Abstract
Mice immunized with either the predominantly vector-stage lipoprotein outer surface protein A (OspA) or the in vivo-expressed lipoprotein decorin binding protein A (DbpA) are protected against Borrelia burgdorferi challenge. DbpA-OspA combinations protected against 100-fold-higher challenge doses than did either single-antigen vaccine and conferred significant protection against heterologous B. burgdorferi, B. garinii, and B. afzelii isolates, suggesting that there is synergy between these two immunogens.
Lyme disease (20), or Lyme borreliosis, is a tick-borne illness of humans and domestic animals caused by at least three antigenically diverse species of spirochetes (Borrelia burgdorferi sensu stricto, B. garinii, and B. afzelii) classified collectively as B. burgdorferi sensu lato. Recent clinical trials showed that monovalent recombinant subunit vaccines composed of the B. burgdorferi outer surface protein A (OspA) lipoprotein were efficacious through two Lyme disease transmission seasons (19, 21).
At the start of feeding, B. burgdorferi spirochetes in ticks are highly vulnerable to OspA antibodies imbibed from immunized hosts (9), but after adaptation to the mammalian host environment following natural or experimental inoculation, most spirochetes down-regulate OspA expression and become resistant to OspA antibodies (3, 5, 9, 14). Protection from tick-borne transmission of B. burgdorferi appears to depend on OspA immunization achieving a critical threshold level of circulating antibodies prior to the tick bite (8).
The addition of mammalian-host-stage antigens may extend the duration, or enhance the level, of protective efficacy of transmission-blocking OspA vaccines against tick-borne Lyme borreliosis. Decorin binding protein A (DbpA) is another B. burgdorferi surface-exposed lipoprotein that has shown vaccine efficacy against experimental infection in the mouse model (5, 10, 13, 14). B. burgdorferi continues to express DbpA, but not OspA, after dermal inoculation and remains vulnerable to DbpA antibodies during the early stages of local and disseminating infection in mice (5, 14); additionally, DbpA is immunogenic during human Lyme disease (6). OspC (25) and other antigens (1, 11) on mammalian-host-adapted B. burgdorferi also represent potential targets for protective or disease-resolving antibodies. We compared the protective efficacies of DbpA and OspA, singly and in combination, against dermal challenge of mice as a first step in the evaluation of second-generation DbpA-OspA combination vaccines for Lyme disease.
The recombinant fusion lipoproteins Lpp2:OspAN40 (OspAN40) and Lpp2:DbpAN40(His)6 (DbpAN40) were used as vaccine antigens and had been previously described (5, 14). A detergent extract of Escherichia coli membrane proteins (5) was used as a negative-control antigen preparation.
In one vaccine experiment, four groups of 20 female 7-week-old C3H/HeJ mice (The Jackson Laboratory, Bar Harbor, Maine) were immunized by intraperitoneal injection of 10 μg of DbpAN40, 10 μg of OspAN40, 5 μg of DbpAN40 plus 5 μg of OspAN40, or 2.5 μg of E. coli protein extract with complete Freund's adjuvant and then, 4 weeks later, were given a second immunization of protein in incomplete Freund's adjuvant. At week 6, five of the mice in each immunization group were challenged by subcutaneous injection, into the dorsolateral thorax, of cloned B. burgdorferi N40 (2) from an exponentially growing culture diluted with BSKII medium (14) to give escalating doses of 103, 104, 105, or 106 spirochetes in 0.1-ml volumes. Other experiments used vaccines prepared by adsorbing antigens to the aluminum hydroxide adjuvant Alhydrogel (Superfos Biosector, Kvistgård, Denmark). Mice were immunized by subcutaneous injection of 0.1 ml of vaccine at weeks 0, 4, and 8 and challenged with spirochetes at week 10. A challenge dose of 104 was used for B. burgdorferi N40 and Sh-2-82; a dose of 105 was used for B. garinii G25 and B. afzelii IPF. The median infective doses for these isolates were determined to be approximately 3 × 102 for N40 (14), 6 × 102 for Sh-2-82 (14), 3 × 103 for G25, and 2 × 104 for IPF. Two weeks after challenge, the mice were killed by CO2 asphyxiation and samples of the inoculation site skin, blood, ear, urinary bladder, and both tibiotarsal joints were cultured in BSKII plus antibiotics to detect spirochetal infection (14).
An enzyme-linked immunosorbent assay was used as previously described (14) to determine the prechallenge DbpA and OspA immunoglobulin G (IgG) endpoint titers of antisera from individual mice and antisera pooled from mice within each immunization group. The borreliacidal activity of the prechallenge antisera was determined, in a microtiter plate format, as the dilution of antiserum from each individual mouse giving 50% growth inhibition by a [3H]adenine metabolic labeling method (15) or as the dilution of pooled antiserum giving a >90% reduction in spirochete numbers (14).
Antigen expression by the spirochetes was evaluated by a direct immunofluorescence assay using combined DbpAN40- and OspAB31 (14)-specific purified rabbit polyclonal IgG antibodies conjugated with the fluorochromes Alexa 546 and Alexa 488, respectively, according to the manufacturer's protocol (Molecular Probes, Inc., Eugene, Oreg.). Double-labeled slides were viewed at 1,000× magnification, using a Nikon E600 epifluorescence microscope (Nikon, Melville, N.Y.), and images were acquired with a Sony DKC-5000 digital photo camera (Sony Electronics, Inc., Park Ridge, N.J.).
DbpA and OspA in combination protect against higher B. burgdorferi challenge doses than single-antigen vaccines.
The possibility that dual immunity to both DbpA and OspA could provide more effective protection than either single antigen alone was addressed in two complementary ways. First, we attempted to exceed the B. burgdorferi challenge dose at which the single antigens provided protection, and second, we asked whether DbpA-OspA combinations provided protection at a lower vaccine dose that was ineffective for either single immunogen. Nearly all mice immunized with 10 μg of either DbpAN40 or OspAN40 were protected from challenge with 103 or 104 spirochetes (Table 1), as expected from our earlier observations (14). At a challenge dose of 105 or 106 spirochetes, mice immunized with DbpAN40 or OspAN40 alone were protected only partially or not at all. In contrast, all mice immunized with the combined DbpAN40-OspAN40 vaccine (5 μg of each antigen) were protected against even the highest challenge dose. The 106 challenge inoculum is at least 3,000 times higher than the median infectious dose for this B. burgdorferi strain (14). At each challenge dose, all mice vaccinated with the E. coli extract were infected, with at least three of the five tissues tested being culture positive for B. burgdorferi. The antisera from all mice immunized with either DbpAN40 or OspAN40, or the combination of both proteins, inhibited the in vitro growth of B. burgdorferi N40, and antisera from E. coli-immunized mice were not borreliacidal. The in vitro killing potency of the antisera from DbpAN40-immunized mice was about 20-fold lower than that of OspAN40, probably because DbpA is expressed at much lower levels than OspA in vitro (5, 14). Interestingly, the potency of OspAN40 antisera was not significantly different from that of the combined vaccine (P = 0.43, Student's two-tailed t test) in this in vitro assay. Our observations clearly showed that the in vitro and in vivo potencies of DbpAN40 and OspAN40 antibodies were divergent and that surrogate in vitro assays were inadequate to predict the relative effectiveness of single-antigen and combined-antigen vaccines. The enhanced in vivo activity of DbpAN40 antibodies is likely due to prolonged or increased vulnerability of the spirochetes to DbpAN40 antibodies (5, 14), the potentiating effects of immune effector functions toward DbpAN40 but not OspAN40 antibodies, or a combination of the two effects.
TABLE 1.
Comparison of the immunogenicities and in vitro potencies of antisera from mice vaccinated with DbpAN40 and OspAN40, singly and in combination, and their protective efficacies against challenge with escalating B. burgdorferi N40 doses
| Immunogen(s) (dose) | Antiserum geometric mean titera
|
No. of mice protected/total no. challenged at challenge dose:
|
|||||
|---|---|---|---|---|---|---|---|
| DbpA IgG | OspA IgG | Causing 50% growth inhibition | 103 | 104 | 105 | 106 | |
| DbpA (10 μg) | 1,910,852 | 500 | 2,263 | 0/5b | 0/5b | 2/5 | 2/5 |
| OspA (10 μg) | 966 | 4,389,984 | 67,559 | 0/5b | 1/5b | 3/5 | 5/5 |
| DbpA (5 μg) + OspA (5 μg) | 2,048,000 | 4,389,984 | 53,006 | 0/5b | 0/5b | 0/5b | 0/5b |
| E. coli extract (2.5 μg) | 3,364 | 27,858 | <50c | 5/5 | 5/5 | 5/5 | 5/5 |
Values are the geometric means of data for the 20 mice in each immunization group.
P < 0.05 versus controls (Fisher's exact test).
No inhibition at lowest dilution tested (1:50).
DbpA and OspA in combination are more effective than single-antigen vaccines against heterologous B. burgdorferi sensu lato isolates.
Next, mice were immunized with single-antigen or DbpAN40-OspAN40 combination vaccines formulated with Alhydrogel, an adjuvant approved for use in humans. Antisera from mice immunized with either 1.0- or 10-μg doses inhibited the in vitro growth of B. burgdorferi N40, and again, DbpAN40 antiserum was less potent for killing in vitro than antisera against OspAN40 or the DbpAN40-OspAN40 combination vaccine (Table 2). Mice were well protected against challenge with a 104 dose of the homologous B. burgdorferi N40 strain when immunized with the 10-μg dose regimen of DbpAN40 (9 of 10), OspAN40 (10 of 10), or the DbpAN40-OspAN40 combination vaccine (10 of 10). However, the DbpAN40-OspAN40 combination vaccine also elicited significant protection (8 of 10) at the 1.0-μg dose, a level at which the single-antigen vaccines were only partially protective. Nearly identical results were also obtained with a 0.1-μg dose and with adjuvant-free formulations of these antigens, demonstrating the intrinsic immunogenicity of the recombinant lipoproteins (data not shown).
TABLE 2.
Comparison of the relative protective efficacies of immunizations with DbpAN40 and OspAN40, singly and in combination and at either of two doses, against challenge with homologous and heterologous B. burgdorferi sensu lato isolates
| Immunogen(s) | Dose (μg) | Result with challenge isolate:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
B. burgdorferi N40
|
B. burgdorferi Sh-2-82a
|
B. garinii G25a
|
B. afzelii IPFa
|
||||||
| Growth inhibition titerb | Infection prevalence | Growth inhibition titerb | Infection prevalence | Growth inhibition titerb | Infection prevalence | Growth inhibition titerb | Infection prevalence | ||
| DbpA | 10 | 1,600 | 1/10c | 1,600 | 10/10 | 200 | 6/10d | 800 | 7/10 |
| 1 | 200 | 6/10d | 200 | 10/10 | NDe | 8/10 | ND | 9/10 | |
| OspA | 10 | 12,800 | 0/10c | 12,800 | 6/10d | 800 | 5/10d | 200 | 10/10 |
| 1 | 3,200 | 7/10 | 3,200 | 9/10 | ND | 7/10 | ND | 9/10 | |
| DbpA + OspA | 5 + 5 | 6,400 | 0/10c | 12,800 | 0/10c | 3,200 | 2/10c | 800 | 4/10d |
| 0.5 + 0.5 | 3,200 | 2/10c | 3,200 | 0/10c | ND | 3/10c | ND | 7/10 | |
| E. coli | 2.5 | <50 | 10/10 | <50 | 10/10 | <100 | 10/10 | <50 | 9/10 |
Sequence identities for DbpA versus N40: Sh-2-82, 66.5%; G25, 53.3%; IPF, 33.7%. Sequence identities for OspA versus N40: Sh-2-82, 99.6%; G25, 80.7%.
Values are the means of duplicate determinations on antisera pooled from the 10 mice within each immunization group. The growth inhibition titer is the antiserum dilution factor for a >90% reduction of the spirochete number.
P < 0.01 versus control (Fisher's exact test).
P < 0.05 versus control (Fisher's exact test).
ND, not determined.
We next examined the relative efficacies of single-antigen and combination vaccines formulated with Alhydrogel against heterologous challenge with B. burgdorferi sensu stricto Sh-2-82, B. garinii G25, and B. afzelii IPF, isolates that express DbpA and OspA proteins whose sequences are substantially divergent (12, 17, 18, 22) (Table 2) from those of the DbpAN40 and OspAN40 immunogens. The DbpAN40-OspAN40 combinations were more effective against all these heterologous challenges than single-antigen vaccines (Table 2). Only partial protection (6 of 10) at the 10-μg dose was achieved against the most divergent isolate, B. afzelii IPF, but this reached significance (P = 0.027).
In each vaccination experiment, we consistently observed that the various formulations of the DbpAN40-OspAN40 combination (Freund's, Alhydrogel, or adjuvant free) were more effective than the same dose of either single antigen. Since the single-antigen and combined-antigen vaccines were compared at doses of equivalent total mass, the improved effectiveness of the DbpAN40-OspAN40 combination was not merely due to an additive effect of the two immunogens but rather provided evidence of vaccine synergy between DbpA and OspA in this model.
Heterogeneity of OspA and DbpA expression by B. burgdorferi may limit efficacy of single-antigen vaccines.
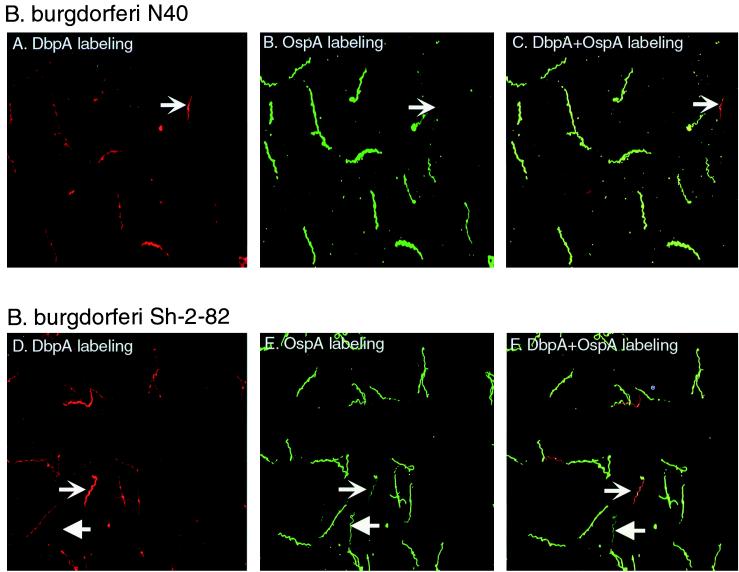
Cultures of cloned B. burgdorferi N40 and uncloned B. burgdorferi Sh-2-82 were found to be heterogeneous for DbpA and OspA expression when double labeled and examined by direct immunofluorescence microscopy (Fig. 1). One percent of B. burgdorferi N40 spirochetes appeared to express little or no OspA, and 2 to 3% expressed little or no DbpA. For B. burgdorferi Sh-2-82, the OspA and DbpA variants represented approximately 9 and 11% of the population, respectively. It is likely that phenotypic heterogeneity in the inoculum contributed to the limited efficacy of the single-antigen vaccines (Tables 1 and 2), particularly for B. burgdorferi Sh-2-82. This phenotypic heterogeneity may be relevant to tick-transmitted B. burgdorferi, since one recent study reported that spirochetes in salivary glands of feeding ticks expressed host-stage OspC predominantly but some still expressed OspA (7).
FIG. 1.
Detection of B. burgdorferi phenotypic variants deficient in DbpA or OspA by direct immunofluorescence assay. B. burgdorferi N40 and Sh-2-82 spirochetes from standard cultures were fixed to microscope slides and double labeled with a combination of two fluorochrome-conjugated IgGs, prepared against DbpA and OspA. Staining of a representative microscopic field for B. burgdorferi N40 (A to C) and for B. burgdorferi Sh-2-82 (D to F) is shown. Images were acquired alternately with the green filter (A and D) (DbpA labeling), the blue filter (B and E) (OspA labeling), or the triple-band-pass filter (C and F) (DbpA and OspA labeling). Leftward-pointing arrows indicate representative DbpA-deficient spirochetes, and rightward-pointing arrows indicate representative OspA-deficient spirochetes.
OspC is another in vivo-expressed B. burgdorferi antigen that has been evaluated with OspA as a combination vaccine. In that study (4), the addition of OspC did not improve upon the vaccine efficacy of OspA alone, but it was also shown that OspC immunization was ineffective against challenge with this particular B. burgdorferi strain (N40). The serological heterogeneity of OspC (23, 24) complicates vaccine design, and thus far only strain-specific protection has been reported with this immunogen (16).
We have shown that a vaccine combining the predominantly vector-stage OspA and the mammalian-host-stage DbpA is superior to either antigen alone against experimental B. burgdorferi challenge of mice. The enhanced efficacy of the DbpAN40- OspAN40 combination was not merely due to the additive mass of the two immunogens but appeared to be mediated, at least in part, by sustained vulnerability of the spirochetes to DbpA antibodies during early disseminating infection (5, 14). We recog- nize that dermal injections of cultured spirochetes do not recapitulate the B. burgdorferi inoculum delivered by the natural route of infection, and tick challenge studies are ultimately required to validate Lyme disease vaccine candidates. The effectiveness of combined DbpA-OspA immunity against experimental infection now provides the impetus for performing studies, possibly of a more complicated nature, using the natural route of infection. Given the several possible mechanisms of complementary interactions between DbpA and OspA immune responses, DbpA-OspA combinations may have a role as second-generation Lyme disease vaccines. Vaccinees receiving DbpA-OspA may also benefit from an anamnestic immune response to DbpA upon natural infection that is unlikely to occur with OspA alone.
Acknowledgments
We thank Alan Barbour, Stephen Barthold, and Russell Johnson for providing Borrelia isolates. We thank Christine Bachy and Christine Fazenbaker for technical assistance and Luis Branco for advice on fluorescence microscopy. We also thank Scott Koenig and Syd Johnson for helpful discussions and for critical review of the manuscript, and we are grateful to Donni Leach for assistance in its preparation.
ADDENDUM
While the manuscript was being reviewed, a paper by Hagman et al. (13a) reporting that DbpA immunity was ineffective at preventing tick-borne transmission of B. burgdorferi infection to mice was published. These authors suggest that DbpA is a host stage antigen but is not a target for protective antibodies, a theory that conflicts with our earlier reports (5, 14). Differences in the potencies of the DbpA immunogens used in the two studies may be a factor contributing to this apparent discordance. Hagman et al. used a recombinant cytosolically expressed form of DbpA, lacking posttranslational modifications, that conferred only partial protection against experimental B. burgdorferi challenge (13) even with much higher vaccine doses (20 to 50 μg in Freund's adjuvant) than those that were effective for our acylated and secreted DbpA in the present study. We have found that conformational epitopes contribute substantially to DbpA immunity (N. D. Ulbrandt, N. K. Patel, and M. S. Hanson, unpublished data), as has been reported for other B. burgdorferi vaccine antigens (9a, 11a).
REFERENCES
- 1.Barthold S W, de Souza M, Feng S. Serum-mediated resolution of Lyme arthritis in mice. Lab Investig. 1996;74:57–67. [PubMed] [Google Scholar]
- 2.Barthold S W, de Souza M S, Janotka J L, Smith A L, Persing D H. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol. 1993;143:959–971. [PMC free article] [PubMed] [Google Scholar]
- 3.Barthold S W, Fikrig E, Bockenstedt L K, Persing D H. Circumvention of outer surface protein A immunity by host-adapted Borrelia burgdorferi. Infect Immun. 1995;63:2255–2261. doi: 10.1128/iai.63.6.2255-2261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bockenstedt L K, Hodzic E, Feng S, Bourrel K W, de Silva A, Montgomery R R, Fikrig E, Radolf J D, Barthold S W. Borrelia burgdorferi strain-specific Osp C-mediated immunity in mice. Infect Immun. 1997;65:4661–4667. doi: 10.1128/iai.65.11.4661-4667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cassatt D R, Patel N K, Ulbrandt N D, Hanson M S. DbpA, but not OspA, is expressed by Borrelia burgdorferi during spirochetemia and is a target for protective antibodies. Infect Immun. 1998;66:5379–5387. doi: 10.1128/iai.66.11.5379-5387.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cinco M, Ruscio M, Rapagna F. Evidence of Dbps (decorin binding proteins) among European strains of Borrelia burgdorferi sensu lato and in the immune response of LB patient sera. FEMS Microbiol Lett. 2000;183:111–114. doi: 10.1111/j.1574-6968.2000.tb08942.x. [DOI] [PubMed] [Google Scholar]
- 7.Coleman J L, Gebbia J A, Piesman J, Degen J L, Bugge T H, Benach J L. Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell. 1997;89:1111–1119. doi: 10.1016/s0092-8674(00)80298-6. [DOI] [PubMed] [Google Scholar]
- 8.de Silva A M, Zeidner N S, Zhang Y, Dolan M C, Piesman J, Fikrig E. Influence of outer surface protein A antibody on Borrelia burgdorferi within feeding ticks. Infect Immun. 1999;67:30–35. doi: 10.1128/iai.67.1.30-35.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Silva A M, Telford III S R, Brunet L R, Barthold S W, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.Exner M M, Wu X, Blanco D R, Miller J N, Lovett M R. Protection elicited by native outer membrane protein Oms66 (p66) against host-adapted Borrelia burgdorferi: conformational nature of bastericidal epitopes. Infect Immun. 2000;68:2647–2654. doi: 10.1128/iai.68.5.2647-2654.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng S, Hodzic E, Stevenson B, Barthold S W. Humoral immunity to Borrelia burgdorferi N40 decorin binding proteins during infection of laboratory mice. Infect Immun. 1998;66:2827–2835. doi: 10.1128/iai.66.6.2827-2835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fikrig E, Barthold S W, Sun W, Feng W, Telford III S R, Flavell R A. Borrelia burgdorferi P35 and P37 proteins, expressed in vivo, elicit protective immunity. Immunity. 1997;6:531–539. doi: 10.1016/s1074-7613(00)80341-6. [DOI] [PubMed] [Google Scholar]
- 11a.Gilmore R D, Jr, Mbow M L. Infect. Immun. 67:5463–5469. 1999. Conformational nature of the Borrelia burgdorferi B31 outer surface protein C protective epitope. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godfroid E, Ben Messaoud A, Poliszczak A, Lobet Y, Bollen A. Assignment of Borrelia burgdorferi strains G25 and VS461 to the Borrelia garinii, Borrelia burgdorferi, and Borrelia afzelii genospecies, respectively: a comparison of OspA protein sequences. DNA Sequence. 1995;54:251–254. doi: 10.3109/10425179509030975. [DOI] [PubMed] [Google Scholar]
- 13.Hagman K E, Lahdenne P, Popova T G, Porcella S F, Akins D R, Radolf J D, Norgard M V. Decorin-binding protein of Borrelia burgdorferi is encoded within a two-gene operon and is protective in the murine model of Lyme borreliosis. Infect Immun. 1998;66:2674–2683. doi: 10.1128/iai.66.6.2674-2683.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Hagman K E, Yang X, Wikel S K, Schoeler G B, Caimano M J, Radolf J D, Norgard M V. Decorin-binding protein A (DbpA) of Borrelia burgdorferi is not protective when immunized mice are challenged via tick infestation and correlates with the lack of DbpA expression by B. burgdorferi in ticks. Infect Immun. 2000;68:4759–4764. doi: 10.1128/iai.68.8.4759-4764.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanson M S, Cassatt D R, Guo B P, Patel N K, McCarthy M P, Dorward D W, Höök M. Active and passive immunity against Borrelia burgdorferi decorin binding protein A (DbpA) protects against infection. Infect Immun. 1998;66:2143–2153. doi: 10.1128/iai.66.5.2143-2153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pavia C S, Kissel V, Bittker S, Cabello F, Levine S. Antiborrelial activity of serum from rats injected with the Lyme disease spirochete. J Infect Dis. 1991;163:656–659. doi: 10.1093/infdis/163.3.656. [DOI] [PubMed] [Google Scholar]
- 16.Probert W S, Crawford M, Cadiz R B, LeFebvre R B. Immunization with outer surface protein (Osp) A, but not OspC, provides cross-protection of mice challenged with North American isolates of Borrelia burgdorferi. J Infect Dis. 1997;175:400–405. doi: 10.1093/infdis/175.2.400. [DOI] [PubMed] [Google Scholar]
- 17.Roberts W C, Mullikin B A, Lathigra R, Hanson M S. Molecular analysis of sequence heterogeneity among genes encoding decorin binding proteins A and B of Borrelia burgdorferi sensu lato. Infect Immun. 1998;66:5275–5285. doi: 10.1128/iai.66.11.5275-5285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosa P A, Schwan T G, Hogan D. Recombination between genes encoding major outer surface proteins A and B of Borrelia burgdorferi. Mol Microbiol. 1992;6:3031–3040. doi: 10.1111/j.1365-2958.1992.tb01761.x. [DOI] [PubMed] [Google Scholar]
- 19.Sigal L H, Zahradnik J M, Lavin P, Patella S J, Bryant G, Haselby R, Hilton E, Kunkel M, Adler-Klein D, Doherty T, Evans J, Malawista S E The Recombinant Outer-Surface Protein A Lyme Disease Vaccine Study Consortium. A vaccine consisting of recombinant Borrelia burgdorferi outer-surface protein A to prevent Lyme disease. N Engl J Med. 1998;339:216–222. doi: 10.1056/NEJM199807233390402. [DOI] [PubMed] [Google Scholar]
- 20.Steere A C. Lyme disease: a growing threat to urban populations. Proc Natl Acad Sci USA. 1994;91:2378–2383. doi: 10.1073/pnas.91.7.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steere A C, Sikand V K, Meurice F, Parenti D L, Fikrig E, Schoen R T, Nowakowski J, Schmid C H, Laukamp S, Buscarino C, Krause D The Lyme Disease Vaccine Study Group. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. N Engl J Med. 1998;339:209–215. doi: 10.1056/NEJM199807233390401. [DOI] [PubMed] [Google Scholar]
- 22.Will G, Jauris-Heipke S, Schwab E, Busch U, Rössler D, Soutschek E, Wilske B, Preac-Mursic V. Sequence analysis of ospA genes shows homogeneity within Borrelia burgdorferi sensu stricto and Borrelia afzelii strains but reveals major subgroups within the Borrelia garinii species. Med Microbiol Immunol. 1995;184:73–80. doi: 10.1007/BF00221390. [DOI] [PubMed] [Google Scholar]
- 23.Wilske B, Busch U, Fingerle V, Jauris-Heipke S, Preac-Mursic V, Rössler D, Will G. Immunological and molecular variability of OspA and OspC. Implications for Borrelia vaccine development. Infection. 1996;24:208–212. doi: 10.1007/BF01713341. [DOI] [PubMed] [Google Scholar]
- 24.Wilske B, Jauris-Heipke S, Lobentanzer R, Pradel I, Preac-Mursic V, Rössler D, Soutschek E, Johnson R C. Phenotypic analysis of outer surface protein C (OspC) of Borrelia burgdorferi sensu lato by monoclonal antibodies: relationship to genospecies and OspA serotype. J Clin Microbiol. 1995;33:103–109. doi: 10.1128/jcm.33.1.103-109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong W, Stehle T, Museteanu C, Seibers A, Gern L, Kramer M D, Wallich R, Simon M M. Therapeutic passive vaccination against chronic Lyme disease in mice. Proc Natl Acad Sci USA. 1997;94:12533–12538. doi: 10.1073/pnas.94.23.12533. [DOI] [PMC free article] [PubMed] [Google Scholar]