Abstract
Coronavirus disease-19 (COVID-19) is an emerging infectious disease caused by SARS-CoV-2 that has rapidly evolved into a pandemic to cause over 600 million infections and more than 6.6 million deaths up to Nov 25, 2022. COVID-19 carries a high mortality rate in severe cases. Co-infections and secondary infections with other micro-organisms, such as bacterial and fungus, further increases the mortality and complicates the diagnosis and management of COVID-19. The current guideline provides guidance to physicians for the management and treatment of patients with COVID-19 associated bacterial and fungal infections, including COVID-19 associated bacterial infections (CABI), pulmonary aspergillosis (CAPA), candidiasis (CAC) and mucormycosis (CAM). Recommendations were drafted by the 7th Guidelines Recommendations for Evidence-based Antimicrobial agents use Taiwan (GREAT) working group after review of the current evidence, using the grading of recommendations assessment, development, and evaluation (GRADE) methodology. A nationwide expert panel reviewed the recommendations in March 2022, and the guideline was endorsed by the Infectious Diseases Society of Taiwan (IDST). This guideline includes the epidemiology, diagnostic methods and treatment recommendations for COVID-19 associated infections. The aim of this guideline is to provide guidance to physicians who are involved in the medical care for patients with COVID-19 during the ongoing COVID-19 pandemic.
Keywords: COVID-19, COVID-19 associated infections, CABI, CAPA, CAC, CAM, Pulmonary aspergillosis, Candidiasis, Mucormycosis
Introduction
Coronavirus disease-19 (COVID-19) is an emerging infectious disease which rapidly spread to cause a pandemic since the end of 2019. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel beta-coronavirus responsible for the highly transmissible and pathogenic disease, which was believed to originate from Wuhan, China.1 The World Health Organization (WHO) declared COVID-19 outbreak as a Public Health Emergency of International Concern on Jan 30, 2020.
As the disease became worldwide, reports of coinfections or secondary infections with other pathogens, such as COVID-19 associated bacterial infections (CABI), COVID-19 associated pulmonary aspergillosis (CAPA), COVID-19 associated candidiasis (CAC) and COVID-19 associated mucormycosis (CAM) accumulated, however, the epidemiology varied across different countries.2, 3, 4, 5 In addition to bacterial and fungal coinfections, respiratory viruses can also be co-pathogens in COVID-19. According to a systemic review, viral coinfections accounts for 6.6% of the COVID-19 patients and Ebstein-Barr virus is the most common virus found, followed by HHV-6 and influenza virus.6 COVID-19 is a novel infectious disease, and the current knowledge on COVID-19 associated infections remains under research. Due to the success of Taiwan's prevention and control strategy for COVID-19, it was not until May, 2021, that Taiwan experienced its first wave of nationwide pandemic and the ongoing second wave of pandemic caused by the Omicron variant began in late April, 2022. This guideline reviewed the current literature and assimilate the international experience. The aim of this guideline is to describe the epidemiology, diagnostic tools and to provide guidance for the management of COVID-19 associated infections.
Methodology
Panel composition
The 7th “Guidelines Recommendations for Evidenced-based Antimicrobial use in Taiwan” (GREAT) working group was formed in October 2021 under the auspices of the Medical Foundation in the Memory of Deh-Lin Cheng; and was appointed by the Infectious Disease Society of Taiwan to develop treatment guidelines for infectious disease. The “Grading of Recommendations Assessment, Development, and Evaluation” (GRADE) system7 was used for the development of this guideline. The GREAT working group had a steering committee and a guideline working committee. The mission of the steering committee was to set the purpose, scope, and target audience of the guideline, and to invite members of the committee group and expert review panel. The steering committee consisted of 2 infectious disease specialists, 3 clinical pharmacists (2 were epidemiologists and one GRADE system methodologist). The guideline working committee included 18 infectious disease doctors from 13 medical centers and hospitals across Taiwan.
Process of guideline development
The members of the 7th GREAT working group committee held 4 in-person meetings between Oct 2021 and Feb 2022, to present reviewed literature, translate evidence into recommendations, and drafted the guidelines. The quality of evidence and strength of recommendations were critically reviewed by an internal and external expert review panel. The joint consensus meeting with a nationwide expert review panel was held on March 12, 2022, and the full guidelines was endorsed by the board members of Infectious Diseases Society of Taiwan in June, 2022.
Rating of the evidence and recommendation
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system7 , 8 is for developing and presenting summaries of evidence and provides a systemic approach for making clinical practice recommendations. The system has four levels of evidence: high (A), moderate (B), low (C) and very low (D). High quality of evidence indicates that after reviewing the current literature, the authors have abundant confidence that the true effect is similar to the estimated effect. Moderate quality of evidence indicates that the true effect is probably close to the estimated effect. Low quality of evidence indicates that the true effect may be markedly different from the estimated effect and a very low quality of evidence indicates that the true effect is probably markedly different from the estimated effect. Five factors renders the evidence less certain and downgrades the quality of evidence, including risk of bias, consistency of the results, directness of the evidence, precision and publication bias.9 Under rare circumstances, the certainty of the evidence can be rated up if the evidence has a large magnitude of effect, a dose–response gradient, or if all residual confounding may decrease the magnitude of effect. Recommendations in GRADE methodology are classified as strong (1) or weak (2). A strong recommendation is when the panel is confident that the desirable effects of adherence to a recommendation outweigh the undesirable effects and this can be either in favor or against an intervention. In contrast, weak recommendations imply that there is likely to be an important variation in the decision that informed persons are likely to make. The strength of recommendations are actionable: a weak recommendation indicates that engaging in a shared decision making process is essential, while a strong recommendation suggests that it is not usually necessary to present both options.
Literature search
We performed systemic literature searches on two electronic databases of PubMed and EMBASE, limited to English articles and a period from January 1, 2019, to December 31, 2021. Different searching terms were applied in accordance with each PICOs, including: “COVID-19″, “SARS-CoV-2″, “bacterial infection”, “superinfection”, “co-infection”, “secondary infection”, “nosocomial infection”, “mycoplasma”, “Staphylococcus aureus”, “MRSA”, “Pseudomonas”, “MDR bacteria”, “multiplex PCR”, “procalcitonin”, “aspergillosis”, “COVID-19 associated pulmonary aspergillosis”, “invasive pulmonary aspergillosis”, “mycosis”, “fungal infections”, “EORTC/MSG”, “Bulpa”, “AspICU”, “ECMM/ISHAM”, “criteria”, “diagnosis”, “prophyla∗“, “candida”, “COVID-19 associated mucormycosis”, “epidemiology”, “incidence”, “mortality”. Randomized controlled trials (RCTs), meta-analysis, systematic reviews, and observational studies were included for evidence rating and analysis to answer the PICOs. We further conducted a meta-analysis using 32 studies to analyze the role of inflammatory biomarkers in diagnosing CABI (online supplement Table S1).191, 192, 193, 194, 195, 196 Studies from a previous meta-analysis10 were included, and extended to include studies published later, between 01 March 2021 to 31 December 2021. We used the keywords “COVID-19″, “SARS-CoV-2″, “bacterial infection”, “superinfection”, “co-infection”, “secondary infection”, and “nosocomial infection".
Definition
COVID-19 associated bacterial infections (CABI)
COVID-19 associated bacterial infection (CABI) is further subdivided into COVID-19 associated bacterial “coinfection” and COVID-19 associated bacterial “secondary infection”. The diagnosis of a bacterial infection was made either according to microbiology reports, serology testing, syndromic diagnostic testing or by the physician's clinical judgement.
COVID-19 associated bacterial coinfections are defined as simultaneous bacterial infections diagnosed within 48 h of admission.11 , 12 COVID-19 associated secondary bacterial infections are defined as infections occurring during the late period of COVID-19 illness, usually developing after 48 h after hospitalization.4 , 13 , 14
COVID-19 associated pulmonary aspergillosis (CAPA)
The term CAPA is defined as when COVID-19 patients are either coinfected or secondary infected with pulmonary aspergillosis. However, currently, there is no consensus on a definition for pulmonary aspergillosis in COVID-19 patients.15 It remains unknown whether previous criteria for pulmonary aspergillosis is applicable to COVID-19 patients, including EORTC/MSG,16 Bulpa criteria,17 AspICU,18 modified AspICU,19 and ECMM/ISHAM,15 and this guideline will address this issue.
COVID-19 associated candidiasis (CAC)
COVID-19 associated candidiasis (CAC) is defined as the presence of a Candida spp. Isolated from culture in a clinical sample obtained from sterile sites, along with compatible clinical presentations,20 in a COVID-19 patient.
COVID-19 associated mucormycosis (CAM)
COVID-19 associated mucormycosis (CAM) is defined as laboratory identification of Mucorales by either culture, histopathology, or polymerase chain reaction (PCR) in a patient with COVID-19 and clinical symptoms of invasive mucormycosis. This includes both coinfection or superinfection.21
Severity of COVID-19 disease
Patients are considered to have mild illness when presenting with a variety of symptoms and signs such as fever, upper respiratory tract symptoms, gastrointestinal tract symptoms but no signs of respiratory distress nor abnormal imaging. Moderate illness is defined as evidence of lower respiratory disease during clinical assessment or imaging, with SpO2 ≥ 94% in room air at sea level. Severe illness is defined as having a SpO2 < 94% in room air at sea level, a PaO2/FiO2 ratio <300 mmHg, a respiratory rate >30 breaths/min, or lung infiltrates >50%. Critically ill COVID-19 is defined as when a patient fulfills the criteria of admission to an intensive care unit or are mechanically ventilated. They may have acute respiratory distress syndrome or septic shock that may represent as virus-induced distributive shock, cardiac dysfunction, an exaggerated inflammatory response, and/or exacerbation of underlying comorbidities. In addition to pulmonary disease, patients with critical illness may also experience cardiac, hepatic, renal, central nervous system, or thrombotic disease.22
Summary of recommendations (Table 1)
Table 1.
Summary of recommendations for the diagnosis and treatment of COVID-19 associated infections.
| Disease | Recommendation | Strength of Recommendation/Quality of Evidence |
|---|---|---|
| COVID-19 Associated Bacterial Infections (CABI) | I. General principles of treatment recommendations | |
| 1. We recommend against routine prescription of antibiotics in COVID-19 patients. The prescription of antibiotics should be based on clinical justifications, such as disease manifestations, disease severity, radiographic imaging, and laboratory data. | Strong/Moderate (1B) | |
| 2. We recommend a comprehensive microbiologic workup before administration of empirical antibiotics in COVID-19 patients to facilitate adjustment, de-escalation, or discontinuation of antibiotics | Strong/Low (1C) | |
| II. Clinical presentations | ||
| 1. Critically ill COVID-19 patients, including patients who need to be admitted to the ICU or are mechanically ventilated, may have a higher risk of acquiring CABI and may require antibiotic use. | Weak/Moderate (2B) | |
| 2. Higher WBC counts, higher CRP values or a PCT level >0.5 ng/mL may indicate a higher possibility of having CABI. However, we suggest against using serum biomarkers alone to decide when to start antimicrobials, especially when the patient is not critically ill. | Weak/Low (2C) | |
| 3. We do not suggest routine administration of antibiotics for COVID-19 patients receiving immunomodulatory agents, such as corticosteroids and IL-6 inhibitors, given the weak evidence that these agents may predispose to secondary bacterial infections | Weak/Moderate (2B) | |
| III. Choice of antimicrobials in patients with suspected bacterial infections | ||
| 1. We suggest the use of empirical antibiotics to cover both typical and atypical pathogens in CAP when pulmonary bacterial coinfections occur in the non-critically ill or non-ICU settinga | Weak/Low (2C) | |
| 2. We suggest empirical, add-on anti-MRSA antibiotics for pulmonary bacterial coinfections in selected patients who are critically ill or in the ICU settinga | Weak/Moderate (2B) | |
| 3. We recommend routine prescription of a single antipseudomonal antibiotic for pulmonary secondary bacterial infections in the non-critically ill or non-ICU settinga | Strong/Moderate (1B) | |
| 4. Double antipseudomonal antibiotics and/or anti-MRSA antibiotics may be prescribed, based on local epidemiology, for pulmonary secondary bacterial infections in the critically ill or ICU settinga | Weak/Low (2C) | |
| IV. Role of diagnostic tools | ||
| 1. Syndromic diagnostic testing (multiplex PCR) may be performed, if available, to improve, streamline, discontinue, or avoid antimicrobial use in critically ill COVID-19 patients based on its excellent sensitivity, high negative predictive value, and a significantly shorter turnaround time | Weak/Moderate (2B) | |
| 2. Syndromic diagnostic testing (multiplex PCR) should be performed using specimens obtained from the endotracheal tube or BAL, to avoid over diagnosis of pulmonary CABI, and conventional cultures should be systematically performed in parallel | Strong/Moderate (1B) | |
| 3. Syndromic diagnostic testing (multiplex PCR) using specimens obtained from the nasopharyngeal swabs is not recommended to guide antimicrobial treatment during the early phase of COVID-19 patients | Strong/Low (1C) | |
| 4. We suggest restricting the use of antimicrobial drugs in mild-to-moderately ill patients with COVID-19 infection, especially in those with low, initial PCT levels (<0.25 ng/mL) | Weak/Low (2C) | |
| 5. We suggest early de-escalation or discontinuation of antibiotics in COVID-19 patients with low PCT levels (<0.25 ng/mL) | Weak/Low (2C) | |
| 6. We suggest serial PCT measurement in all patients during hospitalization, especially in critically ill or ICU patients under mechanical ventilation | Weak/Low (2C) | |
| COVID-19 Associated Pulmonary Aspergillosis (CAPA) | I. Diagnosis | |
| 1. We suggest modified AspICU or ECMM/ISHAM consensus for the diagnosis of CAPA | Weak/Very low (2D) | |
| 2. Currently, there is no gold standard for the diagnosis of CAPA. We suggest clinicians not to rely entirely on these definitions for the diagnosis of CAPA, and encourage clinical judgment when diagnosing invasive pulmonary aspergillosis in a COVID-19 patient | Weak/Very low (2D) | |
| 3. Considering the feasibility of the diagnostic procedure, non-directed BAL may be an alternative to directed BAL to aid in the diagnosis of CAPA | Weak/Very low (2D) | |
| II. Prophylaxis and treatment | ||
| 1. We suggest against routine antifungal prophylaxis in COVID-19 patients based on currently available data | Weak/Very low (2D) | |
| 2. Antifungal prophylaxis using azoles with activity against molds should be guided by risk stratification, knowledge of the local fungal epidemiology, and the efficacy and tolerability profile of available agents | Weak/Very low (2D) | |
| 3. We recommend antifungal treatment for proven, probable, possible, and putative CAPA. | Strong/Moderate (1B) | |
| 4. Single or sequential monotherapy with voriconazole (VOR), isavuconazole (ISZ), posaconazole (POS), liposomal-amphotericin B (L-ampB) is recommended | Strong/Low (1C) | |
| 5. Amphotericin-B deoxycholate (AmpB-d)b and echinocandinsc may be considered as an alternative therapy | Strong/Low (1C) | |
| 6. We recommend adjustment of antifungal regimen according to the identified Aspergillus speciesd, treatment response, adverse effect, and TDM | Strong/Low (1C) | |
| 7. We suggest reference to the local prevalence rate of resistance or the drug susceptibility test when choosing the drug of choice in antifungal regimens when sequential monotherapy or combination therapy is considered | Weak/Low (2C) | |
| 8. Combination therapy may be considered if drug-resistant fungal infection is a concerne, such as when coinfections may be due to triazole-resistant Aspergillus spp., or when coincidence of triazole-resistant Candida spp. Or mucormycosis occurs in CAPA | Weak/Low (2C) | |
| 9. We suggest that the treatment duration of antifungal agents should be determined by the clinical and laboratory evidence of treatment response, such as serum GM testing and chest imaging, and may be discontinued after 6–12 weeks, after a comprehensive evaluation for risk of recurrence | Weak/Very low (2D) | |
| COVID-19 Associated Candidiasis (CAC) | I. Diagnosis | |
| 1. Candida score may not have a role for early detection of CAC among COVID-19 patients | Weak/Low (2C) | |
| II. Treatment | ||
| 1. Fluconazole is recommended as the first-line, empirical therapy for non-critically ill patients or those with a low risk of azole-resistant Candida speciesf | Strong/High (1A) | |
| 2. Echinocandins are recommended as the first-line, empirical therapy for critically ill patients; those with a history of recent azole exposure; or a high risk of fluconazole-resistant Candida speciesf | Strong/High (1A) | |
| 3. For candidemia caused by C. auris, echinocandins are recommended | Strong/Low (1C) | |
| 4. Liposomal amphotericin B or amphotericin B deoxycholate may be considered if there is persistent candidemia or clinically unresponsiveness to treatment with echinocandins without evidence of resistance to amphotericin Bf | Weak/Low (2C) | |
| 5. Recommendations for treatment of CAC other than bloodstream infection are referred to the “2016 guidelines for the use of antifungal agents in patients with invasive fungal diseases in Taiwan” | ||
| 6. For patients with fungus balls or casts in the pyelum or urinary bladder caused by Candida spp. Requiring surgical intervention, delayed operation may be considered after balancing the risk to the patient and the risk of SARS-CoV-2 transmission | Weak/Low (2C) | |
| COVID-19 Associated Mucormycosis (CAM) | I. Treatment | |
| 1. Strict glycemic control and optimization of corticosteroids use is recommended | Strong/Low (1C) | |
| 2. Both antifungal therapy and immediate surgical debridement are recommended for CAM. For patients who need debridement, surgical intervention should not be delayed, and the operation should be performed with appropriate personal protective equipment in a well-established facility to prevent transmission of SARS-CoV-2 | Strong/Low (1C) | |
| 3. Primary therapy: The panel recommends 4–6 weeks of induction and consolidation treatment. Liposomal amphotericin B is recommended as the primary therapy with a dose of 5 mg/kg/day in patients without CNS involvement, or 10 mg/kg/day for those with CNS involvementg |
Strong/Moderate (1B) | |
| 4. Alternative therapyh: A. Amphotericin B deoxycholate (1–1.5 mg/kg/day) should be administered in 5% dextrose with slow infusion over 6–8 h, at the rate of 0.08 mg/kg/hour. Pre-medication with diphenhydramine or acetaminophen, prior to infusion, to avoid drug-related reaction is recommended. To avoid nephrotoxicity, 1 L of normal saline can be given before and after the infusion. |
Strong/Moderate (1B) | |
| B. Posaconazole is preferably given intravenously, or by oral tablet, at a dose of 300 mg twice daily on day 1, followed by 300 mg once dailyi | Strong/Low (1C) | |
| C. Isavuconazole is preferably given intravenously, or by oral tablet, at a dose of 200 mg three times a day for two days, followed by 200 mg daily starting on day 3 | Strong/Low (1C) | |
| 5. Maintenance therapy: Treatment duration of 3–6 months is recommended, until resolution of clinical signs and symptoms A. Posaconazole is preferably given intravenously, or by oral tablet, at a dose of 300 mg twice daily on day 1, followed by 300 mg once daily |
Strong/Low (1C) | |
| B. Isavuconazole is preferably given intravenously, or by oral tablet, at a dose of 200 mg three times a day for two days, followed by 200 mg daily starting on day 3 | Strong/Low (1C) | |
Abbreviations: ICU: intensive care units, WBC: white blood cell, CRP: C-reactive protein, PCT: procalcitonin, IL-6: interleukin-6, CAP: community-acquired pneumonia, MRSA: Methicillin-resistance Staphylococcus aureus, PCR: polymerase chain reaction, BAL: bronchoalveolar lavage, ECMM/ISHAM: European Confederation for Medical Mycology and the International Society for Human and Animal Mycology, TDM: therapeutic drug monitoring, GM: galactomannon, CNS: central nerve system.
Please refer to “Recommendations and guidelines for the treatment of pneumonia in Taiwan” J Microbiol Immunol Infect 2019 Feb; 52 (1):172–199.
The MIC of amphotericin B for Aspergillus spp. Ranges from 0.75 to 8 mg/L in Taiwan.111 A higher dose is recommended for CAPA or combination regimen should be considered.
Echinocandins should not be used as monotherapy if other options are available, unless when used for salvage therapy.190
May refer to Table 4. “Recommendations for management of COVID-19 associated pulmonary aspergillosis (CAPA) according to Aspergillus species and the risk of drug resistance”.
Combination therapy are usually given to patients on immunosuppressant or in high-risk patients. A higher mortality was observed compared to those given monotherapy/sequential monotherapy.
Please refer to the “2016 guidelines for the use of antifungal agents in patients with invasive fungal diseases in Taiwan”. J Microbiol Immunol Infect 2018 Feb; 51 (1):1–17. https://pubmed.ncbi.nlm.nih.gov/28781150/.
Give in 200 mL 5% dextrose with infusion over 2–3 h.
Listed in the order of strength of recommendation.
Intravenous is preferred over oral formulation.
COVID-19 associated bacterial infections (CABI)
General principles of treatment recommendations
Should routine antibiotics be given to every COVID-19 patient?
Recommendation
-
1.
We recommend against routine prescription of antibiotics in COVID-19 patients. The prescription of antibiotics should be based on clinical justifications, such as disease manifestations, disease severity, radiographic imaging, and laboratory data. (Strong recommendation, moderate quality of evidence) (1B)
-
2.
We recommend a comprehensive microbiologic workup before administration of empirical antibiotics in COVID-19 patients to facilitate adjustment, de-escalation, or discontinuation of antibiotics. (Strong recommendation, low quality of evidence) (1C)
Clinical presentations
What are the clinical presentations suggestive of COVID-19-associated bacterial infection (CABI) that may justify the prescription of antibiotics?
Recommendation
-
1.
Critically ill COVID-19 patients, including patients who need to be admitted to the ICU or are mechanically ventilated, may have a higher risk of acquiring CABI and may require antibiotic use. (Weak recommendation, moderate quality of evidence) (2B)
-
2.
Higher white blood cell (WBC) counts, higher C-reactive protein (CRP) values or a procalcitonin (PCT) level >0.5 ng/mL may indicate a higher possibility of having CABI. However, we suggest against using serum biomarkers alone to decide when to start antimicrobials, especially when the patient is not critically ill. (Weak recommendation, low quality of evidence) (2C)
-
3.
We do not suggest routine administration of antibiotics for COVID-19 patients receiving immunomodulatory agents, such as corticosteroids and interleukin-6 (IL-6) inhibitors, given the weak evidence that these agents may predispose to secondary bacterial infections. (Weak recommendation, moderate quality of evidence) (2B)
Choice of antimicrobials in patients with suspect bacterial infections
CABI may occur in sites other than the respiratory tract, such as the urinary tract, bloodstream, skin and soft tissue and others. Our recommendations are for bacterial pulmonary co-infections or secondary infections in COVID-19 patients.
What is the strategy of antibiotic prescription in COVID-19 patients with clinical suspicion of bacterial pulmonary infection, based on the clinical severity in non-critically ill or non-ICU versus critically ill or ICU setting?
Recommendation
-
1.
We suggest the use of empirical antibiotics to cover both typical and atypical pathogens in community-acquired pneumonia (CAP) when pulmonary bacterial coinfections occur in the non-critically ill or non-ICU setting. (Weak recommendation, low quality of evidence) (2C)
-
2.
We suggest empirical, add-on anti-MRSA antibiotics for pulmonary bacterial coinfections in selected patients who are critically ill or in the ICU setting. (Weak recommendation, moderate quality of evidence) (2B)
-
3.
We recommend routine prescription of a single antipseudomonal antibiotic for pulmonary secondary bacterial infections in the non-critically ill or non-ICU setting. (Strong recommendation, moderate quality of evidence) (1B)
-
4.
Double antipseudomonal antibiotics and/or anti-MRSA antibiotics may be prescribed, based on local epidemiology, for pulmonary secondary bacterial infections in the critically ill or ICU setting. (Weak recommendation, low quality of evidence) (2C)
Role of diagnostic tools
What is the role of syndromic diagnostic testing (multiplex PCR) in the diagnosis of pulmonary CABI and in guiding antibiotics use in COVID-19 patients?
Recommendation
-
1.
Syndromic diagnostic testing (multiplex PCR) may be performed, if available, to improve, streamline, discontinue, or avoid antimicrobial use in critically ill COVID-19 patients based on its excellent sensitivity, high negative predictive value, and a significantly shorter turnaround time. (Weak recommendation, moderate quality of evidence) (2B)
-
2.
Syndromic diagnostic testing (multiplex PCR) should be performed using specimens obtained from the endotracheal tube or bronchoalveolar lavage (BAL), to avoid over diagnosis of pulmonary CABI, and conventional cultures should be systematically performed in parallel. (Strong recommendation, moderate quality of evidence) (1B)
-
3.
Syndromic diagnostic testing (multiplex PCR) using specimens obtained from the nasopharyngeal swabs is not recommended to guide antimicrobial treatment during the early phase of COVID-19 patients. (Strong recommendation, low quality of evidence) (1C)
What is the role of procalcitonin in guiding use of antibiotics in COVID-19 patients?
Recommendation
-
1.
We suggest restricting the use of antimicrobial drugs in mild-to-moderately ill patients with COVID-19 infection, especially in those with low, initial procalcitonin (PCT) levels (<0.25 ng/mL). (Weak recommendation, low quality of evidence) (2C)
-
2.
We suggest early de-escalation or discontinuation of antibiotics in COVID-19 patients with low PCT levels (<0.25 ng/mL). (Weak recommendation, low quality of evidence) (2C)
-
3.
We suggest serial PCT measurement in all patients during hospitalization, especially in critically ill or ICU patients under mechanical ventilation. (Weak recommendation, low quality of evidence) (2C)
COVID-19 associated pulmonary aspergillosis (CAPA)
Diagnosis
Which criteria is recommended for the diagnosis of CAPA?
Recommendation
-
1.
We suggest modified AspICU or ECMM/ISHAM consensus for the diagnosis of CAPA. (Weak recommendation, very low quality of evidence) (2D)
-
2.
Currently, there is no gold standard for the diagnosis of CAPA. We suggest clinicians not to rely entirely on these definitions for the diagnosis of CAPA, and encourage clinical judgment when diagnosing invasive pulmonary aspergillosis in a COVID-19 patient. (Weak recommendation, very low quality of evidence) (2D)
Can non-directed bronchoalveolar lavage (BAL) specimen help in the diagnosis of CAPA?
Recommendation
-
1.
Considering the feasibility of the diagnostic procedure, non-directed BAL may be an alternative to directed BAL to aid in the diagnosis of CAPA. (Weak recommendation, very low quality of evidence) (2D)
Prophylaxis and treatment
Can antifungal prophylaxis reduce the incidence of CAPA or improve the clinical outcomes in patients with COVID-19 with acute respiratory failure?
Recommendations
-
1.
We suggest against routine antifungal prophylaxis in COVID-19 patients based on currently available data. (Weak recommendation, very low quality of evidence) (2D)
-
2.
Antifungal prophylaxis using azoles with activity against molds should be guided by risk stratification, knowledge of the local fungal epidemiology, and the efficacy and tolerability profile of available agents. (Weak recommendation, very low quality of evidence) (2D)
What is the appropriate treatment regimen for CAPA?
Recommendation
-
1.
We recommend antifungal treatment for proven, probable, possible, and putative CAPA. (Strong recommendation, moderate quality of evidence) (1B)
-
2.
Single or sequential monotherapy with voriconazole (VOR), isavuconazole (ISZ), posaconazole (POS), liposomal-amphotericin B (L-ampB) is recommended. (Strong recommendation, low quality of evidence) (1C)
-
3.
Amphotericin-B deoxycholate (AmpB-d) and echinocandins may be considered as an alternative therapy. (Strong recommendation, low quality of evidence) (1C)
-
4.
We recommend adjustment of antifungal regimen according to the identified Aspergillus species, treatment response, adverse effect, and therapeutic drug monitoring (TDM). (Strong recommendation, low quality of evidence) (1C)
-
5.
We suggest reference to the local prevalence rate of resistance or the drug susceptibility test when choosing the drug of choice in antifungal regimens when sequential monotherapy or combination therapy is considered. (Weak recommendation, low quality of evidence) (2C)
What is the role of combination antifungal therapy for CAPA?
Recommendation
-
1.
Combination therapy may be considered if drug-resistant fungal infection is a concern, such as when coinfections may be due to triazole-resistant Aspergillus spp., or when coincidence of triazole-resistant Candida spp., or mucormycosis occurs in CAPA. (Weak recommendation, low quality of evidence) (2C)
What is the optimal antifungal treatment duration for CAPA?
-
1.
We suggest that the treatment duration of antifungal agents should be determined by the clinical and laboratory evidence of treatment response, such as serum galactomannan (GM) testing and chest imaging, and may be discontinued after 6–12 weeks, after a comprehensive evaluation for risk of recurrence. (Weak recommendation, very low quality of evidence) (2D)
COVID-19 associated candidiasis (CAC)
Diagnosis
What is the utility of diagnostic criteria, such as candida score, for the diagnosis of CAC?
Recommendation
-
1.
Candida score may not have a role for early detection of CAC among COVID-19 patients. (Weak recommendation, low quality of evidence) (2C)
Treatment
What is the optimal treatment regimen for COVID-19 associated candidemia?
Recommendation
-
1.
Fluconazole is recommended as the first-line, empirical therapy for non-critically ill patients or those with a low risk of azole-resistant Candida species. (Strong recommendation, high quality of evidence) (1A)
-
2.
Echinocandins are recommended as the first-line, empirical therapy for critically ill patients; those with a history of recent azole exposure; or a high risk of fluconazole-resistant Candida species. (Strong recommendation, high quality of evidence) (1A)
-
3.
For candidemia caused by C. auris, echinocandins are recommended. (Strong recommendation, low quality of evidence) (1C)
-
4.
Liposomal amphotericin B or amphotericin B deoxycholate may be considered if there is persistent candidemia or clinically unresponsiveness to treatment with echinocandins without evidence of resistance to amphotericin B. (Weak recommendation, low quality of evidence) (2C)
What is the optimal treatment regimen for CAC other than bloodstream infections?
Recommendation
-
1.
Recommendations for treatment of CAC other than bloodstream infection are referred to the “2016 guidelines for the use of antifungal agents in patients with invasive fungal diseases in Taiwan”.23
-
2.
For patients with fungus balls or casts in the pyelum or urinary bladder caused by Candida spp. Requiring surgical intervention, delayed operation may be considered after balancing the risk to the patient and the risk of SARS-CoV-2 transmission. (Weak recommendation, low quality of evidence) (2C).
COVID-19 associated mucormycosis (CAM)
Treatment
What's the recommendation for the management of CAM?
Recommendation
-
1.
Strict glycemic control and optimization of corticosteroids use is recommended. (Strong recommendation, low quality of evidence) (1C)
-
2.
Both antifungal therapy and immediate surgical debridement are recommended for CAM. For patients who need debridement, surgical intervention should not be delayed, and the operation should be performed with appropriate personal protective equipment in a well-established facility to prevent transmission of SARS-CoV-2. (Strong recommendation, low quality of evidence) (1C)
-
3.Primary therapy: The panel recommends 4–6 weeks of induction and consolidation treatment.
-
A.Liposomal amphotericin B is recommended as the primary therapy with a dose of 5 mg/kg/day in patients without central nerve system (CNS) involvement, or 10 mg/kg/day for those with CNS involvement. (Strong recommendation, moderate quality of evidence) (1B)
-
A.
-
4.Alternatives for primary therapy: The alternative regimens for induction and consolidation are listed in the order of strength of recommendation.
-
A.Amphotericin B deoxycholate (1–1.5 mg/kg/day) should be administered in 5% dextrose with slow infusion over 6–8 h, at the rate of 0.08 mg/kg/hour. Pre-medication with diphenhydramine or acetaminophen, prior to infusion, to avoid drug-related reaction is recommended. To avoid nephrotoxicity, 1 L of normal saline can be given before and after the infusion. (Strong recommendation, moderate quality of evidence) (1B)
-
B.Posaconazole is preferably given intravenously, or by oral tablet, at a dose of 300 mg twice daily on day 1, followed by 300 mg once daily. (Strong recommendation, low quality of evidence) (1C)
-
C.Isavuconazole is preferably given intravenously, or by oral tablet, at a dose of 200 mg three times a day for two days, followed by 200 mg daily starting on day 3. (Strong recommendation, low quality of evidence) (1C)
-
A.
-
5.Maintenance therapy: Treatment duration of 3–6 months is recommended, until resolution of clinical signs and symptoms.
-
A.Posaconazole is preferably given intravenously, or by oral tablet, at a dose of 300 mg twice daily on day 1, followed by 300 mg once daily. (Strong recommendation, low quality of evidence) (1C)
-
B.Isavuconazole is preferably given intravenously, or by oral tablet, at a dose of 200 mg three times a day for two days, followed by 200 mg daily starting on day 3. (Strong recommendation, low quality of evidence) (1C)
-
A.
COVID-19 associated bacterial infections
The actual prevalence of CABI was unclear and variable during the first wave of COVID-19 global pandemic, as it may be influenced by different diagnostic methods, specimen types, time of specimen collection, as well as seasonal factors. Recent studies indicate that the incidence of coinfections ranged between 3.5% and 8%, and secondary bacterial infections between 13.1% and 20%, regardless of the site of infection.2 , 3 , 10 The most common sites of infection in CABI involve the lower respiratory tracts, bloodstream, and urinary tracts.10 , 24 Klebsiella pneumoniae, Streptococcus pneumoniae, S. aureus, and Haemophilus influenzae were the most common pathogens isolated from clinical specimens. For those with secondary bacterial infections, Acinetobacter spp., Pseudomonas aeruginosa, Escherichia coli, K. pneumoniae, and Enterococcus faecium were the most frequent causative pathogens, irrespective of the site of infection.3 , 10
Physicians frequently prescribed antibacterial antibiotics empirically during the first wave of COVID-19 pandemic, mainly due to the concern for CABI during a time when the prevalence of CABI was unknown and some antibiotics were believed to have an in vitro treatment effect for SARS-CoV-2 virus.25 However, with increasing evidence from published studies, COVID-19 associated bacterial coinfection is now known to be less common than influenza associated bacterial coinfection.26 In contrast, secondary bacterial infections tend to be more common, with a higher mortality in COVID-19 patients than in patients with influenza.27
Both bacterial coinfections and secondary infections are associated with significant increase in mortality and a trend towards a longer length of hospital stay.3 , 24 Prescribing antibiotics in a COVID-19 patient should adhere to the principles of antimicrobial stewardship, and a comprehensive evaluation for CABI, including clinical symptoms, obtaining microbiology evidences and inflammatory biomarkers, is crucial and may help to optimize outcomes.28 , 29
Should routine antibiotics be given to every COVID-19 patient?
Recommendation
-
1.
We recommend against routine prescription of antibiotics in COVID-19 patients. The prescription of antibiotics should be based on clinical justifications, such as disease manifestations, disease severity, radiographic imaging, and laboratory data. (Strong recommendation, moderate quality of evidence) (1B)
-
2.
We recommend a comprehensive microbiologic workup before administration of empirical antibiotics in COVID-19 patients to facilitate adjustment, de-escalation, or discontinuation of antibiotics. (Strong recommendation, low quality of evidence) (1C)
Summary of the evidence
In a meta-analysis that included 171 studies, the prevalence of bacterial coinfection in COVID-19 patients was only 5.1% (95% confidence interval [CI] 3.6–7.1%) and secondary bacterial infections were over 2-fold higher at a rate of 13.1% (95% CI 9.8–17.2%).10 Collectively, the overall incidence of respiratory tract infections and/or bloodstream infections was 8.8%.10 This concurred with previous reports in which the prevalence of CABI ranged between 3.5 and 10%.2 , 4 , 5 , 12 , 14 , 26 , 30, 31, 32, 33, 34, 35, 36 The incidence rate of bacterial coinfections in influenza patients appears to be higher than in COVID-19 patients, ranging from 2% to 65%, with majority of the studies ranging between 11% and 35%.37
Despite a relatively low prevalence of CABI, prescription of antibiotics reached over 70% in COVID-19 patients.12 , 24 , 30 , 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49 Over-prescription of antibiotics may cause unnecessary adverse events, lengthen hospital stay, increase medical expenditure,50, 51, 52 and impact on antimicrobial resistance. In particular, multidrug resistance pathogens are a great concern in the COVID-19 era,53 , 54 and effective antimicrobial stewardship to ensure appropriate use of antibiotics is urgently needed. COVID-19 patients who were given antibiotics with clinical justifications were found to have lower mortality rates, higher discharge rates, and shorter length of hospital stay; compared to those who were given antibiotics without clinical justifications.28 Clinicians should prescribe empirical antibiotics for COVID-19 patients with a more judicious approach based clinically on disease manifestations, disease severity, radiographic imaging, and laboratory data.30 , 55 , 56 Strategies of antimicrobial stewardship programs for hospitalized COVID-19 patients recommended that in the first 48 h of hospitalization, focus is placed on obtaining the relevant microbiologic diagnostic tests. After 48–96 h of hospitalization, it is important to evaluate for antibiotic discontinuation, especially if microbiology results are negative; or de-escalation of antibiotics, based on identified pathogens and results of susceptibility testing.47 , 57
What are the clinical presentations suggestive of COVID-19-associated bacterial infection (CABI) that may justify the prescription of antibiotics?
Recommendation
-
1.
Critically ill COVID-19 patients, including patients who need to be admitted to the ICU or are mechanically ventilated, may have a higher risk of acquiring CABI and may require antibiotic use. (Weak recommendation, moderate quality of evidence) (2B)
-
2.
Higher white blood cell (WBC) counts, higher C-reactive protein (CRP) values or a PCT level of >0.5 ng/mL may indicate a higher possibility of having CABI. However, we suggest against using serum biomarkers alone to decide when to start antimicrobials, especially when the patient is not critically ill. (Weak recommendation, low quality of evidence) (2C)
-
3.
We do not suggest routine administration of antibiotics for COVID-19 patients receiving immunomodulatory agents, such as corticosteroids and interleukin-6 (IL-6) inhibitors, given the weak evidence that these agents may predispose to secondary bacterial infections. (Weak recommendation, moderate quality of evidence) (2B)
Summary of the evidence
It is important for clinicians to identify the risk factors for CABI since high-risk populations would benefit from empirical antimicrobial treatment, while over-prescription of antibiotics in low-risk patients may increase antimicrobial resistance. Advanced age, underlying comorbidities, use of immunomodulators, elevation of certain serum biomarkers, and lobar opacities or consolidations on chest radiography were found to be associated with CABI in several studies.4 , 5 , 13 , 14 , 26 , 32 , 34, 35, 36 , 41 , 44 , 45 , 49 , 58, 59, 60, 61 A meta-regression including 171 studies and 17,262 patients to identify risk factors associated with bacterial coinfection, adjusted by age and severity index, concluded that COVID-19 patients who were admitted to the ICU (adjusted odds ratio [aOR] 18.83, 95% CI 6.48–54.77) and those who were under mechanical ventilation (aOR 1.41, 95% CI 1.30–1.52), had a higher risk of bacterial infections. However, underlying comorbidities such as chronic obstructive pulmonary disease, cardiovascular disease, diabetes mellitus, and the use of systemic corticosteroids and interleukin-6 inhibitors were not associated with greater odds of bacterial infections in COVID-19 patients.10
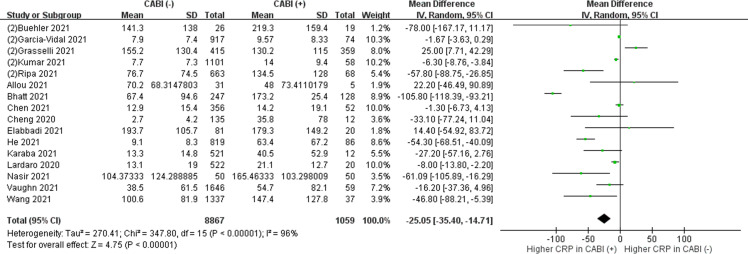
The role of inflammatory biomarkers, such as PCT, CRP and WBC, in the diagnosis of COVID-19 bacterial infections remains uncertain due to its low specificity in non-COVID-19 bacterial infections. Studies were mostly retrospective, with variable cut-off values. We conducted a meta-analysis of 32 studies, and found that higher WBC counts (online supplementary Fig. S1), higher CRP levels (online supplementary Fig. S2), or a PCT >0.5 ng/mL (online supplementary Fig. S3(B)) were more prevalent in those with bacterial coinfections or secondary infections, compared to those without bacterial infections. However, these results must be interpreted with caution since high heterogeneity existed between studies. We advise that the interpretation of laboratory data should take into account the underlying diseases and individual baseline levels. For example, higher values can be observed in patients with autoinflammatory disorders. Due to a low sensitivity and specificity, laboratory data cannot be used alone to diagnose a bacterial infection.31 , 47 In contrast, these biomarkers may have a greater role in excluding bacterial infections. As demonstrated in two large cohort studies including over 1000 patients, low levels of WBC count, CRP and PCT may help to rule out bacterial infections.12 , 31 A negative predictive value exceeding 98% for bacterial infections was obtained using a cut off value for WBC count of <8800/μL, CRP <119.8 mg/dL.12 , 13 Another study demonstrated that a PCT level of <0.1 ng/mL may have a negative predictive value ≥ 98% for bacterial infections.31 The clinical presentations should always remain as the most decisive factor for the diagnosis of bacterial infections.
Choice of antimicrobials in patients with suspected bacterial infections
CABI may occur in sites other than the respiratory tract, such as the urinary tract, bloodstream, skin and soft tissue, and others. Our recommendations are for bacterial pulmonary co-infections or secondary infections in COVID-19 patients. The recommended antimicrobial therapy and treatment duration are shown in Table 2 .
Table 2.
Recommended treatment options for COVID-19 associated bacterial pulmonary infections (CABI).
| Clinical syndrome | Condition | Recommended treatment | Duration |
|---|---|---|---|
| Bacterial Coinfections | Non-ICU or non-critically ill | Empirical antibiotics to cover both typical and atypical pathogens of CAP (2C)a | 7 daysa,b |
| ICU or critically ill | Empirical, add-on, anti-MRSA antibiotics in selected patients (2B)a | 7 daysa,b | |
| Secondary bacterial infections | Non-ICU or non-critically ill | Routine prescription of a single antipseudomonal antibiotic (1B)a | 7 daysa,c |
| ICU or critically ill | Double antipseudomonal and/or anti-MRSA antibiotics may be prescribed based on local epidemiology (2C)a | 7 daysa,c |
Abbreviations: ICU: intensive care units, CAP: community-acquired pneumonia, MRSA: Methicillin-resistance Staphylococcus aureus.
Please refer to “Recommendations and guidelines for the treatment of pneumonia in Taiwan” J Microbiol Immunol Infect 2019 Feb; 52(1):172–199.
If afebrile for 48 h and reached clinical stability.
Treatment should be individualized, and longer treatment course may also be considered in patients with inappropriate initial empirical therapy.
What is the strategy of antibiotic prescription in COVID-19 patients with clinical suspicion of bacterial pulmonary infection, based on the clinical severity (in non-critically ill or non-ICU versus critically ill or ICU setting)?
Should empirical antimicrobials for atypical pneumonia be given to COVID-19 patients when clinical judgment suggests the presence of bacterial pneumonia, in the non-critically ill or non-ICU setting?
Recommendation
-
1.
We suggest the use of empirical antibiotics to cover both typical and atypical pathogens in community-acquired pneumonia (CAP) when pulmonary coinfections occur in the non-critically ill or non-ICU setting. (Weak recommendation, low quality of evidence) (2C)
Summary of the evidence
According to a systemic review and meta-analysis including 118 articles, the pooled prevalence of coinfection was 19% and the most common atypical bacterial infection found in COVID-19 patients was Mycoplasma pneumoniae, accounting for 4.3% of coinfection and 1.3% of secondary infection.3 The incidence rate of Mycoplasma pneumonia ranged from 9.7% to 42% in a mixed ward and ICU settings.6 , 62 One retrospective study conducted in Europe using serology tests found that 26% of the patients were positive for Mycoplasma IgM, 18% for Chlamydia IgM, and both were predictors for more severe symptoms.63 The varying incidence of Mycoplasma coinfection in COVID-19 patients may be due to geographical variation in epidemiology or the use of different diagnostic tools. In addition, the results should be interpreted carefully, as to whether a positive serology test is the consequence of true coinfection or due to cross-reactivity of antibodies during SARS-CoV-2 infection. Similar results was demonstrated in a retrospective study of 139 hospitalized COVID-19 patients, where 79 patient has positive results for Mycoplasma IgM and was associated with a higher mortality (adjusted odds ratio 2.28, 95% confidence interval 1.03 to 5.03).64 Legionella spp. is also a common pathogen causing atypical pneumonia, however, compared with M. pneumoniae and Candida pneumoniae, Legionella spp. is rarely reported to co-infect with SARS-CoV-2 and accounted for 0–1.5% of co-infection in COVID-19 patients.55 , 65
The choice of empirical antimicrobials for CAP should take into consideration the local epidemiology. Based on local guidelines for the treatment of pneumonia in Taiwan, empirical antibiotics prescription should cover both typical and atypical pathogens in CAP patients with moderate severity, defined as CURB-65 score of 2–3. A combination of a beta-lactam antibiotic plus a macrolide is recommended. If resistance to Mycoplasma is a concern, combination of a beta-lactam antibiotic plus doxycycline, or monotherapy with a respiratory fluoroquinolone is suggested.66
Should anti-MRSA antibiotics be given to COVID-19 patients when clinical judgment suggests the presence of bacterial pulmonary co-infection, in the critically ill or ICU setting?
Recommendation
-
1.
We suggest empirical, add-on anti-MRSA antibiotics for pulmonary coinfections in selected patients who are critically ill or in the ICU setting. (Weak recommendation, moderate quality of evidence) (2B)
Summary of the evidence
The prevalence of S. aureus infections among bacterial coinfections in COVID-19 patients, regardless of the site of infection, ranges from 6.5% to 25%.10 , 67 However, there is scant literature describing the incidence and site of methicillin-resistant S. aureus (MRSA) infection.
In a review of 115 COVID-19 patients coinfected with S. aureus, most of the patients (53.9%) were admitted to ICU. Among those patients, 49.6% of the S. aureus were MRSA and the most common clinical syndrome was bacteremia, which accounted for 63.4% of the patients, followed by pneumonia (55.7%) and ventilator-associated pneumonia (VAP) (38.3%). Most of the infections were considered as secondary infections (76.5%), and only 16.5% were coinfections. The mortality rate in this study was high at 61.7%.68
In the United States, risk factors for CAP caused by MRSA including previous influenza infection, end-stage renal disease, lung abscess/empyema, and illicit substance use.69 In Taiwan, nasal colonization rates of S. aureus was 22.1% in patients in the emergency department (ED) and 26.1% in healthcare workers (HCWs). The nasal carriage rates for MRSA was 7.8% in ED patients and 6.1% in HCWs. MRSA accounted for 35.3% of S. aureus isolates in ED patients and 23.4% in HCWs. Patients receiving hemodialysis were significantly associated with MRSA colonization (p = 0.012).70 A higher incidence MRSA infection was observed in bacterial coinfections in the ICU setting and was related to poor outcome. Nasal screening for MRSA may be useful as a strategy for antibiotics stewardship, and is reported to have a high specificity and NPV for ruling out MRSA pneumonia, particularly in cases of CAP or health-care associated pneumonia (HCAP).71 Based on local guidelines for treatment of pneumonia in Taiwan,66 either vancomycin, teicoplanin, or linezolid is recommended for treatment of patients suspected to have MRSA pneumonia.
Should antipseudomonal antibiotics be given to COVID-19 patients when clinical judgements suggest the presence of secondary bacterial pneumonia in the non-critically ill or non-ICU setting?
Recommendation
-
1.
We recommend routine prescription of a single antipseudomonal antibiotic for pulmonary secondary bacterial infections in the non-critically ill or non-ICU setting. (Strong recommendation, moderate quality of evidence) (1B)
Summary of the evidence
The prevalence of P. aeruginosa as a pathogen among secondary infection ranges from 9.8% to 10.8%. In addition to P. aeruginosa, Acinetobacter spp., K. pneumoniae and E. coli also play an important role in COVID-19 associated secondary bacterial infection.3 , 10
According to a retrospective study in the United States which included 64,691 patients, early exposure to corticosteroids and tocilizumab (interleukin-6 (IL-6) inhibitors) increased the risk of secondary infection with incidence rates of 5.7% and 9.9% respectively.24 However, other studies did not report similar findings.10 Further prospective studies are needed to clarify whether COVID-19 patients are at risk for CABI due to use of corticosteroids and/or IL-6 inhibitors, or simply due to the severity of illness.
We suggest to follow the local guidelines for hospital-acquired pneumonia (HAP) to treat pulmonary secondary infections since the definitions are the same; and to take into consideration the local epidemiology. The Taiwan pneumonia guidelines recommend a single agent that can cover P. aeruginosa for treatment of non-severe HAP.66
Should double antipseudomonal antibiotics, or anti-MRSA antibiotics be given to COVID-19 patients when clinical judgements suggest the presence of pulmonary secondary infection in the critically ill or ICU setting?
Recommendation
-
1.
Double antipseudomonal antibiotics and/or anti-MRSA antibiotics may be prescribed, based on local epidemiology for pulmonary secondary infections in the critically ill or ICU setting. (Weak recommendation, low quality of evidence) (2C)
Summary of the evidence
A multicenter, retrospective, European cohort study, conducted in 36 ICUs including 1576 patients, showed that the incidence rate of ventilator-associated lower respiratory tract infections (VA-LRTI) was significantly higher in SARS-CoV-2 pneumonia (50.5%), compared to influenza pneumonia (30.3%) or patients without viral infection (25.3%). Gram-negative bacilli accounted for the majority of isolated pathogens (82%–89.7%), including P aeruginosa, Enterobacter spp., K. pneumoniae, E. coli, and Acinetobacter spp. COVID-19 patients had the lowest rate of infection with multi-drug resistant isolates (23.3%). The incidence rates of MRSA infection were 2.8% in COVID-19, 3.4% in influenza and 3.8% in patients without viral infection.72 S. aureus infections tend to occur as secondary infections in critically ill COVID-19 patients and the overall mortality was 61.7%. The proportion of MRSA among all S. aureus isolates was 49.6%.68
Empirical antibiotics in COVID-19 associated secondary infections in the ICU setting should follow local guidelines for treatment of severe HAP or VAP, since the definitions are the same. The Taiwan pneumonia guidelines recommend empirical use of double anti-pseudomonal agents when there is concern for multi-drug resistant bacteria. Due to the relatively low incidence of HAP due to MRSA in Taiwan compared to the Western countries, empirical anti-MRSA agents should be considered only in high-risk groups, such as patients with a history of MRSA infection or under hemodialysis. We suggest that the gram stains should be obtained as the results have a good negative predictive value for MRSA pneumonia.66
What is the role of syndromic diagnostic testing (multiplex PCR) in the diagnosis of pulmonary CABI and in guiding antibiotics use in COVID-19 patients?
Recommendation
-
1.
Syndromic diagnostic testing (multiplex PCR) may be performed, if available, to improve, streamline, discontinue, or avoid antimicrobial use in critically ill COVID-19 patients based on its excellent sensitivity, high negative predictive value, and a significantly shorter turnaround time. (Weak recommendation, moderate quality of evidence) (2B)
-
2.
Syndromic diagnostic testing (multiplex PCR) should be performed using specimens taken from the endotracheal tube or bronchoalveolar lavage (BAL) to avoid over diagnosis of pulmonary CABI and conventional cultures should be systematically performed in parallel. (Strong recommendation, moderate quality of evidence) (1B)
-
3.
Syndromic diagnostic testing (multiplex PCR) using specimens obtained from the nasopharyngeal swabs is not recommended to guide antimicrobial treatment during the early phase of COVID-19 patients. (Strong recommendation, low quality of evidence) (1C)
Summary of the evidence
Culture-based methods are often insensitive for diagnosing bacterial pneumonia due to antibiotic exposure prior to obtaining specimens, variation in plate growth interpretation, or the challenges with cultivating fastidious organisms.73 , 74 In a systematic review and meta-analysis of seven studies including 558 critically ill, COVID-19 patients, multiplex PCR (mPCR) detected a 33% pooled incidence of co-infections,75 increasing the detection rate almost 2-fold compared to culture (pooled incidence of 18%).76, 77, 78 This was higher than reported in previous meta-analyses.2 , 38 Several prospective and retrospective studies showed good sensitivity and specificity of mPCR in the detection of bacterial pneumonia with an overall sensitivity of 89.3%–100% and specificity ranging between 98.3% and 99%.67 , 77 , 79 One prospective, multicenter study enrolled 99 ICU patients and showed that the sensitivity of mPCR was 100% compared with conventional culture; and the specificity varied from 88.4% to 100% among different pathogens.76 Commercialized mPCR platforms demonstrated a high negative predictive value of 99.7%–100%.67 , 77 Due to its high sensitivity and negative predictive value, mPCR is useful to rule out bacterial coinfections in the context of severe SARS-CoV-2 infection and act as a guide to avoid over-prescription of antibiotics.76 Moreover, some syndromic diagnostic testing also provide the ability to identify SARS-CoV-2 and other respiratory pathogens simultaneously along with antibiotic resistance gene mutations.
An additional advantage of syndromic diagnostic testing is a significantly shorter median turnaround time when using mPCR compared to conventional cultures. The mPCR was associated with an approximately 1 and 2 day reduction in turnaround time for pathogen identification and detection of resistance targets, respectively.67 , 75 , 77 Multiplex PCR had a positive impact on appropriate antibiotic use in critically ill, COVID-19 patients with suspected coinfection or superinfection (67 patients, 112 respiratory samples). Antibiotic use was modified or initiated earlier in 34% (38/112) of the episodes (including 16 withdrawals, 13 initiations, 3 adaptations, 5 de-escalations and one change resulting in inadequacy). Unnecessary use of antibiotics was discontinued in 43% of the cases, and in patients who had a negative mPCR, 28% of the episodes stayed antibiotic-free.79
Molecular methods increased the rate of microbial detection in respiratory samples of COVID-19 patients. Results with ≥ 106 copies/mL can be utilized for early modification of antibiotic therapy,76 however, it is important to discern colonization from infection. Results should be interpreted with caution when bacterial nucleic load is ≤ 105 copies/mL, especially when commensal oral flora are detected. Discordant results with a positive mPCR and negative cultures were mostly characterized by low bacterial loads (104–105 colony forming units (cfu)/mL),67 or the presence of commensal oral flora.76 In contrast, the majority (90%) of positive cultures that was not detected by mPCR were polymicrobial, with bacterial loads that varied between 104 and 105 cfu/mL.67 It is important to remember that some pathogens which play an important role in nosocomial infections are not included in the molecular panel, such as Stenotrophomonas maltophilia. 67
A prospective, cohort study evaluated the mPCR screening approach to detect bacterial coinfections in COVID-19 patients at admission by nasopharyngeal sampling, and found a higher rate (43% vs 28–32%) of positive mPCR results than other studies. A positive result may be due to colonization rather than infection, as it was not correlated with ICU admission, mortality, and inflammatory biomarkers. Antimicrobial treatment in those with a positive mPCR was not associated with reduced rate of ICU admission and mortality, but had a longer hospital stay.65 Therefore, syndromic diagnostic testing using specimens obtained from the nasopharyngeal swabs is not recommended to guide antimicrobial treatment during the early phase of COVID-19 patients.
What is the role of procalcitonin in guiding use of antibiotics in COVID-19 patients?
Recommendation
-
1.
We suggest restricting the use of antimicrobial drugs in mild-to-moderately ill patients with COVID-19 infection, especially in those with low, initial PCT levels (<0.25 ng/mL). (Weak recommendation, low quality of evidence) (2C)
-
2.
We suggest early de-escalation or discontinuation of antibiotics in COVID-19 patients with low PCT levels (<0.25 ng/mL). (Weak recommendation, low quality of evidence) (2C)
-
3.
We suggest serial PCT measurement in all patients during hospitalization, especially in critically ill or ICU patients under mechanical ventilation. (Weak recommendation, low quality of evidence) (2C)
Summary of the evidence
PCT is an inflammatory biomarker that is elevated during bacterial infection and may decline in response to antibiotic therapy. It has been used to differentiate between viral infection with and without bacterial coinfection and may help determine when antibiotic therapy can be discontinued.80 However, some studies suggested that PCT has a limited role in differentiating community-acquired bacterial coinfection from SARS-CoV-2 infection.81, 82, 83 The use of PCT testing to guide antibiotics treatment in COVID-19 patients is confounded by the hyperinflammatory status or cytokine storm induced by SARS-CoV-2 infection, which may result in a higher PCT production than in other viral pneumonia.84, 85, 86, 87
A low PCT level at initial presentation has a high negative predictive value to rule out bacterial coinfections.82 , 88, 89, 90, 91 On the other hand, an initially high PCT level did not provide additional value to traditional clinical criteria or laboratory data, such as fever or hypothermia, white blood cell count ≥12,000/mm3, purulent sputum, need for O2 supplement, imaging consistent with CAP, positive respiratory culture with a respiratory pathogen and/or a positive Streptococcus or Legionella bacterial urinary antigen, in predicting bacterial CAP co-infection.83 Early PCT sampling, defined as within less than 6 h of admission, may have false negative results. The negative predictive value of PCT for bacterial coinfection is expected to be more accurate when sampled on the day after admission, and it is advised to avoid PCT assays on day 0 of admission.92
An elevated PCT may be an indicator of bacterial coinfection. A small, retrospective study including 147 patients with COVID-19 pneumonia found that the majority of patients had low levels of PCT <0.25 ng/mL (101, 69%) and negative cultures (146, 99%). The one patient with a positive bacterial culture had a markedly elevated PCT level.93 Another small, retrospective study demonstrated that patients in the general ward and the ICU who developed secondary bacterial infections (32/99, 32%) had higher PCT and CRP levels at admission and during their hospital stay. Peaking of the PCT and CRP levels corresponded with the time of diagnosis of secondary bacterial infection.90
COVID-19 patients who are critically ill and admitted to the ICU are at a higher risk of nosocomial infection compared to those in the general ward.14 Bacterial pneumonia, especially VAP, is one of the most common secondary bacterial infection in patients with severe COVID-19.94 Elevated PCT level was the only biomarker that differentiated between VAP and non-VAP group (p = 0.001) in a small study including 73 patients.95 Another retrospective, single-center, cohort study showed that a prespecified rise in PCT by 50%, compared to a previous value at any time point, was significantly associated with the occurrence of secondary bacterial infection in critically ill, COVID-19 patients.96 Therefore, serial PCT measurement is recommended in all patients during hospitalization, especially in critically ill or ICU patients, as it may be more predictive of secondary or nosocomial bacterial infection than a single point measurement.
Several studies have investigated the role of PCT levels in antibiotics stewardship in COVID-19 patients with PCT-based algorithms, the most commonly used cut-off value was 0.25 ng/mL.88 , 92 , 93 , 97 , 98 Early de-escalation or discontinuation of antibiotics, within 24 h after admission, in COVID-19 patients with low PCT levels (<0.25 ng/mL) appears to be safe and is associated with a shorter length of hospital stay and potentially lower hospital costs.93
COVID-19 associated pulmonary aspergillosis (CAPA)
Since the emergence of the COVID-19 pandemic, invasive pulmonary aspergillosis (IPA) has been increasingly recognized in patients with severe COVID-19,99 , 100 termed as “COVID-19-associated pulmonary aspergillosis (CAPA)”. The pathophysiology of CAPA is not well understood; however, it is considered the result of interactions between the pathogen, host immunity and environmental factors.101 The incidence of CAPA varies in different countries. In two recent systematic review and meta-analyses, the estimated incidence of CAPA was 10.2%102 and 14.9%103 in patients with severe COVID-19. Apart from Most of the included cohort studies were conducted in European countries, and only one was done in China. The local epidemiology of CAPA in Taiwan requires further research. Patients with CAPA may lack classic host factors for invasive fungal disease, such as underlying hematologic malignancy or being immunocompromised, which is similar to influenza-associated pulmonary aspergillosis (IAPA).104 The median time between diagnosis of influenza and IAPA was short, often within the first 5 days. Most cases of CAPA developed late in the course of admission, which was distinctly different from IAPA, with a median time to diagnosis of 9 days from an initial SARS-CoV-2 positive test.101 The risk factors of CAPA include patients with older age, underlying chronic obstructive pulmonary disease (COPD) and who are receiving long-term corticosteroid treatment.103
A definite diagnosis of IPA is based on pathological or mycological evidence from a lower respiratory tract (LRT) specimen. However, due to concerns with the risk of transmission, bronchoscopy was not routinely performed in patients with COVID-19, impeding the diagnosis of IPA.105 The following diagnostic criteria for CAPA are commonly used in research or clinical practice: (1) European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) definitions,106 (2) AspICU algorithm107 and modified AspICU algorithm,18 , 104 (3) case definitions of influenza-associated pulmonary aspergillosis (IAPA),108 and (4) European Confederation for Medical Mycology and the International Society for Human and Animal Mycology (ECMM/ISHAM) consensus criteria.15
CAPA is associated with poor outcomes, including earlier ICU admission from illness onset, increased mechanical ventilation requirement, multi-organ dysfunction and higher all-cause in-hospital mortality.103 , 109 The mortality of ICU patients with CAPA was as high as 51.2%–54.9%.102 , 110
Local prevalence of drug resistance should be considered when selecting anti-mold agents. In Taiwan, 14.3% of common Aspergillus species in clinical isolates showed resistance to one or two classes of antifungal agents. The rate of azole resistance in clinical isolates of Aspergillus fumigatus was 3–4%.111 , 112 Another single-center study found a 10.2% azole resistant rate in environmental A. fumigatus isolates, but none in the clinical isolates.113 Azole-resistance was not detected in any of the clinical or environmental isolates of other Aspergillus spp. Including Aspergillus flavus, Aspergillus oryzae, Aspergillus niger or Aspergillus terreus.113
Which criteria is recommended for the diagnosis of CAPA?
Recommendation
-
1.
We suggest modified AspICU or ECMM/ISHAM consensus for the diagnosis of CAPA. (Weak recommendation, very low quality of evidence) (2D)
-
2.
Currently, there is no gold standard for the diagnosis of CAPA. We suggest clinicians not to rely entirely on these definitions for the diagnosis of CAPA, and encourage clinical judgment when diagnosing invasive pulmonary aspergillosis in a COVID-19 patient. (Weak recommendation, very low quality of evidence) (2D)
Summary of the evidence
Several criteria are commonly used for diagnosis of CAPA including AspICU107 or modified AspICU,104 IAPA,108 revised EORTC/MSG criteria,106 and ECMM/ISHAM consensus criteria.15 These criteria harbored differences in several aspects, such as host factors, classification and definition of invasive aspergillosis, or the methods used for mycological detection. Only the ECMM/ISHAM consensus was developed specifically for COVID-19 patients. For invasive aspergillosis not belonging to the “proven” category, IAPA, revised EORTC/MSG criteria, and ECMM/ISHAM consensus categorized them as “probable” or “possible,” whereas AspICU and modified AspICU criteria categorized them as “putative” or “colonizer.” Diagnostic fungal biomarkers and molecular methods, such as galactomannan (GM) test or the Aspergillus PCR, were not included in the original AspICU criteria.107 In addition, the GM test in non-BAL specimen was adopted only by the ECMM/ISHAM consensus. A comparison of these criteria is shown in online supplementary Table S2.
In previously published meta-analyses,102 , 103 , 110 the pooled incidence rate of CAPA ranged from 10% to 15%, and the mortality was around 50%. The definition of CAPA used in these studies included a various combination of the above criteria. We performed a meta-analysis of 12 studies to compare the incidence and outcome of CAPA diagnosed by the different criteria.99 , 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124 Overall, the pooled incidence rate of CAPA in ICU patients was 12%. The modified AspICU and IAPA criteria increased detection of CAPA, resulting in a higher incidence rate (15.4% and 15.0%, respectively), while the EORTC/MSG criteria yielded the lowest incidence rate of 5.9%. The incidence of CAPA detected by the AspICU criteria and ECMM/ISHAM consensus was 11.9% and 10.5, respectively. In our analysis, the pooled in-hospital or 30-day mortality was 57% in CAPA patients. In the ICU setting, patients with CAPA diagnosed by the EORTC/MSG criteria (80% vs 33%; risks ratio [RR]: 2.37, 95% confidence interval [CI], 1.81–3.10; p < 0.001) or AspICU criteria (56.1% vs 26.6%; RR: 2.35, 95% CI, 1.68–3.28; p < 0.001) were associated with a higher risk of in-hospital or 30-day mortality, compared to those without CAPA (online supplementary Fig. S4).
Currently, there is no standard criteria for the diagnosis of CAPA. We suggest to consider using the modified AspICU or ECMM/ISHAM consensus for the diagnosis of CAPA, based on the inclusion of entry host factors, the type of fungal biomarkers and molecular method (GM test or Aspergillus PCR), the diagnostic rate, and disease severity identified by these two criteria. However, some of the proposed criteria were developed primarily for research purposes to classify patients homogenously, and not designed for clinical use. Also, most criteria were developed prior to the COVID-19 pandemic and therefore, did not take into account the unique features of CAPA. Therefore, we suggest that the diagnosis of CAPA and the decision-making for management in clinical practice should not rely entirely on these criteria, and management should be tailored to include clinical judgment for the individual patient.
Can non-directed bronchoalveolar lavage (BAL) specimen help in the diagnosis of CAPA?
Recommendation
-
1.
Considering the feasibility of the diagnostic procedure, non-directed BAL may be an alternative to directed BAL to aid in the diagnosis of CAPA. (Weak recommendation, very low quality of evidence) (2D)
Summary of the evidence
Respiratory samples are the preferred specimens for diagnosis of IPA. BAL specimen for GM testing plays a key role in the diagnosis of IPA in the ICU setting, and high levels of GM (GM index >2.5) in the BAL fluid were observed in patients with presumed CAPA.125 Bronchoscopy allows a direct inspection of the trachea and bronchi, which is necessary for the diagnosis of aspergillus tracheobronchitis.126 However, obtaining mycological evidence of CAPA is limited by the concern for the risk of aerosolization and SARS-CoV-2 transmission to healthcare workers during diagnostic bronchoscopy.127, 128, 129 LRT secretions such as sputum or tracheal aspirates are commonly used as a surrogate. But, discerning between colonization from invasive pulmonary disease upon the detection of Aspergillus in specimens of the LRT remains challenging. Therefore, collection of respiratory samples from non-directed BAL has been proposed as an alternative method to aid the diagnosis of CAPA (non-directed BAL fluid is obtained by a blind application of 10–20 mL saline recovered by aspiration via a closed suction system in a patient who is intubated).15 , 120
Potential methods to detect Aspergillus spp. In non-directed BAL fluid include fungal culture, GM testing by enzyme immunoassay (EIA), lateral flow assays (LFAs) or lateral flow devices (LFDs), and PCR.15 Agreement of LFA with the EIA for GM was excellent (κ = 0.702) when testing non-directed BAL or BAL fluid obtained from 23 patients with putative CAPA.130 However, the recommended GM cutoff values for the diagnosis of CAPA using non-directed BAL fluid have not been established. A single center, prospective, cohort study of 42 patients demonstrated a sensitivity of 86% and specificity of 95% for the diagnosis of CAPA, based on AspICU criteria, with a non-directed BAL GM cutoff value of 1 optical density (OD) index.120
To date, there are very few well-designed studies that compare the diagnostic accuracy of non-directed BAL versus directed BAL for the diagnosis of CAPA. A prospective, cohort study compared the performance of GM-EIA and GM-LFA on tracheal aspirate in critically ill COVID-19 patients, for diagnosis of CAPA based on the modified AspICU criteria. With a cutoff value of 2 OD, the sensitivity and specificity of GM-EIA was 57.1% and 81.5%, respectively. In comparison, GM-LFA had a similar sensitivity of 60%, but lower specificity of 72.6%.121
Can antifungal prophylaxis reduce the incidence of CAPA or improve the clinical outcome in patients with COVID-19 in acute respiratory failure?
Recommendations
-
1.
We suggest against routine antifungal prophylaxis in COVID-19 patients based on currently available data. (Weak recommendation, very low quality of evidence) (2D)
-
2.
Antifungal prophylaxis using azoles with activity against molds should be guided by risk stratification, knowledge of the local fungal epidemiology, and the efficacy and tolerability profile of available agents. (Weak recommendation, very low quality of evidence) (2D)
Summary of the evidence
The relatively high prevalence rate of CAPA in critically ill COVID-19 patients requiring invasive ventilation, and the associated high mortality rates of over 50%, raises the important question of whether antifungal prophylaxis with mold-active agents is effective in preventing CAPA in severe COVID-19 patients. The evidence remains controversial as to whether antifungal prophylaxis can reduce mortality due to IPA in these high-risk patients. Currently, there are no well-designed, prospective studies on the efficacy of antifungal prophylaxis to reduce the incidence of CAPA. Neither has the optimal dosing or the class of antifungal agent recommended for antifungal prophylaxis been evaluated. Two observational studies conducted in Europe demonstrated a significant benefit of antifungal prophylaxis in lowering the incidence of CAPA by 85–92%, in critically ill COVID-19 patients who have a high probability of developing fungal infections. However, there was no reduction in mortality rate. One study administered posaconazole 300 mg twice daily for 2 doses then 300 mg once daily for prophylaxis and the other study prescribed inhaled liposomal amphotericin-B 12.5 mg twice weekly.131 , 132 Current guidelines in Taiwan for the use of antifungal agents in hematological malignancies and hematopoietic stem cell transplant recipients recommend preventive strategies to reduce the risk of invasive aspergillosis in high risk patients, even when asymptomatic, due to significant mortality associated with invasive fungal disease.133 Based on limited evidence, we suggest that prophylactic, mold-active, antifungal agents may be considered in selected, high risk patients, if likely to be of clinical benefit after careful risk assessment. A comprehensive, individualized, risk-benefit assessment should be done, taking into account the local epidemiology of CAPA, including the local resistance rates of Aspergillus, the efficacy, tolerability, bioavailability, profiles of adverse reactions and potential drug interactions, availability of expertise and diagnostic tools, and costs. We recommend consulting a microbiologist and infectious disease specialist.
What is the appropriate treatment regimen for CAPA?
Recommendation (Table 3 , Table 4 )
-
1.
We recommend antifungal treatment for proven, probable, possible, and putative CAPA. (Strong recommendation, moderate quality of evidence) (1B)
-
2.
Single or sequential monotherapy with voriconazole (VOR), isavuconazole (ISZ), posaconazole (POS), liposomal-amphotericin B (L-ampB) is recommended. (Strong recommendation, low quality of evidence) (1C)
-
3.
Amphotericin-B deoxycholate (AmpB-d) and echinocandins may be considered as an alternative therapy. (Strong recommendation, low quality of evidence) (1C)
-
4.
We recommend adjustment of antifungal regimen according to the identified Aspergillus species, treatment response, adverse effect, and therapeutic drug monitoring (TDM). (Strong recommendation, low quality of evidence) (1C)
-
5.
We suggest reference to the local prevalence rate of resistance or the drug susceptibility test when choosing the drug of choice in antifungal regimens when sequential monotherapy or combination therapy is considered. (Weak recommendation, low quality of evidence) (2C)
Table 3.
Recommended treatment options for COVID-19 associated pulmonary aspergillosis (CAPA).
| Definition (Criteria) | Diagnostic criteria | Recommendations | Duration |
|---|---|---|---|
| Proven | Histology, Blood culture (+), |
Monotherapy or sequential monotherapy: (1C) Voriconazole: 6 mg/kg IV q12h x 2 doses, then 4 mg/kg IV q12h Isavuconazole: 200 mg IV q8h x 6 doses, then 200 mg IV qd Posaconazole: 300 mg IV q12h x 2 doses, then 300 mg IV qd Liposomal amphotericin B: 3–5 mg/kg IV qda |
6–12 weeks (2D)d |
| Probable (IAPA, EORTC/MSG, ISHAM) Putative (AspICU) |
Respiratory. Aspergillus culture/PCR (+) Blood GM (+), and/or BAL GM (+) |
Alternative monotherapy: (2C) Amphotericin B deoxycholate: 1.0–1.5 mg/kg IV qda Micafunginb: 100 mg IV qd Caspofungin: 70 mg IV loading dose, then 50 mg IV qd Anidulafungin: 200 mg IV loading dose, then 100 mg IV qd Combination Treatment:c (2C) Voriconazole + Anidulafungin Voriconazole + Caspofungin Isavuconazole + Liposomal amphotericin B |
|
| Possible (EORTC/MSG, ISHAM) Putative (AspICU) |
Endotracheal aspirate GM (+)/PCR (+) | ||
| Colonization (AspICU) | Discuss with infectious diseases specialist (antifungal therapy may not be needed) |
Abbreviations: BAL: bronchoalveolar lavage, EORTC/MSG: European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium, GM: galactomannan, IAPA: Influenza-associated pulmonary aspergillosis, ISHAM: The International Society for Human & Animal Mycology, IV: intravenous, MIC: minimum inhibition concentration, PCR: polymerase chain reaction, q12h: every 12 h, q8h: every 8 h, qd: once per day.
The MIC of amphotericin B for Aspergillus spp. Ranges from 0.75 to 8 mg/L in Taiwan.111 A higher dose is recommended for CAPA or combination regimen should be considered.
Echinocandins should not be used as monotherapy if other options are available, unless when used for salvage therapy.190
Combination therapy are usually given to patients on immunosuppressant or in high-risk patients. A higher mortality was observed compared to those given monotherapy/sequential monotherapy. Current clinical evidence is insufficient to recommend any one of the different combination regimens over the other.
Treatment duration of antifungal agents is determined by the clinical and laboratory evidence of response, such as blood GM testing and chest imaging, and may be discontinued after 6–12 weeks after a comprehensive evaluation for risk of recurrence.
Table 4.
Recommendations for management of COVID-19 associated pulmonary aspergillosis (CAPA) according to Aspergillus species and the risk of drug resistance.
| Aspergillus species | Risk of drug resistance | Recommendations |
|---|---|---|
| A. nidulans complex, A. sydowii, A. versicolor complex, A. lentulus, A. luchuenusis | Higher resistance rate to amphotericin B | 1. Avoid amphotericin B, or use with caution. 2. May consider combination treatment if drug resistance is suspected and in severe CAPA (2C) |
| A. fumigatus, A. flavus, A. nidulans, A. versicolor, A. ustus, and others. | Increased resistance to azoles | 1. Close monitoring of treatment response. 2. May consider combination treatment or sequential monotherapy if drug resistance is suspected (2C) |
Summary of the evidence
The overall mortality for CAPA was high, ranging from 43% to 71.4%, across studies with different CAPA definition and epidemiology; two meta-analyses showed that antifungal treatment did not affect mortality.102 , 110 However, a trend towards lower mortality with antifungal treatment was observed in 3 cohort studies. The mortality in CAPA ranged from 59% to 100% without antifungal treatment, and declined to a rate of 38.5% and 62.5% with treatment.19 , 123 , 134 One meta-analysis of 20 studies demonstrated that none of the host risk factors, results of mycological test, therapy for COVID-19 and antifungal therapy affected mortality in patients with CAPA, and suggested that early diagnosis and prompt therapy may be pivotal in optimizing the outcome.110
Before the COVID-19 pandemic, high risk patients for IPA included those with underlying hematological malignancy, organ transplant recipients, neutropenia, ICU patients requiring mechanical ventilation and severe influenza.104 In clinical trials and observational studies of patients with proven or probable invasive aspergillosis, 3-months survival rate was the highest in patients who received isavuconazole or voriconazole based therapy (60–75%), followed by amphotericin B and then echinocandin therapy (less than 50–60%).135, 136, 137 To date, there are no RCTs regarding which regimen is the most appropriate treatment for CAPA. The most common regimens prescribed for CAPA include voriconazole, isavuconazole, and amphotericin B.138 During the first wave of COVID-19 from 2020 to 2021, some patients with CAPA were treated with monotherapy while others received sequential monotherapy with triazoles, echinocandins, and amphotericin B. One prospective, multicenter, cohort study including 30 patients diagnosed with probable CAPA, found that treatment with voriconazole had a trend towards lower mortality (46% vs 59% p = 0.30) and a reduction in GM index over time.123 Most cases received sequential monotherapy and adjustment were made based on the response of the treatment, TDM, side effects, and de-escalation from intravenous infusion to oral tablets.139 To date, there is no study published on the correlation between drug susceptibility of Aspergillus spp. Isolated from clinical specimens and treatment response in CAPA patients, and currently, a high mortality rate is observed despite appropriate antifungal treatment.
What is the role of combination antifungal therapy for CAPA and what is the optimal antifungal treatment duration for CAPA?
Recommendation
-
1.
Combination therapy can be considered if drug-resistant fungal infection is a concern, such as when coinfections may be due to triazole-resistant Aspergillus spp., or when co-incidence of triazole-resistant Candida spp., or mucormycosis occurs in CAPA. (Weak recommendation, low quality of evidence) (2C)
-
2.
We suggest that the treatment duration of antifungal agents should be determined by the clinical and laboratory evidence of treatment response, such as serum GM testing and chest imaging, and may be discontinued after 6–12 weeks, after a comprehensive evaluation for the risk of recurrence. (Weak recommendation, very low quality of evidence) (2D)
Summary of the evidence
Combination treatment was given to 19 patients diagnosed with CAPA during the period of Jan, 2020 to Dec, 2021, and the proportion of patients receiving combination treatment was 10–83% in 3 small case series.118 , 134 , 140 The mortality was high, ranging from 52 to 70%.118 , 134 , 140 There is currently no study that assessed the benefit of combination therapy compared with mono- or sequential therapy on mortality.
To date, there is no RCT that focuses on the optimal treatment duration of the antifungal therapy. Without antifungal treatment, most patients died within 30 days after diagnosis of CAPA.123 , 140 For patients with CAPA who survived, the mortality was similar compared to non-CAPA patients after at least 2 months of follow up.140 A treatment duration of 6–12 weeks is recommended for CAPA by the ECMM/ISHAM expert panel and based on the IDSA guidelines141 for treatment of patients with invasive aspergillosis.
COVID-19 associated candidiasis
Invasive candidiasis is one of the most important healthcare-associated fungal infection caused by Candida species, which results in substantial morbidity and mortality. During the ongoing COVID-19 global pandemic, COVID-19 associated candidiasis (CAC) has been reported frequently among critically ill COVID-19 patients in many countries.142 According to five retrospective studies on the clinical characteristics and outcome of CAC, severe COVID-19 patients who are critically ill or mechanically ventilated had a higher incidence of candidemia compared with non-COVID-19 critically ill patients. Associated risk factors for CAC include longer ICU stay, corticosteroid use, old age, presence of sepsis, mechanical ventilation use, and indwelling central venous catheter. Candida albicans remains the most commonly isolated Candida spp. Among both COVID-19 and non-COVID critically ill patients in several case series.142, 143, 144, 145, 146 However, Candida auris was the most prevalent Candida species reported in some geographical areas such as India, and empirical antifungal therapy should take into consideration the local epidemiology.147
What is the utility of diagnostic criteria, such as candida score, for the diagnosis of CAC?
Recommendation
Candida score may not have a role for early detection of CAC among COVID-19 patients (Weak recommendation, low quality of evidence) (2C).
Summary of the evidence
One of the key challenges to the management of CAC is early recognition of invasive candidiasis among COVID-19 patients. One retrospective study including 236 episodes of candidemia (105 episodes in COVID-19 patients and 131 in non-COVID-19 patients) showed that over one-third of the patients died before receiving antifungal therapy and the 28-day mortality rate was significantly higher in COVID-19 patients (87.5% vs 67.9%, p = 0.02).145 There was no difference in the Candida scores between COVID-19 and non-COVID-19 patients with candidemia.145 Invasive candidiasis should be suspected in patients with known risk factors, or who develop fever with undetermined etiology, and/or poor response to antibacterial therapy.20 , 148 Although previous studies demonstrated that critically ill patients with Candida colonization in multiple sites, i.e. a high Candida score, may benefit from early antifungal treatment.149 , 150 Whether this is applicable to patients with COVID-19 remains uncertain due to the lack of studies on the sensitivity and specificity of Candida score in COVID-19 patients. Based on limited evidence, the panel is in doubt of the role Candida score alone may have for early detection of CAC among COVID-19 patients.
What is the optimal treatment regimen for COVID-19 associated candidemia?
Recommendation (Table 5 )
-
1.
Fluconazole is recommended as the first-line, empirical therapy for non-critically ill patients or those with a low risk of azole-resistant Candida species (Strong recommendation, high quality of evidence) (1A).
-
2.
Echinocandins are recommended as the first-line, empirical therapy for critically ill patients; those with a history of recent azole exposure; or a high risk of fluconazole-resistant Candida species (Strong recommendation, high quality of evidence) (1A).
-
3.
For candidemia caused by C. auris, echinocandins are recommended (Strong recommendation, low quality of evidence) (1C).
-
4.
Liposomal amphotericin B or amphotericin B deoxycholate may be considered if there is persistent candidemia or clinically unresponsiveness to treatment with echinocandins without evidence of resistance to amphotericin B (Weak recommendation, low quality of evidence) (2C).
Table 5.
Recommended treatment options for COVID-19 associated candidiasis (CAC).
| Clinical syndrome | Condition | Recommendations | Duration |
|---|---|---|---|
| Bloodstream infection (BSI) (candidemia) | Non-critically ill or low risk of azole-resistance | Fluconazole IV 6 mg/kg qd (1A) | At least 14 days; refer to the previous guidelinea,b |
| Critically ill or high risk of azole-resistance | Echinocandins (1A) | ||
| C. auris | Echinocandins (1C) Amphotericin B (2C)c | ||
| Sites other than BSIa | Management in general | Antifungal treatmenta | Refer to the previous guidelinea,e |
| Fungus balls or casts in the pyelum or urinary bladder | Surgeryd |
Abbreviations: BSI: blood stream infection, IV: intravenous, qd: once per day.
Please refer to the “2016 guidelines for the use of antifungal agents in patients with invasive fungal diseases in Taiwan”. J Microbiol Immunol Infect 2018 Feb; 51 (1):1–17. https://pubmed.ncbi.nlm.nih.gov/28781150/.
Recommended duration of therapy for candidemia without obvious metastatic complications is 14 days after documented clearance of Candida from the bloodstream and resolution of signs and symptoms attributable to candidemia.
If persistent candidemia or clinically unresponsiveness to treatment with echinocandins.
Delayed operation may be considered after balancing the risk to the patient and the risk of SARS-CoV-2 transmission.
Treatment duration depends on the site of infection.
Summary of the evidence
The diagnosis and management of CAC is based on pre-existing knowledge and the conventional approach before the COVID-19 pandemic. For non-critically ill patients or those with a low risk of azole-resistant Candida species, fluconazole remains the drug of choice for candidemia. For critically ill patients, or those with a history of recent azole exposure, or with a high risk of fluconazole-resistant Candida species, echinocandins are recommended as the first-line therapy.23 , 148
The recent emergence of C. auris to cause sporadic cases or outbreaks in many countries worldwide is of concern due to its extensive resistance to antifungal agents and high mortality (up to 64%).151, 152, 153, 154, 155, 156, 157, 158, 159 The overall susceptibility rate of C. auris to fluconazole was 10.7% and amphotericin B 43.1%, in 75 clinical isolates from patients with COVID-19. In contrast, the overall susceptibility rate of C. auris to caspofungin, micafungin, and anidulafungin was 90%, 98.2%, and 97.2%, respectively.155, 156, 157, 158, 159, 160 Echinocandin- or pan-resistant C. auris has been reported in health-care settings.161 Based on the current published literature, the panel recommends echinocandins as the first-line therapy for candidemia caused by C. auris. For treatment of persistent candidemia under echinocandin treatment, liposomal amphotericin B or amphotericin B deoxycholate can be considered in the absence of resistance.
What is the optimal treatment regimen for CAC other than bloodstream infections?
Recommendation
-
1.
Recommendations for treatment of CAC other than bloodstream infection are referred to the “2016 guidelines for the use of antifungal agents in patients with invasive fungal diseases in Taiwan”.23
-
2.
For patients with fungus balls or casts in the pyelum or urinary bladder caused by Candida requiring surgical intervention, delayed operation may be considered after balancing the risk to the patient and the risk of SARS-CoV-2 transmission (Weak recommendation, low quality of evidence) (2C).
Summary of the evidence
The management of CAC, involving sites other than the bloodstream, is mostly based on pre-existing knowledge before the COVID-19 pandemic. In the current Taiwan guidelines for management of candidiasis, surgical intervention is recommended in adults with fungus balls or casts in the pyelum or urinary bladder.23 However, considering the potential risk of SARS-CoV-2 transmission, the panel suggests that with antifungal treatment, deferral of surgical intervention may be considered after weighing the risk to the patient.
COVID-19 associated mucormycosis
The prevalence of CAM varies in different geographic regions due to differences in humidity and temperature.4 According to a recent systematic review and a large, multicenter, cohort study conducted in India, the estimated prevalence of CAM is around 0.3%, which is much lower than that of CAC and CAPA.162 CAM appears to develop in the late stage of COVID-19; the median time from COVID-19 onset to CAM diagnosis was 13–18 days in different studies and may even develop as late as 42 days following the diagnosis of COVID-19.163
Diabetes mellitus remains the most common risk factor for developing CAM (60%–92%), other risk factors including steroid use, severe COVID-19 and high blood glucose levels (blood glucose >200 mg/dL); on the contrary, zinc therapy was found to be protective (aOR 0.05, 95% CI 0.01–0.19).164 , 165 Risk factors of CAM-associated mortality include ICU admission, pulmonary involvement as well as disseminated infection.166
CAM can be classified into rhino-cerebral-orbital mucormycosis (RCOM), pulmonary, gastrointestinal, cutaneous, and disseminated mucormycosis. RCOM is the most common clinical syndrome (42%), and pulmonary mucormycosis accounted for 10%.163 , 167 In a study of 2826 patients with COVID-19-associated ROCM, the most frequent symptoms were orbital/facial pain (23%), followed by orbital/facial edema (21%), loss of vision (19%), ptosis (11%), and nasal block (9%).168 Symptoms of pulmonary mucormycosis include pyrexia, cough and dyspnea, which was relatively nonspecific and may be difficult to differentiate from symptoms of COVID-19 or bacterial pneumonia.169
What's the recommendations for the management of CAM?
Recommendation (Table 6 )
-
1.
Strict glycemic control and optimization of corticosteroids use is recommended. (Strong recommendation, low quality of evidence) (1C)
-
2.
Both antifungal therapy and immediate surgical debridement are recommended for CAM. For patients who need debridement, surgical intervention should not be delayed, and the operation should be performed with appropriate personal protective equipment in a well-established facility to prevent transmission of SARS-CoV-2. (Strong recommendation, low quality of evidence) (1C)
-
3.Primary therapy: The panel recommends 4–6 weeks of induction and consolidation treatment.
-
A.Liposomal amphotericin B is recommended as the primary therapy with a dose of 5 mg/kg/day in patients without CNS involvement, or 10 mg/kg/day for those with CNS involvement. (Strong recommendation, moderate quality of evidence) (1B)
-
A.
-
4.Alternatives for primary therapy: The alternative regimens for induction and consolidation are listed in the order of strength of recommendation.
-
A.Amphotericin B deoxycholate (1–1.5 mg/kg/day) should be administered in 5% dextrose with slow infusion over 6–8 h, at the rate of 0.08 mg/kg/hour. Pre-medication with diphenhydramine or acetaminophen, prior to infusion, to avoid drug-related reaction is recommended. To avoid nephrotoxicity, 1 L of normal saline can be given before and after the infusion. (Strong recommendation, moderate quality of evidence) (1B)
-
B.Posaconazole is preferably given intravenously, or by oral tablet, at a dose of 300 mg twice daily on day 1, followed by 300 mg once daily. (Strong recommendation, low quality of evidence) (1C)
-
C.Isavuconazole is preferably given intravenously or by oral tablet, at a dose of 200 mg three times a day for two days, followed by 200 mg daily starting on day 3. (Strong recommendation, low quality of evidence) (1C)
-
A.
-
5.Maintenance therapy: Treatment duration of 3–6 months is recommended, until resolution of clinical signs and symptoms
-
A.Posaconazole is preferably given intravenously or by oral tablet, at a dose of 300 mg twice daily on day 1, followed by 300 mg once daily. (Strong recommendation, low quality of evidence) (1C)
-
B.Isavuconazole is preferably given intravenously or by oral tablet, at a dose of 200 mg three times a day for two days, followed by 200 mg daily starting on day 3. (Strong recommendation, low quality of evidence) (1C)
-
A.
Table 6.
Recommended Treatment options for COVID-19 associated mucormycosis (CAM).
| Condition | Preferred | Alternativesb | Duration |
|---|---|---|---|
| Primary therapy (induction & consolidation) | Liposomal amphotericin B IV 5 mg/kg qd, or IV 10 mg/kg qd for those with CNS involvement (1B)a | Amphotericin B deoxycholate 1.0–1.5 mg/kg qd (1B)b,c | 4–6 weeks |
| Isavuconazole 200 mg IV or PO q8h x 6 doses, followed by 200 mg IV/PO qd (1C)b,d | |||
| Posaconazole 300 mg IV or PO q12h x 2 doses, followed by 300 mg IV/PO qd (1C)b,d | |||
| Maintenance therapy | Isavuconazole 200 mg IV or PO q8h for 6 doses, followed by 200 mg IV/PO qd (1C)d | 3–6 monthse | |
| Posaconazole 300 mg IV or PO q12h for 2 doses, followed by 300 mg IV/PO qd (1C)d |
Abbreviation: IV: intravenous, q8h: every 8 h, q12h: every 12 h, qd: once per day.
Give in 200 mL 5% dextrose with infusion over 2–3 h.
Listed in the order of strength of recommendation.
Give in 5% dextrose with slow infusion over 6–8 h, at the rate of 0.08 mg/kg/hour. To avoid nephrotoxicity, 1 L of normal saline can be given before and after infusion. Pre-mediate with diphenhydramine or acetaminophen prior to infusion to avoid drug-related reaction.
Intravenous is preferred over oral formulation.
The duration is usually 3–6 months, until resolution of clinical signs and symptoms, and should be tailored based on the underlying immune status.
Summary of the evidence
The management of CAM is similar to non-COVID-19 patients and generally follows published treatment guidelines.23 , 170 Strict glycemic control and optimization of corticosteroids are highly recommended, since diabetes and the use of corticosteroids have been found to be strongly correlated with the development of mucormycosis.169 A prior study on mucormycosis among patients with hematological malignancy showed that delayed initiation of treatment ≥6 days after diagnosis resulted in a 2-fold increase in mortality at 12 weeks after diagnosis even with an appropriate regimen.171 A retrospective study including 2826 CAM patients in India demonstrated that immediate and extensive radiology-guided surgical intervention in advanced mucormycosis reduced mortality rates from 67% to 39% and slowed disease progression.168 A systematic review including 41 studies concluded that the overall survival rate was higher in patients receiving both antifungal therapy and surgical management, compared to antifungal therapy alone (64.9% vs. 21.7%).172 The panel strongly recommends prompt initiation of antifungals after diagnosis of CAM. For patients who need debridement, surgical intervention should not be delayed, and the operation should be performed with appropriate personal protective equipment in a well-established facility to prevent transmission of SARS-CoV-2.
The panel recommends liposomal amphotericin B as the first-line therapy based on both the guidelines published before the COVID-19 pandemic and several case series and retrospective studies focusing on CAM patients.21 , 168 , 173, 174, 175, 176, 177, 178 Conventional dose is given at 5 mg/kg/day, with 10 mg/kg/day recommended for those with CNS involvement. For patients with endophthalmitis, intravitreal liposomal amphotericin B injection may be considered.176 Amphotericin B deoxycholate 1–1.5 mg/kg/day may be used as an alternative to treat CAM.179 Nephrotoxicity is the major drawback of amphotericin B deoxycholate and can be reduced by isotonic saline infusion and premedication with acetaminophen and diphenhydramine. Electrolyte imbalance such as hypokalemia and hypomagnesemia should be monitored.180 Isavuconazole alone or in combination with amphotericin B has been approved for invasive mucormycosis before the COVID-19 pandemic181 and was shown to be effective for CAM in a case series.173 Posaconazole and itraconazole are shown to be effective in vitro against Mucor spp. Previous studies have shown satisfactory treatment responses with use of posaconazole among non-COVID-19 patients182 or in combination with amphotericin B for CAM.183 Therapeutic drug monitoring should be considered while using posaconazole delayed-release tablet.184 Despite a case series of CAM reporting treatment success by itraconazole,185 the panel does not recommend itraconazole, since clinical studies documenting its effectiveness against mucormycosis remain lacking.
Further research comparing the efficacy of combination therapy versus single antifungal therapy is needed.162 , 186 Oral azoles can be used as sequential therapy for CAM in different clinical settings.183 , 187 , 188 A multicenter, retrospective study among CAM patients in India showed that sequential therapy with posaconazole or isavuconazole after amphotericin B was associated with improved survival.162
There is no clear evidence concerning the optimal duration of treatment for CAM. Code Mucor, a comprehensive guideline for CAM, suggests that RCOM be treated for 3–6 months.189 Based on the recommendations in the previously published guideline,170 the panel suggests that the treatment of CAM be continued until both clinical and radiological resolution occurs and should be tailored to each patient, based on the underlying immune status. A treatment algorithm for CAM is provided (Fig. 1 ).
Figure 1.
Treatment algorithm for COVID-19 association mucormycosis.
Conclusion
The recommendations for management of COVID-19 associated infections including CABI, CAPA, CAC and CAM are included in this guideline to offer guidance to first-line physicians who provide care for COVID-19 patients. The incidence rate of COVID-19 associated infections varied across different countries. The epidemiology and clinical experience in published literature for management of COVID-19 associated infections provided a preliminary framework for guidance and care of these patients. We suggest that local epidemiology should be considered in the implementation of these guidelines. With the rapid evolution of the COVID-19 pandemic, we acknowledge that further studies in the future will shape and modify the current knowledge on and recommendations for management of COVID-19 associated infections.
Declaration of competing interest
The authors declare no potential conflicts of interest with regards to the research, authorship, or publication of this article.
Acknowledgement
This work funded by the Medical Foundation in Memory of Dr. Deh-Lin Cheng, Taiwan and Veterans Affairs Council, Taipei, Taiwan, Republic of China (Grant number: VAC111-005). We thank the members of the internal and external expert review panel, board members of the Infectious Diseases Society of Taiwan (IDST) and specialists in the expert consensus meeting, for providing expert opinion and critical review of the guidelines.
Members of the expert panel and board members of the IDST are listed in alphabetical order: Yu-Jiun Chan, Feng-Yee Chang, Hou-Tai Chang, Yao-Shen Chen, Yee-Chun Chen, Yen-Hsu Chen, Ming-Fang Cheng, Hsin Chi, Cheng-Hsun Chiu, Mao-Wang Ho, Szu-Min Hsieh, Po-Ren Hsueh, Chien-Hsien Huang, Chien-Ching Hung, Kao-Pin Hwang, Kuo-Chin Kao, Wen-Chien Ko, Chien-Feng Kuo, Chung-Hsu Lai, Nan-Yao Lee, Shin-Jung Lee, Hsi-Hsun Lin, Yi-Tsung Lin, Ching-Chuan Liu, Po-Yu Liu, Yung-Ching Liu, Po-Liang Lu, Chun-Yi Lu, Wang-Huei Sheng, Hung-Jen Tang, Hung-Chin Tsai, Fu-Der Wang, Ting-Shu Wu, Chia-Jui Yang.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jmii.2022.12.003.
Contributor Information
Infectious Disease Society of Taiwan,:
Yu-Jiun Chan, Feng-Yee Chang, Hou-Tai Chang, Yao-Shen Chen, Yee-Chun Chen, Yen-Hsu Chen, Ming-Fang Cheng, Hsin Chi, Cheng-Hsun Chiu, Mao-Wang Ho, Szu-Min Hsieh, Po-Ren Hsueh, Chien-Hsien Huang, Chien-Ching Hung, Kao-Pin Hwang, Kuo-Chin Kao, Wen-Chien Ko, Chien-Feng Kuo, Chung-Hsu Lai, Nan-Yao Lee, Shin-Jung Lee, Hsi-Hsun Lin, Yi-Tsung Lin, Ching-Chuan Liu, Po-Yu Liu, Yung-Ching Liu, Po-Liang Lu, Chun-Yi Lu, Wang-Huei Sheng, Hung-Jen Tang, Hung-Chin Tsai, Fu-Der Wang, Ting-Shu Wu, and Chia-Jui Yang
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
figs4.
References
- 1.Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: emergence, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langford B.J., So M., Raybardhan S., Leung V., Westwood D., MacFadden D.R., et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26:1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musuuza J.S., Watson L., Parmasad V., Putman-Buehler N., Christensen L., Safdar N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: a systematic review and meta-analysis. PLoS One. 2021;16 doi: 10.1371/journal.pone.0251170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Vidal C., Sanjuan G., Moreno-García E., Puerta-Alcalde P., Garcia-Pouton N., Chumbita M., et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27:83–88. doi: 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerver S.M., Guy R., Wilson K., Thelwall S., Nsonwu O., Rooney G., et al. National surveillance of bacterial and fungal coinfection and secondary infection in COVID-19 patients in England: lessons from the first wave. Clin Microbiol Infect. 2021;27:1658–1665. doi: 10.1016/j.cmi.2021.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alhumaid S., Al Mutair A., Al Alawi Z., Alshawi A.M., Alomran S.A., Almuhanna M.S., et al. Coinfections with bacteria, Fungi, and respiratory viruses in patients with SARS-CoV-2: a systematic review and meta-analysis. Pathogens. 2021;10 doi: 10.3390/pathogens10070809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guyatt G.H., Oxman A.D., Schünemann H.J., Tugwell P., Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64:380–382. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Guyatt G.H., Oxman A.D., Vist G.E., Kunz R., Falck-Ytter Y., Alonso-Coello P., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balshem H., Helfand M., Schünemann H.J., Oxman A.D., Kunz R., Brozek J., et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Langford B.J., So M., Leung V., Raybardhan S., Lo J., Kan T., et al. Predictors and microbiology of respiratory and bloodstream bacterial infection in patients with COVID-19: living rapid review update and meta-regression. Clin Microbiol Infect. 2022;28:491–501. doi: 10.1016/j.cmi.2021.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elabbadi A., Turpin M., Gerotziafas G.T., Teulier M., Voiriot G., Fartoukh M. Bacterial coinfection in critically ill COVID-19 patients with severe pneumonia. Infection. 2021;49:559–562. doi: 10.1007/s15010-020-01553-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L., Amin A.K., Khanna P., Aali A., McGregor A., Bassett P., et al. An observational cohort study of bacterial co-infection and implications for empirical antibiotic therapy in patients presenting with COVID-19 to hospitals in North West London. J Antimicrob Chemother. 2021;76:796–803. doi: 10.1093/jac/dkaa475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falcone M., Tiseo G., Giordano C., Leonildi A., Menichini M., Vecchione A., et al. Predictors of hospital-acquired bacterial and fungal superinfections in COVID-19: a prospective observational study. J Antimicrob Chemother. 2021;76:1078–1084. doi: 10.1093/jac/dkaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ripa M., Galli L., Poli A., Oltolini C., Spagnuolo V., Mastrangelo A., et al. Secondary infections in patients hospitalized with COVID-19: incidence and predictive factors. Clin Microbiol Infect. 2021;27:451–457. doi: 10.1016/j.cmi.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koehler P., Bassetti M., Chakrabarti A., Chen S.C.A., Colombo A.L., Hoenigl M., et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2021;21:e149–e162. doi: 10.1016/S1473-3099(20)30847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bassetti M., Azoulay E., Kullberg B.J., Ruhnke M., Shoham S., Vazquez J., et al. EORTC/MSGERC definitions of invasive fungal diseases: Summary of Activities of the intensive care Unit working group. Clin Infect Dis. 2021;72 doi: 10.1093/cid/ciaa1751. S121-s7. [DOI] [PubMed] [Google Scholar]
- 17.Huang L., He H., Jin J., Zhan Q. Is Bulpa criteria suitable for the diagnosis of probable invasive pulmonary Aspergillosis in critically ill patients with chronic obstructive pulmonary disease? A comparative study with EORTC/MSG and ICU criteria. BMC Infect Dis. 2017;17:209. doi: 10.1186/s12879-017-2307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gangneux J.P., Reizine F., Guegan H., Pinceaux K., Le Balch P., Prat E., et al. Is the COVID-19 pandemic a good time to include Aspergillus molecular detection to categorize aspergillosis in ICU patients? A Monocentric experience. J Fungi (Basel) 2020;6 doi: 10.3390/jof6030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machado M., Valerio M., Álvarez-Uría A., Olmedo M., Veintimilla C., Padilla B., et al. Invasive pulmonary aspergillosis in the COVID-19 era: an expected new entity. Mycoses. 2021;64:132–143. doi: 10.1111/myc.13213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pappas P.G., Lionakis M.S., Arendrup M.C., Ostrosky-Zeichner L., Kullberg B.J. Invasive candidiasis. Nat Rev Dis Primers. 2018;4 doi: 10.1038/nrdp.2018.26. [DOI] [PubMed] [Google Scholar]
- 21.Selarka L., Sharma S., Saini D., Sharma S., Batra A., Waghmare V.T., et al. Mucormycosis and COVID-19: an epidemic within a pandemic in India. Mycoses. 2021;64:1253–1260. doi: 10.1111/myc.13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.NIH. COVID-19 treatment guidelines. Vol vol. 2022. U.S.A. 2022. [Google Scholar]
- 23.Kung H.C., Huang P.Y., Chen W.T., Ko B.S., Chen Y.C., Chang S.C., et al. 2016 Guidelines for the use of antifungal agents in patients with invasive fungal diseases in Taiwan. J Microbiol Immunol Infect. 2018;51:1–17. doi: 10.1016/j.jmii.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Baghdadi J.D., Coffey K.C., Adediran T., Goodman K.E., Pineles L., Magder L.S., et al. Antibiotic Use and bacterial infection among Inpatients in the first wave of COVID-19: a retrospective cohort study of 64,691 patients. Antimicrob Agents Chemother. 2021;65 doi: 10.1128/AAC.01341-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andreani J., Le Bideau M., Duflot I., Jardot P., Rolland C., Boxberger M., et al. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb Pathog. 2020;145 doi: 10.1016/j.micpath.2020.104228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rouzé A., Martin-Loeches I., Povoa P., Metzelard M., Du Cheyron D., Lambiotte F., et al. Early bacterial identification among intubated patients with COVID-19 or influenza pneumonia: a European multicenter comparative clinical trial. Am J Respir Crit Care Med. 2021;204:546–556. doi: 10.1164/rccm.202101-0030OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shafran N., Shafran I., Ben-Zvi H., Sofer S., Sheena L., Krause I., et al. Secondary bacterial infection in COVID-19 patients is a stronger predictor for death compared to influenza patients. Sci Rep. 2021;11 doi: 10.1038/s41598-021-92220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cong W., Poudel A.N., Alhusein N., Wang H., Yao G., Lambert H. Antimicrobial Use in COVID-19 patients in the first phase of the SARS-CoV-2 pandemic: a scoping review. Antibiotics (Basel) 2021;10 doi: 10.3390/antibiotics10060745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puzniak L., Bauer K.A., Yu K.C., Moise P., Finelli L., Ye G., et al. Effect of Inadequate empiric antibacterial therapy on hospital outcomes in SARS-CoV-2-positive and -negative US patients with a positive bacterial culture: a multicenter evaluation from March to November 2020. Open Forum Infect Dis. 2021;8 doi: 10.1093/ofid/ofab232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karaba S.M., Jones G., Helsel T., Smith L.L., Avery R., Dzintars K., et al. Prevalence of Co-infection at the time of hospital admission in COVID-19 patients, A multicenter study. Open Forum Infect Dis. 2021;8:ofaa578. doi: 10.1093/ofid/ofaa578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaughn V.M., Gandhi T.N., Petty L.A., Patel P.K., Prescott H.C., Malani A.N., et al. Empiric antibacterial therapy and community-onset bacterial coinfection in patients hospitalized with coronavirus disease 2019 (COVID-19): a multi-hospital cohort study. Clin Infect Dis. 2021;72:e533–e541. doi: 10.1093/cid/ciaa1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng L.S., Chau S.K., Tso E.Y., Tsang S.W., Li I.Y., Wong B.K., et al. Bacterial co-infections and antibiotic prescribing practice in adults with COVID-19: experience from a single hospital cluster. Ther Adv Infect Dis. 2020;7 doi: 10.1177/2049936120978095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bashir A., Abdullah M.S., Momin N.R., Chong P.L., Asli R., Ivan B.M., et al. Prevalence of primary bacterial co-infections among patients with COVID-19 in Brunei Darussalam. Western Pac Surveill Response J. 2021;12:65–70. doi: 10.5365/wpsar.2021.12.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anton-Vazquez V., Clivillé R. Streptococcus pneumoniae coinfection in hospitalised patients with COVID-19. Eur J Clin Microbiol Infect Dis. 2021;40:1353–1355. doi: 10.1007/s10096-021-04166-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lardaro T., Wang A.Z., Bucca A., Croft A., Glober N., Holt D.B., et al. Characteristics of COVID-19 patients with bacterial coinfection admitted to the hospital from the emergency department in a large regional healthcare system. J Med Virol. 2021;93:2883–2889. doi: 10.1002/jmv.26795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar G., Adams A., Hererra M., Rojas E.R., Singh V., Sakhuja A., et al. Predictors and outcomes of healthcare-associated infections in COVID-19 patients. Int J Infect Dis. 2021;104:287–292. doi: 10.1016/j.ijid.2020.11.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein E.Y., Monteforte B., Gupta A., Jiang W., May L., Hsieh Y.H., et al. The frequency of influenza and bacterial coinfection: a systematic review and meta-analysis. Influenza Other Respir Viruses. 2016;10:394–403. doi: 10.1111/irv.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rawson T.M., Moore L.S.P., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M., et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to Support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71:2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langford B.J., So M., Raybardhan S., Leung V., Soucy J.R., Westwood D., et al. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin Microbiol Infect. 2021;27:520–531. doi: 10.1016/j.cmi.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asmarawati T.P., Rosyid A.N., Suryantoro S.D., Mahdi B.A., Windradi C., Wulaningrum P.A., et al. The clinical impact of bacterial co-infection among moderate, severe and critically ill COVID-19 patients in the second referral hospital in Surabaya. F1000Res. 2021;10:113. doi: 10.12688/f1000research.31645.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bardi T., Pintado V., Gomez-Rojo M., Escudero-Sanchez R., Azzam Lopez A., Diez-Remesal Y., et al. Nosocomial infections associated to COVID-19 in the intensive care unit: clinical characteristics and outcome. Eur J Clin Microbiol Infect Dis. 2021;40:495–502. doi: 10.1007/s10096-020-04142-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baskaran V., Lawrence H., Lansbury L.E., Webb K., Safavi S., Zainuddin N.I., et al. Co-infection in critically ill patients with COVID-19: an observational cohort study from England. J Med Microbiol. 2021:70. doi: 10.1099/jmm.0.001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bolker A., Coe K., Smith J., Stevenson K., Wang S.H., Reed E. Predictors of respiratory bacterial co-infection in hospitalized COVID-19 patients. Diagn Microbiol Infect Dis. 2022;102 doi: 10.1016/j.diagmicrobio.2021.115558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu C., Wen Y., Wan W., Lei J., Jiang X. Clinical characteristics and antibiotics treatment in suspected bacterial infection patients with COVID-19. Int Immunopharmacol. 2021;90 doi: 10.1016/j.intimp.2020.107157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang M.L., Li Y.Q., Chen X., Lin H., Jiang Z.C., Gu D.L., et al. Co-infection with common respiratory pathogens and SARS-CoV-2 in patients with COVID-19 pneumonia and laboratory Biochemistry findings: a retrospective cross-Sectional study of 78 patients from a single center in China. Med Sci Monit. 2021;27 doi: 10.12659/MSM.929783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buehler P.K., Zinkernagel A.S., Hofmaenner D.A., Wendel Garcia P.D., Acevedo C.T., Gómez-Mejia A., et al. Bacterial pulmonary superinfections are associated with longer duration of ventilation in critically ill COVID-19 patients. Cell Rep Med. 2021;2 doi: 10.1016/j.xcrm.2021.100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Westblade L.F., Simon M.S., Satlin M.J. Bacterial coinfections in coronavirus disease 2019. Trends Microbiol. 2021;29:930–941. doi: 10.1016/j.tim.2021.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Russell C.D., Fairfield C.J., Drake T.M., Turtle L., Seaton R.A., Wootton D.G., et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. Lancet Microbe. 2021;2:e354–e365. doi: 10.1016/S2666-5247(21)00090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nasir N., Rehman F., Omair S.F. Risk factors for bacterial infections in patients with moderate to severe COVID-19: a case-control study. J Med Virol. 2021;93:4564–4569. doi: 10.1002/jmv.27000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dellit T.H., Owens R.C., McGowan J.E., Gerding D.N., Weinstein R.A., Burke J.P., et al. Infectious diseases Society of America and the Society for healthcare epidemiology of America guidelines for developing an Institutional program to Enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 51.Pollack L.A., Srinivasan A. Core elements of hospital antibiotic stewardship programs from the Centers for Disease Control and Prevention. Clin Infect Dis. 2014;59(Suppl 3):S97–S100. doi: 10.1093/cid/ciu542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tamma P.D., Avdic E., Li D.X., Dzintars K., Cosgrove S.E. Association of adverse events with antibiotic Use in hospitalized patients. JAMA Intern Med. 2017;177:1308–1315. doi: 10.1001/jamainternmed.2017.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lai C.C., Chen S.Y., Ko W.C., Hsueh P.R. Increased antimicrobial resistance during the COVID-19 pandemic. Int J Antimicrob Agents. 2021;57 doi: 10.1016/j.ijantimicag.2021.106324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rawson T.M., Moore L.S.P., Castro-Sanchez E., Charani E., Davies F., Satta G., et al. COVID-19 and the potential long-term impact on antimicrobial resistance. J Antimicrob Chemother. 2020;75:1681–1684. doi: 10.1093/jac/dkaa194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rothe K., Feihl S., Schneider J., Wallnöfer F., Wurst M., Lukas M., et al. Rates of bacterial co-infections and antimicrobial use in COVID-19 patients: a retrospective cohort study in light of antibiotic stewardship. Eur J Clin Microbiol Infect Dis. 2021;40:859–869. doi: 10.1007/s10096-020-04063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coenen S., de la Court J.R., Buis D.T.P., Meijboom L.J., Schade R.P., Visser C.E., et al. Low frequency of community-acquired bacterial co-infection in patients hospitalized for COVID-19 based on clinical, radiological and microbiological criteria: a retrospective cohort study. Antimicrob Resist Infect Control. 2021;10:155. doi: 10.1186/s13756-021-01024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Furukawa D., Graber C.J. Antimicrobial stewardship in a pandemic: Picking up the Pieces. Clin Infect Dis. 2021;72:e542–e544. doi: 10.1093/cid/ciaa1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grasselli G., Scaravilli V., Mangioni D., Scudeller L., Alagna L., Bartoletti M., et al. Hospital-acquired infections in critically ill patients with COVID-19. Chest. 2021;160:454–465. doi: 10.1016/j.chest.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allou N., Larsen K., Dubernet A., Traversier N., Masse L., Foch E., et al. Co-infection in patients with hypoxemic pneumonia due to COVID-19 in Reunion Island. Medicine (Baltim) 2021;100 doi: 10.1097/MD.0000000000024524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen S., Zhu Q., Xiao Y., Wu C., Jiang Z., Liu L., et al. Clinical and etiological analysis of co-infections and secondary infections in COVID-19 patients: an observational study. Clin Respir J. 2021;15:815–825. doi: 10.1111/crj.13369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goncalves Mendes Neto A., Lo K.B., Wattoo A., Salacup G., Pelayo J., DeJoy R., 3rd, et al. Bacterial infections and patterns of antibiotic use in patients with COVID-19. J Med Virol. 2021;93:1489–1495. doi: 10.1002/jmv.26441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lansbury L., Lim B., Baskaran V., Lim W.S. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81:266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Francesco M.A., Poiesi C., Gargiulo F., Bonfanti C., Pollara P., Fiorentini S., et al. Co-infection of chlamydia pneumoniae and mycoplasma pneumoniae with SARS-CoV-2 is associated with more severe features. J Infect. 2021;82:e4–e7. doi: 10.1016/j.jinf.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amin D., McKitish K., Shah P.S. Association of mortality and recent Mycoplasma pneumoniae infection in COVID-19 patients. J Med Virol. 2021;93:1180–1183. doi: 10.1002/jmv.26467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rothe K., Spinner C.D., Panning M., Pletz M.W., Rohde G., Rupp J., et al. Evaluation of a multiplex PCR screening approach to identify community-acquired bacterial co-infections in COVID-19: a multicenter prospective cohort study of the German competence network of community-acquired pneumonia (CAPNETZ) Infection. 2021;49:1299–1306. doi: 10.1007/s15010-021-01720-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chou C.C., Shen C.F., Chen S.J., Chen H.M., Wang Y.C., Chang W.S., et al. Recommendations and guidelines for the treatment of pneumonia in Taiwan. J Microbiol Immunol Infect. 2019;52:172–199. doi: 10.1016/j.jmii.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 67.Foschi C., Zignoli A., Gaibani P., Vocale C., Rossini G., Lafratta S., et al. Respiratory bacterial co-infections in intensive care unit-hospitalized COVID-19 patients: conventional culture vs BioFire FilmArray pneumonia Plus panel. J Microbiol Methods. 2021;186 doi: 10.1016/j.mimet.2021.106259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adalbert J.R., Varshney K., Tobin R., Pajaro R. Clinical outcomes in patients co-infected with COVID-19 and Staphylococcus aureus: a scoping review. BMC Infect Dis. 2021;21:985. doi: 10.1186/s12879-021-06616-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lewis P.O. Risk factor evaluation for methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa in community-acquired pneumonia. Ann Pharmacother. 2021;55:36–43. doi: 10.1177/1060028020935106. [DOI] [PubMed] [Google Scholar]
- 70.Wu T.H., Lee C.Y., Yang H.J., Fang Y.P., Chang Y.F., Tzeng S.L., et al. Prevalence and molecular characteristics of methicillin-resistant Staphylococcus aureus among nasal carriage strains isolated from emergency department patients and healthcare workers in central Taiwan. J Microbiol Immunol Infect. 2019;52:248–254. doi: 10.1016/j.jmii.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 71.Parente D.M., Cunha C.B., Mylonakis E., Timbrook T.T. The clinical utility of methicillin-resistant Staphylococcus aureus (MRSA) nasal screening to rule out MRSA pneumonia: a diagnostic meta-analysis with antimicrobial stewardship implications. Clin Infect Dis. 2018;67:1–7. doi: 10.1093/cid/ciy024. [DOI] [PubMed] [Google Scholar]
- 72.Rouzé A., Martin-Loeches I., Povoa P., Makris D., Artigas A., Bouchereau M., et al. Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: a European multicenter cohort study. Intensive Care Med. 2021;47:188–198. doi: 10.1007/s00134-020-06323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Metlay J.P., Waterer G.W., Long A.C., Anzueto A., Brozek J., Crothers K., et al. Diagnosis and treatment of adults with community-acquired pneumonia. An Official clinical practice guideline of the American Thoracic Society and infectious diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jain S., Self W.H., Wunderink R.G., Fakhran S., Balk R., Bramley A.M., et al. Community-acquired pneumonia requiring hospitalization among U.S. Adults. N Engl J Med. 2015;373:415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Timbrook T.T., Hueth K.D., Ginocchio C.C. Identification of bacterial co-detections in COVID-19 critically Ill patients by BioFire® FilmArray® pneumonia panel: a systematic review and meta-analysis. Diagn Microbiol Infect Dis. 2021;101 doi: 10.1016/j.diagmicrobio.2021.115476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kolenda C., Ranc A.G., Boisset S., Caspar Y., Carricajo A., Souche A., et al. Assessment of respiratory bacterial coinfections among severe acute respiratory syndrome coronavirus 2-positive patients hospitalized in intensive care Units using conventional culture and BioFire, FilmArray pneumonia panel plus assay. Open Forum Infect Dis. 2020;7:ofaa484. doi: 10.1093/ofid/ofaa484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Caméléna F., Moy A.C., Dudoignon E., Poncin T., Deniau B., Guillemet L., et al. Performance of a multiplex polymerase chain reaction panel for identifying bacterial pathogens causing pneumonia in critically ill patients with COVID-19. Diagn Microbiol Infect Dis. 2021;99 doi: 10.1016/j.diagmicrobio.2020.115183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kreitmann L., Monard C., Dauwalder O., Simon M., Argaud L. Early bacterial co-infection in ARDS related to COVID-19. Intensive Care Med. 2020;46:1787–1789. doi: 10.1007/s00134-020-06165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maataoui N., Chemali L., Patrier J., Tran Dinh A., Le Fèvre L., Lortat-Jacob B., et al. Impact of rapid multiplex PCR on management of antibiotic therapy in COVID-19-positive patients hospitalized in intensive care unit. Eur J Clin Microbiol Infect Dis. 2021;40:2227–2234. doi: 10.1007/s10096-021-04213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Evans L., Rhodes A., Alhazzani W., Antonelli M., Coopersmith C.M., French C., et al. Surviving sepsis Campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49:e1063–e1143. doi: 10.1097/CCM.0000000000005337. [DOI] [PubMed] [Google Scholar]
- 81.May M., Chang M., Dietz D., Shoucri S., Laracy J., Sobieszczyk M.E., et al. Limited utility of procalcitonin in identifying community-associated bacterial infections in patients presenting with coronavirus disease 2019. Antimicrob Agents Chemother. 2021;65 doi: 10.1128/AAC.02167-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Malinverni S., Nuñez M., Cotton F., Martiny D., Collot V., Konopnicki D., et al. Is procalcitonin a reliable marker of bacterial community-acquired pneumonia in adults admitted to the emergency department during SARS-CoV-2 pandemic? Eur J Emerg Med. 2021;28:312–314. doi: 10.1097/MEJ.0000000000000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fabre V., Karaba S., Amoah J., Robinson M., Jones G., Dzintars K., et al. The role of procalcitonin results in antibiotic decision-making in coronavirus disease 2019 (COVID-19) Infect Control Hosp Epidemiol. 2022;43:570–575. doi: 10.1017/ice.2021.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Han H., Ma Q., Li C., Liu R., Zhao L., Wang W., et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9:1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bhaskar S., Sinha A., Banach M., Mittoo S., Weissert R., Kass J.S., et al. Cytokine storm in COVID-19-Immunopathological Mechanisms, clinical considerations, and therapeutic Approaches: the REPROGRAM Consortium position Paper. Front Immunol. 2020;11:1648. doi: 10.3389/fimmu.2020.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pirofski L.A., Casadevall A. Pathogenesis of COVID-19 from the Perspective of the Damage-response framework. mBio. 2020:11. doi: 10.1128/mBio.01175-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peters C., Williams K., Un E.A., Little L., Saad A., Lendrum K., et al. Use of procalcitonin for antibiotic stewardship in patients with COVID-19: a quality improvement project in a district general hospital. Clin Med. 2021;21:e71–e76. doi: 10.7861/clinmed.2020-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pulia M.S., Wolf I., Schwei R.J., Chen D., Lepak A.J., Schulz L.T., et al. Antibiotic prescribing patterns for coronavirus disease 2019 (COVID-19) in two emergency departments with rapid procalcitonin. Infect Control Hosp Epidemiol. 2021;42:359–361. doi: 10.1017/ice.2020.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pink I., Raupach D., Fuge J., Vonberg R.P., Hoeper M.M., Welte T., et al. C-reactive protein and procalcitonin for antimicrobial stewardship in COVID-19. Infection. 2021;49:935–943. doi: 10.1007/s15010-021-01615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Berkel M., Kox M., Frenzel T., Pickkers P., Schouten J. Biomarkers for antimicrobial stewardship: a reappraisal in COVID-19 times? Crit Care. 2020;24:600. doi: 10.1186/s13054-020-03291-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hughes S., Mughal N., Moore L.S.P. Procalcitonin to guide antibacterial prescribing in patients hospitalised with COVID-19. Antibiotics. 2021;10:1119. doi: 10.3390/antibiotics10091119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roy A., Powers H.R., Craver E.C., Nazareno M.D., Yarrarapu S.N.S., Sanghavi D.K. Antibiotic stewardship: early discontinuation of antibiotics based on procalcitonin level in COVID-19 pneumonia. J Clin Pharm Ther. 2022;47:243–247. doi: 10.1111/jcpt.13554. [DOI] [PubMed] [Google Scholar]
- 94.Cheng K., He M., Shu Q., Wu M., Chen C., Xue Y. Analysis of the risk factors for nosocomial bacterial infection in patients with COVID-19 in a tertiary hospital. Risk Manag Healthc Policy. 2020;13:2593–2599. doi: 10.2147/RMHP.S277963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Côrtes M.F., de Almeida B.L., Espinoza E.P.S., Campos A.F., do Nascimento Moura M.L., Salomão M.C., et al. Procalcitonin as a biomarker for ventilator associated pneumonia in COVID-19 patients: is it an useful stewardship tool? Diagn Microbiol Infect Dis. 2021;101 doi: 10.1016/j.diagmicrobio.2021.115344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Richards O., Pallmann P., King C., Cheema Y., Killick C., Thomas-Jones E., et al. Procalcitonin increase is associated with the development of critical care-acquired infections in COVID-19 ARDS. Antibiotics (Basel) 2021;10 doi: 10.3390/antibiotics10111425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Williams E.J., Mair L., de Silva T.I., Green D.J., House P., Cawthron K., et al. Evaluation of procalcitonin as a contribution to antimicrobial stewardship in SARS-CoV-2 infection: a retrospective cohort study. J Hosp Infect. 2021;110:103–107. doi: 10.1016/j.jhin.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Calderon M., Li A., Bazo-Alvarez J.C., Dennis J., Baker K.F., Schim van der Loeff I., et al. Evaluation of procalcitonin-guided antimicrobial stewardship in patients admitted to hospital with COVID-19 pneumonia. JAC-Antimicrobial Resistance. 2021;3 doi: 10.1093/jacamr/dlab133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alanio A., Dellière S., Fodil S., Bretagne S., Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir Med. 2020;8:e48–e49. doi: 10.1016/S2213-2600(20)30237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Salmanton-García J., Sprute R., Stemler J., Bartoletti M., Dupont D., Valerio M., et al. COVID-19-Associated pulmonary aspergillosis, March-August 2020. Emerg Infect Dis. 2021;27:1077–1086. doi: 10.3201/eid2704.204895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Apostolopoulou A., Esquer Garrigos Z., Vijayvargiya P., Lerner A.H., Farmakiotis D. Invasive pulmonary aspergillosis in patients with SARS-CoV-2 infection: a systematic review of the literature. Diagnostics. 2020;10 doi: 10.3390/diagnostics10100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mitaka H., Kuno T., Takagi H., Patrawalla P. Incidence and mortality of COVID-19-associated pulmonary aspergillosis: a systematic review and meta-analysis. Mycoses. 2021;64:993–1001. doi: 10.1111/myc.13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chong W.H., Saha B.K., Neu K.P. Comparing the clinical characteristics and outcomes of COVID-19-associate pulmonary aspergillosis (CAPA): a systematic review and meta-analysis. Infection. 2022;50:43–56. doi: 10.1007/s15010-021-01701-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schauwvlieghe A.F.A.D., Rijnders B.J.A., Philips N., Verwijs R., Vanderbeke L., Van Tienen C., et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6:782–792. doi: 10.1016/S2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- 105.Koehler P., Cornely O.A., Kochanek M. Bronchoscopy safety precautions for diagnosing COVID-19 associated pulmonary aspergillosis-A simulation study. Mycoses. 2021;64:55–59. doi: 10.1111/myc.13183. [DOI] [PubMed] [Google Scholar]
- 106.Donnelly J.P., Chen S.C., Kauffman C.A., Steinbach W.J., Baddley J.W., Verweij P.E., et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for research and treatment of Cancer and the Mycoses study group Education and research Consortium. Clin Infect Dis. 2020;71:1367–1376. doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Blot S.I., Taccone F.S., Van den Abeele A.M., Bulpa P., Meersseman W., Brusselaers N., et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2012;186:56–64. doi: 10.1164/rccm.201111-1978OC. [DOI] [PubMed] [Google Scholar]
- 108.Verweij P.E., Rijnders B.J.A., Brüggemann R.J.M., Azoulay E., Bassetti M., Blot S., et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med. 2020;46:1524–1535. doi: 10.1007/s00134-020-06091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chong W.H., Saha B.K., Ananthakrishnan R., Chopra A. State-of-the-art review of secondary pulmonary infections in patients with COVID-19 pneumonia. Infection. 2021;49:591–605. doi: 10.1007/s15010-021-01602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Singh S., Verma N., Kanaujia R., Chakrabarti A., Rudramurthy S.M. Mortality in critically ill patients with coronavirus disease 2019-associated pulmonary aspergillosis: a systematic review and meta-analysis. Mycoses. 2021;64:1015–1027. doi: 10.1111/myc.13328. [DOI] [PubMed] [Google Scholar]
- 111.Li Y., Wang H., Zhao Y.P., Xu Y.C., Hsueh P.R. Antifungal susceptibility of clinical isolates of 25 genetically confirmed Aspergillus species collected from Taiwan and Mainland China. J Microbiol Immunol Infect. 2020;53:125–132. doi: 10.1016/j.jmii.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 112.Wu C.J., Liu W.L., Lai C.C., Chao C.M., Ko W.C., Wang H.C., et al. Multicenter study of azole-resistant Aspergillus fumigatus clinical isolates, Taiwan(1) Emerg Infect Dis. 2020;26:804–806. doi: 10.3201/eid2604.190840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen Y.C., Kuo S.F., Wang H.C., Wu C.J., Lin Y.S., Li W.S., et al. Azole resistance in Aspergillus species in Southern Taiwan: an epidemiological surveillance study. Mycoses. 2019;62:1174–1181. doi: 10.1111/myc.13008. [DOI] [PubMed] [Google Scholar]
- 114.Fekkar A., Lampros A., Mayaux J., Poignon C., Demeret S., Constantin J.M., et al. Occurrence of invasive pulmonary fungal infections in patients with severe COVID-19 admitted to the ICU. Am J Respir Crit Care Med. 2021;203:307–317. doi: 10.1164/rccm.202009-3400OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chauvet P., Mallat J., Arumadura C., Vangrunderbeek N., Dupre C., Pauquet P., et al. Risk factors for invasive pulmonary aspergillosis in critically ill patients with coronavirus disease 2019-induced acute respiratory distress syndrome. Crit Care Explor. 2020;2 doi: 10.1097/CCE.0000000000000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.White P.L., Dhillon R., Cordey A., Hughes H., Faggian F., Soni S., et al. A national strategy to diagnose coronavirus disease 2019-associated invasive fungal disease in the intensive care Unit. Clin Infect Dis. 2021;73:e1634–e1644. doi: 10.1093/cid/ciaa1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Segrelles-Calvo G., Araújo G.R.S., Llopis-Pastor E., Carrillo J., Hernández-Hernández M., Rey L., et al. Prevalence of opportunistic invasive aspergillosis in COVID-19 patients with severe pneumonia. Mycoses. 2021;64:144–151. doi: 10.1111/myc.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.van Arkel A.L.E., Rijpstra T.A., Belderbos H.N.A., van Wijngaarden P., Verweij P.E., Bentvelsen R.G. COVID-19-associated pulmonary aspergillosis. Am J Respir Crit Care Med. 2020;202:132–135. doi: 10.1164/rccm.202004-1038LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.van Grootveld R., van Paassen J., de Boer M.G.J., Claas E.C.J., Kuijper E.J., van der Beek M.T. Systematic screening for COVID-19 associated invasive aspergillosis in ICU patients by culture and PCR on tracheal aspirate. Mycoses. 2021;64:641–650. doi: 10.1111/myc.13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Van Biesen S., Kwa D., Bosman R.J., Juffermans N.P. Detection of invasive pulmonary aspergillosis in COVID-19 with non-directed bronchoalveolar lavage. Am J Respir Crit Care Med. 2020;202:1171–1173. doi: 10.1164/rccm.202005-2018LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Roman-Montes C.M., Martinez-Gamboa A., Diaz-Lomelí P., Cervantes-Sanchez A., Rangel-Cordero A., Sifuentes-Osornio J., et al. Accuracy of galactomannan testing on tracheal aspirates in COVID-19-associated pulmonary aspergillosis. Mycoses. 2021;64:364–371. doi: 10.1111/myc.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rouzé A., Lemaitre E., Martin-Loeches I., Povoa P., Diaz E., Nyga R., et al. Invasive pulmonary aspergillosis among intubated patients with SARS-CoV-2 or influenza pneumonia: a European multicenter comparative cohort study. Crit Care. 2022;26:11. doi: 10.1186/s13054-021-03874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bartoletti M., Pascale R., Cricca M., Rinaldi M., Maccaro A., Bussini L., et al. Epidemiology of invasive pulmonary aspergillosis among intubated patients with COVID-19: a prospective study. Clin Infect Dis. 2021;73:e3606–e3614. doi: 10.1093/cid/ciaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dellière S., Dudoignon E., Fodil S., Voicu S., Collet M., Oillic P.A., et al. Risk factors associated with COVID-19-associated pulmonary aspergillosis in ICU patients: a French multicentric retrospective cohort. Clin Microbiol Infect. 2020;27:790.e1–790.e5. doi: 10.1016/j.cmi.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Koehler P., Cornely O.A., Böttiger B.W., Dusse F., Eichenauer D.A., Fuchs F., et al. COVID-19 associated pulmonary aspergillosis. Mycoses. 2020;63:528–534. doi: 10.1111/myc.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Koehler P., Bassetti M., Kochanek M., Shimabukuro-Vornhagen A., Cornely O.A. Intensive care management of influenza-associated pulmonary aspergillosis. Clin Microbiol Infect. 2019;25:1501–1509. doi: 10.1016/j.cmi.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 127.Verweij P.E., Gangneux J.P., Bassetti M., Brüggemann R.J.M., Cornely O.A., Koehler P., et al. Diagnosing COVID-19-associated pulmonary aspergillosis. Lancet Microbe. 2020;1:e53–e55. doi: 10.1016/S2666-5247(20)30027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Torrego A., Pajares V., Fernández-Arias C., Vera P., Mancebo J. Bronchoscopy in patients with COVID-19 with invasive mechanical ventilation: a single-center experience. Am J Respir Crit Care Med. 2020;202:284–287. doi: 10.1164/rccm.202004-0945LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wahidi M.M., Lamb C., Murgu S., Musani A., Shojaee S., Sachdeva A., et al. American association for Bronchology and interventional Pulmonology (AABIP) Statement on the Use of bronchoscopy and respiratory specimen collection in patients with suspected or confirmed COVID-19 infection. Journal of Bronchology & Interventional Pulmonology. 2020;27 doi: 10.1097/LBR.0000000000000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pl W. 2020. ECMM webinar on COVID-19 associated aspergillosis: point of care diagnosis—soon reality? [Google Scholar]
- 131.Hatzl S., Reisinger A.C., Posch F., Prattes J., Stradner M., Pilz S., et al. Antifungal prophylaxis for prevention of COVID-19-associated pulmonary aspergillosis in critically ill patients: an observational study. Crit Care. 2021;25:335. doi: 10.1186/s13054-021-03753-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Van Ackerbroeck S., Rutsaert L., Roelant E., Dillen K., Wauters J., Van Regenmortel N. Inhaled liposomal amphotericin-B as a prophylactic treatment for COVID-19-associated pulmonary aspergillosis/aspergillus tracheobronchitis. Crit Care. 2021;25:298. doi: 10.1186/s13054-021-03728-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ko B.S., Chen W.T., Kung H.C., Wu U.I., Tang J.L., Yao M., et al. Guideline strategies for the use of antifungal agents in patients with hematological malignancies or hematopoietic stem cell transplantation recipients in Taiwan. J Microbiol Immunol Infect. 2018;51:287–301. doi: 10.1016/j.jmii.2017.07.005. 2016. [DOI] [PubMed] [Google Scholar]
- 134.Falces-Romero I., Ruiz-Bastián M., Díaz-Pollán B., Maseda E., García-Rodríguez J. Isolation of Aspergillus spp. in respiratory samples of patients with COVID-19 in a Spanish tertiary care hospital. Mycoses. 2020;63:1144–1148. doi: 10.1111/myc.13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ledoux M.P., Guffroy B., Nivoix Y., Simand C., Herbrecht R. Invasive pulmonary aspergillosis. Semin Respir Crit Care Med. 2020;41:80–98. doi: 10.1055/s-0039-3401990. [DOI] [PubMed] [Google Scholar]
- 136.Enoch D.A., Idris S.F., Aliyu S.H., Micallef C., Sule O., Karas J.A. Micafungin for the treatment of invasive aspergillosis. J Infect. 2014;68:507–526. doi: 10.1016/j.jinf.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 137.Chen F., Qasir D., Morris A.C. Invasive pulmonary aspergillosis in hospital and ventilator-associated pneumonias. Semin Respir Crit Care Med. 2022;43:234–242. doi: 10.1055/s-0041-1739472. [DOI] [PubMed] [Google Scholar]
- 138.Casalini G., Giacomelli A., Ridolfo A., Gervasoni C., Antinori S. Invasive fungal infections complicating COVID-19: a Narrative review. J Fungi (Basel) 2021;7 doi: 10.3390/jof7110921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Feys S., Almyroudi M.P., Braspenning R., Lagrou K., Spriet I., Dimopoulos G., et al. A Visual and comprehensive review on COVID-19-associated pulmonary aspergillosis (CAPA) J Fungi (Basel) 2021;7 doi: 10.3390/jof7121067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Janssen N.A.F., Nyga R., Vanderbeke L., Jacobs C., Ergün M., Buil J.B., et al. Multinational observational cohort study of COVID-19-associated pulmonary aspergillosis(1) Emerg Infect Dis. 2021;27:2892–2898. doi: 10.3201/eid2711.211174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Patterson T.F., Thompson G.R., III, Denning D.W., Fishman J.A., Hadley S., Herbrecht R., et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases Society of America. Clin Infect Dis. 2016;63:e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Seagle E.E., Jackson B.R., Lockhart S.R., Georgacopoulos O., Nunnally N.S., Roland J., et al. The Landscape of candidemia during the coronavirus disease 2019 (COVID-19) pandemic. Clin Infect Dis. 2021;74:802–811. doi: 10.1093/cid/ciab562. [DOI] [PubMed] [Google Scholar]
- 143.Mastrangelo A., Germinario B.N., Ferrante M., Frangi C., Li Voti R., Muccini C., et al. Candidemia in coronavirus disease 2019 (COVID-19) patients: incidence and characteristics in a prospective cohort compared with Historical non-COVID-19 controls. Clin Infect Dis. 2021;73:e2838–e2839. doi: 10.1093/cid/ciaa1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nucci M., Barreiros G., Guimarães L.F., Deriquehem V.A.S., Castiñeiras A.C., Nouér S.A. Increased incidence of candidemia in a tertiary care hospital with the COVID-19 pandemic. Mycoses. 2021;64:152–156. doi: 10.1111/myc.13225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kayaaslan B., Eser F., Kaya Kalem A., Bilgic Z., Asilturk D., Hasanoglu I., et al. Characteristics of candidemia in COVID-19 patients; increased incidence, earlier occurrence and higher mortality rates compared to non-COVID-19 patients. Mycoses. 2021;64:1083–1091. doi: 10.1111/myc.13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Macauley P., Epelbaum O. Epidemiology and Mycology of Candidaemia in non-oncological medical intensive care unit patients in a tertiary center in the United States: overall analysis and comparison between non-COVID-19 and COVID-19 cases. Mycoses. 2021;64:634–640. doi: 10.1111/myc.13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Arastehfar A., Carvalho A., Nguyen M.H., Hedayati M.T., Netea M.G., Perlin D.S., et al. COVID-19-Associated candidiasis (CAC): an Underestimated complication in the absence of Immunological Predispositions? J Fungi (Basel) 2020;6 doi: 10.3390/jof6040211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pappas P.G., Kauffman C.A., Andes D.R., Clancy C.J., Marr K.A., Ostrosky-Zeichner L., et al. Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases Society of America. Clin Infect Dis. 2016;62:e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.León C., Ruiz-Santana S., Saavedra P., Almirante B., Nolla-Salas J., Alvarez-Lerma F., et al. A bedside scoring system ("Candida score") for early antifungal treatment in nonneutropenic critically ill patients with Candida colonization. Crit Care Med. 2006;34:730–737. doi: 10.1097/01.CCM.0000202208.37364.7D. [DOI] [PubMed] [Google Scholar]
- 150.Leroy G., Lambiotte F., Thévenin D., Lemaire C., Parmentier E., Devos P., et al. Evaluation of "Candida score" in critically ill patients: a prospective, multicenter, observational, cohort study. Ann Intensive Care. 2011;1:50. doi: 10.1186/2110-5820-1-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bölükbaşı Y., Erköse Genç G., Orhun G., Kuşkucu M.A., Çağatay A., Önel M., et al. [First case of COVID-19 positive Candida auris fungemia in Turkey] Mikrobiyol Bul. 2021;55:648–655. doi: 10.5578/mb.20219716. [DOI] [PubMed] [Google Scholar]
- 152.Kömeç S., Karabıçak N., Ceylan A.N., Gülmez A., Özalp O. [Three Candida auris case reports from Istanbul, Turkey] Mikrobiyol Bul. 2021;55:452–460. doi: 10.5578/mb.20219814. [DOI] [PubMed] [Google Scholar]
- 153.de Almeida J.N., Jr., Francisco E.C., Hagen F., Brandão I.B., Pereira F.M., Presta Dias P.H., et al. Emergence of Candida auris in Brazil in a COVID-19 intensive care Unit. J Fungi (Basel) 2021;7 doi: 10.3390/jof7030220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Goravey W., Ali G.A., Ali M., Ibrahim E.B., Al Maslamani M., Abdel Hadi H. Ominous combination: COVID-19 disease and Candida auris fungemia-Case report and review of the literature. Clin Case Rep. 2021;9 doi: 10.1002/ccr3.4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Di Pilato V., Codda G., Ball L., Giacobbe D.R., Willison E., Mikulska M., et al. Molecular epidemiological investigation of a nosocomial cluster of C. Auris: evidence of recent emergence in Italy and Ease of transmission during the COVID-19 pandemic. J Fungi (Basel) 2021;7 doi: 10.3390/jof7020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Villanueva-Lozano H., Treviño-Rangel R.J., González G.M., Ramírez-Elizondo M.T., Lara-Medrano R., Aleman-Bocanegra M.C., et al. Outbreak of Candida auris infection in a COVID-19 hospital in Mexico. Clin Microbiol Infect. 2021;27:813–816. doi: 10.1016/j.cmi.2020.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Allaw F., Kara Zahreddine N., Ibrahim A., Tannous J., Taleb H., Bizri A.R., et al. First Candida auris outbreak during a COVID-19 pandemic in a tertiary-care center in Lebanon. Pathogens. 2021;10 doi: 10.3390/pathogens10020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chowdhary A., Tarai B., Singh A., Sharma A. Multidrug-resistant Candida auris infections in critically ill coronavirus disease patients, India, April-July 2020. Emerg Infect Dis. 2020;26:2694–2696. doi: 10.3201/eid2611.203504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hanson B.M., Dinh A.Q., Tran T.T., Arenas S., Pronty D., Gershengorn H.B., et al. Candida auris invasive infections during a COVID-19 case Surge. Antimicrob Agents Chemother. 2021;65 doi: 10.1128/AAC.01146-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rajni E., Singh A., Tarai B., Jain K., Shankar R., Pawar K., et al. A high frequency of Candida auris blood stream infections in coronavirus disease 2019 patients admitted to intensive care Units, Northwestern India: a case control study. Open Forum Infect Dis. 2021;8:ofab452. doi: 10.1093/ofid/ofab452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lyman M., Forsberg K., Reuben J., Dang T., Free R., Seagle E.E., et al. Notes from the Field: transmission of pan-resistant and echinocandin-resistant Candida auris in health care Facilities - Texas and the District of Columbia, January-April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1022–1023. doi: 10.15585/mmwr.mm7029a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Patel A., Agarwal R., Rudramurthy S.M., Shevkani M., Xess I., Sharma R., et al. Multicenter epidemiologic study of coronavirus disease-associated mucormycosis, India. Emerg Infect Dis. 2021;27:2349–2359. doi: 10.3201/eid2709.210934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pal R., Singh B., Bhadada S.K., Banerjee M., Bhogal R.S., Hage N., et al. COVID-19-associated mucormycosis: an updated systematic review of literature. Mycoses. 2021;64:1452–1459. doi: 10.1111/myc.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.John T.M., Jacob C.N., Kontoyiannis D.P. When Uncontrolled diabetes mellitus and severe COVID-19 Converge: the Perfect storm for mucormycosis. J Fungi (Basel) 2021;7 doi: 10.3390/jof7040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Arora U., Priyadarshi M., Katiyar V., Soneja M., Garg P., Gupta I., et al. Risk factors for Coronavirus disease-associated mucormycosis. J Infect. 2022;84:383–390. doi: 10.1016/j.jinf.2021.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Muthu V., Rudramurthy S.M., Chakrabarti A., Agarwal R. Epidemiology and pathophysiology of COVID-19-associated mucormycosis: India versus the rest of the World. Mycopathologia. 2021;186:739–754. doi: 10.1007/s11046-021-00584-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yasmin F., Najeeb H., Naeem A., Dapke K., Phadke R., Asghar M.S., et al. COVID-19 associated mucormycosis: a systematic review from diagnostic challenges to management. Diseases. 2021;9 doi: 10.3390/diseases9040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sen M., Honavar S.G., Bansal R., Sengupta S., Rao R., Kim U., et al. Epidemiology, clinical profile, management, and outcome of COVID-19-associated rhino-orbital-cerebral mucormycosis in 2826 patients in India - Collaborative OPAI-IJO Study on Mucormycosis in COVID-19 (COSMIC), Report 1. Indian J Ophthalmol. 2021;69:1670–1692. doi: 10.4103/ijo.IJO_1565_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Aranjani J.M., Manuel A., Abdul Razack H.I., Mathew S.T. COVID-19-associated mucormycosis: evidence-based critical review of an emerging infection burden during the pandemic's second wave in India. PLoS Negl Trop Dis. 2021;15 doi: 10.1371/journal.pntd.0009921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cornely O.A., Alastruey-Izquierdo A., Arenz D., Chen S.C.A., Dannaoui E., Hochhegger B., et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of medical Mycology in cooperation with the Mycoses study group Education and research Consortium. Lancet Infect Dis. 2019;19:e405–e421. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chamilos G., Lewis R.E., Kontoyiannis D.P. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47:503–509. doi: 10.1086/590004. [DOI] [PubMed] [Google Scholar]
- 172.Hussain S., Baxi H., Riad A., Klugarová J., Pokorná A., Slezáková S., et al. COVID-19-Associated mucormycosis (CAM): an updated evidence Mapping. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph181910340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Seidel D., Simon M., Sprute R., Lubnow M., Evert K., Speer C., et al. Results from a national survey on COVID-19-associated mucormycosis in Germany: 13 patients from six tertiary hospitals. Mycoses. 2022;65:103–109. doi: 10.1111/myc.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chouhan M., Solanki B., Shakrawal N. Rhino-orbital-cerebral mucormycosis: fungal epidemic in a viral pandemic. J Laryngol Otol. 2021;135:981–986. doi: 10.1017/S0022215121002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Panwar P., Gupta A., Kumar A., Gupta B., Navriya S.C. Mucormycosis in COVID diabetic patients: a Horrifying Triad. Indian J Crit Care Med. 2021;25:1314–1317. doi: 10.5005/jp-journals-10071-24025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bayram N., Ozsaygılı C., Sav H., Tekin Y., Gundogan M., Pangal E., et al. Susceptibility of severe COVID-19 patients to rhino-orbital mucormycosis fungal infection in different clinical manifestations. Jpn J Ophthalmol. 2021;65:515–525. doi: 10.1007/s10384-021-00845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dave T.V., Gopinathan Nair A., Hegde R., Vithalani N., Desai S., Adulkar N., et al. Clinical presentations, management and outcomes of rhino-orbital-cerebral mucormycosis (ROCM) following COVID-19: a multi-Centric study. Ophthalmic Plast Reconstr Surg. 2021;37:488–495. doi: 10.1097/IOP.0000000000002030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Singh H., Dua S., Goel A., Dhar A., Bhadauria V., Garg A., et al. Rhino-orbital-cerebral mucormycosis in times of COVID-19: a neurosurgical experience. Surg Neurol Int. 2021;12:538. doi: 10.25259/SNI_772_2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nasir N., Farooqi J., Mahmood S.F., Jabeen K. COVID-19 associated mucormycosis: a life-threatening complication in patients admitted with severe to critical COVID-19 from Pakistan. Clin Microbiol Infect. 2021;27:1704–1707. doi: 10.1016/j.cmi.2021.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Patel R. Antifungal agents. Part I. Amphotericin B Preparations and Flucytosine. Mayo Clin Proc. 1998;73:1205–1225. doi: 10.4065/73.12.1205. [DOI] [PubMed] [Google Scholar]
- 181.Marty F.M., Ostrosky-Zeichner L., Cornely O.A., Mullane K.M., Perfect J.R., Thompson G.R., 3rd, et al. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis. 2016;16:828–837. doi: 10.1016/S1473-3099(16)00071-2. [DOI] [PubMed] [Google Scholar]
- 182.Greenberg R.N., Mullane K., van Burik J.A., Raad I., Abzug M.J., Anstead G., et al. Posaconazole as salvage therapy for zygomycosis. Antimicrob Agents Chemother. 2006;50:126–133. doi: 10.1128/AAC.50.1.126-133.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kaneria M., Baligeri K., Budhe A. Post COVID-19 mucormycosis: a case series. Asian Pacific Journal of Tropical Medicine. 2021;14:517–524. [Google Scholar]
- 184.Patel A., Patel K., Patel K., Shah K., Chakrabarti A. Therapeutic drug monitoring of posaconazole delayed release tablet while managing COVID-19-associated mucormycosis in a real-life setting. Mycoses. 2022;65:312–316. doi: 10.1111/myc.13420. [DOI] [PubMed] [Google Scholar]
- 185.Alfishawy M., Elbendary A., Younes A., Negm A., Hassan W.S., Osman S.H., et al. Diabetes mellitus and coronavirus disease (Covid-19) associated mucormycosis (CAM): a wake-up call from Egypt. Diabetes Metab Syndr. 2021;15 doi: 10.1016/j.dsx.2021.102195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yadav S., Sharma A., Kothari N., Bhatia P.K., Goyal S., Goyal A. Mucormycosis: a case series of patients admitted in non-COVID-19 intensive care Unit of a tertiary care center during the second wave. Indian J Crit Care Med. 2021;25:1193–1196. doi: 10.5005/jp-journals-10071-23986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kumari A., Rao N.P., Patnaik U., Malik V., Tevatia M.S., Thakur S., et al. Management outcomes of mucormycosis in COVID-19 patients: a preliminary report from a tertiary care hospital. Med J Armed Forces India. 2021;77 doi: 10.1016/j.mjafi.2021.06.009. S289-s95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ravani S.A., Agrawal G.A., Leuva P.A., Modi P.H., Amin K.D. Rise of the phoenix: mucormycosis in COVID-19 times. Indian J Ophthalmol. 2021;69:1563–1568. doi: 10.4103/ijo.IJO_310_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Honavar S.G. Code mucor: guidelines for the diagnosis, staging and management of rhino-Orbito-cerebral mucormycosis in the setting of COVID-19. Indian J Ophthalmol. 2021;69:1361–1365. doi: 10.4103/ijo.IJO_1165_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Heinz W.J., Buchheidt D., Ullmann A.J. Clinical evidence for caspofungin monotherapy in the first-line and salvage therapy of invasive Aspergillus infections. Mycoses. 2016;59:480–493. doi: 10.1111/myc.12477. [DOI] [PubMed] [Google Scholar]
- 191.Bhatt P.J., Shiau S., Brunetti L., Xie Y., Solanki K., Khalid S., et al. Risk factors and outcomes of hospitalized patients with severe coronavirus disease 2019 (COVID-19) and secondary bloodstream infections: a multicenter case-control study. Clin Infect Dis. 2021;72:e995–e1003. doi: 10.1093/cid/ciaa1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bonazzetti C., Morena V., Giacomelli A., Oreni L., Casalini G., Galimberti L.R., et al. Unexpectedly high frequency of Enterococcal bloodstream infections in coronavirus disease 2019 patients admitted to an Italian ICU: an observational study. Crit Care Med. 2021;49:e31–e40. doi: 10.1097/CCM.0000000000004748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.De Santis V., Corona A., Vitale D., Nencini C., Potalivo A., Prete A., et al. Bacterial infections in critically ill patients with SARS-2-COVID-19 infection: results of a prospective observational multicenter study. Infection. 2022;50:139–148. doi: 10.1007/s15010-021-01661-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dudoignon E., Caméléna F., Deniau B., Habay A., Coutrot M., Ressaire Q., et al. Bacterial pneumonia in COVID-19 critically ill patients: a case series. Clin Infect Dis. 2021;72:905–906. doi: 10.1093/cid/ciaa762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.He S., Liu W., Jiang M., Huang P., Xiang Z., Deng D., et al. Clinical characteristics of COVID-19 patients with clinically diagnosed bacterial co-infection: a multi-center study. PLoS One. 2021;16 doi: 10.1371/journal.pone.0249668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Saeed N.K., Al-Khawaja S., Alsalman J., Almusawi S., Albalooshi N.A., Al-Biltagi M. Bacterial co-infection in patients with SARS-CoV-2 in the Kingdom of Bahrain. World J Virol. 2021;10:168–181. doi: 10.5501/wjv.v10.i4.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.