Abstract
Basal cell carcinoma (BCC) of the prostate is a rare tumor. Compared with the more common acinar adenocarcinoma (AAC) of the prostate, BCCs show features of basal cell differentiation and are thought to be biologically distinct from AAC. The spectrum of molecular alterations of BCC has not been comprehensively described, and genomic studies are lacking. Herein, whole genome sequencing was performed on archival formalin-fixed, paraffin-embedded specimens of two cases with BCC. Prostatic BCCs were characterized by an overall low copy number and mutational burden. Recurrent copy number loss of chromosome 16 was observed. In addition, putative driver gene alterations in KIT, DENND3, PTPRU, MGA, and CYLD were identified. Mechanistically, depletion of the CYLD protein resulted in increased proliferation of prostatic basal cells in vitro. Collectively, these studies show that prostatic BCC displays distinct genomic alterations from AAC and highlight a potential role for loss of chromosome 16 in the pathogenesis of this rare tumor type.
The prostate is lined by a bilayered epithelium composed of basal cell and luminal cell layers.1 Most prostate cancers show features of prostatic luminal cell differentiation and demonstrate an acinar growth pattern.2 However, a distinct subtype of prostatic carcinomas shows morphologic and molecular similarities with prostatic basal cells, and is termed prostatic basal cell carcinoma (BCC).3, 4, 5, 6 Prostatic BCCs are extremely rare tumors, with only several dozen cases described in the literature. 3, 4, 5,7,8
Although most BCCs are considered indolent, recent studies showed that more than 40% of patients experience disease recurrence after initial therapy. In addition, metastatic spread has been documented in approximately 10% of men diagnosed with BCC.4,9 The metastatic pattern of BCC is distinct, involving liver, lung, and bowel, but not bone, the most common site of acinar adenocarcinoma (AAC) of the prostate. These observations demonstrate that BCC clinically differs from AAC.4
From a molecular perspective, BCCs are characterized by the expression of prostatic basal cell markers, including p63 and high-molecular-weight keratins, and frequent overexpression of the antiapoptotic protein B-cell lymphoma 2 (BCL2).10 Luminal prostate epithelial markers, including androgen receptor or prostate-specific antigen, are usually absent, or are expressed at low levels. In addition, BCCs do not harbor TMPRSS2-ERG gene rearrangements, which are seen in >50% of prostatic AACs.11 Very little is known about the genomic features of BCC, and based on the small number of published studies, it appears that BCCs show limited copy number changes and rare recurrent translocations.12,13
To better characterize the genomic features of BCC, whole genome sequencing was performed on two BCC cases. Although the genomes of BCC are overall quiet, copy number loss of chromosome 16 was present in both cases. In addition, a loss of function mutation was observed in CYLD. In in vitro experiments, CYLD protein loss increased cell proliferation in prostatic basal cells, confirming its putative driver role. Furthermore, the loss of CYLD was associated with morphologic changes that resembled cylindromas, a skin tumor that arises in the context of familial cylindromatosis, which is characterized by CYLD inactivating mutations. Collectively this study provides further insights into the biology of BCCs and highlights the striking genotype-phenotype associations in tumors with CYLD alterations.
Materials and Methods
Patient Samples and Whole Genome Sequencing
Tissues consisted of archival transurethral resection specimens from the consultation files of one of the authors (J.I.E.). Tumor areas were identified by two pathologists (J.I.E. and M.C.H.) on adjacent hematoxylin and eosin–stained slides. These areas were macrodissected, and DNA was extracted from 5 formalin-fixed, paraffin-embedded tissue sections using a DNA formalin-fixed, paraffin-embedded tissue kit (QIAamp, Qiagen, Hilden, Germany) following manufacturer's protocols. Because of the fragmented nature of the transurethral resection specimens and the diffuse infiltrative growth pattern of the tumors, we aimed to achieve a tumor cellularity of >50%. In addition, adjacent, matched, nonneoplastic tissue was collected and used as germline control. DNA concentrations were determined using the a double-stranded DNA broad-range assay kit (Qubit, Invitrogen, Carlsbad, CA). Genomic DNA from tumor and adjacent benign samples was sonicated and further processed (TruSeq Nano DNA library construction kit, Illumina, San Diego, CA). Barcoded libraries were subjected to 151 × 151 paired end sequencing on a HiSeq 2500 Genome Analyzer (Illumina), resulting in a mean coverage of 28.9 (range, 29.5 to 27.22). Reads were aligned against the hg38 genome using the Burrows-Wheeler Alignment Tool version 0.7.7 with default setting.14 Picard tools version 1.119 (Broad Institute, Cambridge, MA; https://github.com/broadinstitute/picard) were used to add read groups as well as remove duplicate reads. The Genome Analysis Toolkit version 3.6.0 (Broad Institute; https://gatk.broadinstitute.org/hc/en-us) base call recalibration steps were used to create the final alignment files.
Somatic Variant Detection
Somatic variants between the tumor-normal pairs were called using Mutect2 (Broad Institute) and Strelka2 (Illumina) and annotated with ANNOVAR.15,16 Variants were further filtered for functional prediction evidence using the dbNSFP, and only deleterious variants supported by one of the prediction databases (SIFT, LRT, MutationTaster, and FATHMM) were considered. Variants were classified based on disease or phenotypes information using ClinVar, InterVar, and COSMIC. Synonymous and nonframeshift indels were excluded. Only variants supported by both callers (Mutect2 and Strelka2) were considered as final call sets.
Copy Number Analysis
Copy number analyses were performed using TitanCNA.17,18 TitanCNA solutions were generated for one to three clonal clusters and ploidy initializations from two to four. Optimal solutions were selected automatically within the pipeline and reselected with manual inspection to confirm tumor ploidy and clonal cluster.
SV Analysis
SvABA and Gridss2 were used to detect structural variants (SVs). The SvABA analysis was performed using tumor-normal paired mode with default parameters.19 SV events were classified into deletions, inversions, tandem duplications, interchromosomal translocations, and intrachromosomal translocations, whereas intrachromosomal translocations were further divided into balanced and unbalanced events based on copy number information as previously described.20 Gene alteration status by genome rearrangements was defined based on the breakpoints of involved SV events. A gene in one whole genome sequencing sample (gene-sample pair) was considered to have gene-transecting events if any breakpoints of SV events were located within the gene body region. Gene coordinates were based on ENSEMBL version 33 (European Bioinformatics Institute, Hinxton, UK).21 Circos plots were generated with shinyCircos (YaoLab-Bioinfo, Guangzhou, China; https://venyao.shinyapps.io/shinyCircos).
In Vitro Experiments
Human telomerase reverse transcriptase (hTERT) immortalized prostate epithelial cells were a gift from John T. Isaacs (Johns Hopkins University, Baltimore, MD) and were grown in keratinocyte serum-free media (Thermo Fisher Scientific, Waltham, MA) supplemented with insulin, epidermal growth factor, and bovine pituitary extract (Thermo Fisher) as described previously.22 CYLD shRNA sequences were obtained from The RNAi Consortium shRNA library (Broad Institute) and cloned into pLKO.1 backbones. Viral particles containing shRNA constructs were generated in HEK293T cells (ATCC, Manassas, VA) and transduced into cells. Seventy-two hours after transduction, cells were harvested for subsequent analyses. Western blot analysis was performed as described previously23 with anti-CYLD (sc-74435, Santa Cruz Biotechnology, Dallas, TX) and anti–glyceraldehyde-3-phosphate dehydrogenase (ab8245, Abcam, Cambridge, UK) antibodies at 1:1000 and 1:10,000 dilutions, respectively. Cell growth assays were performed by seeding CYLD knock down and control cells on poly-l-lysine (Bio-Techne, Minneapolis, MN)–coated 96 well plates. Cell growth was monitored using a Biotek Cytation 5 live cell imager (Winooski, VT), and images were captured every 12 hours for a total of 4 days. Resulting images were analyzed using the Gen5 software version 3.10 (Biotek), and growth curves were plotted with GraphPad Prism software version 8 (GraphPad Inc., San Diego, CA).
Results
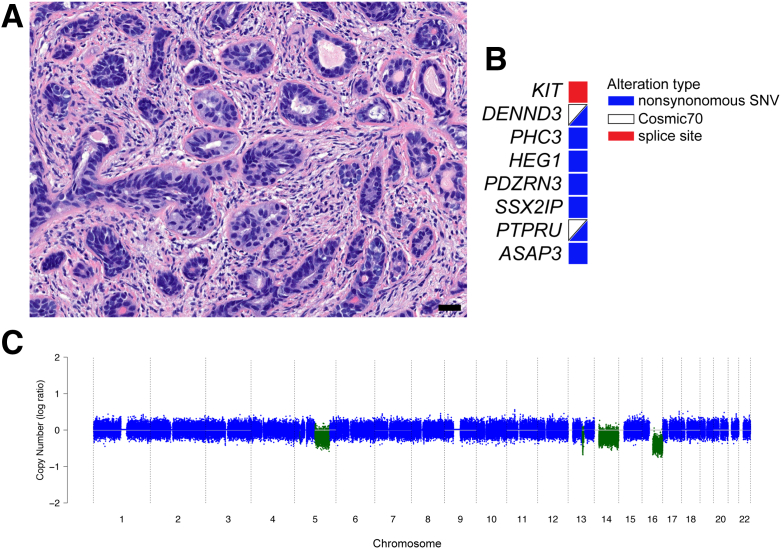
Histomorphologic assessment of case 1 showed nests of basaloid cells with a two-cell pattern and inner tubule formation characteristic of BCC. Cancer cells showed cytologic atypia and eosinophilic cytoplasm with an associated dense desmoplastic stromal reaction and an infiltrative growth pattern that involved 40% of the submitted specimen (Figure 1A). Whole genome sequencing revealed eight consensus protein-coding mutations; two variants in DENND3 and PTPRU were previously described and included in the cosmic database (Figure 1B and Supplemental Table S1). In addition, this case showed a splice site mutation in exon 10 of KIT (Figure 1C and Supplemental Table S2). Shallow subclonal copy number loss of chromosomes 5, 13, and 14 and clonal hemizygous loss of the q-arm of chromosome 16 were noted (Figure 1C). There were 28 intrachromosomal SVs, including a complex rearrangement that involved the ITGA2 gene on chromosome 5 (Supplemental Figure S1, Figure 2, and Supplemental Table S2).
Figure 1.
A: Hematoxylin and eosin–stained micrograph showing histomorphologic features of case 1. Note the nuclear atypia and dense desmoplastic response in this case. B: Table listing consensus single-nucleotide variants as well as insertions and deletions. C: Copy number profile highlights copy number losses (green) that affect chromosomes 5, 13, 14, and 16. Dots represent normalized log ratios for 10-kb windows. Blue indicates copy neutral; green, copy number loss. Scale bar = 50 μm (A).
Figure 2.
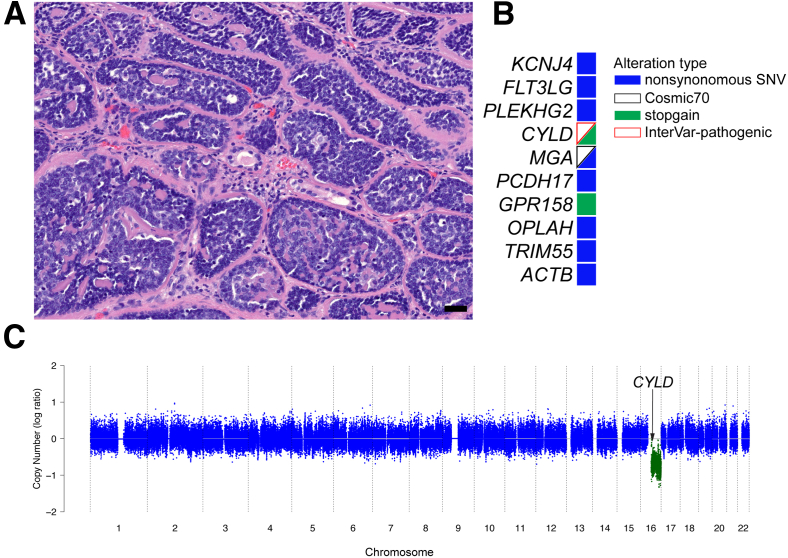
A: Hematoxylin and eosin–stained micrograph showing histomorphologic features of case 2. Note the cribriform-like growth pattern with abundant eosinophilic basement membrane material that resembles cylindromas of the skin. B: Table listing consensus single-nucleotide variants (SNVs) and insertions and deletions. C: Copy number profile highlights copy number loss (green) affecting chromosome 16. Dots represent normalized log ratios for 10-kb windows. Blue indicates copy neutral; green, copy number loss. Scale bar = 50 μm (A).
Case 2 showed expansile tumor nests of variable size and shape with hyperchromatic nuclei at the periphery and larger pale cells in the center. The nests were aligned in a jigsaw pattern and were lined by a dense eosinophilic hyaline rim (Figure 2A). The tumor showed an invasive growth pattern that involved 10% of the submitted specimen with an elevated Ki-67 proliferation index (approximately 10%). A total of 10 protein-coding mutations were detected, which included stop gain mutations in CYLD and GPR158 and a cosmic, annotated, nonsynonymous single-nucleotide variant in MGA (Figure 2B and Supplemental Table S1). Importantly, the CYLD alteration (Y710X) occurred in a region of hemizygous loss on chromosome 16 (Figure 2C and Supplemental Table S1). CYLD is a ubiquitously expressed putative tumor suppressor gene. Although the mutation seen in case 2 has not been previously described, it was predicted to result in a truncation of the ubiquitin carboxyl-terminal hydrolase domain, which is the core catalytic domain responsible for the deubiquitinase function of CYLD, therefore generating a catalytically dead enzyme (Supplemental Table S1). Copy number analyses showed an isolated copy number loss of chromosome 16 (Figure 2C). Three interchromosomal rearrangements were present, of which one directly involved the coding region of ZNF407 (Supplemental Figure S2 and Supplemental Table S2).
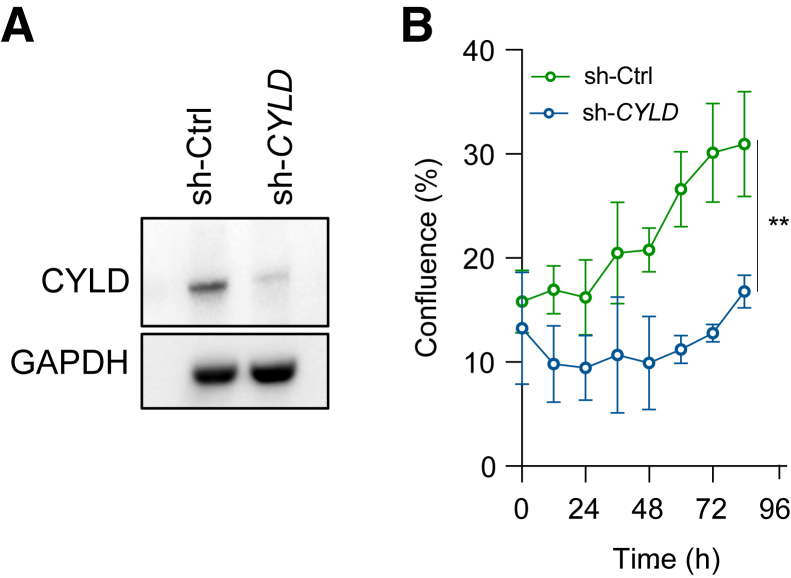
hTERT immortalized prostate epithelial cells, which show basal cell features, were used to model the consequences of loss of CYLD function in prostatic basal cells.22 Expression of a lentiviral shRNA construct targeting CYLD resulted in a robust depletion of CYLD protein levels (Figure 3A). To determine the changes in cell proliferation on CYLD knock down, live cell imaging of short hairpin CYLD and short hairpin control expressing cells was performed. Compared with short hairpin control-transduced cells, CYLD-depleted cells showed a significant increase in cell proliferation (Figure 3B). These data suggest that loss of CYLD can promote basal cell proliferation and validate the functional significance of CYLD loss as seen in case 2.
Figure 3.
A: Western blot analysis of benign basal-like prostate epithelial cells stably transduced with nontargeting control vectors [short hairpin control (sh-Ctrl)] and CYLD targeting shRNAs [short hairpin CYLD (sh-CYLD)] demonstrate effective depletion of CYLD protein levels. B: Cell proliferation assessment based on cellular confluency determined by live cell imaging of sh-CYLD and sh-Ctrl cells. ∗P < 0.01. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Discussion
Prostatic BCCs are rare tumors of the prostate that, as opposed to the much more common AAC, demonstate features of basal cell differentiation. Prior studies suggested that BCCs have a different genomic makeup compared with AACs. However, to date, comprehensive whole genome sequencing studies have not been performed on BCCs. The genomic features of AAC of the prostate have been extensively characterized during the past decade, and key driver gene alterations that involve mutations in TP53, SPOP, FOXA1, and PTEN as well as recurrent rearrangements that involve Ets transcription factors and frequent copy number changes have been described.24, 25, 26, 27
Herein, the first whole genome sequencing study of this rare tumor type was performed to gain insights into the spectrum of genomic changes in BCCs. An overall low rate of single-nucleotide variants, SVs, and copy number changes was observed in the two tumors analyzed. Notably, none of the genomic alterations commonly found in AACs were identified, demonstrating that BCCs are indeed genomically distinct from AACs (Supplemental Figure S3).28,29
Case 1 showed a splice site mutation in KIT with predicted high functional impact as well as mutations in DENND3 and PTPRU. Genomic alterations of KIT are commonly found in gastrointestinal stroma tumors, seminoma, and acute myeloid leukemia.30, 31, 32 Activating missense mutations in the kinase domain are the most common somatic alteration in cancer, but recurrent splice site changes have also been described.33,34 Although the significance of this mutation in this case is unclear, its enrichment in gastrointestinal stroma tumors, which are known to be driven by genomic alterations in KIT, suggests a potential driver function. However, the fact that the KIT mutation seen here is upstream of the kinase domain and that most gastrointestinal stroma tumors harboring this alteration also showed other KIT mutations complicates the assessment of the potential functional consequence.34 PTPRU is part of the R2B receptors and was reported to play a role in gastric cancer and glioma.35,36 DENND3 belongs to the DENN domain–containing protein family of Rab guanine nucleotide exchange factors, which have been shown to be involved in the pathogenesis of familial frontotemporal dementia and amyotrophic lateral sclerosis.37, 38, 39 In addition, case 1 showed several large-scale copy number losses, including a hemizygous loss of chromosome 16q that involved the CYLD locus, but no single-nucleotide alterations in the CYLD gene were observed.
Case 2 had mutations in MGA and GPR158. MAX gene-associated (MGA) protein was shown to bind MYC associated factor X (MAX), which is a critical molecule that dimerizes with MYC oncogenic transcription factors.40,41 Ectopic expression of MGA suppresses growth of lung adenocarcinoma cell lines.42 Conversely, MGA loss promotes lung tumorigenesis in vivo and human colon cancer growth in organoids models.43 The orphan receptor GPR158 is up-regulated in metastatic castration resistant prostate cancer and is thought to promote growth and invasion.44
Although recent studies have demonstrated recurrent translocations encompassing the MYB oncogene in prostatic BCC, these translocations were not present in the two cases studied here.45,46 The t(6;9)(q22-23;p23-24) translocation resulting in the MYB-NFIB fusion protein are commonly found in adenoid cystic carcinoma of the salivary gland, and similar MYB gene rearrangements were detectable in 2/12 adenoid cystic carcinoma–like BCCs but in none of the BCCs with a solid growth pattern.45,47 The tight association between MYB gene alterations and adenoid cystic morphology suggests that certain driver gene alterations can result in histomorphologic features common across different tumor types.
It is worth noting that a somatic stop gain mutation in CYLD with associated copy number loss was observed in case 2. Germline CYLD mutations are associated with familial cylindromatosis, a rare inherited skin tumor syndrome, in which patients have multiple cylindromas.48, 49, 50 Although cylindromas share histologic similarities with adenoid cystic carcinoma, they are characterized by islands of basaloid cells often arranged in a jigsaw pattern separated from the stroma by a thickened basement membrane.48,51 The morphologic features characteristic of cylindromas are remarkably similar to the histomorphologic appearance of case 2. The truncating stop gain mutation in CYLD observed in case 2 is located upstream of the ubiquitin carboxyl-terminal hydrolase domain. Therefore, the resulting protein lacks catalytic activity. CYLD negatively regulates NF-κB, WNT, and JNK signaling, and inactivating mutations can result in aberrant pathway activation, ultimately leading to enhanced cell proliferation, inhibition of apoptosis, and increased cell migration in epidermal cell systems.52, 53, 54, 55, 56 To study the biological consequences of the loss of function alteration in CYLD, shRNA was used to knock down CYLD expression. CYLD depletion resulted in significantly increased cell proliferation. Collectively, these observations suggest that herein, the observed CYLD mutation is likely a driver gene alteration.
In both human and murine prostates, the basal cell compartment harbors stem cells that can contribute to the regeneration of benign prostate epithelia.57 Transformation of isolated prostatic basal cells with oncogenic drivers commonly found in acinar prostate cancer results in tumors with a luminal cell phenotype.58,59 However, different basal cell populations have different propensities to form tumors with a luminal cell phenotype.59 It is therefore possible that subsets of prostatic basal cells with distinct molecular characteristics are capable of giving rise to a prostatic BCC. Alternatively, given the differences in genomic driver alterations observed between AACs and BCCs, the composition of oncogenic drivers, rather than the cell of origin, could determine the lineage phenotype of the tumor.
In summary, this study provides novel insights into the biology of prostatic BCC, highlights potential driver gene alterations, and emphasizes the genotype-morphologic phenotype correlation associated with certain driver gene alterations across different cell lineages.
Acknowledgment
We thank the Sidney Kimmel Comprehensive Cancer Center Experimental and Computational Genomics Core at Johns Hopkins for assistance with the whole genome sequencing studies.
Footnotes
Supported by NIHNational Cancer Institute grants P50CA097186, R01 CA234715, U54CA224079, P30CA015704P30CA006973, PO1CA163227, P50CA58236, and K22 CA237746) NIH Office of Research Infrastructure Programs grant S10OD028685, US Department of Defense Prostate Cancer Research Program grants W81XWH-20-1-0111, W81XWH-21-1-0229, W81XWH-18-1-0347W81XWH-18-1-0756, PC170510; W81XWH-18-1-0356, PC170503P2, and PC200262P1, Doris Duke Charitable Foundation grant 2021184, the Prostate Cancer Foundation, the Safeway Foundation, the Richard M. Lucas Foundation, the Fred Hutchinson Cancer Center/University of Washington Cancer Consortium, the Brotman Baty Institute for Precision Medicine and the University of Washington/Fred Hutchinson Cancer Center Institute for Prostate Cancer Research.
Disclosures: A.M.D.M. and S.Y. serve as consultants for Cepheid Inc. and receive sponsored research funding from Janssen R&D Inc. S.Y. receives sponsored research funding from Cepheid Inc. A.M.D.M. serves as a consultant to Merck Inc. W.G.N. has been a member of the Cancer Scientific Advisory Council, AbbVie Inc., the Scientific Advisory Board, ProQuest Investments Inc., the Board of Directors, Armis Biopharma Inc., and the Scientific Advisory Board, Cepheid Inc.; is a cofounder and adviser for Digital Harmonics and a cofounder and board of directors member for Brahm Astra Therapeutics; and has intellectual property licensed to MDxHealth Inc., Aduro BioTech Inc., Clontech, Bristol Myers Squibb, and Brahm Astra Therapeutics.
Supplemental material for this article can be found at http://doi.org/10.1016/j.ajpath.2022.09.010.
Contributor Information
Jonathan I. Epstein, Email: jepstein@jhmi.edu.
Michael C. Haffner, Email: mhaffner@fredhutch.org.
Supplemental Data
Circos plots depicting structural rearrangements in case 1. Names of genes with rearrangement break sites are shown.
Circos plot depicting structural rearrangements in case 2. Names of genes with rearrangement break sites are shown.
OncoPrint plot showing alteration frequencies of genes mutated in case 1 and case 2 of this study in localized acinar adenocarcinoma (green, The Cancer Genome Atlas), metastatic acinar adenocarcinoma (red/yellow, Fred Hutchinson Cancer Center, SU2C/PFC), and NEPC (blue, Trento/Cornell) extracted from cBioPortal.28,29
References
- 1.Zhang D., Zhao S., Li X., Kirk J.S., Tang D.G. Prostate luminal progenitor cells in development and cancer. Trends Cancer. 2018;4:769–783. doi: 10.1016/j.trecan.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humphrey P.A. Histopathology of prostate cancer. Cold Spring Harb Perspect Med. 2017;7:a030411. doi: 10.1101/cshperspect.a030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali T.Z., Epstein J.I. Basal cell carcinoma of the prostate: a clinicopathologic study of 29 cases. Am J Surg Pathol. 2007;31:697–705. doi: 10.1097/01.pas.0000213395.42075.86. [DOI] [PubMed] [Google Scholar]
- 4.Iczkowski K.A., Ferguson K.L., Grier D.D., Hossain D., Banerjee S.S., McNeal J.E., Bostwick D.G. Adenoid cystic/basal cell carcinoma of the prostate: clinicopathologic findings in 19 cases. Am J Surg Pathol. 2003;27:1523–1529. doi: 10.1097/00000478-200312000-00004. [DOI] [PubMed] [Google Scholar]
- 5.McKenney J.K., Amin M.B., Srigley J.R., Jimenez R.E., Ro J.Y., Grignon D.J., Young R.H. Basal cell proliferations of the prostate other than usual basal cell hyperplasia: a clinicopathologic study of 23 cases, including four carcinomas, with a proposed classification. Am J Surg Pathol. 2004;28:1289–1298. doi: 10.1097/01.pas.0000138180.95581.e1. [DOI] [PubMed] [Google Scholar]
- 6.Grignon D.J., Ro J.Y., Ordoñez N.G., Ayala A.G., Cleary K.R. Basal cell hyperplasia, adenoid basal cell tumor, and adenoid cystic carcinoma of the prostate gland: an immunohistochemical study. Hum Pathol. 1988;19:1425–1433. doi: 10.1016/s0046-8177(88)80235-1. [DOI] [PubMed] [Google Scholar]
- 7.Ayyathurai R., Civantos F., Soloway M.S., Manoharan M. Basal cell carcinoma of the prostate: current concepts. BJU Int. 2007;99:1345–1349. doi: 10.1111/j.1464-410X.2007.06857.x. [DOI] [PubMed] [Google Scholar]
- 8.Thorson P., Swanson P.E., Vollmer R.T., Humphrey P.A. Basal cell hyperplasia in the peripheral zone of the prostate. Mod Pathol. 2003;16:598–606. doi: 10.1097/01.MP.0000073526.59270.6E. [DOI] [PubMed] [Google Scholar]
- 9.Tsuruta K., Funahashi Y., Kato M. Basal cell carcinoma arising in the prostate. Int J Urol. 2014;21:1072–1073. doi: 10.1111/iju.12498. [DOI] [PubMed] [Google Scholar]
- 10.Yang X.J., McEntee M., Epstein J.I. Distinction of basaloid carcinoma of the prostate from benign basal cell lesions by using immunohistochemistry for bcl-2 and Ki-67. Hum Pathol. 1998;29:1447–1450. doi: 10.1016/s0046-8177(98)90014-4. [DOI] [PubMed] [Google Scholar]
- 11.Weier C., Haffner M.C., Mosbruger T., Esopi D.M., Hicks J., Zheng Q., Fedor H., Isaacs W.B., De Marzo A.M., Nelson W.G., Yegnasubramanian S. Nucleotide resolution analysis of TMPRSS2and ERG rearrangements in prostate cancer. J Pathol. 2013;230:174–183. doi: 10.1002/path.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luebke A.M., Schlomm T., Gunawan B., Bonkhoff H., Füzesi L., Erbersdobler A. Simultaneous tumour-like, atypical basal cell hyperplasia and acinar adenocarcinoma of the prostate: a comparative morphological and genetic approach. Virchows Arch. 2005;446:338–341. doi: 10.1007/s00428-004-1199-6. [DOI] [PubMed] [Google Scholar]
- 13.Su X., Long Q., Bo J., Shi Y., Zhao L.-N., Lin Y., Luo Q., Ghazanfar S., Zhang C., Liu Q., Wang L., He K., He J., Cui X., Yang J.Y.H., Han Z.-G., Yang G., Sha J.-J. Mutational and transcriptomic landscapes of a rare human prostate basal cell carcinoma. Prostate. 2020;80:508–517. doi: 10.1002/pros.23965. [DOI] [PubMed] [Google Scholar]
- 14.Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DePristo M.A., Banks E., Poplin R., Garimella K.V., Maguire J.R., Hartl C., Philippakis A.A., del Angel G., Rivas M.A., Hanna M., McKenna A., Fennell T.J., Kernytsky A.M., Sivachenko A.Y., Cibulskis K., Gabriel S.B., Altshuler D., Daly M.J. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim S., Scheffler K., Halpern A.L., Bekritsky M.A., Noh E., Källberg M., Chen X., Kim Y., Beyter D., Krusche P., Saunders C.T. Strelka2: fast and accurate calling of germline and somatic variants. Nat Methods. 2018;15:591–594. doi: 10.1038/s41592-018-0051-x. [DOI] [PubMed] [Google Scholar]
- 17.Ha G., Roth A., Khattra J., Ho J., Yap D., Prentice L.M., Melnyk N., McPherson A., Bashashati A., Laks E., Biele J., Ding J., Le A., Rosner J., Shumansky K., Marra M.A., Gilks C.B., Huntsman D.G., McAlpine J.N., Aparicio S., Shah S.P. TITAN: inference of copy number architectures in clonal cell populations from tumor whole-genome sequence data. Genome Res. 2014;24:1881–1893. doi: 10.1101/gr.180281.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adalsteinsson V.A., Ha G., Freeman S.S., Choudhury A.D., Stover D.G., Parsons H.A., et al. Scalable whole-exome sequencing of cell-free DNA reveals high concordance with metastatic tumors. Nat Commun. 2017;8:1324. doi: 10.1038/s41467-017-00965-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wala J.A., Bandopadhayay P., Greenwald N.F., O'Rourke R., Sharpe T., Stewart C., Schumacher S., Li Y., Weischenfeldt J., Yao X., Nusbaum C., Campbell P., Getz G., Meyerson M., Zhang C.-Z., Imielinski M., Beroukhim R. SvABA: genome-wide detection of structural variants and indels by local assembly. Genome Res. 2018;28:581–591. doi: 10.1101/gr.221028.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viswanathan S.R., Ha G., Hoff A.M., Wala J.A., Carrot-Zhang J., Whelan C.W., Haradhvala N.J., Freeman S.S., Reed S.C., Rhoades J., Polak P., Cipicchio M., Wankowicz S.A., Wong A., Kamath T., Zhang Z., Gydush G.J., Rotem D., PCF/SU2C International Prostate Cancer Dream Team. Love J.C., Getz G., Gabriel S., Zhang C.-Z., Dehm S.M., Nelson P.S., Van Allen E.M., Choudhury A.D., Adalsteinsson V.A., Beroukhim R., Taplin M.-E., Meyerson M. Structural alterations driving castration-resistant prostate cancer revealed by linked-read genome sequencing. Cell. 2018;174:433–447.e19. doi: 10.1016/j.cell.2018.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howe K.L., Achuthan P., Allen J., Allen J., Alvarez-Jarreta J., Amode M.R., et al. Ensembl 2021. Nucleic Acids Res. 2021;49:D884–D891. doi: 10.1093/nar/gkaa942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graham M.K., Principessa L., Antony L., Meeker A.K., Isaacs J.T. Low p16INK4a expression in early passage human prostate basal epithelial cells enables immortalization by telomerase expression alone. Prostate. 2017;77:374–384. doi: 10.1002/pros.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haffner M.C., Aryee M.J., Toubaji A., Esopi D.M., Albadine R., Gurel B., Isaacs W.B., Bova G.S., Liu W., Xu J., Meeker A.K., Netto G., De Marzo A.M., Nelson W.G., Yegnasubramanian S. Androgen-induced TOP2B-mediated double-strand breaks and prostate cancer gene rearrangements. Nat Genet. 2010;42:668–675. doi: 10.1038/ng.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armenia J., Wankowicz S.A.M., Liu D., Gao J., Kundra R., Reznik E., et al. The long tail of oncogenic drivers in prostate cancer. Nat Genet. 2018;50:645–651. doi: 10.1038/s41588-018-0078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson D., Van Allen E.M., Wu Y.-M., Schultz N., Lonigro R.J., Mosquera J.-M., et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–1228. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wedge D.C., Gundem G., Mitchell T., Woodcock D.J., Martincorena I., Ghori M., et al. Sequencing of prostate cancers identifies new cancer genes, routes of progression and drug targets. Nat Genet. 2018;50:682–692. doi: 10.1038/s41588-018-0086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cancer Genome Atlas Research Network The molecular taxonomy of primary prostate cancer. Cell. 2015;163:1011–1025. doi: 10.1016/j.cell.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cerami E., Gao J., Dogrusoz U., Gross B.E., Sumer S.O., Aksoy B.A., Jacobsen A., Byrne C.J., Heuer M.L., Larsson E., Antipin Y., Reva B., Goldberg A.P., Sander C., Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao J., Aksoy B.A., Dogrusoz U., Dresdner G., Gross B., Sumer S.O., Sun Y., Jacobsen A., Sinha R., Larsson E., Cerami E., Sander C., Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corless C.L., Barnett C.M., Heinrich M.C. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11:865–878. doi: 10.1038/nrc3143. [DOI] [PubMed] [Google Scholar]
- 31.Coombs C.C., Tallman M.S., Levine R.L. Molecular therapy for acute myeloid leukaemia. Nat Rev Clin Oncol. 2016;13:305–318. doi: 10.1038/nrclinonc.2015.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Litchfield K., Levy M., Huddart R.A., Shipley J., Turnbull C. The genomic landscape of testicular germ cell tumours: from susceptibility to treatment. Nat Rev Urol. 2016;13:409–419. doi: 10.1038/nrurol.2016.107. [DOI] [PubMed] [Google Scholar]
- 33.Heinrich M.C., Rubin B.P., Longley B.J., Fletcher J.A. Biology and genetic aspects of gastrointestinal stromal tumors: KIT activation and cytogenetic alterations. Hum Pathol. 2002;33:484–495. doi: 10.1053/hupa.2002.124124. [DOI] [PubMed] [Google Scholar]
- 34.Chen L.L., Sabripour M., Wu E.F., Prieto V.G., Fuller G.N., Frazier M.L. A mutation-created novel intra-exonic pre-mRNA splice site causes constitutive activation of KIT in human gastrointestinal stromal tumors. Oncogene. 2005;24:4271–4280. doi: 10.1038/sj.onc.1208587. [DOI] [PubMed] [Google Scholar]
- 35.Zhu Z., Liu Y., Li K., Liu J., Wang H., Sun B., Xiong Z., Jiang H., Zheng J., Hu Z. Protein tyrosine phosphatase receptor U (PTPRU) is required for glioma growth and motility. Carcinogenesis. 2014;35:1901–1910. doi: 10.1093/carcin/bgu123. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y., Zhu Z., Xiong Z., Zheng J., Hu Z., Qiu J. Knockdown of protein tyrosine phosphatase receptor U inhibits growth and motility of gastric cancer cells. Int J Clin Exp Pathol. 2014;7:5750–5761. [PMC free article] [PubMed] [Google Scholar]
- 37.Renton A.E., Majounie E., Waite A., Simón-Sánchez J., Rollinson S., Gibbs J.R., et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nickerson M.L., Warren M.B., Toro J.R., Matrosova V., Glenn G., Turner M.L., Duray P., Merino M., Choyke P., Pavlovich C.P., Sharma N., Walther M., Munroe D., Hill R., Maher E., Greenberg C., Lerman M.I., Linehan W.M., Zbar B., Schmidt L.S. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dubé syndrome. Cancer Cell. 2002;2:157–164. doi: 10.1016/s1535-6108(02)00104-6. [DOI] [PubMed] [Google Scholar]
- 39.Xu J., McPherson P.S. Regulation of DENND3, the exchange factor for the small GTPase Rab12 through an intramolecular interaction. J Biol Chem. 2017;292:7274–7282. doi: 10.1074/jbc.M116.772434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hurlin P.J., Steingrìmsson E., Copeland N.G., Jenkins N.A., Eisenman R.N. Mga, a dual-specificity transcription factor that interacts with Max and contains a T-domain DNA-binding motif. EMBO J. 1999;18:7019–7028. doi: 10.1093/emboj/18.24.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amati B., Brooks M.W., Levy N., Littlewood T.D., Evan G.I., Land H. Oncogenic activity of the c-Myc protein requires dimerization with Max. Cell. 1993;72:233–245. doi: 10.1016/0092-8674(93)90663-b. [DOI] [PubMed] [Google Scholar]
- 42.Llabata P., Mitsuishi Y., Choi P.S., Cai D., Francis J.M., Torres-Diz M., Udeshi N.D., Golomb L., Wu Z., Zhou J., Svinkina T., Aguilera-Jimenez E., Liu Y., Carr S.A., Sanchez-Cespedes M., Meyerson M., Zhang X. Multi-omics analysis identifies MGA as a negative regulator of the MYC pathway in lung adenocarcinoma. Mol Cancer Res. 2020;18:574–584. doi: 10.1158/1541-7786.MCR-19-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mathsyaraja H., Catchpole J., Freie B., Eastwood E., Babaeva E., Geuenich M., Cheng P.F., Ayers J., Yu M., Wu N., Moorthi S., Poudel K.R., Koehne A., Grady W., Houghton A.M., Berger A.H., Shiio Y., MacPherson D., Eisenman R.N. Loss of MGA repression mediated by an atypical polycomb complex promotes tumor progression and invasiveness. Elife. 2021;10:e64212. doi: 10.7554/eLife.64212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel N., Itakura T., Jeong S., Liao C.-P., Roy-Burman P., Zandi E., Groshen S., Pinski J., Coetzee G.A., Gross M.E., Fini M.E. Expression and functional role of orphan receptor GPR158 in prostate cancer growth and progression. PLoS One. 2015;10:e0117758. doi: 10.1371/journal.pone.0117758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bishop J.A., Yonescu R., Epstein J.I., Westra W.H. A subset of prostatic basal cell carcinomas harbor the MYB rearrangement of adenoid cystic carcinoma. Hum Pathol. 2015;46:1204–1208. doi: 10.1016/j.humpath.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Magers M.J., Iczkowski K.A., Montironi R., Grignon D.J., Zhang S., Williamson S.R., Yang X., Wang M., Osunkoya A.O., Lopez-Beltran A., Hes O., Eble J.N., Cheng L. MYB-NFIB gene fusion in prostatic basal cell carcinoma: clinicopathologic correlates and comparison with basal cell adenoma and florid basal cell hyperplasia. Mod Pathol. 2019;32:1666–1674. doi: 10.1038/s41379-019-0297-6. [DOI] [PubMed] [Google Scholar]
- 47.Rettig E.M., Talbot C.C., Sausen M., Jones S., Bishop J.A., Wood L.D., Tokheim C., Niknafs N., Karchin R., Fertig E.J., Wheelan S.J., Marchionni L., Considine M., Ling S., Fakhry C., Papadopoulos N., Kinzler K.W., Vogelstein B., Ha P.K., Agrawal N. Whole-genome sequencing of salivary gland adenoid cystic carcinoma. Cancer Prev Res. 2016;9:265–274. doi: 10.1158/1940-6207.CAPR-15-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rajan N., Ashworth A. Inherited cylindromas: lessons from a rare tumour. Lancet Oncol. 2015;16:e460–e469. doi: 10.1016/S1470-2045(15)00245-4. [DOI] [PubMed] [Google Scholar]
- 49.Verhoeft K.R., Ngan H.L., Lui V.W.Y. The cylindromatosis (CYLD) gene and head and neck tumorigenesis. Cancers Head Neck. 2016;1:10–16. doi: 10.1186/s41199-016-0012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh D.D., Naujoks C., Depprich R., Schulte K.-W., Jankowiak F., Kübler N.R., Handschel J. Cylindroma of head and neck: review of the literature and report of two rare cases. J Craniomaxillofac Surg. 2013;41:516–521. doi: 10.1016/j.jcms.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 51.Bignell G.R., Warren W., Seal S., Takahashi M., Rapley E., Barfoot R., Green H., Brown C., Biggs P.J., Lakhani S.R., Jones C., Hansen J., Blair E., Hofmann B., Siebert R., Turner G., Evans D.G., Schrander-Stumpel C., Beemer F.A., van Den Ouweland A., Halley D., Delpech B., Cleveland M.G., Leigh I., Leisti J., Rasmussen S. Identification of the familial cylindromatosis tumour-suppressor gene. Nat Genet. 2000;25:160–165. doi: 10.1038/76006. [DOI] [PubMed] [Google Scholar]
- 52.Alameda J.P., Moreno-Maldonado R., Navarro M., Bravo A., Ramírez A., Page A., Jorcano J.L., Fernández-Aceñero M.J., Casanova M.L. An inactivating CYLD mutation promotes skin tumor progression by conferring enhanced proliferative, survival and angiogenic properties to epidermal cancer cells. Oncogene. 2010;29:6522–6532. doi: 10.1038/onc.2010.378. [DOI] [PubMed] [Google Scholar]
- 53.Brummelkamp T.R., Nijman S.M.B., Dirac A.M.G., Bernards R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature. 2003;424:797–801. doi: 10.1038/nature01811. [DOI] [PubMed] [Google Scholar]
- 54.Lee C.C., Carette J.E., Brummelkamp T.R., Ploegh H.L. A reporter screen in a human haploid cell line identifies CYLD as a constitutive inhibitor of NF-κB. PLoS One. 2013;8:e70339. doi: 10.1371/journal.pone.0070339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reiley W., Zhang M., Sun S.-C. Negative regulation of JNK signaling by the tumor suppressor CYLD. J Biol Chem. 2004;279:55161–55167. doi: 10.1074/jbc.M411049200. [DOI] [PubMed] [Google Scholar]
- 56.Kovalenko A., Chable-Bessia C., Cantarella G., Israël A., Wallach D., Courtois G. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature. 2003;424:801–805. doi: 10.1038/nature01802. [DOI] [PubMed] [Google Scholar]
- 57.De Marzo A.M., Meeker A.K., Epstein J.I., Coffey D.S. Prostate stem cell compartments: expression of the cell cycle inhibitor p27Kip1 in normal, hyperplastic, and neoplastic cells. Am J Pathol. 1998;153:911–919. doi: 10.1016/S0002-9440(10)65632-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldstein A.S., Huang J., Guo C., Garraway I.P., Witte O.N. Identification of a cell of origin for human prostate cancer. Science. 2010;329:568–571. doi: 10.1126/science.1189992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stoyanova T., Cooper A.R., Drake J.M., Liu X., Armstrong A.J., Pienta K.J., Zhang H., Kohn D.B., Huang J., Witte O.N., Goldstein A.S. Prostate cancer originating in basal cells progresses to adenocarcinoma propagated by luminal-like cells. Proc Natl Acad Sci U S A. 2013;110:20111–20116. doi: 10.1073/pnas.1320565110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Circos plots depicting structural rearrangements in case 1. Names of genes with rearrangement break sites are shown.
Circos plot depicting structural rearrangements in case 2. Names of genes with rearrangement break sites are shown.
OncoPrint plot showing alteration frequencies of genes mutated in case 1 and case 2 of this study in localized acinar adenocarcinoma (green, The Cancer Genome Atlas), metastatic acinar adenocarcinoma (red/yellow, Fred Hutchinson Cancer Center, SU2C/PFC), and NEPC (blue, Trento/Cornell) extracted from cBioPortal.28,29