Abstract
The colon has large capacities for K+ absorption and K+ secretion, but its role in maintaining K+ homeostasis is often overlooked. For many years, passive diffusion and/or solvent drag were thought to be the primary mechanisms for K+ absorption in human and animal colon. However, it is now clear that apical H+,K+-ATPase, in coordination with basolateral K+-Cl− cotransport and/or K+ and Cl− channels operating in parallel, mediate electroneutral K+ absorption in animal colon. We now know that K+ absorption in rat colon reflects ouabain-sensitive and ouabain-insensitive apical H+,K+-ATPase activities. Ouabain-insensitive and ouabain-sensitive H+,K+-ATPases are localized in surface and crypt cells, respectively. Colonic H+,K+-ATPase consists of α (HKCα) and β (HKCβ) subunits which, when coexpressed, exhibit ouabain-insensitive H+,K+-ATPase activity in HEK293 cells, while HKCα coexpressed with the gastric β-subunit exhibits ouabain-sensitive H+,K+-ATPase activity in Xenopus oocytes. Aldosterone enhances apical H+,K+-ATPase activity, HKCα specific mRNA and protein expression, and K+ absorption. Active K+ secretion, on the other hand, is mediated by apical K+ channels operating in a coordinated way with the basolateral Na+-K+−2Cl− cotransporter. Both Ca2+-activated intermediate conductance K+ (IK) and large conductance K+ (BK) channels are located in the apical membrane of colonic epithelia. IK channel-mediated K+ efflux provides the driving force for Cl− secretion, while BK channels mediate active (e.g., cAMP-activated) K+ secretion. BK channel expression and activity are increased in patients with end-stage renal disease and ulcerative colitis. This review summarizes the role of apical H+,K+-ATPase in K+ absorption, and apical BK channel function in K+ secretion in health and disease.
Introduction
Of the average total dietary K+ intake in humans (~90 mEq), 90% is absorbed and only 10% is excreted in the feces (44, 133). Like the kidney, the colon has capacities for both K+ absorption and K+ secretion. In vitro flux studies performed in guinea pig, rabbit and rat colon under voltage clamp conditions (that is, in the absence of passive driving forces) have demonstrated active K+ absorption (63, 116, 206), and a variety of physiological (127, 186), biochemical (45, 74, 101), and molecular (38, 40, 101) studies have shown that active K+ absorption is mediated by apical H+,K+-ATPase in animal colon. Although there is no physiological evidence to support active K+ absorption in human colon, amplification of cDNA fragment suggests that H+,K+-ATPase is expressed in this epithelium (132, 192).
Studies in healthy human and animal colon have also demonstrated active K+ secretion both in vivo and in vitro (8, 10, 53, 79, 114, 151), the magnitude of which is mainly dependent on the expression and activity of Ca2+-activated large conductance apical K+ (BK) channels (94, 156, 159, 176, 178, 209). Enhanced BK expression and active K+ secretion contribute to net K+ hypersecretion during colonic K+ adaptation in patients with end-stage renal disease (114, 152). Increased BK expression and enhanced net K+ secretion also occur in an animal model of colitis and in human ulcerative colitis (94, 158). This review discusses the role and regulation of H+,K+-ATPase-mediated colonic K+ absorption and BK channel-regulated colonic K+ secretion in health and disease.
Potassium Absorption
In healthy individuals, about 90% of the average dietary K+ intake (~90 mEq/day) is absorbed, with a fecal excretion of ~9 mEq/day (44). About 10 mEq K+/day passes from the ileum into the colon, colonic secretion accounting for an additional 5 mEq K+/day (133). Since metabolic studies indicate fecal losses of about 10 mEq K+/day, the human colon appears to absorb about 5 mEq K+/day. Thus, while human small intestine has the capacity to absorb K+, human colon has the capacity to both absorb and secrete K+ (Figure-1). Increasing dietary K+ intake results in only a modest change in fecal K+ excretion, which increases by 5 mEq/day for every 50 mEq increase in dietary K+ in healthy humans (3). In normal individuals, absorbed K+ is mainly excreted via the kidneys (~90 mEq/day), and decreasing the dietary K+ intake (to 9 mEq/day) reduced renal K+ excretion from 81 mEq/day to 16.7 mEq/day (91). Thus, renal K+ excretion mirrors dietary K+ absorption. However, when dietary K+ intake is restricted to zero, the obligatory renal and colonic K+ excretion average about 6.3 mEq/day and 3.6 mEq/day, respectively (182). Since there is no evidence for facilitated K+ absorption in human and animal small intestine, it has been suggested that K+ absorption reflects a passive driving force (so-called solvent drag) (3). As regards to colonic K+ absorption, some of the early studies involving in vivo perfusion did not measure transmucosal electrical gradients and employed a perfusing solution containing a single K+ concentration, making interpretation of the results difficult. However, perfusion of rat colon in vivo using solutions of varying K+ concentrations demonstrated that the K+ absorptive flux was proportional to the K+ concentration, and this flux occurred passively mainly via paracellular pathways, driven by the transmucosal electrical potential difference (PD) (13). Based on in vivo perfusion data from human jejunum and ileum (197) and the whole colon (49), the positive relationship between net K+ transport and luminal K+ concentration indicated that the rate of K+ absorption or secretion was directly proportional to luminal K+ concentration (3). In the colon, the regression line crossed zero at a luminal K+ concentration of 9.5mmol/L, and using the geometric mean of the proximal and distal colonic PD (21.1mV), the predicted Nernst equilibrium K+ concentration in colonic fluid was very similar to those obtained in human colon (18, 49). Based on these results, it was suggested that passive diffusion was the main mechanism of K+ absorption in animal and human colon, despite the paracellular pathway having a relatively low ionic conductance (51).
Figure 1: Role of normal human small intestine and colon in K+ absorption and K+ secretion.
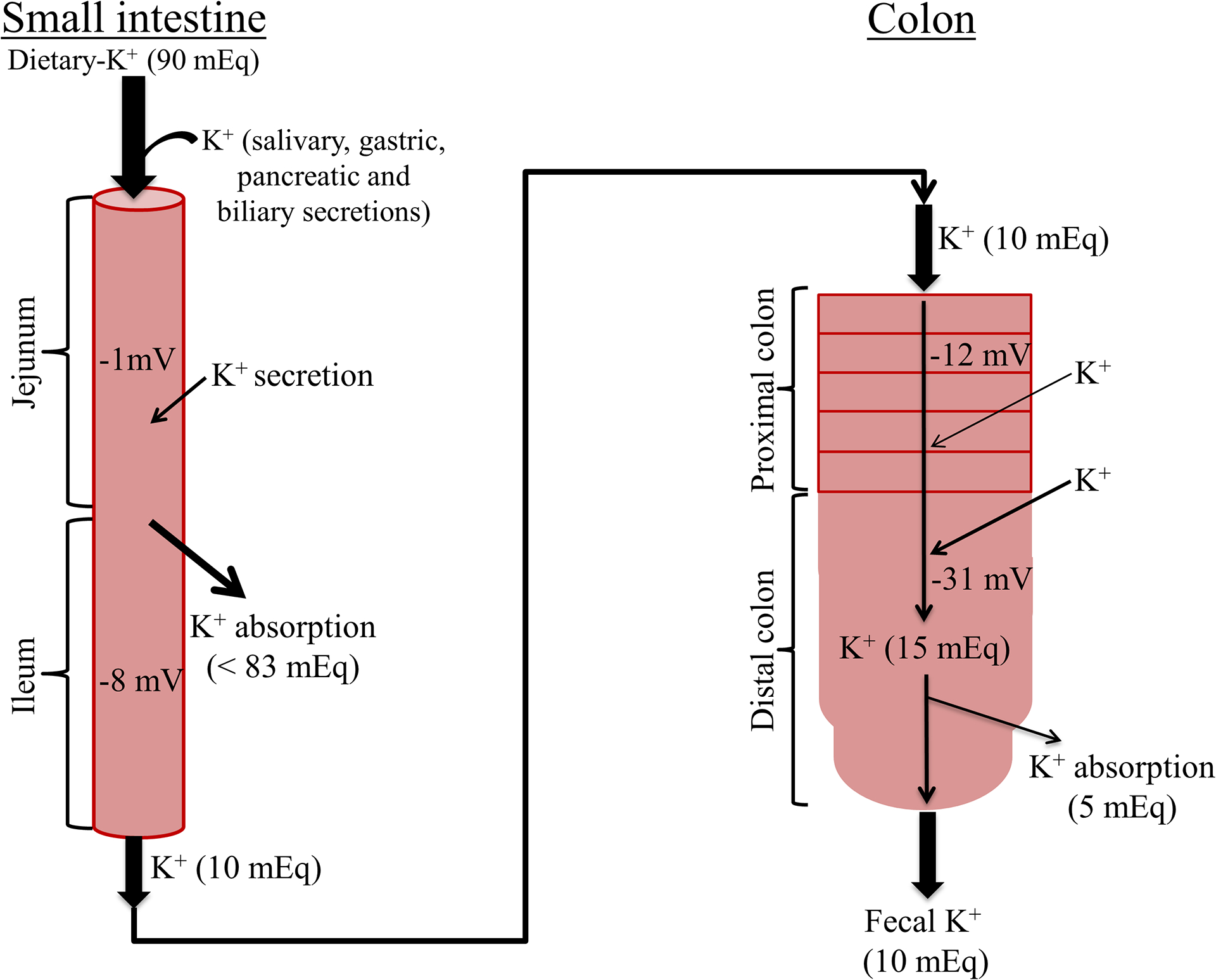
Normal dietary K+ intake is approximately 90 mEq/day. Salivary, gastric, pancreatic and intestinal secretions also contribute to the intestinal K+ content. Thus, approximately 83 mEq K+/day (i.e., 90%) is absorbed in the small intestine, and only 10 mEq K+/day (i.e., ~10%) enters the colon. The colon secretes and absorbs 5 mEq K+/day, and thus fecal K+ excretion is 10 mEq/day. Small intestinal K+ absorption occurs secondary to passive driving forces, such as solvent drag and/or membrane electrical potential. Proximal colon secretes K+, while K+ absorption occurs in distal colon. Average lumen-negative electrical potentials are −1, −8, −12 and −31 mV in the jejunum, ileum, proximal colon and distal colon, respectively (42). Outward and inward arrows indicate K+ absorption and K+ secretion, respectively. Thickness of arrows represent relative rate of absorption and secretion.
Nevertheless, despite the apparent lack of physiological evidence for a mechanism for active K+ absorption in the human colon, K+ flux studies have demonstrated active K+ absorption in guinea pig, rabbit and rat distal colon (52, 63, 66, 76, 116, 134, 142, 185, 206). Under voltage clamp conditions, active K+ secretion (see below) and active K+ absorption in animal colon occur in the proximal and distal segments, respectively (63, 183). Furthermore, both active K+ absorption and active K+ secretion are modulated by cAMP, dietary K+ enhancement, dietary K+ restriction, and dietary Na+ restriction (the latter eliciting marked secondary hyperaldosteronism) (64–66, 77, 142). Active K+ transport (i.e., net absorption and net secretion) are measured in sheets of colonic epithelium maintained under voltage clamp conditions in Ussing chambers. Mucosal to serosal (m-s) and serosal to mucosal (s-m) unidirectional K+ fluxes are measured and net K+ fluxes calculated by subtracting s-m unidirectional fluxes from the m-s unidirectional fluxes. Positive and negative net fluxes therefore represent active K+ absorption and secretion, respectively.
Physiological evidence for active K+ absorption in animal colon
Active K+ absorption reflects K+ uptake across apical membranes and K+ exit across basolateral membranes of epithelial cells in animal colon (Figure-2). Although metabolic studies have shown the presence of K+ absorption, a facilitated transporter has not yet been identified in human colon (3). In contrast, an ATP-driven H+-K+ exchange (i.e., H,+-K+-ATPase) has been shown to mediate K+ uptake across apical membranes in animal colon (127). Either a K+ channel that operates in parallel with a Cl− channel (Figure-2A) or a K+-Cl− cotransporter (KCC) (Figure-2B) may mediate K+ exit through the basolateral membrane in animal colon (21, 45, 76, 162). Colonic H+,K+-ATPase has been identified and characterized using physiological, biochemical, pharmacological and molecular techniques (1, 21, 62, 63, 74, 76, 186). In vitro studies based on unidirectional 42K+ flux measurements under voltage clamp conditions showed that active K+ absorption is electroneutral and Na+-independent, Na+ removal decreasing the short-circuit current (Isc) to zero. However, this electroneutral K+ absorptive process was only partially inhibited under Cl−-free conditions, leading to the suggestion that electroneutral K+ absorption may be mediated by apical K+-H+ exchange (63). Other studies also pointed to the presence of K+-dependent proton (H+) secretion in guinea pig distal colon (185, 186), and the demonstration of outward proton gradientdriven K+ uptake has been interpreted as evidence for K+-H+ exchange in apical membrane vesicles isolated from rat ileum (19). Additional investigations revealed that electroneutral K+ uptake is mediated by an apical ATPase (63, 76, 128, 134). Studies of unidirectional K+ fluxes in normal and dietary Na+ depleted (to produce secondary hyperaldosteronism) rat distal colon demonstrated that Na+ removal from the mucosal and serosal bathing solutions resulted in increased active K+ absorption via an apical K+ uptake mechanism that was: 1) carrier-mediated, as increasing mucosal K+ concentration saturated K+ absorption; 2) competitively inhibited by mucosal Na+; 3) inhibited by mucosal orthovanadate (VO4, a P-type ATPase inhibitor); 4) inhibited partially by mucosal ouabain (a Na+,K+-ATPase inhibitor); and 5) not inhibited by mucosal omeprazole and SCH28080 (gastric H+,K+-ATPase inhibitors) (43). Based on these observations, Sweiry and Binder concluded that apical K+ uptake was mediated by a H+,K+-ATPase that had properties which were unlike those of gastric H+,K+-ATPase, but similar in part to those of Na+,K+-ATPase (190). Further studies in rat distal colon demonstrated that mucosal ouabain completely inhibited active K+ absorption measured in the presence Na+, but only partially inhibited K+ upake in the absence of Na+ (127). These observations suggested that two distinct H+,K+-ATPases mediate apical K+ absorption – one that is ouabain-sensitive (which is also Na+-insensitive), while the other is ouabain-insensitive (which is also Na+-sensitive) (127). This view is consistent with the results from studies in guinea pig distal colon, which demonstrated ouabain-sensitive K+-dependent proton (H+) secretion measured using the pH-stat technique (185, 186). Although these animal studies provide compelling evidence that electroneutral K+ absorption reflects a carrier-mediated processes which is most likely ATP-dependent (i.e., via H+,K+-ATPase), the mechanism of K+ absorption in human colon remains unclear.
Figure 2: Cellular models of electroneutral K+ absorption in animal (guinea pig, mouse and rat) distal colon.
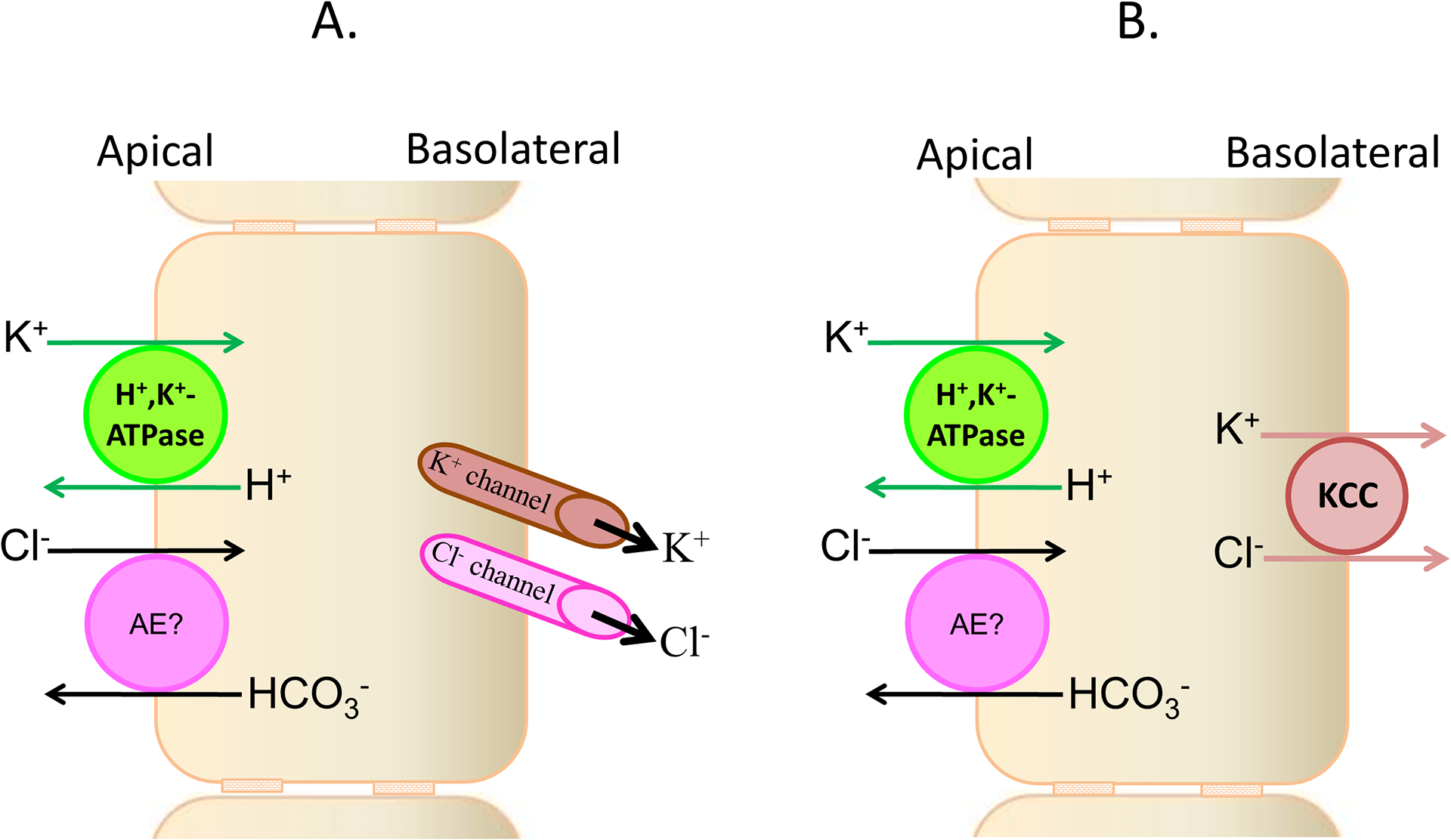
Apical H+,K+-ATPase, and either basolateral parallel exit of K+ and Cl− through respective channels [A] or K+-Cl− cotransport (KCC) [B], mediate electroneutral K+ absorption in animal colon. H+,K+-ATPase-mediated K+ absorption is partially Cl−-dependent. It is not known whether anion exchanger-mediated Cl−-HCO3− exchange plays a role in Cl−-dependent K+ absorption.
Biochemical evidence for colonic H+,K+-ATPase in animal colon
Physiological studies have provided evidence that VO4-sensitive P-type ATPase mediates electroneutral K+ absorption across the mucosal membranes of rat colon (190). Other work demonstrated ATPase activities in epithelial cells which are distinct from Na+,K+-ATPase in animal distal colon (1, 45, 74, 96, 186). Gustin and Goodman identified ouabain-insensitive K+-activated ATPase in apical membranes , while Abrahamse et al demonstrated ouabain-insensitive and SCH28080-sensitive K+-activated ATPases in rabbit distal colon (1, 74). Rabbit distal colon also exhibits two pharmacologically distinct H+-activated ATPase activities – one which is N-ethylmaleimide (NEM)-sensitive and VO4-insensitive, and a second which is NEM-insensitive and VO4-sensitive (96). However, the membrane localization of these two H+-activated ATPases has not been established (96). Suzuki and Kaneko demonstrated ouabain-sensitive 86Rb (K+ surrogate) absorption and mucosal K+-dependent H+ secretion in guinea pig distal colon, consistent with apical membrane H+,K+-ATPase-mediated K+ absorption (185, 186). Del Castillo et al showed that K+-activated ATPase (i.e., H+,K+-ATPase) activities were present in apical, but not in basolateral membranes isolated from rat distal colon (45). In keeping with oubain-sensitive and ouabain-insensitive K+ absorption (127), apical membranes enriched 10-fold with respect to H+,K+-ATPase activity exhibited both ouabain-sensitive (~65%) and ouabain-insensitive (~35%) components (45). Both oubain-sensitive and ouabain-insensitive H+,K+-ATPase activities were inhibited by VO4, but not by omeprazole and SCH2808 (both gastric H+,K+-ATPase inhibitors). Furthermore, isolated apical membranes were enriched 4-fold with respect to Na+,K+-ATPase activity, which was completely inhibited by ouabain. Based on these findings, it was concluded that ouabain-sensitive Na+,K+-ATPase in isolated apical membranes might reflect cross-contamination from basolateral membranes. However, Rajendran et al demonstrated that apical H+,K+-ATPase, in the presence of Na+ and K+ functioned as Na+,K+-ATPase (139). In addition, M1-antibody [a polyclonal antibody raised against a fusion peptide designed from the putative colonic H+,K+-ATPase α-subunit (40, 101)] inhibited H+,K+-ATPase activity, and also inhibited apical but not basolateral Na+,K+-ATPase activity (139), which suggested that apical Na+,K+-ATPase activity was an alternative mode of H+,K+-ATPase activity (139). Based on these observations, it was speculated that colonic apical H+,K+-ATPase, which characteristically differs from basolateral Na+,K+-ATPase and gastric H+,K+-ATPase, may represent a unique and distinct type of ATPase that mediates active K+ absorption in animal colon, and that this novel H+,K+-ATPase has both ouabain-sensitive and ouabain-insensitive components.
Molecular identification of colonic H+,K+-ATPase
Active K+ absorption, mediated by apical H+,K+-ATPase, is a unique function of animal distal colon (45, 63, 76, 128, 139, 186). Since it is inhibited by VO4, colonic H+,K+-ATPase is also a P-type ATPases, and a member of the gene family that includes gastric H+,K+-ATPase and Na+,K+-ATPase (172, 173). P-type ATPases are in general heterodimers, which consist of α- and β-subunits. The α-subunit catalyzes ATP and transports cations (Na+, K+, NH4+ and H+), while the β-subunit regulates structural and functional aspects of the P-type ATPases (69, 72, 90, 117, 188). Molecular studies have cloned the colon-specific H+,K+-ATPase α (HKCα) and β (HKCβ) subunits from rat distal colon (35, 40, 163). Coexpression of these putative HKCα and HKCβ subunit cDNAs in HEK293 cells results in ouabain-insensitive H+,K+-ATPase activity (165). These results indicate that the cloned HKCα and HKCβ subunits encode ouabain-insensitive H+,K+-ATPase present in the apical membranes of the surface cells in rat distal colon.
Molecular cloning of the HKCα subunit
Based on the prediction that colonic H+,K+-ATPase is a P-type ATPase (45, 190), Crowson and Shull used a cDNA probe designed from the human gastric H+,K+-ATPase α-subunit (HKGα; known as ATP4A) gene to screen for and isolate the putative HKCα cDNA which encodes a 1036 deduced amino acid protein. Using the putative HKCα cDNA to probe northern blots, HKCα-specific mRNA was shown to be expressed mainly in surface cells of distal but not proximal colon (40). The HKCα is also known as nongastric K+ transporting ATPase α2 (HKα2), ATP1AL1 and Atp12A (131, 187). HKCα orthologs have also been cloned from guinea pig colon (9), rabbit kidney (57), toad bladder (89), and the human genome (73). These HKCα orthologs exhibited 75 – 85% amino acid identity with each other, and shared 63.3% and 62.9 − 63.6% amino acid identities with the gastric HKGα and Na+,K+-ATPase α-subunit (NaKα1), respectively (40). When expressed in vitro, HKCα exhibited both ouabain-sensitive and/or ouabain-insensitive function (37, 38, 101). In addition to K+ and H+, the in vitro expressed HKCα also showed affinity for Na+ and NH4+ (Table-1) (72, 189). The observation that HKCα was expressed in surface (i.e., absorptive) cells of distal but not proximal colon, suggested that HKCα might mediate electroneutral K+ absorption in animal distal colon.
Table-1: Functional expression of HKCα cDNA in heterologous expression systems.
Functional activity of the putative colonic H+-K+-ATPase α-subunit (HKCα) was determined in vitro using heterologus expression systems. The baculovirus-Sf9 and HEK293, and Xenopus oocyte expression systems, utilized H+,K+-ATPase and 86Rb uptake measurements, respectively. Individually expressed HKCα exhibited ouabain-insenstivie H+,K+-ATPase activity in the Baculovirus-Sf9 expression system (101). cRNA transcribed from HKCα, plus either HKGβ or NaKβ1, exhibited both partial ouabain-sensitive and partial ouabain-insensitive 86Rb uptake in Xenopus oocytes (37). HKCα and HKGβ (and torpedo NaKβ1) coexpression exhibited ouabain-sensitive H+,K+-ATPase (9), while HKCα coexpressed with HKCβ exhibited ouabain-insensitive H+,K+-ATPase activities in HEK293 cells (165). Human HKCα coexpressed with NaKβ1 in baculovirus-Sf9 exhibited ouabain-sensitive H+,NH4+-ATPase activity (188, 189). HKCβ – Colonic H+,K+-ATPase β-subunit; HKGβ – Gastric H+,K+-ATPase β-subunit; NaKβ – Na+,K+-ATPase β-subunit.
| HKCα | β-subunit | Expression system | Function | Ouabain | Reference | |
|---|---|---|---|---|---|---|
| Sensitive | Insensitive | |||||
| Rat | none | Baculovirus-Sf9 | K+-ATPase | - | Yes | (101) |
| Rat | HKGβ | Xenopus oocytes | 86Rb uptake | Yes | Yes | (37) |
| Rat | NaKβ1 | Xenopus oocytes | 86Rb uptake | Yes | Yes | (37) |
| Rat | TBβ | Xenopus oocytes | 86Rb uptake | Yes | - | (38) |
| Guinea pig | HKGβ | HEK-293 cell | H+,K+-ATPase | Yes | - | (9) |
| Guinea pig | Torpedo NaKβ | HEK-293 cell | H+,K+-ATPase | Yes | - | (9) |
| Rat | HKCβ | HEK-293 cell | H+,K+-ATPase | - | Yes | (165) |
| Human | NaKβ1 | Baculovirus-Sf9 | NH4+-ATPase | Yes | - | (188, 189) |
In contrast to animal colon, facilitated K+ absorption and H+,K+-ATPase activities have yet to be indentified in human colon. However, amplification of the ATP1AL1 gene-specific cDNA fragment suggests the presence of an H+,K+-ATPase-like transporter in human colon (132, 192). It is possible that human colonic H+,K+-ATPase has different characteristics, as H+,K+-ATPase-mediated intracellular pH (pHi) recovery was inhibited by the gastric H+,K+-ATPase inhibitor SCH28080 in CaCo2 cells (a human colorectal adenocarcinoma cell line). Colonic H+,K+-ATPase inhibition may therefore contribute to the diarrhea which occurs in some patients treated with proton pump inhibitors (2, 171). However, to establish the presence of facilitated K+ absorption in human colon, additional molecular and functional studies of the human HKCα ortholog and native H+,K+-ATPase are required.
Molecular cloning of the HKCβ subunit
Although P-type ATPases comprise α- and β-subunits, the putative HKCα expressed alone in the baculovirus-Sf9 (Spodoptera frugiperda) cell expression system exhibited H+,K+-ATPase activity (101). However, the β-subunit appears a prerequisite for full HKCα function, as HKCα expressed alone in Xenopus oocytes did not exhibit H+,K+-ATPase activity when assessed by 86Rb uptake (37, 38). By contrast, there was 86Rb uptake by oocytes coinjected with cRNA transcribed from HKCα and either toad bladder β (TBβ), gastric H+,K+-ATPase β (HKGβ), or Na+,K+-ATPase β1 (NaKβ1) subunits (37, 38). Similarly, guinea pig HKCα expressed in HEK293 cells exhibited H+,K+-ATPase activity in the presence of either HKGβ or NaKβ, but not in the absence of a β subunit (9). Thus, the β-subunit is critical for HKCα function and Codina et al showed by co-immunoprecipitation that HKCα coassembles with the NaKβ1-subunit in rat distal colon (36). This is surprising, given that NaKβ1 co-assembles with NaKα1 in epithelial cell basolateral membranes (11, 23). Sangan et al probed a rat colon cDNA library using astrocytoma β-subunit cDNA, which strongly hybridized with rat colonic mRNA, and isolated a putative cDNA (HKCβ) that encoded a 279 deduced amino acid protein (163). The cloned HKCβ had 80% amino acid homology with human NaKβ3, which is expressed in colon (112, 163). Polyclonal antibody raised against HKCβ that localized proteins in the apical membranes co-immunoprecipitated HKCα, while M1 antibody co-immunoprecipitated the HKCβ protein from epithelial cell homogenate of rat distal colon (163, 165). These observations clearly establish that a β-subunit is critical for the functional expression of HKCα, and that the putative HKCβ might compliment HKCα in the rat distal colon.
Functional expression of HKCα and HKCβ subunits
Depending upon the co-expressed β-subunit (i.e., HKCβ, HKGβ or NaKβ1), the putative HKCα exhibits either ouabain-sensitive and/or ouabain-insenstive function (Table-1). The functionality of HKCα in in vitro expression systems (i.e., Xenopus oocytes, Sf9 and HEK293 cells) has been evaluated by measuring 86Rb uptake or H+,K+-ATPase activity (9, 37, 38, 101, 165). HKCα co-expressed with TBβ also exhibited ouabain-sensitive 86Rb uptake (38), but exhibited only partial ouabain-sensitive 86Rb uptake when co-expressed with either HKGβ or NaKβ1 in Xenopus oocytes (37). Lee et al showed that when HKCα was expressed alone in the Sf9 expression system, it exhibited ouabain-insensitive H+,K+-ATPase activity (101). They also used a polyclonal antibody (M1) raised against a HKCα fushion protein to localize a protein to the plasma membranes of HKCα-transfected but not untransfected Sf9 cells (101). The M1-antibody also localized proteins in surface but not crypt cells, and detected a 100 kD protein in mucosal membranes of rat distal but not proximal colon (101). Furthermore, the M1-antibody inhibited expressed (i.e., Sf9) and native (i,e., apical membrane) H+,K+-ATPase activities, but not basolateral Na+,K+-ATPase activity in rat distal colon (101, 139). Thus, these results established that HKCα protein with H+,K+-ATPase activity could be expressed in the plasma membrane of Sf9 cells. By contrast, Asano et al showed that transfection with guinea pig HKCα alone failed to express HKCα protein in the plasma membrane of HEK293 cells, but co-expression with either rabbit HKGβ or Torpedo NaKβ subunit resulted in the expression of plasma membrane HKCα protein with ouabain-sensitive H+,K+-ATPase activity (9). Similarly, Sangan et al showed that when expressed individually, neither HKCα nor HCKβ protein was delivered to the plasma membranes, while both proteins were incorporated into membranes when HKCα and HKCβ were co-expressed in HEK293 cells (165). Although guinea pig HKCα co-expressed with either rabbit HKGβ or Torpedo NaKβ (9) leads to the expression of HKCα protein with ouabain-sensitive H+,K+-ATPase activity, rat HKCα/HKCβ expressed in HEK293 cells exhibited ouabain-insensitive H+,K+-ATPase activity (165). Thus, while HKCβ is the complementary β-subunit for HKCα, HKCα could co-assemble with different β-subunits (e.g., NaKβ1 and HKGβ) to form H+,K+-ATPase proteins with different ouabain sensitivities.
Distribution of ouabain-sensitive and ouabain-insensitive H+,K+-ATPase along surface-crypt cell axis in rat distal colon
Physiological and biochemical studies have shown that both ouabain-sensitive and ouabain-insensitive H+,K+-ATPases are present in the apical membranes that mediate ouabain-sensitive and ouabain-insensitive electroneutral K+ absorption in rat distal colon, respectively (45, 63, 127, 190). However, molecular studies have cloned only HKCα that exhibit either ouabain-insensitive and/or ouabain-sensitive function in different in vitro expresssion systems (9, 37, 40, 165). As previously discussed, the rat HKCα expressed alone in Sf9 cells, or coexpressed with colonic HKCβ-subunt in HEK-293 cells, exhibited ouabain-insensitive H+,K+-ATPase activity (101, 165), while HKCα coexpressed with either NaKβ1 or HKGβ exhibited ouabain-insensitive and partially ouabain-sensitive 86Rb uptake in Xenopus oocytes (9, 37, 38). Further studies of H+,K+-ATPase activity in apical membranes showed that ouabain-sensitive H+,K+-ATPase is present in crypt cells, while other studies evaluating extracellular K+-dependent intracellular pH (pHi) recovery from an acid load established that ouabain-insensitive and ouabain-sensitive H+,K+-ATPase are localized in the apical surface cells and crypt cells of rat distal colon, respectively (Figure-3) (140). In addition, aldosterone upregulated HKCα-specific mRNA abundance and protein expression, and ouabain-insensitive K+ absorption and H+,K+-ATPase activity in rat distal colon (164, 190). The surface cell specific localization of ouabain-insensitive H+,K+-ATPase is in agreement with physiological observations demonstrating increased ouabain-insensitive K+ absorption in aldosterone-treated rat distal colon (127, 190). Thus, although both ouabain-sensitive and ouabain-insensitive H+,K+-ATPases and K+ absorption are present, only HKCα that encodes ouabain-insensitive H+,K+-ATPase has been cloned, while the molecular identity of the ouabain-sensitive H+,K+-ATPase isoform has yet to be identified. Despite H+,K+-ATPase having been established as the mechanism for active K+ absorption in animals, the mechanism for K+ absorption in human colon in unknown, and warrants further study.
Figure-3: Distribution of ouabain-insensitive and ouabain-sensitive H+,K+-ATPase along the surface to crypt cell axis in rat distal colon.
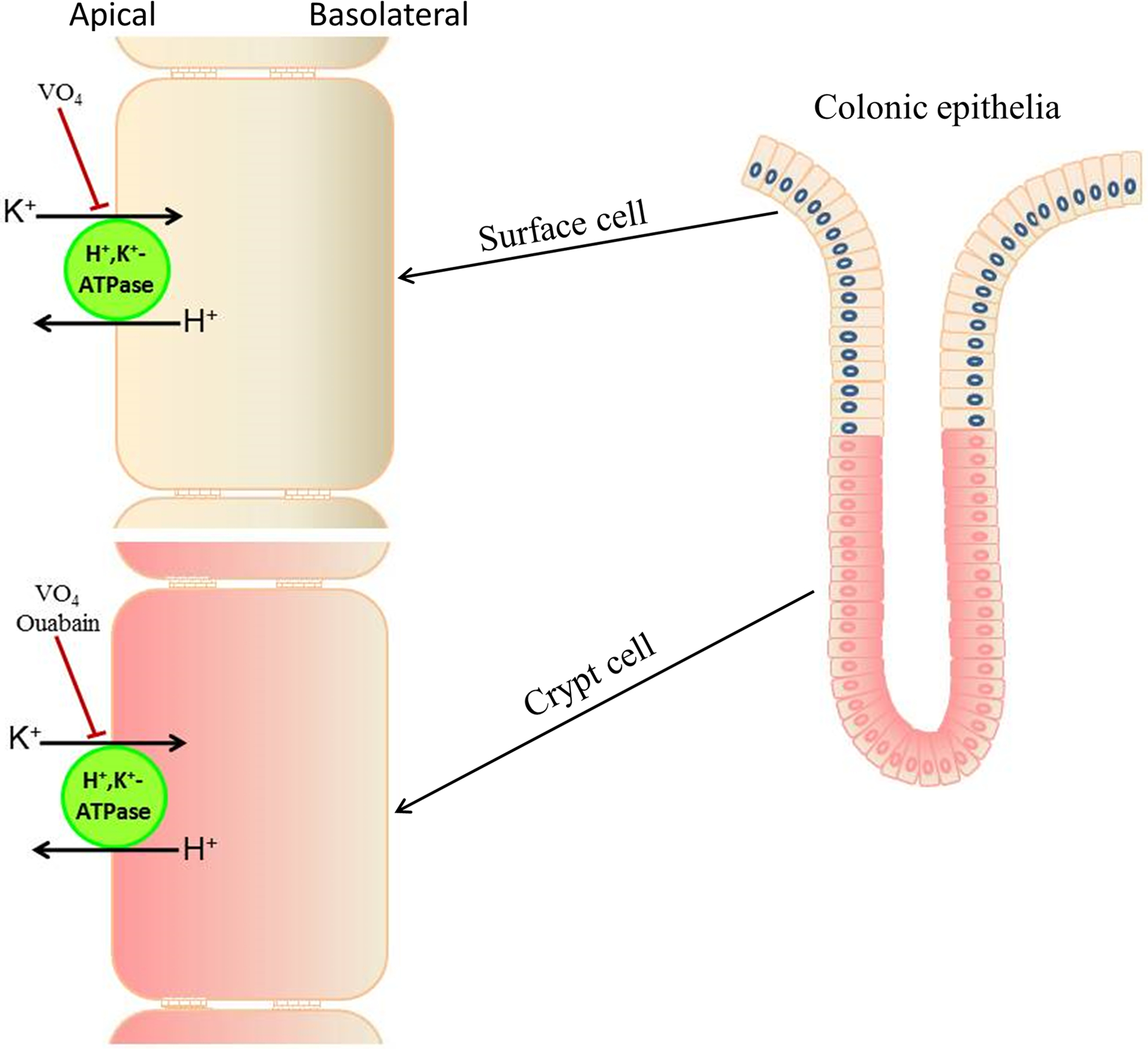
Ouabain-insensitive H+,K+-ATPase is localized in surface cells and upper one-third of matured crypt cells (yellow colored cells), while ouabain-insensitive H+,K+-ATPase is localized mainly to crypt cells (red colored cells). Vanadate (VO4, H+,K+-ATPase inhibitor) inhibits both ouabain-insensitive and ouabain-sensitive H+,K+-ATPase.
Mechanism of K+ exit across the basolateral membrane
Active K+ absorption requires coordinated regulation of K+ uptake and K+ exit across apical and basolateral membranes of epithelial cells, respectively. As discussed, H+,K+-ATPase mediates K+ uptake across apical membranes. Potassium (K+) channels have been suggested as the route for K+ exit across basolateral membranes, as Ba2+ (nonspecific K+ channel blocker) inhibited mucosal to serosal electroneutral K+ fluxes in turtle and rabbit colon (75, 76). Since K+ absorption is electroneutral, the negative membrane potential generated by basolateral K+ exit should be compensated by the exit of an anion (e.g., Cl−) and/or the influx of a cation (e.g., Na+, H+). Ca2+-activated and cAMP-activated K+ channels have been identified in the basolateral membranes of rat and human colon (118, 169). However, Sweiry and Binder suggested that basolateral K+ channels are not involved in electroneutral K+ absorption in rat distal colon, as the serosal addition of K+ channel blockers (TEA, Ba2+, and Cs+) did not inhibit electroneutral K+ absorption in hyperaldosterone-treated rat distal colon (190). In a subsequent study by Sangan et al, northern and western blot analyses identified KCC1-specific mRNA and protein expression in rat distal colon, and KCC1-specific mRNA abundance and protein expression were increased in dietary-K+, but not dietary-Na+ depleted rat distal colon, which suggested that the KCl cotransporter-1 (KCC1) might mediate electroneutral K+ exit across the basolateral membrane in rat distal colon (162). However, despite the evidence that KCC1 mediates basolateral K+ exit during active K+ absorption in rat distal colon, further studies are required to investigate possible species differences in the relative roles of KCC1 and K+ channels.
Role of colonic H+,K+-ATPase
Although ouabain-sensitive and ouabain-insensitive H+,K+-ATPases have been characterized, only HKCα has been cloned, which exhibits both ouabain-sensitive and ouabain-insensitive H+,K+-ATPase function, depending upon different β-subunits (HKCβ, HKGβ or NaKβ) that are coexpressed in vitro (9, 21, 37, 38). Mice lacking HKCα (HKCα−/−) fed a regular (i.e., K+-containing) diet were shown to have fecal K+ losses twice those of normal mice, but nevertheless maintained normal body K+ homostasis (121). However, HKCα−/− mice fed a K+-deprived diet had low intracellular and plasma K+ levels, and lost twice their body weight compared with normal animals (121). Hyperaldosteronism induced by dietary Na+ depletion also increased fecal K+ losses in HKCα−/− mice. In addition, K+ recycled through colonic H+,K+-ATPase has been shown to play critical role in epithelial Na+ channel (ENaC)-mediated Na+ absorption, which is significantly reduced in HKCα−/− mouse colon (181). Thus, it is likely that colonic H+,K+-ATPase may be important for both K+ conservation and ENaC-mediated Na+ absorpion.
Regulation of colonic K+ absorption and H+,K+-ATPase
Hyperaldosteronism secondary to dietary Na+ depletion or dietary K+ loading regulates both active K+ absorption and H+,K+-ATPase activity in rat distal colon (63, 66, 88, 164, 190). Enhanced K+ absorption in response to dietary Na+ depletion is mediated by aldosterone, since aldosterone infused via osmotic mini-pumps and dietary Na+ depletion had similar effects on K+ absorption. Furthermore, both dietary Na+ depletion and aldosterone infusion induce active K+ secretion (191). However, inhibition of the active K+ secretion by serosal bumetanide [Na+-K+−2Cl− cotransport (NKCC) inhibitor] unmasked aldosterone-enhanced active K+ absorption in rat distal colon (190, 191). Thus, aldosterone stimulates both active K+ absorption and active K+ secretion (Figure-4) (64, 127, 190). Bearing in mind that active K+ absorption has both ouabain-sensitive and ouabain-insensitive components (190), it is interesting that aldosterone upregulated ouabain-insensitive K+ absorption, and HKCα-specific mRNA abundance and protein expression, as well as ouabain-insensitive H+,K+-ATPase activity, in rat distal colon (164, 190), although aldosterone had no effect on HKCβ-specific mRNA abundance or protein expression in this epithelium (163). By contrast, aldosterone did not alter ouabain-sensitive K+ absorption and H+,K+-ATPase activity (127, 164, 190). Although active K+ absorption is not present in normal rat proximal colon, aldosterone upregulated HKCα-specific mRNA abundance and protein expression (164). Further studies are necessary to identify whether the enhanced HKCα mRNA and protein expression are also associated with increased active K+ absorption in aldosterone-treated rat proximal colon.
Figure-4: Effect of Na+-free diet (aldosterone) on active K+ absorption and active K+ secretion in rat colon.
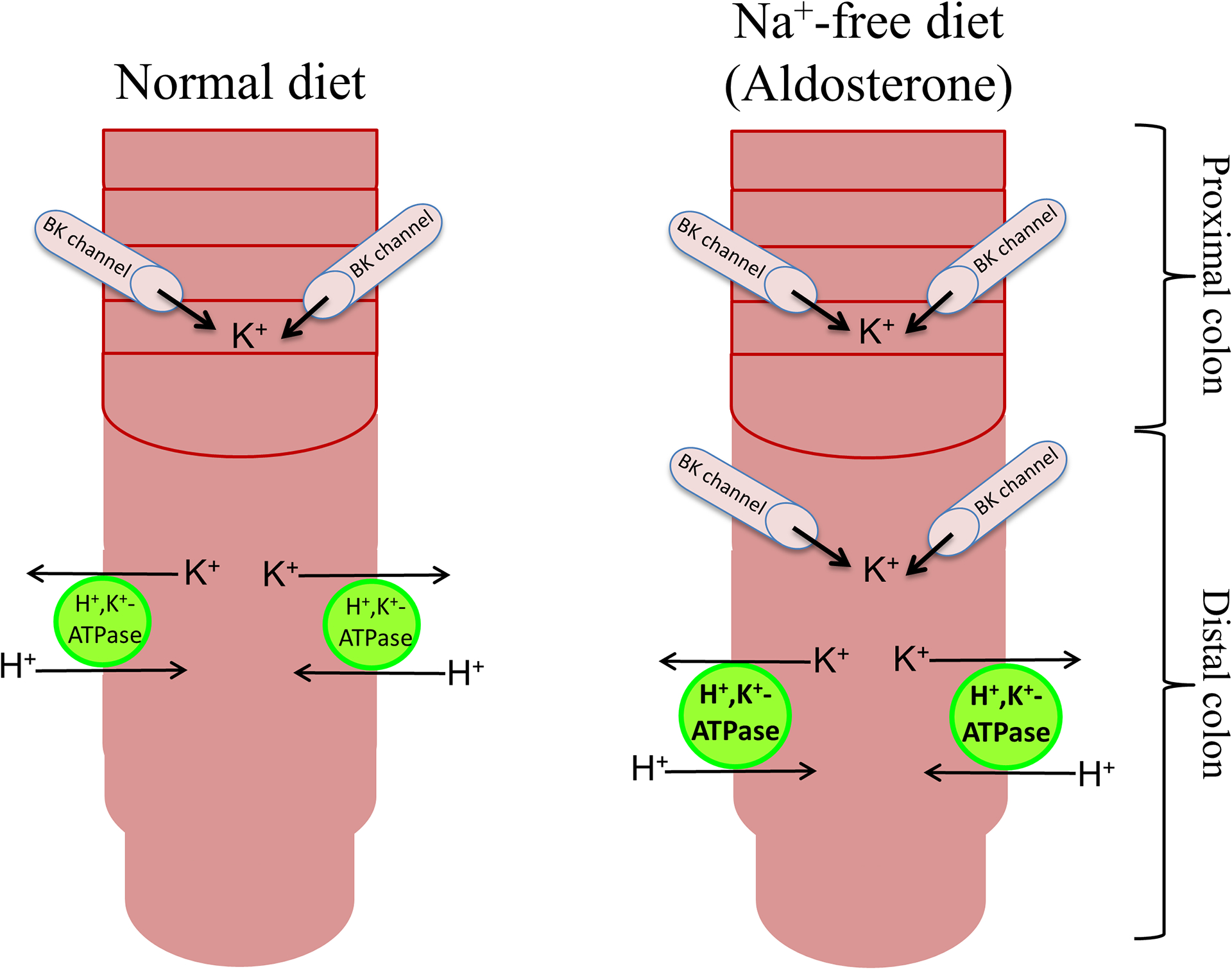
Active K+ secretion and active K+ absorption are present in proximal (lined segment) and distal segments of normal rat colon, respectively. Active K+ absorption is mediated by H+,K+-ATPase, while active K+ secretion is mediated via large conductance K+ (BK) channels. Dietary Na+ depletion induces active K+ secretion in distal colon. Dietary Na+ depletion also stimulates H+,K+-ATPase-mediated active K+ absorption in distal colon. Dietary Na+ depletion enhances BK channel and H+,K+-ATPase-specific protein expression in distal colon. It is not known whether dietary Na+ depletion also stimulates active K+ secretion in proximal colon.
Dietary K+ modulation and aldosterone regulate active K+ absorption and H+,K+-ATPAse by different mechanisms (64, 66, 163, 164). The model of dietary K+ depletion has provided evidence that active K+ absorption is mediated by two different transport processes – one Cl−-dependent, the other Cl−-independent (66). Removal of mucosal Cl− demonstrated that dietary K+ depletion stimulated Cl−-dependent, but not Cl−-independent active K+ absorption, since substitution of Cl− completely inhibited active K+ absorption stimulated by K+ depletion, but did not affect Cl−-independent K+ absorption (66). The effect of Cl− substitution on active K+ absorption stimulated by aldosterone, and on the effect of ouabain on dietary K+ depletion-stimulated active K+ absorption, have yet to be determined. Thus, it is difficult to be certain whether dietary K+ depletion-stimulated active K+ absorption is mediated by ouabain-sensitive or ouabain-insensitive H+,K+-ATPase. Similarly, it is also unclear whether aldosterone-stimulated ouabain-insensitive active K+ absorption is mediated by a Cl−-dependent or Cl−-independent process. However, whereas aldosterone has been shown to stimulate active K+ absorption by enhancing HKCα-specific mRNA abundance and protein expression, dietary K+ depletion stimulated active K+ absorption by enhancing HKCβ-specific mRNA abundance and protein expression, but had no effect on HKCα-specific mRNA abundance and protein expression (163, 164). Thus, aldosterone and dietary K+ depletion stimulate active K+ absorption by enhancing the expression of HKCα and HKCβ, respectively.
Potassium Secretion
Active K+ secretion requires K+ uptake and exit across the basolateral and apical membranes of epithelial cells, respectively (Figure-5). Basolateral K+ uptake is mediated by both Na+,K+-ATPase and NKCC, while K+ exit across the apical membrane is K+ channel-mediated (151, 156, 157). Na+ and Cl− entering cells via NKCC exit across the basolateral membrane through Na+,K+-ATPase and Cl− channels, respectively. In addition to facilitating K+ exit, basolateral K+ channels also help to regulate several important physiological functions (58, 86). Under basal conditions, intracellular K+ recycles via basolateral K+ channels to be delivered to Na+,K+-ATPase (i.e., Na+-pump), a critical active transporter which catalyzes the cellular egress of 3 Na+ ions in exchange for 2 K+ ions entering the cells. By maintaining Na+,K+-ATPase activity, the basolateral K+ channels help maintain low intracellular Na+ and high intracellular K+ concentrations, resulting in an electrochemical gradient that generates a negative (~ −50 mV) membrane potential. This electrochemical gradient provides the driving force for secondary active transporters, such as electroneutral (i.e., Na+-H+ exchange) and electrogenic (i.e., epithelial Na+ channel, ENaC-mediated) Na+ absorptive processes present in the apical membranes of colonic epithelial cells (Figure-6A) (33). In addition to maintaining membrane potential, basolateral K+ channels also help maintain basolateral Na+-K+−2Cl− cotransporter (Cl− loader) activity, which mediates Cl− uptake into cells during active Cl− secretion (e.g., Cl−-driven secretory diarrhea), Cl− exiting from cells via apical CFTR Cl− channels in the colon (Figure-6B) (20, 32, 58). It should be noted that although apical H+-K+-ATPase and apical K+ channels are present in animal colon, channel-mediated K+ secretion does not appear to be linked to H+,K+-ATPase-mediated K+ absorption, but when upregulated makes a major contribution to K+ homeostasis (86, 99).
Figure-5: Cellular model of active K+ secretion.
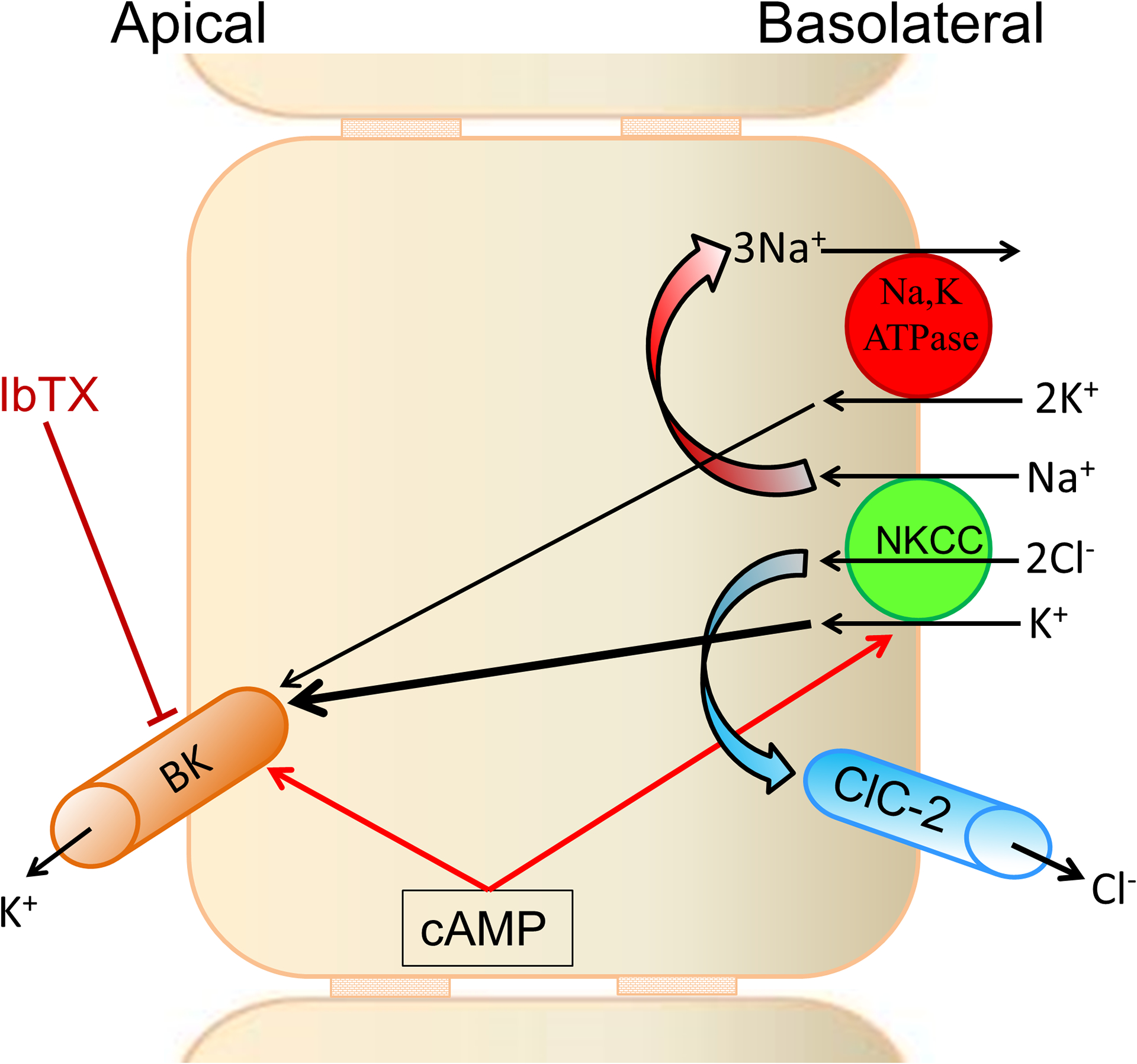
Coordinated activation of apical large conductance K+ (BK) channels, and basolateral Na+-K+−2Cl− cotransporter (NKCC) and Cl− channel 2 (ClC2) regulates active K+ secretion. K+ entering cells via basolateral NKCC (K+ loader) exits via apical BK channels. K+ entering via Na+,K+-ATPase also contributes to apical BK channel-mediated K+ secretion. Continuous K+ secretion depends on CLC2 and Na+,K+-ATPase maintaining low intracellular levels of Cl− and Na+, respectively. cAMP activates both basolateral NKCC and apical BK channel. Mucosal iberiotoxin (IbTX) inhibits active K+ secretion. [Reproduced from (159)].
Figure-6: Colonic epithelial cell models for basolateral membrane K+ channel-regulated transport processes.
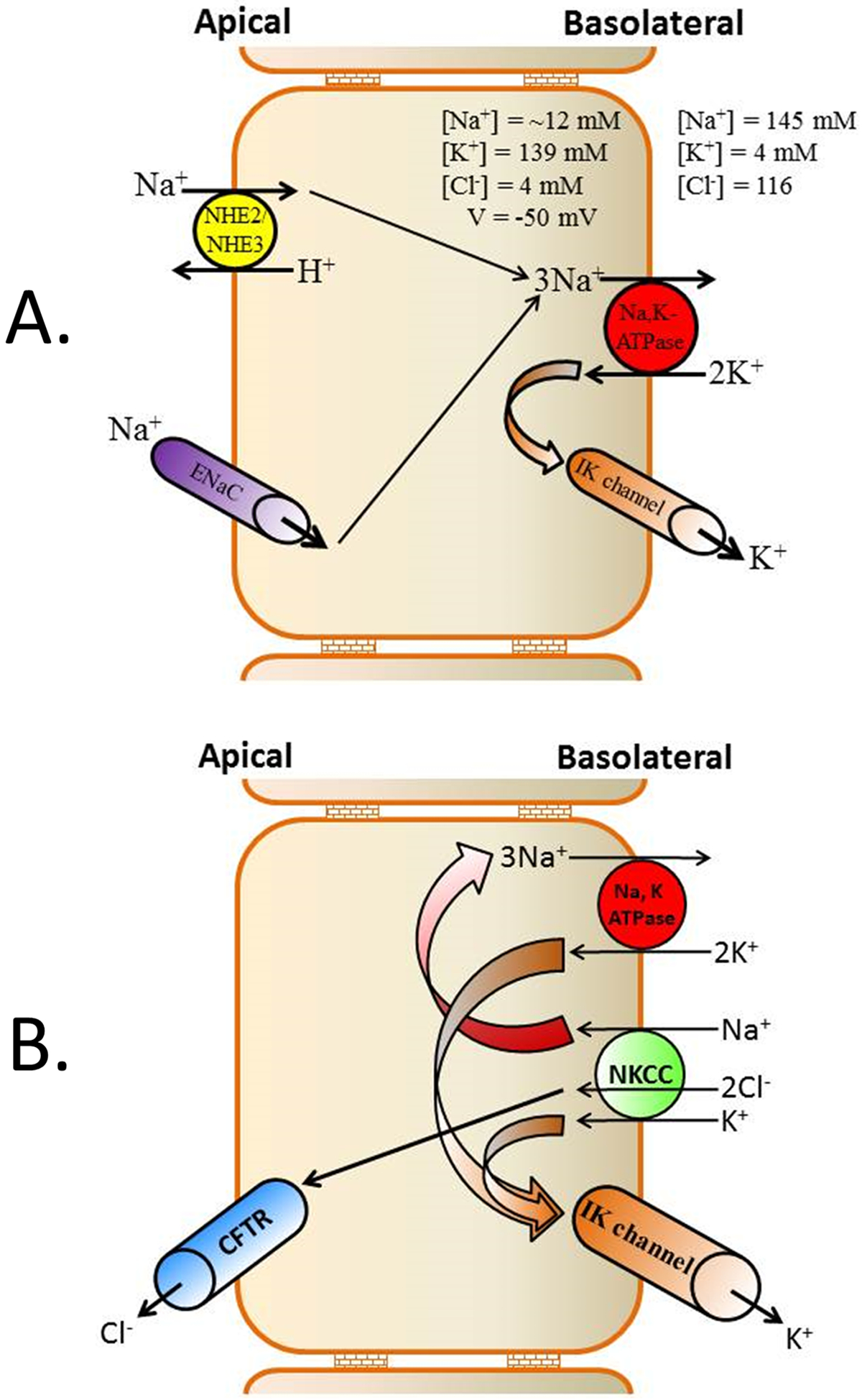
[A] Under basal conditions, K+ recycling across basolateral membranes via K+ channels operating in concert with Na+,K+-ATPase, contributes to the favorable electrochemical gradient (reflecting high intracellular Na+ and low intracellular K+ concentrations, and a negative membrane potential) necessary for the secondary Na+ absorption mediated by apical Na+-H+ exchanger isoforms 2 and 3 (NHE2/NHE3) and epithelial Na+ channel (ENaC). NHE2 and NHE3 mediate electroneutral Na+ absorption, while ENaC mediates electrogenic Na+ absorption [B] Under stimulated condition (e.g., cholera), apical Cl− exit via CFTR (cystic fibrosis transmembrane regulator) Cl− channels following Na+-K+−2Cl− (NKCC) transporter-mediated basolateral Cl− uptake tends to depolarize cells. This is counter-balanced by the hyperpolarizing effect of K+ exit through basolateral intermediate conductance K+ (IK) channels, thus maintaining the electrical gradient required for sustained Cl− secretion.
Molecular identities of K+ channels
The colonic K+ channels include both Ca2+-activated K+ (KCa) channels and cAMP-activated K+ channels. Molecular studies have identified five different KCa channels. These KCa channels include three small conductance K+ channels (SK1–3), an intermediate conductance K+ channel (IK; also known as SK4, KCa3.1 and Kcnn4), and a large conductance K+ channels (BK, also known as hSlo, KCa1.1 and KCNMA1). In the case of colonic epithelial cells, only BK and IK channels are expressed in the apical and/or basolateral membranes and we will discuss their molecular identities and functional roles. The BK channels have conductances ranging between of 150 pS and 230 pS, while IK channels have conductances between 12 pS and 39 pS (Table-2). cAMP-activated K+ channels with conductance of 1 − 3 pS have also been characterized on both apical and basolateral membranes of intestinal epithelial cells (Table-2).
Table-2: Electrophysiological and pharmacological characteristics of basolateral K+ channels in animal and human colon.
Patch clamp studies characterized cAMP- and Ca2+-activated K+ channels with different conductances in basolateral membranes of colon from rat, rabbit, human and the T84 cell line (a human colon carcinoma cell line). cAMP-activated small conductance (< 3 − 6.8 pS) K+ channels are inhibited by chromanol 293B, while Ca2+-activated large conductance (138 − 220 pS) K+ channels are inhibited by Ba2+ and TEA (non-specific K+ channel blockers). Both cAMP and Ca2+ activated intermediate conductance (12 − 28 pS) K+ channels, which are inhibited by Ba2+, TEA and clotrimazole (CLT; an IK channel blocker) in rat and human colon, and inhibited by CLT in T84 cells. Similar detailed information about apical K+ channels are not available in the litrature.
| Species | Agonist | Conductance (pS) | Inhibitor | Reference |
|---|---|---|---|---|
| Rat | cAMP | < 3 | Chromanol 293B | (205) |
| Ca2+ | 12 | Ba2+ and TEA | (27) | |
| Ca2+ | 187 | Ba2+ | (27) | |
| Non-selective | 27 | Not known | (174) | |
| Rabbit | Ca2+, cAMP | 90 – 220 | Ba2+ and TEA | (110) |
| Human | cAMP | 6.8 | Not known | (4) |
| cAMP, Ca2+ | 23 | Ba2+, quinidine and DPC | (109, 157) | |
| Ca2+ | 138 | Ba2+, quinidine and TEA | (109) | |
| T84 cell line | cAMP, Ca2+ | 28 | CLT | (48) |
| Ca2+ | 161 | Ba2+ | (47) |
Ca2+-activated K+ (KCa) channels of colon:
KCa channels are present in both the apical and basolateral membranes of colonic epithelial cells. Ion flux studies indicate that K+ channels mediate K+ secretion across apical membranes, while basolateral membrane K+ channels provide the driving force for active Cl− secretion (21, 32, 48, 50, 113). Patch clamp studies have characterized K+ channels with conductances of approximately 39 pS and 200 pS that are designated intermediate IK and BK channels, respectively (29, 109, 157, 158). BK channels are activated by Ca2+ and cAMP, and inhibited by Ba2+, tetraethyl ammonium (TEA) and quinidine (29, 130). IK channels are activated by Ca2+ and cAMP, and inhibited by Ba2+, diphenylamine-2-carboxylic acid (DPC) and quinidine, but not by TEA (157). Patch clamp studies have also characterized BK and IK channels on the basolateral membranes of rat and guinea pig small intestine (119, 122, 170, 202), and rat, guinea pig, and rabbit colon (27, 29, 145, 157, 198). Immunological studies have also identified IK and BK channel-like proteins in both the apical and basolateral membrane of intestinal epithelial cells (12, 60, 81, 114, 166, 175, 179, 209).
Large conductance K+ (BK) channels:
BK channels are composed of pore-forming α (BKα) and Ca2+/voltage sensing β (BKβ) subunits. Two BKα splice variants (STREX and ZERO) transcribed from a single gene (28), and four BKβ-subunits (β1–4, known as KCNMB1–4) that display tissue-specific distribution, have been cloned (106, 135). Expression of BKα alone results in functional large conductance (~150 − 200 pS) K+ channels, while coexpression with different β-subunits results in large conductance K+ channel activity with different sensitivities to Ca2+ and voltage. The BKα splice variants STREX and ZERO have distinct sensitivities to cAMP, as STREX is inhibited, while ZERO is activated, by cAMP. Exon-18 present in STREX is deleted in ZERO (194). Only ZERO transcripts appear to be expressed in guinea pig and human colon (158, 209). However, both STREX and ZERO transcripts are expressed in mouse colon (179). In addition, BKα (ZERO) has also been shown to exhibit three splice variants of the COOH-terminal in human brain and guinea pig colon (195, 209). The BKα with exon-27 termination resulted in an amino acid sequence ending of QEERL, whereas the termination in exon-28 resulted in an amino acid sequence of EMVYR (61). Splicing of exon-28 resulted in two variants that differed by an omission of three base pairs at the beginning of exon-28 (61). BKα variants ending with amino acid sequences QEERL and EMVYR are known as BKαRL and BKαYR, respectively. Both BKαRL and BKαYR are expressed in guinea pig colon (23). The COOH-terminal variants of BKαRL and BKαYR contribute to the membrane-specific delivery (apical vs basolateral membrane) of BK channels (34, 100, 111). In guinea pig colon, a BKαYR variant that localizes to both the apical and basolateral membrane of immature cells at the crypt base is expressed only at the apical membrane of matured surface cells (209). Although the molecular identities of BKα splice variants of rat and human colon have yet to be established, the demonstration that cAMP activates BK channel activity, and forskolin (FSK; which activates adenylate cyclase and increases intracellular cAMP) activates K+ secretion, suggest that BK channels encoded by ZERO transcripts mediate K+ secretion in rat and human colonic epithelial cells (Figure-7) (130, 159). Hyperaldosteronism induced by both dietary Na+ depletion and chronic dietary K+ loading enhance BKα-specific mRNA abundance and protein expression, and stimulate iberiotoxin (IbTX, BK channel blocker)-inhibitable K+ secretion in rat distal colon (159). Aldosterone appears to stimulate K+ secretion through the ZERO isoform, since FSK (i.e., cAMP) did not inhibit aldosterone-enhanced K+ secretion in rat distal colon (130, 176).
Figure-7: Effect of cellular cAMP increased by forskolin (FSK, adenylate cyclase activator) on K+ fluxes in the presence and absence of mucosal VO4 (P-type ATPase inhibitor) in rat distal colon.
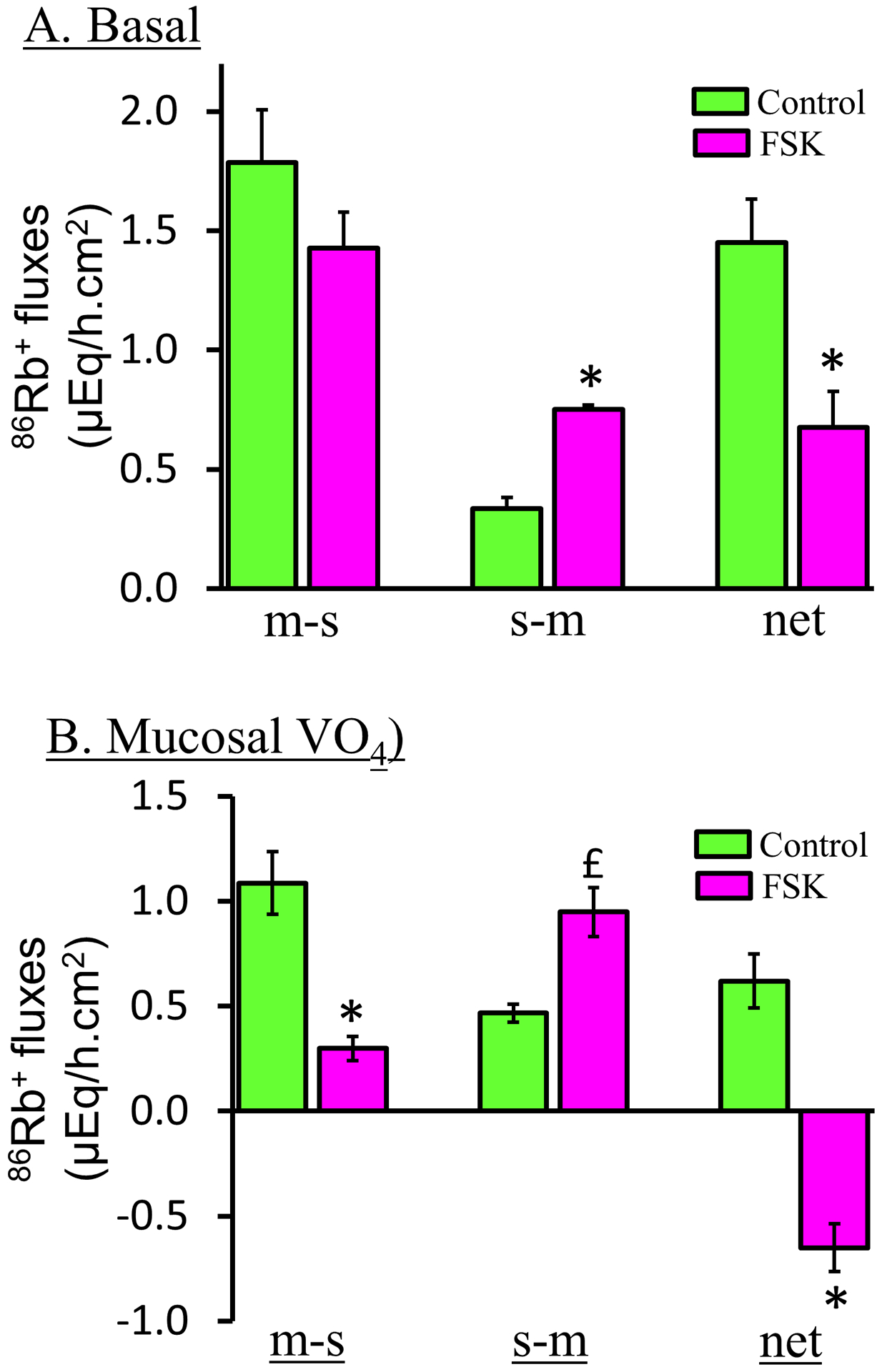
[A] In the absence of mucosal VO4, forskolin (FSK) significantly inhibits active K+ absorption (i.e., net K+ absorption) by stimulating s-m K+ fluxes. [B] Presence of 1 mM mucosal VO4 unmasks FKS-induced active K+ secretion. It is to be noted that mucosal VO4 also inhibits basal K+ absorption in normal colon (see Figure–11). Mucosal to serosal (m-s) and serosal to mucosal (s-m) 86Rb+ fluxes (K+ surrogate) were measured under voltage clamp condition in the absence (green bars) and presence (red bars) of forskolin. Net K+ fluxes were calculated by subtracting s-m fluxes from m-s fluxes. Positive and negative fluxes represent active K+ absorption and active K+ secretion, respectively. *p < 0.001 – compared to respective fluxes in the absence of FSK; £p < 0.05 – compared to in the absence of FSK. [Reproduced from ref. (159)].
The BKα-subunit links with auxiliary β-subunits, which have the ability to modify the activation kinetics (i.e., Ca2+ and voltage sensitivities) and inhibitor sensitivity of the BK channel complex (17, 39, 54, 106, 135). Four isoforms of BKβ subunits have been identified, each of which may associate with the BKα-subunit to modulate BK channel activities in unique ways (54, 201). Earlier studies identified the expression of all four β-subunits (60, 138), while a recent study identified only β2 subunit expression in mouse colon (179). Similarly, although different studies have shown expression of all four β subunits, β1 and β3 subunits were found to be the dominant β subunits in human colon (16, 26, 158). β1 and β4 were the only β subunits detected in guinea pig colon (209). Thus, BKα splice variants (BKαRL and BKαYR) may co-express with different β-subunits in mouse, guinea pig, rat and human colon. Further studies are required to establish whether BKαRL and BKαYR splice variants co-express with the same or different β-subunits, and whether BKαRL and BKαYR expression differs between cell types (i.e., villus vs crypt cells) and different membrane domains (i.e., apical vs basolateral) in colonic epithelial cells.
Intermediate conductance K+ (IK) channels:
The IK channel gene that encodes 427 amino acids was originally cloned from human placenta (93). Human and rat colonic IK orthologues that encode 424 amino acids have been cloned from T84 cells and a rat colonic cDNA library, respectively (92, 204). When expressed in vitro, rat colonic IK channels exhibited Ca2+-activated K+ currents with a single channel conductance of 36 pS, which were inhibited by clotrimazole (CLT, an antifungal inhibitor) (92). Immunofluorescence studies have localized IK channel-like proteins to the apical and basolateral membranes of epithelial cells in rat distal colon, while immunogold labeling studies have localized IK-like proteins to the apical and basolateral membranes of rat and human colon (Figure-8) (12, 68). Extensive cloning studies have isolated two additional IK splice variants that encode 425 and 395 amino acid proteins from a rat colon cDNA library (12). The IK channel isoform mRNAs encoding 425, 424 and 395 amino acid proteins were designated IKa (Kcnn4a), IKb (Kcnn4b) and IKc (Kcnn4c), respectively. Since IKa (i.e., 425 amino acid protein) had 100% homology with the mouse IK channel (mIK1) orthologue that was cloned from smooth muscle (125), it was concluded that IKa encodes smooth muscle IK channels, while IKb and IKc encode basolateral (40 kD) and apical (37 kD) IK channels in epithelial cells, respectively (12).
Figure-8: Immunogold labeling of intermediate conductance K+ (IK) channels in colonic surface epithelial cells of normal rat distal colon (RtDC) and normal human colon (HuC).
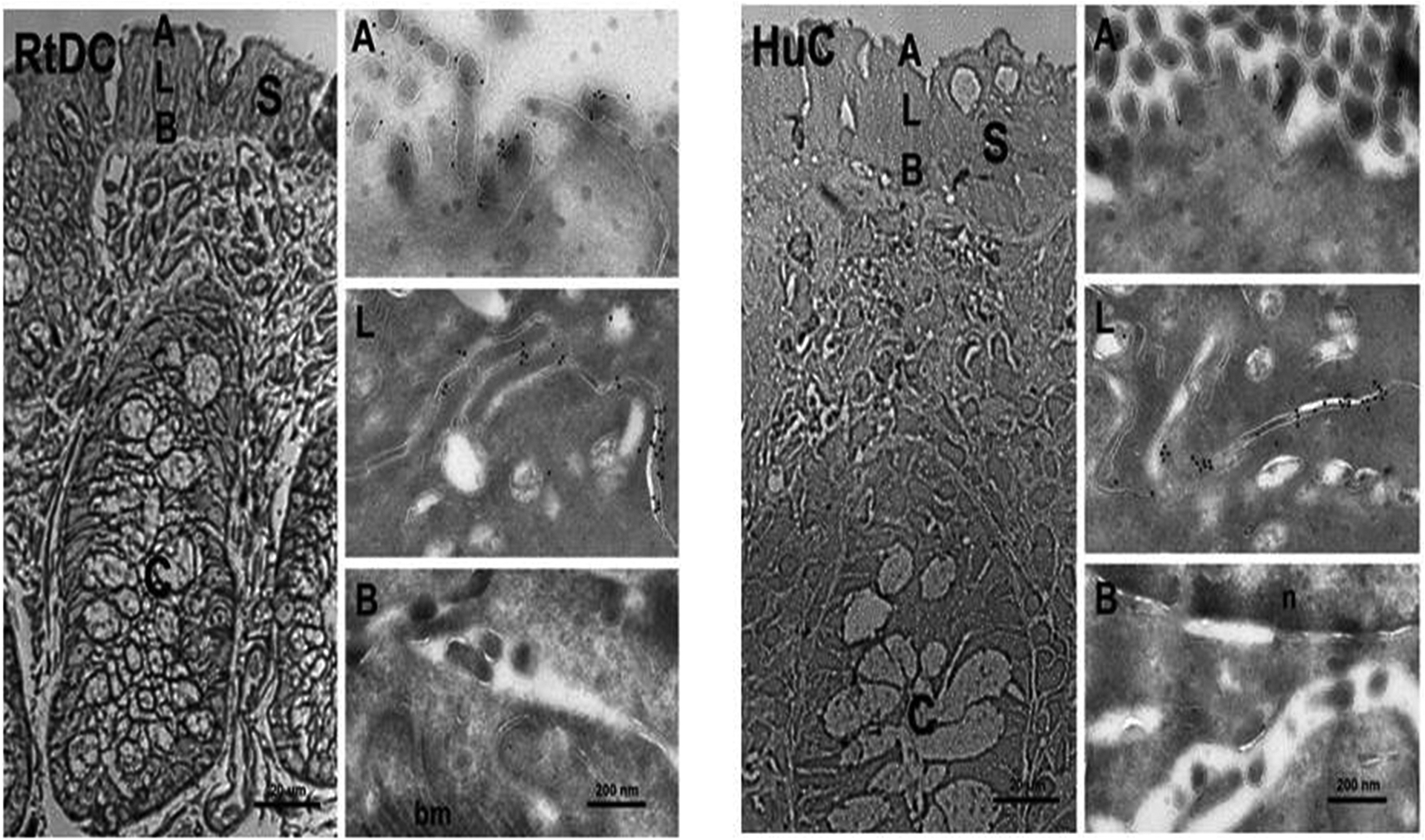
Cryosemithin sections were prepared and trypan blue-stained for orientation (left column of each panel). Tissue specimens were ultratrimmed for cryothin sectioning, focusing on small areas of interest in the colonic surface epithelium to characterize plasma membrane domain-specific localization of IK channel proteins at the ultrastructural level. Cryothin sections were immunolabeled with anti-IKabc and detected with a secondary donkey anti-rabbit 10 nm gold-labeled antibody. Anti-IKabc localized IK-like proteins in apical [A], and lateral [L] plasma membrane domains of rat and human colonic epithelial cells, but not in basal plasma membranes [B] of surface epithelial enterocytes. These results using high resolution immunogold electron microscopy confirm our earlier results using confocal microscopy. [S] Surface epithelium; [C] crypts; [n] nucleus; [bm] basal plasma membrane. Images were acquired either at 400x primary magnification (cryosemithin section, bar = 20 μm) by light microscopy, or at 21,000x primary magnification (cryothin sections, bar = 200 nm) by electron microscopy. Similar results were obtained with two and three different human and rat tissues, respectively. [Reproduced from ref. (12)].
The IKc isoform lacks 29 amino acids, which constitutes the entire second membrane spanning domain (MSD), plus 5 exofacial and 2 endofacial amino acids in the IKc isoform. Thus, the membrane topology of IKc, with five predicted MSDs, differs from IKa and IKb, which have six MSDs. Although even MSDs are common for transport proteins, several transport proteins, including K+ channels with odd MSDs, have been shown to express functional proteins (25, 120, 143). However, although IK protein was readily expressed on the surface membrane of Xenopus oocyte injected with IKb cRNA, IK protein was identified in cytoplasm of oocytes that were injected with IKc cRNA. By contrast, when IKc cRNA was coinjected with β-subunit cRNA transcribed from KCNMB1 (i.e., BKβ1-subunit), IK protein was readily localized on the plasma membranes of oocytes (Figure-9). The IKc and BKβ1 cRNA coinjected oocytes also exhibited TRAM-34 (IK channel specific inhibitor)-sensitive K+ uptake, indicating IK channel functionality (12). These observations indicated that although a specific β-subunit has not yet been identified, IKc requires a β-subunit (i.e., chaperone protein) for its membrane expression. It is not known whether IKc lacking a MSD utilizes BKβ1, or a complementary ‘chaperone’ protein for its membrane expression in colon.
Figure-9: Indirect immunofluorescence imaging of intermediate conductance K+ (IK) channel protein in cRNA-injected Xenopus oocytes.
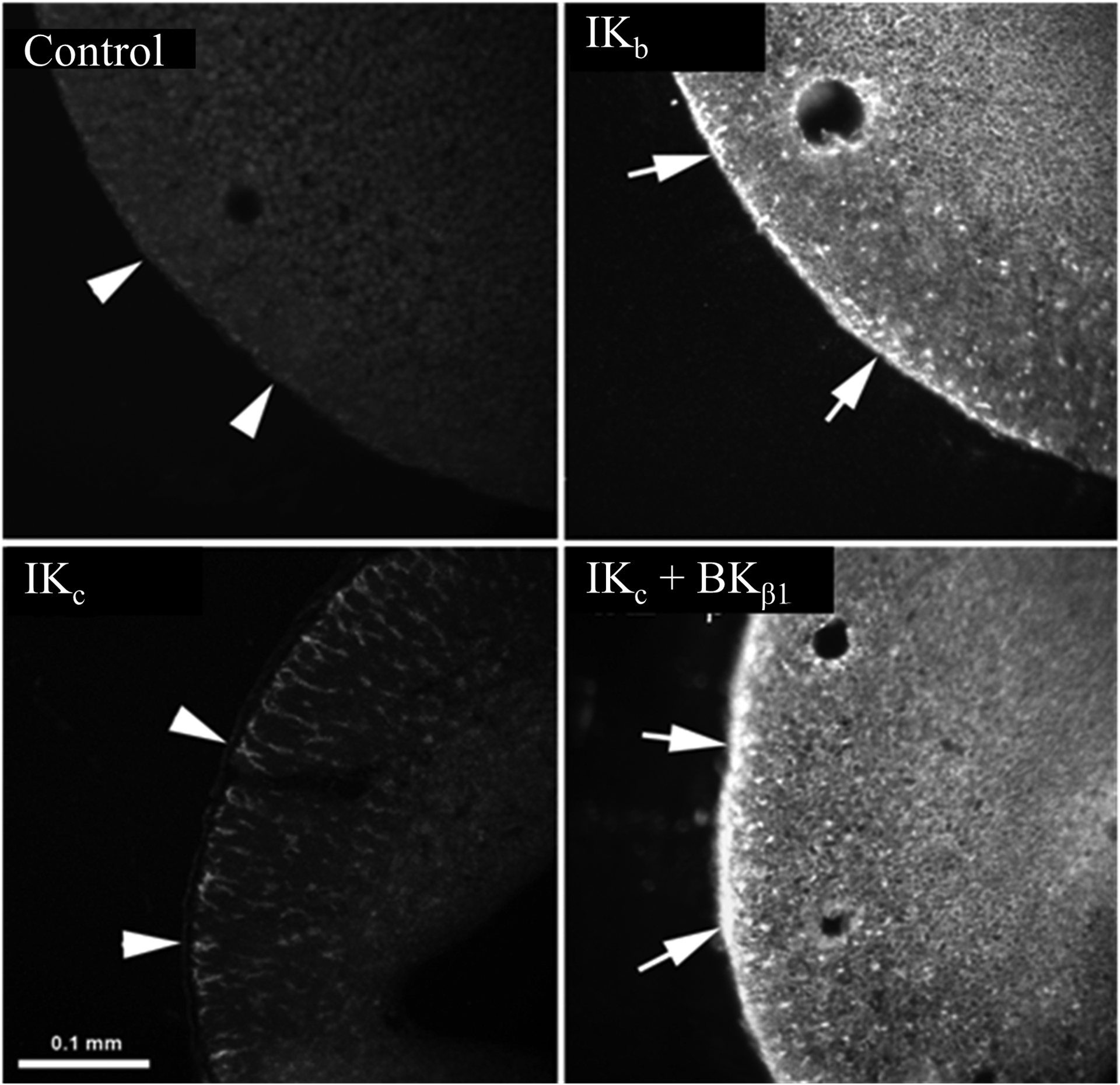
Anti-IKabc antibody localized IK channel proteins to the plasma membrane of IKb cRNA-injected oocytes (IKb), but not water injected (control) oocytes. IK protein was identified only in the cytoplasm, but not in the plasm membrane of IKc cRNA-injected oocytes (IKc). Plasma membrane targeting of IK protein was substantially enhanced in oocytes coinjected with IKc and the BKβ1-subunit (i.e., large conductance K+ channel β-subunit) cRNAs (IKc + BKβ1). Arrowheads indicate the absence (or minimal presence) of IK cahnnel proteins (left panels), while arrows indicate the presence of IK channel proteins (right panels) on the plasma membrane of oocytes. Fluorescence images were acquired using Nikon light microscope. Bar = 0.1 mm. [Adapted from ref. (12)].
cAMP activated K+ channels:
cAMP-activated K+ channels are located in the basolateral membranes of rat and human colon (102, 118, 205). Inhibition by chromanol-293B, a slow delayed rectifier K+ current blocker is the unique feature of these cAMP-activated K+ channels (205). This cAMP-activated K+ channel is encoded by voltage-gated KvLQT1 channel gene (98). KvLQT1 consists of α- (known as KCNQ1) and β- (known as KCNE) subunits. Five different β-subunits (KCNE1–5) have been isolated (123). KCNQ1 complexed with different KCNE isoforms expressed in different cells and membrane domains exhibit different cell functions (123). KCNQ1 complexed with KCNE1 is found in the basolateral membranes of colon (70, 203). KCNQ1/KCNE3 complexes have been identified in the basolateral membranes of mouse epithelial cells (4, 43, 46, 137). Chromanol 293-sensitive KCNQ1-mediated K+ channels play a critical role in cAMP-stimulated Cl− secretion in mouse colon (43), and they appear to be essential for cAMP-stimulated Cl− secretion in guinea pig colon (104). However, since both cAMP- and Ca2+-mediated agonists have been shown to activate IK channels, it is likely that basolateral IK channel-mediated K+ exit maintains the intracellular negative membrane potential which provides the driving force for both cAMP (e.g., cholera toxin) and Ca2+ (e.g., bile acid) agonist-activated secretory diarrhea (118, 149, 157, 160).
Apical membrane K+ channels
Role of apical membrane BK channels in colonic K+ secretion:
Apical membrane K+ channels play a critical role during active K+ secretion (Figure-5), Slow marker perfusion studies have shown that the human colon, but not the small intestine, secretes 4.7 mEq K+ per day (133). Both passive K+ permeation and active K+ secretion contribute to luminal and stool K+ concentration. In the colon, paracellular (passive) K+ permeation is driven by the lumen-negative transepithelial (~20–30 mV) electrical potential difference (18, 141), although the potential difference in the small intestine is much lower. By contrast, transcellular (active) electrogenic K+ secretion is a feature restricted to the salivary gland, gastric mucosa and colon (85, 102, 146). Active K+ secretion contributes significantly to stool K+ losses in diarrheal diseases. For example, whereas normal individuals excrete ~10 mEq K+/day in their stools, cholera patients with severe diarrhea excrete 119 mEq K+/day (3, 200). Active K+ secretion involves K+ uptake across the basolateral membrane and K+ exit across the apical membrane (Figure-5). In addition, electrogenic K+ secretion requires exit mechanisms for Na+ and Cl− across the basolateral membrane. K+ uptake across the basolateral membrane is mediated by Na+,K+-ATPase and Na+-K+−2Cl− cotransport, while K+ exit across the apical membrane is mediated by K+ channels. Basolateral uptake of Na+ reflects Na+-K+−2Cl− cotransport, and Na+ is pumped out by Na+,K+-ATPase. Basolateral Cl− uptake also reflects Na+-K+−2Cl− cotransport, and Cl− exits via Cl− channel-2 (CLC2), which has been identified in the basolateral membrane of mouse and guinea pig colon (30, 31, 208). The use of ouabain to inhibit Na+,K+-ATPase-mediated K+ uptake and bumetanide to inhibit NKCC-mediated K+ uptake across basolateral membranes has established that both Na+,K+-ATPase and NKCC have pivotal roles in the regulation of active K+ secretion (134, 177, 179, 191). Na+,K+-ATPase regulates active K+ secretion under basal conditions, whereas NKCC is the predominant regulator of stimulated active K+ secretion (179, 191). It should be emphasized that K+ uptake across the basolateral membrane far exceeds its transepithelial movement, which points to K+ recycling across the basolateral membrane. Consistent with this view, the addition of Ba2+ (a nonspecific K+ channel blocker) increased net K+ secretion in turtle and rabbit colon (134, 191). However, since the addition of K+ channel blockers to the serosal bath did not alter K+ absorption, it was suggested that K+-Cl− co-transport might be responsible for K+ recycling across the basolateral membranes in rat distal colon (191). However, since active K+ secretion was enhanced in IK channel knockout mice, it seemed likely that IK channels might regulate K+ recycling across basolateral membranes of colon (12, 92, 179, 209). Colonic K+ secretion is activated by cellular second messengers (e.g., cAMP and Ca2+), and stimulated during dietary K+ loading, dietary Na+ depletion (aldosterone), and in an animal model of dextran sulfate sodium (DSS)-induced ulcerative (64, 66, 94, 118, 159, 176, 191). Diarrhea is a major symptom in ulcerative colitis, and while enhanced active Cl− secretion usually underlies diarrhea originating from the colon, defective ENaC-mediated Na+ absorption appears to be the main pathophysiologic mechanism of diarrhea in patients with ulcerative colitis (7, 41). Absence of FSK (cAMP)-activated and CCH (Ca2+)-activated Cl− secretion also suggest that active Cl− secretion is not responsible for diarrhea in DDS-induced ulcerative colitis (Figure-13). Moreover, K+ (but not Na+) enriched diarrhea has been reported in patients with colonic pseudo-obstruction (175, 200), and we therefore predicted that in addition to defective ENaC-mediated Na+ absorption, enhanced K+ secretion might also contribute to diarrhea in ulcerative colitis. Additional studies are therefore required to determine whether K+ channel inhibition decreases K+-driven water secretion in ulcerative colitis, which might ameliorate diarrhea in patients with this disease.
Figure-13: Dextran sulfate sodium (DSS)-induced inflammation stimulates active K+ secretion and abolishes agonist (cAMP and Ca2+)-stimulated Cl− secretion in rat distal colon.
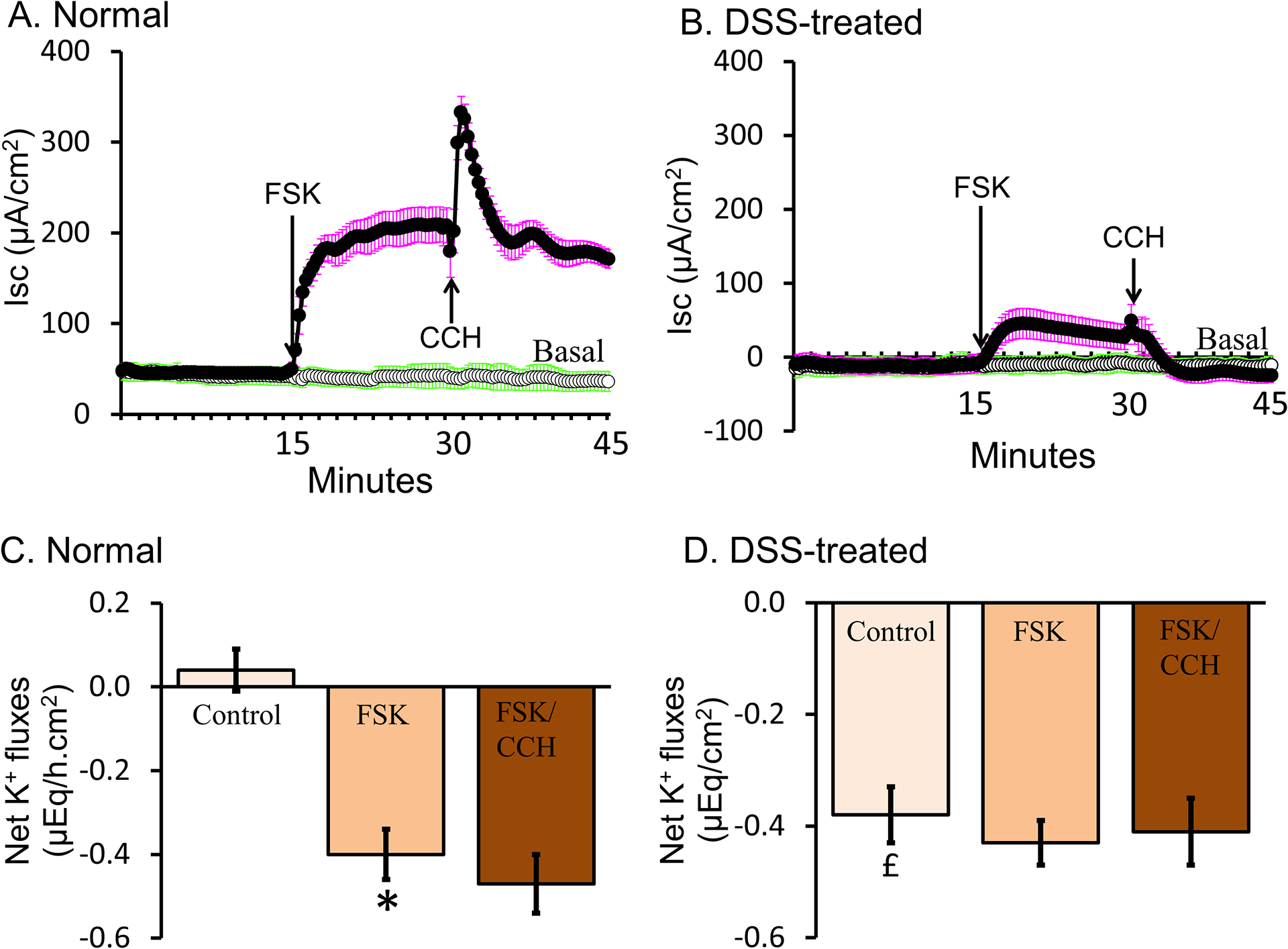
[A] Positive Isc (short circuit current) represents the presence of anion (Cl−/HCO3−) secretion under basal conditions. Increasing intracellular cAMP by forskolin (FSK; adenylate cyclase activator) stimulated active Cl− secretion. Increasing intracellular Ca2+ by carbachol (CCH; adrenergic agonist; FSK/CCH) further transiently stimulated active Cl− secretion. [B] Negative Isc indicates induced cation secretion and absence of anion secretion under basal conditions in DSS-inflamed colon. The minimal increase in Isc induced by FSK, and the absence of a FSK/CCH-induced Isc, indicated that Cl− secretory processes were abolished in DSS-inflamed colon. [C] In the presence of mucosal ortho-VO4 (H+,K+-ATPase inhibitor), minimal K+ absorption was present in normal colon (control). In normal colon, FSK stimulated active K+ secretion, while FSK/CCH had no additional effect on K+ secretion. [D] In DSS-inflamed colon (control), active K+ secretion was present under basal conditions, but neither FSK nor FSK/CCH stimulated active K+ secretion. [Reproduced from (94)].
Ion flux studies performed under voltage clamp condition have shown that the specific BK channel inhibitors iberiotoxin and paxilline block apical membrane K+ channels mediating both basal and stimulated K+ secretion in animal colon (159, 166, 176, 179, 180, 209), while patch clamp studies have characterized K+ channels in rat and human colon (29, 129, 130, 150, 156). The absence of K+ secretion in BKα knockout mice established that BK channels mediate both basal and activated K+ secretion in murine colon (166). It is currently accepted that absorptive and secretory transport processes are distributed differentially along the surface-crypt axis, surface cells being the site of absorption (e.g., ENaC-mediated Na+ absorption) and crypt cells being the site of secretion (e.g., CFTR-mediated Cl− secretion). By contrast, there are conflicting views about BKα protein localization in normal guinea pig, mouse and human colon (114, 158, 166, 175, 179, 209). In normal human colon, BKα-like protein appears to be restricted to the apical membrane of surface cells and cells in the upper third of crypts, while in mouse colon it is seen only in crypt cells. Absence of BKα proteins in BK channel knockout mice supported the presence of BK channels in crypt cells of normal murine colon (166, 179), while BK channel expression in surface cells of normal human colon has been established by patch clamp and immunostaining (114, 158, 175). Unlike mouse and human colon, BKα proteins have been identified in both surface and crypt cells in guinea pig distal colon (209). Despite the species differences in cell localization of BKα protein, it is well established that apical BK channels mediate both basal and active K+ secretion in colon.
Role of apical IK channels in K+ secretion:
Patch clamp studies have characterized IK channels only in the basolateral membranes of crypts from rat and human colon (24, 109, 157, 204). However, immunofluorescence studies have localized IK channel-like proteins to the apical membranes of rat and human colon (6, 12, 68, 92). The apical IK channel is encoded by a IKc transcript in rat distal colon (12), and the role of apical IK channels is starting to emerge (92, 124). The observations that mucosal clotrimazole inhibits carbachol-stimulated K+ secretion (92), and that the IK channel blocker TRAM-34 inhibits K+ secretion induced by the mucosal application of DC-EBIO (IK channel opener), provide strong support for the presence of functional mucosal IK channels in rat colon (Figure-10) (124). In addition, the complete absence of DC-EBIO-induced K+ secretion suggests that down-regulation of apical IK channels may play critical role in K+ conservation in rat distal colon during dietary K+ depletion (171). Although agonist (e.g. cAMP) stimulated K+ secretion is mediated by apical BK channels, apical IK channels may mediate basal K+ secretion, a possibility that could be explored using an appropriate animal knockout model.
Figure-10: Mucosal DC-EBIO [intermediate conductance K+ (IK) channel opener] activates IK channel mediated K+ secretion in normal rat distal colon.
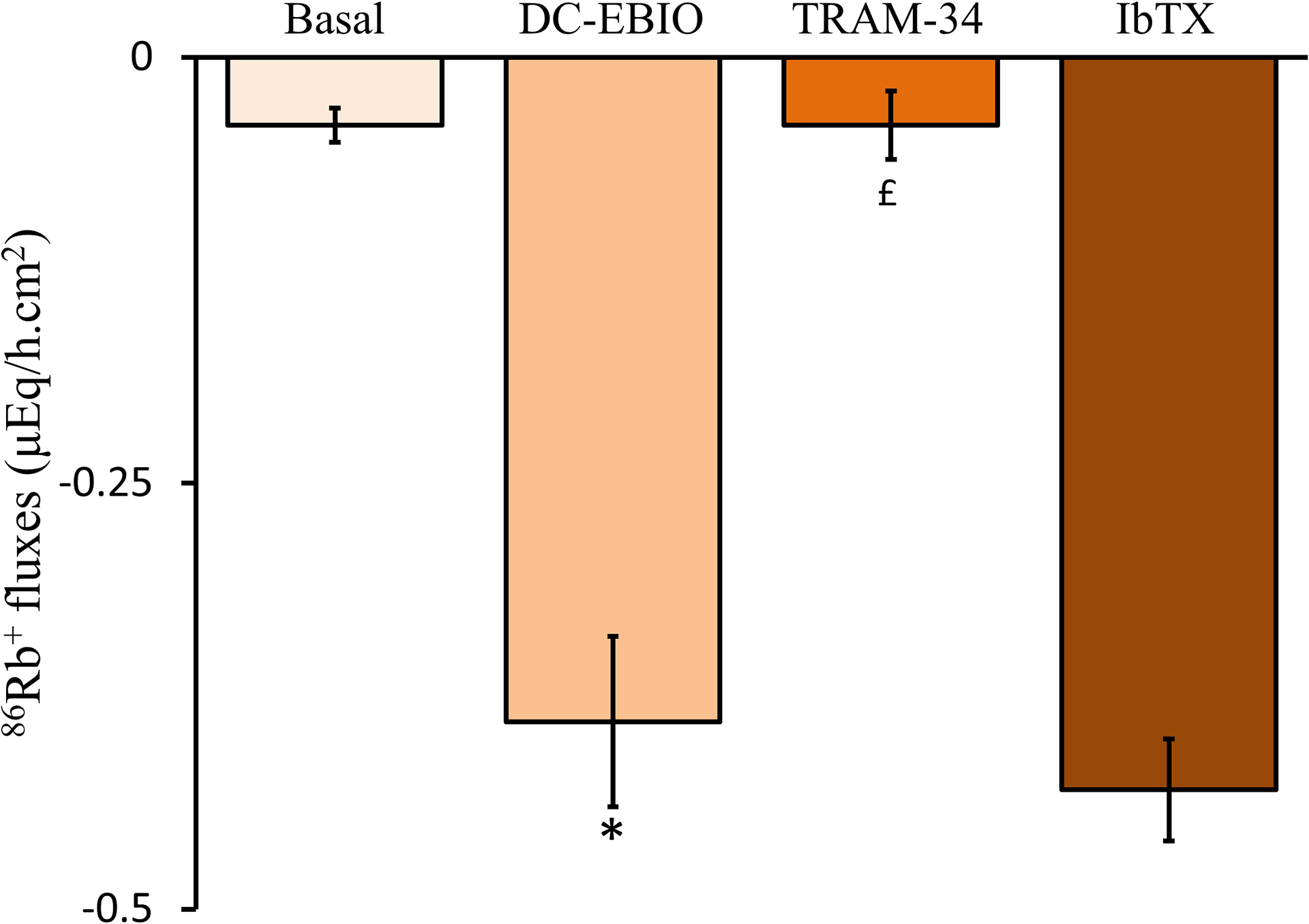
Minimal K+ secretion is present under basal conditions. Mucosal DC-EBIO (IK channel opener) stimulates active K+ secretion. Mucosal TRAM-34 (IK channel blocker) inhibits DC-EBIO-stimulated K+ secretion. DC-EBIO-stimulated K+ secretion is not inhibited by mucosal iberiotoxin [IbTX, large conductance K+ (BK) channel blocker). [Reproduced from ref. (124)].
Role of basolateral membrane K+ channels
By recycling K+ across the basolateral membrane, K+ channels regulate both Na+,K+-ATPase and the Na+-K+−2Cl− cotransporter. In the basal state, constitutively open basolateral K+ channels maintain a negative membrane potential, which is the driving force for Na+-dependent nutrient absorption and Na+/H+-exchanger-mediated electroneutral Na+ absorption in the small intestine. Basolateral K+ exit is counterbalanced by Na+,K+-ATPase-mediated K+ uptake, which maintains the high intracellular K+ concentration. In animal and human distal colon, the electrochemical gradient also provides the driving force for electrogenic Na+ absorption mediated by epithelial Na+ channel (ENaC). By contrast, under stimulated conditions, the Na+-K+−2Cl− cotransporter mediates basolateral Cl− uptake, Cl− exiting through apical cystic fibrosis transmembrane regulator (CFTR) Cl− channels, resulting in membrane depolarization. K+ exits through agonist-activated (e.g., Ca2+ and cAMP) basolateral K+ channels, thereby repolarizing the basolateral membrane to maintain the driving force required for sustained electrogenic Cl− secretion (Figure-6B). Patch clamp studies have identified and characterized different types of basolateral K+ channels in colonic crypt cells in a variety of species (32, 109, 119, 184, 199), and these K+ channels can be divided into three categories: 1) small (< 6 pS), 2) intermediate (~25 pS) and 3) large (~130 pS) conductance K+ channels. Molecular studies have identified that the small, intermediate and large conductance K+ channels correspond to KCNQ1/KCNE3, IK and BK channels, respectively (12, 137, 156, 159). In addition, 27–30 pS nonselective cation channels have also been identified in turtle and rat colon (27, 144), but their molecular identities are not known. Small conductance basolateral (KCNQ1/KCNE3) K+ channels are activated by cAMP, while both Ca2+ and cAMP activate basolateral IK channels. The physiological role of basolateral BK channels is yet to be identified (27, 47, 109, 119, 209). Since KCNQ1/KCNE3 channels make only a small contribution to basolateral K+ conductance in human colonic crypt cells (4), it is likely that the highly abundant basolateral IK channel plays a critical role in regulating Cl−-driven fluid secretion (5, 32).
Regulation of K+ channels and K+ secretion
Corticosteroids (both the glucocorticoids cortisol and corticosterone, and the mineralocorticoid aldosterone) secreted by the adrenal glands are required for basal intestinal electrolyte transport function (103, 148). Although both natural and synthetic glucocorticoids and mineralocorticoids regulate electrolyte transport, aldosterone is the main regulator of electrolyte transport in the colon (148). Glucocorticoids and mineralocorticoids exert different effects on electrolyte transport through specific receptors present in the epithelial cells of the small intestine and colon, respectively (136, 196). Although K+ channels are present throughout the entire intestinal tract (32, 68, 156), only colonic K+ channels are regulated by corticosteroids (20, 148). The distal colon is a major target for mineralocorticoids (15), since mineralocorticoid receptor expression is greater in this colonic segment (67). In vitro studies based on unidirectional ion flux measurement under voltage clamp conditions, and intracellular microelectrodes, demonstrated that hyperaldosteronism produced qualitatively different changes in electrolyte transport by enhancing electroneutral (i.e., Na+-H+ exchange mediated) and inducing electrogenic (i.e., ENaC mediated) Na+ absorption in the proximal and distal segments of rat colon, respectively (63, 87, 153). These segmental variations may reflect different types of corticosteroid receptors in the mucosa (14, 22). Receptor binding studies showed both glucocorticoid and mineralocorticoid receptors to be present throughout the entire colon, but glucocorticoid receptors outnumbered mineralocorticoid receptors in the proximal colon (14, 168). Initial studies regarding the effect corticosteroids on electrolyte transport showed that both glucocorticoids (i.e., dexamethasone) and mineralocorticoids stimulated electrogenic colonic K+ secretion (20, 148). However, additional studies showed that aldosterone, but not RU-28362 (a specific glucocorticoid agonist), stimulated electrogenic K+ secretion in rat proximal and distal colon (108, 196). RU-28362 binds more avidly to glucocorticoid receptors than dexamethasone or corticosterone, and does not compete for radio-labeled aldosterone binding to the mineralocorticoid receptor (193). It seems likely that dexamethasone-stimulated electrogenic K+ secretion reflected crossover binding to mineralocorticoid receptors. Thus, stimulation of electrogenic K+ secretion in animal colon reflects the activation of specific mineralocorticoid receptors, but not specific glucocorticoid receptors.
Ion flux studies have shown that hyperaldosteronism, secondary to exogenous aldosterone administration or chronic dietary Na+ depletion stimulates electrogenic K+ secretion in mouse and rat distal colon (55, 56, 63, 84, 176, 179). Studies with site-directed intracellular microelectrodes showed that aldosterone enhanced the apical K+ conductance of surface epithelial cells in rat distal colon (107, 108). Aldosterone reversed the basal active K+ absorption normally present to active K+ secretion in rat distal colon (Figure-11) (176). Flux measurements under voltage clamp condition have shown that both IK and BK channels contributed to the aldosterone-enhanced K+ secretion, and that the BK made a larger contribution than that of IK channels (64% versus 29%, respectively) (176). Quantitative-PCR and western blot analyses are consistent with aldosterone enhancement of both IKc and BKα specific mRNA abundance and protein expression, respectively. In vitro aldosterone exposure also enhanced IKc and BKα specific mRNA abundance, which was prevented by actinomycin D (DNA-dependent RNA polymerase inhibitor) in normal rat distal colon (Figure-12). These observations strongly suggest that aldosterone induced active K+ secretion by regulating both IKc and KCNMA1α expression at the transcriptional level (176).
Figure-11: Active K+ transport in normal and aldosterone-treated (dietary Na+ depleted) rat distal colon.
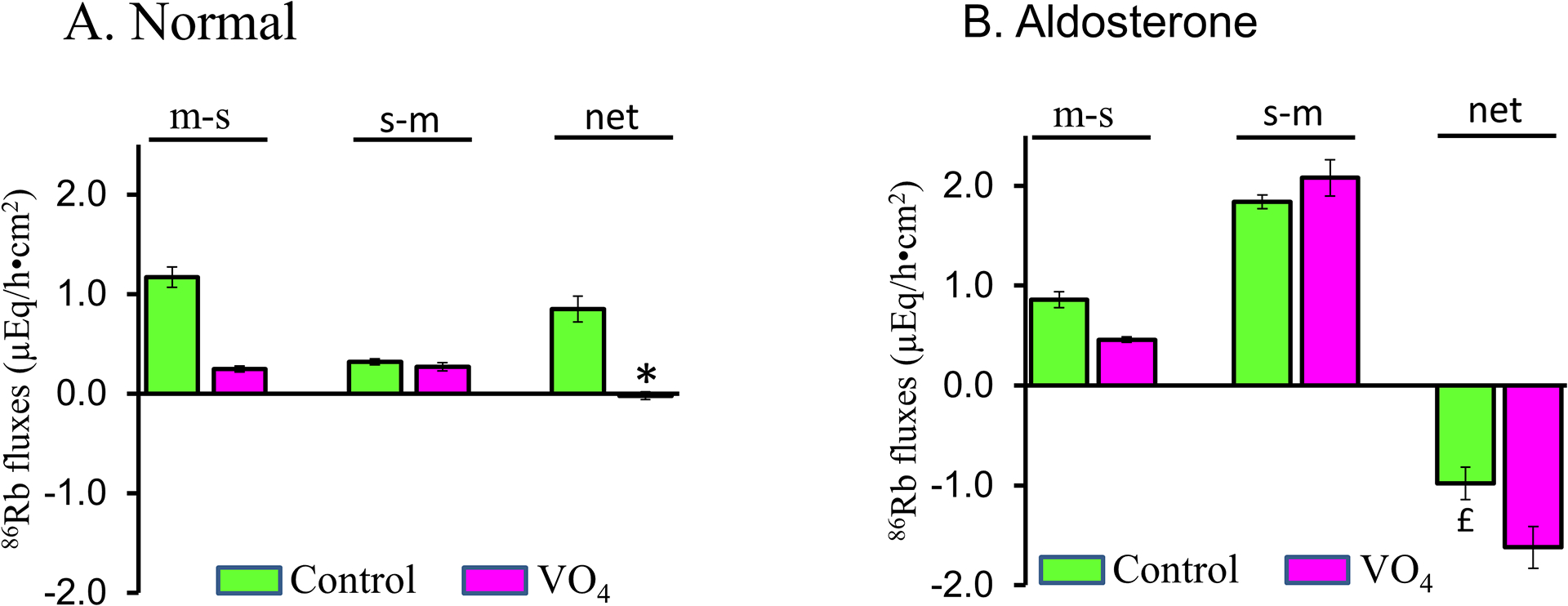
Net K+ transport was determined from the difference between mucosal to serosal (m-s) and serosal to mucosal (s-m) unidirectional fluxes measured under voltage clamp conditions. Net positive value represent active K+ absorption, while net negative value represent active K+ secretion. [A] Active K+ absorption present in normal rat distal colon (green bars) was inhibited by mucosal vanadate (VO4; P-type ATPase inhibitor; red bars). [B] Active K+ secretion in aldosterone-treated rat distal colon was further stimulated by mucosal VO4. *p < 0.001- compared to Control; £p < 0.001 – compared to Control; ‡p < 0.001 – compared to Control. [Reproduced from ref. (176)].
Figure-12: Effect of actinomycin D on aldosterone-induced Isc in normal rat distal colon in vitro.
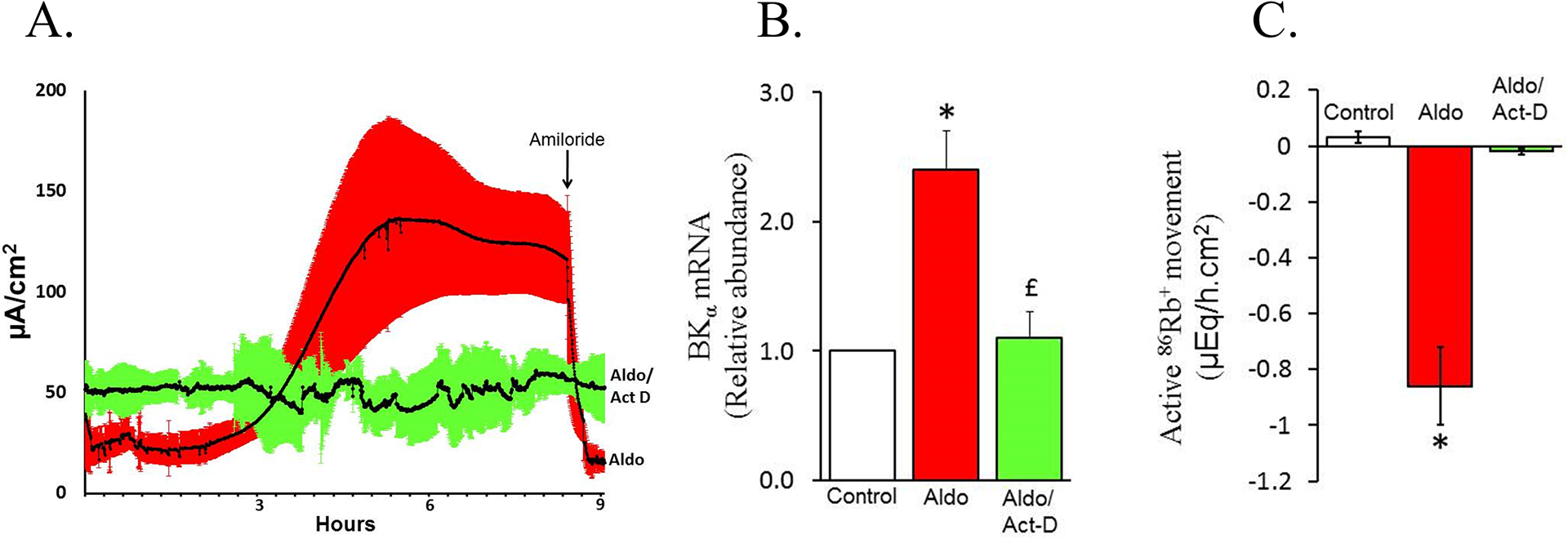
[A] Normal rat distal colonic mucosal layers were mounted under voltage clamp condition in Ussing chambers. Immediately after mounting, either aldosterone (aldo) or aldosterone plus actinomycin D (transcriptional inhibitor) (aldo/act-D) was added to the serosal bath. Isc was measured for up to 9 hr. At the end of 8½ hr, 10 μM amiloride was added to mucosal bath. Enhanced Isc and inhbition of Isc by amiloride indicates that aldosterone induced epithelial Na+ channel (ENaC)-mediated Na+ absorption (aldo). Absence of amiloride-sensitive Isc in the presence of actinomycin D (aldo/act-D) indicates that aldosterone-induced ENaC-mediated Na+ absorption is regulated at transcriptional level. [B] RT-qPCR analyses indicated that aldosterone enhanced the abundance of large conductance K+ channel α-subunit (BKα)-specific mRNA (aldo) , while this change was prevented by actinomycin D (aldo/act-D). [C] K+ fluxes measured under voltage clamp condition indicate that aldosterone stimulated active K+ secretion (aldo), whereas this response was blocked by actinomycin D (aldo/act-D). [Reproduced from ref. (176)].
The ability of aldosterone to increase IKc expression suggests that the IK gene is regulated by aldosterone acting on its cognate receptor (i.e., mineralocorticoid receptor; MR). Chromatin immunoprecipitation (ChIP) assay studies identified the MR response elements (MREs) in regions that spanned 20 kb upstream and 10 kb downstream of the presumed transcription start site in chromatin of colonic epithelial cells from normal and aldosterone-treated rats (126). MREs were immunoprecipitated in a ~5 kb region spanning the first and second introns in aldosterone-treated rats. When co-expressed with MR, these clones exhibited aldosterone-activated enhancer activity in HEK293T and CaCo2 cells. Bioinformatics analyses have identified two MRE regions. These clones lost enhancer activity after mutation of the presumptive MREs, thus establishing the functionality of MREs in the IK gene (126). Although transcriptional regulation IK channel by aldosterone is established, it is not yet known whether MREs are also present in BKα gene.
Pathophysiology of colonic K+ channels
Role of apical and basolateral K+ channels in ulcerative colitis:
Healthy human colon absorbs 1.5 − 2 liters of water a day, which is driven by the net absorption of large amounts of Na+ and Cl−. These net absorptive fluxes of Na+ and Cl− reflect several different transport mechanisms operating in different segments of the colon, which have been described in detail elsewhere (147). Human colon is also capable of water secretion, but in healthy individuals fluid absorption far outweighs fluid secretion. However, in patients with active ulcerative colitis (UC), where the mucosa of the rectum and (to a variable extent) the colon is inflamed, diarrhea is the main and most debilitating symptom. Based on the results of a variety of studies, it is now clear that impaired Na+ and Cl− absorption (rather than increased Cl− secretion) leads to decreased colonic water retention, and this is the main cause of diarrhea in this disease (7, 71, 80, 155).
Basolateral IK channels in ulcerative colitis:
Studies comparing differences in electrolyte transport between healthy individuals and patients with active UC have been done mainly in the distal colon and rectum, where electrogenic Na+ absorption is normally the dominant Na+ absorptive process. This generates a substantial lumen-negative trans-mucosal electrical potential difference (PD) in healthy distal colon (161). By contrast, a marked decrease (depolarization) or loss of this PD is the bioelectric hallmark of active UC, which reflects defective apical Na+ channel function with the virtual disappearance of electrogenic Na+ absorption (7, 71), as well as basolateral membrane depolarization (155). During Na+ absorption across healthy human distal colon and rectum, the basolateral membrane (and thus the cell interior) is maintained in a hyperpolarized state by K+ ions recycling across the membrane via K+ channels. Indeed, the negative intracellular potential is a prerequisite for apical Na+ entry. Several studies in healthy human colon identified intermediate conductance (~25 pS) Ca2+-activated K+ channels encoded by the Kcnn4 gene (referred to as IK channels), as the dominant basolateral K+ channel in colonic crypt cells (24, 105, 157). Immunolabeling revealed basolateral IK channels are distributed uniformly along the surface-crypt axis in healthy individuals, with greatly decreased channel expression in active UC colon (5). Patch clamp analysis showed cell conductance to be dominated by basolateral IK channels in healthy individual, but channel abundance and overall activity were decreased by 53% and 61% respectively in active UC, equating to a 75% decrease in basolateral membrane K+ conductance in this disease (5). Thus, in addition to defective apical Na+ channel function, substantial decreases in basolateral IK channel expression and activity occur in active UC. This loss of IK channel functions most likely accounts for the epithelial cell depolarization that occurs in active UC, resulting in a decreased electrical driving force for electrogenic Na+ absorption across the inflamed mucosa. Interestingly, IK channel expression and activity reverted to normal in UC patients in clinical remission (5), which fits well with results from in vivo rectal dialysis studies, where UC patients in clinical remission had lumen-negative transmucosal PDs and net Na+, Cl−, and water absorptive fluxes identical to those in healthy individuals (154).
Basolateral KCNQ1/KCNE3 channels in ulcerative colitis:
While IK channels dominate basolateral K+ conductance in human colon, basolateral membranes of mouse colonic crypt cells also contain small conductance K+ (KCNQ1/KCNE3) channels. The KCNQ1/KCNE3 complex functions as a constitutively open basolateral K+ channel and, as described earlier, has a critical role in cAMP-stimulated electrogenic Cl− secretion. KCNQ1/KCNE3 (or SK) channels maintain the Cl− secretory response by recycling K+ entering the cell via the basolateral Na+-K+−2Cl− cotransporter, thus hyperpolarizing the cell while Cl− entering basolaterally exits the cell via apical CFTR channels. Although the inflamed mucosa in active UC contains high levels of a number of inflammatory cytokines that increase intracellular cAMP, the low/absent transmucosal PD seen in active UC is inconsistent with electrogenic Cl− secretion (154), and Cl− secretion is not a feature of this disease (80, 154), raising the possibility that the expression/activity of putative SK channels in the inflamed colon might be decreased. In a recent study, however, despite similar levels of KCNQ1 and KCNE3 mRNA expression in colonic crypts from healthy and active UC patients, single cAMP-activated 6.8 pS channels were seen in 36% of basolateral patches in healthy individuals and in 74% of patches in active UC patients, with two or more channels per patch. Furthermore, overall channel activity was 10-fold greater in active UC, with a 20-fold greater contribution to basolateral conductance than in normals. Thus, SK channels appear to make a relatively small contribution to basolateral K+ conductance in normal colonic epithelial cells, and even though enhanced, SK channel activity in active UC is insufficient to prevent cell depolarization. This provides additional evidence that defective Na+ absorption rather than enhanced Cl− secretion is the main pathophysiological mechanism of diarrhea in UC.
Apical BK channels in ulcerative colitis:
Apical BK channels have been studied extensively in mouse colon, where they are present along the entire surface cell-crypt cell axis (166, 179). By contrast, in rat colon, apical BK channels localize to surface cells and cells in the upper 20% of crypts, with relatively low levels of channel abundance (as judged by patch clamp recording) in both the proximal and distal segments, although BK channel expression and abundance are greatly enhanced in the distal colon during chronic dietary K+ loading (129). The distribution of apical BK channels (214 pS) along the crypt axis in normal human colon is similar to that in normal rat colon, without any obvious proximal-distal variation in channel expression (158). However, in patients with UC, the pattern of BK channel distribution is altered, so that BK channel protein is expressed uniformly along the entire surface cell-crypt cell axis, a change that is present irrespective of whether the disease is active or quiescent (158). It is presently unclear whether the wider distribution of BK channel protein along the entire crypt in UC patients results in an increase in luminal (apical) K+ permeability, but if that is indeed the case, it may explain the increased colonic K+ secretion that occurs in some patients with active UC (8, 78, 80), leading to excessive fecal K+ losses and hypokalemia. The fact that the wider cryptal distribution of BK channel expression persists in quiescent UC (where colonic K+ secretion is likely to be normal), suggests that fecal K+ losses in UC are also dependent upon overall BK channel activity, which is likely to be stimulated in active UC by cAMP and/or Ca2+-mediated inflammatory cytokines.
Recent studies using an experimental model of dextran sulfate sodium (DSS)-induced distal colitis in rats, have shown changes in apical BK channel expression and upregulation of active K+ secretion, as well as histological changes, that are remarkably similar to those seen in human active UC (94). Whereas there was zero net K+ transport in control animals, there was active K+ secretion in DSS-treated animals, which was inhibited by 98, 76, and 22% by Ba2+ (a nonspecific K+ channel blocker), iberiotoxin (IbTX; a specific BK channel blocker), and TRAM-34 (a specific IK channel blocker), respectively. Compared to controls, apical BK channel α-subunit mRNA abundance and protein expression were enhanced 6- and 3-fold respectively. Thus, in DSS-induced colitis, active K+ secretion involved upregulation of apical BK channel expression (94). Since similar changes in K+ transport occur in patients with UC, diarrhea in this disease may reflect water secretion driven by increased colonic K+ secretion, in addition to defective Na+ and Cl− absorption. It is worth noting that both Ca2+- and cAMP-stimulated Cl− secretion are defective in DSS-colitis rat distal colon (Figure-13). This experimental model of chronic colitis is likely to be extremely useful for future studies into the intracellular mechanisms that determine the ion transport defects underlying diarrhea in human UC.
Role of apical BK channels in end-stage renal disease:
An important feature of the natural progression of end-stage renal disease (ESRD) is that patients tend to remain normokalemic for long periods in the face of steadily deteriorating renal excretory function. Adaptive changes occur within the remaining functional renal tubules, namely enhanced K+ uptake across the basolateral (peritubular) membrane, which is mediated by increased cortical and outer medullary Na+, K+-ATPase activity (83, 167). However, this response alone cannot entirely explain the maintenance of K+ homeostasis in the pre-dialysis phase (during which there is usually no or only a relatively small restriction of dietary K+ intake), because urinary K+ losses are generally substantially lower than in healthy individuals. This raises the question of how the body continues to excrete K+ when renal K+ excretory capacity is so impaired. A possible clue came from metabolic studies performed nearly 50 years ago, which showed enhanced fecal K+ losses in patients with ESRD, with a strong correlation between dietary K+ intake and fecal K+ output, raising the possibility that some part of the intestinal tract developed an accessory K+ excretory role during progressively deteriorating renal function (82, 207). Indeed, such an adaptive response may account for sustained normokalemia in many patients, before they eventually require additional intervention in the form of continuous ambulatory peritoneal dialysis (CAPD), hemodialysis, and ultimately, renal transplantation. Subsequent studies into intestinal K+ transport in rats with normal renal function fed a high K+ diet for 10–14 days indicated that the colon, but not the small intestine, was capable of increasing its capacity for K+ secretion in response to dietary K+ enrichment (59). Chronic dietary K+ loading stimulated a pan-colonic active K+ secretory process (64, 65, 97), which involved increased Na+,K+- ATPase-mediated K+ uptake across an amplified basolateral membrane, a rise in intracellular K+ concentration, and an increase in apical membrane K+ conductance (95, 150). Furthermore, in vivo dialysis studies in healthy individuals and patients with ESRD indicated that rectal K+ secretion was substantially greater than normal in normokalemic patients with ESRD who were not yet established on dialysis, normokalemic patients maintained on CAPD, and patients undergoing hemodialysis (151, 152). Enhanced rectal K+ secretion in these groups was independent of the transmucosal electrical potential difference, as well as the rate of Na+ absorption and the circulating level of plasma aldosterone, which suggested that altered K+ transport reflected the stimulation of an active K+ secretory process (151).
A critical component of the upregulated active K+ secretory process in rat colon elicited by chronic dietary K+ loading is the induction and/or activation of high-conductance (BK; 220 pS) apical K+ channels in surface colonic epithelial cells (29, 129). Similar if not identical apical BK channels occur in surface cells around the luminal openings of human colonic crypts (114, 158). In a recent study, the apical K+ permeabilities of the proximal rectum in ESRD patients and patients with normal renal function were compared using the rectal dialysis technique, and the expression of BK channels in the distal colon of these two groups of patients evaluated using a specific BK channel antibody (114). Rectal K+ secretion was almost threefold greater in ESRD patients than in patients with normal renal function, and intraluminal Ba2+ (a general inhibitor of K+ channels) decreased K+ secretion in the ESRD patients by 45%, but had no effect on K+ transport in normal patients. Immunostaining with a specific antibody to the BK channel α-subunit demonstrated significantly greater levels of BK channel protein expression in surface colonocytes and crypt cells in ESRD patients than in patients with normal renal function, in whom low levels of expression were mainly restricted to surface colonocytes. Taken together, these results suggest that upregulated colonic K+ secretion in ESRD reflects enhancement of the apical K+ permeability of the large intestinal epithelium, most likely the result of increased expression of apical BK channels (114). What drives this adaptive change in apical BK channel expression in ESRD is unclear, but one possibility is post-prandial increases in plasma K+ concentration. This hypothesis was tested by measuring plasma K+ concentrations in the fasting state, and for 180 minutes after the oral administration of 30 mmol of K+ to control subjects and normokalemic patients with ESRD who were ‘pre-dialysis’ or undergoing CAPD. Plasma K+ concentrations were also monitored in fasting controls and ESRD patients who were not given the oral K+ load. Oral K+ loading caused plasma K+ concentration to rise within the normal range in control subjects, while significantly higher concentrations were achieved in ‘predialysis’ patients and sustained hyperkalemia developed in CAPD patients (115). Thus, by raising the “K+ load” presented to the colonic epithelium, postprandial increases in plasma K+ concentration may be an important signal in maintaining the colon in a state of K+ hypersecretion.
Despite the rise in colonic K+ secretory capacity in ESRD, interdialytic hyperkalemia remains a potentially life-threatening problem in hemodialysis patients. cAMP elicits a greater proximal colonic net K+ secretory response in dietary K+ loaded rats than in normal animals (65). This raises the possibility that interdialytic hyperkalemia might be attenuated by further enhancing colonic K+ secretion using a cAMP-mediated laxative, thus limiting postprandial increases in plasma K+ concentration in hemodialysis patients. This was evaluated by measuring plasma K+ concentrations in control subjects and hemodialysis patients before and during two weeks treatment with bisacodyl (a cAMP-mediated laxative), and in hemodialysis patients before and during two weeks treatment with lactulose (an osmotic laxative) (115). Bisacodyl treatment had no effect on plasma K+ concentrations in control subjects, but significantly decreased the mean interdialytic plasma K+ concentration in hemodialysis patients, whereas there was no change during lactulose treatment (115). Since cAMP stimulates both colonic apical BK channel expression and net colonic K+ secretion (65, 130), these findings suggest that cAMP-mediated laxatives may be a novel approach to the reduction of severe interdialytic hyperkalemia in hemodialysis patients.
Conclusion
The colon plays critical role in K+ homeostasis, as it has enormous adaptive capacities to absorb and secrete K+ in physiological and pathophysiological conditions. This review summarizes the evidence that apical H+,K+-ATPase, composed of catalytic α- (HKCα) and regulatory β- (HKCβ) subunits, and operating in parallel with basolateral K+ and Cl− channels, mediates active K+ absorption in animal colon. Furthermore, dietary Na+ (hyperaldosteronism) and dietary K+ deprivation upregulate H+,K+-ATPase-mediated K+ absorption by regulating HKCα and HKCβ expression, respectively. Although we now have a detailed understanding of how changes in dietary K+ intake regulate colonic H+,K+-ATPase activity and K+ absorption, the effect of disease (e.g., UC and malignant transformation) on H+,K+-ATPase-mediated colonic K+ absorption remains to be established.
Active K+ secretion is mediated by the coordinated regulation of apical K+ channels and basolateral Na+-K+−2Cl− cotransporter in both animal and human colon. To a lesser degree, basolateral Na+,K+-ATPase also participates in active K+ secretion. Although both IK and BK channels are localized to colonic apical membranes, BK channels play a critical role in secretory agonist (i.e., cAMP and Ca2+)- and aldosterone-stimulated colonic K+ secretion. Both BK channel expression and BK channel-mediated K+ secretion are enhanced in patients with ESRD and UC, and in experimental UC in animals. BK channels are composed of α- and β-subunits, there being two BKα (BKαRL and BKαYR) and four BKβ (BKβ1–4) splice variants. BKβ1 and BKβ3 are expressed in human colon. It is not known whether BKαRL and/or BKαYR encode the BK channels that mediate active K+ secretion during physiological and pathophysiological conditions in animal and human colon. It is also unclear whether BKαRL and BKαYR are expressed in same and/or different cell types in animal and human colon. Cellular K+ exit through apical and basolateral IK channels contributes, at least in part, to maintaining membrane hyperpolarization, a prerequisite for sustained agonist-stimulated active Cl− secretion.
Didactic Synopsis.
Major Teaching Points:
The gastrointestinal (GI) tract has enormous capacity to absorb and secrete K+ to maintain K+ homeostasis.
- K+ absorption:
- In vivo perfusion studies to evaluate unidirectional K+ fluxes showed passive K+ absorption is mediated by solvent drag in both animal and human colon.
- In vitro ion fluxes measured under voltage clamp condition, and biochemical and molecular studies of enzyme activities, identified apical H+,K+-ATPase as the mediator of active K+ absorption in animal colon..
- Aldosterone-stimulated active K+ absorption in animal colon reflects enhanced apical H+,K+-ATPase mRNA expression and protein activity.
- Although apical H+,K+-ATPase mediates active K+ absorption in animal colon, the molecular basis for K+ absorption in human colon has yet to be idenitified.
- K+ secretion:
- Active K+ secretion occurs in both animal and human colon.
- Apical K+ channels and basolateral Na+-K+−2Cl− cotransport coordinate active K+ secretion.
- Ca2+-activated large conductance K+ (BK) channels constitute the main apical K+ conductance for K+ exit into the lumen.
- Role and regulation of active K+ secretion:
- Aldosterone and high-K+ diet stimulate apical BK channel expression and active K+ secretion.
- Upregulated BK channel expression is the adaptive mechanism for K+ secretion in patients with end-stage renal disease.
- Increased BK channel expression and K+ secretion likely contribute to diarrhea in patients with ulcerative colitis.
- Potassium channels could be a potential therapeutic target to control diarrhea in ulcerative colitis.
Acknowledgements
This work was supported by NIH/NIDDK RO1DK018777 grant to V. M. Rajendran, and grant support from the Wellcome Trust, Medical Research Council, and Yorkshire Cancer Research to G. I. Sandle.
References
- 1.Abrahamse SI, De Jonge HR, Bindels RJ, and Van Os CH. Two distinct K(+)-ATPase activities in rabbit distal colon. Biochem Biophys Res Commun 207: 1003–1008, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Abrahamse SL, Bindels RJ, and van Os CH. The colon carcinoma cell line Caco-2 contains an H+/K(+)-ATPase that contributes to intracellular pH regulation. Pflugers Arch 421: 591–597, 1992. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal R, Afzalpurkar R, and Fordtran JS. Pathophysiology of potassium absorption and secretion by the human intestine. Gastroenterology 107: 548–571, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Al-Hazza A, Linley J, Aziz Q, Hunter M, and Sandle G. Upregulation of basolateral small conductance potassium channels (KCNQ1/KCNE3) in ulcerative colitis. Biochem Biophys Res Commun 470: 473–478, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Hazza A, Linley JE, Aziz Q, Maclennan KA, Hunter M, and Sandle GI. Potential role of reduced basolateral potassium (IKCa3.1) channel expression in the pathogenesis of diarrhoea in ulcerative colitis. J Pathol 226: 463–470, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Almassy J, Won JH, Begenisich TB, and Yule DI. Apical Ca2+-activated potassium channels in mouse parotid acinar cells. J Gen Physiol 139: 121–133, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amasheh S, Barmeyer C, Koch CS, Tavalali S, Mankertz J, Epple HJ, Gehring MM, Florian P, Kroesen AJ, Zeitz M, Fromm M, and Schulzke JD. Cytokine-dependent transcriptional down-regulation of epithelial sodium channel in ulcerative colitis. Gastroenterology 126: 1711–1720, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Archampong EQ, Harris J, and Clark CG. The absorption and secretion of water and electrolytes across the healthy and the diseased human colonic mucosa measured in vitro. Gut 13: 880–886, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asano S, Hoshina S, Nakaie Y, Watanabe T, Sato M, Suzuki Y, and Takeguchi N. Functional expression of putative H+-K+-ATPase from guinea pig distal colon. Am J Physiol 275: C669–674, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Atwell JD, and Duthie HL. The Absorption of Water, Sodium, and Potassium from the Ileum of Humans Showing the Effects of Regional Enteritis. Gastroenterology 46: 16–22, 1964. [PubMed] [Google Scholar]
- 11.Barada K, Okolo C, Field M, and Cortas N. Na,K-ATPase in diabetic rat small intestine. Changes at protein and mRNA levels and role of glucagon. J Clin Invest 93: 2725–2731, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barmeyer C, Rahner C, Yang Y, Sigworth FJ, Binder HJ, and Rajendran VM. Cloning and identification of tissue-specific expression of KCNN4 splice variants in rat colon. Am J Physiol Cell Physiol 299: C251–263, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnaby CF, and Edmonds CJ. Use of a miniature GM counter and a whole body counter in the study of potassium transport by the colon of normal, sodium-depleted and adrenalectomized rats in vivo. J Physiol 205: 647–665, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bastl CP, Barnett CA, Schmidt TJ, and Litwack G. Glucocorticoid stimulation of sodium absorption in colon epithelia is mediated by corticosteroid IB receptor. J Biol Chem 259: 1186–1195, 1984. [PubMed] [Google Scholar]
- 15.Bastl CP, and Hayslett JP. The cellular action of aldosterone in target epithelia. Kidney Int 42: 250–264, 1992. [DOI] [PubMed] [Google Scholar]
- 16.Behrens R, Nolting A, Reimann F, Schwarz M, Waldschutz R, and Pongs O. hKCNMB3 and hKCNMB4, cloning and characterization of two members of the large-conductance calcium-activated potassium channel beta subunit family. FEBS Lett 474: 99–106, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Berkefeld H, Fakler B, and Schulte U. Ca2+-activated K+ channels: from protein complexes to function. Physiol Rev 90: 1437–1459, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Billich CO, and Levitan R. Effects of sodium concentration and osmolality on water and electrolyte absorption form the intact human colon. J Clin Invest 48: 1336–1347, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Binder HJ, and Murer H. Potassium/proton exchange in brush-border membrane of rat ileum. J Membr Biol 91: 77–84, 1986. [DOI] [PubMed] [Google Scholar]
- 20.Binder HJ, Sandle GI, and Rajendran VM Colonic Fluid and Electrolyte Transport in Health and Disease. In: The Large Intestine, edited by Phillips SF, Shorter R, and Pemberton W New York: Raven Press, 1991, p. pp. 141–168. [Google Scholar]
- 21.Binder HJ, Sangan P, and Rajendran VM. Physiological and molecular studies of colonic H+,K+-ATPase. Semin Nephrol 19: 405–414, 1999. [PubMed] [Google Scholar]
- 22.Binder HJ, White A, Whiting D, and Hayslett J. Demonstration of specific high affinity receptors for aldosterone in cytosol of rat colon. Endocrinology 118: 628–631, 1986. [DOI] [PubMed] [Google Scholar]
- 23.Blanco G, and Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol 275: F633–650, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Bowley KA, Morton MJ, Hunter M, and Sandle GI. Non-genomic regulation of intermediate conductance potassium channels by aldosterone in human colonic crypt cells. Gut 52: 854–860, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breitwieser GE. Mechanisms of K+ channel regulation. J Membr Biol 152: 1–11, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Brenner R, Jegla TJ, Wickenden A, Liu Y, and Aldrich RW. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J Biol Chem 275: 6453–6461, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Burckhardt BC, and Gogelein H. Small and maxi K+ channels in the basolateral membrane of isolated crypts from rat distal colon: single-channel and slow whole-cell recordings. Pflugers Arch 420: 54–60, 1992. [DOI] [PubMed] [Google Scholar]
- 28.Butler A, Tsunoda S, McCobb DP, Wei A, and Salkoff L. mSlo, a complex mouse gene encoding “maxi” calcium-activated potassium channels. Science 261: 221–224, 1993. [DOI] [PubMed] [Google Scholar]
- 29.Butterfield I, Warhurst G, Jones MN, and Sandle GI. Characterization of apical potassium channels induced in rat distal colon during potassium adaptation. J Physiol 501 (Pt 3): 537–547, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Catalan M, Cornejo I, Figueroa CD, Niemeyer MI, Sepulveda FV, and Cid LP. ClC-2 in guinea pig colon: mRNA, immunolabeling, and functional evidence for surface epithelium localization. Am J Physiol Gastrointest Liver Physiol 283: G1004–1013, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Catalan M, Niemeyer MI, Cid LP, and Sepulveda FV. Basolateral ClC-2 chloride channels in surface colon epithelium: regulation by a direct effect of intracellular chloride. Gastroenterology 126: 1104–1114, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Catalan MA, Pena-Munzenmayer G, and Melvin JE. Ca(2)(+)-dependent K(+) channels in exocrine salivary glands. Cell Calcium 55: 362–368, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen L, Tuo B, and Dong H. Regulation of Intestinal Glucose Absorption by Ion Channels and Transporters. Nutrients 8: 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiu YH, Alvarez-Baron C, Kim EY, and Dryer SE. Dominant-negative regulation of cell surface expression by a pentapeptide motif at the extreme COOH terminus of an Slo1 calcium-activated potassium channel splice variant. Mol Pharmacol 77: 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chow DC, and Forte JG. Functional significance of the beta-subunit for heterodimeric P-type ATPases. J Exp Biol 198: 1–17, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Codina J, Delmas-Mata JT, and DuBose TD Jr. The alpha-subunit of the colonic H+,K+-ATPase assembles with beta1-Na+,K+-ATPase in kidney and distal colon. J Biol Chem 273: 7894–7899, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Codina J, Kone BC, Delmas-Mata JT, and DuBose TD Jr. Functional expression of the colonic H+,K+-ATPase alpha-subunit. Pharmacologic properties and assembly with X+,K+-ATPase beta-subunits. J Biol Chem 271: 29759–29763, 1996. [DOI] [PubMed] [Google Scholar]
- 38.Cougnon M, Planelles G, Crowson MS, Shull GE, Rossier BC, and Jaisser F. The rat distal colon P-ATPase alpha subunit encodes a ouabain-sensitive H+, K+-ATPase. J Biol Chem 271: 7277–7280, 1996. [DOI] [PubMed] [Google Scholar]
- 39.Cox DH, and Aldrich RW. Role of the beta1 subunit in large-conductance Ca(2+)-activated K(+) channel gating energetics. Mechanisms of enhanced Ca(2+) sensitivity. J Gen Physiol 116: 411–432, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crowson MS, and Shull GE. Isolation and characterization of a cDNA encoding the putative distal colon H+,K(+)-ATPase. Similarity of deduced amino acid sequence to gastric H+,K(+)-ATPase and Na+,K(+)-ATPase and mRNA expression in distal colon, kidney, and uterus. J Biol Chem 267: 13740–13748, 1992. [PubMed] [Google Scholar]
- 41.Dames P, Bergann T, Fromm A, Bucker R, Barmeyer C, Krug SM, Fromm M, and Schulzke JD. Interleukin-13 affects the epithelial sodium channel in the intestine by coordinated modulation of STAT6 and p38 MAPK activity. J Physiol 593: 5269–5282, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis GR, Santa Ana CA, Morawski SG, and Fordtran JS. Permeability characteristics of human jejunum, ileum, proximal colon and distal colon: results of potential difference measurements and unidirectional fluxes. Gastroenterology 83: 844–850, 1982. [PubMed] [Google Scholar]
- 43.Dedek K, and Waldegger S. Colocalization of KCNQ1/KCNE channel subunits in the mouse gastrointestinal tract. Pflugers Arch 442: 896–902, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Deitrick JE, Whedon GD, and Shorr E. Effects of immobilization upon various metabolic and physiologic functions of normal men. Am J Med 4: 3–36, 1948. [DOI] [PubMed] [Google Scholar]
- 45.Del Castillo JR, Rajendran VM, and Binder HJ. Apical membrane localization of ouabain-sensitive K(+)-activated ATPase activities in rat distal colon. Am J Physiol 261: G1005–1011, 1991. [DOI] [PubMed] [Google Scholar]
- 46.Demolombe S, Franco D, de Boer P, Kuperschmidt S, Roden D, Pereon Y, Jarry A, Moorman AF, and Escande D. Differential expression of KvLQT1 and its regulator IsK in mouse epithelia. Am J Physiol Cell Physiol 280: C359–372, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Devor DC, and Frizzell RA. Modulation of K+ channels by arachidonic acid in T84 cells. II. Activation of a Ca(2+)-independent K+ channel. Am J Physiol 274: C149–160, 1998. [DOI] [PubMed] [Google Scholar]
- 48.Devor DC, Singh AK, Gerlach AC, Frizzell RA, and Bridges RJ. Inhibition of intestinal Cl- secretion by clotrimazole: direct effect on basolateral membrane K+ channels. Am J Physiol 273: C531–540, 1997. [DOI] [PubMed] [Google Scholar]
- 49.Devroede GJ, and Phillips SF. Conservation of sodium, chloride, and water by the human colon. Gastroenterology 56: 101–109, 1969. [PubMed] [Google Scholar]
- 50.Dharmsathaphorn K, and Pandol SJ. Mechanism of chloride secretion induced by carbachol in a colonic epithelial cell line. J Clin Invest 77: 348–354, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diamond JM, Ehrlich BE, Morawski SG, Santa Ana CA, and Fordtran JS. Lithium absorption in tight and leaky segments of intestine. J Membr Biol 72: 153–159, 1983. [DOI] [PubMed] [Google Scholar]
- 52.Dorge A, Beck FX, and Rechkemmer G. Cellular site of active K absorption in the guinea-pig distal colonic epithelium. Pflugers Arch 436: 280–288, 1998. [DOI] [PubMed] [Google Scholar]
- 53.Duthie HL, and Atwell JD. The Absorption of Water, Sodium, and Potassium in the Large Intestine with Particular Reference to the Effects of Villous Papillomas. Gut 4: 373–377, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dworetzky SI, Boissard CG, Lum-Ragan JT, McKay MC, Post-Munson DJ, Trojnacki JT, Chang CP, and Gribkoff VK. Phenotypic alteration of a human BK (hSlo) channel by hSlobeta subunit coexpression: changes in blocker sensitivity, activation/relaxation and inactivation kinetics, and protein kinase A modulation. J Neurosci 16: 4543–4550, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edmonds CJ. Kinetics of potassium in colonic mucosa of normal and sodium-depleted rats. J Physiol 203: 533–554, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Edmonds CJ. Transport of sodium and secretion of potassium and bicarbonate by the colon of normal and sodium-depleted rats. J Physiol 193: 589–602, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fejes-Toth G, Rusvai E, Longo KA, and Naray-Fejes-Toth A. Expression of colonic H-K-ATPase mRNA in cortical collecting duct: regulation by acid/base balance. Am J Physiol 269: F551–557, 1995. [DOI] [PubMed] [Google Scholar]
- 58.Field M Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest 111: 931–943, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fisher KA, Binder HJ, and Hayslett JP. Potassium secretion by colonic mucosal cells after potassium adaptation. Am J Physiol 231: 987–994, 1976. [DOI] [PubMed] [Google Scholar]
- 60.Flores CA, Melvin JE, Figueroa CD, and Sepulveda FV. Abolition of Ca2+-mediated intestinal anion secretion and increased stool dehydration in mice lacking the intermediate conductance Ca2+-dependent K+ channel Kcnn4. J Physiol 583: 705–717, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fodor AA, and Aldrich RW. Convergent evolution of alternative splices at domain boundaries of the BK channel. Annu Rev Physiol 71: 19–36, 2009. [DOI] [PubMed] [Google Scholar]
- 62.Foster ES, Hayslett JP, and Binder HJ. Colonic potassium transport. Kroc Found Ser 17: 47–63, 1984. [PubMed] [Google Scholar]
- 63.Foster ES, Hayslett JP, and Binder HJ. Mechanism of active potassium absorption and secretion in the rat colon. Am J Physiol 246: G611–617, 1984. [DOI] [PubMed] [Google Scholar]
- 64.Foster ES, Jones WJ, Hayslett JP, and Binder HJ. Role of aldosterone and dietary potassium in potassium adaptation in the distal colon of the rat. Gastroenterology 88: 41–46, 1985. [DOI] [PubMed] [Google Scholar]
- 65.Foster ES, Sandle GI, Hayslett JP, and Binder HJ. Cyclic adenosine monophosphate stimulates active potassium secretion in the rat colon. Gastroenterology 84: 324–330, 1983. [PubMed] [Google Scholar]
- 66.Foster ES, Sandle GI, Hayslett JP, and Binder HJ. Dietary potassium modulates active potassium absorption and secretion in rat distal colon. Am J Physiol 251: G619–626, 1986. [DOI] [PubMed] [Google Scholar]
- 67.Fuller PJ, and Verity K. Mineralocorticoid receptor gene expression in the gastrointestinal tract: distribution and ontogeny. J Steroid Biochem 36: 263–267, 1990. [DOI] [PubMed] [Google Scholar]
- 68.Furness JB, Robbins HL, Selmer IS, Hunne B, Chen MX, Hicks GA, Moore S, and Neylon CB. Expression of intermediate conductance potassium channel immunoreactivity in neurons and epithelial cells of the rat gastrointestinal tract. Cell Tissue Res 314: 179–189, 2003. [DOI] [PubMed] [Google Scholar]
- 69.Geering K Functional roles of Na,K-ATPase subunits. Curr Opin Nephrol Hypertens 17: 526–532, 2008. [DOI] [PubMed] [Google Scholar]
- 70.Grahammer F, Warth R, Barhanin J, Bleich M, and Hug MJ. The small conductance K+ channel, KCNQ1: expression, function, and subunit composition in murine trachea. J Biol Chem 276: 42268–42275, 2001. [DOI] [PubMed] [Google Scholar]
- 71.Greig ER, Boot-Handford RP, Mani V, and Sandle GI. Decreased expression of apical Na+ channels and basolateral Na+, K+-ATPase in ulcerative colitis. J Pathol 204: 84–92, 2004. [DOI] [PubMed] [Google Scholar]
- 72.Grishin AV, and Caplan MJ. ATP1AL1, a member of the non-gastric H,K-ATPase family, functions as a sodium pump. J Biol Chem 273: 27772–27778, 1998. [DOI] [PubMed] [Google Scholar]
- 73.Grishin AV, Sverdlov VE, Kostina MB, and Modyanov NN. Cloning and characterization of the entire cDNA encoded by ATP1AL1--a member of the human Na,K/H,K-ATPase gene family. FEBS Lett 349: 144–150, 1994. [DOI] [PubMed] [Google Scholar]
- 74.Gustin MC, and Goodman DB. Isolation of brush-border membrane from the rabbit descending colon epithelium. Partial characterization of a unique K+-activated ATPase. J Biol Chem 256: 10651–10656, 1981. [PubMed] [Google Scholar]
- 75.Halm DR, and Dawson DC. Potassium transport by turtle colon: active secretion and active absorption. Am J Physiol 246: C315–322, 1984. [DOI] [PubMed] [Google Scholar]
- 76.Halm DR, and Frizzell RA. Active K transport across rabbit distal colon: relation to Na absorption and Cl secretion. Am J Physiol 251: C252–267, 1986. [DOI] [PubMed] [Google Scholar]
- 77.Halm DR, and Halm ST. Aldosterone stimulates K secretion prior to onset of Na absorption in guinea pig distal colon. Am J Physiol 266: C552–558, 1994. [DOI] [PubMed] [Google Scholar]
- 78.Harris J, and Shields R. Absorption and secretion of water and electrolytes by the intact human colon in diffuse untreated proctocolitis. Gut 11: 27–33, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hawker PC, Mashiter KE, and Turnberg LA. Mechanisms of transport of Na, Cl, and K in the human colon. Gastroenterology 74: 1241–1247, 1978. [PubMed] [Google Scholar]
- 80.Hawker PC, McKay JS, and Turnberg LA. Electrolyte transport across colonic mucosa from patients with inflammatory bowel disease. Gastroenterology 79: 508–511, 1980. [PubMed] [Google Scholar]
- 81.Hay-Schmidt A, Grunnet M, Abrahamse SL, Knaus HG, and Klaerke DA. Localization of Ca2+ -activated big-conductance K+ channels in rabbit distal colon. Pflugers Arch 446: 61–68, 2003. [DOI] [PubMed] [Google Scholar]
- 82.Hayes CP Jr., McLeod ME, and Robinson RR. An extravenal mechanism for the maintenance of potassium balance in severe chronic renal failure. Trans Assoc Am Physicians 80: 207–216, 1967. [PubMed] [Google Scholar]
- 83.Hayslett JP, and Binder HJ. Mechanism of potassium adaptation. Am J Physiol 243: F103–112, 1982. [DOI] [PubMed] [Google Scholar]
- 84.Hayslett JP, Myketey N, Binder HJ, and Aronson PS. Mechanism of increased potassium secretion in potassium loading and sodium deprivation. Am J Physiol 239: F378–F382, 1980. [DOI] [PubMed] [Google Scholar]
- 85.Heitzmann D, and Warth R. No potassium, no acid: K+ channels and gastric acid secretion. Physiology (Bethesda) 22: 335–341, 2007. [DOI] [PubMed] [Google Scholar]
- 86.Heitzmann D, and Warth R. Physiology and pathophysiology of potassium channels in gastrointestinal epithelia. Physiol Rev 88: 1119–1182, 2008. [DOI] [PubMed] [Google Scholar]
- 87.Ikuma M, Kashgarian M, Binder HJ, and Rajendran VM. Differential regulation of NHE isoforms by sodium depletion in proximal and distal segments of rat colon. Am J Physiol 276: G539–549, 1999. [DOI] [PubMed] [Google Scholar]
- 88.Jaisser F, Escoubet B, Coutry N, Eugene E, Bonvalet JP, and Farman N. Differential regulation of putative K(+)-ATPase by low-K+ diet and corticosteroids in rat distal colon and kidney. Am J Physiol 270: C679–687, 1996. [DOI] [PubMed] [Google Scholar]
- 89.Jaisser F, Horisberger JD, Geering K, and Rossier BC. Mechanisms of urinary K+ and H+ excretion: primary structure and functional expression of a novel H,K-ATPase. J Cell Biol 123: 1421–1429, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jaisser F, Jaunin P, Geering K, Rossier BC, and Horisberger JD. Modulation of the Na,K-pump function by beta subunit isoforms. J Gen Physiol 103: 605–623, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Johnson BB, Lieberman AH, and Mulrow PJ. Aldosterone excretion in normal subjects depleted of sodium and potassium. J Clin Invest 36: 757–766, 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Joiner WJ, Basavappa S, Vidyasagar S, Nehrke K, Krishnan S, Binder HJ, Boulpaep EL, and Rajendran VM. Active K+ secretion through multiple KCa-type channels and regulation by IKCa channels in rat proximal colon. Am J Physiol Gastrointest Liver Physiol 285: G185–196, 2003. [DOI] [PubMed] [Google Scholar]
- 93.Joiner WJ, Wang LY, Tang MD, and Kaczmarek LK. hSK4, a member of a novel subfamily of calcium-activated potassium channels. Proc Natl Acad Sci U S A 94: 11013–11018, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kanthesh BM, Sandle GI, and Rajendran VM. Enhanced K(+) secretion in dextran sulfate-induced colitis reflects upregulation of large conductance apical K(+) channels (BK; Kcnma1). Am J Physiol Cell Physiol 305: C972–980, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kashgarian M, Taylor CR, Binder HJ, and Hayslett JP. Amplification of cell membrane surface in potassium adaptation. Lab Invest 42: 581–588, 1980. [PubMed] [Google Scholar]
- 96.Kaunitz JD, and Sachs G. Identification of a vanadate-sensitive potassium-dependent proton pump from rabbit colon. J Biol Chem 261: 14005–14010, 1986. [PubMed] [Google Scholar]
- 97.Kliger AS, Binder HJ, Bastl C, and Hayslett JP. Demonstration of active potassium transport in the mammalian colon. J Clin Invest 67: 1189–1196, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kunzelmann K, Hubner M, Schreiber R, Levy-Holzman R, Garty H, Bleich M, Warth R, Slavik M, von Hahn T, and Greger R. Cloning and function of the rat colonic epithelial K+ channel KVLQT1. J Membr Biol 179: 155–164, 2001. [DOI] [PubMed] [Google Scholar]
- 99.Kunzelmann K, and Mall M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev 82: 245–289, 2002. [DOI] [PubMed] [Google Scholar]
- 100.Kwon SH, and Guggino WB. Multiple sequences in the C terminus of MaxiK channels are involved in expression, movement to the cell surface, and apical localization. Proc Natl Acad Sci U S A 101: 15237–15242, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee J, Rajendran VM, Mann AS, Kashgarian M, and Binder HJ. Functional expression and segmental localization of rat colonic K-adenosine triphosphatase. J Clin Invest 96: 2002–2008, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee MP, Ravenel JD, Hu RJ, Lustig LR, Tomaselli G, Berger RD, Brandenburg SA, Litzi TJ, Bunton TE, Limb C, Francis H, Gorelikow M, Gu H, Washington K, Argani P, Goldenring JR, Coffey RJ, and Feinberg AP. Targeted disruption of the Kvlqt1 gene causes deafness and gastric hyperplasia in mice. J Clin Invest 106: 1447–1455, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Levin RJ, Newey H, and Smyth DH. The Effects of Adrenalectomy and Fasting on Intestinal Function in the Rat. J Physiol 177: 58–73, 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liao T, Wang L, Halm ST, Lu L, Fyffe RE, and Halm DR. K+ channel KVLQT1 located in the basolateral membrane of distal colonic epithelium is not essential for activating Cl- secretion. Am J Physiol Cell Physiol 289: C564–575, 2005. [DOI] [PubMed] [Google Scholar]
- 105.Linley J, Loganathan A, Kopanati S, Sandle GI, and Hunter M. Evidence that two distinct crypt cell types secrete chloride and potassium in human colon. Gut 63: 472–479, 2014. [DOI] [PubMed] [Google Scholar]
- 106.Lippiat JD, Standen NB, Harrow ID, Phillips SC, and Davies NW. Properties of BK(Ca) channels formed by bicistronic expression of hSloalpha and beta1–4 subunits in HEK293 cells. J Membr Biol 192: 141–148, 2003. [DOI] [PubMed] [Google Scholar]
- 107.Lomax RB, McNicholas CM, Lombes M, and Sandle GI. Aldosterone-induced apical Na+ and K+ conductances are located predominantly in surface cells in rat distal colon. Am J Physiol 266: G71–82, 1994. [DOI] [PubMed] [Google Scholar]
- 108.Lomax RB, and Sandle GI. Comparison of aldosterone- and RU-28362-induced apical Na+ and K+ conductances in rat distal colon. Am J Physiol 267: G485–493, 1994. [DOI] [PubMed] [Google Scholar]
- 109.Lomax RB, Warhurst G, and Sandle GI. Characteristics of two basolateral potassium channel populations in human colonic crypts. Gut 38: 243–247, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Loo DD, and Kaunitz JD. Ca2+ and cAMP activate K+ channels in the basolateral membrane of crypt cells isolated from rabbit distal colon. J Membr Biol 110: 19–28, 1989. [DOI] [PubMed] [Google Scholar]
- 111.Ma D, Nakata T, Zhang G, Hoshi T, Li M, and Shikano S. Differential trafficking of carboxyl isoforms of Ca2+-gated (Slo1) potassium channels. FEBS Lett 581: 1000–1008, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Malik N, Canfield VA, Beckers MC, Gros P, and Levenson R. Identification of the mammalian Na,K-ATPase 3 subunit. J Biol Chem 271: 22754–22758, 1996. [DOI] [PubMed] [Google Scholar]
- 113.Mandel KG, McRoberts JA, Beuerlein G, Foster ES, and Dharmsathaphorn K. Ba2+ inhibition of VIP- and A23187-stimulated Cl- secretion by T84 cell monolayers. Am J Physiol 250: C486–494, 1986. [DOI] [PubMed] [Google Scholar]
- 114.Mathialahan T, Maclennan KA, Sandle LN, Verbeke C, and Sandle GI. Enhanced large intestinal potassium permeability in end-stage renal disease. J Pathol 206: 46–51, 2005. [DOI] [PubMed] [Google Scholar]
- 115.Mathialahan T, and Sandle GI. Dietary potassium and laxatives as regulators of colonic potassium secretion in end-stage renal disease. Nephrol Dial Transplant 18: 341–347, 2003. [DOI] [PubMed] [Google Scholar]
- 116.McCabe R, Cooke HJ, and Sullivan LP. Potassium transport by rabbit descending colon. Am J Physiol 242: C81–86, 1982. [DOI] [PubMed] [Google Scholar]
- 117.McDonough AA, Geering K, and Farley RA. The sodium pump needs its beta subunit. FASEB J 4: 1598–1605, 1990. [DOI] [PubMed] [Google Scholar]
- 118.McNamara B, Winter DC, Cuffe JE, O’Sullivan GC, and Harvey BJ. Basolateral K+ channel involvement in forskolin-activated chloride secretion in human colon. J Physiol 519 Pt 1: 251–260, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McNicholas CM, Fraser G, and Sandle GI. Properties and regulation of basolateral K+ channels in rat duodenal crypts. J Physiol 477 (Pt 3): 381–392, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Meera P, Wallner M, Song M, and Toro L. Large conductance voltage- and calcium-dependent K+ channel, a distinct member of voltage-dependent ion channels with seven N-terminal transmembrane segments (S0-S6), an extracellular N terminus, and an intracellular (S9-S10) C terminus. Proc Natl Acad Sci U S A 94: 14066–14071, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Meneton P, Schultheis PJ, Greeb J, Nieman ML, Liu LH, Clarke LL, Duffy JJ, Doetschman T, Lorenz JN, and Shull GE. Increased sensitivity to K+ deprivation in colonic H,K-ATPase-deficient mice. J Clin Invest 101: 536–542, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Morris AP, Gallacher DV, and Lee JA. A large conductance, voltage- and calcium-activated K+ channel in the basolateral membrane of rat enterocytes. FEBS Lett 206: 87–92, 1986. [DOI] [PubMed] [Google Scholar]
- 123.Nakajo K, and Kubo Y. KCNQ1 channel modulation by KCNE proteins via the voltage-sensing domain. J Physiol 593: 2617–2625, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nanda Kumar NS, Singh SK, and Rajendran VM. Mucosal potassium efflux mediated via Kcnn4 channels provides the driving force for electrogenic anion secretion in colon. Am J Physiol Gastrointest Liver Physiol 299: G707–714, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Neylon CB, Lang RJ, Fu Y, Bobik A, and Reinhart PH. Molecular cloning and characterization of the intermediate-conductance Ca(2+)-activated K(+) channel in vascular smooth muscle: relationship between K(Ca) channel diversity and smooth muscle cell function. Circ Res 85: e33–43, 1999. [DOI] [PubMed] [Google Scholar]
- 126.O’Hara B, Alvarez de la Rosa D, and Rajendran VM. Multiple mineralocorticoid response elements localized in different introns regulate intermediate conductance K+ (Kcnn4) channel expression in the rat distal colon. PLoS One 9: e98695, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pandiyan V, Rajendran VM, and Binder HJ. Mucosal ouabain and Na+ inhibit active Rb+(K+) absorption in normal and sodium-depleted rat distal colon. Gastroenterology 102: 1846–1853, 1992. [DOI] [PubMed] [Google Scholar]
- 128.Perrone RD, and McBride DE. Aldosterone and PCO2 enhance rubidium absorption in rat distal colon. Am J Physiol 254: G898–906, 1988. [DOI] [PubMed] [Google Scholar]
- 129.Perry MD, Rajendran VM, MacLennan KA, and Sandle GI. Segmental differences in upregulated apical potassium channels in mammalian colon during potassium adaptation. Am J Physiol Gastrointest Liver Physiol 311: G785–G793, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Perry MD, and Sandle GI. Regulation of colonic apical potassium (BK) channels by cAMP and somatostatin. Am J Physiol Gastrointest Liver Physiol 297: G159–167, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pestov NB, Korneenko TV, Shakhparonov MI, Shull GE, and Modyanov NN. Loss of acidification of anterior prostate fluids in Atp12a-null mutant mice indicates that nongastric H-K-ATPase functions as proton pump in vivo. Am J Physiol Cell Physiol 291: C366–374, 2006. [DOI] [PubMed] [Google Scholar]
- 132.Pestov NB, Romanova LG, Korneenko TV, Egorov MV, Kostina MB, Sverdlov VE, Askari A, Shakhparonov MI, and Modyanov NN. Ouabain-sensitive H,K-ATPase: tissue-specific expression of the mammalian genes encoding the catalytic alpha subunit. FEBS Lett 440: 320–324, 1998. [DOI] [PubMed] [Google Scholar]
- 133.Phillips SF, and Giller J. The contribution of the colon to electrolyte and water conservation in man. J Lab Clin Med 81: 733–746, 1973. [PubMed] [Google Scholar]
- 134.Plass H, Gridl A, and Turnheim K. Absorption and secretion of potassium by rabbit descending colon. Pflugers Arch 406: 509–519, 1986. [DOI] [PubMed] [Google Scholar]
- 135.Pongs O, and Schwarz JR. Ancillary subunits associated with voltage-dependent K+ channels. Physiol Rev 90: 755–796, 2010. [DOI] [PubMed] [Google Scholar]
- 136.Pressley L, and Funder JW. Glucocorticoid and mineralocorticoid receptors in gut mucosa. Endocrinology 97: 588–596, 1975. [DOI] [PubMed] [Google Scholar]
- 137.Preston P, Wartosch L, Gunzel D, Fromm M, Kongsuphol P, Ousingsawat J, Kunzelmann K, Barhanin J, Warth R, and Jentsch TJ. Disruption of the K+ channel beta-subunit KCNE3 reveals an important role in intestinal and tracheal Cl- transport. J Biol Chem 285: 7165–7175, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Puntheeranurak S, Schreiber R, Spitzner M, Ousingsawat J, Krishnamra N, and Kunzelmann K. Control of ion transport in mouse proximal and distal colon by prolactin. Cell Physiol Biochem 19: 77–88, 2007. [DOI] [PubMed] [Google Scholar]
- 139.Rajendran VM, Sangan P, Geibel J, and Binder HJ. Ouabain-sensitive H,K-ATPase functions as Na,K-ATPase in apical membranes of rat distal colon. J Biol Chem 275: 13035–13040, 2000. [DOI] [PubMed] [Google Scholar]
- 140.Rajendran VM, Singh SK, Geibel J, and Binder HJ. Differential localization of colonic H(+)-K(+)-ATPase isoforms in surface and crypt cells. Am J Physiol 274: G424–429, 1998. [DOI] [PubMed] [Google Scholar]
- 141.Rask-Madsen J Simultaneous measurement of electrical polarization and electrolyte transport by the entire normal and inflamed human colon during in vivo perfusion. Scand J Gastroenterol 8: 327–336, 1973. [PubMed] [Google Scholar]
- 142.Rechkemmer G, and Halm DR. Aldosterone stimulates K secretion across mammalian colon independent of Na absorption. Proc Natl Acad Sci U S A 86: 397–401, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Reyes R, Duprat F, Lesage F, Fink M, Salinas M, Farman N, and Lazdunski M. Cloning and expression of a novel pH-sensitive two pore domain K+ channel from human kidney. J Biol Chem 273: 30863–30869, 1998. [DOI] [PubMed] [Google Scholar]
- 144.Richards NW, and Dawson DC. Selective block of specific K(+)-conducting channels by diphenylamine-2-carboxylate in turtle colon epithelial cells. J Physiol 462: 715–734, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Richards NW, and Dawson DC. Single potassium channels blocked by lidocaine and quinidine in isolated turtle colon epithelial cells. Am J Physiol 251: C85–89, 1986. [DOI] [PubMed] [Google Scholar]
- 146.Roepke TK, Anantharam A, Kirchhoff P, Busque SM, Young JB, Geibel JP, Lerner DJ, and Abbott GW. The KCNE2 potassium channel ancillary subunit is essential for gastric acid secretion. J Biol Chem 281: 23740–23747, 2006. [DOI] [PubMed] [Google Scholar]
- 147.Sandle GI. Salt and water absorption in the human colon: a modern appraisal. Gut 43: 294–299, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sandle GI, and Binder HJ. Corticosteroids and intestinal ion transport. Gastroenterology 93: 188–196, 1987. [DOI] [PubMed] [Google Scholar]
- 149.Sandle GI, Butterfield I, Higgs NB, and Warhurst G. Direct inhibitory effect of nicardipine on basolateral K+ channels in human colonic crypts. Pflugers Arch 437: 596–602, 1999. [DOI] [PubMed] [Google Scholar]
- 150.Sandle GI, Foster ES, Lewis SA, Binder HJ, and Hayslett JP. The electrical basis for enhanced potassium secretion in rat distal colon during dietary potassium loading. Pflugers Arch 403: 433–439, 1985. [DOI] [PubMed] [Google Scholar]
- 151.Sandle GI, Gaiger E, Tapster S, and Goodship TH. Enhanced rectal potassium secretion in chronic renal insufficiency: evidence for large intestinal potassium adaptation in man. Clin Sci (Lond) 71: 393–401, 1986. [DOI] [PubMed] [Google Scholar]
- 152.Sandle GI, Gaiger E, Tapster S, and Goodship TH. Evidence for large intestinal control of potassium homoeostasis in uraemic patients undergoing long-term dialysis. Clin Sci (Lond) 73: 247–252, 1987. [DOI] [PubMed] [Google Scholar]
- 153.Sandle GI, Hayslett JP, and Binder HJ. Effect of chronic hyperaldosteronism on the electrophysiology of rat distal colon. Pflugers Arch 401: 22–26, 1984. [DOI] [PubMed] [Google Scholar]
- 154.Sandle GI, Hayslett JP, and Binder HJ. Effect of glucocorticoids on rectal transport in normal subjects and patients with ulcerative colitis. Gut 27: 309–316, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sandle GI, Higgs N, Crowe P, Marsh MN, Venkatesan S, and Peters TJ. Cellular basis for defective electrolyte transport in inflamed human colon. Gastroenterology 99: 97–105, 1990. [DOI] [PubMed] [Google Scholar]
- 156.Sandle GI, and Hunter M. Apical potassium (BK) channels and enhanced potassium secretion in human colon. QJM 103: 85–89, 2010. [DOI] [PubMed] [Google Scholar]
- 157.Sandle GI, McNicholas CM, and Lomax RB. Potassium channels in colonic crypts. Lancet 343: 23–25, 1994. [DOI] [PubMed] [Google Scholar]
- 158.Sandle GI, Perry MD, Mathialahan T, Linley JE, Robinson P, Hunter M, and MacLennan KA. Altered cryptal expression of luminal potassium (BK) channels in ulcerative colitis. J Pathol 212: 66–73, 2007. [DOI] [PubMed] [Google Scholar]
- 159.Sandle GI, and Rajendran VM. Cyclic AMP-induced K+ secretion occurs independently of Cl-secretion in rat distal colon. Am J Physiol Cell Physiol 303: C328–333, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sandle GI, Warhurst G, Butterfield I, Higgs NB, and Lomax RB. Somatostatin peptides inhibit basolateral potassium channels in human colonic crypts. Am J Physiol 277: G967–975, 1999. [DOI] [PubMed] [Google Scholar]
- 161.Sandle GI, Wills NK, Alles W, and Binder HJ. Electrophysiology of the human colon: evidence of segmental heterogeneity. Gut 27: 999–1005, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sangan P, Brill SR, Sangan S, Forbush B 3rd, and Binder HJ. Basolateral K-Cl cotransporter regulates colonic potassium absorption in potassium depletion. J Biol Chem 275: 30813–30816, 2000. [DOI] [PubMed] [Google Scholar]
- 163.Sangan P, Kolla SS, Rajendran VM, Kashgarian M, and Binder HJ. Colonic H-K-ATPase beta-subunit: identification in apical membranes and regulation by dietary K depletion. Am J Physiol 276: C350–360, 1999. [DOI] [PubMed] [Google Scholar]
- 164.Sangan P, Rajendran VM, Mann AS, Kashgarian M, and Binder HJ. Regulation of colonic H-K-ATPase in large intestine and kidney by dietary Na depletion and dietary K depletion. Am J Physiol 272: C685–696, 1997. [DOI] [PubMed] [Google Scholar]
- 165.Sangan P, Thevananther S, Sangan S, Rajendran VM, and Binder HJ. Colonic H-K-ATPase alpha- and beta-subunits express ouabain-insensitive H-K-ATPase. Am J Physiol Cell Physiol 278: C182–189, 2000. [DOI] [PubMed] [Google Scholar]
- 166.Sausbier M, Matos JE, Sausbier U, Beranek G, Arntz C, Neuhuber W, Ruth P, and Leipziger J. Distal colonic K(+) secretion occurs via BK channels. J Am Soc Nephrol 17: 1275–1282, 2006. [DOI] [PubMed] [Google Scholar]
- 167.Schon DA, Silva P, and Hayslett JP. Mechanism of potassium excretion in renal insufficiency. Am J Physiol 227: 1323–1330, 1974. [DOI] [PubMed] [Google Scholar]
- 168.Schulman G, Miller-Diener A, Litwack G, and Bastl CP. Characterization of the rat colonic aldosterone receptor and its activation process. J Biol Chem 261: 12102–12108, 1986. [PubMed] [Google Scholar]
- 169.Schultheiss G, and Diener M. K+ and Cl- conductances in the distal colon of the rat. Gen Pharmacol 31: 337–342, 1998. [DOI] [PubMed] [Google Scholar]
- 170.Sepulveda FV, Fargon F, and McNaughton PA. K+ and Cl- currents in enterocytes isolated from guinea-pig small intestinal villi. J Physiol 434: 351–367, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shimura S, Hamamoto N, Yoshino N, Kushiyama Y, Fujishiro H, Komazawa Y, Furuta K, Ishihara S, Adachi K, and Kinoshita Y. Diarrhea caused by proton pump inhibitor administration: comparisons among lansoprazole, rabeprazole, and omeprazole. Curr Ther Res Clin Exp 73: 112–120, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shull GE, Greeb J, and Lingrel JB. Molecular cloning of three distinct forms of the Na+,K+-ATPase alpha-subunit from rat brain. Biochemistry 25: 8125–8132, 1986. [DOI] [PubMed] [Google Scholar]
- 173.Shull GE, and Lingrel JB. Molecular cloning of the rat stomach (H+ + K+)-ATPase. J Biol Chem 261: 16788–16791, 1986. [PubMed] [Google Scholar]
- 174.Siemer C, and Gogelein H. Activation of nonselective cation channels in the basolateral membrane of rat distal colon crypt cells by prostaglandin E2. Pflugers Arch 420: 319–328, 1992. [DOI] [PubMed] [Google Scholar]
- 175.Simon M, Duong JP, Mallet V, Jian R, MacLennan KA, Sandle GI, and Marteau P. Over-expression of colonic K+ channels associated with severe potassium secretory diarrhoea after haemorrhagic shock. Nephrol Dial Transplant 23: 3350–3352, 2008. [DOI] [PubMed] [Google Scholar]
- 176.Singh SK, O’Hara B, Talukder JR, and Rajendran VM. Aldosterone induces active K(+) secretion by enhancing mucosal expression of Kcnn4c and Kcnma1 channels in rat distal colon. Am J Physiol Cell Physiol 302: C1353–1360, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Smith PL, and McCabe RD. Mechanism and regulation of transcellular potassium transport by the colon. Am J Physiol 247: G445–456, 1984. [DOI] [PubMed] [Google Scholar]
- 178.Sorensen MV, Matos JE, Praetorius HA, and Leipziger J. Colonic potassium handling. Pflugers Arch 459: 645–656, 2010. [DOI] [PubMed] [Google Scholar]
- 179.Sorensen MV, Matos JE, Sausbier M, Sausbier U, Ruth P, Praetorius HA, and Leipziger J. Aldosterone increases KCa1.1 (BK) channel-mediated colonic K+ secretion. J Physiol 586: 4251–4264, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sorensen MV, Sausbier M, Ruth P, Seidler U, Riederer B, Praetorius HA, and Leipziger J. Adrenaline-induced colonic K+ secretion is mediated by KCa1.1 (BK) channels. J Physiol 588: 1763–1777, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Spicer Z, Clarke LL, Gawenis LR, and Shull GE. Colonic H(+)-K(+)-ATPase in K(+) conservation and electrogenic Na(+) absorption during Na(+) restriction. Am J Physiol Gastrointest Liver Physiol 281: G1369–1377, 2001. [DOI] [PubMed] [Google Scholar]
- 182.Squires RD, and Huth EJ. Experimental potassium depletion in normal human subjects. I. Relation of ionic intakes to the renal conservation of potassium. J Clin Invest 38: 1134–1148, 1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sullivan SK, and Smith PL. Active potassium secretion by rabbit proximal colon. Am J Physiol 250: G475–483, 1986. [DOI] [PubMed] [Google Scholar]
- 184.Supplisson S, Loo DD, and Sachs G. Diversity of K+ channels in the basolateral membrane of resting Necturus oxyntic cells. J Membr Biol 123: 209–221, 1991. [DOI] [PubMed] [Google Scholar]
- 185.Suzuki Y, and Kaneko K. Acid secretion in isolated guinea pig colon. Am J Physiol 253: G155–164, 1987. [DOI] [PubMed] [Google Scholar]
- 186.Suzuki Y, and Kaneko K. Ouabain-sensitive H+-K+ exchange mechanism in the apical membrane of guinea pig colon. Am J Physiol 256: G979–988, 1989. [DOI] [PubMed] [Google Scholar]
- 187.Sverdlov ED, Monastyrskaya GS, Broude NE, Ushkaryov Yu A, Allikmets RL, Melkov AM, Smirnov Yu V, Malyshev IV, Dulobova IE, Petrukhin KE, and et al. The family of human Na+,K+-ATPase genes. No less than five genes and/or pseudogenes related to the alpha-subunit. FEBS Lett 217: 275–278, 1987. [DOI] [PubMed] [Google Scholar]
- 188.Swarts HG, Koenderink JB, Willems PH, and De Pont JJ. The human non-gastric H,K-ATPase has a different cation specificity than the rat enzyme. Biochim Biophys Acta 1768: 580–589, 2007. [DOI] [PubMed] [Google Scholar]
- 189.Swarts HG, Koenderink JB, Willems PH, and De Pont JJ. The non-gastric H,K-ATPase is oligomycin-sensitive and can function as an H+,NH4(+)-ATPase. J Biol Chem 280: 33115–33122, 2005. [DOI] [PubMed] [Google Scholar]
- 190.Sweiry JH, and Binder HJ. Active potassium absorption in rat distal colon. J Physiol 423: 155–170, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sweiry JH, and Binder HJ. Characterization of aldosterone-induced potassium secretion in rat distal colon. J Clin Invest 83: 844–851, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Takahashi Y, Sakai H, Kuragari M, Suzuki T, Tauchi K, Minamimura T, Tsukada K, Asano S, and Takeguchi N. Expression of ATP1AL1, a non-gastric proton pump, in human colorectum. Jpn J Physiol 52: 317–321, 2002. [DOI] [PubMed] [Google Scholar]
- 193.Teutsch G, Costerousse G, Deraedt R, Benzoni J, Fortin M, and Philibert D. 17 alpha-alkynyl-11 beta, 17-dihydroxyandrostane derivatives : a new class of potent glucocorticoids. Steroids 38: 651–665, 1981. [DOI] [PubMed] [Google Scholar]
- 194.Tian L, Duncan RR, Hammond MS, Coghill LS, Wen H, Rusinova R, Clark AG, Levitan IB, and Shipston MJ. Alternative splicing switches potassium channel sensitivity to protein phosphorylation. J Biol Chem 276: 7717–7720, 2001. [DOI] [PubMed] [Google Scholar]
- 195.Tseng-Crank J, Foster CD, Krause JD, Mertz R, Godinot N, DiChiara TJ, and Reinhart PH. Cloning, expression, and distribution of functionally distinct Ca(2+)-activated K+ channel isoforms from human brain. Neuron 13: 1315–1330, 1994. [DOI] [PubMed] [Google Scholar]
- 196.Turnamian SG, and Binder HJ. Aldosterone and glucocorticoid receptor-specific agonists regulate ion transport in rat proximal colon. Am J Physiol 258: G492–498, 1990. [DOI] [PubMed] [Google Scholar]
- 197.Turnberg LA. Potassium transport in the human small bowel. Gut 12: 811–818, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Turnheim K, Plass H, and Wyskovsky W. Basolateral potassium channels of rabbit colon epithelium: role in sodium absorption and chloride secretion. Biochim Biophys Acta 1560: 51–66, 2002. [DOI] [PubMed] [Google Scholar]
- 199.Ueda S, Loo DD, and Sachs G. Regulation of K+ channels in the basolateral membrane of Necturus oxyntic cells. J Membr Biol 97: 31–41, 1987. [DOI] [PubMed] [Google Scholar]
- 200.van Dinter TG Jr., Fuerst FC, Richardson CT, Ana CA, Polter DE, Fordtran JS, and Binder HJ. Stimulated active potassium secretion in a patient with colonic pseudo-obstruction: a new mechanism of secretory diarrhea. Gastroenterology 129: 1268–1273, 2005. [DOI] [PubMed] [Google Scholar]
- 201.Wallner M, Meera P, and Toro L. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: a transmembrane beta-subunit homolog. Proc Natl Acad Sci U S A 96: 4137–4142, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Walters RJ, and Sepulveda FV. A basolateral K+ conductance modulated by carbachol dominates the membrane potential of small intestinal crypts. Pflugers Arch 419: 537–539, 1991. [DOI] [PubMed] [Google Scholar]
- 203.Warth R, Garcia Alzamora M, Kim JK, Zdebik A, Nitschke R, Bleich M, Gerlach U, Barhanin J, and Kim SJ. The role of KCNQ1/KCNE1 K(+) channels in intestine and pancreas: lessons from the KCNE1 knockout mouse. Pflugers Arch 443: 822–828, 2002. [DOI] [PubMed] [Google Scholar]
- 204.Warth R, Hamm K, Bleich M, Kunzelmann K, von Hahn T, Schreiber R, Ullrich E, Mengel M, Trautmann N, Kindle P, Schwab A, and Greger R. Molecular and functional characterization of the small Ca(2+)-regulated K+ channel (rSK4) of colonic crypts. Pflugers Arch 438: 437–444, 1999. [DOI] [PubMed] [Google Scholar]
- 205.Warth R, Riedemann N, Bleich M, Van Driessche W, Busch AE, and Greger R. The cAMP-regulated and 293B-inhibited K+ conductance of rat colonic crypt base cells. Pflugers Arch 432: 81–88, 1996. [DOI] [PubMed] [Google Scholar]
- 206.Wills NK, and Biagi B. Active potassium transport by rabbit descending colon epithelium. J Membr Biol 64: 195–203, 1982. [DOI] [PubMed] [Google Scholar]
- 207.Wilson DR, Ing TS, Metcalfe-Gibson A, and Wrong OM. The chemical composition of faeces in uraemia, as revealed by in-vivo faecal dialysis. Clin Sci 35: 197–209, 1968. [PubMed] [Google Scholar]
- 208.Zdebik AA, Cuffe JE, Bertog M, Korbmacher C, and Jentsch TJ. Additional disruption of the ClC-2 Cl(−) channel does not exacerbate the cystic fibrosis phenotype of cystic fibrosis transmembrane conductance regulator mouse models. J Biol Chem 279: 22276–22283, 2004. [DOI] [PubMed] [Google Scholar]
- 209.Zhang J, Halm ST, and Halm DR. Role of the BK channel (KCa1.1) during activation of electrogenic K+ secretion in guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol 303: G1322–1334, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


