Abstract
Atherosclerosis is a metabolic disorder characterized by chronic inflammation associated with progressive thickening and hardening of the large to medium-sized arteries due to plaque formation. The study aims to evaluate the antioxidative, anti-inflammatory, and hypolipidemic efficacy of Opuntia ficus-indica (OFI) fruit extract against the high-fat-diet associated atherosclerotic rat model. In-vitro qualitative and quantitative phytochemical screening of OFI fruit extract revealed the significant presence of total phenolic content and total flavonoid contents. In-vitro antioxidant activity of fruit extract was determined through 2,2-diphenyl-1-picrylhydrazyl and FRAP assays that have shown their protective efficacy against the overproduction of reactive oxygen species. Results revealed that the level of total oxidant stress was significantly (P < 0.05) reduced and down expression levels of dual oxidases (Duox, Duoxa-1, and Duox-2) in all the treatment groups (I, II, III) as compared with positive control were observed. The total antioxidant capacity was significantly (P < 0.05) increased in all treatment groups in comparison with the positive control group and higher expression level of the Nrf-2 signaling pathway (Nfe-212, NFR-1, and Keap-1) was observed in all the treatment groups compared with the positive control group. Histopathological examination of the aorta showed that high-fat diet markedly increased endothelial lining and thickness of tunica media and adventitia, with irregular media segments having wavy laminae, and a significant increase in entropy of fibers disposition was observed. Conclusively, OFI fruit extract has shown promising protective, anti-oxidative, and anti-inflammatory efficacy through the restoration of normal parenchyma in high-fat dieting-associated oxidative stress and endothelial inflammation.
Keywords: atherosclerosis, Opuntia ficus-indica, high-fat diet, oxidative stress, Nrf-2 signaling cascade, dual oxidases
Graphical abstract
Introduction
Cardiovascular diseases (CVDs) are key responsible for increased death tolls around the globe and are major factors behind acute coronary syndrome, stroke, unstable angina, and myocardial infarctions that may progress toward atherosclerosis.1 Atherosclerosis is a chronic immune-mediated inflammatory and fibro-proliferative vascular disorder that develops mostly due to plaque formation in the large- and medium-sized arteries. The peripheral arterial disease arises from the fatty streaks in the early stage during plaque formation that is prone to rupture, ultimately resulting in atherothrombotic events.2 Several genetic, metabolic, and environmental risk factors contribute to the development of atherosclerosis such as high-fat dieting, hypercholesterolemia, obesity, smoking, diabetes mellitus, hypertension, radiotherapy of the head and neck, coronary artery disease, sedentary lifestyle, as well as prolonged oxidative stress. Moreover, aging also stimulates many pathophysiological processes that are strictly associated with atherosclerosis, i.e. inflammation and oxidative stress-induced endothelial cell dysfunction.3 In addition, recently, inflammation and oxidative stress have become hot research topics on the mechanism of organism tissue and organ damage.4–7
The high-fat diet (HFD), characterized by elevated total cholesterol (TC), triglycerides (TGs), low-density lipoproteins (LDL) levels, and reduced high-density lipoproteins (HDL) levels, is the main cause of oxidative stress by producing reactive oxygen species (ROS) and results into different obesity-related metabolic syndrome manifestations. ROS production leads to the disruption of cellular homeostasis, and hence, adversely affects cell survival via damage to proteins, lipids, and DNA as well as lipid peroxidation that ultimately results in the progression of inflammation, aging, and degenerative dysfunctioning.8,9 Moreover, the generation of ROS can also exert a beneficial role as second messengers via triggering signal transduction pathways involved in several cellular pathways. The major endogenous defense pathways that regulate redox homeostasis are the Nrf2 system and NADPH oxidase systems, which are the generators of endogenous ROS as well as regulate the gene expression of several antioxidants and detoxifying enzymes (expressed in the cardiovascular system) which are very crucial regarding ROS scavenging.10,11 The nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is an NOX family of enzymes such as Nox 1–5 and dual oxygenase Duox 1 and 2. It is a professional superoxide anion (O2−) or hydrogen peroxide (H2O2) generator that is mainly responsible for the regulated ROS production as well as biological functions of these enzymes.12,13 However, the long-term production of ROS damages the cellular structure that leads to cell dysfunction and cell death. Therefore, this damage impairs the Nrf2/ARE pathway, and its efficiency to protect the cells against excessive ROS is reduced substantially.11 Hence, to explore new therapeutic targets, more concern has been shifted toward investigating the role of the endogenous defense system in the pathogenesis of atherosclerosis.
Recently, some medicinal plants have been used in traditional medicine and have shown tremendous potential against hypercholesterolemia. Antihyperlipidemic activities exhibited by these plants are mainly owing to their ability to enhance bile acids in feces as well as increased excretion or decreased biosynthesis of cholesterol and cholesterol esterification. Most plants contain phenolic compounds, dietary fibers, saponins, polysaccharides, alkaloids, and terpenoids that demonstrate hypolipidemic and antioxidant effects and are involved in the improvement of the antioxidant defense systems, with concomitant suppression of lipid peroxidation. Hence, phenolic compounds play a keen role in reducing the impact of oxidative stress on cell integrity as well as tissues.14Opuntia ficus-indica (OFI) has been reported as a medicinal plant that is widely and commercially available as cactus fruit and belongs to the family Cactaceae.15 The Opuntia genus consists of ~1,500 species that are highly valuable due to their nutritional composition and pharmacological properties.16 Cactus is a tropical and subtropical plant that is cultivated in arid to semiarid climates, especially in harsh environmental conditions such as deserts.17 It has been reported that cactus extract and fruits are useful as nutraceuticals that have anti-inflammatory, antioxidant, anti-ulcerogenic, hypoglycemic, and antitumor attributes. Peels and seeds of the cactus fruit are also used to prepare cactus oil that contains a large amount of essential fatty acids and liposoluble antioxidants.18 Flowers of the cactus plant exhibit anti-bacterial, anti-inflammatory, anti-ulcerative, wound healing, and anti-oxidative properties which were mainly attributed to the presence of kaempferol, isorhamnetin, quercetin, and glycosides. The cactus fruit contains various phenolic, alkaloids (betaxanthin, glycosylated flavonoids, and betacyanin).19
Despite the extensive investigations regarding the therapeutic efficacy of OFI bioactive compounds in its cladodes, stem, flowers, seeds, peel, and pulp, scanty data exist in the literature regarding the anti-inflammatory, antioxidant, and lipid-lowering activities of OFI fruits. In this context, the current study aimed to assess the anti-inflammatory, antioxidant, and anti-hypercholesterolemic activities of ethanolic extract prepared from the whole OFI fruit. Furthermore, this study also wanted to evaluate the anti-inflammatory role of OFI fruit extract in controlling the HFD-associated cardiovascular inflammatory responses in rats fed with HFD via regulating Nrf2/Keap1 and dual oxidases signaling pathways.
Materials and methods
Fruit collection and the preparation of fruit extract
Fruit samples (5 kg) of O. ficus-indica (OFI; fruits) were procured from the local market of Faisalabad city in September 2020 and authenticated by Dr Mansoor Hameed (Taxonomist), Chairman, Department of Botany, the University of Agriculture Faisalabad with an herbarium vide No. 197–21-01 for future reference. Fruits were washed, air-dried, as well as dry oven dried up to 45 °C for 15 days and then ground into fine powder. The ethanolic extract was prepared by dissolving 100 g of fine powder of fruit in 1-L ethanol in a flask. These flasks were kept in a rotary evaporator (Heizbad Hei-VAP, Heidolph, Germany) for 24 h at 15,000 rpm. The extract was filtered and reduced under vacuum at 40 °C in an incubator. After freezing at −40 °C, the extract was lyophilized, and fine dried powder was obtained.
Proximate composition
Proximate analysis (fiber contents, crude fat, dry matter, crude proteins, and total ash) was performed in dried powdered fruit as described by Gharbi et al.20 such the crude protein content (N × 6.25) was determined by the Kjehldal Method; the ash content was estimated by incineration at 600 ± 15 °C; the total lipids were extracted by the Folch method and the total sugars were estimated by Dubois Method.
Mineral analysis
Calcium (Ca), sodium (Na), potassium (K), magnesium (Mg), zinc (Zn), copper (Cu), manganese (Mn), iron (Fe), phosphorus (P), lead (Pb), and cadmium (Cd) contents were analyzed for mineral analysis in fruit extracts by using atomic absorption spectrophotometer according to AOAC guidelines.21
Qualitative phytochemical screening
Harborne’s (1980) standard method was used for the qualitative analysis of phytochemicals in the ethanolic extract of fruit described by Minhaj et al.22
Quantitative phytochemical analysis
The ethanolic extract of fruit was subjected to quantitative analysis for the confirmation of phytochemicals (total phenolic and total flavonoids contents) presence in the extract using a high-performance liquid chromatography (HPLC) instrument (Shimadzu, Japan) accompanied by a C18 column and UV–visible detector (Shimadzu, Japan), and whole fruit total phenolic and total flavonoids contents were determined by using standard methods of Folin–Ciocalteu and aluminum chloride method, respectively.23
In vitro antioxidant assays
2,2-diphenyl-1-picrylhydrazyl radical-scavenging assay
The method of Clarke et al.24 was followed to perform a 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay, with slight modifications. In short, plant extract (200 μL) was diluted in different concentration ranges (0.24–500 μg mL−1), mixed with 0.2-mM DPPH (100 μL) inside 96-well plates, and absorbance was measured at 515 nm.
Ferric reducing antioxidant power assay
Ferric reducing antioxidant power (FRAP) assay was performed based on an earlier reported procedure with few modifications. According to this method, the extract was dissolved in methanol to prepare the stock solution and then an FRAP reagent was prepared. About 20 μL of the extract was mixed with a 180-μL FRAP reagent. Later, incubation of the mixture was done at 37 °C for 6 min and absorbance was measured at 595 nm.25
Experimental design
Adult Wistar rats n = 40 weighing about (180–200 g) of either sex were purchased and housed in standard laboratory conditions of the Institute of Physiology & Pharmacology, UAF. The research trial was carried out strictly following the guidelines approved by the institutional committee (GSRB) and Institutional Biosafety and Bioethics Committee (IBC) with D. No. 3668/ORIC, UAF, on the use and care of animals. Rats were acclimatized under standard conditions for 1 week, i.e. temperature (26 ± 2 °C) and humidity (40–60%), during which rats were fed a balanced diet and water ad libitum. Animals were divided into 5 groups after the completion of the adaptation period.
The experimental design was followed as: Group 1: Negative control; Group 2: Positive control (45% HFD); Group 3: Treatment I (45% HFD + Standard drug [Atorvastatin; mg kg−1 day−1 BW orally]); Group 4: Treatment II (45% HFD + Fruit Extract [OFI; 600 mg kg−1 day−1 BW orally]); and Group 5: Treatment III (45% HFD + Fruit Extract [OFI; 800 mg kg−1 day−1 BW orally]). The negative control group received a routine diet and the other four groups received HFD for 8 weeks to induce atherosclerotic alterations, and after 6 weeks of HFD, induction treatment was given to the 3 treatment groups as coadministration with HFD.
Sample collection
At the end of the experimental trial (8 weeks), rats were decapitated by cervical dislocation. For hematology and biochemical analysis, blood samples were taken in EDTA and gel clot activator tubes, respectively, via cardiac puncture inducing mild anesthesia. Blood samples were centrifuged for 10–15 min at 4,000 rpm for separating serum. Then, the separated serum was stored at −20 °C and used for further biochemical analysis. Aortic tissues of each decapitated rat were collected and immediately stored in RNAlater™ for mRNA extraction to perform gene expression analysis.
Determination of lipid profile
Serum TC, high-density (HDL-C), very low-density lipoprotein cholesterol (VLDL-C), and triglycerides (TG) in serum were determined by using the Dia-Sys Diagnostic Systems USA reagent kit.
Determination of biochemical analysis
Total oxidant status and total antioxidant capacity (TAC) were estimated from the serum sample by following novel colorimetric methods described by Erel.26,27
Gene expression analysis
mRNA isolation from the aorta was performed by using TRIzol™ (Thermo Fisher Scientific, Massachusetts, USA). The mRNA quantification of obtained samples was done through nanodrop, and then, the samples were stored at −80 °C. cDNA synthesis was done by using revert aid cDNA Synthesis Kit by using a standard protocol. Quantitative real-time (PCR) was performed on an iQ5 Bio-Rad machine with the help of a Maxima SYBR Green (Thermo Fisher Scientific) kit. The expression levels of the following genes were analyzed: Duox, Duox-1, Duox-2, Nfe212, NFR1, and Keap-1 by keeping beta-actin as a housekeeping gene. For the amplification of genes, following the oligonucleotides (Primers) sequence were used.
Beta-actin-sense 5’CGAGTACAACCTTCTTGCAGC’3.
Beta-actin-antisense, 5’TATCGTCATCCATGGCGAACTG’3.
Duox-sense 5’TCATGTGGATGGCACCAGAC’3.
Duox-antisense 5’CGAAACCACAACAAGCCCAG’3.
Duox-1-sense 5’GAAAGGAAGCATCAACACCCC’3.
Duox-1-antisense 5’GTGGTGTCCATTGGGAAGGT’3.
Duox-2-sense 5’CTCTCCAGCTCTCAGGGTCT’3.
Duox-2-antisense 5’GCGAGAGTATCTGTCTGGGC ‘3.
NFR1-sense 5’CTCCGACTAGCCATTGACGC’3.
NFR1-antisense 5’CAAATCCATGTCCTGCTGGGA’3.
Keap1-sense 5’CATGTGATGAACGGGGCAGT’3.
Keap1-antisense 5’AGAACTCCTCCTCCCCGAAG’3.
Nfe 212 -sense 5’CTTGTCCCGTCCCTAGGTC’3.
Nfe 212 -antisense 5’GGAGTGGCAGGCCAGTCTTA’3.
Histopathological examination
Aortic tissue samples were collected for histopathological examination and preserved in neutral buffered 10% formalin solution. After fixation, tissue samples were processed through a number of processes, e.g. dehydration, clearing, infiltration, embedding, sectioning, and staining. Hematoxylin and Eosin (H/E) stains were used to stain the slides and then examined under a compound microscope by using Toupview 3.7 software for a digital camera.
Statistical analysis
All the data were subjected to the analysis of variance (ANOVA) and means were compared using post hoc Tukey’s test at a P ≤ 0.05 level of significance. Moreover, graph pad prism 6 was used to explain the results in graphical representation.
Results
Proximate analysis
The proximate analysis of OFI fruit powder was performed and the results were presented as mean ± SE in Table 1. Dry weight, moisture content, crude proteins, fats, carbohydrates, fiber, and total ash contents were determined to estimate the nutritive values of the fruit.
Table 1.
Proximate composition of OFI fruit powder.
| Tests | Percentage composition |
|---|---|
| Moisture content | 70 |
| Dry weight | 30 |
| Total carbohydrates | 10.28 |
| Crude proteins | 2.30 |
| Crude fats | 1.62 |
| Crude fiber | 13.40 |
| Total ash | 2.4 |
Mineral analysis
The mineral analysis of OFI fruit powder was performed, and the highest concentration of Ca, K, Mg, Mn, Fe, Zn, and Na was found, while traces of P and Cu were determined (Table 2).
Table 2.
Mineral analysis of OFI fruit (mg 100 g−1, dry matter).
| Minerals | Composition (mg 100 g −1 ) |
|---|---|
| Ca | 31.4 |
| Mg | 6.4 |
| K | 106.4 |
| Fe | 23.8 |
| P | 0.04 |
| Mn | 34.4 |
| Zn | 13 |
| Cu | 0.01 |
| Na | 16.5 |
Qualitative phytochemical analysis of OFI fruit extract
The ethanolic extract of OFI fruit was processed for qualitative phytochemical investigation. Qualitative phytochemical results revealed the presence of phenolic contents, flavonoids, glycosides, carbohydrates, and proteins (Table 3).
Table 3.
Qualitative phytochemical constituents of OFI fruit extract.
| Phytochemicals | Ethanolic extract of the fruit |
|---|---|
| Phenolic compounds | + |
| Flavonoids | + |
| Alkaloids | + |
| Saponins | + |
| Fixed oil | − |
| Glycosides | + |
| Phlobatannins | − |
| Carbohydrates | + |
| Proteins | + |
| Quinones | − |
(+) = Present, (−) = Absent.
Quantitative phytochemical analysis of OFI fruit extract
Total flavonoid and phenolic contents
Total phenolic content in the fruit extract was found to be 23.69 ± 2.86 mg GAE g−1, while total flavonoid contents were 542.76 ± 0.058 mg ce g−1.
Quantitative analysis through HPLC
HPLC analysis revealed the presence of different phytoconstituents in the ethanolic fruit extract including quercetin, gallic acid, p-coumaric acid, caffeic acid, benzoic acid, ferulic acid, cinnamic acid, chlorogenic acid, vanillic acid, m-coumaric acid, sinapic acid, and syringic acid as shown in Fig. 1, respectively, whereas the retention time of each phytoconstituent against concentration is defined in Table 4.
Fig. 1.
A chromatographic spectrum of OFI fruit extract.
Table 4.
Concentrations and retention times of phytocompounds detected in OFI fruit extract.
| Compounds | Retention time (min) | Area (mV.s) | Area (%) | Concentration (ppm) |
|---|---|---|---|---|
| Quercetin | 3.413 | 4,872.134 | 3.2 | 258.22 |
| Gallic acid | 4.680 | 6,488.407 | 4.2 | 233.57 |
| Caffeic acid | 12.820 | 1,052.514 | 0.7 | 48.45 |
| Vanillic acid | 13.407 | 3,815.564 | 2.5 | 236.53 |
| Chlorogenic acid | 15.307 | 3,711.768 | 2.4 | 289.46 |
| Syringic acid | 16.560 | 3,377.656 | 2.2 | 84.43 |
| p-Coumaric acid | 17.647 | 1,522.393 | 1.0 | 19.79 |
| m-Coumaric acid | 20.507 | 1,426.429 | 0.9 | 17.12 |
| Ferulic acid | 22.127 | 652.035 | 0.4 | 46.95 |
| Sinapic acid | 25.953 | 1,177.659 | 0.8 | 15.49 |
In-vitro antioxidant activity
DPPH and FRAP assay
DPPH assay was performed to evaluate the antioxidant potential of fruit extract (Table 5). The in-vitro DPPH assay revealed that OFI fruit extract demonstrated 69.72% inhibition and results showed that almost similar free radical scavenging activity was previously reported by Roghelia and Panchal.28 FRAP assay was performed in-vitro to estimate the antioxidant reducing power of the fruit extract (Table 5), which revealed a substantial potential of scavenging the free radical (1.47 ± 0.09).
Table 5.
Antioxidant potential of OFI fruit extract.
| DPPH (% Inhibition) | 69.72 ± 0.35 |
|---|---|
| FRAP (nmol Fe 2+ Eq. mg −1 extract) | 1.47 ± 0.09 |
Effect of OFI fruit extract on lipid profile
Serum TC was significantly higher in positive control as well as treatment I, II, and III. Similarly, the effect of HFD on triglycerides was nonsignificant in the positive control, while triglycerides levels were high in treatment groups and VLDL levels were significantly increased in the positive control group compared with the negative control group, while the levels were significantly (P < 0.05) restored in treatments II and III in comparison with the positive control group (Fig. 2). However, serum HDL level was found to be slightly increased in treatment II and treatment III groups. In short, the cholesterol-depressing properties of the fruit extract were found to be significant (P < 0.05).29
Fig. 2.
Serum TC, HDL, TG, and vLDL (mg dl−1 ± SE) of negative control group (NC), positive control group (45% HFD), treatment I group (SD + 45% HFD), treatment II group (OFI; 600 mg/kg + 45% HFD), and treatment III group (OFI; 800 mg/kg + 45% HFD). Data are presented as mean ± SEM and P ≤ 0.05.
Effect of OFI fruit extract on HFD-induced oxidative stress markers
Serum TAC levels showed nonsignificant (P < 0.05) differences among control (positive and negative) groups. Similarly, TAC levels among treatment I, treatment II, and treatment III groups were observed to be statistically nonsignificant in comparison with the control (positive and negative) group (Fig. 3), where maximum TAC levels were exhibited by Treatment I and III (800 mg kg−1 day−1 B.W.). Similarly, the total oxidant stress (TOS) level showed significant differences among the control (positive and negative) groups. Moreover, among different treatments, treatment II was statistically nonsignificant to the positive control rat group and treatment III was nonsignificant to the negative control in terms of TOS levels. Maximum TOS levels were found in treatment I in comparison with all other treatment groups (Fig. 3).
Fig. 3.
Serum TAC (mmol L−1 ± SE) and total oxidant status (TOS; μmol L−1 ± SE) negative control group (NC), positive control group (45% HFD), treatment I group (SD + 45% HFD), treatment II group (OFI; 600 mg/kg + 45% HFD), and treatment III group (OFI; 800 mg/kg + 45% HFD). Data are presented as mean ± SEM and P ≤ 0.05.
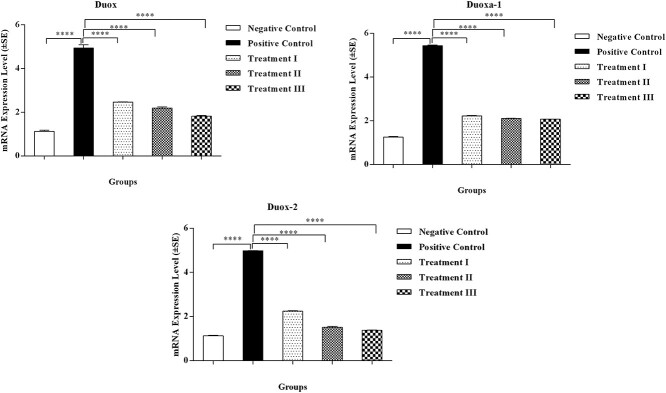
Effect of OFI fruit extract on dual oxidases cascade
Dual oxidases (DUOXs) pathway generates free radicals such as hydrogen peroxide via moving electrons from intracellular NADPH toward extracellular oxygen. They carry out several essential biological processes and are involved in various human diseases, especially thyroid diseases.30 In the current study, the expression level of Duox, Duoxa-1, and Duox-2 genes was analyzed for the activation of dual oxidases. The expression level of Duox, Duoxa-1, and Duox-2 genes showed a significant (P < 0.001) difference among control (positive and negative) groups (Table 3Fig. 4).
Fig. 4.
mRNA expression level of Duox, Duoxa-1, and Duox-2 genes (±SE) of negative control group (NC), positive control group (45% HFD), treatment I group (SD + 45% HFD), treatment II group (OFI; 600 mg/kg + 45% HFD), and treatment III group (OFI; 800 mg/kg + 45% HFD). Data are presented as mean ± SEM and P ≤ 0.05.
The effect of OFI fruit extract on Nrf-2 signaling cascade
Nrf2 (nuclear factor erythroid 2-related factor 2) is actively involved in the regulation of cellular resistance toward oxidants. It regulates the basal and induced expression of some genes stimulated in response to antioxidant production to regulate the pathophysiological effects of oxidant exposure.31 The expression level of Nfe 212, NFR1, and Keap-1 genes was analyzed for the Nrf-2 signaling pathway. The expression level of Nfe 212, NFR1, and Keap-1 genes showed a significant (P < 0.001) difference among the control (positive and negative) groups. As the expression level of Nfe 212, NFR1, and Keap-1 genes was analyzed among treatment I, treatment II, and treatment III groups, a statistically significant (P < 0.05) down expression was obtained in treatment groups in comparison to the positive control group (Fig. 5).
Fig. 5.
mRNA expression level of Nfe-212, NFR-1, and Keap-1 gene (±SE) of negative control group (NC), positive control group (45% HFD), treatment I group (SD + 45% HFD), treatment II group (OFI; 600 mg/kg + 45% HFD), and treatment III group (OFI; 800 mg/kg + 45% HFD).
Effect of OFI fruit extract on aortic histopathology
Histopathology of the aortic tissue for the negative control group showed normal architecture, size, parenchyma, and normal morphological posture. All the layers, e.g. tunica media (TM), tunic adventitia (TA), and tunic intima (TI) were visible with normal appearance and diameter. The positive control group demonstrated proliferation in the tunica intima layer, disorientation of smooth muscle cells with undefined borders, and loss of elastic lamellae architecture when compared with negative control group. This micrograph also showed markedly increased endothelial lining and thickness of tunica media and tunica adventitia with the irregular medial segment having wavy laminae. Increased wall thickness is attributed to increased density of tunica media smooth muscle nuclei and in numbers of elastin bands, while aortic histology of treatment groups showed mild rupture of the intima and mild diffused thickening and fragmentation of media. Opuntia ficus indica fruit extract at low and high doses prevented the thickening of the endothelium, repairing the tissue and collagen accumulation (Fig. 6).
Fig. 6.
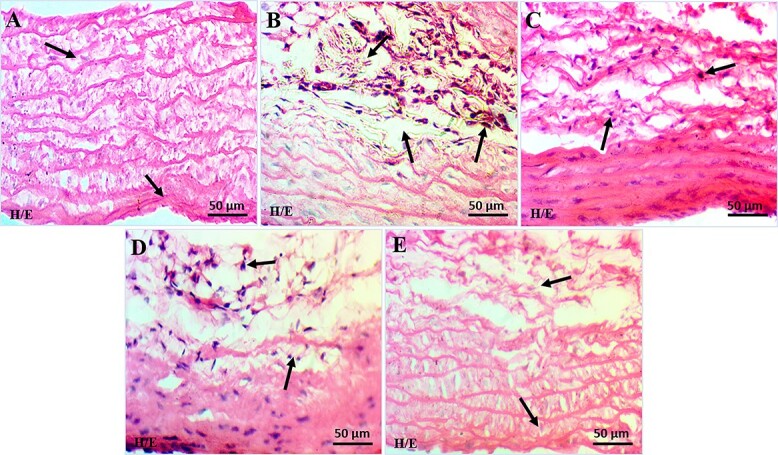
Photomicrographs showing histopathology of aortic tissue (H&E staining; 40X); a) negative control group (NC); b) positive control group (45% HFD); c) Treatment I group (SD + 45% HFD); d) Treatment II group (OFI; 600 mg/kg + 45% HFD); e) Treatment III group (OFI; 800 mg/kg + 45% HFD).
Discussion
In recent decades, the incidence of CVDs has increased to such an extent and has become the principal cause of death around the globe. Atherosclerosis among CVDs has acquired higher morbidity and mortality rates, particularly in high- and intermediate-income countries.3 Atherosclerosis is a multifactorial disease and is widely linked to lifestyle and dietary habits. Several research groups are investigating the possible role of food and dietary supplements that may prevent, mitigate, or even reverse atherosclerosis including hyperlipidemia.32 The current study revealed the effects of an HFD on different oxidative pathophysiological aspects specifically during atherosclerosis. In vitro analysis of OFI fruit extract, acute oral toxicity testing, antioxidant enzymes, and gene expression level for oxidative and antioxidants markers were performed to evaluate the lipid-lowering and preventive or mitigating properties of OFI fruit extract in pathophysiological alterations in an experimental model of HFD-associated atherosclerosis.
Medicinal plants and natural products serve as a significant source of therapeutic efficacy for disease prevention as well as high nutritive values for sustaining human life.33 In recent years, the valorization of under-utilized food for its antioxidant potential has gained great attention.34 Results revealed that the crude powder of OFI fruit extract has shown significant moisture contents, which can contribute to maintaining body fluids.35 Carbohydrates are essential elements in diet and produce significant energy to fulfill body requirements. The estimated carbohydrate contents of OFI fruit extract were high and provide energy for the proper functioning of vital organs especially the brain.36 Proteins are the essential source for the biosynthesis of structural and functional components of the body, including fluid homeostasis, body development, synthesis of enzymes, and hormones, and sustaining the immune system. Similarly, crude fibers also help in the absorption of many trace elements and assist in the removal of waste materials that reveal the nutritional value of a plant. Fats provide adequate energy (~1–2%) of the human requirement.37 Study results showed high protein, fat, and crude fiber contents in OFI fruit extract. OFI fruit extract lowers the triglycerides, and cholesterol levels and protects potentially maintaining a healthy weight, gut health, managing constipation, hemorrhoids, coronary heart diseases, cancer, and glucose modulatory mechanism.38 Phytochemical screening of OFI fruit extract showed the presence of phytoconstituents, including alkaloids, glycosides, carbohydrates, flavonoids, phenolic compounds, phlobatannins, and quinones, saponins, proteins, and fixed oil. Bioactive compounds include quercetin, ferulic acid, gallic acid, benzoic acid, cinnamic acid, chlorogenic acid, caffeic acid, m-coumaric acid, vanillic acid, p-coumaric acid, sinapic acid, and syringic acid. Flavonoids and phenols are considered valuable due to their attributed antioxidant potential. Free radical scavenging property of flavonoids and phenolic contents increase their importance under oxidative stress, cardiac, and inflammatory disorders.35 Mineral analysis depicted that the fruits are a good natural reservoir of essential minerals such as Mg, Ca, and K.39
Antioxidants have been considered the key inhibitors for both the initiation and propagation of oxidizing chain reactions or delaying the oxidative process. In every organism, certain antioxidants and enzyme systems exist for their protection against oxidative stress, i.e. catalase (CAT), glutathione reductase (GR), glutathione peroxidase (GPX), and superoxide dismutase (SOD). Therefore, antioxidant supplements or diets possessing antioxidants contribute to reducing oxidative damage through free radical scavenging activity.40 Ferric ion reducing antioxidant power assay (FRAP) and DPPH free radical scavenging activity assay were performed for possible antioxidant activity. The in-vitro DPPH assay revealed that OFI fruit extract exhibited 69.72% of the free radical scavenging properties. The in-vitro FRAP assay showed that the OFI fruit extract demonstrated promising free radical scavenging properties over the range of 1.47 nmol Fe2+ eq. mg−1 extract and similar findings were reported earlier.41 In vitro, antioxidant assays suggested that OFI fruit extract possesses bioactive compounds correlated to their antioxidant potential.
A HFD is responsible for atherosclerotic plaque formation. Results revealed that HFD significantly increased TC, very-LDL cholesterol, and triglycerides levels, while HDL levels were lowered and key responsible for the development of atherosclerosis as evidenced by previous researchers.42–45 LDL and VLDL are major atherosclerotic plaque formation inducers and are responsible for lipid peroxidation in coronary arteries.46 Current study results showed that the main determinants of atherosclerosis development are triglycerides, TC levels, and VLDL cholesterol which were significantly decreased in treatment I, II, and III groups in comparison to the positive control group. Results illustrated that treatment III had strong lipid-lowering properties and ameliorated hepatic lipid accumulation.
Oxidative stress due to the generation of ROS and decline in innate antioxidant defense systems47 alter the cell growth, vascular tone, inflammatory responses, apoptosis, and LDL-cholesterol oxidation, and it holds native-LDL in atherogenesis. Total oxidative stress and total antioxidant levels were significantly altered (P < 0.05) among the control negative and control positive groups, while a significant level of both TOS and TAC was observed among all the treatment groups.
Dual oxidases belong to the family of NADPH oxidases and are mainly involved in the production of ROS such as H2O2 outside the cells and functional components of the innate host defense system.48,49 The mRNA expression levels of the Nrf-2 signaling cascade and dual oxidases were significantly expressed and cell stress pathways are known to be responsible for apoptosis.50 Earlier studies have confirmed the fact that dietary modulation remains the key reason behind the induction of oxidative stress and affects the development of metabolic syndrome.51 The current study showed a significantly (P < 0.05) higher expression level of Duox, Duoxa-1, and Duox-2 genes in positive control in comparison to the negative control groups. While the expression level of Duox, Duoxa-1, and Duox-2 genes among treatments I, II, and III, a statistically significant (P < 0.05) down expressed in all the treatment groups in comparison with the positive control group.
Transcription factor Nrf2 is involved in the regulation of the expression of phase II detoxifying enzymes including NADPH, ferritin, glutathione peroxidase, NADPH quinone oxidoreductase 1, heme oxygenase-1 (HO−1), and antioxidant genes that possess anti-inflammatory effects and protect the cells from different injuries.52 Nrf2 plays a central role in drug metabolism, the response to oxidative stress as well as involved in the development of atherosclerosis. Nrf2 signaling modulates numerous physiological and pathophysiological processes such as foam cell formation, lipid homeostasis regulation, redox regulation, macrophage polarization, and inflammation during atherosclerosis progression. Interestingly, Nrf2 displays pro as well as anti-atherogenic effects in experimental animal models.53 These findings together underscore the cardioprotective effect of the Nrf2 signaling pathway. Considering that high-fat dieting can elevate ROS in the endothelial cells, it is conceivable that dysregulation of Nrf2 signaling may mediate the role of high-fat dieting in triggering endothelial disruption. Results revealed the analysis of Nef 212, NFR1, and Keap-1 genes for the possible involvement of Nrf-2 signaling pathway. Results indicated that the expression level of Nef 212, NFR1, and Keap-1 genes showed significant (P < 0.05) differences among positive and negative control groups. High-fat dieting has shown activation of Nrf-2 signaling genes which strengthen oxidative stress production. While the expression level of Nef 212, NFR1, and Keap-1 genes among treatments (I, II, and III), a statistically significant (P < 0.05) down expression was observed in comparison to the positive control group which strengthened that antioxidant defense system has been activated due to the activation of Nrf2 signaling genes. Hence, the given results suggest that the OFI fruit extract exerts good antioxidant efficacy through Nrf2 signaling pathway upregulation.
Histopathological examination of the aorta showed that HFD markedly increased the thickening of endothelial lining and thickness of tunica media and tunica adventitia with irregular media segments having wavy laminae. Tunica adventitia showed a significant increase in entropy of fibers disposition in the positive control group.42,54–57 While histopathological examination for both the treatments II and III groups showed mild rupture of the intima and mild diffused thickening and fragmentation of media. Our results revealed that Opuntia ficus indica fruit extract showed a regenerative and preventive role as less accumulation of high-fat in the endothelium. Mild disorder and stretching of tunica intima layer and disorientation of smooth muscle cells with minor disturbances in the elastic lamellae architecture were observed while compared with positive control group. The results suggest that fruit extracts may prevent the initial development of vascular disease by modulating cardiovascular risk factors.
In summary, based on the results of serum analysis, histopathological examination, and genes expression analysis, it was concluded that OFI fruit extract has antioxidant, anti-inflammatory, and athero-protective potential under HFD-associated pathophysiological alterations in atherosclerosis.
Conclusion
The current study provides scientific evidence of anti-oxidative, anti-inflammatory underlying mechanisms as well as hypolipidemic properties of the OFI fruit extract in an HFD associated atherosclerotic rat model. Nrf2 and dual oxidases signaling pathways were used as pharmacological targets to analyze the possible upregulation of antioxidant defense enzymes of endothelial cells against HFD-induced oxidative stress. Our results demonstrated that OFI extract acts as a modifier of signaling cascades to excite cytoprotective responses by inhibiting the activation of stress-induced proteins and also increasing the dissociation of Keap1 from the Nrf2 signal transduction pathway in response to oxidative stress. Therefore, the OFI fruit extract can be effectively used for the prevention of oxidative stress and treatment of HFD-induced inflammatory responses and oxidative stress-induced cellular damage. However, further investigations on Opuntia ficus indica are needed to better understand its pharmacological and physiological mechanisms of action to provide clear scientific proof regarding its traditional uses, also to identify its therapeutic potential, particularly in the field of chronic diseases.
Acknowledgments
The authors are also thankful to the Institute of Physiology and Pharmacology, the University of Agriculture for providing the technical support.
Contributor Information
Noreen Aslam, Institute of Physiology and Pharmacology, Faculty of Veterinary Science, University of Agriculture, Faisalabad-38040, Pakistan.
Muhammad Naeem Faisal, Institute of Physiology and Pharmacology, Faculty of Veterinary Science, University of Agriculture, Faisalabad-38040, Pakistan.
Junaid Ali Khan, Institute of Physiology and Pharmacology, Faculty of Veterinary Science, University of Agriculture, Faisalabad-38040, Pakistan.
Wafa Majeed, Department of Pharmacy, Faculty of Veterinary Science, University of Agriculture, Faisalabad-38040, Pakistan.
Funding
There are no funding sources for this manuscript.
Conflict of interest statement: There are no competing interests in this manuscript.
Author’s contribution
NA: Conceptualization, investigation, writing—original draft preparation, methodology, software, funding acquisition, data curation, formal analysis.
MNF: Conceptualization, supervision, project administration, resources, writing—review & editing, methodology, validation.
JAK: Supervision, Investigation, methodology, writing—reviewing, and editing.
WM: Supervision, Investigation, software, writing—reviewing, and editing.
Data availability
The dataset used and analyzed during the current study is available as supplementary material.
Ethical approval
The study protocols were approved by the Ethical Standards of Animal Care and Institutional Bioethical Committee (IBC) of UAF (D. No. 3668/ORIC). All animals were cared for and treated according to the NIH guidelines for the care and use of laboratory animals (NIH Publication No. 85–23, revised 2002).
Patient consent
Not applicable.
Permission to reproduce material from other sources
Not applicable.
References
- [1]. Li B, Xia Y, Hu B. Infection and atherosclerosis: TLR-dependent pathways. Cell Mol Life Sci. 2020:77(14):2751–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Milutinović A, Šuput D, Zorc-Pleskovič R. Pathogenesis of atherosclerosis in the tunica intima, media, and adventitia of coronary arteries: an updated review. Bosn J Basic Med Sci. 2020:20(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Wu M, Liu L, Xing Y, Yang S, Li H, Cao Y. Roles and mechanisms of hawthorn and its extracts onatherosclerosis: a review. Front Pharmacol. 2020:11(2):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Sun Q, Liu Y, Teng X, Luan P, Teng X, Yin X. Immunosuppression participated in complement activation-mediatedinflammatory injury caused by 4-octylphenol via TLR7/IκBα/NF-κB pathway incommon carp (Cyprinus carpio) gills. Aquat Toxicol. 2022:249:106211. [DOI] [PubMed] [Google Scholar]
- [5]. Miao Z, Zhang K, Bao R, Li J, Tang Y, Teng X. Th1/Th2 imbalance and heat shock protein mediated inflammatory damage triggered by manganese via activating NF-κB pathway in chicken nervous system in vivo and in vitro. Environ Sci Pollut Res. 2021:28(32):44361–44373. [DOI] [PubMed] [Google Scholar]
- [6]. Li Z, Shah SWA, Zhou Q, Yin X, Teng X. The contributions of miR-25-3p, oxidative stress, and heat shock protein in a complex mechanism of autophagy caused by pollutant cadmium in common carp (Cyprinus carpio L.) hepatopancreas. Environ Pollut. 2021:287:117554. [DOI] [PubMed] [Google Scholar]
- [7]. Liu Y, Yu M, Cui J, Du Y, Teng X, Zhang Z. Heat shock proteins took part in oxidative stress-mediated inflammatory injury via NF-κB pathway in excess manganese-treated chicken livers. Ecotoxicol Environ Saf. 2021:226:112833. [DOI] [PubMed] [Google Scholar]
- [8]. Milagro FI, Campión J, Martínez JA. Weight gain induced by high-fat feeding involves increased liver oxidative stress. Obes. 2006:14(7):1118–1123. [DOI] [PubMed] [Google Scholar]
- [9]. Noeman SA, Hamooda HE, Baalash AA. Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetol Metab Syndr. 2011:3(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Lee MT, Lin WC, Yu B, Lee TT. Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals—a review, Asian-australas. J Anim Sci. 2017:30(3):299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Elrashidy RA. Dysregulation of nuclear factor erythroid 2-related factor 2 signaling and activation of fibrogenic pathways in hearts of high fat diet-fed rats. Mol Biol Rep. 2020:47(4):2821–2834. [DOI] [PubMed] [Google Scholar]
- [12]. DeVallance E, Li Y, Jurczak MJ, Cifuentes-Pagano E, Pagano PJ. The role of NADPH oxidases in the etiology of obesity and metabolic syndrome: contribution of individual isoforms and cell biology. Antioxid Redox Signal. 2019:31(10):687–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Petheő GL, Kerekes A, Mihálffy M, Donkó A, Bodrogi L, Skoda G, Baráth M, Hoffmann OI, Szeles Z, Balázs B, et al. Disruption of the NOX5 gene aggravates atherosclerosis in rabbits. Circ Res. 2021:128(9):1320–1322. [DOI] [PubMed] [Google Scholar]
- [14]. Belguith-Hadriche O, Ammar S, Contreras MDM, Turki M, Segura-Carretero A, Makni-Ayedi F, Bouaziz FM. Antihyperlipidemic and antioxidant activities of edible Tunisian Ficus carica L. fruits in high fat diet-induced hyperlipidemic rats. Plant Foods Hum Nutr. 2016:71(2):183–189. [DOI] [PubMed] [Google Scholar]
- [15]. Elsawi SA, Radwan RR, Elbatanony MM, El-Feky AM, Sherif NH. Prophylactic effect of opuntia ficus indica fruit peel extract against irradiation-induced colon injury in rats. Planta Med. 2020:86(01):61–69. [DOI] [PubMed] [Google Scholar]
- [16]. Ammar I, Ennouri M, Attia H. Phenolic content and antioxidant activity of cactus (Opuntia ficus-indica L.) flowers are modified according to the extraction method. Ind Crop Prod. 2015:64:97–104. [Google Scholar]
- [17]. El-Hawary SS, Sobeh M, Badr WK, Abdelfattah MA, Ali ZY, El-Tantawy ME, Rabeh MA, Wink M. HPLC-PDAMS/MS profiling of secondary metabolites from Opuntia ficus-indica cladode, peel and fruit pulp extracts and their antioxidant, neuroprotective effect in rats with aluminum chloride induced neurotoxicity. Saudi J Biol Sci. 2020:27(10):2829–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Barba FJ, Garcia C, Fessard A, Munekata PE, Lorenzo JM, Aboudia A, Ouadia A, Remize F. Opuntia ficus indica edible parts: a food and nutritionalsecurity perspective. Food Rev Int. 2020:38(5):930–952. [Google Scholar]
- [19]. Septembre-Malaterre A, Remize F, Poucheret P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: changes in bioactive compounds during lactic fermentation. Int Food Res J. 2018:104:86–99. [DOI] [PubMed] [Google Scholar]
- [20]. Gharbi S, Renda G, La Barbera L, Amri M, Messina CM, Santulli A. Tunisian tomato by-products, as a potential source of natural bioactive compounds. Nat Prod Res. 2017:31(6):626–631. [DOI] [PubMed] [Google Scholar]
- [21]. Ammar I, Ennouri M, Bouaziz M, Amira AB, Attia H. Phenolic profiles, phytchemicals and mineral content of decoction and infusion of Opuntia ficus-indica flowers. Plant Foods Hum Nutr. 2015:70(4):388–394. [DOI] [PubMed] [Google Scholar]
- [22]. Minhaj M, El Jemli Y, Taourirte M, BOUYAZZA L. Preliminary phytochemical screening, total phenolic, flavonoids and polysaccharides contents and antioxidant capacity of aqueous and hydroalcoholic extracts of Opuntia ficus-barbarica flowers. J Mater Environ Sci. 2019:10:1369–1381. [Google Scholar]
- [23]. Zourgui MN, Hfaiedh M, Brahmi D, Affi W, Gharsallah N, Zourgui L, Amri M. Phytochemical screening, antioxidant and antimicrobial activities of Opuntia streptacantha fruit skin. J Food Meas Charact. 2020:14(5):2721–2733. [Google Scholar]
- [24]. Clarke G, Ting KN, Wiart C, Fry J. High correlation of 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging, ferric reducing activity potential and total phenolics content indicates redundancy in use of all three assays to screen for antioxidant activity of extracts of plants from the Malaysian rainforest. Antioxidants. 2013:2(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Ghareeb MA, Mohamed T, Saad AM, Refahy LAG, Sobeh M, Wink M. HPLC-DAD-ESI-MS/MS analysis of fruits from Firmiana simplex (L.) and evaluation of their antioxidant and antigenotoxic properties. J Pharm Pharmacol. 2018:70(1):133–142. [DOI] [PubMed] [Google Scholar]
- [26]. Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004:37(2):112–119. [DOI] [PubMed] [Google Scholar]
- [27]. Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005:38(12):1103–1111. [DOI] [PubMed] [Google Scholar]
- [28]. Roghelia V, Panchal J. Antioxidant capacity of cactus pear fruit, world. J Pharm Res. 2016:5(5):1298–1307. [Google Scholar]
- [29]. Ghasi S, Nwobodo E, Ofili JO. Hypocholesterolemic effects of crude extract of leaf of Moringa oleifera lam in high-fat diet fed Wistar rats. J Ethnopharmacol. 2000:69(1):21–25. [DOI] [PubMed] [Google Scholar]
- [30]. Wu JX, Liu R, Song K, Chen L. Structures of human dual oxidase 1 complex in low-calcium and high-calcium states. Nat Commun. 2021:12(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Ma Q. The expanding role of mitochondria in apoptosis. Annu Rev Pharmacol Toxicol. 2013:53:401–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Wei T, Liu J, Zhang D, Wang X, Li G, Ma R, Chen G, Lin X, Guo X. The relationship between nutrition and atherosclerosis. Front Bioeng Biotechnol. 2021:9:635504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Boutakiout A, Elothmani D, Hanine H, Mahrouz M, Le Meurlay D, Hmid I, Ennahli S. Effects of different harvesting seasons on antioxidant activity and phenolic content of prickly pear cladode juice. J Saudi Soc Agric Sci. 2018:17(4):471–480. [Google Scholar]
- [34]. Prasad KN, Kong KW, Ramanan RN, Azlan A, Ismail A. Selection of experimental domain using two-level factorial design to determine extract yield, antioxidant capacity, phenolics, and flavonoids from Mangifera pajang Kosterm. Sep Sci Technol. 2012:47(16):2417–2423. [Google Scholar]
- [35]. Angulo-Bejarano PI, Martínez-Cruz O, Paredes-López O. Phytochemical content, nutraceutical potential and biotechnological applications of an ancient Mexican plant: nopal (Opuntia ficus-indica). Curr Nutr Food Sci. 2014:10(3):196–217. [Google Scholar]
- [36]. Ejelonu BC, Lasisi AA, Olaremu AG, Ejelonu OC. The chemical constituents of calabash (Crescentia cujete). Afr J Biotechnol. 2011:10(84):19631–19636. [Google Scholar]
- [37]. Emebu PK, Anyika JU. Proximate and mineral composition of kale (Brassica oleracea) grown in Delta state, Nigeria. Pak J Nutr. 2011:10(2):190–194. [Google Scholar]
- [38]. Madhu C, Krishna KM, Reddy KR, Lakshmi PJ, Kelari EK. Estimation of crude fibre content from natural food stuffs and its laxative activity induced in rats. Int J Pharma Res Health Sci. 2017:5(3):1703–1706. [Google Scholar]
- [39]. Chiteva R, Wairagu N. Chemical and nutritional content of Opuntia ficus-indica (L.). Afr J Biotechnol. 2013:12(21):3309–3312 [Google Scholar]
- [40]. Shah P, Modi HA. Comparative study of DPPH, ABTS and FRAP assays for determination of antioxidant activity, Int. J. Res. Appl. Sci. Eng Technol. 2015:3(6):636–641. [Google Scholar]
- [41]. El-Guezzane C, El-Moudden H, Harhar H, Chahboun N, Tabyaoui M, Zarrouk A. A comparative study of the antioxidant activity of two Moroccan prickly pear cultivars collected in different regions. Chem Data Collect. 2021:31:100637. [Google Scholar]
- [42]. Subramani C, Rajakkannu A, Rathinam A, Gaidhani S, Raju I, Singh DVK. Anti-atherosclerotic activity of root bark of Premna integrifolia Linn. In high fat diet induced atherosclerosis model rats. J Pharm Anal. 2017:7(2):123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Ai C, Duan M, Ma N, Sun X, Yang J, Wen C, Sun Y, Zhao N, Song S. Sulfated polysaccharides from pacific abalone reduce diet-induced obesity by modulating the gut microbiota. J Funct Foods. 2018:47:211–219. [Google Scholar]
- [44]. Shrivastava AK, Thapa S, Shrestha L, Mehta RK, Gupta A, Koirala N. Phytochemical screening and the effect of Trichosanthesdioica in high-fat diet induced atherosclerosis in Wistar rats. Food Frontiers. 2021:2(4):527–536. [Google Scholar]
- [45]. Geng X, Liu H, Yuwen Q, Wang J, Zhang S, Zhang X, Sun J. Protective effects of zingerone on high cholesteroldiet-induced atherosclerosis through lipid regulatory signaling pathway. Hum Exp Toxicol. 2021:40(10):1732–1745. [DOI] [PubMed] [Google Scholar]
- [46]. Zhang J, Almoallim HS, Alharbi SA, Yang B. Anti-atherosclerotic activity of Betulinic acid loaded polyvinyl alcohol/methylacrylate grafted lignin polymer in high fat diet induced atherosclerosis model rats. Arab J Chem. 2021:14(2):102934. [Google Scholar]
- [47]. Kattoor AJ, Pothineni NVK, Palagiri D, Mehta JL. Oxidative stress in atherosclerosis. Curr Atheroscler Rep. 201718:19(11):42. [DOI] [PubMed] [Google Scholar]
- [48]. Willenberg HS, Schott M. Activation of dual oxidases Duox1 and Duox2: differential regulation mediated by camp-dependent protein kinaseand protein kinase C-dependent phosphorylation Rigutto S, Hoste C, GrasbergerH, et al (Université Libre de Bruxelles, Belgium; Univ of Chicago, IL) J Biol Chem, 2009:284:6725–6734. Year Book of Endocrinology. 2010:195–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Sirokmány G, Donkó A, Geiszt M. Nox/Duox family of NADPH oxidases: lessons from knockout mouse models. Trends Pharmacol Sci. 2016:37(4):318–327. [DOI] [PubMed] [Google Scholar]
- [50]. Bhattacharya S, Manna P, Gachhui R, Sil PC. D-saccharic acid-1, 4-lactone ameliorates alloxan-induced diabetes mellitus and oxidative stress in rats through inhibiting pancreatic beta-cells from apoptosis via mitochondrial dependent pathway. Toxicol Appl Pharmacol. 2011:257(2):272–283. [DOI] [PubMed] [Google Scholar]
- [51]. Meza-Miranda ER, Camargo A, Rangel-Zuñiga OA, Delgado-Lista J, Garcia-Rios A, Perez-Martinez P, Tasset-Cuevas I, Tunez I, Tinahones FJ, Perez-Jimenez F, et al. Postprandial oxidative stress is modulated by dietary fat in adipose tissue from elderly people. Age. 2014:36(2):507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52]. Ahmed SMU, Luo L, Namani A, Wang XJ, Tang X. Nrf2 signaling pathway: pivotal roles in inflammation. Biochim Biophys Acta Mol basis Dis. 2017:1863(2):585–597. [DOI] [PubMed] [Google Scholar]
- [53]. Mimura J, Itoh K. Role of Nrf2 in the pathogenesis of atherosclerosis. Free Radic Biol Med. 2015:88:221–232. [DOI] [PubMed] [Google Scholar]
- [54]. Jayachandran M, Chandrasekaran B, Namasivayam N. Effect of geraniol, a plant derived monoterpene on lipids and lipid metabolizing enzymes in experimental hyperlipidemic hamsters. Mol Cell Biochem. 2015:398(1):39–53. [DOI] [PubMed] [Google Scholar]
- [55]. Safi MM, Abou-Nazel MW, Karawya FS, Omar AM. The possible protective effects of inegy versus cinnamon oil on the aorta of albino rats with experimentally induced hyperlipidemia. Int J Clin Exp Med. 2016:1(4):78–91. [Google Scholar]
- [56]. Shatoor AS, Al Humayed S, Alkhateeb MA, Shatoor KA, Aldera H, Alassiri M, Shati AA. Crataegus Aronia protects and reverses vascular inflammation in a high fat diet rat model by an antioxidant mechanism and modulating serum levels of oxidized low-density lipoprotein. Pharm Biol. 2019:57(1):37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57]. Chaiwong S, Chatturong U, Chanasong R, Deetud W, To-on K, Puntheeranurak S, Chulikorn E, Kajsongkram T, Raksanoh V, Chinda K, et al. Dried mulberry fruit ameliorates cardiovascular and liver histopathological changes in high-fat diet-induced hyperlipidemic mice. J Tradit Complement Med. 2021:11(4):356–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset used and analyzed during the current study is available as supplementary material.