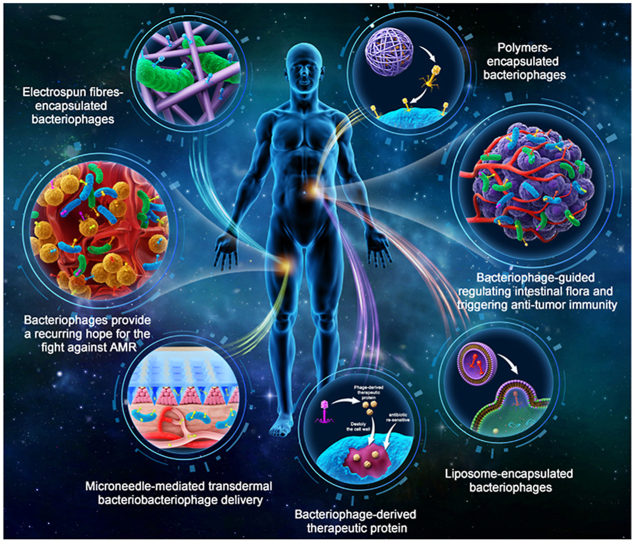
Abstract
Antibiotic resistance is one of the biggest threats to global health, as it can make the treatment of bacterial infections in humans difficult owing to their high incidence rate, mortality, and treatment costs. Bacteriophage, which constitutes a type of virus that can kill bacteria, is a promising alternative strategy against antibiotic-resistant bacterial infections. Although bacteriophage therapy was first used nearly a century ago, its development came to a standstill after introducing the antibiotics. Nowadays, with the rise in antibiotic resistance, bacteriophage therapy is in the spotlight again. As bacteriophage therapy is safe and has significant anti-bacterial activity, some specific types of bacteriophages (such as bacteriophage phiX174 and Pyo bacteriophage complex liquid) entered into phase III clinical trials. Herein, we review the key points of the antibiotic resistance crisis and illustrate the factors that support the renewal of bacteriophage applications. By summarizing recent state-of-the-art studies and clinical data on bacteriophage treatment, we introduced (i) the pharmacological mechanisms and advantages of antibacterial bacteriophages, (ii) bacteriophage preparations with clinical potential and bacteriophage-derived anti-bacterial treatment strategies, and (iii) bacteriophage therapeutics aimed at multiple infection types and infection-induced cancer treatments. Finally, we highlighted the challenges and critical perspectives of bacteriophage therapy for future clinical development.
Key words: Antibiotic resistance, Bacteriophage therapy, Bacteriophage preparations, Bacteriophage-derived anti-bacterial treatment strategies
Graphical abstract
The diverse formulation strategies of bacteriophage therapies (liposomes, polymeric biological particles, microneedles, and electrospun fibres) and bacteriophage-derived antibacterial strategies to treat various antimicrobial resistance infections and infection-induced cancer therapy are reviewed.
1. Introduction
Antibiotics, among the most successful therapeutic interventions in the history of medicine, have saved millions of lives and are crucial for the establishment of medical breakthroughs, such as anti-infection, organ transplantation, and even cancer chemotherapy1. The use of antibiotics has expanded to many medical conditions; thus, it would be disastrous if the effectiveness of antibiotics in medicine were lost or lowered. Unfortunately, we are rapidly stepping into such a bad epoch, the so-called “post-antibiotic era”2.
Antimicrobial resistance (AMR) is a growing threat to global public health. According to statistics from the World Health Organization (WHO), approximately 700,000 people die from AMR every year, and this number is anticipated to rise rapidly in the next few years. This statistic reveals the current AMR-related challenges and urgency to find new effective antimicrobial therapies. Unfortunately, in the past 20 years, the U.S. Food and Drug Administration (FDA) and the European Drug Administration have only approved two new antibiotic classes that work against Gram-positive pathogens but have little effect on Gram-negative bacteria3. As many pharmaceutical companies no longer develop new antibiotics, it is difficult to meet the urgent demand for new therapeutic agents against AMR.
Although bacteriophage therapy is not a new treatment strategy, it seems to provide recurring hope for the fight against AMR. A century ago, the first report on the efficacy of bacteriophage therapy received great attention. However, after World War II, interest in bacteriophage-based therapies declined with the advent of antibiotics4. The development of bacteriophage-based therapies seems to have entered a period of stasis. Owing to the extensive use of antibiotics, the resurgence of AMR forces scientists to revisit bacteriophage therapy. In 2017, the Wellcome Trust commissioned 24 scientists from academia and industries to propose a landmark document aimed at identifying prospective therapeutic alternatives to antibiotics. Several key points were considered under the current crisis: (a) the practicability of informative clinical trials, (b) the size of medical potential, (c) the possibility and results of drug resistance, (d) the actual research and practice level, and (e) possible time for registration. Bacteriophage therapy was included in the top ten strategies that the team considered noteworthy4.
As recently emphasized, state-of-the-art research has been conducted on the development of personalized bacteriophage therapy, which is regarded as highly hopeful for patients infected with antimicrobial-resistant bacteria. This review aims to summarize the key roles of bacteriophage therapy in the AMR crisis. First, we introduce the pharmacological mechanisms and advantages of bacteriophage antibacterial agents. Next, multiple formulation strategies of bacteriophage therapy (cocktail therapy, liposomes, polymeric biological particles, microneedles, and electrospun fibers) and bacteriophage-derived antibacterial strategies are summarized. In addition, novel bacteriophage therapeutics against various infection types and infection-induced cancer therapy are discussed. Moreover, we highlight the challenges and critical perspectives of bacteriophage therapy in future clinical development.
2. AMR: Natural or human-made?
Antibiotic resistance is a natural phenomenon, even before the discovery and use of antibiotics in humans. From a molecular biology perspective, it is not surprising that ancient microbial populations identified in the unpolluted arctic permafrost contained modern antibiotic resistance genes5, 6, 7. This is sufficient to prove that competition between bacteria and antibiotics has appeared long before human intervention.
The overuse of antibiotics in human activities, especially in clinical, agricultural, and animal husbandry settings, has significantly intensified the “arms race” between bacteria and antibiotics. It is estimated that 180 mg of agricultural antibiotics is used for each kilogram of meat produced by animal husbandry in the U.S. The consumption in other countries is similar or even higher8. In addition, millions of tons of antibiotic-containing wastewater from agriculture are discharged into the environment or reservoirs, which has caused unexpected consequences. Microbial communities are constantly exposed to various antibiotics, resulting in the accelerated selection, evolution, and transmission of antibiotic resistance genes in the natural environment9. Furthermore, with the development of globalization, travel, trade, and the frequent spread of disease, resistant genes have been readily exchanged among various pathogens.
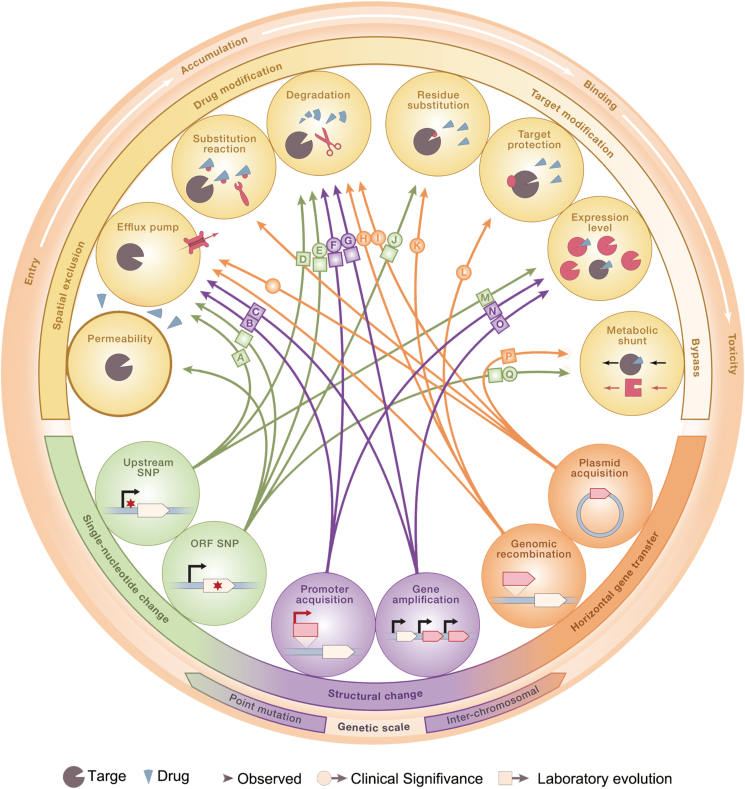
Numerous mechanisms of drug resistance have appeared in almost every step of antibiotic action: (a) the structure of the bacterial cell membrane can be changed to prevent drugs from entering bacteria, or the efflux pumps on the bacterial cell membrane are overexpressed to efflux drugs; (b) antibiotics can be modified or even destroyed by enzymes, such as β-lactamases, or the generation of key enzymes for antibiotic activation can be inhibited; (c) the target of drugs can also be modified, concealed, or adjusted10,11. At the molecular level, the main mechanism of antimicrobial resistance involves genetic changes. Except for gene mutations selected by drug pressure, changes at the gene level can also transform sensitive bacteria into drug-resistant bacteria by ingesting exogenous drug-resistant genes via transformation, conjugation, or transduction12. Additionally, bacterial cells can produce transient, non-gene-coded resistance through biofilm growth, colony adaptation, metabolic dormancy, and persistence13. Many studies have been performed to summarize the mechanisms of bacterial resistance (Fig. 1)14, 15, 16. Thus, we will not go into detail in this topic.
Figure 1.
The mechanism of antibiotic resistance. Arrows shows the decisive relationship between specific genotype changes and specific resistance mechanisms. Reprinted with permission from Ref. 16. Copyright © 2018, Elsevier Inc.
3. The history and anti-bacterial mechanisms of bacteriophage therapy
Phages were independently discovered by Frederick Twort in 1915 and were named “bacteriophages,” meaning that they can kill bacteria. Professor Twort's team evaluated the bacteriophages' antimicrobial properties at an early stage. Unfortunately, the uncertain mechanisms of action led to early clinical failure.
During the same period, antibiotics began to emerge because their mechanisms of action were clear. Following the actual rise of antibiotics, bacteriophage therapy was restricted in Eastern European countries and only applied in the Former Soviet Union and Georgia. At present, with the rise of the bacterial resistance crisis, bacteriophage therapy has been revitalized worldwide.
Bacteriophages are viruses that infect only bacteria. Bacteriophages are ubiquitous, and the ratio of bacteriophages to bacteria in aquatic environments is more than 10:1. It is well known that bacteriophages are antibacterial substances found in abundance in nature. The reproduction of bacteriophages is closely related to the bacterial host. Bacteriophages can be divided into two types: lytic and lysogenic bacteriophages. Lytic bacteriophages can (a) attach to the receptors on the surface of bacteria, (b) transfer the genome content to bacteria, (c) carry out viral replication in the cytoplasm through bacterial transcription, translation, and replication, and (d) release from the host cells. Then, new lytic bacteriophage particles will repeat this process in new susceptible hosts. Similar to lytic bacteriophages, lysogenic bacteriophages are common in nature. After being ingested by host bacteria, lysogenic bacteriophages integrate their genes into the host genome, which can be inherited by progeny cells in the process of binary division. Under environmental disturbance or other physiological stressors, the genes of lysogenic phages have been isolated from the genome of the host. These genes carry out viral replication in the cytoplasm and quickly form new progeny virions, leading to the lysis of infected host cells.
4. The advantages and disadvantages and clinical applications of bacteriophage therapy
4.1. Advantages
Bacteriophage therapy is an attractive antibacterial strategy in which a specific type of virus is used to inhibit or kill harmful bacteria. Bacteriophages exhibit a significant bactericidal effect by increasing the number of self-reproductions, but only minimally disrupting the normal flora. For antibiotic-sensitive and antibiotic-resistant bacteria, the therapeutic effects of bacteriophages are similar, but their inherent toxicity in vivo is low. It can be concluded that bacteriophage therapy has advantages over chemical antibiotics.
4.1.1. Bactericidal agents
Bacteria infected with lytic bacteriophages are unable to regain their viability. In comparison, some antibiotics, such as tetracycline, are bacteriostatic, rather than bactericidal. Consequently, antibiotics may push bacterial evolution towards resistance.
4.1.2. Formulation and applicable universality
With the rapid development of formulations, bacteriophage-based preparations are diverse in their forms, such as liquids, microneedles, and electrospun fibers. Different bacteriophages can be mixed to expand the antibiogram and achieve a wide range of antibacterial activities.
4.1.3. Reproductive capacity
Bacteriophages can increase their numbers while killing bacteria. This reproductive capacity plays a positive role in antibacterial therapy.
4.1.4. Differentiated toxicity
Bacteriophages are mainly composed of nucleic acids and proteins, which are essentially non-toxic. Owing to their host specificity, bacteriophages can infect only a few relatively pathogenic bacteria and are harmless to most normal tissues and probiotics.
4.1.5. Lack of cross-resistance with antibiotics
As the infection and killing mechanisms of bacteriophages are different from those of antibiotics, the specific resistance mechanisms of antibiotics cannot be transformed into those of bacteriophages. Thus, bacteriophages can be used to treat antibiotic-resistant infections, such as multidrug-resistant Staphylococcus aureus.
4.1.6. Rapid discovery
Bacteriophages that eliminate particular pathogenic bacteria are easily found in sewage and other wastes that contain high concentrations of bacteria. Bacteriophages with little or no toxicity can be isolated from most target bacterial communities.
4.2. Disadvantages
4.2.1. Safety
At present, a potential problem with phage therapy is that specific phages can modify host bacteria to make them pathogenic17. For example, temperate phages display a characteristic called lysogeny, that is, the phages can incorporate their genome into infected bacteria, rather than killing hosts immediately and producing phage offspring. This generates a long-term symbiotic relationship. Phages exist as bacterial components that form lysogens. Lysogen-infected bacteria do not die from infection. Bacterial lysogens tend to be resistant to the same lysogen infection and phage type. Even if the same type of phage later infects bacteria, it does not lead to bacterial death18. Temperate phages usually lead to lysogenic transformation, which means that the bacterial phenotype changes and sometimes bacterial toxicity increases. Temperate phages are associated with certain forms of transduction that can easily obtain genes from infected bacteria and subsequently transfer them into the infected bacteria to avoid killing new bacteria. Certain gene types such as drug resistance and bacterial virulence factor genes are easily spread through this process19. Therefore, it is important to avoid the use of temperate phages for treatment20.
Another concern is that phage-mediated bacterial lysis may result in the release of bacteria-encoded toxins21. The release of endotoxins increases along with the phage-mediated rupture of bacterial cells. Many antibiotics also have the ability to cleave target bacteria. It is unclear whether the use of bacteriophages can accelerate the release of exotoxins. In particular, from the perspective of gene expression, phage infection leads to the closure of bacterial gene expression, thereby preventing the expression of bacterial exotoxins21.
4.2.2. Stability
Similar to other live microbial agents, phages are susceptible to heat and chemical denaturation. The poor stability of phages causes challenges in their production, storage, and management. The current manufacturing process may expose phages to harsh solvents, temperatures, and pressures22. For example, electrospinning has been proven to affect the activity of phage products. Furthermore, in terms of storage stability, most phage products are stored in cold chains to maintain their effectiveness. In specific cases, they must be stored at cryogenic temperatures23.
4.2.3. Self-renewal
Phages are a type of living microbial agent that can evolve and self-renew during production or utilization. Self-renewal phages have the potential to produce significant long-term therapeutic effects by administering only one dose23. Although this ability might be key to the development of vaccine-like treatments for chronic diseases, self-renewal phages significantly complicate pharmacokinetics. In addition, some patient-related factors, such as food intake, native microbiota, and disease status, may affect the rate of microbial self-renewal. Thus, self-renewing phages may introduce distinct variability, which is a severe challenge for establishing a standard administration scheme based on clinical trials24.
4.2.4. Bacteriophage-resistance mutants
A major problem with phage therapy is the emergence of phage-insensitive mutants, which may hinder the success of this therapy. Previous studies have shown that the emergence of phage-resistant mutants is frequent and almost inevitable25. The resistance mechanisms include: (a) modification of bacterial receptors to prevent phage adsorption; (b) preventing phage DNA from entering the bacterial exclusion system; (c) degrading phage DNA using a restriction-modification system, CRISPR-Cas system, and other related systems; (e) blocking phage DNA replication, transcription, or translation through abortion infection systems and anti-phage signaling systems26,27.
4.3. Clinical realities of phage therapies
Recently, a wave of successful bacteriophage therapies has been reported in the U.S. and Europe. In the U.S., the FDA approved a clinical trial from the United States Bacteriophage Therapy Center for the first time, where an intravenous bacteriophage treatment was used in patients with drug-resistant S. aureus infection. Several patients in the center were treated successfully, and nearly all patients tolerated the treatment well and did not have significant side effects28. The FDA has also approved bacteriophage therapy for patients with acute diseases, such as prosthetic joint infections, bone and joint infections, implant infections, wound infections, diabetic foot infections, and acute tonsillitis. Currently, the most readily obtainable bacteriophages come from Poland, Russia, and Georgia. They have gained satisfactory safety and various degrees of success in different types of infections. Belgium recently adopted the “magistral bacteriophage” method and allowed the production of personalized bacteriophage preparations based on doctor prescriptions29. Additionally, bacteriophage therapy has been clinically licensed against multidrug-resistant bacteria. Furthermore, some clinical trials, including the treatment of urinary tract infections, are underway, and some preliminary results are encouraging, as is shown in Table S1.
5. Access to bacteriophage-based therapy: Pharmaceutical preparations
Bacteriophage therapy is still limited from the workbench to the clinic30. Previous studies31 have shown that the stability of bacteriophages in solutions is limited, and bacteriophage titers significantly decrease during processing and storage. If bacteriophages serve as commercial agents, a stable and precise dose is required. In addition, the concentration of bacteriophages at the lesions directly contributes to therapeutic efficacy. Therefore, good stability and efficient on-site concentration are the two key determinants for efficient medical bacteriophages. Simple bacteriophage suspensions have recently been used in clinical trials, and only a few bacteriophage-based formulations have been developed. Therefore, stable and reproducible targeted delivery technologies could promote the clinical application of bacteriophage therapy, some bacteriophages preparation characteristics were summarized in Table 1.
Table 1.
Summary of bacteriophages preparation characteristics.
| Preparation | Action mechanism | Advantage | Challenge |
|---|---|---|---|
| Bacteriophages cocktail |
|
|
|
| Liposome-encapsulated bacteriophages |
|
|
|
| Polymers-encapsulated bacteriophages |
|
|
|
| Microneedles |
|
|
|
| Electrospun fibres-encapsulated bacteriophages |
|
|
|
5.1. Bacteriophages cocktail therapy
Owing to the host specificity of bacteriophages, the practicability of a single bacteriophage therapy in clinical settings might be hindered4,32. A key factor in bacteriophage therapy is the exact match between the pathogens and bacteriophages. However, the in vitro screening and in vivo lysis characteristics of bacteriophages may not always be consistent. Although bacteriophages can overcome antibiotic-induced AMR, bacteriophage monotherapy cannot fully satisfy the clinical requirements. Thus, a bacteriophage cocktail treatment is essential to solve the problem of the narrow antibiogram of bacteriophage monotherapy. Bacteriophage cocktails can be directed to battle only a single bacterial strain, multiple strains of a single bacterial species, or even multiple species. However, owing to the complexity of cocktail composition, the preparation and purification processes are complex. In addition, the pharmacokinetic and pharmacodynamic characteristics of bacteriophages derived from cocktails are unpredictable33. The comparative effectiveness of each component in cocktails should be evaluated, and low-efficacy bacteriophages should be discarded34.
The concept of bacteriophage cocktail therapy could be expanded; that is, each bacteriophage can be used in sequence rather than with other bacteriophages in a cocktail. Owing to continuous administration, even if resistance occurs, new non-resistant bacteriophages will continue to work. Animal models have indicated that the sequential administration of bacteriophage cocktails might bring promising results in reducing bacterial populations and bacteriophage resistance35. In Russia and Georgia, bacteriophage cocktails are available as over-the-counter drugs for the treatment of bacterial infections. The Eliava Institute, founded in 1923 by bacteriophage researcher Professor George Eliava, produced two half-year updated cocktails: Pyo bacteriophage (Pyo) and intestibacteriophage36. Pyo has been used in the treatment of suppurative and intestinal diseases against S. aureus, Escherichia coli, Pseudomonas aeruginosa, Proteus vulgaris, Proteus mirabilis, and Streptococcus pyogenes31,37,38.
5.2. Liposome-encapsulated bacteriophages
The poor stability of phages in gastric acid conditions and insufficient retention time in the intestine are the two main problems for the low therapeutic efficiency of oral phages, requiring the frequent administration of free phages. Frequent administration is time-consuming and expensive, resulting in poor patient compliance39. The use of positively charged liposomes (Fig. 2B) can solve both problems simultaneously. As proton barriers, liposomes can protect phages from gastric acids40. In addition, the intestinal retention time of phages can be prolonged owing to the positive charge on their surface41,42. Colom et al.31 designed enterobacteria phages encapsulated in cationic liposomes. Cationic liposome-loaded bacteriophages can achieve two objectives: (a) preventing the inactivation of bacteriophages in gastric acid conditions and (b) acting as promoters of mucus adhesion, owing to their positively charged surfaces, thereby prolonging the intestinal residence time of bacteriophages43.
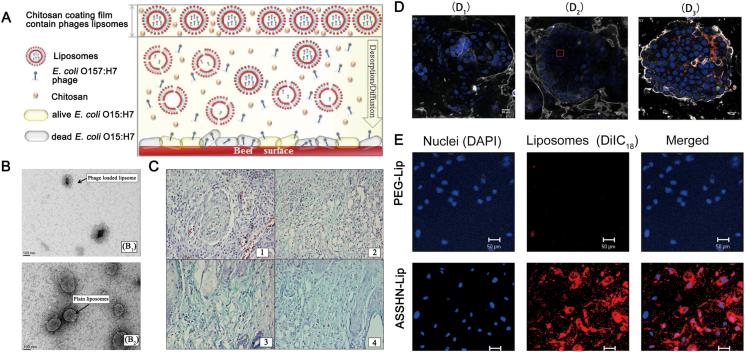
Figure 2.
(A) Liposome-encapsulated phage with chitosan film against E. coli in beef. Reprinted with permission from Ref. 41. Copyright © 2017, Elsevier Ltd (B) Transmission electron micrograph (TEM) images of liposomes. Phage loaded liposomes (B1) and plain liposomes (B2). Reprinted with permission from Ref. 89. Copyright © 2018 by the authors (C) Collagen formation and treatment in wound tissue of mice on day 5 post-infection indicated by Masson's Trichrome staining (C1): Diabetic untreated mice (infected control mice) displayed infiltration of neutrophils along with fibroblastic condensation (C2): Free cocktail of phages (FCP)-treated mice indicated intermediate stage of presence of mature collagen fibrils along with collagen formation (C3): Liposome cocktail of phages (LCP)-treated mice showed the reduction of inflammatory cells and thin fibrils of mature collagen present with decreased vascularity (C4): Clarithromycin-treated mice displayed the presence of mature collagen fibers along with negligible inflammation, indicating wound healing. Reprinted with permission from Ref. Copyright © 2018 by the authors (D) The confocal laser scanning microscope (CLSM) images of the cells (D1), phages (D2), and PPE (D3). Reprinted with permission from Ref. 40. Copyright © 2019 by the authors (E) The CLSM images depicting the association of ASSHN-Lip with hEPCs. Red and Blue fluorescence display the liposomes and nuclei respectively. Reprinted with permission from Ref. 44. Copyright © 2017, Elsevier Ltd.
Another benefit of liposome-encapsulated phages is that smaller particles can increase the possibility of cell uptake through endocytosis or membrane fusion (Fig. 2A, D and E)44. Once active phages are transported to the host cytoplasm, intracellular pathogens (strains of enteroinvasive E. coli, Listeria, and Mycobacterium) can be inactivated. Furthermore, liposomes can be functionalized to increase the probability of bacteriophage clinical application by: (a) attaching particular targeting ligands to the surfaces of liposomes; (b) increasing the in vivo circulation time and slowing the release of contents; (c) encapsulating various probes to monitor the pharmacokinetics of liposome-loaded bacteriophages; and (d) changing the charge distribution of liposomes to prolong the retention time. However, liposomes can adhere to each other and even fuse under certain conditions, which is unfavorable for storage and clinical applications45.
5.3. Polymers-encapsulated bacteriophages
Disturbances in the intestinal flora may be associated with ulcerative colitis, Crohn's disease, colorectal cancer, and food poisoning. The concentration of bacteria in the upper digestive tract is approximately 103–104 CFU/mL and mainly consists of Gram-positive facultative anaerobes. However, the bacterial concentration increases significantly up to 1011–1012 CFU/mL in the colon, which mainly consists of anaerobic bacteria such as Bacillus, Bifidobacterium, Eubacterium, Clostridium, Enterococcus, and Enterobacter. The literature on polymer-encapsulated bacteriophages has mainly focused on the treatment of gastrointestinal tract infections. To design oral bacteriophage formulations for human or animal use, some factors must be considered, including harsh pH conditions in the gastrointestinal tract, effect of digestive ferments (pepsin, protease, lipase, amylase, and trypsinogen), the impact of bile salts, and the retention time of distinct intestinal segments, such as the duodenum and ileum. The purpose of polymeric encapsulation is to protect bacteriophages from the adverse gastric environment to avoid inactivation and decrease the bacteriophage titer46,47. Polymeric carriers can be designed to respond to specific pH values, for example, the pH of stomach (pH 1–3), small intestine (pH 5.5–6.5), and colon (pH 6.5–7.2).
Chemically modified biopolymers (including aliphatic polyesters, polyamides, polycarbonates, and polyamino acids) are commercially available and licensed for the preparation of microspheres or their coatings. For example, alginate (Fig. 3B and C) has been widely used to encapsulate various bacteriophages for oral delivery. When alginate is exposed to two valence cations, cross-linked gels are formed, and microencapsulation can be encapsulated by extrusion (a physical encapsulation method) (Fig. 3A). To enhance acid resistance, many studies have shown that adding calcium carbonate to alginate gel beads can remarkably improve the acid-resistance ability of encapsulated bacteriophages46,48. Compared to free bacteriophages, bacteriophages encapsulated in polymers with anti-acid agents are stable when exposed to acidic conditions. Neutral gum, guar gum, pectin, chitosan, and whey protein have also been used to enhance the acidic stability of alginate microparticles. In addition, to utilize the undigested substrate in the small intestine, the intestinal flora can produce β-glucosidase, α-arabinosidase, β-xylosidase, β-galactosidase, nitroreductase, and urea dehydroxygenase. Enzyme-sensitive polymers, including cellulose-, hydroxypropyl-methylcellulose-, and pectin-derived biopolymers, can respond to the aforementioned enzymes and exert positioning release, resulting in the location regulation of specific flora48.
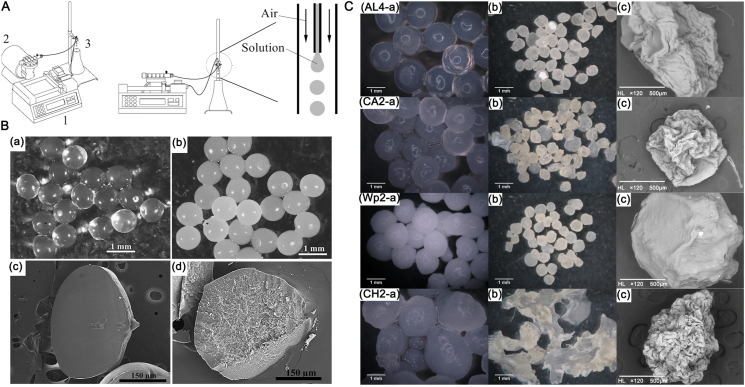
Figure 3.
(A) Sketch map of the encapsulation system consist of a syringe pump (1), air compressor (2) and needle (3). Reprinted with permission from Ref. 47. Copyright © 2020, Elsevier Ltd (B) Optical micrographs of alginate microspheres and calcium carbonate-merged; scanning electron microscope (SEM) images of the cross sections of alginate microspheres with (B3) and without (B4) calcium carbonate particles. Reprinted with permission from Ref. 46. Copyright © 2010, Elsevier Ltd (C) Micrographs of phages-loaded microspheres before (1) and after (2) drying and SEM images of the surface of the microspheres. Reprinted with permission from Ref. 47. Copyright © © 2020, Elsevier Ltd.
5.4. Microneedle-mediated transdermal bacteriophage delivery
Oral bacteriophages have only been used for the topical treatment of infectious microorganisms in the intestine. Bacteriophages cannot enter systemic circulation from the digestive system. In addition, bile salts and intestinal carbohydrates may block the bivalent metal ions required for bacteriophage replication. Therefore, it is difficult for oral bacteriophages to play a systemic therapeutic role. Parenteral administration is an effective way to allow bacteriophages to exert systemic therapeutic effects. However, the direct intravenous injection of bacteriophages has certain disadvantages, such as the need for supervision by health professionals, the possibility of cross-contamination, the need to maintain a costly “cold chain,” and relatively poor compliance. Microneedle-based bacteriophage therapeutics are of great interest because they can overcome these problems. Ryan et al.49 prepared a hollow polycarbonate microneedle array. The average height and bottom diameter were approximately 995 and 750 μm, respectively, and the hollow aperture was 100 μm. The device can completely penetrate the skin and deliver bacteriophages. Skin bioavailability can reach up to 100%. The depth and width of the residual skin pores are 210 and 600 μm, respectively, and they can be closed quickly after treatment. Epithelial barrier function is not damaged in this process. However, it is difficult for microneedles to deliver stock solutions through all the skin layers. Research has shown that microneedles do not penetrate all skin layers and yield a liquid pool on the skin surface4.
5.5. Electrospun fibres-encapsulated bacteriophages
Electrospun biopolymer fibers are considered reliable carrier/delivery devices for bioactive agents. Phages can be wrapped on their surfaces or fixed internally (Fig. 4B and D)50,51. Fibers produced by electrospinning have many advantages, including a large surface area, softness, flexibility, and porosity. They can be used to easily form “bioactive surfaces,” which are very suitable for local administration in the form of bandages and wound dressings or packaging materials with antibacterial properties (Fig. 4A). The “bioactive surfaces” not only target and inactivate bacterial pathogens, but also allow the detection, identification, and phage-mediated immobilization of targeted microbes52,53. The advantage of electrospun fibers is that they allow for the controlled release of phage particles, which is controlled by the selection of materials. The release of phages is mediated by fiber expansion or material disintegration (by polymer erosion or the simple dissolution of the polymer) (Fig. 4C). Mixed fiber polymers with different molecular weights can be used to customize the release kinetics of phages54.
Figure 4.
(A) Electrospun fibers-encapsulated phages capture, infect the bacteria and replicate. Reprinted with permission from Ref. 53. Copyright © 2021, American Chemical Society (B) TEM images of phages in the inner surface of the electrospun and the influence of the positive charge distribution on the surface of polyethylene glycol (PEG) for the phage distribution. Reprinted with permission from Ref. 50. Copyright © 2018 by the authors (C) SEM images of the release of T4 phage loaded electrospun fibers for different formulations. Reprinted with permission from Ref. 51. Copyright © 2013, Elsevier Ltd (D) SEM images of electrospun loaded with Phagestaph (D1) and Fersis (D2) freeze-dried preparations. And the diameter distributions are displayed on the right of the corresponding image. Reprinted with permission from Ref. 50. Copyright © 2018 by the authors.
6. Expansion of access to bacteriophage-derived treatments
6.1. Combination of bacteriophages and antibiotics: Two are better than one
Clinically, the use of a single drug usually does not have a good therapeutic effect. Combination therapy via various mechanisms has better curative efficacy than monotherapy. As shown in Table S2, a common strategy in current anti-infection treatments is to use two or more antibiotics in combination to achieve a cooperative effect55. In some instances, it was also beneficial to add adjuvants without antimicrobial activities, because these adjuvants played an important role in blocking drug resistance or improving the pharmacokinetic effect of drugs56. In some instances, it is also beneficial to add adjuvants without antimicrobial activities because they play an important role in blocking drug resistance or improving the pharmacokinetic effect of drugs57. In addition, the combined effect of the two therapeutic strategies is better than that of each individual strategy. The combination of bacteriophages and antibiotics to treat AMR infections is promising. The bacterial biosynthetic potential increases under antibiotic-rich conditions. This is why bacterial division is inhibited, but the cells do not die. Comeau et al58. first used the bacteriophage‒antibiotic synergy (PAS) strategy to describe the sublethal concentration of antibiotics that help lytic bacteriophages reproduce rapidly and greatly promote their antibacterial effect. Moreover, when bacteriophages are used in combination with antibiotics, there may be a profound order effect; a bacteriophage treatment implemented before a drug treatment results in maximum bacterial killing. The results of the aforementioned study showed that optimizing the timing of bacteriophage–antibiotic combination therapy can improve its therapeutic efficacy59,60.
There are two benefits to the clinical practice of PAS. The limited use of antibiotics can help manage them and reduce the evolution of drug resistance. Additionally, the curative effect against AMR pathogens is enhanced by combining bacteriophages with other antibiotics57. Systemic immunosuppression is one of the most common characteristics of diabetes. Sanjay et al.61 described that the combination of bacteriolytic bacteriophages and linezolid could manage AMR infection in diabetic foot ulcers more effectively than monotherapy. Angiopoietin is a characteristic of endocarditis, which helps bacteria escape antibiotics and the host immune response. Oechslin et al.62 demonstrated that a combination of ciprofloxacin and bacteriophage cocktails had a synergistic effect on experimental endocarditis in rats. In addition, Valerio et al.63 observed a synergistic effect of single bacteriophage therapy and fungicides cultured in human urine on E. coli, and the emergence of monoclonal and double antibody bacterial mutants was reduced in combination therapy. Chan et al.64 found that, under the selection pressure of bacteriophages, antibiotic-sensitive bacteria occupied a dominant population. According to the work of Chan et al.64 on bacterial re-sensitivity to antibiotics, bacteriophage receptor binding sites are involved in multidrug efflux systems. If bacteria acquire phage resistance by changing the structure of bacteriophage receptor-binding sites, the efflux pump function and antibiotic resistance would be lost.63
6.2. Bacteriophage-derived therapeutic proteins
Some of the crucial proteins and enzymes used to destroy bacterial cells during infection are encoded by the bacteriophage genomes. Two groups of proteins, virus-associated peptidoglycan hydrolases and polysaccharide depolymerizing enzymes, are essential for bacteriophages to adsorb and infect host bacteria65. Virus-associated peptidoglycan hydrolases are usually located in the outermost layer of the phage protein shells, which is the structural component of the virus and acts on the local degradation of the peptidoglycan layer so that the bacteriophage tail tube structure ejects its genome into the host. Polysaccharide depolymerizing enzymes, which can degrade polysaccharose components on the surface of the bacterial cell membrane, such as Gram-negative lipopolysaccharides, are also encoded by bacteriophages. The depolymerase of bacteriophages digests the polysaccharides on the cell membrane of bacteria, thereby facilitating the bacteriophages in entering the host receptors; they may degrade biofilms66. Bacteriophage-derived cell wall hydrolases or lysins have become potential alternatives to antimicrobial therapy in recent years. Owing to their rapid bactericidal effect, low drug resistance, and differential virulence, lysins have become attractive clinical candidates67. Bacteriophage-derived lysins have enzymatic properties and can kill non-divided bacterial cells, providing a feasible choice against drug-resistant or recurrent infections. The two main lysine enzymes are endolysins and ectolysins. Endolysins are expressed to decompose the peptidoglycans of the cell wall at the end of phage replication. Ectolysins decompose peptidoglycans externally to bacteria during bacteriophage DNA invasion. Lysine enzymes, defined as glycosidases, peptidases, or amidases, can split different chemical bonds based on the specificity of lysins. Adding lysin micrograms to turbid suspended Gram-positive bacterial cultures (>108 CFU/mL) can rapidly reduce the optical density of the culture within minutes68. In addition, as murein hydrolases only exist in bacteria, lysine does not act on eukaryotic cells. For example, bacteriophage k-derived chimeric recombinant exolysin, p128, can inactivate completely prokaryotic cells and has no cytotoxicity on eukaryotic cell lines Vero and Hep 2, even at a high concentration of 2.5 mg/mL.
The capsule structure and biofilm of bacteria are both key resistance factors that prevent the effects of antibiotics and disinfectants. In Streptococcus pneumoniae, Haemophilus influenzae, E. coli, and other pathogenic bacteria, the capsule structures can help pathogens escape the role of phagocytes and complement the system and promote epithelial colonization, cell invasion, and intravascular survival. Similarly, the biofilm structure can protect the bacterial population against immune effects. Therefore, targeting and removing these structures using bacteriophage-derived depolymerase is a feasible treatment. Bedi et al.69 demonstrated that the synergistic effect of depolymerase and amoxicillin could eradicate Klebsiella pneumoniae biofilms. The function of depolymerase is closely associated with bacteriophage tails70. The enzyme can also degrade the capsule of Erwinia amylovora (a plant pathogen) to resume sensitivity against resistant bacteriophages.
6.3. A new attempt: Bioengineering
With the advanced development of genetic technologies, natural biological entities can be modified or created to perform tasks that cannot be performed naturally71. The directed evolution and reformation of bioengineered bacteriophages can significantly improve the therapeutic potential of bacteriophages through the following mechanisms: (a) expanding the host range, (b) switching host orientation, (c) delivery of foreign genes, and (d) modification of bacteriophage capsids. For example, the host range of E. coli for bacteriophage T2 would be expanded if the long tail fiber gene from bacteriophage IP008 homologous was recombined in T2. Mahichi et al.72 established a chimeric bacteriophage with a wider host range of bacteriophage IP008 and strong lytic effect of bacteriophage T2. In addition, Collins and Lu73 modified the E. coli bacteriophage T7 to express enzyme protein B, a key component that can degrade bacterial biofilms and infect bacteria in host cells. Compared with the wild-type bacteriophage, the engineered bacteriophages can reduce the cellular biofilm count by more than 100 times. Bioengineering bacteriophages can also provide a possibility for improving the specificity of antibiotics or against multidrug-resistant bacterial pathogens. In a study, a pediatric patient with cystic fibrosis and double lung transplantation received a cocktail of three genetically engineered bacteriophages to treat a life-threatening antibiotic-resistant Mycobacterium abscess infection after all standard treatment options were exhausted74. The patient had a good prognosis, without obvious side effects.
With the development of synthetic biology and drug delivery, bacteriophages have been developed into new theranostic platforms. For example, Yacoby et al.75 designed a targeted drug delivery system to directly deliver antibiotics into bacteria by attaching chloramphenicol molecules to lysate bacteriophages. The in vitro efficacy increased by 2000 times, and the side effects caused by interactions between human cells and microflora could be eliminated. Similarly, bacteriophages can be used as targeting carriers to deliver photosensitizers. Pathogenic bacteria are more likely to be photodynamically inactivated and normal flora is less likely to be harmed.
In vivo experimental results demonstrated that this system effectively treats infections caused by antimicrobial-resistant bacteria and the fungal pathogen Candida albicans76,77. In addition, bacteriophage display technology has revealed specific molecular determinants of tumor cells and tumor-related microenvironment factors by displaying antibodies, peptides, or proteins on different bacteriophage surfaces. Bacteriophages have proven to be good carriers for imaging molecules and therapeutics. In tumor immunology, bacteriophages have been used to directly induce tumor immunity or apply their immunogenic characteristics to produce vaccines. Moreover, editing bacteriophages to carry suicide genes in cancer cells has greatly enhanced the prospect of gene therapy in anti-tumor treatment.
7. Access to treating AMR infection
The most recent bacteriophage therapies (using small vertebrates) have focused on AMR infections. Appropriate rapid diagnostic methods can also be used to determine the specific AMR bacteria that cause infection. When the source of infection is identified, specific bacteriophages would provide an appropriate therapeutic alternative to front-line antibiotic resistance in organisms. The use of broad-spectrum antibiotics can be reduced, greatly contributing in dealing with the drug resistance crises. Animal studies have shown that bacteriophage therapies are effective in some cases, including acute respiratory tract infections caused by P. aeruginosa, systemic infections caused by S. aureus, gastrointestinal tract infections, respiratory tract infections, and skin and wound infections78, 79, 80, 81. Studies on bacteriophage therapy in animal models have revealed that bacteriophage therapy may reduce the density of the infected AMR bacterial population to a certain level, thereby enabling the host immune response to successfully defend or eliminate infection78,79. A variety of in vivo bacteriophage studies (both animal and human) have indicated that bacteriophage therapies might also be beneficial for the treatment of intractable antibiotic-resistant pulmonary infections, such as pneumonia and cystic fibrosis78,82,83. The summary of phage therapy strategies is listed in Table 2.
Table 2.
Summary of phage therapy strategies.
| Disease | Pathogenic microbe | Treatment drug | Action mechanism |
|---|---|---|---|
| Superficial bacterial infections | PA and Klebsiella pneumoniae |
|
|
| Diabetic wound healing | Staphylococcus aureus, PA and Acinetobacter baumannii |
|
|
| Burn wound infection | PA,Klebsiella pneumoniae |
|
|
| Tumor | Tumor cells |
|
|
7.1. Potential treatments for superficial bacterial infections
The skin is the largest and outermost organ covering the entire body. Thus, skin injuries are among the most common physical injuries. The wound healing process occurs immediately after the injury to restore the structure and function of the skin84. However, following the emergence of AMR bacteria, infections are major obstacles to wound healing, resulting in at least 10,000 deaths per million injured patients. Animal experiments and clinical trials have proven the safety and efficiency of bacteriophages against AMR bacteria, including the treatment of P. aeruginosa and K. pneumoniae79. In 2015, the first randomized controlled trial (phase I/II) of a bacteriophage cocktail (pp1131) was designed against a P. aeruginosa infection. However, this trial was terminated because of insufficient therapeutic efficacy. One of the key problems in the trial was the poor stability of pp1131, where the bacteriophage titer decreased by 3 log within 15 days after manufacture. Yan et al.85 designed a bacteriophage-containing thermosensitive hydrogel for the treatment of skin infections. The thermosensitive hydrogel turned into a semisolid gel at skin temperature. Relevant research has also illustrated that IME-AB2 bacteriophages in around 18% (w/w) polyoxyamine 407 hydrogel solution have good storage stability, and the titer loss is zero within 24 months at 4 °C. The release of bacteriophages is continuous, with a cumulative release of 60% in the first 24 h. The biofilm-removing abilities of the bacteriophage gel and bacteriophage suspension are 59% and 45%, respectively. Adding bacteriophages to hydrogels not only has no significant effect on bacteriophage killing efficiency, but also maintains high bacteriophage titers at the infected sites to obtain effective treatment. In addition, the bacteriophage LysGH15 shows high lytic activity against methicillin-resistant S. aureus (AMR) and methicillin-sensitive S. aureus. Cheng et al.86 added LysGH15 and an anti-inflammatory apigenin (API) to Aquaphor to form a LysGH15-API-Aquaphor (LAA) ointment. In a mouse AMR-infected skin wound model treated with LAA, the average colony count decreased by approximately 100 CFU/mg 18 h after treatment, and the bacteria could not be detected after 96 h of treatment. In comparison, the average count of untreated mice was approximately 105 CFU/mg of tissue after 18 h. Concurrently, the LAA ointment reduced the levels of pro-inflammatory cytokines and accelerated wound healing. At present, liquid formulations are good carriers for the delivery of bacteriophages to wound infection sites. Theoretically, the preparation of liquid bacteriophage formulations is simple. Mature bacteriophage liquid preparations have been certified and listed by relevant institutions, such as the production of several liquid bacteriophage cocktails (Pyo, intestinal, staphylococcal, and SES bacteriophages) using Eliava bioremediation (Tbilisi, Georgia)87.
7.2. Potential treatments for diabetic wound healing
Refractory chronic wounds are a common complication of diabetes and the most common cause of non-traumatic lower-limb amputation. Unfortunately, bacteria develop resistance, which makes it difficult for doctors to treat. Therefore, it is necessary to find alternative treatments, such as bacteriophage therapy (Fig. 2C). The antibacterial activity and wound healing ability of bacteriophages against chronic S. aureus, P. aeruginosa, and Acinetobacter baumannii infections in rodent and pig models were researched by Mendes et al88. Their results showed that bacteriophages were effective in reducing the population density of S. aureus and P. aeruginosa in wound healing. Bacteriophage therapy is more effective in rodent models than in pig models, and the local administration of a bacteriophage therapy may effectively treat chronic infections, especially in wound debridement.
Bacteriophage cocktails can be encapsulated in liposomes to maintain their activity. Liposomes can mimic the structure and performance of biofilms, and can easily penetrate the epidermal barrier. In vitro stability and in vivo bacteriophage titer results showed that the liposome-entrapping bacteriophage cocktail has good bacteriophage persistence ability at the wound site. Compared with free bacteriophage cocktails, the titer of liposome-encapsulated bacteriophages at the wound site increases remarkablely. Persistent bacteriophage reproduction is observed inside and outside the wound site. Liposome-coated bacteriophage cocktails have also been investigated for their ability to address S. aureus-induced diabetic excision wound infection89. The results revealed that mice treated with free S. aureus-specific lytic bacteriophage cocktails had a significantly lower wound bioburden, good wound contraction, and quick tissue healing.
7.3. Potential treatments for burn wound infection
Although considerable progress has been made in antibacterial treatment, severe sepsis remains the primary cause of death in patients with burns. It was reported that more than 75% of the mortality of 78 patients with burns could be attributed to bacterial infections90. P. aeruginosa plays an important role as a cause of severe infections in burn patients. Acute burns can destroy the protective skin barrier and inhibit the immune system, resulting in bacterial infections. The P. aeruginosa colonization of severe burn wounds and its rapid diffusion into damaged tissues often lead to disseminated infection, bacteremia, septic shock, and high morbidity and mortality91. Refractory P. aeruginosa infections are common in burn patients. Hence, the use of bacteriophages as treatment options is currently being assessed. McVay et al.92 used a heat injury mouse model to test the efficacy of bacteriophages in the treatment of refractory P. aeruginosa infections. Burn-injured mice infected with P. aeruginosa were administered a single dose of P. aeruginosa phagocytic monomer cocktails via intramuscular, subcutaneous, and intraperitoneal injections. The results demonstrated that a single dose of the P. aeruginosa bacteriophage cocktail can significantly reduce mortality in mice. The survival rate of mice infected with P. aeruginosa after treatment was as high as 87%. However, the survival rate without treatment was as low as 6%. In addition, the route of administration had a significant impact on therapeutic efficacy, with intraperitoneal injections producing the best protection rate (>87%). The study also found no recurrence in cured mice, suggesting that bacteriophage cocktails may prevent the emergence of bacteriophage-resistant mutants92.
K. pneumoniae is one of the most common pathogens causing morbidity and mortality in patients with burns, accounting for 15.2% of burn wound infections93. The emergence of multidrug-resistant strains has made the treatment process for Klebsiella infections complex. Epidemiological studies have shown that the frequency of nosocomial infections caused by Klebsiella has increased significantly over the past decade. Chadha et al.94 studied the efficacy of bacteriophage cocktails in treating K. pneumoniae-mediated burn wound infections in mice. The efficacy of a single bacteriophage (kpn 1‒kpn 5) and bacteriophage cocktails was assessed in the treatment of burn wound infections in mice. Compared to the untreated control mice (8.81 log CFU/mL), the bacterial load of animals treated with kpn1‒kpn5 decreased significantly to 4.32, 4.64, 4.42, 4.11, and 4.27 log CFU/mL on peak day (the third day) respectively. However, the group treated with bacteriophage cocktails showed the greatest reduction in the bacterial load of the skin tissue. On the peak day, the bacterial load decreased significantly to 3.01 log CFU/mL and followed by steady decrease thereafter compared with the untreated control group, this result account for a significant reduction of 6 log cycles (P < 0.01). Bacteriophage cocktails effectively hindered the entire infection process (bacterial load, wound contraction, skin myeloperoxidase activity, collagen formation, and histopathological analysis). More importantly, compared to bacteriophage monotherapy, the bacteriophage cocktail significantly prevented the emergence of drug-resistant mutants.
7.4. Anti-tumors: New field of bacteriophage application
Intestinal microorganisms include approximately 100 trillion bacterial cells, which are crucial for human physiological functions. This microbiota plays a key role in the development of acute infections, chronic diseases, and tumors, and affects the regulation of the host immune system. Current evidence suggests that the intestinal microbiota can influence the effect of anti-tumor immunotherapy profoundly95,96. The regulation of intestinal flora can have a considerable effect on tumor treatment. Previous studies have shown that microorganisms are involved in the pathogenesis and prognosis of colorectal cancer (CRC). However, the relationship between the intestinal microflora and CRC is complex. Some microbial populations promote tumorigenesis, whereas others inhibit tumor growth. For example, Fusobacterium is highly resistant to chemical and immunological treatments97,98. Clinical data have shown that the abnormal proliferation of Fusobacterium can directly lead to chemotherapy failure 181. In contrast, CRC growth can be inhibited and the anti-tumor immune response can be induced by short-chain fatty acids produced by bacterial fermentation75,76. Owing to the non-selective killing of pro- and anti-tumor bacteria, it is clear that fecal bacterial transplantation and the antibiotic regulation of intestinal flora are difficult to use in the treatment of CRC97, 98, 99.
To precisely regulate the intestinal flora for the treatment of CRC, a gut microbiota modulatory method using bacteriophage-guided biotic-abiotic hybrid nanomaterials was designed by Dong et al100. Irinotecan (IRT) was encapsulated within dextran nanoparticles (DNPs) to form IRT-loaded DNPs (IDNPs). To construct a bacteriophage-guided biotic-abiotic hybrid nanosystem, azodibenzo-cycloctyne-modified IDNPs were linked to azide-modified bacteriophages, creating a bacteriophage-guided biotic-abiotic hybrid nanosystem via a biorthogonal reaction. This composite nanosystem eliminated Fusobacterium nucleatum. The accumulation of DNPs in tumors can be enhanced through a biorthogonal strategy, and DNPs can promote the proliferation of Clostridium butyricum and ameliorate the colon immunosuppressive tumor microenvironment. The safety and effectiveness of the hybrid system were further proven using a piglet model101, 102, 103.
In addition to regulating intestinal flora and triggering antitumor immunity, bacteriophages can be used as therapeutic carriers. Overexpressed and secreted angiogenic proteins in tumors strongly trigger vascular growth and plunder nutrients from surrounding tissues, contributing to subsequent tumor growth103. Anti-angiogenesis is a promising cancer treatment strategy, but is limited by the lack of the tumor localization ability of anti-angiogenesis drugs104. To ameliorate this problem, Li et al.105 designed a tumor homolog, angiogenin-binding bacteriophage-derived bio-nanofiber, to capture and block tumor-derived angiopoietin to achieve tumor regression (Fig. 5A). Bacteriophage nanofibers exist naturally in healthy humans and can be injected into animals and humans for treatment without obvious toxicity or immune responses (Fig. 5B)106,107. Filamentous phage fd388, a biocompatible nanofiber, can target breast tumors and prevent tumor angiogenesis. To produce nanofibers that can selectively inhibit breast tumor angiogenesis, bacteriophages produce two peptides through genetic engineering, for example, the MCF-7-breast tumor-homing peptide and the angiogenin-binding peptide WV. Engineered bacteriophages can display multiple copies of identified angiopoietin-binding peptides on their side walls and tumor homologous peptides at their tips. Hematoxylin and eosin staining showed necrosis in tumors treated with engineered bacteriophages, but it was rare in the other control groups108. In addition, no microvessels in the group treated with fd388 were found around the tumor necrosis area. As the tumor-homing peptide can be discovered and customized toward any specific tumor through bacteriophage display, angiopoietin-binding bacteriophages would be a general “plug and play” tumor-homing cancer therapeutic105.
Figure 5.
(A) Schematic of using tumor-homing angiogenin-binding engineered bacteriophage nanofibers (fd388-AR-WV) to inhibit the orthotopic breast tumor in the mouse model through targeted anti-angiogenesis therapy. Reprinted with permission from Ref. 105. Copyright © 2020, WILEY-VCH Verlag Gmbh & Co KGAA, Weinheim (B) Immunofluorescent staining image of average integrin expression in brain sections including intracranial tumor and the surrounding healthy brain (C) Co-staining of CD31 (red) and phage (green) in brain sections comprising tumor and healthy brain. Reprinted with permission from Ref. 107. Copyright © 2019 by the authors.
8. Possible challenges in the popularization of phage therapies
Bacteriophage therapies have been considered as an alternative treatment approach to deal with the impending crisis of antibiotic resistance. This is because not only can the bacteriophages save countless drug-resistant bacteria-infected patients, but also exhibit a number of undeniable benefits. Recently, the use of bacteriophages in treating animal infections has made an early strike. For example, bacteriophages have been used to treat diarrhea and mammary gland inflammation in domestic animals, such as pigs and cattle. Despite great progress, the clinical application of bacteriophages in medicine still presents multiple challenges that need to be addressed, particularly within the regulatory framework. In most countries, there is uncertainty regarding the regulatory status of bacteriophage therapy. At present, European regulations only allow sporadic clinical trials under the framework of the Helsinki Declaration and the responsibility and supervision of the medical ethics committee and frontier application. Thus, a regulatory framework must be constructed. Another challenge in the large-scale application of bacteriophage therapy is the financial cost. Only a few institutions in a few countries, such as Georgia and the U.S., qualify for bacteriophage therapies. Patients must bear the high cost of travel and medical treatment as well as spend considerable time. Furthermore, as biological agents, bacteriophages have high requirements for transportation and storage, as they require a complete cold-chain transportation system.
8.1. Quality control and safety requirements
For large-scale applications, phages must be mass-produced under good manufacturing practices (GMP) approved by regulatory authorities. However, there are no specific guidelines for phage production109. A group of phage researchers has set quality and safety standards for sustainable phage therapy products110. One of the criteria is to avoid phages encoding lysogenic and virulence factors, antibiotic resistance, and impurities, such as endotoxins. Although many purification methods have been used to remove these toxic elements from phage preparations, none have been satisfactory111. The clinical applications of bacteriophages are limited. Even for phage reserve preparations, quality control should be conducted periodically by checking the stability (shelf life), sterility, cytotoxicity, and periodic pH measurements.
8.2. Stability of phage preparations
A good stability of bacteriophages is vital for treatment. A potential therapeutic candidate phage preparation should have a good shelf life; that is, phages should be stored in the formulation to ensure that their activity does not decrease significantly. Another key problem of phage stability is the spontaneous mutation in the phage population when stored or accumulated for a long time during the manufacturing process of phage production, which may damage the fitness of the virus109. This fact reminds us to predict the evolution of phages and establish a manufacturing process to reduce the mutation rate of the phage genome112.
8.3. Fast phage screening methods
To target a specific strain, a large number of phage collections need to be screened owing to the high species specificity of phages. The traditional method for detecting bacteriophage activity is double-layer agar (DLA)113. However, the DLA method is not convenient for rapid diagnosis. According to the growth rate of specific strains, the results may take up to 48 h to display. Under these circumstances, high-throughput screening is expected to rapidly identify phages that can effectively infect target strains.
Bacteriophages are often detected and quantified by direct or indirect measurements in the laboratory, but there seems to be little possibility for their clinical application. For example, real-time fluorescence quantitative PCR has been developed for the rapid and sensitive detection of phages. However, PCR requires customized primers for each phage strain. When a large number of phages are collected from the target strain, it is neither a high flux nor feasible114. Based on optical density dynamics analysis in bacterial cultures, a simple method for detecting and quantifying phages has been proposed115. This method can detect a small number of phages with a response time of 3.5 h and is easy to miniaturize and automate for high-throughput applications. It can be implemented in conventional analysis. One possible disadvantage is that it depends only on the change in the optical density of the bacterial culture, which cannot always be observed for cleaved phages. Flow cytometry has also been used to detect phage infection by detecting cells with low-density cell walls (low-density cell walls have been observed as a result of phage infection). This method allows the rapid and early detection of phage infection; however, its flux is low and may not be applicable to all bacterial species or phages116. Other studies have indirectly detected phage reproduction by measuring the enzyme release from bacterial cells caused by phage-induced cell lysis. Enzyme release is detected by generating a bioluminescence or color signal after the cleavage of a specific substrate117. These detections are highly sensitive and can produce detectable signals within a short time (3 h). This method is high throughput and is theoretically applicable to any phage, but may need to be optimized for each bacterial species.
8.4. Regulatory framework of phage therapy
Relevant regulatory authorities classify bacteriophages as biological substances. Bacteriophages are part of the scope of drug legislation118. The regulatory frameworks of the European Union and the U.S. stipulate that these biological drugs prepared by industries or produced through industrial processes require sales authorization. Therefore, phage products need to be safe, effective, and comply with GMP standards. Meeting GMP standards requires considerable financial support, which is undoubtedly a key obstacle for hospitals and non-profit phage therapy centers119. Current legislation also requires predetermined qualitative and quantitative evaluations of each drug component. The recommended standards for phages include the dissolution and specific activity of a single phage to the target phage, the limitation of impurities (such as endotoxins and residual reagents) in phage preparations, and the test of phage efficacy and purity120. To some extent, this strict regulation is applicable to phage preparations with fixed components produced on an industrial scale, but it is certainly not sufficient to meet the requirements of phage preparations customized based on the patient's condition, whose components are variable. Therefore, a more flexible regulatory therapeutic framework is required.
9. Perspective of phage therapy and conclusions
The number of cases in which phage therapy has been successfully used to treat life-threatening infections is increasing121,122. One of the most exciting cases concerned a 68-year-old man who suffered from A. baumannii multidrug-resistant infection and had necrotizing pancreatitis. Despite several rounds of antibiotic treatment, the patient's condition did not improve, but worsened over time. By screening phages in the laboratory and customizing cocktails for the patient, the infection was eliminated and the physical condition of the patient gradually improved123. Meanwhile, the demand for phages around the world is also increasing, which requires the establishment of characterized phage libraries. Some phage libraries have already been constructed to provide fruitful territories for international co-production, such as the Félix d’Hérelle Reference Center for Bacterial Viruses at the University of Laval and the National Collection of Type Cultures122. With the renewed attention of all sectors of society to phage therapies, bacteriophages will certainly leave a strong mark in the “post antibiotic era”.
In this study, we demonstrated that bacteriophages are returning to the spotlight as a valid alternative to classical antibiotics in the post-antibiotic era. Recent studies have shown that bacteriophages have the potential to fill this AMR-induced gap. However, the lack of mature preparations for existing bacteriophage therapies has hindered their popularization. Thus, researchers are focusing on the development of bacteriophage preparations, such as bacteriophage cocktails, liposome-encapsulated bacteriophages, polymer-encapsulated bacteriophages, electrospun fiber-encapsulated bacteriophages, and microneedle-mediated transdermal bacteriophage delivery systems. However, bacteriophages have much more potential. To achieve a better effect on AMR infections, one approach is to combine bacteriophages with other antibiotics to produce synergistic efficacy. Another option is to use bacteriophages edited by genetic engineering; relevant clinical trials are ongoing. Bacteriophages can also serve as novel theranostic platforms. A variety of in vivo bacteriophage studies (both animal and human) have indicated that bacteriophage therapies may be effective for the treatment of intractable antibiotic-resistant pulmonary infections. Furthermore, bacteriophages can be used as carriers of other drugs (e.g., anticancer drugs) to increase their therapeutic effect. To standardize bacteriophage therapy in the clinical treatment, several gaps are needed to be filled, including (a) continuously enriching the reference bacteriophage library; (b) developing efficient bacteriophage screening methods for rapid identification of therapeutic bacteriophages; (c) establishing efficient bacteriophage treatment strategies for infectious biofilms; (d) establishing bacteriophage production specifications to ensure the quality, and safety of bacteriophage agents; (e) ensuring the stability of bacteriophage agents during storage and transportation.
Currently, great progress has been made in bacteriophage research. With a more reasonable design, bacteriophage therapy could develop into one of the most powerful weapons in the battle against AMR infections in the future. Although the promotion of bacteriophage therapy may be a challenging process, we firmly believe that the stumbling blocks will be overcome, and this process will incur medical, economic, commercial, and even political benefits to the society.
Acknowledgments
This work was supported by National Key R&D Program of China (No. 2021YFA0909900), National Natural Science Foundation of China (Nos. 82073777 and 81803442), Liaoning Revitalization Talents Program (No. XLYC180801), Shenyang Youth Science and Technology Innovation Talents Program (No. RC190454), China Postdoctoral Science Foundation (No. 2020M680986) and General Project of Liaoning Provincial Department of Education (Nos. LJKZ0927 and LJKQZ2021034).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2022.05.007.
Contributor Information
Mengchi Sun, Email: smc_1990@aliyun.com.
Jin Sun, Email: sunjin@syphu.edu.cn.
Authors contributions
Hao Ling and Xinyu Lou: Data collection and writing up of the first draft of the paper. Jin Sun and Mengchi Sun: Writing-Reviewing and Editing and Funding Support. Qiuhua Lou and Zhonggui He: Conceptualization and Investigation.
Conflicts of interest
The authors declare no competing financial interest.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Aminov R.I. A brief history of the antibiotic era: lessons learned and challenges for the future. Front Microbiol. 2010;1:134. doi: 10.3389/fmicb.2010.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Şen K.D., Ercan U.K., Bakay E., Topaloğlu N., Rosenholm J.M. Evolving technologies and strategies for combating antibacterial resistance in the advent of the postantibiotic Era. Adv Funct Mater. 2020;30:1908783. [Google Scholar]
- 3.Tacconelli E., Carrara E., Savoldi A., Harbarth S., Mendelson M., Monnet D.L., et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 4.Levin B.R., Bull J.J. Population and evolutionary dynamics of phage therapy. Nature Nat Rev Microbiol. 2004;2:166–173. doi: 10.1038/nrmicro822. [DOI] [PubMed] [Google Scholar]
- 5.Perron G.G., Whyte L., Turnbaugh P.J., Goordial J., Hanage W.P., Dantas G., et al. Functional characterization of bacteria isolated from ancient arctic soil exposes diverse resistance mechanisms to modern antibiotics. PLoS One. 2015;10 doi: 10.1371/journal.pone.0069533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen H.K., Moe L.A., Rodbumrer J., Gaarder A., Handelsman J. Functional metagenomics reveals diverse beta-lactamases in a remote Alaskan soil. ISME J. 2009;3:243–251. doi: 10.1038/ismej.2008.86. [DOI] [PubMed] [Google Scholar]
- 7.D'Costa V.M., King C.E., Kalan L., Morar M., Sung W.W., Schwarz C., et al. Antibiotic resistance is ancient. Nature. 2011;477:457–461. doi: 10.1038/nature10388. [DOI] [PubMed] [Google Scholar]
- 8.Price L.B., Newland J., Bole A., Bortolaia V., Larsen J., Loneragan G.H., et al. 2017. Combating antibiotic resistance—a policy roadmap to reduce use of medically important antibiotics in livestock.http://battlesuperbugs.com/sites/battlesuperbugs.com/files/Final%20Report%208.25.17.pdf Available from: [Google Scholar]
- 9.Aminov R.I. The role of antibiotics and antibiotic resistance in nature. Environ Microbiol. 2009;11:2970–2988. doi: 10.1111/j.1462-2920.2009.01972.x. [DOI] [PubMed] [Google Scholar]
- 10.Lowy F.D. Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Invest. 2003;111:1265–1273. doi: 10.1172/JCI18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frieri M., Kumar K., Boutin A. Antibiotic resistance. J Infect Public Heal. 2017;10:369–378. doi: 10.1016/j.jiph.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 12.D'Costa V.M., McGrann K.M., Hughes D.W., Wright G.D. Sampling the antibiotic resistome. Science. 2006;311:374–377. doi: 10.1126/science.1120800. [DOI] [PubMed] [Google Scholar]
- 13.Olivares Pacheco J., Bernardini A., Garcia-Leon G., Corona F., Sanchez M.B., Martinez J. The intrinsic resistome of bacterial pathogens. Front Microbiol. 2013;4:103. doi: 10.3389/fmicb.2013.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh C. Molecular mechanisms that confer antibacterial drug resistance. Nature. 2000;406:775–781. doi: 10.1038/35021219. [DOI] [PubMed] [Google Scholar]
- 15.Lopatkin Allison J., Bening Sarah C., Manson Abigail L., Stokes Jonathan M., Kohanski Michael A., Badran Ahmed H., et al. Clinically relevant mutations in core metabolic genes confer antibiotic resistance. Science. 2021;371 doi: 10.1126/science.aba0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yelin I., Kishony R. Antibiotic resistance. Cell. 2018;172:1136. doi: 10.1016/j.cell.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Krylov V.N. Phage Therapy in terms of bacteriophage genetics: hopes, prospects, safety, limitations. Russ J Genet. 2001;37:715–730. [PubMed] [Google Scholar]
- 18.Sengun I.Y., Karabiyikli S. Importance of acetic acid bacteria in food industry. Food Control. 2011;22:647–656. [Google Scholar]
- 19.Letkiewicz S., Międzybrodzki R., Kłak M., Jończyk E., Weber-Dąbrowska B., Górski A. The perspectives of the application of phage therapy in chronic bacterial prostatitis. FEMS Immunol Med Microbiol. 2010;60:99–112. doi: 10.1111/j.1574-695X.2010.00723.x. [DOI] [PubMed] [Google Scholar]
- 20.Abedon S.T., LeJeune J.T. Why bacteriophage encode exotoxins and other virulence factors. Evol Bioinf Online. 2005;1:97–110. [PMC free article] [PubMed] [Google Scholar]
- 21.Skurnik M., Pajunen M., Kiljunen S. Biotechnological challenges of phage therapy. Biotechnol Lett. 2007;29:995–1003. doi: 10.1007/s10529-007-9346-1. [DOI] [PubMed] [Google Scholar]
- 22.Hyman P. Phages for phage therapy: isolation, characterization, and host range breadth. Pharmaceuticals. 2019;12:35. doi: 10.3390/ph12010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurtz Caroline B., Millet Yves A., Puurunen Marja K., Perreault M., Charbonneau Mark R., Isabella Vincent M., et al. An engineered E. coli Nissle improves hyperammonemia and survival in mice and shows dose-dependent exposure in healthy humans. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aau7975. [DOI] [PubMed] [Google Scholar]
- 24.Adamik K.-N., Yozova I.D. Colloids yes or no?—a “Gretchen Question” answered. Front Vet Sci. 2021;8:566. doi: 10.3389/fvets.2021.624049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J.W., Chan C.T.Y., Slomovic S., Collins J.J. Next-generation biocontainment systems for engineered organisms. Nat Chem Biol. 2018;14:530–537. doi: 10.1038/s41589-018-0056-x. [DOI] [PubMed] [Google Scholar]
- 26.Egido J.E., Costa A.R., Aparicio-Maldonado C., Haas P.J., Brouns S.J.J. Mechanisms and clinical importance of bacteriophage resistance. FEMS Microbiol Rev. 2022;46:fuab048. doi: 10.1093/femsre/fuab048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernheim A., Sorek R. The pan-immune system of bacteria: antiviral defence as a community resource. Nat Rev Microbiol. 2020;18:113–119. doi: 10.1038/s41579-019-0278-2. [DOI] [PubMed] [Google Scholar]
- 28.Gupta P., Singh H.S., Shukla V.K., Nath G., Bhartiya S.K. Bacteriophage Therapy of Chronic nonhealing wound: clinical study. Int J Low Extrem Wounds. 2019;18:171–175. doi: 10.1177/1534734619835115. [DOI] [PubMed] [Google Scholar]
- 29.Rajesh Kumar S., Chelvaretnam S., Tan Y., Prabakaran M. Broadening the H5N3 vaccine immunogenicity against H5N1 virus by modification of neutralizing epitopes. Viruses. 2017;10:2. doi: 10.3390/v10010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abedon S., Kuhl S., Blasdel B., Kutter E. Phage treatment of human infections. Bacteriophage. 2011;1:66–85. doi: 10.4161/bact.1.2.15845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colom J., Cano-Sarabia M., Otero J., Cortés P., Maspoch D., Llagostera M., et al. Liposome-encapsulated bacteriophages for enhanced oral phage therapy against salmonella spp. Appl Environ Microbiol. 2015;81:4841–4849. doi: 10.1128/AEM.00812-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blount Zachary D., Lenski Richard E., Losos Jonathan B. Contingency and determinism in evolution: replaying life's tape. Science. 2018;362 doi: 10.1126/science.aam5979. [DOI] [PubMed] [Google Scholar]
- 33.Monteiro R., Pires D.P., Costa A.R., Azeredo J. Phage therapy: going temperate? Trends Microbiol. 2019;27:368–378. doi: 10.1016/j.tim.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Abedon S.T. Information phage therapy research should report. Pharmaceuticals. 2017;10 doi: 10.3390/ph10020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall A.R., De Vos D., Friman V.P., Pirnay J.P., Buckling A. Effects of sequential and simultaneous applications of bacteriophages on populations of Pseudomonas aeruginosa in vitro and in wax moth larvae. Appl Environ Microbiol. 2012;78:5646–5652. doi: 10.1128/AEM.00757-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kutateladze M., Adamia R. Phage therapy experience at the Eliava Institute. Med Maladies Infect. 2008;38:426–430. doi: 10.1016/j.medmal.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 37.Villarroel J., Larsen M.V., Kilstrup M., Nielsen M. Metagenomic analysis of therapeutic PYO phage cocktails from 1997 to 2014. Viruses. 2017;9:328. doi: 10.3390/v9110328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCallin S., Alam Sarker S., Barretto C., Sultana S., Berger B., Huq S., et al. Safety analysis of a Russian phage cocktail: from metaGenomic analysis to oral application in healthy human subjects. Virology. 2013;443:187–196. doi: 10.1016/j.virol.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 39.Singla S., Harjai K., Raza K., Wadhwa S., Katare O.P., Chhibber S. Phospholipid vesicles encapsulated bacteriophage: a novel approach to enhance phage biodistribution. J Virol Methods. 2016;236:68–76. doi: 10.1016/j.jviromet.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Otero J., García-Rodríguez A., Cano-Sarabia M., Maspoch D., Marcos R., Cortés P., et al. Biodistribution of liposome-encapsulated bacteriophages and their transcytosis during oral phage therapy. Front Microbiol. 2019;10:689. doi: 10.3389/fmicb.2019.00689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuerban K., Gao X., Zhang H., Liu J., Dong M., Wu L., et al. Doxorubicin-loaded bacterial outer-membrane vesicles exert enhanced anti-tumor efficacy in non-small-cell lung cancer. Acta Pharm Sin B. 2020;10:1534–1548. doi: 10.1016/j.apsb.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cui H., Yuan L., Lin L. Novel chitosan film embedded with liposome-encapsulated phage for biocontrol of Escherichia coli O157:H7 in beef. Carbohydr Polym. 2017;177:156–164. doi: 10.1016/j.carbpol.2017.08.137. [DOI] [PubMed] [Google Scholar]
- 43.Ma Y.-H., Islam G.S., Wu Y., Sabour P.M., Chambers J.R., Wang Q., et al. Temporal distribution of encapsulated bacteriophages during passage through the chick gastrointestinal tract. Poultry Sci. 2016;95:2911–2920. doi: 10.3382/ps/pew260. [DOI] [PubMed] [Google Scholar]
- 44.Fukuta T., Asai T., Kiyokawa Y., Nakada T., Bessyo-Hirashima K., Fukaya N., et al. Targeted delivery of anticancer drugs to tumor vessels by use of liposomes modified with a peptide identified by phage biopanning with human endothelial progenitor cells. Int J Pharm (Amst) 2017;524:364–372. doi: 10.1016/j.ijpharm.2017.03.059. [DOI] [PubMed] [Google Scholar]
- 45.Nieth A., Verseux C., Barnert S., Süss R., Römer W. A first step toward liposome-mediated intracellular bacteriophage therapy. Expet Opin Drug Deliv. 2015;12:1411–1424. doi: 10.1517/17425247.2015.1043125. [DOI] [PubMed] [Google Scholar]
- 46.Ma Y., Pacan J.C., Wang Q., Sabour P.M., Huang X., Xu Y. Enhanced alginate microspheres as means of oral delivery of bacteriophage for reducing Staphylococcus aureus intestinal carriage. Food Hydrocolloids. 2012;26:434–440. [Google Scholar]
- 47.Silva Batalha L., Pardini Gontijo M.T., Vianna Novaes de Carvalho Teixeira A., Meireles Gouvêa Boggione D., Soto Lopez M.E., Renon Eller M., et al. Encapsulation in alginate-polymers improves stability and allows controlled release of the UFV-AREG1 bacteriophage. Food Res Int. 2021;139:109947. doi: 10.1016/j.foodres.2020.109947. [DOI] [PubMed] [Google Scholar]
- 48.Colom J., Cano-Sarabia M., Otero J., Aríñez-Soriano J., Cortés P., Maspoch D., et al. Microencapsulation with alginate/CaCO3: a strategy for improved phage therapy. Sci Rep-UK. 2017;7:41441. doi: 10.1038/srep41441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryan E., Garland M.J., Singh T.R.R., Bambury E., O'Dea J., Migalska K., et al. Microneedle-mediated transdermal bacteriophage delivery. EUR J Pharm Sci. 2012;47:297–304. doi: 10.1016/j.ejps.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Díaz A., Del Valle L.J., Rodrigo N., Casas M.T., Chumburidze G., Katsarava R., et al. Antimicrobial activity of poly(ester urea) electrospun fibers loaded with bacteriophages. Fibers. 2018;6:33. [Google Scholar]
- 51.Korehei R., Kadla J.F. Encapsulation of T4 bacteriophage in electrospun poly(ethylene oxide)/cellulose diacetate fibers. Carbohydr Polym. 2014;100:150–157. doi: 10.1016/j.carbpol.2013.03.079. [DOI] [PubMed] [Google Scholar]
- 52.Nogueira F., Karumidze N., Kusradze I., Goderdzishvili M., Teixeira P., Gouveia I.C. Immobilization of bacteriophage in wound-dressing nanostructure. Nanomed-Nanotechnol. 2017;13:2475–2484. doi: 10.1016/j.nano.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 53.Chen S.Y., Harrison M., Ng E.K., Sauvageau D., Elias A. Immobilized reporter phage on electrospun polymer fibers for improved capture and detection of Escherichia coli O157:H7. ACS Food Sci Technol. 2021;1:1085–1094. [Google Scholar]
- 54.Anany H., Chen W., Pelton R., Griffiths M.W. Biocontrol of listeria monocytogenes and Escherichia coli O157:H7 in meat by using phages immobilized on modified cellulose membranes. Appl Environ Microbiol. 2011;77:6379–6387. doi: 10.1128/AEM.05493-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Worthington R.J., Melander C. Combination approaches to combat multidrug-resistant bacteria. Trends Biotechnol. 2013;31:177–184. doi: 10.1016/j.tibtech.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wright G.D. Antibiotic adjuvants: rescuing antibiotics from resistance. Trends Microbiol. 2016;24:862–871. doi: 10.1016/j.tim.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 57.Torres-Barceló C., Hochberg M.E. Evolutionary rationale for rhages as complements of antibiotics. Trends Microbiol. 2016;24:249–256. doi: 10.1016/j.tim.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 58.Comeau A.M., Tétart F., Trojet S.N., Prère M.-F., Krisch H.M. Phage-Antibiotic Synergy (PAS): beta-lactam and quinolone antibiotics stimulate virulent phage growth. PLoS One. 2007;2:e799. doi: 10.1371/journal.pone.0000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chaudhry W.N., Concepción-Acevedo J., Park T., Andleeb S., Levin B.R. Synergy and order effects of antibiotics and phages in killing Pseudomonas aeruginosa biofilms. PLoS One. 2017;12 doi: 10.1371/journal.pone.0168615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Torres-Barceló C., Arias-Sánchez F.I., Vasse M., Ramsayer J., Kaltz O., Hochberg M.E., et al. A window of opportunity to control the bacterial pathogen pseudomonas aeruginosa combining antibiotics and phages. PLoS One. 2014;9 doi: 10.1371/journal.pone.0106628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanjay Chhibber, Tarsem Kaur. Co-therapy using lytic bacteriophage and linezolid: effective treatment in eliminating methicillin resistant Staphylococcus aureus (MRSA) from diabetic foot infections. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oechslin F., Piccardi P., Mancini S., Gabard J., Moreillon P., Entenza J.M., et al. Synergistic interaction between phage therapy and antibiotics clears pseudomonas aeruginosa infection in endocarditis and reduces virulence. J Infect Dis. 2017;215:703–712. doi: 10.1093/infdis/jiw632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valério N., Oliveira C., Jesus V., Branco T., Pereira C., Moreirinha C., et al. Effects of single and combined use of bacteriophages and antibiotics to inactivate Escherichia coli. Virus Res. 2017;240:8–17. doi: 10.1016/j.virusres.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 64.Chan B.K., Sistrom M., Wertz J.E., Kortright K.E., Narayan D., Turner P.E. Phage selection restores antibiotic sensitivity in MDR Pseudomonas aeruginosa. Sci Rep-UK. 2016;6:26717. doi: 10.1038/srep26717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roach D.R., Leung C.Y., Henry M., Morello E., Singh D., Di Santo J.P., et al. Synergy between the host immune system and bacteriophage is essential for successful phage therapy against an acute respiratory pathogen. Cell Host Microbe. 2017;22:38–47. doi: 10.1016/j.chom.2017.06.018. e4. [DOI] [PubMed] [Google Scholar]
- 66.Maciejewska B., Olszak T., Drulis-Kawa Z. Applications of bacteriophages versus phage enzymes to combat and cure bacterial infections: an ambitious and also a realistic application? Appl Microbiol Biotechnol. 2018;102:2563–2581. doi: 10.1007/s00253-018-8811-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharma U., Vipra A., Channabasappa S. Phage-derived lysins as potential agents for eradicating biofilms and persisters. Drug Discov Today. 2018;23:848–856. doi: 10.1016/j.drudis.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 68.Pastagia M., Schuch R., Fischetti V.A., Huang D.B. Lysins: the arrival of pathogen-directed anti-infectives. J Med Microbiol. 2013;62:1506–1516. doi: 10.1099/jmm.0.061028-0. [DOI] [PubMed] [Google Scholar]
- 69.Bedi M.S., Verma V., Chhibber S. Amoxicillin and specific bacteriophage can be used together for eradication of biofilm of Klebsiella pneumoniae B5055. World J Microbiol Biotechnol. 2009;25:1145–1151. [Google Scholar]
- 70.Born Y., Fieseler L., Klumpp J., Eugster M.R., Zurfluh K., Duffy B., et al. The tail-associated depolymerase of Erwinia amylovora phage L1 mediates host cell adsorption and enzymatic capsule removal, which can enhance infection by other phage. Environ Microbiol. 2014;16:2168–2180. doi: 10.1111/1462-2920.12212. [DOI] [PubMed] [Google Scholar]
- 71.Cameron D.E., Bashor C.J., Collins J.J. A brief history of synthetic biology. Nat Rev Microbiol. 2014;12:381–390. doi: 10.1038/nrmicro3239. [DOI] [PubMed] [Google Scholar]
- 72.Mahichi F., Synnott A.J., Yamamichi K., Osada T., Tanji Y. Site-specific recombination of T2 phage using IP008 long tail fiber genes provides a targeted method for expanding host range while retaining lytic activity. FEMS Microbiol Lett. 2009;295:211–217. doi: 10.1111/j.1574-6968.2009.01588.x. [DOI] [PubMed] [Google Scholar]
- 73.Lu T.K., Collins J.J. Dispersing biofilms with engineered enzymatic bacteriophage. Proc Natl Acad Sci U S A. 2007;104:11197. doi: 10.1073/pnas.0704624104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abbasi J. Patient receives first genetically engineered phage treatment. JAMA. 2019;322:107. doi: 10.1001/jama.2019.9394. [DOI] [PubMed] [Google Scholar]
- 75.Yacoby I., Bar H., Benhar I. Targeted drug-carrying bacteriophages as antibacterial nanomedicines. Antimicrob Agents Chemother. 2007;51:2156–2163. doi: 10.1128/AAC.00163-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hope C.K., Packer S., Wilson M., Nair S.P. The inability of a bacteriophage to infect Staphylococcus aureus does not prevent it from specifically delivering a photosensitizer to the bacterium enabling its lethal photosensitization. J Antimicrob Chemother. 2009;64:59–61. doi: 10.1093/jac/dkp157. [DOI] [PubMed] [Google Scholar]
- 77.Dong S., Shi H., Zhang X., Chen X., Cao D., Mao C., et al. Difunctional bacteriophage conjugated with photosensitizers for Candida albicans-targeting photodynamic inactivation. Int J Nanomed. 2018;13:2199–2216. doi: 10.2147/IJN.S156815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Debarbieux L., Leduc D., Maura D., Morello E., Criscuolo A., Grossi O., et al. Bacteriophages can treat and prevent Pseudomonas aeruginosa lung infections. J Infect Dis. 2010;201:1096–1104. doi: 10.1086/651135. [DOI] [PubMed] [Google Scholar]
- 79.Alemayehu D, Casey Pat G, McAuliffe O, Guinane Caitriona M, Martin James G, Shanahan F, et al. Bacteriophages φMR299-2 and φNH-4 can eliminate Pseudomonas aeruginosa in the murine lung and on cystic fibrosis lung Airway cells. mBio 3:e00029-12. [DOI] [PMC free article] [PubMed]
- 80.Morello E., Saussereau E., Maura D., Huerre M., Touqui L., Debarbieux L. Pulmonary bacteriophage therapy on Pseudomonas aeruginosa cystic fibrosis strains: first steps towards treatment and prevention. PLoS One. 2011;6 doi: 10.1371/journal.pone.0016963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Capparelli R., Parlato M., Borriello G., Salvatore P., Iannelli D. Experimental phage therapy against Staphylococcus aureus in Mice. Antimicrob Agents Chemother. 2007;51:2765–2773. doi: 10.1128/AAC.01513-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Singla S., Harjai K., Katare O.P., Chhibber S. Bacteriophage-loaded nanostructured lipid carrier: improved pharmacokinetics mediates effective resolution of Klebsiella pneumoniae–induced lobar pneumonia. J Infect Dis. 2015;212:325–334. doi: 10.1093/infdis/jiv029. [DOI] [PubMed] [Google Scholar]
- 83.Chhibber S., Kaur S., Kumari S. Therapeutic potential of bacteriophage in treating Klebsiella pneumoniae B5055-mediated lobar pneumonia in mice. J Med Microbiol. 2008;57:1508–1513. doi: 10.1099/jmm.0.2008/002873-0. [DOI] [PubMed] [Google Scholar]
- 84.Adriaenssens E.M., Lehman S.M., Vandersteegen K., Vandenheuvel D., Philippe D.L., Cornelissen A., et al. CIM® monolithic anion-exchange chromatography as a useful alternative to CsCl gradient purification of bacteriophage particles. Virology. 2012;434:265–270. doi: 10.1016/j.virol.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yan W., Banerjee P., Liu Y., Mi Z., Bai C., Hu H., et al. Development of thermosensitive hydrogel wound dressing containing Acinetobacter baumannii phage against wound infections. Int J Pharm (Amst) 2021;602:120508. doi: 10.1016/j.ijpharm.2021.120508. [DOI] [PubMed] [Google Scholar]
- 86.Cheng M., Zhang L., Zhang H., Li X., Wang Y., Xia F., et al. An ointment consisting of the Phage lysin LysGH15 and apigenin for decolonization of methicillin-resistant Staphylococcus aureus from skin wounds. Viruses. 2018;10:244. doi: 10.3390/v10050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chang R.Y.K., Morales S., Okamoto Y., Chan H.-K. Topical application of bacteriophages for treatment of wound infections. Transl Res. 2020;220:153–166. doi: 10.1016/j.trsl.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mendes J.J., Leandro C., Corte-Real S., Barbosa R., Cavaco-Silva P., Melo-Cristino J., et al. Wound healing potential of topical bacteriophage therapy on diabetic cutaneous wounds. Wound Repair Regen. 2013;21:595–603. doi: 10.1111/wrr.12056. [DOI] [PubMed] [Google Scholar]
- 89.Chhibber S., Kaur J., Kaur S. Liposome entrapment of bacteriophages improves wound healing in a diabetic mouse MRSA infection. Front Microbiol. 2018;9:561. doi: 10.3389/fmicb.2018.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mundha Da S., Waghmare P., Rathod P., Ingole K. Bacterial and fungal profile of burn wound infections in tertiary care center. Indian J Burns. 2015;23:71. [Google Scholar]
- 91.Church D., Elsayed S., Reid O., Winston B., Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006;19:403–434. doi: 10.1128/CMR.19.2.403-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McVay C.S., Velásquez M., Fralick J.A. Phage therapy of Pseudomonas aeruginosa infection in a mouse burn wound model. Antimicrob Agents Chemother. 2007;51:1934–1938. doi: 10.1128/AAC.01028-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chung The H., Karkey A., Pham Thanh D., Boinett C.J., Cain A.K., Ellington M., et al. A high-resolution genomic analysis of multidrug-resistant hospital outbreaks of Klebsiella pneumoniae. EMBO Mol Med. 2015;7:227–239. doi: 10.15252/emmm.201404767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chadha P., Katare O.P., Chhibber S. In vivo efficacy of single phage versus phage cocktail in resolving burn wound infection in BALB/c mice. Microb Pathog. 2016;99:68–77. doi: 10.1016/j.micpath.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 95.Vétizou M., Pitt J.M., Daillère R., Lepage P., Waldschmitt N., Flament C., et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gopalakrishnan V., Helmink B.A., Spencer C.N., Reuben A., Wargo J.A. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell. 2018;33:570–580. doi: 10.1016/j.ccell.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kostic Aleksandar D., Chun E., Robertson L., Glickman Jonathan N., Gallini Carey A., Michaud M., et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14:207–215. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mima K., Sukawa Y., Nishihara R., Qian Z.R., Yamauchi M., Inamura K., et al. Fusobacterium nucleatum and T cells in colorectal carcinoma. JAMA Oncol. 2015;1:653–661. doi: 10.1001/jamaoncol.2015.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Louis P., Hold G.L., Flint H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12:661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 100.Dong X., Pan P., Zheng D.-W., Bao P., Zeng X., Zhang X.-Z. Bioinorganic hybrid bacteriophage for modulation of intestinal microbiota to remodel tumor-immune microenvironment against colorectal cancer. Sci Adv. 2020;6 doi: 10.1126/sciadv.aba1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Albac S, Medina M, Labrousse D, Hayez D, Bonnot D, Anzala N, et al. Efficacy of bacteriophages in a Staphylococcus aureus nondiabetic or diabetic foot infection murine model. Antimicrob Agents Chemother 64:e01870-19. [DOI] [PMC free article] [PubMed]
- 102.Ran B., Yuan Y., Xia W., Li M., Yao Q., Wang Z., et al. A photo-sensitizable phage for multidrug-resistant Acinetobacter baumannii therapy and biofilm ablation. Chem Sci. 2021;12:1054–1061. doi: 10.1039/d0sc04889e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miyake M., Goodison S., Lawton A., Gomes-Giacoia E., Rosser C.J. Angiogenin promotes tumoral growth and angiogenesis by regulating matrix metallopeptidase-2 expression via the ERK1/2 pathway. Oncogene. 2015;34:890–901. doi: 10.1038/onc.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chung A.S., Lee J., Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer. 2010;10:505–514. doi: 10.1038/nrc2868. [DOI] [PubMed] [Google Scholar]
- 105.Li Y., Qu X., Cao B., Yang T., Bao Q., Yue H., et al. Selectively suppressing tumor angiogenesis for targeted breast cancer therapy by genetically engineered phage. Adv Mater. 2020;32:2001260. doi: 10.1002/adma.202001260. [DOI] [PubMed] [Google Scholar]
- 106.Wang J., Yang M., Zhu Y., Wang L., Tomsia A.P., Mao C. Phage nanofibers induce vascularized osteogenesis in 3D printed bone scaffolds. Adv Mater. 2014;26:4961–4966. doi: 10.1002/adma.201400154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Przystal J.M., Waramit S., Pranjol M.Z.I., Yan W., Chu G., Chongchai A., et al. Efficacy of systemic temozolomide-activated phage-targeted gene therapy in human glioblastoma. EMBO Mol Med. 2019;11 doi: 10.15252/emmm.201708492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Loi M., Di Paolo D., Soster M., Brignole C., Bartolini A., Emionite L., et al. Novel phage display-derived neuroblastoma-targeting peptides potentiate the effect of drug nanocarriers in preclinical settings. J Control Release. 2013;170:233–241. doi: 10.1016/j.jconrel.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mutti M., Corsini L. Robust approaches for the production of active ingredient and drug product for human phage therapy. Front Microbiol. 2019;10:2289. doi: 10.3389/fmicb.2019.02289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pirnay J.-P., Blasdel B.G., Bretaudeau L., Buckling A., Chanishvili N., Clark J.R., et al. Quality and safety requirements for sustainable phage therapy products. Pharm Res-dordr. 2015;32:2173–2179. doi: 10.1007/s11095-014-1617-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hietala V., Horsma-Heikkinen J., Carron A., Skurnik M., Kiljunen S. The removal of endo- and enterotoxins from bacteriophage preparations. Front Microbiol. 2019;10:1674. doi: 10.3389/fmicb.2019.01674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.García R., Latz S., Romero J., Higuera G., García K., Bastías R. Bacteriophage production models: an overview. Front Microbiol. 2019;10:1187. doi: 10.3389/fmicb.2019.01187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kropinski A.M., Mazzocco A., Waddell T.E., Lingohr E., Johnson R.P. Enumeration of bacteriophages by double agar overlay plaque assay. Methods Mol Biol. 2009;501:69–76. doi: 10.1007/978-1-60327-164-6_7. [DOI] [PubMed] [Google Scholar]
- 114.Ly-Chatain M.H., Durand L., Rigobello V., Vera A., Demarigny Y. Direct quantitative detection and identification of lactococcal bacteriophages from milk and whey by real-time PCR: application for the detection of lactococcal bacteriophages in goat's raw milk whey in France. Internet J Microbiol. 2011;2011:594369. doi: 10.1155/2011/594369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rajnovic D., Munoz-Berbel X., Mas J. Fast phage detection and quantification: an optical density-based approach. PLoS One. 2019;14 doi: 10.1371/journal.pone.0216292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Michelsen O., Cuesta-Dominguez Á., Albrechtsen B., Jensen Peter R. Detection of bacteriophage-infected cells of lactococcus lactis by using flow cytometry. Appl Environ Microbiol. 2007;73:7575–7581. doi: 10.1128/AEM.01219-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guzmán Luna C., Costán-Longares A., Lucena F., Jofre J. Detection of somatic coliphages through a bioluminescence assay measuring phage mediated release of adenylate kinase and adenosine 5′-triphosphate. J Virol Methods. 2009;161:107–113. doi: 10.1016/j.jviromet.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 118.Reindel R., Fiore C.R. Phage therapy: considerations and challenges for development. Clin Infect Dis. 2017;64:1589–1590. doi: 10.1093/cid/cix188. [DOI] [PubMed] [Google Scholar]
- 119.Jault P., Leclerc T., Jennes S., Pirnay J.P., Que Y.-A., Resch G., et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): a randomised, controlled, double-blind phase 1/2 trial. Lancet Infect Dis. 2019;19:35–45. doi: 10.1016/S1473-3099(18)30482-1. [DOI] [PubMed] [Google Scholar]
- 120.Pelfrene E., Willebrand E., Cavaleiro Sanches A., Sebris Z., Cavaleri M. Bacteriophage therapy: a regulatory perspective. J Antimicrob Chemother. 2016;71:2071–2074. doi: 10.1093/jac/dkw083. [DOI] [PubMed] [Google Scholar]
- 121.Expert round table on a, re-implementation of bacteriophage t. Sybesma W., Rohde C., Bardy P., Pirnay J.P., et al. Silk route to the acceptance and re-implementation of bacteriophage therapy—part II. Antibiotics. 2018;7:35. doi: 10.3390/antibiotics7020035. 7: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McCallin S., Sacher J.C., Zheng J., Chan B.K. Current state of compassionate phage therapy. Viruses. 2019;11:343. doi: 10.3390/v11040343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schooley Robert T, Biswas B, Gill Jason J, Hernandez-Morales A, Lancaster J, Lessor L, et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant acinetobacter baumannii Infection. Antimicrob Agents Chemother61:e00954-17. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.