Abstract
Purpose:
High-field magnetic resonance-linear accelerators (MR-Linacs), linear accelerators combined with a diagnostic magnetic resonance imaging (MRI) scanner and online adaptive workflow, potentially give rise to novel online anatomic and response adaptive radiation therapy paradigms. The first high-field (1.5T) MR-Linac received regulatory approval in late 2018, and little is known about clinical use, patient tolerability of daily high-field MRI, and toxicity of treatments. Herein we report the initial experience within the MOMENTUM Study (NCT04075305), a prospective international registry of the MR-Linac Consortium.
Methods and Materials:
Patients were included between February 2019 and October 2020 at 7 institutions in 4 countries. We used descriptive statistics to describe the patterns of care, tolerability (the percentage of patients discontinuing their course early), and safety (grade 3-5 Common Terminology Criteria for Adverse Events v.5 acute toxicity within 3 months after the end of treatment).
Results:
A total 943 patients participated in the MOMENTUM Study, 702 of whom had complete baseline data at the time of this analysis. Patients were primarily male (79%) with a median age of 68 years (range, 22-93) and were treated for 39 different indications. The most frequent indications were prostate (40%), oligometastatic lymph node (17%), brain (12%), and rectal (10%) cancers. The median number of fractions was 5 (range, 1-35). Six patients discontinued MR-Linac treatments, but none due to an inability to tolerate repeated high-field MRI. Of the 415 patients with complete data on acute toxicity at 3-month follow-up, 18 (4%) patients experienced grade 3 acute toxicity related to radiation. No grade 4 or 5 acute toxicity related to radiation was observed.
Conclusions:
In the first 21 months of our study, patterns of care were diverse with respect to clinical utilization, body sites, and radiation prescriptions. No patient discontinued treatment due to inability to tolerate daily high-field MRI scans, and the acute radiation toxicity experience was encouraging.
Introduction
High-precision external beam radiation therapy may allow treatment intensification without increasing toxicity, potentially leading to improved clinical outcome, quality of life, or overall survival.1–4 Magnetic resonance linear accelerators (MR-Linacs)—a combination of a linear accelerator (linac), magnetic resonance imaging (MRI) scanner, and an online adaptive workflow—hold promise for high-precision radiation therapy using magnetic resonance (MR)-guided radiation therapy (MRgRT).5,6 Visualization of anatomy during MRgRT on the MR-Linac enables daily adaptation of radiation therapy treatment plans, thereby compensating for motion and shape changes of targets and organs at risk (OARs).7–9 Consequently, this may allow for treatment with smaller safety margins and therefore treatment with higher doses.10,11 Furthermore, serial imaging with quantitative MRI sequences during treatments may facilitate response monitoring. In the future, this information may guide adaptation of treatments to tumor or normal tissue responses.12–15 High-field MR-Linacs represent the most recent implementation of MRgRT. By integrating a 1.5 T MR magnet, high-field MR-Linacs may generate improved signal-to-noise ratios that facilitate both anatomic and biological response adaptive therapy.16,17
MR-Linacs are generally more expensive than computed tomography based linacs and introduce new patient-related complexities to the radiation therapy workflow, such as MR-safety and claustrophobia, as well as treatment-related challenges. The latter includes geometric distortions, alterations of visible anatomy or the target due to nonlinearities of the gradients and inhomogeneities in B0 field, along with dosimetric impacts of the magnetic field, called the electron return effect (ERE), which results in curvature of electrons’ pathways, particularly at tissue-air barriers.14,18–20 Despite mitigation strategies for these complexities, early clinical studies are needed to confirm patient tolerability and toxicity outcomes associated with the use of this novel device. To make rational decisions about health care investments and individual treatment decisions, patients, providers, and payers need timely information about safety and efficacy profiles.21
Historically, during the implementation of new radiation therapy devices, research on these devices has been ad hoc, usually encompassing proof of concept and limited patient safety studies.22,23 Furthermore, evaluation of efficacy in larger populations treated on novel radiation therapy devices often occurs many years after their introduction, long after broad implementation in clinical practice.24–26 Systematic prospective collection of clinical, toxicity, and patient-reported outcome (PRO) data concurrent with the implementation of a novel device and treatment paradigm would facilitate early evaluation of the device.27 Such data would also provide the opportunity to identify indications wherein patients are most likely to benefit from the innovations, warranting further evaluation in randomized controlled trials.
In line with this systematic approach to data collection, an international prospective registry, called the Multi-Outcome Evaluation of radiation therapy using the MR-Linac (MOMENTUM) Study (NCT04075305) was designed to facilitate evidence-based implementation of the world’s first commercially available high-field MR-Linac (The Elekta Unity, Elekta AB, Stockholm, Sweden) and capture its initial experience.28,29,30 MOMENTUM serves as a research infrastructure enabling the various steps necessary for evidence-based implementation of high-field MRgRT through systematic collection of treatment and outcome data of patients treated on the Elekta Unity, in accordance with R-IDEAL, a methodological tool for the implementation of radiotherapy devices.27
Herein we report the initial 21 months’ experience with high-field MRgRT: how clinicians are using it, how patients tolerate daily high-field MRI, and the grade 3+ toxicity patterns of treatments.
Methods and Materials
The MOMENTUM Study is an academic industrial collaboration between international institutions and the manufacturer of the high-field MR-Linac (Unity, Elekta AB, Stockholm, Sweden) within the context of the MR-Linac Consortium.31 All patients >18 years old treated on an MR-Linac at participating institutions were eligible for inclusion. Patients consented to prospective collection of clinical and technical patient data. Patients had the option to consent to additional collection of PROs and additional MRI scans for research purposes for up to 90 minutes of additional table time per week. Patients in the Netherlands had the choice to share their data with the industry partner, whereas patients at the other institutions automatically shared their data when consenting to the study. These data are used by the industry partner to improve the device. All data were pseudonymized at the institution before storage in the registry.
Clinical and technical patient data
Trained clinical research coordinators collected clinical data upon entry into the cohort (baseline) and at 3, 6, 12, and 24 months after treatment on the MR-Linac. These data included demographics, tumor characteristics, treatment details, toxicity, and disease-response assessment data.
To assess the tolerability of daily high-field MRI, we captured whether and why a patient discontinued an MR-Linac course earlier than initially planned. Acute toxicity was defined as new-onset adverse event of grade 3, 4, or 5 (as per the National Cancer Institute’s Common Terminology Criteria for Adverse Events v 5.0) that was classified as related or unrelated to radiation and that was apparent within 3 months of completion of radiation therapy. For this study, the safety of the MR-Linac treatment was determined by the acute radiation-related toxicity rate.
MOMENTUM also captured technical data, defined as all data generated and used by the MR-Linac, including the treatment details: total gray (in cGy), number of fractions, and fractions on conventional linacs in cases where patients were treated using conventional linacs. Treatment fractions are described as adapt-to-shape (ATS), adapt-to-position (ATP), or a combination of these workflows. In the ATS workflow, target and/or OAR contours are deformed or recontoured to the daily anatomy of the patient, and the radiation plan is reoptimized. The ATP workflow refers to fractions where the MRI is only used for image guidance and where the reference plan is shifted to account for position changes of the target volume, akin to a couch shift in conventional therapy. Contours are not modified or deformed in the ATP workflow. In ATP, instead of shifting the couch, multileaf collimators are shifted to change the dose distribution in space. More detailed descriptions of the ATS and ATP workflow have been published elsewhere.7,32,33
Statistical analysis
Descriptive statistics were used to describe patient characteristics and treatment details. Patient, tumor, and treatment characteristics were presented as means with standard errors, median with (interquartile) range, or frequencies with percentages, depending on their distribution. Data were visualized using Statistical Package for Social Sciences version 25 (released 2017, IBM SPSS Statistics for Windows, Version 25.0; IBM Corp, Armonk, NY).
In our analysis, we report on several distinct subgroups within the MOMENTUM Study. Because MOMENTUM is a prospective registry and patient accrual and follow-up is a continuous process, the number of patients may differ for the various analyses, with more patients available when analyzing baseline characteristics, than when analyzing 3 months toxicity. The MOMENTUM Study allows retrospective inclusion and therefore includes patients treated on the MR-Linac before the start of MOMENTUM. Data presented are based on data received in the registry up to November 2020.
Results
Between February 1, 2019 and November 1, 2020, 934 patients were enrolled in the MOMENTUM Study. Based on publicly available global treatment volume data collected by Elekta, we estimate that the MOMENTUM cohort represents approximately 50% of all patients treated globally with high-field MRgRT.34 Within our cohort and at the time of this analysis, 702 patients had complete baseline data, of whom 6 received multiple courses on the MR-Linac (Table 1). Of the 702 patients, 486 (69%) consented to collection of additional PROs and 491 (70%) to acquisition of additional research MRI scans, and almost all patients (695, 99%) shared their data with the industry partner.
Table 1.
Radiation courses and tumor characteristics of patients with baseline data (n = 702), complete treatment data (n = 516), and toxicity data (n = 415)
| n (%)* | ||
|---|---|---|
| Patients with baseline data | Total patients | 702 |
| Patients with multiple courses | 6† | |
| Male | 554 (79%) | |
| Age, y‡ | 68 (22-93) | |
| Good performance score§ (n = 384) | 353 (92%) | |
| Clinical indication | Primary tumor/tumor bed | 491 (70%) |
| Regional lymph nodes | 110 (16%) | |
| Metastasis | 70 (10%) | |
| Recurrence primary site | 31 (4%) | |
| Treatment intent | Curative | 523 (75%) |
| Palliative | 164 (23%) | |
| Unknown | 15 (2%) | |
| Tumor sites treated | 39 | |
| Patients per tumor site║ | Prostate | 281 (40%) |
| Lymph nodes | 117 (17%) | |
| Brain | 85 (12%) | |
| Rectum | 70 (10%) | |
| Liver# | 33 (5%) | |
| Pancreas | 29 (4%) | |
| Oropharynx | 13 (2%) | |
| Lung | 8 (1%) | |
| Esophagus | 7 (1%) | |
| Bladder | 6 (1%) | |
| Patients with treatment Data | Total patients | 516** |
| Patients with multiple courses | 6 | |
| Male | 418 (81%) | |
| Age, y‡ | 69 (25-93) | |
| Good performance score§ (n = 244) | 221 (91%) | |
| Patients per tumor site | Prostate | 223 (42%) |
| Lymph nodes | 106 (21%) | |
| Rectum | 57 (11%) | |
| Liver# | 30 (6%) | |
| Pancreas | 21 (4%) | |
| Oropharynx | 12 (2%) | |
| Brain | 7 (1%) | |
| Patients with toxicity Data | Total patients | 415** |
| Patients with multiple courses | 5 | |
| Male | 331 (80%) | |
| Age, y‡ | 68 (25-93) | |
| Good performance score§ (n = 287) | 261 (91%) | |
| Patients per tumor site | Prostate | 167 (40%) |
| Lymph nodes | 92 (22%) | |
| Rectum | 43 (10%) | |
| Liver# | 27 (7%) | |
| Pancreas | 19 (5%) | |
| Oropharynx | 12 (3%) | |
Numbers (percentage) except where indicated.
For the treatments and toxicity data all courses were analyzed.
For clarity, the table only summarizes the characteristics of a patient’s first course within MOMENTUM, except where indicated.
Frequencies given in median (range).
Good performance score defined as Karnofsky Performance Score (KPS) ≥80%, Eastern Cooperative Oncology Group (ECOG) score 0, or Charlson Comorbidity Index (CCI) score ≤2.
Total number of patients (%) for the 10 most frequently treated tumor sites.
The liver tumor site includes patients with cancers of the intrahepatic bile ducts.
Median age of patients was 68 (range, 22-93) years; 554 (79%) patients were male, and most patients (523, 70%) received treatment with a curative intent (Table 1). There were 39 different tumor sites treated, the most common being prostate (281, 40%), oligometastatic lymph node (117, 17%), brain (85, 12%), and rectum (70, 10%) cancers. Radiation of the primary tumor (70%) was the most common clinical indication, followed by (regional) lymph node (16%), distant metastases (10%), and tumor recurrence at the primary tumor site (4%).
At the time of this analysis, we had complete (re)irradiation treatment data for 516 treatment courses (of 510 patients). Of these, 497 (96%) courses were delivered entirely using the MR-Linac, whereas 19 (4%) were delivered partly on an MR-Linac and partly on a conventional device (Table 2). In these mixed-modality courses, the MR-Linac was typically used for the boost phase of a treatment. For the most frequently treated tumor sites, the median number of fractions was 29 for oropharynx and brain and 5 for prostate, oligometastatic lymph node, rectum, and pancreas (Fig. 1).
Table 2.
Descriptive parameters for 516 MR-Linac courses
| n (%)* | ||
|---|---|---|
| Course details | Total courses | 516 |
| Mixed modalities† | 19 (4%) | |
| Fractions (median [range]) | 5 (1-35) | |
| Plan adaptation strategy‡ | ATS | 328 (64%) |
| ATP | 152 (29%) | |
| Mixed (ATP and ATS) | 35 (7%) | |
| Unknown | 1 (0.2%) | |
| Discontinued Courses | 6 (1%) | |
| MR-Linac downtime Clinical deterioration# |
3 3 |
Abbreviations: ATP = adapt-to-position; ATS = adapt-to-shape; MR-Linac = magnetic resonance-linear accelerators.
Numbers (percentage) except where indicated.
Course was delivered using the MR-Linac and a conventional linac or other radiation therapy method.
In the ATS strategy the plan is adapted based on daily anatomy, whereas during ATP the plan is adapted based on daily patient position.
The reason to discontinue treatment was not related to the MR-Linac treatment in these patients.
Fig. 1.
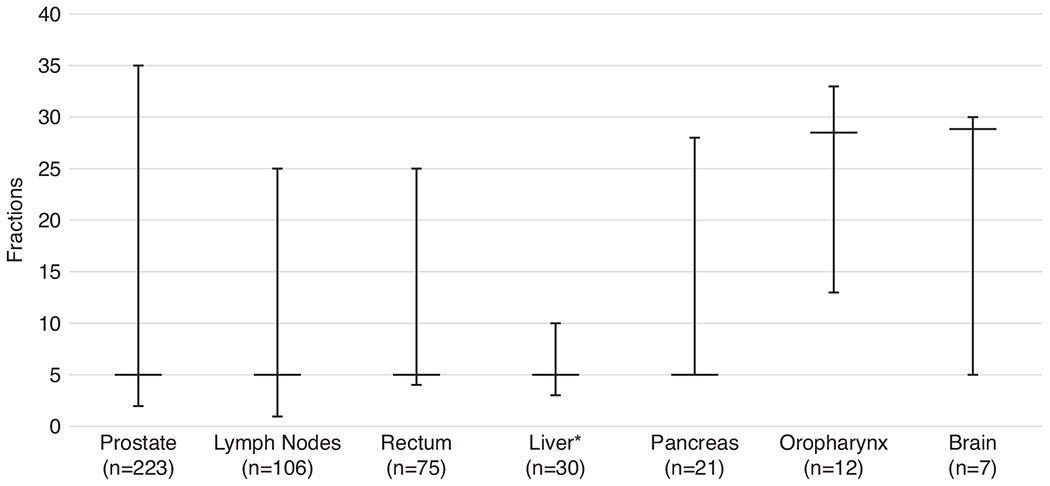
Median number of fractions (and range) for the most frequently treated tumor sites. Number of patients per tumor site is the total number of patients with complete treatment data.*The liver tumor site includes patients with cancers of the intrahepatic bile ducts.
Of the 516 courses, 152 (29%) used ATP for every fraction, 328 (64%) used ATS for every fraction, 35 (7%) used both ATP and ATS, and 1 (0.2%) was unknown. For the most frequently treated tumor sites, both adaptation strategies were used except for patients with brain tumors, in whom treatment was adapted using an ATP strategy only (Table 3).
Table 3.
Treatment strategy for the most frequently treated disease sites
| ATS* | ATP† | Mixed (ATS and ATP) | |
|---|---|---|---|
| Brain (n = 7) | - | 7 (100%) | - |
| Oropharynx (n = 12) | 1 (33%) | 4 (8%) | 7 (58%) |
| Pancreas (n = 21) | 16 (76%) | 5 (24%) | - |
| Liver‡ (n = 30) | 5 (17%) | 25 (83%) | - |
| Rectum (n = 75) | 40 (70%) | 14 (25%) | 3 (5%) |
| Lymph nodes (n = 106) | 87 (82%) | 19 (18%) | - |
| Prostate (n = 223) | 144 (5%) | 61 (27%) | 18 (8%) |
Abbreviations: ATS = adapt-to-shape; ATP = adapt-to-position.
Treatment adaptation strategy based on daily anatomy.
Treatment adaptation strategy based on daily patient position.
The liver tumor site includes patients with cancers of the intrahepatic bile ducts.
Six of 516 (1%) MR-Linac courses were discontinued earlier than expected (Table 2). Three courses were discontinued due to machine downtime: In the first case, the patient received treatment on a conventional linac for 4 days while the MR-Linac was down before returning to the MR-Linac; in the second case, the patient received a final fraction of the prescribed radiation course on a conventional linac when the MR-Linac went down; and in the third case, the treating physician cancelled the final fraction after the machine went down. Two courses were discontinued due to clinical progression: In the first case, the patient was found to have progressive cancer outside of the radiation field, and in the second case, the patient did not experience an improvement in pain control and was transitioned to an alternative therapy. One course of therapy was discontinued when the patient was hospitalized at a remote facility for side effects that were attributed to chemotherapy.
At the time of this analysis, 415 patients had complete data on acute toxicity. Of these, 18 (4%) patients experienced a new-onset grade 3+ adverse event within 3 months of treatment that was deemed possibly, probably, or definitely related to radiation. The details of all recorded adverse events are provided in Table 4.
Table 4.
Tumor and treatment details of patients with any recorded maximum grade 3 to 4 adverse event and whether the event was classified as possibly, probably, or definitely related to radiation therapy
| Tumor site | Radiation therapy | Fractionation (cGy/F) | Adverse event†† | Grade | n n = 26) | RT related (n = 18) |
|---|---|---|---|---|---|---|
| Prostate | MRL only | 6000/20 | Erectile dysfunction | 3 | 8 | 8/8 |
| Prostate | MRL only | 6000/20 | Stroke, erectile dysfunction* | 3 | 1 | 1/1 |
| Prostate | MRL only | 3625/5 | Erectile dysfunction | 3 | 1 | 1/1 |
| Prostate | MRL only | 3625/5 | Progression of multiple myeloma | 3 | 1 | 0/1 |
| Prostate | MRL only | 3862/5 | Respiratory failure, sepsis, flu-like symptoms† | 3 and 4 | 1 | 0/1 |
| Bladder | MRL only | 3600/6 | Bronchial infection | 3 | 1 | 0/1 |
| Bladder | MRL only | 3600/6 | Erectile dysfunction | 3 | 1 | 1/1 |
| Pancreas | MRL only | 5205/28 | Decreased lymphocyte count | 3 | 1 | 0/1 |
| Pancreas | MRL only | 3500/5 | Anemia | 3 | 1 | 0/1 |
| Pancreas | MRL only | 5040/28 | Nausea, diarrhea, abdominal pain, malabsorption, pancreatic enzymes decreased, enterocolitis infection, dehydration‡ | 3 | 1 | 1/1 |
| Peritoneum§ | MRL only | 1400/2 | Heart failure | 3 | 1 | 0/1 |
| Liver | MRL only | 5000/5 | Umbilical hernia | 3 | 1 | 0/1 |
| Liver | MRL only | 4775/5 | Peripheral ischemia | 3 | 1 | 0/1 |
| Hypopharynx | MRL only | 5319/25 | Dysphagia and dry mouth | 3 | 1 | 1/1 |
| Oropharynx | MRL only | 7118/33 | Dysphagia, dry mouth, esophagitis, and oral mucositis | 3 | 1 | 1/1 |
| Oropharynx | MRL only | 5977/28 | Dysphagia, dry mouth, and esophagitis | 3 | 1 | 1/1 |
| Oropharynx | MRL only | 7074/33 | Dry mouth, esophagitis, and oral mucositis | 3 | 1 | 1/1 |
| Oropharynx | Mixed modalities║ | 4991/23# | Lung infection, dysphagia, esophagitis, and dry mouth** | 3 | 1 | 1/1 |
| Larynx | Mixed modalities║ | 3417/17# | Dysphagia, dry mouth, esophagitis, and oral mucositis | 3 | 1 | 1/1 |
Abbreviations: MRL = magnetic resonance-linear accelerators; RT = radiation therapy.
This patient with prostate cancer had a stroke that was classified as unrelated to the MRL treatment.
This patient with prostate cancer experienced respiratory failure and sepsis grade 4 and flu-like symptoms grade 3 due to an infection with the influenza virus, which was classified as unrelated to the radiation therapy.
This patient with pancreatic cancer was dehydrated due to an enterocolitis infection, which was classified as unrelated to the radiation therapy.
Includes retroperitoneal tumor sites.
Course is delivered using the MRL and a conventional linac or other RT method.
Fractionation scheme of MRL treatment, not of the entire course.
This patient with oropharyngeal cancer had a lung infection, which was classified as unrelated to the radiation therapy.
Adverse events were defined as new-onset adverse event grade 3, 4, or 5 according to the National Cancer Institute’s (NCI) Common Terminology Criteria for Adverse Events (CTCAE) v 5.0.
Discussion
In an effort to accelerate both the clinical development and evaluation of MRgRT according to the R-IDEAL frame-work, the MR-Linac Consortium opened the MOMENTUM clinical registry, which has captured approximately half of the first cohort of high-field MR-Linac patients treated globally.27 In this initial report, we observed 3 important findings: First, early in its clinical implementation, the MR-Linac has been used in a wide variety of clinical indications; second, no patients discontinued their MR-Linac treatment course due to claustrophobia or discomfort; and third, the high-grade acute toxicity rates were acceptable, with 4% (n = 18) reporting radiation-related acute grade 3 toxicity.
Twenty-one months since the start of this study, MOMENTUM comprises a substantial amount of all known patients treated on a high-field MR-Linac. These patients represent a specific population because patient selection for MRgRT is determined by the treating physician. However, with the aim to include every patient treated on the MR-Linac irrespective of treatment outcomes, our study prospectively collects data of consecutively treated patients and facilitates objective reports on this population.27 Moreover, MOMENTUM can be used to design clinical studies and randomized trials with appropriate assumptions and suitable power for specific subgroups. These studies can guide identification of the treatment indications with the most clinical value. At the time of writing, several initiatives within the Consortium have requested data for several disease sites, which suggests that investigators indeed appreciate the potential of this registry in optimizing patient selection for high-field MRgRT.
In this study, patients were treated for a wide variety (39) of clinical indications. This variety illustrates the keenness of investigators to apply MRgRT for the different treatment indications but might also reflect the diversity of technical challenges this new device is meant to address by visualizing targets and OARs.12,20,35 Also, the large number of indications might be a result of the systematic clinical evaluation strategy that was used to introduce this technology, which facilitated rapid diversification of the use of this tool.27,31 It is worth noting that in the literature of a recently released low field MR-Linac (MRidian; Viewray Inc, Oakwood, USA), the dominant initial indications are similar to the sites treated on the MR-Linac.36–40 In 2 large single-center studies evaluating their initial experience and patterns of care with the MRIdian, we see distinct treatment indications per center: Sahin et al primarily treated patients with prostate (n = 24, 33%) and lung cancer (n = 15, 21%), whereas Klüter et al primarily treated lymph node metastases (n = 19, 42%) and liver lesions (n = 8, 14%).41,42 The overlap of the treatment indications with the results from this study suggests that similar clinical advantages are explored irrespective of MR-Linac field strength.
Our study also shows that in more than half of total fractions, patients underwent an ATS treatment strategy, meaning the attending radiation oncologist felt it was necessary to adapt contours on a daily basis. This process is associated with a cost in clinic and clinician time, but it was consistently implemented across multiple institutions. These findings are in line with literature exploring the potential of adaptive radiation therapy (ART) using low-field devices; both Sahin and Klüter mention that daily image guidance or ART was used in the majority of patients.43–45 Furthermore, in a recent retrospective study by Güngör et al44 that reports on patients treated through ART strategies on a low-field MR-Linac, 774 (80.4%) of the total 962 fractions were delivered using ART. Moreover, daily adaptation using MRgRT has many postulated benefits, in particular reduction of uncertainties and enabling physicians to reduce treatment margins. The early results of this study and the available literature on low-field MR-Linacs reflect a broad appetite and willingness to perform this activity on the part of radiation oncologists.
Another important finding was that no patients discontinued their own MR-Linac treatments. When MR-Linacs were first proposed, there were concerns that patients may not tolerate repeated exposures to high-field MRI due to claustrophobia or discomfort from peripheral nerve stimulation or specific absorption rate heating (heating of patient tissue by energy absorption of said tissue).46,47 Our results indicate that the features of the device evaluated in this report meant to mitigate these potential effects (eg, a wide and short bore to reduce claustrophobia and anxiety, controls on radiofrequency pulse rate and gradient slew rate to minimize physiological stress) are indeed effective: No patient discontinued MR-Linac treatment themselves. However, physicians may not offer MR-Linac treatment to patients with known claustrophobia, or these patients may decline. Also, undiagnosed claustrophobia may become apparent during preparatory MRI scans in the MRgRT workflow. These patients would likely be deemed unsuitable for MRgRT and not be captured in MOMENTUM. In our current analysis, we did not examine the number of patients with unknown claustrophobia, but from the retrospective analysis by Klüter et al that evaluated 1 year of implementing a low-field MR-Linac we know that this number is low (n = 2 of 23 screen failures). Alternatively, it is also possible that with repeated exposures patients may grow accustomed to the experience—even patients who have less severe forms of claustrophobia. Moreover, the results of 3 studies reporting on patient-reported experience on low-field devices are promising, and overall patient tolerability is good.41,48,49 In the future, we expect more indepth analysis of patient experience on the MR-Linac as several patient experience studies started within the MR-Linac Consortium.
This study showed an overall reassuring radiation-related acute toxicity pattern in a diverse and international cohort. For this study, we have described and analyzed all registered adverse events and whether they were classified as related to radiation (Table 3). Although toxicity attribution is always a subjective matter, our approach maximizes completeness and transparency. Further indication-specific toxicity analyses will be needed to make more nuanced observations on the dosimetric impact of ERE. In this initial cohort, treatments within the pelvis, where ERE is probably least important, were the most common body site; treatments of the thorax, where ERE is likely to be most important, were the least common body site (Table 1), suggesting that clinicians may be taking a cautious approach with this new technology. Publication of more detailed disease-specific analyses with longer follow-up that include both clinician-reported toxicity and PROs are expected.
Our study has limitations. We have not captured the experience of patients who were treated at non-MOMENTUM institutions over the study period. Our cohort represents approximately half of the initial global experience with high-field MRgRT, but all the patients included so far are from Europe and North America. The initial patterns of care and experience at institutions from Asia and Australia may be different. In addition, because MOMENTUM is a registry without a comparison arm, we can only estimate safety and tolerability; we cannot draw conclusions about how these outcomes compare to a conventional radiation therapy cohort. Furthermore, we do not have a view in MOMENTUM of which subsets of patients are being selected for MR-Linac treatment or the proportion of patients who screen out due to contraindications to MRI (eg, metallic implants, cardiac devices, claustrophobia) or other reasons that may be important for understanding the cohort presented here. Lastly, we presently only report the acute toxicity experience, and longer follow-up is required to draw definitive conclusions about safety and efficacy. Furthermore, because this registry captures maximum toxicity since the previous assessment and the first follow-up assessment is 3 months after end of treatment, it is possible that some acute toxicities are not fully captured. We believe that our registry is well suited to capturing major toxicities (ie, grade 3+) or other major safety events (the primary purpose of this manuscript); however, in capturing maximum toxicity scores, more subtle low-grade toxicity patterns may not be captured by the patient or provider. In the future, we plan to implement electronic PRO assessment that patients can complete at home, which should allow us to more cost-effectively implement more frequent evaluations.
Conclusions
The large, diverse, and international cohort of patients treated using MRgRT allowed us to analyze patients and treatments in the initial phase of high-field MR-Linac implementation. We observed that a wide variety of clinical indications have been treated and that the tolerability and clinician-reported acute toxicity profile are encouraging. In the future, the MR-Linac Consortium intends to continue to follow this initial cohort at least 2 years posttreatment to estimate the late toxicity experience and cancer control outcomes. In addition, comparative effectiveness studies will be needed to evaluate the performance of high-field MRgRT relative to alternative radiation therapy paradigms.
Acknowledgments—
A.C. is supported by the NIHR Manchester Biomedical Research Centre. S.H., K.H., E.H., R.H., A.K., S.L., U.O., and A. T. acknowledge the support of the NIHR Biomedical Research Centre at The Royal Marsden NHS Foundation Trust and The Institute of Cancer Research. The views expressed are those of the authors and not necessarily those of the NIHR or the Department for Health and Social Care.
Disclosures:
M.P.W.I. reports personal fees from Elekta. H.A., K.B., J. P.C., D.E., and J.G. report being employed at Elekta. A.C., B.A.E., C.F.-R, R.A.H., K.J.H., M.E.N., C.J.S., R.J.H.A.T., A.C.T., and H.M.V. report (institutional) research funding from Elekta. C.D.F. reports grants, personal fees, nonfinancial support, and other from Elekta AB; grants from the National Institutes of Health (NIH) National Institute of Dental and Craniofacial Research (NIDCR), the NIH National Institute of Biomedical Imaging and Bioengineering, the NIH National Cancer Institute (NCI), the National Science Foundation, and the NIH/NSF/NCI Interagency Institute, during the conduct of the study; and grants from NIH NCI, grants from Patient-Centered Outcomes Research Institute, grants from the NSF and NIH NIDCR, outside the submitted work. S.H. reports grants from National Institute for Health Research (NIHR) Biomedical Research Centre at The Royal Marsden NHS Foundation Trust and The Institute of Cancer Research, nonfinancial support from Elekta (Elekta AB, Stockholm, Sweden), personal fees and nonfinancial support from Roche, and nonfinancial support from Merck Sharp & Dohme (MSD), outside the submitted work. E.H. reports grants from Accuray Inc and Varian Medical Systems Inc, outside the submitted work. U.A.v.d.H. reports grants from Elekta and Philips Healthcare. S.L. reports grants from Elekta. K.O. was an external resource for Elekta at time of writing. A.S. was an advisor/consultant with Abbvie, Merck, Roche, Varian (medical advisory group), Elekta (gamma knife icon), BrainLAB, and VieCure (medical advisory board). A.S. was a board member for the International Stereotactic Radiosurgery Society (ISRS); had past educational seminars with Elekta AB, Accuray Inc, Varian (CNS teaching faculty), BrainLAB, and Medtronic Kyphon; reprots a research grant with Elekta AB; and reports travel accommodations/expenses from Elekta, Varian, and BrainLAB. A.S. also belongs to the Elekta MR Linac Research Consortium, Elekta Spine, Oligometastases, and Linac Based SRS Consortia. C.L.T. reports travel accommodations/expenses and honoraria for educational seminars from Elekta, outside the submitted work. W.A.H. received institutional research funding and travel support from Elekta.
The MOMENTUM Study is financially supported by Elekta AB and through in-kind contributions from all participating institutions.
Footnotes
Data sharing statement: Research data are not available at this time.
References
- 1.Hall MD, Schultheiss TE, Smith DD, Fakih MG, Wong JYC, Chen YJ. Effect of increasing radiation dose on pathologic complete response in rectal cancer patients treated with neoadjuvant chemoradiation therapy. Acta Oncol (Madr) 2016;55:1392–1399. [DOI] [PubMed] [Google Scholar]
- 2.MacHtay M, Bae K, Movsas B, et al. Higher biologically effective dose of radiotherapy is associated with improved outcomes for locally advanced non-small cell lung carcinoma treated with chemoradiation: An analysis of the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 2012;82:425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bentzen SM. Radiation therapy: Intensity modulated, image guided, biologically optimized and evidence based. Radiother Oncol 2005;77:227–230. [DOI] [PubMed] [Google Scholar]
- 4.Jaffray D, Kupelian P, Djemil T, Macklis RM. Review of image-guided radiation therapy. Expert Rev Anticancer Ther 2007;7:89–103. [DOI] [PubMed] [Google Scholar]
- 5.Raaymakers BW, Lagendijk JJW, Overweg J, et al. Integrating a 1.5 T MRI scanner with a 6 MV accelerator: Proof of concept. Phys Med Biol 2009;54. [DOI] [PubMed] [Google Scholar]
- 6.Lagendijk JJW, Raaymakers BW, Van Den Berg CAT, Moerland MA, Philippens ME, Van Vulpen M. MR guidance in radiotherapy. Phys Med Biol 2014;59:R349–R369. [DOI] [PubMed] [Google Scholar]
- 7.Winkel D, Bol GH, Kroon PS, et al. Adaptive radiotherapy: The Elekta Unity MR-linac concept. Clin Transl Radiat Oncol 2019;18:54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Werensteijn-Honingh AM, Kroon PS, Winkel D, et al. Feasibility of stereotactic radiotherapy using a 1.5 T MR-linac: Multi-fraction treatment of pelvic lymph node oligometastases. Radiother Oncol 2019;134:50–54. [DOI] [PubMed] [Google Scholar]
- 9.Dunlop A, Mitchell A, Tree A, et al. Daily adaptive radiotherapy for patients with prostate cancer using a high field MR-linac: Initial clinical experiences and assessment of delivered doses compared to a C-arm linac. Clin Transl Radiat Oncol 2020;23:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kontaxis C, Bol GH, Lagendijk JJW, Raaymakers BW. Towards adaptive IMRT sequencing for the MR-linac. Phys Med Biol 2015;60:2493–2509. [DOI] [PubMed] [Google Scholar]
- 11.Corradini S, Alongi F, Andratschke N, et al. MR-guidance in clinical reality: Current treatment challenges and future perspectives. Radiat Oncol 2019;14:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall WA, Paulson ES, van der Heide UA, et al. The transformation of radiation oncology using real-time magnetic resonance guidance: A review. Eur J Cancer 2019;122:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones KM, Michel KA, Bankson JA, Fuller CD, Klopp AH, Venkatesan AM. Emerging magnetic resonance imaging technologies for radiation therapy planning and response assessment. Int J Radiat Oncol Biol Phys 2018;101:1046–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Herk M, McWilliam A, Dubec M, Faivre-Finn C, Choudhury A. Magnetic resonance imaging–guided radiation therapy: A short strengths, weaknesses, opportunities, and threats analysis. Int J Radiat Oncol Biol Phys 2018;101:1057–1060. [DOI] [PubMed] [Google Scholar]
- 15.Lorenz JW, Schott D, Rein L, et al. Serial T2-weighted magnetic resonance images acquired on a 1.5 Tesla magnetic resonance linear accelerator reveal radiomic feature variation in organs at risk: An exploratory analysis of novel metrics of tissue response in prostate cancer. Cureus 2019;1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, Cao M, Sheng K, et al. Longitudinal diffusion MRI for treatment response assessment: Preliminary experience using an MRI-guided tri-cobalt 60 radiotherapy system. Med Phys 2016;43:1369–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Heide UA, Houweling AC, Groenendaal G, Beets-Tan RGH, Lambin P. Functional MRI for radiotherapy dose painting. Magn Reson Imaging 2012;30:1216–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurz C, Buizza G, Landry G, et al. Medical physics challenges in clinical MR-guided radiotherapy. Radiat Oncol 2020;15:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grégoire V, Guckenberger M, Haustermans K, et al. Image guidance in radiation therapy for better cure of cancer. Mol Oncol 2020;14:1470–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liney GP, Moerland MA. Magnetic resonance imaging acquisition techniques for radiotherapy planning. Semin Radiat Oncol 2014;24:160–168. [DOI] [PubMed] [Google Scholar]
- 21.Bitterman DS, Cagney DN, Singer LL, Nguyen PL, Catalano PJ, Mak RH. Master protocol trial design for efficient and rational evaluation of novel therapeutic oncology devices. J Natl Cancer Inst 2020;112:229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brada M, Pijls-Johannesma M, De Ruysscher D. Proton therapy in clinical practice: Current clinical evidence. J Clin Oncol 2007;25:965–970. [DOI] [PubMed] [Google Scholar]
- 23.De Ruysscher D, Mark Lodge M, Jones B, et al. Charged particles in radiotherapy: A 5-year update of a systematic review. Radiother Oncol 2012;103:5–7. [DOI] [PubMed] [Google Scholar]
- 24.Mishra MV, Aggarwal S, Bentzen SM, Knight N, Mehta MP, Regine WF. Establishing evidence-based indications for proton therapy: An overview of current clinical trials. Int J Radiat Oncol Biol Phys 2017;97:228–235. [DOI] [PubMed] [Google Scholar]
- 25.Ofuya M, McParland L, Murray L, Brown S, Sebag-Montefiore D, Hall E. Systematic review of methodology used in clinical studies evaluating the benefits of proton beam therapy. Clin Transl Radiat Oncol 2019;19:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Loon J, Grutters J, Macbeth F. Evaluation of novel radiotherapy technologies: What evidence is needed to assess their clinical and cost effectiveness, and how should we get it? Lancet Oncol 2012;13:e169–e177. [DOI] [PubMed] [Google Scholar]
- 27.Verkooijen HM, Kerkmeijer LGW, Fuller CD, et al. R-IDEAL: A framework for systematic clinical evaluation of technical innovations in radiation oncology. Front Oncol 2017;7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Mol van Otterloo SR, Christodouleas JP, Blezer ELA, et al. The MOMENTUM Study: An International registry for the evidence-based introduction of MR-Guided Adaptive Therapy. Front Oncol 2020;10:1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lagendijk JW, Raaymakers BW, van Vulpen M. The Magnetic Resonance Imaging-Linac System. Semin Radiat Oncol 2014;24:207–209. [DOI] [PubMed] [Google Scholar]
- 30.Raaymakers BW, Jürgenliemk-Schulz IM, Bol GH, et al. First patients treated with a 1.5 T MRI-Linac: Clinical proof of concept of a high-precision, high-field MRI guided radiotherapy treatment. Phys Med Biol 2017;62:L41–L50. [DOI] [PubMed] [Google Scholar]
- 31.Kerkmeijer LGW, Fuller CD, Verkooijen HM, et al. The MRI-Linear accelerator consortium: Evidence-based clinical introduction of an innovation in radiation oncology connecting researchers, methodology, data collection, quality assurance, and technical development. Front Oncol 2016;6:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winkel D, Kroon PS, Werensteijn-Honingh AM, Bol GH, Raaymakers BW, Jürgenliemk-Schulz IM. Simulated dosimetric impact of online replanning for stereotactic body radiation therapy of lymph node oligometastases on the 1.5T MR-linac. Acta Oncol (Madr) 2018;57:1705–1712. [DOI] [PubMed] [Google Scholar]
- 33.Intven MPW, de Mol van Otterloo SR, Mook S, et al. Online adaptive MR-guided radiotherapy for rectal cancer; feasibility of the workflow on a 1.5T MR-linac: Clinical implementation and initial experience. Radiother Oncol 2021;154:172–178. [DOI] [PubMed] [Google Scholar]
- 34.Elekta BV Elekta annual report 2019/20. Available at: https://mb.cision.com/Main/35/3152151/1277620.pdf. Accessed January 20, 2021.
- 35.Chin S, Eccles CL, McWilliam A, et al. Magnetic resonance-guided radiation therapy: A review. J Med Imaging Radiat Oncol 2019;1–15. [DOI] [PubMed] [Google Scholar]
- 36.Spieler B, Samuels SE, Llorente R, Yechieli R, Ford JC, Mellon EA. Advantages of radiation therapy simulation with 0.35 Tesla magnetic resonance imaging for stereotactic ablation of spinal metastases. Pract Radiat Oncol 2020;10:339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boldrini L, Romano A, Placidi L, et al. Case report: First in human online adaptive MR guided SBRT of peritoneal carcinomatosis nodules: A new therapeutic approach for the oligo-metastatic patient. Front Oncol 2020;10601739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finazzi T, van Sörnsen de Koste JR, Palacios MA, et al. Delivery of magnetic resonance-guided single-fraction stereotactic lung radiotherapy. Phys Imaging Radiat Oncol 2020;14:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tocco BR, Kishan AU, Ma TM, Kerkmeijer LGW, Tree AC. MR-guided radiotherapy for prostate cancer. Front Oncol 2020;10 616291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boldrini L, Piras A, Chiloiro G, et al. Low Tesla magnetic resonance guided radiotherapy for locally advanced cervical cancer: First clinical experience. Tumori 2020;106:497–505. [DOI] [PubMed] [Google Scholar]
- 41.Klüter S, Katayama S, Spindeldreier CK, et al. First prospective clinical evaluation of feasibility and patient acceptance of magnetic resonance-guided radiotherapy in Germany. Strahlentherapie und Onkol 2020;196:691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sahin B, Zoto Mustafayev T, Gungor G, et al. First 500 fractions delivered with a magnetic resonance-guided radiotherapy system: Initial experience. Cureus 2019;11:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bohoudi O, Bruynzeel AME, Senan S, et al. Fast and robust online adaptive planning in stereotactic MR-guided adaptive radiation therapy (SMART) for pancreatic cancer. Radiother Oncol 2017;125:439–444. [DOI] [PubMed] [Google Scholar]
- 44.Güngör G, Serbez I, Temur B, et al. Time analysis of online adaptive magnetic resonance–guided radiation therapy workflow according to anatomical sites. Pract Radiat Oncol 2021;11:e11–e21. [DOI] [PubMed] [Google Scholar]
- 45.van Timmeren JE, Chamberlain M, Krayenbuehl J, et al. Treatment plan quality during online adaptive re-planning. Radiat Oncol 2020;15:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eising EG, Hughes J, Nolte F, Jentzen W, Bockisch A. Burn injury by nuclear magnetic resonance imaging. Clin Imaging 2010;34:293–297. [DOI] [PubMed] [Google Scholar]
- 47.Hu Q, Yu VY, Yang Y, et al. Practical safety considerations for integration of magnetic resonance imaging in radiation therapy. Pract Radiat Oncol 2020;10:443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sayan M, Serbez I, Teymur B, et al. Patient-reported tolerance of magnetic resonance-guided radiation therapy. Front Oncol 2020;10:1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tetar S, Bruynzeel A, Bakker R, et al. Patient-reported outcome measurements on the tolerance of magnetic resonance imaging-guided radiation therapy description of the SMART procedure. Cureus 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]


