Abstract
Microbial infections remain a grave threat to global health security due to increasing antibiotic resistance. The coronavirus pandemic has increased the risk of microbial infection. To combat these infections, the search for new therapeutic agents is in high demand. A series of new 3-(2-(3-(substituted benzyloxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline (9a–i) derivatives have been synthesized. The structure of synthesized compounds was analyzed by spectroscopic methods. The newly synthesized oxetanyl-quinoline derivatives were evaluated for in vitro antibacterial activity against Escherichia coli (NCIM 2574), Proteus mirabilis (NCIM 2388), Bacillus subtilis (NCIM 2063), Staphylococcus albus (NCIM 2178), and in vitro antifungal activity against Aspergillus niger (ATCC 504) and Candida albicans (NCIM 3100). Six oxetanyl-quinoline derivatives 9a, 9b, 9c, 9d, 9e, and 9h have shown good antibacterial activity against P. mirabilis with MIC 31.25–62.5 μM, 3-(((3-(2-fluoro-6-((8-fluoro-2-methylquinolin-3-yl)oxy)phenyl)oxetan-3-yl)oxy)methyl)benzonitrile (9f) reporting comparable activity against P. mirabilis with respect to the standard drug streptomycin. Compound 9a also showed good activity against B. subtilis with MIC 31.25 μM. The eight compounds 9a, 9b, 9d, 9e, 9f, 9g, 9h, and 9i have shown good antifungal activity against A. niger. The synthesized compounds were also screened for antimycobacterial activity against Mycobacterium tuberculosis H37Rv by MTT assay. Among the nine derivatives, compounds 9b, 9c, 9d, 9f, 9g, 9h, and 9i showed excellent antimycobacterial activity with MIC 3.41–12.23 μM, and two derivatives showed good activity with MIC 27.29–57.73 μM. All the derivatives were further evaluated for cytotoxicity against the Vero cell line and were found to be nontoxic. The in silico study of compounds 9a–i was performed against ATP synthase (PDB ID: 4V1F) and most of the compounds showed the stable and significant binding to ATP synthase, confirming their plausible mode of action as ATP synthase inhibitors. Thus, the significant antimycobacterial activity of 3-(2-(3-(substituted benzyloxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline derivatives has suggested that the oxatenyl-quinoline compounds could assist in the development of lead compounds to treat mycobacterial infections.
1. Introduction
Tuberculosis (TB), an infection caused by Mycobacterium tuberculosis (MTB), remains a grave threat to global health security and is now a preeminent cause of mortality from a single infectious agent, after COVID-19. According to the World Health Organization (WHO) TB report 2021, 10 million people developed TB in 2020 and 1.5 million died.1 Over the years, the extensive emergence of drug resistance in the causative pathogen, MTB, has been an encumbrance of global commitments to end TB.2,3 The current treatment regimens for TB disease rely on a combination of drugs (isoniazid, rifampicin, ethambutol and pyrazinamide) and are associated with suboptimal efficacy, toxicity, long duration, and poor adherence which may ultimately lead to drug-resistant cases.4−7 Multidrug-resistant (MDR) or extensively drug-resistant (XDR) TB therapy includes much more toxic and expensive drugs and is tainted by a diminished chance of success.8,9 There is an urgent need to develop effective new anti-TB drugs with better efficacy and reduced duration of action, along with improved patient compliance.
Quinoline pharmacophores containing natural and synthetic compounds (Figure 1) have fulfilled the medicinal needs of society for the last five decades. The modification of quinoline by different functional groups has an immense impact on biological activities.10,11 Many quinoline derivatives as antimycobacterial, antimalarial, and anticancer agents have been successfully marketed, the quinoline and quinolone compounds are endowed with a broad spectrum of biological activities such as antituberculosis,12,13 antimicrobial,14,15 anticancer,16 antimalarial,17,18 anti-inflammatory,19 and antiviral.20
Figure 1.
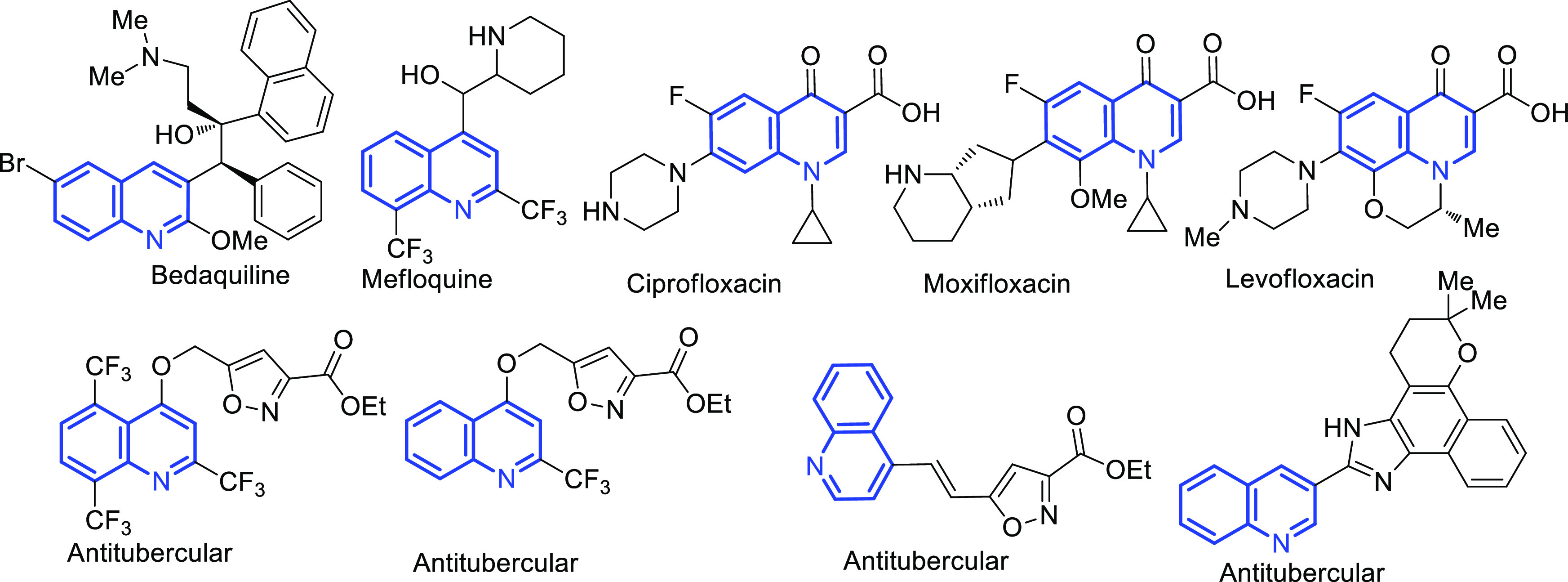
Quinoline pharmacophore containing TB drugs and lead antitubercular molecules.
Oxetane, a four-membered ring containing an oxygen atom, is a key part of the many natural and synthetic bioactive compounds (Figure 2). 3,3-Disubstituted oxetanes are used as the biomimics for the gem-dimethyl groups in medicinal chemistry. The oxetane ring-containing compounds showed a variety of biological activities such as antibacterial,21,22 anticancer,23 antiviral,24,25 and antitumor,26 phosphodiesterase inhibitors,27 Glucokinase activators,28 hypocholesterolemic activity,29 pin kinase inhibitors,30 G-protein coupled receptors,31 protein kinase activity modulators,32 and anti-infective activities.33
Figure 2.
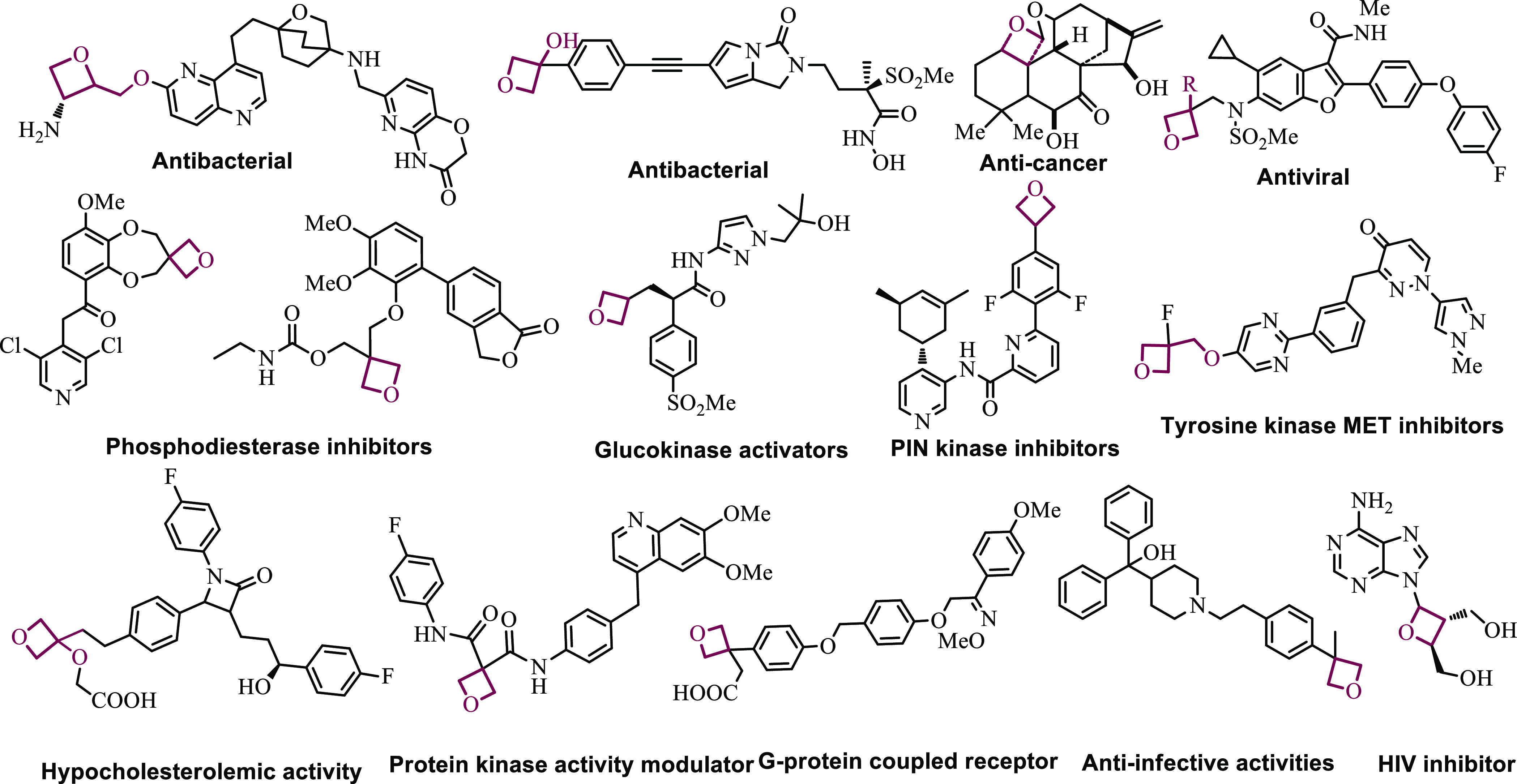
Oxetane nucleus containing bioactive compounds.
The literature reveals that quinoline clubbed with oxygen-containing rings was reported to show promising antitubercular activity,34,35 whereas the quinoline clubbed with an oxetane ring has not been explored for antimicrobial activity. These findings prompted us to substitute the oxygen-containing rings with oxetane rings. In the present work, 3-(2-(3-(substituted benzyloxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline derivatives were synthesized and screened against antimicrobial activity. To learn the plausible mode of action, in silico studies of synthesized derivatives were also performed.
2. Results and Discussion
2.1. Chemistry
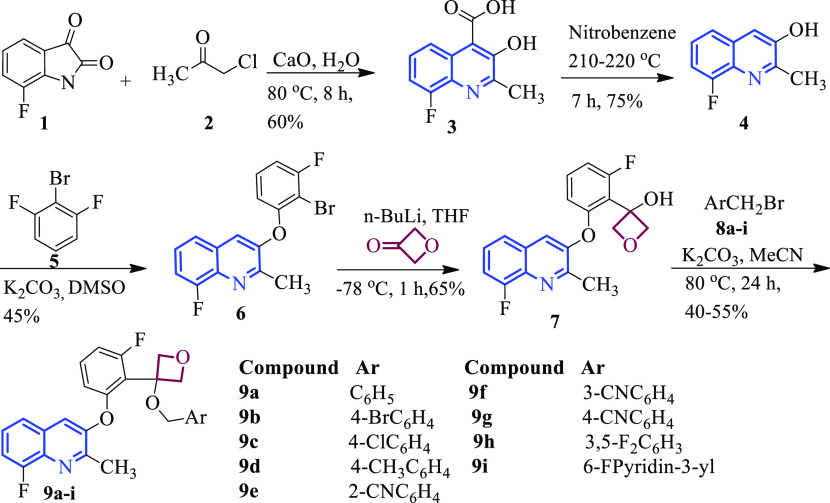
The synthetic route for 3-(2-(3-(substituted benzyloxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline (9a–i) derivatives has been described in Scheme 1. The preparation of 8-fluoro-3-hydroxy-2-methylquinoline-4-carboxylic acid (3) was achieved via the Pfitzinger reaction using 1-chloropropan-2-one (2) and 7-fluoroindoline-2,3-dione (1) in the presence of aqueous calcium oxide at 80 °C.36 Several methods were used for the decarboxylation of the substituted phenyl and quinoline rings. The decarboxylation of 8-fluoro-3-hydroxy-2-methylquinoline-4-carboxylic acid (3) was achieved in nitrobenzene at 210 °C and gave 8-fluoro-2-methylquinolin-3-ol (4).37 The literature revealed the selective aromatic nucleophilic substitution reaction of phenoxy nucleophiles with substituted fluorobenzene gave diphenyl ether formation.38 Compound 4 upon aromatic nucleophilic substitution reaction with 2-bromo-1,3-difluorobenzene (5) gave 3-(2-bromo-3-fluorophenoxy)-8-fluoro-2-methylquinoline (6). The reaction of organolithium or organomagnesium compounds upon nucleophilic addition reaction with oxetan-3-one gave alcohol.39 3-(2-Bromo-3-fluorophenoxy)-8-fluoro-2-methylquinoline (6) upon reaction with n-butyllithium followed by nucleophilic addition reaction with oxetan-3-one gave 3-(2-fluoro-6-((8-fluoro-2-methylquinolin-3-yl)oxy)phenyl)oxetan-3-ol (7). Compound 7 upon nucleophilic substitution reaction40 with substituted benzyl bromide (8a–i) furnished 3-(2-(3-(substituted benzyloxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline (9a–i).
Scheme 1. Synthesis of 3-(2-(3-(Substituted benzyloxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline (9a–i).
The structure of all synthesized 3-(2-(3-(substituted benzyloxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline derivatives (9a–i) was confirmed by spectral analysis. As a representative spectral analysis, the 1H NMR spectrum of compound 8-fluoro-3-(3-fluoro-2-(3-((2-cyanobenzyl)oxy)oxetan-3-yl)phenoxy)-2-methylquinoline 9e revealed a singlet at δ 2.75 integrated for three protons was assigned to the methyl protons of the quinoline C-2 methyl group. A singlet at δ 4.73 integrated for two protons was assigned to methylene protons of the O–CH2–Ar group. Two doublets at δ 5.29 and 5.03 integrated for two protons each were assigned to two methylene protons of the oxetane ring. A doublet at δ 6.64 integrated for one proton was assigned to the ortho proton of the Ph-O-quinolone group. The other protons resonated from δ 7.59 to 6.96 and were assigned quinoline and aromatic ring protons. The 13C NMR spectrum of compound 9e showed a signal at δ 20.82 assigned to methyl carbon was attached to the C-2 position of the quinoline ring. A signal at δ 63.52 was assigned to the methylene carbon of the benzyl group, and a signal at δ 79.10 was assigned to the methylene carbons of the oxetane ring. The quaternary carbon of the oxetane ring showed two signals at δ 80.51 and 80.46 due to characteristic C–F coupling. The aromatic carbons of phenyl and quinoline ring and nitrile carbon have appeared from δ 110.86 to 163.01. The structure of compound 9e was further confirmed by molecular ion peaks (HRMS) at m/z = 459.1533 (M + H)+.
2.2. Biology
2.2.1. Antimicrobial Activity
Synthesized 3-(2-(3-(substituted benzyloxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline (9a–i) derivatives were evaluated for antibacterial activity against Gram-negative strains P. mirabilis (NCIM2388) and E. coli (NCIM 2065) and Gram-positive strains B. subtilis (NCIM2063) and S. albus (NCIM 2178) using the well diffusion method.36,37 Standard drugs streptomycin and DMSO were used as the positive and negative control, respectively. The in vitro antifungal activity was performed against C. albicans (NCIM 3100) and A. niger (ATCC 504) using the well diffusion method.41,42 The antifungal drugs fluconazole and ravuconazole were used as references. All the test solutions were prepared in DMSO at 1000 μM concentrations and the wells were filled with 80 μL (80 μM) of the samples. The results of the antimicrobial activity in the zone of inhibition (mm) are presented in Table S1. All the synthesized compounds were further evaluated for minimum inhibitory concentration (MIC) ranging from 250 to 3.90 μM. The in vitro antimicrobial screening result of MIC in μM is presented in Table 1.
Table 1. Antimicrobial Activity in MIC (μM) of Compounds 9a–i.
| Compd | R | P. mirabilis | E. coli | B. subtilis | S. albus | C. albicans | A. Niger |
|---|---|---|---|---|---|---|---|
| 9a | C6H5 | 31.25 | >250 | 31.5 | 250 | >250 | 31.25 |
| 9b | 4-BrC6H4 | 62.5 | >250 | >250 | 62.5 | 250 | 62.50 |
| 9c | 4-ClC6H4 | 62.5 | >250 | >250 | 250 | >250 | 125 |
| 9d | 4-CH3C6H4 | 62.5 | >250 | >250 | 250 | >250 | 62.50 |
| 9e | 2-CNC6H4 | 31.25 | 125 | >250 | 250 | >250 | 62.50 |
| 9f | 3-CNC6H4 | 15.62 | 250 | 250 | 125 | >250 | 62.50 |
| 9g | 4-CNC6H4 | >250 | >250 | >250 | 250 | 62.50 | |
| 9h | 3,5-F2C6H3 | 31.25 | >250 | 250 | >250 | 250 | 62.50 |
| 9i | 6-FPyridin-3-yl | >250 | >250 | 250 | 250 | 125 | 62.50 |
| Streptomycin | 15.62 | 7.81 | 7.81 | 7.81 | NA | NA | |
| Fluconazole | NAa | NA | NA | NA | 7.81 | 15.62 | |
| Ravuconazole | NA | NA | NA | NA | 15.62 | 31.25 | |
NA = Not applicable.
It was noteworthy that most of the 3-(2-(3-(substituted benzyloxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline (9a–i) derivatives showed good activity against P. mirabilis and A. niger with MIC 15.62–62.5 μM. From the structure–activity relationship (SAR) analysis, it was noted that the unsubstituted, 4-Br, 4-Cl, 4-CH3, or 2-CN 3,5-difluoro substituents on the benzyloxy group showed good activity against P. mirabilis and A. niger.
The SAR analysis revealed that the unsubstituted benzyloxy group at the 3-position of oxetane in compound 3-(2-(3-(benzyloxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline (9a) showed good activity against P. mirabilis, B. subtilis, and A. niger with MIC 31.25 μM. The benzyloxy group was substituted by the 4-bromobenzyloxy in compound 3-(2-(3-((4-bromobenzyl)oxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline 9b which resulted in 2-fold decreased activity against P. mirabilis and A. niger. Compounds 9b showed good activity for P. mirabilis, S. albus, and A. niger with MIC 62.5 μM. The benzyloxy group was substituted by the 4-chlorobenzyloxy in compound 3-(2-(3-((4-chlorobenzyl)oxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline (9c) which showed good activity against P. mirabilis with MIC 62.5 μM and moderate activity against A. niger. The benzyloxy group was substituted by 4-methylbenzyloxy in the compound 8-fluoro-3-(3-fluoro-2-(3-((4-methylbenzyl)oxy)oxetan-3-yl)phenoxy)-2-methylquinoline (9d) which showed good activity against P. mirabilis and A. niger with MIC 62.5 μM.
The benzyloxy group was substituted by the 2-cyanobenzyloxy in the compound 8-fluoro-3-(3-fluoro-2-(3-((2-cyanobenzyl)oxy)oxetan-3-yl)phenoxy)-2-methylquinoline (9e), the activity against P. mirabilis was retained, whereas against A. niger the activity decreased by two-fold. The 2-cyanobenzyloxy group of compound 9e was substituted by 3-cyanobenzyloxy in 8-fluoro-3-(3-fluoro-2-(3-((3-cyanobenzyl)oxy)oxetan-3-yl)phenoxy)-2-methylquinoline (9f), and activity against P. mirabilis increased by two-fold. It showed excellent activity with MIC 15.62 μM, which was comparable with respect to the reference drug streptomycin. Against A. niger compound 9f showed good activity with MIC 62.5 μM. The 2-cyanobenzyloxy group of compound 9e was substituted by 4-cyanobenzyloxy in 8-fluoro-3-(3-fluoro-2-(3-((4-cyanobenzyl)oxy)oxetan-3-yl)phenoxy)-2-methylquinoline (9g), and the activity decreased against P. mirabilis and was retained against A. niger. The benzyloxy group was substituted by the 3,5-difluorobenzyloxy group in compound 3-(2-(3-((3,5-difluorobenzyl)oxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline (9h), which showed good activity against P. mirabilis and A. niger with MIC 31.25 and 62.5 μM, respectively. The benzyloxy group was substituted by 6-fluoropyridin-3-yl-methoxy in 8-fluoro-3-(3-fluoro-2-(3-((6-fluoropyridin-3-yl)methoxy)oxetan-3-yl)phenoxy)-2-methylquinoline (9i), which showed good activity against A. niger with MIC 62.5 μM.
2.2.2. Antitubercular Activity
All newly synthesized 3-(2-(3-(substituted benzyloxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline (9a–i) derivatives were screened for antitubercular activity against Mycobacterium tuberculosis, H37 RV strain (ATCC No-27294), using Microplate Alamar Blue assay (MABA).43,44 The antitubercular drugs pyrazinamide, isoniazid, and rifampicin were used as the positive control. The MIC was defined as the lowest drug concentration which prevented the change of color from blue to pink. The result of the antitubercular activity in Minimum Inhibitory Concentration (MIC) is presented in Table 2.
Table 2. Antitubercular Activity in MIC in μM (μg/mL) and 9a–i.
| Compd | R | M. tuberculosis H37Rv μM (μg/mL) |
|---|---|---|
| 9a | C6H5 | 57.73 (25) |
| 9b | 4-BrC6H4 | 12.23 (6.25) |
| 9c | 4-ClC6H4 | 6.68 (3.12) |
| 9d | 4-CH3C6H4 | 6.98 (3.12) |
| 9e | 2-CNC6H4 | 27.29 (12.5) |
| 9f | 3-CNC6H4 | 6.81 (3.12) |
| 9g | 4-CNC6H4 | 3.49 (1.6) |
| 9h | 3,5-F2C6H3 | 3.41 (1.6) |
| 9i | 6-F-pyridin-3-yl | 3.53 (1.6) |
| pyrazinamide | 25.36 (3.12) | |
| isoniazid | 11.67 (1.6) | |
| rifampicin | 0.97 (0.8) | |
The antitubercular activity result analysis revealed that the 3-(2-(3-(substituted benzyloxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline (9a–i) derivatives showed significant activity against M. tuberculosis H37Rv. Furthermore, the Br, Cl, CH3, CN, and F substituted benzyloxy have an influence on the activity. The structure–activity relationship (SAR) study revealed that the unsubstituted benzyloxy group at the 3-position of oxetane in compounds 3-(2-(3-(benzyloxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline (9a), showed good activity with MIC 57.73 μM, which was 2-fold less with respect to the antitubercular drug pyrazinamide. The benzyloxy group was substituted by 4-bromobenzyloxy in compound 3-(2-(3-((4-bromobenzyl)oxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline 9b, the activity increased by four folds. Compounds 9b showed good activity with MIC 12.23 μM which is two times more potent than the reference drug pyrazinamide and comparable with respect to the drug isoniazid. The benzyloxy group was substituted by 4-chlorobenzyloxy in compound 3-(2-(3-((4-chlorobenzyl)oxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline (9c) the activity increased more than eight folds. Compound 9c showed excellent activity with MIC 6.68 μM which is two times more potent than the reference drug isoniazid. The 4-chlorobenzyloxy group was substituted by 4-methylbenzyloxy in the compound 8-fluoro-3-(3-fluoro-2-(3-((4-methylbenzyl)oxy)oxetan-3-yl)phenoxy)-2-methylquinoline, (9d) the antitubercular activity retained. Compound 9d showed excellent activity with MIC 6.68 μM which is two times more potent than the reference drug isoniazid.
The benzyloxy group was substituted by the 2-cyanobenzyloxy in the compound 8-fluoro-3-(3-fluoro-2-(3-((2-cyanobenzyl)oxy)oxetan-3-yl)phenoxy)-2-methylquinoline, (9e) activity increased by two folds. Compound 9e showed good activity with MIC 27.29 μM, which was comparable activity with respect to the reference drug pyrazinamide. The 2-cyanobenzyloxy group of compound 9e was substituted by 3-cyanobenzyloxy in 8-fluoro-3-(3-fluoro-2-(3-((3-cyanobenzyl)oxy)oxetan-3-yl)phenoxy)-2-methylquinoline, (9f) the activity increased by four folds. Compound 9f showed excellent activity with MIC 6.81 μM, which was 2-fold more potent with respect to the reference drug isoniazid. The 2-cyanobenzyloxy group of compound 9e was substituted by the 4-cyanobenzyloxy in the 8-fluoro-3-(3-fluoro-2-(3-((4-cyanobenzyl)oxy)oxetan-3-yl)phenoxy)-2-methylquinoline, (9g) here the activity increased by eight folds. Compound 9g showed excellent activity with MIC 3.49 μM, which was three times more potent with respect to the reference drug isoniazid. The benzyloxy group was substituted by the 3,5-difluorobenzyloxy group in compound 3-(2-(3-((3,5-difluorobenzyl)oxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline (9h) activity increased significantly. Compound 9h showed excellent activity with MIC 3.49 μM, which was 3-fold more potent respective to the reference drug isoniazid. The benzyloxy group was substituted by 6-fluoropyridin-3-yl-methoxy in 8-fluoro-3-(3-fluoro-2-(3-((6-fluoropyridin-3-yl)methoxy)oxetan-3-yl)phenoxy)-2-methylquinoline (9i) showed excellent activity with MIC 3.53 μM, which was 3-fold more potent with respect to reference drug isoniazid.
It is worth noting that all nine derivatives showed good to excellent antitubercular activity with MIC 3.41–57.73 μM. From the SAR analysis, it is worth mentioning that the substitution of the Br, Cl, CH3, or CN group at the 4-position of the benzyloxy increases the antitubercular activity. Also, the 3-CN or 3,5-difluoro benzyloxy showed excellent activity. It was noted that 4-cyanobenzyloxy at the 3-position of oxetane was found more potent than the 2-cyanobenzyloxy and 3-cyanobenzyloxy groups. The 6-fluoropyridin-3-ylmethoxy at the 3-position of the oxetane showed excellent activity.
2.2.3. Cytotoxicity
Cytotoxicity tests of 3-(2-(3-(substituted benzyloxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline (9a–i) derivatives were performed at 12.5 μg/mL concentration against Vero cell lines by MTT assay (Figure S1). Untreated cells were used as a negative control, and DMSO was used as a positive control for cytotoxicity. It was observed that in all compounds survival is above 50% and 9a, 9b and 9d showed survival above 90% at 12.5 μg/mL concentration.
2.3. In Silico Studies
2.3.1. Molecular Docking
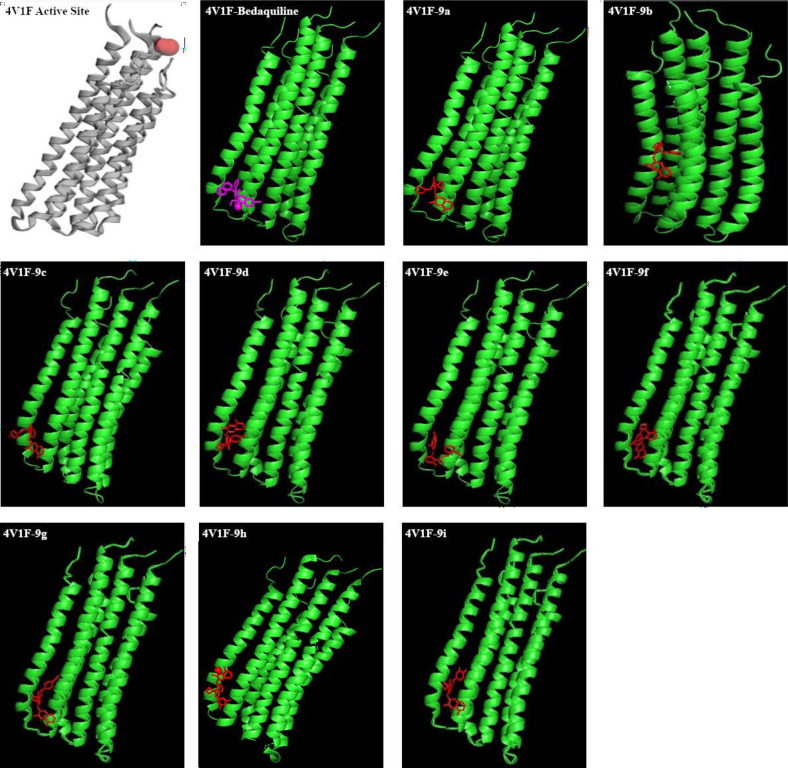
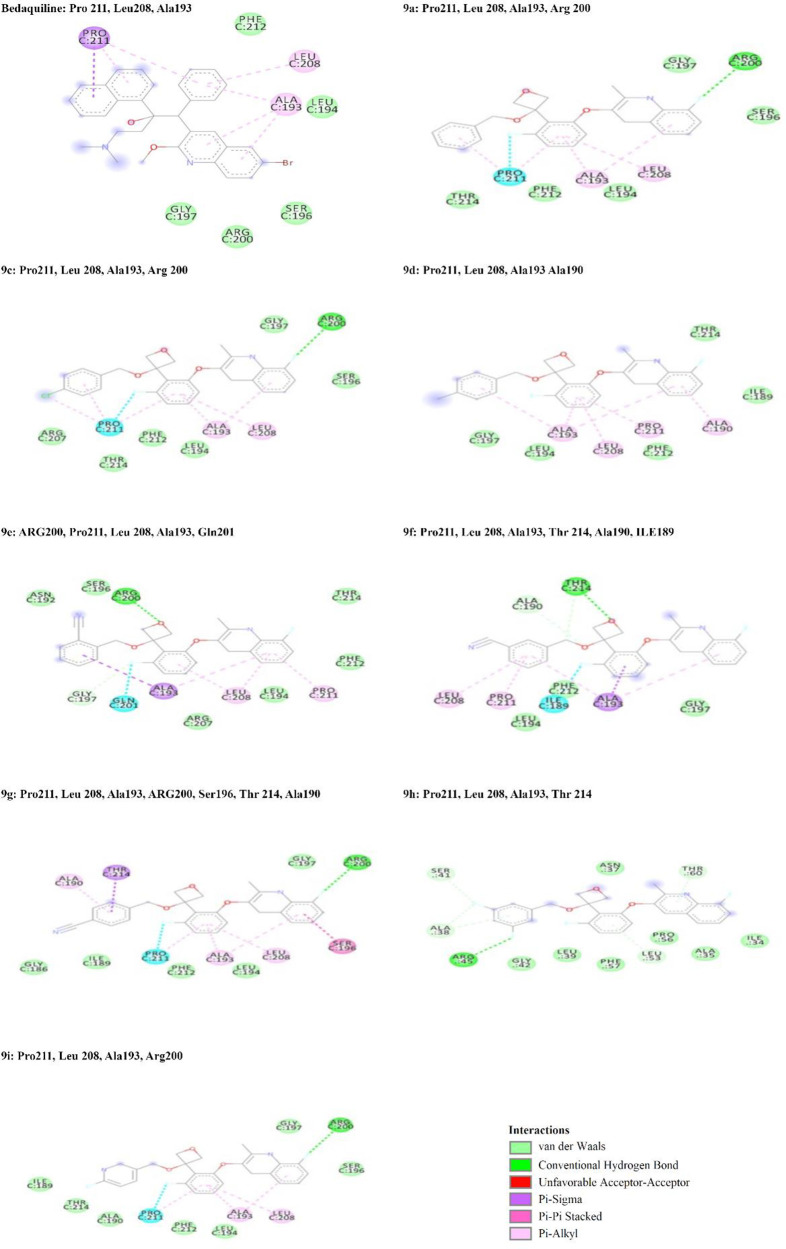
ATP synthase is one of the target proteins of quinoline pharmacophore-containing anti-TB drugs.45,46 3-Fluorophenoxy)-8-fluoro-2-methylquinoline derivatives (9a–i) were studied. All the synthesized compounds 9a–i were docked47,48 against the 3D Crystal structure of ATP synthase (PDB ID: 4V1F). It is clear from Table 3 that all compounds from 9a to 9i are interacting with this protein. Bedaquiline, the ATP synthase inhibitor drug having structural similarity with the synthesized compound, was taken as a reference for molecular docking. The binding energy of the compounds 9a–i is better than that of the bedaquiline. The binding positions of all these compounds are shown in Figure 3, and amino acid interactions are shown in Figure 4. Bedaquiline is interacting with three amino acids Pro C211, LeuC208, and Ala193. It was noticed that all interacting compounds except 9b were sharing three amino acids Pro C211, LeuC208, Ala193 similar to the reference drug bedaquiline. This clearly showed the stable and significant binding of all compounds to ATP synthase indicating their plausible mode of action as ATP synthase inhibitors.
Table 3. Binding Energies (kcal/mol) and Interacting Amino Acids of Compounds 9a–i with ATP Synthase (4V1F).
| Compound | Binding energy (kcal/mol) | Interacting amino acid |
|---|---|---|
| bedaquiline | –6.2 | Pro211, Leu 208, Ala193 |
| 9a | –7.1 | Pro211, Leu 208, Ala193, Arg 200 |
| 9c | –7.3 | Pro211, Leu 208, Ala193, Arg 200 |
| 9d | –6.3 | Pro211, Leu 208, Ala193 Ala190 |
| 9e | –7.8 | ARG200, Pro211, Leu 208, Ala193, ln201 |
| 9f | –6.7 | Pro211, Leu 208, Ala193, Thr 214, Ala190, ILE189 |
| 9g | –7.8 | Pro211, Leu 208, Ala193, ARG200, Se196, Thr 214, Ala190 |
| 9h | –7.5 | Pro211, Leu 208, Ala193 Thr 214 |
| 9i | –7.4 | Pro211, Leu 208, Ala193, Arg200 |
Figure 3.
Molecular docking interactions of reference compound bedaquiline and the synthesized derivatives 9a–i against ATP synthase protein (PDB id 4V1F).
Figure 4.
Binding positions and amino acid interaction against ATP synthase of compounds 9a–i.
2.3.2. ADME
The synthesized 3-(2-(3-(substituted benzyloxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline (9a–i) derivatives were subjected to ADME study for pharmacological analysis. Lipinski’s rule gives us a good approach to predicting drug likeness. Lipinski’s rule of five represents the general predictions such as that the molecular weight is from 160 to 500 Da, the octanol–water partition coefficient (log P) is between −0.4 and +5.6, the molar refractivity is from 40 to 130 cm3, the hydrogen donor is less than 5 atoms, the hydrogen acceptor is less than 10 atoms, and the topological polar surface area is not more than 140 Å. All synthesized compounds (9a–i) are following Lipinski’s rule and therefore have the potential to be developed as lead candidates (Table 4). Positive log p values represent the lipophilicity which will be helpful for GI absorption. Compounds 9a, 9e, 9f, 9g, and 9i are showing high GI absorption. They show overlapping substrate specificity with Pgp and cytochrome P450 (CYP) 3A4. If the bioactivity score of the compound is >0, then it is biologically more active. If the compounds’ bioactivity score values between–0.50 and 0.00 are expected to be moderately active and if the score is <−0.50, it is presumed to be inactive. All these ADME data predict that all compounds have the potential to be developed as lead candidates.
Table 4. ADME Properties of Compounds 9a–i.
| Compd | MW (Da) | No. of H-bond acceptors | No. of H-bond donors | MR cm3 | TPSA (Å) | iLOGP | GI absorption | Pgp substrate | CYP3A4 inhibitor | Bio availability score |
|---|---|---|---|---|---|---|---|---|---|---|
| 9a | 433.45 | 6 | 0 | 116.95 | 40.58 | 3.54 | High | Yes | Yes | 0.55 |
| 9b | 512.34 | 6 | 0 | 124.65 | 40.58 | 4.03 | Low | Yes | Yes | 0.17 |
| 9c | 467.89 | 6 | 0 | 121.96 | 40.58 | 3.94 | Low | Yes | Yes | 0.55 |
| 9d | 447.47 | 6 | 0 | 121.92 | 40.58 | 3.81 | Low | Yes | Yes | 0.55 |
| 9e | 458.46 | 7 | 0 | 121.67 | 64.37 | 3.26 | High | Yes | Yes | 0.55 |
| 9f | 458.46 | 7 | 0 | 121.67 | 64.37 | 3.72 | High | Yes | Yes | 0.55 |
| 9g | 458.46 | 7 | 0 | 121.67 | 64.37 | 3.65 | High | Yes | Yes | 0.55 |
| 9h | 469.43 | 8 | 0 | 116.87 | 40.58 | 3.64 | Low | Yes | Yes | 0.55 |
| 9i | 452.43 | 8 | 0 | 114.71 | 53.47 | 3.46 | High | Yes | Yes | 0.55 |
3. Conclusions
In the present study, a series of 3-(2-(3-(substituted benzyloxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline, (9a–i) derivatives have been synthesized and screened for antimicrobial and antitubercular activities. Seven compounds exhibited good antibacterial activity against P. mirabilis with MIC 62.5–15.62 μM, and eight derivatives showed good antifungal activity against A. niger. Against P. mirabilis, compound 9f showed comparable activity with respect to the drug streptomycin and against A. niger compound 9a showed comparable activity with respect to the drug ravuconazole. All the synthesized derivatives showed good to excellent antitubercular activity against M. tuberculosis, H37 RV with MIC 57.73–3.41 μM. Against M. tuberculosis, H37 RV the compounds 3-(2-(3-((4-bromobenzyl)oxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline (9b), 3-(2-(3-((4-chlorobenzyl)oxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline (9c), 8-fluoro-3-(3-fluoro-2-(3-((4-methylbenzyl)oxy)oxetan-3-yl)phenoxy)-2-methylquinoline (9d), 3-(((3-(2-fluoro-6-((8-fluoro-2-methylquinolin-3-yl)oxy)phenyl)oxetan-3-yl)oxy)methyl)benzonitrile (9f), 4-(((3-(2-fluoro-6-((8-fluoro-2-methylquinolin-3-yl)oxy)phenyl)oxetan-3-yl)oxy)methyl)benzonitrile (9g), 3-(2-(3-((3,5-difluorobenzyl)oxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline (9h), and 8-fluoro-3-(3-fluoro-2-(3-((6-fluoropyridin-3-yl)methoxy)oxetan-3-yl)phenoxy)-2-methylquinoline (9i) showed comparable activity with respect to standard drug isoniazid. From the molecular docking analysis, it was noted that the synthesized oxatenyl-quinoline derivatives showed significant binding to ATP synthase (PDB ID: 4 V1F) and thus confirm the plausible mode of action as ATP synthase inhibitors.
4. Experimental Section
The chemicals and solvents used laboratory grade and were purified as per literature methods. All reactions have been monitored by thin layer chromatography (TLC). TLC was performed on Merck 60 F-254 silica gel plates. 1H NMR and 13C NMR spectra were recorded at either 500 or 400 MHz (1H NMR) and 126 or 100 MHz (13C NMR) on the Bruker spectrometer instruments. The HRMS spectra were recorded on the Bruker Compass Data Analysis 4.2. The column chromatography was performed on silica gel for column chromatography (100–200mesh) which was supplied by Thermo Fisher Scientific India Pvt. Ltd.
4.1. Chemistry
4.1.1. General Procedure for the Synthesis of 8-Fluoro-3-hydroxy-2-methylquinoline-4-carboxylic Acid, 3
To the stirred solution of calcium oxide (67.8 g, 1.212 mmol) in water (1000 mL) was added 7-fluoroindoline-2,3-dione 1 (100 g, 0.60 mmol), and the reaction mixture was stirred at 80 °C for 1 h and cooled to room temperature. Then 1-chloropropan-2-one, 2 (54.6 mL, 0.66 mmol) was added slowly within 15 min. The reaction mixture was heated at 80 °C for 7 h. After completion of the reaction, the reaction mixture was cooled to 10 °C and then acidified with concd HCl. Yellow solid precipitated out, was filtered, and washed with water followed by washing with n-hexane and diethyl ether to give the 8-fluoro-3-hydroxy-2-methylquinoline-4-carboxylic acid, 3 (80 g, 60%), as a yellowish solid. Yield: 60%; 1H NMR (500 MHz, DMSO-d6) δ 8.95 (d, J = 8.4 Hz, 2H), 7.37–7.32 (m, 1H), 7.15–7.10 (m, 1H), 2.53 (s, 3H); LCMS (m/z): 221.9 (M + H)+.
4.1.2. General Procedure for the Synthesis of 8-Fluoro-2-methyl-quinolin-3-ol, 4
A solution of 8-fluoro-3-hydroxy-2-methylquinoline-4-carboxylic acid, 3 (100 g, 452.48 mmol), in nitrobenzene (800 mL) was stirred at 210–220 °C for 7 h. After completion of the reaction (TLC) reaction mixture was cooled to room temperature and filtered. The solid obtained was washed with n-hexane followed by water and dried under vacuum. This crude compound was washed with acetone to give 8-fluoro-2-methyl-quinolin-3-ol (60 g, 75%) as a brown solid. Yield: 75%; 1HNMR (400 MHz, DMSO-d6): δ 10.59 (s, 1H), 7.55 (d, J = 8.4 Hz, 1H), 7.45 (s, 1H), 7.34–7.40 (m, 1H,), 7.21–7.26 (m, 1H), 2.54 (s, 3H). LCMS (m/z): 178.0 [M + H]+.
4.1.3. General Procedure for the Synthesis of 3-(2-Bromo-3-fluorophenoxy)-8-fluoro-2-methylquinoline, 6
To a solution of 8-fluoro-2-methyl-quinolin-3-ol, 4 (25 g, 0.14 mol) and potassium carbonate (23.39 g, 0.17 mol), in dry DMSO (250 mL) 2-bromo-1,3-difluoro-benzene (47 g, 0.243 mol) was added at room temperature. The reaction mixture was heated in an oil bath at 150 °C for 7 h. After the completion of the reaction (TLC), the reaction mixture was cooled at room temperature and filtered. Water (800 mL) was added to the filtrate and extracted with ethyl acetate (4 × 250 mL). The organic layer was dried over anhydrous sodium sulfate, filtered and then concentrated under a rotary evaporator. The crude product was then purified by column chromatography (100–200 mesh silica) using ethyl acetate:hexane as eluent obtained an off-white solid of 3-(2-bromo-3-fluorophenoxy)-8-fluoro-2-methylquinoline, 6 (22 g, 45%). Yield: 45%; 1H NMR (500 MHz, CDCl3) δ 7.40–7.37 (m, 2H), 7.35–7.28 (m, 2H), 7.26 (d, J = 1.4 Hz, 1H), 7.06 (td, J = 8.3, 1.3 Hz, 1H), 6.84 (dt, J = 8.3, 1.3 Hz, 1H), 2.82 (s, 3H); 13C NMR (126 MHz, CDCl3) δ 161.70, 159.72, 158.68, 156.65, 153.91, 153.75, 153.74, 150.21, 134.46, 134.37, 129.75, 129.73, 129.12, 129.04, 126.50, 126.44, 122.37, 122.33, 118.40, 118.38, 116.21, 116.19, 112.83, 112.65, 112.39, 112.24, 103.47, 103.29, 20.77; LC-MS (m/z): 349.5 [M-H]+.
4.1.4. Synthesis of 3-(2-fluoro-6-((8-fluoro-2-methylquinolin-3-yl)oxy)phenyl)oxetan-3-ol, 7
The solution of 3-(2-bromo-3-fluoro-phenoxy)-8-fluoro-2-methyl-quinoline, 6 (11.0 g, 31.42 mmol) in dry THF (110 mL) under nitrogen atmosphere was cooled to −78 °C (dry ice bath) and n-BuLi (15.08 mL, 37.7 mmol, 1.2 equiv) was added slowly. The resulting solution was aged for 15 min at −78 °C and then 3-oxetanone (1.93 mL, 33 mmol, 1.05 equiv) was added. The reaction mixture was allowed to cool at 0 °C for 30 min and quenched with saturated NH4Cl. The mixture was diluted with water, extracted with EtOAc and the organic layer was then washed with brine, dried over Na2SO4, filtrated and concentrated on a rotary evaporator. The crude product was then purified by flash chromatography (100–200 silica gel) in 40% ethyl acetate in hexane as eluent gave 3-(2-fluoro-6-((8-fluoro-2-methylquinolin-3-yl)oxy)phenyl)oxetan-3-ol, 7 as pale yellow solid (7 g, 65%). Yield: 65%; 1H NMR (500 MHz, DMSO-d6) δ 7.77 (d, J = 1.0 Hz, 1H), 7.67 (dd, J = 7.9, 1.5 Hz, 1H), 7.53–7.43 (m, 2H), 7.39 (td, J = 8.3, 6.7 Hz, 1H), 7.10 (ddd, J = 10.0, 8.4, 0.8 Hz, 1H), 6.82 (d, J = 8.3 Hz, 1H), 6.50 (s, 1H), 5.16–5.08 (m, 2H), 4.63 (d, J = 8.1 Hz, 2H), 2.66 (s, 3H); 13C NMR (126 MHz, DMSO-d6) δ 167.02, 165.04, 163.24, 161.21, 159.34, 159.27, 158.55, 155.04, 139.13, 139.03, 134.84, 134.76, 134.63, 131.16, 127.36, 126.31, 126.17, 124.94, 124.92, 119.00, 117.08, 116.93, 116.62, 116.44, 88.76, 88.72, 77.88, 25.51; LC-MS (m/z): 344.3 [M – H]+
4.1.5. Synthesis of 3-(2-(3-(Benzyloxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline, 9a
To a solution 3-(2-fluoro-6-((8-fluoro-2-methylquinolin-3-yl)oxy)phenyl)oxetan-3-ol, 7 (5 mmol), potassium carbonate (10 mmol), and tetrabutylammonium bromide (1 mmol) in dry acetonitrile (25 mL) was added benzyl bromide (6 mmol), and the reaction mixture was refluxed for 24 h. After completion of the reaction (TLC), the solvent was distilled under a vacuum, and the residue was dissolved in water and extracted with ethyl acetate. The organic layer was washed with brine and dried over anhydrous sodium sulfate, filtered, and concentrated on a rotary evaporator. The crude product was purified by column chromatography (100–200 mesh silica) using 20% ethyl acetate in hexane as eluent to give 3-(2-(3-(benzyloxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline, 9a, yield 55%. Compounds 9b–i were synthesized by using a similar experimental protocol.
3-(2-(3-(Benzyloxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline, 9a
Off-white solid; yield: 55%; mp: 136–140 °C; 1H NMR (500 MHz, CDCl3) δ 7.41–7.36 (m, 2H), 7.32 (dd, J = 10.0, 6.9 Hz, 3H), 7.26 (s, 1H), 7.22 (s, 4H), 6.97 (t, J = 9.1 Hz, 1H), 6.63 (d, J = 8.3 Hz, 1H), 5.26 (d, J = 7.9 Hz, 2H), 5.01 (d, J = 7.6 Hz, 2H), 4.54 (s, 2H), 2.74 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 162.96, 160.98, 158.65, 156.61, 155.53, 155.47, 153.92, 149.80, 137.83, 134.80, 134.71, 130.88, 130.79, 129.72, 129.70, 128.29, 127.55, 127.32, 126.59, 126.53, 122.42, 122.38, 120.58, 120.56, 117.74, 117.61, 113.88, 113.85, 112.68, 112.52, 111.65, 111.47, 80.70, 80.65, 78.68, 66.01, 20.74. HRMS (ESI, m/z): calculated for C26H22F2NO3, [M + H]+, 434.1562 found 434.1522.
3-(2-(3-((4-Bromobenzyl)oxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline, 9b
Off-white solid; yield: 50%; mp: 110–112 °C; 1H NMR (500 MHz, CDCl3) δ 7.32–7.18 (m, 7H), 6.99 (d, J = 8.4 Hz, 2H), 6.95–6.86 (m, 1H), 6.58 (d, J = 8.3 Hz, 1H), 5.18 (d, J = 7.2 Hz, 2H), 4.90 (d, J = 8.7 Hz, 2H), 4.41 (s, 2H), 2.66 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 162.91, 160.93, 158.63, 156.59, 155.36, 155.29, 153.69, 149.87, 136.83, 134.72, 134.63, 131.34, 131.03, 130.95, 129.65, 129.64, 128.82, 126.67, 126.61, 122.34, 122.31, 121.37, 120.21, 120.19, 114.21, 114.19, 112.70, 112.55, 111.78, 111.60, 80.59, 80.54, 78.77, 65.22, 20.72. HRMS (ESI, m/z): calculated for C26H21BrF2NO3, [M + H]+, 512.0673found 512.0693.
3-(2-(3-((4-Chlorobenzyl)oxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline, 9c
Pale yellow solid; yield: 50%; mp: 122–126 °C; 1H NMR (500 MHz, CDCl3) δ 7.41–7.36 (m, 2H), 7.35–7.29 (m, 3H), 7.23 (d, J = 8.9 Hz, 1H), 7.17 (dt, J = 7.9, 1.6 Hz, 1H), 7.14 (t, J = 7.6 Hz, 1H), 7.09 (d, J = 7.3 Hz, 1H), 7.00–6.94 (m, 1H), 6.62 (d, J = 8.3 Hz, 1H), 5.26 (d, J = 7.2 Hz, 2H), 4.99 (d, J = 8.8 Hz, 2H), 4.52 (s, 2H), 2.74 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 162.92, 160.94, 158.65, 156.61, 155.52, 155.45, 153.84, 149.66, 139.92, 134.85, 134.76, 134.20, 131.05, 130.96, 129.68, 129.67, 129.53, 127.63, 127.26, 126.67, 126.61, 125.18, 122.41, 122.37, 120.66, 120.64, 117.34, 117.21, 113.80, 113.78, 112.75, 112.60, 111.63, 111.44, 80.58, 80.54, 78.82, 65.23, 20.75. HRMS (ESI, m/z): calculated for C26H21ClF2NO3, [M + H]+, 468.1178 found 468.1199.
8-Fluoro-3-(3-fluoro-2-(3-((4-methylbenzyl)oxy)oxetan-3-yl)phenoxy)-2-methylquinoline, 9d
White solid; yield: 50%; mp: 160–162 °C; 1H NMR (500 MHz, CDCl3) δ 7.41–7.36 (m, 2H), 7.34–7.28 (m, 3H), 7.10 (d, J = 8.0 Hz, 2H), 7.02 (d, J = 7.9 Hz, 2H), 6.99–6.95 (m, 1H), 6.63 (d, J = 8.3 Hz, 1H), 5.29–5.22 (m, 2H), 5.00 (d, J = 8.7 Hz, 2H), 4.49 (s, 2H), 2.73 (s, 3H), 2.29 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 162.95, 160.97, 158.65, 156.61, 155.53, 155.46, 153.94, 149.85, 137.26, 134.76, 130.81, 130.73, 129.73, 129.30, 129.24, 128.98, 127.48, 126.53, 126.46, 122.42, 122.39, 120.55, 120.53, 117.88, 117.74, 113.92, 112.63, 112.47, 111.66, 111.48, 80.74, 80.69, 78.59, 65.93, 21.11, 20.73. HRMS (ESI, m/z): calculated for C27H24F2NO3, [M + H]+, 448.1724 found 448.1744.
8-Fluoro-3-(3-fluoro-2-(3-((2-cyanobenzyl)oxy)oxetan-3-yl)phenoxy)-2-methylquinoline, 9e
White solid; yield: 45%; mp: 154–156 °C; 1H NMR (500 MHz, CDCl3) δ 7.59–7.55 (m, 3H), 7.47 (td, J = 7.8, 1.1 Hz, 1H), 7.40–7.30 (m, 5H), 7.01–6.96 (m, 1H), 6.64 (d, J = 8.4 Hz, 1H), 5.29 (d, J = 8.1 Hz, 2H), 5.03 (d, J = 8.9 Hz, 2H), 4.73 (s, 2H), 2.75 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 163.01, 161.03, 158.61, 156.57, 155.62, 155.56, 153.92, 149.51, 141.27, 134.91, 134.81, 132.92, 132.44, 131.28, 131.19, 129.79, 129.77, 128.40, 127.99, 126.59, 126.53, 122.57, 122.54, 121.10, 121.07, 117.13, 116.54, 116.40, 113.58, 113.55, 112.74, 112.59, 111.73, 111.55, 110.86, 80.51, 80.46, 79.10, 63.52, 20.82. HRMS (ESI, m/z): calculated for C27H21F2N2O3, [M + H]+, 459.1520 found 459.1533.
8-Fluoro-3-(3-fluoro-2-(3-((3-cyanobenzyl)oxy)oxetan-3-yl)phenoxy)-2-methylquinoline, 9f
White solid; yield: 55%; mp: 150–152 °C; 1H NMR (500 MHz, CDCl3) δ 7.56–7.52 (m, 1H), 7.51–7.44 (m, 2H), 7.43–7.37 (m, 2H), 7.33 (tdd, J = 7.6, 5.3, 3.9 Hz, 4H), 6.98 (ddd, J = 9.5, 8.5, 0.8 Hz, 1H), 6.64 (d, J = 8.3 Hz, 1H), 5.26 (d, J = 7.3 Hz, 2H), 4.98 (d, J = 8.9 Hz, 2H), 4.58 (s, 2H), 2.74 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 162.90, 160.92, 158.65, 156.61, 155.41, 155.34, 153.69, 149.62, 139.46, 134.86, 134.77, 131.30, 131.23, 131.14, 130.48, 129.63, 129.61, 129.07, 126.82, 126.75, 122.34, 122.30, 120.49, 120.47, 118.62, 117.09, 116.96, 113.93, 113.90, 112.85, 112.70, 112.39, 111.74, 111.56, 80.47, 80.43, 78.99, 64.80, 20.75. HRMS (ESI, m/z): calculated for C27H21F2N2O3, [M + H]+, 459.1520 found 459.1533.
8-Fluoro-3-(3-fluoro-2-(3-((4-cyanobenzyl)oxy)oxetan-3-yl)phenoxy)-2-methylquinoline, 9g
Off-white solid; yield: 50%; mp: 118–120 °C; 1H NMR (500 MHz, CDCl3) δ 7.47 (d, J = 8.3 Hz, 2H), 7.41–7.32 (m, 3H), 7.32–7.28 (m, 3H), 7.24 (s, 1H), 7.02–6.96 (m, 1H), 6.68 (d, J = 8.3 Hz, 1H), 5.26 (d, J = 7.3 Hz, 2H), 4.98 (d, J = 8.9 Hz, 2H), 4.59 (s, 2H), 2.74 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 162.91, 160.92, 158.63, 156.59, 155.20, 155.14, 153.55, 149.91, 143.27, 132.02, 131.26, 131.17, 129.60, 127.24, 126.78, 126.72, 122.23, 122.19, 119.87, 118.71, 117.34, 117.21, 114.44, 112.79, 112.64, 111.91, 111.73, 111.20, 80.44, 80.40, 78.96, 64.91, 20.71. HRMS (ESI, m/z): calculated for C27H21F2N2O3, [M + H]+, 459.1520 found 459.1522.
3-(2-(3-((3,5-Difluorobenzyl)oxy)oxetan-3-yl)-3-fluorophenoxy)-8-fluoro-2-methylquinoline, 9h
Off-white solid; yield: 40%; mp: 136–138 °C; 1H NMR (500 MHz, CDCl3) δ 7.43–7.37 (m, 2H), 7.36–7.29 (m, 3H), 6.99–6.93 (m, 1H), 6.77 (d, J = 5.9 Hz, 2H), 6.62 (d, J = 8.2 Hz, 2H), 5.25 (d, J = 7.3 Hz, 2H), 4.97 (d, J = 8.9 Hz, 2H), 4.54 (s, 2H), 2.74 (s, 3H). HRMS (ESI, m/z): calculated for C26H20F4NO3, [M + H]+, 470.1379 found 470.1385..
8-Fluoro-3-(3-fluoro-2-(3-((6-fluoropyridin-3-yl)methoxy)oxetan-3-yl)phenoxy)-2-methylquinoline, 9i
White solid; yield: 45%; mp: 166–178 °C; 1H NMR (500 MHz, CDCl3) δ 7.54 (d, J = 8.8 Hz, 1H), 7.48–7.27 (m, 6H), 6.98–6.93 (m, 1H), 6.66 (t, J = 9.1 Hz, 1H), 5.89 (d, J = 8.7 Hz, 1H), 5.29 (t, J = 8.3 Hz, 2H), 4.82 (t, J = 9.0 Hz, 2H), 2.76 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 162.26, 160.28, 158.66, 156.62, 154.86, 154.79, 153.87, 153.86, 153.82, 149.72, 134.87, 134.77, 130.64, 130.55, 130.10, 130.00, 129.78, 129.77, 129.73, 129.72, 126.74, 126.71, 126.68, 126.64, 122.59, 122.55, 122.47, 122.43, 120.79, 120.76, 120.08, 119.94, 115.36, 115.34, 113.74, 113.71, 113.02, 112.89, 112.79, 112.63, 111.93, 111.76, 83.69, 83.65, 74.26, 20.74. HRMS (ESI, m/z): calculated for C25H20F3N2O3, [M + H]+, 453.1426 found 453.1135.
4.2. Biological Evaluation
4.2.1. Antibacterial Activity
The in vitro antibacterial screening of the synthesized derivatives was done by the well diffusion method41,42 against the standard strains of Gram-negative bacteria E. coli and P. mirabilis and Gram-positive bacteria B. subtilis and S. albus. All the strains were procured from the National Collection of Industrial Microorganisms (NCIM) NCL, Pune, India. The Minimum Inhibitory Concentration (MIC) was evaluated at 250, 125, 62.5, 31.25, 15.62, 7.81, and 3.90 μM concentrations. The lowest concentration showing no growth was considered as minimum inhibition concentration (MIC).
4.2.2. Antifungal Activity
The in vitro antifungal activity of the synthesized derivatives was done by the well diffusion method41,42 against Candida albicans (NCIM 3100) and Aspergillus niger (NCIM 504). The fungal strains were obtained from NCIM, NCL, Pune, India.
The microdilution susceptibility test in Sabouraud Liquid Medium (Oxoid) was used for the determination of minimum inhibition concentration (MIC). The stock solutions of the test compounds, fluconazole and ravuconazole were prepared in the DMSO at a concentration of 1000 μM. 2-fold serial dilutions of the test compounds solutions were prepared using a broth. The final concentration of the solutions was 500, 250, 125, 62.5, 31.25, 15.62, 7.81, and 3.90 μM. The tubes were inoculated with the test organisms, grown in Potato-Dextrose broth. The tubes were kept for incubation for 48–72 h at 30 °C. The lowest concentration showing no growth was considered as minimum inhibition concentration (MIC). All experiments were carried out in triplicates.
4.2.3. Antitubercular Assay
The antitubercular activity of compounds was assessed against the M. tuberculosis H37 RV (ATCC No. 27294) vaccine strain using the microplate Almar Blue assay (MABA).43,44 This methodology is nontoxic and uses a thermally stable reagent and shows a good correlation with proportional and BACTEC radiometric methods. The 96 wells plate received 100 μL of the Middlebrook 7H9 broth and serial dilution of compounds was made directly on the plate. The final drug concentrations tested were 100 to 0.2 μg/mL. The plates were covered and sealed with parafilm and incubated at 37 °C for 5 days. 25 μL of freshly prepared 1:1 mixture of Almar Blue reagent and 10% tween 80 was added to the plate and incubated for 24 h. The blue color in the well was interpreted as no bacterial growth, and the pink color was scored as growth. Further, the MIC was defined as the lowest drug concentration which prevented the color change from blue to pink.
4.2.4. Cytotoxicity Assay
The MTT (3-(4,5-dimethylthiazole-2yl)-2,5-biphenyltetrazolium bromide) assay was used to determine the cytotoxicity of all the compounds upon using Vero (Monkey kidney) cells. The cells were harvested with trypsin and seeded to the final concentration of 10,000 cells/per well in a 96-well plate. 12.5 μg/mL compound concentrations were added to each well. After 24 h of incubation, 40 μL of MTT solution (2.5 mg/mL) was added to each well, and the plate was further incubated for 4 h, allowing viable cells to convert the yellow-colored MTT into dark-blue formazan crystals. 100 μL of dimethyl sulfoxide (DMSO) was added to each well to dissolve the formazan crystals. The absorbance was recorded at a 570 nm wavelength (multimode plate reader, Hedix, Germany).
4.2.5. Molecular Docking
Proteins/Macromolecules
3D Crystal structure of ATP synthase (PDB ID: 4 V1F) was downloaded from the protein data bank (https://www.rcsb.org/), in .pdb format.47 The complexes bound to the protein molecule were removed. The protein structure was prepared by removing water molecules using pymol version 2.3.3.
Preparation of Ligand
The 3D structures of the ligands were drawn, and the energy was minimized via an open babel, an open-source platform.
Molecular Docking
The ligand and protein optimization were done using the PyMOL version 2.3.3 [The PyMOL Molecular Graphics System, Version 2.0 Schrödinger, LLC]. For the ligand optimization, the geometry of ligands was cleaned, whereas, for protein, the water was removed. The docking was performed by using PyRx 0.8.48 The docking analyses were performed using both the Pymol as well as the Biovia Discovery Studio 4.5.49
Pharmacological Analysis
SwissADME was done to predict pharmacological properties and bioactivities of these compounds, the in silico studies were done by using the SwissADME online tool developed by Daina et al. 2017.50
Acknowledgments
A.D.S. expresses his gratefulness to the CSIR-SRF fellowship for the financial support (File No. 08/319(000 4)/2017-EMR-1). The authors would like to acknowledge S. P. Mandali Pune and Late Dr. T. R. Ingale’s family for providing infrastructure facilities. The authors would like to thank CIF-SPPU, Pune, for spectral analysis. S. P. Mandali’s Bhide Foundation Pune has been acknowledged for lending support to their biological activities and https://biorender.com/ for drawing figures.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c06245.
Biological activity and spectral data of synthesized compounds (PDF)
Author Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Abhijit Shinde, Sandip Ugale, Yogesh Nandurkar, and Abhijit Chavan. Molecular docking was performed by Manisha Modak. The final version of the manuscript was written by Manisha Modak and Pravin Mhaske.
The authors declare no competing financial interest.
Supplementary Material
References
- Global tuberculosis report 2021. World Health Organization: Geneva, 2021. License: CC BY-NC-SA 3.0 IGO. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2021.
- Mabhula A.; Singh V. Drug-resistance in Mycobacterium tuberculosis: Where we stand. Medchemcomm. 2019, 10, 1342. 10.1039/C9MD00057G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh B. A.; Bhat B. A.; Mehraj U.; Mir W.; Hamadani S.; Mir M. A. Development of new therapeutics to meet the current challenge of drug resistant tuberculosis. Curr. Pharm. Biotechnol. 2021, 22, 480. 10.2174/1389201021666200628021702. [DOI] [PubMed] [Google Scholar]
- Nguyen L. Antibiotic resistance mechanisms in M. tuberculosis: an update. Arch. Toxicol. 2016, 90, 1585. 10.1007/s00204-016-1727-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A.; De Rosa M.; Singla N.; Singh G.; Barnwal R. P.; Pandey A. Tuberculosis: An overview of the immunogenic response, disease progression, and medicinal chemistry efforts in the last decade toward the development of potential drugs for extensively drug-resistant tuberculosis strains. J. Med. Chem. 2021, 64, 4359. 10.1021/acs.jmedchem.0c01833. [DOI] [PubMed] [Google Scholar]
- Tiberi S.; du Plessis N.; Walzl G.; Vjecha M. J.; Rao M.; Ntoumi F.; Mfinanga S.; Kapata N.; Mwaba P.; McHugh T. D.; Ippolito G.; Migliori G. B.; Maeurer M. J.; Zumla A. Tuberculosis: progress and advances in development of new drugs, treatment regimens, and host-directed therapies. Lancet Infect. Dis. 2018, 18, e183. 10.1016/S1473-3099(18)30110-5. [DOI] [PubMed] [Google Scholar]
- Bald D.; Villellas C.; Lu P.; Koul A. Targeting energy metabolism in mycobacterium tuberculosis, a new paradigm in antimycobacterial drug discovery. mBio 2017, 8, 1. 10.1128/mBio.00272-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global tuberculosis report 2020. World Health Organization: Geneva, 2020. License: CC BY-NC-SA 3.0 IGO. https://www.who.int/publications/i/item/9789240013131.
- . Meeting Report of the WHO expert consultation on the definition of extensively drug-resistant tuberculosis. World Health Organization: Geneva, 2020. https://www.who.int/publications/i/item/meeting-report-of-the-who-expert-consultation-on-the-definition-of-extensively-drug-resistant-tuberculosis.
- Nayak N.; Ramprasad J.; Dalimba U. New INH-pyrazole analogs: Design, synthesis and evaluation of antitubercular and antibacterial activity. Bioorg. Med. Chem. Lett. 2015, 25, 5540. 10.1016/j.bmcl.2015.10.057. [DOI] [PubMed] [Google Scholar]
- a Rakesh; Bruhn D. F.; Scherman M. S.; Singh A. P.; Yang L.; Liu J.; Lenaerts A. J.; Lee R. E. Synthesis and evaluation of pretomanid (PA-824) oxazolidinone hybrids. Bioorg. Med. Chem. Lett. 2016, 26, 388. 10.1016/j.bmcl.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Cohen J. Infectious disease. Approval of novel TB drug celebrated--with restraint. Science 2013, 339, 130. 10.1126/science.339.6116.130. [DOI] [PubMed] [Google Scholar]
- Keri R. S.; Patil S. A. Quinoline: a promising antitubercular target. Biomed Pharmacother 2014, 68, 1161. 10.1016/j.biopha.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Gonçalves R. S. B.; Kaiser C. R.; Lourenço M. C. S.; de Souza M. V. N.; Wardell J. L.; Wardell S. M. S. V.; da Silva A. D. Synthesis and antitubercular activity of new mefloquine-oxazolidine derivatives. Eur. J. Med. Chem. 2010, 45, 6095. 10.1016/j.ejmech.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Marella A.; Tanwar O.; Saha R.; Ali M. R.; Srivastava S.; Akhter M.; Shaquiquzzaman M.; Alam M. Quinoline: A versatile heterocyclic. Saudi Pharmaceutical Journal 2013, 21, 1. 10.1016/j.jsps.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y.; Zhang S.; Xu Z.; Lv Z.; Liu M.; Feng L. 4-Quinolone hybrids and their antibacterial activities. Eur. J. Med. Chem. 2017, 141, 335. 10.1016/j.ejmech.2017.09.050. [DOI] [PubMed] [Google Scholar]
- Bollu R.; Banu S.; Kasaboina S.; Bantu R.; Nagarapu L.; Polepalli S.; Jain N. Potential anti-proliferative agents from 1,4-benzoxazinone-quinazolin-4(3H)-one templates. Bioorg. Med. Chem. Lett. 2017, 27, 5481. 10.1016/j.bmcl.2017.10.044. [DOI] [PubMed] [Google Scholar]
- Kalaria P. N.; Karad S. C.; Raval D. K. A review on diverse heterocyclic compounds as the privileged scaffolds in antimalarial drug discovery. Eur. J. Med. Chem. 2018, 158, 917. 10.1016/j.ejmech.2018.08.040. [DOI] [PubMed] [Google Scholar]
- Hu Y.; Gao C.; Zhang S.; Xu L.; Xu Z.; Feng L. Quinoline hybrids and their antiplasmodial and antimalarial activities. Eur. J. Med. Chem. 2017, 139, 22. 10.1016/j.ejmech.2017.07.061. [DOI] [PubMed] [Google Scholar]
- Kumar Gupta S.; Mishra A. Synthesis, characterization & screening for anti-inflammatory & analgesic activity of quinoline derivatives bearing azetidinones scaffolds. Antiinflamm Antiallergy Agents Med. Chem. 2016, 15, 31. 10.2174/1871523015666160210124545. [DOI] [PubMed] [Google Scholar]
- De la Guardia C.; Stephens D. E.; Dang H. T.; Quijada M.; Larionov O. V.; Lleonart R. Antiviral activity of novel quinoline derivatives against dengue virus serotype-2. Molecules 2018, 23, 672. 10.3390/molecules23030672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y.; Jr. Kaelin D. E.; Singh S. B. (Kyorin Pharmaceutical Co., Ltd.; Merck Sharp & Dohme Corp. ) Bridged bicyclic compounds as antibacterial agents and their preparation and use for the treatment of bacterial infections. International Patent WO 2013003383 A1, 2013.
- Chapoux G.; Gauvin J.-C.; Panchaud P.; Specklin J.-L.; Surivet J.-P.; Schmitt C. (Actelion Pharmaceuticals Ltd. ) Preparation of dihydropyrrolo[1,2-c]imidazol-3-one derivatives useful as antibacterial agents. International Patent WO 2015132228 A1, 2015.
- Young J.; Czako B.; Altman M.; Guerin D.; Martinez M.; Rivkin A.; Wilson K.; Lipford K.; White C.; Surdi L.; et al. Merck Sharp & Dohme Corp. Pyridazinones as tyrosine kinase inhibitors and their preparation and use in the treatment of cancer. International Patent WO 2011084402 A1, 2011.
- Labadie S. S.; Lin C. J. J.; Talamas F. X.; Weikert R. J. (F. Hoffmann-La Roche AG ) Benzofuran-3-carboxamide derivatives and their pharmaceutical compositions as antiviral agents useful in the treatment of hepatitis c infection and preparation thereof. International Patent WO 2009101022 A1, 2009.
- AIciro C.; Steadman V. A.; Pettit S. N.; Poullennec K. G.; Lazarides L.; Dean D. K.; Dunbar N. A.; Highton A. J.; Keats A. J.; Siegel D. S.. et al. (Gilead Sciences, Inc.; Selcia Ltd. ) Preparation of macrocyclic peptides as inhibitors of flaviviridae viruses. International Patent WO 2013185103 A1, 2013.
- Michels P. C.; Khmelnitsky Y. L.; Gutterman J.; Haridas V.; Mozhaev V. M. (Research Development Foundation ) Preparation of avicin d derivatives as antitumor agents. International Patent WO 2013126730 A1, 2013.
- Nielsen S. F.; Horneman A. M.; Lau J. F.; Larsen J. C. H. (Leo Pharma A/S ) Biaryl derivatives as phosphodiesterase inhibitors and their preparation and use in the treatment of diseases. International Patent WO 2011134468 A1, 2011.
- Berthel S. J.; Kester R. F.; Murphy D. E.; Prins T. J.; Ruebsam F.; Sarabu R.; Tran C. V.; Vourloumis D. (Hoffmann-La Roche Inc. ) Preparation of pyrazole derivatives as glucokinase activators. U.S. Patent US 20080021032 A1, 2008.
- Fessard T.; Li D.-B.; Barbaras D.; Wolfrum S.; Carreira E. (Lipideon Biotechnology AG ) Preparation of azetidinone-containing compounds for pharmaceutical hypocholesterolemic compositions. International Patent WO 2010100255 A1, 2010.
- Burger M.; Nishiguchi G.; Rico A.; Simmons R. L.; Jr. Tamez V.; Tanner H.; Wan L. (Novartis AG ) N-(3-Pyridyl)biarylamides as kinase inhibitors and their preparation. International Patent WO 2014033631 A1, 2014.
- Sharma R.; Halder S.; Kumar S.; Mascarenhas M. (Piramal Enterprises Ltd. ) Substituted oxetane derivatives as gpr40 agonists and their preparation and use for the treatment of gpr40- mediated diseases. International Patent WO 2015028960 A1, 2015.
- Chen X.-T. (New Hope R & D Bioscience, Inc. ) Preparation of oxetane dicarboxamide derivatives for use as protein kinase activity modulators. International Patent WO 2013032797 A2, 2013.
- Dunman P. M.; Krysan D. J.; Flaherty D. P. (University of Rochester; University of Kansas ) Substituted piperidine derivatives and their preparation. methods and compositions for treating infection. International Patent WO 2014052836 A2, 2014.
- Lilienkampf A.; Mao J.; Wan B.; Wang Y.; Franzblau S. G.; Kozikowski A. P. Structure-activity relationships for a series of quinoline-based compounds active against replicating and nonreplicating Mycobacterium tuberculosis. J. Med. Chem. 2009, 52, 2109. 10.1021/jm900003c. [DOI] [PubMed] [Google Scholar]
- Lilienkampf A.; Pieroni M.; Franzblau S. G.; Bishai W. R.; Kozikowski A. P. Derivatives of 3-isoxazolecarboxylic acid esters - a potent and selective compound class against replicating and nonreplicating Mycobacterium tuberculosis. Current Topics in Medicinal Chemistry 2012, 12, 729. 10.2174/156802612799984544. [DOI] [PubMed] [Google Scholar]
- a Shvekhgeimer M. G. A. The Pfitzinger Reaction. Chem. Heterocycl. Compd. 2004, 40 (3), 257. 10.1023/B:COHC.0000028623.41308.e5. [DOI] [Google Scholar]; b Fang X.-N.; Li J.; Yi X.-G.; Luo Q.; Chen J.-Y.; Li Y.-X. Preparation, structure, photoluminescent and semiconductive properties, and theoretical calculation of a mononuclear nickel complex with 3-hydroxy-2- methylquinoline-4-carboxylato ligand. Acta Chim. Slov. 2019, 66, 414. 10.17344/acsi.2018.4885. [DOI] [PubMed] [Google Scholar]
- Li Q.; Woods K. W.; Zhu G.; Fischer J. P.; Gong J.; Li T.; Gandhi V.; Thomas S. A.; Packard G. K.; Song X.; Abrams J. N.; Diebold R.; Dinges J.; Hutchins C.; Stoll V. S.; Rosenberg S. H.; Giranda V. L. US patent US2003/187026, A1, 2003.
- Rajan R.; Rendler S.; Weiss M.; Bou H. F.; Trah S.; Quaranta L. International Patent WO2017/178408, A1, 2017.
- a Croft R. A.; Dubois M. A. J.; Boddy A. J.; Denis C.; Lazaridou A.; VoisinChiret A. S.; Bureau R.; Choi C.; Mousseau J. J.; Bull J. A. Catalytic Friedel-Crafts reactions on saturated heterocycles and small rings for sp3-sp2 coupling of medicinally relevant fragments. Eur. J. Org. Chem. 2019, 2019, 5385. 10.1002/ejoc.201900498. [DOI] [Google Scholar]; b Sharma R.; Halder S.; Kumar S. International Patent WO2014/170842, A2, 2014.
- Hett E. C.; Xu H.; Geoghegan K. F.; Gopalsamy A.; Kyne R. E.; Menard C. A.; Narayanan A.; Parikh M. D.; Liu S.; Roberts L.; Robinson R. P.; Tones M. A.; Jones L. H. Rational Targeting of Active-Site Tyrosine Residues Using Sulfonyl Fluoride Probes. ACS Chem. Biol. 2015, 10 (4), 1094. 10.1021/cb5009475. [DOI] [PubMed] [Google Scholar]
- Wayne P. A. Approv. Stand. 2002, M100-S12. [Google Scholar]
- Joshi A. B.; Mali M.; Kulkarni V. Phytochemical screening and antimicrobial activity of stevia rebaudiana leaves. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4 (10), 678. [Google Scholar]
- Lourenço M. C. S.; de Souza M. V. N.; Pinheiro A. C.; Ferreira M. de L.; Gonçalves R. S. B.; Nogueira T. C. M.; Peralta M. A. Evaluation of anti-Tubercular activity of nicotinic and isoniazid analogs. ARKIVOC 2007, xv, 181. 10.3998/ark.5550190.0008.f18. [DOI] [Google Scholar]
- Franzblau S. G.; Witzig R. S.; McLaughlin J. C.; Torres P.; Madico G.; Hernandez A.; Degnan M. T.; Cook M. B.; Quenzer V. K.; Ferguson R. M.; Gilman R. H. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J. Clin. Microbiol. 1998, 36, 362. 10.1128/JCM.36.2.362-366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andries K.; Verhasselt P.; Guillemont J.; Göhlmann H. W.; Neefs J. M.; Winkler H.; Van Gestel J.; Timmerman P.; Zhu M.; Lee E.; Williams P.; de Chaffoy D.; Huitric E.; Hoffner S.; Cambau E.; Truffot-Pernot C.; Lounis N.; Jarlier V. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science. 2005, 307, 223. 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- Palomino J. C.; Martin A. TMC207 becomes bedaquiline, a new anti-TB drug. Future Microbiol. 2013, 8, 1071. 10.2217/fmb.13.85. [DOI] [PubMed] [Google Scholar]
- Berman H. M.; Westbrook J.; Feng Z.; Gilliland G.; Bhat T. N.; Weissig H.; Shindyalov I. N.; Bourne P. E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235. 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott O.; Olson A. J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455. 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biovia D. S.Discovery Studio Visualizer, San Diego, 2019.
- Daina A.; Michielin O.; Zoete V. Swiss ADME: A free web tool to evaluate pharmacokinetics, drug likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.