Abstract
Murine bone marrow-derived dendritic cells (DC) can phagocytose and process Salmonella enterica serovar Typhimurium for peptide presentation on major histocompatibility complex class I (MHC-I) and MHC-II molecules. To investigate if a serovar Typhimurium encounter with DC induces maturation and downregulates their ability to present antigens from subsequently encountered bacteria, DC were pulsed with serovar Typhimurium 24 h prior to coincubating with Escherichia coli expressing the model antigen Crl-OVA. Quantitating presentation of OVA epitopes contained within Crl-OVA showed that Salmonella-pulsed DC had a reduced capacity to process Crl-OVA-expressing E. coli for OVA(257-264)/Kb and OVA(265-277)/I-Ab presentation. In addition, time course studies of DC pulsed with Crl-OVA-expressing serovar Typhimurium showed that OVA(257-264)/Kb complexes could stimulate CD8OVA T-hybridoma cells for <24 h following a bacterial pulse, while OVA(265-277)/I-Ab complexes could stimulate OT4H T-hybridoma cells for >24 but <48 h. The phoP-phoQ virulence locus of serovar Typhimurium also influenced the ability of DC to process Crl-OVA-expressing serovar Typhimurium for OVA(265-277)/I-Ab presentation but not for OVA(257-264)/Kb presentation. Furthermore, pulsing of DC with serovar Typhimurium followed by incubation for 24 or 48 h altered surface expression of MHC-I, MHC-II, CD40, CD54, CD80, and CD86, generating a DC population with a uniform, high expression level of these molecules. Finally, neither the serovar Typhimurium phoP-phoQ locus nor lipopolysaccharides (LPS) containing lipid A modifications purified from phoP mutant strains had a different effect on DC maturation from that of wild-type serovar Typhimurium or purified wild-type LPS. Thus, these data show that Salmonella or Salmonella LPS induces maturation of DC and that this process is not altered by the Salmonella phoP virulence locus. However, phoP did influence OVA(265-277)/I-Ab presentation by DC infected with Crl-OVA-expressing serovar Typhimurium when quantitated after 2 h of bacterial infection.
Initiating a specific immune response to bacterial pathogens requires that bacterial antigens be captured, processed, and presented by antigen-presenting cells (APC) that activate naive T cells. Dendritic cells (DC) are the most potent APC for stimulating naive T cells (reviewed in reference 3) and thus are critical in initiating an immune response to a previously unencountered antigen. Immature DC can internalize and process bacteria for antigen presentation on both major histocompatibility complex class I (MHC-I) and MHC-II molecules (13, 41, 47, 48). This capacity of immature DC combined with their ability to migrate to lymphoid tissues after antigen capture (reviewed in reference 3) suggests that DC play a key role in initiating an immune response to bacterial infections. During migration, DC that have encountered inflammatory stimuli undergo a process of maturation in which they develop into fully competent APC. DC maturation involves downregulating their ability to capture and present antigens (44, 45, 56), up regulating MHC molecule synthesis (9, 41), altering MHC-II trafficking (9, 40), increasing the stability and surface expression of MHC molecules (9, 40, 41), increasing costimulatory molecule surface expression (13, 22, 41, 44, 45, 56), and enhancing cytokine secretion (10, 13, 22, 41, 56).
In order to survive the hostile environment encountered during the course of infection, bacterial pathogens coordinately regulate their gene expression (21, 31, 33). One such regulon, phoP-phoQ (34), promotes Salmonella enterica serovar Typhimurium virulence (15, 34, 35). The phoP-phoQ virulence regulon is a bacterial two-component regulatory system consisting of a membrane-associated sensor kinase (PhoQ) and a cytoplasmic transcriptional regulator (PhoP) (34). PhoP and PhoQ both positively and negatively regulate more than 40 gene products (4, 5, 37). Activation of the phoP-phoQ regulatory system is induced by Mg2+ limitation (16) and the low pH of the phagosomal environment within macrophages (Mφ) (2). PhoP-PhoQ regulates modifications of the lipid A moiety of lipopolysaccharide (LPS) (18), affects tumor necrosis factor alpha (TNF-α) expression by monocytes (18), regulates antimicrobial peptide resistance (15, 17), represses invasion genes (37), influences formation of spacious phagosomes (1) and bacterial survival within Mφ (35) after antibody-mediated opsonic uptake, and alters the efficiency of phagocytic processing of serovar Typhimurium by activated Mφ for peptide presentation on MHC-II molecules (54).
The previous observation that murine bone marrow-derived DC can process virulent serovar Typhimurium for peptide presentation on MHC-I and MHC-II molecules (47) led us to further investigate the properties of these Salmonella-pulsed DC. Here we examine the ability of serovar Typhimurium to induce maturation in murine bone marrow-derived DC and the effects of Salmonella phoP-regulated genes on DC maturation. This was assessed by analyzing the ability of DC pulsed with wild-type, phoP null (phoP), or phoP constitutive (phoPc) serovar Typhimurium or of LPS purified from phoP mutant serovar Typhimurium strains containing lipid A modifications to present antigens from subsequently encountered bacteria. In addition, the influence of Salmonella infection of DC or of DC interaction with wild-type or mutant LPS on interleukin-12 (IL-12) production and surface expression of MHC and costimulatory molecules was analyzed. Finally, the influence of the phoP-phoQ locus, which controls numerous aspects of the pathogenesis of this bacterium, on antigen presentation by serovar Typhimurium-pulsed DC was investigated.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were bred in animal facilities at Lund University or purchased from Charles River Laboratories (Sulzfeld, Germany) and were used at 6 to 10 weeks of age.
Bacterial strains, plasmids, and culture conditions.
Bacterial strains used in this study are the wild-type serovar Typhimurium strain ATCC 14028, the phoP strains CSO15 (34) or MS7953 (15), and the phoPc strain CSO22 (35). Unless otherwise indicated, the serovar Typhimurium strains used throughout this study had smooth LPS, as defined by sensitivity to bacteriophage P22c2 and resistance to BR60 (55). When rough-LPS serovar Typhimurium was used, these strains were resistant to P22c2 and sensitive to BR60.
For antigen-processing experiments, the bacteria harbored pJLP-2H (38), pJLP-2H-Kan (47), or pJLP-1E (39). pJLP-2H and pJLP-2H-Kan encode the fusion protein Crl-OVA, which contains residues 257 to 277 of ovalbumin (OVA), including the Kb-binding (257 to 264) epitope and the I-Ab-binding (265 to 277) epitope. pJLP-1E encodes the fusion protein Crl-HEL, which contains the I-Ak-binding (52 to 61) epitope from hen egg lysozyme (HEL). These proteins are expressed in the cytoplasm of the bacteria (39). Bacteria expressing green fluorescent protein (GFP) harbored pSK-XhoGFP, which contains the GFP-encoding cDNA from the jellyfish Aequoera victoria (11) cloned into pBluescript SK. Bacteria containing pJLP-2H and pJLP-1E were grown on Luria-Bertani (LB) agar supplemented with 50 μg of carbenicillin/ml, and the bacteria containing pJLP-2H-Kan were grown on agar supplemented with 50 μg of kanamycin/ml. Bacteria containing pSK-XhoGFP were grown in LB broth supplemented with 50 μg of ampicillin/ml. After overnight incubation at 37°C, a bacterial suspension was made in phosphate-buffered saline (PBS) (pH 7.4). Bacteria were quantified spectrophotometrically by determining the optical density at 600 nm, washed once in PBS, and resuspended at 109 cells/ml in Iscove's modified Dulbecco's medium (IMDM; Gibco BRL, Gaithersburg, Md.). Unless otherwise indicated, IMDM was used without antibiotics. Heat-killed bacteria were prepared by incubating bacterial suspensions at 65°C for 40 min. A lack of remaining viable bacteria was confirmed by plating an aliquot of heat-killed bacteria on LB agar plates.
Cell culture conditions and DC activation assays.
DC were cultured from murine bone marrow in the presence of granulocyte-Mφ colony-stimulating factor (GM-CSF) as described elsewhere (47). On days 6 and 7 of culture, DC were enriched for the CD11c-expressing population by using magnetic cell sorting with N418 magnetic beads (48). Approximately 90% of the enriched population stained positive for MHC-II, CD86, and CD11c when the antibodies M5/114 (I-Ab) and GL-1 (CD86) and either N418 (CD11c) or HL3 (CD11c) were used in flow cytometry analysis.
DC were resuspended in IMDM (without antibiotics) containing 5% fetal calf serum (FCS) and 5% GM-CSF and were seeded at 106 cells per well in 24-well plates. DC were stimulated with viable serovar Typhimurium, heat-killed bacteria, bacteria in the presence of cytochalasin D (CCD; Sigma Chemical, Co., St. Louis, Mo.), 1-μm polystyrene beads (Polyscience, Warrington, Pa.), LPS purified from E. coli (Sigma), or LPS purified from serovar Typhimurium wild-type (ATCC 14028), phoP (CSO15), or phoPc (CSO22) bacteria. Unless otherwise indicated, bacteria without plasmids were used to assess DC activation. The bacterium- or bead-to-cell ratio was 15:1. Plates containing DC pulsed with bacteria or beads were centrifuged at 270 × g for 4 min and were incubated for 2 h at 37°C. After 2 h, DC were washed three times with Hank's balanced salt solution (HBSS; Gibco BRL); 1.0 ml of IMDM containing 5% FCS, 5% GM-CSF, and 25 μg of gentamicin/ml was added; and the cells were incubated for an additional 22 or 46 h. LPS was used at 1 μg/ml and was present throughout the 24- or 48-h stimulation. Culture supernatants were collected at 2, 24, or 48 h of total incubation time and were used to quantify IL-12 or NO2− as described below. The cells were also collected at these times and after washing with HBSS were used in antigen-processing experiments, flow cytometry analysis, and bacterial survival assays. When CCD was used, it was added at 10 μg/ml to the cell cultures approximately 1 h before addition of bacteria and was present at 10 μg/ml for the initial 2 h of the bacterium-DC coincubation.
Antigen-processing and presentation assays.
Antigen-processing assays were performed as described previously (47, 48). Briefly, DC were resuspended in IMDM (without antibiotics) and seeded at 2 × 105 cells per well in 96-well plates. DC were either fixed in 1% paraformaldehyde or were coincubated with E. coli expressing Crl-OVA or Crl-HEL before fixing the cells. For DC pulsed with bacteria, the plates were centrifuged at 270 × g for 4 min and were incubated for 2 h at 37°C. The cultures were then washed thoroughly, and antigen processing was terminated by fixing the cells. After fixing, OT4H (28) or CD8OVA (38) T-hybridoma cells, which secrete IL-2 upon specific recognition of the OVA(265-277)/I-Ab or OVA(257-264)/Kb complex, respectively, were added for 24 h. IL-2 secreted by the T-hybridoma cells was quantitated by measuring [3H]thymidine incorporation by IL-2-dependent CTLL cells.
Bacterial internalization quantitated by using bacteria expressing GFP.
DC were seeded at 106 cells per well in 24-well plates, and serovar Typhimurium expressing GFP was added at a bacterium-to-cell ratio of 15:1. The plates were centrifuged at 270 × g for 4 min and were incubated at 37°C for 2 h. The wells were washed three times with HBSS to remove noninternalized bacteria, and the cells were used in flow cytometry. To determine the number of bacteria associated with but not internalized by DC, DC were preincubated with 10 μg of CCD/ml 60 min prior to adding the bacteria. The inhibitor was present throughout the duration of the infection at 10 μg/ml.
Flow cytometry.
Flow cytometry was performed using a Becton Dickinson FACSort flow cytometer (Becton Dickinson and Co., Mountain View, Calif.), and data were collected on 10,000 or 30,000 cells. Antibodies from hybridomas 2.4.G2 (FcγRIII/II) (50), N418 (CD11c) (32), GL-1 (CD86) (20), M5/114 (I-Ab) (6), K9.178 (Kb) (19), FGK (CD40) (42), H57-597 (αβTCR) (27), RA6-3A2 (B220) (12), GK 1.5 (CD4) (14), and MR10.2 (Vβ9TCR) (52) were used. Antibodies from the hybridomas H57-597, RA6-3A2, GK 1.5, and MR10.2 were used as isotype-matched control antibodies. Antibody supernatants were purified using a γ-bind plus column (Pharmacia-Biotech, Uppsala, Sweden) and labeled with biotin (Sigma) or fluorescein isothiocyanate (Sigma). Biotinylated anti-CD54 (KAT-1) was purchased from Caltag laboratories (Burlingame, Calif.), and biotinylated anti-CD80 and phycoerythrin-labeled anti-CD11c (HL3) antibodies were purchased from Pharmingen (San Diego, Calif.). Phycoerythrin-, fluorescein isothiocyanate-, and Tricolour-streptavidin were all from Vector Laboratories (Burlingame, Calif.) and were used as second-step reagents. Dead cells were excluded by staining with 7-amino-actinomycin D (Sigma) at 1 μg/ml. All incubations with antibodies or reagents were for 20 min on ice in HBSS containing 3% FCS, 2 mM EDTA, and 0.01% sodium azide.
Cytokine measurement.
A sandwich enzyme-linked immunosorbent assay (ELISA) to detect the p40 chain of IL-12 was performed as described (46) by using monoclonal antibody C17.8 (57) as the capture antibody and biotinylated monoclonal antibody C15.6 (57) as the detection antibody. Assays were developed by adding streptavidin-peroxidase (Sigma) and 3,3′,5,5′-tetramethylbenzidine (Sigma) as the substrate. Absorbance was read at 450 nm using a microplate reader (Molecular Devices Corp., Sunnyvale, Calif.). IL-12 p70 was measured as described for the IL-12 p40 subunit, except that 9A5 (Pharmingen) and biotinylated C17.8 (57) monoclonal antibodies were used as the capture and detection antibodies, respectively. Recombinant IL-12 was used as the standard, and the concentration of IL-12 in test samples was calculated using the linear part of a standard curve run in parallel with the samples. The sensitivity of the assay was 45 pg/ml.
Quantitation of Crl-OVA expression by bacteria.
An OVA-specific ELISA was used to quantitate the level of Crl-OVA expression by the different Salmonella strains. Two milliliters of a bacterial suspension at 109/ml was centrifuged at 1,700 × g for 5 min, and the pellet was resuspended in 0.4 ml of PBS (pH 7.4) and lysed by sonication on ice using a Vibra Cell sonicator (Sonics and Material, Danbury, Conn.). The sonicated samples were centrifuged to remove cell debris, and the total protein content of the cleared lysate was determined using the bicinchoninic acid protein determination system (Sigma). Samples were normalized for protein content, serially diluted in PBS, and then seeded in 96-well plates (Nalgen Nunc International, Roskilde, Denmark). The ELISA was performed using an antiserum raised in mice immunized with OVA as the primary antibody. Goat anti-mouse immunoglobulin G (IgG) peroxidase conjugate (Sigma) was used as a secondary antibody, and the absorbance at 450 nm was determined after developing with 3,3′,5,5′-tetramethylbenzidine (Sigma) as the substrate.
Bacterial survival assays.
DC were pulsed with the bacterial strains and were washed and treated with gentamicin as described for DC activation assays. After coincubation of DC with Salmonella for 2, 24, or 48 h, DC were lysed with 0.5 ml of 0.2% Triton X-100. After vigorous pipetting, the plates were incubated at room temperature until the cells were lysed (assessed microscopically for approximately 10 min). Bacteria released into the supernatant after cell lysis were serially diluted in LB broth, and 100 μl of several dilutions was plated onto LB agar plates. After overnight incubation at 37°C, the number of colonies was counted and the total number of bacteria recovered from wells was calculated. As controls for determining the number of viable bacteria present in the wells but not internalized by DC, cells were preincubated with 10-μg/ml CCD prior to addition of the bacteria, which was present for the initial 2 h of bacterium-cell coincubation.
iNOS assay.
Nitrite (NO2−), the stable end product of l-arginine oxidation by inducible nitric oxide synthase (iNOS) (24), was quantitated in the cell culture supernatant by using the Griess diazotization reaction with NaNO2 as the standard. Culture supernatants from bacterial incubations with DC were assayed by mixing 100 μl of supernatant 1:1 with Griess reagent (1% sulfanilamide, 1% naphthylethylene diamine dihydrochloride, 2.5% H3PO4) and incubating at room temperature for 10 min. The absorbance was measured at 540 nm in a microtiter plate reader (Molecular Devices).
RESULTS
DC exposed to Salmonella have a reduced capacity for MHC-I and MHC-II presentation of antigens derived from subsequently encountered bacteria.
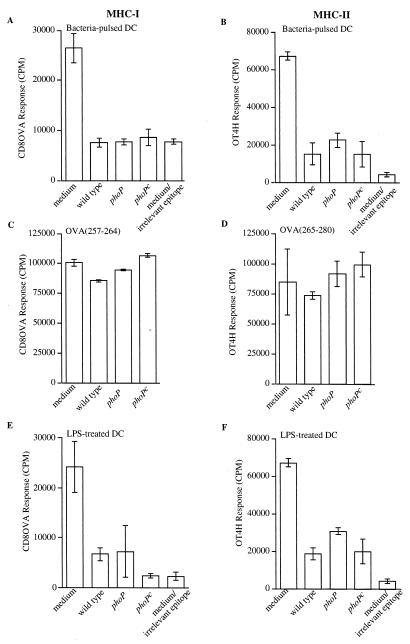
To investigate if exposure to live serovar Typhimurium down modulates DC's ability to process subsequently encountered bacteria for peptide presentation on MHC-I and MHC-II molecules, murine bone marrow-derived DC were pulsed for 2 h with wild-type serovar Typhimurium and cultured for 24 h before pulsing for 2 h with E. coli expressing the model antigen Crl-OVA. Preexposure of DC to serovar Typhimurium reduced the cells' ability to process subsequently added Crl-OVA-expressing E. coli for presentation of OVA(257-264) on Kb as well as presentation of OVA(265-277) on I-Ab (Fig. 1A and B). In addition, we investigated if exposure to phoP or phoPc serovar Typhimurium strains also down modulates DC's ability to process subsequently encountered bacteria for peptide presentation on MHC-I and MHC-II molecules. These results revealed that pulsing of DC with phoP or phoPc serovar Typhimurium showed an ability similar to that of pulsing with wild-type serovar Typhimurium to decrease the capacity of DC to present OVA(257-264)/Kb (Fig. 1A) or OVA(265-277)/I-Ab (Fig. 1B) processed from subsequently encountered Crl-OVA-expressing E. coli. The reduced presentation capacity of DC exposed to bacteria is not due to loss of ability to stimulate T-hybridoma cells, since presentation of exogenously added OVA(257-264) or OVA(265-280) peptide is not affected by bacterial preincubation (Fig. 1C and D). Furthermore, coincubation of Salmonella with DC for 24 h had no effect on DC viability, as determined by trypan blue staining.
FIG. 1.
Reduced presentation of bacterial antigens on MHC-I and MHC-II molecules by DC exposed to serovar Typhimurium or LPS with modified lipid A. DC were pulsed with bacteria for 2 h and after washing and gentamicin treatment were incubated for another 22 h (A to D), or DC were treated with purified LPS for 24 h (E and F). The DC were then pulsed with E. coli expressing Crl-OVA or E. coli expressing Crl-HEL (containing an epitope irrelevant for the T-cell hybridoma [medium/irrelevant epitope]) (A, B, E, and F), OVA(257-264) peptide (C), or OVA(265-280) peptide (D) for 2 h. Following paraformaldehyde fixation, OVA(257-264)/Kb (A, C, and E) or OVA(265-277)/I-Ab presentation (B, D, and F) was quantitated by adding CD8OVA or OT4H T-hybridoma cells, respectively. (A to D) The x axis indicates that the DC were incubated in medium alone during the initial 2 h (medium or medium/irrelevant epitope) or were pulsed for 2 h with bacterial strains as follows: wild type, serovar Typhimurium ATCC 14028; phoP, serovar Typhimurium CS015; phoPc, serovar Typhimurium CS022. (E and F) The x axis indicates that the DC were incubated in medium alone for 24 h (medium or medium/irrelevant epitope) or indicates the type of purified LPS (1 μg/ml) present during the initial 24-h incubation: wild type, LPS purified from serovar Typhimurium ATCC 14028; phoP, LPS purified from serovar Typhimurium CS015; phoPc, LPS purified from serovar Typhimurium CS022. Data are presented as the means of triplicate samples ± 1 standard deviation. Maximum CTLL proliferation after exposure to recombinant IL-2 was 160,000 cpm. Similar results were obtained in at least three independent experiments.
LPS purified from serovar Typhimurium also down modulated the ability of DC to process subsequently encountered Crl-OVA-expressing E. coli for OVA(257-264)/Kb and OVA(265-277)/I-Ab peptide presentation (Fig. 1E and F). Since phoP regulates structural modifications of serovar Typhimurium lipid A and alters the biological effects of the LPS, including TNF-α production by infected monocytes (18), we also tested whether this altered LPS induced DC maturation similar to that observed with purified wild-type Salmonella LPS. Purified LPS containing lipid A modifications controlled by PhoP resulted in a reduced capacity of DC to process subsequently encountered E. coli expressing Crl-OVA for OVA(257-264)/Kb and OVA(265-277)/I-Ab presentation similar to that seen with LPS purified from wild-type serovar Typhimurium (Fig. 1E and F). The reduced presentation capacity of DC exposed to LPS is not due to loss of the ability to stimulate the T-hybridoma cells, since DC stimulated with any of the LPS preparations presented exogenously added OVA(265-280) or OVA(257-264) peptide equally well (data not shown). In addition, trypan blue staining showed that no reduction in viability of LPS-treated DC occurred during the 24-h incubation.
DC encounter with Salmonella alters surface expression of MHC and costimulatory molecules.
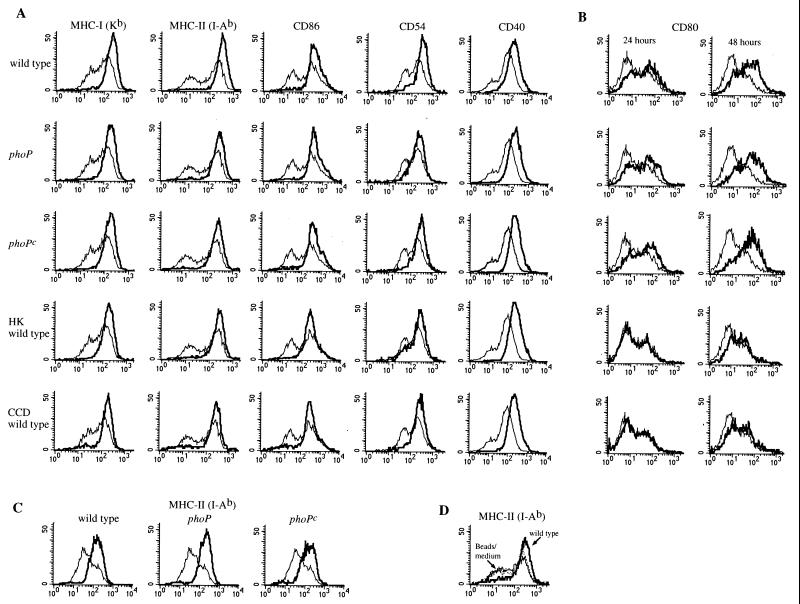
In addition to reduced antigen-capture capacity, another hallmark of murine DC maturation is increased surface expression of MHC and costimulatory molecules (40, 41, 44, 45, 56). To investigate if Salmonella PhoP-regulated genes alter the DC surface expression of molecules important in stimulating T cells, DC were pulsed with viable wild-type, phoP, or phoPc serovar Typhimurium for 2 h and surface expression of MHC-I, MHC-II, CD80 (B7-1), CD86 (B7-2), CD40, and CD54 (ICAM-1) was analyzed following further incubation up to 48 h. At 24 h of total incubation time, the DC population shifted from one with heterogenous levels of surface MHC-I, MHC-II, CD86, CD40, and CD54 to a more uniform population expressing a similar, high level of these molecules (Fig. 2A). In addition, the phoP mutants influenced surface expression of MHC-I, MHC-II, CD86, CD40, and CD54 in a fashion similar to that observed for wild-type bacteria (Fig. 2A). This altered surface molecule expression was also apparent when DC were infected with heat-killed wild-type bacteria or with wild-type bacteria in the presence of CCD (Fig. 2A), demonstrating that neither bacterial viability nor internalization was required for the observed effects. The expression pattern of the surface molecules shown in Fig. 2A on Salmonella-infected DC was similar at 24 h (Fig. 2A) and 48 h (not shown) of total incubation time.
FIG. 2.
DC coincubation with serovar Typhimurium alters expression of surface molecules important in signaling the immune system. (A and B) DC were coincubated with either viable wild-type, phoP, or phoPc serovar Typhimurium; viable wild-type serovar Typhimurium in the presence of CCD; or heat-killed (HK) wild-type serovar Typhimurium, as indicated to the left of each row of histograms. After an initial 2-h pulse with bacteria, the cells were washed, treated with gentamicin, and incubated an additional 22 h (A and B) or 46 h (B) before flow cytometry was performed. (C) DC were coincubated with LPS purified from either wild-type, phoP, or phoPc serovar Typhimurium as indicated. The surface expression of MHC-II molecules on DC after 24 h of stimulation with LPS (thick line) compared to that on DC incubated in medium only (thin line) is shown. (D) The surface expression of MHC-II molecules on DC 48 h after the addition of latex beads (dotted line) compared to that on DC incubated in medium only (thin line) or DC incubated with wild-type serovar Typhimurium (thick line) is shown. The upregulation of the different surface markers was not due to unspecific binding of Ig used for the fluorescence-activated cell sorter analysis, as appropriate Ig isotype subclass controls showed no difference in expression levels for infected and uninfected cells (not shown). Similar results were obtained in at least four independent experiments.
The surface expression of CD80 on DC was also influenced by Salmonella infection (Fig. 2B). However, unlike the case for the other surface molecules studied here, almost no alteration in surface expression of CD80 was detected at 24 h, while at 48 h after infection, the DC population consisted of more cells with a higher level of CD80 expression; the alteration of CD80 expression was similar regardless of the PhoP phenotype of the bacteria (Fig. 2B). In addition, little if any alteration in surface expression of CD80 was detected at 24 or 48 h after DC infection with heat-killed wild-type bacteria or when the initial pulse with bacteria occurred in the presence of CCD (Fig. 2B). This is in marked contrast to the effect of either heat-killed bacteria or inhibition of phagocytosis on the other surface molecules examined (Fig. 2A) and shows that bacterial viability and internalization are required for Salmonella-induced effects on CD80 surface expression but not for the effects observed on CD86, CD54, CD40, and MHC molecule expression.
Furthermore, coincubation of DC with LPS purified from the three Salmonella strains resulted in effects on surface molecule expression similar to those observed with intact bacteria at 24 h (Fig. 2C), that is, a shift from a population with heterogenous levels of expression of MHC-II (Fig. 2C) and MHC-I and CD86 and CD40 (data not shown) to a more uniform population expressing a similar, high level of these molecules. Finally, a phagocytic stimulus per se was not responsible for the observed alteration in surface molecule expression following DC coincubation with serovar Typhimurium, as DC coincubated with 1-μm polystyrene beads did not alter surface expression of MHC-II (Fig. 2D), CD80, CD86, CD54, or CD40 (data not shown).
T-cell-stimulatory capacity of peptide-MHC complexes on the surface of Salmonella-pulsed DC.
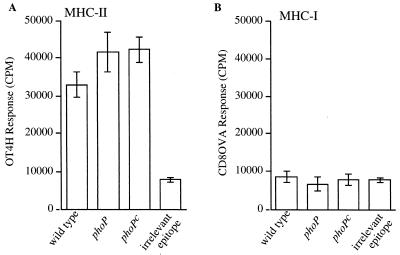
We next investigated the ability of MHC-I and MHC-II containing bacterium-derived peptides formed during a bacterial pulse to stimulate T-hybridoma cells after removing external bacteria. Thus, DC were pulsed with viable wild-type, phoP, or phoPc serovar Typhimurium expressing Crl-OVA for 2 h and were then washed to remove noninternalized bacteria. After 22 or 46 h of additional incubation, DC were fixed and OVA peptide presentation on MHC-I and MHC-II molecules was measured. Significant OVA(265-277)/I-Ab presentation by DC was detectable at 24 h (Fig. 3A), while no OVA(257-264)/Kb presentation above background was detectable at this time point (Fig. 3B). Furthermore, OVA(265-277)/I-Ab complexes generated from the phoP mutants stimulated OT4H T-hybridoma cells to a level similar to that observed for wild-type bacteria, suggesting that PhoP does not affect the level of surface peptide-MHC complexes remaining at this time point. After 48 h of total coincubation time following the pulse of DC with bacteria, neither OVA(265-277)/I-Ab nor OVA(257-264)/Kb presentation by DC could be detected (data not shown). Thus, OVA(265-277)/I-Ab complexes are present on the DC cell surface for OT4H T-hybridoma cell stimulation for at least 24 h, while OVA(257-264)/Kb complexes are available on the cell surface at levels sufficient to stimulate CD8OVA T-hybridoma cells for less than 24 h following exposure to bacteria expressing Crl-OVA. Coincubation of Salmonella with DC for 48 h had no effect on DC viability, as determined by trypan blue staining.
FIG. 3.
Differential stimulatory capacity of OVA(257-264)/Kb and OVA(265-277)/I-Ab complexes after DC processing of Crl-OVA-expressing bacteria. DC were coincubated for 2 h with bacteria expressing Crl-OVA, as indicated on the x axis (see Fig. 1 legend). DC were then washed and treated with gentamicin, and incubations continued for an additional 22 h. After this 24-h period, either OVA(265-277)/I-Ab-specific OT4H T-hybridoma cells (A) or OVA(257-264)/Kb-specific CD8OVA T-hybridoma cells (B) were added for 24 h to quantitate OVA peptide presentation. Data are presented as the means of triplicate samples ± 1 standard deviation. Similar results were obtained in at least three independent experiments.
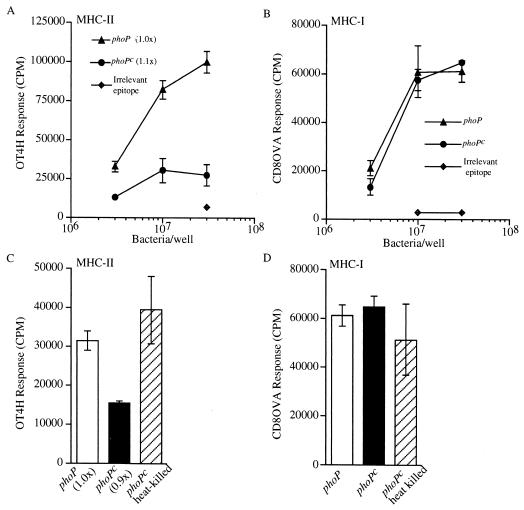
Although PhoP did not influence the T-cell-stimulatory capacity of DC exposed to Crl-OVA-expressing serovar Typhimurium 24 h prior to quantitation of OVA(265-277)/I-Ab and OVA(257-264)/Kb presentation, it did, however, affect OVA(265-277)/I-Ab presentation by DC when quantitated after 2 h of infection with serovar Typhimurium (Fig. 4). These data show that phoP serovar Typhimurium was processed with greater efficiency for OVA(265-277)/I-Ab presentation than were phoPc bacteria expressing the same antigen (Fig. 4A). In contrast, both the phoP and phoPc bacteria were processed by DC with equal efficiency for OVA(257-264)/Kb presentation (Fig. 4B). The observed difference in processing efficiency for MHC-II presentation of the two bacterial strains was not due to lower antigen expression in the phoPc strain, as phoP and phoPc bacteria showed relatively equal amounts of reactivity in an OVA-specific ELISA (Fig. 4A). Furthermore, it is likely that the difference in antigen-processing efficiency for MHC-II presentation following Salmonella internalization by DC is due to the phoP locus, since heat killing phoPc bacteria restore the level of OVA(265-277)/I-Ab presentation to that observed for viable phoP bacteria (Fig. 4C). In contrast, heat killing the phoPc strain did not alter the observed level of OVA(257-264)/Kb presentation (Fig. 4D). Finally, the observed difference in phagocytic processing efficiency for MHC-II presentation is not due to differences in internalization of the two bacterial strains, as equal numbers of phoP and phoPc Salmonella were recovered after a 2-h coincubation of bacteria with DC (Table 1).
FIG. 4.
PhoP influences antigen-processing efficiency for MHC-II presentation when quantitated 2 h following Salmonella infection of DC. DC were infected with either phoP or phoPc serovar Typhimurium expressing Crl-OVA or with phoPc serovar Typhimurium expressing Crl-HEL (irrelevant epitope). After 2 h, the cells were washed and fixed, and either OT4H (A and C) or CD8OVA (B and D) T-hybridoma cells were added. (A) The OVA(265-277)I-Ab-specific OT4H T-hybridoma response to DC coincubated with viable phoP or phoPc Salmonella expressing Crl-OVA or wild-type bacteria expressing Crl-HEL (irrelevant epitope) is shown. The amount of Crl-OVA expressed in the phoPc Salmonella, relative to the amount expressed in the phoP Salmonella as determined by ELISA, is shown within parentheses. (B) The OVA(257-264)/Kb-specific CD8OVA T-hybridoma response to DC coincubated with viable phoP or phoPc Salmonella expressing Crl-OVA or wild-type Salmonella expressing Crl-HEL (irrelevant epitope) is shown. (C) The OT4H T-hybridoma response to DC coincubated with 107 viable phoP or viable phoPc or heat-killed phoPc Salmonella expressing Crl-OVA is shown. (D) The CD8OVA T-hybridoma response to DC coincubated with 107 viable phoP or viable phoPc or heat-killed phoPc Salmonella expressing Crl-OVA is shown. Rough-LPS Salmonella strains were used in these experiments. Data are presented as the means of triplicate samples ± 1 standard deviation. Similar results were obtained in at least three independent experiments.
TABLE 1.
Similar quantities of phoP and phoPc bacteria are recovered following coincubation with serovar Typhimurium for 2 ha
| Bacterium/DC ratio | No. of recovered bacteria
|
|
|---|---|---|
| phoP | phoPc | |
| 15:1 | 2.5 × 106 | 2.1 × 106 |
| 50:1 | 8.8 × 106 | 12.0 × 106 |
| 50:1 (+CCD) | 2.5 × 104 | 4.0 × 104 |
We coincubated 106 DC with phoP (MS7953) or phoPc (CSO22) bacteria with rough LPS at the indicated bacterium/DC ratio for 2 h before lysing the cells and plating the bacteria onto Luria agar plates. The data are representative of at least three independent experiments.
Salmonella is most efficiently phagocytosed by the CD11c+ MHC-IIlow DC subpopulation.
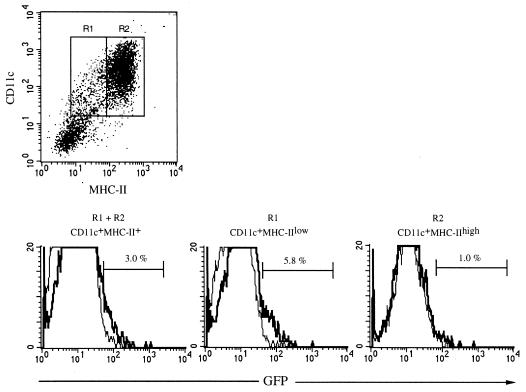
Murine bone marrow cultured in the presence of GM-CSF results in CD11c+ cells with different levels of surface expression of MHC-II which correlate with their stage of maturation (25, 40) (Fig. 2). To define the DC subpopulation (CD11c+MHC-IIlow or CD11c+MHC-IIhigh) active in phagocytosing Salmonella, DC were pulsed with Salmonella expressing GFP and were analyzed for GFP fluorescence and MHC-II and CD11c expression by flow cytometry. Approximately 3% of the total cells expressing both CD11c and MHC-II were infected with GFP-expressing bacteria after 2 h of bacterium-cell coincubation (Fig. 5). Furthermore, the CD11c+MHC-IIlow cells were significantly more active in internalizing bacteria than the CD11c+MHC-IIhigh cells, with 5.8 and 1.0% of the cells, respectively, containing internalized bacteria (Fig. 5). Thus, the DC population with an immature phenotype (CD11c+MHC-IIlow) present in GM-CSF bone marrow cultures is most actively engaged in phagocytosing serovar Typhimurium, and only a minor part of the CD11c+MHC-IIlow cells become infected at a bacterium-to-cell ratio of 15:1 when internalization is quantitated after 2 h of bacterium-DC coculture.
FIG. 5.
The CD11c+MHC-IIlow DC subpopulation is most active in phagocytosing serovar Typhimurium. DC were coincubated with Salmonella phoPc bacteria expressing GFP at a bacterium-to-cell ratio of 15:1. After 2 h, extracellular bacteria were washed away. The DC were subsequently labeled with monoclonal antibodies, cells were fixed in 1% paraformaldehyde and flow cytometry was performed. The dot plot shows the MHC-II and CD11c expression on infected DC and the regions R1 and R2, which were used to analyze the uptake of Salmonella phoPc bacteria by CD11c+ cells. Histograms of flow cytometry analysis of CD11c+MHC-II+ (R1 + R2), CD11c+MHC-IIlow (R1), and CD11c+MHC-IIhigh (R2) cells are shown. The y axis represents the number of DC, and the x axis represents log fluorescence intensity. Infections were done in the absence (thick line) or presence (thin line) of CCD. The numbers shown represent the percentage of DC infected with bacteria in the absence of CCD (i.e., actively internalized bacteria). In this gate 0.5% of the cells was infected when CCD was present. Similar results were obtained in at least four independent experiments.
Intracellular survival of serovar Typhimurium within DC.
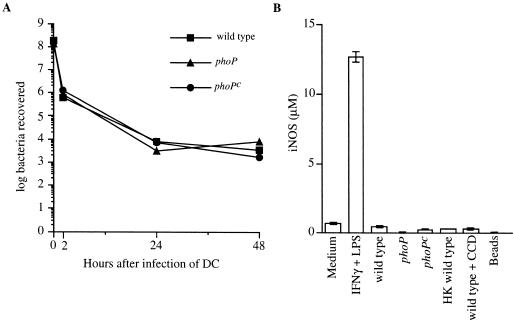
Wild-type serovar Typhimurium can survive and replicate inside phagosomal compartments of infected Mφ, whereas phoP and phoPc mutants are impaired in their ability to survive inside Mφ following antibody-mediated opsonic uptake (35). However, little is known about the replication capacity of these strains in DC. To address the ability of infected DC to control intracellular replication of wild-type, phoP, and phoPc serovar Typhimurium, DC were pulsed with bacteria for 2 h. After washing and gentamicin treatment to kill extracellular bacteria, intracellular survival was followed for 48 h. These data showed that the number of viable Salmonella recovered from infected DC did not increase over the 48-h time period examined (Fig. 6A). In addition, the number of bacteria recovered was similar regardless of the PhoP phenotype. Coincubation of Salmonella with DC for 48 h had no effect on DC viability, while incubations of DC with or without bacteria for 72 h resulted in decreased DC viability. This latter result prevented obtaining meaningful data on bacterial survival in DC for more than 48 h of incubation.
FIG. 6.
DC control intracellular replication of serovar Typhimurium within a 48-h period. (A) Following a 2-h pulse with either wild-type, phoP, or phoPc serovar Typhimurium, DC were washed and were either lysed to determine the number of bacteria recovered at the 2-h time point or treated with gentamicin before continuing the incubation for a total of 24 or 48 h. At these time points, DC were lysed and the number of viable bacteria remaining was determined by plating on LB agar plates. The actual initial bacterium-to-DC infection ratios were 15:1, 13:1, and 17:1 for wild-type, phoP, and phoPc bacteria, respectively, as determined by viable counts. (B) NO2− was quantitated in the supernatants of DC that were incubated in medium alone or in medium containing IFN-γ (300 U/ml) and LPS (10 μg/ml); or infected with wild-type, phoP, or phoPc serovar Typhimurium or with wild-type serovar Typhimurium in the presence of CCD or with heat-killed (HK) wild-type serovar Typhimurium; or incubated with 1-μm polystyrene beads, as indicated. DC were pulsed with bacteria for 2 h. The cells were washed and treated with gentamicin, and incubation was continued for an additional 46 h. At this time point the level of NO2− in the culture supernatant was quantitated by using the method of Greiss with NaNO2 as the standard. Data are presented as the means of triplicate samples ± 1 standard deviation. Similar results were obtained in at least three independent experiments.
To investigate if production of reactive nitrogen intermediates such as nitric oxide (NO) was a mechanism contributing to the ability of immature DC to restrict intracellular replication of serovar Typhimurium, the activity of iNOS was quantitated. Supernatants from DC infected with any of the three Salmonella strains did not increase iNOS activity, as assessed by quantifying NO2− accumulation (Fig. 6B), even though these cells were capable of iNOS induction, as demonstrated by NO2− accumulation after stimulation with gamma interferon (IFN-γ) and LPS (Fig. 6B). Thus, although DC can control serovar Typhimurium replication, at least within the 48-h time frame studied here, significant levels of NO do not appear to be induced by Salmonella infection of DC.
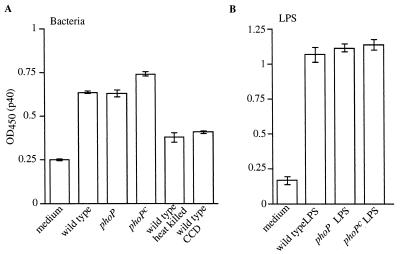
IL-12 is produced by Salmonella-infected DC.
IL-12 promotes the development of T-helper cell type 1 (Th1) responses and is a powerful inducer of IFN-γ production by T cells and NK cells (reviewed in reference 49). Since both IL-12 and IFN-γ are essential for resistance to Salmonella infection in mice (23, 30), we tested the effect of Salmonella PhoP-regulated genes on production of IL-12 by DC. Thus, DC were pulsed with wild-type, phoP, or phoPc serovar Typhimurium, and supernatant samples were tested for the presence of the p40 subunit or the biologically active form of IL-12, the p70 heterodimer, following a total incubation time of 24 or 48 h. Although little IL-12 p40 was detected in culture supernatants from DC pulsed with bacteria and incubated for a total of 24 h, p40 was detected after 48 h of total incubation time (Fig. 7A). Neither bacterial internalization nor viability was required for production of IL-12 p40, as evident from the moderate levels of p40 detected when DC were coincubated with heat-killed wild-type bacteria or when the initial bacterial pulse was performed in the presence of CCD (Fig. 7A). However, although bacterial viability or internalization was not required to elicit IL-12 p40, the level of p40 produced was consistently higher when viable bacteria were used and when bacterial internalization occurred. Similar to the effects on DC maturation presented above, purified LPS from the bacteria was sufficient to elicit p40 production (Fig. 7B). Furthermore, all forms of LPS tested (LPS from wild-type serovar Typhimurium as well as LPS containing lipid A modifications purified from the phoP and phoPc strains [18]) elicited IL-12 p40 secretion. Finally, eliciting p40 production by DC required stimulation with bacteria or LPS, as a phagocytic stimulus per se (1-μm polystyrene beads) was not sufficient to elicit p40 production by DC (data not shown).
FIG. 7.
IL-12 p40 is produced by DC coincubated with serovar Typhimurium or with purified LPS. (A) DC were stimulated with viable wild-type, phoP, or phoPc serovar Typhimurium; viable wild-type serovar Typhimurium in the presence of CCD; or heat-killed wild-type serovar Typhimurium or were incubated in medium alone, as indicated. After an initial 2-h pulse with the bacteria, the cells were washed, treated with gentamicin, and incubated an additional 46 h. At this time point, the relative level of the IL-12 p40 subunit in the culture supernatant was quantitated by sandwich ELISA. (B) DC were either incubated in medium only or in the presence of 1-μg/ml LPS purified from wild-type, phoP, or phoPc serovar Typhimurium, as indicated. After 48 h of total incubation time, the relative level of the IL-12 p40 subunit was quantitated in culture supernatant by sandwich ELISA. Data are presented as the means of triplicate samples ± 1 standard deviation. Similar results were obtained in at least three independent experiments.
Despite IL-12 p40 production by Salmonella-pulsed DC, only a modest increase in IL-12 p70 production was detected in culture supernatants after 48 h of DC coincubation (details in Fig. 7 legend) with wild-type, phoP, or phoPc serovar Typhimurium (Table 2). Furthermore, pulsing of DC with purified LPS from wild-type, phoP, or phoPc strains elicited levels of IL-12 p70 only slightly above those detected in culture supernatants of DC incubated in medium alone. Thus, although exposure of DC to serovar Typhimurium or purified LPS resulted in production of the p40 subunit, only a slight increase in secretion of the biologically active p70 heterodimer was induced, particularly when 1 μg of purified LPS/ml was the stimulus.
TABLE 2.
IL-12 p70 production by DC 48 h after a bacterial pulsea
| Stimulus | IL-12 p70 production (pg/ml)
|
||
|---|---|---|---|
| Wild type | phoP | phoPc | |
| Bacteria | 154 ± 6 | 164 ± 16 | 188 ± 6 |
| LPS | 115 ± 7 | 103 ± 5 | 106 ± 7 |
See the legend to Fig. 7 for details of the incubation conditions. IL-12 p70 production in medium alone was 90 ± 5 pg/ml. The values shown are determined from a standard curve run in parallel and represent the means ± 1 standard deviation of triplicate supernatant aliquots from a single well infected with the indicated bacterial strain or stimulated with purified LPS from the indicated bacterial strain. The data are representative of three independent experiments.
DISCUSSION
Immature DC are specialized in capturing and processing antigens into peptides for MHC presentation. Upon maturation, which can be triggered by microbial products or inflammatory cytokines, these cells function as initiators and modulators of the immune system (reviewed in reference 3). The DC activation process that results in a mature phenotype appears to be a crucial step in generating a specific immune response. It is therefore important to understand how DC are affected when they encounter pathogens. For example, it has been shown that the pathogens Plasmodium falciparum (51), Trypanosoma cruzi (53), and herpes simplex virus (43) prevent DC maturation and that the bacteria Mycobacterium tuberculosis (22), Staphylococcus aureus (56), Streptococcus gordonii (13, 41), and Chlamydia psittaci (36) induce activation and maturation of DC. In the present study, we also show that the facultative intracellular bacterium S. enterica serovar Typhimurium or LPS purified from this bacterium induces maturation of murine bone marrow-derived DC. For example, Salmonella-stimulated DC showed a reduced capacity to process subsequently encountered bacteria for peptide presentation on MHC-I and MHC-II molecules. In addition, DC that have encountered serovar Typhimurium have increased surface expression of MHC-I, MHC-II, CD40, CD54, CD80, and CD86 as well as enhanced production of IL-12.
Although it has previously been shown that one feature of DC maturation is reduced ability to process exogenous proteins for peptide presentation on MHC-I and MHC-II molecules (7, 36, 45, 56), the present study is the first demonstration that an encounter with serovar Typhimurium or purified Salmonella LPS reduces the ability of DC to process antigens from a subsequent bacterial exposure for either MHC-I or MHC-II peptide presentation. This finding along with the observed Salmonella-induced alteration in surface molecule expression suggests that an encounter with serovar Typhimurium or purified Salmonella LPS indeed triggers maturation of murine bone marrow-derived DC.
Increased surface expression of MHC and costimulatory molecules following a pulsing of DC with Salmonella is consistent with that observed using other stimuli to induce DC maturation, such as exposure to TNF-α (44, 45, 56), gram-positive bacteria (56), or M. tuberculosis (22). Alteration in surface expression of MHC-I, MHC-II, CD86, CD40, and CD54 was maximal by 24 h of total incubation time. Neither viable serovar Typhimurium nor bacterial internalization was required to influence surface expression of these molecules. These results suggest that the observed increase in expression is due to surface interaction of bacteria with DC or soluble mediators released by bacterium-DC contact rather than phagocytic uptake of bacteria. This is consistent with the finding that only a small percentage of immature DC internalizes bacteria during a 2-h time frame (Fig. 5), while the whole population of immature DC undergoes alteration in MHC-I, MHC-II, CD86, CD40, and CD54 surface expression when analyzed at 24 h (Fig. 2).
In contrast, alteration in surface expression of CD80 had requirements different from those for the other surface molecules examined here. For example, alteration in surface expression of CD80 required that the DC be coincubated with viable serovar Typhimurium and also required active internalization of the bacteria. Furthermore, upregulation of CD80 expression was not significant until 48 h of total incubation time, with only a slight alteration in CD80 expression being apparent after 24 h. The mechanism for upregulation of surface molecules on DC following a pulse with serovar Typhimurium or Salmonella LPS is presently not known. However, TNF-α, a stimulus that induces DC maturation (44, 45, 56), was detected in culture supernatants of DC stimulated with bacteria or LPS (data not shown). It is possible that TNF-α produced by DC following a serovar Typhimurium infection may be involved in the maturation process. Clearly the regulation of surface molecule expression, and in particular the apparently differential regulation of CD80 expression, on DC following a serovar Typhimurium infection deserves further attention.
We and others (29) have found that bioactive IL-12 is produced following DC exposure to Salmonella. Although the quantity of IL-12 p70 detected was low and only detectable in supernatants collected after 48 h of incubation, both viable bacteria and purified LPS induced IL-12 p70 secretion by DC. IL-12 production by DC that encountered serovar Typhimurium would be favorable to the defense against this bacterium, as IL-12 production favors development of Th1 CD4+ T cells (reviewed in reference 49). Salmonella-specific T cells developing under the influence of IL-12 would produce cytokines such as IFN-γ that in turn enhance the microbicidal effects of phagocytic cells and facilitate bacterial elimination.
It has also been demonstrated that DC maturation induces increased synthesis and stability of MHC-I and MHC-II (9, 40, 41). These observations, combined with previous data showing that DC display OVA(257-264)/Kb complexes for at least 4 h following a 2-h pulse with Crl-OVA-expressing serovar Typhimurium (47), led us to investigate the duration that peptide-MHC complexes are present on the DC surface following phagocytic processing of the bacteria. T-cell stimulation assays revealed detectable levels of OVA(265-277)/I-Ab but not OVA(257-264)/Kb complexes on the DC surface after at least 24 h following an initial pulse of DC with bacteria expressing Crl-OVA. OVA(265-277)/I-Ab complexes were not, however, present at sufficient levels to stimulate the T-hybridoma cells 48 h after an initial bacterial encounter. Although several features of peptide–MHC–T-cell receptor interaction, such as the sensitivity of the two hybridomas, the quantity of specific peptide-MHC complexes formed, and/or the affinity of the T-cell receptor for the ligands, could influence the relative level of T-hybridoma cell stimulation, our data are consistent with biochemical data that measured an increase in the stability of total MHC-I and MHC-II molecules from 3 h to 9 h (41) and from 12 h to 36 h to 40 h (40), respectively, following the maturation of murine DC. DC exposure to maturation stimuli, including gram-positive (41) and gram-negative (Fig. 1 through 3) bacteria, results in DC expressing surface peptide-MHC complexes and costimulatory molecules enabling T-cell stimulation for a fairly short period following the antigen encounter. This suggests that to be able to activate both CD4+ and CD8+ Salmonella-specific T cells, DC need to meet specific T cells within ∼24 h after the bacterial encounter to ensure productive triggering of a Salmonella-specific immune response.
Bacterial pathogens synthesize numerous proteins that contribute to the ability of the bacteria to escape immune surveillance and persist inside host cells. For example, serovar Typhimurium expresses several genes upon contact with eukaryotic host cells (2, 8, 26). Furthermore, expression of some of these loci is under the control of the transcription regulator PhoP (34), which is critical for the virulence of serovar Typhimurium in murine models (15, 34, 35). PhoP also regulates lipid A modifications on LPS (18), affects the level of TNF-α production by monocytes (18), influences bacterial survival within Mφ after antibody-mediated opsonic uptake (35), and alters the efficiency by which Mφ process serovar Typhimurium for peptide presentation on MHC-II molecules (54). In the present study, we also show that PhoP affects the ability of DC to process Crl-OVA-expressing serovar Typhimurium for OVA(265-277)/I-Ab presentation when quantitated following a short (2-h) coincubation of bacteria with DC. In contrast, no effect of PhoP on OVA(265-277)/I-Ab presentation was apparent when peptide presentation was quantitated after 24 h of total Salmonella-DC coincubation. Furthermore, despite its effect on numerous aspects of bacterium-APC interactions as mentioned above, PhoP does not appear to influence IL-12 production by DC or surface expression of molecules important in signaling the immune system by Salmonella-infected DC. Finally, our results with LPS purified from wild-type or phoP mutant serovar Typhimurium strains suggest that the lipid A modifications controlled by PhoP do not affect the maturation program induced in immature DC propagated from murine bone marrow that encounter serovar Typhimurium.
Together our data show that an encounter with serovar Typhimurium stimulates DC to mature and undergo events critical for becoming activators and modulators of T cells. Furthermore, this occurs independently of PhoP and suggests that PhoP may play only a minor role in altering the capacity of infected DC to stimulate bacterium-specific T cells. These data further our understanding of the ability of serovar Typhimurium and specific virulence loci, such as phoP, to influence the processes taking place in DC prior to activation of T cells. Such information is crucial to developing effective vaccines that use attenuated versions of bacteria, such as serovar Typhimurium, to induce specific immune responses to recombinant antigens.
ACKNOWLEDGMENTS
We gratefully acknowledge Hongwei Yu and Vojo Deretic, University of Michigan, Ann Arbor, for pSK-Xho-GFP; Sam Miller, University of Washington, Seattle, for purified serovar Typhimurium LPS; Giorgio Trinchieri, Wistar Institute of Anatomy and Biology, Philadelphia, Pa., for hybridomas C15.6 and C17.8; and Judith A. Kapp, Emory University School of Medicine, Atlanta, Ga., for the OT4H T-cell hybridoma.
This work was supported by the Swedish Natural Sciences Research Council (project 650-19981154/2000), The Swedish Foundation for Strategic Research Infection and Vaccinology Program, The Österlund Foundation, Kock's Foundation, Kungliga Fysiografiska Foundation, The Crafoord Foundation, Åke Wiberg's Foundation, and the Lund University Medical Faculty.
REFERENCES
- 1.Alpuche-Aranda C M, Racoosin E L, Swanson J A, Miller S I. Salmonella stimulate macrophage macropinocytosis and persist within spacious phagosomes. J Exp Med. 1994;179:601–608. doi: 10.1084/jem.179.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alpuche-Aranda C M, Swanson J A, Loomis W P, Miller S I. Salmonella typhimurium activates virulence gene transcription within acidified macrophage phagosomes. Proc Natl Acad Sci USA. 1992;89:10079–10083. doi: 10.1073/pnas.89.21.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.Behlau I, Miller S I. A phoP-repressed gene promotes Salmonella typhimurium invasion of epithelial cells. J Bacteriol. 1993;175:4475–4484. doi: 10.1128/jb.175.14.4475-4484.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belden W J, Miller S I. Further characterization of the PhoP regulon: identification of new PhoP-activated virulence loci. Infect Immun. 1994;62:5095–5101. doi: 10.1128/iai.62.11.5095-5101.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharya A, Dorf M E, Springer T A. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J Immunol. 1981;127:2488–2495. [PubMed] [Google Scholar]
- 7.Brossart P, Bevan M J. Presentation of exogenous protein antigens on major histocompatibility complex class I molecules by dendritic cells: pathway of presentation and regulation of cytokines. Blood. 1997;90:1594–1599. [PMC free article] [PubMed] [Google Scholar]
- 8.Buchmeier N A, Heffron F. Induction of Salmonella stress proteins upon infection of macrophages. Science. 1990;248:730–732. doi: 10.1126/science.1970672. [DOI] [PubMed] [Google Scholar]
- 9.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of class II complexes on dendritic cells. Nature. 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 10.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chalfie M, Tu Y, Euskirchen G, Ward W W, Prasher D C. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 12.Coffman R L. Surface antigen expression and immunoglobulin gene rearrangement during mouse pre-B cell development. Immunol Rev. 1982;69:5–23. doi: 10.1111/j.1600-065x.1983.tb00446.x. [DOI] [PubMed] [Google Scholar]
- 13.Corinti S, Medaglini D, Cavani A, Rescigno M, Pozzi G, Ricciardi-Castagnoli P, Girolomoni G. Human dendritic cells very efficiently present a heterologous antigen expressed on the surface of recombinant Gram-positive bacteria to CD4+ T lymphocytes. J Immunol. 1999;163:3029–3036. [PubMed] [Google Scholar]
- 14.Dialynas D P, Wilde D B, Marrack P, Pierres A, Wall K A, Havran W, Otten G, Loken M R, Pierres M, Kappler J, Fitch F W. Characterization of the murine antigenic determinant designated L3T4a, recognised by monoclonal antibody GK1.5: expression of L3Ta by functional T cell clones appears to correlate primarily with class II MHC antigen reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 15.Fields P I, Groisman E A, Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science. 1989;243:1059–1062. doi: 10.1126/science.2646710. [DOI] [PubMed] [Google Scholar]
- 16.Garcia Véscovi E, Soncini F C, Groisman E A. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell. 1996;84:165–174. doi: 10.1016/s0092-8674(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 17.Gunn J S, Miller S I. PhoP-PhoQ activates transcription of pmrAB, encoding a two-component regulatory system involved in Salmonella typhimurium antimicrobial peptide resistance. J Bacteriol. 1996;178:6857–6864. doi: 10.1128/jb.178.23.6857-6864.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo L, Lim K B, Gunn J S, Bainbridge B, Darveau R P, Hackett M, Miller S I. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science. 1997;276:250–253. doi: 10.1126/science.276.5310.250. [DOI] [PubMed] [Google Scholar]
- 19.Hämmerling G J, Rüsch E, Tada N, Kimura S, Hämmerling U. Localization of allodeterminants on H-2Kb antigens determined with monoclonal antibodies and H-2 mutant mice. Proc Natl Acad Sci USA. 1982;79:4737–4741. doi: 10.1073/pnas.79.15.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hathcock K S, Laszlo G, Dickler H B, Bradshaw J, Linsley P, Hodes R J. Identification of an alternative CTLA-4 ligand costimulatory for T cell activation. Science. 1993;262:905–907. doi: 10.1126/science.7694361. [DOI] [PubMed] [Google Scholar]
- 21.Heithoff D M, Conner C P, Hentschel U, Govantes F, Hanna P C, Mahan M J. Coordinate intracellular expression of Salmonella genes induced during infection. J Bacteriol. 1999;181:799–807. doi: 10.1128/jb.181.3.799-807.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hendersson R A, Watkins S C, Flynn J L. Activation of human dendritic cells following infection with Mycobacterium tuberculosis. J Immunol. 1997;159:635–643. [PubMed] [Google Scholar]
- 23.Hess J, Ladel C, Miko D, Kaufmann S H E. Salmonella typhimurium aroA− infection in gene-targeted immunodeficient mice. J Immunol. 1996;156:3321–3326. [PubMed] [Google Scholar]
- 24.Hibbs J B, Jr, Taintor R R, Vavrin Z. Macrophage cytotoxicity: role for l-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987;235:473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- 25.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman R M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keliher-Burns L, Nickerson C A, Morrow B J, Curtiss R., III Cell-specific proteins synthesized by Salmonella typhimurium. Infect Immun. 1998;66:856–861. doi: 10.1128/iai.66.2.856-861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kubo R T, Born W, Kappler J W, Marrack P, Pigeon M. Characterization of a monoclonal antibody which detects all murine αβ T cell receptors. J Immunol. 1989;142:2736–2742. [Google Scholar]
- 28.Li Y, Ke Y, Gottlieb P D, Kapp J A. Delivery of exogenous antigen into the major histocompatibility complex class I and class II pathway by electroporation. J Leukoc Biol. 1994;56:616–624. doi: 10.1002/jlb.56.5.616. [DOI] [PubMed] [Google Scholar]
- 29.Marriott I, Hammond T G, Thomas E K, Bost K L. Salmonella efficiently enter and survive within cultured CD11c+ dendritic cells initiating cytokine expression. Eur J Immunol. 1999;29:1107–1115. doi: 10.1002/(SICI)1521-4141(199904)29:04<1107::AID-IMMU1107>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 30.Mastroeni P, Harrison J A, Robinson J H, Clare S, Khan S, Maskell D J, Dougan G, Hormaeche C E. Interleukin-12 is required for control of the growth of attenuated aromatic-compound-dependent salmonellae in BALB/c mice: role of gamma interferon and macrophage activation. Infect Immun. 1998;66:4767–4776. doi: 10.1128/iai.66.10.4767-4776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mekalanos J J. Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol. 1992;174:1–7. doi: 10.1128/jb.174.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metlay J P, Witmer-Pack M D, Agger R, Crowley M T, Lawless D, Steinman R M. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J Exp Med. 1990;171:1753–1771. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller J F, Mekalanos J J, Falkow S. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science. 1989;243:916–922. doi: 10.1126/science.2537530. [DOI] [PubMed] [Google Scholar]
- 34.Miller S I, Kukral A M, Mekalanos J J. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci USA. 1989;86:5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller S I, Mekalanos J J. Constitutive expression of the PhoP regulon attenuates Salmonella virulence and survival within macrophages. J Bacteriol. 1990;172:2485–2490. doi: 10.1128/jb.172.5.2485-2490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ojcius D M, Bravo de Alba Y, Kanellopoulos J M, Hawkins R A, Kelly K A, Rank R G, Dautry-Varsat A. Internalization of Chlamydia by dendritic cells and stimulation of Chlamydia-specific T cells. J Immunol. 1998;160:1297–1303. [PubMed] [Google Scholar]
- 37.Pegues D A, Hantman M J, Behlau I, Miller S I. PhoP/PhoQ transcriptional repression of Salmonella typhimurium invasion genes: evidence for a role in protein secretion. Mol Microbiol. 1995;17:169–181. doi: 10.1111/j.1365-2958.1995.mmi_17010169.x. [DOI] [PubMed] [Google Scholar]
- 38.Pfeifer J D, Wick M J, Roberts R L, Findlay K, Normark S J, Harding C V. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 39.Pfeifer J D, Wick M J, Russell D G, Normark S J, Harding C V. Recombinant Escherichia coli express a defined, cytoplasmic epitope that is efficiently processed in macrophage phagolysosomes for class II MHC presentation to T lymphocytes. J Immunol. 1992;149:2576–2584. [PubMed] [Google Scholar]
- 40.Pierre P, Turley S J, Gatti E, Hull M, Meltzer J, Mirza A, Inaba K, Steinman R M, Mellman I. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- 41.Rescigno M, Citterio S, Thèry C, Rittig M, Medaglini D, Pozzi G, Amigorena S, Ricciardi-Castagnoli P. Bacteria-induced neo-biosynthesis, stabilization, and surface expression of functional class I molecules in mouse dendritic cells. Proc Natl Acad Sci USA. 1998;95:5229–5234. doi: 10.1073/pnas.95.9.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rolink A, Melchers F, Andersson J. The SCID but not the RAG-2 gene product is required for Sμ-Sɛ heavy chain class switching. Immunity. 1996;5:319–330. doi: 10.1016/s1074-7613(00)80258-7. [DOI] [PubMed] [Google Scholar]
- 43.Salio M, Cella M, Suter M, Lanzavecchia A. Inhibition of dendritic cell maturation by herpes simplex virus. Eur J Immunol. 1999;29:3245–3253. doi: 10.1002/(SICI)1521-4141(199910)29:10<3245::AID-IMMU3245>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 44.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulation by tumor necrosis factor α. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skeen M J, Miller M A, Shinnick T M, Ziegler H K. Regulation of murine macrophage IL-12 production. Activation of macrophages in vivo, restimulation in vitro and modulation by other cytokines. J Immunol. 1996;156:1196–1206. [PubMed] [Google Scholar]
- 47.Svensson M, Stockinger B, Wick M J. Bone marrow-derived dendritic cells can process bacteria for MHC-I and MHC-II presentation to T cells. J Immunol. 1997;158:4229–4236. [PubMed] [Google Scholar]
- 48.Svensson M, Wick M J. Classical MHC-I peptide presentation of a bacterial fusion protein by bone marrow-derived dendritic cells. Eur J Immunol. 1999;29:180–188. doi: 10.1002/(SICI)1521-4141(199901)29:01<180::AID-IMMU180>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 49.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 50.Unkeless J C. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979;150:580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Urban B C, Ferguson D J, Pain A, Willcox N, Plebanski M, Austyn J M, Roberts D J. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature. 1999;400:73–77. doi: 10.1038/21900. [DOI] [PubMed] [Google Scholar]
- 52.Utsunomiya Y, Kosaka H, Kanagawa O. Differential reactivity of Vβ9 T cells to minor lymphocyte stimulating antigen in vitro and in vivo. Eur J Immunol. 1991;21:1007–1011. doi: 10.1002/eji.1830210422. [DOI] [PubMed] [Google Scholar]
- 53.van Overtvelt L, Vanderheyde N, Verhasselt V, Ismaili J, de Vos L, Goldman M, Willems F, Vray B. Trypanosoma cruzi infects human dendritic cells and prevents their maturation: inhibition of cytokines, HLA-DR, and costimulatory molecules. Infect Immun. 1999;67:4033–4040. doi: 10.1128/iai.67.8.4033-4040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wick M J, Harding C V, Twesten N J, Normark S J, Pfeifer J D. The phoP locus influences processing and presentation of S. typhimurium antigens by activated macrophages. Mol Microbiol. 1995;16:465–476. doi: 10.1111/j.1365-2958.1995.tb02411.x. [DOI] [PubMed] [Google Scholar]
- 55.Wilkinson R G, Gemski P J, Stocker B A D. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J Gen Microbiol. 1972;70:527–554. doi: 10.1099/00221287-70-3-527. [DOI] [PubMed] [Google Scholar]
- 56.Winzler C, Rovere P, Rescigno M, Granucci F, Penna G, Adorini L, Zimmermann V S, Davoust J, Ricciardi-Castagnoli P. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J Exp Med. 1997;185:317–328. doi: 10.1084/jem.185.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wysocka M, Kubin M, Vieira L, Ozwen L, Garotta L, Scott P, Trinchieri G. Interleukin-12 is required for interferon-γ production and lethality in lipopolysaccharide-induced shock in mice. Eur J Immunol. 1995;25:672–676. doi: 10.1002/eji.1830250307. [DOI] [PubMed] [Google Scholar]