Abstract
Anaplasma marginale is a tick-borne pathogen, one of several closely related ehrlichial organisms that cause disease in animals and humans. These Ehrlichia species have complex life cycles that require, in addition to replication and development within the tick vector, evasion of the immune system in order to persist in the mammalian reservoir host. This complexity requires efficient use of the small ehrlichial genome. A. marginale and related ehrlichiae express immunoprotective, variable outer membrane proteins that have similar structures and are encoded by polymorphic multigene families. We show here that the major outer membrane protein of A. marginale, MSP2, is encoded on a polycistronic mRNA. The genomic expression site for this mRNA is polymorphic and encodes numerous amino acid sequence variants in bloodstream populations of A. marginale. A potential mechanism for persistence is segmental gene conversion of the expression site to link hypervariable msp2 sequences to the promoter and polycistron.
Ehrlichiae are major causes of tick-borne diseases, including the recently emergent human monocytic and two granulocytic ehrlichioses and the most prevalent tick-borne infection in cattle worldwide, anaplasmosis (6). These pathogens, classified in genogroups I and II of the tribe Ehrlichieae, have a complex life cycle characterized by acute and persistent infection in the mammalian host and several replicative and developmental stages within the tick vector (10, 17). Notably, this complexity is achieved using a small genome, only 0.8 to 1.5 Mb in a single chromosome (1, 25). Persistence of Anaplasma marginale in cattle, which is fundamental for continued transmission, reflects sequential expression of antigenically variant outer membrane proteins that are encoded by the msp2 multigene family (21). The outer membrane proteins of different ehrlichial organisms are significantly similar to one another in amino acid sequence, are all encoded by multigene families, and possess one to four variable regions (8, 13, 18–20, 22, 26, 29, 32). In A. marginale-infected cattle, three to six MSP2 variants are expressed in each sequential rickettsemic cycle, which recur every 4 to 8 weeks during persistent infection (8, 9, 15, 16). Thus, over the 7-year period in which A. marginale has been shown to persist, over 500 variants may be expressed. Although the cyclic emergence and immune control of A. marginale is similar to that occurring in African trypanosomiasis, the mechanisms used by the organism to persist in mammalian hosts are unknown. We show here that variation of msp2 in erythrocyte stages of A. marginale proceeds through the formation of different sequence mosaics in a polycistronic expression site.
MATERIALS AND METHODS
Isolation and sequencing of A. marginale genomic DNA.
Florida and South Idaho strains of A. marginale were maintained as liquid nitrogen-cryopreserved stabilates of infected bovine erythrocytes in dimethyl sulfoxide–phosphate-buffered saline that were then used to infect cattle (20). A. marginale genomic DNA was isolated from highly rickettsemic (>50% rickettsemia) bovine blood by lysis with sodium dodecyl sulfate and lysozyme, treatment with proteinase K and RNase, phenol-chloroform extraction, and ethanol precipitation (5). In some cases, for PCR amplification and analysis of low rickettsemias, genomic DNA was isolated from 200 μl of infected blood by using a QIAamp DNA mini kit (Qiagen, Valencia, Calif.). To obtain the sequence of the complete msp2 coding region, the sequence flanking orf2 and msp2 was obtained in both 5′ and 3′ directions and then confirmed on both strands following PCR amplification of the entire locus (14, 28). Sequencing was performed at the University of Florida DNA Sequencing Core Laboratory (Gainesville, Fla.) using ABI Prism dye terminator cycle sequencing protocols developed by Applied Biosystems (Perkin-Elmer Corp., Foster City, Calif.). The fluorescently labeled extension products were analyzed on an Applied Biosystems model 373 Stretch DNA Sequencer (Perkin-Elmer Corp.). Oligonucleotide primers were designed using OLIGO 5.0 (Molecular Biology Insights, Cascade, Colo.) software and synthesized by Genosys Biotechnologies (The Woodlands, Tex.). Nucleotide sequences were analyzed using the GCG programs (Genetics Computer Group, University of Wisconsin) available through the Biological Computing core facilities of the Interdisciplinary Center for Biotechnology Research at the University of Florida. Sequence alignments were made using PILEUP and GAP, and similarities were displayed using PLOTSIMILARITY. Prokaryotic factor-independent RNA polymerase terminator sequences were predicted using TERMINATOR.
Analysis of msp2 mRNA.
Total RNA was isolated from whole blood obtained during acute rickettsemia with the FL strain of A. marginale by extraction with 6 M urea–3M LiCl (27). For reverse transcription-PCR (RT-PCR) analysis, RNA was digested with RQ1 DNase (Promega, Madison, Wis.) and the reactions were conducted with or without reverse transcriptase (Retroscript kit; Ambion, Austin, Tex.) in a total volume of 20 μl. Then 1.5 μl of the RT reaction mixture was used in primary PCRs and 2.5 μl of the primary PCR mixture was used in secondary or nested PCRs. For primer extension analysis, the oligonucleotide primer was 5′-end labeled with 32P using T4 polynucleotide kinase and extended in sequencing reactions with reverse transcriptase, using as template 50 μg of RNA isolated from A. marginale-infected blood or 0.5 μg of a 3.9-kb PCR product DNA containing msp2, orf2 to orf4, and flanking regions (12). RNase protection assays were conducted with an antisense RNA probe prepared from msp2 cDNA clone AR11 (7), which contains coding sequence for both msp2 and orf2 (see below). AR11 plasmid DNA was linearized with XhoI and labeled with [32P]UTP using the Maxiscript kit (Ambion) and SP6 RNA polymerase, and the 722-nucleotide probe was purified by gel isolation. The labeled probe was hybridized with different amounts of A. marginale RNA or carrier yeast RNA and then subjected to digestion with RNase A-RNase T1 as described by the manufacturer (RPAIII kit; Ambion). Protected probe fragments were analyzed by electrophoresis in an 8 M urea–5% acrylamide gel with 32P-labeled RNA Century Marker Plus standards (Ambion).
Southern blotting of A. marginale genomic DNA.
Probes for Southern blots were derived as follows: msp2 probe, insert DNA from msp2 cDNA clone AR3 (7) that includes nucleotides 18 to 555 of msp2; orf2 probe, PCR amplification product from msp2 cDNA clone AR9 (7) with oligonucleotide primers AB688 and AB689 that contains nucleotides 28 to 275 of orf2; orf3 probe, PCR amplification product from msp2 cDNA clone AR9 with primers AB690 and AB691 and with nucleotides 618 to 877 of orf3; orf4 probe, PCR amplification product from Florida strain A. marginale genomic DNA with primers AB783 and AB747 that contains nucleotides 467 to 896 of orf4. DNA probes were labeled with fluorescein-dUTP, hybridized, washed under high-stringency conditions (0.1× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate] plus 0.1% sodium dodecyl sulfate at 60°C) and detected by chemiluminescence (Illuminator chemiluminescent detection system; Stratagene, La Jolla, Calif.). Oligonucleotide probes were synthesized with a 5′ fluorescein end label (Genosys Biotechnologies), hybridized, washed, and detected similarly, using final wash temperatures of 5°C below their respective Tm values. Molecular size standards were Illuminator nonradioactive markers (Stratagene).
Determination of msp2 expression site structure in progenitor and progeny populations of A. marginale.
Bovine no. 196 was infected with A. marginale (Florida strain). At 40 days after the initial infection, the animal experienced a peak of acute rickettsemia of 6% (the percentage of erythrocytes containing A. marginale), which subsequently resolved, and the animal became an asymptomatic carrier of infection. At 107 days after initial infection, the animal was splenectomized, which caused recrudescence of a microscopically detectable rickettsemia. The animal was exsanguinated 19 days after splenectomy with a rickettsemia of 95% to obtain the Florida-relapse strain.
Synthetic oligonucleotides.
The synthetic oligonucleotides used in this study were (5′-3′) AB192 (CTATCCTTGAAGCTAATCTTG), AB198 (AAGGCAAACCTAACACCCAAC), AB688 (GGACTGCTTGCCTTCACGCTGTT), AB689 (TGAGCTGGGGAAAAGACGCTTGG), AB690 (CGGCGGCGTGGAGTTCCTTGA), AB691 (TGCCTGCTTCGACGCCAAGGT), AB747 (ATACAAACCCGACCACAAAATCC), AB750 (GGATTTTGTGGTCGGGTTTGTAT), AB752 (CACCGGTTGATGAAGTTTGC), AB764 (GCGTTCGGCAGGCATTTTGG), AB765 (GGAACAACCCCAATACCATC), AB766 (GTATGTCGATTCGCGGAAGAGCCTGTTGT), AB767 (ACGCGCTTGAATAAATCGTT), AB783 (AGTATCACATTGGGGAGGTTT), AB784 (GGAGGGAAAGCCGAAGTTG), AB847 (ACAGACACACTGCTTTCTGTTGAGGGGAACAAAGAC), AB871 (GTACCACTGGCAGTGGTGTCCATTGAT), and AB872 (ATTGTTGGTGCTGCCACTGGTGGCGTTAAC).
Nucleotide sequence accession numbers.
The sequences reported have been assigned GenBank accession numbers AF200925 to AF200927.
RESULTS
MSP2 is encoded on a polycistronic mRNA transcript.
Examination of over 250 msp2 transcripts expressed during acute or persistent A. marginale rickettsemia revealed a single, central hypervariable region that encodes the approximately 100-amino-acid antigenically variable domain (8, 9). The hypervariable msp2 region, characterized by nucleotide insertions, deletions, and substitutions, is flanked by highly conserved 5′ and 3′ ends. This structure is shared by the MSP2 homolog (Blastp E value of 10−102) in a causative agent of human granulocytic ehrlichiosis (13, 18, 32). Using cDNA clones derived from A. marginale during acute rickettsemia, we identified transcripts that extended 5′ to the ATG initiation codon for MSP2 (7). In the longest cDNA clone, AR9, this 5′ sequence extended 726 bp beyond the msp2 ATG and contained two additional open reading frames (ORFs). The immediately 5′-proximal ORF (orf2) was separated from the msp2 ATG by only 12 bp and was separated from orf3 (Fig. 1) by 23 bp. This structure is typical of prokaryotic polycistronic mRNAs, where the ORFs are generally separated by <30 bp. To obtain the structure of a complete transcriptional unit, we sequenced genomic DNA (14, 28) and identified a single locus containing msp2 linked to orf2 and orf3 and a fourth ORF (orf4) immediately 5′ to orf3 (Fig. 1). Numbering from msp2, orf2 encodes a polypeptide with a molecular weight of 13,219 that is predicted by the PSORT (http://psort.nibb.ac.jp) algorithm to be an outer membrane protein. orf2 did not resemble any sequence in the databases. orf3 and orf4 also encoded predicted outer membrane proteins with molecular weights of, respectively, 31,118 and 29,061; both were significantly similar (Blastp E values of 2 × 10−19 and 1 × 10−12, respectively) to the outer membrane protein OMP1b of Ehrlichia chaffeensis, the causative agent of human monocytic ehrlichiosis (19). There were no other significant ORFs within 130 bp 5′ or 240 bp 3′ to these four ORFs in the genome. Thus, the structure in Fig. 1 appeared to represent the entire coding region for a polycistronic msp2 transcript.
FIG. 1.
Structure and variability of the msp2 polycistronic expression site. (A) Diagram of the four ORFs comprising the polycistronic expression site for msp2, showing the molecular weights (M.W.) of encoded proteins, primary (1°) and secondary (2°) RT-PCR products generated from msp2 mRNA, location of probe used in the RNase protection assay (RPA probe, see Fig. 2), and FspI cleavage sites (see Fig. 4 and 5). P, predicted promoter region, 72 to 108 bp 5′ to orf4; T, predicted prokaryotic terminator sequence, GTAGACCAGC....TAGTCGTCAC, 149 to 200 bp 3′ to msp2. (B) PLOTSIMILARITY profile of nucleotide sequence variability between msp2 expression sites of Florida (F) and South Idaho (I-1) strains of A. marginale. A similarity score of 1.0 indicates identical sequence in a sliding window of 10 nucleotides, and a decreasing score from 1.0 to 0.0 indicates increasing variation. (C) PLOTSIMILARITY profile of expression site variability in the South Idaho strain of A. marginale examined at two time points 10 days apart in acute rickettsemia (I-1 and I-2).
To confirm that the genomic region shown in Fig. 1 encoded a polycistronic transcript, RNA obtained from A. marginale during acute rickettsemia was analyzed by RT-PCR, RNase protection, and primer extension (Fig. 2). RNA encoding MSP2 and also containing orf2 to orf4 was present as a polycistronic transcript that could be amplified by RT-PCR. No amplified products were present in control reactions without RT. The sequenced RT-PCR product of 2 kbp (Fig. 2) contained the regions of msp2 and orf2 to orf4 expected from the genomic locus. An RNase protection assay was performed to investigate whether msp2 was transcribed primarily as a polycistronic mRNA. An antisense RNA probe of 624 bases (317 bases at the 5′ end of msp2 and 307 bases of the intercistronic sequence and orf2 [Fig. 1]) was protected from RNase digestion by hybridization with total A. marginale RNA (Fig. 2), suggesting that the majority of msp2 transcripts were polycistronic and linked to orf2 when expressed during acute rickettsemia. The transcription start site (+1) was identified by primer extension analysis (Fig. 2) as 74bp 5′ to the ATG initiation codon of orf4 within the sequence: −35 ATTTAAATTGCAAAATATATTGCCAGCTCTCTTGACT −10+1 TAATTCTTTTTTAGTATACCTTATGGGTGTGTGGG
FIG. 2.
Analysis of msp2 transcript structure by RT-PCR, primer extension analysis, and RNase protection assay (RPA). For RT-PCR, total DNase-treated RNA of Florida strain A. marginale was reverse transcribed into DNA using oligonucleotide primer AB198, which anneals to the 3′ end of msp2. The cDNA was amplified in a primary PCR with primers AB765 and AB766, which generated a product of 3.2 kbp. The primary PCR product was amplified in a secondary or nested PCR with primer combinations AB192 and AB764, AB689 and AB764, and AB192 and AB688 to generate products of 2.0, 1.5, and 0.7 kbp, respectively. See Fig. 1 for the locations of RT-PCR products. S, molecular size standards; +, with reverse transcriptase; −, negative control reactions without reverse transcriptase. For primer extension, oligonucleotide primer AB784, which anneals 153 nucleotides 3′ to the ATG initiation codon of orf4, was radiolabeled with 32P and extended in sequencing reactions using reverse transcriptase and either total RNA of A. marginale or denatured, PCR-amplified genomic DNA containing orf2 to orf4, msp2, and flanking regions as templates. The order of sequencing reactions T, G, C, and A, is shown above the DNA lanes and was the same in the RNA lanes. A strong stop was detected in RNA at the A (underlined) in sequence TGCAACCCACACACCCATAAGG, with evidence also for a minority of transcripts continuing to the next base, C (italic). This corresponds to the coding-strand sequence CCTTATGGGTGTGTGGGTTGCA (see the text). For the RNase protection assay, a 32P-labeled antisense RNA probe of 722 nucleotides (317 nucleotides of the msp2 gene, 307 nucleotides of orf2 and intercistronic spacer, 98 nucleotides of plasmid vector) was allowed to hybridize to various amounts (10, 3, and 0.1 μg) of total RNA of A. marginale and carrier yeast RNA or to yeast RNA alone (lane 0) and then unprotected single-stranded probe was digested with RNase and analyzed by denaturing polyacrylamide gel electrophoresis. C, probe plus yeast RNA, not digested with RNase. The positions of molecular size standards are shown on the left. A band of 624 bp containing the A. marginale sequences within the probe (msp2 and orf2) was the predominant fragment protected.
This sequence contained elements that may be −35 and −10 promoter regions (underlined). The consensus Escherichia coli −35 region is TTGACA and −10 is TATAAT, with an optimal spacing between them of 17 ± 1 bp (11). The three of six identities at −10 were the most highly conserved first, second, and sixth positions of the E. coli consensus sequence. There was also an AT-rich region upstream of −35 in the A. marginale sequence typical of prokaryotic promoters (11). Interestingly, a second region was found (double underline) with a similar level of sequence identity to the consensus E. coli −35 and −10 sequences, separated from the first by only 6 bp. The significance of this potential dual-promoter structure in transcriptional control is unknown.
This msp2 expression site encodes hypervariable sequences expressed in vivo.
To analyze the variability of MSP2 encoded by the polycistronic expression site, we sequenced the expression site in two genetically and biologically distinct A. marginale strains obtained during acute rickettsemia (Fig. 1B) and at two different time points in a single acute infection (Fig. 1C). Alignment of complete expression site sequences revealed minor substitutions in orf2 and orf3 and no changes in the predicted promoter region (orf2 gene product, 98% amino acid identity between the Florida [F] and South Idaho [I-1] strains; orf3 gene product, 99% identity). There were more substitutions in orf4, and these were distributed throughout the orf4 sequence (96% identity between the F and I-1 strains). The greatest differences were in msp2 and included substitutions, insertions, and deletions (92% identity between F and I-1). Essentially similar results were obtained comparing expression site structure at two sequential time points in a single acute infection (Fig. 1C). In msp2, most of the expression site variability was localized to the region encoding the known MSP2 hypervariable region (8, 9) (Fig. 1B and C). We sequenced this hypervariable region in multiple, independent DNA clones of the genomic expression site and compared the encoded MSP2 peptide sequences (Fig. 3). Each population of A. marginale, whether taken from animals with acute rickettsemia with the Florida or South Idaho strains or from different time points in a single infection, was extremely polymorphic in the part of the expression site encoding the MSP2 hypervariable region.
FIG. 3.
Multiple different msp2 variants are present in the polycistronic expression site in each population of A. marginale. The expression site was amplified by PCR using primers which annealed 288 bp 3′ to the termination codon of msp2 (AB752) and to the intercistronic sequence between orf3 and orf4 (AB750) to generate a product of 2.9 kbp from A. marginale genomic DNA that contained msp2, orf2, and orf3. The PCR product was cloned in pCR-XL-TOPO vector (Invitrogen), and independent colonies containing a 2.9-kbp insert were selected for sequencing of cloned plasmid DNA. The hypervariable region of the msp2 gene was sequenced on both strands in 106 independent clones derived by PCR amplification from genomic DNA of the F, I-1, and I-2 A. marginale populations. DNA sequences were translated to amino acids, and the different variant sequences were aligned with PILEUP. The predominant sequence variants are shown. The percentage of each sequence variant in that population is indicated in brackets, e.g., the major sequence variant detected in the South Idaho A. marginale population I-1 was variant A, which was found in 48% of the independent clones of the expression site. Identical amino acids shared between all variants are indicated by dashes and shown on the bottom row of the alignment.
Do the expressed msp2 RNA transcripts reflect the sequence of this genomic locus? The major variant type in msp2 cDNA clones (7) derived from animals with acute rickettsemia with the Florida strain of A. marginale was identical to the expression site variant, F VarA (Fig. 3). The major msp2 variants present in cDNA clones from acute rickettsemia with the South Idaho strain were SGV1 and SGV2 (24). These transcribed variants were identical to I-1 VarA (SGV1) and I-1 VarB (SGV2), which represent the predominant genomic polycistronic expression site sequences at this time point (Fig. 3). We also identified less frequent sequence variants, including I-1 VarD and I-2 VarC, in mRNA by RT-PCR with oligonucleotide primers designed from the expression site DNA sequence followed by cloning and sequencing. Thus, both major and minor variant msp2 transcripts have corresponding genomic expression sites. Importantly, sequencing of cDNA clones derived from RT-PCR confirmed the linkage of the msp2 variants to orf2 to orf4 on polycistronic transcripts.
Genomic mechanism of msp2 variation.
How does this extensive msp2 expression site polymorphism occur? There are multiple genes containing msp2-related sequences in A. marginale (20). Restriction enzyme digests of A. marginale genomic DNA probed with msp2 identified these multiple msp2 copies in both the Florida and South Idaho strains but showed only a single band when probed with orf2, orf3, or orf4 (Fig. 4). Therefore, while msp2 and orf2 genes are contiguous in the expression site, multiple msp2 sequences are dispersed throughout the chromosome and are not contiguous with orf2. Both A. marginale and the human granulocytic ehrlichiosis agent contain incomplete msp2 genes that have the hypervariable and flanking conserved sequences but lack 5′ or 3′ coding regions (2, 31). Hybridizing Southern blots of A. marginale genomic DNA with different regions of msp2 (20) shows that the majority of msp2-containing genomic sequences lack the conserved 5′ end that is present in msp2 mRNA (8). This is consistent with recombination into the polycistronic expression site as a mechanism for activation of msp2 genes, including incomplete genes. Further support for this mechanism is provided by the presence of msp2 mosaic sequences within the expression site. For example, F VarC is identical to F VarA from amino acids 28 to 84 and to F VarB from amino acids 69 to 136 (Fig. 3). Specific MSP2 sequence motifs encoded in the expression site, for example, NAI, KAV, or NAV (amino acids 34 to 36 [Fig. 3]) and TNGEKVSQ or TSGDELSK (amino acids 43 to 50), are present outside the expression site in other msp2 copies (2, 20, 31). Extensive intragenic recombination between the expression site and other msp2 copies, employing flanking conserved regions, would result in the formation of the different sequence mosaics observed in the msp2 hypervariable region and link variable msp2 sequences to orf2 to orf4 and the promoter.
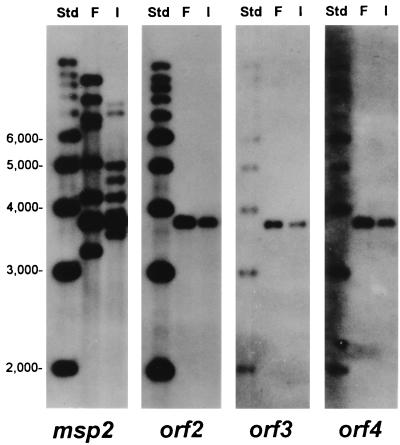
FIG. 4.
Structure of msp2 and orf2 to orf4 in genomic DNA of Florida and South Idaho strains of A. marginale. Southern blots of Florida (F) or South Idaho (I) genomic DNA digested with the restriction enzyme FspI and hybridized with probes specific for msp2, orf2, orf3, or orf4 are shown. FspI cleaves 41 nucleotides 5′ to orf4 and 268 nucleotides 3′ to msp2 to release a fragment of 3.76 kbp containing the complete polycistronic msp2 expression site sequence (see Fig. 1) from both Florida and South Idaho genomic DNAs. Molecular size standards are shown in the left lane of each blot. Multiple msp2-related sequences are detected in genomic DNA of both strains; only msp2 sequences located in the expression site are contiguous with orf2 to orf4.
Data supporting this hypothesis are shown in Fig. 5, comparing the expression site structure in Florida strain A. marginale (progenitor) with that in a Florida-relapse strain (progeny) derived after >3 months of persistent bovine infection. Oligonucleotide probes to msp2 hypervariable region sequences present only in the expression site of the progeny strain detect an extra band in the progeny population. Probes to the hypervariable region sequence present in the expression site in both the progenitor and progeny strains detect an expression site band in both populations, as expected (Fig. 5). Non-expression-site-associated bands remain unchanged in both the progenitor and progeny strains; these may be templates for segmental gene conversion of the expression site.
FIG. 5.
Comparison of genes encoding the msp2 hypervariable region in Florida (F) and Florida relapse (F-rel) strains of A. marginale. Hypervariable-region sequences were obtained from independent clones of the msp2 expression site (ES) in F (10 clones) and F-rel (11 clones) genomic DNA. From this DNA sequence information, different oligonucleotide probes to the hypervariable region were synthesized to use in Southern blotting. These probes contained sequence either unique to one set of expression site clones (the AB871 and AB872 sequences were both observed in 2 of 11 clones of the F-rel expression site and 0 of 10 clones of the F expression site) or were present in different proportions (the AB847 sequence was present in 5 of 10 expression site clones in the F strain and 1 of 11 expression site clones in the F-rel strain). A. marginale genomic DNA of the F or F-rel strains was digested with FspI to release the 3.76-kbp expression site (arrow), separated by gel electrophoresis, and probed with the indicated fluorescein-labeled synthetic oligonucleotides. With probe AB872, an extra (expression site) band of 3.76 kbp is detected in F-rel compared to F genomic DNA probed with the same sequence. A 4.4-kbp band is detected in both F and F-rel DNA, which may contain a template copy for conversion of the expression site. The expression site band is less intense than the 4.4-kbp band because the AB872 sequence is present in the expression site of only some organisms in the F-rel population of A. marginale (approximately 18% [2 of 11] of the total would be predicted from the fraction of F-rel expression site clones containing the AB872 sequence). This figure is approximate because of the small number of clones analyzed and the possibility of representation bias following amplification and cloning of the expression site. A similar result was obtained with a different F-rel-specific probe, AB871, except that there are two potential template copies of the AB871 sequence on 4.4- and 3.5-kbp fragments. The AB847 blot is an example of the converse situation: the AB847 sequence is well represented in the expression site of the F population of A. marginale but has nearly disappeared from the expression site in F-rel A. marginale. Potential template copies for the AB847 expression site sequence are on 11- and 7.2-kbp fragments.
DISCUSSION
A mechanism involving recombination into a polycistronic expression site, especially one containing other outer membrane protein genes, is unusual and represents efficient use of a small genome to generate diversity. Since orf3 and orf4 are distantly related members of the msp2 gene family, they, too, could vary by recombination with partially homologous sequences elsewhere in the genome. Comparison of orf3 and orf4 in different A. marginale populations shows that variation in these genes occurs at a lower rate than in msp2. The large number of diverse sequences found in the expression site of a single A. marginale population at a single time is consistent with the extensive variation of msp2 during the multiple rickettsemia cycles that comprise a single infection.
Recombination of portions of silent genes into a monocistronic expression site has been described for other prokaryotes, including the tick-borne Borrelia burgdorferi (30). However, B. burgdorferi uses plasmid-encoded genes to generate this diversity, an option unavailable to A. marginale (1). In some respects, A. marginale msp2 variation resembles the formation of mosaic genes in the polycistronic expression sites encoding the variable surface glycoprotein (VSG) of African trypanosomes. Complete genes and otherwise inactive pseudogenes are used to generate these mosaics in chronic trypanosomiasis (4, 23). The sequence mosaics encompass most regions of the VSG and are not targeted to a single hypervariable region as in A. marginale msp2. The trypanosome, because of its larger genome size, also has sufficient VSG-encoding genes to produce considerable variation without needing combinatorial mechanisms.
Rickettsiae, including the ehrlichial pathogens, are thought to be closely related to the original bacterial endosymbionts of eukaryotic cells that evolved into mitochondria by loss of genes whose function has been replaced by the host genome (3). Rickettsia prowazekii contains a high proportion of noncoding DNA (24%), despite a size of only 1.1 Mb. Rickettsial genes are postulated to be in the process of elimination from the genome by a series of steps comprising mutation to pseudogene, to unrecognizable sequence, to small fragments, to extinction (3). The data reported herein suggest that some pseudogene sequences may not be on their way to extinction but play a critical role in adaptive mechanisms of small-genome pathogens.
In conclusion, the MSP2 outer membrane protein is encoded on a polycistronic RNA transcript in erythrocyte stages of A. marginale. In addition to msp2, three other genes are present on this transcript that also are predicted to encode outer membrane proteins. All but one of these genes are significantly similar to outer membrane protein genes present in other ehrlichial pathogens, including those that infect humans. There is extensive polymorphism in the genomic expression site for this msp2 polycistronic transcript and evidence for gene rearrangement during persistent infection. Availability of the expression site sequence should allow the determination of the MSP2 variants expressed from this site during cyclical transmission of A. marginale between ticks and cattle. These data provide a possible molecular basis for persistence of A. marginale and may permit identification of similar mechanisms of variation in other ehrlichial pathogens. Common mechanisms of variable surface protein expression may be used by diverse pathogens to evade the host immune response and cause persistent infections.
ACKNOWLEDGMENTS
We thank Carlos R. Sulsona and Carla Robertson for excellent technical assistance.
This investigation was supported by USDA grant 95-372204-2348 and NIH grants AI45580 and AI44005.
REFERENCES
- 1.Alleman A R, Kamper S M, Viseshakul N, Barbet A F. Analysis of the Anaplasma marginale genome by pulsed-field electrophoresis. J Gen Microbiol. 1993;139:2439–2444. doi: 10.1099/00221287-139-10-2439. [DOI] [PubMed] [Google Scholar]
- 2.Alleman A R, Palmer G H, McGuire T C, McElwain T F, Perryman L E, Barbet A F. Anaplasma marginale major surface protein 3 is encoded by a polymorphic, multigene family. Infect Immun. 1997;65:156–163. doi: 10.1128/iai.65.1.156-163.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson S G, Zomorodipour A, Andersson J O, Sicheritz-Ponten T, Alsmark U C, Podowski R M, Naslund A K, Eriksson A S, Winkler H H, Kurland C G. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature. 1998;396:133–140. doi: 10.1038/24094. [DOI] [PubMed] [Google Scholar]
- 4.Barbet A F, Kamper S M. The importance of mosaic genes to trypanosome survival. Parasitol Today. 1993;9:63–66. doi: 10.1016/0169-4758(93)90039-i. [DOI] [PubMed] [Google Scholar]
- 5.Barbet A F, Palmer G H, Myler P J, McGuire T C. Characterization of an immunoprotective protein complex of Anaplasma marginale by cloning and expression of the gene coding for polypeptide Am105L. Infect Immun. 1987;55:2428–2435. doi: 10.1128/iai.55.10.2428-2435.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dumler J S, Bakken J S. Human ehrlichioses: newly recognized infections transmitted by ticks. Annu Rev Med. 1998;49:201–213. doi: 10.1146/annurev.med.49.1.201. [DOI] [PubMed] [Google Scholar]
- 7.Eid G, French D M, Lundgren A M, Barbet A F, McElwain T F, Palmer G H. Expression of major surface protein 2 antigenic variants during acute Anaplasma marginale rickettsemia. Infect Immun. 1996;64:836–841. doi: 10.1128/iai.64.3.836-841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.French D M, Brown W C, Palmer G H. Emergence of Anaplasma marginale antigenic variants during persistent rickettsemia. Infect Immun. 1999;67:5834–5840. doi: 10.1128/iai.67.11.5834-5840.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.French D M, McElwain T F, McGuire T C, Palmer G H. Expression of Anaplasma marginale major surface protein 2 variants during persistent cyclic rickettsemia. Infect Immun. 1998;66:1200–1207. doi: 10.1128/iai.66.3.1200-1207.1998. . (Erratum, 66:2400.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ge N L, Kocan K M, Blouin E F, Murphy G L. Developmental studies of Anaplasma marginale (Rickettsiales: Anaplasmataceae) in male Dermacentor andersoni (Acari: Ixodidae) infected as adults by using nonradioactive in situ hybridization and microscopy. J Med Entomol. 1996;33:911–920. doi: 10.1093/jmedent/33.6.911. [DOI] [PubMed] [Google Scholar]
- 11.Hawley D K, McClure W R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983;11:2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollingshead S K, Fischetti V A, Scott J R. Size variation in group A streptococcal M protein is generated by homologous recombination between intragenic repeats. Mol Gen Genet. 1987;207:196–203. doi: 10.1007/BF00331578. [DOI] [PubMed] [Google Scholar]
- 13.Ijdo J W, Sun W, Zhang Y, Magnarelli L A, Fikrig E. Cloning of the gene encoding the 44-kilodalton antigen of the agent of human granulocytic ehrlichiosis and characterization of the humoral response. Infect Immun. 1998;66:3264–3269. doi: 10.1128/iai.66.7.3264-3269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston S L, Strausbauch M, Sarkar G, Wettstein P J. A novel method for sequencing members of multi-gene families. Nucleic Acids Res. 1995;23:3074–3075. doi: 10.1093/nar/23.15.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kieser S T, Eriks I S, Palmer G H. Cyclic rickettsemia during persistent Anaplasma marginale infection of cattle. Infect Immun. 1990;58:1117–1119. doi: 10.1128/iai.58.4.1117-1119.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knowles D, Torioni D E, Palmer G, McGuire T, Stiller D, McElwain T. Antibody against an Anaplasma marginale MSP5 epitope common to tick and erythrocyte stages identifies persistently infected cattle. J Clin Microbiol. 1996;34:2225–2230. doi: 10.1128/jcm.34.9.2225-2230.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kocan K M, Stiller D, Goff W L, Claypool P L, Edwards W, Ewing S A, McGuire T C, Hair J A, Barron S J. Development of Anaplasma marginale in male Dermacentor andersoni transferred from parasitemic to susceptible cattle. Am J Vet Res. 1992;53:499–507. [PubMed] [Google Scholar]
- 18.Murphy C I, Storey J R, Recchia J, Doros-Richert L A, Gingrich-Baker C, Munroe K, Bakken J S, Coughlin R T, Beltz G A. Major antigenic proteins of the agent of human granulocytic ehrlichiosis are encoded by members of a multigene family. Infect Immun. 1998;66:3711–3718. doi: 10.1128/iai.66.8.3711-3718.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohashi N, Zhi N, Zhang Y, Rikihisa Y. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect Immun. 1998;66:132–139. doi: 10.1128/iai.66.1.132-139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer G H, Eid G, Barbet A F, McGuire T C, McElwain T F. The immunoprotective Anaplasma marginale major surface protein 2 is encoded by a polymorphic multigene family. Infect Immun. 1994;62:3808–3816. doi: 10.1128/iai.62.9.3808-3816.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer G H, Rurangirwa F R, Kocan K M, Brown W C. Molecular basis for vaccine development against the ehrlichial pathogen Anaplasma marginale. Parasitol Today. 1999;15:281–286. doi: 10.1016/s0169-4758(99)01469-6. [DOI] [PubMed] [Google Scholar]
- 22.Reddy G R, Sulsona C R, Barbet A F, Mahan S M, Burridge M J, Alleman A R. Molecular characterization of a 28 kDa surface antigen gene family of the tribe Ehrlichiae. Biochem Biophys Res Commun. 1998;247:636–643. doi: 10.1006/bbrc.1998.8844. [DOI] [PubMed] [Google Scholar]
- 23.Roth C, Bringaud F, Layden R, Baltz T, Eisen H. Active late-appearing variable surface antigen genes in Trypanosoma equiperdum are constructed entirely from pseudogenes. Proc Natl Acad Sci USA. 1989;86:9375–9379. doi: 10.1073/pnas.86.23.9375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rurangirwa F R, Stiller D, French D M, Palmer G H. Restriction of major surface protein 2 (MSP2) variants during tick transmission of the ehrlichia Anaplasma marginale. Proc Natl Acad Sci USA. 1999;96:3171–3176. doi: 10.1073/pnas.96.6.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rydkina E, Roux V, Raoult D. Determination of the genome size of Ehrlichia spp., using pulsed field gel electrophoresis. FEMS Microbiol Lett. 1999;176:73–78. doi: 10.1111/j.1574-6968.1999.tb13644.x. [DOI] [PubMed] [Google Scholar]
- 26.Sulsona C R, Mahan S M, Barbet A F. The map1 gene of Cowdria ruminantium is a member of a multigene family containing both conserved and variable genes. Biochem Biophys Res Commun. 1999;257:300–305. doi: 10.1006/bbrc.1999.0459. [DOI] [PubMed] [Google Scholar]
- 27.Van Der Ploeg L H T, Liu A Y C, Michels P A M, De Lange T, Borst P, Majumder H K, Weber H, Veeneman H G, Van Boom J H. RNA splicing is required to make the messenger RNA for a variant surface antigen in trypanosomes. Nucleic Acids Res. 1982;10:3591–3604. doi: 10.1093/nar/10.12.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weber K L, Bolander M E, Sarkar G. Rapid acquisition of unknown DNA sequence adjacent to a known segment by multiplex restriction site PCR. BioTechniques. 1998;25:415–419. doi: 10.2144/98253st02. [DOI] [PubMed] [Google Scholar]
- 29.Yu X, McBride J W, Zhang X, Walker D H. Characterization of the complete transcriptionally active Ehrlichia chaffeensis 28 kDa outer membrane protein multigene family. Gene. 2000;248:59–68. doi: 10.1016/s0378-1119(00)00147-5. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Hardham J M, Barbour A G, Norris S J. Antigenic variation in Lyme disease Borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell. 1997;89:275–285. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]
- 31.Zhi N, Ohashi N, Rikihisa Y. Multiple p44 genes encoding major outer membrane proteins are expressed in the human granulocytic ehrlichiosis agent. J Biol Chem. 1999;274:17828–17836. doi: 10.1074/jbc.274.25.17828. [DOI] [PubMed] [Google Scholar]
- 32.Zhi N, Ohashi N, Rikihisa Y, Horowitz H W, Wormser G P, Hechemy K. Cloning and expression of the 44-kilodalton major outer membrane protein gene of the human granulocytic ehrlichiosis agent and application of the recombinant protein to serodiagnosis. J Clin Microbiol. 1998;36:1666–1673. doi: 10.1128/jcm.36.6.1666-1673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]