Abstract
Heart failure (HF) treatment has changed substantially over the last 30 years, leading to significant reductions in mortality and hospital admissions in patients with HF with reduced ejection fraction (HFrEF). Currently, the optimization of guideline‐directed chronic HF therapy remains the mainstay to further improve quality of life, mortality, and HF hospitalizations for patients with HFrEF. The angiotensin receptor‐neprilysin inhibitor sacubitril/valsartan (S/V) has an important role in the treatment of patients with HFrEF. The PARADIGM‐HF (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) randomized controlled trial has established solid evidence for the treatment of HFrEF in various subgroups. Apart from HFrEF, several studies have been conducted using S/V in various indications: patients hospitalized with acute decompensated HF, HF with preserved ejection fraction, acute myocardial infarction with reduced ejection fraction, uncontrolled and resistant hypertension, and chronic kidney disease. Data from the German Institute for Drug Use Evaluation reveal that implementation of S/V has increased steadily over time and, by the end of 2021, an estimated 266 000 patients were treated with S/V in Germany. The estimated cumulative real‐world patient exposure is >5.5 million patient‐treatment years worldwide. The number of patients treated with S/V largely exceeds the number of patients treated in clinical trials, and the current indication for S/V is larger than the strict inclusion/exclusion criteria of the randomized trials. Especially elderly patients, women, and patients with more and more severe comorbidities are underrepresented in the clinical trials. We therefore aimed to summarize the importance of S/V in HF in terms of efficacy and safety in clinical trials and daily clinical practice.
Keywords: Heart failure, Sacubitril/valsartan, Efficacy, Effectiveness, Safety, Implementation
Introduction
Heart failure (HF) is one of the most rapidly growing cardiovascular (CV) conditions, imposing a substantial burden on healthcare systems worldwide. 1 , 2 Over the last 15–20 years, a remarkable development of HF pharmacotherapies has been achieved. 2 , 3 However, HF remains a global epidemic with more than 64 million patients worldwide, accounting for 9.91 million years lost due to disability (YLDs) and 346.17 billion US $ expenditure. 4 For illustration, in Germany, HF prevalence has been estimated to be 3.9%, 5 and the number of HF hospitalizations increased continuously and has almost doubled between 2000 and 2017. 6 Additionally, HF continues to be the most common cause of hospitalization and in‐hospital death. 5 , 6 , 7
Over the last 10 years, new HF drugs have merged targeting various pathways, such as those that simultaneously suppress the renin‐angiotensin‐aldosterone system (RAAS) and the breakdown of endogenous natriuretic peptides [e.g. sacubitril/valsartan (S/V)]. 2 , 3 More recently, other potential treatment mechanisms have been explored, such as the sodium/glucose co‐transporter inhibitors (SGLT2i), the guanylate cyclase stimulators, and the cardiac myosin activators. 2 However, because the transferability of the results from randomized controlled trials (RCTs) into clinical practice is challenging, and inertia and resilience to implement even highly effective novel treatment options are known constraints, 8 we aimed to summarize treatment with S/V in HF in terms of efficacy/effectiveness and safety in clinical trials and daily clinical practice.
Methods
We systematically searched the bibliographic database MEDLINE (via PubMed) from 1 January 1990 until 31 December 2021 for clinical trials, real‐world data from observational studies, and registries and/or systematic reviews/meta‐analyses reporting efficacy/effectiveness and/or safety/pharmacovigilance data for S/V. We included articles in English, German, and French. We conducted searches using subject terms and keywords searching. We used the pre‐specified MESH, title/abstract, and publication type terms (Supporting Information S1).
Regarding efficacy/effectiveness, we focused on the indication as per European Medicines Agency (EMA) approval, that is, heart failure with reduced ejection fraction (HFrEF). Regarding safety and tolerability, again, we included preferentially data on patients with HFrEF, but we also took HF with mildly reduced ejection fraction (HFmrEF), HF with preserved ejection fraction (HFpEF), and other indications and aggregated data from pharmacovigilance into account, assuring a holistic understanding of S/V's safety and tolerability profile.
We disregarded individual case reports and case series as well as real‐world studies already included in comprehensive systematic reviews/meta‐analyses and Periodic Safety Update Reports (PSURs) in order to avoid undue granularity. In case of manifold systematic reviews/meta‐analyses accruing over time, mostly, the newest one has been taken into account, in order to present the most up‐to‐date data and to avoid outdated or redundant statements.
The data collated from literature were complemented by and correlated to previously unpublished data that are novel and/or were not yet publicly available. The latter includes, firstly, proprietary information regarding, for example, drug approvals and patient numbers/exposure in clinical trials and in real‐world practice and, secondly, fundamental insights into the PSURs for S/V provided by Novartis Pharma AG to drug regulatory agencies [e.g. EMA, the U.S. Food and Drug Administration (FDA), the Swiss Agency for Therapeutic Products (Swissmedic), or the U.K. Medicines and Healthcare products Regulatory Agency (MHRA)] and national competent authorities [e.g. the Federal Institute for Drugs and Medical Devices (BfArM) in Germany or the Austrian Agency for Health and Food Safety (AGES)]. PSURs are being mandated by and shared with health authorities and/or drug regulatory agencies for all approved medicinal products on a regular basis, and they include, along with other safety data, also the case reports as well as the case series that were disregarded above. They represent comprehensive pharmacovigilance assessments that critically analyse the totality of worldwide safety data and are intended to provide an evaluation of the risk–benefit balance of a medicinal product at defined time points after its marketing authorization. 9
Thirdly, we analysed the database of the German Institute for Drug Use Evaluation (Deutsches Arzneiprüfungsinstitut e.V., DAPI) in order to analyse and elucidate the implementation of S/V in real‐world clinical practice in Germany between Q1/2016 (product launch) and Q4/2021 (latest quarter available). The DAPI database contains anonymized dispensing data from more than 80% (until June 2019) and more than 95% (from July 2019 onwards) of Germany's community pharmacies, claimed at the expense of the statutory health insurance (SHI) funds. Data were extrapolated by regional factors to 100% of the SHI‐insured population, which accounts for approximately 88% of Germany's population, that is, approximately 73.3 million subjects. 10 Fourthly, we report data obtained from IQVIA, 11 a healthcare data provider offering the IQVIA Analytic Platform that covers ~80% of the German prescription market. Prescription data were collected on a physician level and aggregated towards a practice level in order to inform on the share of general practitioners' (GPs') practices and office‐based cardiologists' (OBCs') practices that prescribed S/V in Q4/2021. All data were fully anonymized. Fifthly, we analysed the IQVIA™ longitudinal prescription (LRx) database (data status as of January 2022) that covers approximately 80% of SHI claims in Germany. Anonymized treatment courses are longitudinal across prescribers and pharmacies and allow accurate description and quantification of treatments and basic patient demographics such as age and gender. To quantify patients on S/V treatment, the number of distinct treatment histories with at least 1 day of supply each quarter was extrapolated to total national retail pharmacy sales. Constants to extrapolate LRx patient counts to national retail levels were determined from the ratio of observed claimed packs in LRx vs. total national claims in the IQVIA™ PharmaScope database. The share of S/V patients with concurrent SGLT2i therapy (dapagliflozin, empagliflozin, canagliflozin, or ertugliflozin as mono or fixed‐dose combination product) was approximated by determining the number of treatment days with supply of S/V, SGLT2i, and both, respectively. In each quarter, patients were classified S/V, S/V + SGLT2i, or SGLT2i alone, based on the regimen with most treatment days. The fraction of patients with co‐treatment was defined as (S/V + SGLT2i) divided by S/V.
Pharmacology and clinical development programme
Sacubitril/valsartan (LCZ696) is a first‐in‐class angiotensin receptor‐neprilysin inhibitor (ARNI). By simultaneous inhibition of neprilysin and blocking the angiotensin (AT1) receptor, S/V complements the beneficial effects of inhibition of the maladaptive effects of the activated RAAS with the feature of enhancing the adaptive effects of the endogenous natriuretic peptide system. The latter's vasodilatory, natriuretic, and diuretic actions 12 co‐determine the antiproliferative, antihypertrophic, and antifibrotic effects of ARNI 13 and ultimately their superior effect on beneficial reverse cardiac remodelling over angiotensin‐converting enzyme inhibitors (ACEi)/angiotensin receptor blocker (ARB). 14
Sacubitril/valsartan has been extensively studied in a comprehensive clinical development programme that focuses on HF ‘Fortifying Heart Failure clinical evidence and patient quality of life’ [FortiHFy programme that, albeit focusing on HFrEF, covers the whole spectrum of ejection fraction (EF)] and also addresses hypertension 15 and post‐myocardial infarction (MI) care. 16 Overall, more than 30 000 subjects have been exposed to S/V in clinical trials, 17 , 18 , 19 among many others, with >22 500 patients participating in HF trials (Figure 1 ). Because we are capitalizing on its EMA approved indication, particularly, the HFrEF outcomes of S/V will be outlined and discussed below.
Figure 1.
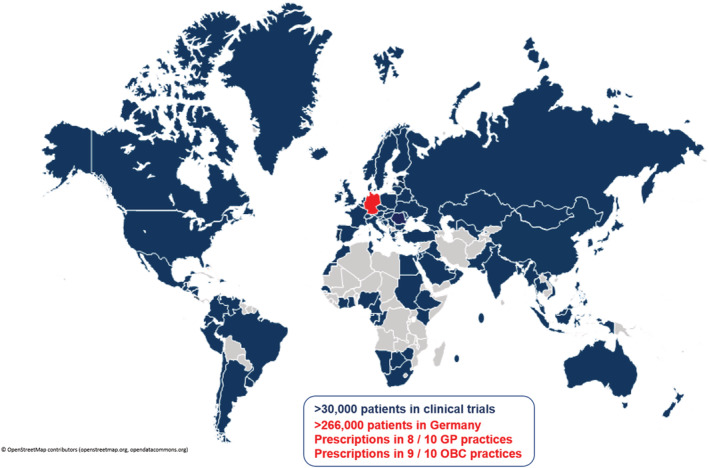
Sacubitril/valsartan is approved in 117 countries. Sacubitril/valsartan in randomized clinical trials and real‐world practice in Germany. GP, general practitioner; OBC, office‐based cardiologist.
Heart failure with reduced ejection fraction: efficacy in and outside clinical trials
Efficacy of sacubitril/valsartan in the PARADIGM‐HF trial
PARADIGM‐HF (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) was an RCT designed to test the superiority of S/V compared with enalapril in improving morbidity and mortality in patients with HFrEF. 17 Overall and after a median follow‐up of 27 months, S/V was superior to the standard‐of‐care enalapril in reducing HF hospitalizations by 21%, CV mortality by 20%, and all‐cause mortality by 16%. 17
Further sub‐analysis from this trial showed a reduction in sudden cardiac death rate, similarly in patients without as well as in those with an implantable cardioverter defibrillator (ICD) 20 and prolonged estimated survival and event‐free survival. 21 S/V was superior to enalapril in improving quality of life (QoL) 17 , 22 as well as functional and social activity. 23 Additionally, S/V demonstrated a reduction in the incidence of diabetes requiring insulin treatment, 24 slowing decline in estimated glomerular filtration rate (eGFR), 25 a lower rate of hyperkalaemia, 26 and a lower requirement of loop diuretics. 27 Interestingly, patients on S/V showed a reduction in biomarkers known to predict clinical outcomes, for example, N‐terminal pro‐b‐type natriuretic peptide (NT‐proBNP) as well as troponin T 28 and soluble suppression of tumorigenesis‐2 (sST2). 29 Of note, S/V was able to demonstrate an early clinically relevant benefit already at 30 days. 28
The superiority of S/V over ACEi in the PARADIGM‐HF trial was independent of the aetiology and HF duration, age, background medications, EF, blood pressure, liver function, the presence of diabetes, chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), or pulmonary congestion and previous HF hospitalization as well as geography. 25 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41
Based on the above and according to the 2021 European as well the American guidelines for the treatment of HF, S/V has a class I recommendation as a first‐line therapy for stable HFrEF patients. 42 , 43
Acute decompensated and severe heart failure
The initiation of S/V in patients hospitalized for acute decompensated HF (ADHF) shortly after haemodynamic stabilization is feasible and safe. 44 , 45 The PIONEER‐HF (Comparison of Sacubitril–Valsartan vs. Enalapril on Effect on NT‐proBNP in Patients Stabilized from an Acute Heart Failure Episode) trial included patients during hospitalization for ADHF. 44 S/V led to a greater unloading of the heart suggested by a stronger reduction of NT‐proBNP concentration and a reduction of exploratory outcomes (HF re‐hospitalizations, left ventricular assist device implantation, death, and heart transplantation) compared with enalapril therapy without safety concerns. 44 Notably, this trial was not powered for clinical endpoints but still provides signals for risk reduction.
In a recent systematic review, the management of clinically stabilized patients hospitalized for ADHF with S/V significantly reduces the risk of serious clinical events and reduces NT‐proBNP concentrations. 46
As such, initiation of S/V in ACEi‐naïve patients with HFrEF may be considered (class of recommendation IIb, level of evidence B). 43
In the recent LIFE investigator‐initiated trial, performed in patients with advanced HFrEF who experienced New York Heart Association (NYHA) class IV symptoms within the previous 3 months or required chronic inotropic therapy, no statistically significant differences between S/V and valsartan with respect to reducing NT‐proBNP levels (primary outcome) and clinical outcomes (secondary and tertiary outcomes) were observed. 47 However, the trial was underpowered to detect differences in clinical outcomes and has been prematurely discontinued due to the COVID‐19 pandemic. 47
Post‐myocardial infarction
Recently, the PARADISE‐MI (Prospective ARNI vs. ACE inhibitor trial to Determine Superiority in reducing heart failure Events after Myocardial Infarction) trial investigated the efficacy and safety of S/V compared with ramipril initiated early after acute myocardial infarction (AMI) in patients without previous HF but with reduced left ventricular EF and/or transient pulmonary congestion. 18 S/V did not reduce the primary endpoint (CV death, first HF hospitalization, or outpatient HF visit) in a contemporary enriched AMI population, compared with ramipril. Rates were numerically lower in the S/V arm, and the composite endpoints that included all HF events, not just the first one, or investigator‐reported HF events, respectively, showed a benefit with S/V. 48 Remarkably, in this vulnerable high‐risk post‐AMI patient population (76% with ST‐elevation myocardial inarction (STEMI)), included in a trial with early treatment initiation (mean time from hospitalization to randomization 4.3 days) and without a run‐in phase, comparable safety and tolerability was shown. Treatment discontinuations overall and because of adverse events (AEs) or severe AEs (SAEs) were all similar between S/V and ramipril groups, as was the safety profile obtained from extensive serum monitoring for hyperkalaemia, renal function, and liver‐enzyme abnormalities. Hypotension was more common with S/V, and cough was more common with ramipril (both P < 0.001), as were hepatotoxic effects (P = 0.04). The rates of angioedema (0.5% vs. 0.6%, P = 0.59) and cognitive impairment (1.9% vs. 2.1%, P = 0.57) were not significantly different. 18
Effectiveness outside clinical trials
Effectiveness of ARNI compared with RAASi (ACEi/ARB) was demonstrated in a cohort study of 51 208 HFrEF patients ≥65 years in clinical daily practice in the USA. 49 In a propensity score‐matched analysis, the primary endpoint of CV death/HF hospitalization was lower in S/V‐treated patients with a hazard ratio (HR) of 0.84 (95% confidence interval 0.80–0.89, P = 0.001). No evidence of treatment effect heterogeneity was observed across pre‐specified subgroups. Regarding the primary endpoint, the HR for S/V patients without and with prior RAASi therapy was below 1. All‐cause mortality was significantly lower in S/V patients in both subgroups, that is, in patients with and without prior RAASi. 49
Additionally, in a propensity score‐matched database analysis from Taiwan, 502 ARNI users and 489 ARB users were included in clinical outcome analyses. 50 Patients who received ARNI therapy had a significantly lower risk of the primary composite outcome of CV death/HF hospitalization than patients who received ARBs only. 50
Moreover, a recent systematic review incorporating 68 unique studies 51 assessed the effectiveness of S/V in stable HFrEF. Most of the studies performing comparisons reported superior effectiveness of S/V in reducing the risk of HF hospitalization, all‐cause hospitalization, and all‐cause mortality as compared with standard of care. A meta‐analysis was conducted only for all‐cause mortality, showing that S/V use was associated with a significant reduction of 25% compared with ACEi/ARBs based on concordant findings in all three comparative real‐world studies reporting this endpoint 51 (Figure 2 ).
Figure 2.
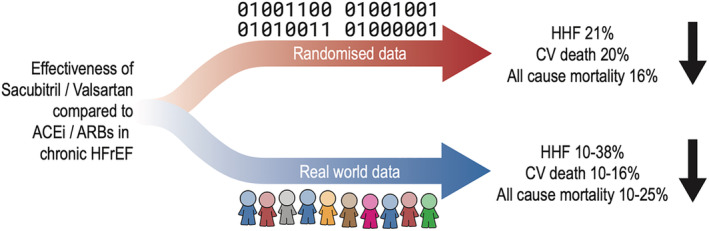
Effectiveness of sacubitril/valsartan compared with ACEi/ARBs in chronic HFrEF in randomized clinical trials and real‐world practice. ACEi, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CV, cardiovascular; HFrEF, heart failure with reduced ejection fraction; HHF, heart failure hospitalization.
Safety and tolerability in and outside clinical trials
The superiority of S/V over enalapril in PARADIGM‐HF was not accompanied by major safety issues with an overall safety and tolerability comparable with ACEi. 17 S/V was associated with significantly less drug discontinuations due to AEs or renal impairment, less hyperkalaemia > 6 mmol/L, and less cough compared with enalapril. Conversely, patients on S/V were more likely to have symptomatic hypotension, but discontinuation of study drug due to hypotension was infrequent and comparable between both groups. 17
The initiation of S/V in patients hospitalized for ADHF shortly after haemodynamic stabilization is also safe. 44 , 45 In the PIONEER‐HF trial, rates of worsening renal function (WRF), hyperkalaemia, symptomatic hypotension, and angioedema did not differ significantly between the enalapril and S/V groups. 44 This was also true in numerous high‐risk subgroups including patients with low baseline systolic blood pressure (SBP) (〈=118 mmHg) and CKD (<60 mL/min/1.73 m2) 52 as well as in patients with new‐onset HF and those naïve to RAASi pre‐treatment. 53
In the TRANSITION trial, S/V initiation in the hospital or within 2 weeks after discharge showed similar tolerability, and discontinuation rates due to AEs remained low in both groups. 45 Moreover, TRANSITION trial data support tolerability of S/V in patients with de novo HF, of whom many were not pre‐treated with RAASi, as these patients were more likely to obtain target dose and less likely to experience SAEs or to discontinue treatment due to AEs compared with patients with prior HFrEF diagnosis. 54
In the LIFE trial, there were no differences with respect to the development of symptomatic hypotension or WRF between the treatment arms, whereas significantly more patients developed hyperkalaemia in the S/V arm (17%) compared with the valsartan arm (9%) (P = 0.04). There were no significant differences in other secondary tolerability endpoints or SAEs between the treatment arms in a vulnerable population of patients with severe advanced HF in whom medical treatment options are limited. 47
Kim et al. explored the safety profile of S/V in 15 538 HFrEF patients, by comparing AEs in RCTs and real‐world use. 55 They showed that in clinical trials, there was no statistical difference in the composite of hypotension, renal dysfunction, hyperkalaemia, and angioedema between S/V and its comparators (ACEi or ARBs). With regard to individual AEs, hypotension was more frequent with S/V, whereas renal dysfunction was less frequent. Similar patterns were found in real‐world pharmacovigilance analyses based on FDA data: while hypotension exhibited a stronger association to S/V compared with other commonly used HF medications, the opposite was true for renal dysfunction, hyperkalaemia, and angioedema that showed stronger association with ACEi (enalapril and lisinopril) and spironolactone than with S/V. The authors concluded that risks of hypotension, renal dysfunction, hyperkalaemia, and angioedema appear low and acceptable in both RCTs and global clinical practice. 55
Moreover, a recent meta‐analysis of cohort studies assessed sex‐specific data on major adverse outcomes and long‐term safety in 8981 patients treated with S/V. AEs were infrequent in both men and women and comparable with the findings of the pivotal trials, indicating that the safety of S/V can be replicated outside the clinical trial context. 56
Efficacy and safety in patients with heart failure with preserved ejection fraction
The PARAGON‐HF (Prospective Comparison of ARNI with ARB Global Outcomes in HF with Preserved Ejection Fraction) trial evaluated the effect of S/V in patients with HFpEF compared with valsartan. 19 S/V did not result in a significantly lower rate of total hospitalizations for HF and death from CV causes among patients with HF and an EF of 45% or higher. In this trial, AEs and SAEs of S/V were similar to valsartan. Rates of hypotension and angioedema were higher; however, a lower incidence of hyperkalaemia and serum creatinine ≥ 2 mmol/L were observed. 19 Furthermore, the decline in eGFR was less for S/V than for valsartan. Reduction in adverse renal outcomes was independent of baseline eGFR [<60 vs. ≥60 mL/min/1.73 m2 (P‐interaction = 0.92)]. 57
In the PARALLAX‐HF trial, 2572 patients with HF, EF > 40%, elevated NT‐proBNP levels, and reduced QoL were enrolled and randomized to S/V or standard medical therapy, in order to assess changes in NT‐proBNP and 6 min walk test. 58 S/V was associated with a reduction in NT‐proBNP. However, ARNI was not associated with improvements in 6 min walk distance, Kansas City Cardiomyopathy Questionnaire—Clinical Summary Score (KCCQ‐CSS), or NYHA class. Regarding safety, SAEs were reported in similar proportions of patients in both groups. However, AEs and study drug‐related AEs were significantly more frequent in the S/V group, driven by hypotension and albuminuria. Angioedema was infrequent and not different between treatment groups (0.3% vs. 0.2%). Patients in the S/V group had a significantly lower decline in renal function (eGFR) at 24 weeks. 58
Recently, data from PARAGON‐HF and PARADIGM‐HF were pooled in order to examine S/V treatment effects across the spectrum of EF. 59 This showed that above an EF of approximately 55%, there was no detectable effect of S/V and that in the previously unstudied ‘EF gap’ of 40–50%, S/V significantly reduced hospitalization and mortality rates. Therefore and according to the 2021 HF European guidelines, S/V may be considered for patients with HFmrEF to reduce the risk of HF hospitalization and death. 43
Pharmacovigilance, pursuant to Periodic Safety Update Reports
From 7 July 2015 up to 31 July 2021 (last available PSUR cut‐off date), the estimated cumulative post‐marketing patient exposure (excluding clinical trial exposure) was 5 501 708 patient‐treatment years. The critical analysis of the worldwide safety data has revealed that the overall benefit–risk profile remains favourable and the benefit–risk balance of S/V in patients with HFrEF has remained positive and unchanged. Indeed, the identified risks of hypotension, renal impairment, hyperkalaemia, and angioedema have been well characterized during the post‐marketing period and the reporting rates of these risks have been decreasing constantly from PSUR 1 (2015) to PSUR 9 (2021) (Figure 3 ). The higher reporting rates of these risks observed in the period from PSUR 1 to PSUR 4 are consistent with a Weber effect, which is a phenomenon of increased volume of reported AEs for a new drug within its first years of approval. Indeed, the cumulative exposure‐adjusted reporting rate provides a more accurate estimator because it reflects the totality of reported cases related to the overall number of patient‐treatment years over the entire investigation period from PSUR 1 to PSUR 9. This also applies with respect to the potential risk of cognitive impairment that had been raised before S/V approval, based on theoretical considerations tracing back to basic research 60 (Figure 3 ).
Figure 3.
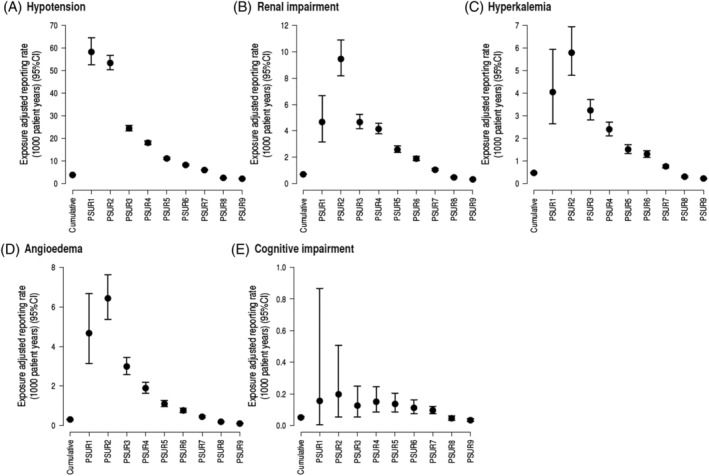
Exposure‐adjusted reporting rate of (A) hypotension cases, (B) renal impairment cases, (C) hyperkalaemia cases, (D) angioedema cases, and (E) cognitive impairment cases. CI, confidence interval; PSUR, Periodic Safety Update Report.
In line with data from recent preclinical and clinical studies 48 , 61 , 62 , 63 and analyses of data from Novartis and EMA pharmacovigilance databases, the last PSUR concludes that, based upon the totality of data available, to date, there is no clinical evidence to suggest a causal association between S/V and cognitive impairment.
In accordance with a requirement of the FDA, the ongoing Prospective Evaluation of Cognitive Function in Heart Failure: Efficacy and Safety of Entresto compared to Valsartan on Cognitive Function in Patients with Chronic Heart Failure and Preserved Ejection Fraction (PERSPECTIVE; NCT02884206) multicentre, randomized, double‐blinded trial is assessing the long‐term neurocognitive effects and safety of S/V, and results are expected in the second half of 2022. Considering the potentially low incremental impact on the individual HF patient's functioning and QoL, the impact of this potential risk in the benefit–risk balance is deemed low.
Thus, the extensive post‐marketing experience has revealed no additional important identified or potential risk and portends a replicable long‐term safety of S/V outside the clinical trial context.
Efficacy and safety in subgroups of interest
Combination of sacubitril/valsartan with other heart failure drugs
The superiority of S/V over ACEi in the PARADIGM‐HF trial was independent of background medication. 33 Initiation of S/V, even when titrated to the target dose, did not lead to greater discontinuation or dose down‐titrations of other key guideline‐directed medical therapies and was associated with fewer discontinuations of mineralocorticoid receptor antagonists (MRA). 64 Moreover, severe hyperkalaemia was more likely during treatment with enalapril than with S/V among MRA‐treated patients with symptomatic HFrEF in PARADIGM‐HF. 26 The combination of S/V and SGLT2i is efficacious, safe, and well tolerated. 65 , 66 , 67 , 68 , 69 Sub‐analyses from the two large HFrEF outcome trials with the SGLT2i dapagliflozin and empagliflozin 65 , 66 demonstrate that patients treated with S/V derive at least the same additional benefit from SGLT2i treatment as patients not on S/V. 3 Administration of SGLT2i on top of S/V was well tolerated, with similar rates of treatment discontinuations (overall and because of AEs), SAEs and AEs related to hyperkalaemia, hypotension, or WRF, as compared with placebo. 65 , 66 Thus, the combination of the ‘fantastic four’ drugs in HFrEF for maximum benefit on mortality, HF hospitalizations, and symptoms consists of S/V, beta‐blocker, MRA, and SGLT2i. 3 , 43 Real‐world data from a propensity score‐matched retrospective observational study with a median follow‐up of 27.6 months showed that combination of ARNI and SGLT2i was associated with a lower risk of HF hospitalization and CV mortality as compared with using either S/V or SGLT2i or ACEi/ARB. Combination of S/V and SGLT2i was also associated with echocardiographic improvements that were more prominent after the initiation of S/V, compared with the initiation of SGLT2i. 67 Similarly, real‐world data from Taiwan showed that a combination of SGLT2i and S/V in diabetic patients with HFrEF was well tolerated and associated with a significantly lower risk of HF hospitalization and a composite of all‐cause death or HF hospitalization as compared with patients with conventional therapy (ACEi/ARB). 68 In a Spanish registry that included 144 HFrEF patients who were treated with ARNI and SGLT2i, co‐administration was associated with a slight eGFR reduction, in line with findings from large, published trials with SGLT2i separately. None of the patients developed hyperkalaemia. With regard to clinical endpoints, combination therapy was associated with a statistically significant improvement in NYHA class throughout the 6 months of the study. 69
Safety in chronic kidney disease patients
The UK HARP‐III RCT included 414 patients with CKD (eGFR 20 to 60 mL/min/1.73 m2) who were randomly assigned to S/V 97/103 mg twice daily vs. the ARB irbesartan 300 mg once daily. Renal effects were similar with regard to efficacy and safety. S/V reduced SBP and diastolic blood pressure, levels of troponin I, and NT‐proBNP. 70
In PARADIGM‐HF, a post hoc composite renal outcome [≥50% reduction in eGFR or end‐stage renal disease (ESRD)] was reduced in the overall cohort (P = 0.028), with consistent effects in patients with and without CKD at screening (P‐interaction = 0.97). Similarly, the number of patients stopping study drug because of a renal AE was significantly lower in the S/V group (P = 0.002), comparably in patients with and without CKD (P‐interaction = 0.54). Likewise, the decline in eGFR was lower with S/V compared with enalapril (P < 0.001), once again with similar effects in patients with and without CKD at screening (P‐interaction = 0.54). 25
Complementary results have also been reported in real‐world HFrEF patients. 71 , 72 , 73 In an observational US study among patients with systolic HF, renal safety was assessed in 4667 matched pairs receiving S/V or ACEi/ARB. After a mean follow‐up period of 7.8 months, the risk of adverse renal outcomes was similar between patients prescribed S/V and those prescribed ACEi/ARB, independent from baseline eGFR. 71 Among normotensive HFrEF patients (SBP ≥ 100 mmHg) with different CKD stages, treatment with S/V showed more favourable clinical outcomes than treatment with standard HF care without ARNI, both in patients with CKD stages I–III and in patients with CKD stages IV and V. 72
An Italian real‐world study assessed the clinical relevance of transient WRF after initiation of S/V in 202 HFrEF outpatients. Early WRF defined as a >20% decrease in eGFR occurring after 1 month of ARNI therapy occurred in one‐third of patients. However, early WRF had no impact on clinical outcomes during a mean follow‐up of 650 days. In addition, the renal function recovered in patients with early WRF at 3 months, with an improvement in eGFR at 1 year compared with baseline value (62 ± 9.3 vs. 69 ± 8.6 mL/min/1.73 m2; P < 0.01). 73
In a retrospective observational study from Korea, 23 HFrEF patients with ESRD on dialysis initiated with S/V were identified. After a median follow‐up of 132 days, S/V treatment was associated with a reduction of high‐sensitive troponin T (hsTnT) and sST2 levels and an improvement in EF (all P < 0.01) and regarded as safe. Five patients experienced AEs and down‐titration, but none discontinued S/V therapy. 74
An observational study from China aiming to analyse the efficacy and pharmacokinetic (PK) properties of S/V in HF patients on dialysis provides extensive PK as well as clinical data on 11 haemodialysis patients with HFrEF (n = 6) or HFmrEF (n = 5) who were treated with S/V and followed up for 4 to 18 months. Sacubitrilat, the active metabolite of the prodrug sacubitril, and valsartan were not removed by dialysis filtration, but their levels remained within the safe ranges on the interdialytic interval days. Moreover, S/V up to 100 mg twice daily was safe and associated with a significant improvement in EF and reductions in NT‐proBNP and hsTnT (all P < 0.05) in HF patients undergoing haemodialysis. 75
Implementation of sacubitril/valsartan in clinical practice
Sacubitril/valsartan has received FDA and EMA approvals for the treatment of HFrEF in 2015 and is currently approved in 117 countries 76 , 77 (Figure 1 ). In the USA, the estimated proportion of HFrEF patients prescribed S/V was 3.6% (1.5–6.8%) for Medicare and 13.7% (4.9–31.8%) for commercial plan populations. 78
Up to mid‐2021, the estimated cumulative real‐world patient exposure was >5.5 million patient‐treatment years worldwide, thereof >1.3 million patient‐treatment years in the European Union and >500 000 patient‐treatment years in Germany. In Germany, implementation of S/V in routine care has increased steadily over time, soaring to a supply of >18 million defined daily doses (DDD) in Q4/2021 in statutory health‐insured patients (Figure 4 A ). Correspondingly, >266 000 patients received S/V in Q4/2021 (Figure 1 ). A total of 24.1% of these patients were prescribed concomitantly with an SGLT2i. The number of new S/V patients also markedly increased over time. Gender was known for ~92% of newly prescribed S/V patients throughout the entire 6 years' observation period (Figure 4 B ).
Figure 4.
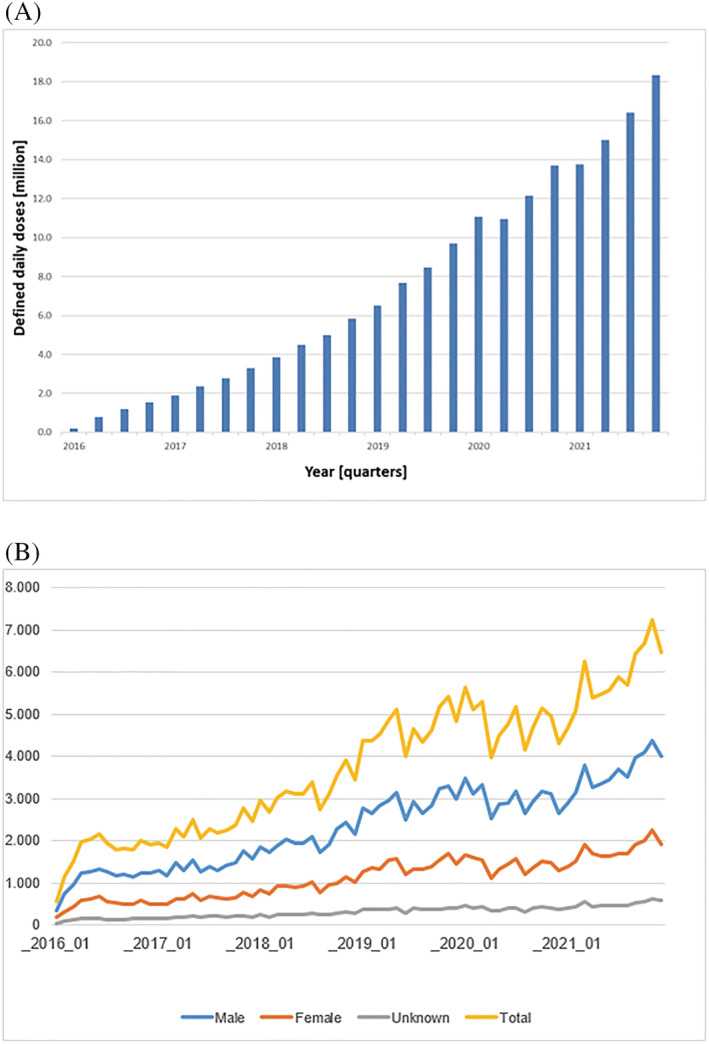
(A) Dispensings of sacubitril/valsartan (S/V) (Entresto®) to statutory health‐insured patients in defined daily doses in Germany from 2016 to 2021 (Source: DAPI database). (B) Number of first‐time S/V patients by month and gender in the IQVIA LRx panel, from January 2016 to December 2021. Absolute numbers as provided in the y‐axis refer to the IQVIA LRx panel that reflects ~80% of the German SHI market. To derive the total number of first‐time S/V patients per month, a correction factor has to be applied (multiply with ~1.5).
In Q1/2021, 76% of the GP practices and 90% of the OBC practices prescribed S/V, further increasing to 81% (GP practices) and 92% (OBC practices) in Q4/2021 (Figure 1 ). Moreover, 16% of the GP and 27% of the OBC practices co‐prescribed S/V and an SGLT2i in at least one patient in Q1/2021, with a marked increase to 39% (GP practices) and 61% (OBC practices) in Q4/2021. These increases in S/V utilization reflect that extensive evidence from clinical trials and accumulating experiential knowledge from real‐world clinical practice now indicate that selecting S/V as first choice, in place of ACEi/ARBs and early in the treatment pathway, is feasible and associated with an incremental prognostic benefit in the majority of patients with HFrEF. Based on the totality of data and supported by recent guidelines, we predicate that ARNIs are preferred agents and should be used ahead and instead of ACEi (or ARB) in symptomatic HFrEF patients, including in RAASi‐naïve patients, in the ambulatory as well as in the hospital setting. Concordantly, ACEi or ARB should only be considered in patients where ARNI administration is not possible due to intolerance, issues of availability, or contraindications.
Early use of S/V has been associated with significant lifetime benefits compared with conventional therapy (ACEi or ARB). 1 , 2 In order to further implement the combination of ARNI, beta‐blocker, MRA, and SGLT2i and to shorten the time to initiation of optimal medical care, every single treating physician should overcome inertia, because HF is an urgency needing rapid intervention. 1 , 2 In addition, an interprofessional healthcare team approach should be implemented to start and adjust therapy as needed as well as to streamline transition of care. This will result in optimized patient outcomes—that is, increased effectiveness and safety while minimizing potential adverse effects.
Conflict of interest
A.A. declares no conflicts of interest.
M.S. has received speaker honoraria from Bristol Myers Squibb, CardioMedLive, Chambers of Pharmacists, DGK Academy, MSD Sharp & Dohme, and Pfizer and consulting fees from MSD Sharp & Dohme and Vifor, all outside the submitted work.
U.R. is an employee of Novartis Pharma GmbH, Germany.
B.H. is an employee of Novartis Pharma AG, Basel, Switzerland.
R.W. received honoraria for lectures/consulting from Abbott, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, CVRx, Medtronic, Novartis, Pfizer, Servier, SOBI, and Vifor, all outside of this work, and research support from Bundesministerium für Bildung und Forschung, Deutsche Forschungsgemeinschaft, Deutsches Zentrum für Herz‐Kreislauf‐Forschung, European Union, and Medtronic, all outside of the present article.
U.L. received honoraria for lectures/consulting from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Novartis, Pfizer, and Sanofi.
J.B. received honoraria for lectures/consulting from Novartis, Vifor, Bayer, Pfizer, Boehringer Ingelheim, AstraZeneca, Cardior, CVRx, BMS, Amgen, and Corvia, not related to the present article, and research support for the department from Zoll, CVRx, and Abiomed, not related to the present article.
I.K. reports personal fees from Akcea Therapeutics Germany GmbH, AstraZeneca, Bayer Vital GmbH, Boehringer Ingelheim, Bristol‐Myers‐Squibb Company, Hexal AG, Novartis, Pfizer Pharma GmbH, Servier Deutschland GmbH, Vifor, and Daiichi Sankyo Deutschland GmbH.
D.V. declares no conflicts of interest.
M.B. reports personal fees from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Medtronic, Novartis, Servier, and Vifor.
Supporting information
Data S1. Supporting Information.
Acknowledgements
MB is supported by the Deutsche Forschungsgemeinschaft (German Research Foundation; TTR 219, project number 322900939). We are grateful to Armin Schweitzer for his technical and artwork help.
Open Access funding enabled and organized by Projekt DEAL.
Abdin, A. , Schulz, M. , Riemer, U. , Hadëri, B. , Wachter, R. , Laufs, U. , Bauersachs, J. , Kindermann, I. , Vukadinović, D. , and Böhm, M. (2022) Sacubitril/valsartan in heart failure: efficacy and safety in and outside clinical trials. ESC Heart Failure, 9: 3737–3750. 10.1002/ehf2.14097.
References
- 1. Abdin A, Anker SD, Butler J, Coats AJS, Kindermann I, Lainscak M, Lund LH, Metra M, Mullens W, Rosano G, Slawik J, Wintrich J, Böhm M. ‘Time is prognosis’ in heart failure: time‐to‐treatment initiation as a modifiable risk factor. ESC Heart Fail. 2021; 8: 4444–4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abdin A, Bauersachs J, Frey N, Kindermann I, Link A, Marx N, Lainscak M, Slawik J, Werner C, Wintrich J, Böhm M. Timely and individualized heart failure management: need for implementation into the new guidelines. Clin Res Cardiol. 2021; 110: 1150–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bauersachs J. Heart failure drug treatment: the fantastic four. Eur Heart J. 2021; 42: 681–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lippi G, Sanchis‐Gomar F. Global epidemiology and future trends of heart failure. AME Med J. 2020; 5: 15. [Google Scholar]
- 5. Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats A. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. 2022: cvac013. [DOI] [PubMed] [Google Scholar]
- 6. Dörr M, Riemer U, Christ M, Bauersachs J, Bosch R, Laufs U, Neumann A, Scherer M, Störk S, Wachter R. Hospitalizations for heart failure: still major differences between East and West Germany 30 years after reunification. ESC Heart Fail. 2021; 8: 2546–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seferović PM, Vardas P, Jankowska EA, Maggioni AP, Timmis A, Milinković I, Polovina M, Gale CP, Lund LH, Lopatin Y, Lainscak M, Savarese G, Huculeci R, Kazakiewicz D, Coats AJS, in collaboration with the National Heart Failure Societies of the ESC member countries (see Appendix) , Berger R, Jahangirov T, Kurlianskaya A, Troisfontaines P, Droogne W, Hudić LD, Tokmakova M, Glavaš D, Barberis V, Spinar J, Wolsk E, Uuetoa T, Tolppanen H, Kipiani Z, Störk S, Bauersachs J, Keramida K, Parissis J, Habon T, Gotsman I, Weinstein JM, Ingimarsdottir IJ, Crowley J, Dalton B, Aspromonte N, Nodari S, Volterrani M, Rakisheva A, Mirrakhimov E, Kamzola G, Skouri H, Čelutkiene J, Jovanova S, Vataman E, Cobac IP, van Pol P, de Boer R, Lueder T, Straburzynska‐Migaj E, Moura B, Chioncel O, Fomin I, Begrambekova J, Lopatin Y, Mareev Y, Goncalvesova E, Seferović P, Milinković I, Lainščak M, Pinilla JMG, Lindmark K, Mueller C, Cavusoglu Y, Gardner R, Voronkov L. The Heart Failure Association Atlas: heart failure epidemiology and management statistics 2019. Eur J Heart Fail. 2021; 23: 906–914. [DOI] [PubMed] [Google Scholar]
- 8. Seferović PM, Polovina M, Adlbrecht C, Bělohlávek J, Chioncel O, Goncalvesová E, Milinković I, Grupper A, Halmosi R, Kamzola G, Koskinas KC, Lopatin Y, Parkhomenko A, Põder P, Ristić AD, Šakalytė G, Trbušić M, Tundybayeva M, Vrtovec B, Yotov YT, Miličić D, Ponikowski P, Metra M, Rosano G, Coats AJS. Navigating between Scylla and Charybdis: challenges and strategies for implementing guideline‐directed medical therapy in heart failure with reduced ejection fraction. Eur J Heart Fail. 2021; 23: 1999–2007. [DOI] [PubMed] [Google Scholar]
- 9. Periodic Safety Update Reports (PSURs) . https://www.ema.europa.eu/en/medicines/human/EPAR/entresto
- 10. Rudolph UM, Enners S, Kieble M, Mahfoud F, Böhm M, Laufs U, Schulz M. Impact of angiotensin receptor blocker product recalls on antihypertensive prescribing in Germany. J Hum Hypertens. 2020; 35: 903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. The IQVIA Institute for Human Data Science . https://www.iqvia.com/solutions/therapeutics/cardiovascular
- 12. Gu J, Noe A, Chandra P, al‐Fayoumi S, Ligueros‐Saylan M, Sarangapani R, Maahs S, Ksander G, Rigel DF, Jeng AY, Lin TH, Zheng W, Dole WP. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual‐acting angiotensin receptor‐neprilysin inhibitor (ARNi). J Clin Pharmacol. 2010; 50: 401–414. [DOI] [PubMed] [Google Scholar]
- 13. Zile MR, O'Meara E, Claggett B, Prescott MF, Solomon SD, Swedberg K, Packer M, McMurray JJV, Shi V, Lefkowitz M, Rouleau J. Effects of sacubitril/valsartan on biomarkers of extracellular matrix regulation in patients with HFrEF. J Am Coll Cardiol. 2019; 73: 795–806. [DOI] [PubMed] [Google Scholar]
- 14. Wang Y, Zhou R, Lu C, Chen Q, Xu T, Li D. Effects of the angiotensin‐receptor neprilysin inhibitor on cardiac reverse remodeling: meta‐analysis. J Am Heart Assoc. 2019; 8: e012272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chua SK, Lai WT, Chen LC, Hung HF. The antihypertensive effects and safety of LCZ696 in patients with hypertension: a systemic review and meta‐analysis of randomized controlled trials. J Clin Med. 2021; 10: 2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jering KS, Claggett B, Pfeffer MA, Granger C, Køber L, Lewis EF, Maggioni AP, Mann D, McMurray JJV, Rouleau JL, Solomon SD, Steg PG, Meer P, Wernsing M, Carter K, Guo W, Zhou Y, Lefkowitz M, Gong J, Wang Y, Merkely B, Macin SM, Shah U, Nicolau JC, Braunwald E. Prospective ARNI vs. ACE inhibitor trial to determine superiority in reducing heart failure events after myocardial infarction (PARADISE‐MI): design and baseline characteristics. Eur J Heart Fail. 2021; 23: 1040–1048. [DOI] [PubMed] [Google Scholar]
- 17. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM‐HF Investigators and Committees . Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 18. Pfeffer MA, Claggett B, Lewis EF, Granger CB, Køber L, Maggioni AP, Mann DL, McMurray J, Rouleau JL, Solomon SD, Steg PG, Berwanger O, Cikes M, de Pasquale CG, East C, Fernandez A, Jering K, Landmesser U, Mehran R, Merkely B, Vaghaiwalla Mody F, Petrie MC, Petrov I, Schou M, Senni M, Sim D, van der Meer P, Lefkowitz M, Zhou Y, Gong J, Braunwald E, PARADISE‐MI Investigators and Committees . Angiotensin receptor–neprilysin inhibition in acute myocardial infarction. N Engl J Med. 2021; 385: 1845–1855. [DOI] [PubMed] [Google Scholar]
- 19. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen D, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin‐Colet J, Cleland J, Düngen HD, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP, PARAGON‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019; 381: 1609–1620. [DOI] [PubMed] [Google Scholar]
- 20. Desai AS, McMurray JJ, Packer M, Swedberg K, Rouleau JL, Chen F, Gong J, Rizkala AR, Brahimi A, Claggett B, Finn PV, Hartley LH, Liu J, Lefkowitz M, Shi V, Zile MR, Solomon SD. Effect of the angiotensin‐receptor‐neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur Heart J. 2015; 36: 1990–1997. [DOI] [PubMed] [Google Scholar]
- 21. Claggett B, Packer M, McMurray JJ, Swedberg K, Rouleau J, Zile MR, Jhund P, Lefkowitz M, Shi V, Solomon SD, PARADIGM‐HF Investigators . Estimating the long‐term treatment benefits of sacubitril‐valsartan. N Engl J Med. 2015; 373: 2289–2290. [DOI] [PubMed] [Google Scholar]
- 22. Lewis EF, Claggett BL, McMurray JJV, Packer M, Lefkowitz MP, Rouleau JL, Liu J, Shi VC, Zile MR, Desai AS, Solomon SD, Swedberg K. Health‐related quality of life outcomes in PARADIGM‐HF. Circ Heart Fail. 2017; 10: e003430. [DOI] [PubMed] [Google Scholar]
- 23. Chandra A, Lewis EF, Claggett BL, Desai AS, Packer M, Zile MR, Swedberg K, Rouleau JL, Shi VC, Lefkowitz MP, Katova T, McMurray JJV, Solomon SD. Effects of sacubitril/valsartan on physical and social activity limitations in patients with heart failure: a secondary analysis of the PARADIGM‐HF trial. JAMA Cardiol. 2018; 3: 498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seferovic JP, Claggett B, Seidelmann SB, Seely EW, Packer M, Zile MR, Rouleau JL, Swedberg K, Lefkowitz M, Shi VC, Desai AS, McMurray JJV, Solomon SD. Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: a post‐hoc analysis from the PARADIGM‐HF trial. Lancet Diabetes Endocrinol. 2017; 5: 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Damman K, Gori M, Claggett B, Jhund PS, Senni M, Lefkowitz MP, Prescott MF, Shi VC, Rouleau JL, Swedberg K, Zile MR, Packer M, Desai AS, Solomon SD, McMurray JJV. Renal effects and associated outcomes during angiotensin‐neprilysin inhibition in heart failure. JACC Heart Fail. 2018; 6: 489–498. [DOI] [PubMed] [Google Scholar]
- 26. Desai AS, Vardeny O, Claggett B, McMurray JJ, Packer M, Swedberg K, Rouleau JL, Zile MR, Lefkowitz M, Shi V, Solomon SD. Reduced risk of hyperkalemia during treatment of heart failure with mineralocorticoid receptor antagonists by use of sacubitril/valsartan compared with enalapril: a secondary analysis of the PARADIGM‐HF trial. JAMA Cardiol. 2017; 2: 79–85. [DOI] [PubMed] [Google Scholar]
- 27. Vardeny O, Claggett B, Kachadourian J, Desai AS, Packer M, Rouleau J, Zile MR, Swedberg K, Lefkowitz M, Shi V, McMurray JJV, Solomon SD. Reduced loop diuretic use in patients taking sacubitril/valsartan compared with enalapril: the PARADIGM‐HF trial. Eur J Heart Fail. 2019; 21: 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Packer M, McMurray JJ, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile M, Andersen K, Arango JL, Arnold JM, Bělohlávek J, Böhm M, Boytsov S, Burgess LJ, Cabrera W, Calvo C, Chen CH, Dukat A, Duarte YC, Erglis A, Fu M, Gomez E, Gonzàlez‐Medina A, Hagège AA, Huang J, Katova T, Kiatchoosakun S, Kim KS, Kozan Ö, Llamas EB, Martinez F, Merkely B, Mendoza I, Mosterd A, Negrusz‐Kawecka M, Peuhkurinen K, Ramires FJ, Refsgaard J, Rosenthal A, Senni M, Sibulo AS Jr, Silva‐Cardoso J, Squire IB, Starling RC, Teerlink JR, Vanhaecke J, Vinereanu D, Wong RC, PARADIGM‐HF Investigators and Coordinators . Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015; 131: 54–61. [DOI] [PubMed] [Google Scholar]
- 29. O'Meara E, Prescott MF, Claggett B, Rouleau JL, Chiang LM, Solomon SD, Packer M, McMurray JJV, Zile MR. Independent prognostic value of serum soluble ST2 measurements in patients with heart failure and a reduced ejection fraction in the PARADIGM‐HF trial (Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure). Circ Heart Fail. 2018; 11: e004446. [DOI] [PubMed] [Google Scholar]
- 30. Balmforth C, Simpson J, Shen L, Jhund PS, Lefkowitz M, Rizkala AR, Rouleau JL, Shi V, Solomon SD, Swedberg K, Zile MR, Packer M, McMurray JJV. Outcomes and effect of treatment according to etiology in HFrEF: an analysis of PARADIGM‐HF. JACC Heart Fail. 2019; 7: 457–465. [DOI] [PubMed] [Google Scholar]
- 31. Yeoh SE, Dewan P, Desai AS, Solomon SD, Rouleau JL, Lefkowitz M, Rizkala A, Swedberg K, Zile MR, Jhund PS, Packer M, McMurray JJV. Relationship between duration of heart failure, patient characteristics, outcomes, and effect of therapy in PARADIGM‐HF. ESC Heart Fail. 2020; 7: 3355–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jhund PS, Fu M, Bayram E, Chen CH, Negrusz‐Kawecka M, Rosenthal A, Desai AS, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, McMurray J, Packer M, PARADIGM‐HF Investigators and Committees . Efficacy and safety of LCZ696 (sacubitril‐valsartan) according to age: insights from PARADIGM‐HF. Eur Heart J. 2015; 36: 2576–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Okumura N, Jhund PS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Swedberg K, Zile MR, Solomon SD, Packer M, McMurray J, PARADIGM‐HF Investigators and Committees* . Effects of sacubitril/valsartan in the PARADIGM‐HF trial (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) according to background therapy. Circ Heart Fail. 2016; 9: e003212. [DOI] [PubMed] [Google Scholar]
- 34. Solomon SD, Claggett B, Desai AS, Packer M, Zile M, Swedberg K, Rouleau JL, Shi VC, Starling RC, Kozan Ö, Dukat A, Lefkowitz MP, McMurray JJV. Influence of ejection fraction on outcomes and efficacy of sacubitril/valsartan (LCZ696) in heart failure with reduced ejection fraction: the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM‐HF) trial. Circ Heart Fail. 2016; 9: e002744. [DOI] [PubMed] [Google Scholar]
- 35. Böhm M, Young R, Jhund PS, Solomon SD, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Swedberg K, Zile MR, Packer M, McMurray JJV. Systolic blood pressure, cardiovascular outcomes and efficacy and safety of sacubitril/valsartan (LCZ696) in patients with chronic heart failure and reduced ejection fraction: results from PARADIGM‐HF. Eur Heart J. 2017; 38: 1132–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Suzuki K, Claggett B, Minamisawa M, Packer M, Zile MR, Rouleau J, Swedberg K, Lefkowitz M, Shi V, McMurray JJV, Zucker SD, Solomon SD. Liver function and prognosis, and influence of sacubitril/valsartan in patients with heart failure with reduced ejection fraction. Eur J Heart Fail. 2020; 22: 1662–1671. [DOI] [PubMed] [Google Scholar]
- 37. Ehteshami‐Afshar S, Mooney L, Dewan P, Desai AS, Lang NN, Lefkowitz MP, Petrie MC, Rizkala AR, Rouleau JL, Solomon SD, Swedberg K, Shi VC, Zile MR, Packer M, McMurray JJV, Jhund PS, Hawkins NM. Clinical characteristics and outcomes of patients with heart failure with reduced ejection fraction and chronic obstructive pulmonary disease: insights from PARADIGM‐HF. J Am Heart Assoc. 2021; 10: e019238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Selvaraj S, Claggett B, Pozzi A, McMurray JJV, Jhund PS, Packer M, Desai AS, Lewis EF, Vaduganathan M, Lefkowitz MP, Rouleau JL, Shi VC, Zile MR, Swedberg K, Solomon SD. Prognostic implications of congestion on physical examination among contemporary patients with heart failure and reduced ejection fraction: PARADIGM‐HF. Circulation. 2019; 140: 1369–1379. [DOI] [PubMed] [Google Scholar]
- 39. Solomon SD, Claggett B, Packer M, Desai A, Zile MR, Swedberg K, Rouleau J, Shi V, Lefkowitz M, McMurray JJV. Efficacy of sacubitril/valsartan relative to a prior decompensation: the PARADIGM‐HF trial. JACC Heart Fail. 2016; 4: 816–822. [DOI] [PubMed] [Google Scholar]
- 40. Kristensen SL, Preiss D, Jhund PS, Squire I, Cardoso JS, Merkely B, Martinez F, Starling RC, Desai AS, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, McMurray J, Packer M, PARADIGM‐HF Investigators and Committees . Risk related to pre‐diabetes mellitus and diabetes mellitus in heart failure with reduced ejection fraction: insights from Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure trial. Circ Heart Fail. 2016; 9: e002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kristensen SL, Martinez F, Jhund PS, Arango JL, Bĕlohlávek J, Boytsov S, Cabrera W, Gomez E, Hagège AA, Huang J, Kiatchoosakun S, Kim KS, Mendoza I, Senni M, Squire IB, Vinereanu D, Wong RCC, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, Packer M, McMurray JJV. Geographic variations in the PARADIGM‐HF heart failure trial. Eur Heart J. 2016; 37: 3167–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW. 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022; 21: 08394. [DOI] [PubMed] [Google Scholar]
- 43. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, ESC Scientific Document Group , de Boer RA, Christian Schulze P, Abdelhamid M, Aboyans V, Adamopoulos S, Anker SD, Arbelo E, Asteggiano R, Bauersachs J, Bayes‐Genis A, Borger MA, Budts W, Cikes M, Damman K, Delgado V, Dendale P, Dilaveris P, Drexel H, Ezekowitz J, Falk V, Fauchier L, Filippatos G, Fraser A, Frey N, Gale CP, Gustafsson F, Harris J, Iung B, Janssens S, Jessup M, Konradi A, Kotecha D, Lambrinou E, Lancellotti P, Landmesser U, Leclercq C, Lewis BS, Leyva F, Linhart A, Løchen ML, Lund LH, Mancini D, Masip J, Milicic D, Mueller C, Nef H, Nielsen JC, Neubeck L, Noutsias M, Petersen SE, Sonia Petronio A, Ponikowski P, Prescott E, Rakisheva A, Richter DJ, Schlyakhto E, Seferovic P, Senni M, Sitges M, Sousa‐Uva M, Tocchetti CG, Touyz RM, Tschoepe C, Waltenberger J, Adamo M, Baumbach A, Böhm M, Burri H, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gardner RS, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Piepoli MF, Price S, Rosano GMC, Ruschitzka F, Skibelund AK. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 44. Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E, PIONEER‐HF Investigators . Angiotensin–neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019; 380: 539–548. [DOI] [PubMed] [Google Scholar]
- 45. Wachter R, Senni M, Belohlavek J, Straburzynska‐Migaj E, Witte KK, Kobalava Z, Fonseca C, Goncalvesova E, Cavusoglu Y, Fernandez A, Chaaban S, Bøhmer E, Pouleur AC, Mueller C, Tribouilloy C, Lonn E, A.L. Buraiki J, Gniot J, Mozheiko M, Lelonek M, Noè A, Schwende H, Bao W, Butylin D, Pascual‐Figal D, TRANSITION Investigators , Gniot J, Mozheiko M, Lelonek M, Dominguez AR, Horacek T, del Rio EG, Kobalava Z, Mueller CE, Cavusoglu Y, Straburzynska‐Migaj E, Slanina M, von Dahl J, Senni M, Ryding A, Moriarty A, Robles MB, Villota JN, Quintana AG, Nitschke T, Manuel Garcia Pinilla J, Bonet LA, Chaaban S, Filali zaatari S, Spinar J, Musial W, Abdelbaki K, Belohlavek J, Fehske W, Bott MC, Hoegalmen G, Leiro MC, Ozcan IT, Mullens W, Kryza R, al‐Ani R, Loboz‐Grudzien K, Ermoshkina L, Hojerova S, Fernandez AA, Spinarova L, Lapp H, Bulut E, Almeida F, Vishnevsky A, Belicova M, Pascual D, Witte K, Wong K, Droogne W, Delforge M, Peterka M, Olbrich HG, Carugo S, Nessler J, McGill TH, Huegl B, Akin I, Moreira I, Baglikov A, Thambyrajah J, Hayes C, Barrionuevo MR, Yigit Z, Kaya H, Klimsa Z, Radvan M, Kadel C, Landmesser U, di Tano G, Lisik MB, Fonseca C, Oliveira L, Marques I, Santos LM, Lenner E, Letavay P, Bueno MG, Mota P, Wong A, Bailey K, Foley P, Hasbani E, Virani S, Massih TA, al‐Saif S, Taborsky M, Kaislerova M, Motovska Z, Praha, Cohen AA, Logeart D, Endemann D, Ferreira D, Brito D, Kycina P, Bollano E, Basilio EG, Rubio LF, Aguado MG, Schiavi LB, Zivano DF, Lonn E, Sayed AE, Pouleur AC, Heyse A, Schee A, Polasek R, Houra M, Tribouilloy C, Seronde MF, Galinier M, Noutsias M, Schwimmbeck P, Voigt I, Westermann D, Pulignano G, Vegsundvaag J, Alexandre da Silva Antunes J, Monteiro P, Stevlik J, Goncalvesova E, Hulkoova B, Juan Castro Fernandez A, Davies C, Squire I, Meyer P, Sheppard R, Sahin T, Sochor K, de Geeter G, Wachter R, Schmeisser A, Weil J, Soares AO, Vasilevna OB, Oshurkov A, Sunderland SJ, Glover J, Exequiel T, Decoulx E, Meyer S, Muenzel T, Frioes F, Arbolishvili G, Tokarcikova A, Karlstrom P, Carles Trullas Vila J, Perez GP, Sankaranarayanan R, Nageh T, Alasia DC, Refaat M, Demirkan B, al‐Buraiki J, Karabsheh S. Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: primary results of the randomised TRANSITION study. Eur J Heart Fail. 2019; 21: 998–1007. [DOI] [PubMed] [Google Scholar]
- 46. Mohyeldin M, Tavares LB, Boorenie M, Abureesh D, Ejaz S, Durrani L, Khan S. Efficacy of sacubitril/valsartan in the setting of acute heart failure: a systematic review. Cureus. 2021; 13: e18740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mann DL, Givertz MM, Vader JM, Starling RC, Shah P, McNulty SE, Anstrom KJ, Margulies KB, Kiernan MS, Mahr C, Gupta D. Effect of treatment with sacubitril/valsartan in patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA Cardiol. 2021: e214567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pfeffer MA, Claggett B, Lewis EF, Granger CB, Køber L, Maggioni AP, Mann DL, McMurray JJV, Rouleau JL, Solomon SD, Steg PG, Berwanger O, Cikes M, de Pasquale CG, Fernandez A, Filippatos G, Jering K, Landmesser U, Menon V, Merkely B, Petrie MC, Petrov I, Schou M, Senni M, Sim D, van der Meer P, Lefkowitz M, Zhou Y, Wang Y, Braunwald E. Impact of sacubitril/valsartan versus ramipril on total heart failure events in the PARADISE‐MI trial. Circulation. 2022; 145: 87–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Desai RJ, Patorno E, Vaduganathan M, Mahesri M, Chin K, Levin R, Solomon SD, Schneeweiss S. Effectiveness of angiotensin‐neprilysin inhibitor treatment versus renin‐angiotensin system blockade in older adults with heart failure in clinical care. Heart. 2021; 107: 1407–1416. [DOI] [PubMed] [Google Scholar]
- 50. Chang PC, Wang CL, Hsiao FC, Wen MS, Huang CY, Chou CC, Chu PH. Sacubitril/valsartan vs. angiotensin receptor inhibition in heart failure: a real‐world study in Taiwan. ESC Heart Fail. 2020; 7: 3003–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Proudfoot C, Studer R, Rajput T, Jindal R, Agrawal R, Corda S, Senni M. Real‐world effectiveness and safety of sacubitril/valsartan in heart failure: a systematic review. Int J Cardiol. 2021; 331: 164–171. [DOI] [PubMed] [Google Scholar]
- 52. Berg DD, Samsky MD, Velazquez EJ, Duffy CI, Gurmu Y, Braunwald E, Morrow DA, DeVore AD. Efficacy and safety of sacubitril/valsartan in high‐risk patients in the PIONEER‐HF trial. Circ Heart Fail. 2021; 14: e007034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ambrosy AP, Braunwald E, Morrow DA, DeVore AD, McCague K, Meng X, Duffy CI, Rocha R, Velazquez EJ, PIONEER‐HF Investigators . Angiotensin receptor‐neprilysin inhibition based on history of heart failure and use of renin‐angiotensin system antagonists. J Am Coll Cardiol. 2020; 76: 1034–1048. [DOI] [PubMed] [Google Scholar]
- 54. Senni M, Wachter R, Witte KK, Straburzynska‐Migaj E, Belohlavek J, Fonseca C, Mueller C, Lonn E, Chakrabarti A, Bao W, Noe A, Schwende H, Butylin D, Pascual‐Figal D, TRANSITION Investigators . Initiation of sacubitril/valsartan shortly after hospitalisation for acutely decompensated heart failure in patients with newly diagnosed (de novo) heart failure: a subgroup analysis of the TRANSITION study. Eur J Heart Fail. 2020; 22: 303–312. [DOI] [PubMed] [Google Scholar]
- 55. Kim YS, Brar S, D'Albo N, Dey A, Shah S, Ganatra S, Dani SS. Five years of sacubitril/valsartan—a safety analysis of randomized clinical trials and real‐world pharmacovigilance. Cardiovasc Drugs Ther. 2021. [DOI] [PubMed] [Google Scholar]
- 56. Nuechterlein K, AlTurki A, Ni J, Martínez‐Sellés M, Martens P, Russo V, Backelin CN, Huynh T. Real‐world safety of sacubitril/valsartan in women and men with heart failure and reduced ejection fraction: a meta‐analysis. CJC. 2021; 3: S202–S208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mc Causland FR, Lefkowitz MP, Claggett B, Anavekar NS, Senni M, Gori M, Jhund PS, McGrath MM, Packer M, Shi V, van Veldhuisen DJ, Zannad F, Comin‐Colet J, Pfeffer MA, McMurray JJV, Solomon SD. Angiotensin‐neprilysin inhibition and renal outcomes in heart failure with preserved ejection fraction. Circulation. 2020; 142: 1236–1245. [DOI] [PubMed] [Google Scholar]
- 58. Pieske B, Wachter R, Shah SJ, Baldridge A, Szeczoedy P, Ibram G, Shi V, Zhao Z, Cowie MR, PARALLAX Investigators and Committee members , Prado AC, Wenetz LMM, Brasca DG, Albisu JP, Fernandez AA, Liberman A, Echeverria G, Bartolacci I, Ranz M, Pedrotti M, Tapia D, Robertson M, Exposito S, Barrionuevo M, Resk JH, Mercau G, Fuente RL, Avaca H, Poy C, Hominal MA, Cursack G, Vogelmann OA, Casas MM, Costantino M, Chiriffe J, Zweiker R, Motloch LJ, Auer J, Siostrzonek P, Grander W, Derthoo D, Mullens W, Vandekerckhove H, Maamar R, Delforge M, Mulleners T, Rassi S, Kormann APM, Moraes A, Manenti ERF, Reis G, Maia LN, Simoes MV, Silva RP, Danzmann LC, Saraiva JFK, Sternieri MCVBB, de Souza WB, Figueiredo EL, Tzekova ML, Boeva B, Vasilev D, Gospodinova STT, Milanova MH, Kabakchieva VM, Valchanova PI, Dimov B, Petrovsky P, Georgiev PG, Spasova N, Mazhdrakov G, Konteva M, Benov HO, Denchev SV, Parent M, Demers C, Dion D, Bourgeois RL, Heffernan ML, Silgado GAM, Quiroz F, Cadena A, Mendoza JLA, Saldarriaga C, Arana C, Nechvatal L, Linhartova K, Klimsa Z, Busak L, Marek D, Slaby J, Polasek R, Koleckar P, Carda J, Kaislerova M, Trestik P, Zajicek P, Kuchar J, Vondrak J, Krupicka J, Brychta T, Lang P, Rickers H, Hove JD, Gustafsson I, Nielsen OW, Uuetoa T, Noodla S, Rosenthal A, Vettus R, Fauchier L, Aboyans V, Dutoiu TM, Decoulx E, Geeter GD, Khanoyan P, Donal E, Rosamel Y, Berthelot E, Seronde MF, Winkelmann BR, Grosskopf W, Haas J, Schmitt B, Jahnke N, Wilke A, Simonis G, Bosiljanoff P, Proskynitopoulos N, Axthelm C, Schenkenberger I, Proepper F, Frey N, Edelmann F, Fechtrup C, Graf T, Seidel M, Fehske W, Prohaska M, Unsoeld B, Birner C, Jungmair W, Hoelscher A, Kadel C, Lahiri K, Mehling H, Hagenow A, Naudts IFK, Winkler J, Salbach P, Naumann R, Killat H, Bastian D, Baeumer AT, Goldmann B, Cuneo A, Hoepfner F, Noutsias M, Beermann J, Rieker W, Buckert D, Woehrle J, Westphal J, Bekfani T, Stoerk S, Schnupp S, Stoehring R, Neumann T, Knapp M, Bourgeois J, Edel K, Sarnighausen HE, Nitschke T, Jeserich M, Ebelt H, Linke A, Gaub R, Sueselbeck T, Stratmann M, Toursarkissian N, Steinebach I, Krackhardt F, Schueler R, Brandt M, Placke J, Stangl V, Schoebel C, Tschoepe D, Overhoff U, Stenzel G, Stellbrink C, Schwefer M, Lorenz E, Uebel P, Gluesing D, Weissbrodt M, John F, Arango JL, Castellanos JM, Chang C, Haase F, Lopez EM, Ovando A, Paniagua V, Vogel M. Effect of sacubitril/valsartan vs standard medical therapies on plasma NT‐proBNP concentration and submaximal exercise capacity in patients with heart failure and preserved ejection fraction: the PARALLAX randomized clinical trial. JAMA. 2021; 326: 1919–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Solomon SD, Vaduganathan M, Claggett BL, Packer M, Zile M, Swedberg K, Rouleau J, Pfeffer MA, Desai A, Lund LH, Kober L, Anand I, Sweitzer N, Linssen G, Merkely B, Luis Arango J, Vinereanu D, Chen CH, Senni M, Sibulo A, Boytsov S, Shi V, Rizkala A, Lefkowitz M, McMurray JJV. Sacubitril/valsartan across the spectrum of ejection fraction in heart failure. Circulation. 2020; 141: 352–361. [DOI] [PubMed] [Google Scholar]
- 60. Marks N, Berg MJ, Chi LM, Choi J, Durrie R, Swistok J, Makofske RC, Danho W, Sapirstein VS. Hydrolysis of amyloid precursor protein‐derived peptides by cysteine proteinases and extracts of rat brain clathrin‐coated vesicles. Peptides. 1994; 15: 175–182. [DOI] [PubMed] [Google Scholar]
- 61. Langenickel TH, Tsubouchi C, Ayalasomayajula S, Pal P, Valentin MA, Hinder M, Jhee S, Gevorkyan H, Rajman I. The effect of LCZ696 (sacubitril/valsartan) on amyloid‐β concentrations in cerebrospinal fluid in healthy subjects. Br J Clin Pharmacol. 2016; 81: 878–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cannon JA, Shen L, Jhund PS, Kristensen SL, Køber L, Chen F, Gong J, Lefkowitz MP, Rouleau JL, Shi VC, Swedberg K, Zile MR, Solomon SD, Packer M, McMurray J, PARADIGM‐HF Investigators and Committees . Dementia‐related adverse events in PARADIGM‐HF and other trials in heart failure with reduced ejection fraction. Eur J Heart Fail. 2017; 19: 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schoenfeld HA, West T, Verghese PB, Holubasch M, Shenoy N, Kagan D, Buono C, Zhou W, DeCristofaro M, Douville J, Goodrich GG, Mansfield K, Saravanan C, Cumin F, Webb RL, Bateman RJ. The effect of angiotensin receptor neprilysin inhibitor, sacubitril/valsartan, on central nervous system amyloid‐β concentrations and clearance in the cynomolgus monkey. Toxicol Appl Pharmacol. 2017; 323: 53–65. [DOI] [PubMed] [Google Scholar]
- 64. Bhatt AS, Vaduganathan M, Claggett BL, Liu J, Packer M, Desai AS, Lefkowitz MP, Rouleau JL, Shi VC, Zile MR, Swedberg K, Vardeny O, McMurray JJV, Solomon SD. Effect of sacubitril/valsartan vs. enalapril on changes in heart failure therapies over time: the PARADIGM‐HF trial. Eur J Heart Fail. 2021; 23: 1518–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Solomon SD, Jhund PS, Claggett BL, Dewan P, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Inzucchi SE, Desai AS, Bengtsson O, Lindholm D, Sjostrand M, Langkilde AM, McMurray JJV. Effect of dapagliflozin in patients with HFrEF treated with sacubitril/valsartan: the DAPA‐HF trial. JACC Heart Fail. 2020; 8: 811–818. [DOI] [PubMed] [Google Scholar]
- 66. Packer M, Anker SD, Butler J, Filippatos G, Ferreira JP, Pocock SJ, Rocca HPBL, Janssens S, Tsutsui H, Zhang J, Brueckmann M, Jamal W, Cotton D, Iwata T, Schnee J, Zannad F, for the EMPEROR‐Reduced Trial Committees and Investigators . Influence of neprilysin inhibition on the efficacy and safety of empagliflozin in patients with chronic heart failure and a reduced ejection fraction: the EMPEROR‐Reduced trial. Eur Heart J. 2021; 42: 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kim HM, Hwang IC, Choi W, Yoon YE, Cho GY. Combined effects of ARNI and SGLT2 inhibitors in diabetic patients with heart failure with reduced ejection fraction. Sci Rep. 2021; 11: 22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hsiao FC, Lin CP, Tung YC, Chang PC, McMurray JJV, Chu PH. Combining sodium‐glucose cotransporter 2 inhibitors and angiotensin receptor‐neprilysin inhibitors in heart failure patients with reduced ejection fraction and diabetes mellitus: a multi‐institutional study. Int J Cardiol. 2021; 330: 91–97. [DOI] [PubMed] [Google Scholar]
- 69. Jiménez‐Blanco Bravo M, Valle A, Gayán Ordás J, del Prado Díaz S, Cordero Pereda D, Morillas Climent H, Bascompte Claret R, Seller Moya J, Zamorano Gómez JL, Alonso Salinas GL. Safety and efficacy of the combination of sacubitril/valsartan and SGLT2i in HFrEF patients (SECSI Registry). J Cardiovasc Pharmacol. 2021; 78: e662–e668. [DOI] [PubMed] [Google Scholar]
- 70. Haynes R, Judge PK, Staplin N, Herrington WG, Storey BC, Bethel A, Bowman L, Brunskill N, Cockwell P, Hill M, Kalra PA, McMurray JJV, Taal M, Wheeler DC, Landray MJ, Baigent C, UK HARP‐III Collaborative Group . Effects of sacubitril/valsartan versus irbesartan in patients with chronic kidney disease. Circulation. 2018; 138: 1505–1514. [DOI] [PubMed] [Google Scholar]
- 71. Tan NY, Deng Y, Yao X, Sangaralingham LR, Shah ND, Rule AD, Burnett JC Jr, Dunlay SM, Sangaralingham SJ. Renal outcomes in patients with systolic heart failure treated with sacubitril‐valsartan or angiotensin converting enzyme inhibitor/angiotensin receptor blocker. Mayo Clin Proc Innov Qual Outcomes. 2021; 5: 286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chang HY, Feng AN, Fong MC, Hsueh CW, Lai WT, Huang KC, Chong E, Chen CN, Chang HC, Yin WH. Sacubitril/valsartan in heart failure with reduced ejection fraction patients: real world experience on advanced chronic kidney disease, hypotension, and dose escalation. J Cardiol. 2019; 74: 372–380. [DOI] [PubMed] [Google Scholar]
- 73. Masarone D, Melillo E, Errigo V, Valente F, Pacileo G. Clinical relevance of transient worsening renal function after initiation of sacubitril/valsartan. Curr Med Res Opin. 2021; 37: 9–12. [DOI] [PubMed] [Google Scholar]
- 74. Lee S, Oh J, Kim H, Ha J, Chun KH, Lee CJ, Park S, Lee SH, Kang SM. Sacubitril/valsartan in patients with heart failure with reduced ejection fraction with end‐stage of renal disease. ESC Heart Fail. 2020; 7: 1125–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Feng Z, Wang X, Zhang L, Apaer R, Xu L, Ma J, Li X, Che H, Tang B, Xiong Y, Xia Y, Xiao J, Su X, Wang Y, Dou X, Chen J, Mei L, Xue Z, Kong Y, Li S, Zhang H, Lin T, Wen F, Fu X, Tao Y, Fu L, Li Z, Huang R, Ye Z, He C, Shi W, Liang X, Ke G, Liu S. Pharmacokinetics and pharmacodynamics of sacubitril/valsartan in maintenance hemodialysis patients with heart failure. Blood Purif. 2022; 51: 270–279. [DOI] [PubMed] [Google Scholar]
- 76. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207620Orig1s000Approv.pdf
- 77. https://www.ema.europa.eu/en/documents/overview/entresto‐epar‐summary‐public_en.pdf
- 78. Ozaki AF, Krumholz HM, Mody FV, Jackevicius CA. National trends in the use of sacubitril/valsartan. J Card Fail. 2021; 27: 839–847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.


