice, indicating a strict requirement of the CsMBE for the NRF2-mediated stress response and cytoprotection.
III. DEREPRESSION REGULATION OF NRF2 BY KEAP1
A. KEAP1-Based Ubiquitin E3 Ligase Complex and Proteasomal Degradation of NRF2
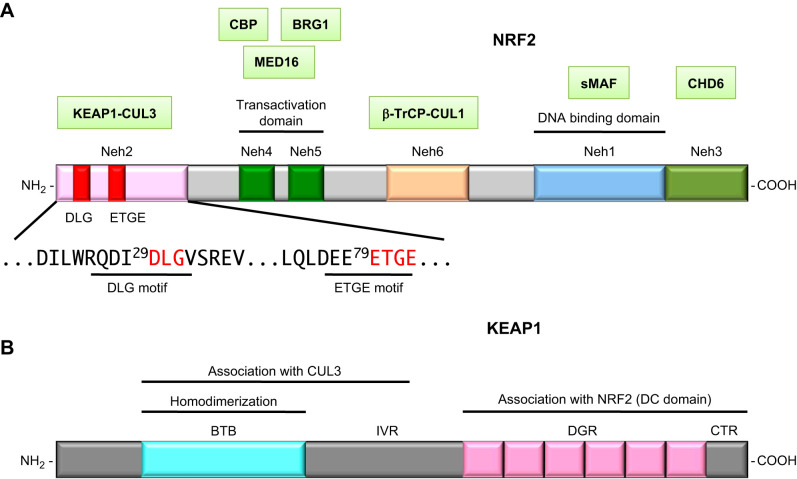
Phylogenetic conservation of the structure of NRF2 among species revealed the presence of six functional domains: Neh1 (NRF2-ECH homology domain-1) to Neh6 (79) (FIGURE 5A). Neh1 contains the CNC and bZIP domains that mediate DNA binding and dimer formation, whereas Neh3, Neh4, and Neh5 are transactivation domains. Of the transactivation domains, Neh4 and Neh5 make a major contribution to transcriptional activation by recruiting histone acetyl-transferase cAMP responsive element binding protein (CBP) (93) and Mediator complex (197).
FIGURE 5.
Domain structures of NRF2 (A) and KEAP1 (B). NRF2-interacting molecules are shown in green boxes and placed above their interacting domains.
Truncated NRF2 lacking the Neh2 domain exhibited markedly increased transcriptional activity, suggesting the existence of a repressor molecule that interacts with Neh2. To this end, KEAP1 was isolated as this Neh2-interacting molecule by yeast two-hybrid screening (79) (FIGURE 5B). Soon after its identification, KEAP1 was found to be a component of ubiquitin E3 ligase (108). Thus Neh2 was found to be a KEAP1-specific degron of NRF2, and this led to the discovery of a unique derepression mechanism. Neh6 is a KEAP1-independent degron of NRF2 as it harbors a cluster of serine residues that are phosphorylated by glycogen synthase kinase 3 (GSK-3), resulting in the facilitation of NRF2 degradation.
The most important feature of NRF2 is its inducibility. KEAP1 creates the inducible nature of NRF2 function by serving as a substrate recognition component of the E3 ubiquitin ligase complex in cooperation with CUL3 and RBX (32, 58, 108, 266). Under normal conditions, ubiquitinated NRF2 undergoes proteasomal degradation, and it is this constitutive degradation of NRF2 that maintains the quantity and activity of NRF2 at a low level. The E3 ubiquitin ligase activity of the KEAP1-CUL3 complex is disrupted on exposure to electrophiles and ROS that modify the cysteine residues of KEAP1, allowing newly synthesized NRF2 to accumulate in the nucleus and activate transcription (109). Thus the possession of highly reactive cysteine residues renders KEAP1 an efficient and sensitive biosensor of redox disturbance through switching on or off the ubiquitin E3 ligase activity of the KEAP1-CUL3 complex.
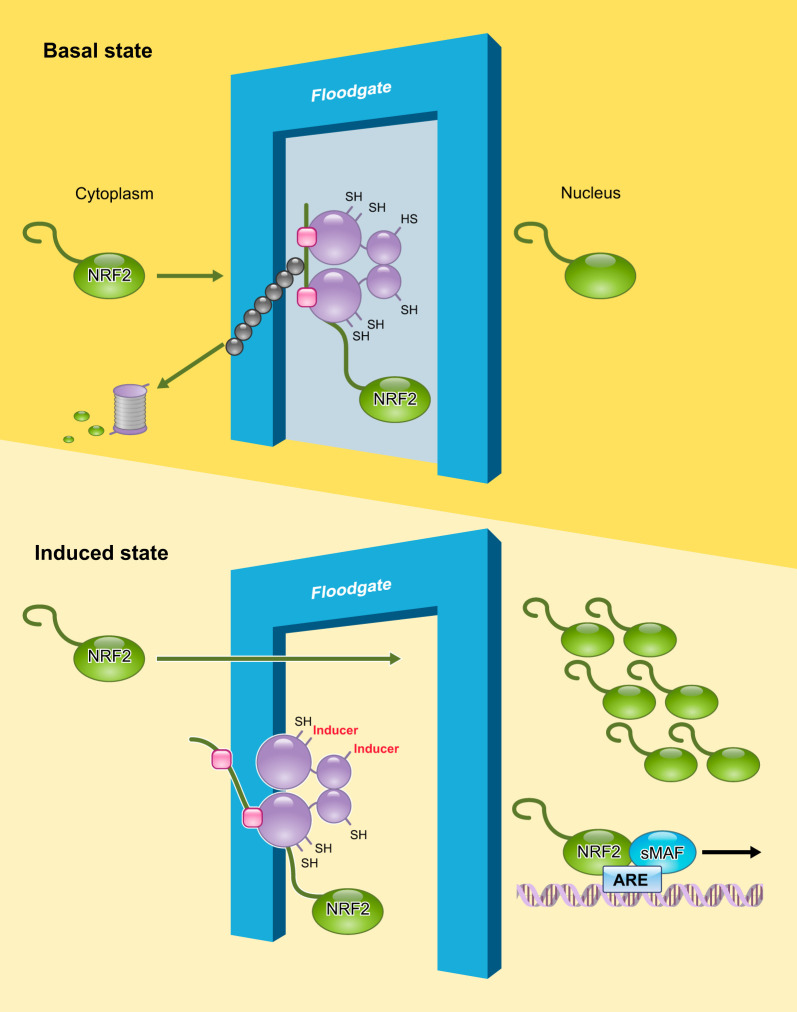
Quantitative immunoblot analyses indicated that, in the basal state, the cellular level of NRF2 was kept lower than the levels of KEAP1 and CUL3 proteins. Challenge with an electrophilic agent dramatically increased NRF2 to a level greater than that of KEAP1 and CUL3, resulting in the accumulation of NRF2 in the nucleus (76). By contrast, KEAP1 and CUL3 did not display any changes in their abundance, subcellular localization, or interaction in response to electrophilic stimuli. Higher resolution immunostaining examination revealed that KEAP1 localizes in the perinuclear cytoplasm (248) with loose attachment to the actin cytoskeleton (88). This localization leads to a model in which KEAP1 acts as a floodgate (76) (FIGURE 6). NRF2 moves into nucleus only when the floodgate (KEAP1) is modified and opened. In summary, the regulation of levels of NRF2 protein during a stress response is mediated by the activity, but not the composition, of the KEAP1-CUL3 ubiquitin ligase complex.
FIGURE 6.
Molecular dynamics of the NRF2-KEAP1-CUL3 complex in cells. KEAP1 acts as a floodgate for NRF2 and usually blocks NRF2 entry to the nucleus by facilitating its degradation. The KEAP1 floodgate opens when cells are assaulted by stresses, and NRF2 translocates into nucleus to activate cytoprotective gene expression.
B. Structure of CUL3-KEAP1-NRF2 Complex Is Permissive for NRF2 Ubiquitination
To elucidate how NRF2 is ubiquitinated under normal conditions, multiple biochemical and biophysical analyses were conducted. Three domains have been identified in KEAP1: a BTB, an intervening region (IVR), and a double glycine repeat and COOH-terminal region (DC) domain (FIGURE 5B). Structure-function and molecular-dissection studies have shown that the NRF2 Neh2 domain directly interacts with the KEAP1 DC domain (79). Two discrete motifs in the NRF2 Neh2 are critical for KEAP1-dependent NRF2 degradation (136, 233), implying that these two motifs mediate the interaction between the NRF2 Neh2 domain and the KEAP1 DC domains.
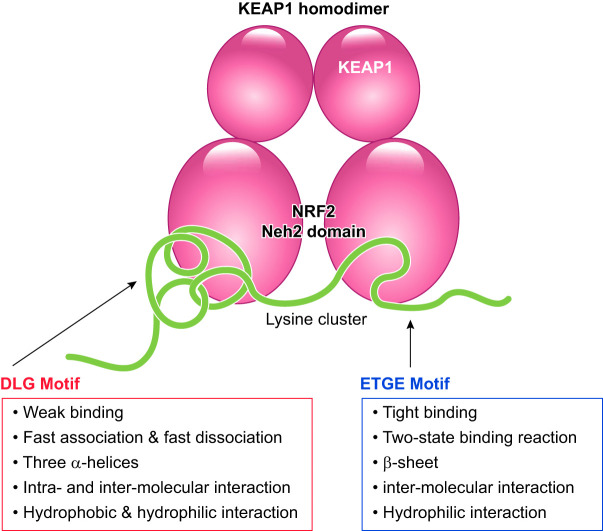
The NRF2 Neh2 and KEAP1 DC domains show a unique mode of interaction, referred to as the two-site binding model. This binding model was first suggested from studies of isothermal calorimetry and subsequently verified by NMR structural analyses and molecular dissection analyses (233, 234). The two distinct motifs DLG and ETGE in the Neh2 domain are critical for its interaction with the KEAP1 DC domain. Together, the DLG and ETGE motifs bind to a KEAP1 homodimer, with each motif interacting individually with its own molecule of KEAP1 (57, 76). Thermodynamic examination revealed a significant difference between the motifs when they interact with the KEAP1 DC domain: the ETGE motif exclusively utilizes hydrogen bonding, whereas the DLG motif relies on both hydrophobic interactions and hydrogen bonding (57).
Kinetic analysis using surface plasmon resonance further consolidated the qualitative difference suggested by the thermodynamic examination (57). Surface plasmon resonance signals indicated that the DLG and ETGE motifs form low- and high-affinity binding sites, respectively, for the KEAP1 DC domain (234). Detailed analyses of association and dissociation rate constants indicated that the DLG exhibits a single-state binding with fast association and fast dissociation. In contrast, the ETGE shows two-state binding with slow association and slow dissociation steps, suggesting that a quick transient binding conformation is slowly transferred to a fully stable binding conformation (57). The qualitative difference in the two binding sites provides an important clue for understanding how NRF2 ubiquitination starts and stops in response to electrophilic signals.
Structural analyses of the KEAP1-NRF2 system were carried out using NMR, X-ray, and electron microscopy. The solution structure of NRF2 Neh2 was examined using NMR (233) and showed that NRF2 Neh2 adopts a rodlike conformation with a central single α-helix flanked by the DLG and ETGE motifs. Seven lysine residues that are ubiquitinated by the KEAP1-CUL3 E3 ubiquitin ligase complex are clustered in the α-helix. Six of these lysines are aligned on a single half-side of the helix surface, resulting in their optimal presentation for high-efficiency ubiquitination.
The crystal structure of the KEAP1 DC domain obtained by X-ray disclosed a β-barrel structure with six blades (57, 125, 170). Cocrystallization of the KEAP1 DC domain with a peptide containing either the DLG motif or the EGTE motif showed that the peptides interact at the bottom of the β-barrel structure. The ETGE motif forms a β-hairpin structure by hydrogen bonding and binds to the KEAP1 DC domain in a key (ETGE) and keyhole (pocket in KEAP1 DC domain) manner, whereas the extended DLG motif forms a triple-helix structure using both hydrogen bonding and hydrophobic interaction and loosely attaches onto the bottom surface of the KEAP1 DC domain (57). The interface between the DLG motif and the KEAP1 DC domain is wider than the binding surface between the ETGE and the KEAP1 DC domain, which is in harmony with the kinetic and thermodynamic differences between the two interactions.
Single-particle analysis using electron microscopy revealed a breathtaking structure of the KEAP1 homodimer and rendered all of the existing biological, biochemical, and biophysical results to be consistent with each other (161). Two large spheres are joined at the end of their stems, just like a cherry-bob (FIGURE 7). The stemlike structure and the globular portion of KEAP1 correspond to the BTB and DC domains, respectively. Superimposition of the DC crystal structure to the globular portion of the cherry-bob indicated that the NRF2 binding sites are located on the bottom of the two globular portions. The distance between the two binding pockets in the two globular domains is ~80 Å (161), which is very close to the expected distance between the DLG and ETGE motifs, as determined by the NMR solution structure analysis of Neh2 (233).
FIGURE 7.
Schematic illustration of the formation of the complex between the KEAP1 homodimer and the Neh2 domain of NRF2. Biophysical characteristics and structural features are summarized.
The next step was to find out how CUL3 is integrated into the KEAP1-NRF2 complex. The interaction between KEAP1 and CUL3 is another important issue for understanding the molecular mechanism underlying NRF2 ubiquitination. Interaction between the NH2-terminal region of CUL3 and the IVR domain of KEAP1 was detected in a molecular dissection analysis (108). Analysis of the crystal structure of KLHL11, a BTB-Kelch family protein that is closely related to KEAP1, was consistent with this observation (20). A hydrophobic groove called the “3-box” motif located between the BTB and IVR domains of KLHL11 associates with the NH2-terminal domain of CUL3. Further analysis is necessary to clarify the composite structure of the CUL3-KEAP1-NRF2 ternary complex.
C. Functional Analyses of KEAP1 Domain Structures
Transgenic complementation rescue is a powerful and sophisticated approach for establishing the in vivo functions of proteins (145, 148, 150). Lethality in Keap1-null mice occurs before weaning due to marked stenosis in the esophagus, thus providing a phenotype for in vivo evaluation of structure-function (148, 240). The esophageal lesions and subsequent lethality observed in Keap1-null mice were completely rescued by the simultaneous disruption of Nrf2 (240). Phenotypes resulting from Keap1 deficiency can also be rescued by transgene-derived KEAP1 expressed under the influence of the Keap1 gene regulatory region (260). This approach of transgenic complementation rescue enabled us to evaluate the function of KEAP1 as a repressor of NRF2 by monitoring the phenotypes caused by Keap1 deficiency. Replacing wild-type KEAP1 with mutant KEAP1 in this experimental setting provided reliable answers concerning the in vivo functions of various domains in KEAP1. For instance, KEAP1 C273S mutant (Cys273 substituted with serine) did not rescue the lethality of Keap1-null mice, indicating that Cys273 is essential for KEAP1 to repress NRF2 activity (260).
The essential functional contribution of the KEAP1 BTB domain, which mediates homodimer formation, was also demonstrated by transgenic complementation rescue experiments. Cross-breeding with transgenic mice expressing wild-type KEAP1 rescued the lethality of Keap1-null mice, whereas cross-breeding with transgenic mice expressing a BTB-deficient KEAP1 did not (260). Five amino acids in the BTB domain that are critical for dimerization were mutated, and the resulting KEAP1 BTB mutant failed to restore the function of KEAP1 (218). These results, in combination with recognition that the stoichiometry of the interaction between NRF2 Neh2 and KEAP1 is 1:2 (233), consolidate the critical significance of homodimer formation of KEAP1 through the BTB domain for the ubiquitination and degradation of NRF2. Collectively, these results have been integrated into the “two-site binding model” of NRF2 and KEAP1 that defines the structural basis for the efficient ubiquitination of NRF2 (FIGURE 7).
D. Phylogenic Distribution of the KEAP1-NRF2 System
The KEAP1-NRF2 system is very well conserved among vertebrates, from fish to mammals. An NRF2 ortholog in zebrafish heterodimerizes with zebrafish sMAF, and the heterodimer binds to DNA and activates genes involved in the adaptive stress response. Zebrafish KEAP1 regulates the activity of the zebrafish NRF2-sMAF heterodimer in a manner similar to that in mammals (112). The system is also well conserved in invertebrates. For example, the NRF2 ortholog in Drosophila is called cap ’n’ collar (CNC). The cnc gene in Drosophila produces multiple alternative splice variants, with the products CncA, CncB, and CncC being the major isoforms. These isoforms possess different lengths of NH2-terminal regions and a common COOH-terminal region containing a CNC domain and a bZIP motif. CncC is the longest isoform and best resembles mammalian NRF2. Similar to vertebrate NRF2, Drosophila CncC requires sMAF for carrying out its NRF2-like functions. A KEAP1 ortholog in Drosophila interacts with CncC and acts as a negative regulator, just like KEAP1 regulates NRF2 in mammals (172).
In contrast, C. elegans possesses an NRF2 ortholog called SKN-1 and operates through a different system of defense against oxidative stress (13). Whereas SKN-1 possesses a CNC domain and a basic region that highly resembles those of Drosophila and vertebrate CNC proteins, it lacks a leucine zipper for dimerization with sMAF. Thus SKN-1 binds to DNA as a monomer that recognizes the sequence GTCAT (12), which coincides with the CNC-side half-site of CsMBE (GCTGAG/CTCAC/T). Therefore, it is not surprising that there is no ortholog of sMAF in C. elegans. In addition, there is no KEAP1 ortholog in C. elegans. Instead of electrophilic signaling, which is directly sensed by KEAP1 in Drosophila and vertebrates, SKN-1 activity is regulated by phosphorylation signals mediated by p38 and AKT/mammalian target of rapamycin (mTOR). SKN-1 translocates to the nucleus when it is phosphorylated by p38 MAPK in response to oxidative stress (73), whereas nuclear localization of SKN-1 is inhibited when it is phosphorylated by GSK-3 (3).
It should be noted that, due to their short life span, Drosophila and C. elegans are often utilized for aging studies. Activation of CncC by KEAP1 mutation extends the life span of Drosophila (221). Disruption of SKN-1 in C. elegans reduces resistance to stress and shortens longevity (236). The contribution of SKN-1 to longevity is more prominent in the presence of oxidative stress than in unstressed conditions, suggesting that reinforcement of an oxidative stress response is an effective antiaging mechanism. A recent study using naked mole-rats, rodents with naturally long life spans, revealed that a higher NRF2 activity is closely associated with their longevity (123). Naked mole-rats have lower levels of KEAP1 protein and higher levels of NRF2 target gene than mice with shorter life spans. Maximum lifetime correlated positively with the ARE/CsMBE-binding activity of NRF2. These reports strongly support the notion that a higher NRF2 activity is advantageous for longevity.
IV. MULTIPLE MECHANISMS REGULATING NRF2 ACTIVITY
A. Cysteine Code and Two-Site Binding Mechanism
KEAP1 is a thiol-rich protein containing many cysteine residues, some of which are adjacent to basic amino acids that foster reactive anionic forms of the sulfhydryl group. Covalent binding of electrophilic inducers to cysteine residues has been observed using mass spectrometry (42, 63). The significant contribution of the cysteine residues to the function of KEAP1 as a sensor has been demonstrated in cultured cells (53, 113, 137, 227, 265), zebrafish (113), and mice (191, 218, 260). These studies revealed three major cysteine residues of KEAP1 that are critical for modulating the ubiquitin E3 ligase activity of the KEAP1-CUL3 complex. These critical cysteine residues are Cys151 in the BTB domain and Cys273 and Cys288 in the IVR (FIGURE 8).
FIGURE 8.
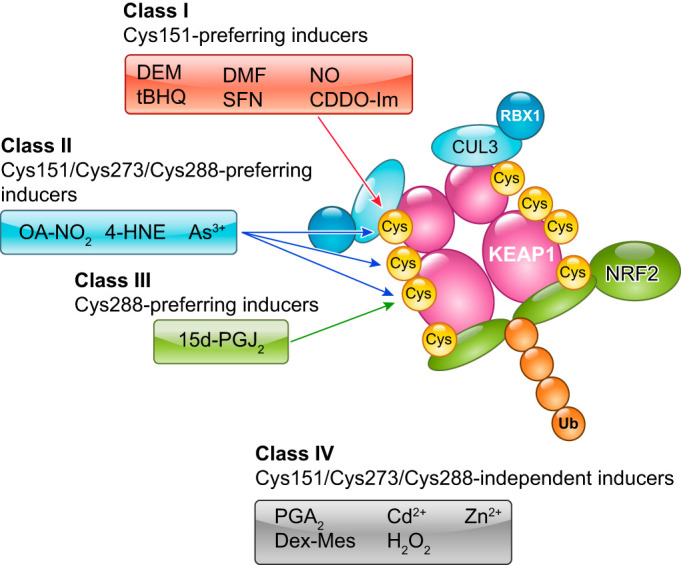
Cysteine codes of the KEAP1-based response to various redox disturbances. Three key cysteine residues, Cys151, Cys273, and Cys288, of KEAP1 are responsible primarily for the responses to a variety of electrophiles. OA-NO2, nitro-fatty acids; 4HNE, 4-hydroxynonel.
Substitution of Cys151 with serine did not affect the ubiquitin conjugating activity of the KEAP1-CUL3 complex. However, the KEAP1 C151S mutant did not respond to a large group of electrophiles, including diethylmaleate, tert-butylhydroquinone, dimethylfumarate, sulforaphane, and nitric oxide, which led to continuous ubiquitination and degradation of NRF2 (227). When Cys151 was substituted with tryptophane, the KEAP1 C151W mutant failed to associate with the CUL3 complex and did not support the ubiquitin E3 ligase activity of the KEAP1-CUL3 complex, leading to the accumulation of NRF2 (47, 113). Considering the bulky nature of a tryptophane residue, KEAP1 C151W is likely to mimic an electrophile-conjugated inactive state, whereas KEAP1 C151S is likely to mimic a conjugation-free active state.
Derivatives of the triterpenoid compound 2-cyano-3,12-dioxooleana-1,9-dien-28-oic-acid (CDDO) are exceedingly potent inducers of the electrophile counterattack response. These derivatives bind to Cys151 and disrupt the interaction between KEAP1 and CUL3 (31, 76). Small molecules possessing similar backbone structures to CDDO (e.g., CDDO-Im) have been developed as stereoselective KEAP1 Cys151-binding reagents (66). Although KEAP1 C151W was shown to lose its association with CUL3 (47), most NRF2-inducing electrophilic chemicals do not directly disrupt the KEAP1-CUL3 interaction, with the only exceptions being CDDO-Im and possibly its derivatives (76). Irrespective of the precise structural mechanisms involved in the KEAP1-CUL3 interaction, these results strongly support the contention that Cys151 serves as a primary sensor cysteine by switching off KEAP1 activity on direct modification by electrophiles.
Substitution of Cys273 and Cys288 with alanine or serine abrogated the ubiquitin E3 ligase activity of the KEAP1-CUL3 complex, leading to the accumulation of NRF2 (218). Notably, substitution of Cys273 with methionine or tryptophane retained the KEAP1 activity that represses NRF2. Similarly, substitution of Cys288 with glutamate, asparagine, or arginine retained KEAP1 activity (191). The KEAP1 C288E mutant molecule did not respond to 15d-PGJ2 (15-deoxy-Δ12,14-prostaglandin J2), indicating that KEAP1 uses Cys288 for sensing 15d-PGJ2 (191, 227) (FIGURE 8). For sensing nitro-fatty acids, 4-hydroxynonenal, and As3+, all three major cysteine residues of KEAP1, i.e., Cys151, Cys273, and Cys288, are involved (191). All of the functionally substitutable amino acids for Cys273 and Cys288 required to maintain KEAP1 activity possess relatively bulky characteristics. This suggests that the mechanism by which KEAP1 responds to electrophiles through Cys273 and Cys288 differs from that of the Cys151-dependent response. The molecular basis for the Cys273- and Cys288-dependent response remains to be clarified.
In addition to Cys151, Cys273, and Cys288, a few other cysteine residues are suggested to participate in the sensor functions of KEAP1. Cys226 and Cys613, together with His-225, are required for sensing Cd2+, As3+, Se4+, and Zn2+ (137). Cys226 and Cys613 formed intramolecular disulfide bonds when cells were treated with hydrogen peroxide, implying that Cys226 and Cys613 are located in close proximity in the fully folded state of KEAP1 (53). However, the cysteine residues responsible for sensing hydrogen peroxide also remain to be fully clarified.
Partial dissociation of NRF2 from KEAP1 appears to be an alternative mechanism for the inhibition of NRF2 ubiquitination. As described above, biochemical and biophysical analyses revealed that one molecule of NRF2 associates with a KEAP1 homodimer using two discrete motifs. The ETGE and DLG motifs utilize high-affinity and low-affinity binding sites, respectively, with almost two magnitudes of difference in their binding affinities (233). The KEAP1-NRF2 association is not completely disrupted following inactivation of KEAP1 by modification with electrophiles (109). Therefore, a fascinating model is that the ETGE and DLG motifs serve as a hinge and latch, respectively, with NRF2 ubiquitination ceasing when just the latch is released or relaxed. Precisely how the latch is released is not clear and may require a complete crystallographic rendering of the complex for an answer.
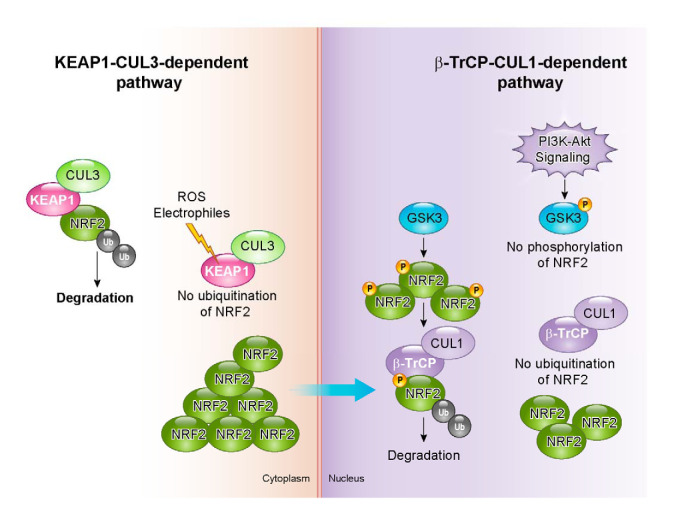
B. KEAP1-Independent Mechanism of NRF2 Degradation
Although NRF2 activity is primarily regulated by KEAP1 in response to electrophiles, several investigators realized that NRF2 signaling can be activated through the PI3K-AKT signaling pathway (85, 124, 132). A key mediator in this action is GSK-3β, which is inhibited by AKT-mediated phosphorylation (192). NRF2 is phosphorylated by GSK-3β, enabling its recognition by β-transducin repeats-containing protein (β-TrCP) that in turn marks NRF2 for ubiquitination. Following its ubiquitination by the β-TrCP-CUL1 E3 ubiquitin ligase complex, NRF2 is degraded through the proteasome (174, 175). As already described, Neh2 serves as the degron for KEAP1-CUL3-dependent degradation of NRF2. By contrast, Neh6 is the degron exploited in β-TrCP-CUL1-dependent degradation of NRF2, as it contains serine residue targets for phosphorylation by GSK-3β (30) (FIGURE 9).
FIGURE 9.
Two degradation pathways of NRF2. KEAP1-CUL3-mediated degradation and β-TrCP-CUL1-mediated degradation are considered to operate in the cytoplasm and nucleus, respectively. The latter is influenced by the activation of PI3K-AKT signaling.
The functional interaction and contributions of the two degradation mechanisms of NRF2 have been examined in the context of postnatal liver development in mice (140, 224). When murine Keap1 and Pten (phosphatase and tensin homolog deleted on chromosome 10) genes are concomitantly disrupted in a hepatocyte-specific manner, the mice die within 3 wk after birth. PTEN is a phosphatase that antagonizes PI3K. In the event of Pten deficiency, inositol-3-phosphate levels increase and AKT is activated, while GSK-3β is inhibited. Inhibition of GSK-3β reduces NRF2 phosphorylation, such that NRF2 escapes KEAP1-independent β-TrCP-CUL1-dependent degradation in the nucleus. Thus a Keap1::Pten double-deficient condition is a state in which both pathways for NRF2 degradation are inactivated. Indeed, Keap1::Pten double-deficient mouse liver showed greater increases in NRF2 accumulation and upregulation of NRF2 target genes than seen in only Keap1-deficient mouse liver. Notably, during the first 3 wk after birth, Pten single-deficient liver did not show any clear increase in NRF2 accumulation, suggesting that KEAP1-dependent degradation of NRF2 in the cytoplasm is the primary mechanism to regulate levels of NRF2 (140, 224). Inactivation of KEAP1-independent degradation while preserving the KEAP1-dependent pathway led to only a marginal difference in NRF2 protein levels in this experimental setting.
C. KEAP1-NRF2 System and Autophagy
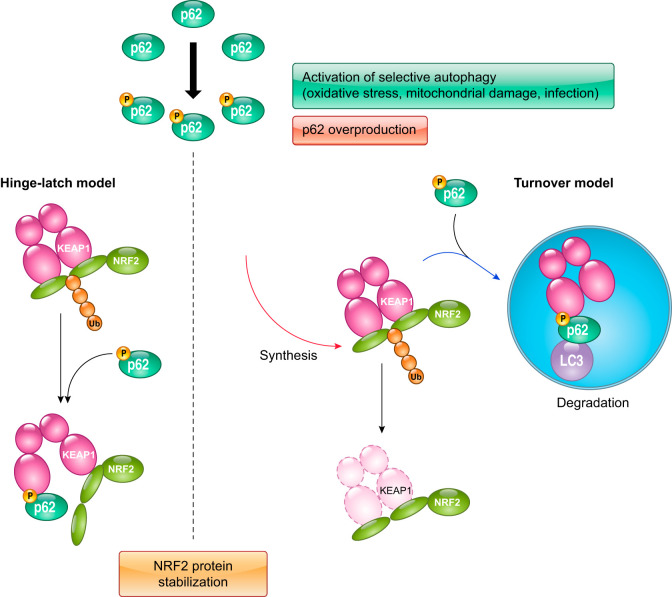
In addition to cross talk with the PI3K-AKT signaling pathway, the KEAP1-NRF2 system has an intriguing functional interaction with autophagy (52, 82, 114, 120, 187). KEAP1 directly interacts with p62, which is a cargo receptor for selective autophagy. This interaction enables p62 to compete with NRF2 for KEAP1 binding and activation of the NRF2 pathway (114, 120). Of note, p62 binds to KEAP1 at the bottom of the DC domain and dislocates NRF2 from KEAP1, leading to the stabilization of NRF2. Defective autophagy in liver impairs the turnover of p62, causing severe liver injury. This is accompanied by the formation of inclusion bodies containing p62, KEAP1, and ubiquitinated proteins and leads to an elevation in the expression of NRF2 target genes (114, 187). This liver injury model can be rescued by NRF2 disruption, indicating that constitutive activation of NRF2 in the selective autophagy-defective condition is deleterious for the functional integrity of hepatocytes. Since p62 acts as an NRF2 target gene, a positive feedback loop between p62 and NRF2 operates: p62 accumulation promotes NRF2 activation, and in turn activated NRF2 further increases p62 (82).
Detailed analyses showed that a serine residue in the phylogenetically conserved “STGE” motif in p62 is phosphorylated, and that this phosphorylated STGE (pSTGE) motif has a higher affinity for the KEAP1 DC domain than unmodified STGE (67) (FIGURE 10). The kinase mTOR complex 1 is responsible for phosphorylating p62, although the involvement of other kinases has also been suggested (67). When the pSTGE motif is compared with the ETGE and DLG motifs of NRF2 in relation to affinity for the KEAP1 DC domain, pSTGE is lower than the ETGE and higher than the DLG. Thus phosphorylated p62 may modulate the interaction between NRF2 and KEAP1.
FIGURE 10.
Cross talk between autophagy and the KEAP1-NRF2 pathway. Phosphorylated p62 possessing pSTGE has a high affinity for KEAP1, resulting in impaired ubiquitination and consequent stabilization of NRF2. Increased production of phosphorylated p62 disrupts the KEAP1-NRF2 interaction and sequesters KEAP1 to the autophagosome, resulting in the stabilization of NRF2.
Several stimuli promoting autophagy-related conditions enhance the phosphorylation of p62 (67). When mitophagy is induced by valinomycin, a mitochondrial uncoupling agent, p62 is phosphorylated in concert with an increased expression of NRF2 target genes. Similarly, p62 is phosphorylated and NRF2 is activated when xenophagy is induced on invasion by microbes. In both cases, mTOR complex 1 is responsible for phosphorylating p62, and the level of KEAP1 protein is reduced as a consequence of its autophagic degradation. This decline in KEAP1 protein level may also account for NRF2 stabilization, in addition to effects on the hinge and latch mechanism described above (sect. IVA).
The regulation of levels of NRF2 protein has been carefully analyzed; however, only recent studies have begun in the case of KEAP1. KEAP1 is degraded through autophagy in a p62-dependent manner (223). The half-lives of NRF2 and KEAP1 degradation are ~20 min and 12 h, respectively, indicating that the turnover rate of KEAP1 is considerably slower than for NRF2 (80, 223). However, treatment of cells with electrophiles such as tert-butylhydroquinone shortened the half-life of KEAP1, implying that electrophile-modified KEAP1 becomes a preferential substrate for autophagy with an accelerated turnover rate (223). As a consequence of KEAP1 degradation, NRF2 is stabilized and activates Keap1 gene transcription, thereby enabling the replenishment of de novo synthesized KEAP1. The precise destiny of KEAP1 following modification by electrophiles, whether it is degraded or recycled, is currently not well elucidated. Therefore, the mechanism by which KEAP1 turnover is accelerated by electrophiles remains a potentially fascinating, but untold story.
D. Intranuclear Players for Regulating NRF2 Activity
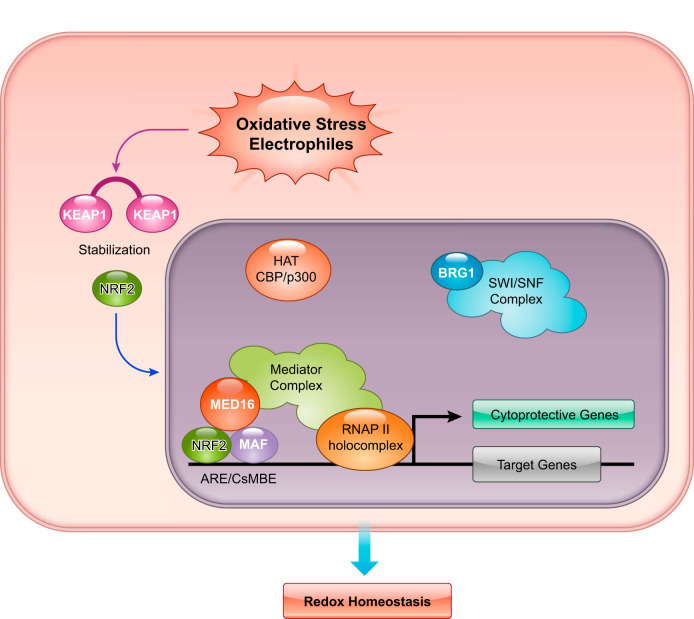
NRF2 was validated as a potent activator of transcription compared with other members of the CNC family in a reporter cotransfection-transactivation assay using cultured cells (107, 149). The transactivation domains Neh4 and Neh5 of NRF2 interact with CBP and BRG1 (93, 267). CBP is a histone acetyltransferase, and BRG1 is a component of the SWI/SNF chromatin remodeling complex. The COOH-terminal domain Neh3 of NRF2 interacts with CHD6, which is a DNA-dependent ATPase and localizes at nuclear sites of mRNA synthesis. CHD6 knockdown reduces the basal and induced expression of NRF2 target genes (157). Whereas these coactivators are important for transactivation, they may not explain fully the strong transactivation mediated by NRF2.
MED16, a subunit of Mediator complex, is a newly discovered member of coactivators that interact with the Neh4 and Neh5 transactivation domains (197). MED16 tethers Mediator complex to NRF2 binding sites through its interaction with NRF2 (FIGURE 11). MED16 serves as a conduit for Mediator complex in a manner that is specific for NRF2-activating signals that include ROS and electrophiles. In the absence of MED16, the inducible expression of nearly three-fourths of NRF2 target genes is diminished (197). Consistent with this result, MED16-deficient cells are as susceptible to oxidative stress as are NRF2-deficient cells.
FIGURE 11.
Intranuclear players cooperating with NRF2 in its potent transcriptional activity.
Comprehensive studies have defined the direct target genes of NRF2 (29, 62, 131), with much attention given to those with an expression that is elevated by NRF2. However, early gene expression studies using microarray analyses also indicated that many genes are repressed by NRF2 (119). It should be noted that several proinflammatory cytokine genes are repressed by NRF2 (111). In particular, the NRF2-mediated suppression of IL6 and IL1b gene expression appears to be critical for the anti-inflammatory effect of NRF2. This observation reinforces the need to investigate this aspect of NRF2 function and to identify the additional factors involved. It is also necessary to determine whether or not NRF2 directly represses transcription in a locus-specific manner, and, if so, how NRF2 inhibits the recruitment of RNA polymerase II to the genes.
V. CONTRIBUTION OF NRF2 TO REDOX HOMEOSTASIS AND CYTOPROTECTION
A. Mouse Models for Analyzing the Contribution of the KEAP1-NRF2 Pathway to Various Diseases
The contribution of the KEAP1-NRF2 pathway to organismal homeostasis has been demonstrated in many studies using Nrf2-deficient and Keap1-deficient mice, either singly or combined with other transgenic and gene knockout mice. Studies representative of the pathological conditions that are alleviated by genetic or pharmacological means through NRF2 activation are shown in Table 1.
Table 1.
Pathological conditions that are alleviated by NRF2 activation or exacerbated by NRF2 disruption
| Modifying Strategy of NRF2 Activity |
||||
|---|---|---|---|---|
| Disease | Species | Pharmacological | Genetic | Reference Nos. |
| Chronic kidney disease | Human | Bardoxolone methyl (CDDO-Me) | 33, 171 | |
| Acute kidney disease | Mouse | Bardoxolone methyl (CDDO-Me) | 251 | |
| Diabetic nephropathy | Mouse | Nrf2–/– mice | 83 | |
| Hyperoxic acute lung injury | Mouse | CDDO-Im | Nrf2–/– mice | 26, 181 |
| Pleurisy | Mouse | 15D-PGJ2 | Nrf2–/– mice | 81 |
| Emphysema | Mouse | CDDO-Im | Nrf2–/– mice | 216 |
| Allergic asthma | Mouse | Keap1F/F:CAG-CreERT2 mice + tamoxifen | 217 | |
| Lung carcinogenesis | Mouse | CDDO-Im, CDDO-Me | 231 | |
| Pulmonary hypertension | Mouse | Oltipraz | Nrf2–/– mice, Keap1F/F (knockdown; KD) mice | 46 |
| Cardiac ischemia-reperfusion | Mouse | 15D-PGJ2 | Nrf2–/– mice | 95 |
| Cardiac hypertrophy | Mouse | Dihydro-CDDO-TEA | 252 | |
| Multiple sclerosis | Mouse | Fumaric acid esters | Nrf2–/– mice | 127 |
| Multiple sclerosis | Human | BG2 (dimethyl fumarate) | 54, 59 | |
| Alzheimer’s disease | Mouse | Lentiviral expression of NRF2 | 91 | |
| Parkinson’s disease | Mouse | Triterpenoids | 86 | |
| Huntington’s disease | Mouse | Triterpenoids | 214 | |
| Amyotrophic lateral sclerosis | Mouse | Triterpenoids | 155 | |
| Cerebral ischemia-reperfusion | Mouse | Neurite outgrowth-promoting prostaglandin (NEPPs) | 196 | |
| Type 2 diabetes | Mouse | CDDO-Im | Nrf2–/– mice, Keap1F/– (KD) mice | 237 |
| Type 1 diabetes | Mouse | Sulforaphane | 212 | |
| Obesity | Mouse | CDDO-Im | Nrf2–/– mice | 203 |
| Acetaminophen hepatotoxicity | Mouse | CDDO-Im | Nrf2–/– mice | 50, 183 |
| Gastric carcinogenesis | Mouse | Oltipraz, sulforaphane | Nrf2–/– mice | 51, 178 |
| Aflatoxin-induced liver carcinogenesis | Rat | CDDO-Im | Nrf2–/– mice | 84, 225, 263 |
| Helicobacter pylori colonization | Mouse | Sulforaphane | Nrf2–/– mice | 262 |
| UV-induced dermatitis | Mouse | TBE-31 | Nrf2–/– mice, Keap1F/F (KD) mice | 106 |
| X-ray irradiation-induced dermatitis | Mouse | Synthetic triterpenoid RTA 408 | 184 | |
| Diabetic retinopathy | Mouse | Nrf2–/– mice | 253 | |
| Fuchs endothelial corneal dystrophy (FECD) | Human tissue culture | Sulforaphane | 269 | |
| Noise-induced hearing loss | Human, mouse | CDDO-Im | Nrf2–/– mice | 64 |
| Rheumatoid arthritis | Mouse | Nrf2–/– mice | 130 | |
| Sepsis | Mouse | CDDO-Im | Nrf2–/– mice | 230 |
| Renal ischemia-reperfusion | Mouse | CDDO-Im | Nrf2–/– mice, Keap1F/F (KD) mice | 156 |
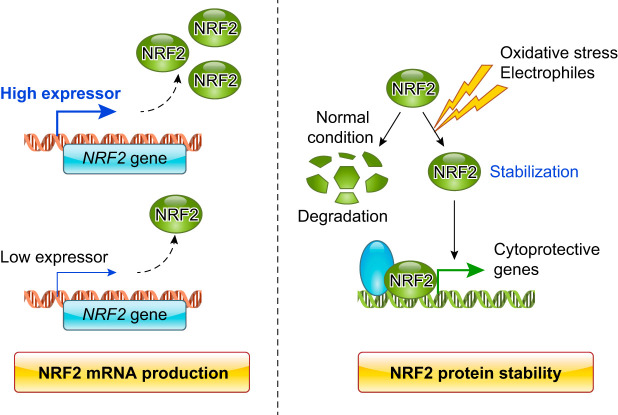
The first report on Nrf2-deficient mice in 1996 described no apparent abnormalities in the birth, growth, or fertility of the Nrf2-null mice (22). In 1997, a study using an independently generated line of Nrf2-null mice made the seminal discovery that NRF2 acts as a key regulator in the inducible expression of cytoprotective genes in vivo (78). Subsequently, these mice have been distributed to laboratories worldwide for further research. The 1997 pioneering discovery has served as the foundation for revealing the critical roles of NRF2 in stress defense mechanisms and remains the most highly cited paper in the field. The initial studies also instigated progress in the theory that a wide range of human diseases may be caused by dysregulation or impaired NRF2 function during times of oxidative stress. The model has been refined as conditional Nrf2 knockout mice were generated. This feature enabled the examination of loss-of-function effects of NRF2 in a cell lineage-specific manner (182, 254).
Subsequently, Keap1-null mice were generated and reported in 2003 (240). Contrary to an initial expectation that Keap1 disruption would render these mice as exceedingly robust due to constitutive stabilization of NRF2, Keap1-null mice died before weaning due to impaired feeding. The epithelial layers of the esophagus and forestomach of Keap1-null pups were dramatically thickened, resulting in stenosis of these tissues. Importantly, the abnormal thickening of the epithelial layers and lethality at the weaning stage were completely abrogated by simultaneous deletion of the Nrf2 gene. This indicates that constitutive stabilization of NRF2 was responsible for the phenotypes.
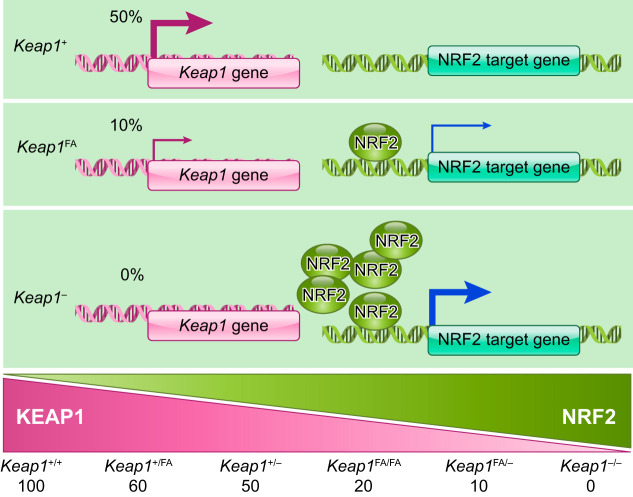
Conditional Keap1 knockout mice with a hepatocyte-specific Keap1 disruption were generated in 2006 and demonstrated that the resultant increase in NRF2 signaling conferred resistance to acetaminophen hepatotoxicity (162). These mice turned out to be hypomorphic as the floxed allele led to a lower level of Keap1 gene expression than seen with the wild-type allele (222). This knockdown allele Keap1FA (238), in combination with the knockout allele of Keap1, enabled the generation of a series of mice with graded expression levels of the Keap1 gene (222). Since a single Keap1FA allele appears to express one-fifth of the Keap1 mRNA level of a single wild-type allele (10% and 50%, respectively), the bi-allelic Keap1 expression levels are ~0, 10, 20, 50, and 60% of wild-type levels in Keap1–/–, Keap1FA/–, Keap1FA/FA, Keap1+/–, and Keap1FA/+ mice, respectively (FIGURE 12). Another conditional Keap1 knockout mouse line was established in 2010 (14) with a floxed allele termed Keap1FB that does not exhibit knockdown phenotypes (238). The Keap1FB allele is useful for analysis of cell lineage-specific contributions of the Keap1 gene, whereas Keap1FA is useful for changing Keap1 levels in the whole body. In this regard, the Keap1FA knockdown mouse serves as a useful tool for modeling the pharmacological actions of pathway inducers in vivo.
FIGURE 12.
Graded expression of KEAP1 can be generated in mice by appropriate combinations of the Keap1-null allele and the Keap1-knockdown allele.
B. Detoxification and Antioxidant Activities of NRF2 Alleviate Various Pathological Conditions
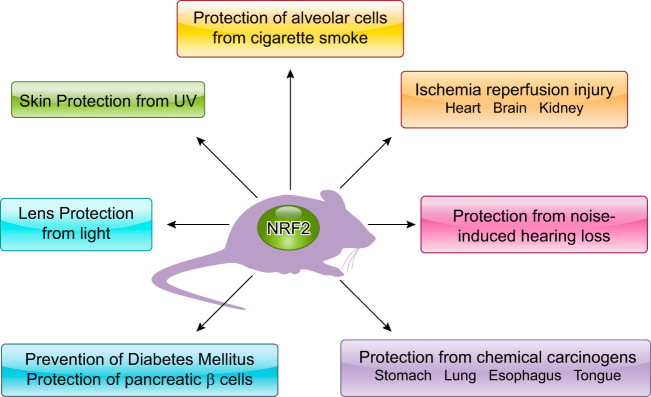
NRF2 was originally identified as a key regulator of phase II detoxication enzymes in response to electrophiles, so not surprisingly the first phenotypes found in Nrf2-null mice reflected enhanced susceptibility to xenobiotics (FIGURE 13). Nrf2-null mice easily succumbed to acute respiratory distress syndrome after exposure to butylated hydroxytoluene (23) and suffered acute liver toxicity following acetaminophen administration (50). Pharmacological activation of NRF2 signaling in wild-type mice effectively attenuated acetaminophen hepatotoxicity (183) and cigarette smoke-induced emphysema (216). NRF2 plays a central role in cancer prevention, as indicated by an exacerbated susceptibility to chemical carcinogenesis and lost efficacy in chemoprevention in carcinogen-challenged Nrf2-null mice (178, 263).
FIGURE 13.
Beneficial effects of NRF2. Various pathological conditions are alleviated by NRF2 activation and often exacerbated in NRF2-null mice.
Since NRF2 was also shown to activate oxidative stress inducible genes (74), Nrf2-null mice were used to analyze various pathological conditions related to oxidative stress. Typical instances of oxidative tissue damage are ischemia-reperfusion injuries of the brain, heart, and kidney. Exogenous and endogenous NRF2 inducers alleviate tissue damage following ischemia-reperfusion (95, 128, 156, 196, 198, 243). Noise-induced hearing loss is also considered an ischemia-reperfusion injury and similarly is effectively prevented by pretreatment with NRF2 inducers (64). Hyperoxic conditions give rise to oxidative tissue damage, particularly in the lung, and can be effectively prevented by NRF2 activation (26, 181). Ionizing radiation, including UV and X-rays, is another important cause of oxidative stress. NRF2 inducers protect skin from irradiation-induced dermatitis (106, 184).
Metabolic disorders, such as obesity and diabetes and its complications, are closely related to dysregulation of oxidative stress and are effectively treated with NRF2 inducers (83, 203, 212, 237, 253). NRF2 activation in multiple organs appears to orchestrate antidiabetic effects. For example, NRF2 induction in pancreatic β-cells suppresses oxidative damage of pancreatic islets and strongly restores insulin secretion in diabetic conditions (255). NRF2 induction in skeletal muscles alters glycogen metabolism and improves glucose tolerance (238). NRF2 induction in the hypothalamus reduces oxidative stress in astrocytes and protects leptin-secreting neurons, thereby improving control of systemic metabolism and obesity (256).
Neurodegenerative disorders, such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis, are another important category of diseases with etiopathogeneses likely linked to oxidative stress. NRF2 activation effectively alleviates the neuronal signs of these diseases in murine models (17, 86, 91, 155, 214). The administration of NRF2 inducers reduces oxidative damage in neural tissues in mouse models of Parkinson’s disease and Huntington’s disease (86, 214). However, because neuroinflammation underlies these neurodegenerative disorders, it is likely that the anti-inflammatory aspect of NRF2 contributes substantially to improving these pathological conditions.
C. Excessive Activation of NRF2 Causes Reductive Stress
G6PD is one of the major enzymes required for NADPH production, and an increase in its activity causes reductive stress. This reductive stress impairs cardiac function, as suggested by studies on inheritable human disorders linked to mutations in genes encoding αB-crystalline. The studies also suggest that constitutive activation of NRF2 in the myocardium is responsible for the reductive stress and consequent protein aggregation and cardiomyopathy (176, 177). Mutant αB-crystalline binds to KEAP1 and stabilizes NRF2. Transgenic mice expressing mutant αB-crystalline in heart exhibit ventricular dysfunction (FIGURE 14). The cardiomyopathy and protein aggregation caused by the transgene is ameliorated by an Nrf2-null background (90). The human LMNA gene that causes muscular dystrophy increases cellular levels of p62/SQSTM1, activates the KEAP1-NRF2 pathway, and leads to reductive stress, as described in a recent report on myopathic lamin mutations (40). This implies that aberrant activation of NRF2 is also deleterious by causing proteotoxicity.
FIGURE 14.
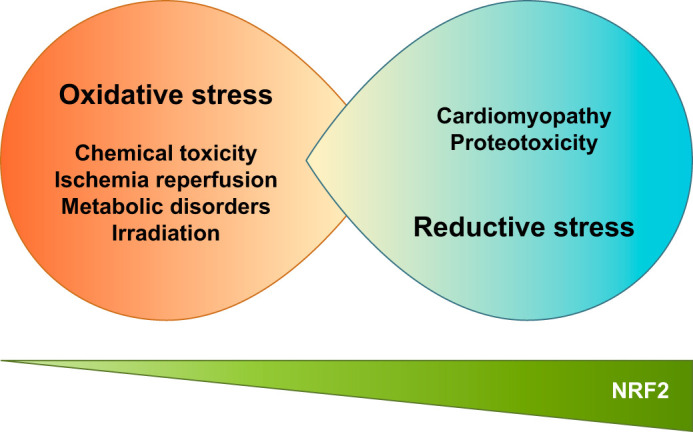
Impaired NRF2 function causes oxidative stress, whereas excessive NRF2 function generates reductive stress.
In contrast, transgenic mice with cardiomyocytic overexpression of NRF2 were resistant to myocardial oxidative stress, as well as cardiac apoptosis, fibrosis, hypertrophy, and dysfunction, under the sustained pressure overload induced by transverse aortic arch constriction (246). The study analyzed transgenic mice with a modest NRF2 expression, so their NRF2 accumulation levels might have been less than in mice with genetic mutations in αB-crystalline and LMNA. Alternatively, simple overexpression of NRF2 was not sufficient to cause myopathy, but NRF2 was required for the development of myopathy under specific conditions.
VI. NRF2 CONTRIBUTION TO ANTI-INFLAMMATORY EFFECTS
A. NRF2 Promotes the Resolution of Acute Inflammation
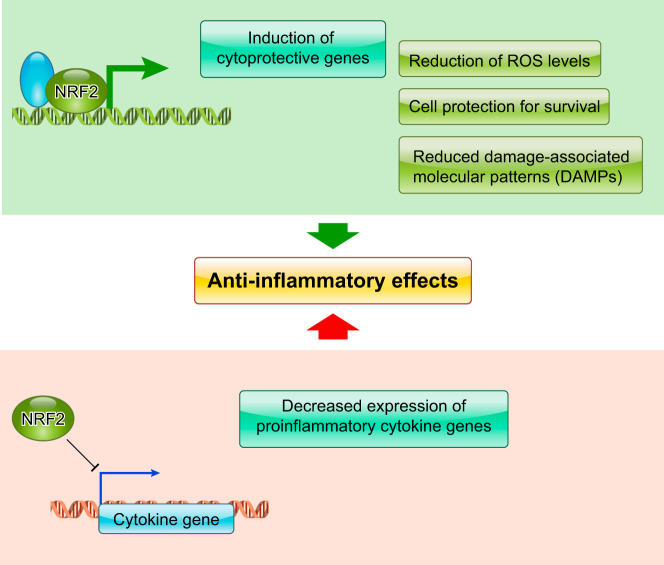
Besides increasing cellular antioxidant capacities, NRF2 evokes strong anti-inflammatory activity. Since NRF2 confers resistance to oxidative and electrophilic insults on cells, damage-associated molecular patterns are suppressed by NRF2 in affected tissues. This action appears to be one of the critical mechanisms behind the anti-inflammatory activity of NRF2 (FIGURE 15). In Nrf2-null mice, the following disease outcomes were exacerbated and protracted: pleurisy induced by carrageenan; emphysema induced by elastase and cigarette smoke; and pulmonary fibrosis induced by bleomycin (28, 75, 81, 180). Nrf2-null mice are also quite susceptible to lipopolysaccharide-induced acute inflammation, whereas NRF2 activation effectively protects against mortality after lipopolysaccharide challenge (230). When Nrf2 or Keap1 genes were disrupted specifically in mouse myeloid cells, the Nrf2 mutants were susceptible to microbial infection and subsequent sepsis, whereas the Keap1 mutants were resistant (115). These results indicate that NRF2 activation protects tissues by modulating innate immunity. The endogenous NRF2 inducer 15d-PGJ2 plays an important role in the anti-inflammatory function of NRF2 by binding to KEAP1 and activating NRF2 (141). In a model of concanavalin A-induced T-cell-mediated acute immune hepatitis, NRF2 disruption enhanced susceptibility, whereas KEAP1 disruption resulted in resistance. This is a similar pattern to that observed above in myeloid cells. Similarly, almost complete protection was achieved by pretreatment with the NRF2 inducer CDDO-Im in wild-type, but not in Nrf2-null mice (167).
FIGURE 15.
Two anti-inflammatory effects mediated by NRF2. NRF2 induces antioxidant response genes. In contrast, transcriptional expression of pro-inflammatory cytokine genes is efficiently inhibited by NRF2.
B. NRF2 Ameliorates Chronic Inflammation
Tissue damage and inflammation are closely related to each other. When the root cause of tissue damage is persistent, inflammatory reactions can become chronic. An example is sickle cell disease, which is caused by a point mutation in the β-globin gene. Abnormally shaped red blood cells are prone to hemolysis and easily release heme into plasma, which provokes oxidative stress and inflammation. Systemic activation of NRF2 promotes degradation of the released heme and suppresses inflammation, which leads to the dramatic alleviation of organ damage in a sickle cell disease mouse model (98). In a mouse model of asthma, NRF2 protects airway epithelia and effectively reduces allergic inflammation and airway hyperresponsiveness (217).
The NRF2 inducer dimethyl fumarate exerts protective effects against neuroinflammation in a mouse model of chronic multiple sclerosis (127). Importantly, dimethyl fumarate became a Food and Drug Administration-approved drug after successful clinical trials for relapsing multiple sclerosis, which is an autoimmune-based inflammation of neural tissues (54, 59). Whether or not NRF2 inducers modulate adaptive immunity is unclear in these cases, although interesting studies have been published. T-cell-specific activation of NRF2 elevates the frequency of regulatory T cells (Tregs), which seems to be critical for protecting kidney after ischemia-reperfusion (160). Another report describes that systemic activation of NRF2 alleviates a lethal autoimmune condition due to Treg deficiency, suggesting that NRF2 suppresses effector T-cell activities independent of Tregs (220). The implication is that NRF2 influences the activity of adaptive immunity.
C. Molecular Basis of NRF2-Mediated Anti-Inflammatory Effects
Although NRF2-mediated tissue protection likely involves reducing damage-associated molecular patterns, which drive secondary inflammatory reactions, NRF2 directly antagonizes the induction of proinflammatory genes, such as IL6 and IL1b, to exert some of its anti-inflammatory functions (111). When the cell-autonomous effects of NRF2 activation were examined in bone marrow-derived macrophages, NRF2 inhibited the expression of proinflammatory cytokines induced by polarization toward M1 type macrophages. NRF2 appears to interfere with the transcriptional activation machinery of proinflammatory genes in response to induction signals (FIGURE 15). While the precise mechanisms as to how NRF2 inhibits the transcriptional activation of proinflammatory cytokine genes remain to be elucidated, this study clearly demonstrates that elements of the anti-inflammatory function of NRF2 can be distinguished from its classical antioxidant function.
VII. THE KEAP1-NRF2 SYSTEM IN CARCINOGENESIS
A. Cancer Chemoprevention Through Activation of NRF2
Many animal models and clinical trials have targeted the NRF2 pathway for preventing chemical carcinogenesis since the late 1990s, which was before NRF2 was identified as a master regulator of cellular defense mechanisms. In the early 1970s, phenolic antioxidants, such as butylated hydroxyanisole and butylated hydroxytoluene, were found to effectively suppress carcinogenesis in rodents (249). Many studies have shown that induction of cytoprotective enzymes is a critical and sufficient mechanism for protection against carcinogenesis provoked by exogenous and endogenous factors. Now a plethora of natural and synthetic compounds are known to activate NRF2 by inactivating KEAP1. Clinically relevant classes of compounds include dithiolethiones (e.g., oltipraz; Ref. 48), isothiocyanates (e.g., sulforaphane; Ref. 43), and triterpenoids (e.g., CDDO-Im; Refs. 126 and 264). Oltipraz, sulforaphane, and CDDO-Im demonstrated a large dynamic range in potency, with a 1,000-fold difference in concentrations required for target gene induction in cell culture (100). This difference in potency may reflect in part their chemical mechanism of interaction with sensor cysteines, as all of these chemicals bind Cys151 of KEAP1 (44, 113). Among these chemicals, oltipraz and sulforaphane have been actively tested in clinical trials as chemoprevention agents. Triterpenoids are in the process of clinical development as therapeutic agents, but are also very promising agents for disease prevention, in part due to their remarkable potency.
NRF2 plays an important role in the detoxication of benzo[a]pyrene, a procarcinogen formed by the incomplete combustion of carbon. The preventive effects of NRF2 activation on benzo[a]pyrene-induced carcinogenesis have been demonstrated in mice treated with multiple NRF2 inducers, such as oltipraz (178, 179) and sulforaphane (51). NRF2 activation increases the efficiency of conjugation and excretion of benzo[a]pyrene, resulting in a reduced toxicological impact. Pretreatment with NRF2 inducers reduces tumor incidence in the forestomach. This protection is lost in the Nrf2-null background, indicating that the pharmacological activation of NRF2 is responsible. Moreover, wild-type mice treated with vehicle developed fewer forestomach tumors than Nrf2-null mice, highlighting their intrinsic sensitivity to carcinogenesis. NRF2 also modulates the pulmonary response to various toxicological insults, including exposures to diesel exhaust and cigarette smoke (69, 216). Nrf2-null mice are predisposed to oxidative DNA damage on exposure to diesel exhaust (2). NRF2 protects epithelial cells of the respiratory tract from the cytotoxicity and genotoxicity of cigarette smoke. Nrf2-null mice are significantly more susceptible to cigarette smoke-induced emphysema and pulmonary damage compared with wild-type mice (69, 180).
The mycotoxin aflatoxin B1, produced by the fungus Aspergillus flavus, is a potent hepatic carcinogen in humans, rats, and infant mice (101). This carcinogen is often found as a contaminant in staple foods, especially corn and peanuts. Food contamination by aflatoxin is very difficult to eradicate, so methods for minimizing the detrimental health effects of aflatoxin is a critical issue in aflatoxin endemic areas of South Asia, sub-Saharan Africa, and Central America. Once ingested, aflatoxin is metabolically activated to aflatoxin-8–9-exo epoxide by cytochrome P-450. This reactive intermediate rapidly forms an N7-guanine DNA adduct that leads to mutations within the cell. CDDO-Im, a potent NRF2-inducing triterpenoid, confers complete lifetime protection against aflatoxin-induced hepatocellular carcinogenesis in rats (84, 263). The protective actions of CDDO-Im against aflatoxin DNA adduct formation and acute toxicity are markedly attenuated in Nrf2 knockout rat, which validates NRF2 as a key player in this protective action (225). Additional studies using carcinogens targeting other tissues further demonstrate the important role of NRF2 in cancer chemoprevention, as reviewed in Slocum and Kensler (211).
B. Somatic Mutations in the KEAP1-NRF2 System in Human Cancers
NRF2 activation is normally beneficial for the health and survival of organisms, including worms, flies, and mammals. However, cancer cells can acquire a similar advantage for their survival through mechanisms that lead to constitutive activation of NRF2 signaling. The first reports describing somatic mutations in KEAP1 and NRF2 genes in human non-small cell lung cancers were published in 2006 and 2008, respectively (170, 201, 206). Since then, somatic mutations involved in the KEAP1-NRF2 system have been described in other cancers, such as breast (158), gallbladder (200), esophagus, and skin cancers (103). Recent cancer genome analyses exploiting deep sequencing revealed that KEAP1 and NRF2 are frequently mutated in solid tumors arising from tissues susceptible to environmental exposures, such as head and neck, lung, liver, bladder, and the upper digestive tract (18, 70). Missense mutations in CUL3 were found in sporadic papillary renal cell carcinoma and head and neck carcinoma, which also leads to activated NRF2 signaling (134, 166).
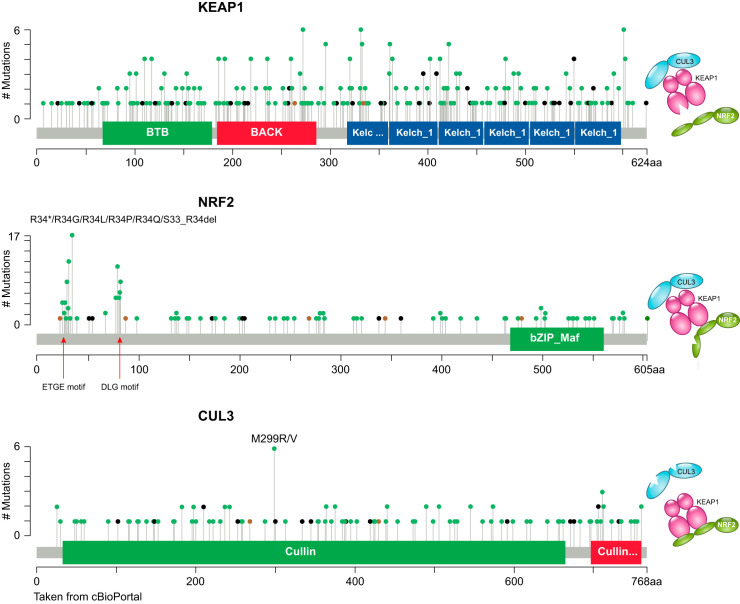
Mutations in the KEAP1, NRF2, and CUL3 genes appear to be mutually exclusive (18, 122), suggesting that the consequences of these mutations converge on the same pathway. Indeed, according to the COSMIC (Catalogue of Somatic Mutations in Cancer) database (FIGURE 16) (7), somatic nonsynonymous mutations in KEAP1 and CUL3 were found throughout the coding region. These mutations are expected to abrogate the activity of the KEAP1-CUL3 complex, thus ameliorating the degradation of NRF2. In contrast, mutations in NRF2 are almost exclusively found in the ETGE and DLG motifs in the Neh2 domain, which are essential for the binding of NRF2 to KEAP1. The pattern of NRF2 mutations in human cancers serves as solid evidence for the importance of the ETGE and DLG motifs in regulating NRF2 stability through the two-site binding interaction for NRF2 with KEAP1, as described in the hinge-latch model for NRF2 activation introduced earlier in this review.
FIGURE 16.
Somatic mutations found in KEAP1, NRF2, and CUL3 genes in cancer. KEAP1 and CUL3 mutations are distributed in a wide range over the coding region, whereas NRF2 mutations are mostly clustered in the ETGE and DLG motifs in Neh2.
These mutations disrupt the interaction between NRF2 and KEAP1 without affecting the transcriptional activation function of NRF2, resulting in constitutive stabilization of NRF2 and persistent activation of its target genes. Therefore, mutations in either the KEAP1, NRF2, or CUL3 gene result in the constitutive activation of NRF2 signaling in cancers. Cancer cells with high levels of NRF2 activity undergo metabolic reprogramming that drives aggressive proliferation (140) as well as chemo- and radio-resistance to anticancer therapy (226), thereby becoming extremely malignant and more difficult to manage clinically. These cells are referred to as NRF2-addicted cancer cells (105, 226).
C. Pathways Leading to KEAP1-CUL3 Complex Dysfunction and NRF2 Induction in Cancers
Clinical studies of tumor tissues using histology revealed high levels of NRF2 accumulation in certain subsets of cancers (i.e., NRF2-addicted cancers) and found that NRF2 was persistently activated, as reflected in elevated target gene expression. These studies have been conducted in multiple research institutes around the world and consistently demonstrated that abundant and persistent activation of NRF2 was associated significantly with poor prognoses in various cancers, including lung, gallbladder, esophagus, breast, head and neck, and renal cancers (19, 72, 87, 134, 164, 201, 202, 244). This association was expected as NRF2 coordinately activates prosurvival genes through its detoxication and antioxidant functions, thereby conferring resistance to chemotherapy and radiotherapy in cancers.
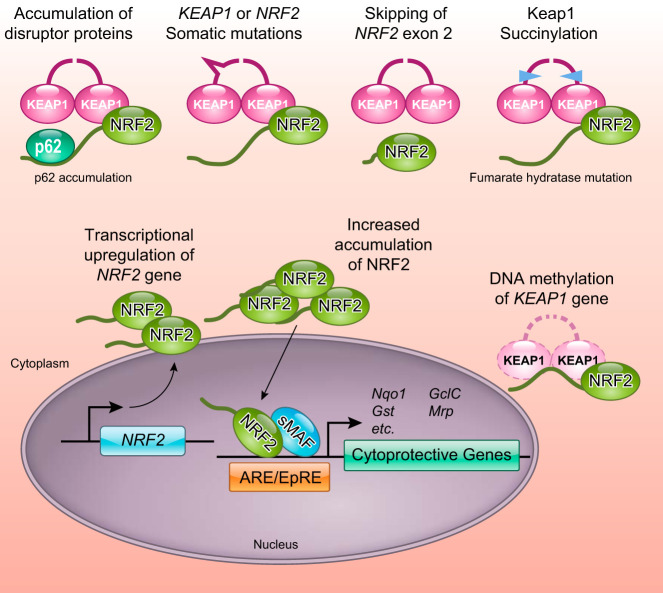
Besides the somatic mutations in the KEAP1 and NRF2 genes, several other mechanisms have been described for the constitutive stabilization of NRF2 (FIGURE 17). First, DNA hypermethylation at the promoter region of KEAP1 in cancer cells decreases KEAP1 expression, leading to NRF2 stabilization. Such epigenetic abnormalities in the KEAP1 gene in lung cancers and malignant gliomas are also associated with poor clinical outcomes (151, 152). In papillary thyroid carcinoma patients, promoter methylation of not only KEAP1 but also CUL3 has been observed, which increased the NRF2 signature of gene expression (133). Second, aberrant accumulation of p62/SQSTM1, which disrupts the KEAP1-NRF2 interaction, causes persistent activation of NRF2. The abnormal accumulation of p62/SQSTM1 is often observed in hepatocellular carcinoma (71, 129, 215), suggesting that increased NRF2 activity contributes to the malignant progression of this cancer. Third, loss of function of fumarate hydratase (FH) in cancers causes fumarate (an intermediate of the TCA cycle) to pool and leads to elevated accumulation of NRF2. Patients carrying heterozygous germline mutations in the FH gene exhibit elevated levels of fumarate and develop hereditary leiomyomatosis and renal cell cancer, a syndrome characterized by smooth muscle tumors and papillary renal cell carcinoma type 2 (232). Fumarate is weakly electrophilic and modifies the cysteine residues of KEAP1, thereby stabilizing NRF2 and leading to the elevated expression of NRF2 target genes (1, 165). This action offers an explanation, at least in part, for the highly malignant papillary renal cell carcinoma type 2 phenotype seen in patients.
FIGURE 17.
Multiple mechanisms lead to excessive activation of NRF2 in cancers.
Fourth, exon skipping of the NRF2 gene results in the production of NRF2 protein lacking a KEAP1-interaction domain (60). In lung cancers and head and neck cancers, which frequently express high levels of NRF2, the recurrent exclusion of exon 2 from NRF2 mRNA has been observed. This results in deletion of the stretch of amino acids D16-Q104 that span the DLG and ETGE motifs, as well as the lysine clusters targeted for ubiquitination. Fifth, transcriptional upregulation of the NRF2 gene by KRAS or BRAF-driven oncogenic pathways involving MYC and JUN leads to increased NRF2 activity (35). To summarize, the six known mechanisms that lead to increased NRF2 activation and accelerated cancer progression are as follows: somatic mutation of KEAP1 or NRF2; aberrant accumulation of p62/SQSTM1; epigenetic silencing of the KEAP1 or CUL3 gene; germline mutations in FH; exon skipping within the NRF2 gene; and oncogene-mediated transcriptional upregulation of the NRF2 gene.
D. Roles of NRF2 in Cancer Initiation, Progression, and Metastasis
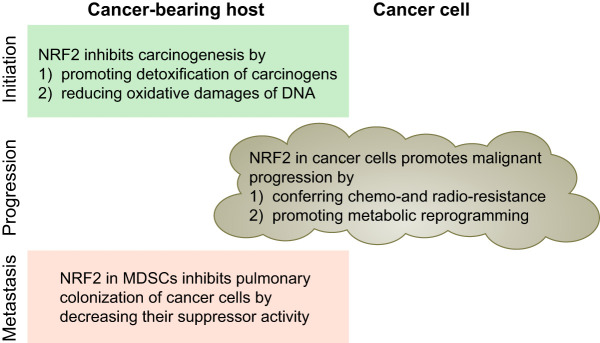
NRF2 protects cells from DNA damaging insults, such as ROS and electrophilic toxicants, thereby inhibiting the initiation of cancer. However, once a cell is transformed by acquiring one or likely several oncogenic mutations, NRF2 in the pre-neoplastic or transformed cells contributes to their resistance to stress and survival, thereby supporting the establishment of cancers (FIGURE 18). An important observation is that NRF2 activity is required for oncogenic KRAS (KRASG12D)-driven lung carcinogenesis in a mouse model (35). In the Nrf2-null background, neoplastic nodules in lung tissues were fewer in number and smaller in size, contained fewer Ki67-positive proliferating cells, and survival of the mice was extended compared with wild type. Similar outcomes were observed in a urethane-induced lung carcinogenesis murine model in which KRAS mutations are frequent (194). Nrf2-null mice are prone to developing lung nodules after being treated with urethane, but the nodules do not acquire malignant characteristics. In contrast, wild-type mice are resistant to the initial development of lung nodules, but the nodules evolve into malignant adenocarcinoma. Consistent with this outcome, Keap1 knockdown mice exhibited greater resistance to the initial development of nodules, but cells isolated from pulmonary nodules in Keap1-knockdown mice grew aggressively when engrafted subcutaneously into immuno-deficient nude mice (195). Similarly, activation of NRF2 by antioxidant antidiabetic agents accelerated tumor metastasis in a xenograft model using nude mice (245). These results indicate that NRF2 may play at least two roles during carcinogenesis: preventing cancer initiation and promoting malignant progression.
FIGURE 18.
Roles of NRF2 in carcinogenesis highlighting the importance of context. NRF2 activation is beneficial and deleterious for the cancer-bearing host, depending on the time (initiation, promotion, and metastasis) and place (cancer cells or microenvironment). MDSCs, myeloid-derived suppressor cells.
From the viewpoint of NRF2 function in the tumor microenvironment, NRF2 activation appears to be beneficial for the cancer-bearing host. When cancer cells are injected into tail veins of mice, Nrf2-null mice exhibit a higher number of lung metastatic nodules than wild-type mice (193). Nrf2 deficiency, particularly in the myeloid cell linage, elevates the activity of myeloid-derived suppressor cells, which inhibits T-cell-mediated antitumor immunity (61). Therefore, it is plausible that systemic administration of NRF2 inducers to cancer-bearing hosts would effectively reinforce antitumor immunity to suppress cancer metastasis. However, administration of NRF2 inducers would need to be very carefully controlled in the case of cancer bearing hosts with immunosuppression (245).
E. NRF2 Contribution to Metabolic Reprogramming of Cancer Cells
The KRAS-driven carcinogenesis model revealed that NRF2 contributes to the establishment and progression of cancer cells (35). This effect differs from acquisition of elevated detoxication capacity (radio- and chemoresistance), as this phenomenon can be seen even in the absence of exogenous anticancer treatments. Cancer cells with high NRF2 activity aggressively proliferate, which undoubtedly contributes to their malignant nature (207, 268). The means by which NRF2 promotes the proliferation of cancer cells does not involve the canonical antioxidant and anti-inflammatory functions of NRF2.
In this regard, detailed analyses of NRF2 target genes in cancer cells identified metabolic enzymes that are involved in the pentose phosphate pathway and glutamine metabolism (140). In particular, in proliferating cells, NRF2 redirects glucose and glutamine into anabolic pathways and supports metabolic activities that are advantageous for proliferation (140, 208). Comprehensive metabolomic profiling of lung cancer cells revealed that the serine synthesis pathway is also facilitated by NRF2 through ATF-4 activation (36). CRISPR library screening identified glutamine transporter as a key factor supporting the growth of Keap1-mutant lung cancer cells, leading to the discovery of dependence on glutaminolysis of Keap1-mutant cancers (188). These findings are particularly intriguing, as the pentose phosphate pathway provides ribose for nucleotide synthesis and NADPH for lipid synthesis, whereas glutamine is important for glutathione synthesis and anaplerosis (replenishing) of TCA cycle intermediates that have been diverted for the synthesis of amino acids and other biomolecules. In addition, serine is an important one-carbon source for nucleotide synthesis and methylation reactions.
Notably, NRF2 plays a major role in these metabolic pathways in the presence of active proliferative signals rather than in quiescent cells. PI3K-AKT signaling is a major proliferative signal that inactivates GSK-3 by phosphorylation, resulting in NRF2 accumulation through inhibition of KEAP1-independent degradation (see FIGURE 9). As described above, an increased abundance in NRF2 enables activation of metabolic genes and promotes anabolic metabolism. This functional expansion of NRF2 is not limited to cancer cells, but operates in normal cells within the transient and physiological magnitude of PI3K-AKT signal activation. Examples include reactive hypertrophy of liver following portal vein branch ligation (204) and possibly liver regeneration after partial hepatectomy (11, 241). In the latter setting, the NRF2-NOTCH axis, a reciprocal activation of NRF2 and NOTCH signaling pathways, plays an important role (241, 242). Still another intriguing recent discovery is a contribution of NRF2 to translation (25). NRF2 deficiency induces cysteine oxidation of subunits of the translational machinery, resulting in impaired mRNA translation. With these recent reports, it is clear that the regulatory layers for NRF2-mediated effects on cell proliferation have been expanded further than previously anticipated.
VIII. THERAPEUTIC APPLICATIONS OF NRF2 AND MODULATION OF ITS ACTIVITY
A. Development of NRF2 Inducers
Based on the profound contributions of NRF2 in the prevention and alleviation of a wide variety of pathological conditions in mouse models (TABLE 1), worldwide efforts have been made to isolate from natural sources or develop potent and potentially specific NRF2-inducing chemicals. Among them, dimethyl fumarate (Tecfidera) has been approved by the Food and Drug Administration and Pharmaceuticals and Medical Devices Agency and is clinically prescribed to patients with multiple sclerosis. CDDO-Me, a member of the exceedingly potent class of oleanane triterpenoid NRF2 inducers, is undergoing clinical trials in Japan for diabetic nephropathy treatment, although its development was paused in the United States due to the occurrence of cardiac complications in patients with end-stage renal disease (33, 171). Inducers with less potency and specificity are also in clinical development, in particular nitro fatty acids (37) and the broccoli-derived isothiocyanate sulforaphane (45). It is possible that modification of additional cysteine residues beyond KEAP1 may trigger complementary signaling that enhances protective effects. Several small trials in autism (210) and air pollution (49) using sulforaphane in the form of broccoli extracts have shown promising effects likely to be NRF2 dependent, at least in part. Although the cysteine residues of KEAP1 are highly reactive and efficiently modified by these electrophiles, strategies that do not require specificity of electrophilic attack still need to be considered. Therefore, NRF2 inducers with higher specificity might be found in the form of non-electrophilic chemicals that disrupt the interaction between KEAP1 and NRF2 by filling a KEAP1 pocket that forms an interface with NRF2 (186).
B. Development of NRF2 Inhibitors
Cancers with persistent activation of NRF2 exhibit high dependency on NRF2 function for drug resistance and cell proliferation. In the treatment of these cancers, NRF2 inhibitors are expected to antagonize cancer progression and sensitize cancer cells to therapy. However, compared with NRF2 inducers, the development of NRF2 inhibitors is in its infancy, so very few NRF2 inhibitors have been reported. The plant-based product brusatol has been reported to inhibit NRF2 (185). Brusatol effectively decreases the protein levels of NRF2 and sensitizes cancer cells to chemotherapy and radiotherapy. Recently, another NRF2 inhibitor, halofuginone, was found to exert a chemosensitizing effect on cancer cells exhibiting constitutive NRF2 stabilization (235). Interestingly, brusatol inhibits global protein synthesis, possibly by targeting the ribosome (239), whereas halofuginone inhibits proline tRNA synthetase and activates the amino acid response pathway (99). This suggests that the inhibition of protein synthesis is a particularly effective means for antagonizing NRF2 activity. This approach is possible because of the short half-life of NRF2. In contrast, ML385 exploits an alternative mechanism by binding to the DNA binding domain of NRF2 and shows significant chemosensitizing activity, specifically in non-small cell lung cancer cells harboring KEAP1 mutations (209). An important point to consider when developing NRF2 inhibitors is the fact that systemic administration of NRF2 inhibitors would reduce both canonical detoxication and antioxidant defense responses, thereby changing the profiles or thresholds of dose-limiting toxicities associated with standard-of-care therapies. Therefore, a drug delivery system specific to cancerous tissue and/or a well-designed medication protocol are necessary to achieve the best efficacy. Alternatively, finding a therapeutic target that is selectively expressed in NRF2-addicted cancers is a new opportunity as represented by identification of an orphan nuclear receptor NR0B1 as a unique therapeutic target for KEAP1-mutant non-small cell lung cancers (8).
C. Diagnostic Use of Regulatory Single Nucleotide Polymorphisms in the NRF2 Promoter and NRF2 Binding Sites
NRF2 activity is regulated through protein stabilization, primarily by KEAP1, but is also regulated at the transcriptional level. Thus two layers of regulation exist that control the NRF2 activity that impacts on the susceptibility of mice and humans to various pathological conditions (27, 64, 135, 219, 258) (FIGURE 19). Importantly, NRF2 autoregulates transcription of the Nrf2 gene (118).
FIGURE 19.
Transcriptional and posttranslational regulation of NRF2 activity. In addition to regulation of NRF2 protein stability, the transcription level of the NRF2 gene is an important determinant of the overall activity of NRF2.
A single nucleotide polymorphism (SNP) in the promoter region of the mouse Nrf2 gene was first described in 2002 from a linkage analysis of susceptibility to hyperoxic challenges of different strains of mice (27). The susceptible strain C57BL/6J and the resistant strain C3H/HeJ were compared in that study. A decreased expression of lung Nrf2 mRNA and a T to C substitution in the promoter region of the Nrf2 gene were found in C57BL/6J mice, which cosegregated with susceptibility phenotypes in the genomewide linkage analysis. Indeed, Nrf2-null mice were susceptible to hyperoxia (26). Thus it is concluded that the Nrf2 promoter SNP influences the transcription level of the Nrf2 gene, which affects susceptibility to hyperoxia.
In humans, similar to the mouse Nrf2 gene, a NRF2 promoter SNP located 617 bp upstream from the transcription start site lowers the level of NRF2 transcription (258) (FIGURE 20). Of note, individuals who possess this SNP are more susceptible to acute lung injury and related diseases (135). The presence of this SNP was also found to significantly correlate with the incidence of non-small cell lung cancer (219). The NRF2 SNP is also associated with susceptibility to noise-induced hearing loss, for which oxidative damage is the major underlying cause (64).
FIGURE 20.
SNPs in the NRF2 promoter and AREs. SNPs in the NRF2 promoter alter the transcription level of the NRF2 gene. SNPs in AREs alter the binding affinity of NRF2-sMAF heterodimers and transcription levels of NRF2 target genes.
In addition to the SNP in the NRF2 promoter, those in NRF2 target sites (AREs/CsMBEs) may have substantial biological impacts (FIGURE 20). Integration of genomewide maps of the NRF2 occupancy with disease-susceptibility loci revealed several associations between polymorphic AREs/CsMBEs and disease-risk SNPs (247). Bioinformatic analysis constructed an NRF2 binding prediction model that successfully identified polymorphisms in the AREs/CsMBE that affect NRF2 target gene expression (116). Thus the NRF2 SNP and those in the NRF2 binding sites are likely to be used for personalized medicine in the future as indicators for predicting disease susceptibility and drug toxicity.
IX. CONCLUSIONS: PERSPECTIVE ON THE KEAP1-NRF2 PATHWAY
Discovery of the Keap1-Nrf2 system revealed a revolutionary concept that has led to new approaches toward improving human health and combating diseases. One of the most salient questions regarding the molecular mechanism of the KEAP1-NRF2 system is how KEAP1 senses electrophilic and oxidative stimuli and transduces them into stabilized NRF2 and subsequent downstream signaling. To this end, elucidation of the overall structure of active and inactive CUL3-KEAP1-NRF2 complexes is underway. The specific positions and numbers of modified cysteine residues differ among chemical inducers, which likely leads to multiple means for structural alterations in the CUL3-KEAP1-NRF2 complex. We believe that full details on the structural features of inactivated or active KEAP1 are key to understanding the whole structure-function basis of this critical cysteine-based biosensor.
Other important questions focus on the roles that NRF2 plays in carcinogenesis. For instance, how and when in their etiology do cancers with constitutive NRF2 activation highjack and capitalize on the contributions of NRF2? Is NRF2 an actual oncogene or, as seems more likely, is it an important, facilitative susceptibility determinant? Long-term observations of Keap1 knockdown mice and conditional Keap1 knockout mice strongly suggest that simple Keap1 deficiency is not sufficient for the establishment of cancers. In other words, NRF2 is not a driver of cancer nor a passive passenger, but rather a copilot and navigator. The presence of activated oncogenes in cells provides NRF2 a role for promoting the malignant evolution of cancers. An intriguing question here is, during the multistep carcinogenesis, when does NRF2 change its role from a faithful guardian to a betrayer? The answer to this question will provide a theoretical basis as to how NRF2 inducers could be used for cancer prevention and in which at-risk cohorts and when and in what settings should NRF2 inhibitors be applied for the treatment of cancer patients or prevention of recurrence. It is also necessary to recognize that there are settings of oncogene-driven carcinogenesis where constitutive activation of NRF2 signaling is protective. Thus context is the critical determinant of the risk and benefit mediated through NRF2 that must be considered (213).
From a clinical point of view, NRF2 inducers and inhibitors both have their own advantages and disadvantages. NRF2 inducers are expected to activate anticancer immunity, but confer resistance to anticancer therapy in cancers. NRF2 inhibitors are expected to reduce proliferation and increase the sensitivity of cancer cells to anticancer drugs. However, when they act within a cancer microenvironment, NRF2 inhibitors are expected to promote cancer metastasis by activating the repressor activity of myeloid-derived suppressor cells. We believe that the appropriate use of NRF2 inducers and inhibitors will greatly reduce the incidence of cancers and improve the prognosis of cancer patients. In addition to appreciating context, it is important to understand the nature of dose-response curves of such agents, whether they are linear or “S” or “U” shaped (100). Moreover, as NRF2 touches the etiopathogenesis of other acute and chronic diseases, ongoing clinical trials (https://clinicaltrials.gov/) using multiple classes of inducers should provide insights into the efficacy and optimization of interventions.
Health is a reflection of the ability of an organism to adapt to stress. How one responds to environmental and endogenous stress is inevitably a critical issue in maintaining health. Various diseases and aging processes must be analyzed and evaluated under the influence of these stress factors. The KEAP1-NRF2 system, a thiol-based sensor-effector apparatus, plays a central role in responses for better adaptation. Thus informed harnessing of this pathway is key to the prosperity and continuation of the human species.
GRANTS
This work was supported in part by the AMED (Japan Agency for Medical Research and Development)-P-CREATE (M. Yamamoto), AMED-P-DIRECT (M. Yamamoto), and AMED-CREST (M. Yamamoto); National Cancer Institute Grant R35 CA-197222 (T. W. Kensler); Japan Society for the Promotion of Science KAKENHI Grants 24249015 and 26111002 (M. Yamamoto) and 15H04692 (H. Motohashi); the Naito Foundation (H. Motohashi); and the Princess Takamatsu Cancer Research Fund 15–24728 (H. Motohashi).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank colleagues in our laboratories and collaborators all over the world who have been actively contributing to the KEAP1-NRF2 study. We also thank Motoko Konno and Drs. Akira Uruno, Takafumi Suzuki, Miki Yamamoto, and Tania Bezak for help in editing this review article.
Address for reprint requests and other correspondence: M. Yamamoto, Dept. of Medical Biochemistry, Tohoku University Graduate School of Medicine, Sendai 980-8575, Japan (e-mail: masiyamamoto@med.tohoku.ac.jp).
REFERENCES
- 1.Adam J, Hatipoglu E, O’Flaherty L, Ternette N, Sahgal N, Lockstone H, Baban D, Nye E, Stamp GW, Wolhuter K, Stevens M, Fischer R, Carmeliet P, Maxwell PH, Pugh CW, Frizzell N, Soga T, Kessler BM, El-Bahrawy M, Ratcliffe PJ, Pollard PJ. Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer Cell 20: 524–537, 2011. doi: 10.1016/j.ccr.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoki Y, Sato H, Nishimura N, Takahashi S, Itoh K, Yamamoto M. Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol Appl Pharmacol 173: 154–160, 2001. doi: 10.1006/taap.2001.9176. [DOI] [PubMed] [Google Scholar]
- 3.An JH, Vranas K, Lucke M, Inoue H, Hisamoto N, Matsumoto K, Blackwell TK. Regulation of the Caenorhabditis elegans oxidative stress defense protein SKN-1 by glycogen synthase kinase-3. Proc Natl Acad Sci USA 102: 16275–16280, 2005. doi: 10.1073/pnas.0508105102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews NC, Erdjument-Bromage H, Davidson MB, Tempst P, Orkin SH. Erythroid transcription factor NF-E2 is a haematopoietic-specific basic-leucine zipper protein. Nature 362: 722–728, 1993. doi: 10.1038/362722a0. [DOI] [PubMed] [Google Scholar]
- 5.Andrews NC, Kotkow KJ, Ney PA, Erdjument-Bromage H, Tempst P, Orkin SH. The ubiquitous subunit of erythroid transcription factor NF-E2 is a small basic-leucine zipper protein related to the v-maf oncogene. Proc Natl Acad Sci USA 90: 11488–11492, 1993. doi: 10.1073/pnas.90.24.11488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baird L, Tsujita T, Kobayashi EH, Funayama R, Nagashima T, Nakayama K, Yamamoto M. A Homeostatic shift facilitates endoplasmic reticulum proteostasis through transcriptional integration of proteostatic stress response pathways. Mol Cell Biol 37: e00439-16, 2017. doi: 10.1128/MCB.00439-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bamford S, Dawson E, Forbes S, Clements J, Pettett R, Dogan A, Flanagan A, Teague J, Futreal PA, Stratton MR, Wooster R. The COSMIC (Catalogue of Somatic Mutations in Cancer) database and website. Br J Cancer 91: 355–358, 2004. doi: 10.1038/sj.bjc.6601894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bar-Peled L, Kemper EK, Suciu RM, Vinogradova EV, Backus KM, Horning BD, Paul TA, Ichu TA, Svensson RU, Olucha J, Chang MW, Kok BP, Zhu Z, Ihle NT, Dix MM, Jiang P, Hayward MM, Saez E, Shaw RJ, Cravatt BF. Chemical proteomics identifies druggable vulnerabilities in a genetically defined cancer. Cell 171: 696–709.e23, 2017. doi: 10.1016/j.cell.2017.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benkhelifa S, Provot S, Lecoq O, Pouponnot C, Calothy G, Felder-Schmittbuhl MP. mafA, a novel member of the maf proto-oncogene family, displays developmental regulation and mitogenic capacity in avian neuroretina cells. Oncogene 17: 247–254, 1998. doi: 10.1038/sj.onc.1201898. [DOI] [PubMed] [Google Scholar]
- 10.Benson AM, Hunkeler MJ, Talalay P. Increase of NAD(P)H:quinone reductase by dietary antioxidants: possible role in protection against carcinogenesis and toxicity. Proc Natl Acad Sci USA 77: 5216–5220, 1980. doi: 10.1073/pnas.77.9.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beyer TA, Xu W, Teupser D, auf dem Keller U, Bugnon P, Hildt E, Thiery J, Kan YW, Werner S. Impaired liver regeneration in Nrf2 knockout mice: role of ROS-mediated insulin/IGF-1 resistance. EMBO J 27: 212–223, 2008. doi: 10.1038/sj.emboj.7601950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blackwell TK, Bowerman B, Priess JR, Weintraub H. Formation of a monomeric DNA binding domain by Skn-1 bZIP and homeodomain elements. Science 266: 621–628, 1994. doi: 10.1126/science.7939715. [DOI] [PubMed] [Google Scholar]
- 13.Blackwell TK, Steinbaugh MJ, Hourihan JM, Ewald CY, Isik M. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic Biol Med 88, Pt. B: 290–301, 2015. doi: 10.1016/j.freeradbiomed.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blake DJ, Singh A, Kombairaju P, Malhotra D, Mariani TJ, Tuder RM, Gabrielson E, Biswal S. Deletion of Keap1 in the lung attenuates acute cigarette smoke-induced oxidative stress and inflammation. Am J Respir Cell Mol Biol 42: 524–536, 2010. doi: 10.1165/rcmb.2009-0054OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blank V. Small Maf proteins in mammalian gene control: mere dimerization partners or dynamic transcriptional regulators? J Mol Biol 376: 913–925, 2008. doi: 10.1016/j.jmb.2007.11.074. [DOI] [PubMed] [Google Scholar]
- 16.Brown JR, Thornton JL. Percivall Pott (1714-1788) and chimney sweepers’ cancer of the scrotum. Br J Ind Med 14: 68–70, 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burton NC, Kensler TW, Guilarte TR. In vivo modulation of the Parkinsonian phenotype by Nrf2. Neurotoxicology 27: 1094–1100, 2006. doi: 10.1016/j.neuro.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Hammerman PS, Lawrence MS, Voet D, Jing R, Cibulskis K, Sivachenko A, Stojanov P, McKenna A, Lander ES, Gabriel S, Getz G, Sougnez C, Imielinski M, Helman E, Hernandez B, Pho NH, Meyerson M, Chu A, Chun H-JE, Mungall AJ, Pleasance E, Gordon Robertson A, Sipahimalani P, Stoll D, Balasundaram M, Birol I, Butterfield YSN, Chuah E, Coope RJN, Corbett R, Dhalla N, Guin R, He A, Hirst C, Hirst M, Holt RA, Lee D, Li HI, Mayo M, Moore RA, Mungall K, Ming Nip K, Olshen A, Schein JE, Slobodan JR, Tam A, Thiessen N, Varhol R, Zeng T, Zhao Y, Jones SJM, Marra MA, Saksena G, Cherniack AD, Schumacher SE, Tabak B, Carter SL, Pho NH, Nguyen H, Onofrio RC, Crenshaw A, Ardlie K, Beroukhim R, Winckler W, Hammerman PS, Getz G, Meyerson M, Protopopov A, Zhang J, Hadjipanayis A, Lee S, Xi R, Yang L, Ren X, Zhang H, Shukla S, Chen P-C, Haseley P, Lee E, Chin L, Park PJ, Kucherlapati R, Socci ND, Liang Y, Schultz N, Borsu L, Lash AE, Viale A, Sander C, Ladanyi M, Todd Auman J, Hoadley KA, Wilkerson MD, Shi Y, Liquori C, Meng S, Li L, Turman YJ, Topal MD, Tan D, Waring S, Buda E, Walsh J, Jones CD, Mieczkowski PA, Singh D, Wu J, Gulabani A, Dolina P, Bodenheimer T, Hoyle AP, Simons JV, Soloway MG, Mose LE, Jefferys SR, Balu S, O’Connor BD, Prins JF, Liu J, Chiang DY, Neil Hayes D, Perou CM, Cope L, Danilova L, Weisenberger DJ, Maglinte DT, Pan F, Van Den Berg DJ, Triche T Jr, Herman JG, Baylin SB, Laird PW, Getz G, Noble M, Voet D, Saksena G, Gehlenborg N, DiCara D, Zhang J, Zhang H, Wu C-J, Yingchun Liu S, Lawrence MS, Zou L, Sivachenko A, Lin P, Stojanov P, Jing R, Cho J, Nazaire M-D, Robinson J, Thorvaldsdottir H, Mesirov J, Park PJ, Chin L, Schultz N, Sinha R, Ciriello G, Cerami E, Gross B, Jacobsen A, Gao J, Arman Aksoy B, Weinhold N, Ramirez R, Taylor BS, Antipin Y, Reva B, Shen R, Mo Q, Seshan V, Paik PK, Ladanyi M, Sander C, Akbani R, Zhang N, Broom BM, Casasent T, Unruh A, Wakefield C, Craig Cason R, Baggerly KA, Weinstein JN, Haussler D, Benz CC, Stuart JM, Zhu J, Szeto C, Scott GK, Yau C, Ng S, Goldstein T, Waltman P, Sokolov A, Ellrott K, Collisson EA, Zerbino D, Wilks C, Ma S, Craft B, Wilkerson MD, Todd Auman J, Hoadley KA, Du Y, Cabanski C, Walter V, Singh D, Wu J, Gulabani A, Bodenheimer T, Hoyle AP, Simons JV, Soloway MG, Mose LE, Jefferys SR, Balu S, Marron JS, Liu Y, Wang K, Liu J, Prins JF, Neil Hayes D, Perou CM, Creighton CJ, Zhang Y, Travis WD, Rekhtman N, Yi J, Aubry MC, Cheney R, Dacic S, Flieder D, Funkhouser W, Illei P, Myers J, Tsao M-S, Penny R, Mallery D, Shelton T, Hatfield M, Morris S, Yena P, Shelton C, Sherman M, Paulauskis J, Meyerson M, Baylin SB, Govindan R, Akbani R, Azodo I, Beer D, Bose R, Byers LA, Carbone D, Chang L-W, Chiang D, Chu A, Chun E, Collisson E, Cope L, Creighton CJ, Danilova L, Ding L, Getz G, Hammerman PS, Neil Hayes D, Hernandez B, Herman JG, Heymach J, Ida C, Imielinski M, Johnson B, Jurisica I, Kaufman J, Kosari F, Kucherlapati R, Kwiatkowski D, Ladanyi M, Lawrence MS, Maher CA, Mungall A, Ng S, Pao W, Peifer M, Penny R, Robertson G, Rusch V, Sander C, Schultz N, Shen R, Siegfried J, Sinha R, Sivachenko A, Sougnez C, Stoll D, Stuart J, Thomas RK, Tomaszek S, Tsao M-S, Travis WD, Vaske C, Weinstein JN, Weisenberger D, Wigle DA, Wilkerson MD, Wilks C, Yang P, John Zhang J, Jensen MA, Sfeir R, Kahn AB, Chu AL, Kothiyal P, Wang Z, Snyder EE, Pontius J, Pihl TD, Ayala B, Backus M, Walton J, Baboud J, Berton DL, Nicholls MC, Srinivasan D, Raman R, Girshik S, Kigonya PA, Alonso S, Sanbhadti RN, Barletta SP, Greene JM, Pot DA, Tsao M-S, Bandarchi-Chamkhaleh B, Boyd J, Weaver JE, Wigle DA, Azodo IA, Tomaszek SC, Christine Aubry M, Ida CM, Yang P, Kosari F, Brock MV, Rogers K, Rutledge M, Brown T, Lee B, Shin J, Trusty D, Dhir R, Siegfried JM, Potapova O, Fedosenko KV, Nemirovich-Danchenko E, Rusch V, Zakowski M, Iacocca MV, Brown J, Rabeno B, Czerwinski C, Petrelli N, Fan Z, Todaro N, Eckman J, Myers J, Kimryn Rathmell W, Thorne LB, Huang M, Boice L, Hill A, Penny R, Mallery D, Curley E, Shelton C, Yena P, Morrison C, Gaudioso C, Bartlett JMS, Kodeeswaran S, Zanke B, Sekhon H, David K, Juhl H, Van Le X, Kohl B, Thorp R, Viet Tien N, Van Bang N, Sussman H, Duc Phu B, Hajek R, Phi Hung N, Khan KZ, Muley T, Mills Shaw KR, Sheth M, Yang L, Buetow K, Davidsen T, Demchok JA, Eley G, Ferguson M, Dillon LAL, Schaefer C, Guyer MS, Ozenberger BA, Palchik JD, Peterson J, Sofia HJ, Thomson E, Hammerman PS, Neil Hayes D, Wilkerson MD, Schultz N, Bose R, Chu A, Collisson EA, Cope L, Creighton CJ, Getz G, Herman JG, Johnson BE, Kucherlapati R, Ladanyi M, Maher CA, Robertson G, Sander C, Shen R, Sinha R, Sivachenko A, Thomas RK, Travis WD, Tsao M-S, Weinstein JN, Wigle DA, Baylin SB, Govindan R, Meyerson M; Cancer Genome Atlas Research Network . Comprehensive genomic characterization of squamous cell lung cancers. Nature 489: 519–525, 2012. [Erratum. Nature 491: 288, 2012.] doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linehan WM, Spellman PT, Ricketts CJ, Creighton CJ, Fei SS, Davis C, Wheeler DA, Murray BA, Schmidt L, Vocke CD, Peto M, Al Mamun AA, Shinbrot E, Sethi A, Brooks S, Rathmell WK, Brooks AN, Hoadley KA, Robertson AG, Brooks D, Bowlby R, Sadeghi S, Shen H, Weisenberger DJ, Bootwalla M, Baylin SB, Laird PW, Cherniack AD, Saksena G, Haake S, Li J, Liang H, Lu Y, Mills GB, Akbani R, Leiserson MD, Raphael BJ, Anur P, Bottaro D, Albiges L, Barnabas N, Choueiri TK, Czerniak B, Godwin AK, Hakimi AA, Ho TH, Hsieh J, Ittmann M, Kim WY, Krishnan B, Merino MJ, Mills Shaw KR, Reuter VE, Reznik E, Shelley CS, Shuch B, Signoretti S, Srinivasan R, Tamboli P, Thomas G, Tickoo S, Burnett K, Crain D, Gardner J, Lau K, Mallery D, Morris S, Paulauskis JD, Penny RJ, Shelton C, Shelton WT, Sherman M, Thompson E, Yena P, Avedon MT, Bowen J, Gastier-Foster JM, Gerken M, Leraas KM, Lichtenberg TM, Ramirez NC, Santos T, Wise L, Zmuda E, Demchok JA, Felau I, Hutter CM, Sheth M, Sofia HJ, Tarnuzzer R, Wang Z, Yang L, Zenklusen JC, Zhang J, Ayala B, Baboud J, Chudamani S, Liu J, Lolla L, Naresh R, Pihl T, Sun Q, Wan Y, Wu Y, Ally A, Balasundaram M, Balu S, Beroukhim R, Bodenheimer T, Buhay C, Butterfield YS, Carlsen R, Carter SL, Chao H, Chuah E, Clarke A, Covington KR, Dahdouli M, Dewal N, Dhalla N, Doddapaneni HV, Drummond JA, Gabriel SB, Gibbs RA, Guin R, Hale W, Hawes A, Hayes DN, Holt RA, Hoyle AP, Jefferys SR, Jones SJ, Jones CD, Kalra D, Kovar C, Lewis L, Li J, Ma Y, Marra MA, Mayo M, Meng S, Meyerson M, Mieczkowski PA, Moore RA, Morton D, Mose LE, Mungall AJ, Muzny D, Parker JS, Perou CM, Roach J, Schein JE, Schumacher SE, Shi Y, Simons JV, Sipahimalani P, Skelly T, Soloway MG, Sougnez C, Tam A, Tan D, Thiessen N, Veluvolu U, Wang M, Wilkerson MD, Wong T, Wu J, Xi L, Zhou J, Bedford J, Chen F, Fu Y, Gerstein M, Haussler D, Kasaian K, Lai P, Ling S, Radenbaugh A, Van Den Berg D, Weinstein JN, Zhu J, Albert M, Alexopoulou I, Andersen JJ, Auman JT, Bartlett J, Bastacky S, Bergsten J, Blute ML, Boice L, Bollag RJ, Boyd J, Castle E, Chen YB, Cheville JC, Curley E, Davies B, DeVolk A, Dhir R, Dike L, Eckman J, Engel J, Harr J, Hrebinko R, Huang M, Huelsenbeck-Dill L, Iacocca M, Jacobs B, Lobis M, Maranchie JK, McMeekin S, Myers J, Nelson J, Parfitt J, Parwani A, Petrelli N, Rabeno B, Roy S, Salner AL, Slaton J, Stanton M, Thompson RH, Thorne L, Tucker K, Weinberger PM, Winemiller C, Zach LA, Zuna R; Cancer Genome Atlas Research Network . Comprehensive molecular characterization of papillary renal-cell carcinoma. N Engl J Med 374: 135–145, 2016. doi: 10.1056/NEJMoa1505917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canning P, Cooper CD, Krojer T, Murray JW, Pike AC, Chaikuad A, Keates T, Thangaratnarajah C, Hojzan V, Marsden BD, Gileadi O, Knapp S, von Delft F, Bullock AN. Structural basis for Cul3 protein assembly with the BTB-Kelch family of E3 ubiquitin ligases. J Biol Chem 288: 7803–7814, 2013. [Erratum. J Biol Chem 388: 28304, 2013.] doi: 10.1074/jbc.M112.437996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan JY, Han XL, Kan YW. Cloning of Nrf1, an NF-E2-related transcription factor, by genetic selection in yeast. Proc Natl Acad Sci USA 90: 11371–11375, 1993. doi: 10.1073/pnas.90.23.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan K, Lu R, Chang JC, Kan YW. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc Natl Acad Sci USA 93: 13943–13948, 1996. doi: 10.1073/pnas.93.24.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan K, Kan YW. Nrf2 is essential for protection against acute pulmonary injury in mice. Proc Natl Acad Sci USA 96: 12731–12736, 1999. doi: 10.1073/pnas.96.22.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Z, Hu M, Shivdasani RA. Expression analysis of primary mouse megakaryocyte differentiation and its application in identifying stage-specific molecular markers and a novel transcriptional target of NF-E2. Blood 109: 1451–1459, 2007. doi: 10.1182/blood-2006-08-038901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chio IIC, Jafarnejad SM, Ponz-Sarvise M, Park Y, Rivera K, Palm W, Wilson J, Sangar V, Hao Y, Öhlund D, Wright K, Filippini D, Lee EJ, Da Silva B, Schoepfer C, Wilkinson JE, Buscaglia JM, DeNicola GM, Tiriac H, Hammell M, Crawford HC, Schmidt EE, Thompson CB, Pappin DJ, Sonenberg N, Tuveson DA. NRF2 promotes tumor maintenance by modulating mRNA translation in pancreatic cancer. Cell 166: 963–976, 2016. doi: 10.1016/j.cell.2016.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Kleeberger SR. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol 26: 175–182, 2002. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- 27.Cho HY, Jedlicka AE, Reddy SP, Zhang LY, Kensler TW, Kleeberger SR. Linkage analysis of susceptibility to hyperoxia. Nrf2 is a candidate gene. Am J Respir Cell Mol Biol 26: 42–51, 2002. doi: 10.1165/ajrcmb.26.1.4536. [DOI] [PubMed] [Google Scholar]
- 28.Cho HY, Reddy SP, Yamamoto M, Kleeberger SR. The transcription factor NRF2 protects against pulmonary fibrosis. FASEB J 18: 1258–1260, 2004. doi: 10.1096/fj.03-1127fje. [DOI] [PubMed] [Google Scholar]
- 29.Chorley BN, Campbell MR, Wang X, Karaca M, Sambandan D, Bangura F, Xue P, Pi J, Kleeberger SR, Bell DA. Identification of novel NRF2-regulated genes by ChIP-Seq: influence on retinoid X receptor alpha. Nucleic Acids Res 40: 7416–7429, 2012. doi: 10.1093/nar/gks409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chowdhry S, Zhang Y, McMahon M, Sutherland C, Cuadrado A, Hayes JD. Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene 32: 3765–3781, 2013. doi: 10.1038/onc.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cleasby A, Yon J, Day PJ, Richardson C, Tickle IJ, Williams PA, Callahan JF, Carr R, Concha N, Kerns JK, Qi H, Sweitzer T, Ward P, Davies TG. Structure of the BTB domain of Keap1 and its interaction with the triterpenoid antagonist CDDO. PLoS One 9: e98896, 2014. doi: 10.1371/journal.pone.0098896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cullinan SB, Gordan JD, Jin J, Harper JW, Diehl JA. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol Cell Biol 24: 8477–8486, 2004. doi: 10.1128/MCB.24.19.8477-8486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, McMurray JJ, Meyer CJ, Parving HH, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Wittes J, Wrolstad D, Chertow GM; BEACON Trial Investigators . Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med 369: 2492–2503, 2013. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeBaun JR, Smith JY, Miller EC, Miller JA. Reactivity in vivo of the carcinogen N-hydroxy-2-acetylaminofluorene: increase by sulfate ion. Science 167: 184–186, 1970. doi: 10.1126/science.167.3915.184. [DOI] [PubMed] [Google Scholar]
- 35.DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES, Scrimieri F, Winter JM, Hruban RH, Iacobuzio-Donahue C, Kern SE, Blair IA, Tuveson DA. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 475: 106–109, 2011. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeNicola GM, Chen PH, Mullarky E, Sudderth JA, Hu Z, Wu D, Tang H, Xie Y, Asara JM, Huffman KE, Wistuba II, Minna JD, DeBerardinis RJ, Cantley LC. NRF2 regulates serine biosynthesis in non-small cell lung cancer. Nat Genet 47: 1475–1481, 2015. doi: 10.1038/ng.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delmastro-Greenwood M, Freeman BA, Wendell SG. Redox-dependent anti-inflammatory signaling actions of unsaturated fatty acids. Annu Rev Physiol 76: 79–105, 2014. doi: 10.1146/annurev-physiol-021113-170341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deveaux S, Cohen-Kaminsky S, Shivdasani RA, Andrews NC, Filipe A, Kuzniak I, Orkin SH, Roméo PH, Mignotte V. p45 NF-E2 regulates expression of thromboxane synthase in megakaryocytes. EMBO J 16: 5654–5661, 1997. doi: 10.1093/emboj/16.18.5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dhakshinamoorthy S, Jain AK, Bloom DA, Jaiswal AK. Bach1 competes with Nrf2 leading to negative regulation of the antioxidant response element (ARE)-mediated NAD(P)H:quinone oxidoreductase 1 gene expression and induction in response to antioxidants. J Biol Chem 280: 16891–16900, 2005. doi: 10.1074/jbc.M500166200. [DOI] [PubMed] [Google Scholar]
- 40.Dialynas G, Shrestha OK, Ponce JM, Zwerger M, Thiemann DA, Young GH, Moore SA, Yu L, Lammerding J, Wallrath LL. Myopathic lamin mutations cause reductive stress and activate the nrf2/keap-1 pathway. PLoS Genet 11: e1005231, 2015. doi: 10.1371/journal.pgen.1005231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dinkova-Kostova AT, Massiah MA, Bozak RE, Hicks RJ, Talalay P. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc Natl Acad Sci USA 98: 3404–3409, 2001. doi: 10.1073/pnas.051632198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci USA 99: 11908–11913, 2002. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dinkova-Kostova AT, Fahey JW, Wade KL, Jenkins SN, Shapiro TA, Fuchs EJ, Kerns ML, Talalay P. Induction of the phase 2 response in mouse and human skin by sulforaphane-containing broccoli sprout extracts. Cancer Epidemiol Biomarkers Prev 16: 847–851, 2007. doi: 10.1158/1055-9965.EPI-06-0934. [DOI] [PubMed] [Google Scholar]
- 44.Dinkova-Kostova AT, Talalay P, Sharkey J, Zhang Y, Holtzclaw WD, Wang XJ, David E, Schiavoni KH, Finlayson S, Mierke DF, Honda T. An exceptionally potent inducer of cytoprotective enzymes: elucidation of the structural features that determine inducer potency and reactivity with Keap1. J Biol Chem 285: 33747–33755, 2010. doi: 10.1074/jbc.M110.163485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dinkova-Kostova AT, Fahey JW, Kostov RV, Kensler TW. KEAP1 and done? targeting the NRF2 pathway with sulforaphane. Trends Food Sci Technol 69, Pt. B: 257–269, 2017. doi: 10.1016/j.tifs.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eba S, Hoshikawa Y, Moriguchi T, Mitsuishi Y, Satoh H, Ishida K, Watanabe T, Shimizu T, Shimokawa H, Okada Y, Yamamoto M, Kondo T. The nuclear factor erythroid 2-related factor 2 activator oltipraz attenuates chronic hypoxia-induced cardiopulmonary alterations in mice. Am J Respir Cell Mol Biol 49: 324–333, 2013. doi: 10.1165/rcmb.2011-0396OC. [DOI] [PubMed] [Google Scholar]
- 47.Eggler AL, Small E, Hannink M, Mesecar AD. Cul3-mediated Nrf2 ubiquitination and antioxidant response element (ARE) activation are dependent on the partial molar volume at position 151 of Keap1. Biochem J 422: 171–180, 2009. doi: 10.1042/BJ20090471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Egner PA, Kensler TW, Prestera T, Talalay P, Libby AH, Joyner HH, Curphey TJ. Regulation of phase 2 enzyme induction by oltipraz and other dithiolethiones. Carcinogenesis 15: 177–181, 1994. doi: 10.1093/carcin/15.2.177. [DOI] [PubMed] [Google Scholar]
- 49.Egner PA, Chen JG, Zarth AT, Ng DK, Wang JB, Kensler KH, Jacobson LP, Muñoz A, Johnson JL, Groopman JD, Fahey JW, Talalay P, Zhu J, Chen TY, Qian GS, Carmella SG, Hecht SS, Kensler TW. Rapid and sustainable detoxication of airborne pollutants by broccoli sprout beverage: results of a randomized clinical trial in China. Cancer Prev Res (Phila) 7: 813–823, 2014. doi: 10.1158/1940-6207.CAPR-14-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Enomoto A, Itoh K, Nagayoshi E, Haruta J, Kimura T, O’Connor T, Harada T, Yamamoto M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol Sci 59: 169–177, 2001. doi: 10.1093/toxsci/59.1.169. [DOI] [PubMed] [Google Scholar]
- 51.Fahey JW, Haristoy X, Dolan PM, Kensler TW, Scholtus I, Stephenson KK, Talalay P, Lozniewski A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc Natl Acad Sci USA 99: 7610–7615, 2002. doi: 10.1073/pnas.112203099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fan W, Tang Z, Chen D, Moughon D, Ding X, Chen S, Zhu M, Zhong Q. Keap1 facilitates p62-mediated ubiquitin aggregate clearance via autophagy. Autophagy 6: 614–621, 2010. doi: 10.4161/auto.6.5.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fourquet S, Guerois R, Biard D, Toledano MB. Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation. J Biol Chem 285: 8463–8471, 2010. doi: 10.1074/jbc.M109.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fox RJ, Miller DH, Phillips JT, Hutchinson M, Havrdova E, Kita M, Yang M, Raghupathi K, Novas M, Sweetser MT, Viglietta V, Dawson KT; CONFIRM Study Investigators . Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 367: 1087–1097, 2012. doi: 10.1056/NEJMoa1206328. [DOI] [PubMed] [Google Scholar]
- 55.Friling RS, Bensimon A, Tichauer Y, Daniel V. Xenobiotic-inducible expression of murine glutathione S-transferase Ya subunit gene is controlled by an electrophile-responsive element. Proc Natl Acad Sci USA 87: 6258–6262, 1990. doi: 10.1073/pnas.87.16.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fujita R, Takayama-Tsujimoto M, Satoh H, Gutiérrez L, Aburatani H, Fujii S, Sarai A, Bresnick EH, Yamamoto M, Motohashi H. NF-E2 p45 is important for establishing normal function of platelets. Mol Cell Biol 33: 2659–2670, 2013. doi: 10.1128/MCB.01274-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fukutomi T, Takagi K, Mizushima T, Ohuchi N, Yamamoto M. Kinetic, thermodynamic, and structural characterizations of the association between Nrf2-DLGex degron and Keap1. Mol Cell Biol 34: 832–846, 2014. doi: 10.1128/MCB.01191-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Furukawa M, Xiong Y. BTB protein Keap1 targets antioxidant transcription factor Nrf2 for ubiquitination by the Cullin 3-Roc1 ligase. Mol Cell Biol 25: 162–171, 2005. doi: 10.1128/MCB.25.1.162-171.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, Tornatore C, Sweetser MT, Yang M, Sheikh SI, Dawson KT; DEFINE Study Investigators . Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 367: 1098–1107, 2012. doi: 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- 60.Goldstein LD, Lee J, Gnad F, Klijn C, Schaub A, Reeder J, Daemen A, Bakalarski CE, Holcomb T, Shames DS, Hartmaier RJ, Chmielecki J, Seshagiri S, Gentleman R, Stokoe D. Recurrent loss of NFE2L2 exon 2 is a mechanism for Nrf2 pathway activation in human cancers. Cell Reports 16: 2605–2617, 2016. doi: 10.1016/j.celrep.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 61.Hiramoto K, Satoh H, Suzuki T, Moriguchi T, Pi J, Shimosegawa T, Yamamoto M. Myeloid lineage-specific deletion of antioxidant system enhances tumor metastasis. Cancer Prev Res (Phila) 7: 835–844, 2014. doi: 10.1158/1940-6207.CAPR-14-0094. [DOI] [PubMed] [Google Scholar]
- 62.Hirotsu Y, Katsuoka F, Funayama R, Nagashima T, Nishida Y, Nakayama K, Engel JD, Yamamoto M. Nrf2-MafG heterodimers contribute globally to antioxidant and metabolic networks. Nucleic Acids Res 40: 10228–10239, 2012. doi: 10.1093/nar/gks827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hong F, Freeman ML, Liebler DC. Identification of sensor cysteines in human Keap1 modified by the cancer chemopreventive agent sulforaphane. Chem Res Toxicol 18: 1917–1926, 2005. doi: 10.1021/tx0502138. [DOI] [PubMed] [Google Scholar]
- 64.Honkura Y, Matsuo H, Murakami S, Sakiyama M, Mizutari K, Shiotani A, Yamamoto M, Morita I, Shinomiya N, Kawase T, Katori Y, Motohashi H. NRF2 is a key target for prevention of noise-induced hearing loss by reducing oxidative damage of cochlea. Sci Rep 6: 19329, 2016. doi: 10.1038/srep19329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang C, Geng H, Boss I, Wang L, Melnick A. Cooperative transcriptional repression by BCL6 and BACH2 in germinal center B-cell differentiation. Blood 123: 1012–1020, 2014. doi: 10.1182/blood-2013-07-518605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huerta C, Jiang X, Trevino I, Bender CF, Ferguson DA, Probst B, Swinger KK, Stoll VS, Thomas PJ, Dulubova I, Visnick M, Wigley WC. Characterization of novel small-molecule NRF2 activators: structural and biochemical validation of stereospecific KEAP1 binding. Biochim Biophys Acta 1860: 2537–2552, 2016. doi: 10.1016/j.bbagen.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 67.Ichimura Y, Waguri S, Sou YS, Kageyama S, Hasegawa J, Ishimura R, Saito T, Yang Y, Kouno T, Fukutomi T, Hoshii T, Hirao A, Takagi K, Mizushima T, Motohashi H, Lee MS, Yoshimori T, Tanaka K, Yamamoto M, Komatsu M. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol Cell 51: 618–631, 2013. doi: 10.1016/j.molcel.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 68.Igarashi K, Kataokat K, Itoh K, Hayashi N, Nishizawa M, Yamamoto M. Regulation of transcription by dimerization of erythroid factor NF-E2 p45 with small Maf proteins. Nature 367: 568–572, 1994. doi: 10.1038/367568a0. [DOI] [PubMed] [Google Scholar]
- 69.Iizuka T, Ishii Y, Itoh K, Kiwamoto T, Kimura T, Matsuno Y, Morishima Y, Hegab AE, Homma S, Nomura A, Sakamoto T, Shimura M, Yoshida A, Yamamoto M, Sekizawa K. Nrf2-deficient mice are highly susceptible to cigarette smoke-induced emphysema. Genes Cells 10: 1113–1125, 2005. doi: 10.1111/j.1365-2443.2005.00905.x. [DOI] [PubMed] [Google Scholar]
- 70.Imielinski M, Berger AH, Hammerman PS, Hernandez B, Pugh TJ, Hodis E, Cho J, Suh J, Capelletti M, Sivachenko A, Sougnez C, Auclair D, Lawrence MS, Stojanov P, Cibulskis K, Choi K, de Waal L, Sharifnia T, Brooks A, Greulich H, Banerji S, Zander T, Seidel D, Leenders F, Ansén S, Ludwig C, Engel-Riedel W, Stoelben E, Wolf J, Goparju C, Thompson K, Winckler W, Kwiatkowski D, Johnson BE, Jänne PA, Miller VA, Pao W, Travis WD, Pass HI, Gabriel SB, Lander ES, Thomas RK, Garraway LA, Getz G, Meyerson M. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell 150: 1107–1120, 2012. doi: 10.1016/j.cell.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Inami Y, Waguri S, Sakamoto A, Kouno T, Nakada K, Hino O, Watanabe S, Ando J, Iwadate M, Yamamoto M, Lee MS, Tanaka K, Komatsu M. Persistent activation of Nrf2 through p62 in hepatocellular carcinoma cells. J Cell Biol 193: 275–284, 2011. doi: 10.1083/jcb.201102031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Inoue D, Suzuki T, Mitsuishi Y, Miki Y, Suzuki S, Sugawara S, Watanabe M, Sakurada A, Endo C, Uruno A, Sasano H, Nakagawa T, Satoh K, Tanaka N, Kubo H, Motohashi H, Yamamoto M. Accumulation of p62/SQSTM1 is associated with poor prognosis in patients with lung adenocarcinoma. Cancer Sci 103: 760–766, 2012. doi: 10.1111/j.1349-7006.2012.02216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Inoue H, Hisamoto N, An JH, Oliveira RP, Nishida E, Blackwell TK, Matsumoto K. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev 19: 2278–2283, 2005. doi: 10.1101/gad.1324805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem 275: 16023–16029, 2000. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- 75.Ishii Y, Itoh K, Morishima Y, Kimura T, Kiwamoto T, Iizuka T, Hegab AE, Hosoya T, Nomura A, Sakamoto T, Yamamoto M, Sekizawa K. Transcription factor Nrf2 plays a pivotal role in protection against elastase-induced pulmonary inflammation and emphysema. J Immunol 175: 6968–6975, 2005. doi: 10.4049/jimmunol.175.10.6968. [DOI] [PubMed] [Google Scholar]
- 76.Iso T, Suzuki T, Baird L, Yamamoto M. Absolute amounts and status of the Nrf2-Keap1-Cul3 comple within cells. Mol Cell Biol 36: 3100–3112, 2016. doi: 10.1128/MCB.00389-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Itoh K, Igarashi K, Hayashi N, Nishizawa M, Yamamoto M. Cloning and characterization of a novel erythroid cell-derived CNC family transcription factor heterodimerizing with the small Maf family proteins. Mol Cell Biol 15: 4184–4193, 1995. doi: 10.1128/MCB.15.8.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236: 313–322, 1997. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 79.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 13: 76–86, 1999. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Itoh K, Wakabayashi N, Katoh Y, Ishii T, O’Connor T, Yamamoto M. Keap1 regulates both cytoplasmic-nuclear shuttling and degradation of Nrf2 in response to electrophiles. Genes Cells 8: 379–391, 2003. doi: 10.1046/j.1365-2443.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- 81.Itoh K, Mochizuki M, Ishii Y, Ishii T, Shibata T, Kawamoto Y, Kelly V, Sekizawa K, Uchida K, Yamamoto M. Transcription factor Nrf2 regulates inflammation by mediating the effect of 15-deoxy-Delta(12,14)-prostaglandin J(2). Mol Cell Biol 24: 36–45, 2004. doi: 10.1128/MCB.24.1.36-45.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jain A, Lamark T, Sjøttem E, Bowitz Larsen KB, Awuh JA, Øvervatn A, McMahon M, Hayes JD, Johansen T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem 285: 22576–22591, 2010. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jiang T, Huang Z, Lin Y, Zhang Z, Fang D, Zhang DD. The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes 59: 850–860, 2010. doi: 10.2337/db09-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johnson NM, Egner PA, Baxter VK, Sporn MB, Wible RS, Sutter TR, Groopman JD, Kensler TW, Roebuck BD. Complete protection against aflatoxin B(1)-induced liver cancer with a triterpenoid: DNA adduct dosimetry, molecular signature, and genotoxicity threshold. Cancer Prev Res (Phila) 7: 658–665, 2014. doi: 10.1158/1940-6207.CAPR-13-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Joung EJ, Li MH, Lee HG, Somparn N, Jung YS, Na HK, Kim SH, Cha YN, Surh YJ. Capsaicin induces heme oxygenase-1 expression in HepG2 cells via activation of PI3K-Nrf2 signaling: NAD(P)H:quinone oxidoreductase as a potential target. Antioxid Redox Signal 9: 2087–2098, 2007. doi: 10.1089/ars.2007.1827. [DOI] [PubMed] [Google Scholar]
- 86.Kaidery NA, Banerjee R, Yang L, Smirnova NA, Hushpulian DM, Liby KT, Williams CR, Yamamoto M, Kensler TW, Ratan RR, Sporn MB, Beal MF, Gazaryan IG, Thomas B. Targeting Nrf2-mediated gene transcription by extremely potent synthetic triterpenoids attenuate dopaminergic neurotoxicity in the MPTP mouse model of Parkinson’s disease. Antioxid Redox Signal 18: 139–157, 2013. doi: 10.1089/ars.2011.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kanamori M, Higa T, Sonoda Y, Murakami S, Dodo M, Kitamura H, Taguchi K, Shibata T, Watanabe M, Suzuki H, Shibahara I, Saito R, Yamashita Y, Kumabe T, Yamamoto M, Motohashi H, Tominaga T. Activation of the NRF2 pathway and its impact on the prognosis of anaplastic glioma patients. Neuro-oncol 17: 555–565, 2015. doi: 10.1093/neuonc/nou282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kang MI, Kobayashi A, Wakabayashi N, Kim SG, Yamamoto M. Scaffolding of Keap1 to the actin cytoskeleton controls the function of Nrf2 as key regulator of cytoprotective phase 2 genes. Proc Natl Acad Sci USA 101: 2046–2051, 2004. doi: 10.1073/pnas.0308347100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kannan MB, Solovieva V, Blank V. The small MAF transcription factors MAFF, MAFG and MAFK: current knowledge and perspectives. Biochim Biophys Acta 1823: 1841–1846, 2012. doi: 10.1016/j.bbamcr.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 90.Kannan S, Muthusamy VR, Whitehead KJ, Wang L, Gomes AV, Litwin SE, Kensler TW, Abel ED, Hoidal JR, Rajasekaran NS. Nrf2 deficiency prevents reductive stress-induced hypertrophic cardiomyopathy. Cardiovasc Res 100: 63–73, 2013. doi: 10.1093/cvr/cvt150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kanninen K, Heikkinen R, Malm T, Rolova T, Kuhmonen S, Leinonen H, Ylä-Herttuala S, Tanila H, Levonen AL, Koistinaho M, Koistinaho J. Intrahippocampal injection of a lentiviral vector expressing Nrf2 improves spatial learning in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA 106: 16505–16510, 2009. doi: 10.1073/pnas.0908397106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kataoka K, Noda M, Nishizawa M. Maf nuclear oncoprotein recognizes sequences related to an AP-1 site and forms heterodimers with both Fos and Jun. Mol Cell Biol 14: 700–712, 1994. doi: 10.1128/MCB.14.1.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Katoh Y, Itoh K, Yoshida E, Miyagishi M, Fukamizu A, Yamamoto M. Two domains of Nrf2 cooperatively bind CBP, a CREB binding protein, and synergistically activate transcription. Genes Cells 6: 857–868, 2001. doi: 10.1046/j.1365-2443.2001.00469.x. [DOI] [PubMed] [Google Scholar]
- 94.Katoh Y, Ikura T, Hoshikawa Y, Tashiro S, Ito T, Ohta M, Kera Y, Noda T, Igarashi K. Methionine adenosyltransferase II serves as a transcriptional corepressor of Maf oncoprotein. Mol Cell 41: 554–566, 2011. doi: 10.1016/j.molcel.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 95.Katsumata Y, Shinmura K, Sugiura Y, Tohyama S, Matsuhashi T, Ito H, Yan X, Ito K, Yuasa S, Ieda M, Urade Y, Suematsu M, Fukuda K, Sano M. Endogenous prostaglandin D2 and its metabolites protect the heart against ischemia-reperfusion injury by activating Nrf2. Hypertension 63: 80–87, 2014. doi: 10.1161/HYPERTENSIONAHA.113.01639. [DOI] [PubMed] [Google Scholar]
- 96.Katsuoka F, Motohashi H, Tamagawa Y, Kure S, Igarashi K, Engel JD, Yamamoto M. Small Maf compound mutants display central nervous system neuronal degeneration, aberrant transcription, and Bach protein mislocalization coincident with myoclonus and abnormal startle response. Mol Cell Biol 23: 1163–1174, 2003. doi: 10.1128/MCB.23.4.1163-1174.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Katsuoka F, Motohashi H, Ishii T, Aburatani H, Engel JD, Yamamoto M. Genetic evidence that small maf proteins are essential for the activation of antioxidant response element-dependent genes. Mol Cell Biol 25: 8044–8051, 2005. doi: 10.1128/MCB.25.18.8044-8051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Keleku-Lukwete N, Suzuki M, Otsuki A, Tsuchida K, Katayama S, Hayashi M, Naganuma E, Moriguchi T, Tanabe O, Engel JD, Imaizumi M, Yamamoto M. Amelioration of inflammation and tissue damage in sickle cell model mice by Nrf2 activation. Proc Natl Acad Sci USA 112: 12169–12174, 2015. doi: 10.1073/pnas.1509158112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Keller TL, Zocco D, Sundrud MS, Hendrick M, Edenius M, Yum J, Kim YJ, Lee HK, Cortese JF, Wirth DF, Dignam JD, Rao A, Yeo CY, Mazitschek R, Whitman M. Halofuginone and other febrifugine derivatives inhibit prolyl-tRNA synthetase. Nat Chem Biol 8: 311–317, 2012. doi: 10.1038/nchembio.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kensler TW, Wakabayashi N. Nrf2: friend or foe for chemoprevention? Carcinogenesis 31: 90–99, 2010. doi: 10.1093/carcin/bgp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kensler TW, Roebuck BD, Wogan GN, Groopman JD. Aflatoxin: a 50-year odyssey of mechanistic and translational toxicology. Toxicol Sci 120, Suppl 1: S28–S48, 2011. doi: 10.1093/toxsci/kfq283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kerppola TK, Curran T. A conserved region adjacent to the basic domain is required for recognition of an extended DNA binding site by Maf/Nrl family proteins. Oncogene 9: 3149–3158, 1994. [PubMed] [Google Scholar]
- 103.Kim YR, Oh JE, Kim MS, Kang MR, Park SW, Han JY, Eom HS, Yoo NJ, Lee SH. Oncogenic NRF2 mutations in squamous cell carcinomas of oesophagus and skin. J Pathol 220: 446–451, 2010. doi: 10.1002/path.2653. [DOI] [PubMed] [Google Scholar]
- 104.Kimura M, Yamamoto T, Zhang J, Itoh K, Kyo M, Kamiya T, Aburatani H, Katsuoka F, Kurokawa H, Tanaka T, Motohashi H, Yamamoto M. Molecular basis distinguishing the DNA binding profile of Nrf2-Maf heterodimer from that of Maf homodimer. J Biol Chem 282: 33681–33690, 2007. doi: 10.1074/jbc.M706863200. [DOI] [PubMed] [Google Scholar]
- 105.Kitamura H, Onodera Y, Murakami S, Suzuki T, Motohashi H. IL-11 contribution to tumorigenesis in an NRF2 addiction cancer model. Oncogene 36: 6315–6324, 2017. doi: 10.1038/onc.2017.236. [DOI] [PubMed] [Google Scholar]
- 106.Knatko EV, Ibbotson SH, Zhang Y, Higgins M, Fahey JW, Talalay P, Dawe RS, Ferguson J, Huang JT, Clarke R, Zheng S, Saito A, Kalra S, Benedict AL, Honda T, Proby CM, Dinkova-Kostova AT. Nrf2 activation protects against solar-simulated ultraviolet radiation in mice and humans. Cancer Prev Res (Phila) 8: 475–486, 2015. doi: 10.1158/1940-6207.CAPR-14-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kobayashi A, Ito E, Toki T, Kogame K, Takahashi S, Igarashi K, Hayashi N, Yamamoto M. Molecular cloning and functional characterization of a new Cap ’n’ collar family transcription factor Nrf3. J Biol Chem 274: 6443–6452, 1999. doi: 10.1074/jbc.274.10.6443. [DOI] [PubMed] [Google Scholar]
- 108.Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol 24: 7130–7139, 2004. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kobayashi A, Kang MI, Watai Y, Tong KI, Shibata T, Uchida K, Yamamoto M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol 26: 221–229, 2006. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kobayashi A, Tsukide T, Miyasaka T, Morita T, Mizoroki T, Saito Y, Ihara Y, Takashima A, Noguchi N, Fukamizu A, Hirotsu Y, Ohtsuji M, Katsuoka F, Yamamoto M. Central nervous system-specific deletion of transcription factor Nrf1 causes progressive motor neuronal dysfunction. Genes Cells 16: 692–703, 2011. doi: 10.1111/j.1365-2443.2011.01522.x. [DOI] [PubMed] [Google Scholar]
- 111.Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Hayashi M, Sekine H, Tanaka N, Moriguchi T, Motohashi H, Nakayama K, Yamamoto M. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun 7: 11624, 2016. doi: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kobayashi M, Itoh K, Suzuki T, Osanai H, Nishikawa K, Katoh Y, Takagi Y, Yamamoto M. Identification of the interactive interface and phylogenic conservation of the Nrf2-Keap1 system. Genes Cells 7: 807–820, 2002. doi: 10.1046/j.1365-2443.2002.00561.x. [DOI] [PubMed] [Google Scholar]
- 113.Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, Nakayama Y, Eguchi M, Wada Y, Kumagai Y, Yamamoto M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol 29: 493–502, 2009. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol 12: 213–223, 2010. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 115.Kong X, Thimmulappa R, Craciun F, Harvey C, Singh A, Kombairaju P, Reddy SP, Remick D, Biswal S. Enhancing Nrf2 pathway by disruption of Keap1 in myeloid leukocytes protects against sepsis. Am J Respir Crit Care Med 184: 928–938, 2011. doi: 10.1164/rccm.201102-0271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kuosmanen SM, Viitala S, Laitinen T, Peräkylä M, Pölönen P, Kansanen E, Leinonen H, Raju S, Wienecke-Baldacchino A, Närvänen A, Poso A, Heinäniemi M, Heikkinen S, Levonen AL. The effects of sequence variation on genome-wide NRF2 binding—new target genes and regulatory SNPs. Nucleic Acids Res 44: 1760–1775, 2016. doi: 10.1093/nar/gkw052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kurokawa H, Motohashi H, Sueno S, Kimura M, Takagawa H, Kanno Y, Yamamoto M, Tanaka T. Structural basis of alternative DNA recognition by Maf transcription factors. Mol Cell Biol 29: 6232–6244, 2009. doi: 10.1128/MCB.00708-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kwak MK, Itoh K, Yamamoto M, Kensler TW. Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: role of antioxidant response element-like sequences in the nrf2 promoter. Mol Cell Biol 22: 2883–2892, 2002. doi: 10.1128/MCB.22.9.2883-2892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kwak MK, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, Kensler TW. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J Biol Chem 278: 8135–8145, 2003. doi: 10.1074/jbc.M211898200. [DOI] [PubMed] [Google Scholar]
- 120.Lau A, Wang XJ, Zhao F, Villeneuve NF, Wu T, Jiang T, Sun Z, White E, Zhang DD. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Mol Cell Biol 30: 3275–3285, 2010. doi: 10.1128/MCB.00248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lecine P, Italiano JE Jr, Kim SW, Villeval JL, Shivdasani RA. Hematopoietic-specific beta 1 tubulin participates in a pathway of platelet biogenesis dependent on the transcription factor NF-E2. Blood 96: 1366–1373, 2000. [PubMed] [Google Scholar]
- 122.Leiserson MD, Vandin F, Wu HT, Dobson JR, Eldridge JV, Thomas JL, Papoutsaki A, Kim Y, Niu B, McLellan M, Lawrence MS, Gonzalez-Perez A, Tamborero D, Cheng Y, Ryslik GA, Lopez-Bigas N, Getz G, Ding L, Raphael BJ. Pan-cancer network analysis identifies combinations of rare somatic mutations across pathways and protein complexes. Nat Genet 47: 106–114, 2015. doi: 10.1038/ng.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lewis KN, Wason E, Edrey YH, Kristan DM, Nevo E, Buffenstein R. Regulation of Nrf2 signaling and longevity in naturally long-lived rodents. Proc Natl Acad Sci U S A 112: 3722–3727, 2015. doi: 10.1073/pnas.1417566112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li MH, Cha YN, Surh YJ. Peroxynitrite induces HO-1 expression via PI3K/Akt-dependent activation of NF-E2-related factor 2 in PC12 cells. Free Radic Biol Med 41: 1079–1091, 2006. doi: 10.1016/j.freeradbiomed.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 125.Li X, Zhang D, Hannink M, Beamer LJ. Crystal structure of the Kelch domain of human Keap1. J Biol Chem 279: 54750–54758, 2004. doi: 10.1074/jbc.M410073200. [DOI] [PubMed] [Google Scholar]
- 126.Liby K, Hock T, Yore MM, Suh N, Place AE, Risingsong R, Williams CR, Royce DB, Honda T, Honda Y, Gribble GW, Hill-Kapturczak N, Agarwal A, Sporn MB. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Res 65: 4789–4798, 2005. doi: 10.1158/0008-5472.CAN-04-4539. [DOI] [PubMed] [Google Scholar]
- 127.Linker RA, Lee DH, Ryan S, van Dam AM, Conrad R, Bista P, Zeng W, Hronowsky X, Buko A, Chollate S, Ellrichmann G, Brück W, Dawson K, Goelz S, Wiese S, Scannevin RH, Lukashev M, Gold R. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 134: 678–692, 2011. doi: 10.1093/brain/awq386. [DOI] [PubMed] [Google Scholar]
- 128.Liu M, Reddy NM, Higbee EM, Potteti HR, Noel S, Racusen L, Kensler TW, Sporn MB, Reddy SP, Rabb H. The Nrf2 triterpenoid activator, CDDO-imidazolide, protects kidneys from ischemia-reperfusion injury in mice. Kidney Int 85: 134–141, 2014. doi: 10.1038/ki.2013.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lu M, Nakamura RM, Dent ED, Zhang JY, Nielsen FC, Christiansen J, Chan EK, Tan EM. Aberrant expression of fetal RNA-binding protein p62 in liver cancer and liver cirrhosis. Am J Pathol 159: 945–953, 2001. doi: 10.1016/S0002-9440(10)61770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Maicas N, Ferrándiz ML, Brines R, Ibáñez L, Cuadrado A, Koenders MI, van den Berg WB, Alcaraz MJ. Deficiency of Nrf2 accelerates the effector phase of arthritis and aggravates joint disease. Antioxid Redox Signal 15: 889–901, 2011. doi: 10.1089/ars.2010.3835. [DOI] [PubMed] [Google Scholar]
- 131.Malhotra D, Portales-Casamar E, Singh A, Srivastava S, Arenillas D, Happel C, Shyr C, Wakabayashi N, Kensler TW, Wasserman WW, Biswal S. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res 38: 5718–5734, 2010. doi: 10.1093/nar/gkq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Martin D, Rojo AI, Salinas M, Diaz R, Gallardo G, Alam J, De Galarreta CM, Cuadrado A. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J Biol Chem 279: 8919–8929, 2004. doi: 10.1074/jbc.M309660200. [DOI] [PubMed] [Google Scholar]
- 133.Martinez VD, Vucic EA, Pikor LA, Thu KL, Hubaux R, Lam WL. Frequent concerted genetic mechanisms disrupt multiple components of the NRF2 inhibitor KEAP1/CUL3/RBX1 E3-ubiquitin ligase complex in thyroid cancer. Mol Cancer 12: 124, 2013. doi: 10.1186/1476-4598-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Martinez VD, Vucic EA, Thu KL, Pikor LA, Lam S, Lam WL. Disruption of KEAP1/CUL3/RBX1 E3-ubiquitin ligase complex components by multiple genetic mechanisms: Association with poor prognosis in head and neck cancer. Head Neck 37: 727–734, 2015. doi: 10.1002/hed.23663. [DOI] [PubMed] [Google Scholar]
- 135.Marzec JM, Christie JD, Reddy SP, Jedlicka AE, Vuong H, Lanken PN, Aplenc R, Yamamoto T, Yamamoto M, Cho HY, Kleeberger SR. Functional polymorphisms in the transcription factor NRF2 in humans increase the risk of acute lung injury. FASEB J 21: 2237–2246, 2007. doi: 10.1096/fj.06-7759com. [DOI] [PubMed] [Google Scholar]
- 136.McMahon M, Thomas N, Itoh K, Yamamoto M, Hayes JD. Redox-regulated turnover of Nrf2 is determined by at least two separate protein domains, the redox-sensitive Neh2 degron and the redox-insensitive Neh6 degron. J Biol Chem 279: 31556–31567, 2004. doi: 10.1074/jbc.M403061200. [DOI] [PubMed] [Google Scholar]
- 137.McMahon M, Lamont DJ, Beattie KA, Hayes JD. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc Natl Acad Sci USA 107: 18838–18843, 2010. doi: 10.1073/pnas.1007387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mignotte V, Eleouet JF, Raich N, Romeo PH. Cis- and trans-acting elements involved in the regulation of the erythroid promoter of the human porphobilinogen deaminase gene. Proc Natl Acad Sci USA 86: 6548–6552, 1989. doi: 10.1073/pnas.86.17.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Miller EC. Some current perspectives on chemical carcinogenesis in humans and experimental animals: Presidential Address. Cancer Res 38: 1479–1496, 1978. [PubMed] [Google Scholar]
- 140.Mitsuishi Y, Taguchi K, Kawatani Y, Shibata T, Nukiwa T, Aburatani H, Yamamoto M, Motohashi H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell 22: 66–79, 2012. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 141.Mochizuki M, Ishii Y, Itoh K, Iizuka T, Morishima Y, Kimura T, Kiwamoto T, Matsuno Y, Hegab AE, Nomura A, Sakamoto T, Uchida K, Yamamoto M, Sekizawa K. Role of 15-deoxy delta(12,14) prostaglandin J2 and Nrf2 pathways in protection against acute lung injury. Am J Respir Crit Care Med 171: 1260–1266, 2005. doi: 10.1164/rccm.200406-755OC. [DOI] [PubMed] [Google Scholar]
- 142.Mohler J, Vani K, Leung S, Epstein A. Segmentally restricted, cephalic expression of a leucine zipper gene during Drosophila embryogenesis. Mech Dev 34: 3–9, 1991. doi: 10.1016/0925-4773(91)90086-L. [DOI] [PubMed] [Google Scholar]
- 143.Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci USA 91: 9926–9930, 1994. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Motohashi H, Shavit JA, Igarashi K, Yamamoto M, Engel JD. The world according to Maf. Nucleic Acids Res 25: 2953–2959, 1997. doi: 10.1093/nar/25.15.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Motohashi H, Katsuoka F, Shavit JA, Engel JD, Yamamoto M. Positive or negative MARE-dependent transcriptional regulation is determined by the abundance of small Maf proteins. Cell 103: 865–876, 2000. doi: 10.1016/S0092-8674(00)00190-2. [DOI] [PubMed] [Google Scholar]
- 146.Motohashi H, O’Connor T, Katsuoka F, Engel JD, Yamamoto M. Integration and diversity of the regulatory network composed of Maf and CNC families of transcription factors. Gene 294: 1–12, 2002. doi: 10.1016/S0378-1119(02)00788-6. [DOI] [PubMed] [Google Scholar]
- 147.Motohashi H, Katsuoka F, Engel JD, Yamamoto M. Small Maf proteins serve as transcriptional cofactors for keratinocyte differentiation in the Keap1-Nrf2 regulatory pathway. Proc Natl Acad Sci USA 101: 6379–6384, 2004. doi: 10.1073/pnas.0305902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Motohashi H, Katsuoka F, Miyoshi C, Uchimura Y, Saitoh H, Francastel C, Engel JD, Yamamoto M. MafG sumoylation is required for active transcriptional repression. Mol Cell Biol 26: 4652–4663, 2006. doi: 10.1128/MCB.02193-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Motohashi H, Kimura M, Fujita R, Inoue A, Pan X, Takayama M, Katsuoka F, Aburatani H, Bresnick EH, Yamamoto M. NF-E2 domination over Nrf2 promotes ROS accumulation and megakaryocytic maturation. Blood 115: 677–686, 2010. doi: 10.1182/blood-2009-05-223107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Motohashi H, Fujita R, Takayama M, Inoue A, Katsuoka F, Bresnick EH, Yamamoto M. Molecular determinants for small Maf protein control of platelet production. Mol Cell Biol 31: 151–162, 2011. doi: 10.1128/MCB.00798-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Muscarella LA, Barbano R, D’Angelo V, Copetti M, Coco M, Balsamo T, la Torre A, Notarangelo A, Troiano M, Parisi S, Icolaro N, Catapano D, Valori VM, Pellegrini F, Merla G, Carella M, Fazio VM, Parrella P. Regulation of KEAP1 expression by promoter methylation in malignant gliomas and association with patient’s outcome. Epigenetics 6: 317–325, 2011. doi: 10.4161/epi.6.3.14408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Muscarella LA, Parrella P, D’Alessandro V, la Torre A, Barbano R, Fontana A, Tancredi A, Guarnieri V, Balsamo T, Coco M, Copetti M, Pellegrini F, De Bonis P, Bisceglia M, Scaramuzzi G, Maiello E, Valori VM, Merla G, Vendemiale G, Fazio VM. Frequent epigenetics inactivation of KEAP1 gene in non-small cell lung cancer. Epigenetics 6: 710–719, 2011. doi: 10.4161/epi.6.6.15773. [DOI] [PubMed] [Google Scholar]
- 153.Muto A, Hoshino H, Madisen L, Yanai N, Obinata M, Karasuyama H, Hayashi N, Nakauchi H, Yamamoto M, Groudine M, Igarashi K. Identification of Bach2 as a B-cell-specific partner for small maf proteins that negatively regulate the immunoglobulin heavy chain gene 3′ enhancer. EMBO J 17: 5734–5743, 1998. doi: 10.1093/emboj/17.19.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nagai T, Igarashi K, Akasaka J, Furuyama K, Fujita H, Hayashi N, Yamamoto M, Sassa S. Regulation of NF-E2 activity in erythroleukemia cell differentiation. J Biol Chem 273: 5358–5365, 1998. doi: 10.1074/jbc.273.9.5358. [DOI] [PubMed] [Google Scholar]
- 155.Neymotin A, Calingasan NY, Wille E, Naseri N, Petri S, Damiano M, Liby KT, Risingsong R, Sporn M, Beal MF, Kiaei M. Neuroprotective effect of Nrf2/ARE activators, CDDO ethylamide and CDDO trifluoroethylamide, in a mouse model of amyotrophic lateral sclerosis. Free Radic Biol Med 51: 88–96, 2011. doi: 10.1016/j.freeradbiomed.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nezu M, Souma T, Yu L, Suzuki T, Saigusa D, Ito S, Suzuki N, Yamamoto M. Transcription factor Nrf2 hyperactivation in early-phase renal ischemia-reperfusion injury prevents tubular damage progression. Kidney Int 91: 387–401, 2017. doi: 10.1016/j.kint.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 157.Nioi P, Nguyen T, Sherratt PJ, Pickett CB. The carboxy-terminal Neh3 domain of Nrf2 is required for transcriptional activation. Mol Cell Biol 25: 10895–10906, 2005. doi: 10.1128/MCB.25.24.10895-10906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nioi P, Nguyen T. A mutation of Keap1 found in breast cancer impairs its ability to repress Nrf2 activity. Biochem Biophys Res Commun 362: 816–821, 2007. doi: 10.1016/j.bbrc.2007.08.051. [DOI] [PubMed] [Google Scholar]
- 159.Nishizawa M, Kataoka K, Goto N, Fujiwara KT, Kawai S. v-maf, a viral oncogene that encodes a “leucine zipper” motif. Proc Natl Acad Sci USA 86: 7711–7715, 1989. doi: 10.1073/pnas.86.20.7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Noel S, Martina MN, Bandapalle S, Racusen LC, Potteti HR, Hamad AR, Reddy SP, Rabb H. T lymphocyte-specific activation of Nrf2 protects from AKI. J Am Soc Nephrol 26: 2989–3000, 2015. doi: 10.1681/ASN.2014100978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ogura T, Tong KI, Mio K, Maruyama Y, Kurokawa H, Sato C, Yamamoto M. Keap1 is a forked-stem dimer structure with two large spheres enclosing the intervening, double glycine repeat, and C-terminal domains. Proc Natl Acad Sci USA 107: 2842–2847, 2010. doi: 10.1073/pnas.0914036107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Okawa H, Motohashi H, Kobayashi A, Aburatani H, Kensler TW, Yamamoto M. Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem Biophys Res Commun 339: 79–88, 2006. doi: 10.1016/j.bbrc.2005.10.185. [DOI] [PubMed] [Google Scholar]
- 163.Onodera K, Shavit JA, Motohashi H, Yamamoto M, Engel JD. Perinatal synthetic lethality and hematopoietic defects in compound mafG:mafK mutant mice. EMBO J 19: 1335–1345, 2000. doi: 10.1093/emboj/19.6.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Onodera Y, Motohashi H, Takagi K, Miki Y, Shibahara Y, Watanabe M, Ishida T, Hirakawa H, Sasano H, Yamamoto M, Suzuki T. NRF2 immunolocalization in human breast cancer patients as a prognostic factor. Endocr Relat Cancer 21: 241–252, 2014. doi: 10.1530/ERC-13-0234. [DOI] [PubMed] [Google Scholar]
- 165.Ooi A, Wong JC, Petillo D, Roossien D, Perrier-Trudova V, Whitten D, Min BW, Tan MH, Zhang Z, Yang XJ, Zhou M, Gardie B, Molinié V, Richard S, Tan PH, Teh BT, Furge KA. An antioxidant response phenotype shared between hereditary and sporadic type 2 papillary renal cell carcinoma. Cancer Cell 20: 511–523, 2011. doi: 10.1016/j.ccr.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 166.Ooi A, Dykema K, Ansari A, Petillo D, Snider J, Kahnoski R, Anema J, Craig D, Carpten J, Teh BT, Furge KA. CUL3 and NRF2 mutations confer an NRF2 activation phenotype in a sporadic form of papillary renal cell carcinoma. Cancer Res 73: 2044–2051, 2013. doi: 10.1158/0008-5472.CAN-12-3227. [DOI] [PubMed] [Google Scholar]
- 167.Osburn WO, Yates MS, Dolan PD, Chen S, Liby KT, Sporn MB, Taguchi K, Yamamoto M, Kensler TW. Genetic or pharmacologic amplification of nrf2 signaling inhibits acute inflammatory liver injury in mice. Toxicol Sci 104: 218–227, 2008. doi: 10.1093/toxsci/kfn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Otsuki A, Suzuki M, Katsuoka F, Tsuchida K, Suda H, Morita M, Shimizu R, Yamamoto M. Unique cistrome defined as CsMBE is strictly required for Nrf2-sMaf heterodimer function in cytoprotection. Free Radic Biol Med 91: 45–57, 2016. doi: 10.1016/j.freeradbiomed.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 169.Oyake T, Itoh K, Motohashi H, Hayashi N, Hoshino H, Nishizawa M, Yamamoto M, Igarashi K. Bach proteins belong to a novel family of BTB-basic leucine zipper transcription factors that interact with MafK and regulate transcription through the NF-E2 site. Mol Cell Biol 16: 6083–6095, 1996. doi: 10.1128/MCB.16.11.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Padmanabhan B, Tong KI, Ohta T, Nakamura Y, Scharlock M, Ohtsuji M, Kang MI, Kobayashi A, Yokoyama S, Yamamoto M. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol Cell 21: 689–700, 2006. doi: 10.1016/j.molcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 171.Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, Krauth M, Ruiz S, Audhya P, Christ-Schmidt H, Wittes J, Warnock DG; BEAM Study Investigators . Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med 365: 327–336, 2011. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
- 172.Pitoniak A, Bohmann D. Mechanisms and functions of Nrf2 signaling in Drosophila. Free Radic Biol Med 88, Pt. B: 302–313, 2015. doi: 10.1016/j.freeradbiomed.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Prestera T, Zhang Y, Spencer SR, Wilczak CA, Talalay P. The electrophile counterattack response: protection against neoplasia and toxicity. Adv Enzyme Regul 33: 281–296, 1993. doi: 10.1016/0065-2571(93)90024-8. [DOI] [PubMed] [Google Scholar]
- 174.Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, Cuadrado A. SCF/beta-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol Cell Biol 31: 1121–1133, 2011. doi: 10.1128/MCB.01204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rada P, Rojo AI, Evrard-Todeschi N, Innamorato NG, Cotte A, Jaworski T, Tobón-Velasco JC, Devijver H, García-Mayoral MF, Van Leuven F, Hayes JD, Bertho G, Cuadrado A. Structural and functional characterization of Nrf2 degradation by the glycogen synthase kinase 3/β-TrCP axis. Mol Cell Biol 32: 3486–3499, 2012. doi: 10.1128/MCB.00180-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rajasekaran NS, Connell P, Christians ES, Yan LJ, Taylor RP, Orosz A, Zhang XQ, Stevenson TJ, Peshock RM, Leopold JA, Barry WH, Loscalzo J, Odelberg SJ, Benjamin IJ. Human alpha B-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell 130: 427–439, 2007. doi: 10.1016/j.cell.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rajasekaran NS, Varadharaj S, Khanderao GD, Davidson CJ, Kannan S, Firpo MA, Zweier JL, Benjamin IJ. Sustained activation of nuclear erythroid 2-related factor 2/antioxidant response element signaling promotes reductive stress in the human mutant protein aggregation cardiomyopathy in mice. Antioxid Redox Signal 14: 957–971, 2011. doi: 10.1089/ars.2010.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci USA 98: 3410–3415, 2001. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ramos-Gomez M, Dolan PM, Itoh K, Yamamoto M, Kensler TW. Interactive effects of nrf2 genotype and oltipraz on benzo[a]pyrene-DNA adducts and tumor yield in mice. Carcinogenesis 24: 461–467, 2003. doi: 10.1093/carcin/24.3.461. [DOI] [PubMed] [Google Scholar]
- 180.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest 114: 1248–1259, 2004. doi: 10.1172/JCI200421146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Reddy NM, Suryanaraya V, Yates MS, Kleeberger SR, Hassoun PM, Yamamoto M, Liby KT, Sporn MB, Kensler TW, Reddy SP. The triterpenoid CDDO-imidazolide confers potent protection against hyperoxic acute lung injury in mice. Am J Respir Crit Care Med 180: 867–874, 2009. doi: 10.1164/rccm.200905-0670OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Reddy NM, Potteti HR, Mariani TJ, Biswal S, Reddy SP. Conditional deletion of Nrf2 in airway epithelium exacerbates acute lung injury and impairs the resolution of inflammation. Am J Respir Cell Mol Biol 45: 1161–1168, 2011. doi: 10.1165/rcmb.2011-0144OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Reisman SA, Buckley DB, Tanaka Y, Klaassen CD. CDDO-Im protects from acetaminophen hepatotoxicity through induction of Nrf2-dependent genes. Toxicol Appl Pharmacol 236: 109–114, 2009. doi: 10.1016/j.taap.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Reisman SA, Lee CY, Meyer CJ, Proksch JW, Sonis ST, Ward KW. Topical application of the synthetic triterpenoid RTA 408 protects mice from radiation-induced dermatitis. Radiat Res 181: 512–520, 2014. doi: 10.1667/RR13578.1. [DOI] [PubMed] [Google Scholar]
- 185.Ren D, Villeneuve NF, Jiang T, Wu T, Lau A, Toppin HA, Zhang DD. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc Natl Acad Sci USA 108: 1433–1438, 2011. doi: 10.1073/pnas.1014275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Richardson BG, Jain AD, Speltz TE, Moore TW. Non-electrophilic modulators of the canonical Keap1/Nrf2 pathway. Bioorg Med Chem Lett 25: 2261–2268, 2015. doi: 10.1016/j.bmcl.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Riley BE, Kaiser SE, Shaler TA, Ng AC, Hara T, Hipp MS, Lage K, Xavier RJ, Ryu KY, Taguchi K, Yamamoto M, Tanaka K, Mizushima N, Komatsu M, Kopito RR. Ubiquitin accumulation in autophagy-deficient mice is dependent on the Nrf2-mediated stress response pathway: a potential role for protein aggregation in autophagic substrate selection. J Cell Biol 191: 537–552, 2010. doi: 10.1083/jcb.201005012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Romero R, Sayin VI, Davidson SM, Bauer MR, Singh SX, LeBoeuf SE, Karakousi TR, Ellis DC, Bhutkar A, Sánchez-Rivera FJ, Subbaraj L, Martinez B, Bronson RT, Prigge JR, Schmidt EE, Thomas CJ, Goparaju C, Davies A, Dolgalev I, Heguy A, Allaj V, Poirier JT, Moreira AL, Rudin CM, Pass HI, Vander Heiden MG, Jacks T, Papagiannakopoulos T. Keap1 loss promotes Kras-driven lung cancer and results in dependence on glutaminolysis. Nat Med 23: 1362–1368, 2017. doi: 10.1038/nm.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rushmore TH, Pickett CB. Transcriptional regulation of the rat glutathione S-transferase Ya subunit gene. Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J Biol Chem 265: 14648–14653, 1990. [PubMed] [Google Scholar]
- 190.Rushmore TH, Morton MR, Pickett CB. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem 266: 11632–11639, 1991. [PubMed] [Google Scholar]
- 191.Saito R, Suzuki T, Hiramoto K, Asami S, Naganuma E, Suda H, Iso T, Yamamoto H, Morita M, Baird L, Furusawa Y, Negishi T, Ichinose M, Yamamoto M. Characterizations of three major cysteine sensors of Keap1 in stress response. Mol Cell Biol 36: 271–284, 2015. doi: 10.1128/MCB.00868-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Salazar M, Rojo AI, Velasco D, de Sagarra RM, Cuadrado A. Glycogen synthase kinase-3beta inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2. J Biol Chem 281: 14841–14851, 2006. doi: 10.1074/jbc.M513737200. [DOI] [PubMed] [Google Scholar]
- 193.Satoh H, Moriguchi T, Taguchi K, Takai J, Maher JM, Suzuki T, Winnard PT Jr, Raman V, Ebina M, Nukiwa T, Yamamoto M. Nrf2-deficiency creates a responsive microenvironment for metastasis to the lung. Carcinogenesis 31: 1833–1843, 2010. doi: 10.1093/carcin/bgq105. [DOI] [PubMed] [Google Scholar]
- 194.Satoh H, Moriguchi T, Takai J, Ebina M, Yamamoto M. Nrf2 prevents initiation but accelerates progression through the Kras signaling pathway during lung carcinogenesis. Cancer Res 73: 4158–4168, 2013. doi: 10.1158/0008-5472.CAN-12-4499. [DOI] [PubMed] [Google Scholar]
- 195.Satoh H, Moriguchi T, Saigusa D, Baird L, Yu L, Rokutan H, Igarashi K, Ebina M, Shibata T, Yamamoto M. NRF2 intensifies host defense systems to prevent lung carcinogenesis, but after tumor initiation accelerates malignant cell growth. Cancer Res 76: 3088–3096, 2016. doi: 10.1158/0008-5472.CAN-15-1584. [DOI] [PubMed] [Google Scholar]
- 196.Satoh T, Okamoto SI, Cui J, Watanabe Y, Furuta K, Suzuki M, Tohyama K, Lipton SA. Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophillic phase II inducers. Proc Natl Acad Sci USA 103: 768–773, 2006. doi: 10.1073/pnas.0505723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sekine H, Okazaki K, Ota N, Shima H, Katoh Y, Suzuki N, Igarashi K, Ito M, Motohashi H, Yamamoto M. The Mediator Subunit MED16 Transduces NRF2-Activating Signals into Antioxidant Gene Expression. Mol Cell Biol 36: 407–420, 2016. doi: 10.1128/MCB.00785-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Shah ZA, Li RC, Thimmulappa RK, Kensler TW, Yamamoto M, Biswal S, Doré S. Role of reactive oxygen species in modulation of Nrf2 following ischemic reperfusion injury. Neuroscience 147: 53–59, 2007. doi: 10.1016/j.neuroscience.2007.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Shavit JA, Motohashi H, Onodera K, Akasaka J, Yamamoto M, Engel JD. Impaired megakaryopoiesis and behavioral defects in mafG-null mutant mice. Genes Dev 12: 2164–2174, 1998. doi: 10.1101/gad.12.14.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shibata T, Kokubu A, Gotoh M, Ojima H, Ohta T, Yamamoto M, Hirohashi S. Genetic alteration of Keap1 confers constitutive Nrf2 activation and resistance to chemotherapy in gallbladder cancer. Gastroenterology 135: 1358–1368.e4, 2008. doi: 10.1053/j.gastro.2008.06.082. [DOI] [PubMed] [Google Scholar]
- 201.Shibata T, Ohta T, Tong KI, Kokubu A, Odogawa R, Tsuta K, Asamura H, Yamamoto M, Hirohashi S. Cancer related mutations in NRF2 impair its recognition by Keap1-Cul3 E3 ligase and promote malignancy. Proc Natl Acad Sci USA 105: 13568–13573, 2008. [Erratum. Proc Natl Acad Sci USA 106: 10393, 2009.] doi: 10.1073/pnas.0806268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Shibata T, Kokubu A, Saito S, Narisawa-Saito M, Sasaki H, Aoyagi K, Yoshimatsu Y, Tachimori Y, Kushima R, Kiyono T, Yamamoto M. NRF2 mutation confers malignant potential and resistance to chemoradiation therapy in advanced esophageal squamous cancer. Neoplasia 13: 864–873, 2011. doi: 10.1593/neo.11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Shin S, Wakabayashi J, Yates MS, Wakabayashi N, Dolan PM, Aja S, Liby KT, Sporn MB, Yamamoto M, Kensler TW. Role of Nrf2 in prevention of high-fat diet-induced obesity by synthetic triterpenoid CDDO-imidazolide. Eur J Pharmacol 620: 138–144, 2009. doi: 10.1016/j.ejphar.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Shirasaki K, Taguchi K, Unno M, Motohashi H, Yamamoto M. NF-E2-related factor 2 promotes compensatory liver hypertrophy after portal vein branch ligation in mice. Hepatology 59: 2371–2382, 2014. doi: 10.1002/hep.27020. [DOI] [PubMed] [Google Scholar]
- 205.Shivdasani RA, Rosenblatt MF, Zucker-Franklin D, Jackson CW, Hunt P, Saris CJ, Orkin SH. Transcription factor NF-E2 is required for platelet formation independent of the actions of thrombopoietin/MGDF in megakaryocyte development. Cell 81: 695–704, 1995. doi: 10.1016/0092-8674(95)90531-6. [DOI] [PubMed] [Google Scholar]
- 206.Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E, Brock MV, Biswal S. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med 3: e420, 2006. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Singh A, Boldin-Adamsky S, Thimmulappa RK, Rath SK, Ashush H, Coulter J, Blackford A, Goodman SN, Bunz F, Watson WH, Gabrielson E, Feinstein E, Biswal S. RNAi-mediated silencing of nuclear factor erythroid-2-related factor 2 gene expression in non-small cell lung cancer inhibits tumor growth and increases efficacy of chemotherapy. Cancer Res 68: 7975–7984, 2008. doi: 10.1158/0008-5472.CAN-08-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Singh A, Happel C, Manna SK, Acquaah-Mensah G, Carrerero J, Kumar S, Nasipuri P, Krausz KW, Wakabayashi N, Dewi R, Boros LG, Gonzalez FJ, Gabrielson E, Wong KK, Girnun G, Biswal S. Transcription factor NRF2 regulates miR-1 and miR-206 to drive tumorigenesis. J Clin Invest 123: 2921–2934, 2013. doi: 10.1172/JCI66353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Singh A, Venkannagari S, Oh KH, Zhang YQ, Rohde JM, Liu L, Nimmagadda S, Sudini K, Brimacombe KR, Gajghate S, Ma J, Wang A, Xu X, Shahane SA, Xia M, Woo J, Mensah GA, Wang Z, Ferrer M, Gabrielson E, Li Z, Rastinejad F, Shen M, Boxer MB, Biswal S. Small molecule inhibitor of NRF2 selectively intervenes therapeutic resistance in KEAP1-deficient NSCLC tumors. ACS Chem Biol 11: 3214–3225, 2016. doi: 10.1021/acschembio.6b00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Singh K, Connors SL, Macklin EA, Smith KD, Fahey JW, Talalay P, Zimmerman AW. Sulforaphane treatment of autism spectrum disorder (ASD). Proc Natl Acad Sci USA 111: 15550–15555, 2014. doi: 10.1073/pnas.1416940111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Slocum SL, Kensler TW. Nrf2: control of sensitivity to carcinogens. Arch Toxicol 85: 273–284, 2011. doi: 10.1007/s00204-011-0675-4. [DOI] [PubMed] [Google Scholar]
- 212.Song MY, Kim EK, Moon WS, Park JW, Kim HJ, So HS, Park R, Kwon KB, Park BH. Sulforaphane protects against cytokine- and streptozotocin-induced beta-cell damage by suppressing the NF-kappaB pathway. Toxicol Appl Pharmacol 235: 57–67, 2009. doi: 10.1016/j.taap.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 213.Sporn MB, Liby KT. NRF2 and cancer: the good, the bad and the importance of context. Nat Rev Cancer 12: 564–571, 2012. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Stack C, Ho D, Wille E, Calingasan NY, Williams C, Liby K, Sporn M, Dumont M, Beal MF. Triterpenoids CDDO-ethyl amide and CDDO-trifluoroethyl amide improve the behavioral phenotype and brain pathology in a transgenic mouse model of Huntington’s disease. Free Radic Biol Med 49: 147–158, 2010. doi: 10.1016/j.freeradbiomed.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Strnad P, Zatloukal K, Stumptner C, Kulaksiz H, Denk H. Mallory-Denk-bodies: lessons from keratin-containing hepatic inclusion bodies. Biochim Biophys Acta 1782: 764–774, 2008. doi: 10.1016/j.bbadis.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 216.Sussan TE, Rangasamy T, Blake DJ, Malhotra D, El-Haddad H, Bedja D, Yates MS, Kombairaju P, Yamamoto M, Liby KT, Sporn MB, Gabrielson KL, Champion HC, Tuder RM, Kensler TW, Biswal S. Targeting Nrf2 with the triterpenoid CDDO-imidazolide attenuates cigarette smoke-induced emphysema and cardiac dysfunction in mice. Proc Natl Acad Sci USA 106: 250–255, 2009. doi: 10.1073/pnas.0804333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Sussan TE, Gajghate S, Chatterjee S, Mandke P, McCormick S, Sudini K, Kumar S, Breysse PN, Diette GB, Sidhaye VK, Biswal S. Nrf2 reduces allergic asthma in mice through enhanced airway epithelial cytoprotective function. Am J Physiol Lung Cell Mol Physiol 309: L27–L36, 2015. doi: 10.1152/ajplung.00398.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Suzuki T, Maher J, Yamamoto M. Select heterozygous Keap1 mutations have a dominant-negative effect on wild-type Keap1 in vivo. Cancer Res 71: 1700–1709, 2011. doi: 10.1158/0008-5472.CAN-10-2939. [DOI] [PubMed] [Google Scholar]
- 219.Suzuki T, Shibata T, Takaya K, Shiraishi K, Kohno T, Kunitoh H, Tsuta K, Furuta K, Goto K, Hosoda F, Sakamoto H, Motohashi H, Yamamoto M. Regulatory nexus of synthesis and degradation deciphers cellular Nrf2 expression levels. Mol Cell Biol 33: 2402–2412, 2013. doi: 10.1128/MCB.00065-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Suzuki T, Murakami S, Biswal SS, Sakaguchi S, Harigae H, Yamamoto M, Motohashi H. Systemic activation of NRF2 alleviates lethal autoimmune inflammation in scurfy mice. Mol Cell Biol 37: e00063-17, 2017. doi: 10.1128/MCB.00063-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sykiotis GP, Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell 14: 76–85, 2008. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Taguchi K, Maher JM, Suzuki T, Kawatani Y, Motohashi H, Yamamoto M. Genetic analysis of cytoprotective functions supported by graded expression of Keap1. Mol Cell Biol 30: 3016–3026, 2010. doi: 10.1128/MCB.01591-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Taguchi K, Fujikawa N, Komatsu M, Ishii T, Unno M, Akaike T, Motohashi H, Yamamoto M. Keap1 degradation by autophagy for the maintenance of redox homeostasis. Proc Natl Acad Sci USA 109: 13561–13566, 2012. doi: 10.1073/pnas.1121572109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Taguchi K, Hirano I, Itoh T, Tanaka M, Miyajima A, Suzuki A, Motohashi H, Yamamoto M. Nrf2 enhances cholangiocyte expansion in Pten-deficient livers. Mol Cell Biol 34: 900–913, 2014. doi: 10.1128/MCB.01384-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Taguchi K, Takaku M, Egner PA, Morita M, Kaneko T, Mashimo T, Kensler TW, Yamamoto M. Generation of a new model rat: Nrf2 knockout rats are sensitive to aflatoxin B1 toxicity. Toxicol Sci 152: 40–52, 2016. doi: 10.1093/toxsci/kfw065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Taguchi K, Yamamoto M. The KEAP1–NRF2 system in cancer. Front Oncol 7: 85, 2017. doi: 10.3389/fonc.2017.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Takaya K, Suzuki T, Motohashi H, Onodera K, Satomi S, Kensler TW, Yamamoto M. Validation of the multiple sensor mechanism of the Keap1-Nrf2 system. Free Radic Biol Med 53: 817–827, 2012. doi: 10.1016/j.freeradbiomed.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Takayama M, Fujita R, Suzuki M, Okuyama R, Aiba S, Motohashi H, Yamamoto M. Genetic analysis of hierarchical regulation for Gata1 and NF-E2 p45 gene expression in megakaryopoiesis. Mol Cell Biol 30: 2668–2680, 2010. doi: 10.1128/MCB.01304-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Talalay P, De Long MJ, Prochaska HJ. Identification of a common chemical signal regulating the induction of enzymes that protect against chemical carcinogenesis. Proc Natl Acad Sci USA 85: 8261–8265, 1988. doi: 10.1073/pnas.85.21.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Thimmulappa RK, Scollick C, Traore K, Yates M, Trush MA, Liby KT, Sporn MB, Yamamoto M, Kensler TW, Biswal S. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem Biophys Res Commun 351: 883–889, 2006. doi: 10.1016/j.bbrc.2006.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.To C, Ringelberg CS, Royce DB, Williams CR, Risingsong R, Sporn MB, Liby KT. Dimethyl fumarate and the oleanane triterpenoids, CDDO-imidazolide and CDDO-methyl ester, both activate the Nrf2 pathway but have opposite effects in the A/J model of lung carcinogenesis. Carcinogenesis 36: 769–781, 2015. doi: 10.1093/carcin/bgv061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Tomlinson IP, Alam NA, Rowan AJ, Barclay E, Jaeger EE, Kelsell D, Leigh I, Gorman P, Lamlum H, Rahman S, Roylance RR, Olpin S, Bevan S, Barker K, Hearle N, Houlston RS, Kiuru M, Lehtonen R, Karhu A, Vilkki S, Laiho P, Eklund C, Vierimaa O, Aittomäki K, Hietala M, Sistonen P, Paetau A, Salovaara R, Herva R, Launonen V, Aaltonen LA; Multiple Leiomyoma Consortium . Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet 30: 406–410, 2002. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 233.Tong KI, Katoh Y, Kusunoki H, Itoh K, Tanaka T, Yamamoto M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model. Mol Cell Biol 26: 2887–2900, 2006. doi: 10.1128/MCB.26.8.2887-2900.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Tong KI, Padmanabhan B, Kobayashi A, Shang C, Hirotsu Y, Yokoyama S, Yamamoto M. Different electrostatic potentials define ETGE and DLG motifs as hinge and latch in oxidative stress response. Mol Cell Biol 27: 7511–7521, 2007. doi: 10.1128/MCB.00753-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Tsuchida K, Tsujita T, Hayashi M, Ojima A, Keleku-Lukwete N, Katsuoka F, Otsuki A, Kikuchi H, Oshima Y, Suzuki M, Yamamoto M. Halofuginone enhances the chemo-sensitivity of cancer cells by suppressing NRF2 accumulation. Free Radic Biol Med 103: 236–247, 2017. doi: 10.1016/j.freeradbiomed.2016.12.041. [DOI] [PubMed] [Google Scholar]
- 236.Tullet JM, Hertweck M, An JH, Baker J, Hwang JY, Liu S, Oliveira RP, Baumeister R, Blackwell TK. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell 132: 1025–1038, 2008. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Uruno A, Furusawa Y, Yagishita Y, Fukutomi T, Muramatsu H, Negishi T, Sugawara A, Kensler TW, Yamamoto M. The Keap1-Nrf2 system prevents onset of diabetes mellitus. Mol Cell Biol 33: 2996–3010, 2013. doi: 10.1128/MCB.00225-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Uruno A, Yagishita Y, Katsuoka F, Kitajima Y, Nunomiya A, Nagatomi R, Pi J, Biswal SS, Yamamoto M. Nrf2-mediated regulation of skeletal muscle glycogen metabolism. Mol Cell Biol 36: 1655–1672, 2016. doi: 10.1128/MCB.01095-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Vartanian S, Ma TP, Lee J, Haverty PM, Kirkpatrick DS, Yu K, Stokoe D. Application of mass spectrometry profiling to establish Brusatol as an inhibitor of global protein synthesis. Mol Cell Proteomics 15: 1220–1231, 2016. doi: 10.1074/mcp.M115.055509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wakabayashi N, Itoh K, Wakabayashi J, Motohashi H, Noda S, Takahashi S, Imakado S, Kotsuji T, Otsuka F, Roop DR, Harada T, Engel JD, Yamamoto M. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet 35: 238–245, 2003. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- 241.Wakabayashi N, Shin S, Slocum SL, Agoston ES, Wakabayashi J, Kwak MK, Misra V, Biswal S, Yamamoto M, Kensler TW. Regulation of notch1 signaling by nrf2: implications for tissue regeneration. Sci Signal 3: ra52, 2010. doi: 10.1126/scisignal.2000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wakabayashi N, Skoko JJ, Chartoumpekis DV, Kimura S, Slocum SL, Noda K, Palliyaguru DL, Fujimuro M, Boley PA, Tanaka Y, Shigemura N, Biswal S, Yamamoto M, Kensler TW. Notch-Nrf2 axis: regulation of Nrf2 gene expression and cytoprotection by notch signaling. Mol Cell Biol 34: 653–663, 2014. doi: 10.1128/MCB.01408-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wang J, Fields J, Zhao C, Langer J, Thimmulappa RK, Kensler TW, Yamamoto M, Biswal S, Doré S. Role of Nrf2 in protection against intracerebral hemorrhage injury in mice. Free Radic Biol Med 43: 408–414, 2007. doi: 10.1016/j.freeradbiomed.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wang J, Zhang M, Zhang L, Cai H, Zhou S, Zhang J, Wang Y. Correlation of Nrf2, HO-1, and MRP3 in gallbladder cancer and their relationships to clinicopathologic features and survival. J Surg Res 164: e99–e105, 2010. doi: 10.1016/j.jss.2010.05.058. [DOI] [PubMed] [Google Scholar]
- 245.Wang H, Liu X, Long M, Huang Y, Zhang L, Zhang R, Zheng Y, Liao X, Wang Y, Liao Q, Li W, Tang Z, Tong Q, Wang X, Fang F, Rojo de la Vega M, Ouyang Q, Zhang DD, Yu S, Zheng H. NRF2 activation by antioxidant antidiabetic agents accelerates tumor metastasis. Sci Transl Med 8: 334ra51, 2016. doi: 10.1126/scitranslmed.aad6095. [DOI] [PubMed] [Google Scholar]
- 246.Wang W, Li S, Wang H, Li B, Shao L, Lai Y, Horvath G, Wang Q, Yamamoto M, Janicki JS, Wang XL, Tang D, Cui T. Nrf2 enhances myocardial clearance of toxic ubiquitinated proteins. J Mol Cell Cardiol 72: 305–315, 2014. doi: 10.1016/j.yjmcc.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wang X, Campbell MR, Lacher SE, Cho HY, Wan M, Crowl CL, Chorley BN, Bond GL, Kleeberger SR, Slattery M, Bell DA. A polymorphic antioxidant response element links NRF2/sMAF binding to enhanced MAPT expression and reduced risk of Parkinsonian disorders. Cell Reports 15: 830–842, 2016. doi: 10.1016/j.celrep.2016.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Watai Y, Kobayashi A, Nagase H, Mizukami M, McEvoy J, Singer JD, Itoh K, Yamamoto M. Subcellular localization and cytoplasmic complex status of endogenous Keap1. Genes Cells 12: 1163–1178, 2007. doi: 10.1111/j.1365-2443.2007.01118.x. [DOI] [PubMed] [Google Scholar]
- 249.Wattenberg LW. Inhibition of carcinogenic and toxic effects of polycyclic hydrocarbons by phenolic antioxidants and ethoxyquin. J Natl Cancer Inst 48: 1425–1430, 1972. [PubMed] [Google Scholar]
- 250.Wattenberg LW. Protective effects of 2(3)-tert-butyl-4-hydroxyanisole on chemical carcinogenesis. Food Chem Toxicol 24: 1099–1102, 1986. doi: 10.1016/0278-6915(86)90294-2. [DOI] [PubMed] [Google Scholar]
- 251.Wu QQ, Wang Y, Senitko M, Meyer C, Wigley WC, Ferguson DA, Grossman E, Chen J, Zhou XJ, Hartono J, Winterberg P, Chen B, Agarwal A, Lu CY. Bardoxolone methyl (BARD) ameliorates ischemic AKI and increases expression of protective genes Nrf2, PPARγ, and HO-1. Am J Physiol Renal Physiol 300: F1180–F1192, 2011. doi: 10.1152/ajprenal.00353.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Xing Y, Niu T, Wang W, Li J, Li S, Janicki JS, Ruiz S, Meyer CJ, Wang XL, Tang D, Zhao Y, Cui T. Triterpenoid dihydro-CDDO-trifluoroethyl amide protects against maladaptive cardiac remodeling and dysfunction in mice: a critical role of Nrf2. PLoS One 7: e44899, 2012. doi: 10.1371/journal.pone.0044899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Xu Z, Wei Y, Gong J, Cho H, Park JK, Sung ER, Huang H, Wu L, Eberhart C, Handa JT, Du Y, Kern TS, Thimmulappa R, Barber AJ, Biswal S, Duh EJ. NRF2 plays a protective role in diabetic retinopathy in mice. Diabetologia 57: 204–213, 2014. doi: 10.1007/s00125-013-3093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Xue P, Hou Y, Chen Y, Yang B, Fu J, Zheng H, Yarborough K, Woods CG, Liu D, Yamamoto M, Zhang Q, Andersen ME, Pi J. Adipose deficiency of Nrf2 in ob/ob mice results in severe metabolic syndrome. Diabetes 62: 845–854, 2013. doi: 10.2337/db12-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Yagishita Y, Fukutomi T, Sugawara A, Kawamura H, Takahashi T, Pi J, Uruno A, Yamamoto M. Nrf2 protects pancreatic β-cells from oxidative and nitrosative stress in diabetic model mice. Diabetes 63: 605–618, 2014. doi: 10.2337/db13-0909. [DOI] [PubMed] [Google Scholar]
- 256.Yagishita Y, Uruno A, Fukutomi T, Saito R, Saigusa D, Pi J, Fukamizu A, Sugiyama F, Takahashi S, Yamamoto M. Nrf2 improves leptin and insulin resistance provoked by hypothalamic oxidative stress. Cell Reports 18: 2030–2044, 2017. doi: 10.1016/j.celrep.2017.01.064. [DOI] [PubMed] [Google Scholar]
- 257.Yamagiwa K, Ichikawa K. On the experimental production of papillomas. Verh Jpn Pathol Ges 5: 142–148, 1915. [Google Scholar]
- 258.Yamamoto T, Yoh K, Kobayashi A, Ishii Y, Kure S, Koyama A, Sakamoto T, Sekizawa K, Motohashi H, Yamamoto M. Identification of polymorphisms in the promoter region of the human NRF2 gene. Biochem Biophys Res Commun 321: 72–79, 2004. doi: 10.1016/j.bbrc.2004.06.112. [DOI] [PubMed] [Google Scholar]
- 259.Yamamoto T, Kyo M, Kamiya T, Tanaka T, Engel JD, Motohashi H, Yamamoto M. Predictive base substitution rules that determine the binding and transcriptional specificity of Maf recognition elements. Genes Cells 11: 575–591, 2006. doi: 10.1111/j.1365-2443.2006.00965.x. [DOI] [PubMed] [Google Scholar]
- 260.Yamamoto T, Suzuki T, Kobayashi A, Wakabayashi J, Maher J, Motohashi H, Yamamoto M. Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Mol Cell Biol 28: 2758–2770, 2008. doi: 10.1128/MCB.01704-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Yamazaki H, Katsuoka F, Motohashi H, Engel JD, Yamamoto M. Embryonic lethality and fetal liver apoptosis in mice lacking all three small Maf proteins. Mol Cell Biol 32: 808–816, 2012. doi: 10.1128/MCB.06543-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Yanaka A, Fahey JW, Fukumoto A, Nakayama M, Inoue S, Zhang S, Tauchi M, Suzuki H, Hyodo I, Yamamoto M. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori-infected mice and humans. Cancer Prev Res (Phila) 2: 353–360, 2009. doi: 10.1158/1940-6207.CAPR-08-0192. [DOI] [PubMed] [Google Scholar]
- 263.Yates MS, Kwak MK, Egner PA, Groopman JD, Bodreddigari S, Sutter TR, Baumgartner KJ, Roebuck BD, Liby KT, Yore MM, Honda T, Gribble GW, Sporn MB, Kensler TW. Potent protection against aflatoxin-induced tumorigenesis through induction of Nrf2-regulated pathways by the triterpenoid 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole. Cancer Res 66: 2488–2494, 2006. doi: 10.1158/0008-5472.CAN-05-3823. [DOI] [PubMed] [Google Scholar]
- 264.Yates MS, Tauchi M, Katsuoka F, Flanders KC, Liby KT, Honda T, Gribble GW, Johnson DA, Johnson JA, Burton NC, Guilarte TR, Yamamoto M, Sporn MB, Kensler TW. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Mol Cancer Ther 6: 154–162, 2007. doi: 10.1158/1535-7163.MCT-06-0516. [DOI] [PubMed] [Google Scholar]
- 265.Zhang DD, Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol 23: 8137–8151, 2003. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol 24: 10941–10953, 2004. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Zhang J, Hosoya T, Maruyama A, Nishikawa K, Maher JM, Ohta T, Motohashi H, Fukamizu A, Shibahara S, Itoh K, Yamamoto M. Nrf2 Neh5 domain is differentially utilized in the transactivation of cytoprotective genes. Biochem J 404: 459–466, 2007. doi: 10.1042/BJ20061611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Zhang P, Singh A, Yegnasubramanian S, Esopi D, Kombairaju P, Bodas M, Wu H, Bova SG, Biswal S. Loss of Kelch-like ECH-associated protein 1 function in prostate cancer cells causes chemoresistance and radioresistance and promotes tumor growth. Mol Cancer Ther 9: 336–346, 2010. doi: 10.1158/1535-7163.MCT-09-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Ziaei A, Schmedt T, Chen Y, Jurkunas UV. Sulforaphane decreases endothelial cell apoptosis in fuchs endothelial corneal dystrophy: a novel treatment. Invest Ophthalmol Vis Sci 54: 6724–6734, 2013. doi: 10.1167/iovs.13-12699. [DOI] [PMC free article] [PubMed] [Google Scholar]