Abstract
The ability to change from yeast to hyphal morphology is a major virulence determinant of Candida albicans. Mutants with defined defects in filamentation regulatory pathways have reduced virulence in mice. However, is it poorly understood why hyphal formation is critical for C. albicans to cause hematogenously disseminated infections. We used recently constructed mutants to examine the role of hyphal formation in the interactions of C. albicans with endothelial cells in vitro. These interactions included the ability of the mutants to invade and injure endothelial cells. Because the formation of hyphae may influence the host inflammatory response to C. albicans, we also investigated the capacity of these mutants to stimulate endothelial cells to express E-selectin and intercellular adhesion molecule 1. We infected endothelial cells with C. albicans strains containing homozygous null mutations in the following filamentation regulatory genes: CLA4, CPH1, EFG1, and TUP1. Whereas the wild-type strain formed true hyphae on endothelial cells, we found that neither the Δefg1 nor the Δcph1 Δefg1 double mutant germinated. The Δtup1 mutant formed only pseudohyphae. We also found that the Δefg1, Δcph1 Δefg1, and Δtup1 mutants had significantly reduced capacities to invade and injure endothelial cells. Therefore, Efg1p and Tup1p contribute to virulence by regulating hyphal formation and the factors that enable C. albicans to invade and injure endothelial cells. With the exception of the Δcph1 Δefg1 mutant, all other mutants stimulated endothelial cells to express at least one of the leukocyte adhesion molecules. Therefore, the combined activities of Cph1p and Efg1p are required for C. albicans to stimulate a proinflammatory response in endothelial cells.
Candida species are the fourth most common cause of nosocomial bloodstream infections (5). In humans with hematogenously disseminated candidiasis, it has been found that the microabscesses contain both blastospores and hyphae (6). This observation has prompted investigations into the role of hyphal formation during the development of candidal infections. Over the past several decades it has been recognized that the transition from yeast to hyphal morphology is a major virulence factor of Candida albicans.
Because the transition from a yeast to a hyphal morphology is important for candidal virulence, the signal transduction pathways that regulate this transition have been the subject of intense investigation. Multiple different pathways that regulate this process have been identified (reviewed in reference 2). These pathways include the mitogen-activated protein kinase (MAPK) and the cyclic AMP (cAMP)-protein kinase A (PKA) pathway. Tup1p is a general transcriptional repressor that may act on the MAPK pathway, and it is currently unknown if it acts on the cAMP-PKA pathway.
In C. albicans, the MAPK pathway terminates in the transcription factor Cph1p (23, 24). Homozygous cph1 mutants exhibit reduced filamentation on some solid media, yet they retain normal virulence in the murine model of hematogenously disseminated infection (Table 1). Disruption of the genes encoding most proteins in the MAPK pathway that are upstream of Cph1p results in similar defects in hyphal formation in vitro (3, 18, 19). Strains carrying some of these mutations have decreased virulence in mice; however, none of these mutants have been shown to have diminished hyphal formation in vivo (3, 19). Thus, the MAPK pathway likely regulates other virulence factors in addition to hyphal formation. Although Cla4p is almost certainly a MAPK, deletion of CLA4 results in a unique phenotype. Unlike other MAPK pathway mutants, homozygous cla4 mutants form aberrantly shaped cells that produce only short germ tubes in both liquid and solid media (Table 1). Also, these mutants have reduced virulence in mice (20). Therefore, Cla4p may regulate hyphal formation by a pathway that is independent of Cph1p.
TABLE 1.
Strains of C. albicans used in these experiments
| Strain | Genotype | In vitro phenotype | Virulence in mice | Source | Reference |
|---|---|---|---|---|---|
| SC5314 | Wild type | Forms hyphae on liquid and solid media | High | W. A. Fonzi | 11 |
| CLJ1 | ura3::1imm434/ura3::1imm434 cla4::hisG/ cla4::hisG-URA3-hisG | Forms short, aberrantly shaped hyphae on liquid and solid media | Low | M. Whiteway | 20 |
| CAN16 | ura3::1imm434/ura3::1imm434 cph1::hisG/cph1::hisG-URA3-hisG | Does not germinate on some solid media | High | G. R. Fink | 23 |
| CAN33 | ura3::1imm434/ura3::1imm434 efg1::hisG/ egf1::hisG-URA3-hisG | Forms pseudohyphae on solid media, does not germinate on liquid media | Low | G. R. Fink | 24 |
| CAN37 | ura3::1imm434/ura3::1imm434 efg1::hisG/ efg1::hisG EFG1 URA3 | Similar to that of SC5314 | Not reported | G. R. Fink | 24 |
| CAN34 | ura3::1imm434/ura3::1imm434 cph1::hisG/cph1::hisG efg1::hisG/efg1::hisG-URA3-hisG | Does not germinate on liquid or solid media | Very low | G. R. Fink | 24 |
| BCa2-10 | ura3::1imm434/ura3::1imm434 tup1::hisG/tup1::hisG-URA3-hisG | Grows as pseudohyphae on both liquid and solid media | Low | B. R. Braun | 1 |
| BCa2-11 | ura3::1imm434/ura3::1imm434 tup1::hisG/ tup1::hisG TUP1 URA3 | Similar to that of SC5314 | Not reported | B. R. Braun | 1 |
The cAMP-PKA pathway likely terminates in the transcription factor Efg1p. Homozygous efg1 mutants form only pseudohyphae on solid media and do not germinate at all in liquid media (Table 1) (24, 30). These mutants exhibit a moderate reduction in virulence in mice. Strains with the greatest defect in filamentation are those with homozygous deletions of both efg1 and cph1. These mutants form short rod-like cells and do not germinate under most conditions. They have very low virulence in mice (24).
Tup1p functions as a general transcriptional repressor and is required to maintain the organism as a blastospore. Disruption of both alleles of TUP1 results in cells that grow only as pseudohyphae, even under conditions that normally induce the organism to form blastospores (Table 1). A preliminary study indicates that homzygous tup1 mutants have decreased virulence in mice (1).
Although the signal transduction pathways that regulate the transition from yeast to hyphal morphology are clearly essential for full virulence in C. albicans, it is less well understood why this transition is critical for the development of a hematogenously disseminated infection. One hypothesis is that the formation of hyphae is necessary for the organism to invade and injure host cells. In addition, the transition from yeast to hyphal morphology may alter the host inflammatory response to the organism. To investigate these hypotheses, we used mutants with defined defects in the above-named filamentation regulatory pathways to examine the role of hyphal formation in the interactions of C. albicans with vascular endothelial cells in vitro. These interactions included the ability of different mutants to invade and injure endothelial cells. We also investigated the capacity of these mutants to stimulate a proinflammatory response in endothelial cells, as manifested by the expression of the leukocyte adhesion molecules E-selectin and intercellular adhesion molecule 1 (ICAM-1).
MATERIALS AND METHODS
Organisms.
The strains of C. albicans used in this study are listed in Table 1. All mutants were constructed in CAI4, which is a homozygous ura3 mutant of SC5314 (11). For use in the experiments, the organisms were grown overnight at 20°C on a rotating drum in yeast nitrogen base (YNB) broth (Difco Laboratories, Detroit, Mich.) supplemented with 2% glucose as described previously (8, 10). The organisms were collected by centrifugation, washed twice in Dulbecco's phosphate-buffered saline (PBS) (Irvine Scientific, Santa Anna, Calif.), and enumerated using a hemacytometer.
In some experiments, blastospores of C. albicans SC5314 were pregerminated by incubation in RPMI 1640 medium (Irvine Scientific) for 90 min in a shaking incubator at 37°C (9). Greater than 90% of the organisms germinated under these conditions. To kill either blastospores or the germinated cells, the organisms were incubated in methanol for 2 min and then washed extensively in PBS prior to being added to the endothelial cells.
Endothelial cells.
The endothelial cells were harvested from human umbilical cord veins by the method of Jaffe et al. (16). They were cultured in M-199 (Gibco, Grand Island, N.Y.) containing 10% fetal bovine serum (Gemini Bio-Products, Inc., Calabasas, Calif.), 10% defined bovine calf serum (Hyclone, Logan, Utah), and 2 mM l-glutamine with penicillin and streptomycin (Irvine Scientific). The cells were grown to confluence on fibronectin (Collaborative Biomedical Products, Bedford, Mass.), either in multiwell tissue culture plates (Falcon, Lincoln Park, N.J.) or on 12-mm-diameter glass coverslips. All incubations were at 37°C in 5% CO2.
Endocytosis assay.
The number of organisms internalized by the endothelial cells was determined using a differential fluorescence assay (12, 13). Briefly, endothelial cells on glass coverslips were infected with 105 cells of each strain of C. albicans in RPMI 1640 medium. After incubation for 3 h, the cells were fixed with 3% paraformaldehyde. The noninternalized cells were stained with anti-C. albicans rabbit serum (Biodesign International, Kennebunk, Maine) that had been conjugated with Alexa 594 (Molecular Probes, Eugene, Oreg.). Next, the endothelial cells were permeablized in 0.1% (vol/vol) Triton X-100 in PBS, after which both the internalized and the noninternalized organisms were stained with 1% (vol/vol) Uvitex (22). The coverslips were mounted inverted on a microscope slide and viewed under epifluorescence. The number of organisms endocytosed by the endothelial cells was determined by subtracting the number of noninternalized organisms (which fluoresced red) from the total number of organisms (which fluoresced blue). At least 100 organisms were counted on each coverslip, and all experiments were performed in triplicate on at least three separate occasions.
Measurement of endothelial cell injury.
The extents of endothelial cell injury caused by the different strains of C. albicans were measured by the release of 51Cr using a slight modification our standard assay (8, 10). Endothelial cells were grown to confluence in 96-well plates containing detachable wells. These cells were incubated overnight with 1 μCi of Na251CrO4 (ICN Biomedicals, Irvine, Calif.) per well. The following day, the unincorporated tracer was aspirated and the wells were rinsed twice with Hanks' balanced salt solution (Irvine Scientific). Next, the endothelial cells were infected with 4 × 104 C. albicans cells per well in 150 μl of RPMI 1640 medium. After a 3-h incubation, the upper 50% of medium was removed from each well and then the wells were manually detached from one another. The amounts of 51Cr in the aspirates and the wells were determined by gamma counting. To measure the spontaneous release of 51Cr, uninfected endothelial cells exposed to medium alone were processed in parallel. After we corrected for differences in the levels of incorporation of 51Cr in the wells, the specific release of 51Cr was calculated using the following formula: (2 × experimental release − 2 × spontaneous release)/(total incorporation − 2 × spontaneous release). Each experiment was performed in triplicate at least three different times.
Detection of leukocyte adhesion molecule expression.
The surface expression of E-selectin and ICAM-1 by endothelial cells infected with the different strains of C. albicans was determined by whole-cell enzyme-linked immunosorbent assay by the method of Noel et al. (25) as described previously (26). The endothelial cells were grown in 96-well tissue culture plates, and inocula of both 4 × 104 and 8 × 104 organisms per well were tested. Because the endothelial cells were exposed to the organisms for 8 h, the RPMI 1640 medium was supplemented with 1% pooled human serum (Gemini Bio-Products, Inc.) to maintain endothelial cell viability. E-selectin expression was detected using the monoclonal antibody clone 1.2B6 (Biodesign International), and ICAM-1 expression was measured using the monoclonal antibody clone 15.2 (Biodesign International). The experiments were performed in triplicate using endothelial cells from at least three different umbilical cords.
Statistical analysis.
Differences among the various strains of C. albicans were assessed using analysis of variance with the Bonferroni correction for multiple comparisons. P values of ≤0.05 were considered to be significant.
RESULTS
Different filamentation mutants had various morphologies after exposure to endothelial cells.
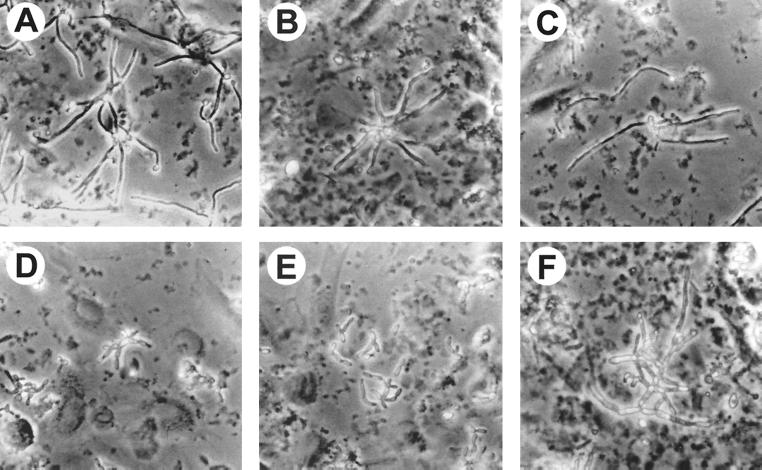
We first examined the morphologies of the different mutants after they had been in contact with the endothelial cells for 3 h in RPMI 1640 medium. All strains of C. albicans except for the Δtup1 mutant (which grows only as pseudohyphae) were added to the endothelial cells as blastospores. The wild-type strain, SC5314, germinated and produced long hyphae with almost no branches (Fig. 1A). The Δcla4 mutant also formed hyphae. However, these hyphae tended to clump together and were generally shorter than those of the parent strain, SC5314 (Fig. 1B). In addition, occasional bizarrely shaped cells were seen. Hyphae of the Δcph1 strain appeared to be identical to those of the wild-type parent (Fig. 1C). Both the Δefg1 mutant and the Δcph1 Δefg1 double mutant formed short chains of rod-like cells (Fig. 1D and E). Neither of these mutants produced germ tubes while they were in contact with the endothelial cells. Finally, the Δtup1 mutant grew as branching pseudohyphae, which in aggregate were much longer than the hyphae of the wild-type strain (Fig. 1F).
FIG. 1.
Photomicrographs of the different strains of C. albicans after growth on endothelial cells in RPMI 1640 medium for 3 h. (A) SC5314; (B) Δcla4 mutant; (C) Δcph1 mutant; (D) Δefg1 mutant; (E) Δcph1 Δefg1 mutant; (F) Δtup1 mutant. The original magnification of all photomicrographs was ×150.
Mutants with abnormal filamentation had reduced capacities to invade endothelial cells.
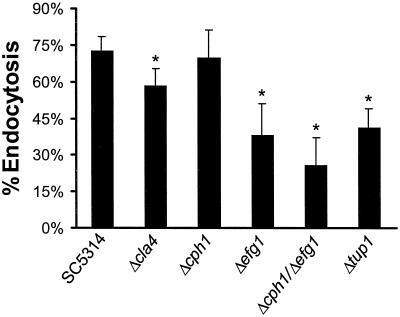
We have shown previously that the predominant method by which C. albicans invades endothelial cells in vitro is by inducing its own endocytosis (10). Therefore, the ability of the different strains to induce their own endocytosis was determined. In general, all mutants that had visible abnormalities in filamentation had at least some diminution in their ability to induce endocytosis (Fig. 2). The mutants with the greatest reduction were the Δefg1, Δcph1 Δefg1, and Δtup1 strains. Endothelial cell endocytosis of the Δcla4 mutant was only slightly reduced, and the endocytosis of the Δcph1 strain was not significantly different from that of strain SC5314. These findings suggest that factors regulated by Efg1p and Tup1p are important for C. albicans to induce its own endocytosis by endothelial cells.
FIG. 2.
Endothelial cell endocytosis of the different strains of C. albicans. Results are means ± standard deviations of results of at least three independent experiments, each performed in triplicate. ∗, P < 0.001 compared to values for SC5314.
Hyphal formation occurs via the protrusion of a germ tube from the blastospore. Because germ tube formation is the earliest phase in the transition from yeast to hyphal morphology, we investigated the link between germination and endocytosis. We germinated C. albicans SC5314 by incubating blastospores of this strain in RPMI 1640 medium at 37°C for 90 min. Next, we fixed the germ tubes in methanol to prevent them from progressing to hyphae. These germ tubes were approximately two blastospore diameters in length. We then investigated the uptake of these fixed germ tubes.
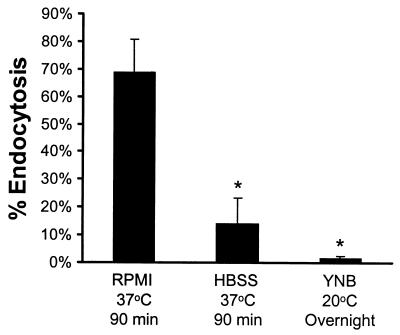
We found that almost 70% of the germinated organisms were taken up by the endothelial cells (Fig. 3). In contrast, there was very little endocytosis of blastospores of strain SC5314 that had been fixed after overnight growth in YNB broth at 20°C. We also examined the possibility that heat shock rather than germination caused the change(s) in the candidal cell wall that enabled the organism to induce its own endocytosis (17, 27). We incubated blastospores in Hanks' balanced salt solution at 37°C for 90 min prior to fixing them. These organisms did not germinate, and significantly fewer of them were endocytosed (Fig. 3). From these results, we conclude that the endocytosis of C. albicans can be induced by factors that are expressed as early as germ tube formation in the yeast-to-hypha transition.
FIG. 3.
Effects of germination on the endocytosis of methanol-fixed C. albicans. C. albicans cells were grown under the indicated conditions and then fixed in methanol prior to being added to the endothelial cells. Results are means ± standard deviations of results of three independent experiments. ∗, P < 0.0001 compared to values for organisms grown in RPMI 1640 medium at 37°C for 90 min. HBSS, Hanks' balanced salt solution.
The poorly endocytosed mutants had a reduced ability to injure endothelial cells.
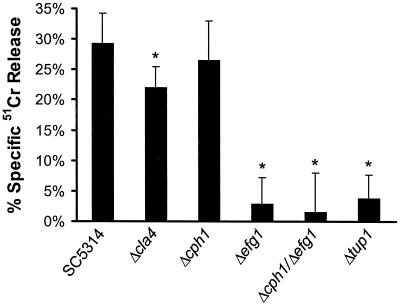
Next, the ability of the different mutants to injure endothelial cells was investigated. We found that the extent of endothelial cell injury caused by these strains paralleled the extent of endothelial cell endocytosis (Fig. 4). However, even though all of the mutants were endocytosed to some extent, the Δefg1, Δcph1 Δefg1, and Δtup1 strains caused virtually no damage to the endothelial cells. These findings suggest that the Efg1p and Tup1p signaling pathways may also regulate the synthesis and/or release of factors required for C. albicans to injure endothelial cells.
FIG. 4.
Amount of endothelial cell injury caused by the different strains of C. albicans. Results are means ± standard deviations of results of at least three separate experiments. ∗, P < 0.001 compared to values for SC5314.
To confirm that disruption of EFG1 and TUP1 was the cause of the decreased ability of the mutants to injure endothelial cells, we assessed the phenotypes of EFG1 and TUP1 revertant strains. These strains had been constructed by reintroducing a copy of the gene that had been disrupted back into the homozygous null mutant (Table 1). When added to the endothelial cells, both revertants produced hyphae that were indistinguishable from those of the wild-type strain, SC5314. In these experiments, the specific release of 51Cr induced by strain SC5314 was 23% ± 3%. The EFG1 revertant caused slightly less endothelial cell injury, and the TUP1 revertant induced slightly more injury than did strain SC5314 (specific releases of 51Cr, 17% ± 4% and 30% ± 4%, respectively; P < 0.001 versus the specific release for SC5314 for both comparisons).
Most mutants stimulated normal leukocyte adhesion molecule expression on endothelial cells.
Endothelial cells respond to contact with C. albicans by expressing leukocyte adhesion molecules and secreting proinflammatory cytokines (9, 26). These immunomodulatory factors have the potential to influence the host response to C. albicans. Because the magnitude of the host inflammatory response determines the outcome of a hematogenously disseminated candidal infection, we examined the ability of the different mutants to stimulate the endothelial cells to express E-selectin and ICAM-1.
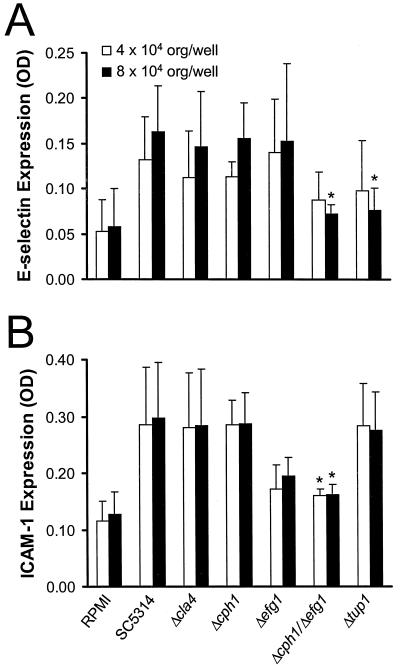
We found significant day-to-day variation in the absolute amount of E-selectin that was expressed by endothelial cells infected with the different strains. Despite this variation, the relative amounts of E-selectin expression induced by the different mutants were consistently observed. When the inoculum was 4 × 104 organisms per well, the amounts of E-selectin expression induced by the mutants were not significantly different from that induced by SC5314 (Fig. 5A). When the endothelial cells were infected with 8 × 104 organisms per well, both the Δcph1 Δefg1 and the Δtup1 mutant stimulated significantly less E-selectin expression than did SC5314.
FIG. 5.
Stimulation of endothelial cell expression of E-selectin (A) and ICAM-1 (B) by the different strains of C. albicans. Results are means ± standard deviations of results of at least three different experiments. ∗, P < 0.001 compared to values for SC5314. OD, optical density; org, organisms.
The only strain with a significantly decreased ability to stimulate ICAM-1 expression was the Δcph1 Δefg1 double mutant (Fig. 5B). There was a trend towards reduced ICAM-1 expression by the endothelial cells infected with the Δefg1 mutant, but this difference was not significant (P = 0.2). Interestingly, even though the Δtup1 mutant had a reduced capacity to stimulate E-selectin expression, its ability to induce ICAM-1 expression was normal. Therefore, endothelial cells react with a vigorous proinflammatory response to all mutants of C. albicans except those with the most severe defects in hyphal formation.
DISCUSSION
Our results demonstrate that the Efg1p and Tup1p signal transduction pathways are particularly important in the interactions of C. albicans with endothelial cells in vitro. In contrast, the MAPK pathway is less significant in these interactions.
The Δefg1 mutant did not germinate on endothelial cells, was only weakly endocytosed, and caused virtually no endothelial cell injury. Therefore, one or more factors that are regulated by Efg1p contribute significantly to the ability of C. albicans to invade and damage endothelial cells.
The Δtup1 mutant was also markedly deficient in its ability to invade and injure endothelial cells. However, this mutant formed extensive pseudohyphae on endothelial cells. These findings indicate that the ability to assume an elongated morphology per se is not sufficient for C. albicans to be endocytosed by and cause damage to endothelial cells under the conditions tested. One notable difference between the Δtup1 mutant and SC5314 was that the former strain grew as pseudohyphae on endothelial cells rather than as hyphae. Thus, one or more factors associated with the formation of true hyphae are likely required for C. albicans to invade and injure endothelial cells in vitro.
We also determined that both the EFG1 revertant and the TUP1 revertant were able to cause significant endothelial cell injury. Because C. albicans must be endocytosed by endothelial cells in order to injure them, the capacity of the revertants to injure the endothelial cells indicates that these strains were also able to induce their own endocytosis by these cells. Although there were minor differences in the amounts of injury induced by the revertants, compared to that induced by strain SC5314, these differences were likely of little biological significance. We therefore conclude that Efg1p and Tup1p are important for C. albicans to invade and injure endothelial cells in vitro.
The Δtup1 mutant stimulated endothelial cells to express ICAM-1 but not E-selectin. Previously, we determined that C. albicans induces the expression of these two leukocyte adhesion molecules via different mechanisms. E-selectin expression is mediated by the endothelial cell synthesis of tumor necrosis factor alpha, whereas ICAM-1 expression occurs independently of this cytokine (26). Our current results provide additional evidence for the differential regulation of E-selectin and ICAM-1 expression in response to C. albicans. Moreover, they suggest that Tup1 regulates the candidal factor(s) required to induce endothelial cells to express E-selectin but not ICAM-1.
The MAPK signal transduction pathway was less important in regulating the interactions of C. albicans with endothelial cells. The Δcph1 mutant was indistinguishable from SC5314 in all endothelial cell interactions studied. Also, the Δcph1 Δefg1 double mutant was similar to the Δefg1 mutant in its diminished capacity to germinate on, invade, and injure endothelial cells. One difference between the Δcph1 Δefg1 and Δefg1 mutants was in their abilities to stimulate endothelial cells to express leukocyte adhesion molecules. The Δcph1 Δefg1 mutant induced significantly less expression of E-selectin and ICAM-1 than did SC5314, whereas the Δefg1 mutant did not. These findings suggest that a candidal factor controlled by the MAPK pathway is important in stimulating endothelial cells to express leukocyte adhesion molecules.
Interestingly, although the Δcph1 Δefg1 mutant causes no mortality in mice following tail vein injection (24), the kidneys from these mice actually contain greater numbers of organisms than do the kidneys of mice infected with SC5314 (Woods Hole Molecular Mycology Course 1999, unpublished data). The weak proinflammatory response induced by the Δcph1 Δefg1 strain in vitro suggests that the high number of organisms in the murine kidneys may be the result of diminished clearance of the organisms due to a reduced host inflammatory response. However, additional studies are required to evaluate this possibility.
The Δcla4 mutant formed aberrantly shaped cells when it was exposed to endothelial cells. However, the majority of the filaments appeared to be hyphae rather than pseudohyphae. This finding may explain why the Δcla4 mutant had an only slightly decreased ability to invade and injure endothelial cells. The relatively minor defects of the Δcla4 mutant in vitro do not correspond to its markedly reduced virulence in the murine model of disseminated infection (20). A possible explanation for these divergent results is that candidal factors regulated by Cla4p may be important during the interaction of the organism with host cells other than endothelial cells. An additional explanation may be that our in vitro model of the interactions of C. albicans with endothelial cells does not completely mimic the conditions that exist in vivo. However, the Cla4p mutant did exhibit a similar morphology upon exposure to endothelial cells in vitro, as it does in the murine kidney during a hematogenous infection (20).
Taken together, our results suggest that the signal transduction pathways that regulate the yeast-to-hypha transition, especially those containing Efg1p and Tup1p, significantly influence the expression of candidal factors required for the organism to invade, injure, and stimulate endothelial cells. How these pathways regulate these putative candidal virulence factors is unclear. Furthermore, the candidal factors that mediate endothelial cell invasion, injury, and stimulation have not been delineated completely.
We have shown previously that secreted aspartyl proteinase 2 contributes to endothelial cell injury by C. albicans (15). However, whether Egf1p and Tup1p regulate the expression of the various secreted aspartyl proteinase genes is unknown. Other lytic enzymes, such as phospholipases may also play a role in causing cellular damage (7, 21). One candidal phospholipase that probably does not contribute significantly to endothelial cell injury under the conditions tested is encoded by PLB1. Hoover et al. reported that this gene is highly expressed in a Δtup1 mutant (14), whereas we found that this mutant caused virtually no damage to endothelial cells. Nevertheless, other phospholipases of C. albicans may well be a cause of endothelial injury.
Although endothelial cell injury is likely caused by factors secreted by C. albicans, induction of endocytosis is not. We have found previously that killed C. albicans cells are endocytosed by endothelial cells but that they do not cause detectable injury to these cells (10). Our present results suggest that the candidal factors that induce endothelial cell endocytosis are expressed by C. albicans cells shortly after they germinate. Because the yeast-to-hypha transition is accompanied by a significant alteration in the candidal cell surface (4, 31), it is probable that the germinated organisms express molecules on their surfaces that trigger the process of endocytosis.
In the near future, the ability to use DNA microarrays to measure gene expression on a genome-wide scale will provide much information about which virulence factor genes are expressed during the different phases of the yeast-to-hypha transition (28, 29). Applying this technique to the different signal transduction pathway mutants will also yield important data on how these virulence factors are regulated. Finally, combining the gene expression data with the findings of in vitro studies similar to the ones performed here will very likely result in the identification of additional virulence factors of C. albicans.
ACKNOWLEDGMENTS
We thank the nurses at Harbor-UCLA Medical Center for collecting umbilical cords and Angela Sanchez for assistance with tissue culture. The Olympus phase-contrast microscope used for this study was generously donated by Toyota U.S.A. We appreciate the helpful discussions with B. J. Eng, and we are grateful to G. R. Fink, W. A. Fonzi, and M. Whiteway for providing the strains of C. albicans used in our experiments.
This work was supported by Public Health Service grants R01 AI19990, P01 AI37194, R29 AI040636, and MO1 RR00425 from the National Institutes of Health. S. G. Filler was supported by the Burroughs Wellcome Fund New Investigator Award in molecular pathogenic mycology.
REFERENCES
- 1.Braun B R, Johnson A D. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science. 1997;277:105–109. doi: 10.1126/science.277.5322.105. [DOI] [PubMed] [Google Scholar]
- 2.Brown A J, Gow N A. Regulatory networks controlling Candida albicans morphogenesis. Trends Microbiol. 1999;7:333–338. doi: 10.1016/s0966-842x(99)01556-5. [DOI] [PubMed] [Google Scholar]
- 3.Csank C, Schroppel K, Leberer E, Harcus D, Mohamed O, Meloche S, Thomas D Y, Whiteway M. Roles of the Candida albicans mitogen-activated protein kinase homolog, Cek1p, in hyphal development and systemic candidiasis. Infect Immun. 1998;66:2713–2721. doi: 10.1128/iai.66.6.2713-2721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deslauriers N, Michaud J, Carre B, Leveillee C. Dynamic expression of cell-surface antigens probed with Candida albicans-specific monoclonal antibodies. Microbiology. 1996;142:1239–1248. doi: 10.1099/13500872-142-5-1239. [DOI] [PubMed] [Google Scholar]
- 5.Edmond M B, Wallace S E, McClish D K, Pfaller M A, Jones R N, Wenzel R P. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin Infect Dis. 1999;29:239–244. doi: 10.1086/520192. [DOI] [PubMed] [Google Scholar]
- 6.Edwards J E., Jr . Candida species. In: Mandell G L, Bennett J E, Dolin R, editors. Mandell, Douglas and Bennett's principals and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingston; 1995. pp. 2289–2306. [Google Scholar]
- 7.Filler S G, Ibe B O, Ibrahim A S, Ghannoum M A, Raj J U, Edwards J E., Jr Mechanisms by which Candida albicans induces endothelial cell prostaglandin synthesis. Infect Immun. 1994;62:1064–1069. doi: 10.1128/iai.62.3.1064-1069.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filler S G, Ibe B O, Luckett P M, Raj J U, Edwards J E., Jr Candida albicans stimulates endothelial cell eicosanoid production. J Infect Dis. 1991;164:928–935. doi: 10.1093/infdis/164.5.928. [DOI] [PubMed] [Google Scholar]
- 9.Filler S G, Pfunder A S, Spellberg B J, Spellberg J P, Edwards J E., Jr Candida albicans stimulates cytokine production and leukocyte adhesion molecule expression by endothelial cells. Infect Immun. 1996;64:2609–2617. doi: 10.1128/iai.64.7.2609-2617.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filler S G, Swerdloff J N, Hobbs C, Luckett P M. Penetration and damage of endothelial cells by Candida albicans. Infect Immun. 1995;63:976–983. doi: 10.1128/iai.63.3.976-983.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fonzi W A, Irwin M Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fratti R A, Belanger P H, Ghannoum M A, Edwards J E, Jr, Filler S G. Endothelial cell injury caused by Candida albicans is dependent on iron. Infect Immun. 1998;66:191–196. doi: 10.1128/iai.66.1.191-196.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fratti R A, Ghannoum M A, Edwards J E, Jr, Filler S G. Gamma interferon protects endothelial cells from damage by Candida albicans by inhibiting endothelial cell phagocytosis. Infect Immun. 1996;64:4714–4718. doi: 10.1128/iai.64.11.4714-4718.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoover C I, Jantapour M J, Newport G, Agabian N, Fisher S J. Cloning and regulated expression of the Candida albicans phospholipase B (PLB1) gene. FEMS Microbiol Lett. 1998;167:163–169. doi: 10.1111/j.1574-6968.1998.tb13223.x. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim A S, Filler S G, Sanglard D, Edwards J E, Jr, Hube B. Secreted aspartyl proteinases and interactions of Candida albicans with human endothelial cells. Infect Immun. 1998;66:3003–3005. doi: 10.1128/iai.66.6.3003-3005.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaffe E A, Nachman R L, Becker C G, Minick C R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Investig. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapteyn J C, Van Egmond P, Sievi E, Van Den Ende H, Makarow M, Klis F M. The contribution of the O-glycosylated protein Pir2p/Hsp150 to the construction of the yeast cell wall in wild-type cells and beta 1,6-glucan-deficient mutants. Mol Microbiol. 1999;31:1835–1844. doi: 10.1046/j.1365-2958.1999.01320.x. [DOI] [PubMed] [Google Scholar]
- 18.Kohler J R, Fink G R. Candida albicans strains heterozygous and homozygous for mutations in mitogen-activated protein kinase signaling components have defects in hyphal development. Proc Natl Acad Sci USA. 1996;93:13223–13228. doi: 10.1073/pnas.93.23.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leberer E, Harcus D, Broadbent I D, Clark K L, Dignard D, Ziegelbauer K, Schmidt A, Gow N A, Brown A J, Thomas D Y. Signal transduction through homologs of the Ste20p and Ste7p protein kinases can trigger hyphal formation in the pathogenic fungus Candida albicans. Proc Natl Acad Sci USA. 1996;93:13217–13222. doi: 10.1073/pnas.93.23.13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leberer E, Ziegelbauer K, Schmidt A, Harcus D, Dignard D, Ash J, Johnson L, Thomas D Y. Virulence and hyphal formation of Candida albicans require the Ste20p-like protein kinase CaCla4p. Curr Biol. 1997;7:539–546. doi: 10.1016/s0960-9822(06)00252-1. [DOI] [PubMed] [Google Scholar]
- 21.Leidich S D, Ibrahim A S, Fu Y, Koul A, Jessup C, Vitullo J, Fonzi W, Mirbod F, Nakashima S, Nozawa Y, Ghannoum M A. Cloning and disruption of caPLB1, a phospholipase B gene involved in the pathogenicity of Candida albicans. J Biol Chem. 1998;273:26078–26086. doi: 10.1074/jbc.273.40.26078. [DOI] [PubMed] [Google Scholar]
- 22.Levitz S M, DiBenedetto D J, Diamond R D. A rapid fluorescent assay to distinguish attached from phagocytized yeast particles. J Immunol Methods. 1987;101:37–42. doi: 10.1016/0022-1759(87)90213-4. [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Kohler J, Fink G R. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science. 1994;266:1723–1726. doi: 10.1126/science.7992058. [DOI] [PubMed] [Google Scholar]
- 24.Lo H J, Kohler J R, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink G R. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 25.Noel R F, Jr, Sato T T, Mendez C, Johnson M C, Pohlman T H. Activation of human endothelial cells by viable or heat-killed gram-negative bacteria requires soluble CD14. Infect Immun. 1995;63:4046–4053. doi: 10.1128/iai.63.10.4046-4053.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orozco A S, Zhou X, Filler S G. Mechanisms of the pro-inflammatory response of endothelial cells to Candida albicans infection. Infect Immun. 2000;68:1134–1141. doi: 10.1128/iai.68.3.1134-1141.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russo P, Kalkkinen N, Sareneva H, Paakkola J, Makarow M. A heat shock gene from Saccharomyces cerevisiae encoding a secretory glycoprotein. Proc Natl Acad Sci USA. 1992;89:3671–3675. doi: 10.1073/pnas.89.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schena M, Shalon D, Davis R W, Brown P O. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science. 1995;270:467–470. doi: 10.1126/science.270.5235.467. [DOI] [PubMed] [Google Scholar]
- 29.Schena M, Shalon D, Heller R, Chai A, Brown P O, Davis R W. Parallel human genome analysis: microarray-based expression monitoring of 1000 genes. Proc Natl Acad Sci USA. 1996;93:10614–10619. doi: 10.1073/pnas.93.20.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoldt V R, Sonneborn A, Leuker C E, Ernst J F. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 1997;16:1982–1991. doi: 10.1093/emboj/16.8.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torosantucci A, Gomez M J, Bromuro C, Casalinuovo I, Cassone A. Biochemical and antigenic characterization of mannoprotein constituents released from yeast and mycelial forms of Candida albicans. J Med Vet Mycol. 1991;29:361–372. doi: 10.1080/02681219180000591. [DOI] [PubMed] [Google Scholar]