Abstract
Background:
Lumbar spinal stenosis (LSS) is a common reason for spine surgery in which ligamentum flavum is resected. Transthyretin (TTR) amyloid is an often unrecognized and potentially modifiable mechanism for LSS that can also cause transthyretin cardiac amyloidosis. Accordingly, older adult patients undergoing lumbar spine (LS) surgery were evaluated for amyloid and if present, the precursor protein, as well as comprehensive characterization of the clinical phenotype.
Methods:
A prospective, cohort study in 2 academic medical centers enrolled 47 subjects (age 69±7 years, 53% male) undergoing clinically indicated LS decompression. The presence of amyloid was evaluated by Congo Red staining and in those with amyloid, precursor protein was determined by laser capture microdissection coupled to mass spectrometry (LCM-MS). The phenotype was assessed by disease specific questionnaires (Swiss Spinal Stenosis Questionnaire and Kansas City Cardiomyopathy Questionnaire) and the 36-question short-form health survey, as well as biochemical measures (TTR, retinol binding protein and TTR stability). Cardiac testing included technetium-99m-pyrophosphate scintigraphy, electrocardiograms, echocardiograms, and cardiac biomarkers as well as measures of functional capacity.
Results:
Amyloid was detected in 16 samples (34% of participants) and was more common in those age ≥75 years of age (66.7%) compared with those <75 years (22.3%, p <0.05). LCM-MS demonstrated TTR as the precursor protein in 62.5% of participants with amyloid while 37.5% had an indeterminant type of amyloid. Demographic, clinical, quality-of-life measures, electrocardiographic, echocardiographic, and biochemical measures did not differ between those with and without amyloid. Among those with TTR amyloid (n=10), one subject had cardiac involvement by scintigraphy.
Conclusions:
Amyloid is detected in more than a third of older adults undergoing LSS. Amyloid is more common with advancing age and is particularly common in those >75 years old. No demographic, clinical, biochemical, or cardiac parameter distinguished those with and without amyloid. In more than half of subjects with LS amyloid, the precursor protein was TTR indicating the importance of pathologic assessment.
Keywords: Lumbar Spinal Stenosis, Amyloidosis, Transthyretin, Cardiac
Lumbar spinal stenosis (LSS) disproportionately affects older adults and is the most common reason for lumbar spine surgery.1 The biological mechanism underlying degeneration in lumbar spinal stenosis has not been fully elucidated. Emerging data suggest that transthyretin (TTR) amyloidosis, characterized by deposition of the misfolded protein TTR, is age-dependent, unrecognized, and commonly present in surgical specimens (e.g., ligamentum flavum) in LSS2–5 that may precede the development of cardiac involvement.6,7
Transthyretin cardiac amyloidosis (ATTR-CA) is causes by misfolded monomers of the protein transthyretin (also known as prealbumin) which deposit as amyloid fibrils in the myocardium causing a restrictive cardiomyopathy, atrial and ventricular arrhythmias, heart failure and premature death.8 ATTR-CA is an under diagnosed cause of heart failure with a preserved ejection fraction (HFpEF) affecting almost exclusively adults over the age of 60. It has been reported that 13% of older adults hospitalized with HFpEF and an increased wall thickness have ATTR-CA9 and that 6% of community dwelling older adults with HFpEF have ATTR-CA.10 Additionally, 16% of patients who undergo a transcatheter aortic valve replacement have ATTR-CA.11 Identification of older adults with ATTR-CA early in the course of their disease is critical, as emerging disease modifying therapies have been shown to meaningfully reduce morbidity and mortality from this condition and are more effective before significant cardiac dysfunction has ensued.7
Uncovering an association between lumbar spinal stenosis and TTR amyloidosis may provide a modifiable biological mechanism for acquired degenerative lumbar spinal stenosis and could lead to further clinical trials of novel compounds that prevent amyloid formation. Additionally, since TTR amyloidosis may result in a cardiomyopathy, leveraging non-invasive imaging (Tc99m pyrophosphate scintigraphy or Tc99-PYP) that can identify TTR cardiac amyloidosis without the need for histology may enable us to identify a group of patients with TTR cardiac amyloidosis before the development of overt heart failure. Early identification of TTR cardiac amyloidosis is critical since approved therapies prevent new amyloid deposition but do not address existing amyloid.12
Accordingly, we conducted a prospective pilot cohort study in patients age >=55 years old who had undergone clinically indicated LSS surgery, in which we screened surgical specimens for transthyretin amyloid (ATTR) using pathologic techniques including tissue typing with mass spectrometry based proteomic analysis. In patients with ligamentum flavum amyloid and in a subset of those without tissue evidence of amyloid, we characterized their cardiac phenotype using biomarkers, transthoracic echocardiography and Tc-99m pyrophosphate cardiac imaging and evaluated for biochemical associations between disease phenotype and TTR instability. The aims of this study are: (1) to confirm the previously reported high prevalence of TTR amyloid deposits in older adult patients undergoing lumbar spinal stenosis surgery, (2) to verify that amyloid deposits in such patients are due to transthyretin and (3) to characterize the cardiac phenotype and establish biochemical associations of TTR stability in patients with pathologically defined TTR amyloidosis.
Methods:
Study Design:
We conducted a prospective, two-center cohort pilot study at Columbia University Irving Medical Center and Tufts Medical Center. In this study, older adults who had undergone lumbar spine surgery for standard clinical indications were evaluated to determine whether amyloid was present in the surgically obtained spinal specimens, and if so, determine the precursor protein by laser capture microdissection coupled to mass spectrometry (LCM-MS). In those with amyloid, we sought to further characterize the cardiac and orthopedic phenotype using disease specific questionnaires, cardiac biomarkers, echocardiography with speckle-tracking strain analysis (GE Vivid 9 and EchoPAC), and TTR stability using a novel assay.13
Study Subjects:
Older adults (age ≥ 55 years) who were undergoing clinically indicated lumbar spine surgery were invited to participate (See CONSORT Chart, Supplementary Figure 1). Exclusion criteria included confirmed transthyretin, primary amyloidosis (AL) or secondary amyloidosis (AA), lumbar surgery for indications other than spinal stenosis, and active malignancy. All subjects who were enrolled reviewed and signed informed consent documents. The respective IRBs of both participating institutions approved the protocol.
Protocol:
Patients were screened and enrolled prior to LSS. Informed consent, complete history, physical examination, and medication list were obtained prior to LSS surgery. LSS specimens were reviewed by a pathologist with experience in detecting amyloidosis with Congo Red staining. In those subjects who had amyloid identified, further characterization of the precursor protein was determined using laser microdissection and tandem mass spectrometry-based analysis of proteomics.14 After LSS surgery, subjects who agreed completed several questionnaires to assess symptoms and quality of life (QOL): Kansas City Cardiomyopathy Questionnaire15, Swiss Spinal Stenosis Questionnaire16, and SF-36.17 Functional assessment was performed using 6MWT distance and short physical performance battery (SPPB)18. Additional cardiovascular assessments included an electrocardiogram with a focus on features of amyloidosis (pseudo-infarcts, low voltage), echocardiogram with speckle tracking strain imaging, and Tc-99m pyrophosphate planar imaging with concomitant single photon emission computed tomography (SPECT) if there was significant uptake on planar imaging to ensure that uptake of 99mTc-PYP was myocardial in nature and not due to blood pool.19 Genotyping on DNA extracted from white blood cells obtained from participants with amyloid was performed using PCR-based assay with all 4 exomes probed for a mutation. Stability of TTR was determined by a subunit exchange method for quantifying the kinetic stability of endogenous TTR in human plasma as previously described.13
Statistical Analysis:
Data were entered into a secure REDCap database (Vanderbilt University, Nashville, TN) designed for this study. Descriptive statistics were used to characterize variables of the study participants. Comparisons were made between those with and without amyloid detected on pathologic specimens, with the former stratified by the type of amyloidosis (TTR vs. indeterminate), and by demographic, clinical, QOL, and electrocardiographic / echocardiographic parameters. Given the small sample size of group, non-parametric testing was employed (SAS Studio 3.8, Enterprise Edition, 2020).
Results:
Forty-seven participants (69±7 years of age, 51% male) underwent pathologic assessment for the presence of amyloid in spinal specimens and further assessment of symptoms, QOL, functional testing and cardiac evaluation was performed as shown (Supplementary Figure 1, consort chart). Amyloid in the ligamentum flavum by Congo Red staining was detected in 34% (Figure 1, Panel A) and was significantly more common in those participants who were 75 years of age or older (66.7%) compared to those younger than 75 years (26.3%), p <0.05 (Figure 1, Panel B). LCM-MS demonstrated TTR as the precursor protein in 62.5% of participants with amyloid, the remaining 37.5% had an indeterminant type of amyloid (Figure 1, Panel A).
Figure 1:

Panel A. Percent of subjects with amyloid in spinal specimens and type of precursor protein detected in those with amyloid. Panel B: Prevalence of Amyloid in Lumbar Spine specimens in those ≤75 years of age and those ≥75 years of age.
Across a wide range of a) demographic and clinical characteristics (Table 1), b) disease specific, general QOL measures and functional capacity (Table 2), c) biochemical parameters (Table 3) and d) electrocardiographic and echocardiographic variables (Supplementary Table 1), participants with and without amyloidosis detected on pathologic specimens did not differ. Furthermore, there were no differences between those with TTR amyloid compared to those with indeterminant type of amyloid. Among the 10 participants who had TTR amyloidosis detected on surgical spine specimens, nine underwent Tc99-PYP scintigraphy and one had cardiac involvement (Figure 2).
Table 1:
Demographic and Clinical Characteristics
| Parameter | Overall (N=47) | Amyloid Absent (N=31)* | Amyloid Present (N=16) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TTR (N=10) | Indeterminant (N=6) | |||||||||||
| n | Mean ± SD | Median (Q1 - Q3) | n | Mean ± SD | Median (Q1 - Q3) | n | Mean ± SD | Median (Q1 - Q3) | n | Mean ± SD | Median (Q1 - Q3) | |
| Age (years) | 47 | 68.5±6.8 | 69 (65, 72) | 31 | 67.8±6.1 | 69 (65, 71.8) | 10 | 72.4±8 | 73 (65, 78.8) | 6 | 66±7.4 | 67 (60.3, 70) |
| Gender (% Male) | 47 | 25 (53.2%) | 31 | 14 (45.2%) | 10 | 8 (80%) | 6 | 3 (50%) | ||||
| Race | ||||||||||||
| White | 47 | 32 (78.7%) | 31 | 24 (77.4%) | 10 | 10 (100%) | 6 | 3 (50%) | ||||
| Black | 47 | 4 (8.5%) | 31 | 3 (9.7%) | 10 | 0 (0%) | 6 | 1 (16.7%) | ||||
| Asian | 47 | 3 (6.4%) | 31 | 2 (6.5%) | 10 | 0 (0%) | 6 | 1 (16.7%) | ||||
| Unknown | 47 | 3 (6.4% | 31 | 2 (6.5%) | 10 | 0 (0%) | 6 | 1 (16.7%) | ||||
| Ethnicity | ||||||||||||
| Hispanic | 47 | 7 (14.9%) | 31 | 6 (19.1%) | 10 | 0 (0%) | 6 | 1 (16.7%) | ||||
| Non-Hispanic | 47 | 37 (78.7%) | 31 | 23 (74.2%) | 10 | 10 (100%) | 6 | 4 (66.7%) | ||||
| Unknown | 47 | 3 (6.4%) | 31 | 2 (6.5%) | 10 | 0 (0%) | 6 | 1 (16.7%) | ||||
| BMI | 38 | 31.6±6.7 | 31.1 (27.8, 34.7) | 22 | 31.3±5.6 | 30.4 (27.9, 33) | 10 | 29.3±4.7 | 30.8 (25.9, 31.7) | 6 | 36.4±11.1 | 34.9 (29.4, 37.4) |
| Co-morbidities | ||||||||||||
| -DM | 38 | 9 (23.7%) | 22 | 7 (31.8%) | 10 | 0 (0%) | 6 | 2 (33.3%) | ||||
| -HTN | 38 | 24 (63.2%) | 22 | 14 (63.6%) | 10 | 7 (70%) | 6 | 3 (50%) | ||||
| -COPD | 38 | 2 (5.3%) | 22 | 1 (4.5%) | 10 | 1 (10%) | 6 | 0 (0%) | ||||
| -CKD | 38 | 5 (13.2%) | 22 | 4 (18.2%) | 10 | 1 (10%) | 6 | 0 (0%) | ||||
| -Stroke | 38 | 2 (5.3%) | 22 | 1 (4.5%) | 10 | 0 (0%) | 6 | 1 (16.7%) | ||||
| -Arthritis | 38 | 12 (31.6%) | 22 | 6 (27.3%) | 10 | 5 (50%) | 6 | 1 (16.7%) | ||||
| -Cancer | 38 | 4 (10.5%) | 22 | 1 (4.5%) | 10 | 2 (20%) | 6 | 1 (16.7%) | ||||
| -Total # comorbidities | 38 | 1.6±1.2 | 1 (1, 2) | 22 | 1.6±1.2 | 1 (1, 2.3) | 10 | 1.6±1 | 1.5 (1, 2) | 6 | 1.3±1.5 | 1 (0.3, 1.8) |
| NYHA Class | ||||||||||||
| -Class I | 37 | 26 (70.3%) | 22 | 15 (68.2%) | 10 | 6 (60%) | 5 | 5 (100%) | ||||
| -Class III | 37 | 9 (24.3%) | 22 | 6 (27.3%) | 10 | 3 (30%) | 5 | 0 (0%) | ||||
| -Class IIII | 37 | 2 (5.4%) | 22 | 1 (4.5%) | 10 | 1 (10%) | 5 | 0 (0%) | ||||
TUFTS completed procedures for (+) amyloid subjects only.
Table 2:
Disease specific, General QOL Measures and Functional Capacity
| Questionnaire | Overall (N=47) | Amyloid Absent (N=31) | Amyloid Present (N=16) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TTR (N=10) | Indeterminant (N=6) | |||||||||||
| n | Mean ± SD | Median (Q1 - Q3) | n | Mean ± SD | Median (Q1 - Q3) | n | Mean ± SD | Median (Q1 - Q3) | n | Mean ± SD | Median (Q1 - Q3) | |
| Swiss Spinal Stenosis Questionnaire | ||||||||||||
| Symptom severity (range 1 to 5) | 27 | 2.7±0.9 | 2.7 (2.1, 3.2) | 14 | 2.9±1.1 | 2.8 (2.3, 3.8) | 9 | 2.5±0.7 | 2.7 (2.1, 3) | 4 | 2.6±0.8 | 2.5 (2.1, 3.1) |
| Functional disability (range 1 to 4) | 27 | 2.2±0.7 | 2.2 (1.6, 2.7) | 14 | 2.3±0.9 | 2.6 (1.5, 3) | 9 | 2±0.7 | 2 (1.6,2.2) | 4 | 2.4±0.2 | 2.4 (2.4, 2.5) |
| Oswestry Low Back Pain Questionnaire | ||||||||||||
| Total percent score (range 0 to 100) | 24 | 38±19.6 | 41 (23.5, 53) | 15 | 41.5±18.8 | 42 (25.5, 54.5) | 6 | 22.8±18.1 | 24 (12.8, 26.3) | 3 | 51±9.6 | 47 (45.5, 54.5) |
| KCCQ scores (range 0 to 100) | ||||||||||||
| Physical limitation | 24 | 71.4±26.2 | 77.1 (54.2, 91.7) | 12 | 74.3±27.9 | 83.3 (65.7, 93.8) | 8 | 68.8±27.1 | 77.1 (40.7, 91.7) | 4 | 67.7±24.6 | 64.6 (54.2, 78.1) |
| Symptom stability | 26 | 51.9±9.6 | 50 (50, 50) | 14 | 53.3±12.9 | 50 (50, 50) | 8 | 50±0 | 50 (50, 50) | 4 | 50±0 | 50 (50, 50) |
| Symptom frequency | 27 | 83.3±24.8 | 92.7 (79.2, 100) | 14 | 83.2±27.9 | 95.8 (85.4, 100) | 9 | 84.3±25.5 | 100 (87.5, 100) | 4 | 81.3±13.3 | 78.2 (75, 84.4) |
| Symptom burden | 27 | 83.6±20 | 91.7 (75, 100) | 14 | 83.9±23 | 91.7 (83.3, 100) | 9 | 82.4±18.8 | 91.7 (75, 100) | 4 | 85.4±12.5 | 83.4 (75, 93.8) |
| Total symptom | 27 | 83.5±21.7 | 89.6 (81.3, 100) | 14 | 83.6±24.8 | 91.7 (82.3, 99) | 9 | 83.4±21.5 | 89.6 (81.3, 100) | 4 | 83.4±12.5 | 80.8 (75.1, 89.1) |
| Self-efficacy | 27 | 83±25.7 | 100 (75, 100) | 14 | 89.2±20 | 100 (87.5, 100) | 9 | 76.4±33.3 | 87.5 (75, 100) | 4 | 75±27 | 81.3 (65.6, 90.6) |
| Quality of life | 24 | 88.3±16.7 | 91.7 (83.3, 100) | 14 | 91.1±12.4 | 100 (87.5, 100) | 7 | 78.6±24 | 91.7 (62.5, 95.9) | 3 | 97.2±4.8 | 100 (95.9, 100) |
| Social limitation | 15 | 75.3±33.1 | 93.8 (59.4, 100) | 8 | 70.8±39 | 100 (37.5, 100) | 4 | 79.7±32.8 | 93.8 (73.5, 100) | 3 | 82.7±16.7 | 81.3 (74, 90.7) |
| Clinical summary | 27 | 78.8±22 | 87.5 (70.8, 94.1) | 14 | 80.3±24.5 | 87.5 (81.1, 94.3) | 9 | 77.8±21.2 | 87.5 (67.8, 90.7) | 4 | 75.6±17.4 | 71.7 (68.3, 78.9) |
| Overall summary | 24 | 82.1±18 | 88.9 (74.5, 95.3) | 14 | 82.9±17.7 | 88.9 (77.9, 93.5) | 7 | 79.8±21.7 | 82.3 (75.5, 94.5) | 3 | 83.5±15.6 | 81.3 (75.2, 90.7) |
| SF-36 (range 0 to 100) | ||||||||||||
| Physical functioning | 27 | 46.7±24.2 | 50 (27.5, 60) | 14 | 42.5±21.6 | 42.5 (26.3, 60) | 9 | 53.9±31.3 | 50 (25, 70) | 4 | 45±14.7 | 45 (33.8, 56.3) |
| Role limitations due to physical health | 27 | 34.3±42.3 | 0 (0, 75) | 14 | 42.9±40.9 | 37.5 (0, 75) | 9 | 36.1±48.6 | 0 (0, 100) | 4 | 0±0 | 0 (0, 0) |
| Role limitations due to emotional problems | 27 | 59.3±46.5 | 100 (0, 100) | 14 | 59.5±45.6 | 83.3 (8.3, 100) | 9 | 74.1±43.4 | 100 (66.7, 100) | 4 | 25±50 | 0 (0, 25) |
| Energy/fatigue | 27 | 55±18.4 | 60 (42.5, 65) | 14 | 57.9±18.1 | 60 (46.3, 72.5) | 9 | 53.3±20.8 | 55 (45, 60) | 4 | 48.8±16.5 | 50 (37.5, 61.3) |
| Emotional well-being | 27 | 76.7±15.6 | 84 (68, 88) | 14 | 77.4±15.3 | 84 (68, 87) | 9 | 76.4±16.2 | 80 (76, 84) | 4 | 75±20 | 80 (66, 89) |
| Social functioning | 27 | 70.8±32.9 | 75 (56.3, 100) | 14 | 61.6±37.5 | 75 (31.3, 87.5) | 9 | 94.4±11 | 100 (100, 100) | 4 | 50±17.7 | 56.3 (43.8, 62.5) |
| Pain | 27 | 47.5±26.9 | 45 (27.5, 68.8) | 14 | 40.4±28.5 | 37.5 (22.5, 57.5) | 9 | 63.9±22.4 | 60 (57.5, 77.5) | 4 | 35.6±13.8 | 32.5 (30, 38.1) |
| General health | 27 | 59.4±18.1 | 60 (47.5, 72.5) | 14 | 60±18.8 | 57.5 (50, 73.8) | 9 | 58.3±21.9 | 50 (45, 75) | 4 | 60±4.1 | 60 (58.8, 61.3) |
| Physical function | ||||||||||||
| SPPB total score | 22 | 8±3.4 | 9 (7, 10) | 8 | 8.4±3.6 | 10 (8.5, 10) | 9 | 7.3±4.2 | 7 (6, 11) | 5 | 8.8±1.1 | 9 (9, 9) |
| 6MWT distance (m) | 21 | 292±103 | 304 (220, 346) | 8 | 256±93 | 240 (220, 290) | 8 | 316±138 | 332 (166, 428) | 5 | 311.2±7.6 | 310 (304, 318) |
Table 3.
Biochemical parameters and TTR gene sequencing
| Laboratory | Overall (N=47) | Amyloid Absent (N=31) | Amyloid Present (N=16) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TTR (N=10) | Indeterminant (N=6) | |||||||||||
| n | Mean ± SD | Median (Q1 - Q3) | n | Mean ± SD | Median (Q1 - Q3) | n | Mean ± SD | Median (Q1 - Q3) | n | Mean ± SD | Median (Q1 - Q3) | |
| Cardiac Biomarkers | ||||||||||||
| NT-proBNP (pg/ml) | 26 | 301±409 | 121 (45, 435) | 13 | 300±401 | 98 (46, 404) | 8 | 443±511 | 282 (98, 559) | 5 | 78±51 | 69 (48, 109) |
| Troponin T (ng/L) | 24 | 12.5±21.6 | 0 (0, 13.8) | 12 | 7.5±8.8 | 6.5 (0, 12) | 7 | 29.9±33.4 | 36 (0, 41) | 5 | 0±0 | 0 (0, 0) |
| TTR/Vitamin A Biomarkers | ||||||||||||
| Prealbumin (mg/dl) | 24 | 26±6 | 27 (23, 30) | 12 | 25.1±6.9 | 27 (21, 30) | 8 | 27.2±4.6 | 28 (24, 30) | 4 | 26.3±5.2 | 26 (22, 30) |
| RBP4 in plasma (ug/ml) | 17 | 3.7±1 | 3.9 (3.5, 4.3) | 8 | 3.6±0.5 | 3.6 (3.4, 4) | 7 | 3.9±1.5 | 4.1 (3.8, 4.7) | 2 | 3.5±0.7 | 3.5 (3.2, 3.7) |
| RBP4 in urine (ug/ml) | 11 | 3.5±1.5 | 3.5 (2.7, 4.5) | 5 | 3.6±1.9 | 3.5 (3.1, 4.3) | 5 | 3.4±1.4 | 3.8 (2.2, 4.8) | 1 | 3.4 | 3.4 (3.4, 3.4) |
| Vitamin A in plasma (μM) | 16 | 2.3±0.8 | 2.3 (2, 2.6) | 8 | 2±0.6 | 2.1 (1.7, 2.3) | 7 | 2.5±0.9 | 2.5 (2.1, 3.1) | 1 | 2.8 | 2.8 (2.8, 2.8) |
| TTR gene sequencing (% wild type) | 17 | 17 (100%) | 8 | 8 (100%) | 7 | 7 (100%) | 2 | 2 (100%) | ||||
| TTR kinetic stability (h−1) | 18 | 0.0156±0.005 | 0.0163 (0.0141, 0.0193) | 8 | 0.0141±0.0066 | 0.0163 (0.0124, 0.0186) | 8 | 0.0166±0.0033 | 0.0158 (0.0143, 0.0197) | 2 | 0.0175±0.0033 | 0.0175 (0.0164, 0.0187) |
Figure 2: Tc99-PYP Scan of Patient with TTR amyloid in LS tissue and evidence of ATTRwt Cardiac Amyloidosis.
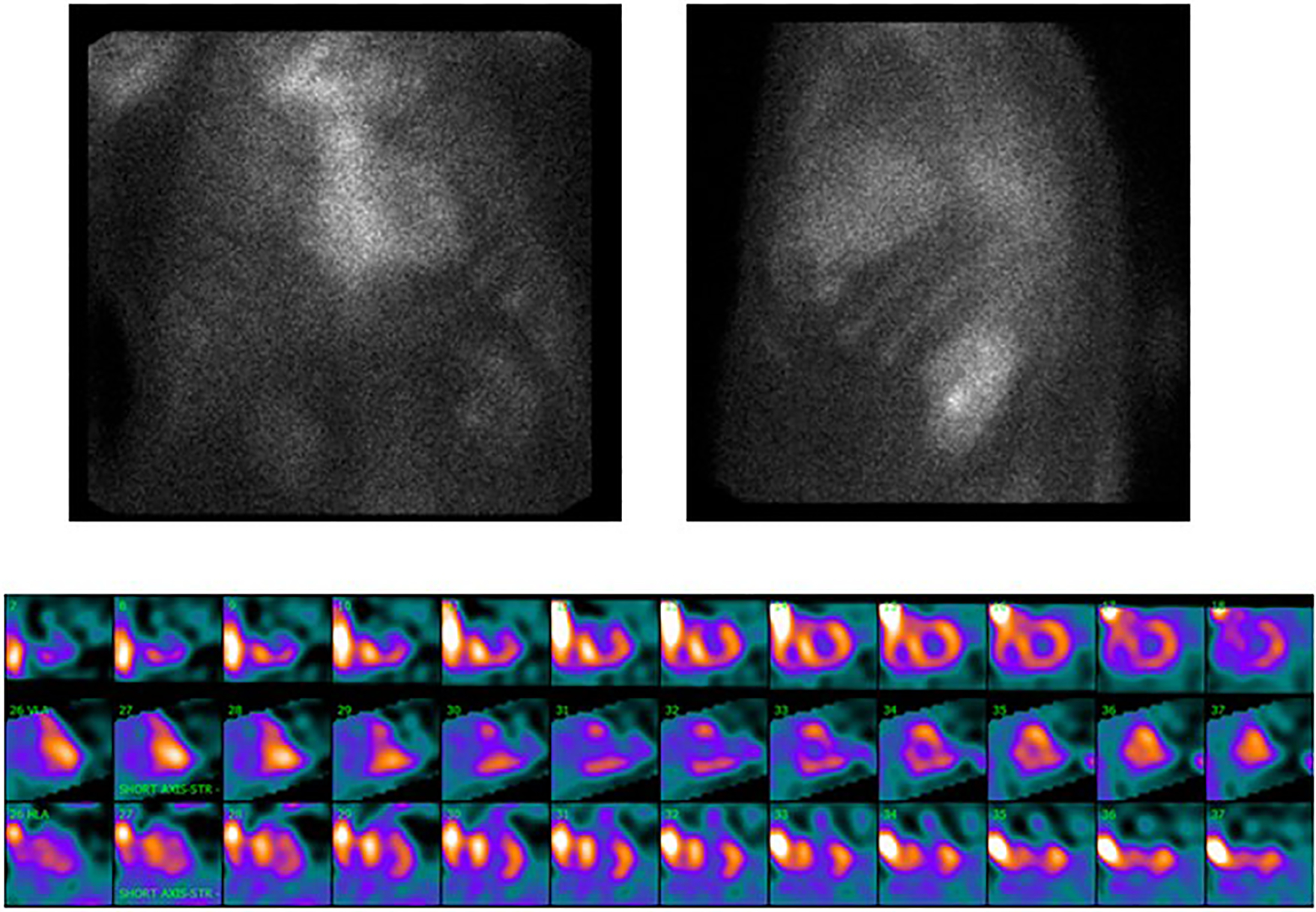
PYP scintigraphy: Top left panel: Planar anteroposterior image. Top right panel: Planar lateral image. Bottom panel: SPECT short axis images from apex to base, vertical long axis images from septum to lateral wall, and horizontal long axis images from inferior to anterior. Note that amyloid uptake on SPECT is in the inferior and lateral wall as the subject has a history or coronary artery disease and had a previous antero-septal myocardial infarction.
Discussion:
The principal findings of this prospective study are: (1) 34% of subjects undergoing clinically indicated lumbar spine surgery have amyloid detected on explanted tissue by Congo red staining; (2) demographic, clinical, biomarker, electrocardiographic, and echocardiographic measures do not differ between those with and without amyloid on pathologic evaluation; and (3) among those with amyloid detected in spinal tissue, 62.5% had transthyretin amyloidosis while the rest were indeterminant and (4) the prevalence of transthyretin amyloid was about 2.5 fold higher in persons ≥75 years of age versus those under 75 years of age. Finally, of participants with TTR amyloid detected in spinal specimens, one had concomitant cardiac amyloidosis at the time of surgical decompression.
Spinal claudication was originally described in systemic amyloidosis by Benson and colleagues.20 Emerging data from single center studies have demonstrated that amyloid is present in the spinal specimens of patients with LSS undergoing surgery. Data from these studies reveal amyloid in 96%3,100%4, 88.4%21 and in the largest study to date among 324 patients undergoing LSS, 43 patients (13%) had ATTR in the ligamentum flavum with wild-type TTR gene sequences.5 While a high prevalence of amyloid was found in small studies3, 4, 21, this may be because one study restricted their analysis to subjects with known systemic amyloidosis21. In another study, only a third of subjects had TTR as the precursor protein by immunohistochemistry3 while another showed that those with transthyretin amyloid deposits had thicker ligamentum flavum and more lumbar spinal segmental instability.4 Our data add to these previous studies and further highlight that older age is a risk factor for the presence of amyloidosis in lumbar spine specimens. Amyloid has been detected in cervical and thoracic spinal tissue as well as in the lumbar spine.22 Additionally, the ligamentum flavum burden as assessed pathologically is greater in patients with ATTRwt amyloid compared to non-ATTRwt patients.23 These data coupled with the association of LSS with ATTR cardiac amyloidosis have led to the hypothesis that screening programs focused on orthopedic manifestations could identify affected subjects early in the course of their disease at a time when emerging disease-modifying therapy may be most beneficial.6, 7
Our data suggest that there are no significant differences in demographics, clinical characteristics, or lumbar spinal disease symptom severity, nor differences in electrocardiographic, echocardiographic, or biomarker-based studies between those with and those without amyloid in spinal specimens at the time of surgery. While limited by a small sample size, these data do not suggest that any variable will be suitable to identify older individuals with LSS who have concomitant amyloid. Accordingly, pathologic evaluation of spinal specimens including assessment for amyloid by Congo red staining and further evaluation of the precursor protein by LDMS will be essential to identify affected individuals. Such testing will require ongoing collaboration between orthopedists, neurologists, neurosurgeons, pathologists, and amyloidosis experts.
While routine testing for amyloid with Congo Red staining or other amyloid specific techniques is relatively inexpensive, our data demonstrate that in a significant percentage of those with amyloid, the detected the precursor protein is not TTR but rather is indeterminant by LDMS. While LDMS is expensive, such testing appears essential to accurately identity the precursor protein causing amyloid. Indeed, if screening programs are aimed at appropriately targeting patients with TTR amyloid for ongoing assessment for cardiac involvement, then defining that the amyloid is due to misfolded transthyretin will be essential. Given the potential for misdiagnosis as part of screening programs24 as well as the high cost of therapies for TTR cardiac amyloidosis25, consideration of more careful pathologic evaluation of specimens appears reasonable and potentially cost effective.
The prevailing paradigm regarding the pathophysiology of wild type transthyretin amyloidosis is that with aging, transthyretin becomes unstable and dissociates into monomers that deposit in affected organs. Clearly, a transthyretin stabilizer has been shown to be an effective therapy for this condition.12 However, we were not able to demonstrate any difference in TTR kinetic stability in those who had amyloid versus those who did not. This is similar to data in patients with carpal tunnel syndrome26 and in those with ATTRwt cardiac amyloidosis versus controls.13 Whether other factors such as shear forces27 and proteolytic cleavage28 or tissue specific epitopes that attract misfolded TTR explain the tissue tropism observed and contribute to the pathogenesis of disease requires further study.
We detected one participant (~10%) with ATTRwt cardiac amyloidosis at the time of lumbar spinal surgery from the 10 participants who had TTR amyloid on pathologic evaluation and nine of whom underwent nuclear scintigraphy. This is quite similar to the percentage of subjects who have been found to have ATTRwt cardiac amyloidosis among those hospitalized for heart failure with a preserved ejection fraction9 and among those undergoing a transcatheter aortic valve replacement for severe aortic stenosis.11 Whether cardiovascular evaluation of patients with TTR amyloid in pathologic spinal specimens 5 to 15 years after surgery would result in a higher clinical yield is unknown but a ripe area for future study. A recently conducted study, the CACTUS-R study (NCT04276220) found that among older adult male patients >70 years who had bilateral carpal tunnel surgery 5–15 years previously, more than 1 in 4 had ATTR cardiac amyloidosis if their BMI was <30 kg/m2. Almost all of the subjects identified in the CACTUS-R study with ATTR cardiac amyloidosis had early-stage disease, demonstrating that such an approach can facilitate early identification of older adults affected by ATTRwt cardiac amyloidosis for whom disease modifying therapy has been shown to meaningfully reduce morbidity and mortality.12 Additionally, whether to treat patients with TTR amyloid in spinal tissue in order to prevent the development of transthyretin cardiac amyloidosis with diflunisal, a non-steroidal anti-inflammatory agent, which when given at low doses has transthyretin stabilizing properties29, 30 is unknown.
Our data are limited by small sample size, and further evaluation in larger samples will be needed to determine if risk factors for amyloid in spinal specimens can guide who should undergo Congo Red staining of tissue samples. Given that subjects in this study were recovering from spinal surgery and it was conducted during the COVID-19 pandemic, several participants were reluctant to undergo comprehensive phenotyping especially when they did not have amyloid detected in their spinal tissue, limiting our ability to compare those affected with amyloidosis with controls. Accordingly, the study design hindered our ability to comprehensively phenotype all subjects. In the absence of demographic and clinical differences between those with and without amyloid, we would advocate Congo Red staining for routine assessment of spinal specimens obtained during surgery. Such an analysis can facilitate the identification of patients whose LSS is due to amyloid and who may be at risk for the development of transthyretin cardiac amyloidosis. We performed pathologic evaluation at the time of surgery and did comprehensive phenotyping within weeks or months of the surgical intervention. However, most patients with transthyretin cardiac amyloidosis have had previous spine surgery 5–15 years prior to their diagnosis of cardiac amyloidosis. Future studies that ‘call back’ patients after spinal surgery would be more likely to detect ATTR cardiomyopathy but to appropriately target patients (e.g., those with TTR amyloid) will require pathologic evaluation as was performed in this study.
In summary, more than a third of older adult patients undergoing lumbar spine surgery for LSS have amyloid detected. Amyloid was 2.5-fold more common in those >75 years of age than those <75 years of age. In almost two thirds the precursor protein is transthyretin. While routine analysis of spinal specimens is recommended, these data suggest that proteomic analysis to determine the precursor protein will be needed to appropriately target those who will require ongoing surveillance for the development of cardiac amyloidosis.
Supplementary Material
Supplementary Figure 1: CONSORT Chart of Study participants. Flow chart of study population showing subjects who were consented and those that underwent clinically indicated LSS surgery with pathologic assessment for amyloidosis. Additional testing is noted in those who did and did not have amyloid detected.
Key Points.
In this prospective pilot study, more than a third of subjects undergoing lumbar spinal stenosis surgery had amyloid detected on pathologic evaluation of the ligamentum flavum by Congo red staining and amyloid in lumbar spine specimens was more common with advancing age.
Amyloid was 2.5-fold more common in those >75 years of age than those <75 years of age.
The precursor protein causing amyloid was transthyretin by mass spectrometry in a majority but not all cases. It was indeterminant in the remainder.
Why does this paper matter?
Pathologic assessment of the ligamentum flavum is essential to identify individuals with transthyretin amyloid who may require ongoing surveillance for transthyretin cardiac amyloidosis.
Acknowledgements
Conflicts of Interest:
The following authors have conflicts that they acknowledge related to this manuscript. Dr Maurer has received funding from the National Institutes of Health (HL139671-01, AG R21AG058348, and AG K24AG036778), and consulting income from Akcea, Alnylam, Eidos Therapeutics, Pfizer, and Prothena; his institution has also received funding for clinical trials for Alnylam, Eidos Therapeutics, Pfizer, and Prothena. Dr. Patel has received consulting income from Pfizer and her institution has received educational grant funding from Pfizer. Dr. Einstein received a speaker’s fee from Ionetix, consulting fees from W. L. Gore & Associates, and authorship fees from Wolters Kluwer Healthcare - UpToDate; his institution has grants/grants pending from Attralus, Canon Medical Systems, Eidos Therapeutics, GE Healthcare, Pfizer, Roche Medical Systems, W. L. Gore & Associates, and XyloCor Therapeutics. None of the other authors reported any conflicts.
Sponsor’s Role:
The sponsor, Dr. Maurer at Columbia University Irving Medical Center, designed the study and recruited subjects at that site. Additionally, the sponsor collected data in collaboration with investigators at Tufts Medical Center and analyzed the data and prepared the manuscript.
This research was supported by a grant from the National Institute on Aging (NIA R21 AG058348). Dr. Maurer was supported by a K24 Grant from NIA (AG036778-11)
Abbreviations:
- ATTR
transthyretin amyloidosis
- LCM-MS
laser capture microdissection coupled to mass spectrometry
- TTR
transthyretin
- LSS
lumbar spine surgery
- LS
lumbar spine
- QOL
quality of life
- SF-36
Short Form – 36 items
- Tc99m-PYP
technetium-99m pyrophosphate
References:
- [1].Lurie J, Tomkins-Lane C. Management of lumbar spinal stenosis. BMJ. 2016;352: h6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Eldhagen P, Berg S, Lund LH, Sorensson P, Suhr OB, Westermark P. Transthyretin amyloid deposits in lumbar spinal stenosis and assessment of signs of systemic amyloidosis. J Intern Med. 2021;289: 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Westermark P, Westermark GT, Suhr OB, Berg S. Transthyretin-derived amyloidosis: probably a common cause of lumbar spinal stenosis. Ups J Med Sci. 2014;119: 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yanagisawa A, Ueda M, Sueyoshi T, et al. Amyloid deposits derived from transthyretin in the ligamentum flavum as related to lumbar spinal canal stenosis. Mod Pathol. 2015;28: 201–207. [DOI] [PubMed] [Google Scholar]
- [5].Godara A, Riesenburger RI, Zhang DX, et al. Association between spinal stenosis and wild-type ATTR amyloidosis. Amyloid. 2021;28: 226–233. [DOI] [PubMed] [Google Scholar]
- [6].Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin Amyloid Cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73: 2872–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nativi-Nicolau JN, Karam C, Khella S, Maurer MS. Screening for ATTR amyloidosis in the clinic: overlapping disorders, misdiagnosis, and multiorgan awareness. Heart Fail Rev. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Griffin JM, Rosenblum H, Maurer MS. Pathophysiology and Therapeutic Approaches to Cardiac Amyloidosis. Circ Res. 2021;128: 1554–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gonzalez-Lopez E, Gallego-Delgado M, Guzzo-Merello G, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36: 2585–2594. [DOI] [PubMed] [Google Scholar]
- [10].AbouEzzeddine OF, Davies DR, Scott CG, et al. Prevalence of Transthyretin Amyloid Cardiomyopathy in Heart Failure With Preserved Ejection Fraction. JAMA Cardiol. 2021;6: 1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Castano A, Narotsky DL, Hamid N, et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J. 2017;38: 2879–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N Engl J Med. 2018;379: 1007–1016. [DOI] [PubMed] [Google Scholar]
- [13].Rappley I, Monteiro C, Novais M, et al. Quantification of transthyretin kinetic stability in human plasma using subunit exchange. Biochemistry. 2014;53: 1993–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vrana JA, Gamez JD, Madden BJ, Theis JD, Bergen HR 3rd, Dogan A. Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood. 2009;114: 4957–4959. [DOI] [PubMed] [Google Scholar]
- [15].Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35: 1245–1255. [DOI] [PubMed] [Google Scholar]
- [16].Pratt RK, Fairbank JC, Virr A. The reliability of the Shuttle Walking Test, the Swiss Spinal Stenosis Questionnaire, the Oxford Spinal Stenosis Score, and the Oswestry Disability Index in the assessment of patients with lumbar spinal stenosis. Spine (Phila Pa 1976). 2002;27: 84–91. [DOI] [PubMed] [Google Scholar]
- [17].Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305: 160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332: 556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hanna M, Ruberg FL, Maurer MS, et al. Cardiac Scintigraphy With Technetium-99m-Labeled Bone-Seeking Tracers for Suspected Amyloidosis: JACC Review Topic of the Week. J Am Coll Cardiol. 2020;75: 2851–2862. [DOI] [PubMed] [Google Scholar]
- [20].Harats N, Worth R, Benson MD. Spinal claudication in systemic amyloidosis. J Rheumatol. 1989;16: 1003–1006. [PubMed] [Google Scholar]
- [21].Bergstrom J, Gustavsson A, Hellman U, et al. Amyloid deposits in transthyretin-derived amyloidosis: cleaved transthyretin is associated with distinct amyloid morphology. J Pathol. 2005;206: 224–232. [DOI] [PubMed] [Google Scholar]
- [22].George KM, Dowd RS, Nail J, et al. Wild-Type Transthyretin Amyloidosis Occurring in the Ligamentum Flavum of the Cervicothoracic Spine. World Neurosurg. 2020;142: e325–e330. [DOI] [PubMed] [Google Scholar]
- [23].George KM, Hernandez NS, Breton J, et al. Increased thickness of lumbar spine ligamentum flavum in wild-type transthyretin amyloidosis. J Clin Neurosci. 2021;84: 33–37. [DOI] [PubMed] [Google Scholar]
- [24].Maurer MS, Ruberg FL. Cardiac Scintigraphy and Screening for Transthyretin Cardiac Amyloidosis: Caveat Emptor. Circulation. 2021;144: 1005–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gurwitz JH, Maurer MS. Tafamidis-A Pricey Therapy for a Not-So-Rare Condition. JAMA Cardiol. 2020;5: 247–248. [DOI] [PubMed] [Google Scholar]
- [26].Sperry BW, Reyes BA, Ikram A, et al. Tenosynovial and Cardiac Amyloidosis in Patients Undergoing Carpal Tunnel Release. J Am Coll Cardiol. 2018;72: 2040–2050. [DOI] [PubMed] [Google Scholar]
- [27].Marcoux J, Mangione PP, Porcari R, et al. A novel mechano-enzymatic cleavage mechanism underlies transthyretin amyloidogenesis. EMBO Mol Med. 2015;7: 1337–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Slamova I, Adib R, Ellmerich S, et al. Plasmin activity promotes amyloid deposition in a transgenic model of human transthyretin amyloidosis. Nat Commun. 2021;12: 7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Berk JL, Suhr OB, Obici L, et al. Repurposing diflunisal for familial amyloid polyneuropathy: a randomized clinical trial. JAMA. 2013;310: 2658–2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sekijima Y, Dendle MA, Kelly JW. Orally administered diflunisal stabilizes transthyretin against dissociation required for amyloidogenesis. Amyloid. 2006;13: 236–249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: CONSORT Chart of Study participants. Flow chart of study population showing subjects who were consented and those that underwent clinically indicated LSS surgery with pathologic assessment for amyloidosis. Additional testing is noted in those who did and did not have amyloid detected.


