SUMMARY
Current influenza vaccines predominantly induce immunity to the hypervariable hemagglutinin (HA) head, requiring frequent vaccine reformulation. Conversely, the immunosubdominant yet conserved HA stem harbors a supersite which is targeted by broadly neutralizing antibodies (bnAbs), representing a prime target for universal vaccines. Here, we showed that co-immunization of two HA stem immunogens derived from group 1 and 2 influenza A viruses elicits cross-group protective immunity and neutralizing antibody responses in mice, ferrets, and nonhuman primates (NHPs). Immunized mice were protected from multiple group 1 and 2 viruses, and all animal models showed broad serum neutralizing activity. A bnAb isolated from an immunized NHP broadly neutralized and protected against diverse viruses, including H5N1 and H7N9. Genetic and structural analyses revealed strong homology between macaque and human bnAbs, illustrating common biophysical constraints for acquiring cross-group specificity. Vaccine-elicitation of stem-directed cross-group protective immunity represents a step towards the development of broadly protective influenza vaccines.
Graphical Abstract
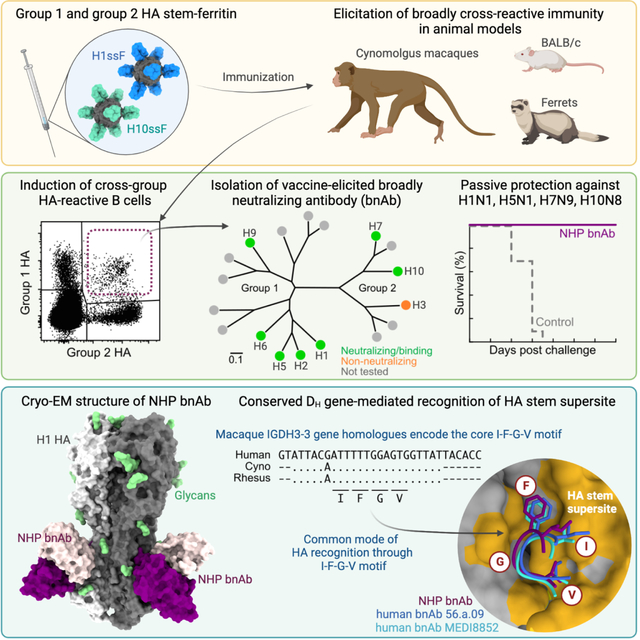
eTOC
Current vaccine-induced influenza immunity targets the hypervariable HA head, requiring frequent vaccine reformulation. Moin et al. show that co-immunization with HA stem immunogens of group 1 and group 2 influenza A viruses elicits broadly cross-protective antibodies in animals and leads to the discovery of a cross-group protective monoclonal antibody in a macaque, offering a blueprint for broadly protective influenza vaccines.
INTRODUCTION
Influenza virus causes a substantial global health and economic burden despite the availability of commercial vaccines. Moreover, it poses a unique biological challenge by evading host immune responses through its genetic plasticity leading to antigenic variation, making the virus a constantly moving target. Current influenza vaccines provide narrow-spectrum protection against antigenically closely matched strains, resulting in inconsistent 10–60% vaccine efficacy against symptomatic disease in humans(McLean and Belongia, 2021). This also necessitates annual updates of vaccine strains in order to match the predicted circulating viruses. Furthermore, these vaccines are not expected to confer meaningful protection against zoonotic virus strains from animal reservoirs that cause sporadic outbreaks in humans and have pandemic potential.
Current influenza vaccines target the most abundant influenza viral glycoprotein, hemagglutinin (HA). HA is a homotrimeric class I fusion protein which densely decorates virions along with another glycoprotein neuraminidase (NA). To date, 18 phylogenetically different HA subtypes and 11 NA subtypes have been identified in influenza A viruses. These 18 HA subtypes are categorized in 2 distinct groups (group 1 and 2). The HA glycoprotein is expressed as a precursor HA0 which is subsequently cleaved by cellular proteases into HA1 and HA2(Lazarowitz and Choppin, 1975). This cleavage is required for virus infectivity and HA1 and HA2 are covalently linked by disulfide bonds(Boonstra et al., 2018). HA has two functionally and structurally distinct regions, head and stem, that mediate host cell attachment and viral membrane fusion processes, respectively. The hypervariable head region contains antigenic sites that are highly neutralization sensitive and immunodominant, whereas the stem region is considerably more conserved within each group and recognized by broadly neutralizing antibodies (bnAbs) that are typically less potent than head-directed monoclonal antibodies (mAbs). These antibodies also utilize Fc-mediated effector functions such as antibody dependent cell-mediated cytotoxicity to broaden protection against diverse influenza strains(DiLillo et al., 2014, 2016; Wang et al., 2015). Multiple broadly neutralizing, stem-directed monoclonal antibodies have been isolated from humans that are capable of neutralizing different group 1 or group 2 subtypes or viruses from both groups(Andrews et al., 2017; Joyce et al., 2016). Hence, one major strategy for universal influenza vaccine efforts has been to focus on the HA stem domain to achieve broad protection against seasonal and pandemic influenza viruses without yearly reformulation. We and others have shown previously that rationally-designed immunogens based on the HA stem elicit broadly cross-reactive antibody responses and confer heterosubtypic protection against H5N1 infection in animal models(Impagliazzo et al., 2015; Lu et al., 2014; Steel et al., 2010; Wang et al., 2010; Yassine et al., 2015). However, cross-group protection against heterosubtypic group 2 viruses has not been reported for HA stem-based immunogens. Recently, parallel approaches have been pursued to generate group 2 HA stem immunogens based on H3 and H7(Corbett et al., 2019; Sutton et al., 2017). Although these stem immunogens elicited intra-group cross-reactive antibody responses and homotypic protective immunity in small animals, the induction of bnAb responses that resemble human bnAbs has not been demonstrated. Group 2 H3 and H7 HA stem displayed on ferritin nanoparticles were shown to activate B cells expressing B cell receptors of unmutated common ancestors (UCAs) for human cross-group bnAbs representing two multi-donor classes, suggesting that these immunogens have potential to activate such precursor cells in humans. Joyce et al.(Joyce et al., 2016) showed that H5 HA immunization in human clinical trials resulted in bnAbs against both group 1 and group 2 influenza viruses. Co-crystal structures with HA demonstrated recognition of overlapping epitopes on the HA stem by these human classes of bnAbs using germline genes and convergent sequence motifs. One such antibody class utilized VH6–1 and DH3–3 (VH6–1+DH3–3) genes, which resulted in recognition of the HA stem in a way that avoided the conserved group-specific HA1 glycans of the respective group 1 and group 2 subtypes. In each case, a somatically mutated CDR H3 residue was inserted into the conserved Trp21HA2 pocket in the hydrophobic groove of HA2. Small animal models such as mice and ferrets have been widely used for studying vaccine responses and antibody mediated protection. However, these animal models do not recapitulate the human immune repertoire due to the lack of sequence homology with human immunoglobulin genes. Non-human primates (NHPs) are evolutionarily close to humans and can generate immune responses similar to humans, which makes them a preferred model for vaccine studies especially when focused on specific antibody lineages. Moreover, NHPs have recently been shown to elicit bnAbs against influenza viruses(Darricarrère et al., 2021). Although NHPs lack VH genes possessing the hydrophobic CDR H2 tip as seen in the human immunoglobulin germline VH1–69, the VH gene most commonly used by group 1 HA stem-recognizing human bnAbs(Avnir et al., 2016), there are some NHP VH genes with human resemblance, including those similar to genes contributing to the VH6–1+DH3–3 class of human multi-donor bnAbs that can facilitate HA stem recognition in monkeys(Darricarrère et al., 2021).
Here, we extend our ability to design additional group 2 stabilized HA stem trimers displayed on ferritin nanoparticles using the HA sequence of an avian H10N8 virus (H10ssF) which causes sporadic infections in humans. We observed that H10ssF immunization elicited antibody responses broadly protective against challenge with multiple heterosubtypic group 2 viruses in mice. We also explored co-immunization with the HA stem of group 1 (H1ssF) and group 2 (H10ssF) as a method to elicit cross-group protective immunity in mice, ferrets, and NHPs. Further, we isolated and characterized vaccine-elicited monoclonal antibodies for their specificity and protective efficacy. We observed that a combined immunization of group 1 and group 2 HAssFs conferred not only contiguous group-specific neutralizing and protective antibody responses but also cross-group reactive bnAb responses that recognized the stem supersite(Cheung et al., 2022) in a manner reminiscent of the human multi-donor VH6–1+DH3–3 class bnAbs, providing a step towards developing broadly protective influenza vaccines.
RESULTS
Design and characterization of H10 HA stabilized stem nanoparticle (H10ssF)
We have previously developed a method to structurally stabilize group 2 HA stem such as H3 and H7 in native-like trimers and display on self-assembling ferritin nanoparticles (H3ssF and H7ssF, respectively) as potential immunogens for eliciting antibody responses to the highly conserved HA stem(Corbett et al., 2019). While H3ssF and H7ssF induced stem-directed protective immune responses to homosubtypic viruses in mice, neither of them had shown to elicit heterosubtypic protective immunity that spans across multiple group 2 subtype viruses. To overcome this hurdle of conferring heterosubtypic protective immunity, we sought to improve the group 2 stem immunogen by redesigning it with different HA subtype sequences. To identify a candidate group 2 HA that has a better cross-reactive potential, we determined the conservation of primary sequences as well as solvent-exposed residues among different group 2 HA stems (Figures S1A and S1B). We found that the H10 stem is uniquely positioned between H3 and H7 phylogenetically and possesses slightly higher sequence and solvent-exposed residue identities both to H3 and H7 than those between H3 and H7 (Figures S1C and S1D). We hypothesized that the H10-based group 2 stem immunogen might have a better antigenic surface to elicit broadly cross-reactive antibody responses. We employed the same design principle to stabilize the H10 HA stem and generate H10ssF using A/Jiangxi-Donghu/346/2013 (H10N8) as previously described for H3 and H7(Corbett et al., 2019). The self-assembled H10ssF was readily purified from the culture supernatant of transfected mammalian cells which gave a distinct peak around 1.2 MDa corresponding to a size of fully assembled particles on size exclusion chromatography (Figure S1E), with monomeric subunits corresponding to ~45 kDa as predicted (Figure S1F). The purified H10ssF showed homogeneous and well-formed particles when analyzed by negative stain electron microscopy (nsEM) (Figure S1G). A reference-free 2D class averaging of nsEM dataset resulted in particles with spherical cores of ~12 nm diameter and regularly spaced spikes of ~7–8 nm in height, consistent with the design and previously reported group 2 HAssFs(Corbett et al., 2019). Single-particle cryo-electron microscopy (cryo-EM) reconstruction of the full H10ssF particle resulted in a 4.8 Å resolution structure that revealed structural elements of the ferritin core and the spikes of HA-stem trimers (Figures 1A and S1H–S1K). Although the ferritin core could be further resolved through focused refinement, the density for the stem trimers became increasingly diffuse, suggesting flexibility relative to the core, likely facilitated by the short 3-amino acid (SGG) linker. Low-pass filtering of the density map to 8 Å was able to resolve the stem domain and its angular orientation, while also suggesting the magnitude of the stem domain flexibility. Dynamic light scattering (DLS) showed homogeneous particles with a radius of ~10 nm (Figure S2A). We measured the thermostability of H10ssF by differential scanning fluorometry (DSF) and found that H10ssF was equivalent or more thermally stable than other group 2 HAssFs or group 1 H1ssF(Corbett et al., 2019; Yassine et al., 2015) (Figure S2B).
Figure 1. H10-based group 2 influenza HA stem ferritin nanoparticles (H10ssF) elicits broad protective immunity against group 2 influenza A viruses.
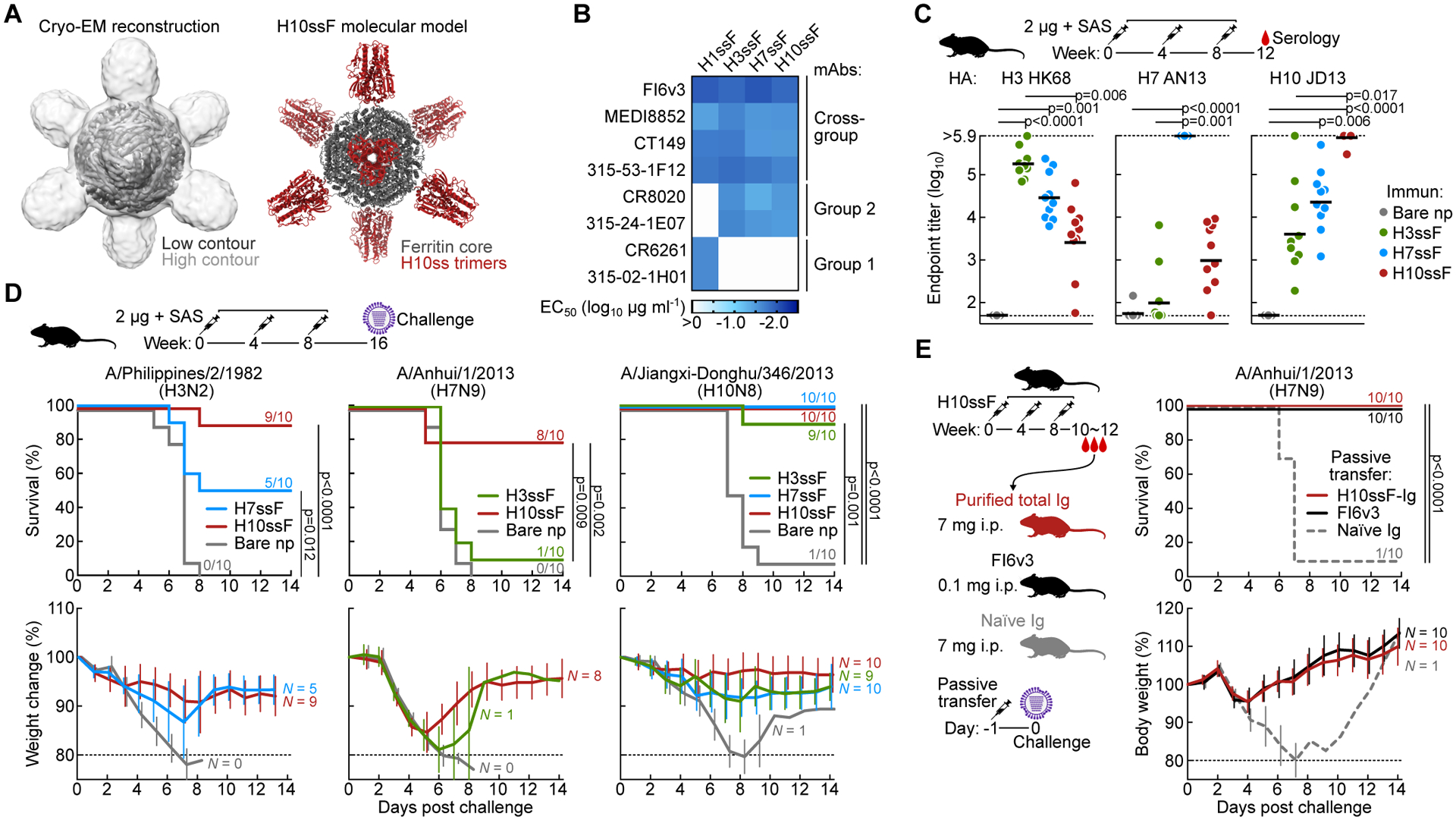
(A) Single-particle cryo-EM structure of H10ssF. 3D reconstruction density map of H10ssF (left). The outer contour in light gray was low-pass filtered to 8 Å to reveal the HA-stem trimers, while the inner contour in dark gray indicates the 4.8 Å map of the full complex, contoured to show the ferritin core. Molecular model of the designed H10ssF showing different structural domains (right). Ferritin core and H10ss spikes are colored in gray and dark red, respectively. Cryo-EM experiment was performed once.
(B) Antigenicity of HAssF determined by ELISA of HA stem-directed mAbs. mAbs FI6v3, MEDI8852, CT149, and 315–53-1F12 recognize both group 1 and group 2 HAs. mAbs CR8020 and 315–24-1E07 recognize group 2 HAs; whereas, mAbs CR6261 and 315–02-1H01 recognize group 1 HAs. Data shown are representative of three experiments.
(C) Immunogenicity of group 2 HAssF in BALB/c mice. Mice (N = 10) were immunized 3 times with 2 μg H3ssF, H7ssF, or H10ssF and sera was analyzed at week 12. ELISA antibody titers to A/Hong Kong/1/1968 HA (H3 HK68), A/Anhui/1/2013 HA (H7 AN13), and A/Jiangxi-Donghu/346/13 HA (H10 JD13) are shown. Each dot represents an individual animal and the horizontal bars indicate the geometric mean of each immunization group (N = 10). Horizontal dotted lines denote higher and lower limits of detection. The mouse experiments were independently performed at least three times, and representative data are shown. Statistical analysis was performed by Kruskal-Wallis test with Dunn’s multiple comparison post-hoc test.
(D) Protective efficacy of group 2 HAssF in mice. BALB/c mice immunized with H3ssF, H7ssF, or H10ssF (N = 10) were experimentally infected intranasally with either A/Philippines/2/1982 (H3N2), A/Anhui/1/2013 (H7N9), or A/Jiangxi-Donghu/346/2013 (H10N8) viruses 8 weeks post the last immunization. Data shown are representative of at least two independent experiments.
(E) Passive transfer experiment of purified hyperimmune Igs followed by experimental H7N9 infection. Igs (7 mg) of H10ssF-immunized or naïve mice were passively given to naïve BALB/c mice (N = 10) intraperitoneally 24 h prior to infecting with A/Anhui/1/2013 (H7N9). mAb FI6v3 (5 mg kg−1) was used as positive control. The passive transfer experiment was performed once. Multiple comparisons of Kaplan-Meier curves were performed by the log-rank test with Bonferroni correction (D and E).
See also Figures S1, S2, and S3.
We next evaluated the antigenic integrity of H10ssF by assessing binding of cross-group, stem-specific, conformation-dependent bnAbs FI6v3(Corti et al., 2011), MEDI8552(Kallewaard et al., 2016), CT149(Wu et al., 2015), 315-53-1F12(Andrews et al., 2017), group 2 stem-specific CR8020(Ekiert et al., 2011) and 315-24-1E07(Andrews et al., 2017), and group 1 stem-specific CR6261(Throsby et al., 2008)) and 315-02-1H01(Andrews et al., 2017) by ELISA. Analogous to H3ssF and H7ssF, H10ssF was recognized by all the stem-directed mAbs except for the group 1-specific mAbs, CR6261 and 315-02-1H01 (Figures 1B and S2C). We also measured the binding kinetics of the antigen-binding fragment of antibody (Fab) of MEDI8852, CT149 and CR8020 to H10ssF by biolayer interferometry (BLI) and found that H10ssF bound all three Fabs with high affinities (KD) of 0.4–4 nM, that were equivalent or of higher affinity than the corresponding H10 HA trimer or other group 2 HAssFs (Figure S2D)(Corbett et al., 2019). To evaluate the ability of H10ssF to activate B cells expressing B-cell receptors (BCRs) of the inferred UCA of human bnAbs, we measured Ca2+ flux by flow cytometry using recombinant Ramos B cells as described previously(Villar et al., 2016; Weaver et al., 2016). We found that H10ssF but not H1ssF activated the recombinant Ramos B cells expressing BCR of the 16.a.26 UCA which engages group 2 HA stem (multi-donor VH1–18 Q-x-x-V class)(Corbett et al., 2019; Joyce et al., 2016) (Figure S2E). In contrast, B cells expressing a group 1-specific BCR were activated by H1ssF but not by H10ssF (Figure S2F). These results demonstrate that H10ssF displays conformationally and antigenically intact trimeric headless HA stem spikes that are capable of engaging and activating B cells expressing BCR of human bnAb precursors.
Group 2 H10ssF elicits broad heterosubtypic protective immunity in mice
To assess the immunogenicity of H10ssF and compare it with other group 2 HAssF, we immunized mice with H3ssF, H7ssF, or H10ssF in the presence of adjuvant (Sigma Adjuvant System (SAS)). All three immunogens were immunogenic and elicited antibody responses that were cross-reactive to more than one group 2 HA subtype although the highest antibody titers were observed against HAs matched to the immunogen subtype (Figure 1C). When we measured virus neutralizing activity using the HA/NA pseudotyped lentiviral reporter assay(Kong et al., 2006), as expected, we observed serum neutralizing activity against the pseudovirus with matched HA/NA subtype (Figure S3A). There was low but detectable neutralizing activity against heterosubtypic pseudoviruses in H7ssF- and H10ssF-immunized mice (Figure S3A), although non-neutralizing antibodies can also mediate protection in mice from lethal influenza virus infections(Henry Dunand et al., 2016). To evaluate protective efficacy of the group 2 HAssF, immunized mice were challenged with a lethal dose of A/Philippines/2/1982 (H3N2), A/Anhui/1/2013 (H7N9) or A/Jiangxi-Donghu/346/2013 (H10N8) influenza viruses. Nearly all of the control mice (97% or 29 of 30 mice) receiving bare ferritin nanoparticles succumbed to infection and were euthanized; in contrast, mice immunized with H10ssF exhibited substantial protection against all 3 viruses tested (93% or 27 of 30 mice survived overall) (Figure 1D). While both H3ssF and H7ssF were previously demonstrated to confer protection against homologous subtype viruses(Corbett et al., 2019), they also provided a near-complete (90–100%) protection to heterosubtypic H10N8 virus (Figure 1D). In contrast, H3ssF and H7ssF conferred only minimal to partial protection against heterosubtypic H7N9 and H3N2 viruses, respectively (10% and 50%, respectively) (Figure 1D). These results illustrate that H10ssF induces a broader protective immunity against group 2 subtype viruses than either H3ssF or H7ssF in mice. To better understand the basis for this stem-directed protective immunity to group 2 viruses, we tested the specificity of both binding and neutralizing antibodies by using an HA variant which possesses an added N-linked glycan at the epicenter of the canonical stem supersite (Δstem HA) so that it blocks access of vast majority of stem-directed bnAbs(Wei et al., 2010). Binding antibody titers of sera from H10ssF immunized mice to Δstem H10 HA were significantly lower than to the wild-type H10 HA (p = 0.0028). Antibody titers to heterosubtypic H7 HA were almost completely abrogated when the Δstem mutation was introduced (Figure S3B), suggesting that H10ssF immunization elicited antibodies that target the canonical stem supersite surrounding the Trp21HA2 hydrophobic groove(Wu and Wilson, 2020) and that those antibodies were responsible for heterosubtypic cross-reactivity. Similarly, sera from H7ssF-immunized mice showed reduced binding to both homologous and heterologous HAs in the presence of the Δstem mutation (Figure S3B). Importantly, the serum neutralizing activity of H10ssF-immunized mice was not substantially diminished by the excess Δstem HA protein unlike the wild-type HA which fully depleted the activity (Figure S3C). In addition, serum immunoglobulins (Igs) purified from H10ssF-immunized mice (H10ssF-Igs) reacted with H10 and H7 HA, and the binding was substantially lower to Δstem H10 HA and nearly abolished to Δstem H7 HA as seen in immune sera (Figure S3D). The H10ssF-Igs neutralized homologous H10N8 or heterosubtypic H3N2 and H7N9 pseudoviruses (Figure S3E). To confirm that the heterosubtypic group 2 protection is antibody-mediated, H10ssF-Igs were passively administered to naïve mice prior to infecting them with a lethal dose of H7N9 virus. Mice receiving the H10ssF-Igs were fully protected from the virus with no measurable weight loss (Figure 1E), while all mice receiving Igs purified from naïve mice succumbed to infection. This result shows that the heterosubtypic group 2 protection can be achieved by stem-directed antibodies elicited by H10ssF.
Co-immunization of group 1 and group 2 HAssF confers cross-group heterosubtypic immunity
To achieve the goal of eliciting protective immune responses to influenza A viruses carrying either group 1 and group 2 subtype HAs, we evaluated a cocktail vaccine approach consisting of H1ssF and H10ssF. Groups of mice, ferrets and cynomolgus macaques were immunized with a cocktail of H1ssF and H10ssF (termed herein H1+10ssF) with a squalene oil-in-water emulsion adjuvant (AddaVax®). Immunization of H1+10ssF elicited HA-binding and neutralizing antibody responses to several group 1 and group 2 subtype viruses in mice (Figures 2A and 2B), ferrets (Figure 2C) and nonhuman primates (NHPs) (Figures 2D and 2E). The serum antibody titer to group 1 viruses were comparable to that induced by H1ssF alone (Figures 2A–2C), and likewise, the responses to group 2 viruses were comparable to that induced by H10ssF alone (Figures 2A–2C). These findings confirm that the co-immunization of group 1 and group 2 HAssF does not cause antigenic competition between the two HAssF immunogens. Importantly, co-immunization also conferred substantial protection in immunized mice from heterosubtypic H5N1 and H3N2 lethal virus challenges (Figures S3F and S3G). Although heterosubtypic virus neutralizing antibody responses in mice and ferrets could be measured by pseudotyped lentiviral neutralization assays, serum neutralizing activity in immunized NHPs were also readily detectable in influenza reporter virus-based microneutralization assays (Figure 2D)(Creanga et al., 2021). Mouse and ferret immune sera neutralized all 5 pseudotyped viruses that represent group 1 and group 2 human influenza viruses (Figures 2B and 2C). While NHP immune sera did not show detectable neutralization against the H3N2 virus by microneutralization assay (Figure 2D), they showed neutralizing activity against H3N2 by pseudovirus neutralization assays (Figure 2E). These results suggest that immunizations with H1+10ssF can elicit broadly neutralizing serum antibody responses that are capable of neutralizing multiple group 1 and group 2 subtype viruses although the breadth may be more limited in NHPs.
Figure 2. Co-immunization of group 1 and group 2 HAssF elicits broadly cross-reactive and neutralizing antibody responses in mice, ferrets, and NHPs.
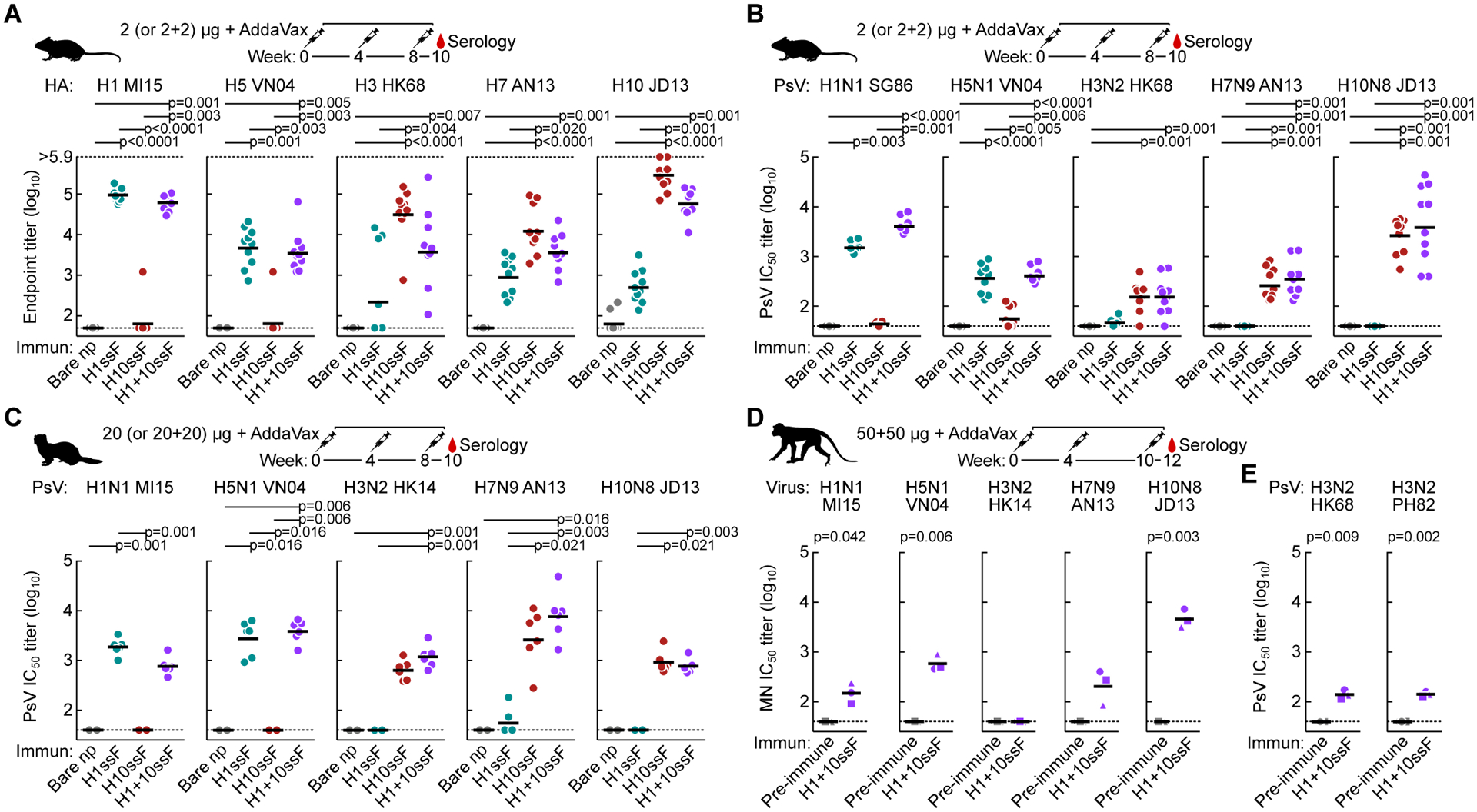
(A) Cross-reactive HA-binding antibody responses to multiple group 1 and group 2 HAs. Sera (N = 10) were collected after 3 immunizations with H1ssF, H10ssF, H1+10ssF, or bare nanoparticles. ELISA antibody responses were assessed for A/Michigan/45/2015 (H1 MI15), A/Vietnam/1203/2004 (H5 VN04), H3 HK68, H7 AN13, and H10 JD13 HA.Mouse experiments were performed at least twice, and representative data are shown. All assays were repeated twice, and representative data are shown.
(B and C) Neutralizing antibody responses to pseudotyped lentiviruses expressing multiple group 1 and group 2 HAs in mice (B) and ferrets (N = 6) (C). Pseudotype neutralization assays were performed by using HA and NA from A/Singapore/1/1986 (H1N1 SG86), H1N1 MI15, H5N1 VN04, H3N2 HK68, H7N9 AN13, and H10N8 JD13. Mouse experiments were performed at least twice, and representative data are shown. Ferret experiment was performed once. All assays were repeated twice, and representative data are shown.
(D and E) Neutralizing antibody responses in NHPs. Cynomolgus macaques (N = 3) were immunized with H1+10ssF. Serum neutralizing activity as measured by the reporter-based microneutralization assay (D)(Creanga et al., 2021) or Pseudotype neutralization assay (E). H1N1 MI15, H5N1 VN04, A/Hong Kong/4801/2014 (H3N2 HK14), H7N9 AN13, and H10N8 JD13 reporter influenza viruses were used for microneutralization assay. Pseudotype viruses were made using HA and NA from H3N2 HK68 and A/Philippines/2/1982 (H3N2 PH82) (E). Different symbols are used to identify individual animals. Black horizontal lines indicate geometric mean titers for each respective group.
Horizontal dotted lines denote higher and lower limits of detection (A) or lower limit of detection (B to D). Statistical analysis was performed by Kruskal-Wallis test with Dunn’s multiple comparison post-hoc test (A to C) or by paired t-test (D). NHP experiment was performed once. All assays were repeated twice and representative data are shown.
See also Figure S3.
Co-immunization induces heterosubtypic and cross-group HA-specific B cells in nonhuman primates
To better understand the basis for cross-reactive antibody responses observed in NHPs immunized with H1+10ssF, we assessed the B cell responses to multiple HAs in peripheral blood mononuclear cells (PBMCs) by flow cytometry. Two weeks after the second immunization (week 6), B cells binding to homologous H1 MI15 HA and H10 JD13 HA were readily detectable (Figures 3A and S4). Frequencies of IgG+ B cells recognizing homologous H1 and H10 HA were substantially increased after the third immunization (Figure 3B). At week 12, a large fraction (~50%) of the H1+ IgG+ B cells showed cross-reactivity to H5 VN04 HA and likewise, approximately half of the H10+ B cells bound H7 AN13 HA; whereas, cross-reactivity between H10 and H3 HK14 HAs was markedly lower than between H10 and H7 (Figure 3C). B cells that recognize both group 1 (H1) and group 2 (H10) HAs were rare yet detectable (Figure 3A). We next assessed the kinetics for the development of cross-reactive B cell responses in the animal (M08145, circle) which had the highest cross-group HA-reactive B cells. The frequency of B cells recognizing homologous HAs (i.e., H1 and H10) were low but detectable as early as one week after the first H1+10ssF immunization (week 1) (Figure 3D). Frequencies of both homologous H1+ and H10+ B cells were substantially increased after the second and the third immunizations (week 5 and week 11, respectively). A disproportional increase of H5+ B cells compared to H1+ only B cells was observed after the second and the third immunization, indicating that selective induction of cross-reactive B cells occurred after each H1+10ssF booster (Figure 3D). Notably, B cells cross-reacting with both H1 and H10 (cross-group) emerged after the second immunization, while the relative frequency of these cells were substantially enriched after the third immunization (Figure 3E). These results demonstrate that a cocktail of H1ssF and H10ssF is capable of inducing a diverse repertoire of cross-reactive HA-specific B cells including some that cross-react with both group 1 and group 2 HAs.
Figure 3. Co-immunization with group 1 and group 2 HAssF induces cross-reactive HA-specific B cells in NHPs.
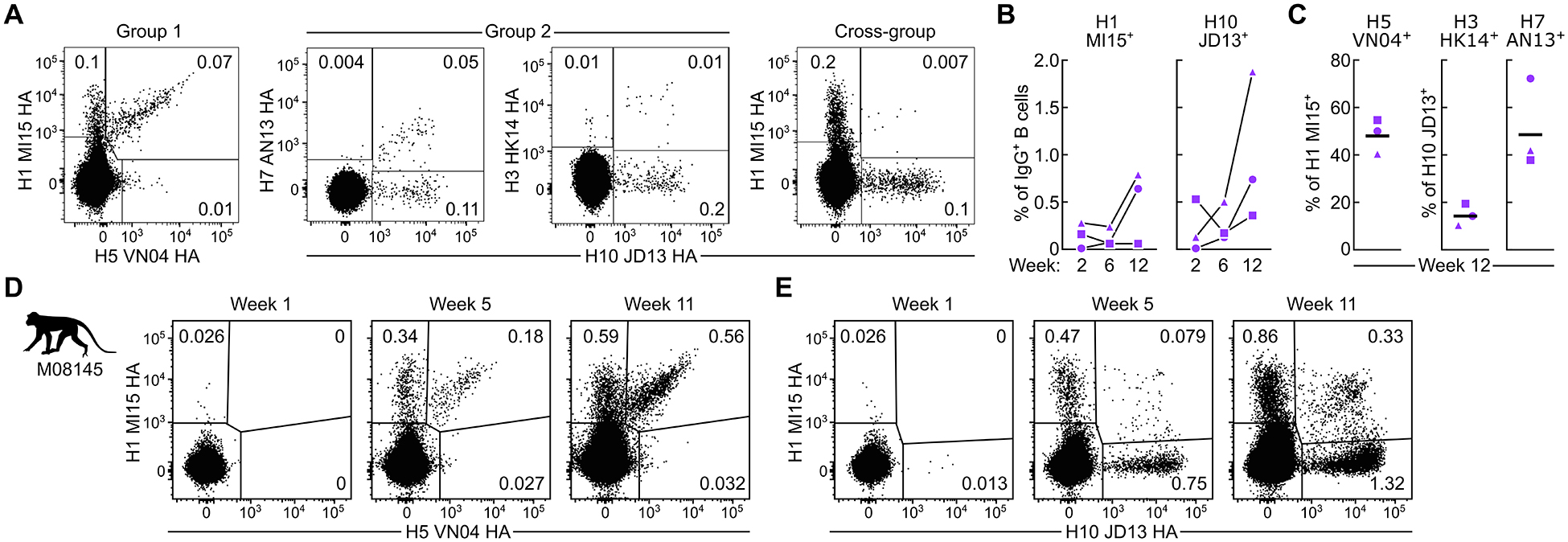
(A) Detection of HA-specific B cells by flow cytometry. PBMCs from NHPs (N = 3) at two weeks after the second immunization were analyzed by using fluorescently-labeled HA trimer probes.
(B) Frequencies of homologous H1+ and H10+ B cells in PBMCs. Percentage of H1+ or H10+ among IgG+ B cells are shown.
(C) Frequencies of cross-reactive B cells. Percentage of H5 positivity among H1+ B cells (left), and percentages of H7+ or H3+ among H10+ B cells (right) are shown. Horizontal lines indicate the geometric mean of a group.
(D,E) Kinetics of cross-reactive B cell responses in macaque M08145. PBMCs taken at one week after each immunization were analyzed by flow cytometry with H1 MI15 and H5 VN04 (D), or H1 MI15 and H10 JD13 (E) HA trimer probes. Flow cytometry experiments were performed twice, and representative data are shown.
See also Figure S4.
We next investigated the functionality of cross-group HA-specific B cells by performing B cell sorting and sequencing. We single-cell sorted H1+H10+ B cells from PBMCs (week 12) of the animal M08145 and sequenced their immunoglobulin (Ig) genes. Recombinant antibodies were produced by co-expressing Ig heavy and light chain genes encoding Ig variable domains recovered from the single-cell sorted B cells.
Among the mAbs derived from cross-group HA-specific B cells, we found an antibody clone 789-203-3C12 that bound multiple subtype HAs and neutralized a wide range of influenza viruses in the reporter-based microneutralization assays (Figures 4A and 4B). Binding affinities of the 789-203-3C12 Fab to various subtypes of HA varied ranging from 6.6 to 534 nM (Figure 4A). Although the mAb 789-203-3C12 did not show detectable binding to H3 HA nor did it neutralize H3N2 viruses, it bound and neutralized multiple group 1 and group 2 HA subtype viruses (Figure 4B). 789-203-3C12 was highly protective against both group 1 (A/California/04/2009 (H1N1) and H5N1 VN04) and group 2 (H7N9 AN13 and H10N8 JD13) virus infections when the antibody was given prophylactically to naïve mice (Figure 4C). These results establish that the co-immunization of H1+10ssF can induce robust B cell responses that are cross-reactive to heterosubtypic HAs not only within either group 1 or group 2, but also across the two HA groups. In addition, the mAb 789-203-3C12 isolated from H1+10ssF-immunized NHP showed cross-group neutralizing activity as well as protective efficacy to multiple group 1 and group 2 subtype influenza viruses.
Figure 4. The NHP mAb 789–203-3C12 shows broad neutralization breadth, potency, and protective efficacy against group 1 and group 2 influenza A viruses.
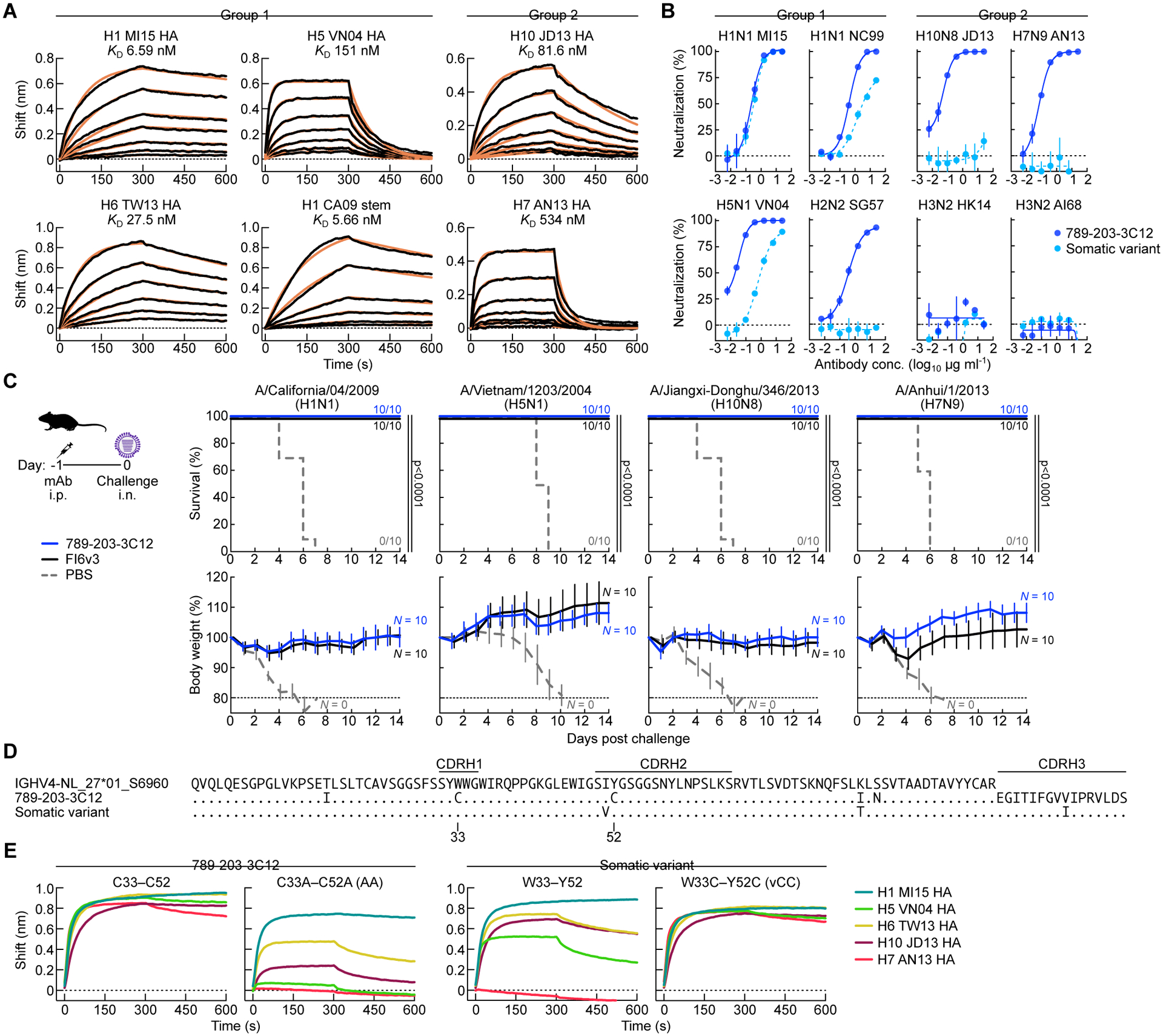
(A) HA-binding kinetics of Fab 789–203-3C12 determined by BLI. Recombinant HA of multiple group 1 and group 2 subtypes or the stabilized HA stem were immobilized on biosensors. Experimental datasets were fitted with the binding equations describing a 1:1 interaction to obtain apparent affinities. Experiments were performed twice, and representative data are shown.
(B) Neutralization profile of mAb 789–203-3C12 and its somatic variant antibody. Neutralization curves were generated by the reporter-based microneutralization assay(Creanga et al., 2021). Experiments were performed twice, and representative data are shown.
(C) Protective efficacy of mAb 789–203-3C12 in mice. 789–203-3C12 or control FI6v3 antibodies were passively administered (10 mg kg−1) to recipient BALB/c mice (N = 10) intraperitoneally 24 h prior to infecting with multiple group 1 and group 2 subtype viruses. Multiple comparisons of Kaplan-Meier curves were performed by the log-rank test with Bonferroni correction. Mouse experiment was performed once. (D) Sequence alignment of the germline-encoded IGHV4-NL_27*01_S6960 heavy chain, 789–203-3C12, and its somatic variant. Dots denote identical residues to the IGHV4-NL_27*01_S6960 whereas the residues that are different from the IGHV4-NL_27*01_S6960 are indicated. CDR loop regions are shown by horizontal lines (Kabat).
(E) Binding profile of mAb 789–203-3C12 and the somatic variant antibody with their CDR H1 and CDR H2 variants. HA-binding was measured using BLI. Recombinant HAs were immobilized on biosensors. All the mAbs used in the assay were tested as IgG. Experiments were performed twice, and representative data are shown.
Vaccine-elicited cross-group bnAb 789-203-3C12 acquires Cys substitutions during somatic hypermutation critical for breadth
To study the molecular basis for broad cross-group neutralization, we characterized immunogenetic composition of 789-203-3C12. The VH region of 789-203-3C12 is highly similar to the M. fascicularis IGHV4-NL_27*01_S6960 gene (97.3%), suggesting it acquired a pair of Cys substitutions within CDR H1 and H2 regions (Tyr33Cys and Tyr52Cys, respectively) alongside a few other substitutions in its framework regions during somatic hypermutation (SHM) (Figure 4D). Among the sequences of single-cell sorted B cells, we found a somatic variant heavy chain sequence clonally related to 789-203-3C12 which did not possess the double Cys substitutions in its CDR H1 and H2 loops (Figure 4D). When the recombinant antibody was produced using the somatic variant heavy chain paired with 789-203-3C12 light chain, the resultant mAb had comparable neutralizing activity against H1N1 MI15 virus (Figure 4B); however, this somatic variant mAb had a much reduced neutralizing activity against heterologous pre-pandemic H1N1 (A/New Caledonia/20/1999) and heterosubtypic H5N1 VN04 viruses. Moreover, its neutralizing activity against the group 1 H2N2 or the group 2 subtype viruses was completely abolished (Figure 4B). To test whether the Cys substitutions were critical for its binding and neutralization breadth and potency, we generated a variant 789-203-3C12 heavy chain by substituting the Cys residues to the smaller side-chain Ala (Cys33Ala/Cys52Ala, AA). The AA variant mAb produced by pairing with the 789-203-3C12 light chain bound H1 HA in a manner analogous to the parental 789-203-3C12 (Cys33/Cys52, CC), but showed reduced or no binding to H5, H6, H7 and H10 HAs (Figure 4E). Introducing the double Cys substitutions in the CDR H1 and H2 of the somatic variant heavy chain (Trp33Cys/Tyr52Cys, vCC) was sufficient to convert the binding profile of the resultant mAb (vCC) from that of the somatic variant to resemble 789-203-3C12 (Figure 4E). These results suggest that the acquisition of double Cys in the CDR H1 and H2 loops during SHM was critical to the optimal cross-group breadth and neutralization potency of the mAb 789-203-3C12.
Structure of the cross-group bnAb 789-203-3C12 in complex with HA reveals common mode of HA recognition between NHP and human bnAbs
To understand the structural basis for HA recognition by 789-203-3C12, we determined the structure of 789–203-3C12 Fab in complex with recombinant stabilized HA trimer(Lee et al., 2015) of H1N1 virus (A/Solomon Islands/3/2006, SI06) to 3.85 Å resolution by single-particle cryo-EM (Figures 5A and S5). While 60,632 particles showed C3 symmetry, the dataset for the HA–Fab complex structure was collected based on the 116,041 particles which showed C1 symmetry (Table S1). 789–203-3C12 bound to the canonical HA stem supersite(Cheung et al., 2022), with HA1 and HA2 subunits providing 15% (143 Å2) and 85% (845 Å2), respectively, of a total buried surface area (BSA) of 988 Å2 (Table S2). The heavy chain dominated the binding interface and contributed 75% of the 1,066 Å2 paratope surface (Table S2). In the CDR H3, Arg100f formed a salt bridge with the HA residue Asp19HA2, and Val100c interacted with the Val18HA2 through hydrophobic contacts (Figure 5B and Table S3). Phe100 at the tip of CDR H3 bound in a hydrophobic groove formed by Trp21HA2, Ile45HA2, and Ile48HA2 (Figure 5B). Overall, CDR H3 contributed ~51% of the total paratope surface. As expected, Cys33 and Cys52 formed an intra-chain disulfide bond, with well-resolved electron density (Figure S5E), linking CDR H1 and CDR H2. This CDR H1-H2 disulfide bond provided perfect shape complementarity to accommodate the loop consisting of HA2 residues 16–21, and helped orient Val18HA2 and Asp19HA2 to interact with CDR H3 (Figure 5B). In addition, CDR H2 Gly55 and Lys64 formed hydrogen bonds and salt bridges with Tyr34HA2 and Asp146HA2, respectively (Figure 5B and Table S3). The light chain Arg50, Ser91, and Thr92 formed hydrogen bonds with Gln42HA2, Asn46HA2, and Thr49HA2 (Figure 5B and Table S3).
Figure 5. Structural characterization of NHP mAb 789–203-3C12 identifies conserved DH motifs between human and NHP bnAbs for HA recognition.
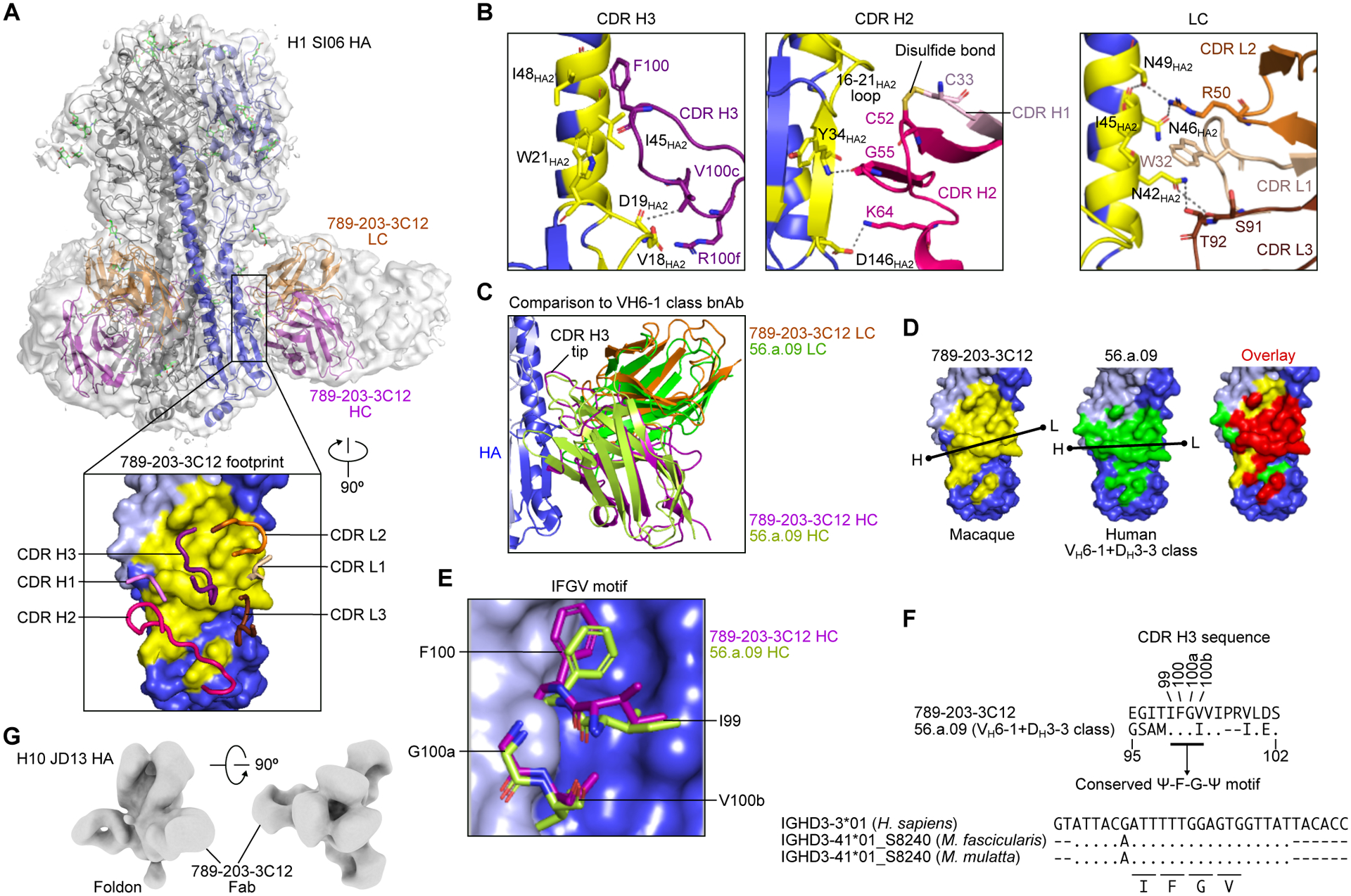
(A) Overall structure of the 789–203-3C12 Fab bound to the H1 SI06 HA trimer. The HA trimer is shown in gray, with the protomer with bound antibody colored blue. HA1, HA2, and antibody heavy and light chains are depicted as ribbons colored light blue, blue, purple, and orange, respectively. A semitransparent surface representation of the entire complex is also shown in gray. A 90° rotated view of the interface with interacting CDR loops shown as worms and HA as surface representation (inset). The epitope of 789–203-3C12 is colored in yellow. Cryo-EM experiment was performed once.
(B) Detailed interactions of 789–203-3C12 CDR loops with HA. The formation of a disulfide bond between CDR H1 and H2 loops are shown in yellow (middle panel). Hydrogen bonds and salt bridges are indicated as dashed lines.
(C) Comparison of the binding mode of 789–203-3C12 antibody with the human VH6–1+DH3–3 class bnAb human 56.a.09 on HA.
(D) The footprints for 789–203-3C12 (yellow) and 56.a.09 (green) are mapped onto the HA surface. A black line indicates the orientation of the antibody defined by the line connecting Cα atoms of heavy chain Cys22 (dot with letter H) and light chain Cys23 (dot with letter L). The overlapping epitope was colored in red (right).
(E) Structural arrangement of the “Ψ-F-G-Ψ” motif in the 789–203-3C12 and 56.a.09. Ψ is I, V, L, or M.
(F) Sequence comparison of the CDR H3 of 789–203-3C12 and 56.a.09 (top) and sequence alignment of human IGHD3–3*01 and IGHD3–41*01_S8240 genes from macaque species (bottom). Amino acid positions shown are based on the Kabat numbering. Dots denote identical nucleotides to the human IGHD3–3*01 whereas the nucleotides that are different from the human IGHD3–3*01 are indicated. Codons encoding the IFGV motif are underlined.
(G) Negative stain EM 3D reconstruction of 789–203-3C12 Fab bound to the H10 JD13 HA trimer. EM experiment was performed once.
We noted that the macaque-derived 789–203-3C12 recognized the HA stem in a manner similar to the convergent VH6–1+DH3–3 class of human bnAbs(Joyce et al., 2016). One such human VH6–1+DH3–3 class antibody, 56.a.09 (PDB 5K9K), shared a very similar approach to the HA stem, where the heavy and light chains were superimposable with nearly identical orientations (Figure 5C). The epitopes for 789–203-3C12 and 56.a.09 showed a high degree of overlap, sharing 21 residues (Figure 5D). Although the structures of CDR H1, H2 and CDR L1, L2, and L3 are substantially different between 789–203-3C12 and 56.a.09, the CDR H3 of both antibodies contained the conserved Ile-Phe-Gly-Val/Ile (Ψ-F-G-Ψ) motif, enabling both antibodies to interact with HA in a similar fashion (Figures 5E and 5F). Additionally, nsEM analyses of the 789–203-3C12 Fab in complex with H10 HA (A/Jiangxi-Donghu/346/2013) confirmed that the Fab bound the group 2 HA stem in a manner analogous to human VH6–1+DH3–3 class bnAbs (Figure 5G). Structures of group 2 HA–Fab complex (MEDI8852–H7 HA (PDB 5JW3) and 56.a.09–H3 HA (PDB 5K9K) fit snugly into the reconstructed nsEM density of 789–203-3C12–H10 complex (Figures S5G and S5H), further highlighting the similarity between macaque and human cross-group bnAbs. While the conserved Ile-Phe-Gly-Val/Ile motif in the VH6–1+DH3–3 class bnAbs is entirely encoded by the human IGHD3–3 gene, this same motif was completely conserved in the CDR H3 of 789–203-3C12 (Figure 5F). Indeed, the DH gene usage of 789–203-3C12 is the M. fascicularis IGHD3–41*01_S8240 allele, which is highly homologous to the human IGHD3–3 gene, and importantly, it encodes the core “Ile-Phe-Gly-Val” motif (Figure 5F). Moreover, the “Ile-Phe-Gly-Val” motif-encoding IGHD3–41*01_S8240 is also present in the M. mulatta genome, suggesting that the “Ile-Phe-Gly-Val” motif is conserved in the macaque species and is utilized by influenza HA stem-directed bnAbs, as in humans.
DISCUSSION
The ongoing global SARS-CoV-2 pandemic underscores the difficult public health challenges encountered when fighting against highly contagious respiratory pathogens. Vaccines remain the most effective countermeasure to reduce disease severity and slow down transmission as demonstrated by the highly efficacious vaccines against SARS-CoV-2. Though parenterally administered vaccines will have limited effect in preventing infection, they should reduce virus load and the length of virus shedding thereby reducing transmission. Even partially effective vaccines deployed early or prior to the pandemic could have a sizable positive impact on the epidemic curve(Kanekiyo and Graham, 2021). This is particularly relevant to influenza as it is not possible to precisely predict the next pandemic virus with the available technologies and research tools. Therefore, the development of vaccines for influenza pandemic preparedness that are effective against future pandemic viruses and their deployment immediately after a new virus emerges would be immensely valuable for maintaining global public health. To this end, a vaccine that induces broad immunity against group 1 and 2 influenza A viruses, could be stockpiled and made available for deployment during the early stages of a pandemic before strain-matched vaccines can be developed and tested. Our study demonstrated the feasibility of such a vaccine approach using a cocktail of the group 1 and group 2 headless HA stem-nanoparticles(Corbett et al., 2019; Yassine et al., 2015) and showed that it could elicit broadly neutralizing and protective immune responses against a wide range of influenza A viruses in three different animal models. Markedly, this cocktail vaccine approach induced not only within-group (group 1 or group 2) cross-reactive antibodies but also the cross-group bnAbs in NHPs. This study reports the vaccine-elicitation and isolation of cross-group bnAbs resembling a public human bnAb clonotype in NHPs, providing a key step to the development of vaccines that elicit bnAbs in humans. To date, such cross-group bnAbs induced by infections and/or vaccinations in humans are rarely found(Andrews et al., 2017; Corti et al., 2011; Dreyfus et al., 2012; Joyce et al., 2016; Kallewaard et al., 2016; Lang et al., 2017).
For more than a century, small animal models have been indispensable to gain insights on fundamental biological processes and study immunological mechanisms. These animal models have been widely used in preclinical studies for evaluating vaccine immunogenicity; however, results obtained from these models do not always recapitulate complex immune responses in humans. For example, in humans, the predominant bnAb responses to group 1 HA stem are largely constrained by the usage of VH1–69 genes, which do not exist in nonhuman animal species(Lingwood et al., 2012; Sui et al., 2009). To better address this issue, transgenic animals that carry human immune receptor genes have been developed and utilized to reproduce human-like immune response(Chen and Murawsky, 2018; Sangesland et al., 2019; Tian et al., 2016; Zhu et al., 2019). Despite the advances in the development of a variety of humanized transgenic and transchromosomic mice over the last few decades, it is still exceedingly difficult to model the complexity of human immune responses. NHPs and in particular macaques are the preferred animal species for vaccine evaluation due to their evolutionary proximity to humans. While NHPs do not possess the human VH1–69 gene which is by far the most prevalent gene utilized for group 1 HA stem-directed bnAbs, they use a different set of VH genes (i.e., IGHV3S5 and IGHV3S25) to target the same stem epitope(Darricarrère et al., 2021). Analogous to humans, these group 1 HA stem-directed antibodies were highly dominant and genetically constrained public clonotypes in NHPs. In addition to the group 1 HA stem-directed antibodies, NHPs have been shown to generate group 2 HA stem-directed antibodies following H3 stem-nanoparticle immunizations(Darricarrère et al., 2021). Noteworthily, these NHP antibodies were shown to target the membrane proximal epitope similar to the human group 2 HA stem-directed antibodies CR8020 and CR8043(Ekiert et al., 2011; Friesen et al., 2014). Despite the fact that NHPs were capable of generating human-like bnAb responses to either group 1 or group 2 HA stem following infection or vaccination(Clemens et al., 2020, 2021; Darricarrère et al., 2021; Jegaskanda et al., 2019), there are no NHP bnAbs that broadly neutralize both group 1 and group 2 influenza viruses reported to date. In the present study, we identified the NHP bnAb 789–203-3C12 as an example of such a cross-group bnAb.
Although the bnAb 789–203-3C12 was isolated from a cynomolgus macaque, the antibody was very similar to the human VH6–1+DH3–3 class of bnAbs(Joyce et al., 2016; Kallewaard et al., 2016). The canonical CDR H3 motif of this class of bnAbs is entirely encoded by the DH3–3 gene in humans. We found a homolog of the human IGHD3–3 gene in both rhesus and cynomolgus macaques (i.e., a specific allele of IGHD3–41, IGHD3–41*01_S8240) that was nearly identical to the human counterpart. While the IGHD3–41 gene may have been conserved in macaques for unrelated reasons, it is astounding to observe the convergent solution to recognize the influenza HA stem between humans and macaques by utilizing this gene out of many DH genes with a particular reading frame. It is unclear at the moment whether or not the VH gene (IGHV4-NL_27) poses an immunogenetic bottleneck in macaques like the human IGHV6–1 gene does for the VH6–1+DH3–3 public clonotype. We also have limited understanding on why bnAb 789–203-3C12 does not neutralize H3N2 viruses and if this limitation is common amongst NHP cross-group bnAbs. Further antibody discovery efforts would be needed to address these questions and leverage the NHP model for studying the germline gene-endowed immunological processes in response to the HA stem.
Although we and others have shown that the HA stem-directed broadly cross-reactive antibody responses can be elicited in multiple animal models through active immunization using designed immunogens(Boyoglu-Barnum et al., 2020, 2021; Corbett et al., 2019; Krammer et al., 2013; Sutton et al., 2017; Yassine et al., 2015), the potential impacts of preexisting influenza immunity on vaccine-elicitation of the stem-directed antibody responses have not been rigorously studied. A recent natural history study found a strong association of early life influenza exposures on protective immunity against heterosubtypic influenza virus infections in humans(Gostic et al., 2016). The observed protective immunity appeared to be HA group-specific and determined by the subtype of influenza virus that the individual was first exposed to in their early childhood (H1N1, H2N2, or H3N2). This “immunological imprint” introduces birth year-dependent bias in immune repertoire and may influence group preference of the vaccine-induced immunity, posing another layer of difficulty for achieving universal influenza immunity in humans(Viboud et al., 2020). Whether a designed vaccine can override and reprogram the imprinted group-biased immunity in humans remains to be determined, although there are vaccine candidates in active clinical development that show some promise in phase 1 human clinical trials by eliciting the HA stem-directed antibody responses in adults with preexisting influenza immunity(Bernstein et al., 2020; Houser et al., 2022; Nachbagauer et al., 2021). Establishing a stem-directed broadly cross-reactive immunological imprint through vaccination in infants might also be a potential approach if reshaping an existing immune repertoire in adults is not possible. In addition, the building block of our stem-nanoparticles is encoded by a single gene and its assembly is precise and efficient, making it a candidate for genetic vaccine delivery approaches such as mRNA and recombinant viral vectors. These vaccine modalities have been pivotal for combating the ongoing SARS-CoV-2 pandemic and are anticipated to play major roles in other vaccines onward. These modalities along with innovative new adjuvants would offer not only manufacturing options and dose sparing but also different immunological properties including durability and cellular immunity. The present study provides a proof-of-concept for the vaccine-elicitation of broad cross-group protective immunity and would foster future studies addressing outstanding challenges such as preexisting immunity and durability.
Limitation of the study
Our study used animals that do not have any preexisting influenza immunity unlike humans. The lack of complex preexisting influenza immunity induced via repeated exposure and/or vaccination in those animal models severely limits our understanding on vaccine-elicited immune response in humans. While we observed substantial cross-group reactivity of B cells in NHPs and isolated a bnAb 789–203-3C12, there was insufficient neutralizing activity against H3N2 viruses, suggesting that the H10ssF does not efficiently prime B cells that cross-react with H3. However, the H10ssF might be an ideal immunogen to selectively induce cross-reactive responses for individuals who have a pre-established repertoire of H3-reactive (and potentially cross-reactive) B cells through natural H3N2 infection or vaccination. Both group 1 HA stem- and group 2 HA stem-nanoparticles reported herein have entered phase 1 clinical trials (NCT03814720 and NCT04579250, respectively) to evaluate safety and immunogenicity in humans. Results from these trials would inform the feasibility of the HA stem-based vaccine strategies for eliciting bnAb responses and reshaping the immune repertoire in humans in the face of preexisting influenza immunity.
STAR⋆Methods
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Masaru Kanekiyo (kanekiyom@nih.gov).
Materials availability
All unique and stable materials generated in this study are available from the lead contact under a Materials Transfer Agreement.
Data and code availability
Structural data generated from this study were deposited in public data repositories and the accession numbers are listed in the key resources table. All these data are publicly available as of the date of publication.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon reasonable request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| FI6v3 | Produced in house (Corti et al., 2011) | N/A |
| CT149 | Produced in house (Wu et al., 2015) | N/A |
| CR6261 | Produced in house (Throsby et al., 2008) | N/A |
| CR8020 | Produced in house (Ekiert et al., 2011) | N/A |
| MEDI8852 | Produced in house (Kallewaard et al., 2016) | N/A |
| 315-53-1F12 | Produced in house (Andrews et al., 2017) | N/A |
| 315-24-1E07 | Produced in house (Andrews et al., 2017) | N/A |
| 315-02-1H01 | Produced in house (Andrews et al., 2017) | N/A |
| 789-203-3C12 | Produced in house (This study) | N/A |
| Goat Anti-Human IgG -HRP conjugated | Southern Biotech | Cat# 2040-05 |
| Goat Anti-Mouse IgG -HRP conjugated | Southern Biotech | Cat# 1080-05 |
| Goat Anti-Ferret IgG -HRP conjugated | Abcam | Cat# Ab112770 |
| PerCP-Cy 5.5 Mouse Anti-Human IgM | BD Biosciences | Cat# 561285; RRID: AB_10611998 |
| BV421 Mouse Anti-Human IgG | BD Biosciences | Cat# 562581; RRID: AB_2737665 |
| BV510 Mouse Anti-Human CD3 | BD Biosciences | Cat# 740187; RRID: AB_2739940 |
| Brilliant Violet 510 anti-human CD14 | Biolegend | Cat# 301842; RRID: AB_2561946 |
| BV510 Mouse Anti-Human CD16 | BD Biosciences | Cat# 563830; RRID: AB_2744296 |
| ECD anti-CD19 | Beckman Coulter | Product# IM2708U |
| APC/Cyanine7 anti-human CD20 | Biolegend | Cat# 302314; RRID: AB_314262 |
| Brilliant Violet 605 anti-human CD27 | Biolegend | Cat# 302830; RRID: AB_2561450 |
| anti-CD38 | Produced in-house | N/A |
| BV510 Mouse Anti-Human CD56 | BD Biosciences | Cat# 740171; RRID: AB_2739924 |
| CD185 (CXCR5) Monoclonal Antibody (MU5UBEE), PE-Cyanine7 | ThermoFisher-eBioscience | Cat# 25-9185-42; RRID: AB_2573540 |
| 7AAD | Thermofisher Scientific | Cat# A1310 |
| Bacterial and virus strains | ||
| A/California/04/2009 (H1N1) | International Reagent Resource | 58841493/FR-201 |
| A/Vietnam/1203/2004 (H5N1) | CDC | CDC# 2004706280 |
| A/Philippines/2/1982 (H3N2) | FDA | C6066 |
| A/Anhui/1/2013 (H7N9) | CDC | CDC# 2013759189 |
| A/Jiangxi-Donghu/346/2013 (H10N8) | BEI Resources | NR-49439/63777209 |
| Influenza reporter viruses | Produced in house (Creanga et al., 2021) | N/A |
| Experimental models: cell lines | ||
| Expi293F | ThermoFisher | Cat# A14527 |
| 293A | ThermoFisher | Cat# R70507 |
| MDCK-SIAT1-PB1 | (Bloom et al., 2010) | N/A |
| MDCK-SIAT1 | MilliporeSigma | SKU# 05071502 |
| Experimental models: organisms/strains | ||
| BALB/c (Figures 1C, 1D, 2A, and 2B) | The Jackson Laboratory | BALB/cJ Strain 000651 |
| BALB/c (Figures 1E and 4C) | Envigo | BALB/cAnNHsd |
| Ferrets | Triple F Farms | N/A |
| Mauritian cynomolgus macaques | NIH | N/A |
| Deposited data | ||
| Cryo-EM structure of H10ssF | This study | EMD-26044 |
| Cryo-EM structure of 789-203-3C12 Fab-HA complex | This study | EMD-22302, PDB 6XSK |
| Chemicals, peptides, and recombinant proteins | ||
| H.pylori ferritin | Produced in house (PDB 3EGM) | N/A |
| H1ssF | Produced in house (Yassine et al., 2015) | N/A |
| H10ssF | Produced in house (This study) | N/A |
| H3ssF | Produced in house (Corbett et al., 2019) | N/A |
| H7ssF | Produced in house (Corbett et al., 2019) | N/A |
| H1 HA Trimer (MI15) | Produced in house (Boyoglu-Barnum et al., 2021) | N/A |
| H5 HA Trimer (VN04) | Produced in house (Boyoglu-Barnum et al., 2021) | N/A |
| H3 HA Trimer (HK68) | Produced in house (Boyoglu-Barnum et al., 2021) | N/A |
| H3 HA Trimer (HK14) | Produced in house (Boyoglu-Barnum et al., 2021) | N/A |
| H3 HA Trimer (PH82) | Produced in house (Boyoglu-Barnum et al., 2021) | N/A |
| H7 HA Trimer (AN13) | Produced in house (Boyoglu-Barnum et al., 2021) | N/A |
| H10 HA Trimer (JD13) | Produced in house (Boyoglu-Barnum et al., 2021) | N/A |
| Sigma Adjuvant System | Sigma-Aldrich | SKU# S6322 |
| AddaVax | Invivogen | Cat# vac-adx-10 |
| Ni-Sepharose excel resin | Cytiva | SKU# GE17-3712-02 |
| Galanthus nivalis agglutinin (GNA) gel | EY Laboratories | SKU# A-7401-2 |
| Methyl α-D-mannopyranoside | MilliporeSigma | SKU# 67770 |
| Protein G Sepharose | GE Healthcare | Product# 17061802 |
| IgG elution buffer | Pierce | Cat# 21009 |
| ExpiFectamine 293 transfection kit | ThermoFisher Scientific | Cat# A14524 |
| Protein A Sepharose | MilliporeSigma | SKU# GE17-1279-03 |
| Protein G Sepharose | MilliporeSigma | SKU# GE17-0618-05 |
| Software and algorithms | ||
| Flowjo v10 | TreeStar | https://www.flowjo.com; RRID: SCR_008520 |
| Prism v.8.4.3 | GraphPad | https://www.graphpad.com; RRID: SCR_002798 |
| MotionCor2 | (Zheng et al., 2017) | N/A |
| RELION2 | (Kimanius et al., 2016) | N/A |
| CTFFIND4 | (Rohou and Grigorieff, 2015) | N/A |
| Gautomatch v0.56 | https://www2.mrc-lmb.cam.ac.uk/download/gautomatch-056/ | |
| SPIDER | (Shaikh et al., 2008) | N/A |
| EMAN2 | (Tang et al., 2007) | N/A |
| LOOPY | (Xiang et al., 2002) | N/A |
| USCF Chimera | (Pettersen et al., 2004) | N/A |
| LSQMAN | (Kleywegt, 1996) | N/A |
| RELAX | (Nivon et al., 2013) | N/A |
| DDG_MONOMER (ROSETTA), | (Kellogg et al., 2011) | N/A |
| PyMOL | Schrodinger, LLC | https://pymol.org/2/ |
| Octet Analysis v11 | Fortebio | https://www.moleculardevices.com/ |
| Inkscape v1.0.0 | Inkscape | https://inkscape.org |
| Biorender | Biorender | https://biorender.com |
| Other | ||
| Superdex 200 10/300 Column | Cytiva | Cat# 17517501 |
| Superose 6 10/300 Column | Cytiva | Cat# 17517201 |
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
BALB/cJ and BALB/cAnNHsd female mice between 4–6 weeks of age were purchased from The Jackson Laboratory and Envigo, respectively, and were housed either in a conventional or specific pathogen free (SPF) animal facility on a 12-hour light/dark cycle under ambient conditions with free access to food and water. Animals were randomly assigned to experimental groups. All experiments were done in accordance with approved guidelines, regulations, and protocols as determined and approved by the Institutional Animal Care and Use Committee at Vaccine Research Center, NIH. The VRC research facility is AAALAC International accredited and standards for all animal care (acquisition, breeding, and experimental protocols), biosafety, and personnel occupational health and safety conform to all Federal, State and local regulations
Ferrets
Male and female ferrets (Mustela putorious furo) of 8 months of age were purchased from TripleF farm. Animals were housed in a controlled temperature, light and humidity environment at BioQual Inc, Rockville, USA. All experimental procedures, protocols, and care of the animals were approved by the Institutional Animal Care and Use Committee at BioQual Inc. and Vaccine Research Center, NIH.
NHPs
Male and female Macaca fascicularis of 9–13 years of age were used in this study. The animals were housed in a temperature-controlled environment with a light/dark cycle of 12 hours at Division of Veterinary Resources at NIH, Bethesda, USA. All experimental procedures, protocols, and care of the animals were approved by the Institutional Animal Care and Use Committee at Vaccine Research Center, NIH. Animals were used in the studies in accordance with all federal regulations, NIH guidelines, AAALAC, and IACUC approval.
Cell lines
Expi293 cells and 293A cells were obtained from Thermo Fisher Scientific under catalog numbers listed in the key resources table. MDCK-SIAT1-PB1 cells(Bloom et al., 2010) are generous gifts from Dr. Jesse Bloom at Fred Hutchinson Cancer Research Center. All cell lines were maintained in a humidified 5% CO2 incubator at 37°C and confirmed to be mycoplasma free. The sex of cell lines used in the study are unknown.
METHOD DETAILS
Structure-based immunogen design
The complete sequence for A/Jiangxi-Donghu/346/2013 (H10N8) including the signal sequence, was obtained from the GISAID accession number EPI497477. The crystal structure of A/Jiangxi-Donghu/346/2013 (H10N8) HA (PDB ID 4QY0) was used as a template for modeling using the “C” version of HAssF as previously described(Corbett et al., 2019). Loops were modeled with the program LOOPY(Xiang et al., 2002), superpositions were performed using UCSF Chimera(Pettersen et al., 2004) or LSQMAN(Kleywegt, 1996), energy minimization was performed using RELAX(Nivón et al., 2013) and the free energy changes from point mutations were calculated using the program DDG_MONOMER(Kellogg et al., 2011) from the ROSETTA suite. PyMOL (Schrodinger, LLC) and UCSF Chimera(Pettersen et al., 2004) were used for detailed visualization of structures and structural figure generation.
Expression and purification of immunogens, antibodies, and probes
The H3ssF, H7ssF and H10ssF sequences each incorporated the “C” version of group 2 HAssF as described previously(Corbett et al., 2019). The HAssF nanoparticles, H3ssF, H7ssF, H10ssF and H1ssF, monoclonal antibodies (mAbs) FI6v3(Corti et al., 2011), CT149(Wu et al., 2015), CR8020(Ekiert et al., 2011), CR6261(Throsby et al., 2008), MEDI8852(Kallewaard et al., 2016), 315–53-1F12, 315–54-1G07, 315–04-1D02, 315–09-1B12(Andrews et al., 2017), 789–203-3C12 (this study), and recombinant foldon-trimerized HA ectodomain trimers of H1 MI15 (A/Michigan/45/2015), H5 VN04 (A/Vietnam/1203/2004), H3 HK14 (A/Hong Kong/4803/2014), H7 AN13 (A/Anhui/1/2013), H10 JD13 (A/Jiangxi-Donghu/346/2013), and H1 SI06 (A/Solomon Islands/3/2006) were produced by transient transfection of the corresponding plasmid(s) in Expi293 cells (Life Technologies) using Expifectamine293 transfection kit (ThermoFisher Scientific) according to manufacturer’s instruction. For every 100mL transfection of Expi293 cells with respective plasmid, cells were grown and diluted to a density of 2.5 × 106 cells/mL. Diluted 0.27 mL Expifectamine 293 transfection reagent in 5 mL Opti-MEM medium. Diluted 0.1 mg of DNA in 5 mL Opti-MEM medium and filtered through 0.2 micron filter. Diluted Expifectamine and diluted DNA were mixed and incubated for 20 minutes. The DNA-reagent complex was added to the cells and incubated in a humidified shaker incubator for 4–5 days. All HAssF and mAbs were purified with Galanthus nivalis lectin (GNA-gel, EY Laboratories) and protein A (rmp Protein A Sepharose, Cytiva), respectively as previously described(Yassine et al., 2015). Briefly, filtered 500 mL HAssF supernatant was incubated with 2 mL Galanthus nivalis lectin resin at 4°C overnight with shaking. The resin was washed with PBS and eluted with 1M Methyl α-D-mannopyranoside. Recombinant HA proteins were purified by Ni-NTA through C-terminal hexa-histidine tag. Affinity purified HAssF and HA proteins were further purified by size exclusion chromatography using Superose 6 increase 10/300 GL and Superdex 200 increase 10/300 GL columns, respectively (Cytiva). To produce Fabs, MEDI8852, CR8020, and 789–203-3C12 IgG was digested with endoproteinase Lys-C (New England Biolabs) overnight at room temperature. The Fab of CT149 was produced by digesting CT149 IgG with an engineered HRV-3C cleavage site(McLellan et al., 2011) using HRV-3C enzyme (MilliporeSigma) overnight at RT. Upon completion, the reactions were quenched by adding protease inhibitor cocktail (MilliporeSigma), and Fc and Fabs were separated by passing through protein A column.
Immunizations
All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the Vaccine Research Center, NIAID, NIH. All animals were housed and cared for in compliance with the pertinent US National Institutes of Health regulations and policies and American Association for Accreditation of Laboratory Animal Care. Female BALB/cJ mice (N = 10) aged between 6 and 8 weeks (Jackson Laboratory) were immunized with 2 μg of H3ssF, H7ssF, H10ssF or H1ssF formulated with the Sigma adjuvant system at weeks 0, 4, and 8 with 100 μl via intramuscular route, given as 50 μl into each hind leg. Two weeks post second (week 6) and final immunization (week 10), sera were collected for immunological assays. For experimental virus challenge studies, mice were inoculated intranasally with a 10 × 50% lethal dose (LD50) of H3N2 A/Philippines/2/1982, 20 × LD50 of H7N9 A/Anhui/1/2013, 10 × LD50 of H10N8 A/Jiangxi-Donghu/346/13 or 25 × LD50 of H5N1 A/Vietnam/1203/2004 virus at BioQual. Infected animals were monitored daily for 14 days post-challenge for their weight change and symptoms. For ferrets (female and male ~6 months old fitch ferrets, TripleF Farm), animals (N = 6) were given 20 μg of HAssF formulated with SAS in 500 μl via intramuscular route at weeks 0, 4 and 8. Serum samples were collected 2 weeks after each immunization for serological assays. For NHP study, cynomolgus macaques (M. fascicularis, N = 3) were immunized with a mixture of 50 μg each of H1ssF and H10ssF formulated with AddaVax (Invivogen) in 1 ml via intramuscular route at weeks 0, 4, and 10. Blood samples were periodically collected after each immunization for immunological assays. For immunoglobulin (Ig) passive transfer studies, serum Igs were purified from H10ssF-immunized mice (combined 2–4 weeks after the third immunization) by using protein A/G (Pierce Thermo Scientific). Seven mg of purified heperimmune Igs or control Igs or 100 μg (5 mg kg−1) of FI6v3 IgG were given via intraperitoneal route (i.p.) to naïve recipient BALB/cAnNHsd mice (N = 10) aged between 4 and 6 weeks (Envigo) 24 h prior to the experimental intranasal challenge with 20 × LD50 of H7N9 A/Anhui/1/2013 virus. Infected animals were monitored daily for 14 days post-challenge for their weight change and symptoms. For mAb passive transfer studies, 200 μg of mAb 789–203-3C12 or FI6v3 were given i.p. to naïve recipient mice as described above. Twenty-four hours later, mice were experimentally challenged with 20 × LD50 of H1N1 A/California/04/2009, 25 × LD50 of H5N1 A/Vietnam/1203/2004, 20 × LD50 of H7N9 A/Anhui/1/2013, or 10 × LD50 of H10N8 A/Jiangxi-Donghu/346/13 virus at BioQual. Infected animals were monitored daily for 14 days post-challenge for their weight change and symptoms.
ELISA
ELISA was performed to assess anti-HA antibodies in response to HAssF immunization. The plates were coated with 200 ng well−1 of recombinant full-length HA-foldon proteins and incubated overnight at 4°C. The plates were then blocked with 5% skim milk in PBS at 37°C for 1 h. The plates were incubated with 4-fold serially diluted mAbs with dilution series starting at 10 μg ml−1 or immune sera dilutions starting at 1:50. Appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies (Southern Biotech) were added and incubated for 1 h followed by a colorimetric detection assay utilizing 3,3′,5′,5-tetramethylbenzidine (TMB) substrate (KPL). The colorimetric reaction was stopped by addition of 1 M H2SO4 and the absorbance recorded at 450 nm (OD450). Serum endpoint titers were recorded as serum dilution resulting in 4-fold increase in OD450 value above the background.
Biolayer interferometry
The biolayer interferometry binding assays were done as previously described(Corbett et al., 2019; Kanekiyo et al., 2019). All biosensors were hydrated in PBS prior to use. CR9114(Dreyfus et al., 2012) was used at 10 μg ml−1 in PBS containing 1% bovine serum albumin (PBS-BSA) to coat AHC biosensors (fortéBio) to capture H10ssF. The CR9114-coated biosensors were dipped in H10ssF (10 μg ml−1 in PBS-BSA). After equilibrated for 60 s in PBS-BSA, the biosensors were then dipped in a 2-fold dilution series of MEDI8852, CT149, or CR8020 Fab for 300 s, followed by incubation in PBS-BSA for 300 s to allow for dissociation. For 789–203-3C12, recombinant HAs (10 μg ml−1) were immobilized on HIS1K biosensors through hexa-histidine tag. After briefly equilibration in PBS-BSA, the biosensors were dipped in a 2-fold dilution series of 789–203-3C12 Fab for 300 s followed by dissociation in PBS-BSA for 300 s. All assay steps were performed at 30°C with agitation set at 1,000 rpm in the Octet HTX instrument (fortéBio). Correction to subtract non-specific baseline drift was carried out by subtracting the measurements recorded for a bare sensor. Data analysis and curve fitting were carried out using Octet analysis software (v9). Experimental data were fitted with the binding equations describing a 1:1 interaction. Global analyses of the complete data sets assuming binding was reversible (full dissociation) were carried out using nonlinear least-squares fitting allowing a single set of binding parameters to be obtained simultaneously for all concentrations used in each experiment.
Negative stain electron microscopy
Purified H3ssF, H7ssF, and H10ssF were diluted to 0.02 mg ml−1 with buffer containing 10 mM HEPES, pH 7.0, and 150 mM NaCl and adsorbed onto glow-discharged carbon-coated copper mesh grids for 15 s. The grids were washed with the buffer and stained with 0.7% uranyl formate. Images were obtained at a magnification of 50,000 semi-automatically on an FEI Tecnai T20 transmission electron microscope (TEM) operated at 200 kV, equipped with a 2,048- by 2,048-pixel Eagle charge-coupled-device (CCD) camera using SerialEM(Mastronarde, 2005). The pixel size was 0.44 nm and particles were picked automatically using software developed in-house. Reference-free 2D classification was performed using SPIDER(Shaikh et al., 2008) and Relion 1.4(Scheres, 2012). For 3D reconstruction of the H10 JD13 HA trimer in complex with 789–203-3C12 Fab, the purified components were mixed at a molar ratio of 1.1 Fab to 1 HA protomer, briefly incubated on ice, and negatively stained as described above. A dataset containing 144 micrographs was collected using the same TEM at a nominal magnification of 100,00x, corresponding to a pixel size of 0.22 nm, and 4746 particle images were selected using e2boxer from EMAN2(Tang et al., 2007) and extracted into 128- by 128-pixel boxes. After 2D classification in Relion 3.0, selected 2D class averages were used to generate an ab-initio 3D model in EMAN2. This was followed by 3D classification and 3D auto-refinement in Relion with imposed C3 symmetry. The final dataset contained 1173 particles, and the resolution at the Fourier shell correlation threshold of 0.5 was 24.8 Å.
Cryo-EM
To obtain cryo-EM structure of H10ssF, purified H10ssF was applied to glow discharged Quantifoil R 2/1 300 mesh grids (Ted Pella, Redding) at a concentration of 1 mg ml−1. Using a Leica EM GP plunge freezer (Leica Microsystems), the grid was blotted for 1 s at 37°C and 68% humidity, then plunge-frozen in liquid ethane. Plunged grids were loaded into a Polara electron microscope (ThermoFisher Scientific) operated at 300 keV, equipped with a Falcon 2 direct electron detector (ThermoFisher Scientific), and EPU data acquisition software (ThermoFisher Scientific). Total of 4,027 micrographs, dose fractionated into 7 frames, were collected at a magnification of 78,000 ×, with 1.43 Å2 pixel−1 and a total dose of 47 e− pixel−1. Movie frames were aligned using MotionCor2(Zheng et al., 2017), and the contrast transfer function for each micrograph was fitted using CTFFIND4(Rohou and Grigorieff, 2015). Total of 262 particles were manually picked to generate class averages with RELION2(Kimanius et al., 2016), which were used for autopicking of 693,018 particles by Gautomatch v0.56 (https://www2.mrc-lmb.cam.ac.uk/download/gautomatch-056/).
Further curation and alignment of particle sets was performed with RELION2. Particles were 2D classified in batches of 30,000–60,000, culling overlapping particles and false-positive particle picks from the analysis. For 3D analysis, an initial model of a solid sphere of diameter 220 Å was used, combined with an outer spherical mask of diameter 325 Å. Iterative 3D refinement of 118,473 particles against the model using “gold-standard” refinement of 2 independent half-maps as implemented in RELION2 generated a map at a resolution of 4.8 Å, using a 0.143 FSC cutoff and post-processed with an ad-hoc B-factor of −300 Å2. While antigenic spikes on H10ssF were observed during initial low-resolution refinement, their density became unresolved as the refined resolution improved. The location of the antigenic spikes could again be visualized by low-pass filtering the final map back to 8 Å resolution.
To obtain cryo-EM structure of 789–203-3C12 Fab in complex with stabilized HA trimer of H1N1 virus (A/Solomon Islands/3/2006, SI06), binary complex was produced by mixing the Fab and HA trimer in a 6:1 molar ratio, with a final trimer concentration of 0.2 mg ml−1, followed by incubation on ice for 1 hr. The protein complex was purified by a Superdex 200 16/60 column in a buffer containing 5 mM HEPES pH 7.5 (Life Technologies) and 150 mM NaCl (Quality Biological, Inc.). To prevent aggregation during vitrification, 0.005% (w/v) n-dodecyl β-D-maltoside was added to the sample prior to plunge freezing. Cryo-EM grids were prepared by applying 2 ml of sample to a freshly glow-discharged carbon-coated copper grid (CF 1.2/1.3 300 mesh); the sample was vitrified in liquid ethane using a Vitrobot Mark IV with a wait time of 30 s and a blot time of 3 s. Cryo-EM data were collected using the Leginon software(Suloway et al., 2005) installed on a Titan Krios electron microscope operating at 300 kV, equipped with a Gatan K3-BioQuantum direct detection device. The total dose was fractionated for 3 s over 60 raw frames. Motion correction, CTF estimation, particle extraction, 2D classification, ab initio model generation, 3D refinements and local resolution estimation for all datasets were carried out in cryoSPARC 2.15(Punjani et al., 2017). 3D classification of the HA–Fab complex showed two sets of particles: 116,041 particles with C1 symmetry and 60,634 particles with C3 symmetry. 3D refinement with the C1-symmetric particles produced a 3.85 Å resolution map while the C3-symmetric particles produced a 4.02 Å map. Given the higher resolution, we performed the analysis on the C1-symmetric structure. HA trimer density was modeled using PDB entry 6FYT(Laursen et al., 2018), as initial template. The heavy chain variable region of 789–203-3C12 Fab was modeled using PDB entry 4FQQ(Mouquet et al., 2012) while the light chain variable region was modeled using PDB entry 5OD8(Ge et al., 2019). Automated and manual model building were iteratively performed using real space refinement in Phenix(Adams et al., 2004) and Coot(Emsley and Cowtan, 2004), respectively. Half maps were provided to the Resolve Cryo-EM tool in Phenix to support manual model building. Geometry validation and structure quality assessment were performed using EMRinger(Barad et al., 2015) and Molprobity(Barad et al., 2015; Davis et al., 2004). Map-fitting cross correlation (Fit-in-Map tool) and figures preparation were carried out using PyMOL and UCSF Chimera(Pettersen et al., 2004) and ChimeraX(Pettersen et al., 2004, 2021). A summary of the cryo-EM data collection, reconstruction and refinement statistics is shown in Table S1. The buried surface area and atomic interactions in the 789–203-3C12–HA complex were measured using PDBePISA(Krissinel and Henrick, 2007) at https://www.ebi.ac.uk/msd-srv/prot_int/cgi-bin/piserver.
Differential scanning fluorimetry
Thermal unfolding of purified HAssF in PBS was measured by nano differential scanning fluorometry (nDSF) using Prometheus NT.48 instrument (NanoTemper technologies). Temperature gradient used for the assay was 20°C to 95°C with an increment of 1°C min−1. Thermal unfolding curves are plotted slope (Δ(F350 nm/F330 nm)/ΔT) against temperature (°C) generated from fluorescence measurements at 350 and 330 nm. Thermal transition midpoint temperature (Tm) was calculated from the curves and reported.
Dynamic light scattering
Dynamic light scattering (DLS) measurements were performed using a DynaPro Plate Reader II (Wyatt Technology). Protein samples of 0.5 mg ml−1 were centrifuged at 13,000 rpm in a tabletop refrigerated microfuge at 4°C for 45 minutes prior to aliquoting 30 μl of each into clear flat bottom 384-well plate (Corning). Measurements for each sample were taken at 25°C in the laser auto-attenuation mode and a wavelength of 830 nm from a total of 10 data acquisitions. DLS data was processed using Dynamics 7.8.2 software using a Rayleigh spheres model.
Influenza pseudovirus and microneutralization assays
Pseudoviruses used for the assay were based on lentivirus vectors encoding the luciferase gene and expressing influenza HA and NA as previously described(Wei et al., 2010). Replication-restricted reporter influenza viruses used in this study were previously described(Creanga et al., 2021). Animal serum was treated with receptor-destroying enzyme, RDE (Denka Seiken) for 37°C, 16 h followed by RDE denaturation at 56°C for 1 h. For pseudovirus neutralization assay, a four-fold serial dilution of mouse sera starting at 1:40 were mixed with either H3N2 (A/Hong Kong/1/1968, HK68), H7N9 (A/Anhui/1/2013, AN13), H10N8 (A/Jiangxi-Donghu/346/2013, JD13), H1N1 (A/Michigan/45/2015, MI15) or H5N1 (A/Vietnam/1203/2004, VN04) pseudoviruses for 30 min at room temperature (RT). The serum-pseudovirus mixture was added to previously plated 293A cells (10,000 cells well−1 in a 96-well plate) in triplicate. After 2 h fresh Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, and 1% penicillin–streptomycin was added. Cells were lysed at 72 h, and luciferase assay (Promega) was performed according to manufacturer’s protocol. Luciferase activity was measured as RLU at 570 nm on a SpectramaxL (Molecular Devices). For influenza neutralization assay, a four-fold serial dilution starting at 1:40 (serum samples) or 25 μg ml−1 (mAbs) were mixed with one of these replication-restricted reporter viruses: H1N1 (A/Michigan/45/2015, MI15); H3N2 (A/Hong Kong/4801/2014, HK14); H5N1 (A/Vietnam/1203/2004, VN04); H7N9 (A/Anhui/1/2013, AN13); H10N8 (A/Jiangxi-Donghu/346–2/2013, JD13) for 60 min at RT. The serum-virus mixtures were added to previously plated MDCK-SIAT1 cells stable expressing influenza PB1 gene (3,000 cells well−1 in a 384-well plate with transparent bottom) in quadruplicate. Plates were scanned at 18–22 h post infection and fluorescence foci were counted using Celigo Image Cytometer (Nexcelom). In Celigo operation and analysis software v5.0, Target 1 protocol was used to detect and count fluorescent foci(Creanga et al., 2021). IC50 titers were calculated considering uninfected cells as 100% and cells transduced with only virus as 0% neutralization.
B cell activation assays
The B cell activation assay was performed as described before(Corbett et al., 2019; Villar et al., 2016). To test the ability of HAssF to engage BCR and induce calcium flux, Ramos cells expressing inferred unmutated common ancestor (UCA) sequence of bnAbs as IgM on their surface were utilized. 106 cells/test were stained with 0.35 microliter fura red (Thermo Fisher Scientific) in 100 ml serum-free medium at room temperature in the dark for 30 min. After washing, cells were resuspended in 300 μl of serum-free medium and warmed to 37°C for 5 min in a heated water bath before being acquired on a FACSymphony interfaced to the FACSDiva software (BD Biosciences). To record baseline intracellular calcium levels, cells were acquired for 30 s without HAssF, followed by addition of HAssF, vortexing and placing it back on acquisition for a total time of 180 s. Nanoparticles were used at the final concentration of 50 nM. The anti-IgM was used as positive control to assess maximal calcium flux induction measured as change in emission by bound versus unbound fura red to calcium over time. The inferred UCA for 01.a.44 was chosen for group 1 specificity while inferred UCAs for 16.a.26 was utilized for group 2 HAs recognition.
PBMC isolation, staining and single-cell sorting
Cryopreserved peripheral blood mononuclear cells (PBMCs) were used. A panel of antibodies were used to stain the cells and gate for particular cell subsets. Antibodies to CD16 (clone 3G8), CD3 (clone SP34–2), CD56 (clone B159), IgM (clone G20–127), IgG (clone G18–145) (BD Biosciences); CD20 (clone 2H7), CD14 (clone M5E2) (Biolegend); and CD19 (clone J3–119) (Beckman Coulter) were used for cell surface staining. Various group 1 (H1 MI15, H5 VN04) and group 2 (H3 HK14, H7 AN13, H10 JD13) full-length ectodomain HA probes with the sialic acid-binding site mutation (Y98F) were prepared as previously described(Whittle et al., 2014). HA probes were conjugated with fluorescently-labeled streptavidin through biotin ligated to the Avi-tag on each probe. The cells were stained with a panel of antibodies to the surface markers and HA-streptavidin probes. The viability dye 7AAD was used for excluding dead cells. The samples were analyzed on a FACS Aria II (BD Biosciences) and single-cell sorted into 96-well plates. Index sorting was applied to identify specificity of individual cells for multiple HA probes. Data analysis and subsequent flowplots were generated following cell sorting using FlowJo (v10). PCR amplification of immunoglobulin heavy and light chain genes and cloning into expression vectors were performed as previously described(Sundling et al., 2012). Immunoglobulin gene assignment was performed by using the Karolinska Macaque database (KIMDB 1.1) (http://kimdb.gkhlab.se/)(Vázquez Bernat et al., 2021).
Illustration tool
The schematic images are created with Inkscape (v1.1) and Biorender.com.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analysis
The details of the replicates for each experiment are listed in the respective figure legends. In brief, unless otherwise indicated, for most in vitro and in vivo studies, the data shown are representative of at least two independent experiments. Conclusions from the repeated experiments that are not shown in the manuscript are the same as those from the shown experiments. The sample size in each experiment was determined based on the expected heterogeneity of the samples, the significance threshold (chosen at 0.05), the expected or observed difference, as well as previous publications and our pilot studies. The chosen sample size in each experiment is sufficient to generate statistically significant results. In all experiments, unless otherwise indicated, data are shown as geometric mean ± s.d., and statistical analyses were performed using GraphPad Prism (v9). Statistical tests used to analyze the data are indicated in figure legends. P values less than or equal to 0.05 were considered significant. All statistical tests used were two tailed.
Supplementary Material
HIGHLIGHTS.
Broad group 2 protective immunity was induced by H10-based group 2 HA stem immunogen
Co-immunization with group 1 and 2 HA stem immunogens elicits cross-protective antibodies
A bnAb isolated from an immunized NHP neutralizes both group 1 and 2 influenza A viruses
A common mode of HA recognition via the DH gene-encoded motif among NHP and human bnAbs
ACKNOWLEDGEMENTS
The authors thank R. Woodward, D. Scorpio, J.-P. Todd, E. McCarthy, A. Taylor, H. Bao, C. Chiedi, M. Dillon, L. Gilman, and G. Sarbadorand (VRC) for help with animal studies; K. Foulds, A. Noe, S.-F. Kao, V. Ficca, N. Nji, and D. Flebbe (VRC) for help with nonhuman primate samples; H. Andersen, N. Jones, and G. Patel (Bioqual) for help with influenza challenge studies; V. Nair and E. Fisher (NIAID) for cryo-electron microscopy data collection for H10ssF; and S. Andrews and members of VRC influenza program and Viral Pathogenesis Laboratory for helpful discussion. This work used the computational resources of the NIH HPC Biowulf cluster (http://hpc.nih.gov) and the Office of Cyber Infrastructure and Computational Biology (OCICB) High Performance Computing (HPC) cluster at the NIAID, NIH. This work was supported in part by Intramural Research Program of the Vaccine Research Center, NIAID, NIH; Intramural Research Program of Division of Intramural Research, NIAID, NIH; federal funds from the Frederick National Laboratory for Cancer Research, National Institutes of Health, under contract number HHSN261200800001E, and by Leidos Biomedical Research, Inc. L.S. and G.C. were supported by a Bill and Melinda Gates Foundation Universal Flu Grand Challenge Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at XXX
DECLARATION OF INTERESTS
S.M.M. J.C.B., P.D.K., J.R.M., B.S.G., and M.K. are named inventors of U.S. patents 9,441,019, 10,137,190, 10,363,301, and 11,338,033 on influenza hemagglutinin nanoparticle vaccines and stabilized hemagglutinin stem trimers and of several pending applications on related technologies filed by the U.S. Department of Health and Human Services (National Institutes of Health).
REFERENCES
- Adams PD, Gopal K, Grosse-Kunstleve RW, Hung L-W, Ioerger TR, McCoy AJ, Moriarty NW, Pai RK, Read RJ, Romo TD, et al. (2004). Recent developments in the PHENIX software for automated crystallographic structure determination. J. Synchrotron Radiat 11, 53–55. 10.1107/s0909049503024130. [DOI] [PubMed] [Google Scholar]
- Andrews SF, Joyce MG, Chambers MJ, Gillespie RA, Kanekiyo M, Leung K, Yang ES, Tsybovsky Y, Wheatley AK, Crank MC, et al. (2017). Preferential induction of cross-group influenza A hemagglutinin stem-specific memory B cells after H7N9 immunization in humans. Sci Immunol 2, eaan2676. 10.1126/sciimmunol.aan2676. [DOI] [PubMed] [Google Scholar]
- Avnir Y, Watson CT, Glanville J, Peterson EC, Tallarico AS, Bennett AS, Qin K, Fu Y, Huang C-Y, Beigel JH, et al. (2016). IGHV1–69 polymorphism modulates anti-influenza antibody repertoires, correlates with IGHV utilization shifts and varies by ethnicity. Sci. Rep 6, 20842. 10.1038/srep20842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barad BA, Echols N, Wang RY-R, Cheng Y, DiMaio F, Adams PD, and Fraser JS (2015). EMRinger: side chain–directed model and map validation for 3D cryo-electron microscopy. Nat. Methods 12, 943–946. 10.1038/nmeth.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DI, Guptill J, Naficy A, Nachbagauer R, Berlanda-Scorza F, Feser J, Wilson PC, Solórzano A, Van der Wielen M, Walter EB, et al. (2020). Immunogenicity of chimeric haemagglutinin-based, universal influenza virus vaccine candidates: interim results of a randomised, placebo-controlled, phase 1 clinical trial. Lancet Infect. Dis 20, 80–91. 10.1016/S1473-3099(19)30393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom JD, Gong LI, and Baltimore D (2010). Permissive secondary mutations enable the evolution of influenza oseltamivir resistance. Science 328, 1272–1275. 10.1126/science.1187816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra S, Blijleven JS, Roos WH, Onck PR, van der Giessen E, and van Oijen AM (2018). Hemagglutinin-Mediated Membrane Fusion: A Biophysical Perspective. Annu. Rev. Biophys 47, 153–173. 10.1146/annurev-biophys-070317-033018. [DOI] [PubMed] [Google Scholar]
- Boyoglu-Barnum S, Hutchinson GB, Boyington JC, Moin SM, Gillespie RA, Tsybovsky Y, Stephens T, Vaile JR, Lederhofer J, Corbett KS, et al. (2020). Glycan repositioning of influenza hemagglutinin stem facilitates the elicitation of protective cross-group antibody responses. Nat. Commun 11, 791. 10.1038/s41467-020-14579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyoglu-Barnum S, Ellis D, Gillespie RA, Hutchinson GB, Park Y-J, Moin SM, Acton OJ, Ravichandran R, Murphy M, Pettie D, et al. (2021). Quadrivalent influenza nanoparticle vaccines induce broad protection. Nature 592, 623–628. 10.1038/s41586-021-03365-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WC, and Murawsky CM (2018). Strategies for Generating Diverse Antibody Repertoires Using Transgenic Animals Expressing Human Antibodies. Front. Immunol 9, 460. 10.3389/fimmu.2018.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CS-F, Gorman J, Andrews SF, Rawi R, Reveiz M, Shen C-H, Wang Y, Harris DR, Nazzari AF, Olia AS, et al. (2022). Structure of an influenza group 2-neutralizing antibody targeting the hemagglutinin stem supersite. Structure 30, 993–1003.e6. 10.1016/j.str.2022.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens E, Angeletti D, Holbrook BC, Kanekiyo M, Jorgensen MJ, Graham BS, Yewdell J, and Alexander-Miller MA (2020). Influenza-infected newborn and adult monkeys exhibit a strong primary antibody response to hemagglutinin stem. JCI Insight 5, e135449. 10.1172/jci.insight.135449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens EA, Holbrook BC, Kanekiyo M, Yewdell JW, Graham BS, and Alexander-Miller MA (2021). An R848-Conjugated Influenza Virus Vaccine Elicits Robust Immunoglobulin G to Hemagglutinin Stem in a Newborn Nonhuman Primate Model. J. Infect. Dis 224, 351–359. 10.1093/infdis/jiaa728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett KS, Moin SM, Yassine HM, Cagigi A, Kanekiyo M, Boyoglu-Barnum S, Myers SI, Tsybovsky Y, Wheatley AK, Schramm CA, et al. (2019). Design of Nanoparticulate Group 2 Influenza Virus Hemagglutinin Stem Antigens That Activate Unmutated Ancestor B Cell Receptors of Broadly Neutralizing Antibody Lineages. MBio 10, e02810–e02818. 10.1128/mBio.02810-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti D, Voss J, Gamblin SJ, Codoni G, Macagno A, Jarrossay D, Vachieri SG, Pinna D, Minola A, Vanzetta F, et al. (2011). A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 333, 850–856. 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]
- Creanga A, Gillespie RA, Fisher BE, Andrews SF, Lederhofer J, Yap C, Hatch L, Stephens T, Tsybovsky Y, Crank MC, et al. (2021). A comprehensive influenza reporter virus panel for high-throughput deep profiling of neutralizing antibodies. Nat. Commun 12, 1722. 10.1038/s41467-021-21954-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darricarrère N, Qiu Y, Kanekiyo M, Creanga A, Gillespie RA, Moin SM, Saleh J, Sancho J, Chou T-H, Zhou Y, et al. (2021). Broad neutralization of H1 and H3 viruses by adjuvanted influenza HA stem vaccines in nonhuman primates. Sci. Transl. Med 13, eabe5449. 10.1126/scitranslmed.abe5449. [DOI] [PubMed] [Google Scholar]
- Davis IW, Murray LW, Richardson JS, and Richardson DC (2004). MolProbity: structure validation and all-atom contact analysis for nucleic acids and their complexes. Nucleic Acids Res. 32, W615–W619. 10.1093/nar/gkh398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLillo DJ, Tan GS, Palese P, and Ravetch JV (2014). Broadly neutralizing hemagglutinin stalk-specific antibodies require FcγR interactions for protection against influenza virus in vivo. Nat. Med 20, 143–151. 10.1038/nm.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLillo DJ, Palese P, Wilson PC, and Ravetch JV (2016). Broadly neutralizing anti-influenza antibodies require Fc receptor engagement for in vivo protection. J. Clin. Invest 126, 605–610. 10.1172/JCI84428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfus C, Laursen NS, Kwaks T, Zuijdgeest D, Khayat R, Ekiert DC, Lee JH, Metlagel Z, Bujny MV, Jongeneelen M, et al. (2012). Highly conserved protective epitopes on influenza B viruses. Science 337, 1343–1348. 10.1126/science.1222908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekiert DC, Friesen RHE, Bhabha G, Kwaks T, Jongeneelen M, Yu W, Ophorst C, Cox F, Korse HJWM, Brandenburg B, et al. (2011). A highly conserved neutralizing epitope on group 2 influenza A viruses. Science 333, 843–850. 10.1126/science.1204839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, and Cowtan K (2004). Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr 60, 2126–2132. 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Friesen RHE, Lee PS, Stoop EJM, Hoffman RMB, Ekiert DC, Bhabha G, Yu W, Juraszek J, Koudstaal W, Jongeneelen M, et al. (2014). A common solution to group 2 influenza virus neutralization. Proc. Natl. Acad. Sci. U. S. A 111, 445–450. 10.1073/pnas.1319058110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge C, Xu B, Liang B, Lönnblom E, Lundström SL, Zubarev RA, Ayoglu B, Nilsson P, Skogh T, Kastbom A, et al. (2019). Structural Basis of Cross-Reactivity of Anti-Citrullinated Protein Antibodies. Arthritis Rheumatol 71, 210–221. 10.1002/art.40698. [DOI] [PubMed] [Google Scholar]
- Gostic KM, Ambrose M, Worobey M, and Lloyd-Smith JO (2016). Potent protection against H5N1 and H7N9 influenza via childhood hemagglutinin imprinting. Science 354, 722–726. 10.1126/science.aag1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry Dunand CJ, Leon PE, Huang M, Choi A, Chromikova V, Ho IY, Tan GS, Cruz J, Hirsh A, Zheng N-Y, et al. (2016). Both Neutralizing and Non-Neutralizing Human H7N9 Influenza Vaccine-Induced Monoclonal Antibodies Confer Protection. Cell Host Microbe 19, 800–813. 10.1016/j.chom.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser KV, Chen GL, Carter C, Crank MC, Nguyen TA, Burgos Florez MC, Berkowitz NM, Mendoza F, Starr Hendel C, Gordon I, et al. (2022). Safety and immunogenicity of a ferritin nanoparticle H2 influenza vaccine in healthy adults: a phase 1 trial. Nat. Med 28, 383–391. 10.1038/s41591-021-01660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impagliazzo A, Milder F, Kuipers H, Wagner MV, Zhu X, Hoffman RMB, van Meersbergen R, Huizingh J, Wanningen P, Verspuij J, et al. (2015). A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science 349, 1301–1306. 10.1126/science.aac7263. [DOI] [PubMed] [Google Scholar]
- Jegaskanda S, Andrews SF, Wheatley AK, Yewdell JW, McDermott AB, and Subbarao K (2019). Hemagglutinin head-specific responses dominate over stem-specific responses following prime boost with mismatched vaccines. JCI Insight 4, e129035. 10.1172/jci.insight.129035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce MG, Wheatley AK, Thomas PV, Chuang G-Y, Soto C, Bailer RT, Druz A, Georgiev IS, Gillespie RA, Kanekiyo M, et al. (2016). Vaccine-Induced Antibodies that Neutralize Group 1 and Group 2 Influenza A Viruses. Cell 166, 609–623. 10.1016/j.cell.2016.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallewaard NL, Corti D, Collins PJ, Neu U, McAuliffe JM, Benjamin E, Wachter-Rosati L, Palmer-Hill FJ, Yuan AQ, Walker PA, et al. (2016). Structure and Function Analysis of an Antibody Recognizing All Influenza A Subtypes. Cell 166, 596–608. 10.1016/j.cell.2016.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekiyo M, and Graham BS (2021). Next-Generation Influenza Vaccines. Cold Spring Harb. Perspect. Med 11, a038448. 10.1101/cshperspect.a038448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekiyo M, Joyce MG, Gillespie RA, Gallagher JR, Andrews SF, Yassine HM, Wheatley AK, Fisher BE, Ambrozak DR, Creanga A, et al. (2019). Mosaic nanoparticle display of diverse influenza virus hemagglutinins elicits broad B cell responses. Nat. Immunol 20, 362–372. 10.1038/s41590-018-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg EH, Leaver-Fay A, and Baker D (2011). Role of conformational sampling in computing mutation-induced changes in protein structure and stability. Proteins 79, 830–838. 10.1002/prot.22921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimanius D, Forsberg BO, Scheres SH, and Lindahl E (2016). Accelerated cryo-EM structure determination with parallelisation using GPUs in RELION-2. Elife 5, e18722. 10.7554/eLife.18722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleywegt GJ (1996). Use of non-crystallographic symmetry in protein structure refinement. Acta Crystallogr. D Biol. Crystallogr 52, 842–857. 10.1107/S0907444995016477. [DOI] [PubMed] [Google Scholar]
- Kong W-P, Hood C, Yang Z-Y, Wei C-J, Xu L, García-Sastre A, Tumpey TM, and Nabel GJ (2006). Protective immunity to lethal challenge of the 1918 pandemic influenza virus by vaccination. Proc. Natl. Acad. Sci. U. S. A 103, 15987–15991. 10.1073/pnas.0607564103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F, Pica N, Hai R, Margine I, and Palese P (2013). Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J. Virol 87, 6542–6550. 10.1128/JVI.00641-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krissinel E, and Henrick K (2007). Inference of macromolecular assemblies from crystalline state. J. Mol. Biol 372, 774–797. 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Lang S, Xie J, Zhu X, Wu NC, Lerner RA, and Wilson IA (2017). Antibody 27F3 Broadly Targets Influenza A Group 1 and 2 Hemagglutinins through a Further Variation in V1–69 Antibody Orientation on the HA Stem. Cell Rep 20, 2935–2943. 10.1016/j.celrep.2017.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen NS, Friesen RHE, Zhu X, Jongeneelen M, Blokland S, Vermond J, van Eijgen A, Tang C, van Diepen H, Obmolova G, et al. (2018). Universal protection against influenza infection by a multidomain antibody to influenza hemagglutinin. Science 362, 598–602. 10.1126/science.aaq0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowitz SG, and Choppin PW (1975). Enhancement of the infectivity of influenza A and B viruses by proteolytic cleavage of the hemagglutinin polypeptide. Virology 68, 440–454. 10.1016/0042-6822(75)90285-8. [DOI] [PubMed] [Google Scholar]
- Lee PS, Zhu X, Yu W, and Wilson IA (2015). Design and Structure of an Engineered Disulfide-Stabilized Influenza Virus Hemagglutinin Trimer. J. Virol 89, 7417–7420. 10.1128/JVI.00808-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingwood D, McTamney PM, Yassine HM, Whittle JRR, Guo X, Boyington JC, Wei C-J, and Nabel GJ (2012). Structural and genetic basis for development of broadly neutralizing influenza antibodies. Nature 489, 566–570. 10.1038/nature11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Welsh JP, and Swartz JR (2014). Production and stabilization of the trimeric influenza hemagglutinin stem domain for potentially broadly protective influenza vaccines. Proc. Natl. Acad. Sci. U. S. A 111, 125–130. 10.1073/pnas.1308701110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde DN (2005). Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol 152, 36–51. 10.1016/j.jsb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- McLean HQ, and Belongia EA (2021). Influenza Vaccine Effectiveness: New Insights and Challenges. Cold Spring Harb. Perspect. Med 11, a038315. 10.1101/cshperspect.a038315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan JS, Pancera M, Carrico C, Gorman J, Julien J-P, Khayat R, Louder R, Pejchal R, Sastry M, Dai K, et al. (2011). Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature 480, 336–343. 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouquet H, Scharf L, Euler Z, Liu Y, Eden C, Scheid JF, Halper-Stromberg A, Gnanapragasam PNP, Spencer DIR, Seaman MS, et al. (2012). Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc. Natl. Acad. Sci. U. S. A 109, E3268–E3277. 10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachbagauer R, Feser J, Naficy A, Bernstein DI, Guptill J, Walter EB, Berlanda-Scorza F, Stadlbauer D, Wilson PC, Aydillo T, et al. (2021). A chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial. Nat. Med 27, 106–114. 10.1038/s41591-020-1118-7. [DOI] [PubMed] [Google Scholar]
- Nivón LG, Moretti R, and Baker D (2013). A Pareto-optimal refinement method for protein design scaffolds. PLoS One 8, e59004. 10.1371/journal.pone.0059004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, and Ferrin TE (2004). UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem 25, 1605–1612. 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Meng EC, Couch GS, Croll TI, Morris JH, and Ferrin TE (2021). UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82. 10.1002/pro.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punjani A, Rubinstein JL, Fleet DJ, and Brubaker MA (2017). cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296. 10.1038/nmeth.4169. [DOI] [PubMed] [Google Scholar]
- Rohou A, and Grigorieff N (2015). CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol 192, 216–221. 10.1016/j.jsb.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangesland M, Ronsard L, Kazer SW, Bals J, Boyoglu-Barnum S, Yousif AS, Barnes R, Feldman J, Quirindongo-Crespo M, McTamney PM, et al. (2019). Germline-Encoded Affinity for Cognate Antigen Enables Vaccine Amplification of a Human Broadly Neutralizing Response against Influenza Virus. Immunity 51, 735–749.e8. 10.1016/j.immuni.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres SHW (2012). RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol 180, 519–530. 10.1016/j.jsb.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh TR, Gao H, Baxter WT, Asturias FJ, Boisset N, Leith A, and Frank J (2008). SPIDER image processing for single-particle reconstruction of biological macromolecules from electron micrographs. Nat. Protoc 3, 1941–1974. 10.1038/nprot.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel J, Lowen AC, Wang TT, Yondola M, Gao Q, Haye K, García-Sastre A, and Palese P (2010). Influenza virus vaccine based on the conserved hemagglutinin stalk domain. MBio 1, e00018–10. 10.1128/mBio.00018-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J, Hwang WC, Perez S, Wei G, Aird D, Chen L-M, Santelli E, Stec B, Cadwell G, Ali M, et al. (2009). Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol 16, 265–273. 10.1038/nsmb.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suloway C, Pulokas J, Fellmann D, Cheng A, Guerra F, Quispe J, Stagg S, Potter CS, and Carragher B (2005). Automated molecular microscopy: the new Leginon system. J. Struct. Biol 151, 41–60. 10.1016/j.jsb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Sundling C, Phad G, Douagi I, Navis M, and Karlsson Hedestam GB (2012). Isolation of antibody V(D)J sequences from single cell sorted rhesus macaque B cells. J. Immunol. Methods 386, 85–93. 10.1016/j.jim.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Sutton TC, Chakraborty S, Mallajosyula VVA, Lamirande EW, Ganti K, Bock KW, Moore IN, Varadarajan R, and Subbarao K (2017). Protective efficacy of influenza group 2 hemagglutinin stem-fragment immunogen vaccines. NPJ Vaccines 2, 35. 10.1038/s41541-017-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Peng L, Baldwin PR, Mann DS, Jiang W, Rees I, and Ludtke SJ (2007). EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol 157, 38–46. 10.1016/j.jsb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Throsby M, van den Brink E, Jongeneelen M, Poon LLM, Alard P, Cornelissen L, Bakker A, Cox F, van Deventer E, Guan Y, et al. (2008). Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS One 3, e3942. 10.1371/journal.pone.0003942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M, Cheng C, Chen X, Duan H, Cheng H-L, Dao M, Sheng Z, Kimble M, Wang L, Lin S, et al. (2016). Induction of HIV Neutralizing Antibody Lineages in Mice with Diverse Precursor Repertoires. Cell 166, 1471–1484.e18. 10.1016/j.cell.2016.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez Bernat N, Corcoran M, Nowak I, Kaduk M, Castro Dopico X, Narang S, Maisonasse P, Dereuddre-Bosquet N, Murrell B, and Karlsson Hedestam GB (2021). Rhesus and cynomolgus macaque immunoglobulin heavy-chain genotyping yields comprehensive databases of germline VDJ alleles. Immunity 54, 355–366.e4. 10.1016/j.immuni.2020.12.018. [DOI] [PubMed] [Google Scholar]
- Viboud C, Gostic K, Nelson MI, Price GE, Perofsky A, Sun K, Sequeira Trovão N, Cowling BJ, Epstein SL, and Spiro DJ (2020). Beyond clinical trials: Evolutionary and epidemiological considerations for development of a universal influenza vaccine. PLoS Pathog. 16, e1008583. 10.1371/journal.ppat.1008583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar RF, Patel J, Weaver GC, Kanekiyo M, Wheatley AK, Yassine HM, Costello CE, Chandler KB, McTamney PM, Nabel GJ, et al. (2016). Reconstituted B cell receptor signaling reveals carbohydrate-dependent mode of activation. Sci. Rep 6, 36298. 10.1038/srep36298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TT, Tan GS, Hai R, Pica N, Ngai L, Ekiert DC, Wilson IA, García-Sastre A, Moran TM, and Palese P (2010). Vaccination with a synthetic peptide from the influenza virus hemagglutinin provides protection against distinct viral subtypes. Proc. Natl. Acad. Sci. U. S. A 107, 18979–18984. 10.1073/pnas.1013387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TT, Maamary J, Tan GS, Bournazos S, Davis CW, Krammer F, Schlesinger SJ, Palese P, Ahmed R, and Ravetch JV (2015). Anti-HA Glycoforms Drive B Cell Affinity Selection and Determine Influenza Vaccine Efficacy. Cell 162, 160–169. 10.1016/j.cell.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver GC, Villar RF, Kanekiyo M, Nabel GJ, Mascola JR, and Lingwood D (2016). In vitro reconstitution of B cell receptor–antigen interactions to evaluate potential vaccine candidates. Nat. Protoc 11, 193–213. 10.1038/nprot.2016.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C-J, Boyington JC, McTamney PM, Kong W-P, Pearce MB, Xu L, Andersen H, Rao S, Tumpey TM, Yang Z-Y, et al. (2010). Induction of broadly neutralizing H1N1 influenza antibodies by vaccination. Science 329, 1060–1064. 10.1126/science.1192517. [DOI] [PubMed] [Google Scholar]
- Whittle JRR, Wheatley AK, Wu L, Lingwood D, Kanekiyo M, Ma SS, Narpala SR, Yassine HM, Frank GM, Yewdell JW, et al. (2014). Flow cytometry reveals that H5N1 vaccination elicits cross-reactive stem-directed antibodies from multiple Ig heavy-chain lineages. J. Virol 88, 4047–4057. 10.1128/JVI.03422-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu NC, and Wilson IA (2020). Influenza Hemagglutinin Structures and Antibody Recognition. Cold Spring Harb. Perspect. Med 10, a038778. 10.1101/cshperspect.a038778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Cho M, Shore D, Song M, Choi J, Jiang T, Deng Y-Q, Bourgeois M, Almli L, Yang H, et al. (2015). A potent broad-spectrum protective human monoclonal antibody crosslinking two haemagglutinin monomers of influenza A virus. Nat. Commun 6, 7708. 10.1038/ncomms8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z, Soto CS, and Honig B (2002). Evaluating conformational free energies: the colony energy and its application to the problem of loop prediction. Proc. Natl. Acad. Sci. U. S. A 99, 7432–7437. 10.1073/pnas.102179699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassine HM, Boyington JC, McTamney PM, Wei C-J, Kanekiyo M, Kong W-P, Gallagher JR, Wang L, Zhang Y, Joyce MG, et al. (2015). Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat. Med 21, 1065–1070. 10.1038/nm.3927. [DOI] [PubMed] [Google Scholar]
- Zheng SQ, Palovcak E, Armache J-P, Verba KA, Cheng Y, and Agard DA (2017). MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332. 10.1038/nmeth.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F, Nair RR, Fisher EMC, and Cunningham TJ (2019). Humanising the mouse genome piece by piece. Nat. Commun 10, 1845. 10.1038/s41467-019-09716-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Structural data generated from this study were deposited in public data repositories and the accession numbers are listed in the key resources table. All these data are publicly available as of the date of publication.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon reasonable request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| FI6v3 | Produced in house (Corti et al., 2011) | N/A |
| CT149 | Produced in house (Wu et al., 2015) | N/A |
| CR6261 | Produced in house (Throsby et al., 2008) | N/A |
| CR8020 | Produced in house (Ekiert et al., 2011) | N/A |
| MEDI8852 | Produced in house (Kallewaard et al., 2016) | N/A |
| 315-53-1F12 | Produced in house (Andrews et al., 2017) | N/A |
| 315-24-1E07 | Produced in house (Andrews et al., 2017) | N/A |
| 315-02-1H01 | Produced in house (Andrews et al., 2017) | N/A |
| 789-203-3C12 | Produced in house (This study) | N/A |
| Goat Anti-Human IgG -HRP conjugated | Southern Biotech | Cat# 2040-05 |
| Goat Anti-Mouse IgG -HRP conjugated | Southern Biotech | Cat# 1080-05 |
| Goat Anti-Ferret IgG -HRP conjugated | Abcam | Cat# Ab112770 |
| PerCP-Cy 5.5 Mouse Anti-Human IgM | BD Biosciences | Cat# 561285; RRID: AB_10611998 |
| BV421 Mouse Anti-Human IgG | BD Biosciences | Cat# 562581; RRID: AB_2737665 |
| BV510 Mouse Anti-Human CD3 | BD Biosciences | Cat# 740187; RRID: AB_2739940 |
| Brilliant Violet 510 anti-human CD14 | Biolegend | Cat# 301842; RRID: AB_2561946 |
| BV510 Mouse Anti-Human CD16 | BD Biosciences | Cat# 563830; RRID: AB_2744296 |
| ECD anti-CD19 | Beckman Coulter | Product# IM2708U |
| APC/Cyanine7 anti-human CD20 | Biolegend | Cat# 302314; RRID: AB_314262 |
| Brilliant Violet 605 anti-human CD27 | Biolegend | Cat# 302830; RRID: AB_2561450 |
| anti-CD38 | Produced in-house | N/A |
| BV510 Mouse Anti-Human CD56 | BD Biosciences | Cat# 740171; RRID: AB_2739924 |
| CD185 (CXCR5) Monoclonal Antibody (MU5UBEE), PE-Cyanine7 | ThermoFisher-eBioscience | Cat# 25-9185-42; RRID: AB_2573540 |
| 7AAD | Thermofisher Scientific | Cat# A1310 |
| Bacterial and virus strains | ||
| A/California/04/2009 (H1N1) | International Reagent Resource | 58841493/FR-201 |
| A/Vietnam/1203/2004 (H5N1) | CDC | CDC# 2004706280 |
| A/Philippines/2/1982 (H3N2) | FDA | C6066 |
| A/Anhui/1/2013 (H7N9) | CDC | CDC# 2013759189 |
| A/Jiangxi-Donghu/346/2013 (H10N8) | BEI Resources | NR-49439/63777209 |
| Influenza reporter viruses | Produced in house (Creanga et al., 2021) | N/A |
| Experimental models: cell lines | ||
| Expi293F | ThermoFisher | Cat# A14527 |
| 293A | ThermoFisher | Cat# R70507 |
| MDCK-SIAT1-PB1 | (Bloom et al., 2010) | N/A |
| MDCK-SIAT1 | MilliporeSigma | SKU# 05071502 |
| Experimental models: organisms/strains | ||
| BALB/c (Figures 1C, 1D, 2A, and 2B) | The Jackson Laboratory | BALB/cJ Strain 000651 |
| BALB/c (Figures 1E and 4C) | Envigo | BALB/cAnNHsd |
| Ferrets | Triple F Farms | N/A |
| Mauritian cynomolgus macaques | NIH | N/A |
| Deposited data | ||
| Cryo-EM structure of H10ssF | This study | EMD-26044 |
| Cryo-EM structure of 789-203-3C12 Fab-HA complex | This study | EMD-22302, PDB 6XSK |
| Chemicals, peptides, and recombinant proteins | ||
| H.pylori ferritin | Produced in house (PDB 3EGM) | N/A |
| H1ssF | Produced in house (Yassine et al., 2015) | N/A |
| H10ssF | Produced in house (This study) | N/A |
| H3ssF | Produced in house (Corbett et al., 2019) | N/A |
| H7ssF | Produced in house (Corbett et al., 2019) | N/A |
| H1 HA Trimer (MI15) | Produced in house (Boyoglu-Barnum et al., 2021) | N/A |
| H5 HA Trimer (VN04) | Produced in house (Boyoglu-Barnum et al., 2021) | N/A |
| H3 HA Trimer (HK68) | Produced in house (Boyoglu-Barnum et al., 2021) | N/A |
| H3 HA Trimer (HK14) | Produced in house (Boyoglu-Barnum et al., 2021) | N/A |
| H3 HA Trimer (PH82) | Produced in house (Boyoglu-Barnum et al., 2021) | N/A |
| H7 HA Trimer (AN13) | Produced in house (Boyoglu-Barnum et al., 2021) | N/A |
| H10 HA Trimer (JD13) | Produced in house (Boyoglu-Barnum et al., 2021) | N/A |
| Sigma Adjuvant System | Sigma-Aldrich | SKU# S6322 |
| AddaVax | Invivogen | Cat# vac-adx-10 |
| Ni-Sepharose excel resin | Cytiva | SKU# GE17-3712-02 |
| Galanthus nivalis agglutinin (GNA) gel | EY Laboratories | SKU# A-7401-2 |
| Methyl α-D-mannopyranoside | MilliporeSigma | SKU# 67770 |
| Protein G Sepharose | GE Healthcare | Product# 17061802 |
| IgG elution buffer | Pierce | Cat# 21009 |
| ExpiFectamine 293 transfection kit | ThermoFisher Scientific | Cat# A14524 |
| Protein A Sepharose | MilliporeSigma | SKU# GE17-1279-03 |
| Protein G Sepharose | MilliporeSigma | SKU# GE17-0618-05 |
| Software and algorithms | ||
| Flowjo v10 | TreeStar | https://www.flowjo.com; RRID: SCR_008520 |
| Prism v.8.4.3 | GraphPad | https://www.graphpad.com; RRID: SCR_002798 |
| MotionCor2 | (Zheng et al., 2017) | N/A |
| RELION2 | (Kimanius et al., 2016) | N/A |
| CTFFIND4 | (Rohou and Grigorieff, 2015) | N/A |
| Gautomatch v0.56 | https://www2.mrc-lmb.cam.ac.uk/download/gautomatch-056/ | |
| SPIDER | (Shaikh et al., 2008) | N/A |
| EMAN2 | (Tang et al., 2007) | N/A |
| LOOPY | (Xiang et al., 2002) | N/A |
| USCF Chimera | (Pettersen et al., 2004) | N/A |
| LSQMAN | (Kleywegt, 1996) | N/A |
| RELAX | (Nivon et al., 2013) | N/A |
| DDG_MONOMER (ROSETTA), | (Kellogg et al., 2011) | N/A |
| PyMOL | Schrodinger, LLC | https://pymol.org/2/ |
| Octet Analysis v11 | Fortebio | https://www.moleculardevices.com/ |
| Inkscape v1.0.0 | Inkscape | https://inkscape.org |
| Biorender | Biorender | https://biorender.com |
| Other | ||
| Superdex 200 10/300 Column | Cytiva | Cat# 17517501 |
| Superose 6 10/300 Column | Cytiva | Cat# 17517201 |


