Abstract
Plasmodium ookinetes secrete chitinases to penetrate the acellular, chitin-containing peritrophic matrix of the mosquito midgut en route to invasion of the epithelium. Chitinases are potentially targets that can be used to block malaria transmission. We demonstrate here that chitinases of Plasmodium falciparum and P. gallinaceum are concentrated at the apical end of ookinetes. The chitinase PgCHT1 of P. gallinaceum is present within ookinete micronemes and subsequently becomes localized in the electron-dense area of the apical complex. These observations suggest a pathway by which ookinetes secrete proteins extracellularly.
The Plasmodium ookinete is the developmental stage of the malaria parasite that invades the mosquito midgut. Within minutes after a mosquito ingests sexually differentiated gametocytes from the vertebrate host, male and female gametes fuse to form zygotes, which develop into ookinetes over the subsequent 10 to 25 h. The ookinete is extracellular and motile and constitutively expresses cell surface proteins, including the 25- and 28-kDa epidermal growth factor-like domain-containing family of proteins of unknown function (3, 7, 10, 15, 21, 22), and the microneme-associated circumsporozoite protein/thrombospondin-related anonymous protein (TRAP)-related protein (CTRP) thought to be involved in motility (6, 20, 25, 26). The ookinete also constitutively secretes chitinolytic activity into the extracellular milieu (9, 17, 24). Chitinases are thought to be required for the ookinete to traverse the acellular, chitin-containing peritrophic matrix and are potential targets that can be used to block malaria transmission from vertebrate hosts to mosquitoes (9, 17, 24).
Current concepts of the cellular and molecular mechanisms by which the Plasmodium parasite invades its diverse targets rest largely on how the parasite stages interact with vertebrate cells, primarily erythrocytes and hepatocytes. The mechanism of host cell invasion by apicomplexans, epitomized by Toxoplasma gondii (14) but not yet demonstrated for Plasmodium spp., seems to be mediated by sequential secretion of three types of organelles, micronemes, rhoptries, and dense granules. Organellar secretion in T. gondii is triggered by specific signals, particularly cell-to-cell contact (5). To date, Plasmodium micronemes have only been shown to contain adhesive-domain-containing proteins thought to be involved in host cell recognition, binding, and motility. These proteins include the circumsporozoite protein (13), TRAP (18), and CTRP (6, 20). These proteins contain presumptive adhesive motifs such as vertebrate-type thrombospondin-like sulfatide-binding domains and von Willebrand factor-like A domains in TRAP (12, 13, 19, 20) and Plasmodium-specific adhesive domains (1).
Malaria parasite chitinases, recently identified and characterized at the molecular level (23, 24), are secreted into the extracellular milieu, in contrast to other micronemal Plasmodium proteins involved in invasion processes that are membrane associated (1, 12, 13, 19, 20). Since chitinase has been reported to be critical for passage of the ookinete through the peritrophic matrix (17), understanding of the precise cellular mechanisms by which the ookinete secretes this enzyme may have implications for the development of transmission-blocking strategies. In this study, the chitinases PgCHT1 in in vitro-developed Plasmodium gallinaceum ookinetes and PfCHT1 in in vivo-developed P. falciparum ookinetes were localized.
P. gallinaceum ookinetes were obtained in vitro as previously described (11). To obtain P. falciparum ookinetes, starved female Anopheles freeborni mosquitoes were fed in vitro-cultured gametocytes of P. falciparum strain 3D7 (16). At 22 h after a blood meal, midguts were homogenized in phosphate-buffered saline (PBS) and fixed on glass slides with 100% methanol. Murine monoclonal antibody (MAb) 1C3 (isotype immunoglobulin G2b [IgG2b]) was developed against recombinant PfCHT1 (23; R. C. Langer and J. M. Vinetz, unpublished data). Polyclonal murine antisera were raised against synthetic peptides derived from the active site and chitin-binding domain of PgCHT1 and previously characterized (24). Polyclonal murine antisera raised against recombinant PgCHT1 (rPgCHT1) recognized both rPgCHT1 and native, ookinete-produced PgCHT1 (data not shown). A rabbit antiserum against a yeast-produced recombinant fusion protein of two P. falciparum zygote-ookinete surface antigens, Pfs25 and Pfs28, has previously been described (8). For immunofluorescence microscopy, slides were blocked in PBS–3% bovine serum albumin (BSA)–3% Triton X-100 and stained with anti-rPgCHT1 or normal mouse serum. For confocal microscopy, slides were blocked in PBS–10% BSA and then incubated with mouse MAb 1C3 (or an isotype control) and anti-Pfs25-Pfs28 rabbit antiserum in PBS–10% BSA. Secondary antibodies were rhodamine-conjugated donkey anti-rabbit IgG and fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Kirkegaard & Perry, Gaithersburg, Md.) (1/100 dilution in PBS–3% BSA–3% Triton X-100 for immunofluorescence assay; 1/1,000 dilution in PBS–10% BSA for confocal microscopy). Slides were mounted with Permafluor (Immunon Shandon) for immunofluorescence assay or Vectashield (Vector Labs, Inc.) for confocal microscopy. Images were collected on a Zeiss Axiophot 2 immunofluorescence microscope or a Leica TCS-NT/SP confocal microscope (Leica Microsystems). With the confocal microscope, Z stacks of images were collected in 0.203-μm increments. Images were processed using Leica TCS-NT/SP software (version 1.6.551) and Imaris 3.0.2 (Bitplane AG). For immunoelectron microscopy, cultured P. gallinaceum ookinetes (11) were fixed in 1% paraformaldehyde–0.1% glutaraldehyde in PBS (pH 7.4) and embedded in LR White resin (Polysciences, Warrington, Pa.). Sections were blocked in PBS containing 5% nonfat milk–0.01% Tween 20 and incubated with primary antibodies diluted in PBS-milk-Tween 20. Grids were incubated with 15-nm gold particle-labeled goat anti-mouse IgG diluted 1/20 with PBS-milk-Tween 20, stained in 2% uranyl acetate in 50% methanol, rinsed with 50% methanol, and stained with Reynold's lead citrate. Sections were then carbon coated in a vacuum evaporator and observed in a Hitachi H-800 electron microscope.
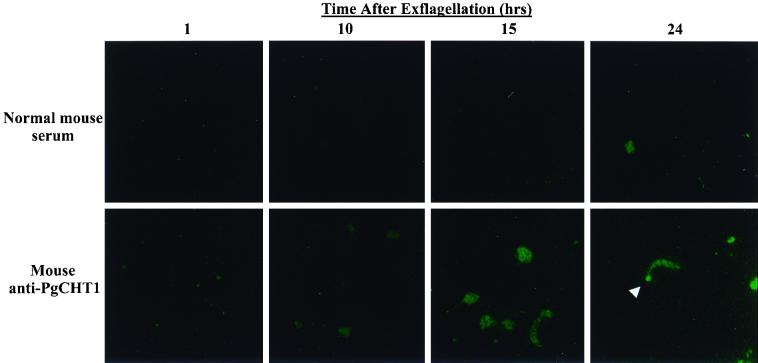
Consistent with previous findings that P. gallinaceum chitinase expression and secretion are developmentally regulated and not detectable biochemically or immunologically until 10 h after zygote formation (24), immunofluorescence microscopy showed a time-dependent appearance of PgCHT1 (Fig. 1). PgCHT1 first became apparent 10 h after exflagellation and was found in retort forms at 15 h. At 24 h, PgCHT1 was present in a nondiffuse, lumpy-granular pattern throughout the cytoplasm of mature-appearing ookinetes and was concentrated in the apical end of the parasite (Fig. 1, arrowhead). PgCHT1 was concentrated in the apical end of ookinetes only in morphologically mature ookinetes at 24 h, and not at 15 h or earlier, after exflagellation.
FIG. 1.
Immunofluorescence localization of P. gallinaceum chitinase PgCHT1 in in vitro-developed mosquito midgut stage parasites. Parasites were stained at the indicated time points with either normal mouse serum or anti-P. gallinaceum chitinase (PgCHT1) antibodies and visualized with FITC-labeled goat anti-mouse antibody. The white arrowhead indicates the apical end of the mature ookinete.
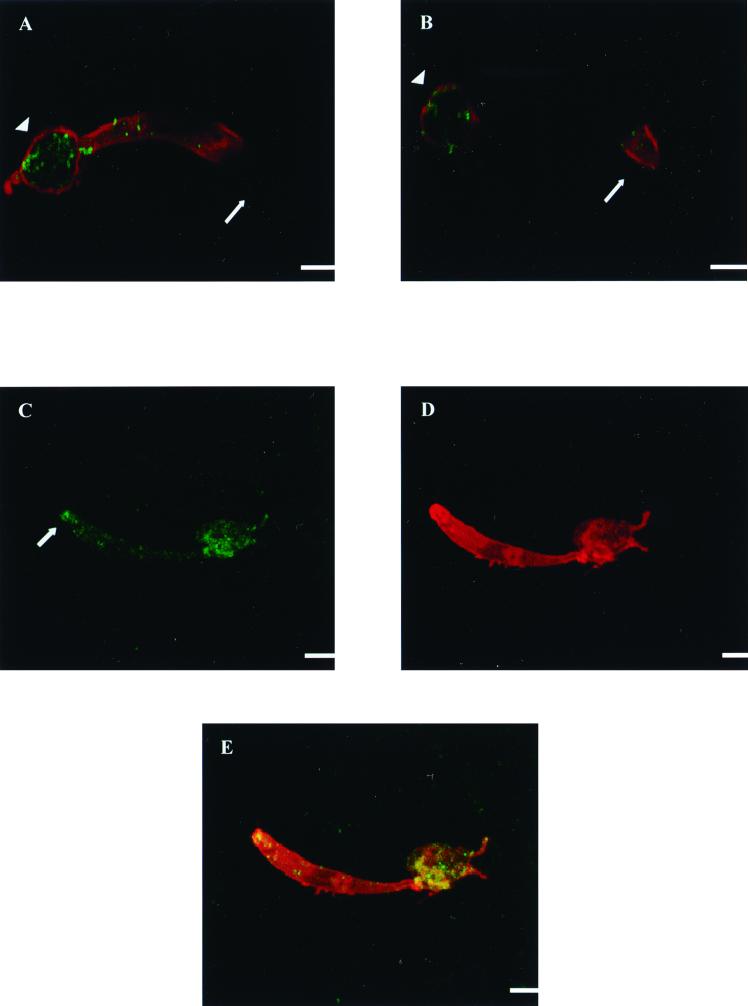
Confocal immunofluorescence microscopy, performed on P. falciparum ookinetes obtained from mosquito midguts 22 h after a blood meal, showed that PfCHT1 is synthesized in the zygote remnant out of which the immature ookinete is emerging (Fig. 2A and B). PfCHT1 was also present anteriorly in immature ookinetes, with labeling reaching near the apical end. In retorts, there was a higher concentration of PfCHT1 in the zygote remnant than elsewhere in the cell. The granular labeling pattern of PfCHT1 throughout the cell is consistent with an organellar distribution of the protein and shows that PfCHT1's localization is distinct from parasite surface membranes, as delineated by rhodamine labeling of surface proteins Pfs25 and Pfs28 (Fig. 2). The timing of synthesis and trafficking of PfCHT1 are distinct from those of Pfs25 and Pfs28, which are expressed earlier after exflagellation. Like PgCHT1, PfCHT1 was observed throughout the parasite cytoplasm but was concentrated at the apical end of a morphologically mature ookinete (Fig. 2C and E).
FIG. 2.
Confocal immunofluorescence microscopy of immature P. falciparum ookinetes stained simultaneously with mouse anti-P. falciparum chitinase (PfCHT1) MAb 1C3 (green) and rabbit polyclonal antiserum to a recombinant P. falciparum zygote-ookinete surface antigen (Pfs25-Pfs28) fusion protein (8) (red). (A and B). In two different planes of focus, a maturing ookinete is shown exiting the zygote remnant of the retort toward the right. The surface of the parasite is delineated by rhodamine staining of surface proteins Pfs25 and Pfs28. Staining with MAb 1C3 demonstrates the granular appearance of PfCHT1, which is most concentrated in the zygote remnant but also appears to be present anteriorly as well, reaching near the apical end. (C to E) An ookinete in panel C shows a higher concentration of PfCHT1 at the apical end than the ookinete in panels A and B. C, staining with MAb 1C3 (FITC); D, staining with rabbit anti-Pfs25-Pfs28; E, colocalization of panels A and B. Staining with an isotype-matched control (not shown) showed no fluorescence. Each arrow indicates the apical end of the parasite; the arrowhead indicates a zygote remnant. Bars, 1 μm.
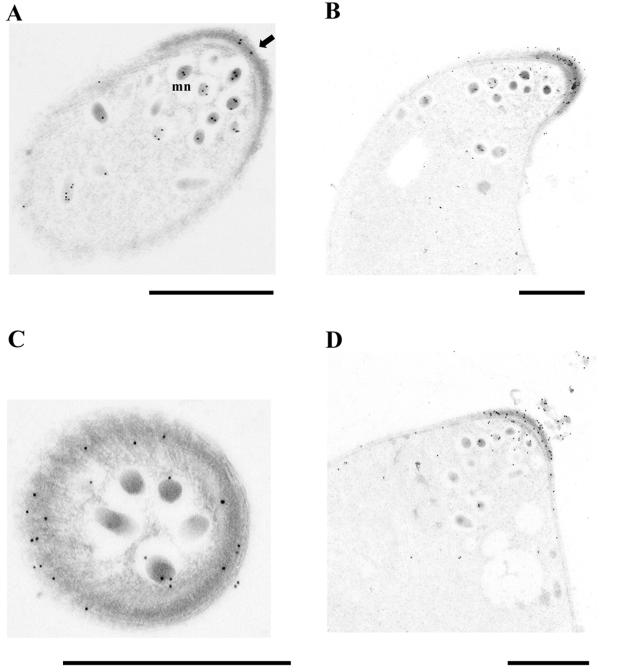
Immunoelectron microscopy was performed to determine more precisely the subcellular localization of PgCHT1 in P. gallinaceum ookinetes. PgCHT1 was found associated with micronemes of maturing ookinetes (Fig. 3A). In fully mature P. gallinaceum ookinetes, PgCHT1 continued to be localized in micronemes and became highly concentrated in the electron-dense subpellicular region just beneath the very apical tip (Fig. 3B to D). This chitinase-containing electron-dense region seemed to be circumferentially distributed around the apical pole (Fig. 3C). The electron-dense area in which immunogold-stained PgCHT1 was seen had the same density as the material in which extracellular PgCHT1 is found (Fig. 3D). This observation suggests that the electron-dense subpellicular region in the apical complex is involved in PgCHT1 secretion.
FIG. 3.
Immunoelectron microscopy of in vitro-developed mosquito midgut stage P. gallinaceum parasites stained with anti-P. gallinaceum chitinase (PgCHT1) antibodies. (A) Longitudinal section of a maturing ookinete in which PgCHT1 is associated with micronemes (mn) at the apical third of the parasite (arrow). The primary antibody was mouse polyclonal antiserum raised to full-length recombinant PgCHT1. (B) Longitudinal section of a mature ookinete stained with anti-PgCHT1 chitin-binding domain antiserum. (C) Cross section of the apical end of a mature ookinete stained with anti-PgCHT1, anti-active-site serum. (D) Longitudinal section of a mature ookinete stained with anti-PgCHT1 chitin-binding domain serum showing extracellular PgCHT1. Bars, 1 μm.
DISCUSSION
We have demonstrated the presence of the chitinases PgCHT1 and PfCHT1 within ookinetes of two species of malaria parasite, P. gallinaceum and P. falciparum, respectively. The nondiffuse, lumpy-granular staining pattern of these proteins demonstrated by routine and confocal immunofluorescence microscopy suggested that their subcellular localization is associated with an organelle. Immunoelectron microscopy of P. gallinaceum ookinetes confirmed this suggestion and showed that PgCHT1 was associated with micronemes. Further, PgCHT1 was found concentrated within the electron-dense area in the apical end of mature ookinetes. The latter finding suggests that this electron-dense area is involved in extracellular secretion. Further, since chitinase is synthesized in the posterior end and other parts of the ookinete and is found within micronemes, we suggest that PgCHT1 and, by analogy, PfCHT1 might be trafficked anteriorly to the apical end of the parasite via micronemes. Our observation that PgCHT1 is highly concentrated in the electron-dense area of the apical complex of 24-h (mature) P. gallinaceum ookinetes is consistent with this hypothesis. Previous transmission electron micrograph studies have observed a “collar”-like structure (4), also called a “canopy” (2), in the P. gallinaceum ookinete apical complex. The immunolocalization results presented here strongly suggest that this apical collar is involved in chitinase secretion.
The mechanisms by which Plasmodium proteins are directed to their various intra- and extracellular fates are not well understood for any stage of the malaria parasite. For example, what mechanisms direct proteins into micronemes? Do all ookinete micronemes contain the same proteins? For example, since CTRP is micronemal, do CTRP and chitinase colocalize? Are mechanisms of microneme formation similar or different in different Plasmodium developmental stages? What signals regulate the intracellular movement of micronemes to the apical complex? The chitinases of P. falciparum and P. gallinaceum promise to be useful markers for answering such questions about the Plasmodium ookinete and related questions of the cell biology of other apicomplexan parasites.
Acknowledgments
We thank Sanat Dave (UTMB), David Keister, Olga Muratova, and Owen Schwartz (NIH) for their expertise and Thomas J. Templeton, Vsevolod Popov, Gard Ward, and Vern Carruthers for critical reading of the manuscript.
R.C.L. is supported by Public Health Service grant T32-AI07536 (Training in Emerging and Reemerging Infectious Diseases) from the National Institute of Allergy and Infectious Diseases. J.M.V. is a Culpeper Medical Sciences Scholar supported by the Rockefeller Brothers Fund. This work was supported by Public Health Service grant RO1-AI 45999 from the National Institute of Allergy and Infectious Diseases (to J.M.V.); Grants-in-Aid for Scientific Research 11147220 and 12557026 (to T.T.) from the Ministry of Education, Science, Sports and Culture, Japan; and Grant-in-Aid for Scientific Research on Priority Areas 08281104 (to M.T.) from the Ministry of Education, Science, Sports and Culture, Japan.
REFERENCES
- 1.Adams J H, Sim B K, Dolan S A, Fang X, Kaslow D C, Miller L H. A family of erythrocyte binding proteins of malaria parasites. Proc Natl Acad Sci USA. 1992;89:7085–7089. doi: 10.1073/pnas.89.15.7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aikawa M, Carter R, Ito Y, Nijhout M M. New observations on gametogenesis, fertilization, and zygote transformation in Plasmodium gallinaceum. J Protozool. 1984;31:403–413. doi: 10.1111/j.1550-7408.1984.tb02987.x. [DOI] [PubMed] [Google Scholar]
- 3.Barker G C, Rodriguez M H, Sinden R E. Attempted isolation of the gene encoding the 21 Kd Plasmodium berghei ookinete transmission blocking antigen from Plasmodium yoelli and Plasmodium vivax. Mem Inst Oswaldo Cruz. 1994;89(Suppl. 2):37–41. doi: 10.1590/s0074-02761994000600010. [DOI] [PubMed] [Google Scholar]
- 4.Canning E U, Sinden R E. The organization of the ookinete and observations on nuclear division in oocysts of Plasmodium berghei. Parasitology. 1973;67:29–40. doi: 10.1017/s0031182000046266. [DOI] [PubMed] [Google Scholar]
- 5.Carruthers V B. Armed and dangerous: Toxoplasma gondii uses an arsenal of secretory proteins to infect host cells. Parasitol Int. 1999;48:1–10. doi: 10.1016/s1383-5769(98)00042-7. [DOI] [PubMed] [Google Scholar]
- 6.Dessens J T, Beetsma A L, Dimopoulos G, Wengelnik K, Crisanti A, Kafatos F C, Sinden R E. CTRP is essential for mosquito infection by malaria ookinetes. EMBO J. 1999;18:6221–6227. doi: 10.1093/emboj/18.22.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duffy P, Pimenta P, Kaslow D. Pgs28 belongs to a family of epidermal growth factor-like antigens that are targets of malaria transmission-blocking antibodies. J Exp Med. 1993;177:505–510. doi: 10.1084/jem.177.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gozar M M, Price V L, Kaslow D C. Saccharomyces cerevisiae-secreted fusion proteins Pfs25 and Pfs28 elicit potent Plasmodium falciparum transmission-blocking antibodies in mice. Infect Immun. 1998;66:59–64. doi: 10.1128/iai.66.1.59-64.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huber M, Cabib E, Miller L H. Malaria parasite chitinase and penetration of the mosquito peritrophic membrane. Proc Natl Acad Sci USA. 1991;88:2807–2810. doi: 10.1073/pnas.88.7.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaslow D C, Quakyi I A, Syin C, Raum M G, et al. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature. 1988;333:74–76. doi: 10.1038/333074a0. [DOI] [PubMed] [Google Scholar]
- 11.Kaushal D C, Carter R. Characterization of antigens on mosquito midgut stages of Plasmodium gallinaceum. II. Comparison of surface antigens of male and female gametes and zygotes. Mol Biochem Parasitol. 1984;11:145–156. doi: 10.1016/0166-6851(84)90061-6. [DOI] [PubMed] [Google Scholar]
- 12.McCutchan T F, Kissinger J C, Touray M G, Rogers M J, Li J, Sullivan M, Braga E M, Krettli A U, Miller L H. Comparison of circumsporozoite proteins from avian and mammalian malarias: biological and phylogenetic implications. Proc Natl Acad Sci USA. 1996;93:11889–11894. doi: 10.1073/pnas.93.21.11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menard R, Sultan A A, Cortes C, Altszuler R, Van Dijk M P, Janse C J, Waters A P, Nussenzweig R S, Nussenzweig V. Circumsporozoite protein is required for development of malaria sporozoites in mosquitoes. Nature. 1997;385:336–340. doi: 10.1038/385336a0. [DOI] [PubMed] [Google Scholar]
- 14.Ngo H M, Hoppe H C, Joiner K A. Differential sorting and post-secretory targeting of proteins in parasitic invasion. Trends Cell Biol. 2000;10:67–72. doi: 10.1016/s0962-8924(99)01698-0. [DOI] [PubMed] [Google Scholar]
- 15.Paton M G, Barker G C, Matsuoka H, Ramesar J, Janse C J, Waters A P, Sinden R E. Structure and expression of a post-transcriptionally regulated malaria gene encoding a surface protein from the sexual stages of Plasmodium berghei. Mol Biochem Parasitol. 1993;59:263–275. doi: 10.1016/0166-6851(93)90224-l. [DOI] [PubMed] [Google Scholar]
- 16.Quakyi I A, Carter R, Rener J, et al. The 230-kDa gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies. J Immunol. 1987;139:4213–4217. [PubMed] [Google Scholar]
- 17.Shahabuddin M, Toyoshima T, Aikawa M, Kaslow D C. Transmission-blocking activity of a chitinase inhibitor and activation of malarial parasite chitinase by mosquito protease. Proc Natl Acad Sci USA. 1993;90:4266–4270. doi: 10.1073/pnas.90.9.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sultan A, Thathy V, Frevert U, Robson K, Crisanti A, Nussenzweig V, Nussenzweig R, Menard R. TRAP is necessary for gliding motility and infectivity of Plasmodium sporozoites. Cell. 1997;90:511–522. doi: 10.1016/s0092-8674(00)80511-5. [DOI] [PubMed] [Google Scholar]
- 19.Templeton T J, Kaslow D C. Cloning and cross-species comparison of the thrombospondin-related anonymous protein (TRAP) gene from Plasmodium knowlesi, Plasmodium vivax and Plasmodium gallinaceum. Mol Biochem Parasitol. 1997;84:13–24. doi: 10.1016/s0166-6851(96)02775-2. [DOI] [PubMed] [Google Scholar]
- 20.Templeton T J, Kaslow D C, Fidock D A. Developmental arrest of the human malaria parasite Plasmodium falciparum within the mosquito midgut via CTRP gene disruption. Mol Microbiol. 2000;36:1–9. doi: 10.1046/j.1365-2958.2000.01821.x. [DOI] [PubMed] [Google Scholar]
- 21.Tsuboi T, Cao Y-M, Hitsumoto Y, Yanagi T, Kanbara H, Torii M. Two antigens on zygotes and ookinetes of Plasmodium yoelii and Plasmodium berghei that are distinct targets of transmission-blocking immunity. Infect Immun. 1997;65:2260–2264. doi: 10.1128/iai.65.6.2260-2264.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuboi T, Cao Y M, Kaslow D C, Shiwaku K, Torii M. Primary structure of a novel ookinete surface protein from Plasmodium berghei. Mol Biochem Parasitol. 1997;85:131–134. doi: 10.1016/s0166-6851(96)02821-6. [DOI] [PubMed] [Google Scholar]
- 23.Vinetz J, Dave S, Specht C, Brameld K, Hayward R, Fidock D. The chitinase PfCHT1 from the human malaria parasite Plasmodium falciparum lacks proenzyme and chitin-binding domains and displays unique substrate preferences. Proc Natl Acad Sci USA. 1999;96:14061–14066. doi: 10.1073/pnas.96.24.14061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vinetz J M, Valenzuela J G, Specht C A, Aravind L, Langer R C, Ribeiro J M, Kaslow D C. Chitinases of the avian malaria parasite Plasmodium gallinaceum, a class of enzymes necessary for parasite invasion of the mosquito midgut. J Biol Chem. 2000;275:10331–10341. doi: 10.1074/jbc.275.14.10331. [DOI] [PubMed] [Google Scholar]
- 25.Yuda M, Sakaida H, Chinzei Y. Targeted disruption of the Plasmodium berghei CTRP gene reveals its essential role in malaria infection of the vector mosquito. J Exp Med. 1999;190:1711–1716. doi: 10.1084/jem.190.11.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuda M, Sawai T, Chinzei Y. Structure and expression of an adhesive protein-like molecule of mosquito invasive-stage malarial parasite. J Exp Med. 1999;189:1947–1952. doi: 10.1084/jem.189.12.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]