Abstract
As we look forward to the bright future of immune checkpoint blockade (ICB) therapy, there is still lacking a pharmacokinetic marker to understand the inter-individual differences in ICB response. ICB therapy is based on IgG antibodies that share the same homeostatic pathway with serum albumin. Therefore, serum albumin level could reflect IgG catabolic rate that directly impacts the clearance of therapeutic IgG antibodies. Through interrogating a large, clinically representative pan-cancer cohort of 1,479 ICB-treated patients, this study found that higher baseline albumin levels were significantly associated with stepwise improvements in overall survival (OS), progression-free survival (PFS), and objective response rate (ORR) (p<0.001), with the variability and reproducibility confirmed in 1,000 bootstrap-resampled cohorts. Furthermore, these findings were also confirmed in most subgroups defined by patient demographics, baseline characteristics, treatments, and cancer types, even in those with low ICB-responsive cancer types and low tumor mutation burden (TMB) (TMB≤10 mut/Mb) that most of which have not been approved by the US Food and Drug Administration (FDA) for ICB therapy. In summary, this study highlights the importance of pretreatment pharmacokinetic modeling for predicting ICB treatment outcomes. Based on serum albumin—an inexpensive, non-invasive, and easily accessible biomarker of IgG pharmacokinetics, we could take a step further towards optimizing ICB therapy.
Keywords: Immunotherapy; Biomarkers, Tumor
Introduction
In the course of tumor progression, tumors harness the immune checkpoint pathways to escape immunosurveillance and suppress antitumor immunity. With growing efforts to block immune checkpoints to reinvigorate antitumor immunity, immune checkpoint blockade (ICB) therapies are now approved by the US Food and Drug Administration (FDA) in a broad range of cancers, with approval likely for more cancer types in the foreseeable future.1 ICB-based immunotherapy could induce durable disease remission and prolonged patient survival,2 3 but such superior efficacy was only achieved in a minority of patients with cancer.4 To solve this critical issue, many precision medicine markers have been developed to predict ICB response.4 However, as we look forward to the future of expanding ICB therapy to more patients with cancer, there is still a lack of data using a pharmacokinetic marker to understand the interindividual differences in ICB response.
Current ICB therapy is based on IgG antibody drugs targeting CTLA-4, PD-1, and PD-L1. As for any therapeutic drug, the treatment efficacy is directly affected by drug pharmacokinetics. The interindividual variability in the pharmacokinetics of ICB antibodies has previously been characterized at the population level, and one of the most important factors for predicting IgG pharmacokinetics is the baseline serum albumin level.5 6 It has been reported that serum albumin is associated with the pharmacokinetics of several FDA-approved antibody drugs, including infliximab,7 bevacizumab,8 ustekinumab,9 and pertuzumab.10 Moreover, a higher baseline level of serum albumin is directly correlated with an increasing trough level of therapeutic IgG antibodies in vivo.7
Albumin and IgG are the first and second most abundant serum proteins in plasma, because both of their uniquely long half-lives depend on the same homeostatic regulation pathway by neonatal Fc receptor (FcRn). FcRn receptor salvages albumin and IgG from intracellular degradation and recycles them into extracellular fluid.11 Since albumin and IgG share the same FcRn salvaging pathway, serum albumin level could reflect the IgG catabolic rate that directly impacts the clearance of IgG antibodies.12 Indeed, it is well acknowledged that the clearance of IgG antibodies is faster in patients with lower serum albumin levels.13 14 Therefore, there is a strong rationale for using serum albumin as a mechanism-based biomarker to present the pharmacokinetics of therapeutic IgG antibodies. This study sought to propose serum albumin as a potential pharmacokinetic marker in ICB therapy, while conducting an in-depth investigation of the dose-dependent relationship of serum-albumin-based pharmacokinetics with patient survival, disease progression, and treatment response under the therapeutic context of ICB drugs.
Methods
Patients with cancer
This study obtained the complete clinical and laboratory data of 1,479 patients with cancer who were diagnosed from 2015 to 2018 at Memorial Sloan Kettering Cancer Center (MSKCC).15 These patients received ICB therapy by targeting PD-1/PD-L1 and CTLA-4 without neoadjuvant and adjuvant settings. Treatment response was categorized according to the Response Evaluation Criteria in Solid Tumors (RECIST) V.1.116 or best radiographic response.15 The ICB-treated patients who underwent serum albumin assessment were used for analyzing overall survival (OS), progression-free survival (PFS), and objective response rate (ORR). Tumor mutation burden (TMB) was measured by next-generation sequencing (NGS), defined as the total number of somatic tumor non-synonymous mutations normalized to the exonic coverage of MSK-IMPACT panel in mutations/megabase (mut/Mb).17
Prognostic association analysis
The survival analysis was performed by the survival R package (https://CRAN.R-project.org/package=survival). The OS and PFS were analyzed by Cox proportional hazards regression analysis. The multivariate Cox regression analysis was performed with covariates including cancer type, sex, age, prior chemotherapy history, ICB drug class, tumor stage, cancer type, and TMB.
The non-linear model of hazard ratio (HR) by spline-based smoothing method
To model the dose-dependent relationship of serum albumin with OS and PFS, the non-linear model of HR was introduced into Cox proportional hazards regression analysis using the spline-based smoothing method.18 19 By taking the median value of serum albumin (3.9 g/dL) as a reference, HRs were estimated across the continuous spectrum of serum albumin levels. A statistical method known as smoothHR20 was applied to construct the natural cubic regression spline curve of HR with 95% confidence intervals (CIs), as previously reported.21 The log-HR curve with 95% CIs presented a straightforward interpretation that the 95% CIs above or below 0 is equivalent to a significance of two-sided p<0.05.
Bootstrap-resampled cohorts
The bootstrap resampling method22 23 was applied to generate 1,000 randomly resampled cohorts with replacement from the original cohort. Next, corresponding statistical estimates, including the mean and 95% CIs, were calculated using 1,000 bootstrap-resampled cohorts. The bootstrap resampling analysis was conducted using the sample function of the base package in R (The R Foundation for Statistical Computing, Vienna, Austria, http://www.r-project.org).
Statistical analysis
All statistical analyses were performed by R V.3.6.1. Survival analysis was conducted by the survival R package. The χ2 test was performed using 1,000 Monte Carlo simulations.24 All statistical tests used 0.05 as the significance level, p≥0.05 was considered not significant (ns), and p<0.05 was considered a statistically significant difference, indicated with asterisks (*p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001).
Result
Baseline serum albumin level is associated with the clinical response to ICB therapy in a dose-dependent manner
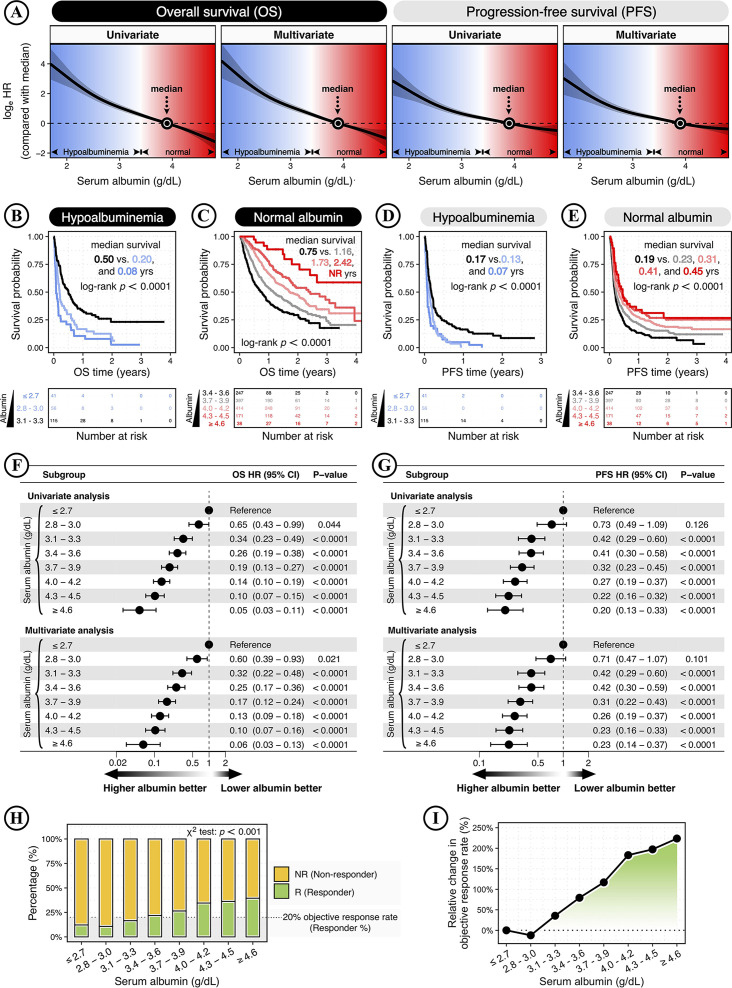
Using the baseline serum albumin as a consistent surrogate of pharmacokinetics for ICB drugs, this study investigated the relationship between baseline serum albumin levels and clinical responses to ICB therapy in patients with cancer. Through interrogating a pan-cancer cohort of 1,479 patients (online supplemental figure S1), this study analyzed the dynamics of OS and PFS following ICB therapy across the continuous spectrum of serum albumin levels. As previously reported, the non-linear HR was modeled by taking the median albumin level as a reference and plotted as log-HR curve.25 Based on Cox proportional hazards regression model, multivariate analysis was conducted using the covariates of cancer type, sex, age, prior chemotherapy history, ICB drug class, tumor stage, cancer type, and TMB. The non-linear HR analysis showed the dose-dependent relationships of serum albumin levels with OS and PFS (figure 1A).
Figure 1.
Baseline serum albumin level is associated with the clinical response to immune checkpoint blockade (ICB) therapy in a dose-dependent manner. (A) The smoothing estimate of HR with 95% CIs across the continuous spectrum of serum albumin levels. The log-HR curves (solid line) with 95% CIs (shading) show the impact of serum albumin increase on the overall survival (OS) and progression-free survival (PFS) of ICB-treated patients with cancer. The HRs were fitted by univariate and multivariate analyses based on Cox proportional hazards regression model. Multivariate analysis was performed using the covariates of cancer type, sex, age, prior chemotherapy history, ICB drug class, tumor stage, cancer type, and tumor mutation burden (TMB). The x-axis shows the serum albumin level (g/dL), and the y-axis shows the log2-HR taking the median albumin level as a reference. The 95% CIs above or below 0 is equivalent to a significance of two-sided p<0.05. Background colors indicate the serum albumin level within or outside its normal range: 3.4–5.4 g/dL. The arrow and dot show the median value of 3.9 g/dL. (B–E) The Kaplan-Meier curves show the OS (B–C) and PFS (D–E) in ICB-treated patients. Patients were divided into hypoalbuminemia (< 3.4 g/dL) and normal albumin (≥3.4 g/dL). Next, patients were stratified by the albumin cutoffs ranging from 2.8 to 4.6 g/dL in increments of 0.3 g/dL. The significance was analyzed by the log-rank test. The bottom panel shows the number of patients at risk every one year. (F–G) The forest plots show the association of serum albumin with OS (F) and PFS (G) following ICB therapy. HRs and 95% CIs were calculated by Cox proportional hazards regression analysis, taking the subgroup with an albumin level of ≤2.7 g/dL as the reference. Multivariate analysis was performed using the covariates of cancer type, sex, age, prior chemotherapy history, ICB drug class, tumor stage, cancer type, and TMB. (H–I) Serum albumin and ICB treatment response. (H) The stacked bar plot shows the percentage of ICB responses (responder vs non-responder) in patients with different serum albumin levels, with color coding according to different ICB responses. The results were considered statistically significant when p<0.05 and insignificant when p≥0.05 using the χ2 test with 1,000 Monte Carlo simulations. The black dashed line indicates a 20% objective response rate (ORR). (I) The relative changes in ORR with increasing serum albumin levels, using the subgroup with the lowest serum albumin level (≤ 2.7 g/dL) as a reference. NR, not reached.
jitc-2022-005670supp001.pdf (5.3MB, pdf)
In the wide spectrum of serum albumin levels ranging from 1.6 to 4.9 g/dL, patients were divided into hypoalbuminemia (<3.4 g/dL) and normal albumin (≥3.4 g/dL), and patients were further stratified into eight subgroups by albumin cutoffs ranging from 2.8 to 4.6 g/dL in increments of 0.3 g/dL. Next, Kaplan-Meier analyses showed that higher albumin levels were significantly associated with stepwise improvements in OS and PFS (p<0.0001; figure 1B–E). In addition, similar trends of OS and PFS improvement could be observed in patients with hypoalbuminemia and normal albuminemia (online supplemental figure S2A–B). Moreover, the multivariate analysis also showed a significant impact of increasing albumin levels on OS and PFS following ICB therapy, indicating serum albumin as an independent prognostic factor (figure 1 F–G).
Next, the efficacy of ICB therapy was assessed by ORR—the percentage of patients who experienced a complete response (CR) or partial response (PR). Among total patients, only 27.7% of patients were responders with CR and PR, while the remaining 72.3% of patients were non-responders of stable disease (SD) and progressive disease (PD). With increasing albumin levels, the ORR showed significantly stepwise improvements (p<0.001; figure 1H). Compared with low-albumin patients (≤ 2.7 g/dL) with an ORR of 12.2%, patients with high albumin levels of ≥4.6 g/dL had an ORR of 39.5%, achieving a maximum relative change of 223.7% increase (figure 1I).
Variability and reproducibility of the remarkably improved clinical benefits associated with increasing serum albumin levels for ICB therapy
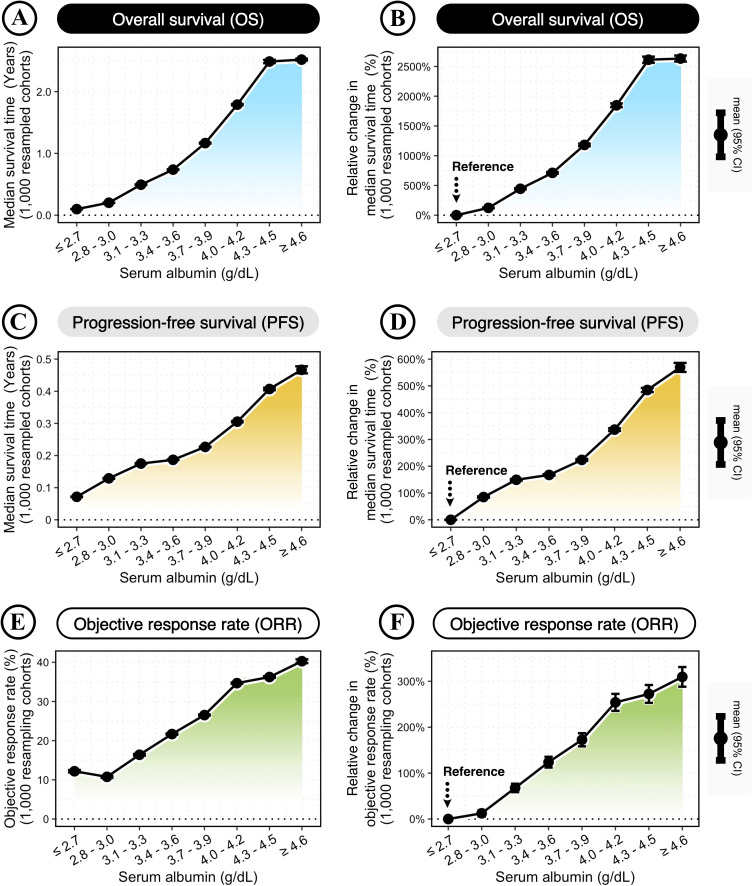
Next, this study confirmed the variability and reproducibility of serum albumin for predicting the clinical benefit of ICB therapy. The variability and reproducibility were estimated by 1,000 randomly resampled cohorts, which were generated by the bootstrap method as previously reported.22 23 The clinical benefits were measured by the median survival times of OS and PFS, and the ORR. In general, increasing serum albumin levels had a cumulative impact on improved clinical benefits of OS, PFS, and ORR (figure 2A–F). According to increasing serum albumin levels, the median survival times ranged from 0.10 to 2.51 years for OS (figure 2A) and 0.07 to 0.46 years for PFS (figure 2C), and the ORR ranged from 10.85% to 39.80% (figure 2E).
Figure 2.
Improved clinical benefit of immune checkpoint blockade (ICB) therapy with increasing serum albumin levels. (A, C, E) Changes in the median survival times of overall survival (OS) (A) and progression-free survival (PFS) (C), and the objective response rate (ORR) (E) across different serum albumin levels. (B, D, F) Relative changes in the median survival times of OS (B) and PFS (D), and the ORR (F) across different serum albumin levels, compared with the reference group of the lowest serum albumin level (≤ 2.7 g/dL). Bootstrap was applied to generate 1,000 randomly resampled cohorts. The dot-line plots show the changes across increasing serum albumin levels, with error bars representing the mean±95% CI calculated by 1,000 bootstrap-resampled cohorts.
Moreover, the relative changes in clinical benefits were calculated in each bootstrap-resampled cohort to estimate the variability and reproducibility. Compared with low-albumin patients (≤ 2.7 g/dL), patients with high albumin levels of ≥4.6 g/dL could achieve maximum relative changes of 2679.92% increase in OS (95% CI: 2633.97% to 2725.86%; figure 2B), 561.38% increase in PFS (95% CI: 545.22% to 577.54%; figure 2D), and 309.73% increase in ORR (95% CI: 288.33% to 331.13%; figure 2F).
Landscape of clinical benefits associated with increasing serum albumin levels for ICB therapy across patient demographics, baseline characteristics, treatment, and cancer type
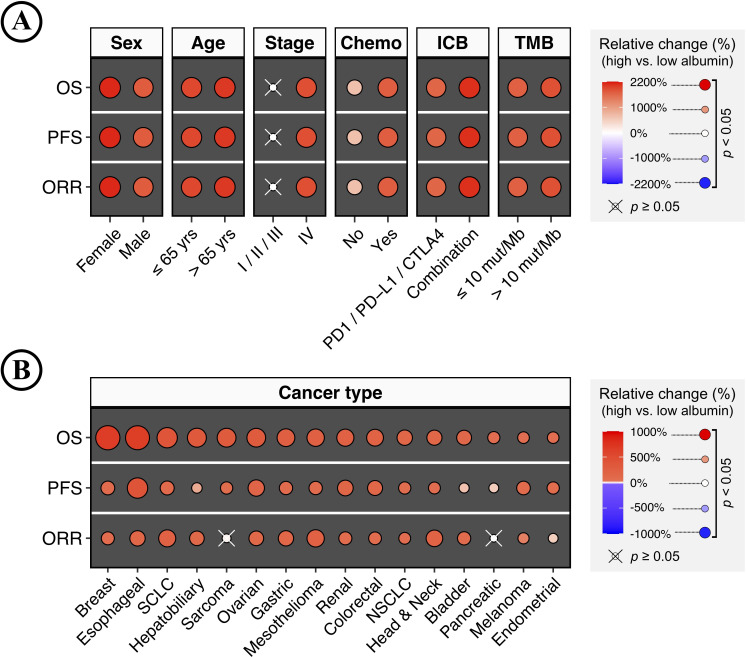
To further confirm the impact of increasing serum albumin levels on ICB therapy, this study extensively analyzed patients of different subgroups defined by demographics, baseline characteristics, treatment, and cancer type. For each subgroup, bootstrap was applied to generate 1,000 randomly resampled cohorts as described above. Patients were separated by the median albumin level, and the clinical benefits were measured by OS, PFS, and ORR (high vs low albumin). An overall trend of significantly improved clinical benefits was observed in patients with higher serum albumin levels (figure 3A–B). Insignificant associations were only observed in less-advanced tumors, sarcoma, and pancreatic cancer. Tumors with less advanced stages (stages I, II, and III) showed insignificant but slightly positive associations (figure 3A). Improved clinical benefits were observed in OS and PFS rather than ORR in patients with sarcoma and pancreatic cancer (figure 3B). In summary, this study presented the landscape of clinical benefits associated with increasing serum albumin levels for ICB therapy, strongly suggesting its excellent reproducibility across patient demographics, baseline characteristics, treatment, and cancer type.
Figure 3.
Landscape of clinical benefits associated with increasing serum albumin levels for immune checkpoint blockade (ICB) therapy across different patient demographics, baseline characteristics, treatments, and cancer types. (A) Heatmap plots show the relative changes in the clinical benefits associated with increasing serum albumin levels for ICB therapy in different subgroups defined by patient demographics, baseline characteristics, and treatments (high vs low albumin). The clinical benefits were measured by overall survival (OS), progression-free survival (PFS), and objective response rate (ORR). Patients were separated by the median albumin level. Bootstrap was applied to generate 1,000 randomly resampled cohorts for each subgroup. The color and size of the dots represent the mean of relative changes from 1,000 bootstrap-resampled cohorts. The positive value means an increased clinical benefit in patients with higher serum albumin levels. The results were evaluated by the one-sample Wilcoxon signed-rank test and considered statistically significant when the false discovery rate (FDR)-adjusted p<0.05, and the ‘×’ represents insignificant results. (B) Relative changes in the clinical benefits associated with increasing serum albumin levels for ICB therapy in different cancer types (high vs low albumin). FDR, false discovery rate.
In addition, it is worth noting that the improved clinical benefits associated with increasing serum albumin levels were also observed in low-TMB tumors (≤10 mutations/megabase [mut/Mb]). Low-TMB tumors are expected to have a statistically lower tumor immunogenicity, and are thus largely refractory to ICB therapy.26 27 As shown in figure 3A, the magnitude of association in low-TMB tumors was comparable to those with high-TMB (>10 mut/Mb). Therefore, the above finding suggests the possibility that low-TMB tumors, harboring lower tumor immunogenicity and reduced ICB responsiveness, could also achieve improved clinical benefits of ICB therapy.
Impact of increasing serum albumin levels on ICB therapy in pooled patients with cancer types categorized by ICB responsiveness
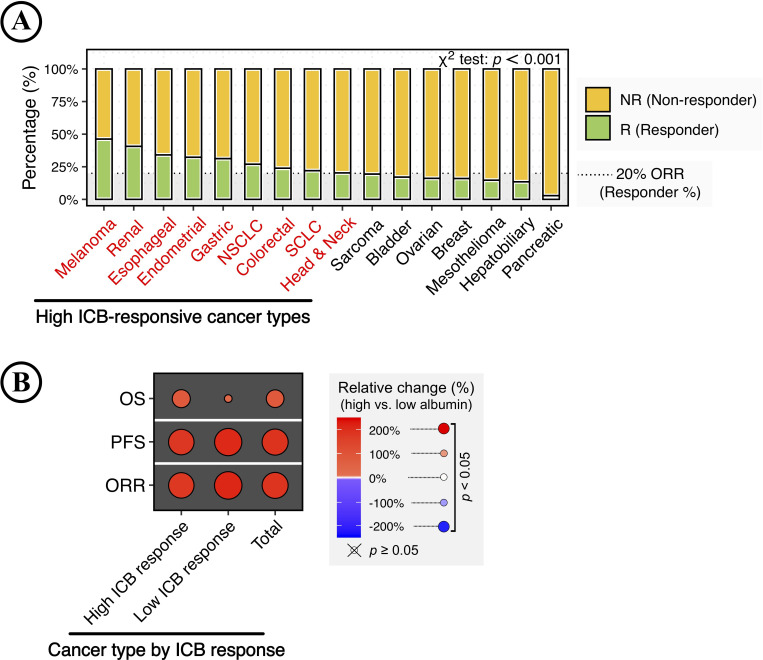
Different cancer types showed a significant difference in ICB response, and the ORR could range from 2.86% in pancreatic cancer to 46.23% in melanoma (p<0.001; figure 4A). In this study, cancer types with ≥20% ORR were defined as high ICB-responsive cancer types, including melanoma, renal, esophageal, endometrial, gastric, non-small cell lung cancer (NSCLC), colorectal, small-cell lung carcinoma (SCLC), and head and neck cancer, while the rest were defined as low ICB-responsive cancer types.
Figure 4.
Impact of increasing serum albumin levels on immune checkpoint blockade (ICB) therapy in pooled patients with cancer types categorized by ICB responsiveness. (A) ICB response in different cancer types. The stacked bar plot shows the percentage of ICB responses (responder vs non-responder) in different cancer types, with color coding according to ICB response. The result was considered statistically significant when p<0.05 and insignificant when p≥0.05 using the χ2 test with 1,000 Monte Carlo simulations. The black dashed line indicates a 20% objective response rate (ORR). Cancer types with ≥20% ORR were defined as high ICB-responsive cancer types. (B) Relative changes in the clinical benefits of ICB therapy associated with increasing serum albumin levels in pooled patients categorized by ICB responsiveness.
Next, this study analyzed the impact of increasing serum albumin levels on ICB therapy in pooled patients with cancer types categorized by ICB responsiveness. High albumin level was still associated with improved clinical benefits of ICB therapy in the analyses of pooled patients (figure 4B). It is worth noting that the albumin-related OS improvement was significant but relatively limited in patients with cancer types of low ICB responsiveness. In contrast, the albumin-related improvements in PFS and ORR were comparable across cancer-type-specific ICB responsiveness (figure 4B).
Discussion
This retrospective study, based on the MSKCC database of a large, clinically representative cohort of ICB-treated patients across 16 cancer types,15 presented interesting findings particularly relevant for clinical practice. First, increasing serum albumin level was dose-dependently associated with better clinical benefits of ICB therapy, leading to increased patient survival, delayed disease progression, and improved treatment response. Second, the dose-dependent relationship between serum albumin and ICB response was validated among those patients presenting serum albumin within or outside the normal range. Third, the positive correlation between serum albumin and ICB response could be confirmed in most subgroups of various demographics, baseline characteristics, treatments, and cancer types, even in low ICB-responsive cancer types and low-TMB (≤ 10 mut/Mb) tumors, most of which have not been FDA-approved for ICB therapy.28 The above findings may help translate the progress and success of currently available immunotherapy to additional cancer types and patients. Moreover, as a widely-used routine hematology test, serum albumin has the clear advantage of being inexpensive, non-invasive, and easily accessible in clinical practice.
The association between serum albumin and ICB treatment outcome has been previously reported in lung cancer,29–32 ovarian cancer,33 gastric cancer,34 and hepatocellular cancer.35 Previous studies used different cutoffs of serum albumin to assess its clinical relevance, making it difficult to analyze these fragmented studies systematically. In addition, previous studies did not analyze the cumulative impact of increasing serum albumin levels. Comparatively, this study has several strengths relative to prior reports. Different from previous efforts, this study analyzed the data of serum albumin collected and harmonized across different types of cancer, while analyzing the cumulative impact on ICB therapy. To the best of my knowledge, this is the first adequately powered post-hoc analysis revealing the dose-dependent serum-albumin-related improvements of ICB response in 16 types of cancer. These cancer types include breast, lung, and colorectal cancers that account for the leading causes of cancer incidence and death. Furthermore, their representative sampling of the general cancer population was reasonably estimated. Here, according to the cancer statistics of the USA in 2022,36 the cancer types analyzed in this study contribute to approximately 1.2 million new cancer cases and 0.4 million new cancer deaths, accounting for about two-thirds of total cancer cases and deaths (online supplemental figure S3A–B).
It should be noted that the sample size in this study is relatively small for the subgroup analyses, such as the subgroups of less-advanced stage (stage I/II/III), breast and ovarian cancers (online supplemental figure S1). To solve this problem, the bootstrap resampling method—a well-recognized approach for small sample size research—has been applied to evaluate the reproducibility of major findings in the subgroup analyses. Nevertheless, further studies in larger cohorts of specific populations might be required to confirm this study and to finally determine whether a serum albumin threshold needs to be reached to achieve a beneficial therapeutic effect of ICB therapy.
Understanding drug pharmacokinetics is essential for the prediction of drug efficacy and the adjustment of drug dose. ICB therapy is based on IgG-antibody drugs. The catabolism and recycling of IgG antibodies are regulated by the FcRn-mediated mechanism—the same as for albumin.11 IgG and albumin can bind to FcRn simultaneously via distinct, non-cooperative, and non-competitive binding sites.37 In addition, FcRn interacts with IgG rather than other immunoglobulin isotypes.38 Therefore, the serum albumin level, reflecting the abundance and efficiency of FcRn, could be a simplified surrogate of the general catabolic rate of therapeutic IgG antibodies,12 13 which ultimately contributes to the inter-individual variability of treatment efficacy and clinical outcome.
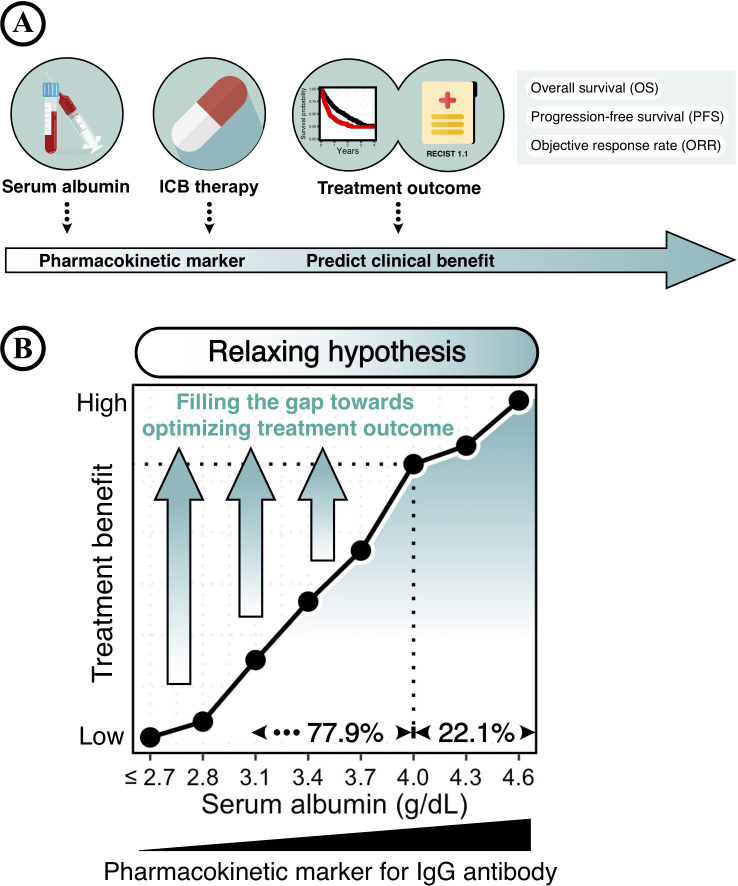
This study proposed serum albumin as a mechanism-based pharmacokinetic marker for ICB therapy, while discovering the cumulative impact of drug pharmacokinetics on treatment outcomes (figure 5A). However, optimizing treatment outcomes were only achieved in a minority of patients with a given high serum albumin level of >4.0 g/dL (22.1%), whereas a majority of patients (77.9%) still had a huge gap towards satisfactory treatment outcomes (figure 5B). Therefore, it is reasonable to assume that filling the gap in IgG pharmacokinetics could further optimize the efficacy of ICB therapy. Currently, several approaches have been developed to manipulate IgG pharmacokinetics. For example, antibody engineering of the IgG Fc-region has been conducted to extend the therapeutic IgG half-lives by optimizing the IgG-FcRn interaction.39–41 The prolonged persistence of therapeutic IgG antibodies is expected to increase the chance of targeting specific tissues/cells, leading to improved therapeutic efficacy.
Figure 5.
Graphical illustration of serum albumin and treatment outcome of immune checkpoint blockade (ICB). (A) Baseline serum albumin levels, a widely-used routine hematology test, could be a pharmacokinetic marker for predicting the clinical benefit of ICB therapy. (B) Relaxing hypothesis of ‘filling the gap towards optimizing ICB treatment outcome.’ Serum albumin level reflects the IgG catabolic rate that directly impacts the clearance of IgG antibodies. An improved clinical benefit of ICB therapy is associated with a given high level of serum albumin (> 4.0 g/dL), which accounts for only a minority of patients (22.1%). Therefore, filling the gap in pharmacokinetics might further optimize the efficacy of ICB therapy.
In summary, this study discovered that the serum albumin level—an inexpensive, non-invasive, and easily-accessible biomarker of IgG pharmacokinetics—has a remarkable clinical value for ICB therapy. These findings shed light on the importance of pretreatment pharmacokinetic modeling for predicting treatment outcomes, providing a novel insight that understanding IgG pharmacokinetics might help us take a step further towards optimizing ICB therapy. Moreover, since IgG antibodies have been used for treating diverse human diseases, including chronic inflammation, infections, autoimmune diseases, and cancers,42 43 this study also has universal implications for a broad range of IgG-antibody-based treatments.
jitc-2022-005670supp002.pdf (2.8MB, pdf)
Acknowledgments
I thank Dr. Luc G. T. Morris and his colleagues for the clinical and laboratory data of cancer patients from their previously published study.
Footnotes
Contributors: MZ conceived the project, developed the method, conducted data analysis, wrote the manuscript, and is the guarantor of this manuscript.
Funding: This project was supported by the National Natural Science Foundation of China (32100739) to MZ.
Disclaimer: The funders had no role in the study design, data analysis, data interpretation, and writing of this manuscript. MZ supervised this project and is responsible for the overall content.
Competing interests: This study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
The data that support the findings of this study will be available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
No human subjects were directly involved in this study. All the data used in this study were derived from existing deidentified biological samples and data from prior studies. Thus, ethical and patient consent were not required in this study.
References
- 1. Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 2018;8:1069–86. 10.1158/2159-8290.CD-18-0367 [DOI] [PubMed] [Google Scholar]
- 2. Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 2015;33:1889–94. 10.1200/JCO.2014.56.2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123–35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Topalian SL, Taube JM, Anders RA, et al. Mechanism-Driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 2016;16:275–87. 10.1038/nrc.2016.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bajaj G, Suryawanshi S, Roy A, et al. Evaluation of covariate effects on pharmacokinetics of monoclonal antibodies in oncology. Br J Clin Pharmacol 2019;85:2045–58. 10.1111/bcp.13996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dirks NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet 2010;49:633–59. 10.2165/11535960-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 7. Fasanmade AA, Adedokun OJ, Olson A, et al. Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int J Clin Pharmacol Ther 2010;48:297–308. 10.5414/CPP48297 [DOI] [PubMed] [Google Scholar]
- 8. Lu J-F, Bruno R, Eppler S, et al. Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother Pharmacol 2008;62:779–86. 10.1007/s00280-007-0664-8 [DOI] [PubMed] [Google Scholar]
- 9. Zhu Y, Hu C, Lu M, et al. Population pharmacokinetic modeling of ustekinumab, a human monoclonal antibody targeting IL-12/23p40, in patients with moderate to severe plaque psoriasis. J Clin Pharmacol 2009;49:162–75. 10.1177/0091270008329556 [DOI] [PubMed] [Google Scholar]
- 10. Garg A, Quartino A, Li J, et al. Population pharmacokinetic and covariate analysis of pertuzumab, a HER2-targeted monoclonal antibody, and evaluation of a fixed, non-weight-based dose in patients with a variety of solid tumors. Cancer Chemother Pharmacol 2014;74:819–29. 10.1007/s00280-014-2560-3 [DOI] [PubMed] [Google Scholar]
- 11. Chaudhury C, Mehnaz S, Robinson JM, et al. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med 2003;197:315–22. 10.1084/jem.20021829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu C, Yu J, Li H, et al. Association of time-varying clearance of nivolumab with disease dynamics and its implications on exposure response analysis. Clin Pharmacol Ther 2017;101:657–66. 10.1002/cpt.656 [DOI] [PubMed] [Google Scholar]
- 13. Dostalek M, Gardner I, Gurbaxani BM, et al. Pharmacokinetics, pharmacodynamics and physiologically-based pharmacokinetic modelling of monoclonal antibodies. Clin Pharmacokinet 2013;52:83–124. 10.1007/s40262-012-0027-4 [DOI] [PubMed] [Google Scholar]
- 14. Gaudreault J, Lieberman G, Kabbinavar F. Pharmacokinetics (pK) of bevacizumab (bv) in colorectal cancer (CRC). Clinical Pharmacology & Therapeutics 2001;69:P25. [Google Scholar]
- 15. Chowell D, Yoo S-K, Valero C, et al. Improved prediction of immune checkpoint blockade efficacy across multiple cancer types. Nat Biotechnol 2022;40:499–506. 10.1038/s41587-021-01070-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 17. Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 2017;23:703–13. 10.1038/nm.4333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Boor C, De Boor C. A practical guide to splines: springer-verlag New York, 1978. [Google Scholar]
- 19. Eilers PHC, Marx BD. Flexible smoothing with B-splines and penalties. Statistical Science 1996;11:89–121. 10.1214/ss/1038425655 [DOI] [Google Scholar]
- 20. Meira-Machado L, Cadarso-Suárez C, Gude F, et al. smoothHR: an R package for pointwise nonparametric estimation of hazard ratio curves of continuous predictors. Comput Math Methods Med 2013;2013:1–11. 10.1155/2013/745742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cadarso-Suárez C, Meira-Machado L, Kneib T. Flexible hazard ratio curves for continuous predictors in multi-state models: an application to breast cancer data. Statistical Modelling 2010;10:291–314. [Google Scholar]
- 22. Efron B. Nonparametric estimates of standard error: the jackknife, the bootstrap and other methods. Biometrika 1981;68:589–99. 10.1093/biomet/68.3.589 [DOI] [Google Scholar]
- 23. Efron B. Better bootstrap confidence intervals. J Am Stat Assoc 1987;82:171–85. 10.1080/01621459.1987.10478410 [DOI] [Google Scholar]
- 24. Patefield WM. Algorithm as 159: an efficient method of generating random R × C tables with given row and column Totals. Appl Stat 1981;30:91–7. 10.2307/2346669 [DOI] [Google Scholar]
- 25. Zheng M. The gradient of Immune/Inflammatory response and COVID-19 prognosis with therapeutic implications. Front Immunol 2021;12:739482. 10.3389/fimmu.2021.739482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 2020;21:1353–65. 10.1016/S1470-2045(20)30445-9 [DOI] [PubMed] [Google Scholar]
- 27. Zheng M. Dose-Dependent effect of tumor mutation burden on cancer prognosis following immune checkpoint blockade: causal implications. Front Immunol 2022;13:853300. 10.3389/fimmu.2022.853300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. FDA . Fda approves pembrolizumab for adults and children with TMB-H solid tumors, 2020. Available: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-adults-and-children-tmb-h-solid-tumors
- 29. Minami S, Ihara S, Ikuta S, et al. Gustave Roussy immune score and Royal Marsden Hospital prognostic score are biomarkers of Immune-Checkpoint inhibitor for non-small cell lung cancer. World J Oncol 2019;10:90–100. 10.14740/wjon1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu Z, Diao Y, Li X. Body mass index and serum markers associated with progression-free survival in lung cancer patients treated with immune checkpoint inhibitors. BMC Cancer 2022;22:824. 10.1186/s12885-022-09744-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stares M, Ding TE, Stratton C, et al. Biomarkers of systemic inflammation predict survival with first-line immune checkpoint inhibitors in non-small-cell lung cancer. ESMO Open 2022;7:100445. 10.1016/j.esmoop.2022.100445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guo Y, Wei L, Patel SH, et al. Serum albumin: early prognostic marker of benefit for immune checkpoint inhibitor monotherapy but not chemoimmunotherapy. Clin Lung Cancer 2022;23:345–55. 10.1016/j.cllc.2021.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boland JL, Zhou Q, Martin M, et al. Early disease progression and treatment discontinuation in patients with advanced ovarian cancer receiving immune checkpoint blockade. Gynecol Oncol 2019;152:251–8. 10.1016/j.ygyno.2018.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen L, Sun H, Zhao R, et al. Controlling nutritional status (CONUT) predicts survival in gastric cancer patients with immune checkpoint inhibitor (PD-1/PD-L1) outcomes. Front Pharmacol 2022;13:836958. 10.3389/fphar.2022.836958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li Y, Pan Y, Lin X, et al. Development and validation of a prognostic score for hepatocellular carcinoma patients in immune checkpoint inhibitors therapies: the hepatocellular carcinoma modified Gustave Roussy immune score. Front Pharmacol 2021;12:819985. 10.3389/fphar.2021.819985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7–33. 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 37. Chaudhury C, Brooks CL, Carter DC, et al. Albumin binding to FcRn: distinct from the FcRn-IgG interaction. Biochemistry 2006;45:4983–90. 10.1021/bi052628y [DOI] [PubMed] [Google Scholar]
- 38. Patel DD, Bussel JB. Neonatal Fc receptor in human immunity: function and role in therapeutic intervention. J Allergy Clin Immunol 2020;146:467–78. 10.1016/j.jaci.2020.07.015 [DOI] [PubMed] [Google Scholar]
- 39. Dall'Acqua WF, Kiener PA, Wu H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn). J Biol Chem 2006;281:23514–24. 10.1074/jbc.M604292200 [DOI] [PubMed] [Google Scholar]
- 40. Yeung YA, Leabman MK, Marvin JS, et al. Engineering human IgG1 affinity to human neonatal Fc receptor: impact of affinity improvement on pharmacokinetics in primates. J Immunol 2009;182:7663–71. 10.4049/jimmunol.0804182 [DOI] [PubMed] [Google Scholar]
- 41. Zalevsky J, Chamberlain AK, Horton HM, et al. Enhanced antibody half-life improves in vivo activity. Nat Biotechnol 2010;28:157–9. 10.1038/nbt.1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol 2010;10:301–16. 10.1038/nri2761 [DOI] [PubMed] [Google Scholar]
- 43. Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol 2010;10:317–27. 10.1038/nri2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2022-005670supp001.pdf (5.3MB, pdf)
jitc-2022-005670supp002.pdf (2.8MB, pdf)
Data Availability Statement
The data that support the findings of this study will be available from the corresponding author on reasonable request.