Abstract
Aims
In people at risk of heart failure (HF) enrolled in the Heart ‘OMics’ in AGEing (HOMAGE) trial, spironolactone reduced circulating markers of collagen synthesis, natriuretic peptides, and blood pressure and improved cardiac structure and function. In the present report, we explored factors associated with dyskalaemia.
Methods and results
The HOMAGE trial was an open‐label study comparing spironolactone (up to 50 mg/day) versus standard care in people at risk for HF. After randomization, serum potassium was assessed at 1 and 9 months and was defined as low when ≤3.5 mmol/L (hypokalaemia) and high when ≥5.5 mmol/L (hyperkalaemia). Multivariable logistic regression models were constructed to identify clinical predictors of dyskalaemia. A total of 513 participants (median age 74 years, 75% men, median estimated glomerular filtration rate 71 mL/min/1.73 m2) had serum potassium available and were included in this analysis. At randomization, 88 had potassium < 4.0 mmol/L, 367 had potassium 4.0–5.0 mmol/L, and 58 had potassium > 5.0 mmol/L. During follow‐up, on at least one occasion, a serum potassium < 3.5 mmol/L was observed in 6 (1.2%) and <4.0 mmol/L in 46 (9%) participants, while a potassium > 5.0 mmol/L was observed in 38 (8%) and >5.5 mmol/L in 5 (1.0%) participants. The median (percentile25−75) increase in serum potassium with spironolactone during the study was 0.23 (0.16; 0.29) mmol/L. Because of the low incidence of dyskalaemia, for regression analysis, hypokalaemia and hyperkalaemia thresholds were set at <4.0 and >5.0 mmol/L, respectively. The occurrence of a serum potassium > 5.0 mmol/L during follow‐up was positively associated with the presence of diabetes mellitus {odds ratio [OR]: 1.21 [95% confidence interval (CI) 2.14; 3.79]} and randomization to spironolactone (OR: 2.83 [95% CI 1.49; 5.37]). Conversely, the occurrence of a potassium concentration < 4.0 mmol/L was positively associated with the use of thiazides (OR: 2.39 [95% CI 1.32; 4.34]), blood urea concentration (OR: 2.15 [95% CI 1.34; 3.39] per 10 mg/dL), and history of hypertension (OR: 2.32 [95% CI 1.02; 5.29]) and negatively associated with randomization to spironolactone (OR: 0.30 [95% CI 0.18; 0.52]).
Conclusions
In people at risk for developing HF and with relatively normal renal function, spironolactone reduced the risk of hypokalaemia and, at the doses used, was not associated with the occurrence of clinically meaningful hyperkalaemia.
Keywords: Hyperkalaemia, Hypokalaemia, Steroidal mineralocorticoid receptor antagonist, Heart failure prevention
Background
Serum potassium plays a key role in cellular function and its homeostasis is mainly achieved by modulation of renal excretion. 1 Both hypokalaemia and hyperkalaemia can cause alterations in cellular membrane potential, which may be arrhythmogenic, potentially accounting for their association with excess morbidity and mortality. 2 , 3 In people at risk for heart failure (HF), the steroidal mineralocorticoid receptor antagonist (MRA), spironolactone, has beneficial effects on blood pressure, collagen turnover, and cardiac structure and function. 4 However, MRA may induce hyperkalaemia. In a large metanalysis of randomized clinical trials on proteinuric kidney disease, the use of MRAs was associated with a 2.6‐fold increase in hyperkalaemia risk compared with placebo/active control. 5 In another study, assessing the efficacy of aldosterone antagonists in patients with resistant hypertension and mild to moderate chronic kidney disease (CKD), the incidence of hyperkalaemia (potassium ≥ 5.5 mmol/L) was 17.3%. 6 On the other hand, spironolactone may reduce the occurrence of hypokalaemia, especially for patients treated with diuretics. 7
The aim of the present study was to investigate the factors associated with dyskalaemia in people at increased risk of developing HF who were assigned to receive spironolactone or not in the Heart ‘OMics’ in AGEing (HOMAGE) trial.
Methods
The HOMAGE trial was a prospective, randomized, open‐label, blinded‐endpoint (PROBE) trial comparing spironolactone to standard care for up to 9 months in people with clinical risk factors for developing HF. The protocol and the outcomes of the HOMAGE trial have been previously described. 8 The main inclusion criteria were age > 60 years, high natriuretic peptides, coronary artery disease, or at least two of the following: diabetes mellitus, hypertension, microalbuminuria, and abnormal electrocardiogram. The key exclusion criteria were an estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2, serum potassium > 5.0 mmol/L, left ventricular ejection fraction < 45%, atrial fibrillation, a diagnosis of HF, or treatment with loop diuretics. After randomization, serum potassium was assessed at 1 and 9 months. Spironolactone was initiated at 25 mg/day. Doses could be increased up to 50 mg/day or reduced to 25 mg every other day or stopped with or without re‐initiation according to serum potassium and renal function. In particular, the dose was adjusted to keep serum potassium in a range between 4.5 and 5.4 mmol/L. 8
Serum potassium was defined as low when ≤3.5 mmol/L (hypokalaemia) and high when ≥5.5 mmol/L (hyperkalaemia), the latter being a prespecified secondary endpoint (Clinical‐Trials.gov: NCT02556450), as reported. 4 Multivariable logistic regression models were constructed to identify clinical predictors of dyskalaemia. Because of the low incidence of dyskalaemia using the cut‐offs described earlier, for regression analysis hypokalaemia and hyperkalaemia thresholds were set at <4.0 and >5.0 mmol/L, respectively. The impact of spironolactone treatment on serum potassium throughout follow‐up was assessed using mixed random effects models. The study was conducted in accordance with the Declaration of Helsinki and approved by site ethics committees. All participants gave written informed consent to participate in the trial. All analyses were performed using Stata Version 15.1 (Stata Corporation, College Station, TX, USA), with two‐sided P < 0.05 considered significant.
Results
The baseline characteristics of the population at randomization are given in Table 1 . Of the 527 patients enrolled in the HOMAGE cohort, potassium value was missing in 14. Therefore, 513 patients were considered in the final analysis. At randomization, 88 had potassium < 4.0 mmol/L, 367 had potassium 4.0–5.0 mmol/L, and 58 had potassium > 5.0 mmol/L. Groups were largely similar in terms of mean age, proportion of men, left ventricular ejection fraction and mass, renal function, and natriuretic peptides. Participants with hypokalaemia had the lowest prevalence of coronary artery disease and the highest prevalence of hypertension. The prevalence of diabetes was similar between the cohort with potassium < 4.0 and >5.0 mmol/L. There were no significant differences between groups in medical treatment (included angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers) except for thiazides that were more commonly used in hypokalaemic patients.
Table 1.
Characteristics of the study population at baseline
| Serum potassium < 4.0 mmol/L (N = 88) | Serum potassium 4.0–5.0 mmol/L (N = 367) | Serum potassium > 5.0 mmol/L (N = 58) | P‐value a | |
|---|---|---|---|---|
| Age, years | 74 [69; 80] | 73 [69; 78] | 74 [68; 79] | 0.41 |
| Men | 63 (72) | 276 (75) | 47 (81) | 0.43 |
| Body mass index, kg/m2 | 27.3 [24.7; 31.7] | 28.0 [25.6; 31.3] | 29.5 [26.8; 32.9] | 0.13 |
| Coronary artery disease | 51 (58) | 277 (75.5) | 43 (74.1) | 0.004 |
| Hypertension | 80 (91) | 275 (74.9) | 46 (79.3) | 0.005 |
| Diabetes mellitus | 47 (53) | 131 (35.7) | 32 (55.2) | <0.001 |
| Systolic blood pressure, mmHg | 144 [130; 159] | 140 [126; 155] | 141 [128; 155] | 0.19 |
| Heart rate, b.p.m. | 63 [55; 68] | 60 [55; 66] | 62 [55; 69] | 0.52 |
| LV ejection fraction, % | 64 [60; 67] | 63 [57; 66] | 62 [58; 66] | 0.28 |
| LV mass index, g/m2 | 100 [85; 115] | 93 [81; 111] | 96 [82; 112] | 0.29 |
| Left atrial volume index, mL/m2 | 29 [25; 38] | 31 [26; 36] | 29 [25; 34] | 0.39 |
| E/E′ ratio | 9.7 [8.2; 11.6] | 9.1 [7.4; 11.4] | 10.0 [8.0; 12.0] | 0.19 |
| eGFR, mL/min/1.73 m2 | 74 [62; 87] | 73 [62; 84] | 67 [56; 79] | 0.068 |
| eGFR < 60 mL/min/1.73 m2 | 20 (23) | 75 (20) | 17 (29) | 0.31 |
| Urea, mmol/L | 13.2 [9.6; 15.9] | 7.7 [5.6; 13.2] | 8.4 [5.9; 12.9] | <0.001 |
| Haemoglobin, g/dL | 14.3 [13.2; 15.3] | 14.0 [13.2; 14.9] | 13.8 [12.9; 14.6] | 0.16 |
| Sodium, mmol/L | 140 [139; 142] | 139 [138; 141] | 139 [137; 141] | 0.016 |
| NT‐proBNP, ng/L | 24.4 [15.7; 34.5] | 25.6 [16.2; 42.0] | 23.9 [13.1; 46.8] | 0.76 |
| ACEi/ARB | 75 (85) | 280 (76) | 48 (83) | 0.13 |
| Thiazides | 28 (32) | 47 (13) | 9 (16) | <0.001 |
| Beta‐blockers | 66 (75) | 247 (67) | 45 (78) | 0.14 |
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blockers; eGFR, estimated glomerular filtration rate; LV, left ventricular; NT‐proBNP, N‐terminal pro‐b‐type natriuretic peptide.
Values are mean ± standard deviation, n (%), or median [interquartile range].
P‐values are for trend values.
During follow‐up, hypokalaemia (both potassium ≤ 3.5 and <4.0 mmol/L) and hyperkalaemia (both potassium > 5.0 and ≥5.5 mmol/L) were infrequent. Serum potassium < 3.5 mmol/L was found, on at least one occasion, in 6 patients (1.2%; 1 in spironolactone group and 5 in control group) while potassium < 4.0 mmol/L was found in 46 patients (9%; 7 in spironolactone group and 39 in control group). Serum potassium > 5.0 mmol/L was found in 38 patients (8%; 29 in spironolactone group and 9 in control group) while potassium > 5.5 mmol/L was found in 5 patients (1.0%; 4 in spironolactone group and 1 in control group). Participants who developed hyperkalaemia were more likely to be treated with spironolactone compared with normokalaemic and hypokalaemic subjects (potassium > 5.0 mmol/L, 75.9% vs. 4.0–5.0 mmol/L, 51.8% vs. <4.0 mmol/L, 27.3%; P < 0.001).
During the entire trial duration, spironolactone (mean daily dose: 33.8 ± 13.9 mg) increased serum potassium on average (percentile25−75) by 0.23 (0.16; 0.29) mmol/L. Risk factors for developing potassium > 5.0 mmol/L during the trial included the presence of diabetes mellitus {odds ratio [OR]: 1.21 [95% confidence interval (CI) 2.14; 3.79]} and randomization to spironolactone (OR: 2.83 [95% CI 1.49; 5.37]). Conversely, the occurrence of a potassium concentration < 4.0 mmol/L was directly associated with use of thiazides (OR: 2.39 [95% CI 1.32; 4.34]), blood urea concentration (OR: 2.15 [95% CI 1.34; 3.39] per 10 mg/dL increase), and history of hypertension (OR: 2.32 [95% CI 1.02; 5.29]) and inversely with spironolactone treatment (OR: 0.30 [95% CI 0.18; 0.52]) (Figure 1 ).
Figure 1.
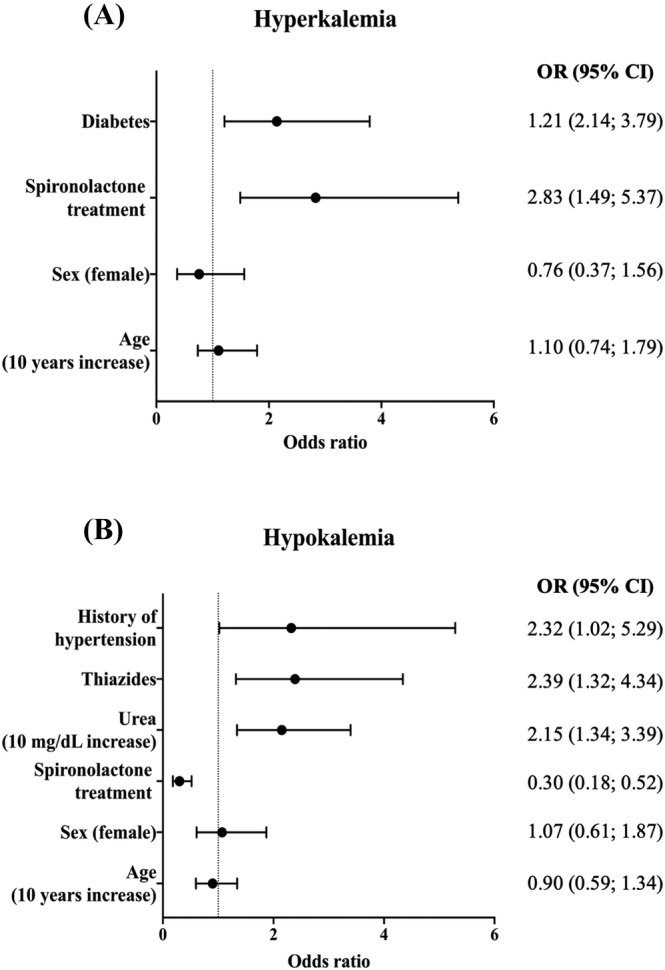
Forest plot of odds ratio (OR) for the predictors of (A) hyperkalaemia (potassium > 5.0 mmol/L) and (B) hypokalaemia (potassium < 4.0 mmol/L) at follow‐up. CI, confidence interval.
Discussion
In patients with risk factors for HF, the relationship between serum potassium concentrations and adverse outcomes has previously been demonstrated to be U‐shaped, with both low and high serum potassium associated with adverse outcomes. 9
Those treated for hypertension had twice the risk of incident hypokalaemia in our population, which could largely be attributed to thiazide‐type diuretic therapy, a not unexpected finding. 10 , 11 In hypertensive patients, reductions in serum potassium may increase the arrhythmia burden. In the Multiple Risk Factor Intervention Trial (MRFIT), a 1 mmol/L reduction in serum potassium produced a 28% increase in ventricular arrhythmias. 12 The MRFIT data also suggested that hypokalaemia increased the risk of sudden cardiac death (SCD) in hypertensives only if they were receiving high doses of thiazide diuretic agents. 12 Danish registry data (8976 patients with hypertension and plasma potassium ≤ 3.7 mmol/L) showed that persistent hypokalaemia was frequent and associated with increased all‐cause and cardiovascular mortality. In this cohort, correcting hypokalaemia was associated with a good prognosis. 13 We found that spironolactone consistently reduced the risk of hypokalaemia, which may translate into a reduction in arrhythmias and SCD as suggested by previous case–control studies in hypertension 7 and in large trials of patients with a reduced left ventricular ejection fraction after myocardial infarction or with HF. 14 Whether potassium supplements can correct hypokalaemia and if so whether this would also reduce SCD is not known. Potassium‐sparing diuretics other than MRA can certainly correct hypokalaemia and reduce blood pressure, but their effects on morbidity and mortality are unknown. 15
In our study, diabetes and being assigned to MRA were independent predictors of serum potassium > 5.0 mmol/L, consistent with previous reports. 16 We demonstrated that the steroidal MRA spironolactone at doses up to 50 mg/day only increased serum potassium concentration slightly, with a low risk of severe hyperkalaemia. In this trial population, spironolactone also reduced circulating markers of fibrosis and blood pressure and improved cardiac structure and function, therefore suggesting a positive benefit–risk ratio. 4 Similar antifibrotic features were also found with high doses (100–200 mg/day) of eplerenone in another trial of patients with type 2 diabetes and a high cardiovascular risk, 17 , 18 , 19 as well as several trials of HF. 8
Patients with CKD show a high risk for hyperkalaemia, which in general is more frequent the lower the eGFR. 20 In our study, renal dysfunction was not associated with an increased risk of dyskalaemia. This finding was probably due to overall preserved renal function in our population (median eGFR: 71.3 mL/min/1.73 m2) with a low prevalence of CKD (eGFR < 60 mL/min/1.73 m2: n = 112 [22%]).
Altogether, this efficacy and safety profile sets the stage for trials of MRA aiming to reduce cardiovascular (including HF) and renal morbidity and mortality in patients at risk of HF. Importantly, two trials with the non‐steroidal MRA finerenone have already demonstrated benefits on renal function and cardiovascular morbidity with an acceptable safety profile in patients with proteinuric diabetic kidney disease. 21 , 22 In particular, a recent analysis of the FIDELIO‐DKD study reported that finerenone was associated with a low absolute risk of clinically relevant hyperkalaemia (incidence of moderate hyperkalaemia 4.5%) across the trial. 23 To date, it is not yet clear if non‐steroidal MRA have any advantage over spironolactone or eplerenone in terms of hyperkalaemia risk or, indeed, whether they share similar benefits. 24
Our analysis has some limitations. HOMAGE was conducted open‐label, a pragmatic decision because the primary endpoint was changes in biomarkers of collagen metabolism measured in a laboratory blind to treatment assigned. Double‐blind, placebo‐controlled trials cause a huge increase in costs and logistic complexity. The dose of spironolactone was not fixed but adapted according to serum potassium. Plasma potassium level was assessed only at 1 and 9 months after randomization; consequently, we cannot exclude fluctuation of plasma potassium during the intercurrent periods. The proportion of patients prescribed renin‐angiotensin system inhibitors was similar across serum potassium groups, but we did not collect their dose in our dataset.
In conclusion, for people at risk of developing HF and with relatively normal renal function, spironolactone, mostly at doses of 25–50 mg/day, reduced the risk of hypokalaemia and caused only a small increase in serum potassium of uncertain clinical significance.
Conflict of interest
Patrick Rossignol reports (outside this manuscript) consulting for Bayer, CinCor, G3P, Idorsia, and KBP; honoraria from Ablative Solutions, AstraZeneca, Bayer, Boehringer‐Ingelheim, Corvidia, CVRx, Fresenius, Grunenthal, Novartis, Novo Nordisk, Relypsa Inc., a Vifor Pharma Group Company, Sanofi, Sequana Medical, Servier, Stealth Peptides, and Vifor Fresenius Medical Care Renal Pharma; and cofounder: CardioRenal. John Cleland reports (outside this manuscript) funding and personal honoraria from Abbott, Amgen, Bayer, Boehringer‐Ingelheim, Bristol Myers Squibb, Medtronic, Novartis, Servier, and Vifor Pharma. All other authors have no conflicts of interest to declare.
Funding
The HOMAGE trial (Clinical‐Trials.gov: NCT02556450, EudraCT‐No. 2015‐000413‐48) was funded by the European Commission under the Health Cooperation Work Programme of the 7th Framework Programme, under the Grant Agreement no. 305 507.
Monzo, L. , Ferreira, J. P. , Cleland, J. G. F. , Pellicori, P. , Mariottoni, B. , Verdonschot, J. A. J. , Hazebroek, M. R. , Collier, T. J. , Cuthbert, J. J. , Pieske, B. , Edelmann, F. , Petutschnigg, J. , Khan, J. , Ahmed, F. Z. , Girerd, N. , Bozec, E. , Díez, J. , González, A. , Clark, A. L. , Cosmi, F. , Staessen, J. A. , Heymans, S. , Rossignol, P. , and Zannad, F. (2022) Dyskalemia in people at increased risk for heart failure: findings from the heart ‘OMics’ in AGEing (HOMAGE) trial. ESC Heart Failure, 9: 4352–4357. 10.1002/ehf2.14086.
[Correction added on 14 December 2022, after first online publication: Six authors and their affiliations have been added in this version.]
References
- 1. Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol. 2015; 10: 1050–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferreira JP, Butler J, Rossignol P, Pitt B, Anker SD, Kosiborod M, Lund LH, Bakris GL, Weir MR, Zannad F. Abnormalities of potassium in heart failure: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2020; 75: 2836–2850. [DOI] [PubMed] [Google Scholar]
- 3. Macdonald JE, Struthers AD. What is the optimal serum potassium level in cardiovascular patients? J Am Coll Cardiol. 2004; 43: 155–161. [DOI] [PubMed] [Google Scholar]
- 4. Cleland JGF, Ferreira JP, Mariottoni B, Pellicori P, Cuthbert J, Verdonschot JAJ, Petutschnigg J, Ahmed FZ, Cosmi F, Brunner La Rocca HP, Mamas MA, Clark AL, Edelmann F, Pieske B, Khan J, McDonald K, Rouet P, Staessen JA, Mujaj B, Gonzalez A, Diez J, Hazebroek M, Heymans S, Latini R, Grojean S, Pizard A, Girerd N, Rossignol P, Collier TJ, Zannad F, Committees HT, Investigators . The effect of spironolactone on cardiovascular function and markers of fibrosis in people at increased risk of developing heart failure: the heart ‘OMics’ in AGEing (HOMAGE) randomized clinical trial. Eur Heart J. 2021; 42: 684–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alexandrou ME, Papagianni A, Tsapas A, Loutradis C, Boutou A, Piperidou A, Papadopoulou D, Ruilope L, Bakris G, Sarafidis P. Effects of mineralocorticoid receptor antagonists in proteinuric kidney disease: a systematic review and meta‐analysis of randomized controlled trials. J Hypertens. 2019; 37: 2307–2324. [DOI] [PubMed] [Google Scholar]
- 6. Khosla N, Kalaitzidis R, Bakris GL. Predictors of hyperkalemia risk following hypertension control with aldosterone blockade. Am J Nephrol. 2009; 30: 418–424. [DOI] [PubMed] [Google Scholar]
- 7. Siscovick DS, Raghunathan TE, Psaty BM, Koepsell TD, Wicklund KG, Lin X, Cobb L, Rautaharju PM, Copass MK, Wagner EH. Diuretic therapy for hypertension and the risk of primary cardiac arrest. N Engl J Med. 1994; 330: 1852–1857. [DOI] [PubMed] [Google Scholar]
- 8. Pellicori P, Ferreira JP, Mariottoni B, Brunner‐La Rocca HP, Ahmed FZ, Verdonschot J, Collier T, Cuthbert JJ, Petutschnigg J, Mujaj B, Girerd N, Gonzalez A, Clark AL, Cosmi F, Staessen JA, Heymans S, Latini R, Rossignol P, Zannad F, Cleland JGF. Effects of spironolactone on serum markers of fibrosis in people at high risk of developing heart failure: rationale, design and baseline characteristics of a proof‐of‐concept, randomised, precision‐medicine, prevention trial. The Heart OMics in AGing (HOMAGE) trial. Eur J Heart Fail. 2020; 22: 1711–1723. [DOI] [PubMed] [Google Scholar]
- 9. Kovesdy CP, Matsushita K, Sang Y, Brunskill NJ, Carrero JJ, Chodick G, Hasegawa T, Heerspink HL, Hirayama A, Landman GWD, Levin A, Nitsch D, Wheeler DC, Coresh J, Hallan SI, Shalev V, Grams ME, Consortium CKDP . Serum potassium and adverse outcomes across the range of kidney function: a CKD Prognosis Consortium meta‐analysis. Eur Heart J. 2018; 39: 1535–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schnaper HW, Freis ED, Friedman RG, Garland WT, Hall WD, Hollifield J, Jain AK, Jenkins P, Marks A, McMahon FG, Sambol NC, Williams RL, Winer N. Potassium restoration in hypertensive patients made hypokalemic by hydrochlorothiazide. Arch Intern Med. 1989; 149: 2677–2681. [PubMed] [Google Scholar]
- 11. Krogager ML, Mortensen RN, Lund PE, Boggild H, Hansen SM, Kragholm K, Aasbjerg K, Sogaard P, Torp‐Pedersen C. Risk of developing hypokalemia in patients with hypertension treated with combination antihypertensive therapy. Hypertension. 2020; 75: 966–972. [DOI] [PubMed] [Google Scholar]
- 12. Cohen JD, Neaton JD, Prineas RJ, Daniels KA. Diuretics, serum potassium and ventricular arrhythmias in the Multiple Risk Factor Intervention Trial. Am J Cardiol. 1987; 60: 548–554. [DOI] [PubMed] [Google Scholar]
- 13. Krogager ML, Sogaard P, Torp‐Pedersen C, Boggild H, Lee CJ, Bonde A, Thomassen JQ, Gislason G, Pareek M, Kragholm K. Impact of plasma potassium normalization on short‐term mortality in patients with hypertension and hypokalemia or low normal potassium. BMC Cardiovasc Disord. 2020; 20: 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shen L, Jhund PS, Petrie MC, Claggett BL, Barlera S, Cleland JGF, Dargie HJ, Granger CB, Kjekshus J, Kober L, Latini R, Maggioni AP, Packer M, Pitt B, Solomon SD, Swedberg K, Tavazzi L, Wikstrand J, Zannad F, Zile MR, McMurray JJV. Declining risk of sudden death in heart failure. N Engl J Med. 2017; 377: 41–51. [DOI] [PubMed] [Google Scholar]
- 15. Williams B, MacDonald TM, Morant SV, Webb DJ, Sever P, McInnes GT, Ford I, Cruickshank JK, Caulfield MJ, Padmanabhan S, Mackenzie IS, Salsbury J, Brown MJ, British Hypertension Society Programme of P , Treatment of Hypertension With Algorithm based Therapy Study G . Endocrine and haemodynamic changes in resistant hypertension, and blood pressure responses to spironolactone or amiloride: the PATHWAY‐2 mechanisms substudies. Lancet Diabetes Endocrinol. 2018; 6: 464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bandak G, Sang Y, Gasparini A, Chang AR, Ballew SH, Evans M, Arnlov J, Lund LH, Inker LA, Coresh J, Carrero JJ, Grams ME. Hyperkalemia after initiating renin‐angiotensin system blockade: the Stockholm Creatinine Measurements (SCREAM) project. J Am Heart Assoc. 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johansen ML, Ibarrola J, Fernandez‐Celis A, Schou M, Sonne MP, Refsgaard Holm M, Rasmussen J, Dela F, Jaisser F, Faber J, Rossignol P, Lopez‐Andres N, Kistorp C. The mineralocorticoid receptor antagonist eplerenone suppresses interstitial fibrosis in subcutaneous adipose tissue in patients with type 2 diabetes. Diabetes. 2021; 70: 196–203. [DOI] [PubMed] [Google Scholar]
- 18. Brandt‐Jacobsen NH, Johansen ML, Rasmussen J, Forman JL, Holm MR, Faber J, Rossignol P, Schou M, Kistorp C. Effect of high‐dose mineralocorticoid receptor antagonist eplerenone on urinary albumin excretion in patients with type 2 diabetes and high cardiovascular risk: data from the MIRAD trial. Diabetes Metab. 2021; 47: 101190. [DOI] [PubMed] [Google Scholar]
- 19. Brandt‐Jacobsen NH, Lav Madsen P, Johansen ML, Rasmussen JJ, Forman JL, Holm MR, Rye Jorgensen N, Faber J, Rossignol P, Schou M, Kistorp C. Mineralocorticoid receptor antagonist improves cardiac structure in type 2 diabetes: data from the MIRAD trial. JACC Heart Fail. 2021; 9: 550–558. [DOI] [PubMed] [Google Scholar]
- 20. Moranne O, Froissart M, Rossert J, Gauci C, Boffa JJ, Haymann JP, M'Rad MB, Jacquot C, Houillier P, Stengel B, Fouqueray B, NephroTest Study G . Timing of onset of CKD‐related metabolic complications. J Am Soc Nephrol. 2009; 20: 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, Joseph A, Kolkhof P, Nowack C, Schloemer P, Ruilope LM, Investigators F‐D. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021; 28: 2252–2263. [DOI] [PubMed] [Google Scholar]
- 22. Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, Kolkhof P, Nowack C, Schloemer P, Joseph A, Filippatos G, Investigators F‐D. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020; 383: 2219–2229. [DOI] [PubMed] [Google Scholar]
- 23. Agarwal R, Joseph A, Anker SD, Filippatos G, Rossing P, Ruilope LM, Pitt B, Kolkhof P, Scott C, Lawatscheck R, Wilson DJ, Bakris GL, Investigators F‐D. Hyperkalemia risk with finerenone: results from the FIDELIO‐DKD trial. J Am Soc Nephrol. 2022; 33: 225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Agarwal R, Joseph A, Anker S, Filippatos G, Rossing P, Ruilope L, Pitt B, Kolkhof P, Scott C, Lawatscheck R, Wilson D, Bakris G. Hyperkalemia risk with finerenone: results from the FIDELIO‐DKD trial. J Am Soc Nephrol. 2022; 3: 225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]


